Abstract
Background
Raised intraocular pressure is a risk factor for glaucoma. One treatment option is glaucoma drainage surgery (trabeculectomy). Antimetabolites are used during surgery to reduce postoperative scarring during wound healing. Two agents in common use are mitomycin C (MMC) and 5‐Fluorouracil (5‐FU).
Objectives
To assess the effects of MMC compared to 5‐FU as an antimetabolite adjunct in trabeculectomy surgery.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015 Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2015), EMBASE (January 1980 to October 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 October 2015.
Selection criteria
We included randomised controlled trials where wound healing had been modified with MMC compared to 5‐FU.
Data collection and analysis
Two review authors independently selected trials and collected data. The primary outcome was failure of a functioning trabeculectomy one year after surgery. Secondary outcomes included mean intraocular pressure at one year. We considered three subgroups: high risk of trabeculectomy failure (people with previous glaucoma surgery, extracapsular cataract surgery, African origin and people with secondary glaucoma or congenital glaucoma); medium risk of trabeculectomy failure (people undergoing trabeculectomy with extracapsular cataract surgery) and low risk of trabeculectomy failure (people who have received no previous surgical eye intervention).
Main results
We identified 11 trials that enrolled 687 eyes of 679 participants. The studies were conducted in the United States, Europe, Asia and Africa. Five studies enrolled participants at low risk of trabeculectomy failure, five studies enrolled participants at high risk of failure, and one study enrolled people with both high and low risk of failure. None of the included trials enrolled participants with combined trabeculectomy/cataract surgery.
We considered one study to be at low risk of bias in all domains, six studies to be at high risk of bias in one or more domains, and the remaining four studies to be at an unclear risk of bias in all domains.
The risk of failure of trabeculectomy at one year after surgery was less in those participants who received MMC compared to those who received 5‐FU, however the confidence intervals were wide and are compatible with no effect (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.30 to 1.00; studies = 11; I2 = 40%). There was no evidence for any difference between groups at high and low risk of failure (test for subgroup differences P = 0.69).
On average, people treated with MMC had lower intraocular pressure at one year (mean difference (MD) ‐3.05 mmHg, 95% CI ‐4.60 to ‐1.50), but the studies were inconsistent (I2 = 52%). The size of the effect was greater in the high‐risk group (MD ‐4.18 mmHg, 95% CI ‐6.73 to ‐1.64) compared to the low‐risk group (MD ‐1.72 mmHg, 95% CI ‐3.28 to ‐0.16), but again the test for interaction was not statistically significant (P = 0.11).
Similar proportions of eyes treated with MMC lost 2 or more lines of visual acuity one year after surgery compared to 5‐FU, but the confidence intervals were wide (RR 1.05, 95% CI 0.54 to 2.06).
Adverse events occurred relatively rarely, and estimates of effect were generally imprecise. There was some evidence for less epitheliopathy in the MMC group (RR 0.23, 95% CI 0.11 to 0.47) and less hyphaema in the MMC group (RR 0.62, 95% CI 0.42 to 0.91).
None of the studies reported quality of life.
Overall, we graded the quality of the evidence as low largely because of risk of bias in the included studies and imprecision in the estimate of effect.
Authors' conclusions
We found low‐quality evidence that MMC may be more effective in achieving long‐term lower intraocular pressure than 5‐FU. Further comparative research on MMC and 5‐FU is needed to enhance reliability and validity of the results shown in this review. Furthermore, the development of new agents that control postoperative scar tissue formation without side effects would be valuable and is justified by the results of this review.
Plain language summary
Mitomycin C versus 5‐Fluorouracil for wound healing in glaucoma surgery
Review question Does mitomycin C (MMC) offer any advantage in comparison to 5‐Fluorouracil (5‐FU) as the antimetabolite used to augment glaucoma surgery (trabeculectomy)? Does MMC help to achieve lower rates of trabeculectomy failure than 5‐FU at one year postoperatively?
Background Raised intraocular pressure is a risk factor for glaucoma. One treatment option is glaucoma drainage surgery (trabeculectomy) to help lower intraocular pressure. Antimetabolites are medicines used during surgery to help reduce scarring after surgery during wound healing. If scarring occurs it can lead to treatment failure because the drainage channel no longer works. Two agents in common use are MMC and 5‐FU.
Search date The evidence is up to date to October 2015.
Study characteristics We included 11 randomised controlled trials conducted in the United States, Europe, Asia and Africa in this review. In total, 687 eyes of 679 participants underwent routine trabeculectomy for glaucoma control. Some participants were at a higher risk of failure than others, for example if they had had previous glaucoma surgery, were of African origin, or if they had secondary glaucoma. Five studies enrolled participants at low risk of trabeculectomy failure, five studies enrolled participants at high risk of failure, and one study enrolled people with both high and low risk of failure. None of the included trials enrolled participants with combined trabeculectomy/cataract surgery.
Key results Our review showed that the risk of failure of trabeculectomy at one year after surgery was slightly less in those participants treated with MMC compared to 5‐FU. All of the included randomised controlled trials contributed to this result, with a mixed study population of high‐ and low‐risk participants and varied methodology of antimetabolite application. We did not detect any significant differences between the subgroups of participants at low and high risk of failure, but the power of this analysis was low.
We identified no difference between the visual outcomes of the group that received MMC and the group that received 5‐FU at one year postoperatively nor in the number of drops used postoperatively. However, we found evidence to suggest that MMC was more effective at lowering intraocular pressure than 5‐FU in both high‐ and low‐risk participants, achieving a lower mean intraocular pressure postoperatively than in those who were treated with 5‐FU at one year. This effect seemed to be greater in the high‐risk populations.
Evaluating the overall complications across all studies revealed a slight favour toward using MMC, particularly with the incidence of epitheliopathy and hyphaema. There was a trend towards bleb leaks, wound leaks, late hypotony and cataract formation in the MMC‐treated group.
None of the studies reported quality of life.
Quality of the evidence We graded the quality of the evidence as low, mostly due to the risk of bias in the included studies. One bias we commonly encountered came from the different techniques of antimetabolite administration, making it difficult to conceal which medicine was being used. Furthermore, most studies only had a few complications to report, which meant that there were low numbers overall to include in the analysis of complications.
Summary of findings
Summary of findings for the main comparison. MMC compared to 5‐FU for wound healing in glaucoma surgery.
| MMC compared to 5‐FU for wound healing in glaucoma surgery | ||||||
| Patient or population: wound healing in glaucoma surgery Settings: Intervention: MMC Comparison: 5‐FU | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants/eyes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU | MMC | |||||
| Failure of functioning trabeculectomy at 1 year | Study population | Low‐risk population RR 0.65 (95% CI 0.19 to 2.20) High‐risk population RR 0.49 (95% CI 0.22 to 1.08) |
634 (11 RCTs: 6 including low‐risk population and 5 including high‐risk population) | ⊕⊕⊝⊝ LOW 1,2 | ||
| Low‐risk population: 74 per 1000 High‐risk population: 272 per 1000 |
Low‐risk population: 50 per 1000 High‐risk population: 137 per 1000 |
|||||
| Intraocular pressure at 1 year | The mean intraocular pressure at 1 year ranged across 5‐FU groups. Low‐risk population: 10.9 to 14.3 mmHg High‐risk population: 14.8 to 16.3 mmHg |
The mean intraocular pressure at 1 year in the MMC groups had a range of values. Low‐risk population: 9.9 to 11.6 mmHg High‐risk population: 8.6 to 13.7 mmHg |
‐ | 386 (7 RCTs: 3 including low‐risk population and 4 including high‐risk population) | ⊕⊕⊝⊝ LOW 1,3 | |
| Loss of 2 or more lines of Snellen visual acuity at 1 year | Study population | Low‐risk population RR 2.00 (95% CI 0.53 to 7.59) High‐risk population RR 0.81 (95% CI 0.36 to 1.80) |
328 (5 RCTs: 2 including low‐risk population and 3 including high‐risk population) | ⊕⊕⊝⊝ LOW 2,4 | ||
| Low‐risk population: 47 per 1000 High‐risk population: 115 per 1000 |
Low‐risk population: 94 per 1000 High‐risk population: 96 per 1000 |
|||||
| Postoperative complications: late hypotony | Study population | RR 1.37 (95% CI 0.41 to 4.63) | 211 (4 RCTs) | ⊕⊕⊝⊝ LOW 2,4 | ||
| 37 per 1000 | 59 per 1000 | |||||
| Postoperative complications: choroidal detachment | Study population | RR 0.86 (95% CI 0.45 to 1.63) | 494 (8 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 68 per 1000 | 70 per 1000 | |||||
| Postoperative complications: endophthalmitis | Study population | RR 3.89 (95% CI 0.44 to 34.57) | 315 (4 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 0 per 1000 | 19 per 1000 | |||||
| Quality of life at 1 year | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 5‐FU: 5‐Fluorouracil; CI: confidence interval; MMC: mitomycin C; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for risk of bias: only one study at low risk of bias in all domains
2Downgraded for imprecision: wide confidence intervals
3Downgraded for inconsistency: I2 = 60%
4Downgraded for risk of bias: no study at low risk of bias in all domains
Background
Description of the condition
Glaucoma is a chronic, progressive optic neuropathy characterised by a progressive loss of ganglion cells that leads to a characteristic visual function loss. Intraocular pressure (IOP) is often considered to be a major risk factor for glaucoma, and it is the only factor that can be modified to try to change the course of the condition. The publication of a series of randomised controlled trials (RCTs) has established the evidence for treating glaucoma with IOP reduction (AGIS 1998; CNTGS 1998; Heijl 2002; Kass 2002; Maier 2005; Vass 2007).
Glaucoma drainage surgery remains an important treatment option for the control of IOP despite the addition of several new IOP‐lowering drugs. Some evidence suggests that trabeculectomy is more effective than either medicine or laser treatment alternatives (Migdal 1994). However, a Cochrane systematic review from 2012 found that visual field deterioration up to five years is not significantly different whether treatment is initiated with medication or trabeculectomy (Burr 2012).
Optimum success rates are achieved when the eye has been exposed to no previous interventions, either surgical or medical, although this is not the usual situation in high‐income countries. Risk factors for trabeculectomy failure are thought to be those that increase the scarring response and include previous exposure to topical medication, previous surgical manipulation of the conjunctiva or other injury, young age, African origin, a history of uveitis and neovascular glaucoma (EGS 2003).
Presentation and diagnosis
The diagnosis of glaucoma is made by the identification of a progressive optic neuropathy or a characteristic visual field defect. There are subgroups of glaucoma, primary open angle glaucoma being most common in European and African populations. A person with primary open angle glaucoma is often unaware of any symptoms until the late stages of the disease, making early diagnosis essential.
Description of the intervention
Treatment is usually initiated with topical treatment, and surgical options are considered if topical treatment fails to prevent progression of the disease. The trabeculectomy produces a guarded fistula between the anterior chamber and the subconjunctival space. There have been numerous modifications since its first description (Cairns 1968), including the use of antimetabolites to reduce fibroblast activity and postoperative scarring at the site of the scleral flap and the subconjunctival space.
