Abstract
As the outermost layer of plants, the epidermis serves as a critical interface between plants and the environment. During leaf development, the differentiation of specialized epidermal cell types, including stomatal guard cells, pavement cells, and trichomes, occurs simultaneously, each providing unique and pivotal functions for plant growth and survival. Decades of molecular-genetic and physiological studies have unraveled key players and hormone signaling specifying epidermal differentiation. However, most studies focus on only one cell type at a time, and how these distinct cell types coordinate as a unit is far from well-comprehended. Here we provide a review on the current knowledge of regulatory mechanisms underpinning the fate specification, differentiation, morphogenesis, and positioning of these specialized cell types. Emphasis is given to their shared developmental origins, fate flexibility, as well as cell cycle and hormonal controls. Furthermore, we discuss computational modeling approaches to integrate how mechanical properties of individual epidermal cell types and entire tissue/organ properties mutually influence each other. We hope to illuminate the underlying mechanisms coordinating the cell differentiation that ultimately generate a functional leaf epidermis.
This review synthesizes the regulatory mechanisms underpinning the coordination of fate specification, differentiation, morphogenesis, and positioning of specialized leaf epidermal cell types.
Introduction
The epidermis is the outermost cell layer surrounding the plant body and serves a range of essential functions, including protection, regulation of growth, and interactions with the environment (Glover, 2000; Javelle et al., 2011). In the leaf, the epidermis plays many important roles including regulating the exchange of gases, water, and nutrients with the surroundings, responding to external threats such as pathogens, herbivores and abiotic stresses, resisting mechanical strain, detoxifying xenobiotics, and contributing to mechanical strength while allowing the flat and flexible shape necessary for maximum light capture (Glover, 2000; Javelle et al., 2011; Staff et al., 2012; Jacques et al., 2014; Sotiriou et al., 2018; Cavé-Radet et al., 2020).
To achieve these many functions, leaf epidermal cells are specialized for specific roles according to their size and shape. This tissue, therefore, develops as a patchwork of cells with various sizes and shapes, not just positioned precisely the across the leaf surface, but also varying in their degree of 3D protrusion according to the cell type (Figure 1). An additional complexity is the presence of a multilayered leaf epidermis in certain species such as Ficus and Peperomia (Becraft, 1999). Nevertheless, these cells fit together in an impressively organized manner to contribute to survival and fitness of the plant. Furthermore, the positioning and shapes of leaf epidermal cells are aligned with the underlying mesophyll and vascular tissues. This requires intricate coordination of cell division, specification, and expansion. However, for the most part, these cell types have been studied more or less individually, with little focus on how such highly orchestrated processes are regulated at the tissue/organ level.
Figure 1.
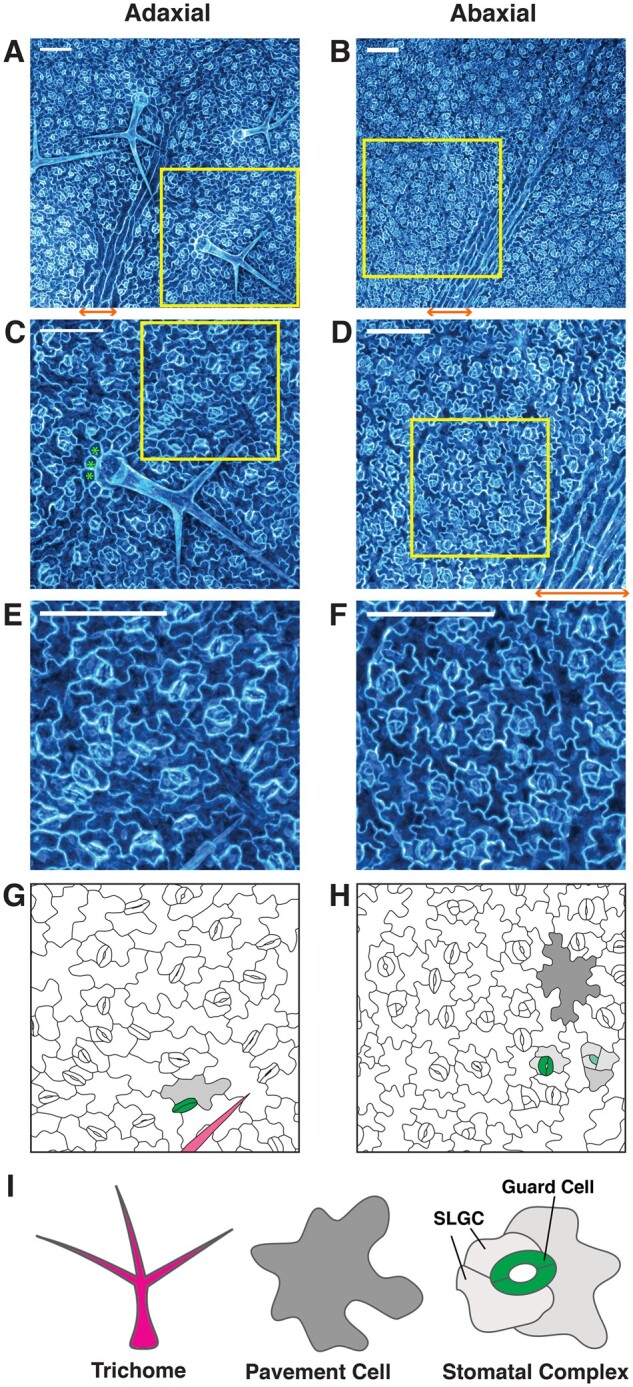
Arabidopsis leaf epidermis and cell types. A–F, Representative images of the adaxial (A), (C), and (E) and abaxial (B), (D), and (F) epidermis of the third true leaf of 2-week-old Arabidopsis seedlings. Maximum fluorescence intensity projections of confocal z-stacks of the endoplasmic reticulum marker line 35S:GFP-HDEL (Haseloff et al., 1997) are shown for cell visualization. Scale bars represent 100 µm. The images in (C–F) correspond to the yellow insets in (A–D), respectively. G and H, Drawings of the cell outlines shown in (E) and (F), respectively, with representative cell types false colored to match with (I). Green asterisks in (C) indicate representative socket cells. Orange double arrows in (A), (B), and (D) indicate the location of a midvein, where pavement cells are rectangular and stomatal development is restricted. I, Representative drawings of trichome (magenta), pavement cell (gray), and stoma with paired guard cells (green)/stomatal complex (with SLGCs light gray). SLGCs are arranged in a spiral manner to form an anisocytic stomatal complex.
Many kinds of epidermal cell types exist in diverse plant species to achieve specialized functions and/or adaptation to extreme environments. Such cell types include hydathode pores for water pressure adjustment (Torii, 2021), bulliform cells in grasses contributing to leaf rolling/unrolling (Mader et al., 2020), subsidiary cells supporting efficient stomatal turgor movement in grasses (Gray et al., 2020), and salt glands of halophytes for secreting excess salt (Yuan et al., 2015), among others. Herein, we will discuss what we know so far about cell fate specification and coordination of division and growth amongst the three main leaf epidermal cell types: pavement cells, guard cells, and trichomes (Torii, 2021). We will summarize the main findings regarding the origins, specification, positioning, and shaping of these cells, as well as their relations to one another, to shed light on the coordinated development of the leaf epidermis as a whole.
Cells constituting the leaf epidermis
All epidermal cells of a plant arise from the embryonic protoderm. The protodermal cells of a young leaf may differentiate directly into pavement cells or trichomes, or instead differentiate into meristemoid mother cells (MMCs) that indirectly give rise to pavement cells and stomata by generating stomatal cell lineages (Larkin et al., 1996; Geisler et al., 2000) (see “Coordination of Leaf Epidermal Cell Patterning”). In the developing Arabidopsis (Arabidopsis thaliana) leaf, a wave of evolving epidermal cell stages is found along the surface, from the base toward the tip (Pyke et al., 1991; Becraft, 1999; Gonzalez et al., 2012). While the base is composed mainly of dividing, undifferentiated protodermal cells, distal to this region, differentiating cells start to become more and more prominent. Meanwhile, cell expansion begins to occur, increasing with proximity to the leaf tip, while fully developed cells become more commonplace. The first fully differentiated cells, therefore, appear toward the leaf tip (Larkin et al., 1996) and the wave of cell differentiation and development moves its way along the leaf surface toward the base until the entire leaf is fully expanded and all epidermal cells are fully developed (Liu et al., 2021).
Pavement cells
Pavement cells can be considered the basic or ground cells of the leaf epidermis, which can grow to be quite large and can vary in shape from isodiametric or rectangular forms to complex, lobed forms—often referred to as jigsaw puzzle shapes. Pavement cells do not appear to have specialized to play one particular role, but have been suggested to possess a range of functions, including spacing out other epidermal cells and contributing to epidermal integrity, mechanical strength, and resistance to mechanical stresses (Staff et al., 2012; Jacques et al., 2014; Szymanski, 2014; Sapala et al., 2018). In Arabidopsis seedlings, the pavement cells overlying the midvein are elongated, while the socket cells, those surrounding the trichome bases, are more square or rectangular in shape (Figure 1). However, the vast majority of the pavement cells in this species develop lobes and necks that interlock perfectly with those of their neighbors, creating a fascinating jigsaw puzzle-like effect (Figure 1). Highly lobed pavement cells also occur in a number of other species, including maize (Zea mays), rice (Oryza sativa), and many ferns (Sotiriou et al., 2018; Vőfély et al., 2019; Liu et al., 2021). However, a quantitative survey of pavement cells in vascular plants revealed that species displaying highly lobed pavement cell margins are actually rather rare across most clades, including eudicots, monocots, and gymnosperms (Vőfély et al., 2019).
Stomatal guard cells
Dispersed amongst a sea of protective “air-tight” pavement cells, pairs of stomatal guard cells form microscopic valves in the leaf surface through which photosynthetic machinery in the leaf’s interior can interface with the external environment. A pair of mirror-symmetric guard cells control each stomatal aperture (Figure 1) to regulate gas and water exchange in response to hormonal, metabolic, and environmental inputs (Becraft, 1999; Pillitteri and Dong, 2013; Qi and Torii, 2018). Additionally, stomata serve as an important first line of defense against pathogens as well as adverse abiotic insults (Sawinski et al., 2013). Because stomatal shape and size, as well as density and spatial arrangement throughout the leaf, can impact gas exchange and water-use efficiency (Bertolino et al., 2019), the development of stomata is a tightly regulated process while their density and patterning can be flexibly adjusted by diverse environmental input (Qi and Torii, 2018). In Arabidopsis, each stoma forms in an anisocytic complex, with several nonstomatal epidermal cells (known as stomatal lineage ground cells [SLGCs]) surrounding the paired guard cells (Figure 1I).
Trichomes
In general, trichomes are large, rigid, hair-like protrusions (Figure 1) that extend from the shoot epidermis, including the stem and adaxial (upper) leaf epidermis, creating a protective physical barrier against insects and abiotic stresses (Becraft, 1999). Trichomes exhibit a range of features, functions, and localization patterns among different species and within an organism or even a single leaf. Glandular trichomes produce and secrete a variety of metabolites, which serve various functions including growth/maturation regulation, pathogen defense, insecticidal or insect repelling (or insect trapping, in the case of carnivorous plants), irritants against larger herbivores, and UV light protection (Huchelmann et al., 2017). In Arabidopsis, trichomes are nonglandular and are unicellular with three branches (Figure 1I).
Diversity of leaf epidermal cell patterning
Most plant species have bifacial leaves, meaning that they display different epidermal cell arrangements, distributions, sizes, and shapes on the adaxial (upper) and abaxial (lower) leaf sides (Becraft, 1999; Figure 1). This is associated with different internal mesophyll tissue architectures (palisade versus spongy) in typical dicot leaves and reflects the important evolutionary influence of environmental conditions on epidermal cell patterning. For instance, the adaxial surface is usually more prone to pathogen, insect, and herbivore attack, and therefore often develops trichomes; in Arabidopsis, trichomes are predominantly on this side (Figure 1). In contrast, the more shaded abaxial side often develops more stomata to avoid excessive moisture loss, as occurs in Arabidopsis. In some species, such as poplar, stomata develop only on this side (Liu et al., 2021). Furthermore, in many species with lobed pavement cells, including Arabidopsis, interdigitation is more pronounced on the abaxial side (Vőfély et al., 2019; Liu et al., 2021; Figure 1). The wide variety of leaf epidermal cell patterns among different species is likely the result of evolutionary adaptations to different growth conditions and lifestyles. However, leaf epidermal cell densities, positions, shapes, and sizes can also display a high level of plasticity within a single plant or organ and often develop differently among individuals of the same species according to various growth conditions.
