Abstract
Exercise-associated muscle cramps (EAMCs) are common and frustrating for athletes and the physically active. We critically appraised the EAMC literature to provide evidence-based treatment and prevention recommendations. Although the pathophysiology of EAMCs appears controversial, recent evidence suggests that EAMCs are due to a confluence of unique intrinsic and extrinsic factors rather than a singular cause. The treatment of acute EAMCs continues to include self-applied or clinician-guided gentle static stretching until symptoms abate. Once the painful EAMCs are alleviated, the clinician can continue treatment on the sidelines by focusing on patient-specific risk factors that may have contributed to the onset of EAMCs. For EAMC prevention, clinicians should obtain a thorough medical history and then identify any unique risk factors. Individualizing EAMC prevention strategies will likely be more effective than generalized advice (eg, drink more fluids).
Keywords: best practice, dehydration, electrolytes, fatigue, risk factors
Exercise-associated muscle cramps (EAMCs) are painful, involuntary contractions of a skeletal muscle during or shortly after exercise. Exercise-associated cramps typically occur in muscles that span multiple joints and are frequently used during exercise (eg, quadriceps).1 Severity ranges from a “cramp-prone state” to mild (ie, usually self-treatable and not limiting exercise performance) to serious (require exercise cessation, occur with other systemic signs or symptoms requiring medical intervention; Table 1) EAMCs.1–3
Table 1.
Clinical Severity of EAMCsa
| Cramp-prone state or precramping9,48 |
|
| Mild or moderate (benign)9 |
|
| Severe or serious or EAMCs as a sign of a systemic condition2 |
|
Abbreviations: EAMC, exercise-associated muscle cramp; EMG, electromyography.
The EAMCs can progress in severity from mild to severe (as a continuum) during exercise.
Heat cramps is a common but inaccurate term for EAMCs. Exercise-associated muscle cramps are not related to body temperature; they occur in a variety of ambient conditions and environments, are not associated with passive heating, and are not immediately relieved by the application of cooling modalities.4–6 Similarly, spasms, contractures, tics, fasciculations, and tremors are inaccurate terms for EAMCs because they are either not painful or are associated with neuromuscular abnormalities.4
An EAMC is one of the most common conditions (or clinical syndromes) affecting athletes. The incidence varies considerably by sport, age, and sex.2 Cooper et al7 observed that EAMCs were most frequent during the hottest months and had an incidence of 3.07/1000 athlete-exposures in a single American football season. In a 4-year study,2 the incidence of serious EAMCs ranged from 1.01/1000 to 2.2/1000 race starters in a 56-km ultramarathon. Less experienced runners, older runners, and those running at a faster pace were at the most risk.2
Confusion and debate persist regarding EAMC pathophysiology, treatment, and prevention.4,8,9 Therefore, the purpose of our review was to critically appraise the literature on the pathophysiology, treatment, and prevention of EAMCs and provide evidence-based recommendations to aid clinicians' diagnosis and management of this common condition.
METHODS
We performed a computerized search of published articles written in English from 1900 to 2020 pertaining to the pathophysiology, treatment, and prevention of EAMCs. We searched the PubMed, Cochrane, CINAHL, SPORTDiscus, and PEDRo databases using Boolean operators combined with the following search terms: exercise associated muscle cramp, cramping, exercise, dehydration, electrolytes, fatigue, rehydration, treatment, and prevention. We also reviewed reference lists for further information on the topic. From this review, we developed 16 recommendations for treating and preventing EAMCs and graded each recommendation using the Strength of Recommendation Taxonomy (SORT) grading scale (Table 2).10
Table 2.
Treatment and Prevention Recommendations for EAMCs With Strength of Recommendation Taxonomy Grading10
| Recommendation |
Grade |
| Treatment | |
| 1. Athletes or clinicians should apply gentle static stretching to the EAMC until it abates.1,5,22,26,35 | A |
| 2. Food items containing acetic acid (ie, vinegar) or transient receptor potential activators (eg, capsaicin) may help relieve acute EAMCs. If used, these should be administered infrequently and in small volumes (<100 mL). Moreover, they should only be attempted in patients without related food allergies.41,42,44,45 | B |
| 3. Once EAMCs have abated and the athlete has been removed from exercise, clinicians may attempt or encourage the following: | |
| (a) Rest in a position of comfort in which treatments can be applied.6 | C |
| (b) Continued gentle stretching, as necessary.6 | C |
| (c) Oral ingestion of fluids containing carbohydrates and electrolytes ad libitum.6 | C |
| (d) Intravenous fluid administration and emergency transport if the athlete cannot tolerate fluids orally or has severe gastrointestinal distress (eg, vomiting, diarrhea).6,39 | C |
| (e) Interventions that reduce muscle pain or soreness in the cramping muscles (eg, cryotherapy, massage, electrical stimulation).6 | C |
| 4. The EAMC treatments should continue in the hours after an initial episode to reduce risk of recurrence.29 | C |
| 5. No medications should be administered for EAMCs without a physician's consent or in a physician's absence.35 | C |
| 6. Quinine or products containing quinine (eg, tonic water) should not be administered for EAMC treatment.47 | A |
| Prevention | |
| 1. Thoroughly evaluate athletes for underlying general medical conditions, allergies, or medication usage that may contribute to EAMC occurrence.35 | C |
| 2. Thoroughly question athletes with an EAMC history to identify their unique extrinsic and intrinsic risk factors and then target those risk factors with appropriate interventions.4 | C |
| 3. Incorporate neuromuscular reeducation, plyometrics, or strength training into training sessions when neuromuscular fatigue has been identified as a factor in an athlete's EAMC.3,18,31 | B |
| 4. Train at intensities and in environments (eg, temperature, altitude) similar to those in competition.3,22,30,31 | B |
| 5. Include suitable rest periods after training and competition to allow recovery and minimize the residual effects of muscle damage.6,31 | C |
| 6. Educate athletes about the various causes of EAMCs and safe hydration and drinking behaviors before beginning an EAMC prevention strategy.38 | C |
| 7. Encourage athletes to consume a nutritious, well-balanced diet that accounts for their unique carbohydrate, electrolyte, and fluid needs before training and competitions.38 | C |
| 8. Advise athletes to consume carbohydrates or a carbohydrate-electrolyte drink during exercise to help stave off fatigue and promote greater absorption and retention of ingested fluids.6,38 | C |
| 9. Identify athletes' fluid and electrolyte needs based on sweat rates and composition to avoid underhydration or overhydration or insufficient or excessive electrolyte supplementation if fluid and electrolyte monitoring is included in an EAMC prevention strategy.38 | C |
| 10. Intravenous fluids should not be administered before events for the sole purpose of preventing EAMCs.6 | C |
Abbreviation: EAMC, exercise-associated muscle cramp.
