Abstract
Although the health effects of exposure to low-dose ionizing radiation have been the focus of many studies, the affected biological functions and underlying regulatory mechanisms are not well-understood. In particular, the influence of radiation exposure at doses of less than 200 mGy on the regulation of genes and pathways remains unclear. To investigate the molecular alterations induced by varying doses of low-dose radiation (LDR), transcriptomic analysis was conducted based on ribonucleic acid (RNA) sequencing following exposure to 50 and 150 mGy doses. Human peripheral blood was collected, and the samples were divided into three groups, including two treatments and one control (no radiation). A total of 876 (318 upregulated and 558 downregulated) and 486 (202 upregulated and 284 downregulated) differentially expressed genes (DEGs) were identified after exposure to 50 mGy and 150 mGy, respectively. Most upregulated genes in both the 50 mGy and 150 mGy groups were associated with ‘antigen processing and presentation,’ which appeared to be the major targets affected by LDR exposure. Several interacting genes, including HLA-DQA1, HLA-DQA2, HLA-DQB2, HLA-DRB1, and HLA-DRB5 were mapped to ‘antigen processing and presentation,’ ‘immune system-related diseases’ and the ‘cytokine-mediated signaling pathway,’ suggesting that these genes might drive the downstream transmission of these signal transduction pathways. Our results suggest that exposure to LDR may elicit changes in key genes and associated pathways, probably helping further explore the biological processes and molecular mechanism responsible for low-dose occupational or environmental exposures in humans.
Keywords: differentially expressed genes (DEGs), low-dose radiation (LDR), radiation exposure, pathway enrichment, transcriptome level
INTRODUCTION
The United Nations Scientific Committee on the Effects of Atomic Radiation defines low-dose radiation (LDR) that may cause deterministic effects (tissue effects) as those of 200 mGy or less and low dose rates as 0.1 mGy.min−1 (averaged over one hour or less) for radiation such as external X-rays and gamma rays [1]. Humans are continuously exposed to low doses of natural and anthropogenic ionizing radiation every day. The increasing use of ionizing radiation in medical procedures, industrial applications, and scientific research continues to increase the potential for exposure. In America, England and Australia, the average yearly medical exposure dose increased dramatically between 1982 and 2006 [2]. In America, for example, the average yearly medical exposure dose increased from 0 to 5 mGy in 1982 to 30 mGy in 2006; and the average yearly medical exposure dose doubled in England and tripled in Australia between 1982 and 2006 [2]. In China, with the increasing use of diagnostic and therapeutic procedures in the fields of radiology and nuclear medicine, the annual per capita dose has doubled over the past two decades [3]. Exploring the biological effects of exposure to low-dose/low-dose-rate ionizing radiation is therefore necessary for adequately protecting both individuals and the environment [4–5]. A better understanding of the biological processes affected by of LDR could lead to advances in patient care and reduce the detrimental impact of radiation therapy on patients [6].
Since the 1970s, the linear no-threshold (LNT) model has been used to estimate the health risks of LDR by extrapolating the risks determined in high-dose studies [7–8]. However, advances in LDR biology and cell molecular techniques have demonstrated that the LNT model does not appropriately reflect the biological or health effects in the low-dose range [9–12]. In fact, LDR can positively affect biological processes such as immunity, DNA repair, and cellular stress resistance [11, 13–15], and the dose–effect relationship could be hormesis, which is a biphasic dose response relationship in which there are beneficial effects at low doses and harmful effects at high doses [16–17]. There have been many large-scale epidemic studies in populations exposed to low doses of radiation, including atomic bomb survivors [18–20], those who have worked with high-background radiation [21–23] and those who have received medical diagnosis and disease treatment [24–26], but the association between LDR and carcinogenesis is still controversial. For example, among the atomic bomb survivors or radiation workers, LDR exposure has a positive association with leukemia, but no radiation-associated risks for either Hodgkin lymphoma or multiple myeloma were found [18, 22–23]. Low-dose exposure from medical X-ray examination and treatment is a risk factor of disease, such as atherosclerosis, leukemia and brain tumors [24–25]. However, other studies have shown that mortality among both medical professionals and nuclear workers who are exposed to LDR is similar to that among non-exposed workers [27–31]. Moreover, even though epidemiological studies of exposed human cohorts have found that some diseases appear under LDR exposure, such as visual disturbances, leukemia and cataract [22, 32–33], there are still uncertainties regarding the determining mechanisms associated with low-dose occupational or environmental exposures in humans. The issue remains controversial, and the exact health risks of exposure to LDR are unclear [34].
Advanced genomic tools have provided new insights related to the hazard assessment of radiation exposure; however, most radiation-inducible gene expression profiling has been performed using hybridization-based microarrays [35–37]. Microarray technology presents some limitations, including indirect quantification from hybridization-signal intensities [38], background and cross-hybridization problems [39] and reproducibility issues [40]. In addition, previously published work has mainly focused on differentially expressed genes (DEG) and their potential to be used as biomarkers or for individualized biodosimetry [36, 41–44]. There are very few existing studies on the regulation of genes and related pathways, especially the molecular alterations caused by exposure to a dose of less than 200 mGy. The effects of radiation on gene expression have biological consequences [45–47]. Recently published data show that LDR can elicit changes in metabolic and immune pathways, potentially increasing the risk of immune dysfunctions and metabolic disorders [14, 37]. Further investigation is needed to elucidate the possible transcriptional regulation mechanisms in response to LDR.
Here, we present the whole-transcriptome gene expression profiling of LDR-induced human peripheral blood through RNA sequencing and pathway enrichment analysis. RNA-Seq presents several advantages over microarrays, including the following: (i) RNA-Seq is not dependent on prior knowledge about the target sequence, (ii) it exhibits a large dynamic range and high sensitivity because of its digital nature, and (iii) the quantification of the transcriptome is more accurate because the quantification of each transcript is directly based on the digital counts of the transcript [48]. This study provides novel insights into specific gene expression and pathway-level differences that are associated with different radiation doses based on technological improvements and combined approaches. The overarching goal of this study is to use global transcriptomic and pathway analysis to identify candidate genes and pathways for understanding the biological processes and molecular mechanisms that occur in human peripheral blood exposed to LDR.
