Abstract
Acute respiratory distress syndrome (ARDS) is a major cause of patient mortality in intensive care units (ICUs) worldwide. Considering that no causative treatment but only symptomatic care is available, it is obvious that there is a high unmet medical need for a new therapeutic concept. One reason for a missing etiologic therapy strategy is the multifactorial origin of ARDS, which leads to a large heterogeneity of patients. This review summarizes the various kinds of ARDS onset with a special focus on the role of reactive oxygen species (ROS), which are generally linked to ARDS development and progression. Taking a closer look at the data which already have been established in mouse models, this review finally proposes the translation of these results on successful antioxidant use in a personalized approach to the ICU patient as a potential adjuvant to standard ARDS treatment.
Keywords: ARDS, antioxidant, Nrf2, GSH, NADPH oxidase, SOD, electron transfer chain
1. Introduction
Acute respiratory distress syndrome (ARDS) was originally described by Ashbaugh et al. in 1967 [1]. Based on 12 patients with an acute onset of tachypnoea, hypoxemia, and diminished compliance due to various causes, the authors postulated a connection between the disease pattern and involvement of the alveolar surface. Positive end-expiratory pressure (PEEP) improved atelectasis and hypoxemia, whereas corticosteroids were helpful in medicating patients suffering from fat embolism or viral pneumonia. In the absence of a uniform definition of ARDS based on its multiple origins, the American-European Consensus Committee on ARDS was established in 1994. Conferences on ARDS took place in Miami, Florida, United States of America, and Barcelona, Spain. Integrating American and European studies, the ARDS experts provided a new definition of ARDS distinguishing between acute lung injury (ALI) and ARDS as its severe form [2]. An acute onset as well as bilateral infiltrates detected by a frontal chest radiograph and a pulmonary artery wedge pressure of above 18 mm Hg or missing evidence of left atrial hypertension were set as common characteristics. Only oxygenation, estimated by the Horovitz index [3], was different in ALI (PaO2/FiO2 < 300 mm Hg) compared to ARDS (PaO2/FiO2 < 200 mm Hg). Because of difficulties in adhering to the definition of the chest radiograph and only moderate accordance with the definitions, novel criteria were required when comparing ARDS clinical criteria with autopsy findings [4]. In 2005, Ferguson et al. developed a new clinical ARDS definition based on the Delphi consensus method [5]. Focusing on ARDS as a putative inclusion criterion or endpoint of clinical trials dealing with ALI, the former clinical definition of ARDS was altered. Defined characteristics were hypoxemia with PaO2/FiO2 < 200 mm Hg and PEEP ≥ 10 mm Hg, acute onset in <72 h, radiographic abnormalities consisting of a bilateral airspace disease involving ≥ two quadrants on frontal chest radiograph, and non-cardiogenic origin, i.e., without clinical signs of congestive heart failure. Moreover, a reduced lung compliance of <50 mL/cm H2O (tranquilized patients were adapted to a tidal volume (Vt) of 8 mL/kg, with an ideal body weight and PEEP ≥ 10) and direct or indirect predisposing factors linked to lung injury were defined [5]. In 2011, Raghavendran and Napolitano discussed the existing ARDS definitions and concluded that a new definition was again mandatory. This definition should include pulmonary vs. nonpulmonary risk factors, PaO2/FiO2 ratio, and standard ventilator settings (PEEP/MAP) < 200 mm Hg and exclude heart failure determined by cardiac echocardiogram [6]. So, one year later the new so-called Berlin Definition was published [7]. With the Berlin definition, ARDS patients are grouped according to disease burden into three states: mild, moderate, and severe. Shared features are acute onset within 1 week, bilateral shadows determined by radiography or computed tomography (CT), and respiratory failure independent of cardiac failure. Differences were now made regarding oxygenation: “mild” is associated with Horovitz 200 mm Hg < PaO2/FiO2 ≤ 300 mm Hg with PEEP or continuous positive airway pressure (CPAP) ≥ 5 cm H2O that can be delivered noninvasively. “Moderate” is characterized by Horovitz of 100 mm Hg < PaO2/FiO2 ≤ 200 mm Hg with PEEP ≥ 5 cm H2O. Finally, “severe” patients are defined by Horovitz index of PaO2/FiO2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O [7]. As a conclusion, the ALI term was removed. Originally, the Berlin definition was developed for use in high-income countries, with well-equipped intensive care units (ICUs) only. To adapt this setting for less well equipped ICUs, an appropriate ARDS definition was drawn up with modified criteria [8]. These included a SpO2/FiO2 ≤ 315 because arterial blood gas diagnostics required to examine the PaO2/FiO2 ratio are often missing. Since mechanical ventilators are also rare, PEEP is not required. Similarly, the lack of X-ray apparatus was taken into account by allowing ultrasonic testing instead.
To date, there is still no appropriate pharmacological treatment regimen available focusing on disease etiology. Intervention is limited to supportive therapy such as lung-protective mechanical ventilation [9] and possibly extracorporeal membrane oxygenation (ECMO) [10], prone positioning [11], positive end-expiratory pressure (PEEP) [12], recruitment maneuvers (RM) [13], neuromuscular blockers [14], and application of systemic corticosteroids [15]. Although high-end medicine techniques are pursued, mortality is still up to 50–80%; therefore, new approaches should be intensively evaluated. To elucidate putative starting points for an improved care strategy, preclinical studies are necessary to prove the efficacies of the tested interventions.
2. Materials and Methods
2.1. Mouse Models of ARDS/ALI
Mouse models of ARDS are widely established because breeding and generation times are short. Humanized models as well as gene knockout and knockin mice are available. Last but not least, mouse maintenance is relatively cheap. To induce ARDS in the murine system, two settings can be chosen (Table 1). Lung injury can be provoked directly by targeting the alveolar epithelium or indirectly by damaging the vascular endothelial cells (for a review see [16]). According to the workshop report published in 2011 by a commission, appointed by the American Thoracic Society (ATS), four ARDS features have been assembled for animal models [17]. Focusing on mouse models, the first of these features is histological determination of tissue injury, i.e., neutrophil immigration into the alveolar or interstitial space, formation of proteinaceous debris, and thickening of the alveolar wall. The second feature is the histological breakdown of the epithelial pulmonary capillary barrier, which can be determined by analyzing the extravascular lung water content, using tracers to follow barrier damage or to show an increase in protein content, especially of high-molecular-weight proteins, in bronchoalveolar lavage fluid (BALF) and an augmented coefficient of microvascular filtration. The third aspect focuses on the inflammatory response, which is characterized by an increased number of neutrophils in BALF, raised expression or activity of lung myeloperoxidase (MPO), and enhanced expression of proinflammatory cytokines in BALF or lung tissue. Finally, the fourth relevant attribute is physiological lung failure, indicated by hypoxemia-accelerated alveolar–arterial oxygen differences including ventilation/perfusion mismatches. Three of these four criteria should be detectable to assure ARDS in the mouse. However, in a mouse model, some ARDS characteristics that are obvious in the human patient, such as formation of hyaline membranes, hemorrhage, atelectasis, enhanced lung lymph flow, or high protein concentration in the lymph, are barely detectable. This must be considered in the selection of a mouse ARDS model. As shown in Table 1, models directly linked to lung damage use intratracheal or intranasal application of lipopolysaccharide (LPS) or bacteria, intratracheal instillation of acids or bleomycin, 100% O2 and mechanical ventilation (MV) [18], or pulmonary ischemia/reperfusion injury. Whereas the last-mentioned intervention requires surgery, all the other methods depend on intranasal or intratracheal application, which is easier to achieve. The classically used treatment is LPS inhalation, acting via Toll-like receptor (TLR) 4 signaling, leading to symptoms similar to a bacterial infection, as obvious with accumulation of neutrophils in the lung and induction of proinflammatory cytokines. However, due to the missing infectious pathogen, only aspects of a bacterial infection are represented. To induce active infection, living bacteria (such as E. coli, S. aureus, or P. aeruginosa) are administered via the identical route [19,20]. Intratracheal instillation of HCl resembles the aspiration of gastric contents by ICU patients. The use of intratracheal bleomycin mimics a longer-lasting lung damage, which is useful for examining mechanisms leading to fibrosis. Pulmonary ischemia/reperfusion is a frequently used model because it depicts the processes found in patients requiring a lung transplantation.
Table 1.
Mouse models in ARDS research.
| Direct Lung Damage [17,22,23] | Route of Application | ARDS-Like Affects | Antioxidant Approaches Already Used |
Ref. |
|---|---|---|---|---|
| LPS [24,25,26] | intranasal/intratracheal instillation |
lung accumulation of neutrophils, induction of proinflammatory cytokines | NAC, SAMC | [27,28,29] |
| Bacteria [30,31,32] | intratracheal instillation | lung accumulation of neutrophils, induction of proinflammatory cytokines | CDC | [33] |
| HCl [34,35,36] | intratracheal instillation | neutrophil infiltration, damage of alveolar/ vascular barrier |
apocynin, MitoTempo | [37,38] |
| Hyperoxia (HALI) [39,40,41] | intratracheal | damage of epithelial cells, neutrophil infiltration | AA, BNF, SFN, MnSOD | [42,43,44] |
| MV (VILI) [45,46,47,48] | intratracheal | inflammasome-mediated proinflammatory cytokine expression | NAC, Nrf2+/+, Nrf2−/−, PIP-2; PC-SOD |
[49,50,51,52] |
| Bleomycin [53,54,55] | intratracheal instillation | invertible fibrosis | BRNPs, adelmidrol, EC-SOD |
[56,57,58] |
| Pulmonary ischemia/reperfusion [59,60,61] |
surgery; mesenteric artery clamping or hilar ligation and reperfusion | neutrophil infiltration, damage of alveolar/ vascular barrier |
irisin | [60] |
| Indirect lung damage [17,22,23] | ||||
| Sepsis (live bacteria, CASP, CLP, CSI) [62,63,64,65,66,67] |
i.p.¸ peritonitis | damage of alveolar/ vascular barrier |
PC-SOD, SOD mimetic, Prdx6−/− | [51,68,69] |
| Endotoxemia [70,71,72] | i.v. or i.p. | damage of alveolar/ vascular barrier |
NAC, EUK-8, CypD | [73,74,75] |
| Oleic acid [70,76,77] | i.v. | mimics fat embolism | BAY 60-6583, leptin | [76,78] |
| Multiple transfusions (TRALI) [79,80,81,82] | i.v.; syngeneic or allogenic | acute onset; underlying a 2-hit onset, pulmonary neutrophil sequestration, involvement of MΦ |
MΦ depletion, C3−/−, C5−/−, C5aR−/− | [83,84,85] |
| Multiple trauma [86,87,88] |
externally received | neutrophil infiltration, complement activation | p47phox−/− | [89] |
| H2O2 [90,91,92] | i.v. | increased vascular permeability and fluid retention, edema formation | AA, TP | [93] |
| Nonpulmonary ischemia/reperfusion [94,95,96] | surgery; liver, gut, kidney | neutrophil sequestration, acceleration of microvascular permeability | CypDPlt−/−, SB239063, FK866, LY333531 | [97,98] |
| Two-hit models | ||||
| LPS + MV [99,100,101] | intratracheal, i.v., i.p. | inflammasome-dependent | ATF3 OE/KD; HIF1α−/−, enoxaparin, DJ-1, paracoxib |
[102,103,104,105] |
| Sepsis + MV [106,107,108] | i.p., peritonitis, intratracheal | augment sepsis-mediated organ damage | AM | [109] |
| HCl + MV [100,110,111,112] |
intratracheal | enhanced HCl impact | IL-6−/− | [113] |
+/+, wild-type mice; −/−, knockout mice; AA, ascorbic acid; AM, adrenomedullin; ATF3, activating transcription factor 3; BAY 60-6583, adenosine A2B receptor agonist; BNF, β-naphthoflavone; BRNPs, bilirubin-derived nanoparticles; C, complement; CASP, colon ascendens stent peritonitis; CDC, water-soluble curcumin formulation; CLP, cecal ligation and puncture; CSI, cecal slurry injection; CybD, cyclophilin D; DJ-1, Daisuke-Junko protein 1; EC-SOD, extracellular SOD; R, receptor; FK866, competitive visfatin inhibitor; HALI, hyperoxia-induced lung injury; HIF, hypoxia-inducible factor; IL, interleukin; i.p., intraperitoneal; i.v., intravenously; KD, knock-down; LPS, lipopolysaccharide; LY333531, PKCβ inhibitor; MΦ, macrophage; MV, mechanical ventilation; NAC, N-acetylcysteine; OE, overexpression; PCI, peritoneal cavity infection; PC-SOD, lecithinized SOD; PIP-2, peroxiredoxin 6 inhibitor peptide-2; PLT−/−, platelet-conditional knockout mice; SAMC, S-allylmercaptocysteine; SB239063, p38 MAPK inhibitor; SFN, sulforaphane; TP, α-tocopherol; TRALI, transfusion-induced acute lung injury; VILI, ventilator-induced lung injury.
