Abstract
Nicotinic receptors of the cholinergic system are ligand-gated ion channels, responding to the excitatory neurotransmitter, acetylcholine, and the addictive component of tobacco, nicotine. They help to transduce salient information in the environment by activating specific neural circuitry in normal and disease states. While nicotinic receptors are promising neurological and neuropsychiatric disorder targets, they have fallen out of favor after several late-stage clinical failures. Targeting the complex of the nicotinic receptor, including lynx1 accessory proteins, could be the key to unlocking the intractable nAChR for therapeutic development. Lynx1 binds to the extracellular face of the nAChR and acts as a critical modulator, suppressing memory, learning, and plasticity. Lynx1 removal in animal models leads to memory and plasticity enhancements, some of which have therapeutic relevance for neuropsychiatric and neurological disease. A review of the lynx1 accessory modulator and its role in modulating neuronal nAChRs will be discussed.
The promise and challenge of targeting nicotinic receptors
The cholinergic system is an excitatory neurotransmitter system involved in the modulation of circuits associated with a range of complex functions and there has been considerable interest in developing cholinergic drugs for a range of neurological and neuropsychiatric indications [1•]. Three of the four major therapeutics prescribed to Alzheimer’s patients target the cholinergic system [2]. Raised acetylcholine levels in the brain can be achieved by inhibiting the enzyme acetylcholinesterase, which breaks down acetylcholine. Clinical improvements associated with this treatment, however, are limited [3]. This is due partly to loss of efficacy due to tachyphylaxis by agonist-based compensatory mechanisms such as receptor desensitization, downregulation, etc. Several therapeutic programs to develop agents specifically targeting nicotinic receptors have been actively pursued, with a number of pharmacological classes (e.g. agonists, partial agonists, allosteric modulators, inverse agonists and blockers), of multiple nicotinic receptor subtypes for multiple indications [1•,4]. After much effort, however, clinical failures have blunted enthusiasm for targeting nicotinic receptors, with only a couple of successful approvals. The reasons are manifold: nAChRs are part of a large family of highly related genes (α1–10, β2–4) which exist in a number of combinations of heteropentameric or homopentameric subtypes. This makes the select targeting of individual subtypes difficult and prone to off-target effects. The most abundant subtypes, α4β2 and α7 nAChRs, are distributed widely throughout the CNS, leading to on-target side effects from actions outside the intended brain regions [1•]. Some of the biophysical properties of nAChRs also present a challenge to therapeutic development: desensitization after channel opening is a feature of most nAChR subtypes [1•]. An agonist-based therapeutic targeting orthosteric binding will be working against these factors.
Complexes of nAChRs with lynx1 – an allosteric approach
Inclusion of accessory molecules within the nAChR complex could circumvent some of these issues because it more faithfully recapitulates the nAChR as they exist in the brain. Lynx1 is part of a large gene family, the Ly6/uPAR/neurotoxin superfamily, that codes for proteins exhibiting a highly successful receptor binding fold, the three-finger toxin fold, many of which bind tightly to nAChRs and modulate their function (Refs. [5–7], reviewed in Refs. [8,9•]; and Ref. [10]). Lynx1 has been shown to bind tightly to nAChRs in vitro [11] in vivo [12•], and to the invertebrate nAChR homolog, AChBP, at nM affinity [13]. As the most well-characterized member of the toxin-like prototoxins, lynx1 is emerging as a significant factor in complex and disease brain states, and will be the focus of this review.
Lynx1 exerts allosteric effects by promoting receptor desensitization and slowing recovery from desensitization of α4β2 nAChRs [11]. Removal of lynx1 leads to nondesensitizing and hypersensitive nAChRs so presumably a lynx1 inhibitor would be less subject to tachyphylaxis.
There is a 10-fold higher EC50 between brain nicotinic responses [14] versus heterologously expressed nAChR ones in cells without lynx1, whereas addition of lynx1 in heterologous systems resembles brain nAChR function [11]. Cell-based screening assays including the entire receptor complex are likely to yield better leads, as compared to screening nAChRs alone, with potentially few drop-outs during pre-clinical development.
