Abstract
Introduction
The study measured the hormonal and protein markers of acute stress, those of oxidative stress and total antioxidant capacity (TAC) in swine oral fluid, determined which of these parameters would be the most appropriate for future livestock welfare assessment and established the time when the samples should be taken.
Material and Methods
Stress was induced in 7 out of 14 castrated six-week-old Danbred×Duroc pigs by immobilisation on a nasal snare at 8 a.m., 1 p.m., and 6 p.m. and samples were taken both directly after the stressor was applied and 30 min later. The remaining pigs were the control group, which were not immobilised; their samples were taken at the same times. The concentrations of hormones and malondialdehyde (MDA) were measured using liquid chromatography with tandem mass spectrometry, while those of alpha-amylase and TAC were measured using spectrophotometry.
Results
The levels of cortisol and cortisone increased with statistical significance immediately after the acute stress response and 30 min later. A cut-off value set at 0.25 ng/mL cortisol concentration was capable of distinguishing between the stressed and control groups with 100% accuracy in evening samples and 95% accuracy overall. Prednisolone was not present, and the levels of testosterone and corticosterone were low and not distinctive. Alpha-amylase became significantly more concentrated during stress induction and 30 min later. The TAC and MDA levels rose after the stress but without statistical significance.
Conclusion
The most suitable markers of acute stress were cortisol, cortisone and alpha-amylase. Oral fluid is a reliable material for monitoring the level of pigs’ stress and should be collected in the evening.
Keywords: swine oral fluid, acute stress markers, oxidative stress, cortisol, alpha-amylase
Introduction
Farm animals, including pigs, are exposed to many stressors associated with being handled or chased or with overcrowding (20). Stress negatively influences the welfare and health of farm animals and can lead to economic losses by raising the percentage of deaths, reducing weight gain, impairing reproduction and degrading meat quality. The monitoring of stress should therefore be an important determinant of animal welfare and play a significant role in their husbandry.
During periods of stress, the heart rate, blood pressure, and the behaviour of the animal vary , but these factors are an insufficient basis on which to accurately assess the actual extent of the stress and its injurious effects. These parameters depend on age, sex, health status, and breed. Moreover, animal behaviour does not always change because of stress. That is why quantifiable stress markers should be measured for an appropriate representation of animal welfare on a farm. However, there is no “gold standard” or procedure for which parameters should be taken into consideration.
The simple definition of stress is: the biological response elicited when an individual perceives a threat to its homeostasis (22). Stress occurs when an animal cannot cope with a stressor that could be dangerous to its health or life (18). There are different classifications of stress depending on its duration (acute or chronic) and causes (social, environmental, or immunological) (20).
As the first reaction to a stressful situation, the sympathetic-adrenal-medullary axis (SAM) is activated and catecholamines are released. Next, the hypothalamic-pituitary-adrenal axis (HPA) releases glucocorticoids, and the hypothalamus-pituitary-gonadal axis stimulates the gonads and adrenal glands to produce testosterone (24).
The catecholamines (epinephrine and norepinephrine) are produced in the chromaffin cells of the adrenal medulla. Determination of their presence is difficult because they are unstable (11), but their blood level may be estimated indirectly by measuring other parameters activated by the SAM axis. One of them is alpha-amylase – an enzyme studied as a biomarker of both physical and psychological stress in humans (23). Its level in saliva is correlated with catecholamine concentrations in plasma and indicates SAM activation (13). Alpha-amylase activity was measured as a biomarker of acute stress in pigs (5) and other animals, including bonobos, dogs (6) and horses (12). After the SAM axis, the HPA axis becomes active and does so through glucocorticoids, which play the main role in the response to stressful situations. The centrality of glucocorticoids to stress response is the reason why cortisol is still one of the most frequently studied concentration parameters in the investigation of animal stress (24).
Oxidative stress is an unavoidable consequence of stress of all kinds, especially when the stressor affects the animal for a prolonged time. Glucocorticoids stimulate gluconeogenesis, lipolysis, and many other metabolic processes that are the source of reactive oxygen species (ROS). The inductive influence of glucocorticoids on oxidative stress, including when they are experimentally administered, has been previously widely described in humans and various animals (1, 8, 20, 28). Chronic stress in humans inflicts greater oxidative stress (16). The data concerning the influence of acute stress on oxidative stress in animals are not as extensive, but in one study, an increase in oxidative stress level was confirmed (20).
Hormonal stress markers are usually measured in the blood because this technique reflects the response to the stressor in real time. However, drawing blood samples may be a severe stressor that confounds the assessment of adrenocortical responses. Moreover, cortisol concentration in blood fluctuates according to the circadian rhythm (29). Cortisol may also be measured in other matrices, which provide information about the average concentration over a time period of a few hours (urine, faeces) or weeks (hair) (7). Recently, oral fluid has been proposed as an alternative to blood because it shows the biomarker levels in real time without the drawbacks of stressful sampling.