Often, ARDS is not directly caused by pulmonary damage, but as a secondary event following a different initial insult. Therefore, ARDS is frequently found in patients suffering from sepsis. In mice, this can be achieved by an intraperitoneal (i.p.) injection of bacteria or of a stool suspension (peritoneal cavity infection/PCI), or, with skilled surgical interventions, by cecal ligation and puncture (CLP), the gold standard of sepsis mouse models, or a similar technique requiring a stent implantation into the colon, which is called colon ascendens stent peritonitis (CASP). All of them result in peritonitis. To induce endotoxemia, it is also possible to inject LPS i.p., with the limitations described above. A disease pattern with high proinflammatory cytokine expression as well as strong activation of the immune system results. Depending on the LPS amount applied, the model can also be lethal. Besides sepsis, a model using i.v. oleic acid provokes fat embolism, which sometimes can be found in patients as secondary to bone fracture. ARDS can also occur after multiple traumata, or sometimes when patients receive multiple transfusions. In this case, it is named TRALI for transfusion-induced acute lung injury [21]. Ischemia/reperfusion injuries can also be produced in nonpulmonary tissues such as gut, kidney, or liver, which might be secondary to ARDS. The i.v. injection of H2O2 can cause ARDS as well. Especially interesting for the ICU patient are so-called two-hit models. These involve a first insult, e.g., development of sepsis or aspiration of gastric content, demanding the use of artificial ventilation as a second hit. This technique takes over active breathing from the lung. Thus, the method has to be adapted to the physiological respiration variables. This means that the pressure used to inflate the lung has to be strong enough to avoid atelectasis but should not be too great to damage pulmonary tissue by overstretching. Because this requires very sensitive pressure setting, ventilation-induced lung injury (VILI) cannot be completely prevented. Hence, modeling these two-hit situations in mice is of great interest for setting up new treatment strategies.
2.2. Oxidative Stress in Pathogenesis of ARDS/ALI
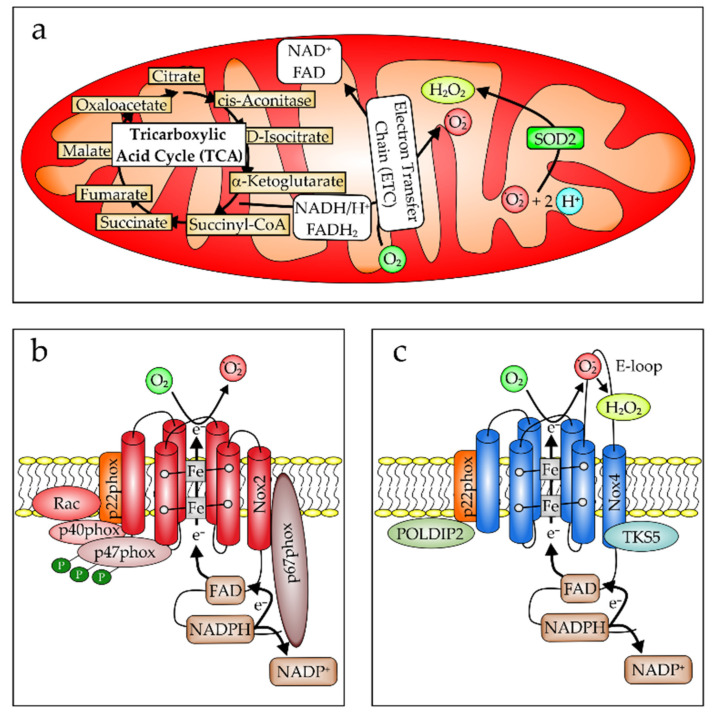
Oxidative stress can be divided into two separate categories. When the intracellular hydrogen peroxide (H2O2) concentration is lower than 100 nM, this is then called “oxidative eustress”, which is a physiological process important for proliferation, differentiation, migration, and angiogenesis (for a review see [114]). This is opposed to an intracellular reactive oxygen species (ROS) level above 100 nM and up to 10 µM, which is pathological or related to host defense and named “oxidative distress”. It is already well established that the generation of oxidative distress is causative in the pathogenesis and progression of ARDS (for recent reviews see [115,116,117]). In brief, ROS can be formed by dying cells, i.e., apoptotic or necrotic cell death [118], as a product of the mitochondrial respiratory chain (Figure 1a) [119] or to fight infections by cells of the innate immune system, such as neutrophil granulocytes or macrophages, by activation of the NADPH oxidase, which in this case is the Nox2 (Figure 1b) [120]. In the lung, accordingly, excessive ROS are formed by damaged pulmonary endothelial and epithelial cells as well as by infiltrating leukocytes, which are predominantly neutrophils [121]. Moreover, alveolar and airway epithelial cells as well as vascular endothelial cells express Nox4 (Figure 1c) [122,123]. This NADPH oxidase, in contrast to Nox2, generates H2O2 and not O2− [128]. Although Nox4-produced H2O2 is an important second messenger, when its expression is induced or its function is activated, it also contributes to lung injury [124].
Figure 1.
Intracellular ROS production. (a) Mitochondria are an important source of intracellular ROS production (mod. from [125]). Being responsible for cellular ATP generation, mitochondria contain the electron transport chain (ETC), and when uncoupled or damaged, ROS can be formed accidentally. The ETC is located in the inner mitochondrial membrane. However, complexes I and III of the respiratory chain also mainly produce O2− in intact mitochondria [126], contributing to the cellular redox load [127]. (b) Phagocytes such as neutrophils and monocytes/MΦ express Nox2. This is a component of a multiprotein complex, formed in the cell membrane upon cell activation. Besides Nox2, which is also named gp91phox, the subunits p40phox, p47phox, p22phox, and p67phox are required to transfer an electron from NADPH to FAD and then via the Fe of the two associated heme groups to O2, leading to generation of the superoxide radical O2−. (c) In contrast, Nox4 is expressed mainly in endothelial and epithelial cells, where it is located at the endoplasmic reticulum and mitochondria. Nox4 only requires the additional subunit p22phox for ROS production, which, in contrast to Nox2, is situated on the E-loop and associated with a direct dismutation of O2− to O2 and H2O2 (mod. from [128]). Because it is constitutively active, Nox4 is regulated by its expression and by binding to factors such as Poldip2 and tyrosine kinase substrate with five SH3 domains (TKS5) (mod. from [129,130]).
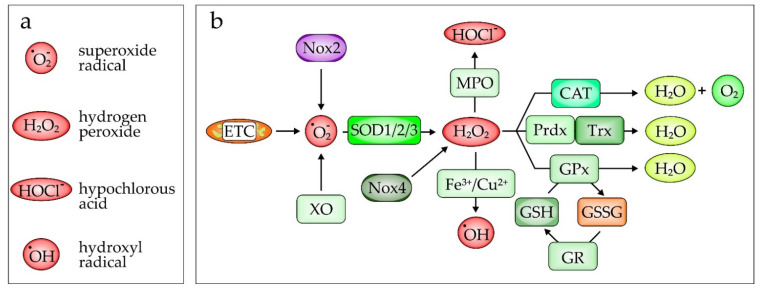
Altering the redox balance by increasing the amount of generated ROS is counterbalanced in part by detoxifying enzymes such as superoxide dismutases (Figure 2). Three isoforms of this enzyme family exist: SOD1 (CuZnSOD), SOD2 (MnSOD), and SOD3 (EC-SOD). These enzymes catalyze the transition from superoxide O2− to H2O2. Although all three enzymes catalyze the same reaction, the proteins differ in their cellular localization [131]. SOD1 is located in the cytoplasm and is important for the removal of O2− mainly derived from NADPH oxidases [132]. SOD2 is predominantly found in mitochondria and is responsible for the conversion of O2− generated from oxidative phosphorylation reactions [133]. Finally, SOD3 is an extracellular enzyme found in the blood and attached to the extracellular matrix, mainly expressed in the lung. SOD3 is important to reduce pulmonary ROS [134,135]. H2O2 can be further metabolized to the hydroxyl radical by the Fenton reaction (Fe2+ + H2O2 → Fe3+ + ˙OH + OH−), the second part of the Haber–Weiss mechanism, leading to the hydroxyl radical (˙OH), which is highly antimicrobial and injurious to cells [136]. To prevent cellular damage, especially in macrophages, it can be completely detoxified by catalase to H2O and O2 (Figure 2b) [137]. In neutrophils, it can be converted by myeloperoxidase (MPO), in the presence of a chloride anion (Cl−), to hypochlorous acid (HOCl−) [138]. Besides enzymes, the redox-sensitive tripeptide γ-L-glutamyl-L-cysteinyl-glycine (glutathione/GSH) is also an important cellular antioxidant [139]. However, the capacity of these naturally occurring antioxidants is limited [140]. This explains why excessive ROS formation cannot be adequately compensated, consequently leading to cell and organ damage [141,142,143]. In ARDS, the breakdown of the endothelial/epithelial barrier is an important hallmark of disease progression [144,145]. Dysfunction of this barrier in ARDS (depicted in Figure 3) is associated with the release of danger-associated molecular patterns (DAMPs), such as HMGB1, from damaged cells [146] or pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides (LPS), originating from the outer membrane of Gram-negative bacteria, or lipoteichoic acid (LTA), arising from Gram-positive bacteria acting via Toll-like receptor-4 or -2 dependent signaling (Figure 3b) [147,148,149]. Originally located in the alveoli, these DAMPs/PAMPs can now diffuse into the vasculature, leading to systemic inflammation (Figure 3c–f). In return, neutrophil granulocytes can more easily pass the endothelial layer when recruited from the blood vessels into the alveoli (Figure 3b) [121]. Thus, as shown in Figure 3c,d, excessive fluids can provoke pulmonary edema, which accumulates in the lung interstitium, inhibiting gas exchange, which is closely associated with hypoxemia, requiring artificial respiration. When edema clearance is appropriately initiated, resolution of the inflammation phase follows (Figure 3e), characterized by phagocytosis of apoptotic neutrophils by MΦ and proliferation or differentiation of alveolar epithelial cells (AT-I and -II), and the function of alveoli is restored (Figure 3f).
Figure 2.
Reactive oxygen species (ROS) and ROS-generating and -scavenging enzymes. (a) ROS involved in ARDS. (b) The superoxide radical O2− is generated by the NADPH oxidase 2 (Nox2), the xanthine oxidase (XO), and the electron transfer chain (ETC) located in the mitochondria. O2− is dismutated to hydrogen peroxide (H2O2) by one of three superoxide dismutases (SODs), which are located in the cytosol (SOD1 ≙ CuZnSOD), in mitochondria (SOD2 ≙ MnSOD), or extracellularly, often associated with the extracellular matrix (SOD3 ≙ EC-SOD). One further source of H2O2 is Nox4, which is located in mitochondria or endoplasmic reticulum (ER) of endothelial as well as epithelial cells. H2O2 is the substrate for the myeloperoxidase-(MPO)-derived oxidant hypochlorous acid (HOCl−), known to cause tissue injury. Stored in neutrophil granules, MPO is released following neutrophil activation. In the Fenton reaction, H2O2 is further metabolized to the highly antimicrobial hydroxyl radical (˙OH). ROS-scavenging enzymes, such as catalase (CAT) or glutathione peroxidase (GPx), detoxify H2O2 to H2O and O2. To achieve this, GPx oxidizes GSH to GSSG, which in return is reduced via the glutathione reductase to GSH. Similarly, peroxiredoxin (Prx), belonging to a small family of peroxidases, reduces H2O2 by oxidizing thioredoxin (Trx), which then is restored to the reduced form by the thioredoxin reductase (not shown).
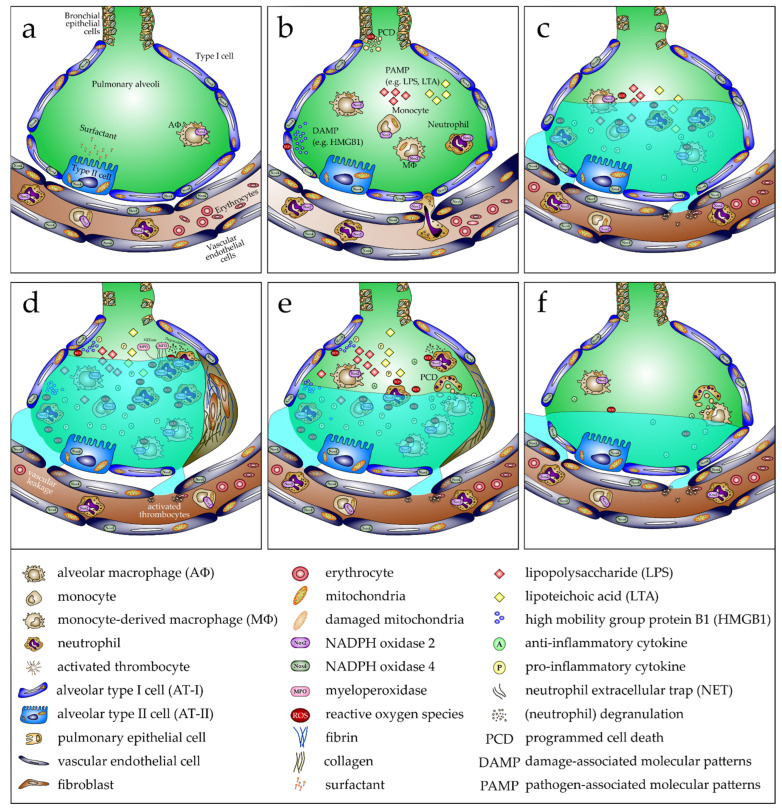
Figure 3.
