Abstract
The three classical core technologies for the preservation of live mammalian biospecimens — slow-freezing, vitrification, and hypothermic storage — limit the biospecimens’ biomedical applications. In this Review, we summarize the principles and procedures of these three technologies, highlight how their limitations are being addressed via the combination of microfabrication and nanofabrication, materials science and thermal-fluid engineering, and discuss the remaining challenges.
Mammalian biospecimens — cells, tissues and organs — are widely used in scientific research and in clinical applications1. They are procured either from animal or human donors via direct isolation, or via in vitro fabrication and modification, and typically used at later time points and at different geographical locations2,3. By maintaining and extending the life and functions of biospecimens outside their native environment and conditions, biopreservation bridges the spaciotemporal gap between the sources and acquisition times of biospecimens and their destinations and times of use, enabling their widespread distribution, transportation and application4 (Fig. 1). For instance, the preservation of reproductive cells and tissues (such as oocytes, spermatozoa, ovaries and embryos) is essential for assisted-reproductive technology, whose global market value is forecasted to reach over US $45.4 billion by 2025 (ref.5). Moreover, the preservation of tissues and organs for transplantation and replacement saves or improves millions of lives every year6. In 2019, there were 11,3000 patients on the waiting list for lifesaving organ transplants in the United States alone7, and the true need for transplantation is estimated to be more than ten times that number8. Biopreservation also aids the provision of reliable, accessible and abundant mammalian-cell sources, especially stem cells, blood cells and genetically engineered cells, which are indispensable for cell-based medicine9,10.
Fig. 1 |. Preservation of mammalian biospecimens.
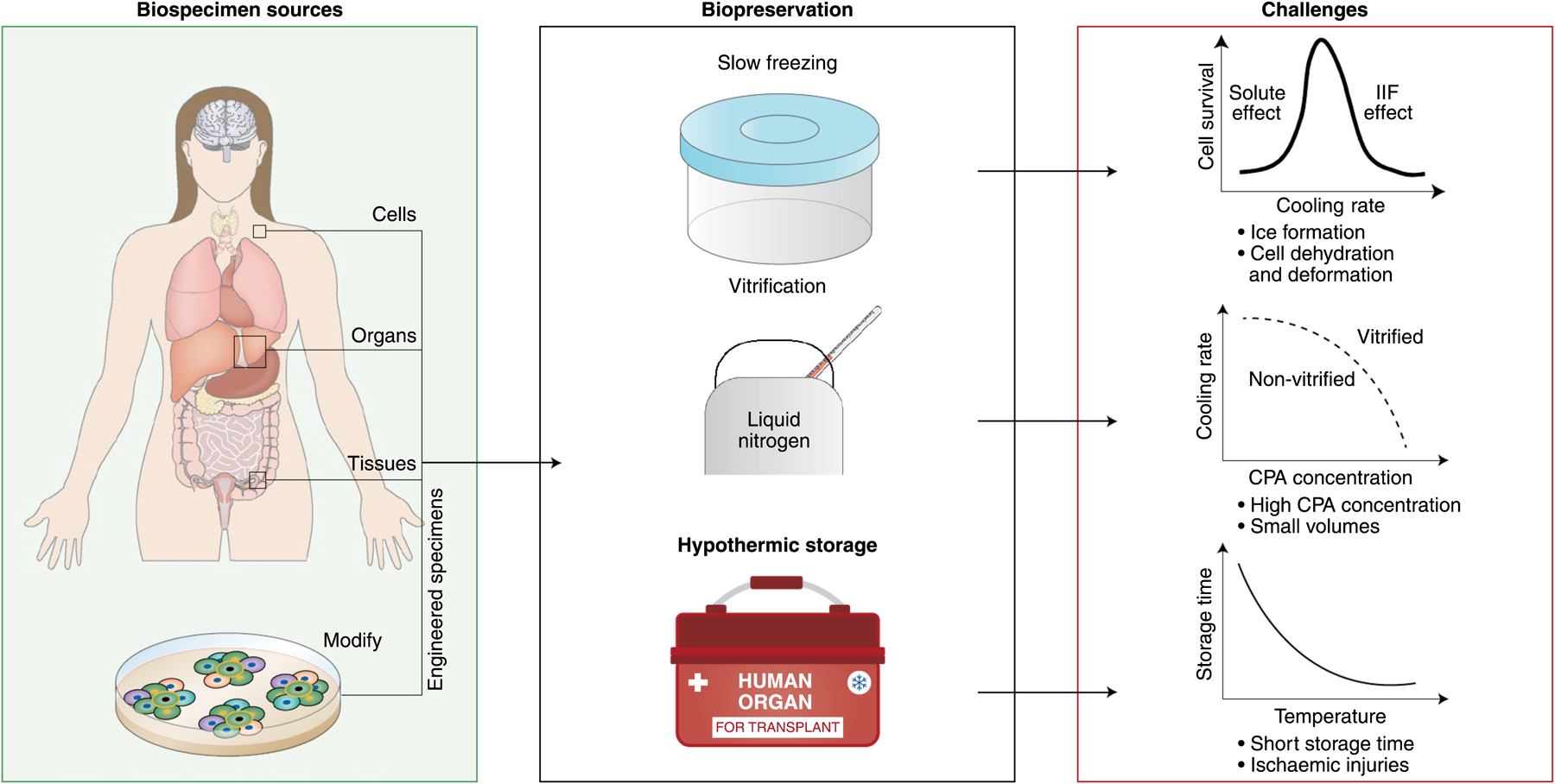
The preservation of cells, tissues and organs from human donors, and of engineered specimens from them, involves slow freezing, vitrification or hypothermic storage (with machine perfusion). Preserved biospecimens enable a wide range of biomedical applications. CPA, cryoprotective agent.
Biopreservation involves keeping biospecimens at low temperatures, so as to reduce or suspend their physiochemical and metabolic activities, and then recovering them back to physiological states at a desired future time. For long-term preservations (more than one week for suspended cells), the classical technology is cryopreservation — that is, the storage of biospecimens at cryogenic temperatures (below −60 °C) to completely arrest their bioactivities. To attenuate adverse effects during cryopreservation (such as ice formation), one or more cryoprotective agents (CPAs; such as dimethyl sulfoxide) are added to the biospecimens. There are two common methods of cryopreservation: slow freezing, and vitrification. Slow freezing is commonly used for the preservation of mammalian cells and microtissues11,12, either in an insulated container at –80 °C with cooling rates of about 1 °C min−1, or in a programmable freezer with controllable cooling rates, followed by storage in a −80 °C freezer or in a −196 °C liquid-nitrogen tank13. Vitrification directly transforms biospecimens from a liquid state into a glassy state through non-equilibrium cooling to minimize or eliminate ice formation. Hence, vitrification is generally considered to be superior to slow freezing for banking stress-sensitive biospecimens, such as oocytes, stem cells and some tissues14–16. Vitrification employs either a high concentration (6–8 M) of CPA (conventional vitrification)17, or an increasing cooling rate with a reduced CPA concentration (low-CPA vitrification)18.
For short-term preservations (typically less than 12 hours, in particular for human livers), hypothermia or static cold storage are typically used. These methods involve the storage of biospecimens at subnormothermic temperatures (usually 0–4 °C) to reduce their metabolic and degradation rates and to avoid ice formation. Also, because hypothermic storage doesn’t trigger substantial thermal stresses, nor needs cytotoxic CPAs, it is preferably used to preserve large-volume tissues and organs with complex and delicate structures (such as microcapillaries) that are highly susceptible to such ‘cryoinjuries’6. Hypothermia has been widely adopted for the preservation of solid tissues and organs for transplantation (in particular, corneas19, livers20, kidneys21 and pancreases22).
In this Review, we examine the processes of these classical biopreservation methods, the associated mechanisms of cryoinjury, and limitations in the applicability of the methods, and highlight recent advances for overcoming these limitations. In particular, we focus on recent progress in preservation strategies for the reduction of cellular injuries and for improving the outcomes of biopreservation. The optimization of the compositions and concentrations of CPAs and of solutions for biopreservation have been summarized eleswhere23,24.
Preservation processes
Depending on the temperature and concentration of solutes (including CPAs), biospecimens can be liquid or vitrified, which are stable states, or supercooled or supersaturated, which are thermodynamically unstable phases (Fig. 2a). For preservation, biospecimens are typically kept in one of the stable regions: either the vitrified state for long-term cryopreservation, or the liquid state for short-term hypothermic storage. The thermodynamic paths of biopreservation processes are depicted in Figs. 2b,c. During slow freezing, biospecimens follow the thermodynamic path A→C→E→F→G→I→L→Z. Ice crystals initiate at E and then propagate and grow (E→F→G), a process known as ‘freeze concentration’, as it elevates extracellular osmolality (or solute concentration), driving cell dehydration and deformation. Conventional vitrification follows the path A→D→II→M→Z; high-concentration CPAs are loaded at non-freezing temperatures, and a rapid and non-equilibrium cooling process leads to vitrification. The higher the CPA concentration, the lower the cooling rate required to inhibit ice formation. Therefore, low-CPA vitrification, which should follow the path A→C→III→N→Z, uses such a high cooling rate that the required CPA concentration is comparable to that of slow freezing18,25. Hypothermic storage typically follows the path A→B→IV→O→Y, with a storage temperature usually above the equilibrium freezing line. Supercooling storage follows the path A→B→V→P→X, with a storage temperature below the equilibrium freezing point, so as to extend preservation time. Notwithstanding their simplified diagrams of phase transition, thermal and osmotic time courses provide a comprehensive view of the procedures of biopreservation, and illustrate their risks and limitations.
Fig. 2 |. Phase diagrams, and thermal and osmotic time courses, for five main biospecimen-preservation methods.
