ABSTRACT
Pseudomonas aeruginosa, an opportunistic bacterial pathogen, can synthesize and catabolize several small cationic molecules known as polyamines. In several clades of bacteria, polyamines regulate biofilm formation, a lifestyle-switching process that confers resistance to environmental stress. The polyamine putrescine and its biosynthetic precursors, l-arginine and agmatine, promote biofilm formation in Pseudomonas spp. However, it remains unclear whether the effect is a direct effect of polyamines or occurs through a metabolic derivative. Here, we used a genetic approach to demonstrate that putrescine accumulation, either through disruption of the spermidine biosynthesis pathway or the catabolic putrescine aminotransferase pathway, promoted biofilm formation in P. aeruginosa. Consistent with this observation, exogenous putrescine robustly induced biofilm formation in P. aeruginosa that was dependent on putrescine uptake and biosynthesis pathways. Additionally, we show that l-arginine, the biosynthetic precursor of putrescine, also promoted biofilm formation but did so by a mechanism independent of putrescine or agmatine conversion. We found that both putrescine and l-arginine induced a significant increase in the intracellular level of bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) (c-di-GMP), a bacterial second messenger widely found in Proteobacteria that upregulates biofilm formation. Collectively these data show that putrescine and its metabolic precursor, arginine, promote biofilm and c-di-GMP synthesis in P. aeruginosa.
IMPORTANCE Biofilm formation allows bacteria to physically attach to a surface, confer tolerance to antimicrobial agents, and promote resistance to host immune responses. As a result, the regulation of biofilm formation is often crucial for bacterial pathogens to establish chronic infections. A primary mechanism of biofilm promotion in bacteria is the molecule c-di-GMP, which promotes biofilm formation. The level of c-di-GMP is tightly regulated by bacterial enzymes. In this study, we found that putrescine, a small molecule ubiquitously found in eukaryotic cells, robustly enhances P. aeruginosa biofilm and c-di-GMP. We propose that P. aeruginosa may sense putrescine as a host-associated signal that triggers a lifestyle switch that favors chronic infection.
KEYWORDS: Pseudomonas aeruginosa, arginine, biofilm, c-di-GMP, polyamines, putrescine
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative gammaproteobacterium that opportunistically causes disease in both animals and plants (1–3). P. aeruginosa frequently forms biofilm-associated infections in cystic fibrosis (CF) airways, contributing to its adaptive resistance to antimicrobials and long-term colonization of the CF lung (4). While attachment to biotic surfaces is required for successful host colonization in both animal and plant roots (5, 6), an increasing body of evidence suggests that increased biofilm formation and attachment to host cells may trigger a more robust host immune response, potentially leading to clearance of the biofilm-associated microbes in plants and human epithelial cells (7, 8). We previously demonstrated that Pseudomonas must modulate its biofilm in the rhizosphere to evade triggering a plant immune response (8).
One mechanism through which P. aeruginosa regulates biofilm formation (9) and virulence (10) is the modulation of bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP), a ubiquitous second messenger in Proteobacteria (11). The turnover of c-di-GMP is mediated by diguanylate cyclase (DGC) enzymes and c-di-GMP phosphodiesterase (PDE) enzymes. P. aeruginosa encodes 41 putative c-di-GMP-modulating enzymes (CMEs) (11), suggesting that intricate spatiotemporal regulation of CME activities is in place to maintain c-di-GMP homeostasis. Many of the CMEs contain ligand-binding domains, such as Per-Arnt-Sim (PAS) or cyclases/histidine kinase associated sensory extracellular (CHASE) domains (12), suggesting that these CMEs may regulate their enzymatic activities in response to specific ligands. Vibrio cholerae, a gastrointestinal pathogen, downregulates its c-di-GMP levels in response to gastrointestinal tract-related molecules, such as bile salt and bicarbonate (13). However, it is not known whether Pseudomonas sense similar host-associated signals to trigger changes in c-di-GMP and bacterial physiology.
We previously identified the putrescine aminotransferase gene spuC as a negative regulator of Pseudomonas fluorescens biofilm formation and a plant rhizosphere fitness determinant (8). Furthermore, similar paradigms have been observed in divergent bacterial taxa, where polyamines either positively or negatively regulate biofilm formation. However, the responses to polyamines differ between taxa. For example, spermine is a biofilm inhibitor in Vibrio cholerae while norspermidine is a robust inducer of pellicle formation in Bacillus subtilis and spermidine and putrescine negatively regulate biofilm formation in Agrobacterium tumefaciens (14–16). These observations suggest that putrescine and related molecules involved in polyamine metabolism may act as a host-associated signal that triggers Pseudomonas lifestyle switching. Importantly, P. aeruginosa has an intricate network of enzymes involved in polyamine metabolism (17), and previous studies identified l-arginine and agmatine, which are known precursors of putrescine biosynthesis (17), as robust signals that upregulate biofilm formation in Pseudomonas (12, 18, 19). In this study, we used systematic mutagenesis of genes involved in polyamine metabolism coupled with exogenous polyamine application and c-di-GMP quantification to disentangle the roles of putrescine derivatives and biosynthetic precursors in biofilm regulation in P. aeruginosa.
RESULTS
Genes predicted to encode proteins required for polyamine uptake, biosynthesis, and catabolism are conserved across the genus Pseudomonas.
We previously demonstrated that putrescine catabolism inhibits P. fluorescens biofilm formation (8). P. aeruginosa has an extensive polyamine metabolism network that includes catabolic and biosynthetic pathways of putrescine, spermidine, and spermine (17). Furthermore, P. aeruginosa is a well-studied model organism for biofilm formation with available genetic tools that allow for the investigation of biofilm and c-di-GMP regulation (20–22). As a result, we chose to study P. aeruginosa to elucidate the mechanisms underpinning the polyamine-mediated biofilm enhancement that is observed in P. fluorescens.