It seems that oral fluid is a perfect material for examining biomarkers of stress and has various advantages. First of all, its collection is more comfortable for the animals, it does not cause stress, it is easy and cheap, and it shows the real concentration of stress markers during the impact of stressors. That is why it is becoming more popular and widely used in veterinary medicine (25) and has already been used in the monitoring of hormonal (9, 26), protein (11), or all biomarkers of stress in pigs (10). The material is collected using an absorbent material, which is chewed and bitten by the pigs because of their natural curiosity (7). Although various studies have been conducted, there are still no guidelines about how surveillance of animal stress should be implemented, including the most suitable time for sample collection.
The aim of this study was to analyse various markers of stress: hormonal (cortisol, cortisone, corticosterone, prednisolone, and testosterone) and protein (alpha-amylase), oxidative stress parameters (malondialdehyde – MDA), and concentrations of antioxidant substances (total antioxidant capacity – TAC) to assess which of them are the most suitable for animal welfare examination and when the sample should be taken. In order to achieve that purpose, male pigs were subjected to stress, and blood and oral fluid were sampled from them and examined.
Material and Methods
Animals. For the experiment, 14 castrated male pigs (DanBred×Duroc) were used at the age of six weeks. Before the experiment, the animals were quarantined for five weeks to exclude infection with any diseases and establish a baseline for the stress level. Water and feed were made available to the animals ad libitum throughout the study. The animals were divided into a control and experimental group (with seven animals each) and housed in two separate pens (5×5 m) to prevent them from having an influence on each other. There were no aggression or steretypies observed in the animals before the start of the experiment. The environmental conditions were the same for each group during the experiment: solid floor with rubber mats, >3.5 m2/pig, a ventilation rate of 200–300 m3/h and a temperature of 20–22°C. The light–dark cycle was 12 h. No environmental stress symptoms such as aggression or cannibalism were observed during the experiment in either group.
Experimental design. Every other day at 8 a.m., 1 p.m., and 6 p.m. for two weeks, the animals in the experimental group were stressed by being immobilised with a nasal snare for a few minutes. During that time, blood samples from the external jugular vein were collected. Oral fluid was sampled using pads chewed by each animal for a short time. After that, a rope was hung in the experimental animals’ pen for 30 min, which they were allowed to chew. At the same times as the experimental group experienced stress, a rope was also left with the control group for 30 min.
Sample collection and pre-treatment. Blood samples were collected in anticoagulant tubes and centrifuged at 5,000 × g for 5 min to obtain plasma. The plasma from each animal was aliquoted separately and kept at −20°C until analysis. Oral fluid was collected from individual experimental animals as follows: a cotton swab held in metal tweezers was placed in the mouth of the animal and they were allowed to bite and chew for a few minutes to moisten it. The cotton swabs were squeezed into a small plastic bag. The samples of oral fluid collected from all of the animals were pooled, centrifuged at 5,000 × g for 10 min at 4°C, aliquoted, and stored at −20°C for further analysis. In order to collect a group sample, a cotton rope was hung in the pig pen for 30 min in a place accessible by all animals but at some distance from feed and water. The rope was then taken away from the animals, placed in a plastic bag, and squeezed to collect oral fluid, which was transferred to tubes. The tubes were centrifuged at 5,000 × g for 10 min at 4°C, and the supernatant was aliquoted and stored at −20°C for further analysis.
In the control group, the oral fluid was only collected from the cotton rope. This took place at the same times as it did for the experimental group (at 8 a.m., 1 p.m., and 6 p.m.). The chewed swab and the larger part of the blood sampling were omitted to avoid stress because the sample collection had not to be invasive or disturb the baseline level of stress marker. Blood drawing itself being a stressor, it was only taken on the last three consecutive days of the experiment and only once day (first at 8 a.m., on the following day at 1 p.m., and on the final day at 6 p.m.). Oral fluid was not collected on those days. Sparing the control group blood drawing earlier than at the end of the experiment was an experimental design decision: it could not have been sampled earlier because it might have had a great impact on the experiment’s results.
Determination of alpha-amylase and antioxidant capacity. Alpha-amylase and TAC were measured using a commercial assay kit (Sigma-Aldrich, Darmstadt, Germany) for spectrophotometric examination, for which a Synergy HTX Multi-Mode Microplate Reader was used (BioTek, Winooski, VT, USA). The analysis was performed according to the manufacturer’s instructions. A standard curve was prepared with every run.
Alpha-amylase activity was assessed by measuring the amount of p-nitrophenol at 405 nm cleaved by the amylase from the reaction substrate ethylidene pNP-G7. One unit (U) is the amount of amylase that generates 1.0 mmol of p-nitrophenol per min at 25°C. All samples were diluted twofold and tested in two replications in 96-well flat-bottom plates.
The TAC kit measures the concentration of all protein and small molecule antioxidants in the sample. They reduce the Cu2+ ion to Cu+, which creates chelates with a colorimetric ingredient directly proportional to the TAC and is measured at 570 nm. The antioxidant capacity was calculated based on the trolox equivalent. All samples were diluted 50-fold and tested in two replications in 96-well flat-bottom plates.
