Abstract
The coronavirus pandemic is the most challenging incident that people have faced in recent years. Despite the time-consuming and expensive conventional methods, point-of-care diagnostics have a crucial role in deterrence, timely detection, and intensive care of the disease’s progress. Hence, this detrimental health emergency persuaded researchers to accelerate the development of highly-scalable diagnostic devices to control the propagation of the virus even in the least developed countries. The strategies exploited for detecting COVID-19 stem from the already designed systems for studying other maladies, particularly viral infections. The present report reviews not only the novel advances in portable diagnostic devices for recognizing COVID-19, but also the previously existing biosensors for detecting other viruses. It discusses their adaptability for identifying surface proteins, whole viruses, viral genomes, host antibodies, and other biomarkers in biological samples. The prominence of different types of biosensors such as electrochemical, optical, and electrical for detecting low viral loads have been underlined. Thus, it is anticipated that this review will assist scientists who have embarked on a competition to come up with more efficient and marketable in-situ test kits for identifying the infection even in its incubation time without sample pretreatment. Finally, a conclusion is provided to highlight the importance of such an approach for monitoring people to combat the spread of such contagious diseases.
Keywords: COVID-19, SARS-CoV-2, point-of-care diagnostics, portable biosensors
I. Introduction
According to the latest data, more than 55.6 million people have been infected by coronavirus disease 2019 (COVID-19) all around the globe since its first emergence in December 2019 [1]. This hazardous pandemic impacted all aspects of peoples’ everyday lives and hindered the most routine activities to a level that was not even foreseeable before [2]. Besides the considerable limitations of international transportation, quarantine and social distancing policies have been applied in most of the nations [3]. However, these strategies cannot be a long-lasting solution. They will have detrimental impacts on the economy, education, food system, and even mental health [4]. It has devastated jobs and positioned millions out of employment. It is noteworthy that, even the strictest rules are not controllable and governments are not able to prevent the violations in the long-term [5]. It has become a challenge to control its ever-increasing transmission speed and make long-term plans to manage the problems it causes [6]. This problem becomes even more vital when talking about health care professionals [7]. Without accessible rapid diagnostic technologies, it is not easy to govern this lethal outbreak [8]. The currently used methodology for detecting COVID-19 is Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) which targets the viral genome in the nasal and nasopharyngeal samples. Although, it is considered the key standard in sensing the virus, a rapid, cheap, and easily available system is needed to determine the presence of the infection during the incubation period to provide enough time for adapting the desirable treatment process and curb its high spread rate [9], [10]. The other diagnostic methods such as CT imaging, nucleic acid tests, gene sequencing, and serological assays also lack the required promptness, cost-effectiveness, and ease of use in a point-of-care (POC) condition [11]. More importantly, their false-negative results necessitate the use of a combinatory detection technique which itself increases the complexity and cost [12]. Given the mentioned constraints of the conventional approaches and the demand for a real-time, portable, and ultra-sensitive alternative for early detecting and monitoring the progression of the infection, it will worth introducing the biosensor technology which is one of the evolving studies throughout the recent years [13], [14]. Knowing that this epidemic can be intelligently regulated by the advantages offered by the novel tailor-made biosensors, researchers can think of innovative ways for designing a highly specific system for detecting SARS-CoV-2-related biomarkers [15]–[17]. This crown-shaped particle is an enveloped, single-stranded RNA virus that encodes spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins [18]. The surface S protein is considered one of the main biomarkers of the virus since it facilitates the virus’s entry to the host cells [19]. Whereas the integral E protein which is responsible for the viral life cycles seems to be the most antigenic target for biosensing [20]. Also, the produced antibodies in the patients’ biological fluids against the virus can be one other alternative target [21]. Similarly, the viral genome is another potential indicator of the virus’s presence [22]. By employing specific biorecognition elements (BREs) like antibodies, antigens, proteins, whole viruses, nucleic acids, and aptamers, precise biosensing systems can be designed for spotting the SARS-CoV-2 [23], [24]. There are several classifications of biosensors that are capable of detecting tiny amounts of the target using low volumes of the sample [25]. Here we reviewed different categories of portable biosensing platforms which are developed recently for detecting the novel coronavirus 2019 and also the previously proposed systems for identifying other types of viruses. Their general aspects and core strategies were discussed comprehensively based on the most recent research outcomes. These portable devices have the required adaptability for being redesigned to recognize not only COVID-19 but also other disease-specific biomarkers and even future pandemic-causing pathogens. Furthermore, their portability, scalability, and simple fabrication process pave the way for their facile mass production. They can be easily available even for people from developing countries. It facilitates home diagnostics and allows remote detection without the need for skilled laboratory personnel and expensive equipment. Different techniques such as electrochemical, optical, surface plasmon resonance (SPR-based), and field-effect transistor (FET-based) biosensors were addressed. Lastly, a conclusion was provided summarizing the remaining challenges and prospects.
II. Different Types of Biosensors
A. Electrochemical
One of the most commonly used biosensors for detecting viral particles is electrochemical sensors [26]. Owing to their strong correlation with advancements in cost-effective microelectronic circuit designs and interrelatedness with standard readout systems, they can be fabricated through a simple and low-cost process [27]. Besides, these reliable and compact tools enable the detection of an analyte in a small volume of complex samples in a highly accurate, POC, and real-time manner which is desirable for medical diagnosis applications [28]. In order to boost the efficiency of the biosensor, it is vital to increase the signal-to-noise ratio which is possible by shrinking the size of the system by using nanostructures in their design [29]. The cooperation of nanotechnology and bioelectronics paved the way for the emergence of nanoscale devices with higher accuracy in comparison to conventional systems [30]. By reducing the size of substances toward the atomic levels, their electrical characteristics become more responsive to external variables [31]. Their miniaturized sizes which are proportionate to the dimensions of the target, enable the identification of single molecules. Generally, this electrochemical reaction would result in a change in current, potential, impedance, or conductive properties of the medium which is categorized in amperometric, potentiometric, impedimetric, and conductometric types of sensors, respectively. There is also one other category called, field-effect, in which the current is measured at the gate electrode of a transistor. One of the most effective factors in the enactment of the electrochemical biosensor is the electrodes. Since the investigated reaction is occurring in their immediate vicinity [32]. That is why it is essential to choose the appropriate size, material, and surface functionalization in order to optimize the recognition capability of the electrode [33]. Typically an electrochemical measurement is conducted by three electrodes namely, reference (sustains a constant potential), auxiliary (facilitates the appliance of a current to the working electrode by connecting it to the electrolyte), and working (serves as the transducer) electrodes. Gold, carbon, platinum, fluorine-doped tin oxide, and silicon compounds are among the most favored materials for fabricating electrodes [34]. The importance of choosing the best material for the electrode is revealed when it comes to optimizing the double layer capacitance, the speed of the electron shuttle, and the surface modification [35].
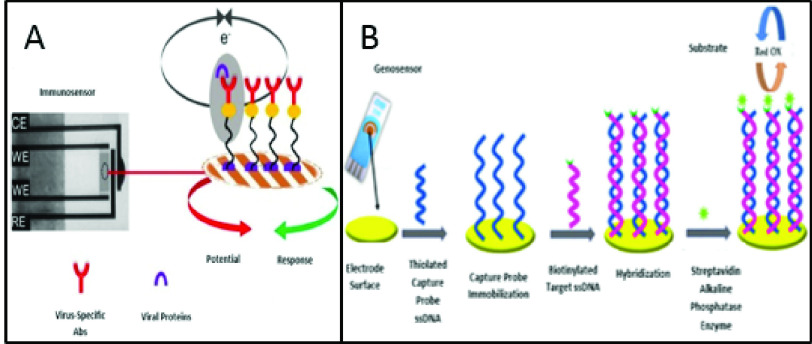
Having said the general aspects of electrochemical biosensors, they are frequently utilized for the identification of viral particles [36]. Due to their portability, short response time, ultra-sensitivity, ease of use, cheapness, compact size, and low limits of detection (LOD), they are one of the most preferred and favorable choices in detecting the recent SARS-CoV-2 [37]. LOD – the lowest detectable concentration of the analyte-is an important factor in evaluating the efficiency of the biosensor. Fig. 1 illustrates the operation of an immunosensor and a genosensor designed for detecting the viral surface proteins, whole viruses, and viral genomes, respectively. These strategies can be employed for detecting the novel coronavirus by modifying the surface of the sensor with virus-specific proteins or oligonucleotides.
Fig. 1.
Configuration of A. an electrochemical immunosensor for detecting viral particles, B. a genosensor for identifying viral genome [41], [42].
