Abstract
Women with valvular heart disease may be more likely to have adverse obstetric and cardiovascular complications during pregnancy. Most current recommendations focus on stenotic lesions with less guidance regarding regurgitant lesions. We aimed to compare adverse events at delivery for women with various stenotic and regurgitant valvular diseases. We used the 2016–2018 National Inpatient Sample data to compare demographics, comorbidities, and obstetric and cardiovascular complications during delivery hospitalizations. After adjusting for clinical and socioeconomic factors, logistic regression was performed to investigate associations between valvular disease and outcomes. Among >11.2 million deliveries, 20,349 were in women with valvular disease. Women with valvular disease were older, had longer length of stays, and higher costs associated with delivery. They had higher prevalence of underlying cardiovascular comorbidities compared to women without valvular disease (hypertension: 5.1 vs 0.25%; pulmonary hypertension: 7.0 vs <0.1%). At delivery, they had higher adjusted odds of obstetric events including preeclampsia/eclampsia (aOR 1.9 [1.8–2.2]) and intrapartum/postpartum hemorrhage (aOR 1.4 [1.2–1.6]), and cardiovascular events including peripartum cardiomyopathy (aOR 65 [53–78]), pulmonary edema (aOR 17 [13–22]), acute ischemic heart disease (aOR 19 [12–30]) and arrhythmias (aOR 22 [19–27]). There were valve lesion-specific differences in the magnitude of risk but both stenotic and regurgitant lesions were associated with elevated risk of cardiovascular complications. In conclusion, pregnant women with stenotic and regurgitant valvular disease have a greater burden of cardiovascular comorbidities and increased odds of obstetric and cardiovascular events at delivery. These women may benefit from specialized care from a Cardio-Obstetrics team.
Keywords: pregnancy, valvular disease, regurgitation, stenosis
INTRODUCTION
Cardiovascular disease (CVD) is a leading cause of maternal morbidity and mortality worldwide, in part due to increased heart disease complicating pregnancy1. During pregnancy, multiple physiologic changes occur, including increased cardiac output, plasma volume and heart rate, with concomitant decline in maternal systemic vascular resistance. Women with valvular heart disease are at unique risk for complications, as these changes can worsen valvular hemodynamics. The three commonly used risk estimators for pregnancy in women with CVD are not specific for women with valvular heart disease,2–4 and there is a paucity of large, nationally representative studies reporting maternal outcomes, particularly for regurgitant lesions3,5–8. In this study, we investigate the obstetric and cardiovascular outcomes of deliveries in pregnancies complicated by stenotic and regurgitant valvular lesions. We hypothesize that women with valvular heart disease will have worse outcomes compared to women without valvular disease, and that stenotic lesions will confer greater risk than regurgitation lesions.
METHODS
Data were obtained for analysis from the 2016–2018 National Inpatient Sample (NIS), which was created by the Agency for Healthcare Research and Quality (AHRQ) for the Healthcare Cost and Utilization Project (HCUP). As the largest, publicly available all-payer national dataset in the US, the NIS comprises a stratified-sample of ~20% of all hospitalizations in a year, comprising greater than 7 million hospitalizations. Hospitals across 48 states and the District of Columbia are included. All data available from the NIS are deidentified and anonymized, with any hospital identifiers removed9,10. Given that the NIS is a public dataset with no identifying information, Institutional Review Board approval was not required.
Diagnosis-related groups were used to identify delivery-related hospitalizations (Supplement, Table 1). Comorbidities and obstetric and cardiovascular events were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) codes (Supplement, Tables 2–4) associated with the hospitalization.
The primary aim of the study was to assess obstetric and cardiovascular events at the time of parturition in women with valvular heart disease compared to those without valvular heart disease.
The primary exposure of interest was valvular heart disease. Valvular heart disease included any form of valve disease, rheumatic and non-rheumatic, and any degree of severity. Secondary exposures of interest included subtypes of valvular heart disease, specifically aortic stenosis, aortic regurgitation, mitral stenosis, mitral regurgitation and tricuspid regurgitation. Of note, women with “mixed valvular disease” were included in the primary analysis evaluating outcomes in women with any valve disease, but were excluded from subgroup analyses to maintain purity of specific lesions for analysis.
The primary outcomes were obstetric/fetal and cardiovascular events. Obstetric complications included preeclampsia/eclampsia, gestational hypertension, gestational diabetes, placenta previa, placental abruption, intra/postpartum hemorrhage and premature rupture of membranes. Fetal complications included fetal growth restriction and stillbirth or fetal death. Cardiovascular complications included peripartum cardiomyopathy, heart failure, pulmonary edema, ischemic stroke, cardiac arrest, pulmonary embolism, acute renal failure, acute ischemic heart disease, arrhythmias and death.
Patient demographics included were age, length of stay, hospital admission cost, race/ethnicity, median household income of residents in the patient’s zip code and primary payer/insurance. We also considered the following cardiovascular comorbidities: hypertension, diabetes, dyslipidemia, coronary artery disease, congestive heart failure, pulmonary hypertension, chronic kidney disease, aortopathy (aortic aneurysm or dissection) and connective tissue disease.
Survey weights, provided by HCUP, were used for all analyses. Patient demographics were compared between women with and without valvular heart disease, and between the various types of valvular lesions. Patient comorbidities, obstetric and fetal outcomes, and cardiovascular complications were then compared. For all analyses, continuous variables that were non-normally distributed are reported as median and interquartile range, and comparison was performed with Wilcoxon rank sum test. Categorical variables are reported as number (frequency) with comparison performed with Pearson’s chi-squared test.
