Abstract
Cancer is the second most common cause of death worldwide. It is a generic name for a large group of diseases that can affect any part of the body. Cancer affects both energy intake through the diet and the total energy expenditure (TEE) through the changes in energy metabolism, resulting in negative or positive energy balance. Determining daily energy requirement is very important in the regulation of the nutrition therapy in a cancer patients. Due to the difficulty in directly measuring the TEE, resting energy expenditure, which is the largest component of the TEE, is often used in the determination of the energy requirement. In this study, the effects of disease-specific factors such as tumor burden, inflammation, weight loss and cachexia on energy metabolism in cancer patients were investigated.
Keywords: Cancer, cancer cachexia, cancer care, energy metabolism, nutrition, resting metabolic rate
INTRODUCTION
Cancer is a general term for a large group of diseases that can affect any part of the body. Other common terms used are malignant tumors and neoplasms. Cancer is the growth of abnormal cells beyond the normal limits that can spread to different organs in the body. This process which is the main cause of cancer related deaths is known as metastasis.[1]
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020.[2] Every one out of 5 men and 6 women in the world are diagnosed with cancer at a time in their lives and one out of 8 men and one out of 11 women lose their lives due to this disease. By sex, men are commonly diagnosed with lung, prostate, colorectal, stomach, and liver cancers while breast, colorectal, lung, cervix, and thyroid cancers are the most common types in women.[3] In addition, leukemia is the most common type in younger ages.[4]
The type and stage of cancer, changes in metabolism associated with the disease, and inflammation can lead to a change in energy metabolism and therefore patients’ resting energy expenditure (REE).[5] REE is defined as the energy, required by the body in resting state.[6] The contribution of REE to the daily energy expenditure is approximately 55%–75%, and it constitutes the largest part of the total energy expenditure (TEE).[7,8] For this reason, the accurate estimation of REE is highly important in determining the total daily energy requirements and planning the medical nutrition therapy for the patients effectively.[9,10]
The changes in REE in cancer patients are conflicting in the literature. Literature suggests that there is a tendency for an increase in REE in cancer patients,[11] but also REE may not change[12] or decrease.[13] The accelerated weight loss related to inadequate dietary intake, as well as the increase in REE, should be considered due to its potential role in malnutrition and cancer cachexia in patients.[14] Therefore, an accurate estimation of energy expenditure is important for planning the nutritional therapy of a cancer patient. In this context, the factors leading to a change in energy metabolism and REE in cancer patients such as the tumor burden, type of cancer, and the altered metabolism related to the disease are discussed in depth in this review.
RESTING ENERGY EXPENDITURE IN CANCER
Energy balance is the compatibility between the energy intake and TEE, including REE, activity energy expenditure, and the thermic effects of food.[15] The metabolic changes due to cancer can affect both REE and the energy intake, which can cause negative or positive energy balance. As it is difficult to determine the TEE directly, REE, which is the largest component of TEE, is often measured in the estimation of energy expenditure.[16]
There are different methods to determine REE. Numerous equations have been developed to estimate REE over the years.[17] Many of the predictive equations for the estimation of REE include the components such as height, weight, sex, age and other factors that can affect the REE. However, determining accurate REE extends far beyond these factors in critically ill patients.[18] Therefore, using predictive equations to estimate REE for dietary recommendations has not been accepted as an accurate assessment of energy requirements in patients with cancer.[19,20]
The method of measuring energy expenditure by heat loss is named direct calorimetry, which includes the equipment that monitors the amount of heat produced by the individual inside the chamber or room. This method is limited, technically difficult, and expensive; thus, indirect calorimetry is the most frequently used technique to determine REE.[21] Indirect calorimetry has the advantage of mobility and low equipment cost and frequently used in clinical practice. Indirect calorimetry is considered as the gold standard to measure REE, by measuring whole-body oxygen (VO2) and carbon dioxide (VCO2) gas exchange.[17,22]
The main determinants of REE in cancer patients are the changes in body composition, tumor burden, inflammation, and brown adipose tissue activation.[16]
Body composition and resting energy expenditure
