Abstract
As COVID-19 hounds the world, the common cause of finding a swift solution to manage the pandemic has brought together researchers, institutions, governments, and society at large. The Internet of Things (IoT), artificial intelligence (AI)—including machine learning (ML) and Big Data analytics—as well as Robotics and Blockchain, are the four decisive areas of technological innovation that have been ingenuity harnessed to fight this pandemic and future ones. While these highly interrelated smart and connected health technologies cannot resolve the pandemic overnight and may not be the only answer to the crisis, they can provide greater insight into the disease and support frontline efforts to prevent and control the pandemic. This article provides a blend of discussions on the contribution of these digital technologies, propose several complementary and multidisciplinary techniques to combat COVID-19, offer opportunities for more holistic studies, and accelerate knowledge acquisition and scientific discoveries in pandemic research. First, four areas, where IoT can contribute are discussed, namely: 1) tracking and tracing; 2) remote patient monitoring (RPM) by wearable IoT (WIoT); 3) personal digital twins (PDTs); and 4) real-life use case: ICT/IoT solution in South Korea. Second, the role and novel applications of AI are explained, namely: 1) diagnosis and prognosis; 2) risk prediction; 3) vaccine and drug development; 4) research data set; 5) early warnings and alerts; 6) social control and fake news detection; and 7) communication and chatbot. Third, the main uses of robotics and drone technology are analyzed, including: 1) crowd surveillance; 2) public announcements; 3) screening and diagnosis; and 4) essential supply delivery. Finally, we discuss how distributed ledger technologies (DLTs), of which blockchain is a common example, can be combined with other technologies for tackling COVID-19.
Keywords: Artificial intelligence (AI), big data, blockchain, COVID-19, digital twin, eHealth, healthcare, Internet of Things (IoT), pandemic, robotics, wearable
I. Introduction
The global COVID-19 pandemic, caused by the SARS-CoV-2 virus, has adversely affected all aspects of daily life and tested the functioning of our societies. As virologists work to develop a vaccine rapidly, a multidisciplinary approach has become vitally important for the appropriate tracing, monitoring, and diagnosis of coronavirus patients. The turn of the decade was expected to bring significant medical and scientific advancement due to the development of digital technologies capable of addressing large clinical issues or major diseases. Promising smart and connected health (SCH) technologies, such as the Internet of Things (IoT), artificial intelligence (AI), robotics, and distributed ledger technologies (DLTs), are increasingly becoming important in almost all healthcare processes [1]–[3], [65]. This paradigm shift, characterized by the convergence of these technologies, has generated new opportunities and advantages, such as the availability and accessibility, the ability to personalize and tailor content, and cost-effective just-in-time delivery. The rapid growth of wearable IoT (WIoT), as well as the public embracement of miniature wearable biosensors, supports the creation of a highly connected personalized patient-centric health ecosystem. Such a system facilitates the collection, integration, and harmonization of real-time data utilized by deep learning and AI to analyze healthcare trends, project potential risks, forecast possible outcomes, accelerate scientific discoveries, and improve decision making [4], [5]. These capabilities are amplified by the ability of DLT to overcome the weaknesses and vulnerabilities of today’s client/server cloud IoT models, such as security, privacy, and traceability, by providing a shared, decentralized, and immutable database ledger based on peer-to-peer networks.
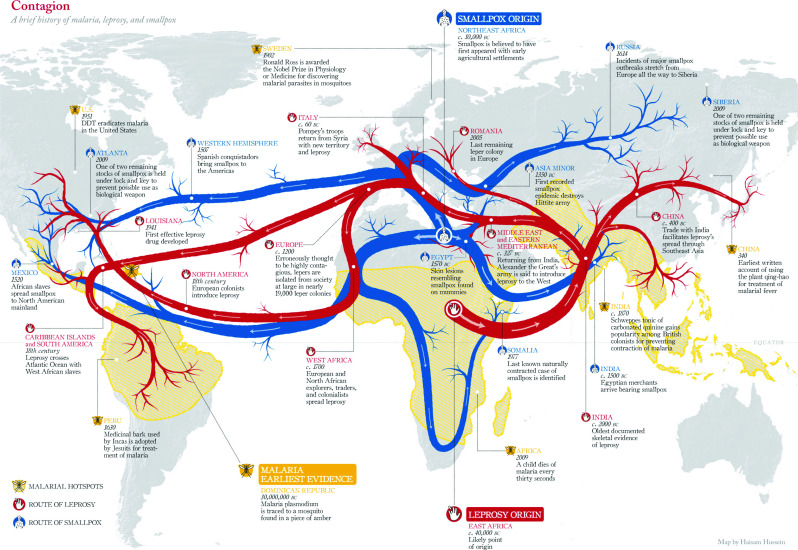
With the transition of human life from a nomadic lifestyle to living in larger groups or cities, humans have experienced epidemics. The gathering of people creates an ideal environment for virus transmission from person to person, resulting in an epidemic. A greater physical distance between groups is a means of slowing the spread of viruses, because an epidemic only grows if a host travels between groups. As illustrated in Fig. 1, epidemics have generally grown along land, water, or commerce passageways. In the past, travel was much slower and infrequent; therefore, epidemics did not grow into pandemics for several years. In today’s world, groups of people in cities and towns are much larger than in the past, and travel between groups occurs much more quickly and often, facilitating the growth of epidemics and pandemics [6].
Fig. 1.
Origin and spread of smallpox, leprosy, and malaria around the globe. Macrorepresentation. Image credit: Doug Belshaw blog [6].
Over the last 100 years, the world has experienced several epidemics and outbreaks, with the majority of them caused by influenza viruses, such as H1N1, H2N2, and H3N2, as well as coronaviruses [7]. In fact, H1N1 has caused two pandemics: 1) the Spanish flu of 1918 and 2) the swine flu pandemic of 2009. The H2N2 virus caused the Asian flu of 1957, and H3N2 caused the Hong Kong flu of 1968. Over the last 20 years, multiple coronavirus outbreaks have also occurred, including the 2002 SARS-CoV outbreak and the 2012 MERS-Co-V outbreak. These viruses are spherical, positive-sense RNA viruses with diameters ranging from 60 to 140 nm [8]. Protruding proteins appear as spikes, giving the virus a crown-like appearance when viewed through an electron microscope. The SARS-CoV outbreak started in China’s Guangdong province and spread to 37 countries through more than 8000 infections and almost 800 deaths. The initial middle east respiratory syndrome coronavirus (MERS-CoV) case was recognized in Saudi Arabia and led to a large outbreak across the Middle East, resulting in almost 900 deaths [7]. The COVID-19 outbreak began in Wuhan, China, in December 2019, and it was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020. The WHO indicates that viral infections, including coronaviruses, will continue to appear, posing serious threats to public health. The epicenter of the outbreak was traced back to an outdoor wholesale market in Wuhan, China, where animals, such as bats, snakes, and marmots, were sold. COVID-19 is distinguished by a long incubation period of up to 14 days and a highly contagious nature. During the incubation period, infected individuals do not necessarily demonstrate symptoms and can unknowingly infect others, resulting in the high basic reproduction number of COVID-19 [7].
Due to the absence of a solid treatment solution, social distancing has been recommended as the best preventative measure for battling COVID-19. However, social distancing requirements have resulted in lockdowns worldwide and negatively impacted economies around the globe. The shutdown of nonessential services has resulted in the disruption of supply chains and job losses across multiple sectors. The rapid spread of the virus has also resulted in trade restrictions that put international trade in danger of collapse. For example, JP Morgan Chase estimated that the current pandemic will cost the U.S. economy more than $5.5 trillion over the following 18–24 months [7]. In light of the challenges posed by COVID-19 to our societies and healthcare systems, there is an imperative need for immediate countermeasures. To this end, SCH technologies can be utilized to mitigate the adverse impacts of the pandemic. For instance, a highly vigilant investigation using currently available data in conjunction with expressive predictions may prove valuable in future policy development and decision making. The massive volume of epidemiological and scientific big data is empowering frontline healthcare workers, strategists, scientists, and epidemiologists to make smart decisions during the COVID-19 pandemic. AI may also play a vital role in understanding and suggesting the development of a vaccine for COVID-19. The efficacy and efficiency of clinical trials is another main contribution of big data, as the world prepares against possible future pandemics. The integration of IoT and AI enables scientists to gather, combine, and fully evaluate global incident data, perform proper screening, and analyze, predict, and track the current patients and likely future patients, allowing healthcare systems to handle the impacts of the pandemic better [1]–[8]. This article consolidates several key technological enablers, evaluates various novel SCH solutions to combat COVID-19, and provides opportunities for more holistic research toward solutions to benefit all of humanity. We will also propose and present a set of complementary techniques to tackle the COVID-19 pandemic, ranging from novel contact tracing to early diagnosis.
The remainder of this article is organized as follows. In Section II, the role of digital twins is discussed. In Section III, we discuss the benefits of IoT for the healthcare industry and the challenges and barriers to be tackled in this regard. Section IV presents ten novel cases of AI use. Section V describes the main techniques and models for integrating robotics and drone technology into healthcare scenarios. Section VI explores the use of DLT and blockchain technologies to help tackle the impact of the COVID-19 pandemic. Section VII demonstrates a holistic use case. Finally, Section VIII concludes this article.
II. Personal Digital Twins and the Promise of Personalized Health
While the modern world is a more fertile ground for epidemics, humanity has also developed tools, such as vaccines, to protect against viruses. However, it takes a significant amount of time to create a vaccine, which is problematic when a virus unexpectedly emerges. This is the case with the coronavirus pandemic of 2020 that likely jumped from bats to humans only months ago. Unfortunately, tools, such as vaccines, can sometimes be in short supply, making it vital that potential epidemics be identified as quickly as possible in order to slow their spread. The illustration in Fig. 1 was developed using historical contagion data. However, such a data graphic needs to be created in real time to forecast the development of an epidemic. Modern tools utilize data from various sources and interpret the data using epidemic models that consider the method and speed of the spread of contagions across different communities and areas [6].
Different techniques can be used to monitor the spread of viruses and identify habits that increase exposure. For instance, the 2020 coronavirus pandemic is being monitored by several organizations, including Northeastern University’s Network Science Institute, through social media using big-data analytics. While social media can be utilized as a sensor, their level of sensitivity and resolution is not ideal. Thus, personal digital twins (PDTs) can be useful in this regard (see Fig. 2). A digital twin is a virtual and digital copy/replica of a tangible entity (a physical object). Although the concept of a digital twin has its roots mostly in the manufacturing industry, several institutes have recently utilized this concept in the domains of medicine and healthcare to develop the digital twin models of human organs [9]. PDTs provide 360-degree health information by synchronizing all sources of data, from electronic health records (EHRs) to clinical data, public records, patient portals, smartphones, wearables, IoT devices, social media, etc. In general, PDTs can be combined with machine learning (ML) algorithms to predict different user contexts, detect early warning signs for preventive measures, forecast the transition from baseline states, and enable the deduction of optimal treatment and personalized medicine. The main advantages of PDTs can be summarized as follows [6].
-
1)
Self-Generation of Alerts: The autogeneration of alerts allows people to be more aware of a possible critical situation.
-
2)
Widespread Analytics: Analytics across a vast community or country help anticipate a significant spread.
-
3)
Clearer Focus: A clearer focus leads to fewer widespread restrictions, where the risk is lower and more robust restrictions in high-risk areas.
-
4)
Lower Cost: More focused restrictions reduce the economic impact.
-
5)
Greater Adaptability: This approach is more dynamic and allows groups or individuals to react to specific situations
-
6)
Better Personal Awareness: PDTs enable greater personal awareness and prompt behavior appropriate to the situation.
-
7)
Quicker Feedback: PDTs allow for real-time or near-real-time feedback regarding actions taken using the data gathered and shared.
-
8)
Reduced Effort: PDTs require lesser effort and offer a lower cost means of monitoring people.
-
9)
Service Development: PDTs facilitate the development of services aimed at infected individuals by creating virtual groups or communities.
-
10)
Better Resource Use: PDTs enable the efficient use of resources considering the availability of resources and competing needs.
-
11)
Faster Triage: Individuals can gain access to necessary support services more appropriately through the cyberspace.
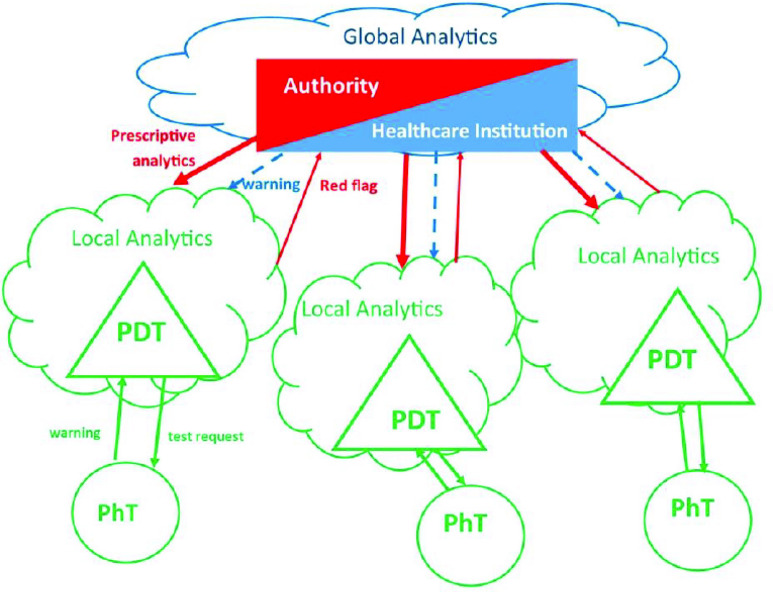
Fig. 2.
Schematic representation of the use of PDTs in epidemic control. The healthcare institution sets the parameters to be monitored by the PDTs, based on global data analytics. The authority enforces the behavioral framework proposed by the healthcare institution (e.g., when Rt is greater than 1.5, in a given area, people have to stay home; if it is between 1.1 and 1.5, people must not share cars; if a physical twin is COVID positive, he/she has to stay home). This framework becomes the reference for the PDT that will ensure awareness of its physical twin and signal the authority if any deviation from the framework occurs. Thus, privacy is protected. The authority is notified only when a behavior is inconsistent with the framework. A person notifies the healthcare institution of a positive test result, not the authority, thus preserving privacy. However, a behavior that is not consistent with the authority-enforced framework will result in the generation of a report.
In the context of COVID-19, PDTs can represent different personal aspects, such as a person’s movement, health status, and interaction with others in geophysical locations. For instance, a person’s physical location and movements can be tracked through smartphone data, and their health status can be monitored using smartwatch sensors. As another example, PDTs could be designed to send an alert if the gathered data form a pattern, such as increased resting body temperature and rapid breathing, which are known to be possible indicators of coronavirus infection. Government healthcare organizations could receive these alerts generated by PDTs, analyze the linked data, including the prevalence of alerts in a specific location, and review the movements of individuals over previous weeks and months to correlate with other emerging alerts.
