Abstract
Melittin (MEL) is a 26-amino acid polypeptide with a variety of pharmacological and toxicological effects, which include strong surface activity on cell lipid membranes, hemolytic activity, and potential anti-tumor properties. However, the clinical application of melittin is restricted due to its severe hemolytic activity. Different nanocarrier systems have been developed to achieve stable loading, side effects shielding, and tumor-targeted delivery, such as liposomes, cationic polymers, lipodisks, etc. In addition, MEL can be modified on nano drugs as a non-selective cytolytic peptide to enhance cellular uptake and endosomal/lysosomal escape. In this review, we discuss recent advances in MEL’s nano-delivery systems and MEL-modified nano drug carriers for cancer therapy.
Keywords: melittin, hemolysis, stable loading, nano-delivery system, tumor therapy
1. Introduction
MEL is a major component of honeybee (Apis mellifera) venom with various biological and pharmacological properties [1]. Its strong surface activity on lipid membranes, anti-microbial, anti-inflammatory, and anti-cancer properties has been widely studied and proved. However, the use and application of MEL in clinical experiments is hindered because of its intense toxic side effects. Multiple strategies have been adopted and optimized to develop a safe and stable MEL delivery system. This review focusses on the recent progress of MEL carriers and drug delivery systems with MEL as a functional molecule in cancer therapy. Measures to lower the side effects of MEL are also discussed, along with the improvements and challenges relevant to each strategy.
1.1. Structure of Melittin (MEL) and Its Interactions with Membrane
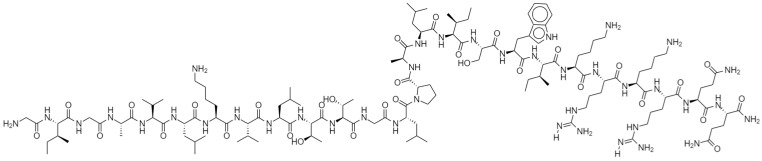
MEL was originally isolated and purified from bee venom (BV), and is the main active ingredient in BV, accounting for approximately 50% of its dry weight [1]. Briefly, MEL has been fractionated and isolated from BV through gel filtration, high-performance liquid chromatography (HPLC), or capillary electrophoresis (CE), and analyzed by ultraviolet assay, reverse-phase HPLC and amino acid analysis [2,3]. This 26-amino peptide (Figure 1) is sequenced as follows: Gly Ile-Gly Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly Leu-Pro-Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-GlnNH2 [4]. Its N-terminal region is hydrophobic, and its C-terminal region is hydrophilic because of the presence of positively charged amino acids [5], which carry a net charge of +6 at physiological pH, leading to its amphiphilic property. Under natural conditions, MEL is in a tetrameric state and dissociates into monomers during ion intensity change [6,7]. The α-helix of MEL is an essential structure that produces its lytic effects. Under different physiological conditions, the α-helix forms a perpendicular or parallel conformation to the membrane surface. When anchored parallel to the membrane surface, the MEL molecule is inactive, and this inserting form prevents other peptides from inserting into the lipid bilayer. In the other case, MEL is inserted perpendicularly into the lipid bilayer and causes pore formation and membrane rupture, leading to the leakage of hemoglobin or other intracellular contents [8,9]. However, the specific mechanism of MEL and cell membrane remains controversial, as one study pointed out that the amphiphilic α-helix structure is formed after MEL insertion to cell membrane [10].
Figure 1.
Chemical structure of MEL.
1.2. Pharmacological Effect of MEL
In addition to its strong surface activity on cell lipid membranes and hemolytic activity [11], MEL has tremendous biological and pharmacological effects, including anti-bacterial [12], anti-virus [13], anti-fungal [14,15] (Table 1), anti-inflammatory [16], and anti-tumor properties [4] (Table 2).
Table 1.
In vitro anti-microbial effects of MEL.
| Type of Microbial | Treatment or Method | Result | Reference | |
|---|---|---|---|---|
| Virus | HIV-1 | MEL | ID50 values was in the range 0.9–1.5 μM | [17] |
| HSV-1 and HSV-2 | MEL | CC50 ranges 1.35–2.05 μM | [18] | |
| SARS-CoV-2 | Sitagliptin-MEL nano-conjugate | IC50 values 8.439 μM | [19] | |
| Bacteria | Pseudomonas aeruginosa | MEL | MIC 10 µg/mL and MBC 20 µg/mL | [20] |
| Methicillin-resistant Staphylococcus aureus | MEL | MIC 6.7 μg/mL and MBC 26 μg/mL. | [21] | |
| Multidrug-resistant Acinetobacter baumannii | MEL | MIC ranges 0.50–32 μg/mL | [22] | |
| E. coli and Staphylococcus aureus | MEL and ionic liquids combination |
E. coli: MIC value was 0.52 μM MEL with 10 μM [Pyr C12]Br− S. aureus: MIC value was 0.62 μM MEL with 20 μM [Pyr C10] Br−. |
[23] | |
| Multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa | Combination of MEL and conventional antibiotics | MDR A. baumannii isolates: MIC for MEL and doripenem were reduced by 61.5 and 51.5 folds, respectively. MDR P. aeruginosa isolates: MIC was reduced by 63.5 and 58 folds for MEL–doripenem, respectively, and by 16 and 11 folds for MEL–ceftazidime, respectively. |
[24] | |
| Fungus | Aspergillus flavus, Aspergillus fumigatus, and Aspergillus parasiticus | MEL | MIC values was 1.25 μM, 1.25 μM, and 2.5 μM for Aspergillus flavus, Aspergillus fumigatus, and Aspergillus parasiticus strains respectively. | [25] |
| Candida albicans | MEL | MIC values for different strains of Candida albicans ranges from 8 μM to 32 μM. | [26] | |
Table 2.
Anti-tumor effects of MEL.
| Tumor Type | Cell Lines | Treatment | Result or Mechanism | Reference |
|---|---|---|---|---|
| Lung cancer | A549 and NCI-H460 cell | MEL | IC50 values were 2 μg/mL, 3 μg/mL, respectively | [45] |
| A549 cell | Antinucleolin aptamer–MEL conjugate | Viability for A549 cells after treatment was 51.2 ± 3.5%, | [46] | |
| Hepatocellular carcinoma | SMMC-7721 cells | MEL | MEL inhibits G0/G1 cell cycle progression by down-regulating MeCP2 through Shh signaling. | [47] |
| HepG2 cells | MEL | HDAC2-mediated PTEN upregulation, Akt inactivation, and inhibition of PI3K/Akt signaling pathways. | [48] | |
| SMMC-7721 and BEL-7402 cells | MEL | MEL sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IκBαkinase-NFκB. | [49] | |
| Breast cancer | MDA-MB-231 cells | MEL | MEL inhibits the EGF-induced MMP-9 expression via blocking the NF-κB and PI3K/Akt/mTOR pathway | [50] |
| SUM159 and SKBR3 | BV or MEL | MEL reduces the level of the PD-L1 immune-checkpoint protein and the immune-suppressive effects of the tumor microenvironment. IC50 values for MEL was 4.24 ng/μL for SUM159 and 3.59 ng/μL for SKBR3. |
[51] | |
| Prostate cancer | LNCaP, DU145, and PC-3 cells | BV or MEL | MEL induces cell apoptosis by activating the caspase pathway via NF-κB inactivation. IC50 for LNCaP cells: MEL 2.9 and BV 14.2 µg/mL, DU145 cells: MEL 1.5 and BV 6.3 µg/mL IC50 for PC-3 cells: MEL 1.8 and BV 6.1 µg/mL, respectively |
[52] |
| Leukemia | CCRF-CEM and K-562 cells | MEL | MEL induces apoptosis via the intrinsic/mitochondrial pathway. | [53] |
The anti-microbial effects of bee venom (BV) and MEL have been reported since the 1940s [27]. With growing concerns regarding drug-resistant bacteria, MEL has become a promising efficacious agent due to its properties of pore formation and bacterial destruction [28]. Evidence has confirmed the anti-bacterial effect of MEL, especially against drug-resistant bacteria that conventional antibiotics fail to inhibit or kill [20,24]. The combination of MEL with other antibiotics is widely evaluated [23,29]. One study evaluated MEL against methicillin-resistant Staphylococcus aureus (MRSA) strains [21] and reported a minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of 6.7 and 26 μg/mL, respectively. Meanwhile, the MIC and MBC values for oxacillin were both 32 μg/mL. MEL in combination with oxacillin also showed synergistic results on MRSA strains.
MEL can also inhibit various viruses [13,30], including human immunodeficiency virus (HIV) [17,31], herpes simplex virus (HSV) [32,33], and respiratory syncytial virus (RSV) [13]. Its mechanism may include suppressing the gene expression of virus and impeding the multiplication process [17,18]. As an antiviral peptide, MEL is also a potential candidate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [34,35]; a survey in beekeepers revealed the potential preventive effect of BV on coronavirus disease 2019 (COVID-19) [36]. A Sitagliptin (SIT)-MEL nano-conjugate was developed [19], and showed better antiviral potential against SARS-CoV-2 isolates than SIT and MEL alone, thus proving MEL to be one of the promising candidates.
In traditional medicine, BV has been used in the treatment of inflammatory-related illnesses [37]. MEL binds non-competitively with phospholipase A2 (PLA2) to inhibit its enzymatic activity, and thus can be used for the treatment of inflammation caused by the production or enhanced activity of secreted PLA2 [38]. MEL also has inhibitory effects on sodium nitroprusside, IκB kinase (IKK) activity, nitric oxide (NO), inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), and other inflammatory mediators due to its high binding affinity with IKKs [39], which suppresses IκB degradation and thereby blocks the nuclear factor kappa-light-chain-enhancer of the activated B cells (NF-κB) signaling pathway. MEL can inhibit the signaling pathways of toll-like receptor 2 (TLR2), TLR4, CD14, NF-κB essential modulator (NEMO), and platelet-derived growth factor receptor beta (PDGFRβ) [40,41,42,43], consequently reducing the activation of p38 mitogen-activated protein kinases, extracellular signal-regulated protein kinase (ERK1/2), protein kinase B (Akt), phospholipase C, gamma 1 (PLCγ1) and the transport of NF-κB into the nucleus. This inhibitory effect can also reduce inflammation of the skin, aorta, joints, liver, and neuronal tissues [16,44].
