Abstract
Unprecedented advances have been made in cancer treatment with the use of immune checkpoint blockade (ICB). However, responses are limited to a subset of patients, and immune-related adverse events (irAEs) can be problematic, requiring treatment discontinuation. Iterative insights into factors intrinsic and extrinsic to the host that impact ICB response and toxicity are critically needed. Our understanding of the impact of host-intrinsic factors (such as the host genome, epigenome, and immunity) has evolved substantially over the past decade, with greater insights on these factors and on tumor and immune co-evolution. Additionally, we are beginning to understand the impact of acute and cumulative exposures--both internal and external to the host (i.e., the exposome)--on host physiology and response to treatment. Together these represent the current day hallmarks of response, resistance, and toxicity to ICB. Opportunities built on these hallmarks are duly warranted.
Wargo eTOC blurb
Immune checkpoint blockade has revolutionized cancer therapeutics, but it doesn’t work for everyone. It may cause unacceptable immune-related adverse events, or tumors may fail to respond or develop resistance. We’re beginning to understand the biological reasons why.
Introduction
Over the course of cancer development and progression, tumors evolve and may exhibit a variety of mechanisms to evade tumor immunosurveillance and to suppress anti-tumor immune responses. A major mechanism underlying tumor immune evasion involves engagement of the immune checkpoint pathways. Under physiological conditions, immune checkpoint molecules regulate the immune system through stimulation and inhibition of immune responses in order to dampen the immune response following successful mitigation of an infection or other threats. However, these immune checkpoint interactions may also be engaged in the setting of cancer, with growing efforts to target these to enhance anti-tumor immunity (Pardoll, 2012; Wykes and Lewin, 2018). In this review, we provide a summary of progress made over the past decade with regard to our understanding of mechanisms of response and resistance to immune checkpoint blockade (ICB). We describe different immune checkpoints and ICB strategies and discuss the role of various host-intrinsic and host-extrinsic factors in developing resistance to ICB and also provide insights into potential determinants of toxicity to ICB. Lastly, we explore the growing diagnostic and therapeutic strategies to enhance response to ICB and abrogate toxicity.
1. Immune checkpoints and checkpoint blockade strategies
1.1. Cytotoxic T lymphocyte-associated protein 4 (CTLA-4)
The immune system operates in a dynamic state of equilibrium. During the initial steps of T cell activation in lymphoid tissues, naïve T cells encounter new antigens through interaction of T cell receptors (TCRs) with major histocompatibility complex (MHC)-bound antigens on dendritic cells (DCs). Successful activation of T cells relies on the amplification of the antigen recognition signal through the interaction of a co-stimulatory checkpoint, CD28, on T cells with ligands CD80 (B7.1) and CD86 (B7.2) on DCs (Rudd et al., 2009). To prevent uncontrolled expansion of activated T cells, this activation signal is counteracted by an inhibitory checkpoint, CTLA-4 (a.k.a. CD152) on T cells, which binds to CD80/86 ligands with an affinity higher than that of CD28 and suppresses the signal (Rudd et al., 2009). The CTLA-4 regulatory effects mainly temper the activation of CD4+ helper T cells while boosting regulatory T cells (Tregs) (Doyle et al., 2001; Wing et al., 2008), leading to a pro-tumor immunosuppressive phenotype. As such, strategies to target and antagonize CTLA-4 have emerged as promising options to enhance anti-tumor immunity. Initial preclinical studies demonstrated that blockade of CTLA-4 with an antibody led to an enhanced and a long-lasting anti-tumor immune response and regression of immunogenic tumors (Leach et al., 1996; van Elsas et al., 1999). While significant autoimmunity was observed in pre-clinical models in which CTLA-4 was completely absent (Tivol et al., 1995; Waterhouse et al., 1995), CTLA-4 blockade did not demonstrate substantial autoimmunity (Leach et al., 1996; Rowshanravan et al., 2018). Based on these preclinical findings, several clinical trials were initiated to evaluate the therapeutic effect of humanized CTLA-4 antibodies such as Ipilimumab and Tremelimumab for advanced melanoma, eventually leading to the US Food and Drug Administration (FDA) approval of Ipilimumab (Camacho et al., 2009; Hodi et al., 2003; Hodi et al., 2010; Kirkwood et al., 2010; O'Day et al., 2010; Ribas et al., 2005). At a time when no other therapeutic option could increase the survival of advanced melanoma patients, Ipilimumab was associated with long-term survival effects (1-year and 2-year survival rate of 45.6% and 23.5%, respectively). Treatment with Ipilimumab was associated with immune-related adverse events (irAEs) in a surprisingly high 60% of patients (Hodi et al., 2010); this demonstrates a limitation of current-day preclinical models in predicting rates of irAEs in patients.
1.2. Programmed cell death protein 1 (PD-1)
Regulatory checkpoint pathways are also active in peripheral tissues where they act on a variety of immune cell types to prevent autoimmunity and tissue damage from inflammation. PD-1 (a.k.a. CD279) is expressed on activated T cells as well as other cells including but not limited to B cells, natural killer (NK) cells, and myeloid cells (Hsu et al., 2018; Nam et al., 2019). Upon interaction with its ligands, programmed death-ligand 1 (PD-L1; a.k.a. B7-H1 or CD274) and PD-L2 (a.k.a. B7-DC or CD273), it can diminish immune responses (Freeman et al., 2000; Latchman et al., 2001). In the tumor microenvironment, PD-L1 and to a lesser extent PD-L2 are expressed by tumor cells, although their expression pattern is heterogeneous and varies between different tumor types (Yearley et al., 2017). Interaction of tumor PD-L1 and PD-L2 with PD-1 on tumor infiltrating lymphocytes (TILs) has been recognized as a major mechanism of tumor immune evasion and therefore, an appealing target for therapeutic implications. Furthermore, the high expression of PD-1 and its ligands on TILs and tumor cells receptively, suggested that blockade of this pathway would potentially lead to less severe immune toxicity compared to CTLA-4 blockade. Initial clinical trials with PD-1 antibodies, Nivolumab and Pembrolizumab, demonstrated potent and durable anti-tumor activity and limited immune toxicity in a broad group of cancer types including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma and colorectal cancer (Brahmer et al., 2015; Garon et al., 2015; Patnaik et al., 2015; Robert et al., 2015; Topalian et al., 2014). While toxicity observed with the PD-1 blockade was less than what was observed in clinical trials of CTLA-4 blockade, the rates were higher than predicted by pre-clinical models (Hirano et al., 2005). With a similar therapeutic rationale, anti-PD-L1 antibodies such as Atezolizumab, Avelumab, and Durvalumab, have been developed and proven effective for treatment of a variety of cancers including NSCLC, urothelial carcinoma, triple negative breast cancer (TNBC) and Merkel cell carcinoma (Iwata et al., 2019; Kaufman et al., 2018; Powles et al., 2018; Rittmeyer et al., 2017). To date, these antibodies have been approved for treatment of various cancer types (Table 1), with many more currently under investigation.
Table 1.
List of different immune checkpoint inhibitors and indications.
| Disease | Stage/Disease Characteristics |
Line of Therapy |
Year approved |
Study Name NCT Number |
Phase | Response Data |
High Grade (3-5) Treatment- related Adverse Event Rate |
Reference (PMID) |
|---|---|---|---|---|---|---|---|---|
| Anti CTLA-4 Ipilimumab (Yervoy) | ||||||||
| Melanoma | Metastatic melanoma | 2nd line | 2011 | MDX010-20 NCT00094653 |
Phase 3 | ORR 10.9% mOS 10.1 mos (HR 0.66) |
10-15% | 20525992 |
| Resected Stage 3 melanoma | Adjuvant therapy | 2015 | EORTC 18071 NCT00636168 |
Phase 3 | mRFS 26.1 mos (HR 0.76) | 54% | 25840693 | |
| Anti PD-1 Nivolumab (Opdivo) | ||||||||
| Melanoma | Advanced metastatic melanoma progressed after ipilimumab or ipilimumab + targeted therapy | 2nd line | 2014 | CheckMate-037 NCT01721746 |
Phase 3 | ORR 31.7% | 9% | 25795410 |
| Resected Stage III melanoma | Adjuvant therapy | 2017 | CheckMate-238 NCT02388906 |
Phase 3 | ORR n/a mRFS not reached |
14.4% | 28891423 | |
| NSCLC | Advanced squamous NSCLC | 2nd line | 2015 | CheckMate-063 NCT01721759 |
Phase 2 | ORR 14.5% mPFS 1.9 mos mOS 8.2 mos |
17% | 25704439 |
| 2015 | CheckMate-017 NCT01642004 |
Phase 3 | ORR 20% mPFS 3.50 mos mOS 9.2 mos |
7% | 26028407 | |||
| Metastatic non-squamous NSCLC | 2nd line | 2015 | CheckMate-057 NCT01673867 |
Phase 3 | ORR 19% mPFS 2.30 mos mOS 12.2 mos |
10% | 26412456 | |
| RCC | Advanced RCC | 2nd line | 2015 | CheckMate-025 NCT01668784 |
Phase 3 | ORR 25% mPFS 4.6 mos mOS 20 mos |
19% | 26406148 |
| SCC of head and neck | Recurrent/metastatic HNSCC progressed on platinum-based therapy | 2nd line | 2016 | Checkmate-141 NCT02105636 |
Phase 3 | ORR 13.3% mPFS 2.0 mos mOS 7.5 mos |
13.1% | 27718784 |
| cHL | Relapsed cHL | 2nd line | 2016a | CheckMate-205 NCT02181738 |
Phase 2 | ORR 69% mPFS 14.7 mos mOS not reached |
21% | 29584546 |
| CRC | Relapsed/refractory MSI-hi or dMMR CRC | 2nd line | 2017a | CheckMate-142 NCT02060188 |
Phase 2 | ORR 31% | 20% | 28734759 |
| HCC | HCC previously treated with sorafinib | 2nd line | 2017a | CheckMate-040 NCT01658878 |
Phase 1/2 | ORR 14.3% mPFS 4.0 mos mOS 15 mos |
25% | 28434648 |
| Urothelial Carcinoma | Advanced urothelial carcinoma | 2nd line | 2017a | CheckMate-275 NCT02387996 |
Phase 2 | ORR19.6% mPFS 2.0 mos mOS 8.74 mos |
18% | 28131785 |
| Small Cell Lung Cancer | Metastatic SCLC | 3rd line | 2018a | CheckMate-032 NCT01928394 |
Phase 1/2 | ORR 12% | 45% | 27269741 |
| Esophageal | Esophageal SCC (advanced unresectable or metastatic after prior FU- or platinum based chemo) | 2nd line | 2020 | ATTRACTION-3 NCT02569242 |
Phase 3 | ORR 21.5% mPFS 1.70 mos (HR 1.1) OS 10.9 mos (HR 0.77) |
18% | 31582355 |
| Anti PD-1 PEMBROLIZUMAB (KEYTRUDA) | ||||||||
| Melanoma | Advanced or unresectable melanoma | 2nd line | 2014 a | KEYNOTE-001 NCT01295827 |
Phase 1 | ORR 33% mPFS 4 mos mOS 23 mos |
14% | 27092830 |
| Advanced or unresectable melanoma | 2nd line | 2015 | KEYNOTE-002 NCT01704287 |
Phase 2 | ORR 25% mPFS 5.60 mos |
11% | 26115796 | |
| Advanced or unresectable melanoma | 1st line | 2015 | KEYNOTE-006 NCT01866319 |
Phase 3 | ORR 33.7% mPFS 5.5 mos HR (OS) 0.63 |
10-13% | 25891173 | |
| Resected Stage III Melanoma | Adjuvant therapy | 2019 | KEYNOTE-054 NCT02362594 |
Phase 3 | HR (RFS) 0.57 | 14.7% | 29658430 | |
| NSCLC | Advanced NSCLC | 2nd line | 2015 | KEYNOTE-001 NCT01295827 |
Phase 1/2 | |||
| KEYNOTE-010 NCT01905657 |
Phase 2/3 | ORR 19.4% mPFS 4.00 mos mOS 12.7 mos |
16% | 26712084 | ||||
| Advanced NSCLC with PD-L1 | 1st line | 2016 | KEYNOTE-024 NCT02142738 |
Phase 3 | ORR 45% mPFS 10.3 mos |
26.6% | 27718847 | |
| Advanced non-squamous NSCLC (in combination with) carboplatin-pemetrexed | 1st line | 2017a | KEYNOTE-021 NCT02039674 |
Phase 2 | ORR 55% mPFS 13 mos |
39% | 27745820 | |
| Advanced non-squamous NSCLC (in combination with pemetrexed and cisplatin/carboplatin) | 1st line | 2018 | KEYNOTE-189 NCT02578680 |
Phase 3 | ORR 47.6% mPFS 8.80 mos |
67.2% | 29658856 | |
| Advanced squamous NSCLC (in combination with carboplatin and paclitaxel or nab-paclitaxel) | 1st line | 2018 | KEYNOTE-407 NCT02775435 |
Phase 3 | ORR 58% mPFS 6.40 mos mOS 15.9 mos |
69.8% | 30280635 | |
| Advanced NSCLC with PD-L1 > 1% | 1st line | 2019 | KEYNOTE-042 NCT02220894 |
Phase 3 | ORR 27% mOS 16.7 mos HR(OS) 0.81 |
18% | 30955977 | |
| HNSCC | Recurrent/metastatic HNSCC (with PD-L1) | 2nd line | 2016a | KEYNOTE-012 NCT01848834 |