Functional modulation of circuits by lynx1
The ability of lynx1 to influence circuit function has implications for the cognitive aspects of neurological and neurodevelopmental diseases [15•,16••]. Removal of the lynx1 brake leads to enhanced cognitive effects, such as augmented associative learning ability [14], and reopening of the critical period of plasticity in the visual cortex [17–19], although subcortical effects have also been reported [12•,16••,20]. The cell type-specific expression of lynx1 [21], for instance, PV-positive GABAergic neurons in the visual cortex [17], and 5HT-3 positive neurons in the auditory cortex [22••], influences the relative weighting of the nAChR response to acetylcholine to emphasize some nodes in a circuit among others. The inhibitory influence of lynx1 within circuits may amplify gain modulation, which is associated with cortical nicotinic activation [23], and even modify the plasticity window of experience-based circuit modification, which has implications for brain repair [17,22••,24].
Lynx1 influences on receptor stoichiometry, specificity and number
The influences of lynx1 on nAChRs are long-term and numerous. In addition to functional effects on gating, lynx1 can also influence receptor stoichiometry. At a single channel level, the co-expression of lynx1 on α4β2 results in an increase in the larger amplitude, faster inactivating openings of agonist-induced responses, associated with the low sensitivity, LS, (α4)3(β2)2 stoichiometry [11]. Lynx1 has been shown to interact in the endoplasmic reticulum (ER) in cells only expressing α4 nAChRs, or at α4:α4 dimers, an interface which exists in only the LS, (α4)3(β2)2 stoichiometry [25]. Removal of the GPI-anchor in this latter case did not influence nAChR responses suggesting that some of the effects occur during receptor maturation [25]. Such long-term interactions can influence a number of features of nAChR biology, including maturation, receptor number and stoichiometry. Gating effects of lynx1 can be revealed more clearly if the stoichiometry can be fixed to a single population, which has been accomplished by concatemerization, synthesizing the entire pentamer through fusion of nAChR subunits into a single polypeptide.
Single channel studies using concatemeric α3β4 nAChRs indicate nAChRs are more often in the closed state when cells are co-transfected with lynx1 [26]. There are different effects depending on which cDNA concatemer, representing the different stoichiometry of the receptor, was used. When coexpressed with lynx1, there was a reduction in the number of receptors of the (α3)2(β4)3–nAChR stoichiometry [26]. For the (α3)3(β4)-nAChR stoichiometry, on the other hand, the effects were largely functional, reducing unitary conductance, and enhancing closed dwell times. In heterologous expression systems, lynx1 has also been shown to influence α5α3β4 nAChRs [26] and α6β2 nAChRs [20]. In vivo, lynx1 has been shown through genetic studies to influence α6* nAChRs in the striatum, α6β2 nAChR in the colliculus [20], and β2* and α7 nAChRs [14].
Three dimensional structure of lynx1
The NMR solution structure of lynx1 has indicated that it exhibits a three-fingered fold common to α-neurotoxins [27]. The disulfide bridges between the cysteine residues stabilize the head of the protein, from which three beta-sheet rich loops emerge. The secondary structure of ws-lynx1 represents two antiparallel β-sheets, one consisting of two strands in loop I, and four strands going across loops I–III. The second part of loop III is relatively disordered. While the 3D-topology of elapid neurotoxins and lynx1 is evident, lynx1 and α-neurotoxins share distinct features as well. Within the Ly6/uPAR/toxin superfamily, structural data of toxin/nAChR complexes support interfacial binding for three-finger fold proteins, exemplified in ɑneurotoxins (Ref. [28], reviewed in Ref. [8]). Functional studies demonstrate binding of lynx1 in the receptor extracellular domain, globally similar to toxin binding, although mutagenesis studies with a synthetic, secreted, version of lynx1, ws-lynx1, indicate more subtle features which are distinct from those of neurotoxins [29].
