Abstract
Nucleoside analogues are among the most successful bioactive classes of druglike compounds in pharmaceutical chemistry as they are well-known for their numerous effective bioactivities in humans, especially as antiviral and anticancer agents. Coronavirus disease 2019 (COVID-19) is still untreatable, with its causing virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continuing to wreak havoc on the ground everywhere. This complicated international situation urged all concerned scientists, including medicinal chemists and drug discoverers, to search for a potent anti-COVID-19 drug. Cordycepin (3′-deoxyadenosine) is a known natural adenosine analogue of fungal origin, which could also be synthetically produced. This bioactive phytochemical compound is characterized by several proven strong pharmacological actions that may effectively contribute to the comprehensive treatment of COVID-19, with the antiviral activities being the leading ones. Some new studies predicted the possible inhibitory affinities of cordycepin against the principal SARS-CoV-2 protein targets (e.g., SARS-CoV-2 spike (S) protein, main protease (Mpro) enzyme, and RNA-dependent RNA polymerase (RdRp) enzyme) based on the computational approach. Interestingly, the current research showed, for the first time, that cordycepin is able to potently inhibit the multiplication of the new resistant strains of SARS-CoV-2 with a very minute in vitro anti-SARS-CoV-2 EC50 of about 2 μM, edging over both remdesivir and its active metabolite GS-441524. The ideal pharmacophoric features of the cordycepin molecule render it a typical inhibitor of SARS-CoV-2 replication, with its flexible structure open for most types of derivatization in the future. Briefly, the current findings further support and suggest the repurposing possibility of cordycepin against COVID-19 and greatly encourage us to confidently and rapidly begin its preclinical/clinical evaluations for the comprehensive treatment of COVID-19.
1. Introduction
About 2 years now and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) still represents a major global threat and concern to human beings since its first emergence in the famous Chinese city Wuhan.1 The acute disease caused by this virus, the coronavirus disease 2019 (COVID-19), is also still ongoing with confirmed infections and deaths to more than 260 and 5.18 million, respectively, people worldwide due to this universal pandemic.2 The increasing evolution of very resistant and virulent novel variants/strains of the COVID-19 virus, especially in the current year 2021, puts a very heavy load on the concerned scientific communities in all of the countries to become much more active in searching for and finding successful medicines and vaccines effective in inhibiting and fighting this irritating virus, along with finding drugs having the abilities to counteract all, or most of, the very severe effects of the COVID-19 on human bodies; thus, searching for efficient comprehensive or dual-action anti-SARS-CoV-2/anti-COVID-19 therapeutic agents will be extremely advantageous as an excellent solution for this untreatable infection and its health sequelae.3 Tens of new and repurposed promising compounds are nowadays under broad international/multinational investigations (including in vitro, in vivo, and clinical trials) to be biologically evaluated as effective candidate anti-COVID-19 drugs, e.g., remdesivir (GS-5734; FDA-approved administration for emergent use), GS-441524, GS-443902, favipiravir, cyanorona-20, hydroxychloroquine, chloroquine, CoViTris2020, taroxaz-104, ChloViD2020, teriflunomide, leflunomide, ivermectin, arbidol, and colchicine, but none of them has proved its successful eventual broad-spectrum effectiveness until now (i.e., the final results of almost all of these worldwide investigations are not disclosed to date).4−20
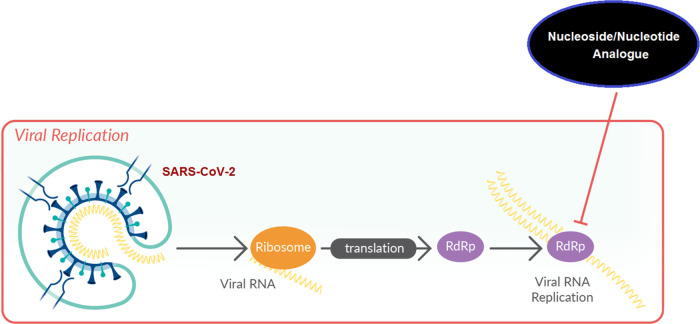
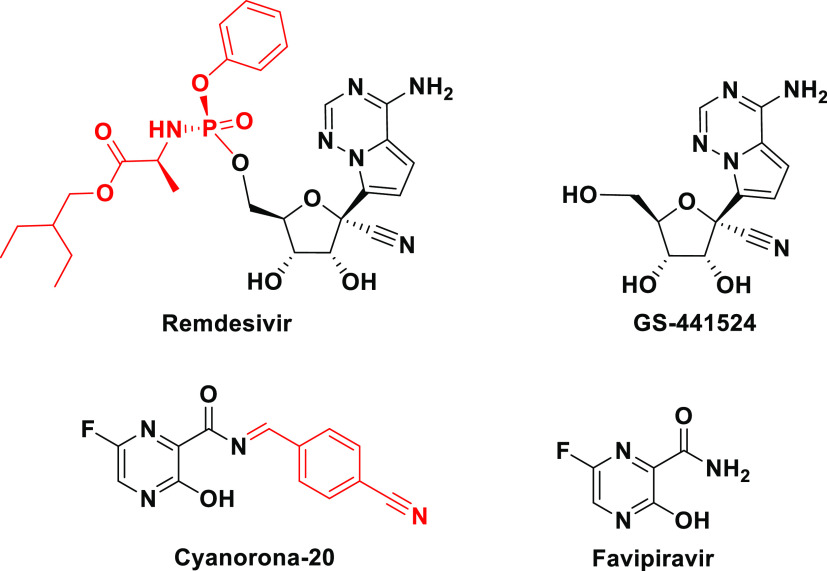
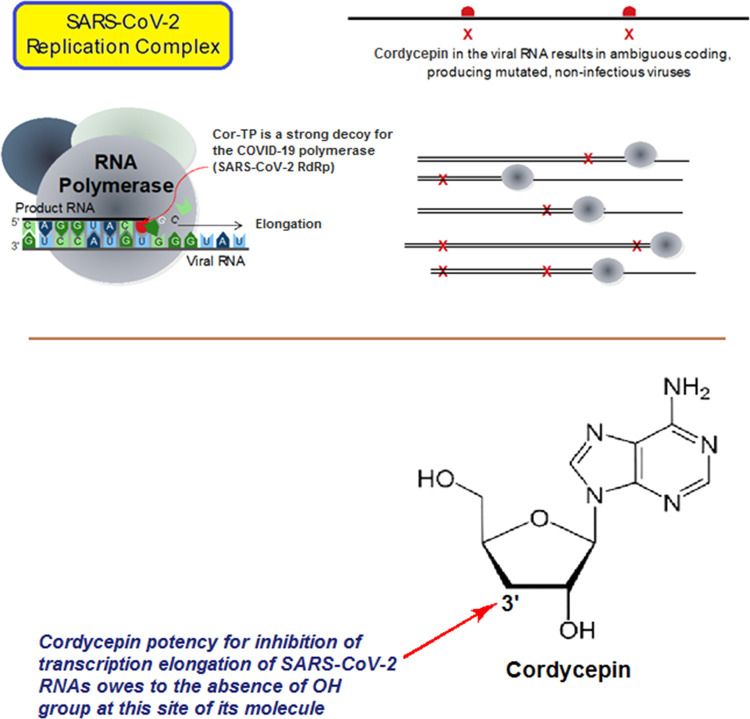
Nucleoside and nucleotide analogism is among the top therapeutic options in drug designers’ and medicinal chemists’ minds to combat and stop coronavirus replication inside the human body.4−9,21 In this COVID-19 therapeutic option or strategy, the natural or synthetic nucleoside/nucleotide analogue uses its resemblance to the normal nucleosides and nucleotides to mislead and trick the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) through incorporation and embedding in the viral genetic strands instead (i.e., in place) of the normal naturally occurring nucleosides and nucleotides, leading to repeated excessive ambiguous coding and premature termination of mRNA synthesis with the generation of vague RNA strands at the end; these pseudostrands, in turn, form abnormal noninfectious and inactive viral particles, and hence no further replication of the virus occurs (Figure 1).21 Some of the aforementioned investigational anti-COVID-19 agents, such as remdesivir and cyanorona-20 and their active metabolites GS-441524 and favipiravir, respectively, depend on this tactical mechanism in their inhibitory bioactivities on the SARS-CoV-2 (Figure 2).4−9 Unfortunately, all the four compounds are synthetic and did not give satisfactory results in the in vitro/in vivo anti-SARS-CoV-2 activity assays (except cyanorona-20, which has a significant anti-SARS-CoV-2 EC50 value of 0.45 μM); therefore, searching for more potent natural anti-COVID-19 drugs is very demanding.4−9
Figure 1.