Phase 1b | ORR 16% | 17% | 28533473 |
| metastatic or recurrent unresectable HNSCC (stand-alone) | 1st line | 2019 | KEYNOTE-048 NCT02358031 |
Phase 3 | ORR 23% mPFS 2.3 mos |
7% | 31679945 | |
| metastatic or recurrent unresectable HNSCC (combined with platinum and FU) | 1st line | 2019 | KEYNOTE-048 NCT02358031 |
Phase 3 | ORR 36% mPFS 4.9 mos |
5% | 31679945 | |
| Urothelial Carcinoma | Advanced urothelial carcinoma progressed after platinum-based chemo | 2nd line | 2017 | KEYNOTE-045 NCT02256346 |
Phase 3 | ORR 21.1% mPFS 2.10 mos mOS 10.3 mos |
15% | 28212060 |
| High risk BCG-unresponsive non-muscle invasive bladder cancer (in situ) who decline cystectomy | 2nd line | 2020 | KEYNOTE-057 NCT02625961 |
Phase 2 | ORR 40.6% | 12.7% | DOI:10.1200/JCO.2020.38.15_suppl.5041 | |
| All solid tumors classified as MSI-high or dMMR | Metastatic | 2nd line | 2017a | KEYNOTE-016 NCT01876511 |
Phase 2 | ORR 53% | 20% | 26028255 |
| cHL | Refractory/relapsed cHL | 2nd line | 2017a | KEYNOTE-087 NCT02453594 |
Phase 2 | ORR 69% | ~10% | 28441111 |
| Esophageal | Advanced/recurrent stomach and gastroesophageal cancer | 3rd line | 2017a | KEYNOTE-059 NCT02335411 |
Phase 2 | ORR 11.6% mPFS 2.0 mos |
17.8% | 29543932 |
| Advanced esophageal | 2nd line | 2019 | KEYNOTE-180 NCT02558687 |
Phase 2 | ORR 9.9% in patients with PD-L1 CPS > 10 mPFS 2.0 mos mOS 5.8 mos |
12.4% | 30570649 | |
| KEYNOTE-18 NCT02564263 |
Phase 3 | ORR 22% In patients with PD-L1 CPS > 10 mPFS 2.6 mos HR (PFS) 0.69 mOS 9.3 mos HR (OS) 0.64 |
18% | 33026938 | ||||
| Cervical Cancer | Previously treated advanced cervical cancer | 2nd line | 2018a | KEYNOTE-158 NCT02628067 |
Phase 2 | ORR 12.2 % | 12.2% | 30943124 |
| MCC | Recurrent or locally advanced MCC | 1st line | 2018a | CITN-09/KEYNOTE-017 NCT02267603 |
Phase 2 | ORR 56% mPFS 16.8 mos |
28% | 30726175 |
| PMBCL | Relapsed/refractory PMBCL | 3rd line | 2018a | KEYNOTE-170 NCT02576990 |
Phase 2 | ORR 45% | 23% | 31609651 |
| RCC | Advanced RCC (with axitinib) | 1st line | 2019 | KeyNote-426 NCT02853331 |
Phase 3 | ORR 59.3 PFS 15.1 mos HR (PFS) 0.69 HR (OS) 0.53 |
75.6% | 30779529 |
| CRC | MSI high dMMR colorectal cancer | 1st line | 2020 | KEYNOTE-177 NCT02563002 |
Phase 3 | ORR 44% mPFS 16.5 mos |
22% | 33264544 |
| Cutaneous SCC | Recurrent/metastatic cutaneous SCC | 1st line | 2020 | KEYNOTE-629 NCT03284424 |
Phase 2 | ORR 34% mPFS 6.9 mos |
5.7% | 32673170 |
| Solid tumors | Solid tumors with high TMB | 2nd line | 2020 | KEYNOTE-158 NCT02628067 |
Phase 2 | ORR 29% mPFS 2.1 mos mOS 11.7 mos |
15% | 32919526 |
| Anti PD-1 Cemiplimab (Libtayo) | ||||||||
| SCC | Cutaneous SCC | 1st line | 2018a |
NCT02383212
NCT02760498 |
Phase 1 Phase 2 |
ORR 46.3% | 19.2% |
29863979
31952975 |
| Anti PD-L1 Atezolizumab (Tecentriq) | ||||||||
| NSCLC | Metastatic resistant NSCLC progressive on platinum therapies | 2nd line | 2016 | POPLAR NCT01903993 |
Phase 2 | ORR 15% mPFS 2.70 mOS 12.6 mos |
11% | 26970723 |
| OAK NCT02008227 |
Phase 3 | mOS 13.8 mos | 15% | 27979383 | ||||
| Metastatic non-squamous NSCLC (combined with bevacizumab and carboplatin/paclitaxel) | 1st line | 2018 | IMpower150 NCT02366143 |
Phase 3 | ORR 56% mPFS 8.50 mos HR 19.2 mos (HR0.71) |
57% | 29863955 | |
| Metastatic resistant NSCLC with carboplatin/nab-paclitaxel | 1st line | 2019 | IMpower130 NCT02367781 |
Phase 3 | ORR49.2% mOS 18.6 mos (0.80) |
73.2% | 31122901 | |
| Metastatic resistant NSCLC | 1st line | 2020 | IMpower110 NCT02409342 |
Phase 3 | mPFS 5.7 mos mOS 17.5 mos |
30.1% (Grade 3-4) 3.8% (Grade 5) |
32997907 | |
| Urothelial carcinoma | Urothelial carcinoma failed treatment with cisplatin | 2nd line | 2016a | IMvigor210 (Cohort 2) NCT02108652 |
Phase 2 | ORR 14.8% mPFS 2.1 mos mOS 11.4 mos |
16% | 26952546 |
| Urothelial carcinoma Carcinoma unable to receive cisplatin | 1st line | 2017a | IMvigor 210 (Cohort 1) NCT02951767 |
Phase 2 | ORR 23.5% mPFS 2.70 mos mOS 15.9 mos |
16% | 27939400 | |
| Small Cell Lung Cancer | Advanced SCLC (combined with carboplatin/etoposide) | 1st line | 2019 | IMpower133 NCT02763579 |
Phase 3 | ORR 60.2% mPFS 5.20 mos HR (PFS) 0.77 mOS 12.3 mos HR OS 0.7 |
37% | 30280641 |
| Breast cancer | Unresectable or metastatic TNBC (combined with nab-paclitaxel) | 1st line | 2019a | IMpassion NCT02425891 |
Phase 3 | ORR 53% mPFS 7.50 mos (HR 0.62) mOS 21.3 mos |
48.7% | 30345906 |
| HCC | HCC (unresectable or metastatic) combined with bevacizumab | 1st line | 2020 | IMbrave150 NCT03434379 |
Phase 3 | ORR 65% mPFS 6.80 mos HR (PFS) 0.59 HR (OS) 0.58 |
56.5% | 32402160 |
| Melanoma | Advanced melanoma (combined with cobimetinib and vemurafenib) | 1st line | 2020 | IMspire150 NCT02908672 |
Phase 3 | ORR 66% mPFS 15.1 mos HR (mPFS) 0.7800 |
79% | 32534646 |
| Anti PD-L1 Avelumab (Bavencio) | ||||||||
| Urothelial Carcinoma | Locally advanced/metastatic urothelial carcinoma after failure with platinum agents | 2nd line | 2017a | JAVELIN Solid Tumor NCT01772004 |
Phase dose expansion | ORR 17% mPFS 1.50 mos mOS 6.5 mos |
8% | 29217288 |
| Locally advanced/metastatic urothelial carcinoma without progression on platinum | 1st line maintenance | 2020 | JAVELIN Bladder 100 NCT02603432 |
Phase 2 (Phase 3) |
mPFS 3.7 mos (HR 0.62) mOS 21.4 mos (HR 0.69) |
47.4% | DOI:10.1200/JCO.2020.38.18_suppl.LBA1 | |
| MCC | MCC after failed chemotherapy | 2nd line | 2017a | JAVELIN Merkel 200 NCT02155647 |
Phase 2 | OR 33% mOS 12.9 mos |
20.5% | 27592805, 29347993 |
| RCC | Advanced RCC (combined with axitinib) | 1st line | 2019 | JAVELIN Renal 10 NCT02684006 |
Phase 3 | ORR 51.4% mPFS 13.80 mos (HR 0.69) |
71.2% | 30779531 |
| Anti PD-L1 Durvalumab (Imfinzi) | ||||||||
| Urothelial Carcinoma | Locally advanced/metastatic urothelial carcinoma | 2nd line | 2017a | NCT01693562 | Phase 1/2 | ORR 17.8% mPFS 1.5 mos mOS 18.2 mos |
6.8% | 28817753 |
| NSCLC | Unresectable, stage 3 NSCLC (stable following definitive chemoradiation) | Adjuvant | 2018 | PACIFIC NCT02125461 |
Phase 3 | mPFS 16.8 mos | 29.9% | 28885881 |
| SCLC | Extensive SCLC (with etoposide and carbo-/cis-platin) | 1st line | 2020 | CASPIAN NCT03043872 |
Phase 3 | ORR 68% mPFS 5.10 mos OS 13 mos HR (OS) 0.73 |
62% | 31590988 |
| Combination Nivolumab and Ipilimumab | ||||||||
| Melanoma | Advanced melanoma | 1st line | 2015a | CheckMate-069 NCT01844505 |
Phase 2 | ORR 53% | 36% | 25891304 |
| Advanced melanoma | 1st line | 2016 | CheckMate-067 NCT01844505 |
Phase 3 | ORR 57.6% mPFS 11.5 mos mOS > 36 mos |
55% |
26027431,
28889792 |
|
| CRC | Relapsed/refractory CRC dMMR or MSI high | 3rd line | 2018 a | CheckMate-142 NCT02060188 |
Phase 2 | ORR 55% | 32% | 29355075 |
| RCC | Advanced RCC | 1st line | 2018 | CheckMate-214 NCT02231749 |
Phase 3 | ORR 42% mPFS 11.60 mos mOS not reached |
46% | 29562145 |
| NSCLC | Metastatic or recurrent NSCLC (+PDL1 expression with no ALK or EGFR mutations) | 1st line | 2020 | CheckMate-227 NCT02477826 |
Phase 3 | ORR 35.9% mPFS 5.1 mos mOS 17.1 mos (HR 0.70) |
32.8% | 31562796 |
| Metastatic or recurrent NSCLC in combination with 2 cycles of platinum doublet therapy | 1st line | 2020 | CheckMate-9LA NCT03215706 |
Phase 3 | ORR 38% mPFS 6.7 mos (HR 0.68) mOS 15.6 mos (HR 0.66) |
47% | 33476593 | |
| HCC | HCC previously treated with sorafenib | 2nd line | 2020a | CheckMate-040 NCT01658878 |
Phase 1/2 | ORR 31% | 29-53% | 33001135 |
| Pleural mesothelioma | Metastatic | 1st line | 2020 | CheckMate-743 NCT02899299 |
Phase 3 | ORR 40% mPFS 6.8 (HR 1.0) mOS 18.1 mos (HR 0.74) |
30.3% | 33485464 |
ORR, overall or objective response rate, mPFS median progression-free survival, mOS median overall survival, HR hazard ratio, mos months, FDA Food and drug administration, SCLC small cell lung cancer, HCC hepatocellular carcinoma, CRC colorectal cancer, SCC squamous cell carcinoma, HNSCC head and neck squamous cell carcinoma, HCC hepatocellular carcinoma, cHL classical Hodgkin’s lymphoma, RCC renal cell carcinoma, NSCLC non-small cell lung cancer, MCC Merkel cell carcinoma, PMBCL primary mediastinal B-cell lymphoma, MSI-hi microsatellite instability high, dMMR mismatch repair deficient, TMB tumor mutational burden, EGFR epidermal growth factor receptor, ALK anaplastic lymphoma kinase, SCC squamous cell carcinoma, TNBC triple-negative breast cancer,
Indicates accelerated approval
1.3. Other immune checkpoints: negative immune regulation
Apart from these well-studied molecules, several novel immune checkpoint molecules have been introduced/revisited over the past decade (Figure 1) with mechanistic insights gained and the potential for therapeutic targeting. The majority of these molecules exhibit a negative immunoregulatory effect in the context of cancer. Lymphocyte activation gene-3 (LAG-3 or CD223) is expressed on a variety of immune cells including activated T cells, Tregs, B cells, NK cells and DCs (Andreae et al., 2002; Triebel et al., 1990) and is also active in a soluble form when shed by a disintegrin and metalloproteinase domain-containing proteins (ADAMs). LAG3 interacts with several molecules including MHCII, Galectin-3 and α-synuclein (Baixeras et al., 1992; Mao et al., 2016); it is known to have an inhibitory role on CD8+ T cell function (Matsuzaki et al., 2010) and increases the immunosuppressive behavior of Tregs (Yano et al., 2019). Different approaches for blockade of LAG-3 in combination with anti-PD-1 treatment are currently under evaluation in a number of clinical trials as potential novel ICBs (NCT02614833, NCT03625323, NCT01968109, NCT03470922, among others). Initial data from the Phase2/3 RELATIVITY-047 trial (NCT03470922) shows improved progression-free survival in patients with metastatic or unresected melanoma treated with anti-Lag-3 combined with anti-PD-1 therapy as compared to anti-PD-1 therapy alone).
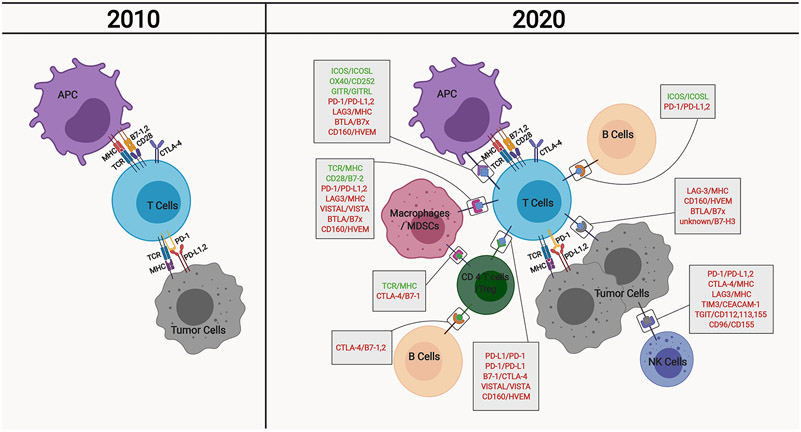
Figure 1. Evolution of our understanding of cellular interactions contributing to tumor immunity.
The basic description of anti-tumor immunity encompasses tumor antigen presentation to T cells via antigen presenting cells (APCs) or tumor cells, followed by T cell activation against tumor cells, which involves a number of costimulatory and inhibitory molecules including CD28, CTLA-4, and PD-1. Over the years, our understanding of anti-tumor immunity has evolved tremendously, owing to the identification of several other regulatory molecules on these and other immune cell types. APC, antigen presenting cells; MDSCs, myeloid-derived suppressor cells; Treg, regulatory T cells; NK cells, natural killer cells; MHC, major histocompatibility complex; TCR, T cell receptor; CTLA4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1,2, programmed death-ligand 1,2; ICOS, Inducible T-cell COStimulator; ICOSL, ICOS ligand; GITR, Glucocorticoid-Induced TNFR-Related; GITRL, GITR ligand; LAG3, lymphocyte activation gene 3; BTLA, B- and T-lymphocyte attenuator; HVEM, Herpes Virus Entry Mediator ; VISTA, V-domain Ig suppressor of T cell activation; VISTAL, VISTA ligand; TIM3, T-cell immunoglobulin domain and mucin domain 3; CEACAM-1, carcinoembryonic antigen-related cell adhesion molecule 1; TGIT, T cell Ig and ITIM domain.
T cell immunoglobulin and mucin-domain containing-3 (TIM-3) is another immune checkpoint molecule that can be expressed on a number of immune cells including CD4+ and CD8+ T cells, Tregs, DCs, and NK cells (de Mingo Pulido et al., 2018; Monney et al., 2002; Xu et al., 2015) as well as non-immune cells such as tumor-associated endothelial cells (Huang et al., 2010). Interaction between TIM-3 and its four ligands (galectin 9, high mobility group protein B1, phosphatidyl serine, and carcinoembryonic antigen cell adhesion molecule 1) can diminish anti-tumor immunity through a variety of mechanisms including inducing CD8+ T cell death and exhaustion (Huang et al., 2015). Several antibodies against TIM-3 are in phase I trials (NCT03307785, NCT03680508, NCT02608268, among others) and the reported initial results demonstrate tolerability and promising efficacy when used in combination with anti-PD-1 treatment (NCT02817633).