Next, multivariable logistic regression was performed to evaluate the unadjusted and adjusted odds ratios of obstetric and cardiovascular complications with valvular disease. Model 1 included adjustment for age, race, insurance status, median residential income and hospital location. Model 2 added the following cardiovascular comorbidities: history of chronic hypertension, pre-existing diabetes, coronary artery disease, and chronic kidney disease. Given that congestive heart failure had a high prevalence among women with valvular disease, additional sensitivity analyses were then performed excluding any women with pre-existing congestive heart failure. Similarly, pulmonary hypertension also had a high prevalence with valvular heart disease and additional sensitivity analyses were also performed excluding any women with pulmonary hypertension and concomitant right-sided valvular lesions. For this analysis, women with pulmonary hypertension and left-sided valvular lesions were included, since pulmonary hypertension in the setting of left-sided valvular lesions is more likely to be a result of severe valvular disease rather than a confounder.
The frequency of obstetric and cardiovascular comorbidities and complications were also calculated stratified by lesion type. Multivariable adjusted logistic regression was used for the association of each lesion type with complications, adjusting for age, race/ethnicity and insurance status. Logistic regression was used for analyses in this study, although given the low overall prevalence (<5%) for events, odds ratios may be interpreted as risk. All analyses were performed using Stata Version 16 (StataCorp, College Station, Texas 77845, USA).
RESULTS
Among a total of 11,284,712 delivery hospitalizations, 20,349 were in women with valvular heart disease. Median age, length of stay, and hospital admission costs were higher for women with valvular disease (Table 1). There were also significant differences in race/ethnicity, hospital region, median residential income, and insurance status (Table 1). Baseline characteristics were also different across the various valvular lesions (Supplement Table 5). Women with mitral valve disorders were older. A greater relative frequency of mitral stenosis and tricuspid regurgitation were seen in Black women (25% for both). There were also differences in hospital location, residential income and insurance status (Supplement Table 5).
Table 1.
Baseline Demographics in Women with and without Valvular Disorders*
| Variable | Valvular Disorders | ||
|---|---|---|---|
| No (n=11,284,712) | Yes (n=20,349) | p-value | |
| Median age (years) | 29 (24–33) | 31 (27–35) | <0.001 |
| Median length of stay (days) | 2 (2–3) | 3 (2–4) | <0.001 |
| Median hospital admission cost ($) | 16,081 (10,766–24,444) | 21,325 (13,526–34,154) | <0.001 |
| Race/Ethnicity | <0.001 | ||
| White | 5,643,321 (50%) | 12,275 (59%) | |
| Black | 1,637,634 (14%) | 3,500 (17%) | |
| Hispanic | 2,230,923 (20%) | 2,355 (11%) | |
| Asian/pacific islander | 669,164 (6%) | 875 (4%) | |
| Native American | 78,991 (0.7%) | 151 (0.7%) | |
| Other | 504,412 (4%) | 796 (4%) | |
| Missing | 520,210 (5%) | 726 (4%) | |
| Hospital Region | <0.001 | ||
| New England | 441,219 (4%) | 769 (4%) | |
| Middle Atlantic | 1,357,512 (12%) | 3,550 (17%) | |
| East North Central | 1,592,227 (14%) | 3,120 (15%) | |
| West North Central | 785,393 (7%) | 1,191 (6%) | |
| South Atlantic | 2,159,831 (19%) | 4,720 (23%) | |
| East South Central | 696,247 (6%) | 1,530 (7%) | |
| West South Central | 1,557,245 (14%) | 1,946 (9%) | |
| Mountain | 851,971 (8%) | 1,131 (5%) | |
| Pacific | 1,842,740 (16%) | 2,721 (13%) | |
| Median household income of residents in patient’s zip code | <0.001 | ||
| ≤$43,999 | 3,149,472 (28%) | 5,320 (26%) | |
| $44,000–55,999 | 2,841,408 (25%) | 4,840 (23%) | |
| $56,000–73,999 | 2,750,005 (24%) | 5,220 (25%) | |
| ≥$74,000 | 2,435,170 (22%) | 5,051 (24%) | |
| Expected primary payer/insurance | <0.001 | ||
| Medicare | 84,633 (0.7%) | 366 (2%) | |
| Medicaid | 4,855,671 (43%) | 7,460 (36%) | |
| Private insurance | 5,732,468 (51%) | 11,946 (58%) | |
| Self-pay | 287,752 (3%) | 339 (2%) | |
| No charge | 7,222 (<0.1%) | 15 (<0.1%) | |
| Other | 303,550 (3%) | 504 (2%) | |
Numbers included are survey weight-adjusted.
All cardiovascular comorbidities were significantly more common in women with valvular heart disease, including hypertension, coronary artery disease, congestive heart failure and pulmonary hypertension (Figure 1, Supplement Table 6). Among obstetric and fetal complications, preeclampsia/eclampsia, placental abruption, hemorrhage and fetal growth restriction were more common in women with valvular heart disease (Figure 2, Supplement Table 6). All cardiovascular complications similarly occurred with greater frequency in women with valvular heart disease (Figure 2, Supplement Table 6). In sensitivity analyses excluding women with pre-existing heart failure, comparable increased risk for obstetric, fetal and cardiovascular complications were noted (Supplement Table 7). Similarly, sensitivity analyses excluding women with pulmonary hypertension in the setting of right-sided valvular lesions also noted a comparable increased risk of complications (Supplement Table 8).
Figure 1.