Representation of the nucleoside/nucleotide analogism approach used for the potent blockade of the SARS-CoV-2 replication in COVID-19 therapy.
Figure 2.
Chemical structures of remdesivir, GS-441524, cyanorona-20, and favipiravir.
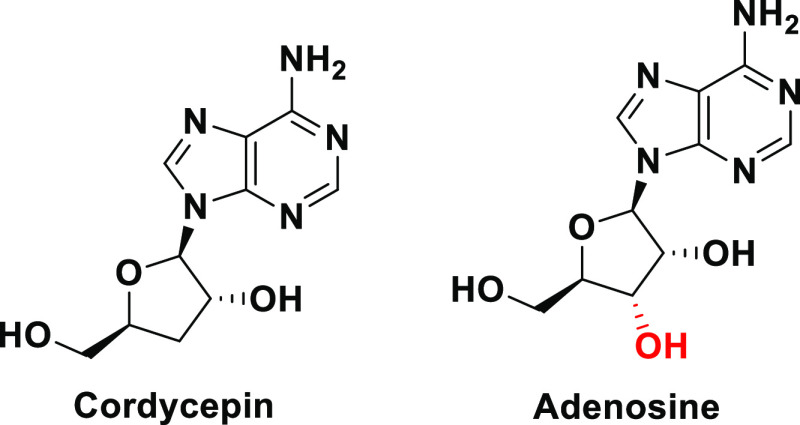
Cordycepin is a known unique natural adenosine analogue found and extracted from various species in the fungal kingdom, mainly those from the two genera Cordyceps (e.g., the species Cordyceps militaris) and Ophiocordyceps (e.g., the species Ophiocordyceps sinensis).22−25 Chemically, the compound cordycepin is 3′-deoxyadenosine (3-dA), which is IUPACally named as (2R,3R,5S)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolan-3-ol or 9-(3-deoxy-β-d-ribofuranosyl)adenine (Figure 3).26 Recently, the natural cordycepin content was biologically increased to more than twofold in the entomopathogenic medicinal fungus C. militaris using newly designed techniques of optimized cultivation and fermentation, which significantly enhanced the biosynthesis of this precious bioactive metabolite.27 Cordycepin could also be easily synthesized from many readily available simple starting materials, such as d-glucose, d-xylose, and adenosine, using different chemical synthetic routes.28,29 Cordycepin molecule is specifically a nucleosidic adenosine molecule that lacks only one hydroxyl group at the 3′ position of the five-membered ring of its ribose moiety (Figure 3).26,30 This extreme structural similarity with the cellular nucleoside adenosine renders cordycepin biologically acting on adenosine receptors and very similar to this pivotal nucleoside in all its biological activities in living organisms. Additionally, this structural analogism also provides camouflage abilities to the cordycepin molecule to be able to antagonize/block and impair/disrupt many of the biological actions of the adenosine molecule itself. Cordycepin has a very broad spectrum of diverse important pharmacological activities since it effectively acts as, for example, antiviral (also in viral myocarditis), antifungal, antibacterial, antitubercular, antimalarial, antiprotozoal, antimicrobial, insecticidal, larvicidal, antiinflammatory, antioxidant (and in several oxidative stress statuses of many different diseases), immunomodulatory/immunoregulatory, antileukemic (since it has exhibited promising in vitro cytotoxicities against various leukemic cell lines), antitumor/anticancer (antineoplastic), antiproliferative, antifibrotic, antimetastatic (in cancer therapy), apoptosis inducer (in cancer cells), antihyperglycemic/antidiabetic, antihyperlipidemic (as in cardiovascular diseases and vascular disorders), antihypercholesterolemic, antiarrhythmic, antitachycardic, coronary vasodilator, antihypertensive, angiogenic, antiatherosclerotic, antiplatelet aggregation, antithrombotic/thrombolytic/fibrinolytic, anti-ischemic (as in myocardial infarction/ischemia and cerebral ischemia injuries), reperfusion therapy, antistroke, hepatoprotective (as in hepatitis and liver cirrhosis), renal functions improver/nephroprotective (renoprotective and in renal fibrosis and chronic kidney disease (CKD)), chondrogenesis promoter, antiarthritic (as in osteoarthritis), antiosteoporotic (in bone loss), antihyperuricemic, antirheumatic (as in multiple sclerosis (MS) and rheumatoid arthritis (RA)), intervertebral disc regenerator, bronchial asthma reliever, acute lung inflammation/injury healer (as in acute respiratory distress syndrome (ARDS), cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD)), pneumoprotective, cough/common cold suppressant, antihypoxic, antidepressant, nootropic, energizer, tonic, stimulant, mood modulator, natural endurance booster, pain killer/analgesic, antifatigue, anticognitive dysfunction, erythropoiesis stimulator, zincophoric (zinc ion carrier), neuronal regenerator, antiparkinsonian, antisleep disorders, antiaging, sexual enhancer, aphrodisiac (prosexual), spermatogenic, anti-infertility, benign prostatic hyperplasia (BPH) inhibitor, steroidogenetic (as in testosterone/estrogens biosyntheses), intestinal irritation suppressor (as in acute colitis conditions), some toxins antidote, and cosmeceutical (in skin photoaging and other cosmeceutical skin/hair problems, also in inflammatory skin conditions/diseases like atopic dermatitis).31−36 All of the aforementioned interesting biological activities make cordycepin one of the most important promising phytotherapeutic agents. Cordycepin is already being clinically investigated as a potential effective antileukemic/anticancer chemotherapeutic agent of the antimetabolites class since 1997 until now in several clinical settings worldwide to pass the clinical phases 1 and 2 (e.g., clinical trials NCT00003005 and NCT00709215).37,38
Figure 3.
Chemical structures of cordycepin and adenosine.
