Abstract
The possible role of the naturally occurring deuterium in the regulation of cell division was first described in the 1990s. To investigate the mechanism of influence of deuterium (D) on cell growth, expression of 236 cancer-related and 536 kinase genes were tested in deuterium-depleted (40 and 80 ppm) and deuterium-enriched (300 ppm) media compared to natural D level (150 ppm). Among genes with expression changes exceeding 30% and copy numbers over 30 (124 and 135 genes, respectively) 97.3% of them was upregulated at 300 ppm D-concentration. In mice exposed to chemical carcinogen, one-year survival data showed that deuterium-depleted water (DDW) with 30 ppm D as drinking water prevented tumor development. One quarter of the treated male mice survived 344 days, the females 334 days, while one quarter of the control mice survived only 188 and 156 days, respectively. In our human retrospective study 204 previously treated cancer patients with disease in remission, who consumed DDW, were followed. Cumulative follow-up time was 1024 years, and average follow-up time per patient, 5 years (median: 3.6 years). One hundred and fifty-six patients out of 204 (77.9%) did not relapse during their 803 years cumulative follow-up time. Median survival time (MST) was not calculable due to the extremely low death rate (11 cancer-related deaths, 5.4% of the study population). Importantly, 8 out of 11 deaths occurred several years after stopping DDW consumption, confirming that regular consumption of DDW can prevent recurrence of cancer. These findings point to the likely mechanism in which consumption of DDW keeps D-concentration below natural levels, preventing the D/H ratio from increasing to the threshold required for cell division. This in turn can serve as a key to reduce the relapse rate of cancer patients and/or to reduce cancer incidence in healthy populations.
Keywords: deuterium-depleted water (DDW), cancer prevention, median survival time (MST), metabolism, anticancer drug development, cancer recurrence, gene expression, D/H ratio, production of metabolic water, NanoString technology
Introduction
In 2018, global cancer statistics showed 18.1 million new cancer cases. According to forecasts, this number will rise to 29.5 million by 2040. This means that without new breakthrough strategies in prevention and treatment, the number of deaths from cancer will probably increase from 9.6 million up to about 15.6 million in 20 years. 1
Significant but very limited achievements have been earned in overall survival associated with new cancer drugs. Between 2000 and 2016, 92 novel cancer drugs were approved for 100 indications based on data from 127 clinical trials. The 127 clinical trials included a median of 191 participants (106-448 participants) but the median absolute survival benefit was not more than 2.4 months. 2 This apparent failure of anticancer drug development brings prevention into focus.
However, there is no consensus regarding the ultimate causes of cancer; therefore, there is no well-accepted protocol for cancer prevention. The mechanisms of carcinogenesis are still under debate. Cancer is considered either as a metabolic, 3 or as a genetic4,5 disease. According to Otto Warburg, 6 the hallmark of cancer is the shift in metabolism when the ATP-synthesis is dislocated from mitochondria to cytosol even in the presence of available oxygen, but the results associating mutation of a specific gene with a cancer phenotype provide a not less strong argument.
A broad range of agents, such as antioxidants, drugs (Aspirin, Metformin, and Raloxifene) used for different indications, but also commonly used cancer drug (Tamoxifen) have been tested for cancer preventive effect.7,8
The final outcome of the numerous studies on effects of antioxidants on incidence and mortality of cancer was that caution should be exercised with high-dose antioxidant preparations, such as beta-carotene or vitamin E, because these can increase the risk of smoking-related tumors (lung, head, neck, upper gastrointestinal tract, and bladder cancer). Clinical trials of selenium and vitamin E for prostate cancer also failed to confirm a risk-reducing effect; moreover, vitamin E was found to increase the risk of prostate cancer. 9
Certain cancer therapeutic drugs have been also tried for chemoprevention. Tamoxifen, for example, blocks the effects of estrogen on tumor growth, and has also been shown to lower the risk of breast-cancer recurrence, but drugs that may lower the risk of cancer frequently cause severe side effects. People with a higher risk of developing cancer may be willing to accept specific side effects but others may not want to use a drug that gives them side effects when they are healthy.10-12 Summarized, even though data confirm the preventive effect of certain approaches, the benefits are limited while the resulting adverse events are too severe to use them routinely.
Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) as COX-2 inhibitors, 13 Metformin as oral medication and first-line treatment for type 2 diabetes mellitus, 14 have been proved to reduce cancer risk and decrease mortality in various types of malignancies.
