ABSTRACT
Infections caused by Naegleria fowleri, Acanthamoeba spp., and Balamuthia mandrillaris result in a variety of clinical manifestations in humans. These amoebae are found in water and soil worldwide. Acanthamoeba spp. and B. mandrillaris cause granulomatous amoebic encephalitis (GAE), which usually presents as a mass, while N. fowleri causes primary amoebic meningoencephalitis (PAM). Acanthamoeba spp. can also cause keratitis, and both Acanthamoeba spp. and B. mandrillaris can cause lesions in skin and respiratory mucosa. These amoebae can be difficult to diagnose clinically as these infections are rare and, if not suspected, can be misdiagnosed with other more common diseases. Microscopy continues to be the key first step in diagnosis, but the amoeba can be confused with macrophages or other infectious agents if an expert in infectious disease pathology or clinical microbiology is not consulted. Although molecular methods can be helpful in establishing the diagnosis, these are only available in referral centers. Treatment requires combination of antibiotics and antifungals and, even with prompt diagnosis and treatment, the mortality for neurological disease is extremely high.
KEYWORDS: Acanthamoeba, Balamuthia, Naegleria, clinical presentation, diagnostics, free-living amoeba, neurological disease, treatment
INTRODUCTION
Naegleria fowleri, Acanthamoeba spp., and Balamuthia mandrillaris are free-living amoeba (FLA) that cause severe, fatal central nervous disease in humans and other animals (1). The name Balamuthia was derived in honor of the parasitologist William Balamuth, while Naegleria was named after the zoologist Mathieu Naegler. Acanthamoeba spp. receive their name from the spine projections, known as acanthopodia (“acanth” means “spine” in Greek), that are present in the trophozoites. The genus Sappinia is also included as a FLA, though only one human case has been published to date. These protozoa can also cause keratitis, as well as skin and mucosal infections. FLA produce infections as opportunistic pathogens in both immunocompetent and immunosuppressed persons. These amoebae can be difficult to diagnose clinically, as these infections are rare; if not suspected, they can go misdiagnosed or altogether undiagnosed until an autopsy is performed, as many of them are fatal. Microscopy continues to be the main diagnostic method; however, experience with these pathogens is infrequent, and requires consultation with experts, which frequently delays the diagnosis.
EPIDEMIOLOGY
Free-living amoeba (FLA) are found in water and soil on all continents. Defining the source of Acanthamoeba spp. and B. mandrillaris infections is usually difficult as, in many cases, the course is prolonged, and the precise time of acquisition may therefore be unclear. However, in a few cases, there may be an association with floods, or suspected exposure to contaminated water or soil sources. The two instances in which the source is usually known include (i) freshwater exposure, in cases of N. fowleri, and (ii) association with contact lenses or ocular trauma, in the case of Acanthamoeba spp keratitis. N. fowleri tolerates temperatures of up to 45°C; thus, exposures occur during the summer months, in children and young adults that play in contaminated freshwater or following nasal irrigation with devices such as a neti pot. Particular risk factors associated with B. mandrillaris and Acanthamoeba spp. infections in the central nervous system and skin include diabetes and immunosuppressive conditions, such as living with HIV or having an organ transplant. In the case of B. mandrillaris, Hispanics appear to be predisposed to infection (2). Although infections in humans appear to be rare, their true frequency is unknown, as diagnosis may not be possible in all settings (3). Many cases are fatal, and autopsies may not be performed. In addition, health care providers and diagnosticians may be unaware or unsuspecting of these clinical entities. The Centers for Disease Control and Prevention (CDC) estimates that many cases of undiagnosed meningoencephalitis in the US are due to N. fowleri infections (4).
PATHOGENESIS
All genera of FLA have two stages: cyst and trophozoite. N. fowleri, additionally, has a third flagellate stage (1). Trophozoites divide by binary fission, feed actively, and are the infectious stage of the organism. In the environment, acanthopodia allow trophozoites to attach to the surfaces of the bacteria, fungi, algae, and detritus on which they feed (3). Acanthamoeba spp. appear to maintain an endosymbiotic relationship with some of the bacteria they consume. Many of these bacteria, such as Legionella, Pseudomonas, Vibrio, and some mycobacteria, seem to acquire virulence factors while living inside the amoebae, enabling them to survive and thrive inside other phagocytic cells (e.g., human macrophages). FLA also provide these “amoeba-resistant bacteria” with protection from human immune responses when they are inhaled or ingested, thus acting as Trojan horses for infectious agents such as Legionella and others.