1.3. Anti-Tumor Effects of MEL
Given that MEL attacks lipid membranes and leads to substantial cytotoxicity, it has been widely studied in anti-tumor treatments (Table 2).
BV suppresses COX-2 mRNA expression and PGE 2 synthesis; hence, MEL may also exert anti-tumor effects [54]. MEL inhibits proliferation of the cancer cells via induction of apoptosis through multiple investigated mechanisms. One possible mechanism is that MEL causes changes in the permeability of cell membranes, which leads to the elevation of intracellular Ca2+, an important regulator in the apoptosis process, and activation of PLA2 [55], resulting in cell death. MEL presents a significant anti-tumor effect through the NF-κB pathway, which is involved in multiple physiological processes including tumor [56]. Its other mechanism of apoptosis includes decreasing methyl-CpG binding protein 2 [47] and PI3K/Akt/mTOR signaling pathway [48,50].
MEL also inhibits the invasion and metastasis of cancer cells. MEL prevents hepatocellular carcinoma cell metastasis via inhibition of ras-related C3 botulinum toxin substrate 1 (Rac1) [57], which participates in the c-Jun N-terminal kinase (JNK) and JNK-dependent cell motility processes and induces metastasis. In addition, MEL selectively inhibits expression of matrix metalloproteinase-9 (MMP-9), which plays an important role in the migration of cancer cells [50,58,59] via down-regulating activator protein-1 (AP-1) and NF-κB expression.
1.4. Obstacles to the Applications of MEL
Despite its multiple pharmacological potentials, the clinical applications of MEL are limited due to its strong surface activity and cytotoxicity. MEL results in 50% hemolysis of human red blood cells at 2 μM concentration and 100% at 7 μM [60]. Studies examining the antimicrobial activity of MEL in vivo [61] have found an increased mortality rate in mice directly injected with MEL. LD50 value of intraperitoneal MEL to mice is around 5 mg/kg, and drops to about 3 mg/kg via intravenous injection [62,63,64]. Preclinical and clinical research on BV therapy has indicated that the main adverse reactions of MEL include allergic reactions and pain at the administration site [65,66], which limits BV’s application in acupuncture therapies. Further applications of MEL are also restricted due to its degradability [67], low bioavailability [68], and non-specific lytic effects.
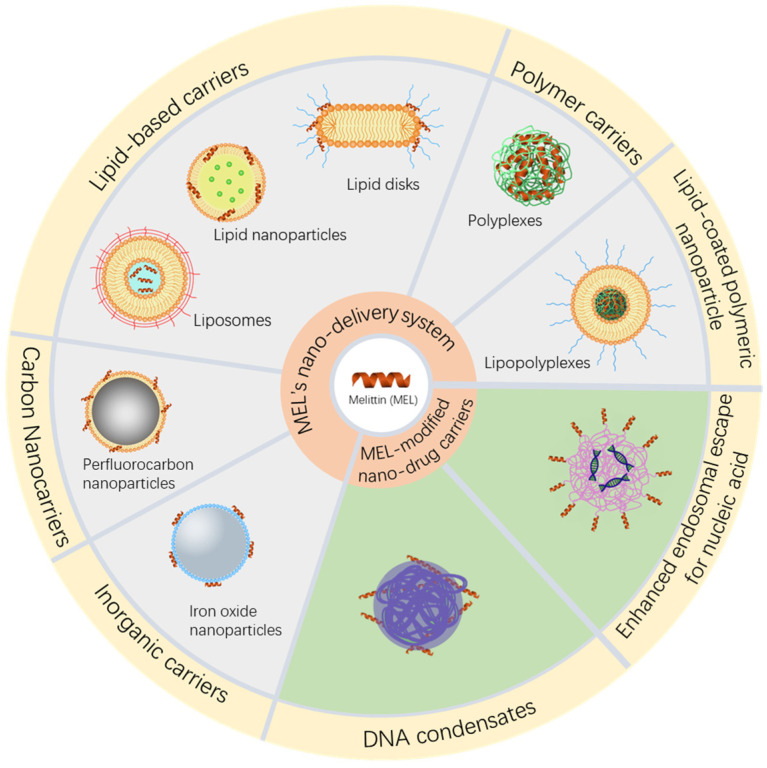
Multiple strategies have been applied to stably and safely load MEL to obtain effective anti-tumor as well as other therapeutic effects. The designated purposes of MEL delivery systems are approximately the same. Other than increasing the delivery efficiency of MEL, delivery systems conceal MEL to prevent it from interacting with cell membranes as well as to mask its positive charge so as not to bind to other proteins in vivo. Additionally, delivery systems enable targeting delivery to the lesion areas via the enhanced permeability and retention (EPR) effect or stimulus responsive designs. With surface activity and affinity for lipid membranes, MEL is also applied in drug delivery system to enhance cell uptake and endosomal escape (Figure 2).
Figure 2.
Strategies of MEL nano-delivery systems and MEL-modified nucleic acid nanocarriers for cancer therapy.
2. Delivery Vehicles for Melittin
The following two main strategies are adopted to overcome the cytotoxicity and hemolysis effects of MEL: incorporating nanoparticles that can safely deliver a substantial amount of MEL through the intravenous route, and modifying MEL to reduce its toxicity.
2.1. Modified MEL and Conjugates
Changing the amino acid sequence of MEL or linking it with polypeptides or other molecules provides it with certain properties, such as in vivo stability or targeting. The hemolysis of MEL decreases after phosphorylation [69] or modification of amino acid sequences to obtain MEL analogs [70,71] has been proved. The derived MEL peptides have been designed and developed to exert more desirable properties, such as lower hemolysis effects [72,73,74], enhanced therapeutic properties [75,76] and even controllable activation providing targeting ability to tumor tissue [70,77]. Substituting alanine for leucine drastically reduces the hemolytic activity on human red blood cells, which is only about 1–2% of the hemolytic activity of MEL, while the antibacterial activity remains equivalent to MEL [73]. Meanwhile, modifications on the C-terminal region (R22A, K23A, R24Q) reduce the net charge of the protein and increase its pore-forming ability (20-fold more potent than MEL) [78].
In one study of MEL conjugate development, 2,3-dimethylmaleic anhydride (DMMA) was used to modify the active amino groups in MEL to obtain ultra pH sensitivity [79]. DMMA concealed the original positive charge of MEL and significantly reduced the hemolysis and clearance of reticulo-endothelial system. Other modification methods include immunoconjugates [46,80] and hybrid peptides [81]. MEL covalently links to the anti-nucleolar protein aptamer AS1411 and forms a conjugate that can achieve targeted delivery to multiple cancer cells. Compared with free MEL, this modified MEL has significantly reduced hemolytic activity and greater cytotoxicity in A549 cells [46]. The bacteria-killing efficacy of MEL is improved after being decorated on graphene (Gra) or graphene oxide (GO) nanosheets, which increase its ability to permeate the cell membrane and cause rapid bacterial leakage [82].
2.2. Nano Delivery Vehicles
Nano drug delivery systems have been widely applied in MEL delivery. Attempts such as inorganic carriers (including quantum dots, Fe3O4 nanoparticles, and perfluorocarbon nanoparticles), polymer carriers (including PLGA nanoparticles and β-cyclodextrin nanoparticles), and lipid carriers (including lipid disks, lipid nanoparticles, MEL–lipid conjugate nanoparticles and liposomes) greatly reduce the toxicity of MEL and provide the possibility of targeting to the intended sites (Table 3).
Table 3.
Summary of MEL-loading nano-delivery systems and applications.
