Abstract
The Coronavirus Disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected 305 million individuals worldwide and killed about 5.5 million people as of January 10, 2022. SARS-CoV-2 is the third major outbreak caused by a new coronavirus in the previous two decades, following SARS-CoV and MERS-CoV. Even though vaccination against SARS-CoV-2 is considered a critical strategy for preventing virus spread in the population and limiting COVID-19 clinical manifestations, new therapeutic drugs, and management strategies are urgently needed, particularly in light of the growing number of SARS-CoV-2 variants (such as Delta and Omicron variants). However, the use of conventional antibodies has faced many challenges, such as viral escape mutants, increased instability, weak binding, large sizes, the need for large amounts of plasma, and high-cost manufacturing. Furthermore, the emergence of new SARS-CoV-2 variants in the human population and recurrent coronavirus spillovers highlight the need for broadly neutralizing antibodies that are not affected by an antigenic drift that could limit future zoonotic infection. Bovine-derived antibodies and camelid-derived nanobodies are more potent and protective than conventional human antibodies, thanks to their inbuilt characteristics, and can be produced in large quantities. In addition, it was reported that these biotherapeutics are effective against a broad spectrum of epitopes, reducing the opportunity of viral pathogens to develop mutational escape. In this review, we focus on the potential benefits behind our rationale for using bovine-derived antibodies and camelid-derived nanobodies in countering SARS-CoV-2 and its emerging variants and mutants.
Keywords: COVID-19, SARS-CoV-2, Antibodies, Nanobodies, Camelide, Bovine, Variants, Vaccines, Omicron
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has posed a serious threat to global public health security, claiming the lives of over 5.5 million people from more than 305 million confirmed cases as of January 10, 2022 [1]. We should not only apply the term “pandemic” to the original virus causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) but also important to the variants of SARS-CoV2 that have recently emerged [[2], [3], [4]]. Recently, SARS-CoV-2 variants are divided into four classes: Variant of concern (VOC) includes Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529); Variant of Interest (VOI) includes Lambda (C.37) and Mu (B.1.621); Variants under monitoring (VUM) and Formerly monitored variants [5] (as shown in Table 1 ). SARS-CoV-2 variants have played a critical role in the surges of COVID-19 cases at the global level, contributing to waves of the ongoing COVID-19 pandemic owing to their higher transmissibility, potentially high virulence, and vaccine breakthrough events in vaccinated individuals. These vaccine-escape variants and fast-growing mutations are possibly posing threats to the currently available vaccines and antibody-based therapies. Therefore, appropriate prevention and control strategies need to be adequately implemented and modified to manage COVID-19 patients efficiently [[6], [7], [8], [9], [10]]. According to currently available data, the Omicron variant is among the most widely transmissible, yet it causes mild symptoms. These emerging variants and mutations make it pragmatic to use the entire Spike (S) protein as a platform for developing therapies against SARS-CoV-2 and not just the receptor-binding domain (RBD) of the S protein.
Table 1.
SARS-CoV-2 variants of concerns (VOCs).
| WHO label | Pango lineage | Country of first detection | Date of designation | Earliest documented samples |
|---|---|---|---|---|
| Alpha | B.1.1.7 | United Kingdom | December 18, 2020 | September 2020 |
| Beta | B.1.351 | South Africa | December 18, 2020 | May 2020 |
| Gamma | P.1 | Brazil | January 11, 2021 | November 2020 |
| Delta | B.1.617.2 | India | April 4, 2021 (VOI) May 11, 2021 (VOC) |
October 2020 |
| Omicron | B.1.1.529 | Botswana South Africa |
November 11, 2021 November 26, 2021 |
November 2021 |
Some antiviral drugs, therapies, immunomodulatory agents, and alternative/supportive therapies were approved for emergency use to reduce disease severity and mortality in COVID-19 patients. In contrast, many antiviral drugs failed to display beneficial impacts or revealed mixed results. Numerous ongoing clinical trials could propose effective drugs of choice [[11], [12], [13], [14], [15]]. Meanwhile, rapid and highly collaborative research efforts have resulted in the approval of COVID-19 vaccines, spawning a global vaccination campaign to vaccinate a large population at the earliest for limiting the spread of COVID-19 [[16], [17], [18], [19]]. We note that neutralizing antibodies (nAbs) that target the SARS-CoV-2 S protein is a common feature of vaccines [[20], [21], [22]], monoclonal antibodies (mAbs) [23,24], and convalescent plasma [[25], [26], [27]] being used for prophylaxis and therapeutic purposes against COVID-19 [11,28,29]. Conversely, drugs and medicines being used for alleviating the severity of SARS-CoV-2 in COVID-19 patients, apart from Dexamethasone, showed limited efficacy against the disease. Despite attempts to develop vaccines against SARS-CoV-2, viral escape mutants could compromise their effectiveness. Recent studies have revealed a varied level of protection of existing COVID-19 vaccines in vaccinated individuals. Therefore larger cases of breakthrough infection are being reported in vaccinated people getting infected with emerging variants, such as the recently emerged Omicron variant, thus posing a potential risk of infection and illness even after vaccination [6,7,9,[30], [31], [32], [33]]. Likewise, monoclonal antibodies and convalescent sera, show less efficacy by inducing resistance to neutralization [[34], [35], [36], [37], [38], [39], [40]] even as new SARS-CoV-2 variants emerge (as shown in Table 2 ). Antigenic diversity, limited effectiveness, tiny produced quantities, and short-term immune responses are the barriers that need addressing before vaccinations generally become available [41,42]. The emergence of new SARS-CoV-2 variants in the human population and recurrent coronavirus spillovers (e.g. mink-to-human transmission) underline the need for broadly neutralizing antibodies that are not affected by the continuous antigenic drift could limit prevention or treatment of future zoonotic infections [28,[43], [44], [45]].
Table 2.
Polyclonal and Monoclonal antibodies.
| Polyclonal (pAb) | Monoclonal (mAb) |
|---|---|
| Low cost | Expensive |
| Short (3–4 months) | Time-consuming (up to a year) |
| Require considerable skills and training | |
| Has high stability | Has moderate stability |
| Moderate specificity | High specificity |
| Has variable sensitivity | Has moderate to high sensitivity |
| Very easy to be obtained. | Difficult to be obtained. |
| Other drawbacks | |
|
1. Weak tissue penetration 2. Slow kinetics | |
Potent and reliable immunotherapies are the need of the current time to limit the lethal impacts of the COVID-19 pandemic. However, some limitations are raised about the conventional antibodies. As such the conventional antibody CR3022 cannot effectively inhibit ACE2 binding and viral entry because it only binds to the receptor-binding domain (RBD) under restricted conformations. Additionally, increased instability leads to a shorter half-life, weak binding due to a steric hindrance linked with large antibody sizes of the conventional antibodies [46]. Other challenges are that they are heat-sensitive, it is difficult to recruit convalescent human donors, and significant amounts of plasma from convalescent human donors with high titers (hpAbs) are needed [47,48]. Monoclonal antibodies (mAbs) have the disadvantage of being directed against a single epitope, making them vulnerable to pathogen mutational escape. Furthermore, the cost of producing mAb products is very high [49] and requires intravenous administration, which necessitates patient hospitalization. Moreover, the potency of a single neutralizing antibody may be reduced due to somatic mutations and antibody-dependent enhancement (ADE) [50]. The US Food and Drug Administration (FDA) has given emergency use authorization (EUA) to three combination intravenous monoclonal antibody medicines in ambulatory individuals with COVID-19 to reduce medical visits and hospitalizations. The logistical limitations of intravenous and subcutaneous medications, on the other hand, hinder timely delivery to patients who could benefit the most from these crucial treatments [51,52]. See summary in Table 3 . Variants of SARS-CoV-2, particularly those with mutations in the S protein, may impair the clinical efficacy of monoclonal antibody treatment. The FDA has restricted monotherapy with bamlanivimab, stating that it should only be used in conjunction with etesevimab due to the rising prevalence of SARS-CoV-2 variants resistant to the antibody [52]. To establish the efficacy of these medicines in the context of the emergence of SARS-CoV-2 variants, more clinical investigations among individuals infected with SARS-CoV-2 variants are required.
Table 3.
Limitations of the conventional antibodies against SARS-CoV-2 Variants.
| 1. | Shorter half-life (due to increased instability). |
| 2. | Weak binding (due to steric hindrance). |
| 3. | Large antibody sizes. |
| 4. | Heat sensitivity. |
| 5. | Scarcity of serum from convalescent human donors. |
| 6. | Vulnerable to pathogen mutational escape (mAbs being directed against a single epitope). |
| 7. | Very high cost. |
| 8. | Necessitates patient hospitalization (mAbs required intravenous administration). |
| 9. | Reduced potency (due to somatic mutations and ADE). |
Polyclonal antibodies are created by injecting an antigen of interest into an animal and then collecting the serum as a source of antibodies many weeks later. Rabbits, mice, rats, hamsters, and guinea pigs are the most widely used animal species. Larger farms animals, such as cattle, sheep, or goats, are frequently used when larger numbers of antibodies are required [53]. In this direction, animal-derived antibody therapies are an appealing option, as they are effective and safe in various human illnesses [[54], [55], [56], [57]]. Their most attractive feature is their simplicity and broad applicability and can be manufactured in huge quantities at a low cost, especially in low-income nations ( Fig. 1 A and B). Recently, several studies have suggested the potential of animal-based antibodies against SARS-CoV-2 and the emerging variants and can serve as a valuable option for managing the COVID-19 patients. However, further explorative research is required for their optimal utility [46,50,54,[58], [59], [60], [61], [62], [63]], which may extend towards protecting humans via protecting animals [64]. This review discusses the potential benefits of employing bovine-derived antibodies and camelid-derived nanobodies in countering SARS-CoV-2 and its emerging variants.
