Abstract
Objectives:
To present the recommendations and consensus statements with supporting literature for plasma and platelet transfusions in critically ill neonates and children undergoing cardiac surgery with cardiopulmonary bypass (CPB) or supported by extracorporeal membrane oxygenation (ECMO) from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB).
Design:
Systematic review and consensus conference of international, multidisciplinary experts in platelet and plasma transfusion management of critically ill children.
Setting:
Not applicable.
Patients:
Critically ill neonates and children following CPB or supported by ECMO
Interventions:
None
Measurements and Main Results:
A panel of 9 experts developed evidence-based and, when evidence was insufficient, expert based statements for plasma and platelet transfusions in critically ill neonates and children following CPB or supported by ECMO. These statements were reviewed and ratified by the 29 TAXI-CAB experts. A systematic review was conducted using MEDLINE, EMBASE, and Cochrane Library databases, from inception to December 2020. Consensus was obtained using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Results were summarized using the Grading of Recommendations Assessment, Development, and Evaluation method. We developed 1 good practice statement, 2 recommendations and 3 expert consensus statements.
Conclusions:
Whereas viscoelastic testing and transfusion algorithms may be considered, in general, evidence informing indications for plasma and platelet transfusions in neonatal and pediatric patients undergoing cardiac surgery with CPB or those requiring ECMO support is lacking.
Keywords: child, critical care, evidence-based, guidelines, intensive care, cardiopulmonary bypass, extracorporeal membrane oxygenation, ECMO, pediatrics, plasma, platelet transfusion
INTRODUCTION
Neonates and children undergoing cardiac surgery requiring cardiopulmonary bypass (CPB) or those requiring extracorporeal membrane oxygenation (ECMO) support for any reason are uniquely vulnerable to significant bleeding and/or arterial/venous thrombosis as a result of complex interactions between blood cells, the vascular endothelium, and CPB and ECMO circuitry, leading to altered hemostasis [1–4]. Presence of intra-cardiac shunts and arterial cannula increases the risk of life- and limb-threatening thromboembolic events (TEE), and vascular insufficiency [5–7]. The need for systemic anticoagulation to maintain CPB and ECMO circuit patency, as well as that of prosthetic valves, conduits and shunts, increases bleeding risk and limits anti-thrombotic therapies [8,9]. Bleeding risk can be further compounded by poor surgical hemostasis, hepatic dysfunction, sepsis, and disseminated intravascular coagulopathy (DIC) [3,10]. Developmental differences in the neonatal and infant coagulation system, and inherent size discrepancy in circulating blood volume and circuit volume causing dilution, further increase bleeding and thrombotic risk in the youngest (and smallest) patients, complicating their transfusion management [11–16].
Blood products, specifically plasma and platelets, are transfused commonly to control or avoid bleeding in children who require ECMO support or CPB [17–21]; management must be balanced with avoidance of thromboembolic complications. Bleeding and transfusion of coagulant products is associated with worse clinical outcomes in these populations [22–25]. Standard laboratory measures of coagulation and platelet count do not always indicate the cause of coagulopathy, and additional measures to assess fibrinolysis and platelet function may help guide the practitioner in their transfusion decision-making [26,27]. The goal is for judicious and targeted plasma and platelet transfusions to manage bleeding, without initiating or propagating thrombosis that can threaten both the patient’s circulation and mechanical circuit’s patency. In this report from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) experts, we report the CPB/ECMO subgroups’ assessment of the literature, along with recommendations and consensus statements.
METHODS
The search strategy, item selection and recommendation generation used to identify and select references for systematic review and to develop recommendation are detailed in the general manuscript of TAXI-CAB [28]. Briefly, we searched Ovid MEDLINE®, Ovid EMBASE, and Cochrane Library (Wiley) from inception through December 2020 using a combination of medical subject heading terms and text words to define concepts of plasma or platelet transfusion, transfusion triggers, laboratory tests to assess efficacy of transfusion in children admitted to the pediatric intensive care unit (PICU) following CPB or supported by ECMO. For articles selected for inclusion, reference lists and citing articles were selected from Scopus (Elsevier) and screened. Two reviewers independently reviewed all citations and performed data extraction and assessments of bias. Literature was reviewed for relevance to this subgroup. Research Electronic Data Capture (REDCap) hosted at Weill Cornell Medicine was used for standardized data extraction. We used a standardized data extraction form to construct evidence tables and graded the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [29].
Nine experts participated in the development of recommendations from this subgroup. A panel of 29 experts convened in an on-line format over 18 months to develop good practice statements, recommendations and, when evidence was lacking, expert consensus statements. Good practice statements are those in which there is high-level of certainty that the practice will do more good than harm, but there is little in the way of supporting literature evidence. Expert consensus statements are based on the expert opinion of the group, but in areas where research is likely needed. All statements from each subgroup were reviewed by the full panel of experts and voted on using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Agreement was defined a priori as >80% of all experts. The recommendations and statements are intended to apply to infants, children and adolescents. For cardiac surgery with CPB, only interventions that occurred following heparin reversal and protamine administration were included, therefore excluding CPB priming or transfusions during CPB. Priming strategies and composition were considered outside of the scope of our search. ECMO was defined according to the Extracorporeal Life Support Organization (ELSO) [30]. ECMO-related articles were included for any support mode (venovenous [VV], venovenoarterial [VVA], or venoarterial [VA]) and for cardiac, cardiac and respiratory, respiratory, and extracorporeal cardiopulmonary resuscitation ECMO applications. Prophylactic transfusions are those prescribed to patients at risk of bleeding, whereas therapeutic transfusions are given to those with active bleeding.
RESULTS
Searching CPB and ECMO identified 3332 and 3219 abstracts, respectively. After duplicates were removed, a total of 2937 and 2829 abstracts were screened. Then, out of 120 and 72 full text manuscripts about CPB and ECMO, respectively, we selected 36 and 30 papers for detailed review (see Figures 1 and 2). These papers underwent data extraction and assessment of bias in order to generate recommendations and statements (see Supplemental Data Tables 1 and 2). One good practice statement, two recommendations and three expert consensus statements were produced. The voting data, including the number of voting experts and median score, are provided for each statement and recommendation.
