Supplemental Digital Content is available in the text.
Keywords: COVID-19, deep venous thrombosis, platelet count, pulmonary embolism, thromboelastography, venous thromboembolism
OBJECTIVES:
To test the hypothesis that relatively lower clot strength on thromboelastography maximum amplitude (MA) is associated with development of venous thromboembolism (VTE) in critically ill patients with COVID-19.
DESIGN:
Prospective, observational cohort study.
SETTING:
Tertiary care, academic medical center in Nashville, TN.
PATIENTS:
Patients with acute respiratory failure from COVID-19 pneumonia admitted to the adult medical ICU without known VTE at enrollment.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Ninety-eight consecutive critically ill adults with laboratory-confirmed COVID-19 were enrolled. Thromboelastography parameters and conventional coagulation parameters were measured on days 0 (within 48 hr of ICU admission), 3, 5, and 7 after enrollment. The primary outcome was diagnosis of VTE with confirmed deep venous thrombosis and/or pulmonary embolism by clinical imaging or autopsy. Twenty-six patients developed a VTE. Multivariable regression controlling for antiplatelet exposure and anticoagulation dose with death as a competing risk found that lower MA was associated with increased risk of VTE. Each 1 mm increase in enrollment and peak MA was associated with an 8% and 14% decrease in the risk of VTE, respectively (enrollment MA: subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.87–0.97; p = 0.003 and peak MA: SHR, 0.86; 95% CI, 0.81–0.91; p < 0.001). Lower enrollment platelet counts and fibrinogen levels were also associated with increased risk of VTE (p = 0.002 and p = 0.01, respectively). Platelet count and fibrinogen level were positively associated with MA (multivariable model: adjusted R2 = 0.51; p < 0.001).
CONCLUSIONS:
When controlling for the competing risk of death, lower enrollment and peak MA were associated with increased risk of VTE. Lower platelet counts and fibrinogen levels at enrollment were associated with increased risk of VTE. The association of diminished MA, platelet counts, and fibrinogen with VTE may suggest a relative consumptive coagulopathy in critically ill patients with COVID-19.
COVID-19 predisposes patients to developing thromboses with approximately 30% of critically ill patients developing a venous thromboembolism (VTE) despite prophylactic anticoagulation (1–4). Thromboelastography has been suggested as a clinical assay for evaluating the coagulopathy associated with COVID-19 (1, 5–7). Hypercoagulable thromboelastography parameters and fibrinolysis shut down have been reported in critically ill patients with COVID-19 (1, 2, 8–10). The association between hypercoagulable thromboelastography parameters and the development of clinically significant VTE, however, has not been established in prospective studies (11). Prior studies of thromboelastography in this population have been retrospective (selecting for patients with a clinical suspicion for coagulopathy), lacked serial measurement, or did not account for the competing risk of death (1, 5, 9).
In 2020, we conducted an exploratory pilot study examining the association between thromboelastography parameters and development of VTE (4). We found that measures indicating relatively less hypercoagulability, specifically lower clot strength measured by thromboelastography maximum amplitude (MA), were associated with development of VTE. Initially, this appeared paradoxical; however, when thromboelastography results were examined in the context of platelets, we found that lower platelet counts correlated closely with diminished MA and were associated with development of VTE (4). This pattern of consumption and the high rates of VTE raise the suspicion for disseminated intravascular coagulation (DIC); however, patients with COVID-19 rarely meet International Society on Thrombosis and Haemostasis (ISTH) criteria for overt DIC based on criteria, suggesting the existence of a similar but distinct pathology described as a COVID-19–associated coagulopathy (12–14).
Severe COVID-19 results in a profound hyperinflammatory state typically associated with broadly elevated platelets, fibrinogen, and thromboelastography parameters (1, 2, 9). The development of a COVID–associated consumptive coagulopathy superimposed on the severe inflammatory state of COVID-19 may result in relatively lower platelets, fibrinogen, and thromboelastography parameters despite being above or within the reference ranges. This study was conducted to test the hypothesis that relatively lower thromboelastography MA is associated with increased risk of VTE among critically ill patients with COVID-19.
MATERIALS AND METHODS
A prospective cohort study was conducted at a single-tertiary care academic medical center in Nashville, Tennessee, between April 29, 2020, and January 21, 2021, using methods previously described (4). Enrollment measurements from the initial 40 patients in this cohort were included in a pilot study published in 2020 (4). This work includes serial measurements from the initial 40 patients not previously reported and results from an additional 58 patients including 14 additional VTE events. This work was conducted as public health surveillance as defined in 45 Code of Federal Regulations 46.102(l)(2) and approved by the Vanderbilt Institutional Review Board (Committee HS3, Number 200591).
Study Population
We included patients with COVID-19 and respiratory failure using the following eligibility criteria: 1) greater than or equal to 18 years old; 2) admitted to an ICU; 3) positive severe acute respiratory syndrome coronavirus 2 reverse transcriptase-polymerase chain reaction during the index hospitalization; 4) chest imaging with pulmonary infiltrate; and 5) peripheral oxygen saturation below 90% on room air. Patients were enrolled within 48 hours of ICU admission. Patients diagnosed with acute venous or arterial thromboembolism on imaging prior to enrollment were excluded. All patients were followed for development of primary and secondary outcomes from enrollment to the earlier of hospital discharge or death.