How the intervention might work
Once trabeculectomy has been selected, the treatment decisions are whether to augment the surgery with antiscarring agents such as antimetabolites. Antimetabolites are applied to the surgical site to inhibit fibroblast activity and reduce postoperative scarring; the two agents commonly in use are mitomycin C (MMC) and 5‐Fluorouracil (5‐FU). Due to reported side effects such as increased risk of bleb leak, hypotony and endophthalmitis (DeBry 2002), there is concern that use of these agents should be restricted to high‐risk cases only. A number of RCTs have reported the use of MMC (Andreanos 1997; Carlson 1997; Cohen 1996; Costa 1996; Martini 1997; Robin 1997; Shin 1995; Shin 1998; Wu 1996). A Cochrane systematic review concluded that compared to placebo, MMC reduces mean IOP at 12 months in all groups of participants (Wilkins 2010). Apart from increase in cataract formation, there was insufficient power to detect any increase in other serious side effects. Postoperative 5‐FU injections to augment trabeculectomy have also been assessed with RCTs (FFSSG 1989; Goldenfeld 1994; Ophir 1992; Ruderman 1987), and also confer an improvement in IOP control at one year compared to placebo (Green 2014). Clinically, MMC and 5‐FU can be applied intraoperatively on a sponge placed for one to five minutes between the conjunctiva and sclera at the start of the operation. Alternatively, 5‐FU may be given as one or more postoperative subconjunctival injections. There is marked variation in the concentrations of both drugs used, the time of intraoperative application and the position and volume of postoperative injections.
Why it is important to do this review
The results of two Cochrane reviews comparing MMC, in Wilkins 2010, and 5‐FU, in Green 2014, to placebo suggest a similar effect for the two agents in inhibiting scarring after trabeculectomy. However, there is no direct comparative evidence to influence which antimetabolite a surgeon should choose. The purpose of this review was to systematically summarise the RCTs in which MMC was compared to 5‐FU in an attempt to clearly identify treatment benefits of one agent over the other.
Objectives
To assess the effects of MMC compared to 5‐FU as an antimetabolite adjunct in trabeculectomy surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs where wound healing had been modified with one of the antimetabolites in one group of people undergoing trabeculectomy, compared to the other antimetabolite in the other group.
Types of participants
There were three separate subgroup populations:
High risk of trabeculectomy failure: people with previous glaucoma or extracapsular cataract surgery, people of African origin and people with secondary glaucoma or congenital glaucoma.
Medium risk of trabeculectomy failure: (combined surgery) people undergoing trabeculectomy with extracapsular cataract surgery.
Low risk of trabeculectomy failure: (primary trabeculectomy): people who have received no previous surgical eye intervention. People who underwent previous laser procedures may be included in this group.
For the purpose of this review, there were no restrictions regarding age or gender.
Types of interventions
We included the following interventions:
Use of intraoperative MMC versus intraoperative 5‐FU.
Use of intraoperative MMC versus postoperative 5‐FU.
Use of intraoperative MMC versus intraoperative and postoperative 5‐FU.
Use of intraoperative MMC and postoperative MMC versus intraoperative and postoperative 5‐FU.
Types of outcome measures
Primary outcomes
The primary outcome was failure of a functioning trabeculectomy at one year from surgery (dichotomous).
We used the following definitions:
Success: adequate pressure control (< 22 mmHg) without additional treatment.
Failure: need for repeat filtration surgery or uncontrolled IOP (= or > 22 mmHg).
Secondary outcomes
Survival analysis (time to event) for the previously given definition of failure
Mean IOP for each group at one year from surgery
Quality‐of‐life measures
Economic data
Adverse outcomes
Adverse events in either group with reference to choroidal detachment, hypotony and late endophthalmitis were reported. Adverse events were reported at any time during the follow‐up period.
We used the following definitions:
Bleb leakage: presence of a positive Seidel test (visible aqueous flow with the tear film stained with fluorescein).
Hypotony: IOP below 5 mmHg and/or associated with complications such as macular oedema and sight loss or choroidal detachments.
Endophthalmitis: an infection of the globe contents that even with prompt aggressive treatment results in substantial loss of visual function.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015 Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2015), EMBASE (January 1980 to October 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 October 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We contacted investigators as necessary to identify additional published and unpublished studies.
Data collection and analysis
Selection of studies
Three review authors (JC/EC/JE) independently reviewed the titles and abstracts resulting from the searches. We obtained full copies of any report referring to possibly or definitely relevant trials and assessed them according to the definitions in the Criteria for considering studies for this review section. We assessed only trials meeting these predefined criteria for methodological quality. We resolved any disagreements by discussion.
Data extraction and management
Three review authors (JC/EC/JE) independently extracted data with relation to the outcome measures outlined above. We resolved discrepancies by discussion. One review author entered the data into Review Manager (RevMan 2014), and the other review authors checked the data entry.
Assessment of risk of bias in included studies
Three review authors (JC/EC/JE) independently assessed risk of bias according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered six domains: random sequence generation, allocation concealment, masking, incomplete outcome data, selective reporting and any other identified bias. We graded each domain as low risk of bias, high risk of bias, or unclear risk of bias. For example, in allocation concealment the grading was low risk if there was central randomisation of subjects, high risk if there was simple alternating methods used to allocate subjects and unclear if there was no real qualifying statement. We resolved disagreements by discussion. Review authors were not masked to trial details during the assessment. We excluded trials scoring 'high risk' on allocation concealment. In cases where missing or confusing data did not permit a clear grading of the trial, we contacted the study authors in order to obtain further information.
Measures of treatment effect
We measured the effect of dichotomous data by risk ratio; continuous data by difference in means; and time to event data by hazard ratio.
Unit of analysis issues
All studies were parallel‐group RCTs. In the majority of studies, one eye per person was enrolled, and therefore there were no unit of analysis issues. In Lamping 1995, WuDunn 2002 and Xinyu 2001, both eyes of some participants were enrolled, but in most cases this was less than 10%, and overall less than 5% of the data would be affected by this. None of the trials took into account the potential correlation between eyes, and we have analysed the data from the trials as reported.
Dealing with missing data
We did an available case analysis. This assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group, if reported. We collected this information as part of the assessment of attrition bias.
Assessment of heterogeneity
We examined the overall characteristics of the studies, in particular the types of participants and interventions, in order to assess the extent to which the studies were similar enough to make pooling study results sensible.
We looked at the forest plot of study results to see how consistent the studies were, in particular looking at the size and direction of effects.
We calculated I2, which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2002). We considered I2 values over 50% to indicate substantial inconsistency or heterogeneity. We also considered Chi2 P values; when the number of studies was few we used P less than 0.1 to indicate statistical significance of the Chi2 test.
Assessment of reporting biases
We planned to do a 'funnel plot' to investigate reporting (publication) bias, but there were not enough included trials (fewer than 10 in each meta‐analysis) to make this possible.
Data synthesis
If there was inconsistency between individual study results such that a pooled result may not have been a good summary of the individual trial results, for example the effects were in different directions, or I2 was greater than 50% and P less than 0.1, we did not pool the data but did describe the pattern of the individual study results.
If I2 was greater than 50%, but all the effect estimates were in the same direction such that a pooled estimate would seem to have provided a good summary of the individual trial results, we did pool the data.
If there was inconsistency between individual study results such that a pooled result may not have been a good summary of the individual trial results, for example the effects were in different directions, or I2 was greater than 50% and P less than 0.1, we did not pool the data but did describe the pattern of the individual study results.
If I2 was greater than 50%, but all the effect estimates were in the same direction such that a pooled estimate would have provided a good summary of the individual trial results, we did pool the data.
Subgroup analysis and investigation of heterogeneity
We compared the effect of intervention in a pre‐planned analysis comparing effects in groups at high and low risk of failure.
Sensitivity analysis
We conducted sensitivity analyses to determine the impact of risk of bias on effect size. We repeated the analyses excluding trials at high risk of bias in one or more domains.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 446 references (Figure 1). The Trials Search Co‐ordinator scanned the search results, removed 58 duplicates and then removed 298 references that were not relevant to the scope of the review. We screened the remaining 90 reports and discarded 69 reports as not relevant. After assessing the reports, we identified a further two studies studies for potential inclusion in the review (Oh 1994; Uva 1996). In total, we obtained 23 full‐text reports for potential inclusion in the review. After consideration of each report, we included a total of 13 reports of 11 studies in the final review; see Characteristics of included studies and excluded seven studies; see Characteristics of excluded studies for reasons. We have categorised three studies as awaiting assessment, two of which we are unable to source copies of the reports and one is awaiting a response from the authors regarding information on methods of randomisation (Liu 2015).
1.
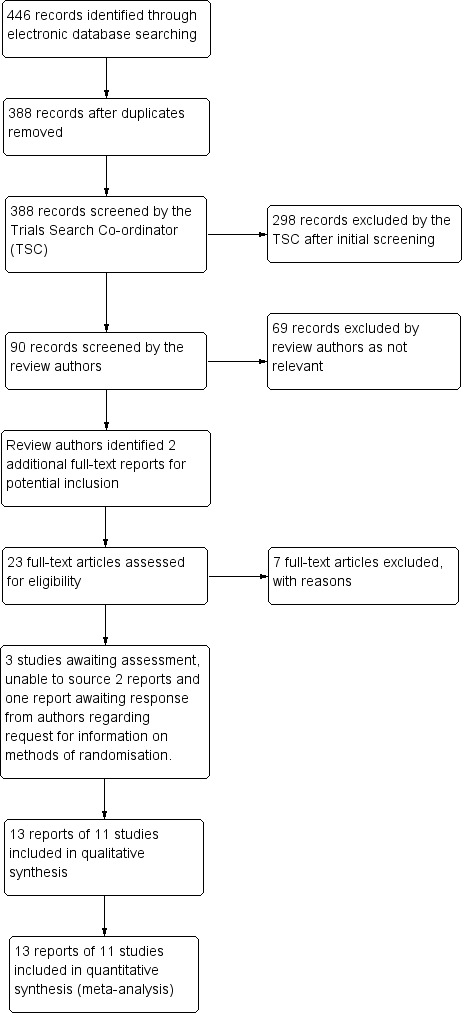
Study flow diagram
Included studies
Design
We included a total of 11 studies in this Cochrane Review and summarised them in the Characteristics of included studies. All 11 studies were designed as a prospective RCTs. One study in this review was a multicentre study (Singh 2000); the rest were single‐centre.
Setting
Four studies were based in the United States (Katz 1995; Lamping 1995; Singh 2000; WuDunn 2002), two in Italy (Sisto 2007; Uva 1996) and the remainder in Ghana (Singh 1997), Japan (Kitazawa 1991), China (Xinyu 2001), Israel (Zadok 1995) and Iran (Mostafaei 2011). All research was carried out in clinical ophthalmic institutes.
Participants and sample sizes
In total, 687 eyes of 679 participants underwent routine trabeculectomy for glaucoma control. The smallest study was of 20 eyes of 20 participants (Zadok 1995), and the largest study included 115 eyes of 103 people (WuDunn 2002). Five studies included high‐risk cases only (Katz 1995; Kitazawa 1991; Lamping 1995; Singh 1997; Sisto 2007), one study enrolled both high‐ and low‐risk cases (Xinyu 2001), and the participants in the remaining five studies were low risk. Participants across the studies were a mixture of male and female; the percentage female ranged from 19% to 67%. The average age in the studies ranged from 47 years to 71 years, with a median average age of 62 years. One study had a significant age difference (P = 0.01), with a mean age of 41.2 in the MMC group and 54.2 in the 5‐FU group (Kitazawa 1991).
Interventions
We have summarised the interventions in Table 2.