The nAChRs twist: clues from molecular dynamics simulations
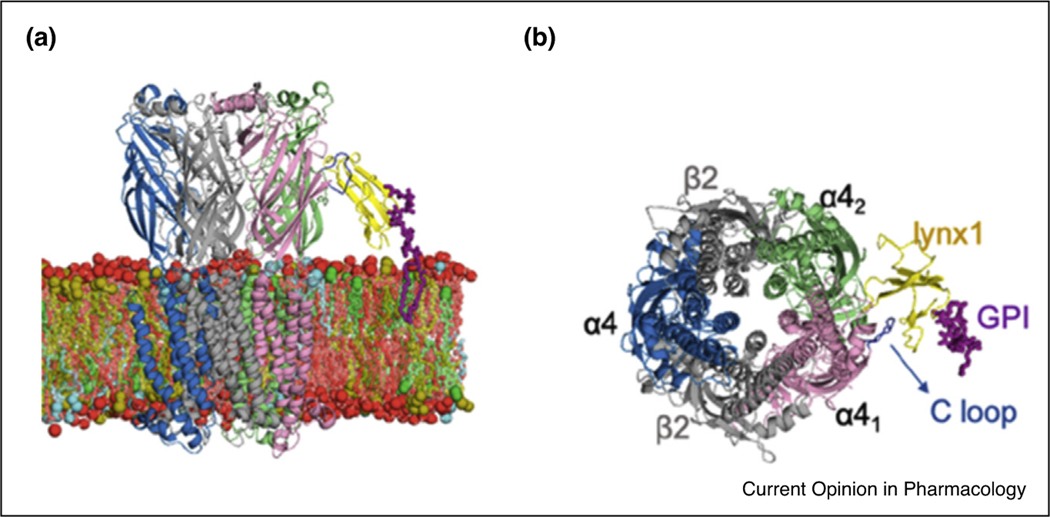
nAChRs transition through several functional states, open, closed and desensitized, etc. [30–32]. Global changes in the pentameric receptor are thought to involve a coordinated twist in the five subunits leading to an increase in the channel diameter, thus allowing for ion flow through this enlarged pore [30]. Molecular dynamics simulations of the GPI-anchored lynx1 bound to α4:α4 interfaces of α4β2 nAChRs embedded in the membrane (Figure 1) indicate prolonged interaction of lynx1 with the receptor C-loop [33••]. The C-loop is involved in the transition from the closed (agonist unbound) state to the open (agonist bound) state [30] by capping the agonist binding site. In molecular dynamics simulations with lynx1, the C-loop is restricted in either the open or bound state, as compared to the nAChR alone, which transitions freely between open, closed, and desensitized states [33••]. Unimpeded, the C-loop may not influence channel opening greatly, per se [34], but binding to an accessory protein could impede the twist. This supports a ‘lock and key’ notion for lynx1 function, and the idea that brain nAChR are restricted in these state transitions by lynx1 binding.
Figure 1.
Model of lynx1 and α4β2 nAChRs embedded in the membrane.
Lynx1 (yellow), GPI anchor of lynx1 (purple), α4 nAChR C loop (blue).
(a) Side view.
(b) En face view.
Reprinted with permission from Dong et al., J Phys Chem B 2020 May 21;124(20):4017–4025 Copyright 2020 American Chemical Society. Lynx1-based on 2L03, from Ref. [27].
Functional studies using soluble lynx1
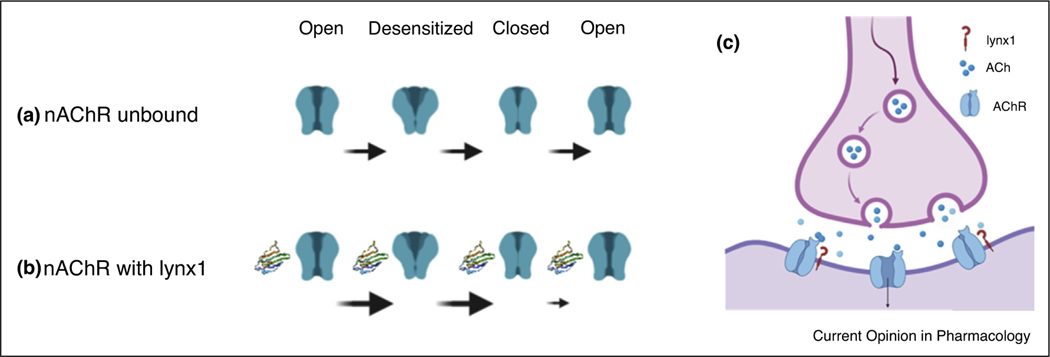
Our current understanding of lynx1 function is that it is a membrane-bound, GPI-linked protein. Variations in effects have been reported when lynx1 is co-expressed with nAChRs in its GPI-anchored form, as opposed to when applied externally to the nAChR-containing cell in a soluble form [11,27,29,35]. Studies of the secreted variant of lynx1, ws-lynx1, have been illuminating for isolating the gating capability of lynx1 independent of receptor stoichiometry. Removal of the GPI-attachment site allows for straightforward purification of lynx1, ws-lynx1 [27,35], with subtle differences in function as compared to the native GPI-anchored lynx1 [11,27,35,36]. The application of ws-lynx1 can influence peak currents of α4β2 [27,35] and α7 nAChRs in a dose-dependent manner [27,29]. It has been shown to inhibit proliferative activity in non-neuronal cells [37]. Ws-lynx1 has been shown to bind to multiple nAChRs from brain extracts, α3–7, β2,and β4 nAChR, subunits [38] as well as a bacterial homolog of the nAChR, GLIC [13] and mAChRs [27]. In vivo demonstration of native lynx1: nAChR complexes have only been shown as yet with β2*-nAChRs [12•], and α7 nAChRs [39••] so the specificity of lynx1 binding needs to be considered in the context of expression in the brain. A model that encompasses the experimental differences between ws-lynx1 versus native, GPI-lynx1, is that the tethering of the nAChR to the membrane slows the rate of transitions of the nAChR (Figure 2).