Coagulation Parameters
Thromboelastography and coagulation tests were completed at enrollment within 48 hours of ICU admission (day 0) and on days 3, 5, and 7. Thromboelastography was measured on the TEG5000 Thromboelastograph Hemostasis Analyzer System (Haemonetics, Boston, MA) and included MA, α angle (α), reaction time (R), shear elastic modulus (complete clot strength) (G) parameter, clotting index, and percentage of clot lysis at 30 minutes (LY30) (1, 4). Coagulation tests included prothrombin time (PT)/international normalized ratio, activated partial thromboplastin time (aPTT), fibrinogen, platelet counts, and d-dimer. Follow-up measurements were completed if the patient remained in the hospital (in the ICU or elsewhere in the hospital) but were not completed if the patient had been discharged from the hospital. The maximum measurement between day 0 and 7 prior to the development of VTE was designated the peak measurement. Immature platelet fraction (IPF) and platelet counts from the same day were retrieved from the medical record when available (11). DIC scores were calculated based on ISTH guidelines (15, 16).
The study protocol for thromboelastography included the use of citrated kaolin with heparinase to remove the effect of anticoagulation. Occasionally, thromboelastography was completed without heparinase due to a laboratory processing error. Measures without heparinase for patients on prophylactic anticoagulation were included in the primary analysis and excluded in a sensitivity analysis. Measures run without heparinase for patients on therapeutic anticoagulation were excluded due to concerns about potentially inaccurate results.
Outcomes
The primary outcome was development of acute VTE during the index hospitalization. Evaluation for VTE was performed by treating clinicians without influence by the study protocol. Institutional guidelines recommended against routine screening for VTE, emphasizing symptom-guided assessment. VTE was defined as acute deep venous thrombosis (DVT) and/or pulmonary embolism (PE) diagnosed by imaging or at autopsy if death occurred during the index hospitalization. Classification of VTE followed the ISTH guidelines (4, 17). Secondary outcomes included the initiation of extracorporeal membrane oxygenation (ECMO), new renal replacement therapy (RRT), major bleeding, and death (18). All outcomes were assessed during the index hospitalization.
Anticoagulation and Antiplatelet Treatments
The study protocol did not control any treatment decisions. The treating clinicians determined all treatments including anticoagulation and antiplatelet therapies. Institutional guidelines recommended that patients chronically on direct oral anticoagulants were continued on their home medications or transitioned to therapeutic unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH); it was recommended that all other patients receive prophylactic anticoagulation with UFH or LMWH. Indications for initiation of therapeutic anticoagulation by treating clinicians included 1) diagnosis of VTE or another illness treated with therapeutic anticoagulation, 2) initiation of ECMO, or 3) frequent circuit clotting while on RRT. Some patients with a high clinical suspicion for VTE were started on therapeutic anticoagulation while awaiting confirmatory testing with de-escalation to prophylactic dosing if the evaluation returned negative.
Data Collection
Data from the electronic medical record were collected by research personnel and directly entered into an electronic case report form using a research electronic data capture platform (19, 20).
Statistical Analysis
The distributions of continuous exposure variables were compared between patients with and without VTE using Mann-Whitney U tests. The Fisher exact test was used to determine the association of categorical variables with development of VTE. A Fine-Gray subdistribution hazards model was fit controlling for the predetermined covariates of antiplatelet exposure and anticoagulation dose at the time of enrollment or peak MA (21). Patients diagnosed with VTE the day of enrollment were excluded in the time to event analysis because they were no longer at risk. Separate multivariable Fine-Gray subdistribution hazards models including minimum platelets and minimum fibrinogen within 7 days of ICU admission controlling for anticoagulation dose and exposure to antiplatelet medications were performed. The cumulative incidence function was predicted from the multivariable model for MA while holding covariates at their medians. Predicted relative subdistribution hazards for MA, platelets, and fibrinogen compared with their medians were built using univariable models. Separate sensitivity analyses were planned as follows: 1) excluding patients who underwent thromboelastography without heparinase, 2) excluding patients on therapeutic anticoagulation at the time of enrollment, and 3) excluding patients who did not undergo extremity duplex ultrasonography or CT pulmonary angiography (CTPA). The associations between MA, platelet counts, and fibrinogen were assessed using linear regression. Analyses were conducted with Stata/SE, Version 17.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
For the 98 enrolled patients, the median age was 59 years and 75 patients (76.5%) were male; 30 patients (30.6%) were on mechanical ventilation at time of enrollment with a median Fio2 of 95% (interquartile range [IQR], 70–100%); 12 patients (12.2%) were being treated with inhaled epoprostenol (Table 1). The median Sequential Organ Failure Assessment (SOFA) score was 5. At the time of enrollment, 83 patients (84.7%) were on prophylactic anticoagulation, 14 (14.3%) were on therapeutic anticoagulation, and 1 (1.0%) was not on anticoagulation due to a prolonged PT; 23 patients (23.5%) had been exposed to antiplatelet medications at enrollment (Table 1).
TABLE 1.
