Abstract
This guidance is designed to assist risk assessors and applicants when quantifying potential non‐dietary, systemic exposures as part of regulatory risk assessment for plant protection products (PPPs). It is based on the Scientific Opinion on ‘Preparation of a Guidance Document on Pesticide Exposure Assessment for Workers, Operators, Residents and Bystanders’ developed by the EFSA Panel on Plant Protection Products and their Residue (PPR) in 2010. Highlighting some inconsistencies between the approaches adopted by regulatory authorities, the PPR Panel proposed a number of changes to the practices in use (i.e. use of deterministic methods for individual PPPs; need to perform an acute risk assessment for PPPs that are acutely toxic; use of appropriate percentile for acute or longer term risk assessments). In the first version of the guidance, issued in 2014, several scenarios for outdoor uses were included, with an annexed calculator, as well as recommendations for further research. The guidance has been updated in 2021 with the inclusion of additional scenarios and revision of default values, on the basis of the evaluation of additional evidence. To support users in performing the assessment of exposure and risk, an online calculator, reflecting the guidance content, has been further developed.
Keywords: exposure, operator, worker, bystander, resident, plant protection products, calculator
Summary
The EFSA Guidance on the assessment of exposure of operators, workers, residents and bystanders, issued in 2014 and updated in 2021, adopted the following principles: the routine risk assessment for individual PPPs should continue to use deterministic methods, and a tiered approach to exposure assessment remains appropriate; an acute risk assessment for operators, workers and bystanders should be introduced when PPPs are acutely toxic; for acute risk assessments, exposure estimates should normally be based on 95th percentiles of relevant data sets, whereas, for longer term risk assessments, the starting point should be a 75th percentile. The guidance (and annexed online calculator) covers exposure scenarios for outdoor uses falling into a category for which standardised exposure assessment can be applied. For scenarios that are not covered by these standardised methods, the risk assessor will need to follow an ad hoc approach that is judged to be the most appropriate. An ad hoc, higher tier, exposure assessment may also be used for exposure scenarios that are covered by a standardised first‐tier method. However, this should be done only if there are good grounds for concluding that the ad hoc method will provide a more reliable and realistic estimate than the standard method for exposures arising from the proposed uses under good agricultural practices. The guidance also identifies those scenarios for which exposure estimates are the least satisfactory and provides recommendations for further research that would reduce current uncertainties.
In 2017, EFSA was asked by the European Commission to update the guidance issued in 2014 based on new relevant information, collected mainly through an open call. In particular, the greenhouse uses have been included and default values for crop parameters have been revised. Also, default values for human parameters have been updated based on more recent information from international and EU organisations as well as for harmonisation with EU Regulations and EFSA guidance documents. Moreover, the online calculator for exposure assessment has been revised by developing an online user‐friendly tool that includes new scenarios, updated default values, revised crop groupings, as well as improved functionalities such as exposure estimates for several active substances in a product, calculation of safe re‐entry interval and generation of a report. Recommendations for the design, conduct and interpretation of higher tier field studies have also been provided in the updated guidance.
The guidance should hereafter be reviewed periodically, when relevant new data become available, and, if appropriate be amended or revised.
1. Introduction
In accordance with Regulation (EC) No 1107/2009, risk assessments must be carried out for all scenarios of exposure of operators, workers, residents and bystanders that can be expected to occur as a consequence of the proposed uses of a plant protection product (PPP) according to Good Agricultural Practices (GAP). To assist risk assessors and applicants when quantifying non‐dietary exposure, the EFSA Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products was issued in 2014 (EFSA, 2014), on the basis of a preparatory opinion of the EFSA PPR Panel (EFSA PPR Panel, 2010). The guidance has been revised in 2021 by an EFSA Working Group (hereafter ‘WG’) on the basis of new available information.
In the guidance issued in 2014, only scenarios for operators during outdoor uses were available. Within the updated guidance (2021), greenhouse scenarios have been included, and revised considerations have been given to default values for crop and human parameters. Additionally, the calculation tool, available online, has been updated and complemented with new features, reflecting current regulatory needs.
1.1. Background and Terms of Reference as provided by the requestor
The EFSA Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for PPPs, and the annexed calculator, were published in October 2014. It was taken note by the Standing Committee with specific implementing provisions (European Commission, 2017a,b), lastly modified on 24 January 2017.
Based on the known limitations and recommendations from the guidance, the potential availability of new data/reports, together with suggestions from users for an improved online calculator, EFSA received a mandate of the EC in December 2017 indicating the need to revise the guidance document.
The mandate included reference to numerous aspects deserving additional considerations:
Inclusion of greenhouse uses based on new data and their available assessment (BfR, 2015)
Recent data on bystander/resident exposure from spray applications to vineyards and orchards and on worker re‐entry exposure in vineyards
BROWSE (Bystanders, Residents, Operators and WorkerS Exposure models for plant protection products) report, developed via the EU 7th Framework Programme (BROWSE, 2016)
Experience gained at EFSA and by Member States during the assessment of active substances or PPPs with the new procedures defined under SANTE‐10832‐2015 to derive the Acute Acceptable Operator Exposure Level (AAOEL)
Update of the default values used in the guidance (and online calculator), in particular under consideration of the updated guidance document on dermal absorption (EFSA, 2017), but also with respect to other parameters (e.g. crop related)
Update of the scenarios under consideration of new information as regards personal protective equipment (PPE) and technical equipment or packaging which leads to a reduction of exposure
Where possible, incorporation of additional scenarios, e.g. for treated seeds, dustable powder formulations, single plant treatments, indoor treatments including post‐harvest, amateur use
Relevance of available data and development of exposure scenarios by 3rd countries and/or at international level
Update of the online calculator under particular consideration of user friendliness and transparency of the respective assessment, with additional features such as user manual, display of results of several risk mitigation options on one page, and calculation of safe re‐entry intervals
For the update of the guidance and the online calculator additional relevant data were needed. Therefore, an open call for data was organised at the beginning of the process in order to gather relevant available data in areas of particular interest (amateur uses, seed treatment, post‐harvest treatments, dustable powder formulations, single plant treatment, bare soil application, exposure reduction by personal/collective protective equipment, exposure reduction by technical equipment or packaging, drift values, foliar half‐life values, crop groupings, re‐entry scenarios for workers, multiple applications).
1.2. Interpretation of the Terms of Reference
Within the submitted mandate (2017), the WG was asked to update the guidance document as well as the online calculator in Annex E. In order to address the terms of reference, all available information was considered, including a preliminary feedback from a workshop at ANSES (ANSES, 2018) (through questionnaire to Member States participants) as well as relevant information from an open call (EFSA, 2018).
Notwithstanding the open call, only few raw data (with original study reports) were obtained. Therefore, the WG was only able to work on a limited number of the open issues identified in the mandate. As a consequence, and as agreed with the European Commission in 2020, the update of the guidance was finalised with the inclusion of the greenhouse scenarios and revised crop and human parameters; also, a user‐friendly and transparent online calculator was implemented, including more functionalities. The pending terms of references, for which insufficient new information was available, will be addressed in following revisions of the guidance once the relevant raw data have been made available to EFSA.
The WG addressed the agreed terms of reference as follows:
Greenhouse scenario:
On the basis of the model developed by BfR (BfR, 2015, 2020), including supportive field data provided by Crop Life Europe (CLE) (formerly European Crop Protection Association, ECPA) as well as new data from three field studies which were conducted in 2012 and 2016 in different EU member states (France, Spain and Greece), performed partly within the framework of the BROWSE project, greenhouse scenarios were included in the EFSA Guidance and online calculator. In particular, the following sections of the guidance were amended:
-
–
the section for operators (2.5.1) to include the new exposure models for greenhouse uses. This amendment is based on the detailed evaluation of the greenhouse model (BfR) and supportive data as described in Appendix A.
-
–
the section on workers (2.5.2) to include considerations on worker exposure during uses in greenhouse.
-
–
the section on bystanders and residents (2.5.3) to include considerations of the specific exposure pathways for greenhouse uses. The basis for these considerations is described in Appendix B.
Default values:
For the update of default values for crop parameters, new evidence from literature and data submitted during the open call (Annex F) were considered during the revision of the guidance. In particular, the following sections of the guidance were amended:
-
–
the section on workers (2.5.2) to include a revised assessment of default values for dislodgeable foliar residue (DFR), dissipation rates (DT50) and transfer coefficients (TC). The evaluation of the available evidence is presented in the following appendices:
Appendix C on the evaluation of DFR data
Appendix D on the evaluation of DT50 data
Appendix E on the evaluation of worker re‐entry activities from EU surveys
Appendix F on the evaluation of TC from US data
Appendix G on the evaluation of TC values for bolting beet
Appendix H on the evaluation of TC values for harvesting peaches
-
–
the section on residents and bystanders (2.5.3), to include the amendments for DFR and DT50
-
–
the section on default values (2.4) to reflect more recent information from international (US EPA, 2011a,b) and EU organisations (European Commission, 2017a,b), from EU regulations (for protective equipment) and from EFSA recommendations (EFSA Guidance on dermal absorption (EFSA, 2017), EFSA Guidance on default values (EFSA Scientific Committee, 2012)).
-
–
Updated calculator:
For the update of the calculator, an online tool was developed with the aim to increase transparency and user‐friendliness. In particular, the following aspects were further developed and implemented:
-
○
new greenhouse scenarios, and related update of the mixing/loading data for outdoor uses
-
○
updated crop parameters (TC for bolting sugar beet and harvesting peaches)
-
○
updated human default values (inhalation rates, default surface area of body parts)
-
○
revision of crop grouping and extension of general re‐entry activity (i.e. inspection) to all crops
-
○
inclusion of exposure to soil‐borne residue (with revised description in Appendix I)
-
○
inclusion of additional functionalities for worker re‐entry and dermal absorption
-
○
generation of a report with detailed results
All formulas included in the online calculator are described in Annex E, together with an impact assessment of the changes brought to the formulas for outdoor uses.
Moreover, relevant instructions on how to use the tool were included in the tool.
Furthermore, the WG collected the relevant references related to the performance of higher tier field studies, and developed recommendations for the design, conduct and interpretation of this type of studies (for human exposure and experimental refinement of crop parameters) under Appendix J.
2. Assessment
2.1. Background data
Basic principles of the present guidance and the related online calculator are the transparency of data, the traceability of information and the reproducibility of the outcomes. Therefore, it was decided to consider only databases of raw data or peer‐reviewed publications offering more adequate protection on the basis of a precautionary approach (see Table 1).
Table 1.
Overview of available database and models
| Exposed category | Database/model | Availability of supporting data | Reference | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| 1 | Operator (field) | Agricultural operator exposure model (AOEM) | X | BfR (2013) | |
| 2 | Operator (greenhouse) | Greenhouse Agricultural operator exposure model (Greenhouse AOEM) | X |
BfR (2015) BfR (2020) |
|
| 3 | Operator (field) | EUROPOEM II | X | van Hemmen (2002) | |
| 4 | Operator (field) | Pesticide Handler Exposure Database (PHED) | X | PHED (1992) | |
| 5 | Operator (field) | Biocides (ECHA) | x | ECHA (2015) | |
| 6 | Amateur | ConsExpo Web | x | https://www.rivm.nl/en/consexpo | |
| 7 | Amateur | French data | x | UPJ (Union des Entreprises pour la Protection des Jardins), unpublished | |
| 8 | Amateur | German | x |
1909‐03 Bullet point ‐ Working document ‐ Amateur non‐professional use in home gardens.pdf https://circabc.europa.eu/ui/group/0b40948d‐7247‐4819‐bbf9‐ecca3250d893/library/c3d31ed6‐6eb4‐4108‐8fca‐8cfc25d0f074/details (restricted access e.g. for MS) |
|
| 9 | Operator (seed treatment) | SeedTropex | x | Unpublished, UK – FR, Industry data (1996) | |
| 10 | Worker | EUROPOEM II | x | van Hemmen et al. (2002) | |
| 11 | Worker (fork lift driver, sowing) | SeedTropex | x | Unpublished, UK – FR, Industry data (1996) | |
| 12 | Worker | Transfer coefficient | x | US EPA (2000, 2011a, 2017) | |
| 13 | Worker | Dislodgeable foliar residue | X | Lewis et al. (2017a) | |
| 14 | Worker | Dissipation rates (DT50) | X | Lewis et al. (2017b) | |
| 15 | Worker | Transfer coefficient | X | Baumann et al. (2019), Urtizberea (2002) | |
| 16 | Residents and bystanders | EUROPOEM II | X | van Hemmen et al. (2002) | |
| 17 | Residents and bystanders | BREAM (Bystander and Resident Exposure Assessment Model) | x( a ) |
Silsoe Spray Application Unit, The Arable Group, https://randd.defra.gov.uk/Document.aspx?Document=11392_PS2005Finalreportforpublication.pdf, Butler Ellis et al. (2010a,b) Butler Ellis and Miller (2010) Glass et al. (2010, 2012, 2012,2010, 2012, 2012) Kennedy et al. (2012) |
|
| 18 | Residents and bystanders | ConsExpo Web | x | https://www.rivm.nl/en/consexpo | |
| 19 | Residents and bystanders | Lloyd and Bell (1983, 1987) (spray drift values) | x( a ) |
Lloyd and Bell (1983) Lloyd et al. (1987) |
|
| 21 | Residents and bystanders |
Ganzelmeier Spray drift data |
x( a ) |
Ganzelmeier and Rautmann (1995) Rautmann et al. (2001) |
|
Public data only.
The guidance is aimed at standardising exposure assessments and providing updated procedures based on new data, where available. The guidance should subsequently be reviewed periodically and, if appropriate, amended or revised when new data become available. Because of the limitations of data currently available, the deterministic methods in routine risk assessment for individual PPPs and a tiered approach to exposure assessment remain appropriate. In addition, the available exposure data for workers are still not sufficient to propose an approach for acute risk assessment. This therefore remains a deficiency in the guidance and the need for further information to address the issue is identified. As regards combined exposure, resulting from the presence of two or more active substances in the PPP, dose addition is assumed. This is a default assumption, unless it can be shown that the substances contained in the same product do not share the same toxicological profile. The justification for different toxicological interactions should be included in the respective assessment report. EFSA is working on a number of activities aimed at implementing risk assessment due to cumulative exposures as well to exposure to mixtures (see https://www.efsa.europa.eu/en/topics/topic/chemical‐mixtures).
2.2. Definitions of exposed groups
For the purpose of this guidance, the following definitions have been adopted:
-
○
Operators are persons who are involved in activities relating to the application of a PPP; such activities include mixing/loading the product into the application machinery, operation of the application machinery, repair of the application machinery whilst it contains the PPP and/or the spray dilution and emptying/cleaning the machinery/PPP containers after use. Operators may be either professionals (e.g. farmers or contract applicators engaged in commercial crop production) or amateur PPP users (e.g. home garden users; it is noted that this guidance does not include an assessment for the scenario of PPP use by amateurs).
-
○
Workers are persons who, as part of their employment, enter an area that has been treated previously with a PPP or who handle a crop that has been treated with a PPP.
-
○
Bystanders are persons who could be located within or directly adjacent to the area where PPP application or treatment is in process or has recently been completed; whose presence is quite incidental and unrelated to work involving PPPs, but whose position may lead them to be exposed to PPP during a short period of time (acute exposure); and who take no action to avoid or control exposure.
-
○
Residents are persons who live, work or attend school or be in any other institution adjacent to an area that is or has been treated with a PPP; whose presence is unrelated to work involving PPPs but whose position might lead them to be exposed; who take no action to avoid or control exposure; and who might be in the location for up to 24 h per day (short‐term exposure).
Bystanders can also be residents and vice versa, but the two categories have been clearly differentiated for the purpose of this guidance and the related exposure estimates.
Operators, workers, residents and bystanders may be exposed to PPPs either directly through contact with the concentrate, with spray dilution, spray drift or dust (via dermal or inhalation routes) or indirectly through contact with drift deposits (dermal or ingestion) or vapour drift (assumed only relevant for vapour exposure) arising from volatilisation of deposits. Exposure is expected to decline over time.
Therefore, the total exposure from application of an active substance results from different exposure routes. However, few data are available to provide quantification of most non‐dietary exposure pathways other than direct dermal or inhalation contact. Indirect contact, apart from hand‐to‐mouth transfer for toddlers, is unlikely to contribute significantly to the overall estimates obtained following this guidance. This guidance is therefore expected to assess the major exposure pathways and provide estimates that adequately account for minor exposure pathways. Nevertheless, and since for many parameters worst‐case default values have been considered, further research will contribute to perform a more representative exposure assessment.
2.3. Overall approach
2.3.1. Step 1: identification of risk assessments that are required
The first step is to establish the risk assessments that will be required. This will depend upon who can be expected to incur exposure as a consequence of the intended use of the PPP (operators, workers, residents, bystanders), and also on whether the PPP has potential for systemic toxicity from exposure during a single day (acute exposure) in addition to systemic toxicity from repeated exposure (short‐term exposure) (see Table 2).
Table 2.
Risk assessment as required (adapted from EFSA PPR Panel, 2010)
| Exposed group | Risk assessments that may be required | |
|---|---|---|
| Acute risk assessment* | Short‐term*** risk assessment | |
| Operators | X | X |
| Workers | –** | X |
| Residents | – (covered by bystanders) | X |
| Bystanders | X | – (covered by residents) |
To be performed if an AAOEL has been set.
An acute assessment is in principle needed, but in the current guidance, insufficient data were available to perform it.
With use of the AOEL, a reference value that is usually based on oral short‐term repeat dose toxicity studies.
Acute exposures are important for substances that have the potential to induce an adverse health effect after a single exposure event (on one day); while short‐term exposures are important where adverse effects may be caused by longer periods of contact ranging from weeks to months (these exposures are also referred to as subchronic or less than lifetime exposures). These acute and short‐term exposure estimates are then compared to the EU relevant health‐based guidance values, respectively, the acceptable operator exposure level (AOEL) and the acute acceptable exposure level (AAOEL), which are derived for active substances during their toxicological evaluation. Given the use patterns of PPPs (seasonal agricultural activities) and typical metabolism and excretion kinetics, there is no evidence of long‐term continuous exposures to substances arising directly from application. If, however, a long‐term risk assessment for a group in this guidance was identified as necessary it would be appropriate to use a lifetime average or near lifetime average exposure (and compare this to a health‐based guidance value equivalent to the acceptable daily intake (ADI)). Therefore, it is likely that the exposure value would be much lower than that proposed for acute and short‐term exposures.
When an acute risk assessment is triggered (i.e. for PPPs containing active substances that are acutely toxic, and for which an AAOEL has been set), upper estimates of exposure in a single day for operators, workers, residents and bystanders should be considered. The exposure assessment for bystanders should cover the upper estimate of exposure that a resident could reasonably be expected to incur in a single day. Therefore, any risk to residents from exposures that can take place within a single day, and may produce adverse effects, would be covered by the risk assessment for bystanders, and there would be no need for a separate acute risk assessment for residents.
When an acute risk assessment is not triggered (i.e. for PPPs containing active substances that are not acutely toxic, and for which the setting of an AAOEL was not necessary), no bystander risk assessment is required. Exposure in this case will be determined by average exposure over a longer duration, and higher exposures on one day will tend to be offset by lower exposures on other days. Therefore, exposure assessment for residents also covers bystander exposure.
2.3.2. Step 2: use of standardised first‐tier methods of exposure assessment where available
For each risk assessment that is deemed necessary, potential daily exposures should be assessed using standardised methods based on measured data where available. These methods have been defined for the most commonly occurring exposure scenarios, which are specified in terms of:
-
○
The category of individual exposed – operator, worker, resident or bystander.
-
○
The type of the PPP – e.g. whether it is formulated as a solid or a liquid.
-
○
The operations that will be carried out with the PPP and the equipment that will be used – e.g. mixing and loading, application by tractor‐mounted equipment, outdoor/indoor application with handheld application equipment.
-
○
The intended uses.
In some cases, it may be necessary to combine exposures from two or more activities to obtain a figure for the total potential daily group exposure – e.g. an operator might be exposed both during preparation of the spray solution (mixing and loading) and spraying. Also, individuals may receive different group exposures on the same day (e.g. an operator doing mixing/loading, application and cleaning and also re‐entering a treated field as a worker). However, it is considered sufficient to assume the exposure from the highest exposed group over a single day represents the exposure from two or more activities.
In the case of professional operators and workers, it may be determined that it is necessary to reduce exposure effectively through the use of protective measures. If so, the exposure of these groups should, where possible, be assessed both with and without the proposed protection(s). Measured values from exposure studies should be used, as in the AOEM for example. Otherwise, the multiplying factors by which protection can be assumed to reduce exposures in Table 8 can be used as discussed in Section 2.4.7.1.
Table 8.
Default PPE (modified from EFSA PPR Panel, 2010, based on Gerritsen‐Ebben et al., 2007; van Hemmen, 2008)
| Technical control/PPE item | Protection factor (by which exposure is reduced) | Specific exposure value affected | |
|---|---|---|---|
| Protective (chemical‐resistant) gloves | Operators, liquids 90%; operators, solids 95%; workers, solids 90% | Dermal exposure – hands only | |
| Protective coverall | Operators 90% | Dermal exposure – body only | |
| Protective coated coverall | Operators certified protective coverall 95% | Dermal exposure – body only | |
| Hood and face shield( a ) | Operators 95% | Dermal exposure – head only | |
| Hood | Operators 50% | Dermal exposure – head only | |
| RPE mask type( b ) | Filter type | ||
| Half and full‐face masks | FFP1, P1 and similar |
75% 20% |
Inhalation exposure Dermal exposure – head only |
| FFP2, P2 and similar |
90% 20% |
Inhalation exposure Dermal exposure – head only |
|
Hood and face shield are considered as an alternative option to respiratory protective equipment (RPE). The hood must be certified to be protective against pesticides (i.e. the hood is usually attached to the protective coverall).
RPE can be either half mask particle filters (FFP1 and FFP2) or full‐face mask particle filters (P1 and P2).
2.3.3. Step 3: higher tier exposure assessment
Where risk assessments using standardised methods give inadequate reassurance of safety, or where no standardised first‐tier method of exposure assessment is available, it will be necessary to apply an ad hoc method that can be shown to be scientifically most appropriate. If there are good grounds for concluding that the ad hoc method will provide a more reliable and realistic estimate of exposures arising from the proposed intended use than the standard method, a higher tier exposure assessment may also be applied for exposure scenarios that are covered by a standardised first‐tier method. This conclusion must take into account the quality and quantity of data underpinning the ad hoc assessment compared with the standard method, and also the closeness with which these data relate to the exposure scenario under consideration. Where a non‐standardised higher tier exposure assessment is adopted, the justification should be clearly documented. Finally, these ad hoc methods will normally be based on higher tier field studies measuring direct human exposure or another related parameter (e.g. DFR). Due to lack of harmonised guidance for conduct and interpretation of such studies, some recommendations are included in Appendix J.
For risk assessments in relation to acute exposures (i.e. those that could occur in a single day), exposure estimates should, as a default, be derived as the higher of: (a) the 95th percentile of the distribution of measurements in the sample (the level of exposure an individual in the population can experience over a single day); or (b) a statistical estimate of the 95th percentile for the theoretical population of measurements from which the sample was derived, under the assumption that this population has a log‐normal distribution. Where the result exceeds the sample maximum, the sample maximum may be used (EFSA PPR Panel, 2010).
For risk assessments in relation to longer term exposures, exposures should, as a default, be derived as the higher of: (a) the 75th percentile of the distribution of measurements in the sample (the level of exposure an individual in the population can experience repeatedly each day over a season); or (b) a statistical estimate of the 75th percentile for the theoretical population of measurements from which the sample was derived, under the assumption that this population has a log‐normal distribution. Where the result exceeds the sample maximum, the sample maximum may be used (EFSA PPR Panel, 2010).
Statistical estimates of percentiles for the theoretical populations from which samples were derived can be made, following EFSA PPR Panel (2010), using the formula:
which is based on the standard prediction interval for a further observation when sampling from a normal distribution. In the formula, n is the number of measurements in the sample, and s are, respectively, the usual sample mean and standard deviation of the natural logarithms of the measurements, and tn‐1,a is the relevant percentile (α = 0.75 or 0.95) of the t‐distribution with n – 1 degrees of freedom.
The reason for including the statistical method based on estimates of the mean and standard deviation of the logarithm of exposure is that sample percentiles may, by chance, be unrepresentatively low, especially when the sample is relatively small, and it is a high percentile that is being estimated. However, it would be reasonable to depart from this default statistical method if, e.g. there were good evidence that the assumption of an underlying log‐normal distribution was inappropriate (e.g. a demonstration that the sample measurements deviated significantly (in statistical terms) and importantly (not just because of a single outlying value) from log‐normality).
Where the quality and relevance of the supporting data set can be clearly established, statistical methods should be used to explore possible relationships between observed exposure and other variables. Quantile regression (Koenker, 2005) is a non‐parametric method which gives an independent estimate for every percentile. As long as the percentile is well within the range of measured data, the resulting fit can be expected to be more robust than one obtained from ordinary least squares regression. In particular, it will not depend on the actual choice of the value substituted for non‐detects and does not assume the variability to be independent of the amount of active substance handled. Therefore, quantile regression is preferred over least squares regression when these issues arise.
Where only a small sample of relevant exposure measurements in operator or worker exposure studies is available (less than 10 which is the minimum specified in OECD No 9 (OECD, 1997)), a decision must be made whether or not the data set is adequate to support a valid risk assessment. If it is used, it may be necessary to make additional allowance for uncertainty in percentile estimates (e.g. by using upper confidence limits for estimated percentiles or a higher than normal percentile from the sample of measurements).
2.4. Default values proposed for the assessment
The following default values have been originally based on the opinion of the EFSA Panel on Plant Protection Products and their Residue (EFSA PPR Panel, 2010), unless otherwise specified. The default values were updated reflecting more recent data from US EPA Exposure Handbook (US EPA, 2011b) and aiming towards more harmonisation with other EU regulatory frameworks such as the biocidal product assessments. The following grouping of age categories was identified to cover the most vulnerable categories in the exposed groups mentioned in Table 2:
Children: Toddlers from 1 to 3 years old representing all age groups up to 14 years old
Adults: Adolescents from 14 to 18 years old representing all age groups from 14 years old
2.4.1. Body weights
In all calculations, it should be assumed, as a default, that adults have a body weight (bw) of 60 kg. For children, the body weight of 10 kg for toddlers is used in the calculations (Table 3).
Table 3.
Default body weight values and age categories (EFSA, 2012)
| Age category | Body weight |
|---|---|
| Infants (0–1 years) | Not needed as toddlers exposures are expected to be greater |
| Toddlers (1–3 years) | 10 kg from the group (1–3 year), protective for all age groups up to 14 year |
| Other children (3–10 years) | Not needed as toddler’s exposures are expected to be greater |
| Adolescents (10–14 years) | Not needed as toddler’s exposures are expected to be greater |
| Adolescents (14–18 years) | 60 kg (14–65 year) covering adults (18–65 year) and adolescents (14–18 year) of both genders |
| Adults (18–65 years) |
According to the EFSA Guidance on default values (EFSA Scientific Committee, 2012), a body weight of 70 kg should be used as default for the European adult population for consumer dietary risk assessment (over 18 years old). However, when a particular subpopulation is identified as a focus for the risk assessment, actual data for this specific group should be used instead of the proposed default value. Therefore, a default body weight value of 60 kg is proposed in this guidance to be protective for the non‐dietary risk assessment of all adults, including females and teenagers from 14 to 18 years, exposed from professional use of PPPs. The proposed value is in line with the approaches for Biocides (European Commission, 2017a).
Selection of the 10 kg bw value for children is assumed to represent a worst‐case scenario for the risk assessment for children up to 14 years old exposed as residents and bystanders. Children less than a year old, which would be represented by a lower body weight, are not expected to be exposed through entry into treated fields (especially not via the dermal route). Nevertheless, exposure of this age group may occur via hand‐to‐mouth transfer by playing on lawns. However, intensity of contact considering overall exposure will be significantly higher for children > 1 year of age, so that these children are assumed to be the ‘worst‐case’.
2.4.2. Inhalation rates
Where values for potential inhalation exposure are given as concentrations per cubic metre of air, an assumption must be made about the person’s inhalation rate in order to derive an estimate of the inhaled amount and systemic exposure.
For operator and worker, the exposure by inhalation needs to be estimated for a whole working day. Therefore, an average inhalation rate of 1.25 m3/h should be used (European Commission, 2017a) and the daily exposure calculated by multiplying the inhalation rate/h by the working hours per day.
For resident exposure to vapours (longer term exposure), the daily inhalation rate should be taken as shown in Table 4.
Table 4.
Daily inhalation rates (for longer term exposures) (modified from European Commission, 2017a; based on US EPA, 2011b Section 6)
| Age category (based on EFSA 2012) |
Daily inhalation rate (Mean) (m³/day) |
Daily inhalation rate, adjusted for group body weight (Mean) (m³/day per kg bw) | Comment |
|---|---|---|---|
| < 1 year | 1–< 2 years: 8.0 | 1–< 2 years: 0.80 | Selected worst‐case scenario across the available ages up to 14‐year‐old children |
| 1–< 3 years | |||
| 3–10 years | Not needed as toddler’s exposures are expected to be greater | ||
| 10–14 years | Not needed as toddler’s exposures are expected to be greater | ||
| 14–18 years | 31–< 41, 41–< 51 years: 16.0 | Adults (including adolescents ≥ 14 years old): 0.27 | Selected worst‐case scenario across the adult ages |
| Adults | |||
As for body weight, the daily inhalation rate of children aged 1 year to less than 3 years of 0.8 m3/day per kg bw was selected to represent the worst‐case scenario across the available scenarios up to 14‐year‐old children and to be protective for other age groups.
For bystander, inhalation exposure could occur predominantly over a shorter period (i.e. typically less than 30 min in duration) and during which activity could be markedly more intense than the daily average; therefore, higher values should be assumed, as shown in Table 5.
Table 5.
Hourly inhalation rates (for acute exposures) (modified from European Commission, 2017a; based on US EPA, 2011b Section 6)
| Age group (based on EFSA 2012) |
High intensity short‐term inhalation rate (m³/minute) |
Hourly inhalation rate, adjusted for group body weight (m³/hour per kg bw) | Comment |
|---|---|---|---|
| < 1 year | 1–< 2 years: 0.038 | 1–< 3 years: 0.228 | Worst‐case scenario across the available scenarios up to 14‐year‐old children |
| 1–< 3 years | |||
| 3–10 years | Not needed as toddler’s exposures are expected to be greater | ||
| 10–14 years | Not needed as toddler’s exposures are expected to be greater | ||
| 14–18 years | 51–61 years: 0.053 | Adults (including adolescents ≥ 14 years old): 0.053 | Worst‐case scenario across adult ages |
| Adults | |||
As for daily inhalation rate, the hourly inhalation rate of children aged 1 year to less than 3 years of 0.228 m3/hour per kg bw was selected to be the worst‐case scenario across the available scenarios up to 14‐year‐old children and to be protective for other age groups.
2.4.3. Average air concentrations
To estimate 24‐h average concentrations of volatilised pesticides that may be inhaled, different approaches can be used for active substances with vapour pressure (at 20 or 25°C) lower than 10‐2 Pa.
As first approach, average air concentrations in the 24 h following application are estimated using surrogate field data (California EPA, 1998; Siebers et al., 2003; PSD, 2008; European Commission, 2011) as follows:
-
○
Substances with low volatility having a vapour pressure of < 5 × 10–3 Pa, the surrogate default average concentration in air for the 24 h after application is 1 µg/m3, derived from Siebers et al. (2003).
-
○
Moderately volatile substances with a vapour pressure between 5 × 10–3 Pa and 10–2 Pa, the default average concentration in air for the 24 h after application is 15 µg/m3, derived from California EPA, 1998.
As alternative approach, the saturated vapour concentration (SVC) can be calculated and is assumed to be the worst‐case scenario, as it is not possible for the concentrations in air to exceed the SVC at a given temperature. Such estimates are likely to be very conservative (overprotective) compared to actual concentrations in air (note exposure from breathing in spray, mist or aerosol is assessed separately). This approach is the same as adopted for Biocides (European Commission, 2011).
The SVC should be calculated from the substance’s measured or estimated vapour pressure as follows
where
– SVC = saturated vapour concentration (in mg/m³)
– mw = molecular weight of the active substance (in g/mol)
– vp = vapour pressure (in Pascal)
– R = gas constant = 8.31451 J × mol‐1 × K‐1 (physical constant)
– T = temperature = 293 K (assumed room temperature = 20°C)
Resulting in SVC = 0.41 × mw × vp [mg/m³].
Estimates based on this method for substances with very low vapour pressures (i.e. below 10−5 Pa) are likely to more realistic, although still conservative, than estimates based on the surrogate field data mentioned above.
For active substances with vapour pressures ≥ 10–2 Pa, since no default value is available, the risk assessments should be based on the SVC approach. If it indicates exposures above the health‐based guidance values, specific measurements of concentrations in air under conditions representative of intended use are required. Alternatively, or possibly in addition, if available, suitably validated dispersion models may be employed to support an exposure assessment.
2.4.4. Hectares treated per day
Table 6 shows default values for area treated per day, in hectares, depending on the type of crop and the application technique. The area treated reflects the technical standard of the equipment used in the original studies underpinning exposure data. In practice, the treated area will depend on the type of equipment used. The assessments proposed for operators, given modern equipment, are also considered to cover the assessment of less modern equipment in correlation with smaller areas treated per day and using smaller amounts of PPPs. The values used for the proposed models should not be adjusted for smaller areas treated with less modern equipment.
Table 6.
Area treated per day
| Crop groups | Cultivation( a ) | Area treated per day (ha) | |
|---|---|---|---|
| Handheld equipment( b ) | Vehicle‐mounted equipment | ||
| Field crops | Outdoor | – | 50 |
| Low vegetables | Outdoor | 4/1 | 50 |
| Indoor | 1 | – | |
| High vegetables | Outdoor | 4/1 | 10( c ) |
| Indoor | 1 | – | |
| Low berries | Outdoor | 4/1 | 50 |
| Indoor | 1 | – | |
| Cane fruit/High berries | Outdoor | 4/1 | 10( c ) |
| Indoor | 1 | – | |
| Orchards | Outdoor | 4/1 | 10( c ) |
| Oil fruits | Outdoor | 4/1 | 10( c ) |
| Amenity grassland ( d ) | Outdoor | 4/1 | 50 |
| Agricultural grassland ( e ) | Outdoor | – | 50 |
| Viticulture | Outdoor | 4/1 | 10( c ) |
| Hops ( f ) | Outdoor | 4/1 | 10( c ) |
| Low ornamentals | Outdoor | 4/1 | 50 |
| Indoor | 1 | – | |
| High ornamentals | Outdoor | 4/1 | 10( c ) |
| Indoor | 1 | – | |
| Bare arable land ( g ) | Outdoor | – | 50 |
| Indoor | 1 | – | |
| Bare non‐arable land ( h ) | Outdoor | 4/1 | 50 |
‘Outdoor’ is understood to mean all areas that are outdoors and have little or no barrier to the free distribution of PPP into the environment when it is applied (e.g. direct cover if covered after application of PPP). ‘Indoor’ means all areas that offer a certain barrier to free distribution of the PPP in the environment when applied (e.g. high and low technology greenhouses). Please see EFSA Guidance Document for details on the individual structures (EFSA, 2014a). For partially protected or entirely protected crops, the greenhouse model should be used for operators, workers, residents and bystanders.
The first value should be used for handheld application using tank sprayers with lances and the second value for other equipment (e.g. knapsack sprayers in low or high crops); for upwards spraying with handheld equipment on dense foliage, the area treated is 1 ha. For indoor uses, the area treated is always 1 ha without tractor‐mounted application. Note: Dense/normal scenario is a parameter only relevant for operators (depending on e.g. growth stage and crop cultivation) and based upon measured exposure values from the AOEM/Greenhouse AOEM studies. It is applicable to outdoor uses in orchards and cane fruits and for all indoor uses.
Also applicable to herbicide application; data based on subset of high crop treatment with small area downward spraying equipment (e.g. equipment with smaller spray booms and normally only a few nozzles).
Amenity grassland and managed amenity turf – includes e.g. semi‐natural or planted grassland such as golf course roughs, frequently mown areas, grass grown for turf production, public parks, sports turf, golf greens, tees and fairways.
Agricultural grassland – includes grass fodder crops and similar forage crops e.g. short‐ and long‐term grass leys, permanent pasture, lucerne or alfalfa and clovers.
Hops are typically treated using vehicle‐mounted sprayers; however, occasionally localised spot treatments within the hop yard are applied. These applications can involve handheld sprayers. To account for such applications the default areas for handheld equipment shown should be assumed. However, where the estimate indicates high exposure, this should be considered carefully, and reliable use information should be provided to support a refined estimate as the default areas are likely to overestimate application by spot treatments.
In the online calculator, there are no specific data on bare soil; however, it was considered that for spraying application downwards on soil (e.g. herbicides in pre‐emergence), the same data as for application in low crops, tractor‐mounted, can be used. Planting activities in a bare soil are not covered by the present guidance; however, exposure to soil‐borne residue occurring in the absence of contact with treated foliage is provided.
‘Outdoor’ is understood to mean all areas that are outdoors and have little or no barrier to the free distribution of PPP into the environment when it is applied (e.g. direct cover if covered after application of PPP). ‘Indoor’ means all areas that offer a certain barrier to free distribution of the PPP in the environment when applied (e.g. high and low technology greenhouses). Please see EFSA Guidance Document for details on the individual structures (EFSA, 2014a). For partially protected or entirely protected crops, the greenhouse model should be used for operators, workers, residents and bystanders.
For crops not reported in Table 6, further justification has to be provided by the applicant to show the most appropriate scenario to bridge the information to.
The WG agreed that the areas in Table 6 were also applicable to granular formulations.
In the online calculator, the selection of the scenario will automatically select the appropriate treated area per day.
2.4.5. Exposure durations
-
○
Operator: 8 h.
-
○
Worker: 2 h (default inspection or irrigation activities); 8 h (other activities, e.g. hand harvesting, thinning, tying, etc.).
-
○
Resident and bystander: 2 h (dermal, surface deposits), 0.25 h (dermal, entry into treated crops) and 24 h (inhalation from vapour).
2.4.6. Absorption values
Dermal and oral absorption percentages should be taken from the toxicological evaluation.
-
○
Oral: if less than 80%, the specific value should be entered in the online calculator; if above 80%, the online calculator will automatically consider 100% oral absorption (note that an oral absorption value lower than 80% is also taken into account during the derivation of the toxicological reference value).
-
○
Dermal: to be determined according to the EFSA Guidance Document on Dermal Absorption, as in force, on the basis of a dermal absorption study or, if no specific study is available, as an appropriate default value. For worker, resident and bystander exposure towards surface deposits and re‐entry into treated crops, the higher of the values for the undiluted product and the in‐use dilution should be taken from the dermal absorption study. The use of higher dermal absorption value is based on the precautionary principle as currently no validated method to measure dermal absorption of dried residue after application of dilutions is available.
-
○
Inhalation: 100%
2.4.7. Default surface area of body parts
In Table 7, the default surface areas for body parts are reported:
Table 7.
Default values for surface area of the various parts of the body at different ages (European Commission, 2017a)
| INFANT irrespective of gender (based on female 6–< 12 months old) | TODDLER irrespective of gender (based on female 1–< 2 years old) | CHILD( a ) irrespective of gender (based on female 6–< 12 years old) | ADULT irrespective of gender (based on female 30–< 40 years old) | |
|---|---|---|---|---|
| Body part surface areas (cm2) | ||||
| Hands (palms and backs of both hands) | 196.8 | 230.4 | 427.8 | 820 |
| Arms (both) |
Upper = 352.6 Lower = 229.6 Total = 582.2 |
Upper = 412.8 Lower = 268.8 Total = 681.6 |
Upper = 772.8 Lower = 496.8 Total = 1,269.6 |
Upper = 1,141.2 Lower = 1,128.8 Total = 2,270 |
| Head | 344.4 | 403.2 | 531.3 | 1,110 |
| Trunk (bosom, neck, shoulders, abdomen, back, genitals and buttocks) | 1,689.2 | 1,977.6 | 3,624.8 | 5,940 |
| Legs (both legs and thighs) | 1,041.4 | 1,219.2 | 2,741.6 | 5,330 |
| Feet (both) | 246 | 288 | 604.9 | 1,130 |
| Total body surface area | 4,100 | 4,800 | 9,200 | 16,600 |
Please note that the age categories for body surface areas correspond to the categories defined in the Recommendation No. 14 of the Biocidal Products Committee (BPC) Ad hoc Working Group on Human Exposure (ECHA, 2017), whereas those for body weight correspond to the age categories defined in EFSA Guidance on Default Values (2012), as well as in the EFSA Food consumption database. Therefore, minor mismatches may occur, e.g. category child in Table 3 corresponds to the category toddler in this table.
2.4.7.1. Use of personal protective equipment
The handling and application of PPPs require the setting of minimum health and safety requirements at the workplace, covering the risks arising from exposure of workers to such products, as well as general and specific preventive measures to reduce those risks (European Commission, 1998,2004). The implementation of adequate preventive measures relies mainly on national regulations in the respective Member States. PPE is part of these measures and should also fulfil the requirements set under Regulation (EU) 2016/425 (European Commission, 2016) to be considered as certified, taking into account appropriate standards, such as:
ISO 18889:2019 – Protective gloves for pesticide operators and re‐entry workers
EN ISO 27065:2017 – Protective clothing — Performance requirements for protective clothing worn by operators applying pesticides and for re‐entry workers
EN 149:2001+A1:2009 – Respiratory protective devices. Filtering half masks to protect against particles.
In practice, trained (professional) operators should at least wear workwear irrespective of the actual risk. Hence, first‐tier exposure assessments should be performed for trained operators using workwear. In the online calculator, reduction of operator exposure by workwear in case of spray applications is based on measured data from the AOEM studies. In these studies, the non‐certified workwear1 used by operators consisted of long‐sleeved shirt and long trousers or coveralls (single layer of work clothing covering arms, body and legs) and is considered as within the scope of the EN ISO 27065 certified protective coverall (level C1‐2). The effect of wearing garments providing greater protection instead of workwear has to be considered separately from the online calculator and in discussion with Member State authorities, as there is no harmonised classification of proposed factors.
Accounting for protection from engineering/technical control items can be used in case additional measures are needed to reduce exposure to an acceptable level (e.g. 50% drift reduction, use of water‐soluble bags, closed cabin in case of tractor‐mounted application in high crops are implemented in the online calculator). Engineering/technical control measures would be preferred above PPE in the occupational hygiene hierarchy, but limited data are currently available to identify suitable equipment and protection factors. Default protection factors for PPE are identified in Table 8 below.
The protection factors (PF) in Table 8 are appropriate for generating estimates of exposure where available data were measured either without protective equipment or outside such equipment. Actual measurements show variability in the levels of protection provided, and the factors were chosen to give estimates that would be unlikely to underestimate true exposures. The PF are not appropriate for estimating the level of potential exposure from measurements below clothing or PPE, as the inverse calculation will only provide a low estimate of the potential exposure. In such cases, a higher PF is required. For example, for estimating dermal exposures during granule applications, the available data are limited to measurements under protective gloves and coveralls, and to estimate the exposures for the no PPE scenario the values are multiplied by 100 (i.e. PF = 99%).
In the online calculator, the PF attributed to PPE items and/or workwear are mostly based on actual study data from the underlying exposure studies. Where the online calculator only estimates exposure for unprotected workers, this is because there are insufficient data to support the use of a PF. Therefore, the online calculator exposure estimates should not be refined by these default PF (e.g. it is not accepted to refine a worker exposure in grapes using gloves).
The PF for inhalation exposure have not been determined in the AOEM studies, but default values have been applied in the online calculator to allow for the use of respiratory protective equipment (RPE) according to the requirements outlined in Regulation (EU) 2016/4252 and in EN 149.
Further refinements with different factors could be considered at Member State level based on national conditions.
It is noted that it is not in the remit of the present guidance document to cover local effects from non‐dietary exposure to pesticides and recommend PPE to protect operator and worker from any potential local effects, such as skin sensitisation. It is acknowledged, however, that independently of the operator/worker exposure assessment outcome related to systemic effects, additional PPE might be needed based on the classification of a pesticide for local effects.
2.5. Methods for first‐tier exposure assessment
2.5.1. Operator exposure
Exposure is estimated for the recommended conditions of use of the PPP. Exposure estimation for mixing/loading (ML) and application is normally done separately. Both dermal and inhalation exposures are considered.
Dermal exposure is converted into systemic dose using appropriate dermal absorption percentages, while absorption via inhalation is considered to be complete (100%). Exposure estimates for individual tasks are the sum of the dermal exposure and the inhalation exposure. Where an operator is expected to be engaged in both ML and application, exposures from these tasks are summed. The total exposure is divided by a standard body weight of 60 kg and then compared to the relevant reference values.
For outdoor spray application uses, the AOEM is considered as a suitable exposure model for operator exposure assessment, as it reflects updated agricultural practices, including protective measures; furthermore, the criteria for the selection of the studies are transparent and allow reproducibility of the outcomes.
For indoor spray application uses, a greenhouse model for operator exposure to pesticides has been developed by BfR (BfR, 2015) on the basis of seven field studies contracted by CLE, and then updated with new greenhouse exposure data from three studies conducted in 2012 and 2016 in different EU Member States (BfR, 2020).
The assessment of this model together with the supporting raw data has been performed by the WG (see Appendix A). The database as well as the model is subject to certain limitations (e.g. for knapsack mixing/loading and low crop application); nevertheless, it reflects current practices and techniques for an acceptable approach to estimate exposure of operators in greenhouses.
For the assessment of operator exposure, in general, the 75th percentile was considered appropriate for short‐term exposure, in addition, a model based on the 95th percentile was developed for the assessment of acute exposure. Available models include application techniques and scenarios for outdoor treatment of low and high crops, by vehicle‐mounted/trailed or self‐propelled sprayers or by handheld spray guns and knapsack sprayers and for indoor treatment of low and high crops by handheld spray guns, knapsack and trolley sprayers (see Tables 2 and 3 in Annex E).
Furthermore, the possibility of using water‐soluble bags was also considered. Exposure to PPPs during ML is likely to be limited but not negligible. Based on expert judgement and approaches at the national level, the WG decided that the default exposure deriving from ML activities of water‐soluble bag should be assumed to be 10% of the corresponding formulation. In case of automated applications, exposure cannot be limited to ML since maintenance and cleaning activities during application should not be excluded and no data are available for this scenario.
Mixing/loading values for spray equipment may also be considered representative of other application methods in which product handling and equipment preparation tasks are comparable (e.g. weed wipers) as long as no further data are available. As a default, intended uses with handheld application equipment should be calculated using knapsack and tank ML scenarios.
For granular formulations, further models (see Tables 7 and 8 in Annex E) are available (adapted from EFSA PPR Panel, 2010) covering partly additional application scenarios. It should be taken into account that these data are relatively old (PHED, 1992). However, to cover these additional scenarios, these models are the only available option. The exposure data from tractor‐mounted granular applications were monitored outdoor and the data are considered only to be appropriate for outdoor vehicle applications. The handheld applications providing exposure data for both carried and push along equipment were also monitored outdoors. However, in the absence of specific indoor data, it is considered appropriate to use the outdoor handheld model to estimate exposures from indoor applications of granular formulations using similar equipment.
The estimated exposures from defined work tasks with granular formulations are assumed to depend on the amount of active substance handled in the tasks (in a few cases, as indicated in Table 8 of Annex E, specific exposures cover a combination of ML and application, in which case the summation exercise is not required). The estimated exposure is the product of the specific exposure in mg (or μg) exposure/kg a.s. handled (see Annex E, Table 5), the area treated (ha/day) (Table 6 above) and the recommended amount of active substance applied (kg a.s./ha).
2.5.2. Worker exposure
Exposure of workers must be estimated for activities that involve contact with treated crops. Such contact may occur when workers re‐enter treated areas after application of a PPP (e.g. for crop inspection or harvesting activities). In addition, worker exposure can arise from other activities such as crop maintenance or packaging, sorting and bundling.
