Abstract
Objectives:
To present consensus statements and supporting literature for plasma and platelet transfusions in critically ill children following non-cardiac surgery and critically ill children undergoing invasive procedures outside the operating room from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB).
Design:
Systematic review and consensus conference of international, multidisciplinary experts in platelet and plasma transfusion management of critically ill children.
Setting:
Not applicable.
Patients:
Critically ill children undergoing invasive procedures outside of the operating room or non-cardiac surgery.
Interventions:
None
Measurements and Main Results:
A panel of 10 experts developed evidence-based and, when evidence was insufficient, expert based statements for plasma and platelet transfusions in critically ill children following non-cardiac surgery or undergoing invasive procedures outside of the operating room. These statements were reviewed and ratified by the 29 TAXI-CAB experts. A systematic review was conducted using MEDLINE, EMBASE, and Cochrane Library databases, from inception to December 2020. Consensus was obtained using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Results were summarized using the Grading of Recommendations Assessment, Development, and Evaluation method. We developed 8 expert consensus statements focused on the critically ill child following non-cardiac surgery and 10 expert consensus statements on the critically ill child undergoing invasive procedures outside the operating room.
Conclusions:
Evidence regarding plasma and platelet transfusion in critically ill children in this area is very limited. The TAXI-CAB Consensus Conference developed eighteen pediatric specific consensus statements regarding plasma and platelet transfusion management in these critically ill pediatric populations.
MeSH Terms: plasma, platelet transfusion, consensus conference, critically ill child, invasive procedure, bleeding, guidelines
INTRODUCTION
Bleeding is common in the pediatric critical care setting. In an observational study of 450 patients admitted to a pediatric intensive care unit (PICU), 10% exhibited clinically significant bleeding at admission, and 8% developed clinically significant bleeding during their PICU course [1]. Bleeding was associated with longer time on vasopressor therapy and longer PICU length of stay.
Platelet and plasma transfusions are commonly administered to critically ill children before, during and after non-cardiac surgery and in those undergoing invasive procedures to improve the coagulation profile and to prevent periprocedural bleeding. However, the ideal platelet count, prothrombin time (PT), PT ratio (or international normalized ratio, INR) and activated partial thromboplastin time (aPTT) values at which procedures may be safely performed have not been identified and optimal transfusion thresholds following surgery are unknown. Plasma and platelet transfusion decisions affect both clinical outcomes and costs and require consideration of the risks and benefits involved. Children are at least twice as likely as adults to have febrile non-hemolytic or allergic transfusion reactions and many acute transfusion reactions develop following plasma and platelet transfusions [2,3]. Transfusion has been independently associated with increased 30-day mortality and postoperative infection in children undergoing non-cardiac surgery [4,5].
We sought to critically review the current literature on plasma and platelet transfusions in critically ill children having undergone non-cardiac surgery, as well as those undergoing invasive procedures outside the operating room (OR) and to develop evidence-based recommendations or expert-based consensus statements for plasma and platelet transfusion.
METHODS
The search strategy, item selection and recommendation generation used to identify and select references for systematic review and to develop recommendation are detailed in the general manuscript of TAXI-CAB [6]. Briefly, we searched Ovid MEDLINE®, Ovid EMBASE, and Cochrane Library (Wiley) from inception through December 2020 using a combination of medical subject heading terms and text words to define concepts of plasma or platelet transfusion, transfusion triggers, laboratory tests to assess efficacy of transfusion in children admitted to the pediatric intensive care unit (PICU) following non-cardiac surgery or undergoing invasive procedures outside of the OR. For articles selected for inclusion, reference lists and citing articles were selected from Scopus (Elsevier) and screened. Two reviewers independently reviewed all citations and performed data extraction and assessments of bias. Literature was reviewed for relevance to this subgroup. Research Electronic Data Capture (REDCap) hosted at Weill Cornell Medicine was used for standardized data extraction. We used a standardized data extraction form to construct evidence tables and graded the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [7].
Ten experts participated in the development of recommendations from this subgroup. A panel of 29 experts convened in an on-line format over 18 months to develop good practice statements, recommendations and, when evidence was lacking, expert consensus statements. Good practice statements are those in which there is high-level of certainty that the practice will do more good than harm, but there is little in the way of supporting literature evidence. Expert consensus statements are based on the expert opinion of the group, but in areas where research is likely needed. All statements from each subgroup were reviewed by the full panel of experts and voted on using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Agreement was defined a priori as >80% of all experts. The recommendations and statements are intended to apply to infants, children and adolescents. The Bleeding Assessment Scale in Critically Ill Children (BASIC) definition was used to describe bleeding severity and defined as no/minimal, moderate and severe [8]. Prophylactic transfusions are those prescribed to patients at risk of bleeding, whereas therapeutic transfusions are given to those with active bleeding.
RESULTS
Searching non-cardiac surgery and invasive procedures outside of the OR identified 3915 and 2781 abstracts, respectively. After duplicates were removed, a total of 3437 and 2395 abstracts were screened. Then, out of 183 and 94 full text manuscripts about non-cardiac surgery and invasive procedures outside the OR, respectively, we selected 30 and 12 papers for detailed review (see Figures 1 and 2). These papers underwent data extraction and assessment of bias in order to generate recommendations and statements (see Supplemental Data Tables 1 and 2); eighteen expert consensus statements were produced. The voting data, including the number of voting experts and median score, are provided for each statement and recommendation.