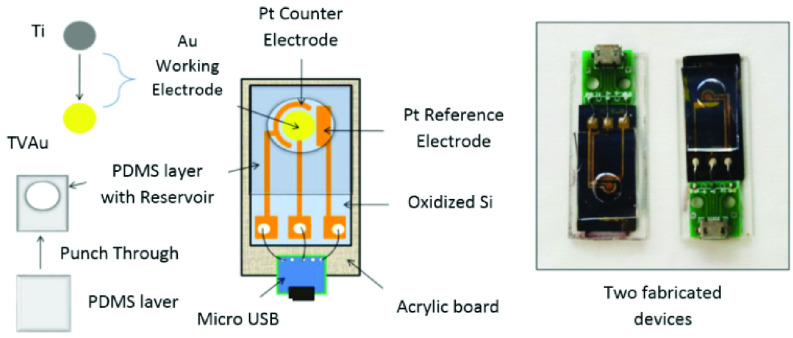
As presented in TABLE I, in a recent study done by Chandra et al., a tailored smart phone-assisted electrochemical impedance spectroscopy (EIS)-based biosensing system was designed for detecting COVID-19. The use of metal nanoparticles (NPs), nano-dendroids, and graphene oxide (GO) nanocomposites for functionalizing the screen-printed carbon electrode (SPCE) provided an extraordinary electrical property and a large surface area for immobilizing a considerable amount of antibodies for capturing higher amounts of the biomarker. They also indicated the feasibility of redesigning this system for recognizing the recently found COVID-19 related markers [38]. Another smartphone-assisted DNA hybridization-based electrochemical genosensor was fabricated to detect the genetic material of the COVID-19. This system did not require complicated arrangements of data gathering from cumbersome electrochemical devices. Thus is an ideal candidate for POC detection (See Fig. 2) [39]. The use of electrochemical biosensors for detecting viral particles is not a recent topic [40]. It has been evolving since the last decades and numerous research studies are aiming at designing an efficient system for quantifying the amount of viral infection-related biomarkers in human biofluids [61]. According to TABLE II, in Hsu and coworkers’ study, a scalable electrochemical Complementary metal-oxide semiconductor (CMOS)-based biosensor array was designed for detecting the Zika virus genome. This miniaturized on-chip sensor enables a mostly digitalized polar-mode measurement which boosts the SNR considerably. The operation principle of this structure is based on assessing the alterations resulted from DNA hybridization on the sensor’s surface employing a trans-impedance enhancer, a detector, and a primary converter. This EIS-based biosensing system holds the potential for accurate and reliable biosensing in POC applications [47]. In another work (See Fig. 3), an economical paper-based sensor was proposed for identifying influenza virus H1N1 antigen in 30 minutes. The surface of the paper was amended with silica nanoparticles which increased the hydrophobicity. This is an imperative feature in designing paper-based biosensors. Besides, stencil-printed carbon electrodes were modified by single-walled carbon nanotubes and chitosan to enhance the preciseness of the sensor by amplifying the signal. In the final stage, the antibodies were immobilized using GA. The sensitivity of the designed system was evaluated by differential pulse voltammetry techniques. The results showed that this structure was able to recognize down to 113 PFU mL−1 of the viral particles in Phosphate-buffered saline (PBS) and saliva samples [49].
TABLE I. Recently Developed Electrochemical Biosensors for Recognizing COVID-19.
| Assay Structure | Target | Readout | Sample | LR | LOD | RT | Ref. |
|---|---|---|---|---|---|---|---|
| SPCE/NPs/nano-Dendroids/GO/Ab | S protein | Impedimetric | Clinical sample | [38] | |||
| SPE/carbon black nanomaterial/anti-mouse-IgG-MB/MAb anti-S or MAb anti-N/ PAb anti-S or PAb anti-N/PAb anti-rabbit-AP | S protein or N protein | Electrochemical | Saliva | 19 and
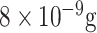 .ml−1 .ml−1
|
30 mins | [43] | |
| FTO/AuNPs/nCOVID-19 Ab | nCOVID-19 Ag | Potentiometric | Spiked saliva |
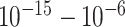 |
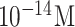 |
10–30 s | [44] |
| SiO2/Ti/AuNPs/Thiolated ssDNA | viral RNA, or c-DNA | Electrochemical (CMOS) | Spiked buffer and serum | [39] | |||
| SPCE/Au@SCX8-RGO-TB/CP/Au@Fe3O4/Probe | Viral RNA | Electrochemical | Clinical samples | 200 copies/mL | [45] |
LR: linear range, RT: response time, Ab: antibody, MB: methylene blue, IgG: immunoglobulin G, MAb: monoclonal antibody, PAb: polyclonal antibody, AP: Alkaline Phosphatase, NP: Nucleocapsid protein, FTO: fluorine-doped tin oxide electrode, SPE: screen-printed electrodes, SCX8-RGO: p-sulfocalix(8)arene functionalized graphene, TB: toluidine blue
Fig. 2.
A configuration of the compact electrochemical system presenting different constituents and a real image of the constructed devices [39].
TABLE II. Recently Developed Electrochemical Biosensors for Recognizing Other Viral Infections.
| Application | Target | RE | Linker | Surface | Sensor | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| AIV detection | Viral DNA | DNA | APTES GA | SiO2 | Impedimetric (On-chip) |
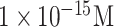 |
Buffer | [46] |
| Zika Virus detection | Viral oligonucleotide | ssDNA | Al/Ni/Au | EIS (On-chip) | Buffer | [47] | ||
| EBV detection | viral oligonucleotide | DNA | PATP EtBr | Graphite | Electrochemical |
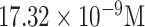 |
Buffer | [48] |
| IV H1N1 detection | Whole virus | Ab | silica NPs SWCNT Chitosan GA | SPCE | DPV | 113 PFU mL−1 | Saliva | [49] |
| WSSV detection | Whole virus | Ab | CB@Ses-Qn HRP | GCE | CV |
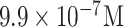 |
Real samples | [50] |
| FLUAV detection | HA | Anti- H5N1 | PANI-coated EAM NPs AuNPs Glycan | SPCE | Electrochemical - SPR |
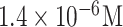 |
Buffer | [51] |
| DENV detection | NS1 | DGV BP1 | MUA EDC/NHS | GE | SWV, EIS |
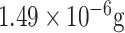 /mL /mL |
Human plasma | [52] |
| MERS-CoV and HCoV detection | MERS-CoV protein | Ab | AuNPs | CE | SWV |
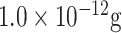 .mL−1 .mL−1
|
Spiked nasal samples | [53] |
| swine virus H1N1 detection | HA | Anti-HA | His6-H1 HA MBT DPM-Cu | Gold | SWV | Diluted vaccinated mice sera | [54] | |
| IV detection | M1 protein | anti-M1 | PABA EDC/NHS | Boron doped diamond electrode | Electrochemical |
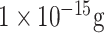 /ml /ml |
Saliva buffer | [55] |
| DENV-3 detection | DENV-3 sequences | DNA | PGE | DPV |
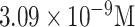 |
Human serum | [56] | |
| Dengue virus detection | Dengue toxin | Ab | CNT AuNP | GE | Electrochemical |
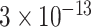 g/mL g/mL |
Human serum | [57] |
| Dengue virus detection | ss-31 mer DNA | 59-aminated DNA probes | nanoporous alumina | platinum wire electrode | Electrochemical |
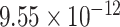 M M |
genomic DNA derived from PCR | [58] |
| CHIGV detection | CHIGV DNA | Probe DNA | MoS2 NSs MB | SPGEs | Electrochemical (voltammetric) |
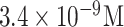 |
Serum sample | [59] |
| Dengue virus detection | Dengue virus gene 1 | 18-mer ssDNA | PGE | DPV |
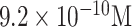 |
Buffer | [60] |
RE: recognition element, AIV: avian influenza virus, APTES: (3-Aminopropyl)triethoxysilane, GA: glutaraldehyde, ssDNA: single stranded DNA, Al: aluminum, EBV: Epstein-Barr virus, SWCNT: single-walled carbon nanotubes, DPV: differential pulse voltammetry, WSSV: white spot syndrome virus, HRP: horseradish peroxidase, CB: carbon nanoblack, Ses-Qn: sesamol-quinone, GCE: glassy carbon electrode, CV: cyclic voltammetry, FLUAV: Influenza A virus, PANI: polyaniline, HA: Hemagglutinin, EAM: electrically active magnetic, DENV: Dengue virus, NS1: nonstructural 1 protein, MUA: 11-mercaptoundecanoic Acid, EDC: N-ethyl-
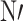 -dimethyl aminopropyl carbodiimide, NHS: N-hydroxysuccinimide, GE: gold electrode, SWV: square wave voltammetry, MERS-COV: Middle East respiratory syndrome corona virus, HCoV: Human coronavirus NL63, AuNPs: gold nanoparticles, His6-H1 HA: His-tagged hemagglutinin, PABA: 4-aminobenzoic acid, MBT: 4-mercaptobutanol, DPM: thiol derivative of dipyrromethene, IV: influenza virus, DENV-3: dengue virus serotype 3, MB: magnetic bead, SPGE: screen printed gold electrodes, PGE: pencil graphite electrode, MoS2 NSs: molybdenum disulphide nanosheets
-dimethyl aminopropyl carbodiimide, NHS: N-hydroxysuccinimide, GE: gold electrode, SWV: square wave voltammetry, MERS-COV: Middle East respiratory syndrome corona virus, HCoV: Human coronavirus NL63, AuNPs: gold nanoparticles, His6-H1 HA: His-tagged hemagglutinin, PABA: 4-aminobenzoic acid, MBT: 4-mercaptobutanol, DPM: thiol derivative of dipyrromethene, IV: influenza virus, DENV-3: dengue virus serotype 3, MB: magnetic bead, SPGE: screen printed gold electrodes, PGE: pencil graphite electrode, MoS2 NSs: molybdenum disulphide nanosheets
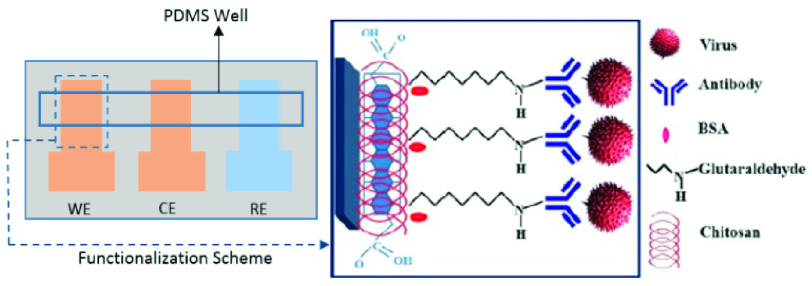
Fig. 3.
The paper-based immunosensor, and the modification of the working electrode (Reconstructed from [49]).