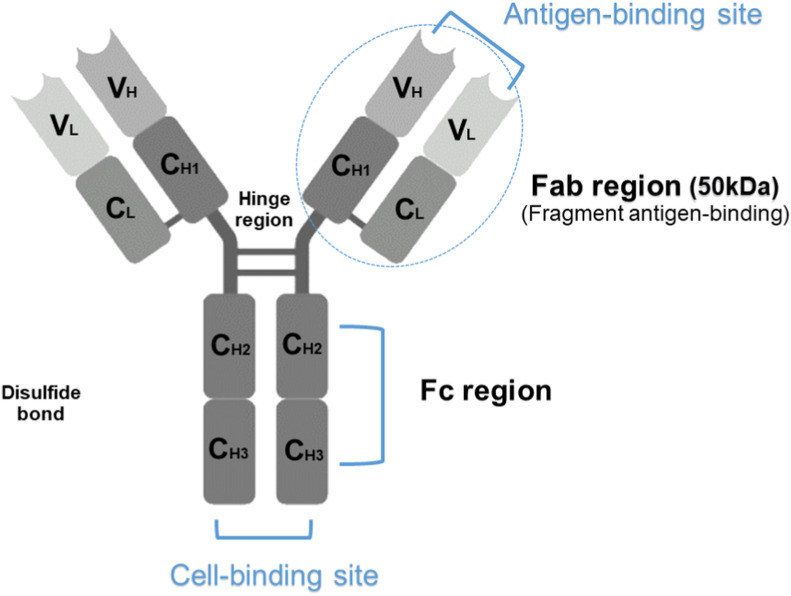
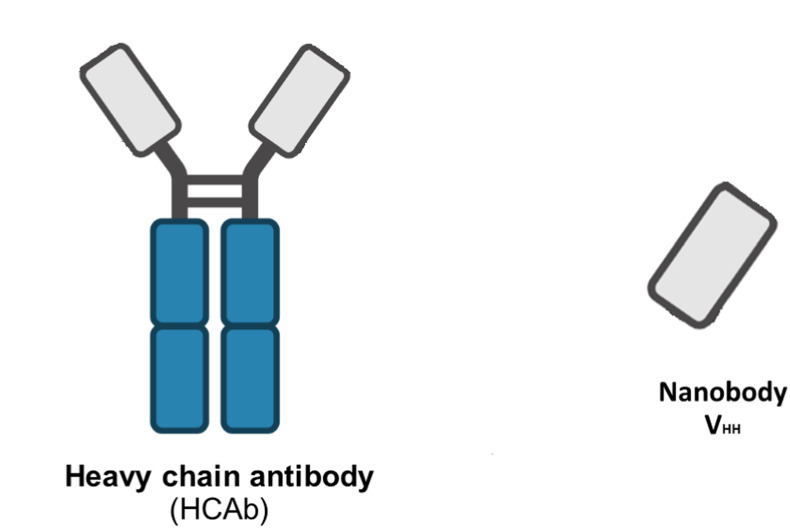
Fig. 1.
A. Four peptide chains, two identical heavy chains, and two light chains make up the entire 150 kDa IgG antibody (Conventional IgG antibodies). The antigen-binding sites are produced by the two variable domains (VL and VH), while the stem of the antibody molecule is generated by the constant Fc-region.
B. Sera of camelids contain a unique functional heavy (H)-chain antibody (HCAbs) in addition to conventional antibodies. The VHH or nanobody (15 kDa), a single-domain antibody generated from HCAb, is the smallest available antibody fragment with functional antigen binding. HCAb is devoid of light chains and is capable of antigen recognition solely by one single domain, the variable heavy domain (VHH).
Fab; fragment antigen binding, CL; light chain constant region, CH; heavy chain constant region, VL; light chain variable region. VH; heavy chain variable region, VHH; variable heavy domain, HCAbs; heavy (H)-chain antibody.
2. Transchromosomic bovines (TcB's) based antibodies
Transchromosomic bovines generate powerful neutralizing human antibodies. These TcB's have limited long-term hyperimmunization, unlike vaccines, their adjuvants, or other immune stimulators. TcB's are currently used to produce fully human antibodies (polyclonal human IgG) against Hantavirus (HTNV) [65,66], Middle East Respiratory Syndrome coronavirus (MERS-CoV) [67,68], Ebola virus [69], Venezuelan equine encephalitis virus (VEEV) [70], Puumala virus (PUUV) [65] and Zika virus [71,72]. Recently, besides polyclonal antibodies, monoclonal antibodies have been produced in TcB against Influenza A Virus [73]. These antibodies are more potent and protective than conventional human antibodies and could be produced in large quantities (Table 4 ). Immunization of Tc bovine triggers the bovine adaptive immune response, enabling secretion of human polyclonal Ig from Tc bovine B lymphocytes. Polyclonal antibodies are effective against a broad spectrum of epitopes, reducing the opportunity of viral pathogens to develop mutational escape. Besides viral neutralization, these antibodies also contribute to public health infection control. They promote virus clearance by inducing antibody-dependent cellular cytotoxicity (ADCC) mechanism in natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). This immune mechanism kills the virus-infected cells, eliminating virus reservoirs. Strikingly, Oshiro et al. [74] reported that bovine IgG enriched fraction has the potential to neutralize SARS-CoV-2 through specific binding to the RBD of SARS-CoV-2 S protein, but it showed less potency activity against SARS-CoV-2 N [74]. Additionally, FM-CBAL74 (cow's milk fermented with the probiotic L. paracasei CBAL74) exerted a preventive action against SARS-CoV-2 [75]. In a similar vein, Kangro et al. [76], reported immunoglobulins-rich colostrum after immunizing pregnant cows with SARS-CoV-2 spike protein in a surprisingly short time. Therefore, colostrum immunoglobulin preparation has a lot of potential for use in SARS-CoV-2 prevention regimens.
Table 4.
Advantages of Tc bovine–based system for producing therapeutic hPABs.
| 1. | Production of large amounts of humanized antibodies. (No human donors) |
| 2. | Possibility of hyperimmunization against almost any human pathogen or other peptide antigens. |
| 3. | Easily tested upon a large number of antigens. |
| 4. | No need for the isolation of a target virus as with vaccine development. |
| 5. | At any stage of antibody development, no patient intervention is required. |
| 6. | A short time from immunization to antibodies purification (3–5 months). |
| 7. | Low cost (compared to mAb development). |
| 8. | Binding to multiple targets. |
| 9. | Theoretical resistance to escape mutation/reduction the potential for escape mutants. |
| 10. | Potential intervention to solve infections epidemic/pandemic outbreaks |
In two recent studies, Liu et al. used TcBs to produce fully human anti-SARS-CoV-2 (TchIgG-SARS-CoV-2) immunoglobulin [63]. TcBs were hyperimmunized twice with plasmid DNA encoding the SARS-CoV-2 Wuhan-Hu-1 strain Spike (S) gene [77], then repeatedly immunized with S protein purified from insect cells. The Tc-hIgG-SARS-CoV-2, termed SAB-185, efficiently neutralizes SARS-CoV-2 and vesicular stomatitis virus (VSV) SARS-CoV-2 chimeras in vitro [63]. Furthermore, it retained neutralizing potency against S variants, including S477 N, E484K, and N501Y substitutions, found in current SARS-CoV-2 variants of concern, implying that it is an effective COVID-19 treatment. Gilliland et al. [60], used SAB-185 to examine the neutralizing capacity of SAB-185 in vitro and the protective efficacy of passive SAB-185 antibody transfer in vivo. In vitro, SAB-185 neutralized five variant SARS-CoV-2 strains: Munich (Spike D614G), UK (B.1.1.7), Brazil (P.1), and South Africa (SA) (B.1.3.5) variants, and a variant isolated from a chronically infected immunocompromised patient (Spike Δ144-146). On Vero E6 cells, SAB-185 neutralized all the three SARS-CoV-2 strains equally, although a control convalescent human serum sample was less effective at neutralizing the SA variant. The animal model used was a new human ACE2 (hACE2) transgenic Syrian hamster. Prophylactic SAB-185 treatment protected the hamsters from a fatal disease and minimized clinical signs of infection, suggesting that SAB-185 may be an effective treatment for patients infected with SARS CoV-2 variants [60]. Although the data is not yet published, SAB-185 was found to be safe and well-tolerated in Phase 1 (healthy adult) and Phase 1b (non-hospitalized SARS-CoV-2 infected) clinical trial (ClinicalTrials.gov nos. NCT04468958 and NCT0446917, respectively) besides its potent neutralization efficacy in vitro [63]. Recently, SAB-185 showed efficacy against Delta (VOC) and Lambda (VBM) variants [59].