Most of the previously mentioned diverse biological activities of cordycepin are extremely needed in the comprehensive treatment of COVID-19; this makes cordycepin a very promising potential comprehensive anti-COVID-19 drug rather than being only a candidate SARS-CoV-2 inhibitor (the complementary part that was proven in the current study).17 The antiviral activities of cordycepin are very potent and broad since they cover several species of human viruses (including almost all virulent RNA viruses, especially those belonging to the genus Flavivirus), e.g., adenoviruses (AVs), dengue virus (DENV), Epstein-Barr virus (EBV), hepatitis B virus (HBV), hepatitis C virus (HCV), Herpes simplex virus 1 and 2 (HSV-1 and HSV-2), human immunodeficiency virus (HIV), human poliovirus (HPV), human rhinovirus (HRV), influenza viruses (IVs), Kyasanur Forest disease virus (KFDV), murine leukemia viruses (MLVs or MuLVs), murine sarcoma viruses (MSVs or MuSVs), Newcastle disease virus (NDV), Omsk hemorrhagic fever virus (OHFV), Powassan virus (POWV), rotaviruses (RV), vaccinia virus (VACV or VV), West Nile virus (WNV), yellow fever virus (YFV), and Zika virus (ZIKV).33,34,39−43 These strong inhibitory activities extend to some plant viruses, such as cowpea chlorotic mottle virus (CCMV) and tobacco mosaic virus (TMV).44,45 The strong antiviral actions of cordycepin are mediated through specific molecular mechanisms that mainly originate due to the extreme structural analogism with the nucleoside adenosine, as this analogism renders the human biological system unable to recognize this molecule, e.g., many enzymes fail to discriminate between it and the endogenous adenosine.33,36,46 Through this camouflage, administered cordycepin could easily be involved in successfully inhibiting/blocking certain several biochemical pathways and reactions in the human body and viral particles; for example, it could cause potent poly(A) polymerase inhibition (i.e., acts as a polyadenylation inhibitor), severe shortening of poly(A) tails, continuous destabilization of mRNAs, strong purine biosynthesis inhibition, and also premature termination of protein synthesis.33,36,46
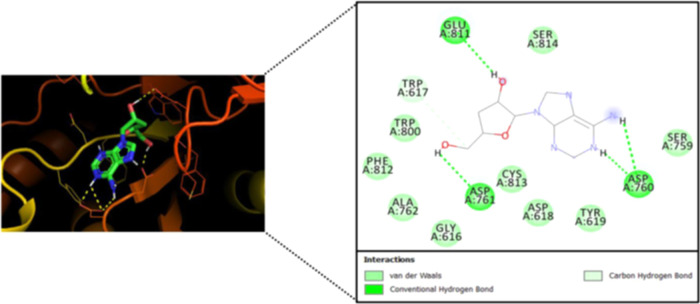
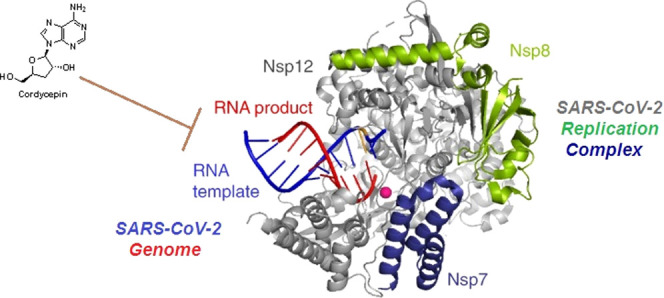
A recently published study sheds light on the possible potent inhibitory effects of cordycepin against SARS-CoV-2 RdRp using an in silico computational molecular docking approach.47 The evaluation results of this validated simulation protocol clearly revealed the very strong intermolecular interactions with the most vital amino acid residues of the principal active site of the SARS-CoV-2 RdRp, proposing that cordycepin molecule actively interacts with the giant molecule of RdRp (Figure 4).47 These strong inhibitory interactions were reflected in the resultant relatively low binding energy, which reaches about −8.2 kcal/mol at the best pose of docking, with the formation of a very stable cordycepin–RdRp complex.47 Interestingly, it was found that the cordycepin molecule strikes and binds to the two pivotal catalytic amino acid residues, Asp760 and Asp761, of the active site of the SARS-CoV-2 RdRp via both strong hydrogen bonds and hydrophobic interactions.47 Additionally, the cordycepin molecule was found to bind also to some residues that are very close to the catalytic ones in the active site, e.g., Trp617, Asp618, Trp800, and Glu811 residues.47 These additional intermolecular interactions are mediated via diverse bonds/interactions, such as nonbonding interactions, hydrogen bonds, and hydrophobic interactions.47
Figure 4.
Molecular docking output image showing the best expected binding mode of cordycepin to SARS-CoV-2 RdRp according to the novel hypothesis of Bibi et al.47
Herein, we suggest that cordycepin could effectively inhibit the coronaviral replication (i.e., act as a SARS-CoV-2 replication inhibitor) by significantly reducing the number of replicated coronaviral copies, which is expected to result from locking mainly the genomic RNA synthesis occurred via the SARS-CoV-2 RdRp, using the nucleoside analogism strategy (as previously mentioned). In this effective mechanism of anti-RNA action, the nucleoside-like cordycepin molecule is first readily phosphorylated to its mono-, di-, and triphosphate forms (i.e., its nucleotide analogues) intracellularly, then the superactive nucleotide analogue cordycepin triphosphate (Cor-TP) can be easily incorporated into RNA in place of the endogenous chemicosimilar bionucleotide adenosine triphosphate (ATP); this inhibits and terminates transcription elongation and synthesis of viral RNA strands in all stages (i.e., acts as an RNA elongation inhibitor due to the hydroxyl moiety absence at the 3′ position of the molecule, this one-hydroxyl group deficiency significantly antagonizes the SARS-CoV-2 RdRp activity as previously mentioned), giving incomplete disrupted premature RNAs in the developing mRNA strands and viral genomes, and finally, this ambiguous coding leads to strong inhibition of SARS-CoV-2 copying/replication and generation of inactive, noninfectious, mutated, useless, nonpathogenic, and nonviral/non-SARS-CoV-2 particles instead of the active, parent, correct, original, infectious, pathogenic, and virulent SARS-CoV-2 particles (Figure 5). This currently presented logic hypothesis, which proposes the potential strong anti-SARS-CoV-2/anti-COVID-19 properties of the cordycepin molecule, was actually tested and evaluated in this current work through a validated anti-SARS-CoV-2 bioassay (along with a full toxicological evaluation). Based on all of the previous promising literature data together with the very interesting in silico and in vitro (i.e., biological) findings of the current research, cordycepin can be repurposed to in vivo evaluate its protective and inhibitory activities specifically against SARS-CoV-2 particles’ invasion (as an anti-RNA virus agent) and to clinically evaluate its protective and inhibitory activities comprehensively against COVID-19 status as a whole (as an anti-COVD-19 condition agent) in COVID-19 patients.
Figure 5.
Illustration of the newly proposed mechanism of anti-SARS-CoV-2 action of cordycepin.
2. Results and Discussion
2.1. In Silico Predictive Toxicological and Stability Properties of Cordycepin
The prediction of toxicities and adverse/side effects of any new potential medicine is a very critical issue of the drug discovery journey. Interestingly, modern virtual toxicity predictions have several favorable merits over the practical classic toxic dose determinations in living creatures, as they afford, e.g., more rapid data, lower research costs, and a lower number of diverse animal experiments. The data obtained from using the ProTox-II (Prediction of Toxicity of Chemicals) Virtual Laboratory disclosed the predicted relative biosafety of the compound cordycepin (over many investigational potential anti-COVID-19 drugs).48 The results revealed that cordycepin lies in the second toxicity class (toxicity class II) with a predicted lethal dose 50 (LD50) of 8 mg/kg of the body weight (BWt) (with an excellent average similarity of 97.27% and a high prediction accuracy of 72.9%). With a relatively small molecular weight of about 251.24 (which is approaching the mean molecular weight of the dataset, 319.67), cordycepin resides in the molecular weight region of the safest druglike compounds in the dataset of this virtual laboratory (Figure S1). The resulting data also predicted that cordycepin is cytotoxicologically inactive (with a probability percentage of more than 58%) (Table 1 and Figure S2). Interestingly, analysis of all diverse toxicity endpoints using the different ProTox-II toxicity model tests showed that cordycepin has high levels of safety with respect to the diverse organ toxicities and adverse outcome pathways since it exhibits very high percentages of inactivities, reaching 100% in some examples, against almost all of them (e.g., cordycepin is predicted to be extremely immunotoxicologically inactive with a probability percentage of more than 99%) (Table 1 and Figure S2). The only exception is the mutagenicity since cordycepin is predicted to be mutagenic with a moderate probability percentage of 69% (Table 1 and Figure S2). According to the newly established PredMS Application methodology, cordycepin is supposed to be metabolically stable in human liver microsomes with a probability percentage of more than 91%.49 All of the previous predictive toxicological/stability speculations are in line by a significant degree with the previous respective literature data about cordycepin.