Energy expenditure is the result of the metabolic activities of tissues and organs in the body. Fat-free mass (FFM) energy expenditure accounts for 53%–88% of the total REE and thus has the largest share in REE.[23] Large organs such as the lungs and the liver constantly perform for metabolic processes, resulting in high energy expenditure and taking a large share in REE.[24] Depending on the type and the stage of cancer, weight loss is frequently reported in cancer patients. Weight loss is observed both in fat tissue and FFM and may occur due to the inadequate dietary intake, inflammation and other metabolic factors.[25,26]
Proteolysis-inducing factor (PIF) secreted by tumor first activates the adenosine triphosphate (ATP)-dependent proteolytic system and thereby increases protein catabolism and seriously decreases FFM.[27,28] Since FFM is the biggest determinant of REE, the decrease in FFM causes a decrease in REE as well.[7] However, some findings suggest that there is an increase in REE due to increased catabolism and inflammation in cancer patients despite the body weight and FFM loss.[16,29]
Tumor burden and resting energy expenditure
Tumor burden is an important factor affecting the energy metabolism of cancer patients. Tumor location, size, central tumor lesion and response rate to chemotherapy are highly correlated with an increase in REE.[16]
Despite their small size, high rates of glycolysis and lactate production are observed in tumors. Tumors are generally described as lactate-producing tissues despite the presence of adequate oxygen (Warburg Effect).[30,31] Large amounts of lactate are observed in tumor cells as a result of anaerobic glycolysis.[32] Excess lactate transported to the liver and converted to glucose again (Cori cycle), and this cycle causes ATP consumption.[33] When energy is produced anaerobically by the tumor via glycolysis, glucose is converted into lactate, net 2 ATP is produced, but 6 ATPs are needed to convert to lactate back into glucose again.[34] Thus, the Cori cycle increases energy consumption. In cancer cases, the Cori cycle has accelerated, which leads to a negative energy balance. The increased glucose turnover has a significant effect on the increase in REE and muscle catabolism in cancer patients.[16,33,35]
Depending on the tumor burden, the additional energy expenditure is estimated to be 100–1400 kcal/day.[16,36] The size of the tumor determines the rate of anaerobic energy production, and energy expenditure increases by 190–470 kcal/tumor kg/day. Increased energy demand depending on the tumor significantly affects REE because theoretically additional 400 kcal/day energy expenditure is 25% of REE of a patient with a daily REE of 1600 kcal. For this reason, the energy demand of the tumor significantly affects the energy expenditure in some patients.[33]
Another factor that may contribute to high REE is the tumor metastases in the liver. Liver energy expenditure accounts for about 20% of total REE (~200 kcal/day) in healthy individuals.[37] A study which investigated the relationship between liver weight and REE pointed to a positive correlation between liver mass and REE. The study also revealed that every 1 kg increase in liver mass due to metastases leads to an increase in REE of approximately 343 kcal/day.[38] Other studies have also shown that the type of cancer, its pathological stage, and the duration of disease affect REE.[39,40,41,42]
Jatoi et al.[43] suggested that after adjustment either for lean body mass determined by dual-energy X-ray absorptiometry, or for body cell mass determined by total-body potassium measurement, patients with nonmetastatic nonsmall-cell lung cancer are hypermetabolic compared to control subjects. In terms of tumor location evaluation, hypermetabolism was seen in patients with various cancer types such as esophagus, stomach, pancreas, and lung cancer,[44] while normal metabolism, hypometabolism or hypermetabolism may occur in patients with colorectal cancer.[41,45] The more aggressive and advanced stages of the disease are associated with higher REE.[16]
Cancer cachexia, inflammation, and resting energy expenditure
In addition to the increased energy requirement of the tumor, various metabolic disorders occur in the body due to the tumor. These metabolic changes are the ones that induce the release of inflammatory mediators such as cytokines and eicosanoids. Cancer cachexia is characterized by systemic inflammation, negative protein and energy balance, and weight loss.[16,46] The most obvious symptom of cancer cachexia is FFM loss, while adipose tissue loss may or may not occur.[47]
Decreased appetite due to tumor and so reduced dietary intake and therefore the changed energy and protein metabolism because of the release of inflammatory cytokines are the major causes of cachexia physiopathology.[39] Cachexia is characterized by chronic inflammation. Inflammatory cytokines act through local and systemic mechanisms to regulate energy metabolism.[48] Increased energy expenditure and decreased food consumption due to increased carbohydrate, lipid, protein cycle, and Cori cycle activity, create negative energy balance, and consequently, cachexia occurs.[49]