SAB-185 showed a broad neutralization to Omicron and other VOCs in an in vitro pseudovirus model, and currently, SAB-185 is being evaluated in the NIH1 -sponsored Phase 3 COVID trial that has been enrolling patients since October 2021. These results were compiled by scientists at the US FDA Center for Biologics Evaluation and Research (CBER), using a lentiviral-based pseudovirus assay conducted in a BSL2 environment that incorporates a stable 293T cell line expressing human angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). SAB-185 retains a potent ability to neutralize recombinant S protein lentiviral pseudovirus that imitates the SARS-CoV-2 Omicron variant. When compared to the wild-type SARS-CoV-2, SAB-185 was still able to neutralize the Omicron form, but it had a mild-moderate loss in potency. SAB Biotherapeutics is hopefully working to optimize SAB-185 by adding activity directed to the Omicron variant. Importantly, pAbs can effectively block receptors utilized for viral entry by binding to multiple epitopes on the RBD, and also may activate immune effector cells, enhancing the individual's immunological response. SAB-185 is also substantially more powerful than human-derived convalescent IgG, according to preclinical research [78].
3. Camel nanobodies
The therapeutic potential of camel nanobodies against COVID-19 has been recently emphasized and proposed to be useful as Dromedary camels could act as a natural source of SARS-CoV-2 neutralizing nanobodies [46,50,54,79] ( Table 5 ). Nanobodies are one of the most intriguing biotherapeutics in this era, with promising outcomes in recent studies [79]. Several SARS-CoV-2 epitopes were found highly immunogenic in humans, and some epitopes can be wholly targeted by camel antibodies. However, SARS-CoV-2 cross-neutralizing camel antibodies, though not highly useful for COVID-19 treatment, are present in nonimmunized camels suggesting a need for further studies [54]. Antibodies from camelids have been found to be more similar to human antibodies than antibodies from other routinely immunized animals, such as mice [80]. Therefore, fears regarding eliciting immune responses are greatly diminished. Camels produce relatively unique heavy homodimeric chain-only antibodies IgG, namely nanobodies (Nbs), heavy-chain antibodies (HcAbs), single-domain antibodies (sdAbs), or antigen-specific variable domains (VHH). VHH is structurally and functionally identical to an IgG Fv but has only three complementarity determining regions (CDR) loops defining the antigen-binding sites [81] and enabling them to bind concave epitopes that cannot be recognized by traditional antibodies [82,83]. These domains are characterized by high specificity, chemo- and thermostability, solubility, lower susceptibility to steric hindrances, an ability to build multimers to strengthen the binding [46] in vitro, as well as small size (15 kDa), which allows them to recognize unusual antigenic sites.
Table 5.
Characteristics of single-domain antibodies in comparison to conventional antibodies.
| 1. | Small molecular weight (12–15 kDa) (Their long, heavy-chain regions make them capable of targeting specific epitopes, such as the receptor-binding site of the S protein) |
| 2. | High penetration in tissues due to small size. |
| 3. | Ease of manipulation. (Can be used for new immunobiotechnological medication). |
| 4. | High solubility and stability in harsh environments, such as high temperatures or denaturing conditions. |
| 5. | Low Immunogenicity. |
| 6. | Easily selected by Phage Display. |
| 7. | Easy production in bacteria and yeast. (Because they lack the glycan-harboring Fc domain, making them easier to make than the standard monoclonal antibodies). |
| 8. | High specificity. (Recognize native epitopes, which are rare for classical antibodies). |
| 9. | Easy production and suitable cost. |
| 10. | Low risk of antibody-dependent enhancement (ADE) of infection. (Due to the absence of the Fc region. However, it shortens the half-life of these molecules, a disadvantage that could be overcome by attaching them to polyethylene glycol or human albumin). |
They are, therefore, inaccessible to conventional antibodies and thus penetrate tissues deeply [[84], [85], [86]]. For example, when nanobodies are used as an inhalant, high pulmonary drug concentrations are achieved, but systemic drug concentrations are kept low, and systemic adverse effects are kept to a minimum [50,79]. Nanobodies can be used in different formats, and currently, nearly fifteen Nanobodies developed against SARS-CoV-2 during the COVID-19 pandemic [79], mostly targeted receptor-binding domain (RBD) [79]. Among wellsprings of these nanobodies are camels immunized with RBD, llama, and/or Alpaca immunized with spike protein and/or RBD, and Naïve library from llamas and/or alpacas [79]. Nanobodies are efficiently produced in various prokaryotic and eukaryotic hosts, including bacteria (E. coli), yeast (Pichia pastoris), and mammalian cells [79]. Multivalent Nbs can be further classified into bivalent (two identical Nbs), biparatropic (two different Nbs), or trivalent (three identical Nbs) [14]. Multivalent formats have the potential to produce extremely high neutralization potency, preventing mutational escape and neutralizing a wide spectrum of SARS-CoV-2 variants. It has been shown that these Nbs can be easily engineered without loss of functionality [79]. They have a high antigen affinity and stability in the gastrointestinal tract, allowing them to be administered orally [50]. Traditional nanobodies are isolated from immunized camelids that efficiently neutralize both the SARS-CoV-2 pseudovirus and live virus [87]. Synthetic nanobodies (sybodies) libraries, on the other hand, are a new technology for rapid drug development that produces highly selective binders with neutralizing potentials in a short [79].
Developing Nbs is a promising approach against respiratory pathogens, such as a respiratory syncytial virus (RSV) [[88], [89], [90]] and SARS-CoV-2. Their small size makes them easily nebulized and delivered directly to the lungs via an inhaler [91,92]. Additionally, Nbs have high yields in bacterial, yeast, and mammalian expression systems [86,93]. Many reports have attested the therapeutic efficacy of VHH against viruses [94,95], such as dengue virus [96], hepatitis C virus (HCV) [97,98], poliovirus [99], norovirus [100], rotavirus A [101], MERS-CoV [102,103], SARS-CoV-2, SARS-CoV-1, Influenza A virus [104,105], respiratory syncytial virus (RSV), hepatitis B virus (HBV), human immunodeficiency virus (HIV) [[106], [107], [108]], Chikungunya virus (CHIKV) [109] and Ebola virus [50]. Cablivi™ (Caplacizumab; ALX-0081, Sanofi company) is the first nanobody approved by the FDA to treat thrombotic thrombocytopenic purpura and thrombosis [110]. ALX-0171, a novel trivalent nanobody, showed inhibitory activity of viral replication in 87% of viruses tested versus 18% observed with Palivizumab, a commercially available neutralizing monoclonal antibody. Moreover, Nbs can target pulmonary surfactant protein A (SPA) and accumulate in the lungs [111]. Nanobodies have good biophysical potential and are less expensive and easier to produce than normal monoclonal antibodies due to their small size [112].
Xu et al. [61] have isolated anti-RBD nanobodies from llamas and mice, engineered to produce cloned VHHs from alpacas, dromedaries, and Bactrian camels. They identified two groups of highly neutralizing nanobodies. Group 1 recognized the highly conserved RBD region in coronaviruses, while Group 2 was almost entirely focused on the RBD–ACE2 interface and could not neutralize SARS-CoV-2 variants that carry E484K or N501Y substitutions. They proposed that multivalent nanobodies overcome SARS-CoV-2 mutations via two distinct mechanisms: enhanced avidity for the ACE2-binding domain and recognition of conserved epitopes that are largely inaccessible to human antibodies. Koenig et al. [112] identified nanobodies that specifically bind to the SARS-CoV-2 RBD, which isolated from alpacas and llamas immunized with SARS-CoV-2 S protein. These nanobodies neutralize the virus by causing a premature structural transition from a pre-fusion to an irreversible post-fusion conformation, which is unable to bind to ACE2 and hence prevents SARS-CoV-2 from causing membrane fusion and entering the host cell. By fusing nanobodies targeting distinct epitope areas, Koenig et al. were able to create biparatopic nanobodies, which have two antigen-binding sites in one molecule [112]. Wagner et al. have generated, detailed epitope mapping, two biparatopic Nbs (NM1267 and NM1268) targeting a conserved epitope outside and two different epitopes inside the RBD:ACE2 interface. Both bipNbs bind all currently circulating VOCs with high affinities and are capable to neutralize cellular infection with Beta and Delta variants in vitro. In vivo, human ACE2 transgenic mice are treated intranasally before infection with a lethal dose of SARS-CoV-2 Beta or Delta variants. bipNbs have conferred protection against SARS-CoV-2 variants, significantly reduced disease progression, and increased survival rates [113]. Because of their potent and broad neutralizing actions, outstanding biophysical characteristics, stability, solubility, and long extended half-lives, multivalent VHH-72 Fc fusion proteins are promising therapeutic candidates [114]. It was successfully attached to spike proteins in alpha and beta SARS-CoV-2 variants.