Determination of corticoids: standards and reagents. The reference standards of cortisone, testosterone, prednisolone, corticosterone, cortisone-2,2,4,6,6,9,12,12-d8 and corticosterone-9,11,11,12-d4 were purchased from Sigma-Aldrich. Cortisol, cortisol-D4 (9,11,12,12-D4), and testosterone-2,3,4-13C3 were supplied by Cerilliant Corporation (Round Rock, TX, USA). Acetonitrile was provided by J.T. Baker (Deventer, the Netherlands), p.a. grade ethyl acetate was sourced from POCh (Gliwice, Poland), HiPerSolv CHROMANORM 99.9% formic acid for liquid chromatography–mass spectrometry (LC-MS) was ordered from VWR (Radnor, PA, USA), and Bakerbond disposable silica gel (SiOH) extraction columns (500 mg) were manufactured by J.T. Baker. Ultrapure water was filtered through a Millipore Milli-Q system (MilliporeSigma, Billerica, MA, USA). Nanosep MF 0.22 μm filters were supplied by Pall (Port Washington, NY, USA).
Stock standard solutions were prepared at a concentration of 0.1 mg/mL for cortisol and cortisone-2,2,4,6,6,9,12,12-D8; other standards were prepared at a concentration of 1.0 mg/mL in methanol and stored at −20°C. A mixed solution of the working standards was prepared in methanol and also stored at −20°C.
Sample extraction and purification. After the transfer of 0.5 mL of oral fluid to a polypropylene tube, an internal standard (IS) was added, followed by 2.5 mL of ethyl acetate after 15 min. The samples were vortexed and centrifuged at 4,500 × g for 15 min. The upper layer was transferred to silica cartridges (500 mg), which had previously been conditioned with 2 mL of ethyl acetate. The filtrate was collected together with the eluate (1 mL of ethyl acetate). The eluates were evaporated to dryness in a heating block at 45°C under a gentle stream of nitrogen. The residue was reconstituted in 100 μL of 20% acetonitrile (ACN) in water and filtered through Nanosep MF 0.22 μm filters before injection into the LC column.
For plasma, 0.1 mL of the sample was transferred to a polypropylene tube, and 50 μL of IS was added. This was followed by the addition of 2.5 mL of ethyl acetate after 15 min. The samples were vortexed and centrifuged at 4,500 × g for 15 min. An aliquot of 0.5 mL of the upper layer was collected in a glass tube and evaporated at 45°C to dryness. The residue was dissolved in 0.5 mL of 20% ACN in water.
Ultra-high performance liquid chromatography– tandem mass spectrometry (UHPLC-MS/MS). The UHPLC-MS/MS system consisted of an Exion LC UHPLC system connected to a 5500 Qtrap mass spectrometer (MDS Sciex, Concord, ON, Canada). Analyst 1.6.3 software (Sciex, Framingham, MA, USA) controlled the UHPLC-MS/MS system, and Multiquant 3.2 (Sciex) was used to process the data. Chromatographic separations were performed in an Eclipse Plus C18 column of 3.5 μm 150 × 2.1 mm (Agilent Technologies, Santa Clara, CA, USA) using the gradient elution of ACN (phase A) and 0.1% formic acid in water (phase B). The mobile phase composition was maintained at 20% A for 3 min, then gradually increased to 50% A at 10 min and 95% A at 14 min. The maximum elution power was retained for 2 min, and then the system was re-equilibrated for 8 min. Desolvation was set to take place at a temperature of 450°C, gas 1 (air) to flow at 40 psi, gas 2 (air) at 30 psi, collision gas (N2) at medium flow, nebuliser gas (N2) at 30 psi, and curtain gas (N2) at 25 psi. The voltage of the electron multiplier and the electrospray capillary were set at 2,100 V and 4,500 V, respectively. The ions were monitored in MRM mode (Table 1).
Table 1.
