Key Points
Question
Is synthetic mesh superior to biologic mesh in reducing 2-year risk of hernia recurrence during single-stage repair of clean-contaminated and contaminated ventral hernias?
Findings
In this multicenter randomized clinical trial, 253 patients were randomized to synthetic mesh (n = 126) and biologic mesh (n = 127) and the follow-up rate was 92% at 2 years. Compared with biologic mesh, synthetic mesh significantly reduced the risk of hernia recurrence (hazard ratio, 0.31; 95% CI, 0.23-0.42; P < .001).
Meaning
Synthetic mesh demonstrated significantly lower hernia recurrence risk than biologic mesh in patients undergoing single-stage repair of clean-contaminated and contaminated ventral hernias.
This randomized clinical trial evaluates whether synthetic mesh results in superior reduction in risk of hernia recurrence compared with biologic mesh during single-stage repair of clean-contaminated and contaminated ventral hernias.
Abstract
Importance
Biologic mesh is widely used for reinforcing contaminated ventral hernia repairs; however, it is expensive and has been associated with high rates of long-term hernia recurrence. Synthetic mesh is a lower-cost alternative but its efficacy has not been rigorously studied in individuals with contaminated hernias.
Objective
To determine whether synthetic mesh results in superior reduction in risk of hernia recurrence compared with biologic mesh during the single-stage repair of clean-contaminated and contaminated ventral hernias.
Design, Setting, and Participants
This multicenter, single-blinded randomized clinical trial was conducted from December 2012 to April 2019 with a follow-up duration of 2 years. The trial was completed at 5 academic medical centers in the US with specialized units for abdominal wall reconstruction. A total of 253 adult patients with clean-contaminated or contaminated ventral hernias were enrolled in this trial. Follow-up was completed in April 2021.
Interventions
Retromuscular synthetic or biologic mesh at the time of fascial closure.
Main Outcomes and Measures
The primary outcome was the superiority of synthetic mesh vs biologic mesh at reducing risk of hernia recurrence at 2 years based on intent-to-treat analysis. Secondary outcomes included mesh safety, defined as the rate of surgical site occurrence requiring a procedural intervention, and 30-day hospital direct costs and prosthetic costs.
Results
A total of 253 patients (median [IQR] age, 64 [55-70] years; 117 [46%] male) were randomized (126 to synthetic mesh and 127 to biologic mesh) and the follow-up rate was 92% at 2 years. Compared with biologic mesh, synthetic mesh significantly reduced the risk of hernia recurrence (hazard ratio, 0.31; 95% CI, 0.23-0.42; P < .001). The overall intent-to-treat hernia recurrence risk at 2 years was 13% (33 of 253 patients). Recurrence risk with biologic mesh was 20.5% (26 of 127 patients) and with synthetic mesh was 5.6% (7 of 126 patients), with an absolute risk reduction of 14.9% with the use of synthetic mesh (95% CI, −23.8% to −6.1%; P = .001). There was no significant difference in overall 2-year risk of surgical site occurrence requiring a procedural intervention between the groups (odds ratio, 1.22; 95% CI, 0.60-2.44; P = .58). Median (IQR) 30-day hospital direct costs were significantly greater in the biologic group vs the synthetic group ($44 936 [$35 877-$52 656] vs $17 289 [$14 643-$22 901], respectively; P < .001). There was also a significant difference in the price of the prosthetic device between the 2 groups (median [IQR] cost biologic, $21 539 [$20 285-$23 332] vs synthetic, $105 [$105-$118]; P < .001).
Conclusions and Relevance
Synthetic mesh demonstrated superior 2-year hernia recurrence risk compared with biologic mesh in patients undergoing single-stage repair of contaminated ventral hernias, and both meshes demonstrated similar safety profiles. The price of biologic mesh was over 200 times that of synthetic mesh for these outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02451176
Introduction
Ventral hernia repair is one of the most commonly performed general surgery operations, costing the US more than $3.2 billion annually.1 Treatment of contaminated ventral hernia cases (US Centers for Disease Control and Prevention [CDC] classes II and III), such as those with need for concomitant gastrointestinal, gynecologic, or urologic procedures, is controversial. Biologic mesh materials were originally developed and marketed as a scaffolding structure, which allows for cellular ingrowth, and are thought to thereby resist infection. They have long been considered the mesh material of choice for contaminated hernias over permanent synthetic mesh, which is feared to lead to chronic infection and further mesh-related complications and/or reoperation. Notably, existing literature on biologic mesh suggests high rates of wound morbidity and 40% to 80% long-term hernia recurrence rates in contaminated cases.2,3 Although the safety and efficacy of biologic mesh (approximately $20 000 per 900 cm2) in contaminated fields has never, to our knowledge, been rigorously studied,4 such devices add $500 million to hernia-related health care expenditure annually.5
While many surgeons fear complications associated with permanent synthetic mesh use in contaminated fields, macroporous monofilament polypropylene mesh has demonstrated favorable performance in contaminated animal models and small retrospective cohorts,6,7,8,9,10 suggesting that the contemporary engineering specifications of these materials may allow host immune cells to clear bacterial contamination. The possibility that a synthetic mesh could perform as well or better than a device that costs 200 times more warrants rigorous investigation. The goal of our study was to compare the risk of hernia recurrence with permanent synthetic mesh vs biologic mesh in the single-stage repair of clean-contaminated (CDC class II) or contaminated (CDC class III) ventral hernia repairs. We hypothesized that synthetic mesh would result in superior hernia recurrence risk with similar safety and lower cost compared with biologic mesh.
