Abstract
Background
Air pollution exposure may make people more vulnerable to COVID-19 infection. However, previous studies in this area mostly focused on infection before May 2020 and long-term exposure.
Objective
To assess both long-term and short-term exposure to air pollution and COVID-19 incidence across four case surges from 03/1/2020 to 02/28/2021.
Methods
The cohort included 4.6 million members from a large integrated health care system in southern California with comprehensive electronic medical records (EMR). COVID-19 cases were identified from EMR. Incidence of COVID-19 was computed at the census tract-level among members. Prior 1-month and 1-year averaged air pollutant levels (PM2.5, NO2, and O3) at the census tract-level were estimated based on hourly and daily air quality data. Data analyses were conducted by each wave: 3/1/2020–5/31/2020, 6/1/202–9/30/2020, 10/1/2020–12/31/2020, and 1/1/2021–2/28/2021 and pooled across waves using meta-analysis. Generalized linear mixed effects models with Poisson distribution and spatial autocorrelation were used with adjustment for meteorological factors and census tract-level social and health characteristics. Results were expressed as relative risk (RR) per 1 standard deviation.
Results
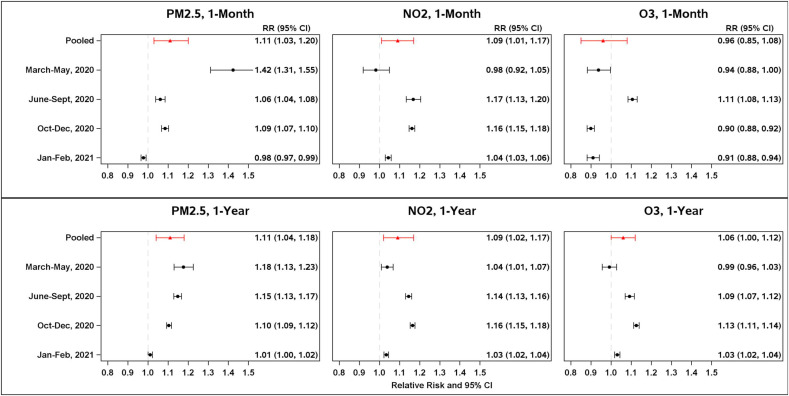
The cohort included 446,440 COVID-19 cases covering 4609 census tracts. The pooled RRs (95% CI) of COVID-19 incidence associated with 1-year exposures to PM2.5, NO2, and O3 were 1.11 (1.04, 1.18) per 2.3 μg/m3,1.09 (1.02, 1.17) per 3.2 ppb, and 1.06 (1.00, 1.12) per 5.5 ppb respectively. The corresponding RRs (95% CI) associated with prior 1-month exposures were 1.11 (1.03, 1.20) per 5.2 μg/m3 for PM2.5, 1.09 (1.01, 1.17) per 6.0 ppb for NO2 and 0.96 (0.85, 1.08) per 12.0 ppb for O3.
Conclusion
Long-term PM2.5 and NO2 exposures were associated with increased risk of COVID-19 incidence across all case surges before February 2021. Short-term PM2.5 and NO2 exposures were also associated. Our findings suggest that air pollution may play a role in increasing the risk of COVID-19 infection.
Keywords: Air pollution, COVID-19, Incidence, PM2.5, NO2, O3
1. Introduction
The new Coronavirus Disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in China in December 2019 (Zhu et al., 2020a). Since then, there have been over 266 million cases and over 5.2 million deaths caused by COVID-19 infection all over the world (Dong et al., 2020; −19 Dashboard. G, 2020). In the United States, there have been consecutive case waves in 2020–2021 across seasons with the highest peak case numbers in December to January 2021 (Coronavirus in the:, 2021). A growing number of reports support a role for ambient air pollutant exposures on COVID-19 prevalence and mortality (Bontempi, 2020; Conticini et al., 2020; Fattorini and Regoli, 2020; Setti et al., 2020; Zoran et al., 2020a; Wu et al., 2020; Bianconi et al., 2020; Yao et al., 2020; Travaglio et al., 2021; Pozzer et al., 2020; Hutter et al., 2020; Cole et al., 2020; Azuma et al., 2020; De Angelis et al., 2021; Zang et al., 2021; Marques and Domingo, 2022). Ecological studies reported before May 2020 concluded that chronic exposure to ambient air pollutants was associated with increased COVID-19 incidence (Hutter et al., 2020; Jiang et al., 2020; Li et al., 2020; Wang et al., 2020; Yao et al., 2021; Andrée, 2020). Long-term air pollution exposure may increase the risk of COVID infection through the chronic effect on preexisting comorbidities (Langrish et al., 2012; An et al., 2018; Lawal, 2017) and immune alterations (Lin et al., 2018). However, most previous studies used aggregated air pollution exposure over long-term and COVID-19 cumulative case or incidence data from Europe and China and only investigated the associations in the early pandemic period. Few have investigated the role of air pollution from early 2020 to the more recent case peak in year 2021. One national study in Israel assessed the long-term averaged PM2.5 exposure with COVID-19 cases from 2020 to January 2021 (Levi and Barnett-Itzhaki, 2021). A second study assessed NO2 exposure in 2016 and COVID-19 case rate in Los Angeles County from March 2020 to February 2021 (Lipsitt et al., 2021).
