Significance Statement
This systematic review summarized evidence from randomized controlled trials concerning benefits and risks of noncalcium-based phosphate-lowering treatment in nondialysis CKD compared with placebo, calcium-based phosphate binders, and no study medication. Noncalcium-based phosphate-lowering therapy reduced serum phosphate and urinary phosphate excretion, but with unclear effect on clinical outcomes and intermediate cardiovascular end points. There was an associated increase risk of constipation and vascular calcification with noncalcium-based phosphate binders compared with placebo. This study highlights the need for more adequately powered trials to evaluate the benefits and risks of phosphate-lowering therapy on patient-centered outcomes in people with CKD.
Keywords: phosphate binders, phosphate, mineral metabolism, cardiovascular disease
Abstract
Background
Benefits of phosphate-lowering interventions on clinical outcomes in patients with CKD are unclear; systematic reviews have predominantly involved patients on dialysis. This study aimed to summarize evidence from randomized controlled trials (RCTs) concerning benefits and risks of noncalcium-based phosphate-lowering treatment in nondialysis CKD.
Methods
We conducted a systematic review and meta-analyses of RCTs involving noncalcium-based phosphate-lowering therapy compared with placebo, calcium-based binders, or no study medication, in adults with CKD not on dialysis or post-transplant. RCTs had ≥3 months follow-up and outcomes included biomarkers of mineral metabolism, cardiovascular parameters, and adverse events. Outcomes were meta-analyzed using the Sidik–Jonkman method for random effects. Unstandardized mean differences were used as effect sizes for continuous outcomes with common measurement units and Hedge’s g standardized mean differences (SMD) otherwise. Odds ratios were used for binary outcomes. Cochrane risk of bias and GRADE assessment determined the certainty of evidence.
Results
In total, 20 trials involving 2498 participants (median sample size 120, median follow-up 9 months) were eligible for inclusion. Overall, risk of bias was low. Compared with placebo, noncalcium-based phosphate binders reduced serum phosphate (12 trials, weighted mean difference -0.37; 95% CI, -0.58 to -0.15 mg/dl, low certainty evidence) and urinary phosphate excretion (eight trials, SMD -0.61; 95% CI, -0.90 to -0.31, low certainty evidence), but resulted in increased constipation (nine trials, log odds ratio [OR] 0.93; 95% CI, 0.02 to 1.83, low certainty evidence) and greater vascular calcification score (three trials, SMD, 0.47; 95% CI, 0.17 to 0.77, very low certainty evidence). Data for effects of phosphate-lowering therapy on cardiovascular events (log OR, 0.51; 95% CI, -0.51 to 1.17) and death were scant.
Conclusions
Noncalcium-based phosphate-lowering therapy reduced serum phosphate and urinary phosphate excretion, but there was an unclear effect on clinical outcomes and intermediate cardiovascular end points. Adequately powered RCTs are required to evaluate benefits and risks of phosphate-lowering therapy on patient-centered outcomes.
Cardiovascular disease is a significant cause of mortality and morbidity in people with CKD, and the increased burden of cardiovascular disease in CKD is, in part, attributed to pathophysiological changes in bone mineralization.1 CKD is characterized by abnormalities in mineral metabolism, termed CKD–Mineral and Bone Disorder (CKD-MBD), especially involving derangements in phosphate homeostasis.2
Elevated serum phosphate levels are risk factors for cardiovascular disease, and are associated with vascular calcification, arterial stiffness, left ventricular hypertrophy and all- cause mortality in CKD.3–5 Observational studies in healthy subjects or those with cardiovascular disease, but normal kidney function, have also reported an independent association between serum phosphate levels within, and above, the upper normal range and increased risk of incident cardiovascular disease, all-cause mortality, and progression of kidney impairment.6–9
Positive phosphate balance contributes to rising levels of fibroblast growth factor-23 (FGF23).10 FGF23 is the most potent hormone regulating phosphate homeostasis, increasing urinary excretion of phosphate by inhibiting phosphate reabsorption in the renal proximal tubule. Thus, serum phosphate levels remain normal, regardless of dietary variability of phosphate intake, until the late stages of CKD. In contrast, serum FGF23 levels are increased even with modest degrees of kidney impairment, before serum phosphate levels rise.11 Increased serum levels of FGF23, like serum phosphate, are associated with increased risks of cardiovascular events and mortality in patients with CKD.12–14
Although observational studies have shown associations between higher serum phosphate and FGF23 levels and worse clinical outcomes, causality has never been demonstrated. Serum phosphate is not a reliable measure of overall phosphate balance or risk of soft tissue calcification in patients with CKD, and benefits of phosphate lowering are yet to be clearly demonstrated. Therapeutic strategies aimed at normalizing phosphate balance and reducing serum phosphate and FGF23 levels in patients with CKD might be beneficial. However, evidence for the benefit of such strategies on cardiovascular disease, bone, or CKD progression remains elusive.
Treatment of hyperphosphatemia in patients with CKD involves dietary phosphate restriction and phosphate-lowering medication, predominantly intestinal phosphate binders. However, the efficacy of these medications has predominantly been focused on improvement in biochemical parameters, and most randomized controlled trials (RCTs) have involved patients with kidney failure on dialysis. The recently updated Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Guidelines15 highlighted the lack of trial data in the nondialysis CKD population demonstrating that lowering serum phosphate improves patient-centered outcomes, and questioned the efficacy and safety of phosphate binders in this population.
Systematic reviews and meta-analyses evaluating the effects of CKD-MBD medications, such as phosphate binders, have been reported but have predominantly involved studies of patients on dialysis and usually compared calcium-based to noncalcium-based phosphate binders.16–21 More recent RCTs of noncalcium-based phosphate-lowering therapy have involved patients with mild-to-moderate or advanced (nondialysis) CKD, including several placebo-controlled studies.22–27 Therefore, we conducted this systematic review of RCTs to evaluate benefits and harms of noncalcium-based phosphate-lowering therapy compared with placebo, no study medication or calcium-based phosphate binders in patients with nondialysis CKD with respect to biochemical and clinical outcomes.
Methods
This systematic review has been conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.28 The protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO 2020 CRD42020149203) (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=149203).
Search Strategy, Study Selection, and Data Extraction
Studies were eligible for inclusion if they (1) were RCTs; (2) included adults aged >18 years with CKD (eGFR ≤60 ml/min per 1.73 m2 and/or proteinuria for a period of ≥3 months) and not on dialysis; (3) compared interventions of noncalcium-based phosphate-lowering therapy with placebo, no study medication or other phosphate-lowering therapy including calcium-based phosphate binders; (4) followed participants for ≥3 months postrandomization; (5) included ≥20 participants; and (6) reported any of the following outcomes: differences in biochemical parameters (serum phosphate, calcium, parathyroid hormone [PTH], FGF23, urinary phosphate); differences in surrogate cardiovascular parameters (pulse wave velocity [PWV], vascular calcification); major adverse cardiovascular events (defined as nonfatal stroke, nonfatal myocardial infarction, and cardiovascular death); gastrointestinal adverse events (constipation, diarrhea, nausea, vomiting); or mortality. Trials involving pediatric patients, patients with kidney failure requiring dialysis, or kidney transplant recipients were excluded.
Noncalcium-based phosphate-lowering therapy in eligible studies included sevelamer (carbonate or hydrochloride), lanthanum carbonate, bixalomer, aluminum hydroxide, nicotinamide, colestilan, and iron-based phosphate binders (ferric citrate and sucroferric oxyhydroxide). Trials evaluating other interventions to lower phosphate, such as dietary phosphate restriction alone, were not eligible. Meta-analysis of RCTs was primarily divided into two parts: (1) comparisons between noncalcium-based phosphate binders and placebo; and (2) comparisons between noncalcium phosphate binders and combined placebo or no study medication. In addition, we also assessed (3) comparisons between noncalcium-based phosphate-lowering therapy (binders and nicotinamide) and placebo, and (4) outcomes for studies involving noncalcium-based phosphate binders versus calcium-based binders.
Potentially relevant studies were identified initially in January 2020 using highly sensitive electronic searches of Medline, EMBASE, and the Cochrane Central Register of Controlled Trials databases with English language restriction (different to the registered protocol, which stated no restriction to search language, see Supplemental Table 1 for complete search strategy). Systematic reviews were screened to identify any studies not retrieved by the electronic database search. The literature search was updated in November 2020. If a trial included ≥2 groups of the same experimental intervention, data from these groups were combined so each trial arm was included only once in the respective analyses. If multiple secondary publications of the same dataset were identified, the most complete data were used. Missing, incomplete, or unpublished data from the clinical trials were requested from the investigators.