PATHOPHYSIOLOGY OF EAMCS
Dehydration and Electrolyte Imbalance Theory
The dehydration and electrolyte imbalance theory is the oldest theory and is primarily based on clinical observations. It proposes that EAMCs occur when sweating causes a contracture of the interstitial fluid space, increasing excitatory neurochemicals and mechanical pressure on motor-nerve terminals.8
Many observations over the last 100 years seemingly supported the dehydration and electrolyte imbalance theory. First, early researchers11 speculated that EAMCs were due to 3 factors: sweat-induced fluid and electrolyte (primarily chloride) losses, hard work by people unacclimated to the heat, and exposure to high temperatures. However, fluid and electrolyte balance measures were often unreported, and many patients were experiencing an acute gastrointestinal illness (eg, vomiting or diarrhea). Regardless, EAMCs were linked to work-induced fluid losses. Second, EAMCs diminished with saline administration in a small case series.11 Third, tennis players (n = 17)12 and American football players with a cramp history (n = 5)13 tended to have higher sweat rates (∼2.5 L/h) and sodium losses (up to 2.7 g/h) than noncrampers. Similarly, American football players with sweat sodium losses >1.18 g or sweat chloride losses >2.3 g during a workout were approximately 9 times more likely to be EAMC prone.14 Finally, Ohno et al15 counted the number of volitionally induced hamstrings cramps after 9 men lost 0.5% (control group), 1%, 2%, or 3% of their body mass passively in a sauna. They observed no cramps with 0.5% or 1% body mass reductions. When dehydrated to −2% or −3% of body mass, 3 participants cramped in each condition.
Conversely, expert opinion4,6,9 and other evidence from experimental16,17 and observational studies1,3,18–21 did not support the dehydration and electrolyte imbalance theory. First, the theory proposes a conflicting physiological argument.9 Athletes with EAMCs lose large volumes of sweat, which would result in significant losses of plasma volume and lead to increased plasma osmolality. Consequently, fluid should leave the interstitial fluid space and enter the vasculature. However, crampers also purportedly lose significant amounts of sodium as “salty sweaters.”8 The electrolyte loss would decrease plasma osmolality, resulting in little or no fluid moving out of the interstitial space.9 Second, plasma characteristics in athletes with and those without EAMCs were often within normal limits and comparable after exercise.1,20 Several authors1,20,22 demonstrated no differences in plasma volume, red cell volume, body mass lost, or plasma electrolyte concentrations during competition between athletes with and those without EAMCs. The comparable blood characteristics between groups would suggest similar osmotic pressures systemically, though some researchers8 argued that blood samples did not reflect conditions near the cramping muscles. Yet when participants were dehydrated to −6% of their body mass, no changes in muscle resting membrane potential occurred.23 Third, even though sodium and chloride losses and the sweat rate predicted EAMC-prone athletes in American football, this relationship was not clinically meaningful in 10 other sports.14 Moreover, when diet, hydration status, environmental conditions, and exercise intensity and duration were controlled, no differences in sweat characteristics were identified among athletes with various EAMC susceptibilities.16 Fourth, because dehydration and electrolyte losses are systemic, if the dehydration and electrolyte imbalance theory was true, then EAMCs should occur in any muscle, not only the working muscles. Fifth, athletes prone to EAMCs consumed similar volumes of fluid during exercise as noncrampers.13 Sixth, stretching relieved cramping but did not alter fluid or electrolyte levels.5 Finally, electrically induced cramp susceptibility was unchanged when participants lost 3% to 5% of their body mass and approximately 4 g of sodium and when fatigue was minimized,17 suggesting that dehydration and electrolyte losses had minimal effects on cramp susceptibility.
Altered Neuromuscular Control Theory
The altered neuromuscular control theory was proposed in 1997 and updated in 2009 as a new explanation for EAMCs.9 This theory suggests that EAMCs occur when fatigue and other risk factors contribute to a final common pathophysiological pathway to produce an imbalance between excitatory and inhibitory stimuli at the α motor nerve. The theory arose out of observations that fatigue altered muscle spindle and Golgi tendon organ firing rates in an animal model.24,25 These afferent activity changes could lead to overexcitation of the motor nerves. Authors1,9 of prospective cohort studies of athletes with EAMCs have subsequently identified risk factors more consistent with fatigue-induced alterations in nervous system excitability (eg, poor conditioning, higher exercise intensities) than dehydration or electrolyte losses. Most EAMCs occur in exercising or actively contracting muscles5 that cross multiple joints (eg, gastrocnemius, hamstrings).1 Muscles that cross multiple joints often contract in already shortened positions; thus, the amount of muscle inhibition coming from Golgi tendon organs is reduced.26 Exercise-associated muscle cramps also typically occur near the end of competitions, when fatigue is likely greatest.1,20 In laboratory studies, investigators confirmed the importance of muscle afferents27 and spinal pathways in cramp development,28 and noncrampers produced greater amounts of inhibition than crampers.27 Moreover, cramps could be induced volitionally in the absence of dehydration or electrolyte losses.29 Collectively, these findings indicated that nervous system alterations were needed for EAMCs to occur and that the final common pathway to EAMCs was altered in neuromuscular control.
This theory better explains clinical and laboratory observations of cramping but is not without limitations and questions. When first proposed, the theory emphasized the importance of fatigue-induced alterations in afferent activity, resulting in overexcitation at the α motor neuron. However, it is unclear how fatigue alters this signaling: if a fatigue threshold must be reached before patients experience EAMCs, or whether fatigue-induced changes in excitability can be modulated by other factors. What is known is that well-trained and conditioned athletes still develop EAMCs. Also, training history does not always predict EAMC occurrence.30,31 Thus, fatigue cannot be the sole generator of EAMCs, and other factors can alter neuromuscular control. Contrary to the theory, in 1 laboratory study, researchers32 noted that localized, fatiguing contractions decreased electrically induced cramp susceptibility. Lastly, authors of many of the laboratory studies17,24–27 that supported this theory used low-frequency electrical stimulation to induce cramps. This technique allows cramp susceptibility in different muscles33 to be examined and can discriminate between crampers and noncrampers.34 However, the muscles studied are often small (eg, flexor hallucis brevis) and different than the muscles that usually develop EAMCs during exercise. Thus, questions exist about the applicability of the findings to EAMCs.