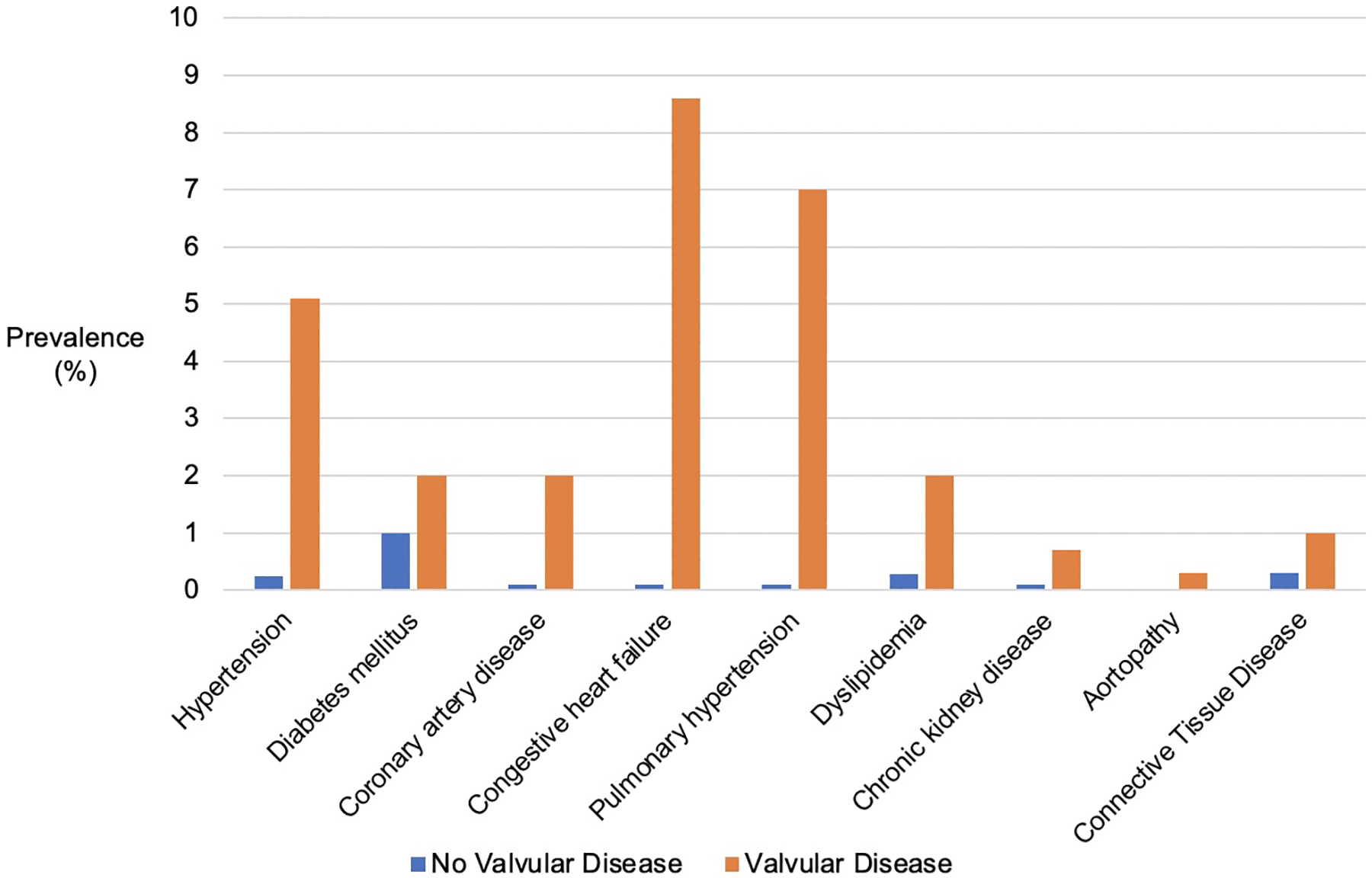
Cardiovascular Comorbidities in Women with and without Valvular Disease
Figure 2.