To our knowledge, the role of short-term air pollution exposure on COVID-19 incidence remained unclear. Limited evidence suggested that nitrogen dioxide (NO2), fine particulate matter (PM2.5), and ozone (O3) exposures up to 21 lagged days were associated with increased COVID-19 incidence (Zoran et al., 2020a; Yao et al., 2021; Adhikari and Yin, 2020; Xing et al., 2021). Since COVID-19 intervention policies may influence the ambient air quality during the pandemic year, the role of short-term air pollution may change over the time. A better understanding of the effects of short-term ambient air pollutant exposures on COVID-19 risk over the long period of COVID-19 pandemic is needed as such exposure are modifiable and interventions to reduce exposures have the potential to reduce COVID-19 incidence.
Additional limitations of previous studies of air pollution and COVID-19 incidence included limited diversity in geographic regions and population and potentially mismatching the exposure time windows and case diagnosis date limited by the publicly available population data.
The purpose of this study was to assess both long-term and short-term air pollution exposure and COVID-19 incidence across four case surges from 03/01/2020 to 02/28/2021. Data were derived from a large integrated health care system with comprehensive electronic medical records (EMR) and 4.6 million members covering southern California and over 446,000 COVID-19 incident cases. COVID-19 case ascertainment was optimally defined using clinical diagnosis and date information from the EMR. The study is novel by investigating associations of both short- and long-term air pollution exposures with COVID-19 incidence in the large population of Southern California which is diverse in age, sex, and race/ethnicity. Also, comparing the air pollution effects between later and earlier COVID-19 pandemic periods is important to confirm the consistent effects of air pollution on COVID-19 incidence across dynamically changing conditions of pandemic, policy, environment, and society.
2. Materials and methods
2.1. Population and COVID-19 case identification and incidence by month and census tract
The study population is members of Kaiser Permanente Southern California (KPSC) between March 1, 2020 and February 28, 2021. KPSC is an integrated healthcare system with 4.6 million members across Southern California, representing approximately 20% of the southern California population. Social demographic characteristics of KPSC members are similar to the diverse population in southern California (Koebnick et al., 2012).
KPSC members diagnosed with COVID-19 between March 1, 2020 and February 28, 2021 were identified based on positive SARS-CoV-2 polymerase chain reaction (PCR) lab test result or a diagnosis code (ICD-10 and internal KPSC codes) for COVID-19 (Supplement table 1). To avoid false positives, individuals with a negative lab result within two weeks following an asymptomatic COVID-19 diagnosis code were not considered as COVID-19 cases. The diagnosis date was the earliest lab order date for those with lab data or the earliest COVID-19 diagnosis code for those with only diagnosis codes. Individuals with COVID-19 diagnosis were aggregated by census tract of residence by the month of diagnosis. No individuals had repeat COVID-19 infection within the study period. Incidence of COVID-19 at the month and census tract level was then calculated where the denominator was the KPSC member size at each month by census tracts and the numerator was the KPSC members with COVID-19 diagnosis in the corresponding month and census tract.
2.2. Regional air pollution exposure
Daily averages for air pollutant levels (PM2.5, NO2, and O3) by census tract were estimated based on hourly and daily air quality data from ambient monitoring stations reported to the U.S. Environmental Protection Agency's Air Quality System (Environmental Protec, 2021) and the California Air Resources Board's Air Quality and Meteorological Information System. Air monitoring stations in California are spaced 20–30 km (km) apart in populated areas, which provides a good characterization of regional air pollution gradients. PM2.5 is measured through Federal Reference Method (FRM) and Federal Equivalent Method (FEM) monitors, while NO2 and O3 are measured by the FRM monitors. Location data and daily air quality data were used in inverse distance-squared weighting algorithms to map exposures. The algorithm spatially interpolates air quality data from up to four monitoring stations within a 50 km radius of the tract centroid. Daily residential air quality data at the census tract level was further averaged to assign exposure levels during shorter- and longer-term periods prior to the month of COVID-19 diagnosis. 1-month averaged exposures were used as representative of shorter-term exposure (Chen et al., 2016; Fouladi et al., 2020; Pegoraro et al., 2021) and 1-year averaged exposures were used as longer-term exposures.
2.3. Meteorological factors
Daily meteorological data was extracted from the national Gridset regional-scale reanalysis that provides spatially and temporally complete, high-resolution (4-km) gridded surface meteorological variables (temperature and relative humidity) (Abatzoglou, 2013). Average 1-month temperature and minimum relative humidity at the census tract level prior to each month of COVID-19 diagnosis were obtained from the daily measures and were treated as covariates in the data analysis.
2.4. Neighborhood characteristics
Population density and public transportation use within the census tract were extracted from the 2019 US census American community survey (ACS). Neighborhood race/ethnicity, age, gender, and Medicaid status for the study population were extracted from KPSC patient self-reported and administrative data and aggregated to the census tract level. The neighborhood deprivation index (NDI), a composite measure including education, unemployment, crowding, female head of household, income, poverty, public assistance, and occupation was calculated based on 2018 ACS data and divided into quintiles (Andrews et al., 2020; Messer et al., 2006). The higher the NDI value, the higher the level of deprivation in the neighborhood (Andrews et al., 2020; Messer et al., 2006). Tree canopy coverage was extracted from the California Healthy Places Index tree canopy measure based on the national land cover database. (Delaney et al., 2018; Homer et al., 2015). Percentage of smokers within the census tract was extracted from self-reported EMR data. Prevalence of obesity, diabetes, asthma, and hypertension were based on electronic medical records (EMR) and calculated based on healthcare effectiveness data and information set (HEDIS) definitions for the Kaiser Permanente Geographically Enriched Member Sociodemographic (GEMS) data-mart. All these neighborhood characteristics were treated as covariates in data analysis.