These examples verify the possibility of reusing the strategies employed in the recently designed biosensing systems for detecting COVID-19 related biomarkers. Although electrochemical biosensors have been thoroughly studied in lab-based arrangements, they are not fully commercialized thus far. Already established electrochemical genosensors like the ePlex platform by GenMark Diagnostics are comprised of PCR with microfluidic systems. It can rapidly identify not only SARS-CoV-2 but also other respiratory pathogens by its multiple nucleic-acid-modified gold electrodes [62]. Another marketable system was developed by Roche diagnostics to detect SARS-CoV-2 antibodies based on electrochemiluminescence technique. This methodology represents a similar selectivity with slightly lower sensitivity in comparison to ELISA [63]. However, at this time, there is not a commercially-available system using electrochemical biosensors with electrochemical readout techniques for spotting SARS-CoV-2-related antibodies. With the ever-increasing research work and growing knowledge regarding the novel coronavirus, an electrochemical-based SARS-CoV-2 diagnostic test can be developed and commercialized as an easily-available alternative. For example, immunosensors can be designed to exploit antibodies against the surface proteins of the novel coronavirus. Or in the same way, researchers can focus on fabricating geno or aptasensors for identifying the genetic material of the pandemic-causing virus.
B. Optical
The other category of rapid, and cost-effective biosensing systems are the optical biosensors [64]. They are composed of a target-specific BRE and an optical transduction unit for producing a signal which is in proportion with the concentration of an analyte [65]. Their functionality in interdisciplinary methods has attracted great attention in recent years. Therefore, a large number of research studies have focused on this important technology during the last few decades [66]. Optical techniques offer more advantages in comparison to electrochemical ones since the detection of the target molecule is done with low energy consumption [67]. Besides, the high sampling speed, extraordinary LODs, real-time and multiplex assessment, simple fabrication process, small size, low reactant usage, and short response time make them one of the most preferred POC approaches [68]. In order to quantify the concentration of the target analyte in a precise manner, it is imperative to immobilize a great quantity of BREs on the sensor’s surface [69]. The analyzed biomolecule which has a higher refractive index links to the immobilized BRE with a lower refractive index and causes a local change that is recordable by the transducer. It converts this variation to a quantifiable electric signal [68]. In general, optical platforms are classified into two subgroups of label-free and label-based. The first category exploits the direct interaction between the target and the transducer, whereas the latter employs a reporter for distinguishing the generation of a signal through a fluorescent, luminescent, or colorimetric technique [70]. A variety of labels can be used for this purpose including, gold nanoparticles [71], upconversion nanoparticles [72], and quantum dots [73]. This tag is usually linked to one of the biological elements, however, it can sometimes influence the coupling event and cause some malfunctions in the system. A comparison of label-based and label-free biosensing is summarized in Table III. Generally, in comparison to electrochemical techniques, color-change-based methods are considered simple to read, since the result is observable without any intricate equipment. However, the tagging process is costly, laborious, and time-consuming. Additionally, a signal bias can occur as a result of the uncontrolled quantity of fluorophore labels on the biomolecules [74]. Though, they still display an acceptable performance and are very popular in the early detection of disease [75]. As summarized in TABLE IV, Chen et al. reported a swift lateral flow immunoassay (LFIA) based on lanthanide-doped polysterene nanoparticles (LNPs) for spotting produced IgG antibodies in human serum against SARV-CoV-2 in 10 minutes. In this order, the surface of the device was coated by a specific viral phosphoprotein to determine the presence of IgG in the sample. Additionally, IgG antibodies were marked by LNPs to be detectable. Since the obtained results were analogous to RT-PCR results, this approach can be used for early detection, monitoring the progression, and treatment of viral infections [76]. Another similar platform which can be seen in Fig. 4 was developed by Feng et al. who constructed an immunofluorescent assay for detecting SARS-CoV-2-specific IgM and IgG in human serum in less than 10 minutes. The viral nucleocapsid (N) protein acted as the probe of this system, where Lanthanide,
TABLE III. Comparison of Label-Based and Label-Free Biosening.
| Label-based biosensing | Label-free biosensing | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Multiplex quantification | Interference in coupling events | Simple, rapid, low-cost, low consumption of reagents, portable, possibility of detecting small biomolecules in their natural conformation in a multiplex manner | Inaccuracies due to environment |
TABLE IV. Recently Developed Label-Based Optical Biosensors for Recognizing COVID-19.
| Assay Structure | Target | Readout | Sample | RT | Ref. |
|---|---|---|---|---|---|
| LFIA(nitrocellulose membrane/recombinant N protein of SARS-CoV-2/Mouse anti-human IgG antibody labeled with LNP) | IgG | Chromatography | Human serum | 10 mins | [76] |
| Nitrocellulose membrane/NP conjugated fluorescent microsphere | IgM and IgG | Fluorescent | Human serum | 10 mins | [77] |
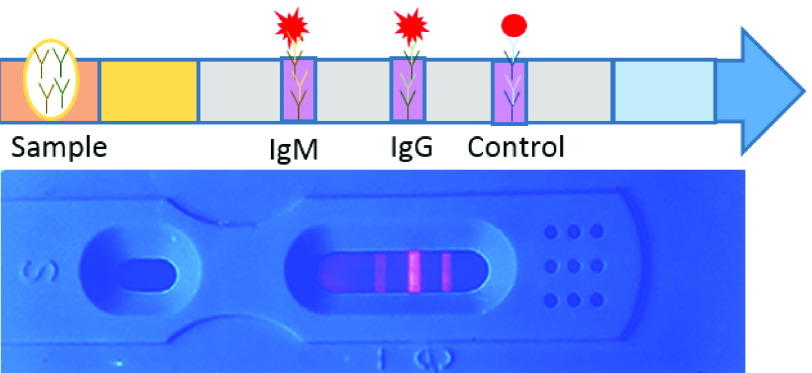
Fig. 4.
Illustration of SARS-CoV-2 IgG/IgM chromatographic test and an actual image of human serum testing results under a UV lamp which shows a high concentration of IgM and IgG (Reconstructed from [77]).
Fig. 4. Illustration of SARS-CoV-2 IgG/IgM chromatographic test and an actual image of human serum testing results under a UV lamp which shows a high concentration of IgM and IgG (Reconstructed from [77]).
Eu(III) fluorescent microsphere was employed as the reporter. The high sensitivity of this biosensing system enables its use in serodiagnostic applications [77]. The exploitation of optical biosensing platforms for virus detection can be seen in TABLE V. For instance, Donaldson and colleagues’ introduced a speedy and accurate method to identify Variola virus (smallpox). This sandwich-type system used anti-Vaccinia antibodies labeled with cyanine 5 dye. The generated signal was sensed utilizing the Analyte 2000 biosensor which provided an excitation light by its laser diode. As a result of the fluorescent molecules’ excitation and the emission of a portion of their energy into the waveguide, the target analyte was recognized. The LOD of the system was
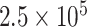 pfu/ml in swab samples [78]. Fig. 5 presents an immunofluorescence biosensing microsystem for the detection of AIV based on ZnO nanorods. The structure and extraordinary attributions of these nanomaterials enhanced the sensing power of this device substantially.
pfu/ml in swab samples [78]. Fig. 5 presents an immunofluorescence biosensing microsystem for the detection of AIV based on ZnO nanorods. The structure and extraordinary attributions of these nanomaterials enhanced the sensing power of this device substantially.
TABLE V. Recently Developed Label-Based Optical Biosensors for Recognizing Other Viral Infections.
| Application | Target | RE | Linker | Surface | Sensor | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vaccinia virus detection | Whole virus | Ab | Cyanine 5 dye | Fluorescent |
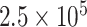 pfu/ml pfu/ml |
seeded throat culture swab specimens | [78] | |
| SARS-CoV detection | N protein | RNA aptamer | QDs ProLinker™ | Glass chip | Fluorescent (On-chip) |
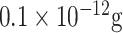 mL−1 mL−1
|
Buffer | [79] |
| AIV detection | H5N2 AIV | mAb | GPTMS SH-PEG Biotin Cy3-SA | ZnO nanorod | Fluorescent |
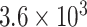 EID50mL−1 EID50mL−1
|
Buffer | [80] |
| HCV detection | HCV RNA | Nucleic acid probe | Thiol Citrate AuNPs cysteamine CTAB | Colorimetric/spectrophotometric | 4.57 IU/
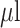
|
Clinical sample | [81] |
QDs: quantum dots, PEG: polyethyleneglycol, HCV: Hepatitis C virus
Fig. 5.
Simultaneous identification of subtypes of AIV in one device. Microchannels II, III, and V that was modified with specific antibodies for AIV recognition [80].
In addition, this highly-selective microfluidic-based immunosensor facilitated multiplex detection of viral targets concurrently. Therefore, it is an appropriate candidate for a cheap and easy to use detection method [80]. By replacing the BRE of these successful systems with the target-specific biomolecules which are capable of detecting COVID-19 biomarkers, pioneering rapid tests can be developed.