Table 1. ProTox-II Toxicity Model Report Which Demonstrates the Expected Probabilities of the Main Toxicities Computationally Analyzed for the Cordycepin Molecule inside the Human Body (Using ProTox-II Virtual Laboratory Methodology).
| classification | target | prediction | probability |
|---|---|---|---|
| organ toxicity | hepatotoxicity | inactive | 0.63 |
| toxicity endpoints | carcinogenicity | inactive | 0.68 |
| toxicity endpoints | immunotoxicity | inactive | 0.99 |
| toxicity endpoints | mutagenicity | active | 0.69 |
| toxicity endpoints | cytotoxicity | inactive | 0.58 |
| Tox21-nuclear receptor signaling pathways | aryl hydrocarbon receptor (AhR) | inactive | 0.89 |
| Tox21-nuclear receptor signaling pathways | androgen receptor (AR) | inactive | 0.99 |
| Tox21-nuclear receptor signaling pathways | androgen receptor ligand-binding domain (AR-LBD) | inactive | 1.0 |
| Tox21-nuclear receptor signaling pathways | aromatase | inactive | 0.91 |
| Tox21-nuclear receptor signaling pathways | estrogen receptor α (ER) | inactive | 0.98 |
| Tox21-nuclear receptor signaling pathways | estrogen receptor ligand-binding domain (ER-LBD) | inactive | 1.0 |
| Tox21-nuclear receptor signaling pathways | peroxisome proliferator-activated receptor γ (PPAR-γ) | inactive | 0.80 |
| Tox21-stress response pathways | nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | inactive | 0.99 |
| Tox21-stress response pathways | heat shock factor response element (HSE) | inactive | 0.99 |
| Tox21-stress response pathways | mitochondrial membrane potential (MMP) | inactive | 0.97 |
| Tox21-stress response pathways | phosphoprotein (tumor suppressor) p53 | inactive | 0.81 |
| Tox21-stress response pathways | ATPase family AAA domain-containing protein 5 (ATAD5) | inactive | 0.71 |
2.2. In Vitro Anti-SARS-CoV-2 and Cytotoxic Bioactivities of Cordycepin
Table 2 shows the resulting values from both the in vitro anti-SARS-CoV-2 and cytotoxicity bioassays in detail. The SARS-CoV-2 strain used in the anticoronaviral assay is one of the newest strains, the first Variant of Concern from December 2020 (VOC-202012/01), which is a very virulent and resistant strain of the virus. The data demonstrated in the table interestingly disclosed the considerably higher antiviral effectiveness of cordycepin against the newly appeared variants of SARS-CoV-2 in comparison to that of each of the two positive control reference drugs remdesivir and GS-441524 (the placebo drug dimethyl sulfoxide “DMSO” showed extremely weak activities, i.e., negligible results). Cordycepin was found to successfully impair and block the entire coronaviral replication/transcription in Vero E6 cells with EC50 considerably smaller than the stock concentration 100 μM. Importantly, cordycepin (EC50 = 2.01 μM) was found to be about 10.5 and 7.8 times as effective as the two reference drugs remdesivir (EC50 = 21 μM) and GS-441524 (EC50 = 15.60 μM), respectively, with respect to the in vitro anti-VOC-202012/01/anti-SARS-CoV-2 bioactivity tested. According to the cytotoxicity assay, in vitro CC50 of cordycepin is significantly greater than 100 μM, and therefore, this nucleoside analogue is supposed to have a very beneficial high clinical selectivity index “SI” (SIcordycepin > 49.75), while remdesivir and GS-441524 have narrower SIs (SIremdesivir > 4.76 and SIGS-441524 > 6.41), reflecting the specific/selective anti-RNA actions (RNA-disrupting activities) of the cordycepin molecule against the new coronaviral-2 genomes rather than the human genome. Cordycepin exhibited a significantly small value of the concentration that results in 100% in vitro inhibition of the coronaviral-2 VOC-202012/01 cytopathic effects (CPEIC100 = 5.98 μM), which is less than the corresponding values of remdesivir (CPEIC100 = 25.17 μM) and GS-441524 (CPEIC100 = 17.40 μM). In line with its potent antiviral-RNA activities, cordycepin also demonstrated a very slight value of the concentration that is needed for 50% in vitro lowering in the number of RNA copies of the VOC-202012/01 strain of SARS-CoV-2 (2.35 μM), which is clearly smaller than the corresponding values of both remdesivir and GS-441524 (22.92 and 16.04 μM, respectively).
Table 2. Anti-SARS-CoV-2/Anti-COVID-19 Activities (along with Cytotoxicities) of the Target Drug Cordycepin (Using Both Remdesivir and GS-441524 as the Positive Control/Reference Drugs, and DMSO as the Negative Control/Placebo Drug) against SARS-CoV-2 (VOC-202012/01 strain) in Vero E6 Cells.
| inhibition
of SARS-CoV-2 replication in vitro (anti-VOC-202012/01
bioactivities) (μM) |
|||||
|---|---|---|---|---|---|
| classification | compound name | CC50a (μM) | 100% CPE inhibitory concentration (CPEIC100)b | 50% reduction in infectious virus (EC50)c | 50% reduction in viral RNA copy (EC50)d |
| target compound | cordycepin | >100 | 5.98 ± 0.41 | 2.01 ± 0.12 | 2.35 ± 0.15 |
| reference drugs | remdesivir | >100 | 25.17 ± 2.51 | 21.00 ± 1.97 | 22.92 ± 1.99 |
| GS-441524 | >100 | 17.40 ± 1.83 | 15.60 ± 0.76 | 16.04 ± 0.81 | |
| placebo solvent | DMSO | >100 | >100 | >100 | >100 |
CC50 or 50% cytotoxic concentration is the concentration of the tested compound that kills half of the cells in an uninfected cell culture. CC50 was determined with serially diluted compounds in Vero E6 cells at 48 h post incubation using the CellTiter-Glo Luminescent Cell Viability Assay (Promega).
CPEIC100 or 100% CPE inhibitory concentration is the lowest concentration of the tested compound that causes 100% inhibition of the cytopathic effects (CPE) of SARS-CoV-2 VOC-202012/01 virus in Vero E6 cells under increasing concentrations of the tested compound at 48 h post infection. Compounds were serially diluted from 100 μM concentration.
EC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in infectious SARS-CoV-2 VOC-202012/01 virus particles in vitro. EC50 is determined by infectious virus yield in culture supernatant at 48 h post infection (log10 TCID50/mL).
EC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in SARS-CoV-2 VOC-202012/01 viral RNA copies in vitro. EC50 is determined by viral RNA copies number in culture supernatant at 48 h post infection (log10 RNA copies/mL).
