Abstract
Bortezomib (BTZ) is the first proteasome inhibitor approved by the Food and Drug Administration. It can bind to the amino acid residues of the 26S proteasome, thereby causing the death of tumor cells. BTZ plays an irreplaceable role in the treatment of mantle cell lymphoma and multiple myeloma. Moreover, its use in the treatment of other hematological cancers and solid tumors has been investigated in numerous clinical trials and preclinical studies. Nevertheless, the applications of BTZ are limited due to its insufficient specificity, poor permeability, and low bioavailability. Therefore, in recent years, different BTZ-based drug delivery systems have been evaluated. In this review, we firstly discussed the functions of proteasome inhibitors and their mechanisms of action. Secondly, the properties of BTZ, as well as recent advances in both clinical and preclinical research, were reviewed. Finally, progress in research regarding BTZ-based nanoformulations was summarized.
Keywords: bortezomib, proteasome inhibitor, cancer therapy, nanoformulations
1. Introduction
Bortezomib (BTZ; trade name: Velcade®), an antineoplastic agent developed by the Millennium Corporation in the United States of America, is the first proteasome inhibitor approved by the Food and Drug Administration [1,2,3,4]. This drug can block the ubiquitin-proteasome system (UPS) pathway, thereby hindering the degradation of antitumor-related protein products and exerting an antitumor effect [5,6,7]. Currently, BTZ has been approved for the treatment of multiple myeloma (MM) and mantle cell lymphoma (MCL) [8]. BTZ also showed inhibitory effects on other hematological cancers and numerous solid tumors in preclinical studies and clinical trials [9,10,11]. Nevertheless, due to its low solubility, poor specificity, and significant toxicity, the application of BTZ in solid tumors is limited [12,13,14,15]. With the development of nanomaterials, a series of BTZ-based drug delivery systems have been developed to improve its therapeutic efficacy [16,17,18,19]. In this review, we summarized the functions of proteasome inhibitors and their mechanisms of action, the clinical and preclinical applications of BTZ, and the progress in research concerning BTZ-based nanoformulations.
2. The UPS and Proteasome Inhibitors
The UPS and lysosomal autophagy are the major metabolic pathways in eukaryotic cells, which can degrade a variety of protein products and damage senescent organelles [20,21,22,23]. The former plays an important role in regulating the biological processes of cells and maintaining homeostasis, it also participates in the hydrolysis of >80% proteins in cells [24,25]. It has been demonstrated that many proteins or cytokines involved in cell growth, proliferation, apoptosis, gene expression, and immune response are degraded by the UPS pathway [26,27].
The UPS pathway is the key pathway through which proteasome inhibitors exert their pharmacological effects. Proteasome inhibitors work by blocking the UPS, which is closely related to the degradation of caspase, the blockage of the nuclear factor-kappa B (NF-κB) signal pathway, the regulation of B-cell lymphoma-2 (Bcl-2) protein, the promotion of the expression of tumor suppressor genes, etc., [28,29,30,31,32,33].
2.1. Functions of the UPS
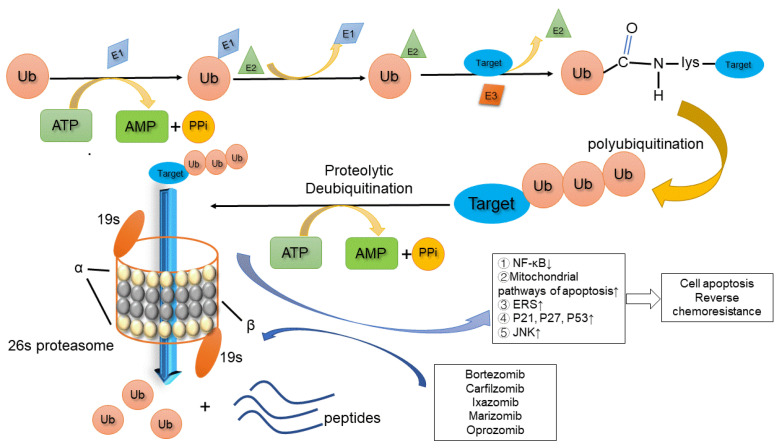
The degradation process of the UPS consists of two steps: substrate ubiquitin labeling and ubiquitinated protein degradation by proteasomes [34,35]. The lysine residues of substrate protein are labeled by forming isopeptide bonds with ubiquitin under the action of ubiquitin-activating enzyme (E1), ubiquitin-binding enzyme (E2), and ubiquitin-linker enzyme (E3). Finally, the residues are degraded by the 26S proteasome. The 26S proteasome consists of a 20S catalytic core and two 19S regulatory subunits that recognize ubiquitination substrates. The 20S catalytic core consists of four rings formed by two groups of α and β subunits [36]. Seven different α subunits form two outer rings, while seven different β subunits form two inner rings. The α subunit ensures the unfolded protein enters the catalytic core, while the β subunits have catalytic activity to hydrolyze the protein substrate [37]. β subunits can be further divided into different types; for example, β1 has caspase-like activity with the ability to dissociate acid residues, β2 has trypsin-like activity and can remove alkaline residues, and β5 has chymotrypsin-like activity and can lyse hydrophobic residues [38,39,40].
UPS abnormalities are closely related to the occurrence, development, and prognosis of malignant tumors. Incorrect degradation of target proteins by the UPS can lead to the aggregation of oncoproteins and abnormal degradation of tumor suppressor proteins. These effects will cause apoptotic blockage and accelerated proliferation of mutant cells, ultimately inducing the occurrence of tumors [41,42].
2.2. Proteasome Inhibitors
With the in-depth understanding of the UPS pathway, proteasome inhibitors have become a reliable option for the treatment of cancer. In the past few decades, a variety of proteasome inhibitors have been developed, including boric acid peptide, epoxy-based peptide, and non-peptide lactones [43]. These inhibitors effectively block the UPS pathway by covalently binding to the β subunit of the 20S core [44].
Boric acid peptide and epoxy-peptide are the most thoroughly studied proteasome inhibitors and have been successfully marketed. BTZ is a representative drug of boric acid proteasome inhibitors. In these agents, boric acid (as the pharmacophore group) can reversibly bind with threonine residues on the β subunit to form a tetrahedral complex, thereby effectively blocking the UPS [45,46]. However, epoxy-peptide drugs differ from boric acid peptide proteasome inhibitors. The epoxy ketone group of drugs can form a stable morpholine ring structure with hydroxyl and amino groups of proteasome Thrl-1. This permits irreversible binding to the proteasome, thus avoiding the off-target effects of boric acid proteasome inhibitors [47]. Currently, there are three commercially available proteasome inhibitors, namely BTZ, carfilzomib, and ixazomib. In addition, various other compounds (e.g., oprozomib, delanzomib, marizomib, etc.) are currently under investigation in clinical trials. Some representative proteasome inhibitors are summarized in Table 1.
Table 1.
Proteasome inhibitors that are approved or currently investigated in clinical trials.
| Proteasome Inhibitor | Company | Phase | Structure Type | Targets | Mechanism | Administration Route | Covalent Bond |
|---|---|---|---|---|---|---|---|
| Bortezomib | Millennium | Approved by the FDA in 2003 | Dipeptide boronic acid |
β1, β5 | Covalently bonds with the N-terminal threonine of the β subunit | Intravenous | Reversible |
| Carfilzomib | Kyprolis | Approved by the FDA in 2012 | Epoxy-peptide | β5 | Combines with threonine at the active site of the β5 subunit to form a stable morpholine ring [48] | Intravenous | Irreversible |
| Ixazomib | Takeda | Approved by the FDA in 2015 | Dipeptide leucine |
β5 | Forms a covalent bond with the N-terminal threonine of the trypsin-like active site [49,50] | Intravenous /oral | Reversible |
| Oprozomib | Amgen | Phase I/II | Tripeptide epoxy ketone | β5 | Combines with threonine at the active site of the β5 subunit to form a stable morpholine ring [51] | Oral | Irreversible |
| Delanzomib | Teva | Phase I/II | Threonine Boric Acid | β1, β5 | Forms a covalent bond with the N-terminal threonine of the trypsin-like active site [52] | Intravenous | Reversible |
| Marizomib | Celgene | Phase III | β-lactone-γ-lactam | β1, β2, β5 | Thr1O γ on the subunit is covalently bound to the carbonyl group derived from the β-lactone ring of the inhibitor [53] | Intravenous | Irreversible |
2.3. Mechanisms of Proteasome Inhibitors
In addition to hematological cancers (e.g., myeloma), the UPS is extremely active in solid tumors (e.g., prostate and breast cancer) [54,55,56]. Accordingly, proteasome inhibitors inhibit the function of the UPS, thus inducing the accumulation of antitumor-related protein products (e.g., tumor suppressors, cyclins, and apoptosis suppressors). Various signaling pathways are involved in the antitumor functions of proteasome inhibitors (Figure 1), which mainly include: (i) blockage of the activation of the NF-κB signaling pathway (currently the most recognized mechanism of action) [57,58], (ii) induction of mitochondrial endogenous apoptosis (change in the permeability of the mitochondrial membrane leads to the release of apoptotic proteins from mitochondria) [59], (iii) promotion of the expression of tumor suppressor genes p53, p21, and p27 [60,61], (iv) activation of the endoplasmic reticulum stress (ERS)-mediated apoptosis pathway, and (v) activation of the c-Jun NH2 terminal kinase (JNK) pathway [62].
Figure 1.
The target protein is ubiquitinated and transported to the 26S proteasome for degradation. Proteasome inhibitors bind to the β subunit of the catalytic core, thereby inhibiting the degradation of antitumor-related protein products. E1: ubiquitin-activating enzyme; E2: ubiquitin-binding enzyme; E3: ubiquitin-linker enzyme; Ub: ubiquitin.