The underlying studies for the worker exposure model show a high level of uncertainty in terms of quality and reliability of data. Therefore, for the online calculator, only the short‐term exposure was considered.
In the guidance issued in 2014, the available data allowed calculations for re‐entry only immediately after the application solution has dried. In the online calculator, a safe re‐entry interval can be estimated based on the formula/approach provided in Section 2.5.2.3. The ‘safe re‐entry interval’ is defined as the specific time point post application, after which the worker exposure levels calculated for the relevant re‐entry tasks are lower than the AOEL considering the different clothing and PPE cases depending on the TC availability.
The main routes of exposure during post‐application activities are dermal and inhalation, and the sources of exposure are contact with foliage (including usually fruits as well as leaves), soil and possibly dust. Oral exposure may occur secondarily to dermal exposure, through hand‐to‐mouth transfer. However, for workers, potential exposure by this route is generally assumed to be negligible in comparison with that via skin and inhalation.
Most crop maintenance and harvesting activities include frequent contacts with the foliage of the crop. Therefore, dermal exposure is the most important exposure route during these re‐entry activities. The level of resultant exposure (for a default activity) depends on the amount of residue on foliage, the intensity of contact with the foliage and the overall duration of contact. The same considerations regarding dermal exposure of workers are valid for both outdoor and indoor scenarios. So far, there is no evidence to substantiate that the parameters and the corresponding default values used to estimate worker exposure via the dermal route outdoor should be different in case of indoor applications.
Inhalation exposure may be to vapour and/or airborne aerosols (including dust). After outdoor application of PPPs and after the spray solution has dried, there will be more rapid dissipation of vapour and aerosols, leading to lower inhalation potential than from indoor treatments (where the inhalation route could be a relevant route for re‐entry workers), such as those made to crops grown in greenhouses. Therefore, worker exposure estimates for the inhalation route after outdoor applications are only necessary in exceptional cases (e.g. for volatile substances). In these cases, an ad hoc approach is necessary. Regarding indoor applications, inhalation exposure data are available only regarding re‐entry activities to greenhouse ornamentals, and therefore, these data have been extrapolated to similar activities in other greenhouse crops and included in the calculator.
There are also some re‐entry situations where significant potential for exposure to soil‐borne residue is possible in the absence of contact with treated foliage, e.g. workers handling compost treated with an insecticide, or during manual harvesting of root crops. In situations where workers may be in contact with treated compost or soil containing quantifiable residue, without foliage contact, the approach in Appendix I is appropriate (and has been included in the online calculator). However, in most situations, the contribution of soil residue to the total exposure is expected to be significantly less than that from DFR. Where there is concomitant exposure to DFR, exposure from contact with soil residue can be ignored. In situations where there is no foliage or no foliage contact and only limited potential for contact with soil, such as re‐entry after pre‐emergence or early post emergence spray applications to arable crops (e.g. up to BBCH 11–12 one or two leaves emerged), the potential for dermal exposure is likely to be very low and is not estimated.
With the first‐tier methods described in this section (and included in the online calculator), only short‐term exposure is considered for the worker. However, if worker exposure is estimated from ad hoc data, then the exposure estimates used for acute and short‐term risk assessments will normally be different.
To derive a total estimate of worker exposure, it is necessary to sum the components of exposure from each relevant source and route. The methods for estimating exposures should assume that the worker will wear no PPE (Commission Regulation (EU) No 284/2013). Normal workwear comprises coverall or long‐sleeved shirt and trousers (arms, body and legs covered). If TC data for protected body and hands for re‐entry activities in the corresponding crop are available, then consideration for this scenario can be made in exposure estimation by application of respective TC as specified in Table 10.
Table 10.
Transfer coefficients (TCs, in cm²/h) (modified from EUROPOEM II (Van Hemmen et al., 2002) considering US EPA, 2012, 2017; for both outdoor and indoor scenarios)
| Crop groups | Nature of task | Main body parts in contact with foliage | TC, total potential exposure | TC assuming arms, body and legs covered (workwear; bare hands) | TC, covered body (workwear) and gloves (PPE) | TC, potential body exposure and gloves (PPE) | Applicable for the following crops |
|---|---|---|---|---|---|---|---|
| Field crops | Hand harvesting | Hand and body | n.a. | 23,000 | n.a. | n.a. | Sweet corn |
| Low/High vegetables | Reach/pick | Hand and body | 5,800 | 2,500 | 580 | n.a. | Fruiting/bulb/legume/leaf vegetables and fresh herbs |
| Low vegetables | Harvest/maintenance | Hand and body | n.a. | 5,000 | n.a. | n.a. | Brassica vegetables |
| Low vegetables (root and tuber) | Bolting beet removal | Hand and body | 18,600 | 4,400 (4,500 for long trousers and T‐shirt) | 430 (530 for long trousers, T‐shirt and gloves) | 14,300( a ) | (Sugar) beets, seed potato |
| Orchards | Maintenance/thinning | Hand and body | 22,500 | 4,500 | 2,250 | n.a. | Citrus/cane/oil/pome/stone fruits, tree nuts, berries (high crops)( b ) |
| Search/reach/pick | Hand and body | 12,500 | 3,500 | 1,250 | n.a. | (same) | |
| Viticulture( c ) | Harvesting and other activities (e.g. leaf pulling and tying) | Hand and body | 30,000 | 10,100 | No justified proposal (data missing) | n.a. | Grapes, hops( d ), and Kiwifruit |
| Low berries | Reach/pick | Hand and forearm | 5,800( e ) | 3,000 | 750 | n.a. | Berries and other small fruit, low( f ) |
| Ornamentals (low/high) | Cut/sort/bundle/carry | Hand and body | 14,000 | 5,000 | 1,400 | n.a. | Ornamentals and nursery |
| Amenity grassland | Turf harvesting, cutting and handling | Hand and body | n.a. | 8,800 | n.a. | n.a. | n.a. |
| All crops | Inspection, irrigation | Hand and body |
12,500( g ) 7,500( h ) |
1,400( g ) | 1,250 | n.a. | Including agricultural grassland, not bare land |
n.a.: not available.
Dermal exposure was measured considering different levels of protection by clothing and protective equipment (i.e. gloves). The combinations of different dosimeters were used to estimate the dermal exposure for different levels of skin protection (T‐shirt, shorts and gloves: 14,300 cm²/h).
Strawberries and other berries that are cultivated at multiple heights indoor are considered high crops.
US EPA data were used even if the underlying data are not available as it is clear that grape harvesting might be a scenario of concern for which EU data are missing. As for inspection activities, the US EPA values are considered to be appropriate, in the absence of supporting data, when compared with the exposure values for other tasks.
TCs from grapes are proposed as surrogate for hops. Relevant tasks are for example training/tying or inserting the hop bines into the picking machine after harvest.
No reliable data for this scenario are available; therefore, the TC of vegetable potential exposure is proposed as surrogate.
Strawberries cultivated outdoor are considered low crops
US Re‐entry Agricultural TF data were used, recalculated by Health and Safety Executive to account for 75th percentile instead of arithmetic mean (see technical report comment 211; EFSA, 2014b).
US Re‐entry Agricultural TF data were used; the value proposed is the arithmetic mean of the 75th percentiles from the two studies considered, lower legs and arms uncovered (see technical report comment 211; EFSA, 2014b).
2.5.2.1. Dermal exposure of workers
Dermal exposure from contact with residue on foliage should be estimated based on the following equation:
where:
– PDE = potential dermal exposure (mg a.s./day)
– DFR = dislodgeable foliar residue (µg/cm2) (consider MAF, if necessary)
– TTR = turf transferable residue (µg/cm2) in the case of amenity grassland
– TC = transfer coefficient (cm2/h)
– T = task duration (h/day).
The default value for time of exposure should be taken as 8 h for harvesting and maintenance type activities and 2 h for crop inspection and irrigation type activities.
To convert estimated dermal exposures to corresponding systemic exposures, the potential dermal exposure should be multiplied by a dermal absorption value (see Section 2.4.6).
2.5.2.2. Dislodgeable foliar residue (DFR)
The amount of initial residue on foliage is presumed to depend on multiple factors, including among others the application rate and water volume, properties of droplets (size, distribution, velocity, adhesion energy, etc.), application technique and efficiency (how much reaches and is retained on the target), crop type/architecture and leaf texture (waxy, smooth, hairy) and the amount of foliage (leaf area index) (see Appendix C).
Where experimentally determined DFR data are not available, the initial DFR (DFR0 is the DFR just after application, assuming that no dissipation has taken place at this time point) in a first‐tier assessment should be assumed to be 3 (μg active substance/cm2 of foliage)/(kg a.s. applied/ha). This value is regarded as highly conservative (van Hemmen et al., 2002; Lewis and Tzilivakis, 2017a). Yet, as the DFR value depends upon multiple parameters and none of them could be estimated as a sole or most critical driver for the worst‐case DFR0 level, no refinement of the default value can be currently proposed. The evaluation of parameters qualitatively investigated for their effect on DFR0 in papers collected in Lewis and Tzilivakis (2017a) and in BROWSE project, considered to be reliable, is included in Appendix C.
The online calculator provides the possibility of entering specific DFR values when available from adequate experimental data (see Appendix J).
Experimental DFR value for another (reference) formulation and/or another intended use can be used if the formulation for which DFR needs to be determined is sufficiently similar and the application scheme is closely related. This would occur when at least the following conditions are met:
-
○
The same active substance is investigated;
-
○
The application was performed on the same crop with a higher or equal application rate;
-
○
The application is performed at a similar growth stage (e.g. data for lower growth stages may not be used for later growth stages because growing and maturing of the leaves as well as the changing density of the foliage might affect DFR level);
-
○
The application is performed under similar application and growth conditions (e.g. for outdoor applications: climatic zone, similar meteorological conditions, temperature; for indoor application: watering technique, temperature).
A justification should be provided in all cases when DFR data of a similar formulation and/or another intended use are considered. In such cases, justification should contain information why the data used represent the worst‐case scenario and do not underestimate the DFR level and/or DT50.
It can be questioned if workers entering the crops (e.g. orchards, vineyards) where herbicides are applied will be exposed to residue on the weeds or grass equal to the default value of 3 µg/cm2 per kg or if this value might provide an overestimation for manual re‐entry activities. However, since there is no validated model to estimate any deposition on the crop foliage, through drift or possibly volatilisation, the use of the default value might still be appropriate. Although it is not clear if the nature of the deposited residue in such a case would behave similar to a DFR. To account for presumably lower deposits than the default value on such crops, an appropriate TC could be applied to reflect the activity and level of contact. This can be the default activity of inspection and irrigation with a low TC value whose applicability has been extended to all crops (see Table 10).
Additional consideration is required for scenarios involving contact with residue on lawns or equivalent amenity grassland surfaces which have been directly treated or are subject to deposition from pesticide drift as in the non‐occupational assessments discussed below (see Section 2.5.3). Here, the generic TC are derived from residue assessments that employ a roller technique, rather than a dislodging solution, to sample the foliar residue. Consequently, the denominator of TC ratio in these cases is not the usual DFR value but is the turf transferable residue (TTR). The default TTRs, as a percentage of the applied application rate, for products applied as liquid sprays, is 5%, and for products applied as granules, 1%. These values come from data obtained using the Modified Californian Roller Method (Fuller et al., 2001; Rosenheck et al., 2001) and represent the upper end of the range from a number of studies with different compounds. The DFR remains the appropriate parameter for agricultural grasslands.
2.5.2.3. Dissipation rate (DT50)
In the absence of experimental data on the degree of dissipation, it may be assumed that active substances which are organic chemicals, and for which there is evidence of breakdown e.g. by photolysis or hydrolysis in soil or water, will dissipate with a DT50 of 30 days (default value in the online calculator). For other categories of active substances with no evidence of breakdown (e.g. inorganic chemicals), only DFR0 (i.e. the residue available directly after application when dry) can be used for calculations since the default DT50 is considered not applicable. In such cases, the multiple application factor (MAF) value is not appropriate and the number of applications without refinement for DT50 should be used (see Section 2.5.2.4).
For the update of the guidance, new dissipation data were investigated (Lewis and Tzilivakis, 2017b), in order to explore if current default DT50 value of 30 days can be refined. Based upon the detailed evaluation of these data, included in Appendix D, no new default DT50 can be proposed.
Where valid experimental data for a dissipation of an active substance on a specific crop are available, these data can be used to refine the exposure assessment.
Dissipation of residue on crop foliage over time depend on a range of physical and chemical properties of the active substance and involve various processes. Physical parameters like volatilisation or wash‐off, physico‐chemical factors like photolysis, abiotic chemical degradation as well as biological factors like uptake through the cuticular layer, biotic biotransformation and dilution due to plant growth have all an impact on the degradation of foliar residue. These processes will also be influenced by the presence of co‐formulants (adjuvants, carriers, surfactants, efficacy improvers, etc.) and by the environmental conditions (rain, air humidity, wind erosion, droplet abrasion, temperature, etc.).
The integrated result of these processes is usually visible in the form of an initial rapid decline in surface residue followed by a phase of slower dissipation (Willis and McDowell, 1987). In principle, the assumption of first‐order kinetics is less appropriate for such type of processes. However, only very few data are typically available on the decline of residues over the initial few hours. Yet, these would be required for achieving more accurate fit of a more complex kinetical model. Since the DT50 from first‐order kinetics tends to underestimate dissipation at earlier time points for the described overlap of partly very rapid processes, but will not overestimate it, this approach is recommended to ensure a more conservative estimate for the earlier period. Thus, in most cases, a first‐order kinetics model is suitable for describing the dissipation of residue. Biphasic approaches may be considered, if at later periods, the dissipation is overestimated when using first‐order kinetics due to a slower dissipation. This becomes even more important, when a specific DFR level of interest lies beyond the last sampling day (predicted DFR levels may then be underestimated, and thus, the risk assessment may not be sufficiently protective).
For the determination of the DT50 value, acceptable DFR studies can be used (see Section 2.5.2.2 and Appendix J). The standard procedures recommended by FOCUS (2014) should be followed, including the assessment of the goodness of fit (e.g. estimates to the measured residue data should be evaluated visually (concentration vs. time plots and residual plots) and statistically (Chi‐square test)). More recommendations on the fitting of DT50 data and the statistical validation of the fit can also be found in the EFSA Technical Report (EFSA, 2019).
For estimation of safe re‐entry interval, with or without workwear and/or gloves, the following equation has been introduced in the online calculator (Hou et al., 2017; Zongmao and Haibin, 1997; FOCUS, 2014):
where
– t = safe re‐entry interval (days)
– PDE = potential dermal exposure (mg a.s./day)
– DFR0 = initial DFR just after application, assuming that no dissipation has taken place at this time point (µg/cm2)
– MAF = multiple application factor
– k = ln(2)/DT50 (rate constant)
– TC = transfer coefficient (cm2/h)
– T = task duration (h/day).
Further explanation on how the online calculator evaluates exposure for extended re‐entry intervals is given in Annex E.
It is noted that the acceptability of the calculated safe re‐entry interval for worker should be examined on a case‐by‐case basis, since this depends on the specific needs for re‐entry tasks of each crop at the time of application.
2.5.2.4. Multiple application factor (MAF)
Multiple applications of a compound may cause a build‐up of residue levels and must be taken into account in the exposure assessment. As long as only peak concentrations are considered in the risk assessment, residue dynamics can be expressed by an MAF. The MAF is a function of the number of applications, the application interval and the decline of residue, typically expressed as a DT50 assuming first‐order kinetics (single first order (SFO‐DT50)) (EFSA, 2010a).
The MAF for average residue levels (i.e. MAFm) is calculated using the following equation (also included in the online calculator):
where:
– k = ln(2)/DT50 (rate constant)
– n = number of applications
– i = application interval (d).
By forming the limit value, lim n→∞, of the equation above, the term e–nki becomes zero and a ‘plateau’ MAFm for an infinite number of applications can be calculated.
Examples of MAF values, calculated on the basis of the default DT50 value of 30 days, can be found in Table 9. When exposure estimates in the first tier are exceeding the established trigger, refined calculations can be performed by introducing specific DT50 values (e.g. DT50 determined experimentally) in the online calculator.
Table 9.
Multiple application factors, assuming a default dissipation DT50 of 30 days (EFSA PPR Panel, 2010)
| Interval between applications (days) | Number of applications | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 7 | 1.0 | 1.9 | 2.6 | 3.2 | 3.7 | 4.2 | 4.5 | 4.9 | 5.1 | 5.4 | 5.6 | 5.7 |
| 10 | 1.0 | 1.8 | 2.4 | 2.9 | 3.3 | 3.6 | 3.9 | 4.1 | 4.2 | 4.4 | 4.5 | 4.5 |
| 14 | 1.0 | 1.7 | 2.2 | 2.6 | 2.9 | 3.1 | 3.2 | 3.3 | 3.4 | 3.5 | 3.5 | 3.5 |
| 21 | 1.0 | 1.6 | 2.0 | 2.2 | 2.4 | 2.5 | 2.5 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 |
2.5.2.5. Transfer coefficient
The transfer coefficient (TC) is related to the transfer of residue from the plant surface to the clothes or skin of the worker, regardless of the product applied. The TC depends on the level of exposure which in turn depends on the intensity of contact with the foliage of the culture under consideration. This is determined by the nature and duration of the activity during re‐entry. Therefore, it is possible to group various crop types and re‐entry activities.
The TC should be calculated with the following equation, where dermal exposure can reflect different levels of clothing or protection, resulting in respective TC values:
where:
– dermal exposure (mg/h)
– TC = transfer coefficient (cm2/h)
– DFR = dislodgeable foliar residue (mg/cm2).
The indicative TC values reported in Table 10 are adapted from EUROPOEM II (van Hemmen et al., 2002), with due consideration of US EPA values (Appendix F) and new experimental data for bolting beet and harvesting peaches (Appendices G and H); they apply to both outdoor and indoor scenarios. Up to four sets of TC values are given per crop group, according to whether or not it can be assumed that the worker will wear clothing that covers the arms, body and legs. It is assumed that harvesting is performed with bare hands or with gloves, and that dermal exposure to the body is reduced 10‐fold (i.e. 90% protection) by clothing covering the arms, body and legs. For cases where no workwear or gloves can be reasonably considered to be worn, exposures may be higher than these estimates and potential exposure should be estimated using the values in the fourth column of Table 10 (total potential exposure).
With regard to activities in sugar beet, the assessment of worker exposure during inspection activities after the application of PPPs in (sugar) beets does not reasonably cover the crop‐specific task of manually removing bolting beet, in particular with respect to task duration and intensity of contact with the crop foliage. Therefore, an adjustment was required, i.e. specific TC values were needed to ensure that the assessment delivers more reasonable exposure estimation. Based on the combined experimental data for exposure and DFR, crop‐ and task‐specific TC values were derived for workers removing bolting beet manually. A work rate of 8 h per day should be assumed for the exposure assessment. Because the task is relevant for growth stage BBCH 39 and beyond, the removal of bolting beets should not be considered for the use of PPPs (particularly herbicides) at early growth stages (application until the BBCH 19) as the remaining residue level at BBCH 39 is assumed to be very low. For these early applications in (sugar) beets, the use of a work rate of 2 h per day with the general TC values for inspection and irrigation is still applicable for the estimation of worker exposure. For any intended use of PPPs in (sugar) beets beyond BBCH 19, the removal of bolting beets should be considered in the risk assessment. These newly proposed TC values could also be used for similar activities in closely related crops, e.g. manual removal of infested plants for virus control in seed potatoes.
With regard to harvesting activities in orchards, the TC value for potential body exposure included in EUROPOEM II was determined to be 20,000 cm2/h according to the 90th percentile of a small database. Considering that this value could be overestimated, more recent field data collected during harvesting peaches have been assessed in addition. It is concluded that TC for body exposure during harvesting can be reduced to 10,000 cm2/h while the TC for potential hand exposure remains 2,500 cm2/h. Furthermore, the WG agreed that the previous TC for the activity of search/reach/pick in orchards could still be applied to maintenance activities (e.g. thinning) in orchards. Resulting TC values for different levels of protection are considered to be conservative enough and comparable to all the US EPA data for orchard crop activities.
These TC values may be extrapolated to other re‐entry scenarios, where the intensity and duration of contact with the foliage are judged to be similar (e.g. inspection activities for rice and cotton can be considered sufficiently similar to other field crops).
Access to the scientific data underlying the TC values is in many cases very limited, as was the ability of the WG to access all the relevant original data (e.g. both the US EPA (2000) and data reported in the EUROPOEM II report). No data from Bystander Resident Orchard Vineyard (BROV) re‐entry project could be considered for this update, since submitted data did not allow an independent analysis by the WG (see Annex F).
Based on collection and assessment of data from EU surveys (see Appendix E), no additional re‐entry worker activities and related TC could be identified (Glass et al., 2012).
2.5.2.6. Inhalation exposure of workers
Potential exposure to a volatilised active substance (a.s.) decreases with time as its concentration is reduced, by absorption into the plant, degradation or loss to the environment. Although, in many cases, inhalation exposure is likely to contribute less to total potential exposure than that arising by the dermal route, inhalation exposure should be estimated for crops grown in enclosed spaces, e.g. in permanent greenhouses.
Exposure via inhalation of airborne residue in enclosed spaces may arise through:
spray, mist or fog droplets remaining airborne at the time of worker entry,
resuspension of pesticide particles in the air as a result of the work activity and
volatilisation of pesticide after application.
Four indicative inhalation task‐specific factors (TSF) have been estimated for a small set of exposure data for harvesting and re‐entry in ornamental greenhouse (van Hemmen et al., 2002), as set out in Table 11.
Table 11.
Indicative inhalation task‐specific factors (TSF) for protected crops
| Task | TSF (mg a.s./h)/(kg a.s./ha) |
|---|---|
| Ornamentals cutting | 0.1 |
| Ornamentals sorting and bundling | 0.01 |
| Re‐entering 8 h after low volume mist (LVM) application | 0.03 |
| Re‐entering 16 h after roof fogging application | 0.15 |
Two scenarios apply: exposure during specific work tasks involved in harvesting ornamentals (cutting, sorting and bundling); and general exposure to airborne droplets/particles after low volume misting (LVM) or roof fogging applications. These TSF should be used in the first‐tier assessment, where exposure is estimated as follows:
where:
– PIE = potential inhalation exposure (mg a.s./h inhaled)
– AR = application rate (kg a.s./ha)
– TSF = task‐specific factor [(mg a.s./h)/(kg a.s./ha)].
Given the limited data supporting the ornamental harvest TSFs, and uncertainties associated with differentiating harvest work tasks (e.g. if it is considered realistic that workers will often be engaged in both activities rather than limited to just cutting or sorting and bundling), a single TSF of 0.1 (mg a.s./h)/(kg a.s./ha) should be used for all harvesting activities. However, other work tasks such as crop, inspection, general maintenance (e.g. watering) and sorting will have lower exposures and the TSF of 0.01 (mg a.s./h)/(kg a.s./ha) should be used where these are done in the absence of harvesting or similar activities.
Although no data are available for harvesting other crops, it is likely that similar mechanisms creating airborne residue could occur where workers reach and pick within well‐developed plant foliage; therefore, the harvest TSF derived from ornamentals should also be used for non‐ornamental crops. Likewise for other work tasks in other crops, the TSF of 0.01 (mg a.s./h)/(kg a.s./ha) should also be used.
Similar issues regarding the amount and quality of data and differentiating between LVM and roof fogging applications also exist for the other TSF reported by van Hemmen et al. (2002). Although mists are often regarded as having volume median diameters (VMD) between 50 and 100 µm, and fogs are defined as having VMDs less than 50 µm, so‐called LVM typically involves application of droplets < 50 µm (e.g. one leading manufacturer states a VMD of 14 µm). Therefore, a single TSF of 0.15 (mg a.s./h)/(kg a.s./ha) should be used to estimate potential exposure for any re‐entry exposure task within 16 h of an application involving VMD < 50 µm (i.e. LVM, cold fogging and hot fogging). For any re‐entry task occurring after 16 h following the application the harvest TSF of 0.1 (mg a.s./h)/(kg a.s./ha) or the sorting TSF of 0.01 (mg a.s./h)/(kg a.s./ha) should be used as described above for hydraulic sprays.
The above‐recommended TSF are summarised in Table 12. Airborne residue following typical hydraulic spray applications are much lower than those following applications with VMDs < 50 µm (Kirknel and Emde, 1997), and it is not necessary to consider them separately.
Table 12.
Recommended TSF for different scenarios
| Crops and application method | Tasks | TSF (mg a.s./h)/(kg a.s./ha) |
|---|---|---|
| Spray applications – All greenhouse crops | Cutting, bundling and other harvest activity | 0.1 TSF |
| Spray applications – All greenhouse crops | Inspection, general maintenance, sorting, watering | 0.01 TSF |
| LVM, Cold fogger, hot fogger, etc. | All tasks before 16 h have elapsed | 0.15 TSF |
| LVM, Cold fogger, hot fogger, etc. | After 16 h have elapsed cutting, bundling and other harvest activity | 0.1 TSF |
| LVM, Cold fogger, hot fogger, etc. | After 16 h have elapsed, inspection, general maintenance, sorting | 0.01 TSF |
Measurements made in greenhouses with three active substances demonstrate the potential for volatilisation to lead to diurnal fluctuations of measurable vapour residue over days following applications (Doan Ngoc et al., 2015). Therefore, an estimation of potential worker inhalation exposure to vapour residue should also be made. Vapour concentrations should follow the approach described in Section 2.4.3. Additional data may be required to estimate inhalation exposures for products applied as vapours and for volatile pesticides, which are outside the scope of this guidance.
The above recommendations for estimating vapour and particulate air concentrations are assumed to give estimates that are applicable during re‐entry tasks soon after application. Where re‐entry occurs at significantly later times, e.g. a number of days later, dissipation or degradation (or a combination of both) are expected to result in lower air concentrations: Even where compounds are relatively stable, greenhouse ventilation (necessary for maintaining acceptable growing conditions) will remove airborne residue. As an interim measure, before further review of information relating to the estimation of such exposures, it is recommended that as a default it can be assumed that the decline in daily concentrations of either vapour or resuspended particulates will be correlated with the decline in DFR, and therefore, the default foliar DT50 can be used to generate estimates of vapour and task‐related particulate exposures to take account of pesticide dissipation after day 1. A similar formula, to that shown in Section 2.5.2.3, is applied to the inhalation exposures. Further details are provided in Annex E.
2.5.3. Resident and bystander exposure
The data set available for assessing resident and bystander exposure is rather limited, being based on only a few studies, some of which performed in the 1980s. Furthermore, some of the US EPA values used to conclude on these assessments are not completely reported (raw data missing).
For exposure through treatment of nearby crops, four pathways of exposure are considered (EFSA PPR Panel, 2010):
-
○
spray drift (at the time of application)
-
○
vapour (may occur after the PPP has been applied)
-
○
surface deposits
-
○
entry into treated crops.
Summing all the exposure pathways, each one being high percentile of exposure, would result in an overly conservative and unrealistic result. This is particularly true for bystanders, considering that it is extremely unlikely that all exposures occur together. However, for residents, it might be appropriate to sum up the mean exposures from each pathway, where available.
For assessing resident and bystander exposure after application in low crops, the Bystander and Resident Exposure Assessment Model (BREAM) calculator was developed in the UK (Kennedy et al., 2012). As the data from the BREAM calculator were considered more appropriate for this scenario than those reported by Lloyd and Bell (1983), they and the scenarios investigated were adopted by the WG and are set out in Table 13.
Table 13.
Data derived using the BREAM calculator and the scenario specified
| BREAM calculator input | Value | Notes |
|---|---|---|
| Nozzle type | FF03110 | Conventional flat fan nozzle. It is the only data set currently available. From other drift data, it is clearly not the worst‐case nor the best case |
| Number of nozzles | 48 | Represents single pass of a 24‐m boom. Further upwind passes could possibly contribute additional drift, but the wind conditions will not be identical and the additional contribution from including more upwind nozzles or passes is relatively small |
| Boom height | 0.7 m | The optimum height is 0.5 m, but anecdotal evidence suggests modern practice involving large sprayers travelling at fast forward speeds exceeds this. Spray drift increases with boom height |
| Forward speed | 12.6 km/h | Considered to be the upper end of the current ‘average’ in the UK based on expert opinion (i.e. 3.5 m/s, hence 12.6 km/h). A 2004 UK survey showed that between 15% and 20% of the area treated by large or self‐propelled sprayers was done using average speeds in the range 13–16 km/h |
| Spray concentration | 1 g a.s./L spray | Used to generate unit values which can be adjusted by product‐specific values |
| Crop height | Short | The model does not yet support estimation of exposure from spraying other crops |
| Wind speed | 2.7 m/s | Upper limit of what is considered acceptable for spraying in the UK Code of Practice |
| Bystander type | Child and adult | Data collected on adult and child mannequins. Adult were 1.87 m tall. Child mannequins were 1.03 m tall (i.e. about median height for a 4‐year‐old child) |
| Exposure route | Dermal and inhalation | Taking into account the surface areas of body parts (Table 7) and the updated values for breathing rates (Tables 4 and 5) |
| Dermal absorption | 100% | Used to give an estimate of the external dose, which later can be adjusted by appropriate dermal absorption values |
| Inhalation rate | Bystanders | Inhalation reflective of high‐intensity activity |
| Children 2.28 m3/h | The body weight assumed in this guidance is 10 kg, which is representative of children around 1 year old. Therefore, to be compatible with this body weight, an average high activity breathing rate of 0.228 m3/h/kg bw should be used, and the rate per hour becomes 0.228 m3/h/kg bw × 10 kg = 2.28 m3/h | |
| Adults 3.18 m3/h | i.e. 0.053 m3/h per kg bw × 60 kg | |
| Residents | Daily average inhalation rate | |
| Children 0.33 m3/h | The body weight assumed in the guidance is 10 kg, which is representative of children around 1 year old. Therefore, to be compatible with this body weight, an average breathing rate of 0.80 m3/day/kg bw should be used, and the rate per hour becomes 0.80 m3/day per kg bw × 10 kg bw/24 h = 0.33 m3/h | |
| Adults 0.675 m3/h | i.e. 0.27 m3/kg bw per day × 60 kg bw/24 h | |
| Distance from source | 2 m | Considered to represent a realistic worst‐case distance. For example, this could represent a sprayer operating at the edge of a field with a resident/bystander in a garden separated from the field by a simple wire fence and with both the spray operator and resident/bystander unaware of each other’s actions |
Note: A typical F11003 nozzle operating at 3 bar, at the above forward speed would apply about 120 L/ha which is 12 mL/m2 and at the spray concentration of 1 g/L. Assuming above, this would deliver 120 g/ha or 12 mg/m2. The model is a good predictor for short crop and short vegetation.
For estimating exposure from surface deposits, ground sediments based on drift for application on high crops are taken from Rautmann et al. (2001); for arable crops, respective data are from the BREAM project.
The online calculator allows adjustments based on drift reduction for upwards and downwards spraying for both residents and bystanders, and based on increases of the distance from the source (5 and 10 m).
An adjustment for light clothing for residents and bystanders is proposed: Assuming that the trunk is covered, that the trunk represents 36% of the body surface area and that the clothing gives 50% protection (in line with the EUROPOEM 1996 report for clothes), there would be a reduction of 18% for adults and 18% for children (trunk represents 35.7% of the body surface area). This adjustment is applied for estimates of potential dermal exposure arising from spray drift only.
Considering the available evidence for pesticide emissions from applications in greenhouses to the surrounding environment, the current practice of disregarding the potential exposure of bystanders and residents living in immediate vicinity of greenhouse areas is not adequate. In the absence of established and commonly accepted models for the risk assessment, bridging from the risk assessment for residents and bystanders for outdoor application is considered as a suitable first‐tier approach, with the difference that a re‐entry into treated areas is deemed not to be appropriate for uninvolved persons. Thus, the following pathways should be covered: direct dermal and inhalation exposure based on spray drift, exposure towards deposits (caused by spray drift) and exposure towards volatilised residue in air (see Appendix B).
2.5.3.1. Resident exposure
In principle, residential exposure should be based on the 75th percentile estimates. However, summing the individual 75th percentile exposures does not seem appropriate, whereas summing the means does seem reasonable for assessing repeated exposure. On this basis, both the 75th percentile and mean values need to be calculated for each exposure pathway (currently only available for spray drift and drift deposit), the 75th percentile will be assessed separately, and the means will be summed up (each calculated exposure is likely providing a conservative estimate; therefore, the final resident exposure should be the sum of the mean values of each exposure pathway).
For repeated applications on tree crops, it may not be possible to specify the ‘season’ in the data entry of the online calculator as ‘with’ or ‘without leaves’. The online calculator will default to the worst‐case scenario.
-
○
Spray drift ‐ resident
The exposures from spray drift should be calculated using the following equation:
Spray drift resident exposure = Dermal exposure × dermal absorption percentage + inhalation exposure.
where the dermal absorption percentage is the value for the in‐use dilution taken from the toxicological evaluation, and the dermal and inhalation exposures are those shown in Tables 14 and 15, taking into account the surface areas of body parts (Table 7) and the updated breathing rates (Table 4).
Table 14.
Dermal and inhalation exposures for residents (75th percentile from data on potential dermal and inhalation exposures) (adapted and amended from EFSA PPR Panel, 2010)
| Method of application (distance from sprayer) | These values are the 75th percentiles for residents (assuming average breathing rates for inhalation exposures) | |||
|---|---|---|---|---|
| Dermal (mL spray dilution/person) | Inhalation (mL spray dilution/person) | |||
| Adults | Children | Adults | Children | |
| Arable/ground boom sprayer | ||||
| 2 m | 0.47 | 0.33 | 0.00012 | 0.00016 |
| 5 m | 0.24 | 0.22 | 0.00011 | 0.00012 |
| 10 m | 0.20 | 0.18 | 0.00010 | 0.00010 |
| Orchard/broadcast air‐assisted applications( a ) | ||||
| 2–3 m | n.a. | n.a. | n.a. | n.a. |
| 5 m | 5.63 | 1.689 | 0.0021 | 0.00103 |
| 10 m | 5.63 | 1.689 | 0.0021 | 0.00103 |
n.a.: not available.
The only available values are for the 8‐m distance downwind from the middle of the tree trunk, which are assumed to represent a 5‐m distance from the edge of the orchard; the same value is used for 5 and 10 m.
Table 15.
Dermal and inhalation exposures for residents (mean data on potential dermal and inhalation exposures) (adapted and amended from EFSA PPR Panel, 2010)
| Method of application (distance from sprayer) | These values are the mean values (assuming average breathing rates for inhalation exposures) | |||
|---|---|---|---|---|
| Dermal (mL spray dilution/person) | Inhalation (mL spray dilution/person) | |||
| Adults | Children | Adults | Children | |
| Arable/ground boom sprayer | ||||
| 2 m | 0.22 | 0.18 | 0.00011 | 0.00012 |
| 5 m | 0.12 | 0.12 | 0.00009 | 0.00010 |
| 10 m | 0.11 | 0.10 | 0.00008 | 0.00008 |
| Orchard/broadcast air‐assisted applications ( a ) | ||||
| 2–3 m | n.a. | n.a. | n.a. | n.a. |
| 5 m | 3.68 | 1.11 | 0.00170 | 0.00083 |
| 10 m | 3.68 | 1.11 | 0.00170 | 0.00083 |
n.a.: not available.
The only available values are for the 8‐m distance downwind from the middle of the tree trunk, which are assumed to represent a 5‐m distance from the edge of the orchard; the same value is used for 5 and 10 m.
For arable crops, BREAM data provide drift data for children (using mannequins representative of 4‐year‐old children). The BREAM results do not provide values for upwards spraying.
For orchard crops and vines, the most appropriate data set out of the three presented is the data set for conventional nozzles (no drift reduction technologies) applying 470 L/ha from a report by Lloyd et al. (1987) for an 8‐m distance downwind from the middle of the tree trunk. This data set gave the highest drift exposures in that report. No adjustment to the exposure values for orchard crops and vines is proposed, since the measurements in the report by Lloyd et al. (1987) relate to application across an entire orchard, and the layout of orchards and vineyards and the way equipment is operated (e.g. when at the edge of the orchard, spray is directed only into the crop) makes the values suitable for a resident located about 5 m from the edge of a field, assuming the space from the tree trunk to the edge of the field is at least 3 m. These data form a significant part of those included in EUROPOEM for this scenario, and are preferred to the others, as they were generated under more representative conditions.
However, it should be taken into account that these data are relatively old and that data for different distances are not available. The WG recommends that further data are produced to refine the proposed assessment.
It is noted that no data are available for manual application. The WG proposes that the same data be used for manual application as for vehicle application as a first‐tier assessment (i.e. deposition values for broadcast air‐assisted sprayers for upwards manual application, and field crop sprayer values for downwards manual application). Further refinement could be needed on a case‐by‐case basis.
The BREAM calculator provides dermal and inhalation exposure estimates from arable applications for adults and children. Based on the scenario above (Table 13), the 75th percentile values at 2 metres from the sprayer in Table 14 are based on the following:
-
–
dermal exposure: adults 0.47 mg and children 0.33 mg. Note, for these examples, 1 mg a.s. = 1 mL spray solution (concentration spray solution 1 g a.s./L; see Table 13)
-
–
inhalation exposure: adults (breathing rate 0.675 m3/h) 0.00012 mg; and children (breathing rate 0.33 m3/h) 0.00016 mg.
Lloyd et al. (1987) provide values measured for orchard applications for adults only. The 75th percentile values for adults in Table 14 were re‐calculated for children:
-
–
dermal exposure = 5.63 mL × 0.3 (child/adult body area) = 1.689 mL
-
–
inhalation exposure = 0.0021 mL × (0.33 m3/h (child breathing rate)/0.675 m3/h (adult breathing rate)) = 0.00103 mL
The average values in Table 15 are derived from the corresponding data in the same manner.
Without additional data, no adjustment of data from Lloyd et al. (1987) for further distances is possible. However, drift‐reducing nozzles and other certified drift reduction technologies or techniques (DRT) can be considered as a risk mitigation measure. Corresponding safety instructions on the label are necessary. An adjustment of drift based on 50% drift reduction was agreed by the WG, considering 50% as a reliable factor from experimental data showing from 50 to 90% drift reduction (e.g. Guidelines for the testing of PPPs Part VII, April 2000. Federal Biological Research Centre for Agriculture and Forestry Federal Republic of Germany). However, these tests are performed measuring drift up to a height of 50 cm only. Further drift measurements are required for implementation of DRTs considering > 50% drift reduction.
-
○
Vapour – resident
Exposures to vapour should be estimated using the method that has been developed in the UK (CRD, 2008) and Germany (Martin et al., 2008), based on the highest time‐weighted average exposure for a 24‐h period, according to the volatility of the active substance:
where
– SERI = systemic exposure of residents via the inhalation route (mg/kg bw per day)
– VC = vapour concentration (mg/m3)
– IR = inhalation rate (m3/day)
– IA = inhalation absorption (%)
– BW = body weight (kg).
For moderately volatile compounds (vapour pressure ≥ 0.005 Pa and < 0.01 Pa), exposures should be calculated assuming a default concentration in the air of 15 μg/m3 and daily average breathing rates as reported in Table 4, resulting in:
-
–
an adult value of 15 μg/m3 × 0.27 m3/day per kg = 4.05 μg/day per kg × 60 kg = 243 μg/day
-
–
a child value of 15 μg/m3 × 0.8 m3/day per kg = 12 μg/day per kg × 10 kg = 120 μg/day.
For compounds with low volatility (vapour pressure < 0.005 Pa), exposures should be calculated assuming a default concentration in the air of 1 μg/m3 and daily average breathing rates as reported in Table 4, resulting in:
-
–
an adult value of 1 μg/m3 × 0.27 m3/day per kg = 0.27 μg/day per kg × 60 kg = 16.2 μg/day
-
–
a child value of 1 μg/m3 × 0.8 m3/day per kg = 0.8 μg/day per kg × 10 kg = 8 μg/day.
Especially for substances with low vapour pressure, the SVC can be used as a screening tool for refinement (see Section 2.4.3).
Any future possibility of modifying the vapour pressure value and the concentration in the air will allow a refinement of the exposure calculations.
-
○
Surface deposits ‐ resident
Dermal exposure from surface deposits based on spray drift should be based on the following equation (EFSA PPR Panel, 2010):
where:
– SERD = systemic exposure of residents via the dermal route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%) (if multiple applications have to be taken into account, a lower percentile could be considered for risk refinement)
– TTR = turf transferable residue (%) (for products applied in liquid sprays, 5%, and for products applied as granules, 1% (these values come from data obtained using the Modified Californian Roller Method (Fuller et al., 2001; Rosenheck et al., 2001) and represent the upper end of the range from a number of studies with different compounds))
– TC = transfer coefficient (cm2/h) (default values of 7,300 cm2/h for adults and 2,600 cm2/h for children are recommended, TC values for minimal protection from clothes)
– H = exposure duration (hours) (a default value of 2 h is recommended by US EPA, 2001)
– DA = dermal absorption (%) (higher of the values for the undiluted product and the in‐use dilution)
– BW = body weight (kg).
Exposure from surface deposits for children aged less than 3 years should be calculated using the following equation:
Dermal exposure + hand‐to‐mouth transfer + object‐to‐mouth transfer.
Children’s hand‐to‐mouth transfer should be calculated using the following equation:
where:
– SOEH = systemic oral exposure via the hand to mouth route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%) (if multiple applications have to be taken into account, a lower percentile could be considered for risk refinement)
– TTR = turf transferable residue (%) (for products applied in liquid sprays, 5% is used, and, for products applied as granules, 1% is used (these values come from data obtained using the Modified Californian Roller Method (Fuller et al., 2001; Rosenheck et al., 2001), and represent the upper end of the range from a number of studies with different compounds)
– SE = saliva extraction factor (%) (a default value of 50% is recommended by US EPA, 2001; it refers to the fraction of pesticide extracted from a hand/object via saliva. It is a median value from a study on the fraction of pesticide extracted by saliva from hands (Camann et al., 1995))
– SA = surface area of hands (cm2) (the assumption used here is that 20 cm2 of skin area is contacted each time a child puts a hand in his or her mouth (US EPA, 2001))
– Freq = frequency of hand‐to‐mouth (events per hour) (for short‐term exposures, a value of 9.5 events per hour is recommended; this is the average of observations ranging from 0 to 70 events per hour (US EPA, 2001))
– H = exposure duration (hours) (a default value of 2 h is recommended by US EPA, 2001)
– OA = oral absorption (%)
– BW = body weight (kg).
Children’s object‐to‐mouth transfer should be calculated using the following equation:
–
where:
– SOEO = systemic oral exposure via the object to mouth route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%)
– DRP = dislodgeable residue percentage (%) (a default value of 20% transferability for object‐to‐mouth assessments is recommended by US EPA, 2001)
– IgR = ingestion rate for mouthing of grass/day (cm2) (a default value of 25 cm2 of grass/day is recommended by US EPA, 2001)
– OA = oral absorption (%)
– BW = body weight (kg).
Values for drift percentage should be taken from Table 16, as appropriate.
Table 16.
Ground sediments based on drift as a percentage of the application rate
| Distance | Field crops (%)( a ) | Fruit crops, early stages b , (c) | Fruit crops, late stages b , (c) | Grapes b , (d) | Hops( b ) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P75 | Median | P77 | Median | P77 | Median | P77 | Median | P77 | |
| 2–3 m | 4.1 | 5.6 | 18.96 | 23.96 | 6.96 | 11.01 | 5.25 | 6.90 | 9.95 | 15.93 |
| 5 m | 1.8 | 2.3 | 11.69 | 15.79 | 3.73 | 6.04 | 2.32 | 3.07 | 5.91 | 8.57 |
| 10 m | 1.0 | 1.3 | 6.07 | 8.96 | 1.6 | 2.67 | 0.77 | 1.02 | 2.91 | 3.70 |
P75: 75th percentile; P77: 77th percentile.
From BREAM. These drift values for field crops are also applied in the online calculator to low berries, low vegetables and low ornamentals (outdoor and indoor).
From Ganzelmeier/Rautmann (the 75th percentile is not published).
Early/late season (stage) is a parameter only relevant for bystanders and residents and is based upon measured drift deposits (Ganzelmeier/Rautmann), in which the values for orchards were displayed separately for early and late stages (without leaves and with leaves). This differentiation applies also to cane fruit/high berries (outdoor) but does not apply to oil fruits or citrus crops, which are not directly comparable to orchards since these crops are evergreen plants. For oil fruits and citrus crops only, late season is considered relevant and realistic as regards exposure of bystanders and residents by deposits based on drift.
The drift values for grapes are also applied in the online calculator to cane fruit/high berries (indoor), high vegetables, high ornamentals (outdoor and indoor).
Different risk mitigation measures for the assessment of surface deposits can be applied. For example, safety distances of > 2–3 m can be used for the risk assessment. Furthermore, drift‐reducing nozzles of 50% can be considered as a risk mitigation measure in this guidance (see e.g. Guidelines for the testing of PPPs Part VII, April 2000. Federal Biological Research Centre for Agriculture and Forestry Federal Republic of Germany). Corresponding safety instructions on the label are necessary. Any further risk mitigation measures need to be supported by data (including an assessment of the conditions used to derive the proposed measures compared with the conditions used to estimate the drift values proposed in this guidance).
Based on the limited availability of data, for products applied as granules, drift from applications of granules should be assumed to be 3% for broadcast and manual applications. Further refinements could be considered based on new data. Dust drift for in‐furrow applications is considered to be negligible.
-
○
Entry into treated crops ‐ resident
Entry into treated crops is based on exposure from activities such as walking in treated fields for adults.
The method used should be the same as for workers, with the same DFR and a TC based on data for inspection activities (75th percentile: 7,500 cm2/h, mean: 5,980 cm2/h), and with a 15‐min exposure. TC values are only available for adults. A factor of 0.3 has been applied to the adult TC for children re‐entering treated crops.
For entry onto amenity grassland (e.g. during outdoor activities on treated lawns), an extra scenario of recreational exposure is also calculated, including only surface deposits (see above) with a deposition percentage of 100% (exposure to drift fallout being considered as not relevant when residents enter directly into the treated area). For children, all the pathways of exposure to surface deposits are relevant. Currently, for adults, object‐to‐mouth and hand‐to‐mouth transfer of surface deposits are considered less important and are not considered in the online calculator.
2.5.3.2. Bystander exposure
Exposures for the four pathways for bystanders should be assessed in the same way as for residents, except that dermal and inhalation exposures to spray drift should be taken as the 95th percentile values derived from the underpinning data sets. However, the four pathway exposures should not be summed because, based on the available data, the WG considers that it is unlikely and unrealistic that 95th percentile exposures from the different pathways will occur at the same time.
-
○
Spray drift ‐ bystander
The exposure from spray drift should be calculated using the following equation:
(Dermal exposure × dermal absorption percentage) + inhalation exposure.
where the dermal absorption percentage is that for the in‐use dilution taken from the toxicological evaluation, and dermal and inhalation exposures are those shown in Table 17, taking into account the surface areas of body parts (Table 7) and the updated breathing rates (Table 5).
Table 17.
Dermal and inhalation exposures for bystanders (95th percentile) (adapted and amended from EFSA PPR Panel, 2010)
| Method of application/distance from sprayer | 95th percentiles for bystanders (assuming high breathing rates for inhalation exposures) | |||
|---|---|---|---|---|
| Dermal (mL spray dilution/person) | Inhalation (mL spray dilution/person) | |||
| Adults | Children | Adults | Children | |
| Arable/ground boom sprayer | ||||
| 2 m | 1.21 | 0.74 | 0.00066 | 0.00135 |
| 5 m | 0.57 | 0.48 | 0.00064 | 0.00100 |
| 10 m | 0.48 | 0.39 | 0.00068 | 0.00091 |
| Orchard/broadcast air assisted applications ( a ) | ||||
| 2–3 m | n.a. | n.a. | n.a. | n.a. |
| 5 m | 12.9 | 3.87 | 0.0044 | 0.0032 |
| 10 m | 12.9 | 3.87 | 0.0044 | 0.0032 |
n.a.: not available.