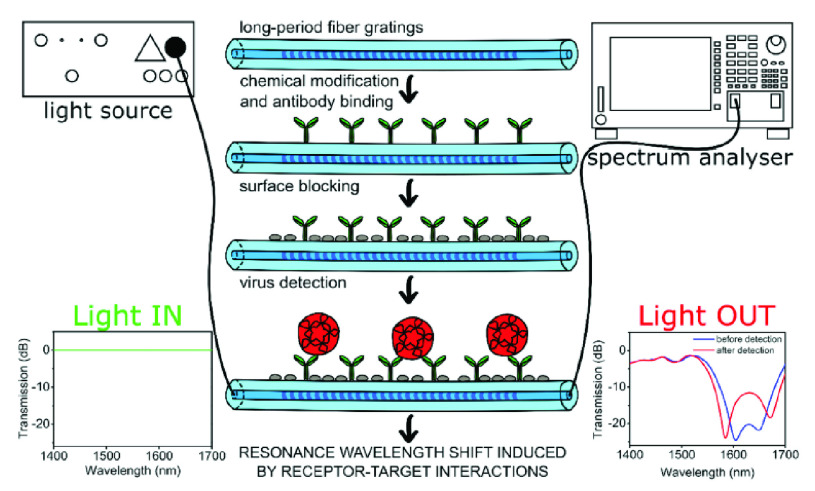
The second group of optical biosensors -Label-free detection systems- provide a fast and easy-to-use method for biochemical and biological applications [66], [82], [83] including the identification of the viruses [84]. They not only necessitate minimum sample preparation steps but also facile and steadfast recognition. This attribution becomes essential in a pandemic situation since rapid management of the virus’s transmission speed is of high importance [85]. Hence, these innovative devices are one of the appropriate choices for detecting COVID-19. For instance, Murugan et al. discussed the possibility of fabricating a handy plasmonic fiber-optic absorbance biosensor (P-FAB) system for detection of SARS-CoV-2’ N protein in the saliva sample within 15 minutes. Based on their previous research works, this well-documented biosensor (Matrix/AuNPs/thiol-PEG-NHS/anti-N protein) has been successfully utilized for detecting different biomolecules such as proteinaceous antigens and endotoxins. It is noteworthy that, they attained LODs down to 10−18 M. Owing to the adaptability of this device, they believe it is feasible to redesign this system for identifying SARS-CoV-2’ N protein in saliva samples with slight modifications in its matrix. This pioneering technology is very promising in the early detection and control of the current and future pandemics [87]. Over the past few decades, several optical sensing devices have been projected for virus detections [88]. As listed in TABLE VI, Nagy and colleagues proposed a CMOS-based immunosensor for multiplex detection of anti-HIV (human immunodeficiency virus) antibodies in serum samples exploiting metal nanoparticles as signal amplifiers. The LOD of the system was reported
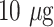 /ml. Using a device based on CMOS technology offers numerous benefits including, facile large-scale production, high-throughput sensing, high sensitivity, and superior selectivity [89]. As can be seen in Fig. 6, a very recent study done by Janczuk-Richter et al., an optical fiber-based immunosensor was reported for norovirus virus-like particles (VLPs) quantification. It could successfully spot 1 ng/mL of the target analyte in a short time (40 minutes).
/ml. Using a device based on CMOS technology offers numerous benefits including, facile large-scale production, high-throughput sensing, high sensitivity, and superior selectivity [89]. As can be seen in Fig. 6, a very recent study done by Janczuk-Richter et al., an optical fiber-based immunosensor was reported for norovirus virus-like particles (VLPs) quantification. It could successfully spot 1 ng/mL of the target analyte in a short time (40 minutes).
TABLE VI. Recently Developed Label-Free Optical Biosensors for Recognizing Other Viral Infections.
| Application | Target | RE | Linker | Surface | Sensor | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| HIV detection | gp120 antigen, and mouse IgG | RAM Ab and anti-HIV | SU-8 AuNPs, silver | Optical (Off-chip) |
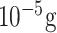 /mL /mL |
Rabbit Serum | [89] | |
| Dengue virus detection | DENV-2 DNA | DNA | (APTS)- PSiNs | Plastic | Optical (Off-chip) |
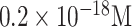 |
Saliva and urine | [90] |
| Ebola virus detection | Viral RNA | oligonucleotide | 4FB- MBs | ARROW (On-chip) | 0.021 pfu/mL | Clinical sample | [91] | |
| VLP detection | main coat protein of the norovirus | Ab | TESPA APTES EDC-modified GFP | SiO2 | Optical (LPFG) |
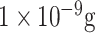 /mL /mL |
Buffer | [86] |
| Dengue virus detection | Virus genome | DNA | AuNPs PSA succinimide | (poly(nBA-NAS)) microspheres | Optical |
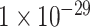 M M |
clinical samples | [92] |
| HIV detection | HIV-1 gp120 | MLV | EDC | Gold | Optical | Buffer | [93] | |
| Papillomavirus detection | VLPs | anti-VLP | APDMES GA | SiO2 | Optical (PhC) |
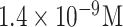 |
10% serum | [94] |
| HCV detection | HCV NS5B | RNA aptamer | Streptavidin biotin | Octet platform | Optical |
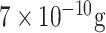 mL−1 mL−1
|
Buffer solution | [95] |
RAM: rabbit anti-mouse, DENV-2: dengue virus serotypes 2, PSiNs: porous silica nanospheres, linker: N,N’-bis-4-(hydroxysalicylidene)-phenylenediamine-nickel(II), 4FB: 4-formyl benzamide, ARROW: antiresonant reflecting optical waveguide, TESPA: 3-(Triethoxysilyl) propylsuccinicanhydride, GFP: Green fluorescent protein, LPFG: long-period fibre gratings, PSA: polyelectrolyte-coated poly(styrene-co-acrylic acid), BA: butyl acrylate, MLV: Murine leukemia virus, APDMES: 3-aminopropyldimethylethoxysilane, PhC: photonic crystal
Fig. 6.
Outline of the operation and surface functionalization for spotting norovirus [86].
This compact and low weight device can be utilized in detecting other types of viral particles as well as vaccine research [86]. The success of these investigations demonstrates their adaptability of being redesigned for identifying SARS-CoV-2.
C. Surface Plasmon Resonance (SPR)
The other well-established and frequently employed category of biosensors is the surface plasmon resonance-based devices [96]. These optical sensing systems which require no labeling operate based on the affinity interactions between an immobilized bioreceptor on the sensor’s surface and the target biomolecule in the sample solution. After the occurrence of this bioreaction, the change in the refractive index is recorded and proportionated to the concentration of the analyte [97]. Due to their high accuracy and LODs down to picomolar levels, they have turned into one of the most powerful and trusted tools in examining the interrelationship between the biological particles [98]. For instance, they have been broadly utilized in detecting disease-specific biomarkers in diagnostic research studies. Like any other biosensing system, the main body of these structures comprises three subassemblies namely, the readout system, BRE, and delivery system [99]. A light wave in the optical readout platform of an SPR sensor stimulates a distinctive type of electromagnetic field which is called a surface plasmon. Because of its dissemination alongside a thin metal film, it can analyze the nearby environment. The binding of the target biomolecules to the BREs immobilized on the surface of the sensor increase the refractive index which accordingly alters the speed of the surface plasmon. This change can be assessed by the optical reader [97]. The employment of SPR-based biosensors for the early detection of viral infections has been highlighted in numerous research articles [100]. Their real-time, label-free, and noninvasive nature make them one of the suitable techniques for speedy and precise detection of coronavirus-related particles [101], [102]. As demonstrated in TABLE VII, Nag et al. discussed the possibility of using an evanescent wave absorbance (EWA)-based optical fibre and localized surface plasmon resonance (LSPR)-based sensor for swift recognition of SARS-CoV-2. Nanostructures such as AuNPs or polyaniline can be used as signal enhancers on the surface of the sensor prior to immobilizing specific antibodies (against the viral particles) or surface proteins of the virus which can sense the produced IgG or IgM in the patient’s serum. The interaction between the probe and target alters the localized charge distribution, refractive index, and accordingly the light intensity and output signal [104]. Qiu and colleagues designed an LSPR-based biosensor implementing the plasmonic photothermal (PPT) effect for detecting the SARS-CoV-2’s genome (See Fig. 7). The DNA probes were modified by 2-D gold nanoislands (AuNIs) for ultra-sensitive detection. The employment of the localized PPT heat enabled precise differentiation of alike sequences. They reported a LOD 0.22 pM in a multiplexed sample. This successful research work shed light on the applicability of thermoplasmonic enhancement in viral disease detection [103]. SPR-based sensors are among the most sensitive systems for recognizing viruses (See TABLE VIII).
TABLE VII. Recently Developed SPR Biosensors for Recognizing COVID-19.
| Assay Structure | Target | Readout | Sample | LOD | RT | Ref. |
|---|---|---|---|---|---|---|
| Optical fibers/AuNPs or PANI/anti- IgG or anti-IgM | IgG or IgM | EWA- and LSPR | swab samples | 100 units.ml−1 | 1 hour | [104] |
| Complementary AuNI chip/Thiol-cDNA | SARS-CoV-2 sequences | PPT-LSPR |
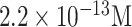 |
[103] |
LSPR: localized surface plasmon resonance
Fig. 7.
A) Configuration of LSPR-based biosensor in combination with the PPT effect for COVID-19 identification, B) the setup of the biosensor [103].
TABLE VIII. Recently Developed SPR Biosensors for Recognizing Other Viral Infections.
| Application | Target | RE | Linker | Surface | Sensor | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Influenza B virus detection | HA | Sialic acid | colloidal AuNPs | SPR | 0.156 vol% | Buffer | [108] | |
| SARS detection | Anti-SCVme | SCVme | GBPs EGFP | Glass/Gold-micropatterned chip | SPR |
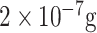 .mL−1 .mL−1
|
Buffer | [109] |
SCVme: SARS coronaviral surface antigen, GBPs: gold binding polypeptides, EGFP: enhanced green fluorescent protein
Besides, this technique is recently being used in plasmon-driven ultrafast photonic PCR and facilitates rapid detection of SARS-CoV-2 RNA. Currently, several studies have focused on designing portable devices for implementing PCR in around 15 minutes. This portable system incorporates reverse transcription, swift thermocycling, and on-site fluorescence-based recognition. The use of magneto-plasmonic photothermal nanoparticles speeds up the thermocycling process via plasmonic heating which decreases the required time and energy, significantly [105]–[107].
