Abstract
Objective:
To determine the association of socioeconomic status at the census block group level with chronic conditions, and to determine whether the associations differ by age, sex, race, or ethnicity.
Patients and Methods:
Adults aged ≥20 years on April 1, 2015 from 7 counties in Southern Minnesota were identified using the Rochester Epidemiology Project records-linkage system. We estimated the prevalence of 19 chronic conditions (7 cardiometabolic, 7 other somatic, and 5 mental health conditions) at the individual level, and a composite measure of neighborhood socioeconomic disadvantage (the area deprivation index; ADI) at the census block group level (n=249).
Results:
Among the 197,578 persons in our study, 46.7% were male, 49.5% were aged ≥50 years, 12.3% were of non-White race, and 5.3% were Hispanic. The risk of most chronic conditions increased with increasing ADI. For each cardiometabolic condition and most other somatic and mental health conditions, the pattern of increasing risk across ADI quintiles was attenuated, or there was no association across quintiles of ADI in the oldest age group (ages ≥70 years). Stronger associations between ADI and several cardiometabolic, other somatic, and mental health conditions were observed in women.
Conclusion:
Higher ADI was associated with increased risk of most chronic conditions, with more pronounced associations in younger persons. For some chronic conditions, the associations were stronger in women. Our findings underscore the importance of recognizing the overall and potentially differential impact of area-level deprivation on chronic disease outcomes for diverse populations.
Keywords: socioeconomic status, area deprivation index, geocoding, US Census, chronic conditions, demographic variables
INTRODUCTION
Over the past 25 years, health equity has not improved in the United States. The Behavioral Risk Factor Surveillance System showed that measures of health equity and justice have declined over time, and income disparities have worsened.1 Persons with lower socioeconomic status generally experience higher prevalence of chronic conditions and multimorbidity, as well as poorer outcomes including increased mortality.2–6 Individual measures of socioeconomic status, including income and education, have been widely studied in relation to health; however, the effect of neighborhood context on health has been gaining attention more recently.
We and others have previously shown that lower neighborhood socioeconomic status is associated with higher prevalence of multimorbidity.7–15 Furthermore, our study in a large cohort of nearly 200,000 persons found that a measure of neighborhood socioeconomic disadvantage, the area deprivation index (ADI), was independently associated with multimorbidity after adjustment for individual level of education.12 We also found differences in association by age and sex, with stronger associations in younger persons and in women.12 However, we did not investigate whether associations differed by race or ethnicity, and did not determine whether certain individual chronic conditions exhibited stronger associations with neighborhood deprivation than others.
Neighborhood context can affect safety, access to food, health behaviors, education, social connections, and stress,16 and may affect a person’s overall health beyond the effect of individual measures of socioeconomic status.17 The social-ecological theory recognizes that individuals are embedded within larger social systems, and that multiple levels of influence beyond biological processes including social, physical, and cultural aspects of an environment interact to effect health.18 Furthermore, the influence of the environmental context on individual health may differ depending on unique beliefs and practices of individuals.18 Thus, we used the social-ecological theory as a conceptual framework to guide our study. The purpose of the current study was to investigate the associations of a measure of neighborhood socioeconomic deprivation, the ADI, with individual chronic conditions in a 7-county region in Southern Minnesota. Acknowledging that the unique beliefs, practices, and experiences of individuals may differentially impact the influence of neighborhood context with health, we determined whether the associations of ADI with chronic conditions differed by age, sex, race, or ethnicity.
METHODS
Study Population
This study was conducted using the expanded Rochester Epidemiology Project medical records-linkage system (E-REP).19 The E-REP captures electronic medical record data for the population residing in a 27-county region in Southern Minnesota and Western Wisconsin. For this study, we utilized a 7-county high capture region of the E-REP, which includes data from 93.8% of the population in the following counties in Southern Minnesota: Olmsted, Wabasha, Dodge, Mower, Steele, Waseca, and Freeborn. All adults (aged ≥20 years) who resided in the 7-county region on April 1, 2015 and who provided authorization to use their medical records for research (Minnesota Research Authorization; obtained from 92% of the REP population) were included in this study (n=206,849). This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Ascertainment of Chronic Conditions
We electronically searched the diagnostic indices of the REP to identify the International Classification of Diseases, Ninth & Tenth Revision codes associated with any health care visit (ICD-9 or ICD-10 inpatient or outpatient codes from all providers indexed in the REP) from April 1, 2010 through March 31, 2015 (5-year capture frame). The ICD-9 or ICD-10 diagnostic codes were used to identify a list of 20 chronic conditions defined by the United States Department of Health and Human Services (DHHS).20,21 Two conditions, autism and human immunodeficiency virus, were later excluded from the analyses due to low prevalence in adults (<0.5%). In addition, anxiety was added, because it is common in the United States population. The diagnostic codes used to define the 19 chronic conditions have been defined previously.12 We required 2 occurrences of a code (within the disease code set) separated by more than 30 days to decrease the chance of false-positive diagnoses. In the presentation of results, the 19 individual conditions were grouped into cardiometabolic conditions (hypertension, congestive heart failure, coronary artery disease, cardiac arrhythmias, hyperlipidemia, stroke, and diabetes), other somatic conditions (arthritis, asthma, cancer, chronic kidney disease, chronic obstructive pulmonary disease, hepatitis, and osteoporosis), and mental health conditions (dementia, depression, schizophrenia, substance abuse disorders, and anxiety).