2.3.1. Blockage of the NF-κB Signaling Pathway
NF-κB is an anti-apoptotic factor closely related to tumor growth, and the earliest verified antitumor pathway linked to proteasome inhibitors [63]. It is most commonly present as a dimer of p65-p50. I kappa B (IκB), an inhibitor protein of the p65-p50 heterodimer, can inactivate NF-κB [64,65]. Following the stimulation of cells by cytokines, antigens, viruses, etc., signal transduction is triggered to promote the phosphorylation and ubiquitination of IκB. Subsequently, ubiquitinated IκB is degraded by the proteasome, resulting in the release of NF-κB and translocation into the nucleus. NF-κB binds to promoter regions of genes encoding anti-apoptotic factors, thereby increasing the expression of anti-apoptotic proteins. Proteasome inhibitors, such as BTZ, could block the degradation of IκB by inhibiting the activity of the UPS. This process leads to inhibition of the activation of anti-apoptotic factor NF-κB [66]. The loss of NF-κB activity inhibits the survival and proliferation of tumor cells, thereby exerting antitumor effects.
2.3.2. Regulation of Bcl-2 Protein and the Signal Transduction Pathway
The mitochondrial endogenous pathway, which is mediated by the Bcl-2 protein family, plays a key role in cell apoptosis [67]. Bcl-2 protein, which is located in the membrane of mitochondria, nucleus, and endoplasm, regulates cell apoptosis. Members of the Bcl-2 protein family can be divided into three categories according to their structures: (i) anti-apoptotic proteins, including Bcl-2, Bcl-XL, and Bcl-w, (ii) pro-apoptotic proteins, mainly including Bcl-2 proteins, Bcl-2-associated X protein (Bax), and Bcl-2 antagonist killer 1 (Bak), and (iii) special members of pro-apoptotic proteins, including Bcl-2-associated agonist of cell death (Bad), Bcl-2-interacting killer (Bik), Bcl-2 homology domain 3 (BH3)-interacting domain death agonist (Bid), Bcl-2-interacting mediator of cell death (Bim), Bcl-2-modifying factor (Bmf), etc. All of these proteins have homologous sequences in the BH3 domain. Under the stress induced by proteasome inhibitors, the homologous BH3 domain acts as a sensing element to directly inhibit anti-apoptotic proteins or activate pro-apoptotic proteins (e.g., Bax). This increases the permeability of the mitochondrial membrane, leading to the release of cytochrome C and the activation of caspase-3. Finally, the caspase cascade leads to apoptosis. Letai et al. found that proteasome inhibitor BTZ could inhibit tumor growth. This effect was accompanied by decreased expression of Bcl-2 and Bcl-XL, enhanced expression of Bax, and activation of caspase-3. The investigators confirmed that BTZ could inhibit tumor growth through the mitochondrial apoptosis pathway [6].
2.3.3. Induction of ERS
ERS occurs when protein accumulation exceeds the rate of protein folding or degradation. Tumor cells are strongly dependent on ERS and can maintain their stability through self-compensation of ERS [68]. Proteasome inhibitors can inhibit the degradation of proteins by blocking proteasome activity, resulting in the accumulation of a large number of proteins in cells [69]. This intracellular protein overload breaks the self-compensatory balance of tumor cells, increasing ERS. Consequently, ERS promotes the activation of apoptosis-related proteins, such as caspase-12 and calpain, and leads to cell apoptosis [6,70].
2.3.4. Promotion of the Expression of Tumor Suppressor Genes
The p53 gene is a tumor suppressor gene in humans, and its transcription product (i.e., p53 protein) exhibits a strong tumor suppressor effect. However, studies have found that p53 protein has a very short half-life and is rapidly degraded by the UPS. Interestingly, it has been shown that proteasome inhibitors directly inhibit tumor growth by blocking the UPS to reduce the degradation of p53 protein [71]. In addition to p53, proteasome inhibitors can also regulate the expression of cyclins, such as p27 and p21, thus inhibiting the cell cycle and inducing apoptosis [72,73].
2.3.5. Activation of the JNK
Following an increase in the levels of misfolded proteins, the JNK protein promotes cell death. The JNK pathway promotes apoptosis mainly through TNF-α and caspase-8. Inhibition of JNK activation prevents the release of cytochrome C and the activation of caspase-8, thereby inhibiting apoptosis. Proteasome inhibitors enable JNK accumulation by inhibiting JNK phosphorylation, while activating TNF-α and caspase-8 to promote tumor apoptosis [74,75].
3. Properties and Applications of BTZ
3.1. Physical and Chemical Properties
BTZ is a hydrophobic drug which is easily soluble in organic solvents; the poor solubility of BTZ in aqueous solution limits its application in clinical practice. BTZ has an aminoalkyl dipeptide boronic acid, which contains pyrazinyl, L-phenylalanine, and L-boroleucine [76,77].
The boronic acid is an important functional group of BTZ and the key to its therapeutic effects [4,78,79]. The boron atom is sp2 hybridized, has a defect center, and can interact with the electron-rich system [8,80]. Consequently, BTZ can covalently bind to the 20s chymotrypsin-like active site in the 26s proteasome (the hydroxyl group of Thr1 at the N-terminus) to form a four-sided adductor [81,82].
In a neutral or alkaline solution, the dissociated BTZ can undergo esterification with 1-2 or 1-3 adjacent diol groups, resulting in the formation of pH-sensitive boronic acid ester bonds [82]. In contrast, BTZ exhibits a stable planar triangular structure under acidic conditions, which is difficult to combine with other substances. In recent years, researchers have discovered that acidity is a typical characteristic of the tumor microenvironment [83]. Therefore, pH-sensitive and reversible borate ester bonds can be selected to achieve the localized release of BTZ and improve the bioavailability. In addition, it has been reported that borate ester bonds are highly sensitive to glucose, adenosine triphosphate, H2O2, and other substances in the biological body. This evidence provided new directions for the efficient release of BTZ [84,85].
3.2. Clinical Applications of BTZ
BTZ is approved for the first-line treatment of refractory MM and MCL [86]. The combination of BTZ with other antitumor drugs in the treatment of MM and MCL has been clinically implemented. Representative clinical therapeutic regimens include: (i) RVD (BTZ, lenalidomide plus dexamethasone), (ii) VD (BTZ plus dexamethasone), (iii) VCD (plus cyclophosphamide on the basis of VD), (iv) VR-CAP (BTZ, rituximab, cyclophosphamide, doxorubicin, and prednisone), and (v) RVD+D (plus daratumumab on the RVD regimen). Clinical data verified that BTZ-based combination regimens exerted beneficial therapeutic effects and improved the quality of life of patients with MCL and MM.
Chang et al. used a therapeutic regimen termed VCR-CAVD (i.e., a combination of BTZ, rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone) for the treatment of MCL. This was followed by 2 years of consolidation and maintenance therapy. The follow-up survey demonstrated that treatment with the VCR-CAVD regimen was effective and well tolerated in treatment-naive patients with MCL. The rates of complete response (CR), overall response rate (ORR), and 3-year progression-free survival (PFS) were 68%, 95%, and 72%, respectively [87,88].
Another clinical study examined the efficacy of BTZ or high-dose dexamethasone in patients with recurrent MM after receiving one to three lines of treatment. The 669 patients involved in this study were randomly assigned to receive BTZ alone or a combination of dexamethasone and BTZ. Surprisingly, monotherapy with BTZ was associated with better prognosis compared with the combination therapy (1-year survival: 80% vs. 66%; partial response: 38% vs. 18%; and CR: 6% vs. 1%, respectively [89]).
3.3. Clinical Trials of BTZ
The potential effectiveness of BTZ on other types of cancer has been investigated extensively in clinical trials and preclinical studies. Among those, hematological cancers are the most widely studied, mainly including Waldenstrom’s macroglobulinemia (WM), diffuse large B-cell lymphoma (DLBCL), peripheral T-cell lymphoma (PTCL), and acute myelogenous leukemia (AML). Additionally, the role of BTZ in the treatment of solid tumors (e.g., colon, non-small cell lung, and breast cancer) has been evaluated. Some representative clinical research is summarized in Table 2.
Table 2.
The application of BTZ in clinics and clinical trials.
| Disease | Regimen | Status | Outcomes |
|---|---|---|---|
| MCL | VCR-CAVD | Approved | CR 68%, ORR 95%, three-year PFS 72% |
| MM | BTZ | Approved | one-year survival was 80%, CR 6%, PR 38% |
| WM | BDR | Phase II | CR 3%, PR 58%, PFS 42 months, three-year survival rate 81% |
| WM | BER | Phase I/II | CR 5.6%, PR 47.2%, PFS 21 months |
| DLBCL | R-CHOP+BTZ | Phase II | ORR 88%, CR/Cru 75%, two-year overall survival rate 70%, two-year progression-free survival rate 64% |
| PTCL | CHOP+BTZ | Phase II | ORR 76%, CR 65%, three-year overall survival rate 47%, PFS 35% |
| AML | BTZ+idarubcin+ cytarabine | Phase II | The overall effective rate 83% CR 58% |
| Breast cancer | BTZ+ pegylated liposomal doxorubicin | Phase II | PR 8%, median overall survival 4.3 months |
| Lung cancer | BTZ+ docetaxel | Phase II | One-year survival was 33%, Disease control rates were 54% |
Abbreviations: WM: Waldenstrom’s Macroglobulinemia; DLBCL: Diffuse Large B-cell Lymphoma; PTCL: Peripheral T-cell Lymphoma; AML: acute myelogenous leukemia; BDR: BTZ, dexamethasone and rituximab; BER: BTZ, everolimus and rituximab; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone; CR: complete response rate; PFS: progression-free survival; ORR: overall response rate; PR: partial response.
3.3.1. Hematological Cancers
Hematological cancers, also termed blood tumors, mainly originate in the bone marrow or lymphatic tissue, they include WM, DLBCL, PTCL, AML etc. Blood tumors mainly exist in blood circulation and are characterized by rapid growth and poor response to surgical treatment. The effectiveness of BTZ in the treatment of MCL and MM offers promise for potential use against other hematologic tumors.
WM is a malignant hyperproliferative disease of inert B lymphocytes, characterized by massive secretion of monoclonal immunoglobulin M (IgM). In a phase II clinical study, 59 patients with WM were treated with a combination of BTZ, dexamethasone, and rituximab (BDR). The BDR regimen showed excellent therapeutic activity with good tolerance and no obvious myelotoxicity. The rate of CR, PR, and 3-year survival rate were 3%, 58%, and 81%, respectively. Progression-free survival (PFS) was 42 months. In addition, it can induce a persistent response in previously untreated patients with WM.
