Abstract
Nucleosides and their derivatives are a well-known and well-described class of compounds with antiviral activity. Currently, in the era of the COVID-19 pandemic, scientists are also looking for compounds not related to nucleosides with antiviral properties. This review aims to provide an overview of selected synthetic antiviral agents not associated to nucleosides developed against human viruses and introduced to preclinical and clinical trials as well as drugs approved for antiviral therapy over the last 10 years. The article describes for the first time the wide classification of such antiviral drugs and drug candidates and briefly summarizes the biological target and clinical applications of the compounds. The described compounds are arranged according to the antiviral mechanism of action. Knowledge of the drug's activity toward specific molecular targets may be the key to researching new antiviral compounds and repositioning drugs already approved for clinical use. The paper also briefly discusses the future directions of antiviral therapy. The described examples of antiviral compounds can be helpful for further drug development.
Keywords: Non-nucleosides, Antiviral agents, Antiviral therapy, Clinical trials, COVID-19
Graphical abstract
1. Introduction
Viruses cause a broad spectrum of diseases, ranging from chronic to severe ones that may lead to sudden death. Antiviral drug development began six decades ago, and the first antiviral drug, nucleoside analogue - idoxuridine, was described by William “Bill” Prusoff [1]. Idoxuridine was used in the treatment of infant keratitis, which is caused by herpes simplex virus (HSV, Herpesviridae family), after the drug was approved by the U.S. Food and Drug Administration (FDA) in 1963 [1]. Since then, many antiviral medications have been developed for clinical use. However, the AIDS (Acquired Immune Deficiency Syndrome) epidemic in the 1980s, caused by the human immunodeficiency virus (HIV, Retroviridae family), was a breakthrough in recognizing antiviral agents which led to an increase in research interest, and consequently, the emergence of novel antiviral drugs. Unfortunately, many of the antiviral drugs that have been approved so far have some limitations, such as poor bioavailability, severe adverse effects, or drug resistance. Long-acting and extended-release formulations are one of the most important considerations to improving the treatment and prevention of HIV infection. The latest trends, including those involving the application of nanotechnology, have been published recently [[2], [3], [4]].
Two approaches play an essential role in the development of new antiviral therapies—de novo drug discovery and repositioning of the currently approved drugs for the treatment of well-known viral diseases. Preclinical research is the first stage of development for drug candidates. It involves especially assessment of biological properties. After obtaining the beneficial results, the tested compound may enter clinical trials (CT). FDA drug approval process is presented on Fig. 1 . Combined antiviral therapy might also be beneficial [5]. Especially at present, in the face of novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2, Coronaviridae family), there is an urgent need to develop effective antiviral therapies. Numerous commonly used drugs, as well as new compounds, are applied or being developed for the treatment of COVID-19 [6,7]. Up-to-date information on the COVID-19 pandemic, in particular, the epidemiology, pathogenesis, prevention and treatment strategies has been described in the following publications [8,9].
Fig. 1.
FDA drug approval process. Preclinical studies and clinical trial phases (according to: The Drug Development and Approval Process [15]).
It is worth mentioning, that crystallographic methods are very important in research of new antiviral compounds, also against COVID-19. Crystal structures of biological targets alone and in complex with ligands and inhibitors provide crucial information about the mechanisms of actions of enzymes and their conformational changes upon ligand binding. Recently, this method has been extended and now often include the identification of hits by structure-based virtual screening methods. Several biologically active compounds discovered by structure-based design are now commercially available, confirming the important role played by structural biology in drug development process [10]. The well-known examples are anti-HIV drugs, such as amprenavir (trade name Agenerase) and nelfinavir (trade name Viracept), which were developed using the crystal structure of HIV protease [11]. Crystal structures are currently used in the design of an anti-COVID-19 drug based on SARS-CoV-2 main protease structure (SARS-CoV-2 Mpro) [12,13]. Such methods are also helpful in the study of the mechanisms of action of compounds, including the mechanism of drug resistance – for example crystal structures of influenza virus polymerase basic protein 2 (PB2) and pimodivir [14].
2. Nucleoside analogues
Nucleoside analogues are the most common group among the FDA-approved antiviral drugs. Several nucleoside-based antiviral drugs have been validated for therapy, and many are being tested in preclinical and clinical trials. Inside the cell, nucleosides are first phosphorylated by a nucleoside kinase, resulting in the production of monophosphate metabolite. The second and third phosphorylation processes are performed by cellular kinases (Fig. 2 ). Active forms of nucleosides—mono-, di-, and triphosphorylated ones—act as inhibitors of intracellular enzymes and are also incorporated into newly synthesized DNA and RNA molecules [16].
Fig. 2.
Nucleoside analogue phosphorylation process.
Examples of the latest nucleoside analogues with excellent and promising results in clinical trials are islatravir and galidesivir (Fig. 3 ). Islatravir (MK-8591, 4′-ethynyl-2-fluoro-2′-deoxyadenosine) is a novel nucleoside reverse transcriptase translocation inhibitor (NRTTI), which appears to be promising and is currently evaluated in a clinical trial for the treatment of HIV-1 infection. The active form of islatravir, islatravir triphosphate, leads to an efficient incorporation of islatravir into the viral DNA [17]. Previous in vitro and preclinical studies suggested that islatravir is characterized by a high barrier to resistance [18,19]. In another preclinical study, islatravir used in combination with two other antiviral drugs—doravirine and lamivudine—demonstrated high viral suppression in HIV-infected individuals [20]. The results of the phase 1b single-dose trial conducted in HIV-1-infected adults confirmed the high antiviral potency, beneficial physical properties, and promising resistance profile of islatravir [17].
Fig. 3.
Islatravir (1), galidesivir (2), remdesivir (3) and molnupiravir (4) – chemical structures of nucleoside drug and drug candidates.
Another adenosine analogue, galidesivir (Immucillin-A, BCX4430, (2S,3S,4R,5R)-2-(4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-5-(hydroxymethyl) pyrrolidine-3,4-diol), was shown to inhibit the function of viral RNA polymerase by inducing RNA chain termination. Warren and colleagues [21] reported that galidesivir is active against a wide range of RNA viruses, including Filoviridae, Togaviridae, Bunyaviridae, Arenaviridae, Paramyxoviridae, Flaviviridae, Orthomyxoviridae, Picornaviridae, and Coronaviridae families. Currently, this drug is considered as a promising medication for COVID-19, as it was found to be effective in binding to the RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 [22,23]. Galidesivir was evaluated in a clinical trial for the assessment of safety, pharmacokinetics, and antiviral effects against yellow fever virus (YFV, Flaviviridae) or SARS-CoV-2 and completed phase 1 for the treatment of the Marburg Virus Disease (Filoviridae family).
Another nucleoside compounds worth mentioning are remdesivir (GS-5734, 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate) and molnupiravir ([(2R,3S,4R,5R)-3,4-dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl 2-methylpropanoate) (Fig. 3). Remdesivir was approved by FDA as an antiviral drug for the treatment of COVID-19 in adult and pediatric patients requiring hospitalization (trade name Veklury). Veklury is the first drug to receive FDA approval for the treatment of COVID-19 [24]. Molnupiravir – a broad-spectrum antiviral drug candidate is a prodrug of the nucleoside analogue β-D-N4-hydroxycytidine (NHC). Molnupiravir targets the SARS-CoV-2 RdRp. The drug increases the frequency of SARS-CoV-2 RNA mutations and impairs SARS-CoV-2 replication. Molnupiravir is currently in phase 3 clinical trials for the treatment of COVID-19 [[25], [26], [27]].
There have been many publications describing the action of nucleoside analogues in antiviral therapy. In 2016, De Clercq and Li [28] presented a comprehensive overview of 90 antiviral drugs approved for the treatment of 9 human infectious diseases over the past 50 years. Additionally, review highlights several forthcoming antiviral regimens in phase 3 clinical trials.
Particularly noteworthy is the review published by Lin and colleagues [29] in 2021, describing individual modifications of the structure of nucleoside analogues and the mechanism of their antiviral activity. Importantly, the authors describe the influence of particular structural modifications on the biological activity of compounds.
Fig. 4 describes selected antiviral nucleoside analogues currently investigated in preclinical or clinical trials [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
Fig. 4.
Selected antiviral nucleoside derivatives currently tested in preclinical or clinical trials [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
The present review aims to provide an overview of selected antiviral agents not related to nucleosides. The drugs described in this paper were developed over the past 10 years. The compounds have been ordered according to the mechanism of action. A brief description is also made of future directions of antiviral therapy.
3. Molecular mechanisms of action of non-nucleoside structured compounds
Another approach for the development of new antiviral drugs involves the use of compounds which structure is not derived from nucleosides. This strategy aims to overcome the resistance resulting from treatment with nucleoside-based antiviral drugs. Numerous agents have entered clinical trials (Table 1 ) and many of them were approved by FDA for use in antiviral therapy (Table 2 ). Molecular mechanisms of action of antiviral drugs/drug candidates mentioned in the article are presented on Fig. 5 .
Table 1.
Antiviral agents tested in preclinical or clinical trials.
| Name | Structure | Mechanism of action | Phase of development |
|---|---|---|---|
| Chloroquine | 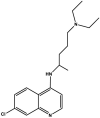 |
Cell entry inhibitor [40,41] | Phase 4 clinical trials: NCT01980745, NCT02463331, NCT02058173, NCT04316377, NCT04286503, NCT02564471 |
| Presatovir/GS-5806 | 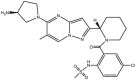 |
Cell entry inhibitor [[42], [43], [44], [45]] | Phase 2 clinical trials: NCT01756482, NCT02534350, NCT02254408, NCT02135614, NCT02254421 |
| Fosdevirine/FDV/GSK2248761/IDX89a |  |
Polymerase inhibitor (NNRTI) [[46], [47], [48]] | Discontinued after phase 2 clinical trial NCT01199731 |
| ABT-072 | 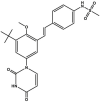 |
Polymerase inhibitor (RdRp) [49,50] | Phase 2 clinical trials: NCT01221298, NCT00872196, NCT01074008 |
| Radalbuvir/GS9669 | 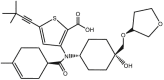 |
Polymerase inhibitor (RdRp) [[51], [52], [53]] | Phase 2 clinical trials: NCT01984294, NCT01260350, NCT01805882, NCT01826981 |
| Tegobuvir/GS-9190 |  |
Polymerase inhibitor (RdRp) [[54], [55], [56], [57]] | Phase 2 clinical trials: NCT01435226, NCT01434498, NCT01353248, NCT01371578, NCT01271790, NCT01072695, NCT01225380, NCT00743795 |
| Setrobuvir/ANA598 | 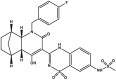 |
Polymerase inhibitor (RdRp) [[58], [59], [60]] | Phase 2 clinical trials: NCT01903954, NCT00978497 |
| PC-786 | 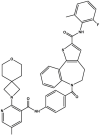 |
Polymerase inhibitor (RdRp) [61,62] | Phase 2 clinical trial: NCT03382431 |
| Pimodivir/JNJ-63623872c |  |
Polymerase inhibitor (RdRp) [[63], [64], [65], [66]] | Phase 2 clinical trial: NCT02342249 |
| RG7834 |  |
Polymerase inhibitorb [67,68] | Preclinical |
| Vesatolimod/GS-9620c |  |
TLR-7d agonist [[69], [70], [71], [72]] | Phase 2 clinical trials: NCT04364035, NCT02579382, NCT02166047 |
| Selgantolimod/GS-9688 | 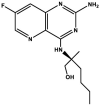 |
TLR-8d agonist [73] | Phase 2 clinical trials: NCT03491553, NCT03615066 |
| Morphothiadin/GLS-4 | 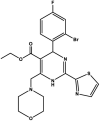 |
Capsid assembly inhibitor [[74], [75], [76], [77]] | Phase 2 clinical trials: NCT04147208, NCT03638076 |
| ABX464 |  |
Viral RNA biogenesis inhibitor [78,79] | Phase 3 clinical trial: NCT04393038; Phase 2 clinical trials: NCT02990325, NCT02735863, NCT02452242 |
Table 2.
