Abstract
Background:
Intake of conventionally grown fruits and vegetables (FVs) is an important route of exposure to pesticide residues in the general population. However, whether health risk stemming from exposure to pesticides through diet could offset benefits of consuming FVs is unclear.
Objective:
We assessed the association of FV intake, classified according to their pesticide residue status, with total and cause-specific mortality.
Methods:
We followed 137,378 women (NHS, 1998–2019, and NHSII, 1999–2019) and 23,502 men (HPFS, 1998–2020) without cardiovascular disease, cancer, or diabetes at baseline. FV intake was assessed using validated food frequency questionnaires and categorized as having high- or low-pesticide-residues using data from the USDA Pesticide Data Program. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for total and cause-specific mortality associated with high- and low-pesticide-residue FV intake.
Results:
A total of 27,026 deaths, including 4,318 from CVD and 6,426 from cancer, were documented during 3,081,360 person-years of follow-up. In multivariable-adjusted analyses, participants who consumed ≥4 servings/day of low-pesticide-residue FVs had 36% (95% CI: 32%-41%) lower mortality risk compared to participants who consumed <1 serving/day. The corresponding estimate for high-pesticide residue FV intake was 0.93 (95% CI: 0.81–1.07). This pattern was similar across the three most frequent causes of death (cardiovascular disease, cancer and respiratory diseases).
Conclusions:
High-pesticide-residue FV intake was unrelated whereas low-pesticide residue FV intake was inversely related to all-cause mortality, suggesting that exposure to pesticide residues through diet may offset the beneficial effect of FV intake on mortality.
1. Introduction
Fruit and vegetables (FVs) are considered an integral part of a healthy diet and thus recommended by major organizations and government guidelines across the world; mostly for their well-documented benefits in the prevention of cardiovascular disease (CVD) (Millen et al., 2016; Eckel et al., 2014). Nevertheless, the main exposure route for pesticide residues in the general population is diet, (Xue et al., 2014; Yu et al., 2012; Fortes et al., 2013; Lu et al., 2008) particularly through the consumption of conventionally grown FVs. Data from the USDA Pesticide Data Program (PDP), which systematically surveys the presence of pesticide residues in foods sold in supermarkets, shows that in 2018 more than 50% of FVs consumed in the U.S. contained detectable pesticide residues and more than 30% had two or more pesticides (Ams, 2018).
Occupational exposure to pesticides used in agriculture is known to increase morbidity and mortality (Suratman et al., 2015). In fact, several pesticides have been banned in the last 50 years, but they can still persist on water and soil (Jones and de Voogt, 1999; Lovecka et al., 2015; Pouch et al., 2018). Moreover, risk assessments by the International Agency for Research on Cancer (IARC) have concluded that occupational exposure to certain pesticides used in agriculture is carcinogenic to humans. (IARC, 1991) Although tolerance levels of pesticides are regulated by the Environmental Protection Agency (EPA), these do not necessarily imply lack of adverse health effects in humans and therefore long-term deleterious effects on human health of pesticide residues through diet remain unclear. We have previously reported that exposure to pesticide residues through FV intake may reduce the benefits of FV intake on CVD incidence (Chiu et al., 2019) and is associated with adverse reproductive outcomes. (Chiu et al., 2018; Chiu et al., 2015) Others have reported benefits of consuming organic produce on cancer risk, (Baudry et al., 2018) whereas overall exposure to pesticides through FV intake was not associated with cancer risk. (Sandoval-Insausti et al., 2021) However, it is not clear whether these apparent benefits on the incidence of major chronic non-communicable diseases can impact mortality. To address this question, we examined the association of FV intake, considering their pesticide residue status based on surveillance data, with total and cause-specific mortality in three cohorts of U.S. health professionals.
2. Methods
2.1. Study population
We included participants from three ongoing U.S. based prospective cohorts: the Nurses’ Health Study (NHS) which included 121,700 female registered nurses aged 30–55 years in 1976; the Nurses’ Health Study II (NHSII) which included 116, 671 female registered nurses, aged 25–42 in 1989; and the Health Professionals Follow-up Study (HPFS) which included 51,529 male health professionals aged 40–75 in 1986. (Bao et al., 2016) At baseline and biennially thereafter, participants of all cohorts were asked to complete a self-administered questionnaire to obtain information on demographic factors, lifestyle, and medical history. Habitual food consumption was recorded every four years using an extensively validated food frequency questionnaire (FFQ) with 131 food items, of which 27 were fruits and vegetables. (Rimm et al., 1992; Yuan et al., 2018,2018; Yuan et al., 2017) The follow-up rates in each cycle were around 90%. In the current analysis, we followed individuals from 1998 (in NHS and HPFS) and 1999 (in NHSII) based on the ability to couple information on FV intake from prospectively collected FFQs with contemporaneous national surveillance data on pesticide residues in food. We excluded participants with cardiovascular disease, cancer, or diabetes at baseline. We also excluded participants with missing or invalid total energy intakes (<500 or greater than 3500 kcal/day for women and < 800 or greater than 4200 kcal/day for men) or who lacked data on more than 50% of the FVs related questions. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
2.2. Pesticide residue assessment
FVs reported in the FFQ were classified as having high- or low-pesticide-residues using the Pesticide Residue Burden Score (PRBS), a validated score system used to ascertain pesticide residues in FVs. (Hu et al., 2016; Chiu et al., 2018) Briefly, we linked period-matched data on pesticide residues in FVs as reported in surveillance data from the PDP, (Ams, 2018) with individual level FV intake data as reported in the FFQ. PDP database includes approximately 420 pesticides and their metabolites. In 2018, detectable levels of at least one pesticide was reported in more than 50% of conventionally grown FVs in and 2 or more pesticides were detected in 31% of the fruits and vegetables analyzed. (Ams, 2018) PDP data from 1996 to 1999 was paired to FFQ data from 1998 (NHS/HPFS) and 1999 (NHSII); PDP data from 2000 to 2003 was paired to FFQ data from 2002 and 2003; until 2012–2013. Each FV included in the FFQ was ranked according to three measures obtained from the PDP: 1) the percentage of samples with any detectable pesticide residues; 2) the percentage of samples with any pesticide residues above tolerance levels; and 3) the percentage of samples with three or more individual detectable pesticides. For each contamination measure, a score of 0 was assigned for FVs in the lowest tertile, 1 for FVs in the middle tertile, and 2 for FVs in the highest period-specific tertile. The PRBS was the sum of scores across the three measures on a scale of 0 to 6. Then, for each period, FVs with PRBS ≥ 4 were classified as having high-pesticide-residue status, FVs with PRBS < 4 as having low-pesticide-residue status, and FVs without matching PDP data as having undetermined -pesticide residue status. Finally, intakes of high-, low-, and undetermined pesticide-residue FVs were summed for each participant in the three cohorts (Table S1). (Chiu et al., 2019)
2.3. Covariates
In the questionnaires, participants self-reported diagnoses of hypertension, hypercholesterolemia, cardiovascular disease, cancer, and diabetes as well as information on race, body mass index (BMI), physical activity, family history of cancer and cardiovascular disease, smoking status, and smoking in package-years. Further information on total energy intake, alcohol intake, and the Alternate Healthy Eating Index, excluding criteria for intake of FVs and alcohol, was collected every four years. (McCullough and Willett, 2006; Chiuve et al., 2012) In the NHS and NHSII, updated data on postmenopausal status and current hormone therapy use were also gathered.