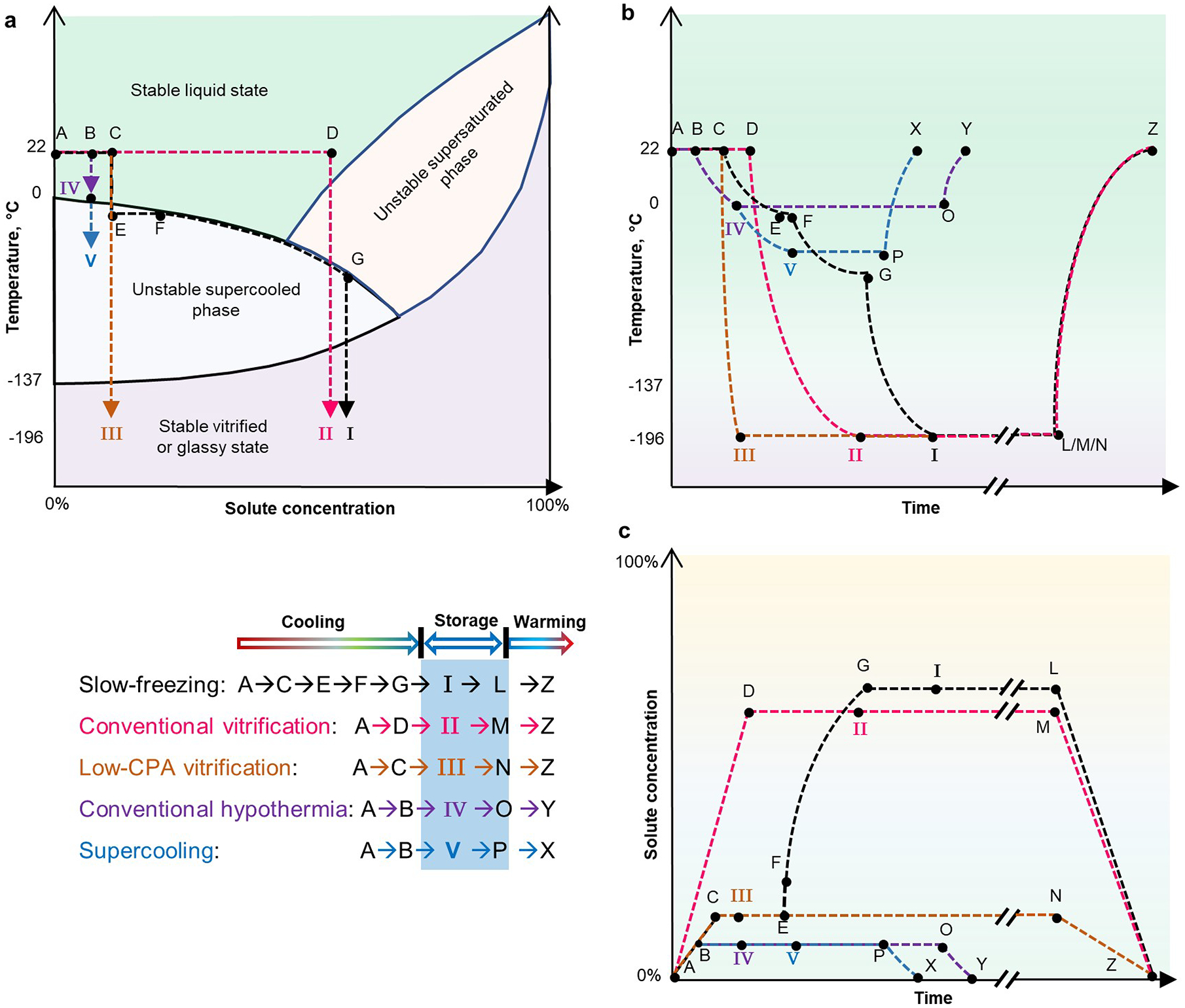
For each preservation method — I, slow freezing; II, conventional vitrification; III, low-CPA vitrification; IV, conventional hypothermia; and V, supercooling — the thermodynamic paths involved in the cooling, storage and warming steps are indicated. a, Diagram of the phases of matter of biospecimens processed with different preservation methods. Arrows indicate the direction of the preservation process. b, Thermal time course. c, Time course of the solute concentration (or osmolality). Axes not drawn to scale. The rates of temperature change and of osmolality change are indicated by the slopes of the curves. Long-term storage is indicated by ‘//’. For simplicity, panel a doesn’t show the steps of storage and warming, and panels a and b do not show temperature fluctuations arising from the release (during cooling) and absorption (during heating) of latent heat associated with phase changes. It is assumed that there is no solute precipitation during freeze concentration (path E→F→G) and during plunging into liquid nitrogen (path G→I). Temperature profiles during cooling are approximated as convex curves (in paths C→I, D→II, C→III, B→IV, and B→V) because cooling rates decrease as the temperature of the biospecimen drops, and as concave curves (in paths L/M/N→Z, O→Y, and P→X) during warming because the warming rate decreases as the biospecimen temperature increases179. The solute-concentration paths in c are drawn as straight lines because CPA loading and unloading can be controlled arbitrarily before and after preservation.
Current preservation technology
All the classical preservation approaches have intrinsic limitations. During slow freezing, the biospecimens are subject to ice formation, freeze concentration and morphological deformation (E→F→G in Fig. 2). Extracellular ice crystals can damage the plasma membrane as a result of ‘crushing’, and any appreciable formation of intracellular ice is almost always fatal to the cells, as it disrupts subcellular organelles and their cellular membrane as well as the cytoskeleton. Freeze concentration causes significant osmotic stress, leading to cell dehydration and deformation under a high concentration of solutes (point G in Fig. 2a,c). A classical two-factor theory of such cryoinjuries that considers solute effects under slow cooling and intracellular ice formation under fast cooling26 suggests an optimal cooling rate for each specific type of cells that minimizes both types of injury, and indicates a reversed U-shape relationship curve between cell survival and cooling rate27. The optimal cooling rate for cells depends on the cells’ characteristics, especially cell size and membrane-transport properties28–31.
Conventional vitrification uses a high concentration of CPA so as to increase the biospecimen’s glass-transition temperature and to decrease its diffusion coefficient of water molecules (and thus increasing the biospecimen’s viscosity). However, because highly concentrated CPA at high temperatures (path A→D in Fig. 2) can be highly toxic to mammalian cells, the exposure time is often minimized and the temperature decreased32. Therefore, it is almost mandatory to use a multistep protocol to load CPAs before preservation (path A→D in Fig. 2c) and to unload them after preservation (path M→Z in Fig. 2c). This is a time-consuming, tedious and stressful process33,34. However, low-CPA vitrification relies on such a high cooling rate that water molecules do not have time to relocate, realign and reorganize to form ice crystals before they are immobilized in a glassy state (which is akin to the liquid state in microstructure). Moreover, to prevent devitrification and ice recrystallization, the required heat-transfer rate is higher during warming than during cooling35,36. This is probably due to the fact that the warming rate would be slowed down, owing to the absorbance of latent heat from −15 °C to 0 °C (path N→Z in Fig. 2b), and to the presence of myriad tiny ice embryos and interfaces in vitrified biospecimens, which can drive the nucleation of large ice crystals37. Hence, high surface-to-volume ratios and small sample volumes are required to obtain high cooling rates and warming rates (as indicated by the high slopes for the paths C→ III and N→Z in Fig. 2b). This is achieved through conventional heat transfer via conduction and convection in liquid nitrogen or in a water bath25.
During conventional hypothermic storage (path A→B→IV→O→Y in Fig. 2) above the equilibrium freezing temperatures, biospecimens undergo substantial physiochemical and metabolic activities under suboptimal conditions, consuming nutrients and oxygen, depleting energy, and producing noxious metabolites (such as tumour necrosis factor alpha and nitric oxide)38–41. In addition, the cut-off from circulation systems and the lack of nutrient and metabolite transport aggravate ischaemic injuries42. Furthermore, when hypothermic tissues or organs are rewarmed to physiological temperatures on reperfusion, the suddenly available oxygen produces overwhelming oxygen-free radicals beyond cellular-scavenge capacity, inducing pro-apoptotic signal transduction and concomitant damages (ischaemia-reperfusion injury)43. Therefore, biospecimens in hypothermic storage usually last for short periods (path IV→O in Fig. 2b), from several hours (4–6 hours for hearts and lungs) to few days (1 day for human kidneys and 3 days for rat kidneys)44–46. Hence, the shortcomings of the classical approaches of biopreservation demand innovation and optimization.
Advances in slow freezing
Slow freezing involves cell-specific cooling rates so as to avoid excessive solute effects and intracellular ice formation (Fig. 3a). Such cryoinjuries depend on the quality and quantity of ice crystals, and on the cells’ characteristics. For many cell types and for tissues with more than one cell type, optimal cooling rates remain elusive. A number of methods and devices are being used to investigate and address these issues.
Fig. 3 |. Slow freezing.