1. Interventions.
| Study | MMC* | 5‐FU | ||||||
| Dose | Duration (minutes) | Location | Intraoperative or postoperative | Dose | Number of injections | Duration | Location | |
| Katz 1995 | 0.5 mg/ml | 5 | Between the conjunctiva and the episclera | Postoperative | 5 mg | 10 (daily for 1 week, 3 times following week) | NA (injection) | Subconjunctival injection |
| Kitazawa 1991 | 0.4 mg/ml | 5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (each day for 1 week and every other day for the following week) | NA (injection) | Subconjunctival injections, 90 to 180 degrees away from the surgical site |
| Lamping 1995 | 0.4 mg/ml | 2.5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (first 10 days) | NA (injection) | Subconjunctival injection, 180 degrees from operating site |
| Mostafaei 2011 | 0.02 mg | not stated | Subconjunctival injection, 180 degrees away from operating site | Intraoperative | 5 mg | NA | Not stated | Subconjunctival injection |
| Singh 1997 | 0.5 mg/ml | 3.5 | Between scleral flap and conjunctiva | Intraoperative | 50 mg/ml | NA | 5 | Between scleral flap and conjunctiva |
| Singh 2000 | 0.4 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated |
| Sisto 2007 | 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Postoperative | 0.1 ml of 50 mg/ml | 10 (starting on day 7, 2 injections per week for 2 weeks and then 1 injection per week for 6 weeks | NA (injection) | Subconjunctival injections near the bleb |
| Uva 1996 | 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Intraoperative | 50 mg/ml | NA | 5 | Between the sclera and the Tenon's capsule |
| WuDunn 2002 | 0.2 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated |
| Xinyu 2001 | 0.2 mg/ml | 5 | Not stated | Postoperative | 5 mg | 6 to 8 (alternate days, starting on day 3) | NA (injection) | Subconjunctival, 180 degrees away from the site of scleral flap |
| Zadok 1995 | 0.2 mg/ml | 5 | Between the conjunctiva and episclera | Postoperative | 5 mg (0.5 ml of 10 mg/ml solution) | 7 (once daily up to 7 times in the first week after surgery) | NA (injection) | Subconjunctival, 180 degrees from site of surgery |
NA: not applicable
* All MMC only one intraoperative application
The majority of trials applied MMC using an intraoperative sponge; the exception was Mostafaei 2011, where 0.02mg MMC was applied by intraoperative subconjunctival injection. Subconjunctival application of MMC is not consistent with current practice (Dhingra 2009). The MMC dose given by intraoperative sponge varied between studies:
Two studies used 0.5 mg/ml applied for 5 minutes, in Katz 1995, or 3.5 minutes, in Singh 1997.
Three studies used 0.4 mg/ml applied for 5 minutes (Kitazawa 1991), 2.5 minutes (Lamping 1995), or 2 minutes (Singh 2000).
Five studies used 0.2 mg/ml applied for 5 minutes, in Xinyu 2001 and Zadok 1995, or 2 minutes, in Sisto 2007, Uva 1996 and WuDunn 2002.
The method of administration of the 5‐FU varied between studies: four studies used an intraoperative sponge technique similar to that of MMC application (Singh 1997; Singh 2000; Uva 1996; WuDunn 2002), six trials used a series of postoperative subconjunctival injections (Katz 1995; Kitazawa 1991; Lamping 1995; Sisto 2007; Xinyu 2001; Zadok 1995), and one study used intraoperative subconjunctival 5‐FU 5 mg (Mostafaei 2011). All four studies with a group receiving intraoperative sponge‐applied 5‐FU used 50 mg/ml for 5 minutes, which is consistent with current practice (Dhingra 2009).
Different dosing regimens were used for the postoperative injections.
-
Four studies used 10 postoperative injections
daily for 1 week, 3 times the following week (Katz 1995);
each day for 1 week, every other day for the following week (Kitazawa 1991);
first 10 days (Lamping 1995);
starting on day 7, 2 injections per week for 2 weeks and then 1 injection per week for 6 weeks (Sisto 2007).
-
Two studies used approximately 7 postoperative injections
once daily up to 7 times in the first week after surgery (Zadok 1995);
6 to 8 (alternate days, starting on day 3) (Xinyu 2001)
Outcomes
All of the 11 included studies stated an optimal postoperative IOP to achieve in order to accept success: five studies used a level of below 21 mmHg as desirable (Kitazawa 1991; Singh 1997; Singh 2000; Sisto 2007; Zadok 1995), two studies used equal to or less than 21 mmHg (Lamping 1995; WuDunn 2002), two used equal to or less than 12 mmHg (Katz 1995; Uva 1996), one used less than 21.06 mmHg (Xinyu 2001), and one study used 6 to 22 mmHg (Mostafaei 2011). Each study group reported their findings either as a percentage success or mean IOP.
Excluded studies
We excluded seven studies from the review: Ashworth 2003; Dreyer 1995; Li 2001; Membrey 2000; Membrey 2001; Rodriguez‐Bermejo 1993; Oh 1994. For further details please see Characteristics of excluded studies.
Risk of bias in included studies
2.
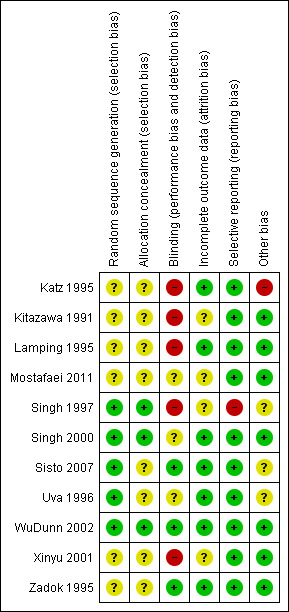
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
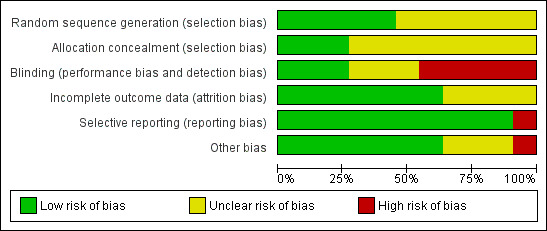
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
The review authors individually assessed the risk of bias. If the relative point was addressed in a study’s manuscript, then a true assessment of ‘high’ or ‘low’ risk was carried out. If we deemed the risk as unclear, then this indicated we could make no true assessment because the required information was not given either in the published manuscript or after making contact with the lead author.
Allocation
Five studies reported adequate methods to generate a random allocation sequence: Singh 1997 tossed a coin in the operating theatre to allocate participants; Uva 1996 used a table of random numbers; and the remaining three studies used computer‐generated allocation sequences (Singh 2000; Sisto 2007; WuDunn 2002).
It was unclear how the allocation schedule was generated in the remaining six studies.
Five studies reported adequate methods of allocation concealment (Kitazawa 1991; Singh 1997; Singh 2000; Uva 1996; WuDunn 2002)
Blinding
In four of the included studies, 5‐FU was administered using a different technique to that of MMC, and no report was given about whether or not the follow‐up information was gathered from masked assessors. We classified all these studies as high risk of performance and detection bias (Katz 1995; Kitazawa 1991; Lamping 1995; Xinyu 2001).
In one study, the method of 5‐FU administration was the same as for MMC, but information gathered in the follow‐up period was not from masked assessors. We therefore classified this study as high risk (Singh 1997).
Two studies used different techniques for antimetabolite administration but assessors were masked during the follow‐up period. We graded these two studies as low risk of performance and detection bias for the primary outcome of this review (Sisto 2007; Zadok 1995).
Only one study used a placebo to mask allocation, which we graded as at low risk of perfomance and detection bias (WuDunn 2002).
We graded the other three studies as unclear because the surgical administration of the antimetabolites was the same, but there was no mention of masking during follow‐up (Mostafaei 2011; Singh 2000; Uva 1996).
Incomplete outcome data
Four studies did not comment on the exclusion or inclusion of participants in their analysis (Kitazawa 1991; Mostafaei 2011; Singh 1997; Xinyu 2001); we classified these as unclear risk. We classified the other seven studies as low risk as participants were clearly identified as included or not. No studies raised any concern over their intention to include or exclude participants.
Selective reporting
Singh 1997 did not specify in the methods of the paper what outcomes they considered, thus we cannot be certain that all the intended outcomes were addressed; we highlighted this as high risk. All other studies commented on all stated outcomes.
Other potential sources of bias
The only other sources of bias identified were that of postoperative care with regard to what other care or medications participants received and the varied amount of 5‐FU a participant would receive with an incomplete postoperative regimen. This was highlighted in the study by Katz 1995. Other studies had no other clear identifiable bias.
Effects of interventions
See: Table 1
Failure of a functioning trabeculectomy at one year from surgery (primary outcome)
All 11 studies reported failure of a functioning trabeculectomy at approximately one year, which was defined as IOP above (approximately) 22 mmHg or more (Analysis 1.1).
1.1. Analysis.
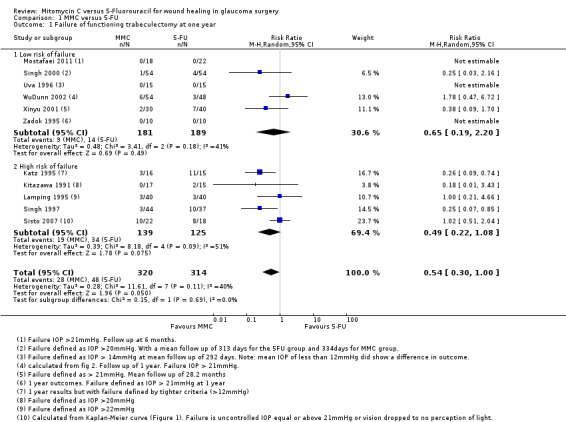
Comparison 1 MMC versus 5‐FU, Outcome 1 Failure of functioning trabeculectomy at one year.
The risk of failure of trabeculectomy at one year after surgery was lower in those treated with MMC compared to 5‐FU (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.30 to 1.00; studies = 11; I2 = 40%). However, the confidence intervals of the studies were wide, and we cannot exclude important differences.
There was no evidence for any difference between groups at high and low risk of failure (test for subgroup differences P = 0.69), but with only a few trials in each group, the power of the analysis to detect any differences was low.
The dose of MMC varied across the studies included in the review, and consequently we performed a dose‐response analysis. We identified a trend showing that studies increasingly favoured the use of MMC rather than 5‐FU as the intraoperative exposure to MMC increased (Analysis 1.2). Overall exposure was calculated by multiplying the concentration of MMC by the duration of exposure for each study. We then listed the studies in descending order of MMC exposure to view the overall effect. We excluded one study that administered the MMC by subconjunctival injection from this analysis.
1.2. Analysis.
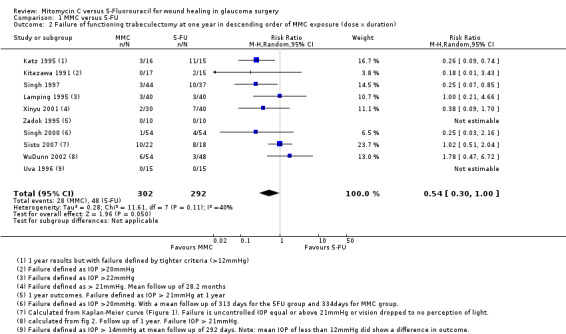
Comparison 1 MMC versus 5‐FU, Outcome 2 Failure of functioning trabeculectomy at one year in descending order of MMC exposure (dose x duration).