While LAG-3 and TIM-3 exhibit a broader expression pattern, T cell immunoglobulin and ITIM domain (TIGIT) is an immune checkpoint molecule that is almost exclusively expressed on T cells and NK cells and interacts with its ligands CD155 and CD112 to exert immunosuppressive effects (Stanietsky et al., 2009; Yu et al., 2009). TIGIT can also induce immunosuppressive activity in DCs (Yu et al., 2009) and suppresses the cytotoxicity of NK cells (Liu et al., 2013). Monoclonal antibodies against TIGIT are being tested as single agents or combination therapy with anti-PD-1 and anti-PD-L1 (NCT04294810, NCT04256421 among others). Combination of Tiragolumab (anti-TIGIT) and atezolizumab (PD-L1 antibody) improved overall response rate (37% compared to 21% for atezolizumab alone) in metastatic NSCLC patients with high tumor PD-L1 expression and was recently granted Breakthrough Therapy Designation by the FDA (CITYSCAPE, NCT03563716).
VISTA is another inhibitory checkpoint molecule that is largely and constitutively expressed on myeloid cells and also on T cells, and NK cells (Blando et al., 2019; Gao et al., 2017; Wang et al., 2019a). VSIG-3 has been reported as a ligand for VISTA (Wang et al., 2019a), however other interacting partners are yet to be discovered. VISTA is known as a PD-1 homolog; however, it acts through a non-redundant pathway to exert immunosuppressive effects on T cells (Liu et al., 2015) and, therefore, presents an opportunity as a target for immunotherapy. Other inhibitory checkpoint molecules that are being studied as potential therapeutic targets include B7-H3 (CD276) (NCT02628535, NCT03406949), B and T lymphocyte attenuator (BTLA or CD272) (NCT04137900), and sialic acid-binding immunoglobulin-like lectin 15 (Siglec-15) (NCT03665285).
1.4. Other immune checkpoints: positive immune regulation
Checkpoint molecules with positive immunoregulatory effects have also been considered for cancer immunotherapy applications. Immune co-stimulator (ICOS) is a co-stimulatory molecule expressed on T cells, which enhances function and expansion of CD8+ T cells and Tregs (Fu et al., 2011; Hutloff et al., 1999; Liakou et al., 2008). ICOS agonist monoclonal antobodies are currently under investigation as single agents and in combination with anti-PD-1 or anti-CTLA-4 treatments (NCT02904226, NCT02723955, NCT03251924). Checkpoint molecules belonging to the tumor necrosis factor receptor (TNF) superfamily such as glucocorticoid-induced TNFR-related gene (GITR) and OX40 have also been introduced as stimulatory factors and are being assessed as therapeutic targets. GITR is expressed by effector T cells and Tregs, NK cells and to a lower extent by B cells and myeloid cells. GITR can reduce T cell apoptosis and increase T cell activity through its interaction with its ligand (GITRL) (McHugh et al., 2002; Shimizu et al., 2002). OX40 is expressed on T cells transiently after T cell antigen recognition and can also be expressed on a variety of immune and non-immune cells such as endothelial cells (Calderhead et al., 1993). OX40 plays a complex role in promoting anti-tumor immunity. It enhances T cell expansion at a later stage compared to CD28 and further regulates T helper responses (Flynn et al., 1998; Sharpe and Freeman, 2002). To date, several agonist antibodies for GITR (NCT02598960, NCT01239134, NCT02628574, among others) and OX40 (NCT01862900, NCT02315066, NCT02410512, among others) have been developed and are currently under investigation.
The mechanisms underlying the effects of these positive immune regulators and their interactomes have yet to be thoroughly described. Further basic and translational studies are encouraged to unravel the unknowns including potential roles in homeostatic or active immune responses and to provide opportunities for novel therapeutic strategies.
2. Factors impacting response and resistance to ICB
The scientific discoveries in tumor immunology and the resultant breakthrough concept of harnessing the immune system to treat cancer have brought considerable clinical benefits to cancer patients and tremendously advanced the field of oncology. Nevertheless, several challenges remain associated with immune checkpoint inhibitors that need to be addressed in order to broaden their application. One major shortcoming of current immune checkpoint inhibitors is the lack of response in certain cancers such as glioblastoma and pancreatic cancer, potentially attributed to their low inherent immunogenicity. Within those cancer types for which ICB has proven efficacy such as melanoma, potent and durable response has only been limited to a subgroup of patients, with several patients demonstrating a lack of initial response to treatment (i.e., primary resistance). Furthermore, patients with initial promising response to treatment can develop resistance overtime (i.e., acquired resistance), necessitating a change in therapeutic strategies. Our understanding of the mechanisms of resistance to ICB is continuously evolving as more insight is gained into the multi-dimensional interactions between the tumor, the immune system, and other systemic factors. Importantly, it is also becoming increasingly appreciated that the exposure of patients--the hosts--to environmental factors can affect their immune responses. In this review, we explore the mechanisms underlying resistance to checkpoint inhibitors under two major categories: (1) Host (patient)-intrinsic, including factors tumor-specific and systemic factors and (2) Host (patient)-extrinsic factors, including environmental factors-a.k.a, exposome.
2.1. Host-intrinsic factors
When we consider forces affecting anti-tumor immune responses, we broadly consider the tumor and the patient. The tumor itself contains several components including tumor cells and their secretome, non-tumor cells (immune cells and stromal cells) and also microbes, all of which may affect tumor immunity and response to ICB. Furthermore, we will discuss systemic factors that alter the systemic immunity of patients as contributors to response to ICB.
2.1.1. Tumor-intrinsic factors
Genetic and epigenetic defects
The genetic status of the tumor is one of the primary determinants of response to treatment with immune checkpoint inhibitors (Figure 2). Over the course of tumor development, tumor cells acquire several mutations leading to the production of mutated proteins and peptides; these mutated peptides can serve as novel antigens, termed neoantigens, that are distinct from selfantigens. In many cases, neoantigens can be immunogenic and not protected by mechanisms of self-tolerance (Schumacher and Schreiber, 2015). Moreover, tumor genetic aberrations can promote expression of self-antigens at a higher-than-normal level or at locations where those antigens are absent under normal physiological conditions. The expression of neoantigens and aberrant self-antigens within the tumor tissue can attract T cells for elimination of tumor cells and further reinforce the anti-tumor immune response elicited by immune checkpoint inhibitors. As such, tumor mutational burden (TMB), quantified as the total number of distinct mutations per coding area of tumor genome, has been used as a criterion to determine tumor antigenicity and to explain response or resistance to immune checkpoint inhibitors. Melanoma, lung, and bladder cancer, tumors commonly associated with an increased number of mutations due to environmental DNA damage, exhibit a stronger response to ICB (Yarchoan et al., 2017). TMB was shown to be higher in melanoma patients with durable responses to Ipilimumab and Tremelimumab (CTLA-4 antibody) compared to melanoma patients who did not gain clinical benefit (Snyder et al., 2014; Van Allen et al., 2015).
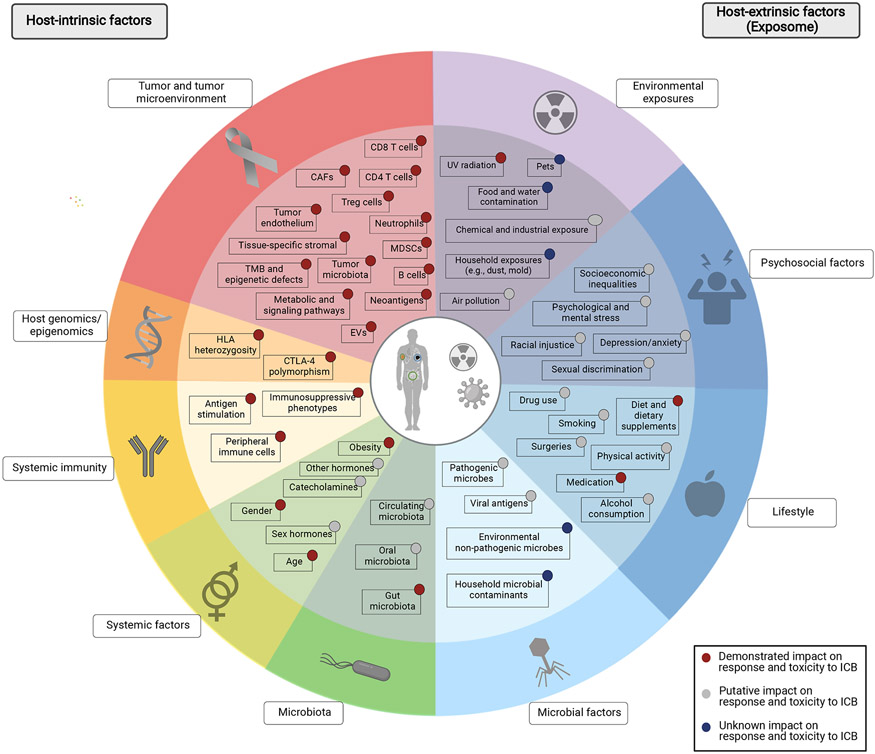
Figure 2. Factors impacting anti-tumor immunity and immunotherapy response.
Numerous factors regulate the dynamic process of tumor immunity and response to immune checkpoint blockade. Host-intrinsic factors including those inherent to the tumor cells and the tumor microenvironment (red), host genomics and epigenomics (orange), host immunity (yellow), as well as other immune-regulating factors (systemic factors, light green; microbiota, dark green) have been evaluated through a rapidly growing body of evidence. More recently, the importance of host-extrinsic factors, i.e., the exposome (shown in blue and purple) in modulating the tumor immunity and their potential impact on response to checkpoint blockade is being recognized increasingly and calls for comprehensive, albeit complicated, studies on this matter. TMB, tumor mutational burden; Treg, regulatory T cells; MDSCs, myeloid-derived suppressor cells; CAFs, cancer associated fibroblasts; EVs, extracellular vesicles; HLA, human leukocyte antigen; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; UV, ultraviolet.
Genetic defects such as DNA mismatch repair deficiencies (dMMR) and microsatellite instability (MSI) predispose tumor cells to the accumulation of somatic mutations and are associated with increased TMB (Lengauer et al., 1998) and increased susceptibility to ICB (Le et al., 2017). While these studies suggest that high TMB plays a major role in tumor response to ICB, the response to ICB is far more nuanced. In fact, it has been demonstrated that the landscape and composition of neoantigens within tumors is a stronger indicator of response to treatment in melanoma and NSCLC patients (McGranahan et al., 2016). Furthermore, genetic and epigenetic defects can induce several mechanisms of immune evasion in tumor cells that further affect response to ICB. For instance, genetic and epigenetic aberrations leading to defects in antigen presentation can promote primary and acquired resistance to ICB regardless of TMB (Snahnicanova et al., 2020; Sucker et al., 2014).
Signaling defects
Oncogenic signaling and metabolic pathways and their associated mutations have also been proven to drive immunogenic responses in various cancer types (Figure 2). Interferon (IFN) signaling through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway plays a prominent role in tumor immunity. Defects associated with this pathway and its downstream effectors have shown both negative and positive correlations with response to ICB, suggesting a dual role for this pathway in tumor immunity (Ayers et al., 2017; Gao et al., 2016).
Several studies have shown that phosphatidylinositol-3-kinase (PI3K)-activating mutations can be associated with increased expression of PD-L1 on tumor cells leading to immune evasion in glioma, breast, prostate, lung, and pancreatic cancer (Crane et al., 2009). PI3K activation as a result of phosphatase and tensin homolog (PTEN) loss was correlated with a poor response to PD-1 antibodies in melanoma and could be reversed by PI3Kβ inhibition. PTEN loss decreased the number and the cytolytic activity of CD8+ T cells in tumors and also promoted resistance to T cell-induced tumor apoptosis (Peng et al., 2016).
Activating mutations in the Wnt/β-catenin pathway can also induce resistance to ICB through altering the expression of PD-L1 and PD-L2 in a broad group of tumors such as melanoma, breast cancer, adenoid cystic carcinoma, and medulloblastoma (Bockmayr et al., 2018; Castagnoli et al., 2019). Mechanistic studies revealed that Wnt-induced decrease in the expression of the chemokine CCL4 hindered the recruitment of CD103+ DCs and T cells to the tumor microenvironment (Spranger et al., 2015).
While these and several other studies strongly support the role of signaling-associated mutations in tumor resistance to ICB, it is important to acknowledge that the signaling landscape of tumor cells is extremely complex with some overlapping and opposing pathways. The complexity of signaling is further amplified by the continuous cross talk between tumor cells and stromal and immune cells within the tumor microenvironment. Nevertheless, the large body of evidence on the role of signaling in tumor immune responses has formed the rationale to combine inhibition of signaling pathways with ICB to enhance response, which will be discussed in detail later in this review.
Extracellular vesicles
Recent studies have demonstrated a potential role for extracellular vesicles (EVs), in particular, the exosome subset of EVs, in tumor immunity and resistance to ICB (Figure 2). EVs derived from a variety of tumor types including melanoma, glioblastoma, breast, and head and neck cancer contain functional PD-L1 on their surface (Chen et al., 2018; Ricklefs et al., 2018; Theodoraki et al., 2018). Exosomal PD-L1 suppressed CD8+ T cell activity and induced T cell exhaustion in draining lymph nodes and promoted tumor growth in an anti-PD-L1 blockade-resistant prostate cancer model (Poggio et al., 2019). In cancer patients undergoing anti-PD-1 treatment, pre-treatment levels of circulating exosomal PD-L1 were higher in those who did not benefit from the treatment, reflecting the role of exosomal PD-L1 in tumor immunity and its potential association with T cell exhaustion (Chen et al., 2018). Levels of circulating PD-L1+ EVs can mirror the dynamic interaction between tumor and immune system and may serve as a promising biomarker for ICB response.
2.1.2. Tumor microenvironment: stromal cells
The tumor microenvironment harbors several non-immune stromal components including endothelial cells, fibroblasts, and tissue-specific cells, all of which contribute tremendously to the different hallmarks of cancer such as angiogenesis, invasion into the extracellular matrix (ECM) and metastasis. Growing evidence suggests that these stromal components can also contribute to mechanisms of immune evasion and resistance to ICB (Figure 2).
The high rates of angiogenesis in the tumor microenvironment and the resultant abnormal vasculature and high interstitial pressure within the tumor (Folkman, 1971) can impair the infiltration of immune cells and the penetrance of checkpoint inhibitors (Fukumura et al., 2018). Moreover, endothelial cells can express PD-L1, which can further attenuate T cell function within the tumor microenvironment (Eppihimer et al., 2002). Consistent with these studies, strategies to combine anti-angiogenic antibodies with checkpoint inhibitors have shown promising results in enhancing the anti-tumor immune response (Fukumura et al., 2018).
Cancer-associated fibroblasts (CAFs) can have dual effects on the tumor immune responses. CAF-derived transforming growth factor beta (TGFβ) signaling in the tumor microenvironment was associated with dysregulation of the ECM genes resulting in a distinct signature that correlated with higher CD8+ T cells and M1:M2 macrophage ratio. This signature was enriched in immunologically “hot” tumors across different cancer types within TCGA (Chakravarthy et al., 2018). In contrast, fibroblast activation protein (FAP)+ CAFs inhibited anti-tumor function of T cells in gastric cancer and pancreatic cancer and targeting these FAP+ subtypes enhanced tumor response to ICB (Feig et al., 2013).