Complete epidemic monitoring and control using PDTs is still several years away. Nevertheless, as will be explained in Section VII, the government of South Korea and the Korean Center for Disease Control have already gathered a massive amount of data from smartphone locations using local telecommunications and public security cameras to develop a contagion map using microlevel human interactions. Rapid testing combined with the interaction map enabled the appropriate isolation of specific hosts rather than the large-area lockdowns enforced in China and other countries, including Italy, Austria, and Spain. Outlining the different processes can clarify the differences and similarities between South Korea’s model and the use of PDTs; however, the goal of containing an epidemic is the same in both scenarios [6].
-
1)South Korea’s Model:
-
a)Possible Symptoms Emerge: An individual with possible symptoms of a coronavirus infection is tested. If positive, the individual is quarantined.
-
b)Contact Tracing: The contact of the infected individual with others is traced through technology, including smartphone movements and security camera footage.
-
c)Data Analysis: The contact data of the individual are reviewed using data analytics to determine the likelihood of exposure for others. The contacts are located and tested. If positive, these individuals are quarantined. The sequence is repeated to identify any other likely exposure.
-
a)
-
2)PDT Model:
-
a)Prescriptive Analytics: All PDTs are notified by the healthcare organization of a need to send alerts based on specific conditions, such as increased resting body temperature, elevated heart rate (HR), rapid breathing at rest, and other infection indicators.
-
b)Global Analytics: The healthcare organization obtains the data through PDTs and uses global analytics to detect the development of patterns. The organization then alerts PDTs that are part of a visible pattern or in a location with a high likelihood of exposure to request that the individual be tested.
-
c)Trigger Action: The coronavirus test results trigger specific action, such as quarantine for those infected, and provides additional updates to affected PDTs.
-
d)Contact Tracing: The PDTs of those who test positive then report the movement and contact history of the infected individual.
-
e)Dynamic Updating: PDTs continue to update the healthcare organization and communicate with the PDTs of the people nearby, resulting in warnings of proximity that can help reduce risk.
-
a)
PDTs have several advantages and disadvantages. In such a situation, healthcare organizations and governmental agencies may use data analytics and alerts on all PDTs to gather the required data, increase awareness of likely epidemics, and allow for a better forecasting based on the movement of individuals and groups. This would provide a more accurate and timely picture of the global situation. While these would be positive improvements, issues around data privacy and organizational/governmental control would also be raised, as these measures would push society into unprecedented areas. In the world of healthcare, actions taken are shaped by multiple factors, including social concerns, cost, ethics, and resource availability. Even now, newspapers are exploring the importance of protecting privacy while monitoring people to identify infections. Nations and businesses that did not previously favor lockdowns are now adjusting their guidelines and policies. The West adopted a macrolevel approach, whereas South Korea adopted a microlevel approach. To date, it appears that South Korea’s model is better at reducing the spread of the virus and protecting business operations. The tradeoff between the two models lies between civil rights and privacy. Every society operates on a system of tradeoffs between community and personal rights and societal versus personal advantages. The larger issue on which there is no global agreement is where personal rights end and societal rights begin [6].
Technology can be beneficial in identifying the line between personal privacy and the needs of society by protecting privacy as much as possible while still meeting societal safety needs. PDTs can serve to separate social and private spheres by protecting the privacy of personal data and sending metadata to the social sphere. This generates a buffer zone that can be defined by a regulator. PDTs may be capable of developing a privacy shield that can transmit only the information required to meet community needs. In this context, blockchain is a promising technology for monitoring data flow and safeguarding privacy [10].
From a practical point of view, various COVID-19-related systems, applications, and services that are currently developed and used are likely to disappear after temporary use. Previously, several systems have been created to respond to infectious diseases or disasters. However, after the incidents were resolved, these systems became difficult to maintain further due to the decrease in the number of users and were terminated. For example, in the case of a mask inventory management application currently used in South Korea, several people used it in the first half of 2020, when it was difficult to purchase a mask; however, now that the mask supply-and-demand situation has stabilized, there are no more users of this application. On the other hand, the epidemic investigation support system (EISS) platform has shown the possibility of operating as a hub of various kinds of data, as it was expanded by interlocking the platforms of telecommunication companies and card companies based on the smart city system that was already being developed. In other words, it is necessary to expand the developed system into a more general system, not a service for COVID-19 alone, to maintain its sustainability and practicality.
III. Role of IoT in Managing COVID-19
The IoT revolution is reshaping the healthcare landscape, generating new opportunities and advantages, particularly in the age of COVID-19, such as increasing the availability and accessibility of diagnosis and treatments, reducing hospital visits, reducing the fatigue of healthcare workers, reducing the risk of infection for medical staff, and lowering interactions and costs. The major applications of IoT for the COVID-19 pandemic in three main phases, namely, early diagnosis, quarantine time, and after recovery, include [7], [11] the following.
-
1)
Contact Tracing: Identifying those who have had contact with an infected individual.
-
2)
Rapid Screening and Early Diagnosis: Rapid screening and early diagnosis are the key to prevent the spread of COVID-19. WIoT can be utilized to monitor remotely and understand the corresponding symptoms (e.g., fever, dry cough, tiredness, aches and pains, sore throat, diarrhea, headache, loss of taste or smell, skin rash, discoloration of fingers or toes, and difficulty breathing or shortness of breath) in a faster and more efficient manner than traditional techniques while reducing the risk of infection for medical staff.
-
3)
Remote Monitoring: Remote monitoring (RM) is utilized to collect medical data from biosensors or WIoT devices to monitor the status of a patient (e.g., HR variability, pressure, and temperature) outside a clinical setting. RM is a vital component of treating patients while preventing medical staff from being infected and reducing the fatigue of healthcare workers.
-
4)
Alerting: IoT can be used to alert the authorities, healthcare providers, and families in case of an emergency.
-
5)
Controlling Social Distancing: IoT can also be utilized to monitor and enforce social distancing policies.
A. Contact Tracing
The identification of those who have been exposed to or infected by the virus is known as contact tracing. The extended incubation period of COVID-19 and the lack of extensive testing have made it difficult for authorities to quantify the number of infections accurately. The WHO indicates that contact tracing includes the following three steps [7].
-
1)
Identifying those who have had contact with an infected individual.
-
2)
Documenting the details of the contacted individuals.
-
3)
Testing those individuals as quickly as possible.
The state-of-the-art contact tracing solutions can be classified into the following categories.
The spring of 2020 witnessed the emergence of several smartphone contact-tracing application projects, championed in a large part by TraceTogether, an application developed by Singapore’s government. TraceTogether utilizes Bluetooth to support anonymous, close-proximity smartphone-to-smartphone communication. Several tracing application projects also center on Bluetooth technology. One of the primary issues in the development of a contact-tracing application is the precise perception of distance and discernment using a meter scale. Most developers have determined that a 2-m standard distance for contact-tracing applications is ideal.
The implementation of contact-tracing applications requires one of the technologies indicated below.
-
1)
Bluetooth: Applications using Bluetooth measure the distance between two parties by calculating the space between devices with the received signal strength indicator (RSSI). The applications are capable of storing a device’s previous Bluetooth connection history, including data about the amount of time the devices were connected. If an individual is diagnosed with COVID-19, tracing applications can use the Bluetooth connection history to trace all the individuals exposed to the virus through the infected person [7].
-
2)
GPS: Governmental agencies can monitor the location of COVID-19 patients in real time and view historical GPS data, which can be useful in tracing coronavirus exposure.
-
3)
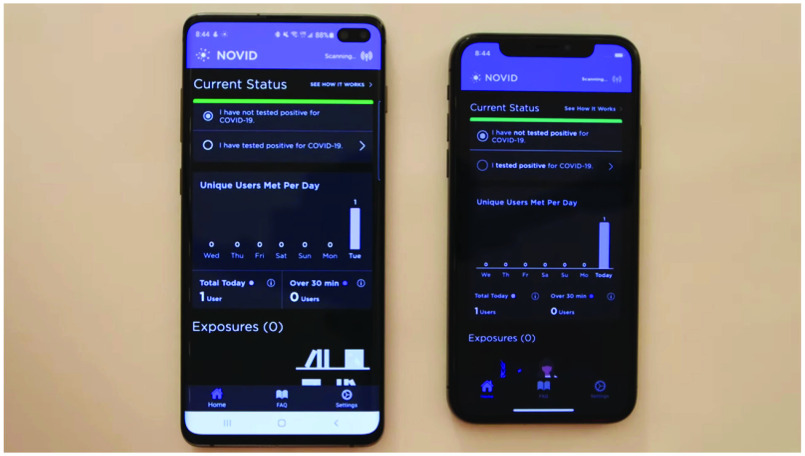
Ultrasonic: It has been argued that Bluetooth cannot precisely calculate the distance. In addition, GPS and Bluetooth are susceptible to inappropriate logging interactions between parties located in separate rooms when signals traverse ceilings or walls. We propose the use of ultrasonic technology in conjunction with Bluetooth. As ultrasound measures the time required for sound to travel, it is capable of measuring device distances more precisely. The NOVID application was launched in April 2020 (Please See Fig. 3). An experiment using the publicly accessible features of the application allowed for systemic testing in various real-world settings. The data suggest that a 9-foot threshold for distance measurement is highly effective. NOVID was tested in challenging environments. Consequently, the distances in 99.6% of 225 interactions, where the devices were 12 or more feet apart were correctly categorized as greater than 9 feet. The distances in more than 50% of the 187 interactions where the devices were less than 6 feet apart were accurately categorized as less than 9 feet. The experiment indicates that contact-tracing applications can substantially benefit from ultrasound technology.
Fig. 3.
NOVID application.
B. COVID-19 and the Rise of WIoT-Based Remote Patient Monitoring
Due to the increasing number of patients and the lack of appropriate medication, several nations have been seriously affected by COVID-19. During pandemic outbreaks, such as COVID-19, wearable health devices can play a very important role by providing unique patient-centric insights into health and wellbeing in everyday settings. Unlike conventional monitoring solutions, which are performed in a noncontinuous manner a few times a year, WIoT provides continuous access to real-time physiological data, thus changing the healthcare landscape. In addition, wearables can offer new methods to incentivize or “game-ify” self-monitoring. Stay-at-home orders and social distancing have accelerated the adoption of WIoT. Indeed, in the age of COVID-19, WIoT devices have been increasingly piloted in clinical trials to track remotely those individuals who might require hospital admission or who have recently been discharged from hospitals, or to monitor the vital signs of people in quarantine [12]–[15]. WIoT can be utilized in the early diagnosis of diseases and for tracking by inspecting systemic infection sources. Wearable devices may alert patients and doctors of possible COVID-19 symptoms prior to a severe illness. The desire for a noninvasive device to detect continuously and track coronavirus infections at home and in the hospital makes wearable devices attractive. After a rapid diagnosis, having the capability to monitor and track vital biological signs can encourage doctors to proceed with the appropriate action for a quick recovery or reduce severe deterioration.
The continuous expansion of monitoring from the hospital to the home represents an optimism in overcoming COVID-19 infections. Wearable technologies with clinical-grade accuracy may illustrate the degree of this benefit through ongoing clinical analyses. An emphasis on information sharing and interoperability empowers the growth of predictive algorithms, which can be generalized within various populations. Along with the worldwide development of successful drugs and vaccines to treat and counteract COVID-19, compatible skin-integrated devices and sensors, placed at the optimal positions on the body, resolve the critical and ongoing requirement for continuous, objective, and sensitive systems to identify COVID-19 symptoms.
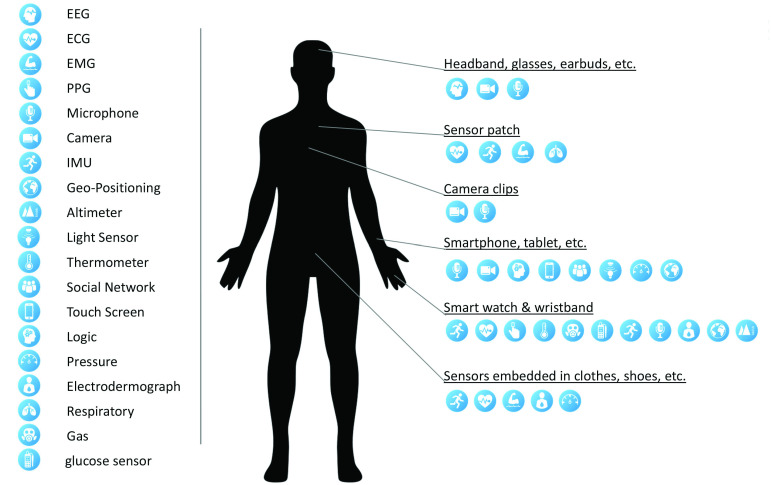
Generally, after exposure to this virus, the human body undergoes various physiological changes that can be monitored for treatment purposes. These signs can be in the form of biochemical, electrical, and biosignals, which are derived from different body parts [16], [17]. These physiological signs help predict the health status of a patient or individual easily. Consequently, based on these measurements, the appropriate medication can be administered, or people can be transferred to the hospital when necessary. Current commercial wearables and WIoT technologies can monitor and collect only a small portion of the physiological data and biomarkers (see Fig. 4) [1], [18]–[27]. The sensor modalities implemented to measure physiological symptoms at the onset of COVID-19 include (but are not limited to) the temperature, HR using an electrocardiogram (ECG); pulse plethysmography (PPG); HR variability using ECG and PPG; blood pressure using PPG; respiratory rate using ECG, PPG, and an accelerometer; oxygen saturation (SpO2) using PPG; sleep using an accelerometer; and cough using mechanical or piezoelectric sensing.
Fig. 4.
Currently available wearables or portable platforms [4].
While wearables, implantable devices, and smart textiles have received considerable attention in the literature, and numerous consumer wearables are commercially available in the marketplace, the potential of WIoT has yet to be fully realized. This state of affairs can be attributed to specific technological shortcomings, including [28]–[33] the following.
-
1)
The available wearables are limited in measurement precision and modalities. For instance, commercial gadgets typically do not provide a measurement of body temperature and high-fidelity respiratory rate, or pulse oximetry.
-
2)
Existing devices are typically not medical grade. For example, popular Oura Ring and FitBit sensors still lack FDA approval for RM. On the other hand, FDA-approved devices, such as the Apple Watch Series 4, can be used for episodic ECG and may provide notifications for uneven heart rhythm for people aged over 22. However, with such limited specifications, this device is not a substitute for clinical diagnostic systems.
-
3)
The current solutions do not fulfill support interoperability (i.e., effectively connect, exchange data, and function with each other) criteria.