When considering the method of 5‐FU administration as in Analysis 1.3, there was no significant effect on the overall outcome whether the 5‐FU was administered by postoperative subconjunctival injections or by the more current method of intraoperative sponge application (subgroup difference P = 0.93)
1.3. Analysis.
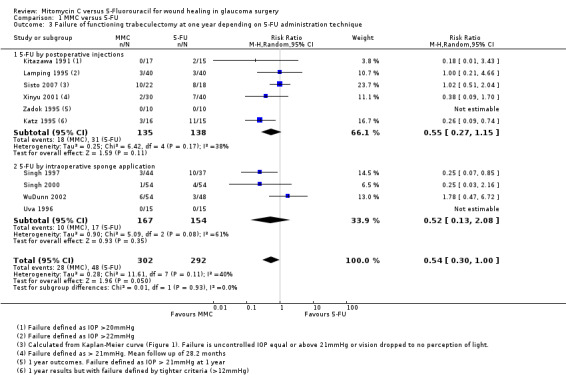
Comparison 1 MMC versus 5‐FU, Outcome 3 Failure of functioning trabeculectomy at one year depending on 5‐FU administration technique.
Time to failure of functioning trabeculectomy
No trial reported this outcome.
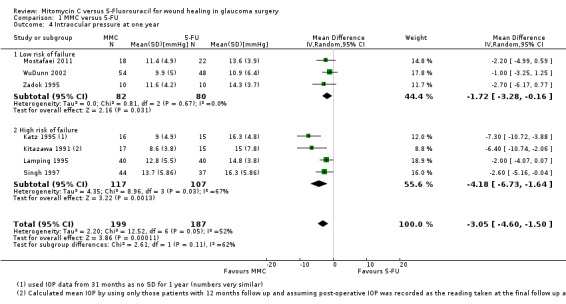
Mean IOP one year from surgery
Seven studies reported mean IOP at 12 months (range 6 to 18 months). On average, people treated with MMC had lower IOP at one year (mean difference (MD) ‐3.05 mmHg, 95% CI ‐4.60 to ‐1.50) Analysis 1.4. There was inconsistency between trials (I2 = 52%), the MD showing a large range in the studies.
1.4. Analysis.
Comparison 1 MMC versus 5‐FU, Outcome 4 Intraocular pressure at one year.
The size of the effect was greater in the high‐risk group (MD ‐4.18 mmHg, 95% CI ‐6.73 to ‐1.64) compared to the low‐risk group (MD ‐1.72 mmHg, 95% CI ‐3.28 to ‐0.16), but the test for interaction was not statistically significant (P = 0.11).
Postoperative use of antiglaucoma medications
Seven studies reported on the frequency of postoperative use of antiglaucoma medications. Similar proportions of people treated with MMC and 5‐FU required postoperative medication to control pressure (RR 1.03, 95% CI 0.57 to 1.85) (Analysis 1.5). There was no evidence for any difference in effect between high‐risk and low‐risk groups (P = 0.88). The low‐risk group trials were consistent (I2 = 0%), but we saw different results in the three higher‐risk group trials (I2 = 74%).
1.5. Analysis.
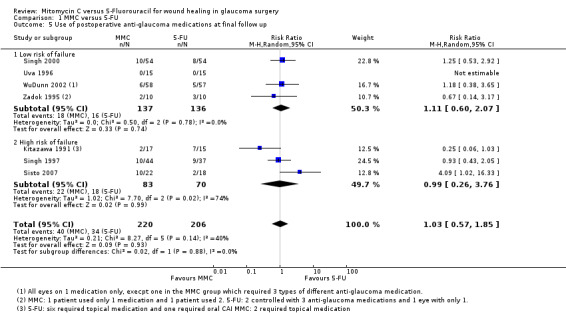
Comparison 1 MMC versus 5‐FU, Outcome 5 Use of postoperative anti‐glaucoma medications at final follow up.
Four studies reported the mean number of antiglaucoma medications used. On average, people receiving MMC used fewer antiglaucoma medications (MD ‐0.33, 95% CI ‐0.70 to 0.05), but the effect was uncertain (CIs include 0.00), and the studies were inconsistent (I2 = 71%) (Analysis 1.6). The inconsistency in the trials came from those with a higher risk of failure (I2 = 62%). However, there was a difference between the trials including participants at high risk of failure and those including participants at low risk of failure with a greater relative effect of MMC in the higher‐risk groups (test for interaction P = 0.06). The main caveat was that there were only two trials in each group of the analysis.
1.6. Analysis.
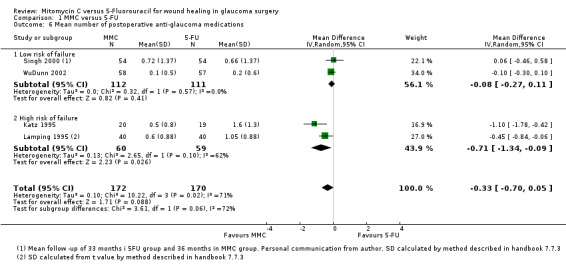
Comparison 1 MMC versus 5‐FU, Outcome 6 Mean number of postoperative anti‐glaucoma medications.
Reduction in visual acuity
Five studies reported postoperative visual acuity. The proportion of eyes treated with MMC that lost 2 or more lines of visual acuity one year after surgery was similar to that of 5‐FU, but the CIs were wide (RR 1.05, 95% CI 0.54 to 2.06) Analysis 1.7.
1.7. Analysis.
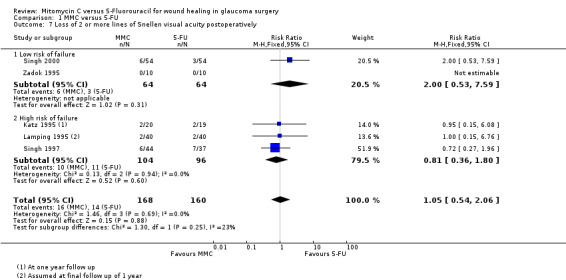
Comparison 1 MMC versus 5‐FU, Outcome 7 Loss of 2 or more lines of Snellen visual acuity postoperatively.
Quality of life
No trial reported this outcome.
Economic data
No trial reported this outcome.
Adverse outcomes
Bleb leak
Two studies reported bleb leak as a complication encountered following trabeculectomy. Participants receiving MMC were more likely to have a postoperative bleb leak, although the CI was wide, and only two studies reported this outcome (RR 1.22, 95% CI 0.32 to 4.68; I2 = 0%).
Six studies used the term 'wound leak' rather than 'bleb leak' in their assessment of postoperative complications. These studies also showed, with similar statistics, that participants receiving MMC were more likely to have a postoperative wound leak than those receiving 5‐FU, although the CI was wide (RR 1.17, 95% CI 0.51 to 2.71; I2 = 0%).
Late hypotony
Five studies reported hypotony post‐trabeculectomy. Participants receiving MMC were more likely to have postoperative hypotony compared to those participants who received 5‐FU, however the effect was uncertain with wide CIs compatible with no effect or increased hypotony in the 5‐FU group (RR 1.37, 95% CI 0.41 to 4.63; I2 = 0%).
Maculopathy
Four studies reported maculopathy following trabeculectomy. Participants receiving MMC were more likely to encounter maculopathy postoperatively than those receiving 5‐FU, but the effect was uncertain and CI compatible with no effect or increased maculopathy in the 5‐FU group (RR 1.71, 95% CI 0.35 to 8.33; I2 = 0%).
Cataract
Four studies reported the incidence of postoperative cataract development. Participants receiving MMC were more likely to develop cataract than those receiving 5‐FU, but again the CIs include 1 (null effect) (RR 1.73, 95% CI 0.65 to 4.61; I2 = 24%).
Shallow anterior chamber
Five studies noted postoperative shallowing of the anterior chamber. Those participants receiving MMC were more likely to present with a shallow anterior chamber than those who received 5‐FU. The statistical analysis showed a wide CI (RR 1.22, 95% CI 0.67 to 2.21; I2 = 0%).
Choroidal detachment
Nine studies (549 eyes) reported a choroidal detachment as a postoperative complication following trabeculectomy. Three studies (303 eyes) reported the same event as a 'suprachoroidal haemorrhage'. The former group of studies found no difference in the rate of events between those participants who received MMC and those who received 5‐FU (RR 0.86, 95% CI 0.45 to 1.63; I2 = 0%). The latter group of studies favoured those participants who received MMC, although the CI was wide (RR 0.73, 95% CI 0.09 to 5.66; I2 = 18%).
Epitheliopathy
Nine studies (474 eyes) reported this complication following trabeculectomy. Those participants who received MMC were less likely to have an epitheliopathy following surgery than those who received 5‐FU, which is most likely a result of the differences in the technique of antimetabolite application (RR 0.23, 95% CI 0.11 to 0.47; I2 = 0%).
Tenon's cyst
Four studies (232 eyes) reported Tenon's cysts in their postoperative complication analysis. Those participants who received MMC were less likely to have a Tenon's cyst following surgery, although the CI was wide (RR 0.94, 95% CI 0.20 to 4.38; I2 = 34%).
Hyphaema
Four studies (250 eyes) documented postoperative hyphaema during their follow‐up of participants. Participants who received MMC were less likely to have a postoperative hyphaema than those who received 5‐FU, which may be a consequence of antimetabolite application differences (RR 0.62, 95% CI 0.42 to 0.91; I2 = 0%).
Endophthalmitis
Four studies (315 eyes) published rates of postoperative endophthalmitis. Participants receiving MMC were more likely to have endophthalmitis following trabeculectomy than those who received 5‐FU. The CI was wide (RR 3.89, 95% CI 0.44 to 34.57; I2 = 0%).
Sensitivity analyses (excluding studies at high risk of bias)
An interesting feature of these analyses was that the trials at high risk of bias were also the trials recruiting participants at high risk of failure. In general, excluding these studies improved the consistency (reduced I2). Although the estimate of effect changed in these analyses, in general the conclusions (of uncertainty in most cases) did not.
| Outcome | Name | All trials | Excluding trials at high risk of bias in 1 or more domains |
| Analysis 1.1* | Failure of functioning trabeculectomy at 1 year | RR 0.54, 95% CI 0.30 to 1.00 | RR 1.02, 95% CI 0.50 to 2.04 |
| Analysis 1.4* | Mean intraocular pressure at 1 year | MD ‐3.05 mmHg, 95% CI ‐4.60 to ‐1.50 | MD ‐1.72 mmHg, 95% CI ‐3.28 to ‐0.16 |
| Analysis 1.5** | Use of postoperative medication at 1 year | RR 1.03, 95% CI 0.57 to 1.85 | RR 1.39, 95% CI 0.75 to 2.57 |
| Analysis 1.6* | Mean number of postoperative medications at 1 year | MD ‐0.33, 95% CI ‐0.70 to 0.05 | MD ‐0.08, 95% CI ‐0.27 to 0.11 |
| Analysis 1.7** | Loss of 2 or more lines of visual acuity at 1 year | RR 1.05, 95% CI 0.54 to 2.06 | RR 2.00, 95% CI 0.53 to 7.59 |
CI: confidence interval; MD: mean difference; RR: risk ratio
* The trials at high risk of bias were also the trials of the subgroup at high risk of failure.