The trophozoites of Acanthamoeba spp. attach to host surface cells using a mannose-binding protein, while those of B. mandrillaris attach to endothelial cells using a galactose binding protein (3, 5). Trophozoites of these amoebae are inoculated into the skin or eyes, or are inhaled into the respiratory tract, and can produce disease at the entry sites (1). These amoebae produce a variety of enzymes, including neuraminidase, plasminogen activator, metalloproteinase, phospholipase, and serine and cysteine protease; all of these destroy cells, creating a cytopathic effect and facilitating the invasion of different structures (6). Hematogenous dissemination is likely mediated by the phosphorylation of host myosin light chains, which alter cell tight junctions, allowing increased blood-brain barrier permeability and the entry of amoebae into the central nervous system. Trophozoites mount an antioxidant defense by producing two superoxide dismutases. They survive in tissues by ingesting nutrients using “food cups,” or amoebastosomes, which are large cytoplasmic projections that engulf material to be phagocytosed.
The trophozoites of N. fowleri enter through the cribriform plate, in both immunocompetent and immunocompromised individuals, and have selective chemotaxis toward neural tissue. Acetylcholine released by neurons in the olfactory region and the base of the frontal lobe causes the amoeba to rapidly enter the brain, with little damage occurring in the nasal mucosa. N. fowleri produce phospholipases, neuraminidase, metalloproteinases, and perforin-like substances that trigger a cytopathic effect with apoptosis in cell cultures.
In the cyst stage, amoebae are dormant and resistant to environmental stressors. Amoebic cysts can survive for more than 20 years (7). These cysts probe the environment through pores known as ostioles so that when the conditions are appropriate, they can change to trophozoites. The cysts of Acanthamoeba spp. and B. mandrillaris are double-walled with a wrinkled ectocyst wall, while the double-walled cysts of N. fowleri are motile. Acanthamoeba spp. and N. fowleri have only one nucleus, while B. mandrillaris can occasionally have two nuclei. Sappinia’s cyst and trophozoite forms are both binucleate.
Host susceptibility likely plays a role in the pathogenesis of FLA, since many people come in contact with them but only a few exposures result in disease. The initial immune response to invading FLA involves neutrophils and complement (innate immunity). Tumor necrosis factor alpha and cytokines (IL-6 and others) are released by host inflammatory cells. Antibody production and transition to a cell-mediated immune response with granuloma formation occurs in Acanthamoeba spp. and B. mandrillaris infections, though there is no time for this to happen in N. fowleri infections
CLINICAL PRESENTATION
FLA infections have distinct clinical presentations, including Acanthamoeba spp. keratitis, cutaneous and mucosal infections, and disease of the central nervous system. The last can be further subdivided into granulomatous amoebic encephalitis (GAE) and primary amoebic meningoencephalitis (PAM).
Keratitis caused by Acanthamoeba spp. was originally described in a schoolteacher in the United Kingdom in 1974, and has since been found worldwide, predominantly in contact lens-wearers who swim or bathe while wearing them, or who clean their contact lenses with tap water (8, 9). Outbreaks have been associated with contaminated contact lens solutions (10, 11). They are also observed in patients who have sustained corneal injuries or those who have had ocular exposure to contaminated dirt or water. Clinical symptoms include eye pain, redness, blurry vision, photophobia, tearing, and foreign body sensation. Signs observed by ophthalmological examination may include epithelial, stromal, and ring infiltrates, as well as perineuritis (5, 6). When using in vivo confocal microscopy, Acanthamoeba spp. cysts appear as spherical, hyperreflective structures. Ocular complications may develop and range in severity, and include secondary glaucoma, iris atrophy, and cataracts. In rare cases, scleritis, anterior uveitis, chorioretinitis, and retinal vasculitis can also occur. While most patients experience unilateral infection, bilateral infection has been reported in 2% to 15% of cases (12). Bilateral infection can occur in both eyes at the same time, suggesting exposure to the same source; or at a later time, suggesting continued unsafe practices. Risk factors associated with poor outcomes include delayed diagnosis or misdiagnosis of another infectious etiology (often herpes), an infection unrelated to swimming, poor baseline visual acuity, the presence of an epithelial defect, and initial treatment with corticosteroids (13, 14).