Calculation of the Area Deprivation Index (ADI)
Each person’s geolocation was calculated by linking addresses to the TIGER/Line address range shapefile provided by the United States Census.22 We successfully geocoded 198,941 of 206,849 (96.2%) persons. Geocoded data were linked to census block groups (251 census block groups), and publicly available data were obtained for ADI at the census block group level.23,24 The ADI was not available for 2 of the census block groups, and thus we retained 249 of the census block groups for our analysis (N=197,578 persons). Specifically, we used Version 3 2018 ADI estimates for the year 2015. We obtained the United States ADI percentile rankings (1-100), and the distribution of these ADI values were stratified into quintiles for analyses. Higher values of the ADI score indicate greater disadvantage.
Statistical Analysis
Guided by the social-ecological theory framework, we examined individual and area level factors and evaluated interactions between levels. Descriptive characteristics at the individual level were summarized overall and by age group using number and % for categorical variables. Hierarchical logistic regression, with a random effect of census block group to adjust for clustering,25,26 was used to model the association of each individual condition with the ADI quintiles (with quintile 1 serving as the reference group). For models with rare events, Firth penalized likelihood methods were used. Results were presented as odds ratios (OR) and 95% confidence intervals (CI). An unadjusted model and a model adjusted for age (20-39, 40-49, 50-59, 60-69, 70-79, ≥80 years), sex, race (White, Black, Asian, other/unknown), ethnicity (Hispanic, non-Hispanic), and education (high school or less, some college, college or advanced degree, unknown) were developed. Two-way interactions were tested between ADI and age, sex, race, and ethnicity. Significance of the interactions was determined after adjustment for multiple comparisons using the false discovery rate (FDR).27 For interactions that reached significance, forest plots were used to display the fully-adjusted ORs in graphical form in strata by age (for the age by ADI interaction), by sex (for the sex by ADI interaction), by race (for the race by ADI interaction), and by ethnicity (for the ethnicity by ADI interaction). For completeness, the fully-adjusted ORs in strata by age, sex, race, and ethnicity were presented in tabular format for all of the chronic conditions. Analyses were performed using ArcGIS 10.3, SAS 9.4 (SAS Institute Inc., Cary, NC), and R version 3.2.3. Tests of statistical significance were conducted at the two-tailed alpha level of 0.05, and a FDR of 0.05 was used for interaction tests.
RESULTS
Of the 206,849 persons in our population, we excluded 6,480 (3.1%) persons because we could not geocode their address, and 1,363 (0.7%) persons who resided in the 2 census block groups missing ADI. The persons not geocoded were older (median age 53 years) whereas persons missing ADI were younger (median age 46 years) compared to persons included in our study (median age 49 years). In addition, persons in the census block groups missing ADI were more likely to be Hispanic (10.0%) compared to persons included in our study (5.3%). Finally, the proportion of persons with multimorbidity (≥2 chronic conditions) was lower for persons not geocoded (34.0%) and higher for persons missing ADI (42.9%) compared to persons in the final study cohort (39.5%).
Among the 197,578 persons in our study, 46.7% were male, 49.5% were aged 50 years and older, 12.3% were of non-White race (3.4% Black, 3.1% Asian, 3.6% other, and 2.2% unknown race), and 5.3% were Hispanic (Supplemental Table 1). The prevalence of all cardiometabolic and most other somatic conditions increased with increasing age. By contrast, most of the mental health conditions did not increase in prevalence with increasing age except for dementia which was most common among those aged ≥70 years.
The distribution of ADI in our 7-county region was less diverse than in the entire United States. Only 7.0% of our population (25 census block groups) were in the most deprived quintile using the nationally-ranked ADI. After adjusting for age, sex, race, ethnicity, and individual level of education, an increasing risk of each cardiometabolic condition and each mental health condition was observed with increasing ADI (Table 1). All tests for a linear trend across ADI quintiles were significant with one exception. No significant trend across ADI quintiles was observed for cancer.
Table 1.