Multifactorial Theory of EAMCs
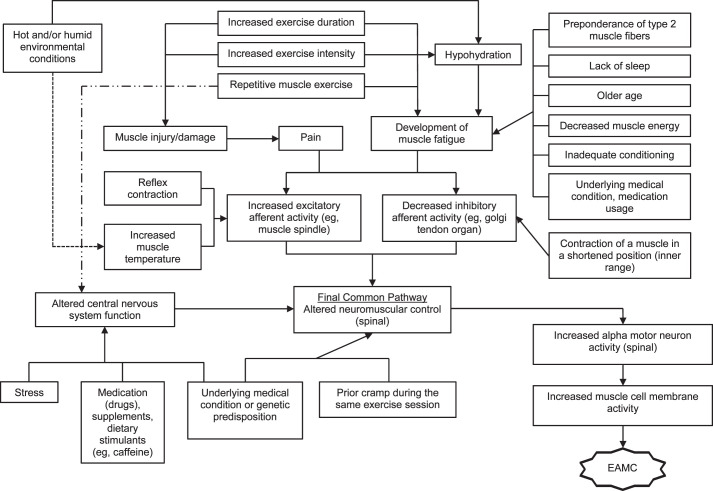
Building on the Schwellnus theory,9 Miller4 recently proposed an EAMC pathophysiology model focused on how multiple risk factors interact to elicit a chain reaction that alters neuromuscular control and induces EAMCs (Figure 1). In this model,4 he theorized that numerous unique intrinsic and extrinsic factors coalesce through different pathways and elicit EAMCs. For example, consider an athlete who sustains a muscle injury. This injury may cause deconditioning, pain, and an inability to tolerate the same exercise intensities and durations as before the injury. Consequently, these risk factors coalesce, alter neuromuscular control, and elicit EAMCs.
Figure 1.
Multifactorial theory for pathogenesis of exercise-associated muscle cramps (EAMCs). Dashed arrows used to help with clarity of understanding the hot, humid, or both environmental conditions and repetitive muscle exercise pathways. Reprinted by permission from Miller KC. Exercise-associated muscle cramps. In: Adams WM, Jardine JF, eds. Exertional Heat Illness: A Clinical and Evidence-Based Guide. Springer; 2020:117–136.4
This model also proposes that a factor threshold must be reached before EAMCs occur and that this threshold may be positively or negatively mitigated by other risk factors. Therefore, when predisposed individuals with intrinsic risk factors are exposed to extrinsic factors and exceed their factor threshold, EAMCs occur. This multifactorial theory and factor threshold may explain why EAMCs occur in some individuals and some situations but not others. Further examination is needed to clarify which factors contribute to EAMCs, how these factors coalesce, and whether some factors are more or less important to EAMC development.
ACUTE EAMC DIAGNOSIS AND TREATMENT
Exercise-associated muscle cramps normally occur acutely during or after exercise. The diagnosis of EAMCs is based on a thorough clinical examination and history.35 Acutely, patients present in noticeable pain, often resulting in slowing or ceasing activity altogether.1,3 Patients often report subtle muscle twitching before the EAMCs began (cramp-prone state). Cramping muscles appear rigid, with the joint often locked in its end range of motion. Clinicians observe visible and palpable knotting or tautness, a key sign differentiating EAMCs from exertional sickling cramps. Fasciculations that wander over the muscle are also possible.35 Athletes with more serious EAMCs that occur concomitantly with other serious medical conditions (eg, hyponatremia) warrant additional evaluation and diagnosis and should be immediately referred for further medical care (Table 1).2,35
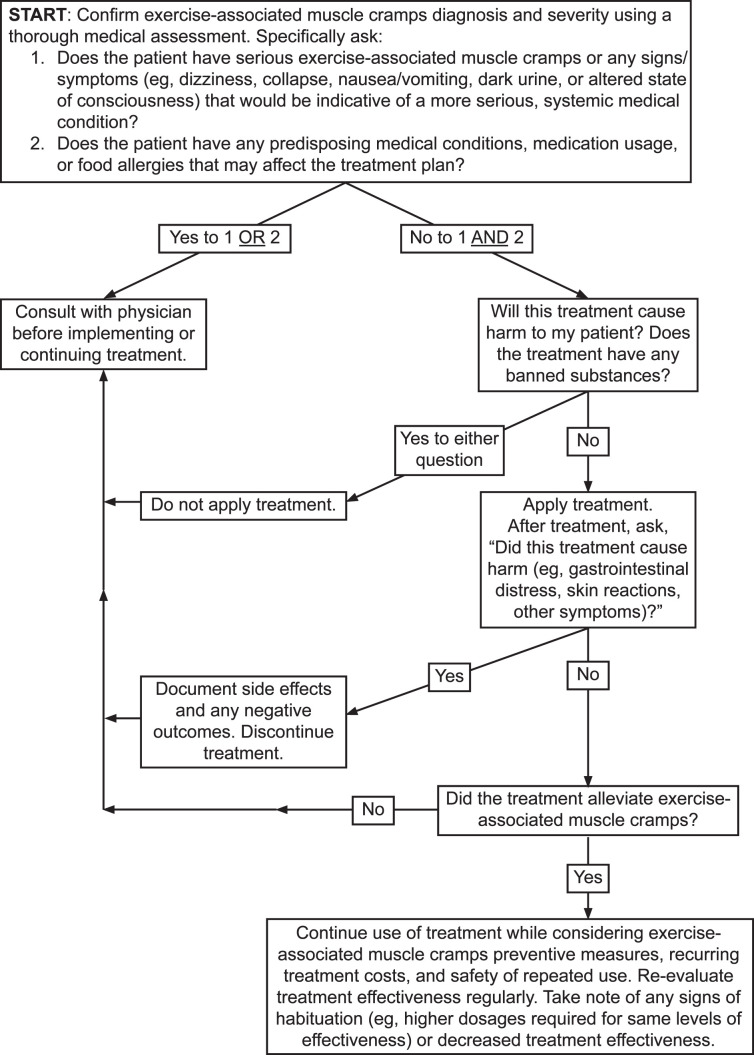
Clinicians should develop treatment protocols for acute EAMCs. Ideally, the treatment approach is individualized (Figure 2 and Table 3) and EAMC treatments are continued for up to 1 hour because susceptibility to EAMCs remains high even after cessation.29
Figure 2.
Decision tree for the individualized treatment of exercise-associated muscle cramps.
Table 3.