Clinical Characteristics and Laboratory Measures on Day 0 Grouped by Venous Thromboembolism
| Laboratory Measures | Reference Range | All Patients, n = 98 | VTE, n = 26 | No VTE, n = 72 | p |
|---|---|---|---|---|---|
| Age (yr) | 59 (53–66) | 60.5 (56–63) | 57.5 (51–67) | 0.33 | |
| Sex: male | 75 (76.5) | 21 (80.8) | 54 (75) | 0.79 | |
| Comorbidities | 78 (79.6) | 18 (69.2) | 60 (83.3) | 0.16 | |
| Diabetes | 53 (54.1) | 12 (46.2) | 41 (56.9) | 0.37 | |
| Hypertension | 64 (65.3) | 13 (50) | 51 (70.8) | 0.09 | |
| Heart disease | 20 (20.4) | 1 (3.9) | 19 (26.4) | 0.02 | |
| Chronic kidney disease | 12 (12.2) | 1 (3.9) | 11 (15.3) | 0.17 | |
| Prior transplant | 13 (13.3) | 3 (11.5) | 10 (13.9) | 1.0 | |
| Body mass index (kg/m2) (n = 95) | 32.4 (29.4–38.7) | 32.9 (29.8–36.7) | 32.2 (29.4–39.1) | 0.96 | |
| Anticoagulation | |||||
| Prophylactic | 83 (84.7) | 22 (84.6) | 61 (84.7) | 1.0 | |
| UFH | 21 (25.3) | 8 (36.4) | 13 (21.3) | 0.25 | |
| LMWH | 62 (74.7) | 14 (63.6) | 48 (78.7) | 0.25 | |
| Therapeutic | 14 (14.3) | 4 (15.4) | 10 (13.9) | 1.0 | |
| UFHa | 7 (50) | 3 (75) | 4 (40) | 0.11 | |
| LMWHb | 1 (7.1) | 1 (25) | 0 (0) | 0.11 | |
| Direct oral anticoagulant | 6 (42.9) | 0 (0) | 6 (60) | 0.11 | |
| No anticoagulationc | 1 (1.0) | 0 (0) | 1 (1.4) | 1.0 | |
| Exposure to antiplatelet agent | 23 (23.5) | 3 (11.5) | 20 (27.8) | 0.11 | |
| Aspirin | 16 (69.6) | 3 (100) | 13 (65) | 0.59 | |
| P2Y12 inhibitor | 1 (4.4) | 0 (0) | 1 (5) | 0.59 | |
| Dual antiplatelet therapy | 6 (26.1) | 0 (0) | 6 (30) | 0.59 | |
| Epoprostenol use | 12 (12.2) | 6 (23.1) | 6 (8.3) | 0.08 | |
| Extracorporeal membrane oxygenation (venovenous) | 2 (2.0) | 1 (3.9) | 1 (1.4) | 0.46 | |
| Level of oxygen support | |||||
| Mechanical ventilation | 30 (30.6) | 9 (34.6) | 21 (29.2) | 0.10 | |
| Bilevel positive airway pressure | 27 (27.6) | 11 (42.3) | 16 (22.2) | 0.10 | |
| High-flow nasal cannula oxygen | 31 (31.6) | 4 (15.4) | 27 (37.5) | 0.10 | |
| Low-flow nasal cannula oxygen | 10 (10.2) | 2 (7.7) | 8 (11.1) | 0.10 | |
| Sepsis-related Organ Failure Assessment score | 5 (4–8) | 6 (5–8) | 5 (4–7) | 0.04 | |
| Fibrinogen (mg/dL) (n = 96) | 188–450 | 678 (574.5–843.5) | 583 (452–787) | 688 (604–851) | 0.01 |
| Platelet count (×103/μL) (n =97) | 135–371 | 242 (169–300) | 174 (130–263) | 260 (196–313) | 0.002 |
| Creatinine (mg/dL) | 0.72–1.25 | 0.97 (0.77–1.47) | 0.97 (0.74–1.41) | 0.97 (0.78–1.5) | 0.83 |
| International normalized ratio (n = 96) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.94 | |
| Prothrombin time (s) (n = 96) | 11.9–14.5 | 14.6 (14.0–15.7) | 15.1 (14–15.7) | 14.6 (13.9–15.7) | 0.77 |
| Activated partial thromboplastin time (s) (n = 94) | 23.5–33.5 | 31.1 (28.2–35.3) | 30.2 (28.1–35) | 32.2 (28.3–35.4) | 0.73 |
| d-dimer (μg/mL fibrinogen equivalent units) | 0.27–0.49 | 2.16 (0.96–6.81) | 13.5 (1.48–20) | 1.61 (0.80–3.32) | < 0.001 |
| Disseminated intravascular coagulation score (International Society on Thrombosis and Haemostasis) | 2 (2–3) | 3 (2–3) | 2 (0–3) | 0.004 | |
| Thromboelastography (n = 95) | |||||
| Maximum amplitude (mm) | 50–70 | 67 (63.7–70.7) | 62.8 (57.8–67.2) | 68.1 (65–71.6) | < 0.001 |
| Alpha angle (degrees) | 53–72 | 73.2 (68.7–76.4) | 68.2 (63.2–73) | 74.6 (70.6–76.8) | < 0.001 |
| Reaction time (min) | 5–10 | 3.7 (3.2–4.8) | 3.7 (3–4) | 3.8 (3.2–4.8) | 0.44 |
| Shear elastic modulus (complete clot strength) (dyne/cm2)d | 4.5–11 | 10.2 (8.8–12.1) | 8.5 (6.8–10.3) | 10.7 (9.3–12.6) | < 0.001 |
| Clotting index | –3.0 to +3.0 | 3.4 (2.9–3.9) | 2.9 (1.9–3.4) | 3.6 (3.0–4.1) | < 0.001 |
| Percent lysis at 30 min (%) | 0–8 | 0.6 (0–1.4) | 0.9 (0.1–1.6) | 0.5 (0–1.2) | 0.16 |
LMWH = low-molecular-weight heparin (enoxaparin), UFH = unfractionated heparin, VTE = venous thromboembolism.