Odds ratio (95% confidence interval)a of each chronic condition considered separately across national quintiles of area deprivation indexb
| Chronic condition | Quintile 2 (21-40%) |
Quintile 3 (41-60%) |
Quintile 4 (61-80%) |
Quintile 5 (81-100%) |
P-trendc |
|---|---|---|---|---|---|
| Cardiometabolic conditions | |||||
| Hypertension | 1.35 (1.15,1.58) | 1.66 (1.42,1.94) | 1.89 (1.62,2.21) | 2.57 (2.15,3.06) | <0.001 |
| Hyperlipidemia | 1.14 (0.97,1.34) | 1.36 (1.15,1.59) | 1.42 (1.21,1.68) | 1.88 (1.57,2.26) | <0.001 |
| Diabetes | 1.12 (1.00,1.25) | 1.24 (1.11,1.38) | 1.37 (1.23,1.53) | 1.71 (1.52,1.94) | <0.001 |
| Cardiac arrhythmias | 1.05 (0.93,1.19) | 1.14 (1.01,1.28) | 1.30 (1.15,1.47) | 1.55 (1.34,1.78) | <0.001 |
| Coronary artery disease | 1.13 (0.98,1.31) | 1.33 (1.15,1.53) | 1.51 (1.30,1.75) | 1.88 (1.59,2.22) | <0.001 |
| Congestive heart failure | 1.36 (1.08,1.72) | 1.56 (1.24,1.96) | 1.94 (1.54,2.45) | 2.48 (1.92,3.21) | <0.001 |
| Stroke | 1.17 (0.97,1.40) | 1.28 (1.07,1.53) | 1.47 (1.23,1.77) | 1.73 (1.40,2.13) | <0.001 |
| Other somatic conditions | |||||
| Arthritis | 0.96 (0.87,1.07) | 1.03 (0.93,1.14) | 1.07 (0.96,1.19) | 1.12 (0.99,1.27) | <0.001 |
| Osteoporosis | 0.87 (0.70,1.06) | 0.86 (0.71,1.05) | 1.00 (0.82,1.23) | 1.12 (0.89,1.42) | 0.0040 |
| Cancer | 0.93 (0.83,1.05) | 0.94 (0.84,1.06) | 0.94 (0.84,1.06) | 0.98 (0.86,1.13) | 0.8005 |
| Chronic kidney disease | 1.30 (1.09,1.55) | 1.57 (1.32,1.87) | 1.83 (1.54,2.17) | 2.58 (2.13,3.14) | <0.001 |
| Hepatitis | 0.86 (0.61,1.23) | 1.17 (0.83,1.65) | 1.58 (1.12,2.24) | 2.60 (1.77,3.82) | <0.001 |
| Asthma | 0.97 (0.86,1.09) | 1.11 (0.98,1.25) | 1.30 (1.15,1.47) | 1.75 (1.53,2.01) | <0.001 |
| Chronic obstructive pulmonary disease | 1.38 (1.10,1.73) | 1.80 (1.44,2.25) | 2.52 (2.01,3.15) | 4.24 (3.32,5.41) | <0.001 |
| Mental health conditions | |||||
| Dementia | 0.96 (0.74,1.25) | 0.99 (0.77,1.29) | 1.29 (0.99,1.67) | 1.36 (1.01,1.83) | <0.001 |
| Anxiety | 1.13 (0.98,1.29) | 1.34 (1.17,1.53) | 1.67 (1.46,1.92) | 2.41 (2.07,2.80) | <0.001 |
| Depression | 1.22 (1.07,1.39) | 1.50 (1.32,1.71) | 1.87 (1.64,2.13) | 2.54 (2.20,2.94) | <0.001 |
| Schizophrenia | 0.99 (0.66,1.48) | 1.21 (0.82,1.80) | 2.00 (1.35,2.97) | 3.14 (2.05,4.83) | <0.001 |
| Substance abuse disorders | 1.16 (0.90,1.49) | 1.46 (1.14,1.87) | 2.15 (1.68,2.76) | 3.57 (2.72,4.69) | <0.001 |
Adjusted for age, sex, race, ethnicity, and individual level of education.
The area deprivation index was estimated at the census block group level. Quintiles were defined using national area deprivation index rankings from the Neighborhood Atlas (https://www.neighborhoodatlas.medicine.wisc.edu/). Quintile 1 (1-20%) served as the reference (OR=1.00).
Differences across quintiles were tested using a test for linear trend of the ORs. The P-value for trend was adjusted for the false discovery rate.
Differences in associations of ADI with chronic conditions by age, sex, race, and ethnicity were tested using two-way interactions. Results stratified by age (Supplemental Table 2), sex (Supplemental Table 3), race (Supplemental Table 4), and ethnicity (Supplemental Table 5) are provided for all conditions regardless of statistical significance of the interactions. By contrast, figures 1–4 summarize the stratified results only for the interactions that reached statistical significance.
Figure 1.