Coordination of leaf epidermal cell patterning
While trichomes and about half of pavement cells develop directly from protodermal cells, stomata result from a multi-stage differentiation program, initiating in the protoderm as meristematic stomatal lineage cells (Larkin et al., 1996; Geisler et al., 2000). Divisions within the stomatal lineage are responsible for generating mature stomata as well as the remaining ∼50% and 70% of pavement cells in the leaf and the cotyledon, respectively, in Arabidopsis (Geisler et al., 2000). The first stomatal lineage cell arises from the asymmetric division of a single MMC, which results in a meristemoid and SLGC (Zhao and Sach, 1999; Shpak et al., 2005). The meristemoid then continues to divide asymmetrically in a spiral manner, each time producing a meristemoid and SLGC (Zhao and Sach, 1999; Figure 1). Variability in the number of asymmetric divisions of each meristemoid provides an important means of flexibility in epidermal cell patterning, allowing plasticity in response to environmental conditions. While the meristemoid ultimately differentiates into a guard mother cell (GMC; guard cell precursor), SLGCs begin to form lobes and transition into pavement cells. The GMC divides once symmetrically to form the two guard cells of a stoma (Zhao and Sach, 1999). In this way, the surface of a developing leaf becomes peppered with spiral anisocytic stomatal complexes (Figure 1) which, together, control the distribution and positioning of the various epidermal cell types. Interestingly, SLGCs maintain pluripotency, as they too can divide asymmetrically to generate a new meristemoid that leads to the formation of a satellite stoma, a process that is also important for stomatal positioning (Geisler et al., 2000) and which also contributes to the plasticity of epidermal patterning.
Inner tissues subtending the epidermis play an important role in epidermal development, promoting stomata above photosynthetic mesophyll to facilitate gas exchange, while restricting stomatal development directly over major veins where pavement cells are less interdigitated and more elongated (see Figure 1). In Arabidopsis, the developing mesophyll layer produces STOMAGEN, a secreted paracrine signaling peptide that promotes stomatal development in the adjacent epidermis (Sugano et al., 2010). The relationship between subepidermal bundles and stomatal patterning is more evident in grass species, where stoma-producing epidermal cell files usually arise above both sides of vascular bundles (Nunes et al., 2020). This suggests that mobile signals emanating from vascular bundles may promote stomatal development in the epidermis. Indeed, heterologous overexpression of the maize mobile transcription factor ZmSHORTROOT in rice vasculature conferred excessive rows of stoma-producing cell files (Schuler et al., 2018).
The degree to which different epidermal cells impact each other’s development is largely unknown, but several observations offer a few key insights. For instance, pavement cells seemingly arise in the absence of trichome or stomatal lineage-activating signals, suggesting that the balance of epidermal cell types is at least partially controlled by signal-dependent fate segregation in the protoderm, although the flexible supply of pavement cells relies on stomatal cell lineages (Geisler et al., 2000). Further, the number, organization, and shape of pavement cells are impacted by cells of the stomatal lineage, which regulate auxin flow to control differentiation and lobe development (Grones et al., 2020), while also generating roughly half of the pavement cell population through spiraled asymmetric divisions. Trichomes may also directly impact other epidermal cells, as the shapes of socket, cells suggest that trichomes restrict both the local expansion of pavement cells and the formation of stomata in this zone.
Epidermal cell development and morphogenesis
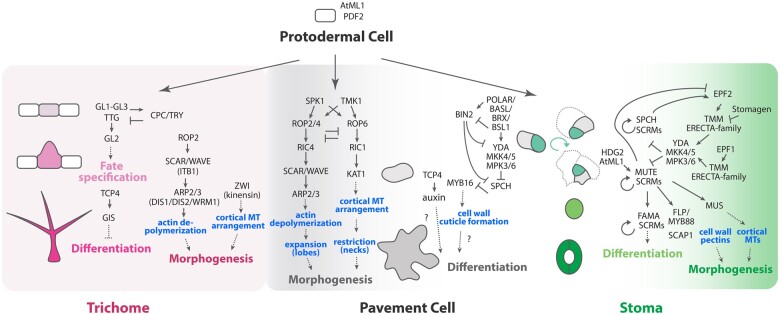
Over the past few decades, intensive research into the genetic regulation of stoma and trichome development, primarily in Arabidopsis, has revealed many details of feedback-regulated molecular signaling pathways controlling cell fate, patterning, and differentiation. Homeodomain-leucine zipper IV transcription factors MERISTEM LAYER1 and PROTODERMAL FACTOR2 were identified as critical regulators specifying the multipotent protodermal cell fate (Lu et al., 1996; Abe et al., 2003; Figure 2). Subsequently, stomatal lineage cells initiate and differentiate from protodermal cells in response to the sequential action of three basic Helix–Loop–Helix (bHLH) transcription factors, and, independently, trichome initials are specified by a regulatory complex composed of Myb, bHLH, and WD-40 proteins (Schellmann and Hülskamp, 2005; Lee and Bergmann, 2019; Torii, 2021).
Figure 2.
Regulatory pathways of epidermal cell fate specification, differentiation, and morphogenesis. Trichomes (magenta), pavement cells (gray), and stomata (green) as well as stomatal precursor cells (meristemoids, jade green, GMCs, light green) are all originated from protodermal cells (white). Genes (dark gray) and key regulatory components (blue) specifying each process (activation as arrows; inhibition as T-bars) are indicated. Solid arrows/T-bars represent known direct regulation, and dotted arrow/T-bars represent indirect relationships. Stomatal differentiation program gives rise to SLGCs (light gray) which can generate both pavement cells and stomatal precursors, and are therefore placed in between these two developmental programs. For details of individual genes and cellular processes, see the text. Fate Specification: a process in which an undifferentiated precursor cell acquires a specific fate. Differentiation: a process whereby a given cell becomes a specific (different) cell type. Morphogenesis: a cellular process to elaborate specific cell shape, size, and morphology to function as a specific cell type.
It is less clear whether pavement cell specification requires a specific gene regulatory program. Several genes preferentially expressed in pavement cells, such as those encoding epicuticular wax and cutin components, have been identified (Aarts et al., 1995; Bird and Gray, 2003). A more recent study shows that the transcription factor TEOSINTE BRANCHED 1, CYCLOIDEA, and PROLIFERATING CELL FACTOR1/2 4 (TCP4) promotes pavement cell differentiation via auxin-dependent and independent pathways (Challa et al., 2019). It would be interesting to test whether TCP4-mediated gene regulatory cascades feed into the pavement cell morphogenesis pathways (Figure 2).
Master regulators of stomatal development
Stomatal development is governed by the sequential action of the three master regulatory bHLH transcription factors SPEECHLESS (SPCH), MUTE, and FAMA, together with their heterodimeric binding partners SCREAM1 (SCRM1) and SCRM2 (Ohashi-Ito and Bergmann, 2006; MacAlister et al., 2007; Pillitteri et al., 2007; Kanaoka et al., 2008). Together, SPCH–SCRM, MUTE–SCRM, and FAMA–SCRM complexes coordinate the differentiation of sequential stomatal stages to create a symmetric pair of stomatal guard cells surrounding a pore (Figure 2).
A subset of protodermal cells express and accumulate SPCH proteins at a sufficient level to enter the stomatal lineage, becoming MMCs (meristemoid precursors). SPCH–SCRM heterodimers induce a subset of genes encoding peptide-receptor signaling components, such as TOO MANY MOUTHS (TMM) and EPIDERMAL PATTERNING FACTOR2 (EPF2), that repress meristemoid fate in neighboring cells (Lau et al., 2014; Han et al., 2018). Initial decisions to enter the stomatal cell lineage are coordinated by the mesophyll-derived peptide EPF-LIKE9/STOMAGEN as well as EPF2, both of which competitively bind to the same receptor complex, ERECTA and TMM (Nadeau and Sack, 2002; Shpak et al., 2005; Sugano et al., 2010; Lee et al., 2015). Perception of EPF2 leads to the activation of a MAPK cascade comprised a MAPKKK (YODA [YDA]), MAPKKs (MPK4/5), and MAPKs (MPK3/6) which phosphorylate and destabilize SPCH–SCRMs in cells neighboring MMCs and meristemoids, thereby limiting the density of incipient MMCs (Wang et al., 2007; Lampard et al., 2008; Horst et al., 2015; Figure 2). MMCs initiate asymmetric cell divisions (ACDs), maintaining the meristemoid stem cell-like fate in the smaller daughter cell via retained SPCH protein accumulation, while SPCH–SCRM complexes are destabilized in the larger daughter cell (the SLGC) by local sequestration and activation of the YDA MAPK cascade (see “Bistability in the stomatal lineage”; Zhang et al., 2015, 2016; Putarjunan et al., 2019; Figure 2).
The second bHLH protein, MUTE, drives the transition from stem cell-like meristemoid to differentiating GMC (Pillitteri et al., 2007; Figure 2). MUTE-SCRMs activate the expression of a large cohort of differentiation-related and cell cycle genes, and together with FAMA, control the timely differentiation and symmetric division creating a functional stoma (Han et al., 2018). FAMA, along with Myb protein FOUR LIPS (FLP), epigenetically represses SPCH and MUTE expression, thereby stabilizing the mature guard cell fate (Hachez et al., 2011; Lee et al., 2014b; Matos et al., 2014; Figure 2) (see “Coordination of terminal guard cell division and differentiation”).
Bistability in the stomatal lineage
Asymmetric fate acquisition during meristemoid divisions reflects the dual potential of stomatal lineage cells, which can also be observed in nascent SLGCs—either assuming pavement cell fate or undergoing another round of asymmetric divisions to produce a secondary meristemoid. Asymmetric division within the stomatal lineage is mediated by the intrinsic polarity complex (Guo et al., 2021a; Figure 2). In the MMC, this protein complex includes POLAR LOCALIZATION DURING ASYMMETRIC DIVISION AND REDISTRIBUTION, BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL), and BREVIS RADIX, which likely prevent nuclear localization of BIN2, thus “protecting” SPCH protein accumulation (Dong et al., 2009; Pillitteri et al., 2011; Houbaert et al., 2018; Rowe et al., 2019). During the asymmetric division, these polarity complexes are maintained and inherited by the SLGC daughter cell. In SLGCs, the BSL1 phosphatase likely displaces BIN2, allowing it to localize to the nucleus to inhibit SPCH, and at the same time, dephosphorylate YDA to resume the active state of the MAPK cascade (Guo et al., 2021b). Both BIN2 and MAPKs phosphorylate SPCH, and this leads to SPCH protein degradation, which is generally followed by the differentiation into a pavement cell (Zhang et al., 2015, 2016; Gong et al., 2021; Figure 2). It is thus likely that the strength and sustained duration of BIN2/MAPK activity are critical for SLGCs to maintain their bipotent state.
Whereas these intrinsic polarity proteins are central to the ACD of stomatal-lineage cells, their localization and function are influenced by the supracellular environment. For example, BASL localization shifts to the membrane adjacent to the existing stoma prior to the asymmetric spacing division to maintain one cell spacing (Dong et al., 2009). This process is under the influence of EPF-(ERECTA)/TMM cell–cell signaling pathways, given that BASL mis-localizes to a “wrong” position in the epf1 and tmm mutant backgrounds (Dong et al., 2009). In addition, tissue-wide mechanical forces, as well as long-range chemical signals, play a role in orienting the polarity of asymmetric divisions (Bringmann and Bergmann, 2017).
Is there any molecular link between cell–cell signaling and cellular mechanics? Very recent work has identified that the expression of MYB16, a regulator of cutin biosynthesis, is enriched in SLGCs and negatively regulated by SPCH (Yang et al., 2021). Ectopic expression of MYB16 in meristemoids led to stomatal clustering, thereby supporting a decades-old hypothesis that cuticular wax plays a role in restricting local cell–cell signaling or maintenance of a specific mechanical framework between stomatal lineage cells (Bird and Gray, 2003; Figure 2). Through a subtractive approach, genes enriched in SLGCs have been reported, and their characteristics imply a bistable “latent” state of SLGCs (Ho et al., 2021). It remains to be seen whether specific molecular regulators of pavement cell specification and differentiation can be inferred from such studies.
Genetic regulation of trichome specification and differentiation
Trichomes are initiated in an evenly distributed manner from the pool of protodermal cells in a developing leaf. Trichome fate is specified and directed by thehomeoodomain-leucine zipper (HD-ZIP) transcription factor GLABRA2 (GL2), which reaches sufficient levels to specify trichome initials with an average spacing of about three cells between each developing trichome (Hulskamp et al., 1994; Larkin et al., 1996). The regular, 2D spatial pattern of trichome development is established by lateral inhibition, whereby a core transcription factor complex consisting of GL1 (an MYBR2R3 protein), GL3 (a bHLH protein), and its paralogs ENHANCER OF GL3 (EGL3) and MYC1, as well a WD40-repeat protein, TRANSPARENT TESTA GL1 (TTG1), induce the expression of the trichome activator GL2, as well as a family of short MYBR3 proteins that function as diffusible repressors of trichome initiation (Larkin et al., 1996; Walker et al., 2000; Schellmann and Hülskamp, 2005; Zhao et al., 2012; Figure 2). The short MYBR3 proteins TRIPTYCHON (TRY) and CAPRICE (CPC) are capable of moving to neighbor cells via plasmodesmata, where they competitively displace the transcriptional activator GL1 from the GL1–GL3/EGL3–TTG1 complex, switching the complex from an activator to a repressor of trichome fate (Schellmann and Hülskamp, 2005; Figure 2).