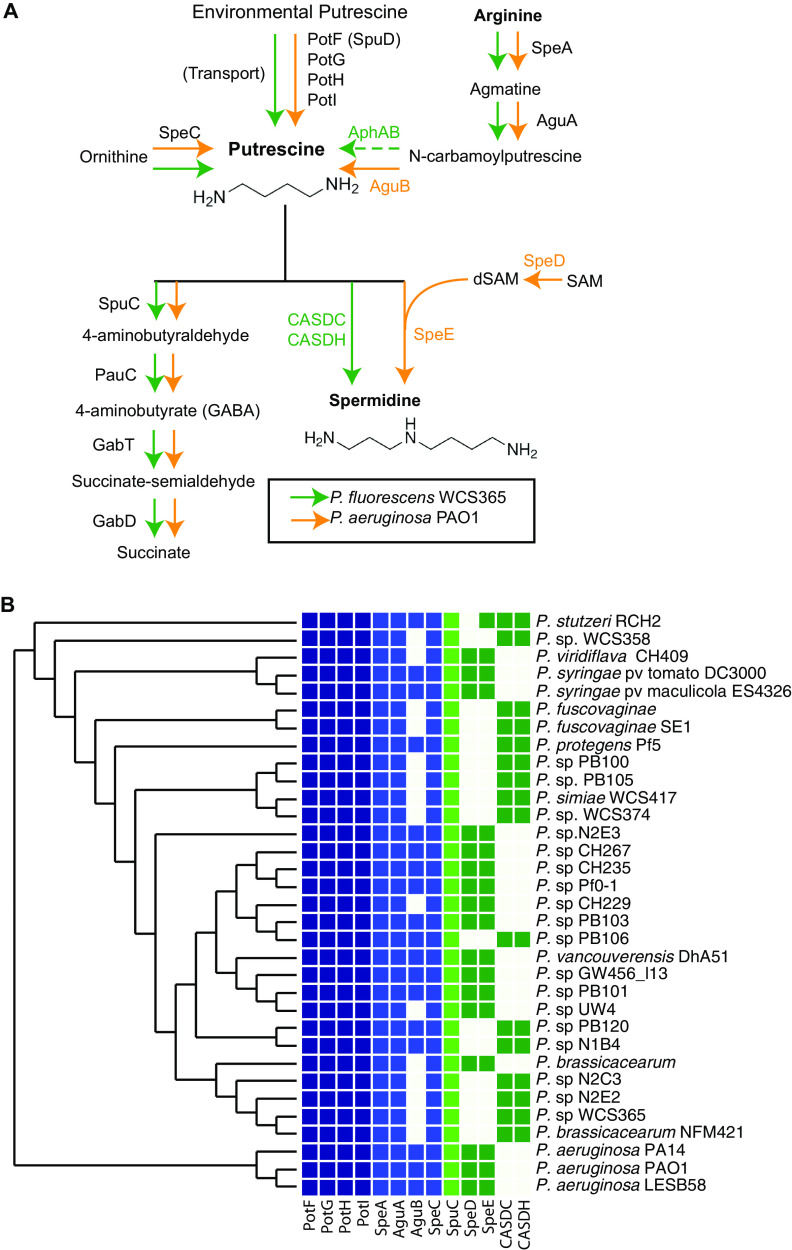
To determine how similar putrescine metabolism is across Pseudomonas strains, we identified the orthologous genes involved in putrescine uptake, biosynthesis, and catabolism pathways in both P. fluorescens WCS365 and P. aeruginosa PAO1. Based on what is known in P. aeruginosa (17), we identified orthologs in P. fluorescens WCS365 (Fig. 1A). The P. aeruginosa genome encodes genes required for putrescine biosynthesis (speA and speC), uptake (spuD), catabolism (spuC), and conversion to spermidine (speD and speE). Interestingly, speD and speE are absent in P. fluorescens WCS365, so we queried whether carboxyspermidine dehydrogenase (CASDH) and decarboxylase (CASDC), which catalyzes an alternate spermidine biosynthesis pathway from putrescine (23), were present. We identified CASDH/C homologs in P. fluorescens WCS365 but not in PAO1 (Fig. 1A). We used a previously described comparative genomics platform (24) to query the presence and absence of these polyamine biosynthesis genes in diverse Pseudomonas spp. We found that the majority of polyamine metabolism genes are conserved across diverse Pseudomonas. Interestingly, all strains contained predicted enzymes to convert putrescine to spermidine either through speD/E or CASDH/C but never both (Fig. 1B). This suggests that Pseudomonas strains have either a CASDH/C or speD/E pathway to generate spermidine from putrescine, but they do not maintain both in their genomes. These findings show that the potential for putrescine uptake, biosynthesis and catabolism, and spermidine biosynthesis is broadly conserved across the Pseudomonas genus (Fig. 1B).
FIG 1.
Polyamine metabolism pathway in Pseudomonas spp. (A) Predicted polyamine metabolism pathways in P. aeruginosa PAO1 and P. fluorescens WCS365. The pathway was reconstructed based on the work of Lu et al. (17). P. fluorescens WCS365 genes were identified by BLASTp using P. aeruginosa homologs as query sequences. (B) Phylogenetic tree of Pseudomonas spp. including the presence and absence of genes involved in polyamine metabolism. Genes predicted to encode proteins involved in putrescine uptake (PotFGHI), putrescine biosynthesis (SpeA, AguA, AguB, SpeC), putrescine catabolism (SpuC), or spermidine biosynthesis (SpeDE and CADSC/H) are shown.
Exogenous putrescine promotes biofilm formation in P. aeruginosa.
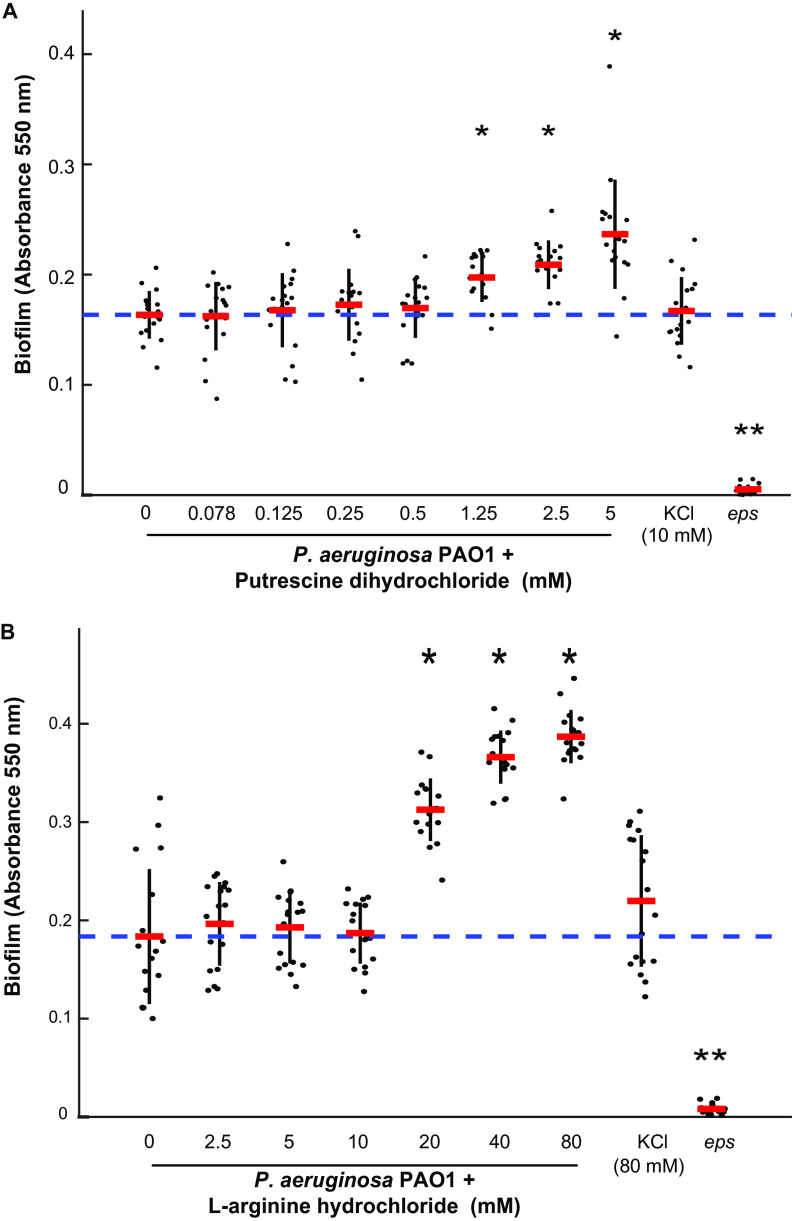
To test whether the accumulation of putrescine promotes biofilm formation, we performed crystal violet biofilm assays in P. aeruginosa subline H103 (P. aeruginosa PAO1) with and without the presence of exogenous putrescine. We titrated the concentration of exogenous l-arginine hydrochloride or putrescine dihydrochloride and observed that exogenous putrescine promotes biofilm at 1.25 mM (Fig. 2A) and l-arginine promotes biofilm at 20 mM (Fig. 2B). We found that KCl does not affect biofilm in P. aeruginosa PAO1 at concentrations up to 80 mM, ruling out an effect of chlorine ions in l-arginine hydrochloride or putrescine dihydrochloride in modulating biofilm (Fig. 2). While previous reports show that the concentration of putrescine in bacterial cultures is around 50 μM (25), bacteria routinely colonize environments with much higher putrescine concentrations. Putrescine is estimated at 0.5 mM in cystic fibrosis sputum (26), 1 mM in the human gut (27), and up to 11 mM in tomato root exudate (28). These data show that the addition of putrescine or arginine is sufficient to promote biofilm formation in wild-type P. aeruginosa PAO1 at concentrations they may encounter during associations with hosts (Fig. 2).
