Abstract
Panax notoginseng (Burk.) F. H. Chen is a Chinese medicinal plant of the Araliaceae family used for the treatment of cardiovascular and cerebrovascular diseases in Asia. P. notoginseng is vulnerable to root rot disease, which reduces the yield of P. notoginseng. In this study, we analyzed the rhizosphere soil and root endophyte microbial communities of P. notoginseng from different geographical locations using high-throughput sequencing. Our results revealed that the P. notoginseng rhizosphere soil microbial community was more diverse than the root endophyte community. Rhodopseudomonas, Actinoplanes, Burkholderia, and Variovorax paradoxus can help P. notoginseng resist the invasion of root rot disease. Ilyonectria mors-panacis, Pseudomonas fluorescens, and Pseudopyrenochaeta lycopersici are pathogenic bacteria of P. notoginseng. The upregulation of amino acid transport and metabolism in the soil would help to resist pathogens and improve the resistance of P. notoginseng. The ABC transporter and gene modulating resistance genes can improve the disease resistance of P. notoginseng, and the increase in the number of GTs (glycosyltransferases) and GHs (glycoside hydrolases) families may be a molecular manifestation of P. notoginseng root rot. In addition, the complete genomes of two Flavobacteriaceae species and one Bacteroides species were obtained. This study demonstrated the microbial and functional diversity in the rhizosphere and root microbial community of P. notoginseng and provided useful information for a better understanding of the microbial community in P. notoginseng root rot. Our results provide insights into the molecular mechanism underlying P. notoginseng root rot and other plant rhizosphere microbial communities.
Keywords: Panax notoginseng, root rot, rhizosphere soil, root endophytes, metagenomic sequencing, microbial community
Introduction
Panax notoginseng (Burk.) F. H. Chen, belonging to the Araliaceae family, is a Chinese medicinal plant that is mainly grown in Yunnan and Guangxi Provinces of China and is commonly used for the treatment of coronary heart and cardiovascular diseases (Hong et al., 2010; Fan et al., 2016). P. notoginseng is often used in a large variety of traditional Chinese medicine. P notoginseng has been widely used in China and other Asian countries as a therapeutic strategy or health care product, such as hemostatic, anti-thrombotic, and anti-atherosclerotic agent, as well as used to reduce blood pressure and relieve pain, and presents neuroprotection effects (Yang et al., 2019; Guo et al., 2020). Same as the root of the medicinal part of ginseng, the root of P. notoginseng has been used as a raw medicinal material in more than 400 products in 1,300 companies in China (Miao et al., 2006). The World Health Organization has reported that approximately one-third of all deaths worldwide are attributed to cardiovascular diseases (Santhakumar et al., 2018). As the incidence of cardiovascular diseases has increased, and with the modernization of Chinese medicine, the demand for root material of P. notoginseng has been increasing. However, P. notoginseng is vulnerable to various plant diseases, of which root rot is the most serious, limiting P. notoginseng production.
Approximately 70% of medicinal plants have different degrees of pathogen infection related to continuous cropping obstacles (Wu et al., 2013; Zhao Y. P. et al., 2016). The death rates of P. notoginseng seedlings were 2.0–39.4 and 2.6–81.2% in continuous cropping and replanting systems, respectively (Dong et al., 2016). Root rot can reduce P. notoginseng yield by 70% during the entire growing period (Dong et al., 1988). Previous studies showed that Cylindrocarpon destructans var. destructans causes root rot in P. notoginseng (Mao et al., 2014); Fusarium oxysporum and Fusarium solani are associated with root rot disease in P. notoginseng (Ma et al., 2019). Using the internal transcribed spacer (ITS) sequencing technology, the dominant microorganisms in root rot of P. notoginseng was identified as Ilyonectria mors-panacis (Mi et al., 2017), and found several potential pathogenic fungi in the rhizosphere soil of 2-year-old healthy P. notoginseng plants (Miao et al., 2016). Owing to the influence of various plant diseases, continuous cropping obstacles, and the Daodi (the distinctively higher quality of the medicinal materials that grow in a certain area) of P. notoginseng (Liu et al., 2020), the available planting area of P. notoginseng is decreasing. Therefore, to increase the production of P. notoginseng, it is necessary to identify the key pathogens associated with root rot in this species to provide support for research on corresponding prevention methods.
The microbial community plays important roles in nutrient absorption and resistance to environmental pressures, and the rhizosphere microbial community is also essential for plant health and disease suppression in rhizosphere soil. Previous studies have shown that the rhizosphere soil microbial diversity of healthy plants is greater than diseased plants (Brussaard et al., 2007; Bakker et al., 2010). Recently, it has been shown that plant stress responses are linked to the composition of beneficial plant microbial communities (Bakker et al., 2018). The relationship between soybeans and rhizosphere microbial communities shows that resistant soybeans are rich in genes encoding phenazines, phospholipids, and other antifungal-related genes. Furthermore, more rhizosphere bacteria resistant to the Fusarium genotype can be synthesized (Mendes et al., 2018). The microbial communities in the rhizosphere soil and roots of healthy and diseased P. notoginseng have been analyzed through bacterial 16S rRNA and fungal 18S rRNA genes. In the rhizosphere soil of diseased P. notoginseng, microbial communities showed a decrease in alpha diversity, the bacterial community dissimilarity increased, and fungal community dissimilarity decreased. However, the bacterial and fungal community dissimilarity in the roots were not markedly different between healthy and diseased P. notoginseng (Wu et al., 2015). It has also been shown that following root rot infection of P. notoginseng, the content of phenolic acid in the soil is lower in diseased P. notoginseng, and increase the phenolic acid content can effectively prevent the root rot of P. notoginseng (Zhang et al., 2018). Phenolic acid can inhibit the growth of pathogenic bacteria, while also stimulating the production of ferric acid; therefore, adjusting the content of phenolic acid can effectively prevent root rot disease occurrence in P. notoginseng (Zhao et al., 2018).
Metagenomics refers to the total DNA of the microbial community investigated at one point, allowing the identification of all microorganisms in an environment, especially those that are difficult to cultivate in a laboratory (Chen and Pachter, 2005). Metagenomics technology is an effective approach to find unculturable microorganisms and overcome the limitation of amplicons (Wang P. P. et al., 2020). Several studies have applied metagenomics technology to help solve disease-related problems in plants, such as potato (Goss et al., 2014), peas (Gaulin et al., 2007; Taheri et al., 2017), citrus (Duan et al., 2009), and tomatoes (Kwak et al., 2018). Previous studies have investigated root rot in P. notoginseng using the amplicon technologies, such as 16S/18S rRNA sequencing and ITS, and mostly focused on fungi. Even though other species such as Panax ginseng and Panax quinquefolius have been studied (Xing et al., 2010; Vendan et al., 2012; Ji et al., 2018; Deng et al., 2020; Wang Q. et al., 2020), reports on the endophytes and rhizosphere microorganisms of P. notoginseng remain limited and no studies have investigated the microbial community of root rot P. notoginseng using metagenomics.
In this study, the high-throughput sequencing technology was used to sequence the rhizosphere soil and root endophytes of healthy and root rot diseased P. notoginseng. We aimed to reveal the structure and functional difference of microbial community through metagenomic analysis and report the pathogenic and antagonistic bacteria of root rot diseased P. notoginseng. Investigating the correlation between the root rot and microbial community will provide a theoretical basis for the prevention and treatment of root rot in P. notoginseng. Consequently, the findings of this study will ensure the sustainable development of P. notoginseng.
Materials and Methods
Sample Collection and DNA Isolation
In this study, Lijiang City (LJ) (26°49′59″ N, 100°3′24″ E, altitude: 2,640 m) and Qiubei County (QB) (24°2′49″ N, 103°58′40″ E, altitude: 2,020 m) in Yunnan Province were selected as sampling points of P. notoginseng. We set three biological replicates in each sampling point. In each biological replicate, one diseased (the aboveground parts were wilted and yellowed, and the roots were necrotic) and one healthy plant (no signs of root rot) were selected (Figure 1A). In each plant, the root endophyte and corresponding rhizosphere soil were collected. A total of 24 samples were collected for further analysis.
FIGURE 1.
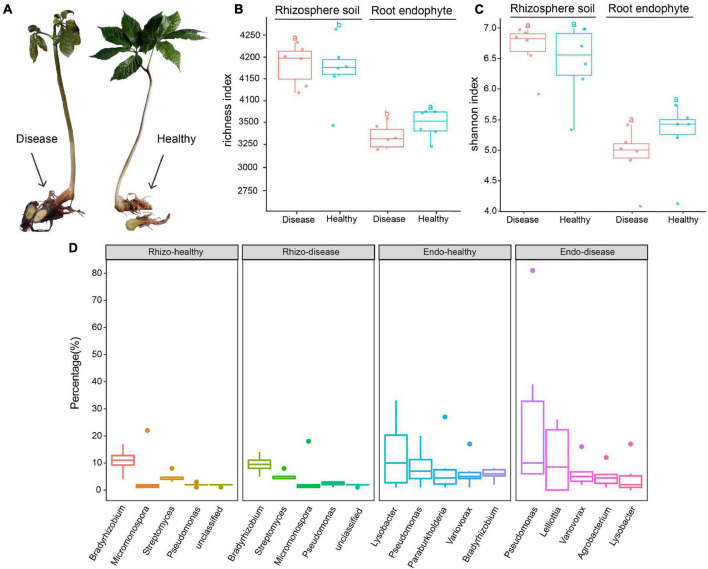
Characteristics of healthy and root rot diseased Panax notoginseng plants. (A) Diseased and healthy P. notoginseng plant parts. (B) Observed OTUs box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (C) Shannon index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (D) Boxplots of the top five species in each of the rhizosphere soil and root endophyte groups. Tukey’s HSD test is used to compare the differences between two groups and add the difference group letters.
When collecting the root endophyte of P. notoginseng, the plant was gently lifted from the soil and shaken to remove the dirt and other impurities attached to the roots. Thereafter, the roots were carefully rinsed with sterile water until the root surfaces were free of impurities. A prepared sterile scalpel was used to cut off the cleaned roots (∼100 g) into 50 mL sterile centrifuge tubes and were immediately placed into liquid nitrogen for preservation. Then, the corresponding rhizosphere soil of the plant was collected. A standard soil ring knife was used to collect the rhizosphere soil (∼20 cm under the ground) from each plant and placed into 50 mL sterile centrifuge tubes that were stored in liquid nitrogen for preservation.