Cutaneous amoebiasis occurs with both Acanthamoeba spp. and B. mandrillaris infections (15, 16). It can be diagnosed as an initial lesion in patients who later develop disseminated disease, and it can be seen in immunocompromised or immunocompetent hosts. Lesions are usually multiple and are most commonly described as being nodular, ulcerated, or necrotic, but they may also have papulo-pustular components and are occasionally tender. Red indurated plaques have also been described. Several cases of isolated cutaneous acanthamoebiasis have been noted to have lower extremity distribution and an association with trauma. In immunocompromised hosts, cutaneous distribution is often widespread. Formal diagnosis of cutaneous amoebiasis is made with tissue biopsy and is extremely challenging in the absence of either a suspected exposure and/or a confirmed infection from another body site (concurrent encephalitis or disseminated infection). Rare cases involving the nasopharyngeal and respiratory sinus mucosa associated with Acanthamoeba spp. infections have been reported (17). These have primarily been in patients living with HIV who present with signs and symptoms of chronic sinusitis (rhinorrhea, congestion, epistaxis), and, upon imaging studies, have thickened mucosa in the nasal cavity; some have disseminated infection by the time of diagnosis.
Involvement of the central nervous system is responsible for the greatest degree of mortality from FLA infections. Acanthamoeba spp and B. mandrillaris can both cause GAE, a subacute to chronic disease initially characterized by headache, low-grade fever, dizziness, hallucinations, and progressively worsening neurological deficits; these can include visual disturbances, focal neurological findings, cranial nerve palsies, seizures, personality changes, and coma (7). Symptoms can take weeks to months to develop (1). GAE occurs in patients with organ transplants, HIV infection, hematological malignancies, diabetes, systemic lupus erythematosus, malnutrition, or a history of skin/soft tissue trauma, among others (7). Amoebic pneumonitis, osteomyelitis, and endophthalmitis, and leukocytoclastic vasculitis have been reported with Acanthamoeba spp. infections in patients with HIV (3). The disease can also occur in immunocompetent hosts. Imaging (CT scan or MRI) demonstrates one or several hyperintense lesions with rim enhancement. Death typically occurs due to increased intracranial pressure and ensuing uncal or tonsillar herniation that has built up over a period of weeks to months. The mortality for GAE approaches 100%, with very few published cases of survivors in the medical literature. The few recorded favorable outcomes have typically been associated with early diagnosis and initiation of combination therapy (18, 19). Autopsies of deceased patients with GAE have also found involvement outside the central nervous system which was not diagnosed antemortem (20). To date, only one known human encephalitis case due to Sappinia has been described (21). This patient, a 38-year-old farmer from Texas, had no history of an immunocompromising condition. He developed worsening neurological symptoms following a recent frontal sinus infection, and was found to have a free-living amoeba, subsequently classified as Sappinia diploidea, upon pathological review of an excised left temporal lobe mass. He fully recovered after treatment.
PAM is caused by N. fowleri and has a mean time from symptom onset to death of approximately 5 to 7 days (1). At its onset, patients present with flu-like symptoms such as fever, fatigue, nausea, vomiting, and earache (22), followed by restlessness, severe headache located bilaterally to the frontal and/or temporal areas, and signs of meningismus; these can include nausea, vomiting, nuchal rigidity, and photophobia. As the infection progresses, the patient may experience seizures, diplopia, confusion, and eventually coma. Uncal herniation and death occur rapidly. Complete blood cell counts show leukocytosis, while imaging demonstrates enhancement of leptomeninges, cerebral edema, a decrease in ventricle size, and areas of hemorrhage, necrosis, and herniation. To date, four survivors of PAM, all children aged 8 to 16 years, have been reported in the United States, with three of them experiencing full or nearly full neurological recovery (22).
The differential diagnoses for amoebic infections vary widely based on the site of infection and on host risk factors for disease. Due to the rarity of clinically encountered FLA infections, even among those with epidemiological risk factors, diagnosis is appropriately considered a major challenge a priori; indeed, diagnosis is usually performed following the unexpected findings of a pathological review of tissue specimens obtained for a suspected infection of unclear etiology. For sinus and cutaneous disease, appropriate considerations include other common bacterial, fungal, and parasitic infections, as well as noninfectious etiologies. For GAE, the differential diagnosis is quite wide due to the subacute manifestations of numerous other infectious diseases of the central nervous system, which may include bacterial abscesses, tuberculosis, nocardiosis, fungal infections such as cryptococcosis and histoplasmosis, and parasitic infections such as neurocysticercosis and toxoplasmosis. The differential diagnosis of GAE should also include infarcts and some neoplasias. For PAM, the differential is narrower, primarily due to the speed of its progression; for this reason, bacterial meningitis should be considered concurrently pending additional diagnostic work-up for FLA infections.
