Abstract
“Diffuse midline glioma (DMG), H3K27M-mutant” was newly classified in the revised World Health Organization (WHO) 2016 classification of central nervous system tumors. Spinal cord DMG, H3K27M-mutant is relatively rare, with poor prognosis, and there are no effective treatment protocols. In this study, we report two cases of spinal cord DMG, H3K27M-mutant treated with bevacizumab. The two patients were women in their 40s who initially presented with sensory impairment. MRI showed spinal intramedullary tumors, and each patient underwent laminectomy/laminoplasty and biopsy of the tumors. Histological examination initially suggested low-grade astrocytoma in case 1 and glioblastoma in case 2. Upon further immunohistochemical examination in case 1 and molecular examination in case 2, however, both cases were diagnosed as DMG, H3K27M-mutant. Case 1 was treated with radiation therapy and temozolomide (TMZ) chemotherapy, which induced a transient improvement of symptoms; 3 months after surgery, however, the patient’s symptoms rapidly deteriorated. MRI showed tumor enlargement with edema to the medulla. Triweekly administration of bevacizumab improved her symptoms for the following 12 months. Case 2 was treated with bevacizumab from the beginning because of acute deterioration of breathing. After bevacizumab administration, both cases showed tumor regression on MRI and drastic improvement of symptoms within a few days. Although spinal cord DMG, H3K27M-mutant has an aggressive clinical course and poor prognosis, bevacizumab administration may offer the significant clinical benefit of alleviating edema, which improves patient’s capacity for activities of daily life.
Keywords: diffuse midline glioma, H3K27M, bevacizumab
Introduction
Spinal intramedullary tumors are relatively rare lesions, accounting for only 2%–4% of all central nervous system tumors in adults.1) Diffuse midline glioma (DMG) with histone H3K27M mutation (DMG, H3K27M-mutant) is a newly described entity in the revised World Health Organization classification (WHO, 2016) that corresponds to a grade IV diagnosis regardless of tissue histological features.2) The intersection of these two categories is extremely uncommon: fewer than 100 adult cases of spinal cord DMG, H3K27M-mutant have been reported in the literature,3) and only 4.3% of all DMG, H3K27M-mutant cases are located in the spinal cord.4) In general, DMG, H3K27M-mutant often results in rapid neurological deterioration and has a poor prognosis,5) with a 2-year survival rate under 10%.6) The standard treatment for spinal cord DMG, H3K27M-mutant includes gross total resection or biopsy followed by radiation and chemotherapy,7) but due to the rarity and severity of the condition, no optimal treatment has been established with certainty.
We have experienced two cases of spinal cord DMG, H3K27M-mutant in which bevacizumab temporarily stopped tumor progression and alleviated edema. Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF)8) that has been clinically applied as a molecular-targeted agent for primary and recurrent high-grade glioma in Japan. Although bevacizumab does not improve overall survival in glioblastoma,9,10) previous trials have shown that it does extend progression-free survival. However, its effectiveness against spinal cord DMG, H3K27M-mutant has not been clarified. In this study, we report on our successful use of bevacizumab against spinal cord DMG, H3K27M-mutant in two patients.