Antiviral drugs approved by FDA in 2010–2020a.
| Generic name | Trade name | Structure | Mechanism of action | FDA approval year |
|---|---|---|---|---|
| Rilpivirine hydrochloride | Component of Complera® | 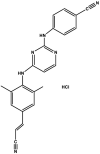 |
Polymerase inhibitor (NNRTI) [87] | 2011 |
| Type of treatment: in combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-naïve adult patients | ||||
| Doravirine | Pifeltro™, component of Delstrigo™ | 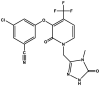 |
Polymerase inhibitor (NNRTI) [88] | 2018 |
| Type of treatment: treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history | ||||
| Dasabuvir | Component of Viekira Pak | 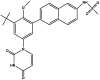 |
Polymerase inhibitor (RdRp) [89] |
2014 |
| Type of treatment: as monotherapy or in combination with ribavirin for the treatment of patients with chronic genotype 1 Hepatitis C Virus (HCV, Flaviviridae family) infections | ||||
| Baloxavir marboxil | Xofluza™ |  |
Polymerase inhibitor (RdRp) [90] | 2018 |
| Type of treatment: treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 h | ||||
| Boceprevir | Victrelis™ | 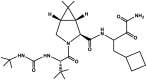 |
Protease inhibitor [91] | 2011 |
| Type of treatment: treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients, 18 years of age and older | ||||
| Simeprevir sodium | Olysio™ |  |
Protease inhibitor [92] | 2013 |
| Type of treatment: treatment of CHC infection, as a component of a combination antiviral treatment regimen | ||||
| Voxilaprevir | Component of Vosevi™ |  |
Protease inhibitor [93] | 2016 |
| Type of treatment: treatment of adult patients with chronic HCV infection without cirrhosis or with compensated cirrhosis | ||||
| Grazoprevir | Component of Zepatier™ | 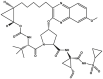 |
Protease inhibitor [94] | 2016 |
| Type of treatment: indicated with or without ribavirin for the treatment of chronic HCV genotype 1 or 4 infections in adults | ||||
| Glecaprevir | Component of Mavyret™ |  |
Protease inhibitor [95] | 2017 |
| Type of treatment: treatment of chronic HCV genotype 1, 2, 3, 4, 5, or 6 infections without cirrhosis or with compensated cirrhosis; for patients with HCV genotype 1 infection who previously have been treated with a regimen containing a HCV nonstructural protein 5A (NS5Ab) inhibitor or an nonstructural protein 3–4A (NS3/4A) protease inhibitor | ||||
| Cobicistat | Component of Stribild® |  |
Cytochrome P450 inhibitor [96] | 2012 |
| Type of treatment: complete regimen for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older who have no antiretroviral treatment history or to replace the current antiretroviral regimen | ||||
| Dolutegravir sodium | Tivicay™ |  |
Integrase inhibitor (INSTI) [97] | 2013 |
| Type of treatment: in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients | ||||
| Elvitegravir | Component of Stribild® | 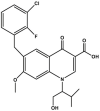 |
Integrase inhibitor (INSTI) [98] | 2012 |
| Type of treatment: complete regimen for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older who have no antiretroviral treatment history or to replace the current antiretroviral regimen | ||||
| Peramivir | Rapivab™ | 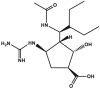 |
Neuraminidase inhibitor [99] | 2014 |
| Type of treatment: treatment of acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than 2 days | ||||
| Letermovir | Prevymis™ | 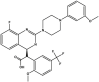 |
Terminase inhibitorc [100] | 2017 |
| Type of treatment: for the prophylaxis of cytomegalovirus (CMV, Herpesviridae family) infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant | ||||
| Ombitasvir | Component of Viekira Pak | 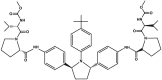 |
NS5A inhibitor [101] | 2014 |
| Type of treatment: as monotherapy or in combination with ribavirin for the treatment of patients with chronic genotype 1 HCV infections | ||||
| Elbasvir | Component of Zepatier™ | 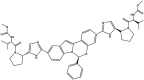 |
NS5A inhibitor [94] | 2016 |
| Type of treatment: indicated with or without ribavirin for the treatment of chronic HCV genotype 1 or 4 infections in adults | ||||
| Pibrentasvir | Component of Mavyret™ | 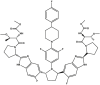 |
NS5A inhibitor [95] | 2017 |
| Type of treatment: treatment of chronic HCV genotype 1, 2, 3, 4, 5, or 6 infections without cirrhosis or with compensated cirrhosis; for patients with HCV genotype 1 infection who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor | ||||
| Daclatasvir | Daklinza™d |  |
NS5A inhibitor [102] | 2015 |
| Type of treatment: in combination with sofosbuvir for the treatment of HCV genotype 3 infections | ||||
| Ledipasvir | Component of Harvoni™ |  |
NS5A inhibitor [103] | 2014 |
| Type of treatment: treatment of chronic HCV genotype 1 infections in adults | ||||
| Velpatasvir | Component of Epclusa® | 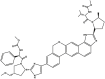 |
NS5A inhibitor [104] | 2015 |
| Type of treatment: treatment of adult patients with chronic HCV genotypes 1, 2, 3, 4, 5, or 6 | ||||
INSTI - integrase strand transfer inhibitor.
According to the FDA Drug Approvals and Databases [105].
Essential component of HCV replication complex.
CMV DNA terminase complex inhibitor.
Discontinued.
Fig. 5.
Molecular mechanisms of antiviral agents action.
3.1. Cell entry/fusion inhibitors
Viral entry/fusion pathways represent promising targets for antiviral drugs. Most viral pathogens are membrane-enveloped viruses, characterized by a lipid bilayer (envelope), covering the virus particle. Viruses replicate within the host cell, disrupting various of cellular proteins and chemical pathways necessary to survive and replicate. At the first stage, enveloped viruses non-covalently bind to specific surface receptors of the target host cell membrane, which is followed by fusion between virus and host cell. Fusion is catalyzed by one type of viral glycoproteins or combinations of multiple viral glycoproteins (fusogens), activated by non-covalent interactions with surface receptors and/or by acidic endosomal pH, resulting in a conformational change, which drives the fusion process [81,82].
Chloroquine (4-N-(7-chloroquinolin-4-yl)-1-N,1-N-diethylpentane-1,4-diamine) (Table 1) is well-known FDA-approved antimalarial drug which synthesis was described more than seventy years ago [83]. One of the mechanisms of its action involves the inhibition of receptor binding and fusion of cell membrane. Chloroquine were found to inhibit novel coronavirus in vitro [40] and have shown clinical benefits in COVID-19 patients. Reports on the possible efficacy of chloroquine in the treatment of SARS-CoV-2 infections, in the absence of a drug for the COVID-19 disease, have resulted in a huge amount of research. Extended research sheds new light on the effects of chloroquine and its derivatives on COVID-19 patients. The pharmacokinetic and safety properties of chloroquine suggest that the drug usage should be limited to an acute virus infection, and patients ought to be routinely monitored for cardiovascular conditions to prevent lethal adverse events [[84], [85], [86]]. Before using chloroquine, an individual risk-benefit assessment should be taken into account, especially in COVID-19 patients with cardiovascular conditions and injury as well as kidney and liver diseases [86].
Presatovir (GS-5806, N-[2-[(2S)-2-[5-[(3S)-3-aminopyrrolidin-1-yl]-6-methylpyrazolo [1,5-a]pyrimidin-2-yl]piperidine-1-carbonyl]-4-chlorophenyl]methanesulfonamide) (Table 1) is an orally bioavailable antiviral compound. Its mechanism of action is based on the inhibition of respiratory syncytial virus (RSV, Paramyxoviridae family) fusion with host cell membranes. The piperidine ring has been revealed to facilitate the formation of an appropriate dihedral angle between the pyrazolo [1,5-a]pyrimidine scaffold and the plane of the amide bond for exertion of anti-RSV activity [106]. Presatovir showed potent antiviral activity in vitro against 75 RSV clinical isolates [42] and dose-dependent antiviral efficacy in vivo in the Sprague-Dawley rat model of RSV infection. The drug was found to be safe and showed beneficial antiviral effects with a reduction in disease severity in phase 2 clinical trial (Table 1, NCT01756482) in healthy adults [43,44]. However, a phase 2b trial conducted in hematopoietic cell transplant recipients with RSV infection of the lower respiratory tract revealed that presatovir did not improve viral or clinical outcomes versus placebo (Table 1, NCT02254421) [45].
3.2. Polymerase inhibitors
Viral DNA and RNA polymerases responsible for the replication are the main components in the life cycle of viruses. The reverse transcriptase (RT) of the retroviruses and the hepadnaviruses is the crucial viral enzyme required for the synthesis of DNA from viral RNA. Another well-known target is NS5B (nonstructural protein 5B) – a RdRp that catalyzes the replication of HCV RNA. Viral polymerases are therefore an extremely favorable target for the development of antiviral therapy [107].
3.2.1. Reverse transcriptase inhibitors
Rilpivirine hydrochloride (4-[[4-[4-[(E)-2-cyanoethenyl]-2,6-dimethylanilino]pyrimidin-2-yl]amino]benzonitrile hydrochloride) is a second-generation NNRTI approved for use with NRTIs (nucleoside reverse transcriptase inhibitors) in treatment-naïve HIV-1 patients (Table 2) [87]. NNRTIs represent one of the most significant classes of drugs designed for the treatment of HIV infection and are a crucial component of current antiretroviral therapy. NNRTIs block HIV-1 replication by preventing RT from completing reverse transcription of the viral single-stranded RNA genome into DNA [108]. Rilpivirine hydrochloride (component of Complera®) was approved by FDA in 2011. Synthesis has been developed but is constantly being refined [109].
Another NNRTI – doravirine (3-chloro-5-[1-[(4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-2-oxo-4-(trifluoromethyl)pyridin-3-yl]oxybenzonitrile) (Table 2), is a safe and well-tolerated compound used so far in treatment switch without the development of resistance. Doravirine yields an advantageous safety profile and may be most beneficial to patients who wish to reduce pill burden or toxicity of other treatment regimens. In 2018, doravirine was approved by the FDA (trade name Pifeltro™) for treatment of adults living with HIV [88].