Figure 1:
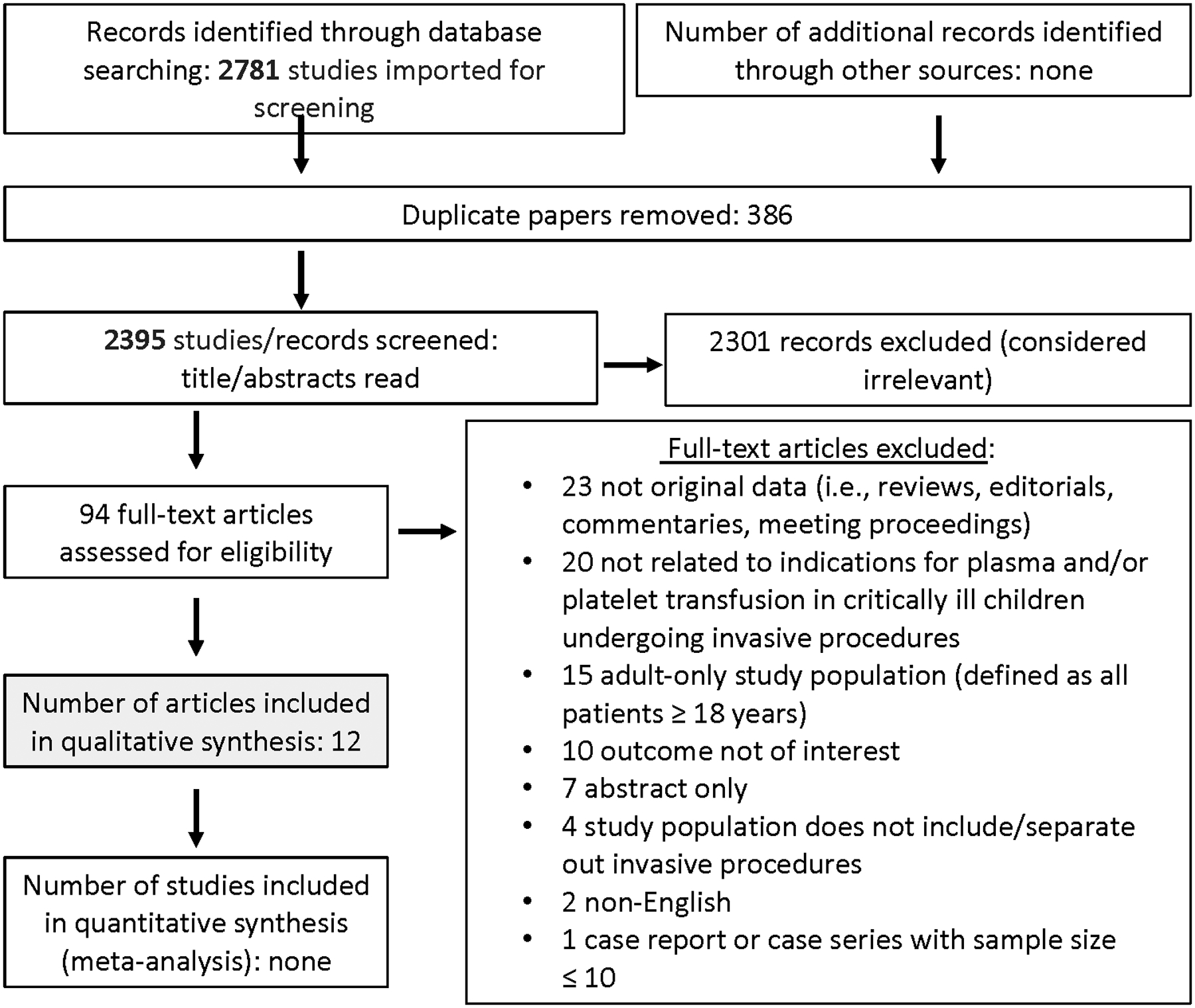
Papers Flow Chart for critically ill children undergoing invasive procedures outside of OR
Figure 2.
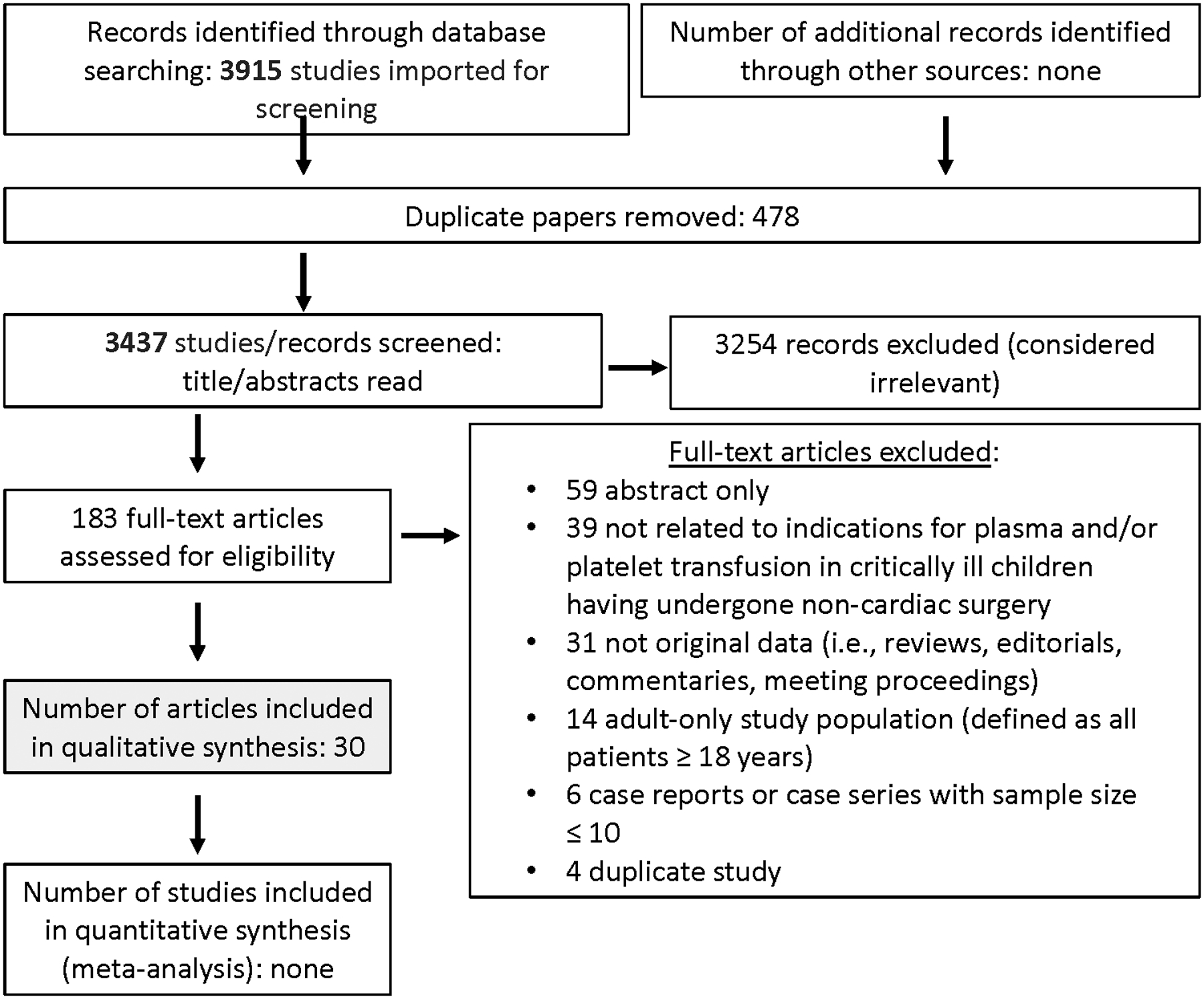
Papers Flow Chart for critically ill children following non-cardiac surgery
Indications for Plasma or Platelet Transfusions in Critically Ill Children Undergoing Invasive Procedures Outside the OR
Expert Consensus Statements
1. In critically ill pediatric patients undergoing invasive procedures outside of the OR, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma and platelet transfusion. 100% Agreement (n=22), Median 8 (IQR 8–9).