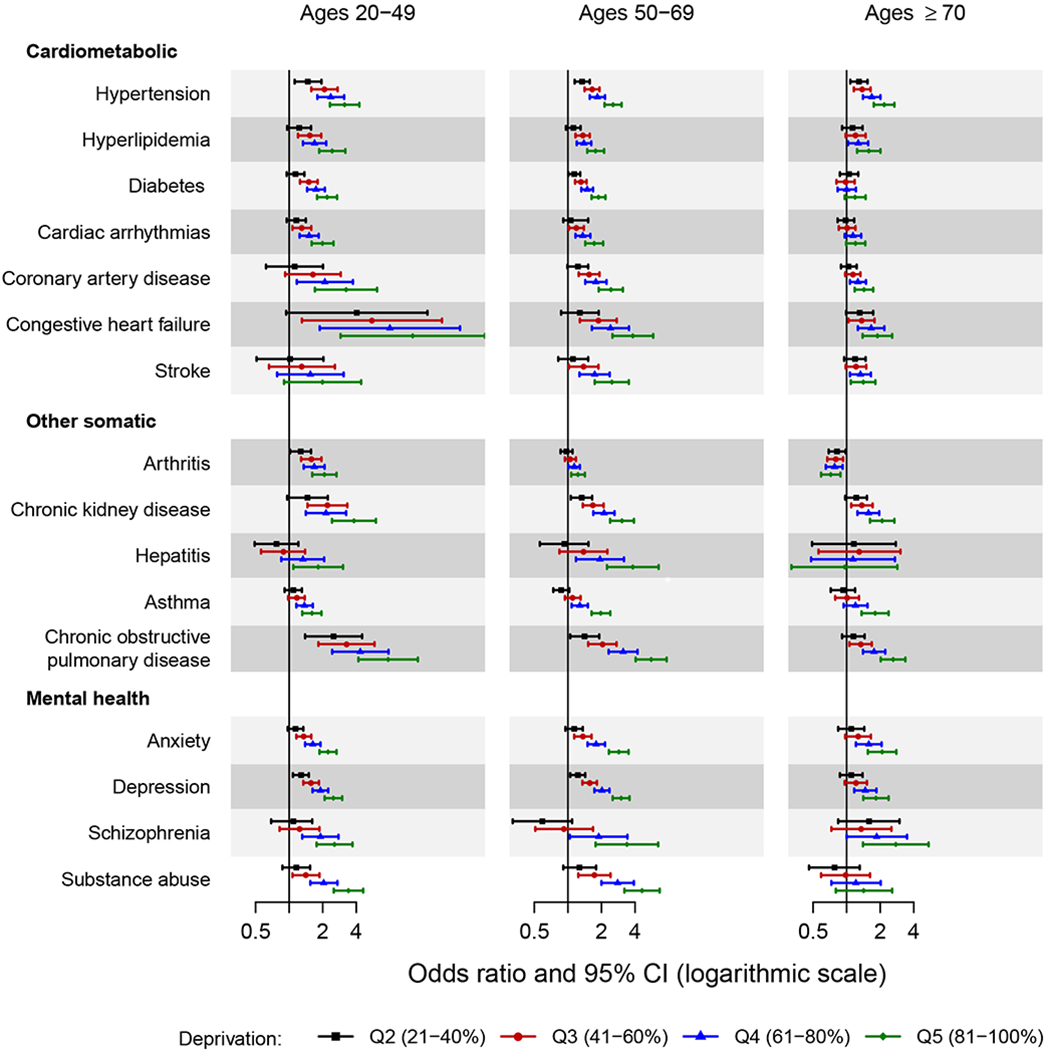
Forest plot showing the odds ratio and 95% confidence interval of chronic conditions across quintiles of area deprivation index (ADI) by age groups.
The area deprivation index was estimated at the census block group level. Quintiles were defined using national area deprivation index rankings from the Neighborhood Atlas (https://www.neighborhoodatlas.medicine.wisc.edu/). Quintile 1 (1-20%) served as the reference.
Only the chronic conditions for which the two-way interactions of area deprivation index by age reached statistical significance are presented in the figure (16 chronic conditions). Odds ratios were adjusted for sex, race, ethnicity, and individual level of education.
CI, confidence interval.
Figure 4.
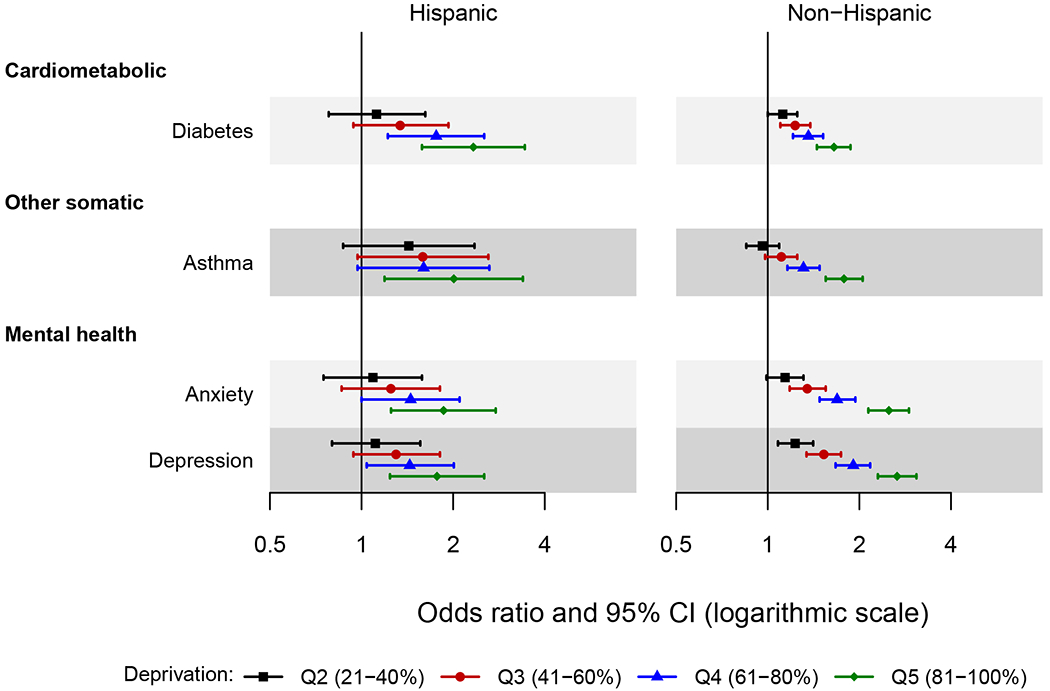
Forest plot showing the odds ratio and 95% confidence interval of chronic conditions across quintiles of area deprivation index (ADI) by ethnicity.