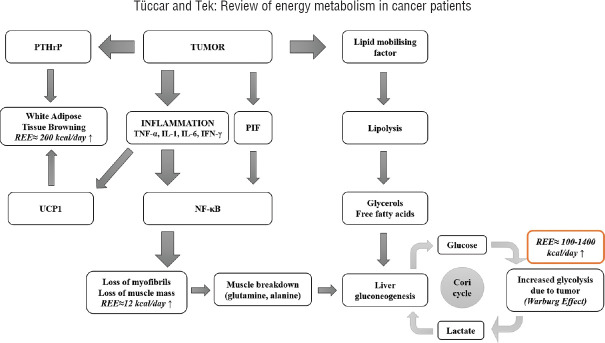
However, because of their rapid proliferations, the energy and nutritional needs of cancer cells are high. Accordingly, adipose tissue and skeletal muscle are used to meet the increased energy need in cachectic patients. Cancer cells use fatty acids for energy production, and accordingly, lipid oxidation increases.[50] The cytokines in the muscles stimulate protein catabolism so that amino acids can be released. Amino acids are used in the protein synthesis of cancer cells or they are used to obtain glucose with gluconeogenesis.[51] With glucose and glyconeogenesis, glucose production increases in cancer cachexia. Impaired insulin release and insulin resistance lead to a decrease in the use of glucose in tissues and result in glucose intolerance.[52] The majority of the glucose is consumed by tumors and fermented to lactate rather than oxidized in the pathways (Warburg effect).[53] These metabolic changes are categorized into two groups. The first is the factors that are produced and released by the tumor cells. The most important ones are the lipid-mobilizing factor that lead to lipolysis and PIF which initiates protein catabolism from skeletal muscles.[54] The second group includes the proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-1 (IL-1), IL-6, and interferon-gamma, which are synthesized by the immune system in response to the tumor. Inflammatory cytokines are the signals from the cancer tissue, which induce catabolism in adipose and muscle tissue.[46,55,56,57] Chronic inflammation contributes to energy expenditure, and thus, REE increases in cachexia.[55,56,57,58] Mechanisms of full metabolism and the effect on energy expenditure in the tumor-bearing state are summarized in Figure 1.
Figure 1.
Mechanisms of full metabolism and the effect on energy expenditure in the tumor-bearing state. REE = Resting energy expenditure; PTHrP = Parathyroid hormone-related protein; UCP1 = Uncoupling protein 1; TNF-α = Tumor necrosis factor-alpha; IL-1 = Interleukin-1; IL-6 = Interleukin-6; PIF = Proteolysis-inducing factor; NF-κB = Nuclear factor kappa B
In addition to the studies showing the increase in REE in cancer, some of the findings suggest that there is hypometabolism in cancer. It has been shown that approximately 30% of the patients are hypermetabolic, commonly seen in patients diagnosed with breast and prostate cancer.[59] It is thought that hypometabolism may be associated with decreased appetite and inadequate dietary intake.[5]
Brown adipose tissue and resting energy expenditure
Another mechanism that may account for the increased energy expenditure and cancer cachexia is the activation of brown adipose tissue or browning of the white adipose tissue. Since the brown fat tissue is a heat-producing tissue, it contributes to increased energy expenditure. Recently, brown adipose tissue activation or browning of the white fat tissue has been evaluated within the context of cancer cachexia.[60,61]
The tumor may produce the parathyroid hormone-related protein, which induces the browning of white fat tissue.[62] The presence of both a tumor and the related activated immune system triggers the energy demand reaction. Energy is obtained from the liver (via glycogenogenesis) as glucose, from muscles as protein (protein catabolism), from the adipose tissue as lipids (lipolysis), and from the liver as ketones. As a part of the energy demand signal, proinflammatory cytokines increase melanocortin 4 receptor activation in the brain, leading to decreased appetite and increased energy expenditure. In this way, the use of energy sources and energy expenditure increase.[50]
The browning of the white fat tissue is associated with increased expression of uncoupling protein 1 (UCP1). This protein uncouples mitochondrial respiration toward thermogenesis rather than ATP synthesis. This was found to cause increased lipid mobilization and energy expenditure in cachectic mice. Chronic inflammation and IL-6 increase the expression of UCP1 in white fat tissue. Treatments that decrease inflammation or β-adrenergic blockade reduce white fat tissue browning and the severity of cachexia.[50]
White fat tissue browning is responsible for an increase in TEE. It was observed that with fully activated brown fat tissue, the rate of REE increases by 203 ± 40 kcal/day.[63] The inhibition of white fat tissue browning is thought to be a promising approach to cure cachexia in cancer patients.[64]
DISCUSSION
Many factors can alter energy metabolism in cancer patients as discussed in this review. For this reason, the importance of accurate determination of REE becomes important. Considering the factors such as age, body composition, type of cancer, pathological stage, and the duration of the disease that can affect REE, it is recommended that patients should be assesed individually.