Establishment of the trichome initial patterning is reminiscent of Turing reaction–diffusion dynamics, whereby trichomes can initiate a pattern in a self-sufficient manner through the cell–cell movement of long-range transcriptional inhibitors and short-range transcriptional activators (Torii, 2012). Does cell-to-cell trafficking of trichome regulatory molecules operate independently of other epidermal fate specification processes? Interestingly, differentiation of multiple epidermal cell types can be regulated by a single transcription factor. For example, in addition to pavement cell differentiation (see above), TCP4 regulates trichome branching by directly activating GLABROUS INFLORESCENCE STEMS, which inhibits trichome branching independent of the endoreduplication pathway (Vadde et al., 2018; Figure 2). Therefore, coordinated differentiation of multiple epidermal cell types may involve shared transcription factor modules.
Coordinated genetic regulation of leaf epidermal cells
Very little evidence has been produced regarding the integration and antagonism between gene regulatory networks governing development of the different epidermal cell types. In the leaf protoderm, the sequence of cellular specification is unclear, but the rigid evenly-spaced activation of trichome fate by the GL1–GL3/EGL3–TTG1 complex seems to be unaffected by stoma position, suggesting that trichome initiation may precede (or supersede) the formation of the other, more plastic cell types. It would logically follow that nontrichome cell types are able to differentiate into meristemoids and enter the stomatal lineage pathway by activation/stabilization of SPCH, or, in the absence of SPCH, differentiate into pavement cells. This sequential fate segregation logic implies a mutually exclusive, hierarchical role for master regulatory transcription factors in the protoderm.
However, phenotypic analyses of mutants in which individual developmental pathways are affected have highlighted a complex regulatory relationship that may fine-tune the balance between epidermal cell types. For example, constitutive activation of SCRM in the scrm-D mutant, which promotes the accumulation of stomatal bHLH master regulatory heterodimers, produces leaves composed entirely of stomata, suggesting that stomata specification is not subsequent to trichome patterning (Kanaoka et al., 2008). Moreover, the SPCH protein directly binds to the promoter regions for and upregulates the transcription of the trichome-associated factors MYC1 and ENHANCER OF TRY AND CPC3 (ETC3), a seemingly contradictory finding that suggests SPCH may function to initiate both trichome and stomatal lineage cell development (Adrian et al., 2015). However, spch null mutants, which are devoid of stomata, produce trichomes in the epidermis of rosette leaves (MacAlister et al., 2007; Hara et al., 2009). Interestingly, trichome-associated factor ETC2 is highly expressed in stomata and stomatal lineage cells and loss of MYC1 results in slightly higher stomatal index (Adrian et al., 2015), suggesting further overlap. On the contrary, cotyledons of etc2 mutants and try cpc etc2 triple mutants do not appear to show stomatal patterning defects (Kirik et al., 2001). These conflicting observations suggest that the molecular logic underlying these pathways may have once been integrated, but have since become uncoupled as the differentiation pathways diverged.
In contrast, a very recent study in maize proposed that leaf epidermal stomata and trichomes share a common precursor cell, and the two fates are segregated by the activity of SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE proteins ZmSPL10/14/26 in incipient trichomes. In ZmSPL10/14/26 triple mutants, the epidermis not only lacks trichomes, but the cells that would normally become trichomes instead differentiate into stomata, suggesting that stomatal development may be the default in maize (Kong et al., 2021).
Intriguingly, a reciprocal relationship is observed in Brachypodium, whereby stomataless mutants, which lack the functional SCRM ortholog BdICE1, produce other epidermal cell types normally, including hairs, pavement cells, and silica cells (Raissig et al., 2016). In Arabidopsis, a failure to maintain trichome fate by the ectopic expression of cyclin-dependent kinase inhibitor (CKI) KIP-RELATED PROTEIN1 results in transdifferentiation from trichomes into pavement cell-like cells (Bramsiepe et al., 2010). These lines of evidence suggest that different species and tissues likely specify a default ground cell, and that the core regulatory logic underlying each of these different cell fates is at least partially integrated.
The pavement cell may represent the ground state cell. For instance, the spch mutant epidermis differentiates jigsaw puzzle-shaped pavement cells despite the absence of meristemoids and stomata (MacAlister et al., 2007; Pillitteri et al., 2007). Yet, SPCH activities are required to increase the pavement cell numbers via supplying SLGCs (see above), suggesting regulatory overlap with the stomatal lineage. Additionally, studies have shown that pavement cells share downstream morphogenetic factors with trichomes, including the transcription factor TCP4 and the small GTPase family Rho of Plants (ROPs; Fu et al., 2002, 2005) (see Figure 2 and “Genetic Coordination of Pavement Cell Interdigitation” below).
Genetic coordination of pavement cell interdigitation
To date, investigations into molecular signaling in pavement cells have primarily focused on the regulation of the unique puzzle shape they form in Arabidopsis. Our current understanding of the genetic regulation of pavement cell shape primarily centers on two distinct ROP signaling pathways: one controlling the formation of lobe regions and the other restricting growth of the neck regions of the cell perimeter in Arabidopsis. In the lobes, the redundant ROPs 2 and 4 are thought to promote cell expansion by inducing local cortical F-actin assembly via the activation of the ROP-interactive CRIB motif-containing (RIC) protein, RIC4 (Fu et al., 2005, 2009; Figure 2). Downstream of ROP2/4-RIC4, the suppressor of cyclic AMP receptor/Wiskott–Aldrich syndrome protein family verprolin homologous (SCAR/WAVE) complex activates the actin-related protein2/3 (ARP2/3) complex (Frank et al., 2004; Basu et al., 2005; Zhang et al., 2008), a nucleator of actin filament polymerization (Le et al., 2003; Li et al., 2003; Mathur et al., 2003; Figure 2). On the other hand, in the neck regions, ROP6 is thought to locally inhibit cell growth by activating RIC1, which interacts with the KATANIN1 subunit of the microtubule-severing enzyme katanin, thereby promoting cortical transverse microtubule alignment (Fu et al., 2009; Lin et al., 2013; Figure 2).
Upstream of ROPs, the guanine nucleotide exchange factor (GEF) SPIKE1, a member of the dedicator of cytokinesis GEF family, is thought to act as an activator of these signaling pathways during pavement cell shape regulation (Qiu et al., 2002; Basu et al., 2008; Figure 2). Importantly, evidence suggests that the ROP2/4-RIC4 and ROP6-RIC1 mechanisms are antagonistic, with ROP2 inhibiting RIC1-induced microtubule alignment in the lobes and RIC1 suppressing ROP2-RIC4 in the necks (Fu et al., 2005, 2009; Figure 2). This provides feedback-mediated coordination of the spatial regulation needed for seamless interdigitation of neighboring pavement cells.
It should be noted that a role for these ROP2/4-RIC4 and ROP6-RIC1 antagonistic mechanisms in lobe initiation has been challenged recently by a failure to observe stable microtubule arrays in future neck regions or a pavement cell phenotype in a ric4 mutant (Belteton et al., 2018). When considering the roles of regulatory mechanisms in pavement cell shape, it is rather difficult to dissect the initial lobe initiation event from subsequent expansion and maintenance of the lobe. Whether ROP6-RIC1-mediated alignment of cortical microtubules, which is in turn thought to lead to alignment of cellulose microfibrils in the cell wall and consequent growth inhibition at the neck region (Panteris and Galatis, 2005; Liu et al., 2021), actually leads to lobe initiation, controls expansion of a preexisting lobe, or a combination of both, remains rather controversial. As discussed later (see “Mechanical Modeling at the Cellular Scale”), cellulose microfibril alignment is only one of several means by which the cell wall is proposed to drive pavement cell shape, and a more detailed understanding of the steps of lobe initiation and expansion is needed to fully understand the roles of these regulatory mechanisms in generating the jigsaw puzzle shape.
Trichome morphogenesis
The fundamental mechanisms of trichome morphogenesis mirror those of pavement cells, with important roles for the spatial organization of microtubules and cortical actin filaments (Figure 2). The regulation of incipient branchpoints requires ZWICHEL (ZWI), a kinesin-like protein (Oppenheimer et al., 1997). zwi mutants occasionally produce unbranched trichomes, which can be partially rescued by treatment with microtubule-stabilizing drugs (Mathur and Chua, 2000). Extension of trichome branches requires proper cortical actin polymerization, as a suite of loss-of-function mutants exhibiting F-actin organization defects have been isolated on the basis of short, stunted, and “distorted” trichomes. In fact, DISTORTED (DIS) genes encode components of the Arp2/3 complex, including DIS1/ARP3, DIS2/ARPC2, and WRUM1 (WRM1)/ARP2 (Le et al., 2003; El-Din El-Assal et al., 2004). Likewise, a loss-of-function mutation in a gene encoding the SCAR/WAVE complex subunit, IRREGULAR TRICHOME BRANCHED1, results in distorted trichomes (Zhang et al., 2005; Figure 2). Like pavement cells, trichome morphology becomes defective when ROP2 activity is misregulated (Fu et al., 2002). Here, ROP2 regulates directional cell expansion via organizing F-actin arrangements during the early phase of trichome growth, when microtubules are not yet aligned transversely (Fu et al., 2002).
Stoma and guard cell morphogenesis
Unlike pavement cells and trichomes, mature guard cells constituting a stoma must perform reversible turgor-based movement to open and close the stomatal aperture. Owing to the importance of stomatal movement for photosynthesis and water-use efficiency, stomatal mechanics have been modeled extensively (Woolfenden et al., 2017). The morphogenesis and function of stomata rely on cytoskeletal and cortical microtubule dynamics as well as cell wall remodeling (Aylor et al., 1975). Microtubule arrays during stomatal development have been characterized in detail using GFP-labeled α-tubulin (Lucas et al., 2006). During the transition from triangular-shaped meristemoids to round GMCs, cortical microtubule arrays remodel dramatically from mesh-like to longitudinal, forming a preprophase band orienting the symmetric division site. Following the symmetric division, the microtubules within each immature guard cell exhibit radial patterns with mirror symmetry. A characteristic cell wall thickening occurs during the final stage of differentiation, which is critical for stomatal morphology (Lucas et al., 2006) (see “Mechanical Modeling at the Cellular Scale” for further discussion of the cell wall role).
Molecular-genetic regulation of stomatal morphogenesis is not fully understood. The key bHLH driving GMC differentiation, MUTE, significantly induces a suite of genes involved in microtubule-based movement (Han et al., 2018). Importantly, MUTE upregulates MUSTACHE (MUS), which encodes a receptor-like kinase required for proper microtubule movement and bilateral symmetry of stomata. Loss-of-function mus mutants exhibit deformed stomata with irregularly shaped guard cells and occasional incomplete pore formation (Keerthisinghe et al., 2015). The later-acting Dof transcription factor gene STOMATA CARPENTER1 (SCAP1) is required for proper guard cell morphogenesis, and its loss-of-function mutation confers skewed stomata with impaired function (Negi et al., 2013). Importantly, SCAP1 controls the expression of genes regulating stomatal morphogenesis and movement, including those encoding a K+-channel and the cell wall remodeling enzyme pectin methyltransferase (Negi et al., 2013). A very recent study integrating experimental force measurement and finite element modeling of turgor-driven guard cell movement highlighted the critical role of the pectin modifying enzyme PECTATE LYASE2 in stomatal function (Chen et al., 2021). How the dynamic force generated by individual stomata influences, or is influenced by the physiology and function of the entire leaf epidermis is an important future question.
Cell cycle in the leaf epidermis
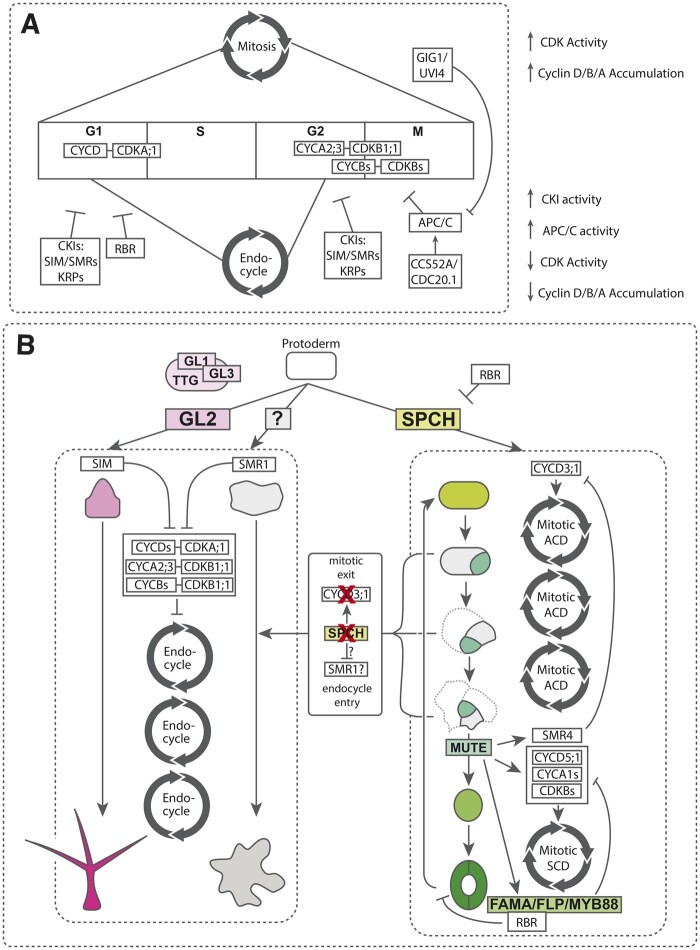
Each epidermal cell type requires a specific cell cycle mode to properly differentiate, and as such, cell cycle regulation is intricately woven into the developmental logic of the epidermis. Two primary cell cycle modes are employed in the developing epidermis: mitosis and endoreduplication. On the one hand, canonically, mitosis drives proliferative divisions. On the other hand, endoreduplication is a truncated version of the mitotic program in which cells repeatedly undergo DNA synthesis without cytokinesis, generating a single polyploid cell. The ploidy shift is concomitant with an increase in cellular volume and is associated with the development of large cell types (Li et al., 2012; Tsukaya, 2013; Robinson et al., 2018). Of the three mature Arabidopsis epidermal cell types discussed herein, pavement cells and trichomes require endoreduplication to physically expand and properly differentiate, whereas stomatal precursors require complete mitotic cycles to maintain proliferative asymmetric divisions and to subsequently orchestrate a final symmetric division (Guimil and Dunand, 2007; Torii, 2021; Figure 3).