Figure 1:

Papers Flow Chart for Critically ill children following cardiopulmonary bypass
Figure 2:
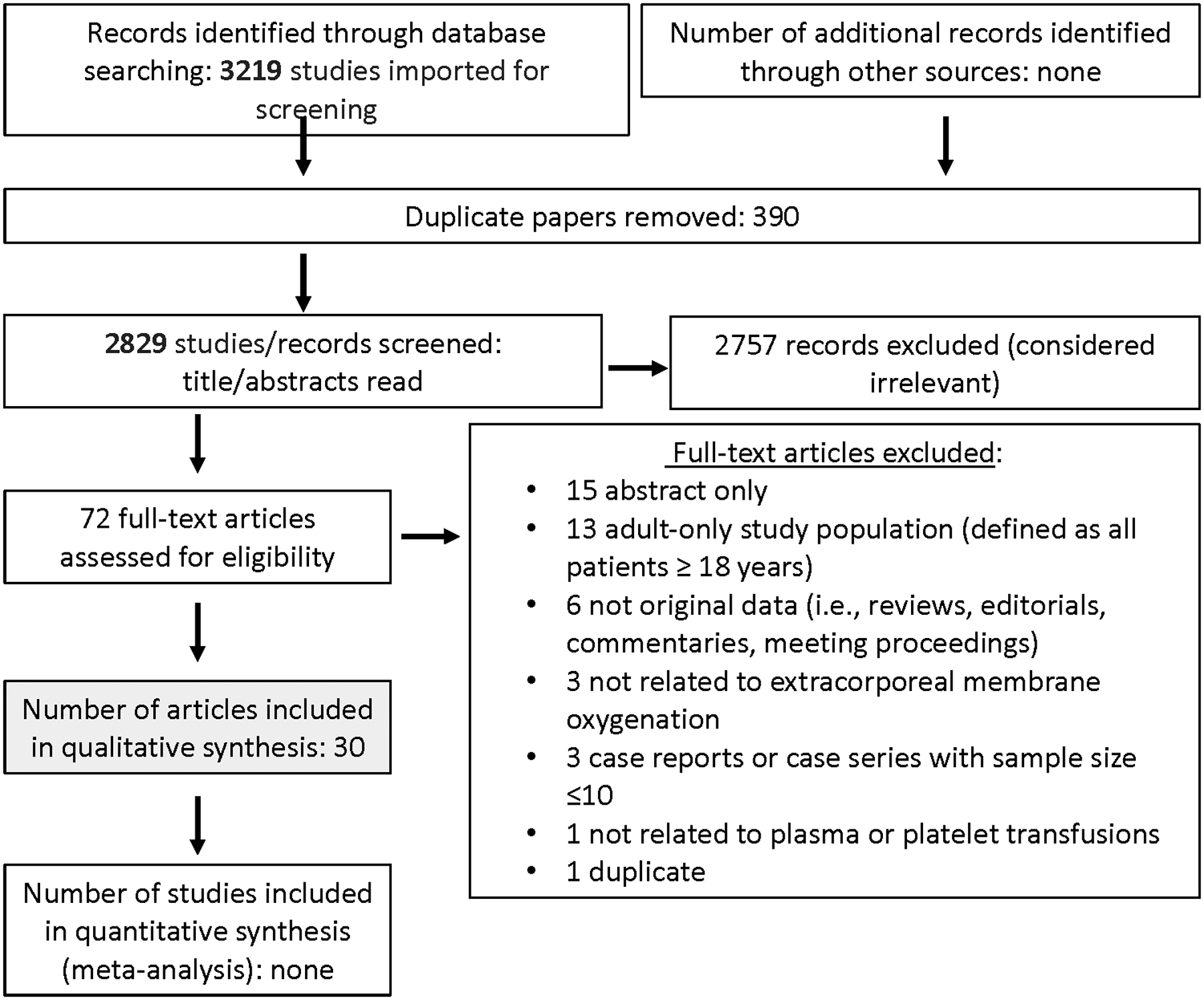
Papers Flow Chart for Critically ill children supported by ECMO
Indications for Plasma or Platelet Transfusions in Critically Ill Neonates and Children Following Cardiopulmonary Bypass Surgery
Clinical Recommendations
3.1. In neonatal and pediatric patients undergoing cardiac surgery with CPB, viscoelastic testing (VET) might be considered as an adjunct to standard hemostatic testing (in the operating room after rewarming and protamine, and in the intensive care unit) to inform decisions regarding prophylactic and therapeutic transfusions of plasma and/or platelets. Weak recommendation, Moderate quality level of evidence (2B), 82% Agreement (n=22), Median 8, IQR 7–9.
Rationale:
The use of intra operative VET is increasingly used for anticoagulation monitoring during the course of CPB and to guide the choice of blood product transfusion postoperatively [31,32]. The implementation of VET in the pediatric population has lagged behind the adult experience in part due to the limitations in implementing pediatric transfusion trials. Despite these challenges, significant progress has been made in understanding the differences in clot formation of children compared to adults, and the role of VET during pediatric cardiac surgery. Mendeloff et al presented thromboelastography (TEG) data from an observational cohort of 112 children (< 6 months of age) undergoing cardiac surgery compared to historical retrospective controls [33]. In the absence of a formal transfusion algorithm, they found a significant decrease in the use of platelet transfusions (1.7 vs. 3.7 mL/kg; p = 0.006) post operatively, based on TEG results.
In a randomized controlled trial (RCT) Cui et al. evaluated the use of TEG-guided transfusion of fibrinogen concentrate, plasma and platelets compared to clinically guided transfusion in children with cyanotic heart disease. While this study did not identify significant differences in the intraoperative volume of transfusion of platelets, the use of TEG was associated with shorter hospital ICU length of stay and less transfusion of plasma in the ICU [34].
A subsequent RCT conducted by Nakayama et al. evaluated the effect of post-bypass Rotational Thromboelastometry (ROTEM) results on the decision to transfuse platelets and plasma in 78 pediatric patients. EXTEM and FIBTEM at the A10 time point with values of 30 mm and 5 mm were identified as targets to maintain or reduce post-operative bleeding, red blood cell transfusion and ICU length of stay [1]. More recently Kane et al. sought to determine the cost-effectiveness of TEG implementation at reducing blood product utilization in a cohort of pediatric patients undergoing cardiac surgery. In this study, the TEG-guided transfusion cohort experienced a significant reduction in the volume of transfused platelets (1 unit vs. 2.2 units, p < 0.0001) and cryoprecipitate (0.7 vs. 1.7 units, p < 0.0001), with associated reduced cost. Mortality and other major outcomes were similar between the two groups [35].
In 2018, Emani et al. identified specific TEG cut-off values for transfusion in patients undergoing cardiac surgery with CPB, in a large retrospective cohort of pediatric patients that included neonates to children younger than 18 years of age. This single center study examined the association between prophylactic transfusion of platelets in the operating room and the presence of a composite outcome (extended bleeding/transfusion requirements and/or surgical exploration) in the ICU. In patients with low MA, platelet transfusion was associated with lower proportion of cases meeting the outcome (7/93 vs 8/33, 95%CI of the difference 3.2 to 33.9%, p = 0.02) [36].
Overall, the available data for VET in pediatric trials are sufficient to make a weak recommendation regarding the use of TEG or ROTEM in the ICU after cardiac surgery. Although VET provides accurate depiction of the characteristics of clot formation in a dynamic manner, the lag time between the start of assay to the reporting of results may be a limitation guiding transfusion, given the rapid clinical changes encountered in pediatric heart surgery particularly in the immediate post-bypass period. The statistical power of most studies published to date along with significant differences in practice between institutions also limits the applicability of the results. Monitoring coagulation with point of care VET technology during CPB regularly and adjusting the criteria for transfusion at individual intuitions may provide sufficient evidence to develop patient specific recommendations for transfusion. VET-guided protocols may help in maximizing hemostasis, reducing the amount of wasted blood products, institutional costs, and associated complications.