aTwo patients on venovenous extracorporeal membrane oxygenation, two were started on UFH after being diagnosed with VTE the day of enrollment, two patients were transitioned to UFH to continue home anticoagulation, and one patient was diagnosed with a non-ST-elevation myocardial infarction after enrollment.
bThe patient was initially on therapeutic anticoagulation while awaiting extremity venous duplex ultrasounds that returned as negative the day of ICU admission laboratories. The patient was transitioned back to prophylactic dosing of LMWH and developed an acute pulmonary embolism 26 d later.
cAnticoagulation was held due to a prolonged prothrombin time.
dShear elastic modulus (complete clot strength) = (5,000 × amplitude)/(100–amplitude) with a unit of force represented as dyne/cm2 (1 dyne/cm2 = 0.1 Pa).
Measures represent number (column percent) or median (interquartile range) as appropriate. n = 98 except where noted.
p values from two-sided Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables.
Outcomes
Among the 98 critically ill patients, 26 (26.5%) developed VTE, a median of 5.5 days after ICU admission (IQR, 3–11 d). Isolated DVT occurred in 17 patients (15 proximal, two distal) and nine patients developed PE (four central, two lobar, one segmental, and two subsegmental) (Table 2). Four of the nine patients with PE also had DVT. One patient was diagnosed with PE on autopsy; all other patients were diagnosed with VTE prior to death. The median SOFA score at enrollment was 1 point higher in the group who developed VTE (SOFA score = 6) compared with those without VTE (SOFA score = 5).
TABLE 2.
Outcome Data Grouped by Development of Venous Thromboembolism
| Outcome Variable | All Patients, n = 98 | VTE, n = 26 | No VTE, n = 72 | p |
|---|---|---|---|---|
| VTE | 26 (26.5) | 26 (100) | ||
| DVT | 17 (17.4) | 17 (65.4) | ||
| Proximal | 15 (88.2) | 15 (57.7) | ||
| Distal | 2 (11.8) | 2 (7.7) | ||
| Isolated PE | 5 (5.1) | 5 (19.2) | ||
| PE and proximal DVT | 4 (4.1) | 4 (15.4) | ||
| Renal replacement therapy | 27 (27.6) | 15 (57.7) | 12 (16.7) | < 0.001 |
| Major bleedinga | 11 (11.2) | 6 (23.1) | 5 (6.9) | 0.06 |
| Extracorporeal membrane oxygenation (venovenous) | 7 (7.1) | 5 (19.2) | 2 (2.8) | 0.01 |
| Inhospital death (%) | 50 (51.0) | 18 (69.2) | 32 (44.4) | 0.04 |
| Time from ICU admit to death (d) | 13 (8–17) | 14 (6–28) | 13 (9–16) | 0.61 |
| ICU length of stay (d) | 13 (7–21) | 23.5 (8–37) | 11.5 (7–15) | 0.01 |
| Hospital length of stay (d) | 14 (11–28) | 23.5 (8–42) | 13 (11–20) | 0.08 |
DVT = deep venous thrombosis, PE = pulmonary embolism, VTE = venous thromboembolism.
aClassified by International Society on Thrombosis and Haemostasis guidelines: nine patients were on therapeutic anticoagulation, one was on prophylactic anticoagulation, and one had a prolonged prothrombin time of unknown etiology; one of the patients on therapeutic anticoagulation received tissue plasminogen activator prior to the bleed due to clotting of the extracorporeal membrane oxygenation circuit.
Measures represent number (column percent) or median (interquartile range) as appropriate.
New RRT was initiated in 27 patients (27.6%) overall, including 15 of 26 patients (57.7%) with VTE and 12 of 72 (16.7%) without VTE (p < 0.001). Major bleeding occurred in 11 patients (11.2%) overall, including six of 26 patients (23.1%) with VTE and five of 72 (6.9%) without VTE (p = 0.06). The association between thromboelastography and conventional coagulation tests with bleeding events can be found in Table S1 (http://links.lww.com/CCX/A895). The median ICU and hospital lengths of stay were 13 and 14 days, respectively. Inhospital death occurred in 50 patients (51.0%) overall, including 18 of 26 patients (69.2%) with VTE and 32 of 72 (44.4%) without VTE (p = 0.04). Time from ICU admission to in hospital death was 13 days overall (IQR, 8–17 d), 14 days in patients with VTE (IQR, 6–28 d), and 13 days in patients without VTE (IQR, 9–16 d). There was no significant difference in enrollment or peak thromboelastography MA between patients who died during the hospitalization compared with survivors (median enrollment: 66.3 vs 67.3; p = 0.47 and peak: 70.5 vs 72.1; p = 0.33).