FIG 2.
The biofilm-promoting effect of l-arginine and putrescine in P. aeruginosa PAO1 is dosage dependent. Putrescine can promote biofilm at 1.25 mM, and l-arginine enhances biofilm formation at 20 mM. The eps mutant has decreased biofilm and was used as a control. * indicates a P value of <0.0001 by Student's t test. Error bars represent standard deviation. Data points show all technical replicates from 3 biological replicates.
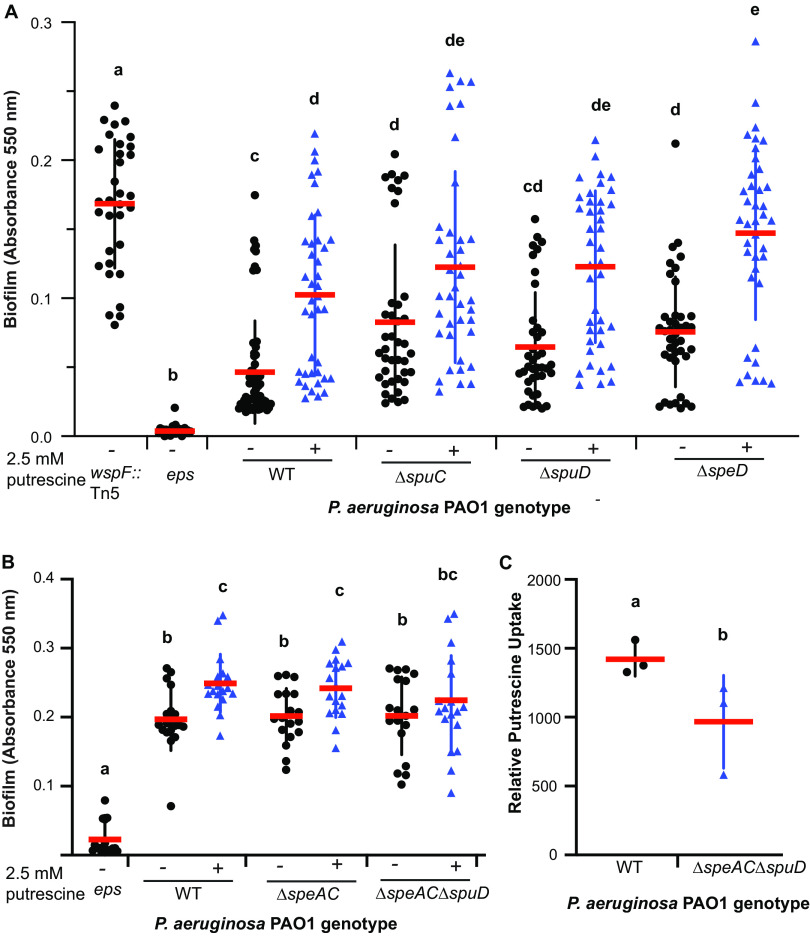
To determine whether putrescine accumulation promotes biofilm formation in P. aeruginosa PAO1, we generated clean deletions of genes encoding key enzymes involved in putrescine biosynthesis (speA and speC), catabolism (spuC), and spermidine biosynthesis (speD) in P. aeruginosa PAO1 (Fig. 1A). We first validated that these mutations affected polyamine biosynthesis through targeted metabolomics and found that the PAO1 ΔspuC, ΔspeAC, and ΔspeD mutants all had significantly reduced intracellular putrescine when grown in the absence of exogenous putrescine and arginine (see Fig. S1A in the supplemental material) and spermidine (Fig. S1B) levels compared to wild-type PAO1. This indicates that, in the absence of precursors, disruption of putrescine biosynthesis (ΔspeAC), catabolism (ΔspuC), and spermidine biosynthesis (ΔspeD) affects putrescine and spermidine accumulation in P. aeruginosa PAO1.
To determine if putrescine accumulation promotes biofilm, we tested whether mutants predicted to accumulate putrescine had enhanced biofilm formation capacity using crystal violet staining with and without exogenous putrescine. Consistent with the hypothesis that the loss of putrescine catabolism results in putrescine accumulation and increased biofilm and the previous observation that deletion of spuC in P. fluorescens enhances biofilm formation, we found that deletion of PAO1 spuC leads to a significant increase in biofilm formation (Fig. 3A). This response was further enhanced by the addition of exogenous putrescine (Fig. 3A), suggesting that putrescine accumulation robustly enhances biofilm in P. aeruginosa PAO1 through the loss of putrescine catabolism.
FIG 3.
Uptake of exogenous putrescine and l-arginine is sufficient to promote biofilm formation in P. aeruginosa. (A) Wild-type P. aeruginosa PAO1 and ΔspuC, ΔspuD, and ΔspeD mutants were treated with 2.5 mM putrescine dihydrochloride. Mutants predicted to accumulate putrescine (ΔspuC and ΔspeD) had enhanced biofilm that was further enhanced by exogenous putrescine. Putrescine induction of biofilm formation is independent of SpuD, a periplasmic substrate-binding protein involved in putrescine uptake, and SpeD, an enzyme required for the production of spermidine through putrescine aminopropylation. (B) Putrescine-mediated biofilm induction is reduced in a ΔspuD ΔspeAC triple mutant. (C) The triple ΔspuD ΔspeAC mutant had reduced levels of intracellular putrescine. Cells were grown with exogenous putrescine and washed followed by targeted metabolomics. (A to C) Error bars represent standard deviation. Data represent at least 3 biological replicates. Letters indicate significant differences by one-way ANOVA followed by Tukey’s honest significant difference (HSD).
P. aeruginosa can synthesize spermidine from putrescine via an aminopropylation reaction using decarboxylated S-adenosyl methionine (dSAM) as an aminopropyl donor. To test whether putrescine conversion to spermidine is necessary for enhanced biofilm formation, we generated a deletion mutant in speD, which is required for spermidine biosynthesis from putrescine. SpeD decarboxylates S-adenosyl methionine to generate dSAM. Similar to the deletion of spuC, deletions of speD enhanced biofilm formation in P. aeruginosa (Fig. 3A), which was further enhanced by exogenous putrescine, suggesting that the putrescine-induced enhancement of biofilm formation is not mediated by the conversion of putrescine to spermidine via speD/E. Collectively, these data showed that the accumulation of putrescine promotes biofilm formation or that putrescine catabolism inhibits biofilm formation in P. aeruginosa.
