Abstract
Objectives:
To present consensus statements and supporting literature for plasma and platelet product parameters and related laboratory testing for transfusions in general critically ill children from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB).
Design:
Systematic review and consensus conference of international, multidisciplinary experts in platelet and plasma transfusion management of critically ill children.
Setting:
Not applicable.
Patients:
Critically ill pediatric patients at risk of bleeding and receiving plasma and/or platelet transfusions.
Interventions:
None
Measurements and Main Results:
A panel of 10 experts developed evidence-based and, when evidence was insufficient, expert based statements for laboratory testing and blood product attributes for platelet and plasma transfusions. These statements were reviewed and ratified by the 29 TAXI-CAB experts. A systematic review was conducted using MEDLINE, EMBASE, and Cochrane Library databases, from inception to December 2020. Consensus was obtained using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Results were summarized using the Grading of Recommendations Assessment, Development, and Evaluation method. We developed 5 expert consensus statements and 2 recommendations in answer to two questions: what laboratory tests and physiologic triggers should guide the decision to administer a platelet or plasma transfusion in critically ill children; and what product attributes are optimal to guide specific product selection?
Conclusions:
The TAXI-CAB program provides some guidance and expert consensus for the laboratory and blood product attributes used for decision making for plasma and platelet transfusions in critically ill pediatric patients.
MeSH Terms: platelet transfusion, plasma, hemostasis, critical illness, child, blood grouping and crossmatching, platelet count, coagulopathy
INTRODUCTION
The Joint Commission on Accreditation of Health Care Organization has identified blood transfusion as a top five overused treatment that reduced patient safety [1]. Historically, platelet and plasma transfusions have been used to treat or prevent bleeding. However, balancing transfusion associated risks and the potential of overuse with benefit from treatment is challenging. As reported in a large national survey [2], platelet transfusions are the second most commonly prescribed blood product in pediatric patients with 19.3% of pediatric inpatients receiving platelets. Notably, platelet usage in all inpatients has increased nationwide from 1993 to 2014 [3].
Adverse events related to blood transfusion are an important clinical concern and result in increased healthcare cost [4]. Pediatric patients are more at risk for acute transfusion reactions than adults. In a study of >130,000 blood transfusions, given to pediatric and adult patients, the rate of acute transfusion reactions was higher in the pediatric patients compared to the adult patients (6.2 versus 2.1 per 1000 transfusions, respectively) [5]. The excess incidence of transfusion related reactions included three of the most common events; 1.9 vs 0.47 per 1000 transfusions for febrile non-hemolytic, 0.29 vs 0.078 per 1000 transfusions for hypotension, and 2.7 vs 1.1 per 1000 transfusions for allergic transfusion reactions, respectively. Transfusion reactions are most common following platelet transfusions, possibly as a result of the plasma contained in the products. However, the current literature for these reactions is limited in children [6, 7].
Multiple reports describe specific laboratory or clinical physiologic parameters to guide transfusion decisions in children admitted to the Pediatric Intensive Care Unit. Large-scale studies using objective measures evaluating laboratory and product attributes as efficacious in reducing bleeding are limited, as is research into development of functional hemostatic or coagulation parameters. Numerous studies have demonstrated that protocols, guidelines and/or decision trees improve clinical management and/or patient outcomes of critically ill children [8, 9]. The objective of the ‘Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding’ (TAXI-CAB)” was to rigorously and methodologically develop guidelines that can support clinicians (e.g. pediatric intensivists, cardiologists, anesthesiologists, transfusion medicine specialists, etc.) in their decision-making about platelet and plasma transfusion in critically ill children. This sub-group of TAXI-CAB evaluated and summarized the current literature and ‘state of the science’ on the questions: what laboratory tests and physiologic triggers should guide the decision to administer a platelet or plasma transfusion in critically ill children and what product attributes are optimal to guide specific product selection?
METHODS
The search strategy, item selection and recommendation generation used to identify and select references for systematic review and to develop recommendation are detailed in the general manuscript of TAXI-CAB [10]. Briefly, we searched Ovid MEDLINE®, Ovid EMBASE, and Cochrane Library (Wiley) from inception through December 2020 using a combination of medical subject heading terms and text words to define concepts of plasma or platelet transfusion, transfusion triggers, laboratory tests to assess efficacy of transfusion in children admitted to the pediatric intensive care unit (PICU). For articles selected for inclusion, reference lists and citing articles were selected from Scopus (Elsevier) and screened. Two reviewers independently reviewed all citations and performed data extraction and assessments of bias. Literature was reviewed for relevance to laboratory testing and blood product attributes. Research Electronic Data Capture (REDCap) hosted at Weill Cornell Medicine was used for standardized data extraction. We used a standardized data extraction form to construct evidence tables and graded the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [11].
Ten experts participated in the development of recommendations from this subgroup. A panel of 29 experts convened in an on-line format over 18 months to develop good practice statements, recommendations and, when evidence was lacking, expert consensus statements. Good practice statements are those in which there is high-level of certainty that the practice will do more good than harm, but there is little in the way of supporting literature evidence. Expert consensus statements are based on the expert opinion of the group, but in areas where research is likely needed. All statements from each subgroup were reviewed by the full panel of experts and voted on using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Agreement was defined a priori as >80% of all experts. The recommendations and statements are intended to apply to infants, children and adolescents. Prophylactic transfusions are those prescribed to patients at risk of bleeding, whereas therapeutic transfusions are given to those with active bleeding.
RESULTS
Searching laboratory parameters and blood product attributes identified 4031 and 5476 abstracts, respectively. After duplicates were removed, a total of 3446 and 4921 abstracts were screened. Then, out of 291 and 462 full text manuscripts about laboratory parameters and blood product attributes, respectively, we selected 49 and 88 papers for detailed review (see Figures 1 and 2). These papers underwent data extraction and assessment of bias in order to generate recommendations and statements (see Supplemental Data Tables 1 and 2); five 5 expert consensus statements and 2 graded recommendations were produced. The voting data, including the number of voting experts and median score, are provided for each statement and recommendation.
Figure 1. papers flow chart.
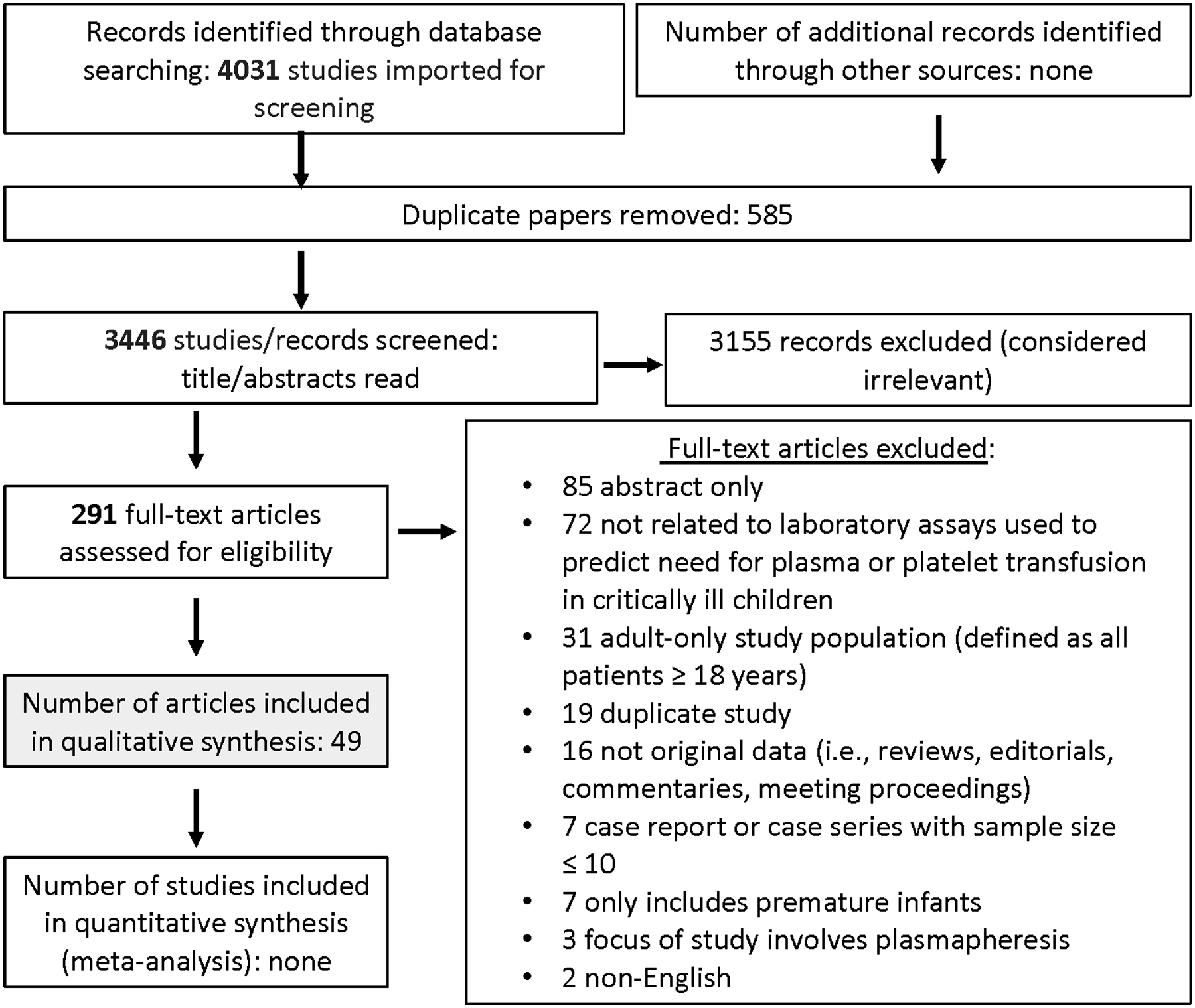
PRISMA diagram for studies on laboratory assays to guide plasma and platelet transfusion
Figure 2. papers flow chart.