MATERIALS AND METHODS
Sample collection and radiation
In this study, blood samples from nine healthy adult donors (five males and four females; aged 20–48 years) were collected in PAXgene Blood RNA tubes for transcriptomic sequencing. The eligibility of the donors was evaluated using questionnaires and regular medical examinations. Peripheral blood samples (5 ml) were collected from each subject. The subjects had no history of chronic disease, substance abuse, smoking, or toxic chemical exposure. In addition, they had not been exposed to radiation nor did they have a history of viral infections during the months preceding the study. The present study was approved by the Ethics Committee. Written informed consent was obtained from all human subjects prior to enrollment in the present study.
Irradiation with 137Cs γ-rays was performed in the Beijing Radiation Center (Beijing, China). The exposure setup was calibrated by physical measurement using a tissue-equivalent ionizing chamber. The radiation dose rate was calculated using the source radioactivity and the distance between the source and samples: dose rate of 58.4 cGy/h corresponds to a source-sample distance of 70 cm. The homogeneous irradiation field was 30 × 30 cm; the samples were placed within a 5 cm-radius circle, and the uncertainty of the calibration was 1%. A control group (non-radiation) and two different radiation groups were used (50 and 150 mGy). Nine blood samples were divided into three dose groups, and each group included three biological replicates. Following irradiation, blood samples were incubated for 6 hours at 37°C in RPMI-1640 medium of equal volume (Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA), see Supplementary Fig. S1.
RNA-seq and analysis
Red blood cell (RBC) lysis was performed before RNA extraction. Total RNA was extracted using Trizol reagent (Invitrogen, Gaithersburg, MD) following the manufacturer’s instructions and then treated with DNase I (Fermentas, Hanover, MD). We checked the purity of the samples using a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA), the concentration was assessed in a Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA), and RNA integrity was verified using an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA). In this study, the RNA integrity number (RIN) ranged between 7.9 and 9.2, which is greater than 7 and could be acceptable for further analysis.
Library construction and sequencing were performed in a BGISEQ-500 system at the Beijing Genomic Institution (www.genomics.org.cn, BGI, Shenzhen, China). Clean reads were mapped to human reference gene and genome hg19 available in the NCBI database (https://www.ncbi.nlm.nih.gov/assembly) by using Bowtie2 and HISAT [49–50], respectively. The original sequence data have been submitted to the NCBI Sequence Read Archive (Accession number: PRJNA622427). For gene expression analysis, the matched reads were calculated and then normalized to RPKM values using RSEM software [51]. The differential expression analysis between treatments and controls was performed using Cuffdiff program, and the significant DEGs for further data analysis were filtered with false discovery rate (FDR) corrected P value ≤0.001 and fold change ≥1.5.
Bioinformatics analysis
DEGs were used as the input for a series of bioinformatics analyses performed with the WEB-based GEneSeTAnaLysis Toolkit (WebGestalt) [52–53]. WebGestalt is an open online analytical platform that integrates Gene Ontology (GO) [54], KEGG [55], and protein interaction networks for a variety of functional enrichment analyses (http://www.webgestalt.org). The GO, pathway annotation and enrichment analyses were based on the GO database (http://www.geneontology.org/) and the KEGG pathway database (http://www.genome.jp/kegg/).
Quantitative real-time PCR and data analysis
In the above mentioned selected DEGs, 10 newly discovered radiation-related genes were selected to be validated. Validations of the mRNA levels of DEGs were performed using quantitative real-time PCR (qRT-PCR). Peripheral blood samples (30 ml) were collected from another three healthy adult subjects (two males and one female: aged 28–30 years). The health status of the sample and sample irradiation were same as the above materials for RNA-seq. Then, blood samples were incubated at 37°C for 6 hours in RPMI-1640 medium of equal volume (Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). After RBC lysis, total RNA for qPCR was obtained using Trizol, treated with DNase (Fermentas, Hanover, MD), as described above (also see Supplementary Fig. S1). First-stranded cDNA was synthesized by RevertAid First Strand cDNA synthesis Kit (Thermo Scientific, Waltham, MA, USA). QRT-PCR was performed on an ABI7500 Real-Time PCR System using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and gene primer sequences are provided in Supplementary Table S3. The relative expression levels of each gene were normalized according to β-actin gene expression and analyzed using the 2-ΔΔCt method. Each group, composed of three parallel samples, was analyzed after irradiation. All experiments were performed three times.
Each data represents the means ± standard deviation (SD) of three independent biological experiments. Data management and statistical analysis was performed using MedCalc version 15.2.2 (MedCalc Software bvba, Ostend, Belgium). Comparisons were analyzed using Student’s t test with statistical significance at P < 0.05.
RESULTS
Identification of differentially express genes (DEGs)
High-throughput sequencing was used to identify mRNA expression in the radiation-treated and control groups. The clean reads obtained from the control, 50 and 150 mGy groups totaled 24.11, 23.87 and 24.11 Mb, respectively, and all Q30 values were higher than 90%. The comparison of differential gene expression between the radiation and control groups revealed 318 upregulated DEGs and 558 downregulated DEGs in the 50 mGy group, and the number of DEGs decreased to 202 upregulated and 284 downregulated genes in the 150 mGy group. In addition, 489 DEGs were found in the 150 vs 50 mGy groups, among which 263 DEGs were upregulated and 225 DEGs were downregulated. The complete DEG list can be found in Table 1. The Venn diagram analysis (Supplementary Fig. S2) showed that only 112 upregulated and 136 downregulated DEGs were altered by both gamma doses, whereas large numbers of DEGs were uniquely regulated by the different gamma doses, especially at the 50 mGy dose.
Table 1.