DIAGNOSTIC TESTS
Acanthamoeba spp. cultures of corneal scrapings, contact lenses, and brain tissue can be performed on non-nutrient agar (dilute peptone–yeast extract–glucose agar) covered with a lawn of nonencapsulated, nonpigmented bacteria such as Escherichia coli or Enterobacter spp.(1). When observing these cultures, one sees the tracks left by the trophozoites as they eat the bacteria, and under magnification, one can observe trophozoites (Fig. 1A) and cysts if nutrients have been depleted. The sensitivity for this method reaches 77% (5). This same method can be used to culture N. fowleri. However, B. mandillaris cannot be grown in axenic media, requiring cell cultures such as human lung fibroblast, Vero E6, and human brain microvascular endothelial cells.
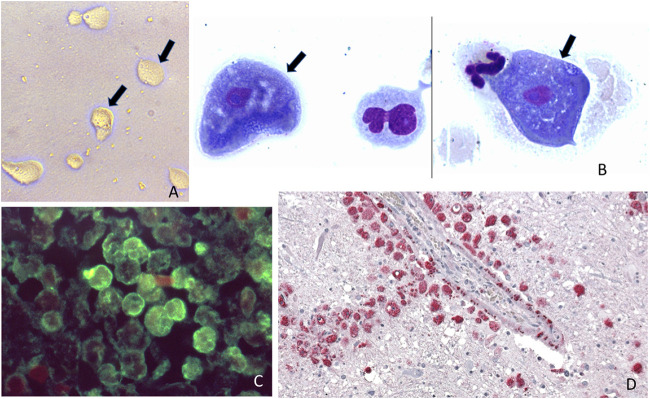
FIG 1.
Different diagnostic methods used for free living amoeba: (A) Free-living amoeba trophozoites observed in a culture using a non-nutrient agar and a bacterial lawn (arrows mark the trophozoites). (B) Giemsa stain of cerebrospinal fluid in a patient with Acanthamoeba spp. granulomatous amoebic encephalitis (arrows mark 2 trophozoites from the same slide but from different locations). (C) Immunofluorescence assay in brain tissue of a patient with N. fowleri (amoeba stained green). (D) Immunohistochemical assay in brain tissue of a patient with B.mandrillaris granulomatous amoebic encephalitis, red staining corresponds to the amoeba. Note that the amoeba surrounds a blood vessel. Panels A and C are from the Public Health Image Library, CDC.
In patients with GAE caused by Acanthamoeba spp. or B. mandrillaris, trophozoites or cysts are rarely found in the cerebrospinal fluid (CSF). When this occurs, the amoebae are stained with a Romanowsky stain, such as Giemsa, since this is the routine stain used for CSF cell counts (Fig. 1B). In GAE patients, the CSF demonstrates an increased white blood cell count, mostly composed of lymphocytes and monocytes, as well as high protein and moderately low glucose (1). However, PAM patients (N. fowleri), the CSF pressure is elevated, trophozoites are frequently observed together with neutrophils and red blood cells, and the protein concentration is high while glucose tends to be low (22). To distinguish trophozoites from macrophages, which can look similar to one another, microscopists should look for the characteristic nuclear features of amoebae: a round, prominent karyosome, finely dispersed nuclear chromatin, and a well-demarcated nuclear membrane. By contrast, macrophages have several clumps of darker nuclear material and several nucleoli.
Corneal scrapings can be stained directly with calcofluor white, lactophenol cotton blue, or acridine orange, which will demonstrate the cysts (6). Corneal scrapings and skin or brain biopsies can be formalin-fixed, paraffin-embedded, and studied using histopathology. In these specimens, the trophozoites and cysts can be found in a background of necrosis and a granulomatous inflammatory infiltrate containing multinucleated giant cells, macrophages, lymphocytes, and varying degrees of neutrophils. Several stains can be used to highlight the cyst wall, including fungal silver stains (7). As trophozoites may be difficult to distinguish from macrophages, paraffin blocks can be further examined using antibodies against the different amoebae with either immunofluorescence (Fig. 1C) or immunohistochemistry (Fig. 1D), or with PCR to amplify and detect amoebic nucleic acids (16). In the case of corneal scrapings, histopathology reaches a diagnostic sensitivity of 31% to 65% while PCR has a sensitivity of 84% to 100% (5).
The pathological finding of amoebae in skin (Fig. 2A to D) or brain (Fig. 2E to H) tissue can occur if a biopsy or postmortem is performed as part of an autopsy. The basic histopathology of GAE is a necrotizing, granulomatous inflammation with giant cells prominently centered around blood vessels (23). Admixed with the necrotic and inflammatory material, trophozoites (Fig. 2D and H) and cysts (Fig. 2C, E, and G) can be observed. To distinguish trophozoites from macrophages, microscopists need to look for cells with a prominent round karyosome and finely dispersed chromatin of the amoeba (Fig. 2C, D, G, and H), as opposed to the clumped chromatin in macrophages. Acute inflammatory infiltrate with neutrophils can also be observed. Blood vessels in the lesions show thrombi and an infarct can be present. In autopsy cases, a macroscopic examination of the brain reveals focal lesion(s) with softened parenchyma and variable amounts of hemorrhage. In addition to the lesions described in the brain, autopsies have found amoebae in other tissues, such as lungs, skin lesions, and other organs.