** Two trials with a high risk of failure and one trial with a low risk of failure were excluded.
Discussion
Summary of main results
We have summarised the results in the Table 1.
We identified 11 trials conducted in the United States, Europe, Asia and Africa. Five studies enrolled participants at low risk of trabeculectomy failure, five studies enrolled participants at high risk of failure, and one study enrolled people with both high and low risk of failure. None of the included trials enrolled participants with combined trabeculectomy/cataract surgery.
We considered one study to be at low risk of bias in all domains, six studies at high risk of bias in one or more domains, and the remaining four studies at an unclear risk of bias.
Our review showed that the risk of failure of trabeculectomy at one year after surgery was lower in those participants treated with MMC compared to those treated with 5‐FU. However, the estimate of effect was imprecise, and we cannot exclude important differences. All 11 RCTs contributed to this finding with an overall mixed study population and varied methodology of antimetabolite application. Although MMC appeared to have a greater success and more of an IOP‐lowering effect in the higher‐risk populations, we detected no significant difference between the subgroups of participants at low and high risk of failure in these analyses. We identified no difference between the visual outcomes of the people receiving MMC or 5‐FU at one year postoperatively nor in the number of drops used postoperatively.
Evaluation of postoperative complications showed that there was a higher incidence of epitheliopathy and hyphaema when using 5‐FU compared to MMC. However, we found those participants who received MMC to have more reported bleb leaks, wound leaks, late hypotony and cataract formation than those who received 5‐FU. The quality of the evidence was low given that in general adverse outcomes were rare, and hence estimates of effect were imprecise. Although there were trends, any real significance cannot be determined from this review alone.
None of the studies reported quality of life.
Overall completeness and applicability of evidence
This review is limited owing to the small numbers of and large variability between studies, for example in participant demographics, methodology, masking of participants and varied follow‐up. Some of the included studies had only 20 or 30 participants in their study population, which contrasts with the largest study, which had 115 participants.
After many years of widespread use of antimetabolite agents, uncertainty remains about the relative benefits and harms of their use in trabeculectomy surgery. Newer agents and techniques may be developed and evaluated to then eventually take over the role of antimetabolites.
The majority of studies were carried out in the United States, although we included studies from European, Asian, African and Middle Eastern countries. Two of the included papers, one in Chinese and one in Italian, were translated. The analysis has taken into account the risk of failure of each study population and reported the risk as high or low. This is important when interpreting the results in a clinical setting in order to reflect the practice population. However, results showed a similar trend between high‐ and low‐risk participants, which perhaps may be due to the inclusion of poor‐quality evidence as discussed.
Quality of the evidence
Overall, we graded the quality of the evidence as low, in most cases because of risk of bias in the included studies and imprecision in the estimate of effect. One commonly encountered bias came from the difficulty in masking participants and surgeons owing to the different techniques of antimetabolite administration. All studies included in the review were RCTs, but the variability in outcome reporting reduced the quality of the evidence for some outcomes. Each study group reported few complications, which subsequently led to small numbers being incorporated into the analysis of complications.
Potential biases in the review process
We identified no obvious bias from the review process.
Agreements and disagreements with other studies or reviews
Fendi 2013 completed a meta‐analysis that showed significantly higher success rates with the use of MMC when compared with 5‐FU. This analysis included only five studies with participants who had recieved previous surgical treatment. Lin 2012 found in an analysis of eight studies that MMC achieved a significantly lower postoperative IOP than 5‐FU, but MMC and 5‐FU were comparable in achieving success. Likewise, Abdu 2010 found similar results to both Lin 2012 and this review with little difference between the two antimetabolites at achieving success. Abdu 2010 also also found that there was no difference in the mean postoperative IOP between participants who received MMC and participants who received 5‐FU and suggest that further research in this area would enhance results to determine any true superiority of either MMC or 5‐FU.
Authors' conclusions
Implications for practice.
This review provided low‐quality evidence that to achieve lower IOP following trabeculectomy MMC may be a more effective antimetabolite than 5‐FU across both high‐ and low‐risk populations. The risk associated with using either MMC or 5‐FU as an antimetabolite in a routine trabeculectomy was low given the infrequent reporting of adverse outcomes.
Implications for research.
Antimetabolites are a widely used adjunct in trabeculectomy surgery to help achieve lower postoperative IOP. However, the use of these medications may be associated with an increased risk of sight‐threatening complications, predominantly due to the toxic effects on the conjunctiva and Tenon's capsule.
Further comparative research on MMC and 5‐FU would be required to enhance reliability and validity of the results shown in this review. However, the development of newer, safer agents to control wound healing in glaucoma surgery may be of more benefit to patients in the longer term. These future agents would require full evaluation with well‐designed trials to become integrated into clinical practice, particularly through the inclusion of trials with higher power to detect minimally important clinical differences and to consider cost and patient‐orientated outcomes.
History
Protocol first published: Issue 4, 2006 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 10 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Eyes and Vision Group created and executed the electronic searches. We thank Catey Bunce, Tianjing Li and Mark Wilkins for providing comments on the protocol for this review and Richard Wormald and Anupa Shah for their assistance on the protocol. We thank Marie Diener‐West and Anthony King for their comments on the review and also Hsin‐wen Amanda Wu for translation services for the review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Glaucoma] explode all trees #2 MeSH descriptor: [Filtering Surgery] explode all trees #3 MeSH descriptor: [Trabeculectomy] explode all trees #4 MeSH descriptor: [Sclerostomy] explode all trees #5 ((surg* near glaucoma) or filter* or filtrat*) #6 surg* near intra ocular pressure #7 trabeculectom* #8 sclerostom* #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Mitomycin] explode all trees #11 mitomycin* #12 mytomycin* #13 mitomicin* #14 mytomicin* #15 #10 or #11 or #12 or #13 or #14 #16 MeSH descriptor: [Fluorouracil] explode all trees #17 fluorouracil #18 flourouracil #19 fluoro uracil #20 5FU* #21 5 FU* #22 #16 or #17 or #18 or #19 or #20 or #21 #23 #9 and #15 and #22
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp glaucoma/ 14. exp intraocular pressure/ 15. exp filtering surgery/ 16. trabeculectom$.tw. 17. sclerostom$.tw. 18. ((surg$ or filter$ or filtrat$) adj5 glaucoma$).tw. 19. (surg$ adj5 intra?ocular pressure$).tw. 20. or/13‐19 21. exp mitomycin/ 22. mitomycin$.tw. 23. mytomycin$.tw. 24. mitomicin$.tw. 25. mytomicin$.tw. 26. or/21‐25 27. exp fluorouracil/ 28. fluorouracil$.tw. 29. flourouracil$.tw. 30. fluoro uracil$.tw. 31. 5FU$.tw. 32. 5 FU$.tw. 33. or/27‐32 34. 20 and 26 and 33 35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp glaucoma/ 34. exp filtering surgery/ 35. exp trabeculectomy/ 36. exp sclerostomy/ 37. ((surg$ or filter$ or filtrat$) adj5 glaucoma$).tw. 38. (surg$ adj2 intra?ocular pressure$).tw. 39. trabeculectom$.tw. 40. sclerostom$.tw. 41. or/33‐40 42. exp mitomycin/ 43. mitomycin$.tw. 44. mytomycin$.tw. 45. mitomicin$.tw. 46. mytomicin$.tw. 47. or/42‐46 48. exp fluorouracil/ 49. fluorouracil.tw. 50. flourouracil.tw. 51. fluoro uracil.tw. 52. 5FU$.tw. 53. 5 FU$.tw. 54. or/48‐53 55. 41 and 47 and 54 56. 32 and 55
Appendix 4. LILACS search strategy
glaucom$ OR intraoccular presure OR trabeculectom$ and mitomycin or mytomycin or mitomicin or mytomicin and fluorouracil or flourouracil or fluoro uracil or 5FU
Appendix 5. ISRCTN search strategy
"( Condition: glaucoma AND Interventions: mitomycin OR mytomycin OR mitomicin OR mytomicin )"
Appendix 6. ClinicalTrials. gov search strategy
Glaucoma AND (mitomycin OR mytomycin OR mitomicin OR mytomicin) AND (fluorouracil OR flourouracil OR fluoro uracil OR 5FU OR 5 FU)
Appendix 7. ICTRP search strategy
Condition = Glaucoma AND Intervention mitomycin AND fluorouracil
Data and analyses
Comparison 1. MMC versus 5‐FU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of functioning trabeculectomy at one year | 11 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 1.1 Low risk of failure | 6 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.19, 2.20] |
| 1.2 High risk of failure | 5 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 2 Failure of functioning trabeculectomy at one year in descending order of MMC exposure (dose x duration) | 10 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 3 Failure of functioning trabeculectomy at one year depending on 5‐FU administration technique | 10 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 3.1 5‐FU by postoperative injections | 6 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.27, 1.15] |
| 3.2 5‐FU by intraoperative sponge application | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.13, 2.08] |
| 4 Intraocular pressure at one year | 7 | 386 | Mean Difference (IV, Random, 95% CI) | ‐3.05 [‐4.60, ‐1.50] |
| 4.1 Low risk of failure | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐3.28, ‐0.16] |
| 4.2 High risk of failure | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐4.18 [‐6.73, ‐1.64] |
| 5 Use of postoperative anti‐glaucoma medications at final follow up | 7 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.57, 1.85] |
| 5.1 Low risk of failure | 4 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.60, 2.07] |
| 5.2 High risk of failure | 3 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.26, 3.76] |
| 6 Mean number of postoperative anti‐glaucoma medications | 4 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.70, 0.05] |
| 6.1 Low risk of failure | 2 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 6.2 High risk of failure | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.34, ‐0.09] |
| 7 Loss of 2 or more lines of Snellen visual acuity postoperatively | 5 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.06] |
| 7.1 Low risk of failure | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.59] |
| 7.2 High risk of failure | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.80] |
| 8 Postoperative Complications | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Bleb leak | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.32, 4.68] |
| 8.2 Wound leak | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.51, 2.71] |
| 8.3 Late hypotony | 4 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.41, 4.63] |
| 8.4 Maculopathy | 4 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.35, 8.33] |
| 8.5 Cataract | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.65, 4.61] |
| 8.6 Shallow anterior chamber | 5 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.21] |
| 8.7 Choroidal detachment | 8 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.63] |
| 8.8 Epitheliopathy | 8 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.11, 0.47] |
| 8.9 Tenon cyst | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.38] |
| 8.10 Hyphaema | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.91] |
| 8.11 Suprachoroidal haemorrhage | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.09, 5.66] |
| 8.12 Endophthalmitis | 4 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 3.89 [0.44, 34.57] |