Another clinical trial examined the use of BTZ in combination with everolimus and rituximab (BER) for the treatment of relapsed and refractory macroglobulinemia. Ghobrial et al. investigated the safety and effectiveness of this regimen. The results showed that this treatment was well tolerated, and the median PFS was prolonged. The rate of CR, PR, and PFS were 5.6%, 47.2%, and 21 months, respectively. This evidence suggests that the BER regimen is a new model for the clinical treatment of WM [90].
DLBCL is a type of non-Hodgkin’s lymphoma. A phase II trial of the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen in combination with rituximab and incremental doses of BTZ was carried out in patients with DLBCL. The ORR, 2-year PFS, and 2-year overall survival rates were 88%, 64%, and 70%, respectively [91].
PTCL is a heterogeneous aggressive lymphoma derived from mature T cells and natural killer cells. In a phase I clinical trial, BTZ combined with CHOP was used to treat 46 patients with PTCL. The results showed that the ORR, CR rate, and overall 3-year survival rate associated with the combined chemotherapy regimen were 76%, 65%, and 47%, respectively, indicating significant therapeutic effects. Moreover, Fader et al. reported that standard doses of idarubicin, cytarabine, and BTZ were effective in 83% of patients with AML, and the CR rate was 58% [92].
3.3.2. Solid Tumors
In recent years, studies on BTZ have provided a new approach to the treatment of solid tumors. A phase II study investigated the efficacy of a combination of BTZ and pegylated liposomal doxorubicin in patients with metastatic breast cancer. Patients received BTZ through intravenous administration on days one, four, eight, and eleven, and doxorubicin on days one and eight of the 21-day treatment cycle (partial response: 8%; median overall survival: 4.3 months). Due to the limited number of trials, the efficacy of BTZ combined with pegylated liposomal doxorubicin in the treatment of metastatic breast cancer remains to be explored [93].
A phase II trial was performed to investigate the effects of BTZ in combination with docetaxel in 80 patients with advanced non-small cell lung cancer. The 1-year survival rate was 33%, and the median survival was 7.8 months. This combination regimen was more effective than monotherapy with BTZ [94].
3.4. Pharmacokinetics of BTZ
The pharmacokinetics of BTZ are characterized by a two-compartment model. BTZ has a rapid initial distribution stage with an elimination half-life of >40 h [95,96]. Following intravenous administration, BTZ can be rapidly distributed throughout the whole body, except the brain and adipose tissue; of note, relatively high concentrations of the drug are detected in the liver and gastrointestinal tract [97]. The pharmacokinetic properties of BTZ were confirmed by Reece and Moreau et al. in the treatment of relapsed MM [98,99]. After one or more repeated administrations, the distribution volume of BTZ was 498–1884 L/m2, the plasma protein binding rate was 83%, and the total body clearance rate was 1095–1866 mL/min. BTZ is mainly metabolized by oxidative deborization of the cytochrome P450 enzyme and is completely cleared 72 h after injection [96,100].
The safety, tolerability, and pharmacokinetic characteristics of BTZ in patients with different degrees of renal and liver impairment were evaluated. At the dosage of 1.3 mg/m2, BTZ was well tolerated without significant changes observed on the maximum plasma concentration and half-life [101,102]. Moreau et al. investigated the pharmacokinetics of BTZ after subcutaneous and intravenous administration. The data showed that the systemic exposure and therapeutic effects of the two approaches were similar. Nevertheless, subcutaneous injection is favored due to the lower incidence of blood toxicity and peripheral neuropathy [58].
3.5. Adverse Reactions and Drug Resistance Induced by BTZ
BTZ exerts significant therapeutic effects on both hematological cancers and solid tumors. Nonetheless, the toxicity and side effects of BTZ cannot be ignored. Representative adverse drug reactions associated with BTZ mainly include: (i) fatigue (the most common adverse reaction), (ii) digestive tract reaction (nausea, diarrhea, constipation, vomiting, etc.) [103,104,105], (iii) peripheral neuropathy (e.g., numbness, pain, and paresthesia) [106,107], (iv) thrombocytopenia, neutropenia, and anemia [108], and (v) hypotension (in some patients during the treatment period) [109].
Numerous studies have confirmed that BTZ can cause drug resistance during administration. Franke et al. found that mutations in the β subunit weakened the binding of BTZ to the β5 subunit, leading to the development of drug resistance in patients [110]. This may be attributed to mutations caused by BTZ in key sites of proteasome subunit beta 5 (PSMB5). PSMB5 encodes a protein termed the β5 subunit. Changes in PSMB5 lead to an increase in the β5 subunit, thereby counteracting the inhibitory effect of β5 binding to BTZ [111]. In addition, the researchers found that the expression of neural precursor cell expressed developmentally downregulated 4-1 (NEDD4-1) E3s (member of the NEED4 protein family) and the enhancement of autophagy may be the cause of BTZ resistance [112,113].
4. Research Progress Regarding BTZ-Based Nanoformulations
Currently, the disadvantages of BTZ (e.g., high hydrophobicity, poor stability, low bioavailability, high toxicity to normal tissues, and drug resistance) limit its clinical applications and efficacy. In addition, BTZ binds nonspecifically to proteins in serum and is rapidly cleared in the liver, impairing its accumulation and penetration into solid tissues. This limits the use of BTZ for the treatment of solid tumors.
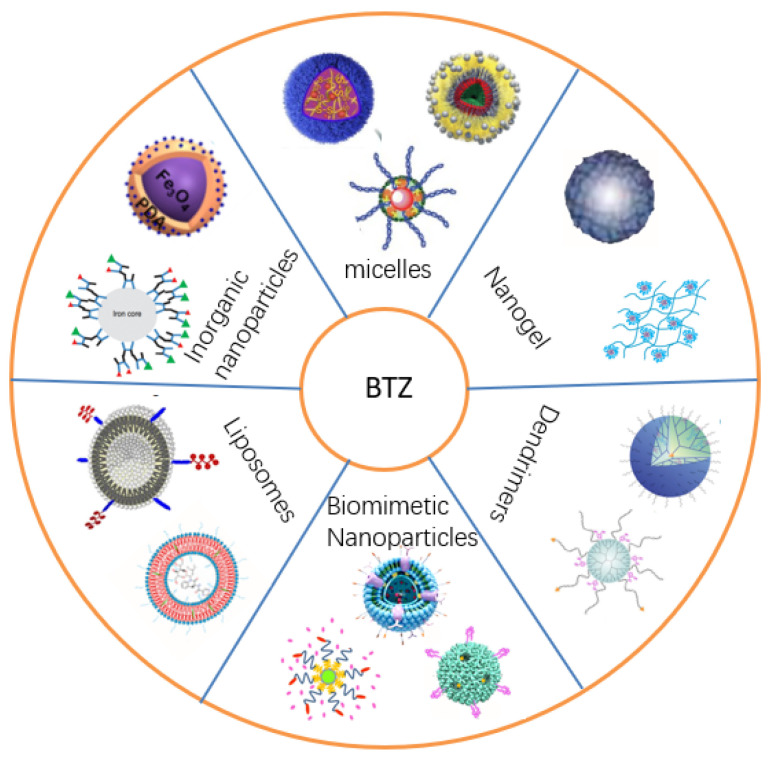
With the rapid development of nanotechnology, drug delivery systems (e.g., liposomes, polymer micelles, inorganic nanoparticles, and biomimetic nanoparticles) have become a research hotspot (Figure 2). The nano-delivery platform can increase the accumulation of drugs at the targeted site and realize controlled drug release, thereby improving the therapeutic effects and reducing the occurrence of side effects. Thus far, numerous BTZ-based nanoformulations have been developed and investigated to further improve druggability and enhance the therapeutic effects (Table 3). In this section, research progress regarding BTZ-based nanoformulations is summarized.
Figure 2.
The nanocarriers for the delivery of BTZ.
Table 3.
Representative BTZ-based nanoformulations in cancer therapy.
| Drug Delivery Systems | Nanocarriers | Drug | Ligand/Target | Cancers | Ref. |
|---|---|---|---|---|---|
| Liposomes | DODEAC, DEX, cholesterol | BTZ, DEX | DEX/GR | WM | [114] |
| Liposomes | PSGL-1, DPPC, Cholesterol | BTZ, Y27632 | PSGL-1/P-selectin | MM | [115] |
| Liposomes | HSPC, Cholesterol, DSPE-PE2000-MAL | BTZ | NGR/aminopeptidase N | Neuroblastoma | [26] |
| Polymeric micelles | catechol-functionalized PC, PEG | BTZ | / | Breast cancer | [116] |
| Polymeric micelles | PEG-b-PCL | BTZ | / | Hepatocellular carcinoma | [117] |
| Polymeric micelles | HA-P(TMC-co-DTC) | BTZ | HA/CD44 | Multiple myeloma | [118] |
| Polymeric micelles | DSPE-PEG, polydopamine | BTZ, DOX | / | Breast cancer | [119] |
| Dendrimers | PEG-PAMAM-Cat | BTZ | cRGD/Integrin αvβ3 | Breast cancer | [120] |
| Dendrimers | G5-PAMAM-KAC-Cat | BTZ | / | Breast cancer | [121] |
| Nanogels | HA, acylate anhydride, dopamine | BTZ | HA/CD44 | Hepatocellular carcinoma | [122] |
| Nanogels | pNIPAAm-co-pAAm | BTZ | / | Colon cancer | [123] |
| Nanogels | PLL−P(LP-co-LC), mPEG-b-PLL/DMMA | BTZ, CA4P | / | Colon cancer | [124] |
| Nanogels | P(Gu)5-PEG-P(Gu)5, P(Bor)5-PEG-(Bor)5, | BTZ | / | Multiple myeloma | [125] |
| Inorganic nanoparticles | MSNs | BTZ | HP/MMP2/9 | Lung cancer | [126] |
| Inorganic nanoparticles | chitosan, magnetic iron oxide | BTZ | / | Breast cancer | [127] |
| Inorganic nanoparticles | mSiO2-H2A | BTZ | / | Cervical cancer | [128] |
| Inorganic nanoparticles | p(HEMA-co-DMA), superparamagnetic iron oxide | BTZ | / | Squamous-cell carcinoma | [129] |
| Biomimetic nanoparticles | macrophage membrane | BTZ | T7/TFR | Breast cancer | [130] |
| Biomimetic nanoparticles | phage P22 virus capsids | BTZ | / | Hepatocellular carcinoma | [131] |
4.1. Liposomes
Liposomes are closed vesicles with a bilayer structure, which are composed of phospholipid and cholesterol [132]. Owing to the similar biochemical structure to that of the cell membrane, liposomes have a favorable safety profile and biocompatibility [133]. Thus far, liposomes have been widely utilized for the delivery of drugs, and numerous products have become commercially available. Hydrophilic and hydrophobic drugs can be wrapped into the hydrophilic core and phospholipid bilayer, respectively [134].