The following data were extracted using a standardized form: patient demographic details, study design and conduct, outcomes (baseline and end-of-study values of serum phosphate, serum calcium, PTH, FGF23, urinary phosphate, PWV, and vascular calcification scores), and adverse events and other clinical outcomes. The certainty of evidence was assessed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach, with each outcome being estimated as high, moderate, low, or very low level of evidence.29 The methodological quality of each included study was also assessed using the risk of bias assessment tool developed by the Cochrane Bias Methods Group.30 The following six items were assessed: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants, investigators, and outcome assessors; (4) incomplete outcome data; (5) selective outcome reporting; and (6) any other bias (e.g., insufficient rationale, study design). Study selection, data extraction, risk of bias assessment and GRADE assessment were all carried out independently by two authors (N.M.L. and N.D.T.). Disagreements were resolved via consultation with two other authors (S.V.B. and C.M.H.).
Outcomes Assessed
The primary outcome assessed was the difference in serum phosphate between groups at the end of study follow-up. This measure was chosen as the primary outcome as the vast majority of RCTs assessed biochemical markers (predominantly either serum phosphate or FGF23) as the primary end point, and because most studies were of short duration (≤12 months), such that only limited data evaluating cardiovascular outcomes and mortality were available. Secondary outcomes assessed included differences in urinary phosphate, serum calcium, PTH, FGF23, eGFR, PWV, vascular calcification scores, cardiovascular events, all-cause mortality, and adverse events, including gastrointestinal side effects.
Statistical Analysis
For each study, the unstandardized mean difference in treatment effect on continuous outcomes at final measurement between treatment groups was calculated together with the 95% confidence interval (95% CI). Where measurement units varied across studies, Hedge’s g standardized mean differences (SMDs) were calculated. Mean differences in treatment effects across all studies were summarized as weighted mean differences (WMD) or SMD and 95% CI. For dichotomous outcomes, the results were expressed as log odds ratios (OR) with 95% CI. Treatment effect estimates were obtained by random-effects models using the Sidik–Jonkman method.31 Heterogeneity across the studies was estimated using the Cochran's Q and I2 statistics.32 I2 values of 25%, 50%, and 75% corresponded to low, moderate, and high levels of heterogeneity, respectively. Metaregression was conducted on the primary outcome and urinary phosphate to investigate whether the following variables were a source of statistical heterogeneity: study size and duration of follow-up. Publication bias was assessed using Egger’s test for funnel plot asymmetry.33 All analyses were conducted using the meta package in Stata (version 16.1, StataCorp, College Station, Texas).
Results
Study Selection and Description
Our search strategy identified 2301 studies, with 745 duplicates (Figure 1). Of these, 20 trials involving 2498 participants (median sample size 120, range 32–278 patients; median follow-up 9 months, range 3–36 months) were included in the meta-analyses (Table 1). In total, 12 trials enrolled participants with CKD stages 3–4 only,22–27,34–39 whereas eight trials enrolled participants with CKD stages 3–5.40–47 One of these enrolled only participants with eGFR ≤20 ml/min per 1.73m2.47 All trials except two42,46 included participants with baseline mean serum phosphate levels within the normal serum phosphate range (<4.9 mg/dl), so the vast majority of participants included in the systematic review were normophosphatemic. One trial excluded participants with diabetes mellitus,24 and one excluded participants with cardiovascular disease.40 Median age was 63 years (range 54–69 years). One study, an RCT evaluating the effects of sevelamer versus calcium carbonate on advanced glycation end-products in 117 patients with diabetes and stage 2–4 CKD, was excluded from the meta-analyses because there were no end-of-study measurements reported.48
Figure 1.
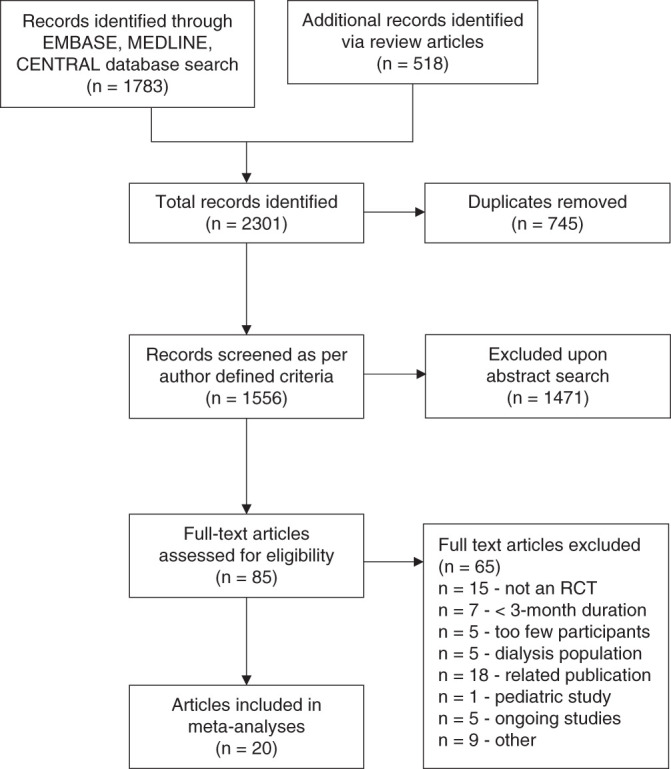
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing selection of studies.
Table 1.
Summary of studies included in the meta-analysis
| Study | Inclusion Criteria | n | Intervention | Control | Mean Age (yrs) | Primary Outcome | Baseline Kidney Function | Mean Baseline Serum Phosphate (mg/dl) | Follow- Up (mos) | Industry-sponsored Funding |
|---|---|---|---|---|---|---|---|---|---|---|
| Russo 200740 | CKD Stage 3–5; without cardiovascular disease | 90 | Low phosphate diet versus sevelamer | Calcium carbonate | 54 | Coronary artery calcification | eGFR 26.2–33.4 ml/min per 1.73 m2 | 3.9–4.6 | 24 | Nil |
| Block 2012 (PNT)22 | eGFR 20–40ml/m in per 1.73m2; serum phosphate >3.5 mg/dl, <6.0 mg/dl | 148 | Lanthanum carbonate versus calcium versus sevelamer | Placebo | 68 | Serum phosphate | eGFR 32 ml/min per 1.73 m2 | 4.2 | 9 | Genzyme, Davita, Fresenius, Novartis |
| Di Iorio 201234 | CKD Stage 3–4 | 239 | Sevelamer | Calcium carbonate | 57.9 | All-cause Mortality | CrCl 32.7 ml/min | 4.8 | 36 | Nil |
| Chue 2013 (CRIB- PHOS)24 | CKD Stage 3; serum phosphate 2.5– 5.6 mg/dl | 120 | Sevelamer carbonate | Placebo | 55 | Left ventricular mass (MRI) | eGFR 50 ml/min per 1.73 m2 | 3.2 | 10 | Genzyme |
| Isakova 201335 | CKD Stage 3–4; serum phosphate 2.5– 4.6 mg/dl | 39 | Low phosphate diet (900 mg)/placebo versus normal diet/lanthanum versus low phosphate diet/lanthanum | Placebo/normal diet | 55.4 | Serum FGF23 | eGFR 37.8 ml/min per 1.73 m2 | 3.6 | 3 | Shire |