Questions to Aid in Identifying EAMC Risk Factorsa
| Question |
Justification |
Risk Factor |
| 1. Do you have any illnesses, allergies, or medical conditions? | Muscle cramping may be associated with allergies or diseases.35 | Underlying illness |
| 2. Did EAMCs occur after a change or start of medication or drug use? | Muscle cramping may be associated with medication use (eg, β2-agonists).35 | Medication side effect |
| 3. Do you regularly cramp during exercise? | Muscle cramping has a genetic component. Asking about a history of EAMC is helpful for identifying recurrent crampers.4,29,48 | Multiple mechanisms |
| 4. How intensely and long were you exercising before you developed an EAMC? | Athletes with the fastest actual and predicted race times are most likely to experience EAMCs.2,22,30 | Fatigue |
| 5. When did the EAMC occur during exercise (ie, beginning, middle, or end)? | Near the end of exercise or competition is most common.1,20 | Fatigue |
| 6. How much sleep did you get the night before your exercise session when the EAMC occurred? | Sleep loss reduces muscle glycogen and time to exhaustion.49 | Premature fatigue |
| 7. How hot and humid was it when you developed the EAMC? | Though EAMCs occur most frequently in summer months (eg, August),7 they can occur under any environmental conditions. | Hypohydration, premature fatigue, unacclimatized to environment |
| 8. Was the exercise session during which the EAMC occurred novel in any way? | Untrained individuals and those participating in events that are more strenuous than anticipated are more prone to EAMCs.2,31 | Overexertion, fatigue, or both |
| 9. What was your diet like in the days preceding the EAMC? Was your diet nutritious, balanced (ie, containing the major essential nutrients such as protein, fat, and carbohydrates), and varied (ie, nutrients coming from fruits, vegetables, meats)? | Diets low in carbohydrates may reduce muscle glycogen and the amount of work or exercise performed.38 Athletes' diets should be well balanced and include electrolytes and carbohydrates to stave off fatigue.35 | Premature fatigue |
| 10. Did you consume supplements or stimulants (eg, caffeine) before or during your exercise session? | Stimulants and supplements can increase the excitability of the nervous system.55 | Overexcitation of nervous system |
| 11. Were you recently injured? | Pain21 and prior injury30,31 predict cramping. | Overexcitation of nervous system |
| 12. What was your psychological state during the exercise session when the EAMC developed? | Stress or unrealistic expectations may increase nervous system excitability.22,30 | Overexcitation of nervous system |
| 13. Did you consume enough fluids, electrolytes, or both to replace your sweat losses? | The contribution of sweat losses to EAMCs is minor for most athletes.13,14,16 However, American football players with an EAMC history may benefit from fluid and electrolyte monitoring.14 | Hypohydration, premature fatigue |
| 14. Did the EAMC tend to stop once you stopped the activity? | Activity cessation, reduced pace, or both can relieve EAMCs.1,3 | Overexcitation of nervous system |
| 15. Do the EAMCs tend to occur only in the muscles that are doing the most work? | Working muscles tend to be most affected, and EAMCs may be mitigated with reeducation of synergistic muscles.3 | Overexcitation of nervous system |
| 16. Did the EAMC occur during training or during competition? | Although EAMCs can occur at any time and in any sport, they tend to affect athletes who run faster or exercise harder during competitions than during training.2,22,30,31 | Overexcitation due to stress of competition, premature fatigue due to overexertion |
Abbreviation: EAMC, exercise-associated muscle cramp.
After questions 1 and 2, the items appear in no particular order or level of importance. Adapted from Miller.4
Rest and Pain-Relieving Agents
Although most patients can finish exercising after mild EAMCs, some athletes cannot complete their competitions because of EAMCs.3,36 In these cases, rest helps to normalize neuromuscular activity and allows treatments to address the underlying risk factors that contributed to EAMC development (eg, depleted muscle energy).29 The patient should be placed in a position of comfort. Similarly, pain-relieving agents (eg, cryotherapy, massage, electrical stimulation) may provide relief from the EAMCs by interrupting the pain-spasm-pain cycle.4 As the athlete rests, clinicians should identify and address the factors that may have precipitated EAMC development.
Stretching
The fastest, safest, and most effective treatment for an active EAMC is self-administered or clinician-administered gentle stretching.5,22,35 No researchers have examined which type of stretching most effectively relieves active EAMCs, but static stretching increases tendon tension, and elongated muscles produce the greatest Golgi tendon organ inhibition.26 This may help restore the balance between excitatory and inhibitory signaling and explain the reduction in muscle activity observed when cramping muscles are stretched.9 Stretching also physically separates the contractile proteins, which is beneficial as muscle shortening is required for cramping.5 If stretching fails to relieve EAMCs, clinicians should seek advanced medical care.
Rehydration: Beverage Type
Athletes can drink water or carbohydrate-electrolyte beverages ad libitum during EAMC treatment if tolerated because these liquids both restore plasma volume and osmolality over time and rehydrate effectively.37,38 However, stretching will alleviate EAMCs more quickly than rehydration. Oral fluids require ≥13 minutes to be absorbed into the bloodstream though this timing can be prolonged depending on the beverage's contents (eg, acidity, electrolyte, and carbohydrate content).38 Also, these drinks are hypotonic compared with plasma, so it can be dangerous to try and fully replace the electrolytes lost during exercise by consuming only sport drinks (Table 4). Ingesting such large volumes of hypotonic fluids will dilute the blood and could result in life-threatening hyponatremia.
Table 4.
Dangerous Volumes of Some Popular Sports Drinks an Athlete With EAMCs Would Need to Ingest to Completely Replace Sweat Sodium and Potassium Losses During Exercisea
| Sports Drink |
[Na+] of Beverage, (g/L) |
Volume Needed to Fully Replace Na+ Lost, L (gal) |
[K+] of Beverage, (g/L) |
Volume Needed to Fully Replace K+ Lost, L (gal) |
| A-Game | 0.49 | 10.0 (2.7) | 0.32 | 2.9 (0.8) |
| Accelerade (Pacific HealthLabs)b | 0.62 | 7.9 (2.1) | 0.25 | 3.8 (1.0) |
| All Sport (Jel Sert) | 0.24 | 20.5 (5.4) | 0.25 | 3.8 (1.0) |
| Body Armor SuperDrink (BA Sports Nutrition, LLC) | 0.09 | 54.6 (14.4) | 1.48 | 0.64 (0.2) |
| Gatorade, Original, Fierce, G2, Organic, Flow, Zero, or Frost | 0.46 | 10.7 (2.8) | 0.13 | 7.23 (1.9) |
| Infuse Thirst Quencher | 0.42 | 11.7 (3.1) | 0.13 | 7.23 (1.9) |
| K+ Organic Sports Drink | 0.25 | 19.6 (5.2) | 0.15 | 6.3 (1.7) |
| Monster Energy Hydro Sports Drink | 0.21 | 23.4 (6.2) | 0.17 | 5.53 (1.5) |
| PowerAde, Original or Zero (The Coca-Cola Co) | 0.42 | 11.6 (3.1) | 0.10 | 9.4 (2.5) |
| Propel Fitness Water | 0.46 | 10.7 (2.8) | 0.12 | 7.8 (2.1) |
| SoBe Water | 0.13 | 37.8 (9.9) | 0.15 | 6.3 (1.7) |
| Sqwincher (Northern Safety Co) | 0.23 | 21.3 (5.6) | 0.19 | 4.9 (1.3) |
| Staminade (Steric Trading Pty Ltd) | 0.38 | 12.9 (3.4) | 0.19 | 4.9 (1.3) |
| Vitamin Water, Active Werk It (The Coca-Cola Co) | 0.51 | 9.8 (2.6) | 0.18 | 5.2 (1.4) |
Abbreviations: EAMC, exercise-associated muscle cramp; [K+], potassium concentration; [Na+], sodium concentration.
Estimates are based on the following assumptions and data from the literature on cramp-prone athletes: (1) an average sweat sodium concentration of 48.4 mmol/L (1.1 g/L),12,13,51 (2) an average sweat potassium concentration of 5.4 mmol/L (0.21 g/L),51 (3) an average sweat rate of 2.23 L/h,12,13,51 and (4) a 2-hour, continuous exercise bout. Based on these assumptions, the athlete would need to replace 4.91 g of Na+ and 0.94 g of K+. Adapted from Miller.4
Product sold as a powder and made per manufacturer instructions.