P. aeruginosa can synthesize putrescine via arginine decarboxylation or ornithine decarboxylation pathways or take up environmental putrescine through the putative putrescine transporter system encoded by spuABCDEFGH-spuI (17, 29). To test whether putrescine biosynthesis and uptake promote biofilm formation in P. aeruginosa, we tested a deletion mutant in spuD (encoding a putative periplasmic putrescine substrate-binding protein) and a double deletion mutant in the putrescine biosynthesis genes speAC (speA encodes an arginine decarboxylase [17] and speC encodes an ornithine decarboxylase [17]) for biofilm formation. We found that abolishing the biosynthesis of putrescine from l-arginine and l-ornithine decarboxylation did not impair biofilm formation (Fig. 3B) nor did disrupting the putrescine uptake pathway with spuD deletion (Fig. 3A). This suggests that disrupting a single intrinsic putrescine biosynthesis or uptake system is not sufficient to impair biofilm formation and that either redundancy or additional pathways are required.
To test whether abolishing both putrescine uptake via SpuD and putrescine biosynthesis through SpeAC would result in a loss of biofilm in the presence of exogenous putrescine, we tested a P. aeruginosa PAO1 ΔspuD ΔspeAC triple mutant (predicted to be impaired in both putrescine uptake and biosynthesis). We performed a biofilm assay and found that the triple mutant had a reduced response to putrescine, suggesting that the reduction of intracellular putrescine in this mutant results in lower enhancement of biofilm (Fig. 3B). To quantify intracellular levels of putrescine in the triple mutant ΔspuD ΔspeAC, we grew PAO1 WT and the triple mutant in the presence of 2.5 mM putrescine then spun down and washed the cells before performing targeted metabolomics. We found that the triple mutant has a significant reduction in intracellular putrescine (Fig. 3C), suggesting that these three pathways provide a significant contribution to putrescine uptake and biosynthesis.
Exogenous arginine promotes biofilm formation in P. aeruginosa independent of conversion to putrescine.
Previous studies reported that l-arginine is a strong inducer of biofilm formation in P. aeruginosa PA14 and in P. fluorescens WCS365 within a modified M63 medium that uses l-arginine as the sole carbon and nitrogen source (8, 12, 18). The addition of exogenous putrescine has been shown to inhibit transcription of aguAB (30), an operon encoding enzymes that convert agmatine to putrescine in the l-arginine decarboxylation pathway (31). As a result, exogenous supplementation of putrescine could lead to the accumulation of agmatine, l-arginine, and l-ornithine, given their roles as putrescine biosynthetic precursors (Fig. 1A). Importantly, agmatine and l-arginine are known to promote biofilm formation in Pseudomonas (12, 18, 19). To investigate whether putrescine causes biofilm induction by feedback inhibition of l-arginine conversion or by l-arginine triggering biofilm formation through putrescine biosynthesis, we tested the P. aeruginosa PAO1 ΔspeAC double mutant, which cannot convert l-arginine (in ΔspeA strain) to agmatine (and subsequently, putrescine) or l-ornithine (in ΔspeC strain) to putrescine via decarboxylation pathways (Fig. 1A). Notably, the ΔspeAC double mutant does not have a biofilm formation defect (Fig. 3B), suggesting that neither the depletion of putrescine nor the accumulation of l-arginine, achieved by abolishing the putrescine biosynthesis pathway, interferes with biofilm formation.
Previous studies that reported l-arginine as a robust biofilm-promoting molecule used a defined, modified M63 medium in which l-arginine was used at 0.4% (wt/vol) as the sole carbon and nitrogen source in M63 salts (dM63-Arg [12, 18]). However, because dM63-Arg replaces Casamino Acids and d-glucose with an excess of l-arginine, this medium may have limited the intracellular availability of amino acids other than l-arginine because P. aeruginosa would need to synthesize these amino acids de novo. Amino acid unavailability has been shown to trigger RelA-mediated production of (p)ppGpp via ribosome stalling (32, 33). (p)ppGpp is a stringent response second messenger that mediates physiological changes, including biofilm formation, in Proteobacteria (33, 34). To avoid the confounding effect of nutrient starvation on biofilm formation, we used a modified medium that contains the standard nutrients in M63 medium with additional l-arginine supplementation to test the effect of l-arginine as a biofilm-promoting agent. We found that while 2.5 mM putrescine robustly induced biofilm formation (Fig. 2A and 3A), equimolar l-arginine HCl did not affect the biofilm formation in the wild-type or an l-arginine-accumulating ΔspeAC background (Fig. S2). As previous studies that examined the effect of l-arginine and agmatine used concentrations consistant with our titration results (22.69 mM for l-arginine [12, 18] or 10 mM for agmatine [19]), we confirmed the effect of l-arginine as a biofilm-promoting agent at 20 mM. At 20 mM, l-arginine robustly increased biofilm formation in wild-type P. aeruginosa PAO1 (Fig. S2). Importantly, we also noticed that l-arginine-induced biofilm enhancement is independent of speA and speC (Fig. S2). This suggested that l-arginine rather than its downstream decarboxylation pathway metabolites, such as agmatine or putrescine, is responsible for the enhanced biofilm formation.
Because l-arginine can be used as a carbon and nitrogen source by P. aeruginosa, we examined whether the increased biofilm formation with the addition of 20 mM l-arginine was the result of increased growth. Because previous reports suggested that P. aeruginosa exopolysaccharide (EPS) production can confound the measured optical density at 600 nm (OD600) in growth assays (22), we measured the growth of P. aeruginosa PAO1 with l-arginine supplementation in both wild-type and an EPS-defective background (Δeps::FRT). The addition of l-arginine in M63 medium at 20 mM did not alter the maximum growth rate achieved by either the wild-type or Δeps::FRT strains (Fig. S3). Furthermore, while growth curves showed that l-arginine supplementation increased both the maximum OD600 reached by P. aeruginosa PAO1 during the growth assay (Fig. S3) and the OD600 at 8 h (Fig. S3), a time point consistent with our biofilm assay measurement, we noted that these increases in OD600 are observed only in the wild-type background and are dependent on exopolysaccharide (EPS) biosynthesis genes (Fig. S3). This is consistent with our hypothesis that l-arginine supplementation led to increased OD600 in an EPS-dependent manner via biofilm enhancement rather than bacterial growth promotion. Collectively these data suggest that putrescine and l-arginine promote biofilm in P. aeruginosa independently of their role as carbon and nitrogen sources.
Putrescine and arginine promote the intracellular accumulation of c-di-GMP.