Fosdevirine (FDV, GSK2248761; IDX899, 5-chloro-3-[[3-[(E)-2-cyanoethenyl]-5-methylphenyl]-methoxyphosphoryl]-1H-indole-2-carboxamide), seems to be a very potent, selective reverse transcriptase inhibitor of HIV-1 (Table 1). The compound was active at low nanomolar concentrations in vitro and effective against a broad range of HIV-1 strains [46]. In phase 1 clinical trials, FDV was well tolerated with no significant adverse effects [47]. Unfortunately, in a phase 2b clinical trial (NCT01199731), five subjects experienced new-onset seizures after at least 4 weeks of treatment, and as a result, the clinical development of FDV was terminated [48].
3.2.2. RdRp inhibitors
RNA viruses encode a unique class of RdRps to carry out their fully RNA-based genome replication and transcription. Well-known HCV NS5B is a viral-specific enzyme that is essential for HCV replication (the catalytic subunit of the replicase complex) which contains all the sequence motifs highly conserved among all the known RdRps. HCV RdRp is considered an important target for anti-HCV drug development [110,111].
Dasabuvir (N-[6-[3-tert-butyl-5-(2,4-dioxopyrimidin-1-yl)-2-methoxyphenyl]naphthalen-2-yl]methanesulfonamide) (Table 2) is a non-nucleoside NS5B inhibitor of HCV used in combination with ombitasvir for the treatment of chronic HCV infections. It is primarily metabolized by cytochrome P450. Biotransformation of dasabuvir forms the M1 metabolite, which retains antiviral activity. Dasabuvir (trade name Viekira Pak) was approved by FDA in 2014 for the treatment of patients with chronic genotype 1 HCV infections [89].
ABT-072 (N-[4-[(E)-2-[3-tert-butyl-5-(2,4-dioxopyrimidin-1-yl)-2-methoxyphenyl]ethenyl]phenyl]methanesulfonamide) (Table 1) is another promising compound for the treatment of HCV infections. This trans-stilbene analogue acts as an NS5B polymerase inhibitor. It was developed by Randolph's team [49] and selected among other compounds for clinical evaluation. ABT-072 was characterized by excellent oral bioavailability. Phase 2 clinical studies (NCT01221298) showed ABT-072 as effective in the treatment of HCV genotype 1-infected patients in combination with ABT-450, ritonavir (ABT-450/r), and ribavirin [50].
Radalbuvir (GS-9669, 5-(3,3-dimethylbut-1-ynyl)-3-[[4-hydroxy-4-[[(3S)-oxolan-3-yl]oxymethyl]cyclohexyl]-[(1R)-4-methylcyclohex-3-ene-1-carbonyl]amino]thiophene-2-carboxylic acid) (Table 1), an inhibitor of HCV NS5B thumb site II, was discovered in 2013 by Lazerwith and co-workers [51]. The drug was active against the clinically relevant NS5B M423T mutant, relative to the wild type. Lazerwith's group reported that this activity was related to both the N-alkyl substituent and N-acyl group [51]. Radalbuvir completed phase 2 clinical trials for the treatment of HCV and was found to be generally well tolerated [53].
Tegobuvir (GS-9190, 5-[[6-[2,4-bis(trifluoromethyl)phenyl]pyridazin-3-yl]methyl]-2-(2-fluorophenyl)imidazo [4,5-c]pyridine) (Table 1) exhibits anti-HCV activity by targeting NS5B polymerase and inhibiting viral replication [54]. The compound undergoes cytochrome P450-mediated activation, and the resulting metabolite specifically interacts with NS5B. A study indicated that tegobuvir showed high potency in vitro [55]. According to reports from phase 1 clinical trials [56,57], the drug induced QT prolongation at high doses. Therefore, its further development was limited to a dose of 40 mg [56].
Basing on the promising results observed in the in vitro studies of tegobuvir, Liu's team [57] designed and synthesized a series of novel inhibitors of HCV NS5B polymerase to improve the antiviral activity and reduce the adverse effects of tegobuvir. However, only one of the investigated compounds showed beneficial effects and safety and was selected for further studies [57].
Inhibitor of the palm pocket of HCV polymerase, setrobuvir (ANA598, N-[3-[(1R,2S,7R,8S)-3-[(4-fluorophenyl)methyl]-6-hydroxy-4-oxo-3-azatricyclo[6.2.1.02,7]undec-5-en-5-yl]-1,1-dioxo-4H-1λ6,2,4-benzothiadiazin-7-yl]methanesulfonamide) (Table 1), showed moderate-to-high bioavailability and low clearance in clinical trials [59,112].
Coates and co-workers [61] described the in vitro and in vivo activity of the novel inhaled anti-RSV drug, PC-786 (N-(2-fluoro-6-methylphenyl)-6-(4-(5-methyl-2-(7-oxa-2-azaspiro[3.5]nonan-2-yl)nicotinamido)benzoyl)-5,6-dihydro-4H-benzo[b]thieno [2,3-d]azepine-2-carboxamide) (Table 1). Their in vitro studies on Hep-2 cells indicated that PC-786 potently inhibited the activity of RSV RdRp. In addition, sustained antiviral effects were observed in vivo in human bronchial epithelial cells and in lung homogenates from RSV-infected mice and cotton rats. Brookes [62] reported that PC-786 caused concentration-dependent inhibition of viral replication with better effects compared to the nucleoside analogue ALS-8112. PC-786 has completed phase 2 clinical trials (Table 1).
Pimodivir (JNJ-63623872, (2S,3S)-3-[[5-fluoro-2-(5-fluoro-1H-pyrrolo [2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino]bicyclo[2.2.2]octane-2-carboxylic acid) (Table 1) is an inhibitor of PB2 [14]. It prevents the polymerase from binding the 7-methyl GTP cap structures on the host capped RNA, thus causing the inhibition of viral gene transcription. It is worth noting that pimodivir acts selectively against influenza A but not influenza B virus, due to the structural differences in the PB2 cap-binding pocket [63]. In mice influenza model, pimodivir was found to increase survival and reduce lung infections. Smee and co-workers [64] observed that lung viral titers were 100–10,000-fold lower in pimodivir-treated mice than those exposed to placebos. In phase 2 clinical trials, pimodivir was well tolerated, and patients did not experience any complications typically associated with influenza, such as bronchitis, sinusitis, or otitis [65]. These beneficial results led to recent research on its analogues. In the search for novel influenza inhibitors, McGowan and his team evaluated 7-fluoro-substituted indoles as bioisosteric replacements for the 7-azaindole scaffold of pimodivir [66].
Baloxavir marboxil is a single dose oral agent for the treatment of influenza A and B. It suppresses influenza replication by inhibition of cap-dependent endonuclease (CEN, an enzyme required for initiation of influenza mRNA synthesis). CEN is a part of a PA subunit, which constitutes the RdRp of influenza virus. Baloxavir marboxil inhibits mRNA synthesis by binding to RdRp and prevents the replication of the influenza virus [90,113]. Baloxavir marboxil was approved by FDA in 2018 (trade name Xofluza™, Table 2) for the treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 h.
In the face of the COVID-19 pandemic, a huge increase of interest in RdRp was observed, as it is an important therapeutic target in RNA virus-caused diseases, including SARS-CoV-2. For this reason, many known RdRp inhibitors (such as described in this paragraph dasabuvir [114], pimodivir [115], radalbuvir [116,117] and tegobuvir [118]) have been also included in the research of an effective drug for COVID-19. Elfiky and colleagues [119] made attempts to assess the possibility of using several antiviral drugs or drug candidates (including setrobuvir and mentioned earlier galidesivir) for the treatment of SARS-CoV-2 virus infections (in silico drug-repurposing). In this study, the RdRp of the SARS-CoV-2 was modeled, validated, and after that, molecular docking was performed. Both galidesivir and setrobuvir were able to bind the SARS-CoV-2 RdRp (galidesivir was able to bind with binding energies comparable to those of native nucleotides). However, it should be taken into account that such studies are the first, preliminary step in the search for compounds active against the SARS-CoV-2 virus, and their results may not be consistent with the results of further in vivo studies [60,119]. Researchers have also made attempts to synthetize new RdRp inhibitors. The current state of the knowledge, including both nucleoside analogues and non-nucleoside compounds, has been summarized in a comprehensive review published by Tian and colleagues [107].
3.2.3. Host noncanonical poly(A) RNA polymerase inhibitor
In 2018, Han and co-workers discovered RG7834 [67] ((6S)-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6-propan-2-yl-6,7-dihydrobenzo[a]quinolizine-3-carboxylic acid) (Table 1) as a lead compound from the dihydroquinolizinone chemical series based on a phenotypic screening. RG7834 reduced viral messenger RNA and accelerated RNA degradation by targeting host noncanonical poly(A) RNA polymerase-associated domain containing protein 5 and 7 (PAPD5 and PAPD7). Both PAPD5 and PAPD7 are very important host components that are required for hepatitis B virus (HBV, Hepadnaviridae family) RNA stabilization, identified as the protein targets of dihydroquinolizinone series [120]. Interestingly, screening studies showed that RG7834 was highly specific for HBV and showed no inhibitory effects against the other 15 DNA or RNA viruses in the panel. RG7834 more strongly inhibited HBV DNA production in vitro in HepaG2.2.15 cell line compared to lamivudine. The drug was well tolerated in vivo studies in rats and monkeys and was found to be orally bioavailable and highly selective. In the same year, RG7834 was investigated by Mueller and colleagues [68]. The researchers confirmed that RG7834 is highly selective for HBV and inhibits the expression of viral proteins specifically by reducing HBV mRNAs.
3.3. Protease inhibitors
Viral proteases are essential in the life cycle of many viruses by affecting the cleavage of viral polyprotein precursors to yield functional products or by catalyzing the processing of the structural proteins necessary for assembly and morphogenesis of virus particles. Most recently, several of the HCV-encoded enzymes, specifically the NS3 protease, have been the focus of intensive research [121]. The NS3/4A serine protease is a non-covalent, heterodimer complex formed by the catalytic subunit of the N-terminal serine protease domain of NS3 and the activation subunit of the NS4A cofactor. The multi-functional property of NS3/4A protease has been an attractive target for drug development [91].
NS3/4A protease inhibitors (PIs) constitute the first class of direct antiviral agents (DAAs) that were approved for HCV treatment. Boceprevir ((1R,2S,5S)–N-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-[(2S)-2-(tert-butylcarbamoylamino)-3,3-dimethylbutanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide), component of the drug Victrelis™, is an oral HCV NS3 protease inhibitor that had been approved by FDA in May 2011 for treating chronic genotype 1 HCV infection in combination with peginterferon alfa and ribavirin, in adult patients, 18 years of age and older (Table 2).
Simeprevir sodium (sodium; cyclopropylsulfonyl-[(1R,4R,6S,7Z,15R,17R)-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carbonyl]azanide) (Table 2) – a small molecular drug targeting the NS3/4A protease of HCV was approved by FDA in 2013 as a component of Olysio™, for the treatment of chronic hepatitis C (CHC) infection [92]. Chemical work on the industrial-scale synthesis of this drug using ring-closing metathesis [122], for example, is still in progress.