1.8. Analysis.
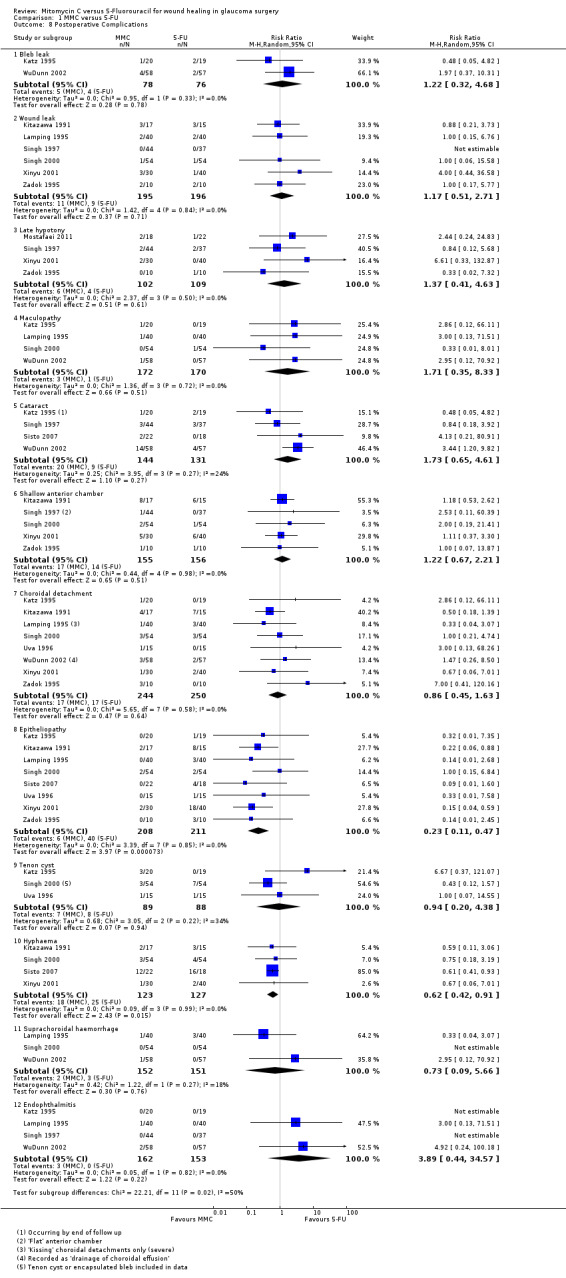
Comparison 1 MMC versus 5‐FU, Outcome 8 Postoperative Complications.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Katz 1995.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: USA Number of participants (eyes): 39 (39) % women: 67% Average age: 63 years (range not reported) Risk of trabeculectomy failure: high Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
All surgeries involved a limbus‐based conjunctival flap. Scleral flap was closed by 10‐0 nylon sutures. Postoperative topical steroids were used in all participants and tapered over several weeks. 4 surgeons were involved in the study |
|
| Outcomes | Postoperative IOP Number of glaucoma medications used Change in visual acuity Follow‐up: 1 and 2 years |
|
| Notes | Date study conducted: May 1990 to March 1991 Conflict of interest: None declared Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised" but no elaboration of methods used |
| Allocation concealment (selection bias) | Unclear risk | No mention of patient concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Surgical method varied between both the groups, so masking for the surgeon and participant was impossible. No mention as to masking during the follow‐up period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up recorded for all participants with recognition of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were identified and discussed |
| Other bias | High risk | Participants may have received different postoperative treatment: "The use of antibiotics, cycloplegics, digital massage and laser suture lysis were left to the discretion of the surgeon". Participants received different doses of 5‐FU (average 46.0 mg, +/‐ 4.9 mg) |
Kitazawa 1991.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Japan Number of participants (eyes): 32 (32) % women: 38% Average age: 47 years (range 22 to 81) Risk of trabeculectomy failure: high Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Following the trabeculectomy, 10‐0 monofilament nylon suture was used for the scleral flap, and 10‐0 nylon shoelace suture was used for the conjunctival wound closure. Postoperatively 1.2 mg of dexamethasone was injected subconjunctivally. Topical atropine and antibiotics were given at the time of surgery. 0.1% betamethasone, 1% atropine sulfate and 0.3% ofloxacine were used as a standard for all participants postop |
|
| Outcomes | Mean IOP at 12 months Category 1 success: IOP controlled without antiglaucoma medication Category 2 success: IOP controlled with or without topical eye drops Category 3 success: IOP controlled without any medication or with oral carbonic anhydrase inhibitors in addition to topical medication Success was defined as IOP equal to or less than 20 mmHg without any medication. Follow‐up: 7 to 12 months |
|
| Notes | Date study conducted: December 1989 to November 1990 Conflict of interest: None declared Funding source: Research grant for Aging and Health from the Ministry of Health and Welfare, Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear methods described. Significant difference between the ages of each group (P = 0.01) |
| Allocation concealment (selection bias) | Unclear risk | Randomly allocated to intervention groups, but no elaboration on method used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking due to nature of 2 techniques of administration for the interventions, and no mention of follow‐up masking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statements about attrition or exclusion made |
| Selective reporting (reporting bias) | Low risk | Stated outcome measures were reported |
| Other bias | Low risk | The study appears to be free from other bias |
Lamping 1995.
| Methods | Parallel‐group randomised controlled trial, 1 or both eyes included | |
| Participants | Country: USA Number of participants (eyes): 74 (80) % women: 41% Average age: 71 years (range not reported) Risk of trabeculectomy failure: high Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
No steroid or antibiotic was used at the time of surgery, but topical prednisolone, tobramycin and dexamethasone and atropine were applied postoperatively. Single surgeon |
|
| Outcomes | Postoperative IOP Follow‐up: week 1, week 2 and months 1, 2, 3, 6, 9 and 12 |
|
| Notes | Date study conducted: Not reported Conflict of interest: None declared Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consecutive eyes were selected, no random sequence generation mentioned |
| Allocation concealment (selection bias) | Unclear risk | Randomised allocation to each intervention group, but no elaboration of methods used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No masking due to different methods of application of the 2 interventions, and no mention of follow‐up masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Recognition of 1 postop complication that stopped the use of antimetabolite therapy in this participant. This participant was not excluded from the study |
| Selective reporting (reporting bias) | Low risk | Stated outcome measures were reported |
| Other bias | Low risk | Participants in the 5‐FU group received varied amounts of antimetabolite due to withholding of treatments if indicated by complications. 8 participants did not receive the full dose. This was taken into account in the data analysis |
Mostafaei 2011.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Iran Number of participants (eyes): 40 (40) % women: 19% Average age: 68 years (range 48 to 83) Risk of trabeculectomy failure: low Inclusion criteria:
Exclusion criteria: none reported |
|
| Interventions |
|
|
| Outcomes | Primary outcome of successful surgery defined as an IOP of 6 to 22 mmHg at 6 months postoperatively. Secondary outcome: complications identified at the 6‐month follow‐up IOP using Goldmann applanation Complications Follow‐up: baseline, 2 weeks postoperatively, 1, 3 and 6 months |
|
| Notes | Date study conducted: Not reported Conflict of interest: None declared Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Parallel trial design, but the details not described |
| Allocation concealment (selection bias) | Unclear risk | No report on concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No report on masking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No report on loss to follow‐up. One reported complication of surgery was observed but it was not made clear to which study group this participant belonged to. |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were identified and reported |
| Other bias | Low risk | The study appeared to be free from other bias |
Singh 1997.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Ghana Number of participants (eyes): 81 (81) % women: 40% Average age: 54 years (range not reported) Risk of trabeculectomy failure: high Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Limbal‐based conjunctival flaps. Antimetabolite delivered with a sponge and thoroughly irrigated after required time. 5 surgeons with small variation on technique. Day 1 postop is when topical gentamycin, prednisolone acetate and atropine therapy started |
|
| Outcomes | IOP outcomes: < 21 mmHg, < 18 mmHg and < 15 mmHg Visual acuity Postoperative complications Follow‐up: Post‐operative days 1, 3, 7 and 14 and then average longer term follow up of 10 months (+/‐ 4.41) |
|
| Notes | Date study conducted: Reported completed in 1995 Conflict of interest: Not reported Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treated decided by the flick of a coin in the operating theatre |
| Allocation concealment (selection bias) | Low risk | Surgeons masked from allocation up until time of surgery (minimal influence) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Surgeons were masked up until time of surgery but not thereafter. Method of administration of treatment similar between groups. Follow‐up team were not masked as to which antimetabolite the participant had received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attempts made to contact missing participants. No description about the number of participants lost to follow‐up. 81/85 participants had at least 3 months' follow‐up |
| Selective reporting (reporting bias) | High risk | No stated outcomes in the methods |
| Other bias | Unclear risk | Insufficient information |
Singh 2000.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person Multicentre |
|
| Participants | Country: USA Number of participants (eyes): 108 (108) % women: not reported Average age: 66 years (range not reported) Risk of trabeculectomy failure: low Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Limbal‐based conjunctival flaps, closed with 8‐0 or 9‐0 polyglactin 910 (Vicryl) suture. Antimetabolite delivered with a sponge and thoroughly irrigated after required time. 18 surgeons were involved in the study. 8 centres |
|
| Outcomes | This is the preliminary report with plans to extend follow‐up time. Main outcome measures were IOP and proportion of participants achieving successful outcomes, with varying IOP criteria for success (< 21 mmHg, < 18 mmHg, < 15 mmHg and < 12 mmHg). Post‐operative visual acuity, complications and use of IOP‐lowing medications were also included in the follow up data. |
|
| Notes | Date study conducted: December 1996 Conflict of interest: None declared Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a modified Moses‐Oakford algorithm, and the randomisation envelope mailed to the study co‐ordinators at the respective sites |
| Allocation concealment (selection bias) | Low risk | Participating surgeons were masked with regard to antimetabolite use until after participant enrolment into the study and written informed consent |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Surgeons were masked with regard to antimetabolite use until after participant enrolment into the study. Participants and follow‐up period were not masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants not included in analysis due to lack of pre‐ or intraoperative information |
| Selective reporting (reporting bias) | Low risk | Stated outcome measures in the methods were reported in the results |
| Other bias | Low risk | The study appears free of other sources of bias |
Sisto 2007.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Italy Number of participants (eyes): 40 (40) % women: 35% Average age: 61 years (range 36 to 75) Risk of trabeculectomy failure: high Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Fornix‐based conjunctival flaps with single surgeon. No releasable sutures or suture lysis employed |
|
| Outcomes | Success defined as IOP < 21 mmHg at final postoperative visit. Qualified success defined if IOP < 21 mmHg with addition of topical treatment. Failure is uncontrolled IOP equal or above 21 mmHg or vision dropped to no perception of light. Follow‐up: every 3 months in the first year, every 6 months thereafter to maximum 60 months (5 years) |
|
| Notes | Date study conducted: January 1993 to November 2000 Conflict of interest: Not reported Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 40 consecutive people with neovascular glaucoma selected. "All eyes had been assigned with a computer generated randomization code." |
| Allocation concealment (selection bias) | Unclear risk | No statement made |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Surgeon not masked due to technique, but the follow‐up staff were masked on collecting postoperative data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participant data included in analysis. However, length of follow‐up was variable and no statement was made regarding the participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Success criteria defined in the methods |
| Other bias | Unclear risk | No attempted power calculations |
Uva 1996.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Italy Number of participants (eyes): 30 (30) % women: 47% Average age: 54.1 years (range 45 to 60) Risk of trabeculectomy failure: low Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Limbal flap was used that was closed with 10‐0 nylon suture. 1% atropine, antibiotic and steroid was applied at the time of surgery. Conjunctiva was closed with 8‐0 polyglactin synthetic suture |
|
| Outcomes | Postoperative IOP Visual acuity Postoperative complications Follow‐up: Mean follow‐up 292 days +/‐ 46.1 days |
|
| Notes | Date study conducted: Not reported Conflict of interest: Not reported Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with a "table of numbers" technique |
| Allocation concealment (selection bias) | Unclear risk | No statement made |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant masking carried out given same surgical procedure for both antimetabolite interventions. Surgeons are presumed to not be masked given different duration of antimetabolite application. No mention of follow‐up masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Commented on all intended outcomes. Short period of follow‐up with all participants recorded within similar follow‐up period |
| Selective reporting (reporting bias) | Low risk | Stated outcome measures in the methods were reported in the results |
| Other bias | Unclear risk | No obvious further bias |
WuDunn 2002.