DNA isolation performed using the modified CTAB method (Doyle and Doyle, 1987). Briefly, we piped 1,000 μL CTAB lysate into a 2.0 mL EP tube; added lysozyme and 3 g sample to the lysate; placed the EP tube into a 65°C water bath; inverted and mixed several times to fully mix the sample. Then, we centrifuged to collect the supernatant, added phenol: chloroform: isoamyl alcohol (25:24:1), mixed, and centrifuged at 12,000 rpm for 10 min. Subsequently, we removed the supernatant, added chloroform: isoamyl alcohol (24:1), inverted and mixed, and centrifuged again at 12,000 rpm for 10 min. We placed the supernatant into a 1.5 mL centrifuge tube, added isopropanol, mixed, and left to precipitate at −20°C. Then, we centrifuged at 12,000 rpm for 10 min, removed the liquid and washed the precipitate with 1 mL 75% ethanol. After washing twice, the remaining liquid could be collected after centrifugation and removed with a pipette. We placed the final sample in an ultra-clean workbench to dry at 25°C. We added ddH2O to dissolve the DNA sample, 1 μL RNase A was added to digest the RNA, and placed the sample at 37°C for 15 min. The collected DNA sample was used for sequencing.
Metagenomic Sequencing and Quality Control
A Qubit 2.0 fluorometer (Thermo Fisher Scientific Inc., Waltham, MA, United States) was used to accurately quantify the DNA concentration. After the DNA samples were quantified, they were randomly interrupted using the Covaris Focused-ultrasonicator (Covaris, Inc., Woburn, MA, United States), and then the library was prepared following the steps of end repair, A-tailing, sequencing adapters, purification, and PCR amplification followed by Illumina library construction protocol (Illumina Inc., San Diego, CA, United States). Initially, library was constructed, and the inserted fragments of the library were detected using the Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA, United States). After the size of the inserted fragments were standardized (500 bp), the effective concentration of the library was accurately quantified using the Q-PCR method to ensure the quality of the library. Secondly, libraries were pooled to flow cells according to the effective concentration and target data volume requirements. At last, the cBOT was clustered, a NovaSeq 6000 high-throughput sequencing platform (Illumina, Inc., San Diego, CA, United States) was used for sequencing. Each root endophyte sample had approximately 20–30 Gb of sequencing data, and each rhizosphere soil sample had approximately 10–30 Gb of sequencing data.
The FastQC software was used to estimate the quality of the original sequences of 24 samples to generate a quality evaluation report (Andrews, 2010). The Kneaddata tool1 was used to perform quality control and de-hosting the P. notoginseng genome sequence. The Kneaddata process relies on Trimmomatic to remove adaptors (Bolger et al., 2014), linkers, and low-quality sequences (parameters: sequence quality ≥ 20, minimum sequence length ≥ 50 bp). Bowtie2 (Langmead and Salzberg, 2012) was used to mapping the reads to the genome of P. notoginseng (Zhang et al., 2017), and reserved the unmapped reads as finally clean sequences (Table 1). Cleaned reads were used for downstream analyses.
TABLE 1.
Summary table of samples and sequencing data.
| Sample type | Plant status | Samples number | Raw reads (Gb) | Clean reads (Gb) |
| Root endophyte | Disease | LJ-1 | 25.7 | 10.9 |
| LJ-2 | 32.1 | 24.9 | ||
| LJ-3 | 25.5 | 4.16 | ||
| QB-1 | 33.3 | 17.4 | ||
| QB-2 | 30.4 | 22.8 | ||
| QB-3 | 31.8 | 28.1 | ||
| Healthy | LJ-1 | 12.8 | 1.7 | |
| LJ-2 | 27.9 | 4.0 | ||
| LJ-3 | 28.6 | 6.5 | ||
| QB-1 | 28.5 | 8.6 | ||
| QB-2 | 27.0 | 13.6 | ||
| QB-3 | 30.3 | 9.2 | ||
| Rhizosphere soil | Disease | LJ-1 | 13.5 | 12.8 |
| LJ-2 | 13.2 | 12.8 | ||
| LJ-3 | 13.0 | 12.5 | ||
| QB-1 | 13.0 | 12.4 | ||
| QB-2 | 12.7 | 12.3 | ||
| QB-3 | 13.1 | 12.6 | ||
| Healthy | LJ-1 | 33.9 | 32.5 | |
| LJ-2 | 10.5 | 10.1 | ||
| LJ-3 | 13.0 | 12.5 | ||
| QB-1 | 12.2 | 11.7 | ||
| QB-2 | 12.8 | 12.3 | ||
| QB-3 | 12.9 | 12.3 |
Raw reads are the data volume of paired-end sequencing, and clean reads are sequences that are still paired after quality control and de-hosting.
Analysis of the Microbial Diversity of Root Rot Panax notoginseng
The HUMAnN2 software was utilized to calculate the species and functional composition of the samples (Franzosa et al., 2018). HUMAnN2 does not consider paired-end information in the analysis, therefore the paired-end sequence after quality control is combined as one input file, while the other parameters are the default parameters. MetaPhlAn2 was used to calculate the species composition (Truong et al., 2015). We mapped the clean reads to the HUMAnN2 nucleic acid database using Bowtie2 software (Langmead and Salzberg, 2012), and generated species abundance composition table for each group. We used GraPhlAn to analyze the top 100 representative species in the species composition table at the phylum level (Asnicar et al., 2015). To display the species circle map, R software version 3.4.3 was used to display the heat map of the species at the genus level (R Core Team, 2015, 2020), and to compare the differences in species composition between groups. Finally, LEfSe was used for the species difference analysis (Segata et al., 2011). The LEfSe used the Kruskal–Wallis and Wilcoxon signed-rank tests, signed linear judgment analysis (LDA) logarithmic score, and related P-values to identify species with significant differences in each group (LDA > 4 was considered as significantly different species) (Zuo et al., 2021).
Kraken2 is a high-precision metagenomic sequence classification software based on the k-mer algorithm (Wood et al., 2019). Kraken2 is used for species annotation at the read level, which can rapidly classify sequencing reads for species classification. We compared our data to the Kraken2 standard library, fungi, protozoa, and plasmid databases. The bacteria, archaea, fungi, protozoa, plasmids, and virus libraries were provided by the RefSeq database2. The species composition table was used for species diversity analysis, the species level annotations were extracted and combined to the minimum sequencing amount, and alpha diversity was calculated using the R. In addition, alpha diversity boxplots and the top 30 genera of each group were used to compare the differences.
The EukDetect method (Lind and Pollard, 2021) was used to detect eukaryotic microorganisms in the clean data. The EukDetect pipeline uses a Snakemake workflow engine (Kster and Rahmann, 2018). Microbial eukaryotic genomes were downloaded from NCBI GenBank for all species designated as fungi, protists, other non-vertebrate metazoans, and non-streptophyta archaeplastida species. In addition to the GenBank genomes, 314 genomes and transcriptomes comprising 282 protists, 30 Archaeplastida species, and 2 metazoans curated by the EukProt project were downloaded (Richter et al., 2020).
Assembly and Binning Analysis of Metagenomic Data
MEGAHIT (Li et al., 2015) was used for each sample assembly. The contigs of each sample were mixed by group, and the quality of contigs was assessed by QUAST (Alexey et al., 2013). MetaProdigal (Hyatt et al., 2012) was used for gene prediction, and cd-hit-est (Fu et al., 2012) to cluster and de-redundancy the predicted genes to construct a non-redundant gene set (similarity ≥ 95%, coverage ≥ 90%). The non-redundant gene set obtained was used for subsequent analysis. The nucleic acid sequences were quantified using Salmon (Patro et al., 2017). The parameter was set to the metagenomic model (–meta) for all reads that could be binned, and the abundance of genes in each sample was obtained.
We used MetaWRAP (Uritskiy et al., 2018) to excavate the draft genome of a single strain. This process integrates three popular binning software. We selected the MaxBin2 (Wu et al., 2016) and MetaBAT2 (Kang et al., 2019) software for binning. The de-redundant contigs of each group were binned, and the bins were purified. We evaluated and comprehensively analyzed the results to obtain better results. The Checkm database (Parks et al., 2015) requires completeness of more than 70% and a pollution rate of less than 5%, which was used for purification. After purification, the Blobology module was used to visualize the GC content and abundance of contigs by comparing the NCBI nt and taxonomy database was obtained. Samlon (Patro et al., 2017) was used to quantify the bins and calculate the abundance of a single bacterial genome in each sample. Subsequently, each contig in the bin was annotated, and the species of each bin was estimated. Krona was used to visualize the results of bin annotation (Ondov et al., 2011). Finally, Bins were classified using a concatenated set of 122 universal marker proteins by GTDB-Tk to find new species (Chaumeil et al., 2019).
Functional Annotation
Diamond (Huson and Buchfink, 2015) was used to compare contigs to the UniProt protein library3, which includes the UniRef90 protein database; the MetaCyc metabolic pathway database was used to predict gene pathways4. MinPath5 defines the minimum set of pathways, producing results at the protein, gene, and pathway levels, and produces a functional composition table. The STAMP (Parks et al., 2014) software was used to calculate the functional pathways and to produce a histogram. Significant functional differences between the groups were set at P < 0.05.
The nucleic acid sequence was translated into a protein sequence and compared to the eggNOG (Jaime et al., 2016) database using the Diamond (Huson and Buchfink, 2015). The eggNOG database integrates annotation information, such as Gene Ontology (GO), clusters of orthologous genes (COG), and KEGG orthologs (KO) information. We performed multiple sequence alignment between the query and the eggNOG database to determine the conserved sites and analyze their evolutionary relationships (Jaime et al., 2016). We selected the possible gene names of each sequence predicted by the eggNOG database and eliminated the duplicated results. The eggNOG annotation results were used for subsequent analysis. Diamond (Huson and Buchfink, 2015) was used to align the protein sequence with the carbohydrate-active enzymes database (CAZy) (Vincent et al., 2013). The CAZy database provides an understanding of the nature and extent of the complex carbohydrate metabolism focuses on the differences in carbon source metabolism between species, and was used to compare to the Resfams database using Diamond (Gibson et al., 2015), we predicted the unknown resistance gene and obtained the mechanism and function of the resistance genes in each sample.