2.5. Statistical analysis
Data analysis used generalized linear mixed effects models with a Poisson distribution where the outcome variable was the number of COVID cases at each month for each census tract with the log of total KPSC member at the month and census tract as the offset, and the exposure variable was air pollution levels 1-month or 1-year prior to the month of COVID-diagnosis. Moran's I was used to assess spatial autocorrelation among census tract and results (I = 0.53, p<0.0001) support clustered autocorrrelation (Mitchell, 2005); thus, the models included a random intercept of census tract and a spatial autocorrelation based on the latitude and longitude of the tract centroid with an exponential spatial covariance structure. To account for different COVID-19 surge patterns that could be influenced by various factors such as policy and lifestyle changes, the study cohort was divided into four waves: March to May 2020, June to September 2020, October to December 2020, and January to February 2021. The classification of waves was based on the peak number of cases. The cut between December and January was to account for vaccine availability that started in the beginning of 2021. Data analysis was conducted by each wave and results were pooled across all waves using meta-analysis techniques. All models were adjusted for month, minimum relative humidity and average temperature in the prior month, population density, public transportation use, race/ethnicity, age, gender, NDI, tree canopy, and prevalence of smoking, obesity, diabetes, asthma, and hypertension within the census tract. For each air pollutant, heterogeneities of the associations between air pollutant and rates of COVID-19 cases across four waves were assessed using the I2 statistic (Deeks et al., 2021). The I2 statistic were all above 75%, suggesting strong heterogeneities thus results were pooled across four waves with random effects meta-analysis using the DerSimonian-Laird estimators (DerSimonian RaL, 1986; Spiegelman EHaD, 2021). Results are presented as relative risks (RR) and 95% confidence intervals (CI) for an increase of one standard deviation (SD) of the corresponding exposure across all waves. Analyses were performed using ArcGIS Pro (Environmental Systems Research Institute (ESRI) Redlands, CA, USA) and SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
3. Results
A total of 446,440 KPSC members were identified as having a COVID-19 diagnosis during the study period. KPSC members live across 4609 Southern California census tracts. The mean population per square mile in these tracts was 10,023 (SD 9532) people (Table 1 ). The average proportion of public transportation use was 4.1% (SD 5.9%) and 48.3% of tracts were predominantly Hispanic (Table 1). Also, 969 tracts (21%) had the highest level of neighborhood deprivation, while the mean adult obesity and diabetes prevalence of KPSC members across these census tracts were 38.1% and 9.3%, respectively (Table 1).
Table 1.
Census tract characteristics for Kaiser Permanente Southern California members.
| N = 4609 Census Tracts | |
|---|---|
| Population per square mile a | 10,023 (9532) |
| Public transportation users, percentage a | 4.1 (5.9) |
| Neighborhood race/ethnicity, highest percentage b | |
| Asian/Pacific Islander | 272 (5.9) |
| Black | 128 (2.8) |
| Hispanic | 2225 (48.3) |
| Non-Hispanic White | 1984 (43) |
| Age 65+, percentage a | 15.5 (7.9) |
| Males, percentage a | 48.6 (3.5) |
| Medicaid, percentage a | 7.4 (4.2) |
| Neighborhood deprivation index (NDI) quintile b | |
| 1 (Lowest level of deprivation) | 654 (14.2) |
| 2 | 988 (21.4) |
| 3 | 982 (21.3) |
| 4 | 1015 (22) |
| 5 (Highest level of deprivation) | 969 (21) |
| Tree canopy, percentage a | 4.9 (2.8) |
| Current smokers, percentage a | 7.3 (2.5) |
| Adult obesity, percentage a | 38.1 (10.98) |
| Adult diabetes, percentage a | 9.3 (3.15) |
| Adult hypertension, percentage a | 10.9 (3.23) |
| Asthma, percentage a | 1.0 (0.52) |
Mean (Standard Deviation).
Number of census tracts (%).