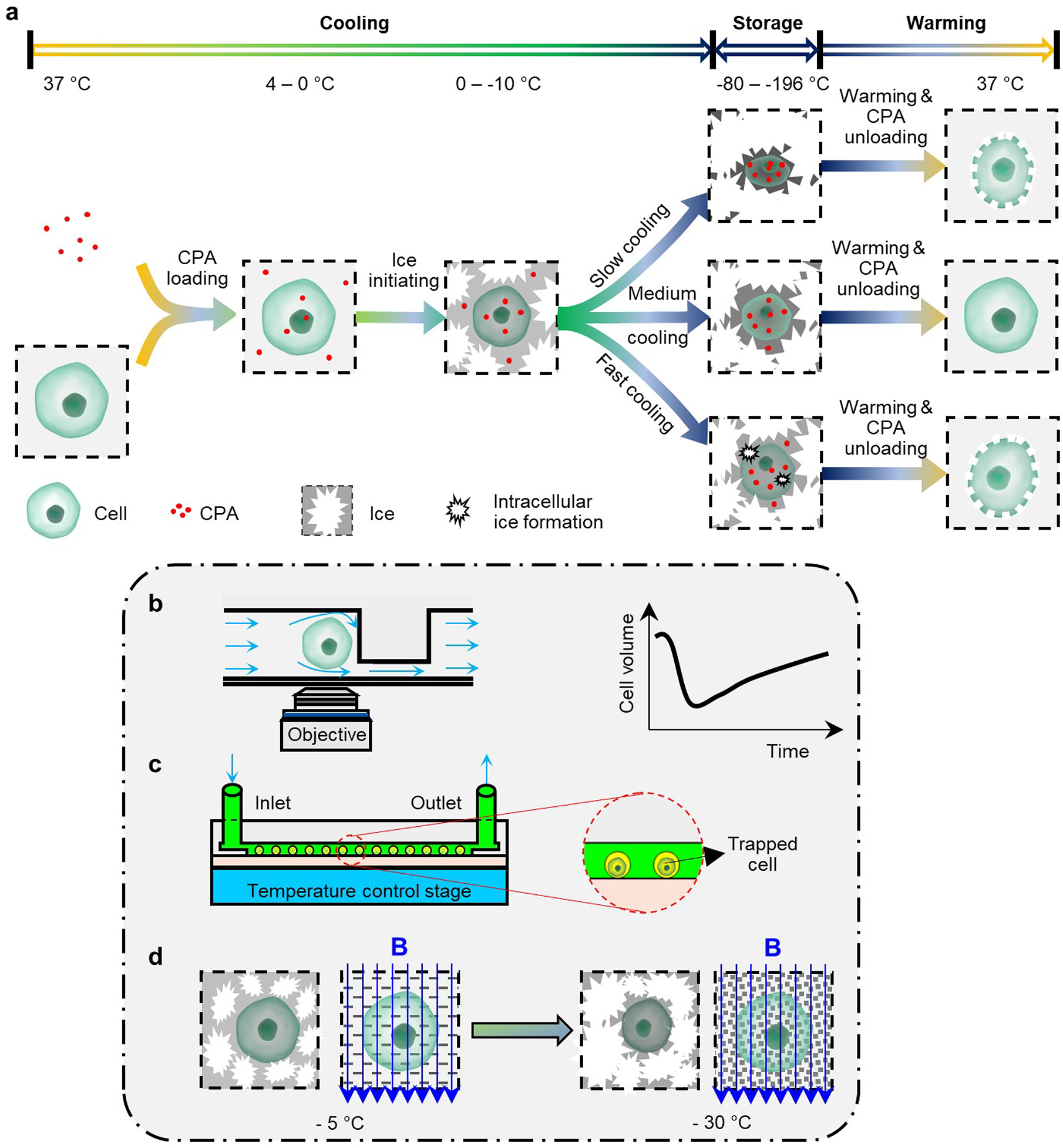
a, Steps in slow freezing, and mechanisms of the cryoinjuries that it can cause. For specific types of cell, there are optimal cooling rates that minimize cell injury, owing to excessive cell dehydration and intracellular ice formation. b, Microfluidic perfusion for the investigation of the cell-transport properties of water and of the cryoprotectant agents (CPAs). c, On-chip cryopreservation allowing for the visualization and study of cell responses at the single-cell level. d, Magnetic cryopreservation for the modulation of the kinetics of ice formation. Magnetic fields can drive the formation of finer ice crystals and thus less cellular damage.
Dynamic measurement of membrane transport.
Understanding the osmotic behaviour of cells and quantifying the transport properties of water and CPAs across cell membranes (in particular the osmotically inactive volume, the cell-membrane permeability coefficient of water, and the associated activation energy) are prerequisites for the development of optimal slow-freezing procedures. Methods based on Coulter Counters have been used to determine cell volumes and osmotic responses by measuring the impedance of cell suspensions, but these methods cannot be used to study the osmotic responses of specific cells, and are influenced by the presence of debris and agglomerates47–50. However, polydimethylsiloxane or glass microfluidic devices bearing microchannels and perfusion chambers for trapping the cells (Fig. 3b) can measure these properties at single-cell-resolution. These devices also enable the real-time monitoring of cell volumes on changes in the extracellular solutions for a range of cell types (as has been demonstrated for rat basophilic leukaemia cells51, human umbilical vein endothelial cells52 and mouse dendritic cells53). Also, because these methods use monolayers of immobilized individual cells, they avoid the measurement ambiguities associated with cells cultured on conventional Petri dishes, as they often overlap and move out of the imaging focus plane. And with the assistance of cryostages54 or thermocouples55, these systems can quantify the transport properties of cell membranes at supra-zero and sub-zero temperatures. This enabled the discovery of lipid phase transitions occurring in the cell membrane at temperatures of 0–12 °C, as indicated by a discontinuity in the activation energy54. Furthermore, accompanied by hydrodynamic switching, on-chip perfusion can be used to change the extracellular solution at a controllable rate56. Therefore, these microfluidic devices allow for the optimization of the choice of CPAs, their concentrations and the cooling rate, to reduce osmotic stresses, control water and CPA transport, and minimize intracellular ice formation52 (that is, they modify the path E→F→G in Fig. 2).
On-chip cryopreservation.
Microfluidic chips can also be used for improved on-chip cryopreservation efficiency, accuracy and safety. For instance, microfluidic channels have been used to freeze, thaw, culture and analyse suspensions of cells in a streamlined manner to save time, labour and costs57,58, and glass slides with etched two-dimensional arrays of picometric wells have been designed to hold individual cells in each well (Fig. 3c). In such ‘cryo-chips’, the cells retain their positions during the freeze–thaw cycle, allowing for comparisons of individual cells before and after cryopreservation. Cryo-chips enable the investigation of correlations between cell characteristics and cryopreservation outcomes, and hence provide insights than can’t be obtained via conventional bulk cryopreservation (because of the inherent heterogeneity of cell populations)59. Cryo-chips also allow for multiparametric analyses of the cryopreserved cells (such as apoptotic rate, mitochondrial membrane potential, and intracellular metabolism60). For example, single-cell arrays have revealed that primary hepatocytes with a high mitochondrial membrane potential before freezing are significantly less likely to survive the freeze–thaw cycle61. Also, on-chip cryopreservation provides precise control of the cooling and warming procedures. For instance, microchannels allowed for the cryopreservation of human spermatozoa without the need for CPAs, because of the microchannels’ high heat-transfer rate62. And the insertion of microheaters into microfluidic devices, to temporarily hold them at −25 °C for 3 mins before plunging them into liquid nitrogen, improved yeast-cell viability after cryopreservation63. Moreover, fluid flow over a monolayer of adherent cells on microfluidic devices can induce shear stress on the cells, increasing the number of focal-point adhesions between cells and the surface, enhancing cellular survival during cryopreservation64. Therefore, microfluidic devices can offer higher levels of manoeuvrability for sample preparation and analysis, of controllability of the cooling and warming procedures, and of the optimization of the cryopreservation conditions.
Magnetic cryopreservation.
The formation of intracellular ice, especially of large intracellular crystals, is almost always fatal to mammalian cells65,66. The application of a magnetic field to biospecimens can alter the kinetics of ice nucleation and propagation to improve slow freezing. For instance, by imposing an external magnetic field, human periodontal ligament cells and pulp tissues were preserved via slow freezing with a significantly reduced CPA concentration, and then transplanted into patients to heal normal periodontal ligaments67–69. These studies suggest that biospecimens can be preserved by maintaining them under a magnetic field of 0.01 mT and at a temperature of −5 °C for 15 min, and then by slowly cooling the biospecimens to approximately −30 °C before plunging them into liquid nitrogen (Fig. 3d). It has been shown that cells or tissues exposed to a magnetic field have, after cryopreservation, improved viability, adhesion and proliferation, osteogenic and adipogenic differentiation, and histological integrity67,68,70,71. These findings imply that slow freezing with a magnetic field is a feasible strategy for the banking and transplantation of teeth. Although the precise working mechanisms remain to be fully understood72, it appears that magnetic fields can act directly on water molecules by re-orientating, vibrating or spinning them, preventing them from clustering and nucleating and facilitating supercooling or intracellular vitrification (which would correspond to a decrease in the temperature of point E in Fig. 2a). Hence, subjecting biospecimens to a magnetic field of adequate strength during slow freezing can reduce the usage of CPAs and enhance cryopreservation outcomes, especially for dental stem cells and dental tissues71.
Advances in vitrification
In principle, vitrification avoids all cryoinjuries associated with ice formation during slow freezing. However, vitrification requires a high concentration of cytotoxic CPAs to prevent ice formation. Traditionally, CPAs are loaded and unloaded via tedious and time-consuming multistep procedures. In addition, the use of CPAs restricts sample sizes to be in the order of 100 μl so as to obtain sufficiently high cooling and warming rates (Fig. 4a). These shortcomings have been alleviated by innovations in biomedical devices, biomaterials and thermal-fluid engineering.
Fig. 4 |. Vitrification.