| Type | Loading Strategy | Size | Applications | Reference |
|---|---|---|---|---|
| Quantum dots | MEL was modified to CdSe/ZnS core quantum dots | 5–10 nm | Quantum dots were used to study the interaction between protein and membrane, and had potential to deliver MEL in vivo. | [83] |
| Iron oxide nanoparticles | MEL and doxorubicin (DOX) were co-loaded to citric acid-functionalized Fe3O4 magnetic nanoparticles (CA-MNPs) | 20 nm | The release of both MEL and DOX was strongly enhanced at pH 4.5 and the nanoparticles were potentially applied in magnetically targeted cancer therapy. | [84] |
| Perfluorocarbon (PFC) nanoparticles | MEL was added to the PFC nanoparticles | ~290 nm | PFC nanoparticles retained their structural integrity after the addition and contribute to the stability and slow dissociation of MEL from the stabilizing monolayer. | [85] |
| MEL was mixed and incubated with PFC nanoparticles | 227 nm | The growth of the tumors was inhibited by 24.68% in MDA-MB-435 human breast cancer. | [86] | |
| MEL derivative peptide was incubated with PFC nanoparticles composed of egg phosphatidylcholineand dipalmitoylphosphatidylglycerol | ~280 nm | This MEL derivative is activated by matrix metalloproteinase-9 (MMP-9), a protease overexpressed in many tumor cells. In addition, treatment of PFC nanoparticles resulted in ~54% reduction in melanoma tumor size in vivo. | [70] | |
| Poly (d,l-lactic acid-coglycolic acid) (PLGA) nanoparticles | BV-loaded PLGA/PVA nanoparticles | 180 nm | PLGA nanoparticles reduced side effects by slowing down BV release, and prolonged suppression of nociceptive behavior in rats with formalin-induced pain. | [87] |
| MEL was modified with sodium dodecyl sulfate and then formulated into PLGA nanoparticles | ~130 nm | MEL was loaded with a high encapsulation efficiency in the nanoparticles and the concentration of half the cell growth (GI50) in breast cancer MCF-7 cells was 4.42 μg/mL in vitro. | [88] | |
| Tetrameric MEL binds avidly to PLGA-NPs | 110 nm | Biodegradable tetrameric MEL is encapsulated in nanoparticles at efficiency of 97% and retains lytic activity. | [89] | |
| β-cyclodextrin(β-CDP) nanoparticles | 5 different functional monomer adamantane derivatives (Ad-Ds) incubated with β-CDPs respectively, and then mixed with MEL | 30–200 nm | The percentage of hemolytic toxicity neutralization reached 100% at the concentration of 100 μM. The cytotoxicity of 30 μg/mL MEL with 2 mmol/mL nanoparticle decreased by sixfold compared with that of free MEL in CCRF-CEM cells. | [90] |
| Lipodisks | MEL incubated with PEG-stabilized lipid disks which composed of POPC/cholesterol/ceramide-PEG5000 | 20–100 nm | PEGylated lipodisks allowed stable loading of MEL, and retained anti-bacterial activity of MEL in E. coli, but extended the actions by slowing down releasing rate. | [91] |
| Lipid disks was modified by c(RGDyK)-PEG3400-DSPE | 50 nm | The disks induced no hemoglobin release at maximum tested concentration (100 μg/mL) and presented significate targeting and in vivo anti-tumor effect towards U87 glioma cells. | [92] | |
| MEL loaded lipodisks contained EGF-conjugated PEG-lipids. | ~20 nm | The EGF-targeted lipodisks binded specifically to A-431 tumor cells, and resulted in a improved cell-killing effect, as cell viability decreased 20% compared to free MEL. | [93] | |
| MEL and paclitaxel were co-loaded within 9G-A7R modified lipodisks. | ~50 nm | Co-loading prevented leakage of MEL from the disks and improved cytotoxicity on U87 cells in vitro and anti-tumor effect in intracranial glioma models. The synergistic effect of MEL and paclitaxel was proved as combination index values was 0.45. | [94] | |
| Lipid nanoparticles | MEL was linked to an amphipathic peptide then loaded in ultrasmall lipid nanoparticles | 14 nm | The ultrasmall lipid nanoparticles significantly reduced the hemolysis of MEL and showed obvious anti-tumor effect in malignant melanoma B16F10 cells, with IC50 values being 11.26 μM. | [81] |
| MEL-lipid conjugate nanoparticles | MEL-phospholipid scaffold | 10–20 nm | The nanoparticles induced tumor cell apoptosis, releasing whole-tumor antigens in situ, and targeting to lymph nodes. | [95] |
| Liposomes | MEL was loaded in PEGylated anti-HER2 immunoliposomes modified by the complete antibody (trastuzumab) | 139 nm | The immunoliposomes decreased cancer cells viability in a dose–response manner and in correlation to the level of HER2 expression in human breast cancer cells. | [96] |
| MEL loaded liposomes was modified by antibodies against the fish viral hemorrhagic septicemia rhabdovirus (VHSV) glycoprotein G (gpG) | ~140 nm | The in vitro antiviral studies showed that the liposomes inhibited the infectivity by 95.2% through inactivating VHSV. | [97] | |
| MEL was modified with 2% poloxamer 188 then loaded in nano-liposomes. | NA | Multiple hepatic carcinoma cell lines (Bel-7402, BMMC-7721, HepG2, LM-3, and Hepa 1–6 cells) were sensitive to the liposomes, and the IC50 value was close to free MEL, indicating efficient anti-tumor effect. | [98] | |
| Hyaluronic acid (HA) modified MEL-loading liposomes | 133 nm | HA enhanced the sustained-release effect of MEL from the liposomes and provide targeting ability via specific binding with CD44, which is highly expressed on the surface of melanoma B16F10 cells. | [99] | |
| Lipid-coated polymeric Nanoparticles | MEL and poly γ-glutamic acid (γ-PGA) formed nanoparticles which then coated by cationic liposomes modified by PEG and DSPE-PEG-RGD | ~100 nm | The hemolytic activity and nonspecific cytotoxicity of MEL were remarkably reduced by the lipid-coated polymeric nanoparticles and the RGD-modified RGD modified nanoparticles effectively induced apoptosis in A549 cells. | [100] |
| Stimulus-responsive delivery systems | MEL was grafted to nanodiamonds coated with PEGylated PGA. | 220 nm | The nanoparticles were pH sensitive and steady able to released MEL in an acidic environment. Toxicity to breast cancer MFC-7 cells was enhanced than free MEL in a concentration-dependent manner. | [101] |
| D-MEL was conjugated with PEG which is polymerized with DIPAMA and PDSEMA, to form micelles. | 33 nm | The pH sensitive micellar formulations unsheathes MEL only at endosomal pH, remarkably reducing hemolytic effects of MEL, and IC50 for the micelles in 3T3, A549, CT26 cancer cells were 8.5 μM, 6.9 μM, 11.6 μM, respectively. | [102] | |
| MEL was loaded in negatively charged nanospheres consisting of NIR-absorbing molecule cypate and HA. | ∼50 nm | The nanospheres responsive to both pH and near-infrared (NIR) laser irradiation changes into net-like nanofibers and small nanospheres (~25 nm) when stimulated and induce cancer cell death, inhibit the metastatic dissemination of tumor cells, and facilitated deep tumor penetration o | [103] | |
| Serum albumin (SA)-coated boehmite scaffold was loaded with photosensitizer chlorin e6 (Ce6) and MEL. | 184 nm | The nanocarrier exerted high encapsulation efficiency of MEL and low hemocompatibility. In vivo phototreatment of the scaffold eliminated 4T1 cells remarkably in subcutaneous breast tumor models. | [104] | |
| MEL loaded in redox-sensitive nanocomplexes | 357 nm | The nanocomplexes decreased hemolysis of MEL and released MEL responding to high redox potential environment, and showed an enhanced cytotoxicity on both HCT 116 colon cancer cells and MCF-7 breast cancer cells. | [105] |
2.2.1. Inorganic Carriers
Quantum dots are small semiconductor particles (size of a few nanometers) with unique optical and electronic properties as well as potential anti-tumor and photosensitive properties [106,107]. Dang et al. [83] modified CdSe/ZnS core/shell quantum dots by using the high-affinity interaction between phosphorylcholine and MEL. A fluorescence resonance energy transfer (FRET) system was formed between the Cy3b label on MEL and the quantum dots. This system was used to study the interaction between protein and membrane, and has potential applications in cancer therapy. The tumor-targeting and anti-tumor effects of quantum dots were demonstrated in lung cancer [108,109] and pancreatic cancer cells [110]. However, it is only in primary stage and cannot stably carry MEL for a long time to avoid MEL hemolysis.
Studies have proved the feasibility of loading MEL on inorganic metal nanoparticles such as iron oxide [111,112] and gold nanoparticles [113,114]. Hematyar et al. [84] developed a magnetic-responsive co-delivery system for effective cancer therapy. Doxorubicin (DOX) and MEL were loaded onto the surface of citric acid-functionalized Fe3O4 magnetic nanoparticles (CA-MNPs) through electrostatic interaction. CA-MNPs possess superparamagnetic nature and have potential to be directed and localized to tumor targets by external magnetic fields. The advantage of metal nanoparticles as MEL vectors is that several metal materials possess stimulus-responsive ability, which enhances targeting transportation of MEL, while some metal-based (gold or silver) nanoparticles have been proven to exert anti-cancer effects [115], and may obtain a better therapeutic effect in combination with MEL.
2.2.2. Carbon Nanocarriers
Perfluorocarbon (PFC) nanoparticles are composed of a hydrophobic PFC core surrounded by a phospholipid monolayer where MEL can be stably inserted into the phospholipid monolayer without destroying the nanoparticle structure [70,85,116]. The toxicity of MEL to sperm and vaginal epithelial cells is reduced fivefold when delivered in PFC nanoparticles; to some extent, this characteristic guarantees the safety of MEL as an anti-HIV agent [116]. PFC nanoparticles extends the half-life of MEL in plasma from 24 min to more than 300 min and improves safety by promoting the clearance of circulating peptides through the reticuloendothelial system [86]. However, the PFC-containing MEL nanoformulation has a large particle size (250~300 nm), which may not be conducive to diffusion and cellular uptake on sites [117].
2.2.3. Polymer Carriers
Poly (d, l-lactic acid-co-glycolic acid) (PLGA) is a common biodegradable polyester that can carry MEL in nanoparticles [87,88,89,118]. Yang et al. [88] paired MEL with the anionic agent sodium lauryl sulfate. The formed complex was highly soluble in organic solvents and formulated into MEL-PLGA nanoparticles, resulting in improved drug loading efficiency (~90%) and drug content (6–7%). The in vivo experimental study of Jeong et al. [87] on PLGA-coated BV preparations for pain inhibition showed that PLGA-coated MEL induced by acupuncture therapy significantly prolonged the time of pain suppression in rats and decreased the side effects by reducing the rate of release from nanoparticles. The MEL-loaded PLGA microspheres produced in high encapsulation can achieve a controlled release rate correlated with polymer degradation rate [119]. However, MEL-polymer nanoparticles formed based on charge interaction face obstacles in the stable loading of MEL under relatively complicated physiological conditions, and remain unsuitable for systemic circulation.
β-Cyclodextrin (β-CDP), an easily available polymer material, has a ring-shaped truncated cone topology with a hydrophobic cavity to non-covalently contain diverse guest molecules [120]. Xu et al. [90] established a library of self-assembled MEL nanoparticles based on β-CDPs and functional monomer adamantane derivatives (Ad-Ds). The cytotoxicity of 30 μg/mL MEL with 2 mmol/mL nanoparticle decreased by sixfold compared with that of free MEL.
2.2.4. Lipid-Based Carriers
Lipodisk (or lipid disk) is a flat circular lipid bilayer structure where PEGylated lipid forms the highly curved edges of the lipodisk. Owing to the large curvature of the edge and hydrophobic interactions, MEL has high affinity with the edge of the lipodisk that allows it to bind to the disk preferentially; the interaction between MEL and PEG chains is negligible [91,121]. Gao et al. [92] established a cyclic RGD peptide (c (RGDyK))-modified lipid disk as a MEL carrier. In vivo experiments suggested that the lipodisk loaded with MEL significantly reduced the hemolysis effect and effectively inhibited tumor growth in mice. c (RGDyK) modification of the lipodisk increases its distribution in solid tumors and its anti-cancer efficiency. Ahlgren et al. [93] reported that EGF-targeted lipodisks had high MEL-loading efficiency and improved the specificity and cytotoxicity of MEL on tumor cells. Although the co-loading of MEL and paclitaxel in lipodisks did not induce hemolysis, the lipodisks loaded with MEL alone exhibited hemolytic toxicity at high concentrations [94], which may imply that the safety of lipid disks loaded with MEL needs to be further improved.