3.2. In neonatal and pediatric patients undergoing cardiac surgery with CPB, the development of institution-specific transfusion algorithms for post-bypass (after rewarming and protamine, and in intensive care unit) transfusion management might be considered to reduce individual case and global overall blood product usage. Weak recommendation, Moderate quality level of evidence (2B), 96% Agreement (n=23), Median 8, IQR 8–9.
Rationale:
Transfusion practices vary across and within pediatric cardiac surgical centers and are influenced by patient factors (age, weight, gestation, hemodynamics, oxygen saturation, cardiac morphology, comorbidities), surgical factors (type of procedure or palliation), anesthesia and perfusion factors (volumes, duration, temperature), conventional laboratory indices, and clinical assessment of the presence and risk of bleeding [3]. Transfusion of plasma and platelets is often empiric, highly variable, and lacking standardization in cardiac surgery [37–39].
Whitney et al. instituted a blood product transfusion algorithm based on conventional coagulation studies in a single institution’s pediatric cardiac surgical program and compared number of blood transfusions for the 12 months preceding (N=303) and 11 months following its implementation (N = 246) [40]. Their transfusion algorithm reduced operating room and ICU total transfusions when assessed per patient case (p < 0.001) and per month (p = 0.019) without an increase in bleeding. After controlling for surgical severity and CPB duration, their transfusion algorithm was associated with a 0.247 relative risk of mortality (p = 0.013).
Addition of point-of-care (POC) testing that more broadly reflects hemostasis (including qualitative platelet function and fibrinolysis) will allow for targeted transfusion therapy reducing blood transfusions in adult cardiac surgery [41,42]. Benefits of transfusion algorithms that incorporate POC VET testing have also been described in pediatric cardiac [43] and craniosynostosis surgery [44]. Faraoni et al. created a VET based transfusion algorithm and concluded that reserving VET testing for bleeding patients increases the sensitivity and specificity of these tests and avoids unnecessary transfusions in non-bleeding patients [45].
In an RCT of 170 children > 10 years of age with cyanotic heart disease undergoing cardiac surgery with CPB, subjects were randomized to a conventional or ROTEM transfusion algorithm group. Karanjkar et al found that children managed with the ROTEM algorithm received fewer plasma and platelet transfusions (p < 0.001), with reduced ICU, ventilator, and hospital duration [46]. Patients were included if after heparin reversal they had diffuse capillary bed bleeding requiring hemostatic therapy, or post-operative blood loss > 2 mL/kg/hr during the first 24 hours post-op.
These studies provide weak evidence for pediatric cardiac surgical centers to implement blood transfusion guidelines that incorporate hemostatic testing in patients with clinically significant bleeding after protamine reversal. Adoption of transfusion guidelines may reduce individual and institutional blood transfusions and so they may be considered. Conventional coagulation testing may be all that is required in the non-bleeding patient, but additional POC (e.g., VET) hemostatic testing should be available to guide transfusion decisions in the bleeding patient.
Expert Consensus Statement
3.3. In neonatal and pediatric patients undergoing cardiac surgery, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 87% Agreement (n=23), Median 8, IQR 8–9.
Rationale:
The end goal of clinical trials investigating indications and strategies to direct plasma or platelet transfusion following cardiac surgery is to control bleeding [47–49]. However, trials have been limited by the lack of universally accepted bleeding definitions for use in CPB. Adult and pediatric clinical trials have thus far reported various outcomes including allogenic transfusion exposures, anastomotic hemostasis time, postoperative drainage output, reoperation, or combinations of clinical composites or bleeding scores [35, 36, 50–54]. Limitations and challenges include the heterogeneity of bleeding risk and management, age/gestational age, weight, primary cardiac diagnoses, pre-existing conditions, end-organ compromise, hemostatic alterations due to CPB, operative characteristics (such as procedure complexity, re-operation(s), single vs. multiple procedures), surgical technique variability and surgical hemostatic control, and CPB characteristics [54–57]. Bleeding resulting in transfusion in neonatal and pediatric patients is associated with poor outcomes, and reoperation due to excessive hemorrhage is associated with increased morbidity, mortality, and expense [58,59]. While standardized management algorithms have been shown to decrease bleeding, transfusions, and surgical re-exploration, others have reported no differences, and findings may be center-specific [35,36,40,43,44,46,60–62]. Thus, variable institutional standards and published guidelines across adult and pediatric populations recommend different algorithms, including incorporating VET and newer hemostatic agents, and none have been rigorously studied to be widely embraced.
Indications for Plasma or Platelet Transfusions in Critically Ill Neonates and Children on ECMO Support
Good Practice Statement
4.1. In critically ill neonatal and pediatric patients on ECMO, we suggest measuring platelet counts and coagulation system dysfunction before all platelet and plasma transfusions, unless the patient experiences life-threatening bleeding. Consensus Panel Expertise, 92% Agreement (n=24), Median 9, IQR 7.25–9.
Rationale:
In the absence of clinically significant bleeding and/or laboratory results predictive of bleeding and response to transfusion products, transfusion of platelets and/or plasma may expose patients to the risks of transfusion without potential benefit [63,64]. Therefore, we suggest that laboratory assays be used to help direct the decision to transfuse.
Expert Consensus Statements
4.2. In critically ill neonatal and pediatric patients on ECMO, there is insufficient evidence to make a recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. Consensus Panel Expertise, 96% Agreement (n=23), Median 8, IQR 8–9.
Rationale:
Pediatric transfusion guidelines published in 2002 acknowledged the lack of interventional trial data to recommend platelet transfusion thresholds in children supported on ECMO. General practice at most centers was to transfuse platelets to keep platelet count >100 × 109/L or transfuse to higher levels during active bleeding [65]. General practice for pediatric plasma transfusion included presence of bleeding or planned invasive procedure in patients with “significantly prolonged” PT and/or aPTT or a documented coagulation factor. No specific reference to plasma transfusion indications during neonatal or pediatric ECMO was made.
To date, despite increased use of VET with conventional coagulation tests, absence of data from interventional trials has remained. The 2018 ELSO guidelines cited that it is “usual practice” to transfuse platelets to keep platelet count >80 × 109/L, while acknowledging that platelet function may be impaired at this, or higher platelet counts [66]. This guideline included recommendations for platelet transfusions to maintain platelet count >100 × 109/L “if bleeding,” and plasma transfusion if deficiencies in clotting factors are demonstrated.
The rationale for platelet transfusions to prevent bleeding on ECMO derives from studies describing platelet dysfunction in neonatal and pediatric ECMO patients [63,64]. Prophylactic platelet transfusions are common, with thresholds that ranging from 50 to 100 ×109/L [67,68]. An international point prevalence study of platelet transfusion practices in critically ill children found that neonates and children supported with ECMO received a median (IQR) of 8 (3, 18) platelet transfusions with a median platelet transfusion volume of 92 ml/kg over the course of their ICU stay [69]. Median (IQR) pre-transfusion platelet count was 70 (52, 90) ×109/L; 83% of responding centers reported having platelet transfusion protocols, with a reported median (IQR) platelet transfusion threshold of 84 (57, 100) ×109/L for non-bleeding patients.