The only available values are for the 8‐m distance downwind from the middle of the tree trunk, which are assumed to represent a 5‐m distance from the edge of the orchard; the same value is used for 5 and 10 m.
Using the BREAM calculator, the values for arable crops at 2 metres from the sprayer in Table 17 should be based on the following:
-
–
dermal exposure: adults 1.21 mg and children 0.74 mg (for this case, mg = mL)
-
–
inhalation exposure: adults (breathing rate 3.18 m3/h) 0.00066 mg and children (breathing rate 2.28 m3/h) 0.00135 mg (for this case 1 mg a.s. = 1 mL spray solution).
For orchard applications, Lloyd et al. (1987) provide 95th percentile exposures: dermal, 12.9 mL (maximum) and inhalation, 0.0044 mL. These figures are for adults. Assuming that the vertical spray drift profile is uniform for both adult and child heights, child values can be estimated as follows:
-
–
dermal = 12.9 mL × 0.3 (child/adult body area) = 3.87 mL
-
–
inhalation = 0.004 mL × (2.28 m3/h child/3.18 m3/h adult) = 0.0032 mL.
-
○
Vapour ‐ bystander
Vapour concentrations should be estimated as for residents (see Section 2.5.3.1), and exposures calculated in the same way taking into account duration, inhalation rate and body weight.
-
○
Surface deposits ‐ bystander
Dermal exposures from surface deposits based on spray drift should be based on the following equation (EFSA PPR Panel, 2010):
where:
– SEBD = systemic exposure of bystander via the dermal route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%) (if multiple applications have to be taken into account, a lower percentile could be considered for risk refinement)
– TTR = turf transferable residue (%) (for products applied in liquid sprays, 5% is used, and, for products applied as granules, 1% is used. These values come from data obtained using the Modified Californian Roller Method (Fuller et al., 2001; Rosenheck et al., 2001), and represent the upper end of the range from a number of studies with different compounds
– TC = transfer coefficient (cm2/h) (default values of 14,500 cm2/h for adults and 5,200 cm2/h for children are recommended; TC values take into account minimal protection from clothes)
– H = exposure duration (hours) (a default value of 2 h to cover bystander dermal exposure)
– DA = dermal absorption (%) (higher of the values for the undiluted product and the in‐use dilution)
– BW = body weight (kg).
Exposure from surface deposits for children less than 3 years old should be calculated using the following equation:
Children’s hand‐to‐mouth transfer should be calculated using the following equation:
where:
– SOEH = systemic oral exposure via the hand to mouth route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%) (if multiple applications have to be taken into account, a lower percentile could be considered for risk refinement)
– TTR = turf transferable residue (%) (for products applied in liquid sprays, 5% is used, and, for products applied as granules, 1% is used). These values come from data obtained using the Modified Californian Roller Method (Fuller et al., 2001; Rosenheck et al., 2001) and represent the upper end of the range from a number of studies with different compounds
– SE = saliva extraction factor (%) (a default value of 50% is recommended by US EPA, 2001; it refers to the fraction of pesticide extracted from a hand/object via saliva. It is a median value from a study by Camann and colleagues on the fraction of pesticide extracted by saliva from hands (Camann et al., 1995))
– SA = surface area of hands (cm2) (the assumption used here is that 20 cm2 of skin area is contacted each time a child puts a hand in his or her mouth (US EPA, 2001))
– Freq = frequency of hand‐to‐mouth (events per hour) (for short‐term exposures, the value of 20 events per hour is recommended; this is the 95th percentile of observations ranging from 0 to 70 events per hour (US EPA, 2001))
– H = exposure duration (hours) (a default value of 2 h to cover bystander exposure)
– OA = oral absorption (%)
– BW = body weight (kg).
Children’s object‐to‐mouth transfer should be calculated using the following equation:
where:
– SOEO = systemic oral exposure via the object to mouth route (mg/kg bw per day)
– AR = application rate (mg/cm2) (consider MAF, if necessary)
– D = drift (%)
– DRP = dislodgeable residue percentage (%) (a default value of 20% transferability for object‐to‐mouth assessments is recommended by US EPA, 2001)
– IgR = ingestion rate for mouthing of grass/day (cm2) (a default value of 25 cm2 of grass/day is recommended by US EPA, 2001)
– OA = oral absorption (%)
– BW = body weight (kg).
Values for drift percentage should be taken from Table 18, as appropriate.
Table 18.
Ground sediments as a percentage of the application rate
| Distance | Field crops( a ) | Fruit crops, early stages ( b , c ) | Fruit crops, late stages ( b , c ) | Grapes( b ,( d | Hops( b ) |
|---|---|---|---|---|---|
| 95th percentile | 90th percentile | 90th percentile | 90th percentile | 90th percentile | |
| 2–3 m | 8.5% | 29.20 | 15.73 | 8.02 | 19.33 |
| 5 m | 3.5% | 19.89 | 8.41 | 3.62 | 11.57 |
| 10 m | 1.9% | 11.81 | 3.60 | 1.23 | 5.77 |
From BREAM. These drift values for field crops are also applied in the online calculator to low berries, low vegetables and low ornamentals (outdoor and indoor).
From Ganzelmeier/Rautmann.
Early/late season (stage) is a parameter only relevant for bystanders and residents and is based upon measured drift deposits (Ganzelmeier/Rautmann), in which the values for orchards were displayed separately for early and late stages (without leaves and with leaves). This differentiation applies also to cane fruit/high berries (outdoor) but does not apply to oil fruits or citrus crops, which are not directly comparable to orchards since these crops are evergreen plants. For oil fruits and citrus crops only late season is considered relevant and realistic as regards exposure of bystanders and residents by drift.
The drift values for grapes are also applied in the online calculator to cane fruit/high berries (indoor), high vegetables, high ornamentals (outdoor and indoor).
Different risk mitigation measures for the assessment of surface deposits can be applied at the Member State level. For example, safety distances of > 2–3 m can be used for the risk assessment. Furthermore, drift‐reducing nozzles of 50% can be considered as a risk mitigation measure in this Guidance (see Guidelines for the testing of PPPs Part VII, April 2000. Federal Biological Research Centre for Agriculture and Forestry Federal Republic of Germany). Corresponding safety instructions on the label are necessary. Any further risk mitigation measures need to be supported by data (including an assessment of the conditions used to derive the proposed measures compared with the conditions used to estimate the drift values proposed in this guidance).
Drift from agricultural applications of granules (general granule application, e.g. slug pellets) is assumed to be 3% for broadcast and manual applications (‘worst‐case’). Dust drift for in‐furrow applications is considered to be negligible.
-
○
Entry into treated crops ‐ bystander
For entry into crops, refer to Section 2.5.3.1.
For entry onto treated lawns, exposures should be calculated in the same way as for surface deposits (see above) but using a deposit (% of application rate) of 100%.
When estimating the maximum exposure that a bystander might reasonably be expected to incur in a single day by higher tier methods, account must be taken of the possibility that a bystander could be a resident.
3. Conclusions and recommendations
The update of this guidance represents a significant development towards the harmonisation of the pesticide exposure assessment for operators, workers, residents and bystanders at the EU level. However, many gaps still remain and, when relevant new data will become available, where appropriate, the guidance should be further amended or revised. It is noted that all raw data and original study reports should be provided to EFSA in order to guarantee a transparent and independent assessment (such supporting data are also required for models that are submitted to EFSA).
The following topics/issues should be addressed:
-
○
Operator
-
–
The greenhouse use has been implemented; however, additional scenarios of plant protection uses are still not covered (e.g. seed treatment including handling of treated seeds, post‐harvest treatments, single plant treatment, paintbrush application, home and allotment garden uses and other minor scenarios).
-
–
-
○
Workers
-
–
Additional data for worker re‐entry activities are necessary to improve the exposure estimates for workers (e.g. for acute exposure, for re‐entry in vineyards, for sowing of treated seed, for dislodgeable boll residue in case of harvesting cotton activities)
-
–
New TC values have been implemented; however, further collection/production of data on specific TC and DFR values is necessary to produce more realistic exposure assessments (including acute exposure assessment where appropriate).
-
–
Good quality data from DFR/DT50 studies should be provided for the evaluation of the factors relevant to conclude on possibility to extrapolate results between crops and formulations
-
–
Additional data are necessary to improve the inhalation exposure estimates for workers re‐entering greenhouses.
-
–
-
○
Residents and bystanders
-
–
Additional data and/or models for bystander/resident exposure (e.g. related to drift from spray application in high crops, to relevant daily air concentrations of substances during/after application, to dust exposure from sowing treated seed) are still necessary to produce more realistic exposure assessments.
-
–
Further qualitative and quantitative information on the different pathways of resident and bystander exposure is necessary in order to produce more realistic exposure assessments.
-
–
Further data and/or information on human parameters (e.g. inhalation rates and activities intensity) for the different age categories in the exposed groups.
-
–
-
○
Risk mitigation measures
-
–
Further experimental data are necessary to support the reduction of exposure by wearing PPE in realistic conditions of use.
-
–
Further experimental data are necessary to support the reduction of exposure by applying specific technical equipment or packaging (e.g. water‐soluble packages, closed transfer systems, closed cabins, drift reducing technology) in realistic conditions of use.
-
–
-
○
General
-
–
Additional data/information are necessary for the consideration of oral exposure secondary to dermal exposure (through hand‐to‐mouth transfer) for operators, workers and adult bystanders/residents.
-
–
The combined exposure to several active substances in one product is implemented in the online calculator; however, additional data/information are necessary to address the more complex issue of conducting an aggregate assessment covering multiples sources of exposure for a single product, or to address combined exposure to multiples products.
-
–
Additional considerations should be given to the statistical analysis of small data sets (with sample size as low as 10) in higher tier field studies for the purpose of acute risk assessment.
-
–
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- AAOEL
Acute Acceptable Exposure Level
- AAOEL
Acute Acceptable Operator Exposure Level
- ADE
Actual Dermal Exposure
- ADI
Acceptable Daily Intake
- AEPLA
Asociación Empresarial Para La Protección De Las Plantas (Spanish Crop Protection Association)
- ANSES
Agence Nationale de SEcurité Sanitaire de l'alimentation, de l’environnement et du travail (French Agency for Food, Environmental and Occupational Health & Safety)
- AOEM
Agricultural operator exposure model
- AR
Application Rate
- ARTF
Agricultural Re‐entry Task Force
- AUC
Area Under the Curve
- BBCH
Biologische Bundesanstalt, Bundessortenamt and CHemical industry
- BfR
Bundesinstitut für Risikobewertung (German Federal Institute for Risk Assessment)
- BPI
Benaki Phytopathological Institute
- BREAM
Bystander and Resident Exposure Assessment Model
- BROV
Bystander Resident Orchard Vineyard
- BROWSE
Bystanders, Residents, Operators and WorkerS Exposure models for plant protection products
- BTR
Boll Transferrable Residue
- bw
body weight
- CAKE
Computer Assisted Kinetic Evaluation
- CDI
Data‐Call‐In
- CLE
Crop Life Europe
- CTS
Closed Transfer Systems
- DAA
Days After Application
- DAT
Days After Treatment
- DBA
Days Before Application
- DFR
Dislodgeable Foliar Residue
- DFR0
Initial DFR
- DRT
Drift Reduction Technologies or Techniques
- DT50
Dissipation Rates
- EC
Emulsifiable Concentrate
- ECPA
European Crop Protection Association
- FOCUS
FOrum for Co‐ordination of pesticide fate models and their USe
- FS
Facial Swabs
- GAP
Good Agricultural Practices
- GLP
Good Laboratory Practice
- HAT
Hours After Treatment
- HCHH
High Crop HandHeld
- HCVM
High Crop Vehicle‐Mounted
- HPLC‐MS
High Performance Liquid Chromatography‐Mass Spectrometry
- HW
Handwash
- IA
Inhalation Absorption
- ID
Inner Dosimeters
- IR
Inhalation Rate
- ISO
International Organization for Standardization
- LAI
Leaf Area Index
- LCHH
Low Crop Handheld
- LCVM
Low Crop Vehicle‐Mounted
- LOD
Limit Of Detection
- LOQ
Limit Of Quantification
- LVM
Low Volume Misting
- MAF
Multiple Application Factor
- ML
Mixing and Loading
- MS
Member State
- OD
Outer Dosimeters
- PDE
Potential Dermal Exposure
- PEC soil
Predicted Environmental Concentrations in soil
- PHED
Pesticide Handler Exposure Database
- PIE
Potential Inhalation Exposure
- PPE
Personal Protective Equipment
- PPP
Plant Protection Product
- RL50
Residual Lifetime
- RPE
Respiratory Protective Equipment
- SEBD
Systemic Exposure Of Bystander Via The Dermal Route
- SERI
Systemic Exposure Of Residents Via The Inhalation Route
- SFO
Single First Order
- SOEH
Systemic Oral Exposure Via The Hand To Mouth Route
- SOEO
Systemic Oral Exposure Via The Object To Mouth Route
- SVC
Saturated Vapour Concentration
- T
Task Duration
- TDE
Total Dermal Exposure
- TC
Transfer Coefficients
- TNO
Toegepast Natuurwetenschappelijk Onderzoek (Netherlands Organisation for Applied Scientific Research)
- TPS
Theoretical Profile Shape Method
- TSF
Task Specific Factors
- TTR
Turf Transferable Residue
- TWD
Time Within a Day
- UIPP
Union Des Industries De La Protection Des Plantes (French Crop Protection Industry Association)
- USDA‐ARS
United States Department of Agriculture ‐ Agricultural Research Service
- US EPA
United States Environmental Protection Agency
- VC
Vapour Concentration
- VMD
Volume Median Diameters
- WP
Wettable Powder
Glossary
- Acceptable Daily Intake (ADI)
The ADI of a chemical is the estimate of the amount of a substance in food or drinking water, expressed on a body weight basis, then can be ingested daily over a lifetime without appreciable health risks to the consumer on the basis of all known facts at the time of the evaluation (WHO, 1987).
- Acceptable Operator Exposure Level (AOEL)
The reference value against which non‐dietary exposures to pesticides are currently assessed, expressed in milligrams of the chemical per kilogram body weight of the operator (covering also worker, resident and bystander). It is intended to define a level of daily systemic exposure throughout a spraying season, below which no adverse systemic health effects would be expected. The AOEL is normally derived by applying an uncertainty factor (most often 100) to a no observed adverse effect level (NOAEL) (corrected if appropriate for incomplete absorption) from a toxicological study in which animals were dosed daily for 90 days or longer. Less often, the critical NOAEL comes from a study with a shorter or longer dosing period (e.g. a developmental study) in the most sensitive relevant animal species.
- Actual dermal exposure
Exposure to the skin that would occur in the presence of clothing and/or personal protective equipment.
- Acute Acceptable Operator Exposure Level (AAOEL)
A term used to describe a reference value against which acute non‐dietary exposures (i.e. those that might be incurred in a single day) could be assessed. This would be relevant only to those plant protection products for which such exposures might produce significant toxicity.
- Ad hoc exposure assessment
An assessment of exposures incorporating data specific to one or more uses of a particular plant protection product, which is considered to provide a more reliable estimate of potential exposure than the normal first‐tier approach using more generic data.
- Aggregate risk assessment
Risk assessment that takes into account all pathways and routes of exposure to a single chemical.
- Bystanders
Persons who could be located directly adjacent to the area where PPP application or treatment is in process or has recently been completed; whose presence is quite incidental and unrelated to work involving PPPs, but whose position might lead them to be exposed; and who take no action to avoid or control exposure.
- Cumulative risk assessment
Risk assessment for combined exposure to two or more chemicals by all relevant pathways and routes.
- Dense crop
Crops (high or low) for which the spray operator technically cannot avoid contact with treated foliage during spray operations.
- Dislodgeable foliar residue (DFR)
The residue of a pesticide following deposition on foliage, which can be transferred to a person through contact with the foliage or fruit.
- Drift (expressed as percentage of areic mass)
The deposition of a substance per unit receiving (non‐target) surface, expressed as a percentage of the amount applied per unit area target surface. For example, at 1% drift, the deposition per square metre is 1 mg when the dosage is 1 kg per ha (100 mg per square metre).
- Drift reduction Technology
Refers to spray application technologies that have scientifically demonstrated to reduce drift compared to standard applications, and which have been officially recognised as meeting specific standards of drift reduction.
- Engineering controls
Methods of reducing exposure to pesticides (or other hazardous agents) through appropriately designed equipment (e.g. a closed tractor cab with air filtration).
- Filtration unit (on a tractor cab)
A device that removes pesticide residue from the air that enters a closed tractor cab.
- Formulation
The composition of a pesticide product as supplied.
- Good Agricultural Practices
‘Practices that address environmental, economic and social sustainability for on‐farm processes, and result in safe and quality food and non‐food agricultural products’; see https://www.fao.org/docrep/meeting/006/y8704e.htm
- Hand‐to‐mouth transfer
Transfer of pesticide residue from contaminated surfaces to the mouth via the hand
- In‐use preparation
The form in which a pesticide is applied after any dissolution, dilution or mixing of the product as supplied.
- IOM Sampler
A sampling head that houses a reusable two‐part filter cassette with specified filter for the collection of inhalable airborne particles, developed by the Institute of Occupational Medicine
- Least squares regression
Ordinary least squares regression is the common method for fitting linear regression models to data. Once fitted, the expected value (mean) can be predicted, as can any required percentile (by adding the respective variation to the predicted value). However, the method assumes normality of the distribution at each exposure level and uniform variation over the whole range. Least squares regression is also sensitive to outliers and in particular to the assumed values of measurements below the limit of quantification. These assumptions may be violated by peculiarities of a given data set, especially by the presence of non‐detected values (see quantile regression).
- Log‐normality
The nature of a statistical distribution in which the logarithms of individual measurements have a Gaussian or ‘normal’ distribution. For a given scenario, measurements of individual exposures often have a log‐normal distribution.
- Non‐professional operators
People who use PPPs for their own benefit not part of a commercial activity; e.g. home gardeners.
- Normalisation (of exposure)
Adjustment of exposure estimates to take account of the amount of a product handled or applied.
- Object‐to‐mouth transfer
Transfer of pesticide residue to the mouth from contaminated objects through placement of the object in the mouth—a pathway of exposure of greatest importance in infants and toddlers.
- Operators
Persons who are involved in activities relating to the application of a plant protection product; such activities include mixing/loading the product into the application machinery, operation of the application machinery, repair of the application machinery whilst it contains the plant protection product, and emptying/cleaning the machinery/containers after use. Operators may be either professionals (e.g. farmers or contract applicators engaged in commercial crop production) or amateur users (e.g. home garden users).
- Parametric
Relating to a summary characteristic of the (theoretically infinite) population from which a sample is derived. Population parameters can be estimated from corresponding sample statistics. For example, a sample mean may provide an estimate of the mean of the population from which the sample was derived.
- Percentile
Value in a distribution below which a specified percentage of values falls. For a continuous distribution without gaps, the value is unique for each percentage. For samples of data, the value is generally not unique and there are competing ways to estimate percentiles. The PERCENTILE function in Excel uses the method in Definition 7 of Hyndman and Fan (1996) which assumes, for n data sorted in increasing order, that 1/(n – 1) probability lies between any two successive data values and is uniformly distributed between them. This is also the default method used by the quantile function in R.
- Personal protective equipment (PPE)
Certified equipment worn by an operator or worker that is designed to reduce hazardous exposures (e.g. gloves, coveralls, face masks).
- Potential dermal exposure
Exposure to the skin that would occur in the absence of clothing or personal protective equipment.
- Product
A pesticide preparation as supplied. It includes not only the active substance(s) but also co‐formulants such as emulsifiers, solvents and safeners.
- Protected hand exposure
All residues that were found on the hands of operators protected in any case of exposure; this is considered identical to hand exposure using personal protective equipment.
- Quantile regression
A non‐parametric method which gives an independent estimate for every percentile providing a view of possible relationships between variables (Koenker, 2005). As long as the percentile is well within the range of measured data, the resulting fit can be expected to be more robust than the least squares fit. In particular, it will not depend on the actual choice of the value substituted for non‐detects and does not assume the variability to be independent of the quantity of predictor variable(s) (see least squares regression).
- Residents
Persons who live, work or attend school or any other institution adjacent to an area that is or has been treated with a plant protection product; persons whose presence is quite incidental and unrelated to work involving plant protection products but whose position might lead them to be exposed; persons who take no action to avoid or control exposure; or persons who might be in the location for 24 h per day.
- Safe re‐entry interval
The specific time point post application, from which the worker exposure levels calculated for the relevant re‐entry tasks are lower than the AOEL considering the different PPE cases depending on the TC availability.
- Saliva extraction percentage
The fraction (expressed as a percentage) of pesticide extracted from a contaminated hand or object via saliva.
- Systemic exposure
Exposure of organs and tissues that occurs following absorption and distribution of a chemical in the body.
- Task‐specific factor (for worker re‐entry)
A factor (with units ha/h × 10–3) relating to a specified task carried out by a re‐entry worker (e.g. cutting ornamentals). It is multiplied by the rate at which a pesticide was applied to derive an estimate of potential exposures through inhalation.
- Total (potential) body exposure
All residues that were found on an inner layer of clothing (‘inner’ body exposure) and on an outer layer of clothing (‘outer’ body exposure), excluding head and hands; this is considered identical to potential body exposure.
- Total (potential) hand exposure
All residues that were found on the hands and gloves of the operator; this is considered identical to potential hand exposure and exposure without using any personal protective equipment.
- Transfer coefficient
The rate at which dislodgeable foliar residue can be transferred to a worker during a specified activity (expressed in terms of the area of contaminated foliage or fruit from which residues are transferred per hour).
- Turf transferable residue
Equivalent to a dislodgeable foliar residue for residue of plant protection products deposited on lawns.
- Workwear (non‐certified)
Normal workwear consists of coveralls or long‐sleeved shirt and trousers that are made of cotton (≥ 300 g/m2) or of cotton and polyester with at least 65% polyester (≥ 245 g/m2). It is noted that C1 level based on performance criteria in ISO 27065:2017 can be considered equivalent.
- Workers
In the context of this opinion, the term worker refers to persons who, as part of their employment, enter an area that has been treated previously with a plant protection product, or who handle a crop that has been treated with a plant protection product.
Appendix A – Greenhouse Agricultural Operator Exposure Model
A.1 Introduction
In the absence of a harmonised EU model for operator exposure during greenhouse applications, a new greenhouse model for operator exposure to pesticides has been developed by BfR (BfR, 2015) on the basis of seven field studies sponsored by CLE. Despite the relatively large number of data, the model had some limitations, and it was recommended that further studies should be performed and/or provided to improve the model.
In 2018, new greenhouse exposure data from studies conducted in 2012 and 2016 in different EU member states were made available to the BfR. The data were considered to be suitable for further analysis and used for an update of the greenhouse model (BfR, 2020). This appendix includes an overview of the available data and of the methodology applied by BfR for data processing and modelling, as well as considerations by the WG. This is followed by the assessment of the WG of the developed models and applied statistical analysis, with description of related uncertainties.
A.2 Overview of the Greenhouse AOEM (BfR, 2020 )
A.2.1 Data
A.2.1.1 Exposure studies
The first greenhouse model project (BfR, 2015) included a database containing seven exposure studies with in total 70 replicates for individuals performing mixing/loading and 102 replicates for application. In all studies, the operators used either lance sprayers or spray guns that were connected to a large static tank located at the edge of the greenhouse. Information on body exposure during mixing/loading of the tank were not available in the studies. In addition, no data for liquid formulations were in the database.
Three new studies that were not available at the time of this first project but also fulfilled the data quality criteria defined for the outdoor model (BfR, 2013) were afterwards included in the database to improve the model.
Two of these new studies, sponsored by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) and the Spanish Crop Protection Association (AEPLA), contain data for additional spray equipment typical for application in greenhouses, i.e. knapsack sprayers and trolley sprayers. In the French study, the trolley sprayers (connected via a hose to a static tank) were pushed during spraying while in the Spanish study, the trolley sprayers were pulled during spraying leaving a spray cloud behind the trolley, and avoiding contact of the operator with treated foliage (INSST, 2020). The third study, conducted in Greece by Benaki Phytopathological Institute (BPI) in collaboration with TNO and FERA, in the frame of the FP7 BROWSE project (Tsakirakis, 2014), contains data for spray guns connected via a hose to a static tank, but in contrast to the first set of greenhouse studies, a liquid formulation was used and body exposure was monitored also during mixing and loading of the tank.
The majority of the protected structures where the new studies took place were similar to the greenhouses in the studies already included in the database. They were made of large wooden or steel constructions covered with plastic and fulfilling the criteria of greenhouses as defined in the EFSA Guidance on protected crops (EFSA, 2014b). However, for seven of 10 operators in the French study, exposure was monitored in plastic tunnels (or walk‐in tunnels according to the definition of the EFSA Guidance on protected crops) of approximately 4–5 metre width.
The greenhouses or walk‐in tunnels were either fully closed or partly open, i.e. gaps between plastic sheets, covers or panels on the side or on the roof partially raised or tunnel ends fully opened. The greenhouses were located in Spain, in the south of France, south of Greece and Italy.
The study characteristics of the greenhouse database are visualised in Figure A.1. Vegetables, strawberries and ornamentals were tested. Strawberries were tested either as low crops or as high crops (grown on so‐called racks). When operators cannot avoid contact with the treated crop, the model conditions are considered as ‘dense’.
Figure A.1.
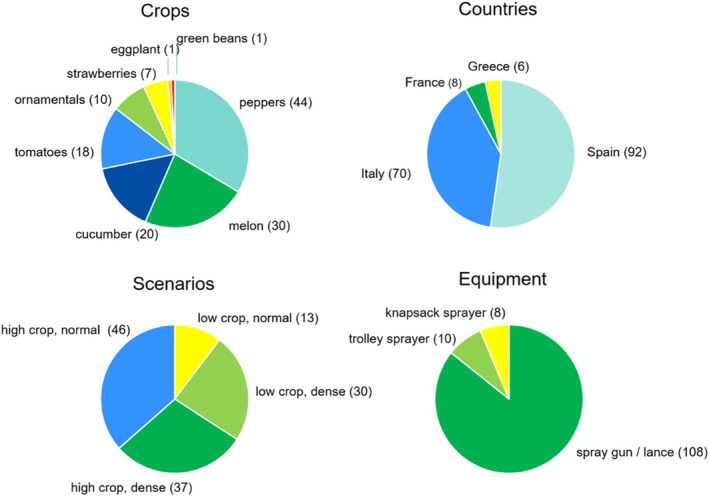
Overview of the study characteristics and different scenarios in the greenhouse database (BfR, 2020)
The exposure data cover a broad range of total amount applied per day, from 0.003 up to 1.5 kg of active substance (see Figure A.2). The spraying duration in the studies ranged from 8 to 206 min in which an area of 0.04–1.10 ha was treated. According to the study reports, the monitoring duration reflected a typical application in greenhouses. In the model, exposure data were related to the amount of active substance applied which correlates with the area treated per day. Thereby, a normalisation for the area is included.
Figure A.2.
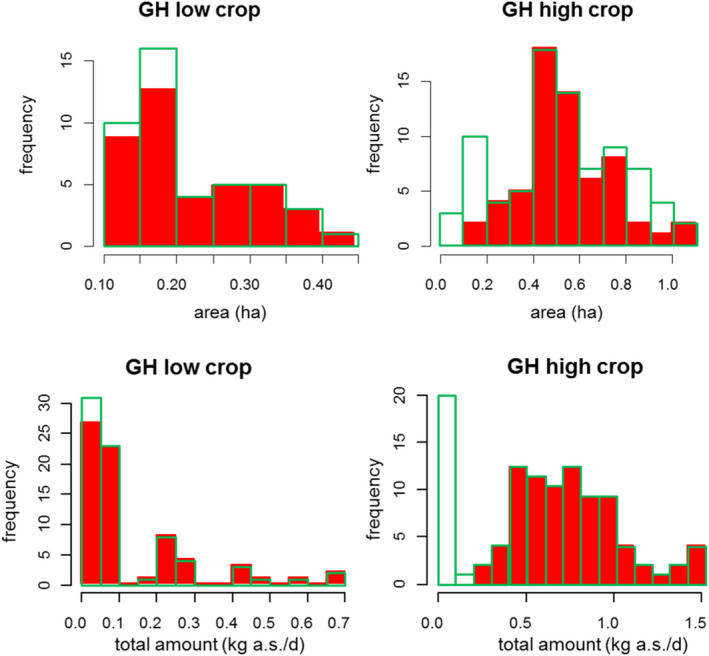
Distribution of the area treated and the total amount of active substance applied on one day in the old greenhouse studies (red columns) and in both old and new greenhouse studies (green columns) (BfR, 2020)
A.2.1.2 Exposure sampling
Exposure in the greenhouse studies was monitored with whole body dosimetry for dermal exposure and personal air sampling for inhalation exposure.
Two layers of clothing were used for body exposure (long underwear made from 100% cotton or ca. 50% polyester/50% cotton and long workwear made from 100% cotton or ca. 65% polyester/35% cotton). Raincoats, rain pants or a protective overall (Cat. 3 Type 6) (AENOR, 2009) were worn in two studies. Protective clothing (cat. 3 type 4) was worn in the French study. When hand exposure was measured, users were allowed to wash their hands as needed (hands were always washed at the end of the studies). In the new Spanish study, hands were washed more frequently (every 20–25 min). In the new Greek study, cotton gloves were also used as inner dosimeter. Different dosimeters were used to determine head exposure (e.g. caps or face/neck wipes). When inhalation was determined, air samplers were used (pumps with a flow rate of about 2 L/min and IOM sampling units with a glass fibre filter). Cleaning of equipment was monitored as part of the application but performed only in eight of 128 replicates.
A.2.1.3 Data selection and processing
BfR excluded the data for two operators of the French study using trolley sprayers, since the application scenario differed from that of the Spanish data where the trolley sprayers were pulled instead of pushed. The data for these two operators pushing trolleys were not sufficient for a separate scenario to be modelled. In another case, where the operator treated low crops and high crops in the same trial, it was decided to categorise the data set as high crop since twice as much rows with high crops than with low crops were sprayed.
Before modelling the data needed to be processed to adjust for differences in the analytical quality and methodology. For the greenhouse data, following the OECD Guidance (OECD, 1997), a threshold of 95% was used for the correction for recovery. Additionally, values below the LOQ were replaced by 1/2 limit of quantification (LOQ) in statistical modelling. For non‐detected values (indicated as ‘zero’), 0.01 µg/sample was used for the statistical analysis. Since quantile regression was used for modelling, this approach is not expected to have a significant effect on the result. A breathing rate of 1.25 m3/h was used to calculate inhalation exposure. An additional correction factor of 2 was used for whole head exposure when only part of the head exposure was determined. Those values that were derived from operators using face shields were considered separately as they do not reflect the whole exposure to the face.
The WG considered as appropriate the selection and processing of data as proposed by BfR.
A.2.2 Methodologies
A.2.2.1 Protocol for quality and plausibility check of the database
Data from the 10 available field studies of acceptable quality were extracted and collected into an MS Excel file for the development of the greenhouse model (BfR, 2020). A quality check was performed by the WG to validate the correctness of data entry in this file (plausibility check) and to verify the data processing according to criteria defined in the BfR report and agreed by the WG.
In details, the agreed protocol consists of two steps:
Quality check: It was performed for all data reported for two randomly selected operators per study
-
Plausibility check: It was performed on the following aspects of data processing:
-
○
correction for field recovery with threshold 95% instead of 70%
-
○
values < LOQ were considered as 1/2 LOQ for further evaluation
-
○
values reported as ‘zero’ (not detected) were considered as 0.01 µg/sample to facilitate data processing and statistical analysis
-
○
breathing rate of 1.25 m3/h to adjust inhalation exposure
-
○
head exposure determined by using a correction factor of 2 for face/neck wipe data and for hood/cap data (no correction when head exposure sampled with both face/neck wipes and hats)
-
○
A.2.2.2 Exposure scenarios
For spray application in greenhouses, the following two scenarios were defined3:
Low crop handheld application (LCHH)
High crop handheld application (HCHH)
For both scenarios, an impact of the application equipment, protective equipment, etc. was examined and, if statistically confirmed, addressed by an additional factor included in the individual formulas for indoor low crops and indoor high crops (e.g. for face shields included in one formula, see Table A.1 below).
Table A.1.
Exposure models predicting the 75th percentile; in case no model could be derived the 75th percentile was calculated (normal scenario/dense scenario/dense scenario with rain trousers); exposure is given in µg/person (BfR, 2020)
| Tank ML | log exp = α log TA + [formulation type] + constant | |
| Total hands | log DML(H) = 0.64 log TA + 0.64 [liquid] + 1.28 [WP] + 1.17 [WPS] – 0.47 [glove wash] + 3.27 | |
| Prot. hands | log DML(Hp) = 0.46 log TA + 0.32 [liquid] + 1.66 [WP] + 0.20 [WPS] + 1.46 | |
| Total body | log DML(B) = 0.74 log TA + 0.52 [liquid] + 1.85 [WP] + 3.04 | |
| Inner body | log DML(Bp) = 0.62 log TA + 0.12 [liquid] + 1.84 [WP] + 1.58 | |
| Head | log DML(C) = log TA + 0.34 [liquid] + 0.70 [WP] – 1.67 [face shield] + 1.46 | |
| Inhalation | log IML = 0.38 log TA – 0.87 [liquid] + 1.96 [WP] – 0.03 [WPS] + 1.38 | |
| Knapsack ML | 75th percentile (above 1.5 kg linear extrapolation) | |
| Total hands | 9497 | |
| Prot. hands | 21 | |
| Total body | 803 | |
| Inner body | 25 | |
| Head | 5.5 | |
| Inhalation | 35 | |
| GH HCHH | log exp = α log TA + [dense] + [trolley] + constant | |
| Total hands | log DA(H) = 0.83 log TA + 0.17 [dense] – 0.62 [trolley] + 4.40 | |
| Prot. hands | log DA(Hp) = log TA + 1.32 [dense] – 1.04 [trolley] + 1.71 | |
| Total body | log DA(B) = log TA + 0.67 [dense] − 0.81 [trolley] + 5.59 | |
| Inner body (1) | log DA(Bp) = log TA + 1.64 [dense] − 2.42 [dense with rain suit] – 0.54 [dense with protective coverall] – 1.23 [trolley] + 4.19 | |
| Head | log DA(C) = 0.18 log TA + 0.29 [dense] – 0.41 [trolley] + 2.70 | |
| Inhalation | log IA = log TA + 0.08 [dense] – 0.19 [trolley] + 2.69 | |
| GH LCHH | 75th percentile (above 0.60 kg a.s./0.075 kg a.s./0.086 kg a.s. linear extrapolation) | |
| Total hands | 1323 | |
| Prot. hands | 1.5 | |
| Total body | 16,797 (normal)/55,521 (dense) | |
| Inner body | 132 (normal)/12,180 (dense)/80 (dense with rain trousers) | |
| Head | 21 | |
| Inhalation | 47 |
GH: greenhouse; ML: mixing and loading.
Rain suit and protective coverall are only applicable to exposure in dense foliage. In that case, either ‘dense with rain suit’ or ‘dense with protective coverall’ may be selected.
For mixing/loading (ML), the two different scenarios from the outdoor AOEM (BfR, 2013) are considered appropriate for greenhouses as well:
Tank mixing/loading (indoor + outdoor)
Knapsack mixing/loading (indoor + outdoor)
There were only few data available for mixing/loading tanks indoors and no significant differences were obtained between mixing/loading data for indoor and outdoor applications. Therefore, the data for ML were combined, since relevant differences are not expected for outdoor or indoor applications. Furthermore, for the ML model, a threshold value of < 95% was used to correct for recovery.
A.2.2.3 Exposure model
In analogy to the outdoor AOEM, the following exposure variables contributing to the overall exposure for the respective exposure scenario were defined:
inhalation exposure
head exposure
inner body exposure
total body exposure
protected hand exposure
total hand exposure
The same approach for modelling was used as for the outdoor model, i.e. assuming a log linear model:
where
– X = exposure variable
– α = coefficient estimated from the available data set
– A = total amount of active substance applied per day
–F = numerical adjustment to log‐exposure for the presence of specific factor, e.g. specific product formulation type or nature of contact with foliage (dense or normal scenario).
Contact with treated foliage (dense or normal scenario) was used as factor F as significant differences between the two scenarios were determined. Statistical evaluation of further factors was not possible due to the limited data. However, the application with trolleys was considered as a separate scenario, since this application technique can reduce the exposure of operators. Raincoat (high crops) or rain trousers (low crops) or a certified protective suit can be taken into account for the dense scenario.
For the model for mixing/loading, the data for greenhouse and outdoor applications were combined.
A.2.2.4 Statistical method
The modelling approach was the same as in the original model (Greenhouse AOEM, BfR, 2015). Quantile regression was used to determine the 75th percentile (for longer term exposure) and the 95th percentile (for acute exposure). It is expected that this non‐parametric method provides better fit than least squares regression as long as the percentile is within the range of the measured data. The method is particularly robust against the non‐detects and does not use the same standard deviation over the entire range. When a statistical model could not be derived due to the lack of dependence on the exposure factors, the empirical percentiles with the quantile regression were determined.
A.2.2.5 Results
The model equations are presented in Tables A.1, A.2 and A.1, A.2. Where a coefficient is followed by a factor name between square brackets [], e.g. [liquid], this indicates that the coefficient is to be added to the log exposure when the factor is present. The factors that appear in this way are: [liquid] (liquid formulation for product), [WP] (wettable powder formulation for product), [WPS] (wettable powder formulation for product in small non‐soluble packages), [glove wash] (gloves washed after mixing and loading operation), [face shield] (face shield worn during mixing and loading), [dense] (application in dense crop), [trolley] (application using trolley), [dense with rain suit] (application in dense crop wearing a rain suit), [dense with protective coverall] (application in dense crop wearing a protective coverall), [normal] (application in normal crop), [dense with rain trousers] (application in dense crop wearing rain trousers).
Table A.2.
Exposure models predicting the 95th percentile; in case no model could be derived the 95th percentile was calculated (normal scenario/dense scenario/with rain trousers); exposure is given in µg/person (BfR, 2020)
| Tank ML | log exp = α log TA + [formulation type] + constant | |
| Total hands | log DML(H) = 0.69 log TA + 0.71 [liquid] + 1.21 [WP] + 1.30 [WPS] – 0.72 [glove wash] + 3.74 | |
| Prot. Hands | log DML(Hp) = 0.53 log TA + 0.83 [liquid] + 1.39 [WP] + 0.38 [WPS] + 2.29 | |
| Total body | log DML(B) = 0.69 log TA + 0.72 [liquid] + 1.29 [WP] + 3.87 | |
| Inner body | log DML(Bp) = 0.78 log TA + 0.44 [liquid] + 1.58 [WP] + 2.09 | |
| Head | log DML(C) = log TA + 0.39 [liquid] + 0.11 [WP] – 1.16 [face shield] + 2.19 | |
| Inhalation | log IML = 0.49 log TA – 0.92 [liquid] + 1.54 [WP] + 0.19 [WPS] + 1.81 | |
| Knapsack ML | 95th percentile (above 1.5 kg linear extrapolation) | |
| Total hands | 25,490 | |
| Prot. Hands | 164 | |
| Total body | 2,787 | |
| Inner body | 103 | |
| Head | 11 | |
| Inhalation | 36 | |
| GH HCHH | log exp = α log TA + [dense] + [trolley] + constant | |
| Total hands | log DA(H) = 0.84 log TA + 0.14 [dense] – 0.82 [trolley] + 4.81 | |
| Prot. Hands | log DA(Hp) = 0.67 log TA + 0.76 [dense] – 1.19 [trolley] + 2.36 | |
| Total body | log DA(B) = log TA + 0.48 [dense] – 0.92 [trolley] + 6.10 | |
| Inner body (1) | log DA(Bp) = log TA + 1.07 [dense] – 2.20 [dense with rain suit] – 0.64 [dense with protective coverall] – 1.71 [trolley] + 5.07 | |
| Head | log DA(C) = 0.33 log TA + 0.79 [dense] + 0.25 [trolley] + 3.10 | |
| Inhalation | log IA = log TA + 0.63 [dense] – 0.26 [trolley] + 2.82 | |
| GH LCHH | 95th percentile (above 0.60 kg a.s./0.075 kg a.s./0.086 kg a.s. linear extrapolation) | |
| Total hands | 4,159 | |
| Prot. Hands | 12 | |
| Total body | 28,082 (normal)/85,382 (dense) | |
| Inner body | 640 (normal)/27,958 (dense)/154 (dense with rain trousers) | |
| Head | 39 | |
| Inhalation | 80 |
GH: greenhouse; ML: mixing and loading.
Rain suit and protective coverall are only applicable to exposure in dense foliage. In that case, either ‘dense with rain suit’ or ‘dense with protective coverall’ may be selected.
Tank mixing/loading: Most revised models do not differ substantially for the combined model compared to the original outdoor AOEM model. Values for recoveries < 95% were corrected. However, in particular in the case of inner hand and inner body exposure, significant differences were found, particularly at low application rates, since more measured data for low application rates are now available from the greenhouse studies. For checking the validity of the models, the 75th percentile and the 95th percentile were compared. Two deviations from the expected relation occurred: actual body exposure with powder formulations and inhalation exposure with powder formulations. Due to the limited data availability, the prediction in the first case for low application rates for the 95th percentile was below the prediction of the 75th percentile. In the second case, the prediction of the 95th percentile was slightly below the prediction of the 75th percentile even at higher amounts. In order to avoid a lower prediction for the 95th percentile compared to the 75th percentile, the higher of the two values will be automatically selected.
Knapsack mixing/loading: The new percentiles were calculated based on additional data. Data with < 95% recovery were corrected accordingly. Most percentiles have been only slightly changed except for inhalation, which, however, does not contribute much to the overall exposure.
HCHH greenhouse: This model also included the application with trolleys. Due to the limited data situation, significant differences of exposure due to the application with lance or knapsack sprayer could not be determined. Data with < 95% recovery were corrected accordingly.
Rain suits or certified protective coveralls can be used to reduce exposure, whereby waterproof rain suits reduce exposure by a greater amount. For the normal scenario, there is no data for rain suits or certified protective suits. However, for the normal scenario data are also available for applications with trolley. This equipment reduces exposure further provided that the row width is sufficiently large. Reduced exposure is expected since operators pull the trolley in the opposite direction to the spray cloud.
LCHH greenhouse: The data situation for hand, head and inhalation exposure does not allow differentiation of normal and dense scenarios. Here, the data were combined into one scenario. For the dense scenario, exposure can be reduced by wearing rain trousers. For low crops, the lance and knapsack scenarios are available. The trolley scenario is only available for high crops (Tables A.1 and A.2).
A.2.2.6 Uncertainties
Models are usually subject to limitations in their range of applicability and uncertainties due to data gaps and knowledge of relevant parameters. Therefore, in the BfR project report (BfR, 2020), the underlying uncertainties were discussed based on the principles described in Section 7 of the EFSA Guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018).
The aim of the model is to provide a realistic worst‐case exposure estimate for handheld application technique in greenhouses. Dermal and inhalation exposure during mixing/loading and during application was considered. Other scenarios (e.g. automated application techniques) have not been taken into account.
Table A.3 summarises relevant sources of uncertainty, characterises their overall impact on exposure assessment and provides recommendations for impact reduction where applicable (BfR, 2020).
Table A.3.
Sources and impact of potentially protective and underprotective predictions on exposure assessment (BfR, 2020)
| Source of uncertainty | Potential to be protective | Potential to be underprotective | Impact on exposure assessment |
|---|---|---|---|
| Data set | |||
| Cultivation systems (high and low crops) are not well characterised | Crops between 0.6 and 1.1 m height are considered as high crops | Crops between 0.6 and 1.1 m height are considered as low crops |
High Crops above 0.6 m height should be considered as high crops, leading to sufficiently conservative exposure estimation (height of 0.6 m is based on values presented in exposure studies for greenhouses) |
| Normal and dense scenarios are not well characterised | Dense scenario is calculated as worst case | Normal scenario is falsely applied to dense scenarios |
High Unless dense scenario can be excluded (e.g. trolley sprayer application), it should be used as a worst case |
| Application techniques in this model are limited to spray lance/gun as well as knapsack and trolley sprayers | Handheld data provide a worst‐case exposure estimation for operators in greenhouses | Other greenhouse application techniques result in higher operator exposure |
High Other application techniques than those included in the evaluated studies could have a relevant impact on exposure assessment. However, such techniques would either be considered outside the applicability domain of the model or covered by comparably conservative handheld application techniques. |
| Variability of products and active substances applied | The tested formulations (application) adequately predict exposure for all formulation types and active substances | The tested formulations (application) are insufficient to adequately predict exposure for all formulation types and active substances |
Moderate Variability between formulation types and different active substances is unknown due to the limited data set. However, the impact of formulation type during application is low. Moreover, volatile active substances should be considered outside the applicability domain of the model. |
| Studies conducted in Southern Europe (F, GR, ES, IT) | Application practices in Europe are similar or, alternatively, application in Southern Europe is worst case | Application practices in Central and Northern Europe differ/lead to higher operator exposure |
Low Differences in area treated, application duration, rate and practices as well as climatic conditions are unknown/uncharacterised. Since they may be considered worst case, e.g. application area of 1 ha per operator and day, uncertainty is deemed low. |
| Variability of greenhouses | Wood and steel constructions covered with plastic foil or glass provide a conservative scenario for other types of greenhouses | Application in other types of greenhouses leads to higher exposure |
Low Uncertainty by the type of greenhouse is considered low in comparison to other relevant factors, such as application technique or cultivation system |
| Variability of crop types | The studied crop types adequately predict exposure for all relevant crops in greenhouses | The studied crop types are insufficient to adequately predict exposure for all relevant crops in greenhouses |
Low Crop type has a lower impact on exposure assessment than the cultivation system used for the crop, i.e. high or low crop |
| Model | |||
| Model robustness | The available data are sufficient to produce a robust model | The data set does not provide a sufficiently robust model |
Moderate Model robustness has been supported by cross validation. Especially for application in low crops, it is affected by the limited data set with regard to application rates and resulting extrapolation beyond the rates used in the studies. |
| Combination of indoor and outdoor data for mixing/loading | Exposure during mixing/loading is comparable with regard to indoor and outdoor application scenarios | Mixing/loading for indoor application leads to higher operator exposure |
Low The use of similar equipment for indoor and outdoor application leads to comparable mixing/loading scenarios. This was confirmed by statistical analysis. |
| Extrapolation of head exposure data (in case only face/neck wipe or hood/cap data) | Correction factor of 2 sufficient to account for missing exposure data | Correction factor of 2 not sufficient to account for missing exposure data |
Low Head exposure is generally low and the overall impact on total exposure is marginal |
| Operator variability | Body weight normalisation to 60 kg is conservative for lower body weights | Dermal exposure of operators with high body surface areas, e.g. tall persons, is underestimated |
Low In general, operators weighed more than 60 kg. The normalisation to lower body weights while using dermal exposure data as measured is reasonably conservative. |
| Correction of data with insufficient recovery | The correction of low recovery data (< 95%) is sufficiently conservative | N/A |
Low Data correction is sufficiently conservative |
| Choice of regression model | Quantile regression is adequate to describe exposure | Quantile regression underestimates exposure |
Low Quantile regression is robust since it is non‐parametric and thus independent of non‐detects and heterogeneous standard deviation. The quantiles used are the current general agreement for longer term (75th percentile) and acute (95th percentile) exposure. |
| Combination of 75th percentiles (long term) and 95th percentile (acute) for different body parts modelled | The selected percentiles are sufficiently protective to estimate total exposure | The selected percentiles underestimate total exposure in a relevant number of cases |
Low The addition of the selected percentiles is considered conservative and thus sufficiently protective |
N/A: not applicable.
Most of the uncertainties have rather a small impact on the exposure assessment for greenhouse applications. Uncertainties exist in particular with regard to cultivation systems and application technology. Furthermore, data availability varies greatly depending on the scenario. For example, the low‐crop model lacks sufficient data for a wider range of application rates. Therefore, more data on low crop applications could reduce these uncertainties. Furthermore, data for other application techniques could expand the application domain, e.g. application via drip irrigation, automated spraying, tractor‐mounted equipment. However, it can be assumed that the handheld application technique is usually a very conservative scenario.