-
4)
The performance of the sensors varies over time. The collected data are often unreliable due to motion artifacts and measurement. These inadequacies may undermine the potential benefits of wearable technologies for monitoring, predicting, and tracking coronavirus patients.
-
5)
WIoT technologies for continuous monitoring present opportunities and challenges in data management and data analytics, due to the remarkably large amount and broad range of health data produced through each device. Consequently, wearable sensor systems must incorporate accessible information backends that securely store, transfer, process, and provide the required patient data in an amenable way. In addition, the necessity to associate this information with additional distinct sources (e.g., EHRs) to improve the data content encourages the expansion of strategies for interoperability. With the help of ML techniques, by associating such physiological information along with clinical results, the outcomes of investigational therapeutics and molecular assessments will provide a treasured large-scale source to identify asymptomatic COVID-19 infections. This will institute digital biomes of anticipated recuperation specific to the health status of a patient and offer recommendations for staff to continue working carefully.
ECGs are extensively used in wearable technology to monitor cardiac function [34], [35]. ECG measures the electrical activity of the heart [34]. Although ECG sensors are generally mounted as epidermal patches that are attached to the surface of the skin (e.g., Zio Patch) by using benchtop equipment, the commercialization of predictive algorithm-based wrist-worn devices has empowered the heart activity measurement from wearable devices, for example, Apple Watch 4 and 5 [36]. ECG has the potential to provide meaningful insight into the onset of COVID-19, as it measures the heart function directly. The early indications of COVID-19 infections are high fever (98%), coughing (65%), and breathing difficulties (55%) [37], [38]. There are also cases, where symptoms were obtained from mobile applications (e.g., loss of taste and smell), suggesting a more analytical advantage. Another indication of COVID-19 contamination and deterioration is silent hypoxemia [39]. There is evidence to suggest that COVID-19 is accompanied by a greater chance of arrhythmic incidents [40]. Upon analysis of 138 COVID-19 patients by Driggin et al. [41], arrhythmias, such as ventricular tachycardia/fibrillation, accounted for prominent impediments (19.6%) after severe respiratory distress syndrome, predominantly in intensive care unit (ICU) patients, in whom the prevalence increased to 44.4%.
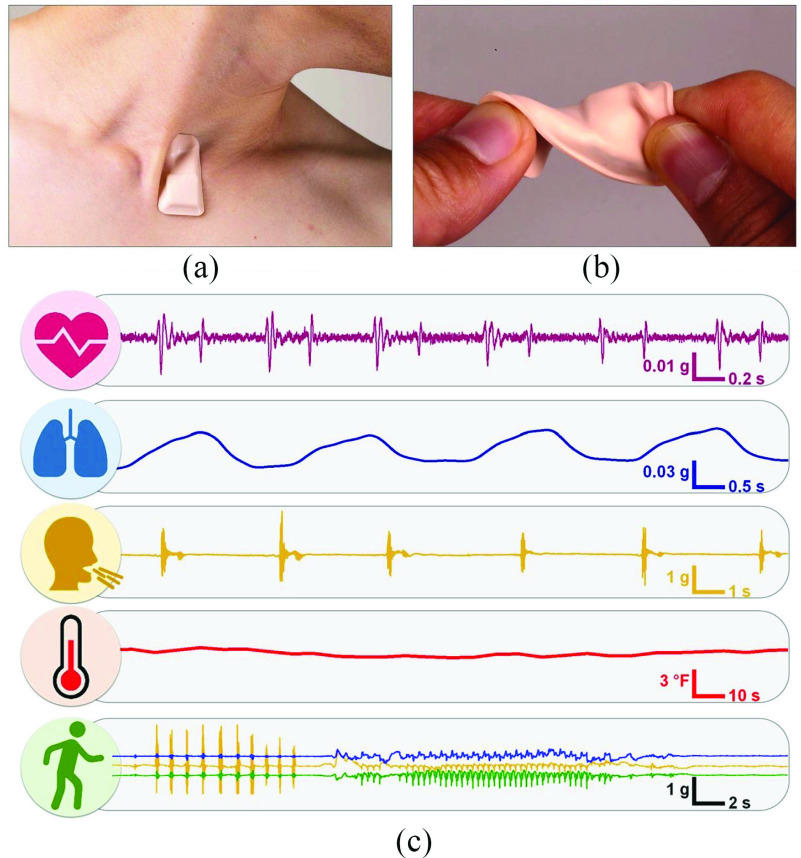
In assessing the signs and treatment of COVID-19, soft and flexible electronic systems that can be attached comfortably to the skin at positions outside the finger or wrist present crucial benefits. As COVID-19 is a respiratory disease, measuring numerous respiratory biomarkers, such as cough intensity/frequency/sound, respiratory effort, and rate, directly from the thorax may provide vital information [42]. Similarly, it is critical to ensure the maintenance of clinical-grade standards for measuring blood oxygenation through pulse oximetry. In [15], a soft sensor was mounted close to the skin near the suprasternal notch, including precision temperature and high-bandwidth accelerometer sensor mounts, as shown in Fig. 5 [42]. This small area of the body close to the neck offers an exceptional interface for recordings of high-fidelity respiratory activities through cough rate, duration, or frequency, and respiratory features linked with sneezing and wheezing. Heart sounds, cardiac amplitude, and HR were also included in the same data streams. To measure skin temperature, a temperature sensor with thermal insulation is used, which is correlated to the core body temperature to ensure that the ambient conditions do not influence the measurement. As they are soft and flexible, these sensors can support the natural movements of the neck. Fig. 5 shows the wearable device and some illustrative data gathered from a COVID-19 patient.
Fig. 5.
Soft, wireless, skin-interfaced wearable ECG patch placed on the suprasternal notch. (a) Representation of the ability of the gadget to follow natural neck movements. (b) Illustration of the mechanical deformation of the device. (c) Data recorded from a COVID-19 patient. Recreated from [42].
IV. Role of AI/ML in Managing COVID-19
During this global public health crisis, the healthcare industry is seeking technology capable of monitoring and controlling the spread of COVID-19. AI/ML is capable of tracing the virus, identifying at-risk individuals, and controlling infection rates in real time. AI/ML can also predict the risk of mortality by analyzing the previous medical data of patients. AI can also aid in population screening and notification, and enhance the treatment and outcomes of COVID-19 patients as an evidence-based healthcare tool. In this section, we review, present, and discuss the 10 most important AI/ML use cases—including our proposed solutions—to tackle the COVID-19 pandemic.
A. COVID-19 Open Research Data Set
Scientific literature is an important source of technical information about COVID-19. Most findings about the progression, diagnostics, treatment, vaccines, and social impacts of COVID-19 are eventually disseminated to the scientific audience and health officials through published research papers and preprints. The rate and speed of publication around COVID-19 have been unprecedented. Several hundreds of new papers or preprints have been released every day since March 2020, and they continue to be released. AI-powered text mining systems and systems that leverage natural language processing (NLP) techniques to provide search, discovery, and summarization of the literature are urgently required. Several corpora of structured, machine-readable scientific literature have emerged to assist in the development of these systems. This includes the COVID-19 Open Research Data Set1 [43], LitCovid2 [44], and other organization-specific databases, such as the WHO’s COVID-19 database.3
CORD-19 was the earliest corpus released for this purpose, and it has been used in the majority of COVID-related automated text mining systems. The CORD-19 corpus was released by the Allen Institute for AI in conjunction with seven other institutions. CORD-19 is a fairly comprehensive data set of coronavirus and COVID-19 papers, incorporating papers and preprints from PubMed Central, PubMed, the WHO’s Covid-19 database, bioRxiv, medRxiv, and arXiv. Metadata are collected from these sources, harmonized, and deduplicated, and the full text of open-access publications is extracted and represented in the S2ORC JSON format [45] to support downstream text mining applications. Detailed descriptions of the data processing pipeline and design motivations of CORD-19 can be found in [43].
CORD-19 has been incorporated into dozens of COVID search and discovery systems; a survey of these text mining resources and applications is provided in [46]. Of these resources, some have integrated the literature data of CORD-19 with other documents (patents and clinical trial documentation) and biomedical and clinical knowledge bases (e.g., CovidGraph).4 Other systems focus on tasks, such as: 1) search, e.g., Neural Covidex [47]; 2) question-answering, e.g., COVIDASK [48]; 3) summarization, e.g., CAiRE-COVID [49]; 4) scientific claim verification, e.g., SciFact [50]; and 5) assistive literature review, e.g., ASReview [51]. The CORD-19 corpus has also been leveraged as the foundation of several community shared tasks: the Kaggle CORD-19 Challenge,5 TREC-COVID ad hoc retrieval challenge6 [52], [53] at TREC 2020, and the Epidemic Question Answering challenge7 at TAC 2020, which aim to evaluate and compare the performances of various text mining and NLP systems.
Challenges, such as the availability of open-access full text (over 60% of COVID-19-related papers from 2020 do not have an associated license allowing redistribution), and difficulties in PDT parsing and metadata harmonization have impeded our ability to expand and improve the quality of CORD-19. We believe that these challenges are surmountable, and we continue to work with publishers and the community to improve the data set. Based on preliminary responses, the CORD-19 corpus offers a strong example of how text mining and NLP can be used to address and respond to major scientific challenges such as COVID-19.
B. Multimodal Diagnosis of COVID-19
With the global spread of COVID-19, the early diagnosis and identification of the risk of infection in potential patients who may not be showing visible critical symptoms can assist medical staff when allocating limited resources. In this section, we propose solutions using ML methods that can help doctors improve the diagnosis and further prognosis of patients using the knowledge extracted from the available data of all patients. Accordingly, a data set comprising more than 2000 samples from individual triaged patients was constructed. Data were collected during triage and follow-ups by medical experts at Sina Hospital in Tehran. In addition, there was a subsequent validation step to minimize the entry-level error in the collected data.
Through feature selection methods, the large initial feature set was reduced to simplify the process for both patients and medical staff. ML techniques were employed to create prediction models for identifying the risk of COVID-19 in patients. This system is currently deployed at Sina Hospital for the diagnosis and clinical monitoring of COVID-19 patients. A key strength of this work is the close continuous collaboration with the medical team at Sina Hospital and regular information updates about the patients. This helps create more robust models that are less prone to bias and overfitting. The proposed framework, adopted methods, and highlighted preliminary results are briefly presented in the following sections.
1). System Overview:
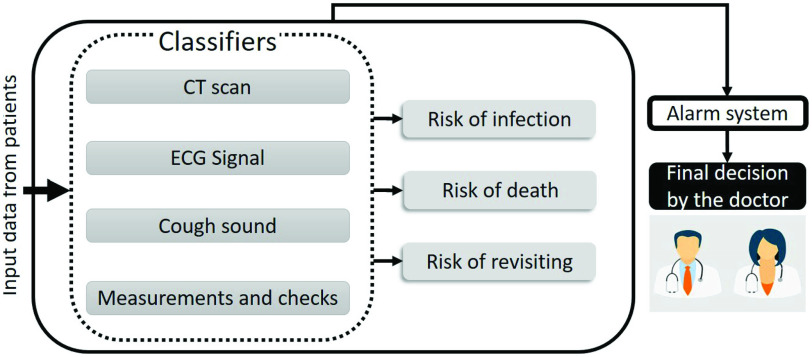
Our proposed system is composed of four classifiers that are fused to achieve more robustness, as shown in Fig. 6. The four data modalities used in our system are: 1) the information gathered during triage and the following tests; 2) cough sound recordings; 3) CT scans; and 4) ECG signals. In this section, we focus on estimating the risk of infection among the risks shown in Fig. 6, as this is the most vital information requested by doctors. This risk measure can be used as a tool to consult with doctors in the diagnosis stage, and also for activating an alarm system in case the clinical conditions of a hospitalized patient dramatically change.
Fig. 6.
Overview of the main components of the proposed framework.
2). Data Collection:
In the first step, we gathered information on more than 2000 patients in three categories: 1) the first follow-up (F1); 2) hospitalization (H); and 3) the second follow-up (F2). All the data were entered and verified by the medical staff and were later checked again using our scripts for data cleaning. The data collection procedure is briefly explained as follows: after visiting the hospital, the patients will undergo triage. In some cases, they are discharged and a follow-up occurs between one and four weeks later with information contributing to the (
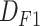 ) data set. In some cases, the patients undergo hospitalization. In such cases, more information is added to the patients’ records, resulting in a more complete entry in the (
) data set. In some cases, the patients undergo hospitalization. In such cases, more information is added to the patients’ records, resulting in a more complete entry in the (
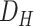 ) data set. Unfortunately, some patients pass away. The remaining patients return home and are contacted in 1–4 weeks for another follow-up, leading to the (
) data set. Unfortunately, some patients pass away. The remaining patients return home and are contacted in 1–4 weeks for another follow-up, leading to the (
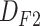 ) data set. The selected features comprise the clinical information collected upon arrival, CT scan image information, the laboratory test results of patients admitted to the ICU, the additional information of patients in that unit, and patient medications and the corresponding reactions. Table I lists the specifications of the data sets.
) data set. The selected features comprise the clinical information collected upon arrival, CT scan image information, the laboratory test results of patients admitted to the ICU, the additional information of patients in that unit, and patient medications and the corresponding reactions. Table I lists the specifications of the data sets.
TABLE I. Data Set Specifications.
| Dataset | #Features | Deceased patients | Recovering patients |
|---|---|---|---|
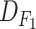 |
81 | 15 | 1985 |
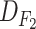 |
81 | 4 | 196 |
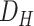 |
1186 | 104 | 507 |
3). Feature Selection:
Due to the different conditions of the patients and the corresponding treatments and medications, not all data fields are present in the collected samples, and there are several missing values for some of the patients. Therefore, we selected the features that are more frequently available while considering the inherent importance of some less frequent features. We choose a threshold that will ensure that the top k% of the features are preserved by considering the histogram of their weighted frequencies. Thus, a feature selection step is performed prior to training our models based on: 1) a cutoff threshold in a weighted histogram of features and 2) the expertise of the doctors. We further refined the features using the chi-squared method, eventually narrowing down the features to 17 for
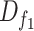 , and 18 for
, and 18 for
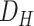 .
.
4). Trained Models:
We trained two sets of models for the diagnosis of COVID-19 (M-I) and the clinical condition classification of virus-infected patients (M-II). M-I is trained based on
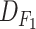 and
and
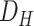 , with two class labels of low and high risks. Patients who have died of coronavirus, who have been hospitalized, have suspicious CT scans (diagnosed by a radiologist based on bronchopneumonia, ground glass opacity, and patchy airspace), or have been revisiting the hospital for COVID-19-related symptoms are identified as high-risk patients (695 cases), and the others are considered low-risk patients (1916 cases).