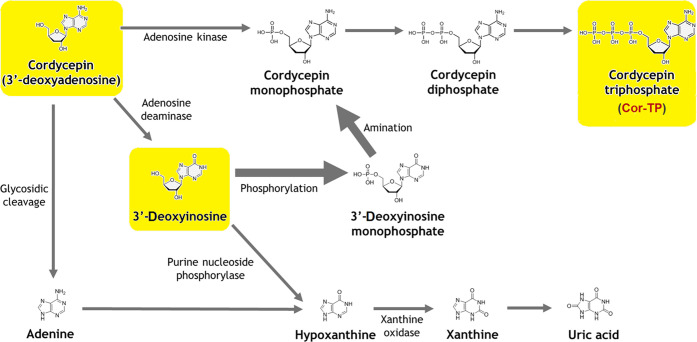
It was surprisingly observed that cordycepin acts against the coronavirus in a time-dependent manner since it reaches its maximal potency against the SARS-CoV-2 particles within 36–48 h of treatment. This observation revealed the expected clinically sustained action (long-acting activities) against COVID-19; this, in turn, supports the virtually predicted high metabolic stability of this bioactive drug. In spite of the expected high metabolic stability of cordycepin in the liver, a considerable portion of its molecules could be biologically transformed through metabolic phosphorylation inside the human cells to other active forms (e.g., its mono/di/triphosphate nucleotidic forms, with Cor-TP as the major metabolite among them).50 This type of intracellular metabolism, specifically, would not hinder the potential anti-COVID-19 activities of cordycepin and would not cause any clinical/therapeutic problem of cordycepin use since it is an advantageous chemical transformation that modifies the nucleoside analogue to a more biocompatible form of nucleotide analogue (the more required form) without any metabolic hydroxylation of the 3′ position of the molecule (as previously mentioned, the nucleotide analogue Cor-TP could be successfully merged into viral RNA and strongly inhibits the transcription elongation and RNA generation because of the absence of a hydroxyl moiety at the 3′ position).50 Clinically, when cordycepin enters the blood circulation after absorption from oral administration, a portion of its amount may be broken down to other structurally related nucleosides, mainly 3′-deoxyinosine by the enzymes adenosine deaminases (which are present in blood plasma, erythrocytes, and vessel walls).50 It was previously thought that 3′-deoxyinosine is a biologically inactive metabolite; thus, it was clinically suggested to coadminister a potent adenosine deaminase inhibitor, e.g., the antimetabolite pentostatin (PTN), with cordycepin to protect it from deamination by the adenosine deaminase since this inhibitor mimics the nucleoside analogue cordycepin and therefore inhibits the enzyme adenosine deaminase and interferes with the ability of the human biological system to break down cordycepin molecules.51−53 Surprisingly, some recent studies refuted the need for adenosine deaminase inhibitor coadministration to achieve the ideal benefits of cordycepin since these studies proved that 3′-deoxyinosine could be intracellularly reconverted to cordycepin again in the form of the very active Cor-TP; thus, the metabolite 3′-deoxyinosine previously considered inactive is now considered as one of the biological metabolites that are well-responsible for the therapeutic actions of cordycepin after its oral administration, and the use of an adenosine deaminase inhibitor is no more a must (Figure 6).50 Another suggestion (in addition to the optional use of a potent adenosine deaminase inhibitor) to increase the blood level of cordycepin and resist any rapid metabolism is the use of a nonspecific inhibitor of nucleoside transporters (including adenosine nucleoside transporters), like dipyridamole (DPM), to solve this issue and allow cordycepin accumulation in the human blood, since this nucleoside transport inhibitor will block the cellular reuptake of cordycepin into platelets, erythrocytes, and endothelial cells, resulting in elevated concentrations of cordycepin extracellularly (i.e., extended clinical half-life of cordycepin).54 This, on the other hand, causes an increase in coronary vasodilatation, which is an extra beneficial effect needed in the comprehensive treatment of COVID-19.54 It should be taken into consideration that DPM has very useful biological effects that can help to prevent or suppress the SARS-CoV-2 exacerbation of respiratory/cardiovascular status and combat the complications of the comprehensive COVID-19 condition.54 Additionally, the two adjuvant drugs DPM and PTN can also have some inhibitory activities against the coronaviral RNA synthesis and replication.51−54
Figure 6.
Scheme of the expected human biological metabolic cycle of cordycepin after oral administration.
Cordycepin is an ideal druglike compound since it fully obeys Lipinski’s rule of five (Ro5) with no violations.47 Cordycepin is also a highly biocompatible molecule with enhanced abilities to pass through biologic membranes when compared with the adenosine molecule due to the relatively higher lipophilic characters resulting from the shortage of a hydroxyl group (i.e., the cordycepin molecule is less hydrophilic than the adenosine molecule). Metabolic similarity with the biosimilar adenosine molecule greatly helps cordycepin in tricking the human biological system and doing its intended therapeutic roles. The experimental findings of the present research are in full agreement with the previously proven effects of cordycepin to partially inhibit and impair mRNA translation.55 These current findings are also in agreement with almost all of the computational predictions previously presented and discussed in Section 1.
3. Conclusions and Future Recommendations
COVID-19 treatment is still a critical challenge to all respective scientists. Cordycepin is a promising potential comprehensive anti-COVID-19 agent that should be put under the microscope in the coming days. Some new studies reported the in silico inhibitory activities of cordycepin on SARS-CoV-2 spike (S) protein and main protease (Mpro) enzyme,46,56,57 and, recently, another computational study showed the in silico inhibitory activities of cordycepin on the SARS-CoV-2 RdRp enzyme.47 In light of the findings of the previous in silico studies and the current in vitro study, it is proven that the cordycepin molecule has interesting superiority and advantage over most of the other investigational anti-SARS-CoV-2 molecules, especially those acting only on the S protein and/or Mpro enzyme, as, first, it acts via the expected synergistic triple anti-SARS-CoV-2 mode of action (i.e., it acts on the three different target proteins, thus inhibiting all the three major stages of the SARS-CoV-2 infection in humans, which are the viral entry, replication/multiplication, and pathogenic phases) and, second, it acts as nonspecific/nonselective anti-SARS-CoV-2, i.e., it is capable of acting on all SARS-CoV-2 strains and variants since its anti-SARS-CoV-2 bioactivity does not depend only on the predicted action on the changeable and mutated S protein (cordycepin has general broad-spectrum anti-SARS-CoV-2 activities effective on all SARS-CoV-2 strains and variants with different mutations).
The expected comprehensive nature of cordycepin in COVID-19 treatment mainly comes from two practically proven pathways. First, it can inhibit the replication and survival of the COVID-19-causing virus itself with potent broad-spectrum activities (including activities against the most recent strains of SARS-CoV-2), which reach an EC50 of about 2 μM. This anti-SARS-CoV-2 action of cordycepin is mainly due to its chemical similarity/analogism with the human adenosine. During viral replication, the SARS-CoV-2 RdRp incorporates Cor-TP into the newly synthesized RNA strands instead of using real ATP. Consequently, when the SARS-CoV-2 RdRp attempts to copy the destabilized RNA containing Cor-TP, it either falsely interprets it or fails to interpret it. This interrupted and hindered interpretation and the consequent errors in the viral genetic code cause a very significant number of mutations in all downstream coronaviral-2 copies that much exceeds the threshold the virus can survive, an effect known in virology as lethal mutagenesis or viral error catastrophe. Second, it can mitigate and relieve COVID-19 characteristic severe symptoms and sequelae that are mainly related to the SARS-CoV-2 invasion of the respiratory and cardiovascular systems of the patients since this attack results primarily in acute biological disruptions of immunologic/inflammatory origin (see the numerous diverse and broad pharmacological activities of cordycepin in Section 1).