In vitro Validation of Pseudomonas fluorescens
The Pseudomonas fluorescens strain (Mingzhou Biotechnology Co., Ltd., Ningbo, China) was expanded using NA (Nutrient Agar) culture medium. Before the experiment, the healthy P. notoginseng was scratched with a sterile knife to facilitate the entry of P. fluorescens into the plant. A control and an experimental group were set up in this study. The diluted P. fluorescens strain was added into the culture bottle with healthy P. notoginseng in the experimental group. The control group was filled with sterile water with the same volume, and each group had three biological replicates, all samples were cultured at 25°C, and the results were observed each day.
Results
Metagenomic Sequencing Data Quality Control and Saturation Verification
A total of 333.90 Gb raw data were produced from the 12 root endophyte samples, with an average of 27.83 Gb raw data per sample. Twelve rhizosphere soil samples produced a total of 173.80 Gb raw data with an average of 14.48 Gb raw data per sample. After assessing the quality of the original data, the Q30 values of all samples were >93%. Quality controls were performed on the raw data to remove host contamination and obtain clean data. After removing the host-related sequences of P. notoginseng, each rhizosphere soil and root endophyte sample had an average of 13.90 and 12.66 Gb of clean data (Table 1 and Supplementary Table 1). Based on the alpha diversity chao1 and observed dilution curves (Supplementary Figure 1) of rhizosphere soil and root endophyte samples, Supplementary Figures 1A,B show that the data were saturated at 500,000 sequences (0.075 Gb) of rhizosphere soil sample. Similarly, Supplementary Figures 1C,D show saturation at 1,000,000 sequences (0.15 Gb) of the root endophyte sample. These results confirm that the sequencing depth of each sample reached saturation, enabling us to perform the next steps of this study.
Microbial Community Characteristics Between Healthy and Diseased Panax notoginseng
Kraken2 is a high-precision metagenomic sequence classification software based on the k-mer algorithm, which can classify sequencing data by species (Wood et al., 2019). In this study, the species diversity of each group was analyzed, the alpha diversity and Shannon index boxes were plotted (Figures 1B,C). The other diversity index boxes are shown in Supplementary Figure 2. Boxplots of the top five genera are shown in Figure 1D. The results showed that the overall richness of the rhizosphere soil was higher than the root endophytes. The number of operational taxonomic units (OTUs) in the rhizosphere soil of diseased plants was higher than healthy plants, whereas, in the root endophyte samples, the number of OTUs of healthy plants was higher than diseased plants (Figure 1B). The abundance of Bradyrhizobium in healthy rhizosphere soil was higher than in diseased rhizosphere soil (Figure 1D). In the healthy rhizosphere soil, the top three genera were Bradyrhizobium, Micromonospora, and Streptomyces. The genus-level differences were more evident in the root endophytes between healthy and diseased plants. The three most abundant genus in the healthy root endophytes were Lysobacter, Pseudomonas, and Paraburkholderia. The top three genera in diseased root endophytes were Pseudomonas, Lelliottia, and Variovorax. Lysobacter and Paraburkholderia were more abundant in healthy samples than in root rot diseased samples, whereas Pseudomonas and Lelliottia were more abundant in diseased root endophyte samples (Figure 1D).
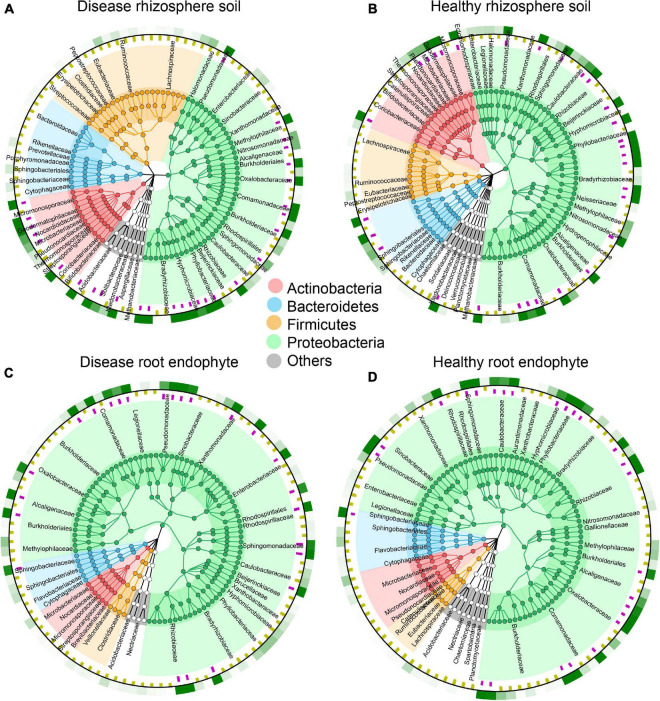
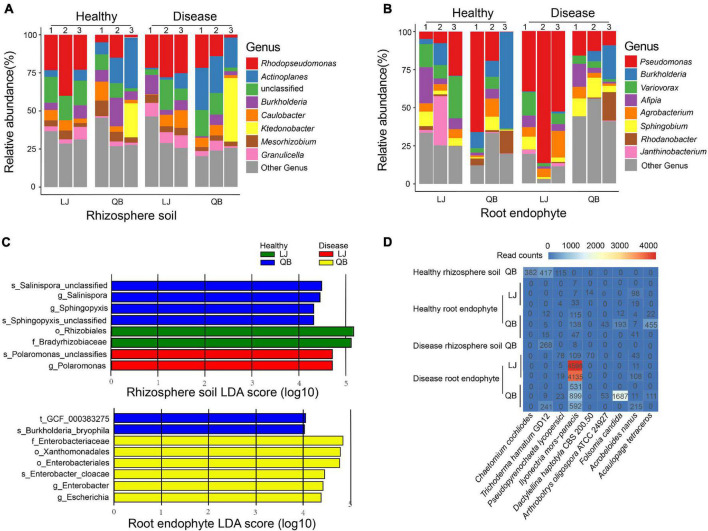
We used the species annotation results of MetaPhlAn2, which is more accurate. There are 143 OTUs in rhizosphere soil and 187 OTUs in root endophyte, and we chose the top 100 species, which is more representative and classified them by phylum. As indicated by the colors in the species circle diagrams, there were four main phyla: Actinobacteria, Bacteroides, Firmicutes, and Proteobacteria. Species that did not classify into these four phyla were grouped as others in the circle diagrams. The circle diagrams showed that Proteobacteria occurred in the highest proportion in both the rhizosphere soil and root endophyte samples. The relative occurrence of the phyla in the rhizosphere soil and root endophyte samples are Actinobacteria, 14 and 7%; Bacteroides, 10 and 7%; Firmicutes, 20 and 6%; Proteobacteria, 50 and 77%; others, is 6 and 3%, respectively (Figure 2). The species composition histograms showed the differences at the genus level between different health statuses (Figure 3). A total of 87 genera were detected in the rhizosphere soil and the top seven most abundant genera were Rhodopseudomonas, Actinoplanes, Burkholderia, Caulobacter, Ktedonobacter, Mesorhizobium, and Granulicella (Figure 3A). The rhizosphere soil bacterial community in the diseased and healthy rhizosphere soil samples from LJ and QB showed contrasting results. The abundance of Rhodopseudomonas was higher in the healthy rhizosphere soil at the LJ site, whereas it was more abundant in the root rot diseased rhizosphere soil at the QB site. Ktedonobacter was found only in the rhizosphere soil from QB and was more abundant in the root rot diseased soil sample. In total, 108 genera were detected in the root endophyte samples (Figure 3B). The eight most abundant genera were Pseudomonas, Burkholderia, Variovorax, Afipia, Agrobacterium, Sphingobium, Rhodanobacter, and Janthinobacterium. Pseudomonas showed a high abundance in the diseased root endophyte samples from LJ, and a high abundance in the healthy root endophyte samples from QB, which may be related to the ecological environment of the different locations. The abundance of Burkholderia in the healthy root samples was higher than in diseased samples. Variovorax and Afipia showed high abundance in the healthy root endophyte samples from LJ. Overall, the abundance of Agrobacterium and Sphingobium in the diseased root endophyte samples was higher than in healthy samples. There were eight species with significant differences in the root endophyte and rhizosphere soil samples (LDA > 4 for significant differences; Figure 3C).
FIGURE 2.
Species composition circle diagrams based on the top 100 most abundant species in rhizosphere soil and root endophyte samples. The colors represent the four phyla that contain these species. The purple squares in the first ring indicate the species with an abundance greater than 5 per 1,000. The yellow squares in the second ring indicate the species with an abundance less than 5 per 1,000. The third ring indicates the average value for all samples, where the different shades of green show the different species abundances. (A) Top 100 most abundant species in disease rhizosphere soil. (B) Top 100 most abundant species in healthy rhizosphere soil. (C) Top 100 most abundant species in disease root endophyte. (D) Top 100 most abundant species in healthy root endophyte.
FIGURE 3.
Genus composition heatmaps and corresponding histograms for the microbial communities associated with healthy and root rot Panax notoginseng. (A,B) Relative abundance of the top eight species in each sample. (C) Species differences in the rhizosphere soil and root endophyte microbial communities associated with healthy and root rot P. notoginseng. Display the species with significant differences (LDA > 4) between the groups. Panel (D) shows the top nine species of eukaryotes present in the samples. Lijiang (LJ) and Qiubei (QB) and 1, 2, and 3 represent biological replicate in each location.
We also analyzed the composition of fungi in all samples and a total of 12 eukaryote species in the rhizosphere soil, and 29 in the root endophyte samples were present (Supplementary Table 2). Acaulopage tetraceros, Acrobeloides nanus, Arthrobotrys oligospora ATCC 24927, Dactylellina haptotyla CBS 200.50, and Folsomia candida were unique to root endophytes; A. tetraceros and Chaetomium cochliodes were more abundant in the healthy samples; A. nanus, A. oligospora ATCC 24927, F. candida, and Pseudopyrenochaeta lycopersici were more abundant in the diseased samples; I. mors-panacis was the most abundant species in the diseased samples (Figure 3D).