Association of Laboratory Parameters With Risk of VTE
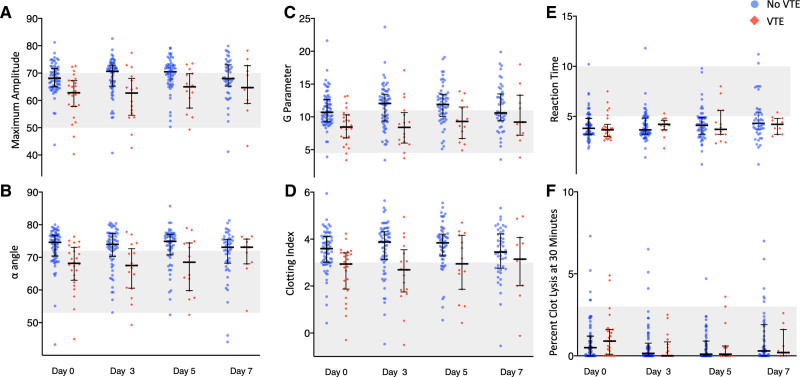
In laboratory studies obtained at enrollment, 28.4% of patients had an MA above the upper limit of normal, considered a hypercoagulable measurement in prior studies (1, 5, 9, 11). The primary outcome of VTE occurred in three of 27 patients (11.1%) with hypercoagulable enrollment MA compared with 23 of 68 (33.8%) with MA not in the hypercoagulable range. The median MA at enrollment was 67.0 overall, 62.8 for patients who developed VTE and 68.1 for patients who did not develop VTE (p < 0.001). The median MA for patients with VTE compared with those without VTE were 62.7 versus 70.7 on day 3, 65.0 versus 70.5 on day 5, and 64.7 versus 68 on day 7, respectively. Patients who developed VTE had lower measures of clotting on thromboelastography MA, α, G, and clotting index on both enrollment and serial measurements compared with those who did not develop VTE (Table 1 and Fig. 1); there was no difference in R or Ly30 between patients who developed VTE and those who did not. Enrollment and peak d-dimer were higher in patients who developed VTE compared with those who did not (p < 0.001). d-dimer was negatively correlated with thromboelastography MA with higher d-dimer associated with lower MA (R2 = 0.27; p < 0.001). Patients who developed VTE had higher DIC scores at the time of enrollment (p = 0.01), but only six patients (6.5%) met criteria for overt DIC defined as a DIC score of greater than 5 that was not associated with VTE (p = 0.49). Results were similar when the 40 patients with previously reported values were excluded (Table S2, http://links.lww.com/CCX/A895).
Figure 1.
Dot plot of thromboelastography measurements on days 0, 3, 5, and 7 after enrollment grouped by patients who did not develop a venous thromboembolism (VTE) (blue circles) and those who did develop a VTE (red diamond) during the index hospitalization. Each dot represents a single measurement. The median (bolded center line) and interquartile range (brackets) are also denoted for measurements at each time point. The shaded area represents the normal reference range. A, Maximum amplitude (mm); (B) alpha angle (α) (degrees); (C) shear elastic modulus (complete clot strength) (G parameter) (dynes/cm2); (D) clotting index; (E) reaction time (R) (s); (F) percentage of clot lysis at 30 min (Ly30) (one measure is not shown on day 3: Ly30 of 20.7 in a patient that did not develop a VTE).
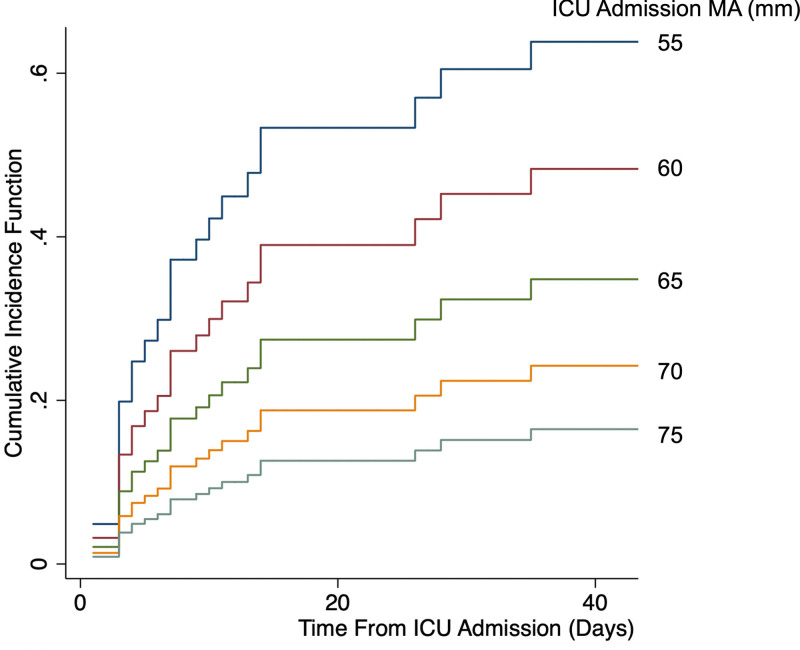
In the Fine-Gray subdistribution hazards model controlling for anticoagulation dose and exposure to antiplatelet medication, patients with lower enrollment MA had a higher predicted cumulative incidence of VTE (Fig. 2). Each 1 mm increase in enrollment and peak MA was associated with an 8% and 14% decrease in the risk of VTE, respectively (enrollment MA: subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.87–0.97; p = 0.003 and peak MA: SHR, 0.86; 95% CI, 0.81–0.91; p < 0.001; Fig. 2). The predicted relative subhazard for development of VTE compared with the median MA increased as enrollment MA decreased (Fig. S1, http://links.lww.com/CCX/A895).