Promisingly, the net distinction, in almost all anti-COVID-19 parameters/properties/activities, of cordycepin over the potent antiviral agents remdesivir and GS-441524 supports cordycepin candidacy to be a superior potential comprehensive therapeutic agent against COVID-19. It is also worth mentioning that the cordycepin molecule has more than 10 sites that are considered as very active positions for chemical reactions; thus, hundreds of possible derivatives with required enhanced pharmacokinetic and/or pharmacodynamic properties can be designed and synthesized based on the biocompatible scaffold of this golden multitask molecule, which belongs to the nucleoside analogues’ class of antiviral/antineoplastic therapeutic agents. Last but not the least, it is highly suggested to the scientific community to start the cordycepin repurposing journey against COVID-19 by conducting extensive worldwide preclinical and clinical studies to seriously evaluate the efficacy and safety of cordycepin (as a monotherapy or, optionally, as either a double-combination therapy with DPM or a triple-combination therapy with both DPM and PTN) to be used for the comprehensive treatment and prevention of all types of COVID-19 infections.
4. Materials and Methods
4.1. In Silico Predictive Toxicological and Stability Properties of Cordycepin
There is an increasing need for such type of in silico toxicological and metabolic studies for cordycepin due to their absence in the previous literature. Therefore, a complete simulative toxicological study of cordycepin was implemented in the current work using the ProTox-II web server, which is a speculative web-based laboratory for the prediction of toxicities/adverse effects of small chemical molecules.48 ProTox-II web server computationally integrates the principles of molecular similarity, fragment propensities, fragment similarity-based cluster cross-validation machine learning, and most frequent features of artificial intelligence (AI) with each other, establishing a diverse collection of more than 33 models for the analysis and prediction of diverse toxicity endpoints, e.g., cytotoxicity, general acute toxicity, organ toxicity “mainly hepatotoxicity”, carcinogenicity, immunotoxicity, mutagenicity, adverse outcomes (Tox21) pathways, and several toxicity targets.48,58 Oral toxic doses in this validated virtual laboratory are given as LD50 values in mg/kg BWt.48,58−60 Practically, the LD50 can be defined as the experimental dose at which 50% of test subjects die upon exposure to a compound.48,58−60 This interesting virtual web server classifies the different chemicals into six classes of decreasing order of toxicity (according to the Globally Harmonized System of Classification and Labeling of Chemicals “GHS”) as follows: toxicity class I “fatal if swallowed” (LD50 ≤ 5 mg/kg BWt), toxicity class II “fatal if swallowed” (5 mg/kg BWt < LD50 ≤ 50 mg/kg BWt), toxicity class III “toxic if swallowed” (50 mg/kg BWt < LD50 ≤ 300 mg/kg BWt), toxicity class IV “harmful if swallowed” (300 mg/kg BWt < LD50 ≤ 2000 mg/kg BWt), toxicity class V “may be harmful if swallowed” (2000 mg/kg BWt < LD50 ≤ 5000 mg/kg BWt), and toxicity class VI “nontoxic” (LD50 > 5000 mg/kg BWt).48,58−60 A small complementary simulative/speculative study was also done to determine the expected degree of metabolic stability of cordycepin in its free form, using the PredMS Application.49 This application is a new web server utilized to predict the metabolic stability of a certain chemical compound, where the compound is said to be either stable (if ≥50% was predicted to remain intact after 30 min) or unstable (if <50% was predicted to remain intact after 30 min) in human liver microsomes.49
4.2. In Vitro Anti-SARS-CoV-2 and Cytotoxic Bioactivities of Cordycepin
This credible and robust in vitro anti-COVID-19 test (including the cytotoxicity assay) is based mainly upon the validated procedures of Rabie.8,9,12−15 The complete procedures were carried out in a specialized biosafety level 3 (BSL-3) laboratory. The assayed new strain of SARS-CoV-2 virus, VOC-202012/01, was isolated from the fresh nasopharynx aspirate and throat swab from a 50-year-old man with confirmed COVID-19 infection using Vero E6 cells (ATCC CRL-1586) on September 21, 2021. Stock virus (107.25 TCID50/mL) was prepared after three serial passages in Vero E6 cells in infection media (Dulbecco’s modified Eagle medium (DMEM) supplemented with 4.5 g/L d-glucose, 100 mg/L sodium pyruvate, 2% fetal bovine serum (FBS), 100 000 U/L penicillin–streptomycin, and 25 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES)). Cordycepin (3′-deoxyadenosine, CAS registry number: 73-03-0) and remdesivir (GS-5734, CAS registry number: 1809249-37-3) were purchased from Biosynth Carbosynth (Carbosynth Ltd., Berkshire, U.K.) (for cordycepin, product code: ND02930, purity: ≥98%; for remdesivir, product code: AG170167, purity: ≥98%), while the other reference compound GS-441524 (CAS registry number: 1191237-69-0) was purchased from MedChemExpress (MCE, MedChemExpress LLC, New Jersey, U.S.A.) (catalog number: HY-103586, purity: 99.77%). The ultrapure solvent DMSO (CAS registry number: 67-68-5) was purchased from a local distributor, El-Gomhouria Company For Drugs (El-Gomhouria Co. For Trading Drugs, Chemicals & Medical Supplies, Mansoura Branch, Egypt) (purity: ≥99.9% “anhydrous”). Preliminary pilot assays were performed mainly to determine the best concentration of cordycepin, remdesivir, and GS-441524 to start the in vitro anti-COVID-19 and cytotoxicity tests with. Accordingly, the stocks of the tested compounds were accurately prepared by dissolving each of the three compounds in DMSO to obtain a 100 μM concentration of each compound. Additionally, DMSO was used for the purpose of a negative control comparison to make the study placebo-controlled. To evaluate the in vitro anti-SARS-CoV-2 activity of the target compound, cordycepin, in comparison to that of each of the two positive control drugs, remdesivir and GS-441524, along with that of the negative control solvent, DMSO, Vero E6 cells were pretreated with the four compounds diluted in infection media for 1 h prior to infection by the new variant of the SARS-CoV-2 virus at MOI = 0.02. The four tested compounds were maintained with the virus inoculum during the 2 h incubation period. The inoculum was removed after incubation, and the cells were overlaid with infection media containing the diluted test compounds. After 48 h of incubation at 37 °C, supernatants were immediately collected to quantify viral loads by TCID50 assay or quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) “qRT-PCR” (TaqMan Fast Virus 1-Step Master Mix). Viral loads in this assay were fitted in a logarithmic scale (log10 TCID50/mL and log10 viral RNA copies/mL), not in a linear scale, under increasing concentrations of the tested compounds. Four-parameter logistic regression (GraphPad Prism) was used to fit the dose–response curves and determine the EC50 of the tested compounds that inhibit SARS-CoV-2 viral replication (CPEIC100 was also determined for each compound). Cytotoxicity of each of the four tested compounds was also evaluated in Vero E6 cells using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). The final results were represented as the mean (μ) ± the standard deviation (SD) from the triplicate biological experiments. Statistical analysis was performed using SkanIt 4.0 Research Edition software (Thermo Fisher Scientific) and Prism V5 software (GraphPad). All reported data were significant at p < 0.05.
Acknowledgments
This new discovery did not receive any external funding. The author gratefully thanks and deeply acknowledges anyone who helped to make this new discovery and work coming out to light.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05998.
Graphic comparison of the molecular weight of cordycepin with the mean value of molecular weights of all the dataset compounds (Figure S1); screenshot of the output table for the ProTox-II Toxicity Model Report (Figure S2) (PDF)
The author declares no competing financial interest.
Supplementary Material
References
- Hui D. S.; Azhar E. I.; Madani T. A.; Ntoumi F.; Kock R.; Dar O.; Ippolito G.; Mchugh T. D.; Memish Z. A.; Drosten C.; Zumla A.; Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Map. Available from Johns Hopkins Coronavirus Research Center, https://coronavirus.jhu.edu/map.html (accessed Nov 25, 2021).