PRISMA diagram for studies on product selection
Laboratory Tests for Guiding Plasma and Platelet Transfusions
Expert Consensus Statements
1. In critically ill pediatric patients, there is insufficient evidence to recommend a specific laboratory test as a threshold or as a target for platelet transfusion for prophylactic or therapeutic indications. 92% Agreement (n=24), Median 8, IQR 7–9.
Rationale: Over 50% of platelet transfusions in the pediatric ICU are given as prophylaxis [12,13]. Thus, platelet count alone determines whether a platelet transfusion will be administered. The risks associated with bleeding from thrombocytopenia have been evaluated in numerous studies with inconclusive findings [14–17]. Furthermore, the understanding of bleeding in certain types of adult thrombocytopenic patients suggests that patients only require a platelet level of approximately 7×109 cells/L to maintain effective hemostasis [18]. Likewise, to minimize bleeding, a specific platelet count to trigger a prophylactic platelet transfusion has been controversial. Although studies have included children with cancer or undergoing hematopoietic stem cell transplant, very few recommendations have been made that are relevant to critically ill children. However, guidance from the American Association of Blood Banks (AABB) state platelet transfusions are indicated in adults for: 1) prophylactic transfusion when total platelet count (TPC) < 10×109 cells/L due to hypoproliferative thrombocytopenia; 2) prophylactic transfusion when elective central venous catheter placement < 20×109 cells/L; 3) prophylactic transfusion when elective lumbar puncture < 50×109 cells/L; and 4) prophylactic transfusion when patient having major elective non-neuraxial surgery < 50×109 cells/L. In addition, the AABB recommends against routine prophylactic transfusion in adult patients who are not thrombocytopenic and undergoing cardiopulmonary bypass surgery, but recommends platelet transfusions when bleeding or when there is evidence of platelet function defect [19]. Nonetheless, studies to assess quantitative platelet counts as targets to stop bleeding are lacking, particularly in the pediatric population.
Recent advances in platelet function testing (PFT) instead of reliance on platelet number alone, have made this assessment more widely available, albeit not at all centers. These have previously included bleeding time by various methodologies, platelet aggregometry, and studies of activated platelets via flow cytometry [20]. Although these tests may become available in the future, at present there is insufficient information to support a recommendation or consensus statement about routine use. Moreover, the platelet count does not correlate well with risk of bleeding or represent a functional hemostatic marker [21].
We evaluated studies with platelet transfusion alone (n=34), platelet and RBC (n=2), or platelet and plasma (n=7) using platelet count (n=26), platelet aggregation (n=2), thrombin generation (n=1), closure time (n=1), or platelet mass index (n=2) in surgical, neonatal, pediatric ICU, oncology, trauma, extracorporeal membrane oxygenation (ECMO), or dengue patients. Given the available laboratory testing, platelet count has been incorporated into decision trees based on expert consensus to help practitioners in the decision to transfuse platelets to critically ill children in eight pre-defined clinical settings [10]. However, similar to current British guidelines [22], we concluded that pediatric intensivists should not use platelet count alone to drive the decision to administer platelet transfusions in PICU patients as the overall general health and acuity of the patient may govern bleeding tolerance more than a pretransfusion threshold. The tolerance of thrombocytopenia and risk of bleeding varies with contextual patient circumstances, e.g., a child following a traumatic brain injury with intracranial hemorrhage is different from a patient with disseminated intravascular coagulation or bone marrow failure. It is the presence of thrombocytopenia, and other risk factors that should prompt the practitioner to prescribe a platelet transfusion, such as presence of severe bleeding, severity and type of illness, patient trajectory of illness (deteriorating or recovering), hemodynamic instability, and other related conditions.
2. In critically ill pediatric patients, there is insufficient evidence to recommend a specific laboratory test as a threshold or as a target for plasma transfusion for prophylactic or therapeutic indications. 96% Agreement (n=24), Median 8.5, IQR 7.25–9.
Rationale: Plasma contains all coagulation factors present in whole blood. Thirty to 50% of plasma transfusions given in the PICU are prophylactic (3, 23–26). In patients on extracorporeal membrane oxygenation, almost 60% of plasma transfusions are given prophylactically even though conventional coagulation tests (prothrombin time (PT), international normalized ratio (INR) or activated partial thromboplastin time (aPTT)) are unchanged after transfusion [12]. A retrospective study found that plasma transfusion is independently associated with adverse outcomes including transfusion-related acute lung injury, anaphylaxis, and venous thrombosis [27]. Finding relevant laboratory tests to indicate which patients may benefit from plasma transfusion to minimize bleeding risk or stop active bleeding is paramount.
PT and/or aPTT are the conventional clot-based tests that measure the time it takes for blood to form a clot after in vitro incubation with thromboplastin. INR is calculated from PT and used to monitor the intensity of warfarin-induced anticoagulation. The PT and aPTT results are used as an estimation of coagulation efficiency. Viscoelastic tests (VET) are clot-based assays (thromboelastometry (ROTEM™) or thromboelastography (TEG™)) that measure various parameters regarding clot formation. However, studies evaluating the use of traditional and viscoelastic test parameters in critically ill children to minimize bleeding are inconclusive and these values alone may not be reliable triggers for transfusion. Furthermore, due to changes in hemostasis during development, the neonatal coagulation values differ from those in children and adults and therefore measured values must be interpreted against age-specific norms.
After evaluation of studies reporting plasma transfusion alone (n=13) or platelet and plasma (n=7) using INR (n=6), VET (n=4), in surgical, neonatal, pediatric ICU, oncology, trauma, ECMO, or dengue patients, we agreed that pediatric intensivists should not use PT (INR) cutoffs or VET R times alone to drive the decision to administer plasma transfusions in PICU patients; they should be guided by clinical assessment. Given the available laboratory testing, PT (INR) has been incorporated into decision trees based on expert consensus to help practitioners in the decision to transfuse plasma to critically ill children in eight pre-defined clinical settings [10]. Nonetheless, current level of evidence is insufficient to recommend a PT (INR) threshold as a guide for plasma transfusion. Plasma transfusion may be indicated in clinical situations such as invasive procedures in patients with major alterations in coagulopathy testing (INR, PT, and VET).
Blood Product Attributes for Plasma and Platelet Components
Clinical Recommendations
1. In critically ill neonatal and pediatric patients, the use of leukocyte reduced cellular blood components is recommended. GRADE 1C (strong recommendation, Low quality of pediatric evidence), 96% Agreement (n=23), Median 9, IQR 8–9.
Rationale: Leukocyte reduction for cellular blood products is now standard in transfusion practice due to compelling evidence of safety improvements they produce. Specifically, studies have demonstrated leukocyte reduced blood products are associated with 1) decreased alloimmunization to foreign human leukocyte antigens (HLA), 2) fewer febrile transfusion reactions and 3) less risk of transfusion transmitted cytomegalovirus (CMV) compared to non-leukocyte reduced blood products [28–30]. Studies in adult patients not included in the selected articles find similar results [31]. Lastly, leukocyte reduction is also a reliable mechanism to decrease the rate of febrile non-hemolytic transfusion reactions and should be employed for this reason alone whenever possible [32]. The timing of leukocyte reduction is important; products leukocyte reduced before storing (i.e. prestorage) are less likely to cause a febrile reaction than those that are filtered after storage [33]. In pediatric patients, there is an association of allergic and febrile reactions with non-leukoreduced blood product administration, thus attributing the reaction to the leukocytes or secreted mediators [34].
2. In a critically ill RhD negative neonate, infant or child in need of a platelet transfusion, RhD positive platelets should be used if an RhD negative platelet component is not available. GRADE 1C (strong recommendation, Low quality of pediatric evidence), 95% Agreement (n=20), Median 8, IQR 8–9.
Rationale: RhD is a membrane protein restricted to the surface of RBCs that functions to support membrane integrity and transport. Approximately 7–15% of the population is lacking the RhD protein on the RBC, i.e. are “Rh negative” and are at risk of forming anti-D antibody if exposed to RhD positive RBCs through transfusion or pregnancy [35]. While platelets do not express the RhD protein, platelet concentrates can provide RhD via RBC contamination during donation or by shed antigen adsorbed to the platelet membrane. Whole blood derived platelet concentrates are estimated to have an average of 0.036 mL per full adult dose, while apheresis platelets have 0.00043 mL [36]. There is a theoretical concern that the very low amount of RhD positive RBCs in a platelet product could cause the patient to become alloimmunized to RhD (i.e., form anti-D antibodies).