According to the data in Nb phage display libraries, 381 Nbs were identified to recognize SARS-CoV-2-RBD, which was derived from four camels immunized with the SARS-CoV-2 Spike receptor-binding domain (RBD). Seven Nbs blocked human ACE2 interaction with SARS-CoV-2-RBD variants, while two Nbs blocked human ACE2 interaction with bat-SL-CoV-WIV1-RBD and SARS-CoV-1-RBD. Nb11-59 had the best efficacy against real SARS-CoV-2, and it can be produced on a large scale in Pichia pastoris [115,116]. According to a naïve VHH library, Nb91-hFc and Nb3-hFc demonstrated antiviral activity by neutralizing the spike of SARS-CoV-2 pseudoviruses in vitro. Therefore, the heterodimer nanobody Nb91–Nb3-hFc was constructed to improve its neutralizing capacity and exhibits a strong RBD-binding affinity against SARS-CoV-2 pseudoviruses [117].
Synthetic nanobodies (sybodies) are small, aerosolizeable, and heat stable, making them a viable candidate for COVID-19 prevention and treatment [79]. Stefan et al. [118] created a massive synthetic VHH library (3.18 × 1010) and identified more than 50 VHH candidates that can bind to SARS-2 [6]. Schoof et al. [119] used a synthetic Nbs library to identify a panel of Nbs that can bind to multiple epitopes on Spike. These Nbs are split into two categories. On human cell surfaces, Class I binds directly to the RBD and competes with the ACE2 receptor. Class II, which has been identified as a distinct binding location, contributes to the RBD's structural conformation being altered, preventing it from recognizing the ACE2 receptor. SR31, a novel synthetic Nb identified by researchers, has shown stronger binding affinities and neutralizing actions in fusion with other synthetic nanobodies. Therefore, SR31 could also be combined with monoclonal antibodies or other antibody fragments to improve affinity and efficacy [120]. Chen et al. [121] developed CeVICA, a cell-free nanobody technique that uses ribosome display to isolate nanobodies from a vast library in vitro and thereafter produce high-affinity nanobodies that bind to RBD. They discovered 30 RBD binder families and 11 families that prevent the pseudovirus infection. CeVICA nanobodies have good biophysical features that are equivalent to nanobodies derived from animals. Many recent reports documented the promising antiviral efficacy of nanobodies and their potential as biotherapeutics against SARS-CoV-2 [54,58,87,92,112,[122], [123], [124], [125], [126], [127]]. Nanobodies are currently engineered into different multivalent forms, fused to Fc domains, and their affinity matured to increase neutralization potency [58,112,114,[125], [126], [127]]. Bivalent Fc–VHH variants, recently recovered in both immune and pre-immune nanobody libraries, had a 10-fold higher neutralizing activity than humanized nanobodies from a synthetic library [122]. Recently, nanobodies identified using a synthetic yeast display library recognized different antigen epitopes and prevented ACE2-virus interaction through distinct mechanisms [119]. Additional studies showed that storage and nebulization conditions did not affect the stability of anti-SARS-CoV-2 neutralizing nanobodies. That makes them promising candidates for localized treatment of COVID-19 patients by inhalation delivery directly to the airway epithelia [127]. The promising effect of Nanobodies extends from neutralizing to anti-inflammatory action, such as anti-IL-6R Nanobody® ALX-0061 (Ablynx) that primarily indicated for rheumatoid arthritis. Nanobody® ALX-0061 could be used in COVID-19 to reduce pulmonary inflammation, similar to Tocilizumab (Actemera®), an anti-IL-6R monoclonal antibody with extensive use in COVID-19 [79]. Using integrative proteomics, Xiang Y et al. [128] isolated a vast repertoire of unique ultrahigh-affinity nanobodies (super-immunity), a camelid extensively immunized with the SARS-CoV-2 RBD, from that bind strongly to all known sarbecovirus clades, which can bind strongly and specifically to all sarbecoviruses. These pan-sarbecovirus nanobodies (psNbs) are highly effective against SARS-CoV and SARS-CoV-2 variants, including the Omicron, with the best median antiviral potency at the single-digit ng/ml range. PiN-31 (a highly powerful, inhalable, and bispecific psNb) was produced as a multivalent construct. PiN-31 targets different and conserved epitopes, and a cocktail of such multivalent compounds could give broad protection against future SARS-CoV-2 antigenic drift and novel sarbecovirus threats [128].
4. Omicron variant
On November 24, South Africa issued an international alert about the latest SARS-CoV-2 variant, omicron (B.1.1.529). The omicron variant differs from prior variants in that it has 50 mutations in its genomic sequence, compared to the delta variant's (B.1.617.2) 13 mutations [129]. Almost instantly, the global health community was concerned about omicron's transmissibility and capacity to evade both vaccine-induced and natural immunity [129]. Most significantly, it is suggested that Omicron was recognized and detected before the variant's formal discovery, as was the case with wild-type SARS-CoV-2. The Omicron variant is expected to be three times more contagious than the original SARS-CoV-2 strain, and maybe even more so than the delta variant [130].
The omicron variant has been widely distributed to 150 countries in less than two months with over 0.55 million confirmed cases and caused 115 deaths (https://newsnodes.com/nu_tracker). Several countries, notably the United Kingdom, Denmark, and Norway, are experiencing rapid spread, , and in other countries, it is spreading speedily owing to its very high transmissibility and infectivity. Omicron has been reported to cause mild infection and lesser hospitalization requirements. However, any severity of disease and deaths linked with this variant is yet to be known, much as genomic sequencing is required to confirm it, and presently only a limited proportion of samples that are found positive for COVID-19 by RT-PCR testing are being subjected to gene sequencing. Therefore the real magnitude of infection with Omicron is to be explored by enhancing genomic surveillance and tracking for this newer variant. Cases of infection with Omicron are rising in several countries, with the incidences of hospitalization going up owing to its high transmission rate (https://newsnodes.com/nutracker). The most recent evidence showed that the existing COVID-19 vaccines give less immunity to the omicron variant than other VOCs, and Omicron is resistant to imdevimab and casirivimab. However, sotrovimab, which targets the conserved area to limit viral escape, is effective against Omicron in the same way that it was against the original SARS-CoV-2 [131]. Additionally, the sera from vaccinated individuals had about 41-fold lower neutralizing abilities against the Omicron variant than the wild-type SARS-CoV-2 [33]. Given together, the current COVID-19 vaccines may not be as effective against the Omicron variant as they are against other SARS-CoV-2 variants. More information about the efficacy of current COVID-19 vaccines will need to be gathered [132]. SARS-CoV-2 mutations may alter the neutralizing activity of vaccine-elicited antibodies and monoclonal antibodies (mAbs), resulting in a mild-to-significant loss of effectiveness [133]. Multivalent nanobodies resist the mutations of the SARS-CoV-2 variant through two mechanisms: increased avidity for the ACE2 binding domain and identification of preserved epitopes that are not accessible to human antibodies [134]. Furthermore, oligomerization of nanobodies could improve serum half-life [85]. Bi-specific VHH-Fc antibodies are substantially more effective than monoclonal VHH-Fc antibodies in inhibiting SARS-CoV-2's S1 RBD and S/ACE2 [135]. Seven anti-RBD Nbs isolated from alpacas inoculated with SARS-CoV-2 RBD, were combined with two Nbs with different epitopes, resulting in two hetero-bivalent Nbs with strong affinity and considerable SARS-CoV-2 neutralizing activity [136]. Thus Nanobodies could thus be a useful technique for neutralizing SARS-CoV-2 variants, even if new mutations emerge [137]. Synergism of nanobodies together with anti-viral drugs could serve as useful tools in the armamentarium against COVID-19 [138].
5. Conclusion and future prospects
It appears that SARS-CoV-2 will inevitably continue to adapt to human transmission and immune escape. Notwithstanding, the rapid global deployment of licensed and effective vaccines remains an urgent and vital public health priority [139]. There is mounting evidence that COVID-19 vaccines lose potency over time [140,141]. The recent emergence of the omicron (B.1.1.529) variant has emphasized the need for a 3rd dose of COVID-19 vaccines to boost protective antibody levels in vaccinated individuals. Therefore, some governments are rushing to deploy vaccine boosters. The mitigation of this crisis by vaccines, therapeutics, and non-pharmaceutical interventions need to be comprehensively applied. New therapeutics that are effective against SARS-CoV-2 variants and provide an alternative to intravenous monoclonal antibody medicines are eagerly awaited [142].