D. Field Effect Transistor (FET)
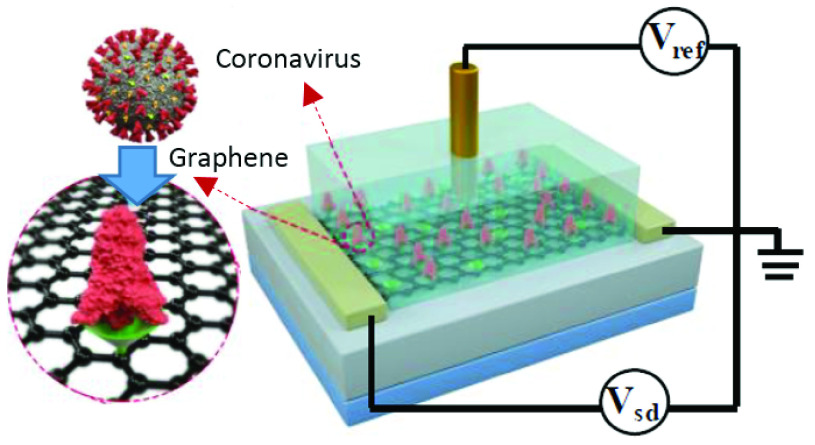
The next group of functional biosensing platforms is the field-effect transistor (FET)-based biosensors [110]. They are usually made from the unification of a BRE and an ion-sensitive field-effect transistor (ISFET) which is a popular form of electrical biosensors from the researchers’ viewpoint [111]. A standard FET device is composed of a semiconductor channel that is interrelated to the source (S) and drain (D) electrodes. After the immobilization of BREs on this section, a potential is applied which controls the current flow between the S and D via the gate electrode. By measuring the varying conductivity of the channel which is dependent on the captured biomolecules, the concentration of the analyte is quantified [112]. In general, the charge transporters are electrons or holes. In the first circumstance, the device is categorized as an n-type, wherein the latter, is considered a p-type. If the target biomolecules are positively charged, we will have an accumulation of the electrons in an n-type system which causes an upward trend in the conductance. Reversely, this feature decreases as a result of electron depletion, if the captured species contain negative charges [113], [114]. The evolutions in the field of nanotechnology lead to the emergence of devices with higher performances and of course, FET biosensors are not an exception. The employment of nanostructures such as nanowires, nanorods, nanotubes, and metal nanoparticles provide a large specific area for immobilizing BREs [115]–[117]. Another important factor to consider is the type of transducer. Silicon nanowires, carbon nanotubes, and nanorods are among the most preferred alternatives because they offer high preciseness, physical stamina, high surface to volume ratio, chemical durability, superior conductivity, and scalability. They can be easily produced on a large scale which is a key parameter in designing a bioFET [119], [120]. Consequently, they have attracted the attention of scientists in the field of early disease detection. Especially, the recent pandemic situation triggered the need for a cheap and highly scalable device for the early detection of COVID-19 [15], [110]. Therefore, several studies have addressed this problem which is summarized in TABLE IX. A FET-based immunosensor was proposed by Seo and coworkers for the measurement of SARS-CoV-2 in biofluids. To increase the biocompatibility of the sensor’s surface and functionalize it with desired antibodies, it was coated with sheets of graphene. This device could successfully detect
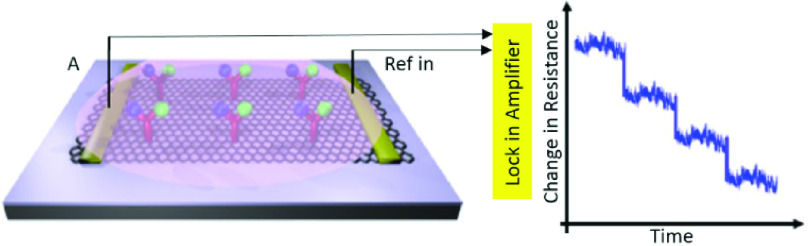
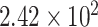 copies/ml of the whole virus in clinical samples. Because this biosensor requires no sample pretreatment or labeling, it holds the potential for being one of the alternative approaches in controlling viral infections [121]. As illustrated in Fig. 8, a similar Gr-FET –based immunosensing platform was developed by Zhang et al. which can recognize SARS-CoV-2’S S1 protein in around 2 minutes with a LOD of 0.2 pM [118]. Thus it facilitates early diagnosis, monitoring, and decreasing the transmission rate of this infectious disease. A wide range of other viral infections has been monitored using FET-based biosensors over the past few years. TABLE X depicts some of them. For instance, a CMOS-based silicon nanowire (SiNW) FET biosensor was established using Cytidine-50-monophospho-N-acetylneuraminic acid (CMP-NANA) as a specific receptor for the surface protein of the influenza virus. This methodology demonstrated high sensitivity, acceptable linear response, and desirable SNR which enable its large- scale production for being used in POC detection of viral infections [123]. Another miniaturized and straightforward FET-based immunosensing assay was fabricated for measuring JEV and AIV which was modified by carboxy functionalized graphene and target-specific antibodies (See Fig. 9). Real-time monitoring of changes in resistance showed the formation of the antibody-antigen complex. The accuracy, selectivity, simplicity, reproducibility, and probability of being integrated into standard FET-based devices specify the possibility of its mass-production for cheap and POC diagnosis of virus-related disorders [127].
copies/ml of the whole virus in clinical samples. Because this biosensor requires no sample pretreatment or labeling, it holds the potential for being one of the alternative approaches in controlling viral infections [121]. As illustrated in Fig. 8, a similar Gr-FET –based immunosensing platform was developed by Zhang et al. which can recognize SARS-CoV-2’S S1 protein in around 2 minutes with a LOD of 0.2 pM [118]. Thus it facilitates early diagnosis, monitoring, and decreasing the transmission rate of this infectious disease. A wide range of other viral infections has been monitored using FET-based biosensors over the past few years. TABLE X depicts some of them. For instance, a CMOS-based silicon nanowire (SiNW) FET biosensor was established using Cytidine-50-monophospho-N-acetylneuraminic acid (CMP-NANA) as a specific receptor for the surface protein of the influenza virus. This methodology demonstrated high sensitivity, acceptable linear response, and desirable SNR which enable its large- scale production for being used in POC detection of viral infections [123]. Another miniaturized and straightforward FET-based immunosensing assay was fabricated for measuring JEV and AIV which was modified by carboxy functionalized graphene and target-specific antibodies (See Fig. 9). Real-time monitoring of changes in resistance showed the formation of the antibody-antigen complex. The accuracy, selectivity, simplicity, reproducibility, and probability of being integrated into standard FET-based devices specify the possibility of its mass-production for cheap and POC diagnosis of virus-related disorders [127].
TABLE IX. Recently Developed FET Biosensors for Recognizing COVID-19.
| Assay Structure | Target | Readout | Sample | LR | LOD | RT | Ref. |
|---|---|---|---|---|---|---|---|
| Si/SiO2/graphene/PBASE/anti- SARS-CoV-2 | SARS-CoV-2 | FET | Clinical sample |
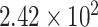 copies/ml copies/ml |
[121] | ||
| Graphene/CSAb | S1 protein | FET |
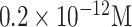 |
2 mins | [118] | ||
| Silicon TFT/Al layer/aptamer | S protein | Electrical | Buffer sample (PBS) |
 |
[122] |
PBASE: 1-pyrenebutanoic acid succinimidyl ester, CSAb: SARS-COV spike S1 subunit protein antibody, TFT: thin film transistor
Fig. 8.
Configuration of the FET-based biosensor modified with the graphene and S1 protein-specific antibodies (Reconstructed from [118].
TABLE X. Recently Developed FET Biosensors for Recognizing Other Viral Infections.
| Application | Target | RE | Linker | Surface | Sensor | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| IV diagnosis | GST-tagged-HA | CMP-NANA/ Biotin-tagged GST Ab | APTES GA Au-streptavidin | SiO2/SiNW | FET (Off-chip) |
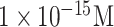 |
Buffer | [123] |
| SARS diagnosis | N protein | AMPs | EDC/NHS/fibronectin | Si/ SiO2/In2O3NWs | FET (off-chip) |
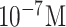 |
Buffer | [124] |
| AIV H1N1 detection | synthetic viral antigen (H1N1) | anti-H1N1 | APTES SBP | SiO2-SiN-bottom SiO2 ONO dielectric stack | ISFET | Buffer | [125] | |
| Dengue Virus detection | RT-PCR product of DEN-2 | PNA | APTES GA | Si/ SiO2/SiNW | FET (Off-chip) | below
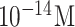
|
Buffer | [126] |
| JEV and AIV detection | JEV and AIV | Ab | carboxy Graphene EDC/NHS | SiO2 | FET |
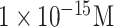 and and
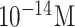
|
Buffer | [127] |
GST: glutathione S-transferase, AMP: Antibody mimic proteins, ONO: oxide-nitride-oxide, PNA: peptide nucleic acid, JEV: Japanese Encephalitis Virus
Fig. 9.
Configuration of a FET-based immunosensor functionalized with graphene, EDC/NHS, and specific antibodies to target JEV and AIV and the resistance measurement for different concentrations of the target biomolecules using an amplifier (Reconstructed from [127]).