Hypermetabolism is defined as the increase in REE.[65] When evaluated in terms of the cancer type, normal rate of REE was observed in patients with breast, melanoma, gastric, or colorectal cancer in some studies,[39,66] while hypermetabolism was seen in patients with pancreatic or lung cancer.[66] In addition, in studies in which REE was evaluated by indirect calorimetry, it was reported that 57% of the 140 patients with head and neck cancer[67] and 56.9% of 109 patients with advanced gastrointestinal cancer[68] were hypermetabolic and most were malnourished. In addition to the type of cancer, its stage also affects REE. In urologic cancers, REE changes were shown in relation to the tumor type and stage.[40] In a study, the variability in REE in patients with stage III or IV colorectal cancer was associated with age, FFM, inflammation, and disease stage.[18] Cao et al.[41] reported that advanced stage cancer patients have increased REE compared to the early stages of the disease. Purcell et al.[69] also detected hypermetabolism in patients with newly diagnosed stage II-III colorectal cancer.
A study revealed that 50% of the cancer patients with weight loss were found to be hypermetabolic compared to the control group with similar physical activity, body composition, age, and weight loss.[5] In a study, it was found that 48% of newly diagnosed cancer patients were hypermetabolic compared to the control group and that REE estimated per kg FFM was higher.[41] In line with the previous studies, a recent meta-analysis of 27 studies presented that REE increased by 9.66 kJ/kg FFM/day in cancer patients.[29]
In many studies, REE has been shown to increase in cancer patients.[5,29,58,70] However, while REE was found to be increased, TEE was found to be lower in advanced stage cancer patients compared to healthy individuals. This has been attributed to reduced daily physical activity in advanced stage cancer patients.[71]
According to the European Society for Clinical Nutrition and Metabolism experts, it is recommended to measure REE with indirect calorimetry in order to determine the energy requirement and to plan nutrition therapy that can prevent cancer-related malnutrition.[66]
CONCLUSIONS
Cancer patients have metabolic changes depending on the stage and the type of the disease, and these metabolic changes may cause alterations in energy expenditure. Determining the changes in energy expenditure is important to estimate the individual energy requirements and to plan an energy-balanced nutrition therapy, which is important to prevent malnutrition in patients. Considering the changing energy metabolism and metabolic differences of patients, the error margin of using the predictive equations to determine the energy requirements of patients with different types of cancer may be high. For this reason, if possible, the main approach should be to measure energy expenditure using indirect calorimetry and each patient should be evaluated individually. REE tends to increase in advanced stage cancer patients, but increased fatigue and decreased physical activity also lead to limited physical activity energy expenditure in these patients. It will be more accurate to plan an individual nutrition therapy program by measuring REE with indirect calorimetry to prevent negative energy balance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization: Cancer. 2018. [Last accessed on 2021 May 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer .
- 2.Ferlay JE, Lam F, Colombet M, Mery L, Piñeros M. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. [Last accessed on 2021 May 09]. Available from: https://gco.iarc.fr/today/home .
- 3.International Agency for Research on Cancer. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018. [Last accessed on 2021 May 10]. Available from: https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf .
- 4.Keramatinia A, Mohseny M, Akbari ME, Mosavi-Jarrahi A, Monfared ED, Amanpour F, et al. Determinants of survival of common childhood cancers in Iran. J Res Med Sci. 2018;23:101. doi: 10.4103/jrms.JRMS_835_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–383. doi: 10.1002/ijc.1332. [DOI] [PubMed] [Google Scholar]
- 6.Blasco Redondo R. Resting energy expenditure; assessment methods and applications. Nutr Hosp. 2015;31(Suppl 3):245–54. doi: 10.3305/nh.2015.31.sup3.8772. [DOI] [PubMed] [Google Scholar]
- 7.Soares MJ, Müller MJ. Resting energy expenditure and body composition: Critical aspects for clinical nutrition. Eur J Clin Nutr. 2018;72:1208–14. doi: 10.1038/s41430-018-0220-0. [DOI] [PubMed] [Google Scholar]
- 8.McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: A public health perspective. Med Sci Sports Exerc. 2014;46:1352–8. doi: 10.1249/MSS.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, et al. Energy requirements in nonobese men and women: Results from CALERIE. Am J Clin Nutr. 2014;99:71–8. doi: 10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoeller DA, Westerterp M. Advances in the Assessment of Dietary Intake. CRC Press: USA; 2017. Biomarker for energy intake: Resting energy expenditure and physical activity; pp. 199–200. [Google Scholar]
- 11.Hyltander A, Drott C, Körner U, Sandström R, Lundholm K. Elevated energy expenditure in cancer patients with solid tumours. Eur J Cancer. 1991;27:9–15. doi: 10.1016/0277-5379(91)90050-n. [DOI] [PubMed] [Google Scholar]
- 12.Ravasco P, Monteiro-Grillo I, Camilo M. Colorectal cancer: Intrinsic characteristics modulate cancer energy expenditure and the risk of cachexia. Cancer Invest. 2007;25:308–14. doi: 10.1080/07357900701208873. [DOI] [PubMed] [Google Scholar]
- 13.Langius JA, Kruizenga HM, Uitdehaag BM, Langendijk JA, Doornaert P, Leemans CR, et al. Resting energy expenditure in head and neck cancer patients before and during radiotherapy. Clin Nutr. 2012;31:549–54. doi: 10.1016/j.clnu.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca GWPD, Farkas J, Dora E, von Haehling S, Lainscak M. Cancer cachexia and related metabolic dysfunction. Int J Mol Sci. 2020;21:2321. doi: 10.3390/ijms21072321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westerterp KR. Control of energy expenditure in humans. Eur J Clin Nutr. 2017;71:340–4. doi: 10.1038/ejcn.2016.237. [DOI] [PubMed] [Google Scholar]
- 16.Purcell SA, Elliott SA, Baracos VE, Chu QS, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70:1230–8. doi: 10.1038/ejcn.2016.96. [DOI] [PubMed] [Google Scholar]
- 17.Schlein KM, Coulter SP. Best practices for determining resting energy expenditure in critically ill adults. Nutr Clin Pract. 2014;29:44–55. doi: 10.1177/0884533613515002. [DOI] [PubMed] [Google Scholar]
- 18.Purcell SA, Wallengren O, Baracos VE, Lundholm K, Iresjö BM, Chu QSC, et al. Determinants of change in resting energy expenditure in patients with stage III/IV colorectal cancer. Clin Nutr. 2020;39:134–40. doi: 10.1016/j.clnu.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Purcell SA, Baracos VE, Chu QS, Sawyer MB, Severin D, Mourtzakis M, et al. Profiling determinants of resting energy expenditure in colorectal cancer. Nutr Cancer. 2020;72:431–8. doi: 10.1080/01635581.2019.1635172. [DOI] [PubMed] [Google Scholar]
- 20.Mazzo R, Ribeiro FB, Vasques ACJ. Accuracy of predictive equations versus indirect calorimetry for the evaluation of energy expenditure in cancer patients with solid tumors-An integrative systematic review study. Clin Nutr ESPEN. 2020;35:12–9. doi: 10.1016/j.clnesp.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Ireton-Jones C. Intake: Energy. In: Kathleen Mahan JL, editor. Krause‘s Food and The Nutrition Care Process. USA: Elsevier; 2019. pp. 17–27. [Google Scholar]
- 22.Delsoglio M, Achamrah N, Berger MM, Pichard C. Indirect calorimetry in clinical practice. J Clin Med. 2019;8:1387. doi: 10.3390/jcm8091387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen S, Hensrud DD, Romanski S, Levine JA, Burguera B, Jensen MD. Body composition and resting energy expenditure in humans: Role of fat, fat-free mass and extracellular fluid. Int J Obes Relat Metab Disord. 2000;24:1153–7. doi: 10.1038/sj.ijo.0801317. [DOI] [PubMed] [Google Scholar]
- 24.Elia M. Organ and Tissue Contribution to Metabolic Rate. Raven Press: New York; 1992. Organ and tissue contribution to metabolic rate; pp. 61–80. [Google Scholar]
- 25.Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract. 2016;12:1163–71. doi: 10.1200/JOP.2016.016832. [DOI] [PubMed] [Google Scholar]