As RhD negative individuals are relatively uncommon in the population, ensuring an ample supply of RhD negative platelets for RhD negative patients can be difficult. Additionally, the platelet supply is often low due to seasonal and local shortages, further affecting availability of all platelet products. When this occurs, transfusion services may issue RhD positive platelets to RhD negative patients, particularly when a patient requires multiple platelet transfusions.
Real world, clinical studies have found the risk of RhD alloimmunization due to platelet transfusion to be very low. In a single center study, 315 RhD negative patients (aged 0–103 years) were tested for anti-D antibodies 4+ weeks after receiving a RhD positive product, 89% of which were platelet concentrates [36]. There were 12 of 303 recipients who formed anti-D antibodies for a 3.8% alloimmunization rate. In a retrospective multicenter study of RhD negative patients (aged 2 – 100 years) who received RhD+ platelets, only 7 of 485 patients (1.44%; 95% CI 0.58–2.97%) had evidence of anti-D alloimmunization following RhD+ platelet transfusion. Diagnoses included hematologic (203/485, 42%), oncologic (64/485, 13%) and other disorders (218/485, 45%). There were no differences between patients who formed anti-D antibodies from non-responders (gender, age, immunosuppressive therapy, type and number of platelet products) [36]. In a study of RhD negative pediatric oncology and stem cell transplant patients receiving over 200 RhD positive apheresis platelet “units”, authors found no evidence of anti-D alloimmunization [37]. Using available evidence, the transfusion of RhD positive platelets, especially apheresis products to RhD negative patients who are bleeding or have met clinical or laboratory criteria for a platelet transfusion, appears to be safe, particularly if platelet supply is limited.
Some centers use Rh immune globulin (RhIg) when RhD positive platelets are administered to certain RhD negative patients (for example, in females of childbearing potential and/or those who are pre-stem cell transplant). Given the low rate of RhD alloimmunization and limited evidence, this practice is variable. In a recent survey of 28 cancer centers who are members of the National Comprehensive Cancer Network, fifty-three percent of centers stated they would consider using RhIg in an RhD negative female patient of childbearing potential receiving RhD positive platelets [38]. Each institution may consider the development of institutional guidelines in collaboration with the transfusion service medical leadership to direct the use of RhIg.
Expert Consensus Statements
1. When considering pathogen reduction and selecting products for plasma transfusion, products may be selected that balance risk of transfusion-transmitted infection, hemostatic effects, and clinical outcomes, as well as feasibility. 92% Agreement (n=24), Median 9, IQR 8–9.
Rationale: Since coagulation factors are highly variable between single-donor plasma units pooling plasma from many donors provides a method to maintain a “normal” concentration of these factors [39]. However, pooling donors increases the odds of transmission of infectious diseases. To mitigate this risk, multiple pathogen inactivation methods are available including solvent/detergent (S/D) treatment, methylene blue (MB), ultraviolet light with riboflavin, and psoralens (amotosalen) [40].
Some coagulation factors are reduced in various products, including factor VIII (25% lower in MB plasma and 45% lower in amotosalen and S/D plasma), and fibrinogen (30% lower in MB and amotosalen plasma) [41]. Additionally, both Protein S and alpha-2 antiplasmin are lower in S/D plasma causing this product to be contraindicated in patients with Protein S deficiency. However, S/D plasma has been shown to have a better thrombin generation profile (total, lag time to peak, and peak thrombin formation), compared with regular plasma [42].
Safety of these products is supported by hemovigilance studies of over 10 million units administered to patients in Europe, although detailed information regarding children is not included [39]. In a retrospective study of adults with thrombotic thrombocytopenic purpura, patients younger than 40 years who underwent plasma exchange with S/D plasma exhibited a shorter time to platelet recovery [43]. Conversely, one randomized controlled trial (RCT) in adults undergoing liver transplantation, demonstrated lower transfusion volumes with regular plasma than with S/D or MB plasma [44]. In critically ill children, a secondary analysis of a prospective observational study suggested S/D plasma to be independently associated with a lower risk of mortality [45]. However, there are no multicenter RCTs comparing the efficacy of regular plasma compared to pooled products in critically ill children. Pathogen-reduced plasma may be safe and have appropriate hemostatic effects, but studies are needed in children for confirmation.
2. When considering pathogen reduction and selecting products for platelet transfusion, products may be selected that balance risk of transfusion-transmitted infection, hemostatic effects, and clinical outcomes, as well as feasibility. 87% Agreement (n=23), Median 9, IQR 8–9.
Rationale: Platelets are typically stored at a room temperature (20– 24°C) under gentle agitation, which, when compared to cold storage, improves circulation time. However, room temperature storage increases the risk of bacterial contamination [46]. Therefore, several pathogen reduction techniques have been developed to mitigate infection risk [47]. For example, amotosalen, a light-activated, DNA-, RNA-crosslinking psoralen compound, is able to neutralize pathogens, by preventing replication of DNA or RNA present in pathogens but not in the blood components being treated [48]. In addition to decreasing the risk of infections, pathogen reduction obviates the need for blood product irradiation to decrease the risk of transfusion associated graft versus host disease, as the pathogen reduction process irreversibly blocks DNA and RNA replication.
Studies of pathogen reduced platelets compared to conventional (untreated) platelets have not demonstrated an increase in acute transfusion adverse events. However, two retrospective cohort studies in children and infants suggested that pathogen reduction of platelets is associated with decreased post-transfusion platelet increments as compared to untreated platelet products. One study from the USA found that pediatric patients ages 1–18 years who were given Intercept® platelets (Cerus, CA, USA) required additional platelet transfusion(s) within 48 hours after the index transfusion suggesting that the target platelet count had not been achieved [49]. No such difference was found in infants < 1yr whether treated in a NICU or PICU. Additionally, the authors did not find any difference in bleeding in any of the age groups. There was no difference in the rate of allergic or febrile non-hemolytic transfusion reactions in those infants and children who received either conventional or Intercept® platelets. A second study from Spain found that neonatal patients exhibited increased usage of Mirasol® (Terumo BCT, CO, USA) pathogen reduced platelets (transfusion events and dose) when compared to historic controls who received conventional platelets [50]. The authors also found that adults and children had the same rate of acute transfusion reactions (1.3%) when using Mirasol® pathogen reduced platelets.
There are prospective studies that further support the retrospective studies in children. A RCT in adult hematology-oncology patients with chemotherapy-induced thrombocytopenia comparing untreated platelets with Mirasol® pathogen reduced platelets showed a 50% lower platelet count increment in the pathogen-reduced platelet arm (p<0.001), and shorter intervals between platelet transfusion (p<0.001) [51]. Nonetheless, pathogen-reduced platelets did not lead to increased bleeding events. Another RCT has just finished enrolling 3,070 children and adults with hematology-oncology disorders who were randomized to conventional vs pathogen-reduced platelet transfusions (ClinicalTrials.gov NCT02549222). The primary outcome was the proportion of patients requiring mechanical ventilation. A 2017 Cochrane systematic review, based on 12 trials and 1,981 participants, concluded that pathogen-reduced platelet transfusions do not affect all-cause mortality, risk of clinically significant or severe bleeding, or risk of a serious adverse event [52]. Therefore, while there is a benefit in terms of decreased risk of transfusion transmitted infection, pathogen-reduced platelets might have lower hemostatic effects, as measured by platelet increment count and requiring more frequent transfusions.
3. When a critically ill pediatric patient has persistently poor platelet count increments following platelet transfusion, a clinical and laboratory assessment for platelet refractoriness is suggested to elucidate the cause. 96% Agreement (n=23), Median 9, IQR 8–9.
Rationale: Platelet transfusion refractoriness (PTR) is generally defined as persistently insufficient post-transfusion platelet count increments following platelet transfusion from random donors [53] and is typically considered when the corrected count increment (CCI) is ≤7500/μL and absolute count increment less than 5000 per unit of platelets measured at 1-hour post-transfusion. PTR has been associated with adverse clinical outcomes including risk of bleeding, length of stay, survival and hospital cost [54,55].
PTR can be due to either immune or non-immune causes, with non-immune causes being more common. A detailed history, physical examination and laboratory testing help to distinguish between these potential causes. Non-immune factors include both patient and product related factors. Patient related factors include bleeding, medications, splenomegaly, or a consumptive etiology, e.g., diffuse intravascular coagulation. Product related factors may include the type of product received (buffy coat or apheresis), blood group ABO compatibility, or age [56]. The relative contribution of immune or non-immune factors can be challenging to separate when treating complex critically ill patients with underlying co-morbidities.
Prevalence estimates of PTR in adults are widely variable depending on the underlying disease and comorbidities and have been estimated to range from 1 in 20 to 1 in 5 in various observational studies [14,57]. The prevalence of PTR in critically ill children has not been assessed [58]. Fewer than ten studies have been published on PTR in children and rates of PTR have varied widely between 8–100% [56,58]. Experts suggest that PTR occurs less frequently in children when compared to adults due to decreased previous exposure to transfusions and pregnancy, which typically precedes immune PTR.