III. Conclusion
Undoubtedly, the COVID-19 pandemic will be restrained in the immediate future. However, we should learn from this disastrous experience of the current century and be better prepared for upcoming hazards by adapting appropriate diagnostic interventions. Though, there is still a laborious way in front of the novel detection technologies. Currently, the dominant method of clinical detection is RT-PCR-based test kits. Although they are sensitive, selective, and well-established, they are time-consuming and necessitate costly instrumentation and experienced laboratory staff. These shortcomings underline a vital need for some rapid and user-friendly alternatives. POC biosensors can be one of the potent candidates. They provide rapid analysis, convenient use, affordable fabrication, multiplex detection, and facile mass production. The present review summarized the recently proposed biosensing platforms specialized to detect SARS-CoV-2-related biomarkers. Furthermore, it compared the core strategies of already designed biosensors for detecting other types of viruses and argued their potential to be used for identifying COVID-19. Especially, the significance of electrochemical, optical, SPR- and FET-based biosensors in the recognition of COVID-19 is underscored. They hold huge potential for being used as self-sufficient devices for COVID-19 identification in asymptomatic cases beyond laboratory settings, in houses, hospitals, airports, and even in remote areas. It is worth noting that the integration of microfluidic technology into the structure of biosensors facilitates the concurrent assessment of multiple biomarkers which increases the accuracy of the test. Besides, advances in bioengineering for designing unique BREs would enable highly-selective sensing platforms. Also, the employment of nanostructures decreases the background noise by boosting the available surface area. However, there are still challenges to be tackled to optimize the performance of these miniaturized devices. More examinations are required to ease the using procedure and uniting all the compartments into a single device at the lowest possible expense. Utilizing smartphones and particular apps that make analyzing the data and tracking the progress of the disease easier is another functional approach that should be improved in future studies. Also, removable power supplies like batteries can be added to the structure of biosensors to enhance their practicality specifically in places with restricted electricity sources. To conclude, considering that such pandemics are probable to reoccur, investing in attentiveness against these global threats is highly imperative. This cooperative and universal duty cannot be fulfilled without the collaboration of universities, companies, funding agents, and the government. We expect that well-timed screening based on robust biosensing strategies might relax severe quarantine and social distancing rules.
References
- [1].Worldometer. (2020). Covid-19 Coronavirus Pandemic. [Online]. Available: https://www.worldometers.info/coronavirus/
- [2].Haleem A., Javaid M., and Vaishya R., “Effects of COVID-19 pandemic in daily life,” Current Med. Res. Pract., vol. 10, no. 2, pp. 78–79, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheval S., Adamescu C. M., Georgiadis T., Herrnegger M., Piticar A., and Legates D. R., “Observed and potential impacts of the COVID-19 pandemic on the environment,” Int. J. Environ. Res. Public Health, vol. 17, no. 11, p. 4140, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li H. O.-Y. and Huynh D., “Long-term social distancing during COVID-19: A social isolation crisis among seniors?” Can. Med. Assoc. J., vol. 192, no. 21, p. E588, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nicola M.et al. , “The socio-economic implications of the coronavirus pandemic (COVID-19): A review,” Int. J. Surg., vol. 78, pp. 185–193, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perez G. I. P. and Abadi A. T. B., “Ongoing challenges faced in the global control of COVID-19 pandemic,” Arch. Med. Res., vol. 51, no. 6, pp. 574–576, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ehrlich H., McKenney M., and Elkbuli A., “Protecting our healthcare workers during the COVID-19 pandemic,” Amer. J. Emergency Med., vol. 38, no. 7, pp. 1527–1528, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhai P., Ding Y., Wu X., Long J., Zhong Y., and Li Y., “The epidemiology, diagnosis and treatment of COVID-19,” Int. J. Antimicrobial Agents, vol. 55, no. 5, May 2020, Art. no. 105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tahamtan A. and Ardebili A., “Real-time RT-PCR in COVID-19 detection: Issues affecting the results,” Expert Rev. Mol. Diag., vol. 20, no. 5, pp. 453–454, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Emery S. L.et al. , “Real-time reverse transcription–polymerase chain reaction assay for SARS-associated coronavirus,” Emerg. Infectious Disease, vol. 10, no. 2, pp. 311–316, Feb. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Udugama B.et al. , “Diagnosing COVID-19: The disease and tools for detection,” ACS Nano, vol. 14, no. 4, pp. 3822–3835, Apr. 2020. [DOI] [PubMed] [Google Scholar]
- [12].Giri B., Pandey S., Shrestha R., Pokharel K., Ligler F. S., and Neupane B. B., “Review of analytical performance of COVID-19 detection methods,” Anal. Bioanal. Chem., vol. 413, no. 1, pp. 35–48, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laghrib F.et al. , “Current progress on COVID-19 related to biosensing technologies: New opportunity for detection and monitoring of viruses,” Microchem. J., vol. 160, Jan. 2021, Art. no. 105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asif M.et al. , “The role of biosensors in coronavirus disease-2019 outbreak,” Current Opinion Electrochem., vol. 23, pp. 174–184, Oct. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Samson R., Navale G. R., and Dharne M. S., “Biosensors: Frontiers in rapid detection of COVID-19,” 3 Biotech, vol. 10, no. 9, pp. 1–9, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu L., Li D., Ramadan S., Li Y., and Klein N., “Facile biosensors for rapid detection of COVID-19,” Biosensors Bioelectron., vol. 170, Dec. 2020, Art. no. 112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Choi J. R., “Development of point-of-care biosensors for COVID-19,” Frontiers Chem., vol. 8, p. 517, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boopathi S., Poma A. B., and Kolandaivel P., “Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment,” J. Biomolecular Struct. Dyn., vol. 39, pp. 1–10, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Naqvi A. A. T.et al. , “Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach,” Biochimica et Biophysica Acta (BBA)-Mol. Basis Disease, vol. 1866, no. 10, Oct. 2020, Art. no. 165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ouassou H.et al. , “The pathogenesis of coronavirus disease 2019 (COVID-19): Evaluation and prevention,” J. Immunol. Res., vol. 2020, pp. 1–7, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L. and Guo H., “Biomarkers of COVID-19 and technologies to combat SARS-CoV-2,” Adv. Biomarker Sci. Technol., vol. 2, pp. 1–23, Jul. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kermali M., Khalsa R. K., Pillai K., Ismail Z., and Harky A., “The role of biomarkers in diagnosis of COVID-19—A systematic review,” Life Sci., vol. 254, Aug. 2020, Art. no. 117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ponti G., Maccaferri M., Ruini C., Tomasi A., and Ozben T., “Biomarkers associated with COVID-19 disease progression,” Crit. Rev. Clin. Lab. Sci., vol. 57, no. 6, pp. 389–399, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Antiochia R., “Developments in biosensors for CoV detection and future trends,” Biosensors Bioelectron., vol. 173, Feb. 2021, Art. no. 112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ji T.et al. , “Detection of COVID-19: A review of the current literature and future perspectives,” Biosensors Bioelectron., vol. 166, Oct. 2020, Art. no. 112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cesewski E. and Johnson B. N., “Electrochemical biosensors for pathogen detection,” Biosensors Bioelectron., vol. 159, Jul. 2020, Art. no. 112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li H., Liu X., Li L., Mu X., Genov R., and Mason A., “CMOS electrochemical instrumentation for biosensor microsystems: A review,” Sensors, vol. 17, no. 12, p. 74, Dec. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cho I.-H., Kim D. H., and Park S., “Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis,” Biomater. Res., vol. 24, no. 1, pp. 1–12, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carpenter A., Paulsen I., and Williams T., “Blueprints for biosensors: Design, limitations, and applications,” Genes, vol. 9, no. 8, p. 375, Jul. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaya S. I., Karadurmus L., Ozcelikay G., Bakirhan N. K., and Ozkan S. A., “Electrochemical virus detections with nanobiosensors,” in Nanosensors for Smart Cities: Micro and Nano Technologies. Amsterdam, The Netherlands: Elsevier, 2020, pp. 303–326. [Google Scholar]
- [31].Chamorro-Garcia A. and Merkoçi A., “Nanobiosensors in diagnostics,” Nanobiomedicine, vol. 3, pp. 1–26, Jan. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kimmel D. W., LeBlanc G., Meschievitz M. E., and Cliffel D. E., “Electrochemical sensors and biosensors,” Anal. Chem., vol. 84, no. 2, pp. 685–707, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singh P., Pandey S. K., Singh J., Srivastava S., Sachan S., and Singh S. K., “Biomedical perspective of electrochemical nanobiosensor,” Nano-Micro Lett., vol. 8, no. 3, pp. 193–203, Jul. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thévenot D. R., Toth K., Durst R. A., and Wilson G. S., “Electrochemical biosensors: Recommended definitions and classification1International union of pure and applied chemistry: Physical chemistry division, commission I.7 (biophysical chemistry); analytical chemistry division, commission V.5 (electroanalytical chemistry).1,” Biosensors Bioelectron., vol. 16, nos. 1–2, pp. 121–131, Jan. 2001. [DOI] [PubMed] [Google Scholar]