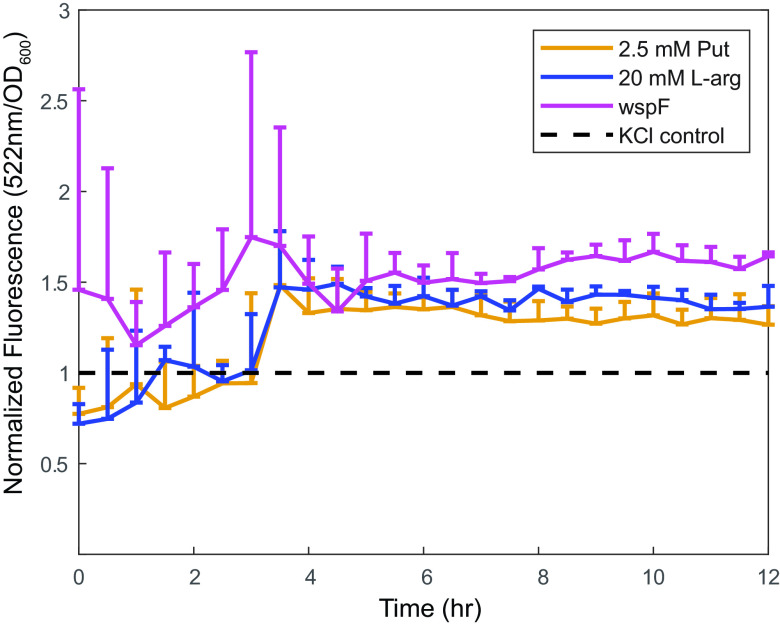
c-di-GMP is a second messenger ubiquitous in Proteobacteria that governs lifestyle switching between biofilm and planktonic cells (16). Exogenous l-arginine can lead to increased c-di-GMP levels in Pseudomonas putida (35). Therefore, we hypothesized that putrescine and l-arginine modulate P. aeruginosa biofilm formation by regulating intracellular c-di-GMP levels. We tested this hypothesis using a c-di-GMP-dependent GFP reporter (22). We found that the addition of exogenous putrescine or l-arginine in M63 medium resulted in a significant increase in fluorescence signal, suggesting that exogenous polyamines and the polyamine biosynthetic precursor l-arginine induce c-di-GMP biosynthesis in a timescale similar to that of the biofilm assays starting at 4 h (Fig. 4). These data indicated that putrescine and l-arginine may promote biofilm formation by regulating intracellular levels of c-di-GMP.
FIG 4.
Addition of exogenous l-arginine and putrescine induces c-di-GMP accumulation. P. aeruginosa carrying a reporter pCdrA-gfpS was grown in M63 medium supplemented with 20 mM l-arginine hydrochloride or 2.5 mM putrescine dihydrochloride. wspF::Tn5, a genetic background with constitutive high levels of c-di-GMP, was used as a control for the pCdrA-gfpS reporter. Putrescine dihydrochloride or l-arginine HCl supplementation promotes GFP expression relative to equimolar KCl, suggesting that polyamines upregulate intracellular c-di-GMP levels. Error bars represent standard deviation. Each time point represents at least 3 biological replicates with 8 technical replicates in each biological replicate.
DISCUSSION
We previously demonstrated that biofilm downregulation and putrescine catabolism via SpuC are required for successful plant rhizosphere colonization by Pseudomonas fluorescens (8). Additionally, putrescine and spermidine are components of tomato rhizosphere exudate (28, 36), suggesting a role of putrescine in biofilm regulation in a host-associated context. However, no mechanistic link exists between putrescine metabolism and biofilm attenuation. We found that putrescine-mediated enhancement of biofilm formation coincided with increased intracellular level of c-di-GMP in P. aeruginosa PAO1 as reflected by a c-di-GMP-induced GFP expression reporter. This is consistent with the previous observation that polyamine regulation of Agrobacterium tumefaciens biofilm formation is dependent on the intracellular c-di-GMP pool (16) and that l-arginine-induced c-di-GMP increase correlates with biofilm enhancement in P. putida (35). It is unclear how putrescine supplementation is linked to the increase of c-di-GMP. Our data suggest that SpuD-mediated uptake of putrescine is not necessary for biofilm promotion. One possibility is that the P. aeruginosa PAO1 genome encodes an unknown, redundant periplasm substrate-binding protein for putrescine uptake. However, SpuE, the periplasm polyamine substrate-binding protein with the highest homology to SpuD in P. aeruginosa PAO1, has been shown to have no detectable putrescine-binding ability (29).
An alternative hypothesis is that there may be a periplasmic sensor for putrescine that triggers the downstream signaling that leads to biofilm enhancement. A recent study in Vibrio cholerae proposes a model in which norspermidine and spermidine can competitively bind a periplasmic protein, NspS, allowing NspS to regulate the antagonistic activities of a dual activity diguanylate cyclase/c-di-GMP phosphodiesterase, MbaA (37). Additionally, previous reports suggested that c-di-GMP-modulating enzymes (CMEs) containing ligand-binding domains may be regulated by the direct binding of exogenous signals (38). As a result, our current hypothesis is that one or more P. aeruginosa CMEs respond to polyamine compounds either through their ligand-binding domains or through another periplasmic sensor protein. Interestingly, a prior study demonstrated that, while a diguanylate cyclase may only contribute to a subtle increase in the intracellular global concentration of c-di-GMP, its effect on biofilm formation is much more pronounced (39, 40). This is due to the localization of diguanylate cyclase, which allows direct “loading” of c-di-GMP into a receptor protein that mediates adhesion protein secretion (39, 40). Hence, it should be noted that, while the change in c-di-GMP level induced by exogenous polyamines is modest as reflected by the relative GFP signal, polyamines can still lead to a robust physiological response in Pseudomonas.
While we demonstrated that l-arginine and putrescine serve as environmental signals that promote biofilm in Pseudomonas, the identities of potential polyamine sensors are unknown. Our current hypothesis is that CMEs can directly bind polyamines via ligand-binding domains. In addition to PAS domain- and CHASE domain-containing CMEs, Pseudomonas fluorescens also encodes Ca2+ channel and chemotaxis (CACHE) domain-containing CMEs that can recognize other ligands that modulate their enzymatic activities (12, 38). Therefore, a comprehensive screening of a Pseudomonas aeruginosa CME deletion library (12) could identify genes required for sensing and responding putrescine. This work lays the foundation for future mechanistic studies that can identify such polyamine metabolite sensors.
MATERIALS AND METHODS
Strains, media, and culture conditions.
All strains and plasmids used in this study are in Table 1. For routine culture, P. aeruginosa PAO1 H103 subline (P. aeruginosa PAO1 [41, 42]), Escherichia coli DH5α λpir, and E. coli SM10 λpir strains were grown on lysogeny broth (LB) agar or in LB medium at 37°C with shaking at 200 rpm. When appropriate, antibiotics and counterselection agents were supplemented at the following concentrations: 10% (wt/vol) sucrose, 5 μg/mL (E. coli) or 50 μg/mL (P. aeruginosa) gentamicin (Gm), 100 μg/mL (E. coli) or 250 μg/mL (P. aeruginosa) carbenicillin (Cb), and 10 μg/mL Irgasan.
TABLE 1.