Functional Composition and Pathways of Healthy and Root Rot Panax notoginseng
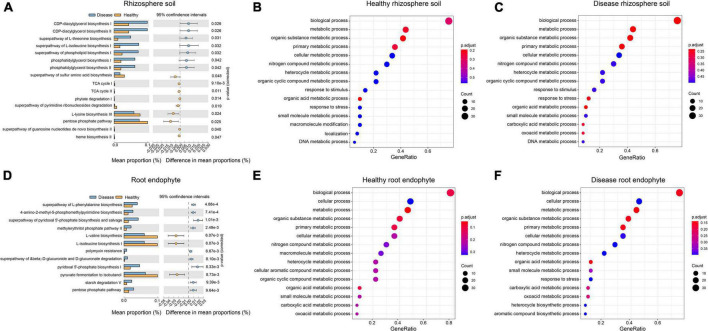
There were 16 significantly different pathways in the rhizosphere soil. The pathways of the CDP-diacylglycerol biosynthesis I/II, superpathway of L-threonine biosynthesis, and superpathway of L-isoleucine biosynthesis I were significantly up-regulated in the diseased rhizosphere soil samples, whereas the L-lysine biosynthesis III and pentose phosphate pathway were significantly up-regulated in the healthy rhizosphere soil (Figure 4A). There were 12 significantly different functional pathways in the root endophyte samples. Superpathway of L-phenylalanine biosynthesis, 4-amino-2-methyl-5-phosphomethylpyrimidine biosynthesis and pyridoxal 5′-phosphate biosynthesis I was significantly up-regulated in the root endophyte diseased sample, whereas in the healthy root endophyte samples L-valine biosynthesis, L-isoleucine biosynthesis I, and pyruvate fermentation to isobutanol were significantly up-regulated (Figure 4D). The GO term annotation results were mainly divided into three categories: molecular function (MF), cellular component (CC), and biological process (BP). The top 50 GO terms of each group were classified and enriched, and the identified GO terms belonged to the BP category (Figure 4). The enrichment degree of the GO terms in the root endophytes (Figures 4E,F) was higher than that in the rhizosphere soil (Figures 4B,C). The pathways of the top three enrichments in the rhizosphere soil were the BP, metabolic process, and organic substance metabolic process. The carboxylic acid metabolic process and the oxoacid metabolic process were enriched in the root rot diseased soil group and did not appear in the top 20 processes associated with the healthy rhizosphere soil (Figures 4B,C). The macromolecule metabolic process, cellular aromatic compound metabolic process, and organic cyclic compound metabolic process were significantly enriched in healthy root endophytes and did not occur in the top 20 processes of the diseased root endophytes (Figures 4E,F).
FIGURE 4.
Differences in the functional composition of the rhizosphere soil and root endophyte microbial communities associated with healthy and root rot P. notoginseng. Panels (A,D) show the functional pathways with significant differences between healthy and diseased samples, respectively. Panels (B,C,E,F) represent the Gene Ontology (GO) enrichment annotation results for each group.
We de novo assembled the clean data, evaluated the quality of the contig, and then performed de-redundancy, quantification and obtained pure contig for subsequent functional annotation. We obtained 11.22 Gb contigs with an average contig N50 of 1, 287 bp (Supplementary Table 3). We compared the contigs to the eggNOG (Jaime et al., 2016) database, integrated the annotation results, and counted the annotated COG orthologous proteins in each group. The COG classified 24 functional descriptions, and the annotation implied no significant differences among the 24 functional descriptions of the diseased and healthy rhizosphere samples (P > 0.05). The three dominant functions with higher abundance in the rhizosphere soil samples were S: function unknown (healthy rhizosphere soil = 20.61%, diseased rhizosphere soil = 20.85%), E: amino acid transport and metabolism (healthy rhizosphere soil = 9.82%, diseased rhizosphere soil = 9.54%), and C: energy production and conversion (healthy rhizosphere soil = 8.69%, diseased rhizosphere soil = 8.22%; Supplementary Table 3). Among the 24 functional descriptions of the root endophytes, four of them were significantly different between the diseased and healthy samples (P < 0.05), and all of them showed a higher abundance in the diseased samples: E: amino acid transport and metabolism (P = 0.011), N: cell motility (P = 0.028), O: posttranslational modification, protein turnover, chaperones (P = 0.027), and S: function unknown (P = 0.027) (Supplementary Figure 3A). The three dominant functions with a higher abundance in the root endophyte samples were S: function unknown (healthy root endophyte = 20.73%, diseased root endophyte = 19.38%), E: amino acid transport and metabolism (healthy root endophyte = 8.88%, diseased root endophyte = 9.60%), and P: inorganic ion transport and metabolism (healthy root endophyte = 8.29%, diseased root endophyte = 8.63%), the functional pathway of which was relatively abundant in the root rot diseased samples (Supplementary Table 3).
The Resfam database analysis results indicated that there were 15 classifications of mechanisms with 94 functional descriptions. Among them, the diseased rhizosphere soil group had the most resistance genes (106,554), with an average of 17,759 resistance genes per sample. The resistance genes of the other groups were healthy rhizosphere soil (89,250), diseased root endophyte (84,135), and healthy root endophyte (57,122). Overall, there were more resistance genes in the rhizosphere soil than in the root endophytes, and the number of resistance genes in the diseased group was higher than in the healthy group. The ABC transporter, RND antibiotic efflux and gene modulating resistance functions in the root rot disease group were higher than those in the healthy group (Supplementary Table 3 and Supplementary Figure 3B).
The KO function results for each group were counted. Among them, the healthy rhizosphere soil group had a total of 7,898 KOs, the disease rhizosphere soil group presented 8,078 KOs, the healthy root endophyte group presented 9,886 KOs, and the disease root endophyte group presented 10,736 KOs. The number of KOs in the root endophytes was higher than in the rhizosphere soil, and that of KOs in the diseased group was higher than in the healthy group. There was 6,469 core KOs in the four groups, accounting for 54.4% (Supplementary Figure 3C). In addition, each group had unique KOs, which were 106 (0.9%), 124 (1.0%), 435 (3.7%), 921 (7.7%), sorted by healthy rhizosphere soil, disease rhizosphere soil, healthy root endophyte, and disease root endophyte, respectively. The KO results showed that zearalenone synthase (K15418), lysophospholipid acyltransferase (K13519), Pin2-interacting protein (K11135), G protein-coupled receptor 64 (K08451), and MFS transporter (K08181) are unique KO in disease root endophyte.
The CAZy database includes five categories and one related module. Each category was divided into families. Among them, the glycoside hydrolases (GHs), which mainly hydrolyze and rearrange glycosidic bonds, presented a total number of 167 families (Vincent et al., 2013). We found 123 GH families in the healthy rhizosphere soil, 127 in the diseased rhizosphere soil, 126 in the healthy root endophyte, and 131 in the diseased root endophyte group (Supplementary Table 3). The number of GH families in the diseased samples was slightly higher than that in the healthy group. Similarly, there were 110 families of glycosyltransferases (GTs), mainly involved in the formation of glycosidic bonds. In addition, polysaccharide lyases (PLs) presented 40 families, carbohydrate esterases have 17 families, auxiliary activities have 16 families, and carbohydrate-binding modules have 86 families. These families were observed in slightly higher numbers in the diseased group than in the healthy group. The sequence annotation results of CAZy database showed that healthy rhizosphere soil, disease rhizosphere soil, healthy root endophyte, and disease root endophyte show an increasing trend by turns. The healthy rhizosphere soil group had the lowest number of reads matched to the CAZy database, the diseased root endophyte group had the most reads matched to the CAZy database, and the GT family matched ratio was the highest, followed by that of the GH family (Supplementary Table 3).
Metagenomic Data Binning
After assembling the clean data, de-redundant contigs were binned to mine the single bacterial genome. Ultimately, 9 bins were obtained in the healthy rhizosphere soil group, 24 bins in the diseased rhizosphere soil group, and 2 bins in the diseased rhizosphere soil group with >99% completeness. Thaumarchaeota had 99.51% completeness with the genome length of 1.40 Mb; Clostridium had 99.01% completeness with the genome length of 6.31 Mb in the diseased rhizosphere soil (Supplementary Figure 4 and Supplementary Table 4). A total of 41 bins were in the healthy root endophyte group, two of which were 100% complete, namely Flavobacteriaceae (genome length = 5.00 Mb) and Bacteroidetes (genome length = 6.34 Mb). There were 91 bins in the diseased root endophyte group, one of which was 100% complete (Flavobacteriaceae, genome length = 4.3 Mb). We found that these two 100% complete Flavobacteriaceae genomes were two different species under the Flavobacteriaceae genus through similarity comparison. The GC content results indicated that the abundance of orders in the rhizosphere soil was greater than that in the root endophytes (Supplementary Table 4). These results are consistent with the findings of higher microbial diversity in the rhizosphere soil than root endophytes samples (Figure 1B).
The abundance of the purified single bacterial genome in each sample was determined using bin quantification, and the results are displayed in a heat map (Supplementary Figure 4). Bacteria (bin 2) were enriched in the QB-3 sample and Sphingomonadaceae (bin 13) was enriched in the QB-1 sample (Supplementary Figures 4A,C). Alphaproteobacteria (bin 3) were enriched in the LJ diseased root endophyte samples (Supplementary Figure 4D and Supplementary Table 4). Based on taxonomic annotations from the Genome Taxonomy Database (GTDB), the analysis revealed that the bins cover 34 known species (ANI > 95%). There are 132 bins with <95% ANI of the existing genome, which represented potential new species. The GTDB classification of all the 166 bins is shown in Supplementary Table 4.
Discussion
Microbial Communities in Different Ecological Niches of Root Rot Panax notoginseng
The shape of the dilution curves for the rhizosphere soil and root endophyte samples significantly differed (Supplementary Figure 1). Among the rhizosphere soil samples (Supplementary Figures 1A,B), only the healthy rhizosphere soil dilution curve shape changed, while the dilution curves of the diseased rhizosphere soil samples remained uniform and the shape of the root endophyte dilution curves was more distinct (Supplementary Figures 1C,D). The evident change in the OTU dilution curve of root endophytes may be attributed to the uneven distribution of the microbial community associated with the roots and other plant parts (Gottel et al., 2011). In addition, when comparing the observed OTUs of rhizosphere soil and root endophyte samples (Figure 1B), we found that the microbial diversity of the rhizosphere soil was higher than that of the root endophytes. Studies have shown that the microbial community of rhizosphere soil is more diverse than root endophytes (Johnson and Jumpponen, 2005; Bulgarelli et al., 2013), with a clear compartmental separation between the rhizosphere soil and root endophyte of plants (Bulgarelli et al., 2012, 2015; Lundberg et al., 2012; Peiffer et al., 2013; Zarraonaindia et al., 2015; Yeoh et al., 2016; Hartman et al., 2017). Our results illustrate this uniqueness of the microbial communities (Figures 3A,B). Previous studies have also shown that the bacteria of root endophytes are recruited from the rhizosphere soil (Chaparro et al., 2014; Edwards et al., 2015), which may explain the higher microbial diversity in the rhizosphere soil than root endophyte.