- Jiang S.; Du L.; Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerging Microbes Infect. 2020, 9, 275–277. 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moirangthem D. S.; Surbala L. Remdesivir (GS-5734) in COVID-19 Therapy: The Fourth Chance. Curr. Drug Targets 2021, 22, 1346–1356. 10.2174/1389450121999201202110303. [DOI] [PubMed] [Google Scholar]
- Yan V. C.; Muller F. L. Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment. ACS Med. Chem. Lett. 2020, 11, 1361–1366. 10.1021/acsmedchemlett.0c00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunotte L.; Zheng S.; Mecate-Zambrano A.; Tang J.; Ludwig S.; Rescher U.; Schloer S. Combination Therapy with Fluoxetine and the Nucleoside Analog GS-441524 Exerts Synergistic Antiviral Effects against Different SARS-CoV-2 Variants In Vitro. Pharmaceutics 2021, 13, 1400 10.3390/pharmaceutics13091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q.; Yang M.; Liu D.; Chen J.; Shu D.; Xia J.; Liao X.; Gu Y.; Cai Q.; Yang Y.; Shen C.; Li X.; Peng L.; Huang D.; Zhang J.; Zhang S.; Wang F.; Liu J.; Chen L.; Chen S.; Wang Z.; Zhang Z.; Cao R.; Zhong W.; Liu Y.; Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, 6, 1192–1198. 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie A. M. Discovery of (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20): the first potent and specific anti-COVID-19 drug. Chem. Pap. 2021, 75, 4669–4685. 10.1007/s11696-021-01640-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rabie A. M. Cyanorona-20: The first potent anti-SARS-CoV-2 agent. Int. Immunopharmacol. 2021, 98, 107831 10.1016/j.intimp.2021.107831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ip A.; Ahn J.; Zhou Y.; Goy A. H.; Hansen E.; Pecora A. L.; Sinclaire B. A.; Bednarz U.; Marafelias M.; Sawczuk I. S.; Underwood J. P. III; Walker D. M.; Prasad R.; Sweeney R. L.; Ponce M. G.; La Capra S.; Cunningham F. J.; Calise A. G.; Pulver B. L.; Ruocco D.; Mojares G. E.; Eagan M. P.; Ziontz K. L.; Mastrokyriakos P.; Goldberg S. L. Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study. BMC Infect. Dis. 2021, 21, 72 10.1186/s12879-021-05773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Cao R.; Zhang L.; Yang X.; Liu J.; Xu M.; Shi Z.; Hu Z.; Zhong W.; Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie A. M. Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): successful repurposing against COVID-19 as the first potent multitarget anti-SARS-CoV-2 drugs. New J. Chem. 2021, 45, 761–771. 10.1039/D0NJ03708G. [DOI] [Google Scholar]
- Rabie A. M. CoViTris2020 and ChloViD2020: a striking new hope in COVID-19 therapy. Mol. Diversity 2021, 25, 1839–1854. 10.1007/s11030-020-10169-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rabie A. M. Potent toxic effects of Taroxaz-104 on the replication of SARS-CoV-2 particles. Chem.-Biol. Interact. 2021, 343, 109480 10.1016/j.cbi.2021.109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie A. M. Discovery of Taroxaz-104: The first potent antidote of SARS-CoV-2 VOC-202012/01 strain. J. Mol. Struct. 2021, 1246, 131106 10.1016/j.molstruc.2021.131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H.; Sarma P.; Bhattacharyya A.; Sharma S.; Chhimpa N.; Prajapat M.; Prakash A.; Kumar S.; Singh A.; Singh R.; Avti P.; Thota P.; Medhi B. Efficacy and safety of dihydroorotate dehydrogenase (DHODH) inhibitors “leflunomide” and “teriflunomide” in Covid-19: A narrative review. Eur. J. Pharmacol. 2021, 906, 174233 10.1016/j.ejphar.2021.174233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie A. M. Teriflunomide: A possible effective drug for the comprehensive treatment of COVID-19. Curr. Res. Pharmacol. Drug Discovery 2021, 2, 100055 10.1016/j.crphar.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L.; Druce J. D.; Catton M. G.; Jans D. A.; Wagstaff K. M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020, 178, 104787 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Cao R.; Zhang H.; Liu J.; Xu M.; Hu H.; Li Y.; Zhao L.; Li W.; Sun X.; Yang X.; Shi Z.; Deng F.; Hu Z.; Zhong W.; Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discovery 2020, 6, 28 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif J.-C.; Bouabdallaoui N.; L’Allier P. L.; Gaudet D.; Shah B.; Pillinger M. H.; Lopez-Sendon J.; Da Luz P.; Verret L.; Audet S.; Dupuis J.; Denault A.; Pelletier M.; Tessier P. A.; Samson S.; Fortin D.; Tardif J.-D.; Busseuil D.; Goulet E.; Lacoste C.; Dubois A.; Joshi A. Y.; Waters D. D.; Hsue P.; Lepor N. E.; Lesage F.; Sainturet N.; Roy-Clavel E.; Bassevitch Z.; Orfanos A.; Stamatescu G.; Grégoire J. C.; Busque L.; Lavallée C.; Hétu P.-O.; Paquette J.-S.; Deftereos S. G.; Levesque S.; Cossette M.; Nozza A.; Chabot-Blanchet M.; Dubé M.-P.; Guertin M.-C.; Boivin G.; Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien M.; Anderson T. K.; Jockusch S.; Tao C.; Li X.; Kumar S.; Russo J. J.; Kirchdoerfer R. N.; Ju J. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J. Proteome Res. 2020, 19, 4690–4697. 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. G.; Manson W.; Spring F. S.; Hutchinson S. A. Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Luo L.; Dressel W.; Shadier G.; Krumbiegel D.; Schmidtke P.; Zepp F.; Meyer C. U. Cordycepin is an Immunoregulatory Active Ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008, 36, 967–980. 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- Bibi S.; Wang Y.-B.; Tang D.-X.; Kamal M. A.; Yu H. Prospects for Discovering the Secondary Metabolites of Cordyceps Sensu Lato by the Integrated Strategy. Med. Chem. 2021, 17, 97–120. 10.2174/1573406416666191227120425. [DOI] [PubMed] [Google Scholar]
- Yue K.; Ye M.; Zhou Z.; Sun W.; Lin X. The genus Cordyceps: a chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- Cordycepin. PubChem CID: 6303, 2021. Available from PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/6303 (accessed Oct 6, 2021).
- Wang Y.; Yang Z.; Bao D.; Li B.; Yin X.; Wu Y.; Chen H.; Tang G.; Li N.; Zou G. Improving Hypoxia Adaption Causes Distinct Effects on Growth and Bioactive Compounds Synthesis in an Entomopathogenic Fungus Cordyceps militaris. Front. Microbiol. 2021, 12, 698436 10.3389/fmicb.2021.698436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Yang R.; Ruan Z.; Hu T.; Ding H.; Xiao Q. Total Synthesis of Cordycepin. Chin. J. Org. Chem. 2013, 33, 1340–1344. 10.6023/cjoc201303009. [DOI] [Google Scholar]
- Huang S.; Liu H.; Sun Y.; Chen J.; Li X.; Xu J.; Hu Y.; Li Y.; Deng Z.; Zhong S. An effective and convenient synthesis of cordycepin from adenosine. Chem. Pap. 2018, 72, 149–160. 10.1007/s11696-017-0266-9. [DOI] [Google Scholar]
- Adenosine. PubChem CID: 60961, 2021. Available from PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/60961 (accessed Oct 6, 2021).