Vijay et al. prepared D1X-encapsulated BTZ (D1XB) liposomes, in which BTZ and dexamethasone were loaded. Dexamethasone, a cholesterol analogue, can be modified on the surface of the liposomes to strengthen and stabilize the lipid bilayer. In addition, dexamethasone is a synthetic ligand of glucocorticoid receptors that can specifically bind to tumor cells with high expression of these receptors [114]. Studies have shown that the loading in liposomes improves the accumulation of BTZ into tumor sites. D1XB has demonstrated strong cytotoxicity against WM cells.
Zuccari et al. prepared asparagine-glycine-arginine (NGR) peptide active-targeting liposomes, termed NGR-SL (amino-lactose-BTZ [LM-BTZ]) [26]. BTZ and LM were firstly complexed via a borate ester bond, and subsequently encapsulated into the liposomes. By forming pH-sensitive borate ester bonds, LM can bind the poorly water-soluble BTZ to the hydrophilic core and prevent leakage of free BTZ from the liposomes. This process improves the encapsulation efficiency and achieves specific release of the drug. In a mouse model of neuroblastoma, NGR-SL (LM-BTZ) exhibited a higher therapeutic index and lower toxicity than the free drug.
4.2. Polymeric Micelles
Polymer micelles are a type of nanoparticle with a core-shell structure formed by the self-assembly of amphiphilic block copolymers in aqueous solution. The hydrophobic core can accommodate the insoluble substances to improve solubility. Besides the physical encapsulation manner, the drug can also be chemically conjugated to the amphiphilic block copolymers to realize the drug loading and delivery [135].
Liu et al. designed a dual pH-sensitive drug delivery system termed BTZ-polymer conjugates (BTZ-PC). The copolymers were synthesized by catechol-functionalized PC conjugation with polyethylene glycol (PEG) via acetal bonds, followed by formation of the micelles after self-assembly [116]. BTZ can be encapsulated into the hydrophobic core of the micelles by conjugation with catechol via a borate ester bond. Both acetal and borate ester bonds are acid-unstable and can be broken in the acidic tumor microenvironment to achieve pH-sensitive drug release. In a xenograft mouse model of human breast cancer, BTZ-PC micelles exerted enhanced antitumor effects and reduced toxicity compared with the free drug.
Liu et al. modified dopamine methylacrylamide to the end of PEG-block-poly(D,L-lactic acid) (PEG-b-PCL) using the “graft” method, leading to the formation of the PEG-b-PCL-b-PDMA co-polymer. Afterwards, BTZ was grafted on the polymer chain by conjugation with the catechol group in dopamine to form a pH-sensitive borate ester bond. Subsequently, BTZ-loaded copolymer micelles were formed under self-assembly [117]. The borate ester bond is a dynamic covalent bond, which improves the therapeutic effects of the drug. In a mouse model of hepatocellular carcinoma, the micelles were associated with stronger antitumor effects and a lower incidence of adverse reactions compared with the free drug.
Gu et al. synthesized a novel single copolymer HA-P(TMC-co-DTC) and prepared the biodegradable micelles (HA-curcumin) by self-assembly. In this process, the shell of HA is crosslinked to the core by disulfide bonds to increase the stability of the micelles [118]. BTZ-loaded micelles (HA-core-disulfide-crosslinked biodegradable micelles-BTZ-pinanediol [HA-CCMS-BP]) were prepared by encapsulating alcohol-esterified BTZ in the carriers. HA can actively target solid tumors overexpressing CD44, while disulfide bonds can selectively release the drug in tumor sites with high glutathione expression. The results showed that the cytotoxicity of HA-CCMS-BP was lower than that of the free drug. In a mouse model of MM, HA-CCMS-BP targeted therapy for MM was better tolerated than the free drug.
4.3. Dendrimers
Dendrimers refer to dendritic macromolecules that start from core molecules and repeatedly grow outwards to form highly branched structures [136] The cargoes encapsulated in dendrimers can stably exist due to the spatial effects. Furthermore, the groups on the surface of dendrimers can control the solubility of hybrid nanocomposites and be linked with other molecules for further modification. Dendritic materials, such as polyamidoamine (PAMAM) and branched polyetherimide, have been widely used as carriers for drug delivery [137].
Wang et al. used PEG as a linker to connect cyclic arginine-glycine-aspartic acid (cRGD) to fifth-generation PAMAM and performed modifications with catechol to obtain the nanocarriers DPA-G5-PEG-cRGD [120]. BTZ was subsequently conjugated with catechol by a pH-sensitive borate ester bond to obtain a novel DPA-G5-PEG-cRGD/BTZ formulation. cRGD can target integrin αvβ3, which is highly expressed during tumor angiogenesis, thus achieving effective drug internalization. In healthy mice, the blood toxicity associated with this formulation was lower than that observed with the free drug. Interestingly, cRGD-PEG-PAMAM-Cat/BTZ also showed significant antitumor activity in a mouse model of MM.
Similarly, Wang et al. prepared a novel nanoformulation (G5-KAC-Cat-BTZ), in which dopamine (Cat) functionalized with a neutral shell (acetylated lysine, KAC) was grafted onto G5 PAMAM. Next, BTZ was loaded into the dendrimers through reaction with catechol [121]. In the acidic tumor microenvironment, the borate ester bond formed by BTZ and Cat realized effective drug release.
4.4. Nanogel
Nanogel is a type of water-soluble, three-dimensional, gelatinous nanoformulation formed after the crosslinking of the polymers. The network structure of the nanogel provides convenient space for accommodating a variety of compounds, such as molecular drugs, luciferin, proteins, peptides, and nucleic acids. Advantages of the nanogel include adjustable particle size, large surface area, high water content, and degradability [138,139]. In recent years, the construction of a controlled drug release system using the nanogel has become a research hotspot.
Liu et al. developed a novel method for the encapsulation of BTZ, in which BTZ was loaded with dopamine-grafted HA nanogel via a borate ester bond [122]. Owing to the location of dopamine on the skeleton structure of the nanogel (NSS-BTZ), more BTZ loading was achieved (≤8.58%). In this nanoformulation, HA can actively target CD44, while the borate ester bond is pH-sensitive. These advantages enable accurate and efficient drug release in the acidic tumor microenvironment with high CD44 expression. In an animal model, free BTZ was linked to severe systemic toxicity. In contrast, at the same dosage, BTZ loaded into the nanogel exhibited better antitumor efficacy and was associated with a significantly lower rate of side effects.
In a study performed by Amin et al., the highly efficient photothermal agent pNIPAAm-co-pAAm was synthesized. Afterwards, dopamine nanoparticles (DP) as the carriers of BTZ were incorporated into pNIPAAm-co-pAAm to prepare the temperature-responsive nanogel, termed NIPAM-AM-DP [123]. The nanogel directly kills the tumor cells through photothermal therapy, as well as localizes and releases BTZ for combination therapy.
4.5. Inorganic Nanoparticles
The term inorganic nanoparticles refers to a category of nanocarriers based on inorganic materials. Their advantages include good size control, low toxicity, and a large specific surface area [140,141]. In addition, inorganic nanoparticles have general properties, such as broad availability, easy functionality, good biocompatibility, and controlled release. Many inorganic materials have been widely used in the development of nanodrugs for the diagnosis and treatment of tumors, such as calcium phosphate, gold nanoparticles, carbon materials, mesoporous silica, etc., [142].
Sabine et al. prepared mesoporous silica nanoparticles (MSNs) for the encapsulation of BTZ [126]. The heptapeptide (HP) was combined with anti-biotin protein to block the pores of MSNs, thus yielding a new formulation termed MSNsHP. HP can be specifically recognized and cleaved by matrix metallopeptidase 9 (MMP9), which is highly expressed in tumor tissue, thereby opening the MSN channel to release the drug. The results showed that MSNsHP could remain stable under normal conditions, while it responsively released BTZ in the acidic tumor microenvironment. As the nanocarrier for BTZ, MSNsHP reduced the systemic toxicity of BTZ and selectively released the drug.
Gozde et al. prepared chitosan-coated superparamagnetic iron oxide nanoparticles (CS-MNP) for the encapsulation of BTZ. The nanoparticles were formed by ionic crosslinking in the presence of chitosan and tripolyphosphate molecules [127]. It was found that CS-MNP improved the therapeutic efficacy of BTZ. Because the protonated amino group of chitosan produces a swelling of osmotic pressure at a low pH, CS-MNP has the advantage of pH-responsive release.
4.6. Biomimetic Nanomaterials
Biomimetic nanomaterials are “living” materials with similar functions to those of living organisms. Unlike allogenic compounds (e.g., chemically synthesized polymers or inorganic nanoparticles), biomimetic materials are associated with low toxicity and not recognized by the immune system. This may be beneficial for improving the pharmacokinetics and biodistribution of the drugs [143,144]. Biomimetic nanoparticles are generally developed by coating or mixing the biomimetic nanomaterials with organic or inorganic nanoparticles [145]. At present, biomimetic nanomaterials (e.g., protein polypeptides, DNA, RNA, exosomes, phages, various cells, and cell membranes) have been extensively studied. Among those, albumin, exosomes, and cell membranes are the most widely investigated materials. Specifically, it has been shown that a variety of cell membranes (erythrocyte, platelet, tumor, macrophage, neutrophil, etc.) are useful for drug encapsulation.