Strains and plasmids
| Strains or plasmids | Genotype or description | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α λpir | supE44 ΔlacU169 (ΦlacZΔM15), recA1 endA1 hsdR17 thi-1 gyrA96 relA1 λpir | |
| SM10 λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Km λpir; conjugating strain | |
| P. aeruginosa strains | ||
| PAO1-H103 | H103 subline of PAO1 strain; parental strains of all mutants | 41, 42 |
| PAO1 ΔspeA | speA (PA4839) in frame deletion; l-arginine decarboxylase mutant | This study |
| PAO1 ΔspeC | speC (PA4519) in frame deletion; l-ornithine decarboxylase mutant | This study |
| PAO1 ΔspeD | speD (PA0654) in frame deletion; SAM decarboxylase mutant | This study |
| PAO1 ΔspuC | spuC (PA0299) in frame deletion; putrescine aminotransferase mutant | This study |
| PAO1 ΔspuD | spuD (PA0300) in frame deletion; putrescine periplasmic SBP mutant | This study |
| PAO1 ΔspeAC | speAC in frame deletions | This study |
| PAO1 ΔspeAC ΔspuD | ΔspeAC ΔspuD in frame deletions | This study |
| PAO1 pel | ΔpelA::FRT; markerless FLP-mediated excision of pelA | This study |
| PAO1 psl | ΔpslBCDE::FRT; markerless FLP-mediated excision of pslBCDE | This study |
| PAO1 eps | ΔpelA::FRT ΔpslBCDE::FRT; exopolysaccharide (EPS) mutant | This study |
| PAO1 wspF::Tn5 | wspF(PA3703)::mini-Tn5-luxCDABE; ID PAO1_lux_75_D8; 91/1,008 nt | 42 |
| PAO1 wspF pel | wspF::mini-Tn5-luxCDABE ΔpelA::FRT | This study |
| PAO1 wspF psl | wspF::mini-Tn5-luxCDABE ΔpslBCDE::FRT | This study |
| PAO1 wspF eps | wspF::mini-Tn5-luxCDABE ΔpelA::FRT ΔpslBCDE::FRT | This study |
| PAO1 ZXL001 | ΔpelA::FRT ΔpslBCDE::FRT pCdrA-gfpS | This study |
| PAO1 ZXL002 | wspF::mini-Tn5-luxCDABE ΔpelA::FRT ΔpslBCDE::FRT pCdrA-gfpS | This study |
| Plasmids | ||
| pEXG2 | Vector for Pseudomonas spp. allelic exchange; aacC1 sacB oriT; pBR322 oriC | 43 |
| pΔspeA | pEXG2::speA-flanking; allelic exchange construct | This study |
| pΔspeC | pEXG2::speC-flanking; allelic exchange construct | This study |
| pΔspeD | pEXG2::speD-flanking; allelic exchange construct | This study |
| pΔspeE | pEXG2::speE-flanking; allelic exchange construct | This study |
| pΔspuC | pEXG2::spuC-flanking; allelic exchange construct | This study |
| pΔspuD | pEXG2::spuD-flanking; allelic exchange construct | This study |
| pMPELA | pEX18.Ap::pelA::FRT-aacC1-gfp-FRT | 45 |
| pMPSL-KO1 | pEX18.Ap::pslBCDE::FRT-aacC1-gfp-FRT | 46 |
| pFLP3 | Source of FLP recombinase; Apr Tcr | 47 |
| pCdrA-gfpS | pUCP22Not-PcdrA-RBS-CDS-RNaseIII-gfp(Mut3)-T0-T1 Apr Gmr | 22 |
Strain construction.
All primers used in this study are described in Table S1 in the supplemental material. Construction of ΔspeA, ΔspuC, ΔspuD, ΔspeC, ΔspeD, and ΔspeE mutants in P. aeruginosa was performed as previously described (8, 43). Briefly, 450 to 600 bp of the upstream and downstream flanking regions of the target genes were amplified and joined via overlap extension PCR (44) then ligated into the pEXG2 vector (43). Correct insertions were verified by PCR and Sanger sequencing. The deletion constructs were transformed into calcium competent E. coli SM10 λpir, and subsequently mobilized into P. aeruginosa PAO1 by conjugation (8). To allow for conjugation, E. coli and P. aeruginosa cultures were mixed, plated on solid LB medium, and incubated at 37°C for 4 h. The mating spots were then scraped off, resuspended in 1 mL of 10 mM MgSO4, and plated on LB agar containing 50 μg/mL Gm and 10 μg/mL Irgasan. Second homologous recombination events were selected for using sucrose counterselection (8). Briefly, single recombinant colonies are grown in lysogeny broth with no selection overnight before plating on LB-sucrose to select for the double recombinant colonies that excised the sacB-containing plasmid backbone. Successful deletions were verified by testing for Gm sensitivity and by colony PCR.
To construct ΔpelA::FRT ΔpslBCDE::FRT (Δeps) double mutants in P. aeruginosa PAO1 H103 subline, the gene disruption constructs pMPELA (45) and pMPSL-KO1 (46) that were originally developed based on the Flp-FRT gene disruption system (47) were introduced into P. aeruginosa by E. coli SM10 λpir conjugation. To select for the first homologous recombination events, the mating spots were resuspended and plated on LB agar containing 50 μg/mL Gm and 10 μg/mL Irgasan. To confirm plasmid integration, P. aeruginosa single recombinant colonies were either streaked on LB-Cb or LB-Gm plates. Additionally, single recombinant colonies contain FRT-aacC1-gfp-FRT gene disruption cassettes and, therefore, could be distinguished from the wild type by measuring the green fluorescent protein (GFP) signal from cultures grown overnight (diluted to OD600 of 0.5) using a 96-well plate reader. To remove the pMPELA or pMPSL-KO1 plasmid backbones by second homologous recombination events, single recombinant P. aeruginosa were cultured overnight in LB broth with no selection before plating on LB-sucrose. Double recombinant colonies (ΔpelA::FRT-aacC1-gfp-FRT or ΔpslBCDE::FRT-aacC1-gfp-FRT) were confirmed by selecting for Gm resistance and Cb sensitivity. The FRT-aacC1-gfp-FRT cassettes were excised via Flp-mediated recombination as previously described (48). Briefly, double recombinant colonies were transformed with pFLP3. Cbr colonies were grown overnight on LB agar before patching onto LB-Gm and LB-Cb. Gms Cbr colonies were picked and grown in LB broth with no selection before plating on sucrose to select for colonies cured of the sacB-harboring pFLP3 plasmid. The final colonies carrying ΔpelA::FRT or ΔpslBCDE::FRT scars were further confirmed by the lack of GFP signal in the cultures grown overnight. Double knockout mutants of pelA and pslBCDE were created by performing gene disruption sequentially with pMPELA and pMPSL-KO1.
To create the c-di-GMP reporter strains P. aeruginosa PAO1 ZXL001 and P. aeruginosa PAO1 ZXL002, P. aeruginosa PAO1 Δeps::FRT and P. aeruginosa PAO1 Δeps::FRT wspF::mini-Tn5-luxCDABE (P. aeruginosa PAO1 Δeps wspF::Tn5 [42]) strains were transformed with pCdrA-gfpS (22) by electroporation as previously described (49). Plasmids were confirmed by PCR using GFPmut3-specific primers and maintained in LB-Gm.
Polyamine quantification through targeted metabolomics.
To quantify intracellular spermidine and putrescine levels, wild-type PAO1 and the ΔspuC, ΔspeD, and ΔspeAC mutants were grown overnight in standard M63 medium (comprised of a modified M63 medium with M63 salts, glucose [0.2%] as the carbon source, magnesium sulfate [1 mM], and ammonium chloride [20 mM] as the nitrogen source). The next day, the cultures were spun down at 13,000 × g for 2 min, washed twice, and resuspended in the same medium they were grown in overnight. The bacteria were diluted to a final OD600 of 0.1. To quantify putrescine uptake in the ΔspuD ΔspeAC triple mutant, the triple mutant and the wild-type PAO1 strains were grown overnight in standard M63 medium with the addition of putrescine dihydrochloride (2.5 mM). They were spun down the next day at 13,000 × g for 2 min, washed twice, resuspended in standard M63 medium (without putrescine dihydrochloride), and diluted to a final OD600 of 0.1. Targeted metabolomics to quantify putrescine and spermidine levels was performed by West Coast Metabolomics.