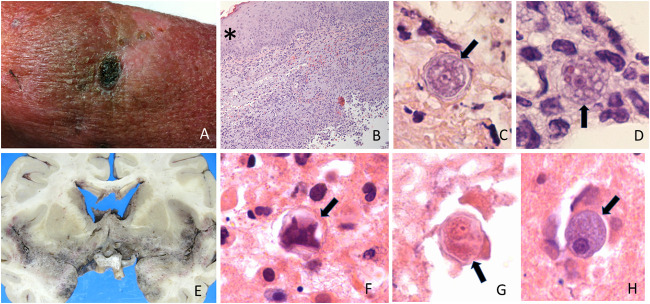
FIG 2.
Skin and brain Acanthamoeba spp. infections. (A) Ulcerated necrotic lesion in leg. (B) Hematoxylin and eosin stain showing thickened epidermis (*) and intense inflammatory infiltrate through the dermis. Normally, the dermis should stain mostly homogeneously pink; in this photomicrograph, the dermis appears to have multiple blue dots corresponding to the inflammatory infiltrate. (C) Hematoxylin and eosin stain showing double-walled cyst with wrinkled external wall (arrow marks the cyst). (D) Hematoxylin and eosin stain showing trophozoite (arrow marks the trophozoite) with multiple vacuoles (stained light pink inside the trophozoite) and two erythrocytes (stained dark red). Note the nuclear characteristics of the cyst and trophozoite: the karyosome is prominent and central inside the nucleus, and the nuclear chromatin is dispersed and not clumping. (E) Macroscopic photograph of brain with granulomatous amoebic encephalitis. In this case, the base of the brain was the most affected, as demonstrated by the brown/gray coloring which is distinct from the rest of the brain parenchyma. (F to H) Microscopic images of cysts and trophozoite, corresponding to the same brain. (F) Collapsed cyst (arrow marks the cyst). (G) Cyst showing the characteristic nuclear features (arrow marks the cyst). (H) Trophozoite (arrow marks the trophozoite).
In the case of PAM, an examination of the brain will show hemorrhagic meningitis, with the base and olfactory bulb being most affected. Histopathology will show abundant trophozoites in pockets, with abundant necrotic material in meninges and Virchow-Robin spaces (23). Cysts are not observed since there is not enough time from inoculation to death for these to be formed. Neutrophils and red cells will be observed in the meninges.
The genus of the amoeba can be determined in tissues by immunohistochemistry, immunofluorescence, or PCR, and in cultures by immunofluorescence, PCR, or MALDI-TOF, though these studies are only performed in referral centers (1, 23, 24). For molecular tests, researchers have used a variety of probes or primers that are part of ribosomal internal transcribed spacer (ITS) regions, mitochondrial DNA, or 18S rRNA. In a study of paraffin blocks from 56 cases, comparing immunofluorescence and PCR results, there was only one discrepant block where the PCR result was positive while the immunofluorescence was negative. The discrepant result occurred in a paraffin block from the lung tissue of a patient with GAE due to B. mandrillaris and suggests that PCR could be more sensitive than other techniques (25). The question of when to consider referring the sample for PCR testing is easy if there are cysts and/or trophozoites in the sample, as identifying the genus will define treatment possibilities. If amoebae are not present in a tissue sample, specific PCR testing for Acanthamoeba spp., B. mandrillaris and N. fowleri could result in many referrals with little gain. However, in cases where clinical suspicion is high and no tissue has been obtained, some have used unbiased next-generation sequencing in blood or CSF to diagnose cases of B. mandrillaris (26). These cases describe sequencing the DNA present in the sample, discarding all human sequences, then aligning the remaining sequences with a database of microorganisms. This technique could uncover other FLA, such as Vermamoeba vermiform, Hartmanella spp., and others, which could be pathogenic to humans or harbor amoeba-resistant bacteria and other pathogens.
Serologic tests for Acanthamoeba spp. and B. mandrillaris are available through the CDC (3). The use of serology is quite limited; FLA are ubiquitous, so exposure occurs frequently in many people, and studies have shown the presence of low-titer antibodies to Acanthamoeba spp. in healthy children and adults (1). In addition, patients that are immunosuppressed and have GAE may have low titers of antibodies. There is no use for serology in the diagnosis of N. fowleri, as patients with PAM progress so rapidly that an antibody response has not had time to be mounted.