Figure 2.
Predicted cumulative incidence function (CIF) for the development of venous thromboembolism at each level of ICU admission maximum amplitude (MA). The Fine-Gray model was used to predict the CIF with death as a competing risk and holding other covariates, anticoagulation dose and antiplatelet exposure, at their medians. The y-axis was truncated at 40 d, as there were no events after that time point.
Platelet count (R2 = 0.38; p < 0.001) and fibrinogen (R2 = 0.27; p < 0.001) were positively associated with MA using enrollment laboratory measurements (Fig. S2, http://links.lww.com/CCX/A895). Patients who developed VTE tended to have lower platelets, lower fibrinogen, and lower MA as shown in Supplemental Figure S2 (http://links.lww.com/CCX/A895). The predicted relative subhazard for development of VTE for fibrinogen and platelets compared with their respective medians increased with lower enrollment fibrinogen and platelet counts (Figs. S3 and S4, http://links.lww.com/CCX/A895). In a multivariable linear regression model accounting for the interaction between platelet count and fibrinogen, lower enrollment platelet count and fibrinogen were associated with lower enrollment MA (adjusted R2, 0.51; p < 0.001).
Patients who developed VTE had lower platelet counts and fibrinogen at enrollment compared with those never diagnosed with VTE (p = 0.002 and p = 0.01, respectively) (Table 1). Platelet counts and fibrinogen were lower in patients who developed VTE on every day of measurement except for day 7 platelets (Fig. S5, http://links.lww.com/CCX/A895). In separate competing risk regressions controlling for anticoagulation dose and exposure to antiplatelet medication, lower minimum platelet count and fibrinogen were associated with an increased risk of VTE (minimum platelets: SHR, 0.94; 95% CI, 0.89–0.99; p = 0.01 and minimum fibrinogen: SHR, 0.96; 95% CI, 0.94–0.97; p < 0.001).
Sensitivity Analyses
Separate sensitivity analyses were performed: 1) excluding measures run without heparinase, 2) excluding patients on therapeutic anticoagulation at the time of enrollment, and 3) excluding patients who did not undergo an extremity ultrasound or CTPA. Each sensitivity analysis was consistent with the primary analysis (Table S3, http://links.lww.com/CCX/A895). Results did not change substantively when the model was adjusted for patient age, sex, and enrollment SOFA score. IPF was measured during the index hospitalization for 16 patients (16.3%) and increased as platelet counts declined, although the number of available measures was limited (Fig. S6, http://links.lww.com/CCX/A895).
DISCUSSION
Relatively lower thromboelastography MA within 48 hours of ICU admission and at peak measurement are associated with development of VTE in critically ill patients with COVID-19 when accounting for the competing risk of death. The multiplatform randomized controlled trial of therapeutic anticoagulation in COVID-19 demonstrated that therapeutic anticoagulation for the entire critically ill COVID-19 population was not beneficial (22); whether subpopulations of critically ill COVID-19 patients may benefit from therapeutic anticoagulation remains unresolved. Hypercoagulable thromboelastography has been suggested as a tool to guide anticoagulation in this population (23, 24). This study suggests that elevated thromboelastography MA, termed hypercoagulable in prior studies, may not accurately identify patients at increased risk of VTE. Instead, relative decreases in thromboelastography MA may be seen in patients at increased risk of VTE.
Thromboelastography and conventional coagulation tests aim to quantify hypercoagulability to identify patients at increased risk of VTE. However, elevated thromboelastography measurements, described as hypercoagulable in previous work, were not associated with development of VTE in this cohort. Instead, decreased measures of clot strength (MA and G), rate of clot propagation (α), and overall measures of coagulation (clotting index) at enrollment and peak measurement were associated with development of VTE in the presence of decreased platelets and decreased fibrinogen. The time from test initiation to first evidence of thrombus formation (R) and the percentage of clot lysis (Ly30) were not associated with VTE. Patients who developed VTE had lower MA, α, G, and clotting index throughout the first 7 days of hospitalization (Fig. 1). The differences between thromboelastography parameters in patients who developed VTE and those who did not narrowed over time, likely due to the exclusion of measurements in patients who developed VTE and/or died over time; yet, lower MA, α, G, and clotting index were more common in patients who subsequently developed VTE compared with those who did not at nearly every timepoint. Prior retrospective studies have shown an association between hypercoagulable thromboelastography and the development of VTE; the differences between our results and those studies may be attributed to differences in methodology including retrospective study design selecting for patients with concern for coagulopathy and contributing to ascertainment bias, use of thromboelastography without heparinase or the TEG®6 machine, and use of the combined endpoints of VTE or death instead of controlling for the competing risk of death (5, 25–27).