2.4. Outcome assessment
Our main outcome was all-cause mortality. Deaths from the three cohorts were assessed from state vital records, National Death Index, and reports from family members or the postal authorities. These methods ascertained more than 98% of the deaths. (Rich-Edwards et al., 1994) The cause of death was classified according to the International Classification of Diseases, Eighth Revision (ICD-8) in four categories: deaths from cardiovascular disease (heart failure, coronary heart disease, stroke, and any other vascular disease; ICD codes: 390–458), deaths from cancer (ICD codes: 140–207), deaths from respiratory diseases (ICD codes: 460–519), and other causes of death (Table S2). We also calculated premature deaths, defined as deaths before age 70 years, in all the three cohorts. (Shiels et al., 2017)
2.5. Statistical analysis
We followed the participants from the date of the return of the baseline questionnaire to the date of death or the end of follow up (June 2019 in NHS and NHSII and June 2020 in HPFS), whichever occurred first. Cumulative average intakes of high- and low-pesticide-residue FVs over updated questionnaires were calculated and modeled in categories of absolute intake (<1, 1–1.9, 2–2.9, 3–3.9, ≥4 servings/day) and as quintiles of intake. The associations between high- and low-pesticide-residue FV intake and all-cause, cause-specific, and premature mortality were summarized with Hazard Ratios (HRs) and their 95% confidence interval (95% CI) obtained from Cox proportional hazards regression models with age as the time scale. In the multivariable analysis, we adjusted for age (years), BMI (quintiles), race (white/non-white), physical activity (quintiles), family history of cancer (yes/no), family history of cardiovascular disease (yes/no), smoking in package-years (never smoker, 1–4.9, 5–19.9, 20–39.9, or greater than 40), baseline hypertension (yes/no), baseline hypercholesterolemia (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, or greater than 30 g/day), and a modified version of the Alternate Healthy Eating Index (quintiles), which excluded scoring for intakes of fruits, vegetables, and alcohol. In the NHS and NHSII, we further adjusted for postmenopausal hormone use (premenopausal/never/past/current). For intakes of high-pesticide-residue FVs, the multivariable model was additionally adjusted for intakes of low-pesticide-residue FVs and other FVs with undetermined-pesticide-residues. Similarly, models for low-pesticide-residue FV intake were further adjusted for intakes of high-pesticide-residue FVs and other FVs with undetermined- pesticide-residue status. Tests for linear trend were assessed by modeling as a continuous variable the median values of each category of consumption. Analyses were carried out separately for each cohort and then were pooled using a fixed-effect model. To test for non-linear associations and examine the dose–response relationships, we depicted restricted cubic splines with four knots using the exposure variables as continuous in fully-adjusted models. Finally, we estimated the effect of substituting high-pesticide-residue FVs with low-pesticide-residue FVs by modeling both as continuous variables in the same model and using the difference of the regression coefficients as the point estimate, and the covariance matrix to estimate 95 %CIs. We also carried out cohort-specific analyses and evaluated the intake of FVs modeled as quintiles, the deaths before age 70 years, and the most recent diet assessment. Moreover, we did not see evidence of effect modification by various established risk factors for premature mortality.
Analyses were performed with SAS software version 9.4 for UNIX (SAS Institute, Cary, NC), at a two sided p<0.05.
3. Results
We identified 27,026 deaths over 3,081,360 person-years of follow-up, including 4,318 from CVD, 6,426 from cancer, 2,012 from respiratory diseases, and 14,222 from other causes. Of these deaths, 3,492 took place before age 70 years. Participants who consumed ≥ 4 servings/day of high-pesticide-residue FV intake were more frequently active, never smokers and were more likely to have hypercholesterolemia, had a lower BMI, and a higher consumption of total calories, total FVs, flavonoids, antioxidant nutrients, and fiber. (Table 1 and Table S3). We observed the same pattern of association between baseline characteristics and intake of low-pesticide-residue FV intake (Table 1 and Table S3). Intakes of low-pesticide-residue and high-pesticide-residue FVs were positively related to each other (rSpearman = 0.63 in NHS, 0.70 in NHSII, and 0.63 in HPFS).
Table 1.
Baseline characteristics of participants according to absolute intakes of high- and low-pesticide-residue fruits and vegetables.