- [35].Hammond J. L., Formisano N., Estrela P., Carrara S., and Tkac J., “Electrochemical biosensors and nanobiosensors,” Essays Biochem., vol. 60, no. 1, pp. 69–80, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sin M. L., Mach K. E., Wong P. K., and Liao J. C., “Advances and challenges in biosensor-based diagnosis of infectious diseases,” Expert Rev. Mol. Diag., vol. 14, no. 2, pp. 225–244, Mar. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eguilaz M. R. D., Cumba L. R., and Forster R. J., “Electrochemical detection of viruses and antibodies: A mini review,” Electrochem. Commun., vol. 116, Jul. 2020, Art. no. 106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chandra P., “Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis,” Sensors Int., vol. 1, Jul. 2020, Art. no. 100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tripathy S. and Singh S. G., “Label-free electrochemical detection of DNA hybridization: A method for COVID-19 diagnosis,” Trans. Indian Nat. Acad. Eng., vol. 5, no. 2, pp. 205–209, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Khan M. Z. H., Hasan M. R., Hossain S. I., Ahommed M. S., and Daizy M., “Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art,” Biosensors Bioelectron., vol. 166, Oct. 2020, Art. no. 112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saylan Y., Erdem Ö., Ünal S., and Denizli A., “An alternative medical diagnosis method: Biosensors for virus detection,” Biosensors, vol. 9, no. 2, p. 65, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaushik A.et al. , “A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein,” Sci. Rep., vol. 8, no. 1, p. 9700, Jun. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fabiani L.et al. , “Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva,” Biosensors Bioelectron., vol. 171, Jan. 2021, Art. no. 112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mahari S., Roberts A., Shahdeo D., and Gandhi S., “eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19,” DBT-Nat. Inst. Animal Biotechnol. (NIAB), Hyderabad, India, Tech. Rep. BT/AAQ/ 01/NIAB-Flagship/2019, 2020. [Google Scholar]
- [45].Zhao H.et al. , “Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone,” Sens. Actuators B, Chem., vol. 327, Jan. 2021, Art. no. 128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lai W.-A., Lin C.-H., Yang Y.-S., and Lu M. S.-C., “Ultrasensitive detection of avian influenza virus by using CMOS impedimetric sensor arrays,” in Proc. IEEE 25th Int. Conf. Micro Electro Mech. Syst. (MEMS), Jan. 2012, pp. 894–897. [Google Scholar]
- [47].Hsu C., Sun A., Zhao Y., Aronoff-Spencer E., and Hall D. A., “A 16×20 electrochemical CMOS biosensor array with in-pixel averaging using polar modulation,” presented at the IEEE Custom Integr. Circuits Conf. (CICC), San Diego, CA, USA, 2018. [Google Scholar]
- [48].Balvedi R., Castro A., Madurro J., and Brito-Madurro A., “Detection of a specific biomarker for epstein-barr virus using a polymer-based genosensor,” Int. J. Mol. Sci., vol. 15, no. 5, pp. 9051–9066, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Devarakonda S., Singh R., Bhardwaj J., and Jang J., “Cost-effective and handmade paper-based immunosensing device for electrochemical detection of influenza virus,” Sensors, vol. 17, no. 11, p. 2597, Nov. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gandhi M., Rajagopal D., Parthasarathy S., Raja S., Huang S.-T., and Kumar A. S., “In situ immobilized sesamol-quinone/carbon nanoblack-based electrochemical redox platform for efficient bioelectrocatalytic and immunosensor applications,” ACS Omega, vol. 3, no. 9, pp. 10823–10835, Sep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kamikawa T. L.et al. , “Pandemic influenza detection by electrically active magnetic nanoparticles and surface plasmon resonance,” IEEE Trans. Nanotechnol., vol. 11, no. 1, pp. 88–96, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kim J. H.et al. , “Development of peptide biosensor for the detection of dengue fever biomarker, nonstructural 1,” PLoS ONE, vol. 14, no. 9, Sep. 2019, Art. no. e0222144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Layqah L. A. and Eissa S., “An electrochemical immunosensor for the corona virus associated with the middle east respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes,” Microchimica Acta, vol. 186, no. 4, p. 224, Mar. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mikuła E., Silva C. E., Kopera E., Zdanowski K., Radecki J., and Radecka H., “Highly sensitive electrochemical biosensor based on redox–active monolayer for detection of anti-hemagglutinin antibodies against swine-origin influenza virus H1N1 in sera of vaccinated mice,” BMC Veterinary Res., vol. 14, no. 1, p. 328, Nov. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nidzworski D.et al. , “A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond,” Sci. Rep., vol. 7, no. 1, p. 15707, Nov. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Oliveira N.et al. , “A sensitive and selective label-free electrochemical dna biosensor for the detection of specific dengue virus serotype 3 sequences,” Sensors, vol. 15, no. 7, pp. 15562–15577, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Palomar Q., Xu X., Gondran C., Holzinger M., Cosnier S., and Zhang Z., “Voltammetric sensing of recombinant viral dengue virus 2 NS1 based on au nanoparticle–decorated multiwalled carbon nanotube composites,” Microchimica Acta, vol. 187, no. 6, p. 363, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rai V., Hapuarachchi H. C., Ng L. C., Soh S. H., Leo Y. S., and Toh C.-S., “Ultrasensitive cDNA detection of dengue virus RNA using electrochemical nanoporous membrane-based biosensor,” PLoS ONE, vol. 7, no. 8, Aug. 2012, Art. no. e42346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Singhal C., Khanuja M., Chaudhary N., Pundir C. S., and Narang J., “Detection of chikungunya virus DNA using two-dimensional MoS₂ nanosheets based disposable biosensor,” Sci. Rep., vol. 8, no. 1, p. 7734, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Souza E.et al. , “Label-free electrochemical detection of the specific oligonucleotide sequence of dengue virus type 1 on pencil graphite electrodes,” Sensors, vol. 11, no. 6, pp. 5616–5629, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Campuzano S., Yáñez-Sede no P., and Pingarrón J., “Molecular biosensors for electrochemical detection of infectious pathogens in liquid biopsies: Current trends and challenges,” Sensors, vol. 17, no. 11, p. 2533, Nov. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].GenMarkDx. (2020). Respiratory Pathogen Panel (RP)1 and NEW Respiratory Pathogen Panel 2 (RP2)2. [Online]. Available: https://genmarkdx.com/panels/eplex-panels/respiratory-pathogen-panel/
- [63].RocheDiagnostics. (2020). Roche to Launch SARS-CoV-2 Rapid Antigen Test in Countries Accepting CE Mark, Allowing Fast Triage Decisions at Point of Care. [Online]. Available: https://www.roche.com/media/releases/med-cor-2020-09-01b.htm
- [64].Ligler F. S., “Perspective on optical biosensors and integrated sensor systems,” Anal. Chem., vol. 81, no. 2, pp. 519–526, Jan. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Geng Z., Zhang X., Fan Z., Lv X., Su Y., and Chen H., “Recent progress in optical biosensors based on smartphone platforms,” Sensors, vol. 17, no. 11, p. 2449, Oct. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Peltomaa R., Glahn-Martínez B., Benito-Peña E., and Moreno-Bondi M., “Optical biosensors for label-free detection of small molecules,” Sensors, vol. 18, no. 12, p. 4126, Nov. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Xu W., Wang D., Li D., and Liu C. C., “Recent developments of electrochemical and optical biosensors for antibody detection,” Int. J. Mol. Sci., vol. 21, no. 1, p. 134, Dec. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Estrela P., Damborský P., Švitel J., and Katrlík J., “Optical biosensors,” Essays Biochem., vol. 60, no. 1, pp. 91–100, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schuck P., Boyd L. F., and Andersen P. S., “Measuring protein interactions by optical biosensors,” Current Protocols Protein Sci., vol. 17, no. 1, pp. 20.2.1–20.2.25, Sep. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Baird C. L. and Myszka D. G., “Current and emerging commercial optical biosensors,” J. Mol. Recognit., vol. 14, no. 5, pp. 261–268, 2001. [DOI] [PubMed] [Google Scholar]
- [71].Hu J.et al. , “Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays,” Lab Chip, vol. 13, no. 22, pp. 4352–4357, Nov. 2013. [DOI] [PubMed] [Google Scholar]
- [72].You M.et al. , “Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-color upconversion nanoparticles,” ACS Nano, vol. 11, no. 6, pp. 6261–6270, Jun. 2017. [DOI] [PubMed] [Google Scholar]
- [73].Hu J.et al. , “Dual-signal readout nanospheres for rapid point-of-care detection of Ebola virus glycoprotein,” Anal. Chem., vol. 89, no. 24, pp. 13105–13111, Dec. 2017. [DOI] [PubMed] [Google Scholar]
- [74].Benito-Peña E., Moreno-Bondi M. C., Glahn-Martínez B., and Valdés M. G., “Fluorescence based fiber optic and planar waveguide biosensors. A review,” Anal. Chim. Acta, vol. 943, pp. 17–40, Nov. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pirzada M. and Altintas Z., “Recent progress in optical sensors for biomedical diagnostics,” Micromachines, vol. 11, no. 4, p. 356, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen Z.et al. , “Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay,” Anal. Chem., vol. 92, no. 10, pp. 7226–7231, May 2020. [DOI] [PubMed] [Google Scholar]
- [77].Feng M.et al. , “Development of a sensitive immunochromatographic method using lanthanide fluorescent microsphere for rapid serodiagnosis of COVID-19,” ACS Sensors, vol. 5, no. 8, pp. 2331–2337, Aug. 2020. [DOI] [PubMed] [Google Scholar]
- [78].Donaldson K. A., Kramer M. F., and Lim D. V., “A rapid detection method for Vaccinia virus, the surrogate for smallpox virus,” Biosensors Bioelectron., vol. 20, no. 2, pp. 322–327, Sep. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Roh C. and Jo S. K., “Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip,” J. Chem. Technol. Biotechnol., vol. 86, no. 12, pp. 1475–1479, Dec. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yu X.et al. , “A nanostructured microfluidic immunoassay platform for highly sensitive infectious pathogen detection,” Small, vol. 13, no. 24, Jun. 2017, Art. no. 1700425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shawky S. M., Awad A. M., EL-Khamisy S. F., Allam W., and Alkordi M. H., “Gold aggregating gold: A novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples,” Biosensors Bioelectron., vol. 92, pp. 349–356, Jun. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Khansili N., Rattu G., and Krishna P. M., “Label-free optical biosensors for food and biological sensor applications,” Sens. Actuators B, Chem., vol. 265, pp. 35–49, Jul. 2018. [Google Scholar]