Development, progression, and resolution of ARDS in response to pathogen-associated molecular patterns (PAMPs). (a) In the healthy lung, alveoli show no neutrophil infiltration and only limited alveolar macrophages (AΦ). Alveolar type II cells (AT-II) produce adequate surfactant to keep the alveolar epithelium covered effectively, thus reducing the surface tension, necessary to prevent a collapse of the alveoli after expiration. Consequently, optimal gas exchange occurs. (b) Following inhalation of bacteria or bacterial components such as lipopolysaccharide (LPS) or lipoteichoic acid (LTA), which are PAMPs, bronchial and alveolar epithelial cells are activated, increasing expression of proinflammatory chemokines and cytokines. This provokes infiltration of immune cells, mainly neutrophils and some monocytes, from the bloodstream. Consequently, the proinflammatory response is enhanced, including proinflammatory cytokines and mediators such as reactive oxygen species (ROS), produced primarily by the phagocytic NADPH oxidase (Nox2) expressed by neutrophils and monocytes/MΦ. Programmed cell death (PCD) of bronchial epithelial cells is induced and HMGB1 as a damage-associated molecular pattern (DAMP) from alveolar type I cells (AT-I) is released, which is also associated with cell demise. (c) Cell death is linked to the damage of the alveolar–capillary barrier, causing lung edema, which significantly reduces lung function with reduced blood gas exchange. (d) High numbers of neutrophils and MΦ in the alveoli, a consequence of cell death and a proinflammatory environment, facilitate proliferation of fibroblasts, expressing fibronectin and collagen. These contribute to fibrosis and reduce the normal function of the alveoli. (e) Reduction of the proinflammatory profile emphasizes an anti-inflammatory response and fibrosis is reversed. Proliferation of alveolar and bronchial cells closes the gap, which arises due to prior cell death. Therefore, lung edema abates. Finally, (f) fibrosis completely reverses, and the cell composition of alveoli is almost completely restored. When edema is also entirely resolved, lung function is reestablished.
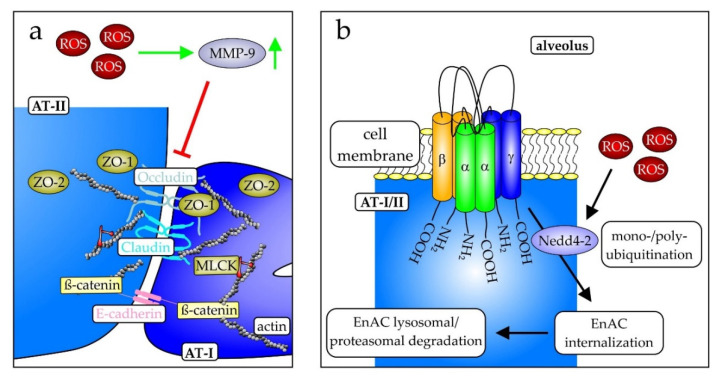
Mechanistically, ROS contribute to the damage of the epithelial barrier. ROS-dependent induction of the matrix metalloproteinase (MMP)-9 causes damage, internalization, and downregulation of proteins of intercellular connections, so-called tight junctions [150], such as claudins, occludins, and E-cadherins, linking the extracellular glycocalyx with the intracellular cytoskeleton (Figure 4a) [151,152]. The loss of cell–cell interactions consequently is associated with an increase in permeability and gap formation [117], leading to edema formation [153]. This is promoted by the similarly reduced expression of the epithelial sodium channel (ENaC), responsible for fluid retention, thus generally counteracting the development of edema [122]. ROS-mediated modification of cysteine 43 of the β-subunit of ENaC permits its ubiquitination by the E3 ubiquitin-protein ligase NEDD4-2, leading to its proteasomal or lysosomal degradation (Figure 4b) [154]. In line with this, a conditional knockout of NEDD4-2 in lung epithelial cells supports this observation [155]. However, the knockout has an adverse effect by lowering the epithelial humidity, favoring fibrosis [155]. Increased ROS, as observed in lungs of PKCα-knockout mice, enhanced ENaC internalization and reduced ENaC expression [156]. The ROS scavenger tempol reversed this effect, further supporting a role of ROS in ENaC regulation [156].
Figure 4.
Important structures in alveolar epithelial cells. (a) The connection between alveolar type I and type II cells is mediated by occludin and claudin, two proteins involved in the formation of tight junctions, and the calcium-dependent cell adhesion protein epithelial (E)-cadherin. These proteins connect cells to the intracellular actin filaments and downstream signaling cascades as exemplified by the myosin light chain kinase (MLCK) via β-catenin and the zona occludens proteins ZO-1 and ZO-2 (mod. from [117]). (b) The epithelial sodium channel (ENaC) is a multimeric protein complex localized in the cell membrane of the pulmonary AT-I and -II cells. It consists of the three homo-dimeric subunits αα, ββ, and γγ and is an important mediator of pulmonary edema clearance and is expressed in two isoforms. One is highly Na+ selective, whereas the other is a cation-nonselective form. During ARDS, several mechanisms provoke downregulation of ENaC expression, apical localization, and activity. ENaC is downregulated by internalization and proteasomal or lysosomal degradation, following Nedd4-2-dependent mono- or poly-ubiquitination. During infection, the downregulation of inflammatory cytokines TNF-α, TGF-β, IL-4, IL-13, and IL-1β contributes to this [mod. from [157,158]).
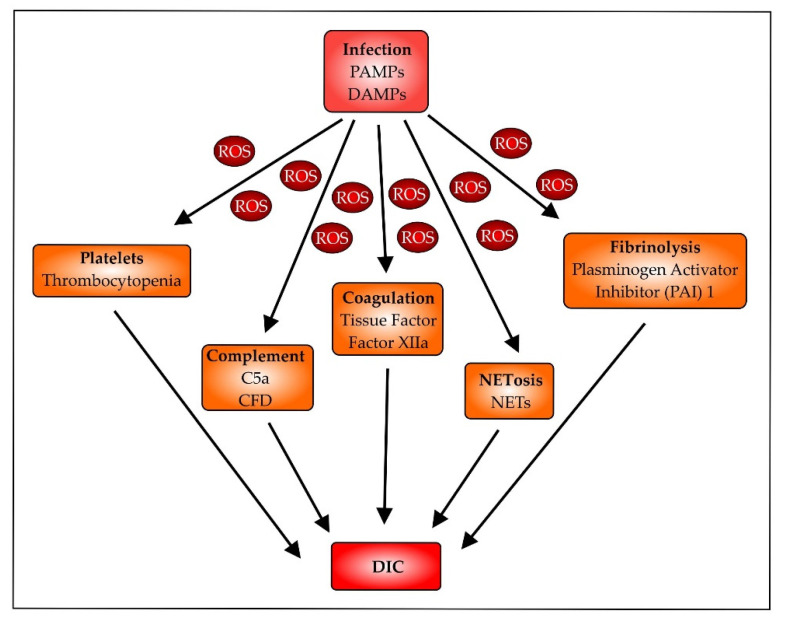
In addition to the effect of ROS on cell–cell interactions, ROS are also involved in altering coagulation and fibrinolysis, contributing to ARDS (Figure 5) [159,160,161]. However, further research is necessary to understand the underlying principles in detail [162]. Studies already performed have shown that ROS mainly contribute to a proinflammatory response, which is associated with activation of platelets and subsequent trapping in the microcirculation leading to thrombocytopenia [163,164]. Nox2 activation is critically involved in platelet activation and thrombosis in response to oxidative stress [165]. The coagulation factors XIIa (FXIIa) and tissue factor (TF) are involved in ROS-mediated effects as well. The contact factor FXIIa, activated by polyphenols released from activated platelets, is an important component in clot formation [166,167], and TF promotes neutrophil extracellular trap (NET) formation, a process which is called NETosis [168], and disease progression [169]. NETosis is directly associated with ROS release, as shown in mice where NADPH oxidase inhibition blocked NETosis and improved thrombosis [163]. Complement activation, a characteristic event in infection, is involved in thrombosis and disseminated intravascular coagulation (DIC). This is especially true when complement activation is unbalanced [170]. The complement factor C5a is an established chemotactic factor attracting immune cells such as neutrophils and macrophages. Subsequently, by binding to the phagocytes, C5a activates a G-protein-coupled signal pathway, leading to Nox2-dependent ROS formation [171]. A similar ROS effect was observed in murine kidney endothelial cells, leading to mitochondria-dependent apoptosis in response to C5a [172]. In addition to compounds leading to coagulation, endogenous fibrinolysis is also reduced. Although fibrinolysis is initially activated, this is counteracted by the simultaneous and sustained upregulation of the plasminogen activator inhibitor-1 (PAI), resulting in enhanced coagulation [173]. Taken together, these mechanisms, in combination, ensure coagulation, thus ensuring that the risk of DIC occurrence remains high [174].
Figure 5.
Disseminated intravascular coagulation (DIC). Infectious pathogens via their associated PAMPs release resultant DAMPs, following cell activation or damage, leading to ROS production. These proinflammatory mediators contribute to the activation of platelets, leading to thrombocytopenia [175]; attract immune cells due to the liberation of the chemotactic complement factor C5a [89]; induce tissue factor (TF) production by endothelial cells [169]; and increase coagulation factor FXIIa [166] and plasminogen activator inhibitor (PAI) I, reducing fibrinolysis [173].
2.3. Antioxidative Treatments
In view of the role of ROS in ARDS development and progression, various possibilities exist to prevent their damaging impact. Pharmacologically, effector proteins, i.e., enzymes involved in ROS formation, can be inhibited, factors important in ROS detoxification can be activated, or their function mimicked. First, we focus on compounds that can be used pharmacologically to reduce ROS formation.
2.3.1. Pharmacological Antioxidants
Pharmacologically, differentiation should be made between factors inhibiting ROS-generating enzymes such as apocynin, developed to inhibit Nox2, thus preventing ARDS in a CLP mouse model [176], or setanaxib, also known as GKT 137831, to block Nox4-dependent H2O2 generation, which in turn reduced lung ischemia/reperfusion injury (LIRI) in mice [177], together with so-called ROS scavengers. These are crucial to detoxify ROS. Among them, classical SOD mimetics such as tempol [178] and EUK-8, which is also a catalase mimetic [73], or SOD-derived compounds such as lecithinized SOD2 (PC-SOD) [51], or recombinant SOD1 [179] and the mitochondria-targeted antioxidants MitoQ, MitoTempo, or tiron are available [180]. The mitochondria-targeted antioxidants are especially suitable when ROS are generated by the mitochondrial ETC and were found to improve VILI in mouse preclinical settings [181]. However, the most important endogenous compound in terms of antioxidant capacity is GSH [182]. This is also true in lung inflammation [183]. As shown in Figure 2b, it is indispensable to the function of glutathione peroxidase (GPx), which detoxifies H2O2 to H2O by oxidizing two GSH to one GSSG, which is accordingly the oxidized form of GSH [139]. Then, the GSH pool is restored by glutathione reductase (GR), which reduces GSSG back to GSH. Based on the pharmacologically available GSH precursor N-acetylcysteine (NAC) (Figure 6), the quantity of GSH can be adjusted therapeutically. This has been investigated also in infectious diseases, where NAC improved the disease pattern and survival in the murine system [49,73,184]. Interestingly, contradictory results have also been observed [185,186]. Therefore, the single use of NAC does not appear to be a sufficient treatment regime in the mouse model [187]. The organoselenium compound ebselen, under development for therapy of a variety of clinical conditions involving oxidative stress, reacts with GSH in a cyclic mechanism similar to that of GPx, thereby inactivating hydroperoxides, including H2O2 and regenerating GSH [188,189,190]. Oral administration of ebselen inhibits ozone-induced lung inflammation in rats [191]. It has also been proposed as a potential treatment for respiratory inflammation in COVID-19 infection, since it also reacts with the free thiol group of the main protease (Mpro) of the coronavirus SARS-CoV-2 to inhibit the protease [192].
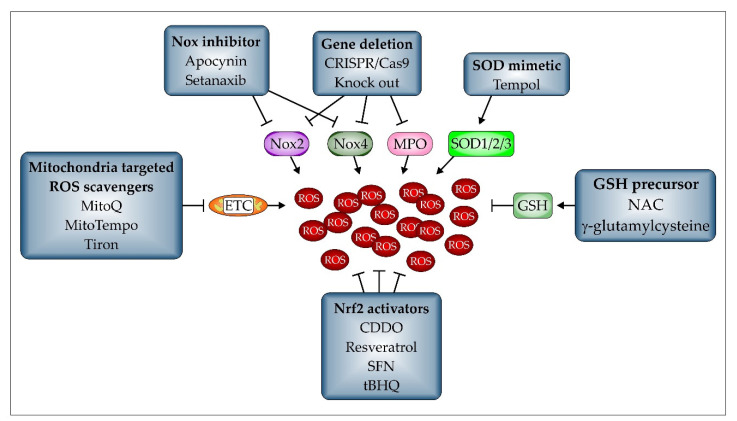
Figure 6.