Modified MEL can be stably loaded into lipid nanoparticles (LNP). The hemolytic properties of MEL were concealed when linked to an amphipathic peptide, and then MEL interacted with phospholipids, self-assemble into lipid nanoparticles with a size of 15 nm [81]. With high MEL encapsulation rate (>80%) and neutral zeta potential, this LNP has a significant tumor inhibitory effect on melanoma cancer models, with an inhibitory rate of 82.8%. Other attempts to load MEL on nanoparticles include the use of a peptide–phospholipid scaffold to form an ultrasmall (10–20 nm) MEL-lipid nanoparticle (α-MEL-NP) [95,122] that targets lymph nodes and elicit an anti-tumor effect and immune response as a nanovaccine. α-MEL-NPs promote the release of whole-tumor antigens in situ. On the other hand, the size of α-MEL-NPs is optimal so that they can efficiently drain into lymphatic capillaries and lymph node, activating resident antigen-presenting cells [95]. As researches have proved a significant increase of affinity of MEL with positively curved lipid surfaces [123,124], both lipodisks and ultrasmall lipid nanoparticles increase the drug loading efficiency and loading stability, and has improved biocompatibility.
Liposomes are closed vesicles with a bilayer structure formed when phospholipid or phospholipid-like substances are dispersed in the aqueous phase [125,126]. MEL is amphiphilic, has a positive charge, and can be loaded into the aqueous phase of liposomes [97]. However, necessary measures must be taken to overcome the interaction between MEL and lipid layer to protect the liposome membrane from this cell-penetrating peptide. The nonionic block linear copolymer poloxamer 188, is applied in the preparation of MEL liposomes to prevent leakage [98,127], resulting in decreased hemolysis and MEL-induced vascular irritation. Mao et al. [98] attached poloxamer 188 to MEL in order to encapsulate MEL and form liposomes. In vitro experiments revealed that this material has a significant inhibitory effect on the survival of hepatocellular carcinoma (HCC) cells and suppresses the growth of subcutaneous and orthotopic liver cancer transplantation tumors in vivo. A recent attempt used a liposome modified with dioleoyl-phosphoethanolamine (DOPE)-coupled HA (HA-DOPE) to deliver MEL; the HA layer on the surface of liposome entraps MEL and prevents it from leakage [99]. However, the outer-surface modification of liposomes may exert a limited effect in preventing MEL from affecting the lipid bilayer structure of liposomes. Further improvement is needed for long-term circulation in vivo.
2.2.5. Lipid-Coated Polymeric Nanoparticles
With the advantages of both the liposomes and polymeric nanoparticles, lipid-coated nanosized drug delivery systems have properties such as high drug loading capacity, high stability and biocompatibility, and prolonged circulation time in vivo [128], which make them promising for targeting delivery of MEL. Ye et al. [100] prepared a nanoparticle inner core with negative charges containing MEL and poly γ-glutamic acid (γ-PGA), an anionic polymer. The core is then coated by the cationic lipid to form liposomes, which effectively prevent the leakage of MEL. The outer shell is composed of PEG and PEG-targeting molecule (DSPE-PEG-RGD), providing stability in long-term circulation, capacity of selective binding with target tumor cells and cytolytic activity via apoptosis induction.
2.2.6. Stimulus-Responsive Delivery Systems
Multiple stimulus-responsive nano delivery systems have been used to deliver MEL to attenuate the cytotoxicity of MEL during systemic circulation and to achieve its targeted delivery, including pH-responsive [101,102,103], magnetic-responsive [84], photosensitive [103,104] and redox-sensitive [105] delivery systems. The stimulus-responsive carrier maintains a stable state during circulation in the body and is stimulated and changes conformation or structure when the nanocarrier reaches the target site and releases MEL to a therapeutic concentration [84,101,104]. Similar to pH-sensitive MEL, the pH-responsive polymer is converted in a lower pH environment and releases active MEL. Lai et al. [101] developed a nanoparticle consisting of nanodiamonds and PEGylated polyglutamic acid, which exhibited enhanced cytotoxicity towards MCF-7 cells. A near-infrared (NIR) laser irradiation responsive nanosystem can be assembled using MEL, NIR-absorbing molecule cypate, and HA [103], of which size and morphology transform successively under changes in pH and NIR laser irradiation.
3. Nano Drug Delivery System with Melittin as a Functional Molecule
3.1. Melittin Enables Efficient Vesicular Escape
The entry of exogenously applied nuclear acid into the cytoplasm and its subsequent transport into the nucleus is a major cellular barrier for nonviral gene delivery vectors. A variety of strategies have been applied with the purposes of targeted delivery to the target sites and improved cellular uptake and endosome release, including polymer-or lipid-based nanoparticles [129] and liposomes [130] etc. As a cell-penetrating peptide, MEL can improve the cellular uptake of therapeutic compounds and the endosomal escape of nanoparticles [131,132]. With its strong surface activity, MEL can work as a penetrating peptide to promote escape from endosomes, thus increasing the bioavailability of nanoparticles [133]. Such features makes MEL popular as an oligonucleotide transfection agent [5], as the intracellular delivery of gene has always been a technical obstacle to overcome urgently [134].
The endosomal escape effect of conjugate of MEL with commonly used polymers for nucleic acid delivery has been widely verified. Transfection experiments on a variety of cell lines have shown that the transfection efficiency of MEL-PEI-luciferase DNA conjugates is up to 700-fold higher than that of controlled group (PEI-DNA conjugates) [133]. Ogris et al. [133] developed a conjugate that covalently attached MEL to poly (ethylenimine) (PEI) condensed DNA into small, discrete particles (<100 nm in diameter). Compared with PEI, the transfection activity of this conjugate was strongly increased within a broad range of cell lines and types. The connection between MEL and polymer has also been explored. PEI and MEL binding experiment [135] showed that the conjugate connected to the N-terminal of MEL (N-mel-PEI) has a low toxicity and a high transfection efficiency, whereas the PEI bound to the C-terminal of MEL (C-mel-PEI) shows a high cytotoxicity. A possible explanation is that the hydrophobic N-terminal of C-mel-PEI is easily inserted into the cell membrane and facilitates water flow into the bilayer, leading to membrane disturbance and instability. By contrast, N-mel-PEI tends to form a parallel conformation. This speculation is not universal; a stearyl attached to the C-terminus of MEL (stearyl-rMel) shows great efficiency, and the stearyl-rMel/p53 plasmid complex exhibits high p53 expression and anti-tumor activity [136].
3.2. Enhanced Drug Delivery of MEL as an Adjuvant
MEL has been widely studied and combined with various polymers to develop new, efficient, and safe non-viral gene drug delivery systems. In addition, studies reveal that MEL is able to act as attractant for certain receptors, such as PLA2 [38,137], for nano drug modification.
The problem with the use of MEL as an endosomal penetrating peptide is its non-specificity to the lipid membrane; the nanocarrier directly inserted with MEL will present a major safety risk. Therefore, MEL must be masked until it reaches the target sites. Oude Blenke et al. [138] explored the coupling of MEL to liposomes via functionalized PEG-lipids following aldehyde–hydrazide chemistry. At endosomal pH, the acid-labile hydrazone bond hydrolyzes and releases the peptide. Similarly, MEL can be applied in the conjugates of siRNA with pH-sensitive polymers by masking it with pH-labile dimethylmaleic anhydride (DMMAn) [139,140,141], or concealing it in micellar structure consisting of pH-sensitive poly (2-diisopropylaminoethyl methacrylate) (p (DIPAMA)) [142,143]. Another alternative strategy is to modify the peptide to reduce cytotoxicity. Changes in hemolytic activity can be achieved by MEL derivative peptides including pH-sensitive peptides [144,145], and light-sensitive peptides [146]. Chen et al. [147] transformed MEL into a sulfhydryl polymerized peptide and incubated with plasmid DNA to obtain peptide DNA condensates. The hemolytic potency of poly MEL was efficiently covered when combined to DNA. Modified MEL peptide p5RHH (sequence VLTTGLPALISWIKRKRQQ) transfects siRNA with an IC50 as low as 25 nM and possesses minimal cytotoxicity at the highest tested dose (10 μM) [71,148]. p5RHH also works as a linker inserted into nanoparticles and liposomes, and incorporates targeting ligands, imaging agents, and therapeutic drugs into particles without affecting their integrity [149].
The immune effects of MEL make it an effective adjuvant for vaccines. Owing to its ability to increase IFN-γ and IL-1β and decrease IL-10, MEL was selected as suitable adjuvant candidate for Helicobacter pylori intranasal vaccine development [150]. After deletion of the last four residues of the C-terminal region, with the aim of lowering the interactions with the cell membranes, the derived MEL peptide was linked to epitopes as an adjuvant. As the vaccine is administrated through the nasal mucosa, the side effect of MEL is largely eliminated. Another study showed an enhanced absorption effect of MEL as a mucosal adjuvant [151]. Compared with the control group and those receiving intranasal administration of free antigen, BALB/c mice administered with 4 μg of MEL and tetanus toxoid/diphtheria toxoid remarkably enhanced the antibody titers and prolonged the immune responses.
4. Conclusions and Prospect
MEL is a polypeptide with various pharmacological properties and has great potential in anti-inflammatory, anti-tumor, and anti-viral applications. Although pure MEL has toxicity and hemolytic properties, the use of protection or packaging greatly reduces its systemic toxicity. New strategies based on MEL can deliver drugs safely and effectively in the body. The modified peptide transduction domain and MEL-based technology are specifically designed to enhance endosomal escape and enable new nanoscale strategies to complete the arduous task of in vivo drug delivery. Upcoming designs such as stimulus responsiveness and masking are adopted in the transportation and functionalization of MEL.
However, challenges with respect to MEL-based nanodrug delivery systems still exist. Current methods cannot completely avoid the side effects of MEL, and the transfection efficiency of gene delivery systems still has room for improvement. Further understanding the mechanism of interaction of MEL with membrane and endosomal escape is crucial for the development of its delivery system.
Despite the limitations and challenges of MEL delivery systems, the development of various novel delivery strategies and clinical trials for various diseases is being undertaken to explore an ideal delivery system for MEL. The main directions of advancement are to design and synthesize modified MEL with weakened toxicity while maintaining its pharmacological effects, and to develop more stable and biocompatible delivery systems with improved targeting and controlled releasing ability. Other measures focused on MEL treatment include construction of tumor targeted gene vector containing MEL coding sequence that induced tumor cell-specific MEL expression.
