Abstract
Under visible light irradiation, o-phosphinophenolate functions as an easily accessible photoredox catalyst to activate trifluoromethyl groups in trifluoroacetamides, trifluoroacetates, and trifluoromethyl (hetero)arenes to deliver corresponding difluoromethyl radicals. It works in relay with a thiol hydrogen atom transfer (HAT) catalyst to enable selective defluoroalkylation and hydrodefluorination. The reaction allows for the facile synthesis of a broad scope of difluoromethylene-incorporated carbonyl and (hetero)aromatic compounds, which are valuable fluorinated intermediates of interest in the pharmaceutical industry. The ortho-diphenylphosphino substituent, which is believed to facilitate photoinduced electron transfer, plays an essential role in the redox reactivity of phenolate. In addition to trifluoromethyl groups, pentafluoroethyl groups could also be selectively defluoroalkylated.
Subject terms: Synthetic chemistry methodology, Photocatalysis
Photoredox catalysis can strongly reduce and cleave unactivated chemical bonds via photoinduced electron transfer. Here the authors use o-phosphinophenolate for photocatalytic C–F activation of a wide range of trifluoromethyl groups in trifluoroacetamides, trifluoroacetates, and trifluoromethyl(hetero)arenes to deliver corresponding difluoromethyl radicals.
Introduction
Photoredox catalysis has demonstrated its strong reducing power to cleave unactivated chemical bonds via photoinduced electron transfer1–5. Among the various photocatalysis-enabled methods of activating inert bonds, the direct selective C–F activation6–11 of trifluoromethyl groups to deliver corresponding difluoromethyl radicals is an ideal transformation for the synthesis of difluoromethylene-incorporated compounds, which are valuable fluorinated intermediates in the pharmaceutical industry12–14. The low cost and ready availability of trifluoroacetamide, acetate, and a variety of trifluoromethylated (hetero)aromatics make this transformation appealing. Ingenious photocatalytic methods for selective hydrodefluorination and defluoroalkylation of trifluoromethyl(hetero)arenes have been developed by Jui15,16, König17, and Gouverneur18. While the photocatalysts prevalently used in these transformations are precious metal-based polypyridyl complexes and π-conjugated organic dyes, we conceived that anionic phenolate19–23, which has strong reductive potential in its excited state, may work as a suitable catalyst for selective C–F functionalization of trifluoromethyls over a broad scope of substrates. Although simple phenolate failed as a catalyst, probably because of its insufficient excited state lifetime and the poor stability of phenoxy radicals19,21, which limited catalyst turnover, we were inspired by the effect of triphenylphosphine that we previously observed in photocatalytic decarboxylative couplings24–27 to facilitate photoinduced electron transfer, and we hypothesized that an ortho-phosphino group easily installed onto phenolate may overcome these problems. We present our catalyst design in Fig. 1a. The installation of ortho-PPh2 has three benefits. First, the ortho-PPh2 substituent redshifts the absorption of the ground state anion (PO–) from the ultraviolet to visible light range. Second, phosphine exerts a heavy atom effect28,29 to facilitate intersystem crossing to access the triplet state and extends its lifetime for efficient photoelectron transfer with substrates. Third, the interaction of phosphine with oxygen radicals is expected not only to facilitate efficient photoelectron transfer in the anionic excited state (*PO–) but also to stabilize the radical double state (PO•).
Fig. 1. o-Phosphinophenolate as photocatalyst for C–F functionalization of –CF3.
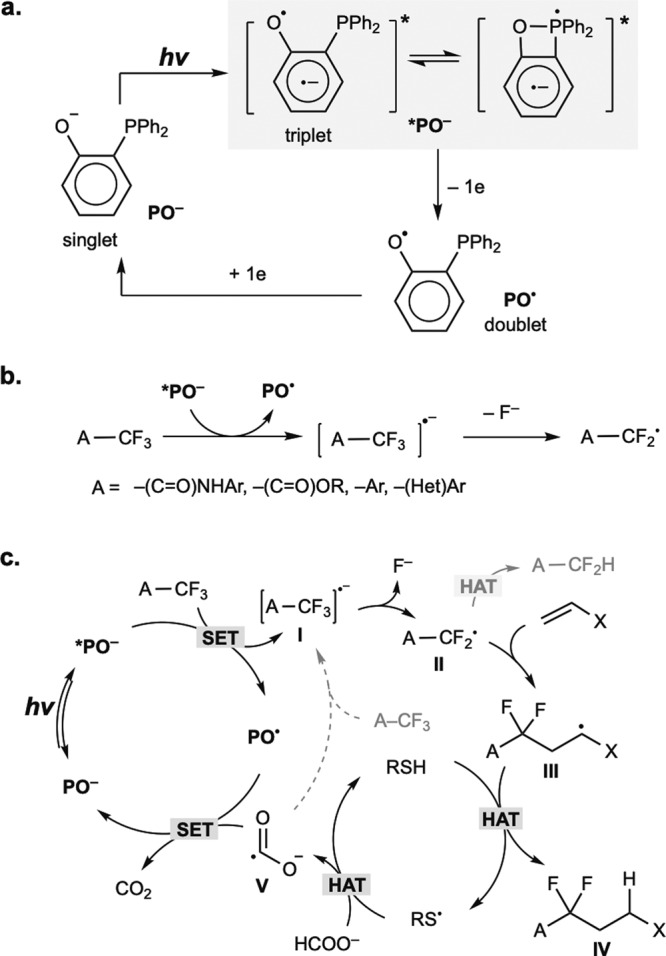
a Design of a system that uses o-phosphinophenolate (PO–) as a photoredox catalyst. b Generation of –CF2• radicals from –CF3 groups using PO–. c Hypothesized photocatalytic cycles for defluoroalkylation and hydrodefluorination. hv light, SET single electron transfer, HAT hydrogen atom transfer.