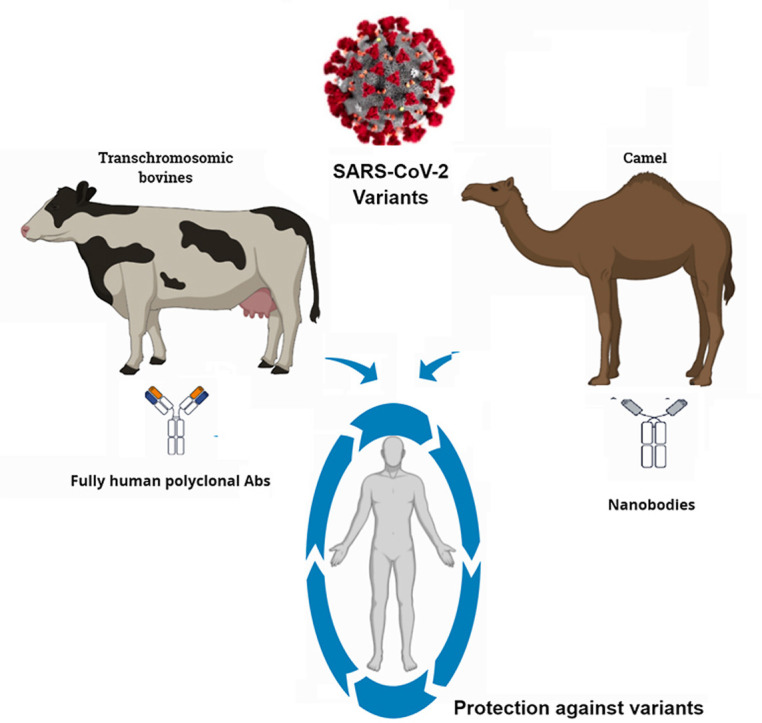
Currently, the SAB-185 antibody is in phase 3 of development, while different therapeutic nanobodies against SARS-CoV-2 are in the discovery or preclinical phase. Using SAB-185 antibody produced in Tc bovines and camels nanobodies such as Nb11-59 combined as two entities or constructed as a single antibody could be a very beneficial strategy against the emergent variants and preventing COVID-19 mortality ( Fig. 2 ). As mentioned previously, human polyclonal antibodies produced in TcB and nanobodies produced in camelids showed safety and efficacy in vivo. Additionally, both can be produced in large quantities, indicating their easiness and wide production and distribution. These methods could solve some challenges facing existing COVID-19 vaccines, monoclonal antibodies, and convalescent sera. Barriers against their actual successful application exist, which must be addressed adequately for their optimal use against COVID-19. Significant immune evasion characteristics of the Omicron variant has resulted in lowering down the efficacy of the available COVID-19 vaccines and reduction in protective abilities of antibodies-based immunotherapies, especially by escaping neutralization potential of therapeutic monoclonal antibodies (mABs), hence some of the mAbs that are presently being used in the clinics may not be useful in treating Omicron-infected patients. This appealing option of broadly neutralizing antibodies such as bovine-derived antibodies and camelid-derived nanobodies would be useful against the rapid emergence of new variants (new variants; IHU variant in France and Deltacron in Cyprus) alongside other disease countermeasures. The success of this therapeutic modality could support us in our battle against the emerging challenges of this ongoing COVID-19 pandemic. Alternative to vaccine coverage, hesitancy, and the restricted access, development of such easily applicable therapeutic approaches to protect and treat predisposed individuals are highly promising strategies and urgently warranted. As argued above, there is significant evidence suggesting that bovine-derived antibodies and camelid-derived nanobodies could work as biotherapeutic weapons against SARS-CoV-2 and its variants. This appealing option is required against the rapid emergence of new variants alongside other disease countermeasures. The success of this therapeutic modality could support us in our battle against the emerging challenges of this ongoing COVID-19 pandemic.
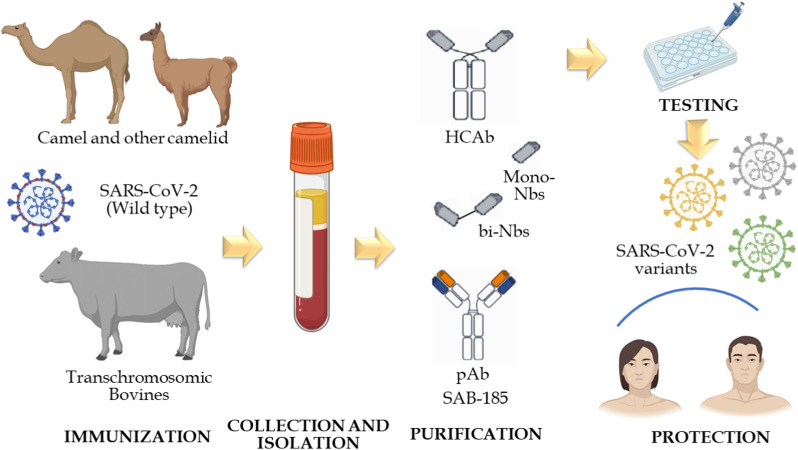
Fig. 2.
Using Cow-derived antibodies in combination with camelid nanobodies could be a powerful biotherapeutics in our armamentarium against SARS-CoV-2 and its emerging variants. It starts by immunizing TcBs and camelid thereafter collecting and isolating polyantibodies (pAbs) and heavy chain antibodies (HCAb), respectively. Consequently, the purification process is done which followed by pABs and VHH testing. Notably, Nanobodies could be used after fusion (Multivalent). Combining SAB-185, which is administrated intravenously, and nanobodies, which are administrated orally or by inhalation, could be a powerful synergism against SARS-CoV-2 and its emergent variants either detected or undetected yet.
Research Registration Unique Identifying Number (UIN)
-
1
Name of the registry:
-
2
Unique Identifying number or registration ID:
-
3
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Declarations of interest
None.
Funding
This compilation is a review article written by its authors and required no substantial funding to be stated.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Author contributions
AAS conceptualized the manuscript and wrote the first draft with input from AAM, MA, JS, KS, and KD, who reviewed and updated the manuscript. All authors have contributed significantly to this manuscript and approved the final manuscript.
Guarantor
AbdulRahman A Saied, Researcher, National Food Safety Authority (NFSA), Aswan, Egypt and Ministry of Tourism and Antiquities, Aswan, Egypt Tel: +02–01060290104; Email: saied_abdulrahman@yahoo.com
Availability of data and materials
The datasets used and analyzed in this research are available from the corresponding author upon reasonable request.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities. I (AAS) would like to dedicate this paper to the soul of my friend Mohamed El Sayed Abdel Rahim, a young veterinarian who died unexpectedly on January 13, 2022, at the age of 31. I have lost an intelligent colleague and a wonderful friend.
Footnotes
National Institutes of Health.
References
- 1.WHO Coronavirus (COVID-19) dashboard - overview. https://covid19.who.int/
- 2.Boehm E., Kronig I., Neher R.A., Eckerle I., Vetter P., Kaiser L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin. Microbiol. Infect. 2021;27:1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet (London, England) 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause P.R., Fleming T.R., Longini I.M., Peto R., Briand S., Heymann D.L., Beral V., Snape M.D., Rees H., Ropero A.-M. SARS-CoV-2 variants and vaccines. N. Engl. J. Med. 2021;385:179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 6.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., Schaefer-Babajew D.J., DaSilva J., Muecksch F., Gaebler C. Vaccine breakthrough infections with SARS-CoV-2 variants. N. Engl. J. Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R., Chen J., Gao K., Wei G.-W. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021;113:2158–2170. doi: 10.1016/j.ygeno.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Zhang L., Chen S., Ji W., Li C., Ren L. Recent progress on the mutations of SARS-CoV-2 spike protein and suggestions for prevention and controlling of the pandemic. Infect. Genet. Evol. 2021;93 doi: 10.1016/j.meegid.2021.104971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farinholt T., Doddapaneni H., Qin X., Menon V., Meng Q., Metcalf G., Chao H., Gingras M.-C., Farinholt P., Agrawal C. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021;19:1–6. doi: 10.1186/s12916-021-02103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y.R., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R. B. 1.1. 529 escapes the majority of SARS-CoV-2 neutralizing antibodies of diverse epitopes. bioRxiv. 2021 doi: 10.1101/2021.12.07.470392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharun K., Tiwari R., Iqbal Yatoo M., Patel S.K., Natesan S., Dhama J., Malik Y.S., Harapan H., Singh R.K., Dhama K. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expet Opin. Biol. Ther. 2020;20:1033–1046. doi: 10.1080/14712598.2020.1796963. [DOI] [PubMed] [Google Scholar]
- 12.Ghareeb D.A., Saleh S.R., Nofal M.S., Kaddah M.M.Y., Hassan S.F., Seif I.K., El-Zahaby S.A., Khedr S.M., Kenawy M.Y., Masoud A.A. Potential therapeutic and pharmacological strategies for SARS-CoV2. J. Pharm. Investig. 2021;51:281–296. doi: 10.1007/s40005-021-00520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav R., Chaudhary J.K., Jain N., Chaudhary P.K., Khanra S., Dhamija P., Sharma A., Kumar A., Handu S. Role of structural and non-structural proteins and therapeutic targets of SARS-CoV-2 for COVID-19. Cells. 2021;10:821. doi: 10.3390/cells10040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouffak S., Shubbar Q., Saleh E., El-Awady R. Recent advances in management of COVID-19: a review. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saied A.A., Metwally A.A., Madkhal N.A., Haque S., Dhama K. Egypt's COVID-19 recent happenings and perspectives: a mini-review. Front. Publ. Health. 2021;9 doi: 10.3389/fpubh.2021.696082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawat K., Kumari P., Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2020;892 doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Montero C., Fraile-Martínez O., Bravo C., Torres-Carranza D., Sanchez-Trujillo L., Gómez-Lahoz A.M., Guijarro L.G., García-Honduvilla N., Asúnsolo A., Bujan J. An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines. 2021;9:433. doi: 10.3390/vaccines9050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal Yatoo M., Hamid Z., Parray O.R., Wani A.H., Ul Haq A., Saxena A., Patel S.K., Pathak M., Tiwari R., Malik Y.S. COVID-19-Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum. Vaccines Immunother. 2020;16:2891–2904. doi: 10.1080/21645515.2020.1788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO COVID-19 vaccine tracker and landscape. Https//Www.Who.Int/Publications/m/Item/Draft-Landscape-of-Covid-19-Candidate-Vaccines