Yu et al. synthesized a copper sulfide/carbon dot nanocomposite (CuSCD) with a hollow structure. CuSCD could be utilized as a multifunctional nanocarrier for both the photothermal conduction and encapsulation of BTZ [130]. Furthermore, the macrophage membrane hybridized with the T7 peptide was coated on the surface of BTZ-loaded CuSCD to form a novel biomimetic drug delivery system termed CuSCDB@MMT7. The T7 peptide binds to the ferritin receptor, which is overexpressed on tumor cells, and mediates the endocytosis of the nanoparticles. In addition, the macrophage membrane avoids immune recognition, thus ensuring that the therapeutic drugs safely reach the target. CuSCDB@MMT7 exerts both phototherapeutic and chemotherapeutic effects and has exhibited greater antitumor efficacy in the breast cancer mouse model.
Min et al. obtained phage P22 virus capsids by high-temperature treatment [131]. Catechol ligands were chemically attached to the interior surface of the P22 viral capsid for subsequent encapsulation of BTZ. A novel nanoplatform was obtained by connecting hepatocellular carcinoma-targeting peptide SP94 (SFSIIHTPILPL) as a ligating agent to the outer surface of the P22 virus capsid nanocomposite. This biomimetic system is characterized by high efficiency for the loading of BTZ. Owing to the presence of SP94, the biomimetic nanoparticles can achieve a localized release of BTZ.
5. Outlook
BTZ is the first proteasome inhibitor approved by the Food and Drug Administration. It has demonstrated unique advantages in the treatment of MCL and MM, and numerous BTZ-based combination regimens have been widely utilized in clinical practice. Moreover, according to preclinical and clinical data, BTZ showed high antitumor activity against different hematological cancers and solid tumors. With the development of nanomaterials and their application in drug delivery, BTZ-based nanoformulations have been developed and widely investigated to improve the antitumor efficacy and reduce the occurrence of side effects. Although great advances have been achieved, the current understanding of BTZ remains insufficient. Further research focusing on the crosstalk between different action pathways, the in vivo metabolic process, and the immune effects on solid tumors is warranted. In addition, the unfavorable safety profile of nanomaterials, unexpected accumulation in normal tissues, poor stability of nanoparticles, etc., pose barriers to the commercialization of BTZ-based nanoformulations. Therefore, further understanding of the mechanism of BTZ in vivo is necessary. Currently, the construction of novel nanomedicines with the desired safety and specific targeting properties remains a challenge. It is expected that this field of research will attract considerable attention in the future.
Author Contributions
J.L. and R.Z. collected the data and wrote the manuscript; X.J. and Z.L. helped collect and analyze the data; B.Z. conceived the study and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Science Foundation of China (No. 81803464) and Natural Science Foundations of Shandong Province (ZR2018BH041). The work was also funded by the Domestic Visiting Scholar Program of Weifang Medical University (20207-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson P.G., Hideshima T., Mitsiades C., Anderson K. Proteasome inhibition in hematologic malignancies. Ann. Med. 2004;36:304–314. doi: 10.1080/07853890410030877. [DOI] [PubMed] [Google Scholar]
- 2.Johnson D.E. The ubiquitin-proteasome system: Opportunities for therapeutic intervention in solid tumors. Endocr. Relat. Cancer. 2015;22:T1–T17. doi: 10.1530/ERC-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hideshima T., Richardson P.G., Anderson K.C. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol. Cancer Ther. 2011;10:2034–2042. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams J., Behnke M., Chen S., Cruickshank A.A., Dick L.R., Grenier L., Klunder J.M., Ma Y.T., Plamondon L., Stein R.L. Potent and selective inhibitors of the proteasome: Dipeptidyl boronic acids. Bioorg. Med. Chem. Lett. 1998;8:333–338. doi: 10.1016/S0960-894X(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 5.Ebert B., Kisiela M., Wsól V., Maser E. Proteasome inhibitors MG-132 and bortezomib induce AKR1C1, AKR1C3, AKR1B1, and AKR1B10 in human colon cancer cell lines SW-480 and HT-29. Chem. Biol. Interact. 2011;191:239–249. doi: 10.1016/j.cbi.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Letai A., Bassik M.C., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 7.Yang M.H., Jung S.H., Chinnathambi A., Alahmadi T.A., Alharbi S.A., Sethi G., Ahn K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules. 2019;10:23. doi: 10.3390/biom10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan D., Singh A., Brahmandam M., Podar K., Hideshima T., Richardson P., Munshi N., Palladino M.A., Anderson K.C. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goy A., Younes A., McLaughlin P., Pro B., Romaguera J.E., Hagemeister F., Fayad L., Dang N.H., Samaniego F., Wang M., et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 10.Papandreou C.N., Logothetis C.J. Bortezomib as a potential treatment for prostate cancer. Cancer Res. 2004;64:5036–5043. doi: 10.1158/0008-5472.CAN-03-2707. [DOI] [PubMed] [Google Scholar]
- 11.Scagliotti G.V., Germonpré P., Bosquée L., Vansteenkiste J., Gervais R., Planchard D., Reck M., De Marinis F., Lee J.S., Park K., et al. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer. 2010;68:420–426. doi: 10.1016/j.lungcan.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Vanderloo J.P., Pomplun M.L., Vermeulen L.C., Kolesar J.M. Stability of unused reconstituted bortezomib in original manufacturer vials. J. Oncol. Pharm. Pract. 2011;17:400–402. doi: 10.1177/1078155210386268. [DOI] [PubMed] [Google Scholar]
- 13.Wei W., Yue Z.G., Qu J.B., Yue H., Su Z.G., Ma G.H. Galactosylated nanocrystallites of insoluble anticancer drug for liver-targeting therapy: An in vitro evaluation. Nanomedicine. 2010;5:589–596. doi: 10.2217/nnm.10.27. [DOI] [PubMed] [Google Scholar]
- 14.Mujtaba T., Kanwar J., Wan S.B., Chan T.H., Dou Q.P. Sensitizing human multiple myeloma cells to the proteasome inhibitor bortezomib by novel curcumin analogs. Int. J. Mol. Med. 2012;29:102–106. doi: 10.3892/ijmm.2011.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S.K., Harrison S.J., Cavo M., de la Rubia J., Popat R., Gasparetto C., Hungria V., Salwender H., Suzuki K., Kim I., et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1630–1642. doi: 10.1016/S1470-2045(20)30525-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen M.Y., Xiao Z.K., Lei X.P., Li J.X., Yu X.Y., Zhang J.Y., Ye G.D., Guo Y.J., Mo G., Li C.W., et al. Preparation, characterization and in vitro-in vivo evaluation of bortezomib supermolecular aggregation nanovehicles. J. Nanobiotechnology. 2020;18:57. doi: 10.1186/s12951-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Dai Z., Wang J., Tu Y., Zhu L. Folate-targeted pH-sensitive bortezomib conjugates for cancer treatment. Chem. Commun. 2019;55:4254–4257. doi: 10.1039/C9CC01344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che F., Chen J., Dai J., Liu X. Inhibition of Multiple Myeloma Using 5-Aza-2′-Deoxycytidine and Bortezomib-Loaded Self-Assembling Nanoparticles. Cancer Manag. Res. 2020;12:6969–6976. doi: 10.2147/CMAR.S255682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoudian M., Valizadeh H., Zakeri-Milani P. Bortezomib-loaded solid lipid nanoparticles: Preparation, characterization, and intestinal permeability investigation. Drug Dev. Ind. Pharm. 2018;44:1598–1605. doi: 10.1080/03639045.2018.1483385. [DOI] [PubMed] [Google Scholar]
- 20.Luza S., Opazo C.M., Bousman C.A., Pantelis C., Bush A.I., Everall I.P. The ubiquitin proteasome system and schizophrenia. Lancet Psychiatry. 2020;7:528–537. doi: 10.1016/S2215-0366(19)30520-6. [DOI] [PubMed] [Google Scholar]
- 21.Varshavsky A. The Ubiquitin System, Autophagy, and Regulated Protein Degradation. Annu. Rev. Biochem. 2017;86:123–128. doi: 10.1146/annurev-biochem-061516-044859. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Mallampalli R.K. Small molecule therapeutics targeting F-box proteins in cancer. Semin. Cancer Biol. 2016;36:105–119. doi: 10.1016/j.semcancer.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins G.A., Goldberg A.L. The Logic of the 26S Proteasome. Cell. 2017;169:792–806. doi: 10.1016/j.cell.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohl C., Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–822. doi: 10.1126/science.aax3769. [DOI] [PubMed] [Google Scholar]
- 25.Kaarniranta K., Uusitalo H., Blasiak J., Felszeghy S., Kannan R., Kauppinen A., Salminen A., Sinha D., Ferrington D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020;79:100858. doi: 10.1016/j.preteyeres.2020.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuccari G., Milelli A., Pastorino F., Loi M., Petretto A., Parise A., Marchetti C., Minarini A., Cilli M., Emionite L., et al. Tumor vascular targeted liposomal-bortezomib minimizes side effects and increases therapeutic activity in human neuroblastoma. J. Control Release. 2015;211:44–52. doi: 10.1016/j.jconrel.2015.05.286. [DOI] [PubMed] [Google Scholar]
- 27.Narayanan S., Cai C.Y., Assaraf Y.G., Guo H.Q., Cui Q., Wei L., Huang J.J., Ashby C.R., Jr., Chen Z.S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updates. 2020;48:100663. doi: 10.1016/j.drup.2019.100663. [DOI] [PubMed] [Google Scholar]