Case Report
Case 1
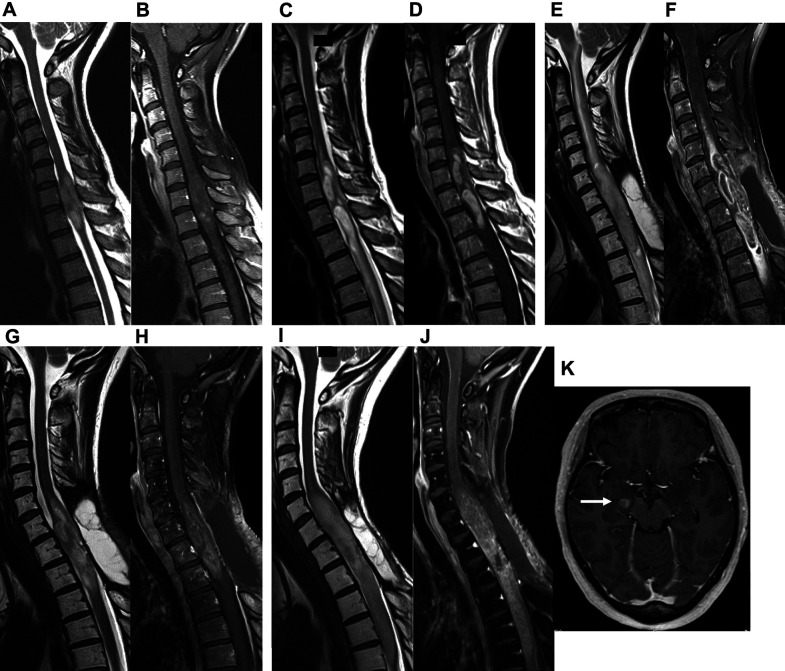
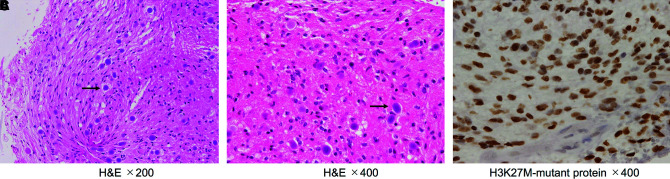
A 47-year-old woman presented with sensory impairment of the right lower extremity that had persisted for 1 month. She was referred to our department because MRI suggested a spinal cord tumor. Her symptoms worsened into right-side/dominant-limb paresis and dysuria over the following month, and she was admitted to the hospital on an emergency basis. MRI at the initial visit had shown an intramedullary tumor at the C6-Th1 level (Fig. 1A and 1B), but MRI on admission revealed tumor growth (Fig. 1C and 1D). The patient underwent laminectomy/laminoplasty and biopsy. Histological examination revealed proliferation of tumor cells with piloid appearance and numerous corpora amylacea. Mitotic figures, endothelial proliferation, and necrosis were not found. A diagnosis of low-grade astrocytoma was made (Fig. 2A and 2B). We then performed immunohistochemistry for H3K27M because of the rapid tumor growth and found that the tumor cells were positive for H3K27M. The definitive diagnosis was DMG, H3K27M-mutant (Fig. 2C). Radiation therapy and chemotherapy with temozolomide (TMZ) were initiated, and the paraparesis and sensory impairment gradually improved. As the patient’s condition stabilizes, she was scheduled to be discharged from the hospital; just before her scheduled discharge, however, bilateral upper-extremity sensory impairment reappeared with nausea. MRI showed tumor growth with severe edema extending to the brain stem (Fig. 1E and 1F). In response, bevacizumab was started. The patient’s symptoms drastically improved with a few days, and she was discharged from the hospital. MRI at 14 days after bevacizumab administration showed tumor regression with significant improvement in edema (Fig. 1G and 1H). She was treated with maintenance therapy (TMZ (a 5-day course every 28 days) + bevacizumab (triweekly)) as an outpatient and lived at home without symptom worsening for over 1 year. Fourteen months after discharge, however, her paralysis gradually became exacerbated, and MRI showed tumor regrowth and intracranial metastases (Fig. 1I–K). She died due to poor general condition 17 months after discharge.
Fig. 1. Changes over time in spinal MRI findings in a spinal intramedullary tumor with edema (case 1). (A) T2-weighted imaging (T2WI) prior to admission showed a high-intensity area at the C6-Th1 level. (B) Contrast-enhanced T1-weighted imaging (CET1WI) prior to admission revealed that the tumor was diffusely enhanced at the C6-Th1 level. (C and D) T2WI and CET1WI on admission showed tumor growth. (E and F) MRI at the time of the deterioration of symptoms showed tumor growth with severe edema extending to the brain stem. (G and H) MRI at 14 days after bevacizumab administration showed tumor regression with significant improvement in edema. (I–K) MRI at 14 months after discharge showed tumor regrowth and intracranial dissemination of the tumor (white arrow).