Methods
Study Design and Oversight
This study was an investigator-initiated, single-blinded multicenter randomized controlled parallel-group trial comparing the use of biologic mesh vs synthetic mesh in the single-stage repair of clean-contaminated and contaminated ventral hernias. The study was registered with the US Food and Drug Administration (FDA) as an investigational device exemption trial (G120130), ClinicalTrials.gov (02451176), and was conducted and analyzed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guideline.11 Each participating institution provided departmental funds of research coordinators and necessary infrastructure to complete the trial. To facilitate long-term follow-up, the protocol was amended after trial initiation to include the post hoc application of the validated hernia recurrence inventory to screen for long-term hernia recurrence.12 Ethical approval for the study was granted by the institutional review board at all 5 sites. All participants provided written informed consent.
Patients and Study Setting
Patients with a contaminated wound (CDC class II or III) undergoing elective single-stage ventral hernia repair were eligible for inclusion. Complete inclusion or exclusion criteria can be found in the eMethods in Supplement 3. Patient recruitment and surgical procedures were performed at 1 of 5 academic medical centers with dedicated abdominal wall reconstruction units. Patients were recruited directly from the respective medical center’s surgery clinics.
Surgical Intervention
All procedures were performed by 1 of 8 qualified general surgeons (M.J.R., D.M.K., C.C.P., A.C., J.W., B.K.P., J.B., A.S.P.) with fellowship training in advanced abdominal wall reconstruction. A retromuscular hernia repair was performed in all patients as previously described and complete details are available in the eMethods in Supplement 3.13
Study Outcomes
The primary outcome of this study was the efficacy of biologic mesh compared with synthetic mesh during single-stage repairs of ventral hernias in a contaminated field. The efficacy of each mesh was assessed by the 2-year hernia recurrence risk. Hernia recurrence was defined a priori (with the exception of the hernia recurrence inventory being added post hoc) as a composite measure based on clinical exam, blinded review of radiographic imaging (abdominal CT or ultrasonography), or patient-reported outcome of a bulge on the hernia recurrence inventory12 (eMethods in Supplement 3). We also sought to determine the safety of synthetic and biologic meshes in a contaminated field as a secondary outcome to investigate undue risk for the study devices. The safety of mesh was assessed by comparing rates of surgical site occurrence requiring procedural intervention between biologic and synthetic mesh groups up to 24 months after hernia repair. Surgical site occurrence requiring procedural intervention was defined as any surgical site occurrence (including surgical site infection) that required procedural intervention, defined as opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.14 Definitions of surgical site occurrence and surgical site infection can be found in the eMethods in Supplement 3.
Other secondary outcomes included postoperative adverse events, quality of life, and cost. Complete definitions are available in the eMethods in Supplement 3. In brief, postoperative adverse events were defined as any adverse medical occurrence and were further categorized and scored based on the comprehensive complication index to report the severity and burden of complications incurred by each patient.15 Quality of life (QOL) assessment included the EuroQoL descriptive system and visual analogue scale (EQ-5D)16 and the hernia-specific quality of life survey (HerQLes).17 Cost data are presented as total 30-day direct hospital costs (not charges), including all patient visits, admissions, prosthetic mesh cost, and procedural costs from the operation through the first 30 days postoperatively abstracted directly from the hospital administration accounting system. These costs include any readmission, reintervention, or management of other complications. Cost analysis did not include indirect institutional costs associated with overhead or professional fees.
Data Collection
Clinical evaluation was documented by the treating physician at a mean (SD) 1 (15) days, 6 (2) months, 12 (2) months, and 24 (4) months after surgery or additionally if complications or follow-up occurred. Consensus definitions and treatment plans for common surgical site occurrences following open complex ventral hernia repair were developed a priori for the purposes of this study protocol (eMethods in Supplement 3). All coinvestigators agreed to follow these consensus definitions and treatment plans for enrolled study participants to maximize objectivity of this study measure. Long-term follow-up was attempted with all patients. Patients were deemed lost to follow-up after a minimum of 6 attempted telephone contacts and an institutional review board–approved, department-funded $10 stipend sent via mail to encourage completion of quality-of-life assessments and hernia recurrence inventory.
Power Calculation
Our study investigated the superiority of synthetic (experimental) vs biologic (control) mesh measured as hernia recurrence through 2 years. In a review of published data and the authors’ experience in contaminated ventral hernia repair, we noted a hernia recurrence risk of 29% for biologic mesh vs 9% for permanent synthetic mesh cohorts.18,19,20 Based on these data, an estimated 253 patients were necessary to adequately power the study to detect a difference between groups. Assuming a maximum 20% loss to follow-up, 201 patients were anticipated to remain in the study sample for a 1:1 randomized allocation to each treatment arm (100 subjects per cohort). At the 2-tailed overall type I error rate of .05, the study would have 92% power to detect a significant difference in the rates of hernia recurrence (29% vs 9%).
Randomization and Blinding
Patients were enrolled by treating physicians or appropriate research personnel. Randomization sequence was generated by a statistician and allocation was executed by local research coordinators. Eligible patients were randomized 1:1 in the operating room to either biologic or synthetic mesh immediately prior to mesh deployment. Randomization was stratified by medical center and CDC wound class. A central concealed randomization scheme was housed in Research Electronic Data Capture (REDCap) and used a random number of blocks. Investigators were blinded until the point of intraoperative device placement, whereas patients remained blinded until the conclusion of the study period. This study was analyzed on an intent-to-treat basis for our primary outcome.