In addition to a comprehensive clinical assessment, laboratory tests are essential to confirm an immune mediated cause [59]. Immune causes include alloimmunization to human leucocyte antigen (HLA) and or human platelet antigen (HPA) antigens with HLA alloimmunization occurring more frequently. Other immune causes include ABO incompatibility, platelet autoantibodies and drug related platelet antibodies. Methods to screen for the presence of HLA antibodies include lymphocytotoxicity, enzyme-linked immunosorbent assay (ELISA), flow cytometric immunofluorescence tests or the newer multiplex flow cytometric bead-based assays. Due to technological aspects of testing, significant amount of discordance exists between methods, and consequently there is no recognized gold standard test.
Standard practice is to provide unselected or random platelet products for initial platelet transfusion support while awaiting results. Typically, ABO-identical, fresh platelet products are issued with assessment of a 1-hour post transfusion increment. If non-immune factors are suspected and treatment is essential, support may be continued with random donor platelets. Immune-based PTR can be managed by either crossmatch-compatible, HLA-matched or HLA-compatible platelet units than using random non-selected units [60,61]. Platelet crossmatching involves a match with antibodies against HLA or HPA bound to the donor platelets seen with indicator red cells coated with anti–immunoglobulin G. In HLA matching, donor HLA-A and HLA-B antigens can be matched with those of the patient aiming for a 4/4 match. When exact HLA matches are unavailable, antibody profile determined by the single-antigen bead test can be used to select donor units that lack the corresponding cognate antigens called as HLA compatible/HLA selected platelets. Response to HLA selected platelet transfusions should be carefully monitored, ideally with one-hour post transfusion platelet assessment. If responses are poor, additional rare etiologies including HPA antibodies may be considered.
DISCUSSION
In the TAXI-CAB program [10], the current report has sought to address the following two questions: 1) what laboratory tests and physiologic triggers should guide the decision to administer a platelet or plasma transfusion in critically ill children; and 2) what product attributes are optimal to guide specific product selection? The ensuing literature review and expert assessment produced two expert consensus statements about laboratory testing and three expert consensus statements and two graded recommendations about blood product attributes best suited for critically ill pediatric patients. Our extensive systematic review resulted in very few studies of high quality related to plasma and/or platelet transfusion strategies in critically ill children. Specifically, there are few RCTs in these two areas of medical science. Thus, many of the statements are derived from a mixture of retrospective studies and single center studies. There are some large population studies about blood safety that helped to develop blood product attribute statements. However, several are hemovigilance studies from European countries that lack detail on children and do not include efficacy outcomes.
CONCLUSIONS
This systematic review and assessment of the literature has yielded a robust set of two recommendations and five expert consensus statements that describe the current state of evidence-based practice of laboratory testing and blood products attributes for platelet and plasma transfusions for critically ill children. Further research, particularly to investigate appropriate laboratory parameters that correlate bleeding risks to guide transfusion therapy are urgently needed and discussed in the companion TAXI-CAB research-gaps article [62].
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all members of TAXI-CAB for their support, especially during the Coronavirus-19 Disease pandemic. In addition, we thank the Chaire Héma-Québec-Bayer en médecine transfusionnelle de l’Université de Montréal, the Society for the Advancement of Blood Management, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, the International Society of Blood Transfusion, the Society for Critical Care Medicine, and the AABB for their support.
Financial Support:
The Transfusion and Anemia EXpertise Initiative - Control/Avoidance of Bleeding (TAXI-CAB) was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute under award number R13 HL154544-01.
Copyright Form Disclosure:
Dr. Goel received funding from the National Heart, Lung, and Blood Institute and Rigel Pharmaceuticals. Dr. Nellis received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
APPENDIX 1. Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members
(* for executive committee) Co-chairs: Marianne E. Nellis, MD, MS*, Weill Cornell Medicine, New York, NY, and Robert I. Parker, MD*, Renaissance School of Medicine, State University of New York at Stony Brook, Stony Brook, NY; Content Experts: Section 1. Laboratory assays used to assess need for plasma and/or platelet transfusions: Scot T. Bateman, MD*, University of Massachusetts Medical School, Worcester, MA, Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Kenneth E. Remy, MD, MHSc, MSCI, Washington University of St. Louis, St. Louis, MO, Katherine Steffen, MD, Stanford University, Palo Alto, CA; Section 2. Traumatic brain injury and intracranial hemorrhage: David F. Bauer, MD, MPH, Baylor College of Medicine, Houston, TX, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA; Section 3. Following cardiopulmonary bypass: Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Sitaram Emani, MD, Harvard Medical School, Boston, MA, Juan Ibla, MD, Harvard Medical School, Boston, MA, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 4. Supported by extracorporeal membrane oxygenation: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 5. Following severe trauma: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 6. With oncologic diagnosis or following hematopoietic stem cell transplantation: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Lani Lieberman, MD, University of Toronto, Canada, Simon J. Stanworth, MD, University of Oxford, UK, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 7. With acute liver failure or following liver transplantation: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 8. Following non-cardiac surgery: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Marisa Tucci, MD, Université de Montréal, Montreal, QC, Canada, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 9. Invasive procedures outside of the operating room: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 10. Sepsis and/or disseminated intravascular coagulation: Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Simon J. Stanworth, MD, University of Oxford, UK, Katherine Steffen, MD, Stanford University, Palo Alto, CA, Stacey L. Valentine, MD, MPH*, University of Massachusetts Medical School, Worcester, MA; Section 11. Product processing and selection: Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH; Evidence-based medicine: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Diana Delgado and Michelle Demetres, Weill Cornell Medicine, New York, NY; Implementation science: Katherine Steffen, MD, Stanford University, Palo Alto, CA.
Footnotes
Conflicts: None to report
Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members are listed in Appendix 1
REFERENCES
- 1.Organization tJCoAoHC: Available at: https://www.jointcommissionjournal.com/article/S1553-7250(17)30198-8/fulltex. Accessed June 1, 2021.
- 2.Slonim AD, Joseph JG, Turenne WM, et al. Blood transfusions in children: a multi-institutional analysis of practices and complications. Transfusion 2008; 48:73–80. [DOI] [PubMed] [Google Scholar]
- 3.Goel R, Chappidi MR, Patel EU, et al. Trends in Red Blood Cell, Plasma, and Platelet Transfusions in the United States, 1993–2014. JAMA. 2018; 319:825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton-Maggs PH. Bullet points from SHOT: key messages and recommendations from the Annual SHOT Report 2013. Transfus Med 2014; 24:197–203. [DOI] [PubMed] [Google Scholar]
- 5.Oakley FD, Woods M, Arnold S, Young PP. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion 2015; 55:563–570. [DOI] [PubMed] [Google Scholar]
- 6.Heddle NM, Blajchman MA, Meyer RM, et al. A randomized controlled trial comparing the frequency of acute reactions to plasma-removed platelets and prestorage WBC-reduced platelets. Transfusion 2002; 42:556–566. [DOI] [PubMed] [Google Scholar]
- 7.Tobian AA, Savage WJ, Tisch DJ, et al. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion 2011; 51:1676–1683. [DOI] [PubMed] [Google Scholar]
- 8.Du Pont-Thibodeau G, Tucci M, Ducruet T, Lacroix J. Survey on stated transfusion practices in PICUs*. Pediatr Crit Care Med 2014; 15:409–416. [DOI] [PubMed] [Google Scholar]