Fig. 2. Histological and immunohistochemical findings in case 1. (A and B) Hematoxylin and eosin staining showed a piloid appearance. Numerous corpora amylacea (black arrow) were seen within the tumor (A: ×200, B: ×400). (C) Immunohistochemistry for H3K27M-mutant protein showed strong nuclear positivity (×400).
Case 2
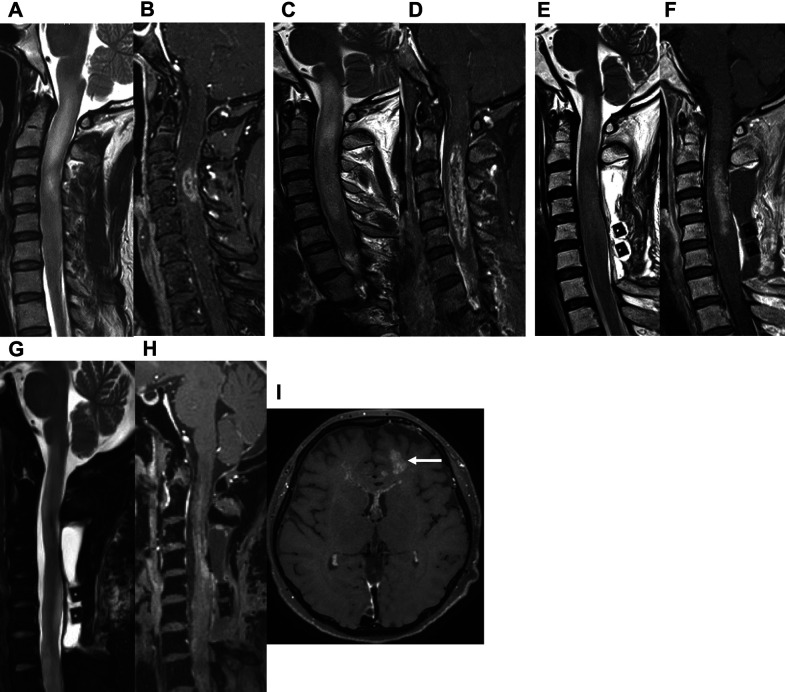
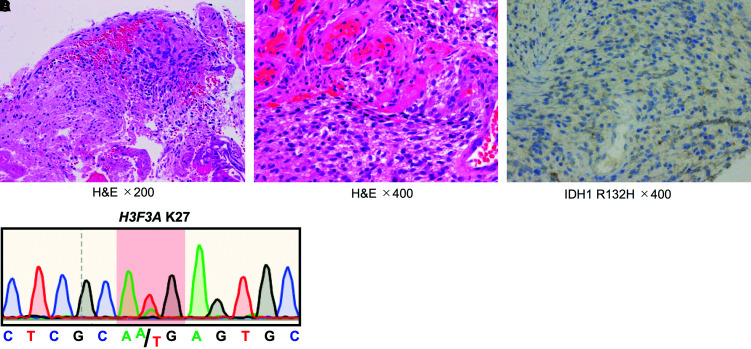
A 46-year-old woman presented with a 5-month history of sensory impairment of the right upper extremity. She was referred to our department for a suspected spinal intramedullary tumor on MRI taken at her previous hospital (Fig. 3A and 3B). Physical findings revealed right hemiparesis (Manual Muscle Test (MMT): 2) and sensory impairment of the right extremities. Two weeks later, her symptoms deteriorated and she was admitted to the hospital on an emergency basis. MRI on admission revealed a spinal intramedullary tumor at the C1–C5 level (Fig. 3C and 3D). The patient underwent laminectomy/laminoplasty and biopsy. Histologically, the tumor showed a dense proliferation of tumor cells with a necrotic focus, and a histological diagnosis of high-grade glioma, suggestive of glioblastoma or DMG, was made (Fig. 4A–C). We also pursued a molecular diagnosis because of her rapid deterioration of symptoms and MRI findings and found a K27M mutation in H3F3A through Sanger sequencing. The patient was diagnosed with DMG, H3K27M-mutant (Fig. 4D). Thirteen days after surgery, her breathing worsened: in response, bevacizumab was administered, followed by radiation therapy and TMZ. MRI 7 days after bevacizumab administration showed tumor regression with improved edema (Fig. 3E and 3F). Her breathing condition and right hemiparesis dramatically improved up to MMT 4 and she was transferred to a regional hospital. She died 11 months after the transfer due to intracranial dissemination of the tumor (Fig. 3G–I).