TREATMENT
Antimicrobial treatment for FLA infections appears to be most effective when administered as early as possible in the course of disease, regardless of whether the treatment is for keratitis, localized skin or nasopharyngeal disease, or more advanced neurological disease. Early detection and combination antimicrobial therapies have been associated with improved outcomes (18). The cyst stage tends to be more resistant to treatment than the trophozoites (6). Where applicable, such as in the setting of focal disease, surgical debridement of focal areas of infection likely plays an important role as well; however, precise indications for this treatment are less well-defined.
Optimal treatment of Acanthamoeba spp. keratitis involves combination antimicrobial topical agents to target both the trophozoite and cyst forms. Biguanides such as 0.02% polyhexamethylene biguanide (PHMB) or 0.02% chlorhexidine are considered the most effective first-line agents, distinguished by their ability to penetrate the amoebic pore and damage the inner cell membrane of the organism (5, 6). These drugs may also be used in combination with aromatic diamidines, such as propamidine or neomycin. Topical drops should be applied every hour, with gradual spacing-out of the application interval over the ensuing days and weeks. Treatment is typically continued for up to 6 to 12 months. While the precise indications for surgical debridement or corneal transplantation are unclear, these are typically performed for severe disease and/or treatment-refractory disease. Corneal transplantation carries some risk of reinfection, depending on the extent to which the organisms have been successfully eradicated prior to surgery. Those wearing contact lenses should follow prevention strategies: wash hands before handling contact lenses; replace lenses as prescribed; if wearing reusable lenses, clean them as instructed and use proper storage containers; remove lenses before performing activities that involve water, including bathing; and have regular eye exams (27).
The optimal combination of antimicrobials to be used in the treatment of GAE and PAM is not well defined in the medical literature. No clinical trials have been performed to evaluate the comparative efficacy of drug regimens, because of the low annual incidence of these diseases and the frequent difficulty in establishing the diagnosis either antemortem or prior to the onset of severe illness. Accordingly, expert recommendations are primarily based on retrospective reviews of individual cases. Unfortunately, regardless of the precise approach used, mortality remains extremely high for these diseases. Combination antimicrobial therapy is considered the gold standard of treatment for both GAE and PAM, although the evidence supporting specific combinations of treatments over others is limited. Miltefosine, an oral alkyl phosphocholine drug originally studied for cancer and approved in the United States for leishmaniasis, has been found to play an important role in the treatment of both GAE and PAM. A retrospective review of 123 cases of Acanthamoeba spp. and B. mandrillaris GAE found survival in a minority of patients to be significantly associated with receiving miltefosine as part of their antimicrobial regimens, compared to patients who did not receive miltefosine (28). A review of 109 cases of B. mandrillaris infection, 99% of which involved encephalitis, found that all patients received combination treatment which included antifungals such as amphotericin B, fluconazole, flucytosine and/or pentamidine, and antibacterial agents such as ceftriaxone, azithromycin, clarithromycin, metronidazole and/or sulfadiazine (2). The most common treatments included amphotericin B, metronidazole, ceftriaxone, rifampin, and isoniazid. In this study, 9 patients survived; however, their regimens included the same antimicrobial agents compared to the regimens of those who died. Additionally, of the 27 patients reviewed who had an established antemortem diagnosis of FLA infection and who received FLA-directed therapy, 3 of the 9 who survived and 12 of the 18 who died all received miltefosine. Based on this limited data in conjunction with available in vitro data, the current favored treatment regimen for B. mandrillaris GAE is a combination of pentamidine, fluconazole, flucytosine, miltefosine, sulfadiazine, and a macrolide antibiotic (either azithromycin or clarithromycin); the same regimen, without the macrolide antibiotic, is preferred for Acanthamoeba spp. GAE (27, 29). The patient with encephalitis due to S. diploidea was successfully treated with azithromycin, pentamidine, itraconazole, and flucytosine for 25 weeks, and experienced a full recovery (21).
For PAM, the CDC’s recommended treatment course is both intravenous and intrathecal amphotericin B in combination with azithromycin, fluconazole, rifampin, miltefosine, and a 4-day dexamethasone course (30). This regimen is based upon anecdotal cases of successful treatment. Notably, despite its substantially increased risk of toxicities, conventional amphotericin B is preferred over the more commonly used liposomal formulation due to 10-fold lower MICs against N. fowleri. Other specific toxicities of combination therapy for PAM and GAE include QTc prolongation, renal toxicity, and others related to the specific antimicrobial agents involved, but are typically considered to be worth the risk given the high degree of mortality in the absence of aggressive treatment. In the search for novel treatments for N. fowleri based on genes that have drug targets in Drug Bank, protein production and crystallographic structure are being compared to human homologues (31). If the targets in the amoeba are sufficiently different from human structures/targets, these drugs could be tested in vitro and in vivo and be potential treatments for patients with FLA infections.