To better understand the association between decreased measures of clotting and development of VTE, we considered the components of whole blood that influence thromboelastography. MA, G, and α are influenced by platelets and fibrinogen with MA and G being more influenced by platelets and α more influenced by fibrinogen (28, 29). Platelet counts and fibrinogen were lower in patients who developed VTE than in those who did not both at enrollment and minimum measurements. Platelet counts and fibrinogen were correlated with MA, and relatively lower measures may drive the association between decreased MA and development of VTE. Lower platelet counts and fibrinogen may be driven by decreased production or increased consumption through the development of micro and macrovascular thrombosis or immune clearance (30). It is possible, for example, that these patients did not mount an adequate immunologic response to develop appropriate elevations in platelets, fibrinogen, and MA and that decreased thromboelastography is identifying a more sick or vulnerable patient population. The association between VTE and increased need for RRT or ECMO and increased rates of mortality may support this hypothesis. However, the lack of an association between lower MA and in hospital death makes it more likely that lower MA is identifying patients at increased risk for VTE and that VTEs are associated with worse outcomes. Additionally, in a subset of patients with available measurements, higher IPFs were seen in patients with lower platelets, suggesting that increased consumption is more likely than decreased production. Unfortunately, the lack of thromboelastography measures at the time of hospital admission, prior to the potential consumptive process, prevents the conclusive identification of a consumptive coagulopathy.
Consistent with prior studies, few patients in this cohort met criteria for overt DIC based on the ISTH criteria (15, 16). Increasing DIC scores, however, were associated with VTE. This may be explained by COVID-19–associated hyperinflammation stimulating increases in platelet counts and fibrinogen (31). Of the four criteria for overt DIC, two include strict cutoff values for platelet counts and fibrinogen. While platelet counts and fibrinogen levels were lower overall in patients who developed VTE, the median values remained above the lower limit of the reference range. Elevated fibrinogen and platelet counts, and, therefore, elevated thromboelastography parameters, may be a physiologic response to systemic inflammation in this population. Relatively lower parameters in this context may mark the development of a consumptive coagulopathy similar to DIC (32). As can be seen in DIC, microvascular immunothrombosis associated with neutrophil extracellular traps have been identified in patients with COVID-19 and may explain the high rates of new RRT and the significant oxygen requirements in this population (33, 34). In contrast to overt DIC, prolongation of the PT/aPTT is often not seen. For this reason, a separate entity termed COVID–associated coagulopathy has been proposed (12). While consumption defined as declines in fibrinogen and platelet counts is common in patients with COVID-19, consumption defined as deficiencies in those parameters is not frequently observed due to thrombocytosis and hyperfibrinogenemia induced by inflammation. Our results suggest a relative consumption of fibrinogen and platelets, resulting in decreased measures of clotting on thromboelastography, correlating with increased rates of VTE.
Our study has limitations. The inclusion of patients on therapeutic anticoagulation at enrollment and the inclusion of thromboelastography parameters without the use of heparinase for patients on prophylactic anticoagulation may have biased our results. However, some patients on therapeutic anticoagulation at enrollment developed VTE supporting the need for these patients to be studied, and the effect of prophylactic doses of anticoagulation on thromboelastography is considered to be minimal. Furthermore, sensitivity analyses excluding each of these groups were reassuring. The thromboelastography parameters were visible to clinicians in this study and may have influenced decision-making regarding the evaluation for VTE (potential ascertainment bias). The lack of laboratory measures prior to ICU admission limited the ability of this study to conclusively determine the etiology of diminished thromboelastography MA, platelet counts, and fibrinogen. Despite being one of the largest reported cohorts measuring thromboelastography parameters in critically ill patients with COVID-19, the sample size was modest, limiting the precision of our findings and the number of covariates used in multivariable models.
CONCLUSIONS
Lower clot strength as measured by thromboelastography MA was associated with an increased risk of VTE. Platelet counts and fibrinogen levels positively correlated with MA and were also associated with the risk of VTE. While fibrinogen levels and platelet counts were typically within or above the normal limits in patients with COVID-19, they were both lower in those who developed VTE than those who did not. The association between decreased measures of clotting on thromboelastography and the development of VTE may suggest a relative consumptive coagulopathy similar to but distinct from DIC.
Supplementary Material
APPENDIX
The members of the IVY network involved in the study not included in the main list of authors are as follows: Jonathan D. Casey, M.D., Adrienne Baughman, Jakea Johnson, Kelsey N. Womack, Kimberly W. Hart.
Footnotes
Members of the Influenza and Other Viruses in the Acutely Ill (IVY) Network are listed in the Appendix.
Supplemental digital content content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grant from the Center for Disease Control and Prevention contract 75D30120C07637. Research electronic data capture was funded by UL1TR000445 from National Center for Advancing Translational Sciences/National Institutes of Health.