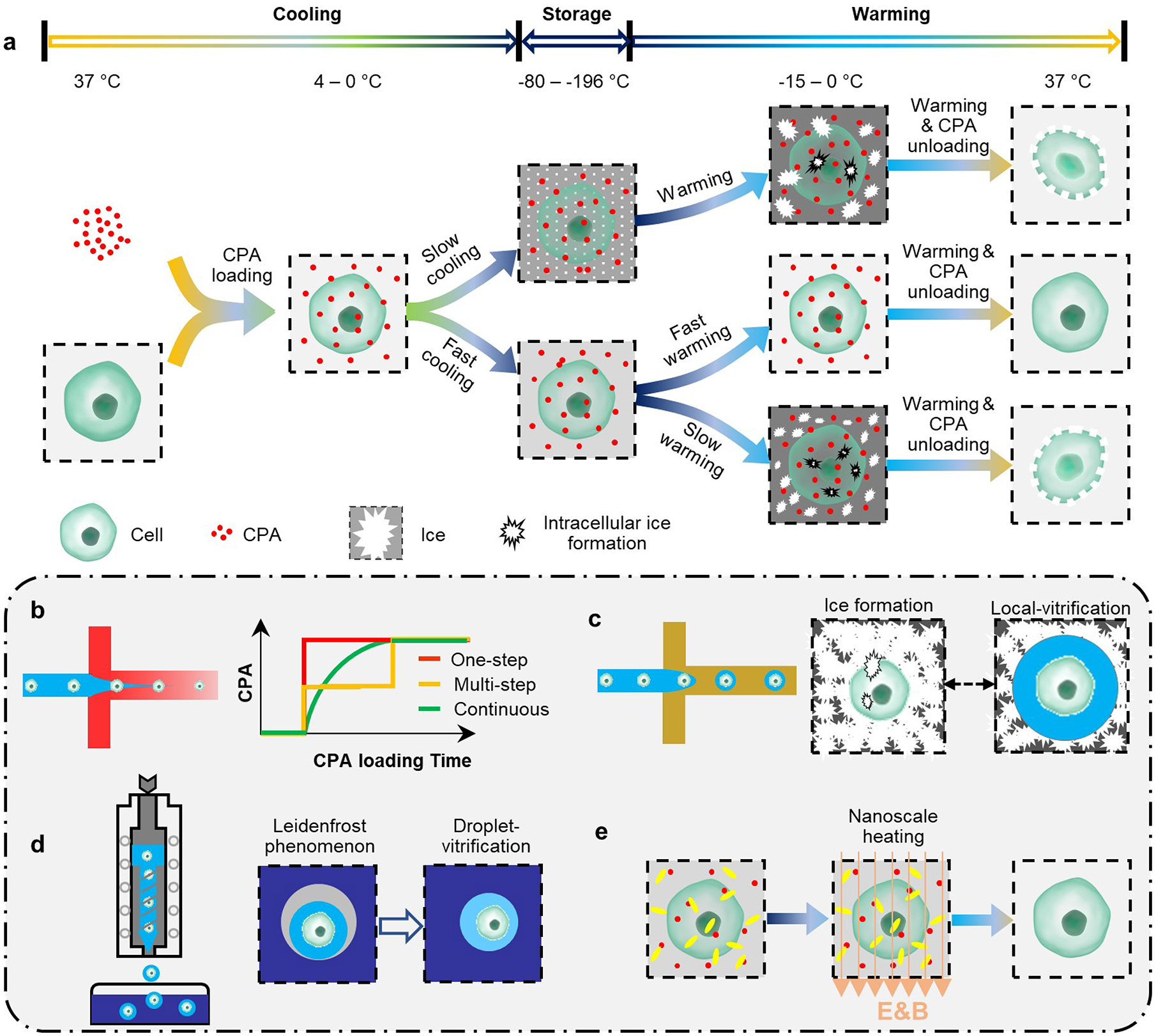
a, Steps in vitrification, and the mechanisms of the cryoinjuries that it can cause. b, Automated loading of cryoprotectant agents (CPAs) to minimize loading time, cell exposure to the agents, and osmotic stresses. c, Local vitrification via hydrogel microencapsulation. Hydrogel microcapsules inhibit devitrification and ice recrystallization during warming, and create an ice-free microenvironment for the encapsulated biospecimens. d, Droplet vitrification by ‘shooting’ cell-laden microdroplets into liquid nitrogen. During rapid cooling, a gas bubble (grey) can form around the microdroplets (Leidenfrost effect), causing reduced cooling rates. e, Nanoscale heating, which combines nanoparticles (yellow) and electromagnetic fields, can significantly increase warming rates, and thus alleviate cryoinjuries and the volume constraints of vitrification.
Automated CPA loading and unloading.
Various types of microfluidic device have been developed to facilitate and improve the outcomes of multistep CPA loading and unloading processes during vitrification. The devices can automatically add and remove CPAs (that is, they can modify the paths A→D and M→Z in Fig. 2c) with smoother osmotic-change profiles than conventional step-wise methods73,74. By capitalizing on laminar flow and on the steady molecular diffusion occurring in the microchannels, these microfluidic devices can reduce CPA loading time and cell-exposure time to cytotoxic CPAs at non-freezing temperatures, and decrease both the rate of cell-volume change and dehydration overshooting to reduce osmotic stress74 (Fig. 4b). As a result, cell viability and cell functionalities after cryopreservation have been improved, and the manoeuvrability, reliability and fidelity of the experiments have been enhanced75. Specifically, a microfluidic device has been designed to steadily and slowly load CPA into bovine and murine oocytes and zygotes, and can decrease cell-shrinkage rate, osmotic stress and sublethal damage to the cells76. Similar microfluidic strategies have been used in the context of assisted reproductive technologies77. Overall, the use of microfluidic technology for automated CPA loading and unloading during vitrification effectively eases the tedious and stressful procedures of CPA loading and unloading, reduces osmotic stresses and the rates of cell-volume changes, and enhances the cryopreservation outcomes78.
Hydrogel microencapsulation.
Besides the use of high CPA concentration and small sample volumes, vitrification can be carried out by changing the biophysical and biochemical properties of the biospecimens to be preserved (that is, by modifying the general phase diagram depicted in Fig. 2a for a particular biospecimen). For instance, water confined in a calcium alginate hydrogel matrix can vitrify more readily than free bulk water79. In fact, ice formation owing to devitrification and ice recrystallization during warming are major obstacles to preservation by vitrification36 (Fig. 4a). Calcium-alginate hydrogels possess a remarkable capacity to inhibit devitrification and recrystallization, and therefore can be used to create localized nanolitre volumes of ice-free microenvironments80 (Fig. 4c). Embryonic stem cells and mesenchymal stem cells encapsulated in microcapsules of calcium alginate hydrogel via droplet-based microfluidics for cryopreservation via ‘local’ vitrification reduced the needed CPA concentration 4 times and increased the maximum sample volumes 100 times80. This method has also been used to preserve ‘ready-to-use’ cell–biomaterial constructs81 and individual pancreatic islets82. Also, the alginate hydrogels enhance the metabolic activity and proliferative capacity of the cells after cryopreservation83. Supramolecular gels have also been used to minimize cryoinjuries during vitrification84. Besides protecting encapsulated cells by confining the nucleation and growth of ice crystals and decreasing osmotic stresses during cryopreservation, hydrogels can also have excellent biocompatibility (particularly, natural polymer hydrogels), controllability (synthetic polymer hydrogels), and versatility (supramolecular hydrogels), and hence their wider use in cell and tissue engineering, transfusion and transplantation applications85.
Droplet vitrification.
Small sample volumes and low throughputs limit the use of low-CPA vitrification86. For low CPA concentrations, vitrification can be achieved at high cooling rates and high throughputs by continuously ‘shooting’ cell-laden microdroplets directly into liquid nitrogen via droplet generators (Fig. 4d). This method has been used to preserve hepatocytes, fibroblasts, cardiomyocytes, embryonic stem cells and mouse embryos87–89. A similar strategy preserved mouse oocytes in microdroplets using low CPA concentrations90, as well as red blood cells91. In droplet-mediated vitrification, the optimal droplet size has been determined via theoretical and numerical analyses of heat transfer and ice formation, which take into account the effects of a gas bubble that forms around the microdroplet on contact with liquid nitrogen (because of the Leidenfrost effect)92. Vitrification of macroscopic cell-laden droplets (3–5 mm in diameter) can also preserve cells, as shown for primary hepatocytes at cooling rates of the order of 103 °C min−1 (ref.93). However, this droplet-vitrification method exposes the cells directly to liquid nitrogen, which can cause pathogenic contamination and cell loss94–96. However, cell-laden droplets and liquid nitrogen can be segregated via cell printing on a silver film97. Incidentally, droplet vitrification is widely used to preserve plant shoot tips and germplasms98–100.
Nanoscale warming.
To effectively suppress devitrification and ice recrystallization, the critical warming rate must exceed the critical cooling rate by at least one order of magnitude35,36. However, warming rates achievable by convective heating in a 37 °C water bath are only 10–100 °C min−1, even for small samples (about 1 ml). Laser beams and a laser absorber material such as carbon black have been used to increase the warming rate101, which allowed the vitrification of mouse oocytes in a CPA solution diluted 3-fold with nearly 100% survival of the cells101. Warming rates of 1.4 × 107 °C min−1 have been achieved for zebrafish embryos at a low concentration of CPA (2 M of propylene glycol) by microinjecting gold nanorods coated with carbon black and exciting them with pulsed laser beams102 (Fig. 4e). Moreover, the uniformity of the warming, which reduces the formation of thermal stresses and mechanical cracks, can be improved via the application of electromagnetic fields103.
The warming rates of large-volume (1–80 ml) biospecimens (in particular, human dermal fibroblast cells, porcine arteries, and porcine aortic heart-valve-leaflet tissues) preserved in standard vitrification solution (VS55) were elevated to 130 °C min−1 or higher by coupling magnetic fields and radiofrequency-excited iron oxide nanoparticles coated with mesoporous silica104. Such nanoscale-heating approach improved the viability of the cells and tissues as well as their biomechanical properties104. The vitrification of mesenchymal stem cells from human umbilical cord has also been enhanced by a similar strategy combining magnetic-induction heating and superparamagnetic Fe3O4 nanoparticles105. The combination of nanoparticles and electromagnetic heating can thus significantly increase the warming rates of cryopreserved biospecimens (the slope of the path M→Z in Fig. 2b), inhibiting the formation of ice owing to devitrification and recrystallization, and hence decreasing the required concentrations of CPAs.
Advances in hypothermic storage
Slow freezing and vitrification have been used to preserve cells and simple or small tissues106,107. Yet most large or complex tissues and organs cannot tolerate the extensive ice formation and deformation triggered by slow freezing, nor the highly concentrated CPA and osmotic stresses of vitrification. Therefore, large tissues are mostly preserved in liquid states, especially hypothermia (Fig. 5a). However, biospecimens in hypothermia can degrade rapidly and undergo ischaemic injuries (such as ischaemia-reperfusion injury). A few strategies can be used to alleviate or avoid these issues.
Fig. 5 |. Hypothermic storage.