Figure 2.
Schematic of possible lynx1 action on transition states of nAChRs.
(a) nAChR (green) states.
(b) nAChR with lynx1 present (2L03, ribbon).
(c) Reduced cholinergic drive at synapses with lynx1-bound nAChR complexes.
Therapeutic potential of lynx1
There have been suggestions that lynx1 inhibition would raise cholinergic tone by releasing the molecular brake on nAChRs [40], indicating its potential as a therapeutic target. Titration of lynx1 dosage will be important, as lynx1 has a neuroprotective effect in aged brains and hence complete lynx1 inhibition would not be warranted as a long-term therapeutic solution [14,41]. A link between Alzheimer’s disease and lynx1 is emerging. Ws-lynx1 can influence synaptic plasticity in the hippocampus, a memory structure associated with early Alzheimer’s pathology [42•] and lynx expression [35]. Lynx1 is subject to transcriptional fluctuations [40] and demonstrates a modest (10%) downregulation in animal models of Alzheimer’s disease [38]. Alzheimer’s disease is associated with an increase in a toxic peptide, soluble β-amyloid (Aβ) [43], which has been shown to enter into neurons via nAChRs [38]. A demonstration of the potential utility of a lynx-based therapeutic, comes from studies on ws-lynx1 which have shown that ws-lynx1 can compete for binding of Aβ to nAChRs, and as such to lower Aβ toxicity [38], and abolish the negative effect of Aβ on synaptic plasticity [42•]. This is suggestive of anti-toxic activity of peptides or proteins derived from lynx-like proteins by binding to nAChRs.
Disruptions of lynx1 and nAChRs present a challenge since protein–protein interactions can be difficult to disrupt by a small molecule. The water-soluble variant of lynx1, ws-lynx1 has potential as a therapeutic because it has been shown to partially displace endogenous lynx1 for binding at nAChRs potentially leading to a partial loss-of-function of lynx1 [39••]. Further, it can breach the blood–brain barrier with intranasal delivery [39••]. Even more promising, synthetic circularized peptides derived from lynx1 and lynx1-like family members have been found to bind to α7 and the ectodomains of α9 nAChR subtypes [44••]. If it too can penetrate the blood–brain barrier, its relatively small size increases its potential as a biologic therapeutic. Other α7 nAChR-binding proteins have been shown to induce transfer of macromolecules across the blood–brain barrier and into cells through α7 nAChR mediated transport [45] or direct binding in the case of viruses [46], indicating a potential for the development of carrier transport peptides-based on nicotinic receptor binding protein sequences.
Lynx1 removal has been associated with schizophrenia [15•] and biological signatures in an autism model [16••], indicating an involvement in neurodevelopmental disorders. The emerging understanding of lynx1 in neurological, neuropsychiatric and neurodevelopmental disorders underscores the immense potential of lynx1 accessory proteins to revive nicotinic receptor therapeutic development.
Acknowledgements
This work was supported by N.I.H.DA043567 and GM123131–01, NSF BCS-1745823. Thanks to Dr. Wonpil Im for helpful discussions and use of co-model coordinate. Thanks to Kristin R. Anderson, Dr. Katie M. Hoffman, and Talulla Palumbo for helpful discussions, as well as Ruhshona Khusenova for editorial assistance.
Footnotes
Conflict of interest statement
The author declares that he is founder of Ophidion, Inc., a biotechnology company.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1. Bertrand D, Terry AV: The wonderland of neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 2018, 151:214–225 10.1016/j.bcp.2017.12.00829248596 • A comprehensive review of the promise and challenges for the development of nicotinic therapeutics.