The area deprivation index was estimated at the census block group level. Quintiles were defined using national area deprivation index rankings from the Neighborhood Atlas (https://www.neighborhoodatlas.medicine.wisc.edu/). Quintile 1 (1-20%) served as the reference.
Only the chronic conditions for which the two-way interactions of area deprivation index by ethnicity reached statistical significance are presented in the figure (4 chronic conditions). Odds ratios were adjusted for age, sex, race, and individual level of education.
CI, confidence interval.
Interactions with Age
The associations of ADI with each cardiometabolic condition varied by age (Figure 1). An increasing risk of the condition was observed with increasing quintile of ADI in the youngest 2 age groups (ages 20-69 years). However, the pattern of increasing risk across ADI quintiles was attenuated, or there was no association across quintiles of ADI in the oldest age group (ages ≥70 years). For example, the risk of hypertension for those residing in the highest deprivation quintile compared to the lowest deprivation quintile (quintile 5 vs. 1) was approximately 3-fold for ages 20-49 years (OR 3.14, 95% CI 2.32-4.26) and 2-fold for ages ≥70 years (OR 2.17, 95% CI 1.75-2.68). For diabetes, a more than 2-fold increased risk was observed for ages 20-49 years (OR 2.19, 95% CI 1.78-2.69), whereas no increased risk was observed for ages ≥70 years (OR 1.19, 95% CI 0.96-1.48). For the other somatic conditions, there was evidence of interactions between age and ADI for arthritis, chronic kidney disease, hepatitis, asthma, and chronic obstructive pulmonary disease. For hepatitis, there was no association across quintiles of ADI in persons aged ≥70 years, but for the other conditions the associations attenuated with increasing age. Finally, for the mental health conditions, interactions between age and ADI were observed for anxiety, depression, schizophrenia, and substance abuse disorders, with attenuated results observed in those ≥70 years of age.
Interactions with Sex
For the cardiometabolic conditions hyperlipidemia, diabetes, cardiac arrhythmias, and coronary artery disease, the patterns were generally similar in men and women, but we found stronger associations with increasing ADI quintile in women compared to men (Figure 2). For example, in women the risk of hyperlipidemia was 2.2-fold higher for those residing in the highest deprivation quintile (quintile 5 vs. 1; OR 2.18, 95% CI 1.79-2.66), whereas in men it was 1.6-fold higher (OR 1.61, 95% CI 1.33-1.95). For other somatic conditions, stronger associations were observed in women for arthritis and osteoporosis. In particular, there was no difference in association across ADI quintiles for arthritis in men. For osteoporosis, the wide confidence intervals in men (due to the low prevalence of osteoporosis) obscured the pattern of association. Finally, for mental health conditions, similar patterns were observed in men and women, with modestly stronger associations in women for depression.
Figure 2.
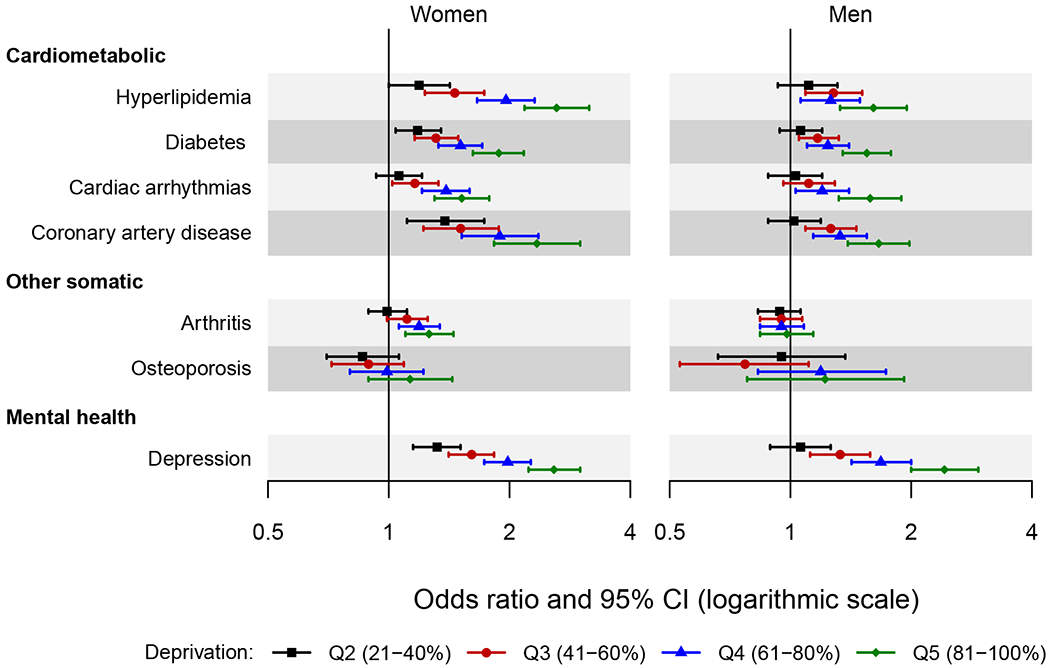
Forest plot showing the odds ratio and 95% confidence interval of chronic conditions across quintiles of area deprivation index (ADI) by sex.