A.3 Assessment
A.3.1 Quality check
Results from the plausibility check demonstrated the correctness of all the evaluated data entries. Concerning data processing, a mistake was only found for the correction for field recovery and this was considered by the WG as not significantly impacting on the modelling results.
With regard to data processing, the approach proposed by BfR was considered appropriate: taking either 1/2 LOQ or the LOQ would not significantly affect the outcome because quantile regression and empirical estimates of 75th and 95th percentiles were used. For values reported as ‘zero’ (not detected) a small positive value was used instead because statistical modelling was conducted using logarithm of exposures. The value of 0.01 µg/sample was considered to be small enough when using quantile regression for the range of values found in the available data set; again, the outcome should not be sensitive to this value due to the choice of statistical methodology.
A.3.2 Statistical analysis
The technical details of the statistical modelling are clearly and transparently presented in the BfR report (BfR, 2020), and it has been possible to reproduce the results. The use of semi‐parametric quantile regression is consistent with the development of the AOEM model. The argument made for using quantile regression is strong although there are alternatives, such as parametric regression modelling for censored data, which might perform better in some regards but might perform less well in others. Expert judgement, informed by the results of multiple regression modelling, was used to decide which factors appear in each model. Expert judgement is needed because there are no satisfactory automatic statistical methods which can take into account contextual information and background knowledge. However, it should be recognised that choices have been made that might be made differently by others.
The decision to focus on the 75th and 95th percentiles of exposure is consistent with the development of the outdoor AOEM model and with the EFSA Guidance issued in 2014 and the EFSA PPR Panel (2010) Scientific Opinion which stated that ‘for acute risk assessments, exposure estimates should normally be based on 95th centiles of relevant data sets, whereas for longer term risk assessments, the starting point should be a 75th centile’. EFSA PPR Panel (2010) also noted that ‘estimates of exposure from small data sets may be liable to major statistical uncertainty’ and recommended that ‘the exposure value used for risk assessment should be the higher of: (a) the appropriate centile in the relevant data set; and (b) a parametric estimate of the corresponding centile in the theoretical population of measurements from which the dataset was derived’. The procedure specified by EFSA PPR Panel (2010) for the parametric estimate is the upper limit of the relevant prediction interval for an individual exposure, based on using normal distributions to model variability of the logarithm of exposure (for 95th percentile exposure, the relevant prediction interval is the central 90% prediction interval). EFSA (2014) adds a restriction that the exposure estimate should not exceed the maximum of the data. This procedure is principle applicable to the data for knapsack mixing/loading and handheld application in low crops where estimates of percentiles are provided without use of regression modelling. However, it was not possible to apply this procedure systematically to those data because the log‐normal distribution is a poor fit to many of those data sets. There is no obvious alternative to apply to the cases of poor fit, and therefore, this option has not been pursued further.
EFSA PPR Panel (2010) did not consider regression modelling to make exposure estimates dependent on covariates. EFSA (2014) considered the use of regression modelling and recommended the use of quantile regression which would seem to be an appropriate equivalent to the above‐mentioned options (a) and made no statement about a parametric calculation corresponding to (b). A natural approach to (b) would seem to be to use the relevant prediction interval for a conventional multiple linear regression model taking the logarithm of exposure as the response variable. However, the conventional linear regression model would make the inappropriate assumption that the residual variation is homogeneous, and therefore, this approach has not been pursued. An alternative approach to allow for uncertainty in the regression estimates might be to use the upper limit of a confidence interval for the estimate of the relevant percentile of exposure. To implement this would require a decision about the appropriate level of confidence to use and this option has not been pursued.
A difficulty with the use of quantile regression is that it does not require that the model for 95th percentile exposure results in higher exposure estimates than the model for 75th percentile exposure. In most cases, this issue does not arise, but there is one case for which it does and the recommendation in the BfR report is to use the 75th percentile estimate as the 95th percentile estimate when the 75th percentile estimate is higher. This is a pragmatic decision in the circumstances.
Although the total number of replicates is reasonably high, the models rely on data from a moderate number of studies and the statistical modelling does not include components to model study differences. This is effectively a judgement that the studies are sufficiently comparable that differences between measured exposures can be accounted for by the factors included in models and are otherwise random. This is an important judgement given that a number of differences between studies are identified that might have an impact on exposure, e.g. differences in protective clothing. However, inclusion of study effects in the models would increase the difficulty of interpreting of the models for use in risk assessment and there is substantial confounding between study and explanatory factors which would not be easy to address. Cross‐validation was used to validate the modelling and the results for omitting 10% of data from each model and assessing resulting predictions for those data are satisfactory. However, the division of the data into 10 subsets for this purpose is random and a cross‐validation based on omitting entire studies might be expected to show larger differences.
Concerning the use of trolley sprayers, as the trolley equipment does not allow the spraying of the outside rows or other areas where the trolley cannot be driven into (considered as representing 10% of the treated area), these areas have to be sprayed by other means, e.g. lance sprayers. As a consequence, it is considered appropriate first to calculate separate exposure estimates using the model for handheld application with 10% of the total amount of active substance applied and the model for trolley application with 90% of the total amount and then to add these two estimates to obtain the overall exposure estimate for trolley application. To ensure that all cases are covered, it is considered appropriate to always use the dense scenario for the handheld application part of the calculation. For exposure during mixing and loading (before trolley application), the data for tank mixing and loading are considered more robust and more realistic.
A.3.3 Summary results
Results of the BfR Project (BfR, 2015, 2020) are summarised in Tables A.1 and A.2. The results in the BfR report were presented in a transparent way. All exposure information has been published. The statistical evaluation and the corresponding results are comprehensible. Based on the current data availability, both the revised models for mixing/loading and the new models for the application of plant protection products in greenhouses are appropriate for the risk assessment.
A.3.4 Uncertainties
Table A.3 includes an analysis of sources of uncertainty relating to the exposure estimates, giving a description of each source, an indication of how it might lead to over‐ or underestimating exposure, and a qualitative score (High/Moderate/Low) and text summarising the impact on risk assessment. The qualitative score should be interpreted as a relative statement of importance of the source of uncertainty. One source of uncertainty that is omitted is the limited size of the data sets used in the regression modelling and the resulting uncertainty of statistical estimates such as quantile regression and empirical estimates of 75th and 95th percentiles of exposure. This is not a problem as long as the overall risk assessment procedure is judged to make adequate allowance for uncertainties affecting the assessment. A second source is uncertainty about which factors should be included in each regression model. As discussed above, the choice was made by expert judgement informed by statistical modelling. One possible approach to addressing the second uncertainty would be some form of statistical model averaging (used, e.g. in EFSA BMD modelling guidance). However, this would need further statistical modelling resource and expert judgement about reasonable alternative choices of factors and the approach has not been pursued.
A.4 Conclusions
The Greenhouse AOEM provides an acceptable approach to estimate exposure of operators in greenhouses. The database as well as the model are subject to certain limitations (e.g. for knapsack mixing/loading and low crop application). In comparison to existent models and approaches, it is based on an appropriate amount of data that reflect current practices and techniques and is fully transparent with respect to the process of model development.
A.5 Recommendation
The Greenhouse AOEM model for operator exposure in greenhouses is based upon available raw data, which allowed an independent assessment. The model is considered suitable for the risk assessment of pesticides, applied in greenhouses. Generation of new data and their implementation in the current model is recommended in order to increase its robustness.
Appendix B – Greenhouse scenarios for residents and bystanders
B.1 Introduction
Article 3 (27) of Regulation (EC) No 1107/2009 defines a greenhouse as ‘a walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products into the environment.’ As a consequence of the anticipated ability to control or even prevent the release of plant protection products from the greenhouse area to the environment, the risk assessments for the application of plant protection products do not consider resident/bystander exposure and respective health risks for these persons staying in close proximity of greenhouse areas.
However, the assumption that plant protection products will not be released to the environment, as stated in Regulation (EC) No 1107/2009, has since been disproved. Considering the mentioned controlled exchange of material and energy, emissions from greenhouse areas are likely, as it was noted in research reports and other literature (Duyzer and Vonk, 2002; Duyzer et al., 2004; Stanghellini, 2009; Vermeulen et al., 2010; EFSA, 2014a), even if the protective structures of the greenhouse were in place. Consequently, a significant fraction of the applied active substances will always reach the environment of the greenhouse area along with the convective flow of material and energy, unless the exchange with the environment can be fully blocked by technical measures or when the active substance has a very short aerial half‐life time (EFSA, 2010b). Two main routes of emissions from greenhouse areas were identified (EFSA, 2010b,2012,2014a):
Leaching, drainage, run‐off of the plant protection product from treated plants or areas, as well as removal of (waste) material (chiefly soil, water or parts of plants) from the greenhouse may lead to contamination of water bodies (ground or surface water) or soil in areas adjacent to enclosed spaces for crop production.
Aerial emissions of aerosols, mists, droplets and volatilised compounds are a consequence of the need for climate control in greenhouses, which is usually accomplished by ventilation.
From these findings, it follows that exposure of residents and bystanders during and after application of pesticides in greenhouses cannot be excluded. Therefore, the development and implementation of harmonised approaches to assess risks, particularly for non‐dietary exposure of residents and bystanders, is required.
According to the available literature, drift and volatilisation are the relevant routes of exposure to be considered for a non‐dietary risk assessment for pesticide applications in greenhouses. The term ‘drift’ includes aerial emissions directly related to the application procedure (e.g. aerial transport and deposition of spray droplets, aerosols or fume), while volatilisation is solely related to vaporisation of residue from treated surfaces. For walk‐in tunnels with ventilation holes or rolled‐up sides, drift is considered as comparable to open field applications (EFSA, 2010b,2012; Beulke et al., 2011). Therefore, the EFSA GD (2014a) proposes to use open‐field methodology as a first tier (worst case), which considers drift and volatilisation, except for applications along with nutrients (e.g. drip application).
B.2 Assessment
Emissions of plant protection products from greenhouses to soil and water bodies are considered as not relevant for the non‐dietary risk assessment, because a direct exposure is rather unlikely. Therefore, the non‐dietary risk assessment for residents and bystanders should focus on aerial emissions. Aerial emissions from greenhouses, as the relevant aspect of the bystander and resident exposure, are mainly a result of the need for air exchange and proper ventilation, respectively, in order to maintain acceptable climate conditions for plant growth. The extent of aerial emissions from greenhouses depends on different factors. On the one hand, there are technical factors such as greenhouse type, ventilation type, mode of application, material properties of the cover materials, but on the other hand also the physico‐chemical properties of the pesticide or the active ingredient (e.g. vapour pressure, potential for short‐ or long‐range transport) are important factors. In addition, the weather conditions outside the greenhouse (e.g. temperature, wind speed) can influence the needs for ventilation and may have an impact on volatilisation. Irrespective of these influencing factors, most relevant categories of aerial emissions for an exposure assessment of uninvolved persons are spray/fume drift and vapour from the greenhouse area and the resulting surface deposits on non‐treated areas.
According to the recent definition in Regulation (EC) No 1107/2009, ‘greenhouse’ is an umbrella term for various types of enclosures for plant growth, ranging from high technology greenhouses to simple plastic covers. As a consequence, various scenarios for application and ventilation must be considered. In order to simplify the evaluation, a common worst‐case scenario may be identified and assessed based on the analogy of existing approaches.
Walk‐in tunnels may be open or have the sides of the tunnel rolled up, which is obviously the worst‐case scenario for emission via the aerial route during and after the application of plant protection products. The same holds true for shelters, shade houses or low tunnels. Hence, it can be assumed that the potential (worst‐case) exposure of residents and bystanders is comparable to field applications, except that a re‐entry into previously treated crops under cover is unlikely. This conclusion is supported by scientific reports (Beulke et al., 2011; EFSA, 2014a, 2015).
However, this approach is suitable for some types of applications but may be overly conservative for the assessment of other types. When applying a pesticide via the irrigation system, as soil injection, as solid granules or when using it for soil treatments, the emission of drift is unlikely, while for applications by fumigation, fogging or spraying a reasonable emission of fume, mist or droplets is expected. At the same time, it should be kept in mind that fogging/fumigation usually creates significantly smaller droplets as opposed to spraying. As smaller droplets precipitate slower than larger droplets, the droplet size may have an impact on emission rates as well, but ventilation is usually stopped during fogging/fumigation (Stanghellini, 2009). However, it is worth to be noted that the air exchange rate of relatively air‐tight modern greenhouses with closed ventilators is still 0.5 volumes per hour (EFSA, 2010b), thus, stopping ventilation will not fully prevent a release of aerial emissions of plant protection products. Yet, in the EFSA Guidance issued in 2014 suitable data is only available (and used) for the exposure assessment of residents and bystanders during and after outdoor spray applications (EFSA, 2014b). Data on other modes of application are scarce or missing (EFSA, 2014a). Consequently, the use of data and procedures from outdoor spray applications for greenhouse applications is a reasonable initial approach to estimate exposure to drift. The same holds true for volatilisation and deposition of active substances, while a re‐entry into treated cultures by residents and bystanders in enclosures is unlikely and should not be included in the calculation for the exposure assessment of residents and bystanders. Nevertheless, the procedures for the assessment of outdoor applications as outlined in the EFSA Guidance issued in 2014 (EFSA, 2014) are recommended as a first‐tier approach in the assessment of the exposure of residents and bystanders to plant protection products applied in crops grown under cover.
As stated earlier, the emissions from greenhouses are influenced by various factors. Thus, some uncertainties remain.
Firstly, huge spatio‐temporal variations may be expected for aerial emissions, mainly driven by the high variability of air exchange rates (e.g. depending on structure of greenhouses, outside climate conditions, etc.) and uneven distribution of emissions outside the greenhouse, which are expected to reach a maximum in the area close to the outlets of the ventilation system.
Secondly, kinetics of degradation or dissipation (e.g. photolysis, wash‐off) may be different under cover in comparison to outdoor applications, as there are different environmental conditions in the greenhouse (e.g. varying light conditions in terms of intensity and spectrum, absence of rain, temperature). These differences may have an impact on rate and duration of the release of volatile substances.
Thirdly, application techniques for greenhouses are different as opposed to field uses. This is not limited to the devices used for application, but also to the modes of application, which may result in different physical properties of spray droplets/aerosols. Thus, drift emissions may be misestimated.
However, based on the available literature discussed in the previous section (B.2), it can be concluded that the use of exposure models for outdoor applications (EFSA, 2014b) is reasonably conservative as an initial approach in the assessment of greenhouse applications of plant protection products. Considering that exposure of residents and bystanders may be overestimated for some scenarios (e.g. for spray application in permanent glasshouses, a reduced emission of spray drift is expected as opposed to application in walk‐in tunnels), further experimental data are required to adjust the available exposure models for greenhouse applications.
B.3 Conclusions
Considering the available evidence for pesticide emissions from applications in greenhouses to the surrounding environment, the current practice of disregarding the possible exposure of bystanders and residents moving and living in immediate vicinity of greenhouse areas is not adequate.
Based on the available literature, the use of models and procedures for the assessment of outdoor applications, as stipulated in the EFSA Guidance issued in 2014 (EFSA, 2014), are recommended as a first‐tier approach in the assessment of the exposure of residents and bystanders towards plant protection products applied in crops grown under cover. The exposure pathways via spray drift and the resulting surface deposits in non‐treated areas, as well as volatilisation of the active substances are considered as relevant routes of non‐dietary exposure for residents and bystanders, which should be addressed in the risk assessment. A re‐entry into treated areas is deemed unlikely for greenhouse applications of plant protection products since it is not an appropriate activity for uninvolved persons. Thus, the risk assessment should cover direct dermal and inhalation exposure based on spray drift, exposure towards deposits (caused by spray drift) and exposure towards volatilised residue in air.
It should be considered that some uncertainties remain. These are related to, e.g. spatio‐temporal variations of emissions, differences in terms of dissipation of the active substances in controlled environments, and changes of physical properties of spray aerosols. According to the available literature, the approach to use exposure models from outdoor applications is deemed sufficiently conservative in order to avoid underestimation of average exposure of residents and bystanders.
Bearing in mind that the suggested use of exposure models for the outdoor application of plant protection products for the risk assessment of uses in greenhouses is a consequence of a lack of suitable exposure data for residents/bystanders from greenhouse applications, the recommended approach is considered as an interim solution.
In addition to the EFSA Guidance issued in 2014, other models to predict exposure of residents and bystanders to plant protection products like BROWSE or BREAM (Kennedy et al., 2012; Butler Ellis et al., 2017; Kennedy and Butler Ellis, 2017) or models to estimate emissions of plant protection products from greenhouse like EVA (European Commission, 2008) areas are available, which may be evaluated for their potential use in the assessment of the exposure for residents and bystanders for greenhouse uses in the future.
Despite the fact that the use of exposure data for residents and bystanders for outdoor applications is considered as reasonably conservative for a risk assessment of uses of plant protection products in greenhouse areas, some uncertainties remain. The approach may be overly conservative for some types of application (e.g. application via drop irrigation or nutrient solutions), and in addition, little is known about spatio‐temporal variations of emissions from greenhouses. Thus, more experimental data are required in order to refine existing or develop new models for the assessment of non‐dietary exposure of residents and bystanders towards active substances resulting from greenhouse applications of plant protection products.
Appendix C – Considerations of DFR studies from open literature
C.1 Introduction
In the EFSA Guidance issued in 2014, and a spreadsheet calculator for the prediction of exposure estimates to pesticides were published. For bystanders and residents, the underlying data set was restricted to a limited number of studies, while for workers, the limited data set also presented statistical uncertainties. As a follow‐up, EFSA has commissioned a review of literature data for the last 25 years related to the exposure to pesticides for residents and bystanders and for environmental risk assessment. In this review (Lewis and Tzilivakis, 2017a), one of the four investigated themes were ‘dislodgeable foliar residue’ (DFR).
In addition to Lewis and Tzilivakis (2017), results from BROWSE project (Doan Ngoc, 2014) were investigated for new data to be considered. One of the primary objectives of the BROWSE project (2011–2014), a European Commission research project funded under the Seventh Framework, was to develop new and improved models for assessing the exposure of operators, workers, residents and bystanders to plant protection products. In this framework, a review of the existing and emerging models of worker exposure and an overview of relevant data in the open/grey literature were also conducted.
C.2 Data and methodologies
C.2.1 Data
In Lewis and Tzilivakis (2017a), as regards dislodgeable foliar residue, 27 articles published in a period of 25 years (from 1 January 1990 onwards) were found to match the quality criteria as set by the authors and data for 49 discrete studies were extracted from these. The data were collected via a systematic and extensive literature review defined and managed according to a predefined ‘review protocol’.
In the BROWSE project, the EFSA report on pesticide exposure assessment (EFSA, 2008), which included EUROPOEM II, BBA model and SeedTropex, was used as a starting point for the review. Furthermore, for the collation of exposure data from open (public) sources, the search database PubMed was used. The publication period chosen was from 1990 (there are nine references published before 1990, included in the list of studies considered within BROWSE) until March 2011. In addition to the PubMed database, the ‘Web of Science’ database was used for the period from January 2010 to March 2011.
As summarised in the BROWSE Report for ‘Deliverable 2.1_Overview of currently used and emerging models and data relevant to worker and data’ (2011), the open literature search revealed the following:
The majority of the publications concerning worker exposure (51) were published in the 90s.
Europe and the USA have supplied a big part of the publications (54 and 30, respectively).
TNO reports (13), California EPA reports (12) and the American Industrial Hygiene Association Journal (11) were the biggest suppliers of publications concerning worker exposure.
Most publications were not included in any database or report, although 27 publications were included in the MS Excel file based on the EFSA report (EFSA, 2008).
The majority of the publications were publicly available; only five entries contain confidential information.
Only a limited number of publications contain raw data (31).
For most entries (66), it was not possible to evaluate – based on the abstract – whether or not the study was conducted by good laboratory practice (GLP). Only five publications were already identified as being GLP compliant.
35 worker exposure studies used liquid formulations; 18 studies used solid formulations. The abstracts of the remaining entries did not mention which formulation was used during the study. WP and EC formulation were the most popular types of liquid/solid formulations.
Chlorothalonil was by far the most commonly studied active substance (14).
Flowers (26 + 6), tomatoes (11) and ornamentals (9) were the most commonly studied crops.
Harvesting was the most commonly studied task.
Most of the identified studies only monitored a single task per measurement. The whole body and patch technique are the most commonly used sampling methods for body or hand exposure. The washing technique was also frequently used to measure hand exposure.
Inhalation exposure was monitored in 51 publications and biological monitoring was performed in 24 publications.
61 publications contained data on DFR, leaf area index (LAI) or TC.
C.2.2 Methodologies
In the ‘Review of the published exposure data to pesticides for residents and bystanders, and for environmental risk assessment: Final report’ by Lewis and Tzilivakis (2017a), the authors first collected works known to them, and then applied a search strategy (part of a review protocol) to numerous literature databases (e.g. using keywords and citation tracking). The results of the literature searches were then subject to relevancy screening (criteria listed below), using the title and abstract only. No full text check has been done at this stage. Those that passed the screening were then compared to specific quality criteria (listed below). Studies that failed these criteria were excluded from the review, and those that passed went on to the next stage, data extraction. A Standard Operating Procedure was used to ensure data were extracted in a systematic way, minimising scope for error.
Screening criteria for public literature from Lewis and Tzilivakis (2017a):
Research articles & studies published on or after 1 January 1990 will be included.
Research articles & studies published in English, Italian, French, Spanish, Dutch and German only will be included. Other languages will be excluded.
-
Studies for inclusion must contain original empirical data i.e. primary research
-
–
Reviews, editorials, articles from the popular media, etc. will be excluded.
-
–
Data that is modelled or inferred will be excluded.
However, reviews and modelling articles will be retained and used for reference snowballing. Their bibliographical data will be recorded.
-
–
-
Only studies relating to plant protection products (PPPs), tracers or surrogates will be included.
If a surrogate is used within a study instead of a pesticide, the surrogate compound must have similar properties to the PPP and/or be fully justified against the study objectives.
-
Studies undertaken in the field, under cover and indoors will be included provided the PPP is used for the protection of plant material including amateur use.
Studies on non‐plant material, e.g. animals, carpets, beds will be excluded.
Studies that do not report quantitative data will be excluded.
Studies must link the PPP application via experimentation of the plant protection substances with a measure of exposure, drift, air concentrations and/or dislodgeable residue.
-
Studies that are not concerned with plant protection substances will be excluded.
The exception being where a surrogate substance or tracer substance is used, and it is appropriate to use that approach.
Outcome data must be presented in a form whereby results can be extracted with reasonable accuracy. Graphical data are acceptable providing the image is sharp and of a reasonable size/resolution.
The quality assessment for inclusion of studies for data collection included the criteria as listed below. The criteria were applied more or less stringently (present – absent – desirable).
Quality criteria from Lewis and Tzilivakis (2017a) for data extraction:
Studies should refer to dislodgeable residue on plant material.
The study includes a thorough and up‐to‐date literature review.
A clear description of the methodology is given and justified.
The residue must be directly linked to a single application of a PPP substance via a realistic experimental study.
The aims, objectives and context are clearly stated and appropriate to the study.
The sampling approach is clearly described and is justifiable, representative and appropriate, and allows for a consistent sample to be collected. As a minimum this must include sampling time, sampling interval, distance from application to sampling point, sampling height, foliage/fruit type.
Plant material should be selected and collected in a consistent manner ‐ avoiding new growth.
The test site should be clearly defined. This should include the location where the experiment was conducted, positioning of sampling points and time of year.
Key experimental data must be reported. As a minimum this should be identification of the plant protection substance, formulation, application rate and crop. If a named commercial product is used, the concentration in the product should also be reported.
Key experimental conditions/application rates, etc. should not be extreme in comparison with normal label uses and conditions.
The meteorological conditions must be fully reported. As a minimum, this must include temperature and humidity.
Rainfall, sunshine/cloud, wind speed and direction would be desirable.
Measurements should be replicated under conditions as similar as can be reasonably expected. A minimum of two replicates are required (see Appendix J). There should be at least two sampling points per site (Iwata et al., 1977).
Statistical analysis is appropriate and must address the variability of the study results.
Laboratory/analytical work should be done using a validated technique. Limit of quantification (LOQ)/limit of detection (LOD) should be reported or identifiable from elsewhere.
Extraction of dislodgeable residue should be done quickly ideally within 4 h and always within 24 h. Sample storage time should be recorded.
Samples should be kept on ice but not frozen (see Appendix J, Iwata et al., 1977; California EPA, 2002).
Extraction should ideally be done using aqueous extraction methods or with methanol. Strong organic solvents should not be used.
If a surrogate is used instead of a pesticide the compound must be clearly identifiable.
Overall, this literature review resulted in a total of 27 studies of acceptable quality, according to quality criteria as defined by Lewis and Tzilivakis (2017a).
Additionally, according to Lewis and Tzilivakis (2017a), the results of a literature review performed during the BROWSE project were also considered. Within this project, the DFR data from the literature were included in the BROWSE transfer coefficient database for the compilation of which specific acceptance criteria were used (see Figure C.1. below) (Doan Ngoc, 2014).
Figure C.1.
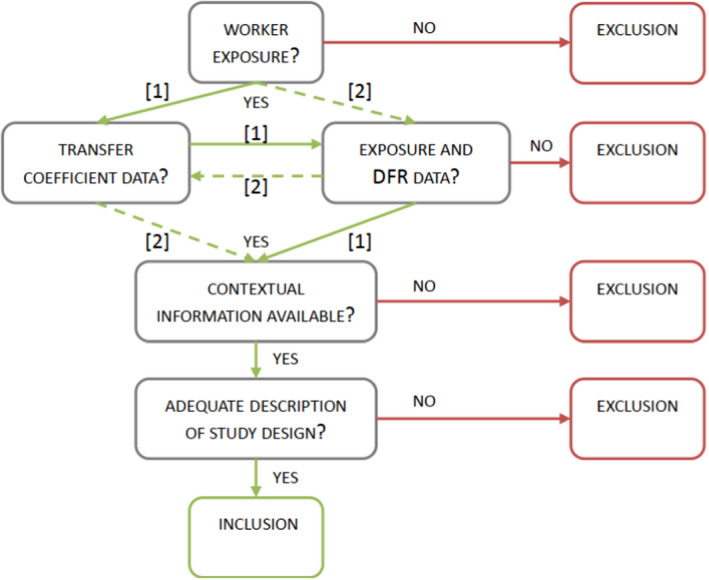
Acceptance criteria used for the BROWSE transfer coefficient database (BROWSE, 2016)
Overall, the literature search resulted in a total of 35 studies of acceptable quality. These studies were used to populate the BROWSE transfer coefficient database and covered a wide range of crops and activities.
The WG assessed all relevant papers, extracting the parameters which were considered by the authors as relevant to potentially influence the level of DFR in field studies. These parameters were compiled in a list (see C.3. Assessment). Additionally, the WG discussed if any of the extracted parameters could be considered as a main driver for the DFR values, based upon the compilation of Lewis and Tzilivakis (2017).
C.3 Assessment
The WG assessed the studies from Lewis and Tzilivakis (2017a) and BROWSE project (Doan Ngoc, 2014).
The main purpose of Lewis and Tzilivakis (2017) was to collate publicly available data on dislodgeable foliar residue. No further evaluation of these data has been done in their review.
Among the 27 studies on DFR from Lewis and Tzilivakis (2017), considered as reliable by the contractor, one of them was already included in Hamey et al. (2009) database and thus considered as being taken into account for the preparation of the EFSA Guidance issued in 2014 (and excluded for any further consideration).
For updating the guidance, the remaining 26 studies on DFR from Lewis and Tzilivakis (2017a) were further reviewed by the WG for new information on potential parameters which were considered there to potentially influence the DFR values, as measured in the field studies (see Annex B).
These 26 studies covered 29 different pesticides and 17 crops, including: Alfalfa; Chrysanthemum; Citrus fruit; Cotton seeds; Cucumbers; Cynodon dactylon (L.) Pers.; Dianthus caryophyllus L.; Fir; Gerbera; Grapes; Lawn; Nectarine; Picea glauca; Rhododendron simsii Planch; Rose; and Tomatoes. There is a significant gap regarding arable and field crops. Most of the studies were conducted on turf, with focus on golf and other recreational activities.
Regarding the review of the existing and emerging models of worker exposure and the overview of relevant data in the open/grey literature conducted within the BROWSE project, it should be noted that although a high number of studies was collated, finally, not all of them have been considered to fulfil the acceptance criteria (as shown in Figure C.1) for further analysis within the BROWSE project and the development of the BROWSE models.
For updating the guidance and after looking in detail the final BROWSE outcome (Final Browse report; Doan Ngoc, 2014), only two studies (see Annex B) have been identified in the BROWSE reports as not already reported by Lewis and Tzilivakis (2017a) or not having been considered for the preparation of the existing Guidance in 2014.
The assessment of these studies retrieved from different projects included the compilation of parameters that might influence the DFR (Table C.1).
Table C.1.
Factors potentially influencing DFR
| Application regime |
|---|
| Application rate |
| Frequency of the treatment |
| Application volume (high, low) |
| Physico‐chemical properties of the chemical: |
| Photolysis, hydrolysis |
| Mode of action (e.g. cellular uptake via hydrophobic diffusion across living cell membranes) |
| Volatility, persistence |
| Properties of the formulation: |
| Components of the formulation (adjuvants, carriers, surfactants, efficacy improvers, etc.) can influence the solubility, deposition, surface retention and penetration of the active ingredient through the cuticular layer |
| Adsorption/binding to the plant surface |
| Impact of formulants on atomisation, droplet formation, transport and target impingement |
| Form of the a.s. particles; particulate vs. emulsion or solution |
| Application techniques: |
| Spraying equipment (e.g. knapsack sprayer, knapsack mist blower, high‐pressure spray gun) |
| Moving direction during application for handheld equipment |
| Size of droplets and droplets dispersion |
| Droplet properties (diameter, impact velocity, adhesion energy, etc.) |
| Weather/cultivation conditions: |
| Post‐application irrigation, rain |
| Wind erosion, droplet abrasion |
| Temperature |
| Humidity of the air (influencing co‐distillation) |
| Crop specific factors: |
| Structure of the leaves |
| Density of plant foliage (canopy) |
| Plant height |
| For greenhouses: |
| Set up of the greenhouse (orientation, structure, isolation and construction material) |
| Structural design (glass, fiberglass, polyvinyl sheeting, rigid acrylic, walls and ceilings) allows for different degrees of light transmission |
| Ventilation systems vary greatly (passive ventilation to mechanical ventilation) |
| Heating system |
| Temperature and humidity (high humidity is assumed to enhance pesticide penetration into the leaf by favouring stomatal opening and by slowing the drying of spray deposits allowing more time for absorption) |
| Some general conditions for validity of DFR studies: |
|
No rain events during the study Replicate samples should be collected on more than 1 day Residue should be dislodged from leaf surfaces with a detergent solution, Application should be at or near the maximum stated on the product label Study performed under climate conditions typical for the crop’s growing season |
| Evaluation of predicted DFR values: |
| Different models can be applied, pending better fit (log‐linear versus log‐quadratic) |
| Inclusion/exclusion of Day 0 DFR values in the calculation of dissipation, pending variability of data |
| Use of biased/non‐biased backtransformations |
C.4 Conclusions
Since DFR were sampled by different techniques, some of them hardly standardised and different units were reported, a direct comparison between the studies is not possible. High variability in reported DFR values was observed. Many of the studies were conducted on turf, with focus on golf and other recreational activities. The collated data confirm the complexity of factors (among others physico‐chemical properties of the chemical and the co‐formulants, properties of the formulation, application techniques, cultivation, weather conditions and crop‐specific factors) influencing the level of DFR. No parameter could be estimated as the major driver for level of DFR, as measured in the field studies.
C.5 Recommendation
For future considerations, the establishment of a harmonised guidance for conduction of DFR studies, as well as of criteria for adequate extrapolation between crops is recommended. Only based upon a broad database, comparisons between the studies can be made and more general considerations could be derived. Until then, there is no justified basis for refinement of current default initial DFR value (DFR0) of 3 (μg a.s./cm2 of foliage)/(kg a.s. applied/ha).
Appendix D – Considerations of DT50 studies from open literature
D.1 Introduction
In the EFSA Guidance issued in 2014 (EFSA, 2014), a half‐life of 30 days was concluded as a default value for the dissipation rate of pesticide residue on crop foliage (DT50) in the absence of specific experimental data. Although it was noted that the half‐life of 30 days was different from that proposed in the PPR Panel Opinion on the science behind the Guidance Document on risk assessment for birds and mammals (EFSA PPR Panel, 2008) this was decided as a more conservative approach based on the available data.
More specifically, the Willis and McDowell (1987) & the United States Department of Agriculture (USDA) Agricultural Research Service (ARS) data sets [Appendices C and D of the EFSA OpEx Guidance issued in (2014), respectively] had been considered as indicating possible DT50 values (the time required for 50% of the initial concentration to dissipate) up to 30 days. Only limited cases of a DT50 higher than 30 days were reported in the USDA‐ARS data set. It is noted that the default value of 10 days, been considered as reasonable to use in the birds and mammals risk assessment (EFSA PPR Panel, 2008), was based on an analysis of the Willis and McDowell (1987) data only and considering the mean values and the respective standard deviations.
Lewis and Tzilivakis (2017b) have recently conducted a literature review of pesticide dissipation data, which was published along with the data set as a supplementary file: ‘Plant dissipation data August 2017.xlxs’. The purpose of Lewis and Tzilivakis (2017b) was to collate a new database in a format compatible with the main online pesticide database resource (the Pesticide Properties Database, PPDB), to validate this database in line with the PPDB protocols and thus ensure that the data are maintained and updated in future. In the Lewis and Tzilivakis (2017b) data set, there was already a distinction of the captured dissipation data to ‘ON’ and ‘IN’ the matrix. The WG agreed that this literature review should be further considered in view of a possible refinement of the default DT50 value of 30 days.
It has been acknowledged that additional data sets, such as Fantke and Juraske (2013), or publications related to dissipation rate of pesticides, such as Ebeling and Wang (2018), are also available.
Fantke and Juraske (2013) reviewed the published dissipation/decline data for 346 pesticides in various parts of 183 plant species. Based upon the available data, which were not representative for all pesticide−plant combinations, their findings included a large variation in half‐lives reported per pesticide. Fantke et al. (2014) used these data as a starting point to develop a predictive regression model for foliar pesticide dissipation to estimate foliar half‐lives from chemical substance properties and crop classes. This model development (Fantke et al. 2014) considered 4442 data points (reported half‐lives) for 333 pesticides where the authors considered there was sufficient information on the chemical substance properties and plant. In the Fantke and Juraske (2013), although there was a distinction between the different plant compartments, there was no distinction of the captured dissipation data to ‘ON’ and ‘IN’ the matrix.
Since the data set by Lewis and Tzilivakis (2017b) was created based on peer‐reviewed literature, building on the work previously done, particularly that of Fantke and Juraske (2013), it was not reviewed in detail by the WG.
The review conducted by Ebeling and Wang (2018) was also acknowledged, who have reassessed foliar dissipation data based on a data set of 396 non‐published residue trials covering 30 compounds. The study aimed at having a better estimation of the foliar DT50 to be used for the risk assessment of herbivorous birds and mammals feeding on sprayed foliage. Thus, the focus of the study authors was to obtain an overview of foliar residue decline under field conditions in the European Union trying also to address those factors that are relevant for wildlife risk assessment and considered only parameters that are measured in standard field residue trials. Although the study authors refer to the pesticide’s dissipation from a leaf surface, the relevance of the assessed data for the non‐dietary exposure assessment and the dissipation of DFR could not be established based solely on the publication. Also considering the non‐availability of the raw data, this review was not further considered for the purpose of the guidance update.
Lahr et al. (2018) have also conducted a study aiming at developing a database with ecological data and residue data to be used for the risk assessment of plant protection products for birds and mammals. While the main sources of data were the information submitted in the context of approval of active substances and authorisation of products, additional information were retrieved through a systematic literature review. However, the gathered data focused on residue detected on matrices mostly not relevant for worker exposure assessment, i.e. whole plant, foliage, grass/weeds, flower heads, insects and worms. Even for the data regarding foliage residue, there is no information on whether these could be considered as dislodgeable. Although the data set created by Larh et al. (2018) was not considered relevant for the reassessment of the DT50 value to be used in the non‐dietary risk assessment, elements of this study have been taken into account for the development of the protocol for the review of relevant DT50 studies.
Considering the above, it was agreed to further focus only on the database established by Lewis and Tzilivakis (2017b).
D.2 Data and methodologies
D.2.1 Data
In the review performed by Lewis and Tzilivakis (2017b), data on dissipation rates were collated using a systematic review approach and considering several scientific databases. A review protocol was predefined, identifying the literature databases to be searched (i.e. Google Scholar, ScienceDirect, Scopus, American Chemical Society Journals Database) and including a rigorous search protocol to retrieve all (insofar as this is reasonably possible) relevant peer‐reviewed published literature available during the period from January 1980 to June 2017. The review protocol applied by Lewis and Tzilivakis (2017b) has been the same as the one applied by Lewis and Tzilivakis (2017a) in the ‘Review of the published exposure data to pesticides for residents and bystanders, and for environmental risk assessment: Final report’.
Based on Lewis and Tzilivakis (2017b), the collated literature was subjected to a quality assessment before being extracted into an MS Excel spreadsheet (from now on referred to as ‘Lewis‐DT50 ’ database). The authors of the study had stated that the same quality assessment criteria were considered, as in case of Lewis and Tzilivakis (2017a) database. These criteria are included in Table D.1.
Table D.1.
Quality criteria considered by Lewis and Tzilivakis (2017b)
| Criteria | Inclusion/Exclusion/Desirable |
|---|---|
| The study includes a thorough and up‐to‐date literature review | Desirable |
| A clear description of the methodology is given and justified. | Present – Include; Absent – Exclude |
| The aims, objectives and context are clearly stated and appropriate to the study. | Present – Include; Absent – Exclude |
| The sampling approach is clearly described and is justifiable, representative and appropriate. As a minimum this must include the sampling technique (e.g. patches, mannequins, etc.), sampling time and interval, and distance from source to sample point/person being exposed, sampling height. | Present – Include; Absent – Exclude |
| The test site should be clearly defined. This should include the location where the experiment was conducted, positioning of sampling points/person being exposed and time of year. | Present – Include; Absent ‐ Exclude |
| Key experimental data must be reported. As a minimum this should be identification of the PPP, application rate, formulation and crop. If a named commercial product is used the concentration of active substance in the product should also be reported. | Present – Include; Absent – Exclude |
| Key experimental conditions/application rates, etc. should not be extreme in comparison with normal label uses and conditions. | Desirable |
|
The meteorological conditions must be fully reported. As a minimum this must include temperature & humidity. Rainfall, sunshine/cloud levels, wind speed & direction would be desirable. |
Present – Include; Absent – Exclude. Desirable |
| Measurements should be replicated under conditions as similar as can be reasonably expected. A minimum of two replicates are required and there should be at least three sampling points per site. | Include if conforms; Exclude if it does not conform |
| Statistical analysis must be appropriate and must address the variability of the study results. | Include if conforms; Exclude if it does not conform |
| Laboratory/analytical work should be done using a validated technique. LOQ/LOD should be reported or identifiable from elsewhere. | Include if conforms; Exclude if it does not conform |
| If a surrogate is used instead of a pesticide the compound must be clearly identifiable. | Include if conforms; Exclude if it does not conform |
Regarding the review articles identified by Lewis and Tzilivakis (2017b), these were primarily used to identify suitable studies and not for data extraction. However, in some instances, particularly where the article was old and obtaining a copy was problematic, the data were recorded as included in the review article itself. Lewis and Tzilivakis (2017b) had recognised that this approach might not have been ideal, as it was not possible to check the data or to judge its quality. Overall, the decision to include data from reviews was based on expert judgement regarding the value of the data in terms of the amount of other data available on the pesticide/plant/matrix combination.
The parameters captured in the Lewis‐DT50 database are shown below in Table D.2.
Table D.2.
Description of parameters captured in the database developed by Lewis and Tzilivakis (2017b)
| Parameter | Description |
|---|---|
| Pesticide common name | The name by which the pesticide active substance is commonly known. Data in this column are listed alphabetically |
| Pesticide chemical name | Chemical name of the pesticide using the Chemical Abstract Services (CAS) nomenclature |
| CAS registry number | The Chemical Abstract Services’ unique identifying number (RN) assigned to the pesticide |
| Plant | Common name of the plant/crop the data relates to |
| Plant scientific name | Scientific name including cultivar or variety where known |
| Matrix | The part of the plant tested |
| On/In | Whether the residue was measured on (O—as a surface residue) on in (I—as total residue in and on) the sample |
| Country | The country (and in some instances region) where the study was undertaken |
| Study conditions | Whether the study was undertaken in the open field (F), undercover (U) or under special conditions (X). In the latter case, the data are accompanied by short qualifying text |
| Min DT50 (days) | Minimum experimental value for the plant dissipation rate expressed as the half‐life (RL50 ( a )) in days |
| Max DT50 (days) | Maximum experimental value for the plant dissipation rate expressed as the half‐life (RL50) in days |
| Mean DT50 (days) | Arithmetic mean experimental value for the plant dissipation rate expressed as the half‐life (RL50) in days |
| Reference | Full bibliographical reference for the publication from which the data were extracted |
| PPDB code | Unique identifier linking the record to the PPDB (see User Notes |
RL50: The pesticide half‐life (residual lifetime), expressing the dissipation rate of pesticides in different crop matrices.
Dissipation rates were reported as arithmetic mean for the pesticide–plant–matrix combination as reported in the published literature. According to Lewis and Tzilivakis (2017b), in cases where sufficient data were provided within an article to calculate half‐lives, only the temporal variation in pesticide concentration within or on the matrix was reported. In cases where more than one experiment on the same pesticide–plant–matrix combination had been reported in one publication, the data range across experiments was captured in the database. Lewis and Tzilivakis noted that the approach followed in their work was in contrast to that of Fantke and Juraske (2013), who reported experimental residual lifetime (RL50) values for each separate experiment/data point.
It is noted that it was identified that Lewis and Tzilivakis (2017b) database contained many of the studies reported by Fantke and Juraske (2013) previously, such as the publication of Willis and McDowell (1987), however, this was not the outcome of a systematic comparison of the data included in the two databases.
Overall, the Lewis‐DT50 database contained data from 1390 published studies:
for 407 different active substances
across 207 different plants
on a wide range of different matrices including leaves, fruits, seeds, root, new shoots, etc. and
including over 2200 records for unique pesticide–plant–matrix combinations.
D.2.2 Methodologies
For the current update of the guidance issued in 2014 and looking into the Lewis‐DT50 database in more detail, it was agreed that not all the records would be relevant for worker exposure.
More specifically, it had been considered that those records that were captured as ‘IN’ the matrix referred to residue measured in the plant sample (as total residue) and it had been decided to exclude these records.
Thus, it was decided not to filter the ‘Matrix’ parameter but exclude the ‘IN’ records for the ‘IN or ON’ parameter. This filtering had resulted in 746 records out of total 1,048,575, corresponding to 295 publications.
As a first step, the set of these 295 publications was decided to be subject to a more detailed review.
Based on a pilot study, the need for a closer and detailed screening of the records entered in the Lewis and Tzilivakis (2017b) database was identified.
It was decided that for further exploitation of these data, the individual publications should be reviewed based on specific additional criteria, while the existing Lewis‐DT50 database should be restructured in order to include additional information. For example, in many entries of the Lewis‐DT50 database, the same value was recorded as DT50 mean, min and max, while it was not possible to identify those entries derived from a review publication. Furthermore, in specific entries, there was a note that the recorded values had been calculated without providing any relevant information.
Thus, it was decided that the abstracts of all 295 publications identified as ‘ON’ matrix should be screened in order to further confirm their relevance for worker exposure.
Following this step, the need to further confirm the validity of the records captured as ‘IN’ matrix, at least for the matrices related to foliage/leaves/skin surface, was identified. Thus, the abstracts of further 362 publications were screened for their relevance to worker exposure. More specifically, the following were considered:
Residue to be considered should be only those which are reliably dislodgeable
Not the total amount of residue but their dissipation rate is crucial
Only studies with measurements ‘ON’ matrix should be included.
The overall outcome of this prescreening of Lewis and Tzilivakis (2017b) database (1st selection step) resulted in the need for further assessment of in total 133 publications, 106 of the 279 ‘ON’ screened and 27 of the 362 ‘IN’ prescreened. The detailed selection procedure is described in Annex C.
It is noted that for 16 references from the 295 ‘ON’, neither the publication was available nor the abstract could be retrieved online.
Based on the outcome of this prescreening pilot study, a detailed protocol was developed for further checking the publications identified as relevant (Annex C).
It is noted that for compiling the structure of the new DT50 database, the BROWSE Reports (BROWSE, 2016) for Work Package 2 (Deliverable 2.4 – Work Package 2: Completed worker exposure models for final scenario & Appendices) and the EFSA external scientific report ‘Data collection for the estimation of ecological data (specific focal species, time spent in treated areas collecting food, composition of diet), residue level and residue decline on food items to be used in the risk assessment for birds and mammals’ of Lahr et al. (2018) have been taken into account. In addition, the captured parameters have been chosen considering their potential influence on DT50 results based on elements highlighted also for the higher tier studies (Appendix J).
As a next step, and in order to consider a possible refinement of the default DT50, a data collection was outsourced by EFSA aiming at reviewing the identified published literature from the Lewis and Tzilivakis data set (2017b) by applying the protocol developed by the WG (Annex C).
For the outsourced data collection, 1304 publications were checked against eight inclusion/exclusion criteria (Table 2 of the protocol in Annex C):
Presence of control samples (excluded if no control samples were gathered);
Number of samples per sampling interval (excluded if less than three);
Storage conditions (excluded if samples were stored frozen or in dry ice);
Dislodging solvent (excluded if organic solvents were used);
Extraction time after sampling (excluded if extraction of DFR was not conducted within 24 h);
Reporting of the dislodgeable extraction method (excluded if not reported);
DT50 calculation clearly reported (excluded if not reported);
Review articles (excluded secondary literature in general)
After the first 20 publications were screened and following consultation with WG members and EFSA, the consideration of one additional screening step was agreed. More specifically, since it was realised that among the 20 studies first checked finally only one concerned the determination of dislodgeable residue, it was decided that before checking for the inclusion/exclusion criteria, the relevance of each publication should be confirmed, i.e. whether the objective of the study was the determination of residues that are reliably dislodgeable and ‘ON’ the matrix. Additional guidance was provided for the data collection regarding the outcome of the prescreening step conducted by EFSA WG, which had been based on reviewing only the abstract for each study.
Finally, 80 out of the 130 checked publications were considered as not relevant to dislodgeable residue on the matrix. Thus, only 50 were the objective of the next step, i.e. the check against the inclusion/exclusion criteria.
Initially, only studies/data fulfilling all eight criteria should have been considered for detailed data extraction in the DT50 database. However, further clarifications were required during the implementation of this check and the need for amending the protocol was identified. More specifically, following a consultation with the WG and EFSA, it was agreed that:
The criterion ‘Storage other than freeze or with dry ice’ was considered as fulfilled if leaf samples were stored in a cooling box on the way to the lab, or put on ice for the transport, but not frozen.
If the time until extraction was not mentioned and/or there was no information regarding storage, then the publication should be further considered for data extraction. However, since actually it would be assumed that the samples were extracted following their arrival in the laboratory, the relevant entries should be flagged for further addressing uncertainty issues during the analysis.
In case there were DFR data available but there was no DT50 calculation, the study was considered acceptable for further review; a DT50 value was calculated by the awarded expert based on the DFR data.
The lack of control samples, or the absence of reference to control samples was not a criterion for excluding the study for further review and data extraction, but the study was flagged, for further addressing uncertainty issues during the analysis.
Overall, 32 of the 50 publications were considered as relevant for further data extraction in accordance with the protocol.
D.2.3 Data extraction
As described in Table 3 of the protocol (Annex C), data were extracted form included papers in a Excel file (Annex D), also capturing relevance assessment and the check against inclusion/exclusion criteria.