Rehydration: Delivery Method
In most situations, rehydration should be oral due to its simplicity, accessibility, and myriad of delivery options (eg, cup, water bottle, prepacked container). Intravenous (IV) fluids are popular among professional athletes, yet they must be administered by a trained person and pose certain risks (eg, infection, air embolism, arterial puncture).39 Authors of numerous studies38,39 have examined oral versus IV fluid administration on hydration status and noted comparable restoration of plasma osmolality, plasma volume, skin blood flow, stroke volume, cardiac output, heart rate, skin temperature, rectal temperature, performance, and fluid regulatory hormone responses. Interestingly, perceptual measures (eg, thirst, thermal sensation, and rating of perceived exertion) are often lower with oral rehydration because IV fluid delivery bypasses fluid volume receptors in the mouth (ie, baroreceptors).40 Although either fluid delivery method can be used to treat EAMCs, IV fluid administration should be saved for time-sensitive situations (ie, <15 minutes) or when patients cannot consume fluids orally (eg, too much pain, repeated vomiting).
Transient Receptor Potential Receptor Agonists
Transient receptor potential (TRP) receptors detect temperature and sensations in the mouth, oropharynx, esophagus, and stomach. Ingredients such as vinegar, cinnamon, capsaicin, and ginger activate these receptors and, in theory, may affect neural function if potent enough.41,42 Two of the more popular TRP agonists are pickle juice and mustard due to their high concentrations of vinegar, salt, or both.43 In 1 single-blinded study,44 ingesting small volumes (<100 mL) of pickle juice relieved cramps 45% faster (68.6 seconds) than no fluids and 37% faster (49.1 seconds) than water. This effect was neither immediate (∼90 seconds) nor the result of electrolytes consumed as the small volumes of pickle juice did not affect plasma volume, plasma electrolyte concentrations, or plasma osmolality.44,45 Instead, the investigators44 hypothesized that vinegar triggered an oropharyngeal reflex that inhibited cramping. Conversely, only anecdotal evidence exists regarding mustard's efficacy in relieving acute EAMCs. However, the efficacy of mustard is not likely due to electrolyte replacement because ingesting large volumes (∼135 g) had no effect on plasma electrolyte concentrations, plasma osmolality, or plasma volume up to 60 minutes postingestion.45
Authors of other studies assessed the effect of spicy, capsaicin-based TRP agonists on cramp susceptibility. While the researchers in 1 study42 reported longer times before cramping, higher contraction forces necessary to induce cramping, and lower muscle activity during cramping, all participants still cramped after ingesting the TRP-agonist drink. Conversely, Behringer et al41 noted insignificant changes in cramp susceptibility, perceived muscle pain, cramp intensity, and maximal isometric force from 15 minutes to 24 hours postingestion of a TRP agonist. Further work is needed on TRP agonists and EAMCs.
The ingestion of TRP agonists is usually benign, even though gastrointestinal tolerance varies considerably. If used, clinicians should provide them in small volumes (<100 mL) and only when oral solutions are tolerated and no food allergies are present. If symptoms are not relieved relatively quickly (<2 minutes), other treatments should be used.
Bananas
Potassium is generally not considered an electrolyte of interest in EAMCs, yet bananas are sometimes used during treatment due to their high potassium and glucose content. However, no evidence exists on their efficacy. Some data suggested they are unlikely to help by increasing blood potassium; dehydrated participants who ingested 1 or 2 servings of bananas postexercise did not experience increases in plasma potassium concentrations or plasma volume until 60 minutes after consumption.46 Interestingly, plasma glucose increased significantly in the 2-servings trial, but this effect occurred 15 minutes postingestion. If poor nutrition is suspected as a risk factor for an athlete's EAMCs, we advise clinicians to advocate for a well-rounded pre-exercise nutrition plan and consult with a registered dietitian before implementing dietary interventions.
Quinine
Quinine and quinine products (eg, tonic water) were once a popular treatment for cramping.47 These have fallen out of favor because they require a prescription, and the United States Food and Drug Administration banned all over-the-counter quinine products for cramping in the mid-1990s. A Cochrane review47 of 23 trials showed that quinine reduced the number and intensity of cramps, though the extent of this reduction was clinically unimpressive, with a mean reduction of less than 2 cramps over a 2-week period (95% CI = −2.2, −1.42). Interestingly, cramp duration, which is the variable of interest in the acute treatment of EAMCs, was not reduced. Importantly, minor and major adverse events were reported in many of the trials (eg, gastrointestinal distress, thrombocytopenia).47 Consequently, we do not recommend using quinine for EAMCs.
CHRONIC OR RECURRENT EAMC DIAGNOSIS AND TREATMENT
The first step in the diagnosis and treatment of a patient presenting with recurrent EAMCs is a thorough medical evaluation to rule out any intrinsic risk factors, including a history of injury, past EAMC history, chronic medical conditions, medication use, or allergies (Figure 2 and Table 3).35 If an underlying condition is identified, that condition should be treated before implementing EAMC prevention strategies.
After ruling out underlying conditions, the clinician should thoroughly question the patient to determine if pertinent extrinsic or intrinsic risk factors exist. Risk factors consistently associated with EAMCs include pain,21 a history or previous occurrence of EAMCs,21,22,48 muscle damage or injury,18,21,31,48 prolonged exercise durations,1,20,30,48 and faster finishing times than anticipated.2,22 Consequently, setting realistic goals, obtaining regular and sufficient sleep,49 incorporating rest and recovery sessions in training schedules, and training at similar intensities and in similar environments to competition may help stave off many risk factors known to contribute to EAMCs.
The strongest and most recent evidence4,9 suggested that EAMCs are due to changes in the neuromuscular system, yet most diagnostic questions revolve around factors that affect nervous system excitability (Table 3). These questions can be asked before and after each EAMC to help clinicians identify consistent risk factors. Targeted prevention strategies for those risk factors can then be attempted.
PREVENTION OF EAMCS
Many EAMC prevention recommendations have been advocated, but unfortunately, most either lack support from strong patient-oriented studies or are based on anecdotes (Table 2). Indeed, much of the published EAMC prevention advice is derived from studies of electrically induced cramps rather than EAMCs, is anecdotal, is often too generic (eg, consume more salt), or fails to account for the complexity of EAMC pathogenesis. Moreover, many patients and clinicians lack an understanding of the possible causes and risk factors for EAMCs and are overly confident about the contributions of hydration and electrolytes to EAMCs.50 Sadly, an overemphasis on hydration and electrolyte consumption to prevent EAMCs contributed to the deaths of 2 high school athletes from hyponatremia in 2014.4 Therefore, we advocate for clinicians to educate athletes about EAMC causes and safe hydration practices before implementing prevention strategies,38 take a multifactorial approach to EAMC prevention, implement EAMC prevention strategies cautiously and with thorough documentation, and at a minimum, perform a thorough medical examination before implementing any of the following strategies.