The area deprivation index was estimated at the census block group level. Quintiles were defined using national area deprivation index rankings from the Neighborhood Atlas (https://www.neighborhoodatlas.medicine.wisc.edu/). Quintile 1 (1-20%) served as the reference.
Only the chronic conditions for which the two-way interactions of area deprivation index by sex reached statistical significance are presented in the figure (7 chronic conditions). Odds ratios were adjusted for age, race, ethnicity, and individual level of education.
CI, confidence interval.
Interactions with Race
An increasing risk of hyperlipidemia with increasing quintile of ADI was observed in whites, but was less apparent in non-whites (Figure 3). The large confidence intervals in non-whites made it difficult to interpret some of the significant interactions for the other somatic and mental health conditions. For example, the associations were generally similar across ADI quintile in whites and non-whites for arthritis. An increasing risk of hepatitis was observed with increasing quintile of ADI in whites, but the pattern was not as pronounced in non-whites. Finally, for the mental health conditions, the increased associations with ADI were not as strong in non-whites, particularly for anxiety and depression. The pattern appeared similar across race groups for substance abuse disorders because the large confidence intervals in non-whites obscured the differences.
Figure 3.
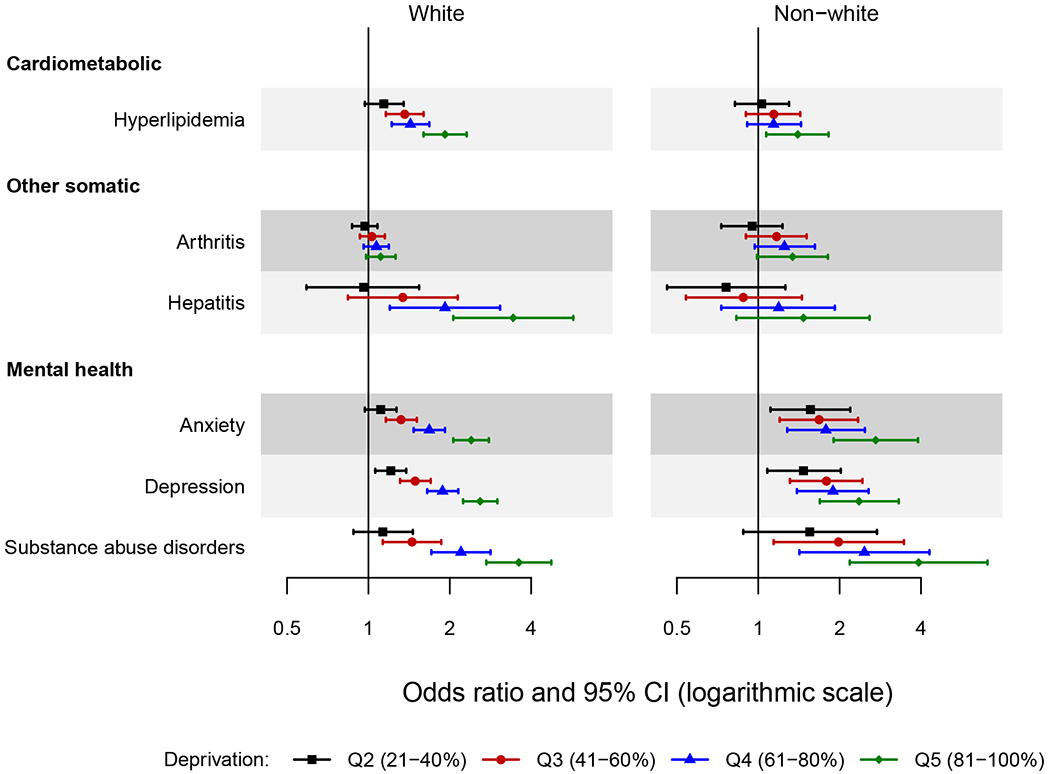
Forest plot showing the odds ratio and 95% confidence interval of chronic conditions across quintiles of area deprivation index (ADI) by race.
The area deprivation index was estimated at the census block group level. Quintiles were defined using national area deprivation index rankings from the Neighborhood Atlas (https://www.neighborhoodatlas.medicine.wisc.edu/). Quintile 1 (1-20%) served as the reference.
Only the chronic conditions for which the two-way interactions of area deprivation index by race reached statistical significance are presented in the figure (6 chronic conditions). Odds ratios were adjusted for age, sex, ethnicity, and individual level of education.
CI, confidence interval.
Interactions with Ethnicity
The large confidence intervals in Hispanics made it difficult to interpret the significant interactions with ethnicity (Figure 4). The pattern of increasing risk across ADI quintiles appeared stronger in Hispanics for diabetes. For asthma, anxiety, and depression, similar patterns were observed across ethnic groups with modestly stronger associations in non-Hispanics.