Tissue-specific stromal cells can also play a role in tumor resistance to ICB. Resistance to ICB in bone metastases from prostate cancer has been attributed, at least to some extent, to the release of TGFβ following osteoclast-induced bone resorption, which reduced the number of T helper type 1 (Th1) cells within the tumor (Jiao et al., 2019). Moreover, phosphoSTAT3+ reactive astrocytes associated with metastatic brain tumors were shown to decrease CD8+ T cell activity and increased the abundance of CD74+ microglia/macrophages, promoting tumor immune evasion and suggesting a potential role in resistance to ICB (Priego et al., 2018).
2.1.3. Tumor microenvironment: immune cells
Various types of innate and adaptive immune cells reside within or infiltrate the tumor microenvironment. The dynamic cross talk between these immune cells and tumor cells define the immune status of the tumor and can promote or hinder the tumor response to ICB. Tumor immune profiles can be classified into “cold” or “hot” tumors or more precisely into “immune-inflamed”, “immune-excluded”, or “immune-desert” (Chen and Mellman, 2017). Immune-inflamed tumors are identified by the abundance of CD4+ and CD8+ T cells and their penetration into the tumor, and are often, but not always, associated with a favorable response to ICB. Immune-excluded and immune-desert tumors are respectively defined by the presence of T cells without infiltration into the tumor or the absence of T cells, and do not respond to ICB (Chen and Mellman, 2017). As tumors evolve, the tumor microenvironment gradually becomes more immunosuppressive with several components of the innate and adaptive immune system contributing to tumor immune evasion and inevitably to resistance to checkpoint inhibitors.
CD8+ effector T cells have a central role in inciting an anti-tumor immune response through the release of cytolytic factors and induction of apoptosis in tumor cells (Figure 2). The presence of CD8+ T cells at tumor margins and within the tumor prior to treatment with checkpoint inhibitors was associated with a stronger response to treatment (Tumeh et al., 2014). Accordingly, Tregs attenuate the activity of CD4+ and CD8+ T cells to maintain self-tolerance, through the secretion of immunosuppressive cytokines (including IL-2, IL-10, IL-35, TGF β) and the expression of checkpoint molecules such as CTLA-4 and PD-1 (Saleh and Elkord, 2019). Identified by the expression of CD4, CD25, and the Forkhead box P3 (FoxP3) transcription factor, Tregs are often found in abundance both in the tumor microenvironment and in circulation (Okita et al., 2005; Woo et al., 2001). The critical role of Tregs in regulation of tumor immunity was verified by preclinical studies where depletion of Tregs in a variety of tumor types could evoke an anti-tumor immune response (Shimizu et al., 1999). Due to the constitutive expression of CTLA-4 on Tregs and the high expression of PD-1 on these cells, anti-CTLA-4 and anti-PD-1 antibodies have been successful in depleting tumor-infiltrating Tregs and increasing the effector T cell (Teff) to Treg ratio in the tumor microenvironment (Curran et al., 2010; Quezada et al., 2006; Simpson et al., 2013). However, compensatory proliferation of Tregs due to incomplete depletion by checkpoint inhibitors (Kavanagh et al., 2008) and upregulation of alternative checkpoint molecules such as TIM-3 and Lag-3 in Tregs are among the Treg-driven mechanisms of resistance to ICB (Ma et al., 2018).
Myeloid-derived suppressor cells (MDSCs) are another subset of immune cells with immunosuppressive activity in the tumor microenvironment. Through different mediators such as arginase 1, Inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and peroxynitrite, MDSCs attenuate the activity of Teffs and NK cells (Gabrilovich and Nagaraj, 2009), regulate the differentiation of Tregs (Huang et al., 2006), and induce an immunosuppressive phenotype in macrophages (Sinha et al., 2007). Moreover, tumor-infiltrating MDSCs exhibit a high expression of inhibitory checkpoints such as PD-L1 in various cancer types including colon, ovarian, and bladder cancer (Lu et al., 2016). Consistent with these reports, MDSCs were shown to be targeted and depleted by ICB, resulting in an increased Teff to MDSC ratio (Retseck et al., 2018). These observations suggest a potential role for MDSCs in development of resistance to ICB. In fact, lower numbers of MDSCs in melanoma patients were associated with a better response to Ipilimumab (CTLA-4 antibody) (Meyer et al., 2014).
Tumor-associated macrophages (TAMs) also play pivotal roles in regulation of tumor immunity. Studies on the complex plasticity of TAMs suggest the presence of a spectrum of phenotypes for these cells with M1 and M2 being the two ends of the spectrum (Xue et al., 2014). While M1 macrophages classically express pro-inflammatory cytokines and promote an anti-tumor immune response, M2 macrophages are characterized by the expression of anti-inflammatory cytokines and chemokines and suppress CD8+ T cell activation, promote recruitment of Tregs, and contribute to tumor immune evasion (Xue et al., 2014). Expression of inhibitory checkpoint molecules such as PD-L1 on these cells further enhances their immunosuppressive effects (Gordon et al., 2017). Several studies have demonstrated that inhibiting the activity of M2-like TAMs and redirecting the polarization of macrophages toward the M1 phenotype can enhance response to ICB (Rodell et al., 2018; Zhu et al., 2019). A low ratio of adaptive immune response to pro-tumorigenic inflammatory gene signatures in phagocytic myeloid cells is another factor shown to be associated with resistance to PD-L1 blockade in urothelial cancer (Wang et al., 2021).
Recent studies have also demonstrated a role for B cells in tumor immunity and response to ICB. Presence of B cells in tumor was associated with a better response to neoadjuvant therapy with ICB in melanoma and renal cell carcinoma (Cabrita et al., 2020; Helmink et al., 2020). B cells were found primarily in tertiary lymphoid structures (TLS). Tumor-infiltrating B cell populations in responder tumors were enriched in memory B cells; in contrast, naïve B cells were more prominent in non-responder tumors (Helmink et al., 2020). Similarly, in soft-tissue sarcomas, the presence of TLS enriched in B cells was associated with a better response to PD-1 blockade (Petitprez et al., 2020). The mechanism(s) underlying the effect of B cells on response to ICB is poorly understood. However, present data suggests a number of potential mechanisms including activation of T cells through antigen presentation by memory B cells and B cell-derived cytokines, as well as potential contribution through producing antibodies against tumor. Future studies are required to determine the precise mechanism of action for these cells as well as the different components of the TLS in the context of immune checkpoint inhibitors.
Other innate immune cells infiltrating the tumor microenvironment such as NK cells, neutrophils and DCs can further impact anti-tumor immune responses. Tumor-infiltrated neutrophils have shown both pro- and anti-tumor phenotypes (Shaul and Fridlender, 2018). The activities of tumor-associated DCs depends significantly on the subtype of DC present. The tumor microenvironment often dictates an immature phenotype in DCs, which are not effective in activating T cells through antigen presentation and further promote an immunosuppressive microenvironment through expanding Tregs (Muenst et al., 2016). In contrast, conventional type I dendritic cells (cDC1) can effectively stimulate CD8+ T cells in tumor-draining lymph node and within the tumor (Roberts et al., 2016), creating a rationale for therapeutic efforts to increase these cells in the tumor microenvironment in order to improve response to ICBs. In line with this, NK cells have been demonstrated to increase cDC1 tumor infiltration through secretion of CCL5 and XCL1 chemokines (Bottcher et al., 2018), and targeting these chemokine pathways was suggested as a potential strategy to improve response to ICBs.
2.1.4. Tumor microenvironment: Metabolic status
The metabolic status of the tumor microenvironment is another factor that can affect tumor immunity through a variety of mechanisms. For instance, hypoxic tumors have been shown to exhibit decreased MHC-I expression in tumor cells and DCs (Ramakrishnan et al., 2014). Exhausted T cells and tumor-infiltrating NK cells also exhibit dysregulated mitochondrial biogenesis, a mechanism that has created interest in using strategies to improve mitochondrial biogenesis to promote tumor immunity (Scharping et al., 2016; Zheng et al., 2019). In addition to the prominent role of hypoxia in tumor immunity, other aspects of the tumor microenvironment that are under the influence of metabolic conditions such as altered source of nutrients (Leone et al., 2019) are actively being explored as novel mechanisms of immune evasion and resistance to ICB.
2.1.5. Tumor microenvironment: Microbial components
More recently, intratumoral microbes--yet another component of the tumor microenvironment that was heretofore underappreciated--has been shown to have significant impact on the anti-tumor immune responses and responses to ICB (Figure 2). Two recent studies demonstrate a high prevalence of microbes within a broad range of tumors, including those not physically associated with the aerodigestive tract and its commensal organisms (Nejman et al., 2020; Poore et al., 2020). Characterization of the tumor microbiota within melanoma, lung, ovarian, glioblastoma, pancreas, bone, breast tumors suggest that these microbes can be localized within the cancer cells themselves or within tumor-associated immune cells. Further, these microbes may be tumor-type specific, suggesting distinct functions that may complement the tumor (Nejman et al., 2020). Intratumoral microbes have been shown to affect nearly all aspects of cancer biology including tumor initiation/growth, invasion and metastasis (Bullman et al., 2017; Riquelme et al., 2019). Long-term survival in pancreatic cancer has been linked to increased alpha-diversity in the tumor microbiome and the presence of a particular intratumoral microbiome signature (Pseudoxanthomonas-Streptomyces-Saccharopolyspora-Bacillus clausii) (Riquelme et al., 2019). Moreover, intratumoral microbes can also alter the tumor immune microenvironment; tumor-associated microbes are associated with decreased immune cell infiltrate and a remodeling towards a more immunosuppressive environment (Helmink et al., 2019). Analyses of human samples suggest differences in the composition of the tumor microbiota between responders and non-responders for a cohort of melanoma patients undergoing immunotherapy (Nejman et al., 2020). Peptides derived from intracellular bacteria can be presented by tumor cells in the context of human HLA-I and -II and recognized by tumor-infiltrating T-cells; this is one possible mechanism by which intratumoral bacteria could directly impact anti-tumor immunity (Kalaora et al., 2021).
2.1.6. Host systemic factors
Evidence suggests a robust systemic immune response is absolutely essential to the success of cancer immunotherapies (Chen and Mellman, 2017; Spitzer et al., 2017). Computer modeling has been utilized to describe overall tumor immune “fitness” by predicting the ability of the host to present a variety of neoantigens (Luksza et al., 2017). As one example, we know that homozygosity at HLA loci leads to less diverse cohort of antigens being expressed; HLA homozygosity has been linked to decreased survival in cancer patients treated with ICB (Chowell et al., 2018).
Other host factors contribute to overall systemic immune function including the gut microbiota (Figure 2). The effects of the gut microbiota on the anti-tumor response has been demonstrated in preclinical models as well as in patients with melanoma, renal cell carcinoma and NSCLC (Chaput et al., 2017; Gopalakrishnan et al., 2018; Matson et al., 2018; Routy et al., 2018). Further, multiple studies demonstrated the negative impact of antibiotics in the context of treatment with ICB, likely due to their detrimental impact on gut microbial diversity (Derosa et al., 2018; Routy et al., 2018; Vetizou et al., 2015). Factors that can alter the gut microbiota may secondarily influence systemic immune function and anti-tumor immune response. A high-fiber diet and exercise are associated with increased diversity of the gut microbiota and enrichment of short chain fatty acids (SCFAs), both of which have been implicated in improved survival following treatment with ICB (Barton et al., 2018; McQuade et al., 2019; McQuade et al., 2020). Clinical trials wherein the gut microbiome is modulated in patients on ICB by fecal microbial transplantation, antibiotics, and/or pre/probiotics or dietary changes are ongoing (McQuade et al., 2019; McQuade et al., 2020)
While obesity is associated with oncogenesis and poor outcomes overall, it has interestingly been associated with improved responses to ICB and survival in patients with melanoma, NSCLC, and other solid tumors (Cortellini et al., 2019; Wang et al., 2019b)(Figure 2). Retrospective analyses suggest that obesity (as defined as BMI > 30) was associated with almost 40% lower risk of death in in patients with melanoma treated with ICB; interestingly, this effect was most predominant in men (McQuade et al., 2018).
Estrogens and androgens affect sex-related and non-sex related physiologic functions including systemic immunity and anti-tumor immune responses (Ozdemir and Dotto, 2019). Men have overall higher susceptibility to malignancy but better responses to ICB, potentially owing to increased PD-L1 expression (Ozdemir and Dotto, 2019). Anti-estrogen therapies are being combined with ICB in clinical trials for breast cancer, while anti-androgen therapies are being combined with ICB in prostate cancer (Ozdemir and Dotto, 2019).
2.2. Host-extrinsic factors (the exposome)
In addition to factors intrinsic to the to the host (both tumor-specific and systemic), factors external to the host--the exposome--may also impact cancer biology and response to therapies including ICB. Defined simply, the exposome includes all non-genetic determinants of health and disease (Wild, 2012); more specifically, the exposome represents “the cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behavior, and endogenous processes” (Miller and Jones, 2014). The exposome incorporates where we live, where we work, what we eat, and the medications and cosmetics we use. Psychosocial factors including chronic stress and depression/anxiety are key factors as well. Thinking even more broadly, these “exposures” themselves are related to more global social constructs including socioeconomic status, educational level, access to health care and food, as well as climate change and even racial injustice and sexual discrimination; these too comprise the exposome (Miller and Jones, 2014; Rappaport and Smith, 2010; Vermeulen et al., 2020; Wild, 2012; Zhang et al., 2019).
An individual’s exposome is in a constant but variable state of change and its total effect can only be understood as the accumulation of exposures over the context of an entire lifetime. Exposures at critical points in development such as those during early childhood may be especially important and may be distant from the point of study or effect (Wild, 2012; Zhang et al., 2019).
The all-encompassing protean nature of the exposome makes analysis a daunting task. However, to continue to limit our study to the “genetic” and ignore the “environmental” is to limit our ability to fully comprehend the complexities of human health and disease. It is only through the recognition, appreciation, and dedicated study of these external factors that we will be able to delineate their individual and cooperative contributions to overall health (Vermeulen et al., 2020)—including immune function and more specifically anti-tumor immune responses.