| Methods | Parallel‐group randomised controlled trial, 1 or both eyes per person | |
| Participants | Country: USA Number of participants (eyes): 103 (115) % women: 44% Average age: 65 years Risk of trabeculectomy failure: low Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Limbal‐based conjunctival flaps, closed with 8‐0 polyglactin 910 (Vicryl) suture. Antimetabolite was delivered with a cellulose sponge and thoroughly irrigated after required time. The application was divided into 2 phases to allow surgeon masking through same time of antimetabolitie/sham application. Corticosteroid, antibiotic ointment and atropine were instilled at the time of surgery. Postoperatively, all eyes received 1% prednisolone acetate, 1% atropine and an antibiotic |
|
| Outcomes |
|
|
| Notes | Date study conducted: 1997 to 2001 Conflict of interest: Not reported Funding source: Research to Prevent Blindness, Inc, New York, New York NCT00346489 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The assignment schedule was generated in blocks of 50 (25 per group) by a study co‐ordinator who was not involved in the surgical procedure or clinical care. If the second eye of the participant was also enrolled, it was assigned to the opposite group of the first eye |
| Allocation concealment (selection bias) | Low risk | Group assignment was made randomly on the day of the surgery by the study co‐ordinator and relayed directly to the operating room circulating nurse who prepared the antimetabolite solutions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the study co‐ordinator and the circulating nurse knew the allocation. The assignment code was kept in a locked drawer in the office of the study coordinator. The code was broken at 6 months postoperative to allow data analysis. Surgeons were kept masked by using the same colour solution and the same duration of sponge application for both intervention groups (the MMC group had 3‐minute application of balanced salt solution to equal the 5‐minute 5‐FU group in total) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clearly identified participants: 1 participant in each group failed to reach 6‐month follow‐up. By 1 year, the lost to follow‐up were 9 in the 5‐FU group and 4 in the MMC group. |
| Selective reporting (reporting bias) | Low risk | Stated outcome measures in the methods were reported in the results |
| Other bias | Low risk | The study appears free of other sources of bias |
Xinyu 2001.
| Methods | Parallel‐group randomised controlled trial, 1 or both eyes included | |
| Participants | Country: China Number of participants (eyes): 98 (108) % women: 57% Average age: 54 years (range 16 to 76) Risk of trabeculectomy failure: Mixed population: mostly low‐risk population, 4 participants with previous surgery were termed "high risk" Inclusion criteria: Of those in the 5‐FU group, 33 eyes were angle‐closure glaucoma, 3 open‐angle glaucoma, 2 eyes had glaucoma recurrent following previous control and 2 with previous glaucoma surgery. Of those in the MMC group, 25 had angle‐closure glaucoma, 2 open‐angle, 1 recurrent and 2 with previous glaucoma surgery. In the control group, 33 had angle‐closure glaucoma, 3 open‐angle and 2 with recurrence. Exclusion criteria: Not mentioned |
|
| Interventions |
All surgeons used same standard surgical technique. Control (untreated) group also included. (n = 38) |
|
| Outcomes | Measure of corneal scarring and corneal staining, postoperative IOP and occurrence of complications (conjunctivitis, vitreous detachment, hyphaema, corneal epithelial defect, hypotony and corneal ulcer). Successful reduction in IOP defined as < 21.06 mmHg. Follow‐up: twice a week for 2 weeks, once a week for the subsequent 4 weeks, and then 1 or 2 times a month thereafter |
|
| Notes | Date study conducted: May 1995 to October 1999 Conflict of interest: Unable to ascertain with manuscript translation Funding source: Unable to ascertain with manuscript translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random division into groups, but no detail given |
| Allocation concealment (selection bias) | Unclear risk | No information of allocation methods |
| Blinding (performance bias and detection bias) All outcomes | High risk | Application methods different for all 3 groups, and therefore difficult to mask surgeons and participants. Follow‐up of participants varied between 3 and 34 months with no clear statement about minimum length of follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of attrition/exclusion |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were addressed |
| Other bias | Low risk | No other obvious bias identified |
Zadok 1995.
| Methods | Parallel‐group randomised controlled trial, 1 eye per person | |
| Participants | Country: Israel Number of participants (eyes): 20 (20) % women: 45% Average age: 69 years (range not reported) Risk of trabeculectomy failure: low Inclusion criteria:
Exclusion criteria: Not mentioned |
|
| Interventions |
Closure of scleral flap by 10‐0 nylon sutures. Conjunctiva closed by running suture. All participants received 1% atropine sulphate twice daily for 4 weeks and dexamethasone 4 times daily, tapered over several weeks |
|
| Outcomes | IOP < 21 mmHg as a primary outcome with or without antiglaucoma medication. Follow‐up: Participants reviewed at 1 week postoperatively, 2 weeks, 1, 2, 3, 6 and 12 months |
|
| Notes | Date study conducted: Not reported Conflict of interest: Not reported Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of method of selection. Randomised |
| Allocation concealment (selection bias) | Unclear risk | No mention of how participants were concealed from their respective allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Surgeons not masked given different administration techniques. Follow‐up completed by masked professionals |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Evidence from Table 3 (IOP distributions at 6 and 12 months) that all participants in study reached full follow‐up period |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in manuscript |
| Other bias | Low risk | No other bias evident |
5‐FU: 5‐Fluorouracil IOP: intraocular pressure MMC: mitomycin C
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashworth 2003 | Prospective, non‐randomised trial |
| Dreyer 1995 | Not a randomised controlled study. No data on intraocular pressure as an outcome, therefore does not match inclusion criteria |
| Li 2001 | Prospective, non‐randomised trial |
| Membrey 2000 | Retrospective study |
| Membrey 2001 | Case‐control; not a randomised controlled study |
| Oh 1994 | Random allocation not mentioned; no reply from authors to request for clarification |
| Rodriguez‐Bermejo 1993 | Manuscript not available for review |
Characteristics of studies awaiting assessment [ordered by study ID]
Liu 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting response from authors regarding our query on methods of randomisation |
Susanna 1995.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to locate copy of the report |
Yamamoto 1997.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to locate copy of the report |
Differences between protocol and review
We did not include outomes 1.4, 1.5 and 1.6 in the protocol. Use of postoperative glaucoma medication is a surrogate for partial trabeculectomy failure and was measured as an outcome in several of the included studies. Visual acuity was another commonly reported outcome in the included studies, in particular a loss of 2 lines of Snellen visual acuity. We therefore considered it appropriate to include these two outcomes in our review given their use in the assessment of trabeculectomy outcomes.
No data were available on time to failure as no studies were found to use Kaplan‐Meier survival analysis as an outcome measure. Additionally, only one study commented clearly on non‐attendance rates. No data were available on quality‐of‐life measures. We therefore did not report outcomes for time to failure, quality of life and non‐attendance rate.
Late hypotony, endophthalmitis and choroidal detachment are of clinical interest and significance when concerning trabeculectomy. We therefore chose these adverse outcomes as priority in the Table 1.
Contributions of authors
Conceiving the review: JCKC, PGS Designing the review: JCKC, PGS Coordinating the review: JCKC, EC Undertaking manual searches: JCKC, PGS Screening search results: JCKC, PGS Organising retrieval of papers: JCKC, PGS, EC Screening retrieved papers against inclusion criteria: JCKC, PGS, EC, JE Appraising quality of papers: JCKC, PGS, EC Abstracting data from papers: JCKC, PGS, EC, JE Writing to authors of papers for additional information: JCKC, PGS, EC Obtaining and screening data on unpublished studies: JCKC, PGS Data management for the review: JCKC, PGS, EC, JE Entering data into RevMan: JCKC, PGS, EC Analysis of data: JCKC, PGS, EC, JE Interpretation of data: JCKC, PGS, EC, JE Writing the review: JCKC, EC, JE Securing funding for the review: JCKC, PGS
Sources of support
Internal sources
-
National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), UK.
Jonathan Clarke acknowledges financial support by the National Institute for Health Research Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
External sources
-
National Institute for Health and Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
Emily Cabourne: None known. Jonathan CK Clarke: None known. Patricio G Schlottmann: None known. Jennifer R Evans: None known.