Structurally similar voxilaprevir ((1R,18R,20R,24S,27S,28S)-24-tert-butyl-N-[(1R,2R)-2-(difluoromethyl)-1-[(1-methylcyclopropyl)sulfonylcarbamoyl]cyclopropyl]-28-ethyl-13,13-difluoro-7-methoxy-22,25-dioxo-2,21-dioxa-4,11,23,26-tetrazapentacyclo[24.2.1.03,12.05,10.018,20]nonacosa-3,5(10),6,8,11-pentaene-27-carboxamide), grazoprevir ((1R,18R,20R,24S,27S)-24-tert-butyl-N-[(1R,2S)-1-(cyclopropylsulfonylcarbamoyl)-2-ethenylcyclopropyl]-7-methoxy-22,25-dioxo-2,21-dioxa-4,11,23,26-tetrazapentacyclo[24.2.1.03,12.05,10.018,20]nonacosa-3,5(10),6,8,11-pentaene-27-carboxamide), and glecaprevir ((1R,14E,18R,22R,26S,29S)-26-tert-butyl-N-[(1R,2R)-2-(difluoromethyl)-1-[(1-methylcyclopropyl)sulfonylcarbamoyl]cyclopropyl]-13,13-difluoro-24,27-dioxo-2,17,23-trioxa-4,11,25,28-tetrazapentacyclo[26.2.1.03,12.05,10.018,22]hentriaconta-3,5,7,9,11,14-hexaene-29-carboxamide) (Table 2) bind reversibly to the NS3/4A protease active site. Voxilaprevir (Table 2), a component of Vosevi™, was approved in 2016 for the treatment of adult patients with chronic HCV infection without cirrhosis or with compensated cirrhosis [93]. Grazoprevir, a component of Zepatier™, was approved the same year by FDA to treat chronic HCV genotype 1 or 4 infections in adults [94].
Glecaprevir is a component of Mavyret™ (Table 2), which FDA approved in 2017 as the cure for chronic HCV genotype 1, 2, 3, 4, 5 or 6 infections without cirrhosis or with compensated cirrhosis as well as for patients with HCV genotype 1 infection, previously treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor [95].
The use of HIV protease as a potential target was documented in experiments that showed that mutations in the protease resulted in the production of defective, noninfectious viral particles. Clinical trials with protease inhibitors in monotherapy demonstrated the potency of this class of drugs, as evidenced by significant reductions in HIV-1 RNA levels in plasma [121]. Mentioned above cobicistat (1,3-thiazol-5-ylmethyl N-[(2R,5R)-5-[[(2S)-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]-4-morpholin-4-ylbutanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate) (Table 2) is a pharmacoenhancer used with HIV protease inhibitors [96].
3.4. Integrase inhibitors
HIV-1 integrase (IN) catalyzes the insertion of the viral DNA into the genome of host cells. IN inhibition is an attractive therapeutic target for HIV-1 treatment because of its essential role in HIV-1 replication and the lack of homologue in human cells. Identification of IN led to the approval of specific drugs [123].
One of the examples of HIV integrase inhibitors is dolutegravir sodium (sodium; (3S,7R)-13-[(2,4-difluorophenyl)methylcarbamoyl]-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.03,8]tetradeca-10,13-dien-11-olate) (trade name Tivicay™, Table 2), a second-generation integrase inhibitor approved by FDA in 2013 in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients. Dolutegravir is also an attractive option for HIV-positive liver transplant recipients because of its minimal dependence on cytochrome P450 (CYP)-3A-mediated metabolism, high potency, and high genetic barrier [97].
Another example is elvitegravir (6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid (Table 2). It was approved by FDA in 2012 as a component of Stribild®, the four-drug combination (elvitegravir/cobicistat/emtricitabine/tenofovir). Elvitegravir requires coadministration with a CYP enzyme inhibitor. The aim of the CYP enzyme inhibitor was to increase the exposure to the study drug. FDA's ClinPharm Review for elvitegravir described the problem of excessive catabolism and also revealed the solution of using a “pharmacokinetic booster” – an inhibitor of cytochrome P450 3A (CYP3A) – mentioned above Stribild® component – cobicistat [124].
3.5. Neuraminidase inhibitors
Neuraminidase (NA) is an attractive target for antiviral therapy because of its crucial role in the pathogenicity of many respiratory viruses. NA removes sialic acid from the surface of virus particles and infected host cells, preventing viral self-aggregation and promoting efficient viral spread. NA also plays a role in the initial penetration of the mucosal lining of the respiratory tract [125].
Neuraminidase inhibitor peramivir ((1S,2S,3S,4R)-3-[(1S)-1-acetamido-2-ethylbutyl]-4-(diaminomethylideneamino)-2-hydroxycyclopentane-1-carboxylic acid) (trade name Rapivab™, Table 2) was approved by FDA in 2014 for the treatment of uncomplicated acute influenza in patients 18 years and older who have been symptomatic for no more than 2 days.
3.6. Terminase inhibitors
CMV genomic replication involves a mechanism that produces multiple genomic units (concatamers). The viral terminase complex cleaves concatameric viral DNA into full-length genomes and packages a single genome into the viral nucleocapsid as part of new virion formation. Thus, terminase complex represents an attractive therapeutic target. Letermovir (2-[(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-4H-quinazolin-4-yl]acetic acid) (Table 2) was discovered by high-throughput screening to have activity against CMV. Letermovir (trade name Prevymis™) was approved by FDA in 2017 for the prophylaxis of CMV infection and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant [100].
3.7. NS5A inhibitors
NS5A is an essential component of the HCV replicase and exerts a wide range of effects on cellular pathways and processes, including innate immunity and host cell growth and proliferation. Therefore, NS5A has been widely investigated because of its ability to subvert the host interferon-induced antiviral response. There are several NS5A inhibitors approved for the treatment of different types HCV genotypes, usually in combination with other antiviral components [126].
Well-known NS5A inhibitors are ombitasvir (methyl N-[(2S)-1-[(2S)-2-[[4-[(2S,5S)-1-(4-tert-butylphenyl)-5-[4-[[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidine-2-carbonyl]amino]phenyl]pyrrolidin-2-yl]phenyl]carbamoyl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate) (Table 2) – a component of mentioned above drug Viekira Pak and elbasvir (methyl N-[(2S)-1-[(2S)-2-[5-[(6S)-3-[2-[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidin-2-yl]-1H-imidazole-5-yl]-6-phenyl-6H-indolo [1,2-c] [1,3]benzoxazin-10-yl]-1H-imidazole-2-yl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate) (Table 2, component of Zepatier™). Other examples are pibrentasvir (methyl N-[(2S,3R)-1-[(2S)-2-[6-[(2R,5R)-1-[3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl]-5-[6-fluoro-2-[(2S)-1-[(2S,3R)-3-methoxy-2-(methoxycarbonylamino)butanoyl]pyrrolidin-2-yl]-3H-benzimidazol-5-yl]pyrrolidin-2-yl]-5-fluoro-1H-benzimidazol-2-yl]pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl]carbamate) (Table 2, component of Mavyret™) and daclatasvir dihydrochloride (methyl N-[(2S)-1-[(2S)-2-[5-[4-[4-[2-[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidin-2-yl]-1H-imidazole-5-yl]phenyl]phenyl]-1H-imidazole-2-yl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate; dihydrochloride) – which is used in combination with mentioned above sofosbuvir for HCV treatment genotype 3 infections (Daklinza™, Table 2). In addition, ledipasvir (methyl N-[(2S)-1-[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-2-azabicyclo[2.2.1]heptan-3-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazole-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate) and veplatasvir (methyl N-[(1R)-2-[(2S,4S)-2-[5-[6-[(2S,5S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-5-methylpyrrolidin-2-yl]-21-oxa-5,7-diazapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-1(13),2,4(8),5,9,11,14(19),15,17-nonaen-17-yl]-1H-imidazole-2-yl]-4-(methoxymethyl)pyrrolidin-1-yl]-2-oxo-1-phenylethyl]carbamate) are compounds that also belong to the group of NS5A inhibitors and were approved as components of drugs approved for the treatment of HCV (Table 2, trade names Harvoni™ and Epclusa®, respectively).
3.8. TLR-7 and TLR-8 inhibitors
TLR-7, a member of the TLR family, is a pathogen recognition receptor. It is expressed in the cell membrane or the endosomal compartments of plasmacytoid dendritic cells and B cells. After a ligand binds TLR-7, recruitment of adaptor proteins occurs and a signal transduction cascade starts, resulting in both innate and adaptive immune response. Furthermore, due to its ability to drive an antiviral response, TLR-7 is considered a therapeutic host-based target for viral infections [69,70].
Vesatolimod (GS-9620, 4-amino-2-butoxy-8-{[3-(pyrrolidin-1-ylmethyl)phenyl]methyl}-5,7-dihydropteridin-6-one) (Table 1) is an oral TLR-7 agonist developed for the treatment of chronic viral hepatitis by Lanford [69]. In vivo studies on chimpanzees showed that vesatolimod activated TLR-7 signaling and caused a rapid reduction of viremia, a decrease in the levels of serum hepatitis B surface antigen (HBsAg) and hepatitis B e-antigen, and an apparent reduction in the number of infected hepatocytes. Pharmacokinetic studies indicated that vesatolimod is safe and generally well tolerated at low oral doses [70]. However, in phase 2 clinical studies (NCT02579382, NCT02166047) conducted with patients suffering from chronic HBV infection, vesatolimod did not result in a clinically significant decline in serum HBsAg level and hence was not tested further [71,72].
Another TLR agonist, selgantolimod (GS-9688, (2R)-2-[(2-amino-7-fluoropyrido [3,2-d]pyrimidin-4-yl)amino]-2-methylhexan-1-ol) (Table 1), was discovered by Mackman's group as a selective oral compound for the treatment of CHB [73]. It is an agonist of TLR-8. Contrary to TLR-7, human TLR-8 is located on the endosomal membrane of myeloid dendritic cells, macrophages, monocytes, and neutrophils and can recognize single-stranded RNA fragments. In vivo, the woodchuck CHB model displays many features of human CHB and is therefore commonly used to evaluate the antiviral efficacy of immunomodulatory compounds. In vivo studies conducted by Mackman's group on this model showed that selgantolimod is a selective TLR-8 agonist and exhibits good oral absorption and high clearance limiting the systemic exposure levels [73]. Selgantolimod completed phase 2 clinical trials.
3.9. Capsid assembly inhibitors
Capsid assembly is a critical step in the propagation of HBV [74]. Based on the previous reports that heteroaryldihydropyrimidines (HAPs) influence the accumulation of HBV capsid [127], Wang and colleagues synthesized another HAP compound, morphothiadin (GLS-4, ethyl 4-(2-bromo-4-fluorophenyl)-6-(morpholin-4-ylmethyl)-2-(1,3-thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate) (Table 1) [74], first-in-class HBV capsid assembly modulator, that can inhibit HBV replication by interfering with the assembly and disassembly of HBV nucleocapsid. Authors observed that the compound exhibited promising in vitro dose-dependent activity against HBV. Moreover, its antiviral activity was found to be higher than lamivudine which is an antiviral drug used for HBV treatment. Adefovir-resistant mutants were also sensitive to the investigated compound. In vivo studies confirmed beneficial results with little evidence of hepatotoxicity as well as overall toxicity in the mice model [75]. In addition, the pharmacokinetic and toxicity profiles of the drug were favorable [76]. Morphothiadin in combination with ritonavir reached a phase 2 clinical trial for the treatment of chronic HBV infections (NCT04147208). Recently, based on the strategy of inhibiting HBV capsid assembly, Ren and co-workers [77] carried out further research on a series of morphothiadin derivatives with promising anti-HBV activity and improved pharmacokinetic properties.