2. In critically ill pediatric patients undergoing invasive procedures outside of the OR (e.g., central line insertion, liver biopsy), plasma transfusion may not be beneficial if the INR is ≤ 1.5. 91% Agreement (n=22), Median 9 (IQR 8–9).
3. In critically ill pediatric patients undergoing invasive procedures outside of the OR, it is uncertain whether there is any benefit of plasma transfusions in correcting an INR between 1.6 and 2.4, and the risks of the plasma transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, other coagulation abnormalities, the type of procedure, the risks associated with procedure, and the risk of bleeding. 85% Agreement (n=20), Median 8 (IQR 8–9).
4. In pediatric patients undergoing invasive procedures outside of the OR in whom the INR is > 2.5, plasma transfusion might be considered, but should be balanced against the risks of the plasma transfusion and the clinical context. 91% Agreement (n=22), Median 8 (IQR 7.75–9).
Rationale:
For plasma transfusion, three articles were relevant and underwent data extraction and bias assessment. These included one prospective observational cross-sectional study of PICU patients who received plasma transfusion [9], and two retrospective studies, one cohort study looked at children (0–17 years) with acute liver failure (n=26) [10] and a case series of infants (0–3-months) undergoing percutaneous liver biopsy (n=63) [11].
Karam et al conducted a multicenter point-prevalence study that included 443 PICU patients who received plasma during the study period with a primary outcome of indication for the plasma transfusion [9]. Twelve percent of patients were given prophylactic plasma prior to a planned surgery or invasive procedure; it was not possible to separate out procedure types. In 281 of 443 critically ill children the median change in INR post plasma transfusion was −0.2 (IQR −0.4–0) and the median aPTT change was -5 seconds (IQR-17-+2). Clinically significant decreases in INR and aPTT following plasma transfusion were only seen when the INR was >2.5 and aPTT >60 seconds [9]. Stanworth et al similarly demonstrated minimal change in INR following plasma transfusion in children receiving plasma with a median INR reduction of 0.1 and PT reduction of 1.2 seconds [12]. This correlates with studies in adults that have demonstrated plasma is ineffective in correcting minor coagulation test abnormalities [13,14].
Liver biopsy
Chapin et al reviewed 26 cases of children with acute liver failure who underwent liver biopsy with an INR ≥1.5 [10]. Median INR prior to biopsy was 2.1 (IQR 1.73–2.9), and 80% of these patients received plasma prior to the procedure. One patient with a pre-biopsy INR of 3.1 who received plasma during the procedure experienced biopsy-associated bleeding requiring blood transfusion [10].
Azzam et al reviewed 66 percutaneous liver biopsies performed in 63 infants (0–3 months) [11]. Three infants had INR >1.5 and received plasma. Three patients with normal platelet count and coagulation studies developed significant bleeding (drop in hemoglobin (Hb) >2.0g/dL) following biopsy.
A further discussion of the considerations in the transfusion of plasma in critically ill children with acute liver failure and/or following liver transplantation is available in this supplement [15].
5. In critically ill pediatric patients undergoing invasive procedures outside of the OR, prophylactic platelet transfusions may not be beneficial when the platelet count is > 50 ×109/L (50,000/mm3). 83% Agreement (n=23), Median 8 (IQR 7–9).
6. In critically ill pediatric patients undergoing invasive procedures outside of the OR, it is uncertain whether there is any benefit of prophylactic platelet transfusion when the platelet count is between 20 and 50 × 109/L (20–50,000/mm3). The risks of the platelet transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, any other coagulation abnormalities, the procedure, the risks associated with procedure, and the risk of bleeding. 86% Agreement (n=22), Median 8 (IQR 7–9).
7. In critically ill pediatric patients undergoing invasive procedures outside of the OR, platelet transfusion might be considered when the platelet count is ≤ 20 × 109/L (20,000/mm3). 87% Agreement (n=23), Median 8 (IQR 7–9).
8. In critically ill pediatric patients, prophylactic platelet transfusion may not be beneficial prior to minor procedures such as peripheral intravenous cannula insertion, central line catheter removal, bone marrow aspirate and bone marrow biopsy. 85% Agreement (n=20), Median 7 (7–8.75).
Rationale:
For platelet transfusion, ten articles were relevant and underwent data extraction and bias assessment (one article is represented in both groups). There were two prospective cohort and eight retrospective cohort studies.
Four studies examined platelet transfusion and/or thrombocytopenia in critically ill children undergoing invasive procedures other than lumbar puncture (LP). One prospective single-center study examined consecutive PICU patients who received platelet transfusion (n= 60), with the objectives of characterizing the indications for platelet transfusion and to review for adverse events [4]. Four patients (7%) received platelet transfusions prior to an invasive procedure, with a mean pre-transfusion platelet count 85±32 ×109/L and post-transfusion count of 147±54 ×109/L. Platelet transfusions were associated with adverse outcomes including increased frequency and severity of organ dysfunction, sepsis, nosocomial infections, prolonged ICU stay and increased mortality.
One prospective cohort multicenter study examined transfusion indications, transfusion thresholds, PICU length of stay, mechanical ventilator-free days and mortality in patients admitted to a mixed cardiac/non-cardiac PICU (n= 559) who received platelets [16]. The indication for platelet transfusion in 17% was preparation for surgery or an invasive procedure; however, procedure types were not subdivided, and pre- and post-transfusion platelet increments and bleeding outcomes were not presented.