Figure 3.
Cell cycle control in the leaf epidermis. A, Schematic of core cell cycle components regulating mitosis (top) and endoreduplication (bottom). Endoreduplication is a truncated, alternate version of mitosis maintained by high CKI activity. B, Schematic of cell cycle integration with trichome (magenta, left), pavement cell (gray, center), and stoma (right, green) developmental pathways. See the text for specific details.
Formative division and bipotency
Stomatal meristemoids are initiated in the protoderm by the accumulation of the bHLH master transcription factor SPCH (MacAlister et al., 2007). Recent evidence has shown that SPCH directly upregulates the expression of CYCLIN D3;1 (CYCD3;1), which complexes with CYCLIN-DEPENDENT KINASE A;1 (CDKA;1) and stimulates the cell cycle by promoting the G1/S transition via CDKA-dependent phosphorylation of RETINOBLASTOMA-RELATED1 (RBR1) (Lau et al., 2014; Adrian et al., 2015; Figure 3). Both CYCD3;1 and CDKA;1 are required for stomatal precursor proliferation, and CYCD3;1 overexpression can induce ectopic divisions in endoreduplicating trichomes, suggesting that sufficient CYCD–CDKA activity can tip the cell cycle balance in favor of mitosis (Schnittger et al., 2002; Figure 3). CDKA;1 also directly phosphorylates SPCH, activating it and possibly stabilizing an SPCH–CYCD3–CDKA;1 feedback loop; however, RBR1, phosphorylated by CDKA;1, represses SPCH and stomatal lineage genes (Nowack et al., 2012; Weimer et al., 2012; Yang et al., 2015). Loss-of-function spch and cdka;1 mutants result in an epidermis composed solely of endoreduplicating pavement cells and trichomes (MacAlister et al., 2007; Weimer et al., 2012; Yang et al., 2015), suggesting that endoreduplication may be the “default” cell cycle mode in the epidermis and that early endocycle onset may be antagonistic to the initiation of stomatal development. A recent study has shown that SPCH may also promote the G2/M transition and suppress endocycle entry by directly inducing the expression of homologous components of the mammalian DREAM complex, SUPPRESSOR OF LLP1 1 (SOL1) and SOL2 (Simmons et al., 2019). Together, these data inform a model wherein SPCH promotes mitosis and prevents endoreduplication in meristemoids through the direct upregulation of both G1/S and G2/M-associated cell cycle components. In line with this, declined SPCH activity in nascent SLGCs likely disengages the mitotic program (Figure 3).
Proper regulation and function of the Anaphase Promoting Complex (APC/C) are critical for formative divisions within the stomatal precursors as well as proper fate segregation. For instance, a suppressor screen for loss-of-function mutants of CCS52A2, an activator of APC/C, identified CYCA3;4 as a substrate (Willems et al., 2020). The overexpression of CYCA3;4 conferred a highly divided leaf epidermis, indicating that downregulation of CYCA3;4 is critical for restriction of formative divisions. Conversely, loss of OMISSION OF SECOND DIVISION or UV-INSENSITIVE4, negative regulators of APC/C, leads to suppressed asymmetric division and inappropriate fate segregation in stomatal lineage cells, whereby cells develop mixed phenotypes resembling both pavement cells and stomata (Iwata et al., 2011; Figure 3). Exactly how stomatal fate acquisition and segregation are linked to these early cell cycle shifts remains an open question.
Coordination of terminal guard cell division and differentiation
MUTE drives the differentiation of meristemoids into GMCs and simultaneous occurrence of a final, single symmetric division to create a mature stoma. In order to do so, MUTE must modulate the existing meristemoid cell cycle dynamics to support the precise single terminal division (Han et al., 2021). A single CKI, SIAMESE-RELATED4 (SMR4) was identified as a direct MUTE target, which specifically interacts with CYCD3;1, thereby reducing SPCH-dependent CYCD3 activity and clearing the path to terminal division. Interestingly, loss of SMR4 function or temporally shifted SMR4 expression results in cells with abnormal morphologies, some displaying properties of both pavement cells and guard cells (Han et al., 2021), suggesting that proper stoma-pavement cell fate segregation requires tightly regulated cell cycle dynamics.
Additional direct MUTE targets include CYCD5;1, and CYCA2;3 and its partner CDKB1;1, which are components driving the symmetric division (Han et al., 2018). MUTE also drives the expression of FAMA and Myb proteins FLP and MYB88, which, together with the help of RBR1, repress the expression of CDKA;1, CYCA2;3, and CDKB1;1 in order to promptly halt the cell cycle after a single division (Xie et al., 2010; Hachez et al., 2011; Vanneste et al., 2011; Matos et al., 2014). In silico modeling predicted that the MUTE-FAMA/FLP network logic forms a type-1 incoherent feed-forward loop, capable of generating a single, narrow pulse of expression of cell cycle genes to orchestrate a single terminal division (Han et al., 2018). Experimental perturbations showed that this feed-forward loop is critical for producing a functional stoma with paired guard cells surrounding a pore.
Further, FAMA and FLP/MYB88 redundantly complex with RBR1 to direct polycomb-repressive complex-mediated chromatin remodeling in order to suppress the expression of cell cycle genes and SPCH, terminally locking down both cell fate and cell cycle, thereby preventing fate reversion (Lee et al., 2014a, 2014b; Matos et al., 2014; Ruijtenberg and van den Heuvel, 2016; Figure 3). Expression of FAMA with a mutated RBR binding motif induced a stoma-in-stoma phenotype, whereby mature guard cells reverted to a meristematic state—dividing, expressing early meristemoid markers, and eventually generating a secondary stoma within the first (Matos et al., 2014). Similarly, ectopic expression of CYCA2;3 in maturing guard cells under control of the FAMA promoter was sufficient to restore both mitotic cell cycles and expression of the stomatal precursor marker TMM, suggesting that even in differentiated cells, specific cell cycle components remain poised and tightly integrated into the stomatal differentiation program (Yang et al., 2014). Counterintuitively, loss of CYCA2;3-CDKB1;1 activity in GMCs prevents the final mitotic division, but does not seem to interfere with differentiation, producing single, and round guard cells (Boudolf et al., 2004). In contrast, the presence of MUTE and GMC differentiation can be disconnected in sol1/2 mutants (Simmons et al., 2019). This may reflect a dependence on M-phase cyclins or suggest that the DREAM complex has targets within the stomatal differentiation pathway. Together, these results highlight the complex relationship between differentiation programs and the cell cycle in stomata.
Endoreduplication: trichomes and pavement cells
Trichomes emerge from the protoderm of rosette leaves following endocycle entry and fate induction by the homeodomain protein GL2, a master trichome regulator (Figure 2). Both endoreduplication and GL2 are required for proper trichome formation, and interestingly, GL2 expression itself may depend on endoreduplication to reach sufficient levels to ensure trichome fate maintenance (Bramsiepe et al., 2010). Mature trichomes require endoreduplication for both cell expansion and morphology, as the number of trichome branches is directly dependent on ploidy (Melaragno et al., 1993; Hulskamp et al., 1994; Perazza et al., 1999; Kirik et al., 2001).
The trichome endocycle is most likely initiated by the CKI SIAMESE (SIM), a direct target of the GL1–GL3–TTG1 complex (Figure 3). Indeed, SIM is required for trichome endocycle entry, and is sufficient to induce endoreduplication when ectopically expressed. The antagonism between mitotic and endoreduplication cycles can be illustrated by ectopic CYCD3;1 expression in trichomes, which results in multicellular trichomes resembling those found in the sim loss-of-function mutant (Schnittger et al., 2002). SIM, as well as its pavement cell-associated paralog SMR1 have been shown to interact with and inactivate a variety of CYC–CDK targets at each phase of the cell cycle, including CYCDs–CDKA;1, CYCA2;3–CDKB1;1, and CYCBs–CDKBs, reducing overall mitotic CDK activity (Walker et al., 2000; Churchman et al., 2006). This likely contributes to a reduced transcriptional repression of CCS52A1/2, which activates APC/C, thereby stabilizing endocycle entry (Figure 3). Indeed, overexpression of CCS52A1/2 is sufficient to drive early endocycle onset in the epidermis (Larson-Rabin et al., 2009; Kasili et al., 2010).
While very little information is available regarding the interplay between differentiation and cell cycle in pavement cells, accumulating evidence has shown that the E2F family and their dynamic binding partner RBR1 are involved in the direct regulation of noncell cycle genes including expansins, which are required for the loosening of cell wall architecture during pavement cell expansion (Ramirez-Parra et al., 2004; Magyar et al., 2012). Together, these findings inform a tightly integrated cell cycle–differentiation network in the epidermis, by which multiple transcriptional and posttranslational inputs likely converge to regulate each core component in order to direct cell-specific developmental outcomes.
Hormonal regulation of leaf epidermal growth
At the interface of a plant and the environment, leaf epidermal development is influenced by numerous environmental conditions, including light, drought, and pathogen attack, and endogenous plant hormones are utilized to respond to such environmental conditions to optimize plant growth and survival (Hauser, 2014; Qi and Torii, 2018). Here, we describe how hormones regulate leaf epidermal cell growth. Among the plant hormones, auxin has essential functions in the epidermis, both promoting cell division through the upregulation of cell cycle components, and promoting cell growth, which it mediates through the enhancement of cell wall loosening and extension. This is achieved partly via the activation of gene expression, including a range of cell wall-related genes (Majda and Robert, 2018), and partly via a process referred to as the “acid growth theory,” which suggests that auxin induces activation of plasma membrane-localized proton pumps that acidify the apoplast (Rayle and Cleland, 1977), together leading to activation of various cell wall proteins and enzymes involved in cell wall softening and extension (Majda and Robert, 2018). In particular, the low apoplastic pH induced by auxin activates expansins, xyloglucan endohydrolases, and cellulases, which loosen connections between cellulose microfibrils, xyloglucans, and other polysaccharides within the cell wall (Majda and Robert, 2018). Auxin-induced acidification also affects cell wall stiffness by inducing pectin methylesterase, which demethylesterifies homogalaturonans (Liu et al., 2021). This highlights the role of pectin polymer modifications in diverse epidermal cell morphogenesis (see “Stoma and Guard Cell Morphogenesis”).
Cytokinin is another hormone controlling epidermal growth, as a major regulator of the cell cycle that plays important role in epidermal cell proliferation during leaf expansion (Werner et al., 2001). In the epidermis of developing Arabidopsis leaves, a biphasic role for cytokinin has recently been demonstrated, whereby cell expansion is inhibited during the cell proliferation phase and promoted during the cell expansion phase (Skalák et al., 2019). The spatial, temporal, and concentration-dependent effects of plant hormones, as well as complex interplay among them, enables fine-tuning of complicated developmental processes in the leaf epidermis. As examples, we highlight our current understanding of the roles of various plant hormones in leaf epidermal development with particular focus on the regulation of pavement cell shape and the coordination of epidermal cell patterning.