In summary, data captured concerned the following parameters:
Crop, growth stage (Crop height, BBCH)
Location (Field/Indoor/other) and Country/Region – Köppen–Geiger climate classification (Main climates)
Row data availability
GLP status
Active substance, Pesticidal mode of action and Product name and type/physical state
Active substance content in product
Spray adjuvant used (Y/N) – Spray adjuvant (product name, if used)
Application type (e.g. spraying, foliar spraying, dusting) and application method/equipment
Application rate and total amount of active substance applied
Number of applications and application interval
Time between last application and sampling
Spray volume used in application
Year and month/season of application
Temperature–Rain during field phase–Wind–Ventilation (any details on ventilation, if indoor)
Sampling matrix
Sampling strategy
Number of sampling sites, sampling plots at sampling site, replicates/plot
Number of leave discs (or whole leaves) per sample
Expression of data on the basis of single‐ or double‐sided leaf area
Control samples (Number of control plots)
Distance between control and sampling plots
Days of sampling post‐application
Number of samples per sampling interval
Dislodging solvent (name)
Extraction time after sampling (within 24h)
Actual field results corrected for field recoveries
Dislodgeable residue extraction method (brief description)
LOQ or LOD for the active substance ‐ matrix combination
DT50 calculation clearly reported (even if no raw data are available)
Dislodgeable residue measurements
Reported DT50 and calculation method indicated in the study
Calculated DT50 (using CAKE software, single first‐order fitting)
Where no DT50 was reported, but DFR values (raw data) were available, the DT50 was calculated using the ‘Computer assisted kinetic evaluation’ (CAKE) software, version 3.4 (Tessella Technology and Consulting. Computer Assisted Kinetic Evaluation CAKE version 3.4. Available online: https://www.tessella.com/showcase/computer‐assisted‐kinetic‐evaluation), a tool which implements both the FOCUS Kinetics (FOCUS, 2006) and the NAFTA Guidance (NAFTA, 2015) to generate degradation kinetics. In cases where DT50 was reported in the study and raw data were available, the DT50 was calculated as well using CAKE.
Regarding the meteorological conditions, it was considered that the climate classification according to Köppen–Geiger should be included for each case in order to have additional information regarding the representativeness of the test conditions in relation to the European climatic conditions. The available ‘Country/Region’ and ‘Location’ data were used for this purpose. The assignment was performed using the Köppen–Geiger high‐resolution map and data of March (2017) available for Google Earth Pro (v.7.3.3.7786) (World maps of Köppen–Geiger climate classification). When available, location data allowed for a more precise classification. In case of the availability of only country/region data, the classification reported all the ‘main climates’ as per Köppen–Geiger.
In addition, any specific remarks were captured if considered to be useful for the analysis.
The parameters/information captured were characterised as A, B and C based on whether they were considered necessary for further evaluation (Table 4 of the protocol in Annex C), i.e.:
Parameters necessary to trust that the DT50, as derived per study, is a reliable value.
Parameters necessary for further evaluation/interpretation/analysis of DT50 values, e.g. for a refinement of the default DT50 (differences indoor/field, applicability for European climate, influence of climate, etc.).
Additional parameters extracted (primarily less relevant for DT50 evaluation, but to be collected for reason of completeness).
The data extraction step led to 198 DT50 values in total.
The DT50 values, concluded as reliable, were analysed in order to provide descriptive statistics information (e.g. mean, median, 75th and 95th percentiles). For the statistical analysis conducted in the context of the outsourced data collection, the software GraphPad Prism (Version 8.4.3) was used. Additional analysis was conducted by the WG using MS Excel and the R software environment for statistical computing and graphics; figures were produced using the ggplot2 package for R.
D.3 Assessment
Purpose of the review of the dissipation data identified as relevant for worker exposure assessment in the Lewis and Tzilivakis (2017b) database was to collate publicly available data on DT50. Pending the outcome of this evaluation, the question if DT50 default value of 30 days, as currently used in first‐tier assessment, is overprotective or not was assessed.
Based on the detailed assessment of relevant studies, sorted out according to the protocol (Annex C), 198 data points related to dislodgeable foliar residue (DFR) were extracted. Among them:
45 data points from seven published papers were assessed as ‘not reliable’. Reasons for non‐reliability were mainly insufficient number of replicates (less than 3) and inadequate information on sampling time point (e.g. ‘sampling intervals ranged from 2 to 4 h). There were, however, cases where the presentation of the results in the publications had raised additional concerns. For example, Study ID 45 was not considered reliable since data origin could not be proven with certainty to be primary data, but rather a collation of several past investigations done by the same research group.
28 data points from 11 published papers were concluded as ‘reliable’ since all criteria A from the protocol were fulfilled and no uncertainties from data as reported were flagged.
42 data points from 10 published papers were considered ‘reliable with flagged uncertainties’. The flagged uncertainties were related to reporting bias on storing/transporting samples before the extraction, not mentioning the time until the extraction and not reporting/including control samples. Either one or more flagged uncertainties were captured in these studies.
83 data points from 10 published papers were concluded ‘reliable with flagged uncertainties*’. The additional uncertainties marked with asterisks derived from e.g. lack of information on total amount of active substance applied, lack of information on number of control plots included in study design, or lack on any or exact information on sampling times, which however could be retrieved from included graphs.
D.3.1 DT50 values: reported vs. calculated
For data points where DT50 values were reported but also DFR raw data were available, DT50 has been calculated using CAKE. In several instances, the reported and the calculated DT50 values were different; e.g. in study ID 104 the reported DT50 was 30 days, while the calculated DT50 was is 22.5 days. For the majority of the studies, however, good agreement between the reported and the calculated DT50 was noted.
Discrepancies between the DT50 values reported in the publications and calculated in the context of the outsourced data extraction could not be easily explained. However, based upon generally spoken minor differences (Table D.3), no further attempts have been undertaken to explore them. Potential reasons of the noted discrepancies might include the following:
In the published papers, no detailed explanation on the regression analysis performed was included.
The calculated DT50 values were based mostly only on averages since raw data were available in limited studies.
It cannot be excluded that typos were present in the published studies.
Table D.3.
Comparison of reported and calculated DT50 values (in days)
| Reported DT50 (days) | Calculated DT50 (days) | |
|---|---|---|
| Number of data points | 46 | 46 |
| Minimum | 0.30 | 0.20 |
| 5th perc. | 0.40 | 0.64 |
| 25th perc. | 1.3 | 1.3 |
| Median | 2.4 | 2.3 |
| Mean (SD) | 4.6 (5.7) | 3.9 (4.6) |
| 75th perc. | 5.6 | 4.0 |
| 95th perc. | 17 | 16 |
| Maximum | 30 | 23 |
Figure D.1 shows the relationship between reported and calculated DT50 values, when both are available (46 data points). The solid line describes the cases where DT50 values are equal and the dashed lines where one is twice the other. While DT50 values from data points considered to be ‘reliable with flagged uncertainties’ tend to have calculated DT50 values greater than reported, the DT50 values from data points concluded ‘reliable with flagged uncertainties*’ tend to have reported DT50 values higher than calculated.
Figure D.1.
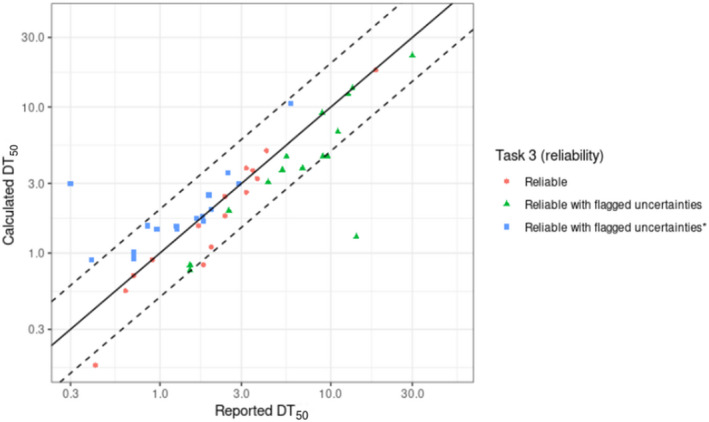
Reported and calculated DT50 values, and differences as regards reliability
D.3.2 General statistics – assessment scenarios
As regards reported vs. calculated DT50 values, different scenarios were initially considered in the evaluations:
A scenario: statistics for the DT50 values reported from the papers only (137 data points);
B scenario: statistics for the DT50 values calculated from DFR data using CAKE software (62 data points);
C scenario: statistics for data set consisting of reported DT50 values and calculated DT50 values where no DT50 values were reported but DFR data available (153 data points);
D scenario: statistics for data set consisting of reported and calculated DT50 values where calculated DT50 always replaced the reported DT50 if DFR data were available to do so (153 data points);
E scenario: statistics for all DT50 values, both reported in the public papers and calculated from DFR data (199 data points).
Based on the simple analysis performed (Table D.4), all scenarios revealed comparable statistics.
Table D.4.
Descriptive statistics of the DT50 values (in days) according to different scenarios
| A scenario | B scenario | C scenario | D scenario | E scenario | |
|---|---|---|---|---|---|
| Number of data points (DT50 values in days) | 137 | 62 | 153 | 153 | 199 |
| Minimum | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 |
| 5th perc. | 0.69 | 0.22 | 0.40 | 0.60 | 0.60 |
| 25th perc. | 1.70 | 1.08 | 1.65 | 1.50 | 1.50 |
| Median | 3.0 | 2.5 | 3.0 | 3.0 | 2.8 |
| Mean (SD) | 4.66 (6.24) | 5.31 (7.84) | 5.16 (7.28) | 4.94 (7.07) | 4.86 (6.77) |
| 75th per. | 4.65 | 4.70 | 5.05 | 4.60 | 4.60 |
| 95th perc. | 14.2 | 22.3 | 19.2 | 19.2 | 18.3 |
| Maximum | 53.0 | 39.2 | 53.0 | 53.0 | 53.0 |
Since the scenario C best reflects the complete database, but without bias of overrepresentation of double entries, this scenario has been taken further for all following evaluations.
D.3.3 Studies as regards their reliability
The DT50 data points building the C scenario were evaluated according to the level of reliability assigned, i.e. ‘reliable’, ‘reliable with flagged uncertainties’ and ‘reliable with flagged uncertainties*’ (Table D.5).
Table D.5.
Descriptive statistics of the DT50 values of C scenario database according to the level of reliability
| Level of reliability | |||
|---|---|---|---|
| Reliable | Reliable with flagged uncertainties | Reliable with flagged uncertainties* | |
| Number of data points (DT50 values in days) | 28 | 42 | 83 |
| Minimum | 0.20 | 0.40 | 0.30 |
| 5th perc. | 0.20 | 0.62 | 0.74 |
| 25th perc. | 0.75 | 1.5 | 1.8 |
| Median | 2.3 | 4.4 | 2.7 |
| Mean (SD) | 3.0 (3.5) | 8.4 (9.5) | 4.3 (6.4) |
| 75th per. | 4.1 | 11 | 3.8 |
| 95th perc. | 13 | 35 | 14 |
| Maximum | 18 | 39 | 53 |
The 28 fully reliable data points have lower summary statistics, while the 42 data points graded as ‘reliable with flagged uncertainties’ had higher summary statistics compared to the other two data sets.
The differences in distribution of DT50 values when evaluated for different reliability levels would be statistically significant (Kruskal–Wallis rank sum test, p = 0.0061) if data were treated as randomly sampled and unstructured (Figure D.1). However, since the variables, such as active substance and crop, are not the same in the three reliability groups, the differences in distribution could be due to a combination of other factors and not due to reliability rating.
Figure D.2 hows the distribution of DT50 values in histogram and highlights the reliability status of data points. As regards the absence of higher DT50 values in the category of ‘reliable’ studies, it cannot be excluded that this is partially due to the lower number of data here (28 data points) (Figure D.3)
Figure D.2.
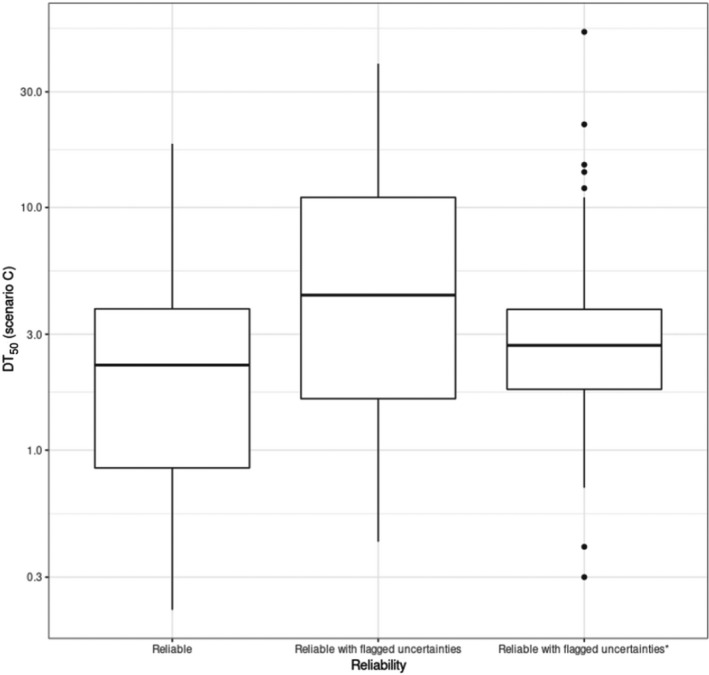
Distribution of DT50 values as regards reliability of data points
Figure D.3.
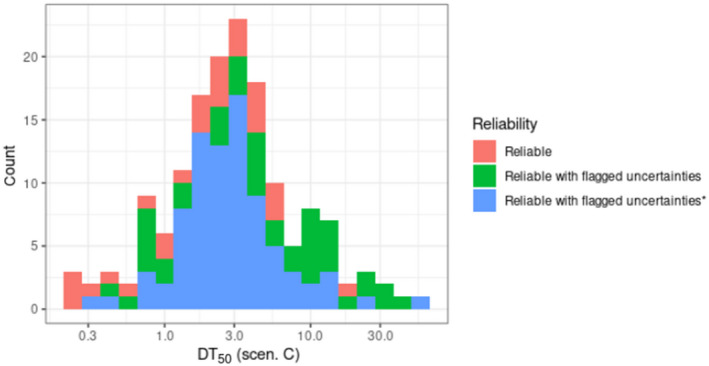
Histogram of DT50 values as regards reliability of data points
No calculated DT50 values are available for data points concluded as ‘reliable with flagged uncertainties*’. Although there is no clear picture as regards differences between the three reliability groups, DT50 of 30 days is a high value regardless which group of data is considered for the calculation, i.e. reliable or reliable with flagged restrictions (with or without asterisk) (Figure D.4).
Figure D.4.
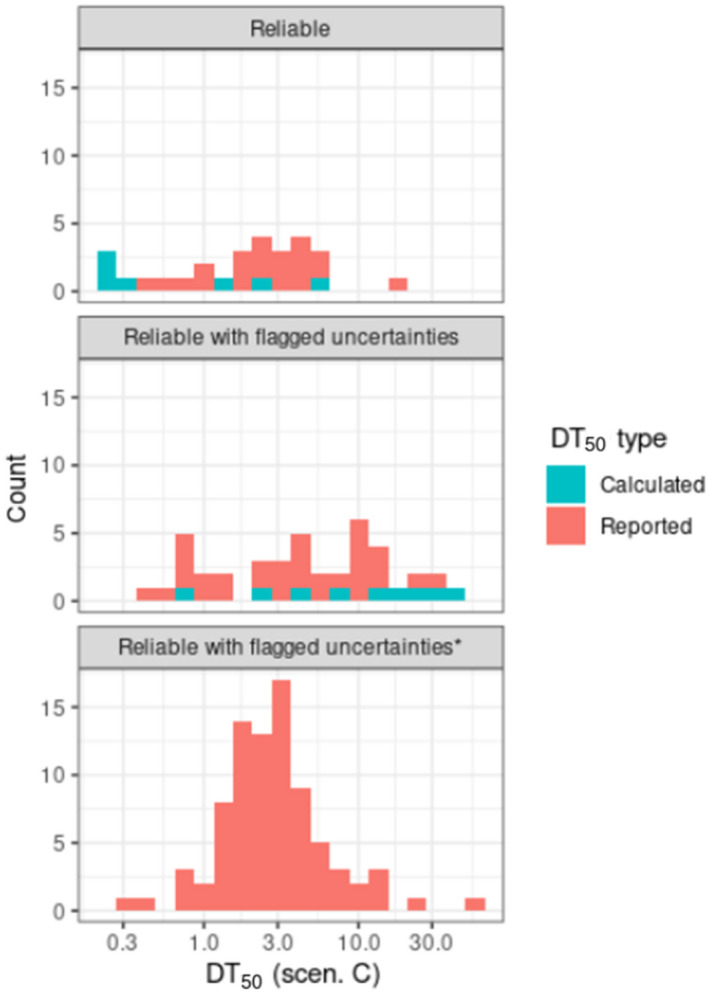
Histogram of DT50 values as regards reliability of data points
D.3.4 Analysis of potential factors influencing DT50 values
The database containing reliable studies contains data for 32 different active substances, and 24 different crops. Among the 32 active substances, only seven are still approved in the EU (status: February 2021), i.e. abamectin, azadirachtin, bupirimate, captan, emamectin, esfenvalerate, ethofumesate (Regulation (EU) 1107/2009). From the 25 active substances in the database which are not approved in the EU, only for endosulfan one the reasons for non‐approval was ‘persistence’. For the other 24 substances either different justifications for non‐approval were listed or no reasoning could be captured in the pesticide database (https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐db_en) without deeper investigations. As regards the 28 fully reliable data points, they cover only six active substances currently approved in the EU.
The distribution of DT50 values reveals statistically significant differences between different crops (Kruskal–Wallis test, p = 4 × 10−12) and/or between different active substances (Kruskal–Wallis test, p = 5 × 10−11), but these two factors are heavily confounded, and it is not possible to distinguish which of these might be driving variation in the DT50. The same applies to whether the study was in the field or indoor, the mode of action and the matrix (leaves, fruits) since these covariates are also quite unbalanced: the vast majority of data are from the field, are for insecticides and are on leaves. Based upon available data, their amount and quality, it is not possible to decide which relationships are real and which are due to confounding. Some general evaluations are included below.
D.3.5 Indoor–outdoor
Among the 153 data points, only 11 covered indoor applications. None of the active substances was tested both outdoor and indoor. Based on the limited number of data points for indoor applications, no evaluation could be further done to reliably analyse the influence of indoor conditions, but 75th and 95th percentiles DT50 values were remarkably higher than for field conditions.
Among the 142 data points from outdoor applications, 122 concerned dislodgeable residue on leaves while 20 concerned dislodgeable residue on fruits. No data points related to dislodgeable residue on fruits for indoor application are available. Although very limited data points were available for fruits, the statistics was not different from the one done on DT50 values on leaves (Table D.6).
Table D.6.
DT50 values from indoor and outdoor applications
| Indoor data points | Outdoor data points | Outdoor data points (leaves only) | |
|---|---|---|---|
| Number of values | 11 | 142 | 122 |
| Minimum | 1.40 | 0.2 | 0.2 |
| 5th perc. | 1.40 | 0.40 | 0.40 |
| 25th perc. | 1.50 | 1.675 | 1.675 |
| Median | 12.20 | 2.85 | 2.95 |
| Mean (SD) | 14.54 (13.49) | 4.43 (6.066) | 4.45 (6.348) |
| 75th per. | 21.20 | 4.60 | 4.45 |
| 95th perc. | 39.20 | 13.91 | 13.91 |
| Maximum | 39.20 | 53.0 | 53.0 |
D.3.6 Crops and matrix (leaves, fruits)
In most of the studies, the crops were in a mature stage, so it can be concluded that no ‘diluting’ effects on DT50 values occurred due to the plant growth after the treatment period. Only for study ID 115, captan was applied through the growing period.
Twelve active substances were tested at least on two crops. The majority of these data points (i.e. 94) was obtained from dislodgeable residue on leaves, while only seven data points were from fruit surface. Fruit surface DT50 values were not available for more than one crop for each active substance. The limited amount of data points available do not allow the conclusion if residue on fruit surfaces was higher than on leaves, as regards the same active substance.
The DT50 values measured for the same active substance from different crops on the same matrix (leaves) were very well comparable between the crops, i.e. they were of same magnitude (Table D.7). Only in case of azinphos‐methyl, where one value was available for peach leaves, whilst nine values were available for pear leaves, the 9 DT50 values for pear leaves spread from 3 to 24 days, showing high variability in dissipation rate in this case.
Table D.7.
Summary table of average DT50 of active substances applied on more than one crop
| Matrix | ||
|---|---|---|
| Active substance | Fruits | Leaves |
| Azinphos‐methyl | ||
| Peach | 30.00 | |
| Pear | 7.47 | |
| Carbaryl | ||
| Lemon | 22.00 | |
| Orange | 14.00 | |
| Carbosulfan | ||
| Grapefruit | 3.20 | |
| Lemon | 3.75 | |
| Orange | 3.10 | 3.08 |
| Chlorobenzilate | ||
| Lemon | 3.30 | |
| Orange | 10.93 | 5.27 |
| Dimethoate | ||
| Lemon | 3.93 | |
| Orange | 3.60 | |
| Emamectin | ||
| Alfalfa | 0.42 | |
| Celery | 0.63 | |
| Endosulfan | ||
| Grape | 1.60 | |
| Melons | 1.80 | |
| Peaches | 0.35 | |
| Methomyl | ||
| Cotton | 0.64 | |
| Grape | 2.78 | |
| Mevinphos | ||
| Broccoli | 0.63 | |
| Cauliflower | 1.42 | |
| Celery | 0.86 | |
| Green onion | 0.42 | |
| Lettuce | 0.88 | |
| Phenthoate | ||
| Lemon | 3.13 | |
| Orange | 10.00 | 3.60 |
| Trichlorfon | ||
| Lemon | 2.60 | |
| Orange | 3.30 | |
It is further noted that the 153 DT50 values cannot be considered as representative for all crops; only data for 23 crops were obtained as reliable. While there are 40 data points for grape/vine and 39 data point for citrus fruits (grapefruit, lemon, orange), there are only one to three data points for crops such as alfalfa, apple, cucumber, cauliflower, green onion, tomato and broccoli.
Table D.8 DT50 data points obtained per crop and matrix
| Crop | Matrix | |
|---|---|---|
| Leaves | Fruit/other | |
| Alfalfa | 1 | – |
| Apple | 2 | – |
| Azalea | 1 | – |
| Broccoli | 3 | – |
| Carnations | 6 | – |
| Cauliflower | 2 | – |
| Celery | 3 | – |
| Cotton | 5 | – |
| Cucumber | 2 | – |
| Gerbera flowers | 2 | – |
| Grape/vine | 40 | – |
| Grapefruit | 2 | |
| Green onion | 1 | – |
| Jasmine flower | ‐ | 9* |
| Lemon | 13 | – |
| Lettuce | 3 | – |
| Melons | 2 | – |
| Orange | 17 | 7 |
| Peach | 7 | 2 |
| Pear | 9 | – |
| Strawberry | 9 | – |
| Tomato | 3 | – |
| Turfgrass | ‐ | 2 |
Unopened flower buds.
D.3.7 Active substance – crop(s)
All crops were evaluated individually, only citrus fruits (oranges, grapefruits, lemons) were combined. The data indicate that dissipation rate is probably less driven by the crop properties than by the active substance properties. However, too few data are available to further substantiate this assumption. In Table D.9 only data points where more than one active–substance–crop combination was available are included.
Table D.9.
Summary table of DT50 values for different crop (groups)
| Row labels | Min DT50 | Max DT50 | Average of DT50 |
Crop DT50 min–max range |
|---|---|---|---|---|
| Apple | ||||
| Parathion | 0.80 | 0.80 | 0.80 | 0.80–2.50 |
| Phosalone | 2.50 | 2.50 | 2.50 | |
| Broccoli | ||||
| Esfenvalerate | 1.00 | 2.10 | 1.55 | 0.63–2.10 |
| Mevinphos | 0.63 | 0.63 | 0.63 | |
| Carnations | ||||
| Methiocarb | 7.12 | 21.20 | 13.51 | 7.12–39.20 |
| Thiophanate‐methyl | 18.40 | 39.20 | 31.07 | |
| Cauliflower | ||||
| Mevinphos | 0.75 | 2.08 | 1.42 | 0.75–2.08 |
| Celery | ||||
| Emamectin | 0.63 | 0.63 | 0.63 | 0.63–1.04 |
| Mevinphos | 0.67 | 1.04 | 0.86 | |
| Citrus fruit (orange, lemon, grapefruit) | ||||
| Acephate | 8.20 | 8.20 | 8.20 | 1.80–22.00 |
| Carbaryl | 14.00 | 22.00 | 18.00 | |
| Carbofuran (applied as Carbosulfan) | 3.20 | 3.70 | 3.45 | |
| Carbosulfan | 1.80 | 4.40 | 3.24 | |
| Chlorobenzilate | 3.30 | 14.10 | 7.85 | |
| Dimethoate | 2.20 | 7.00 | 3.85 | |
| Phenthoate | 2.90 | 10.00 | 4.10 | |
| Phosphamidon | 3.86 | 3.86 | 3.86 | |
| Trichlorfon | 2.60 | 3.30 | 2.95 | |
| Cotton | ||||
| Ethyl parathion | 0.34 | 0.34 | 0.34 | 0.22–0.34 |
| Methomyl | 0.22 | 1.46 | 0.64 | |
| Methyl parathion | 0.25 | 0.25 | 0.25 | |
| Cucumber | ||||
| Bupirimate | 3.50 | 3.50 | 3.50 | 1.40–3.50 |
| Methidation | 1.40 | 1.40 | 1.40 | |
| Grape | ||||
| Dialifor | 8.90 | 13.40 | 11.15 | 0.70–13.40 |
| Endosulfan | 0.70 | 2.50 | 1.60 | |
| Methomyl | 1.00 | 7.70 | 2.78 | |
| Jasmine flower | ||||
| Profenofos | 0.85 | 1.99 | 1.49 | 0.85–1.99 |
| Lettuce | ||||
| Mevinphos | 0.80 | 1.00 | 0.88 | 0.80–1.00 |
| Melons | ||||
| Endosulfan | 0.70 | 2.90 | 1.80 | 0.70–2.90 |
| Peach | ||||
| Azadirachtin A | 1.68 | 3.20 | 2.44 | 1.68–53.00 |
| Azinphosmethyl | 30.00 | 30.00 | 30.00 | |
| Propargite | 11.00 | 53.00 | 22.75 | |
| Peaches | ||||
| Endosulfan | 0.30 | 0.40 | 0.35 | 0.30–0.40 |
| Pear | ||||
| Azinphosmethyl | 3.00 | 24.00 | 7.47 | 3.00–24.00 |
| Strawberry | ||||
| Captan | 2.53 | 12.66 | 6.06 | 2.53–12.66 |
| Tomato | ||||
| Methamidophos | 0.70 | 2.40 | 1.33 | 0.70–2.40 |
| Turfgrass | ||||
| Ethofumesate | 2.39 | 2.39 | 2.39 | 2.39–5.03 |
| Triadimefon | 5.03 | 5.03 | 5.03 | |
D.3.8 Seasons – geographical area
Potential influence of season or geographical area was investigated only for field applications (Table D.10).
Table D.10.
DT50 values referring to different geographical areas and seasons of application (in case of multiple values available, the average has been calculated)
| Application period of the active substances | Arizona (US) | California (US) | Florida (US) | India | Kentucky (US) | Maryland (US) | Orange County, California (US) | Pinal County, Arizona (US) | Not reported |
|---|---|---|---|---|---|---|---|---|---|
| Azinphos‐methyl | |||||||||
| July | 15.00 | ||||||||
| June | 3.70 | ||||||||
| May | 30.00 | ||||||||
| Captan | |||||||||
| April | 5.82 | ||||||||
| July | 5.30 | ||||||||
| November–December | 6.83 | ||||||||
| November–February | 4.02 | ||||||||
| November–January | 12.66 | ||||||||
| Carbofuran (applied as Carbosulfan) | |||||||||
| August–September | 3.70 | ||||||||
| December–January | 3.20 | ||||||||
| Carbosulfan | |||||||||
| August–September | 1.90 | ||||||||
| December–January | 3.30 | ||||||||
| June | 3.20 | ||||||||
| June–July | 3.75 | ||||||||
| May–June | 4.05 | ||||||||
| Dimethoate | |||||||||
| August | 3.10 | ||||||||
| June | 4.60 | ||||||||
| Emamectin | |||||||||
| July | 0.63 | ||||||||
| September | 0.42 | ||||||||
| Esfenvalerate | |||||||||
| June | 1.00 | ||||||||
| October | 2.10 | ||||||||
| Methomyl | |||||||||
| August | 3.30 | 0.22 | |||||||
| July | 0.85 | 1.82 | |||||||
| June | 2.16 | ||||||||
| October | 4.85 | ||||||||
| September | 4.00 | ||||||||
| Mevinphos | |||||||||
| June | 1.07 | ||||||||
| October | 0.72 | ||||||||
| Phenthoate | |||||||||
| April | 4.88 | ||||||||
| October | 3.13 | ||||||||
| Profenofos | |||||||||
| December–March | 1.20 | ||||||||
| May–July | 1.48 | ||||||||
| September–November | 1.80 | ||||||||
For azinphos‐methyl individual DT50 values ranged from 3.7 to 30 days, showing a wide range of dissipation rates for pear leaves. Since data for pears were collected only from one location from May to June, no influence of seasons nor of geographical areas can be attributed to this difference in dissipation values.
The geographical areas captured in this analysis, all from North America, are considered relevant for European conditions. There was only one study conducted in India (equatorial climate according to Köppen–Geiger criteria), which was not considered representative for European climate zone.
D.3.9 Adjuvants; physical state of the formulations
All DT50 values (except two) were determined from spray application of products. Therefore, the impact of different types of application (e.g. spraying vs. dusting, etc.) on the DT50 values cannot be investigated. Effect of adjuvants cannot be determined neither since only the information is very scarce (adjuvant used only in three studies with three different active substances). Only for emamectin, DT50 values were obtained with and without the use of adjuvants, revealing values of 0.6 and 0.4 d, respectively. The database lacks sufficient number of representative values to conclude on the possible effects of adjuvants on DT50 values.
D.3.10 Meteorological conditions
The most frequent information on meteorological conditions was on temperature and rain. Rain occurred either long after the last application when residue levels were low or before the last treatment. Heavy rain was not reported but, generally, light rain episodes were described. The DT50 values, obtained in trials where rain occurred, were considered not to be severely affected by this variable.
Information on wind conditions was scarcely reported. Wind measurements were reported only in two studies and only in one the wind speed was reported. Wind data, when available, were not discussed by the study authors.
Temperature mean data are available for only four active substances as reported in Table D.11 below.
Table D.11.
DT50 values in relationship to mean temperatures
| Temperature °C | DT50 values | |||
|---|---|---|---|---|
| Captan | Chlorobenzilate | Emamectin | Esfenvalerate | |
| 11 | 12.66 | |||
| 13 | 5.3 | |||
| 15 | 5.5 | 2.1 | ||
| 17.8 | 0.63 | |||
| 18 | 6.83 | |||
| 20 | 2.53 | |||
| 22.9 | 1 | |||
| 24.4 | 8.65 | |||
| 25 | 5.82 | |||
| 29.6 | 9.3 | |||
D.3.11 Analytical methods
Information regarding the dislodgeable residue extraction methods (including the non‐organic solvents as aqueous dilutions) was available for all the studies considered during the data extraction phase. On the other hand, methods for quantification of DFR and LOQ/LOD in reliable studies were available only for limited numbers of data points (21 LOQs and 97 LODs).
As regards the correction of field results for recovery, this information was available for 101 DT50 values out of 153 composing the database. For the remaining 52 DT50 values (34%), no information in this regard was retrieved.
D.3.12 Uncertainties
In the assessment conducted, the following uncertainties in the investigated database and DT50 evaluations were identified:
Since only 7 of 32 active substances investigated in reliable entries are approved in the EU, uncertainty is flagged for appropriateness of the data set for current European approval system.
The evaluated data are not representative for the majority of pesticidal chemical classes; this is considered a source of uncertainty.
Lack and diversity of methodological information (LOD/LOQ, field recovery, quantification methodology) within the retrieved data points add an uncertainty in reported/calculated DT50 values.
Due to limited information available in public papers (rather subject to reporting bias than to mistakes in methodology), there is an uncertainty about the quality of reported data comparing to GLP studies conducted for regulatory purposes.
There is currently no harmonised Guideline for the conduction of DFR studies and elaboration of DT50 values; there is an uncertainty when comparing data points that were retrieved using partially different methodologies.
The most recent data from the evaluated database are from 2007 and the oldest date back to 1971; there is uncertainty in comparison of methodologies in studies conducted over 40‐year time period.
It is recognised that where reported DT50 values were recalculated with a different software, different DT50 values were retrieved. Lack of detailed information on regression analysis in the public literature is considered an uncertainty for the potential refinement of DT50 values.
D.4 Conclusions
Based on the conducted assessment, the following conclusions were derived by the WG:
The limited number of reliable data points (28) for DT50, collected from 15 active substances (only six of them currently approved in the EU), could indicate that the current default value of 30 days for DT50 is probably a conservative value;
However, data points from studies flagged with uncertainties but still reliable partially exceed the current DT50 of 30 days;
The pesticides included in the evaluated data set are not a good representative sample of currently authorised active substances and products in the EU, given that most of the active substances are not (anymore) approved under European legislation;
The range of combinations of pesticides and crops as included in the evaluated database is also not good representative samples since only limited pesticide–crop combinations were part of the data set;
There are too many uncertainties as regards reporting, analytical methods used and quantifications of DFR;
For the original question of how likely it is that an unmeasured DT50 would be 30 or more days if it were measured, the data are clearly relevant but do not give a direct answer due to uncertainty about their representativeness.
Overall, it is finally concluded that the evaluated data do not provide any insight to propose a refinement of the default DT50 value currently in place (30 days).
D.5 Recommendation
It is recommended for the future submissions to assess information on DFR and DT50 values from high‐quality studies, either publicly available or submitted for regulatory purposes in order to conclude if a refinement of the current default DT50 of 30 days covers relevant European scenarios and conditions.
Appendix E – Worker Re‐entry Activities from EU surveys
E.1 Introduction
EFSA has funded two projects related to surveys and data collection, i.e.:
Collection and assessment of data relevant for non‐dietary cumulative exposure to pesticides and proposal for conceptual approaches for non‐dietary cumulative exposure assessment (Glass et al., 2012b), and
Collection of pesticide application data in view of performing Environmental Risk Assessments for pesticides (Garthwaite et al., 2015),
referred to as CAPEX I and CAPEX II, respectively.
The main objective of these projects was to collect data related to cumulative exposure, either non‐dietary human or environmental exposure.
More specifically, the aim of CAPEX I, an 18‐month project, was to address cumulative exposure to plant protection products (PPPs) by means of carrying out pilot surveys in six EU Member States (MS), using a specifically designed survey form. The pilot CAPEX I surveys collected information on a wide range of factors for both operators and workers such as the number of hours worked each day for specific operator and worker tasks, personal protective equipment (PPE) used, etc.
The CAPEX II project, built upon on knowledge and experience of the CAPEX I pilot surveys to collate information on cumulative non‐dietary exposure, aimed in addressing cumulative exposure to PPPs and the potential combined non‐target effects of multiple applications of PPPs. For this purpose, surveys using a specifically designed form were conducted in eight EU MSs, representing the northern (Lithuania), central (Belgium, Netherlands, Poland and United Kingdom) and southern (Greece, Italy and Spain) regulatory zones.
The data obtained within the CAPEX I and II projects have been considered in detail by the WG in order to identify any information that could be used in the update of the EFSA Guidance issued in 2014, as regards worker re‐entry activities and related parameters.
E.2 Data and methodologies
E.2.1 Data
E.2.1.1 CAPEX I (CFT/EFSA/PPR/2010/04)
The objective of the CAPEX I project was to establish a database including PPP usage data and information relevant for the non‐dietary exposure of operators and workers to PPPs. The database contains existing data regarding tasks carried out by operators and workers which have been collated and reviewed by the project consortium in addition to new relevant data collected as part of a pilot survey. CAPEX I database aimed in providing EFSA with information relevant for the development of a methodology assessing the risk of cumulative non‐dietary exposure to PPPs for operators and workers, i.e. risk resulting from exposure to multiple active substances used for crop protection and via carrying out different tasks from PPP application to re‐entry tasks, such as inspection, maintenance tasks and harvesting.
As a first step in the collation of available data, a farm questionnaire was designed for the surveys. The questionnaire consisted of six forms, with each form being dedicated in capturing different data/information in order to facilitate further analysis related. Data collected/recorded concerned cropping details, farm business and PPP application data, operator and worker related data.
More specifically, regarding operator data, the data collected related to the principal operator in each farm and ranged from age and gender to the percentage spraying undertaken, the acquired qualifications, the time spent for each task and the PPE worn during mixing/loading, application, sprayer cleaning or other work activities resulting in exposure to PPPs.
The available information regarding the application/spraying equipment used were also collated.
Regarding worker‐related data, all available details of the work activities conducted were collated, such as the activity type, date, crop stage, time since last pesticide application and time (hours) spent.
The surveyed countries and the crops included in the CAPEX I surveys are presented in Table E.1.
Table E.1.
Crop types selected for the pilot surveys within CAPEX I (Glass et al., 2012b)
| Country | Crops | Number of holdings |
|---|---|---|
| Belgium | Greenhouse ornamentals | 48 |
| Outdoor vegetables | 50 | |
| Greece | Greenhouse vegetables | 25 |
| Arable (cotton/maize) | 25 | |
| Italy | Wine Grapes | 50 |
| Poland | Arable (wheat) | 52 |
| Orchard (apple) | 52 | |
| Spain | Greenhouse vegetables | 50 |
| UK | Arable Crops | 49 |
| Soft Fruit Crops | 26 |
The pilot surveys conducted within CAPEX I project covered PPP applications over 3 years (2011–2012) and provided data which could be considered to be representative of the agricultural conditions and PPP use only for specific crops surveyed and for the geographical areas surveyed (Italy, Belgium, Greece and Spain). However, considering taking into account the small sample size surveyed (428 farms, 581 operators, 749 sprayers, 481 workers), it cannot be considered to be a representative national sample, as well, at least for most of the countries surveyed. This has been also noted by the CAPEX I consortium.
It is noted that within CAPEX I the data obtained from a survey amongst workers in flower greenhouses conducted in the Netherlands in 2002—2003 by TNO in collaboration with the Radboud University (Nijmegen, the Netherlands), were also presented. Since only an overview of these surveys had been available to the CAPEX I Consortium, the assessment of the relevant data had not been possible within CAPEX. Thus, it was also not possible for the WG to further check these data and further consider them for the update of the EFSA Guidance issued in 2014.
E.2.1.2 CAPEX II (CTF/EFSA/PRAS/2012/05)
The overall objective of the project was the collection of detailed data on PPP applications from farms in the three regulatory zones of the EU as defined in Annex I to Regulation (EC) 1107/2009, producing crops for direct consumption (such as potatoes and wheat) and crops for processing (such as oilseeds and sugar beet). The data were related not only to PPP applications and additional activities that are considered relevant for operator and worker exposure to pesticides but also related to environmental exposure. In this context, pesticide application data were collected not only over a period of 1 year but also for the preceding 4 years if available.
For the CAPEX II surveys, a more farm detailed than in CAPEX I questionnaire was designed including eight different forms in order to collate all information/data identified as relevant for the project objectives. More specifically the following data were captured in the database (Garthwaite et al., 2015):
Cropping types and area grown in 2013
Farm business details including, size, location, number of spray operators and use of agronomists, buffer strips and Integrated Pest Management (IPM)
Pesticide application details for the principal spray operator on the farm, including date, crop stage, product, method of application, application rate, area treated, start time and duration of application.
Pesticide application details for the environmental field which was collected the same information as Form 3 and off‐ and in‐field margin information and all applications, not just the principal operator and where possible for the application details for the five previous years.
Information on the principal operator, age, gender, percentage spraying undertaken, qualifications, time and PPE worn during mixing and loading, PPE worn during application, time and PPE worn during sprayer cleaning and the PPE worn during other work activities that may contribute to their pesticide exposure.
Details of the sprayers on the farm including make, model, age, tank capacities, filling systems, cab type, age and nozzle sets.
Details of other work activities, including date, crop stage, time since last pesticide application, activity types and number of hours.
Details of non‐crop pesticide application including method of application, product, PPE, mass of product and duration.
Along with any other worker activities that might be considered as resulting to additional pesticide exposure.
The surveys were conducted based on the experience gained within CAPEX I with specific instructions provided to surveyors.
The surveyed countries and the crops are presented in Table E.2.
Table E.2.
The country surveyed per crop selected for the CAPEX II surveys (Garthwaite et al., 2015)
| Crops | Countries |
|---|---|
| Wheat | Lithuania, Poland, UK |
| Potatoes | Belgium, Lithuania, Netherlands |
| Oilseed rape | Lithuania, UK |
| Maize | Belgium, Italy, Poland |
| Sugar beet | Belgium, UK |
| Apples | Italy, Poland, UK |
| Citrus | Greece, Spain |
| Grapes | Greece, Italy, Spain |
| Vegetables | Greece, Italy, Netherlands, Spain, Poland |
A picture of the overall data (both related to operator exposure and the environment) collected within the CAPEX II surveys and all related information per survey form are summarised in Table E.3.
Table E.3.
Summary of the operator exposure and environmental data collated within CAPEX II (Garthwaite et al., 2015)
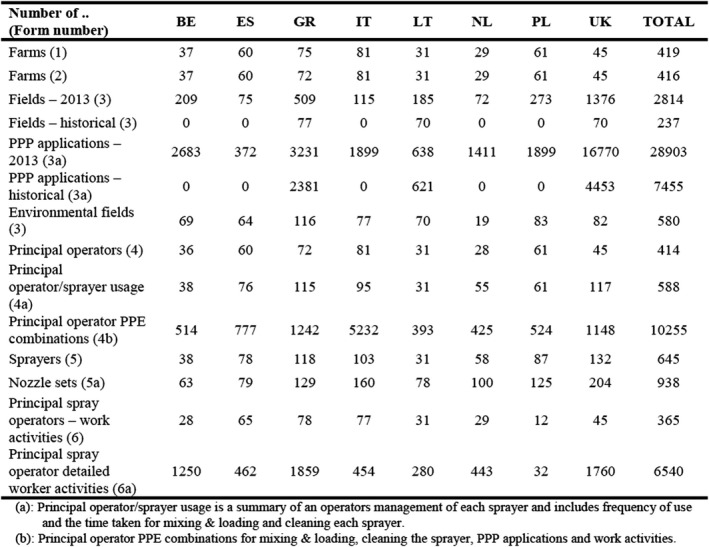
The surveys covered PPP applications over 6 years (2008–2013).
E.2.2 Methodologies
The data collated within both CAPEX I and II, as summarised in the final project reports, were reviewed in order to identify any information that could be used in the update of the guidance issued in 2014. More specifically, the data collated were reviewed with focus on the assessment of worker long‐term exposure and the related re‐entry tasks in order to conclude on whether there should be any changes in the assumptions made in the guidance and/or in case additional parameters/tasks should be taken into account.
It is noted that most of the data collated within these projects related to the cumulative exposure to PPPs, either for non‐dietary human exposure or for environmental exposure assessment purposes. Thus, a significant amount of data such as the number of different PPPs and/or active substances applied in one specific field or by the same operator within a specific time zone, the different consecutive tasks that may be performed by the same person were not further assessed.
E.3 Assessment
Based on the results of the project CAPEX I, a list of re‐entry activities for workers in different countries and for different crops has been identified (see Table E.4). Within the CAPEX II project, limited information is included regarding worker activities; tasks such as crop rogueing, drilling/filling, inspection, vertebrate control measures, fertiliser spreading and spraying have been recorded.
Table E.4.
List of re‐entry activities for workers in different countries and for different crops
| Crop (Country) | Re‐entry task |
|---|---|
|
Melon (Greece) |
Cutting |
| Thinning | |
| Propping/training branches | |
| Watering | |
| Inspection | |
| Removing shoots | |
| Planting | |
|
Tomato (Greece) |
Watering |
| Inspection | |
| Sorting | |
| Planting | |
| Thinning | |
| Packaging | |
| Picking/cutting | |
|
Aubergines (egg plants) (Spain) |
Planting |
| Propping/Training Branches | |
| Removing Shoots | |
| Manual Lifting | |
| Inspection/Maintenance | |
| Leaf Removal | |
|
Pot plants (Belgium) |
Cutting |
| Taking Cuttings | |
| Propping/Training Branches | |
|
Table & wine grapes (Italy) |
Propping/Training Branches |
| Removing Shoots | |
| Leaf Removal | |
| Pruning | |
| Thinning |
With regard to the use of protective equipment by the workers, the results of CAPEX I have identified workers wearing either a T‐shirt, a long‐sleeved shirt, a workwear (cotton) or two‐piece rainwear (vinyl, Gore‐Tex, etc.), leather/fabric boots but no respiratory protective equipment.
E.4 Conclusions
Overall, based on the CAPEX I and II survey data and taking into account the current data on worker activities and related transfer coefficient (TC) data, no revision of the worker re‐entry activities (regarding the ‘nature of task’) and related parameters (e.g. TC) can be proposed at this stage.
E.5 Recommendation
Among the worker activities recorded within CAPEX I and II, at least one re‐entry activity, i.e. planting, has been identified as not covered by those re‐entry tasks already included in the EFSA Guidance (see Table E.4). Considering that planting in a field treated previously with a PPP is an activity that may lead to worker exposure to PPP (herbicides mostly) through contact to soil and not to foliage, this pathway of exposure (that might be considered relevant also for other re‐entry tasks following PPP application to bare soil) should be further considered when relevant data are available (see also Appendix I).
Appendix F – Transfer Coefficients from US data
F.1 Introduction
As discussed in this guidance, limited data are available to EFSA to establish transfer coefficients to estimate exposures for the wide range of crops and activity scenarios in which workers may potentially encounter exposure to pesticide residue. Historically the situation in North America was similar, then in 1995 due to concerns that their screening approach was not adequately addressing the variation in crops and work tasks the US EPA issued a Data‐Call‐In (DCI) to all registrants to support crop entry exposure assessments for agricultural crop registrations. To address the significant demand that the DCI presented the North American industry formed the Agricultural Re‐entry Task Force (ARTF). The ARTF, worked with the authorities in North America (California, Canada, USDA and the US EPA) to develop an agronomically based, task‐specific approach to assessing worker exposure. The approach assumed that tasks can be grouped or ‘clustered’ into similar crop defined activities, that are ergonomically alike with similar potential for contact with pesticide residue. To support this approach, the ARTF conducted 47 exposure studies.
These ARTF data have not been published, nor submitted by industry to EFSA, and are therefore not available for EFSA to review. However, the US EPA has described their use of the data in a published internal guidance document ‘Science Advisory Council for Exposure (ExpoSAC) Policy 3 ‐ Revised January 2017’, (US EPA, 2017). This policy document provides information on the individual ‘clusters’ agreed by the EPA, and the recommended corresponding transfer coefficients, based on the arithmetic mean values of the individual cluster measurements.
The EPA also published some additional information on the data when their FIFRA Scientific Advisory Panel was charged with reviewing the ARTF data and approach in 2008. Although the commercially protected data were not published, the US EPA did issue a summary spreadsheet that provides for each individual monitoring event, the work task duration, the environmental residue concentration and the individual TC value for each individual subject (US EPA, 2008). This extra detail provides some information on the distribution of individual TCs within clusters which is useful to compare with the data available to EFSA.
F.2 Data and methodologies
F.2.1 Data
The US EPA Office of Pesticide Programs, Health Effects Division, Science Advisory Council for Exposure Policy Number 3, January 2017, presents the TC derived by the US EPA from the industry Agricultural Re‐entry Exposure Task Force (ARTF) data submitted in response to US EPA data requests (US EPA, 2017).