DISCUSSION
In this large population-based study in a 7-county region in Southern Minnesota, using the social-ecological theory as a conceptual framework, we studied the associations of an area-level measure of socioeconomic status (the ADI) with 19 chronic conditions, and determined whether the associations differed by age, sex, race, or ethnicity. An increasing risk of most chronic conditions with increasing ADI was observed, except for cancer. We observed some differences in associations by age, sex, race, and ethnicity. In particular, we observed differences by age for the associations of ADI with each cardiometabolic condition and with most other somatic and mental health conditions, with attenuated results or no difference across ADI in persons aged ≥70 years. Stronger associations were observed in women compared to men between ADI and several cardiometabolic, other somatic, and mental health conditions. Some statistically significant interactions by race and ethnicity were observed. However, the limited power, reflected in the wide confidence intervals for non-whites and Hispanics, made it difficult to interpret some of the interactions.
We have previously shown that lower neighborhood socioeconomic status is associated with higher prevalence of multimorbidity, with stronger associations observed in younger persons and in women.12 Similarly, our current findings suggest that for the majority of chronic conditions considered separately, stronger associations were observed for younger persons, with attenuation of the association in persons aged ≥70 years. One possible explanation of the observed differences across age is a selective survival effect where the most disadvantaged persons may die younger compressing the difference in associations across ADI at older ages. In addition, it is possible that as people age, their socioeconomic status has less of an impact on disease burden because age itself becomes a more important risk factor for the development of chronic conditions.28–31
For some chronic conditions, we also observed modestly stronger associations in women compared to men. However, sex differences in the association with ADI were not observed for approximately half of the chronic conditions. This finding is inconsistent with some evidence that the socioeconomic gradient is stronger in men for most conditions, perhaps with the exception of cardiovascular conditions where the socioeconomic gradient is greater in women.32–34
Differences by race and ethnicity in the associations of ADI with chronic conditions were less clear because of the small number of non-whites and Hispanics with diagnoses of certain chronic conditions. It should be noted that racism or implicit bias, as well as access to care, may have differentially affected the diagnosis or treatment of some conditions.35 If the most deprived non-whites and Hispanics were less likely to receive diagnoses, this could explain the stronger associations between ADI and some chronic conditions in whites and non-Hispanics. However, our sample size was limited to further stratify results by race and ethnicity. Future research using more racially and ethnically diverse populations is warranted to better understand these associations.
We adjusted our models for individual level of education, providing evidence that neighborhood socioeconomic status may influence health above and beyond individual measures of socioeconomic status. However, future research accounting for other individual measures of socioeconomic status may be warranted to better understand the contribution of neighborhood and group-level variables vs. individual socioeconomic status on health.
Implications and Future Directions
Disparities in the prevalence of chronic conditions exist in relation to the neighborhood in which a person lives. Thus, neighborhood context may provide for critical information when targeting interventions for prevention, or for the management of chronic conditions.36,37 The ADI is a simple measure that can be calculated as long as a person’s address is known, and has been made available for 2013 and 2015 for all census block groups in the United States by Kind and colleagues at the University of Wisconsin-Madison.23,24 The ADI could be used to ensure that health care systems and providers are prepared to care for populations that have experienced deprivation, racism, or other social determinants of health. The ADI could be used for tailoring patient-centered care, as demonstrated by Intermountain Healthcare where the ADI was added to an existing risk score to identify patients who may benefit from community-based care management interventions.38 In addition, the ADI could be used to identify neighborhoods to solicit stakeholder engagement for guidance on the design of research studies, and for the recruitment and retention of research participants to ensure adequate representation and inclusion of participants who may benefit most from the research findings.
Limitations and Strengths
The following limitations should be acknowledged. First, we required 2 occurrences of a code separated by more than 30 days to define each chronic condition. Although this rule was employed to reduce false positive diagnoses, it may have resulted in some misclassification of chronic conditions. Second, it is important to note that the prevalence of the chronic conditions in our population may be affected by the degree of access to the health care system, and by both diagnostic and coding practices. This may vary by ADI, and may be affected by structural factors in health care systems and providers, which can include implicit biases that affect treatment (and therefore diagnoses). Third, the relatively small number of non-whites and Hispanics in our population limited our power to detect and interpret interactions by race and ethnicity for some chronic conditions. Thus, additional research in more diverse populations may be warranted. Further, the interpretation of differences by race and ethnicity needs to consider the role that racism plays in social determinants of health and health outcomes. Finally, it is important to note that our region is less racially and ethnically diverse and has lower rates of poverty compared to national averages.39 It is possible that the relationships we observed between ADI and chronic conditions would differ in populations with a greater degree of area deprivation. However, to increase the generalizability of our findings, we utilized a nationally-ranked ADI obtained from the Neighborhood Atlas.23,24
The following strengths of our study should be noted. First, our population included nearly 200,000 adults of all ages residing in a 7-county region, which allowed us to explore whether associations of ADI with chronic conditions differed by age, sex, race, or ethnicity. Second, we were able to successfully geocode >95% of the population to link the ADI, a composite area-level measure of socioeconomic status at the census block group level (n=249 census block groups). Third, we were able to link individual data on chronic conditions from the E-REP to the area-level ADI. Finally, although individual measures of socioeconomic status are not widely available in medical records, we had adequate information on education, and we found significant residual associations of ADI with chronic conditions after adjustment for education, suggesting that neighborhood context plays a role in health above and beyond the effect of individual socioeconomic status.