Already, inklings of these “exposomal” effects are beginning to be understood. As noted above, exposure to ultraviolet radiation and/or cigarette smoke can increase TMB and secondarily neoantigen levels which are proposed to explain the relatively high response rate to ICB in melanoma and NSCLC respectively (Snyder et al., 2014; Van Allen et al., 2015; Yarchoan et al., 2017). Further, chronic stress enhances tumor growth and impairs anti-tumor immune response and response to ICB in pre-clinical models potentially through the activation of β2-receptor signaling pathways by the firing of the sympathetic nervous system (Bucsek et al., 2017). Retrospective data suggests that non-selective beta-blocker use in patients being treated with ICB led to improved overall survival (Kokolus et al., 2018), which has prompted clinical studies investigating the use of beta-blockers in combination with ICB (Gandhi et al., 2021). Other cancer-related treatments (e.g., chemotherapeutic agents, anti-angiogenic agents, and radiation therapy) can be considered part of the exposome. A large body of evidence demonstrates that these therapies can induce dose-dependent immune modulating effects through a variety of mechanisms. For instance, low doses of cyclophosphamide and gemcitabine were shown to decrease the number of Tregs and myeloid suppressor cells, respectively (Lutsiak et al., 2005; Suzuki et al., 2005). Several chemotherapeutic agents have also been shown to increase the expression of PD-L1 (Fournel et al., 2019; Peng et al., 2015). Furthermore, the cytotoxic effect of chemotherapeutic agents can facilitate antigen presentation and reinforce anti-tumor immune responses (Nowak et al., 2003). Similarly, irradiation can also increase tumor antigenicity through enriching the pool of antigenic peptides (Reits et al., 2006).
The application of “omics” technologies in a longitudinal fashion in a large number of diseased individuals will be key to understanding the impact of environmental exposures on health. Exposome-wide association studies (EWAS), analogous to genome-wide association studies (GWAS), are slowly evolving (Escher et al., 2017; Jeong et al., 2018; Niedzwiecki et al., 2019; Patel et al., 2010; Vermeulen et al., 2020; Wild, 2012; Zhang et al., 2019). However, success in such endeavors require the interdisciplinary cooperation of experts in fields ranging from medicine to environmental science. Moreover, it will require new technologies and creative strategies to detect and quantify exposures (smartphone technologies, individual monitoring devices) as well as more creative collection of biological samples (including breast milk and cord blood as well as teeth and hair) and the development of robust platforms to handle such complex datasets (Escher et al., 2017; Niedzwiecki et al., 2019; Vermeulen et al., 2020; Wild, 2012; Zhang et al., 2019).
Overall, it should be noted that our rapidly evolving knowledge on the factors associated with response to ICB --both host-intrinsic and -extrinsic factors-- demonstrates the high level of complexity of interactions involved in determining response to ICB. While the relative individual and collective significance of each of these factors within the context of response to ICB remains poorly understood, a number of studies have developed frameworks and mathematical models to create a more comprehensive picture of tumor immunity and response to ICB (Blank et al., 2016; Mpekris et al., 2020). These models are extremely beneficial in guiding future pre-clinical and clinical studies to enhance our understanding of determinants of response to ICB and to improve the diagnostic and therapeutic applications.
3. Tumor evolution in the context of immunotherapy
While the genetic landscape of tumor can help shape anti-tumor immunity, the immune microenvironment may reciprocally impact the tumor genetic evolution (Jamal-Hanjani et al., 2017) (Figure 3). Various mechanisms of immune evasion present in early-stage untreated cancers could impose selection pressure on the evolving tumors through affecting neoantigens and antigen presentation (Rosenthal et al., 2019). Evolving tumor subclones with disruption in antigen presentation or neoantigen depletion at DNA and RNA levels were subject to positive selection. Notably, clonal diversities of different tumor regions in lung adenocarcinomas were negatively correlated with CD8+ T cell infiltration within those regions (Rosenthal et al., 2019). This has also been observed in other cancer types such as melanoma (Mitra et al., 2020; Reuben et al., 2017).
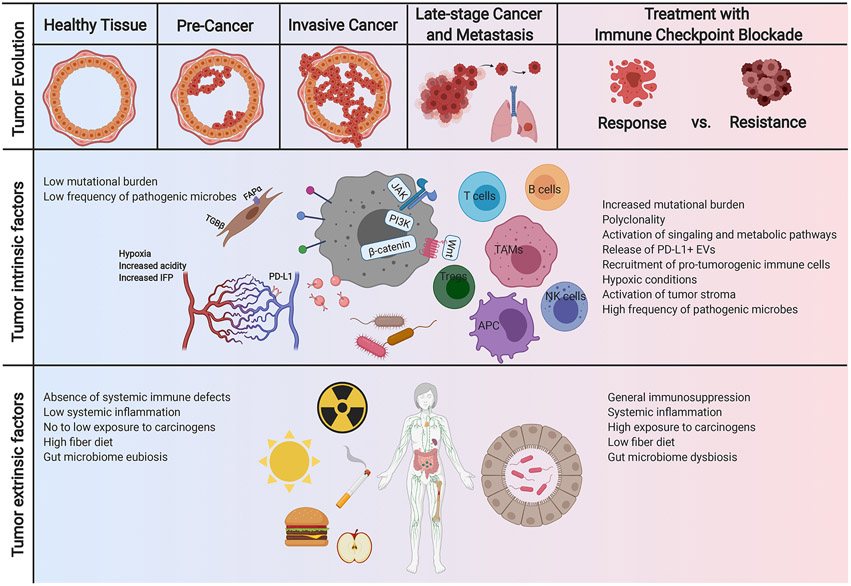
Figure 3. Coevolution of cancer and anti-tumor immunity.
This reciprocal evolution of tumor and the immune microenvironment has important clinical implications within the context of immunotherapy. As the tumor evolves, mechanisms of immune evasion can positively select the tumor subclones with low immunogenicity and disruption in antigen presentation. Furthermore, treatment with immune checkpoint blockade can also change the evolutionary landscape of the tumor, characterized by several factors such as reduction in mutational load in responders and can determine the mechanisms of resistance. TAM, tissue associated macrophages; NK cells, natural killer cells; APC, antigen presenting cells; Tregs, regulatory T cells; PD-L1, programmed death-ligand 1; TGF, tissue growth factor; FAP, fibroblast activation protein; IFP, interstitial fluid pressure; JAK, Janus kinase; PI3K, phosphatidylinositol-3-kinase; EVs, extracellular vesicles.
Furthermore, treatment with ICB has been reported to change the evolutionary landscape of the tumor. Longitudinal evaluation of tumor genetic features through the course of treatment with Nivolumab (PD-1 antibody) demonstrated a reduction in mutational load in responders, due to a reduction of neoantigen-producing mutations as opposed to synonymous mutations (Riaz et al., 2017). Favorable response to anti-PD-1 therapy was associated with reshaping the evolutionary landscape of the tumor with several clonal populations becoming undetectable on treatment, whereas T cell clones were expanded in these patients (Riaz et al., 2017). The reciprocal evolution of tumor and the immune microenvironment and their co-evolution during ICB therapy can not only define mechanisms of resistance to ICBs but can also be leveraged to develop strategies to predict response to therapy.
4. Factors impacting toxicity to ICB
The human immune system relies on a complex system of checks and balances that affords effective response to pathogens (or tumor) while preserving tolerance to non-tumor self as well as some commensal organisms. Perturbation of this homeostatic balance by ICBs can lead to a loss of self-tolerance and errant non-tumor self-directed immune activity resulting in irAEs (Figure 4).
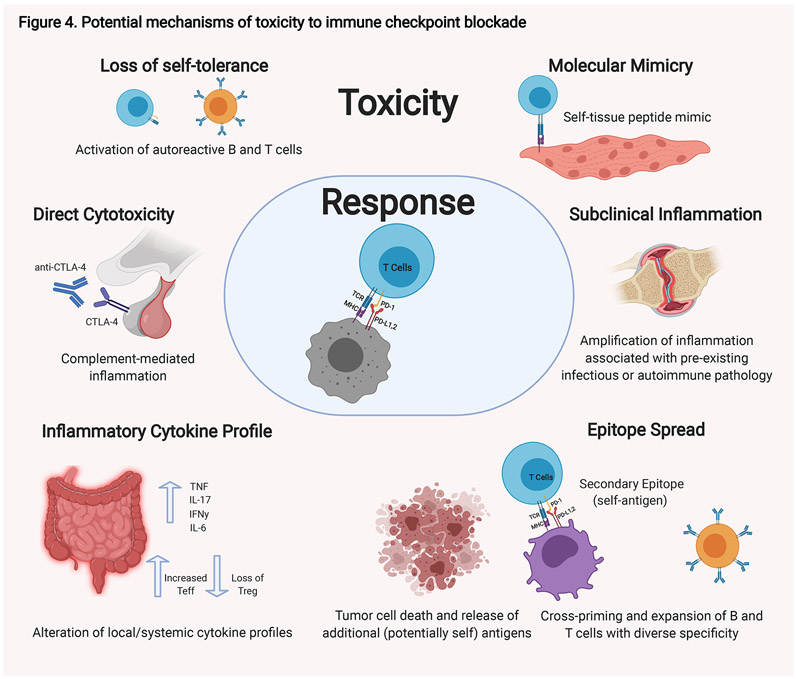
Figure 4. Potential mechanisms of toxicity to immune checkpoint blockade.
There are a number of possible mechanisms that have been proposed that contribute to the toxicities observed in some patients in response to immune checkpoint blockade. These possibilities are not mutually exclusive, and different mechanisms likely exist for different immune-related toxicities. Autoreactive T and B cells are thought to be key moieties in these processes. Autoreactive T cells could be generated de novo. These T cells are activated by professional APCs at the tumor site and reactive to tumor-specific antigens; however, they may coincidentally be reactive to peptides found on normal tissue that mimic the tumor-specific antigens. Alternatively, pre-existing autoreactive T and B cells that have escaped self-tolerance which were quiescent could be activated when self-peptides are presented through epitope spread by antigen presenting cells (APCs) at the tumor site. Immune-checkpoint blockade can result in alterations in the systemic immunity including changes in cytokine profiles. Changes in the cytokine profile within a given tissue can tip the existing balance towards inflammation. Alternative mechanisms also likely exist. For hypopituitarism, direct antibody-mediated cytotoxicity to CTLA-4 normally expressed on the pituitary gland is thought to play a role. Finally, amplification for pre-existing inflammatory or autoimmune pathologies are also possible. TNF, tumor necrosis factor; IFN, interferon; Teff, effector T cells; Treg, regulatory T cells.
4.1. Characteristics of irAEs
irAEs comprise over 70 different pathologies affecting nearly every organ system including the neurologic, genitourinary, gastrointestinal, pulmonary, cardiovascular, and integumentary systems (Pauken et al., 2019; Postow et al., 2018). The severity of pathology varies but irAEs can be severe, even fatal in some cases (Wang et al., 2018a). irAEs are common, with a low-grade (Grade 1-2) effect observed in up to more than 90% of patients, while more severe effects (Grades 3-5) can range from 20-60% (Pauken et al., 2019; Postow et al., 2018). While toxicities associated with other anti-cancer therapies including chemotherapy and radiation therapy often follow a predictable time-course, the onset of irAE varies widely with some starting days to weeks after therapy and others months (Pauken et al., 2019; Postow et al., 2018). The breadth of systems affected, severity and timing of irAEs can all vary between agents, specifically between anti-CTLA-4 agents and anti-PD-1/PD-L1 and combinations thereof (Pauken et al., 2019; Postow et al., 2018). Currently, treatment for irAEs typically involves terminating ICB and initiating a course of high-dose corticosteroids (Haanen et al., 2018; Puzanov et al., 2017; Thompson, 2018); however, more targeted therapeutic regimens are being developed (Esfahani et al., 2020). In all cases, successful treatment of irAEs relies on early recognition of pathology and an aggressive therapeutic approach often coordinated by a multidisciplinary team of specialists (Haanen et al., 2018; Puzanov et al., 2017; Thompson, 2018).
4.2. Mechanism of irAEs
Unfortunately, we lack a full mechanistic understanding of the development of irAEs. Early hints at mechanism came from preclinical mouse models including CTLA-4 knockouts (Tivol et al., 1995; Waterhouse et al., 1995), which succumb to overwhelming autoimmune lymphoproliferative disease and PD-1 knockouts (Nishimura et al., 1999) and exhibit lupus-like autoimmune disease with arthritis and cardiomyopathy. In support of this, haplo-insufficiency of CTLA-4 and polymorphisms in CTLA-4 and PD-1/PD-L1 are associated with some autoimmune diseases (Lo et al., 2016). Further, an autoimmune disease corollary to individual irAEs occasionally exists suggesting that irAEs may, in some situations, represent clinically silent autoimmune disease or autoimmune disease kept in check by normal immunosuppressive mechanisms. However, ICB treatment in pre-clinical models did not lead to overwhelming autoimmune pathology (Leach et al., 1996; Rowshanravan et al., 2018). Moreover, those irAEs with autoimmune correlates do not always share histopathologic findings in the involved tissue, other clinical findings, or demographic factor associations with the respective autoimmune phenomena (June et al., 2017; Shah et al., 2020). Moreover, some patients with clear autoimmune disease have been treated successfully with ICB without exacerbation of disease (Boland et al., 2020). Thus, many believe that irAEs represent truly unique pathologies.
The high rate of irAEs in patients as compared to those observed in early mouse studies highlights one of the limitation of current-day preclinical models, not only in predicting rates of irAEs in patients but also in furthering our mechanistic understanding. Although new preclinical models are being developed, (Liu et al., 2016; Wei et al., 2021), we must rely heavily on clinical data collection and translational research studies utilizing samples from patients on various clinical trials.
Aberrant T cell activity is thought to be a prime factor in the development of irAEs. Shared antigens between the tumor and normal tissue could lead to de novo T cell activation and precipitate on-target off-tumor effects (Figure 4). This has been observed in both myocarditis and rash where infiltrating T cells have been observed in the tumor and in the cardiac muscle or skin, respectively (Berner et al., 2019; Johnson et al., 2016). The scope of this activity can be broadened through epitope spread. Antigen or epitope spread describes the phenomenon by which tumor cell death releases additional antigens; these antigens are presented in an “immune activated” microenvironment and T cells can be activated against normal tissue (June et al., 2017; Rojas et al., 2018)(Figure 4). Finally, in some tissues, pre-existing autoreactive T cells may already exist and be kept in check through checkpoint molecules. Activation or re-activation of tissue-resident autoreactive T cells is thought to be a dominant factor in the development of irAEs (Dougan et al., 2021; June et al., 2017). TCR analysis demonstrates that a large fraction of the cytotoxic effector cells found in ICB-induced colitis derive from tissue-resident CD8+ T cells (Dougan et al., 2021; Luoma et al., 2020).
The role of the humoral immune system and B cells in causing irAEs has also been suggested; early changes in the peripheral B cell repertoire are associated with toxicity (Das et al., 2018). Nearly 25% of patients developed new autoantibodies following treatment with ICB for melanoma; however, the typical antibody targets observed in autoimmune disease are not always seen in irAEs even when the same target is affected (de Moel et al., 2019; Dougan et al., 2021; Luoma et al., 2020).