We show herein the application of o-phosphinophenolate for photocatalytic C–F activation of a wide range of trifluoromethyl groups in trifluoroacetamides, trifluoroacetates, and trifluoromethyl (hetero) arenes to deliver corresponding difluoromethyl radicals (Fig. 1b). In this work, we target two transformations, defluoroalkylation15–17,30,31 with alkenes and hydrodefluorination18,32,33, to prepare valuable carbonyl and aromatic compounds with incorporated difluoromethylene motifs.
Results and discussion
Studies of properties of PO catalysts and reaction parameters
Our mechanistic hypothesis depicted in Fig. 1c was inspired by previous examples of photoredox/HAT synergistic catalysis15,16,18. The photoexcited PO– catalyst (*PO–) reduces the trifluoromethyl substrate (I) to deliver difluoromethyl radicals (II). II can be reduced by a hydrogen donor to produce a hydrodefluorination product or be intercepted by an alkene to generate a new alkyl radical (III). The alkyl radical (III) can be reduced by a polarity-reversal thiol HAT catalyst34 to produce a defluoroalkylation product. The thiol catalyst regenerates through hydrogen abstraction from formate (BDE of formate C–H: 88 kcal/mol) to deliver CO2•–, which is a strong reductant (E1/2 CO2/CO2•– = − 2.2 V vs. SCE), to reduce PO• and complete the PO– redox cycle. Guided by the hypothesis, we first focused on the selective defluoroalkylation of trifluoroacetamide with alkenes, a transformation that has not been successfully developed using photocatalysis. We discovered that a catalytic amount of PO in combination with 1-adamatanethiol (1-AdSH) as the HAT catalyst in the presence of formate catalyzed monoselective defluoroalkylation of N-phenyltrifluoroacetamide with alkenes under irradiation with a 427 nm LED (Kessil®, emission FWHM of ~20 nm)35. A similar transformation was reported by Houk and Wang et al. under thermal conditions using a stoichiometric amount of DMAP-BH3 via a spin-center shift strategy36. Figure 2 shows the key reaction parameters. Under optimal conditions (Fig. 2a), defluoroalkylation product (3) was obtained in 90% yield determined by 1H-NMR, along with the generation of 4 in 5 % yield. The reaction mixture appeared as a light yellow homogeneous solution, suggesting its promising applications in flow photosynthesis37,38. UV–Vis absorption spectra of catalysts, substrates, and reaction mixtures were measured to determine the light absorbing species (Fig. 2b). Both substrates (1 and 2) absorb light only in the ultraviolet range (< 325 nm). PO1 in its phenol form had an absorption onset at ~370 nm (purple line in Fig. 2b). Upon mixing with Cs2CO3, the deprotonated anion of PO1 (PO1–) exhibited redshifted absorption into the visible light range with an absorption onset at ~425 nm. Comparison of the absorption spectra of PO1– and tert-butylphenolate showed that the ortho-PPh2 substituent significantly redshifted the absorption curve by ~70 nm (see Supplementary Information for details). PO1– absorbs visible light with an onset at ~425 nm and shows an emission maximum at 540 nm. The reduction potential of excited PO1– (*PO1–) is estimated to be –2.89 V vs. SCE, a value sufficient to reduce a broad scope of trifluoromethyl aromatic and carbonyl compounds (PhCF3, Ered1/2 = –2.50 V vs. SCE in DMF18; CF3COOEt, Eredp/2 = –2.40 V vs. SCE) (see Supplementary Information S41, for details). Anions generated by the deprotonation of amide 1 maximally absorb light until 350 nm. In the presence of 1, the absorption spectrum of PO1– did not show a bathochromic shift, showing that an electron-donor-acceptor complex39–41 between 1 and PO1– was not formed (blue line). The absorption curve of the reaction mixture (red line) was identical to that of PO1–, indicating that PO1– was the light absorbing species in the reaction mixture. Figure 1c shows the PO catalysts with different structures. 2-Methyl (PO2)-, 2,4-di-tert-butyl (PO3)-, and 2,4-dimethyl (PO4)-substituted o-phosphinophenols all showed performances comparable to that of PO1. o-Phosphinophenol (PO5) without a p-substituent showed reduced catalytic efficacy. p-Phosphinophenol (PO6) gave 3 in only 15% yield, which suggests the essential role of intramolecular P–O interactions in catalytic efficacy. A bulky PO catalyst with a dicyclohexylphosphine substituent (PO7) was ineffective. Regarding to stability, PO1 is bench-stable white powder and can be stored under ambient air for months without apparent decomposition and oxidation.
Fig. 2. Defluoroalkylation of trifluoroacetamide with an alkene.