Statistical Analysis
Continuous data were described using median values with IQRs and comparisons made using Wilcoxon rank sum test. Categorical data were described using counts and proportions with comparisons performed using Pearson χ2 or Fisher exact test. Hernia recurrence was analyzed as a binary outcome at 2 years (yes or no). Time-to-hernia recurrence was assessed with a log-rank test and the Cox proportional hazard model with prespecified covariates described by Kaplan-Meier curves, tested by proportional hazard models, and reported as hazard ratios (HR). Prespecified covariates included mesh type, study center, age, body mass index (calculated as weight in kilograms divided by height in meters squared), hernia width, and history of smoking. Cluster effect of site and surgeon was adjusted in the regression model. Given a baseline difference in available hernia mesh sizes between the 2 groups, mesh width and mesh/defect area ratios were added to the regression models based on prior concerns of adequate mesh overlap association with hernia recurrence.21,22
Surgical site occurence requiring procedural intervention was determined with 4 repeated binary measures in a generalized mixed-effect model analysis with repeated measures to test the difference between biologic mesh and synthetic mesh cohorts over the study time points. For secondary outcomes of QOL, a mixed-effect model analysis with repeated measures was performed to determine the difference in the mean change in score from the preoperative assessment to the postoperative assessments for the EQ-5D and HerQLes QOL instruments after adjusting for baseline differences. Costs were compared as continuous variables. All statistical tests were 2-sided and a P value of <.05 was considered statistically significant. No adjustments were made for multiple comparisons. Data were analyzed using R version 4.0.0 (the R Foundation). An independent data and safety monitoring board reviewed the efficacy and safety data at 50-patient intervals with a predetermined stopping point of 3:1 deep surgical site infection rate in any group. No adjustment for this stopping rule was made in the analysis.
Results
Baseline Characteristics of Patients
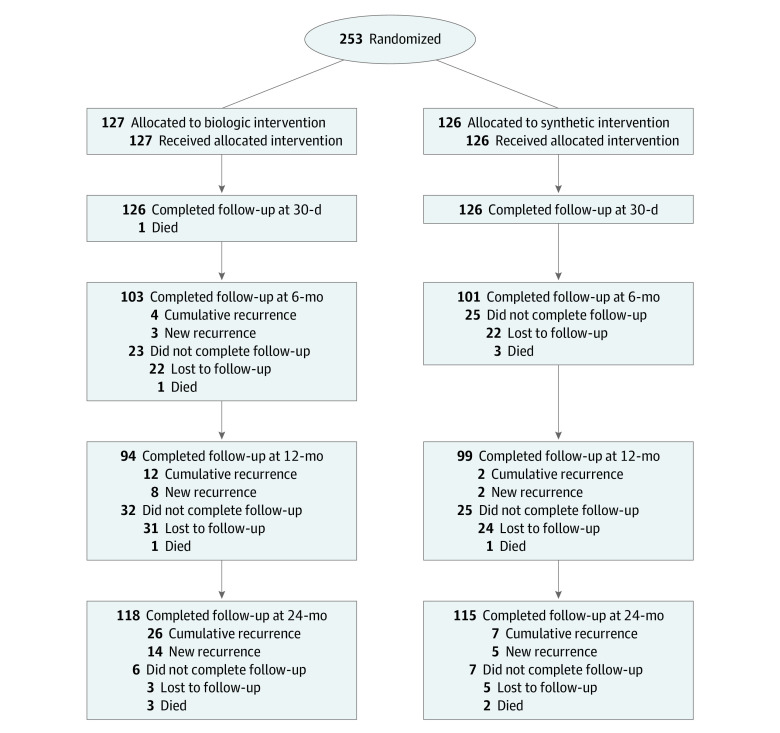
Between December 2012 and April 2019, 253 patients were enrolled and assigned to treatment groups as described in the CONSORT flow diagram (Figure 1). Follow-up was completed in April 2021. The median (IQR) age was 64 (55-70) years, and 117 participants (46%) were male. The 2 groups were similar with respect to baseline medical comorbidities and hernia characteristics (Table 1). There was a significant difference in mesh dimensions between the 2 groups because the largest biologic mesh available at the beginning of the trial was 20 cm × 30 cm. A complete list of mesh sizes used is provided in eTable 1 in Supplement 3. There were no other significant differences in operative characteristics (Table 2).
Figure 1. CONSORT Diagram.
Table 1. Patient Demographics, Comorbid Conditions, Wound, and Hernia Defect Characteristics by Randomizationa.
| Characteristic | No. (%) | |
|---|---|---|
| Biologic mesh (n = 127) | Synthetic mesh (n = 126) | |
| Male | 56 (44) | 61 (48) |
| Age, median (IQR), y | 63.7 (55-70) | 63.7 (55-69) |
| BMI,b median (IQR) | 32.9 (29-35) | 32.3 (28-37) |
| Obesity (BMIb >30) | 81 (64) | 73 (58) |
| Hypertension | 80 (63) | 73 (58) |
| History of ever smoking | 81 (64) | 71 (56) |
| Diabetes | 36 (28) | 29 (23) |
| COPD | 21 (16) | 12 (10) |
| Dyspnea | 8 (6) | 8 (6) |
| CHF | 6 (5) | 3 (2) |
| Prior DVT | 18 (14) | 14 (11) |
| Prior PE | 8 (6) | 15 (12) |
| Crohn disease | 14 (11) | 13 (10) |
| Ulcerative colitis | 12 (9) | 9 (7) |
| History of cancer | 48 (38) | 46 (37) |
| Previous radiotherapy | 15 (12) | 14 (11) |
| Serum HbA1C, median (IQR) | 5.7 (5.4-6.1) | 5.7 (5.5-6.3) |
| Recurrent incisional hernia | 67 (53) | 59 (47) |
| Stoma present | 82 (65) | 77 (61) |
| Recurrent parastomal hernia | 29 (23) | 30 (24) |
| Prior mesh infection | 9 (7) | 7 (6) |
| History of abdominal wall infection | 24 (19) | 25 (20) |
| Nonhealing abdominal wound | 16 (13) | 16 (13) |
| Prior MRSA infection | 9 (7) | 10 (8) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; DVT, deep venous thrombosis; HbA1C, hemoglobin A1C; MRSA, methicillin-resistant Staphylococcus aureus; PE, pulmonary embolus.
All P values >.05.