Author Contributions
A.W. performed the literature research, wrote the manuscript, and prepared the tables and figures. Y.Z., W.Z., L.Y. and Y.Y. helped to perform revisions. J.P. designed, supervised and reviewed the complete manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos: 81571787 and 81690262).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Habermann E. Bee and Wasp Venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 2.Maulet Y., Brodbeck U., Fulpius B.W. Purification from bee venom of melittin devoid of phospholipase A2 contamination. Anal. Biochem. 1982;127:61–67. doi: 10.1016/0003-2697(82)90144-0. [DOI] [PubMed] [Google Scholar]
- 3.Pacáková V., Štulík K., Thi Hau P., Jelínek I., Vinš I., Sýkora D. Comparison of high-performance liquid chromatography and capillary electrophoresis for the determination of some bee venom components. J. Chromatogr. A. 1995;700:187–193. doi: 10.1016/0021-9673(94)01170-J. [DOI] [Google Scholar]
- 4.Chen J., Guan S.M., Sun W., Fu H. Melittin, the Major Pain-Producing Substance of Bee Venom. Neurosci. Bull. 2016;32:265–272. doi: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghuraman H., Chattopadhyay A. Melittin: A Membrane-active Peptide with Diverse Functions. Biosci. Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 6.Hagihara Y., Kataoka M., Aimoto S., Goto Y. Charge repulsion in the conformational stability of melittin. Biochemistry. 1992;31:11908–11914. doi: 10.1021/bi00162a033. [DOI] [PubMed] [Google Scholar]
- 7.Bello J., Bello H.R., Granados E. Conformation and aggregation of melittin: Dependence on pH and concentration. Biochemistry. 1982;21:461–465. doi: 10.1021/bi00532a007. [DOI] [PubMed] [Google Scholar]
- 8.DeGrado W.F., Musso G.F., Lieber M., Kaiser E.T., Kézdy F.J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys. J. 1982;37:329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorilă B., Necula G., Radu M., Bacalum M. Melittin Induces Local Order Changes in Artificial and Biological Membranes as Revealed by Spectral Analysis of Laurdan Fluorescence. Toxins. 2020;12:705. doi: 10.3390/toxins12110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantinescu I., Lafleur M. Influence of the lipid composition on the kinetics of concerted insertion and folding of melittin in bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2004;1667:26–37. doi: 10.1016/j.bbamem.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Sessa G., Freer J.H., Colacicco G., Weissmann G. Interaction of a Lytic Polypeptide, Melittin, with Lipid Membrane Systems. J. Biol. Chem. 1969;244:3575–3582. doi: 10.1016/S0021-9258(18)83408-1. [DOI] [PubMed] [Google Scholar]
- 12.Picoli T., Peter C.M., Zani J.L., Waller S.B., Lopes M.G., Boesche K.N., Vargas G.D.Á., Hübner S.d.O., Fischer G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017;112:57–62. doi: 10.1016/j.micpath.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Uddin M.B., Lee B.H., Nikapitiya C., Kim J.H., Kim T.H., Lee H.C., Kim C.G., Lee J.S., Kim C.J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016;54:853–866. doi: 10.1007/s12275-016-6376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W., Lee D.G. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim. Biophys. Acta. 2015;1848:673–679. doi: 10.1016/j.bbamem.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.G., Park J.H., Shin S.Y., Lee S.G., Lee M.K., Kim K.L., Hahm K.S. Design of novel analogue peptides with potent fungicidal but low hemolytic activity based on the cecropin A-melittin hybrid structure. Biochem Mol. Biol. Int. 1997;43:489–498. doi: 10.1080/15216549700204281. [DOI] [PubMed] [Google Scholar]
- 16.Lee G., Bae H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules. 2016;21:616. doi: 10.3390/molecules21050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wachinger M., Kleinschmidt A., Winder D., von Pechmann N., Ludvigsen A., Neumann M., Holle R., Salmons B., Erfle V., Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 18.Albiol Matanic V.C., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rabia M.W., Alhakamy N.A., Ahmed O.A.A., Eljaaly K., Aloafi A.L., Mostafa A., Asfour H.Z., Aldarmahi A.A., Darwish K.M., Ibrahim T.S., et al. Repurposing of Sitagliptin- Melittin Optimized Nanoformula against SARS-CoV-2: Antiviral Screening and Molecular Docking Studies. Pharmaceutics. 2021;13:307. doi: 10.3390/pharmaceutics13030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shams Khozani R., Shahbazzadeh D., Harzandi N., Feizabadi M.M., Pooshang Bagheri K. Kinetics Study of Antimicrobial Peptide, Melittin, in Simultaneous Biofilm Degradation and Eradication of Potent Biofilm Producing MDR Pseudomonas aeruginosa Isolates. Int. J. Pept. Res. Ther. 2019;25:329–338. doi: 10.1007/s10989-018-9675-z. [DOI] [Google Scholar]
- 21.Marques Pereira A.F., Albano M., Bérgamo Alves F.C., Murbach Teles Andrade B.F., Furlanetto A., Mores Rall V.L., Delazari dos Santos L., de Oliveira Orsi R., Fernandes Júnior A. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020;141:104011. doi: 10.1016/j.micpath.2020.104011. [DOI] [PubMed] [Google Scholar]
- 22.Giacometti A., Cirioni O., Kamysz W., D’Amato G., Silvestri C., Del Prete M.S., Łukasiak J., Scalise G. Comparative activities of cecropin A, melittin, and cecropin A–melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides. 2003;24:1315–1318. doi: 10.1016/j.peptides.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Saraswat J., Aldahmash B., AlOmar S.Y., Imtiyaz K., Rizvi M.M.A., Patel R. Synergistic antimicrobial activity of N-methyl substituted pyrrolidinium–based ionic liquids and melittin against Gram-positive and Gram-negative bacteria. Appl. Microbiol. Biotechnol. 2020;104:10465–10479. doi: 10.1007/s00253-020-10989-y. [DOI] [PubMed] [Google Scholar]
- 24.Akbari R., Hakemi-Vala M., Pashaie F., Bevalian P., Hashemi A., Pooshang Bagheri K. Highly Synergistic Effects of Melittin with Conventional Antibiotics Against Multidrug-Resistant Isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2019;25:193–202. doi: 10.1089/mdr.2018.0016. [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Hwang J.-S., Hwang I.-s., Cho J., Lee E., Kim Y., Lee D.G. Coprisin-induced antifungal effects in Candida albicans correlate with apoptotic mechanisms. Free. Radic. Biol. Med. 2012;52:2302–2311. doi: 10.1016/j.freeradbiomed.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Jia F., Wang J., Peng J., Zhao P., Kong Z., Wang K., Yan W., Wang R. The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids. 2018;50:229–239. doi: 10.1007/s00726-017-2507-1. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Lange W. The germicidal effect of bee venom. Muench Med. Wochenschr. 1941;83:935. [Google Scholar]
- 28.Memariani H., Memariani M., Shahidi-Dadras M., Nasiri S., Akhavan M.M., Moravvej H. Melittin: From honeybees to superbugs. Appl. Microbiol. Biotechnol. 2019;103:3265–3276. doi: 10.1007/s00253-019-09698-y. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X., Qian K., Liu G., Sun L., Zhou G., Li J., Fang X., Ge H., Lv Z. Design and activity study of a melittin-thanatin hybrid peptide. AMB Express. 2019;9:14. doi: 10.1186/s13568-019-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Memariani H., Memariani M., Moravvej H., Shahidi-Dadras M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:5–17. doi: 10.1007/s10096-019-03674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hood J.L., Jallouk A.P., Campbell N., Ratner L., Wickline S.A. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antivir. Ther. 2013;18:95–103. doi: 10.3851/IMP2346. [DOI] [PubMed] [Google Scholar]
- 32.Yasin B., Pang M., Turner J.S., Cho Y., Dinh N.N., Waring A.J., Lehrer R.I., Wagar E.A. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 33.Isaacs Charles E., Jia Jun H., Xu W. A Lipid-Peptide Microbicide Inactivates Herpes Simplex Virus. Antimicrob. Agents Chemother. 2004;48:3182–3184. doi: 10.1128/AAC.48.8.3182-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mousavi Maleki M.S., Rostamian M., Madanchi H. Antimicrobial peptides and other peptide-like therapeutics as promising candidates to combat SARS-CoV-2. Expert Rev. Anti-Infect. Ther. 2021;19:1205–1217. doi: 10.1080/14787210.2021.1912593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasozi K.I., Niedbała G., Alqarni M., Zirintunda G., Ssempijja F., Musinguzi S.P., Usman I.M., Matama K., Hetta H.F., Mbiydzenyuy N.E., et al. Bee Venom-A Potential Complementary Medicine Candidate for SARS-CoV-2 Infections. Front. Public Health. 2020;8:755. doi: 10.3389/fpubh.2020.594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W., Hu F.-L., Xu X.-F. Bee venom and SARS-CoV-2. Toxicon. 2020;181:69–70. doi: 10.1016/j.toxicon.2020.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon Y.B., Lee J.D., Lee H.J., Han H.J., Mar W.C., Kang S.K., Beitz A.J., Lee J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90:271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- 38.Saini S.S., Peterson J.W., Chopra A.K. Melittin binds to secretory phospholipase A2 and inhibits its enzymatic activity. Biochem. Biophys. Res. Commun. 1997;238:436–442. doi: 10.1006/bbrc.1997.7295. [DOI] [PubMed] [Google Scholar]
- 39.Park H.J., Son D.J., Lee C.W., Choi M.S., Lee U.S., Song H.S., Lee J.M., Hong J.T. Melittin inhibits inflammatory target gene expression and mediator generation via interaction with IkappaB kinase. Biochem. Pharmacol. 2007;73:237–247. doi: 10.1016/j.bcp.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Lee W.R., Kim K.H., An H.J., Kim J.Y., Chang Y.C., Chung H., Park Y.Y., Lee M.L., Park K.K. The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014;134:1922–1930. doi: 10.1038/jid.2014.75. [DOI] [PubMed] [Google Scholar]