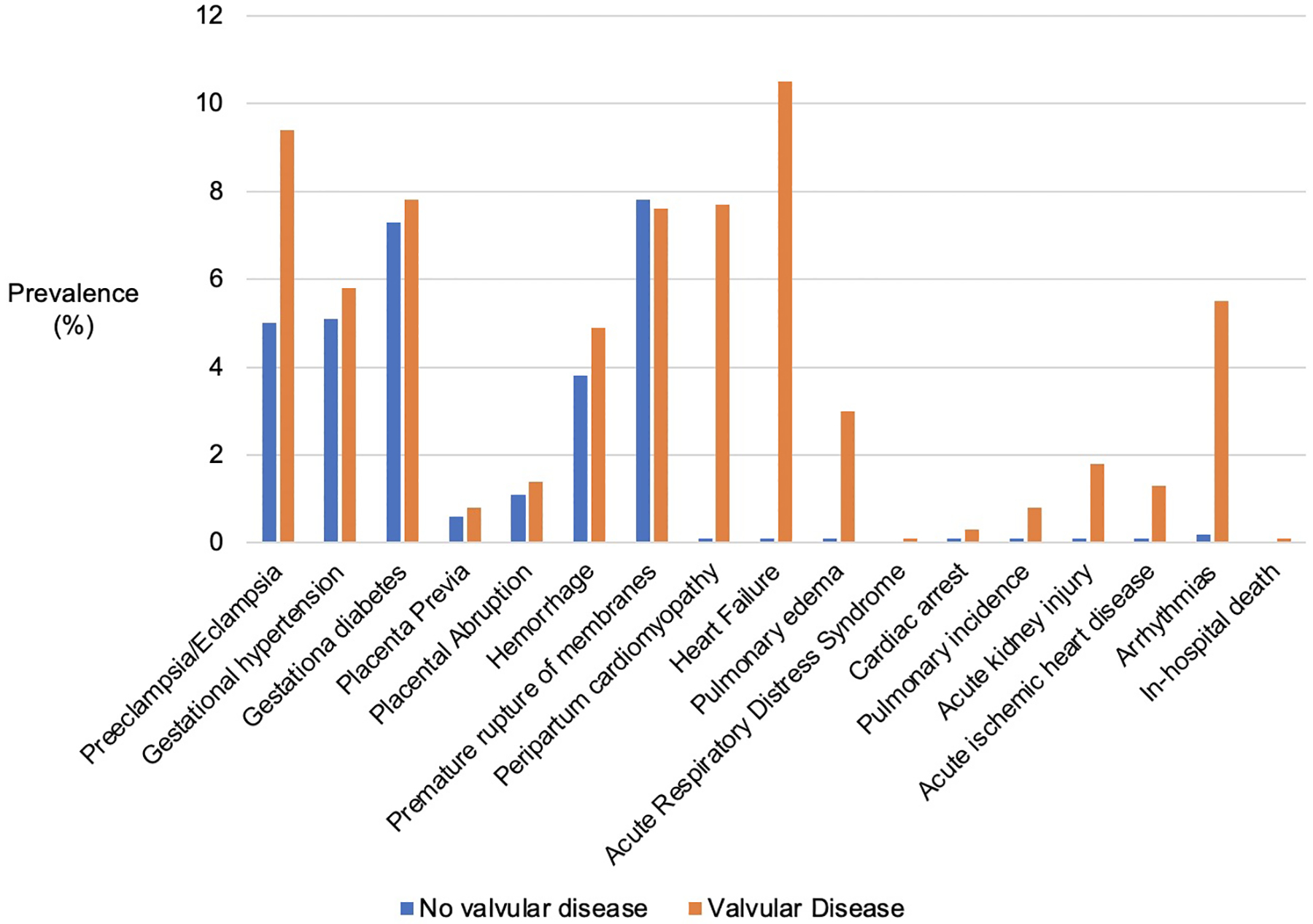
Maternal Obstetric and Cardiovascular Complications in Women with and without Valvular Disease
Following adjustment, valvular disease was significantly associated with obstetric complications, particularly for preeclampsia/eclampsia (aOR 1.9 [1.8–2.2]) in Model 2, placental abruption, and hemorrhage (Table 2). Valvular disease was also significantly associated with fetal growth restriction in the unadjusted and adjusted Model 1, but not in Model 2. For all cardiovascular complications, valvular disease remained significantly associated after adjustment, although the magnitude of risk was lower in the fully adjusted models compared to the unadjusted (Table 2).
Table 2.
Odds Ratios of Obstetric, Fetal and Cardiovascular Complications with Valvular Heart Disease
| Outcomes in Valvular compared to No Valvular Disease | Unadjusted Odds Ratio | p-value | Model 1 (age, race, income, insurance, location) | p-value | Model 2 (Model 1 + hypertension, diabetes, coronary disease, chronic kidney disease) | p-value |
|---|---|---|---|---|---|---|
| OBSTETRIC COMPLICATIONS | ||||||
| Preeclampsia/Eclampsia | 2.1 (1.9–2.3) | <0.001 | 2.0 (1.8–2.3) | <0.001 | 1.9 (1.8–2.2) | <0.001 |
| Gestational Hypertension | 1.1 (1.0–1.3) | 0.124 | 1.0 (0.9–1.2) | 0.480 | 1.0 (0.9–1.2) | 0.511 |
| Gestational Diabetes | 1.1 (1.0–1.2) | 0.179 | 1.0 (0.8–1.1) | 0.490 | 1.0 (0.9–1.1) | 0.609 |
| Placenta Previa | 1.2 (0.8–1.7) | 0.328 | 1.0 (0.7–1.4) | 0.928 | 1.0 (0.7–1.5) | 0.848 |
| Premature rupture of membranes | 0.9 (0.8–1.1) | 0.397 | 0.9 (0.8–1.0) | 0.184 | 0.9 (0.8–1.1) | 0.356 |
| Placental Abruption | 1.3 (1.0–1.7) | 0.039 | 1.3 (1.0–1.7) | 0.036 | 1.3 (0.1–1.7) | 0.044 |
| Hemorrhage | 1.4 (1.2–1.6) | <0.001 | 1.4 (1.2–1.6) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| FETAL COMPLICATIONS | ||||||
| Fetal growth restriction | 1.2 (1.0–1.4) | 0.021 | 1.2 (1.0–1.4) | 0.023 | 1.2 (1.0–1.4) | 0.062 |