Even so, as discussed above, CTLA-4 and PD-1 are not expressed solely on T cells, and their activity may affect other immune cell components. CTLA-4 is also expressed on Tregs; targeting CTLA-4 could theoretically lead to Treg cell dysfunction or depletion as has been demonstrated in mice (Simpson et al., 2013), though not all data support this notion in humans (Dougan et al., 2021; Luoma et al., 2020). PD-1 can be expressed on some myeloid cells as well, and changes in the myeloid compartment could lead to an influx of inflammatory cells into various, distant tissues/organs and inciting organ damage (Nam et al., 2019; Strauss et al., 2020). Patients with ICB-associated colitis and myocarditis exhibit a robust, active macrophage infiltrate (Dougan et al., 2021; Luoma et al., 2020)s; macrophages also play a prominent role in ICB-induced diabetes (Hu et al., 2020). Finally, other less common effects may be secondary to on-target effects on normal tissue. The proposed mechanism for pituitary dysfunction, for example, is binding of anti-CTLA-4 agents to CTLA-4 expressed on normal tissue inciting complement mediated killing (Iwama et al., 2014).
4.3. Correlation of response and toxicity
There is significant data suggesting a correlation between response to and toxicity from ICB, though this data is somewhat mixed and may be specific to the agent used, tumor type, resulting irAE, as well as the kinetics of onset (Das and Johnson, 2019). Interestingly, a polygenic risk score (PRS) designed to calculate risk for vitiligo, psoriasis, and atopic dermatitis was predictive of response to ICBs in bladder cancer (Khan et al., 2020). Further, detection of immune activation in off-target organs by increased metabolic activity as seen on PET imaging was predictive of response (Nobashi et al., 2019).
Our ability to define an association between irAEs and response is muddied by a number of factors. For one, it remains unclear as to whether corticosteroids administered for irAEs have a detrimental effect on anti-tumor response (Das and Johnson, 2019). The effect likely depends on both the timing of administration as well as the dose. While some studies show no effect (Horvat et al., 2015), high-dose steroids administered at the initiation of therapy has been associated with decreased survival (Arbour et al., 2018).
Unfortunately, patients experiencing irAEs often have very limited options for future avenues of immunotherapy which can ultimately affect their oncologic outcome. Although some data suggests that ICBs (specifically anti PD-1/PD-L1) can be successfully reinitiated in some patients following the development and resolution of irAEs (Pollack et al., 2018; Santini et al., 2018), this is not universally true. Patients experiencing severe or recurrent irAEs are often not re-started on ICBs. This represents another confounding factor when assessing the relationship between toxicity and overall survival.
5. Strategies to enhance response and abrogate toxicity
Durable responses to ICB are only seen in a small subset of patients and vary between different cancer types. Moreover, treatment with ICB can be associated with significant side effects, skewing the risk-benefit ratio for these treatments towards an unfavorable balance. These limitations have led to several ongoing efforts to develop predictive and prognostic approaches to identify patients that would benefit from these treatments as well as therapeutic strategies to enhance response to treatment and overcome resistance and toxicity.
5.1. Precision approaches to predict response using known and novel diagnostic strategies
5.1.1. Genetic biomarkers
The predictive value of TMB, MSI, and dMMR as biomarkers of response to ICBs has been supported by several studies. Initial trials on CTLA-4 blockade demonstrated a clear clinical benefit in melanoma patients with high TMB (Snyder et al., 2014), also shown to be true for PD- 1 blockade in NSCLC (Rizvi et al., 2015). In addition, comparison of the clinical efficacy of pembrolizumab (PD-1 antibody) in colorectal and non-colorectal patients with dMMR demonstrated a significantly higher response rate and increased progression free survival (Le et al., 2015).
In 2017, FDA granted approval of pembrolizumab for treatment of advanced pediatric and adult solid tumors with high MSI or dMMR that have not responded to prior treatments and have no other alternative treatment option, a first-of-kind tissue-agnostic approval of an ICB based on a common biomarker across cancer types. This decision was made based on the results of 149 patients with high MSI or dMMR across 5 clinical trials, in whom treatment with pembrolizumab led to an overall response rate of 39.6% with duration of response of 6 months or more in 78% of patients (NCT01876511). A second tissue-agnostic approval for all advanced pediatric and adult solid tumors with high TMB (≥10 mutations/megabase) that have not responded to prior treatments and have no other alternative treatment option (based on the KEYNOTE-158 trial-NCT02628067) was later issued. Pembrolizumab was also granted approval for colorectal cancer patients with high MSI or dMMR as a first line treatment (based on the KEYNOTE-177 trial-NCT02563002).
It should be noted that, despite the mounting evidence on the benefits of these genetic factors as biomarkers of response to ICBs, association between TMB and response to ICB is not observed in all patients (Snyder et al., 2014). Moreover, the association between mutational load and a favorable response to nivolumab (PD-1 antibody) in melanoma patients was only observed in ipilimumab (CTLA-4 antibody) naïve patients, suggesting the limited predictive value of mutational load and a need for alternative biomarkers for patients who had progressed on ipilimumab prior to nivolumab treatment (Riaz et al., 2017). The need for advanced technologies to conduct these analyses also limits the applicability of these biomarkers to certain clinical settings.
5.1.2. Immunological biomarkers
Tumor-infiltrating lymphocytes
The expression level of immune markers in pre-treatment tumor samples has been evaluated as potential predictive biomarkers of response to ICBs. In metastatic melanoma patients, a higher density of intratumoral CD8+ T cells at baseline was shown to predict favorable response to anti-PD-1 treatment (Tumeh et al., 2014). Importantly, this study evaluated the spatiotemporal distribution of T cells within the tumor and demonstrated that abundance of T cells at the invasive tumor margin prior to treatment was associated with a better response, whereas upon treatment, T cell densities were increased both at the margin and within the tumor parenchyma in responding patients (Tumeh et al., 2014). In contrast to PD-1 blockade, the presence of TILs has not proven beneficial for prognostic purposes in anti-CTLA-4 treatments, reflecting the mechanism of action of this drug (Huang et al., 2011). In contrast, early on-treatment levels of TILs could predict response to CTLA-4 blockade in melanoma patients (Chen et al., 2016). Thus, early on-treatment biopsies may be an important biomarker. The International Immuno-Oncology Biomarker Working Group has made significant efforts to standardize the histological methods of assessment of TILs to further improve the reliability and reproducibility of this potential biomarker (Hendry et al., 2017).
Immune checkpoint molecules
Initial trials on anti-PD-1 and anti-PD-L1 inhibitors reported a significant association between pre-treatment intratumoral PD-L1 expression and response to treatment in a variety of tumor types including melanoma and NSCLC (Herbst et al., 2014; Reck et al., 2016; Topalian et al., 2014). Interestingly, association between PD-L1 expression and response to anti-PD-L1 treatment was only significant for PD-L1 expression on tumor-infiltrating immune cells but not tumor cells (Herbst et al., 2014). In contrast to these studies, other reports have shown that durable response can be obtained in the absence of PD-L1 expression in some patients (Daud et al., 2016). It should be noted that variability in the definition of PD-L1 positivity and methods of evaluation could account for inconsistencies in results and calls for further standardization of these criteria.
Emerging strategies
While the value of TILs and PD-L1 expression has been proven in certain cancer types, given the complexity of tumor immune responses and mechanisms of resistance to ICB, these biomarkers alone cannot fulfill the prognostic/predictive needs to improve patient selection for treatment with ICB. Identification of novel markers that can guide the treatment decision making with ICB is therefore, of significant importance and a matter of active investigation. Evaluation of melanoma patients treated with CTLA-4 inhibitor followed by PD-1 blockade, demonstrated that expression of T cell-related markers including CD4, CD3, CD8, FOXP3, and granzyme B as well as checkpoint inhibitors such as PD-1, PD-L1, and LAG3 in early on-treatment samples had a strong correlation with response to treatment (Chen et al., 2016). The AMADEUS trial (NCT03651271, ongoing) has been designed to evaluate the benefit of classifying tumors into immunologically hot and cold tumors based on CD8+ T cell density (≥15% and <15%, respectively) as a predictive biomarker to identify patients that are more likely to respond to Nivolumab (PD-1 antibody) or combination of Nivolumab and Ipilimumab (CTLA-4 antibody). In addition, this prospective exploratory study aims to identify novel biomarkers that can be used as strong predictive indicators of response to ICBs.
A high predictive value has also been demonstrated for exosomal PD-L1 in combination with CD28 (area under curve of 0.85) (Zhang et al., 2020). Further longitudinal studies in larger cohorts are warranted to verify the predictive and prognostic value of exosomal PD-L1 in the context of treatment with ICB.
Another emerging strategy for predicting and monitoring response to ICB is the use of microbiome signatures. The gut and tumor microbiome have been associated with anti-tumor responses and, in some cases, response to immune checkpoint blockade (Gopalakrishnan et al., 2018; Matson et al., 2018; Nejman et al., 2020; Riquelme et al., 2019; Routy et al., 2018). Moreover, analyses of TCGA datasets identified unique microbial signatures in blood that could distinguish between healthy and cancer patients (Poore et al., 2020). These latter findings reveal novel opportunities for the use of the circulating microbiome as a minimally invasive biomarkers in cancer.
5.2. Therapeutic strategies to promote response and overcome resistance
Built upon the growing knowledge of the variable mechanisms of resistance to ICB, several therapeutic strategies have evolved to overcome these and to promote response to ICB.
5.2.1. Modulation of epigenetic status
Different approaches have been introduced to mitigate resistance to ICBs in tumors with low immunogenicity and antigen presentation through epigenetic modulation. Treatment with DNA methyltransferase inhibitors has been shown to reverse the epigenetic suppression of MHC-I, which facilitated antigen presentation, immunogenicity, and tumor immune targeting (Luo et al., 2018). Histone deacetylase (HDAC) inhibitors in combination with DNA methyltransferase inhibitors have also resulted in an increase in anti-tumor immune responses in preclinical models (Topper et al., 2017). The effect of HDAC inhibitors on tumor immunity as a single agent is yet to be determined and their clinical efficacy in combination with ICB is currently under evaluation in a number of clinical trials (e.g., NCT02638090, NCT02619253).
5.2.2. Signaling modulators
The prominent role of tumor signaling provides a rationale for combination treatment strategies with ICB. One such strategy includes treatment of BRAF-mutated melanoma patients with a combination of atezolizumab (PD-L1 antibody) and v-raf murine sarcoma viral oncogene homolog B1 (BRAF)/ Mitogen-activated protein kinase kinase (MEK) inhibitors, which significantly increased progression-free survival (NCT02908672) (Gutzmer et al., 2020). Several other strategies have been tested in preclinical models to reverse signaling defects and metabolic stress in order to enhance response to ICB including PI3K inhibitors (Marijt et al., 2019), and cyclin-dependent kinase 4 and 6 (CDK4/6) (Goel et al., 2017). These and many other studies suggest that targeting signaling pathways holds promise as a potential strategy to improve response to ICB.
5.2.3. Cytokines
Other therapeutic strategies have focused on increasing the abundance of TILs to enhance response to ICBs. Cytokines such as IL-2 and IL-12 were previously introduced to increase intratumoral lymphocyte infiltration and anti-tumor immunity; however, these treatments were associated with severe toxicity (Panelli et al., 2004; Sangro et al., 2004). These complications have triggered several efforts to redesign the next generation cytokines for therapeutic purposes, which are currently being tested and have shown beneficial effects in combination with ICB in preclinical studies (Klein et al., 2017; Sun et al., 2019).
5.2.4. Anti-angiogenic agents
Several clinical trials have assessed the benefits of combining anti-angiogenic therapy with ICB and have demonstrated an increase in immune cell tumor infiltration and improved outcomes in patients with immune suppressed signatures compared to ICB alone (Hodi et al., 2014; McDermott et al., 2018). To date, the use of bevacizumab (anti-angiogenic agent) in combination with atezolimumab (PD-L1 antibody) has been FDA approved for unresectable or metastatic hepatocellular carcinoma in patients who have not received prior systemic therapy (Finn et al., 2020). Indications for this combination therapy is expected to be expanded in the future.
5.2.5. Chemotherapy and radiotherapy
The immune modulatory effects of treatment with chemotherapy and radiotherapy in (reviewed in (Zitvogel et al., 2008)) have formed the basis for combination strategies to boost response to ICB, which are currently under evaluation in several clinical trials. While most of these studies are in the initial safety phase, improvement in overall survival has been reported in unresectable NSCLC patients and TNBC patients treated with anti PD-L1 antibody with chemotherapy (Schmid et al., 2020; West et al., 2019). Preclinical and clinical efforts to determine the optimal treatment schedule and dosing for different cancer types are warranted to further expand the benefits of this treatment strategy.
5.2.6. Oncolytic viruses
Oncolytic viruses target and kill tumor cells through a number of different mechanisms such as inducing lysis, cytotoxicity, and stimulating anti-tumor innate and adaptive immune responses. Due to their immune stimulatory effects and their ability to change a noninflamed tumor to an inflamed microenvironment, strategies to combine oncolytic viruses with ICB have been considered for enhancing response to treatment (Chon et al., 2019). Talimogene laherparepvec is a genetically modified oncolytic virus that has been tested in combination with Ipilimumab (CTLA-4 antibody) for treatment of advanced, unresectable melanoma and was associated with an increase in objective response rate (NCT01740297). Selecting the optimal viral strain, genetic modification, and treatment strategy is key to obtaining positive results from oncolytic viruses in combination with ICB (Rojas et al., 2015).
5.2.7. Vaccines
Vaccines have been used as an approach to enhance anti-tumor immune responses and have demonstrated encouraging results in preclinical and clinical studies, leading to an expansion of the intratumoral T cell infiltration and anti-tumor immune responses (Carreno et al., 2015; Ott et al., 2017). Several efforts are ongoing to improve the efficacy of vaccines. Recent preclinical studies have shown that vaccination with CD103+ cDC1s in combination with CTLA-4 blockade were highly effective in inducing tumor regression in murine models of osteosarcoma and melanoma (Zhou et al., 2020). Furthermore, initial trials testing a combination of vaccines with ICB have shown promise (Massarelli et al., 2019); however randomized clinical trials are required to evaluate the added benefit of this combination strategy.
5.2.8. Other strategies
There is growing interest in developing other novel approaches to overcome resistance to ICB. Mounting evidence on the role of the gut and tumor microbiome, stress, and diet in tumor immunity and response to ICB (Helmink et al., 2020) has created a foundation for emerging adjunct therapies. A better understanding of the underlying mechanisms of these factors along with discovery and thorough validation of actionable targets are prerequisites for the development and successful application of these emerging strategies.
5.3. Diagnostic and therapeutic strategies to abrogate toxicity to ICB
Currently, oncologists prescribing ICB must weigh the risk of development of irAE against the benefit of ICB without any real data to guide that decision. This has fueled intensive efforts to identify potential biomarkers for the development and severity of irAEs, thereby guiding the rational prescribing of these agents or combinations of these agents as well as the development of surveillance strategies for high-risk patients allowing for earlier detection and intervention.
5.3.1. Potential biomarkers of toxicity
While genetic determinants for autoimmune disease are numerous (Hoefsmit et al., 2019), genetic pre-disposition to irAEs is less well-defined. Certain HLA types have been associated with development of various irAEs; these associations seem to be disease-specific (Cappelli et al., 2019; Hasan Ali et al., 2019). Work continues to allow for development of polygenic risk scores of patients at risk for irAEs and will require very large association studies across treatments and malignancies (Hoefsmit et al., 2019). Tumor factors including tumor mutational burden can be associated with irAEs (Bomze et al., 2019).