Hormones regulate pavement cell shape
As auxin is a master regulator of plant growth and development, a major role for auxin in the generation of puzzle-shaped cells is hardly surprising. Auxin is thought to regulate both general pavement cell growth and the formation of the puzzle shape formed by these cells in Arabidopsis, as evidenced by the morphological effects of auxin treatments and auxin-related mutations (Wilmoth et al., 2005; Xu et al., 2010; Grones et al., 2015, 2020). Fluctuating cell-to-cell gradients of auxin response have recently been demonstrated to occur within the spirals of anisocytic stomatal complexes (Grones et al., 2020). These gradients ascend in the direction of increasing SLGC age throughout the spiral when the youngest SLGC is in a nonlobed stage. However, once the youngest SLGC has formed its first lobe, the presence and direction of the gradient becomes rather random, suggesting that fluctuations in these auxin response gradients may regulate first lobe formation in the youngest SLGCs (Grones et al., 2020). The ROP-RIC regulation of lobe and neck formation in developing pavement cells has also been linked to auxin, which is thought to activate ROP2 and RIC4 on the lobe side, and ROP6 on the neck side, leading to growth promotion and inhibition, respectively (Xu et al., 2010, 2014; Figure 2). TRANSMEMBRANE KINASE1 is a receptor-like kinase that has been shown to mediate auxin signaling (Cao et al., 2019) and in particular the auxin-dependent activation of ROP2 and ROP6 at the plasma membranes of pavement cells during interdigitation (Xu et al., 2014; Pan et al., 2020).
Carrier-directed transport of auxin is proposed to be the main process driving auxin gradient formation and polarity establishment during pavement cell interdigitation, with a range of Arabidopsis auxin carrier mutants displaying defective pavement cell shapes (Xu et al., 2010; Guo et al., 2015; Grones et al., 2020). Dynamic localization patterns of auxin carriers such as PIN-FORMED3 (PIN3) and AUXIN-RESISTANT1 on the plasma membranes of anisocytic stomatal complex cells have been linked to the establishment and fluctuations of the auxin gradients involved in SLGC first lobe formation (Grones et al., 2020). Furthermore, polarized localization of PIN1 has been observed at the tips of developing pavement cell lobes (Xu et al., 2010; Li et al., 2011; Guo et al., 2015), which has been suggested to direct the accumulation of extracellular auxin in this region, leading to activation of ROP2/4 and promotion of lobe outgrowth. However, a recent study failed to observe pavement cell phenotypes in cotyledons of several PIN-affected mutants (Belteton et al., 2018). Furthermore, this and a previous study failed to observe polarized localization of PIN1 at cotyledon lobe tips (Le et al., 2014; Belteton et al., 2018).
It has been suggested that differences in the mechanisms regulating pavement cell shape depending on the cell location, developmental stage, and plant growth conditions may explain these apparent discrepancies (Liu et al., 2021). PIN family members display high redundancy, therefore other PIN proteins, or even other auxin carrier family members, might take over this role in tissues from which PIN1 is absent. Furthermore, whether an extracellular accumulation of auxin at lobe tips might play a role in lobe initiation or in the expansion of existing lobes has generated debate. Although many details are still lacking, what does seem clear is that tightly regulated auxin transport plays an important role in regulating the complicated jigsaw puzzle shape of Arabidopsis pavement cells.
Apart from auxin, it is likely that a range of other phytohormones also directly or indirectly play important roles in pavement cell shape regulation. Cytokinin appears to act antagonistically to auxin as an inhibitor of pavement cell lobing, upstream of ROPs (Li et al., 2013). Additionally, brassinosteroids have recently been shown to mediate microtubule stabilization during pavement cell shape regulation (Liu et al., 2018). It is therefore likely that crosstalk among various hormonal pathways plays important roles in coordinating the synchronous interdigitation of pavement cells during leaf growth.
The roles of hormones in coordinating epidermal cell patterning
As dynamic signals that can move from cell to cell and regulate various cellular processes depending on their concentration, it seems logical that hormones should play major roles in coordinating the patterning and development of the various leaf epidermal cell types. Dynamically fluctuating auxin gradients in anisocytic stomatal complexes have been suggested to coordinate the division and development of the different cell types within the complexes (Grones et al., 2020) (see “Hormones Regulate Pavement Cell Shape”). An auxin minimum is proposed to stimulate the transition of the meristemoid into the GMC, resulting in a symmetric cell division (SCD), with auxin levels reducing even further within the young guard cells (Le et al., 2014). Simultaneously, a low auxin level resulting from the efflux of auxin from the youngest SLGC into the older SLGCs of the spiral induces the formation of the first lobe in the youngest SLGC at a specific position opposite to the neighboring meristemoid or stoma (Grones et al., 2020). After formation of the first lobe, auxin may then be redirected back toward the youngest SLGC to raise the auxin level, potentially inhibiting lobe expansion (Grones et al., 2020). In this way, auxin likely plays a highly dynamic, key role in the patterning and shaping of the epidermal tissue in space and time.
Mechanical modeling of epidermal morphogenetic dynamics
Ongoing research is just beginning to shed light on the interwoven nature of epidermis development, whereby individual cells influence tissue pattering and the tissue as a whole reciprocally influences individual cells. As already discussed, harmonized fate, patterning, and morphogenesis amongst the epidermal cell types are partly achieved via shared and coordinated molecular and hormonal signaling. Furthermore, mechanical forces within the individual cells, which reciprocally affect stress patterns across the tissue as a whole, are also being revealed as key players in epidermal cell coordination. Mechanical modeling of epidermal morphogenetic dynamics is increasingly being employed in order to understand and predict these complex behaviors. Here, we describe multiple scale modeling approaches that have helped to form an integrated understanding of epidermal cell and tissue development in cotyledons and leaves.
The mechanical role of the epidermis
Since cells are interconnected within tissues, their growth needs to be highly coordinated with the growth of their neighbors. Individual cells both contribute to and are subjected to mechanical signals and forces, forming stress patterns at the tissue and organ scales. The plant epidermis, as the most external tissue monolayer, is thought to be under considerable tension from the turgor pressure of the cells within. Early studies showed that a stem sliced lengthwise will bend outwards, suggesting that the epidermal cells are under tension (Kutschera and Niklas, 2007). The role of mechanical forces acting at the organ level was further tested in Arabidopsis cotyledons, showing that small cuts on the margins caused the organ to split open due to tensional stress (Sampathkumar et al., 2014a). These observations suggest that epidermis limits the growth of underlying tissues, thereby playing an important role in controlling emergent shape (Savaldi-Goldstein et al., 2007; Bai et al., 2010; Dyson et al., 2014).
To investigate the role of mechanical stress, different techniques have been used. Cell ablations have been performed at the shoot apical meristem (SAM; Hamant et al., 2008), and in cotyledons (Sampathkumar et al., 2014a) and sepals (Hervieux et al., 2016). Mechanical stress has been induced by stretching or compressing hypocotyls (Hejnowics et al., 2000), and by mechanical compression of the SAM (Louveaux et al., 2016). These studies lend support to the idea that plants are able to sense and respond to applied stresses. However, most experiments aimed at changing forces also create a change in cell geometry.
Mechanical modeling at the cellular scale
Tissue-level responses are ultimately controlled by cells, and the mechanics that operate at the cellular level. Turgor pressure generates tensile stress on cell walls that need to be able to grow while still maintaining their structural integrity. The process of making the elastic strain induced by turgor plastic is called cell wall extensibility, and is accomplished by the action of cell wall loosening compounds (Cosgrove, 2018). Thus, growth is a combination of two factors, the elastic strain induced by turgor that depends on cell wall stiffness and geometry, combined with the action of cell wall modifiers that induce extensibility. Cell wall loosening alone would cause the wall to get thinner. To maintain the wall thickness, new polysaccharides must be synthesized and integrated within the matrix. One possibility is that cells are able to sense the changes in the cell wall taking place over growth, either sensing increased cell wall stress or reduced cell wall integrity. It should be mentioned that it is not possible to measure stress directly; rather, it must be deduced from the strains it causes. Trinh et al. (2021) recently reviewed possible mechanotransduction pathways sensing mechanical forces. Potential mechanisms could involve cellulose microfibril loosening and modifications in pectin composition, opening of mechanosensitive channels in the plasma membrane, or changes in cytoskeleton configuration. Another possibility is the cortical microtubules themselves, as they are thought to be more stable and less prone to catastrophe when under tension (Saltini and Mulder, 2021), which could cause them to align with the principal directions of stress (Hejnowics et al., 2000; Hamant et al., 2008).
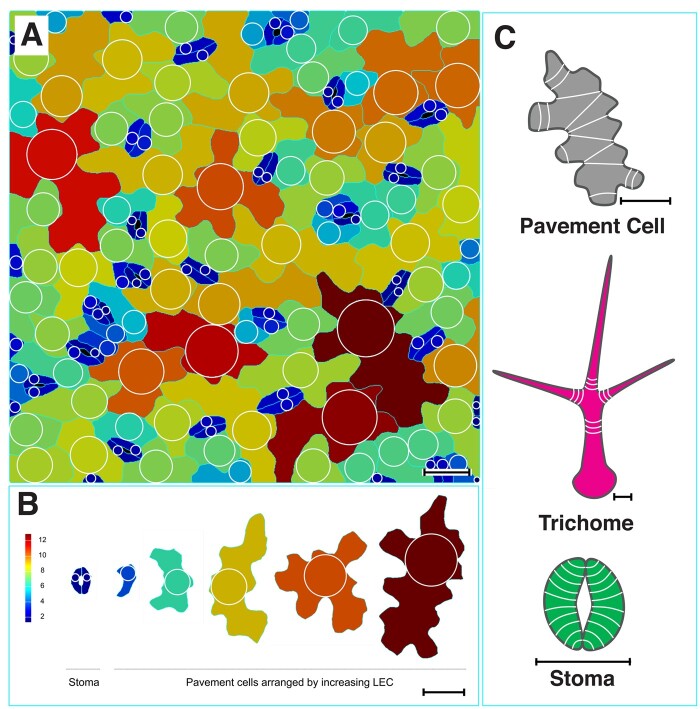
Physical micro-indentation and computational modeling of isolated spherical or elongated cells with uniform cell wall properties demonstrated that stress tends to be cell-shape and cell-size dependent. In spherical cells, stress increases linearly with size. However, elongated cells can become very large (long) without a significant increase in stress, as stress is highest around the cell circumference (Weber et al., 2015; Mosca et al., 2017; Sapala et al., 2018). Mechanical modeling of pavement cells demonstrated that lobed cells display significantly lower stress than equivalent-sized cells with simple shapes (Sapala et al., 2018). This suggests that interdigitation of initially isodiametrically growing cells, such as Arabidopsis leaf epidermal cells, may function in preventing excessive stress as the cells expand. This concept can be visualized by observing the size of the largest empty circle that fits inside each cell, which is a good proxy for the maximal stress in the walls of the cell. This stress can be greatly reduced by the formation of a lobed perimeter (Figure 4, A and B). This measure can also be applied to trichomes, where stress is predicted to be highest at the cell base and lowest at the tips of the branches (Kierzkowski and Kierzkowska, 2019).
Figure 4.
Mechanical stress control of epidermal cell shape. A, Cells segmented in MorphoGraphX (Barbier de Reuille et al., 2015) with each large empty circle (LEC) visualized. B, Cells arranged by the size of the LEC, which indicates the magnitude of stress. Cells in (A) and (B) are colored according to the LEC, from cells with small LEC (2–4) in blue to those with large LEC (10–12) in red or brown. C, The stress patterns in the different cell types (white lines), which correspond with the localization patterns of cortical microtubules and cell wall cellulose microfibrils. Scale bars represent 20 µm.
As described earlier (see “Genetic Coordination of Pavement Cell Interdigitation”), a proposed mechanism of lobe formation in pavement cells is based on the occurrence of growth restriction zones around the perimeter, produced by the local accumulation of cortical microtubules (Fu et al., 2005; Panteris and Galatis, 2005). During the expansion of initially isodiametric cells, these restriction zones would lead to the formation of indentations resulting in interdigitated shapes (Panteris and Galatis, 2005). The stress patterns arising from cell and tissue shape may be what drives the accumulation of the cortical microtubules, which subsequently direct the deposition of cellulose microfibrils (Sampathkumar et al., 2014b; Figure 4C). Localized cellulose deposition stiffens the cell wall, limiting growth where stress is high. Cells would then expand where the stress is lower and the wall softer. After the lobes are formed, the interdigitation may be further enhanced by a feedback mechanism in which local, multi-cell geometry forms new stress patterns that are reciprocally reinforced by the dynamic alignment of cortical microtubules and cell wall microfibrils (Sampathkumar et al., 2014b). A similar mechanism could be associated with growing trichomes. The combination of confocal time lapse microscopy and computational modeling showed that the regions of high stress and growth restriction in trichomes are overlaid by cortical microtubules (Yanagisawa et al., 2015; Figure 4C). Interestingly, the mechanical stress imposed by early emerging trichomes in sepals alters the stress patterns in adjacent cells in much the same way as an ablation (Hervieux et al., 2017), as the faster growing trichrome transfers load onto its neighbors. The neighbor cells respond by reorienting their cortical microtubules to align with a new pattern of stress, suggesting a stress-responsive mechanism in the absence of any ablation-induced wounding.