The parameters of the mass-spectrometry detection of corticoids and their respective internal standards
| Analyte | Precursor ion (m/z) | Fragment ion (m/z) | Declustering potential | Collision energy | Ionisation |
|---|---|---|---|---|---|
| Prednisolone | 405 | 295 | −60 | −45 | |
| 405 | 329 | −60 | −25 | ||
|
|
|||||
| Cortisol | 407 | 331 | −60 | −25 | |
|
|
|||||
| 407 | 297 | −60 | −45 | ||
|
|
|||||
| Cortisone | 405 | 329 | −60 | −20 | Negative mode |
|
|
|||||
| 405 | 301 | −60 | −30 | ||
|
|
|||||
| Cortisol d4 | 411 | 335 | −60 | −60 | |
|
|
|||||
| Cortisone d8 | 413 | 337 | −60 | −24 | |
|
| |||||
| Testosterone | 289 | 109 | 100 | 35 | |
|
|
|||||
| 289 | 97 | 100 | 28 | ||
|
|
|||||
| Testosterone d2 | 291 | 99 | 100 | 31 | Positive mode |
|
|
|||||
| Corticosterone | 347 | 121 | 80 | 32 | |
|
|
|||||
| Corticosterone | 347 | 311 | 80 | 24 | |
|
|
|||||
| Corticosterone d4 | 351 | 121 | 80 | 31 | |
Validation results. The results were calculated based on a standard calibration curve. The slope of the calibration curve reached 88–106% of the slope of the matrix-matched curve in a range of 0–5 ng/mL for the oral fluid and 0–100 ng/mL for the plasma. The recovery was in the ranges of 84.4–116% and 86.9–103% for the oral fluid and plasma, respectively. Repeatability, expressed as the coefficient of variation, was 2.4–5.2% and 4.4– 6.9% for cortisol and cortisone, respectively. The limit of detection ranged from 0.01 ng/mL (for cortisol and cortisone) to 0.05 ng/mL (corticosterone), and the limit of quantification was 0.2 ng/mL for corticosterone and 0.05 ng/mL for the other corticoids.
Determination of MDA: standards and reagents. Reference standards of malondialdehyde tetrabutylammonium salt, trifluoroacetic acid (TFA), and 2,4-dinitrophenylhydrazine (DNPH) were purchased from Sigma-Aldrich. Toronto Research Chemicals (Toronto, ON, Canada) supplied 1,1,3,3-Tetraethoxypropane-1,3-d2 (TEP-d2). Acetonitrile, 99.9% formic acid, and the filtration system for ultrapure water were provided by the same suppliers as previously detailed.
A stock reference standard solution was prepared at 1.0 mg/mL in water and stored at −20°C. Working standard solutions were prepared in methanol and also stored at −20°C. A stock solution of TEP-d2 (1,000 μg/mL) was prepared in acetonitrile and then hydrolysed with 10% hydrochloric acid (POCh) for 1 h at 40°C. The solution was then diluted in methanol.
Sample preparation. In order to extract the MDA from the oral fluid, protein precipitation was required. A 50 μL volume of IS was added to 100 μL of oral fluid and, after 10 min, 20 μL of TFA was added to initiate protein precipitation. Samples were vortexed and centrifuged at 14,500 × g for 5 min. Then, 50 μL of supernatant was transferred to dark glass vials, and 20 μL of DNPH solution (2 mg/mL in ACN:H2O:TFA, 75:25:0.1) was added. The samples were vortexed and incubated at ambient temperature for 1 h. After that time, 430 μL of solvent (ACN:0.1% HCOOH, 6 :40) was added, and the solution was diluted five-fold.
UHPLC-MS/MS. Chromatographic separations were performed in an Eclipse Plus C18 column of 3.5 μm 150 × 2.1 mm using an isocratic elution of acetonitrile (50%) and 0.1% formic acid in water (50%). The flow rate was 0.25 mL/min, the injection volume 20 μL, and the oven temperature 30°C. Detection was in the positive ionisation mode with two transitions monitored for MDA (m/z 235 to m/z 189 and m/z 235 to m/z 159) and one transition for IS (m/z 237 to m/z 161). All other parameters were the same as enumerated for the previous UHPLC-MS/MS procedure.
Statistical analysis. The results were analysed with a one-way ANOVA and Tukey honestly significant difference test.
Results
Hormones. All endogenous corticoids were detected in all samples of oral fluid. However, two of them (testosterone and corticosterone) were observed at levels close to the limit of quantification of the method. Prednisolone was not detected in any sample from either the control or experimental animals. The levels of cortisol and cortisone differed statistically between the control group and the stress-subjected animals directly after immobilisation (Fig. 1). For cortisol, the effect was also observed in samples taken 30 min later, but it was not significant when the sampling times were assessed separately. A circadian rhythm–correlated pattern of cortisol concentrations was observed in the control group, although the differences between morning, afternoon, and evening samplings were not statistically significant.
Fig. 1.