- 28.Rapino F., Naumann I., Fulda S. Bortezomib antagonizes microtubule-interfering drug-induced apoptosis by inhibiting G2/M transition and MCL-1 degradation. Cell Death Dis. 2013;4:e925. doi: 10.1038/cddis.2013.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Lin C., Song J., Chen H., Chen X., Ren L., Zhou Z., Pan J., Yang Z., Bao W., et al. Parkin facilitates proteasome inhibitor-induced apoptosis via suppression of NF-κB activity in hepatocellular carcinoma. Cell Death Dis. 2019;10:719. doi: 10.1038/s41419-019-1881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J., Sun Y., Xu J., Xu T., Xu Z., Liu P. ZHX2 mediates proteasome inhibitor resistance via regulating nuclear translocation of NF-κB in multiple myeloma. Cancer Med. 2020;9:7244–7252. doi: 10.1002/cam4.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller C.P., Ban K., Dujka M.E., McConkey D.J., Munsell M., Palladino M., Chandra J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vriend J., Reiter R.J. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014;115:8–14. doi: 10.1016/j.lfs.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Muenchow A., Weller S., Hinterleitner C., Malenke E., Bugl S., Wirths S., Müller M.R., Schulze-Osthoff K., Aulitzky W.E., Kopp H.G., et al. The BCL-2 selective inhibitor ABT-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring BAX and NOXA. Cell Death Dis. 2020;11:701. doi: 10.1038/s41419-020-02910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D., Xu Q., Yuan Q., Jia M., Niu H., Liu X., Zhang J., Young C.Y., Yuan H. Proteasome inhibition boosts autophagic degradation of ubiquitinated-AGR2 and enhances the antitumor efficiency of bevacizumab. Oncogene. 2019;38:3458–3474. doi: 10.1038/s41388-019-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manasanch E.E., Korde N., Zingone A., Tageja N., Fernandez de Larrea C., Bhutani M., Wu P., Roschewski M., Landgren O. The proteasome: Mechanisms of biology and markers of activity and response to treatment in multiple myeloma. Leuk. Lymphoma. 2014;55:1707–1714. doi: 10.3109/10428194.2013.828351. [DOI] [PubMed] [Google Scholar]
- 36.Orlowski R.Z., Small G.W., Shi Y.Y. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J. Biol. Chem. 2002;277:27864–27871. doi: 10.1074/jbc.M201519200. [DOI] [PubMed] [Google Scholar]
- 37.Hendil K.B., Kriegenburg F., Tanaka K., Murata S., Lauridsen A.M., Johnsen A.H., Hartmann-Petersen R. The 20S proteasome as an assembly platform for the 19S regulatory complex. J. Mol. Biol. 2009;394:320–328. doi: 10.1016/j.jmb.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 38.Cheriyath V., Jacobs B.S., Hussein M.A. Proteasome inhibitors in the clinical setting: Benefits and strategies to overcome multiple myeloma resistance to proteasome inhibitors. Drugs R D. 2007;8:1–12. doi: 10.2165/00126839-200708010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Yerlikaya A., Yöntem M. The significance of ubiquitin proteasome pathway in cancer development. Recent Pat. Anticancer Drug Discov. 2013;8:298–309. doi: 10.2174/1574891X113089990033. [DOI] [PubMed] [Google Scholar]
- 40.Pitts T.M., Morrow M., Kaufman S.A., Tentler J.J., Eckhardt S.G. Vorinostat and bortezomib exert synergistic antiproliferative and proapoptotic effects in colon cancer cell models. Mol. Cancer Ther. 2009;8:342–349. doi: 10.1158/1535-7163.MCT-08-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansour M.A. Ubiquitination: Friend and foe in cancer. Int. J. Biochem Cell Biol. 2018;101:80–93. doi: 10.1016/j.biocel.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Driscoll J.J., Woodle E.S. Targeting the ubiquitin+proteasome system in solid tumors. Semin. Hematol. 2012;49:277–283. doi: 10.1053/j.seminhematol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Genin E., Reboud-Ravaux M., Vidal J. Proteasome inhibitors: Recent advances and new perspectives in medicinal chemistry. Curr. Top. Med. Chem. 2010;10:232–256. doi: 10.2174/156802610790725515. [DOI] [PubMed] [Google Scholar]
- 44.Mitsiades C.S., Mitsiades N., Hideshima T., Richardson P.G., Anderson K.C. Proteasome inhibition as a therapeutic strategy for hematologic malignancies. Expert Rev. Anticancer Ther. 2005;5:465–476. doi: 10.1586/14737140.5.3.465. [DOI] [PubMed] [Google Scholar]
- 45.Gelman J.S., Sironi J., Berezniuk I., Dasgupta S., Castro L.M., Gozzo F.C., Ferro E.S., Fricker L.D. Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib. PLoS ONE. 2013;8:e53263. doi: 10.1371/journal.pone.0053263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kisselev A.F., Callard A., Goldberg A.L. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J. Biol. Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 47.Berkers C.R., Verdoes M., Lichtman E., Fiebiger E., Kessler B.M., Anderson K.C., Ploegh H.L., Ovaa H., Galardy P.J. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat. Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 48.Manasanch E.E., de Larrea C.F., Zingone A., Steinberg S.M., Kwok M., Tageja N., Bhutani M., Kazandjian D., Roschewski M., Wu P., et al. Enzymatic activities of circulating plasma proteasomes in newly diagnosed multiple myeloma patients treated with carfilzomib, lenalidomide and dexamethasone. Leuk. Lymphoma. 2017;58:639–645. doi: 10.1080/10428194.2016.1214953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta N., Hanley M.J., Xia C., Labotka R., Harvey R.D., Venkatakrishnan K. Clinical Pharmacology of Ixazomib: The First Oral Proteasome Inhibitor. Clin. Pharmacokinet. 2019;58:431–449. doi: 10.1007/s40262-018-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirley M. Ixazomib: First Global Approval. Drugs. 2016;76:405–411. doi: 10.1007/s40265-016-0548-5. [DOI] [PubMed] [Google Scholar]
- 51.Caputi F.F., Di Cesare Mannelli L., Rullo L., Micheli L., Stamatakos S., Posa L., Ghelardini C., Romualdi P., Candeletti S. The active second-generation proteasome inhibitor oprozomib reverts the oxaliplatin-induced neuropathy symptoms. Biochem. Pharmacol. 2020;182:114255. doi: 10.1016/j.bcp.2020.114255. [DOI] [PubMed] [Google Scholar]
- 52.Vogl D.T., Martin T.G., Vij R., Hari P., Mikhael J.R., Siegel D., Wu K.L., Delforge M., Gasparetto C. Phase I/II study of the novel proteasome inhibitor delanzomib (CEP-18770) for relapsed and refractory multiple myeloma. Leuk. Lymphoma. 2017;58:1872–1879. doi: 10.1080/10428194.2016.1263842. [DOI] [PubMed] [Google Scholar]
- 53.Singh A.V., Palladino M.A., Lloyd G.K., Potts B.C., Chauhan D., Anderson K.C. Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI-0052 (marizomib) in a human plasmacytoma xenograft murine model. Br. J. Haematol. 2010;149:550–559. doi: 10.1111/j.1365-2141.2010.08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen F.Z., Zhao X.K. Ubiquitin-proteasome pathway and prostate cancer. Onkologie. 2013;36:592–596. doi: 10.1159/000355166. [DOI] [PubMed] [Google Scholar]
- 55.Wei W., Li H., Zhang G., Zhang Y., Wu K., Bao R., Wang G., Zheng H., Xia Y., Li C. Proteasome inhibitors attenuates mitoxantrone-triggered immunogenic cell death in prostate cancer cells. Med. Oncol. 2020;37:116. doi: 10.1007/s12032-020-01445-y. [DOI] [PubMed] [Google Scholar]
- 56.Raninga P.V., Lee A., Sinha D., Dong L.F., Datta K.K., Lu X., Kalita-de Croft P., Dutt M., Hill M., Pouliot N., et al. Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics. 2020;10:5259–5275. doi: 10.7150/thno.42705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boccadoro M., Morgan G., Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005;5:18. doi: 10.1186/1475-2867-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau P., Pylypenko H., Grosicki S., Karamanesht I., Leleu X., Grishunina M., Rekhtman G., Masliak Z., Robak T., Shubina A., et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 59.Fennell D.A., Chacko A., Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27:1189–1197. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 60.Guo N., Peng Z. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac. J. Clin. Oncol. 2013;9:6–11. doi: 10.1111/j.1743-7563.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhu B., Jin Y., Han L., Chen H., Zhong F., Wang W., Chen N. Proteasome inhibitor inhibits proliferation and induces apoptosis in renal interstitial fibroblasts. Pharmacol. Rep. 2013;65:1357–1365. doi: 10.1016/S1734-1140(13)71494-4. [DOI] [PubMed] [Google Scholar]
- 62.Granato M., Romeo M.A., Tiano M.S., Santarelli R., Gonnella R., Gilardini Montani M.S., Faggioni A., Cirone M. Bortezomib promotes KHSV and EBV lytic cycle by activating JNK and autophagy. Sci. Rep. 2017;7:13052. doi: 10.1038/s41598-017-13533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papandreou C.N., Daliani D.D., Nix D., Yang H., Madden T., Wang X., Pien C.S., Millikan R.E., Tu S.M., Pagliaro L., et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J. Clin. Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 64.Sánchez-Serrano I. Success in translational research: Lessons from the development of bortezomib. Nat. Rev. Drug Discov. 2006;5:107–114. doi: 10.1038/nrd1959. [DOI] [PubMed] [Google Scholar]
- 65.Markovina S., Callander N.S., O’Connor S.L., Kim J., Werndli J.E., Raschko M., Leith C.P., Kahl B.S., Kim K., Miyamoto S. Bortezomib-resistant nuclear factor-kappaB activity in multiple myeloma cells. Mol. Cancer Res. 2008;6:1356–1364. doi: 10.1158/1541-7786.MCR-08-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim C., Lee J.H., Ko J.H., Chinnathambi A., Alharbi S.A., Shair O.H.M., Sethi G., Ahn K.S. Formononetin Regulates Multiple Oncogenic Signaling Cascades and Enhances Sensitivity to Bortezomib in a Multiple Myeloma Mouse Model. Biomolecules. 2019;9:262. doi: 10.3390/biom9070262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poulaki V., Mitsiades C.S., Kotoula V., Negri J., McMillin D., Miller J.W., Mitsiades N. The proteasome inhibitor bortezomib induces apoptosis in human retinoblastoma cell lines in vitro. Investig. Ophthalmol. Vis. Sci. 2007;48:4706–4719. doi: 10.1167/iovs.06-1147. [DOI] [PubMed] [Google Scholar]