Thrombocytopenia and platelet transfusion volume have each been associated with adverse clinical outcomes in pediatric ECMO patients [70,71]. In a secondary analysis of 514 neonates and children supported with ECMO from the Bleeding and Thrombosis during ECMO (BATE) study, 97% of patients received at least one platelet transfusion, with lower average daily platelet count (≤ 115 ×109/L) during ECMO being associated with mortality [67]. Platelet counts above 115 ×109/L were not associated with mortality, suggesting that thrombocytopenia below this threshold may be harmful. However, it could not be determined if the increased mortality resulted from thrombocytopenia or from the greater platelet transfusion volume.
These observational studies are challenging to interpret. However, it is notable that a recent RCT of liberal (<50 ×109/L) vs. restrictive (<25 ×109/L) platelet transfusion threshold in preterm infants, those in the liberal transfusion arm experienced a higher rate of the composite outcome of death or major bleeding [72]. Though ECMO patients were not included in this trial, the data suggest that prophylactic platelet transfusion in a high-risk group and may be harmful.
Summarizing the evidence suggests platelet transfusions are likely harmful and should be avoided when possible. However, the optimal thresholds at which platelets should be administered have not been determined. Likewise, we do not know whether the combination of platelet function assays and platelet count and/or VET would provide information for platelet transfusions that would more accurately predict response to bleeding.
Reduction in coagulation factors is common following initiation of ECMO, and identification of these abnormal values results in plasma transfusions in effort to prevent bleeding and bleeding complications [73,74]. Data to inform decisions regarding plasma transfusions in neonates and children supported by ECMO are complex, as anticoagulation strategies, hemostatic monitoring and plasma transfusion practices vary across institutions [75]. A single center RCT testing effect of scheduled plasma transfusion (q48 hrs) vs standard plasma transfusion practice on ECMO circuit life reported similar volume of plasma transfused in the two groups, with no difference in ECMO circuit life, or in platelet or red blood cell transfusion volumes administered during the ECMO course [76]. The rates of thrombotic and bleeding complications were similar between groups. While study design did not allow for conclusions regarding indications for plasma transfusions, results suggest that scheduled plasma transfusions were of no benefit.
4.3. In critically ill neonatal and pediatric patients on ECMO, prophylactic platelet transfusion in the absence of clinically significant bleeding is unlikely to benefit patients if the platelet count is >100 ×109/L (100,000/mm3). Consensus Panel Expertise, 91% Agreement (n=23), Median 9, IQR 8–9.
Rationale:
The majority of reviewed studies reported a platelet transfusion threshold of 50–100 ×109/L to guide platelet transfusion during neonatal and pediatric ECMO support (Supplemental Table 1). Several studies included in this review suggested increased risk of hemorrhage associated with platelet counts <100 ×109/L or <50 ×109/L [77–79]. Two neonatal ECMO studies published in 1994 and 1998 suggested lower rates of hemorrhage and no increase in total volume of platelet transfusions when the threshold for platelet transfusion was raised to 200 ×109/L [80,81]. However, these findings have not been reproduced. In addition, while this statement is focused on platelet count, one might take the platelet function into account when considering platelet transfusion.
DISCUSSION
Prevention and management of bleeding in neonates and children undergoing cardiac surgery with CPB, or those managed with ECMO, are complex clinical challenges. Variations in cardiac morphology (including cyanosis) and/or co-morbidities requiring ECMO support (cardiac v. non-cardiac, VV v. VA, or central v. peripheral cannulation) contribute to the complexity. Our systematic review demonstrated that apart from consideration of the use of VET and transfusion algorithms, evidence does not exist to direct recommendations on plasma and platelet transfusion strategies in these complex children.
In addition to TAXI-CAB, several groups have recognized the importance of guideline development in children following CPB or supported by ECMO. A panel of experts recently convened a workshop sponsored by the National Heart, Lung and Blood Institute (NHLBI) to develop consensus-based recommendations for primary clinical trial outcomes of hemostatic interventions in bleeding children [82]. They described limitations in currently published work similar to the limitations we have described and propose standardized outcomes for children following CPB and ECMO. These include the total allogeneic blood products administered intraoperatively and postoperatively to day 5 in CBP and a 5-point ordinal score of both thrombosis and bleeding severity in ECMO patients. In addition, the Pediatric ECMO Anticoagulation CollaborativE (PEACE), a similarly structured systematic review and expert consensus conference series, is underway and may address some of these limitations [83].
Lastly, one must recognize limitations imposed by grouping children following CPB with those supported by ECMO. Though similarities exist with the effects of the circuitry and anticoagulation on bleeding risk, the underlying pathophysiology of the two groups may be distinct. For example, a child cannulated to ECMO for cardiovascular collapse in the setting of septic shock may have additional and different risk factors for bleeding as compared to the child undergoing cardiac surgery with CPB. These differences must be taken into account when considering the expert consensus statements of this group.
CONCLUSIONS
The use of viscoelastic and platelet function testing, and adoption of institution specific transfusion algorithms may benefit children in the control and avoidance of bleeding, and reduction in plasma and platelet transfusion. However, insufficient evidence precludes the development of further recommendations.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all members of TAXI-CAB for their support and input, especially during the COVID-19 pandemic. In addition, we thank the Chaire Héma-Québec-Bayer en médecine transfusionnelle de l’Université de Montréal, the Society for the Advancement of Blood Management, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, the International Society of Blood Transfusion, the Society for Critical Care Medicine, and the AABB for their support.
Financial Support:
The Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute under award number R13 HL154544-01. Support for this work included funding from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS106292 (MMB).
Copyright Form Disclosure:
Dr. Emani disclosed that he is a consultant for Chiesi Pharmaceuticals. Dr. Steiner received funding from PumpKIN DSMB and HealthCore. Drs. Nellis and Bembea received support for article research from the National Institutes of Health. Dr. Bembea’s institution received funding from the National Institute of Neurological Disorders and Stroke (R01NS106292), the National Institute of Child Health and Human Development, and Grifols Investigator Sponsored Research Grant. The remaining authors have disclosed that they do not have any potential conflicts of interest.
APPENDIX 1. Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members
(* for executive committee) Co-chairs: Marianne E. Nellis, MD, MS*, Weill Cornell Medicine, New York, NY, and Robert I. Parker, MD*, Renaissance School of Medicine, State University of New York at Stony Brook, Stony Brook, NY; Content Experts: Section 1. Laboratory assays used to assess need for plasma and/or platelet transfusions: Scot T. Bateman, MD*, University of Massachusetts Medical School, Worcester, MA, Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Kenneth E. Remy, MD, MHSc, MSCI, Washington University of St. Louis, St. Louis, MO, Katherine Steffen, MD, Stanford University, Palo Alto, CA; Section 2. Traumatic brain injury and intracranial hemorrhage: David F. Bauer, MD, MPH, Baylor College of Medicine, Houston, TX, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA; Section 3. Following cardiopulmonary bypass: Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Sitaram Emani, MD, Harvard Medical School, Boston, MA, Juan Ibla, MD, Harvard Medical School, Boston, MA, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 4. Supported by extracorporeal membrane oxygenation: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 5. Following severe trauma: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 6. With oncologic diagnosis or following hematopoietic stem cell transplantation: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Lani Lieberman, MD, University of Toronto, Canada, Simon J. Stanworth, MD, University of Oxford, UK, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 7. With acute liver failure or following liver transplantation: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 8. Following non-cardiac surgery: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Marisa Tucci, MD, Université de Montréal, Montreal, QC, Canada, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 9. Invasive procedures outside of the operating room: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 10. Sepsis and/or disseminated intravascular coagulation: Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Simon J. Stanworth, MD, University of Oxford, UK, Katherine Steffen, MD, Stanford University, Palo Alto, CA, Stacey L. Valentine, MD, MPH*, University of Massachusetts Medical School, Worcester, MA; Section 11. Product processing and selection: Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH; Evidence-based medicine: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Diana Delgado and Michelle Demetres, Weill Cornell Medicine, New York, NY; Implementation science: Katherine Steffen, MD, Stanford University, Palo Alto, CA.