Total number of DEGs
| Comparison (mGy) | Up-regulated genes | Down-regulated genes |
|---|---|---|
| 50 vs 0 | 318 | 558 |
| 150 vs 0 | 202 | 284 |
| 150 vs 50 | 263 | 225 |
GO and KEGG pathway enrichment analysis of DEGs
To understand the distribution of the DEGs at a macro level, the set of DEGs identified under different doses of radiation were mapped in accordance with GO terms and KEGG pathways. The goal of the GO consortium is to produce a dynamic, controlled vocabulary that can be applied to all eukaryotes even as knowledge of the roles of genes in cells accumulates, and our understanding of these roles changes [54]. The GO enrichment analysis revealed a wide range of cellular components, molecular functions and biological processes, including 54 important functional groups (Fig. 1). KEGG enrichment revealed that 758 DEGs and 1335 DEGs were enriched in 291 pathways in the 50 mGy and 246 pathways in the 150 mGy group, respectively, within six main categories. For both the 50 and 150 mGy groups, ‘human diseases,’ ‘organismal systems’ and ‘metabolism’ were the top three categories with the highest proportions of different DEGs. With a P-value <0.05 as the threshold, the top 20 significant enriched pathways of the DEGs were determined and are shown in Fig. 2. Among these pathways, ‘signal transduction,’ ‘immune system,’ ‘cancer: overview,’ ‘transport and catabolism’ and ‘signaling molecules and interaction’ showed significant enrichment. In addition, several pathways related to metabolism and human diseases were found, including ‘global and overview maps,’ ‘lipid metabolism’ and ‘amino acid metabolism’ as well as ‘infectious diseases.’ The results indicated that the greatest numbers of genes were active in environmental information processing, organismal systems, human diseases, cellular processes and metabolism.
Fig. 1.
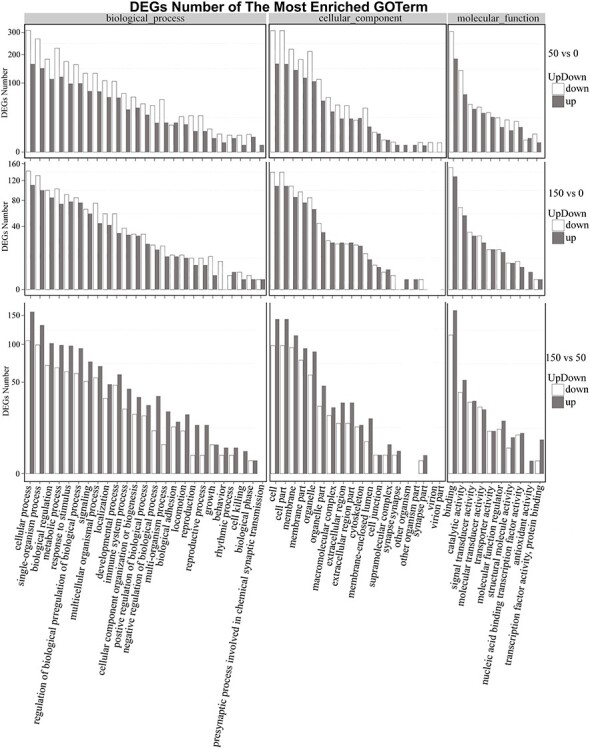
Gene ontology (GO) plot of genes with different expression patterns, including comparisons of the 50 vs 0 mGy, 150 vs 0 mGy and 150 vs 50 mGy groups.
Fig. 2.
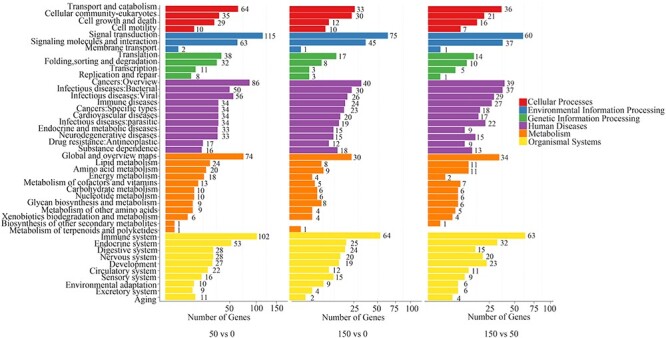
Pathway classifications of DEGs identified in the 50 vs 0 mGy, 150 vs 0 mGy and 150 vs 50 mGy comparisons.
Among the most enriched pathways, 11 pathways were identified under both the 50 and 150 mGy doses, and nine pathways were unique to the 50 and 150 mGy groups (Fig. 3). We found that the most downregulated genes at 150 mGy were related to the ‘rap1 signaling pathway,’ ‘calcium signaling pathway,’ ‘ras signaling pathway,’ ‘cAMP signaling pathway,’ and ‘cGMP-PKG signaling pathway,’ while at 50 mGy, the most downregulated genes were enriched in numerous diseases, including herpes simplex infection, Huntington’s disease, Alzheimer’s disease, Parkinson’s disease and non-alcoholic fatty liver disease and in cytokine-cytokine receptor interaction. At both radiation doses, ‘antigen processing and presentation’ was found to be the most enriched pathway among the upregulated genes.
Fig. 3.
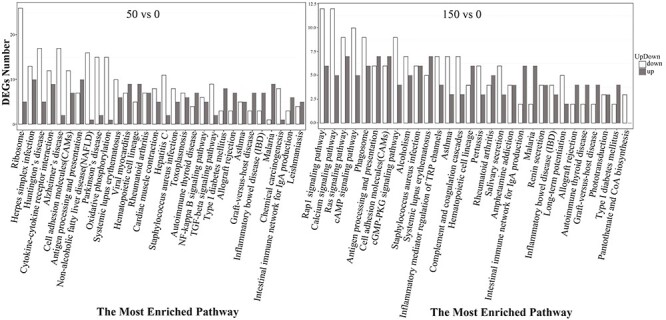
The most enriched pathways of DEGs, including upregulated and downregulated genes under 50 and 150 mGy.