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Operative Details.
| Detail | No. (%) | P value | |
|---|---|---|---|
| Biologic mesh | Synthetic mesh | ||
| ASA classification | .34 | ||
| 2 | 13 (10) | 14 (11) | |
| 3 | 108 (86) | 111 (88) | |
| 4 | 4 (3) | 1 (1) | |
| OR time, median (IQR), min | 265 (193-332) | 260 (186-344) | .71 |
| Abdomen entered midline laparotomy | 126 (99) | 126 (100) | >.99 |
| Source of contamination | |||
| Bowel resection | 38 (30) | 31 (25) | .42 |
| GI fistula repair | 4 (3) | 2 (2) | .41 |
| Cholecystectomy | 14 (11) | 16 (13) | .83 |
| Appendectomy | 4 (3) | 3 (2) | >.99 |
| Ostomy reversal/takedown | 28 (22) | 27 (21) | >.99 |
| Gynecologic procedure | 9 (7) | 3 (2) | .14 |
| Urologic procedure | 12 (9) | 13 (10) | .98 |
| Creation of stoma | 15 (12) | 13 (10) | .86 |
| Enterotomy | 2 (2) | 4 (3) | .45 |
| EHS classification | |||
| M1 | 34 (27) | 25 (20) | .25 |
| M2 | 102 (80) | 101 (80) | >.99 |
| M3 | 115 (91) | 113 (90) | .98 |
| M4 | 100 (79) | 97 (77) | .85 |
| M5 | 28 (22) | 30 (24) | .85 |
| L1 | 3 (2) | 4 (3) | .72 |
| L2 | 20 (16) | 19 (15) | >.99 |
| L3 | 34 (27) | 29 (23) | .59 |
| L4 | 1 (1) | 1 (1) | >.99 |
| CDC wound class | .76 | ||
| 2 | 52 (41) | 54 (43) | |
| 3 | 75 (59) | 72 (57) | |
| Hernia, median (IQR), cm | |||
| Width | 14.0 (12-16) | 14.0 (11-15) | .12 |
| Length | 22 (18-25) | 23 (16-25) | .69 |
| Mesh, median (IQR), cm | |||
| Width | 30 (20-30) | 30 (30-30) | .002 |
| Length | 30 (30-30) | 30 (30-30) | .05 |
| Mesh/defect ratio, median (IQR) | 2.5 (1.9-3.6) | 3.0 (2.4-4.0) | .001 |
| Parastomal hernia component | 73 (60) | 69 (58) | .81 |
| Midline fascia closed | 127 (100) | 126 (100) | >.99 |
| Transversus abdominus release | 115 (94) | 111 (92) | .78 |
| External oblique release | 1 (1) | 0 (0) | >.99 |
| Estimated blood loss, median (IQR), mL3 | 150 (100-250) | 100 (100-200) | .18 |
| Hospital length of stay, median (IQR), d | 7 (6-11) | 7 (5-9) | .21 |
Abbreviations: ASA, American Society of Anesthesiology; CDC, Centers for Disease Control and Prevention; EHS, European Hernia Society; L, lateral; M, midline; OR, operating room.
Primary End Points
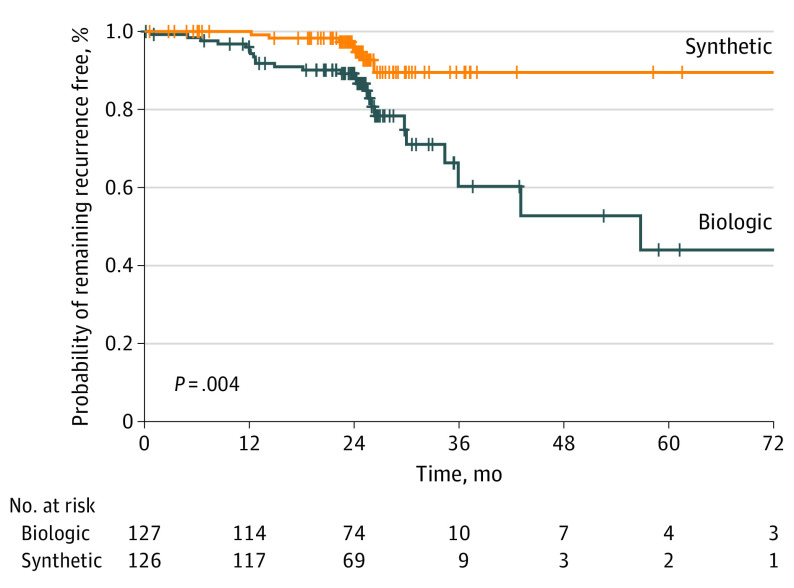
During long-term follow-up, 12 patients died of unrelated causes (6 in the biologic arm; 6 in the synthetic arm) (Figure 1; eResults in Supplement 3). Of the remaining 241 patients, 233 (97%) were available for long-term follow-up, and 8 were lost to follow-up (3 in the biologic arm; 5 in the synthetic arm). The intent-to-treat hernia recurrence risk at 2 years was 13% (33 of 253 patients) overall, 20.5% (26 of 127 patients) in the biologic arm, and 5.6% (7 of 126 patients) in the synthetic arm, with an absolute risk reduction of 14.9% with the use of synthetic mesh (95% CI, −23.8 to −6.1; P = .001). The hernia recurrence risk Kaplan-Meier time-to-event log-rank test revealed a significant difference in hernia recurrence risk favoring synthetic mesh (Figure 2). On multivariate Cox regression model with cluster effect adjusted by site and surgeon, synthetic mesh significantly reduced the risk of hernia recurrence (site adjustment: HR 0.31; CI, 0.23-0.42, P ≤ .001) and (surgeon adjustment: HR, 0.31; 95% CI, 0.13-0.75; P = .009). Defect size, mesh size, and mesh to defect ratio were not significantly associated with an increased risk of hernia recurrence (eTables 2 and 3 in Supplement 3). After adjustment for lower-volume centers, there was an effect on hernia recurrence (HR, 1.33; 95% CI, 1.18-1.39; P < .001), but when analyzed by surgeon, the effect did not persist (HR, 1.33; 95% CI, 0.24-7.37; P = .74).