REFERENCES
- 1.Nakayama Y, Nakajima Y, Tanaka KA, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric surgery. Brit J Anaesth 2015; 114:91–102. [DOI] [PubMed] [Google Scholar]
- 2.Ali U, Goldenberg N, Foreman C, et al. Association between cyanosis, transfusion, and thrombotic complications in neonates and children undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2020; 34:349–55. [DOI] [PubMed] [Google Scholar]
- 3.Cholette JM, Faraoni D, Goobie SM, Ferraris V, Hassan N. Patient blood management in pediatric cardiac surgery: a review. Anesth Analg 2018; 127:1002–1016. [DOI] [PubMed] [Google Scholar]
- 4.Despotis GJ, Gravlee G, Filos K, Levy J. Anticoagulation monitoring during cardiac surgery. Anesthesiology 1999; 91:1122–51. [DOI] [PubMed] [Google Scholar]
- 5.Odegard KC, Zurakowski D, DiNardo JA, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage 1 through Fontan completion. J Thorac Cardiovasc Surg 2009; 137:934–41. [DOI] [PubMed] [Google Scholar]
- 6.Murphy LD, Benneyworth BD, Moser EA, Hege KM, Valentine KM, Mastropietro CW. Analysis of patient characteristics and risk factors for thrombosis after surgery for congenital heart disease. Pediatr Crit Care Med 2018; 19:1146–52. [DOI] [PubMed] [Google Scholar]
- 7.Giglia TM, Massicotte MP, Tweddell JS, et al. American Heart Association Congenital Heart Defects Committee on the Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Stroke Council: Prevention and treatment of thrombosis in pediatric and congenital heart disease: A scientific statement from the American Heart Association. Circulation 2013; 128:2622–2703. [DOI] [PubMed] [Google Scholar]
- 8.Manlhiot C, Menjak IB, Brandao LR, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation 2011; 124:1511–19. [DOI] [PubMed] [Google Scholar]
- 9.Emani S, Zurakowski E, Baird CW, et al. Hypercoagulability markers predict thrombosis in single ventricle neonates undergoing cardiac surgery. Ann Thorac Surg 2013; 96:651–6. [DOI] [PubMed] [Google Scholar]
- 10.Williams GD, Bratton SL, Ramamoorthy C. Factors associated with blood loss and blood product transfusions: A multivariate analysis in children after open-heart surgery. Anesth Analg 1999; 89:57–64. [DOI] [PubMed] [Google Scholar]
- 11.Murray DJ, Pennell BJ, Weinstein SL, Olson JD. Packed red cells in acute blood loss: dilutional coagulopathy as a cause of surgical bleeding. Anesth Analg 1995; 80:336–42. [DOI] [PubMed] [Google Scholar]
- 12.Guzzetta NA, Allen NN, Wilson EC, et al. Excessive bleeding and outcomes in neonates undergoing cardiopulmonary bypass. Anesth Analg 2015; 120:405–10. [DOI] [PubMed] [Google Scholar]
- 13.Andrews M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol 1990; 12:95–104. [DOI] [PubMed] [Google Scholar]
- 14.Ignjatovic V, Mertyn E, Monagle P. The coagulation system in children: developmental and pathophysiological considerations. Semin Thromb Hemost 2011; 37:723–9. [DOI] [PubMed] [Google Scholar]
- 15.Tempe DK, Virmani S. Coagulation abnormalities in patients with cyanotic congenital heart disease. J Cardiovasc Vasc Anesth 2002; 16:752–65. [DOI] [PubMed] [Google Scholar]
- 16.Chan AK, Leaker M, Burrows FA, et al. Coagulation and fibrinolytic profile of paediatric patients undergoing cardiopulmonary bypass. Thromb Haemost 1997; 77:270–77. [PubMed] [Google Scholar]
- 17.Durandy Y Use of blood products in pediatric cardiac surgery. Artificial Organs 2015; 39:21–27. [DOI] [PubMed] [Google Scholar]
- 18.Willems A, Patte P, De Groote F, Van der Linden P. Cyanotic heart disease is an independent predicting factor for fresh frozen plasma and platelet transfusion after cardiac surgery. Transfus Apher Sci 2019; 58:304–9. [DOI] [PubMed] [Google Scholar]
- 19.Petaja J, Lundstrom U, Leijala M, Peltola K, Siimes MA. Bleeding and use of blood products after heart operations in infants. J Thorac Cardiovasc Surg 1995; 109:524–9. [DOI] [PubMed] [Google Scholar]
- 20.Fiser RT, Irby K, Ward RM, et al. RBC transfusion in pediatric patients supported with extracorporeal membrane oxygenation: Is there an impact on tissue oxygenation? Pediatr Crit Care Med 2014; 15:806–13. [DOI] [PubMed] [Google Scholar]
- 21.Mazzeffi M, Greenwood J, Tanaka K, et al. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg 2016; 101:682–89. [DOI] [PubMed] [Google Scholar]
- 22.Iyengar A, Scipione CN, Sheth P, et al. Association of complications with blood transfusions in pediatric cardiac surgery patients. Ann Thorac Surg 2013; 96:910–16. [DOI] [PubMed] [Google Scholar]
- 23.Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013; 28:54–60. [DOI] [PubMed] [Google Scholar]
- 24.Szekely A, Cserep Z, Sapi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing heat operations. Ann Thorac Surg 2009; 87:187–97. [DOI] [PubMed] [Google Scholar]
- 25.Redlin M, Kukucka M, Boettcher W, et al. Blood transfusion determines postoperative morbidity in pediatric cardiac surgery applying a comprehensive blood-sparing approach. J Thorac Cardiovasc Surg 2013; 146:537–42. [DOI] [PubMed] [Google Scholar]
- 26.Maganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass in children. Anesth Analg 2010; 100:995–1002. [DOI] [PubMed] [Google Scholar]
- 27.Segal JB, Dzik WH; Transfusion Medicine/Hemostasis Clinical trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005; 45:1413–25. [DOI] [PubMed] [Google Scholar]
- 28.Nellis ME, Karam O, Valentine S, et al. Executive Summary of Recommendations and Expert Consensus for Plasma and Platelet Transfusion Practice in Critically Ill Children: From the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008, 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrad SA, Broman LM, Taccone FS, et al. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A Position Paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med 2018; 198:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meco M, Montisci A, Giustiniano E, et al. Viscoelastic Blood Tests Use in Adult Cardiac Surgery: Meta-Analysis, Meta-Regression, and Trial Sequential Analysis. J Cardiothorac Vasc Anesth 2020; 34:119–127. [DOI] [PubMed] [Google Scholar]
- 32.Redfern RE, Fleming K, March RL, et al. Thrombelastography-Directed Transfusion in Cardiac Surgery: Impact on Postoperative Outcomes. Ann Thorac Surg 2019; 107:1313–1318. [DOI] [PubMed] [Google Scholar]