Dysbiosis of the gut microbiota is also associated with response as well as toxicity; increased pre-treatment levels of Bacteroidetes and richness in genetic pathways associated with polyamine transport and B vitamin biosynthesis was protective for immunotherapy-related colitis in patients with metastatic melanoma treated with ipilimumab (Dubin et al., 2016). Even more creative strategies to predict irAEs are being considered. In a proof-of-concept study, 1860 radiomic features identified in chest CTs were obtained for each patient using first- and second-order texture analysis prior to therapy; skewness and angular variance of sum of squares (measure of dispersion) were higher in patients who later developed pneumonitis (Colen et al., 2018).
A change in the immune signature of peripheral blood represents an attractive biomarker given ease of assessment. Early diversification of the circulating T cell repertoire has been associated with both response and toxicity (Oh et al., 2017) as has early clonal expansion of large numbers of CD8+ T cells (Subudhi et al., 2016). An increase in CD21-lo B cells and plasmablasts in peripheral blood early after combination therapy has also been associated with irAEs (Das et al., 2018). Increased circulating IL-17 levels at baseline in patients with locoregional metastatic melanoma (Tarhini et al., 2015), and increased IL-6 levels in patients with metastatic melanoma treated with ipilimumab (CTLA-4 antibody) are also associated with irAEs (Valpione et al., 2018). More global cytokine dysregulation as assessed by measuring the circulating levels of 11 cytokines (CYTOX score) at baseline or early on treatment has been shown to be predictive of irAEs in patients treated with anti-PD1 therapies alone or in combination with anti-CTLA-4 therapies (Lim et al., 2019).
The search for appropriate biomarkers is ongoing and include collaborative efforts with large multi-national databases including patients with both low and high-grade toxicity with appropriate controls (healthy individuals as well as those treated with ICB without developing irAEs) (Hoefsmit et al., 2019; Jing et al., 2020).
5.3.2. Therapeutic strategies to abrogate toxicity
The development of unique strategies for the treatment of irAEs, with a focus on alternatives to high-dose corticosteroids, is ongoing (Dougan et al., 2021; Esfahani et al., 2020). Dermatologic conditions can often be managed with topical steroids or other disease-specific drugs (Johnson et al., 2019; Tattersall and Leventhal, 2020); involvement of a dermatologist early for biopsy and diagnosis is key. In some cases, treatments used for some autoimmune disease corollaries of irAEs have been utilized in irAEs with success. TNF-inhibitors have been very successful in the treatment of inflammatory bowel disease as well as severe ICB-induced colitis; in fact, it may become first-line treatment for patients with severe disease (Abu-Sbeih and Wang, 2020). α4β7-integrin modulates immune cell trafficking specifically to the gut mucosa; vedolizumab is an α4β7-integrin inhibitor that has been used in both inflammatory bowel diseases as well as ICB-induced colitis (Abu-Sbeih and Wang, 2020). Finally, fecal microbiota transplantation (FMT) was originally trialed in patients with refractory infectious colitis but has also been used successfully in select cases of severe refractory ICB-induced colitis (Wang et al., 2018b).
Further mechanistic understanding of irAEs will allow us to progress from a generalized approach to the treatment of all irAEs in all patients (corticosteroids) to a more nuanced approach adapted to the immunohistopathogenesis of a particular irAE and various patient factors (Esfahani et al., 2020). Such targeted therapeutic strategies may include modulators of T and B cell activity and trafficking, innate immune components, circulating cytokines, immune-related signaling pathways, and commensal microbiota (Esfahani et al., 2020).
Another intriguing possibility is the prevention of irAEs all together by improving existing immunotherapeutic agents to limit off-target activities. One potential for more targeted immunotherapeutic approaches is the use of bi- and tri-specific antibodies (i.e., synthetic antibody-based molecules that bind to two or three different entities simultaneously. T cell based bi-specific antibodies serve “to bridge” T cells to tumor cells to facilitate more focal T cell activation (Labrijn et al., 2019). However, this is only one of many configuration of bi- specific antibodies, and, in reality, the potential for unique combinations and novel applications of this technology is near limitless (Labrijn et al., 2019). Stimulation of OX40 and CD137, both TNF superfamily costimulatory receptors, results in T cell activation, proliferation and survival; however, mAbs targeting and inhibiting either OX40 and CD137 individual result in poor efficacy and/or promote liver toxicity. Dual agonistic bispecific antibodies binding to both OX40 and CD137, however, promote FcRγ-crosslinking-independent anti-tumor activity and moreover limit liver injury; clinical trials utilizing these dual agonistic bi-specific antibodies are ongoing (Gaspar et al., 2020).
Conclusions
Paradigm-shifting discoveries in the field of cancer immunotherapy and their successful translation to treatment strategies have resulted in long-term survival of cancer patients who would have had limited treatment options otherwise. Nevertheless, the number of patients who gain clinical benefit from these treatments is still limited due to primary or acquired resistance to ICB as well as associated toxicity. Extensive efforts have been made to develop diagnostic approaches that identify patients who would benefit from ICB and therapeutic strategies that enhance response and abrogate toxicity. Nevertheless, expanding the benefits of ICB to a larger population of cancer patients requires an in depth understanding of the mechanisms underlying resistance and toxicity. The role of host-intrinsic factors in response to ICB, including tumor genetics and immune and non-immune components of the tumor microenvironment has been studied extensively and is still subject to active investigation. However, to fully elucidate these mechanisms, it is important to acknowledge that various factors including the microbiome, host systemic factors, as well as environmental exposures (the exposome) can have a prominent role in response and toxicity to ICB. Future studies are warranted to understand the individual and collective role of these factors and will facilitate the development of novel diagnostic, prognostic, and therapeutic strategies to address the current limitations associated with ICB and to improve the outcome of cancer patients.
Acknowledgements
We are grateful to Dr. Stephanie Watowich of MD Anderson Cancer Center for thoughtful comments and helpful suggestions. G.M. is supported by the National Institute of Health (1F32CA260769-01). P.S. is a member of the Parker Institute for Cancer immunotherapy. J.A.W. is supported by the National Institutes of Health (1R01CA219896-01A1), the Melanoma Research Alliance (4022024), American Association for Cancer Research Stand Up To Cancer (SU2C-AACR-IRG-19-17), and the MD Anderson Melanoma Moonshot Program. Schematic images were created with BioRender.com.
Footnotes
Declaration of Interests
B.A.H. report no conflict of interest. G.M. is a co-inventor on US patents (PCT/US2019/022194, PCT/US2020/029556, PCT/US2020/046050) relating to extracellular vesicles. P.S. reports consulting, advisory roles, and/or stocks/ownership for Achelois, Adaptive Biotechnologies, Apricity Health, BioAlta, BioNTech, Codiak Biosciences, Constellation, Dragonfly Therapeutics, Forty-Seven Inc., Hummingbird, ImaginAb, Infinity Pharma, Jounce Therapeutics, Lave Therapeutics, Lytix Biopharma, Marker Therapeutics, Oncolytics, Phenomics, and Polaris: and owns a patent licensed to Jounce Therapeutics. P.S. reports consulting or Stock Ownership or Advisory Board for Achelois, Adaptive Biotechnologies, Apricity, BioAtla, BioNTech, Candel Therapeutics, Codiak, Dragonfly, Earli, Enable Medicine, Hummingbird, ImaginAb, Jounce, Lava Therapeutics, Lytix, Marker, PBM Capital, Phenomic AI, Polaris Pharma, Time Bioventures, Trained Therapeutix, Venn Biosciences for immediate family member. J.A.W. is a co-inventor on US patent (PCT/US17/53,717) relating to the microbiome. JAW reports speaker fees from Imedex, Dava Oncology, Omniprex, Illumina, Gilead, MedImmune and BMS; consultant/advisor roles or advisory board membership for Roche-Genentech, Novartis, AstraZeneca, GSK, BMS, Merck/MSD, Biothera Pharma, and Microbiome DX; and receives clinical trial support from GSK, Roche-Genentech, BMS, and Novartis, all outside the current work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Sbeih H, and Wang Y (2020). Management Considerations for Immune Checkpoint Inhibitor-Induced Enterocolitis Based on Management of Inflammatory Bowel Disease. Inflamm Bowel Dis 26, 662–668. [DOI] [PubMed] [Google Scholar]
- Andreae S, Piras F, Burdin N, and Triebel F (2002). Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J Immunol 168, 3874–3880. [DOI] [PubMed] [Google Scholar]
- Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martinez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al. (2018). Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 36, 2872–2878. [DOI] [PubMed] [Google Scholar]
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. (2017). IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127, 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, and Piatier-Tonneau D (1992). Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 176, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, and O'Sullivan O (2018). The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 67, 625–633. [DOI] [PubMed] [Google Scholar]
- Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, et al. (2019). Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol 5, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, Kim J, Sepulveda AM, Sharp M, Maitra A, et al. (2019). Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A 116, 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Haanen JB, Ribas A, and Schumacher TN (2016). CANCER IMMUNOLOGY. The "cancer immunogram". Science 352, 658–660. [DOI] [PubMed] [Google Scholar]
- Bockmayr M, Mohme M, Klauschen F, Winkler B, Budczies J, Rutkowski S, and Schuller U (2018). Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 7, e1462430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland P, Pavlick AC, Weber J, and Sandigursky S (2020). Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J Immunother Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomze D, Hasan Ali O, Bate A, and Flatz L (2019). Association Between Immune-Related Adverse Events During Anti-PD-1 Therapy and Tumor Mutational Burden. JAMA Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, and Reis e Sousa C (2018). NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 172, 1022–1037 e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, et al. (2017). beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res 77, 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, et al. (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, et al. (2020). Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565. [DOI] [PubMed] [Google Scholar]
- Calderhead DM, Buhlmann JE, van den Eertwegh AJ, Claassen E, Noelle RJ, and Fell HP (1993). Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol 151, 5261–5271. [PubMed] [Google Scholar]
- Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, et al. (2009). Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol 27, 1075–1081. [DOI] [PubMed] [Google Scholar]
- Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, and Shah AA (2019). Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford) 58, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, et al. (2015). Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli L, Cancila V, Cordoba-Romero SL, Faraci S, Talarico G, Belmonte B, Iorio MV, Milani M, Volpari T, Chiodoni C, et al. (2019). WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 38, 4047–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy A, Khan L, Bensler NP, Bose P, and De Carvalho DD (2018). TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 9, 4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. (2017). Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 28, 1368–1379. [DOI] [PubMed] [Google Scholar]
- Chen DS, and Mellman I (2017). Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. (2016). Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 6, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et al. (2018). Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, Hajjar J, Suarez-Almazor ME, Alshawa A, Hong DS, et al. (2018). Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs 36, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, et al. (2019). A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 7, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, and Parsa AT (2009). PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene 28, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, and Allison JP (2010). PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 107, 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, Bacchiocchi A, Kluger H, Wei W, Halaban R, et al. (2018). Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, and Johnson DB (2019). Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. (2016). Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol 34, 4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, and Ruffell B (2018). TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 33, 60–74 e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moel EC, Rozeman EA, Kapiteijn EH, Verdegaal EME, Grummels A, Bakker JA, Huizinga TWJ, Haanen JB, Toes REM, and van der Woude D (2019). Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol Res 7, 6–11. [DOI] [PubMed] [Google Scholar]
- Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. (2018). Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 29, 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Luoma AM, Dougan SK, and Wucherpfennig KW (2021). Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AM, Mullen AC, Villarino AV, Hutchins AS, High FA, Lee HW, Thompson CB, and Reiner SL (2001). Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med 194, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. (2016). Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7, 10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, and Leonard JP (2002). Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, Hackermuller J, Polte T, Scholz S, Aigner A, Altenburger R, Bohme A, Bopp SK, Brack W, Busch W, et al. (2017). From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 99, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, Miller WH Jr., and Calabrese L (2020). Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 17, 504–515. [DOI] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 110, 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. (2020). Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 382, 1894–1905. [DOI] [PubMed] [Google Scholar]
- Flynn S, Toellner KM, Raykundalia C, Goodall M, and Lane P (1998). CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med 188, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J (1971). Tumor angiogenesis: therapeutic implications. N Engl J Med 285, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Fournel L, Wu Z, Stadler N, Damotte D, Lococo F, Boulle G, Segal-Bendirdjian E, Bobbio A, Icard P, Tredaniel J, et al. (2019). Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett 464, 5–14. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. (2000). Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, He Q, and Sharma P (2011). The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res 71, 5445–5454. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Kloepper J, Amoozgar Z, Duda DG, and Jain RK (2018). Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15, 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, and Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Pandey MR, Attwood K, Ji W, Witkiewicz AK, Knudsen ES, Allen C, Tario JD, Wallace PK, Cedeno CD, et al. (2021). Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin Cancer Res 27, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al. (2016). Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 167, 397–404 e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al. (2017). VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 23, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Gaspar M, Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F, Morrow M, Tuna M, and Brewis N (2020). CD137/OX40 Bispecific Antibody Induces Potent Antitumor Activity that Is Dependent on Target Coengagement. Cancer Immunol Res 8, 781–793. [DOI] [PubMed] [Google Scholar]
- Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, et al. (2017). CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. (2017). PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, et al. (2020). Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395, 1835–1844. [DOI] [PubMed] [Google Scholar]
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, and Committee EG (2018). Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29, iv264–iv266. [DOI] [PubMed] [Google Scholar]
- Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, Jorger M, Fruh M, Driessen C, Lenz TL, et al. (2019). Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer 107, 8–14. [DOI] [PubMed] [Google Scholar]
- Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, and Wargo JA (2019). The microbiome, cancer, and cancer therapy. Nat Med 25, 377–388. [DOI] [PubMed] [Google Scholar]
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. (2020). B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. (2017). Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol 24, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. (2005). Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65, 1089–1096. [PubMed] [Google Scholar]
- Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al. (2014). Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. (2003). Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 100, 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsmit EP, Rozeman EA, Haanen J, and Blank CU (2019). Susceptible loci associated with autoimmune disease as potential biomarkers for checkpoint inhibitor-induced immune-related adverse events. ESMO Open 4, e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM, et al. (2015). Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33, 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, et al. (2018). Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest 128, 4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zakharov PN, Peterson OJ, and Unanue ER (2020). Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc Natl Acad Sci U S A 117, 31319–31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, and Chen SH (2006). Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66, 1123–1131. [DOI] [PubMed] [Google Scholar]
- Huang RR, Jalil J, Economou JS, Chmielowski B, Koya RC, Mok S, Sazegar H, Seja E, Villanueva A, Gomez-Navarro J, et al. (2011). CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res 17, 4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Bai X, Cao Y, Wu J, Huang M, Tang D, Tao S, Zhu T, Liu Y, Yang Y, et al. (2010). Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med 207, 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. (2015). CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, and Kroczek RA (1999). ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266. [DOI] [PubMed] [Google Scholar]
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, and Caturegli P (2014). Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 6, 230ra245. [DOI] [PubMed] [Google Scholar]
- Iwata H, Inoue K, Kaneko K, Ito Y, Tsugawa K, Hasegawa A, Nakagawa S, Kuratomi H, and Tamura K (2019). Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn J Clin Oncol 49, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, et al. (2017). Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 376, 2109–2121. [DOI] [PubMed] [Google Scholar]
- Jeong A, Fiorito G, Keski-Rahkonen P, Imboden M, Kiss A, Robinot N, Gmuender H, Vlaanderen J, Vermeulen R, Kyrtopoulos S, et al. (2018). Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environ Int 119, 334–345. [DOI] [PubMed] [Google Scholar]
- Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, and Sharma P (2019). Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 179, 1177–1190 e1113. [DOI] [PubMed] [Google Scholar]
- Jing Y, Liu J, Ye Y, Pan L, Deng H, Wang Y, Yang Y, Diao L, Lin SH, Mills GB, et al. (2020). Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun 11, 4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, Tetzlaff MT, Hwu P, Curry JL, and Diab A (2019). IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer Immunol Res 7, 860–865. [DOI] [PubMed] [Google Scholar]
- Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. (2016). Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 375, 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Warshauer JT, and Bluestone JA (2017). Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med 23, 540–547. [DOI] [PubMed] [Google Scholar]