| Stillbirth or fetal death | 1.3 (0.9–1.9) | 0.211 | 1.1 (1.0–1.3) | 0.051 | 1.1 (0.7–1.7) | 0.681 |
| CARDIOVASCULAR COMPLICATIONS | ||||||
| Peripartum Cardiomyopathy | 123 (108–140) | <0.001 | 107 (93–123) | <0.001 | 65 (53–79) | <0.001 |
| Heart failure | 144 (129–162) | <0.001 | 124 (110–141) | <0.001 | 101 (84–122) | <0.001 |
| Pulmonary edema | 32 (25–38) | <0.001 | 26 (22–33) | <0.001 | 17 (13–22) | <0.001 |
| ARDS | 26 (11–58) | <0.001 | 29 (13–65) | <0.001 | 11 (4–33) | <0.001 |
| Ischemic Stroke | 32 (17–59) | <0.001 | 27 (15–50) | <0.001 | 14 (6–31) | <0.001 |
| Cardiac arrest | 27 (14–49) | <0.001 | 25 (14–46) | <0.001 | 11 (5–24) | <0.001 |
| Pulmonary embolism | 34 (23–49) | <0.001 | 31 (21–45) | <0.001 | 20 (12–32) | <0.001 |
| Acute renal failure | 14 (10–18) | <0.001 | 11.7 (8.9–15.4) | <0.001 | 4.6 (3.2–6.7) | <0.001 |
| Acute ischemic heart disease | 69 (51–93) | <0.001 | 51 (36–71) | <0.001 | 19 (12–30) | <0.001 |
| Arrhythmias | 28 (24–32) | <0.001 | 24 (21–27) | <0.001 | 18 (15–21) | <0.001 |
| In-hospital death | 16 (6.5–39) | <0.001 | 15 (6–37) | <0.001 | 7.3 (2.7–19.8) | <0.001 |
Among the various valve lesions, mitral regurgitation was the most common (n=8,931), followed by mitral stenosis (n=5,313), tricuspid regurgitation (n=2,600), aortic regurgitation (n=678), aortic stenosis (n=667), and pulmonic stenosis (n=396) (Figure 3). The frequency of obstetric and cardiovascular complications with each valve lesion demonstrated elevated events for all stenotic and regurgitant lesions (Supplement, Table 9). Similarly, while there was some heterogeneity in risk between the various valvular lesions, adjusted odds ratios were elevated for several obstetric complications and the majority of cardiovascular complications across both stenotic and regurgitant left and right-sided valvular lesions (Table 3).
Figure 3.
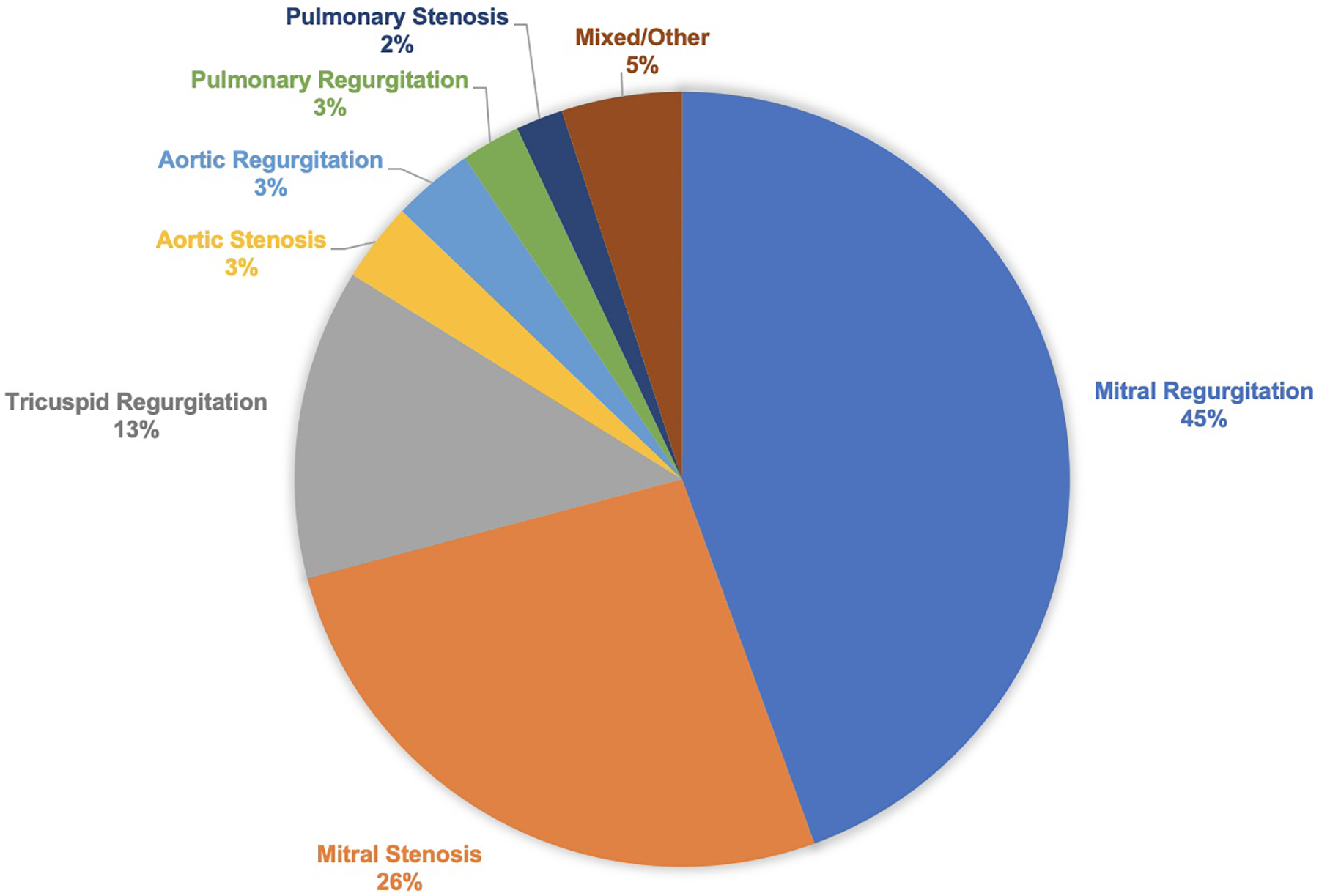
Frequency of Various Valvular Lesions
Table 3.