Fig. 3. Changes over time in spinal MRI findings in a spinal intramedullary tumor with edema (case 2). (A) T2WI prior to admission showed a high-intensity area at the C1–C4 level. (B) CET1WI prior to admission revealed that the tumor was enhanced at the C4 level. (C and D) T2WI and CET1WI on admission showed tumor growth at the C1–C5 level with severe edema extending to the brain stem. (E and F) MRI at 7 days after bevacizumab administration showed tumor regression with significant improvement in edema. (G–I) MRI at 7 months after the transfer out of our hospital showed intracranial dissemination of the tumor (white arrow).
Fig. 4. Histological and immunohistochemical findings in case 2. (A and B) Hematoxylin and eosin staining showed signs of glioblastoma or diffuse midline glioma, including dense proliferation of tumor cells and necrosis (A: ×200, B: ×400). (C) Immunohistochemistry showed no expression of IDH1 R132H-mutant protein (×400). (D) Mutation analysis using Sanger sequencing showed a H3F3A K27M mutation.
Discussion
Due to the distinctly less favorable prognosis for glioma patients harboring the H3K27M mutation, DMG with an H3K27M mutation in either H3F3A or HIST1H3B/C is now considered a novel entity known as “DMG, H3K27M-mutant.” This condition is classified as a grade IV glioma regardless of its other histological features and location, although it can occur in the thalamus, pons, or spinal cord.4,11,12) Chai et al. have reported that, among 35 cases of spinal cord DMG, H3K27M-mutant, the median age was 35 years (range: 9–52 years), with the following locations: cervical vertebrae, 29%; cervicothoracic vertebrae, 20%; thoracic vertebrae, 43%; thoracolumbar vertebrae, 9%.13)
DMG, H3K27M-mutant patients have poor prognoses. The overall median survival time of spinal cord DMG, H3K27M-mutant has been reported to range from 6 to 16 months,14–16) with the longest survival time reported to be 31 months.3) In general, DMG, H3K27M-mutant is associated with shorter survival time than DMG without H3K27M mutation, regardless of tissue histological features.17) When cases in the thalamus, pons, and spinal cord are considered together, DMG with H3K27M mutation has a reported median overall survival of 13.1 months, compared to 23.1 months for DMG without mutation. When cases in the spinal cord are considered alone, however, the results are conflicting: Kleinschmidt- DeMasters et al. have reported that H3K27M mutation is significantly associated with poorer prognosis in spinal cord DMG,5) yet a few recent studies have reported that spinal cord DMG with H3K27M mutation is associated with prolonged overall and progression-free survival.7,18) Thus, the prognostic relevance of the H3K27M mutant for spinal cord tumors is controversial.
In the few years since the category of “DMG, H3K27M-mutant” was established, circumscribed/non-DMG with the same H3K27M mutation have also been observed: the reported types include pilocytic astrocytomas, gangliogliomas, and ependymomas.19–21) In response to these reports, in 2018, the Consortium to Inform Molecular and Practical Approaches to Central Nervous System Tumor Taxonomy-Not Official WHO (cIMPACT-NOW) have clarified the diagnostic criteria for “DMG, H3K27M- mutant,” and suggested that this term should be reserved for tumors that are diffuse (i.e., infiltrating), midline (e.g., thalamus, brainstem, and spinal cord), and gliomas with the H3K27M mutation, and should not be applied to other tumors with the H3K27M mutation.22) According to this definition, circumscribed/non-diffuse glioma harboring the H3K27M mutant do not fall into the DMG, H3K27M-mutant category. Pratt et al. have reported that circumscribed midline glioma with the H3K27M mutation have poorer overall survival rates compared to circumscribed midline glioma without the mutation, but better overall survival rates compared to DMG, H3K27M-mutant.23) More detailed studies are necessary to clarify the prognosis of circumscribed/non-DMG as compared to DMG, H3K27M-mutant.