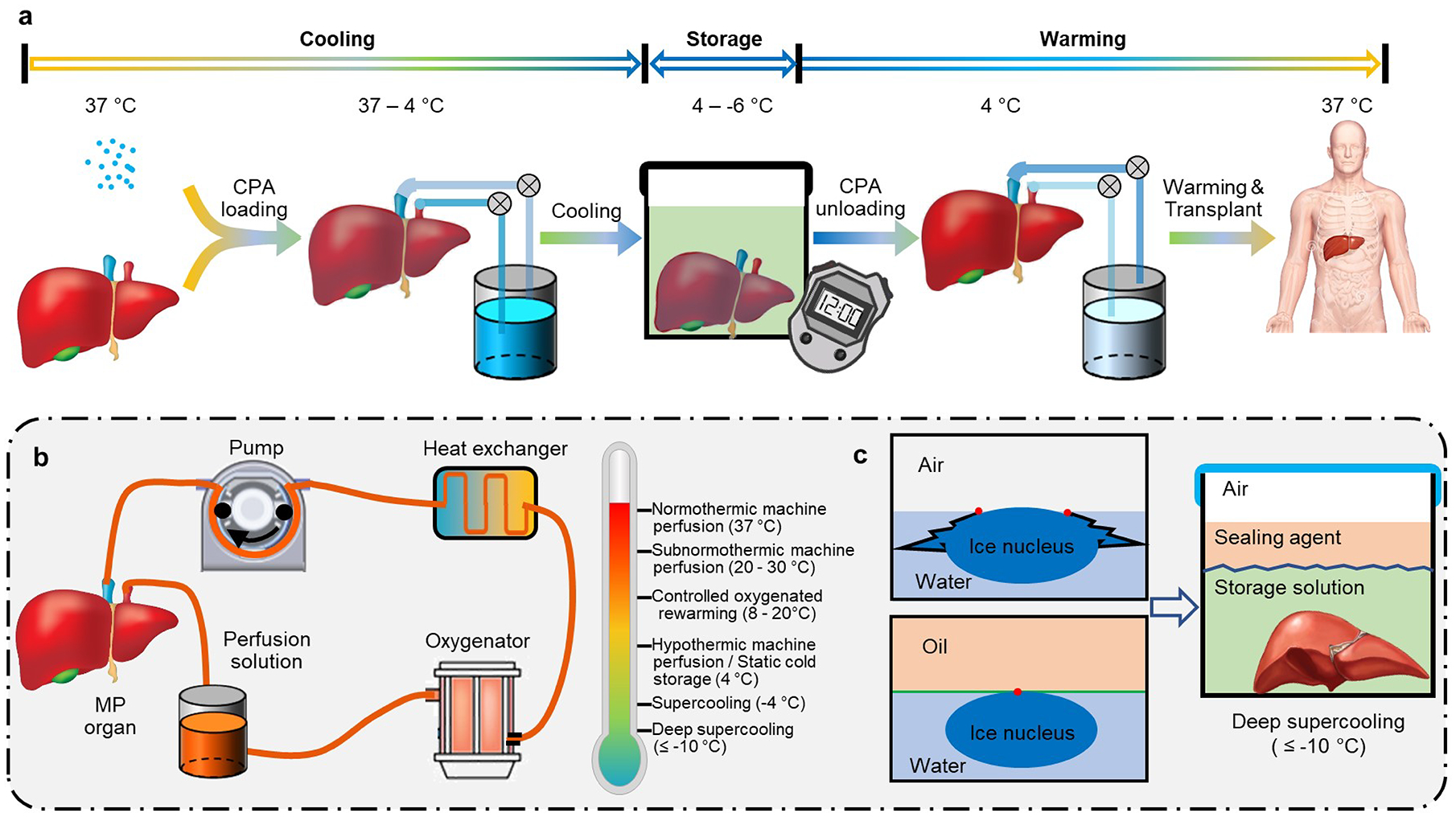
a, Steps in the hypothermic storage of tissues and organs. Owing to their metabolic activities and to fast ‘decaying’ cellular processes, tissues and organs can only be stored for much shorter durations than slow-frozen or vitrified biospecimens. b, In hypothermic storage, oxygenated machine perfusion alleviates ischaemic injuries and reconditions tissues and organs. The temperature range depends on the characteristics and conditions of the tissues and organs to be preserved. c, Deep supercooling (at temperatures lower than −10 °C) extends the duration of hypothermic storage, because it eliminates primary ice-nucleation sites at the water–air interface by ‘sealing’ it with water-immiscible agents.
Temperature-controlled machine perfusion.
Ischaemic injuries can be alleviated by supplying nutrients and removing wastes from the preserved tissue via machine perfusion, with different tissues and organs requiring different perfusates and perfusing temperatures (Fig. 5b). Differently from conventional static cold storage, normothermic machine perfusion (at 37 °C) can reinstitute physiological-like conditions for tissues and organs and circumvent cold injuries, ischaemic injuries and reperfusion injuries, and it allows for the close monitoring of viability and functions108. Therefore, it has been widely used to preserve livers109, hearts110 and lungs111 for transplantation. Normothermic machine perfusion is also effective to assess tissue and organ conditions before transplantation112,113, and can even be used to recondition or restore questionable or high-risk organs114. It maintains preserved organs in a physiological state, avoiding cooling and recovering procedures and therefore significantly decreasing the possibility of graft injury and extending the preservation time of human livers115. Oxygenated sanguineous normothermic machine perfusion has also been used to recover the metabolism and functions of livers after extended warm ischaemia and simple static cold storage116,117. And in combination with the management of glucose, oxygen and waste products, it has been used to preserve the viability and functions of human livers that had been rejected for clinical transplantation for 7 days118, raising the possibility of restoring marginal organs to expand the organ-donor pool.
Alternatively, subnormothermic machine perfusion (at 20–30 °C) has been used to repair damages induced by warm ischaemia in rat liver for orthotopic transplantations119,120, and to maintain human liver function post-ischemia with minimal injury121,122. And hypothermic machine perfusion (at 4–8 °C) can significantly decrease metabolic rates for extended storage123. It has been used to improve transplantation outcomes for human livers124, kidneys125,126, and even ‘orphan’ organs from extended-criteria donors20. Moreover, perfusion via controlled oxygenated rewarming (at 8–20 °C) — that is, the slow rising of temperature and oxygen concentration (which would alter the path O→Y in Fig. 2b,c)127,128 — can gently restore cellular conditions and functions to minimize ischaemia-reperfusion injury, which is mainly caused by abrupt changes in oxygen concentration and temperature127,129. Machine perfusion can also be used to gradually load CPAs (path A→B in Fig. 2c) to solid organs before static cold storage and to gradually unload the CPAs after it123,130 (path O→Y in Fig. 2c).
Supercooling and deep supercooling.
The rates of biophysical and biochemical activities decrease as the storage temperature decreases131 (as per the Arrhenius equation). In conventional hypothermia at 0–4 °C, the metabolic rates of biospecimens are decreased by 90% or more (with respect to the physiological rates at a temperature of 37 °C; refs.132–134). Therefore, hypothermic storage is effective for the short-term preservation of mammalian cell suspensions135,136, two-dimensional and three-dimensional cell aggregates137, and cell–biomaterial constructs138,139. It is in fact the gold-standard method for the preservation of large tissues and organs for clinical transfusion and transplantation. For example, donated human blood is routinely stored at 4 °C in a blood bank for a maximum of 42 days before transfusion or disposal. Excised livers, pancreases and kidneys are usually flushed with a hypothermic storage solution and stored at hypothermic temperatures (0–4 °C) for preservation and shipping before transplantation140,141. Because the limit of hypothermia is largely determined by tissue degradation, decreasing storage temperatures and thus degradation rates extend the duration of preservation via hypothermia. However, decreases in storage temperature that push the sample to the unstable supercooled phase (point H in Fig. 2) risks the viability of the biospecimens, owing to spontaneous ice formation. By staying at high sub-zero temperatures (higher than −6 °C), the risk of freezing is low and supercooling can preserve cells and organs for as long as 4 days39,123,142,143 (path V→P in Fig. 2b,c). The lower the temperature, the higher the probability of ice formation39 and thus of biospecimens in the supercooled state to be affected by stochastic freezing events. However, theoretical, numerical and experimental studies have identified that the primary freezing mechanism for supercooled water and aqueous solutions involves heterogeneous ice nucleation at the water surface144–147. Therefore, sealing the water surface with an immiscible oil phase can ‘remove’ these primary ice-nucleation sites, and stabilize the supercooled state (Fig. 5c). For pure water, such ‘deep supercooling’ state can be maintained at temperatures as low as −20°C for at least 100 days144. Deep supercooling has been used to preserve human red blood cells at −13 °C and −16 °C for 100 days144, as well as adipose-derived stem cells for 7 days at −16 °C, with high cell viability and functionalities maintained148. Deep supercooling of tissues and organs could significantly extend their hypothermic preservation and maintain their quality after storage, for later use in clinical assessment, allocation and transplantation.
Outlook
For small biospecimens, current preservation methods usually handle a million or more cells. Yet the preservation outcomes of individual cells can vary significantly, even for the same protocols, owing to intrinsic heterogeneities in the characteristics of the cells, in the properties of their microenvironment, and in physiochemical conditions149,150. For ‘precision preservation’, it is therefore necessary to scale down methods for biospecimen preservation to the level of single cells. This is particularly important for small quantities of precious biospecimens, such as oocytes, zygotes and embryos. Currently, the actual success rates of cryopreservation for these reproductive cells and tissues are dismal (in particular, the birth rate of cryopreserved human oocytes is less than 5%; refs.151,152). Precision preservation would allow the manipulation and monitoring of the cells at single-cell resolution, with enhanced handling accuracy and preservation efficiency.
A large quantity of cell suspensions, cell–biomaterial constructs, tissues and organs are needed for clinical transfusions and transplantations (for example, billions of chimaeric antigen-receptor T cells are required in immunotherapy for the treatment of acute leukaemia153). Therefore, it is imperative to scale-up preservation methods for large-volume biospecimens. For instance, the vitrification of tissues and organs for long-term preservation can benefit from the optimization of CPA mixtures and the incorporation of nanoscale heating154. And supercooling and deep supercooling can be scaled up to preserve a large quantity of cells suspensions, tissues and organs for extended periods by modulating ice-nucleation mechanisms. Because supercooling approaches do not use cytotoxic CPA and do not trigger the formation of ice crystals, they do not need CPA removal and cell recovery, which is clinically advantageous.