- 20.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 22.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundgren J.D., Grund B., Barkauskas C.E., Holland T.L., Gottlieb R.L., Sandkovsky U., Brown S.M., Knowlton K.U., Self W.H., Files D.C. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. 2020;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., Van Buskirk C., Grossman B.J., Joyner M., Henderson J.P. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focosi D., Franchini M., Pirofski L., Burnouf T., Fairweather D., Joyner M.J., Casadevall A. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental Co-factors. Viruses. 2021;13:1594. doi: 10.3390/v13081594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhawan M., Parmar M., Angural S., Choudhary O.P. Convalescent plasma therapy against the emerging SARS-CoV-2 variants: delineation of the potentialities and risks. Int. J. Surg. 2021;97 doi: 10.1016/j.ijsu.2021.106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du L., Yang Y., Zhang X. Neutralizing antibodies for the prevention and treatment of COVID-19. Cell. Mol. Immunol. 2021;18:2293–2306. doi: 10.1038/s41423-021-00752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang N.Y.-L., Pang A.S.-R., Chow V.T., Wang D.-Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021;8:1–17. doi: 10.1186/s40779-021-00342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tareq A.M., Bin Emran T., Dhama K., Dhawan M., Tallei T.E. Impact of SARS-CoV-2 delta variant (B. 1.617. 2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum. Vaccines Immunother. 2021;2:1–2. doi: 10.1080/21645515.2021.1963601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharun K., Tiwari R., Dhama K., Bin Emran T., Rabaan A.A., Al Mutair A. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum. Vaccines Immunother. 2021;17:3491–3494. doi: 10.1080/21645515.2021.1923350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R., Chen J., Hozumi Y., Yin C., Wei G.-W. Emerging vaccine-breakthrough SARS-CoV-2 variants. ArXiv. 2021 doi: 10.1021/acsinfecdis.1c00557. 2103.08023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cele S., Jackson L., Khan K., Khoury D., Moyo-Gwete T., Tegally H., Scheepers C., Amoako D., Karim F., Bernstein M. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 doi: 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 34.Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I., Dal Monego S., Pantano E., Manganaro N., Manenti A. SARS-CoV-2 escape from a highly neutralizing COVID-19 convalescent plasma. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118:1–7. doi: 10.1073/pnas.2103154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C., Muecksch F., Rutkowska M., Hoffmann H.-H., Michailidis E. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding C., He J., Zhang X., Jiang C., Sun Y., Zhang Y., Chen Q., He H., Li W., Xie J. Crucial mutations of spike protein on SARS-CoV-2 evolved to variant strains escaping neutralization of convalescent plasmas and RBD-specific monoclonal antibodies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.693775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K.H.D. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29:44–57. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z., VanBlargan L.A., Bloyet L.-M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanBlargan L.A., Errico J.M., Halfmann P., Zost S.J., Crowe J.E., Purcell L.A., Kawaoka Y., Corti D., Fremont D.H., Diamond M. An infectious SARS-CoV-2 B. 1.1. 529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies. bioRxiv. 2021 doi: 10.1101/2021.12.15.472828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R. Antibody evasion by the P. 1 strain of SARS-CoV-2. Cell. 2021;184:2939–2954. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloch E.M., Goel R., Wendel S., Burnouf T., Al-Riyami A.Z., Ang A.L., DeAngelis V., Dumont L.J., Land K., Lee C. Guidance for the procurement of COVID-19 convalescent plasma: differences between high-and low-middle-income countries. Vox Sang. 2021;116:18–35. doi: 10.1111/vox.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect. Dis. Ther. 2020;9:255–274. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharun K., Tiwari R., Natesan S., Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. Vet. Q. 2020;41:50–60. doi: 10.1080/01652176.2020.1867776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet. Q. 2021;41:181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tortorici M.A., Czudnochowski N., Starr T.N., Marzi R., Walls A.C., Zatta F., Bowen J.E., Jaconi S., Di Iulio J., Wang Z. Broad sarbecovirus neutralization by a human monoclonal antibody. Nature. 2021;597:103–108. doi: 10.1038/s41586-021-03817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soong D., Leeman R., Pillai A. Finding camel-ot: a holy grail against pandemic SARS-CoV-2? Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemming V.G. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clin. Diagn. Lab. Immunol. 2001;8:859–863. doi: 10.1128/CDLI.8.5.859-863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luke T.C., Casadevall A., Watowich S.J., Hoffman S.L., Beigel J.H., Burgess T.H. Hark back: passive immunotherapy for influenza and other serious infections. Crit. Care Med. 2010;38:e66–e73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 49.Matsushita H., Sano A., Wu H., Wang Z., Jiao J., Kasinathan P., Sullivan E.J., Kuroiwa Y. Species-specific chromosome engineering greatly improves fully human polyclonal antibody production profile in cattle. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bessalah S., Jebahi S., Mejri N., Salhi I., Khorchani T., Hammadi M. Perspective on therapeutic and diagnostic potential of camel nanobodies for coronavirus disease-19 (COVID-19) 3 Biotech. 2021;11:1–14. doi: 10.1007/s13205-021-02647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leenaars M., Hendriksen C.F.M. Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. ILAR J. 2005;46:269–279. doi: 10.1093/ilar.46.3.269. https://doi: 10.1093/ilar.46.3.269 [DOI] [PubMed] [Google Scholar]
- 54.Chouchane L., Grivel J.-C., Abd Farag E.A.B., Pavlovski I., Maacha S., Sathappan A., Al-Romaihi H.E., Abuaqel S.W.J., Ata M.M.A., Chouchane A.I. Dromedary camels as a natural source of neutralizing nanobodies against SARS-CoV-2. JCI Insight. 2021;6 doi: 10.1172/jci.insight.145785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixit R., Herz J., Dalton R., Booy R. Benefits of using heterologous polyclonal antibodies and potential applications to new and undertreated infectious pathogens. Vaccine. 2016;34:1152–1161. doi: 10.1016/j.vaccine.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparrow E., Friede M., Sheikh M., Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017;95:235–237. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saied A.A., Metwally A.A. Bovine-origin human therapy; need more attention. Int. J. Curr. Microbiol. App. Sci. 2019;8:2766–2770. doi: 10.20546/ijcmas.2019.809.318. [DOI] [Google Scholar]
- 58.Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., Tan T.K., Rijal P., Dumoux M., Ward P.N. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020;27:846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- 59.Luke T., Wu H., Egland K.A., Sullivan E.J., Bausch C.L. Fully human antibody immunoglobulin from transchromosomic bovines is potent against SARS-CoV-2 variant pseudoviruses. bioRxiv. 2021 doi: 10.1101/2021.08.09.454215. [DOI] [Google Scholar]
- 60.Gilliland T., Liu Y., Li R., Dunn M., Cottle E., Terada Y., Ryckman Z., Alcorn M., Vasilatos S., Lundy J. Protection of human ACE2 transgenic Syrian hamsters from SARS CoV-2 variants by human polyclonal IgG from hyper-immunized transchromosomic bovines. bioRxiv. 2021 doi: 10.1101/2021.07.26.453840. [DOI] [Google Scholar]
- 61.Xu J., Xu K., Jung S., Conte A., Lieberman J., Muecksch F., Lorenzi J.C.C., Park S., Schmidt F., Wang Z. Nanobodies from camelid mice and llamas neutralize SARS-CoV-2 variants. Nature. 2021;595:278–282. doi: 10.1038/s41586-021-03676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saied A.A., Metwally A.A., Mohamed H.M.A., Haridy M.A.M. The contribution of bovines to human health against viral infections. Environ. Sci. Pollut. Res. 2021;28:46999–47023. doi: 10.1007/s11356-021-14941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z., Wu H., Egland K.A., Gilliland T.C., Dunn M.D., Luke T.C., Sullivan E.J., Klimstra W.B., Bausch C.L., Whelan S.P.J. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. Hum. Vaccin. Immunotherapy. 2021 doi: 10.1080/21645515.2021.1940652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharun K., Tiwari R., Saied A.A., Dhama K. SARS-CoV-2 vaccine for domestic and captive animals: an effort to counter COVID-19 pandemic at the human-animal interface. Vaccine. 2021;39:7119–7122. doi: 10.1016/j.vaccine.2021.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perley C.C., Brocato R.L., Wu H., Bausch C., Karmali P.P., Vega J.B., Cohen M.V., Somerville B., Kwilas S.A., Principe L.M. Anti-HFRS human IgG produced in transchromosomic bovines has potent hantavirus neutralizing activity and is protective in animal models. Front. Microbiol. 2020;11:832. doi: 10.3389/fmicb.2020.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper J.W., Brocato R.L., Kwilas S.A., Hammerbeck C.D., Josleyn M.D., Royals M., Ballantyne J., Wu H., Jiao J., Matsushita H. DNA vaccine–derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3010082. [DOI] [PubMed] [Google Scholar]