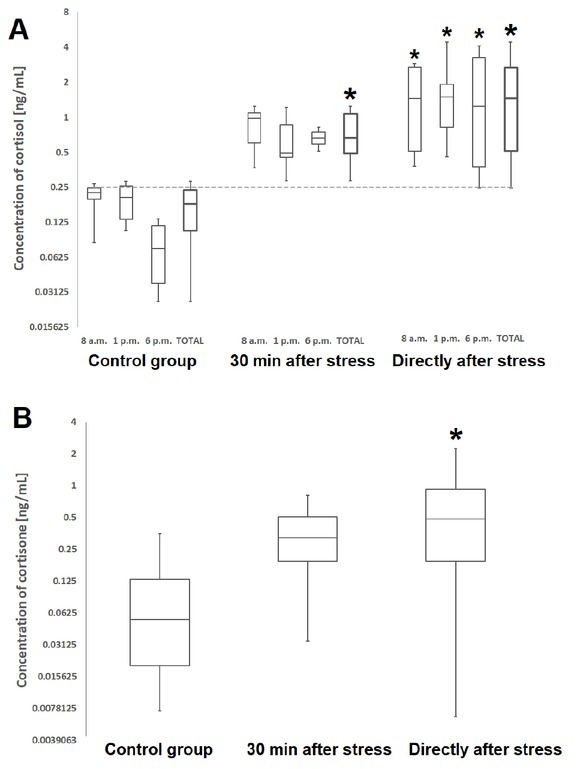
The concentrations of cortisol (A) and cortisone (B) in oral fluid of swine. In the box plots, the boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers below and above the box indicate the minimum and maximum values, respectively. Results that differ statistically from the control are marked with an asterisk (*)
The cortisol concentration was higher than the level of cortisone both in oral fluid and in plasma. The differences between the control and the stress-subjected animals were smaller for plasma (Fig. 2), and a statistically significant result was only observed for cortisone.
Fig. 2.

The concentration of cortisol (A) and cortisone (B) in swine plasma collected at 8 a.m. The results of evaluation of samples from individual animals are presented, together with the mean of all results for the stressed and control groups. Statistically significant difference between the control and treated groups is marked with an asterisk (*). The results are presented for the five pigs which were sampled every other day throughout the experiment. Two other animals were excluded from blood sampling on some days due to extensive swelling of the injection site
Alpha-amylase. The alpha-amylase activity increased with statistical significance in the experimental group compared with the control group. The average alpha-amylase activity levels in the control and experimental groups during the period of stress were 0.021 (±0.009) and 0.089 (±0.025) mU, respectively, but 30 min later, the activity had decreased slightly to 0.059 (±0.034) mU. The influence of the circadian rhythm in the control group was not statistically significant (Fig. 3).
Fig. 3.

Alpha-amylase activity in swine oral fluid expressed in milliunits (mU). Statistically significant difference between the control and experimental group is marked with an asterisk (*)
Total antioxidant capacity. The total antioxidant capacity increased in the experimental group compared to the control group during the stress period and 30 min later but without statistical significance. The average TAC in the control group was 3.73 (±0.71) nmol/μL, but during the stress period it increased to 6.52 (±2.21) nmol/μL and it decreased 30 min later to 4.95 (±1.08) nmol/μL. No impact of the circadian rhythm was observed (Fig. 4).
Fig. 4.

Total antioxidant capacity (TAC) in swine oral fluid, based on the trolox equivalent
MDA level. The oral fluid levels of MDA also increased from 662 ± 333 ng/mL in the control group to 1050 ± 959 ng/mL and 749 ± 524 ng/mL in the stressed animals directly after the stressor was applied and 30 min later, respectively. None of the results differed significantly in comparison with the control group.
Discussion
The physiological reaction of stress is a complex process, during which different mechanisms are activated depending on the type of stressor and the duration of its impact. That is why various parameters involved in the stress response should be measured to assess animal welfare.
In the current study, we elucidated the stress response through measurement of parameters in the oral fluid of pigs at predetermined times to discover which of them can be best used to assess animal welfare, which time period is the most suitable for sample collection and whether oral fluid is a suitable material for such assessments. The experiment was performed at the group level because, under farm conditions, the welfare of the whole herd and not just the individual animals is of particular interest to farmers.
Oral fluid is a mixture of saliva, for moisturising and protection, and transudates, and originates from the circulatory system of the cheek mucosa and gingival tissues. It contains proteins (enzymes, antibodies and glycoproteins), lipids and hormones, the last of which are secreted into saliva at rates which are dependent on the permeability of the lipophilic layer of the capillaries and cells of the glandular epithelium. As a consequence, lipophilic molecules such as steroids pass through these barriers more rapidly than hydrophilic molecules such as peptides (15).
Lipid-soluble steroid hormones passively diffuse through the capillary into gland secretory cells according to the concentration gradient. Their concentration in saliva reflects approximately 10% of the plasma free cortisol concentration and equilibrium is achieved in 2 min (33). In the salivary glands, part of the cortisol is converted to cortisone, an inactive ketone form. Because cortisone may be bound by the antibodies used in immunological tests, the cortisol concentration is often overestimated. That is why LC-MS/MS methods are recommended for assaying this hormone.
The cortisol concentration in blood, and consequently in oral fluid, fluctuates and changes according to the circadian rhythm (29). It is higher in the morning and lower in the afternoon and evening. However, some authors have noted another peak in the afternoon at about 4 p.m. (14). Moreover, the response to the stressors could be different depending on the time of the stimulus, and may disrupt the rhythm.
In our study, the effect of the circadian rhythm was observed in the control group but not in the stressed animals, which confirms previous findings (7). Based on the results, the cut-off value was established at 0.25 ng/mL. When only the samples taken at 6 p.m. were considered, all of the results were correctly assigned to the control or stressed group. However, when all samples were taken into account, some misdetection of stress occurred, albeit below 5%. Although these results are promising, they must be verified using samples collected under farm conditions because the baseline level of cortisol in pig oral fluid may depend on age, sex, breed and other factors (7).