- 9.Lacroix J, Demaret P, Tucci M. Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinatol 2012; 36:225–231. [DOI] [PubMed] [Google Scholar]
- 10.Nellis ME, Karam O, Valentine S, et al. Executive Summary of Recommendations and Expert Consensus for Plasma and Platelet Transfusion Practice in Critically Ill Children: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008, 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nellis ME, Levasseur J, Stribling J, et al. Bleeding Scales Applicable to Critically Ill Children: A Systematic Review. Pediatr Crit Care Med 2019; 20:603–607. [DOI] [PubMed] [Google Scholar]
- 13.Saini A, West AN, Harrell C, et al. Platelet Transfusions in the PICU: Does Disease Severity Matter? Pediatr Crit Care Med 2018; 19:e472–e478. [DOI] [PubMed] [Google Scholar]
- 14.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005; 105:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med 2010; 362:600–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med 2013; 368:1771–1780. [DOI] [PubMed] [Google Scholar]
- 17.Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood 2017; 130:1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood 1985; 66:1105–1109. [PubMed] [Google Scholar]
- 19.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015; 162:205–13. [DOI] [PubMed] [Google Scholar]
- 20.Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag 2015; 11:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephson CD, Granger S, Assmann SF, et al. Bleeding risks are higher in children versus adults given prophylactic platelet transfusions for treatment-induced hypoproliferative thrombocytopenia. Blood 2012; 120:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New HV, Stanworth SJ, Gottstein R, et al. British Society for Haematology Guidelines on transfusion for fetuses, neonates and older children (Br J Haematol. 2016;175:784–828). Addendum August 2020. Br J Haematol 2020; 191:725–727. [DOI] [PubMed] [Google Scholar]
- 23.Goel R, Patel EU, White JL, et al. Factors associated with red blood cell, platelet, and plasma transfusions among inpatient hospitalizations: a nationally representative study in the United States. Transfusion 2019; 59:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn S, Chegondi M, Nellis ME, Karam O. Overview of Plasma and Platelet Transfusions in Critically Ill Children. Front Pediatr 2020; 8:601659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karam O, Demaret P, Duhamel A, et al. Factors influencing plasma transfusion practices in paediatric intensive care units around the world. Vox Sang 2017; 112:140–149. [DOI] [PubMed] [Google Scholar]
- 26.Karam O, Tucci M, Combescure C, et al. Plasma transfusion strategies for critically ill patients. Cochrane Database Syst Rev 2013:CD010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozek-Langenecker S Clinical efficacy of fresh frozen plasma compared with coagulation factor concentrates for treating coagulopathy in patients with massive bleeding. Med Intensiva 2016; 40:371–373. [DOI] [PubMed] [Google Scholar]
- 28.Saarinen UM, Koskimies S, Myllyla G. Systematic use of leukocyte-free blood components to prevent alloimmunization and platelet refractoriness in multitransfused children with cancer. Vox Sang 1993; 65:286–292. [DOI] [PubMed] [Google Scholar]
- 29.Trial to Reduce Alloimmunization to Platelets Study. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997; 337:1861–1869. [DOI] [PubMed] [Google Scholar]
- 30.Ronghe MD, Foot AB, Cornish JM, et al. The impact of transfusion of leucodepleted platelet concentrates on cytomegalovirus disease after allogeneic stem cell transplantation. Br J Haematol 2002; 118:1124–1127. [DOI] [PubMed] [Google Scholar]
- 31.Blajchman MA, Goldman M, Freedman JJ, Sher GD. Proceedings of a consensus conference: prevention of post-transfusion CMV in the era of universal leukoreduction. Transfus Med Rev 2001; 15:1–20. [DOI] [PubMed] [Google Scholar]
- 32.Pruss A, Kalus U, Radtke H, et al. Universal leukodepletion of blood components results in a significant reduction of febrile non-hemolytic but not allergic transfusion reactions. Transfus Apher Sci 2004; 30:41–46. [DOI] [PubMed] [Google Scholar]
- 33.Wang RR, Triulzi DJ, Qu L. Effects of prestorage vs poststorage leukoreduction on the rate of febrile nonhemolytic transfusion reactions to platelets. Am J Clin Pathol 2012; 138:255–259. [DOI] [PubMed] [Google Scholar]
- 34.Yanagisawa R, Shimodaira S, Sakashita K, et al. Factors related to allergic transfusion reactions and febrile non-haemolytic transfusion reactions in children. Vox Sang 2016; 110:376–384. [DOI] [PubMed] [Google Scholar]
- 35.Olsson MRCL-FM. The Blood Group Antigen FactsBook. 3rd Edition Edition. Academic Press, 2012. [Google Scholar]
- 36.Cid J, Harm SK, Yazer MH. Platelet transfusion - the art and science of compromise. Transfus Med Hemother 2013; 40:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar R, Johnson R, Sweat LT, Geiger TL. Absence of D alloimmunization in D- pediatric oncology patients receiving D-incompatible single-donor platelets. Transfusion 2002; 42:177–182. [DOI] [PubMed] [Google Scholar]
- 38.Poston JN, Sugalski J, Gernsheimer TB, Marc Stewart F, Pagano MB. Mitigation strategies for anti-D alloimmunization by platelet transfusion in haematopoietic stem cell transplant patients: a survey of NCCN® centres. Vox Sang 2020; 115:334–338. [DOI] [PubMed] [Google Scholar]
- 39.Hellstern P, Solheim BG. The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma. Transfus Med Hemother 2011; 38:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein HG. Pathogen inactivation technology: cleansing the blood supply. J Intern Med 2005; 257:224–237. [DOI] [PubMed] [Google Scholar]
- 41.Hacquard M, Lecompte T, Belcour B, et al. Evaluation of the hemostatic potential including thrombin generation of three different therapeutic pathogen-reduced plasmas. Vox Sang 2012; 102:354–361. [DOI] [PubMed] [Google Scholar]
- 42.Spinella PC, Frazier E, Pidcoke HF, et al. All plasma products are not created equal: Characterizing differences between plasma products. J Trauma Acute Care Surg 2015; 78:S18–25. [DOI] [PubMed] [Google Scholar]
- 43.Toussaint-Hacquard M, Coppo P, Soudant M, et al. Type of plasma preparation used for plasma exchange and clinical outcome of adult patients with acquired idiopathic thrombotic thrombocytopenic purpura: a French retrospective multicenter cohort study. Transfusion 2015; 55:2445–2451. [DOI] [PubMed] [Google Scholar]
- 44.Bartelmaos T, Chabanel A, Leger J, et al. : Plasma transfusion in liver transplantation: a randomized, double-blind, multicenter clinical comparison of three virally secured plasmas. Transfusion 2013; 53:1335–1345. [DOI] [PubMed] [Google Scholar]
- 45.Camazine MN, Karam O, Colvin R, et al. Outcomes Related to the Use of Frozen Plasma or Pooled Solvent/Detergent-Treated Plasma in Critically Ill Children. Pediatr Crit Care Med 2017; 18:e215–e223. [DOI] [PubMed] [Google Scholar]
- 46.Pidcoke HF, Spinella PC, Ramasubramanian AK, et al. : Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock 2014; 41:51–53. [DOI] [PubMed] [Google Scholar]
- 47.Gathof BS, Tauszig ME, Picker SM. Pathogen inactivation/reduction of platelet concentrates: turning theory into practice. ISBT Sci Ser 2010; 5:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amotosalen: Allogeneic Cellular Immunotherapies system, INTERCEPT Plasma System, INTERCEPT Platelet System, S 59. BioDrugs 2003; 17:66–68. [DOI] [PubMed] [Google Scholar]
- 49.Schulz WL, McPadden J, Gehrie EA, et al. Blood Utilization and Transfusion Reactions in Pediatric Patients Transfused with Conventional or Pathogen Reduced Platelets. J Pediatr 2019; 209:220–225. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Marco T, Garcia-Recio M, Girona-Llobera E. Use and safety of riboflavin and UV light-treated platelet transfusions in children over a five-year period: focusing on neonates. Transfusion 2019; 59:3580–3588. [DOI] [PubMed] [Google Scholar]
- 51.van der Meer PF, Yoma PF, van Geloven N, et al. Hemostatic efficacy of pathogen-inactivated vs untreated platelets: a randomized controlled trial. Blood 2018; 132:223–231. [DOI] [PubMed] [Google Scholar]
- 52.Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2017; 7:CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saris A, Pavenski K. Human Leukocyte Antigen Alloimmunization and Alloimmune Platelet Refractoriness. Transfus Med Rev 2020; 34:250–257. [DOI] [PubMed] [Google Scholar]
- 54.Kerkhoffs JL, Eikenboom JC, van de Watering LM, et al. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008; 48:1959–1965. [DOI] [PubMed] [Google Scholar]
- 55.Meehan KR, Matias CO, Rathore SS, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol 2000; 64:251–256. [DOI] [PubMed] [Google Scholar]
- 56.Lieberman L, Callum J, Cohen R, et al. Impact of red blood cell alloimmunization on fetal and neonatal outcomes: A single center cohort study. Transfusion 2020; 60:2537–2546. [DOI] [PubMed] [Google Scholar]