3.10. Viral biogenesis inhibitor
ABX464 (8-chloro-N-[4-(trifluoromethoxy)phenyl]quinolin-2-amine) (Table 1) was selected as the most promising anti-HIV drug after the functional screening. Its mechanism of action was described for the first time in 2015 [78]. The activity of ABX464 is connected with Rev protein and is based on blocking viral inhibition by preventing the export of unspliced RNA to the cytoplasm, and by interacting with the Cap-Binding Complex (CBC). ABX464 is non-toxic and does not give rise to resistant HIV-1 variants. After favorable results obtained in humanized SCID mice models, ABX464 entered clinical trials. A recent study [79] confirmed that this new compound is characterized by good safety and tolerability. When included in an HIV-1 protease inhibitor-based regimen, it caused a reduction in the levels of HIV-1 DNA in peripheral blood mononuclear cells. ABX464 is currently tested for the treatment of patients with COVID-19 (phase 3) and HIV (phase 2).
4. Conclusions and future directions
The last decade has witnessed remarkable achievements in the field of antiviral drug discovery. Several new antiviral drugs have been introduced for the treatment of HIV, HCV, CMV, and influenza infections. The efforts included introduction of new molecules and repositioning of drugs already in clinical use. The search for new antiviral compounds was performed not only among nucleoside analogues but also not related to nucleosides chemical compounds. The adenosine analogue islatravir is an example of a new nucleoside analogue that has been successfully introduced into phase 3 clinical trials as an anti-HIV agent. ABX464 is an example of a non-nucleoside derivative that has entered phase 3 clinical trials. The antimalarial drug chloroquine, an example of drug repositioning, has currently completed phase 4 clinical trials for treating COVID-19 infection. Most of the tested antiviral agents are inhibitors of specific enzymes involved in various stages of the virus life cycle, such as viral polymerases and proteases.
Scientists are also interested in using biologically active metabolites in antiviral therapy. Such compounds have been considered for antiviral therapy and prophylaxis, due to their antioxidant, antibacterial, antifungal, and antiviral activities [128]. Articles published by Singh and colleagues, and Bibi and co-workers, describe the antiviral efficacy of plant-based therapeutics against SARS-CoV-2 [129,130]. Besides, nanoparticles have been widely investigated as carriers for known antiviral agents [131]. Search for new compounds with antiviral activity is currently supported by bioinformatics methods that enable a better understanding of pathophysiology of diseases and to introduce effective treatment [132], including design of antiviral agents for COVID-19 [133]. Furthermore, monoclonal antibodies [[134], [135], [136]] and the CRISPR-Cas system are considered as biotherapeutic alternatives to synthetic compounds in antiviral therapy [[137], [138], [139]].
At least 20 antiviral drugs have been approved for treatment by the FDA between 2010 and 2020, and 14 compounds described in this review show promising results in clinical or preclinical trials. As an update, it should also be added that on the December 22, 2021, the FDA authorized a new drug against COVID-19 – trade name Paxlovid, which proves the rapid development of antiviral therapies.
Finding an effective antiviral therapy is a challenge for modern medicine. However, the variety of currently available treatment methods may contribute to further advances in this field, and although these methods are not a panacea, they could serve as means to control the broad range of viral infections.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was financed in part by the Ministry of Science and Higher Education within the project POL-OPENSCREEN (decision no. DIR/WK/2018/06 of October 24, 2018). The contribution from the Statutory Found of IMB PAS is also gratefully acknowledge.
References
- 1.Prusoff W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim. Biophys. Acta. 1959;32:295–296. doi: 10.1016/0006-3002(59)90597-9. [DOI] [PubMed] [Google Scholar]
- 2.Flexner C., Owen A., Siccardi M., Swindells S. Long-acting drugs and formulations for the treatment and prevention of HIV infection. Int. J. Antimicrob. Agents. 2021;57:106220. doi: 10.1016/j.ijantimicag.2020.106220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrivastava N., Husain A., Rashid M., Alsabeelah N.F., Karim S., Siddiqui N.A. Recent advances towards treatment of HIV: synthesis and SAR studies. Mini Rev. Med. Chem. 2019;21:471–499. doi: 10.2174/1389557519666190312170158. [DOI] [PubMed] [Google Scholar]
- 4.Obisesan O., Katata-Seru L., Mufamadi S., Mufhandu H. Applications of nanoparticles for herpes simplex virus (HSV) and human immunodeficiency virus (HIV) treatment. J. Biomed. Nanotechnol. 2021;17:793–808. doi: 10.1166/jbn.2021.3074. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam S., Lombardi A., Ouanounou A. COVID-19: a review of the proposed pharmacological treatments. Eur. J. Pharmacol. 2020;886:173451. doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020;72:1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.C.S., Yim A.K.Y., Chan A.K.C., Ng L.P.W., Wong Y.K.E., Pei X.M., Li M.J.W., Wong S.C.C. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti Infect. Ther. 2021;19:877–888. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 9.Anka A.U., Tahir M.I., Abubakar S.D., Alsabbagh M., Zian Z., Hamedifar H., Sabzevari A., Azizi G. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021;93 doi: 10.1111/sji.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassetto M., Massarotti A., Coluccia A., Brancale A. Structural biology in antiviral drug discovery. Curr. Opin. Pharmacol. 2016;30:116–130. doi: 10.1016/j.coph.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blundell T.L., Jhoti H., Abell C. High-throughput crystallography for lead discovery in drug design. Nat. Rev. Drug Discov. 2002;1:45–54. doi: 10.1038/nrd706. [DOI] [PubMed] [Google Scholar]
- 12.Rut W., Groborz K., Zhang L., Sun X., Zmudzinski M., Pawlik B., Wang X., Jochmans D., Neyts J., Młynarski W., Hilgenfeld R., Drag M. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2021;17:222–228. doi: 10.1038/s41589-020-00689-z. [DOI] [PubMed] [Google Scholar]
- 13.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregor J., Radilová K., Brynda J., Fanfrlík J., Konvalinka J., Kožíšek M. Structural and thermodynamic analysis of the resistance development to Pimodivir (VX-787), the clinical inhibitor of cap binding to PB2 subunit of influenza a polymerase. Molecules. 2021;26:1007. doi: 10.3390/molecules26041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The drug development and approval process. https://www.fdareview.org/issues/the-drug-development-and-approval-process/ June 23, 2021)
- 16.Jordheim L.P., Durantel D., Zoulim F., Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 17.Schürmann D., Rudd D.J., Zhang S., De Lepeleire I., Robberechts M., Friedman E., Keicher C., Hüser A., Hofmann J., Grobler J.A., Stoch S.A., Iwamoto M., Matthews R.P. Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration to treatment-naive adults infected with HIV-1: an open-label, phase 1b, consecutive-panel trial. Lancet HIV. 2020;7:e164–e172. doi: 10.1016/S2352-3018(19)30372-8. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K., Desai D.V., Aoki M., Nakata H., Kodama E.N., Mitsuya H. Delayed emergence of HIV-1 variants resistant to 4’-ethynyl-2-fluoro-2’-deoxyadenosine: comparative sequential passage study with lamivudine, tenofovir, emtricitabine and BMS-986001. Antivir. Ther. 2014;19:179–189. doi: 10.3851/IMP2697. [DOI] [PubMed] [Google Scholar]
- 19.Murphey-Corb M., Rajakumar P., Michael H., Nyaundi J., Didier P.J., Reeve A.B., Mitsuya H., Sarafianos S.G., Parniak M.A. Response of simian immunodeficiency virus to the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine in vitro and in vivo, Antimicrob. Agents Chemother. 2012;56:4707–4712. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz M., Grobler J.A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1. Curr. Opin. HIV AIDS. 2020;15:27–32. doi: 10.1097/COH.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 21.Warren T.K., Wells J., Panchal R.G., Stuthman K.S., Garza N.L., Van Tongeren S.A., Dong L., Retterer C.J., Eaton B.P., Pegoraro G., Honnold S., Bantia S., Kotian P., Chen X., Taubenheim B.R., Welch L.S., Minning D.M., Babu Y.S., Sheridan W.P., Bavari S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noor R. Antiviral drugs against severe acute respiratory syndrome coronavirus 2 infection triggering the coronavirus disease-19 pandemic. Tzu Chi Med. J. 2021;33:7–12. doi: 10.4103/tcmj.tcmj_100_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alshaeri H.K., Natto Z.S. A contemporary look at COVID-19 medications: available and potentially effective drugs. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9188–9195. doi: 10.26355/eurrev_202009_22870. [DOI] [PubMed] [Google Scholar]
- 24.FDA's approval of Veklury (remdesivir) for the treatment of COVID-19. https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness June 23, 2021)
- 25.Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Höbartner C., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon C.J., Tchesnokov E.P., Schinazi R.F., Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021;297:100770. doi: 10.1016/j.jbc.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malone B., Campbell E.A. Molnupiravir: coding for catastrophe. Nat. Struct. Mol. Biol. 2021;28:706–708. doi: 10.1038/s41594-021-00657-8. [DOI] [PubMed] [Google Scholar]
- 28.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X., Liang C., Zou L., Yin Y., Wang J., Chen D., Lan W. Advance of structural modification of nucleosides scaffold. Eur. J. Med. Chem. 2021;214:113233. doi: 10.1016/j.ejmech.2021.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takamatsu Y., Tanaka Y., Kohgo S., Murakami S., Singh K., Das D., Venzon D.J., Amano M., Higashi-Kuwata N., Aoki M., Delino N.S., Hayashi S., Takahashi S., Sukenaga Y., Haraguchi K., Sarafianos S.G., Maeda K., Mitsuya H. 4′-modified nucleoside analogs: potent inhibitors active against entecavir-resistant hepatitis B virus. Hepatology. 2015;62:1024–1036. doi: 10.1002/hep.27962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Cao L., Li G., Cong F., Li Y., Sun J., Luo Y., Chen G., Li G., Wang P., Xing F., Ji Y., Zhao J., Zhang Y., Guo D., Zhang X. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mouse models. J. Med. Chem. 2021 doi: 10.1021/acs.jmedchem.0c01929. [DOI] [PubMed] [Google Scholar]