Bacterial Community Differences Between Healthy and Root Rot Panax notoginseng
We compared the bacterial community difference in rhizosphere soil between healthy and diseased samples and found that Rhodopseudomonas was the most abundant in the rhizosphere soil (Figure 3A), especially in the healthy samples from the LJ site. Rhodopseudomonas can promote plant growth, and induce resistance in plants (Hsu et al., 2021). In tobacco, Rhodopseudomonas induces resistance against tobacco mosaic disease (Su et al., 2017). KTSSR54, an active compound produced by Rhodopseudomonas palustris, can help rice inhibit pathogens (Nookongbut et al., 2020). Our results are in line with previous findings showing that Rhodopseudomonas can protect P. notoginseng from root rot. Among the bacteria studied, Actinoplanes have attracted increasing attention since they can inhibit plant pathogens and produce more than 100,000 compounds, such as antifungal compounds, siderophores, hydrocyanic acid, hydrolytic enzymes, and ammonia. Actinoplanes can accelerate the absorption of nutrients and have positively influenced plant growth (Palaniyandi et al., 2013; Sreevidya et al., 2016; Sumaira et al., 2016). Actinoplanes produce many antibiotics, accounting for approximately 45% of the antibiotics on the market (Liu et al., 2012), most of which can treat plant disease (Samac et al., 2003; Cheng et al., 2010; Zucchi et al., 2010; Palaniyandi et al., 2011). In our study, Actinoplanes were more abundant in LJ and QB rhizosphere soil samples (Figure 3A), suggesting that the diseased P. notoginseng plants may have recruited Actinoplanes to resist root rot pathogens, thereby increasing the abundance of Actinoplanes in the soil of diseased plants. Burkholderia was relatively abundant in most samples. Several studies have shown that Burkholderia has antibacterial properties, promotes plant growth and is a potential and effective biological control agent (Jeong et al., 2003; Kunakom and Eustáquio, 2019). Burkholderia is an antagonist of black spot disease in cherries (Ding et al., 2021), and secretes beneficial compounds to protect bananas against Fusarium wilt (Xu et al., 2020). In our study, the abundance of Burkholderia in the healthy root endophyte samples was higher than that in the diseased samples, which is consistent with previous studies (Jeong et al., 2003; Kunakom and Eustáquio, 2019; Xu et al., 2020; Ding et al., 2021), suggesting that Burkholderia is a beneficial bacterium for P. notoginseng. We infer that Burkholderia can protect P. notoginseng from plant diseases.
Pseudomonas, the most abundant dominant bacterial species in the root endophyte samples in our study, was more abundant in the diseased samples from LJ than in other groups (Figure 3B). Pseudomonas has been previously suggested to cause root rot (Miao et al., 2006), and was only identified at the genus level. Herein, we identified Pseudomonas to the species level, with P. fluorescens, known to protect many crop plants against soil-borne diseases caused by phytopathogens (Silby et al., 2009; Liang F. et al., 2020), at the highest abundance. It has also been shown that P. fluorescens, as a biological control agent, can effectively reduce root knot nematode infestation of cucumbers and replace chemical measures for nematode control, thereby increasing cucumber yield and reducing cultivation costs (Panpatte et al., 2020). Screened under in vitro conditions, P. fluorescens has been shown to have antagonistic activity against pea root rot, with high biological control activity (Godebo et al., 2020). A recent report found that Pseudomonas is recruited from roots to resist plant pathogens and was used to verify the disease incidence of plants where it reduces the disease incidence of Arabidopsis (Wen et al., 2020). However, the role of P. fluorescens is still controversial. In this study, we conducted an in vitro experiment to verify whether P. fluorescens has a pathogenic effect on P. notoginseng (Supplementary Figure 5). Combining our data with experimental results, we concluded that P. fluorescens has a pathogenic effect on P. notoginseng. Our results revealed that Variovorax had a higher abundance in most healthy root endophytic samples. We identified Variovorax to the species level and found that the species with the highest abundance was Variovorax paradoxus. Variovorax can facilitate the interaction between plants and microorganisms by manipulating plant hormone levels to balance the growth of plant roots. V. paradoxus can significantly inhibit potato tuber soft rot and is considered to be a new type of potato tuber soft rot biological control agent (Ha et al., 2018). In our study, the abundance of V. paradoxus in healthy root endophyte samples was high. This suggests that V. paradoxus may promote the growth of P. notoginseng and may be an effective biological control agent for root rot disease.
Eukaryotic Microbial Communities Associated With Healthy and Root Rot Panax notoginseng
Our analysis showed that I. mors-panacis had the highest ratio in the diseased root endophyte samples (Figure 3D) corroborating with the findings of a previous study that presented I. mors-panacis as the main pathogen of P. notoginseng root rot (Mi et al., 2017). I. mors-panacis is also the main pathogen of P. ginseng root rot (Desrochers et al., 2020; Farh et al., 2020). P. lycopersici is also a plant fungal pathogen that causes severe root rot and root knot disease (Errico et al., 2019). In our study, P. lycopersici had a higher number of reads in the diseased samples, except for disease rhizosphere soil from QB. P. lycopersici has not previously been reported in association with root rot P. notoginseng, hence we infer that P. lycopersici may be a pathogen of P. notoginseng root rot. A. oligospora ATCC 24927, a fungus that feeds on nematodes, is often used as a biological control agent for plant and animal parasitic nematodes (Ahman et al., 2002). The number of reads of this fungus was relatively high in the diseased samples in our study. A. nanus is a type of nematode that feeds on bacteria and was mainly associated with diseased samples in our study. This could account for the high occurrence of A. oligospora ATCC 24927, which feeds on nematodes, in the diseased samples.
The Interaction of Functional Genes and Pathways Helps Improve Plant Resistance
Amino acids play an important role in plant growth as well as pathogen infection as they are indispensable nitrogen sources for many nutritional pathogens (Douglas, 1993; Rico and Preston, 2008), hence regulating the content of amino acids is critical for the growth and defense of plants. It has been demonstrated that the increased expression of usually multiple acids moving in and out transporters (UMAMIT) can induce plant resistance to pathogens (Besnard et al., 2021). In addition, the existence of the amino acid-sensing mechanism in plants indicates that changing the level of amino acids by changing the metabolism or transport of amino acids may trigger a defense response; furthermore, resistance-related genes are up-regulated (Yang et al., 2014). Our study found higher amino acid transport and metabolism in healthy rhizosphere soil than in diseased rhizosphere soil. Based on previous findings, we concluded that the upregulation of amino acid transport and metabolism in soil and the enhancement of transport activity would help to resist the invasion of pathogens and improve the resistance of P. notoginseng (Supplementary Table 3). In a study on the resistance signal mechanism of the related tomato root knot nematode disease, the protein with the greatest difference in the differentially expressed protein group belonged to energy production and conversion because the defense response is an energy-consuming process, which may play a key role in the plant’s disease resistance process (Zhao W. et al., 2016). In our study, the energy production and conversion process in diseased roots was three times that of healthy roots and may be closely related to the disease resistance process of P. notoginseng (Supplementary Table 3).
The Antibiotic Resistance-Related Gene Can Help Panax notoginseng Resist Root Rot
The misuse of antibiotics has contributed to the widespread development of antimicrobial resistance among clinically significant bacterial species, therefore, understanding the mechanism of antibiotics in P. notoginseng is important for facilitating the healthy growth of this plant species. A previous study has shown that Chinese herbal medicines, such as P. notoginseng, can produce compounds that interfere with quorum sensing, which can effectively inhibit pathogens and help delay antibiotic resistance (Koh and Tham, 2011). In our study, ABC transporter (34.16%), gene modulating resistance (20.55%), and RND antibiotic efflux (16.62%) accounted for the highest proportions and the highest number of diseased samples (Supplementary Figure 3B). ABC transporter reactions play major roles in disease resistance of plants (Devanna et al., 2021). The G and B subfamily of the ABC transporter family have been the most researched on related secondary metabolism and is also the key transport family to the defensive process (Hwang et al., 2016; Borghi et al., 2019; Dhara and Raichaudhuri, 2021). The proportion of ABC transporters in the two groups of diseased samples was higher than that in the corresponding two groups of healthy samples, which may be associated with the disease resistance of P. notoginseng. Previous studies have reported that gene modulating resistance helps plants to inhibit pathogenic bacteria and improve plant resistance (Gong et al., 2020; Liang Y. et al., 2020; Wang J. et al., 2020; Corredor-Moreno et al., 2021; Zia et al., 2021), while the RND antibiotic efflux mechanism has an efflux effect on various antibiotics; inhibiting these pumps can mitigate antibiotic resistance (Siriyong et al., 2017; Liang et al., 2019; Ichinose et al., 2020). Our study showed that the RND antibiotic efflux mechanism in diseased samples was greater than that in healthy samples. We speculate that the RND antibiotic efflux mechanism in the diseased samples was active and had an efflux effect on antibiotics. Thus, the reduction of antibiotics in the diseased samples might have weakened the resistance to pathogens. This phenomenon may induce plant roots to produce antibiotic efflux, making it easier for root rot to invade.
Panax notoginseng Root Rot Carbohydrate-Active Enzymes Genes
According to the CAZy database comparison results, the proportion of GHs in each group was the largest, and the proportion in diseased samples was higher than that of healthy samples. In a study of peanut stem rot caused by fungi, the number of GHs was the highest in the pathogenic secreted protein (Iquebal et al., 2017). In addition, in soybean seed rot, 149 plant cell wall-degrading enzymes were detected, most of which were GHs (Li et al., 2018). Our results are in line with these previous reports. The number of GHs in the diseased samples was higher than healthy samples, suggesting that the root rot-affected parts of P. notoginseng secrete more enzymes related to the GH family. Regarding the GTs, in a study of wheat infection with F. wilt, the GTs in wheat infected with Fusarium graminearum showed an increasing trend (Ha et al., 2016), indicating that infection with P. notoginseng root rot can cause GTs to increase. In short, the increase in the number of GH and GT families can predict the tendency of P. notoginseng to suffer from root rot (Supplementary Table 3).