| High-Pesticide-Residue Fruit and Vegetable Intake (servings/day) |
Low-Pesticide-Residue Fruit and Vegetable Intake (servings/day) |
|||||
|---|---|---|---|---|---|---|
| Characteristics | <1 | ≥2–< 3 | ≥4 | <1 | ≥2–< 3 | ≥4 |
|
| ||||||
| Nurses’ Health Study (1998) | ||||||
| Number of participants | 20,267 | 10,096 | 1,512 | 7,821 | 17,717 | 4,626 |
| High-pesticide-residue FVs (servings/day) | 0.6(0.2) | 2.4(0.3) | 5.2(1.4) | 0.8(0.7) | 1.7(0.9) | 2.6(1.4) |
| Low-pesticide-residue FVs (servings/day) | 1.6(0.9) | 2.8(1.2) | 3.8(1.9) | 0.7(0.2) | 2.4(0.3) | 5.0(1.3) |
| Total FVs (servings/day) | 2.9(1.2) | 6.4(1.5) | 10.7(2.9) | 2.2(1.0) | 5.1(1.3) | 9.0(2.5) |
| Age (y) | 63.6(7.2) | 64.0(6.9) | 62.8(6.5) | 64.0(7.3) | 63.8(7.0) | 63.5(6.7) |
| BMI (kg/m2) | 26.6(5.3) | 26.1(4.9) | 25.4(4.9) | 26.5(5.2) | 26.3(5.0) | 26.0(4.9) |
| Postmenopause (%) | 93.4 | 93.3 | 94.1 | 93.2 | 93.2 | 93.6 |
| White (%) | 97.7 | 97.7 | 96.9 | 97.1 | 97.9 | 96.8 |
| Physical activity (MET/wk) | 13.2(17.9) | 22.8(24.7) | 30.4(30.6) | 13.0(18.1) | 18.9(21.9) | 25.1(27.5) |
| Family history of cancer (%) | 21.5 | 23.0 | 23.7 | 21.0 | 22.2 | 22.7 |
| Family history of cardiovascular disease (%) | 6.6 | 7.0 | 6.8 | 6.8 | 6.4 | 6.7 |
| Never smoker (%) | 43.5 | 50.1 | 48.6 | 42.0 | 48.0 | 51.0 |
| No of pack-years among ever smokers | 15.9 (21.8) | 9.9(16.3) | 9.7 (15.5) | 17.3(22.7) | 11.6(18.0) | 10.4(17.2) |
| Current hormone therapy use (%) | 45.9 | 49.4 | 48.1 | 44.5 | 48.7 | 48.3 |
| Hypertension (%) | 39.6 | 38.1 | 35.3 | 37.3 | 38.9 | 41.7 |
| Hypercholesterolemia (%) | 54.7 | 53.3 | 50.2 | 54.1 | 53.8 | 53.5 |
| Total energy intake (kcal/day) | 1,527(482) | 1,932(521) | 2,166(568) | 1,373(452) | 1,807(477) | 2,227(538) |
| Alcohol intake (g/day) | 5.2(9.7) | 5.1(8.4) | 4.5(7.7) | 4.5(9.4) | 5.5(9.2) | 5.5(9.2) |
| Modified AHEI (score)* | 38.1(8.3) | 40.4(8.5) | 41.9(8.8) | 41.4(8.6) | 38.2(8.3) | 37.9(8.0) |
| Nurses’ Health Study II (1999) | ||||||
| Number of participants | 33,222 | 10,669 | 1,921 | 13,978 | 20,124 | 4,939 |
| High-pesticide-residue FVs (servings/day) | 0.6(0.3) | 2.4(0.4) | 5.4(1.6) | 0.7(0.6) | 1.6(0.9) | 2.6(1.6) |
| Low-pesticide-residue FVs (servings/day) | 1.5(0.9) | 2.8(1.2) | 3.8(2.1) | 0.7(0.2) | 2.4(0.3) | 5.1(1.3) |
| Total FVs (servings/day) | 2.5(1.2) | 6.1(1.6) | 10.5(3.4) | 1.8(0.9) | 4.8(1.3) | 8.9(2.9) |
| Age (y) | 44.4(4.7) | 45.0(4.6) | 45.1(4.6) | 44.5(4.7) | 44.7(4.6) | 45.0(4.6) |
| BMI (kg/m2) | 26.7(6.3) | 25.9(5.7) | 25.4(5.7) | 26.9(6.4) | 26.1(5.9) | 26.0(6.0) |
| Postmenopause (%) | 22.2 | 22.0 | 21.7 | 23.1 | 21.3 | 21.8 |
| White (%) | 96.2 | 97.1 | 95.4 | 95.4 | 97.2 | 95.1 |
| Physical activity (MET/wk) | 14.7(19.2) | 23.3(24.3) | 29.8(31.2) | 14.3(19.4) | 19.9(22.7) | 26.0(30.0) |
| Family history of cancer (%) | 19.3 | 20.0 | 21.9 | 19.4 | 19.8 | 19.5 |
| Family history of cardiovascular disease (%) | 33.9 | 32.8 | 33.1 | 33.9 | 32.7 | 33.1 |
| Never smoker (%) | 65.3 | 67.1 | 64.7 | 64.1 | 67.3 | 68.7 |
| No of pack-years among ever smokers | 5.2(9.8) | 3.8(7.5) | 4.1(7.6) | 5.7(10.4) | 4.0(8.0) | 3.7(7.6) |
| Current hormone therapy use (%) | 1.0 | 1.2 | 1.1 | 1.1 | 0.9 | 1.1 |
| Hypertension (%) | 13.2 | 12.5 | 11.1 | 13.2 | 12.8 | 13.3 |
| Hypercholesterolemia (%) | 25.6 | 22.8 | 22.9 | 24.9 | 23.8 | 22.7 |
| Total energy intake (kcal/day) | 1,632(521) | 2,086(542) | 2,270(578) | 1,458(475) | 1,959(509) | 2,375(542) |
| Alcohol intake (g/day) | 3.7(9.1) | 4.2(6.8) | 3.9(7.2) | 3.2(7.2) | 4.3(7.1) | 4.3(7.5) |
| Modified AHEI (score)* | 37.4(9.1) | 40.2(9.7) | 42.9(10.0) | 39.9(9.2) | 37.8(9.4) | 37.7(9.3) |
| Health Professionals Follow-up Study (1998) | ||||||
| Number of participants | 7,763 | 4,106 | 845 | 2,394 | 7,219 | 2,576 |
| High-pesticide-residue FVs (servings/day) | 0.6(0.2) | 2.4(0.3) | 5.4(1.5) | 0.9(0.7) | 1.6(0.9) | 2.6(1.6) |
| Low-pesticide-residue FVs (servings/day) | 1.8(1.0) | 3.0(1.3) | 4.0(2.0) | 0.7(0.2) | 2.5(0.3) | 5.2(1.3) |
| Total FVs (servings/day) | 3.1(1.3) | 6.6(1.6) | 11.2(2.9) | 2.2(1.0) | 5.1(1.3) | 9.2(2.7) |
| Age (y) | 62.1(8.5) | 64.3(8.7) | 64.5(8.9) | 62.2(8.5) | 63.4(8.7) | 63.2(8.7) |
| BMI (kg/m2) | 26.2(3.4) | 25.6(3.4) | 25.5(3.5) | 26.3(3.5) | 25.9(3.4) | 25.8(3.6) |
| White (%) | 90.4 | 91.6 | 92.5 | 90.1 | 91.7 | 90.6 |
| Physical activity (MET/wk) | 28.3(33.5) | 43.7(44.2) | 54.6(51.4) | 26.9(35.8) | 36.6(38.8) | 48.0(48.5) |
| Family history of cancer (%) | 9.8 | 10.7 | 12.0 | 8.6 | 11.0 | 10.1 |
| Family history of cardiovascular disease (%) | 9.5 | 9.6 | 9.4 | 9.8 | 10.2 | 8.6 |
| Never smoker (%) | 50.8 | 57.7 | 62.6 | 50.4 | 55.6 | 61.1 |
| No of pack-years among ever smokers | 13.3(19.2) | 8.7(14.3) | 7.7(12.7) | 13.7(19.3) | 10.2(16.0) | 8.5(14.8) |
| Hypertension (%) | 33.0 | 29.6 | 28.7 | 31.1 | 31.0 | 32.1 |
| Hypercholesterolemia (%) | 45.5 | 42.5 | 41.6 | 45.1 | 43.5 | 41.0 |
| Total energy intake (kcal/day) | 1,797(558) | 2,198(610) | 2,484(646) | 1,607(513) | 2,046(562) | 2,490(643) |
| Alcohol intake (g/day) | 12.0(15.7) | 10.5(12.8) | 9.3(11.8) | 11.0(15.7) | 11.5(14.0) | 10.5(12.9) |
| Modified AHEI (Score)* | 32.3(8.0) | 37.0(8.0) | 40.3(8.7) | 34.8(8.9) | 34.4(8.1) | 35.8(8.0) |
Values are means (SD) or percentages. All variables except age are age-standardized.