Figure 2. Kaplan-Meier Plot of Time to Hernia Recurrence.
Secondary End Points
Safety
The generalized mixed-effect model analysis with repeated measures of surgical site occurrence requiring a procedural intervention revealed no significant difference between the 2 groups over the 24-month study period (odds ratio, 1.22; 95% CI, 0.60-2.44; P = .58). Comparable risks of surgical site occurrences requiring procedural intervention were found at each time point through the 2-year study period (biologic vs synthetic at 30 days, 27.6% vs 24.6%; P = .70; 6 months, 7.1% vs 4.8%; P = .61; 12 months, 1.6% vs 2.4%; P = .68; 24 months, 0.8% vs 1.6%; P = .62), and types of procedural interventions were similar between the 2 groups (Table 3). Overall, there were comparable rates of surgical site infection; however, the biologic mesh group tended to have a higher risk of deep surgical site infection than the synthetic group (14 [11%] vs 5 [4%], respectively; P = .06). There were no absolute risk reductions with regards to surgical site infection or surgical site occurrence between treatment arms (Table 3).
Table 3. Summary of Wound Complications Throughout the Study Period: Outcomes by Surgical Site Occurrence (SSO) and/or Surgical Site Infection (SSI) Requiring a Procedural Intervention.
| Outcome | No. (%) | Absolute difference, percentage points (95% CI) | P value | ||
|---|---|---|---|---|---|
| All (N = 253) | Biologic (n = 127) | Synthetic (n = 126) | |||
| SSO requiring procedural intervention rates | |||||
| 30 d | 66 (26.1) | 35 (27.6) | 31 (24.6) | 3 (−8 to 14) | .70 |
| 6 mo | 15 (5.9) | 9 (7.1) | 6 (4.8) | NA | .61 |
| 1 y | 5 (2.0) | 2 (1.6) | 3 (2.4) | NA | .68 |
| 2 y | 3 (1.2) | 1 (0.8) | 2 (1.6) | NA | .62 |
| SSO requiring procedural intervention rates details | |||||
| Wound opening | 32 (12.6) | 17 (13.4) | 15 (12) | NA | .87 |
| Wound debridement | 7 (2.8) | 4 (3.2) | 3 (2.4) | NA | >.99 |
| Percutaneous drainage | 17 (6.7) | 11 (8.7) | 6 (4.8) | NA | .32 |
| Mesh removal | 1 (0.4) | 1 (0.8) | 0 (0) | NA | >.99 |
| SSI (30 d) | 46 (18.2) | 27 (21.3) | 19 (15.1) | 6 (−4 to 16) | .27 |
| Superficial | 25 (9.9) | 12 (9.5) | 13 (10.3) | NA | .98 |
| Deep | 19 (7.5) | 14 (11) | 5 (4) | NA | .06 |
| Organ space | 2 (0.8) | 1 (0.8) | 1 (0.8) | NA | >.99 |
| SSO (30 d) | 23 (9.1) | 9 (7.1) | 14 (11.1) | 4 (−11 to 3) | .37 |
| Wound cellulitis | 12 (4.7) | 4 (3.2) | 8 (6.4) | NA | .37 |
| Serous drainage | 2 (0.8) | 1 (0.8) | 1 (0.8) | NA | >.99 |
| Purulent drainage | 1 (0.4) | 1 (0.8) | 0 (0) | NA | >.99 |
| Seroma | 6 (2.4) | 3 (2.4) | 3 (2.4) | NA | >.99 |
| Hematoma | 4 (1.6) | 2 (1.6) | 2 (1.6) | NA | >.99 |
Abbreviation: NA, not applicable.
Adverse Events
At 30 days follow-up, 149 patients (58.9%) had experienced at least 1 postoperative adverse event (eTable 4 in Supplement 3). There were significantly more adverse events in the biologic vs the synthetic mesh group (84 [66.1%] vs 65 [51.6%], respectively; P = .03). Patients receiving synthetic mesh had a 14.5% (95% CI, 1.7-27.3) absolute risk reduction of having an adverse event compared with the biologic mesh group. Most adverse events were either wound morbidity or ileus, and no specific type of adverse event was noted to be responsible for this difference. According to the comprehensive complication index, the 30-day adverse events in the biologic group tended to be more severe than the synthetic group (20.9 [95% CI, 0.0-28.2] vs 8.7 [95% CI, 0.0-22.6], respectively; P = .05). There was no difference in reoperation rates between the 2 groups at any time point. After 6 months, there were no mesh-related reoperations in either group (eResults in Supplement 3). The most common reason for reoperation after 6 months was hernia recurrence (eTable 5 in Supplement 3).
Quality of Life
Patients had similar baseline QOL measurements, with overall improvements in both groups. There were no significant differences between the groups regarding QOL measured by EQ-5D and HerQles scores at any time point (eFigure in Supplement 3).
Cost Analysis
There was a significant difference in the total median (IQR) 30-day hospital direct costs of the biologic group vs the synthetic group ($44 936 [$35 877-$52 656] vs $17 289 [$14 643-$22 901], respectively; P < .001). There was also a significant difference in the price of the prosthetic device between the 2 groups (median [IQR] cost biologic $21 539 [$20 285-$23 332] vs synthetic $105 [$105-$118]; P < .001).