Recognition of the importance of the naturally occurring deuterium (present in 130-150 ppm abundance, equivalent to 15-17 mmol/L concentration) in maintaining normal cell growth opened up a novel form of cancer treatment and prevention. The first report by Somlyai et al. describing an anticancer effect of deuterium depletion in vitro and in vivo 15 appeared in 1993. The in vitro anticancer effect of deuterium-depleted water (DDW) has since been confirmed also by other laboratories.16,17 The key signal to stimulate cell division is probably the increasing D/H ratio, which in turn is the consequence of selective elimination of the light hydrogen by the Na+/H+ antiport after the binding of growth hormone to its receptor, leading to increased, that is, more alkaline, intracellular pH.18-20 Indeed, higher than natural D-concentration (300–600 ppm) was found to stimulate cell growth. 15
Prospective and retrospective clinical studies also confirmed that DDW consumption results in significant tumor regression, 21 increases median survival time, and reduces relapse rate.22-24 Among breast-cancer patients in remission at the start of DDW consumption, only one out of 48 patients died during the cumulative follow-up period of 221.1 years. These data indicated that DDW consumption, in repeated 3-to-4-month cures, was highly beneficial for breast-cancer patients and could lower the risk of cancer recurrence. 24
The likely mechanism of the anticancer effect of deuterium depletion is an increased proton export from the matrix to the intermembrane space of mitochondria, resulting in increased mitochondrial membrane potential, and enhanced production of reactive oxygen species (ROS). The resulting oxidative stress25,26 leads to slower cell growth and may induce apoptosis. The effect of deuterium depletion on the pro-oxidant/antioxidant balance has been confirmed when DDW restored the SOD-3 level, which could be the consequence of DDW-induced radicals, in Mn-exposed Caenorhabditis elegans.27,28 The significant effect of heavy hydrogen below natural concentration on mitochondrial activity also was confirmed by Pomytkin. 29
Studies further revealed that DDW inhibited expression of COX-2 gene 30 which is also the target of NSAID drugs, having been shown to reduce cancer risk and mortality 13 and expression of the oncogenes c-Myc, Ha-ras, and p53 after exposure of mice to the carcinogenic agent 7,12-dimethylbenz(a)anthracene (DMBA). 31
In this paper, results proving the anticancer effect of deuterium depletion from in vitro, as well as in vivo animal and human studies are presented. In the in vitro experiment, the aim was to expand gene expression analysis on other cancer-related and kinase genes (772 genes) in deuterium-depleted and deuterium-enriched media using NanoString technology. The in vivo aims included observation of the effect of D-depletion on survival in DMBA-treated mice, and in patients enrolled in a retrospective human study.
Materials and Methods
Production of Deuterium-Depleted Water
DDW of 25 ppm D-concentration was produced by HYD LLC for Cancer Research and Drug Development (Budapest) from ordinary tap water (150 ppm equivalent to 16.8 mmol/L D) after reverse osmosis purification by fractional distillation using a Good Manufacturing Practice (GMP) conform technology. The final D-concentration was verified by a Liquid-Water Isotope Analyser-24d (manufactured by Los Gatos Research Inc, USA) with ± 1 ppm precision. For gene expression study 40 ppm and 80 ppm as deuterium-depleted, 150 ppm as control and 300 ppm as deuterium-enriched water was applied adding 99.9% heavy water (Sigma Aldrich, Budapest) to get the desired concentration. For the marketed product intended for human consumption (Preventa), DDW was mixed with natural spring water to adjust the exact D-concentration, and to replenish minerals. In the present study, drinking water of 105, 85, 65, 45, and 25 ppm D-concentration was used.
Gene Expression Analysis
In a previous short-term (48 hour) study, carcinogen-induced expression of a few genes with key roles in tumor development was found to be suppressed in mice consuming DDW instead of normal drinking water.22,31
This time, an in vitro approach was used to investigate the possible impact of D-concentration on a wide range of genes. The lung cancer cell line A459 was grown in media containing 40 ppm, 80 ppm, 150 ppm, and 300 ppm deuterium (regarded as depleted, control, and enriched medium, respectively) as published earlier. 32 Sixteen hours before the experiments 400 000 A459 cells were seeded on serum-free media, then the media was removed and replaced with different D-containing media supplemented with fetal calf serum. The cells were incubated for 72 hours and harvested; total RNA was isolated when cells covered 60% of dishes. From each sample, 150 ng was used up for hybridization, the copy number of genes was compared to the control group. The presented data are results of 2 experiments. The data of 2 experiments were compared, the correlation coefficient R-value was .999 confirming the reproducibility of the method.