- 2.Sharma K: Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol Med Rep 2019, 20:1479–1487 10.3892/mmr.2019.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmaleh DR et al. : Developing effective Alzheimer’s disease therapies: clinical experience and future directions. J Alzheimers Dis 2019, 71:715–732 10.3233/JAD190507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry AV, Callahan PM: Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob Res 2019, 21:383–394 10.1093/ntr/nty166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasilyeva NA, Loktyushov EV, Bychkov ML, Shenkarev ZO, Lyukmanova EN: Three-finger proteins from the Ly6/uPAR family: functional diversity within one structural motif. Biochemistry (MOSC) 2017, 82:1702–1715 10.1134/S0006297917130090 PMID: 29523067. [DOI] [PubMed] [Google Scholar]
- 6.Ono H, Sakamoto H, Yoshida T, Saeki N: Prostate stem cell antigen is expressed in normal and malignant human brain tissues. Oncol Lett 2018, 15:3081–3084 10.3892/ol.2017.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu M, Puddifoot CA, Taylor P, Joiner WJ: Mechanisms of inhibition and potentiation of α4β2 nicotinic acetylcholine receptors by members of the Ly6 protein family. J Biol Chem 2015, 290:24509–24518 10.1074/jbc.M115.647248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsetlin V: Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends Pharmacol Sci 2015, 36 10.1016/j.tips.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 9. Miwa JM, Anderson KR, Hoffman KM: Lynx prototoxins: roles of endogenous mammalian neurotoxin-like proteins in modulating nicotinic acetylcholine receptor function to influence complex biological processes. Front Pharmacol 2019, 10:343 10.3389/fphar.2019.0034331114495 • Comprehensive review of the family of lynx prototoxins expressed in the brain.
- 10.Anderson KR, Hoffman KM, Miwa JM: Modulation of cholinergic activity through lynx prototoxins: implications for cognition and anxiety regulation. Neuropharmacology 2020, 174:108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM et al. : Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron 2002, 33:893–903 10.1016/S0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 12. Nissen NI, Anderson KR, Wang H, Lee HS, Garrison C, Eichelberger S, Ackerman K, Im W, Miwa JM: Augmenting the antinociceptive effects of nicotinic acetylcholine receptor activity through lynx1 modulation. PLoS One 2018, 13:e0199643 10.1371/journal.pone.0199643 • Subcortical effects of lynx1, demonstration of lynx1 and β2* nAChRs interactions in the brain and co-model of lynx1/α4β2 nAChR complexes.
- 13.Faure G, Shelukhina IV, Porowinska D et al. : Interaction of three-finger proteins from snake venoms and from mammalian brain with the cys-loop receptors and their models. Dokl Biochem Biophys 2016, 468:193–196 10.1134/S1607672916030091. [DOI] [PubMed] [Google Scholar]
- 14.Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C et al. : The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron 2006, 51:587–600 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 15. Smith MR, Glicksberg BS, Li L, Chen R, Morishita H, Dudley JT: Loss-of-function of neuroplasticity-related. genePac Syms conferp s risk for human neurodevelopmental disorders Biocomput 2018, 23:68–79 Genomic approach to identify risk genes for neurodevelopmental disorders using lynx1KO mice as a model, linked to alleles associated with epilepsy and schizophrenia.
- 16. Artoni P, Piffer A, Vinci V, LeBlanc J, Nelson CA, Hensch TK, Fagiolini M: Deep learning of spontaneous arousal fluctuations detects early cholinergic defects across neurodevelopmental mouse models and patients. Proc Natl Acad Sci U S A 2019, 2019 10.1073/pnas.1820847116 pii:201820847. PMID: 31332003 •• Using lynx1 deficient mice, the authors report alterations in arousal, reflective of autism-like states. They also use a neural network, CovNetAch, to detect pupillary fluctuations which could applied as a biomarker, a signature of cholinergic dysregulation. This is the first report linking the lynx1 to a neurodevelopmental disorders, such as autism.