- 32.Singh U.S., Mulamoottil V.A., Chu C.K. 2′-Fluoro-6′-methylene carbocyclic adenosine and its phosphoramidate prodrug: a novel anti-HBV agent, active against drug-resistant HBV mutants. Med. Res. Rev. 2018;38:977–1002. doi: 10.1002/med.21490. [DOI] [PubMed] [Google Scholar]
- 33.Zmurko J., Marques R.E., Schols D., Verbeken E., Kaptein S.J.F., Neyts J. The viral polymerase inhibitor 7-Deaza-2’-C-Methyladenosine is a potent inhibitor of in vitro Zika virus replication and Delays disease progression in a robust mouse infection model. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qing J., Luo R., Wang Y., Nong J., Wu M., Shao Y., Tang R., Yu X., Yin Z., Sun Y. Resistance analysis and characterization of NITD008 as an adenosine analog inhibitor against hepatitis C virus. Antivir. Res. 2016;126:43–54. doi: 10.1016/j.antiviral.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M., Taguchi K., Ishiguro T., Kohgo S., Imoto S., Yamasaki K., Mitsuya H., Otagiri M. Pharmacokinetics studies of 4′-cyano-2′-deoxyguanosine, a potent inhibitor of the hepatitis B virus, in rats. J. Pharm. Pharmacol. 2018;70:723–731. doi: 10.1111/jphp.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higashi-Kuwata N., Hayashi S., Das D., Kohgo S., Murakami S., ichiro Hattori S., Imoto S., Venzon D.J., Singh K., Sarafianos S.G., Tanaka Y., Mitsuya H. CMCDG, a novel nucleoside analog with favorable safety features, exerts potent activity against wild-type and entecavir-resistant hepatitis B virus. Antimicrob. Agents Chemother. 2019;63:e02143–18. doi: 10.1128/AAC.02143-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L., Wang Q., Yang X., Guo X., Chen L., Tao L., Dong L., Li Y., An H., Yu X., Wang Q., Chang J. Antiviral activity of FNC, 2′-deoxy-2′-β-fluoro-4′- azidocytidine, against human and duck HBV replication. Antivir. Ther. 2012;17:679–687. doi: 10.3851/IMP2094. [DOI] [PubMed] [Google Scholar]
- 38.Patel K., Kirkpatrick C.M., Nieforth K.A., Chanda S., Zhang Q., McClure M., Fry J., Symons J.A., Blatt L.M., Beigelman L., Devincenzo J.P., Huntjens D.R., Smith P.F. Respiratory syncytial virus-A dynamics and the effects of lumicitabine, a nucleoside viral replication inhibitor, in experimentally infected humans. J. Antimicrob. Chemother. 2019;74:442–452. doi: 10.1093/jac/dky415. [DOI] [PubMed] [Google Scholar]
- 39.Peifer M., Berger R., Shurtleff V.W., Conrad J.C., Macmillan D.W.C. A general and enantioselective approach to pentoses: a rapid synthesis of PSI-6130, the nucleoside core of sofosbuvir. J. Am. Chem. Soc. 2014;136:5900–5903. doi: 10.1021/ja502205q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan M.M., Noor A., Madni A., Shafiq M. Emergence of novel coronavirus and progress toward treatment and vaccine. Rev. Med. Virol. 2020;30:e2116. doi: 10.1002/rmv.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perron M., Stray K., Kinkade A., Theodore D., Lee G., Eisenberg E., Sangi M., Gilbert B.E., Jordan R., Piedra P.A., Toms G.L., Mackman R., Cihlar T. GS-5806 inhibits a broad range of respiratory syncytial virus clinical isolates by blocking the virus-cell fusion process. Antimicrob. Agents Chemother. 2016;60:1264–1273. doi: 10.1128/AAC.01497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeVincenzo J.P., Whitley R.J., Mackman R.L., Scaglioni-Weinlich C., Harrison L., Farrell E., McBride S., Lambkin-Williams R., Jordan R., Xin Y., Ramanathan S., O'Riordan T., Lewis S.A., Li X., Toback S.L., Lin S.L., Chien J.W. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 44.Mackman R.L., Sangi M., Sperandio D., Parrish J.P., Eisenberg E., Perron M., Hui H., Zhang L., Siegel D., Yang H., Saunders O., Boojamra C., Lee G., Samuel D., Babaoglu K., Carey A., Gilbert B.E., Piedra P.A., Strickley R., Iwata Q., Hayes J., Stray K., Kinkade A., Theodore D., Jordan R., Desai M., Cihlar T. Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. J. Med. Chem. 2015;58:1630–1643. doi: 10.1021/jm5017768. [DOI] [PubMed] [Google Scholar]
- 45.Marty F.M., Chemaly R.F., Mullane K.M., Lee D.G., Hirsch H.H., Small C.B., Bergeron A., Shoham S., Ljungman P., Waghmare A., Blanchard E., Kim Y.J., McKevitt M., Porter D.P., Jordan R., Guo Y., German P., Boeckh M., Watkins T.R., Chien J.W., Dadwal S.S. A phase 2b, randomized, double-blind, placebo-controlled multicenter study evaluating antiviral effects, pharmacokinetics, safety, and tolerability of presatovir in hematopoietic cell transplant recipients with respiratory syncytial virus infection of the. Clin. Infect. Dis. 2020;71:2787–2795. doi: 10.1093/cid/ciz1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellino S., Groseclose M.R., Sigafoos J., Wagner D., De Serres M., Polli J.W., Romach E., Myer J., Hamilton B. Central nervous system disposition and metabolism of Fosdevirine (GSK2248761), a non-nucleoside reverse transcriptase inhibitor: an LC-MS and matrix-assisted laser desorption/ionization imaging MS investigation into central nervous system toxicity. Chem. Res. Toxicol. 2013;26:241–251. doi: 10.1021/tx3004196. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X.J., Pietropaolo K., Damphousse D., Belanger B., Chen J., Sullivan-Bólyai J., Mayers D. Single-dose escalation and multiple-dose safety, tolerability, and pharmacokinetics of IDX899, a candidate human immunodeficiency virus type 1 nonnucleoside reverse transcriptase inhibitor, in healthy subjects. Antimicrob. Agents Chemother. 2009;53:1739–1746. doi: 10.1128/AAC.01479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margolis D.A., Eron J.J., DeJesus E., White S., Wannamaker P., Stancil B., Johnson M. Unexpected finding of delayed-onset seizures in HIV-positive, treatment-experienced subjects in the phase IIb evaluation of fosdevirine (GSK2248761) Antivir. Ther. 2014;19:69–78. doi: 10.3851/IMP2689. [DOI] [PubMed] [Google Scholar]
- 49.Randolph J.T., Krueger A.C., Donner P.L., Pratt J.K., Liu D., Motter C.E., Rockway T.W., Tufano M.D., Wagner R., Lim H.B., Beyer J.M., Mondal R., Panchal N.S., Colletti L., Liu Y., Koev G., Kati W.M., Hernandez L.E., Beno D.W.A., Longenecker K.L., Stewart K.D., Dumas E.O., Molla A., Maring C.J. Synthesis and biological characterization of Aryl Uracil inhibitors of hepatitis C virus NS5B polymerase: discovery of ABT-072, a trans-stilbene analog with good oral bioavailability. J. Med. Chem. 2018;61:1153–1163. doi: 10.1021/acs.jmedchem.7b01630. [DOI] [PubMed] [Google Scholar]
- 50.Lawitz E., Poordad F., Kowdley K.V., Cohen D.E., Podsadecki T., Siggelkow S., Larsen L., Menon R., Koev G., Tripathi R., Pilot-Matias T., Bernstein B. A phase 2a trial of 12-week interferon-free therapy with two direct-acting antivirals (ABT-450/r, ABT-072) and ribavirin in IL28B C/C patients with chronic hepatitis C genotype 1. J. Hepatol. 2013;59:18–23. doi: 10.1016/j.jhep.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Lazerwith S.E., Lew W., Zhang J., Morganelli P., Liu Q., Canales E., Clarke M.O., Doerffler E., Byun D., Mertzman M., Ye H., Chong L., Xu L., Appleby T., Chen X., Fenaux M., Hashash A., Leavitt S.A., Mabery E., Matles M., Mwangi J.W., Tian Y., Lee Y.J., Zhang J., Zhu C., Murray B.P., Watkins W.J. Discovery of GS-9669, a thumb site II non-nucleoside inhibitor of NS5B for the treatment of genotype 1 chronic hepatitis C infection. J. Med. Chem. 2014;57:1893–1901. doi: 10.1021/jm401420j. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Johnson K.A. Thumb site 2 inhibitors of hepatitis C viral RNA-dependent RNA polymerase allosterically block the transition from initiation to elongation. J. Biol. Chem. 2016;291:10067–10077. doi: 10.1074/jbc.M115.708354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borgia G., Maraolo A.E., Nappa S., Gentile I., Buonomo A.R. NS5B polymerase inhibitors in phase II clinical trials for HCV infection. Expet Opin. Invest. Drugs. 2018;27:243–250. doi: 10.1080/13543784.2018.1420780. [DOI] [PubMed] [Google Scholar]
- 54.Shih I.H., Vliegen I., Peng B., Yang H., Hebner C., Paeshuyse J., Pürstinger G., Fenaux M., Tian Y., Mabery E., Qi X., Bahador G., Paulson M., Lehman L.S., Bondy S., Tse W., Reiser H., Lee W.A., Schmitz U., Neyts J., Zhong W. Mechanistic characterization of GS-9190 (tegobuvir), a novel nonnucleoside inhibitor of hepatitis C virus NS5B polymerase. Antimicrob. Agents Chemother. 2011;55:4196–4203. doi: 10.1128/AAC.00307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hebner C.M., Han B., Brendza K.M., Nash M., Sulfab M., Tian Y., Hung M., Fung W., Vivian R.W., Trenkle J., Taylor J., Bjornson K., Bondy S., Liu X., Link J., Neyts J., Sakowicz R., Zhong W., Tang H., Schmitz U. The HCV non-nucleoside inhibitor tegobuvir utilizes a novel mechanism of action to inhibit NS5B polymerase function. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sofia M.J., Chang W., Furman P.A., Mosley R.T., Ross B.S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 2012;55:2481–2531. doi: 10.1021/jm201384j. [DOI] [PubMed] [Google Scholar]
- 57.Liu M., Xu Q., Guo S., Zuo R., Hong Y., Luo Y., Li Y., Gong P., Liu Y. Design, synthesis, and structure-activity relationships of novel imidazo[4,5-c]pyridine derivatives as potent non-nucleoside inhibitors of hepatitis C virus NS5B. Bioorg. Med. Chem. 2018;26:2621–2631. doi: 10.1016/j.bmc.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 58.Mallalieu N.L., Rahimy M.H., Crowley C.A., Appleman J.R., Smith P.F., Freddo J.L. Pharmacokinetics and pharmacodynamics of setrobuvir, an orally administered hepatitis C virus non-nucleoside analogue inhibitor. Clin. Therapeut. 2014;36:2047–2063. doi: 10.1016/j.clinthera.2014.10.002. e3. [DOI] [PubMed] [Google Scholar]
- 59.Canini L., Lemenuel-Diot A., Brennan B.J., Smith P.F., Perelson A.S. A pharmacokinetic/viral kinetic model to evaluate treatment of chronic HCV infection with a non-nucleoside polymerase inhibitor. Antivir. Ther. 2018;23:353–361. doi: 10.3851/IMP3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coates M., Brookes D., Kim Y.I., Allen H., Fordyce E.A.F., Meals E.A., Colley T., Ciana C.L., Parra G.F., Sherbukhin V., Stockwell J.A., Thomas J.C., Hunt S.F., Anderson-Dring L., Onions S.T., Cass L., Murray P.J., Ito K., Strong P., DeVincenzo J.P., Rapeport G. Preclinical characterization of PC786, an inhaled small-molecule respiratory syncytial virus L protein polymerase inhibitor. Antimicrob. Agents Chemother. 2017;61:e00737–17. doi: 10.1128/AAC.00737-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brookes D.W., Coates M., Allen H., Daly L., Constant S., Huang S., Hows M., Davis A., Cass L., Ayrton J., Knowles I., Strong P., Rapeport G., Ito K. Late therapeutic intervention with a respiratory syncytial virus L-protein polymerase inhibitor, PC786, on respiratory syncytial virus infection in human airway epithelium. Br. J. Pharmacol. 2018;175:2520–2534. doi: 10.1111/bph.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mifsud E.J., Hayden F.G., Hurt A.C. Antivirals targeting the polymerase complex of influenza viruses. Antivir. Res. 2019;169:104545. doi: 10.1016/j.antiviral.2019.104545. [DOI] [PubMed] [Google Scholar]