In clinical contexts, the safety of preserved biospecimens has to be assured. Insufficient assessments of safety can lead to graft rejection, graft-versus-host diseases, and teratoma formation (for pluripotent stem cells) after transfusion and transplantation155. Freeze–thaw cycles in cryopreservation can cause massive losses in cell viability and in cellular functions, owing to apoptosis and necrosis (which can be alleviated via inhibitors of caspases, proteases and kinases156,157). Moreover, cytotoxic CPAs such as dimethyl sulfoxide can sacrifice cell viabilities and alter the cell cycle and cell genetics (for instance, they can cause chromosomal instabilities and aberrations, eventually leading to cell-function alterations and tumorigenesis158). Therefore, the cytogenetic status of preserved biospecimens has to be determined through karyotyping before use in any clinical applications159. Furthermore, CPAs can cause DNA methylation and histone modifications, and thus induce epigenetic changes to cellular functions (such as uncontrollable cell proliferation, cell differentiation and tumour formation160,161). Because epigenetic changes cannot be detected via karyotyping, the tumourigenic potential of preserved biospecimens should be evaluated by tumourigenic assessments of DNA damage and via the expression levels of tumour suppressor genes and oncogenes10. Although non-toxic CPAs (sugars, proteins, peptides, amino acids, and their derivatives162,163) are available, these are often impermeable to the plasma membrane of mammalian cells, and hence their use has to rely on strategies, such as nanoparticle-mediated intracellular delivery, for CPA delivery into mammalian cells164,165. Furthermore, pathogenic contaminations (for example, via Aspergillus sp. and the Hepatitis B virus in liquid nitrogen94–96) from preservation materials and devices in close contact with the biospecimens should be avoided; in fact, biopreservation systems must be sterilized (by ultraviolet radiation or via filtration166) and operated in sterile hoods.
The development of preservation technologies for biospecimens should be driven by the intended applications, most commonly cell therapies and assisted reproduction13,167 in the case of cells, and transplantation in the case of large tissues and organs. For instance, thorough monitoring of the conditions of preserved organs is crucial for the prevention of early graft failure after transplantation45. Also, because the condition and quality of organs donated after brain death differ from those donated after circulatory death (which experience longer warm ischaemia before preservation), preservation protocols should be tailored accordingly115,122,168. Preservation methods should indeed be adjusted for the specific condition, settings, and application purposes of the biospecimen. Also, the choice of animal models for research should be most representative of human physiology. Naturally, the use of pig livers rather than mouse livers in preservation studies bears higher relevance to the preservation of human livers169. However, the choice of a most appropriate animal model is not always obvious, and is organ-specific170. Furthermore, guidance on good manufacturing practices for the collection, processing, preservation, and assessment of each type of biospecimen is urgently needed to ensure the safety, feasibility, reliability and fidelity of the preservation procedures171,172.
The development and implementation of biopreservation are closely related to socioeconomic factors. On the one hand, the costs associated with the clinical use of preserved biospecimens (for stem cell therapy, oocyte cryopreservation and organ transplantation, in particular) is often prohibitively high, especially in developing countries without robust healthcare systems173. The total discounted costs of liver transplantation per patient in the United States approached US $1.5 million in 2014, and are predicted to increase to over US $2 million in 2034 (ref.174); yet household median income is around 55,000 (figure for 2018; ref.175). Also, 25% of the organ transplantations are carried out in the United States, which has 4% of the world’s population; only 0.5% of the organ transplants were carried out in Africa6, which has 16% of the world’s population. Hence, the wider accessibility and affordability of biopreservation methods for clinical applications remain a significant unmet need. On the other hand, regulatory and governance challenges do also bear significant influence on the clinical application of preserved biospecimens. For instance, oocyte cryopreservation is illegal for unmarried women in China176, and few stem cell therapies have received market approvals177,178. Widespread clinical application of biospecimen preservation won’t thus be possible unless technology development seriously considers affordability, accessibility and regulatory challenges.
Acknowledgements
The authors acknowledge partial funding for this work from the NIH grants P41EB002503 and R01EB023632, the NSF grant CBET-1831019, the NSFC grant (52076157), and from Xian Jiaotong University (Young Talent Support Program).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Fischbach MA, Bluestone JA & Lim WA Cell-based therapeutics: The next pillar of medicine. Science Translational Medicine 5, 179ps177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh VK, Kalsan M, Kumar N, Saini A & Chandra R Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology 3, 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell LD et al. Development of the isber best practices for repositories: Collection, storage, retrieval and distribution of biological materials for research. Biopreservation and Biobanking 10, 232–233 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Jing L, Yao L, Zhao M, Peng L. p. & Liu M Organ preservation: From the past to the future. Acta Pharmacologica Sinica 39, 845–857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massarotti C et al. State of the art on oocyte cryopreservation in female cancer patients: A critical review of the literature. Cancer Treat Rev 57, 50–57 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Giwa S et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 35, 530–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health Resources & Services Administration. Organ donation statistics, <https://www.organdonor.gov/statistics-stories/statistics.html> (2019).
- 8.Evans RW Coming to terms with reality: Why xenotransplantation is a necessity. Xenotransplantation, 29–51 (2000). [Google Scholar]
- 9.Aijaz A et al. Biomanufacturing for clinically advanced cell therapies. Nat Biomed Eng 2, 362–376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong KW et al. Cryopreservation of human mesenchymal stem cells for clinical applications: Current methods and challenges. Biopreserv Biobank 13, 231–239 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Chi H-J et al. Cryopreservation of human embryos using ethylene glycol in controlled slow freezing. Human Reproduction 17, 2146–2151 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Pegg DE Principles of cryopreservation. Methods Mol Biol 368, 39–57 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Fuller B et al. Applications and optimization of cryopreservation technologies to cellular therapeutics. Cell & Gene Therapy Insights 3, 359–378 (2017). [Google Scholar]
- 14.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F & Movaghar B Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet 26, 347–354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glujovsky D et al. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database Syst Rev, CD010047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q, Xie Y, Wang Y & Li S Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-anlaysis. Sci Rep 7, 8538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rall WF & Fahy GM Ice-free cryopreservation of mouse embryos at −196-degrees-c by vitrification. Nature 313, 573–575 (1985). [DOI] [PubMed] [Google Scholar]
- 18.He XM, Park EYH, Fowler A, Yarmush ML & Toner M Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: A study using murine embryonic stem cells. Cryobiology 56, 223–232 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gain P et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmology 134, 167–173 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Guarrera JV et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant 15, 161–169 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Penland L, Gokce O, Croote D & Quake SR High fidelity hypothermic preservation of primary tissues in organ transplant preservative for single cell transcriptome analysis. BMC Genomics 19, 140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutts M, Hinton S, Zheng J & Scharp DW Hypothermic storage and preservation of human pancreatic acinar tissue. In Vitro Cell Dev Biol Anim 43, 2–6 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Elliott GD, Wang S & Fuller BJ Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 76, 74–91 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya MS A review on cryoprotectant and its modern implication in cryonics. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J Pharm 10 (2016). [Google Scholar]
- 25.Akiyama Y, Shinose M, Watanabe H, Yamada S & Kanda Y Cryoprotectant-free cryopreservation of mammalian cells by superflash freezing. Proceedings of the National Academy of Sciences 116, 7738–7743 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazur P Freezing of living cells: Mechanisms and implications. American journal of physiology-cell physiology 247, C125–C142 (1984). [DOI] [PubMed] [Google Scholar]
- 27.He X Thermostability of biological systems: Fundamentals, challenges, and quantification. Open Biomed Eng J 5, 47–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner DK, Lane M, Stevens J & Schoolcraft WB Changing the start temperature and cooling rate in a slow-freezing protocol increases human blastocyst viability. Fertil Steril 79, 407–410 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Jang TH et al. Cryopreservation and its clinical applications. Integr Med Res 6, 12–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahadevan M & Trounson AO Effect of cooling, freezing and thawing rates and storage conditions on preservation of human spermatozoa. Andrologia 16, 52–60 (1984). [DOI] [PubMed] [Google Scholar]
- 31.Dumont F, Marechal P-A & Gervais P Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl. Environ. Microbiol 70, 268–272 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Best BP Cryoprotectant toxicity: Facts, issues, and questions. Rejuvenation Res 18, 422–436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson JO, Szurek EA, Higgins AZ, Lee SR & Eroglu A Optimization of cryoprotectant loading into murine and human oocytes. Cryobiology 68, 18–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson AF, Glasscock C, McClanahan DR, Benson JD & Higgins AZ Toxicity minimized cryoprotectant addition and removal procedures for adherent endothelial cells. PLoS One 10, e0142828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyridieu JF et al. Critical cooling and warming rates to avoid ice crystallization in small pieces of mammalian organs permeated with cryoprotective agents. Cryobiology 33, 436–446 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Seki S & Mazur P The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology 59, 75–82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H et al. Predehydration and ice seeding in the presence of trehalose enable cell cryopreservation. ACS Biomater Sci Eng 3, 1758–1768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southard JH, Senzig KA & Belzer FO Effects of hypothermia on canine kidney mitochondria. Cryobiology 17, 148–153 (1980). [DOI] [PubMed] [Google Scholar]
- 39.Usta OB et al. Supercooling as a viable non-freezing cell preservation method of rat hepatocytes. PLoS One 8, e69334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colletti LM et al. The production of tumor necrosis factor alpha and the development of a pulmonary capillary injury following hepatic ischemia/reperfusion. Transplantation 49, 268–272 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Strüber M et al. Inhaled nitric oxide as a prophylactic treatment against reperfusion injury of the lung. The Thoracic and Cardiovascular Surgeon 47, 179–182 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Hausenloy DJ & Yellon DM Ischaemic conditioning and reperfusion injury. Nature Reviews Cardiology 13, 193–209 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Koyama I, Bulkley GB, Williams GM & Im MJ The role of oxygen free radicals in mediating the reperfusion injury of cold-preserved ischemic kidneys. Transplantation 40, 590–595 (1985). [DOI] [PubMed] [Google Scholar]