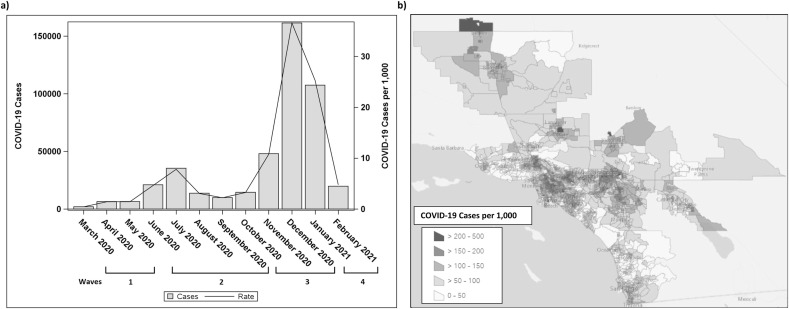
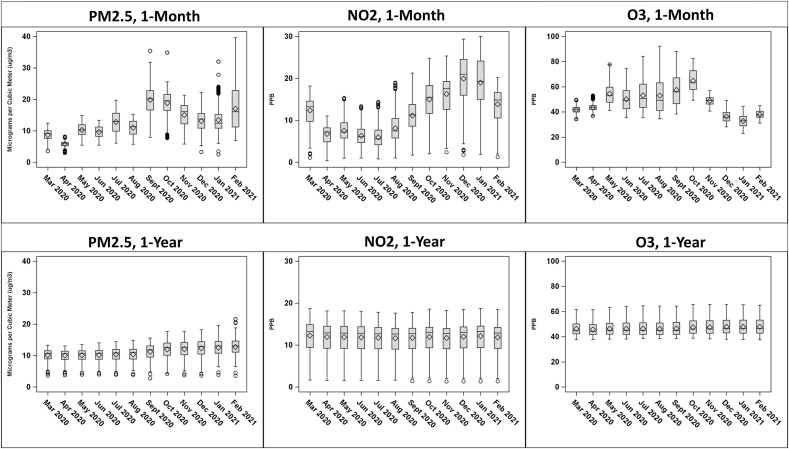
The highest rate of COVID-19 cases was in December 2020 (37 new cases per 1000 members) followed by January 2021 (25 new cases per 1000 members), November 2020 (11 new cases per 1000 members), and July 2020 (8 new cases per 1000 members) (Fig. 1 ). The prior 1-month mean (SD) PM2.5, NO2, and O3 exposures before COVID-19 diagnosis across all the waves were 12.8 (5.2) μg/m3, 11.9 (6.0) ppb, and 47.8 (12.0) ppb respectively. The corresponding prior 1-year means (SD) were 11.2 (2.3) μg/m3 for PM2.5, 11.9 (3.2) ppb for NO2, and 46.9 (5.5) ppb for O3. Fig. 2 presents distribution of 1-month and 1-year PM2.5, NO2, and O3 across study periods by month. The highest correlations observed were between PM2.5 and NO2, with Pearson's correlation coefficient of 0.5 for 1-month exposures and 0.7 for 1-year exposures (Supplement Table 2).
Fig. 1.
a) Number of COVID-19 Cases and COVID-19 case rate per 1000 members in Kaiser Permanente Southern California census tracts by month and b) COVID-19 rate per 1000 members by census tract from March 2020 through February 2021.
Fig. 2.
Distribution of 1-month and 1-year means for PM2.5 (ug/m3), NO2 (ppb), and O3 (ppb) by month.
The wave stratified and pooled adjusted RR of COVID-19 incidence associated with each pollutant are displayed in Fig. 3 . 1-month PM2.5 exposure prior to diagnosis was positively associated with COVID-19 incidence from March to December 2020. However, in January to February 2021, it was negatively associated with COVID-19 incidence. The pooled RR across four waves for a one SD (5.2 mg/m3) increase in PM2.5 was 1.11 (95% CI 1.03, 1.20). 1-month NO2 exposure was positively associated with COVID-19 incidence from June 2020 through February 2021, the pooled RR across 4 waves for a one SD (6.0 ppb) increase was 1.09 (95% CI 1.01, 1.17). 1-month O3 was not associated with COVID-19 incidence in the pooled analysis (RR 0.96, 95% CI 0.85, 1.08), although the associations fluctuated throughout the study periods (Fig. 3).
Fig. 3.
Pooled and 4 time wave stratified (March–May 2020, June–Sept, 2020, Oct–Dec 2020, Jan–Feb 2021) relative risk of COVID-19 incidence at the census trace levels associated with 1 standard deviation (SD) increase in 1-month (top panel) and 1-year (bottom panel) average exposures from PM2.5, NO2, and O3 at the census tract levels, adjusted for month, population density, race/ethnicity, age, sex, neighborhood deprivation index, tree canopy, public transportation use, proportion of smoking, obesity, diabetes, asthma, hypertension, and spatial autocorrelation of census tract. The SDs were 5.2 μg/m3, 6.0 ppb, and 12.0 ppb for 1-month PM2.5, NO2, and O3, and 2.3 μg/m3, 3.2 ppb, and 5.5 ppb for 1-year PM2.5, NO2, and O3.
For 1-year averaged exposures, all three pollutants were significantly associated with COVID-19 incidence in the pooled analysis over four waves. The pooled RR (95% CI) were 1.11 (1.04, 1.18) per one SD (2.3 μg/m3) for PM2.5, 1.09 (1.02, 1.17) per one SD (3.2 ppb) for NO2, and 1.06 (1.00, 1.12) per one SD (5.5 ppb) for O3 (Fig. 3). In the analysis by each wave, all three pollutants were positively associated with COVID-19 incidence in each wave except for O3, which was not associated from March to May 2020. The associations were generally smaller from January to February 2021, compared to the previous 3 waves in 2020.