| Seifert 201323 | CKD Stage 3 | 38 | Lanthanum carbonate | Placebo | 61.5 | Serum phosphate | CrCl 47 ml/min 1.73 m2 | 3.4 | 12 | Shire |
| Soriano 201341 | CKD Stage 4–5; serum phosphate > 4 mg/dl | 32 | Lanthanum, carbonate | Calcium carbonate | 60.4 | Serum FGF23 | eGFR 16.5 ml/min per 1.73 m2 | 4.95 | 4 | Nil |
| Lemos 201336 | CKD Stage 3–5 | 117 | Sevelamer hydrochloride versus rosuvastatin | Standard of care | 56.4 | Coronary artery calcification | eGFR 35.8 ml/min per 1.73 m2 | 3.8 | 24 | Genzyme |
| Yokoyama 201442 | CKD Stage 3–5; serum phosphate ≥5.0-<8.0 mg/dl | 90 | Ferric citrate | Placebo | 65.1 | Serum phosphate | eGFR 9.21 ml/min per 1.73 m2 | 5.63 | 3 | Japan Tobacco |
| Ureña- Torres 201437 | CKD Stage 3; serum phosphate 0.808–1.55 mmol/L) | 35 | Lanthanum carbonate | Placebo | 67 | Serum intact FGF23 | eGFR 44.4 ml/min per 1.73 m2 | NR | 3 | Shire |
| Block 201543 | CKD Stage 3–5; serum phosphate >4.0– 6.0 mg/dl | 149 | Ferric citrate | Placebo | 65 | Serum phosphate and transferrin saturations | eGFR 24.3 ml/min per 1.73 m2 | 4.6 | 3 | Keryx Biopharmaceuticals |
| Akizawa 201646 | CKD Stage 3–5; serum phosphate >4.6 mg/dl | 163 | Bixalomer | Placebo | 64.2 | Serum phosphate | eGFR 10.1 ml/min per 1.73 m2 | 5.28 | 3 | Astellas |
| Fishbane 201747 | CKD Stage 3–5; serum phosphate >3.5 mg/dl | 232 | Ferric citrate | Placebo | 65.4 | Hemoglobin | eGFR 28.6 ml/min per 1.73 m2 | 4.2 | 4 | Keryx Biopharmaceuticals |
| Liabeuf 201738 | CKD Stage 3–4b; serum phosphate >3.1 mg/dl | 78 | Sevelamer carbonate | Placebo | 63 | Serum c- terminal FGF23 | eGFR 27 ml/min per 1.73 m2 | 3.7 | 3 | Sanofi/Genzyme |
| Kovesdy 201825 | CKD Stage 3–4; serum phosphate >4.6 mg/dl | 120 | Lanthanum carbonate | Calcium acetate | 66.1 | Biochemical outcomes (serum phosphate, calcium, PTH) | eGFR 32 ml/min per 1.73 m2 | 3.8 | 12 | Shire |
| Riccio 201844 | CKD Stage 3–5 | 69 | Sevelamer hydrochloride | Placebo | Between 54.7–56.9 | P-cresol levels | eGFR 38.3–39.1 ml/min per 1.73 m2 |
4.3–4.4 | 3 | Nil |
| Block 201945 | eGFR ≤20 ml/min per 1.73 m2 | 203 | Ferric citrate | Standard of care | Between 61.1–63.1 | Biochemical outcomes (serum phosphate, FGF23, | eGFR between 14.2–14.5 ml/min per 1.73 m2 | 4.4–4.5 | 9 | Keryx Biopharmaceuticals |
| PTH) and hemoglobin | ||||||||||
| Ix 201926 | eGFR 20–40 ml/min per 1.73m2; serum phosphate ≥2.8 mg/dl | 205 | Nicotinamide versus lanthanum carbonate | Placebo | 69 | Serum phosphate and FGF23 | eGFR 32 ml/min per 1.73 m2 | 3.7 | 12 | Medications provided by Shire and Endurance |
| Ruggiero 201939 | eGFR >15ml/min per 1.73m2; serum phosphate 2.5–5.5 mg/dl |
53 | Sevelamer carbonate | Standard of care | 55 | 24-hour proteinuria | eGFR 49 ml/min per 1.73 m2 | 3.8 | 3 | Sanofi |
| Toussaint 202027 | CKD Stage 3–4b; serum phosphate ≥ 3.10 mg/dl | 278 | Lanthanum carbonate | Placebo | 63.1 | Carotid- femoral PWV | eGFR 21.8 ml/min per 1.73 m2 | 3.87 | 24 | Shire |
CrCl, creatinine clearance; MRI, magnetic resonance imaging.
By design, noncalcium-based phosphate-lowering therapy was the interventional agent in all trials. In total, 13 trials were placebo controlled22–24,26,27,35,37,38,42–44,46,47 and four trials involved calcium-based phosphate binders as the comparator.25,34,40,41 There was no study medication in the control arm of three trials.36,39,45 One of the placebo-controlled trials involved sevelamer, calcium, and lanthanum carbonate groups as phosphate-lowering interventions in a four-arm study design.22 Otherwise, six trials involved lanthanum carbonate,23,25,27,35,37,41 seven trials involved sevelamer,24,34,36,38,39,40,44, four trials involved ferric citrate,42,43,45,47 one trial involved bixalomer,46 and one trial involved nicotinamide (and a lanthanum carbonate arm in a placebo-controlled factorial study design).26 This latter trial was the only eligible study that involved a phosphate-lowering medication (nicotinamide) that was not a phosphate binder. We did not analyze the combined arm in this trial (nicotinamide plus lanthanum) as part of the meta-analyses. Three trials had an additional third intervention: low dietary phosphate in two trials35,40 and rosuvastatin in one trial.36
There were no studies eligible for the meta-analyses in the nondialysis CKD population which involved colestilan, aluminum hydroxide, or sucroferric oxyhydroxide. One RCT involving calcium-based phosphate binders compared with placebo in patients with nondialysis CKD was excluded from the systematic review.49 In regard to clinical outcomes, all-cause mortality was the primary end-point in one trial,34 left ventricular mass in one trial,24 coronary artery calcification in two studies36,40 and PWV in one trial.27 All other trials involved biochemical or hematologic parameters as the primary end points.
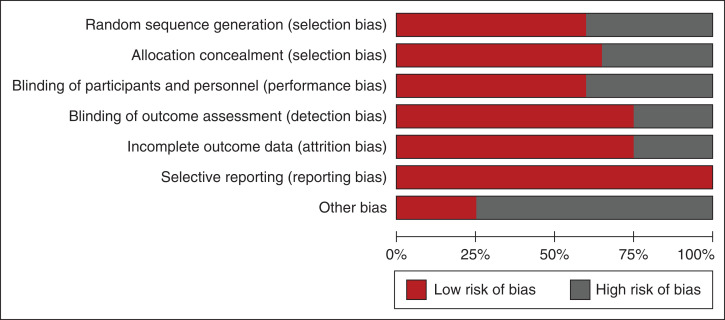
Risk of Bias and GRADE Assessment
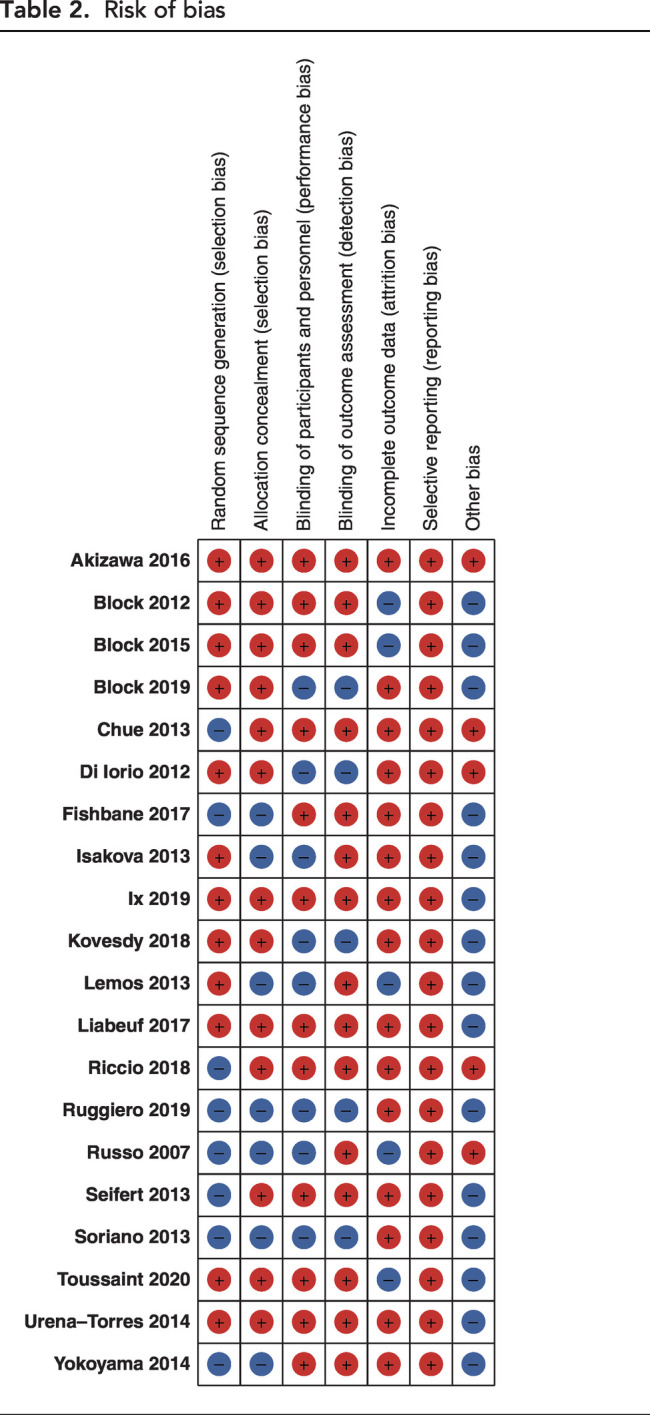
Figure 2 and Table 2 summarize the risk of bias assessment. Random-sequence generation and allocation concealment were reported with low risks of bias in 61% and 68% of trials, respectively. Blinding of participants and investigators to the allocated intervention and blinding to outcomes were reported, with low risks of bias in 61% and 75% of trials, respectively. The majority of analyzes were on the basis of low certainty evidence, primarily in the context of risk of bias, inconsistency of results, and imprecision of reporting (Table 3, Supplemental Tables 2–8). Imprecision grading was on the basis of small event numbers and wide confidence in respective analyzes.