In case 1, the initial histological diagnosis was low-grade astrocytoma, but we suspected an alternate diagnosis because of the drastic deterioration of symptoms and the MRI findings. Accordingly, we followed up with immunohistochemical diagnosis, which enabled the diagnosis of DMG, H3K27M-mutant. This case demonstrates that even when histological findings indicate low-grade glioma, it is important to consider the possibility of DMG with H3K27M-mutation if the MRI findings and symptoms suggest it, and to confirm diagnosis through immunohistochemical and molecular observations.
In terms of treatment, due to the rarity of the disease, no optimal treatment and management for spinal cord DMG, H3K27M-mutant has been established. Most cases are treated similar to intracranial glioblastoma,17) but there have not been enough reported cases for us to evaluate the effectiveness of this treatment at present. In our cases, radiation therapy and chemotherapy were performed after biopsy of the tumors. TMZ was used for initial and maintenance therapy according to the Stupp regimen.24) In case 1, bevacizumab was started after the standard radiation and chemotherapy regimen because of rapid tumor growth to the brain stem and symptom deterioration. In case 2, which was histologically diagnosed as glioblastoma, bevacizumab was emergently administered starting 13 days after surgery and followed by radio-chemotherapy.
In our two cases, the administration of bevacizumab stopped the tumor growth and alleviated edema, providing rapid symptom relief. Similarly, Kumar et al. have reported a pediatric case of spinal cord DMG, H3K27M-mutant treated with radiation therapy and chemotherapy with TMZ followed by bevacizumab after tumor recurrence.25) A certain amount of tumor shrinkage and symptom improvement was observed in that case as well as in our cases. Additionally, the combination of TMZ and bevacizumab against rat spinal intramedullary tumors has been found to decrease tumor growth and improve neurological outcomes.26) As our cases demonstrate, bevacizumab administration for spinal cord DMG, H3K27M-mutant can immediately improve symptoms by alleviating edema, and may extend the period during which quality of life can be maintained. Other cases showing the therapeutic effectiveness of bevacizumab against this tumor should be accumulated and carefully examined in the future. Bevacizumab’s effectiveness at alleviating edema could make it a valuable treatment option, as therapeutic drugs targeting H3F3A and HIST1H3B/C mutations are still under development.27,28)
Conclusion
We present two cases of spinal cord DMG, H3K27M-mutant effectively treated with bevacizumab. DMG, H3K27M-mutant is characterized by aggressive clinical behavior and very poor prognosis, but treatment with bevacizumab might delay tumor progression and alleviate edema, prolonging patient’s ability to engage in activities of daily life, although its therapeutic effects might diminish over time. Although bevacizumab has been shown to have a certain therapeutic effect, its effectiveness should be carefully evaluated as more cases are accumulated.
Footnotes
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (IRB#1911-023) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest Disclosure
The authors declare that they have no conflict of interest.