- 68.Honma Y., Sato-Morita M., Katsuki Y., Mihara H., Baba R., Harada M. Trehalose activates autophagy and decreases proteasome inhibitor-induced endoplasmic reticulum stress and oxidative stress-mediated cytotoxicity in hepatocytes. Hepatol. Res. 2018;48:94–105. doi: 10.1111/hepr.12892. [DOI] [PubMed] [Google Scholar]
- 69.Best S., Hashiguchi T., Kittai A., Bruss N., Paiva C., Okada C., Liu T., Berger A., Danilov A.V. Targeting ubiquitin-activating enzyme induces ER stress-mediated apoptosis in B-cell lymphoma cells. Blood Adv. 2019;3:51–62. doi: 10.1182/bloodadvances.2018026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hideshima T., Bradner J.E., Wong J., Chauhan D., Richardson P., Schreiber S.L., Anderson K.C. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C., Burghardt R., Smith R., Wormke M., Stewart J., Safe S. Peroxisome proliferator-activated receptor gamma agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor alpha in MCF-7 breast cancer cells. Cancer Res. 2003;63:958–964. [PubMed] [Google Scholar]
- 72.Blagosklonny M.V., Wu G.S., Omura S., el-Deiry W.S. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem. Biophys Res. Commun. 1996;227:564–569. doi: 10.1006/bbrc.1996.1546. [DOI] [PubMed] [Google Scholar]
- 73.Pagano M., Tam S.W., Theodoras A.M., Beer-Romero P., Del Sal G., Chau V., Yew P.R., Draetta G.F., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 74.Granato M., Santarelli R., Lotti L.V., Di Renzo L., Gonnella R., Garufi A., Trivedi P., Frati L., D’Orazi G., Faggioni A., et al. JNK and macroautophagy activation by bortezomib has a pro-survival effect in primary effusion lymphoma cells. PLoS ONE. 2013;8:e75965. doi: 10.1371/journal.pone.0075965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li C., Johnson D.E. Bortezomib induces autophagy in head and neck squamous cell carcinoma cells via JNK activation. Cancer Lett. 2012;314:102–107. doi: 10.1016/j.canlet.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M., Wang Y., Hu K., Shao N., Cheng Y. Tumor extracellular acidity activated “off-on” release of bortezomib from a biocompatible dendrimer. Biomater. Sci. 2015;3:480–489. doi: 10.1039/C4BM00365A. [DOI] [PubMed] [Google Scholar]
- 77.Park S.B., Goldstein D., Krishnan A.V., Lin C.S., Friedlander M.L., Cassidy J., Koltzenburg M., Kiernan M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 78.André P., Cisternino S., Chiadmi F., Toledano A., Schlatter J., Fain O., Fontan J.E. Stability of bortezomib 1-mg/mL solution in plastic syringe and glass vial. Ann. Pharmacother. 2005;39:1462–1466. doi: 10.1345/aph.1E620. [DOI] [PubMed] [Google Scholar]
- 79.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/S1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 80.Bedford L., Lowe J., Dick L.R., Mayer R.J., Brownell J.E. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat. Rev. Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brooks W.L., Sumerlin B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016;116:1375–1397. doi: 10.1021/acs.chemrev.5b00300. [DOI] [PubMed] [Google Scholar]
- 82.Li Y., Xiao W., Xiao K., Berti L., Luo J., Tseng H.P., Fung G., Lam K.S. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew. Chem. Int. Ed. Engl. 2012;51:2864–2869. doi: 10.1002/anie.201107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tannock I.F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 84.Avcu F., Ural A.U., Cetin T., Nevruz O. Effects of bortezomib on platelet aggregation and ATP release in human platelets, in vitro. Thromb. Res. 2008;121:567–571. doi: 10.1016/j.thromres.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Weniger M.A., Rizzatti E.G., Pérez-Galán P., Liu D., Wang Q., Munson P.J., Raghavachari N., White T., Tweito M.M., Dunleavy K., et al. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin. Cancer Res. 2011;17:5101–5112. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tibullo D., Giallongo C., Romano A., Vicario N., Barbato A., Puglisi F., Parenti R., Amorini A.M., Wissam Saab M., Tavazzi B., et al. Mitochondrial Functions, Energy Metabolism and Protein Glycosylation are Interconnected Processes Mediating Resistance to Bortezomib in Multiple Myeloma Cells. Biomolecules. 2020;10:696. doi: 10.3390/biom10050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang J.E., Peterson C., Choi S., Eickhoff J.C., Kim K., Yang D.T., Gilbert L.A., Rogers E.S., Werndli J.E., Huie M.S., et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab in mantle cell lymphoma: A Wisconsin Oncology Network study. Br. J. Haematol. 2011;155:190–197. doi: 10.1111/j.1365-2141.2011.08820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang J.E., Li H., Smith M.R., Gascoyne R.D., Paietta E.M., Yang D.T., Advani R.H., Horning S.J., Kahl B.S. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: An Eastern Cooperative Oncology Group study (E1405) Blood. 2014;123:1665–1673. doi: 10.1182/blood-2013-08-523845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richardson P.G., Sonneveld P., Schuster M., Irwin D., Stadtmauer E., Facon T., Harousseau J.L., Ben-Yehuda D., Lonial S., Goldschmidt H., et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 90.Ghobrial I.M., Xie W., Padmanabhan S., Badros A., Rourke M., Leduc R., Chuma S., Kunsman J., Warren D., Poon T., et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenström Macroglobulinemia. Am. J. Hematol. 2010;85:670–674. doi: 10.1002/ajh.21788. [DOI] [PubMed] [Google Scholar]
- 91.Ruan J., Martin P., Furman R.R., Lee S.M., Cheung K., Vose J.M., Lacasce A., Morrison J., Elstrom R., Ely S., et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 92.Kim S.J., Yoon D.H., Kang H.J., Kim J.S., Park S.K., Kim H.J., Lee J., Ryoo B.Y., Ko Y.H., Huh J., et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur. J. Cancer. 2012;48:3223–3231. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Irvin W.J., Jr., Orlowski R.Z., Chiu W.K., Carey L.A., Collichio F.A., Bernard P.S., Stijleman I.J., Perou C., Ivanova A., Dees E.C. Phase II study of bortezomib and pegylated liposomal doxorubicin in the treatment of metastatic breast cancer. Clin. Breast Cancer. 2010;10:465–470. doi: 10.3816/CBC.2010.n.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fanucchi M.P., Fossella F.V., Belt R., Natale R., Fidias P., Carbone D.P., Govindan R., Raez L.E., Robert F., Ribeiro M., et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 95.Richardson P.G., Barlogie B., Berenson J., Singhal S., Jagannath S., Irwin D., Rajkumar S.V., Srkalovic G., Alsina M., Alexanian R., et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 96.Tan C.R.C., Abdul-Majeed S., Cael B., Barta S.K. Clinical Pharmacokinetics and Pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2019;58:157–168. doi: 10.1007/s40262-018-0679-9. [DOI] [PubMed] [Google Scholar]
- 97.Tew K.D. Commentary on “Proteasome Inhibitors: A Novel Class of Potent and Effective Antitumor Agents”. Cancer Res. 2016;76:4916–4917. doi: 10.1158/0008-5472.CAN-16-1974. [DOI] [PubMed] [Google Scholar]
- 98.Reece D.E., Sullivan D., Lonial S., Mohrbacher A.F., Chatta G., Shustik C., Burris H., 3rd, Venkatakrishnan K., Neuwirth R., Riordan W.J., et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother. Pharmacol. 2011;67:57–67. doi: 10.1007/s00280-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moreau P., Karamanesht I.I., Domnikova N., Kyselyova M.Y., Vilchevska K.V., Doronin V.A., Schmidt A., Hulin C., Leleu X., Esseltine D.L., et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin. Pharmacokinet. 2012;51:823–829. doi: 10.1007/s40262-012-0010-0. [DOI] [PubMed] [Google Scholar]
- 100.Pekol T., Daniels J.S., Labutti J., Parsons I., Nix D., Baronas E., Hsieh F., Gan L.S., Miwa G. Human metabolism of the proteasome inhibitor bortezomib: Identification of circulating metabolites. Drug Metab. Dispos. 2005;33:771–777. doi: 10.1124/dmd.104.002956. [DOI] [PubMed] [Google Scholar]
- 101.Leal T.B., Remick S.C., Takimoto C.H., Ramanathan R.K., Davies A., Egorin M.J., Hamilton A., LoRusso P.A., Shibata S., Lenz H.J., et al. Dose-escalating and pharmacological study of bortezomib in adult cancer patients with impaired renal function: A National Cancer Institute Organ Dysfunction Working Group Study. Cancer Chemother. Pharmacol. 2011;68:1439–1447. doi: 10.1007/s00280-011-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LoRusso P.M., Venkatakrishnan K., Ramanathan R.K., Sarantopoulos J., Mulkerin D., Shibata S.I., Hamilton A., Dowlati A., Mani S., Rudek M.A., et al. Pharmacokinetics and safety of bortezomib in patients with advanced malignancies and varying degrees of liver dysfunction: Phase I NCI Organ Dysfunction Working Group Study NCI-6432. Clin. Cancer Res. 2012;18:2954–2963. doi: 10.1158/1078-0432.CCR-11-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y.L. Analysis of bortezomib treatment efficacy and adverse reactions for patients with follicular lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23:119–122. doi: 10.7534/j.issn.1009-2137.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 104.Aghajanian C., Soignet S., Dizon D.S., Pien C.S., Adams J., Elliott P.J., Sabbatini P., Miller V., Hensley M.L., Pezzulli S., et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin. Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 105.Orlowski R.Z., Stinchcombe T.E., Mitchell B.S., Shea T.C., Baldwin A.S., Stahl S., Adams J., Esseltine D.L., Elliott P.J., Pien C.S., et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J. Clin. Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 106.Velasco R., Alberti P., Bruna J., Psimaras D., Argyriou A.A. Bortezomib and other proteosome inhibitors-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019;24((Suppl. 2)):S52–S62. doi: 10.1111/jns.12338. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto S., Egashira N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021;22:888. doi: 10.3390/ijms22020888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuter D.J. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29:282–294. [PubMed] [Google Scholar]