- 33.Mendeloff EN, Glenn GF, Tavakolian P, et al. The role of thromboelastography in directing blood product usage in infant open heart surgery. Innovations 2009; 4:282–90. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, Hei F, Long C, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artif Organs 2010; 34:955–60. [DOI] [PubMed] [Google Scholar]
- 35.Kane LC, Woodward CS, Husain SA, Frei-Jones MJ. Thromboelastography--does it impact blood component transfusion in pediatric heart surgery? J Surg Res 2016; 200:21–7. [DOI] [PubMed] [Google Scholar]
- 36.Emani S, Sleeper LA, Faraoni D, et al. Thromboelastography Is Associated With Surrogates for Bleeding After Pediatric Cardiac Operations. Ann Thorac Surg 2018; 106:799–806. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Fraser CD, Suarez-Pierre A, et al. Variations in platelet transfusion practices in cardiac surgery. Innovations 2019; 14:134–43. [DOI] [PubMed] [Google Scholar]
- 38.Snyder-Ramos SA, Mohnle P, Weng Y, et al. The ongoing variability in blood transfusion practices in cardiac surgery. Transfusion 2008; 48:1284–99. [DOI] [PubMed] [Google Scholar]
- 39.Yanagawa B, Ribeiro R, Lee J, Verma S, Friedrich JO. Platelet transfusion in cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg 2021; 111:607–14. [DOI] [PubMed] [Google Scholar]
- 40.Whitney G, Daves S, Hughes A, et al. Implementation of a transfusion algorithm to reduce blood product utilization in pediatric cardiac surgery. Pediatric Anesth 2013; 23:639–46. [DOI] [PubMed] [Google Scholar]
- 41.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999; 88:312–9. [DOI] [PubMed] [Google Scholar]
- 42.Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thromboelastography during cardiopulmonary bypass. Br J Anaesth 2001; 86:575–8. [DOI] [PubMed] [Google Scholar]
- 43.Romlin BS, Wahlander H, Berggren H, et al. Intraoperative thromboelastometry is associated with reduced transfusion prevalence in pediatric cardiac surgery. Anesth Analg 2011; 112:30–36. [DOI] [PubMed] [Google Scholar]
- 44.Haas T, Goobie S, Spielmann N, et al. Improvements in patient blood management for pediatric craniosynostosis surgery using a ROTEM-assisted strategy: feasibility and costs. Paediatr Anaesth 2014; 24:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraoni D, Willems A, Romlin BS, Belisle S, Van der Liden P. Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery. Eur J Anaesthiol 2015; 32:320–9. [DOI] [PubMed] [Google Scholar]
- 46.Karanjkar A, Kapoor PM, Sharan S, et al. A Prospective Randomized Clinical Trial of Efficacy of Algorithm-Based Point of Care Guided Hemostatic Therapy in Cyanotic Congenital Heart Disease Surgical Patients. J Card Crit Care 2019; 3:8–16. [Google Scholar]
- 47.Mariscalco G, Gherli R, Ahmed AB, et al. Validation of the European Multicenter Study on Coronary Artery Bypass Grafting (E-CABG) Bleeding Severity Definition. Ann Thorac Surg 2016; 101:1782–1788. [DOI] [PubMed] [Google Scholar]
- 48.Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 2019; 38:114–126. [DOI] [PubMed] [Google Scholar]
- 49.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 50.Vivacqua A, Koch CG, Yousuf AM, et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg 2011; 91:1780–1790. [DOI] [PubMed] [Google Scholar]
- 51.STS National Database. STS INTERMACS (The Society of Thoracic Surgeons, Interagency Registry for Mechanically Assisted Circulatory Support). Available from: https://www.uab.edu/medicine/intermacs/intermacs-documents. Accessed June 1, 2021.
- 52.Levy JH, Steiner ME. How to interpret recent restrictive transfusion trials in cardiac surgery: More new data or new more data? J Thorac Cardiovasc Surg 2018; 157:1038–1040. [DOI] [PubMed] [Google Scholar]
- 53.Colson PH, Gaudard P, Fellahi JL, et al. Active bleeding after cardiac surgery: a prospective observational multicenter study. PLoS One 2016; 11:e0162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg 2014; 147:1458–1463. [DOI] [PubMed] [Google Scholar]
- 55.Sniecinski RM, Levy JH. Bleeding and management of coagulopathy. J Thorac Cardiovasc Surg 2011; 142:662–7. [DOI] [PubMed] [Google Scholar]
- 56.D’Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018; 105:15–23. [DOI] [PubMed] [Google Scholar]
- 57.Karkouti K, Wijeysundera DN, Beattie WS, et al. Variability and predictability of large-volume red blood cell transfusion in cardiac surgery: a multicenter study. Transfusion. 2007; 47:2081–2088. [DOI] [PubMed] [Google Scholar]
- 58.Ruel M, Chan V, Boodhwani M, et al. How detrimental is reexploration for bleeding after cardiac surgery? J Thorac Cardiovasc Surg 2017; 154:927–935. [DOI] [PubMed] [Google Scholar]
- 59.Frojd V, Jeppsson A. Reexploration for Bleeding and Its Association With Mortality After Cardiac Surgery. Ann Thorac Surg 2016; 102:109–117. [DOI] [PubMed] [Google Scholar]
- 60.Steiner ME, Despotis GJ. Transfusion algorithms and how they apply to blood conservation: the high-risk cardiac surgical patient. Hematol Oncol Clin North Am 2007; 21:177–184. [DOI] [PubMed] [Google Scholar]
- 61.Karkouti K, Callum J, Wijeysundera DN, et al. Point of care hemostatic testing in cardiac surgery. Circulation 2016; 134:1152–1162. [DOI] [PubMed] [Google Scholar]
- 62.Despotis GJ, Goodnough LT. Management approaches to platelet-related microvascular bleeding in cardiothoracic surgery. Ann Thorac Surg. 2000; 70:S20–32. [DOI] [PubMed] [Google Scholar]
- 63.Yaw HP, Van Den Helm S, MacLaren G, Linden M, Monagle P, Ignjatovic V. Platelet phenotype and function in the setting of pediatric extracorporeal membrane oxygenation (ECMO): A systematic review. Front Cardiovasc Med 2019; 6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung PY, Sawicki G, Salas E, et al. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med 2000; 28:2584–90. [DOI] [PubMed] [Google Scholar]
- 65.Roseff SD, Luban NLC, Manno CS. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion 2002; 42:1398–1413. [DOI] [PubMed] [Google Scholar]
- 66.Pediatric Cardiac Failure, Extracorporeal Life Support Organization, Ann Arbor, MI. [cited 2017 Feb 15]. Available from http://www.elso.org/resources/guidelines.aspx [Google Scholar]