Gene interaction network
The genes and the associated pathways were grouped into categories, which included ‘antigen processing and presentation,’ ‘immune system process,’ ‘immune system disease,’ ‘cytokine-mediated signaling pathway’ and ‘gas transport’ under both doses, the ‘response to stress’ at 50 mGy and the ‘calcium signaling pathway’ at 150 mGy. The interaction network was constructed based on co-expression data and experimental validation. Fig. 4 shows that the radiation-sensitive genes (HLA-DQA1, HLA-DQA2, HLA-DQB2, HLA-DRB1, and HLA-DRB5) located on chromosome 6 were not only mapped to ‘antigen processing and presentation,’ ‘immune system-related diseases’ and ‘cytokine-mediated signaling pathway,’ but may also directly interact with each other, mediating the effects of varying doses of radiation.
Fig. 4.
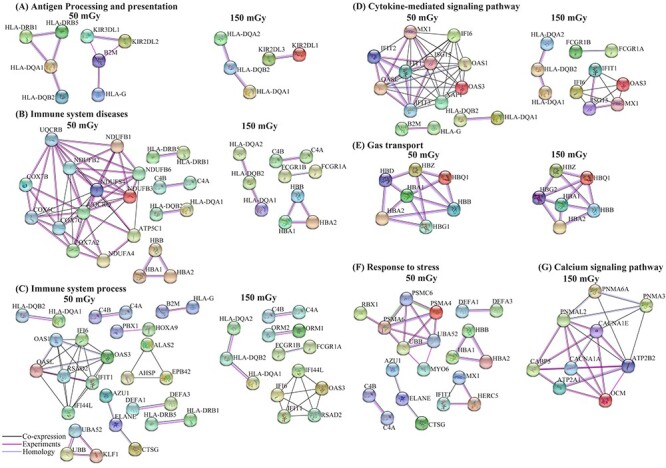
Gene interaction networks specific to varying doses of 137Csγ radiation exposure. (A) Gene interactions associated with antigen processing and presentation; (B) gene interactions associated with immune system diseases; (C) gene interactions associated with immune system processes; (D) gene interactions associated with the cytokine-mediated signaling pathway; (E) gene interactions associated with gas transport; (F) gene interactions associated with the response to stress; (G) gene interactions associated with the calcium signaling pathway.
Key candidate DEGs and pathways involved in the radiation-responsive mechanisms
At both doses, differential expression analysis showed that there were more downregulated DEGs than upregulated DEGs (Table 1), and these downregulated DEGs were enriched in immune system- and human disease-related processes and signaling molecules and interaction pathways (Fig. 3). Interestingly, signaling molecules and interaction pathways such as the‘rap1 signaling pathway,’ ‘calcium signaling pathway,’ ‘ras signaling pathway,’ ‘cAMP signaling pathway’ and ‘cGMP-PKG signaling pathway,’ were specific to the 150 mGy group, which is an indication that molecular signaling may be activated when irradiation reaches a certain dose, which was not observed in the initial stage at 50 mGy. The upregulated DEGs were generally enriched in antigen processing and presentation at both doses (Fig. 3), demonstrating the importance of the immune system after LDR. The top 10 most enriched pathways of the DEGs included antigen processing and presentation, asthma, rheumatoid arthritis, type I diabetes mellitus, malaria, the cytokine-mediated signaling pathway and gas transport (see Supplementary Tables S1 and S2). At both doses, many DEGs such as HLA-DQA1, HLA-DQA2, HLA-DQB2, HLA-DRB1, HLA-DRB5, KIR2DL1, KIR2DL3, TNFSF10, TNFSF13B and TNFRSF4 were shared among the top 10 most enriched pathways (Supplementary Tables S1 and S2) and even directly interacted with each other (Fig. 4), which indicated that these genes could play important roles in the molecular mechanisms responding to LDR.
Verification of the key genes’ expression profiles by qRT-PCR
To verify the expression profiles of the key candidate DEGs (Supplementary Table S3) in the RNA sequencing experiment, we performed qRT-PCR assays using samples of human peripheral blood obtained from three healthy donors. These DEGs were selected for their potential role involving in the radiation-responsive mechanisms, because they were shared among the top 10 most enriched pathways (Supplementary Tables S1 and S2) and directly interacted with each other (Fig. 4). Samples were analyzed at 6 hours following ex vivo irradiation with 0, 50 and 150 mGy of γ-rays. As shown in Fig.5, the qRT-PCR results showed that the expression patterns of the selected genes were similar to those of RNA sequencing experiment (Fig. 5A and B), indicating their signal perception and transcription reactions after receiving radiation. Thus, the specific functions of these genes in human peripheral blood at different dose and different time should be further investigated in the future studies.
Fig. 5.
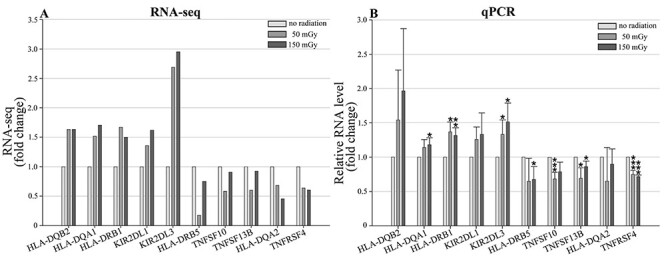
The expression profiles of 10 genes in human peripheral blood by the quantitative RT-PCR. (A) The expression patterns of DEGs under irradiation and no radiation treatment by RNA-seq; (B) the expression patterns of DEGs following irradiation (50 mGy and 150 mGy) and no radiation by qPCR; Data are presented as means ± SD of three independent experiments. The asterisks indicate significant differences compared with the control treatment, *P < 0.05; **P < 0.01; ***P < 0.005, t-test.
DISCUSSION
According to ICRP Publication 90, the lowest experimentally observed doses causing persistent damage at the anatomical and structural level are in the range of 100–300 mGy of acute low-LET radiation [56]. Even evidence of malformations found at considerably lower doses, i.e. within a dose range of 50–250 mGy, cannot be ignored [56]. As mentioned in the ICRP103 report, the occupational exposure from existing and planned exposure sources should meet the requirements of individual dose limit [57]. However, the temporary constraint value of emergency exposure may reach 100 mGy and higher, such as nuclear accident or emergency disposal of nuclear facilities. Moreover, the irradiation of patients in medical irradiation should meet the requirements of medical irradiation guidance level for radiation diagnosis and nuclear medicine diagnosis, but there may be cases where the cumulative dose exceeds 150 mGy in a short time, such as multiple abdominal multi-slice spiral CT diagnosis [57]. The radiation in the range of 50–150 mGy is the focus of effect research, which is closely related to some scenes in emergency and medical irradiation [57]. Therefore, this study focused on the DEGs of transcriptome in peripheral blood of healthy people irradiated by 50 and 150 mGy dose, providing targets for the study of LDR effect mechanism and theoretical reference for the study of radiation protection.