Discussion
In this randomized clinical trial, synthetic mesh added a substantial benefit over biologic mesh during single-stage ventral hernia repair in a clean-contaminated or contaminated surgical field in terms of reducing hernia recurrence risk at 2-year follow-up. Safety profiles were similar between the meshes at up to 2 years; however, there was a significant difference in the prespecified secondary end point of cost between the groups, with biologic mesh costing roughly 200 times as much as synthetic mesh and being the sole driver doubling the total 30-day median hospital costs. While there were significantly more adverse events (also of greater severity) in the biologic group, there was no difference in reoperation rates or health-related and hernia-specific QOL measures between the groups at any time point. Overall, patients undergoing hernia repair with mesh in contaminated fields experienced improved QOL outcomes regardless of the type of mesh device used.
In terms of efficacy, synthetic mesh was associated with a significant absolute risk reduction of hernia recurrence at 2 years compared with biologic mesh. This is in keeping with prior work,18,19 and further supports the notion that synthetic mesh is a superior device for repair of contaminated ventral hernias. Although medium- to long-term recurrence rates after incisional hernia repair with mesh are considered somewhat common and range from 20% to 70% in surgical literature,23,24 these recurrences are far from benign with regard to their ultimate impact on patient well-being and health care resource utilization. Incisional hernia recurrence is well known to be associated not only with high cumulative rates of reoperative repairs,25 but also with a progressive and step-wise increase in the likelihood of a complication associated with each subsequent operation.26 While the exact health care utilization and financial burden associated with these outcomes is not well defined, some authors conservatively estimate an annual savings of $32 million for every 1% reduction in hernia recurrence rates,1 highlighting an urgent need to prioritize durability in the care of patients with incisional hernias.
Long-term mesh-related complications such as chronic mesh infection and the associated reoperations likely drive much of the fear of using a permanent synthetic mesh in these cases.27,28 As such, we defined the safety of mesh devices in a contaminated field as surgical site occurrences requiring procedural intervention for the purposes of this study. We did not find a difference in surgical site occurrences requiring procedural intervention between the 2 groups over the 2-year study period, nor did we find a difference in mesh explantation between groups. Notably, almost two-thirds of the patients in this trial experienced an adverse event during the study period, suggesting significant short-term morbidity for single-stage repair of contaminated hernias regardless of mesh device used. Most adverse events occurred in the biologic group and were also of greater severity than those in the synthetic group, suggesting that several additional factors may contribute to the safety of mesh devices, including the complexity of the operation itself, and that any adverse signals detected were associated more with biologic mesh than with synthetic mesh. Still, the equivalent risks of mesh-related reoperations at every time point and no mesh-related complications requiring reoperation in any group after 6 months suggest that the noted adverse events ultimately resolved without further long-term implications, and at a minimum biologic and synthetic meshes likely perform comparably regarding safety.
Although the true market penetration of biologic mesh remains unknown, there is some evidence to suggest that biologic mesh holds a substantial proportion of the ventral hernia market share,29 with as much a $500 million annual expenditure on biologic mesh alone.5 Despite this expense, our findings align with those of prior systematic reviews, which have failed to identify a clinical benefit to the use of biologic mesh over synthetic mesh in contaminated cases.3,4,29,30,31 Our findings of a nearly 200-fold increase in price of biologic mesh have been previously described and borne out in a burgeoning body of surgical literature.4,32,33,34,35,36 When viewed in the context of the value in health care equation, defined as health outcomes achieved per dollar spent,37,38 our findings of comparable safety, significant increase in efficacy (numerator), and vastly decreased cost (denominator) associated with synthetic mesh are overwhelmingly in favor of synthetic mesh. This is a particularly salient finding given the likely overstated notion that synthetic mesh is contraindicated in contaminated fields, and the likely equally overstated notion that biologic mesh is the preferred device in contaminated fields.
One of the strengths of our study is that all cases were performed in a clean-contaminated or contaminated setting. As such, it is difficult to compare our results to previously published series evaluating the outcomes of biologic or synthetic mesh given the heterogeneity of wound class in prior studies. Furthermore, most prior studies lack a control arm, hernia-specific details, and granular mesh type and location, and are biased by case selection including clean or dirty cases,18,36,39,40,41 limiting comparison with our study.
Limitations
There are several limitations to our trial. Despite the multicenter nature of our trial, it is important for these findings to be confirmed in larger trials at other centers. Additionally, our study only investigated 2 types of materials, medium-weight polypropylene and non–cross-linked porcine dermis, both placed in the retromuscular position. Therefore, we cannot necessarily extrapolate our findings to other materials32,42,43,44 placed in other positions.45 While there were no significant differences in baseline characteristics in this study, we were not powered to control for all these factors and despite randomization, some subtle baseline differences might have confounded our results. In addition, as multiple testing methods were not used, the secondary outcomes of this study should be considered exploratory and hypothesis generating. Our operations were performed by high-volume, fellowship-trained surgeons with experience in abdominal wall reconstruction, and while our outcomes therefore may not be generally reproducible, we believe our clinical outcomes and cost data were thus maximized to best represent both meshes.46 Additionally, while the patients and radiographic assessors were blinded to the treatment allocation, some patients were evaluated clinically by physicians without blinding owing to trial constraints. Although our 2-year follow-up rate was excellent, at 6 months and 1 year follow-up rates were variable, which could have affected some of our repeated measures analysis. Although the patient-reported outcomes in this trial were similar up to the 2-year end point, there may be other patient-reported mesh-related outcomes which are herein unaccounted for and may require further dedicated study. It is also that longer-term follow-up might reveal more prosthetic-related complications that did not present by 2 years follow-up.47 However, we identified no mesh-related complications or reoperations after 6 months other than hernia recurrence. Still, we acknowledge that a randomized clinical trial is likely not powered to identify rare, catastrophic mesh-related complications.
Conclusions
In conclusion, synthetic mesh demonstrated a significantly superior hernia recurrence risk compared with biologic mesh in the single-stage repair of contaminated ventral hernias with similar safety outcomes. Additionally, the secondary end point of cost was greatly reduced with the use of synthetic mesh, suggesting that synthetic mesh should be the device of choice for the repair of contaminated ventral hernias.