Dr. Marvi was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number R38HL143619. Dr. Grijalva was supported in part by National Institute for Allergy and Infectious Diseases K24 AI148459; he has received unrelated consulting fees from Pfizer, Sanofi, and Merck; and he has received research support from Sanofi-Pasteur, Campbell Alliance, the Centers for Disease Control and Prevention, NIH, The Food and Drug Administration, and the Agency for Healthcare Research and Quality. Dr. Rice is supported in part by the NIH U01HL123009; he has received unrelated consulting fees from Cumberland Pharmaceuticals, Cytovale, and Sanofi. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Disease Control and Prevention or the National Institutes of Health.
REFERENCES
- 1.Yuriditsky E, Horowitz JM, Merchan C, et al. : Thromboelastography profiles of critically ill patients with coronavirus disease 2019. Crit Care Med 2020; 48:1319–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maatman TK, Jalali F, Feizpour C, et al. : Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med 2020; 48:e783–e790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. : Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA 2020; 324:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marvi TK, Stubblefield WB, Tillman BF, et al. : Thromboelastography parameters and platelet count on admission to the ICU and the development of venous thromboembolism in patients with coronavirus disease 2019. Crit Care Explor 2021; 3:e0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortus JR, Manek SE, Brubaker LS, et al. : Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open 2020; 3:e2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavoni V, Gianesello L, Pazzi M, et al. : Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis 2020; 50:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng J, Fan BE, Chia YW: In response to “coagulopathy of coronavirus disease 2019.” Crit Care Med 2020; 48:e1159–e1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright FL, Vogler TO, Moore EE, et al. : Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg 2020; 231:193–203.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panigada M, Bottino N, Tagliabue P, et al. : Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020; 18:1738–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann J, Ergang A, Mason D, et al. : The role of TEG analysis in patients with COVID-19-associated coagulopathy: A systematic review. Diagnostics (Basel) 2021; 11:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem N, Atallah B, El Nekidy WS, et al. : Thromboelastography findings in critically ill COVID-19 patients. J Thromb Thrombolysis 2021; 51:961–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iba T, Levy JH, Connors JM, et al. : The unique characteristics of COVID-19 coagulopathy. Crit Care 2020; 24:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Li D, Wang X, et al. : Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asakura H, Ogawa H: COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol 2021; 113:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor FB, Jr, Toh CH, Hoots WK, et al. ; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH): Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001; 86:1327–1330 [PubMed] [Google Scholar]
- 16.Toh CH, Hoots WK; SSC on Disseminated Intravascular Coagulation of the ISTH: The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: A 5-year overview. J Thromb Haemost 2007; 5:604–606 [DOI] [PubMed] [Google Scholar]
- 17.Le Gal G, Carrier M, Castellucci LA, et al. ; ISTH CDE Task Force: Development and implementation of common data elements for venous thromboembolism research: On behalf of SSC Subcommittee on official Communication from the SSC of the ISTH. J Thromb Haemost 2021; 19:297–303 [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis: Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694 [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium: The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. : Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999; 94:496–509 [Google Scholar]
- 22.Goligher EC, Bradbury CA, McVerry BJ, et al. : Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med 2021; 385:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas AV, Lin KP, Stillson JE, et al. : A case series of thromboelastography-guided anticoagulation in COVID-19 patients with inherited and acquired hypercoagulable states. Case Rep Med 2021; 2021:5568982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Hajizadeh N, Shore-Lesserson L: The value of thromboelastography (TEG) in COVID-19 critical illness as illustrated by a case series. J Cardiothorac Vasc Anesth 2021. Oct 16. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunch CM, Thomas AV, Stillson JE, et al. : Preventing thrombohemorrhagic complications of heparinized COVID-19 patients using adjunctive thromboelastography: A retrospective study. J Clin Med 2021; 10:3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurbel PA, Bliden KP, Levy JH, et al. : Thrombogenicity markers for early diagnosis and prognosis in COVID-19: A change from the current paradigm? Blood Coagul Fibrinolysis 2021; 32:544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandel A, Patolia S, Looby M, et al. : Association of D-dimer and fibrinogen with hypercoagulability in COVID-19 requiring extracorporeal membrane oxygenation. J Intensive Care Med 2021; 36:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harr JN, Moore EE, Chin TL, et al. : Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg 2013; 74:756–762; discussion 762–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashuk JL, Moore EE, Sabel A, et al. : Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery 2009; 146:764–772; discussion 772–774 [DOI] [PubMed] [Google Scholar]
- 30.Sjöström A, Wersäll J, Warnqvist A, et al. : Platelet count rose while D-dimer levels dropped as deaths and thrombosis declined, an observational study on anticoagulation shift in COVID-19. Thromb Haemost 2021; 121:1610–1621 [DOI] [PubMed] [Google Scholar]
- 31.Becker RC: COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis 2020; 50:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iba T, Warkentin TE, Thachil J, et al. : Proposal of the definition for COVID-19-associated coagulopathy. J Clin Med 2021; 10:E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levi M, Scully M: How I treat disseminated intravascular coagulation. Blood 2018; 131:845–854 [DOI] [PubMed] [Google Scholar]
- 34.Nicolai L, Leunig A, Brambs S, et al. : Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020; 142:1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.