, with two class labels of low and high risks. Patients who have died of coronavirus, who have been hospitalized, have suspicious CT scans (diagnosed by a radiologist based on bronchopneumonia, ground glass opacity, and patchy airspace), or have been revisiting the hospital for COVID-19-related symptoms are identified as high-risk patients (695 cases), and the others are considered low-risk patients (1916 cases).
Although having a richer data set and higher dimensional feature space leads to more accurate representation and possibly better predictions, providing the required information for constructing these samples can be very expensive in terms of time and the limited availability of resources. Additionally, training such complex models relies on having significantly more samples compared with simpler models and is more prone to overfitting. Therefore, as the first step for COVID-19 diagnosis, our objective is to construct models that take as input the data obtained from the measurements and initial checks by the medical staff and the recorded cough sounds, as these data modalities can be provided quickly and comparatively more conveniently, leading to a faster diagnosis and a larger and more diverse data set.
For clinical condition classification (M-II), we used the hospitalized data set (
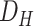 ), with two class labels of mild to moderate, and severe/critical conditions based on the request of doctors. Patients showing mild to moderate symptoms are in the first class (210 cases), and patients showing more severe symptoms, such as respiratory failure and low oxygen levels in their blood (SPO2 < 93), are in the second class (401 cases). The labeling conditions were defined by our medical collaborators.
), with two class labels of mild to moderate, and severe/critical conditions based on the request of doctors. Patients showing mild to moderate symptoms are in the first class (210 cases), and patients showing more severe symptoms, such as respiratory failure and low oxygen levels in their blood (SPO2 < 93), are in the second class (401 cases). The labeling conditions were defined by our medical collaborators.
5). Model Performance:
A series of classification methods was used to find the model with the best performance. Table II lists some methods, along with their complexity and performance for both models. All the models were validated using threefold cross validation. As the doctors prefer better sensitivity, followed by better specificity (stated in our discussions with the medical team), we chose the logistic regression model as our classifier for M-I. The features selected by this model are listed in Table III. For clinical condition classification
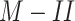 , the best sensitivity was obtained using a multilayer perceptron (MLP) model with two layers, and the best specificity was obtained using the logistic regression model.
, the best sensitivity was obtained using a multilayer perceptron (MLP) model with two layers, and the best specificity was obtained using the logistic regression model.
TABLE II. Performance of the Different Trained Models for COVID-19 Diagnosis and the Clinical Condition Classification Problems.
| M-I | ||||||
|---|---|---|---|---|---|---|
| Method | #Features | Accuracy | F1 Score | Sensitivity | Precision | Specificity |
| MLP (ReLu) | 16 | 81.99 | 62.43 | 56.22 | 70.19 | 91.31 |
| SVM (Linear) | 15 | 79.81 | 65.03 | 70.76 | 60.41 | 83.21 |
| Decision Tree using Entropy | 9 | 74.75 | 59.1 | 68.65 | 52.72 | 77.22 |
| Random Forest using Entropy | 11 | 75.21 | 59.52 | 68.50 | 52.71 | 77.68 |
| lightgray Logistic Regression | 15 | 78.97 | 64.47 | 71.84 | 58.60 | 81.63 |
| SVM (Polynomial) | 14 | 77.05 | 62.29 | 71.22 | 55.43 | 79.2 |
| M-II | ||||||
| SVM (Linear) | 30 | 72.34 | 79.14 | 80.29 | 78.24 | 57.12 |
| Decision Tree using Entropy | 6 | 67.59 | 74.53 | 72.32 | 76.93 | 58.54 |
| Logistic Regression | 28 | 72.01 | 77.88 | 75.32 | 80.84 | 65.71 |
| MLP (ReLu) | 10 | 72.17 | 80.32 | 86.53 | 74.95 | 44.69 |
| AdaBoost | 7 | 71.36 | 77.36 | 74.56 | 80.38 | 65.21 |
| Bagging KNN | 23 | 73.32 | 80.46 | 83.77 | 77.45 | 53.25 |
TABLE III. Features Selected for the Logistic Regression Model.
| Heart diseases | High blood pressure | Diabetes |
|---|---|---|
| Gender | Age | Smoking |
| COPD | Asthma | Rheumatological |
| Malignancy | Body temperature |
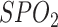 |
| Vaccination background | BMI | Opium |
6). Cough Sound Analysis for COVID-19 Diagnosis:
A respiratory symptom in several COVID-19 patients is dry cough, and having a model that can predict the risk of infection based on cough sounds can be very useful as a preliminary tool for both triaged patients and people who have just started showing symptoms and have not yet visited a doctor. To create models for predicting the infection risk, we constructed a data set composed of cough sound recordings gathered by a nurse using a mobile device, at a distance of 0.3–0.4 m from a patient, recording three incidents per patient.
Such samples collected from diagnosed and hospitalized patients are very limited and expensive, as finding nurses who would be willing to get close to infected patients is very difficult. Moreover, the ward environment is very noisy and uncontrolled in terms of the presence of different sounds. Nonetheless, 133 samples comprising 65 healthy cases, 39 COVID-19-infected cases, and 29 cases with other respiratory diseases were collected. The first 25 mel-frequency cepstral coefficients (MFCCs) [54] of the cough signal were selected as the features for a support vector machine (SVM) model with a radial basis function kernel, which was validated using tenfold cross validation as our classifier. We trained two models, each with two class labels, 1) (
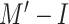 ) with healthy patients and patients who may have any respiratory disease and 2) (
) with healthy patients and patients who may have any respiratory disease and 2) (
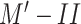 ) with two classes of COVID-19-infected and other patients. Table IV presents the performances of both models. The results show that the performance of
) with two classes of COVID-19-infected and other patients. Table IV presents the performances of both models. The results show that the performance of
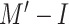 is better than that of
is better than that of
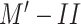 . Moreover, it is easier to differentiate healthy cases from infected cases with any respiratory disease, and correctly classifying COVID-19-infected cases is considerably more difficult.
. Moreover, it is easier to differentiate healthy cases from infected cases with any respiratory disease, and correctly classifying COVID-19-infected cases is considerably more difficult.
TABLE IV. Performance of the Two Trained Models Using the Cough Sounds of Patients.
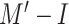 |
||||
|---|---|---|---|---|
| Accuracy | F1 Score | Precision | Sensitivity | |
| Healthy | 0.91 | 0.90 | 0.93 | 0.88 |
| Affected | 0.92 | 0.89 | 0.94 | |
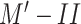 |
||||
| Accuracy | F1 Score | Precision | Sensitivity | |
| COVID-19-infected | 0.80 | 0.68 | 0.66 | 0.70 |
| Other | 0.86 | 0.87 | 0.85 | |
C. Image-Based Diagnosis of COVID-19
AI has the potential to improve the medical diagnosis processes drastically based on imaging. COVID-19 has spread quickly due to the transmission between individuals. SARS is confirmed via laboratory testing with RT-PCR, but this test may require up to 48 h for completion. Chest CT can be an important element in diagnosing and evaluating patients suspected of having SARS. Chest CT results may be normal in some newly infected patients. Therefore, chest CT alone has a limited predictive value for infection. This highlights the need to include clinical information during the diagnosis. AI algorithms may contribute to the diagnostic process by combining chest CT results with symptomology, laboratory testing, and the history of exposure.
We propose a data set composed of 4173 CT scans of 210 different patients, who were divided into 80 patients infected by SARS-CoV-2, 80 patients with other pulmonary diseases, such as non-COVID pneumonia, DPOC, and lung cancer, and 50 patients with healthy lung conditions [55]. Fig. 8 illustrates data that are found in the dataset. Data were collected from March 15, 2020 to June 1, 2020, at the Public Hospital of the Government Employees of Sao Paulo, and the Metropolitan Hospital of Lapa, Sao Paulo, Brazil. Table V details the patients investigated in this study.
Fig. 8.
(a) 27-year-old male patient presenting with fever and headache for two days. CT scans did not show the presence of any pulmonary disease. The RT-PCR test was negative for SARS-CoV-2. (b) 63-year-old female patient presenting with shortness of breath and cough for four days. CT scans showed the presence of a subpulmonic pleural effusion. The RT-PCR test was negative for SARS-CoV-2. (c) 31-year-old female patient presenting with fever, dry cough, and shortness of breath for four days. CT scans revealed multifocal bilateral consolidation with ground-glass opacities with a typical distribution. RT-PCR tested positive for SARS-CoV-2.
TABLE V. Composition of the Data Set. In This Case, We Considered the Data From 80 Patients Infected With SARS-CoV-2, Among Whom 41 Were Male and 39 Were Female. We Also Considered Data From 80 Patients Presenting With Other Pulmonary Diseases, Such as Lung Cancer and DPOC. The Data Set is Also Composed of CT Scans From 50 Patients Who do Not Present Any Pulmonary Disease.
| Condition | No. of Patients | No. of CT-Scans | Average No. of CT-Scans per patient |
|---|---|---|---|
| Healthy | 50 | 758 | 15 |
| COVID-19 | 80 | 2168 | 27 |
| Other pulmonary diseases | 80 | 1247 | 16 |
| TOTAL | 210 | 4173 | 20 |
The inclusion criteria for this study are listed as follows.
-
1)
Patients with a positive new coronavirus nucleic acid antibody test confirmed by the CDC.
-
2)
Patients who underwent thin-section CT.
-
3)
Age >= 18.
-
4)
The presence of lung infection in CT images.
Fig. 7 illustrates the data distribution for patients infected with SARS-CoV-2 considered in this study.
Fig. 7.
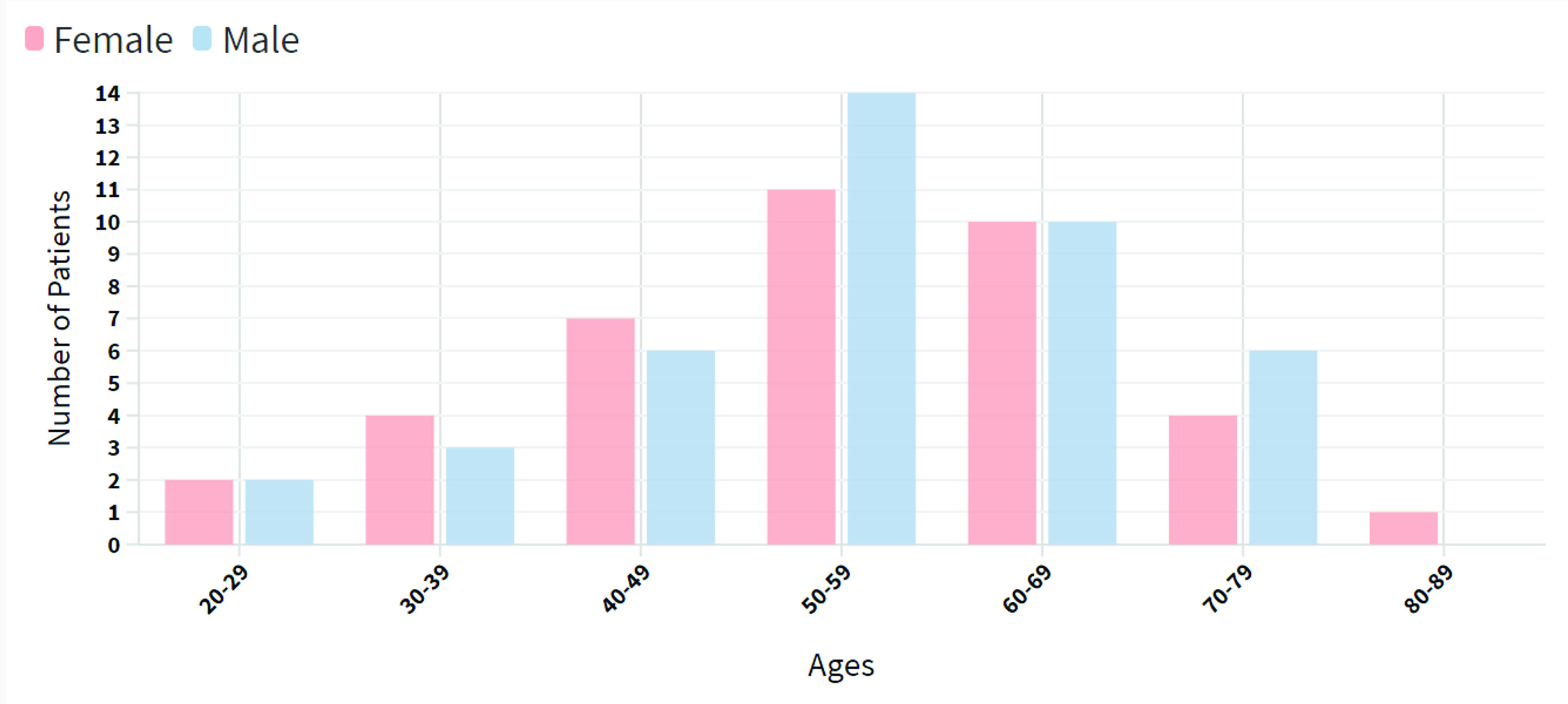
Data distribution for 80 different patients (41 male and 39 female patients). The data revealed that most of the patients were 50–59 years old.
The duration from the onset of illness to the CT scan was in the range of 1–14 days, with a median of five days. The CT protocol was as follows: 120 kV; automatic tube current (180–400 mA); iterative reconstruction, 64-mm detector; rotation time, 0.35 s; slice thickness, 5-mm; collimation, 0.625 mm; pitch, 1.5; matrix,
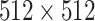 ; and breath hold at full inspiration. The reconstruction kernel used was set as “lung smooth with a thickness of 1 mm and an interval of 0.8 mm.” During reading, the lung window (with a window width of 1200 HU and window level of 600 HU) was used. Fig. 10 illustrates some examples of CT scans found in the data set.
; and breath hold at full inspiration. The reconstruction kernel used was set as “lung smooth with a thickness of 1 mm and an interval of 0.8 mm.” During reading, the lung window (with a window width of 1200 HU and window level of 600 HU) was used. Fig. 10 illustrates some examples of CT scans found in the data set.
Fig. 10.
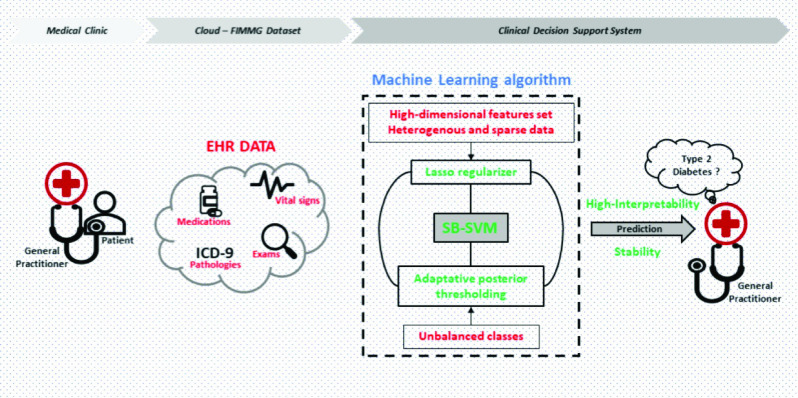
FIMMG data set, in which the general practitioner stores the EHR data. The features were used to train the sparse balanced SVM (SB-SVM) model and to predict T2D. The SB-SVM is formulated with a sparse regularizer and adaptive posterior thresholding.