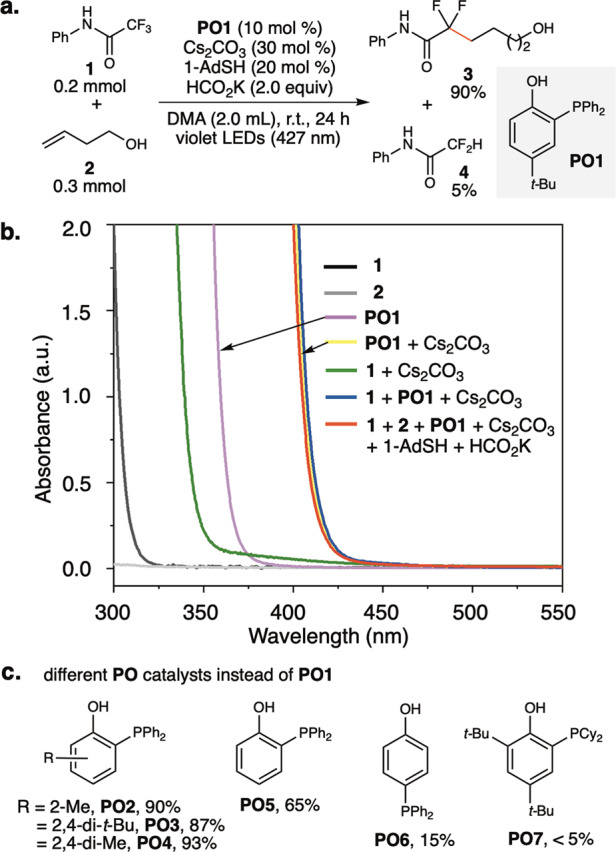
a Optimized reaction condition. b UV–Vis absorption spectra of catalyst, substrates, and reaction mixtures. The concentrations of substances measured were identical to those under optimized reaction conditions in Fig. 2a. Arbitrary units (a.u.) c PO catalysts other than PO1.
Table 1 summaries key reaction parameters. Using cyclohexanethiol as the HAT catalyst reduced the yield (entry 1, Table 1). Using 10 mol % 1-AdSH resulted in decreased yield (entry 2). PO1 (2 mol %) still catalyzed the reaction in 52% yield (entry 3). Increasing the amount of alkene to 2.0 equivalents suppressed hydrodefluorination and increased the defluoroalkylation yield to 96%. Replacing formate with triethylsilane gave 2 in 30% yield (entry 5), indicating that formate is not essential for C–F activation. LEDs with an emission peak at 440 nm (emission range from 415 nm to 470 nm) also promoted the reaction (entry 6), but LEDs with emission peaks at 456 nm and 467 nm, which did not overlap with the absorption of PO1–, were ineffective (entries 7 and 8). PPh3 and 4-tert-butylphenol used alone were both ineffective (entries 9 and 10), while 10% of 3 was generated by using a mixture of them (entry 11), suggesting certain role of P–O interactions in efficient photoelectron transfer. Control experiments showed that PO1, 1-AdSH, and light were all essential parameters (entries 12, 13, 15). In the absence of formate salt, product 3 was detected in 18% yield (entry 14), suggesting the role of formate in catalyst turnover (ref. Fig. 1c). The cation moiety of formate salt affects not only solubility but also reactivity, because the alkali metal cations act as counter cations of both formate and generated fluoride salt, that may affect the rates of HAT and defluorination. Hence, different formate salts (Li, Na, K, Cs) were tested (see Supplementary Table 1 in SI page 6 for details). The quantum yield of 3 was estimated to be 4.4 according to the literature42,43, which suggested that CO2•– generated after HAT may activate –CF3 substrates (e.g., reduction potential of 1,3-bistrifluoromethylbenzene, Ered1/2 = –2.07 V vs. SCE; reduction potential of 1, Eredp/2 = –2.11 V vs. SCE) in relay with the thiol HAT catalyst to deliver 3 (pale dashed arrow in Fig. 1c)44,45. Exposure to air completely killed the catalytic reactivity (entry 16) resulting recovery of starting materials, as air can quench excited triplet state of PO1– and oxidize thiol.
Table 1.
Key reaction-controlling parameters.
 | ||
|---|---|---|
| Entry | Variations from standard condition | Yielda (%) |
| 1 | CySH instead of 1-AdSH | 75 |
| 2 | 1-AdSH (10 mol %) | 72 |
| 3 | PO1 (2 mol %) | 52 |
| 4 | 2 (0.4 mmol) | 96 |
| 5 | Et3SiH instead of HCO2K | 30 |
| 6 | 440 nm instead of 427 nm | 70 |
| 7 | 456 nm instead of 427 nm | trace |
| 8 | 467 nm instead of 427 nm | trace |
| 9 | PPh3 instead of PO1 | trace |
| 10 | 4-t-Bu-C6H4OH instead of PO1 | 0 |
| 11 | 4-t-Bu-C6H4OH+PPh3 (1:1, 10 mol %) | 10 |
| 12 | w/o PO1 | 0 |
| 13 | w/o 1-AdSH | trace |
| 14 | w/o HCO2K | 18 |
| 15 | w/o light | 0 |
| 16 | under air | trace |
w/o without.
aYields measured by 1H-NMR using diphenylmethane as an internal standard.