4. Discussion
Based on this large and diverse population cohort in Southern California we found that both 1-year and 1-month averaged PM2.5 and NO2 exposures were associated with increased risk of COVID-19 incidence in Southern California between March 2020 and February 2021. Significant associations were also observed for 1-year averaged O3 exposure during this period. The associations were lower during the recent case surge wave from January to February 2021 when the vaccination campaign started, compared to the waves from June to December 2020. The associations were also lower at the beginning of the pandemic from March to May 2020 for NO2 and O3, which could be explained by the small number of cases. The associations of 1-month O3 exposure fluctuated over the four waves, and overall was not associated with COVID-19 incidence in the pooled analysis. The fluctuation of the associations might be due to the large seasonal changes in O3 exposure. The air pollution associations were independent of socio-contextual variables such as neighborhood age, race/ethnicity, sex, socio-economic status, neighborhood deprivation (including crowding and poverty), public transportation use, comorbidity prevalence, as well as meteorological factors. Our findings suggest that long-term exposures to ambient air pollutants including PM2.5, NO2 and O3 may contribute to higher risk of COVID-19 infection. Short-term PM2.5 and NO2 exposures may also have an effect.
The finding of air pollution association with COVID-19 incidence is consistent with findings from previous ecological analyses worldwide (Bontempi, 2020; Conticini et al., 2020; Fattorini and Regoli, 2020; Setti et al., 2020; Zoran et al., 2020a, 2020b; Marques and Domingo, 2022; Jiang et al., 2020; Li et al., 2020; Wang et al., 2020; Yao et al., 2021; Fronza et al., 2020; Zhu et al., 2020b). However, most previous studies have only investigated the earlier pandemic period before May 2020. Only two studies included cases during the surge in early 2021 (Levi and Barnett-Itzhaki, 2021; Lipsitt et al., 2021). One large national study in Israel found that long-term PM2.5 exposure was significantly associated with higher COVID-19 case numbers at the first three wave peaks before December 2020 but was not associated at the third wave peak in January 2021. Our findings are consistent with this trend that the associations of 1-year averaged exposures to PM2.5 and NO2 were generally larger from June to December 2020, while the association in January to February 2021 was smaller but still statistically significant. In contrast, another study in Los Angeles, US assessed the association of historical NO2 exposure in 2016 with COVID-19 incidence and found that the effect sizes were similar before September 2020 compared to the later time till February 2021. We extended the analysis to a larger geographic area in Southern California and our meta-analysis pooling the associations over four waves suggest that long-term PM2.5, NO2 and O3 exposure all contribute to the risk of COVID-19 incidence. It is noted that during the later pandemic period, the long-term air pollution exposure effects on COVID-19 infection may be attenuated by the nonpharmaceutical interventions and vaccinations. The dynamic changes in new virus variants and lifestyle and mobility patterns could also influence the associations.
Potential pathophysiological mechanisms related to the long-term air pollution effects on COVID-19 infection include the chronic effects of air pollution on comorbidities such as respiratory and cardiometabolic diseases (Langrish et al., 2012; An et al., 2018; Lawal, 2017). Air pollution exposure could also perturb immune response and increase the susceptibility of virus infection (Lin et al., 2018). Rodent studies have shown that PM2.5 and NO2 exposures increase the expression of coronavirus binding receptor, angiotensin-converting enzyme 2 (ACE-2) in the lung (Aztatzi-Aguilar et al., 2015; Sagawa et al., 2021; Meulenbelt et al., 1992; Patel et al., 1990). The effect of short-term air pollution on COVID-19 incidence is less studied and unclear, though fine particles in the ambient air may help to transport virus. A few studies in earlier pandemic period have shown that short-term up to 21-day prior exposures to PM2.5, O3, and NO2 were associated with increased COVID-19 incidence; however, many time-varying confounders such as the change in air quality due to lock-down policy, wearing masks, changes in mobility, and new variants may all affect the role of short-term air pollution (Coccia, 2021a, 2021b; Saini et al., 2021; Adhikari et al., 2020). Since meteorological factors have temporal correlations with short-term air pollution exposures, temperature and humidity are also key confounders. In this study, after adjusting for sociodemographic, comorbidity, and meteorological confounders, we observed significant associations with 1-month PM2.5 during March to December 2020 and with NO2 exposures during June 2020-Feburary 2021. There was no association with 1-month O3 by pooling the data across four waves. Considering the correlations of short and long-term air pollution exposures, the association with short-term exposure may also be partially driven by the long-term air pollution effects. Taken together, our findings suggest that both long-term and short-term PM2.5 and NO2 may play a role in increasing the risk of COVID-19 infection.
The advantages of this study include a large and multiethnic population cohort based on over 4.6 million members and over 446,000 COVID-19 cases from KPSC medical system in southern California. The analysis covers four major case waves from March 2020 to February 2021, which allows the continuous assessment of air pollution effect in the ever-changing pandemic situations. Uniform guidelines were applied for testing and clinical diagnosis of COVID-19 across all KPSC medical centers, which minimize the bias in case ascertainment. To our knowledge, this is the first study assessing both long-term and short-term ambient air pollution effect on COVID-19 incidence in a large geographic area with diverse population adjusting for detailed socio-contextual, race/ethnicity, comorbidities, and meteorological covariates.