We relied on the xDNN classification approach [56] for the proposed SARS-CoV-2 CT scan data set to detect patients infected with COVID-19. We divided the data set into 80% for training purposes and 20% for validation purposes. However, notably, xDNN does not require full retraining if new data are presented, which retains all the prototypes identified so far and may add new ones if the data pattern requires them [57].
Using the xDNN method, we generated (extracted from the data) linguistic IF
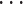 THEN rules that involve actual CT scans of all the classes (COVID-19, other pulmonary diseases, and healthy), as illustrated in Fig. 9. Such transparent rules can be used by specialists to support clear early diagnostics for COVID-19 infection or other diseases. Rapid detection with a high sensitivity to viral infection may allow a better control of viral spread. The early diagnosis of COVID-19 is crucial for disease treatment and control.
THEN rules that involve actual CT scans of all the classes (COVID-19, other pulmonary diseases, and healthy), as illustrated in Fig. 9. Such transparent rules can be used by specialists to support clear early diagnostics for COVID-19 infection or other diseases. Rapid detection with a high sensitivity to viral infection may allow a better control of viral spread. The early diagnosis of COVID-19 is crucial for disease treatment and control.
Fig. 9.
Final rule given by the xDNN classifier for COVID-19 identification. In contrast to typical deep neural networks, xDNN provides highly interpretable rules that can be visualized and used by human experts for the early diagnosis of patients suspected of being infected with COVID-19.
D. Forecasting Spread of COVID-19
Anticipating and monitoring the spread of the disease is vital. Generally, there are three methods for identifying the rise and decline of illnesses such as flu [66].
-
1)
Nowcast: An estimate of the number of current infections; Laboratories collect historical and current data from the CDC and other organizations and data about illness-related Google searches, medical website traffic, and Twitter activity. Such data streams are analyzed using ML algorithms to make predictions.
-
2)
ML Forecasting: This method predicts up to four weeks in advance and anticipates milestones, such as the maximum number of cases and when an outbreak will peak; this information enables healthcare providers and the CDC to anticipate and prepare for capacity needs. ML forecasting considers both the nowcast and previous CDC data. With 20 years of the U.S. flu season data available, the algorithm has a large amount of information.
-
3)
Crowdsourced Opinion Forecasting: This forecasting method utilizes volunteers. Each week, both experts and amateurs log into a system illustrating the trajectory of the current and past flu seasons. These groups of volunteers then forecast the current curve by projecting the number of cases over time. While individuals may not accurately predict the trajectory, in groups, they are as accurate as ML forecasting.
The nowcast and ML forecasts use several common data sources but different prediction models. Algorithms must learn new correlations between the ground truth and the data signals. This is because of the increased panic around COVID-19, which results in different online activity patterns as people search for coronavirus information. As people who are not ill will still search for information, it can be difficult to know who is experiencing symptoms. In the event of a pandemic, there is little historical data available, which can affect forecasting. While the flu occurs cyclically, pandemics are rarer and less predictable. The H1N1 pandemic in 2009 was characteristically different from the COVID-19 pandemic. In contrast to COVID-19, H1N1 affected younger rather than older individuals. In addition, in 2009, the tracking systems were not completely developed. Teams rely on historical data from the current pandemic due to a lack of data from prior pandemics. Researchers include data from countries that experienced earlier cases and will continue to update ML models as data are provided. At the end of each week, the CDC reports on the updated U.S. case trajectory and revises prior numbers. This allows laboratories to update the models and eliminate the gaps in the rolling statistics and original predictions [66].
E. Risk Prediction
AI may be used to predict the risk of COVID-19. In general, risk prediction can be categorized into three areas.
-
1)
Risk of infection.
-
2)
Risk of severe symptom development.
-
3)
Risk of specific treatment use for an infected individual.
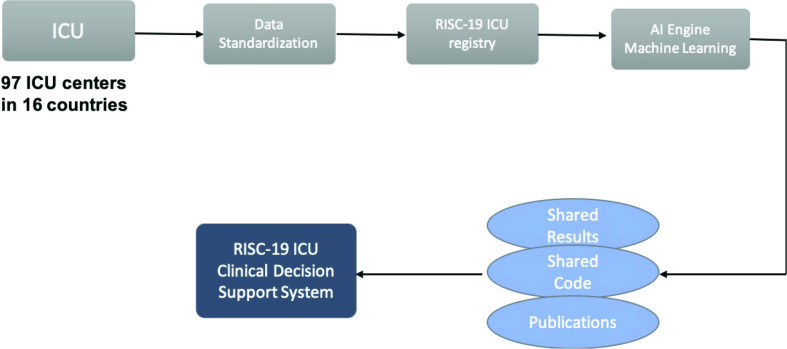
During the flood of COVID cases and the lockdown phase, intensive care in Italy was pushed to the limit, reaching a peak at the end of March, 2020 of approximately 4000 hospitalized patients in ICUs. Italian doctors were forced to choose ICU patients who had the best chance for survival. In particular, the last data on April 8, 2020 confirmed the reaching of the peak and the stabilization of the trend in Italy. The Civil Protection Bulletin reported that 3693 people were admitted to the ICU, 99 fewer than the previous day. A total of 28 485 people were hospitalized with symptoms, 233 fewer than the previous day. The contagion was reduced, with an incidence of 7.4%. On April 8, 2020, 542 people died (there were 604 deaths the day before), reaching a total of 17 669 deaths in Italy. This worldwide emergency has highlighted the need to define a predictive care model that can provide an accurate estimation of resources and preventive medicine. Currently, the conditions that predispose people to develop complications are largely unknown, and the ability to understand them by using patient records is hampered by numerous challenges. These obstacles include the difficulty in finding structured clinical data, nonuniform data sampling leading to several missing values, and a lack of annotation with respect to a target variable that may represent the patient’s own risk level. Understanding and predicting the risk of a particular patient developing complications associated with COVID-19 is a very important and topical challenge. Therefore, we proposed the design and development of ML algorithms for the early-stage prediction of complications and the risk stratification of COVID-19 patients in ICUs using heterogeneous longitudinal EHR data. In particular, the study was performed as part of the collaborative international ICU registry for critically ill COVID-19 patients (RISC-19-ICU). The aim of the registry is to collect real-time data of COVID patients admitted to non-ICU or ICU wards. The registry was launched on March 13, 2020 and it already included 97 ICU centers from 16 countries collecting data. The registry includes more than 1000 patients and more than 400 fields (e.g., laboratory analysis and ICU analysis). The idea behind our project is to better prevent and treat the complications that appear in patients affected by COVID-19 by developing a clinical decision support system (CDSS) that allows computing the following.
-
1)
Risk profiles of individual patients from which a different intensity of care can be deduced, with a consequent modification of the control time according to the patient needs; this approach would shorten the waiting time and improve the appropriateness of care.
-
2)
The prediction of risk of short-term complications, which will activate personalized prevention systems directly addressed to the patient: from targeted recalls to targeted motivational and training activities.
The EHR and ICU data pose different challenges within the ML community. These challenges should be considered when predicting the complications associated with COVID-19. The ML model should be able to achieve a higher predictive performance but simultaneously ensure a high interpretability (i.e., localize the most discriminative features). The model should deal with high-dimensional data, representing irrelevant and redundant features, and the naturalistic unbalanced setting of this task (e.g., larger sample size of the control class with respect to the pathological class). Simultaneously, the temporal evolution of features should be encapsulated. However, the employed EHR/ICU data reflect the clinical use-case scenarios, where not all laboratory examinations were prescribed uniformly over time. This problem leads to a highly sparse data set in which each patient can have missing features and/or sparse annotations of diagnosis over time. Our recent work in this field aimed to overcome these challenges by proposing ML methodologies for predicting type 2 diabetes (T2D) [58] and the early temporal prediction of T2D risk conditions [59] using the EHR data collected by general practitioners. The ML algorithm represents the core of CDSS (see Fig. 10).
The sequential organ failure assessment (SOFA) score is used to track a patient’s status during ICU stay to determine the extent of the organ function or rate of failure of the patient. The SOFA score can be measured daily in all patients admitted to the ICU to determine the level of acuity and mortality risk. The accurate prediction of SOFA may be relevant to the clinical scenario to provide the risk profiles of individual patients, from which a different intensity of care can be deduced, with a consequent modification of the control time according to the patient needs. We aim to predict the worsening or improvement of SOFA on day 5 of the ICU stay of patients by solving a classification task. We are currently adopting a no-temporal approach based on the extreme gradient boosting (XGBoost) algorithm. The predictors consisted of patient characteristics during hospitalization and ICU admission. The model should be capable of being generalized across subjects. Thus, we performed a leave-one-subject-out cross-validation procedure.
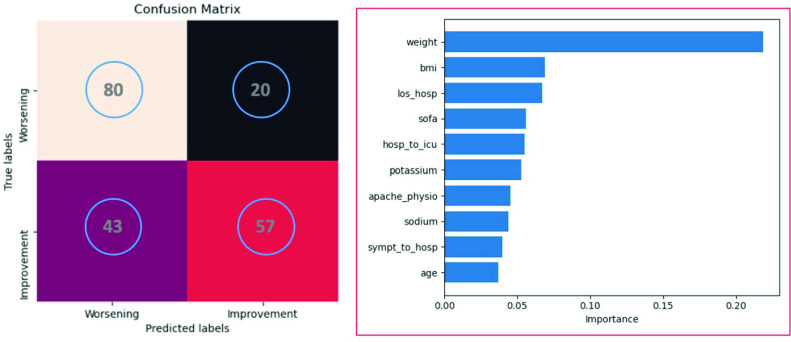
The algorithm was tested on a subset of 100 patients from the RISC-19 ICU registry. Fig. 11 shows the predictive performance and feature importance of the XGBoost algorithm. The model achieved an accuracy, macroprecision, macrorecall, and macro-f1 of 0.69, 0.70, 0.69, and 0.68, respectively.
Fig. 11.
Predictive performance (confusion matrix, left side) and feature importance (right side) of the XGBoost algorithm tested on a subset of 100 patients from the RISC-19 ICU registry.
The proposed framework (Fig. 12) aims to provide a coordinated, evidence-based, fair, and global public-health response. Therefore, the effectiveness and robustness of our framework are not limited to the accuracy of the ML algorithm, but they depend on ICU data-sharing, multidisciplinary collaborations, and the interpretability, reproducibility, and transparency of the extracted results. The continuous expansion of the RISC-19 ICU registry with the collaboration of new ICU centers has led to the creation of a standardized benchmark to support worldwide researchers in the fight against COVID-19. Thus, our framework can be encapsulated in smart healthcare IoT solutions, which may improve the medical service performance and accessibility of preventive medicine.
Fig. 12.
Framework of the proposed risk prediction approach.
F. Voice-Based Diagnosis of COVID-19
Multiple voice detection applications have been developed to assist in COVID-19 screening. These applications analyze a user’s voice sample to detect viral infection symptoms. The Corona Voice Detect app utilizes forensic voice technology and AI to identify patterns in voice, tone, and speaking sounds related to illness [7].
G. Respondent-Driven Sampling
In the SNOWBALL study, which is a CDC-funded contract (BAA 75D301-20-R-68024), we investigated the potential of respondent-driven sampling (RDS) to fill the gaps in understanding SARS-CoV-2 and COVID-19 by relying on the active engagement of respondents in their own close-contact networks to build a self-generating contact trace from persons who test positive for SARS-CoV-2. The key components of the proposed approach include the following.
-
1)
Building a rapid-deployment RDS platform to detect active, undiagnosed cases and determine the spread or distribution of active infection in the community. By developing the capability to deploy targeted testing rapidly into North Carolina (NC) communities, we can provide early, high-impact data for the public health management of future influenza-like illnesses, such as COVID-19, leveraging transmission pathways to recruit community members systematically to complete electronic surveys and present for testing.
-
2)
Testing the effectiveness of RDS sampling in identifying novel positive cases and reaching otherwise underrepresented populations and assessing differential social contact patterns via personal network surveys to evaluate the social determinants of infection risk. We will test whether the RDS platform yields a substantively different population of the epidemic by reacting rapidly with multiple testing modalities and by reaching more distal network contacts that are typically accessed through traditional contact tracing channels.
-
3)
Using RDS to inform and direct molecular epidemiology studies that are representative of the local population. Combining detailed contact patterns with the transmission patterns assessed through molecular epidemiology will permit the estimation of secondary attack rates in multiple settings. Identifying contact patterns with a higher transmission likelihood protects health workers in hospitals and other care settings.
Network-targeted sampling can efficiently sample the community, starting with Duke University Health System (DUHS) patients as COVID-19+ “seeds” (index cases). Its benefits include: 1) locating cases where they are most likely to occur; 2) assessing community spread/distribution; and 3) interrupting transmission by diagnosing infected people before they are infectious to others in the community. SNOWBALL offers a translational toolset combining social-structural insights regarding how the community structure channels infectivity with clinical expertise that can detect, treat, and monitor populations.
1). Rationale and Research Strategy:
In order to “reopen” NC to typical activities, we must develop efficient surveillance designs to understand how widespread SARS-CoV-2 is, where and how people are most at risk of acquiring or transmitting it, and whether recovered individuals are immune and prevent the transmission of the virus. Contact patterns and underlying comorbidities are likely different for the most vulnerable groups, putting them at risk biologically and socially (COVID-19 cases are quickly climbing in the Hispanic/Latinx population, a group that has poor access to care). However, a network-targeted community sampling design can also direct testing to yield a higher proportion of results that indicate active infection and potentially differentiate venues or communities, where transmission is active and undiagnosed. Network-targeted methods require neither intensive contact tracing to achieve a robust, representative sample, nor personnel to circulate through the community to collect samples. Instead, this approach passively recruits community members to complete electronic surveys and present for testing.
We propose a network-targeted, short-term sampling strategy to identify active cases efficiently and reduce SARS-CoV-2 transmission in NC. With the end of statewide shelter-in-place orders, any new COVID-19 case indicates that the case has had sufficient contact to acquire the infection and is likely to continue to have the same types of contacts, which can further spread the infection. Surveying the contact patterns of the case can locate where to deploy testing and where such testing is still limited. This social-network-targeted sampling creates an efficient sample for identifying and diagnosing additional infections. Building on the model of public health contact tracing, a network-targeted method focused on the entire network of an infected person will also include the weaker or incidental contacts often responsible for epidemic spread.