The dose–response relationship is usually nonlinear and difficult to establish under LDR, potentially due to the dynamics between the compensatory mechanisms defending against radiation-induced damage in an organism [56]. In the present study, different dose-related gene expression patterns were observed at the transcriptional level. These changes suggest that radiation, even at low levels, could modulate cellular processes [37], and that gene expression, even at low levels, could be used as a sensitive endpoint for evaluating the effect of radiation under environmentally realistic low-dose exposure. In this study, we found a decreasing number of DEGs that were affected by radiation at doses from 50 mGy to 150 mGy (Table 1), showing that the effect of radiation on gene expression may be dose dependent at the transcriptome level. Similar observations of different dose–response patterns after LDR exposure have been reported at the transcriptional level [56–58]. The high radiation dose resulted in a lower number of DEGs, indicating a possible phase shift in the response to radiation doses above 150 mGy. The genes that were responsive to 50 mGy radiation were mostly associated with the immune system and diseases (e.g. Parkinson’s disease, rheumatoid arthritis, type I diabetes mellitus and asthma), whereas a radiation dose of 150 mGy induced gene expression changes related not only to the immune system and human diseases but also to environmental information processing (e.g. calcium signaling pathway) (Figs 3 and 4; Supplementary Tables S1 and S2). Significant changes in pathways observed at different doses indicated that they may be involved in the response to radiation.
GO terms represent gene product properties, and KEGG enrichment analysis can supply information for understanding gene functions and signaling pathways in metabolism. On the basis of GO analysis, we found that LDR significantly changed cellular components, biological processes, and molecular functions in human peripheral blood (Fig. 1). The relevant genes were included TNFSF10, TNFSF13B and TNFRSF4 (Supplementary Table S1), which belong to the tumor necrosis factor (TNF) superfamily (TNFSF), the TNF cell surface receptors, and the TNF receptor superfamily (TNFRSF), respectively. TNFSF–TNFRSF interactions activate signaling pathways for cell survival, death, and differentiation that control immune function and disease [59–66]. As members of the TNF superfamily, TNFSF10 induces apoptosis in transformed and tumor cells, and TNFSF10 could stimulate the activation of MAPK8/JNK, caspase 8 and caspase 3 by binding to its receptors (TNF-related apoptosis-inducing ligand, TRAIL) [67]. TRAIL has been reported to mediate the calcification of aortic valve interstitial cells through the apoptosis mechanism [68]. Previous reports have shown that TNFRSF4 can be upregulated by activated T cells [62], playing an important role in supporting/sustaining the immune response [69]. In this study, TNFRSF4 was downregulated after LDR by both RNA-seq and qRT-PCR experiments (Fig. 5; Supplementary Table S1), suggesting its impact on the immune process. Based on the pathway enrichment results, DEGs related to human disease were more enriched at 50 mGy (53 genes) than at 150 mGy (31 genes) (Fig. 3; Supplementary Table S2), and most of these genes have been reported to play important roles in human diseases. For instance, the HLA-DQA1 and HLA-DQB2 genes, which regulate type I diabetes and rheumatoid arthritis [70–74], were highly expressed under both the 50 and 150 mGy doses in our study (Fig. 5). Pathways involved in immunity were altered after radiation exposure at both the 50 and 150 mGy doses (Supplementary Table S1), which indicated that radiation exposure, even at low dose levels, may have harmful effects disrupting the immune system. Previous studies have shown that the mature lymphocytes and bone marrow stem cells of atomic bomb survivors were severely damaged, which significantly weakened their immune system, thus reducing their ability to resist microbial invasion [75–76]. Thus, many of these people died from infections. In our study, we found many more upregulated genes involved in antigen processing and presentation, as well as human diseases such as type I diabetes mellitus, malaria, rheumatoid arthritis and systemic lupus erythematosus (Fig. 3; Supplementary Table S2). Researchers also found fewer CD4 T-cells and increased levels of inflammatory proteins in A-bomb survivor blood samples [75–76]. LDR has been shown to impact type I diabetes in a mouse model receiving whole-body radiation [77]. Additionally, the Radiation Effects Research Foundation reported that mortality attributed to heart diseases increased with the radiation dose in a Life Span Study cohort [78]. Metabolic risk factors, including diabetes mellitus and high blood pressure, may increase the risk of coronary heart disease [79], and radiation exposure might enhance the risk of coronary heart disease via metabolic pathway disruption [37]. This is supported by the enriched KEGG pathways, which showed that type I diabetes mellitus was significantly enriched after 50 mGy radiation, and the number of enhanced genes related to type I diabetes mellitus was greater than the number of suppressed genes (Fig. 3, Supplementary Table S2).