- 17.Morishita H, Miwa JM, Heintz N, Hensch TK: Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 2010, 330:1238–1240 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukhari N, Burman PN, Hussein A, Demars MP, Sadahiro M, Brady DM et al. : Unmasking proteolytic activity for adult visual cortex plasticity by the removal of lynx1. J Neurosci 2015, 35:12693–12702 10.1523/JNEUROSCI.431514.2015bcp.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajo M, Ellis-Davies G, Morishita H: Lynx1 limits dendritic spine turnover in the adult visual cortex. J Neurosci 2016, 36:94729478 10.1523/JNEUROSCI.0580-16.2016 PMID: 27605620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker RL, O’Neill H, Henley BM, Wageman CR, Drenan RM, Marks MJ, Miwa JM, Lester HA: Deletion of lynx1 reduces the function of α6*nicotinic receptors. PLoS One 2017, 12: e0188715 10.1371/journal.pone.0188715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomsen MS, Cinar B, Jensen MM et al. : Expression of the Ly-6 family proteins Lynx1 and Ly6H in the rat brain is compartmentalized, cell-type specific, and developmentally regulated. Brain Struct Funct 2014, 219:1923–1934 10.1007/s00429-013-0611-x. [DOI] [PubMed] [Google Scholar]
- 22. Takesian AE, Bogart LJ, Lichtman JW, Hensch TK: Inhibitory circuit gating: of auditory critical-period plasticity. Nat Neurosci 2018, 21:218–227 10.1038/s41593-017-0064-PMID: 2935866629358666 •• lynx1 expression in 5-HT3AR positive neurons in the primary auditory cortex suppressed cholinergic tone and tonotopic plasticity.
- 23.Disney AA, Aoki C, Hawken MJ: Gain modulation by nicotine in macaque v1. Neuron 2007, 56:701–713 10.1016/j.neuron.2007.09.034 PMID: 18031686. PMCID: PMC2875676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadahiro M, Sajo M, Morishita H: Nicotinic regulation of experience-dependent plasticity in visual cortex. J Physiol Paris 2016, 110:29–36 10.1016/j.jphysparis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols WA, Henderson BJ, Yu C, Parker RL, Richards CI, Lester HA, Miwa JM: Lynx1 shifts alpha4beta2 nicotinic receptor subunit stoichiometry by affecting assembly in the endoplasmic reticulum. J Biol Chem 2014, 289:31423–31432 10.1074/jbc.M114.573667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George AA, Bloy A, Miwa JM, Lindstrom JM, Lukas RJ, Whiteaker P: Isoform-specific mechanisms of α3β4 nicotinic acetylcholine receptor modulation by the prototoxin lynx1. FASEB J 2017, 31:1398–1420 10.1096/fj.201600733R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyukmanova EN, Shenkarev ZO, Shulepko MA et al. : NMR structure and action on nicotinic acetylcholine receptors of water-soluble domain of human lynx1. J Biol Chem 2011, 286:10618–10627 10.1074/jbc.M110.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman MM, Teng J, Worell BT, Moviello CM, Lee M, Karlin A, Stowell MHB, Hibbs RE: Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 2020, 106:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyukmanova EN, Shulepko MA, Buldakova SL et al. : Water-soluble lynx1 residues important for interaction with muscle-type and/or neuronal nicotinic receptors. J Biol Chem 2013, 288:15888–15899 10.1074/jbc.M112.436576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Changeux JP: The nicotinic acetylcholine receptor: a typical ‘allosteric machine’. Philos Trans R Soc Lond B Biol Sci 2018, 373:20170174 10.1098/rstb.2017.0174 PMID: 29735728; PMCID: PMC5941169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharpure A, Moviello CM, Hibbs RE: Progress in nicotinic receptor structural biology. Neuropharmacology 2020, 171:108086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathy S, Zheng W, Auerbach A: A single molecular distance predicts agonist binding energy in nicotinic receptors. J Gen Physiol 2019, 151:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong C, Kern NR, Anderson KR, Zhang XF, Miwa JM, Im W Dynamics and interactions of GPI-linked lynx1 protein with/without nicotinic acetylcholine receptor in membrane bilayers. J Phys Chem B 2020, 124:4017–4025 10.1021/acs.jpcb.0c0015932208709 •• The authors perform molecular dynamics simulations of lynx1 with nAChRs and interactions at the receptor C-loop. This is the first depiction of lynx1 interactions of nAChR embedded in the membrane.