Figure 2.
Risk of bias assessment of the included studies according to the Cochrane Collaboration tool.
Table 2.
Risk of bias
|
Table 3.
Summary of GRADE findings: Noncalcium-based phosphate binders compared with placebo
| Outcomes | Illustrative Comparative Outcomes | Relative Effect (95% Cl) | Number of Participants (studies) | Certainty of Evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Effect of non calcium-based phosphate binders | Risk with placeboa | |||||
| Serum phosphate (mg/dl) (3–36 months) | The measure of mean serum phosphate was -0.37 mg/dl lower than the placebo group | The mean serum phosphate was 4.28 mg/dl | −0.58 to -0.15 | 1280 (12) | ⊕⊕OO Due to risk of bias and inconsistency |
|
| Urinary phosphate excretion (SMD) (3–24 months) | A medium effect on lowering urinary phosphate with the use of phosphate binders (Hedges g=0.61) | The mean urinary phosphate was 130.52 standard units | −0.90 to -0.31 | 702 (8) | ⊕⊕OO Due to risk of bias and inconsistency |
Different units of urinary phosphate excretion including ratios added to inconsistency of results |
| Serum PTH (pg/dl) (3–24 months) | The measure of mean serum PTH was unchanged | The mean serum PTH was 432.5 pg/dl | −18.79 to 8.92 | 1080 (10) | ⊕⊕ΟΟ Due to risk of bias and inconsistency of results |
Some inconsistency in results regarding effect on PTH. Heterogeneity of studies (I2=70%) |
| Pulse wave velocity (m/s) (10–24 months) | The PWV was unchanged | The mean PWV was 10.4 m/s | −0.46 to 1.05 | 333 (3) | ⊕OOO Due to risk of bias, imprecision and inconsistency |
Three studies only able to be analyzed, with two sets of mean values identical (likely due to transformation of median to mean for analysis) |
| Vascular calcification (Standard units) (9–24 months) | A low effect of increasing vascular calcification was noted with the use of phosphate binders (Hedge’s g=0.41) | High background risk of vascular calcification in CKD depending on stage (30%–70% of CKD population has defined vascular calcification by stage 3–4 CKD) | 0.17 to 0.77 | 184 (3) | ⊕ΟΟΟ Due to risk of bias, imprecision and inconsistency |
Statistically significant (P<0.01)/ Not likely to be of clinical significance. Three studies only able to be analyzed. |
| Mortality (log OR) (3–24 months) | There was no significant difference in mortality rate between the two groups (log OR -0.51) | Risk with placebo 2/100 person years64 | −2.12–1.11 | 1479 (8) | ⊕OOO Due to risk of bias, imprecision and inconsistency |
Large confidence intervals leading to imprecision. Studies often not powered or long enough follow-up for consideration of mortality |
| Cardiovascular events (log OR) (3–24 mos) | There was no significant difference in cardiovascular events between the two groups (log OR 0.31) | Not estimable | −0.55–1.17 | 873 (7) | ⊕OOO Due to risk of bias, imprecision and inconsistency |
Challenge due to differing follow-up depending on studies |
Calculations for overall weighted means for serum and urinary phosphate, PTH and PWV– sum of weighted placebo means across studies for each variable.
Serum and Urinary Phosphate
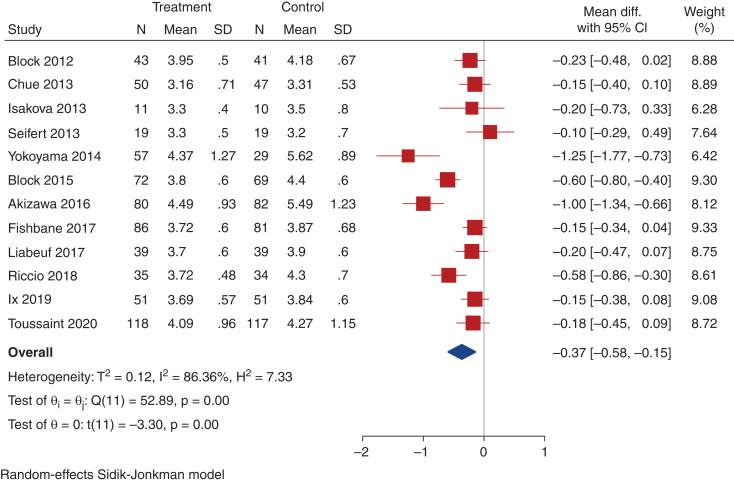
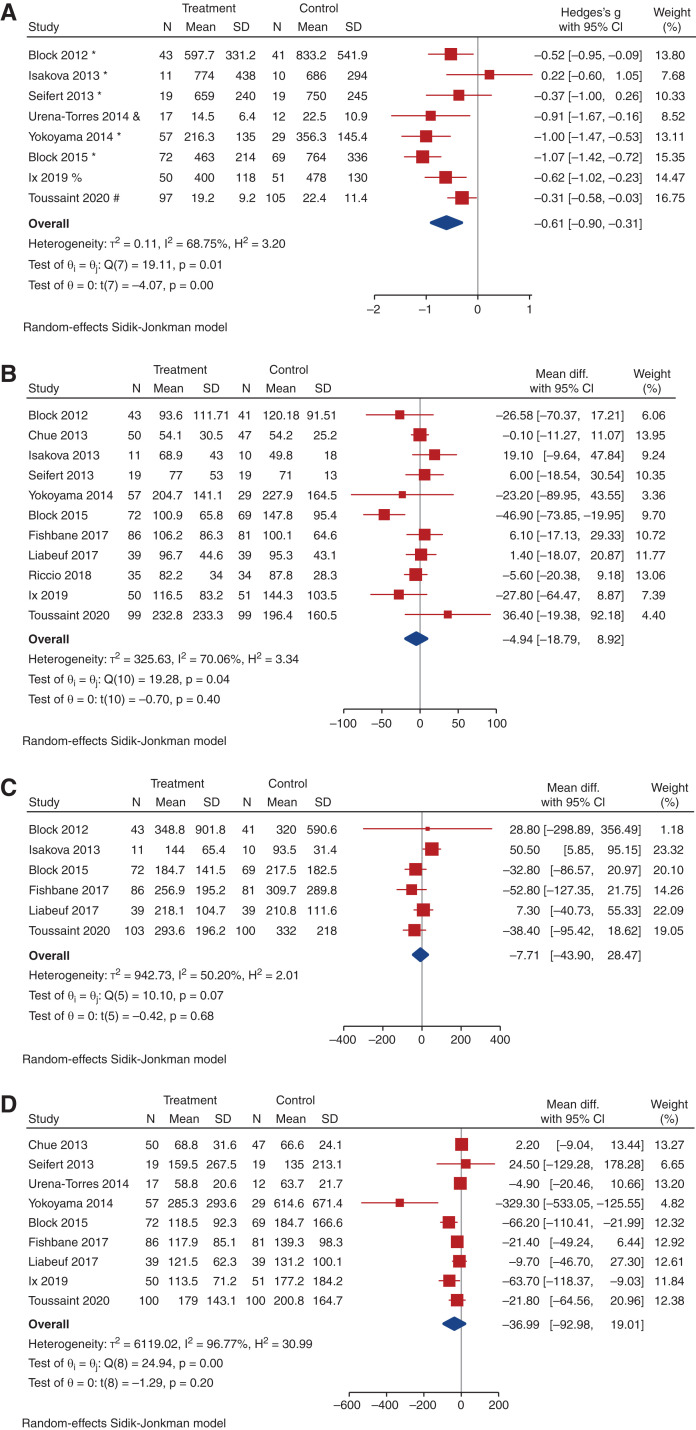
All included trials (2498 participants) reported data on serum phosphate, although one trial did not report end-of-study visit serum phosphate levels, and could not be included in the analysis for this parameter.37 Compared with placebo, noncalcium-based phosphate binders may slightly decrease serum phosphate at trial completion (12 trials, 1260 participants, WMD, -0.37 mg/dl; 95% CI, -0.58 to -0.15; heterogeneity I2=86.4%, moderate certainty evidence, Figure 3). Compared with placebo or no study medication, noncalcium-based phosphate binders may decrease serum phosphate (15 trials, 1596 participants, WMD, -0.29 mg/dl; 95% CI, -0.49 to -0.10; heterogeneity I2=85.3%, low certainty evidence, Supplemental Figure 1). In total, 12 studies included data on urinary phosphate excretion, including eight placebo-controlled trials. Compared with placebo, noncalcium phosphate binders may reduce urinary phosphate excretion at trial completion (eight trials, 702 participants, SMD, -0.61; 95% CI, -0.90 to -0.31; heterogeneity I2=68.8%, low certainty evidence, Figure 4a). Compared with placebo or no study medication, noncalcium-based phosphate binders may reduce urinary phosphate excretion (ten trials, 964 participants, SMD, -0.61; 95% CI, -84 to -38; heterogeneity I2=64.0%, very low-certainty evidence, Supplemental Figure 2). Neither study size nor duration of follow-up were significant predictors of variation in effect size estimates for serum or urinary phosphate for the noncalcium phosphate binder comparisons.