References
- 1). Santi M, Mena H, Wong K, Koeller K, Olsen C, Rushing EJ: Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer 98: 554– 561, 2003 [DOI] [PubMed] [Google Scholar]
- 2). Louis DN, Perry A, Reifenberger G, et al. : The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131: 803– 820, 2016 [DOI] [PubMed] [Google Scholar]
- 3). Peters K, Pratt D, Koschmann C, Leung D: Prolonged survival in a patient with a cervical spine H3K27M- mutant diffuse midline glioma. BMJ Case Rep 12: 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ishi Y, Takamiya S, Seki T, et al. : Prognostic role of H3K27M mutation, histone H3K27 methylation status, and EZH2 expression in diffuse spinal cord gliomas. Brain Tumor Pathol 37: 81– 88, 2020 [DOI] [PubMed] [Google Scholar]
- 5). Kleinschmidt-DeMasters BK, Mulcahy Levy JM: H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol 37: 53– 63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Qiu T, Chanchotisatien A, Qin Z, et al. : Imaging characteristics of adult H3 K27M-mutant gliomas. J Neurosurg 15: 1– 9, 2019 [DOI] [PubMed] [Google Scholar]
- 7). Yi S, Choi S, Shin DA, et al. : Impact of H3.3 K27M mutation on prognosis and survival of grade IV spinal cord glioma on the basis of new 2016 World Health Organization classification of the central nervous system. Neurosurgery 84: 1072– 1081, 2019 [DOI] [PubMed] [Google Scholar]
- 8). Kim KJ, Li B, Winer J, et al. : Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841– 844, 1993 [DOI] [PubMed] [Google Scholar]
- 9). Chinot OL, Wick W, Mason W, et al. : Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709– 722, 2014 [DOI] [PubMed] [Google Scholar]
- 10). Gilbert MR, Dignam JJ, Armstrong TS, et al. : A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699– 708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Karremann M, Gielen GH, Hoffmann M, et al. : Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol 20: 123– 131, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Wang L, Li Z, Zhang M, et al. : H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 78: 89– 96, 2018 [DOI] [PubMed] [Google Scholar]
- 13). Chai RC, Zhang YW, Liu YQ, et al. : The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol Commun 8: 40, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Morais N, Mascarenhas L, Soares-Fernandes JP, Silva A, Magalhães Z, Costa JA: Primary spinal glioblastoma: A case report and review of the literature. Oncol Lett 5: 992– 996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Shen CX, Wu JF, Zhao W, Cai ZW, Cai RZ, Chen CM: Primary spinal glioblastoma multiforme: a case report and review of the literature. Medicine (Baltimore) 96: e6634, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Timmons JJ, Zhang K, Fong J, et al. : Literature review of spinal cord glioblastoma. Am J Clin Oncol 41: 1281– 1287, 2018 [DOI] [PubMed] [Google Scholar]
- 17). Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C: Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128: 573– 581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Alvi MA, Ida CM, Paolini MA, et al. : Spinal cord high- grade infiltrating gliomas in adults: clinico-pathological and molecular evaluation. Mod Pathol 32: 1236– 1243, 2019 [DOI] [PubMed] [Google Scholar]
- 19). Nguyen AT, Colin C, Nanni-Metellus I, et al. : Evidence for BRAF V600E and H3F3A K27M double mutations in paediatric glial and glioneuronal tumours. Neuropathol Appl Neurobiol 41: 403– 408, 2015 [DOI] [PubMed] [Google Scholar]
- 20). Orillac C, Thomas C, Dastagirzada Y, et al. : Pilocytic astrocytoma and glioneuronal tumor with histone H3 K27M mutation. Acta Neuropathol Commun 4: 84, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Pagès M, Beccaria K, Boddaert N, et al. : Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol 28: 103– 111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Louis DN, Giannini C, Capper D, et al. : cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135: 639– 642, 2018 [DOI] [PubMed] [Google Scholar]
- 23). Pratt D, Natarajan SK, Banda A, et al. : Circumscribed/non-diffuse histology confers a better prognosis in H3K27M-mutant gliomas. Acta Neuropathol 135: 299– 301, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005 [DOI] [PubMed] [Google Scholar]
- 25). Kumar A, Rashid S, Singh S, Li R, Dure LS: Spinal cord diffuse midline glioma in a 4-year-old boy. Child Neurol Open 6: 2329048X19842451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Gwak SJ, An SS, Yang MS, et al. : Effect of combined bevacizumab and temozolomide treatment on intramedullary spinal cord tumor. Spine (Phila Pa 1976) 39: E65– 73, 2014 [DOI] [PubMed] [Google Scholar]
- 27). Chheda ZS, Kohanbash G, Okada K, et al. : Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med 215: 141– 157, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Chi AS, Tarapore RS, Hall MD, et al. : Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol 145: 97– 105, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]