Recognising the impracticality of providing data specific to every use, the US EPA accepted an approach that grouped crops, crop growth stages and post‐application activities into clusters that are expected to share similar exposures (as indicated by similar individual TC values). In part, as well as being informed by the results of the large volume of re‐entry exposure data, this approach was constructed using information from detailed surveys of post application activities and advice on agricultural and horticultural activities which are also part of the protected data which are not available for further independent examination.
Table F.1 gives the details of the different clusters recognised by the US EPA and indicates the crops and activities monitored to provide the exposure data to support the derivation of the generic TC values for the clusters. The EPA approach to assessing post application exposures assumes that agricultural field workers wear shoes, socks, long trousers and long‐sleeved shirts and the TC values therefore reflect this clothing assumption. The TC values for most clusters are based on dislodgeable foliar residue (DFR) as measured in the supporting data, except for some mechanical harvesting activities related to cotton (where the TC is based on boll residue) and for sod (i.e. turf) and golf course activities (where the TC is based on turf transferable residue).
Table F.1.
US EPA Crop Activity Clusters and Supporting Crop Activity Data (US EPA, 2017)
| EPA TC Cluster | Supporting data | ||
|---|---|---|---|
| Code | Description | Crop | Activity |
| HH | Hairy‐leaf field crops: hand harvesting and similar contact activities | Cucumbers | Hand Harvesting |
| Summer Squash | Hand Harvesting | ||
| HHt | Hairy‐leaf (Tobacco): hand harvesting and canopy management | Tobacco | Hand harvesting |
| HS | Hairy‐leaf field crops: scouting and similar contact activities | Sunflowers | Scouting |
| SH | Smooth‐leaf field crops: hand harvesting and tying | Tomato | Tying |
| Strawberry | Hand Harvesting | ||
| Tomato | Hand Harvesting | ||
| Strawberry | Hand Harvesting | ||
| SSr | Smooth‐leaf field crops: scouting in row conditions | Cotton | Scouting |
| Tomato | Scouting | ||
| SSs | Smooth‐leaf field crops: scouting in solid stand conditions | Corn | Scouting |
| Dry Pea | Scouting | ||
| SW | Smooth‐leaf field crops: hand weeding, thinning and similar contact activities | Cotton | Hand weeding |
| Cotton (2nd study) | Hand weeding | ||
| Dry Pea | Hand weeding | ||
| Sx (EPA) | Smooth‐leaf field crops: intense contact activities | Sweet Corn | Hand harvesting |
| Sweet Corn (2nd study not reported) | Hand harvesting | ||
| WIH | Waxy‐leaf field crops, low height: hand harvesting and similar contact activities | Cabbage | Hand harvesting |
| WIS | Waxy‐leaf field crops, low height: scouting and similar contact activities | Cauliflower | Scouting |
| Wm | Waxy‐leaf field crops, medium height: all activities, plus full foliage weeding | Cauliflower | Scouting |
| Cauliflower | Hand harvesting | ||
| Cabbage | Hand weeding | ||
| OH (EPA) | Orchard crops: hand harvesting and similar contact activities | Apples | Hand Harvesting |
| Oranges | Hand Harvesting | ||
| Oranges (2nd study) | Hand Harvesting | ||
| Grapefruit | Hand Harvesting | ||
| Peaches | Hand Harvesting | ||
| Peaches (2nd study) | Hand Harvesting | ||
| Peaches (3rd study) | Hand Harvesting | ||
| OT (EPA) | Orchard crops: thinning | Apples | Thinning |
| OHn | Orchard crops: mechanically harvesting nuts | Almonds | Mechanical Harvesting |
| OP | Orchard crops: hand pruning, scouting and similar contact activities | Olives | Hand Pruning |
| Apples | Hand Pruning | ||
| OW | Orchard crops: hand weeding and similar contact activities | Peaches | Propping |
| THb | Trellis crops: hand harvesting cranberries and similar contact activities | Blackberries | Hand harvesting |
| THjg (EPA) | Trellis crops: hand harvesting juice/wine grapes and similar contact activities | Juice/Wine Grapes | Hand harvesting |
| THrg (EPA) | Trellis crops: hand harvesting table/raisin grapes and similar contact activities | Table/Raisin Grapes | Hand harvesting |
| Table/Raisin Grapes (2nd study) | Hand harvesting | ||
| TP | Trellis crops: hand pruning, scouting and similar contact activities | Table/Raisin Grapes | Scouting |
| Tx | Trellis crops: intense contact activities | Table/Raisin Grapes | Cane turning |
| GHf | Greenhouse and nursery floriculture hand harvesting: all flowers and methods | Solidasters, Snapdragons, Lillies | Hand Harvesting |
| GHv | Greenhouse vegetables: hand harvesting and similar contact activities | Blackberries | Hand Harvesting |
| Tomatoes, fresh | Tying | ||
| GN | Greenhouse and nursery crops: all activities | Chrysanthemums | Pinching |
| Nursery Stock Citrus Trees | Hand Pruning | ||
| All crops: transplanting | Nursery Stock Citrus Trees | Hand Harvesting | |
| I | Irrigation, any crop where hand line is possible | Potatoes | Irrigation |
| CHp | Cotton, mechanical harvesting: picker operator and raker (based on boll residue) | Cotton | Mechanical Harvesting |
| CHm | Cotton, mechanical harvesting: module builder operator (based on boll residue) | ||
| CHt | Cotton, mechanical harvesting: tramper (based on boll residue) | ||
| DH | Sod: mechanical harvesting, scouting, transplanting and hand weeding | Sod | Mechanical Harvesting |
| DM | Golf courses: maintenance activities | Golf Course Turf | Maintenance |
The EPA recommendations for the cluster TC values are derived from the arithmetic mean of the individual data in each cluster.
Although, the ExpoSAC document does not provide details on the individual data some further information relating to these have been published by the US EPA in the agency’s submission to the FIFRA SAP in 2008 (US EPA, 2008). This publication takes the form of a spreadsheet that provides for each individual monitoring event in the ARTF data set the following: study identification; crop; crop height (low/high); foliage density (min/full); work task activity (hand harvesting/scouting/hand weeding/mechanical harvesting/irrigation non‐hand set/irrigation hand‐set/transplanting/hand pruning); pesticide active substance; applied dose of pesticide; ARTF proposed cluster; entry day, i.e. days after pesticide application; monitoring unit number (unique monitoring event identifier); subject identifier (single letter label); gender; age; years of experience; hours worked and monitored; residue concentration (i.e. DFR, turf transferrable residue (TTR) or boll transferrable residue (BTR)); and TC.
The individual TC values were derived from a consideration of the individual DFR (or TTR or BTR), the activity duration and the unreported corresponding exposure value.
F.2.2 Methodologies
It should be noted that without access to the data supporting these values, e.g. the detailed study reports, these data cannot be considered to be independently validated by EFSA. Therefore, the objective of this analysis was limited to comparing the distributions of TC within the overall ARTF data set and to consider how at face value these data relate to those available to EFSA.
This was done visually by plotting individual TC data by ‘cluster’ with summary boxplots showing the median, 25th and 75th centiles (the plot whiskers show the smallest and largest values observed within 1.5 times the interquartile range from the median). The distributions of TC within clusters were assumed to be from lognormal distributions (although this was not formally tested) and the boxplots and individual TC data were plotted on log scales.
As already noted, the EPA recommends arithmetic mean values to represent the TC for each cluster. As the residue value used to derive the TC is also a mean value, this suggests the exposure estimates derived using the EPA TC will also be representative of the mean values. This contrasts with the position of EU risk managers who charged EFSA in producing the EFSA Guidance issued in 2014 (EFSA, 2014) to provide estimates of the 75th and 95th centiles of exposure. Therefore, for this comparison 75th and 95th centile empirical, non‐parametric, estimates were calculated for each cluster (using MS Excel).
F.3 Assessment
Figure F.1 provides an overview of all the ARTF data, which the EPA arranged into 30 individual clusters. Cluster codes are given in Table F.1
Figure F.1.
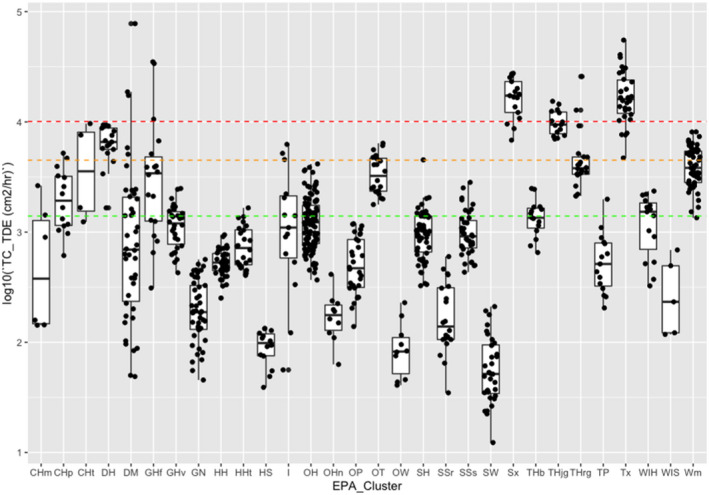
- Cluster group prefixes are: C = cotton; D = turf; G = greenhouse; H = hairy field crops; O = orchard crops; S = smooth field crops; T = trellis crops; and W = waxy field crops. Each group is shown separately in more detail below. The dashed red line is the current EFSA grapes TC, the dashed orange line = EFSA tree fruits TC and the dashed green line is the EFSA general TC. These EFSA TCs, and other values discussed below, assume workwear and bare hands so are analogous to the EPA approach.
Figure F.2 shows the data for tree and bush crops.
Figure F.2.
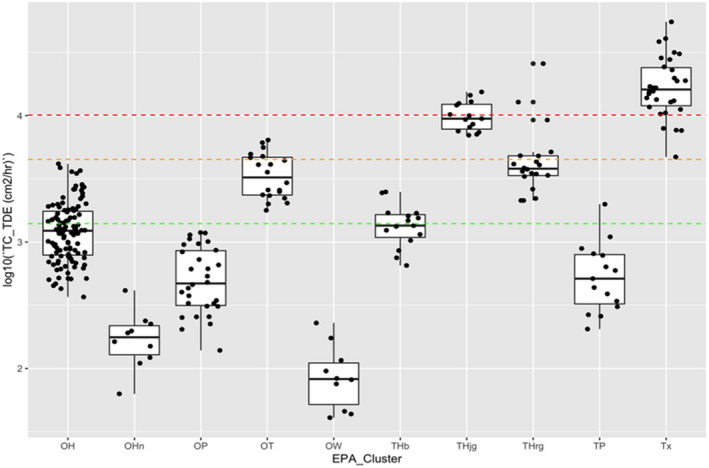
- The orchard Clusters are: OH = hand harvest; OHn = mechanical harvest nuts; OP = hand pruning, scouting, & similar; OT = thinning; and OW = weeding and similar. Trellis clusters are: THb = hand harvest cane berries; THjg = hand harvest juice/wine grapes; THrg = hand harvest raisin grapes; Tp = hand pruning, scouting and similar; and Tx = intense contact (cane turning). The dashed red line = EFSA grapes TC; dashed orange line = EFSA Tree fruits TC, and the dashed green line = EFSA General TC.
Figure F.3 shows the less extensive data for greenhouse crops.
Figure F.3.
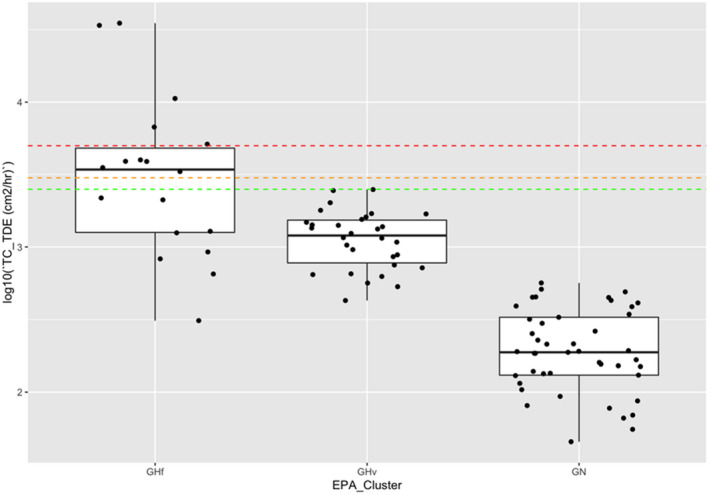
- The three clusters are: GHf = Floriculture hand harvesting; GHv = vegetables hand harvesting and similar; and GN = greenhouse & nursery all activities. For the greenhouse crops, the EFSA TCs are dashed red line = Ornamentals, dashed orange line = Strawberries and dashed green line = Vegetables.
The EPA divided field crops on the basis of leaf type, as these were considered to influence DFR and hence exposure. All these field crop clusters are shown in Figure F.4. Clusters were set for hairy, smooth and waxy leaves as follows.
Figure F.4.
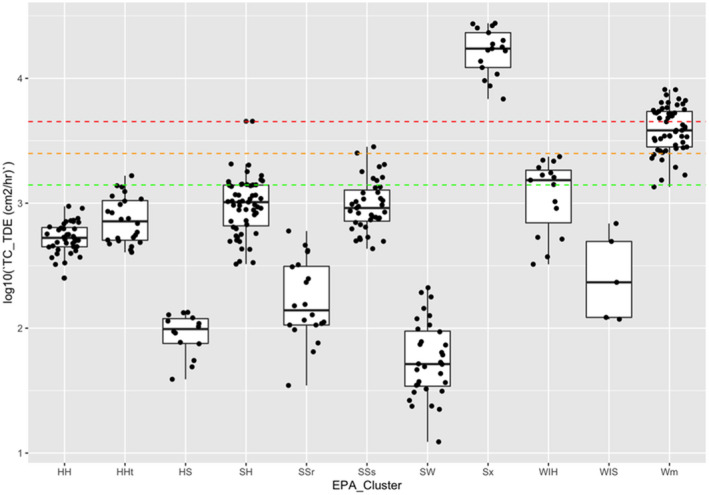
- Hairy leaf clusters: HH = hand harvest; HHt = tobacco hand harvest, canopy management; and HS = scouting and similar. Smooth leaf clusters: SH = hand harvest & tying; SSr = row conditions scouting; SSs = solid stand scouting; SW = weeding, thinning and similar; and Sx = intense contact (e.g. hand harvest sweet corn). Waxy leaf clusters: WIH = low height hand harvest and similar; WIS = low height scouting; and Wm = medium height all activities, full foliage weeding. Dashed lines are: red = EFSA Tree fruit TC, orange = EFSA Vegetables TC and green = EFSA General TC.
Figure F.5 shows the two EPA clusters for turf activities. However, it should be noted that the TC for turf are based on TTR not DFR so it is not appropriate to compare these data to TC derived from DFR values.
Figure F.5.
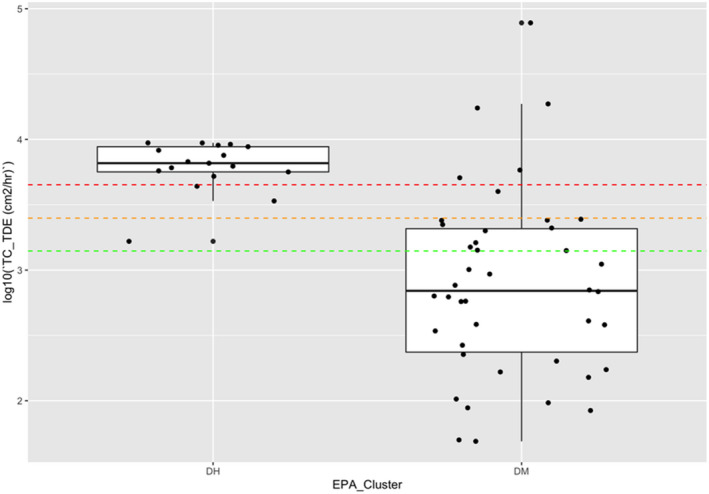
- DH = sod harvesting, scouting, transplanting and hand weeding DM = golf course maintenance. The comparisons are red line = EFSA Tree fruit TC, orange line = EFSA Golf course (Vegetables) TC, and green line = EFSA General TC.
The EPA also established three clusters for cotton mechanical harvesting activities, see Figure F.6.
Figure F.6.
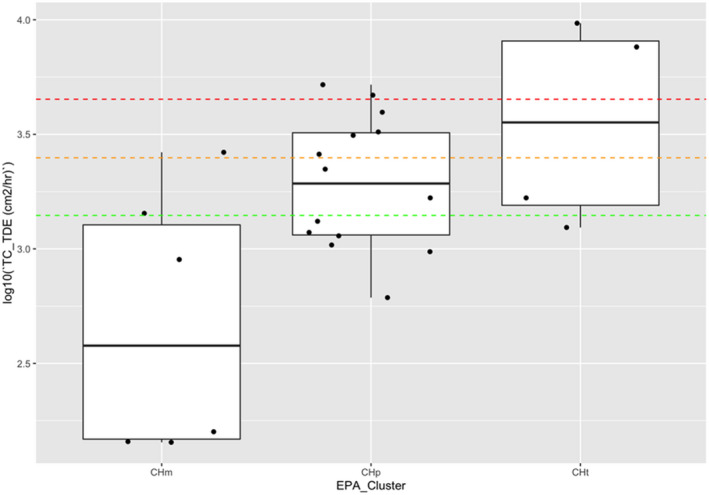
EPA Cotton mechanical harvesting activity clusters, showing individual log10 TC values for total dermal exposure (TDE, cm2/h)
The activities are: CHm = module builder operator; CHp = picker operator & raker; and CHt = tramper. Here the red line = EFSA Tree fruit TC, orange line = EFSA Vegetables TC, and green line = EFSA General TC. Although, again it should be noted that the cotton TCs are derived from boll residue not DFRs.
As already stated, the EPA based their TC value recommendations on the arithmetic average of the individual TC for each cluster. The 75th and 95th centiles of the individual TC values in each cluster calculated here are shown in Table F.2.
Table F.2.
Estimated 75th and 95th centiles TC for the US EPA Crop Activity Clusters
| EPA TC cluster | Supporting data | EFSA estimated Values | ||||
|---|---|---|---|---|---|---|
| Code | Description | Crop | Activity | 75th, 95th centiles & max | ||
| HH | Hairy‐leaf field crops: hand harvesting and similar contact activities | Cucumbers | Hand Harvesting | 640 | 773 | 946 |
| Summer Squash | Hand Harvesting | |||||
| HHt | Hairy‐leaf (Tobacco): hand harvesting and canopy management | Tobacco | Hand harvesting | 1,050 | 1,376 | 1,660 |
| HS | Hairy‐leaf field crops: scouting and similar contact activities | Sunflowers | Scouting | 119 | 133 | 134 |
| SH | Smooth‐leaf field crops: hand harvesting and tying | Tomato | Tying | 1,395 | 1,859 | 4,530 |
| Strawberry | Hand Harvesting | |||||
| Tomato | Hand Harvesting | |||||
| Strawberry | Hand Harvesting | |||||
| SSr | Smooth‐leaf field crops: scouting in row conditions | Cotton | Scouting | 313 | 469 | 600 |
| Tomato | Scouting | |||||
| SSs | Smooth‐leaf field crops: scouting in solid stand conditions | Corn | Scouting | 1,275 | 2,088 | 2,830 |
| Dry Pea | Scouting | |||||
| SW | Smooth‐leaf field crops: hand weeding, thinning, and similar contact activities | Cotton | Hand weeding | 95 | 185 | 211 |
| Cotton (2nd study) | Hand weeding | |||||
| Dry Pea | Hand weeding | |||||
|
Sx (EPA) |
Smooth‐leaf field crops: intense contact activities | Sweet Corn | Hand harvesting | 23,200 | 27,360 | 27,600 |
| Sweet Corn (2nd study not reported) | Hand harvesting | |||||
| WIH | Waxy‐leaf field crops, low height: hand harvesting and similar contact activities | Cabbage | Hand harvesting | 1,840 | 2,255 | 2,360 |
| WIS | Waxy‐leaf field crops, low height: scouting and similar contact activities | Cauliflower | Scouting | 495 | 650 | 689 |
| Wm | Waxy‐leaf field crops, medium height: all activities, plus full foliage weeding | Cauliflower | Scouting | 4,990 | 5,902 | 6,130 |
| Cauliflower | Hand harvesting | |||||
| Cabbage | Hand weeding | |||||
| OH (EPA) | Orchard crops: hand harvesting and similar contact activities | Apples | Hand Harvesting | 1,750 | 2,185 | 55,200 |
| Oranges | Hand Harvesting | |||||
| Oranges (2nd study) | Hand Harvesting | |||||
| Grapefruit | Hand Harvesting | |||||
| Peaches | Hand Harvesting | |||||
| Peaches (2nd study) | Hand Harvesting | |||||
| Peaches (3rd study) | Hand Harvesting | |||||
| OT (EPA) | Orchard crops: thinning | Apples | Thinning | 4,678 | 6,125 | 6,410 |
| OHn | Orchard crops: mechanically harvesting nuts | Almonds | Mechanical Harvesting | 218 | 335 | 414 |
| OP | Orchard crops: hand pruning, scouting and similar contact activities | Olives | Hand Pruning | 857 | 1,162 | 1,190 |
| Apples | Hand Pruning | |||||
| OW | Orchard crops: hand weeding and similar contact activities | Peaches | Propping | 111 | 204 | 292 |
| THb | Trellis crops: hand harvesting caneberries and similar contact activities | Blackberries | Hand harvesting | 1,650 | 2,462 | 2,490 |
| THjg (EPA) | Trellis crops: hand harvesting juice/wine grapes and similar contact activities | Juice/Wine Grapes | Hand harvesting | 12,300 | 14,770 | 15,400 |
|
THrg (EPA) |
Trellis crops: hand harvesting table/raisin grapes and similar contact activities | Table/Raisin Grapes | Hand harvesting | 4,805 | 13,450 | 25,800 |
| Table/Raisin Grapes (2nd study) | Hand harvesting | |||||
| TP | Trellis crops: hand pruning, scouting and similar contact activities | Table/Raisin Grapes | Scouting | 797 | 1,367 | 1,990 |
| Tx | Trellis crops: intense contact activities | Table/Raisin Grapes | Cane turning | 23,975 | 39,710 | 55,200 |
| GHf | Greenhouse and nursery floriculture hand harvesting: all flowers and methods | Solidasters, Snapdragons, Lillies | Hand Harvesting | 4,843 | 34,018 | 35,045 |
| GHv | Greenhouse vegetables: hand harvesting and similar contact activities | Blackberries | Hand Harvesting | 1,498 | 2,214 | 2,490 |
| Tomatoes, fresh | Tying | |||||
| GN | Greenhouse and nursery crops: all activities | Chrysanthemums | Pinching | 328 | 483 | 565 |
| Nursery Stock Citrus Trees | Hand Pruning | |||||
| All crops: transplanting | Nursery Stock Citrus Trees | Hand Harvesting | ||||
| I | Irrigation, any crop where hand line is possible | Potatoes | Irrigation | 2,135 | 5,115 | 6,260 |
| CHp | Cotton, mechanical harvesting: picker operator and raker (based on boll residue) | Cotton | Mechanical Harvesting | 3,213 | 4,872 | 5,210 |
| CHm | Cotton, mechanical harvesting: module builder operator (based on boll residue) | 1,297 | 2,338 | 2,640 | ||
| CHt | Cotton, mechanical harvesting: tramper (based on boll residue) | 7,935 | 9,485 | 9,660 | ||
| DH | Sod: mechanical harvesting, scouting, transplanting and hand weeding | Sod | Mechanical Harvesting | 8,800 | 9,402 | 9,410 |
| DM | Golf courses: maintenance activities | Golf Course Turf | Maintenance |
2,075 2,250* |
16,821 13,929* |
77,900 187,700* |
| *Greens only | ||||||
Finally, the EPA also established a cluster for line irrigation in all crops. See Figure F.7.
Figure F.7.
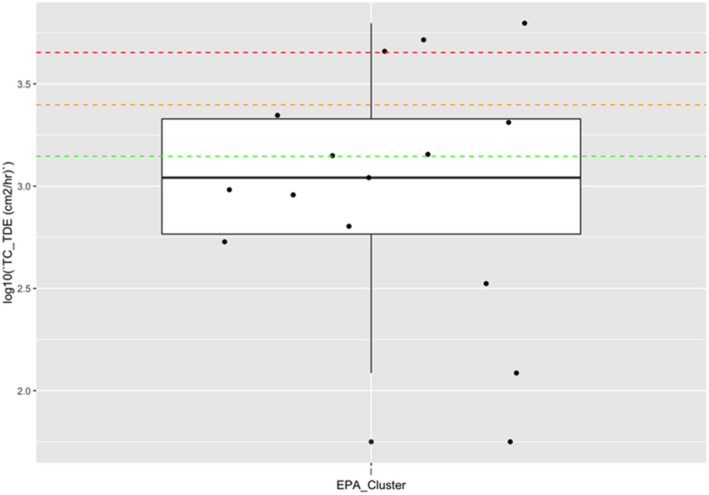
- The red line = EFSA Tree fruit TC, orange line = EFSA Vegetables TC and green line = EFSA General TC.
F.4 Conclusions
The ARTF studies obviously provide substantial information regarding worker exposures to pesticide residue. However, without access to the supporting data, it is not possible to adequately validate the information, nor to achieve the level of transparency required by EFSA’s policy. Therefore, the information can only be considered to be indicative and its use in the context of this guidance limited to a comparative check of the more restricted data and conclusions drawn from the data available to EFSA.
The current guidance includes seven sets of TC recommendations, which are mainly 75th centile‐based values. Considering the TCs for the workwear scenario, these compare to the ARTF data as follows:
F.4.1 Vegetables
The EFSA TC is 2,500 cm2/h, and this applies to harvesting both outdoor and protected (greenhouse) crops. The EPA greenhouse vegetable TC are lower. The EPA hairy‐leaf and smooth‐leaf (except sweet corn) harvest activities are also lower. EPA TC for harvesting low height waxy‐leaf crops are also within the EFSA value.
The EFSA TC appears not to be protective for harvesting sweet corn in cases where there is potential for intense contact. The observed 75th centile was over 920% of the EFSA value. Similarly, the EPA TC for all activities in medium height waxy‐leaf crops frequently exceed the EFSA TC and the 75th centile was about 200% of the EFSA value.
F.4.2 Tree fruits
The EFSA TC is 4,500 cm2/h. Most of the EPA TC observed in orchard crops lie below this value. The thinning activity gave the highest TC, and the observed 75th centile is slightly higher than the EFSA value.
F.4.3 Grapes
The EFSA TC is 10,100 cm2/h. This was established in part by reference to information from the USA. The EPA data for harvesting cane berries are clearly lower than the EFSA value. The data for harvesting raisin grapes also exhibit a 75th centile lower than the EFSA value.
The 75th centile for harvesting juice/wine grapes is about 20% higher than the EFSA value. Although this difference is not considered to be significant. The EPA have also established a cluster for more intense contact activity (cane turning) that has a 75th centile 240% of the EFSA value.
F.4.4 Hops
The US data do not include exposure measurements to support TCs for work activities in hops. However, the US EPA and the Canadian authorities both originally used the TC derived for cane turning in grapes (considered to be the most intense contact cluster in grapes) for hop harvesting. Then, in 2015, the US EPA, based on information regarding the high level of mechanisation in the USA, changed their position to accept a much lower TC, representative of hand and forearm exposure only, for mechanically assisted hop harvesting, and also concluded that no TC was required for fully mechanical hop harvesting (US EPA, 2017). However, Health Canada reviewed harvesting practices in Canada and found less mechanisation and potential for high exposure activities during cutting bines such as loading the bines into the truck/trailer, which included body exposure, loading the bines on the stripping table and stripping by hand. As such activities could involve significant contact between foliage and workers, Health Canada did not revise the TC for mechanically assisted hop harvesting. They did accept in some situations hop harvesting could be completely mechanical.
Information on European hop harvest practices shows similar work practices to those reported in Canada, with a potential for body and hands foliage contact when cutting and loading bines into the trailer, followed by potentially hand and forearm contact when stringing the bines up prior to mechanical picking. As such, mechanically assisted hop harvesting is not considered to be a no or very limited worker exposure scenario. In the absence of specific data measured on hops, the current EFSA TC for grape harvesting is assumed to be a suitable surrogate for harvesting hops.
F.4.5 Strawberry
The EFSA TC is 3,000 cm2/h. Strawberries were one of the crops where exposure was monitored in the smooth‐leaf field crop harvest cluster. The observed 75th centile was less than half the EFSA value.
F.4.6 Ornamentals
The EFSA TC is 5,000 cm2/h. The EPA cluster for floriculture hand harvesting has an observed 75th centile that is marginally below this value.
F.4.7 Golf course, turf or other sports lawns
The EFSA TC is 2,500 cm2/h. The EPA have established two TC clusters for turf. The cluster for golf course maintenance exhibits a very wide spread in the data (the EPA considered there was insufficient data to create subcategories showing less variation and the EPA also noted that daily work routines typically involve mixed maintenance activities, so it may be inappropriate to assume only a limited range of actions). The observed 75th centile is lower than the EFSA TC. The second cluster is for turf farming.
The data available for turf (sod) harvesting show less variation than the golf‐course data, and they appear to be typically higher. The observed 75th centile TC value is 350% times the EFSA TC.
F.4.8 General activities
The EFSA TC is 1,400 cm2/h for crop inspection and irrigation. The EPA clusters include orchard mechanical harvest of nuts, orchard pruning, scouting and similar, and orchard weeding for which all the observed TCs are lower than the EFSA value. The EPA cluster for hand pruning, scouting and similar in trellis crops has one value above the EFSA TC, but the observed 75th centile is clearly lower.
The EPA include a cluster for all activities in greenhouse and nursery crops. The observed 75th centile for these activities is about 330 cm2/h, well within the EFSA TC.
The EPA also include a cluster for line irrigation. This cluster shows wide variation within the data, and the observed 75th centile TC is about 50% more than the EFSA TC.
The EPA also include clusters for mechanical harvesting of cotton. However, the EFSA Guidance does not include equivalent information.
F.5 Recommendation
Taken at face value, i.e. without the expected level of validation and transparency, comparison of the EPA ARTF TCs and the values used by EFSA suggests in the main that values in the EFSA Guidance issued in 2014 are likely to provide the levels of protection that risk managers asked for when considering risks from repeated exposures.
There are, however, indications of a limited number of situations where the predicted levels of exposure may be markedly lower than required. It is considered that, although full supporting data were not available for review, it is appropriate to use the more protective TC values published by a competent authority. The EPA identified two field crop situations where exposures may be higher than suggested in data available to EFSA: harvesting sweet corn in cases where there is potential for intense contact and all activities in medium height waxy‐leaf crops.
The other situation where a higher TC appears to be necessary is for turf harvesting, where it appears that intensive manual handling when staking turves is the issue.
In order to maintain adequate protection of human health for these three scenarios, it is recommended to replace the EFSA TC values with values from Table F.3, as follows:
Table F.3.
Updated Transfer coefficient values
| Activity description and examples | TC workwear and bare hands |
|---|---|
| Smooth‐leaf field crops: intense contact activities hand harvesting sweetcorn | 23,200 cm2/h |
|
Waxy‐leaf crops: medium height crop activities and full foliage activities( a ) Brussel sprouts – inspection, hand harvest, topping, hand weeding in low crop, full foliage; hand weeding in low crop, min foliage; and scouting, and hand harvest, in high crop, full foliage. Cabbage – hand weeding in low crop, full foliage. Cauliflower – inspection, hand harvest, tying/training, hand weeding in low crop, full foliage. Chinese Cabbage, Bok Choy hand weeding in low crop, full foliage. Chinese Cabbage, Napa hand weeding in low crop, full foliage. Onion, Bulb ‐ hand weeding in low crop, full foliage Onion, Green hand weeding in low crop, full foliage Broccoli inspection, hand harvest, hand weeding in low crop, full foliage |
4,990 cm2/h |
|
Turf harvesting Turf cutting and handling |
8,800 cm2/h |
The listed crops and activities are those where the waxy‐leaf crop TC is stipulated by the EPA.
The alternative to adopting these TC based on the US EPA summary values would be to explicitly require that the EFSA Guidance must not be used to be used to support assessments for worker exposure when harvesting sweet corn where there is potential for intense contact, for the listed activities in waxy‐leaf crops, and harvesting turf.
In addition to the above new TC, the current EFSA recommended TC established for harvesting grapes, and inspections and irrigation in various crops should be extrapolated to work tasks in hops as follows:
Inspection and irrigation activities: TC = 1,400 cm2/h
Mechanically‐assisted harvesting: TC = 10,100 cm2/h
It is noted that the proposed TC for mechanically assisted harvesting hops is lower than the value used by Health Canada. The study from which the higher TC was derived from was for cane turning in table grapes which exceeded values from studies involving tying/training, leaf pulling and hand harvesting grapes giving rise to the TC 10,100 cm2/h. Those activities involve a level of body exposure that seems to be commensurate with that observed in mechanically assisted hop harvesting. Therefore, it is recommended to extrapolate the TC 10,100 cm2/h used for manual harvesting of grapes to cover mechanically assisted harvest of hops. This value is higher than the EFSA TC 4,500 cm2/h for harvesting tree fruits which also is a scenario involving body and hand exposure (based on EUROPOEM).
Data are not available to EFSA to establish appropriate (lower) TC values for various maintenance activities, such as weeding, stripping (removing lower leaves and lateral shoots) and tying or training of hops.
The intense contact activities in grapes were previously considered not to be relevant to Europe. However, EFSA expects soon to review some more recent data on worker exposure in viticulture provided by Crop Life Europe (CLE) and this issue should be reconsidered as part of that review.
The above recommendation for hops should be reconsidered when the viticulture data are reviewed.
Appendix G – Transfer Coefficients for Removal of Bolting Beets
G.1 Introduction
Sugar beet is a biennial crop. In the first‐year sugar beet plants remain in the vegetative growth phase. During this period, sugars are produced and translocated into the taproot. Since the production of sugar is the process of interest for this crop, beets are harvested at the end of the vegetative growth phase. Plants which are not harvested at this time may enter the reproductive phase after a cold period (usually winter). The so‐called vernalisation triggers a complex reprogramming at the molecular level, which ultimately leads to the formation of flowers and seed setting in the second year. However, certain genotypes or adverse weather conditions (e.g. periods with low temperatures) may trigger the transition from the vegetative to the reproductive phase in some beet plants already during the first year. As a consequence, so‐called bolting beets emerge on sugar beet fields.
Bolting beets must be eliminated before their seeds attain maturity. Otherwise, economic losses are expected due to reduced yield or contamination of the field with weed beets in the following years. Bolting beet removal takes place between June and September. In case of more than 500 bolting beets/ha, mechanical or chemical treatments are preferred. At infestation rates of less than 500 bolting beets/ha, bolting plants are usually removed manually (Landwirtschaftlicher Informationsdienst Zuckerrübe (Agricultural Information Service, Sugar Beet), 2020). Since the removal period coincides with the time frame for the application of insecticides and fungicides, workers who enter the field to remove the bolters manually after the treatment might be exposed to the applied pesticides. Therefore, this activity is a relevant worker task that needs to be considered in the risk assessment for (PPPs) which will be used in sugar beets.
The removal of bolting beets is not covered by the EFSA Guidance issued in 2014. So far, only a re‐entry for inspection tasks for up to 2 h is considered for the risk assessment of PPPs for field crops, including sugar beet (EFSA, 2014). However, it is expected that the assumed working rate of 2 h per day will be exceeded when removing bolting beets manually. In addition, the TC which are being used to estimate the exposure of the worker during inspection activities in field crops might not be appropriate for this task. Consequently, experimental data were required in order to enable a more accurate estimation of worker exposure.
A worker exposure study with a concomitant determination of DFR was conducted for bolting beet removal (summarised in Baumann et al., 2019) and submitted to EFSA during the Open Call for the update of the guidance. Experimental data from both parts of the field trial, from the determination of worker exposure as well as from the measurement of the DFR, were used to derive TC for different levels of protection.
Results from the concurrent DFR and exposure studies are presented in the section ‘Data’. Tables in this section summarise the unprocessed raw data as shown in Baumann et al. (2019), with the exception that any measurement reading below the LOQ was set to the LOQ. Experimental and analytical procedures, as well as the general study design are depicted in the section ‘Methodologies’. Eventually, details on data evaluation and implications of the results for the risk assessment are provided in Section G.3 (‘Assessment’) and G.4 (‘Conclusions’), respectively.
G.2 Data and methodologies
G.2.1 Data
Experimental data were gathered in a trial that was conducted on a farm in Switzerland in July 2017 (for summary of the results, see Baumann et al., 2019). The test site was assumed to represent a typical sugar beet field in Europe. Samples to determine amounts of DFR of the active substance were collected at different time points after the spray application of the PPP containing it. Worker re‐entry activities took place on the day after treatment. Since the active substance is rapidly converted to a relevant metabolite, both compounds were considered for the assessment of exposure and the determination of DFR.
G.2.1.1 Worker Exposure
The measured amounts of residue on the different dosimeters of each worker (WA to WF) are reported in Table G.1 (for the active substance) and Table G.2 (for the metabolite). Values shown in these tables were not corrected for field recovery or body parameters of the worker. For some samples, the amount of residue was below the LOQ. For further calculations, the WG agreed to use the LOQ as surrogate for the amount of residue in these samples.5 Legs and hands were identified as parts of the body with the highest exposure levels.
Table G.1.
Results of the determination of residue of the active substance (not corrected for field recovery)
| LOQ | Residue [µg active substance/sample] | ||||||
|---|---|---|---|---|---|---|---|
| WA | WB | WC | WD | WE | WF | ||
| Outer jacket sleeves | 20 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Outer jacket torso | 20 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Outer trousers torso | 20 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Outer trousers legs | 20 | 268 | 283 | 242 | 314 | 290 | 171 |
| Sum outer clothing | 328 | 343 | 302 | 374 | 350 | 231 | |
| Underwear (shirt + trousers) | 2 | 14.1 | 9.38 | 15.1 | 23.3 | 8.98 | 18.6 |
| Face/neck wipe | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| 1st handwash | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 4.00 | 2.00 |
| 2nd handwash | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Sum handwash | 4.00 | 4.00 | 4.00 | 4.00 | 6.00 | 4.00 | |
| Working gloves | 20 | 32.7 | 19.1 | 28.6 | 17.4 | 45.4 | 14.4 |
| Potential dermal exposure | 380.8 | 377.5 | 351.7 | 420.7 | 412.4 | 270.0 | |
Values below the LOQ (shown in italics) were considered as 1 x LOQ for further calculations.
WA‐WF: workers A–F.
Table G.2.
Results of the determination of residue of the metabolite (not corrected for field recovery)
| LOQ | Residue [µg metabolite/sample] | ||||||
|---|---|---|---|---|---|---|---|
| WA | WB | WC | WD | WE | WF | ||
| Outer jacket sleeves | 20 | 22.7 | 33.6 | 25.5 | 22.5 | 21.0 | 10.0 |
| Outer jacket torso | 20 | 26.7 | 23.2 | 38.4 | 23.4 | 49.1 | 23.4 |
| Outer trousers torso | 20 | 20.0 | 20.0 | 21.5 | 20.0 | 20.0 | 20.0 |
| Outer trousers legs | 20 | 2,916 | 2,791 | 2,204 | 2,874 | 288 | 1,489 |
| Sum outer clothing | 2,985 | 2,868 | 2,289 | 2,940 | 2,970 | 1,542 | |
| Underwear (shirt + trousers) | 2 | 56.6 | 52.2 | 54.7 | 83.4 | 37.5 | 56.7 |
| Face/neck wipe | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| 1st handwash | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| 2nd handwash | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Sum handwash | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | |
| Working gloves | 20 | 369 | 159 | 288 | 183 | 829 | 129 |
| Potential dermal exposure | 3,417 | 3,085 | 2,638 | 3,212 | 3,843 | 1,734 | |
Values below the LOQ (values shown in italics) were considered as 1 x LOQ for further calculations.
WA‐WF: workers A–F.
For each matrix (outer/inner dosimeters, working gloves, gauze pads for face/neck wipe, handwash), three samples per fortification level were prepared and analysed. Mean recovery rates were in the range between 76% and 98% (low variability), supporting the validity of the analytical method. However, since the field recovery was below 95% for all matrices, the exposure values needed to be corrected for the low field recovery for the assessment (see Table G.6 in Section G.3).
Table G.6.
Summarised dermal exposure figures for workers considering different levels of skin protection (values corrected for field recovery)
| Metabolite [µg] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| WA | WB | WC | WD | WE | WF | 75th | 95th | max | |
| Potential | 3,870 | 3,480 | 2,988 | 3,625 | 4,385 | 1,960 | 3,809 | 4,256 | 4,385 |
| T‐shirt + shorts | 3,817 | 3,431 | 2,921 | 3,576 | 4,308 | 1,911 | 3,757 | 4,185 | 4,308 |
| T‐shirt + shorts + gloves | 3,373 | 3,240 | 2,574 | 3,356 | 3,309 | 1,755 | 3,344 | 3,369 | 3,373 |
| T‐shirt + long trousers | 541.1 | 295.3 | 444.5 | 347.2 | 1,072 | 237.8 | 516.9 | 939.0 | 1,072 |
| Long clothes | 515.6 | 257.6 | 415.8 | 321.9 | 1,048 | 226.5 | 490.7 | 915.0 | 1,048 |
| T‐shirt + long trousers + gloves | 93.5 | 88.5 | 93.0 | 123.9 | 71.8 | 93.6 | 93.6 | 116.4 | 123.9 |
| Long clothes + gloves | 71.0 | 66.0 | 68.9 | 101.5 | 49.3 | 71.1 | 71.1 | 93.9 | 101.5 |
The WG agreed with these considerations and with the correction of exposure values for low recovery.
G.2.1.2 Dislodgeable Foliar Residue
DFR of the active substance and its metabolite were determined before and after the application of the PPP (Tables G.3 and G.4). Due to heavy rainfall on day 3 after the application (after collection of samples), the sampling was terminated before the scheduled end of the study.
Table G.3.
Dislodgeable foliar residue of the active substance (not corrected for recovery)
| Active substance [µg/cm2] | ||||
|---|---|---|---|---|
| DAT | Plot 1 | Plot 2 | Plot 3 | Mean |
| –0 | < LOQ | < LOQ | < LOQ | < LOQ |
| 0 | 0.0328 | 0.0448 | 0.0402 | 0.0393 |
| 1 | 0.00690 | 0.00965 | 0.00855 | 0.00837 |
| 2 | < LOQ | < LOQ | < LOQ | < LOQ |
| 3 | < LOQ | < LOQ | < LOQ | < LOQ |
DAT = Days After Treatment (−0 = before application); LOQ = 0.005 µg/cm2
Table G.4.
Dislodgeable foliar residue of the metabolite (not corrected for recovery)
| Metabolite [µg/cm2] | ||||
|---|---|---|---|---|
| DAT | Plot 1 | Plot 2 | Plot 3 | Mean |
| ‐0 | < LOQ | < LOQ | < LOQ | < LOQ |
| 0 | 0.0920 | 0.138 | 0.109 | 0.113 |
| 1 | 0.0595 | 0.0765 | 0.0483 | 0.0614 |
| 2 | 0.0126 | 0.0159 | 0.0111 | 0.0132 |
| 3 | 0.00835 | 0.0146 | 0.0105 | 0.0112 |
DAT = Days After Treatment; LOQ = 0.005 µg/cm2.
It is worth to be mentioned that measured residue levels for the metabolite were higher than residue levels of the active substance at all sampling points (Tables G.3 and G.4). The low residue levels for the active substance can be explained by a technical problem with the solution used for the dislodging procedure, which should have contained a sufficient amount of a stabilising agent in order to prevent conversion of the active substance to its metabolite. By mistake, the solution contained less than 10% of the stabilising compound, which is not sufficient to block the conversion. Based on this finding, the low field recovery results of the active substance (39–55%) when compared to the metabolite (92–96%) can be explained by an ongoing conversion during sample processing and storage. Since the recovery of the metabolite from spiked samples of the fortification level close to the range of residue levels of the metabolite obtained from the field samples is above 95%, no correction for recovery is required.
The WG agreed with these considerations including correction for low recovery and concluded that the results for the active substance were not reliable enough (due to low field recovery results) and should not be further considered for the assessment of TC.
G.2.2 Methodologies
The worker exposure study was conducted in accordance with the ‘Guidance document on the conduct of studies of occupational exposure to pesticides during agricultural application’ (OECD, 1997). In parallel, DFR were determined as following the principles laid down in the US EPA guideline OPPTS 875.2100 (US EPA, 1996).
The PPP was applied with a standard boom sprayer equipped with standard XR‐110 nozzles at an application rate of 160 g active substance/ha in a water volume of 220 L/ha. No rainfall was reported between application and completion of the exposure sampling. Relevant information is summarised in Table G.5.
Table G.5.
Summary of relevant information for the field trial
| Crop | |
|---|---|
| Crop type | Sugar beet |
| Plant density [plants/ha] | 110,000 |
| Growth stage [BBCH] | 45–48 |
| Plant height [cm] | 40–45 |
| Row spacing [cm] | 50 |
| PPP | |
| PPP type | Fungicide |
| Mode of application | Downward spraying |
| Application equipment | Tractor‐hauled applicator |
| Nozzle type | XR‐110 |
| Application rate [g a.s./ha] | 160 |
| Water rate [L/ha] | 220 |
| Rainfall within 24 h after the application [mm] | 0 |
| First rainfall recorded [h after application] | 48 |
G.2.2.1 Worker Exposure
Re‐entry started one day after the application. While walking through the field the workers manually removed the bolting beets. Due to the low infestation rate also, normal plants were removed in order to reach a representative number of contacts. The working period was approximately 4 h for each worker.
Dermal exposure of the workers was monitored using whole body dosimetry.
Body exposure was monitored with two layers of clothing that was worn throughout the sampling period. The outer layer consisted of a long‐sleeved cotton/polyester jacket and a pair of cotton/polyester trousers; the inner layer consisted of a long‐sleeved cotton T‐shirt and long cotton pants. The clothing was collected at the end of the work and stored in polyethylene bottles.
Hand exposure was monitored by taking handwash samples and collecting the gloves (uvex phynomic foam®, elastan/polyamid gloves with nitrile coating on palm and fingers, EN 388) that were used during work. The handwash were taken at the end of the working day before and after the workers removed their outer clothing. The workers rubbed their hands with Esemtan lotion (ca. 1 mL per hand) and rinsed them in 500 mL water. To avoid further conversion of the active substance to its metabolite in the handwash solution, a stabilising agent (10 g) was added to each sample. Working gloves were put into a prelabelled bag after completion of the task.
Head exposure was determined by using face and neck wipes. The corresponding samples were collected at the end of the working day before the removal of the outer clothing. Face and neck were thoroughly wiped with two cotton gauze pad (10 × 10 cm) which were moistened with 4 mL of 0.2% Esemtan solution prior to wiping. Both pads were combined after wiping to form a single specimen. To avoid further conversion of the active substance to its metabolite after sampling, 2 mL of a solution containing a stabilising agent were added to the face/neck wipes.
In order to assess stability of residue, field recovery samples were taken according to the study protocol. The active substance and its metabolite were extracted from the garments and pads with acetonitrile and from the protective gloves with isopropanol. Both solvents were added on the day of sampling and contained a stabilising agent to avoid degradation of the active substance. The samples were stored frozen at −18°C until measurement with reversed phase high‐performance liquid chromatography‐mass spectrometry (HPLC‐MS/MS). Residue of the metabolite was quantified in electrospray positive mode using stable isotopic labelled internal standards, residue of the active substance was determined in electrospray negative mode using either external matrix‐matched calibration standards (samples, concurrent recoveries) or labelled internal standards (validation recoveries). The LOQ for both compounds was 20 µg/sample for outer dosimeter and working gloves and 2 µg/sample for inner dosimeter, handwash solution and face neck wipes. Field recovery samples were processed as outlined for the dosimeter samples.