We also acknowledge several limitations of this study. First, this is an ecological analysis based on air pollution exposure and COVID-19 incidence rate aggregated by census tract. The aggregated exposure and outcome data may induce bias in the observed associations. Longitudinal cohort studies with individual-level data are needed to corroborate our findings. The estimate of air pollution exposure based on central monitor data at census track level could be biased from individual air pollution exposure. Within census tracks, the level of individual air pollution exposure is not only varied by the residential proximity to different air pollution sources such as industrial, commercial and transportation, but also influenced by individual time activity patterns, residential characteristics, window-opening time, occupation, and meteorological factors. Personal air pollution monitors for specific air pollutants are needed to accurately measure individual air pollution exposure. However, it is not feasible to monitor personal exposures to various air pollutants in a long-term period across a large population as the case in this study. Beyond three air pollutants: NO2, PM2.5 and O3 that were investigated in this study, other air pollutants including carbon monoxide (CO), particulate matter with a diameter less than 10 μm or less (PM10), and sulfur dioxide (SO2) are also important ambient air pollutants that have been investigated for their associations with COVID-19 prevalence and incidence, mostly in Europe and China. Several studies have found associations between exposure to PM10 and increased number of COVID-19 cases (Setti et al., 2020; Bianconi et al., 2020; De Angelis et al., 2021; Jiang et al., 2020; Zhu et al., 2020b; Solimini et al., 2021; Sahoo, 2021; Zheng et al., 2021; Ma et al., 2021; Saez et al., 2020), while fewer studies have investigated the role of CO and SO2 for their associations with COVID-19 incidence and transmissibility and results were inconsistent across studies (Meo et al., 2021; Ran et al., 2020). The associations of short- and long-term exposures to PM10, SO2 and CO in Southern California need to be investigated in future studies. In terms of the choice of short and long-term exposure time windows, we used 1-month and 1-year averaged exposures to represent exposure levels during a relatively shorter and longer-time period before the COVID-19 diagnosis date. We chose this method based on our ecological design. For future cohort studies with longitudinal data of COVID-19 incidence and daily air pollution exposure data, time-series analysis using distributed lag models can be considered to identify critical exposure time windows with consideration of other time-varying covariates such as time activity pattern and meteorological factors. Second, although a spectrum of key socio-characteristics and neighborhood-level covariates were adjusted for in this analysis, several potential confounders such as indoor air pollution, built-environment, traffic exposure, occupation, adherence to intervention policy and variants of virus were not available. Future studies are needed to investigate the role of these covariates in the associations between air pollution exposure and COVID-19 incidence. Third, although the pooled association with 1-month O3 exposure was not statistically significant, negative association between 1-month O3 and COVID-19 incidence was observed for later pandemic periods from October–December 2020 and January–February 2021. It is known that larger measurement error exists in O3 exposure compared to NO2 and PM2.5 exposures when using outdoor exposure level to predict personal exposure level (Geyh et al., 2000). In general, O3 had less robust association with COVID-19 incidence compared to PM2.5 and NO2 in this study. Moreover, considering the negative correlations between O3 and NO2 due to the photochemical reactions and inconsistent associations observed for O3 from short to long-term exposure periods, the negative associations with 1-month O3 need to be interpreted with caution. Lastly, due to the temporal correlations of air pollution exposure, our analyses could not fully dissect the long and short-term air pollution effects. Studies using individual exposure monitoring over the pandemic period will help to explore the independent effect of short-term air pollution exposure beyond the long-term effect.
5. Conclusions
Findings from this large EMR-based population cohort reveals that long-term exposures to PM2.5, NO2, and O3 are associated with increased risk of COVID-19 infection across all case surges before February 2021. The associations were smaller but still statistically significant in the recent surge during January–February 2021 compared to the associations in 2020. Short-term PM2.5 and NO2 exposures may also contribute to the risk. The effect of air pollution was independent of key neighborhood characteristics such as race/ethnicity, age, sex, and socioeconomic status, as well as meteorological factors. Taken together, our findings indicate that reducing exposures and improving air quality need to be considered in the prevention of COVID-19 surges in the future.
Author contributions
M.A.S., Z.C., F.D.G., and A.H.X. were responsible for the study concept and design. A.H.X. and Z.C. obtained funding. A.H.X., Z.C., M.A.S., B.Z.H., T.C., S.P.E., M.P.M., F.L., D.C.T., and F.D.G. conducted the study. B.Z.H., M.A.S., T.C., M.P.M., and A.H.X., acquired data. B.Z.H, M.A.S., T.C., and A.H.X. analyzed data. M.A.S., Z.C., B.Z.H, and A.H.X. drafted the manuscript. All authors revised the manuscript for important intellectual content and approved the final version to be published.
Funding
This study was supported by the National Institute of Environmental Health Sciences (3R01ES029963-01 to AHX and ZC) at the National Institutes of Health, and the Keck School of Medicine Department of Preventive Medicine COVID-19 Pandemic Research Center (CPRC) at the University of Southern California. The funding agencies had no role in the design or conduct of the study; in the analysis or interpretation of the data; or in the preparation, review, or approval of the manuscript.
The project protocol has been reviewed and approved by the Institutional Review Board at Kaiser Permanente Southern California and the University of Southern California.