- 67.Luke T., Wu H., Zhao J., Channappanavar R., Coleman C.M., Jiao J.-A., Matsushita H., Liu Y., Postnikova E.N., Ork B.L., Glenn G., Flyer D., Defang G., Raviprakash K., Kochel T., Wang J., Nie W., Smith G., Hensley L.E., Olinger G.G., Kuhn J.H., Holbrook M.R., Johnson R.F., Perlman S., Sullivan E., Frieman M.B. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 68.Beigel J.H., Voell J., Kumar P., Raviprakash K., Wu H., Jiao J.-A., Sullivan E., Luke T., Davey R.T., Jr. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect. Dis. 2018;18:410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dye J.M., Wu H., Hooper J.W., Khurana S., Kuehne A.I., Coyle E.M., Ortiz R.A., Fuentes S., Herbert A.S., Golding H. Production of potent fully human polyclonal antibodies against Ebola Zaire virus in transchromosomal cattle. Sci. Rep. 2016;6 doi: 10.1038/srep24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner C.L., Sun C., Luke T., Raviprakash K., Wu H., Jiao J., Sullivan E., Reed D.S., Ryman K.D., Klimstra W.B. Antibody preparations from human transchromosomic cows exhibit prophylactic and therapeutic efficacy against venezuelan equine encephalitis virus. J. Virol. 2017;91 doi: 10.1128/JVI.00226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siddharthan V., Miao J., Van Wettere A.J., Li R., Wu H., Sullivan E., Jiao J., Hooper J.W., Safronetz D., Morrey J.D. Human polyclonal antibodies produced from transchromosomal bovine provides prophylactic and therapeutic protections against Zika virus infection in STAT2 KO Syrian hamsters. Viruses. 2019;11:1–15. doi: 10.3390/v11020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stein D.R., Golden J.W., Griffin B.D., Warner B.M., Ranadheera C., Scharikow L., Sloan A., Frost K.L., Kobasa D., Booth S.A. Human polyclonal antibodies produced in transchromosomal cattle prevent lethal Zika virus infection and testicular atrophy in mice. Antivir. Res. 2017;146:164–173. doi: 10.1016/j.antiviral.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Gao R., Sreenivasan C.C., Sheng Z., Hause B.M., Zhou B., Wentworth D.E., Clement T., Rausch D., Brunick C., Christopher-Hennings J. Human monoclonal antibody derived from transchromosomic cattle neutralizes multiple H1 clades of influenza A virus by recognizing a novel conformational epitope in the hemagglutinin head domain. J. Virol. 2020;94 doi: 10.1128/JVI.00945-20. e00945–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oshiro S., Tada T., Mizutani N., Funatogawa K., Sekiguchi J., Takahashi M., Kirikae T. Presence of antibodies against SARS-CoV-2 spike protein in bovine whey IgG enriched fraction. Int. Dairy J. 2021;117 doi: 10.1016/j.idairyj.2021.105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paparo L., Bruno C., Ferrucci V., Punto E., Viscardi M., Fusco G., Cerino P., Romano A., Zollo M., Canani R.B. Protective effects elicited by cow milk fermented with L. Paracasei CBAL74 against SARS-CoV-2 infection in human enterocytes. J. Funct. Foods. 2021;87 doi: 10.1016/j.jff.2021.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kangro K., Kurashin M., Gildemann K., Sankovski E., Zusinaite E., Lello L.S., Pert R., Kavak A., Poikalainen V., Lepasalu L. Bovine colostrum derived antibodies against SARS-CoV-2 show great potential to serve as a prophylactic agent. medRxiv. 2021 doi: 10.1101/2021.06.08.21258069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biospace SAB biotherapeutics announces SAB-185 retains neutralization against omicron SARS-CoV-2 variant in vitro. https://www.biospace.com/article/releases/sab-biotherapeutics-announces-sab-185-retains-neutralization-against-omicron-sars-cov-2-variant-in-vitro/
- 79.Najmeddin A., Shapourabadi M.B., Behdani M., Dorkoosh F. Nanobodies as powerful pulmonary targeted biotherapeutics against SARS-CoV-2, pharmaceutical point of view. Biochim. Biophys. Acta (BBA)-General Subj. 2021:129974. doi: 10.1016/j.bbagen.2021.129974. 1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossotti M.A., Bélanger K., Henry K.A., Tanha J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2021 doi: 10.1111/febs.15809. [DOI] [PubMed] [Google Scholar]
- 81.Guzman E., Montoya M. Contributions of farm animals to immunology. Front. Vet. Sci. 2018;5:307. doi: 10.3389/fvets.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muyldermans S., Atarhouch T., Saldanha J., Barbosa J., Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. Des. Sel. 1994;7:1129–1135. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- 83.Vu K.B., Ghahroudi M.A., Wyns L., Muyldermans S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997;34:1121–1131. doi: 10.1016/S0161-5890(97)00146-6. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y., Fan Z., Shao L., Kong X., Hou X., Tian D., Sun Y., Xiao Y., Yu L. Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications. Int. J. Nanomed. 2016;11:3287–3303. doi: 10.2147/IJN.S107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bannas P., Hambach J., Koch-Nolte F. Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Front. Immunol. 2017;8:1603. doi: 10.3389/fimmu.2017.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 87.Esparza T.J., Martin N.P., Anderson G.P., Goldman E.R., Brody D.L. High affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-79036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larios Mora A., Detalle L., Gallup J.M., Van Geelen A., Stohr T., Duprez L., Ackermann M.R. Delivery of ALX-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs, in: MAbs. Taylor & Francis. 2018;10:778–795. doi: 10.1080/19420862.2018.1470727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Detalle L., Stohr T., Palomo C., Piedra P.A., Gilbert B.E., Mas V., Millar A., Power U.F., Stortelers C., Allosery K. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob. Agents Chemother. 2016;60:6–13. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schepens B., Ibanez L.I., De Baets S., Hultberg A., Bogaert P., De Bleser P., Vervalle F., Verrips T., Melero J., Vandevelde W. Nanobodies® specific for respiratory syncytial virus fusion protein protect against infection by inhibition of fusion. J. Infect. Dis. 2011;204:1692–1701. doi: 10.1093/infdis/jir622. [DOI] [PubMed] [Google Scholar]
- 91.Van Heeke G., Allosery K., De Brabandere V., De Smedt T., Detalle L., de Fougerolles A. Nanobodies® as inhaled biotherapeutics for lung diseases. Pharmacol. Ther. 2017;169:47–56. doi: 10.1016/j.pharmthera.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L. V.-C. COVID, R. Team, Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181:1004–1015. doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pardon E., Laeremans T., Triest S., Rasmussen S.G.F., Wohlkönig A., Ruf A., Muyldermans S., Hol W.G.J., Kobilka B.K., Steyaert J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014;9:674–693. doi: 10.1038/nprot.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Vlieger D., Ballegeer M., Rossey I., Schepens B., Saelens X. Single-domain antibodies and their formatting to combat viral infections. Antibodies. 2019;8:1–22. doi: 10.3390/antib8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rahbarizadeh F., Ahmadvand D., Sharifzadeh Z. Nanobody; an old concept and new vehicle for immunotargeting. Immunol. Invest. 2011;40:299–338. doi: 10.3109/08820139.2010.542228. [DOI] [PubMed] [Google Scholar]
- 96.Fatima A., Wang H., Kang K., Xia L., Wang Y., Ye W., Wang J., Wang X. Development of VHH antibodies against dengue virus type 2 NS1 and comparison with monoclonal antibodies for use in immunological diagnosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarr A.W., Lafaye P., Meredith L., Damier-Piolle L., Urbanowicz R.A., Meola A., Jestin J., Brown R.J.P., McKeating J.A., Rey F.A. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology. 2013;58:932–939. doi: 10.1002/hep.26430. [DOI] [PubMed] [Google Scholar]
- 98.Thueng-In K., Thanongsaksrikul J., Srimanote P., Bangphoomi K., Poungpair O., Maneewatch S., Choowongkomon K., Chaicumpa W. Cell penetrable humanized-VH/VHH that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thys B., Schotte L., Muyldermans S., Wernery U., Hassanzadeh-Ghassabeh G., Rombaut B. In vitro antiviral activity of single domain antibody fragments against poliovirus. Antivir. Res. 2010;87:257–264. doi: 10.1016/j.antiviral.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 100.Koromyslova A.D., Hansman G.S. Nanobodies targeting norovirus capsid reveal functional epitopes and potential mechanisms of neutralization. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maffey L., Vega C.G., Miño S., Garaicoechea L., Parreño V. Anti-VP6 VHH: an experimental treatment for rotavirus A-associated disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He L., Tai W., Li J., Chen Y., Gao Y., Li J., Sun S., Zhou Y., Du L., Zhao G. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses. 2019;11:166. doi: 10.3390/v11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao G., He L., Sun S., Qiu H., Tai W., Chen J., Li J., Chen Y., Guo Y., Wang Y. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol. 2018;92 doi: 10.1128/JVI.00837-18. e00837–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ashour J., Schmidt F.I., Hanke L., Cragnolini J., Cavallari M., Altenburg A., Brewer R., Ingram J., Shoemaker C., Ploegh H.L. Intracellular expression of camelid single-domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization. J. Virol. 