- Tan L.; Song X.; Ren Y.; Wang M.; Guo C.; Guo D.; Gu Y.; Li Y.; Cao Z.; Deng Y. Anti-inflammatory effects of cordycepin: A review. Phytother. Res. 2021, 35, 1284–1297. 10.1002/ptr.6890. [DOI] [PubMed] [Google Scholar]
- Ashraf S. A.; Elkhalifa A. E. O.; Siddiqui A. J.; Patel M.; Awadelkareem A. M.; Snoussi M.; Ashraf M. S.; Adnan M.; Hadi S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Medicinal Fungus Cordyceps with Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735 10.3390/molecules25122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P.; Li X.; Yang H.; Wang Z.-Y.; Lu D. Therapeutic Potential and Biological Applications of Cordycepin and Metabolic Mechanisms in Cordycepin-Producing Fungi. Molecules 2019, 24, 2231 10.3390/molecules24122231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K.; Masuda M.; Sakurai A.; Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Li B.; Hou Y.; Zhu M.; Bao H.; Nie J.; Zhang G. Y.; Shan L.; Yao Y.; Du K.; Yang H.; Li M.; Zheng B.; Xu X.; Xiao C.; Du J. 3′-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway. Int. J. Neuropsychopharmacol. 2016, 19, pyv112 10.1093/ijnp/pyv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli H. S.; Sandhu S. S.; Sharma A. K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. 10.1007/s13205-013-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemotherapy With Cordycepin Plus Pentostatin in Treating Patients with Refractory Acute Lymphocytic or Chronic Myelogenous Leukemia. ClinicalTrials.gov Identifier: NCT00003005, 2021. Available from ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00003005 (accessed Oct 8, 2021).
- Study of Cordycepin Plus Pentostatin in Patients With Refractory TdT-Positive Leukemia. ClinicalTrials.gov Identifier: NCT00709215, 2021. Available from ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00709215 (accessed Oct 8, 2021).
- Panya A.; Songprakhon P.; Panwong S.; Jantakee K.; Kaewkod T.; Tragoolpua Y.; Sawasdee N.; Lee V. S.; Nimmanpipug P.; Yenchitsomanus P.-T. Cordycepin Inhibits Virus Replication in Dengue Virus-Infected Vero Cells. Molecules 2021, 26, 3118 10.3390/molecules26113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E.; Son M.; Lee M.; Lee K.; Cho J. Y.; Cho S.; Lee S. K.; Lee Y. M.; Cho H.; Sung G.-H.; Kang H. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. 10.18632/oncoscience.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E. G.; Weiler B. E.; Charubala R.; Pfleiderer W.; Leserman L.; Sobol R. W.; Suhadolnik R. J.; Schröder H. C. Cordycepin analogues of 2′,5′-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry 1991, 30, 2027–2033. 10.1021/bi00222a004. [DOI] [PubMed] [Google Scholar]
- Mahy B. W. J.; Cox N. J.; Armstrong S. J.; Barry R. D. Multiplication of Influenza Virus in the Presence of Cordycepin, an Inhibitor of Cellular RNA Synthesis. Nat. New Biol. 1973, 243, 172–174. 10.1038/newbio243172a0. [DOI] [PubMed] [Google Scholar]
- Lonai P.; Declève A.; Kaplan H. S. Spontaneous Induction of Endogenous Murine Leukemia Virus-Related Antigen Expression During Short-Term In Vitro Incubation of Mouse Lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1974, 71, 2008–2012. 10.1073/pnas.71.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. L.; Dawson W. O. Effect of Cordycepin Triphosphate on In Vitro RNA Synthesis by Plant Viral Replicases. J. Virol. 1979, 29, 811–814. 10.1128/jvi.29.2.811-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O. Tobacco mosaic virus protein synthesis is correlated with double-stranded RNA synthesis and not single-stranded RNA synthesis. Virology 1983, 125, 314–323. 10.1016/0042-6822(83)90204-0. [DOI] [PubMed] [Google Scholar]
- Verma A. K. Cordycepin: a bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID-19. J. Biomol. Struct. Dyn. 2020, 1–8. 10.1080/07391102.2020.1850352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi S.; Hasan M. M.; Wang Y.-B.; Papadakos S. P.; Yu H. Cordycepin as a Promising Inhibitor of SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp). Curr. Med. Chem. 2022, 29, 152–162. 10.2174/0929867328666210820114025. [DOI] [PubMed] [Google Scholar]
- ProTox-II Virtual Laboratory. https://tox-new.charite.de/protox_II (accessed Oct 5, 2021).
- PredMS Application. https://predms.netlify.app (accessed Oct 5, 2021).
- Lee J. B.; Radhi M.; Cipolla E.; Gandhi R. D.; Sarmad S.; Zgair A.; Kim T. H.; Feng W.; Qin C.; Adrower C.; Ortori C. A.; Barrett D. A.; Kagan L.; Fischer P. M.; de Moor C. H.; Gershkovich P. A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin. Sci. Rep. 2019, 9, 15760 10.1038/s41598-019-52254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.; Nakagome I.; Hirono S.; Itoh T.; Fujiwara R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol. Res. Perspect. 2015, 3, e00121 10.1002/prp2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg M. E.; Masocha W.; Ferella M.; Petitto-Assis F.; Goto H.; Kristensson K.; McCaffrey R.; Wigzell H. Treatment of African Trypanosomiasis with Cordycepin and Adenosine Deaminase Inhibitors in a Mouse Model. J. Infect. Dis. 2005, 192, 1658–1665. 10.1086/496896. [DOI] [PubMed] [Google Scholar]
- Rodman L. E.; Farnell D. R.; Coyne J. M.; Allan P. W.; Hill D. L.; Duncan K. L. K.; Tomaszewski J. E.; Smith A. C.; Page J. G. Toxicity of Cordycepin in Combination with the Adenosine Deaminase Inhibitor 2′-Deoxycoformycin in Beagle Dogs. Toxicol. Appl. Pharmacol. 1997, 147, 39–45. 10.1006/taap.1997.8264. [DOI] [PubMed] [Google Scholar]
- Fata-Hartley C. L.; Palmenberg A. C. Dipyridamole Reversibly Inhibits Mengovirus RNA Replication. J. Virol. 2005, 79, 11062–11070. 10.1128/JVI.79.17.11062-11070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. Y.; Moon A.; Duffin R.; Barthet-Barateig A.; Meijer H. A.; Clemens M. J.; de Moor C. H. Cordycepin Inhibits Protein Synthesis and Cell Adhesion through Effects on Signal Transduction. J. Biol. Chem. 2010, 285, 2610–2621. 10.1074/jbc.M109.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K.; Aggarwal R. Repurposing potential of FDA-approved and investigational drugs for COVID-19 targeting SARS-CoV-2 spike and main protease and validation by machine learning algorithm. Chem. Biol. Drug Des. 2021, 97, 836–853. 10.1111/cbdd.13812. [DOI] [PubMed] [Google Scholar]
- Suwannarach N.; Kumla J.; Sujarit K.; Pattananandecha T.; Saenjum C.; Lumyong S. Natural Bioactive Compounds from Fungi as Potential Candidates for Protease Inhibitors and Immunomodulators to Apply for Coronaviruses. Molecules 2020, 25, 1800 10.3390/molecules25081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P.; Eckert A. O.; Schrey A. K.; Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwal M. N.; Banerjee P.; Dunkel M.; Wettig M. R.; Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. 10.1093/nar/gku401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P.; Dehnbostel F. O.; Preissner R. Prediction Is a Balancing Act: Importance of Sampling Methods to Balance Sensitivity and Specificity of Predictive Models Based on Imbalanced Chemical Data Sets. Front. Chem. 2018, 6, 362 10.3389/fchem.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.