One retrospective single center cohort study examined patient characteristics and indications for platelet transfusion in a non-cardiac PICU (n=232) and described clinical outcomes [5]. Seven patients received a platelet transfusion prior to an invasive procedure. Platelet increment, bleeding outcomes and procedure types were not reported.
The remaining study was a retrospective cohort of major complications in acute liver failure in children 0–17 years undergoing liver biopsy (n = 26) [10]. Median (IQR) platelet count prior to biopsy was 147 (94, 188) x109/L. Two children received a platelet transfusion prior to percutaneous liver biopsy (platelet counts of 32 and 52 ×109/L). Three patients who underwent trans-jugular biopsy had a pre-biopsy platelet count of <50 ×109/L but did not receive platelet transfusions as they were deemed not necessary. These patients underwent biopsy without complication.
9. In critically ill pediatric patients undergoing elective LP, it is uncertain whether there is any benefit of prophylactic platelet transfusion when the platelet count is 20 to 50 × 109/L (20–50,000/mm3). The risks of the platelet transfusion should be balanced against the clinical condition, the presence of bleeding symptoms, other coagulation abnormalities, the procedure, the risks associated with procedure, and the risk of bleeding. 90% Agreement (n=20), Median 8 (IQR 7–8.75).
10. In critically ill pediatric patients undergoing elective LP, prophylactic platelet transfusion might be considered when the platelet count is <20 × 109/L (20,000/mm3). 84% Agreement (n=19), Median 8 (IQR 7–9).
Rationale:
Six studies on platelet transfusion dealt with LP in the setting of thrombocytopenia associated with hematologic malignancy. Though not all the children were critically ill, the data were extrapolated to apply to critically ill children. Five single-center retrospective cohort studies (in six publications) were reviewed with sample sizes of 54, 264, 266, 440 and 958 patients [17–22].
Four studies with a combined total of 1,716 children undergoing 15,315 LPs evaluated for serious procedural complications and no cases of serious neurological or hematological complications were identified [17–21]. Two studies reported outcomes for LPs performed with a platelet count of <50 ×109/L (651 children), <20 ×109/L (291 children) and <10 ×109/L (54 children) with no serious complications reported [18,20]. Platelet transfusion practice varied between studies, from not routinely administering platelets prior to LP [20], to transfusing for platelet count <50 ×109/L (15), <30 ×109/L (17), or >50 ×109/L [17,22].
Five studies examined the relationship between platelet count and traumatic LP (ie 500 red blood cells (RBC)s per high powered field [16] or ≥ 10 RBC/μL of CSF) [19,21,22]. One study reported no relationship between traumatic tap and platelet count [21] while the two largest studies reported that a traumatic tap was associated with a pre-procedure platelet count of <100 ×109/L [19,22]. The final retrospective study reported that the pre-LP platelet count <50 ×109/L, versus not, was associated with five-fold greater odds of traumatic tap (OR 95%CI 3.1 to 9.1, z statistic 6.2, p < 0.0001) [17].
Following non-cardiac surgery
11. In critically ill pediatric patients who have undergone non-cardiac surgery and have no active bleeding or minimal bleeding, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma and platelet transfusion. 100% Agreement (n=24), Median 8, IQR 8–9.
12. In critically ill pediatric patients who have undergone non-cardiac surgery and have moderate bleeding, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma and platelet transfusion. 96% Agreement (n=24), Median 8, IQR 7.25–9.
Rationale:
There is no strong high-quality evidence on the indications for plasma and platelet transfusion in critically ill pediatric patients undergoing non-cardiac surgery. Supplemental Table 2 describes the characteristics and risk of bias of the 28 studies included in this analysis. Among the 3915 studies screened that evaluated plasma transfusion in this population (Figure 2), five small randomized controlled trials (RCTs) [23–27] with several limitations were included. Two large point prevalence studies assessed plasma and platelet transfusion practices in large cohorts of critically ill children but did not independently report results for non-cardiac surgical patients [9,16]. The remaining analyzed literature included historical registry studies [28–30], a case series [31], an audit [32], prospective cohort studies [12,33–37] and retrospective cohort studies [38–46].
13. In critically ill infants or children who have undergone non-cardiac surgery and who are not actively bleeding or have minimal bleeding, routine coagulation testing may not be beneficial. 87% Agreement (n=23), Median 8, IQR 8–9.
Rationale:
Multiple studies have demonstrated that neither preoperative bleeding history nor routine laboratory screening with PT/INR, or aPTT, are reliable predictors of postoperative bleeding [47–50]. A detailed personal and family bleeding history, previous excessive post-operative bleeding, correlation with current medications (e.g. anti-platelet agents), knowledge regarding bleeding risks associated with the surgery and clinical assessment of hemostasis are more important than test results.
14. In critically ill pediatric patients who have undergone non-cardiac surgery and who are not actively bleeding or have minimal bleeding, if any coagulation testing is done and an abnormality is noticed, prophylactic plasma transfusion based solely on abnormal PT, INR or aPTT values may not be beneficial and we encourage formal evaluation by hematology or transfusion medicine in order to ascertain the cause. 86% Agreement (n=22), Median 8, IQR 7–9.
Rationale:
Prophylactic plasma transfusion based solely on abnormal coagulation parameters is not recommended in patients with minimal or no bleeding. RCTs investigating the efficacy of prophylactic plasma transfusion across a broad range of patient ages and clinical settings are limited and have failed to consistently demonstrate benefit [51,52]. Numerous studies in adults have shown that patients transfused prior to invasive procedures for mild or moderate coagulation abnormalities are unnecessarily transfused, that plasma transfusion results in minimal or no correction of PT or INR and, most importantly, result in high levels of inappropriate plasma use in both adults and children [12]. Karam et al corroborated this in critically ill children in a large point-prevalence study in which over one third of plasma transfused with no formal indication resulted in a minimal INR reduction [9]. In a systematic review evaluating clinical effectiveness and safety of prophylactic plasma transfusion in adult patients with coagulation test abnormalities who required non-cardiac surgery or invasive procedures, Huber et al reported uncertainty for the utility and safety of prophylactic plasma use and neither support nor oppose use of prophylactic plasma due to very low-quality evidence reported for the outcomes [53]. When evaluating plasma transfusion compared with no plasma transfusion, no significant association was shown for major bleeding, need for transfusion or harmful effects. When different triggers for plasma transfusion (INR < 1.6 versus 1.6 to 3) were compared, there was no significant association shown between a difference in major bleeding or in harmful effects from plasma transfusion.