Abbreviations: Modified AHEI, Modified Alternate Healthy Eating Index score; BMI, body mass index; METs: metabolic equivalent tasks; FV: fruits and vegetables.
Modified AHEI: AHEI-2010, but excluding criteria for intakes of fruits and vegetables and alcohol.
METs are defined as the ratio of caloric needed per kilogram of body weight per hour of physical activity divided by the caloric needed per kilogram of body weight at rest.
In age-adjusted models, intakes of high- and low-pesticide-residue FVs were inversely associated with all-cause and cause-specific mortality (Table 2). In multivariable-adjusted analyses, however, intake of high-pesticide-residue FVs was unrelated to mortality whereas the inverse association for low-pesticide residue FV intake persisted (Table 2). Most of the change between the age-adjusted and the multivariate-adjusted models was a result of adjustment for physical activity. Relative to individuals consuming <1 serving of high-pesticide-residue FVs/day, the pooled multivariable-adjusted HRs (95% CI) for total mortality were 0.95 (0.92–0.99) for individuals consuming 1–1.9 servings/day, 0.97(0.92–1.02) for individuals consuming 2–3 servings/day, 1.01 (0.93–1.11) for individuals consuming 3–4 servings/day, and 0.93 (0.81–1.07) for individuals consuming ≥ 4 servings/day (P, trend = 0.84). The corresponding estimates for intake of low-pesticide-residue were: 1 (Reference), 0.81 (0.77–0.84), 0.72 (0.68–0.75), 0.65 (0.62–0.69), and 0.64 (0.59–0.68) (P for trend ≤ 0.001). This pattern was similar in analyses of cause-specific mortality (Table 2), when intake was modeled as quintiles (Table S4), in cohort-specific analyses (Table S5), when only deaths before age 70 years were considered (Table S6), and when only the most recent diet assessment was considered (Table S7). Interestingly, although intake of low-pesticide residue FV was inversely related to mortality from causes other than CVD, cancer and respiratory diseases, combined (Table 2), mortality from neurological diseases was unrealated to intake of intake of either low- or high-pesticide residue FVs.
Table 2.
Pooled hazard ratios (95% CI) of total mortality, CVD mortality, cancer mortality, respiratory mortality, and other causes of death according to absolute intakes of high- and low-pesticide-residue fruits and vegetables in the NHS, NHSII, and HPFS.
| High-Pesticide-Residue Fruit and Vegetable Intake (servings/day) |
P, trend | |||||
|---|---|---|---|---|---|---|
| <1 | ≥1–<2 | ≥2–< 3 | ≥3–<4 | ≥4 | ||
|
| ||||||
| Total mortality (n = 27,026) | ||||||
| Person-years | 1,228,473 | 1,280,067 | 426,074 | 102,468 | 44,278 | |
| Cases | 12,133 | 10,777 | 3,185 | 696 | 235 | |
| Age-adjusted | 1 (Ref.) | 0.70 (0.68, 0.72) | 0.62(0.60, 0.65) | 0.63(0.59, 0.69) | 0.59(0.52, 0.68) | <0.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.95 (0.92, 0.99) | 0.97(0.92, 1.02) | 1.01(0.93, 1.11) | 0.93(0.81, 1.07) | 0.84 |
| CVD mortality (n = 4,318) | ||||||
| Cases | 1,861 | 1,764 | 539 | 113 | 41 | |
| Age-adjusted1 | 1 (Ref.) | 0.74(0.70, 0.80) | 0.64(0.58, 0.71) | 0.61(0.50, 0.75) | 0.56(0.41, 0.78) | <0.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 1.03 (0.95, 1.12) | 1.00 (0.88, 1.14) | 1.03(0.82, 1.28) | 0.95(0.67, 1.34) | 0.76 |
| Cancer mortality (n = 6,426) | ||||||
| Cases | 2,722 | 2,583 | 854 | 198 | 69 | |
| Age-adjusted | 1 (Ref.) | 0.76(0.72, 0.81) | 0.72(0.67, 0.78) | 0.72(0.62, 0.83) | 0.67(0.53, 0.85) | <0.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.93(0.87, 1.00) | 1.01(0.92, 1.12) | 1.02(0.86, 1.20) | 0.93(0.72, 1.20) | 0.95 |
| Respiratory mortality (n = 2,012)4 | ||||||
| Cases | 1,009 | 730 | 212 | 48 | 13 | |
| Age-adjusted | 1 (Ref.) | 0.56 (0.51, 0.61) | 0.47 (0.40, 0.55) | 0.51(0.38, 0.68) | 0.38(0.22, 0.66) | <0.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.85 (0.75, 0.96) | 0.90 (0.74, 1.09) | 1.05(0.76, 1.47) | 0.66(0.36, 1.19) | 0.41 |
| Other causes of death (n = 14,222) 5 | ||||||
| Cases | 6,516 | 5,682 | 1,575 | 337 | 112 | |
| Age-adjusted | 1 (Ref.) | 0.69(0.66, 0.71) | 0.59(0.56, 0.63) | 0.63(0.56, 0.70) | 0.63(0.52, 0.76) | <0.0001 |
| Multivariable-adjusted1,2 | 1 (Ref.) | 0.96(0.92, 1.00) | 0.94(0.88, 1.02) | 1.10(0.97, 1.25) | 1.17(1.09, 1.25) | 0.78 |