The KEGG analysis indicated that one of the most important pathways responding to radiation exposure was antigen processing and presentation (Fig. 3; Supplementary Table S2), which has been reported to be related to immune response, transport pathogenesis, secretion, and phagocytosis. As shown in Fig. 4A, in the gene interaction network, the expression of several genes was associated with the antigen processing and presentation pathway, including the HLA-DQA1, HLA-DQA2, HLA-DQB2, HLA-DRB1, HLA-DRB5, KIR2DL1 and KIR2DL3 genes. As protein-coding genes, HLA-DQA1 and HLA-DQB2 belong to the HLA class II alpha chain paralogues and are anchored in the membrane. These genes are expressed in antigen presenting cells and play a central role in the immune system by presenting peptides derived from extracellular proteins [80–81]. Overexpression of HLA-DQA1 and HLA-DQB2 was demonstrated to be associated with diseases including type I diabetes and rheumatoid arthritis [70–74]. Our research also indicated that HLA-DQA1 and HLA-DQB2 were upregulated in type I diabetes and rheumatoid arthritis. The KIR2DL3 gene has been revealed to be associated with protection against rheumatoid arthritis (RA) [82–85]. Previous studies suggested that RA subtypes with different KIRs could result in clinical heterogeneity of the disease. For example, the presence of the KIR2DL3 gene in the absence of the KIR2DL2 gene is associated with seronegative RA, while the presence of the KIR2DL2 gene is associated with seropositive anti-CCP [83]. Interestingly, 17 relevant genes were found to be uniquely enriched in the calcium signaling pathway among the KEGG pathways at 150 mGy (Fig. 3; Supplementary Table S2), and this pathway has been reported to be important in radiation-induced bystander effects [86–89]. As shown by the gene interaction network (Fig. 4G), ATP2B2 (ATPase plasma membrane calcium transporter 2) exhibited co-expression with many other genes in this pathway, which was experimentally validated, indicating its importance in the calcium signaling pathway, especially in bystander effects. ATP2B2 encodes a human Ca2+-pumping ATPase [90] with critical roles in intracellular calcium homeostasis [91]. It has been reported to be expressed predominately in the brain and mammary gland, acting as an emerging player in autism and breast cancer [63, 92–96]. ATP2B2 exhibits the fastest activation and is one of the primary ATP pumps found in the brain and sensory epithelium. Investigations of this gene have highlighted its pivotal role in the normal function of the auditory system as well as the vestibular system. The differentially expressed HLA-DQA1, HLA-DQB2, KIR2DL3 and ATP2B2 genes, which were enriched in the immune system processes and human diseases, probably play important roles in the response to LDR.
In this study, we used RNA-Seq to screen DEGs and signaling pathways involved in LDR impacts and verify the expression patterns of some key candidate genes in the RNA-seq experiment by qRT-PCR. Although our study provides a more complete list of candidate genes or pathways that play important roles in response to LDR and indicates the coregulation of several new signaling pathways, the precise molecular mechanisms of these interacting pathways remain unclear. Furthermore, different tissues respond to radiation differently, the conclusions in this study are only referred from human peripheral blood responsible for LDR. Further efforts are needed to assess more novel DEGs and study how these signaling pathways guide humans to respond to LDR.
Supplementary Material
FUNDING
The present work was supported by President’s Fund of Beijing Institute of Occupational Disease Prevention and Treatment (BHY1701001).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
References
- 1. United Nations Scientific Committee on the Effects of Atomic Radiation . Report of the United Nations Scientific Committee on the Effects of Atomic Radiation 2010. Fifty-Seventh Session, Includes Scientific Report: Summary of Low-Dose Radiation Effects on Health. 2011. https://www.unscear.org/docs/reports/2010/UNSCEAR_2010_Report_M.pdf. (Jul 28, 2021, date last accessed).
- 2. World Nuclear Association . What Is Radiation. World Nuclear Association; 2015. https://www.world-nuclear.org/. (Jul 26, 2021, date last accessed). [Google Scholar]
- 3. Yi Y, Zheng J, Zhuo W et al. Trends in radiation exposure from clinical nuclear medicine procedures in Shanghai, China. Nucl Med Commun 2012;33:331–6. [DOI] [PubMed] [Google Scholar]
- 4. Morgan WF, Bair WJ. Issues in low dose radiation biology: the controversy continues. A perspective. Radiat Res 2013;179:501–10. [DOI] [PubMed] [Google Scholar]
- 5. Gillies M, Kuznetsova I, Sokolnikov M et al. Lung cancer risk from plutonium: a pooled analysis of the Mayak and Sellafield worker cohorts. Radiat Res 2017;188:725–40. [DOI] [PubMed] [Google Scholar]
- 6. Puukila S, Lemon JA, Lees SJ et al. Impact of ionizing radiation on the cardiovascular system: a review. Radiat Res 2017;188:539–46. [DOI] [PubMed] [Google Scholar]
- 7. Calabrese EJ. How the US National Academy of Sciences misled the world community on cancer risk assessment: new findings challenge historical foundations of the linear dose response. Arch Toxicol 2013a;87:2063–81. [DOI] [PubMed] [Google Scholar]
- 8. Calabrese EJ. From Muller to mechanism: how LNT became the default model for cancer risk assessment. Environ Pollut 2018;241:289–302. [DOI] [PubMed] [Google Scholar]
- 9. Shephard AM, Aksenov V, Tran J et al. Hormetic effects of early juvenile radiation exposure on adult reproduction and offspring performance in the cricket (Acheta domesticus). Dose-Response 2018;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang FR, Loke WK, Khoo BC. Low-dose or low-dose-rate ionizing radiation–induced bioeffects in animal models. J Radiat Res 2017;58:165–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tharmalingam S, Sreetharan S, Brooks AL et al. Re-evaluation of the linear no-threshold (LNT) model using new paradigms and modern molecular studies. Chem Biol Interact 2019;301:54–67. [DOI] [PubMed] [Google Scholar]
- 12. Tubiana M, Feinendegen LE, Yang C et al. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 2009;251:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calabrese EJ. Low doses of radiation can enhance insect lifespans. Biogerontology 2013b;14:365–81. [DOI] [PubMed] [Google Scholar]
- 14. Cui J, Yang G, Pan Z et al. Hormetic response to low-dose radiation: focus on the immune system and its clinical implications. Int J Mol Sci 2017;18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luckey TD. Atomic bomb health benefits. Dose-Response 2008;6:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut 2013c;182:452–60. [DOI] [PubMed] [Google Scholar]