Scope of the reactions
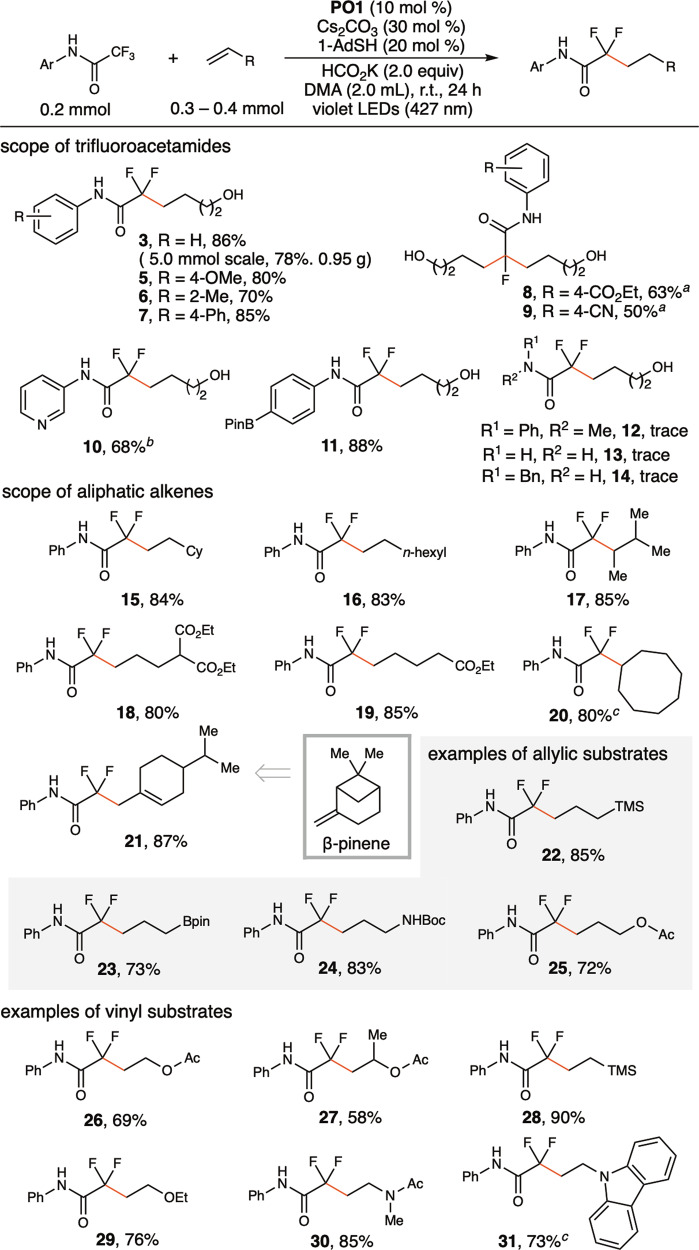
Figure 3 illustrates the scope of the defluoroalkylation of trifluoroacetamides. The reaction can be easily scaled up to the gram scale under batch conditions using Kessil LEDs and a Schlenk flask (3). For trifluoroacetamides possessing electron-neutral and electron-rich N-aryl substituents, monodefluoroalkylation products accompanied by a small amount of hydrodefluorination byproduct (5–10%) were obtained (5, 6, 7). For trifluoroacetamides with strong electron-deficient N-aryl substituents, products of didefluoroalkylation were obtained as major products (8, 9), and monodefluoroalkylation products were observed only in trace amounts (<3%). The recovered amide staring materials accounted for moderate yields (8, 9). For N-3-pyridyl trifluoroacetamide (10), a monodefluoroalkylation product was obtained in 68% yield.
Fig. 3. Scope of defluoroalkylation of trifluoroacetamides.
Yields of isolated products. aalkene (0.5 mmol), HCO2K (0.6 mmol). bPO3 (10 mol %) used instead of PO1. calkene (0.4 mmol).
An N-aryl substituent is essential for defluoroalkylation (13, 14), and the reactivity is not applicable to tertiary amides (12). Measuring reduction potentials of different trifluoroacetamides by cyclic voltammetry revealed that N-phenyltrifluoroacetamide (1) is thermodynamically easier to reduce (Eredp/2 = –2.11 V vs. SCE) than N-benzyltrifluoroacetamide (Eredp/2 = –2.56 V vs. SCE) and primary trifluoroacetamide (Eredp/2 = –2.49 V vs. SCE). We also rationalize that N-aryl substituent stabilizes amide radical anion through charge delocalization, thus suppresses back electron transfer with PO• to facilitate subsequent cleavage of C–F bond. The reaction tolerates a broad scope of aliphatic alkenes (15, 16, 18, 19), including sterically hindered internal alkenes (17) and cyclic alkenes (20). β-Pinene gave ring-opened product (21) in high yield. Allylic-type substrates were also amenable, as allylic silane (22), allylic boronate (23), allylic amine (24), and allylic acetate (25) all reacted well. The amenable alkenes also include vinyl-type substrates. Vinyl acetates (26, 27) are suitable substrates. Hydrolysis of defluoroalkylation products with vinyl acetates can provide fluorinated and methylated γ-hydroxybutyrate (26, 27), which is an active component of XYREM, a drug approved by the FDA to treat symptoms of narcolepsy46. Vinyl silane (28), vinyl ether (29), N-vinyl amide (30), and vinyl carbazole (31) all reacted effectively. However, styrene- and acrylate-type substrates were unsuitable, probably because of the facile addition of these types of alkenes with CO2•– under the reaction conditions47,48. In addition to trifluoroacetamides, trifluoroacetates also reacted in high yield to provide α,α-difluorinated aliphatic carboxylate esters (Fig. 4, 32–35).
Fig. 4. Examples of defluoroalkylation of trifluoroacetates.
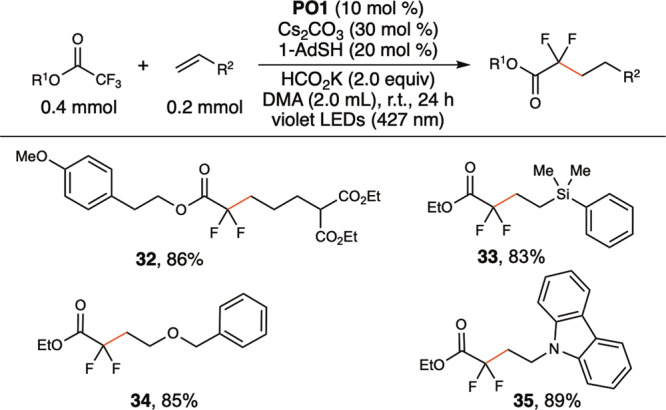
Yields of isolated products.