| Low-Pesticide-Residue Fruit and Vegetable Intake (servings/day) | P, trend | |||||
| <1 | ≥1–<2 | ≥2–< 3 | ≥3– <4 | ≥4 | ||
|
| ||||||
| Total mortality (n = 27,026) | ||||||
| Person-years | 398,732 | 956,163 | 950,927 | 501,456 | 274,083 | |
| Cases | 3,808 | 8,324 | 8,230 | 4,296 | 2,368 | |
| Age-adjusted | 1 (Ref.) | 0.67 (0.65, 0.70) | 0.54 (0.52, 0.56) | 0.48 (0.46, 0.50) | 0.47 (0.44, 0.49) | <0.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.81 (0.77, 0.84) | 0.72 (0.68, 0.75) | 0.65 (0.62, 0.69) | 0.64 (0.59, 0.68) | <0.0001 |
| CVD mortality (n = 4,318) | ||||||
| Cases | 611 | 1,279 | 1,258 | 725 | 445 | |
| Age-adjusted | 1 (Ref.) | 0.66 (0.60, 0.73) | 0.51 (0.46, 0.57) | 0.48 (0.43, 0.54) | 0.49 (0.43, 0.56) | <0.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.77 (0.69, 0.86) | 0.65 (0.57, 0.73) | 0.64 (0.55, 0.74) | 0.70 (0.58, 0.83) | <0.0001 |
| Cancer mortality (n = 6,426) | ||||||
| Cases | 762 | 1,989 | 2,053 | 1,071 | 551 | |
| Age-adjusted | 1 (Ref.) | 0.82(0.76, 0.90) | 0.72(0.66, 0.78) | 0.65(0.59, 0.72) | 0.60(0.54, 0.67) | <0.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.95(0.86, 1.04) | 0.91(0.82, 1.01) | 0.87(0.77, 0.98) | 0.81(0.69, 0.94) | <0.01 |
| Respiratory mortality (n = 2,012) 4 | ||||||
| Cases | 317 | 640 | 589 | 306 | 160 | |
| Age-adjusted | 1 (Ref.) | 0.62 (0.54, 0.71) | 0.46 (0.40, 0.53) | 0.39 (0.33, 0.46) | 0.36 (0.29, 0.44) | <0.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.80 (0.69, 0.93) | 0.71 (0.59, 0.84) | 0.65 (0.52, 0.80) | 0.62 (0.47, 0.82) | <0.0001 |
| Other causes of death (n = 14,222) 5 | ||||||
| Cases | 2,107 | 4,396 | 4,319 | 2,190 | 1,210 | |
| Age-adjusted | 1 (Ref.) | 0.63(0.60, 0.67) | 0.50(0.47, 0.52) | 0.43(0.40, 0.45) | 0.43(0.40, 0.46) | <0.0001 |
| Multivariable-adjusted1,3 | 1 (Ref.) | 0.77(0.73, 0.81) | 0.67(0.62, 0.72) | 0.60(0.55, 0.65) | 0.56(0.51, 0.63) | <0.0001 |
Abbreviations: NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; HPFS, Health Professionals Follow-up Study.
Adjusted for age, body mass index (quintiles), ethnicity (white/non-white), physical activity (quintiles), family history of cancer (yes/no), family history of cardiovascular disease (yes/no), smoking in package-years (never smoker, 1–4.9, 5–19.9, 20–39.9, or ≥ 40), postmenopausal hormone use (premenopausal/never/past/current, in NHS and NHSII), baseline hypertension (yes/no), baseline hypercholesterolemia (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, or ≥ 30 g/day), and Alternate Healthy Eating Index score excluding criteria for intake of fruits and vegetables and alcohol (quintiles).
Additionally adjusted for intakes of low-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues (quintile).
Additionally adjusted for intakes of high-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues (quintile).
Respiratory mortality only included NHS and HPFS.
Other causes of death included diabetes, neurological disease, kidney diseases, injury or poison, suicide, and all other causes.
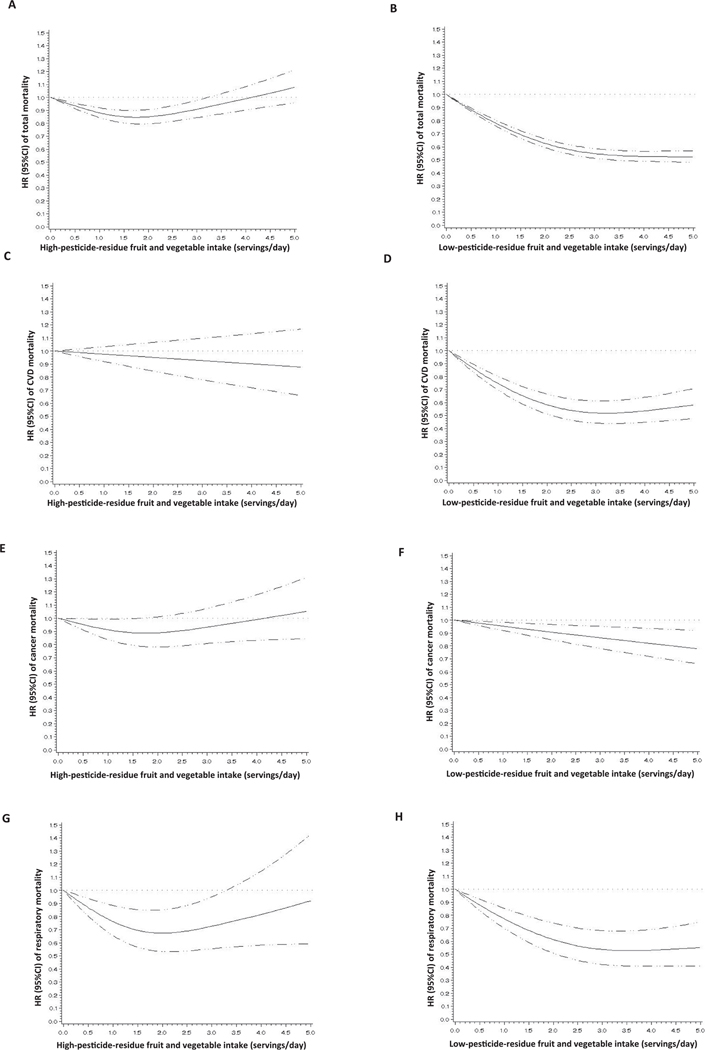
To gain further insights into the shape of the dose–response relation we then modeled intake of FVs as a continuous variable, allowing for non-linear relations (Fig. 1). In these models, the inverse relation of low-pesticide residue FV with all-cause (Fig. 1B), CVD (Fig. 1D), and respiratory mortality (Fig. 1H) plateaued at approximately a 50% reduction in mortality with an intake of 3.5 servings/day, whereas for cancer mortality the benefits were more modest but did not plateau within the observed range of intake (Fig. 1F). The picture was more complex for intake of high-pesticide-residue FV intake. For all-cause mortality, there was a statistically significant non-linear relation where intake of high-pesticide-residue FVs was related to lower mortality rates at modest intake levels, reaching a nadir at intakes of 1.5 servings/day, but without evidence of benefit at higher intake levels (Fig. 1A). This pattern was similar to that observed for cancer mortality (Fig. 1E), and respiratory mortality (Fig. 1G). On the other hand, intake of high-pesticide-residue FVs was completely unrelated to CVD mortality within the observed range of intake (Fig. 1C).