- 34.Purohit P, Auerbach A: Loop C and the mechanism of acetylcholine receptor-channel gating. J Gen Physiol 2013, 141:467–478 10.1085/jgp.201210946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N: Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 1999, 23:105–114 10.1016/S0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 36.Miwa JM, Walz AW: Enhancement in motor learning through genetic manipulation of the lynx1 gene. PLoS One 2012, 7: e43302 10.1371/journal.pone.0043302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bychkov M, Shenkarev Z, Shulepko M, Shlepova O, Kirpichnikov M, Lyukmanova E: Water-soluble variant of human lynx1 induces cell cycle arrest and apoptosis in lung cancer cells via modulation of α7 nicotinic acetylcholine receptors. PLoS One 2019, 14:e0217339 10.1371/journal.pone.0217339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomsen MS, Arvaniti M, Jensen MM, Shulepko MA, Dolgikh DA, Pinborg LH et al. : Lynx1 and Aβ1–42 bind competitively to multiple nicotinic acetylcholine receptor subtypes. Neurobiol Aging 2016, 46:13–21 10.1016/j.neurobiolaging.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 39. Shenkarev ZO, Shulepko MA, Bychkov ML, Kulbatskii DS, Shlepova OV, Vasilyeva NA, Andreev-Andrievskiy AA, Popova AS, Lagereva EA, Loktyushov EV et al. : Water-soluble variant of human lynx1 positively modulates synaptic plasticity and ameliorates cognitive impairment associated with α7-nAChR dysfunction. J Neurochem 2020, 2020 10.1111/jnc.15018 •• Ws-lynx1 inhibits α7-nAChRs with an IC50 of ~50 μM in oocytes. In mice, ws-lynx1 penetrated the blood–brain barrier upon intranasal administration and partially overrides blockade of α7-nAChRs by MLA.
- 40.Miwa JM, Lester HA, Walz A: Optimizing cholinergic tone through lynx modulators of nicotinic receptors: implications for plasticity and nicotine addiction. Physiology 2012, 27:187–199 10.1152/physiol.00002.2012. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi A, Parker RL, Wright AP, Brahem H, Ku P, Oliver KM et al. : Lynx1 supports neuronal health in the mouse dorsal striatum during aging: an ultrastructural investigation. J Mol Neurosci 2014, 53:525–536 10.1007/s12031014-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bychkov ML, Vasilyeva NA, Shulepko MA, Balaban PM, Kirpichnikov MP, Lyukmanova EN: Lynx1 prevents long-term potentiation blockade and reduction of neuromodulator expression caused by Aβ1–42 and JNK activation. Acta Nat 2018, 10:57–61 •• The authors use a recombinant protein, water-soluble (ws-lynx1) and demonstrate that it can be protective against the negative physiological effects of Aβ1–42 on synaptic plasticity such as LTP. The authors also show that the toxic component in Alzheimer’s disease pathology, β-amyloid peptide Aβ1–42, treatment also decreases lynx1 mRNA expression in rat primary cortical neurons.
- 43.Lasala M, Fabiani C, Corradi J, Antollini S, Bouzat C: Molecular modulation of human α7 nicotinic receptor by amyloid-β peptides. Front Cell Neurosci 2019, 13:37 10.3389/fncel.2019.00037 Published 2019 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kryukova EV, Egorova NS, Kudryavtsev DS, Lebedev DS, Spirova EN, Zhmak MN, Garifulina AI, Kasheverov IE, Utkin YN, Tsetlin VI: From synthetic fragments of endogenous three-finger proteins to potential drugs. Front Pharmacol 2019, 10:748 10.3389/fphar.2019.00748 Published 2019 July 331333465 ••The authors synthesize a circularized lynx1-derived peptide, and a circularized peptide of peptides of other members of the prototoxin family, such as SLURP-1 and SLURP-2. This study demonstrates binding at ά7 nAChRs at 147 µM, and the ligand-binding domain of the α9 nAChR subunit at 40 µM. This is the first published report of a physiological function of a short lynx1-derived peptide with inhibitory effects on nAChRs.
- 45.Dos Santos Rodrigues B, Arora S, Kanekiyo T, Singh J: Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res 2020, 1734:146738 10.1016/j.brainres.2020.146738. [DOI] [PubMed] [Google Scholar]
- 46.Huang Q, Chan KY, Tobey IG, Chan YA, Poterba T, Boutros CL, Balazs AB, Daneman R, Bloom JM, Seed C, Deverman BE: Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS One 2019, 14: e0225206 10.1371/journal.pone.0225206. [DOI] [PMC free article] [PubMed] [Google Scholar]