G.2.2.2 Dislodgeable Foliar Residue
DFR of the active substance and its metabolite on sugar beets were determined after a single spray application of a PPP. The study was conducted in parallel to the sampling for the determination of worker exposure described in the previous section. Application of the PPP was conducted according to common practices with typical spraying equipment. Samples were taken at 0 (after the spray solution had dried), 1, 2 and 3 days after application from three different subplots (plot size: 100 m2). No samples were taken after day 3 due to heavy rainfall.
Each sample consisted of 40 leaf discs with a double‐sided surface area of 400 cm2 in total, taken from all the parts of the foliage the worker might come in contact with. Control samples were taken prior to the application. To determine field recovery, solutions from dislodged control samples were fortified at different levels.
Dislodging of the leaf samples was performed as soon as possible but not later than 4 h after collection. A 0.01% AEROSOL OT solution was added to the leaf discs. After shaking the solution was transferred to a fresh container and the dislodging procedure was repeated. The decanted solutions were merged and stored in a deep freezer until analysis. To prevent degradation of the active substance, a solution containing a stabilising agent (by mistake containing only 20 g/L instead of 250 g/L) was added before freezing.
For analysis, aliquots of the filtered samples were analysed with HPLC‐MS/MS. The LOQ was 0.005 µg/cm2.
G.3 Assessment
Chiefly, the exposure of a person working in a crop which has been treated with a PPP depends mainly on three variables: the amount of DFR, the duration of the task and the intensity of the contact with foliage. For the latter, task‐specific TC are used as a proxy in the risk assessment.
In order to derive a TC for a specific task, two data points are required:
An estimate of dermal exposure obtained from workers executing the task in a previously treated crop for a certain period of time.
An estimate of DFR, which was determined at the time when workers executed their tasks.
These data points were provided (see Tables G.1, G.2, G.3–G.4 above) and are deemed appropriate to determine a TC value for the removal of bolting beets.
For the DFR, the DFR data for the metabolite were considered as acceptable for deriving TC values (instead of using the sum of active substance and metabolite). Based on these results the uncorrected mean6 DFR‐value of 0.0614 µg/cm² obtained from samples collected on day 1 after the treatment (Table G.4) can be used to derive TC‐values. Results obtained from samples collected on days 2 and 3 after application may have been compromised by rainfall, thus using these data to assess the time‐resolved dissipation of foliar residue may result in an overestimation of the dissipation rate.
For the dermal exposure of workers towards the metabolite, the residue detected on legs and hands (handwash + gloves) accounted for more than 95% of the total residue detected (Figure G.1). Thus, legs and hands are the most relevant parts for risk mitigation for workers by means of protective garment. This should be addressed by deriving TC‐values for different levels of skin protection.
Figure G.1.
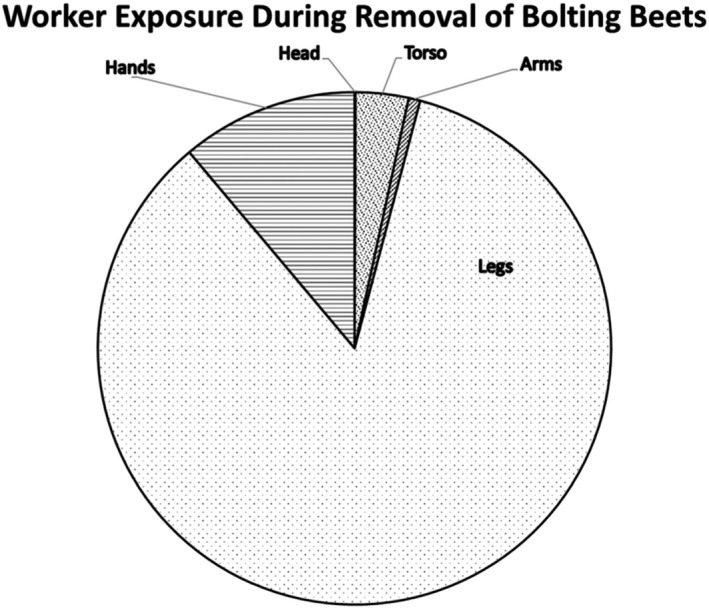
Relative distribution of exposure based on 75th percentile of the measured exposure of workers removing bolting beets. (hands = handwash + gloves)
As presented in Tables G.1 and G.2, dermal exposure can be displayed for different levels of protection by clothing and protective equipment (i.e. gloves). The combinations of different dosimeters can be used to estimate the dermal exposure for different levels of skin protection:
-
○
Potential: sum of all dosimeters
-
○
T‐shirt + shorts: sum of outer jacket sleeves, outer trousers legs, underwear, face/neck wipe, handwash and gloves
-
○
T‐shirt + shorts + gloves: sum of outer jacket sleeves, outer trousers legs, underwear, face/neck wipe, handwash;
-
○
T‐shirt + long trousers: sum of outer jacket sleeves, underwear, face/neck wipe, handwash and gloves;
-
○
Long clothes: sum of underwear, face/neck wipe, handwash and gloves;
-
○
T‐shirt + long trousers + gloves: sum of outer jacket sleeves, underwear, face/neck wipe, handwash;
-
○
Long clothes + gloves: sum of underwear, face/neck wipes, handwash
Exposure figures corrected for field recovery < 95% for above‐mentioned scenarios are presented in Table G.6. As mentioned earlier, protection of legs and hands has a high impact on the dermal exposure, while protection of arms and the torso hardly reduced dermal exposure.
During the field study (see Section G.2.2), worker exposure was monitored one day after the application. Samples to determine amounts of DFR were taken at the beginning of this exposure period. Based on the data shown in Table G.4, DFR declined rapidly with a half‐life time of approximately 24 h. Given that worker exposure was monitored for approximately 4 h, a noticeable decline of foliar residue is expected during this period. Hence, the use of the initial amounts of DFR may lead to an underestimation of the contact intensity (i.e. the TC‐values). Thus, a correction considering the dissipation rate was proposed.
To derive a correction factor, the area under the curve (AUC) may be used in analogy to the use of this procedure to determine bioavailability of drugs (Turner, 2013; DiBartolomeis et al., 2019). The AUC value represents the potential exposure towards a compound depending on its time‐dependent concentration:
By comparing the AUC with and without decline of the amount of DFR, a correction factor can be derived. For this purpose, the dissipation rate (DT50) for DFR was determined. Due to the fact that residue data from days 2 and 3 may have been compromised by rainfall, only data from day 0 and 1 were considered for regression analysis (assuming single first‐order kinetics). Results and the derived equation are shown in Figure G.2. Considering the initial DFR value of 0.113 µg/cm² and a DFR value of 0.0614 µg/cm² 24 h after the application, a DT50 value of 27.27 h was determined.
Figure G.2.
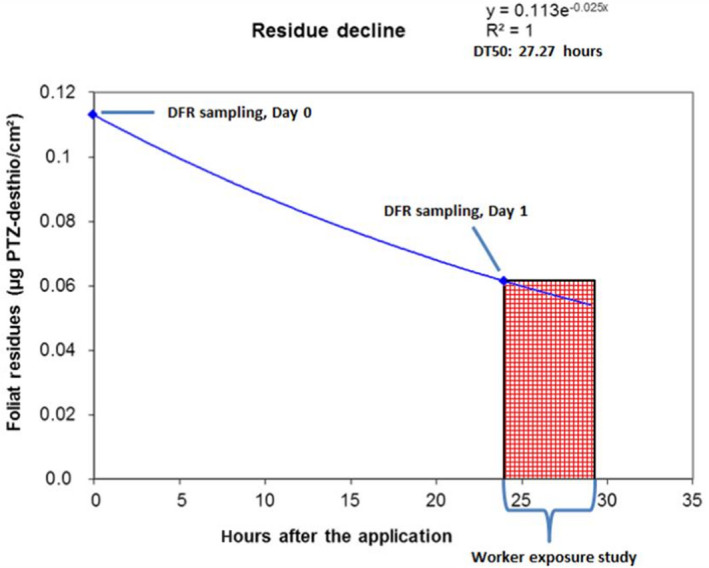
Determination of the decline rate for DFR on leaves of sugar beets. The chequered rectangle represents the AUC without dissipation of residue. With dissipation, only the area of the rectangle below the blue regression curve should be considered. The ratio between both areas was used as correction factor
Considering the equation shown Figure G.2. and assuming a DT50 value of 27.27 h the AUC value was calculated as follows:
Assuming no dissipation [DFR(t) = constant, which means DFR at the end of the exposure period is equal to the value determined at the beginning of the exposure period], the AUC value was calculated as follows
The ratio between both AUC‐values is approximately 0.96. This value was used as correction factor to derive transfer coefficients.
This correction factor can be included in the formula for the calculation of the TC for different levels of skin protection, considering the initial amount of DFR (on day 1, at the beginning of the worker exposure, see Table G.4) in combination with the corrected worker exposure data (Table G.7) as follows:
Table G.7.
Transfer coefficients for different levels of skin protection and different confidence levels
| Transfer coefficient [cm2/h] | ||||
|---|---|---|---|---|
| 75th | 95th | Max | Max (rounded) | |
| Potential | 16,154 | 18,053 | 18,599 | 18,600 |
| T‐shirt + shorts | 15,936 | 17,750 | 18,270 | 18,300 |
| T‐shirt + shorts + gloves | 14,184 | 14,288 | 14,306 | 14,300 |
| T‐shirt + long trousers | 2,193 | 3,983 | 4,545 | 4,500 |
| Long clothes | 2,081 | 3,881 | 4,445 | 4,400 |
| T‐shirt + long trousers + gloves | 397 | 493 | 526 | 530 |
| Long clothes + gloves | 302 | 398 | 430 | 430 |
In addition to different levels of skin protection, different levels of confidence were included in the calculation of TC, as shown in Table G.7. As already indicated by the exposure figures, legs and hands were identified as parts of the body with the most intense contact to the treated crop, while dermal exposure of arms and trunk is low. This observation relies on characteristics of the crop (e.g. plant height app. 45 cm) and the main worker activities when removing bolting beets manually: walking across the field, grabbing and dragging beets. Therefore, long trousers or the combination of long trousers and gloves were selected as reasonable scenarios for a refinement of worker exposure, while covering arms will not significantly contribute to a reduction of exposure.
In contrast to the above‐mentioned limitations, it should be noted that the removal of bolting beets is a simple, straightforward activity. Thus, the low variability of the worker exposure observed in the study may indicate that the observed numbers are representative. In order to account for the uncertainties, the use of the rounded maximum values (as shown in the last column of Table G.7) is recommended for the estimation of worker exposure. This approach is considered as reasonably conservative, even though the values are based on a small set of data.
Uncertainties
Even though experimental data were considered as reliable, care should be taken when drawing general conclusions based on these data, because:
The whole assessment is based on a single study with a limited number of replicates (three samples per time point for DFR, six sets of samples from workers). Thus, the data set is small and there is no independent confirmation of the results and conclusions.
The data were collected for a particular growth stage. Even though the selected growth stage is considered as representative, it cannot be ruled out that exposure would be different at other time points (e.g. lower at earlier growth stages, higher at later time points when all beets get closer to their mature size). Nevertheless, it cannot be estimated if increased plant size would lead to an increased exposure, because it is expected that plant size and DFR are negatively correlated which may compensate for more intense contact with the crop.
The reported planting density within the study (app. 110,000 plants per ha) was higher than the average planting density in practice (80,000–100,000 plants per ha, personal communication7). No data is available to estimate the impact of plant density on the exposure. It expected that the amount of DFR is lower (because of a higher leaf area per ha), while the intensity of the skin contact with the treated cop plants should be higher (because of the higher density and leaf area). As already noted with regard to the impact of the growth stage, it is not predictable if these changes are compensating for each other.
These values can be used as a default approach in the update of the EFSA Guidance issued in 2014. Nevertheless, it is noted that other combinations of skin protection may be of interest due to country‐specific considerations (e.g. if workwear is mandatory).
G.4 Conclusions
A field study on manual removal of bolting beets was performed and experimental data were gathered and evaluated in order to establish task‐specific parameters for the worker exposure assessment.
Given that legs and hands were identified as parts of the body with the highest dermal exposure, the following TC values are proposed for different levels of skin protection:
Potential exposure: 18,600 cm2/h
T‐shirt and long trousers: 4,500 cm2/h
T‐shirt, long trousers and gloves: 530 cm2/h
In addition, rounded maximum values for other levels of skin protection may be used in order to meet country‐specific considerations for refinements:
T‐shirt and shorts: 18,300 cm2/h
T‐shirt, shorts and gloves: 14,300 cm2/h:
Long clothes (workwear): 4,400 cm2/h
Long clothes (workwear) and gloves: 430 cm2/h
Irrespective of the selected TC‐value, a work rate of 8 h per day should be assumed for the exposure assessment.
Because the task is relevant for growth stage BBCH 39 and beyond, the removal of bolting beets should not be considered for the use of PPPs (particularly herbicides) at early growth stages (application until the BBCH 19). For the assessment of these early applications in (sugar) beets, the recent practice of using a work rate of 2 h per day and the general TC‐values for inspection and irrigation are still applicable. For any application of PPPs in (sugar) beets beyond BBCH 19, the above‐mentioned crop‐specific parameter should be used in order to estimate the potential exposure of workers removing bolting beets. Beets are harvested at the BBCH 49 (‘Beet root has reached harvestable size’). It should be noted that this growth stage is reached well before the actual harvest, because ‘harvestable’ does not mean that the beet has reached its maximum size (Meier et al., 1993). Therefore, the new TC‐values should be used for all applications of PPPs in beets between BBCH 19 and BBCH 49. Finally, it is important to note that the risk assessment should also include an evaluation of the worker exposure for tasks like inspection, as it is already done. Risk mitigation measures should be assigned individually to the respective tasks.
G.5 Recommendation
Availability of sufficient data is crucial for the development or refinement of procedures and models for risk assessments. In order to improve or develop models or refine default input parameters for the risk assessment further experimental data must be gathered, as these enhancements are expected to increase the probability that model‐based estimates reflect the real situation as accurately as possible. Further improvements related to more realistic TC values should address e.g. task or growth stage‐dependent TC values for different crops.
Appendix H – Transfer Coefficients for Harvesting Peaches
H.1 Introduction
In 1998, a worker exposure study for peach harvesting with concurrent determination of DFR was conducted by French Crop Protection Industry Association (UIPP) and submitted to EFSA during the Open Call for the update of the Guidance. Both parts of the field trial, the measurement of worker exposure as well as the measurement of the DFR, are analysed in this appendix view of a possible revision of the TC for harvesting tree fruits.
H.2 Data and methodologies
H.2.1 Data
The study was conducted in compliance with good laboratory practice (GLP) standards (OECD, 1997; Urtizberea, 2002).
The field phase took place on orchards, on three sites (covering three different varieties of peaches: spring lay, manon and melida) in France in June 1998.
Dermal exposure was monitored for 15 workers during harvesting of peaches in one site. Re‐entry occurred at 3, 5 and 7 days after the second application of the plant protection product, with five workers monitored each day. When dressed with dosimeters, workers were equipped with crate maintained by means of a leather harness. The work consisted of collecting peaches from the inner and outer part of the peach trees and of setting the fruits into the crate. When the crate was full, workers had to haul the full crate and install an empty one.
DFR were determined in parallel to the sampling of worker exposure and comprised three field trials (3 days after application (DAA) 2, 5 DAA 2 and 7 DAA 2). Also, dislodgeable foliar study was conducted over the three sites several times from the day before the first application and until 21 days after the second application.
In addition to leaf samples, fruits were collected from the same plot during the harvesting days and were dislodged following the same procedure used for leaf samples. Fruits were also collected to determine the total residue by extraction with acetone.
H.2.1.1 Worker exposure
Dermal and inhalation exposure were measured for each worker, no values below the LOQ were obtained. Results were corrected for the field recovery which was below 95% for all specimens. In contrast to the study report, the dermal exposure values were corrected with the mean recovery of the closest fortification level (i.e. geometric mean method) and not with the mean recovery rate over all fortification levels (see Tables H.1 and H.2).
Table H.1.
Residue [µg/sample] in worker samples (corrected for each level of fortification) (Urtizberea, 2002)
| Worker | HW | FS | ID Legs | ID Trunk | ID Arms | OD Legs | OD Trunk | OD Arms | Filter |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,643 | 21.1 | 133 | 76.2 | 326 | 1,208 | 2,934 | 3,054 | 0.402 |
| 2 | 3,277 | 35.7 | 180 | 53.8 | 326 | 567 | 2,196 | 3,580 | 0.578 |
| 3 | 2,992 | 43.5 | 142 | 43.3 | 75 | 743 | 2,426 | 3,777 | 0.341 |
| 4 | 3,521 | 112 | 149 | 73.0 | 182 | 978 | 3,416 | 4,345 | 0.297 |
| 5 | 2,296 | 28.1 | 160 | 64.8 | 137 | 477 | 3,534 | 2,650 | 0.209 |
| 6 | 2,744 | 60.9 | 323 | 106.1 | 160 | 1,222 | 6,938 | 5,857 | 0.426 |
| 7 | 1,562 | 19.4 | 141 | 45.1 | 113 | 471 | 1,875 | 2,711 | 0.263 |
| 8 | 2,035 | 32.1 | 188 | 122.7 | 105 | 746 | 2,732 | 4,350 | 0.303 |
| 9 | 2,041 | 55.5 | 295 | 81.1 | 150 | 1,187 | 3,672 | 4,738 | 0.551 |
| 10 | 1,790 | 101 | 181 | 48.9 | 150 | 1,109 | 3,358 | 3,828 | 0.415 |
| 11 | 3,069 | 86.5 | 113 | 52.9 | 181 | 1,832 | 3,873 | 5,786 | 0.782 |
| 12 | 2,034 | 174 | 213 | 83.6 | 262 | 1,080 | 4,431 | 6,547 | 1.204 |
| 13 | 1,648 | 26.5 | 98.6 | 80.8 | 138 | 951 | 1,632 | 3,059 | 0.410 |
| 14 | 2,907 | 37.9 | 106 | 87.7 | 266 | 1,314 | 3,291 | 4,368 | 0.663 |
| 15 | 2,340 | 56.2 | 175 | 77.4 | 228 | 1,255 | 5,665 | 5,784 | 1.078 |
Note: HW (handwash); FS (Facial swabs); ID (Inner dosimeters); OD (outer dosimeters).
Table H.2.
Field recovery data for different matrices (Urtizberea, 2002)
| Matrix | Fortification level [µg a.s./sample] | n | Mean recovery [%]( a ) (min–max) | RSD | |
|---|---|---|---|---|---|
| Outer dosimeter | 1 | 5 | 88.00 (73–99) | 9.6 | |
| 100 | 6 | 78.33 (71–83) | 3.9 | ||
| 10,000 | 6 | 88.67 (86–95) | 3.0 | ||
| Inner dosimeter | 1 | 5 | 80.20 (72–95) | 8.3 | |
| 100 | 6 | 71.80 (64–79) | 4.6 | ||
| Filter | 0.2 | 6 | 75.7 (66–88) | 6.6 | |
| 20 | 6 | 87.6 (81–93) | 4.7 | ||
| Facial Swab | 0.2 | 6 | 40.33 (36–44) | 2.7 | |
| 20 | 6 | 44.83 (40–50) | 3.7 | ||
| Handwash | 1 | 6 | 82.83 (70–103) | 11.7 | |
| 100 | 6 | 65.67 (55–71) | 5.3 | ||
| 10,000 | 6 | 86.83 (71–100) | 11.2 |
Field recoveries with unusually high or low values were considered faulty and excluded.
These results show that dermal exposure during harvesting of peaches occurs mainly on the trunk, arms and hands of the worker.
H.2.1.2 Dislodgeable Foliar Residue
The site where worker exposure was measured was divided in three plots (two treated and one control plot) for DFR measurements. Leaf samples were collected after the spray solution had dried. Since the worker exposure study was only carried out on one site, only DFR values from this site are considered.
DFR samples from treated and untreated plots were taken the day before the second application, and then at 2, 6, 12 and 24 h, and 3, 5, 7, 14, 17 and 21 days after the second application (DAA 2). Worker exposure was measured at 3, 5 and 7 days after the second application.
To determine the field recovery, samples were collected from the untreated plot after 2 h and 7 days after the second application (last day of worker exposure measurement). No field spikes samples were collected on the two first worker exposure sampling. Two replicate solutions from dislodged control samples were fortified at three different levels (120, 1,200 and 12,000 µg/L).
Mean field recoveries are presented in Table H.3.
Table H.3.
Field recovery data for the leaf samples (Urtizberea, 2002)
| Matrix | Fortification level [µg a.s./l] | n | Mean recovery [%] (min–max) | RSD |
|---|---|---|---|---|
| Leaf samples | 120 | 2 | 88 (71–105) | 17 |
| 1,200 | 2 | 75.5 (70–81) | 5.5 | |
| 12,000 | 2 | 76 (66–86) | 5 | |
The DFR values are shown in Table H.4.
Table H.4.
DFR values (µg a.s./cm2) (not corrected for recovery) (Urtizberea, 2002)
| DAA 2 | Subplot 1R1 (untreated) | Plot 2R1 (treated) | Plot 2R2 (treated) | Mean |
|---|---|---|---|---|
| 3 | 0.009 | 0.411 | 0.460 | 0.4355 |
| 5 | 0.011 | 0.451 | 0.441 | 0.4460 |
| 7 | 0.006 | 0.372 | 0.338 | 0.3550 |
DAA 2 = days after second application.
DFR were not corrected by field spikes although recoveries were below 95%. Despite it is mentioned that residue values greater than LOQ were adjusted for field fortification recoveries this adjustment was not done in the study report.
H.2.2 Methodologies
The worker exposure study was conducted in compliance with the ‘Guidance document on the conduct of studies of occupational exposure to pesticides during agricultural application’ (OECD, 1997). In parallel, the DFR were determined following the US EPA guideline OPPTS 875.2100 (US EPA, 1996).
The pesticide was applied two times according to common practices with a tractor‐mounted HARDI TS3082, on May 29 and on June 12. The application conditions (second application) are summarised in Table H.5.
Table H.5.
UIPP study conditions (Urtizberea, 2002)
| Test material | Confidential |
|---|---|
| Formulation type | SC (suspension concentrate) |
| Application rate | 640 g a.s.ha |
| Water volume | 803 L/ha |
| Culture(s) | Peaches trees |
| Application equipment | HARDI TS3082 |
| Temperature | 17.5°C |
| Rel. humidity | 48% |
| Growth stage | BBCH 86 |
| Wind speed | No wind |
| Rainfall | No rain (no data between 2nd application and sampling) |
| Study site | Southern part of France |
H.2.2.1 Worker exposure
Re‐entry started 3 days after the second application and comprised three field trials (3 days after application (DAA) 2, 5 DAA 2 and 7 DAA 2), monitoring five workers each during a normal working day (ranging from 303 to 384 min).
Dermal exposure of the workers was monitored using whole body dosimetry. Body exposure was sampled with two layers of clothing that were worn throughout the sampling period. The outer layer consisted of a 100% cotton coverall; the inner layer consisted of a long‐sleeved cotton T‐shirt and long cotton pants.
Exposure of the hands was monitored by taking handwash samples with detergent at the end of the working day. The wash was repeated a second time, both handwash were combined adding a buffer solution to avoid any degradation of the active substance.
Head exposure was measured by the use of two detergent‐soaked cloth swabs. The facial and neck areas were wiped thoroughly, after the handwash and removal of the coverall but before the removal of underwear.
Inhalation monitoring was performed using air‐sampling pump, including a pre‐filter Millipore and a filter Millipore held in a cassette which was attached near the breathing zone.
Field recovery samples were taken each day of monitoring. Fortification took place under a shed located in the field where climatic conditions were considered representatives. Samples were exposed to field conditions for the duration of the monitoring period except handwash samples and facial swabs which were capped and placed in a freezer within approximately 10 min after fortification.
For each matrix, two or three levels of fortification were used. For each level and each matrix, three samples were fortified. Two of them were analysed and the other was stored.
All samples were stored under ambient conditions before extraction in the test facility that was conducted not later than 96 h after sampling. The extracts were stored at −18°C until analysis.
Residue were extracted from facial swabs, filters, underwear and coverall with dichloromethane. The extraction of the a.s. from handwash samples was made using solvent partition with dichloromethane. The samples were measured with gas chromatography using electron capture detector (GC‐ECD) detection. The LOQ were 0.3 µg/sample for handwash (600 mL), 0.1 µg/sample for both facial swabs (2 gauze pads) and filters (front and back section) and 0.5 for both underwear and coverall (1,000 cm2).
The analytical method was validated for each matrix using spiked samples before the analysis of test samples.
H.2.2.2 Dislodgeable foliar residue
DFR were determined on three sites. In one of these sites, DFR study was conducted in parallel to the sampling of worker exposure (3 DAA 2, 5 DAA 2 and 7 DAA 2). Orchard density was ~ 555 trees per hectare. The interval between trees in a row was approximately 3 m, the interval between rows being 6 m.
Environmental conditions such as wind speed, temperature or relative humidity were recorded during and between treatments. It is mentioned that moderate rain fell between two treatments, but no rain fell during the sampling period that could have flushed residue from foliage or fruits. No data on the weather conditions were collected between sampling days.
Leaf samples were randomly sampled directly into a pre‐labelled glass jar. Each sample consisted of 65 leaf discs (2 cm diameter) taken from 40 leaves corresponding to a total area of 408.2 cm2. Two punches were taken from each collected leaf using a Birkestrand leaf punch sampler. At each time interval, one sample was collected from the untreated plot and two samples were taken from the treated plots. No samples were taken from the border areas.
Dislodging of the leaf samples was performed as soon as possible but not later than 4 h after collection. The dislodging solution consisted of 100 mL of 0.01% w/v surfactant bis(2‐ethylhexyl) sulfosuccinate sodium salt in water. Each leaf sample was dislodged twice. Samples were stored and shipped in glass jars with Teflon lined caps.
The residue were extracted by a liquid–liquid solvent partition with dichloromethane. The samples were measured with gas chromatography (GC) using electron capture detection.
The analytical method was validated prior to the first sample analysis and the validation was confirmed during each series of sample analysis spiking known concentrations of the active substance in dislodging solutions.
H.2.3 Assessment
The TC for a specific task in a given crop is calculated by dividing the dermal exposure by the amount of DFR and time:
Regarding dermal exposure, the corrected values as reported in Table H.1 were used.
It is also noted that bare hand and head and neck exposure are considered as actual exposure in the UIPP study report, but should have been treated as potential exposure, as defined in the EFSA Guidance issued in 2014.
Regarding DFR results, in the study report, they were not corrected by field spikes although recoveries were below 95%. No field spikes samples were collected on each day of monitoring, they were collected the day before. It may be acceptable to collect a single set of field recovery samples if the environmental conditions are similar on each day and/or at each site (OECD, 1997), but no data on the weather conditions were collected between sampling days. In addition, only one control sample per fortification level from the untreated plot were collected, when three replicates per fortification level are usually required. Regarding the number of DFR samples, only two samples were taken from the treated plots at each time interval, while it is recommended to collect four to six samples at each sampling interval (three samples at least, see SANTE/2020/12830, Rev.1, 24. February 2021).
For all these above‐mentioned reasons, DFR values are kept as they were included in the UIPP report and not corrected, this is considered a conservative approach for TC calculation.
The TC values for the different levels of protection (e.g. potential TC, body actual TC), derived from the respective exposure values for the different levels of protection, are presented in Table H.6. No TC values for protected hand is calculated since handwash method is used.
Table H.6.
DFR, worker exposure and derived TC for harvesting peaches (Urtizberea, 2002)
| Sampling time | Worker | Actual time of working (h) | DFR average a.s. (µg/cm2) | PDE body (inner + outer + head dosimeters) [µg a.s./day] | ADE body [µg a.s./day] | PDE hands | PDE body + hands | TC (PDE body + PDE hand) [cm2/h] | TC (ADE body + PDE hand) [cm2/h] |
|---|---|---|---|---|---|---|---|---|---|
| 3DAA | 1 | 5.07 | 0.44 | 7,751.63 | 534.82 | 2,643,10 | 10,394,72 | 4,710,88 | 1,440,23 |
| 2 | 5.08 | 0.44 | 6,937.88 | 559.33 | 3,276,52 | 10,214,39 | 4,613,98 | 1,732,71 | |
| 3 | 5.08 | 0.44 | 7,250.09 | 260.03 | 2,992,05 | 10,242,15 | 4,626,52 | 1,469,01 | |
| 4 | 5.07 | 0.44 | 9,255.00 | 404.46 | 3,520,67 | 12,775,67 | 5,789,93 | 1,778,87 | |
| 5 | 5.07 | 0.44 | 7,051.82 | 361.84 | 2,296,44 | 9,348,26 | 4,236,63 | 1,204,73 | |
| 5DAA | 6 | 5.48 | 0.45 | 14,667.16 | 589.42 | 2,744,44 | 17,411,60 | 7,119,66 | 1,363,23 |
| 7 | 5.50 | 0.45 | 5,376.14 | 298.89 | 1,561,67 | 6,937,81 | 2,828,30 | 758,48 | |
| 8 | 5.48 | 0.45 | 8,274.41 | 415.32 | 2,035,01 | 10,309,42 | 4,,215,55 | 1,001,95 | |
| 9 | 5.52 | 0.45 | 10,179.09 | 526.74 | 2,040,77 | 12,219,86 | 4,,966,55 | 1,043,52 | |
| 10 | 5.52 | 0.45 | 8,775.95 | 380.36 | 1,789,70 | 10,565,65 | 4,294,22 | 881,99 | |
| 7DAA | 11 | 6.23 | 0.36 | 11,924.36 | 346.80 | 3,069,22 | 14,993,57 | 6,775,74 | 1,543,73 |
| 12 | 6.37 | 0.36 | 12,790.92 | 558.50 | 2,033,86 | 14,824,78 | 6,559,15 | 1,146,98 | |
| 13 | 6.40 | 0.36 | 5,985.89 | 317.27 | 1,648,05 | 7,633,94 | 3,360,01 | 865,02 | |
| 14 | 6.40 | 0.36 | 9,469.84 | 459.47 | 2,906,83 | 12,376,67 | 5,447,48 | 1,481,65 | |
| 15 | 6.40 | 0.36 | 13,241.54 | 481,34 | 2,340,20 | 15,581,75 | 6,858,16 | 1,241,88 | |
| Mean | 5.64 | 0.41 | 9,262.11 | 432,97 | 2,459,90 | 11,722,02 | 5,093,52 | 1,263,60 | |
| Minimum | 5.07 | 0.36 | 5,376.14 | 260,03 | 1,561,67 | 6,937,81 | 2,828,30 | 758,48 | |
| Maximum | 6.40 | 0.45 | 14,667.16 | 589,42 | 3,520,67 | 17,411,60 | 7,119,66 | 1,778,87 | |
| 75th Centile | 6.30 | 0.45 | 11,051.73 | 530,78 | 2,949,44 | 13,800,22 | 6,174,54 | 1,475,33 | |
| Standard Deviation | 0.56 | 0.04 | 2,792.06 | 105,58 | 612,64 | 2,978,24 | 1,301,05 | 314,98 | |
H.2.4 Conclusions
The indicative TC values, as included in the EFSA Guidance issued in 2014, for tree fruit were based on EUROPOEM II (2002) report of the re‐entry WG (van Hemmen II, 2002).
TC for tree fruit were established based on 5 studies, according to the following rationale: TC for hand exposure amounts to about 2,500 cm2/h (75th percentile). However, TC value body exposure was not easy to determine in view of the spread of the data for actual and potential exposures. For actual exposure it may be just below 10,000 cm2/h (75th percentile) and for potential exposure the database is small, but a surrogate value could be up to 20,000 cm2/h (90th percentile). If body exposure were to be reduced by (protective) clothing, with for instance a tenfold reduction of exposure, the total exposure would lead to a TC value of 4,500 cm2/h (with bare hands).
A summary of the analysis of these studies developed for its inclusion in EUROPOEM II report is included in Table H.7.
Table H.7.
TC study reviews for tree fruit in EUROPOEM II (2002) report of the re‐entry WG
| Study | Documentation | Re‐entry conditions | Work activities | Sampling methodology | Chemical analysis and validation | Conclusion |
|---|---|---|---|---|---|---|
| Study 36: Nigg and Stamper ( 1984 ) | Estimation of transfer of chlorobenzilate in citrus tree (oranges) harvesters | Not given (Florida) | Professional harvester crew. 2–3 DAA |
Pad methodology for the body and hand rinses with ethanol. Gunther method |
Not described | The quality of the data is probably acceptable for inclusion in the database |
| Study 45: Schneider et al. ( 1990 ) | Publication of a research project | Only max/min temperatures | 26 nectarine harvesters. Harvesting nectarines | Dermal (Shirt/wipes/handwash) and DFR (Gunther method) | GC with nitrogen/ phosphorous detector. No recovery data | Mean transfer factor of 6,935 cm2/h |
| Study 51: Spencer et al. ( 1993 ) | Internal report. No documentation for review |
Fruit tree plantations (peach and apple), mainly harvesters Climatic conditions not available |
Working procedure well described |
Gunther method Actual exposure on parts of the body (not head and thigh), hands covered with nylon knitted gloves |
Method validation not available for DFR and exposure. Results not adjusted for recovery (bad at handwash: 53 +/− 34%!) | Before entering the database, data on method validation should be retrieved |
| Study 53: Spencer et al. (1991) | Internal report | Peach and apple orchards | 29 male professionals. 100–470 min/day |
Gunther method. Long‐sleeved cotton undershirts and outer shirts. Hand exposure was measured with wipes and/or washes |
Quality analysis is not described | The study is acceptable for extraction of exposure data |
| Study 55: Stamper et al. (1986) | Climatic conditions not available. Citrus harvesters are mentioned | Not described |
Iwata et al. (1977), Gunther et al. (1977) Exposure pads, actual exposure. Hand rinse, 95% ethanol |
Chemical analysis Method validations are not transparent | The study is not suitable for entering a re‐entry database before at least the method validation has been checked | |
| Study 57: Thongsinthusak et al. ( 1989 ) | Internal report. The only checkable item is the calculation of transfer factors for peach harvesting | No details on climate are given. 10 harvesters | No concomitant DE and DFR, different studies | As this report is issued by CDFA the published data and the transfer factors calculated therefrom should be acceptable. |
Results, summarised in EUROPOEM II report, are included in Table H.8.
Table H.8.
EUROPOEM II TC values for fruit trees
| Crop type | Number of publications | Number of records | Hands | Body | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |||
| Fruit trees | 5 | 106 | 1,124 | 0 | 5,281 | 6,892 | 0 | 24,945 |
This value compares reasonably well with the US EPA data (US EPA, 2017) if body exposure is to be reduced by a tenfold factor.
Table H.9 EPA ExpoSAC Policy3
| Crop | ExpoSAC Policy 3 TC crop group | Activity | ARTF data cluster code | TC (cm2/h) arithmetic mean | TC (cm2/h) geometric mean |
|---|---|---|---|---|---|
| Peach | Tree, ‘fruit’, deciduous | Harvesting, Hand | OH (EPA) | 1,721 | 1,400 |
Despite some uncertainties mentioned in the previous assessment section, the UIPP study was well conducted and data were considered as reliable.
Although this study could be considered as being adequately representative, it is a single study, and the issue of which exposure value to be taken from the study has been further considered. Given the small number of subjects involved, usually the maximum exposure is considered most adequate in order to address any uncertainty. Thus, in the case of the UIPP study, the following values are proposed:
TC (cm2/h), total potential exposure: 7,120
TC (cm2/h) assuming arms, body and legs covered (workwear; bare hands): 1,779
It is noted that the UIPP maximum value of TC (ADE body + PDE hands) is similar to the arithmetic mean values included in the existing databases (EUROPOEM II and EPA ExpoSAC Policy 3), when body exposure from EUROPOEM database is reduced considering a tenfold factor for the use of (protective) clothing.
Table H.10 TC arithmetic mean values (cm2/h) from EUROPOEM II, US EPA ExpoSAC Policy 3 and UIPP study (Urtizberea, 2002)
| EUROPOEM II | US EPA | UIPP study | |
|---|---|---|---|
| TC (ADE body + PDE hand) | (689 + 1,124) 1,813 | 1,721 (GM: 1,400) | 1,264 (Maximum: 1,779) |
Table H.11 TC values (Van Hemmen, 2002) included in EFSA (2014) and TC values based on the UIPP study (Urtizberea, 2002)
| TC (cm2/h), Body potential exposure | TC (cm2/h), hand potential exposure | TC (cm2/h), total potential exposure | TC (cm2/h) assuming arms, body and legs covered (workwear; bare hands) | TC (cm2/h), covered body (workwear) and gloves (PPE) | |
|---|---|---|---|---|---|
| EUROPOEM II | 20,000 | 2,500 | 22,500 | 4,500 | 2,250 |
| UIPP Study (Maximum) | 5,138 | 1,233 | 7,120 | 1,779 | – |
Nevertheless, comparing UIPP TC values to the TC values included in EFSA Guidance issued in 2014, it could be concluded that TC for potential body could be overestimated in the database. However, TC for potential hand exposure would be more comparable to the obtained in UIPP study.
Taking into account the EUROPOEM data, the following considerations were made by the WG:
-
The TC for tree fruits included in EFSA (2014) can be considered conservative in particular for potential body exposure TC values, due to the following reasons:
-
–
Pad methodology for the body exposure is used in EUROPOEM II database. As a result, the distribution of worker exposure is not uniform. This may lead to very conservative results and a large standard deviation.
-
–
Mean transfer factor of 6,935 cm2/h was obtained in one of the studies included in EUROPOEM database (Study 45). Shirt/wipes/handwash instead of pads were used in this study. Potential exposure on legs was not measured (it would be equivalent to 10% of the body potential exposure according to UIPP study). Higher results obtained in the UIPP study (7,120 cm2/h) could be explained by these differences.
-
–
Outer and inner shirt were measured in another study included in EUROPOEM II database (Study 53 and published in Toxicology Letters 78 (1995) 17‐24). The mean shirt penetration value of 33% would result in an overestimation of ADE, possibly caused by sweat.
-
–
The UIPP maximum value of TC (ADE body + PDE hands) is similar to the arithmetic mean values included in the existing databases (EUROPOEM II for search/reach/pick activities and EPA ExpoSAC Policy 3 for harvesting trees).
The TC for potential hand exposure included in EFSA (2014) could be conservative enough, considering that hand exposure was determined with handwash method in the UIPP study and there is no large difference, such as in the case of TC for potential body exposure.
Orchard crop thinning may be more contact‐intensive, according to EPA ExpoSAC Policy 3 database (TC (ADE body + PDE hand, geometric mean) = 3,600 cm2/h). No differences in TC values are established in EFSA Guidance (EFSA, 2014) for different crop activities in orchards.
The WG concluded that the TC for body exposure during harvesting orchards should be reduced to a conservative value of 10,000 cm2/h, corresponding to the TC for actual body exposure in EUROPOEM II. TC values established in EFSA Guidance (EFSA, 2014) are proposed to be applied for other activities than harvesting.
Derived TC values, considering a tenfold reduction of exposure for the use of (protective) clothing, are shown in Table H.12.
Table H.12.
Proposal for TC values for orchards (harvesting) to be included in OPEX the update of the EFSA Guidance (2014), according to UIPP study (Urtizberea, 2002)
| TC (cm2/h), Body potential exposure | TC (cm2/h), hand potential exposure | TC (cm2/h), total potential exposure | TC (cm2/h) assuming arms, body and legs covered (workwear; bare hands) | TC (cm2/h), covered body (workwear) and gloves (PPE) | |
|---|---|---|---|---|---|
| Proposal | 10,000 | 2,500 | 12,500* | 3,500** | 1,250*** |
*: 10,000 (body) + 2,500 (hand) = 12,500.
**: 10,000 (body) × 10% (workwear) + 2,500 (hand) = 3,500.
***: 10,000 (body) × 10% (workwear) + 2,500 (hand) × 10% (gloves) = 1,250.
H.2.5 Recommendations
It is recommended to include this study in an updated database including EUROPOEM II data, US EPA ExpoSAC data and a literature review of the more recent studies determining TC for orchards activities (thinning, harvesting, training, pruning, etc.).
Appendix I – Exposure to soil‐borne residue
No data are available to EFSA to establish TC values to estimate exposure following contact with soil‐borne residue. In addition, the US EPA has not derived any TC values for contact with soil. However, for situations in which exposure to soil‐borne residue occurs in the absence of contact with treated foliage, an estimate of potential (dermal) exposure may be derived by considering the concentration in the treated soil, together with soil dermal adherence data. As a default, the hand soil loading for a worker should be taken as 0.44 mg/cm2 (EFSA, 2008). A default value for inhalation exposure should be estimated assuming a total inhalation dust exposure of 98.6 mg/m3 (EFSA, 2008).
For handling compost after admixture treatment, the concentration in compost should be derived from the label‐recommended application rate for the admixture of the product with compost.
For other situations, such as hand planting in soil previously treated with plant protection products or hand harvesting root or bulb crops following desiccation, soil concentration values should be sought from the fate and behaviour evaluation:
for acute assessment, the highest initial predicted environmental concentration (PEC) soil value should be used;
Where exposure occurs at a time significantly after application, a measured estimate of the degradation in soil (DT50, soil) can be used to estimate the PEC in soil at the appropriate re‐entry time (see Annex E for explanation of how the online calculator evaluates exposure for extended re‐entry periods).
if chronic exposure is a concern, an appropriate time‐weighted average (TWA) value may be used;
-
Where values are not available from the fate and behaviour evaluation, soil concentrations for field applications can be estimated assuming:
-
–
the distribution is limited to the top 5 cm layer, or 20 cm when cultivation follows the application;
-
–
soil density is 1.5 g/cm3; and
-
–
100% (worst‐case PEC soil) of the applied dose reaches the soil surface (where ground cover is present, a minimum of 50% of the applied dose reaches the soil surface).
-
–
Appendix J – Recommendations for designing, conducting and assessing higher tier field studies
J.1 Introduction
Where first‐tier methods of exposure assessment based on generic assumptions fails to demonstrate an exposure within acceptable levels or no appropriate exposure model exist, higher tier field studies measuring the human exposure or other related parameter (e.g. DFR or TTR) may be used.
Furthermore, field studies might replace some of the precautionary assumptions incorporated within a first‐tier method, to cope with uncertainties (e.g. in cases where first‐tier models are based on only a few studies), with representative measurements for the specific active substance or plant protection product. However, a field study would not be accepted as being adequately representative unless more reliable and realistic estimate of exposures than first‐tier methods is provided.
However, studies should be conducted taking into account that exposure would be influenced by several factors, such as:
Activity monitored (mixing/loading, application, cleaning, thinning, harvesting, etc.).
Crop (type, growth stage, foliage structure, location (greenhouse, outdoor, etc.), height, row width, leaf wall area, etc.).
Spray equipment (nozzles, spray techniques, etc.).
Formulation (physicochemical properties, state of matter, etc.).
Container (type, size, neck diameter).
Weather and climatic conditions (temperature, humidity, wind, rainfall, etc.).
Mitigation measures (DRT, PPE, etc.).
Also, by considering the above‐mentioned parameters, the range of exposure may vary substantially (e.g. over two orders of magnitude). Therefore, the number of sites monitored, and the number of samples collected would determine the acceptability of the study. In addition, the selection of a suitable sampling and analysis method has also a significant influence on the results.
This appendix discusses the rationale for conducting and assessing higher tier field studies, providing information about the quality requirements, sampling and analytical methodology (study design, sampling parameters and techniques, samples storage, etc.) and detailed information on data analysis used to quantify exposure or other related parameter.
J.2 Reference documents for higher tier field studies
Field studies should be performed according to existing guidance documents or test guidelines, following a minimum set of general and specific requirements.
Non‐exhaustive list of guidance or test guidelines considered and the related exposure parameter (human exposure, DFR/TTR or both) are included in Table J.1.
Table J.1.
Available reference documents for higher tier studies
| Document | Exposure parameter | Reference |
|---|---|---|
| Occupational and Residential Exposure Test Guidelines | All | US EPA (1996) |
| Principles on Good Laboratory Practice | All | OECD (1998) |
| Occupational exposure to pesticides during agricultural application | Exposure (QA/QM) |
OECD (1997) Series on testing and assessment no 9 |
| Strategy for the evaluation of dermal exposure | Exposure (dermal) | CEN/TR 15278:2006 (CEN, 2006a) |
| Measurement of dermal exposure – Principles and method | Exposure (dermal) | CEN/TS 15279:2006 (CEN, 2006b) |
|
EUROPOEM II. Post‐application exposure of workers to pesticides in agriculture. |
DFR | Hemmen et al. (2002) |
| Procedures for the Determination of Dislodgeable Pesticide Residue on Foliage. | DFR (sampling part) | Iwata et al. (1977) |
| Procedures for the Determination of Transferable Pesticide Residue on Turf. | TTR (sampling part) | Fuller et al. (2001) |
| On data requirements for setting maximum residue levels, comparability of residue trials and extrapolation of residue data on products from plant and animal origin | DFR (sampling part) | SANTE/2019/12752 |
| Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post‐approval Control and Monitoring Purposes. | DFR/TTR (analytical part) | SANTE/2020/12830, Rev.1, 24. February 2021 |
| Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration | DFR/TTR (DT50) | FOCUS Work Group on Degradation Kinetics, Version 1.1., 18 December 2014 |
J.3 General requirements for field studies measurements
A non‐exhaustive list of recommended requirements for each target population (operator, worker, bystander or resident) and related parameter (exposure, DFR, TTR, etc.) is included in Table J.2.
Table J.2.