CONCLUSIONS
Infections due to any of the FLA can be challenging to diagnose. Despite the availability of molecular tests for identification of FLA infection, these infections may not be suspected by clinicians, thus, tests will not be ordered. Although the distinguishing morphological characteristics of amoeba are evident to infectious disease pathologists or clinical microbiologists, amoebic forms may be easily mistaken for yeasts, macrophages, or other artifacts. These infections have very high mortality rates, so awareness of these entities is essential to ensure early diagnosis and the initiation of combination therapy, which have been associated with anecdotal case survival and recovery. For clinicians seeking diagnostic and/or therapeutic management assistance with FLA infections, clinical consultation with the CDC is available through the Emergency Operations Center (770-488-7100).
Contributor Information
Jeannette Guarner, Email: jguarne@emory.edu.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, Moser M, Puttagunta R, Yoder JS, Ali IK, Roy SL. 2019. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. Clin Infect Dis 68:1815–1822. 10.1093/cid/ciy813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duggal S, Rongpharpi S, Duggal A, Kumar A, Biswal I. 2017. Role of Acanthamoeba in granulomatous encephalitis: a review. J Infect Dis Immune Ther 1:1. [Google Scholar]
- 4.Matanock A, Mehal J, Liu L, Blau D, Cope J. 2018. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg Infect Dis 24:162–164. 10.3201/eid2401.170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szentmáry N, Daas L, Shi L, Laurik KL, Lepper S, Milioti G, Seitz B. 2019. Acanthamoeba keratitis — Clinical signs, differential diagnosis and treatment. J Curr Ophthalmol 31:16–23. 10.1016/j.joco.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo-Morales J, Khan NA, Walochnik J. 2015. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 22:10. 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra SK, Sharma P, Shyam K, Tejan N, Ghoshal U. 2020. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp Parasitol 208:107788. 10.1016/j.exppara.2019.107788. [DOI] [PubMed] [Google Scholar]
- 8.Scruggs BA, Quist TS, Salinas JL, Greiner MA. 2019. Notes from the field: Acanthamoeba keratitis cases — Iowa, 2002–2017. MMWR Morb Mortal Wkly Rep 68:448–449. 10.15585/mmwr.mm6819a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagington J, Watson PG, Playfair TJ, Mcgill J, Jones BR, Steele ADMG. 1974. Amebic infection of the eye. Lancet 304:1537–1540. 10.1016/S0140-6736(74)90285-2. [DOI] [PubMed] [Google Scholar]
- 10.Fraser MN, Wong Q, Shah L, Holland SP, Morshed M, Isaac-Renton J, Chong M, Kibsey P, Patrick DM. 2012. Characteristics of an Acanthamoeba keratitis outbreak in British Columbia between 2003 and 2007. Ophthalmology 119:1120–1125. 10.1016/j.ophtha.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 11.Verani JR, Lorick SA, Yoder JS, Beach MJ, Braden CR, Roberts JM, Conover CS, Chen S, McConnell KA, Chang DC, Park BJ, Jones DB, Visvesvara GS, Roy SL, Acanthamoeba Keratitis Investigation Team. 2009. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis 15:1236–1242. 10.3201/eid1508.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelmus KR, Jones DB, Matoba AY, Hamill MB, Pflugfelder SC, Weikert MP. 2008. Bilateral Acanthamoeba keratitis. Am J Ophthalmol 145:193–197. 10.1016/j.ajo.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Carnt N, Robaei D, Minassian DC, Dart JKG. 2018. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol 102:1431–1435. 10.1136/bjophthalmol-2017-310806. [DOI] [PubMed] [Google Scholar]