Therapeutic concepts to reduce and prevent ROS formation. Several approaches have been tested in the murine model to reduce and prevent ROS formation during lung inflammation. Considering the major factors involved in ROS formation, such as Nox2, Nox4, MPO, and the ETC, specific inhibitors or compounds targeted to mitochondria were shown to be effective in improving ALI. SOD mimetics, which only reduce the amount of generated O2−, have also been found to have an impact. Additionally, GSH precursors, maintaining a high intra- and extracellular GSH-pool, leading to a more reductive environment, are potent in depleting ROS. Finally, activators leading to the stabilization and thus activation of the transcription factor Nrf2 have been shown to significantly contribute to the expression of factors important in detoxifying ROS. However, one further possibility in the murine system, gene deletion, is still difficult to achieve in the human patient.
Vitamin C has also been identified as an antioxidant with a protective function in murine acute lung injury [193]. The mice received either ascorbic acid (AscA) or dehydroascorbic acid (DHA) 30 min following abdominal sepsis induction by intraperitoneal cecal slurry injection [194]. Both treatments reduced lung injury by differing mechanisms, involving reduced epithelial barrier breakdown, maintaining alveolar fluid clearance, preventing tight junction loss, and inhibiting rearrangement of the cytoskeleton [194].
2.3.2. Nrf2
The most important transcription factor in regulating antioxidant genes is the cap-and-collar type transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2) [195]. Its expression is regulated under healthy conditions by Keap1, which targets Nrf2 for proteasomal degradation by the Cullin3/Rbx1 ubiquitination system [196]. Upon (electrophilic) oxidative stress, cysteines in Keap1 are oxidized and Nrf2 is released [196], consequently leading to its stabilization and subsequent induction of target gene expression [197]. In keeping with its role as a transcription factor, Nrf2 activates the expression of factors involved in detoxifying ROS, such as glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), GPx, GR, heme-oxygenase (HO-1), Prx, and SOD3 [197]. Thus, activation of Nrf2 leads to an antioxidative response, which is generally associated with an improved outcome of lung injury in mouse models [198]. In line with this, Nrf2-deficient mice show enhanced acute lung injury compared to wild-type mice following intestinal ischemia/reperfusion [199]. Considering that Nrf2 binds to antioxidative-response elements (AREs) in the promoter regions of target genes, their activation can also be followed in an ARE-reporter mouse, where the ARE site has been associated with a luciferase (Luc) reporter gene. In these mice, Nrf2 activation provokes luciferase expression in parallel, which can be determined by bioluminescence imaging [200]. In association with its anti-inflammatory function, Nrf2 prevents classical activation of MΦ to an M1 phenotype and promotes alternative activation, resulting in an M2 MΦ phenotype. This was reversed by an Nrf2 siRNA approach knocking down the transcription factor [201]. In these studies, established pharmacological treatments, such as tert-butylhydroquinone (tBHQ) [201], bardoxolone (CDDO) [202], resveratrol [203], and sulforaphane (SFN) [200], stabilized Nrf2 by modulating its binding to Keap1. Interestingly, stabilization of Nrf2 has also been shown to occur indirectly. Among others, the tyrosine kinase inhibitor dasatinib counteracts LPS-dependent ALI. This effect was associated with increased Nrf2 expression and activation as observed by an enhanced expression of the Nrf2 target gene HO-1 in lung tissue [204]. This treatment promotes a similar M2 polarization of MΦ. The above-mentioned mitochondria-targeted ROS scavenger mitoQ reduced ROS formation in an intraperitoneal LPS mouse model by activating Nrf2 and the corresponding expression of target genes [205]. This improvement was largely abolished in Nrf2 knockout mice. Mechanistically, mitoQ-mediated Nrf2 stabilization induced the expression of HO-1, which in turn blocks LPS-upregulated expression of the mitochondria fission factor Drp1 in alveolar epithelial cells [206]. Thus, fission of mitochondria, associated with cytochrome c release, concomitant caspase 3 activation, and apoptosis leading to the breakdown of the epithelial barrier, is prevented [206]. Since sex-specific effects in the response to antioxidative treatments are possible, Callaway et al. [42] demonstrated in a hyperoxic lung injury model that Nrf2 knockout especially in female animals reduced survival. Independently of sex, treatment with the cytochrome P450 (CYP) 1A inducer β-napthoflavone (BNF) improved animal survival, eliminating sex differences [42]. Therefore, these data might open up a novel option for a treatment regime in patients with a relative NRF2 deficiency. Furthermore, it has been shown that Nrf2 induction is associated with a decrease in NF-κB activation [207]. However, recent data suggest that Nrf2 is activated in response to mild hyperoxia (30% O2), whereas following high hyperoxia (100% O2) the increase in oxidative stress is linked to the activation of Nrf2 and NF-κB in parallel. Very high hyperoxia (140% O2) activated NF-κB almost exclusively [208].
2.3.3. PPARγ
This ligand-dependent nuclear hormone receptor PPARγ was identified on the basis of its role in glucose metabolism. Recent studies support a second role of PPARγ as an anti-inflammatory, sometimes proapoptotic factor. It has been shown that activation of PPARγ inhibited activation of Nox2 in macrophages [209]. It is an important regulator of MΦ polarization, which was further substantiated using PPARγ knockout mice [210,211]. Moreover, PPARγ is also a target of direct redox regulation, i.e., cysteine-residue modification [212]. In ARDS research, activation of PPARγ with the ligand rosiglitazone restored surface expression of the ENaC channel on alveolar type II epithelial cells (Figure 3a) and ameliorated acute lung injury in an intratracheal LPS mouse model, showing upregulation of ENaC expression as well [213]. In the study by Wang et al. [214] activation of PPARγ attenuated LPS-induced acute lung injury by preventing HMGB1 release and decreasing RAGE levels, both known to be upregulated in ARDS mouse models [215]. Considering PPARγ as a negative regulator of macrophage activation [216], metabolic and epigenetic changes leading to PPARγ expression or activation can also alter the phenotype of alveolar MΦs, which are important contributors to sepsis-initiated ARDS. In this regard, α-ketoglutarate, originating as an intermediate from the tricarboxylic acid (TCA) cycle, has recently been characterized as a PPARγ agonist [217]. This is especially important in classically activated, so-called M1 MΦ, known to show a reduced TCA circle with an emphasis on the glycolytic metabolism to generate ATP, compared to alternatively activated M2 MΦ, relying on oxidative metabolism to produce ATP [218,219]. Thus, Liu et al. observed an α-ketoglutarate-dependent inhibition of alveolar MΦs, which was corroborated by the cellular localization of PPARγ, which was mainly nuclear after α-ketoglutarate exposure in the murine alveolar MΦ cell line MH-S [220]. In connection with the epigenetic regulation of PPARγ, Bao et al. demonstrated that a key epigenetic regulator, the histone methyltransferase enhancer of zeste homolog 2 (EZH2), alters the expression profile of alveolar MΦ in LPS-mediated ARDS in mice [185]. Genetic knockdown of EZH2 and pharmacological inhibition of EZH2 by 3-dezaneplanocin suppressed an M1 MΦ phenotype, while promoting M2 MΦs, by permitting activation of STAT6 and PPARγ [185]. Correspondingly, inhibition of PPARγ is associated with an enhanced inflammatory immune response, for instance with fibroproliferative ARDS following bleomycin intratracheal instillation [221]. However, endogenously activated PPARγ also provoked T cell depletion in a CLP mouse model, contributing to immune paralysis and a worse outcome [222]. Such interventions in sepsis could, therefore, only be considered with a close assessment of the ongoing immune status.
2.4. Mouse Data Translated to the ARDS Patients’ Situation
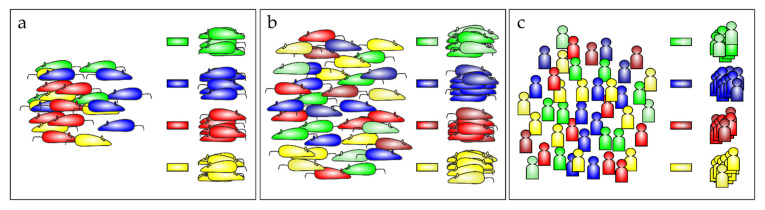
Although antioxidative therapy concepts have been successfully employed in the mouse model as outlined above, translating these approaches to the human patient has not been very successful. One reason for this failure is the heterogeneous multifactorial etiology of ARDS [223,224], which makes it difficult to establish an effective standard treatment regime. Moreover, in the mouse models, mainly young mice of one sex have been used to determine whether the individual treatments improved animal survival. Generally, in animals, this type of study does not require any IC support during the experimental phase, which is of course mandatory in the human ICU. Therefore, disease progression in the mouse model cannot be adequately followed. Experimental settings are planned to control the health state of mice, providing estimates of the time of disease onset, which is crucial to start therapy on time and to draw firm conclusions from the data obtained. However, this rating is vague, and accordingly, the evidence generated is limited. Hence, it would be more appropriate to follow disease progression in the animal with similar equipment to that in the human ICU which facilitates adequate translation of the mouse data to the human situation. In addition, the mouse metabolic rate is significantly different from humans [225], which is even enhanced during sepsis [226]. Moreover, mouse models need to be extended to experiments with aged mice, thus better reflecting the ICU situation. Keeping in mind the different conditions that ultimately result in ARDS, also in the murine system [100], it would be appropriate to group data from the animals according to the specific etiologies, courses of disease, treatments, and subsequent responses. Correspondingly, grouped animals might lead to the identification of different treatment regimens (Figure 7a). Broadening the marker panel would possibly allow the inclusion of slightly differing animals into the same test groups, to determine whether these also respond to the treatment (Figure 7b). Finally, with treatment concepts established in this preclinical setting, data from clinical trials can be reanalyzed in relation to the mouse results, allowing similar group assignments (Figure 7c). Transferring these retrospective analyses to the active ICU may open new therapeutic concepts with a special focus on personalized medicine. First attempts have been proposed and already been made to subphenotype ARDS patients [227,228,229,230], although some data are discouraging [231].
Figure 7.
Precision medicine from mice to men. (a) Grouping animals according to the characteristics of ARDS origin or disease pattern might be advantageous for the optimization of treatment. However, (b) whether a more global classification, integrating some broader aspects, will provide a greater advance, needs to be tested. (c) This approach is also likely to be helpful for patients suffering from ARDS, allowing specific personalized treatment of the corresponding patient group.
3. Conclusions and Outlook
Recently, two reviews summarized pharmacological treatments for patients suffering from ARDS [232,233]. Although, the main message of both reviews is that there is no appropriate pharmacological therapy option, but only supportive care, they agree on the necessity to subgroup ARDS patients to identify personalized treatment concepts. With respect to examples of mouse models of ARDS discussed here, which respond to antioxidative treatment, putative care concepts in the ICU might also include an antioxidative approach as a component of the medical care. This seems especially interesting since, as an antioxidative concept concerning viral infections associated with ARDS described here, the use of NAC as a putative (adjuvant) therapy concept in COVID-19 infection, like that of ebselen mentioned previously, has also been suggested [234] and summarized in [235].