Carbohydrate-Electrolyte Beverages, Electrolyte Supplements, and Stimulants
Sport drink consumption and electrolyte supplementation are frequently touted as effective for preventing EAMCs, though the content of sports drinks and electrolyte supplementation products varies greatly (Table 4).51 Despite the fact that electrolyte tablets and magnesium supplementation are frequently promoted to prevent cramping, the authors52 of a Cochrane review reported that magnesium supplementation offered no clinically meaningful benefits in terms of cramp frequency, intensity, or duration compared with placebo. However, in the 1920s, investigators11 observed that workers prevented EAMCs by consuming saline or adding salt to their beverages. Bergeron51 described a tennis player who prevented EAMCs by increasing dietary salt intake by 6000 to 8000 mg/day, increasing caloric intake by 5000 to 6000 kcal/day, improving pre-event hydration status, and adding salt to sports drinks when the athlete felt EAMCs were imminent. When participants ingested a 6% carbohydrate-electrolyte beverage with added salt during a calf-fatiguing protocol, EAMCs occurred after 36.8 ± 17.3 minutes, compared with 14.5 ± 5.0 minutes when nothing was consumed.53 More recently, researchers54 noted that a 2% carbohydrate-electrolyte beverage decreased electrically induced muscle cramp susceptibility. However, in both studies,53,54 the athletes still experienced cramping, and the experimental designs prohibited identification of the ingredient responsible for this effect because the drink contained multiple ingredients (eg, electrolytes and carbohydrates). Still, the large carbohydrate load (18.3 to 57 g/L) may have been responsible as plasma electrolyte values decreased postingestion.54 In theory, carbohydrate-electrolyte drinks could help prevent EAMCs by increasing muscle glycogen and delaying fatigue,38 but more well-designed studies are needed to identify the active ingredients and minimal and optimal dosages for effectiveness. Clinicians should be wary of sport drinks that contain stimulants (eg, caffeine), which may cause an increase in nervous system excitability and, theoretically, predispose patients to EAMCs.55
Conversely, the authors1,18,20,22 of several studies failed to show differences in plasma electrolyte concentrations in athletes with and those without EAMCs. Sodium supplementation did not differ between ultramarathoners with and those without EAMCs.19,48 Further examination of electrolyte supplementation and EAMCs is needed.
Hydration Assessment
If clinicians suspect hydration is a risk factor for recurrent EAMCs, we recommend sweat testing. Determining the sweat rate is relatively simple and only requires body weight to be measured before and after exercise. Clinicians must also know the duration of exercise and the volume or weight of any fluids ingested or lost (ie, urination).38 Determining the sweat electrolytes lost is more complicated and requires expensive equipment. Nonetheless, sweat electrolyte estimates are available for many sports.14 Ideally, sweat tests would be performed under similar environmental, equipment, and exercise conditions as during competition for accuracy. Combining sweat test results with a well-balanced, nutritious diet that considers the athlete's unique carbohydrate, fluid, and electrolyte needs will better ensure that he or she is prepared for exercise and minimize the risk of hyponatremia.38
Intravenous Fluids
Some clinicians use IV fluids to prevent EAMCs and believe they are effective.39 Unfortunately, no large-scale clinical trials have demonstrated that IV fluid administration reduces the occurrence of EAMCs or is more effective than oral fluid administration.39 Because EAMCs are not life threatening and oral rehydration can safely restore fluid and electrolyte deficits,38 we recommend clinicians avoid using IV fluids prophylactically.
Prophylactic Stretching
Although static stretching effectively treats EAMCs,5,22,35 it appears to be ineffective as a prophylactic strategy. In a laboratory study,56 three 1-minute bouts of static or proprioceptive neuromuscular facilitation (hold-relax with agonist contraction) stretching did not lower cramp susceptibility. In observational studies of athletes, investigators21,22,30 also consistently failed to demonstrate relationships among flexibility, range of motion, and stretching frequency, duration, or timing and EAMC occurrence. Moreover, Golgi tendon organ inhibition was unaffected by a single bout of clinician-applied static stretching to the triceps surae both immediately and up to 30 minutes poststretching.57 Thus, pre-event stretching is unlikely to produce the long-lasting inhibition from Golgi tendon organs that would help prevent the overexcitation of the α motor-neuron pool thought to contribute to EAMC development.
Exercise and Neuromuscular Retraining Protocols
Neuromuscular retraining with exercise shows promise for EAMC prevention. Wagner et al3 found that a triathlete's hamstrings EAMCs were eliminated by lowering hamstrings activity during running and improving gluteal strength and endurance. To achieve this outcome, the patient required few professional visits (once a month for 8 months) and just a short (20-minute) daily at-home protocol.3 More recently, marathoners who experienced EAMCs were less likely to perform once-per-week lower extremity strength training in the 3 months leading up to a race than their noncramping counterparts (25% versus 48%, respectively),18 suggesting that strength training may be helpful in preventing EAMCs. Fatigue is hypothesized to be a main factor in EAMC development and overexertion is often tied to EAMCs,9 so it is vital to ensure that athletes exercise with appropriate work-to-rest ratios.2,31 Future researchers should explore if and how sport-specific exercise or endurance activities may prevent EAMCs.
CONCLUSIONS
Advances in our understanding of EAMC pathogenesis have emerged in the last 100 years and suggested that alterations in neuromuscular excitability and, to a much lesser extent, dehydration and electrolyte losses are the predominant factors in their pathogenesis. Strong evidence supports EAMC treatments that include exercise cessation (rest) and gentle stretching until abatement, followed by techniques to address the underlying precipitating factors. However, little patient-oriented evidence exists regarding the best methods for EAMC prevention. Therefore, rather than providing generalized advice, we recommend clinicians take a multifaceted and targeted approach that incorporates an individual's unique EAMC risk factors when trying to prevent EAMCs.
REFERENCES
- 1.Schwellnus M, Nicol J, Laubscher R, Noakes T. Serum electrolyte concentrations and hydration status are not associated with exercise associated muscle cramping (EAMC) in distance runners. Br J Sports Med . 2004;38(4):488–492. doi: 10.1136/bjsm.2003.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwabe K, Schwellnus MP, Derman W, Swanevelder S, Jordaan E. Less experience and running pace are potential risk factors for medical complications during a 56 km road running race: a prospective study in 26 354 race starters—SAFER study II. Br J Sports Med . 2014;48(11):905–911. doi: 10.1136/bjsports-2014-093471. [DOI] [PubMed] [Google Scholar]
- 3.Wagner T, Behnia N, Ancheta WKL, Shen R, Farrokhi S, Powers CM. Strengthening and neuromuscular reeducation of the gluteus maximus in a triathlete with exercise associated cramping of the hamstrings. J Orthop Sports Phys Ther . 2010;40(2):112–119. doi: 10.2519/jospt.2010.3110. [DOI] [PubMed] [Google Scholar]
- 4.Miller KC. WM Adams, Jardine JF., editors. Exercise-associated muscle cramps. Exertional Heat Illness A Clinical and EvidenceBased Guide . 2020. Springer; 117–136.