In the absence of an alkene, hydrodefluorination products were generated (Fig. 5). Thiol as a polarity reversal catalyst34 is essential for a high yield of hydrodefluorination. Cesium formate used in 1.2 equivalents is critical to ensure high monoselectivity (4, 36–39). The N-phenyltrifluoroacetamide with a para-ester substituent underwent thorough hydrodefluorination to generate acetamide (40). The same reaction conditions are also applicable to the selective hydrodefluorination and defluoroalkylation of pentafluoropropionamide (Eqs. (1) and (2) in Fig. 6), suggesting the further application of PO catalysts in the selective C–F functionalization of polyfluorinated compounds (41, 43)49–51.
Fig. 5. Scope of hydrodefluorination of trifluoroacetamides.
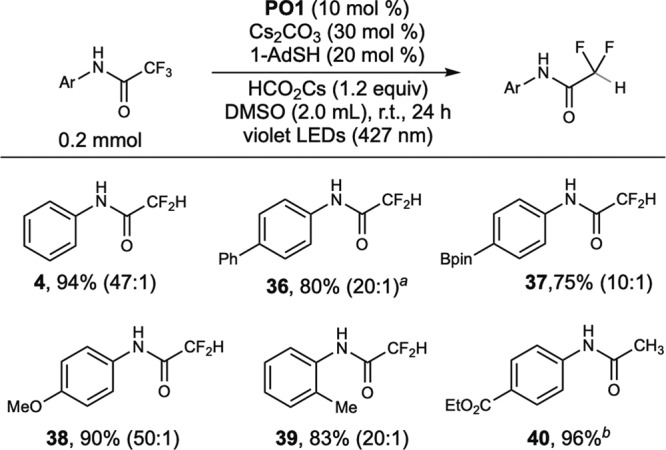
Yields of isolated products. aPO5 (10 mol %), HCO2Cs (0.4 mmol). bHCO2Cs (0.8 mmol). The ratios of monohydrodefluorination and dihydrodefluorination are shown in parentheses.
Fig. 6. Hydrodefluorination and defluoroalkylation of pentafluoroethyl group.
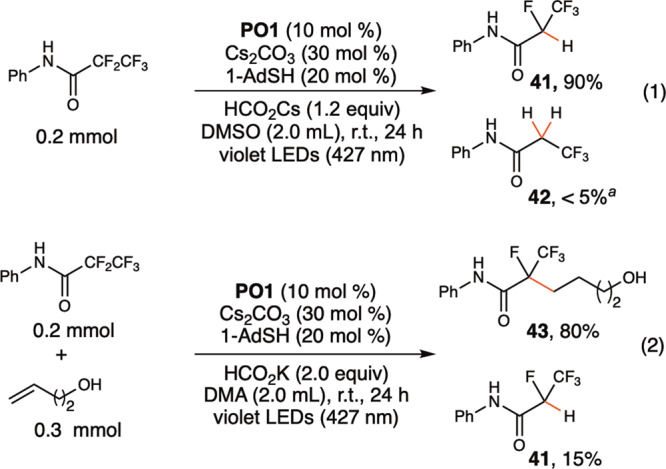
Yields of isolated products. aYield measured by 1H-NMR using diphenylmethane as an internal standard.
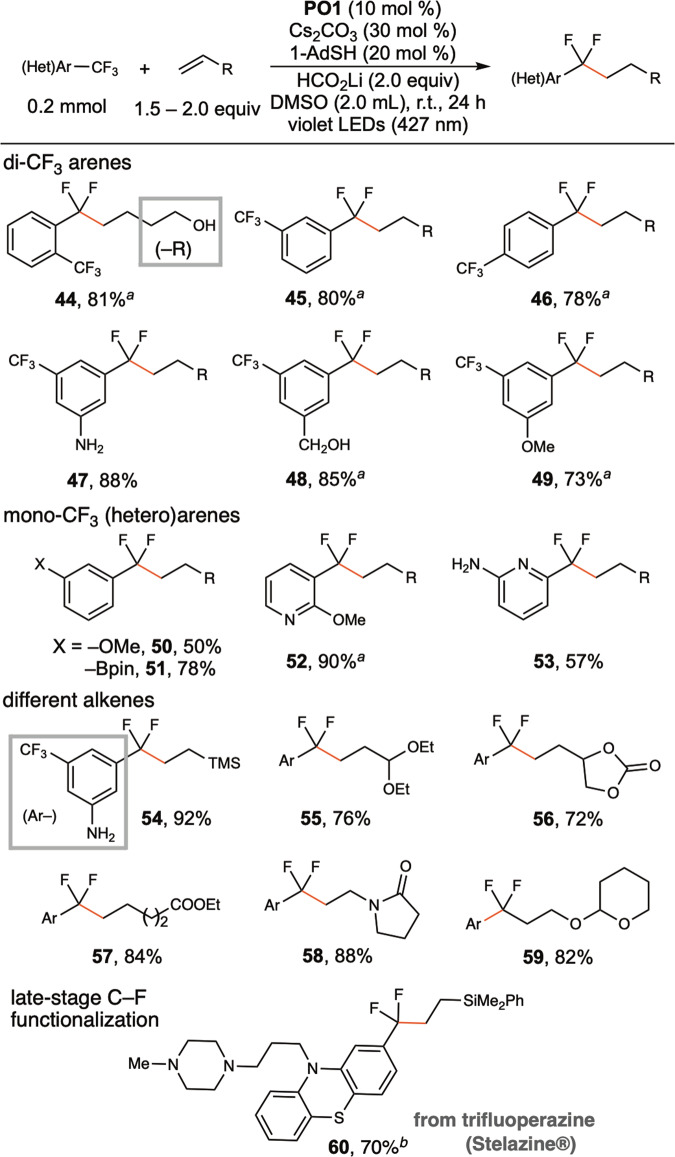
In Fig. 7, the application of PO1 as a catalyst for defluoroalkylation of trifluoromethyl arenes and heteroarenes is demonstrated. Lithium formate was found to be the hydrogen donor of choice, and DMSO was found to be the preferred solvent. The monoselective defluoroalkylation reaction is applicable to a broad scope of trifluoromethyl arenes, including both di-CF3 substituted arenes and mono-CF3 substituted arenes. Potassium tert-butoxide was found to be a better base for di-CF3 arenes than cesium carbonate because it resulted in high selectivity (44, 45, 46, 48, 49). Although trifluoromethylbenzene was an unsuitable substrate (<15% conversion), m-methoxy-substituted trifluoromethylbenzene reacted in 50% yield (50). m-CF3-substituted phenylboronate reacted in 78% to give fluorinated building blocks useful in cross-coupling reactions (51). Pyridine derivatives bearing –CF3 at both the 2- and 3-positions reacted smoothly, generating valuable α,α-difluoroalkylated pyridines (52, 53). For alkene scope, in addition to vinyl silane (54) and aliphatic alkene (57), acetals of acrylaldehyde (55), vinyl ethylene carbonate (56), N-vinylpyrrolidinone (58), and 2-vinyloxytetrahydropyran (59) all reacted smoothly. An FDA-approved antipsychotic drug for schizophrenia, trifluoperazine (Stelazine®), was selectively defluoroalkylated (60), showcasing the practical utility for late-stage functionalization of CF3-containing drugs. Monoselective hydrodefluorination of trifluoromethyl arene could also be achieved in the absence of alkene (61, Fig. 8).
Fig. 7. Scope of defluoroalkylation of trifluoromethyl(hetero)arenes.
Yields of isolated products. aKOtBu (30 mol %). bPO3 (10 mol %) instead of PO1, alkene (0.6 mmol).