Given the lack of evidence to support the development of a recommendation, in cases of abnormal coagulation testing in patients who have no/minimal bleeding, we encourage the consultation of hematology and/or transfusion medicine specialists before prophylactic plasma transfusion is prescribed.
15. In critically ill pediatric patients who have undergone non-cardiac surgery and who have moderate bleeding, coagulation testing might be considered to ascertain the likely etiology of bleeding and to permit appropriate targeted intervention. 87% Agreement (n=23), Median 8, IQR 7–9.
Rationale:
If bleeding and abnormal coagulation tests persist in a post-surgical patient with moderate bleeding, coagulation testing might be considered, to promptly investigate etiology and initiate treatment of bleeding; formal evaluation by hematology or transfusion medicine sub-specialists is suggested. Surgical re-exploration may be necessary based on the amount, rate of bleeding, presumed location or source of bleeding, and potential for a surgical cause [54]. While clinical judgment must be used in children with severe bleeding, a formal recommendation could not be supported by the available literature. Children with hemorrhagic shock should be treated with a balanced resuscitation strategy as described by the TAXI-CAB trauma group [55].
1.16. In critically ill pediatric patients who have undergone non-cardiac surgery and who have moderate bleeding, plasma transfusion might be considered to correct abnormal PT, INR or aPTT values that are ≥ 2.0 times the reference value (as compared to baseline for patient if available or upper limit of normal for local institution). The risk of plasma transfusion should be balanced against the clinical condition, timing from surgery, type of surgery, site of bleeding and the risks associated, pattern or trajectory of bleeding, as well as consideration of coagulation parameters such as plasma fibrinogen level, platelet count and viscoelastic monitoring results. In addition to clinical evaluation for bleeding at the surgical site, we suggest formal evaluation by experts in hematology or transfusion medicine if the etiology of bleeding is unclear. 87% Agreement (n=23), Median 8, IQR 8–9.
Rationale:
There is a paucity of evidence to inform best therapeutic plasma transfusion practice in general as well as in critically ill pediatric patients who have undergone non-cardiac surgery. Numerous adult guidelines for plasma use exist but contain significant variations in guidance and quality of the evidence base [53]. Clinical studies evaluating plasma transfusion strategies are lacking [55] and it is uncertain whether plasma truly improves clinical or laboratory parameters for patients with mild to moderately abnormal coagulation tests [57,58]. Available expert consensus guidelines in adults do not advise plasma be used to treat bleeding or as prophylaxis in patients with INR values ranging from 1.6 to 2.0, due to lack of high-quality data that support its efficacy and given the known risks of complications from plasma transfusion [12,14,49,52,59]. In children, some have suggested that when there is ongoing bleeding and PT/INR or aPTT are more than 1.5 times reference value, plasma transfusion to replete coagulation factor deficiency may be indicated [50]. However, Karam et al reported that plasma transfusion significantly improved INR values only in patients with a baseline INR of >2.5 [9].
Given the available information and lack of data, plasma transfusion may be considered in critically ill pediatric patients who have undergone non-cardiac surgery and have INR value greater than 2.0 and/or clinical status of bleeding is worsening. The primary indication for plasma products is repletion of coagulation factors in the context of abnormal hemostasis associated with clinically relevant bleeding. Plasma should be considered only if the benefit of correcting abnormal hemostasis (reduction of bleeding) is judged to outweigh the risks of transfusion and a safer alternative is unavailable; plasma should not be used for treatment of hypovolemia. Plasma products are only partially effective to replete coagulation factors, have short duration of action, may cause hypervolemia and lead to complications such as transfusion-associated circulatory overload (TACO) or transfusion-related acute lung injury (TRALI) [60].
17. In critically ill pediatric patients who have undergone non-cardiac surgery and have no active or minimal bleeding, platelet transfusion might be considered when the platelet count is ≤ 20 ×109/L (20,000/mm3). The risk of platelet transfusion should be balanced against the clinical condition, timing from surgery, type of surgery and potential risk of bleeding. 96% Agreement (n=23), Median 8, IQR 7–9.
Rationale:
In critically ill children, including patients in ICU after non-cardiac surgery, the majority of platelet transfusions are given prophylactically to prevent bleeding [16]. No high-quality evidence exists to guide best practice for platelet transfusion in the general post-surgical pediatric population. The data for prophylactic platelet transfusion thresholds in children come from relatively stable oncology patients with hypoproliferative thrombocytopenia due to bone marrow suppression, a population for whom platelet transfusion thresholds of 10–20 ×109/L have been proven effective to minimize bleeding but is not comparable to children who have just undergone surgery and are in an ICU [61]. In premature neonates, who are generally not admitted to PICU, one large RCT provided high-quality evidence for appropriate platelet transfusion thresholds and reported a significantly higher rate of death or major bleeding in patients who received platelet transfusions at a threshold of 50 ×109/L versus 25 ×109/L [62]. In adults, the American Association of Blood Banks recommends prophylactic platelet transfusion at 10 ×109/L for hospitalized patients with hypoproliferative thrombocytopenia and at 50 ×109/L for patients with major non-neuraxial surgery (Grade: weak recommendation; very-low-quality evidence) [63].
The decision to transfuse platelets after non-cardiac surgery must factor in clinical context as well as possible side effects of platelet transfusion [4,5]. Given the paucity of evidence and available recommendations in other patient populations, our expert consensus is that transfusion might be considered when thrombocytopenia is at a threshold of 20 ×109/L in a non-bleeding or minimally bleeding pediatric patient having undergone non-cardiac surgery.
18. In critically ill pediatric patients who have undergone non-cardiac surgery and have moderate bleeding, platelet transfusion might be considered when the platelet count is ≤ 50 ×109/L (50,000/mm3). The risk of platelet transfusion should be balanced against the clinical condition, timing from surgery, type of surgery, pattern or trajectory of bleeding, site of bleeding and associated risk, and other coagulation parameters. 96% Agreement (n=23), Median 8, IQR 7–9.