- 5.Bertolasi L, De Grandis D, Bongiovanni LG, Zanette GP, Gasperini M. The influence of muscular lengthening on cramps. Ann Neurol . 1993;33(2):176–180. doi: 10.1002/ana.410330207. [DOI] [PubMed] [Google Scholar]
- 6.Casa DJ, DeMartini JK, Bergeron MF, et al. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train . 2015;50(9):986–1000. doi: 10.4085/1062-6050-50.9.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper ER, Ferrara MS, Broglio SP. Exertional heat illness and environmental conditions during a single football season in the Southeast. J Athl Train . 2006;41(3):332–336. [PMC free article] [PubMed] [Google Scholar]
- 8.Bergeron MF. Muscle cramps during exercise—is it fatigue or electrolyte deficit. Curr Sports Med Rep . 2008;7(4):S50–S55. doi: 10.1249/JSR.0b013e31817f476a. [DOI] [Google Scholar]
- 9.Schwellnus MP. Cause of exercise associated muscle cramps (EAMC)—altered neuromuscular control, dehydration, or electrolyte depletion. Br J Sports Med . 2009;43(6):401–408. doi: 10.1136/bjsm.2008.050401. [DOI] [PubMed] [Google Scholar]
- 10.Ebell MH, Siwek J, Weiss BD, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract . 2004;17(1):59–67. doi: 10.3122/jabfm.17.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Talbott JH, Michelsen J. Heat cramps: a clinical and chemical study. J Clin Invest . 1933;12(3):533–549. doi: 10.1172/JCI100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergeron M. Heat cramps: fluid and electrolyte challenges during tennis in the heat. J Sci Med Sport . 2003;6(1):19–27. doi: 10.1016/s1440-2440(03)80005-1. [DOI] [PubMed] [Google Scholar]
- 13.Stofan JR, Zachwieja JJ, Horswill CA, Murray R, Anderson SA, Eichner ER. Sweat and sodium losses in NCAA football players: a precursor to heat cramps. Int J Sport Nutr Exerc Metab . 2005;15(6):641–652. doi: 10.1123/ijsnem.15.6.641. [DOI] [PubMed] [Google Scholar]
- 14.Miller KC, McDermott BP, Yeargin SW. Sweat characteristics of cramp-prone and cramp-resistant athletes. Int J Sports Nutr Exerc Metab . 2020;30(3):218–228. doi: 10.1123/ijsnem.2019-0308. [DOI] [PubMed] [Google Scholar]
- 15.Ohno M, Lavender AP, Sawai A. Heat-induced body fluid loss causes muscle cramp during maximal voluntary contraction for the knee flexors. Int J Sport Health Sci . 2018;16:191–199. doi: 10.5432/ijshs.201729. [DOI] [Google Scholar]
- 16.Szymanski M, Miller KC, O'Connor P, Hildebrandt L, Umberger L. Sweat characteristics in individuals with varying susceptibilities to exercise associated muscle cramps. J Strength Cond Res . 2020. [DOI] [PubMed]
- 17.Braulick KW, Miller KC, Albrecht JM, Tucker JM, Deal JE. Significant and serious dehydration does not affect skeletal muscle cramp threshold frequency. Br J Sports Med . 2012;47:710–714. doi: 10.1136/bjsports-2012-091501. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Navarro I, Montoya-Vieco A, Collado E, Hernando B, Panizo N, Hernando C. Muscle cramping in the marathon: dehydration and electrolyte depletion vs. muscle damage. J Strength Cond Res . 2020. [DOI] [PubMed]
- 19.Hoffman MD, Stuempfle KJ, Valentino T. Sodium intake during an ultramarathon does not prevent muscle cramping, dehydration, hyponatremia, or nausea. Sports Med Open . 2015;1(1):39–45. doi: 10.1186/s40798-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maughan RJ. Exercise induced muscle cramp: a prospective biochemical study in marathon runners. J Sports Sci . 1986;4(1):31–34. doi: 10.1080/02640418608732095. [DOI] [PubMed] [Google Scholar]
- 21.Summers KM, Snodgrass SJ, Callister R. Predictors of calf cramping in rugby league. J Strength Cond Res . 2014;28(3):774–783. doi: 10.1519/JSC.0b013e31829f360c. [DOI] [PubMed] [Google Scholar]
- 22.Schwellnus MP, Drew N, Collins M. Increased running speed and previous cramps rather than dehydration or serum sodium changes predict exercise associated muscle cramping: a prospective cohort study in 210 Ironman triathletes. Br J Sports Med . 2011;45(6):650–656. doi: 10.1136/bjsm.2010.078535. [DOI] [PubMed] [Google Scholar]
- 23.Costill DL, Coté R, Fink W. Muscle water and electrolytes following varied levels of dehydration in man. J Appl Physiol . 1976;40(1):6–11. doi: 10.1152/jappl.1976.40.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Hutton RS, Nelson DL. Stretch sensitivity of Golgi tendon organs in fatigued gastrocnemius muscle. Med Sci Sports Exerc . 1986;18(1):69–74. [PubMed] [Google Scholar]
- 25.Nelson DL, Hutton RS. Dynamic and static stretch responses in muscle spindle receptors in fatigued muscle. Med Sci Sports Exerc . 1985;17(4):445–450. doi: 10.1249/00005768-198508000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Khan SI, Burne JA. Afferents contributing to autogenic inhibition of gastrocnemius following electrical stimulation of its tendon. Brain Res . 2009;1282:28–37. doi: 10.1016/j.brainres.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Khan SI, Burne JA. Reflex inhibition of normal cramp following electrical stimulation of the muscle tendon. J Neurophysiol . 2007;98(3):1102–1107. doi: 10.1152/jn.00371.2007. [DOI] [PubMed] [Google Scholar]
- 28.Minetto MA, Holobar A, Botter A, Ravenni R, Farina D. Mechanisms of cramp contractions: peripheral or central generation. J Physiol . 2011;589(Pt 23):5759–5773. doi: 10.1113/jphysiol.2011.212332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller KC, Long BC, Edwards JE. Muscle cramp susceptibility increases following a volitionally induced muscle cramp. Muscle Nerve . 2017;56(6):E95–E99. doi: 10.1002/mus.25562. [DOI] [PubMed] [Google Scholar]
- 30.Shang G, Collins M, Schwellnus MP. Factors associated with a self-reported history of exercise-associated muscle cramps in Ironman triathletes: a case-control study. Clin J Sport Med . 2011;21(3):204–210. doi: 10.1097/JSM.0b013e31820bcbfd. [DOI] [PubMed] [Google Scholar]
- 31.Schwellnus MP, Allie S, Derman W, Collins M. Increased running speed and pre-race muscle damage as risk factors for exercise-associated muscle cramps in a 56 km ultra-marathon: a prospective cohort study. Br J Sports Med . 2011;45(8):650–656. doi: 10.1136/bjsm.2010.078535. [DOI] [PubMed] [Google Scholar]