- 67.Cashen K, Dalton H, Reeder RW et al. Platelet transfusion practice and related outcomes in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 2020; 21:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozment CP, Scott BL, Bembea MM, Spinella PC. Anticoagulation and Transfusion Management during neonatal and pediatric extracorporeal membrane oxygenation: a survey of medical directors in the United States. Pediatr Crit Care Med 2021; 22:530–541. [DOI] [PubMed] [Google Scholar]
- 69.Nellis ME, Saini A, Spinella PC et al. Pediatric Plasma and Platelet Transfusions on Extracorporeal Membrane Oxygenation: a subgroup analysis of two large international point-prevalence studies and the role of local guidelines. Pediatr Crit Care Med 2020; 21:267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keene SD, Patel RM, Stansfield BK, Davis J, Josephson CD, Winkler AM. Blood product transfusion and mortality in neonatal extracorporeal membrane oxygenation. Transfusion 2020; 60:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nellis ME, Dalton H, Karam O. Quantifiable bleeding in children supported by extracorporeal membrane oxygenation and outcome. Crit Care Med 2019; 47:e886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 2019; 380:242–51. [DOI] [PubMed] [Google Scholar]
- 73.McManus ML, Kevy SV, Bower LK, Hickey PR. Coagulation factor deficiencies during initiation of extracorporeal membrane oxygenation. J Pediatr 1995; 126:900–904. [DOI] [PubMed] [Google Scholar]
- 74.Arnold P, Jackson S, Wallis J, Smith J, Bolton D, Haynes S. Coagulation factor activity during neonatal extra-corporeal membrane oxygenation. Intensive Care Med 2001; 27:1395–1400. [DOI] [PubMed] [Google Scholar]
- 75.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowiz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013; 14:e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMichael ABV, Zimmerman KO, Kumar KR, Ozment CP. Evaluation of effect of scheduled fresh frozen plasma on ECMO circuit life: a randomized pilot trial. Transfusion 2021; 61:42–51. [DOI] [PubMed] [Google Scholar]
- 77.Sell LL, Cullen ML, Whittlesey GC, et al. Hemorrhagic complications during extracorporeal membrane oxygenation: prevention and treatment. J Pediatr Surg 1986; 21:1087–91. [DOI] [PubMed] [Google Scholar]
- 78.Dela Cruz TV, Stewart DL, Winston SJ, Weatherman KS, Phelps JL, Mendoza JC. Risk factors for intracranial hemorrhage in the extracorporeal membrane oxygenation patient. J Perinatol 1997; 17:18–23. [PubMed] [Google Scholar]
- 79.Nardell K, Annich GM, Hirsch JC, et al. Risk factors for bleeding in pediatric post-cardiotomy patients requiring ECLS. Perfusion 2009; 24:191–7. [DOI] [PubMed] [Google Scholar]
- 80.Zavadil DP, Stammers AH, Willett LD, Deptula JJ, Christensen KA, Sydzyik RT. Hematological abnormalities in neonatal patients treated with extracorporeal membrane oxygenation (ECMO). J Extra Corpor Technol 1998; 30:83–90. [PubMed] [Google Scholar]
- 81.Stallion A, Cofer BR, Rafferty JA, Ziegler MM, Ryckman FC. The significant relationship between platelet count and haemorrhagic complications on ECMO. Perfusion 1994; 9:265–9. [DOI] [PubMed] [Google Scholar]
- 82.Spinella PC, El Kassar N, Cap AP, et al. Recommended primary outcomes for clinical trials evaluating hemostatic blood products and agents in patients with bleeding: Proceedings of a National Heart Lung and Blood Institute and US Department of Defense Consensus Conference. J Trauma Acute Care Surg 2021; 91:S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexander PMA, Muszynski JA. Ongoing Variability in Pediatric Extracorporeal Membrane Oxygenation Anticoagulation Practices-Could Consensus Change the Next Survey Results? Pediatr Crit Care Med 2021; 22:581–584. [DOI] [PubMed] [Google Scholar]
- 84.Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg 1997; 85:1196–202. [DOI] [PubMed] [Google Scholar]
- 85.Williams GD, Bratton SL, Riley EC, Ramamoorthy C. Association between age and blood loss in children undergoing open heart operations. Ann Thorac Surg 1998; 66:870–5. [DOI] [PubMed] [Google Scholar]
- 86.Oliver WC Jr, Beynen FM, Nuttall GA, et al. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Ann Thorac Surg 2003; 75:1506–12. [DOI] [PubMed] [Google Scholar]
- 87.Mou SS, Giroir BP, Molitor-Kirsch EA, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med 2004; 351:1635–44. [DOI] [PubMed] [Google Scholar]
- 88.Andreasen JB, Hvas AM, Christiansen K, Ravn HB. Can RoTEM® analysis be applied for haemostatic monitoring in paediatric congenital heart surgery? Cardiol Young 2011; 21:684–91. [DOI] [PubMed] [Google Scholar]
- 89.Niebler RA, Gill JC, Brabant CP, et al. Thromboelastography in the assessment of bleeding following surgery for congenital heart disease. World J Pediatr Congenit Heart Surg 2012; 3:433–8. [DOI] [PubMed] [Google Scholar]
- 90.Mejak BL, Ing RJ, McRobb C, et al. Cryoprecipitate and platelet administration during modified ultrafiltration in children less than 10 kg undergoing cardiac surgery. J Extra Corpor Technol 2013; 45:107–11. [PMC free article] [PubMed] [Google Scholar]
- 91.Faraoni D, Van der Linden P. Factors affecting postoperative blood loss in children undergoing cardiac surgery. J Cardiothorac Surg 2014; 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romlin BS, Söderlund F, Wåhlander H, Nilsson B, Baghaei F, Jeppsson A. Platelet count and function in paediatric cardiac surgery: a prospective observational study. Br J Anaesth 2014; 113:847–54. [DOI] [PubMed] [Google Scholar]
- 93.Agarwal HS, Barrett SS, Barry K, et al. Association of blood products administration during cardiopulmonary bypass and excessive post-operative bleeding in pediatric cardiac surgery. Pediatr Cardiol 2015; 36:459–67. [DOI] [PubMed] [Google Scholar]
- 94.Gertler R, Hapfelmeier A, Tassani-Prell P, Wiesner G, Martin K. The effect of cyanosis on perioperative platelet function as measured by multiple electrode aggregometry and postoperative blood loss in neonates and infants undergoing cardiac surgery. Eur J Cardiothorac Surg 2015; 48:301–7. [DOI] [PubMed] [Google Scholar]
- 95.Miao X, Liu J, Zhao M, et al. Evidence-based use of FFP: the influence of a priming strategy without FFP during CPB on postoperative coagulation and recovery in pediatric patients. Perfusion 2015; 30:140–7. [DOI] [PubMed] [Google Scholar]