Trial protocol
Trial protocol appendices
eMethods
eTable 1. Mesh Sizes Utilized for the Trial
eTable 2. Multivariate Cox model for hernia recurrence rate with cluster analysis adjusted by Site
eTable 3. Multivariate Cox model for hernia recurrence rate with cluster analysis adjusted by surgeon
eTable 4. 30-day adverse events and complications
eTable 5. Indications for reoperations throughout the study period
eFigure. EQ5D, 3B EQ5D VAS, and 3C HerQles estimates from mixed effect regression model throughout the study period adjusted for baseline differences
eReferences
Data sharing statement
References
- 1.Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16(2):179-183. doi: 10.1007/s10029-011-0879-9 [DOI] [PubMed] [Google Scholar]
- 2.Rosen MJ, Krpata DM, Ermlich B, Blatnik JA. A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg. 2013;257(6):991-996. doi: 10.1097/SLA.0b013e3182849871 [DOI] [PubMed] [Google Scholar]
- 3.Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia. 2018;22(2):249-269. doi: 10.1007/s10029-018-1735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huerta S, Varshney A, Patel PM, Mayo HG, Livingston EH. Biological mesh implants for abdominal hernia repair: US food and drug administration approval process and systematic review of its efficacy. JAMA Surg. 2016;151(4):374-381. doi: 10.1001/jamasurg.2015.5234 [DOI] [PubMed] [Google Scholar]
- 5.United States Securities and Exchange Commission. Tela Bio Annual Report. Published 2020. Accessed March 5, 2021. https://ir.telabio.com/static-files/05ac9577-950e-4607-a4e4-e00cd0851ff7
- 6.Engelsman AF, van Dam GM, van der Mei HC, Busscher HJ, Ploeg RJ. In vivo evaluation of bacterial infection involving morphologically different surgical meshes. Ann Surg. 2010;251(1):133-137. doi: 10.1097/SLA.0b013e3181b61d9a [DOI] [PubMed] [Google Scholar]
- 7.Engelsman AF, van der Mei HC, Busscher HJ, Ploeg RJ. Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br J Surg. 2008;95(8):1051-1059. doi: 10.1002/bjs.6154 [DOI] [PubMed] [Google Scholar]
- 8.Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ. In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg. 2012;16(11):2139-2144. doi: 10.1007/s11605-012-1992-5 [DOI] [PubMed] [Google Scholar]
- 9.Campanelli G, Nicolosi FM, Pettinari D, Avesani EC. Intestinal resection and multiple abdominal hernia mesh repair: is the combination safe and feasible? Chir Ital. 2004;56(6):839-842. [PubMed] [Google Scholar]
- 10.Birolini C, Utiyama EM, Rodrigues AJ Jr, Birolini D. Elective colonic operation and prosthetic repair of incisional hernia: does contamination contraindicate abdominal wall prosthesis use? J Am Coll Surg. 2000;191(4):366-372. doi: 10.1016/S1072-7515(00)00703-1 [DOI] [PubMed] [Google Scholar]
- 11.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-732. doi: 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 12.Baucom RB, Ousley J, Feurer ID, et al. Patient reported outcomes after incisional hernia repair-establishing the ventral hernia recurrence inventory. Am J Surg. 2016;212(1):81-88. doi: 10.1016/j.amjsurg.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Krpata DM, Blatnik JA, Novitsky YW, Rosen MJ. Posterior and open anterior components separations: a comparative analysis. Am J Surg. 2012;203(3):318-322. doi: 10.1016/j.amjsurg.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Haskins IN, Horne CM, Krpata DM, et al. A call for standardization of wound events reporting following ventral hernia repair. Hernia. 2018;22(5):729-736. doi: 10.1007/s10029-018-1748-6 [DOI] [PubMed] [Google Scholar]
- 15.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1-7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 16.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 17.Krpata DM, Schmotzer BJ, Flocke S, et al. Design and initial implementation of HerQLes: a hernia-related quality-of-life survey to assess abdominal wall function. J Am Coll Surg. 2012;215(5):635-642. doi: 10.1016/j.jamcollsurg.2012.06.412 [DOI] [PubMed] [Google Scholar]
- 18.Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. 2013;217(6):991-998. doi: 10.1016/j.jamcollsurg.2013.07.382 [DOI] [PubMed] [Google Scholar]
- 19.Rosen MJ, Bauer JJ, Harmaty M, et al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg. 2017;265(1):205-211. doi: 10.1097/SLA.0000000000001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavallo JA, Criss C, Poulose BK, et al. A multicenter prospective observational cohort study of permanent synthetic mesh versus biologic mesh reinforcement for open ventral hernia repair in clean-contaminated and contaminated surgical sites. J Am Coll Surg. 2013;217(3):S25. doi: 10.1016/j.jamcollsurg.2013.07.041 [DOI] [Google Scholar]
- 21.LeBlanc K. Proper mesh overlap is a key determinant in hernia recurrence following laparoscopic ventral and incisional hernia repair. Hernia. 2016;20(1):85-99. doi: 10.1007/s10029-015-1399-9 [DOI] [PubMed] [Google Scholar]
- 22.Lambrecht J. Overlap-coefficient for the relationship between mesh size and defect size in laparoscopic ventral hernia surgery. Hernia. 2011;15(4):473-474. doi: 10.1007/s10029-011-0817-x [DOI] [PubMed] [Google Scholar]