- 44.Ferng AS et al. Novel vs clinical organ preservation solutions: Improved cardiac mitochondrial protection. J Cardiothorac Surg 12, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wekerle T, Segev D, Lechler R & Oberbauer R Strategies for long-term preservation of kidney graft function. Lancet 389, 2152–2162 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Tomalty HE et al. Kidney preservation at subzero temperatures using a novel storage solution and insect ice-binding proteins. Cryo Letters 38, 100 (2017). [PubMed] [Google Scholar]
- 47.Casula E et al. Osmotic behaviour of human mesenchymal stem cells: Implications for cryopreservation. PLoS One 12, e0184180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross-Rodriguez LU, Elliott JA & McGann LE Characterization of cryobiological responses in tf-1 cells using interrupted freezing procedures. Cryobiology 60, 106–116 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Casula E, Asuni GP, Sogos V & Cincotti A Hmscs from ucb: Isolation, characterization and determination of osmotic properties for optimal cryopreservation. Icheap12: 12th International Conference on Chemical & Process Engineering 43, 265–270 (2015). [Google Scholar]
- 50.Ebertz SL & McGann LE Osmotic parameters of cells from a bioengineered human corneal equivalent and consequences for cryopreservation. Cryobiology 45, 109–117 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Chen HH, Purtteman JJ, Heimfeld S, Folch A & Gao D Development of a microfluidic device for determination of cell osmotic behavior and membrane transport properties. Cryobiology 55, 200–209 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Niu D, Zhao G, Liu X, Zhou P & Cao Y Prevention of osmotic injury to human umbilical vein endothelial cells for biopreservation: A first step toward biobanking of endothelial cells for vascular tissue engineering. Tissue Eng Part C Methods 22, 270–279 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Chen H. h. et al. A microfluidic study of mouse dendritic cell membrane transport properties of water and cryoprotectants. International Journal of Heat and Mass Transfer 51, 5687–5694 (2008). [Google Scholar]
- 54.Tseng HY et al. A microfluidic study of megakaryocytes membrane transport properties to water and dimethyl sulfoxide at suprazero and subzero temperatures. Biopreserv Biobank 9, 355–362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W et al. High-precision approach based on microfluidic perfusion chamber for quantitative analysis of biophysical properties of cell membrane. International Journal of Heat and Mass Transfer 86, 869–879 (2015). [Google Scholar]
- 56.Lyu S-R, Chen W-J & Hsieh W-H Measuring transport properties of cell membranes by a pdms microfluidic device with controllability over changing rate of extracellular solution. Sensors and Actuators B: Chemical 197, 28–34 (2014). [Google Scholar]
- 57.Berthier E et al. Kit-on-a-lid-assays for accessible self-contained cell assays. Lab Chip 13, 424–431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Lv X, Guo H, Shi X & Liu J On-chip direct freezing and thawing of mammalian cells. RSC Advances 4, 34443–34447 (2014). [Google Scholar]
- 59.Deutsch M et al. The individual-cell-based cryo-chip for the cryopreservation, manipulation and observation of spatially identifiable cells. I: Methodology. BMC Cell Biology 11, 54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afrimzon E et al. The individual-cell-based cryo-chip for the cryopreservation, manipulation and observation of spatially identifiable cells. Ii: Functional activity of cryopreserved cells. BMC cell biology 11, 83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roach KL et al. High-throughput single cell arrays as a novel tool in biopreservation. Cryobiology 58, 315–321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou Y, Yin T, Chen S, Yang J & Huang W On-chip cryopreservation: A novel method for ultra-rapid cryoprotectant-free cryopreservation of small amounts of human spermatozoa. PLoS One 8, e61593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S, Liu W & Lin L On-chip cryopreservation of living cells. JALA: Journal of the Association for Laboratory Automation 15, 99–106 (2010). [Google Scholar]
- 64.Bissoyi A, Bit A, Singh BK, Singh AK & Patra PK Enhanced cryopreservation of mscs in microfluidic bioreactor by regulated shear flow. Sci Rep 6, 35416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang G, Zhang A, Xu LX & He X Modeling the cell-type dependence of diffusion-limited intracellular ice nucleation and growth during both vitrification and slow freezing. Journal of Applied Physics 105, 114701 (2009). [Google Scholar]
- 66.Karlsson J, Cravalho E & Toner M A model of diffusion-limited ice growth inside biological cells during freezing. Journal of Applied Physics 75, 4442–4455 (1994). [Google Scholar]
- 67.Kawata T et al. Water molecule movement by a magnetic field in freezing for tooth banking. Biomedical Research 21, 351–354 (2010). [Google Scholar]
- 68.Kaku M et al. Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology 61, 73–78 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Abedini S et al. Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology 62, 181–187 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Lee SY et al. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs 196, 23–33 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Conde MCM et al. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Brazilian dental journal 27, 633–640 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Otero L, Rodríguez AC, Pérez-Mateos M & Sanz PD Effects of magnetic fields on freezing: Application to biological products. Comprehensive Reviews in Food Science and Food Safety 15, 646–667 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Meng L et al. Development of a microfluidic device for automated vitrification human embryo. Fertil Steril 96, S207 (2011). [Google Scholar]
- 74.Heo YS et al. Controlled loading of cryoprotectants (cpas) to oocyte with linear and complex cpa profiles on a microfluidic platform. Lab Chip 11, 3530–3537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song YS et al. Microfluidics for cryopreservation. Lab Chip 9, 1874–1881 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai D, Ding J, Smith GW, Smith GD & Takayama S Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification. Hum Reprod 30, 37–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swain JE, Lai D, Takayama S & Smith GD Thinking big by thinking small: Application of microfluidic technology to improve art. Lab Chip 13, 1213–1224 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Zhao G & Fu J Microfluidics for cryopreservation. Biotechnology advances 35, 323–336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W, Yang G, Zhang A, Xu LX & He X Preferential vitrification of water in small alginate microcapsules significantly augments cell cryopreservation by vitrification. Biomed Microdevices 12, 89–96 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Huang H et al. Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-cpa cell vitrification. Adv Funct Mater 25, 6939–6850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao G, Liu X, Zhu K & He X Hydrogel encapsulation facilitates rapid-cooling cryopreservation of stem cell-laden core-shell microcapsules as cell-biomaterial constructs. Adv Healthc Mater 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W, Shu Z, Gao D & Shen AQ Sensing and sensibility: Single-islet-based quality control assay of cryopreserved pancreatic islets with functionalized hydrogel microcapsules. Adv Healthc Mater 5, 223–231 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Cagol N, Bonani W, Maniglio D, Migliaresi C & Motta A Effect of cryopreservation on cell-laden hydrogels: Comparison of different cryoprotectants. Tissue Eng Part C Methods 24, 20–31 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Lan D et al. Using a novel supramolecular gel cryopreservation system in microchannel to minimize the cell injury. Langmuir 34, 5088–5096 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Zhang C et al. Hydrogel cryopreservation system: An effective method for cell storage. Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang H & He X Microscale materials and devices for cell cryopreservation by vitrification. (2016).
- 87.Demirci U & Montesano G Cell encapsulating droplet vitrification. Lab Chip 7, 1428–1433 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Kim B-H et al. Effect of droplet vitrification on mitochondrial membrane potential and developmental competence in two-cell mouse embryos. Animal Cells and Systems 15, 287–294 (2011). [Google Scholar]
- 89.An L et al. Efficient cryopreservation of mouse embryos by modified droplet vitrification (mdv). Cryobiology 71, 70–76 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Zhang X et al. Nanoliter droplet vitrification for oocyte cryopreservation. Nanomedicine 7, 553–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samot J et al. Blood banking in living droplets. PLoS One 6, e17530 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song YS et al. Vitrification and levitation of a liquid droplet on liquid nitrogen. Proceedings of the National Academy of Sciences 107, 4596–4600 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Vries RJ et al. Bulk droplet vitrification: An approach to improve large-scale hepatocyte cryopreservation outcome. Langmuir 35, 7354–7363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tedder RS et al. Hepatitis b transmission from contaminated cryopreservation tank. Lancet 346, 137–140 (1995). [DOI] [PubMed] [Google Scholar]
- 95.Grout BWW & Morris G Contaminated liquid nitrogen vapour as a risk factor in pathogen transfer. Theriogenology 71, 1079–1082 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Kuleshova L & Shaw JM A strategy for rapid cooling of mouse embryos within a double straw to eliminate the risk of contamination during storage in liquid nitrogen. Human Reproduction 15, 2604–2609 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Shi M et al. High-throughput non-contact vitrification of cell-laden droplets based on cell printing. Sci Rep 5, 17928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marković Z, Chatelet P, Sylvestre I, Kontić J & Engelmann F Cryopreservation of grapevine (vitis vinifera l.) in vitro shoot tips. Open Life Sciences 8, 993–1000 (2013). [Google Scholar]
- 99.Bi W-L, Hao X-Y, Cui Z-H, Volk GM & Wang Q-C Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (vitis spp.). In Vitro Cellular & Developmental Biology-Plant 54, 590–599 (2018). [Google Scholar]
- 100.Souza FVD et al. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell, Tissue and Organ Culture (PCTOC) 124, 351–360 (2016). [Google Scholar]
- 101.Jin B, Kleinhans FW & Mazur P Survivals of mouse oocytes approach 100% after vitrification in 3-fold diluted media and ultra-rapid warming by an ir laser pulse. Cryobiology 68, 419–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khosla K, Wang Y, Hagedorn M, Qin Z & Bischof J Gold nanorod induced warming of embryos from the cryogenic state enhances viability. ACS Nano 11, 7869–7878 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Robinson MP, Wusteman MC, Wang L & Pegg DE Electromagnetic re-warming of cryopreserved tissues: Effect of choice of cryoprotectant and sample shape on uniformity of heating. Phys Med Biol 47, 2311–2325 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Manuchehrabadi N et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Science Translational Medicine 9, eaah4586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J, Zhao G, Zhang Z, Xu X & He X Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hucm-mscs cryopreserved by vitrification. Acta Biomater 33, 264–274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eskandari N, Marquez-Curtis LA, McGann LE & Elliott JAW Cryopreservation of human umbilical vein and porcine corneal endothelial cell monolayers. Cryobiology 85, 63–72 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Jomha NM et al. Vitrification of intact human articular cartilage. Biomaterials 33, 6061–6068 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Vogel T, Brockmann JG, Coussios C & Friend PJ The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplantation reviews 26, 156–162 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Ravikumar R, Leuvenink H & Friend PJ Normothermic liver preservation: A new paradigm? Transpl Int 28, 690–699 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Messer S, Ardehali A & Tsui S Normothermic donor heart perfusion: Current clinical experience and the future. Transpl Int 28, 634–642 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Cypel M et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 364, 1431–1440 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Van Raemdonck D, Neyrinck A, Cypel M & Keshavjee S Ex-vivo lung perfusion. Transpl Int 28, 643–656 (2015). [DOI] [PubMed] [Google Scholar]