We need an approach that can quickly identify and contain new COVID-19 cases to prevent the second wave from overwhelming the state health system of NC. Network-targeted sampling can identify where in the community undiagnosed infections might be present, as confirmed through RT-PCR to diagnose active infections. This scalable sampling strategy would be useful for capturing asymptomatic or minimally symptomatic cases or close network contacts not within the same household. This approach improves public health contact tracing in three important ways.
-
1)
We will leverage the social contact network of those diagnosed with COVID-19 to identify the periphery of the epidemic. Social contact networks comprise multiple ties between people, with strong ties reflecting intensive interaction, which is a clear risk for transmission, and weak ties representing incidental contact through common daily activities. Although weak ties are less likely to pass infection per tie, a much larger number indicates that people are likely to pass through these ties. Importantly, as index patients will be largely aware of the symptoms and recent activities of their strong-tie contacts, they are best positioned to understand where they acquired the disease or whom they may have infected, helping to guide targeted recruitment and sampling.
-
2)
To broaden participation, we will use both in-clinic and at-home testing. We have multiple teams of testers, including at least one with Spanish fluency, who can be mobilized to collect appropriate respiratory samples (nasopharyngeal) for RT-PCR testing for active infection and venous blood for antibody and serologic testing.
-
3)
Testing for both active infection with RT-PCR and for convalescence with serology, combined with a symptom diary, will provide key knowledge about the infection course, symptom prevalence in conjunction with infection prevalence, and transmission related to behavior and contact patterns.
2). Approach:
The transmission of SARS-CoV-2 is most likely to occur with repeated, prolonged, or invasive contact, indicating that close contacts, people in congregate living situations, and healthcare workers are most likely to become infected. Thus, targeting social contacts is likely to yield higher numbers of undiagnosed but positive cases than random sampling would. “Index” cases (seeds) for these link-sampling designs would be patients from Durham County who test positive for SARS-CoV-2 at DUHS. We will also trace weaker contacts who might be the source of infection for the index case, or someone to whom the index spread the infection.
Our goal is to work closely with DUHS to develop a workflow, whereby index cases will be sampled from anyone testing positive for SARS-CoV-2. Once a case is enrolled, we will administer a survey that elicits general information about activities and symptoms and includes a social network module to capture details about social networks, living situations, and activities/venues. Building this capability will create a “SNOWBALL Platform,” allowing public health departments and/or epidemiologic researchers to deploy an easy-to-use platform quickly for physical and electronic coupons and surveys in future pandemics. The platform employs the fast healthcare interoperability resources (FHIR) standard to facilitate access to EHRs and public health surveillance systems.
RDS leverages cases to identify testing candidates. Each index is given 3–5 unique electronic codes to invite peers, together with recommendations to guide their selection, based on survey information about seed–peer contact patterns, peer risk, and sample diversity to achieve representativeness. We excluded peers known by the seed to have been diagnosed with COVID-19. We will aim for 1 close contacts likely to be infected based on index–peer interactions, 1 contacts at risk due to their close interactions, and 1 contacts constituting a central figure at a venue frequented by the index. Indices in congregate living situations will receive 1–2 additional coupons for co-residents. Indices whose samples required fewer than ten RT-PCR cycles until detection received 1–2 additional coupons for people with whom they had sufficient contact during the 48 h prior to sampling. We also aim to maximize diversity in the sample through venue-targeted sampling. Contacts with coupons who complete the survey will be given an appointment for testing (in-clinic or at home). Recruited contacts who test positive via RT-PCR will be given coupons to elicit their own set of contacts, following the same protocols as the seeds.
The primary purposes of selecting network contacts for serological testing were efficiency and sample diversity. Each respondent will provide information on close contacts and venues frequented, acting as key informants for the likelihood of infection risk at each place/type. We will conduct a network analysis of contact patterns and venue-based behaviors aimed at evaluating population diversity and spatial heterogeneity. We anticipate collecting samples for 100 serological tests drawn from persons who tested positive for SARS-CoV-2 at a DUHS clinic. Although this does not provide sufficient power to test effectiveness fully, this pilot will provide information on feasibility and population diversity. Peers will be asked to consent to nasopharyngeal swabbing to diagnose active COVID-19 infection and to consent to blood draws for a serological test.
Serology test results provide us with an assessment of previously infected but recovered cases to help map the extent of infection within the “network neighborhood” of each RT-PCR+ positive SNOWBALL participant. Our goal is to use the network contacts and contact patterns described above to develop our understanding of SARS-CoV-2 transmission, including asymptomatic and presymptomatic transmission likelihood among diverse contacts and with detailed information about previous symptoms, mitigating or exacerbating behaviors, and the social and spatial ranges of Durham County residents.
H. Early Warnings and Alerts: Face Recognition and Body Temperature Scanning
AI is transforming COVID-19 diagnosis and screening. Infrared temperature scanners have been used to screen for fever in public places. However, this technology requires personnel to complete scanning. Cameras with multisensory technology based on AI have begun to be used to limit the potential exposure of frontline staff at airports, hospitals, or healthcare facilities. These enhanced cameras can recognize individuals with elevated body temperatures, recognize corresponding faces, and trace an individual’s movement. During the COVID-19 pandemic, several states have begun to use facial recognition to fight the spread of the virus. This technology assists in monitoring those who disregard quarantine guidelines or assessing the body temperature of infected people in a crowd. Facial recognition and AI provide unparalleled control for quarantine scenarios by using facial recognition in collaboration with other technologies. For example, the FindFace system [67] combined with CCTV cameras can identify individuals in real time, facilitating prompt responses, even as AI assesses individuals’ social networks.
-
1)
Social Interaction Analysis: It uses complex recognition and searches of historical data to assess the number of likely infected individuals.
-
2)
Real-Time Violation Monitoring: It identifies quarantined individuals and notifies authorities if they are recognized on camera, even if wearing a mask or facial covering.
-
3)
Tracking Quarantine Compliance: Algorithms that recognize silhouettes make it possible to monitor individuals using multiple cameras even if facial imaging is not available.
-
4)
Age Detection: AI is highly useful in assessing age, which is helpful in monitoring those aged 60 and over who are encouraged to remain at home during the COVID-19 pandemic because they are more susceptible to infection.
I. Social Control and Fake News Detection
While fighting the actual pandemic, science and medicine must also battle the dissemination of incorrect information online. During the COVID-19 pandemic, inaccurate information spreads quickly. Overcoming misinformation requires the integration of various tools in the arenas of information technology, law, and education. Previously, news was spread by a limited number of organizations; in contrast, today, news is spread via social media and the Internet. Fake news can be defined as deliberately inaccurate information disseminated through traditional or social media. Unfortunately, fake news can seriously mislead or harm individuals and organizations. Fake news is sometimes aimed at profiting from the promotion of specific treatments, supplements, or products. Stressful situations, such as pandemics, are often linked to an influx of information or misinformation. When COVID-19 was designated as a global public health emergency, the WHO Information Network for Epidemics (EPI-WIN), a platform for sharing specific information with targeted groups, was created. In the context of misinformation, AI tools can recognize and monitor incorrect information from questionable sources. Doing so helps focus on fighting the coronavirus, which is vital to an effective response. For example, the JRC created an AI-based classifier capable of identifying misinformation by evaluating the language in news articles. While a 100% detection rate is not possible, the JRC ML algorithm offers a success rate of 80% [69], [70], and [74].
J. Communication and Chatbot
Using triage systems based on AI may reduce the load on physicians. Medical chatbots could assist patients with identifying symptoms, provide vital education about hand hygiene, and refer individuals for treatment if symptoms worsen. In addition, phone software capable of recognizing and recording patient data, such as temperature and advancing symptoms, could prevent patients from seeking unnecessary hospital care if they have mild symptoms. These additional data can also be used with AI algorithms to monitor COVID-19 [65].
K. Vaccine and Drug Development
Fighting COVID-19 effectively over the long term requires the development of vaccines. AI can be utilized to facilitate vaccine production. Emerging studies using the Vaxign-ML tool to forecast two dozen COVID-19 vaccine options using five nonstructural proteins and the S protein focus on exploring the whole proteome of COV-2. Using ML in conjunction with reverse vaccinology allows researchers to predict vaccine targets. Another vaccine trial is using HLA-binding prediction tools that require peptide stability assays. In the study, 777 peptides were evaluated and predicted as acceptable binders for 11 MHC allotypes with elevated prediction binding scores [72] and [73]. The initial results will make key contributions to the creation of an effective COVID-19 vaccine. Another study utilizes DL to create a drug-target interaction model known as the molecule transformer-drug target interaction (MT-DTI). It recognizes available drugs capable of acting on COVID-19 viral proteins. This model suggests that atazanavir, an antiretroviral drug typically used to treat HIV, may be beneficial in developing a drug aimed specifically at COVID-19. The drug must pass through the trial stage before possibly being used to treat coronavirus patients. The study creates a data-driven drug utilization framework using statistical analysis and ML methods to mine large transcriptome data and knowledge graphs. The data supporting analytics to identify antivirals and vaccines rest upon an understanding of the genomic content of SARS-CoV-2, which contains the necessary molecular targets for these health interventions. To support this research, the IBM Functional Genomics Platform has analyzed over 60 000 SARS-CoV-2 genomes and precomputed all gene, protein, functional domains, and biochemical pathways for this virus, presenting the data as an open research asset to aid in the pandemic. This data resource provides important ground-truth data to aid in controlling this global health crisis. Trial results indicate that ML can effectively predict drug SARS candidates, making it a possible means of reframing drugs to combat the global COVID-19 pandemic [68], [72], and [73].
V. Role of Robotics in Managing COVID-19
As the COVID-19 pandemic grows, the potential use of robotics has become evident. Robotics can be utilized during a pandemic to disinfect, deliver food or medication, monitor vital signs, or assist with border control. Generally, robots have been created to handle dangerous, tedious, or dirty jobs. Initially, they were used industrially. Similarly, an infectious disease can result in environments that are unsafe for human interaction. The recent Ebola outbreak indicated various use cases, but interdisciplinary research funding in collaboration with government agencies and industries is still limited. However, the far-reaching impacts of the COVID-19 pandemic may facilitate more expansive research into the use of robotics to mitigate infectious disease risks [7], [60]:
-
1)
Disease Prevention: Currently, robotic ultraviolet (UV) disinfection of surfaces is in use because COVID-19 spreads through respiratory droplets and contaminated surfaces. The virus can live on surfaces, such as glass, plastic, and metal, for days. However, UV light is effective in reducing the contamination of high-touch surfaces in hospital settings. While disinfection normally requires manual work by humans, which increases exposure risks for workers, remote-controlled or autonomous robots may provide effective, fast, and low-cost disinfection. Additional opportunities remain in the sensing of high-touch areas and intelligent navigation. There is potential for the next generation of robots to traverse high-risk areas and constantly sanitize all high-traffic areas.
-
2)
Crowd Surveillance: Containing COVID-19 requires that governments enforce social distancing mandates. Some countries, including India and China, have utilized drones to monitor crowds and gatherings.
-
3)
Public Announcements: Drones are capable of broadcasting vital information, especially in locations without open communication channels.
-
4)
Mass Screening: Drones can utilize thermal cameras, night vision, computer vision systems, or specialized sensors to monitor crowds and screen groups using body temperature and HR data.
-
5)
Essential Supply Delivery: Drone technology can quickly deliver medical or other essential supplies, reducing the strain on healthcare institutions and staff. During pandemics and outbreaks, it is difficult to maintain sufficient staffing to test individuals and process samples. Automated oral or nasal swabbing may accelerate the process, lessen the chance of infection, and allow staff to undertake more complex tasks.
-
6)
Screening and Diagnosis: Mobile robots may be utilized to measure body temperature in public spaces. Automated camera systems are often used to screen crowds in expansive areas. Integrating vision algorithms and thermal sensors with autonomous or remote-controlled robots may enhance screening coverage and efficiency. Mobile robots could also be utilized for monitoring body temperature for hospitalized individuals or outpatients with data automatically linked with healthcare IT systems. It is possible that linking facial recognition software and security systems would enable faster contact tracing; however, appropriate guidelines are required to respect privacy.
-
7)
Drones and UAVs: These technologies offer apparent advantages during public health emergencies. These devices can reach remote areas or reduce human interactions. China has successfully utilized drone technology to combat the COVID-19 pandemic. Thus, several countries are collaborating with researchers to drive innovative drone use in the fight against the coronavirus. Drones offer several benefits for mitigating pandemic effects and stopping further outbreaks [7].
VI. Role of Blockchain in Managing COVID-19
While DLT/blockchain and IoT are distinct forms of technology, the integration of the IoT and blockchain is a massive paradigm shift that will spur the evolution of systems across the health industry. Blockchain has the capability to address IoT vulnerabilities and resolve security issues around the connection of WIoT devices. Blockchain creates and stores data and consensus mechanisms, and uses its decentralized nature to resolve issues found in centralized cloud-based IoT systems. Several use cases have illustrated the applicability of blockchain to all IoT system aspects. Blockchain can be utilized to manage device identities, store cloud data or distributed objects, and confirm and encrypt data within communication networks. It also mitigates risk by using multiple nodes to transmit peer-to-peer data, making data tampering almost impossible. The consensus mechanism of blockchain can also prevent a compromised node from contributing data to the chain, which safeguards data integrity [10], [61].
Blockchain offers the ability to create audit trails for healthcare interactions, protect system access, facilitate data sharing, and support healthcare supply chains, as the health industry moves to embrace a patient-centered, IoT-based paradigm. Healthcare systems created using blockchain technology would benefit from greater data security and accuracy as records could be integrated, and patients would have clearer ownership of their health data. In addition, blockchain lessens the reliance of a system on a centralized third party to manage the sharing of data, handle transactions, and confirm data accuracy or ownership. Blockchain enables chain participants to engage directly in pseudonymous secure transactions. Blockchain enables data owners and eHealth service providers to interact without the need for a third party. The type of data shared and the timeframe of use are controlled by the data owner via smart contracts. The distributed ledger of blockchain allows data owners to observe the transfer of data among chain participants. Cryptocurrencies, such as Bitcoin, also allow for the monetization of eHealth data [4], [10].