- 17. Calabrese EJ, Dhawan G, Kapoor R et al. What is hormesis and its relevance to healthy aging and longevity? Biogerontology 2015;16:693–707. [DOI] [PubMed] [Google Scholar]
- 18. Hsu W, Preston DL, Soda M et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res 2013;179:361–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preston DL, Pierce DA, Shimizu Y et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res 2004;162:377–89. [DOI] [PubMed] [Google Scholar]
- 20. National Research Council . Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2. Washington, DC: National Academies Press, 2005. [PubMed] [Google Scholar]
- 21. Cardis E, Vrijheid M, Blettner M et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res 2007;167:396–416. [DOI] [PubMed] [Google Scholar]
- 22. Gillies M, Haylock R, Hunter N et al. Risk of leukemia associated with protracted low-dose radiation exposure: updated results from the national registry for radiation workers study. Radiat Res 2019;192:527–37. [DOI] [PubMed] [Google Scholar]
- 23. Leuraud K, Richardson DB, Cardis E et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. The Lancet Haematology 2015;2:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boaventura P, Durães C, Mendes A et al. Is Low-dose radiation exposure a risk factor for atherosclerotic disease? Radiat Res 2018;189:418–24. [DOI] [PubMed] [Google Scholar]
- 25. Pearce MS, Salotti JA, Little MP et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang S, Peng H, He L et al. Response of fingernail growth to out-of-field low-dose X ray in cancer patients receiving radiotherapy. Radiat Res 2017;187:682–8. [DOI] [PubMed] [Google Scholar]
- 27. Iwasaki T, Murata M, Ohshima S et al. Second analysis of mortality of nuclear industry workers in Japan, 1986–1997. Radiat Res 2003;159:228–38. [DOI] [PubMed] [Google Scholar]
- 28. Kendall GM, Muirhead CR, MacGibbon BH et al. Mortality and occupational exposure to radiation: first analysis of the National Registry for Radiation Workers. Br Med J 1992;304:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linet MS, Kitahara CM, Ntowe E et al. Mortality in U.S. physicians likely to perform fluoroscopy-guided interventional procedures compared with psychiatrists, 1979 to 2008. Radiology 2017;284:482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohan AK, Hauptmann M, Freedman DM et al. Cancer and other causes of mortality among radiologic technologists in the United States. Int J Cancer 2003;103:259–67. [DOI] [PubMed] [Google Scholar]
- 31. Muirhead CR, O'Hagan JA, Haylock RGE et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Brit J Cancer 2009;100:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaudreau K, Thome C, Weaver B et al. Cataract formation and low-dose radiation exposure from head computed tomography (CT) scans in Ontario, Canada, 1994–2015. Radiat Res 2020;193:322–30. [DOI] [PubMed] [Google Scholar]
- 33. Mao XW, Boerma M, Rodriguez D et al. Acute effect of low-dose space radiation on mouse retina and retinal endothelial cells. Radiat Res 2018;190:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Little MP, Tawn EJ, Tzoulaki I et al. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys 2010;49:139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pirkkanen J, Tharmalingam S, Morais IH et al. Transcriptomic profiling of gamma ray induced mutants from the CGL1 human hybrid cell system reveals novel insights into the mechanisms of radiation-induced carcinogenesis. Free Radical Bio Med 2019;145:300–11. [DOI] [PubMed] [Google Scholar]
- 36. Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys 2008;71:1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee K, Weng JT, Hsu PW et al. Gene expression profiling of biological pathway alterations by radiation exposure. Biomed Res Int 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cassone M, Giordano A, Pozzi G. Bacterial DNA microarrays for clinical microbiology: the early logarithmic phase. Front Biosci 2007;12:2658–69. [DOI] [PubMed] [Google Scholar]
- 39. Okoniewski MJ, Miller CJ. Hybridization interactions between probesets in short oligo microarrays lead to spurious correlation. BMC Bioinformatics 2006;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Draghici S, Khatri P, Eklund AC et al. Reliability and reproducibility issues in DNA microarray measurements. Trends Genet 2006;22:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tucker JD, Joiner MC, Thomas RA et al. Accurate gene expression-based biodosimetry using a minimal set of human gene transcripts. Int J Radiat Oncol Biol Phys 2014;88:933–9. [DOI] [PubMed] [Google Scholar]
- 42. Dressman HK, Muramoto GG, Chao NJ et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med 2007;4:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Badie C, Kabacik S, Balagurunathan Y et al. Laboratory intercomparison of gene expression assays. Radiat Res 2013;180:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amundson SA, Bittner M, Meltzer P et al. Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res 2001;156:657–61. [DOI] [PubMed] [Google Scholar]
- 45. Woloschak GE, Chang-Liu CM. Modulation of expression of genes encoding nuclear proteins following exposure to JANUS neutrons or γ-rays. Cancer Lett 1995;97:169–75. [DOI] [PubMed] [Google Scholar]
- 46. Szumiel I. Monitoring and signaling of radiation-induced damage in mammalian cells. Radiat Res 1998;43:771–89. [PubMed] [Google Scholar]
- 47. Sheikh M, Burns T, Huang Y et al. P53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res 1998;58:1593–8. [PubMed] [Google Scholar]
- 48. Wang Y, Ghaffari N, Johnson CD et al. Evaluation of the coverage and depth of transcriptome by RNA-Seq in chickens. BMC Bioinformatics 2011;12:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015;12:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Langmead B, Trapnell C, Pop M et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 2009;10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 2005;33:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, Duncan D, Shi Z et al. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res 2013;41:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ashburner M, Ball CA, Blake JA et al. Gene ontology: tool for the unification of biology. Nat Genet 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kanehisa M, Araki M, Goto S et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res 2008;36:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song Y, Salbu B, Teien H et al. Dose-dependent hepatic transcriptional responses in Atlantic salmon (Salmo salar) exposed to sublethal doses of gamma radiation. Aquat Toxicol 2014;156:52–64. [DOI] [PubMed] [Google Scholar]