The idea that it is primarily growth restriction that drives cell shape was further tested in a 2D computational model of lobe formation (Sapala et al., 2018). Growth restrictions were placed across the cell to represent the action of cortical microtubules directing cellulose deposition. The restrictions accumulated in the indentations and were reduced in the protrusions, causing lobes and necks to be enhanced in a feedback mechanism based on the local geometry of the cell (Sapala et al., 2018). In this model, the alternating pattern of lobes and necks results purely from the curvature of the cell wall, and the growth restriction in one cell necessarily causes a protrusion in its neighbor without requiring mobile chemical signals. However, a chemical signal is thought to coordinate the ROPs from lobes and indentations in neighboring cells, and modeling work has shown that such a process provides a general framework for coordinated cell polarity (Abley et al., 2013). In the model by Sapala et al. (2018), the formation of elongated or puzzle-shaped cells depends on the amount and anisotropy of growth of the cells. This may provide an explanation for the conflicting results generated when investigating the effects of hormones such as auxin on cell shape. The model predicts that hormones and genes change growth rates and anisotropy in subtle ways, which would, in turn, have subtle effects on puzzle cell shape, and may not be directly involved in the patterning process at all.
One of the reasons for the diversity in plant cell shape is the heterogeneous properties of cell walls, which could be associated with the differential ability to stretch beyond a yield point (Zhang et al., 2021). Although the roles of cortical microtubules and cellulose microfibrils in cell shape formation are well established, the roles of the matrix polysaccharides are largely unknown. A model by Majda et al. (2017) demonstrates that lobing of initially straight anticlinal cell walls (perpendicular to the leaf surface) of neighboring pavement cells requires local cell wall mechanical inhomogeneities along and across the cell wall. Although this model does not consider the periclinal cell walls (parallel to the leaf surface), it could account for the initial mechanisms underlying lobe formation, which could be further amplified by other processes such as feedback from the stress patterns, distribution of cortical microtubules, and cell geometry (Sampathkumar et al., 2014a; Sapala et al., 2018; Majda et al., 2019). More recently, a 3D model of pavement cells was proposed that consists of stiff and soft sections alternating across the width of periclinal walls and stiff bands on anticlinal walls (Bidhendi et al., 2019). The model shows that extending the mechanical heterogeneities from anticlinal to periclinal walls can induce the cells to lobe. Interestingly, the local mechanical inhomogeneities and changes in cell geometry are preceded by buckling of the cell wall, which could trigger microtubule polarization and initiation of pavement cell interdigitation. It has recently also been suggested that puzzle cell shape formation could be the result of pectin swelling rather than by the turgor-driven strain relaxation model of growth (Haas et al., 2020); however, it is unclear if such a mechanism could result in sustained growth as cells increase to many times their original size during the process of forming lobes (Cosgrove and Anderson 2020).
In contrast to pavement cells or trichomes, which are associated with irreversible plastic deformation, stomatal function is an example of a temporal elastic change with large cell wall deformations. Stomatal opening and closing is a mechanical process that involves changes in the turgor pressure, which induce changes in cell geometry (Woolfenden et al., 2018). Nearly half a century ago, it was proposed that under high turgor pressure, the circumferential arrangement of cellulose microfibrils leads to the elongation of guard cells and stomatal pore opening (Aylor et al., 1975). The use of indentation and computational models has since demonstrated that the functional mechanics of stomata are based on the anisotropy of the cellulose microfibrils and polar stiffening of the stomatal wall. A 3D computational model of stomatal dynamics revealed that heterogeneous deformation of the cell walls and high mechanical stress at the tips connecting two neighboring guard cells drives stomatal function (Woolfenden et al., 2017). This was further tested in a model which showed that fixing the tips of guard cells led to changes in the wall properties critical for stomatal movement (Carter et al., 2017).
Conclusions and perspectives
As the leaf epidermis is the primary interface between a plant and the aerial environment, its critical functions must be robust to environmental challenges, and as sessile organisms, plants must be able to adapt their epidermal function to a broad range of harsh and variable environmental conditions. The identity, shape, and function of each cell in the epidermis is determined by a combination of overlapping molecular circuits, including differentiation pathways, cell cycle, mechanical signals, and phytohormone signaling. In Arabidopsis, although each epidermal cell type utilizes a specific regulatory module for its development, pavement cells, trichomes, and stomata are engaged in a tissue-wide series of molecular and mechanical conversations—each impacting the development of the other, and cooperatively shaping the epidermal landscape.
Despite the wealth of current knowledge on the specification of individual epidermal cell types, many outstanding questions remain. Importantly, which cells are “chosen” to obtain the ability to differentiate within the context of the epidermis as a whole? How do leaf organ-wide polarity and development impinge on specialized cell-type differentiation within the epidermis? What are the exact contributions of physiological, molecular, or mechanical signals that link epidermal development to underlying mesophyll and vasculature tissues? Most importantly, the cell types in the epidermis are more complex in some plant species owing to their environmental adaptations and developmental innovations. It will be critically important to apply what we have learned from model systems to other plant species.
The molecular-genetic studies of epidermal cell type differentiation in the past primarily focused on the core regulators, for which loss- or gain-of-function mutations confer “extreme” phenotypes (e.g. epidermis with excessive stomata, devoid of stomata, or devoid of trichomes). As the field moves forward to understand the modulation and re-wiring of developmental regulatory networks by mechanical, hormonal, and environmental inputs, quantitative analyses become increasingly needed to capture subtle and transient phenotypes at high spatiotemporal resolution. New quantitative analysis tools and pipelines have recently been developed, including polarity measurement (POME), which analyzes the stomatal lineage polarity (Gong et al., 2021), stomata patterning autocorrelation on epidermis (SPACE), which applies spatial autocorrelation statistics to quantify the probabilistic distribution of stomatal patterning (Zeng et al., 2020), and image analyses tools based on machine learning (Xie et al., 2021). Together with recent advances in single cell genomics with spatial reconstruction and high-resolution microscopy (Neumann et al., 2021), these new tools and approaches will allow for discrete spatiotemporal events at the cellular and subcellular level to be observed, dissected, and further integrated at the tissue level and beyond (i.e. whole organ/plant level). Many mechanisms underlying cellular plasticity in the epidermis have only recently become accessible/evident (Meyer et al., 2017; Han et al., 2018), as computational approaches provide novel insights into the regulation of poised and transient cell fates.
The increasing role of these powerful tools in developmental biology offers new perspectives into the molecular crosstalk between cellular events and cell types that were previously considered independent or unassociated. Furthermore, the expansion of plant model organisms and the application of these techniques under mimicked changing climate as well as across multiple species will continue to unravel the relationships between pathways controlling tissue patterning, providing new insights into the regulation and roles of cellular plasticity in the leaf epidermis. Understanding the flexibility of proliferation, growth, and development in epidermal cells is fundamental to predicting tissue-scale morphogenetic events and identifying new strategies to enhance plant fitness in a changing environment.
Contributor Information
Daniel T Zuch, Department of Molecular Biosciences, Howard Hughes Medical Institute, The University of Texas at Austin, Austin, Texas 78712, USA.
Siamsa M Doyle, Department of Forest Genetics and Plant Physiology, Umeå Plant Science Centre, Swedish University of Agricultural Sciences, Umeå 90183, Sweden.
Mateusz Majda, Department of Computational and Systems Biology, John Innes Centre, Norwich NR4 7UH, UK.
Richard S Smith, Department of Computational and Systems Biology, John Innes Centre, Norwich NR4 7UH, UK.
Stéphanie Robert, Department of Forest Genetics and Plant Physiology, Umeå Plant Science Centre, Swedish University of Agricultural Sciences, Umeå 90183, Sweden.
Keiko U Torii, Department of Molecular Biosciences, Howard Hughes Medical Institute, The University of Texas at Austin, Austin, Texas 78712, USA.
All authors contributed to initial brainstorming, writing, editing, presenting figures, and finalizing this review article.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are: Keiko U. Torii (ktorii@utexas.edu) and Stéphanie Robert (stephanie.robert@slu.se).
Funding
Our research is supported by the Howard Hughes Medical Institute and the Johnson & Johnson Centennial Chair from The University of Texas at Austin, Molecular Biosciences (to K.U.T.); Vinnova (Verket för Innovationssystem), and the Knut and Alice Wallenberg Foundation funding to Umeå Plant Science Centre (to S.R. and S.M.D.); Institute Strategic Programme Grant from the BBSRC to the John Innes Centre (BB/P013511/1) and ERA–CAPS project V-Morph, German Research Foundation DFG 355722357 (to R.S.S.).
Conflict of interest statement. The authors declare no conflict of interest.
References
- Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A (1995) Molecular characterization of the CER1 gene of arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 7: 2115–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Abley K, Barbier de Reuille P, Strutt D, Bangham A, Prusinkiewicz P, Marée AFM, Grieneisen VA, Coen E (2013) An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development 140: 2061–2074 [DOI] [PubMed] [Google Scholar]
- Adrian J, Chang J, Ballenger CE, Bargmann BO, Alassimone J, Davies KA, Lau OS, Matos JL, Hachez C, Lanctot A, et al. (2015) Transcriptome dynamics of the stomatal lineage: birth, amplification, and termination of a self-renewing population. Dev Cell 33: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylor DE, Parlange JY, Krikorian AD (1975) Comment on some recent models of stomatal mechanics. J Theor Biol 54: 395–397 [DOI] [PubMed] [Google Scholar]
- Bai Y, Falk S, Schnittger A, Jakoby MJ, Hülskamp M (2010) Tissue layer specific regulation of leaf length and width in Arabidopsis as revealed by the cell autonomous action of ANGUSTIFOLIA. Plant J 61: 191–199 [DOI] [PubMed] [Google Scholar]
- Barbier de Reuille P, Routier-Kierzkowska AL, Kierzkowski D, Bassel GW, Schupbach T, Tauriello G, Bajpai N, Strauss S, Weber A, Kiss A, et al. (2015) MorphoGraphX: a platform for quantifying morphogenesis in 4D. eLife 4: 05864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB (2008) A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci USA 105: 4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Le J, El-Essal Sel D, Huang S, Zhang C, Mallery EL, Koliantz G, Staiger CJ, Szymanski DB (2005) DISTORTED3/SCAR2 is a putative Arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. Plant Cell 17: 502–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW (1999) Development of the leaf epidermis. Curr Top Dev Biol 45: 1–40 [DOI] [PubMed] [Google Scholar]
- Belteton SA, Sawchuk MG, Donohoe BS, Scarpella E, Szymanski DB (2018) Reassessing the roles of PIN proteins and anticlinal microtubules during pavement cell morphogenesis. Plant Physiol 176: 432–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino LT, Caine RS, Gray JE (2019) Impact of stomatal density and morphology on water-use efficiency in a changing world. Front Plant Sci 10: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidhendi AJ, Altartouri B, Gosselin FP, Geitmann A (2019) Mechanical stress initiates and sustains the morphogenesis of wavy leaf epidermal cells. Cell Rep 28: 1237–1250 e1236 [DOI] [PubMed] [Google Scholar]
- Bird SM, Gray JE (2003) Signals from the cuticle affect epidermal cell differentiation. New Phytol 157: 9–23 [DOI] [PubMed] [Google Scholar]
- Boudolf V, Barroco R, Engler Jde A, Verkest A, Beeckman T, Naudts M, Inze D, De Veylder L (2004) B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell 16: 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramsiepe J, Wester K, Weinl C, Roodbarkelari F, Kasili R, Larkin JC, Hulskamp M, Schnittger A (2010) Endoreplication controls cell fate maintenance. PLoS Genet 6: e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann M, Bergmann DC (2017) Tissue-wide mechanical forces influence the polarity of stomatal stem cells in Arabidopsis. Curr Biol 27: 877–883 [DOI] [PubMed] [Google Scholar]
- Cao M, Chen R, Li P, Yu Y, Zheng R, Ge D, Zheng W, Wang X, Gu Y, Gelova Z, et al. (2019) TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568: 240–243 [DOI] [PubMed] [Google Scholar]