Figure 3.
Effect of noncalcium phosphate binders versus placebo on change in serum phosphate. Forest plot showing the effect of noncalcium phosphate binders compared with placebo on serum phosphate (mg/dl) at last measurement.
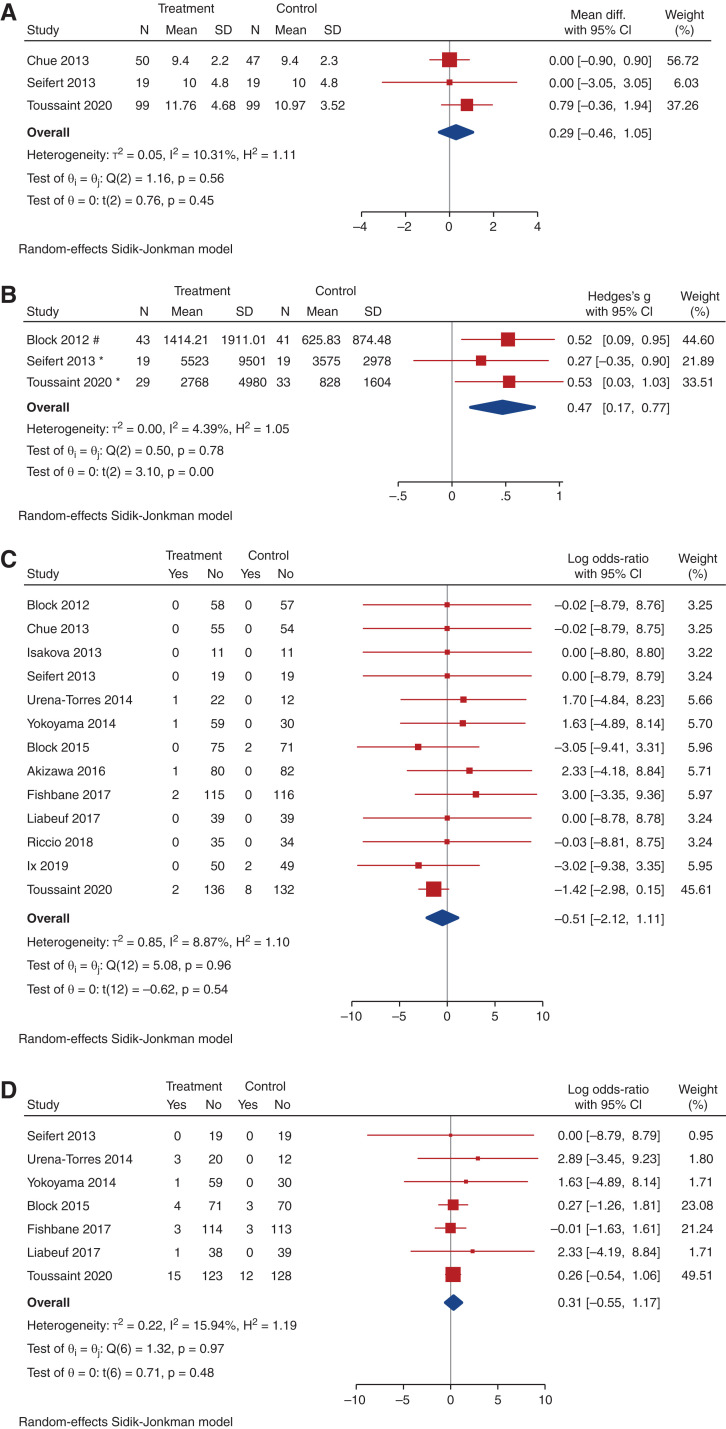
Figure 4.
Effect of noncalcium phosphate binders on change in urinary phosphate, PTH, c-terminal FGF23 (cFGF23), and intact FGF23 (iFGF23). (A) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on urinary phosphate at last measurement. (Units for urinary phosphate: * mg/day; # mmol/L; and mmol/day; % 24hr phosphate:creatinine mg/g; $spot urine phosphate:creatinine ratio mg/mg). (B) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on PTH (pg/ml) at last measurement. (C) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on cFGF23 (RU/ml) at last measurement. (D) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on iFGF23 (pg/ml) at last measurement.
Other Biochemical Outcomes
When compared with placebo, noncalcium-based phosphate binders had uncertain effects on PTH, calcium, intact and c-terminal FGF23 and eGFR (Figures 4b–4d, Supplemental Figures 3a and 4a). Noncalcium-based phosphate binders, when compared with placebo or no study medication, also had uncertain effects at trial completion on these outcomes (Supplemental Figures 3b, 4b, 5, 6a, 6b).
Cardiovascular Outcomes and Mortality
There were uncertain effects on PWV between the noncalcium phosphate binder and placebo arms (very low-certainty evidence, Figure 5a). Noncalcium phosphate binders also had uncertain effects on vascular calcification compared with placebo, with a paucity of studies despite a significant SMD (three trials, 184 participants, SMD, 0.47; 95% CI, 0.17 to 0.77; heterogeneity I2=4.4%; very low-certainty evidence, Figure 5b). When compared with all studies including placebo and no study medication, noncalcium-based phosphate binders also had uncertain effects on PWV (Supplemental Figure 7) but an increased risk of vascular calcification (Supplemental Figure 8). One other RCT also assessed coronary artery calcification; however, only baseline scores were reported, therefore this study was not included in analysis for vascular calcification.40 There was an uncertain effect on all-cause mortality and cardiovascular events (log OR, 0.51; 95% CI, -0.55 to 1.17) between the noncalcium-based phosphate binder and placebo groups (Figure 5c and 5d, respectively, Supplemental Figures 9 and 10, respectively).
Figure 5.
Effect of noncalcium phosphate binders on change in PWV, in vascular calcification, on mortality, and on cardiovascular events. (A) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on PWV (m/s) at last measurement. (B) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on vascular calcification score at last measurement. (C) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on mortality. (D) Forest plot showing the effect of noncalcium phosphate binders compared with placebo on nausea.
Other Adverse Events
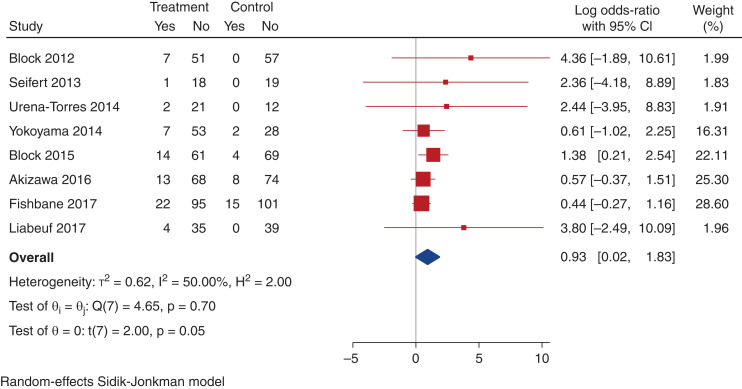
There was an uncertain effect on the risk of nausea and diarrhea (Table 4, Supplemental Figures 11a and 13a). Compared with placebo, there was an increase in risk of constipation with noncalcium-based phosphate binders (nine trials, 928 participants, log OR, 0.93; 95% CI, 0.02 to 1.83; heterogeneity I2=50.0%; moderate certainty evidence, Table 4, Figure 6).
Table 4.
Summary of differences in gastrointestinal adverse effectsa
| Adverse Effect | Noncalcium Phosphate Binders versus Placebo | Noncalcium Phosphate Binders Versus Combined Placebo and No Study Medication |
|---|---|---|
| Constipation | Increased risk, moderate certainty evidence 0.93 (0.02 to 1.83) | Uncertain effect 0.86 (-0.02 to 1.75) |
| Diarrhea | Uncertain effect 0.57 (-0.56 to 1.70) | Uncertain effect 0.57 (-0.56 to 1.70) |
| Nausea | Uncertain effect 0.61 (-01.5 to 1.37) | Uncertain effect 0.58 (-0.14 to 1.29) |
Log OR with 95% CI.