Crystal violet biofilm assays.
Biofilm assays were performed as previously described (18, 50). Overnight cultures of P. aeruginosa PAO1 in LB were spun down at 13,000 × g for 2 min, washed twice, resuspended, and diluted to an OD600 of 0.1 in 1.1× M63 medium (1× M63 salt, 0.2% glucose, 0.5% Casamino Acids, 1 mM MgSO4). Putrescine and l-arginine were supplemented at the indicated concentrations (2.5 mM putrescine dihydrochloride for M63-Put, 2.5 mM l-arginine hydrochloride for M63-Arg, and 20 mM l-arginine for M63-Arg20). When putrescine dihydrochloride or l-arginine hydrochloride were added, the control medium were supplemented with KCl to control for the chloride ion concentration (5 mM KCl for putrescine dihydrochloride control and 20 mM KCl for l-arginine hydrochloride control). 100 μL of the diluted cultures were incubated at 37°C under a static condition in non-tissue culture-treated 96-well plates (Falcon; product no. 351177) for 8 h. After incubation, the plates were rinsed in distilled water twice and stained with 125 μL of 0.1% crystal violet aqueous solution for 10 min. After staining, plates were washed in distilled water 3 times to remove excess stain and dried for at least 6 h before solubilizing the crystal violet with 150 μL of 30% acetic acid for 10 min. One hundred microliters of the resolubilized crystal violet was transferred to a flat bottom 96-well plate for absorbance reading at 550 nm (SpectraMax i3x fluorescence plate reader). Background signals were measured from wells containing 100 μL of 30% unstained acetic acid and subtracted from the absorbance readings.
Growth curves.
Growth curves were performed in tissue culture-treated 96-well plates. Cultures of P. aeruginosa PAO1 grown overnight in LB were spun down at 13,000 × g for 2 min, washed twice, resuspended, and diluted to an OD600 of 0.11 in 1.1× M63 medium. 10 μL of 200 mM l-arginine, 200 mM KCl, or H2O was first added to the appropriate wells before adding 90 μL of the bacterial suspension (OD600 0.11) in 1.1× M63 medium for a final OD600 of 0.1 in 1× M63 medium. Wells on the edge of the plates were not used to minimize the effects of evaporation. The OD600 was measured every 15 min in a plate reader (Molecular Devices, VersaMax) at 37°C with constant shaking for at least 20 h.
Comparative genomics.
The species tree used in Fig. 1B was generated as described using 122 single-copy genes we previously found to be conserved in all Pseudomonas strains (24). Determination of the presence/absence of the polyamine uptake, biosynthesis, and catabolism genes was determined using the PyParanoid comparative genomics tool (24). We used the annotated sequences for potFGHI (PA0300, PA0302-0304), speA (PA4839), aguA (PA0292), aguB (PA0293), spuC (PA0299), speC (PA4519), speD (PA0654), and speE (PA1687) from P. aeruginosa PAO1, and CASDH (WCS365_04584) and CASDC (WCS365_04583) from P. fluorescens WCS365 to query the database and then plotted the presence/absence data against the species tree.
c-di-GMP GFP reporter assays.
Cultures of P. aeruginosa PAO1 ZXL001 and P. aeruginosa PAO1 ZXL002 strains were grown overnight in LB-Gm for 20 h. Bacterial cells were pelleted by centrifugation at 13,000 × g for 2 min, washed three times with M63 medium, resuspended, and diluted to an OD600 of 0.5 in fresh 1.1× M63 medium with no antibiotics. In a black-welled 96-well plate (Corning; cat. no. CLS3603), 90 μL of diluted bacterial cultures was added to each well. When appropriate, each well was supplemented with either 10 μL of 50 mM KCl, 25 mM putrescine dihydrochloride, 200 mM KCl, or 200 mM l-arginine hydrochloride for a final concentration of 2.5 mM putrescine or 20 mM l-arginine with appropriate KCl molarity in the control medium (5 mM KCl for putrescine dihydrochloride and 20 mM KCl for l-arginine hydrochloride). The OD600 and GFP signals of each well were read immediately and again every 30 min for 12 h on a 96-well plate reader (SpectraMax i3x fluorescence plate reader) at 497 nm excitation and 522 nm emission. Relative fluorescence intensity (the ratio of A522 and OD600) was used as a proxy for c-di-GMP levels in the cells.
Data processing and availability.
Growth curves and c-di-GMP GFP reporter assays data were processed and visualized with custom scripts in Python 3.0 and MATLAB. Raw data and scripts used for data analysis and visualization are available at https://github.com/fzxliu/liu_et_al_polyamine_2021.git.
ACKNOWLEDGMENTS
This work was supported by an NSERC CGS-M awarded to Z.L. and by an NSERC Discovery Grant (NSERC-RGPIN-2016-04121) and CIHR Project Grant (PJT − 169051) awarded to C.H.H.
We thank Matthew Parsek’s lab at the University of Washington for the pMPELA, pMPSL-KO1, and the pCdrA-gfpS plasmids. We thank Rachel Fernandez’s lab at the University of British Columbia (UBC) for the pFLP3 plasmid and Robert E. W. Hancock’s lab at UBC for the P. aeruginosa PAO1-H103 subline and the mini-Tn5-lux insertion mutants.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Cara H. Haney, Email: cara.haney@msl.ubc.ca.
George O'Toole, Geisel School of Medicine at Dartmouth.