Blockchain technology is also capable of aiding in the fight against COVID-19 by providing verifiable blockchain certificates. This technology could secure COVID-19 testing results, providing people with a certificate to confirm whether the test results are positive or negative. This would be useful for providing proof of immunity based on antibody testing. Blockchain can help users confirm their health status, which would enable communities to lift restrictions appropriately and target quarantine guidelines better. Blockchain technology also offers the ability to monitor patients infected with contagious viruses, including COVID-19. Infected or exposed individuals can wear an IoT device to monitor movements, enabling effective containment and simultaneously protecting privacy. Remote patient monitoring (RPM) can also benefit from blockchain technology. Reviewing the gathered data incurs both data privacy and security issues. Blockchain provides a new means of accessing, storing, and sharing data in compliance with the GDPR and HIPPA protocols. Blockchain technology can also be used for supply chain management. For instance, VeChain is a blockchain-based platform created to track the vaccine production in China. All aspects related to vaccine production, including codes, materials, and packaging, can be documented and maintained via distributed ledgers. This platform provides a dependable method for mitigating risks around alterations to vaccine data because the records are permanent, enabling researchers to create a high-quality vaccine during the pandemic [10], [71].
VII. Real-Life Use Case: Korean Solution
In South Korea, a large-scale epidemic occurred mainly at the Shincheonji Church in Daegu, followed by sporadic community infections, such as a group infection at a call center in Seoul. At one time, the number of confirmed cases per day was in single digits, but the group infection in a nightclub for young people was at risk of causing a large-scale secondary infection. However, it was possible to induce a rapid diagnosis of the confirmed person through a thorough epidemiological investigation based on the identification of the movement line of the confirmed person using IoT technologies. Furthermore, by inducing voluntary diagnosis and isolation of the citizens through the disclosure of the movement line, large-scale secondary infections were successfully prevented. In this section, we describe how the Korean government uses information and communications technology (ICT) to detect and prevent the spread of COVID-19.
A. IoT Systems for COVID-19 in South Korea
At the time of writing this article, various applications and services are available in South Korea to provide useful information to citizens, as follows.
-
1)
Pandemic Tracking App: There exist approximately ten webpages and 50 mobile apps that disclose the path of confirmed patients. Such apps provide various functions, such as linkage displays between confirmed patients and providing traffic line information using reactive maps. However, there is a limit to the information displayed because only the traffic line information disclosed by the quarantine authorities and local governments is used.
-
2)
Self-Quarantine Safety Protection App: The self-quarantine safety protection app developed by the Ministry of Public Administration and Security needs to be installed on smartphones owned by self-quarantined people. Initially, the installation of this app was voluntary, but as the COVID-19 situation became serious, the installation became mandatory. The main function is to notify the officials in charge automatically when symptoms are developed during quarantine and notify them when leaving the quarantine area.
-
3)
Mask Inventory Checking App: A government organization developed a platform that provides real-time quantities of available masks that can be purchased at pharmacies, post offices, and marts.
-
4)
QR Code-Based Entry Log App: A government organization in South Korea introduced quick response (QR) code-based electronic access lists for facilities at risk of group infection in cooperation with social media companies. Such applications attempt to solve problems caused by false statements of confirmed persons and concerns about the leakage of personal information in handwritten lists.
-
5)
Epidemic Investigation Support System: Government agencies and research centers have developed an IoT-based epidemiological investigation support system. For the epidemiological investigation of infectious diseases, the system systematically acquires access information to the base station of telecom companies and card usage information for the confirmed person authorized by the domestic infectious disease prevention law. It quickly uses the collected information to analyze the travel route of a confirmed person. This system is being used by the epidemiological investigators of local governments for the rapid epidemiological investigations of COVID-19.
Table VI presents the comparison of various available COVID-19 solutions in South Korea.
TABLE VI. Comparison of ICT Services and Applications for COVID-19.
| Service/App | Service Target | Data Type | Supporting Functions | Limitations |
|---|---|---|---|---|
| Pandemic tracking app | Personal | Public data | Linkage display between confirmed patents | Limited route information |
| Self-quarantine safety protection app | Government | GPS | Checking personal health status | Initially voluntary but not mandatory app installation for entering South Korea |
| Mask inventory checking app | Personal | Government data | Mask inventory | No available information for confirmed persons |
| QR code-based entry log app | Government | QR code | Access lists for high-risk places | Only available information for visited persons |
| EISS | Government | Location data Credit card usage | Rapid investigation results on the travel route of confirmed persons | Only government people can use the information |
B. Enhanced Smart City Platform for COVID-19
As EISS provides the most useful information collected from various sectors, we describe the EISS system in detail [62]. Upon the outbreak of COVID-19, a responsible government organization conducted an epidemiological investigation by collecting information on the movement lines of confirmed patients and payment details by credit card based on the epidemic law, which was legislated in 2015 for the MERS-CoV. However, as all the data were collected and handled through e-mail, the analysis of the movement line data requires a long time and a considerable amount of human resources. Therefore, the development of ICT-based systems that enable rapid epidemiological investigations through the systematization of requests for information provision and provision of traffic data analysis functions is strongly required.
The EISS system was developed to collect and analyze information related to COVID-19 by expanding the IoT-based smart city platform called CityHub, which was previously developed as a national Research and Development project. According to the infectious disease prevention method, data related to confirmed patients are collected in real time from mobile carriers and credit card companies, refined and analyzed, and used for epidemiological investigations.
Fig. 13 shows the additional functions and procedures of the EISS system. The EISS system provides three functions: 1) data collection of confirmed patients; 2) refinement of movement data; and 3) analysis based on the movement of confirmed patients. A security function to prevent the leakage of personal information that may occur during this process has also been added to the EISS system.
-
1)
Data Collection of Confirmed Patients: To collect the data of a confirmed case, the epidemiological investigator registers the confirmed case in the system. The EISS system receives the data related to the confirmed case through an interface connected to the National Police Agency, credit card network, and telecommunication systems. EISS analyzes the data and converts them into a standard-based data format referring to oneM2M global IoT standards [63]. The converted data in CityHub can be accessed through a context-aware application programming interface (API), that is, the NGSI-LD API developed by ETSI, which can process semantic data [64].
-
2)
Data Refining of Confirmed Cases: The location data collected from the telecommunication company are the location data of a base station to which the confirmed person is connected. Therefore, they may be different from the actual location data of a confirmed person. To correct this deviation, EISS infers the moving path of the confirmed patient by applying ML technology through various interpolation, clustering, and classification algorithms based on the location data and access time of the base stations to which the confirmed terminal is connected.
-
3)
Analysis of Confirmed Patient Movement: As shown in Fig. 14, EISS provides a map-based location service that supports epidemic investigators to analyze the movement of the confirmed person. EISS also supports a function that analyzes infection hotspots to derive and display the contact area between infected people. Based on the results of epidemiological investigations, the EISS visualizes the connection network between infected people.
-
4)
Security Concerns: EISS provides two-factor authentication using a one-time password (OTP) via a virtual private network (VPN) connection and password-based Web login to allow access only to authorized users. In the case of user accounts and access rights, an indiscriminate use of the system is prevented by creating an account only through official letters issued by an authorized government agency.
Fig. 13.
Smart CityHub architecture with EISS extension.
Fig. 14.
Analysis of movement routes of confirmed cases for epidemiological investigation. (a) Route analysis; (b) infection hotspots; and (c) visualization of connection network.
C. Effectiveness of EISS and Lessons Learned
Simplifying the Collection Process: The process of requesting and collecting data for confirmed cases, which was done through the official letters or e-mails, has been simplified to be possible with just a few clicks in EISS. This improvement reduces unnecessary waiting time so that the processing time is shortened from 2–3 days to within 10 min.
Data Standardization: Existing movement data and card payment details from individual companies are not standardized. Different data formats and storage mechanisms were used by each provider and person in charge. This was one of the factors hindering the rapid analysis of COVID-19 data. EISS purified these data and saved them in a standardized manner in the system according to the international IoT standard, that is, oneM2M, thereby reducing the time related to data processing. Consequently, by enabling an automatic analysis of movement routes and infection hotspots, the epidemiological investigation time could be drastically reduced from 24 h to 10 min.
The IoT-based common smart city platform, CityHub, provides a standardized framework for smart city services based on the convergence of various data, easy connection of new data sources and services, and standardized APIs. A horizontal IoT service platform that can be used in common with various applications plays a crucial role in accepting various services in the city quickly and easily. EISS could quickly respond to COVID-19 by developing and linking the necessary service functions for epidemiological investigation as a separate module by utilizing the standardized interface of CityHub. As such, the use of a common IoT service layer platform in a city using global standards can support various smart city services and connect data. Such systems can be a solution for quickly responding to various urban problems that may arise in the future.
VIII. Conclusion
The COVID-19 pandemic has caused extreme strains on healthcare systems and has shut down almost the entire global economy. As the scientific and research community is struggling to find a swift solution and a cure, SCH has become the core technology for fast prediction, modeling, examination, and evaluation of infected patients. In light of the urgent need for SCH solutions, this article presented a selection of original and innovative techniques in the field to help combat this raging pandemic. We began this article by proposing a set of complementary techniques and providing a comprehensive overview of the role of (W)IoT. Following this, we discussed how AI, ML, and Big Data can be harnessed to stop the epidemic and minimize the loss of human lives. We also took a broad look on the role of robotics and drone technology in managing public health and pandemics. Finally, we examined how DLT/blockchain can improve the shortcomings of the current SCH solutions and help us in the event of pandemics.
Acknowledgment
Farshad Firouzi is with the Electrical and Computer Engineering Department, Duke University, Durham, NC 27708 USA (e-mail: farshad.firouzi@duke.edu).
Bahar Farahani, Zeynab Talebpour, Reza Moradi, Mohsen Goodarzi, and Alireza Talebpour are with the Cyberspace Research Institute, Shahid Beheshti University, Tehran 1983969411, Iran (e-mail: b_farahani@sbu.ac.ir).
Mahmoud Daneshmand is with the Business Intelligence and Analytics, Stevens Institute of Technology, Hoboken, NJ 07030 USA (e-mail: mdaneshm@stevens.edu).
Kathy Grise and Roberto Saracco are with the IEEE Future Directions, Piscataway, NJ 08854 USA.
Jaeseung Song is with the Department of Computer and Information Security, Sejong University, Seoul 15600, South Korea.
Lucy Lu Wang and Kyle Lo are with the Allen Institute for Artificial Intelligence, Seattle, WA 98112 USA.
Plamen Angelov and Eduardo Soares are with the School of Computing and Communications, Lancaster University, Lancashire LA1 4YW, U.K.
Po-Shen Loh is with the Department of Mathematical Sciences, Carnegie Mellon University, Pittsburgh, PA 15213 USA.
Haleh Ashraf and Mohammad Talebpour are with Sina Hospital, Tehran, Iran.
Luca Romeo is with the Department of Information Engineering, Universit Politecnica delle Marche, 60121 Ancona, Italy.
Rupam Das and Hadi Heidari are with the James Watt School of Engineering, University of Glasgow, Glasgow G12 8QQ, U.K.
Dana Pasquale, James Moody, Chris Woods, and Erich S. Huang are with the School of Medicine and Duke Health, Duke University, Durham, NC 27708 USA.
Payam Barnaghi is with Department of Brain Sciences, Imperial College London, London SW7 2AZ, U.K., and also with U.K. Dementia Research Institute, London, U.K.
Majid Sarrafzadeh is with the Computer Science Department & Electrical and Computer Engineering Department, University of California at Los Angeles, Los Angeles, CA 90095 USA.
Ron Li is with the Department of Medicine, Stanford University School of Medicine, Stanford, CA 94305 USA.
Kristen L. Beck is with Almaden Research Center, IBM, San Jose, CA 95120 USA.
Olexandr Isayev is with the Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 USA.
Nakmyoung Sung is with Korea Electronics Technology Institute, Seongnam 13509, South Korea.
Alan Luo is with the Computer Science Department, Stanford University, Stanford, CA 94305 USA.
Funding Statement
The work of Jaeseung Song was supported by the Smart City Research and Development Project of the Korea Agency for Infrastructure Technology Advancement (KAIA) Grant funded by the Ministry of Land, Infrastructure and Transport (MOLIT), Ministry of Science and ICT (MSIT) under Grant 18NSPS-B149388-01. The work of Luca Romeo was supported in part by the Vision Robotics Artificial Intelligence Group; in part by the RISC-19 ICU Board (https://www.risc-19-icu.net); and in part by the Bioengineering Lab Group.
Footnotes
Contributor Information
Farshad Firouzi, Email: farshad.firouzi@duke.edu.
Bahar Farahani, Email: b_farahani@sbu.ac.ir.
Mahmoud Daneshmand, Email: mdaneshm@stevens.edu.