- 32.Stone MB, Edwards JE, Huxel KC, Cordova ML, Ingersoll CD, Babington JP. Threshold frequency of an electrically induced cramp increases following a repeated, localized fatiguing exercise. J Sports Sci . 2010;28(4):399–405. doi: 10.1080/02640410903508854. [DOI] [PubMed] [Google Scholar]
- 33.Minetto MA, Botter A. Elicitability of muscle cramps in different leg and foot muscles. Muscle Nerve . 2009;40(4):535–544. doi: 10.1002/mus.21382. [DOI] [PubMed] [Google Scholar]
- 34.Miller KC, Knight KL. Electrical stimulation cramp threshold frequency correlates well with the occurrence of skeletal muscle cramps. Muscle Nerve . 2009;39(3):364–368. doi: 10.1002/mus.21170. [DOI] [PubMed] [Google Scholar]
- 35.Maquirriain J, Merello M. The athlete with muscular cramps: clinical approach. J Am Acad Orthop Surg . 2007;15(7):425–431. doi: 10.5435/00124635-200707000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman MD, Fogard K. Factors related to successful completion of a 161-km ultramarathon. Int J Sports Physiol Perform . 2011;6(1):25–37. doi: 10.1123/ijspp.6.1.25. [DOI] [PubMed] [Google Scholar]
- 37.McCartney D, Desbrow B, Irwin C. The effect of fluid intake following dehydration on subsequent athletic and cognitive performance: a systematic review and meta-analysis. Sports Med Open . 2017;3(1):13. doi: 10.1186/s40798-017-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott BP, Anderson SA, Armstrong LE, et al. National Athletic Trainers' Association position statement: fluid replacement for the physically active. J Athl Train . 2017;52(9):877–895. doi: 10.4085/1062-6050-52.9.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Givan GV, Diehl JJ. Intravenous fluid use in athletes. Sports Health . 2012;4(4):333–339. doi: 10.1177/1941738112446285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maresh CM, Herrera-Soto JA, Armstrong LE, et al. Perceptual responses in the heat after brief intravenous versus oral rehydration. Med Sci Sports Exerc . 2001;33(6):1039–1045. doi: 10.1097/00005768-200106000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Behringer M, Nowak S, Leyendecker J, Mester J. Effects of TRPV1 and TRPA1 activators on the cramp threshold frequency: a randomized, double-blind placebo-controlled trial. Eur J Appl Physiol . 2017;117(8):1641–1647. doi: 10.1007/s00421-017-3653-6. [DOI] [PubMed] [Google Scholar]
- 42.Craighead DH, Shank SW, Gottschall JS, et al. Ingestion of transient receptor potential channel agonists attenuates exercise-induced muscle cramps. Muscle Nerve . 2017;56(3):379–385. doi: 10.1002/mus.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper-Marosek SE, Antharam V, Dowlatshahi K. Quantitative analysis of the acetic acid content in substances used by athletes for the possible prevention and alleviation of exercise-associated muscle cramps. J Strength Cond Res . 2020;34(6):1539–1546. doi: 10.1519/JSC.0000000000003595. [DOI] [PubMed] [Google Scholar]
- 44.Miller KC, Mack GW, Knight KL, et al. Reflex inhibition of electrically induced muscle cramps in hypohydrated humans. Med Sci Sports Exerc . 2010;42(5):953–961. doi: 10.1249/MSS.0b013e3181c0647e. [DOI] [PubMed] [Google Scholar]
- 45.Miller KC. Electrolyte and plasma responses following pickle juice, mustard, and deionized water ingestion in dehydrated humans. J Athl Train . 2014;49(3):360–367. doi: 10.4085/1062-6050-49.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller KC. Plasma potassium concentration and content changes following banana ingestion in exercised males. J Athl Train . 2012;47(6):648–654. doi: 10.4085/1062-6050-47.6.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Tawil S, Al Musa T, Valli H, et al. Quinine for muscle cramps. Cochrane Database Syst Rev 2015(4)CD005044. [DOI] [PubMed]
- 48.Hoffman MD, Stuempfle KJ. Muscle cramping during a 161-km ultramarathon: comparison of characteristics of those with and without cramping. Sports Med Open . 2015;1(1):24–33. doi: 10.1186/s40798-015-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skein M, Duffield R, Edge J, Short MJ, Mündel T. Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc . 2011;43(7):1301–1311. doi: 10.1249/MSS.0b013e31820abc5a. [DOI] [PubMed] [Google Scholar]
- 50.Stone MB, Edwards JE, Stemmans CL, Ingersoll CD, Palmieri RM, Krause BA. Certified athletic trainers' perceptions of exercise associated muscle cramps. J Sport Rehabil . 2003;12(4):333–342. doi: 10.1123/jsr.12.4.333. [DOI] [Google Scholar]
- 51.Bergeron MF. Heat cramps during tennis: a case report. Int J Sport Nutr . 1996;6(1):62–68. doi: 10.1123/ijsn.6.1.62. [DOI] [PubMed] [Google Scholar]
- 52.Garrison SR, Korownyk CS, Kolber MR, et al. Magnesium for skeletal muscle cramps. Cochrane Database Syst Rev . 2020. 9(9):CD009402. [DOI] [PMC free article] [PubMed]
- 53.Jung AP, Bishop PA, Al-Nawwas A, Dale RB. Influence of hydration and electrolyte supplementation on incidence and time to onset of exercise-associated muscle cramps. J Athl Train . 2005;40(2):71–75. [PMC free article] [PubMed] [Google Scholar]
- 54.Lau WY, Kato H, Nosaka K. Water intake after dehydration makes muscles more susceptible to cramp but electrolytes reverse that effect. BMJ Open Sport Exerc Med . 2019;5(1):e000478–e000484. doi: 10.1136/bmjsem-2018-000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalmar JM, Cafarelli E. Caffeine: a valuable tool to study central fatigue in humans. Exerc Sport Sci Rev . 2004;32(4):143–147. doi: 10.1097/00003677-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Miller KC, Harsen JD, Long BC. Prophylactic stretching does not reduce cramp susceptibility. Muscle Nerve . 2017;57(3):473–477. doi: 10.1002/mus.25762. [DOI] [PubMed] [Google Scholar]
- 57.Miller KC, Burne JA. Golgi tendon organ reflex inhibition following manuallyapplied acute static stretching J Sports Sci. 2014;32(15):1491–1497. doi: 10.1080/02640414.2014.899708. [DOI] [PubMed] [Google Scholar]