Fig. 1.
Fruit and vegetable intake, considering pesticide residue status, and total mortality (panels A and B), CVD mortality (panels C and D), cancer mortality (panels E and F), and respiratory mortality (panels G and H). Adjusted for age, body mass index (quintiles), ethnicity (white/non-white), physical activity (quintiles), family history of cancer (yes/no), family history of cardiovascular disease (yes/no), smoking in package-years (never smoker, 1–4.9, 5–19.9, 20–39.9, or ≥ 40), postmenopausal hormone use (premenopausal/never/past/current, in NHS and NHSII), baseline hypertension (yes/no), baseline hypercholesterolemia (yes/no), total energy intake (quintiles), alcohol intake (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, or ≥ 30 g/day), and Alternate Healthy Eating Index score excluding criteria for intake of fruits and vegetables and alcohol (quintiles). Panels A,C,E,G are additionally adjusted for intakes of low-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues(servings/day). Panels B,D,F,H are additionally adjusted for intakes of high-pesticide-residue fruits and vegetables (servings/day) and other fruits and vegetables with undetermined residues(servings/day).
We then estimated the effect of substituting high-pesticide-residue FVs with low-pesticide-residue FVs (Figure S1). Consuming one daily serving of low-pesticide-residue FVs instead of a serving of high-pesticide residue FVs was associated with 11% lower risk of all-cause mortality (HR: 0.89; 95 %CI: 0.86–0.93). A similar pattern was observed in analyses for disease-specific mortality.
4. Discussion
We evaluated the association of high- and low-pesticide-residue FV intake with all-cause and cause-specific mortality in three large U.S. cohorts. We observed that intakes of FVs with low-pesticide-residue content were associated with lower risk of total mortality and cause-specific mortality. Specifically, consumptions of ≥ 4 servings/day of low-pesticide-residue FVs were linked to 36% lower risk of total mortality relative to consuming < 1 serving/day. Conversely, intake of highpesticide-residue FVs was unrelated to mortality, with some differences for cancer, CVD and respiratory disease mortality. On aggregate, these findings suggest that exposure to pesticide residues through diet may offset some of the well documented benefits of FV consumption on mortality, particularly for CVD mortality.
A recent meta-analysis found that intakes of FVs up to 800 g/day (10 servings/day) were associated with decreased risk of all-cause mortality. (Aune et al., 2017,2017) Another previous meta-analysis investigated the same relationship, reporting higher FV consumption reduced total mortality risk up to around 5 servings/day. (Wang et al., 2014) An inverse relationship was found for cardiovascular mortality whereas no association was reported for cancer mortality. Regarding respiratory disease, although we did not have a prior hypothesis regarding exposure to pesticides through diet in relation to this outcome, a meta-analysis showed FV intake protects against respiratory diseases, such as asthma. (Hosseini et al., 2017) An inverse relationship was also observed between dietary patterns with high plant-based foods and chronic respiratory mortality. (Neelakantan et al., 2018) Moreover, others have shown that diets favoring intake of fruits and vegetables are related to a lower risk of chronic obstructive pulmonary disease. (Varraso et al., 2015; Tabak et al., 2001; Varraso et al., 2010) The 2015 USDA Dietary Guidelines for Americans recommended intake of 4.5 servings/day from fruits and vegetables for adults consuming 2,000 calories/day. (U.s., December 2015) Our results for low-pesticide-residue FV intake suggest a benefit for total and disease specific mortality and are in line with these findings and recommendations.
Despite the consistency between our findings for low-pesticide-residue FVs with the existing literature, the divergent association of high-pesticide-residue FVs with mortality begs for an explanation. Confounding should be considered as a first potential explanation. However, not only were intakes of high- and low-pesticide residues positively related to each other in this study population but also each of these were associated with generally more healthy behaviors including healthier diets, greater engagement in physical activity and less smoking. Another potential explanation is that the nutritional profile of high-and low-pesticide-residue FVs is sufficiently different to result in different relations with mortality. This, however, proved not to be the case in this study population (Table S3). While we did not have direct biomarker measures of exposure to pesticides, we have previously shown that the classification method used in this study is related to biomarkers of exposure to pesticides in a clinical sample from Boston as well as in samples representative of the general population of the U.S. (Hu et al., 2016; Chiu et al., 2018) Based on these considerations, our interpretation of these findings is that the diverging relations of high-and low-pesticide-residue FV intake with mortality are attributable to pesticide contamination of these foods.
To our knowledge, no previous epidemiological study has directly examined the association between exposure to pesticide residues through diet and mortality. There are some studies; however, that have assessed associations with risk of major chronic diseases. Organic foods are less likely to have pesticide residues than conventionally grown foods due to the prohibition of synthetic pesticides by organic food standards. Moreover, interventions switching individuals from conventional to organic diets result in a dramatic decline in urinary pesticide metabolites. (Hyland et al., 2019; Baudry et al., 2019; Lu et al., 2006; Bradman et al., 2015; Oates et al., 2014) In the Nutri-Net Santé study, increasing consumption of organic foods was related to 25% lower cancer risk, driven by inverse relations with post-menopausal breast cancer and Non-Hodgkin lymphoma (NHL). (Baudry et al., 2018) However, in the Million Women Study, organic food intake was unrelated to cancer risk, although it was inversely related to NHL risk. (Bradbury et al., 2014) Using the same method for classifying FVs in the U.S. food supply according to their pesticide residues, we have previously reported no association between intake of low- or high-pesticide residue fruits and vegetables and cancer incidence, (Sandoval-Insausti et al., 2021) raising the question of whether exposure to pesticide residues through diet may be related to disease progression, rather than incidence. This scenario would be consistent with our observations of no elevated risk of cancer but increased cancer mortality, is consistent with previous findings on the relation between diet and incidence of common malignancies, (Chavarro et al., 2008) and should be evaluated in future studies. Also of relevance to our finding of a divergent association pattern for low and high-pesticide residue intake with mortality, we previously reported an inverse association of low-pesticide-residue FV intake with coronary heart disease but no relation between high-pesticide-residue FV intake with this outcome, (Chiu et al., 2019) mirroring our findings for CVD mortality. We have also reported the same pattern of association for different reproductive outcomes, including successful infertility treatment and markers of spermatogenesis (Chiu et al., 2018; Chiu et al., 2015; Chiu et al., 2016).