- 96.Zubair MM, Bailly DK, Lantz G, et al. Preoperative platelet dysfunction predicts blood product transfusion in children undergoing cardiac surgery. Interact Cardiovasc Thorac Surg 2015; 20:24–30. [DOI] [PubMed] [Google Scholar]
- 97.Vida VL, Spiezia L, Bortolussi G, et al. The Coagulative Profile of Cyanotic Children Undergoing Cardiac Surgery: The Role of Whole Blood Preoperative Thromboelastometry on Postoperative Transfusion Requirement. Artif Organs 2016; 40:698–705. [DOI] [PubMed] [Google Scholar]
- 98.Bianchi P, Cotza M, Beccaris C, et al. Early or late fresh frozen plasma administration in newborns and small infants undergoing cardiac surgery: the APPEAR randomized trial. Br J Anaesth 2017; 118:788–796. [DOI] [PubMed] [Google Scholar]
- 99.Faraoni D, Emani S, Halpin E, et al. Relationship Between Transfusion of Blood Products and the Incidence of Thrombotic Complications in Neonates and Infants Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth 2017; 31:1943–1948. [DOI] [PubMed] [Google Scholar]
- 100.Fahlbusch FB, Heinlein T, Rauh M, et al. Influence of factor XIII activity on post-operative transfusion in congenital cardiac surgery-A retrospective analysis. PLoS One 2018; 13:e0199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scott JP, Niebler RA, Stuth EAE, et al. Rotational Thromboelastometry Rapidly Predicts Thrombocytopenia and Hypofibrinogenemia During Neonatal Cardiopulmonary Bypass. World J Pediatr Congenit Heart Surg 2018; 9:424–433. [DOI] [PubMed] [Google Scholar]
- 102.Zwifelhofer NMJ, Bercovitz RS, Cole R, et al. Platelet Function Changes during Neonatal Cardiopulmonary Bypass Surgery: Mechanistic Basis and Lack of Correlation with Excessive Bleeding. Thromb Haemost 2020; 120:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fang ZA, Bruzdoski K, Kostousov V, et al. Low antithrombin levels in neonates and infants undergoing congenital heart surgery result in more red blood cell and plasma transfusion on cardiopulmonary bypass. Transfusion 2020; 60:2841–2848. [DOI] [PubMed] [Google Scholar]
- 104.Gautam NK, Pierre J, Edmonds K, et al. Transfusing Platelets During Bypass Rewarming in Neonates Improves Postoperative Outcomes: A Randomized Controlled Trial. World J Pediatr Congenit Heart Surg 2020; 11:71–76. [DOI] [PubMed] [Google Scholar]
- 105.McCoy-Pardington D, Judd WJ, Knafl P, et al. Blood use during extracorporeal membrane oxygenation. Transfusion 1990; 30:307–9. [DOI] [PubMed] [Google Scholar]
- 106.Minifee PK, Daeschner CW 3rd, Griffin MP, Allison PL, Zwischenberger JB. Decreasing blood donor exposure in neonates on extracorporeal membrane oxygenation. J Pediatr Surg 1990; 25:38–42. [DOI] [PubMed] [Google Scholar]
- 107.Plotz FB, Wildevuur WR, Wildvuur CRH, Delius RE, Bartlett RH. Platelet consumption during neonatal extracorporeal life support (ECLS). Perfusion 1992; 7:27–33. [Google Scholar]
- 108.Bjerke HS, Kelly RE Jr, Foglia RP, Barcliff L, Petz L. Decreasing transfusion exposure risk during extracorporeal membrane oxygenation (ECMO). Transfus Med 1992; 2:43–9. [DOI] [PubMed] [Google Scholar]
- 109.Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Effect of extracorporeal membrane oxygenation on platelets in newborns. Crit Care Med 1993; 21:1029–34. [DOI] [PubMed] [Google Scholar]
- 110.Rosenberg EM, Chambers LA, Gunter JM, Good JA. A program to limit donor exposures to neonates undergoing extracorporeal membrane oxygenation. Pediatrics 1994; 94:341–6. [PubMed] [Google Scholar]
- 111.Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol. 1995; 27:137–45. [PubMed] [Google Scholar]
- 112.Chevuru SC, Sola MC, Theriaque DW, et al. Multicenter analysis of platelet transfusion usage among neonates on extracorporeal membrane oxygenation. Pediatrics 2002; 109:e89. [DOI] [PubMed] [Google Scholar]
- 113.Stiller B, Lemmer J, Merkle F, et al. Consumption of blood products during mechanical circulatory support in children: comparison between ECMO and a pulsatile ventricular assist device. Intensive Care Med 2004; 30:1814–20. [DOI] [PubMed] [Google Scholar]
- 114.Dohner ML, Wiedmeier SE, Stoddard RA, et al. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion 2009; 49:869–72. [DOI] [PubMed] [Google Scholar]
- 115.Muensterer OJ, Laney D, Georgeson KE. Survival time of ECMO circuits on and off bleeding protocol: is there a higher risk of circuit clotting? Eur J Pediatr Surg 2011; 21:30–2. [DOI] [PubMed] [Google Scholar]
- 116.Perry R, Stein J, Young G, et al. Antithrombin III administration in neonates with congenital diaphragmatic hernia during the first three days of extracorporeal membrane oxygenation. J Pediatr Surg 2013; 48:1837–42. [DOI] [PubMed] [Google Scholar]
- 117.Henríquez-Henríquez M, Kattan J, Chang M, et al. Blood component usage during extracorporeal membrane oxygenation: experience in 98 patients at a Latin-American tertiary hospital. Int J Artif Organs 2014; 37:233–40. [DOI] [PubMed] [Google Scholar]
- 118.Northrop MS, Sidonio RF, Phillips SE, et al. The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. Pediatr Crit Care Med 2015; 16:66–74. [DOI] [PubMed] [Google Scholar]
- 119.Doymaz S, Zinger M, Sweberg T. Risk factors associated with intracranial hemorrhage in neonates with persistent pulmonary hypertension on ECMO. J Intensive Care 2015; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saini A, Hartman ME, Gage BF, et al. Incidence of Platelet Dysfunction by Thromboelastography-Platelet Mapping in Children Supported with ECMO: A Pilot Retrospective Study. Front Pediatr 2016; 3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santiago MJ, Gómez C, Magaña I, et al. Hematological complications in children subjected to extracorporeal membrane oxygenation. Med Intensiva (Engl Ed). 2019l; 43:281–289. [DOI] [PubMed] [Google Scholar]
- 122.Phillips RC, Shahi N, Leopold D, et al. Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr Surg Int 2020; 36:1027–1033. [DOI] [PubMed] [Google Scholar]
- 123.Workman JK, Bailly DK, Reeder RW, et al. Risk Factors for Mortality in Refractory Pediatric Septic Shock Supported with Extracorporeal Life Support. ASAIO J 2020; 66:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karam O, Goel R, Dalton H, Nellis ME. Epidemiology of Hemostatic Transfusions in Children Supported by Extracorporeal Membrane Oxygenation. Crit Care Med 2020; 48:e698–e705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