Because of the low levels of glucocorticoids in the oral fluid, it was necessary to use a sensitive analytical method. The in-house method developed was based on the work of Rey-Salgueiro et al. (26) and proved to be sufficient even though the concentrations in the tested samples were lower in our study than in its precedent.
The concentration of cortisone also increased after stress, but it was found to be less suitable as a stress biomarker. The high degree of variation in the results, especially in the samples taken directly after the stress-inducing event, made it impossible to distinguish between the control and the treated animals. Other authors also stated that cortisone may be an additional parameter for analysing the stress response but that the cortisol level is more reliable (24).
We did not observe an increase in the glucocorticoid level in plasma in the group subjected to immobilisation, probably because the sampling process was stressful in itself. It is interesting that the oral fluid : plasma ratio of cortisol concentrations was only 0.0155 (calculated from the means of all observations: 1.69 ng/mL and 109 ng/mL for oral fluid and plasma, respectively) and was significantly lower at approximately 10% less than in an investigation by other authors (7).
Alpha-amylase is a non-hormonal marker of stress in saliva, which has been widely studied in humans because its level increases as a result of physical and psychological stress (13, 23). While cortisol level maybe a potential marker of psychological stress according to some authors (3), it is usually thought to increase due to physical stressors. Alpha-amylase is considered to be a more reliable biomarker of the influence of psychological stress than cortisol.
In the current study, alpha-amylase activity increased almost ten-fold in the experimental group compared with the control group, and 30 min later it had only decreased slightly. Similar results were observed by other authors (5), who examined this activity in the oral fluid of pigs stressed using a nasal snare. However, they observed a wide range of results from a 2-fold to a more than 20-fold increase in activity, which was probably connected with individual reactions to stress in individual animals. Similar results were noted in humans (32).
Many factors may affect alpha-amylase activity in humans, including food intake, exercise, and circadian rhythm. Age and sex also influence its activity in reaction to psychological stress in both humans and bonobos (2, 30). The influence of the circadian rhythm on alpha-amylase activity was only observed in the control group but it was without statistical significance; it was lower in the morning (8 a.m.) but increased in the afternoon (6 p.m.). Similar results were noted in studies with humans (23). Other factors (e.g. feed and water intake) were not monitored in our study because it is not possible to control them under farm conditions.
Reactive oxygen species are an inherent part of aerobic respiration. An imbalance between their production and neutralisation by antioxidants may lead to oxidative stress. They can be detrimental to cell structures and damage them by lipid peroxidation, forming MDA with proven carcinogenic properties. Reactive oxygen species and antioxidants are also present in oral fluid (34) and in humans stress and cortisol levels are known to increase ROS concentrations (1, 16). Oxidative stress was measured in piglets during weaning (27) in response to vaccination and heat stress. Lipid peroxidation significantly increased after vaccination, whereas glutathione peroxidase activity decreased and antioxidant supplementation enhanced the piglets’ protection against oxidative stress. The results showed that during weaning, piglets experience the different environment and diet, separation from sows, and other changes as stressors.
In the current study, there were no significant changes in the concentrations of MDA between the groups or times of sample collection. In stress-induced injuries of pig myocardia, the MDA level was elevated together with other parameters of oxidative stress (4). In another study, nursery pigs under social-mixing stress had MDA levels in plasma of 72 to 1,800 ng/mL, which were closely correlated with cortisol concentrations (30). Our results fall into that range; however, results obtained under different conditions and measured with various analytical methods are difficult to compare and this places some doubt over the observed correlation. The baseline levels observed by other authors in human saliva varied by two orders of magnitude and were from 4.32 to 490 ng/mL (17). For this reason, the concentration of MDA alone cannot indicate the level of oxidative stress.
Another parameter examined was the TAC, which was higher in the experimental group than in the control group but without statistical significance. No influence of the circadian rhythm on TAC was observed. The increased level of TAC in the experimental group indicates a higher level of protection from ROS, the production of which may be enhanced by the stressor. Total antioxidant capacity was also measured in other studies on pigs and increased significantly due to acute stress (28), but during other studies it decreased (19) when the stressor was present for a prolonged time and oxidative stress levels increased. In our study, the TAC increase was not statistically significant and therefore this parameter was found to be unsuitable for the estimation of oxidative stress.