- 57.Hess JR, Trachtenberg FL, Assmann SF, et al. Clinical and laboratory correlates of platelet alloimmunization and refractoriness in the PLADO trial. Vox Sang 2016; 111:281–291. [DOI] [PubMed] [Google Scholar]
- 58.Embaby MM, Rangarajan HG, Abu-Arja R, et al. Refractory Thrombocytopenia Is a Valid Early Diagnostic Criteria for Hepatic Veno-Occlusive Disease in Children. Biol Blood Marrow Transplant 2020; 26:546–552. [DOI] [PubMed] [Google Scholar]
- 59.Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program 2020; 2020:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forest SK, Hod EA. Management of the Platelet Refractory Patient. Hematol Oncol Clin North Am 2016; 30:665–677. [DOI] [PubMed] [Google Scholar]
- 61.Rioux-Masse B, Cohn C, Lindgren B, et al. : Utilization of cross-matched or HLA-matched platelets for patients refractory to platelet transfusion. Transfusion 2014; 54:3080–3087. [DOI] [PubMed] [Google Scholar]
- 62.Nellis ME, Remy KE, Lacroix J, et al. Research Priorities for Plasma and Platelet Transfusion Strategies in Critically Ill Children: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carr R, Hutton JL, Jenkins JA, Lucas GF, Amphlett NW. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol 1990; 75:408–13. [DOI] [PubMed] [Google Scholar]
- 64.Balduini CL, Salvaneschi L, Klersy C, et al. Factors influencing post-transfusional platelet increment in pediatric patients given hematopoietic stem cell transplantation. Leukemia 2001; 15:1885–91. [DOI] [PubMed] [Google Scholar]
- 65.Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IA. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med 2002; 12:35–41. [DOI] [PubMed] [Google Scholar]
- 66.Chaudhary R, Khetan D, Sinha S, et al. Transfusion support to Dengue patients in a hospital based blood transfusion service in north India. Transfus Apher Sci 2006; 35:239–44. [DOI] [PubMed] [Google Scholar]
- 67.Baer VL, Lambert DK, Henry E, Snow GL, Sola-Visner MC, Christensen RD. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol 2007; 27:790–6. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara M, Capozzi L, Coppola A, Save G, Coppola L. Prophylactic platelet transfusion in children with thrombocytopenic disorders: a retrospective review. Hematology 2007; 12:297–9. [DOI] [PubMed] [Google Scholar]
- 69.Saigo K, Sakota Y, Masuda Y, et al. Automatic detection of immature platelets for decision making regarding platelet transfusion indications for pediatric patients. Transfus Apher Sci 2008; 38:127–32. [DOI] [PubMed] [Google Scholar]
- 70.Baer VL, Lambert DK, Henry E, Christensen RD. Severe Thrombocytopenia in the NICU. Pediatrics 2009; 124:e1095–100. [DOI] [PubMed] [Google Scholar]
- 71.Cui Y, Hei F, Long C, et al. Perioperative monitoring of thromboelastograph on hemostasis and therapy for cyanotic infants undergoing complex cardiac surgery. Artif Organs 2009; 33:909–14. [DOI] [PubMed] [Google Scholar]
- 72.Gerday E, Baer VL, Lambert DK, et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion 2009; 49:2034–9. [DOI] [PubMed] [Google Scholar]
- 73.Stanworth SJ, Clarke P, Watts T, et al. ; Platelets and Neonatal Transfusion Study Group. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 2009; 124:e826–34. [DOI] [PubMed] [Google Scholar]
- 74.Belen FB, Okur A, Kulali F, et al. Platelet usage trends in a tertiary care hospital - Could it be less and less expensive? Transfus Apher Sci 2012; 47:101–6. [DOI] [PubMed] [Google Scholar]
- 75.Honohan A, van’t Ende E, Hulzebos C, et al. Posttransfusion platelet increments after different platelet products in neonates: a retrospective cohort study. Transfusion 2013; 53:3100–9. [DOI] [PubMed] [Google Scholar]
- 76.Nosanov L, Inaba K, Okoye O, et al. The impact of blood product ratios in massively transfused pediatric trauma patients. Am J Surg 2013; 206:655–60. [DOI] [PubMed] [Google Scholar]
- 77.Vogel AM, Radwan ZA, Cox CS Jr, Cotton BA. Admission rapid thrombelastography delivers real-time “actionable” data in pediatric trauma. J Pediatr Surg 2013; 48:1371–6. [DOI] [PubMed] [Google Scholar]
- 78.Kahvecioglu D, Erdeve O, Alan S, et al. The impact of evaluating platelet transfusion need by platelet mass index on reducing the unnecessary transfusions in newborns. J Matern Fetal Neonatal Med 2014; 27:1787–9. [DOI] [PubMed] [Google Scholar]
- 79.Lieberman L, Liu Y, Portwine C, Barty RL, Heddle NM. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion 2014; 54:2736–44. [DOI] [PubMed] [Google Scholar]
- 80.Motta M, Del Vecchio A, Perrone B, Ghirardello S, Radicioni M. Fresh frozen plasma use in the NICU: a prospective, observational, multicentred study. Arch Dis Child Fetal Neonatal Ed 2014; 99:F303–8. [DOI] [PubMed] [Google Scholar]
- 81.Soundar EP, Besandre R, Hartman SK, Teruya J, Hui SK. Plasma is ineffective in correcting mildly elevated PT-INR in critically ill children: a retrospective observational study. J Intensive Care 2014; 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karam O, Demaret P, Shefler A, et al. Indications and Effects of Plasma Transfusions in Critically Ill Children. Am J Respir Crit Care Med 2015; 191:1395–402. [DOI] [PubMed] [Google Scholar]
- 83.Kaur A, Sethi GK, Goyal RK, et al. Thrombocytopenia in Paediatric ICU: Incidence, Transfusion Requirement and Role as Prognostic Indicator. J Clin Diagn Res 2015; 9:SC05–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pieters BJ, Conley L, Weiford J, et al. Prophylactic versus reactive transfusion of thawed plasma in patients undergoing surgical repair of craniosynostosis: a randomized clinical trial. Paediatr Anaesth 2015; 25:279–87. [DOI] [PubMed] [Google Scholar]
- 85.Pothapregada S, Kamalakannan B, Thulasingam M. Role of platelet transfusion in children with bleeding in dengue fever. J Vector Borne Dis 2015; 52:304–8. [PubMed] [Google Scholar]
- 86.Raban MS, Harrison MC. Fresh Frozen Plasma Use in a Neonatal Unit in South Africa. J Trop Pediatr 2015; 61:266–71. [DOI] [PubMed] [Google Scholar]
- 87.Ziegler B, Solomon C, Cadamuro J, Jones N. Thromboelastometric Monitoring of the Hemostatic Effect of Platelet Concentrates Transfusion in Thrombocytopenic Children Undergoing Chemotherapy. Clin Appl Thromb Hemost 2015; 21:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du Pont-Thibodeau G, Tucci M, Robitaille N, Ducruet T, Lacroix J. Platelet Transfusions in Pediatric Intensive Care. Pediatr Crit Care Med 2016; 17:e420–9. [DOI] [PubMed] [Google Scholar]
- 89.Kane LC, Woodward CS, Husain SA, Frei-Jones MJ. Thromboelastography--does it impact blood component transfusion in pediatric heart surgery? J Surg Res 2016; 200:21–7. [DOI] [PubMed] [Google Scholar]
- 90.Alsheikh B, Chegondi M, Totapally B. Platelet Transfusion Thresholds Among Children Admitted to a Pediatric Intensive Care Unit. Cureus 2017; 9:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arni D, Wildhaber BE, McLin V, et al. Effects of plasma transfusions on antithrombin levels after paediatric liver transplantation. Vox Sang 2018. May 15. doi: 10.1111/vox.12664. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 92.Chapin CA, Mohammad S, Bass LM, Taylor SA, Kelly S, Alonso EM. Liver Biopsy Can Be Safely Performed in Pediatric Acute Liver Failure to Aid in Diagnosis and Management. J Pediatr Gastroenterol Nutr 2018; 67:441–445. [DOI] [PubMed] [Google Scholar]
- 93.Deng Q, Hao F, Wang Y, Guo C. Rotation thromboelastometry (ROTEM) enables improved outcomes in the pediatric trauma population. J Int Med Res 2018; 46:5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leeper CM, Neal MD, Billiar TR, Sperry JL, Gaines BA. Overresuscitation with plasma is associated with sustained fibrinolysis shutdown and death in pediatric traumatic brain injury. J Trauma Acute Care Surg 2018; 85:12–17. [DOI] [PubMed] [Google Scholar]
- 95.Leibowitz M, Wolfe H, Flynn A, et al. Standardization of prophylactic platelet transfusion dosing in a pediatric oncology population: a quality improvement project. Transfusion 2018; 58:2836–2840. [DOI] [PubMed] [Google Scholar]
- 96.Nellis ME, Karam O, Mauer E, et al. Platelet Transfusion Practices in Critically Ill Children. Crit Care Med 2018; 46:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scott JP, Niebler RA, Stuth EAE, et al. Rotational Thromboelastometry Rapidly Predicts Thrombocytopenia and Hypofibrinogenemia During Neonatal Cardiopulmonary Bypass. World J Pediatr Congenit Heart Surg 2018; 9:424–433. [DOI] [PubMed] [Google Scholar]
- 98.Barker EE, Saini A, Gazit AZ, et al. TEG Platelet Mapping and Impedance Aggregometry to Predict Platelet Transfusion During Cardiopulmonary Bypass in Pediatric Patients. Front Pediatr 2019; 7:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ddungu H, Krantz EM, Kajja I, et al. How low can you go: What is the safe threshold for platelet transfusions in patients with hematologic malignancy in sub-Saharan Africa. PLoS One 2019; 14:e0211648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nellis ME, Goel R, Karam O, et al. Effects of ABO Matching of Platelet Transfusions in Critically Ill Children. Pediatr Crit Care Med 2019; 20:e61–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nellis ME, Goel R, Karam O, et al. International Study of the Epidemiology of Platelet Transfusions in Critically Ill Children With an Underlying Oncologic Diagnosis. Pediatr Crit Care Med 2019; 20:e342–e351. [DOI] [PubMed] [Google Scholar]