REFERENCES
- 1.Tan M-W, Mahajan-Miklos S, Ausubel FM. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA 96:715–720. 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pletzer D, Mansour SC, Hancock REW. 2018. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog 14:e1007084. 10.1371/journal.ppat.1007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahme LG, Tan M-W, Le L, Wong SM, Tompkins RG, Calderwood SB, Ausubel FM. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci USA 94:13245–13250. 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau-Marquis S, Stanton BA, O'Toole GA. 2008. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 21:595–599. 10.1016/j.pupt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez-Dozal A, Hogan D, Gorman C, Quintanal-Villalonga A, Nishiguchi MK. 2012. Multiple Vibrio fischeri genes are involved in biofilm formation and host colonization. FEMS Microbiol Ecol 81:562–573. 10.1111/j.1574-6941.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier J-F, Garneau D, Caudwell L, Vlamakis H, Bais HP, Beauregard PB. 2016. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 7:e01664-16. 10.1128/mBio.01664-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd MS, Pang B, Mishra M, Swords WE, Wozniak DJ. 2010. The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-κB activation in A549 cells. mBio 1:e00140-10. 10.1128/mBio.00140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Beskrovnaya P, Melnyk RA, Hossain SS, Khorasani S, O’Sullivan LR, Wiesmann CL, Bush J, Richard JD, Haney CH. 2018. A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. mBio 9:e00433-18. 10.1128/mBio.00433-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha D-G, O'Toole GA. 2015. c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol Spectr 3:MB. 10.1128/microbiolspec.MB-0003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc Natl Acad Sci USA 103:2839–2844. 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha D-G, Richman ME, O'Toole GA. 2014. Deletion mutant library for investigation of functional outputs of cyclic diguanylate metabolism in Pseudomonas aeruginosa PA14. Appl Environ Microbiol 80:3384–3393. 10.1128/AEM.00299-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koestler BJ, Waters CM. 2014. bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in Vibrio cholerae. Infect Immun 82:3002–3014. 10.1128/IAI.01664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobley L, Kim SH, Maezato Y, Wyllie S, Fairlamb AH, Stanley-Wall NR, Michael AJ. 2014. Norspermidine is not a self-produced trigger for biofilm disassembly. Cell 156:844–854. 10.1016/j.cell.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobe RC, Bond WG, Wotanis CK, Zayner JP, Burriss MA, Fernandez N, Bruger EL, Waters CM, Neufeld HS, Karatan E. 2017. Spermine inhibits Vibrio cholerae biofilm formation through the NspS–MbaA polyamine signaling system. J Biol Chem 292:17025–17036. 10.1074/jbc.M117.801068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Kim SH, Natarajan R, Heindl JE, Bruger EL, Waters CM, Michael AJ, Fuqua C. 2016. Spermidine inversely influences surface interactions and planktonic growth in Agrobacterium tumefaciens. J Bacteriol 198:2682–2691. 10.1128/JB.00265-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C-D, Itoh Y, Nakada Y, Jiang Y. 2002. Functional analysis and regulation of the divergent spuABCDEFGH-spuI operons for polyamine uptake and utilization in Pseudomonas aeruginosa PAO1. J Bacteriol 184:3765–3773. 10.1128/JB.184.14.3765-3773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Toole GA. 2011. Microtiter dish biofilm formation assay. JoVE 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams BJ, Du R-H, Calcutt MW, Abdolrasulnia R, Christman BW, Blackwell TS. 2010. Discovery of an operon that participates in agmatine metabolism and regulates biofilm formation in Pseudomonas aeruginosa. Mol Microbiol 76:104–119. 10.1111/j.1365-2958.2010.07083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ, Parsek MR. 2012. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol 14:1913–1928. 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, Secor PR, Tseng BS, Scian M, Filloux A, Wozniak DJ, Howell PL, Parsek MR. 2015. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci USA 112:11353–11358. 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. 2012. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl Environ Microbiol 78:5060–5069. 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tait GH. 1976. A new pathway for the biosynthesis of spermidine. Biochem Soc Trans 4:610–612. 10.1042/bst0040610. [DOI] [PubMed] [Google Scholar]
- 24.Melnyk RA, Hossain SS, Haney CH. 2019. Convergent gain and loss of genomic islands drive lifestyle changes in plant-associated Pseudomonas. 6. ISME J 13:1575–1588. 10.1038/s41396-019-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama Y, Nakamura A, Matsumoto M, Kanbe A, Sakanaka M, Higashi K, Igarashi K, Katayama T, Suzuki H, Kurihara S. 2016. A novel putrescine exporter SapBCDF of Escherichia coli. J Biol Chem 291:26343–26351. 10.1074/jbc.M116.762450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasemann H, Shehnaz D, Enomoto M, Leadley M, Belik J, Ratjen F. 2012. L-ornithine derived polyamines in cystic fibrosis airways. PLoS One 7:e46618. 10.1371/journal.pone.0046618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto M, Benno Y. 2007. The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol 51:25–35. 10.1111/j.1348-0421.2007.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 28.Oota M, Tsai AY-L, Aoki D, Matsushita Y, Toyoda S, Fukushima K, Saeki K, Toda K, Perfus-Barbeoch L, Favery B, Ishikawa H, Sawa S. 2020. Identification of naturally occurring polyamines as root-knot nematode attractants. Mol Plant 13:658–665. 10.1016/j.molp.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Lim SC, Dong Y, Wu J, Tao F, Zhou L, Zhang L-H, Song H. 2012. Structural basis of substrate binding specificity revealed by the crystal structures of polyamine receptors SpuD and SpuE from Pseudomonas aeruginosa. J Mol Biol 416:697–712. 10.1016/j.jmb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Chou HT, Kwon D-H, Hegazy M, Lu C-D. 2008. Transcriptome analysis of agmatine and putrescine catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol 190:1966–1975. 10.1128/JB.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, Lu C-D. 2007. Functional genomics enables identification of genes of the arginine transaminase pathway in Pseudomonas aeruginosa. J Bacteriol 189:3945–3953. 10.1128/JB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haseltine WA, Block R. 1973. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci USA 70:1564–1568. 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. 2015. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Yu H, Zhang D, Xiong J, Qiu J, Xin R, He X, Sheng H, Cai W, Jiang L, Zhang K, Hu X. 2016. Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol Res 192:84–95. 10.1016/j.micres.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Barrientos-Moreno L, Molina-Henares MA, Ramos-González MI, Espinosa-Urgel M. 2020. Arginine as an environmental and metabolic cue for cyclic diguanylate signalling and biofilm formation in Pseudomonas putida. Sci Rep 10:13623. 10.1038/s41598-020-70675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuiper I, Bloemberg GV, Noreen S, Thomas-Oates JE, Lugtenberg BJ. 2001. Increased uptake of putrescine in the rhizosphere inhibits competitive root colonization by Pseudomonas fluorescens strain WCS365. Mol Plant Microbe Interact 14:1096–1104. 10.1094/MPMI.2001.14.9.1096. [DOI] [PubMed] [Google Scholar]
- 37.Bridges AA, Bassler BL. 2021. Inverse regulation of Vibrio cholerae biofilm dispersal by polyamine signals. Elife 10:e65487. 10.7554/eLife.65487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacalone D, Smith TJ, Collins AJ, Sondermann H, Koziol LJ, O’Toole GA. 2018. Ligand-mediated biofilm formation via enhanced physical interaction between a diguanylate cyclase and its receptor. mBio 9:e01254-18. 10.1128/mBio.01254-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahlstrom KM, Giglio KM, Sondermann H, O'Toole GA. 2016. The inhibitory site of a diguanylate cyclase is a necessary element for interaction and signaling with an effector protein. J Bacteriol 198:1595–1603. 10.1128/JB.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlstrom KM, Giglio KM, Collins AJ, Sondermann H, O'Toole GA. 2015. Contribution of physical interactions to signaling specificity between a diguanylate cyclase and its effector. mBio 6:e01978-15–e01915. 10.1128/mBio.01978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angus BL, Carey AM, Caron DA, Kropinski AM, Hancock RE. 1982. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother 21:299–309. 10.1128/AAC.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewenza S, Falsafi RK, Winsor G, Gooderham WJ, McPhee JB, Brinkman FSL, Hancock REW. 2005. Construction of a mini-Tn5-luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res 15:583–589. 10.1101/gr.3513905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. 2005. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 102:8006–8011. 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 45.Starkey M, Hickman JH, Ma L, Zhang N, Long SD, Hinz A, Palacios S, Manoil C, Kirisits MJ, Starner TD, Wozniak DJ, Harwood CS, Parsek MR. 2009. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol 191:3492–3503. 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirisits MJ, Prost L, Starkey M, Parsek MR. 2005. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 71:4809–4821. 10.1128/AEM.71.8.4809-4821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 48.Choi K-H, Schweizer HP. 2006. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 49.Bloemberg GV, O'Toole GA, Lugtenberg BJ, Kolter R. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 63:4543–4551. 10.1128/aem.63.11.4543-4551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merritt JH, Kadouri DE, O’Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol 1:Unit-1B.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3 and Table S1. Download JB.00297-21-s0001.pdf, PDF file, 0.4 MB (369.1KB, pdf)