Figure 6.
Effect of noncalcium phosphate binders on constipation. Forest plot showing the effect of noncalcium phosphate binders compared with placebo on constipation.
Compared with placebo or no study medication, noncalcium phosphate binders also showed an uncertain effect on adverse events of nausea, constipation, and diarrhea (Supplemental Figures 11b, 12, 13b). When compared with placebo, there was an uncertain effect on cessation of study medication in trials with noncalcium phosphate binders (Supplemental Figure 14).
Test for Small Study Bias
There was no evidence of small study bias for all outcomes except intact FGF23. When the study by Yokoyama et al.42 was removed from the phosphate binder versus placebo comparison for intact FGF23, the P value for Egger’s test increased from 0.0008 to 0.04, and for the phosphate binder versus placebo or no study medication groups the P value increased from 0.02 to 0.27.
Noncalcium Phosphate-lowering Therapy compared with Placebo
With the addition of nicotinamide as a phosphate-lowering agent to the noncalcium phosphate binder group, studies comparing phosphate-lowering therapy to placebo did not differ from those without nicotinamide (Supplemental Table 5). Serum and urinary phosphate may be reduced with all phosphate-lowering therapy compared with placebo, and there may be an increased risk of constipation and vascular calcification, but with uncertain effect.
Noncalcium Binders compared with Calcium-based Binders
Given the paucity of studies comparing noncalcium phosphate binders to calcium-based binders, there is an uncertain effect on all outcomes assessed, including serum calcium, vascular calcification, and mortality (Supplemental Table 7).
Discussion
This systematic review and meta-analysis demonstrated that in patients with nondialysis CKD, noncalcium-based phosphate binders may reduce serum phosphate and urinary phosphate when compared with placebo. Noncalcium phosphate binders also have the same effect when compared with placebo or no study medication combined. However, noncalcium-based phosphate binder use had uncertain effects on other biochemical parameters of mineral metabolism, such as calcium, PTH, or FGF23. We report an increased risk of constipation with noncalcium-based phosphate binders compared with placebo, but an uncertain effect on other gastrointestinal adverse effects, such as nausea or diarrhea. More importantly, there was an increased risk of vascular calcification with noncalcium phosphate binders when compared with placebo, although with very-low level evidence. Data on clinical outcomes and death were reported in fewer trials, with an uncertain effect on cardiovascular events or all-cause mortality.
Our results are consistent with previous systematic reviews that also report reduced serum and urinary phosphate with phosphate-binding agents.19–21,50–52 This systematic review, however, included only patients with CKD stages 3–5 not on dialysis and involved more recent RCTs, including several recent placebo-controlled studies. Lower serum phosphate levels with intestinal phosphate binder use in patients on dialysis and in patients with CKD not on dialysis is not surprising, given the mechanism of action of these phosphate-lowering agents. Physiologically, the hypothesized cardiovascular benefit of phosphate-lowering medications is mediated via a reduction in FGF23, which, in turn, may result in reduction in cardiovascular sequelae. FGF23 has been associated with the development of left ventricular hypertrophy and uremic cardiomyopathy, and reduction of this phosphotonin in animal models has been associated with reduction in cardiovascular adverse outcomes.53,54 A statistically significant reduction in serum phosphate of 0.37 mg/dl with noncalcium binders compared with placebo in our meta-analysis may not be clinically significant, although a previous study in patients with nondialysis CKD did report that a 1 mg/dl increase in serum phosphate was associated with a 26% and 50% increase in all-cause and cardiovascular mortality, respectively.55
A previous systematic review and meta-analysis which evaluated evidence for correlation between the effects of CKD-MBD medications, such as phosphate binders, on biochemical parameters and on patient-level end points of cardiovascular disease and mortality in patients with CKD (28 studies, 6999 participants), reported that effects on serum phosphate levels were weakly and imprecisely correlated with all-cause and cardiovascular death.16
Another recent network meta-analysis of 77 RCTs that assessed effects of different phosphate binders on mortality, cardiovascular disease, and biochemical parameters (involving 12,562 patients with CKD), reported no evidence that any class of phosphate binder lowered mortality or cardiovascular events when compared with placebo.17 The vast majority of studies in the latter, however, involved patients on dialysis (62 trials, 11,009 patients) and most trials were generally of short duration with high risks of bias. Our systematic review primarily assessed RCTs involving participants with nondialysis CKD and although studies of patients on dialysis were excluded, our findings were consistent with previous systematic reviews, with no evidence for benefit on clinical outcomes such as cardiovascular events or mortality.
A recent updated Cochrane systematic review, which included 104 studies (13,744 patients, the majority involving patients on dialysis), also reported that effects of phosphate binders on patient‐important outcomes compared with placebo are uncertain.56 For patients with stages 3–5 CKD not on dialysis, this Cochrane review, similar to our study, concluded that the effects of noncalcium-based phosphate binders on cardiovascular, vascular calcification, and bone outcomes compared with placebo or usual care, are also uncertain and they may incur constipation.
Evidence for any benefit of phosphate-lowering intervention has only been on the basis of epidemiologic studies and biologic plausibility. Although several historical cohort analyses suggest that prescription of phosphate binders in patients on dialysis are associated with improved survival,57–59 with one observational study also reporting an association in patients with nondialysis CKD,60 these are only observational studies and do not prove causation.
Previous meta-analyses have reported reduction in mortality in the dialysis population associated with use of noncalcium-based phosphate binders, but only when compared with calcium-based binders,18,19 and no reduction in adverse clinical events or mortality has been shown in placebo-controlled RCTs. Results of our systematic review assessing RCTs in the nondialysis CKD population question the efficacy and safety of phosphate binders in this population, with no reported benefit apart from biochemical improvement in serum and urinary phosphate, but with associated increased risks of constipation and possibly vascular calcification.
The updated KDIGO CKD-MBD guidelines in 2017 amended a previous recommendation statement to maintain serum phosphate in the normal range for patients with CKD stages 3–5 to suggest treatment should focus on patients with overt hyperphosphatemia (on the basis of weak clinical evidence).15 We highlight that the lack of evidence for a benefit of phosphate-lowering therapy in nondialysis CKD has predominantly involved studies in patients who are normophosphatemic, and studies of relatively short trial duration. Lack of cardiovascular or mortality benefit is not surprising perhaps, because most studies are between 3 and 9 months in duration, and it may, in fact, take several years to see any potential benefit of a sustained reduction in phosphate or FGF23. Longer RCTs, of at least 2–3 years in study design, are required to evaluate benefits on hard clinical outcomes.
Our systematic review also assessed the potential benefits of noncalcium phosphate binders on intermediate cardiovascular markers of PWV and vascular calcification when compared with placebo or no study medication, although few RCTs have assessed these parameters as primary end points in patients with nondialysis CKD. Abnormalities of mineral metabolism in CKD are associated with the development of vascular calcification, increased arterial stiffness, and greater cardiovascular morbidity, and mortality.4,5 Use of intermediate cardiovascular markers in RCTs have allowed for smaller sample sizes than studies assessing “harder” end points of cardiovascular events or death, and PWV and vascular calcification are considered valid surrogates for cardiovascular morbidity and mortality. No evidence for a benefit was seen with phosphate binders compared with placebo, or combined placebo and no study medication, for PWV in our meta-analysis. There was an increased risk of vascular calcification observed with noncalcium phosphate binders compared with placebo, although with very low-level evidence. This finding was first highlighted in the study by Block et al,22 which raised concern about phosphate binder use in the nondialysis CKD population, although that outcome was thought to be largely a result of the calcium-based phosphate binder arm in that RCT. The reason why noncalcium-based phosphate binders would result in greater vascular calcification is unclear, and only three trials assessing this outcome, with significant heterogeneity, were included for analysis in our systematic review. One possible explanation may be the trend toward a higher serum calcium with noncalcium-based binders when compared with placebo.
Most of the RCTs included in our systematic review involved patients who had serum phosphate levels within the normal reference range, but with increased FGF23 levels. Studies have reported that vascular calcification was associated with elevated FGF23 levels independent of serum phosphate,61 and even modest serum phosphate elevations within the normal laboratory range were associated with arterial calcification and cardiovascular events.8 Normalizing a positive phosphate balance, and lowering serum FGF23, is considered a potential strategy to reduce cardiovascular risk in CKD, although a recent meta-analysis did not show an exposure-response relationship between FGF23 and cardiovascular or noncardiovascular outcomes in populations with and without known CKD.62
Interestingly, the three RCTs in our systematic review that included patients with the most advanced CKD (and hyperphosphatemia) reported reduction in serum concentrations of intact FGF2332,45,46 and one also reported reduction in the composite outcome of hospital admission, mortality and cardiovascular outcomes with the use of ferric citrate.45 This composite end-point was a secondary outcome of this study by Block et al. however, and the trial was an open-label, single-center study, with 37% of those in the usual care arm receiving phosphate binders, including calcium-based binders. Perhaps targeting patients with elevated rather than normal serum phosphate may be more appropriate, given the lack of evidence for benefit of phosphate-lowering therapy in patients with nondialysis CKD in whom serum phosphate levels are normal, as highlighted in our systematic review.