- 26.Cooper C, Burden ST, Cheng H, Molassiotis A. Understanding and managing cancer-related weight loss and anorexia: Insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99–111. doi: 10.1002/jcsm.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabal-Manzano R, Bhargava P, Torres-Duarte A, Marshall J, Bhargava P, Wainer IW. Proteolysis-inducing factor is expressed in tumours of patients with gastrointestinal cancers and correlates with weight loss. Br J Cancer. 2001;84:1599–601. doi: 10.1054/bjoc.2001.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skelton WP, 4th, Parekh H, Starr JS, Trevino J, Cioffi J, Hughes S, et al. Clinical factors as a component of the personalized treatment approach to advanced pancreatic cancer: A systematic literature review. J Gastrointest Cancer. 2018;49:1–8. doi: 10.1007/s12029-017-0021-z. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TY, Batterham MJ, Edwards C. Comparison of resting energy expenditure between cancer subjects and healthy controls: A meta-analysis. Nutr Cancer. 2016;68:374–87. doi: 10.1080/01635581.2016.1153667. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin ML, Gladden LB, Nijsten MW, Jones KB. Lactate and cancer: Revisiting the Warburg effect in an era of lactate shuttling. Front Nutr. 2014;1:27. doi: 10.3389/fnut.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 32.Gupta G. Lactate: Metabolic hallmark of cancer in 21st century. Clin Oncol. 2017;2:1375. [Google Scholar]
- 33.Friesen DE, Baracos VE, Tuszynski JA. Modeling the energetic cost of cancer as a result of altered energy metabolism: Implications for cachexia. Theor Biol Med Model. 2015;12:17. doi: 10.1186/s12976-015-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melkonian EA, Schury MP. Biochemistry, Anaerobic Glycolysis. StatPearls. 2021. [Last accessed on 2021 May 10]. Available from: https://www.ncbi.nlm.nih. gov/books/NBK546695/ [PubMed]
- 35.Shyh-Chang N. Metabolic changes during cancer cachexia pathogenesis. Adv Exp Med Biol. 2017;1026:233–49. doi: 10.1007/978-981-10-6020-5_11. [DOI] [PubMed] [Google Scholar]
- 36.Hall KD, Baracos VE. Computational modeling of cancer cachexia. Curr Opin Clin Nutr Metab Care. 2008;11:214–21. doi: 10.1097/MCO.0b013e3282f9ae4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, et al. Specific metabolic rates of major organs and tissues across adulthood: Evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369–77. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89:1173–9. doi: 10.3945/ajcn.2008.27273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvie MN, Howell A, Thatcher N, Baildam A, Campbell I. Energy balance in patients with advanced NSCLC, metastatic melanoma and metastatic breast cancer receiving chemotherapy – A longitudinal study. Br J Cancer. 2005;92:673–80. doi: 10.1038/sj.bjc.6602357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu WP, Cao DX, Lin ZM, Wu GH, Chen L, Zhang JP, et al. Analysis of energy utilization and body composition in kidney, bladder, and adrenal cancer patients. Urol Oncol. 2012;30:711–8. doi: 10.1016/j.urolonc.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Cao DX, Wu GH, Zhang B, Quan YJ, Wei J, Jin H, et al. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr. 2010;29:72–7. doi: 10.1016/j.clnu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Vaisman N, Lusthaus M, Niv E, Santo E, Shacham-Shmueli E, Geva R, et al. Effect of tumor load on energy expenditure in patients with pancreatic cancer. Pancreas. 2012;41:230–2. doi: 10.1097/MPA.0b013e3182264d05. [DOI] [PubMed] [Google Scholar]
- 43.Jatoi A, Daly BD, Hughes VA, Dallal GE, Kehayias J, Roubenoff R. Do patients with nonmetastatic non-small cell lung cancer demonstrate altered resting energy expenditure? Ann Thorac Surg. 2001;72:348–51. doi: 10.1016/s0003-4975(01)02847-8. [DOI] [PubMed] [Google Scholar]
- 44.Jouinot A, Vazeille C, Goldwasser F. Resting energy metabolism and anticancer treatments. Curr Opin Clin Nutr Metab Care. 2018;21:145–51. doi: 10.1097/MCO.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 45.Gangadharan A, Choi SE, Hassan A, Ayoub NM, Durante G, Balwani S, et al. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. 2017;8:24009–30. doi: 10.18632/oncotarget.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Ye J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16:47–54. doi: 10.1007/s11154-014-9306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: Impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4:95–109. doi: 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–47. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. 2018;29:i18–26. doi: 10.1093/annonc/mdx815. [DOI] [PubMed] [Google Scholar]
- 53.Luengo A, Li Z, Heiden MV. National Institute of Health: New clarity on the Warburg Effect; . 2021. [Last accessed on 2021 Aug 25]. Available from: https://www.cancer.gov/research/key-initiatives/ras/ras-central/blog/2021/vander-heiden-warburg-effect .