- 113.Cypel M et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. American Journal of Transplantation 9, 2262–2269 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Jing L, Yao L, Zhao M, Peng LP & Liu M Organ preservation: From the past to the future. Acta Pharmacol Sin 39, 845–857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nasralla D et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018). [DOI] [PubMed] [Google Scholar]
- 116.St Peter SD, Imber CJ, Lopez I, Hughes D & Friend PJ Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg 89, 609–616 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Xu H et al. Excorporeal normothermic machine perfusion resuscitates pig dcd livers with extended warm ischemia. J Surg Res 173, e83–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eshmuminov D et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol 38, 189–198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berendsen TA et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res 1, 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tolboom H et al. Subnormothermic machine perfusion at both 20 degrees c and 30 degrees c recovers ischemic rat livers for successful transplantation. Journal of Surgical Research 175, 149–156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruinsma BG et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant 14, 1400–1409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruinsma BG et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci Rep 6, 22415 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berendsen TA et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat Med 20, 790–793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guarrera JV et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant 10, 372–381 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Moers C et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360, 7–19 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Moers C, Pirenne J, Paul A, Ploeg RJ & Machine Preservation Trial Study, G. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 366, 770–771 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Hoyer DP et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: First clinical application of a new concept. Transplantation 100, 147–152 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Hoyer DP, Paul A & Minor T Prediction of hepatocellular preservation injury immediately before human liver transplantation by controlled oxygenated rewarming. Transplantation direct 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schopp I, Reissberg E, Luer B, Efferz P & Minor T Controlled rewarming after hypothermia: Adding a new principle to renal preservation. Clin Transl Sci 8, 475–478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bruinsma BG et al. Supercooling preservation and transplantation of the rat liver. Nat Protoc 10, 484–494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson WR, Peters RH & Zimmermann J The effects of body size and temperature on metabolic rate of organisms. Canadian Journal of Zoology 61, 281–288 (1983). [Google Scholar]
- 132.Belzer FO & Southard JH Principles of solid-organ preservation by cold storage. Transplantation 45, 673–676 (1988). [DOI] [PubMed] [Google Scholar]
- 133.Weeder PD, van Rijn R & Porte RJ Machine perfusion in liver transplantation as a tool to prevent non-anastomotic biliary strictures: Rationale, current evidence and future directions. Journal of Hepatology 63, 265–275 (2015). [DOI] [PubMed] [Google Scholar]
- 134.de Perrot M et al. Report of the ishlt working group on primary lung graft dysfunction part iii: Donor-related risk factors and markers. J Heart Lung Transplant 24, 1460–1467 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Snyder KK, Baust JM, Van Buskirk RG & Baust JG Enhanced hypothermic storage of neonatal cardiomyocytes. Cell Preservation Technology 3, 61–74 (2005). [Google Scholar]
- 136.Pogozhykh D, Prokopyuk V, Pogozhykh O, Mueller T & Prokopyuk O Influence of factors of cryopreservation and hypothermic storage on survival and functional parameters of multipotent stromal cells of placental origin. PLoS One 10, e0139834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Correia C et al. Effective hypothermic storage of human pluripotent stem cell-derived cardiomyocytes compatible with global distribution of cells for clinical applications and toxicology testing. Stem Cells Transl Med 5, 658–669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Groger M et al. Preservation of cell structure, metabolism, and biotransformation activity of liver-on-chip organ models by hypothermic storage. Adv Healthc Mater 7 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Xu Y, Mawatari K, Konno T, Kitamori T & Ishihara K Spontaneous packaging and hypothermic storage of mammalian cells with a cell-membrane-mimetic polymer hydrogel in a microchip. ACS Appl Mater Interfaces 7, 23089–23097 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Southard James H., D. a. M & Belzer Folkert O., D. M Organ preservation. 46, 235–247 (1995). [DOI] [PubMed] [Google Scholar]
- 141.McAnulty JF Hypothermic organ preservation by static storage methods: Current status and a view to the future. Cryobiology 60, S13–19 (2010). [DOI] [PubMed] [Google Scholar]
- 142.Puts CF et al. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology 71, 125–129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Vries RJ et al. Supercooling extends preservation time of human livers. Nat Biotechnol 37, 1131–1136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang H, Yarmush ML & Usta OB Long-term deep-supercooling of large-volume water and red cell suspensions via surface sealing with immiscible liquids. Nat Commun 9, 3201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cox SJ, Kathmann SM, Slater B & Michaelides A Molecular simulations of heterogeneous ice nucleation. I. Controlling ice nucleation through surface hydrophilicity. J Chem Phys 142, 184704 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Shaw RA, Durant AJ & Mi Y Heterogeneous surface crystallization observed in undercooled water. J Phys Chem B 109, 9865–9868 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Tabazadeh A, Djikaev YS & Reiss H Surface crystallization of supercooled water in clouds. Proceedings of the National Academy of Sciences 99, 15873–15878 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang H, Rey-Bedon C, Yarmush ML & Usta OB Deep-supercooling for extended preservation of adipose-derived stem cells. Cryobiology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Charlton SJ et al. Strong heterogeneity in advances in cryopreservation techniques in the mammalian orders. Zoological science 35, 1–23 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Hunt CJ Technical considerations in the freezing, low-temperature storage and thawing of stem cells for cellular therapies. Transfus Med Hemother 46, 134–150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meyer H Women ‘are being given false hope’ over freezing eggs, <https://www.theguardian.com/society/2015/oct/24/women-false-hope-freezing-eggs> (2015).
- 152.Faulkner K, Bentley P & Smyth S Ivf clinics peddling false hope over egg freezing: Doctors caught on camera making wildly optimistic claims about the method’s success, <https://www.dailymail.co.uk/news/article-4467352/Desperate-women-duped-freezing-eggs.html> (2017).
- 153.Wang X & Riviere I Clinical manufacturing of car t cells: Foundation of a promising therapy. Mol Ther Oncolytics 3, 16015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu D & Pan F Advances in cryopreservation of organs. Journal of Huazhong University of Science and Technology [Medical Sciences] 36, 153–161 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Sharpe ME, Morton D & Rossi A Nonclinical safety strategies for stem cell therapies. Toxicol Appl Pharmacol 262, 223–231 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Bissoyi A, Nayak B, Pramanik K & Sarangi SK Targeting cryopreservation-induced cell death: A review. Biopreserv Biobank 12, 23–34 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Yagi T et al. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology 33, 1432–1440 (2001). [DOI] [PubMed] [Google Scholar]
- 158.Laurent LC et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human escs and ipscs during reprogramming and time in culture. Cell Stem Cell 8, 106–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dittmar KE et al. Quality of cell products: Authenticity, identity, genomic stability and status of differentiation. Transfusion Medicine and Hemotherapy 37, 57–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iwatani M et al. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 24, 2549–2556 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Chatterjee A et al. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 74, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Browne J, Tunnacliffe A & Burnell A Anhydrobiosis - plant desiccation gene found in a nematode. Nature 416, 38–38 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Yang J et al. Exploring the potential of biocompatible osmoprotectants as highly efficient cryoprotectants. Acs Applied Materials & Interfaces 9, 42516–42524 (2017). [DOI] [PubMed] [Google Scholar]
- 164.Stewart S & He XM Intracellular delivery of trehalose for cell banking. Langmuir 35, 7414–7422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang YT et al. Cold-responsive nanoparticle enables intracellular delivery and rapid release of trehalose for organic-solvent-free cryopreservation. Nano Letters 19, 9051–9061 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Parmegiani L, Cognigni GE & Filicori M Ultra-violet sterilization of liquid nitrogen prior to vitrification. Hum Reprod 24, 2969 (2009). [DOI] [PubMed] [Google Scholar]
- 167.Massarotti C, Scaruffi P, Lambertini M, Remorgida V & Anserini P State of the art on oocyte cryopreservation in female cancer patients: A critical review of the literature. Cancer Treatment Reviews 57 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Eren EA et al. Donations after circulatory death in liver transplant. Exp Clin Transplant 14, 463–470 (2016). [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang ZB, Gao W, Liu L, Shi Y & Shen ZY Development and assessment of normothermic machine perfusion preservation for extracorporeal splitting of pig liver. Ann Transplant 22, 507–517 (2017). [DOI] [PubMed] [Google Scholar]
- 170.Ratner BD, Hoffman AS, Schoen FJ & Lemons JE Biomaterials science: An introduction to materials in medicine, (Elsevier, 2004). [Google Scholar]
- 171.Juliano L, Eastwood G, Berard T & Mathew AJ The importance of collection, processing and biopreservation best practices in determining car-t starting material quality. Cell Gene Ther Insights 4, 327–336 (2018). [Google Scholar]
- 172.Hawkins BJ, Abazari A & Mathew AJ Biopreservation best practices for regenerative medicine gmp manufacturing & focus on optimized biopreservation media. Cell Gene Ther Insights 3, 345–358 (2017). [Google Scholar]
- 173.Hong Y et al. A survey on the awareness and knowledge about elective oocyte cryopreservation among unmarried women of reproductive age visiting a private fertility center. Obstetrics & Gynecology Science 62, 438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Habka D, Mann D, Landes R & Soto-Gutierrez A Future economics of liver transplantation: A 20-year cost modeling forecast and the prospect of bioengineering autologous liver grafts. PLoS One 10, e0131764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Household income in the united states, <https://statisticalatlas.com/United-States/Household-Income> (2018).
- 176.Wang L, Huang X & Liu X On the ethiical and legal problems of single women’ “frozen eggs”. Medicine & Jurisprudence 7, 33 (2015). [Google Scholar]
- 177.Poway C Personalized stem cells, inc. Announces fda approval of ind application for treatment of osteoarthritis with stem cells, <https://personalizedstemcells.com/wp-content/uploads/2019/08/Personalized-Stem-Cells-Inc.-Announces-FDA-Approval-of-IND-Application-for-Treatment-of-Osteoarthritis-with-Stem-Cells-July-22-2019.pdf> (2019).
- 178.Pellegrini G et al. Navigating market authorization: The path holoclar took to become the first stem cell product approved in the european union. Stem Cells Translational Medicine 7, 146–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Risco R, Elmoazzen H, Doughty M, He X & Toner M Thermal performance of quartz capillaries for vitrification. Cryobiology 55, 222–229 (2007). [DOI] [PubMed] [Google Scholar]