Globally, calcium-based phosphate binders are the most commonly prescribed phosphate-lowering therapy, although exogenous calcium has been associated with the development and progression of vascular calcification.18,19 Minimizing exogenous calcium through reducing exposure to calcium-based phosphate binders within the CKD population has been suggested to be beneficial, with meta-analyses showing mortality benefits of noncalcium containing phosphate binders over calcium-based binders, predominantly in patients on dialysis.18,19 Our systematic review targeted noncalcium-based phosphate-lowering therapy to eliminate the complication of exogenous calcium in calcium-based binders, although only one placebo-controlled RCT was found in the nondialysis CKD population, which assessed calcium-based binders,48 and this study was excluded from our meta-analyses. Of note, this was only a 12-week trial that showed a reduction in serum phosphate and PTH with calcium-based binders compared with placebo.
We report low-level evidence for an increase in adverse events for phosphate-lowering interventions in contrast to placebo, particularly related to an increased risk of constipation. Noncalcium-based phosphate binders have been associated with gastrointestinal side effects due to the nature of their role in intestinal phosphate binding. Nicotinamide, the only phosphate-lowering treatment included in our study that is not a phosphate binder, also has considerable adverse gastrointestinal effects, as reported in one placebo-controlled RCT included in our systematic review.26 Phosphate-lowering therapy contributes to a high pill burden, associated with poor adherence, and potentially impaired health-related quality of life. Any degree of clinical benefit needs to be assessed against cost and pill burden of phosphate-lowering agents in longer-term follow up trials in the nondialysis CKD population.
Our study reports that noncalcium phosphate binders may reduce urinary phosphate excretion, although Stremke et al. questioned the role and reliability of urinary phosphate measurement as an indicator of dietary phosphate intake and absorption in patients with CKD, reporting that urinary phosphate excretion was more tightly correlated with whole-body phosphate balance than intestinal phosphate absorption.63 Low urinary phosphate excretion in fact may indicate a positive phosphate balance, rather than a low net intestinal load, with phosphate balance influenced by serum phosphate and PTH, and dietetic and pharmacologic interventions; and adding to the argument that changes to biochemical parameters, such as urinary phosphate, do not necessarily relate to clinical or patient-centered outcomes when assessing phosphate-lowering treatments.
The strengths of this review include a comprehensive overview of the evidence, risk of bias assessment, use of the GRADE approach to assess the certainty of evidence and subgroup analysis of outcomes with high heterogeneity. These strengths should be balanced against the limitations of the systematic review, which include a high degree of heterogeneity due to moderately low numbers of study participants and short follow-up times. There was also clinical heterogeneity in participant characteristics of baseline kidney function, diabetes status, serum phosphate levels, and study level characteristics of dosage of phosphate-lowering agents, intervention in the control groups, follow-up duration, and study outcomes. Other limitations of the systematic review include imprecision of estimates, and poor directness of results (short-term studies performed in limited number of countries).
Given the high prevalence of CKD-MBD, which contributes to a disproportionate burden of cardiovascular disease, skeletal complications, and death in patients with CKD, this systematic review is an important contribution, summarizing existing evidence on phosphate-lowering interventions and adding to the clinical trial data. This review raises further concern about the paucity of proven benefits of phosphate binder therapy in the nondialysis CKD population, albeit predominantly with normophosphatemia and mild hyperphosphatemia in the RCTs to date. Specifically, these results question the value of phosphate binders to lower serum phosphate and FGF23 levels in an attempt to reduce the burden of cardiovascular disease. Further studies should be adequately powered, and perhaps targeted to those with positive phosphate balance, to assess the utility of phosphate-lowering strategies on patient-level outcomes.
Disclosures
C. Hawley reports having consultancy agreements with GlaxoSmithKline, Janssen, and Otsuka; reports receiving research funding from Abbott product supply, Amgen, Bayer product supply, Baxter, Baxter product supply, Janssen Cilag, Otsuka, and Shire study grant; reports receiving honoraria from Janssen paid to university, GlaxoSmithKline consultancy fees paid to the university; and other interests/relationships with National Health and Medical Research Council of Australia via the grants panel, Kidney Health Australia (KHA) via involvement with consumers in relation to clinical trials and interact with KHA in these activities, and Polycystic Kidney Disease Australia Foundation as a Board Member. D.W. Johnson reports receiving consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Amgen, AstraZeneca, AWAK, Bayer, Baxter Healthcare, Boehringer Ingelheim, Fresenius Medical Care, Lilly, and ONO; reports being a scientific advisor or member of the American Journal of Kidney Disease, Australian and New Zealand Society of Nephrology Councilor, Clinical Journal of the American Society of Nephrology, Cochrane Kidney and Transplant Group, International Society of Peritoneal Dialysis Immediate Past President, the National Health & Medical Research Council Academy, Peritoneal Dialysis International, and Past International Society of Nephrology Councilor; reports other interests/relationships with Amgen (accommodation sponsorship), Kidney Health Australia (Advisor), Past International Society of Nephrology Councilor, International Society for Peritoneal Dialysis Immediate Past President, and Australian and New Zealand Society of Nephrology President; reports being a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant; and reports being supported by an Australian Government National Health and Medical Research Council Leadership Investigator Grant. G.A. Block reports receiving research grants from Akebia, Ardelyx, GSK, and Keryx Inc.; reports having consultancy agreements with Akebia, Keryx, Kirin, and Reata; reports having an ownership interest in Ardelyx and Reata; reports receiving honoraria from Amgen and Kirin; reports being a scientific advisor or member of Ardelyx, CJASN, Kirin, and Reata; and reports other interests/relationships through previously being on the Executive Steering Committee of KDIGO, Medical Director at Davita, and previous employment at Reata, and is a current Director for Ardelyx. G.J. Elder has received honoraria, travel support and research funding from Amgen, Sanofi, and Shire. N.D. Toussaint has received honoraria, travel support, and research funding from Amgen, Shire, Takeda and Sanofi. N. Lioufas reports receiving research funding from the Australian Commonwealth Government (Research Training Program Scholarship). S.V. Badve has served on the advisory boards of AstraZeneca, Bayer, and Vifor; reports receiving speaker’s honoraria from Amgen, Bayer, Pfizer, Vifor Pharma, outside of the submitted work, all fees paid to his institution; reports receiving research funding from Bayer and reports being supported by a John Chalmers Clinical Research Fellowship with the support of Servier from The George Institute for Global Health, Australia. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
C. Hawley, N. Lioufas, E. Pascoe, and N. Toussaint were responsible for the study design, statistical analysis, and interpretation; N. Lioufas and N. Toussaint were responsible for the literature search, study selection, and data extraction; and S. Badve, G. Block, G. Elder and D. Johnson provided supervision. Each author contributed important intellectual content during manuscript drafting or revision, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The supporting organizations/agencies had no role in the design and conduct of the study, analysis, and interpretation of the data, review, and approval of the manuscript, and decision to submit the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040554/-/DCSupplemental.
Supplemental Table 1. Search strategy.
Supplemental Table 2. GRADE summary of evidence for noncalcium-based phosphate binders vs placebo.
Supplemental Table 3. Summary of GRADE findings. Noncalcium-based phosphate binders compared with combined placebo or no study medication.
Supplemental Table 4. GRADE summary of evidence for noncalcium phosphate binders vs placebo or no study treatment.
Supplemental Table 5. Summary of GRADE findings. Noncalcium phosphate-lowering therapy compared with placebo.
Supplemental Table 6. GRADE summary of evidence for noncalcium phosphate-lowering therapy vs placebo.
Supplemental Table 7. Summary of GRADE findings. Noncalcium-based phosphate binders compared with calcium-based phosphate binders.
Supplemental Table 8. GRADE summary of evidence for noncalcium-based phosphate binders vs calcium-based phosphate binders.
Supplemental Figure 1. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on serum phosphate.
Supplemental Figure 2. Effect of noncalcium phosphate binders compared with placebo or no study treatment on urinary phosphate.
Supplemental Figure 3a. Effect of noncalcium phosphate binder compared with placebo on serum calcium
Supplemental Figure 3b. Effect of noncalcium phosphate binders compared with placebo or no study treatment on serum calcium.