Recommended requirements for performance and analysis of higher tier field studies
| Criteria | Exposure parameter |
|---|---|
| General considerations/Quality | |
| A GLP compliance certificate. | Human exposure/DFR/TTR |
|
A GLP compliance statement. When the field phase and analytical phase are conducted by separate facilities, the appropriate documentation for the laboratory sub‐contracted to perform the analytical work is expected. |
Human exposure/DFR /TTR |
|
QA statement This should provide inspection dates for the key elements of the study (field and laboratory phases). |
Human exposure/DFR /TTR |
| Study design | |
| The study includes a review which shows that the study design used is representative of the scenario to be considered (e.g. currently typical cultivation and application methods in Europe, including demonstration of representative climatic conditions, e.g. with Köppen‐Geiger criteria). | Human exposure/DFR /TTR |
| Representative application methods and application techniques, according to the current agricultural application practices in Europe. Application equipment, tank volume, water volume, pressure, forward speed etc. should be described and reported. | Human exposure/DFR /TTR |
| Representative crop activities should be tested, reflecting current agricultural practices in Europe. Activities carried out by workers should be described in detail. | Human exposure/DFR /TTR |
| The test site is clearly defined, including location and positioning of the sampling points/person being exposed. | Human exposure/DFR /TTR |
| At least three test sites in different locations to capture variation in working/agronomic practices and environmental conditions would be desirable. A justification for the selection of the locations and the working/agronomic practices used in the study shall be provided. | Human exposure/DFR /TTR |
| The meteorological conditions must be fully reported. As a minimum, this must include temperature, humidity and rainfall (for worker exposure and DFR studies, information about date, duration and amount of rainfall is necessary). | Human exposure/DFR /TTR |
| Key experimental data must be reported. As a minimum this should be identification of the plant protection substance, formulation, application rate and crop (BBCH, age of the crop). Sprayer description should also be included. | Human exposure/DFR /TTR |
| It is recommended to consider the worst‐case intended use for each crop investigated (e.g. maximum application rates; multiple applications using the minimum treatment interval; late growth stage). | Human exposure/DFR /TTR |
| The timing of the applications should bracket the time frames when re‐entry activities are anticipated to occur, with a focus on the timeframes where higher exposure activities occur. Likewise, the transferable residue (e.g. DFR/TTR) samples should be collected accordingly. | Human exposure/DFR /TTR |
| Agricultural spray adjuvants should not be used unless they are recommended for the respective product (e.g. in cases where the use of adjuvants is mandatory). | Human exposure/DFR /TTR |
| Only necessary maintenance products (plant protection products and fertilisers) should be used. These products must not interfere with the chemical analysis. | Human exposure/DFR /TTR |
| For studies designed to provide estimate of TC values, the exposure measurements and DFR determinations should be done concurrently in the same crop and at the same sites. | Human exposure/DFR /TTR |
| According to OECD Guideline (OECD, 1997), at least 10 subjects are required for each task performed. It is recommended that a sufficient number of measurements be made in different locations to cover the range of use procedures and conditions, including as an example that variation in harvesting work procedures may be substantial, even within the same crop. | Human exposure |
| Monitoring of professional agricultural operators or workers (e.g. farmers and contractors) working in accordance with Good Agricultural Practices. | Human exposure |
| Representative workwear and PPE used should be described and reported. | Human exposure |
| To measure the exposure of uninvolved persons (e.g. residents and bystanders) to spray‐drift, mannequins (other types of dosimeters are also acceptable if the transferability to humans/mannequins is demonstrated) for adults and children/toddler are to be used (i.e. 2D measurements are no longer acceptable). The body surface of the mannequins should be comparable to that described in 2.4.7. Default surface area of body parts. For the total of the trials, at least ten mannequins are to be used for each distance and group (e.g. ten mannequins each for adults and children/toddler). | Human exposure |
| Mannequins are to be positioned downhill (if applicable) and downwind direction (at application) of the field plots in order to measure the spray‐drift (i.e. ‘worst‐case’ conditions). Thereby, mannequins are to be set up staggered at different distances from the treated culture (e.g. to avoid a ‘spray shadow’). | Human exposure |
| At the test site one or several field plot(s) and one control plot should be established. In order to obtain representative samples from a field plot, it must be divided into at least 3 subplots. Replicate sample should be taken from the different subplots of a field plot. | DFR/TTR |
| The control plot will be positioned upslope (if applicable) and upwind (at application) of the field plots to reduce the potential for contamination due to drift. The separation distance between control and field plots should be sufficient to avoid contamination of the control plot while ensuring that the crop, soil and environmental conditions are the same in field and control plots. | DFR/TTR |
| Since climatic conditions and growing conditions can influence the dissipation rate, studies should be performed at sites representative of the climatic and growing conditions representative of the intended use areas. The Köppen–Geiger criteria may be useful when considering climatic equivalence. (Note: If the intended use is relevant for the entire EU then representativeness of climatic conditions should be covered by multiple field studies, unless comparability of climatic conditions or ‘worst‐case’ conditions for the relevant crop can be justified, based on the residue guidelines (e.g. SANTE/2019/12752) a differentiation for northern and southern studies for outdoor crops should normally be sufficient). | DFR/TTR (DT50) |
| Individual studies should be conducted in areas where the slowest dissipation of residue is assumed, i.e. representing ‘worst‐case’ conditions. There should be no rainfall for 24 h before and after applying the product. If the precipitation during the sampling period is higher than the typical precipitation at the field location, the study may not be acceptable for the estimation of half‐lives (DT50). However, this should be decided on the basis of the resulting dissipation kinetics. | DFR/TTR |
| Sampling parameters | |
| The sampling approach should be clearly described and be justifiable, representative and appropriate, allowing for a consistent sample collection. It should include sampling time, sampling interval, distance from application to sampling point, sampling height, foliage type, etc. | Human exposure/DFR/TTR |
|
To verify the application rate, and the amount of active substance loaded and applied per tank, tank mix samples should be taken and analysed. Various sampling techniques can be used, e.g. samples can be taken directly from the spray nozzles; from a tap attached to the tank or directly from the tank. It is recommended to take at least three samples (e.g. at the beginning in the middle and at the end of each treatment). The nozzles must be calibrated at the beginning of each treatment. Other sampling techniques can also be used if these methods are appropriate for analysing the concentration of the spray solution. |
Human exposure/DFR/TTR |
| The active substance, or any degradation products relevant to the risk assessment, should be sufficiently stable under field conditions to permit reliable estimation of exposure and other values. | Human exposure/DFR/TTR |
| It is recommended that the formulation used in the study should be used for fortification experiments when analytics is assumed to be influenced by co‐formulants (e.g. lower extraction efficiency). | Human exposure/DFR/TTR |
| Worker exposure should only be measured during re‐entry activities. Workers should enter the treated areas only after the foliage has dried off. | Human exposure |
| Ideally, the exposure duration for a single measurement of exposure or absorbed dose should be representative of the typical working day. Actual exposure duration is to be measured and it is expected to be around at least 4–5 h, up to or even exceeding 8 h for operators and workers. For certain tasks, however, shorter exposure durations can also be considered (e.g. ~ 2 h for crop inspection and irrigation tasks). Duration of tasks should be given. | Human exposure |
| In order to measure the exposure of uninvolved person to spray‐drift, the entire plot must be treated with the application technique and application rate specified in the list of intended uses (i.e. not only the outer row of the culture). The application should take place in a growth stage according to the intended uses (Note: in general, data in lower growth stages cover later growth stages, as the growth and the changing density of the foliage can directly influence the spray‐drift). | Human exposure |
| A minimum of three replicate samples should be taken in each field plot and at each sampling interval. However, more are recommended (e.g. four to six) to provide more robust data and a better estimate of the DFR value (see also Criteria below). Where only the minimum are provided, the representative DFR value is likely to be set at the maximum value observed. | DFR/TTR |
| Replicate samples are to be taken from the areas of the plant where contact with workers is expected. Different approaches are available e.g. non‐directed sampling where field technicians enter a treated area and sample at their own discretion; the Iwata approach (Iwata et al., 1977) for tree crops where samples are collected at 45 degree intervals around the circumference of each sampled tree and at varying heights in the tree; the planned approach for row crops where investigators develop a scheme that predetermines sample collection locations. | DFR |
| To characterise dissipation rates of dislodgeable residue (DT50), data should be sufficient to cover several half‐lives (e.g. three half‐lives). Typical sampling intervals are 4 h, 12 h, 1, 3, 7, 14, 21, 28, 35 days after treatment (DAT). If the study involves multiple applications, samples should be taken prior to and after each application on the day of application. It is also suggested that samples are taken in the intervals between the application events at least every 7 days after each application. | DFR/TTR |
| Sampling techniques | |
| Samples should be collected and prepared in the field, if necessary, transported and stored according to OECD 1997 (see also EC Guidance 7029/VI/95 rev. 5). | Human exposure/DFR/TTR |
|
Inhalation exposure should be determined with appropriate inhalation fraction samplers (e.g. personal air sampling). Whole body dosimetry for dermal exposure should be selected. Patch data should not be considered unless uniform exposure can be demonstrated. Absorbent gloves method should be prioritised over hand wash or rinse methods unless efficiency of these methods is determined. |
Human exposure |
|
For sampling and extracting of leaves, the protocol by Iwata et al. (1977) should be followed. In short, leaf samples should be gathered with a mechanical leaf punch device (equal to ~ 200 cm2 single side, or 400 cm2 double‐sided). Some crops do not lend themselves to the use of a leaf punch (e.g. some ornamentals and conifers). Determinations of leaf sample surface areas should be addressed on a case‐by‐case basis. Ideally within 4 h and always within 24 h leaves, samples should be extracted by washing the surface of the leaf with a water/surfactant solution (e.g. a 0.01% dioctyl sulfosuccinate, sodium salt solution). The use of organic solvents should be avoided as they may carry surface residue into the leaf tissues or extract penetrated residue. Non‐extracted samples should not be stored freeze or with dry ice. |
DFR |
| For measuring the amount of transferable residue on turf, the protocol by Fuller et al. (2001) should be followed (Modified California Roller Method). In short, a 100% white cotton percale sheet (0.68 m²; 0.58 m² sampling area) is securely attached to a PVC frame and placed on turf‐covered ground. To collect residue, a weighted roller is pushed five times over the sample area. Visible debris (e.g. grass clipping, thatch, granules) are carefully removed before the cotton cloth is placed in a suitable sample container and sent to an analytical laboratory. | TTR |
| Sample storage | |
|
Samples should be stored in a manner that will minimise deterioration and loss of analyte(s) between collection and analysis. Sample storage time should be recorded. The study investigator is responsible for demonstrating the stability of the samples under the storage duration and conditions used (for further details see ‘quality assurance/quality control’ below). |
Human exposure/DFR/TTR |
| Quality assurance/quality control (pre‐field laboratory considerations) | |
| SANTE/2020/12830, Rev.1, 24. February 2021 should be used when generating and reporting methods of analysis. Any analytical method used to analyse samples from field studies needs to be sufficiently validated regarding all parameters in accordance with the available guidance in force. | Human exposure/DFR/TTR |
|
The stability of analyte(s) should be determined on appropriate sampling matrices under storage conditions similar to those anticipated for storage of field samples. This study is optional if the field recovery samples are stored and analysed with the actual field samples. Storage stability samples should include preparation and analysis of at least three blanks, three low‐level fortifications and three high‐level fortifications. Samples should be stored under the same conditions as planned for field samples and the study duration should be ≥ the likely storage duration of the field samples. These can be done before or in conjunction with the field phase. |
Human exposure |
| Quality assurance/quality control (in field considerations) | |
| Valid field recovery data (and thus, the ability to accurately fortify field recovery samples with a known amount of mass ingredient) is essential to the study, to allow the experimental data to be corrected for losses that occur during all phases of sample collection and analysis. | Human exposure/DFR/TTR |
|
Ideally, a complete set of field recovery samples should be collected at each site and on each day of sampling. If it can be shown that the field recovery does not change over the sampling period, then in the case of DFR studies, a complete set of field recovery may not be required for each sampling day. It may be acceptable to collect a single set of field recovery samples if the environmental conditions are similar on each day and/or at each site. |
Human exposure/DFR/TTR |
|
A complete set of field recovery samples should include 3 (or more) samples, each blank control samples, low level fortification and high level fortification. The high and low fortification should cover the range of the anticipated level of chemical on the respective matrices. If the highest expected level is more than 100X the lowest spiking level, it is recommended that a midlevel of fortification is included. |
Human exposure/DFR/TTR |
|
Field recovery samples should be handled using the same procedures as the actual field samples. They should be collected, handled, transported and stored concurrently with actual field samples. Additionally, field recovery samples should be analysed concurrently with actual field samples to account for residue losses during sample extraction and analysis. |
Human exposure/DFR/TTR |
|
Field recovery results less than 95% should be used to correct the results of field samples. However, if field recoveries are below 70% they must be technically justified. Recovery results greater than 95–100% should be noted but not used to correct the data. Actual field samples should be corrected with the closest spiking level obtained from the fortified samples. |
Human exposure/DFR/TTR |
|
Blank control field samples indicate whether contamination of the field recovery samples has occurred. The report should provide a valid explanation for the occurrence of residue in control samples when results are higher than 30% of LOQ. |
Human exposure/DFR/TTR |
|
Travel recovery samples should be shipped and stored with the field recovery and actual field samples. Travel recovery samples are optional and reflect losses which may occur during shipment and possibly storage. These samples are not used to correct actual field samples but may be useful to determine where losses have occurred. |
Human exposure/DFR/TTR |
| Quality assurance/quality control (post‐field laboratory considerations) | |
|
Laboratory recovery samples are analysed in the analytical laboratory concurrently with the actual field samples to determine the recovery efficiency of the analyte(s) from the respective matrices. It is recommended that the field recovery samples are used as concurrent laboratory samples whenever possible. When used in this manner, field recovery samples can be used to correct actual field samples for losses that occur both in the field and in the laboratory. |
Human exposure/DFR/TTR |
| Presenting and analysing results | |
| Raw data must be provided as well as detailed observations on operators and workers. | Human exposure/DFR/TTR |
| Results should be reported as absolute values (μg or mg active ingredient per sample) as well as mg or μg active ingredient per kg active ingredient applied. | Human exposure/DFR /TTR |
| If residues are below the limit of quantification (LOQ) and above the limit of detection (LOD), they should be reported as below LOQ (e.g. < LOQ), but they should be considered as LOQ. | Human exposure/DFR /TTR |
| If residue are below the limit of detection (LOD), they should be reported below LOD (e.g. < LOD), but they should be considered as LOD. | Human exposure/DFR /TTR |
| A justification for excluding outliers should be clearly stated in the study report and summary text. Although outliers may be excluded from the analysis if well justified, for technical or procedural reasons e.g. part of the sample extract was lost (note a statistical test alone is not sufficient justification), the data must nevertheless be presented. It should be noted that results treated as outliers should include spuriously low values as well as high values. Expert judgement might ultimately be applied on a case‐by‐case basis to increase values compensating for deficiencies in the quality of the study. Justification for choosing a certain increased value should be provided and fully documented in such cases. | Human exposure/DFR /TTR |
| Statistical analysis is appropriate and must be provided addressing the variability of the study results. | Human exposure/DFR /TTR |
| Exposure values must be related to a single intake route (e.g. dermal and inhalation exposure should not be combined). | Human exposure |
| Suitable data form for model development: separately measured hand, head and body exposure; separate measurements for the different activities (mixing/loading, application, cleaning, etc.) and for inner and outer dosimeters. | Human exposure |
| Exposure is reported as the amount of active ingredient the individual person receives; amount as excreted from the body (urine samples) will only be used as supplementary information. | Human exposure |
| When using results of exposure measurements, considering that a sample size as low as 10 subjects is allowed by the respective guideline (OECD No 9, 1997), due consideration should be given to the statistical analysis of such small data set for the purpose of acute risk assessment | Human exposure |
| Correction for background concentration should not be performed. If the worst‐case intended use for each crop investigated is considered, no correction is needed even in the case of multiple applications. If residue are found before the first application, then consideration should be given to use determined DFR/TTR value without correction or rejecting the study entirely. | DFR/TTR |
| The highest DFR/TTR value should be used if only 3 replicate samples were taken from a field plot per sampling interval. When ≥ 4 replicate samples are available per field plot and per sampling interval, the use of a mean might be justified. However, if there is significant variation between these replicate samples (i.e. the standard deviation is equal to or larger than 25% of the mean) the standard deviation should be added to the mean value. | DFR /TTR |
|
DT50 values can only be derived from acceptable DFR studies, therefore all validity criteria for DFR studies must be taken into account. For estimation of DT50 the standard procedures recommended by FOCUS (2014) should be followed, including e.g. the general procedure and the assessment of the goodness‐of‐fit. Since calculated DT50 values are used in models for exposure assessments (e.g. determination of the MAF), single first‐order kinetics should generally be used (EFSA, 2014c). More recommendations on the fitting of DT50 data and the statistical validation of the fit can also be found in the EFSA Technical Report (2019). |
DFR /TTR (DT50) |
|
In case of multiple applications, when a field study is available, but not considered sufficient for the specific DFR estimation, the following should be considered for the DT50 derivation: a) If appropriate data (adequate sampling points) in between the different applications are available then: – each application (and the following points until the next application) can be considered as a standalone trial – a DT50 is calculated for each application and then the geomean (GM) of the calculated DT50 values, – depending on the amount and variability of the data, use either the GM or the highest DT50 value calculated as a worst case. b) If the sampling points for the in between applications are not adequate for the calculation of single DT50 values, the data set after the last application is to be used. |
DT50 |
J.4 Conclusions
In general, field studies are a suitable way to replace generic ‘realistic worst‐case’ values. In cases where Tier 1 methods of exposure assessment do not lead to acceptable levels, field studies measuring the human exposure or other relevant parameters (e.g. DFR) may be used.
During the evaluation of the existing guidance documents diverging recommendations for the implementation and evaluation of field studies were found, e.g. different guidance documents containing different number of replicates, type of dosimeters to be used, acceptance criteria for recovery. To the knowledge of EFSA, there is currently no internationally harmonised guidance document, e.g. for the determination of DFR and TTR. This analysis clearly indicates the need for improved harmonisation among guidance/guideline documents for field studies measuring the human exposure and the development of such for the determination of DFR and TTR.
Due to the lack of harmonisation, different acceptance criteria and rules are currently used for evaluating studies in the various Member States. Therefore, this appendix provides general acceptance criteria for conducting and evaluating field studies and studies for the determination of DFR and TTR with regard to quality requirements, sampling and analysis methods (study design, sampling parameters and techniques, sample storage, etc.) as well as detailed information on data analysis and quantification of exposure. This appendix does not address criteria for extrapolation between crops, since no data are currently available to identify critical parameters.
The information presented here is intended to ensure that this is taken into account when planning and carrying out field studies and studies for the determination of DFR as well as TTR and to harmonise acceptance criteria and rules for the evaluation of studies between the different member states. However, it is not intended to supplement or replace the current guidelines.
Therefore, it is acknowledged that current OECD guidelines should be further developed to support a globally harmonised approach for the conducting of field studies measuring human exposure and for the determination of DFR and TTR.
Annex A – CIPAC formulation codes
Annex B – Public literature on dislodgeable foliar residue
Annex C – Protocol for the review of relevant DT studies
Annex D – DT Data collection
Annex E – New calculator
Annex F – Table overview open call
Annex G – Outcome of Public Consultation on the draft EFSA Guidance
Annex A–G can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7032
Supporting information
CIPAC formulation codes
Public literature on dislodgeable foliar residue
Protocol for the review of relevant DT studies
DT Data collection
New calculator
Table overview open call
Outcome of Public Consultation on the draft EFSA Guidance
Suggested citation: EFSA (European Food Safety Authority) , Charistou A, Coja T, Craig P, Hamey P, Martin S, Sanvido O, Chiusolo A, Colas M and Istace F, 2022. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment of plant protection products. EFSA Journal 2022;20(1):7032, 134 pp. 10.2903/j.efsa.2022.7032
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00274
Correspondence: pesticides.ppr@efsa.europa.eu
Update: This scientific output, approved on 30 November 2021, supersedes the previous output published on 24 April 2015 (EFSA Guidance, 2014). The European Commission will decide the implementation time for the mandatory use of this guidance in the regulatory context.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The EFSA Working Group wishes to acknowledge the hearing experts, Denise Bloch, Isaac Abril Munoz and Thierry Mercier, for the views provided to this scientific output. It is also acknowledged the support of the EFSA AMU Unit in the revision of the online calculator, through the framework contract with Open Analytics NV.
Adopted: 30 November 2021
Notes
Non‐certified workwear was a mixed fabric of cotton and polyester with at least 65% polyester and an average grammage of ≥ 245 g/m².
Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on personal protective equipment and repealing Council Directive 89/686/EEC. Commission communication in the framework of the implementation of Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment and repealing Council Directive 89/686/EEC. The EN ISO 27065:2017 Protective clothing — Performance requirements for protective clothing worn by operators applying pesticides and for re‐entry workers (ISO 27065:2017) is listed in the Commission communication.
Handheld applications are the main application techniques in greenhouses in Europe. In addition, there are no data available on operator exposure from tractor‐mounted application techniques in greenhouses. Therefore, the model presented here only covers exposure from handheld application techniques.
Due to one duplicate publication and two in Chinese, finally 130 publications were the objective of this external outsourcing and not 133 as concluded in the pre‐screening pilot study.
The WG considered the use of the LOQ as more appropriate for this type of analysis. The use of the LOQ as surrogate value for residue is based on the worst‐case assumption that the amount is close to or almost equal to the LOQ, thereby ensuring that derived values are not underestimating the exposure. However, it should be noted that the use of different surrogates (LOQ vs. 1/2 LOQ) does not significantly alter the results of the assessment presented here, but it may be more relevant if all measured values are close to the limit of quantification.
The mean was chosen in order to account for the fact that workers may have been exposed to variable DFR‐levels while moving through the field. Using a maximum or minimum value would lead to significantly lower or higher TC‐values, respectively, which are not considered as adequately reflecting the real experimental situation.
See also recommendations of seed vendors to obtain maximum sugar yields, e.g.: https://www.kws.com/gb/en/consulting/sowing/sowing‐sugarbeet/; https://hort.purdue.edu/newcrop/afcm/sugarbeet.html
References
- AENOR (Asociación Española de Normalización y Certificación) , 2009. UNE‐EN13034:2005+A1:2009: Protective clothing against liquid chemicals ‐ Performance requirements for chemical protective clothing offering limited protective performance against liquid chemicals (Type 6 and Type PB [6] equipment), 23 September 2009.
- ANSES (Agence Nationale Sécurité Sanitaire Alimentaire) , 2018. Report from the Workshop on Toxicological Risk Assessment of Plant Protection Products. Available online: https://www.anses.fr/en/system/files/WorkshoponToxicologicalRiskAssessmentofPPP‐FinalReport.pdf
- Baumann J, Anft T, Doughty KJ and Kuster CJ, 2019. Exposure to pesticide residue during manual removal of bolting sugar beets: determination of transfer coefficients for worker risk assessment. Journal of Consumer Protection and Food Safety, 14, 283–286. [Google Scholar]
- Beulke S, van Beinum W, Glass R, van Os E, Hoterman HJ, Sapounas A, Voogt W, van de Zande J, de Zwart F and Garratt J, 2011. Estimation/calculation of emissions of Plant Protection Products from protected crops greenhouses and cultivations grown under cover to support the Development of risk assessment methodology under Council Directive 91/414/EEC and EU regulation 1107/2009 (EC). EFSA Supporting Publication 2011;8(5):EN‐151, 55 pp. 10.2903/sp.efsa.2011.EN-151 [DOI] [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung, German Federal Institute for Risk Assessment) , 2013. Joint development of a new Agricultural Operator Exposure Model, BfR, Berlin. 259 pp. Available online: https://www.bfr.bund.de/cm/350/joint‐development‐of‐a‐new‐agricultural‐operator‐exposure‐model.pdf
- BfR (Bundesinstitut für Risikobewertung, German Federal Institute for Risk Assess) , 2015. Joint development of a new Greenhouse Agricultural Operator Exposure Model for handheld application. BfR, Berlin. 117 pp. Available online: https://www.bfr.bund.de/cm/350/joint‐development‐of‐a‐new‐greenhouse‐agricultural‐operator‐exposure‐model‐for‐handheld‐application.pdf
- BfR (Bundesinstitut für Risikobewertung, German Federal Institute for Risk Assess) , 2020. Update of the Greenhouse Agricultural Operator Exposure Model – Amendment to Project Report 01/2016. BfR, Berlin. 133 pp. Available online: https://www.bfr.bund.de/cm/350/revision‐of‐the‐greenhouse‐agricultural‐operator‐exposure‐model.pdf
- Butler Ellis MC and Miller PCH, 2010. The Silsoe Spray Drift Model: a model of spray drift for the assessment of non‐target exposures to pesticides. Biosystems Engineering, 107, 169–177. [Google Scholar]
- Butler Ellis MC, Lane AG, O’Sullivan CM, Miller PCH and Glass CR, 2010a. Bystander exposure to pesticide spray drift: new data for model development and validation. Biosystems Engineering, 107, 162–168. [Google Scholar]
- Butler Ellis MC, Underwood B, Peirce MJ, Walker CT and Miller PCH, 2010b. Modelling the dispersion of volatilised pesticides in air after application for the assessment of resident and bystander exposure. Biosystems Engineering, 107, 149–154. [Google Scholar]
- Butler Ellis MC, Van Den Berg F, Van De Zande JC, Kennedy M, Charistou A, Arapaki N, Butler A, Machera K and Jacobs C, 2017. The BROWSE model for predicting exposures of residents and bystanders to agricultural use of pesticides: comparison with experimental data and other exposure models. Biosystems Engineering, 154, 122–136. 10.1016/j.biosystemseng.2016.09.002 [DOI] [Google Scholar]
- Bystanders, Residents, Operators and WorkerS Exposure models for plant protection products (BROWSE) , 2016. Project reports related to worker exposure models. Available online: https://secure.fera.defra.gov.uk/browse/software/ (relevant links: Detailed Technical Report ‐ Worker Exposure Model; Detailed Technical Report ‐ Worker Model ‐ Appendices; Comparison report ; Comparison report appendix II).
- California Environmental Protection Agency, Air Resources Board , 1998. Report for the application and ambient air monitoring for chlorpyrifos (and the oxon analogue) in Tulare County during spring/summer 1996.
- Camann DE, Majumadar TK and Geno P, 1995. Determination of pesticide removal efficiency from human hands wiped with gauze moistened with three salivary fluids. Final Report to EPA by ManTech under Contract 68‐D5‐0049. [Google Scholar]
- CEN (European Committee for Standardization) , 2006a. Assessment of workplace exposure to chemical and biological agents. Workplace Exposure ‐ Strategy for the evaluation of dermal exposure ‐ CEN/TR 15278:2006.
- CEN (European Committee for Standardization) , 2006b. Assessment of workplace exposure to chemical and biological agents. Workplace exposure. Measurement of dermal exposure. Principles and methods ‐ CEN./TR 15279:2006.
- Commission communication in the framework of the implementation of Regulation (EU). 2016/425 of the European Parliament and of the Council on personal protective equipment and repealing Council Directive 89/686/EEC.
- CRD (The Chemical Regulation Directorate, UK) , 2008. Bystander Exposure Guidance. Available online: https://www.pesticides.gov.uk/guidance/industries/pesticides/topics/pesticide‐approvals/enforcement/resident‐and‐bystander‐exposure‐to‐pesticides
- DiBartolomeis M, Kegley S, Mineau P, Radford R and Klein K, 2019. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS One, 14, e0220029. 10.1371/journal.pone.0220029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan Ngoc K, 2014. The development of an improved model to assess worker re‐entry exposure to plant protection products. PhD Thesis. Ghent University, Belgium. Available online: https://biblio.ugent.be/publication/5782963
- Doan Ngoc K, van den Berg F, Houbraken M and Spanoghe P, 2015. Volatilisation of pesticides after application in vegetable greenhouses. Science of the Total Environment, 505, 670–679. 10.1016/j.scitotenv.2014.10.036 [DOI] [PubMed] [Google Scholar]
- Duyzer J and Vonk A, 2002. Atmosferische depositie van pesticiden, PAK en PCB’s in Nederland. TNO‐Milieu, Energie en Procesinnovatie. R 2002/606, 155 pp. In Dutch.
- Duyzer J, Van der Staaij M, Weststrate H, Boertjes B, Hollander K and Verhagen H, 2004. De blootstelling van omwonenden van kassen aan gewasbeschermingsmiddelen via de lucht. TNO‐rapport, R 2004/517: 72 pp. In Dutch.
- Ebeling M and Wang M, 2018. Dissipation of plant protection products from foliage. Environmental Toxicology, 37, 1926–1932. 10.1002/etc.4148 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) , 2015. Biocides Human Health Exposure Methodology. Available online: https://echa.europa.eu/documents/10162/17158508/bpr_exposuremethodbiochh_en.rtf/17e40d4c‐5f48‐4e12‐952b‐5372bfe2403c
- ECHA (European Chemicals Agency) , 2017. Recommendation no. 14 of the BPC Ad hoc Working Group on Human Exposure Default human factor values for use in exposure assessments for biocidal products (revision of HEEG opinion 17 agreed at the Human Health Working Group III on 12 June 2017). Available online: https://echa.europa.eu/documents/10162/1154636/recom_14+_default+human_factor_values_biocidal+products_en.pdf/88354d31‐8a3a‐475a‐9c7d‐d8ef8088d004?t=1498041450529
- EFSA (European Food Safety Authority) , 2008. Agreement number EFSA/PPR/2007/01 ‘Project to assess current approaches and knowledge with a view to develop a Guidance Document for pesticide exposure assessment for workers, operators, bystanders and residents’. 542 pp. Available online: https://www.efsa.europa.eu/en/scdocs/doc/26e.pdf
- EFSA (European Food Safety Authority) , 2008. Project to assess current approaches and knowledge with a view to develop a Guidance Document for pesticide exposure assessment for workers, operators, bystanders and residents. EFSA agreement number EFSA/PPR/2007/01.
- EFSA (European Food Safety Authority) , 2010a. Guidance Document on Risk Assessment for Birds and Mammals on request from EFSA; Appendix H. EFSA Journal 2009;7(12):1438, 45 pp. 10.2903/j.efsa.2009.1438 [DOI]
- EFSA (European Food Safety Authority) , 2010b. Scientific Opinion on emissions of plant protection products from greenhouses and crops grown under cover: outline for a new guidance. EFSA Journal 2010;8(4):1567, 44 pp. 10.2903/j.efsa.2010.1567 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Scientific Opinion on clustering and ranking of emissions of plant protection products from protected crops (greenhouses and crops grown under cover) to relevant environmental. EFSA Journal 2012;10(3):2611, 87 pp. 10.2903/j.efsa.2012.2611 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014a. EFSA Guidance Document on clustering and ranking of emissions of active substances of plant protection products and transformation products of these active substances from protected crops (greenhouses and crops grown under cover) to relevant environmental compartments. EFSA Journal 2014;12(3):3615, 43 pp. 10.2903/j.efsa.2014.3615 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014b. Outcome of the Public Consultation on the draft EFSA Guidance Document on the Assessment of Exposure for Operators, Workers, Residents and Bystanders in Risk Assessment for Plant Protection Products. EFSA supporting publication 2014;EN‐681, 97 pp. 10.2903/j.efsa.2014.681 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014c. Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 60 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in ecotoxicology. EFSA supporting publication 2015;EN‐924, 62 pp. 10.2903/j.efsa.2015.924 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Buist H, Craig P, Dewhurst I, Hougaard Bennekou S, Kneuer C, Machera K, Pieper C, Court Marques D, Guillot G, Ruffo F and Chiusolo A, 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 150 pp. 10.2903/j.efsa.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018. Call for new scientific information/data related to the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. Available online: https://www.efsa.europa.eu/en/consultations/call/180618 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , 2019. Technical report on the outcome of the Pesticides Peer Review Meeting on general recurring issues in ecotoxicology. EFSA supporting publication 2019;EN‐1673, 117 pp. 10.2903/sp.efsa.2019.EN-1673 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residue) , 2008. Scientific Opinion of the Panel on Plant protection products and their Residue (PPR) on the Science behind the Guidance Document on Risk Assessment for birds and mammals. EFSA Journal 2008;6(7):734, 790 pp. 10.2903/j.efsa.2008.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residue) , 2010. Scientific Opinion on preparation of a guidance document on pesticide exposure assessment for workers, operators, bystanders and residents. EFSA Journal 2010;8(2):1501, 65 pp. 10.2903/j.efsa.2010.1501 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579. 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1998. Council Directive 98/24/EC of 7 April 1998 on the protection of the health and safety of workers from the risks related to chemical agents at work (fourteenth individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC).
- European Commission , 2004. Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
- European Commission , 2008. Pesticides in air: considerations for exposure assessment. Report prepared by the FOCUS Working Group on Pesticides in Air (FOCUS Air Group). SANCO/10553/2006 Rev.2 June 2008, 328 pp.
- European Commission , 2011. HEEG opinion 13 Assessment of Inhalation Exposure of Volatilised Biocide Active Substance.
- European Commission , 2016. Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on personal protective equipment and repealing Council Directive 89/686/EEC.
- European Commission , 2017a. Default human factor values for use in exposure assessments for biocidal products. Revision of HEEG opinion 17 agreed at the Human Health Working Group III on 12 June 2017. Available online: https://echa.europa.eu/documents/10162/1154636/recom_14+_default+human_factor_values_biocidal+products_en.pdf/88354d31‐8a3a‐475a‐9c7d‐d8ef8088d004?t=1498041450529
- European Commission , 2017b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products SANTE‐10832‐2015 24, January 2017, rev. 1.7. [DOI] [PMC free article] [PubMed]
- Fantke P and Juraske R, 2013. Variability of pesticide dissipation half‐lives in plants. Environmental Science and Technology, 47, 3548–3562. Available online: http://pubs.acs.org/doi/abs/10.1021/es303525x [Accessed: 3 August 2017]. [DOI] [PubMed] [Google Scholar]
- Fantke P, Gillespie BW, Juraske R and Jolliet O, 2014. Estimating half‐lives for pesticide dissipation from plants. Environmental Science and Technology, 48, 8588–8602. 10.1021/es500434 [DOI] [PubMed] [Google Scholar]
- Federal Biological Research Centre for Agriculture and Forestry, Federal Republic of Germany , 2000.
- FOCUS (Forum for the co‐ordination of pesticide fate models and their use) , 2006. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 version 2.0. 434 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2014. Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. FOCUS Work Group on Degradation Kinetics, Version 1.1., 18 December 2006.
- Fuller R, Klonne D, Rosenheck L, Eberhart D, Worgan J and Ross J, 2001. Modified California Roller for measuring transferable residue on treated turfgrass. Bulletin of Environmental Contamination and Toxicology, 67, 787–794. [DOI] [PubMed] [Google Scholar]
- Ganzelmeier H and Rautmann D, 1995. Studies on the spray drift of plant protection products. Mitteilungen aus der BBA für Land‐und Forstwirtschaft Berlin‐Dahlem, Heft 305, 111 pp. [Google Scholar]
- Garthwaite D, Sinclair C, Glass R, Pote A, Trevisan M, Sacchettini G, Spanoghe P, Ngoc KD, Fevery D, Machera K, Charistou A, Nikolopoulou D, Arapaki N, Tskirakis A, Gerritsen‐Ebben R, Spaan S, González FE, Stobiecki S, Śliwiński W, Stobiecki T and Hakaite P, 2015. Collection of pesticide application data in view of performing environmental risk assessments for pesticides. EFSA Supporting Publication 2015;12(7):EN‐846, 246 pp. 10.2903/sp.efsa.2015.EN-846 [DOI] [Google Scholar]
- Gerritsen‐Ebben RMG, Brouwer DH and van Hemmen JJ, 2007. TNO report V7333, Effective Personal Protective Equipment (PPE). Default setting of PPE for registration purposes of agrochemical and biocidal pesticides. [PubMed]
- Glass CR, Mathers JJ, Harrington P, Miller PCH, Butler Ellis C and Lane A, 2010. Generation of field data for bystander exposure and spray drift with arable sprayers. Aspects of Applied Biology, 99, 271–276. [Google Scholar]
- Glass CR, Mathers JJ, Hetmanski MT, Sehnalova M and Fussell RJ, 2012. Development of techniques to measures vapour concentrations of pesticides to determine potential bystander & resident exposure. Aspects of Applied Biology, 114, 79–86. [Google Scholar]
- Glass R, Garthwaite D, Pote A, Kennedy M, Hart A, Trevisan M, Grasso P, Sacchi A, Spanoghe P, Ngoc KD, Beck B, Machera K, Nikolopoulou D, Arapaki N, Gerritsen‐Ebben R, Spaan S, Goede H, Morgan N, González FE, Stobiecki S, Śliwiński W, van Engelen J and Bokkers B, 2012. Collection and assessment of data relevant for non‐ dietary cumulative exposure to pesticides and proposal for conceptual approaches for non‐dietary cumulative exposure assessment. EFSA Supporting Publications 2012;9(10):EN‐346, 192 pp, 10.2903/sp.efsa.2012.EN-346 [DOI] [Google Scholar]
- Hamey P, Byron N, Hanley L, Leslie W, Morgan N, Steurbaut W, de Backer E and Vergucht S, 2009. Final report: Project to assess current approaches and knowledge with a view to develop a Guidance Document for pesticide exposure assessment for workers, operators, bystanders and residents. EFSA Supporting Publication 2009;6(3):EN‐26, 50 pp. 10.2903/sp.efsa.2009.EN-26 [DOI] [Google Scholar]
- Hou R, Tong M, Gao W, Wang L, Yang T and He L, 2017. Investigation of degradation and penetration behaviors of dimethoate on and in spinach leaves using in situ SERS and LC‐MS. Food Chemistry, 237, 305–311. 10.1016/j.foodchem.2017.05.117 [DOI] [PubMed] [Google Scholar]
- Hyndman RJ and Fan Y, 1996. Sample quantiles in statistical packages. The American Statistician, 50, 361–365. [Google Scholar]
- INSST (Instituto Nacional de Seguridad y Salud en el Trabajo) , 2020. Recommendations guide for the design, construction, inspection and calibration of trolley sprayers for the application of plant protection products in greenhouses.
- Iwata Y, Knaak JB, Spear RC and Foster RJ, 1977. Worker re‐entry into pesticide treated crops. I. Procedures for the determination of dislodgeable pesticide residue on foliage. Bulletin of Environment Contamination and Toxicology, 18, 649. [DOI] [PubMed] [Google Scholar]
- Kennedy MC and Butler Ellis MC, 2017. Probabilistic modelling for bystander and resident exposure to pesticides using the Browse software. Biosystems Engineering, 154, 105–121. [Google Scholar]
- Kennedy MC, Butler Ellis MC and Miller PCH, 2012. BREAM: a probabilistic bystander and resident exposure assessment model of spray drift from an agricultural boom sprayer. Computers and Electronics in Agriculture, 88, 63–71. [Google Scholar]
- Kirknel AR and Emde X, 1997. Pesticides Research No. 31, Pesticide Re‐entry Exposure of Workers in Greenhouses, Danish Institute of Agricultural Sciences, Flakkebjerg, DK‐4200 Slagelse.
- Koenker R, 2005. Quantile Regression. Economic Society Monographs No 38. In: Chesher A and Jackson M (eds.), Cambridge University Press, 368 pp. [Google Scholar]
- Lahr J, Krämer W, Mazerolles V, Poulsen V, Jölli D, Müller M, McVey E, Wassenberg J, Derkx R, Brouwer A, Deneer D, Beltman W, Lammertsma D, Jansman H, Buij R, 2018. Data collection for the estimation of ecological data (specific focal species, time spent in treated areas collecting food, composition of diet), residue level and residue decline on food items to be used in the risk assessment for birds and mammals. EFSA supporting publication 2018;EN‐1513, 155 pp. [Google Scholar]
- Landwirtschaftlicher Informationsdienst Zuckerrübe (Agricultural Information Service, Sugar Beet) , 2020. Rübenschosser (Bolting Beets). Available online: https://www.liz‐online.de/fileadmin/user_upload/pdf/ruebenschosser/ruebenschosser.pdf [Accessed: 4 August 2020]. [Google Scholar]
- Lewis KA and Tzilivakis J, 2017a. Review of the published exposure data to pesticides for residents and bystanders, and for environmental risk assessment: Final Report. EFSA Supporting Publication 2017;14(5):EN‐1204, 101 pp. 10.2903/sp.efsa.2017.EN-1204 [DOI] [Google Scholar]
- Lewis KA and Tzilivakis J, 2017b. Development of a data set of pesticide dissipation rates in/on various plant matrices for the pesticide properties database (PPDB). Data, 2, 28. 10.3390/data203002. Available online: https://www.mdpi.com/2306‐5729/2/3/28/s1 [DOI] [Google Scholar]
- Lloyd GA and Bell GJ, 1983. Hydraulic nozzles: comparative spray drift study. Agricultural Development and Advisory Service, Ministry of Agriculture Fisheries and Food, UK. [Google Scholar]
- Lloyd GA, Bell GJ, Samuels SW, Cross JV and Berry AM, 1987. Orchard sprayers: comparative operator exposure and spray drift study. Agricultural Science Service, Agricultural Development and Advisory Service, Ministry of Agriculture Fisheries and Food, UK. [Google Scholar]
- Martin S, Westphal D, Erdtmann‐Vourliotis M, Dechet F, Schulze‐Rosario C, Stauber F, Wicke H and Chester G, 2008. Guidance for Exposure and Risk Evaluation for Bystanders and Residents Exposed to Plant Protection Products during and after Application Journal Für Verbraucherschutz Und Lebensmittelsicherheit, 3, 272–281. [Google Scholar]
- Meier U, Bachmann L, Buhtz H, Hack H, Klose R, Marlander B and Weber E, 1993. Phänologische Entwicklungsstadien der Beta‐Rüben (Beta vulgaris L. ssp.). Codierung und Beschreibung nach der erweiterten BBCH‐Skala (mit Abbildungen) (Phenological development stages of Beta beet (Beta vulgaris L. ssp.). Coding and description according to the extended BBCH scale (with illustrations). Nachrichtenbl. Deut. Pflanzenschutzd, 45, 37–41. [Google Scholar]
- NAFTA (North American Free Trade Agreement) Technical Working Group on Pesticides , 2015. Guidance for Evaluating and Calculating Degradation Kinetics in Environmental Media. Available online: https://www.epa.gov/sites/production/files/2015‐09/documents/degradation‐kin.pdf
- Nigg HN and Stamper JH, 1984. Dislodgeable residues of Chlorobenzilate in Florida citrus: worker reenry implications. University of Florida. Institute of Food and Agricultural Sciences. Chemosphere, 13, 1143–1156. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1997. OCDE/GD(97)148, Series on Testing and Assessment, No. 9, Guidance Document for the Conduct of Studies of Occupational Exposure to Pesticides During Agricultural Application.
- OECD (Organisation for Economic Co‐operation and Development) , 1998. ENV/MC/CHEM(98)17, OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring Number 1. [Google Scholar]
- PHED (Pesticide Handlers Exposure Database) , 1992. US Environmental Protection Agency, Health and Welfare Canada, National Agricultural Chemicals Association. Vesar Inc., Springfield, IL, USA. [Google Scholar]
- PSD (Pesticide Safety Directorate) , 2008. Bystander exposure guidance. Reissued 2008. Available online: https://www.pesticides.gov.uk/Resources/CRD/Migrated‐Resources/Documents/B/Bystander‐exposure‐guidance.pdf
- Rautmann D, Streloke M, Winkler R, 2001. New drift values in the authorisation procedure for plant protection products. Mitteilungen aus der Biologischen Bundesanstalt für Land‐und Forstwirtschaft (Federal Biological Research Center for Agriculture and Forestry). 383, Berlin, Germany, 133–141. [Google Scholar]
- RIVM (Rijksinstituut voor Volksgezondheid en Milieu) , ConsExpo Web. Available online: https://login‐ext.rivm.nl/nidp/idff/sso?id=57&sid=0&option=credential&sid=0&target=https%3A%2F%2Fesp‐ext.rivm.nl%2FLAGBroker%3F%2522https%3A%2F%2Fwww.consexpoweb.nl%2F%2522
- Rosenheck L, Cowell J, Mueth M, Eberhart D, Klonne D, Norman C and Ross J, 2001. Determination of a standardized sampling technique for pesticide transferable turf residue. Bulletin of Environmental Contamination and Toxicology, 67, 780–786. [DOI] [PubMed] [Google Scholar]
- Schneider F, et al. 1990. Dermal and urinary monitoring of nectarine harvesters exposed to azinphos‐methyl residues in Fresno County California 1988. Report number 1532.
- Siebers J, Binner R and Wittich K‐P, 2003. Investigation of downwind short‐range transport of pesticides after application in agricultural crops. Chemosphere, 51, 397–407. [DOI] [PubMed] [Google Scholar]
- Spencer JR, et al. 1993. Dermal and urinary monitoring of peach and apple harvesters exposed to organophosphate residues in Sutter, Stanislaus and Madera Counties, 1989 and 1990. Report number 1577.
- Stanghellini C, 2009. Emissions by aerial routes from protected crop systems (greenhouses and crops grown under cover): a position paper. Wageningen UR Greenhouse Horticulture, Report No. 224. [Google Scholar]
- Tessella Technology and Consulting. Computer Assisted Kinetic Evaluation CAKE version 3.4. Available online: https://www.tessella.com/showcase/computer‐assisted‐kinetic‐evaluation
- Thongsinthusak T, Meinders D and Krieger R, 1989. Estimation of Exposure of Persons in California to Pesticide Products that Contain Amino‐triazole (Amitrole) Estimation of Effectiveness of Exposure Reduction Measure. Report number 1470.
- Tsakirakis , 2014. BROWSE Report of the Reserve fund experiments conducted in Greece; ‘Collation of data on dermal transfer and efficiency of (protective) clothing and gloves for use in WP1‐3 models. Available online: https://secure.fera.defra.gov.uk/browse/openFile.cfm?dir=deliverables&name=ReserveFund2.pdf
- Turner JR, 2013. Area Under the Curve (AUC). In: Gellman MD and Turner JR (eds.). Encyclopedia of Behavioral Medicine. Springer, New York. 10.1007/978-1-4419-1005-9_986 [DOI] [Google Scholar]
- Urtizberea M, 2002. Post‐application worker exposure study and determination of Transfer Coefficient during harvesting of peaches treated by Rovral Aqua Flo®. Aventis CropScience, Study report n° SA 98151.
- US EPA (United States Environmental Protection Agency) , 1996. Occupational and residential exposure test guidelines. OPPTS 875.2100. Foliar dislodgeable residue dissipation. US EPA Publication No. 712‐C‐96‐267, Office of Prevention, Pesticides and Toxic Substances. Available from the U.S. Government Printing Office. Washington, DC. [Google Scholar]
- US EPA (US Environmental Protection Agency) , 2000. Agricultural transfer coefficients. Policy No 003.1 (dated August 7). Science Advisory Council for Exposure, Health Effects Division, Office of Pesticide Programs, Washington, DC, USA. [Google Scholar]
- US EPA (US Environmental Protection Agency) , 2001. Science Advisory Council for Exposure, policy number 12, recommended revisions to the standard operating procedures (SOPs) for residential exposure assessments. Office of Pesticide Programs, Health Effects Division, Washington, DC, USA. [Google Scholar]
- US EPA (US Environmental Protection Agency) , 2008. Exhibit D of Agency Issue Paper ‐ ARTF TC Data Summary, Document ID EPA‐HQ‐OPP‐2008‐0673‐0004. Available online: https://downloads.regulations.gov/EPA‐HQ‐OPP‐2008‐0673‐0004/attachment_3.xls
- US EPA (US Environmental Protection Agency) , 2011a. Agricultural transfer coefficients. Policy No. 3.1 and 3.2 (dated May 5, 2011. Science Advisory Council for Exposure, Health Effects Division, Office of Pesticide Programs, Washington, DC, USA.
- US EPA (US Environmental Protection Agency) , 2011b. Exposure Factors Handbook 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R‐09/052F, 2011.
- US EPA (US Environmental Protection Agency) , 2012. Health Effects Division Office of Pesticide Programs. Standard Operating Procedures for Residential Pesticide Exposure Assessment. 582 pp. Available online: https://www.epa.gov/opp00001/science/USEPA‐OPP‐HED_Residential%20SOPs_Oct2012.pdf
- US EPA (US Environmental Protection Agency) , 2017. Science Advisory Council for Exposure (ExpoSAC) Policy 3, US Environmental Protection Agency Office of Pesticide Programs, Revised January 2017. Available online: https://www.epa.gov/sites/production/files/2016‐12/documents/usepa‐opp‐hed_exposac_policy_3_january2017.pdf
- van Hemmen JJ, 2008. Addendum to the TNO Report V7333: effective personal protective equipment (PPE). Default setting of PPE for registration purposes of agrochemical and biocidal pesticides. Covering the literature published in the period 2005 to early, 2008. TNO Quality of Life, TNO Chemistry, Food & Chemical Risk Analysis, Chemical Exposure assessment, Zeist, The Netherlands.
- van Hemmen JJ, Chester G, Hamey P, Kangas J, Kirknel E, Maasfeld W, Perkins J, Phillips J and Schulze‐Rosario C, 2002. Post‐application exposure of workers to pesticides in agriculture, report of the re‐entry working group, EUROPOEM II Project, FAIR3‐CT96‐1406, December 2002.
- Vermeulen T, Van der Linden AMA and Van Os EA (eds.), 2010. Emissions of plant protection products from glasshouses to surface water in The Netherlands. Bleiswijk: Wageningen UR Greenhouse Horticulture, RIVM rapport 607407001, 81 pp.
- WHO (World Health Organization), 1987. Principles for the Safety Assessment of Food Additives and Contaminants in Food. Environmental Health Criteria, No. 70, pp. 111.
- Willis GH and McDowell LL, 1987. Pesticide persistence on foliage. Reviews of Environmental Contamination and Toxicology, 100, 23–73. [Google Scholar]
- World maps of Köppen‐Geiger climate classification. Available online: https://koeppen‐geiger.vu‐wien.ac.at/present.htm
- Zongmao C and Haibin W, 1997. Degradation of pesticides on plant surfaces and its prediction ‐ a case study on tea plant. Environmental Monitoring and Assessment, 44, 303–313. 10.1023/A:1005791514357 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CIPAC formulation codes
Public literature on dislodgeable foliar residue
Protocol for the review of relevant DT studies
DT Data collection
New calculator
Table overview open call
Outcome of Public Consultation on the draft EFSA Guidance