2015;89:2792–2800. doi: 10.1128/JVI.02693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei G., Meng W., Guo H., Pan W., Liu J., Peng T., Chen L., Chen C.-Y. Potent neutralization of influenza A virus by a single-domain antibody blocking M2 ion channel protein. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forsman A., Beirnaert E., Aasa-Chapman M.M.I., Hoorelbeke B., Hijazi K., Koh W., Tack V., Szynol A., Kelly C., McKnight A. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J. Virol. 2008;82:12069–12081. doi: 10.1128/JVI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boons E., Li G., Vanstreels E., Vercruysse T., Pannecouque C., Vandamme A.-M., Daelemans D. A stably expressed llama single-domain intrabody targeting Rev displays broad-spectrum anti-HIV activity. Antivir. Res. 2014;112:91–102. doi: 10.1016/j.antiviral.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Weiss R.A., Verrips C.T. Nanobodies that neutralize HIV. Vaccines. 2019;7:77. doi: 10.1016/j.antiviral.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J.L., Shriver-Lake L.C., Zabetakis D., Anderson G.P., Goldman E.R. Selection and characterization of protective anti-chikungunya virus single domain antibodies. Mol. Immunol. 2019;105:190–197. doi: 10.1016/j.molimm.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 110.Peyvandi F., Scully M., Kremer Hovinga J.A., Cataland S., Knöbl P., Wu H., Artoni A., Westwood J.-P., Mansouri Taleghani M., Jilma B. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N. Engl. J. Med. 2016;374:511–522. doi: 10.1056/NEJMoa1505533. [DOI] [PubMed] [Google Scholar]
- 111.Wang S.-M., He X., Li N., Yu F., Hu Y., Wang L.-S., Zhang P., Du Y.-K., Du S.-S., Yin Z.-F. A novel nanobody specific for respiratory surfactant protein A has potential for lung targeting. Int. J. Nanomed. 2015;10:2857–2869. doi: 10.2147/IJN.S77268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koenig P.-A., Das H., Liu H., Kümmerer B.M., Gohr F.N., Jenster L.-M., Schiffelers L.D.J., Tesfamariam Y.M., Uchima M., Wuerth J.D. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371 doi: 10.1126/science.abe6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wagner T.R., Schnepf D., Beer J., Ruetalo N., Klingel K., Kaiser P.D., Junker D., Sauter M., Traenkle B., Frecot D.I. Biparatopic nanobodies protect mice from lethal challenge with SARS-CoV-2 variants of concern. EMBO Rep. 2021 doi: 10.15252/embr.202153865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zupancic J.M., Schardt J.S., Desai A.A., Makowski E.K., Smith M.D., Pornnoppadol G., Garcia de Mattos Barbosa M., Cascalho M., Lanigan T.M., Tessier P.M. Engineered multivalent nanobodies potently and broadly neutralize SARS-CoV-2 variants. Adv. Ther. 2021;4 doi: 10.1002/adtp.202100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martinez-Delgado G. Inhaled nanobodies against COVID-19. Nat. Rev. Immunol. 2020;20:593. doi: 10.1038/s41577-020-00443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gai J., Ma L., Li G., Zhu M., Qiao P., Li X., Zhang H., Zhang Y., Chen Y., Ji W. A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. MedComm. 2021;2:101–113. doi: 10.1002/mco2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu Q., Zhang Z., Li H., Zhong K., Zhao Q., Wang Z., Wu Z., Yang D., Sun S., Yang N. Development of multivalent nanobodies blocking SARS-CoV-2 infection by targeting RBD of spike protein. J. Nanobiotechnol. 2021;19:1–12. doi: 10.1186/s12951-021-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stefan M.A., Light Y.K., Schwedler J.L., McIlroy P.R., Courtney C.M., Saada E.A., Thatcher C.E., Phillips A.M., Bourguet F.A., Mageeney C.M. Development of potent and effective synthetic SARS-CoV-2 neutralizing nanobodies. mAbs. 2021;13 doi: 10.1080/19420862.2021.1958663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schoof M., Faust B., Saunders R.A., Sangwan S., Rezelj V., Hoppe N., Boone M., Billesbølle C.B., Zimanyi M., Deshpande I. An ultra-high affinity synthetic nanobody blocks SARS-CoV-2 infection by locking Spike into an inactive conformation. bioRxiv. 2020 doi: 10.1101/2020.08.08.238469. [DOI] [Google Scholar]
- 120.Yao H., Cai H., Li T., Zhou B., Qin W., Lavillette D., Li D. A high-affinity RBD-targeting nanobody improves fusion partner's potency against SARS-CoV-2. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X., Gentili M., Hacohen N., Regev A. A cell-free nanobody engineering platform rapidly generates SARS-CoV-2 neutralizing nanobodies. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-21171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chi X., Liu X., Wang C., Zhang X., Li X., Hou J., Ren L., Jin Q., Wang J., Yang W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat. Commun. 2020;11:1–7. doi: 10.1038/s41467-020-18387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Custódio T.F., Das H., Sheward D.J., Hanke L., Pazicky S., Pieprzyk J., Sorgenfrei M., Schroer M.A., Gruzinov A.Y., Jeffries C.M. Selection, biophysical and structural analysis of synthetic nanobodies that effectively neutralize SARS-CoV-2. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-19204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong J., Huang B., Wang B., Titong A., Kankanamalage S.G., Jia Z., Wright M., Parthasarathy P., Liu Y. Development of humanized tri-specific nanobodies with potent neutralization for SARS-CoV-2. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-74761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanke L., Perez L.V., Sheward D.J., Das H., Schulte T., Moliner-Morro A., Corcoran M., Achour A., Hedestam G.B.K., Hällberg B.M. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-18174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schoof M., Faust B., Saunders R.A., Sangwan S., Rezelj V., Hoppe N., Boone M., Billesbølle C.B., Puchades C., Azumaya C.M. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science. 2020;370:1473–1479. doi: 10.1126/science.abe3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiang Y., Nambulli S., Xiao Z., Liu H., Sang Z., Duprex W.P., Schneidman-Duhovny D., Zhang C., Shi Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science. 2020;370:1479–1484. doi: 10.1126/science.abe4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiang Y., Huang W., Liu H., Sang Z., Nambulli S., Tubiana J., Williams K.L., Duprex W.P., Schneidman-Duhovny D., Wilson I.A., Taylor D.J., Shi Y. Super-immunity by broadly protective nanobodies to sarbecoviruses. bioRxiv. 2021 doi: 10.1101/2021.12.26.474192. [DOI] [Google Scholar]
- 129.Diseases T.L.I. Emerging SARS-CoV-2 variants: shooting the messenger. Lancet Infect. Dis. 2021;22:1. doi: 10.1016/S1473-3099(21)00770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao S.-J., Guo H., Luo G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J. Med. Virol. 2021:1–2. doi: 10.1002/jmv.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ikemura N., Hoshino A., Higuchi Y., Taminishi S., Inaba T., Matoba S. SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.13.21267761. [DOI] [Google Scholar]
- 132.He X., Hong W., Pan X., Lu G., Wei X. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021;2:838–845. doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dhawan M., Priyanka O.P.C. Omicron SARS-CoV-2 variant: reasons of emergence and lessons learnt. Int. J. Surg. 2022;97 doi: 10.1016/j.ijsu.2021.106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., De Oliveira T., Vermeulen M., Van der Berg K. SARS-CoV-2 501Y. V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 135.Dong J., Huang B., Jia Z., Wang B., Gallolu Kankanamalage S., Titong A., Liu Y. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg. Microb. Infect. 2020;9:1034–1036. doi: 10.1080/22221751.2020.1768806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma H., Zeng W., Meng X., Huang X., Yang Y., Zhao D., Zhou P., Wang X., Zhao C., Sun Y. Potent neutralization of sars-cov-2 by hetero-bivalent alpaca nanobodies targeting the spike receptor-binding domain. J. Virol. 2021;95 doi: 10.1128/JVI.02438-20. e02438–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun D., Sang Z., Kim J., Xiang Y., Cohen T., Belford A.K., Huet A., Conway J.F., Sun J., Taylor D. Potent neutralizing nanobodies resist convergent circulating variants of SARS-CoV-2 by targeting novel and conserved epitopes. bioRxiv. 2021;12:4676. doi: 10.1101/2021.03.09.434592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Karampela Ι., Dalamaga M. Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic. Metab. Open. 2021 doi: 10.1016/j.metop.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cevik M., Grubaugh N.D., Iwasaki A., Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., Al Khatib H.A., Coyle P., Ayoub H.H., Al Kanaani Z. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021;385:1–15. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sasisekharan R. Preparing for the future—nanobodies for Covid-19? N. Engl. J. Med. 2021;384:1568–1571. doi: 10.1056/NEJMcibr2101205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed in this research are available from the corresponding author upon reasonable request.