Supplemental Figure 4a. Effect of noncalcium phosphate binders compared with placebo on eGFR.
Supplemental Figure 4b. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on eGFR.
Supplemental Figure 5. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on PTH.
Supplemental Figure 6a. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on cFGF23.
Supplemental Figure 6b. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on iFGF23.
Supplemental Figure 7. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on PWV.
Supplemental Figure 8. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on vascular calcification.
Supplemental Figure 9. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on mortality.
Supplemental Figure 10. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on cardiovascular events.
Supplemental Figure 11a. Effect of noncalcium-based phosphate binders compared with placebo on nausea.
Supplemental Figure 11b. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on nausea.
Supplemental Figure 12. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on constipation.
Supplemental Figure 13a. Effect of noncalcium-based phosphate binders compared with placebo on diarrhea.
Supplemental Figure 13b. Effect of noncalcium-based phosphate binders compared with placebo or no study treatment on diarrhea.
Supplemental Figure 14. Effect of noncalcium phosphate binders compared with placebo on cessation of study medication.
REFERENCES
- 1.Tonelli M, Pfeffer MA: Kidney disease and cardiovascular risk. Annu Rev Med 58: 123–139, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. : Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann Intern Med 168: 422–430, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Toussaint ND, Pedagogos E, Tan SJ, Badve SV, Hawley CM, Perkovic V, et al. : Phosphate in early chronic kidney disease: Associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 17: 433–444, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, et al. : Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 7.O’Seaghdha CM, Hwang S-J, Muntner P, Melamed ML, Fox CS: Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 26: 2885–2890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Jüppner H: Phosphate and FGF-23. Kidney Int Suppl 79: S24–S27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. : Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu JJ, Katz R, Ix JH, de Boer IH, Kestenbaum B, Shlipak MG: Association of fibroblast growth factor-23 with arterial stiffness in the Multi-Ethnic Study of Atherosclerosis. Nephrol Dial Transplant 29: 2099–2105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozkok A, Kekik C, Karahan GE, Sakaci T, Ozel A, Unsal A, et al. : FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrol 14: 241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada S, Giachelli CM: Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 100: 87–93, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group : KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) 7: 1–59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer SC, Teixeira-Pinto A, Saglimbene V, Craig JC, Macaskill P, Tonelli M, et al. : Association of drug effects on serum parathyroid hormone, phosphorus, and calcium levels with mortality in CKD: A meta-analysis. Am J Kidney Dis 66: 962–971, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Gardner S, Tonelli M, Mavridis D, Johnson DW, Craig JC, et al. : Phosphate-binding agents in adults with CKD: A network meta-analysis of randomized trials. Am J Kidney Dis 68: 691–702, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. : Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Patel L, Bernard LM, Elder GJ: Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: A meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 11: 232–244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habbous S, Przech S, Acedillo R, Sarma S, Garg AX, Martin J: The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: A systematic review and meta-analysis. Nephrol Dial Transplant 32: 111–125, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Sekercioglu N, Thabane L, Díaz Martínez JP, Nesrallah G, Longo CJ, Busse JW, et al. : Comparative effectiveness of phosphate binders in patients with chronic kidney disease: A systematic review and network meta-analysis. PLoS One 11: e0156891, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. : Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, et al. : Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 38: 158–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, et al. : Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 24: 842–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Lu JL, Wall BM, Gyamlani G, Naseer A, Wallick A, et al. : Changes with lanthanum carbonate, calcium acetate, and phoshorus restriction in CKD: A randomized controlled trial. Kidney Int Rep 3: 897–904, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK, et al. : Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE Trial. J Am Soc Nephrol 30: 1096–1108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toussaint ND, Pedagogos E, Lioufas NM, Elder GJ, Pascoe EM, Badve SV, et al. ; IMPROVE-CKD Trial Investigators : A randomized trial on the effect of phosphate reduction on vascular end points in CKD (IMPROVE-CKD). J Am Soc Nephrol 31: 2653–2666, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 62: e1–e34, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. ; GRADE Working Group : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J, Altman DG: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions, edited by Higgins J, Green S, Chichester, West Sussex, John Wiley & Sons Ltd, 2008, pp 187–241 [Google Scholar]
- 31.Sidik K, Jonkman JN: A note on variance estimation in random effects meta-regression. J Biopharm Stat 15: 823–838, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson SG, Higgins JP: How should meta-regression analyses be undertaken and interpreted? Stat Med 21: 1559–1573, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Di Iorio B, Bellasi A, Russo D; INDEPENDENT Study Investigators : Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin J Am Soc Nephrol 7: 487–493, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Isakova T, Barchi-Chung A, Enfield G, Smith K, Vargas G, Houston J, et al. : Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol 8: 1009–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemos MM, Watanabe R, Carvalho AB, Jancikic ADB, Sanches FMR, Christofalo DM, et al. : Effect of rosuvastatin and sevelamer on the progression of coronary artery calcification in chronic kidney disease: A pilot study. Clin Nephrol 80: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Ureña-Torres P, Prié D, Keddad K, Preston P, Wilde P, Wan H, et al. : Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol 15: 71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liabeuf S, Ryckelynck JP, El Esper N, Ureña P, Combe C, Dussol B, et al. ; FRENCH Study collaborators : Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol 12: 1930–1940, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggiero B, Trillini M, Tartaglione L, Rotondi S, Perticucci E, Tripepi R, et al. ; ANSWER Study Organization : Effects of sevelamer carbonate in patients with CKD and proteinuria: The ANSWER randomized trial. Am J Kidney Dis 74: 338–350, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, et al. : The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Soriano S, Ojeda R, Rodríguez M, Almadén Y, Rodríguez M, Martín-Malo A, et al. : The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol 80: 17–22, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, et al. : Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9: 543–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, et al. : A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3-5. Am J Kidney Dis 65: 728–736, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Riccio E, Sabbatini M, Bruzzese D, Grumetto L, Marchetiello C, Amicone M, et al. : Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: Evidence from a randomized controlled trial. Clin Exp Nephrol 22: 529–538, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Block GA, Block MS, Smits G, Mehta R, Isakova T, Wolf M, et al. : A pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol 30: 1495–1504, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akizawa T, Origasa H, Kameoka C, Tsukada J, Kuroishi K, Yamaguchi Y: Bixalomer in hyperphosphatemic patients with chronic kidney disease not on dialysis: Phase 3 randomized trial. Ther Apher Dial 20: 588–597, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, et al. : Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol 28: 1851–1858, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yubero-Serrano EM, Woodward M, Poretsky L, Vlassara H, Striker GE; AGE-less Study Group : Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin J Am Soc Nephrol 10: 759–766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qunibi W, Winkelmayer WC, Solomon R, Moustafa M, Kessler P, Ho CH, et al. : A randomized, double-blind, placebo-controlled trial of calcium acetate on serum phosphorus concentrations in patients with advanced non-dialysis-dependent chronic kidney disease. BMC Nephrol 12: 9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekercioglu N, Angeliki Veroniki A, Thabane L, Busse JW, Akhtar-Danesh N, Iorio A, et al. : Effects of different phosphate lowering strategies in patients with CKD on laboratory outcomes: A systematic review and NMA. PLoS One 12: e0171028, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Wen J, Li Z, Fan J: Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: A systematic review. BMC Nephrol 14: 226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhai CJ, Yu XS, Yang XW, Sun J, Wang R: Effects and safety of iron-based phosphate binders in dialysis patients: A systematic review and meta-analysis. Ren Fail 37: 7–15, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. : FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, et al. : FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep 7: 1993, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, et al. : Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, Strippoli GFM: Phosphate binders for preventing and treating chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Cochrane Database of Systematic Reviews 8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopes AA, Tong L, Thumma J, Li Y, Fuller DS, Morgenstern H, et al. : Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis 60: 90–101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K, et al. : Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20: 388–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Martín JL, Carrero JJ, Benedik M, Bos W-J, Covic A, Ferreira A, et al. : COSMOS: the dialysis scenario of CKD-MBD in Europe. Nephrol Dial Transplant 28: 1922–1935, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K: Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis 56: 842–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UAA: Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant 25: 2679–2685, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, et al. : Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: A meta-analysis. J Am Soc Nephrol 29: 2015–2027, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stremke ER, McCabe LD, McCabe GP, Martin BR, Moe SM, Weaver CM, et al. : Twenty-four-hour urine phosphorus as a biomarker of dietary phosphorus intake and absorption in CKD: A secondary analysis from a controlled diet balance study. Clin J Am Soc Nephrol 13: 1002–1012, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ku E, Yang W, McCulloch CE, Feldman HI, Go AS, Lash J, et al. ; CRIC Study Investigators : Race and Mortality in CKD and Dialysis: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 75: 394–403, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.