Taking into consideration the results from our current study, cortisol, cortisone, and alpha-amylase could be regarded as useful stress biomarkers under farm conditions. Because the circadian rhythm is more pronounced for glucocorticoids, the optimal time for sample collection is 6 p.m., when their baseline concentrations are at their lowest. Oral fluid contains the stress markers which are most suitable for surveillance of stress impact, and for this reason and others it was found to be the perfect matrix for studies on stress in animals in husbandry. Besides its containing several ideal constituents, oral fluid is a strong proposition because sample collection has no impact on animal welfare, which is crucial for the measurement of the concentration of stress markers. Furthermore, the sampling process is straightforward and economical and could easily be applied on farms. Farmers are joined by consumers in concern for animal welfare, who are more aware of meat production conditions and have greater consideration for the wellbeing of farm animals. This proof-of-concept research will be a wide-ranging study of pig welfare in different husbandry systems and may support the development of an accessible tool to enhance the consumer appeal of meat from the pig industry.
Acknowledgements
We thank Beata Korycińska and Magdalena Pyra for support in the development of the method to investigate hormonal markers of stress and samples collection.
Footnotes
Conflict of Interest
Conflict of Interest Statement The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This research was funded by the KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal – Safe Food”, Ministry of Science and Higher Education of Poland, resolution No. 05-1/KNOW2/2015.
Animal Rights Statement: As regards care and use of laboratory animals, the authors declare that the experiments on animals were conducted in accordance with local Ethical Committee laws and regulations and those of Directive 2010/63/EU and were approved by the Ethical Animal Experiments Inspectorate (Lublin, Poland, resolution No. 28/2018).
References
- 1.Aschbacher K., O’Donovan A., Wolkowitz O.M., Dhabhar F.S., Su Y., Epel E.. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behringer V., Deschner T., Möstl E., Selzer D., Hohmann G.. Stress affects salivary alpha-amylase activity in bonobos. Physiol Behav. 2012;105:476–482. doi: 10.1016/j.physbeh.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Burke H.M., Davis M.C., Otte C., Mohr D.C.. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Ceremużyński L., Barcikowski B., Lewicki Z., Wutzen J., Gordon-Majszak W., Famulski K.S., Kłoś J., Herbaczyńska-Cedro K.. Stress-induced injury of pig myocardium is accompanied by increased lipid peroxidation and depletion of mitochondrial ATP. Exp Pathol. 1991;43:213–220. doi: 10.1016/S0232-1513(11)80120-9. [DOI] [PubMed] [Google Scholar]
- 5.Contreras-Aguilar M.D., Escribano D., Martínez-Subiela S., Martínez-Miró S.. Changes in alpha-amylase activity, concentration and isoforms in pigs after an experimental acute stress model: an exploratory study. BMC Vet Res. 2018;14:256. doi: 10.1186/s12917-018-1581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras-Aguilar M.D., Tecles F., Martínez-Subiela S., Escribano D., Bernal L.J., Cerón J.J.. Detection and measurement of alpha-amylase in canine saliva and changes after an experimentally induced sympathetic activation. BMC Vet Res. 2017;13:1–6. doi: 10.1186/s12917-017-1191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook N.J.. Review: Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can J Anim Sci. 2012;92:227–259. doi: 10.4141/cjas2012-045. [DOI] [Google Scholar]
- 8.Costantini D., Marasco V., Møller A.P.. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–456. doi: 10.1007/s00360-011-056. [DOI] [PubMed] [Google Scholar]
- 9.Escribano D., Fuentes-Rubio M., Cerón J.J.. Salivary testosterone measurements in growing pigs: validation of an automated chemiluminescent immunoassay and its possible use as an acute stress marker. Res Vet Sci. 2014;97:20–25. doi: 10.1016/j.rvsc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Escribano D., Gutiérrez A.M., Tecles F., Cerón J.J.. Changes in saliva biomarkers of stress and immunity in domestic pigs exposed to a psychosocial stressor. Res Vet Sci. 2015;102:38–44. doi: 10.1016/j.rvsc.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Escribano D., Soler L., Gutiérrez A.M., Martínez-Subiela S., Cerón J.J.. Measurement of chromogranin A in porcine saliva: Validation of a time-resolved immunofluorometric assay and evaluation of its application as a marker of acute stress. Animal. 2013;7:640–647. doi: 10.1017/S1751731112002005. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes-Rubio M., Fuentes F., Otal J., Quiles A., Tecles F., Cerón J.J., Hevia M.L.. Measurements of salivary alpha-amylase in horse: comparison of 2 different assays. J Vet Behav Clin Appl Res. 2015;10:122–127. doi: 10.1016/j.jveb.2014.12.008. [DOI] [Google Scholar]