The mechanisms underlying these findings have not been reported yet in humans. Concerning animal research, (Gibert and Sargis, 2020) long-term gestational and lactational exposure to six pesticides (Cyromazine, MCPB, Pirimicarb, Quinoclamine, Thiram, and Ziram) was related to metabolic disorders in rats. (Svingen et al., 2018) Likewise, chronic ingestion of six pesticides (boscalid, captan, chlorpyrifos, thiofenate, thiaclopid, and ziram) was associated with metabolic disruption in mice. (Lukowicz et al., 2018) Long-term exposure to eleven pesticides, including pesticides found on food samples, was linked to body weight gain and hepatotoxic parameters in rats. (Docea et al., 2018) In vitro, the incubation for 48 h of McA rat hepatoma cells with chlorpyrifos promoted hepatic steatosis, by increasing lipid accumulation and decreasing triglycerides secretion. (Howell et al., 2016) Nevertheless, no previous animal or in vitro study has tested the effects of dietary pesticide exposure on mortality.
Our study has notable strengths. This is the first epidemiological study that we know of to assess associations between dietary pesticide exposure and mortality. Diet was measured repeatedly with a validated tool during 20 years of follow-up, (Rimm et al., 1992; Yuan et al., 2018; Yuan et al., 2017) and cumulative average intakes were calculated to mitigate within-subject variation. Furthermore, we had a large sample size and number of deaths, providing enough statistical power and allowed to examine different causes of mortality. Some limitations should be noted. First, dietary pesticide exposure was not quantified directly in each participant but obtained by matching self-reported FV intake from FFQ with pesticide residue surveillance data. Second, we could not take into account whether FVs were conventionally grown or organic. Third, it was not possible to match some FVs in the FFQ with the corresponding item in the pesticide surveillance data, particularly in the early years of follow-up when the PDP had yet to collect information on most produce available in the U.S. This could lead to certain misclassification and lack of specificity of pesticide exposure. However, we created a category of undetermined-pesticide-residue FVs we adjusted for in multivariable analyses. Fourth, the PRBS prioritizes the assesment of overall food contamination with pesticides rather than contamination with specific compounds. This may result in observed associations that are weaker than true associations, especially if associations are driven by only one or a small number of specific pesticides and contamination with the causally-relevant pesticide is only weakly related to overall contamination. Although we cannot rule out this possibility, we do know that the overall contamination score correlates well with markers of exposure to organophosphates, organochlorides and 2,4-D. Additional limitations related to the PRBS include the fact that it does not take into consideration cooking methods, which could affect exposure to pesticide residues, and that if focuses exclusively on intake of fruits and vegetables, which are the most important –but not the only– source of pesticide residues in the diet. Fifth, as in most nutritional epidemiological studies, diet was self-reported and measurement error cannot be dismissed. Sixth, some residual confounding cannot be ruled out. However, results remained unchanged after several sensitivity analyses and after adjusting for a large number of potential confounders, including variables associated with the exposure or the outcome in the literature. (Ding et al., 2019)
In conclusion, low-pesticide-residue FV intake was inversely related to mortality whereas high-pesticide-residue FV intake was unrelated to mortality, suggesting pesticide residues may modify the beneficial effect of FV intake on mortality. Nevertheless, given the paucity of data on this topic, more studies should be performed in order to provide further evidence on the long-term health effects of pesticide exposure through diet. Additional evaluation in independent studies is particularly important for respiratory disease mortality and for mortality due to causes other than CVD, cancer or respiratory diseases, since the priors for these two broad disease categories were significantly weaker than the priors regarding CVD and cancer mortality.
Supplementary Material
Acknowledgement
We would like to thank the participants and staff of the NHS, NHSII, and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
5. Funding information
Supported by grants U01 HL145386, R24 ES028521, UM1 CA186107, P01 CA87969, U01 CA176726, R01 HL034594, R01 HL088521, and U01 CA 167552 from the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.107024.
References
- Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Advances in nutrition. 2016;7(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TA, Yanovski SZ, 2014. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Journal of the American College of Cardiology 63 (25), 2960–2984. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Tornero-Velez R, Tulve NS, 2014. EPA’s SHEDS-multimedia model: children’s cumulative pyrethroid exposure estimates and evaluation against NHANES biomarker data. Environment international. 73, 304–311. [DOI] [PubMed] [Google Scholar]
- Yu Y, Li C, Zhang X, Zhang X, Pang Y, Zhang S, Fu J, 2012. Route-specific daily uptake of organochlorine pesticides in food, dust, and air by Shanghai residents. China. Environment international. 50, 31–37. [DOI] [PubMed] [Google Scholar]
- Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C, 2013. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 52, 91–96. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA, 2008. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environmental health perspectives. 116 (4), 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA AMS. U.S. Department of Agriculture, Pesticide Data Program (PDP), annual summary 2018. Available from: www.ams.usda.gov/datasets/pdp.