- 23.Arita NA, Nguyen MT, Nguyen DH, et al. Laparoscopic repair reduces incidence of surgical site infections for all ventral hernias. Surg Endosc. 2015;29(7):1769-1780. doi: 10.1007/s00464-014-3859-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger JWA, Luijendijk RW, Hop WCJ, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240(4):578-585. doi: 10.1097/01.sla.0000141193.08524.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? a population-based analysis. Ann Surg. 2003;237(1):129-135. doi: 10.1097/00000658-200301000-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holihan JL, Alawadi Z, Martindale RG, et al. Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg. 2015;221(2):478-485. doi: 10.1016/j.jamcollsurg.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 27.Sanchez VM, Abi-Haidar YE, Itani KMF. Mesh infection in ventral incisional hernia repair: incidence, contributing factors, and treatment. Surg Infect. 2011;12(3):205-210. doi: 10.1089/sur.2011.033 [DOI] [PubMed] [Google Scholar]
- 28.Jolissaint JS, Dieffenbach B V., Tsai TC, et al. Surgical site occurrences, not body mass index, increase the long-term risk of ventral hernia recurrence. Surgery. 2020;167(4):P765-771. doi: 10.1016/j.surg.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross W, Kumar A, Chandru Kowdley G. Biological mesh in contaminated fields—overuse without data: a systematic review of their use in abdominal wall reconstruction. Am Surg. 2014;80(1):3-8. doi: 10.1177/000313481408000104 [DOI] [PubMed] [Google Scholar]
- 30.Kissane NA, Itani KMF. A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: what have we learned? Plast Reconstr Surg. 2012;130(5)(suppl 2):194S-202S. doi: 10.1097/PRS.0b013e318265a5ec [DOI] [PubMed] [Google Scholar]
- 31.Primus FE, Harris HW. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia. 2013;17(1):21-30. doi: 10.1007/s10029-012-1037-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer JP, Basta MN, Krishnan NM, Wink JD, Kovach SJ. A cost-utility assessment of mesh selection in clean-contaminated ventral hernia repair. Plast Reconstr Surg. 2016;137(2):647-659. doi: 10.1097/01.prs.0000475775.44891.56 [DOI] [PubMed] [Google Scholar]
- 33.Schneeberger S, Phillips S, Huang LC, Pierce RA, Etemad SA, Poulose BK. Cost-utility analysis of biologic and biosynthetic mesh in ventral hernia repair: when are they worth it? J Am Coll Surg. 2019;228(1):66-71. doi: 10.1016/j.jamcollsurg.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 34.Olavarria OA, Bernardi K, Dhanani NH, et al. Synthetic versus biologic mesh for complex open ventral hernia repair: a pilot randomized controlled trial. Surg Infect. 2021;21(5):496-503. doi: 10.1089/sur.2020.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Totten CF, Davenport DL, Ward ND, Roth JS. Cost of ventral hernia repair using biologic or synthetic mesh. J Surg Res. 2016;203(2):459-465. doi: 10.1016/j.jss.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 36.Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W. Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg Infect (Larchmt). 2017;18(2):112-118. doi: 10.1089/sur.2016.106 [DOI] [PubMed] [Google Scholar]
- 37.Yount KW, Turrentine FE, Lau CL, Jones RS. Putting the value framework to work in surgery. J Am Coll Surg. 2015;220(4):596-604. doi: 10.1016/j.jamcollsurg.2014.12.037 [DOI] [PubMed] [Google Scholar]
- 38.Lee TH. Putting the value framework to work. N Engl J Med. 2010;363(26):2481-2483. doi: 10.1056/NEJMp1013111 [DOI] [PubMed] [Google Scholar]
- 39.Bondre IL, Holihan JL, Askenasy EP, et al. ; Ventral Hernia Outcomes Collaborative . Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res. 2016;200(2):488-494. doi: 10.1016/j.jss.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Itani KMF, Rosen M, Vargo D, Awad SS, Denoto G III, Butler CE; RICH Study Group . Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery. 2012;152(3):498-505. doi: 10.1016/j.surg.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 41.Harris HW, Primus F, Young C, et al. Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: the PRICE Randomized Clinical Trial. Ann Surg. 2021;273(4):648-655. doi: 10.1097/SLA.0000000000004336 [DOI] [PubMed] [Google Scholar]
- 42.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12(1):63-69. doi: 10.1177/155335060501200109 [DOI] [PubMed] [Google Scholar]
- 43.Huntington CR, Cox TC, Blair LJ, et al. Biologic mesh in ventral hernia repair: outcomes, recurrence, and charge analysis. Surgery. 2016;160(6):1517-1527. doi: 10.1016/j.surg.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 44.Montgomery A. The battle between biological and synthetic meshes in ventral hernia repair. Hernia. 2013;17(1):3-11. doi: 10.1007/s10029-013-1043-5 [DOI] [PubMed] [Google Scholar]
- 45.Birolini C, de Miranda JS, Tanaka EY, Utiyama EM, Rasslan S, Birolini D. The use of synthetic mesh in contaminated and infected abdominal wall repairs: challenging the dogma—a long-term prospective clinical trial. Hernia. 2020;24(2):307-323. doi: 10.1007/s10029-019-02035-2 [DOI] [PubMed] [Google Scholar]
- 46.Aquina CT, Kelly KN, Probst CP, et al. Surgeon volume plays a significant role in outcomes and cost following open incisional hernia repair. J Gastrointest Surg. 2015;19(1):100-110. doi: 10.1007/s11605-014-2627-9 [DOI] [PubMed] [Google Scholar]
- 47.Kokotovic D, Bisgaard T, Helgstrand F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA. 2016;316(15):1575-1582. doi: 10.1001/jama.2016.15217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Trial protocol appendices
eMethods
eTable 1. Mesh Sizes Utilized for the Trial
eTable 2. Multivariate Cox model for hernia recurrence rate with cluster analysis adjusted by Site
eTable 3. Multivariate Cox model for hernia recurrence rate with cluster analysis adjusted by surgeon
eTable 4. 30-day adverse events and complications
eTable 5. Indications for reoperations throughout the study period
eFigure. EQ5D, 3B EQ5D VAS, and 3C HerQles estimates from mixed effect regression model throughout the study period adjusted for baseline differences
eReferences
Data sharing statement