- 41.Han S.M., Kim J.M., Park K.K., Chang Y.C., Pak S.C. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement. Altern. Med. 2014;14:286. doi: 10.1186/1472-6882-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son D.J., Kang J., Kim T.J., Song H.S., Sung K.J., Yun D.Y., Hong J.T. Melittin, a major bioactive component of bee venom toxin, inhibits PDGF receptor beta-tyrosine phosphorylation and downstream intracellular signal transduction in rat aortic vascular smooth muscle cells. J. Toxicol. Environ. Health A. 2007;70:1350–1355. doi: 10.1080/15287390701428689. [DOI] [PubMed] [Google Scholar]
- 43.Park H.J., Lee H.J., Choi M.S., Son D.J., Song H.S., Song M.J., Lee J.M., Han S.B., Kim Y., Hong J.T. JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-kappaB activation by melittin. J. Inflamm. (Lond.) 2008;5:7. doi: 10.1186/1476-9255-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H.J., Lee S.H., Son D.J., Oh K.W., Kim K.H., Song H.S., Kim G.J., Oh G.T., Yoon D.Y., Hong J.T. Antiarthritic effect of bee venom: Inhibition of inflammation mediator generation by suppression of NF-kappaB through interaction with the p50 subunit. Arthritis Rheum. 2004;50:3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- 45.Choi K.E., Hwang C.J., Gu S.M., Park M.H., Kim J.H., Park J.H., Ahn Y.J., Kim J.Y., Song M.J., Song H.S., et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF-kappa B in NSCLC Cells. Toxins. 2014;6:2210–2228. doi: 10.3390/toxins6082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajabnejad S.H., Mokhtarzadeh A., Abnous K., Taghdisi S.M., Ramezani M., Razavi B.M. Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev. Ind. Pharm. 2018;44:982–987. doi: 10.1080/03639045.2018.1427760. [DOI] [PubMed] [Google Scholar]
- 47.Wu X., Zhao B., Cheng Y., Yang Y., Huang C., Meng X., Wu B., Zhang L., Lv X., Li J. Melittin induces PTCH1 expression by down-regulating MeCP2 in human hepatocellular carcinoma SMMC-7721 cells. Toxicol. Appl. Pharmacol. 2015;288:74–83. doi: 10.1016/j.taap.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H., Zhao B., Huang C., Meng X.M., Bian E.B., Li J. Melittin restores PTEN expression by down-regulating HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS ONE. 2014;9:e95520. doi: 10.1371/journal.pone.0095520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C., Chen T., Zhang N., Yang M., Li B., Lü X., Cao X., Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J. Biol. Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 50.Jeong Y.J., Choi Y., Shin J.M., Cho H.J., Kang J.H., Park K.K., Choe J.Y., Bae Y.S., Han S.M., Kim C.H., et al. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014;68:218–225. doi: 10.1016/j.fct.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Duffy C., Sorolla A., Wang E., Golden E., Woodward E., Davern K., Ho D., Johnstone E., Pfleger K., Redfern A., et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020;4:24. doi: 10.1038/s41698-020-00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park M.H., Choi M.S., Kwak D.H., Oh K.-W., Yoon D.Y., Han S.B., Song H.S., Song M.J., Hong J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate. 2011;71:801–812. doi: 10.1002/pros.21296. [DOI] [PubMed] [Google Scholar]
- 53.Ceremuga M., Stela M., Janik E., Gorniak L., Synowiec E., Sliwinski T., Sitarek P., Saluk-Bijak J., Bijak M. Melittin-A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules. 2020;10:247. doi: 10.3390/biom10020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang M.-H., Shin M.-C., Lim S., Han S.-M., Park H.-J., Shin I., Lee J.-S., Kim K.-A., Kim E.-H., Kim C.-J. Bee Venom Induces Apoptosis and Inhibits Expression of Cyclooxygenase-2 mRNA in Human Lung Cancer Cell Line NCI-H1299. J. Pharmacol. Sci. 2003;91:95–104. doi: 10.1254/jphs.91.95. [DOI] [PubMed] [Google Scholar]
- 55.Sharma S.V. Melittin-induced hyperactivation of phospholipase A2 activity and calcium influx in ras-transformed cells. Oncogene. 1993;8:939–947. [PubMed] [Google Scholar]
- 56.Karin M., Cao Y., Greten F.R., Li Z.-W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Yu M., He Y., Xiao L., Wang F., Song C., Sun S., Ling C., Xu Z. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 58.Jeong Y.-J., Cho H.-J., Whang K., Lee I.-S., Park K.-K., Choe J.-Y., Han S.-M., Kim C.-H., Chang H.-W., Moon S.-K., et al. Melittin has an inhibitory effect on TNF-α-induced migration of human aortic smooth muscle cells by blocking the MMP-9 expression. Food Chem. Toxicol. 2012;50:3996–4002. doi: 10.1016/j.fct.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 59.Park J.H., Jeong Y.-J., Park K.-K., Cho H.-J., Chung I.-K., Min K.-S., Kim M., Lee K.-G., Yeo J.-H., Park K.-K., et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Mol. Cells. 2010;29:209–215. doi: 10.1007/s10059-010-0028-9. [DOI] [PubMed] [Google Scholar]
- 60.Pandey B.K., Ahmad A., Asthana N., Azmi S., Srivastava R.M., Srivastava S., Verma R., Vishwakarma A.L., Ghosh J.K. Cell-Selective Lysis by Novel Analogues of Melittin against Human Red Blood Cells and Escherichia coli. Biochemistry. 2010;49:7920–7929. doi: 10.1021/bi100729m. [DOI] [PubMed] [Google Scholar]
- 61.Choi J.H., Jang A.Y., Lin S., Lim S., Kim D., Park K., Han S.M., Yeo J.H., Seo H.S. Melittin, a honeybee venomderived antimicrobial peptide, may target methicillinresistant Staphylococcus aureus. Mol. Med. Rep. 2015;12:6483–6490. doi: 10.3892/mmr.2015.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rauen H.M., Schriewer H., Ferié F. Alkylans-alkylandum reactions. 10. Anti-alkylanting effect of bee poison, melittine and apamine. Arzneimittelforschung. 1972;22:1921–1922. [PubMed] [Google Scholar]
- 63.Wolfgang Bücherl E.E.B. Venomous Animals and Their Venoms: Venomous Vertebrates. Volume 3. Academic Press; Cambridge, MA, USA: 1971. pp. 157–158. [Google Scholar]
- 64.Askari P., Namaei M.H., Ghazvini K., Hosseini M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021;22:42. doi: 10.1186/s40360-021-00503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J.H., Yim B.K., Lee J.-H., Lee S., Kim T.-H. Risk Associated with Bee Venom Therapy: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0126971. doi: 10.1371/journal.pone.0126971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korošec P., Žiberna K., Šilar M., Dežman M., Čelesnik Smodiš N., Rijavec M., Kopač P., Eržen R., Lalek N., Bajrović N., et al. Immunological and clinical factors associated with adverse systemic reactions during the build-up phase of honeybee venom immunotherapy. Clin. Exp. Allergy. 2015;45:1579–1589. doi: 10.1111/cea.12582. [DOI] [PubMed] [Google Scholar]
- 67.Pan H., Soman N.R., Schlesinger P.H., Lanza G.M., Wickline S.A. Cytolytic peptide nanoparticles (’NanoBees’) for cancer therapy. Wiley Interdiscip Rev. Nanomed Nanobiotechnol. 2011;3:318–327. doi: 10.1002/wnan.126. [DOI] [PubMed] [Google Scholar]
- 68.Maher S., McClean S. Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem. Pharmacol. 2008;75:1104–1114. doi: 10.1016/j.bcp.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 69.Resende V.M.F., Vasilj A., Santos K.S., Palma M.S., Shevchenko A. Proteome and phosphoproteome of Africanized and European honeybee venoms. Proteomics. 2013;13:2638–2648. doi: 10.1002/pmic.201300038. [DOI] [PubMed] [Google Scholar]
- 70.Jallouk A.P., Palekar R.U., Marsh J.N., Pan H., Pham C.T.N., Schlesinger P.H., Wickline S.A. Delivery of a Protease-Activated Cytolytic Peptide Prodrug by Perfluorocarbon Nanoparticles. Bioconjug. Chem. 2015;26:1640–1650. doi: 10.1021/acs.bioconjchem.5b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou K.K., Pan H., Lanza G.M., Wickline S.A. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34:3110–3119. doi: 10.1016/j.biomaterials.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hewish D.R., Barnham K.J., Werkmeister J.A., Kirkpatrick A., Bartone N., Liu S.T., Norton R.S., Curtain C., Rivetta D.E. Structure and activity of D-Pro14 melittin. J. Protein. Chem. 2002;21:243–253. doi: 10.1023/A:1019741202601. [DOI] [PubMed] [Google Scholar]
- 73.Asthana N., Yadav S.P., Ghosh J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004;279:55042–55050. doi: 10.1074/jbc.M408881200. [DOI] [PubMed] [Google Scholar]
- 74.Rex S. A Pro→Ala substitution in melittin affects self-association, membrane binding and pore-formation kinetics due to changes in structural and electrostatic properties. Biophys. Chem. 2000;85:209–228. doi: 10.1016/S0301-4622(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 75.Guha S., Ferrie R.P., Ghimire J., Ventura C.R., Wu E., Sun L., Kim S.Y., Wiedman G.R., Hristova K., Wimley W.C. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 2021;193:114769. doi: 10.1016/j.bcp.2021.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu M., Wang H., Liu L., Wang B., Sun G. Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J. Transl. Med. 2016;14:155. doi: 10.1186/s12967-016-0910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiedman G., Kim S.Y., Zapata-Mercado E., Wimley W.C., Hristova K. pH-Triggered, Macromolecule-Sized Poration of Lipid Bilayers by Synthetically Evolved Peptides. J. Am. Chem. Soc. 2017;139:937–945. doi: 10.1021/jacs.6b11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krauson A.J., He J., Wimley W.C. Gain-of-function analogues of the pore-forming peptide melittin selected by orthogonal high-throughput screening. J. Am. Chem. Soc. 2012;134:12732–12741. doi: 10.1021/ja3042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo L., Wu W., Sun D., Dai H.B., Wang Y., Zhong Y., Wang J.X., Maruf A., Nurhidayah D., Zhang X.J., et al. Acid-Activated Melittin for Targeted and Safe Antitumor Therapy. Bioconjug. Chem. 2018;29:2936–2944. doi: 10.1021/acs.bioconjchem.8b00352. [DOI] [PubMed] [Google Scholar]