- 64.Smee D.F., Barnard D.L., Jones S.M. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antivir. Res. 2016;136:45–50. doi: 10.1016/j.antiviral.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Finberg R.W., Lanno R., Anderson D., Fleischhackl R., Van Duijnhoven W., Kauffman R.S., Kosoglou T., Vingerhoets J., Leopold L. Phase 2b study of pimodivir (JNJ-63623872) as monotherapy or in combination with oseltamivir for treatment of acute uncomplicated seasonal influenza A: TOPAZ trial. J. Infect. Dis. 2019;219:1026–1034. doi: 10.1093/infdis/jiy547. [DOI] [PubMed] [Google Scholar]
- 66.McGowan D.C., Balemans W., Embrechts W., Motte M., Keown J.R., Buyck C., Corbera J., Funes M., Moreno L., Cooymans L., Tahri A., Eymard J., Stoops B., Strijbos R., Van Den Berg J., Fodor E., Grimes J.M., Koul A., Jonckers T.H.M., Raboisson P., Guillemont J. Design, synthesis, and biological evaluation of novel indoles targeting the influenza PB2 cap binding region. J. Med. Chem. 2019;62:9680–9690. doi: 10.1021/acs.jmedchem.9b01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han X., Zhou C., Jiang M., Wang Y., Wang J., Cheng Z., Wang M., Liu Y., Liang C., Wang J., Wang Z., Weikert R., Lv W., Xie J., Yu X., Zhou X., Luangsay S., Shen H.C., Mayweg A.V., Javanbakht H., Yang S. Discovery of RG7834: the first-in-class selective and orally available small molecule hepatitis B virus expression inhibitor with novel mechanism of action. J. Med. Chem. 2018;61:10619–10634. doi: 10.1021/acs.jmedchem.8b01245. [DOI] [PubMed] [Google Scholar]
- 68.Mueller H., Wildum S., Luangsay S., Walther J., Lopez A., Tropberger P., Ottaviani G., Lu W., Parrott N.J., Zhang J.D., Schmucki R., Racek T., Hoflack J.C., Kueng E., Point F., Zhou X., Steiner G., Lütgehetmann M., Rapp G., Volz T., Dandri M., Yang S., Young J.A.T., Javanbakht H. A novel orally available small molecule that inhibits hepatitis B virus expression. J. Hepatol. 2018;68:412–420. doi: 10.1016/j.jhep.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Lanford R.E., Guerra B., Chavez D., Giavedoni L., Hodara V.L., Brasky K.M., Fosdick A., Frey C.R., Zheng J., Wolfgang G., Halcomb R.L., Tumas D.B. An oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517. doi: 10.1053/j.gastro.2013.02.003. GS-9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopatin U., Wolfgang G., Tumas D., Frey C.R., Ohmstede C., Hesselgesser J., Kearney B., Moorehead L., Subramanian G.M., McHutchison J.G. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral Toll-like receptor 7 agonist. Antivir. Ther. 2013;18:409–418. doi: 10.3851/IMP2548. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal K., Ahn S.H., Elkhashab M., Lau A.H., Gaggar A., Bulusu A., Tian X., Cathcart A.L., Woo J., Subramanian G.M., Andreone P., Kim H.J., Chuang W.L., Nguyen M.H. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J. Viral Hepat. 2018;25:1331–1340. doi: 10.1111/jvh.12942. [DOI] [PubMed] [Google Scholar]
- 72.Janssen H.L.A., Brunetto M.R., Kim Y.J., Ferrari C., Massetto B., Nguyen A.H., Joshi A., Woo J., Lau A.H., Gaggar A., Subramanian G.M., Yoshida E.M., Ahn S.H., Tsai N.C.S., Fung S., Gane E.J. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2018;68:431–440. doi: 10.1016/j.jhep.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 73.Mackman R.L., Mish M., Chin G., Perry J.K., Appleby T., Aktoudianakis V., Metobo S., Pyun P., Niu C., Daffis S., Yu H., Zheng J., Villasenor A.G., Zablocki J., Chamberlain J., Jin H., Lee G., Suekawa-Pirrone K., Santos R., Delaney W.E., Fletcher S.P. Discovery of GS-9688 (selgantolimod) as a potent and selective oral toll-like receptor 8 agonist for the treatment of chronic hepatitis B. J. Med. Chem. 2020;63:10188–10203. doi: 10.1021/acs.jmedchem.0c00100. [DOI] [PubMed] [Google Scholar]
- 74.Wang X.Y., Wei Z.M., Wu G.Y., Wang J.H., Zhang Y.J., Li J., Zhang H.H., Xie X.W., Wang X., Wang Z.H., Wei L., Wang Y., Chen H.S. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovirdipivoxil-resistant HBV mutations. Antivir. Ther. 2012;17:793–803. doi: 10.3851/IMP2152. [DOI] [PubMed] [Google Scholar]
- 75.Wu G., Liu B., Zhang Y., Li J., Arzumanyan A., Clayton M.M., Schinazi R.F., Wang Z., Goldmann S., Ren Q., Zhang F., Feitelson M.A. Preclinical characterization of GLS4, an inhibitor of Hepatitis B virus core particle assembly. Antimicrob. Agents Chemother. 2013;57:5344–5354. doi: 10.1128/AAC.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren Q., Liu X., Luo Z., Li J., Wang C., Goldmann S., Zhang J., Zhang Y. Discovery of hepatitis B virus capsid assembly inhibitors leading to a heteroaryldihydropyrimidine based clinical candidate (GLS4) Bioorg. Med. Chem. 2017;25:1042–1056. doi: 10.1016/j.bmc.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 77.Ren Q., Liu X., Yan G., Nie B., Zou Z., Li J., Chen Y., Wei Y., Huang J., Luo Z., Gu B., Goldmann S., Zhang J., Zhang Y. 3-((R)-4-(((R)-6-(2-Bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propanoic acid (HEC72702), a novel hepatitis B virus capsid inhibitor based on clinical candidate GLS4. J. Med. Chem. 2018;61:1355–1374. doi: 10.1021/acs.jmedchem.7b01914. [DOI] [PubMed] [Google Scholar]
- 78.Campos N., Myburgh R., Garcel A., Vautrin A., Lapasset L., Nadal E.S., Mahuteau-Betzer F., Najman R., Fornarelli P., Tantale K., Basyuk E., Séveno M., Venables J.P., Pau B., Bertrand E., Wainberg M.A., Speck R.F., Scherrer D., Tazi J. Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis. Retrovirology. 2015;12:30. doi: 10.1186/s12977-015-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutsaert S., Steens J.M., Gineste P., Cole B., Kint S., Barrett P.N., Tazi J., Scherrer D., Ehrlich H.J., Vandekerckhove L. Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a Phase IIa randomised controlled study. J. Virus Erad. 2019;5:10–22. doi: 10.1016/s2055-6640(20)30273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groaz E., De Clercq E., Herdewijn P. Anno 2021: which antivirals for the coming decade? Annu. Rep. Med. Chem. 2021;57:49–107. doi: 10.1016/bs.armc.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pattnaik G.P., Chakraborty H. Entry inhibitors: efficient means to block viral infection. J. Membr. Biol. 2020;253:425–444. doi: 10.1007/s00232-020-00136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y., Simmons G. Development of novel entry inhibitors targeting emerging viruses. Expert Rev. Anti Infect. Ther. 2012;10:1129–1138. doi: 10.1586/eri.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Surrey A.R., Hammer H.F. Some 7-substituted 4-Aminoquinoline derivatives. J. Am. Chem. Soc. 1946;68:113–116. doi: 10.1021/ja01205a036. [DOI] [PubMed] [Google Scholar]
- 84.Cherian S.S., Agrawal M., Basu A., Abraham P., Gangakhedkar R.R., Bhargava B. Perspectives for repurposing drugs for the coronavirus disease 2019. Indian J. Med. Res. 2020;151:160–171. doi: 10.4103/ijmr.IJMR_585_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smit C., Peeters M.Y.M., van den Anker J.N., Knibbe C.A.J. Chloroquine for SARS-CoV-2: implications of its unique pharmacokinetic and safety properties. Clin. Pharmacokinet. 2020;59:659–669. doi: 10.1007/s40262-020-00891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X.L., Li Z.M., Ye J.T., Lu J., Ye L.L., Zhang C.X., Liu P.Q., Duan D.D. Pharmacological and cardiovascular perspectives on the treatment of COVID-19 with chloroquine derivatives. Acta Pharmacol. Sin. 2020;41:1377–1386. doi: 10.1038/s41401-020-00519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho S., Wong J.G., Ng O.T., Lee C.C., Leo Y.S., Lye D.C.B., Wong C.S. Efficacy and safety of abacavir/lamivudine plus rilpivirine as a first-line regimen in treatment-naïve HIV-1 infected adults. AIDS Res. Ther. 2020;17:23. doi: 10.1186/s12981-020-00272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pham H.T., Xiao M.A., Principe M.A., Wong A., Mesplède T. Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection. Drugs Context. 2020;9:1–11. doi: 10.7573/dic.2019-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.King J.R., Zha J., Khatri A., Dutta S., Menon R.M. Clinical pharmacokinetics of dasabuvir. Clin. Pharmacokinet. 2017;56:1115–1124. doi: 10.1007/s40262-017-0519-3. [DOI] [PubMed] [Google Scholar]
- 90.Lampejo T. Influenza and antiviral resistance: an overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1201–1208. doi: 10.1007/s10096-020-03840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan Q., Peppelenbosch M.P., Janssen H.L.A., de Knegt R.J. Telaprevir/boceprevir era: from bench to bed and back. World J. Gastroenterol. 2012;18:6183–6188. doi: 10.3748/wjg.v18.i43.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Izquierdo L., Helle F., François C., Castelain S., Duverlie G., Brochot E. Simeprevir for the treatment of hepatitis C virus infection. Pharmgenomics. Pers. Med. 2014;7:241–249. doi: 10.2147/PGPM.S52715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Besandre R., Liu H.W. Biochemical basis of Vosevi, a new treatment for hepatitis C. Biochemistry. 2018;57:479–480. doi: 10.1021/acs.biochem.7b01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sulejmani N., Jafri S.-M. Grazoprevir/elbasvir for the treatment of adults with chronic hepatitis C: a short review on the clinical evidence and place in therapy. Hepatic Med. Evid. Res. 2018;10:33–42. doi: 10.2147/hmer.s130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotter T.G., Jensen D.M. Glecaprevir/pibrentasvir for the treatment of chronic hepatitis C: design, development, and place in therapy. Drug Des. Dev. Ther. 2019;13:2565–2577. doi: 10.2147/DDDT.S172512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Capetti A., Cossu M.V., Rizzardini G. Darunavir/cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. Expet Opin. Pharmacother. 2015;16:2689–2702. doi: 10.1517/14656566.2015.1109632. [DOI] [PubMed] [Google Scholar]