CONCLUSIONS
Higher ADI was associated with increased risk of most chronic conditions considered separately. More pronounced associations were generally observed in younger persons, with results attenuated in those aged ≥70 years. In addition, stronger associations were observed in women between ADI and several cardiometabolic, other somatic, and mental health conditions. Differences were less pronounced by race and ethnicity. Our findings underscore the importance of recognizing the overall and potentially differential impact of area-level deprivation on chronic disease outcomes for diverse populations.
Supplementary Material
Funding/Support:
This work was supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, the National Institute on Aging (R01 AG052425), and was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging (AG058738), the Mayo Clinic Research Committee, and by fees paid annually by REP users.
Role of Funder/Sponsor:
The funding sources played no role in the design of the study, the analysis, the interpretation of study results, or the writing of the manuscript.
Abbreviations
- ADI
area deprivation index
- CI
confidence interval
- E-REP
expanded Rochester Epidemiology Project
- FDR
false discovery rate
- OR
odds ratio
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Zimmerman FJ, Anderson NW. Trends in health equity in the United States by race/ethnicity, sex, and income, 1993-2017. JAMA Netw Open. 2019;2(6):e196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42(2):186–194. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Mishra GD, Jones M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev. 2017;37:53–68. [DOI] [PubMed] [Google Scholar]
- 4.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;315(16):1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawshani A, Svensson A-M, Zethelius B, Eliasson B, Rosengren A, Gudbjörnsdottir S. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with type 2 diabetes. JAMA Intern Med. 2016;176(8):1146–1154. [DOI] [PubMed] [Google Scholar]
- 7.Katikireddi SV, Skivington K, Leyland AH, Hunt K, Mercer SW. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the Twenty-07 cohort. BMC Med. 2017;15(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macleod U, Mitchell E, Black M, Spence G. Comorbidity and socioeconomic deprivation: an observational study of the prevalence of comorbidity in general practice. Eur J Gen Pract. 2004;10(1):24–26. [DOI] [PubMed] [Google Scholar]
- 9.Mercer SW, Watt GC. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med. 2007;5(6):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61(582):e12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker AE. Multiple chronic diseases and quality of life: patterns emerging from a large national sample, Australia. Chronic Illn. 2007;3(3):202–218. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain AM, Finney Rutten LJ, Wilson PM, et al. Neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. BMC Public Health. 2020;20(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orueta JF, Nuno-Solinis R, Garcia-Alvarez A, Alonso-Moran E. Prevalence of multimorbidity according to the deprivation level among the elderly in the Basque Country. BMC Public Health. 2013;13:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violan C, Foguet-Boreu Q, Roso-Llorach A, et al. Burden of multimorbidity, socioeconomic status and use of health services across stages of life in urban areas: a cross-sectional study. BMC Public Health. 2014;14:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med. 2011;365(16):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden SD, Earp JA. Social ecological approaches to individuals and their contexts: twenty years of health education & behavior health promotion interventions. Health Educ Behav. 2012;39(3):364–372. [DOI] [PubMed] [Google Scholar]
- 19.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. Multiple chronic conditions - a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. 2010. Washington, DC. [Google Scholar]
- 22.2016 TIGER/Line Shapefiles Technical Documentation. US Census Bureau. Accessed at https://www.census.gov/geo/maps-data/data/tiger-line.html on July 2, 2021. Accessed. [Google Scholar]
- 23.Neighborhood Atlas. University of Wisconsin School of Medicine and Public Health. Available at https://www.neighborhoodatlas.medicine.wisc.edu/ Accessed July 2, 2021. Accessed.
- 24.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36(20):3257–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Nelder JA. Hierarchical generalized linear models. J R Statist Soc B. 1996;58(4):619–678. [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289–300. [Google Scholar]
- 28.Franceschi C, Garagnani P, Morsiani C, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne). 2018;5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–752. [DOI] [PubMed] [Google Scholar]
- 31.Barzilai N, Cuervo AM, Austad S. Aging as a biological target for prevention and therapy. JAMA. 2018;320(13):1321–1322. [DOI] [PubMed] [Google Scholar]
- 32.Phillips SP, Hamberg K. Women’s relative immunity to the socio-economic health gradient: artifact or real? Glob Health Action. 2015;8:27259–27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deguen S, Lalloue B, Bard D, Havard S, Arveiler D, Zmirou-Navier D. A small-area ecologic study of myocardial infarction, neighborhood deprivation, and sex: a Bayesian modeling approach. Epidemiology. 2010;21(4):459–466. [DOI] [PubMed] [Google Scholar]
- 34.Thurston RC, Kubzansky LD, Kawachi I, Berkman LF. Is the association between socioeconomic position and coronary heart disease stronger in women than in men? Am J Epidemiol. 2005;162(1):57–65. [DOI] [PubMed] [Google Scholar]
- 35.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurani S, McCoy RG, Inselman J, et al. Place, poverty and prescriptions: a cross-sectional study using area deprivation index to assess opioid use and drug-poisoning mortality in the USA from 2012 to 2017. BMJ Open. 2020;10(5):e035376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020;3(3):e200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS. 2016;4(3):1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Census Bureau Minnesota. Available at https://data.census.gov/cedsci/profile?g=0400000US27# Accessed July 2, 2021. Accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