- 80.Shin S.Y., Lee M.K., Kim K.L., Hahm K.S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1997;50:279–285. doi: 10.1111/j.1399-3011.1997.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 81.Huang C., Jin H., Qian Y., Qi S., Luo H., Luo Q., Zhang Z. Hybrid melittin cytolytic Peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano. 2013;7:5791–5800. doi: 10.1021/nn400683s. [DOI] [PubMed] [Google Scholar]
- 82.Lu X., Liu J., Gou L., Li J., Yuan B., Yang K., Ma Y. Designing Melittin-Graphene Hybrid Complexes for Enhanced Antibacterial Activity. Adv. Healthc. Mater. 2019;8:1801521. doi: 10.1002/adhm.201801521. [DOI] [PubMed] [Google Scholar]
- 83.Dang Y.-Q., Li H.-W., Wu Y. Construction of a Supramolecular Förster Resonance Energy Transfer System and Its Application Based on the Interaction between Cy3-Labeled Melittin and Phosphocholine Encapsulated Quantum Dots. ACS Appl. Mater. Interfaces. 2012;4:1267–1272. doi: 10.1021/am3000984. [DOI] [PubMed] [Google Scholar]
- 84.Hematyar M., Soleimani M., Es-Haghi A., Rezaei Mokarram A. Synergistic co-delivery of doxorubicin and melittin using functionalized magnetic nanoparticles for cancer treatment: Loading and in vitro release study by LC-MS/MS. Artif. Cells Nanomed. Biotechnol. 2018;46:S1226–S1235. doi: 10.1080/21691401.2018.1536063. [DOI] [PubMed] [Google Scholar]
- 85.Soman N.R., Lanza G.M., Heuser J.M., Schlesinger P.H., Wickline S.A. Synthesis and Characterization of Stable Fluorocarbon Nanostructures as Drug Delivery Vehicles for Cytolytic Peptides. Nano Lett. 2008;8:1131–1136. doi: 10.1021/nl073290r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soman N.R., Baldwin S.L., Hu G., Marsh J.N., Lanza G.M., Heuser J.E., Arbeit J.M., Wickline S.A., Schlesinger P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009;119:2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeong I., Kim B.-S., Lee H., Lee K.-M., Shim I., Kang S.-K., Yin C.-S., Hahm D.-H. Prolonged analgesic effect of PLGA-encapsulated bee venom on formalin-induced pain in rats. Int. J. Pharm. 2009;380:62–66. doi: 10.1016/j.ijpharm.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 88.Yang L., Cui F., Shi K., Cun D., Wang R. Design of high payload PLGA nanoparticles containing melittin/sodium dodecyl sulfate complex by the hydrophobic ion-pairing technique. Drug Dev. Ind. Pharm. 2009;35:959–968. doi: 10.1080/03639040902718039. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Horta A., Matamoros-Acosta A., Chavez-Montes A., Castro-Rios R., Lara-Arias J. Biodegradable nanoparticles loaded with tetrameric melittin: Preparation and membrane disruption evaluation. Gen. Physiol. Biophys. 2017;36:373–381. doi: 10.4149/gpb_2017011. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y., Deng M., Zhang H., Tan S., Li D., Li S., Luo L., Liao G., Wang Q., Huang J., et al. Selection of Affinity Reagents to Neutralize the Hemolytic Toxicity of Melittin Based on a Self-Assembled Nanoparticle Library. ACS Appl. Mater. Interfaces. 2020;12:16040–16049. doi: 10.1021/acsami.0c00303. [DOI] [PubMed] [Google Scholar]
- 91.Zetterberg M.M., Reijmar K., Pränting M., Engström Å., Andersson D.I., Edwards K. PEG-stabilized lipid disks as carriers for amphiphilic antimicrobial peptides. J. Control. Release. 2011;156:323–328. doi: 10.1016/j.jconrel.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 92.Gao J., Xie C., Zhang M., Wei X., Yan Z., Ren Y., Ying M., Lu W. RGD-modified lipid disks as drug carriers for tumor targeted drug delivery. Nanoscale. 2016;8:7209–7216. doi: 10.1039/C5NR05577F. [DOI] [PubMed] [Google Scholar]
- 93.Ahlgren S., Reijmar K., Edwards K. Targeting lipodisks enable selective delivery of anticancer peptides to tumor cells. Nanomed. Nanotechnol. Biol. Med. 2017;13:2325–2328. doi: 10.1016/j.nano.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 94.Wang H., Wang S., Wang R., Wang X., Jiang K., Xie C., Zhan C., Wang H., Lu W. Co-delivery of paclitaxel and melittin by glycopeptide-modified lipodisks for synergistic anti-glioma therapy. Nanoscale. 2019;11:13069–13077. doi: 10.1039/C9NR01820D. [DOI] [PubMed] [Google Scholar]
- 95.Yu X., Dai Y., Zhao Y., Qi S., Liu L., Lu L., Luo Q., Zhang Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020;11:1110. doi: 10.1038/s41467-020-14906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barrajón-Catalán E., Menéndez-Gutiérrez M.P., Falco A., Carrato A., Saceda M., Micol V. Selective death of human breast cancer cells by lytic immunoliposomes: Correlation with their HER2 expression level. Cancer Lett. 2010;290:192–203. doi: 10.1016/j.canlet.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Falco A., Barrajón-Catalán E., Menéndez-Gutiérrez M.P., Coll J., Micol V., Estepa A. Melittin-loaded immunoliposomes against viral surface proteins, a new approach to antiviral therapy. Antivir. Res. 2013;97:218–221. doi: 10.1016/j.antiviral.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Mao J., Liu S., Ai M., Wang Z., Wang D., Li X., Hu K., Gao X., Yang Y. A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J. Hematol. Oncol. 2017;10:71. doi: 10.1186/s13045-017-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y., Ruan S., Wang Z., Feng N., Zhang Y. Hyaluronic Acid Coating Reduces the Leakage of Melittin Encapsulated in Liposomes and Increases Targeted Delivery to Melanoma Cells. Pharmaceutics. 2021;13:1235. doi: 10.3390/pharmaceutics13081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye R., Zheng Y., Chen Y., Wei X., Shi S., Chen Y., Zhu W., Wang A., Yang L., Xu Y., et al. Stable Loading and Delivery of Melittin with Lipid-Coated Polymeric Nanoparticles for Effective Tumor Therapy with Negligible Systemic Toxicity. ACS Appl. Mater. Interfaces. 2021;13:55902–55912. doi: 10.1021/acsami.1c17618. [DOI] [PubMed] [Google Scholar]
- 101.Lai H., Chen F., Lu M., Stenzel M.H., Xiao P. Polypeptide-Grafted Nanodiamonds for Controlled Release of Melittin to Treat Breast Cancer. ACS Macro Lett. 2017;6:796–801. doi: 10.1021/acsmacrolett.7b00389. [DOI] [Google Scholar]
- 102.Lv S., Sylvestre M., Song K., Pun S.H. Development of D-melittin polymeric nanoparticles for anti-cancer treatment. Biomaterials. 2021;277:121076. doi: 10.1016/j.biomaterials.2021.121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jia H.-R., Zhu Y.-X., Liu X., Pan G.-Y., Gao G., Sun W., Zhang X., Jiang Y.-W., Wu F.-G. Construction of Dually Responsive Nanotransformers with Nanosphere–Nanofiber–Nanosphere Transition for Overcoming the Size Paradox of Anticancer Nanodrugs. ACS Nano. 2019;13:11781–11792. doi: 10.1021/acsnano.9b05749. [DOI] [PubMed] [Google Scholar]
- 104.Liu H., Hu Y., Sun Y., Wan C., Zhang Z., Dai X., Lin Z., He Q., Yang Z., Huang P., et al. Co-delivery of Bee Venom Melittin and a Photosensitizer with an Organic–Inorganic Hybrid Nanocarrier for Photodynamic Therapy and Immunotherapy. ACS Nano. 2019;13:12638–12652. doi: 10.1021/acsnano.9b04181. [DOI] [PubMed] [Google Scholar]
- 105.Cheng B., Xu P. Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin. Toxins. 2020;12:582. doi: 10.3390/toxins12090582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clapp A.R., Medintz I.L., Mauro J.M., Fisher B.R., Bawendi M.G., Mattoussi H. Fluorescence Resonance Energy Transfer Between Quantum Dot Donors and Dye-Labeled Protein Acceptors. J. Am. Chem. Soc. 2004;126:301–310. doi: 10.1021/ja037088b. [DOI] [PubMed] [Google Scholar]
- 107.Li X., Vinothini K., Ramesh T., Rajan M., Ramu A. Combined photodynamic-chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Deliv. 2020;27:791–804. doi: 10.1080/10717544.2020.1765431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi Y.J., Kim Y.J., Lee J.W., Lee Y., Lim Y.B., Chung H.W. Cyto-/genotoxic effect of CdSe/ZnS quantum dots in human lung adenocarcinoma cells for potential photodynamic UV therapy applications. J. Nanosci. Nanotechnol. 2012;12:2160–2168. doi: 10.1166/jnn.2012.5781. [DOI] [PubMed] [Google Scholar]
- 109.Cai X., Luo Y., Zhang W., Du D., Lin Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces. 2016;8:22442–22450. doi: 10.1021/acsami.6b04933. [DOI] [PubMed] [Google Scholar]
- 110.He S.J., Cao J., Li Y.S., Yang J.C., Zhou M., Qu C.Y., Zhang Y., Shen F., Chen Y., Li M.M., et al. CdSe/ZnS quantum dots induce photodynamic effects and cytotoxicity in pancreatic cancer cells. World J. Gastroenterol. 2016;22:5012–5022. doi: 10.3748/wjg.v22.i21.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maleki H., Rai A., Pinto S., Evangelista M., Cardoso R.M.S., Paulo C., Carvalheiro T., Paiva A., Imani M., Simchi A., et al. High Antimicrobial Activity and Low Human Cell Cytotoxicity of Core–Shell Magnetic Nanoparticles Functionalized with an Antimicrobial Peptide. ACS Appl. Mater. Interfaces. 2016;8:11366–11378. doi: 10.1021/acsami.6b03355. [DOI] [PubMed] [Google Scholar]