- 54.Laviano A, Muscaritoli M, Fanelli FR. Lipid mobilising factor in cancer cachexi. In: Mantovani G, Anker SD, Inui A, Morley JE, Fanelli FR, Scevola D, et al., editors. Cachexia and Wasting: A Modern Approach. Milan: Springer; 2006. pp. 489–93. [Google Scholar]
- 55.Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. Biomed Res Int 2014. 2014 doi: 10.1155/2014/168407. 168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia – pathophysiology and management. J Gastroenterol. 2013;48:574–94. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 58.Vazeille C, Jouinot A, Durand JP, Neveux N, Boudou-Rouquette P, Huillard O, et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: A prospective study in 390 cancer patients before initiation of anticancer therapy. Am J Clin Nutr. 2017;105:1139–47. doi: 10.3945/ajcn.116.140434. [DOI] [PubMed] [Google Scholar]
- 59.Seyfried T. Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. New Jersey: John Wiley and Sons; 2012. [Google Scholar]
- 60.Beijer E, Schoenmakers J, Vijgen G, Kessels F, Dingemans AM, Schrauwen P, et al. A role of active brown adipose tissue in cancer cachexia? Oncol Rev. 2012;6:e11. doi: 10.4081/oncol.2012.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: Possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol. 1986;111:82–5. doi: 10.1007/BF00402783. [DOI] [PubMed] [Google Scholar]
- 62.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–8. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, et al. Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging. 2014;41:776–91. doi: 10.1007/s00259-013-2611-8. [DOI] [PubMed] [Google Scholar]
- 65.Ishida J, Konishi M, Saito M, Springer J. Hypermetabolism: Should cancer types, pathological stages and races be considered in assessing metabolism and could elevated resting energy expenditure be the therapeutic target in patients with advanced cancer? J Cachexia Sarcopenia Muscle. 2015;6:391–2. doi: 10.1002/jcsm.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 67.Souza MT, Singer P, Ozorio GA, Rosa VM, Alves MM, Mendoza López RV, et al. Resting energy expenditure and body composition in patients with head and neck cancer: An observational study leading to a new predictive equation. Nutrition. 2018;51-52:60–5. doi: 10.1016/j.nut.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Ozorio GA, Souza MT, Singer P, López RV, Alves-Almeida MM, Ribeiro-Junior U, et al. Validation and improvement of the predictive equation for resting energy expenditure in advanced gastrointestinal cancer. Nutrition. 2020;73:110697. doi: 10.1016/j.nut.2019.110697. [DOI] [PubMed] [Google Scholar]
- 69.Purcell SA, Elliott SA, Walter PJ, Preston T, Cai H, Skipworth RJ, et al. Total energy expenditure in patients with colorectal cancer: Associations with body composition, physical activity, and energy recommendations. Am J Clin Nutr. 2019;110:367–76. doi: 10.1093/ajcn/nqz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dev R, Hui D, Chisholm G, Delgado-Guay M, Dalal S, Del Fabbro E, et al. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle. 2015;6:95–8. doi: 10.1002/jcsm.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NE, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–96. doi: 10.1016/j.clnu.2017.06.017. [DOI] [PubMed] [Google Scholar]