Association of Specific Valvular Lesion with Obstetric and Cardiovascular Outcomes
| Aortic Stenosis aOR 95% CI (n=667) | Aortic Regurgitation aOR 95% CI (n=678) | Mitral Stenosis aOR 95% CI (n=5,313) | Mitral Regurgitation aOR 95% CI (n=8,931) | Tricuspid Regurgitation aOR 95% CI (n=2,600) | Pulmonic Stenosis aOR 95% CI (n=396) | |
|---|---|---|---|---|---|---|
| OBSTETRIC COMPLICATIONS | ||||||
| Preeclampsia/Eclampsia | 2.7 (1.6–4.5) | 2.1 (1.2–3.7) | 3.0 (2.5–3.5) | 1.2 (1.0–1.4) | 2.6 (2.0–3.3) | 1.4 (0.8–3.8) |
| Gestational Hypertension | 1.1 (0.5–2.3) | 0.9 (0.4–2.0) | 1.1 (0.8–1.4) | 0.9 (0.8–1.1) | 1.2 (0.8–1.7) | - |
| Gestational Diabetes | 2.1 (1.2–3.4) | 1.4 (0.7–2.5) | 0.9 (0.7–1.1) | 1.0 (0.9–1.2) | 0.6 (0.4–0.9) | 2.5 (1.3–4.8) |
| Placenta Previa | - | 3.7 (1.2–11.5) | 0.9 (0.4–1.8) | 1.0 (0.6–1.7) | 1.6 (0.7–3.5) | - |
| Placental Abruption | 2.2 (0.7–7.0) | - | 1.4 (0.9–2.3) | 1.4 (0.9–2.1) | 1.7 (0.9–3.1) | 3.6 (1.1–11.5) |
| Hemorrhage | 1.0 (0.4–2.4) | 1.2 (0.5–2.8) | 1.6 (1.3–2.1) | 1.1 (0.8–1.4) | 1.6 (1.1–2.2) | 2.5 (1.1–5.4) |
| Premature rupture of membranes | 1.0 (0.5–1.9) | 1.2 (0.7–2.2) | 0.8 (0.6–1.0) | 1.0 (0.8–1.2) | 0.8 (0.6–1.1) | 1.3 (0.6–2.8) |
| FETAL COMPLICATIONS | ||||||
| Fetal growth restriction | 2.6 (1.4–5.0) | 4.0 (2.3–6.7) | 1.1 (0.8–1.6) | 1.1 (0.8–1.4) | 1.5 (1.0–2.2) | - |
| Stillbirth or fetal death | - | 1.6 (0.2–11.6) | 1.2 (0.5–2.4) | 0.7 (0.3–1.8) | 2.0 (0.9–4.6) | - |
| MATERNAL CARDIOVASCULAR COMPLICATIONS | ||||||
| Peripartum Cardiomyopathy | - | 65 (28–149) | 270 (227–323) | 6.4 (3.3–12.4) | 105 (76–145) | - |
| Heart failure | 23.9 (7.6–77) | 103 (54–195) | 271 (229–322) | 8.3 (4.9–13.9) | 140 (104–187) | 28.5 (6.8–119) |
| Pulmonary edema | 17.9 (4.4–72) | 37 (13–99) | 52 (39–69) | 2.4 (0.9–6.5) | 47 (31–72) | - |
| Acute Ischemic Heart Disease | 41 (5.5–300) | 42 (5.7–307) | 57 (34–96) | 10 (3.9–28) | 69 (35–136) | - |
| Arrhythmias | 6.1 (1.5–25) | 21 (9.8–45) | 30 (24–38) | 14 (11–18) | 37 (27–51) | 5.4 (0.7–39) |
Adjusted for age, race/ethnicity and insurance status
Per recommendations from the Healthcare Cost and Utilization Project, any cells with less than 10 events/outcomes were left blank and are marked by “ – ”
DISCUSSION
In this large, nationally representative study, we demonstrate that women with valvular heart disease have a greater frequency of cardiovascular comorbidities and obstetric, fetal and cardiovascular complications at delivery compared to women without valvular heart disease (Central Illustration). These complications are associated with all stenotic and regurgitant lesions and for both right and left-sided valves. More so, elevated risk of cardiovascular complications remains despite excluding women with pre-existing heart failure and pulmonary hypertension. In addition, these women have higher prevalence of cardiovascular comorbidities, including cardiometabolic disorders, structural and ischemic disease, aortopathies, and connective tissue diseases, as reported previously11–14.
Further, women with valvular disease have greater odds of several obstetric complications. In particular, we note a 90% increased risk for preeclampsia/eclampsia, similar in magnitude to the risk reported with congenital heart disease or heart failure12,15. Preeclampsia is particularly important to detect and manage as it is independently associated with acute cardiovascular complications and increases a woman’s future risk for CVD16–18. Future studies should focus on elucidating common pathophysiologic features (such as angiogenic imbalance or endothelial dysfunction) for maternal valvular disease and disorders of pregnancy, including preeclampsia19,20. Consideration should be given into investigating the use of aspirin for preeclampsia prophylaxis in valvular heart disease, comparable to other cardiometabolic conditions as recommended by the American College of Obstetricians and Gynecologists21,22.