- [83].Ciminelli C., Campanella C. M., Dell’Olio F., Campanella C. E., and Armenise M. N., “Label-free optical resonant sensors for biochemical applications,” Prog. Quantum Electron., vol. 37, no. 2, pp. 51–107, Mar. 2013. [Google Scholar]
- [84].Citartan M., Gopinath S. C. B., Tominaga J., and Tang T.-H., “Label-free methods of reporting biomolecular interactions by optical biosensors,” Analyst, vol. 138, no. 13, p. 3576, 2013. [DOI] [PubMed] [Google Scholar]
- [85].Maddali H., Miles C. E., Kohn J., and O’Carroll D. M., “Optical biosensors for virus detection: Prospects for SARS-CoV-2/COVID-19,” ChemBioChem, vol. 21, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Janczuk-Richter M.et al. , “Immunosensor based on long-period fiber gratings for detection of viruses causing gastroenteritis,” Sensors, vol. 20, no. 3, p. 813, Feb. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Murugan D., Bhatia H., Sai V. V. R., and Satija J., “P-FAB: A fiber-optic biosensor device for rapid detection of COVID-19,” Trans. Indian Nat. Acad. Eng., vol. 5, no. 2, pp. 211–215, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Saylan Y. and Denizli A., “Virus detection using nanosensors,” in Nanosensors for Smart Cities: Micro and Nano Technologies. Amsterdam, The Netherlands: Elsevier, 2020, pp. 501–511. [Google Scholar]
- [89].Nagy B., Al-Rawhani M. A., Cheah B. C., Barrett M. P., and Cumming D. R. S., “Immunoassay multiplexing on a complementary metal oxide semiconductor photodiode array,” ACS Sensors, vol. 3, no. 5, pp. 953–959, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ariffin E., Tan L., Karim N. A., and Heng L. Y., “Optical DNA biosensor based on square-planar ethyl piperidine substituted nickel (II) salphen complex for dengue virus detection,” Sensors, vol. 18, no. 4, p. 1173, Apr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Du K.et al. , “Multiplexed efficient on-chip sample preparation and sensitive amplification-free detection of Ebola virus,” Biosensors Bioelectron., vol. 91, pp. 489–496, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jeningsih et al. , “Sandwich-type DNA micro-optode based on gold–latex spheres label for reflectance dengue virus detection,” Sensors, vol. 20, no. 7, p. 1820, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hoffman T. L., Canziani G., Jia L., Rucker J., and Doms R. W., “A biosensor assay for studying ligand-membrane receptor interactions: Binding of antibodies and HIV-1 Env to chemokine receptors,” Proc. Nat. Acad. Sci. USA, vol. 97, no. 21, pp. 11215–11220, Oct. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pal S., Yadav A. R., Lifson M. A., Baker J. E., Fauchet P. M., and Miller B. L., “Selective virus detection in complex sample matrices with photonic crystal optical cavities,” Biosensors Bioelectron, vol. 44, pp. 229–234, Jun. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Roh C., Kim S. E., and Jo S.-K., “Label free inhibitor screening of hepatitis c virus (HCV) NS5B viral protein using RNA oligonucleotide,” Sensors, vol. 11, no. 7, pp. 6685–6696, Jun. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shpacovitch V. and Hergenröder R., “Surface plasmon resonance (SPR)-based biosensors as instruments with high versatility and sensitivity,” Sensors, vol. 20, no. 11, p. 3010, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Piliarik M., Vaisocherova H., and Homola J., “Surface plasmon resonance biosensing,” Methods Mol. Biol., vol. 503, pp. 65–88, Jul. 2007. [DOI] [PubMed] [Google Scholar]
- [98].Gorodkiewicz E. and Lukaszewski Z., “Recent progress in surface plasmon resonance biosensors (2016 to mid-2018),” Biosensors, vol. 8, no. 4, p. 132, Dec. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Nguyen H., Park J., Kang S., and Kim M., “Surface plasmon resonance: A versatile technique for biosensor applications,” Sensors, vol. 15, no. 5, pp. 10481–10510, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Singh P., “Surface plasmon resonance: A boon for viral diagnostics,” Mahatma Gandhi Central Univ., Motihari, India, Tech. Rep. PMC7157476, 2017. [Google Scholar]
- [101].Das C. M., Guo Y., Kang L., Ho H., and Yong K., “Investigation of plasmonic detection of human respiratory virus,” Adv. Theory Simul., vol. 3, no. 7, Jun. 2020, Art. no. 2000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mauriz E., “Recent progress in plasmonic biosensing schemes for virus detection,” Sensors, vol. 20, no. 17, p. 4745, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G. A., and Wang J., “Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection,” ACS Nano, vol. 14, no. 5, pp. 5268–5277, May 2020. [DOI] [PubMed] [Google Scholar]
- [104].Nag P., Sadani K., and Mukherji S., “Optical fiber sensors for rapid screening of COVID-19,” Trans. Indian Nat. Acad. Eng., vol. 5, no. 2, pp. 233–236, Jun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cheong J.et al. , “Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device,” Nature Biomed. Eng., vol. 4, no. 12, pp. 1159–1167, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].You M.et al. , “Ultrafast photonic PCR based on photothermal nanomaterials,” Trends Biotechnol., vol. 38, no. 6, pp. 637–649, Jun. 2020. [DOI] [PubMed] [Google Scholar]
- [107].You M., Cao L., and Xu F., “Plasmon-driven ultrafast photonic PCR,” Trends Biochem. Sci., vol. 45, no. 2, pp. 174–175, Feb. 2020. [DOI] [PubMed] [Google Scholar]
- [108].Lee C., Wang P., Gaston M. A., Weiss A. A., and Zhang P., “Plasmonics-based detection of virus using sialic acid functionalized gold nanoparticles,” Methods Mol. Biol., vol. 1571, pp. 109–116, Mar. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Park T. J., Hyun M. S., Lee H. J., Lee S. Y., and Ko S., “A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome,” Talanta, vol. 79, no. 2, pp. 295–301, Jul. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Vu C. A. and Chen W. Y., “Field-effect transistor biosensors for biomedical applications: Recent advances and future prospects,” Sensors, vol. 19, no. 19, p. 4214, Sep. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pachauri V., Estrela P., and Ingebrandt S., “Biologically sensitive field-effect transistors: From ISFETs to NanoFETs,” Essays Biochem., vol. 60, no. 1, pp. 81–90, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sakata T., “Biologically coupled gate field-effect transistors meet in vitro diagnostics,” ACS Omega, vol. 4, no. 7, pp. 11852–11862, Jul. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lee C.-S., Kim S., and Kim M., “Ion-sensitive field-effect transistor for biological sensing,” Sensors, vol. 9, no. 9, pp. 7111–7131, Sep. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dai X., Vo R., Hsu H.-H., Deng P., Zhang Y., and Jiang X., “Modularized field-effect transistor biosensors,” Nano Lett., vol. 19, no. 9, pp. 6658–6664, Sep. 2019. [DOI] [PubMed] [Google Scholar]
- [115].Zhang A., Zheng G., and Lieber C. M., “Nanowire field-effect transistor sensors,” in Nanowires. Cham, Switzerland: Springer, 2016, pp. 255–275. [Google Scholar]
- [116].Tran D., Pham T., Wolfrum B., Offenhäusser A., and Thierry B., “CMOS-compatible silicon nanowire field-effect transistor biosensor: Technology development toward commercialization,” Materials, vol. 11, no. 5, p. 785, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Syedmoradi L., Ahmadi A., Norton M. L., and Omidfar K., “A review on nanomaterial-based field effect transistor technology for biomarker detection,” Microchimica Acta, vol. 186, no. 11, pp. 1–23, Nov. 2019. [DOI] [PubMed] [Google Scholar]
- [118].Zhang X.et al. , “Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor,” Tsinghua Univ., Beijing, China, Tech. Rep., 2020. [Online]. Available: https://arxiv.org/abs/2003.12529 [Google Scholar]
- [119].Meyyappan M. and Lee J.-S., “Nanowire BioFETs: An overview,” in Nanowire Field Effect Transistors: Principles and Applications. New York, NY, USA: Springer, 2014, pp. 225–240. [Google Scholar]
- [120].Xu J.-J., “Analytical aspects of fet-based biosensors,” Frontiers Biosci., vol. 10, nos. 1–3, p. 420, 2005. [DOI] [PubMed] [Google Scholar]
- [121].Seo G.et al. , “Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor,” ACS Nano, vol. 14, no. 4, pp. 5135–5142, Apr. 2020. [DOI] [PubMed] [Google Scholar]
- [122].Farrow T., Laumier S., Sandall I., and Zalinge H. V., “Silicon thin film transistor-based aptamer sensor for COVID-19 detection,” Univ. Liverpool, Liverpool, U.K., Tech. Rep. CC BY 4.0, 2020, doi: 10.21203/rs.3.rs-74726/v1. [DOI] [Google Scholar]
- [123].Uhm M.et al. , “Ultrasensitive electrical detection of hemagglutinin for point-of-care detection of influenza virus based on a CMP-NANA probe and top-down processed silicon nanowire field-effect transistors,” Sensors, vol. 19, no. 20, p. 4502, Oct. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ishikawa F. N.et al. , “Label-free, electrical detection of the SARS virus N-protein with nanowire biosensors utilizing antibody mimics as capture probes,” ACS Nano, vol. 3, no. 5, pp. 1219–1224, May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kwon D. W., Lee R., Kim S., Mo H.-S., Kim D. H., and Park B.-G., “A novel fabrication method for co-integrating ISFET with damage-free sensing oxide and threshold voltage-tunable CMOS read-out circuits,” Sens. Actuators B, Chem., vol. 260, pp. 627–634, May 2018. [Google Scholar]
- [126].Zhang G.-J.et al. , “Silicon nanowire biosensor for highly sensitive and rapid detection of dengue virus,” Sens. Actuators B, Chem., vol. 146, no. 1, pp. 138–144, Apr. 2010. [Google Scholar]
- [127].Roberts A.et al. , “Graphene functionalized field-effect transistors for ultrasensitive detection of Japanese encephalitis and avian influenza virus,” Sci. Rep., vol. 10, no. 1, p. 14546, Sep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]