Rationale:
When assessing the need for platelet transfusion in the context of postoperative bleeding, clinical judgement is warranted and factors to consider might include whether there is an acquired or inherited platelet function defect, coagulopathy involving platelet consumption such as disseminated intravascular coagulopathy (DIC), or bleeding at the surgical site. Generally, a patient with a platelet count >50 ×109/L does not require treatment unless additional issues that might impair hemostasis and cause further blood loss are expected. Platelet transfusion might be considered in a patient with moderate bleeding and a platelet count <50 ×109/L. Transfusion regardless of platelet count or at a higher threshold may be appropriate depending on the clinical context. No recommendation for severe bleeding could be made based on the available literature. As referenced above, children with hemorrhagic shock following non-cardiac surgery should be treated with a balanced resuscitation strategy as outlined by the TAXI-CAB trauma group [55].
DISCUSSION
The paucity of evidence does not allow us to make recommendations to guide plasma and platelet transfusion in children following non-cardiac surgery or undergoing invasive procedures outside of the OR. However, the 18 expert consensus statements are meant to assist practitioners in their consideration of transfusion in these patient populations.
Many considerations are involved in the decision to transfuse plasma or platelets in children following non-cardiac surgery or undergoing invasive procedures outside of the OR. Conventional coagulation testing (PT, aPTT, INR) is used in the statements. However, other tests of coagulation, such as viscoelastic testing, may be available at some institutions. The utility of assays assessing coagulation is discussed elsewhere in this supplement [64]. The type of surgery may also affect one’s decision to transfuse. Though we have grouped all non-cardiac surgeries together to separate those using cardiopulmonary bypass from those that do not, there are certainly bleeding and thrombosis associated with surgeries involving grafts, including renal transplantation and plastic surgery [65,66]. Whereas liver transplantation is addressed in a separate manuscript [15], future development of transfusion strategies should consider developing different recommendations for different types of non-cardiac surgery.
CONCLUSIONS
There is insufficient high-quality evidence to guide plasma and platelet transfusion practice in critically ill pediatric patients who have undergone non-cardiac surgery or are undergoing invasive procedures outside the operating room. The expert consensus statements proposed are derived mostly from consensus panel expertise, adult data and professional guidelines and from limited available pediatric literature.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all members of TAXI-CAB for their support and input, especially during the COVID-19 pandemic. In addition, we thank the Chaire Héma-Québec-Bayer en médecine transfusionnelle de l’Université de Montréal, the Society for the Advancement of Blood Management, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, the International Society of Blood Transfusion, the Society for Critical Care Medicine, and the AABB for their support.
Financial Support:
The Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute under award number R13 HL154544-01.
APPENDIX 1. Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members
(* for executive committee) Co-chairs: Marianne E. Nellis, MD, MS*, Weill Cornell Medicine, New York, NY, and Robert I. Parker, MD*, Renaissance School of Medicine, State University of New York at Stony Brook, Stony Brook, NY; Content Experts: Section 1. Laboratory assays used to assess need for plasma and/or platelet transfusions: Scot T. Bateman, MD*, University of Massachusetts Medical School, Worcester, MA, Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Kenneth E. Remy, MD, MHSc, MSCI, Washington University of St. Louis, St. Louis, MO, Katherine Steffen, MD, Stanford University, Palo Alto, CA; Section 2. Traumatic brain injury and intracranial hemorrhage: David F. Bauer, MD, MPH, Baylor College of Medicine, Houston, TX, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA; Section 3. Following cardiopulmonary bypass: Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Sitaram Emani, MD, Harvard Medical School, Boston, MA, Juan Ibla, MD, Harvard Medical School, Boston, MA, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 4. Supported by extracorporeal membrane oxygenation: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 5. Following severe trauma: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 6. With oncologic diagnosis or following hematopoietic stem cell transplantation: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Lani Lieberman, MD, University of Toronto, Canada, Simon J. Stanworth, MD, University of Oxford, UK, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 7. With acute liver failure or following liver transplantation: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 8. Following non-cardiac surgery: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Marisa Tucci, MD, Université de Montréal, Montreal, QC, Canada, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 9. Invasive procedures outside of the operating room: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 10. Sepsis and/or disseminated intravascular coagulation: Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Simon J. Stanworth, MD, University of Oxford, UK, Katherine Steffen, MD, Stanford University, Palo Alto, CA, Stacey L. Valentine, MD, MPH*, University of Massachusetts Medical School, Worcester, MA; Section 11. Product processing and selection: Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH; Evidence-based medicine: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Diana Delgado and Michelle Demetres, Weill Cornell Medicine, New York, NY; Implementation science: Katherine Steffen, MD, Stanford University, Palo Alto, CA.