Fig. 8. Monoselective hydrodefluorination of trifluoromethyl arene.

Yield of isolated product. The ratio of monohydrodefluorination and dihydrodefluorination measured by 19F-NMR is shown in parentheses.
In summary, o-phosphinophenolates were developed as efficient photocatalysts for the selective C–F activation of a wide range of trifluoromethyl groups in trifluoroacetamides, trifluoroacetates, and trifluoromethyl (hetero) arenes to deliver corresponding difluoromethyl radicals for defluoroalkylation with alkenes or hydrodefluorination to prepare valuable difluoromethylene-incorporated carbonyl and aromatic compounds. Similar reactivity is also applicable for selective functionalization of pentafluoroethyl groups. In these reactions, o-phosphinophenolate works synergistically with an alkyl thiol HAT catalyst52. This work offers practical methods of synthesizing valuable geminal difluoro-substituted carbonyl and aromatic compounds and demonstrates a new design strategy for developing photoredox catalysts at a low cost (We list prices of typical photoredox catalysts and prices of materials for PO synthesis to justify this statement. From Aldrich® (Japan), 4CzIPN, 250 mg, 1282 USD; (Ir[dF(CF3)ppy]2(dtbpy))PF6, 1 g, 1500 USD; (2-hydroxyphenyl)diphenylphosphine (PO5), 1 g, 135 USD. Materials for synthesis of PO1: 2-bromo-4-tert-butylphenol, 5 g, 62 USD; chlorodiphenylphosphine, 25 g, 60 USD).
Methods
Materials
For the optimization of reaction conditions, see Supplementary Tables 1 and 2, For the experimental procedures and analytic data of compounds synthesized, see Supplementary Methods. For NMR spectra of compounds in this manuscript, see Supplementary Figures 13–179.
General procedure for defluoroalkylation of trifluoroacetamides
Trifluoroacetamide (1.0 equiv, 0.2 mmol), alkene (1.5 equiv., 0.3 mmol), PO1 (10 mol %), Cs2CO3 (30 mol %), 1-adamananethiol (20 mol %), HCO2K (2.0 equiv., 0.4 mmol) were placed in a transparent Schlenk tube equipped with a stirring bar. The tube was evacuated and filled with argon (three times). To the mixture, anhydrous DMA (2 mL) were added via a gastight syringe under argon atmosphere. The reaction mixture was stirred under irradiation with violet LEDs (Kessil® 427 nm) in a HepatoChem photoreactor at room temperature for 24 h. The mixture was quenched with brine and extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and concentrated under vacuo. The product was purified by flash column chromatography on silica gel.
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China and USTC (GG2065010002 and KY2060000119).
Author contributions
R.S. conceived the idea and wrote the manuscript. C.L. performed the experiments. C.L. and N.S. analyzed the data and participated in preparation of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files, or from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28007-2.