- Kalaora S, Nagler A, Nejman D, Alon M, Barbolin C, Barnea E, Ketelaars SLC, Cheng K, Vervier K, Shental N, et al. (2021). Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbe C, Milella M, Brownell I, et al. (2018). Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after >/=1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, and Fong L (2008). CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 112, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z, Di Nucci F, Kwan A, Hammer C, Mariathasan S, Rouilly V, Carroll J, Fontes M, Ley Acosta S, Guardino E, et al. (2020). Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci U S A 117, 12288–12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, Marshall MA, Gomez-Navarro J, Liang JQ, and Bulanhagui CA (2010). Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 16, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, Dunn C, Bolijn M, Benz J, Stihle M, et al. (2017). Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 6, e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, and Schell TD (2018). Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Janmaat ML, Reichert JM, and Parren P (2019). Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18, 585–608. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. (2001). PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2, 261–268. [DOI] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372, 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, and Allison JP (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, and Vogelstein B (1998). Genetic instabilities in human cancers. Nature 396, 643–649. [DOI] [PubMed] [Google Scholar]
- Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, et al. (2019). Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, and Sharma P (2008). CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 105, 14987–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, Breen EJ, Yang JYH, Ghazanfar S, Kefford RF, et al. (2019). Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin Cancer Res 25, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Liu J, Blake SJ, Harjunpaa H, Fairfax KA, Yong MC, Allen S, Kohrt HE, Takeda K, Smyth MJ, and Teng MW (2016). Assessing Immune-Related Adverse Events of Efficacious Combination Immunotherapies in Preclinical Models of Cancer. Cancer Res 76, 5288–5301. [DOI] [PubMed] [Google Scholar]
- Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH, et al. (2015). Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A 112, 6682–6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang H, Li M, Hu D, Li C, Ge B, Jin B, and Fan Z (2013). Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ 20, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B, Fritz JM, Su HC, Uzel G, Jordan MB, and Lenardo MJ (2016). CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood 128, 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Redd PS, Lee JR, Savage N, and Liu K (2016). The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology 5, e1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, et al. (2017). A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Nixon MJ, Gonzalez-Ericsson PI, Sanchez V, Opalenik SR, Li H, Zahnow CA, Nickels ML, Liu F, Tantawy MN, et al. (2018). DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun 9, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, et al. (2020). Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655–671 e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, and Sabzevari H (2005). Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105, 2862–2868. [DOI] [PubMed] [Google Scholar]
- Ma Q, Liu J, Wu G, Teng M, Wang S, Cui M, and Li Y (2018). Co-expression of LAG3 and TIM3 identifies a potent Treg population that suppresses macrophage functions in colorectal cancer patients. Clin Exp Pharmacol Physiol 45, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al. (2016). Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijt KA, Sluijter M, Blijleven L, Tolmeijer SH, Scheeren FA, van der Burg SH, and van Hall T (2019). Metabolic stress in cancer cells induces immune escape through a PI3K-dependent blockade of IFNgamma receptor signaling. J Immunother Cancer 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, et al. (2019). Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol 5, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, and Gajewski TF (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. (2010). Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 107, 7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. (2018). Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. (2016). Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, and Byrne MC (2002). CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16, 311–323. [DOI] [PubMed] [Google Scholar]
- McQuade JL, Daniel CR, Helmink BA, and Wargo JA (2019). Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol 20, e77–e91. [DOI] [PubMed] [Google Scholar]
- McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. (2018). Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JL, Ologun GO, Arora R, and Wargo JA (2020). Gut Microbiome Modulation Via Fecal Microbiota Transplant to Augment Immunotherapy in Patients with Melanoma or Other Cancers. Curr Oncol Rep 22, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, and Speiser DE (2014). Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 63, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GW, and Jones DP (2014). The nature of nurture: refining the definition of the exposome. Toxicol Sci 137, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Andrews MC, Roh W, De Macedo MP, Hudgens CW, Carapeto F, Singh S, Reuben A, Wang F, Mao X, et al. (2020). Spatially resolved analyses link genomic and immune diversity and reveal unfavorable neutrophil activation in melanoma. Nat Commun 11, 1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. (2002). Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541. [DOI] [PubMed] [Google Scholar]
- Mpekris F, Voutouri C, Baish JW, Duda DG, Munn LL, Stylianopoulos T, and Jain RK (2020). Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A 117, 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, and Hoeller S (2016). The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med 279, 541–562. [DOI] [PubMed] [Google Scholar]
- Nam S, Lee A, Lim J, and Lim JS (2019). Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs). Biomol Ther (Seoul) 27, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, and Miller GW (2019). The Exposome: Molecules to Populations. Annu Rev Pharmacol Toxicol 59, 107–127. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, and Honjo T (1999). Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151. [DOI] [PubMed] [Google Scholar]
- Nobashi T, Baratto L, Reddy SA, Srinivas S, Toriihara A, Hatami N, Yohannan TK, and Mittra E (2019). Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin Nucl Med 44, e272–e279. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, and Robinson BW (2003). Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 170, 4905–4913. [DOI] [PubMed] [Google Scholar]
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, et al. (2010). Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 21, 1712–1717. [DOI] [PubMed] [Google Scholar]
- Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, Faham M, and Fong L (2017). Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res 77, 1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita R, Saeki T, Takashima S, Yamaguchi Y, and Toge T (2005). CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep 14, 1269–1273. [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir BC, and Dotto GP (2019). Sex Hormones and Anticancer Immunity. Clin Cancer Res 25, 4603–4610. [DOI] [PubMed] [Google Scholar]
- Panelli MC, White R, Foster M, Martin B, Wang E, Smith K, and Marincola FM (2004). Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CJ, Bhattacharya J, and Butte AJ (2010). An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One 5, e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, et al. (2015). Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res 21, 4286–4293. [DOI] [PubMed] [Google Scholar]
- Pauken KE, Dougan M, Rose NR, Lichtman AH, and Sharpe AH (2019). Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol 40, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et al. (2015). Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res 75, 5034–5045. [DOI] [PubMed] [Google Scholar]
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al. (2016). Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 6, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, et al. (2020). B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560. [DOI] [PubMed] [Google Scholar]
- Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, et al. (2019). Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 177, 414–427 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV, Menzies AM, Eroglu Z, et al. (2018). Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, et al. (2020). Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Postow MA, Sidlow R, and Hellmann MD (2018). Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378, 158–168. [DOI] [PubMed] [Google Scholar]
- Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391, 748–757. [DOI] [PubMed] [Google Scholar]
- Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martinez L, Martinez-Saez E, Ramon YCS, et al. (2018). STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24, 1024–1035. [DOI] [PubMed] [Google Scholar]
- Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. (2017). Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Curran MA, and Allison JP (2006). CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 116, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, et al. (2014). Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol 192, 2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM, and Smith MT (2010). Epidemiology. Environment and disease risks. Science 330, 460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. (2006). Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203, 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retseck J, Nasr A, Lin Y, Lin H, Mendiratta P, Butterfield LH, and Tarhini AA (2018). Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma. J Transl Med 16, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Spencer CN, Prieto PA, Gopalakrishnan V, Reddy SM, Miller JP, Mao X, De Macedo MP, Chen J, Song X, et al. (2017). Genomic and immune heterogeneity are associated with differential responses to therapy in melanoma. NPJ Genom Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Algarra S, Mandal R, Sharfman WH, et al. (2017). Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171, 934–949 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, et al. (2005). Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 23, 8968–8977. [DOI] [PubMed] [Google Scholar]
- Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C, et al. (2018). Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 4, eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. (2019). Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 178, 795–806 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. (2015). Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372, 320–330. [DOI] [PubMed] [Google Scholar]
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, and Krummel MF (2016). Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 30, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, and Weissleder R (2018). TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2, 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JJ, Sampath P, Hou W, and Thorne SH (2015). Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin Cancer Res 21, 5543–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Restrepo-Jimenez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramirez-Santana C, Leung PSC, Ansari AA, Gershwin ME, and Anaya JM (2018). Molecular mimicry and autoimmunity. J Autoimmun 95, 100–123. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanic M, Reading JL, Joshi K, et al. (2019). Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97. [DOI] [PubMed] [Google Scholar]
- Rowshanravan B, Halliday N, and Sansom DM (2018). CTLA-4: a moving target in immunotherapy. Blood 131, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Taylor A, and Schneider H (2009). CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev 229, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R, and Elkord E (2019). Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett 457, 168–179. [DOI] [PubMed] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, et al. (2004). Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol 22, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, et al. (2018). Safety and Efficacy of Retreating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 6, 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, and Delgoffe GM (2016). The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 45, 374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. (2020). Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 382, 810–821. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, and Schreiber RD (2015). Neoantigens in cancer immunotherapy. Science 348, 69–74. [DOI] [PubMed] [Google Scholar]
- Shah KP, Song H, Ye F, Moslehi JJ, Balko JM, Salem JE, and Johnson DB (2020). Demographic Factors Associated with Toxicity in Patients Treated with Anti-Programmed Cell Death-1 Therapy. Cancer Immunol Res 8, 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, and Freeman GJ (2002). The B7-CD28 superfamily. Nat Rev Immunol 2, 116–126. [DOI] [PubMed] [Google Scholar]
- Shaul ME, and Fridlender ZG (2018). Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function. FEBS J 285, 4316–4342. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, and Sakaguchi S (1999). Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163, 5211–5218. [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, and Sakaguchi S (2002). Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3, 135–142. [DOI] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. (2013). Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210, 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, and Ostrand-Rosenberg S (2007). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179, 977–983. [DOI] [PubMed] [Google Scholar]
- Snahnicanova Z, Kasubova I, Kalman M, Grendar M, Mikolajcik P, Gabonova E, Laca L, Caprnda M, Rodrigo L, Ciccocioppo R, et al. (2020). Genetic and epigenetic analysis of the beta-2-microglobulin gene in microsatellite instable colorectal cancer. Clin Exp Med 20, 87–95. [DOI] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. (2014). Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371, 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, et al. (2017). Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 168, 487–502 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, and Gajewski TF (2015). Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235. [DOI] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. (2009). The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 106, 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, Pal R, Yuan M, Asara J, Patsoukis N, et al. (2020). Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L, et al. (2016). Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A 113, 11919–11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucker A, Zhao F, Real B, Heeke C, Bielefeld N, Mabetaen S, Horn S, Moll I, Maltaner R, Horn PA, et al. (2014). Genetic evolution of T-cell resistance in the course of melanoma progression. Clin Cancer Res 20, 6593–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Ren Z, Yang K, Liu Z, Cao S, Deng S, Xu L, Liang Y, Guo J, Bian Y, et al. (2019). A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat Commun 10, 3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, and Albelda SM (2005). Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11, 6713–6721. [DOI] [PubMed] [Google Scholar]
- Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, and Kirkwood JM (2015). Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 3, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall IW, and Leventhal JS (2020). Cutaneous Toxicities of Immune Checkpoint Inhibitors: The Role of the Dermatologist. Yale J Biol Med 93, 123–132. [PMC free article] [PubMed] [Google Scholar]
- Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, and Whiteside TL (2018). Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin Cancer Res 24, 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA (2018). New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J Natl Compr Canc Netw 16, 594–596. [DOI] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, and Sharpe AH (1995). Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. (2014). Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RC, Wenzel A, Hicks J, Ballew M, Stone M, et al. (2017). Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell 171, 1284–1300 e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, and Hercend T (1990). LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171, 1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, and Chiarion-Sileni V (2018). Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med 16, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. (2015). Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A, Hurwitz AA, and Allison JP (1999). Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Schymanski EL, Barabasi AL, and Miller GW (2020). The exposome and health: Where chemistry meets biology. Science 367, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. (2018a). Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 4, 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, Guan J, Singh R, Rollins S, Solorz A, et al. (2019a). VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 156, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, Farkas AM, Gnjatic S, Hake A, Izadmehr S, et al. (2021). Myeloid Cell-associated Resistance to PD-1/PD-L1 Blockade in Urothelial Cancer Revealed Through Bulk and Single-cell RNA Sequencing. Clin Cancer Res 27, 4287–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. (2018b). Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 24, 1804–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et al. (2019b). Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 25, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, and Mak TW (1995). Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988. [DOI] [PubMed] [Google Scholar]
- Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand PY, et al. (2021). A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov 11, 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et al. (2019). Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20, 924–937. [DOI] [PubMed] [Google Scholar]
- Wild CP (2012). The exposome: from concept to utility. Int J Epidemiol 41, 24–32. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, and Sakaguchi S (2008). CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275. [DOI] [PubMed] [Google Scholar]
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, and June CH (2001). Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61, 4766–4772. [PubMed] [Google Scholar]
- Wykes MN, and Lewin SR (2018). Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 18, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, and Zhang Y (2015). Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol 29, 635–641. [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. (2014). Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Andrews LP, Workman CJ, and Vignali DAA (2019). Intratumoral regulatory T cells: markers, subsets and their impact on anti-tumor immunity. Immunology 157, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Hopkins A, and Jaffee EM (2017). Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 377, 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY, et al. (2017). PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res 23, 3158–3167. [DOI] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. (2009). The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 10, 48–57. [DOI] [PubMed] [Google Scholar]
- Zhang C, Fan Y, Che X, Zhang M, Li Z, Li C, Wang S, Wen T, Hou K, Shao X, et al. (2020). Anti-PD-1 Therapy Response Predicted by the Combination of Exosomal PD-L1 and CD28. Front Oncol 10, 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Arora M, Chaleckis R, Isobe T, Jain M, Meister I, Melen E, Perzanowski M, Torta F, Wenk MR, et al. (2019). Tackling the Complexity of the Exposome: Considerations from the Gunma University Initiative for Advanced Research (GIAR) Exposome Symposium. Metabolites 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, Shen Y, Zhang H, Sun R, Tian Z, et al. (2019). Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol 20, 1656–1667. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Slone N, Chrisikos TT, Kyrysyuk O, Babcock RL, Medik YB, Li HS, Kleinerman ES, and Watowich SS (2020). Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103(+) conventional dendritic cells. J Immunother Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, Li CW, Lim SO, Sheng YY, Zhang Y, et al. (2019). Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68, 1653–1666. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, and Kroemer G (2008). Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8, 59–73. [DOI] [PubMed] [Google Scholar]