- Suratman S, Edwards JW, Babina K, 2015. Organophosphate pesticides exposure among farmworkers: pathways and risk of adverse health effects. Reviews on environmental health. 30 (1), 65–79. [DOI] [PubMed] [Google Scholar]
- Jones KC, de Voogt P, 1999. Persistent organic pollutants (POPs): state of the science. Environmental pollution. 100 (1–3), 209–221. [DOI] [PubMed] [Google Scholar]
- Lovecka P, Thimova M, Grznarova P, Lipov J, Knejzlik Z, Stiborova H, Nindhia TGT, Demnerova K, Ruml T, 2015. Study of Cytotoxic Effects of Benzonitrile Pesticides. BioMed research international. 2015, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouch A, Zaborska A, Pazdro K, 2018. The history of hexachlorobenzene accumulation in Svalbard fjords. Environmental monitoring and assessment. 190 (6), 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans. IARC; Lyon, France: 1991. Occupational exposures in insecticide applications, and some pesticides. [Google Scholar]
- Chiu Y-H, Sandoval-Insausti H, Ley SH, Bhupathiraju SN, Hauser R, Rimm EB, Manson JE, Sun Q.i., Chavarro JE, 2019. Association between intake of fruits and vegetables by pesticide residue status and coronary heart disease risk. Environment international. 132, 105113. 10.1016/j.envint.2019.105113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-H, Williams PL, Gillman MW, Gaskins AJ, Mínguez-Alarcón L, Souter I, Toth TL, Ford JB, Hauser R, Chavarro JE, 2018. Association Between Pesticide Residue Intake From Consumption of Fruits and Vegetables and Pregnancy Outcomes Among Women Undergoing Infertility Treatment With Assisted Reproductive Technology. JAMA internal medicine. 178 (1), 17. 10.1001/jamainternmed.2017.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, et al. , 2015. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Human reproduction. 30 (6), 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry J, Assmann KE, Touvier M, Allés B, Seconda L, Latino-Martel P, Ezzedine K, Galan P, Hercberg S, Lairon D, Kesse-Guyot E, 2018. Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Sante Prospective Cohort Study. JAMA internal medicine. 178 (12), 1597. 10.1001/jamainternmed.2018.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Insausti H, Chiu Y-H, Lee DH, Wang S, Hart JE, Mínguez-Alarcón L, Laden F, Ardisson Korat AV, Birmann B, Heather Eliassen A, Willett WC, Chavarro JE, 2021. Intake of fruits and vegetables by pesticide residue status in relation to cancer risk. Environment international. 156, 106744. 10.1016/j.envint.2021.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE, 2016. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. American journal of public health. 106 (9), 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC, 1992. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 135 (10), 1114–1126 discussion 27–36. [DOI] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. , 2018. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol. 187 (5), 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. , 2017. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 185 (7), 570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chiu Y-H, Hauser R, Chavarro J, Sun Qi., 2016. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: A validation study. Environment international. 92–93, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-H, Williams PL, Mínguez-Alarcón L, Gillman M, Sun Q.i., Ospina M, Calafat AM, Hauser R, Chavarro JE, 2018. Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide biomarkers. Journal of exposure science & environmental epidemiology. 28 (1), 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ams, USDA AMS. U.S. Department of Agriculture PDPP, annual summary 2018. Available from: www.ams.usda.gov/datasets/pdp.
- McCullough ML, Willett WC, 2006. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public health nutrition. 9 (1a), 152–157. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. , 2012. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 142 (6), 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Corsano KA, Stampfer MJ, 1994. Test of the National Death Index and Equifax Nationwide Death Search. American journal of epidemiology. 140 (11), 1016–1019. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Chernyavskiy P, Anderson WF, Best AF, Haozous EA, Hartge P, Rosenberg PS, Thomas D, Freedman ND, de Gonzalez AB, 2017. Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet. 389 (10073), 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. International journal of epidemiology. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB, 2014. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Bmj. 349 (jul29 3), g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini B, Berthon BS, Wark P, Wood LG, 2017. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients. 9 (4), 341. 10.3390/nu9040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan N, Koh WP, Yuan JM, van Dam RM, 2018. Diet-Quality Indexes Are Associated with a Lower Risk of Cardiovascular, Respiratory, and All-Cause Mortality among Chinese Adults. The Journal of nutrition. 148 (8), 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA, 2015. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. Bmj. 350 (feb03 7), h286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Heederik D, Ocke MC, Kromhout D, 2001. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 31 (5), 747–755. [DOI] [PubMed] [Google Scholar]
- Varraso R, Willett WC, Camargo CA Jr., 2010. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. American journal of epidemiology. 171 (7), 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/..
- Hyland C, Bradman A, Gerona R, Patton S, Zakharevich I, Gunier RB, Klein K, 2019. Organic diet intervention significantly reduces urinary pesticide levels in U.S. children and adults. Environmental research. 171, 568–575. [DOI] [PubMed] [Google Scholar]
- Baudry J, Debrauwer L, Durand G, Limon G, Delcambre A, Vidal R, et al. , 2019. Urinary pesticide concentrations in French adults with low and high organic food consumption: results from the general population-based NutriNet-Sante. Journal of exposure science & environmental epidemiology. 29 (3), 366–378. [DOI] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R, 2006. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environmental health perspectives. 114 (2), 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Quirós-Alcalá L, Castorina R, Schall RA, Camacho J, Holland NT, Barr DB, Eskenazi B, 2015. Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environmental health perspectives. 123 (10), 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates L, Cohen M, Braun L, Schembri A, Taskova R, 2014. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environmental research. 132, 105–111. [DOI] [PubMed] [Google Scholar]
- Bradbury KE, Balkwill A, Spencer EA, Roddam AW, Reeves GK, Green J, Key TJ, Beral V, Pirie K, 2014. Organic food consumption and the incidence of cancer in a large prospective study of women in the United Kingdom. British journal of cancer. 110 (9), 2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J, 2008. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. The American journal of clinical nutrition. 88 (5), 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Levine H, et al. , 2016. Intake of Fruits and Vegetables with Low-to-Moderate Pesticide Residues Is Positively Associated with Semen-Quality Parameters among Young Healthy Men. The Journal of nutrition. 146 (5), 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert Y NA, Sargis R,. Endocrine disrupters and Metabolism: Luasanne: Frontiers Media SA; 2020. [DOI] [PMC free article] [PubMed]
- Svingen T, Ramhøj L, Mandrup K, Christiansen S, Axelstad M, Vinggaard AM, Hass U, 2018. Effects on metabolic parameters in young rats born with low birth weight after exposure to a mixture of pesticides. Scientific reports. 8 (1) 10.1038/s41598-017-18626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowicz C, Ellero-Simatos S, Régnier M, Polizzi A, Lasserre F, Montagner A, Lippi Y, Jamin EL, Martin J-F, Naylies C, Canlet C, Debrauwer L, Bertrand-Michel J, Al Saati T, Théodorou V, Loiseau N, Mselli-Lakhal L, Guillou H, Gamet-Payrastre L, 2018. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environmental health perspectives. 126 (6), 067007. 10.1289/EHP2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docea AO, Gofita E, Goumenou M, Calina D, Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P, Taitzoglou I, Zlatian O, Rakitskii VN, Hernandez AF, Kouretas D, Tsatsakis A, 2018. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 115, 470–481. [DOI] [PubMed] [Google Scholar]
- Howell GE 3rd, Mulligan C, Young D, Kondakala S, 2016. Exposure to chlorpyrifos increases neutral lipid accumulation with accompanying increased de novo lipogenesis and decreased triglyceride secretion in McArdle-RH7777 hepatoma cells. Toxicology in vitro : an international journal published in association with BIBRA. 32, 181–189. [DOI] [PubMed] [Google Scholar]
- Ding M, Li J, Qi L, Ellervik C, Zhang X, Manson JE, et al. , 2019. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. Bmj. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.