- 14.Kaiserman I, Bahar I, McAllum P, Srinivasan S, Elbaz U, Slomovic AR, Rootman DS. 2012. Prognostic factors in Acanthamoeba keratitis. Can J Ophthalmol 47:312–317. 10.1016/j.jcjo.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Galarza C, Ramos W, Gutierrez EL, Ronceros G, Teran M, Uribe M, Ñavincopa M, Ortega-Loayza AG. 2009. Cutaneous acanthamebiasis infection in immunocompetent and immunocompromised patients. Int J Dermatol 48:1324–1329. 10.1111/j.1365-4632.2008.03786.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Cheng W, Li B, Jian Z, Qi X, Sun D, Gao J, Lu X, Yang Y, Lin K, Lu C, Chen J, Li C, Wang G, Gao T. 2020. Balamuthia mandrillaris infection in China: a retrospective report of 28 cases. Emerg Microbes Infect 9:2348–2357. 10.1080/22221751.2020.1835447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera M, Padhya T. 2002. Acanthamoeba: a rare primary cause of rhinosinusitis. Laryngoscope 112:1201–1203. 10.1097/00005537-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Martínez DY, Seas C, Bravo F, Legua P, Ramos C, Cabello AM, Gotuzzo E. 2010. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 51:e7-11. 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 19.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. 2003. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis 37:1304–1312. 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 20.Kaul DR, Lowe L, Visvesvara GS, Farmen S, Khaled YA, Yanik GA. 2008. Acanthamoeba infection in a patient with chronic graft-versus-host disease occurring during treatment with voriconazole. Transpl Infect Dis 10:437–441. 10.1111/j.1399-3062.2008.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelman BB, Rauf SJ, Nader R, Popov V, Borkowski J, Chaljub G, Nauta HW, Visvesvara GS. 2001. Amoebic encephalitis due to Sappinia diploidea. JAMA 285:2450–2451. 10.1001/jama.285.19.2450. [DOI] [PubMed] [Google Scholar]
- 22.Capewell LG, Harris AM, Yoder JS, Cope JR, Eddy BA, Roy SL, Visvesvara GS, Fox LAM, Beach MJ. 2015. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc 4:e68-75–e75. 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 23.Guarner J, Bartlett J, Shieh W, Paddock C, Visvesvara G, Zaki S. 2007. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol 20:1230–1237. 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 24.Norgan A, Sloa L, Pritt B. 2019. Detection of Naegleria fowleri, Acanthamoeba spp, and Balamuthia mandrillaris in formalin-fixed, paraffin-embedded tissues by real-time multiplex polymerase chain reaction. Am J Clin Pathol 152:799–807. 10.1093/ajcp/aqz103. [DOI] [PubMed] [Google Scholar]
- 25.Yagi S, Schuster FL, Visvesvara GS. 2008. Demonstration of Balamuthia and Acanthamoeba mitochondrial DNA in sectioned archival brain and other tissues by the polymerase chain reaction. Parasitol Res 102:211–217. 10.1007/s00436-007-0749-7. [DOI] [PubMed] [Google Scholar]
- 26.Kalyatanda G, Rand K, Lindner M, Hong D, Albayram M, Gregory J, Kresak J, Ibne K, Cope J, Roy S, Gary J, Reddy V, Ahmed A. 2020. Rapid, noninvasive diagnosis of Balamuthia mandrillaris encephalitis by a plasma-based next-generation sequencing test. Open Forum Infect Dis 7:ofaa189. 10.1093/ofid/ofaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). 2017. Parasites — Acanthamoeba — Granulomatous Amebic Encephalitis (GAE); keratitis. https://www.cdc.gov/parasites/acanthamoeba/index.html. Accessed March 2021.
- 28.Cope JR, Roy SL, Yoder JS, Beach MJ. 2013. Increased patient survival: miltefosine for treatment of free-living ameba infections caused by Acanthamoeba and Balamuthia., abstr ID Week 2013. Infectious Diseases Society of America. October 5, 2013. [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). 2019. Parasites — Balamuthia mandrillaris — Granulomatous Amebic Encephalitis (GAE). https://www.cdc.gov/parasites/balamuthia/index.html. Accessed March 2021.
- 30.Centers for Disease Control and Prevention (CDC). 2017. Parasites — Naegleria fowleri — Primary Amebic Meningoencephalitis (PAM) — amebic encephalitis. https://www.cdc.gov/parasites/naegleria/index.html. Accessed March 2021.
- 31.Tillery L, Barrett K, Goldstein J, Lassner J, Osterhout B, Tran N, Xu L, Young R, Craig J, Chun I, Dranow D, Abendroth J, Delker S, Davies D, Mayclin S, Calhoun B, Bolejack M, Staker B, Subramanian S, Phan I, Lorimer D, Myler P, Edwards T, Kyle D, Rice C, Morris J, Leahy J, Manetsch R, Barrett L, Smith C, Van-Voorhis W. 2021. Naegleria fowleri: protein structures to facilitate drug discovery for the deadly, pathogenic free-living amoeba. PLoS One 16:e0241738. 10.1371/journal.pone.0241738. [DOI] [PMC free article] [PubMed] [Google Scholar]