Author Contributions
This work was conceptualized and written by A.v.K., U.H., V.L., M.J.P., A.U.S. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. M.J.P. is currently an employee of EpiEndo Pharmaceuticals ehf. Iceland.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress syndrome in adults. Lancet. 1967;61:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., LeGall J.R., Morris A., Spragg R., et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. J. Crit. Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 3.Luterman A., Horovitz J.H., Carrico C.J., Canizaro P.C., Heimbach D., Colocousis J. Withdrawal from positive end-expiratory pressure. Surgery. 1978;83:328–332. doi: 10.1097/00132586-197906000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Esteban A., Fernández-Segoviano P., Frutos-Vivar F., Aramburu J.A., Nájera L., Ferguson N.D., Alía I., Gordo F., Ríos F. Comparison of Clinical Criteria for the Acute Respiratory Distress Syndrome With Autopsy Findings. Ann. Intern. Med. 2004;141:440–445. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson N.D., Davis A.M., Slutsky A.S., Stewart T.E. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. J. Crit. Care. 2005;20:147–154. doi: 10.1016/j.jcrc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Raghavendran K., Napolitano L.M. Definition of ALI/ARDS. Crit. Care Clin. 2011;27:429–437. doi: 10.1016/j.ccc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Riviello E.D., Kiviri W., Twagirumugabe T., Mueller A., Banner-Goodspeed V.M., Officer L., Novack V., Mutumwinka M., Talmor D.S., Fowler R.A. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am. J. Respir. Crit. Care Med. 2016;193:52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]
- 9.Fan E., Brodie D., Slutsky A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 10.Ficial B., Vasques F., Zhang J., Whebell S., Slattery M., Lamas T., Daly K., Agnew N., Camporota L. Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure. Membranes. 2021;11:225. doi: 10.3390/membranes11030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin C., Reignier J., Richard J.-C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 12.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., de Moraes Paisani D., Damiani L.P., Guimarães H.P., Romano E.R., de Moraes Regenga M., Taniguchi L.N.T., Teixeira C., et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzumura E.A., Amato M.B.P., Cavalcanti A.B. Understanding recruitment maneuvers. Intensive Care Med. 2016;42:908–911. doi: 10.1007/s00134-015-4025-5. [DOI] [PubMed] [Google Scholar]
- 14.Wei X.-B., Wang Z.-H., Liao X.-L., Guo W.-X., Qin T.-H., Wang S.-H. Role of Neuromuscular Blocking Agents in Acute Respiratory Distress Syndrome: An Updated Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019;10:1637. doi: 10.3389/fphar.2019.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y.-Q., Ding X.-F., Liang H.-Y., Wang D., Zhang X.-J., Li L.-F., Kan Q.-C., Wang L.-X., Sun T.-W. Efficacy and safety of low-dose corticosteroids for acute respiratory distress syndrome: A systematic review and meta-analysis. World J. Emerg. Med. 2021;12:207–213. doi: 10.5847/wjem.j.1920-8642.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aeffner F., Bolon B., Davis I.C. Mouse Models of Acute Respiratory Distress Syndrome: A Review of Analytical Approaches, Pathologic Features, and Common Measurements. Toxicol. Pathol. 2015;43:1074–1092. doi: 10.1177/0192623315598399. [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello G., Downey G., Moore B.B., Groshong S.D., Matthay M.A., Slutsky A.S., Kuebler W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joelsson J.P., Ingthorsson S., Kricker J., Gudjonsson T., Karason S. Ventilator-induced lung-injury in mouse models: Is there a trap? Lab. Anim. Res. 2021;37:30. doi: 10.1186/s42826-021-00108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S., Chen Q., Liu J., Yang X., Zhang Y., Huang F. Sinomenine protects against E. coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed. Pharmacother. 2018;107:696–702. doi: 10.1016/j.biopha.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 20.El Kebir D., Gjorstrup P., Filep J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semple J.W., Rebetz J., Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840–1853. doi: 10.1182/blood-2018-10-860809. [DOI] [PubMed] [Google Scholar]
- 22.Chimenti L., Morales-Quinteros L., Puig F., Camprubi-Rimblas M., Guillamat-Prats R., Gómez M.N., Tijero J., Blanch L., Matute-Bello G., Artigas A. Comparison of direct and indirect models of early induced acute lung injury. Intensive Care Med. Exp. 2020;8:62. doi: 10.1186/s40635-020-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro A.P., Mayer C.A., Wilson C.G., Martin R.J., MacFarlane P.M. Intratracheal LPS administration attenuates the acute hypoxic ventilatory response: Role of brainstem IL-1β receptors. Respir. Physiol. Neurobiol. 2017;242:45–51. doi: 10.1016/j.resp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Peritore A.F., D’amico R., Siracusa R., Cordaro M., Fusco R., Gugliandolo E., Genovese T., Crupi R., Di Paola R., Cuzzocrea S., et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021;22:5533. doi: 10.3390/ijms22115533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khadangi F., Forgues A.-S., Tremblay-Pitre S., Dufour-Mailhot A., Henry C., Boucher M., Beaulieu M.-J., Morissette M., Fereydoonzad L., Brunet D., et al. Intranasal versus intratracheal exposure to lipopolysaccharides in a murine model of acute respiratory distress syndrome. Sci. Rep. 2021;11:7777. doi: 10.1038/s41598-021-87462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Q., Lin L., Chen L., Cheng L., Zhong W. Co-administration of N-acetylcysteine and dexmedetomidine plays a synergistic effect on protection of LPS-induced acute lung injury via correcting Th1/Th2/Th17 cytokines imbalance. Clin. Exp. Pharmacol. Physiol. 2020;47:294–301. doi: 10.1111/1440-1681.13196. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H., Wang Z., Liu R., Qian T., Liu J., Wang L., Chu Y. Reactive oxygen species stimulated pulmonary epithelial cells mediate the alveolar recruitment of FasL+ killer B cells in LPS-induced acute lung injuries. J. Leukoc. Biol. 2018;104:1187–1198. doi: 10.1002/JLB.3A0218-075R. [DOI] [PubMed] [Google Scholar]
- 29.Mo M., Li S., Dong Z., Li C., Sun Y., Li A., Zhao Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharmacol. 2020;81:106273. doi: 10.1016/j.intimp.2020.106273. [DOI] [PubMed] [Google Scholar]
- 30.Gugliandolo E., Fusco R., Ginestra G., D’amico R., Bisignano C., Mandalari G., Cuzzocrea S., Di Paola R. Involvement of TLR4 and PPAR-α Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection With Pseudomonas Aeruginosa. Shock. 2019;51:221–227. doi: 10.1097/SHK.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 31.Aoyagi T., Newstead M.W., Zeng X., Nanjo Y., Peters-Golden M., Kaku M., Standiford T.J. Interleukin-36γ and IL-36 receptor signaling mediate impaired host immunity and lung injury in cytotoxic Pseudomonas aeruginosa pulmonary infection: Role of prostaglandin E2. PLoS Pathog. 2017;13:e1006737. doi: 10.1371/journal.ppat.1006737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh W.M., Russell W.W., Fleszar A.J., Rodenhouse P.E., Rietberg S.P., Sun L., Shanley T.P., Cornell T.T. Protein phosphatase 2A activation attenuates inflammation in murine models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;311:L903–L912. doi: 10.1152/ajplung.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B., Swamy S., Balijepalli S., Panicker S., Mooliyil J., Sherman M.A., Parkkinen J., Raghavendran K., Suresh M.V. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33:13294–13309. doi: 10.1096/fj.201901047RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto S., Amaya F., Oh-Hashi K., Kiuchi K., Hashimoto S. Expression of neutral endopeptidase activity during clinical and experimental acute lung injury. Respir. Res. 2010;11:164. doi: 10.1186/1465-9921-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki-Nagase T., Nagase T., Oh-Hashi Y., Kurihara Y., Yamaguchi Y., Yamamoto H., Nagata T., Kurihara H., Ouchi Y. Calcitonin gene-related peptide mediates acid-induced lung injury in mice. Respirology. 2007;12:807–813. doi: 10.1111/j.1440-1843.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 36.Zarbock A., Schmolke M., Spieker T., Jurk K., van Aken H., Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: A critical role for uremic neutrophils. J. Am. Soc. Nephrol. 2006;17:3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- 37.Puri G., Naura A.S. Critical role of mitochondrial oxidative stress in acid aspiration induced ALI in mice. Toxicol. Mech. Methods. 2020;30:266–274. doi: 10.1080/15376516.2019.1710888. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Q., Basit A., Liang W., Qu R., Luan Y., Ren C., Li A., Xu X., Liu X., Yang C., et al. Pazopanib ameliorates acute lung injuries via inhibition of MAP3K2 and MAP3K3. Sci. Transl. Med. 2021;13:eabc2499. doi: 10.1126/scitranslmed.abc2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey T.C., Martin E.L., Zhao L., Veldhuizen R.A.W. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J. Appl. Physiol. (1985) 2003;94:975–982. doi: 10.1152/japplphysiol.00619.2002. [DOI] [PubMed] [Google Scholar]
- 40.Kawaguchi T., Yanagihara T., Yokoyama T., Suetsugu-Ogata S., Hamada N., Harada-Ikeda C., Suzuki K., Maeyama T., Kuwano K., Nakanishi Y. Probucol attenuates hyperoxia-induced lung injury in mice. PLoS ONE. 2017;12:e0175129. doi: 10.1371/journal.pone.0175129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah D., Das P., Acharya S., Agarwal B., Christensen D.J., Robertson S.M., Bhandari V. Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS) Int. J. Mol. Sci. 2021;22:2573. doi: 10.3390/ijms22052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callaway D.A., Jiang W., Wang L., Lingappan K., Moorthy B. Oxygen-mediated lung injury in mice lacking the gene for NRF2: Rescue with the cytochrome P4501A-inducer, beta-naphthoflavone (BNF), and differential sex-specific effects. Free Radic. Biol. Med. 2020;160:208–218. doi: 10.1016/j.freeradbiomed.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel V., Dial K., Wu J., Gauthier A.G., Wu W., Lin M., Espey M.G., Thomas D.D., Ashby C.R., Mantell L.L. Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1. Int. J. Mol. Sci. 2020;21:977. doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Y.G., Zhang J. Protective effect of SIRT3 on acute lung injury by increasing manganese superoxide dismutase-mediated antioxidation. Mol. Med. Rep. 2018;17:5557–5565. doi: 10.3892/mmr.2018.8469. [DOI] [PubMed] [Google Scholar]
- 45.Szabari M.V., Takahashi K., Feng Y., Locascio J.J., Chao W., Carter E.A., Vidal Melo M.F., Musch G. Relation between Respiratory Mechanics, Inflammation, and Survival in Experimental Mechanical Ventilation. Am. J. Respir. Cell Mol. Biol. 2019;60:179–188. doi: 10.1165/rcmb.2018-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson M.R., Patel B.V., Takata M. Ventilation with “clinically relevant” high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit. Care Med. 2012;40:2850–2857. doi: 10.1097/CCM.0b013e31825b91ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lex D., Uhlig S. One-hit models of ventilator-induced lung injury-benign inflammation versus inflammation as a by-product. Anesthesiology. 2017;126:909–922. doi: 10.1097/ALN.0000000000001605. [DOI] [PubMed] [Google Scholar]
- 48.Dolinay T., Kim Y.S., Howrylak J., Hunninghake G.M., An C.H., Fredenburgh L., Massaro A.F., Rogers A., Gazourian L., Nakahira K., et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papaiahgari S., Yerrapureddy A., Reddy S.R., Reddy N.M., Dodd-O J.M., Crow M.T., Grigoryev D.N., Barnes K., Tuder R.M., Yamamoto M., et al. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 2007;176:1222–1235. doi: 10.1164/rccm.200701-060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher A.B., Dodia C., Chatterjee S. A Peptide Inhibitor of Peroxiredoxin 6 Phospholipase A2 Activity Significantly Protects against Lung Injury in a Mouse Model of Ventilator Induced Lung Injury (VILI) Antioxidants. 2021;10:925. doi: 10.3390/antiox10060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka K.-I., Tamura F., Sugizaki T., Kawahara M., Kuba K., Imai Y., Mizushima T. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. Am. J. Respir. Cell Mol. Biol. 2017;56:179–190. doi: 10.1165/rcmb.2016-0158OC. [DOI] [PubMed] [Google Scholar]
- 52.Veskemaa L., Graw J.A., Pickerodt P.A., Taher M., Boemke W., González-López A., Francis R.C.E. Tert-butylhydroquinone augments Nrf2-dependent resilience against oxidative stress and improves survival of ventilator-induced lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;320:L17–L28. doi: 10.1152/ajplung.00131.2020. [DOI] [PubMed] [Google Scholar]