Similarly, other maternal obstetric and fetal complications also carry increased risk in valvular heart disease, including placental abruption, hemorrhage and fetal growth restriction. Adequate uteroplacental flow is necessary for normal pregnancy outcomes and is regulated by vascular remodeling during placentation. Abnormalities in this placentation process may also contribute to complications in women with valvular heart disease, either due to the valve disease itself or shared comorbidities5,23.
Women with valvular heart disease also had higher odds of all cardiovascular complications assessed. Even after excluding preexisting heart failure or pulmonary hypertension, the risks of peripartum cardiomyopathy and acute heart failure were high. Notably, peripartum cardiomyopathy is diagnosed as novel heart failure in the setting of pregnancy, raising suspicion that perhaps oxidative stress in women with valvular heart disease may be contributing to its development24. These findings are comparable to a recent study from the CARPREG cohort from Canada evaluating cardiovascular events in women with valvular regurgitation, which suggested that there is heterogeneity in risk with specific lesion types25. Our study differs though from this prior study as our current study reports odds ratios and compares risk to women without valvular lesions, is larger in size, and nationally representative of US women at time of delivery.
Current guidelines for maternal cardiovascular risk assessment have emphasized risks with stenotic lesions – mainly mitral and aortic stenosis. In the modified WHO guidelines, for those with severe mitral stenosis or symptomatic aortic stenosis, pregnancy is contraindicated4. For regurgitant lesions, risk categories are less well defined as they are thought to be well tolerated. Consequently, only ZAHARA incorporates systemic valvular regurgitation into the risk score, although even this does not differentiate risk based on regurgitation site4. Since our findings suggest significant obstetric and cardiovascular complications associated with regurgitant valves, this challenges the notion that regurgitant lesions are benign. These findings may spur the need to revise contemporary risk calculators, since cardiovascular complications occur in women with regurgitant lesions at rates comparable to those with stenotic lesions.
Our findings should be considered in the context of several limitations. First, valvular lesions and outcomes were classified by ICD-10 codes and misclassification could occur due to heterogeneity in coding, such as potential inclusion of women with prosthetic valves. Second, this database is comprised of inpatients and post-admission data are unavailable. Third, more granular information such as aspirin use or patient symptoms were not available. Fourth, severity of valvular disease could not be assessed and the degree of disease is likely associated with variations in risk. It remains unclear whether risk is primarily being driven by severe lesions. Lastly, while we performed sensitivity analyses excluding women with prior cardiomyopathy or pulmonary hypertension, residual bias is possible, and there may be some overlap with congenital heart disease. Despite these limitations, our findings remain important as they are derived from the largest US inpatient admissions database and reflect a diverse, nationally representative sample of women.
In conclusion, pregnant women with valvular heart disease have more cardiovascular comorbidities and an increased adverse obstetric and cardiovascular events. Notably, regurgitant lesions are associated with similar risk as stenotic lesions. Thus, the presence of any valvular heart disease should be considered when risk-stratifying pregnancy and developing management plans. Additionally, women with both regurgitant and stenotic lesions should be monitored closely during pregnancy with frequent clinical examinations and echocardiograms. Studies should also be performed evaluating the benefit of aspirin prophylaxis for preeclampsia for women with regurgitant lesions given the higher prevalence of preeclampsia in this group. Women with valvular heart disease may benefit from specialized care from a Cardio-Obstetric or Pregnancy Heart Team.
Supplementary Material
Sources of Funding:
Dr. Minhas was supported by National Heart, Lung, and Blood Institute training grant T32HL007024 and the Lou and Nancy Grasmick Research Fellowship. Dr. Michos is supported by the Amato Fund for Women’s Cardiovascular Health Research at Johns Hopkins University.
Disclosures:
Dr. Resar reports grants from Abbott Vascular Inc, grants from Medtronic Inc, grants from Medtronic Inc, other from Boston Scientific Corp, outside the submitted work. All other authors reports no disclosures or conflicts of interest.