- 112.Vu H.D., Huynh P.T., Ryu J., Kang U.R., Youn S.W., Kim H., Ahn H.J., Park K., Hwang S.K., Chang Y.C., et al. Melittin-loaded Iron Oxide Nanoparticles Prevent Intracranial Arterial Dolichoectasia Development through Inhibition of Macrophage-mediated Inflammation. Int. J. Biol. Sci. 2021;17:3818–3836. doi: 10.7150/ijbs.60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rai A., Pinto S., Evangelista M.B., Gil H., Kallip S., Ferreira M.G., Ferreira L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016;33:64–77. doi: 10.1016/j.actbio.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 114.Brand I., Khairalla B. Structural changes in the model of the outer cell membrane of Gram-negative bacteria interacting with melittin: An in situ spectroelectrochemical study. Faraday Discuss. 2021;232:68–85. doi: 10.1039/D0FD00039F. [DOI] [PubMed] [Google Scholar]
- 115.Chugh H., Sood D., Chandra I., Tomar V., Dhawan G., Chandra R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018;46:1210–1220. doi: 10.1080/21691401.2018.1449118. [DOI] [PubMed] [Google Scholar]
- 116.Jallouk A.P., Moley K.H., Omurtag K., Hu G., Lanza G.M., Wickline S.A., Hood J.L. Nanoparticle incorporation of melittin reduces sperm and vaginal epithelium cytotoxicity. PLoS ONE. 2014;9:e95411. doi: 10.1371/journal.pone.0095411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M., Terada Y., Kano M.R., Miyazono K., Uesaka M., et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 118.Park M.H., Kim J.H., Jeon J.W., Park J.K., Lee B.J., Suh G.H., Cho C.W. Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles. Molecules. 2015;20:15072–15083. doi: 10.3390/molecules200815072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cui F., Cun D., Tao A., Yang M., Shi K., Zhao M., Guan Y. Preparation and characterization of melittin-loaded poly (DL-lactic acid) or poly (DL-lactic-co-glycolic acid) microspheres made by the double emulsion method. J. Control. Release. 2005;107:310–319. doi: 10.1016/j.jconrel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Zhan W., Wei T., Yu Q., Chen H. Fabrication of Supramolecular Bioactive Surfaces via β-Cyclodextrin-Based Host–Guest Interactions. ACS Appl. Mater. Interfaces. 2018;10:36585–36601. doi: 10.1021/acsami.8b12130. [DOI] [PubMed] [Google Scholar]
- 121.Lundquist A., Wessman P., Rennie A.R., Edwards K. Melittin–Lipid interaction: A comparative study using liposomes, micelles and bilayerdisks. Biochim. Biophys. Acta (BBA)-Biomembr. 2008;1778:2210–2216. doi: 10.1016/j.bbamem.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Z., Cao W., Jin H., Lovell J.F., Yang M., Ding L., Chen J., Corbin I., Luo Q., Zheng G. Biomimetic Nanocarrier for Direct Cytosolic Drug Delivery. Angew. Chem. Int. Ed. 2009;48:9171–9175. doi: 10.1002/anie.200903112. [DOI] [PubMed] [Google Scholar]
- 123.Wessman P., Strömstedt A.A., Malmsten M., Edwards K. Melittin-Lipid Bilayer Interactions and the Role of Cholesterol. Biophys. J. 2008;95:4324–4336. doi: 10.1529/biophysj.108.130559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wessman P., Morin M., Reijmar K., Edwards K. Effect of α-helical peptides on liposome structure: A comparative study of melittin and alamethicin. J. Colloid Interface Sci. 2010;346:127–135. doi: 10.1016/j.jcis.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 125.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian J.L., Ke X., Chen Z., Wang C.J., Zhang Y., Zhong T.C. Melittin liposomes surface modified with poloxamer 188: In vitro characterization and in vivo evaluation. Pharmazie. 2011;66:362–367. [PubMed] [Google Scholar]
- 128.Esim O., Hascicek C. Lipid-Coated Nanosized Drug Delivery Systems for an Effective Cancer Therapy. Curr. Drug Deliv. 2021;18:147–161. doi: 10.2174/1567201817666200512104441. [DOI] [PubMed] [Google Scholar]
- 129.Hanafy N.A.N., Quarta A., Di Corato R., Dini L., Nobile C., Tasco V., Carallo S., Cascione M., Malfettone A., Soukupova J., et al. Hybrid polymeric-protein nano-carriers (HPPNC) for targeted delivery of TGFβ inhibitors to hepatocellular carcinoma cells. J. Mater. Sci. Mater. Med. 2017;28:120. doi: 10.1007/s10856-017-5930-7. [DOI] [PubMed] [Google Scholar]
- 130.Zylberberg C., Gaskill K., Pasley S., Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24:441–452. doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- 131.Erazo-Oliveras A., Muthukrishnan N., Baker R., Wang T.-Y., Pellois J.-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals. 2012;5:1177–1209. doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paray B.A., Ahmad A., Khan J.M., Taufiq F., Pathan A., Malik A., Ahmed M.Z. The role of the multifunctional antimicrobial peptide melittin in gene delivery. Drug Discov. Today. 2021;26:1053–1059. doi: 10.1016/j.drudis.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Ogris M., Carlisle R.C., Bettinger T., Seymour L.W. Melittin Enables Efficient Vesicular Escape and Enhanced Nuclear Access of Nonviral Gene Delivery Vectors. J. Biol. Chem. 2001;276:47550–47555. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]
- 134.Overhoff M., Sczakiel G. Phosphorothioate-stimulated uptake of short interfering RNA by human cells. EMBO Rep. 2005;6:1176–1181. doi: 10.1038/sj.embor.7400535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boeckle S., Wagner E., Ogris M. C- versus N-terminally linked melittin-polyethylenimine conjugates: The site of linkage strongly influences activity of DNA polyplexes. J. Gene Med. 2005;7:1335–1347. doi: 10.1002/jgm.783. [DOI] [PubMed] [Google Scholar]
- 136.Zhang W., Song J., Liang R., Zheng X., Chen J., Li G., Zhang B., Yan X., Wang R. Stearylated Antimicrobial Peptide Melittin and Its Retro Isomer for Efficient Gene Transfection. Bioconjug. Chem. 2013;24:1805–1812. doi: 10.1021/bc400053b. [DOI] [PubMed] [Google Scholar]
- 137.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oude Blenke E., Sleszynska M., Evers M.J.W., Storm G., Martin N.I., Mastrobattista E. Strategies for the Activation and Release of the Membranolytic Peptide Melittin from Liposomes Using Endosomal pH as a Trigger. Bioconjug. Chem. 2017;28:574–582. doi: 10.1021/acs.bioconjchem.6b00677. [DOI] [PubMed] [Google Scholar]
- 139.Meyer M., Dohmen C., Philipp A., Kiener D., Maiwald G., Scheu C., Ogris M., Wagner E. Synthesis and Biological Evaluation of a Bioresponsive and Endosomolytic siRNA−Polymer Conjugate. Mol. Pharm. 2009;6:752–762. doi: 10.1021/mp9000124. [DOI] [PubMed] [Google Scholar]
- 140.Keil T.W.M., Baldassi D., Merkel O.M. T-cell targeted pulmonary siRNA delivery for the treatment of asthma. Wiley Interdiscip. Reviews. Nanomed. Nanobiotechnol. 2020;12:e1634. doi: 10.1002/wnan.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meyer M., Zintchenko A., Ogris M., Wagner E. A dimethylmaleic acid-melittin-polylysine conjugate with reduced toxicity, pH-triggered endosomolytic activity and enhanced gene transfer potential. J. Gene. Med. 2007;9:797–805. doi: 10.1002/jgm.1075. [DOI] [PubMed] [Google Scholar]
- 142.Cheng Y., Yumul R.C., Pun S.H. Virus-Inspired Polymer for Efficient In Vitro and In Vivo Gene Delivery. Angew. Chem. (Int. Ed. Engl. ) 2016;55:12013–12017. doi: 10.1002/anie.201605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feldmann D.P., Cheng Y., Kandil R., Xie Y., Mohammadi M., Harz H., Sharma A., Peeler D.J., Moszczynska A., Leonhardt H., et al. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J. Control. Release. 2018;276:50–58. doi: 10.1016/j.jconrel.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang S.-K., Song J.-W., Li S.-B., Gao H.-W., Chang H.-Y., Jia L.-l., Gong F., Tan Y.-X., Ji S.-P. Design of pH-sensitive peptides from natural antimicrobial peptides for enhancing polyethylenimine-mediated gene transfection. J. Gene Med. 2017;19:e2955. doi: 10.1002/jgm.2955. [DOI] [PubMed] [Google Scholar]
- 145.Ahmad A., Ranjan S., Zhang W., Zou J., Pyykkö I., Kinnunen P.K.J. Novel endosomolytic peptides for enhancing gene delivery in nanoparticles. Biochim. Biophys. Acta (BBA)-Biomembr. 2015;1848:544–553. doi: 10.1016/j.bbamem.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 146.Ventura C.R., Wiedman G.R. Substituting azobenzene for proline in melittin to create photomelittin: A light-controlled membrane active peptide. Biochim. Biophys. Acta (BBA)-Biomembr. 2021;1863:183759. doi: 10.1016/j.bbamem.2021.183759. [DOI] [PubMed] [Google Scholar]
- 147.Chen C.-P., Kim J.-s., Steenblock E., Liu D., Rice K.G. Gene transfer with poly-melittin peptides. Bioconjug. Chem. 2006;17:1057–1062. doi: 10.1021/bc060028l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hou K.K., Pan H., Ratner L., Schlesinger P.H., Wickline S.A. Mechanisms of Nanoparticle-Mediated siRNA Transfection by Melittin-Derived Peptides. ACS Nano. 2013;7:8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan H., Myerson J.W., Ivashyna O., Soman N.R., Marsh J.N., Hood J.L., Lanza G.M., Schlesinger P.H., Wickline S.A. Lipid membrane editing with peptide cargo linkers in cells and synthetic nanostructures. FASEB J. 2010;24:2928–2937. doi: 10.1096/fj.09-153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jafari E., Mahmoodi S. Design, expression, and purification of a multi-epitope vaccine against Helicobacter Pylori based on Melittin as an adjuvant. Microb. Pathog. 2021;157:104970. doi: 10.1016/j.micpath.2021.104970. [DOI] [PubMed] [Google Scholar]
- 151.Bramwell V.W., Somavarapu S., Outschoorn I., Alpar H.O. Adjuvant Action of Melittin Following Intranasal Immunisation with Tetanus and Diphtheria Toxoids. J. Drug Target. 2003;11:525–530. doi: 10.1080/10611860410001670080. [DOI] [PubMed] [Google Scholar]