References
- 1.Prier CK, Rankic DA, MacMillan DWC. Visible Light Photoredox Catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh I, Ghosh T, Bardagi JI, König B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science. 2014;346:725–728. doi: 10.1126/science.1258232. [DOI] [PubMed] [Google Scholar]
- 3.Du J, Skubi KL, Schultz DM, Yoon TP. A Dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light. Science. 2014;344:392–396. doi: 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monos TM, McAtee RC, Stephenson CRJ. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science. 2018;361:1369–1373. doi: 10.1126/science.aat2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Wang H, König B. Photo-induced thiolate catalytic activation of inert Caryl-hetero bonds for radical borylation. Chem. 2021;7:1653–1665. [Google Scholar]
- 6.Mattay J, Runsink J, Rumbach T, Ly C, Gersdorf J. Selectivity and charge transfer in photoreactions of α,α,α-trifluorotoluene with olefins. J. Am. Chem. Soc. 1985;107:2557–2558. [Google Scholar]
- 7.Yoshida S, Shimomori K, Kim Y, Hosoya T. Single C–F bond cleavage of trifluoromethylarenes with an ortho-silyl group. Angew. Chem. Int. Ed. 2016;55:10406–10409. doi: 10.1002/anie.201604776. [DOI] [PubMed] [Google Scholar]
- 8.Jaroschik F. Picking One out of three: selective single C−F activation in trifluoromethyl groups. Chem. Eur. J. 2018;24:14572–14582. doi: 10.1002/chem.201801702. [DOI] [PubMed] [Google Scholar]
- 9.Mandal D, Gupta R, Jaiswal AK, Young RD. Frustrated lewis-pair-meditated selective single fluoride substitution in trifluoromethyl groups. J. Am. Chem. Soc. 2020;142:2572–2578. doi: 10.1021/jacs.9b12167. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y-C, Tong F-F, Zhang Y, He C-Y, Zhang X. Visible-light-Induced palladium-catalyzed selective defluoroarylation of trifluoromethylarenes with arylboronic acids. J. Am. Chem. Soc. 2021;143:13971–13979. doi: 10.1021/jacs.1c07459. [DOI] [PubMed] [Google Scholar]
- 11.Yan G, Qiu K, Guo M. Recent advance in the C–F bond functionalization of trifluoromethyl-containing compounds. Org. Chem. Front. 2021;8:3915–3942. [Google Scholar]
- 12.Boyer J, et al. Difluoromethylbenzoxazole pyrimidine thioether derivatives: a novel class of potent non-nucleoside HIV-1 reverse transcriptase inhibitor. J. Med. Chem. 2011;54:7974–7985. doi: 10.1021/jm200766b. [DOI] [PubMed] [Google Scholar]
- 13.Meanwell NA. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 14.Meanwell NA. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Jui NT. Catalytic defluoroalkylation of trifluoromethylaromatics with unactivated alkenes. J. Am. Chem. Soc. 2018;140:163–166. doi: 10.1021/jacs.7b12590. [DOI] [PubMed] [Google Scholar]
- 16.Vogt DB, Seath CP, Wang H, Jui NT. Selective C−F functionalization of unactivated trifluoromethylarenes. J. Am. Chem. Soc. 2019;141:13203–13211. doi: 10.1021/jacs.9b06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, Berg N, Gschwind R, König B. Selective single C(sp3)−F bond cleavage in trifluoromethylarenes: merging visible-light catalysis with lewis acid activation. J. Am. Chem. Soc. 2017;139:18444–18447. doi: 10.1021/jacs.7b10755. [DOI] [PubMed] [Google Scholar]
- 18.Sap JBI, et al. Organophotoredox hydrodefluorination of trifluoromethylarenes with translational applicability to drug discovery. J. Am. Chem. Soc. 2020;142:9181–9187. doi: 10.1021/jacs.0c03881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altwicker ER. The Chemistry of stable phenoxy radicals. Chem. Rev. 1967;67:475–531. [Google Scholar]
- 20.Soumillion JP, Vandereecken P, De Schryver FC. Photodechlorination of chloroaromatics by electron transfer from an anionic sensitizer. Tetrahedron Lett. 1989;30:697–700. [Google Scholar]
- 21.Krauss M, Jensen JO, Hameka HF. Electronic structure of the excited states and phenol fluorescence. J. Phys. Chem. 1994;98:9955–9959. [Google Scholar]
- 22.Liang K, et al. Intermolecular oxyarylation of olefins with aryl halides and TEMPOH catalyzed by the phenolate anion under visible light. Chem. Sci. 2020;11:6996–7002. [Google Scholar]
- 23.Schmalzbauer M, Marcon M, König B. Excited state anions in organic transformations. Angew. Chem. Int. Ed. 2021;60:6270–6292. doi: 10.1002/anie.202009288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu M-C, Shang R, Zhao B, Wang B, Fu Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science. 2019;363:1429–1434. doi: 10.1126/science.aav3200. [DOI] [PubMed] [Google Scholar]
- 25.Noble A, Aggarwal VK. Triphenylphosphine and sodium iodide: a new catalyst combination to rival precious metal complexes in visible light photoredox catalysis. Sci. China Chem. 2019;62:1083–1084. [Google Scholar]
- 26.Fu M-C, Wang J-X, Shang R. Triphenylphosphine-catalyzed alkylative iododecarboxylation with lithium iodide under visible light. Org. Lett. 2020;22:8572–8577. doi: 10.1021/acs.orglett.0c03173. [DOI] [PubMed] [Google Scholar]
- 27.Wang G-Z, Fu M-C, Zhao B, Shang R. Photocatalytic decarboxylative alkylations of C(sp3)–H and C(sp2)–H bonds enabled by ammonium iodide in amide solvent. Sci. China Chem. 2021;64:439–444. [Google Scholar]
- 28.Koziar JC, Cowan DO. Photochemical heavy-atom effects. Acc. Chem. Res. 1978;11:334–341. [Google Scholar]
- 29.Gan S, et al. Heavy atom effect of bromine significantly enhances exciton utilization of delayed fluorescence luminogens. ACS Appl. Mater. Interfaces. 2018;10:17327–17334. doi: 10.1021/acsami.8b05389. [DOI] [PubMed] [Google Scholar]
- 30.Utsumi S, Katagiri T, Uneyama K. Cu-deposits on Mg metal surfaces promote electron transfer reactions. Tetrahedron. 2012;68:1085–1091. [Google Scholar]
- 31.Wolfe MMW, Shanahan JP, Kampf JW, Szymczak NK. Defluorinative functionalization of Pd(II) fluoroalkyl complexes. J. Am. Chem. Soc. 2020;142:18698–18705. doi: 10.1021/jacs.0c09505. [DOI] [PubMed] [Google Scholar]
- 32.Dang H, Whittaker AM, Lalic G. Catalytic activation of a single C–F bond in trifluoromethyl arenes. Chem. Sci. 2016;7:505–509. doi: 10.1039/c5sc03415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz SB, et al. Selective late-stage hydrodefluorination of trifluoromethylarenes: a facile access to difluoromethylarenes. Eur. J. Org. Chem. 2017;2017:2322–2326. [Google Scholar]
- 34.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- 35.Information about emission spectra of Kessil LEDs, see: https://www.kessil.com/science/PR160L.php.