- 109.Schwartz R., Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology. 2004;18:14–21. [PubMed] [Google Scholar]
- 110.Franke N.E., Niewerth D., Assaraf Y.G., van Meerloo J., Vojtekova K., van Zantwijk C.H., Zweegman S., Chan E.T., Kirk C.J., Geerke D.P., et al. Impaired bortezomib binding to mutant β5 subunit of the proteasome is the underlying basis for bortezomib resistance in leukemia cells. Leukemia. 2012;26:757–768. doi: 10.1038/leu.2011.256. [DOI] [PubMed] [Google Scholar]
- 111.Oerlemans R., Franke N.E., Assaraf Y.G., Cloos J., van Zantwijk I., Berkers C.R., Scheffer G.L., Debipersad K., Vojtekova K., Lemos C., et al. Molecular basis of bortezomib resistance: Proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112:2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- 112.Dong H., Chen L., Chen X., Gu H., Gao G., Gao Y., Dong B. Dysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cells. Leuk. Lymphoma. 2009;50:974–984. doi: 10.1080/10428190902895780. [DOI] [PubMed] [Google Scholar]
- 113.Huang X., Gu H., Zhang E., Chen Q., Cao W., Yan H., Chen J., Yang L., Lv N., He J., et al. The NEDD4-1 E3 ubiquitin ligase: A potential molecular target for bortezomib sensitivity in multiple myeloma. Int. J. Cancer. 2020;146:1963–1978. doi: 10.1002/ijc.32615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Madamsetty V.S., Paulus A., Akhtar S., Manna A., Rachamalla H.R., Banerjee R., Mukhopadhyay D., Chanan-Khan A. Novel tumor-targeted liposomes comprised of an MDM2 antagonist plus proteasome inhibitor display anti-tumor activity in a xenograft model of bortezomib-resistant Waldenstrom macroglobulinemia. Leuk. Lymphoma. 2020;61:2399–2408. doi: 10.1080/10428194.2020.1775204. [DOI] [PubMed] [Google Scholar]
- 115.Federico C., Alhallak K., Sun J., Duncan K., Azab F., Sudlow G.P., de la Puente P., Muz B., Kapoor V., Zhang L., et al. Tumor microenvironment-targeted nanoparticles loaded with bortezomib and ROCK inhibitor improve efficacy in multiple myeloma. Nat. Commun. 2020;11:6037. doi: 10.1038/s41467-020-19932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S., Ono R.J., Yang C., Gao S., Ming Tan J.Y., Hedrick J.L., Yang Y.Y. Dual pH-Responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for in Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces. 2018;10:19355–19364. doi: 10.1021/acsami.8b01954. [DOI] [PubMed] [Google Scholar]
- 117.Liu L., Wang S., Qi P., Song S., Yang Y., Shi J., Han G. Dopamine-modified poly(ε-caprolactone) micelles for pH controlled delivery of bortezomib. Int. J. Pharm. 2020;590:119885. doi: 10.1016/j.ijpharm.2020.119885. [DOI] [PubMed] [Google Scholar]
- 118.Gu Z., Wang X., Cheng R., Cheng L., Zhong Z. Hyaluronic acid shell and disulfide-crosslinked core micelles for in vivo targeted delivery of bortezomib for the treatment of multiple myeloma. Acta Biomater. 2018;80:288–295. doi: 10.1016/j.actbio.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 119.Zhang R., Su S., Hu K., Shao L., Deng X., Sheng W., Wu Y. Smart micelle@polydopamine core-shell nanoparticles for highly effective chemo-photothermal combination therapy. Nanoscale. 2015;7:19722–19731. doi: 10.1039/C5NR04828A. [DOI] [PubMed] [Google Scholar]
- 120.Wang M., Cai X., Yang J., Wang C., Tong L., Xiao J., Li L. A Targeted and pH-Responsive Bortezomib Nanomedicine in the Treatment of Metastatic Bone Tumors. ACS Appl. Mater. Interfaces. 2018;10:41003–41011. doi: 10.1021/acsami.8b07527. [DOI] [PubMed] [Google Scholar]
- 121.Wang M., Wang Y., Shao N., Xiao J., Cheng Y. Catechol-grafted dendrimer with a neutral shell allows pH-triggered "off-on" release of bortezomib. J. Control Release. 2015;213:e78–e79. doi: 10.1016/j.jconrel.2015.05.130. [DOI] [PubMed] [Google Scholar]
- 122.Liu L., Luan S., Zhang C., Wang R., Zhang Y., Zhang M., Sheng Q., Han G., Wang T., Song S. Encapsulation and pH-responsive release of bortezomib by dopamine grafted hyaluronate nanogels. Int. J. Biol. Macromol. 2021;183:369–378. doi: 10.1016/j.ijbiomac.2021.04.161. [DOI] [PubMed] [Google Scholar]
- 123.GhavamiNejad A., SamariKhalaj M., Aguilar L.E., Park C.H., Kim C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016;6:33594. doi: 10.1038/srep33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen J., Jiang Z., Xu W., Sun T., Zhuang X., Ding J., Chen X. Spatiotemporally Targeted Nanomedicine Overcomes Hypoxia-Induced Drug Resistance of Tumor Cells after Disrupting Neovasculature. Nano Lett. 2020;20:6191–6198. doi: 10.1021/acs.nanolett.0c02515. [DOI] [PubMed] [Google Scholar]
- 125.Lee A.L.Z., Voo Z.X., Chin W., Ono R.J., Yang C., Gao S., Hedrick J.L., Yang Y.Y. Injectable Coacervate Hydrogel for Delivery of Anticancer Drug-Loaded Nanoparticles in vivo. ACS Appl. Mater. Interfaces. 2018;10:13274–13282. doi: 10.1021/acsami.7b14319. [DOI] [PubMed] [Google Scholar]
- 126.Van Rijt S.H., Bölükbas D.A., Argyo C., Datz S., Lindner M., Eickelberg O., Königshoff M., Bein T., Meiners S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano. 2015;9:2377–2389. doi: 10.1021/nn5070343. [DOI] [PubMed] [Google Scholar]
- 127.Unsoy G., Yalcin S., Khodadust R., Mutlu P., Onguru O., Gunduz U. Chitosan magnetic nanoparticles for pH responsive Bortezomib release in cancer therapy. Biomed. Pharmacother. 2014;68:641–648. doi: 10.1016/j.biopha.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 128.Rong J., Li P., Ge Y., Chen H., Wu J., Zhang R., Lao J., Lou D., Zhang Y. Histone H2A-peptide-hybrided upconversion mesoporous silica nanoparticles for bortezomib/p53 delivery and apoptosis induction. Colloids Surf. B Biointerfaces. 2020;186:110674. doi: 10.1016/j.colsurfb.2019.110674. [DOI] [PubMed] [Google Scholar]
- 129.Sasikala A.R., GhavamiNejad A., Unnithan A.R., Thomas R.G., Moon M., Jeong Y.Y., Park C.H., Kim C.S. A smart magnetic nanoplatform for synergistic anticancer therapy: Manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale. 2015;7:18119–18128. doi: 10.1039/C5NR05844A. [DOI] [PubMed] [Google Scholar]
- 130.Yu Y., Song M., Chen C., Du Y., Li C., Han Y., Yan F., Shi Z., Feng S. Bortezomib-Encapsulated CuS/Carbon Dot Nanocomposites for Enhanced Photothermal Therapy via Stabilization of Polyubiquitinated Substrates in the Proteasomal Degradation Pathway. ACS Nano. 2020;14:10688–10703. doi: 10.1021/acsnano.0c05332. [DOI] [PubMed] [Google Scholar]
- 131.Min J., Moon H., Yang H.J., Shin H.H., Hong S.Y., Kang S. Development of P22 viral capsid nanocomposites as anti-cancer drug, bortezomib (BTZ), delivery nanoplatforms. Macromol. Biosci. 2014;14:557–564. doi: 10.1002/mabi.201300401. [DOI] [PubMed] [Google Scholar]
- 132.Abu Lila A.S., Ishida T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017;40:1–10. doi: 10.1248/bpb.b16-00624. [DOI] [PubMed] [Google Scholar]
- 133.Wang N., Chen M., Wang T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control Release. 2019;303:130–150. doi: 10.1016/j.jconrel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 135.Sun H., Meng F., Cheng R., Deng C., Zhong Z. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin. Drug Deliv. 2013;10:1109–1122. doi: 10.1517/17425247.2013.783009. [DOI] [PubMed] [Google Scholar]
- 136.Kim Y., Park E.J., Na D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018;41:571–582. doi: 10.1007/s12272-018-1008-4. [DOI] [PubMed] [Google Scholar]
- 137.Chauhan A.S. Dendrimers for Drug Delivery. Molecules. 2018;23:938. doi: 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petrova V.A., Elokhovskiy V.Y., Raik S.V., Poshina D.N., Romanov D.P., Skorik Y.A. Alginate Gel Reinforcement with Chitin Nanowhiskers Modulates Rheological Properties and Drug Release Profile. Biomolecules. 2019;9:291. doi: 10.3390/biom9070291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moghassemi S., Hadjizadeh A., Hakamivala A., Omidfar K. Growth Factor-Loaded Nano-niosomal Gel Formulation and Characterization. AAPS PharmSciTech. 2017;18:34–41. doi: 10.1208/s12249-016-0579-y. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y., Bhattarai P., Dai Z., Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019;48:2053–2108. doi: 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anselmo A.C., Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015;17:1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park J.H., Dehaini D., Zhou J., Holay M., Fang R.H., Zhang L. Biomimetic nanoparticle technology for cardiovascular disease detection and treatment. Nanoscale Horiz. 2020;5:25–42. doi: 10.1039/C9NH00291J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao P., Wang M., Chen M., Chen Z., Peng X., Zhou F., Song J., Qu J. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142. doi: 10.1016/j.biomaterials.2020.120142. [DOI] [PubMed] [Google Scholar]
- 145.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P., Gao G., Pan H., Liu L., Ma A., et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.