- 57. Nosel I, Vaurijoux A, Barquinero J et al. Characterization of gene expression profiles at low and very low doses of ionizing radiation. DNA Repair 2013;12:508–17. [DOI] [PubMed] [Google Scholar]
- 58. Amundson S, Anthony Lee R, Koch-Paiz C et al. Differential responses of stress genes to low dose-rate γ irradiation. Mol Cancer Res 2003;1:445–52. [PubMed] [Google Scholar]
- 59. Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005;23:23–68. [DOI] [PubMed] [Google Scholar]
- 60. Ward-Kavanagh LK, Lin WW, Šedý JR et al. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 2016;44:1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. So T, Lee S, Croft M. Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int J Hematol 2006;83:1–11. [DOI] [PubMed] [Google Scholar]
- 62. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- 63. Holbrook J, Lara-Reyna S, Jarosz-Griffiths H et al. Tumour necrosis factor signalling in health and disease [version 1; peer review: 2 approved]. F1000Research 2019;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dondelinger Y, Darding M, Bertrand MJM et al. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol Life Sci 2016;73:2165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009;9:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003;3:745–56. [DOI] [PubMed] [Google Scholar]
- 67. Muller J, Baeyens A, Dustin ML. Tumor necrosis factor receptor superfamily in T cell priming and effector function. Adv Immunol 2018;140:21–57. [DOI] [PubMed] [Google Scholar]
- 68. Galeone A, Brunetti G, Oranger A et al. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int J Cardiol 2013;169:296–304. [DOI] [PubMed] [Google Scholar]
- 69. Chang Y, Wang KC, Chu K et al. Dichotomous expression of TNF superfamily ligands on antigen-presenting cells controls post-priming anti-viral CD4+ T cell immunity. Immunity 2017;47:943–58. [DOI] [PubMed] [Google Scholar]
- 70. Kochi Y, Yamada R, Kobayashi K et al. Analysis of single-nucleotide polymorphisms in Japanese rheumatoid arthritis patients shows additional susceptibility markers besides the classic shared epitope susceptibility sequences. Arthritis Rheum-US 2004;50:63–71. [DOI] [PubMed] [Google Scholar]
- 71. Guo J, Zhang T, Cao H et al. Sequencing of the MHC region defines HLA-DQA1 as the major genetic risk for seropositive rheumatoid arthritis in Han Chinese population. Ann Rheum Dis 2019;78:773–80. [DOI] [PubMed] [Google Scholar]
- 72. Erlich H, Valdes AM, Noble J et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. David L, Gokhale A, Jois S et al. CD74/DQA1 dimers predispose to the development of arthritis in humanized mice. Immunology 2016;147:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Behrens M, Papadopoulos GK, Moustakas A et al. Trans heterodimer between two non-arthritis-associated HLA alleles can predispose to arthritis in humanized mice. Arthritis Rheum-US 2011;63:1552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 2008;84:1–14. [DOI] [PubMed] [Google Scholar]
- 76. Hayashi T, Morishita Y, Kubo Y et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 2005;118:83–6. [DOI] [PubMed] [Google Scholar]
- 77. Zhang C, Jin S, Guo W et al. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res 2011;175:307–21. [DOI] [PubMed] [Google Scholar]
- 78. Shimizu Y, Pierce DA, Preston DL et al. Studies of the mortality of atomic bomb survivors. Report 12, Part II. noncancer mortality: 1950-1990. Radiat Res 1999;152:374–89. [PubMed] [Google Scholar]
- 79. Fujioka S, Matsuzawa Y, Tokunaga K et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987;36:54–9. [DOI] [PubMed] [Google Scholar]
- 80. Pisapia L, Camarca A, Picascia S et al. HLA-DQ2.5 genes associated with celiac disease risk are preferentially expressed with respect to non-predisposing HLA genes: Implication for anti-gluten T cell response. J Autoimmun 2016;70:63–72. [DOI] [PubMed] [Google Scholar]
- 81. Lenormand C, Bausinger H, Gross F et al. HLA-DQA2 and HLA-DQB2 genes are specifically expressed in human langerhans cells and encode a new HLA class II molecule. J Immunol 2012;188:3903–11. [DOI] [PubMed] [Google Scholar]
- 82. Ramírez-De Los Santos S, Sánchez-Hernández PE, Muñoz-Valle JF et al. Associations of killer cell immunoglobulin-like receptor genes with rheumatoid arthritis. Dis Markers 2012;33:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ramirez S, Ramirez MG, Munoz JF et al. Association of KIR2DL2 gene with anti-cyclic citrullinated protein antibodies for serodiagnosis in rheumatoid arthritis. Medicina (B Aires) 2019;79:161–6. [PubMed] [Google Scholar]
- 84. Prakash S, Alam S, Bharadwaj U et al. Associations of killer cell immunoglobulin like receptors with rheumatoid arthritis among North Indian population. Hum Immunol 2014;75:802–7. [DOI] [PubMed] [Google Scholar]
- 85. Nazari M, Mahmoudi M, Rahmani F et al. Association of killer cell immunoglobulin-like receptor genes in Iranian patients with rheumatoid arthritis. PLoS One 2015;10:e143757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mothersill C, Lyng F, Seymour C et al. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat Res 2005;163:391–9. [DOI] [PubMed] [Google Scholar]
- 87. Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Brit J Cancer 2000;83:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat Res 2002;157:365–70. [DOI] [PubMed] [Google Scholar]
- 89. Lyng FM, Maguire P, McClean B et al. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res 2006;165:400–9. [DOI] [PubMed] [Google Scholar]
- 90. Brandt P, Ibrahim E, Bruns GAP et al. Determination of the nucleotide sequence and chromosomal localization of the ATP2B2 gene encoding human Ca2+-pumping ATPase isoform PMCA2. Genomics 1992;14:484–7. [DOI] [PubMed] [Google Scholar]
- 91. Agha G, Mendelson MM, Ward-Caviness CK et al. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation 2019;140:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang W, Liu J, Zheng F et al. The evidence for association of ATP2B2 polymorphisms with autism in Chinese Han population. PLoS One 2013;8:e61021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roberts-Thomson SJ. Plasma membrane calcium pumps and their emerging roles in cancer. World J Biol Chem 2010;1:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peters AA, Milevskiy MJG, Lee WC et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci Rep 2016;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jeong J, VanHouten JN, Dann P et al. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. PNAS 2016;113:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carayol J, Sacco R, Tores F et al. Converging evidence for an association of ATP2B2 allelic variants with autism in male subjects. Biol Psychiatry 2011;70:880–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