Footnotes
Copyright Form Disclosure: Dr. Crighton disclosed that she is employed by Royal Children’s Hospital of Melbourne, Australia and that she was the Australian and New Zealand Society of Blood Transfusion President. Drs. Russel and Nellis received support for article research from the National Institutes of Health. Dr. Haas received funding from Octapharma. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.White LJ, Fredericks R, Mannarino CN, Janofsky S, Faustino EVS. Epidemiology of Bleeding in Critically Ill Children. J Pediatr 2017; 184:114–119. [DOI] [PubMed] [Google Scholar]
- 2.Oakley FD, Woods M, Arnold S, Young PP. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion 2015; 55:563–570. [DOI] [PubMed] [Google Scholar]
- 3.Vossoughi S, Perez G, Whitaker BI, Fung MK, Stotler B. Analysis of pediatric adverse reactions to transfusions. Transfusion 2018; 58:60–69. [DOI] [PubMed] [Google Scholar]
- 4.Du Pont-Thibodeau G, Tucci M, Robitaille N, Ducruet T, Lacroix J. Platelet Transfusions in Pediatric Intensive Care. Pediatr Crit Care Med 2016; 17:e420–429. [DOI] [PubMed] [Google Scholar]
- 5.Saini A, West AN, Harrell C, et al. Platelet Transfusions in the PICU: Does Disease Severity Matter? Pediatr Crit Care Med 2018; 19:e472–e478. [DOI] [PubMed] [Google Scholar]
- 6.Nellis ME, Karam O, Valentine S, et al. Executive Summary of Recommendations and Expert Consensus for Plasma and Platelet Transfusion Practice in Critically Ill Children: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008, 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nellis ME, Tucci M, Lacroix J, et al. Bleeding Assessment Scale in Critically Ill Children (BASIC): Physician-Driven Diagnostic Criteria for Bleeding Severity. Crit Care Med 2019; 47:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karam O, Demaret P, Shefler A, et al. Indications and Effects of Plasma Transfusions in Critically Ill Children. Am J Respir Crit Care Med 2015; 191:1395–1402. [DOI] [PubMed] [Google Scholar]
- 10.Chapin CA, Mohammad S, Bass LM, Taylor SA, Kelly S, Alonso EM. Liver Biopsy Can Be Safely Performed in Pediatric Acute Liver Failure to Aid in Diagnosis and Management. J Pediatr Gastroenterol Nutr 2018; 67:441–445. [DOI] [PubMed] [Google Scholar]
- 11.Azzam R, Alonso EM, Emerick KM, Whitington PF. Safety of percutaneous liver biopsy in infants less than three months old. J Pediatr Gastroenterol Nutr 2005; 41:639–643. [DOI] [PubMed] [Google Scholar]
- 12.Stanworth SJ, Grant-Casey J, Lowe D, et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 2011; 51:62–70. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006; 46:1279–1285. [DOI] [PubMed] [Google Scholar]
- 14.Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol 2006; 126:133–139. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman L, Karam O, Stanworth SJ, et al. Plasma and Platelet Transfusion Strategies in Critically Ill Children with Malignancy, Acute Liver Failure and/or Liver Transplantation, or Sepsis: From the Transfusion and Anemia Expertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nellis ME, Karam O, Mauer E, et al. Platelet Transfusion Practices in Critically Ill Children. Crit Care Med 2018; 46:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung HH, Morjaria S, Frame J, et al. Rethinking the need for a platelet transfusion threshold of 50 × 10(9) /L for lumbar puncture in cancer patients. Transfusion 2020; 60:2243–2249. [DOI] [PubMed] [Google Scholar]
- 18.Foerster MV, Pedrosa Fde P, da Fonseca TC, Couceiro TC, Lima LC. Lumbar punctures in thrombocytopenic children with cancer. Paediatr Anaesth 2015; 25:206–210. [DOI] [PubMed] [Google Scholar]
- 19.Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. JAMA 2002; 288:2001–2007. [DOI] [PubMed] [Google Scholar]
- 20.Howard SC, Gajjar A, Ribeiro RC, et al. Safety of lumbar puncture for children with acute lymphoblastic leukemia and thrombocytopenia. JAMA 2000; 284:2222–2224. [DOI] [PubMed] [Google Scholar]
- 21.Ruell J, Karuvattil R, Wynn R, Will A. Platelet count has no influence on traumatic and bloody lumbar puncture in children undergoing intrathecal chemotherapy. Br J Haematol 2007; 136:347–348. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh F, Voicu L, Tole S, et al. The risk of traumatic lumbar punctures in children with acute lymphoblastic leukaemia. Eur J Cancer 2014; 50:1482–1489. [DOI] [PubMed] [Google Scholar]
- 23.Pieters BJ, Conley L, Weiford J, et al. Prophylactic versus reactive transfusion of thawed plasma in patients undergoing surgical repair of craniosynostosis: a randomized clinical trial. Paediatr Anaesth 2015; 25:279–287. [DOI] [PubMed] [Google Scholar]
- 24.Tejiram S, Sen S, Romanowski KS, Greenhalgh DG, Palmieri TL. Examining 1:1 vs. 4:1 Packed Red Blood Cell to Fresh Frozen Plasma Ratio Transfusion During Pediatric Burn Excision. J Burn Care Res 2020; 41:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galganski LA, Greenhalgh DG, Sen S, Palmieri TL. Randomized Comparison of Packed Red Blood Cell-to-Fresh Frozen Plasma Transfusion Ratio of 4:1 vs 1:1 During Acute Massive Burn Excision. J Burn Care Res 2017; 38:194–201. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri TL, Greenhalgh DG, Sen S. Prospective comparison of packed red blood cell-to-fresh frozen plasma transfusion ratio of 4:1 versus 1:1 during acute massive burn excision. J Trauma Acute Care Surg 2013; 74:76–83. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrandt B, Machotta A, Riess H, et al. Intraoperative fresh-frozen plasma versus human albumin in craniofacial surgery--a pilot study comparing coagulation profiles in infants younger than 12 months. Thromb Haemost 2007; 98:172–177. [PubMed] [Google Scholar]
- 28.Stricker PA, Fiadjoe JE, Davis AR, et al. Reconstituted blood reduces blood donor exposures in children undergoing craniofacial reconstruction surgery. Paediatr Anaesth 2011; 21:54–61. [DOI] [PubMed] [Google Scholar]
- 29.Stricker PA, Fiadjoe JE, Kilbaugh TJ, et al. Effect of transfusion guidelines on postoperative transfusion in children undergoing craniofacial reconstruction surgery. Pediatr Crit Care Med 2012; 13:e357–362. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TT, Hill S, Austin TM, Whitney GM, Wellons JC, Lam HV. Use of blood-sparing surgical techniques and transfusion algorithms: association with decreased blood administration in children undergoing primary open craniosynostosis repair. J Neurosurg Pediatr 2015; 16:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas T, Goobie S, Spielmann N, Weiss M, Schmugge M. Improvements in patient blood management for pediatric craniosynostosis surgery using a ROTEM((R))-assisted strategy - feasibility and costs. Paediatr Anaesth 2014; 24:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman L, Lin Y, Cserti-Gazdewich C, et al. Utilization of frozen plasma, cryoprecipitate, and recombinant factor VIIa for children with hemostatic impairments: An audit of transfusion appropriateness. Pediatr Blood Cancer 2018; 65(4). [DOI] [PubMed] [Google Scholar]