Declaration of competing interest
No potential conflicts of interest relevant to this article were reported.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.112758.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- WHO COVID-19 Dashboard. World Health Organization; Geneva: 2020. https://covid19.who.int/ Available online: [Google Scholar]
- Abatzoglou J.T. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 2013;33(1):121–131. [Google Scholar]
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM2.5, and meteorological factors on COVID-19 confirmed cases and deaths in queens, New York. Int. J. Environ. Res. Publ. Health. 2020;17(11) doi: 10.3390/ijerph17114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari S., Pantaleo N.P., Feldman J.M., Ogedegbe O., Thorpe L., Troxel A.B. Assessment of community-level disparities in coronavirus disease 2019 (COVID-19) infections and deaths in large US metropolitan areas. JAMA Netw. Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z., Jin Y., Li J., Li W., Wu W. Impact of particulate air pollution on cardiovascular health. Curr. Allergy Asthma Rep. 2018;18(3):15. doi: 10.1007/s11882-018-0768-8. [DOI] [PubMed] [Google Scholar]
- Andrée B.P.J. Incidence of COVID-19 and connections with air pollution exposure: evidence from The Netherlands. medRxiv. 2020 2020.2004.2027.20081562. [Google Scholar]
- Andrews M.R.T.K., Claudel S.E., et al. Geospatial analysis of neighborhood deprivation index (NDI) for the United States by County. J. Maps. 2020;16:101–112. doi: 10.1080/17445647.2020.1750066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aztatzi-Aguilar O.G., Uribe-Ramirez M., Arias-Montano J.A., Barbier O., De Vizcaya-Ruiz A. Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: angiotensin-II type-I receptor as a molecular target of particulate matter exposure. Part. Fibre Toxicol. 2015;12:17. doi: 10.1186/s12989-015-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Kagi N., Kim H., Hayashi M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ. Res. 2020;190:110042. doi: 10.1016/j.envres.2020.110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi V., Bronzo P., Banach M., Sahebkar A., Mannarino M.R., Pirro M. Particulate matter pollution and the COVID-19 outbreak: results from Italian regions and provinces. Arch. Med. Sci. 2020;16(5):985–992. doi: 10.5114/aoms.2020.95336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Salam M.T., Toledo-Corral C., et al. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care. 2016;39(4):547–554. doi: 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the deja vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ. Sci. Pollut. Res. Int. 2021;28(15):19147–19154. doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2021;78(1):1–27. [Google Scholar]
- Cole M.A., Ozgen C., Strobl E. Air pollution exposure and covid-19 in Dutch municipalities. Environ. Resour. Econ. 2020:1–30. doi: 10.1007/s10640-020-00491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus in the U.S.: latest map and case count. 2021. https://www.nytimes.com/interactive/2021/us/covid-cases.html Available online: Published.
- De Angelis E., Renzetti S., Volta M., et al. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021;195:110777. doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J.H.J., Altman D.G. In: Higgins J.P.T.T.J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Vol. 6.2. 2021. Chapter 10: analysing data and undertaking meta-analyses. (Cochrane Handbook for Systematic Reviews of Interventions). Cochrane. [Google Scholar]
- Delaney T., Dominie W., Dowling H., Naizlish N. 2018. Healthy Places Index. Healthy Places Index.https://healthyplacesindex.org/wp-content/uploads/2018/07/HPI2Documentation2018-07-08-FINAL.pdf Accessed July 8. [Google Scholar]
- DerSimonian RaL N. Meta-analysis in clinical trials. Contr. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental protection Agency's air quality system. https://aqs.epa.gov/aqsweb/airdata/download_files.html Available at: Accessed March 9th, 2021.
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi F., Bailey M.J., Patterson W.B., et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ. Int. 2020;138:105604. doi: 10.1016/j.envint.2020.105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronza R., Lusic M., Schmidt M., Lucic B. Spatial-temporal variations in atmospheric factors contribute to SARS-CoV-2 outbreak. Viruses. 2020;12(6) doi: 10.3390/v12060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyh A.S., Xue J., Ozkaynak H., Spengler J.D. The Harvard Southern California Chronic Ozone Exposure Study: assessing ozone exposure of grade-school-age children in two Southern California communities. Environ. Health Perspect. 2000;108(3):265–270. doi: 10.1289/ehp.00108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer C.G.D.J., Yang L., Jin S., Danielson P., Xian G., et al. Completion of the 2011 National Land Cover Database for the conterminous United States-Representing a decade of land cover change information. Photogramm. Eng. Rem. Sens. 2015;81(5):345–354. [Google Scholar]
- Hutter H.P., Poteser M., Moshammer H., et al. Air pollution is associated with COVID-19 incidence and mortality in vienna, Austria. Int. J. Environ. Res. Publ. Health. 2020;17(24) doi: 10.3390/ijerph17249275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Wu X.J., Guan Y.J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control Hosp. Epidemiol. 2020;41(9):1011–1015. doi: 10.1017/ice.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C., Langer-Gould A.M., Gould M.K., et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm. J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish J.P., Bosson J., Unosson J., et al. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J. Intern. Med. 2012;272(3):224–239. doi: 10.1111/j.1365-2796.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- Lawal A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf 2 and AhR-mediated pathways. Toxicol. Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Levi A., Barnett-Itzhaki Z. Effects of chronic exposure to ambient air pollutants, demographic, and socioeconomic factors on COVID-19 morbidity: the Israeli case study. Environ. Res. 2021;202:111673. doi: 10.1016/j.envres.2021.111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu X.L., Dai D.W., Huang Z.Y., Ma Z., Guan Y.J. Air pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.I., Tsai C.H., Sun Y.L., et al. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018;14(3):253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitt J., Chan-Golston A.M., Liu J., Su J., Zhu Y., Jerrett M. Spatial analysis of COVID-19 and traffic-related air pollution in Los Angeles. Environ. Int. 2021;153:106531. doi: 10.1016/j.envint.2021.106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Cheng B., Shen J., et al. Association between environmental factors and COVID-19 in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2021;28(33):45087–45095. doi: 10.1007/s11356-021-13834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M., Domingo J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2022;203:111930. doi: 10.1016/j.envres.2021.111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Adnan Abukhalaf A., Sami W., Hoang T.D. Effect of environmental pollution PM2.5, carbon monoxide, and ozone on the incidence and mortality due to SARS-CoV-2 infection in London, United Kingdom. J. King Saud Univ. Sci. 2021;33(3):101373. doi: 10.1016/j.jksus.2021.101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer L.C.L.B., Kaufman J.S., et al. The development of a standardized neighborhood deprivation index. J. Urban Health. 2006;83:1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbelt J., van Bree L., Dormans J.A., Boink A.B., Sangster B. Biochemical and histological alterations in rats after acute nitrogen dioxide intoxication. Hum. Exp. Toxicol. 1992;11(3):189–200. doi: 10.1177/096032719201100307. [DOI] [PubMed] [Google Scholar]
- Mitchell A. vol. 2. ESRI Press; 2005. (The ESRI Guide to GIS Analysis). [Google Scholar]
- Patel J.M., Sekharam K.M., Block E.R. Oxidant injury increases cell surface receptor binding of angiotensin II to pulmonary artery endothelial cells. J. Biochem. Toxicol. 1990;5(4):253–258. doi: 10.1002/jbt.2570050408. [DOI] [PubMed] [Google Scholar]
- Pegoraro V., Heiman F., Levante A., Urbinati D., Peduto I. An Italian individual-level data study investigating on the association between air pollution exposure and Covid-19 severity in primary-care setting. BMC Publ. Health. 2021;21(1):902. doi: 10.1186/s12889-021-10949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Münzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116(14):2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran J., Zhao S., Han L., et al. Initial COVID-19 transmissibility and three gaseous air pollutants (NO2, SO2, and CO): a nationwide ecological study in China. Front. Med. 2020;7:575839. doi: 10.3389/fmed.2020.575839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez M., Tobias A., Barcelo M.A. Effects of long-term exposure to air pollutants on the spatial spread of COVID-19 in Catalonia, Spain. Environ. Res. 2020;191:110177. doi: 10.1016/j.envres.2020.110177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa T., Tsujikawa T., Honda A., et al. Exposure to particulate matter upregulates ACE2 and TMPRSS2 expression in the murine lung. Environ. Res. 2021;195:110722. doi: 10.1016/j.envres.2021.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo M.M. Significance between air pollutants, meteorological factors, and COVID-19 infections: probable evidences in India. Environ. Sci. Pollut. Res. Int. 2021;28(30):40474–40495. doi: 10.1007/s11356-021-12709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini G., Swahn M.H., Aneja R. Disentangling the coronavirus disease 2019 health disparities in african Americans: biological, environmental, and social factors. Open Forum Infect. Dis. 2021;8(3):ofab064. doi: 10.1093/ofid/ofab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., et al. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimini A., Filipponi F., Fegatelli D.A., et al. A global association between Covid-19 cases and airborne particulate matter at regional level. Sci. Rep. 2021;11(1):6256. doi: 10.1038/s41598-021-85751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman EHaD . 2021. The SAS METAANAL Macro.https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/08/metaanal.pdf [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268(Pt A):115859. doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu J., Li Y., et al. Airborne particulate matter, population mobility and COVID-19: a multi-city study in China. BMC Publ. Health. 2020;20(1):1585. doi: 10.1186/s12889-020-09669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6(45) doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X., Xiong Y., Yang R., et al. Predicting the effect of confinement on the COVID-19 spread using machine learning enriched with satellite air pollution observations. Proc. Natl. Acad. Sci. U. S. A. 2021;118(33) doi: 10.1073/pnas.2109098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Wang W., et al. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741:140396. doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Liu Z., et al. Ambient nitrogen dioxide pollution and spreadability of COVID-19 in Chinese cities. Ecotoxicol. Environ. Saf. 2021;208:111421. doi: 10.1016/j.ecoenv.2020.111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang S.T., Luan J., Li L., et al. Ambient air pollution and COVID-19 risk: evidence from 35 observational studies. Environ. Res. 2021;204(Pt B):112065. doi: 10.1016/j.envres.2021.112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Chen Z., Liu Y., et al. Association between coronavirus disease 2019 (COVID-19) and long-term exposure to air pollution: evidence from the first epidemic wave in China. Environ. Pollut. 2021;276:116682. doi: 10.1016/j.envpol.2021.116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between ground levels of ozone (O3) and nitrogen dioxide (NO2) with coronavirus (COVID-19) in Milan, Italy. Sci. Total Environ. 2020;740:140005. doi: 10.1016/j.scitotenv.2020.140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.