- 53.Sun L., Hult E.M., Cornell T.T., Kim K.K., Shanley T.P., Wilke C.A., Agarwal M., Gurczynski S.J., Moore B.B., Dahmer M.K. Loss of myeloid-specific protein phosphatase 2A enhances lung injury and fibrosis and results in IL-10-dependent sensitization of epithelial cell apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L1035–L1048. doi: 10.1152/ajplung.00299.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaver C.M., Grove B.S., Clune J.K., Mackman N., Ware L.B., Bastarache J.A. Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci. Rep. 2016;6:22249. doi: 10.1038/srep22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaRivière W.B., Liao S., McMurtry S.A., Oshima K., Han X., Zhang F., Yan S., Haeger S.M., Ransom M., Bastarache J.A., et al. Alveolar heparan sulfate shedding impedes recovery from bleomycin-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;318:L1198–L1210. doi: 10.1152/ajplung.00063.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keum H., Kim D., Kim J., Kim T.W., Whang C.-H., Jung W., Jon S. A bilirubin-derived nanomedicine attenuates the pathological cascade of pulmonary fibrosis. Biomaterials. 2021;275:120986. doi: 10.1016/j.biomaterials.2021.120986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fusco R., Cordaro M., Genovese T., Impellizzeri D., Siracusa R., Gugliandolo E., Peritore A.F., D’amico R., Crupi R., Cuzzocrea S., et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants. 2020;9:601. doi: 10.3390/antiox9070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allawzi A., McDermott I., Delaney C., Nguyen K., Banimostafa L., Trumpie A., Hernandez-Lagunas L., Riemondy K., Gillen A., Hesselberth J., et al. Redistribution of EC-SOD resolves bleomycin-induced inflammation via increased apoptosis of recruited alveolar macrophages. FASEB J. 2019;33:13465–13475. doi: 10.1096/fj.201901038RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Y., Huang W., Wu F., Shang J., Ping F., Wang W., Li Y., Zhao X., Zhang X. ZFP36 protects lungs from intestinal I/R-induced injury and fibrosis through the CREBBP/p53/p21/Bax pathway. Cell Death Dis. 2021;12:685. doi: 10.1038/s41419-021-03950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K., Xu Z., Liu Y., Wang Z., Li Y., Xu X., Chen C., Xia T., Liao Q., Yao Y., et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017;9:eaao6298. doi: 10.1126/scitranslmed.aao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dodd-o J.M., Hristopoulos M.L., Kibler K., Gutkowska J., Mukaddam-Daher S., Gonzalez A., Welsh-Servinsky L.E., Pearse D.B. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L714–L723. doi: 10.1152/ajplung.00185.2007. [DOI] [PubMed] [Google Scholar]
- 62.Xiong S., Hong Z., Huang L.S., Tsukasaki Y., Nepal S., Di A., Zhong M., Wu W., Ye Z., Gao X., et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020;130:3684–3698. doi: 10.1172/JCI136908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou Y., Bao S., Wang F., Guo L., Zhu J., Wang J., Deng X., Li J. FN14 Blockade on Pulmonary Microvascular Endothelial Cells Improves the Outcome of Sepsis-Induced Acute Lung Injury. Shock. 2018;49:213–220. doi: 10.1097/SHK.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 64.Murando F., Peloso A., Cobianchi L. Experimental Abdominal Sepsis: Sticking to an Awkward but Still Useful Translational Model. Mediat. Inflamm. 2019;2019:8971036. doi: 10.1155/2019/8971036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fallon E.A., Chung C.-S., Heffernan D.S., Chen Y., de Paepe M.E., Ayala A. Survival and Pulmonary Injury After Neonatal Sepsis: PD1/PDL1’s Contributions to Mouse and Human Immunopathology. Front. Immunol. 2021;12:634529. doi: 10.3389/fimmu.2021.634529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollenberg S.M., Dumasius A., Easington C., Colilla S.A., Neumann A., Parrillo J.E. Characterization of a Hyperdynamic Murine Model of Resuscitated Sepsis Using Echocardiography. Am. J. Respir. Crit. Care Med. 2001;164:891–895. doi: 10.1164/ajrccm.164.5.2010073. [DOI] [PubMed] [Google Scholar]
- 67.Neumann B., Zantl N., Veihelmann A., Emmanuilidis K., Pfeffer K., Heidecke C.D., Holzmann B. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 1999;11:217–227. doi: 10.1093/intimm/11.2.217. [DOI] [PubMed] [Google Scholar]
- 68.Constantino L., Gonçalves R.C., Giombelli V.R., Tomasi C.D., Vuolo F., Kist L.W., de Oliveira G.M.T., de Bittencourt Pasquali M.A., Bogo M.R., Mauad T., et al. Regulation of lung oxidative damage by endogenous superoxide dismutase in sepsis. Intensive Care Med. Exp. 2014;2:17. doi: 10.1186/2197-425X-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X., An X., Wang X., Hu X., Bi J., Tong L., Yang D., Song Y., Bai C. Peroxiredoxin 6 knockout aggravates cecal ligation and puncture-induced acute lung injury. Int. Immunopharmacol. 2019;68:252–258. doi: 10.1016/j.intimp.2018.12.053. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Z., Kozlowski J., Schuster D.P. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2005;172:344–351. doi: 10.1164/rccm.200503-343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tesse A., Gena P., Rützler M., Calamita G. Ablation of Aquaporin-9 Ameliorates the Systemic Inflammatory Response of LPS-Induced Endotoxic Shock in Mouse. Cells. 2021;10:435. doi: 10.3390/cells10020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colón D.F., Wanderley C.W., Franchin M., Silva C.M., Hiroki C.H., Castanheira F.V.S., Donate P.B., Lopes A.H., Volpon L.C., Kavaguti S.K., et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care. 2019;23:113. doi: 10.1186/s13054-019-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baboolal H.A., Ichinose F., Ullrich R., Kawai N., Bloch K.D., Zapol W.M. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology. 2002;97:1227–1233. doi: 10.1097/00000542-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 74.Iyer S.S., Jones D.P., Brigham K.L., Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2009;40:90–98. doi: 10.1165/rcmb.2007-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonai F., Priber J.K., Jakus P.B., Kalman N., Antus C., Pollak E., Karsai G., Tretter L., Sumegi B., Veres B. Lack of cyclophilin D protects against the development of acute lung injury in endotoxemia. Biochim. Biophys. Acta. 2015;1852:2563–2573. doi: 10.1016/j.bbadis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Xu X., Zhu Q., Niu F., Zhang R., Wang Y., Wang W., Sun D., Wang X., Wang A. A2BAR activation attenuates acute lung injury by inhibiting alveolar epithelial cell apoptosis both in vivo and in vitro. Am. J. Physiol. Cell Physiol. 2018;315:C558–C570. doi: 10.1152/ajpcell.00294.2017. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Z., Xu D., Li S., He B., Huang Y., Xu M., Ren S., Li S., Wang H., Xie W. Activation of Liver X Receptor Attenuates Oleic Acid-Induced Acute Respiratory Distress Syndrome. Am. J. Pathol. 2016;186:2614–2622. doi: 10.1016/j.ajpath.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong H.-Y., Xu M., Ji Z.-Y., Wang Y.-X., Dong M.-Q., Liu M.-L., Xu D.-Q., Zhao P.-T., Liu Y., Luo Y., et al. Leptin attenuates lipopolysaccharide or oleic acid-induced acute lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2013;49:1057–1063. doi: 10.1165/rcmb.2012-0301OC. [DOI] [PubMed] [Google Scholar]
- 79.Kapur R., Kim M., Rebetz J., Hallström B., Björkman J.T., Takabe-French A., Kim N., Liu J., Shanmugabhavananthan S., Milosevic S., et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Adv. 2018;2:1651–1663. doi: 10.1182/bloodadvances.2018018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hechler B., Maître B., Magnenat S., Heim V., El Mdawar M.B., Gachet C., de la Salle H. Platelets are dispensable for antibody-mediated transfusion-related acute lung injury in the mouse. J. Thromb. Haemost. 2016;14:1255–1267. doi: 10.1111/jth.13335. [DOI] [PubMed] [Google Scholar]
- 81.Kapur R., Kim M., Shanmugabhavananthan S., Liu J., Li Y., Semple J.W. C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury. Blood. 2015;126:2747–2751. doi: 10.1182/blood-2015-09-672592. [DOI] [PubMed] [Google Scholar]
- 82.Fung Y.L., Tung J.P. How different animal models help us understand TRALI. ISBT Sci. Ser. 2018;13:197–205. doi: 10.1111/voxs.12423. [DOI] [Google Scholar]
- 83.Semple J.W., Kim M., Hou J., McVey M., Lee Y.J., Tabuchi A., Kuebler W.M., Chai Z.-W., Lazarus A.H. Intravenous immunoglobulin prevents murine antibody-mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS ONE. 2012;7:e31357. doi: 10.1371/journal.pone.0031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van der Zeeuw Laan E.A.N., van der Velden S., Porcelijn L., Semple J.W., van der Schoot C.E., Kapur R. Update on the pathophysiology of transfusion-related acute lung injury. Curr. Opin. Hematol. 2020;27:386–391. doi: 10.1097/MOH.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 85.Strait R.T., Hicks W., Barasa N., Mahler A., Khodoun M., Köhl J., Stringer K., Witte D., van Rooijen N., Susskind B.M., et al. MHC class I-specific antibody binding to nonhematopoietic cells drives complement activation to induce transfusion-related acute lung injury in mice. J. Exp. Med. 2011;208:2525–2544. doi: 10.1084/jem.20110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langgartner D., Palmer A., Rittlinger A., Reber S.O., Huber-Lang M. Effects of Prior Psychosocial Trauma on Subsequent Immune Response After Experimental Thorax Trauma. Shock. 2018;49:690–697. doi: 10.1097/SHK.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 87.Kalbitz M., Karbach M., Braumueller S., Kellermann P., Gebhard F., Huber-Lang M., Perl M. Role of Complement C5 in Experimental Blunt Chest Trauma-Induced Septic Acute Lung Injury (ALI) PLoS ONE. 2016;11:e0159417. doi: 10.1371/journal.pone.0159417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen Z., Fan L., Li Y., Zou Z., Scott M.J., Xiao G., Li S., Billiar T.R., Wilson M.A., Shi X., et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: Role in posthemorrhagic shock acute lung inflammation. J. Immunol. 2014;193:4623–4633. doi: 10.4049/jimmunol.1400899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barrett C.D., Hsu A.T., Ellson C.D., Miyazawa B.Y., Kong Y.-W., Greenwood J.D., Dhara S., Neal M.D., Sperry J.L., Park M.S., et al. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin. Exp. Immunol. 2018;194:103–117. doi: 10.1111/cei.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christofidou-Solomidou M., Scherpereel A., Wiewrodt R., Ng K., Sweitzer T., Arguiri E., Shuvaev V., Solomides C.C., Albelda S.M., Muzykantov V.R. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 91.Hammerschmidt S., Wahn H. The oxidants hypochlorite and hydrogen peroxide induce distinct patterns of acute lung injury. Biochim. Biophys. Acta. 2004;1690:258–264. doi: 10.1016/j.bbadis.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Kim S.R., Lee K.S., Park S.J., Min K.H., Lee K.Y., Choe Y.H., Hong S.H., Koh G.Y., Lee Y.C. Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury. Exp. Mol. Med. 2008;40:320–331. doi: 10.3858/emm.2008.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mraheil M.A., Toque H.A., La Pietra L., Hamacher J., Phanthok T., Verin A., Gonzales J., Su Y., Fulton D., Eaton D.C., et al. Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia. Antioxid. Redox Signal. 2021;34:962–978. doi: 10.1089/ars.2019.7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hepokoski M., Wang J., Li K., Li Y., Gupta P., Mai T., Moshensky A., Alotaibi M., Crotty Alexander L.E., Malhotra A., et al. Altered lung metabolism and mitochondrial DAMPs in lung injury due to acute kidney injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;320:L821–L831. doi: 10.1152/ajplung.00578.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imtiazul I.M., Asma R., Lee J.-H., Cho N.-J., Park S., Song H.-Y., Gil H.-W. Change of surfactant protein D and A after renal ischemia reperfusion injury. PLoS ONE. 2019;14:e0227097. doi: 10.1371/journal.pone.0227097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gray K.D., MacMillan-Crow L.-A., Simovic M.O., Stain S.C., May A.K. Pulmonary MnSOD is nitrated following hepatic ischemia-reperfusion. Surg. Infect. (Larchmt) 2004;5:166–173. doi: 10.1089/sur.2004.5.166. [DOI] [PubMed] [Google Scholar]
- 97.Li G., Zhang Y., Fan Z. Cellular Signal Transduction Pathways Involved in Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion. Oxid. Med. Cell. Longev. 2021;2021:9985701. doi: 10.1155/2021/9985701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan Y., Alwis I., Wu M.C.L., Kaplan Z., Ashworth K., Bark D., Pham A., Mcfadyen J., Schoenwaelder S.M., Josefsson E.C., et al. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci. Transl. Med. 2017;9:eaam5861. doi: 10.1126/scitranslmed.aam5861. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., Gao T.-T., Xu D.-F., Zhu X.-Y., Dong W.-W., Lv Z., Liu Y.-J., Jiang L. Upregulation of sphingosine kinase 1 contributes to ventilator-associated lung injury in a two-hit model. Int. J. Mol. Med. 2019;44:2077–2090. doi: 10.3892/ijmm.2019.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoegl S., Burns N., Angulo M., Francis D., Osborne C.M., Mills T.W., Blackburn M.R., Eltzschig H.K., Vohwinkel C.U. Capturing the multifactorial nature of ARDS—“Two-hit” approach to model murine acute lung injury. Physiol. Rep. 2018;6:e13648. doi: 10.14814/phy2.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones H.D., Crother T.R., Gonzalez-Villalobos R.A., Jupelli M., Chen S., Dagvadorj J., Arditi M., Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am. J. Respir. Cell Mol. Biol. 2014;50:270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shan Y., Akram A., Amatullah H., Zhou D.Y., Gali P.L., Maron-Gutierrez T., González-López A., Zhou L., Rocco P.R.M., Hwang D., et al. ATF3 protects pulmonary resident cells from acute and ventilator-induced lung injury by preventing Nrf2 degradation. Antioxid. Redox Signal. 2015;22:651–668. doi: 10.1089/ars.2014.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li L.-F., Liu Y.-Y., Lin S.-W., Chang C.-H., Chen N.-H., Hung C.-Y., Lee C.-S. Low-Molecular-Weight Heparin Reduces Ventilation-Induced Lung Injury through Hypoxia Inducible Factor-1α in a Murine Endotoxemia Model. Int. J. Mol. Sci. 2020;21:3097. doi: 10.3390/ijms21093097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amatullah H., Maron-Gutierrez T., Shan Y., Gupta S., Tsoporis J.N., Varkouhi A.K., Teixeira Monteiro A.P., He X., Yin J., Marshall J.C., et al. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2021;38:101796. doi: 10.1016/j.redox.2020.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang C., Hu S., Zosky G.R., Wei X., Shu S., Wang D., Chai X. Paracoxib Alleviates Ventilator-Induced Lung Injury Through Functional Modulation of Lung-Recruited CD11bloLy6Chi Monocytes. Shock. 2021;55:236–243. doi: 10.1097/SHK.0000000000001591. [DOI] [PubMed] [Google Scholar]