Footnotes
This work was performed at the Johns Hopkins University, Baltimore, MD, USA
Data Availability Statement:
The data underlying this article are available from The Healthcare Cost and Utilization Project. The datasets were derived from sources in the public domain: the National Inpatient Sample (https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp)
REFERENCES
- 1.Lima FV, Yang J, Xu J, Stergiopoulos K. National Trends and In-Hospital Outcomes in Pregnant Women With Heart Disease in the United States. The American Journal of Cardiology 2017;119:1694–1700. [DOI] [PubMed] [Google Scholar]
- 2.Anon. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstetrics & Gynecology 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 3.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, Wald RM, Colman JM, Siu SC. Pregnancy Outcomes in Women With Heart Disease. Journal of the American College of Cardiology 2018;71:2419–2430. [DOI] [PubMed] [Google Scholar]
- 4.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJM, Vliegen HW, van Dijk APJ, Voors AA, Yap SC, van Veldhuisen DJ, Pieper PG, On behalf of the ZAHARA Investigators. Predictors of pregnancy complications in women with congenital heart disease. European Heart Journal 2010;31:2124–2132. [DOI] [PubMed] [Google Scholar]
- 5.Ducas RA, Javier DA, D’Souza R, Silversides CK, Tsang W. Pregnancy outcomes in women with significant valve disease: a systematic review and meta-analysis. Heart 2020;106:512–519. [DOI] [PubMed] [Google Scholar]
- 6.Chu R, Chen W, Song G, Yao S, Xie L, Song L, Zhang Y, Chen L, Zhang X, Ma Y, Luo X, Liu Y, Sun P, Zhang S, Fang Y, Dong T, Zhang Q, Peng J, Zhang L, Wei Y, Zhang W, Su X, Qiao X, Song K, Yang X, Kong B. Predicting the Risk of Adverse Events in Pregnant Women With Congenital Heart Disease. JAHA 2020;9. Available at: https://www.ahajournals.org/doi/10.1161/JAHA.120.016371. Accessed January 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hagen IM, Thorne SA, Taha N, Youssef G, Elnagar A, Gabriel H, ElRakshy Y, Iung B, Johnson MR, Hall R, Roos-Hesselink JW. Pregnancy Outcomes in Women With Rheumatic Mitral Valve Disease: Results From the Registry of Pregnancy and Cardiac Disease. Circulation 2018;137:806–816. [DOI] [PubMed] [Google Scholar]
- 8.Elkayam U, Bitar F. Valvular Heart Disease and Pregnancy. Journal of the American College of Cardiology 2005;46:223–230. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality, Rockville, MD. HCUP Databases. Healthcare Cost and Utilization Project (HCUP). 2020. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 26, 2020. [Google Scholar]
- 10.Agency for Healthcare Research and Quality, Rockville, MD. Checklist for Working with the NIS. Healthcare Cost and Utilization Project (HCUP). 2020. Available at: www.hcup-us.ahrq.gov/db/nation/nis/nischecklist.jsp. Accessed December 26, 2020. [Google Scholar]
- 11.Bonnet V, Boisselier C, Saplacan V, Belin A, Gérard J-L, Fellahi J-L, Hanouz J-L, Fischer M-O. The role of age and comorbidities in postoperative outcome of mitral valve repair: A propensity-matched study. Medicine 2016;95:e3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlichting LE, Insaf TZ, Zaidi AN, Lui GK, Van Zutphen AR. Maternal Comorbidities and Complications of Delivery in Pregnant Women With Congenital Heart Disease. Journal of the American College of Cardiology 2019;73:2181–2191. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SA, D’Ottavio A, Spears T, Chiswell K, Hartman RJ, Krasuski RA, Kemper AR, Meyer RE, Hoffman TM, Walsh MJ, Sang CJ, Paolillo J, Li JS. Causes of Death and Cardiovascular Comorbidities in Adults With Congenital Heart Disease. JAHA 2020;9. Available at: https://www.ahajournals.org/doi/10.1161/JAHA.119.016400. Accessed January 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, Enriquez-Sarano M. Incidence of Aortic Complications in Patients With Bicuspid Aortic Valves. JAMA 2011;306:1104. [DOI] [PubMed] [Google Scholar]
- 15.Ruys TPE, Roos-Hesselink JW, Hall R, Subirana-Domènech MT, Grando-Ting J, Estensen M, Crepaz R, Fesslova V, Gurvitz M, De Backer J, Johnson MR, Pieper PG. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart 2014;100:231–238. [DOI] [PubMed] [Google Scholar]
- 16.Bauer ST, Cleary KL. Cardiopulmonary Complications of Pre-eclampsia. Seminars in Perinatology 2009;33:158–165. [DOI] [PubMed] [Google Scholar]
- 17.Minhas AS, Ying W, Ogunwole SM, Miller M, Zakaria S, Vaught AJ, Hays AG, Creanga AA, Cedars A, Michos ED, Blumenthal RS, Sharma G. The Association of Adverse Pregnancy Outcomes and Cardiovascular Disease: Current Knowledge and Future Directions. Curr Treat Options Cardio Med 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minhas AS, Ogunwole SM, Vaught AJ, Wu P, Mamas MA, Gulati M, Zhao D, Hays AG, Michos ED. Racial Disparities in Cardiovascular Complications With Pregnancy-Induced Hypertension in the United States. Hypertension 2021. Available at: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.121.17104. Accessed July 4, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshioka J, Lee RT. Vascularization as a Potential Enemy in Valvular Heart Disease. Circulation 2008;118:1694–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Human Pathology 2003;34:756–763. [DOI] [PubMed] [Google Scholar]
- 21.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-Dose Aspirin for Prevention of Morbidity and Mortality From Preeclampsia: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:695. [DOI] [PubMed] [Google Scholar]
- 22.ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstetrics & Gynecology 2018;132:e44–e52. [DOI] [PubMed] [Google Scholar]
- 23.Pieper PG, Balci A, Aarnoudse JG, Kampman MAM, Sollie KM, Groen H, Mulder BJM, Oudijk MA, Roos-Hesselink JW, Cornette J, van Dijk APJ, Spaanderman ME, Drenthen W, van Veldhuisen DJ. Uteroplacental Blood Flow, Cardiac Function, and Pregnancy Outcome in Women With Congenital Heart Disease. Circulation 2013;128:2478–2487. [DOI] [PubMed] [Google Scholar]
- 24.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand J-L, Desjardins F, Ansari A, Struman I, Nguyen NQN, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A Cathepsin D-Cleaved 16 kDa Form of Prolactin Mediates Postpartum Cardiomyopathy. Cell 2007;128:589–600. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller B, Dave Javier A, Grewal J, Gabarin N, Colman J, Kiess M, Wald RM, Sermer M, Siu SC, Silversides CK. Risk Associated With Valvular Regurgitation During Pregnancy. Journal of the American College of Cardiology 2021;77:2656–2664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from The Healthcare Cost and Utilization Project. The datasets were derived from sources in the public domain: the National Inpatient Sample (https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp)