- 102.Nellis ME, Saini A, Spinella PC, et al. Pediatric Plasma and Platelet Transfusions on Extracorporeal Membrane Oxygenation: A Subgroup Analysis of Two Large International Point-Prevalence Studies and the Role of Local Guidelines. Pediatr Crit Care Med 2020; 21:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cashen K, Dalton H, Reeder RW, et al. Platelet Transfusion Practice and Related Outcomes in Pediatric Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2020; 21:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cunningham AJ, Condron M, Schreiber MA, et al. Rotational thromboelastometry predicts transfusion and disability in pediatric trauma. J Trauma Acute Care Surg 2020; 88:134–140. [DOI] [PubMed] [Google Scholar]
- 105.Dieu A, Van Regemorter V, Detaille T, et al. Combined Use of Rotational Thromboelastometry (Rotem) and Platelet Impedance Aggregometry (Multiplate Analyzer) in Cyanotic and Acyanotic Infants and Children Undergoing Cardiac Surgery With Cardiopulmonary Bypass: Subgroup Analysis of a Randomized Clinical Trial. J Cardiothorac Vasc Anesth 2021; 35:2115–2123. [DOI] [PubMed] [Google Scholar]
- 106.Jonas J, Durila M, Malosek M, et al. Usefulness of perioperative rotational thrombelastometry during scoliosis surgery in children. J Neurosurg Spine 2020; 24:1–6. [DOI] [PubMed] [Google Scholar]
- 107.Lee A, Mendoza J, Brubaker AL, et al. Eliminating international normalized ratio threshold for transfusion in pediatric patients with acute liver failure. Clin Transplant 2020; 34:e13819. [DOI] [PubMed] [Google Scholar]
- 108.Nellis ME, Spinella PC, Tucci M, et al. Effect of platelet storage duration on clinical outcomes and incremental platelet change in critically ill children. Transfusion 2020; 60:2849–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levy L, Woodfield DG. The transfusion of HLA-matched platelets to thrombocytopenic patients resistant to random donor platelets. N Z Med J 1984; 97:719–21. [PubMed] [Google Scholar]
- 110.Shanwell A, Ringdén O, Wiechel B, Rumin S, Akerblom O. A study of the effect of ABO incompatible plasma in platelet concentrates transfused to bone marrow transplant recipients. Vox Sang 1991; 60:23–7. [DOI] [PubMed] [Google Scholar]
- 111.Shanwell A, Larsson S, Aschan J, Ringdén O. A randomized trial comparing the use of fresh and stored platelets in the treatment of bone marrow transplant recipients. Eur J Haematol 1992; 49:77–81. [DOI] [PubMed] [Google Scholar]
- 112.Saarinen UM, Koskimies S, Myllylä G. Systematic use of leukocyte-free blood components to prevent alloimmunization and platelet refractoriness in multitransfused children with cancer. Vox Sang 1993; 65:286–92. [DOI] [PubMed] [Google Scholar]
- 113.Blundell EL, Pamphilon DH, Fraser ID, et al. A prospective, randomized study of the use of platelet concentrates irradiated with ultraviolet-B light in patients with hematologic malignancy. Transfusion 1996; 36:296–302. [DOI] [PubMed] [Google Scholar]
- 114.Gelb AB, Leavitt AD. Crossmatch-compatible platelets improve corrected count increments in patients who are refractory to randomly selected platelets. Transfusion 1997; 37:624–30. [DOI] [PubMed] [Google Scholar]
- 115.Riccardi D, Raspollini E, Rebulla P, et al. Relationship of the time of storage and transfusion reactions to platelet concentrates from buffy coats. Transfusion 1997; 37:528–30. [DOI] [PubMed] [Google Scholar]
- 116.Baudoux E, Margraff U, Coenen A, et al. Hemovigilance: clinical tolerance of solvent-detergent treated plasma. Vox Sang 1998;74 Suppl 1:237–9. [DOI] [PubMed] [Google Scholar]
- 117.Norol F, Bierling P, Roudot-Thoraval F, et al. Platelet transfusion: a dose-response study. Blood 1998; 92:1448–53. [PubMed] [Google Scholar]
- 118.Ness P, Braine H, King K, et al. Single-donor platelets reduce the risk of septic platelet transfusion reactions. Transfusion 2001; 41:857–61. [DOI] [PubMed] [Google Scholar]
- 119.Reinhard H, Seyfert U, Krenn T, Morsdorf S, Aliani S, Graf N. Comparison of Prefiltration and Bedside Filtration in Thrombocytopenic Children with Malignant Diseases. Infus Ther Transfus Med 2001; 28:344–348. [Google Scholar]
- 120.Hiramatsu T, Okamura T, Imai Y, et al. Effects of autologous platelet concentrate reinfusion after open heart surgery in patients with congenital heart disease. Ann Thorac Surg 2002; 73:1282–5. [DOI] [PubMed] [Google Scholar]
- 121.Couban S, Carruthers J, Andreou P, et al. Platelet transfusions in children: results of a randomized, prospective, crossover trial of plasma removal and a prospective audit of WBC reduction. Transfusion 2002; 42:753–8. [DOI] [PubMed] [Google Scholar]
- 122.Ronghe MD, Foot AB, Cornish JM, et al. The impact of transfusion of leucodepleted platelet concentrates on cytomegalovirus disease after allogeneic stem cell transplantation. Br J Haematol 2002; 118:1124–7. [DOI] [PubMed] [Google Scholar]
- 123.McCullough J, Vesole DH, Benjamin RJ, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood 2004; 104:1534–41. [DOI] [PubMed] [Google Scholar]
- 124.Perseghin P, Baldini V, Elli E, et al. Clinical efficacy of plasma-reduced platelet concentrates from multicomponent (MC) collection: A non-randomized prospective study in onco-haematological paediatric patients. Blood Transfusion 2004; 2:290–299. [Google Scholar]
- 125.de Alarcon P, Benjamin R, Dugdale M, et al. Fresh frozen plasma prepared with amotosalen HCl (S-59) photochemical pathogen inactivation: transfusion of patients with congenital coagulation factor deficiencies. Transfusion 2005; 45:1362–72. [DOI] [PubMed] [Google Scholar]
- 126.Snyder E, McCullough J, Slichter SJ, et al. Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation: the SPRINT trial. Transfusion 2005; 45:1864–75. [DOI] [PubMed] [Google Scholar]
- 127.Lapierre V, Mahé C, Aupérin A, et al. Platelet transfusion containing ABO-incompatible plasma and hepatic veno-occlusive disease after hematopoietic transplantation in young children. Transplantation 2005; 80:314–9. [DOI] [PubMed] [Google Scholar]
- 128.Murphy S, Snyder E, Cable R, et al. Platelet dose consistency and its effect on the number of platelet transfusions for support of thrombocytopenia: an analysis of the SPRINT trial of platelets photochemically treated with amotosalen HCl and ultraviolet A light. Transfusion 2006; 46:24–33. [DOI] [PubMed] [Google Scholar]
- 129.Mintz PD, Bass NM, Petz LD, et al. Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease. Blood 2006; 107:3753–60. [DOI] [PubMed] [Google Scholar]
- 130.Chekrizova V, Murphy WG. Solvent-detergent plasma: use in neonatal patients, in adult and paediatric patients with liver disease and in obstetric and gynaecological emergencies. Transfus Med 2006; 16:85–91. [DOI] [PubMed] [Google Scholar]
- 131.Osselaer JC, Cazenave JP, Lambermont M, et al. An active haemovigilance programme characterizing the safety profile of 7437 platelet transfusions prepared with amotosalen photochemical treatment. Vox Sang 2008; 94:315–23. [DOI] [PubMed] [Google Scholar]
- 132.Osselaer JC, Messe N, Hervig T, et al. A prospective observational cohort safety study of 5106 platelet transfusions with components prepared with photochemical pathogen inactivation treatment. Transfusion 2008; 48:1061–71. [DOI] [PubMed] [Google Scholar]
- 133.Heim D, Passweg J, Gregor M, et al. Patient and product factors affecting platelet transfusion results. Transfusion 2008; 48:681–7. [DOI] [PubMed] [Google Scholar]
- 134.Rasonglès P, Angelini-Tibert MF, et al. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a Chikungunya virus epidemic in Ile de La Réunion. Transfusion 2009; 49:1083–91. [DOI] [PubMed] [Google Scholar]
- 135.Diedrich B, Ringdén O, Watz E, Shanwell A. A randomized study of buffy coat platelets in platelet additive solution stored 1–5 versus 6–7 days prior to prophylactic transfusion of allogeneic haematopoietic progenitor cell transplant recipients. Vox Sang 2009; 97:254–9. [DOI] [PubMed] [Google Scholar]
- 136.Julmy F, Ammann RA, Taleghani BM, Fontana S, Hirt A, Leibundgut K. Transfusion efficacy of ABO major-mismatched platelets (PLTs) in children is inferior to that of ABO-identical PLTs. Transfusion 2009; 49:21–33. [DOI] [PubMed] [Google Scholar]
- 137.Cazenave JP, Waller C, Kientz D, et al. An active hemovigilance program characterizing the safety profile of 7483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. Transfusion 2010; 50:1210–9. [DOI] [PubMed] [Google Scholar]
- 138.Marktel S, Napolitano S, Zino E, et al. Platelet transfusion refractoriness in highly immunized beta thalassemia children undergoing stem cell transplantation. Pediatr Transplant 2010; 14:393–401. [DOI] [PubMed] [Google Scholar]