- 36.Yu Y-J, et al. Sequential C–F bond functionalizations of trifluoroacetamides and acetates via spin-center shifts. Science. 2021;371:1232–1240. doi: 10.1126/science.abg0781. [DOI] [PubMed] [Google Scholar]
- 37.Britton J, Raston CL. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017;46:1250–1271. doi: 10.1039/c6cs00830e. [DOI] [PubMed] [Google Scholar]
- 38.Booker-Milburn KI, Noël T. Flow photochemistry. ChemPhotoChem. 2018;2:830–830. [Google Scholar]
- 39.Rosokha SV, Kochi JK. Fresh look at electron-transfer mechanisms via the donor/acceptor bindings in the critical encounter complex. Acc. Chem. Res. 2008;41:641–653. doi: 10.1021/ar700256a. [DOI] [PubMed] [Google Scholar]
- 40.Lima CGS, et al. Organic synthesis enabled by light-irradiation of EDA complexes: theoretical background and synthetic applications. ACS Catal. 2016;6:1389–1407. [Google Scholar]
- 41.Crisenza GEM, Mazzarella D, Melchiorre P. Synthetic methods driven by the photoactivity of electron donor−acceptor complexes. J. Am. Chem. Soc. 2020;142:5461–5476. doi: 10.1021/jacs.0c01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tlahuext-Aca A, Garza-Sanchez RA, Schäfer M, Glorius F. Visible-light-mediated synthesis of ketones by the oxidative alkylation of styrenes. Org. Lett. 2018;20:1546–1549. doi: 10.1021/acs.orglett.8b00272. [DOI] [PubMed] [Google Scholar]
- 43.Woźniak Ł, Murphy JJ, Melchiorre P. Photo-organocatalytic enantioselective perfluoroalkylation of β-ketoesters. J. Am. Chem. Soc. 2015;137:5678–5681. doi: 10.1021/jacs.5b03243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendy CM, Smith GC, Xu Z, Lian T, Jui NT. Radical chain reduction via carbon dioxide radical anion (CO2•−) J. Am. Chem. Soc. 2021;143:8987–8992. doi: 10.1021/jacs.1c04427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chmiel AF, Williams OP, Chernowsky CP, Yeung CS, Wickens ZK. Non-innocent radical ion intermediates in photoredox catalysis: parallel reduction modes enable coupling of diverse aryl chlorides. J. Am. Chem. Soc. 2021;143:10882–10889. doi: 10.1021/jacs.1c05988. [DOI] [PubMed] [Google Scholar]
- 46.Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. doi: 10.1007/s40263-019-00689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Gao Y, Zhou C, Li G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc. 2020;142:8122–8129. doi: 10.1021/jacs.0c03144. [DOI] [PubMed] [Google Scholar]
- 48.Alektiar SN, Wickens ZK. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc. 2021;143:13022–13088. doi: 10.1021/jacs.1c07562. [DOI] [PubMed] [Google Scholar]
- 49.Wettergren J, Ankner T, Hilmersson G. Selective α-defluorination of polyfluorinated esters and amides using SmI2/Et3N/H2O. Chem. Commun. 2010;46:7596–7597. doi: 10.1039/c0cc02009e. [DOI] [PubMed] [Google Scholar]
- 50.Ichitsuka T, Fujita T, Arita T, Ichikawa J. Double C−F bond activation through β-fluorine elimination: nickel-mediated [3 + 2] cycloaddition of 2-trifluoromethyl-1-alkenes with alkynes. Angew. Chem. Int. Ed. 2014;53:7564–7568. doi: 10.1002/anie.201402695. [DOI] [PubMed] [Google Scholar]
- 51.Sugihara N, Suzuki K, Nishimoto Y, Yasuda M. Photoredox-catalyzed C–F bond allylation of perfluoroalkylarenes at the benzylic position. J. Am. Chem. Soc. 2021;143:9308–9313. doi: 10.1021/jacs.1c03760. [DOI] [PubMed] [Google Scholar]
- 52.Dénès F, Pichowicz M, Povie G, Renaud P. Thiyl radicals in organic synthesis. Chem. Rev. 2014;114:2587–2693. doi: 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files, or from the corresponding author upon request.