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: National Center for Biotechnology Information (NCBI) BioProject database under accession number PRJNA753694.
Author Contributions
PW and YL wrote and reviewed the manuscript and draw the picture. PW, LY, JS, and YL collected the samples. YY, YQ, CW, DL, and LH provided the analysis ideas and reviewed the manuscript. XC and YL supervised the work and read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the editor and reviewers for providing valuable comments on the manuscript.
Footnotes
Funding
YL acknowledges the financial support from the National Natural Science Foundation of China (Grant No. 31960134) and Key Project at central government level: the ability establishment of sustainable use for valuable Chinese medicine resources (Grant No. 2060302), and Major Science and Technology Special Project of Yunnan Province (Grant No. 202102AA310034).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.752683/full#supplementary-material
Alpha diversity dilution curves. Each group contained six samples. The abscissa is the total number of sequences, while the ordinate represents the number of OTUs. (A) Healthy and disease rhizosphere soil chao1 alpha diversity dilution curves. (B) Healthy and disease rhizosphere soil observed alpha diversity dilution curves. (C) Healthy and disease root endophyte chao1 alpha diversity dilution curves. (D) Healthy and disease root endophyte observed alpha diversity dilution curves.
Boxplots of diversity in the rhizosphere soil and root endophyte group. (A) ACE index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (B) Chao1 index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (C) Invsimpson index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (D) Simpson index box plot based on the rhizosphere soil and root endophyte bacteria species abundance.
Annotation results of bacteria with significantly different functional descriptions. (A) Amino acid transport and metabolism, cell motility, posttranslational modification, protein turnover, and chaperones, and unknown function. (B) Antibiotic gene percentage of each group. (C) Venn diagram of KEGG orthologs (KO) functional annotation results for each group.
Quantitative heat map of the bins in each group. (A) Quantitative heat map of the bins in healthy rhizosphere soil. (B) Quantitative heat map of the bins in disease rhizosphere soil. (C) Quantitative heat map of the bins in healthy root endophyte. (D) Quantitative heat map of the bins in disease root endophyte.
Vitro infection results of Pseudomonas fluorescens.
Sample and data information.
Eukaryote information for each sample.
Function annotation information.
Genomic and classification information of the bins in each group.
References
- Ahman J., Johansson T., Olsson M., Punt P., van den Hondel C., Tunlid A. (2002). Improving the pathogenicity of a nematode-trapping fungus by genetic engineering of a subtilisin with nematotoxic activity. Appl. Environ. Microbiol. 68 3408–3415. 10.1128/AEM.68.7.3408-3415.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexey G., Vladislav S., Nikolay V., Glenn T. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29 1072–1075. 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews A. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc [Google Scholar]
- Asnicar F., Weingart G., Tickle T. L., Huttenhower C., Segata N. (2015). Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 3:e1029. 10.7717/peerj.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M. G., Glover J. D., Mai J. G., Kinkel L. L. (2010). Plant community effects on the diversity and pathogen suppressive activity of soil streptomycetes. Appl. Soil Ecol. 46 35–42. [Google Scholar]
- Bakker P., Pieterse C. M. J., Jonge R. D., Berendsen R. L. (2018). The soil-borne legacy. Cell 172 1178–1180. [DOI] [PubMed] [Google Scholar]
- Besnard J., Sonawala U., Maharjan B., Collakova E., Okumoto S. (2021). Increased expression of UMAMIT amino acid transporters results in activation of salicylic acid dependent stress response. Front. Plant Sci. 11:606386. 10.3389/fpls.2020.606386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi L., Kang J., Francisco R. D. (2019). Filling the gap: functional clustering of ABC proteins for the investigation of hormonal transport in planta. Front. Plant Sci. 10:422. 10.3389/fpls.2019.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard L., Ruiter P., Brown G. G. (2007). Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 121 33–244. [Google Scholar]
- Bulgarelli D., Garrido-Oter R., Münch P., Weiman A., Droge J., Pan Y., et al. (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17 392–403. 10.1016/j.chom.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgarelli D., Rott M., Schlaeppi K., Van Themaat E., Ahmadinejad N., Assenza F., et al. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488 91–95. 10.1038/nature11336 [DOI] [PubMed] [Google Scholar]
- Bulgarelli D., Schlaeppi K., Spaepen S., Themaat E., Schulze P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64 807–838. 10.1146/annurev-arplant-050312-120106 [DOI] [PubMed] [Google Scholar]
- Chaparro J. M., Badri D. V., Vivanco J. M. (2014). Rhizosphere microbiome assemblage is affected by plant development. ISME J. 8 790–803. 10.1038/ismej.2013.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil P. A., Mussig A. J., Hugenholtz P. (2019). GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 36 1925–1927. 10.1093/bioinformatics/btz848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Pachter L. (2005). Bioinformatics for whole-genome shotgun sequencing of microbial communities. PLoS Comput. Biol. 1 106–112. 10.1371/journal.pcbi.0010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Yang S., Palaniyandi S., Han J., Yoon T., Kim J., et al. (2010). Azalomycin F complex is an antifungal substance produced by Streptomyces malaysiensis MJM1968 isolated from agricultural soil. J. Korean Soc. Appl. Biol. Chem. 53 545–552. 10.3839/jksabc.2010.084 [DOI] [Google Scholar]
- Corredor-Moreno P., Minter F., Davey P., Wegel E., Kular B., Brett P., et al. (2021). The branched-chain amino acid aminotransferase TaBCAT1 modulates amino acid metabolism and positively regulates wheat rust susceptibility. Plant Cell 33 1728–1747. 10.1093/plcell/koab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Mao X., Liu D., Ning X. Q., Luo H. B. (2020). Comparative analysis of physicochemical properties and microbial composition in high-temperature Daqu with different colors. Front. Microbiol. 11:588117. 10.3389/fmicb.2020.588117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers N., Walsh J., Renaud J., Seifert K., Sumarah M. (2020). Metabolomic profiling of fungal pathogens responsible for root rot in American Ginseng. Metabolites 10:35. 10.3390/metabo10010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanna B. N., Jaswal R., Singh P. K., Kapoor R., Jain P., Kumar G., et al. (2021). Role of transporters in plant disease resistance. Physiol. Plant. 171 849–867. 10.1111/ppl.13377 [DOI] [PubMed] [Google Scholar]
- Dhara A., Raichaudhuri A. (2021). ABCG transporter proteins with beneficial activity on plants. Phytochemistry 184:112663. 10.1016/j.phytochem.2021.112663 [DOI] [PubMed] [Google Scholar]
- Ding L., Xu L., Chu X., Yang L., Zhu H., Huang J. (2021). Dissimilarity analysis of microbial communities in the rhizosphere and tissues of diseased and healthy cherry trees (Cerasus pseudocerasus). Can. J. Plant Pathol. 43 612–621. 10.1080/07060661.2020.1861101 [DOI] [Google Scholar]
- Dong F. Z., Liu Z. W., Le L. T. (1988). Panax notoginseng in Yunnan. Kunming, CHN: Yunnan Science and Technology Press, 103–114. [Google Scholar]
- Dong L., Xu J., Feng G., Li X., Chen S. (2016). Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system. Sci. Rep. 6:31802. 10.1038/srep31802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. E. (1993). The nutritional quality of phloem sap utilized by natural aphid populations. Ecol. Entomol. 18 31–38. [Google Scholar]
- Doyle J. J., Doyle J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19 11–15. [Google Scholar]
- Duan Y., Zhou L., Hall D. G., Li W., Gottwald T. (2009). Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol. Plant Microbe Interact. 22 1011–1020. 10.1094/MPMI-22-8-1011 [DOI] [PubMed] [Google Scholar]
- Edwards J., Johnson C., Santos-Medellín C., Lurie E., Sundaresan V. (2015). Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. U. S. A. 112 E911–E920. 10.1073/pnas.1414592112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico G., Marra R., Crescenzi A., Davino S., Fanigliulo A., Woo S., et al. (2019). Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop Prot. 122 159–164. [Google Scholar]
- Fan Z. Y., Miao C. P., Qiao X. G., Zheng Y. K., Chen H. H., Chen Y. W., et al. (2016). Diversity, distribution, and antagonistic activities of rhizobacteria of Panax notoginseng. J. Ginseng Res. 40 97–104. 10.1016/j.jgr.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh E., Kim Y., Abbai R., Singh P., Jung K., Kim Y., et al. (2020). Pathogenesis strategies and regulation of ginsenosides by two species of Ilyonectria in Panax ginseng: power of speciation. J. Ginseng Res. 44 332–340. 10.1016/j.jgr.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa E. A., Mciver L. J., Rahnavard G., Thompson L. R., Schirmer M., Weingart G., et al. (2018). Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 15 962–968. 10.1038/s41592-018-0176-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Niu B., Zhu Z., Wu S., Li W. (2012). CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28 3150–3152. 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin E., Jacquet C., Bottin A., Dumas B. (2007). Root rot disease of legumes caused by Aphanomyces euteiches. Mol. Plant Pathol. 8 539–548. 10.1111/j.1364-3703.2007.00413.x [DOI] [PubMed] [Google Scholar]
- Gibson M. K., Forsberg K. J., Dantas G. (2015). Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 9 207–216. 10.1038/ismej.2014.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godebo A., Germida J., Walley F. (2020). Isolation, identification, and assessment of soil bacteria as biocontrol agents of pea root rot caused by Aphanomyces euteiches. Can. J. Soil Sci. 100 206–216. [Google Scholar]
- Gong C., Cheng M., Li J., Chen H., Zhang Z. Z. (2020). Alpha-subunit of the chloroplast ATP synthase of tomato reinforces the resistance to grey mold and broad-spectrum resistance in transgenic tobacco. Phytopathology 111 485–495. 10.1094/PHYTO-06-20-0242-R [DOI] [PubMed] [Google Scholar]