- 106.Ding X., Tong Y., Jin S., Chen Z., Li T., Billiar T.R., Pitt B.R., Li Q., Zhang L.-M. Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: Role of WISP1-αvβ5 integrin pathway in TLR4-mediated inflammation and injury. Crit. Care. 2018;22:302. doi: 10.1186/s13054-018-2237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng J., Huang Y., Islam D., Wen X.-Y., Wu S., Streutker C., Luo A., Li M., Khang J., Han B., et al. Dual effects of human neutrophil peptides in a mouse model of pneumonia and ventilator-induced lung injury. Respir. Res. 2018;19:190. doi: 10.1186/s12931-018-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu S., Deng M., Pan P., Turnquist H.R., Pitt B.R., Billiar T.R., Zhang L.-M. Mechanical Ventilation With Moderate Tidal Volume Exacerbates Extrapulmonary Sepsis-Induced Lung Injury via IL33-WISP1 Signaling Pathway. Shock. 2021;56:461–472. doi: 10.1097/SHK.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 109.Müller-Redetzky H.C., Will D., Hellwig K., Kummer W., Tschernig T., Pfeil U., Paddenberg R., Menger M.D., Kershaw O., Gruber A.D., et al. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: Protection by adrenomedullin. Crit. Care. 2014;18:R73. doi: 10.1186/cc13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu J., Han B., Fanelli V., Wen X., Huang Y., Luo A., Ghazarian M., Wang D., Khang J., Morriello F., et al. Distinctive Roles and Mechanisms of Human Neutrophil Peptides in Experimental Sepsis and Acute Respiratory Distress Syndrome. Crit. Care Med. 2018;46:e921–e927. doi: 10.1097/CCM.0000000000003265. [DOI] [PubMed] [Google Scholar]
- 111.Zhang R., Pan Y., Fanelli V., Wu S., Luo A.A., Islam D., Han B., Mao P., Ghazarian M., Zeng W., et al. Mechanical Stress and the Induction of Lung Fibrosis via the Midkine Signaling Pathway. Am. J. Respir. Crit. Care Med. 2015;192:315–323. doi: 10.1164/rccm.201412-2326OC. [DOI] [PubMed] [Google Scholar]
- 112.Walker M.G., Yao L.-J., Patterson E.K., Joseph M.G., Cepinskas G., Veldhuizen R.A.W., Lewis J.F., Yamashita C.M. The effect of tidal volume on systemic inflammation in Acid-induced lung injury. Respiration. 2011;81:333–342. doi: 10.1159/000323609. [DOI] [PubMed] [Google Scholar]
- 113.Gurkan O.U., He C., Zielinski R., Rabb H., King L.S., Dodd-o J.M., D’Alessio F.R., Aggarwal N., Pearse D., Becker P.M. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp. Lung Res. 2011;37:575–584. doi: 10.3109/01902148.2011.620680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 115.Checa J., Aran J.M. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. Int. J. Mol. Sci. 2020;21:9317. doi: 10.3390/ijms21239317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) Adv. Exp. Med. Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Picca A., Calvani R., Coelho-Junior H.J., Marzetti E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells. 2021;10:537. doi: 10.3390/cells10030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hernansanz-Agustín P., Enríquez J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants. 2021;10:415. doi: 10.3390/antiox10030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vermot A., Petit-Härtlein I., Smith S.M.E., Fieschi F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants. 2021;10:890. doi: 10.3390/antiox10060890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin W.-C., Fessler M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021;78:4095–4124. doi: 10.1007/s00018-021-03768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peters D.M., Vadász I., Wujak L., Wygrecka M., Olschewski A., Becker C., Herold S., Papp R., Mayer K., Rummel S., et al. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc. Natl. Acad. Sci. USA. 2014;111:E374–E383. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Witten M.L., Chau B., Sáez E., Boitano S., Clark Lantz R. Early life inhalation exposure to mine tailings dust affects lung development. Toxicol. Appl. Pharmacol. 2019;365:124–132. doi: 10.1016/j.taap.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Z.-M., Xu S.-Y., Feng Y.-Z., Cheng Y.-R., Xiong J.-B., Zhou Y., Guan C.-X. The role of NOX4 in pulmonary diseases. J. Cell. Physiol. 2021;236:1628–1637. doi: 10.1002/jcp.30005. [DOI] [PubMed] [Google Scholar]
- 125.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Malley Y., Fink B.D., Ross N.C., Prisinzano T.E., Sivitz W.I. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J. Biol. Chem. 2006;281:39766–39775. doi: 10.1074/jbc.M608268200. [DOI] [PubMed] [Google Scholar]
- 127.Schumacker P.T., Gillespie M.N., Nakahira K., Choi A.M.K., Crouser E.D., Piantadosi C.A., Bhattacharya J. Mitochondria in lung biology and pathology: More than just a powerhouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L962–L974. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diaz B., Shani G., Pass I., Anderson D., Quintavalle M., Courtneidge S.A. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lyle A.N., Deshpande N.N., Taniyama Y., Seidel-Rogol B., Pounkova L., Du P., Papaharalambus C., Lassègue B., Griendling K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Faraci F.M., Didion S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 132.Fridovich I. Superoxide anion radical (O2−.), superoxide dismutases, and related matters. J. Biol. Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 133.Budinger G.R.S., Mutlu G.M., Urich D., Soberanes S., Buccellato L.J., Hawkins K., Chiarella S.E., Radigan K.A., Eisenbart J., Agrawal H., et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am. J. Respir. Crit. Care Med. 2011;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gongora M.C., Lob H.E., Landmesser U., Guzik T.J., Martin W.D., Ozumi K., Wall S.M., Wilson D.S., Murthy N., Gravanis M., et al. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: A potential mechanism underlying adult respiratory distress syndrome. Am. J. Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kinnula V.L., Crapo J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 136.Winterbourn C.C., Kettle A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 137.Glorieux C., Calderon P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017;398:1095–1108. doi: 10.1515/hsz-2017-0131. [DOI] [PubMed] [Google Scholar]
- 138.Davies M.J., Hawkins C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020;32:957–981. doi: 10.1089/ars.2020.8030. [DOI] [PubMed] [Google Scholar]
- 139.Forman H.J., Zhang H., Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rahman I., MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 141.Zuo L., Wijegunawardana D. Redox Role of ROS and Inflammation in Pulmonary Diseases. Adv. Exp. Med. Biol. 2021;1304:187–204. doi: 10.1007/978-3-030-68748-9_11. [DOI] [PubMed] [Google Scholar]
- 142.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 143.Alharbi K.S., Fuloria N.K., Fuloria S., Rahman S.B., Al-Malki W.H., Javed Shaikh M.A., Thangavelu L., Singh S.K., Rama Raju Allam V.S., Jha N.K., et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021;345:109568. doi: 10.1016/j.cbi.2021.109568. [DOI] [PubMed] [Google Scholar]
- 144.Herrero R., Sanchez G., Lorente J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018;6:32. doi: 10.21037/atm.2017.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barabutis N. Unfolded Protein Response: A Regulator of the Endothelial Barrier. Endocr. Metab. Sci. 2021;3:100092. doi: 10.1016/j.endmts.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang M., Gauthier A., Daley L., Dial K., Wu J., Woo J., Lin M., Ashby C., Mantell L.L. The Role of HMGB1, a Nuclear Damage-Associated Molecular Pattern Molecule, in the Pathogenesis of Lung Diseases. Antioxid. Redox Signal. 2019;31:954–993. doi: 10.1089/ars.2019.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020;11:1722. doi: 10.3389/fimmu.2020.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dessing M.C., Schouten M., Draing C., Levi M., Von Aulock S., van der Poll T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 2008;197:245–252. doi: 10.1086/524873. [DOI] [PubMed] [Google Scholar]
- 149.Ciesielska A., Matyjek M., Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021;78:1233–1261. doi: 10.1007/s00018-020-03656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wittekindt O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017;469:135–147. doi: 10.1007/s00424-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Mao Y.-F., Zhao Y., Xu D.-F., Wang Y., Xu C.-F., Dong W.-W., Zhu X.-Y., Ding N., Jiang L., et al. Upregulation of Matrix Metalloproteinase-9 Protects against Sepsis-Induced Acute Lung Injury via Promoting the Release of Soluble Receptor for Advanced Glycation End Products. Oxid. Med. Cell. Longev. 2021;2021:8889313. doi: 10.1155/2021/8889313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee I.-T., Yang C.-M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012;84:581–590. doi: 10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 153.Li J., Qi Z., Li D., Huang X., Qi B., Feng J., Qu J., Wang X. Alveolar epithelial glycocalyx shedding aggravates the epithelial barrier and disrupts epithelial tight junctions in acute respiratory distress syndrome. Biomed. Pharmacother. 2021;133:111026. doi: 10.1016/j.biopha.2020.111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He H., Huang C., Chen Z., Huang H., Wang X., Chen J. An outlined review for the role of Nedd4-1 and Nedd4-2 in lung disorders. Biomed. Pharmacother. 2020;125:109983. doi: 10.1016/j.biopha.2020.109983. [DOI] [PubMed] [Google Scholar]
- 155.Duerr J., Leitz D.H.W., Szczygiel M., Dvornikov D., Fraumann S.G., Kreutz C., Zadora P.K., Seyhan Agircan A., Konietzke P., Engelmann T.A., et al. Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat. Commun. 2020;11:2012. doi: 10.1038/s41467-020-15743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eaton A.F., Yue Q., Eaton D.C., Bao H.-F. ENaC activity and expression is decreased in the lungs of protein kinase C-α knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L374–L385. doi: 10.1152/ajplung.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eaton D.C., Helms M.N., Koval M., Bao H.F., Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu. Rev. Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 158.Wynne B.M., Zou L., Linck V., Hoover R.S., Ma H.-P., Eaton D.C. Regulation of Lung Epithelial Sodium Channels by Cytokines and Chemokines. Front. Immunol. 2017;8:766. doi: 10.3389/fimmu.2017.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yadav H., Kor D.J. Platelets in the pathogenesis of acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hirata N., Ngo D.T., Phan P.H., Ainai A., Phung T.T.B., Ta T.A., Takasaki J., Kawachi S., Nunoi H., Nakajima N., et al. Recombinant human thrombomodulin for pneumonia-induced severe ARDS complicated by DIC in children: A preliminary study. J. Anesth. 2021;35:638–645. doi: 10.1007/s00540-021-02971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wake H., Mori S., Liu K., Morioka Y., Teshigawara K., Sakaguchi M., Kuroda K., Gao Y., Takahashi H., Ohtsuka A., et al. Histidine-Rich Glycoprotein Prevents Septic Lethality through Regulation of Immunothrombosis and Inflammation. EBioMedicine. 2016;9:180–194. doi: 10.1016/j.ebiom.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Middleton E.A., Rondina M.T., Schwertz H., Zimmerman G.A. Amicus or Adversary Revisited: Platelets in Acute Lung Injury and Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2018;59:18–35. doi: 10.1165/rcmb.2017-0420TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leung H., Perdomo J., Ahmadi Z., Yan F., McKenzie S.E., Chong B.H. Inhibition of NADPH oxidase blocks NETosis and reduces thrombosis in heparin-induced thrombocytopenia. Blood Adv. 2021;5:5439–5451. doi: 10.1182/bloodadvances.2020003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Delaney M.K., Kim K., Estevez B., Xu Z., Stojanovic-Terpo A., Shen B., Ushio-Fukai M., Cho J., Du X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:846–854. doi: 10.1161/ATVBAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fuentes E., Gibbins J.M., Holbrook L.M., Palomo I. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018;28:429–434. doi: 10.1016/j.tcm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 166.Renné T., Pozgajová M., Grüner S., Schuh K., Pauer H.-U., Burfeind P., Gailani D., Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith S.A., Mutch N.J., Baskar D., Rohloff P., Docampo R., Morrissey J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vorobjeva N.V., Chernyak B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry. 2020;85:1178–1190. doi: 10.1134/S0006297920100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang H., Zhou Y., Qu M., Yu Y., Chen Z., Zhu S., Guo K., Chen W., Miao C. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell. Infect. Microbiol. 2021;11:677902. doi: 10.3389/fcimb.2021.677902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mannes M., Schmidt C.Q., Nilsson B., Ekdahl K.N., Huber-Lang M. Complement as driver of systemic inflammation and organ failure in trauma, burn, and sepsis. Semin. Immunopathol. 2021;43:773–788. doi: 10.1007/s00281-021-00872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsai I.-J., Lin W.-C., Yang Y.-H., Tseng Y.-L., Lin Y.-H., Chou C.-H., Tsau Y.-K. High Concentration of C5a-Induced Mitochondria-Dependent Apoptosis in Murine Kidney Endothelial Cells. Int. J. Mol. Sci. 2019;20:4465. doi: 10.3390/ijms20184465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Levi M., Schultz M.J., Rijneveld A.W., van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit. Care Med. 2003;31:S238–S242. doi: 10.1097/01.CCM.0000057849.53689.65. [DOI] [PubMed] [Google Scholar]