New
References
References to studies included in this review
Katz 1995 {published data only}
- Katz GJ, Higginbotham EJ, Lichter PR, Skuta GL, Musch DC, Bergstrom TJ, et al. Mitomycin C versus 5‐fluorouracil in high‐risk glaucoma filtering surgery. Extended follow‐up. Ophthalmology. 1995;102(9):1263‐9. [DOI] [PubMed] [Google Scholar]
- Skuta GL, Beeson CC, Higginbotham EJ, Lichter PR, Musch DC, Bergstrom TJ, et al. Intraoperative mitomycin versus postoperative 5‐fluorouracil in high‐risk glaucoma filtering surgery. Ophthalmology 992;99(3):438‐44. [DOI] [PubMed] [Google Scholar]
Kitazawa 1991 {published data only}
- Kitazawa Y, Kawase K, Matsushita H, Minobe M. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Archives of Ophthalmology. 1991;109(12):1693‐8. [DOI] [PubMed] [Google Scholar]
Lamping 1995 {published data only}
- Lamping KA, Belkin JK. 5‐Fluorouracil and mitomycin C in pseudophakic patients. Ophthalmology 1995;102(1):70‐5. [DOI] [PubMed] [Google Scholar]
Mostafaei 2011 {published data only}
- Mostafaei A. Augmenting trabeculectomy in glaucoma with subconjunctival mitomycin C versus subconjunctival 5‐fluorouracil: A randomized clinical trial. Clinical Ophthalmology 2011;5(1):491‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1997 {published data only}
- Singh K, Byrd S, Egbert PR, Budenz D. Risk of hypotony after primary trabeculectomy with antifibrotic agents in a black west African population. Journal of Glaucoma. 1998;7(2):82‐5. [PubMed] [Google Scholar]
- Singh K, Egbert PR, Byrd S, Budenz DL, Williams AS, Decker JH, et al. Trabeculectomy with intraoperative 5‐fluorouracil vs mitomycin C. American Journal of Ophthalmology. 1997;123(1):48‐53. [DOI] [PubMed] [Google Scholar]
Singh 2000 {published data only}
- Singh K, Mehta K, Shaikh NM, Tsai JC, Moster MR, Budenz DL, et al. Trabeculectomy with intraoperative mitomycin C versus 5‐fluorouracil. Prospective randomized clinical trial. Ophthalmology. 2000;107:2305‐9. [DOI] [PubMed] [Google Scholar]
Sisto 2007 {published data only}
- Sisto D, Vetrugno M, Trabucco T, Cantatore F, Ruggeri G, Sborgia C. The role of antimetabolites in filtration surgery for neovascular glaucoma: intermediate‐term follow‐up. Acta Ophthalmologica Scandinavica 2007;85(3):267‐71. [DOI] [PubMed] [Google Scholar]
Uva 1996 {published data only}
- Uva MG, Panta G, Avitabile T, Ott JP, Reibaldi A. Comparative study on intraoperative low dose MMC versus intraoperative 5‐FU in primary open angle glaucoma [Studio comparativo sull'uso intra‐operatorio di mitomicina C a basso dosaggio versus 5‐FU nel glaucoma primario ad angolo aperto]. Bollettino di Oculistica 1996;75(2):209‐19. [Google Scholar]
WuDunn 2002 {published data only}
- WuDunn D, Cantor LB, Palanca‐Capistrano AM, Hoop J, Alvi NP, Finley C, et al. A prospective randomized trial comparing intraoperative 5‐fluorouracil vs mitomycin C in primary trabeculectomy. American Journal of Ophthalmology. 2002;134(4):521‐8. [DOI] [PubMed] [Google Scholar]
Xinyu 2001 {published data only}
- Xinyu C, Xiaoning L, Denglei W. Application of mitomycin C and 5‐Fluorouracil in the glaucoma filtering surgery. Chinese Ophthalmic Research 2001;19:347‐9. [Google Scholar]
Zadok 1995 {published data only}
- Zadok D, Zadok J, Turetz J, Krakowski D, Nemet P. Intraoperative mitomycin versus postoperative 5‐fluorouracil in primary glaucoma filtering surgery. Annals of Ophthalmology Glaucoma 1995;27(6):336‐40. [Google Scholar]
References to studies excluded from this review
Ashworth 2003 {published data only}
- Ashworth JL, Mahmood S, Spencer AF, Grigg J. Assessment and modulation of bleb vascularity after enhanced trabeculectomy in high‐risk patients. Clinical and Experimental Ophthalmology 2003;31:24‐8. [Google Scholar]
Dreyer 1995 {published data only}
- Dreyer EB, Chaturvedi N, Zurakowski D. Effect of mitomycin C and fluorouracil‐supplemented trabeculectomies on the anterior segment. Archives of Ophthalmology 1995;113(5):578‐80. [DOI] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li J, Pang L. Influence on tear film postoperative 5‐fluourouracil and intraoperative mitomycin C in glaucoma filtration surgery. Chinese Journal of Ophthalmology 2001;37(1):43‐7. [PubMed] [Google Scholar]
Membrey 2000 {published data only}
- Membrey WL, Poinoosawmy DP, Bunce C, Hitchings RA. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 1 Intraocular pressure control and complications. British Journal of Ophthalmology 2000;84(6):586‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Membrey 2001 {published data only}
- Membrey WL, Bunce C, Poinoosawmy DP, Fitzke FW, Hitchings RA. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 2 Visual field progression. British Journal of Ophthalmology 2001;85(6):696‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 1994 {published data only}
- Oh SY, Youn DH, Kim DM, Hong C. The effects of intraoperative mitomycin‐C or 5‐fluorouracil on glaucoma filtering surgery. Korean Journal of Ophthalmology 1994;8(1):6‐13. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Bermejo 1993 {published data only}
- Rodriguez‐Bermejo C, Montero P, Perez‐Santonja JJ, Meza J, Gasco JL, Zato Gomez De Liano MA. Comparative study of chemotherapy agents as contribution in chronic simple glaucoma surgery [Estudio Comparativo De Agents Quimiotherapicos Como Coadyuvantes A La Cirugia Del Glaucoma Cronico Simple]. Archivos de la Sociedad Espanola de Oftalmologia 1993;65(5):441‐8. [Google Scholar]
References to studies awaiting assessment
Liu 2015 {published data only}
- Liu LJ, Xiao BW. Clinical efficacy and safety of glaucoma filtration surgery combined with the application of 5‐fluorouracil. International Eye Science 2015;15(1):38‐40. [Google Scholar]
Susanna 1995 {published data only}
- Susanna Jr R, Takahashi WY. A comparative study between mitomycin and 5‐FU to combined surgery. [Portuguese]. Revista Brasileira De Oftalmologia 1995;54:485‐8. [Google Scholar]
Yamamoto 1997 {published data only}
- Yamamoto S, Suzuki K, Fujita K, Yuguchi T, Kaiya T, Iwata K. Prognosis for trabeculectomy with mitomycin C. [Japanese]. Folia Ophthalmologica Japonica 1997;48:140‐3. [Google Scholar]
Additional references
Abdu 2010
- Abdu M, Chen XY, Kadir J, Arkin A. Mytomycin C versus 5‐fluorouracil for trabeculectomy: A systematic review. Chinese Journal of Evidence‐Based Medicine 2010;10(6):730‐9. [Google Scholar]
AGIS 1998
- The Advanced Glaucoma Intervention Study. The Advanced Glaucoma Intervention Study (AGIS): 4. Comparison of treatment outcomes within race. Seven‐year results. Ophthalmology 1998;105(7):1146‐64. [DOI] [PubMed] [Google Scholar]
Andreanos 1997
- Andreanos D, Georgopoulos GT, Vergados J, Papaconstantinou D, Liokis N, Theodossiadis P. Clinical evaluation of the effect of mitomycin‐C in re‐operation for primary open angle glaucoma. European Journal of Ophthalmology 1997;7(1):49‐54. [DOI] [PubMed] [Google Scholar]
Burr 2012
- Burr J, Azuara‐Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004399.pub3] [DOI] [PubMed] [Google Scholar]
Cairns 1968
- Cairns JE. Trabeculectomy. Preliminary report of a new method. American Journal of Ophthalmology 1968;66(4):673‐9. [PubMed] [Google Scholar]
Carlson 1997
- Carlson DW, Alward WL, Barad JP, Zimmerman MB, Carney BL. A randomized study of mitomycin augmentation in combined phacoemulsification and trabeculectomy. Ophthalmology 1997;104(4):719‐24. [DOI] [PubMed] [Google Scholar]
CNTGS 1998
- Collaborative Normal‐Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal‐tension glaucoma and patients with therapeutically reduced intraocular pressures. American Academy of Ophthalmology 1998;126:487‐97. [DOI] [PubMed] [Google Scholar]
Cohen 1996
- Cohen JS, Greff LJ, Novack GD, Wind BE. A placebo‐controlled, double‐masked evaluation of mitomycin C in combined glaucoma and cataract procedures. Ophthalmology 1996;103(11):1934‐42. [DOI] [PubMed] [Google Scholar]
Costa 1996
- Costa VP, Comegno PE, Vasconcelos JP, Malta RF, Jose NK. Low‐dose mitomycin C trabeculectomy in patients with advanced glaucoma. Journal of Glaucoma 1996;5(3):193‐9. [PubMed] [Google Scholar]
DeBry 2002
- DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC. Incidence of late‐onset bleb‐related complications following trabeculectomy with mitomycin. Archives of Ophthalmology 2002;120(3):297‐300. [DOI] [PubMed] [Google Scholar]
Dhingra 2009
- Dhingra S, Khaw PT. The Moorfields Safer Surgery System. Middle East African Journal of Ophthalmology 2009;16(3):112‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
EGS 2003
- European Glaucoma Society. Terminology and guidelines for glaucoma. www.oogheelkunde.org/uploads/4D/w6/4Dw6MftS95uSq8OajRtEDQ/EGS‐2nd.pdf (accessed 11 April 2012).
Fendi 2013
- Fendi L, Arruda G, Scott I, Paula J. Mitomycin C versus 5‐fluorouracil as an adjunctive treatment for trabeculectomy: a meta‐analysis of randomized clinical trials. Clinical and Experimental Ophthalmology 2013;41(8):798‐806. [DOI] [PubMed] [Google Scholar]
FFSSG 1989
- The Fluorouracil Filtering Surgery Study Group. Fluorouracil Filtering Surgery Study one‐year follow‐up. The Fluorouracil Filtering Surgery Study Group. American Journal of Ophthalmology 1989;108(6):625‐35. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Goldenfeld 1994
- Goldenfeld M, Krupin T, Ruderman JM, Wong PC, Rosenberg LF, Ritch R, et al. 5‐Fluorouracil in initial trabeculectomy. A prospective, randomized, multicenter study. Ophthalmology 1994;101(6):1024‐9. [DOI] [PubMed] [Google Scholar]
Green 2014
- Green E, Wilkins M, Bunce C, Wormald R. 5‐Fluorouracil for glaucoma surgery. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD001132.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heijl 2002
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of Ophthalmology 2002;120(10):1268‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kass 2002
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Archives of Ophthalmology 2002;120(6):701‐13. [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin Z, Li Y, Cheng J, Lu X. Intraoperative mitomycin C versus intraoperative 5‐fluorouracil for trabeculectomy: a systematic review and meta‐analysis. Journal of Ocular Pharmacology and Therapeutics 2012;28(2):166‐73. [DOI] [PubMed] [Google Scholar]
Maier 2005
- Maier PC, Funk J, Schwarzer G, Antes G, Falck‐Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta‐analysis of randomised controlled trials. BMJ 2005;331:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martini 1997
- Martini E, Laffi GL, Sprovieri C, Scorolli L. Low‐dosage mitomycin C as an adjunct to trabeculectomy. A prospective controlled study. European Journal of Ophthalmology 1997;7(1):40‐8. [DOI] [PubMed] [Google Scholar]
Migdal 1994
- Migdal C, Gregory W, Hitchings R. Long‐term functional outcome after early surgery compared with laser and medicine in open‐angle glaucoma. Ophthalmology 1994;101(10):1651‐6. [DOI] [PubMed] [Google Scholar]
Ophir 1992
- Ophir A, Ticho U. A randomized study of trabeculectomy and subconjunctival administration of fluorouracil in primary glaucomas. Archives of Ophthalmology 1992;110(8):1072‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Robin 1997
- Robin AL, Ramakrishnan R, Krishnadas R, Smith SD, Katz JD, Selvaraj S, et al. A long‐term dose‐response study of mitomycin in glaucoma filtration surgery. Archives of Ophthalmology 1997;115(8):969‐74. [DOI] [PubMed] [Google Scholar]
Ruderman 1987
- Ruderman JM, Welch DB, Smith MF, Shoch DE. A prospective, randomized study of 5‐fluorouracil and filtration surgery. Transactions of the American Ophthalmological Society 1987;85:238‐53. [PMC free article] [PubMed] [Google Scholar]
Shin 1995
- Shin DH, Simone PA, Song MS, Reed SY, Juzych MS, Kim C, et al. Adjunctive subconjunctival mitomycin C in glaucoma triple procedure. Ophthalmology 1995;102(10):1550‐8. [DOI] [PubMed] [Google Scholar]
Shin 1998
- Shin DH, Kim YY, Sheth N, Ren J, Shah M, Kim C, et al. The role of adjunctive mitomycin C in secondary glaucoma triple procedure as compared to primary glaucoma triple procedure. Ophthalmology 1998;105:740‐5. [DOI] [PubMed] [Google Scholar]
Vass 2007
- Vass C, Hirn C, Sycha T, Findl O, Bauer P, Schmetterer L. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003167.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 2010
- Wilkins M, Indar A, Wormald R. Intraoperative Mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD002897.pub2] [DOI] [Google Scholar]
Wu 1996
- Wu L, Yin J. The effect of mitomycin C on filtration surgery of glaucoma with poor prognosis. Zhonghua Yan Ke Za Zhi 1996;32:32‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Clarke 2006
- Clarke JCK, Schlottmann PG. Mitomycin C versus 5‐Fluorouracil for wound healing in glaucoma surgery. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006259] [DOI] [PMC free article] [PubMed] [Google Scholar]