- Goss E. M., Tabima J. F., Cooke D., Restrepo S., Fry W. E., Forbes G. A., et al. (2014). The Irish potato famine pathogen Phytophthora infestans originated in central Mexico rather than the Andes. Proc. Natl. Acad. Sci. U. S. A. 111 8791–8796. 10.1073/pnas.1401884111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottel N. R., Castro H. F., Kerley M., Yang Z., Pelletier D. A., Podar M., et al. (2011). Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl. Environ. Microbiol. 77 5934–5944. 10.1128/AEM.05255-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. Q., Chen Y. G., Shi R. Z., He K., Gao J. L. (2020). 20(S)-Protopanaxdiol suppresses the abnormal granule-monocyte differentiation of hematopoietic stem cells in 4T1 breast cancer-bearing mouse. Evid. Based Complement. Alternat. Med. 2020:8747023. 10.1155/2020/8747023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha N. T., Minh T. Q., Hoi P. X., Thuy N., Long H. H. (2018). Biological control of potato tuber soft rot using N-acyl-L-homoserine aactone-degrading endophytic bacteria. Curr. Sci. 115 1921–1927. 10.18520/cs/v115/i10/1921-1927 [DOI] [Google Scholar]
- Ha X., Koopmann B., Von Tiedemann A. (2016). Wheat blast and fusarium head blight display contrasting interaction patterns on ears of wheat genotypes differing in resistance. Phytopathology 106 270–281. 10.1094/PHYTO-09-15-0202-R [DOI] [PubMed] [Google Scholar]
- Hartman K., Van G. A., Roussely-Provent V., Walser J. C., Schlaeppi K. (2017). Deciphering composition and function of the root microbiome of a legume plant. Microbiome 5:2. 10.1186/s40168-016-0220-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B. G., Xiu M. C., An N., Ge P. C. (2010). Sanchi ginseng (Panax notoginseng (Burkill) F. H. Chen) in China: distribution, cultivation and variations. Genet. Resour. Crop Evol. 57 453–460. 10.1007/s10722-010-9531-2 [DOI] [Google Scholar]
- Hsu S., Shen M., Chen J., Lur H., Liu C. (2021). The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front. Plant Sci. 12:573634. 10.3389/fpls.2021.573634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H., Buchfink B. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12 59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- Hwang J., Song W., Hong D., Ko D., Yamaoka Y., Jang S., et al. (2016). Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 9 338–355. 10.1016/j.molp.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Hyatt D., LoCascio P. F., Hauser L., Uberbacher E. (2012). Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 28 2223–2230. 10.1093/bioinformatics/bts429 [DOI] [PubMed] [Google Scholar]
- Ichinose Y., Nishimura T., Harada M., Kashiwagi R., Matsui H. (2020). Role of two sets of RND-type multidrug efflux pump transporter genes, mexAB-oprM and mexEF-oprN, in Virulence of Pseudomonas syringae pv. tabaci 6605. Plant Pathol. J. 36 148–156. 10.5423/PPJ.OA.11.2019.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iquebal M. A., Tomar R. S., Parakhia M. V., Singla D., Jaiswal S., Rathod V. M., et al. (2017). Draft whole genome sequence of groundnut stem rot fungus Athelia rolfsii revealing genetic architect of its pathogenicity and virulence. Sci. Rep. 7:5299. 10.1038/s41598-017-05478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaime H. C., Kristoffer F., Pedro C. L., Damian S., Juhl J. L., Christian V. M. (2016). Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol. Biol. Evol. 34 2115–2122. 10.1093/molbev/msx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y., Kim J., Kim S., Kang Y., Nagamatsu T., Hwang I. (2003). Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 87 890–895. 10.1094/PDIS.2003.87.8.890 [DOI] [PubMed] [Google Scholar]
- Ji W., Xue L., Jin Z., Li H. (2018). Plant growth promoting bacteria increases biomass, effective constituent, and modifies rhizosphere bacterial communities of Panax ginseng. Soil Plant Sci. 69 1–12. 10.1007/978-3-319-13921-0_1 [DOI] [Google Scholar]
- Johnson J., Jumpponen A. (2005). Can rDNA analyses of diverse fungal communities in soil and roots detect effects of environmental manipulations–a case study from tallgrass prairie. Mycologia 97 1177–1194. 10.3852/mycologia.97.6.1177 [DOI] [PubMed] [Google Scholar]
- Kang D., Li F., Kirton E., Thomas A., Egan R., An H., et al. (2019). MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7:e7359. 10.7717/peerj.7359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K. H., Tham F. Y. (2011). Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. 44 144–148. 10.1016/j.jmii.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Kster J., Rahmann S. (2018). Snakemake—a scalable bioinformatics workflow engine. Bioinformatics 28 2520–2522. [DOI] [PubMed] [Google Scholar]
- Kunakom S., Eustáquio A. E. (2019). Burkholderia as a source of natural products. J. Nat. Prod. 82 2018–2037. 10.1021/acs.jnatprod.8b01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M. J., Kong H. G., Choi K., Kwon S. K., Kim J. F. (2018). Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 36 1100–1109. 10.1038/nbt.4232 [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C., Luo R., Sadakane K., Lam T. (2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31 1674–1676. 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- Li S., Bryan M., David L., Ji P. (2018). The interactomic analysis reveals pathogenic protein networks in Phomopsis longicolla underlying seed decay of soybean. Front. Genet. 9:104. 10.3389/fgene.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Zhang B., Yang Q., Zhang Y., Wu X. (2020). Cyclic-di-GMP regulates the quorum-sensing system and the biocontrol activity of Pseudomonas fluorescens 2P24 through RsmA and RsmE proteins. Genet. Mol. Biol. 86 e02016–20. 10.1128/AEM.02016-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Li Z., Zhang Y., Meng F. (2020). Nbnrp1 mediates Verticillium dahliae effector PevD1-triggered defense responses by regulating sesquiterpenoid phytoalexins biosynthesis pathway in Nicotiana benthamiana. Gene 768:145280. 10.1016/j.gene.2020.145280 [DOI] [PubMed] [Google Scholar]
- Liang Z., Huang L., He F., Zhou X., Zhang L. H. (2019). A substrate-activated efflux pump, DesABC, confers zeamine resistance to Dickeya zeae. mBio 10 e00713–19. 10.1128/mBio.00713-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A. L., Pollard K. S. (2021). Accurate and sensitive detection of microbial eukaryotes from whole metagenome shotgun sequencing. Microbiome 9:58. 10.1186/s40168-021-01015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Bolla K., Ashforth E. J., Zhuo Y., Gao H., Pei H., et al. (2012). Systematics-guided bioprospecting for bioactive microbial natural products. Antonie Van Leeuwenhoek 101 55–66. 10.1007/s10482-011-9671-1 [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang Y., Wu M., Ma Z., Huang Z., Tian F., et al. (2020). The scientific elucidation of daodi medicinal materials. Chin. Med. 15 1–11. 10.1186/s13020-020-00367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D. S., Lebeis S. L., Paredes S. H., Yourstone S., Gehring J., Malfatti S., et al. (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488 86–90. 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. N., Chen C. J., Li Q. Q., Xu F. R., Cheng Y. X., Dong X. (2019). Monitoring antifungal agents of artemisia annua against Fusarium oxysporum and Fusarium solani, associated with Panax notoginseng root-rot disease. Molecules 24:213. 10.3390/molecules24010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z. S., Long Y. J., Zhu Y. Y., Zhu S. S., Chen Z. J. (2014). First Report of Cylindrocarpon destructans var. destructans causing black root rot of Sanqi (Panax notoginseng) in China. Plant Dis. 98:162. 10.1094/PDIS-11-12-1104-PDN [DOI] [PubMed] [Google Scholar]
- Mendes L. W., Raaijmakers J. M., Hollander M. D. (2018). Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 12 212–224. 10.1038/ismej.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi C., Yang R., Rao J., Yang S., Wei F., Li O., et al. (2017). Unveiling of dominant fungal pathogens associated with rusty root rot of Panax notoginseng based on multiple methods. Plant Dis. 101 2046–2052. 10.1094/PDIS-01-17-0135-RE [DOI] [PubMed] [Google Scholar]
- Miao C. P., Mi Q. L., Qiao X. G., Zheng Y. K., Zhao L. X. (2016). Rhizospheric fungi of Panax notoginseng: diversity and antagonism to host phytopathogens. J. Ginseng Res. 40 127–134. 10.1016/j.jgr.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z. Q., Li S. D., Liu X. Z., Chen Y. J., Zhang K. Q. (2006). The causal microorganisms of Panax notoginseng root rot disease. Sci. Agric. Sin. 39 1371–1378. [Google Scholar]
- Nookongbut P., Kantachote D., Khuong N., Tantirungkij M. (2020). The biocontrol potential of acid-resistant Rhodopseudomonas palustris KTSSR54 and its exopolymeric substances against rice fungal pathogens to enhance rice growth and yield. Biol. Control 150:104354. 10.1016/j.biocontrol.2020.104354 [DOI] [Google Scholar]
- Ondov B. D., Bergman N. H., Phillippy A. M. (2011). Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12:385. 10.1186/1471-2105-12-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyandi S. A., Yang S. H., Cheng J. H., Meng L., Suh J. W. (2011). Biological control of anthracnose (Colletotrichum gloeosporioides) in yam by Streptomyces sp.MJM5763. J. Appl. Microbiol. 111 443–455. 10.1111/j.1365-2672.2011.05048.x [DOI] [PubMed] [Google Scholar]
- Palaniyandi S. A., Yang S. H., Zhang L., Suh J. W. (2013). Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 97 9621–9636. 10.1007/s00253-013-5206-1 [DOI] [PubMed] [Google Scholar]
- Panpatte D. G., Shelat H. N., Jhala Y. K., Vyas R. V. (2020). Fortified bacterial consortium – A novel approach to control root knot nematode in cucumber (cucumis sativum). Biol. Control 155:104528. 10.1016/j.biocontrol.2020.104528 [DOI] [Google Scholar]
- Parks D. H., Imelfort M., Skennerton C. T., Hugenholtz P., Tyson G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25 1043–1055. 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. H., Tyson G. W., Philip H., Beiko R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30 3123–3124. 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R., Duggal G., Love M., Irizarry R., Kingsford C. (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14 417–419. 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer J. A., Spor A., Koren O., Jin Z., Tringe S. G., Dangl J. L., et al. (2013). Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. U. S. A. 110 6548–6553. 10.1073/pnas.1302837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015). R: a Language And Environment For Statistical Computing. Vienna: R: Foundation for Statistical Computing. [Google Scholar]