- Carter R, Woolfenden H, Baillie A, Amsbury S, Carroll S, Healicon E, Sovatzoglou S, Braybrook S, Gray JE, Hobbs J, et al. (2017) Stomatal opening involves polar, not radial, stiffening of guard cells. Curr Biol 27: 2974–2983.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavé-Radet A, Rabhi M, Gouttefangeas F, El Amrani A (2020) Do specialized cells play a major role in organic xenobiotic detoxification in higher plants? Front Plant Sci 11: 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa KR, Rath M, Nath U (2019) The CIN-TCP transcription factors promote commitment to differentiation in Arabidopsis leaf pavement cells via both auxin-dependent and independent pathways. PLoS Genet 15: e1007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li W, Turner JA, Anderson CT (2021) PECTATE LYASE LIKE12 patterns the guard cell wall to coordinate turgor pressure and wall mechanics for proper stomatal function in Arabidopsis. Plant Cell 33: 3134–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman ML, Brown ML, Kato N, Kirik V, Hulskamp M, Inze D, De Veylder L, Walker JD, Zheng Z, Oppenheimer DG, et al. (2006) SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 18: 3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (2018) Diffuse growth of plant cell walls. Plant Phys 176: 16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Anderson CT (2020) Plant cell growth: do pectins drive lobe formation in Arabidopsis pavement cells? Curr Biol 30: R660–R662 [DOI] [PubMed] [Google Scholar]
- Dong J, MacAlister CA, Bergmann DC (2009) BASL controls asymmetric cell division in Arabidopsis. Cell 137: 1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson RJ, Vizcay-Barrena G, Band LR, Fernandes AN, French AP, Fozard JA, Hodgman TC, Kenobi K, Pridmore TP, Stout M, et al. (2014) Mechanical modelling quantifies the functional importance of outer tissue layers during root elongation and bending. New Phytol 202: 1212–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din El-Assal S, Le J, Basu D, Mallery EL, Szymanski DB (2004) DISTORTED2 encodes an ARPC2 subunit of the putative Arabidopsis ARP2/3 complex. Plant J 38: 526–538 [DOI] [PubMed] [Google Scholar]
- Frank M, Egile C, Dyachok J, Djakovic S, Nolasco M, Li R, Smith LG (2004) Activation of Arp2/3 complex-dependent actin polymerization by plant proteins distantly related to Scar/WAVE. Proc Natl Acad Sci USA 101: 16379–16384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z (2009) A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol 19: 1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Nadeau J, Sack FD (2000) Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell 12: 2075–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover BJ (2000) Differentiation in plant epidermal cells. J Exp Bot 51: 497–505 [DOI] [PubMed] [Google Scholar]
- Gong Y, Varnau R, Wallner ES, Acharya R, Bergmann DC, Cheung LS (2021) Quantitative and dynamic cell polarity tracking in plant cells. New Phytol 230: 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Vanhaeren H, Inzé D (2012) Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci 17: 332–340 [DOI] [PubMed] [Google Scholar]
- Gray A, Liu L, Facette M (2020) Flanking support: how subsidiary cells contribute to stomatal form and function. Front Plant Sci 11: 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grones P, Majda M, Doyle SM, Van Damme D, Robert S (2020) Fluctuating auxin response gradients determine pavement cell-shape acquisition. Proc Natl Acad Sci USA 117: 16027–16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grones P, Chen X, Simon S, Kaufmann WA, De Rycke R, Nodzynski T, Zazimalova E, Friml J (2015) Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J Exp Bot 66: 5055–5065 [DOI] [PubMed] [Google Scholar]
- Guimil S, Dunand C (2007) Cell growth and differentiation in Arabidopsis epidermal cells. J Exp Bot 58: 3829–3840 [DOI] [PubMed] [Google Scholar]
- Guo X, Wang L, Dong J (2021a) Establishing asymmetry: stomatal division and differentiation in plants. New Phytol 232: 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Park CH, Wang ZY, Nickels BE, Dong J (2021b) A spatiotemporal molecular switch governs plant asymmetric cell division. Nat Plants 7: 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Qin Q, Yan J, Niu Y, Huang B, Guan L, Li Y, Ren D, Li J, Hou S (2015) TYPE-ONE PROTEIN PHOSPHATASE4 regulates pavement cell interdigitation by modulating PIN-FORMED1 polarity and trafficking in Arabidopsis. Plant Physiol 167: 1058–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Meyerowitz EM, Peaucelle A (2020) Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367: 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Ohashi-Ito K, Dong J, Bergmann DC (2011) Differentiation of Arabidopsis guard cells: analysis of the networks incorporating the basic helix-loop-helix transcription factor, FAMA. Plant Physiol 155: 1458–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant O, Heisler MG, Jönsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Han SK, Yang J, Arakawa M, Iwasaki R, Sakamoto T, Kimura S, Kim ED, Torii KU (2021) Deceleration of cell cycle underpins a switch from proliferative- to terminal division in plant stomatal lineage. bioRxiv, doi:10.1101/2021.05.17.442671 [DOI] [PMC free article] [PubMed]
- Han SK, Qi X, Sugihara K, Dang JH, Endo TA, Miller KL, Kim ED, Miura T, Torii KU (2018) MUTE directly orchestrates cell-state switch and the single symmetric division to create stomata. Dev Cell 45: 303–315 e305 [DOI] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T (2009) Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol 50: 1019–1031 [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MT (2014) Molecular basis of natural variation and environmental control of trichome patterning. Front Plant Sci 5: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnowics Z, Rusin A, Rusin T (2000) Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J Plant Growth Regulat 19: 34–44 [DOI] [PubMed] [Google Scholar]
- Hervieux N, Dumond M, Sapala A, Routier-Kierzkowska AL, Kierzkowski D, Roeder AHK, Smith RS, Boudaoud A, Hamant O (2016) A mechanical feedback restricts sepal growth and shape in Arabidopsis. Curr Biol S0960–9822: 30180–30184 [DOI] [PubMed] [Google Scholar]
- Hervieux N, Tsugawa S, Fruleux A, Dumond M, Kierzkowska AL, Komatsuzaki T, Boudaoud A, Larkin JC, Smith RS, Li CB, et al. (2017) Mechanical shielding of rapidly growing cells buffers growth heterogeneity and contributes to organ shape reproducibility. Curr Biol 27: 3468–3479.e4 [DOI] [PubMed] [Google Scholar]
- Ho CK, Bringmann M, Oshima Y, Mitsuda N, Bergmann DC (2021) Transcriptional profiling reveals signatures of latent developmental potential in Arabidopsis stomatal lineage ground cells. Proc Natl Acad Sci USA 118: e2021682118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst RJ, Fujita H, Lee JS, Rychel AL, Garrick JM, Kawaguchi M, Peterson KM, Torii KU (2015) Molecular framework of a regulatory circuit initiating two-dimensional spatial patterning of stomatal lineage. PLoS Genet 11: e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaert A, Zhang C, Tiwari M, Wang K, de Marcos Serrano A, Savatin DV, Urs MJ, Zhiponova MK, Gudesblat GE, Vanhoutte I, et al. (2018) POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature 563: 574–578 [DOI] [PubMed] [Google Scholar]
- Huchelmann A, Boutry M, Hachez C (2017) Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiol 175: 6–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulskamp M, Misra S, Jurgens G (1994) Genetic dissection of trichome cell development in Arabidopsis. Cell 76: 555–566 [DOI] [PubMed] [Google Scholar]
- Iwata E, Ikeda S, Matsunaga S, Kurata M, Yoshioka Y, Criqui MC, Genschik P, Ito M (2011) GIGAS CELL1, a novel negative regulator of the anaphase-promoting complex/cyclosome, is required for proper mitotic progression and cell fate determination in Arabidopsis. Plant Cell 23: 4382–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques E, Verbelen JP, Vissenberg K (2014) Review on shape formation in epidermal pavement cells of the Arabidopsis leaf. Fun Plant Biol 41: 914–921 [DOI] [PubMed] [Google Scholar]
- Javelle M, Vernoud V, Rogowsky PM, Ingram GC (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189: 17–39 [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu JK, Torii KU (2008) SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to arabidopsis stomatal differentiation. Plant Cell 20: 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasili R, Walker JD, Simmons LA, Zhou J, De Veylder L, Larkin JC (2010) SIAMESE cooperates with the CDH1-like protein CCS52A1 to establish endoreplication in Arabidopsis thaliana trichomes. Genetics 185: 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe S, Nadeau JA, Lucas JR, Nakagawa T, Sack FD (2015) The Arabidopsis leucine-rich repeat receptor-like kinase MUSTACHES enforces stomatal bilateral symmetry in Arabidopsis. Plant J 81: 684–694 [DOI] [PubMed] [Google Scholar]
- Kierzkowski D, Kierzkowska AL (2019) Cellular basis of growth in plants: geometry matters. Curr Opin Plant Biol 47: 56–63 [DOI] [PubMed] [Google Scholar]
- Kirik V, Bouyer D, Schobinger U, Bechtold N, Herzog M, Bonneville JM, Hulskamp M (2001) CPR5 is involved in cell proliferation and cell death control and encodes a novel transmembrane protein. Curr Biol 11: 1891–1895 [DOI] [PubMed] [Google Scholar]
- Kong D, Pan X, Jing Y, Zhao Y, Duan Y, Yang J, Wang B, Liu Y, Shen R, Cao Y, et al. (2021) ZmSPL10/14/26 are required for epidermal hair cell fate specification on maize leaf. New Phytol 230: 1533–1549 [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ (2007) The epidermal-growth-control theory of stem elongation: an old and a new perspective. J Plant Phys 164: 1395–1409 [DOI] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC (2008) Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks MD (1996) The control of trichome spacing and number in Arabidopsis. Development 122: 997–1005 [DOI] [PubMed] [Google Scholar]
- Larson-Rabin Z, Li Z, Masson PH, Day CD (2009) FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol 149: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Davies KA, Chang J, Adrian J, Rowe MH, Ballenger CE, Bergmann DC (2014) Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science 345: 1605–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, El-Assal Sel D, Basu D, Saad ME, Szymanski DB (2003) Requirements for Arabidopsis ATARP2 and ATARP3 during epidermal development. Curr Biol 13: 1341–1347 [DOI] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M, et al. (2014) Auxin transport and activity regulate stomatal patterning and development. Nat Commun 5: 3090. [DOI] [PubMed] [Google Scholar]
- Lee E, Lucas JR, Sack FD (2014a) Deep functional redundancy between FAMA and FOUR LIPS in stomatal development. Plant J 78: 555–565 [DOI] [PubMed] [Google Scholar]
- Lee E, Lucas JR, Goodrich J, Sack FD (2014b) Arabidopsis guard cell integrity involves the epigenetic stabilization of the FLP and FAMA transcription factor genes. Plant J 78: 566–577 [DOI] [PubMed] [Google Scholar]
- Lee JS, Hnilova M, Maes M, Lin YC, Putarjunan A, Han SK, Avila J, Torii KU (2015) Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LR, Bergmann DC (2019) The plant stomatal lineage at a glance. J Cell Sci 132: jcs228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin D, Dhonukshe P, Nagawa S, Chen D, Friml J, Scheres B, Guo H, Yang Z (2011) Phosphorylation switch modulates the interdigitated pattern of PIN1 localization and cell expansion in Arabidopsis leaf epidermis. Cell Res 21: 970–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu T, Lin D, Wen M, Xie M, Duclercq J, Bielach A, Kim J, Reddy GV, Zuo J, et al. (2013) Cytokinin signaling regulates pavement cell morphogenesis in Arabidopsis. Cell Res 23: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Blanchoin L, Yang Z, Lord EM (2003) The putative Arabidopsis arp2/3 complex controls leaf cell morphogenesis. Plant Physiol 132: 2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu E, Fan C, Zhang C, Fu T, Zhou Y (2012) Developmental, cytological and transcriptional analysis of autotetraploid Arabidopsis. Planta 236: 579–596 [DOI] [PubMed] [Google Scholar]
- Lin D, Cao L, Zhou Z, Zhu L, Ehrhardt D, Yang Z, Fu Y (2013) Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr Biol 23: 290–297 [DOI] [PubMed] [Google Scholar]
- Liu S, Jobert F, Rahneshan Z, Doyle SM, Robert S (2021) Solving the puzzle of shape regulation in plant epidermal pavement cells. Annu Rev Plant Biol 72: 12.11–12.26 [DOI] [PubMed] [Google Scholar]
- Liu X, Yang Q, Wang Y, Wang L, Fu Y, Wang X (2018) Brassinosteroids regulate pavement cell growth by mediating BIN2-induced microtubule stabilization. J Exp Bot 69: 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveaux M, Rochette S, Beauzamy L, Boudaoud A, Hamant O (2016) The impact of mechanical compression on cortical microtubules in Arabidopsis: a quantitative pipeline. Plant J 88: 328–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Porat R, Nadeau JA, O’Neill SD (1996) Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell 8: 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Nadeau JA, Sack FD (2006) Microtubule arrays and Arabidopsis stomatal development. J Exp Bot 57: 71–79 [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC (2007) Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445: 537–540 [DOI] [PubMed] [Google Scholar]
- Mader A, Langer M, Knippers J, Speck O (2020) Learning from plant movements triggered by bulliform cells: the biomimetic cellular actuator. J R Soc Interface 17: 20200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Horvath B, Khan S, Mohammed B, Henriques R, De Veylder L, Bako L, Scheres B, Bogre L (2012) Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO J 31: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majda M, Robert S (2018) The role of auxin in cell wall expansion. Int J Mol Sci 19: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majda M, Krupinski P, Jonsson H, Hamant O, Robert S (2019) Mechanical asymmetry of the cell wall predicts changes in pavement cell geometry. Dev Cell 50: 9–10 [DOI] [PubMed] [Google Scholar]
- Majda M, Grones P, Sintorn IM, Vain T, Milani P, Krupinski P, Zagorska-Marek B, Viotti C, Jonsson H, Mellerowicz EJ, et al. (2017) Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Dev Cell 43: 290–304 e294 [DOI] [PubMed] [Google Scholar]