- 139.Lozano M, Knutson F, Tardivel R, et al. A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. Br J Haematol 2011; 153:393–401. [DOI] [PubMed] [Google Scholar]
- 140.Cazenave JP, Isola H, Waller C, et al. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient outcomes and component utilization during a 3-year period. Transfusion 2011; 51:622–9. [DOI] [PubMed] [Google Scholar]
- 141.Cid J, Carbassé G, Pereira A, et al. Platelet transfusions from D+ donors to D- patients: a 10-year follow-up study of 1014 patients. Transfusion 2011; 51:1163–9. [DOI] [PubMed] [Google Scholar]
- 142.Inaba K, Branco BC, Rhee P, et al. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma 2011; 71:1766–73. [DOI] [PubMed] [Google Scholar]
- 143.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med 2012; 13:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karafin M, Fuller AK, Savage WJ, King KE, Ness PM, Tobian AA. The impact of apheresis platelet manipulation on corrected count increment. Transfusion 2012; 52:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dannaway DC, Noori S. A randomized trial of platelet transfusions over 30 vs 120 minutes: is there an effect on post-transfusion platelet counts? J Perinatol 2013; 33:703–6. [DOI] [PubMed] [Google Scholar]
- 146.Yanagisawa R, Shimodaira S, Kojima S, et al. Replaced platelet concentrates containing a new additive solution, M-sol: safety and efficacy for pediatric patients. Transfusion 2013; 53:2053–60. [DOI] [PubMed] [Google Scholar]
- 147.Xiao W, Tormey CA, Capetillo A, Maitta RW. Allergic transfusion reactions to platelets are more commonly associated with prepooled than apheresis components. Vox Sang 2013; 105:334–40. [DOI] [PubMed] [Google Scholar]
- 148.Elhence P, Chaudhary RK, Nityanand S. Cross-match-compatible platelets improve corrected count increments in patients who are refractory to randomly selected platelets. Blood Transfus 2014; 12:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Politis C, Kavallierou L, Hantziara S, et al. Haemovigilance data on the use of methylene blue virally inactivated fresh frozen plasma with the Theraflex MB-Plasma System in comparison to quarantine plasma: 11 years’ experience. Transfus Med 2014; 24:316–20. [DOI] [PubMed] [Google Scholar]
- 150.Tobian AA, Fuller AK, Uglik K, et al. The impact of platelet additive solution apheresis platelets on allergic transfusion reactions and corrected count increment (CME). Transfusion 2014; 54:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cohn CS, Stubbs J, Schwartz J, et al. A comparison of adverse reaction rates for PAS C versus plasma platelet units. Transfusion 2014; 54:1927–34. [DOI] [PubMed] [Google Scholar]
- 152.Julmy F, Ammann RA, Fontana S, Taleghani BM, Hirt A, Leibundgut K. Transfusion efficacy of apheresis platelet concentrates irradiated at the day of transfusion is significantly superior compared to platelets irradiated in advance. Transfus Med Hemother 2014; 41:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bost V, Chavarin P, Boussoulade F, et al. Independent evaluation of tolerance of therapeutic plasma inactivated by amotosalen-HCl-UVA (Intercept ™) over a 5-year period of extensive delivery. Vox Sang 2015; 109:414–6. [DOI] [PubMed] [Google Scholar]
- 154.Cid J, Lozano M, Ziman A, et al. Low frequency of anti-D alloimmunization following D+ platelet transfusion: the Anti-D Alloimmunization after D-incompatible Platelet Transfusions (ADAPT) study. Br J Haematol 2015; 168:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Knutson F, Osselaer J, Pierelli L, et al. A prospective, active haemovigilance study with combined cohort analysis of 19,175 transfusions of platelet components prepared with amotosalen-UVA photochemical treatment. Vox Sang 2015; 109:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Noorman F, van Dongen TT, Plat MJ, Badloe JF, Hess JR, Hoencamp R. Transfusion: −80°C Frozen Blood Products Are Safe and Effective in Military Casualty Care. PLoS One 2016; 11:e0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhat A, Chowdappa V, Masamatti SS. Effectiveness of Pooled Platelet Transfusion in Concordant and Discordant Groups among Dengue Patients. J Clin Diagn Res 2016; 10:EC21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trakhtman P, Karpova O, Balashov D, et al. Efficacy and safety of pathogen-reduced platelet concentrates in children with cancer: a retrospective cohort study. Transfusion 2016; 56 Suppl 1:S24–8. [DOI] [PubMed] [Google Scholar]
- 159.Kaplan A, Lindgren B, Marschner S, et al. Evaluation of the post-transfusion platelet increment and safety of riboflavin-based pathogen reduction technology (PRT) treated platelet products stored in platelet additive solution for 5 days or less versus 6–7 days. Transfus Apher Sci 2016; 54:248–52. [DOI] [PubMed] [Google Scholar]
- 160.Hao B, Wang Y, Zhou J, Shao S, Dong X. Comparison between the clinical efficacy of platelet concentrates, derived from buffy coat and apheresis in tumor patients. Oncol Lett 2017; 14:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017; 83:25–29. [DOI] [PubMed] [Google Scholar]
- 162.Amato M, Schennach H, Astl M, et al. Impact of platelet pathogen inactivation on blood component utilization and patient safety in a large Austrian Regional Medical Centre. Vox Sang 2017; 112:47–55. [DOI] [PubMed] [Google Scholar]
- 163.Dunbar NM, Yazer MH; Biomedical Excellence for Safer Transfusion (BEST) Collaborative and the STAT Study Investigators. Safety of the use of group A plasma in trauma: the STAT study. Transfusion 2017; 57:1879–1884. [DOI] [PubMed] [Google Scholar]
- 164.Noens L, Vilariño MD, Megalou A, Qureshi H. International, prospective haemovigilance study on methylene blue-treated plasma. Vox Sang 2017; 112:352–359. [DOI] [PubMed] [Google Scholar]
- 165.van Hout FMA, van der Meer PF, Wiersum-Osselton JC, et al. Transfusion reactions after transfusion of platelets stored in PAS-B, PAS-C, or plasma: a nationwide comparison. Transfusion 2018; 58:1021–1027. [DOI] [PubMed] [Google Scholar]
- 166.Kalsi AS, Al-Azzawi O, Gill R. Comparison of the Coagulation Effect Achieved by OctaplasLG Versus Fresh Frozen Plasma in Pediatric Cardiac Surgical Patients. Clin Appl Thromb Hemost 2018; 24:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caram-Deelder C, van der Bom JG, Putter H, et al. Age of platelet concentrates and time to the next transfusion. Transfusion 2018; 58:121–131. [DOI] [PubMed] [Google Scholar]
- 168.Kobayashi J, Yanagisawa R, Ono T, et al. Administration of platelet concentrates suspended in bicarbonated Ringer’s solution in children who had platelet transfusion reactions. Vox Sang 2018; 113:128–135. [DOI] [PubMed] [Google Scholar]
- 169.Kojima S, Yanagisawa R, Tanaka M, Nakazawa Y, Shimodaira S. Comparison of administration of platelet concentrates suspended in M-sol or BRS-A for pediatric patients. Transfusion 2018; 58:2952–2958. [DOI] [PubMed] [Google Scholar]
- 170.Leeper CM, Yazer MH, Cladis FP, Saladino R, Triulzi DJ, Gaines BA. Cold-stored whole blood platelet function is preserved in injured children with hemorrhagic shock. J Trauma Acute Care Surg 2019; 87:49–53. [DOI] [PubMed] [Google Scholar]
- 171.Infanti L, Holbro A, Passweg J, et al. Clinical impact of amotosalen-ultraviolet A pathogen-inactivated platelets stored for up to 7 days. Transfusion 2019; 59:3350–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kingsley S, Chacko MP, Amal P, et al. Frequency of Platelet Crossmatch Positivity and Predictive Value for Poor Platelet Increment Among Paediatric Oncohaematology Patients in India. Indian J Hematol Blood Transfus 2020; 36:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nadler R, Mozer-Glassberg Y, Gaines B, Glassberg E, Chen J. The Israel Defense Forces experience with freeze-dried plasma for the resuscitation of traumatized pediatric patients. J Trauma Acute Care Surg 2019; 87:1315–1320. [DOI] [PubMed] [Google Scholar]
- 174.Saadah NH, Schipperus MR, Wiersum-Osselton JC, et al. Transition from fresh frozen plasma to solvent/detergent plasma in the Netherlands: comparing clinical use and transfusion reaction risks. Haematologica 2020; 105:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lieberman L, Liu Y, Barty R, Heddle NM. Platelet transfusion practice and platelet refractoriness for a cohort of pediatric oncology patients: A single-center study. Pediatr Blood Cancer 2020; 67:e28734. doi: 10.1002/pbc.28734. [DOI] [PubMed] [Google Scholar]
- 176.Malvik N, Leon J, Schlueter AJ, Wu C, Knudson CM. ABO-incompatible platelets are associated with increased transfusion reaction rates. Transfusion 2020; 60:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tanaka M, Yanagisawa R, Yamanaka M, et al. Transfusion outcome for volume- and plasma-reduced platelet concentrates for pediatric patients. Transfus Apher Sci 2020; 59:102776. doi: 10.1016/j.transci.2020.102776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