- 13.Granger D.A., Kivlighan K.T., el-Sheikh M., Gordis E.B.. Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 14.Griffith M.K., Minton J.E.. Free-running rhythms of adrenocorticotrophic hormone (ACTH), cortisol and melatonin in pigs. Domest Anim Endocrinol. 1991;8:201–208. doi: 10.1016/0739-7240(91)90056-P. [DOI] [PubMed] [Google Scholar]
- 15.Gröschl M.. Current Status of Salivary Hormone Analysis. Clin Chem. 2008;54:1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- 16.Joergensen A., Broedbaek K., Weimann A., Semba R.D., Ferrucci L., Joergensen M.B., Poulsen H.E.. Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PLoS ONE. 2011;6:20795. doi: 10.1371/journal.pone.0020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoubnasabjafari M., Ansarin K., Jouyban A.. Salivary malondialdehyde as an oxidative stress biomarker in oral and systemic diseases. J Dent Res Dent Clin Dent Prospect. 2016;10:71–74. doi: 10.15171/joddd.2016.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar B., Manuja A., Aich P.. Stress and its impact on farm animals. Front Biosci. 2012;1:1759–1767. doi: 10.2741/496. [DOI] [PubMed] [Google Scholar]
- 19.Kwang-Woon K., Hyun-Soo K.. Evaluation of oxidative stress status of growing pigs on two pig farms with different performance. J Prev Vet Med. 2017;41:66–70. doi: 10.13041/jpvm.2017.41.2.66. [DOI] [Google Scholar]
- 20.Majer A.D., Fasanello V.J., Tindle K., Frenz B.J., Ziur A.D., Fischer C.P., Fletcher K.L., Seecof O.M., Gronsky S., Vassallo B.G., Reed W.L., Paitz R.T., Stier A., Haussmann M.F.. Is there an oxidative cost of acute stress? Characterization, implication of glucocorticoids and modulation by prior stress experience. Proc R Sci B. 2019;286:20191698. doi: 10.1098/rspb.2019.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Miró S., Tecles F., Ramón M., Escribano D., Hernández F., Madrid J., Orengo J., Martínez-Subiela S., Manteca X., Cerón J.J.. Causes, consequences and biomarkers of stress in swine: an update. BMC Vet Res. 2016;12:171. doi: 10.1186/s12917-016-0791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moberg G.P. Moberg G.P, Mench J.A. The Biology of Animal Stress. CABI Publishing; Oxon,New York: 2000. Biological response to stress: implications for animal welfare; pp. 1–21. edited by. [Google Scholar]
- 23.Nater U.M., Rohleder N.. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Palme R.. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim Welf. 2012;21:331–337. doi: 10.7120/09627286.21.3.331. [DOI] [Google Scholar]
- 25.Prickett J.R., Zimmerman J.J.. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim Health Res Rev. 2010;11:207–216. doi: 10.1017/S1466252310000010. [DOI] [PubMed] [Google Scholar]
- 26.Rey-Salgueiro L., Martínez-Carballo E., Simal-Gándara J.. Liquid chromatography–mass spectrometry method development for monitoring stress-related corticosteroids levels in pig saliva. J Chromatogr B. 2015;990:158–163. doi: 10.1016/j.jchromb.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Royer E., Barbé F., Guillou D., Rousselière Y., Chevaux E.. Development of an oxidative stress model in weaned pigs highlighting plasma biomarkers’ specificity to stress inducers. J Anim Sci. 2016;94:48–53. doi: 10.2527/jas.2015-9857. [DOI] [Google Scholar]
- 28.Rubio C.P., Mainau E., Cerón J.J., Contreras-Aguilar M.D., Martínez-Subiela S., Navarro E., Tecles F., Manteca X., Escribano D.. Biomarkers of oxidative stress in saliva in pigs: analytical validation and changes in lactation. BMC Vet Res. 2019;15:144. doi: 10.1186/s12917-019-1875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruis M.A., Te Brake J.H., Engel B., Ekkel E.D., Buist W.G., Blokhuis H.J., Koolhaas J.M.. The circadian rhythm of salivary cortisol in growing pigs: effects of age, gender and stress. Physiol Behav. 1997;62:623–630. doi: 10.1016/s0031-9384(97)00177-7. [DOI] [PubMed] [Google Scholar]
- 30.Sahu G.K., Upadhyay S., Panna S.M.. Salivary Alpha Amylase Activity in Human Beings of Different Age Groups Subjected to Psychological Stress. Ind J Clin Biochem. 2014;29:485–490. doi: 10.1007/s12291-013-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y.B., Voilqué G., Odle J., Kim S.W.. Dietary L-Tryptophan Supplementation with Reduced Large Neutral Amino Acids Enhances Feed Efficiency and Decreases Stress Hormone Secretion in Nursery Pigs under Social-Mixing Stress. J Nutr. 2012;8:1540–1546. doi: 10.3945/jn.112.163824. [DOI] [PubMed] [Google Scholar]
- 32.Takai N., Yamaguchi M., Aragaki T., Eto K., Uchihashi K., Nishikawa Y.. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Vining R.F., McGinley R.A.. The measurement of hormones in saliva: Possibilities and pitfalls. J Steroid Biochem. 1987;27:81–94. doi: 10.1016/0022-4731(87)90297-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Schipper H.M., Velly A.M., Mohit S., Gornitsky M.. Salivary biomarkers of oxidative stress: A critical review. Free Radic Biol Med. 2015;85:95–104. doi: 10.1016/j.freeradbiomed.2015.04.005. [DOI] [PubMed] [Google Scholar]