References
- [1].Farahani B., Firouzi F., Chang V., Badaroglu M., Constant N., and Mankodiya K., “Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare,” Future Gener. Comput. Syst., vol. 78, pp. 659–676, Jan. 2018. [Google Scholar]
- [2].Farahani B., Firouzi F., and Chakrabarty K., “Healthcare IoT,” in Intelligent Internet of Things. Cham, Switzerland: Springer, 2020, pp. 515–545. [Google Scholar]
- [3].Firouzi F.et al. , “Internet-of-Things and big data for smarter healthcare: From device to architecture, applications and analytics,” Future Gener. Comput. Syst., vol. 78, pp. 583–586, Jan. 2018. [Google Scholar]
- [4].Firouzi F., Farahani B., Barzegari M., and Daneshmand M., “AI-driven data monetization: The other face of data in IoT-based smart and connected health,” IEEE Internet Things J., early access, Sep. 30, 2020, doi: 10.1109/JIOT.2020.3027971. [DOI]
- [5].Firouzi F., Chakrabarty K., and Nassif S., Intelligent Internet of Things: From Device to Fog and Cloud. Cham, Switzerland: Springer, 2020. [Google Scholar]
- [6].Saracco R., Autiosalo J., de Kerckhove D., Flammini F., and Nisiotis L., “Personal digital twins and their role in epidemics control: An IEEE digital reality white paper,” IEEE, New York, NY, USA, White Paper, 2020. [Google Scholar]
- [7].Chamola V., Hassija V., Gupta V., and Guizani M., “A comprehensive review of the COVID-19 pandemic and the role of IoT, drones, ai, blockchain, and 5G in managing its impact,” IEEE Access, vol. 8, pp. 90225–90265, 2020. [Google Scholar]
- [8].Alimolaie A., “A review of coronavirus disease-2019 (COVID-19),” Biol. Sci. Promo., vol. 3, no. 6, pp. 152–157, 2020. [Google Scholar]
- [9].Van Houten H., How a Virtual Heart Could Save Your Real One, Philips, Amsterdam, The Netherlands, 2018. [Google Scholar]
- [10].Farahani B., Firouzi F., and Luecking M., “The convergence of IoT and distributed ledger technologies (DLT): Opportunities, challenges, and solutions,” J. Netw. Comput. Appl., vol. 177, Mar. 2021, Art. no. 102936. [Google Scholar]
- [11].Nasajpour M., Pouriyeh S., Parizi R. M., Dorodchi M., Valero M., and Arabnia H. R., “Internet of Things for current COVID-19 and future pandemics: An exploratory study,” J. Healthc. Informat. Res., vol. 4, pp. 325–364, Nov. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davies H. J., Williams I., Peters N. S., and Mandic D. P., “In-ear SPO2: A tool for wearable, unobtrusive monitoring of core blood oxygen saturation,” Sensors, vol. 20, no. 17, p. 4879, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Singh V., Chandna H., Kumar A., Kumar S., Upadhyay N., and Utkarsh K., “IoT-Q-band: A low cost Internet of Things based wearable band to detect and track absconding COVID-19 quarantine subjects,” EAI Endorsed Trans. Internet Things, vol. 6, no. 21, 2020. [Google Scholar]
- [14].Stojanović R., Škraba A., and Lutovac B., “A headset like wearable device to track COVID-19 symptoms,” in Proc. IEEE 9th Mediterr. Conf. Embedded Comput. (MECO), Budva, Montenegro, 2020, pp. 1–4. [Google Scholar]
- [15].Lee K.et al. , “Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch,” Nat. Biomed. Eng., vol. 4, no. 2, pp. 148–158, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boyett M., Wang Y., and D’Souza A., “Crosstalk opposing view: heart rate variability as a measure of cardiac autonomic responsiveness is fundamentally flawed,” J. Physiol., vol. 597, no. 10, pp. 2599–2601, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ghosh A. M., Halder D., and Hossain S. K. A., “Remote health monitoring system through IoT,” in Proc. 5th Int. Conf. Informat. Electron. Vis. (ICIEV), Dhaka, Bangladesh, 2016, pp. 921–926. [Google Scholar]
- [18].Ghijsen M., Rice T. B., Yang B., White S. M., and Tromberg B. J., “Wearable speckle plethysmography (SPG) for characterizing microvascular flow and resistance,” Biomed. Opt. Exp., vol. 9, no. 8, pp. 3937–3952, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Farzam P., Starkweather Z., and Franceschini M. A., “Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle,” Physiol. Rep., vol. 6, no. 7, 2018, Art. no. e13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hu G., Zhang Q., Ivkovic V., and Strangman G. E., “Ambulatory diffuse optical tomography and multimodality physiological monitoring system for muscle and exercise applications,” J. Biomed. Opt., vol. 21, no. 9, 2016, Art. no. 091314. [DOI] [PubMed] [Google Scholar]
- [21].Zakharov P., Talary M. S., and Caduff A., “A wearable diffuse reflectance sensor for continuous monitoring of cutaneous blood content,” Phys. Med. Biol., vol. 54, no. 17, pp. 5301–5320, 2009. [DOI] [PubMed] [Google Scholar]
- [22].Istfan R., LaRochelle S., Chaudury R., and Roblyer D., “A miniature frequency domain diffuse optical optode for quantitative wearable oximetry,” in Proc. Opt. Tomogr. Spectrosc. Tissue XIII, vol. 10874, 2019, Art. no. 108742B. [Google Scholar]
- [23].Kang J. W.et al. , “Direct observation of glucose fingerprint using in vivo raman spectroscopy,” Sci. Adv., vol. 6, no. 4, 2020, Art. no. eaay5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shokrekhodaei M. and Quinones S., “Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone,” Sensors, vol. 20, no. 5, p. 1251, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu H., Ji Z., and Li M., “Non-invasive continuous blood-pressure monitoring models based on photoplethysmography and electrocardiography,” Sensors, vol. 19, no. 24, p. 5543, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Plarre K.et al. , “Continuous inference of psychological stress from sensory measurements collected in the natural environment,” in Proc. 10th ACM/IEEE Int. Conf. Inf. Process. Sens. Netw., Chicago, IL, USA, 2011, pp. 97–108. [Google Scholar]
- [27].Gjoreski M., Gjoreski H., Luštrek M., and Gams M., “Continuous stress detection using a wrist device: In laboratory and real life,” in Proc. ACM Int. Joint Conf. Pervasive Ubiquitous Comput. Adjunct, 2016, pp. 1185–1193. [Google Scholar]
- [28].Fan R. and Andrew T. L., “Perspective—Challenges in developing wearable electrochemical sensors for longitudinal health monitoring,” J. Electrochem. Soc., vol. 167, no. 3, 2020, Art. no. 037542. [Google Scholar]
- [29].Bandodkar A. J., Jeang W. J., Ghaffari R., and Rogers J. A., “Wearable sensors for biochemical sweat analysis,” Annu. Rev. Anal. Chem., vol. 12, pp. 1–22, Jun. 2019. [DOI] [PubMed] [Google Scholar]
- [30].Ray T. R.et al. , “Bio-integrated wearable systems: A comprehensive review,” Chem. Rev., vol. 119, no. 8, pp. 5461–5533, 2019. [DOI] [PubMed] [Google Scholar]
- [31].Săndulescu R. and Cristea C., “Editorial overview: Exploring new ideas and challenges in electrochemical sensing: Towards implantable or wearable devices and nanorobots,” Current Opinion Electrochem., vol. 10, pp. A4–A7, Aug. 2018. [Google Scholar]
- [32].Heikenfeld J.et al. , “Wearable sensors: Modalities, challenges, and prospects,” Lab Chip, vol. 18, no. 2, pp. 217–248, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yokus M. A., Songkakul T., Pozdin V. A., Bozkurt A., and Daniele M. A., “Wearable multiplexed biosensor system toward continuous monitoring of metabolites,” Biosens. Bioelectron., vol. 153, Apr. 2020, Art. no. 112038. [DOI] [PubMed] [Google Scholar]
- [34].Seshadri D. R.et al. , “Wearable sensors for monitoring the physiological and biochemical profile of the athlete,” NPJ Digit. Med., vol. 2, no. 1, pp. 1–16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seshadri D. R., Drummond C., Craker J., Rowbottom J. R., and Voos J. E., “Wearable devices for sports: New integrated technologies allow coaches, physicians, and trainers to better understand the physical demands of athletes in real time,” IEEE Pulse, vol. 8, no. 1, pp. 38–43, Jan./Feb. 2017. [DOI] [PubMed] [Google Scholar]
- [36].Seshadri D. R.et al. , “Accuracy of the apple watch 4 to measure heart rate in patients with atrial fibrillation,” IEEE J. Transl. Eng. Health Med., vol. 8, pp. 1–4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chow E. J.et al. , “Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in king county, Washington,” J. Amer. Med. Assoc., vol. 323, no. 20, pp. 2087–2089, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Greenhalgh T., Koh G. C. H., and Car J., “COVID-19: A remote assessment in primary care,” Brit. Med. J., vol. 368, p. m1182, Mar. 2020. [DOI] [PubMed] [Google Scholar]
- [39].Tobin M. J., Laghi F., and Jubran A., “Why COVID-19 silent hypoxemia is baffling to physicians,” Amer. J. Respiratory Critical Care Med., vol. 202, no. 3, pp. 356–360, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lazzerini P. E., Boutjdir M., and Capecchi P. L., “COVID-19, arrhythmic risk, and inflammation: Mind the gap!,” Circulation, vol. 142, no. 1, pp. 7–9, 2020. [DOI] [PubMed] [Google Scholar]
- [41].Driggin E.et al. , “Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic,” J. Amer. Coll. Cardiol., vol. 75, no. 18, pp. 2352–2371, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jeong H., Rogers J. A., and Xu S., “Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities,” Sci. Adv., vol. 6, no. 36, 2020, Art. no. eabd4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L. L.et al. , “CORD-19: The COVID-19 open research dataset,” in Proc. 1st Workshop NLP COVID-19 ACL, Jul. 2020. [Online]. Available: https://www.aclweb.org/anthology/2020.nlpcovid19-acl.1 [Google Scholar]
- [44].Chen Q., Allot A., and Lu Z., “Keep up with the latest coronavirus research,” Nature, vol. 579, p. 193, Mar. 2020. [DOI] [PubMed] [Google Scholar]
- [45].Lo K., Wang L. L., Neumann M., Kinney R., and Weld D., “S2ORC: The semantic scholar open research corpus,” in Proc. 58th Annu. Meeting Assoc. Comput. Linguist., Jul. 2020, pp. 4969–4983. [Online]. Available: https://www.aclweb.org/anthology/2020.acl-main.447 [Google Scholar]
- [46].Wang L. L. and Lo K., “Text mining approaches for dealing with the rapidly expanding literature on COVID-19,” Briefings Bioinformat., vol. 22, no. 2, pp. 781–799, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang E., Gupta N., Nogueira R., Cho K., and Lin J., “Rapidly deploying a neural search engine for the COVID-19 open research dataset,” in Proc. 1st Workshop NLP COVID-19 ACL, Jul. 2020. [Online]. Available: https://www.aclweb.org/anthology/2020.nlpcovid19-acl.2 [Google Scholar]
- [48].Lee J.et al. , “Answering questions on COVID-19 in real-time,” 2020. [Online]. Available: https://arxiv.org/abs/2006.15830 [Google Scholar]
- [49].Su D., Xu Y., Yu T., Siddique F. B., Barezi E. J., and Fung P., “CAiRE-COVID: A question answering and query-focused multi-document summarization system for COVID-19 scholarly information management,” 2020. [Online]. Available: https://arxiv.org/abs/2005.03975 [Google Scholar]
- [50].Wadden D.et al. , “Fact or fiction: Verifying scientific claims,” in Proc. Conf. Empirical Methods Nat. Lang. Process., Nov. 2020, pp. 7534–7550. [Google Scholar]
- [51].van de Schoot R.et al. , “Open source software for efficient and transparent reviews,” 2020. [Online]. Available: https://arxiv.org/abs/2006.12166 [Google Scholar]
- [52].Roberts K.et al. , “TREC-COVID: Rationale and structure of an information retrieval shared task for COVID-19,” J. Amer. Med. Informat. Assoc., vol. 27, no. 9, pp. 1431–1436, Jul. 2020. [Online]. Available: https://doi.org/10.1093/jamia/ocaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Voorhees E.et al. , “TREC-COVID: Constructing a pandemic information retrieval test collection,” ACM SIGIR Forum, vol. 54, no. 1, pp. 1–12, Jun. 2020. [Google Scholar]
- [54].Imran A.et al. , “AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an APP,” 2020. [Online]. Available: http://arXiv:2004.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Soares E. and Angelov P.. (2020). A Large Dataset of Real Patients CT Scans for COVID-19 Identification. [Online]. Available: https://doi.org/10.7910/DVN/SZDUQX [Google Scholar]
- [56].Angelov P. and Soares E., “Towards explainable deep neural networks (xDNN),” Neural Netw., vol. 130, pp. 185–194, Oct. 2020. [DOI] [PubMed] [Google Scholar]
- [57].Soares E., Angelov P., Biaso S., Froes M. H., and Abe D. K., “SARS-CoV-2 CT-scan dataset: A large dataset of real patients CT scans for SARS-CoV-2 identification,” medRxiv, vol. 1, pp. 1–8, 2020. [Google Scholar]
- [58].Bernardini M., Romeo L., Misericordia P., and Frontoni E., “Discovering the type 2 diabetes in electronic health records using the sparse balanced support vector machine,” IEEE J. Biomed. Health Inform., vol. 24, no. 1, pp. 235–246, Jan. 2020. [DOI] [PubMed] [Google Scholar]
- [59].Bernardini M., Morettini M., Romeo L., Frontoni E., and Burattini L., “Early temporal prediction of type 2 diabetes risk condition from a general practitioner electronic health record: A multiple instance boosting approach,” Artif. Intell. Med., vol. 105, May 2020, Art. no. 101847. [DOI] [PubMed] [Google Scholar]
- [60].Yang G.-Z.et al. , “Combating COVID-19—The role of robotics in managing public health and infectious diseases,” Sci. Robot., vol. 5, no. 40, 2020, Art. no. eabb5589. [DOI] [PubMed] [Google Scholar]
- [61].Liu X., Farahani B., and Firouzi F., “Distributed ledger technology,” in Intelligent Internet of Things. Cham, Switzerland: Springer, 2020, pp. 393–431. [Google Scholar]
- [62].Park Y. J.et al. , “Development and utilization of a rapid and accurate epidemic investigation support system for COVID-19,” Osong Public Health Res. Perspect., vol. 11, no. 3, pp. 118–127, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hwang J., An J., Aziz A., Kim J., Jeong S., and Song J., “Interworking models of smart city with heterogeneous Internet of Things standards,” IEEE Commun. Mag., vol. 57, no. 6, pp. 74–79, Jun. 2019. [Google Scholar]
- [64].Cirillo F., Solmaz G., Berz E. L., Bauer M., Cheng B., and Kovacs E., “A standard-based open source IoT platform: Fiware,” IEEE Internet Things Mag., vol. 2, no. 3, pp. 12–18, Sep. 2019. [Google Scholar]
- [65].Ting D. S. W., Carin L., Dzau V., and Wong T. Y., “Digital technology and COVID-19,” Nat. Med., vol. 26, no. 4, pp. 459–461, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hao K., “This is how the CDC is trying to forecast Coronavirus’s spread,” MIT Technol. Rev., vol. 13, p. 2020, 2020. [Google Scholar]
- [67].Findface Facial Recognition. [Online]. Available: https://findface.pro/en/
- [68].Ge Y.et al. , A Data-Driven Drug Repositioning Framework Discovered a Potential Therapeutic Agent Targeting COVID-19, Cold Spring Harbor Lab., Laurel Hollow, NY, USA, 2020. [Google Scholar]
- [69].Mesquita C. T., Oliveira A., Seixas F. L., and Paes A., “Infodemia, fake news and medicine: Science and the quest for truth,” Int. J. Cardiovasc. Sci., vol. 33, no. 3, pp. 203–205, 2020. [Google Scholar]
- [70].Martens B., Aguiar L., Gomez-Herrera E., and Mueller-Langer F., “The digital transformation of news media and the rise of disinformation and fake news,” Joint Res. Centre Techn. Rep., Ispra, Italy, Digital Economy Working Paper 2018-02, 2018. [Google Scholar]
- [71].Sharma A., Bahl S., Bagha A. K., Javaid M., Shukla D. K., and Haleem A., “Blockchain technology and its applications to combat COVID-19 pandemic,” Res. Biomed. Eng., to be published.
- [72].Ong E., Wong M. U., Huffman A., and He Y., “COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning,”, Frontiers in immunology, vol. 11, p. 1581, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Prachar M.et al. , “Identification and validation of 174 COVID-19 vaccine candidate epitopes reveals low performance of common epitope prediction tools,”, Scientific reports, vol. 10, no. 1, pp. 1–8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mesquita C. T., Oliveira A., Seixas F., and Paes A., “Infodemia, fake news and medicine: science and the quest for truth,” International Journal of Cardiovascular Sciences, vol. 33, no. 3, pp. 203–205, 2020. [Google Scholar]