- R Core Team (2020). R: a Language And Environment For Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Richter D. J., Berney C., Strassert J., Burki F., Vargas C. D. (2020). EukProt: a database of genome-scale predicted proteins across the diversity of eukaryotic life. Cold Spring Harb. Lab. 2020:180687. [Google Scholar]
- Rico A., Preston G. (2008). Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol. Plant Microbe Interact. 21 269–282. 10.1094/MPMI-21-2-0269 [DOI] [PubMed] [Google Scholar]
- Samac D., Willert A., Mcbride M., Kinkel L. (2003). Effects of antibiotic-producing Streptomyces on nodulation and leaf spot in alfalfa. Appl. Soil Ecol. 22 55–66. 10.1016/s0929-1393(02)00109-9 [DOI] [Google Scholar]
- Santhakumar A., Battino M., Alvarez-Suarez J. M. (2018). Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 113 49–65. 10.1016/j.fct.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silby M. W., Cerde-Tárraga A., Vernikos G. S., Giddens S. R., Jackson R. W., Preston G. M. (2009). Genomic and genetic analyses of diversity and plant interactions of Pseudomonas fluorescens. Genome Biol. 10:R51. 10.1186/gb-2009-10-5-r51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriyong T., Srimanote P., Chusri S., Yingyongnarongkul B. E. (2017). Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Aaltern. Med. 17:405. 10.1186/s12906-017-1913-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevidya M., Gopalakrishnan S., Kudapa H., Varshney R. K. (2016). Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 47 85–95. 10.1016/j.bjm.2015.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P., Tan X., Li C., Zhang D., Cheng J., Zhang S., et al. (2017). Photosynthetic bacterium Rhodopseudomonas palustris GJ-22 induces systemic resistance against viruses. Microb. Biotechnol. 10 612–624. 10.1111/1751-7915.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaira A., Basharat A., Imran S. (2016). Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front. Microbiol. 7:1334. 10.3389/fmicb.2016.01334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri A. E., Chatterton S., Gossen B. (2017). Metagenomic analysis of oomycete communities from the rhizosphere of field pea on the Canadian prairies. Can. J. Microbiol. 63 758–768. 10.1139/cjm-2017-0099 [DOI] [PubMed] [Google Scholar]
- Truong D. T., Franzosa E. A., Tickle T. L., Scholz M., Weingart G., Pasolli E., et al. (2015). MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12 902–903. 10.1038/nmeth.3589 [DOI] [PubMed] [Google Scholar]
- Uritskiy G. V., DiRuggiero J., Taylor J. (2018). MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6:158. 10.1186/s40168-018-0541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendan R. T., Lee S. H., Yu Y. J., Rhee Y. H. (2012). Analysis of bacterial community in the ginseng soil using denaturing gradient gel electrophoresis (DGGE). Indian J. Microbiol. 52 286–288. 10.1007/s12088-011-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent L., Hemalatha G. R., Elodie D., Coutinho P. M., Bernard H. (2013). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42 D490–D495. 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang R., Fang H., Zhang C., Zhang F., Hao Z., et al. (2020). Two VOZ transcription factors link an E3 ligase and an NLR immune receptor to modulate immunity in rice. Mol. Plant 14 253–266. 10.1016/j.molp.2020.11.005 [DOI] [PubMed] [Google Scholar]
- Wang Q., Sun H., Li M., Xu C., Zhang Y. (2020). Different age-induced changes in rhizosphere microbial composition and function of Panax ginseng in transplantation mode. Front. Plant Sci. 11:563240. 10.3389/fpls.2020.563240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. P., Ye Y., Liu D. Q., Cui X. M., Liu Y. (2020). Application of Metagenomics in Plant Diseases Research. Biotechnol. Bull. 36 146–154. [Google Scholar]
- Wen T., Zhao M., Yuan J., Kowalchuk G. A., Shen Q. (2020). Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes. Soil Ecol. Lett. 3 42–51. 10.1007/s42832-020-0057-z [DOI] [Google Scholar]
- Wood D. E., Lu J., Langmead B. (2019). Improved metagenomic analysis with Kraken 2. Genome Biol. 20:257. 10.1186/s13059-019-1891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Li Z., Li J., Khan M. A., Huang W., Zhang Z., et al. (2013). Assessment of shifts in microbial community structure and catabolic diversity in response to Rehmannia glutinosa monoculture. Appl. Soil Ecol. 67 1–9. 10.1016/j.apsoil.2013.02.008 [DOI] [Google Scholar]
- Wu Y. W., Simmons B. A., Singer S. W. (2016). MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32 605–607. 10.1093/bioinformatics/btv638 [DOI] [PubMed] [Google Scholar]
- Wu Z., Hao Z., Zeng Y., Guo L., Huang L., Chen B. (2015). Molecular characterization of microbial communities in the rhizosphere soils and roots of diseased and healthy Panax notoginseng. Antonie Van Leeuwenhoek 108 1059–1074. 10.1007/s10482-015-0560-x [DOI] [PubMed] [Google Scholar]
- Xing X., Guo S., Fu J. (2010). Biodiversity and distribution of endophytic fungi associated with Panax quinquefolium L. cultivated in a forest reserve. Symbiosis 51 161–166. 10.1007/s13199-010-0062-6 [DOI] [Google Scholar]
- Xu Z., Wang M., Du J., Huang T., Chen Y. (2020). Isolation of Burkholderia sp. HQB-1, a promising biocontrol bacteria to protect banana against Fusarium wilt through phenazine-1-carboxylic acid secretion. Front. Microbiol. 11:605152. 10.3389/fmicb.2020.605152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Postel S., Kemmerling B., Ludewig U. (2014). Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ. 37 1404–1414. 10.1111/pce.12244 [DOI] [PubMed] [Google Scholar]
- Yang M., Yuan Y., Huang H., Ye C., Guo C., Xu Y., et al. (2019). Steaming combined with biochar application eliminates negative plant-soil feedback for sanqi cultivation. Soil Tillage Res. 189 189–198. 10.1016/j.still.2019.02.006 [DOI] [Google Scholar]
- Yeoh Y., Paungfoo-Lonhienne C., Dennis P., Robinson N., Ragan M., Schmidt S., et al. (2016). The core root microbiome of sugarcanes cultivated under varying nitrogen fertilizer application. Environ. Microbiol. 18 1338–1351. 10.1111/1462-2920.12925 [DOI] [PubMed] [Google Scholar]
- Zarraonaindia I., Owens S. M., Weisenhorn P., West K., Gilbert J. A. (2015). The soil microbiome influences grapevine-associated microbiota. mBio 6 e02527–14. 10.1128/mBio.02527-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Li W., Xia E., Zhang Q., Liu Y., Zhang Y., et al. (2017). The Medicinal herb Panax notoginseng genome provides insights into ginsenoside biosynthesis and genome evolution. Mol. Plant 10 903–907. 10.1016/j.molp.2017.02.011 [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Cheng Y. X., Ma Y. N., Chen C. J., Xu F. R., Dong X. (2018). Role of phenolic acids from the rhizosphere soils of Panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules 23:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Hao J., Xing J., Rui Y., Wang S. J. (2016). Proteomic analysis of phloem proteins leads to the identification of potential candidates for JA-mediated RKN-resistant elements in Solanum lycopersicum. J. Plant Growth Regul. 36 1–10. [Google Scholar]
- Zhao Y. P., Lin S., Chu L., Gao J. T., Azeem S., Lin W. (2016). Insight into structure dynamics of soil microbiota mediated by the richness of replanted Pseudostellaria heterophylla. Sci. Rep. 6:26175. 10.1038/srep26175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. M., Cheng Y. X., Ma Y. N., Chen C. J., Xu F. R. (2018). Role of phenolic acids from the rhizosphere soils of Panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules 23:819. 10.3390/molecules23040819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia R., Nawaz M., Yousaf S., Amin I., Hakim S., Mirza M., et al. (2021). Seed inoculation of desert-plant growth-promoting rhizobacteria induce biochemical alterations and develop resistance against water stress in wheat. Physiol. Plant. 172 990–1006. 10.1111/ppl.13362 [DOI] [PubMed] [Google Scholar]
- Zucchi T. D., Almeida L. G., Dossi F. (2010). Secondary metabolites produced by Propionicimonas sp. (ENT-18) induce histological abnormalities in the sclerotia of Sclerotinia sclerotiorum. BioControl 55 811–819. 10.1007/s10526-010-9295-9 [DOI] [Google Scholar]
- Zuo J., Zu M., Liu L., Song X., Yuan Y. (2021). Composition and diversity of bacterial communities in the rhizosphere of the Chinese medicinal herb Dendrobium. BMC Plant Biol. 21:127. 10.1186/s12870-021-02893-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alpha diversity dilution curves. Each group contained six samples. The abscissa is the total number of sequences, while the ordinate represents the number of OTUs. (A) Healthy and disease rhizosphere soil chao1 alpha diversity dilution curves. (B) Healthy and disease rhizosphere soil observed alpha diversity dilution curves. (C) Healthy and disease root endophyte chao1 alpha diversity dilution curves. (D) Healthy and disease root endophyte observed alpha diversity dilution curves.
Boxplots of diversity in the rhizosphere soil and root endophyte group. (A) ACE index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (B) Chao1 index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (C) Invsimpson index box plot based on the rhizosphere soil and root endophyte bacteria species abundance. (D) Simpson index box plot based on the rhizosphere soil and root endophyte bacteria species abundance.
Annotation results of bacteria with significantly different functional descriptions. (A) Amino acid transport and metabolism, cell motility, posttranslational modification, protein turnover, and chaperones, and unknown function. (B) Antibiotic gene percentage of each group. (C) Venn diagram of KEGG orthologs (KO) functional annotation results for each group.
Quantitative heat map of the bins in each group. (A) Quantitative heat map of the bins in healthy rhizosphere soil. (B) Quantitative heat map of the bins in disease rhizosphere soil. (C) Quantitative heat map of the bins in healthy root endophyte. (D) Quantitative heat map of the bins in disease root endophyte.
Vitro infection results of Pseudomonas fluorescens.
Sample and data information.
Eukaryote information for each sample.
Function annotation information.
Genomic and classification information of the bins in each group.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: National Center for Biotechnology Information (NCBI) BioProject database under accession number PRJNA753694.