- 97.Cattaneo D., Sollima S., Meraviglia P., Milazzo L., Minisci D., Fusi M., Filice C., Gervasoni C. Dolutegravir-based antiretroviral regimens for HIV liver transplant patients in real-life settings. Drugs R. 2020;20:155–160. doi: 10.1007/s40268-020-00300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komal D., Khushboo J., Aaftaab S., Lakshmi S., Mallika A. Targeting integrase enzyme: a therapeutic approach to combat HIV resistance. Mini Rev. Med. Chem. 2019;20:219–238. doi: 10.2174/1389557519666191015124932. [DOI] [PubMed] [Google Scholar]
- 99.Wester A., Shetty A.K. Peramivir injection in the treatment of acute influenza: a review of the literature. Infect. Drug Resist. 2016;9:201–214. doi: 10.2147/IDR.S86460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hakki M. Moving past ganciclovir and foscarnet: advances in CMV therapy. Curr. Hematol. Malig. Rep. 2020;15:90–102. doi: 10.1007/s11899-020-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krishnan P., Beyer J., Mistry N., Koev G., Reisch T., DeGoey D., Kati W., Campbell A., Williams L., Xie W., Setze C., Molla A., Collins C., Pilot-Matias T. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of hepatitis C virus NS5A. Antimicrob. Agents Chemother. 2015;59:979–987. doi: 10.1128/AAC.04226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kurniawan J., Gani R.A., Hasan I., Sulaiman A.S., Lesmana C.R.A., Jasirwan C.O.M., Kalista K.F., Nababan S.H.H., Zulkifly S. Comparative efficacy of sofosbuvir-ribavirin versus sofosbuvir-daclatasvir for treatment of chronic hepatitis C in an area with limited NS5A inhibitor availability. Indian J. Gastroenterol. 2018;37:520–525. doi: 10.1007/s12664-018-0921-2. [DOI] [PubMed] [Google Scholar]
- 103.Yamazhan T., Turan İ., Ersöz G., Günşar F., Pullukçu H., Danış N., Ünal N.G., Vardar R., Oruç N., Tekin F., Taşbakan M., Sipahi O.R., Akarca U.S. Real-life experience of ledipasvir and sofosbuvir single-tablet regimen among chronic hepatitis C patients in Turkey. Turk. J. Gastroenterol. 2020;31:239–245. doi: 10.5152/tjg.2020.19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson W.E., Everson G.T. Sofosbuvir and velpatasvir for the treatment of hepatitis C. Expet Rev. Gastroenterol. Hepatol. 2017;11:501–505. doi: 10.1080/17474124.2017.1326817. [DOI] [PubMed] [Google Scholar]
- 105.FDA drug approvals and Databases. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases June 23, 2021)
- 106.Yamaguchi-Sasaki T., Tamura Y., Ogata Y., Kawaguchi T., Kurosaka J., Sugaya Y., Iwakiri K., Busujima T., Takahashi R., Ueda-Yonemoto N., Tanigawa E., Abe-Kumasaka T., Sugiyama H., Kanuma K. Design and synthesis of 2-(1-Alkylaminoalkyl)pyrazolo[1,5-a]pyrimidines as new respiratory syncytial virus fusion protein inhibitors. Chem. Pharm. Bull. 2020;68:345–362. doi: 10.1248/cpb.c19-00895. [DOI] [PubMed] [Google Scholar]
- 107.Tian L., Qiang T., Liang C., Ren X., Jia M., Zhang J., Li J., Wan M., YuWen X., Li H., Cao W., Liu H. RNA-dependent RNA polymerase (RdRp) inhibitors: the current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 2021;213:113201. doi: 10.1016/j.ejmech.2021.113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sluis-Cremer N., Tachedjian G. Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors. Virus Res. 2008;134:147–156. doi: 10.1016/j.virusres.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang T., Yang J., Zhou Z., Fu Z., Cherukupalli S., Kang D., Zhan P., Liu X. The development of an effective synthetic route of rilpivirine. BMC Chem. 2021;15 doi: 10.1186/s13065-021-00749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ranjith-Kumar C.T., Cheng Kao C. In: Hepatitis C Viruses: Genomes and Molecular Biology, Horizon Bioscience. Tan S.L., editor. 2006. Biochemical activities of the HCV NS5B RNA-dependent RNA polymerase.http://www.ncbi.nlm.nih.gov/pubmed/21250392 (Chapter 10) [PubMed] [Google Scholar]
- 111.Shu B., Gong P. Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4005–E4014. doi: 10.1073/pnas.1602591113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mallalieu N.L., Rahimy M.H., Crowley C.A., Appleman J.R., Smith P.F., Freddo J.L. Pharmacokinetics and pharmacodynamics of setrobuvir, an orally administered hepatitis C virus non-nucleoside analogue inhibitor. Clin. Therapeut. 2014;36:2047–2063. doi: 10.1016/j.clinthera.2014.10.002. e3. [DOI] [PubMed] [Google Scholar]
- 113.Yoshino R., Yasuo N., Sekijima M. Molecular dynamics simulation reveals the mechanism by which the influenza cap-dependent endonuclease acquires resistance against Baloxavir marboxil. Sci. Rep. 2019;9:17464. doi: 10.1038/s41598-019-53945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohd I., Khalid Bukhari S.U., Alshrari A.S., Hussain Shah S.S., Abida, Bawadekji A. Molecular docking based drug repurposing study of antiviral drugs against COVID-19 virus spike receptor binding domain. Pakistan J. Med. Heal. Sci. 2020;14:1040–1043. [Google Scholar]
- 115.Adebambo K.F. Computational investigation of the interaction of anti-influenza drugs with CoVID-19 protein. Comput. Mol. Biosci. 2020;10:45–60. doi: 10.4236/cmb.2020.102003. [DOI] [Google Scholar]
- 116.Li D., Hu J., Li D., Yang W., Yin S.F., Qiu R. Reviews on biological activity, clinical trial and synthesis progress of small molecules for the treatment of COVID-19. Top. Curr. Chem. 2021;379 doi: 10.1007/s41061-020-00318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piplani S., Singh P.K., Winkler D.A., Petrovsky N. Computationally repurposed drugs and natural products against RNA dependent RNA polymerase as potential COVID-19 therapies. Mol. Biomed. 2021;2 doi: 10.1186/s43556-021-00050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sahoo B.M., Ravi Kumar B.V.V., Sruti J., Mahapatra M.K., Banik B.K., Borah P. Drug repurposing strategy (DRS): emerging approach to identify potential therapeutics for treatment of novel coronavirus infection. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.628144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mueller H., Lopez A., Tropberger P., Wildum S., Schmaler J., Pedersen L., Han X., Wang Y., Ottosen S., Yang S., Young J.A.T., Javanbakht H. PAPD5/7 are host factors that are required for hepatitis B virus RNA stabilization. Hepatology. 2019;69:1398–1411. doi: 10.1002/hep.30329. [DOI] [PubMed] [Google Scholar]
- 121.Patick A.K., Potts K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horváth A., Depre D., Vermeulen W.A.A., Wuyts S.L., Harutyunyan S.R., Binot G., Cuypers J., Couck W., Van Den Heuvel D. Ring-closing metathesis on commercial scale: synthesis of HCV protease inhibitor simeprevir. J. Org. Chem. 2019;84:4932–4939. doi: 10.1021/acs.joc.8b03124. [DOI] [PubMed] [Google Scholar]
- 123.Hajimahdi Z., Zarghi A. Progress in HIV-1 integrase inhibitors: a review of their chemical structure diversity, Iran. J. Pharm. Res. 2016;15:595–628. doi: 10.22037/ijpr.2016.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.FDA's Clinical Pharmacology review, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207561Orig1s000ClinPharmR.pdf June 23, 2021)
- 125.Alymova I.V., Taylor G., Portner A. Neuraminidase inhibitors as antiviral agents. Curr. Drug Targets - Infect. Disord. 2005;5:401–409. doi: 10.2174/156800505774912884. [DOI] [PubMed] [Google Scholar]
- 126.Huang Y., Staschke K., De Francesco R., Tan S.L. Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication? Virology. 2007;364:1–9. doi: 10.1016/j.virol.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 127.Deres K., Schröder C.H., Paessens A., Goldmann S., Hacker H.J., Weber O., Krämer T., Niewöhner U., Pleiss U., Stoltefuss J., Graef E., Koletzki D., Masantschek R.N.A., Reimann A., Jaeger R., Groß R., Beckermann B., Schlemmer K.H., Haebich D. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299:893–896. doi: 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- 128.Zakaryan H., Arabyan E., Oo A., Zandi K. Flavonoids: promising natural compounds against viral infections. Arch. Virol. 2017;162:2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh M., Trivedi D., Mohapatra R., Bagchi T., Durthi C.P., Kuppam C. Phytotherapic drugs for COVID-19 treatment: a scoping review. Curr. Pharmaceut. Des. 2021;27:3389–3398. doi: 10.2174/1381612827666210705163807. [DOI] [PubMed] [Google Scholar]
- 130.Bibi S., Sarfraz A., Mustafa G., Ahmad Z., Zeb M.A., Wang Y.-B., Khan T., Khan M.S., Kamal M.A., Yu H. Impact of traditional plants and their secondary metabolites in the discovery of COVID-19 treatment. Curr. Pharmaceut. Des. 2020;27:1123–1143. doi: 10.2174/1381612826666201118103416. [DOI] [PubMed] [Google Scholar]
- 131.Chen L., Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C. 2020;112:110924. doi: 10.1016/j.msec.2020.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He Y., Zhou Y., Wang H., Yin J., Chang Y., Hu P., Ren H., Xu H. Identifying potential biomarkers in hepatitis B virus infection and its response to the antiviral therapy by integrated bioinformatic analysis. J. Cell Mol. Med. 2021;25:6558–6572. doi: 10.1111/jcmm.16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Majumder J., Minko T. Recent developments on therapeutic and Diagnostic approaches for COVID-19. AAPS J. 2021;23 doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Salazar G., Zhang N., Fu T.M., An Z. Antibody therapies for the prevention and treatment of viral infections. Npj Vaccines. 2017;2 doi: 10.1038/s41541-017-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pelegrin M., Naranjo-Gomez M., Piechaczyk M. Antiviral monoclonal antibodies: can they Be more than simple neutralizing agents? Trends Microbiol. 2015;23:653–665. doi: 10.1016/j.tim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.FDA authorized the use of bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 June 23, 2021)
- 137.Hille F., Charpentier E. CRISPR-cas: biology, mechanisms and relevance. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150496. doi: 10.1098/rstb.2015.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin S.R., Yang H.C., Kuo Y.T., Liu C.J., Yang T.Y., Sung K.C., Lin Y.Y., Wang H.Y., Wang C.C., Shen Y.C., Wu F.Y., Kao J.H., Chen D.S., Chen P.J. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol. Ther. Nucleic Acids. 2014;3:e186. doi: 10.1038/mtna.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanches-Da-Silva G.D.N., Medeiros L.F.S., Lima F.M. The potential Use of the CRISPR-cas system for HIV-1 gene therapy. Int. J. Genomics. 2019:8458263. doi: 10.1155/2019/8458263. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]