- 174.Akinosoglou K., Alexopoulos D. Use of antiplatelet agents in sepsis: A glimpse into the future. Thromb. Res. 2014;133:131–138. doi: 10.1016/j.thromres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Naime A.C.A., Ganaes J.O.F., Lopes-Pires M.E. Sepsis: The Involvement of Platelets and the Current Treatments. Curr. Mol. Pharmacol. 2018;11:261–269. doi: 10.2174/1874467211666180619124531. [DOI] [PubMed] [Google Scholar]
- 176.Gill S.E., Rohan M., Mehta S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury In Vivo. Respir. Res. 2015;16:109. doi: 10.1186/s12931-015-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cui Y., Wang Y., Li G., Ma W., Zhou X.-S., Wang J., Liu B. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice. PLoS ONE. 2018;13:e0209444. doi: 10.1371/journal.pone.0209444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park W.H. Tempol differently affects cellular redox changes and antioxidant enzymes in various lung-related cells. Sci. Rep. 2021;11:14869. doi: 10.1038/s41598-021-94340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu N.-C., Liao F.-T., Cheng H.-M., Sung S.-H., Yang Y.-C., Wang J.-J. Intravenous superoxide dismutase as a protective agent to prevent impairment of lung function induced by high tidal volume ventilation. BMC Pulm. Med. 2017;17:105. doi: 10.1186/s12890-017-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oyewole A.O., Birch-Machin M.A. Mitochondria-targeted antioxidants. FASEB J. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 181.Liu G., Gu C., Liu M., Liu H., Wang D., Liu X., Wang Y. Protective role of p120-catenin on mitochondria by inhibiting NLRP3 in ventilator-induced lung injury. J. Cell. Mol. Med. 2019;23:7360–7371. doi: 10.1111/jcmm.14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Matuz-Mares D., Riveros-Rosas H., Vilchis-Landeros M.M., Vázquez-Meza H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants. 2021;10:1220. doi: 10.3390/antiox10081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Biswas S.K., Rahman I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qian M., Lou Y., Wang Y., Zhang M., Jiang Q., Mo Y., Han K., Jin S., Dai Q., Yu Y., et al. PICK1 deficiency exacerbates sepsis-associated acute lung injury and impairs glutathione synthesis via reduction of xCT. Free Radic. Biol. Med. 2018;118:23–34. doi: 10.1016/j.freeradbiomed.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 185.Bao X., Liu X., Liu N., Zhuang S., Yang Q., Ren H., Zhao D., Bai J., Zhou X., Tang L. Inhibition of EZH2 prevents acute respiratory distress syndrome (ARDS)-associated pulmonary fibrosis by regulating the macrophage polarization phenotype. Respir. Res. 2021;22:194. doi: 10.1186/s12931-021-01785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang Y., Li L., Hang Q., Fang Y., Dong X., Cao P., Yin Z., Luo L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019;20:157–166. doi: 10.1016/j.redox.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghezzi P. Redox regulation of immunity and the role of small molecular weight thiols. Redox Biol. 2021;44:102001. doi: 10.1016/j.redox.2021.102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parnham M.J., Sies H. The early research and development of ebselen. Biochem. Pharmacol. 2013;86:1248–1253. doi: 10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 189.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic. Biol. Med. 1993;14:313–323. doi: 10.1016/0891-5849(93)90028-S. [DOI] [PubMed] [Google Scholar]
- 190.Azad G.K., Tomar R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 191.Ishii Y., Hashimoto K., Hirano K., Morishima Y., Mochizuki M., Masuyama K., Nomura A., Sakamoto T., Uchida Y., Sagai M., et al. Ebselen decreases ozone-induced pulmonary inflammation in rats. Lung. 2000;178:225–234. doi: 10.1007/s004080000026. [DOI] [PubMed] [Google Scholar]
- 192.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tyml K., Li F., Wilson J.X. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit. Care Med. 2008;36:2355–2362. doi: 10.1097/CCM.0b013e31818024f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fisher B.J., Kraskauskas D., Martin E.J., Farkas D., Wegelin J.A., Brophy D., Ward K.R., Voelkel N.F., Fowler A.A., Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303:L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 195.Audousset C., McGovern T., Martin J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021;12:727806. doi: 10.3389/fphys.2021.727806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vomund S., Schäfer A., Parnham M.J., Brüne B., Von Knethen A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017;18:2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee J., Jang J., Park S.-M., Yang S.-R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021;22:8406. doi: 10.3390/ijms22168406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dong H., Xia Y., Jin S., Xue C., Wang Y., Hu R., Jiang H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021;12:1027. doi: 10.1038/s41419-021-04307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liang W., Greven J., Fragoulis A., Horst K., Bläsius F., Wruck C., Pufe T., Kobbe P., Hildebrand F., Lichte P. Sulforaphane-Dependent Up-Regulation of NRF2 Activity Alleviates Both Systemic Inflammatory Response and Lung Injury After Hemorrhagic Shock/Resuscitation In Mice. Shock. 2021 doi: 10.1097/SHK.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 201.Wei J., Chen G., Shi X., Zhou H., Liu M., Chen Y., Feng D., Zhang P., Wu L., Lv X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018;500:790–796. doi: 10.1016/j.bbrc.2018.04.161. [DOI] [PubMed] [Google Scholar]
- 202.Pei X., Zhang X.-J., Chen H.-M. Bardoxolone treatment alleviates lipopolysaccharide (LPS)-induced acute lung injury through suppressing inflammation and oxidative stress regulated by Nrf2 signaling. Biochem. Biophys. Res. Commun. 2019;516:270–277. doi: 10.1016/j.bbrc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 203.De Oliveira M.T.P., Coutinho D.D.S., Guterres S.S., Pohlmann A.R., Martins M.A., Bernardi A. Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice. Pharmaceutics. 2021;13:683. doi: 10.3390/pharmaceutics13050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu Z., Chen S., Zhang X., Liu F., Yang K., Du G., Rui X. Dasatinib protects against acute respiratory distress syndrome via Nrf2-regulated M2 macrophages polarization. Drug Dev. Res. 2021;82:1247–1257. doi: 10.1002/ddr.21839. [DOI] [PubMed] [Google Scholar]
- 205.Cen M., Ouyang W., Zhang W., Yang L., Lin X., Dai M., Hu H., Tang H., Liu H., Xia J., et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936. doi: 10.1016/j.redox.2021.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hou L., Zhang J., Liu Y., Fang H., Liao L., Wang Z., Yuan J., Wang X., Sun J., Tang B., et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic. Biol. Med. 2021;165:219–228. doi: 10.1016/j.freeradbiomed.2021.01.045. [DOI] [PubMed] [Google Scholar]
- 207.Buelna-Chontal M., Zazueta C. Redox activation of Nrf2 & NF-kB: A double end sword? Cell. Signal. 2013;25:2548–2557. doi: 10.1016/j.cellsig.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 208.Fratantonio D., Virgili F., Zucchi A., Lambrechts K., Latronico T., Lafère P., Germonpré P., Balestra C. Increasing oxygen partial pressures induce a distinct transcriptional response in human PBMC: A pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 2021;22:458. doi: 10.3390/ijms22010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.von Knethen A., Tzieply N., Jennewein C., Brüne B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J. Cell Sci. 2010;123:192–201. doi: 10.1242/jcs.055475. [DOI] [PubMed] [Google Scholar]
- 210.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A.W., et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 212.Trümper V., Wittig I., Heidler J., Richter F., Brüne B., von Knethen A. Redox Regulation of PPARγ in Polarized Macrophages. PPAR Res. 2020;2020:8253831. doi: 10.1155/2020/8253831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He J., Qi D., Tang X.-M., Deng W., Deng X.-Y., Zhao Y., Wang D.-X. Rosiglitazone promotes ENaC-mediated alveolar fluid clearance in acute lung injury through the PPARγ/SGK1 signaling pathway. Cell. Mol. Biol. Lett. 2019;24:35. doi: 10.1186/s11658-019-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang G., Liu L., Zhang Y., Han D., Liu J., Xu J., Xie X., Wu Y., Zhang D., Ke R., et al. Activation of PPARγ attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur. J. Pharmacol. 2014;726:27–32. doi: 10.1016/j.ejphar.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 215.Xie K., Chen Y.-Q., Chai Y.-S., Lin S.-H., Wang C.-J., Xu F. HMGB1 suppress the expression of IL-35 by regulating Naïve CD4+ T cell differentiation and aggravating Caspase-11-dependent pyroptosis in acute lung injury. Int. Immunopharmacol. 2021;91:107295. doi: 10.1016/j.intimp.2020.107295. [DOI] [PubMed] [Google Scholar]
- 216.Ricote M., Li A.C., Willson T.M., Kelly C.J., Glass C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 217.Liu P.-S., Wang H., Li X., Chao T., Teav T., Christen S., Di Conza G., Cheng W.-C., Chou C.-H., Vavakova M., et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 218.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Arora S., Dev K., Agarwal B., Das P., Syed M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223:383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu M., Chen Y., Wang S., Zhou H., Feng D., Wei J., Shi X., Wu L., Zhang P., Yang H., et al. α-Ketoglutarate Modulates Macrophage Polarization Through Regulation of PPARγ Transcription and mTORC1/p70S6K Pathway to Ameliorate ALI/ARDS. Shock. 2020;53:103–113. doi: 10.1097/SHK.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 221.Jain M., Budinger G.R.S., Lo A., Urich D., Rivera S.E., Ghosh A.K., Gonzalez A., Chiarella S.E., Marks K., Donnelly H.K., et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am. J. Respir. Crit. Care Med. 2011;183:1490–1498. doi: 10.1164/rccm.201009-1409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schmidt M.V., Paulus P., Kuhn A.M., Weigert A., Morbitzer V., Zacharowski K., Kempf V.A., Brüne B., von Knethen A. Peroxisome proliferator-activated receptor γ-induced T cell apoptosis reduces survival during polymicrobial sepsis. Am. J. Respir. Crit. Care Med. 2011;184:64–74. doi: 10.1164/rccm.201010-1585OC. [DOI] [PubMed] [Google Scholar]
- 223.Menezes S.L.S., Bozza P.T., Neto H.C.C.F., Laranjeira A.P., Negri E.M., Capelozzi V.L., Zin W.A., Rocco P.R.M. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J. Appl. Physiol. (1985) 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 224.Capelozzi V.L., Allen T.C., Beasley M.B., Cagle P.T., Guinee D., Hariri L.P., Husain A.N., Jain D., Lantuejoul S., Larsen B.T., et al. Molecular and Immune Biomarkers in Acute Respiratory Distress Syndrome: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2017;141:1719–1727. doi: 10.5858/arpa.2017-0115-SA. [DOI] [PubMed] [Google Scholar]
- 225.Radermacher P., Haouzi P. A mouse is not a rat is not a man: Species-specific metabolic respsonses to sepsis—A nail in the coffin of murine models for critical care research? Intensive Care Med. Exp. 2013;1:7. doi: 10.1186/2197-425X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zolfaghari P.S., Pinto B.B., Dyson A., Singer M. The metabolic phenotype of rodent sepsis: Cause for concern? Intensive Care Med. Exp. 2013;1:6. doi: 10.1186/2197-425X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Drohan C.M., Nouraie S.M., Bain W., Shah F.A., Evankovich J., Zhang Y., Morris A., McVerry B.J., Kitsios G.D. Biomarker-Based Classification of Patients With Acute Respiratory Failure Into Inflammatory Subphenotypes: A Single-Center Exploratory Study. Crit. Care Explor. 2021;3:e0518. doi: 10.1097/CCE.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Spadaro S., Park M., Turrini C., Tunstall T., Thwaites R., Mauri T., Ragazzi R., Ruggeri P., Hansel T.T., Caramori G., et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019;16:1. doi: 10.1186/s12950-018-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wick K.D., McAuley D.F., Levitt J.E., Beitler J.R., Annane D., Riviello E.D., Calfee C.S., Matthay M.A. Promises and challenges of personalized medicine to guide ARDS therapy. Crit. Care. 2021;25:404. doi: 10.1186/s13054-021-03822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Matthay M.A., Arabi Y.M., Siegel E.R., Ware L.B., Bos L.D.J., Sinha P., Beitler J.R., Wick K.D., Curley M.A.Q., Constantin J.-M., et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med. 2020;46:2136–2152. doi: 10.1007/s00134-020-06296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sweeney T.E., Thomas N.J., Howrylak J.A., Wong H.R., Rogers A.J., Khatri P. Multicohort Analysis of Whole-Blood Gene Expression Data Does Not Form a Robust Diagnostic for Acute Respiratory Distress Syndrome. Crit. Care Med. 2018;46:244–251. doi: 10.1097/CCM.0000000000002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Qadir N., Chang S.Y. Pharmacologic Treatments for Acute Respiratory Distress Syndrome. Crit. Care Clin. 2021;37:877–893. doi: 10.1016/j.ccc.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gonzalez H., Horie S., Laffey J.G. Emerging cellular and pharmacologic therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2021;27:20–28. doi: 10.1097/MCC.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 234.Jaiswal N., Bhatnagar M., Shah H. N-acetycysteine: A potential therapeutic agent in COVID-19 infection. Med. Hypotheses. 2020;144:110133. doi: 10.1016/j.mehy.2020.110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cazzola M., Rogliani P., Salvi S.S., Ora J., Matera M.G. Use of Thiols in the Treatment of COVID-19: Current Evidence. Lung. 2021;199:335–343. doi: 10.1007/s00408-021-00465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.