- 33.Karam O, Lacroix J, Robitaille N, Rimensberger PC, Tucci M. Association between plasma transfusions and clinical outcome in critically ill children: a prospective observational study. Vox Sang 2013; 104:342–349. [DOI] [PubMed] [Google Scholar]
- 34.Chongsrisawat V, Suprajitporn V, Kittikalayawong Y, Poovorawan Y. Platelet count in predicting bleeding complication after elective endoscopy in children with portal hypertension and thrombocytopenia. Asian Biomedicine 2009; 3:731–734. [Google Scholar]
- 35.Camazine MN, Karam O, Colvin R, et al. Outcomes Related to the Use of Frozen Plasma or Pooled Solvent/Detergent-Treated Plasma in Critically Ill Children. Pediatr Crit Care Med 2017; 18:e215–e223. [DOI] [PubMed] [Google Scholar]
- 36.Dogra K, Kaur G, Basu S, Chawla D. Fresh Frozen Plasma and Platelet Transfusion Practices in Neonatal Intensive Care Unit of a Tertiary Care Hospital. Indian J Hematol Blood Transfus 2020; 36:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauvin F, Lacroix J, Robillard P, Lapointe H, Hume H. Acute transfusion reactions in the pediatric intensive care unit. Transfusion 2006; 46:1899–1908. [DOI] [PubMed] [Google Scholar]
- 38.Stricker PA, Shaw TL, Desouza DG, et al. Blood loss, replacement, and associated morbidity in infants and children undergoing craniofacial surgery. Paediatr Anaesth 2010; 20:150–159. [DOI] [PubMed] [Google Scholar]
- 39.van Uitert A, Megens JH, Breugem CC, Stubenitsky BM, Han KS, de Graaff JC. Factors influencing blood loss and allogeneic blood transfusion practice in craniosynostosis surgery. Paediatr Anaesth 2011; 21:1192–1197. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri TL, Sen S, Falwell K, Greenhalgh DG. Blood product transfusion: does location make a difference? J Burn Care Res 2011; 32:61–65. [DOI] [PubMed] [Google Scholar]
- 41.Mulder HD, Augustijn QJ, van Woensel JB, Bos AP, Juffermans NP, Wosten-van Asperen RM. Incidence, risk factors, and outcome of transfusion-related acute lung injury in critically ill children: a retrospective study. J Crit Care 2015; 30:55–59. [DOI] [PubMed] [Google Scholar]
- 42.Thalji L, Thum D, Weister TJ, et al. Incidence and Epidemiology of Perioperative Transfusion-Related Pulmonary Complications in Pediatric Noncardiac Surgical Patients: A Single-Center, 5-Year Experience. Anesth Analg 2018; 127:1180–1188. [DOI] [PubMed] [Google Scholar]
- 43.Alsheikh B, Chegondi M, Totapally B. Platelet Transfusion Thresholds Among Children Admitted to a Pediatric Intensive Care Unit. Cureus 2017; 9:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Ghumlas AK, Al Momen AK, Badri M, Abdel Gader AG. Long-term audit of platelet consumption in a university hospital. Transfus Clin Biol 2017; 24:68–75. [DOI] [PubMed] [Google Scholar]
- 45.Raturi M, Shastry S, Baliga PB. Preponderant use of fresh-frozen plasma in children despite weaker evidence. J Applied Hematol 2019; 10:10–14. [Google Scholar]
- 46.Slonim AD, Joseph JG, Turenne WM, Sharangpani A, Luban NL. Blood transfusions in children: a multi-institutional analysis of practices and complications. Transfusion 2008; 48:73–80. [DOI] [PubMed] [Google Scholar]
- 47.Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. Hematol J 2003; 4:373–378. [DOI] [PubMed] [Google Scholar]
- 48.Goobie SM, Zurakowski D, Isaac KV, et al. Predictors of perioperative complications in paediatric cranial vault reconstruction surgery: a multicentre observational study from the Pediatric Craniofacial Collaborative Group. Br J Anaesth 2019; 122:215–223 [DOI] [PubMed] [Google Scholar]
- 49.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005; 45:1413–1425. [DOI] [PubMed] [Google Scholar]
- 50.Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schochl H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: is there any evidence? Br J Anaesth 2015; 114:217–224. [DOI] [PubMed] [Google Scholar]
- 51.Stanworth SJ. The evidence-based use of FFP and cryoprecipitate for abnormalities of coagulation tests and clinical coagulopathy. Hematology Am Soc Hematol Educ Program 2007:179–186. [DOI] [PubMed] [Google Scholar]
- 52.Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol 2004; 126:139–152. [DOI] [PubMed] [Google Scholar]
- 53.Huber J, Stanworth SJ, Doree C, et al. Prophylactic plasma transfusion for patients without inherited bleeding disorders or anticoagulant use undergoing non-cardiac surgery or invasive procedures. Cochrane Database Syst Rev 2019; 11:CD012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018; 73:1418–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell R, Bauer DF, Goobie SM, et al. Plasma and platelet transfusion strategies in children following severe trauma, traumatic brain injury and/or intracranial hemorrhage: From the Transfusion and Anemia Expertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spitalnik SL, Triulzi D, Devine DV, et al. State of the Science in Transfusion Medicine Working: 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion 2015; 55:2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holland LL, Foster TM, Marlar RA, Brooks JP. Fresh frozen plasma is ineffective for correcting minimally elevated international normalized ratios. Transfusion 2005; 45:1234–1235. [DOI] [PubMed] [Google Scholar]
- 58.Raturi M, Shastry S, Murugesan M, Baliga PB, Chakravarthy K. Effect of plasma component transfusion on conventional coagulation screening tests. Asian J Transfus Sci 2018; 12:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triulzi DJ. The art of plasma transfusion therapy. Transfusion 2006; 46:1268–1270. [DOI] [PubMed] [Google Scholar]
- 60.Parker RI. Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med 2014; 42:675–690. [DOI] [PubMed] [Google Scholar]
- 61.Schiffer CA, Bohlke K, Delaney M, et al. Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2018; 36:283–299. [DOI] [PubMed] [Google Scholar]
- 62.Curley A, Stanworth SJ, Willoughby K, et al. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med 2019; 380:242–251. [DOI] [PubMed] [Google Scholar]
- 63.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015; 162:205–213. [DOI] [PubMed] [Google Scholar]
- 64.Delaney M, Karam O, Lieberman L, et al. What laboratory tests and physiologic triggers should guide the decision to administer a platelet or plasma transfusion in critically ill children and what product attributes are optimal to guide specific product selection? From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goel R, Josephson CD, Patel EU, et al. Perioperative Transfusions and Venous Thromboembolism. Pediatrics 2020; 145:e20192351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Porro Gil L, Leon Vintro X, Lopez Fernandez S, et al. The Effect of Perioperative Blood Transfusions on Microvascular Anastomoses. J Clin Med 2021; 10:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