- Mathur J, Chua NH (2000) Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12: 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Hülskamp M (2003) Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell 15: 1632–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos JL, Lau OS, Hachez C, Cruz-Ramirez A, Scheres B, Bergmann DC (2014) Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. eLife 3: e03271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW (1993) Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5: 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HM, Teles J, Formosa-Jordan P, Refahi Y, San-Bento R, Ingram G, Jonsson H, Locke JC, Roeder AH (2017) Fluctuations of the transcription factor ATML1 generate the pattern of giant cells in the Arabidopsis sepal. Elife 6: e19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca G, Sapala A, Strauss S, Routier-Kierzkowska AL, Smith SS (2017) On the micro-indentation of plant cells in a tissue context. Phys Biol 14: 015003. [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Negi J, Moriwaki K, Konishi M, Yokoyama R, Nakano T, Kusumi K, Hashimoto-Sugimoto M, Schroeder JI, Nishitani K, Yanagisawa S, et al. (2013) A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr Biol 23: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Xu X, Smaczniak K, Schumacher J, Yan W, Blüthgen N, Greb T, Jönsson H, Traas J, Kaufmann K, et al. (2021) A 3D gene expression atlas of the floral meristem based on spatial reconstruction of single nucleus RNA sequencing data. bioRxiv, doi.org/10.1101/2021.1106.1130.450319 [Advance access publication on 1 July 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack MK, Harashima H, Dissmeyer N, Zhao X, Bouyer D, Weimer AK, De Winter F, Yang F, Schnittger A (2012) Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev Cell 22: 1030–1040 [DOI] [PubMed] [Google Scholar]
- Nunes TDG, Zhang D, Raissig MT (2020) Form, development and function of grass stomata. Plant J 101: 780–799 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC (2006) Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18: 2493–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DG, Pollock MA, Vacik J, Szymanski DB, Ericson B, Feldmann K, Marks MD (1997) Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc Natl Acad Sci USA 94: 6261–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Fang L, Liu J, Senay-Aras B, Lin W, Zheng S, Zhang T, Guo J, Manor U, Van Norman J, et al. (2020) Auxin-induced signal protein nanoclustering contributes to cell polarity formation. Nat Commun 11: 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteris E, Galatis B (2005) The morphogenesis of lobed plant cells in the mesophyll and epidermis: organization and distinct roles of cortical microtubules and actin filaments. New Phytol 167: 721–732 [DOI] [PubMed] [Google Scholar]
- Perazza D, Herzog M, Hulskamp M, Brown S, Dorne AM, Bonneville JM (1999) Trichome cell growth in Arabidopsis thaliana can be derepressed by mutations in at least five genes. Genetics 152: 461–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Dong J (2013) Stomatal development in Arabidopsis. Arabidopsis Book 11: e0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU (2007) Termination of asymmetric cell division and differentiation of stomata. Nature 445: 501–505 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU (2011) Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell 23: 3260–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putarjunan A, Ruble J, Srivastava A, Zhao C, Rychel AL, Hofstetter AK, Tang X, Zhu JK, Tama F, Zheng N, et al. (2019) Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat Plants 5: 742–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K, Marrison J, Leech R (1991) Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heynh. J Exp Bot 42: 1407–1416 [Google Scholar]
- Qi X, Torii KU (2018) Hormonal and environmental signals guiding stomatal development. BMC Biol 16: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Jilk R, Marks MD, Szymanski DB (2002) The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14: 101–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Abrash E, Bettadapur A, Vogel JP, Bergmann DC (2016) Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc Natl Acad Sci USA 113: 8326–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Parra E, Lopez-Matas MA, Frundt C, Gutierrez C (2004) Role of an atypical E2F transcription factor in the control of Arabidopsis cell growth and differentiation. Plant Cell 16: 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland R (1977) Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol 11: 187–214 [DOI] [PubMed] [Google Scholar]
- Robinson DO, Coate JE, Singh A, Hong L, Bush M, Doyle JJ, Roeder AHK (2018) Ploidy and size at multiple scales in the Arabidopsis sepal. Plant Cell 30: 2308–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DBM, Dong J, Weimer A, Bergmann DC (2019) A plant-specific polarity module establishes cell fate asymmetry in the Arabidopsis stomatal lineage. bioRxiv, doi.org/10.1101/614636 [Advance access publication on 19 April 2021] [Google Scholar]
- Ruijtenberg S, van den Heuvel S (2016) Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 15: 196–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini M, Mulder BM (2021) A plausible mechanism for longitudinal lock-in of the plant cortical microtubule array after light-induced reorientation. Quantitative Plant Biol 2: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM (2014a) Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3: e01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Yan A, Krupinski P, Meyerowitz EM (2014b) Physical forces regulate plant development and morphogenesis. Curr Biol 24: R475–R483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapala A, Runions A, Routier-Kierzkowska AL, Das Gupta M, Hong L, Hofhuis H, Verger S, Mosca G, Li CB, Hay A, et al. (2018) Why plants make puzzle cells, and how their shape emerges. Elife 7: e32794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nat Lett 446: 199–202 [DOI] [PubMed] [Google Scholar]
- Sawinski K, Mersmann S, Robatzek S, Bohmer M (2013) Guarding the green: pathways to stomatal immunity. Mol Plant Microbe Interact 26: 626–632 [DOI] [PubMed] [Google Scholar]
- Schellmann S, Hülskamp M (2005) Epidermal differentiation: trichomes in Arabidopsis as a model system. Int J Dev Biol 49: 579–584 [DOI] [PubMed] [Google Scholar]
- Schnittger A, Schobinger U, Bouyer D, Weinl C, Stierhof YD, Hulskamp M (2002) Ectopic D-type cyclin expression induces not only DNA replication but also cell division in Arabidopsis trichomes. Proc Natl Acad Sci USA 99: 6410–6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler ML, Sedelnikova OV, Walker BJ, Westhoff P, Langdale JA (2018) SHORTROOT-mediated increase in stomatal density has no impact on photosynthetic efficiency. Plant Physiol 176: 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309: 290–293 [DOI] [PubMed] [Google Scholar]
- Simmons AR, Davies KA, Wang W, Liu Z, Bergmann DC (2019) SOL1 and SOL2 regulate fate transition and cell divisions in the Arabidopsis stomatal lineage. Development 146: dev171066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalák J, Vercruyssen L, Claeys H, Hradilová J, Černý M, Novák O, Plačková L, Saiz-Fernández I, Skaláková P, Coppens F, et al. (2019) Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant J 97: 805–824 [DOI] [PubMed] [Google Scholar]
- Sotiriou P, Giannoutsou E, Panteris E, Galatis B, Apostolakos P (2018) Local differentiation of cell wall matrix polysaccharides in sinuous pavement cells: its possible involvement in the flexibility of cell shape. Plant Biol (Stuttg) 20: 223–237 [DOI] [PubMed] [Google Scholar]
- Staff L, Hurd P, Reale L, Seoighe C, Rockwood A, Gehring C (2012) The hidden geometries of the Arabidopsis thaliana epidermis. PLoS One 7: e43546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara-Nishimura I (2010) Stomagen positively regulates stomatal density in Arabidopsis. Nature 463: 241–244 [DOI] [PubMed] [Google Scholar]
- Szymanski DB (2014) The kinematics and mechanics of leaf expansion: new pieces to the Arabidopsis puzzle. Curr Opin Plant Biol 22: 141–148 [DOI] [PubMed] [Google Scholar]
- Torii KU (2012) Two-dimensional spatial patterning in developmental systems. Trends Cell Biol 22: 438–446 [DOI] [PubMed] [Google Scholar]
- Torii KU (2021) Stomatal development in the context of epidermal tissues. Ann Bot 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh DC, Alonso-Serra J, Asaoka M, Colin L, Cortes M, Malivert A, Takatani S, Zhao F, Traas J, Trehin C, et al. (2021) How mechanical forces shape plant organs. Curr Biol 31: R143–R159 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2013) Does ploidy level directly control cell size? Counterevidence from Arabidopsis genetics. PLoS One 8: e83729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadde BVL, Challa KR, Nath U (2018) The TCP4 transcription factor regulates trichome cell differentiation by directly activating GLABROUS INFLORESCENCE STEMS in Arabidopsis thaliana. Plant J 93: 259–269 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Coppens F, Lee E, Donner TJ, Xie Z, Van Isterdael G, Dhondt S, De Winter F, De Rybel B, Vuylsteke M, et al. (2011) Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO J 30: 3430–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vőfély RV, Gallagher J, Pisano GD, Bartlett M, Braybrook SA (2019) Of puzzles and pavements: a quantitative exploration of leaf epidermal cell shape. New Phytol 221: 540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JD, Oppenheimer DG, Concienne J, Larkin JC (2000) SIAMESE, a gene controlling the endoreduplication cell cycle in Arabidopsis thaliana trichomes. Development 127: 3931–3940 [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Braybrook S, Huflejt M, Mosca G, Routier-Kierzkowska AL, Smith SS (2015) Measuring the mechanical properties of plant cells by combining micro-indentation with osmotic treatments. J Exp Bot 66: 3229–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer AK, Nowack MK, Bouyer D, Zhao X, Harashima H, Naseer S, De Winter F, Dissmeyer N, Geldner N, Schnittger A (2012) Retinoblastoma related1 regulates asymmetric cell divisions in Arabidopsis. Plant Cell 24: 4083–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmulling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems A, Heyman J, Eekhout T, Achon I, Pedroza-Garcia JA, Zhu T, Li L, Vercauteren I, Van den Daele H, van de Cotte B, et al. (2020) The cyclin CYCA3;4 is a postprophase target of the APC/C(CCS52A2) E3-ligase controlling formative cell divisions in Arabidopsis. Plant Cell 32: 2979–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43: 118–130 [DOI] [PubMed] [Google Scholar]
- Woolfenden HC, Baillie AL, Gray JE, Hobbs JK, Morris RJ, Fleming AJ (2018) Models and mechanisms of stomatal mechanics. Trends Plant Sci 23: 822–832 [DOI] [PubMed] [Google Scholar]
- Woolfenden HC, Bourdais G, Kopischke M, Miedes E, Molina A, Robatzek S, Morris RJ (2017) A computational approach for inferring the cell wall properties that govern guard cell dynamics. Plant J 92: 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Fernandes SB, Mayfield-Jones D, Erice G, Choi M, Lipka AE, Leakey ADB (2021) Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for maize QTL mapping. Plant Physiol kiab299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lee E, Lucas JR, Morohashi K, Li D, Murray JA, Sack FD, Grotewold E (2010) Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. Plant Cell 22: 2306–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, et al. (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Desyatova AS, Belteton SA, Mallery EL, Turner JA, Szymanski DB (2015) Patterning mechanisms of cytoskeletal and cell wall systems during leaf trichome morphogenesis. Nat Plants 1: 15014. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang H, Xue S, Qu X, Zou J, Le J (2014) Requirement for A-type cyclin-dependent kinase and cyclins for the terminal division in the stomatal lineage of Arabidopsis. J Exp Bot 65: 2449–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KZ, Jiang M, Wang M, Xue S, Zhu LL, Wang HZ, Zou JJ, Lee EK, Sack F, Le J (2015) Phosphorylation of serine 186 of bHLH transcription factor SPEECHLESS promotes stomatal development in Arabidopsis. Mol Plant 8: 783–795 [DOI] [PubMed] [Google Scholar]
- Yang SL, Tran N, Tsai MY, Ho CMK (2021) Misregulation of MYB16 causes stomatal cluster formation by disrupting polarity in asymmetric cell division. bioRxiv, doi.org/10.1101/2021.1105.1103.442461 [Advance access publication on 3 May 2021] [Google Scholar]
- Yuan F, Lyu MJ, Leng BY, Zheng GY, Feng ZT, Li PH, Zhu XG, Wang BS (2015) Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell Environ 38: 1637–1657 [DOI] [PubMed] [Google Scholar]
- Zeng SM, Lo EKW, Hazelton BJ, Morales MF, Torii KU (2020) Effective range of non-cell autonomous activator and inhibitor peptides specifying plant stomatal patterning. Development 147: dev192237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Mallery EL, Schlueter J, Huang S, Fan Y, Brankle S, Staiger CJ, Szymanski DB (2008) Arabidopsis SCARs function interchangeably to meet actin-related protein 2/3 activation thresholds during morphogenesis. Plant Cell 20: 995–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dyachok J, Krishnakumar S, Smith LG, Oppenheimer DG (2005) IRREGULAR TRICHOME BRANCH1 in Arabidopsis encodes a plant homolog of the actin-related protein2/3 complex activator Scar/WAVE that regulates actin and microtubule organization. Plant Cell 17: 2314–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo X, Dong J (2016) Phosphorylation of the polarity protein BASL differentiates asymmetric cell fate through MAPKs and SPCH. Curr Biol 26: 2957–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang P, Shao W, Zhu JK, Dong J (2015) The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev Cell 33: 136–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu J, Wang X, Durachko DM, Zhang S, Cosgrove DJ (2021) Molecular insights into the complex mechanics of plant epidermal cell walls. Science 372: 706–711 [DOI] [PubMed] [Google Scholar]
- Zhao L, Sach FD (1999) Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Am J Bot 86: 929–939 [PubMed] [Google Scholar]
- Zhao X, Harashima H, Dissmeyer N, Pusch S, Weimer AK, Bramsiepe J, Bouyer D, Rademacher S, Nowack MK, Novak B, et al. (2012) A general G1/S-phase cell-cycle control module in the flowering plant Arabidopsis thaliana. PLoS Genet 8: e1002847. [DOI] [PMC free article] [PubMed] [Google Scholar]