Changes in the expression of 236 cancer-related, and 536 kinase genes were examined, relative to the control medium, applying the nCounter Analysis System from NanoString technology as published earlier. 33
NanoString technology is an enzyme-free method, based on a solution hybridization principle, and can be used for gene-expression profiling. The system employs unique fluorescent barcodes that enable direct, digital detection of hundreds ( ≤ 800) of different target molecules in a single run. NanoString can detect targets as small as 100 base pairs, and also mRNAs. It requires only nanoscale amounts of RNA and has a detection sensitivity down to 1 copy per cell.
The method is to hybridize RNA extracted from a given biological sample with complementary, synthetic single-stranded DNA. One strand contains the biotin-labeled DNA terminus, and through this, the hybridized molecule will be fixed to a solid carrier. The other strand is not complementary; it contains a so-called reader, which consists of a coded sequence of known repeats. To these repeating sections, we hybridize complementary “stainer” sections marked with different colors. Each color-coded hybridized molecular complex attached to the solid support is subjected to an electric current, and then the surface of the solid support is scanned using a high-resolution camera to identify individually the molecular complexes for each gene. In this way, molecules with a unique code (unique color code) for each gene are counted one by one. Thus, they can perform digital gene expression analysis (determine the number of mRNA molecules associated with each gene).
The question addressed was whether subnormal or elevated D-concentration will affect gene expression in comparison to control, and which of the altered D-levels will have stronger impact on gene expression. We also wanted to investigate if the altered D-concentration has an impact on a few specific genes or influences the expression of a wide range of genes.
To minimize bias, only the data of those genes were evaluated, for which the change in gene expression was ≥ 30% than the control and the number of copies, that is the number of mRNA molecules, was ≥ 30.
Animal Experiment
In the previous short-term mouse experiment mentioned above,22,31 triggering the expression of certain genes with a key role in cancer development—by the carcinogen agent DMBA—proved to be inhibited in the mice consuming DDW.
The aim of the present animal experiment was to see whether consumption of DDW can prevent tumor development in the long term. Eight-week-old (20 ± 4 g weight) male and female CBA/Ca mice were treated with DMBA, which is known to activate cancer-related genes in this mouse strain. 22 The animals (24 males and 24 females) after DMBA treatment randomly were divided into 2 groups and kept under standard conditions conforming to ARRIVE guidelines 34 and the “Guide for the Care and Use of Laboratory Animals". 35 Animals were fed a conventional dry rodent diet. DMBA (Sigma Aldrich, Budapest, Hungary) was dissolved in corn oil and delivered to each of the mice by a single intraperitoneal injection at a dose of 20 mg/kg body weight. Water was provided ad libitum, with 150 ppm deuterium content (natural level) for the control animals (12 males and 12 females), and 25 ppm deuterium content for the DDW-treated mice (12 males and 12 females). Survival of the control and DDW-treated mice after the carcinogen exposure was observed for one year.
Administration of Deuterium-Depleted Water in Humans and Data Collection
The aim of the treatment was to reduce the D-concentration in the patients’ bodies by replacing the normal fluid intake with DDW, taking 1.5-2 L per day. DDW consumption generally started at 105 ppm D level and was changed for 85 ppm, 65 ppm, 45 ppm, and 25 ppm every 1 to 3 months. DDW consumption was suspended for 3 to 6 months and started again for further repeated 4-to-6-months treatment durations. Over time, the treatment periods were shortened, although each had to be at least 3-to-4 months long and later the 3-to-6 month-long breaks were prolonged. To prove the cancer-preventive effect of any therapy or agent on a healthy population would require the follow-up of several thousands of subjects for long periods, since general cancer incidence is 442.4 per 100 000 persons per year, based on data for 2013–2017. 36 Recurrence of cancer in tumor-free patients after completion of conventional therapy is, on the contrary, much more likely, and so more easily measurable, depending on the type of tumor, the stage at diagnosis, and the received therapy.
So, out of over 2000 case histories from the period April 1993 to October 2019, patients who were in tumor-free state at the start of DDW-consumption after receiving the appropriate therapy were selected for a retrospective analysis. Finally, data from 204 cases were used.
For each patient, we recorded age, body weight, gender, type of cancer, date of diagnosis, date of the start, and the end of DDW-consumption, date of the last available information and the status of being alive or dead.
Statistical Evaluation
In the mice study, MedCalc for Windows version 11.1.1.0 (MedCalc Software, Mariakerke, Belgium) was used for calculating Kaplan–Meier cumulative survival curves, and the log-rank test and Wilcoxon-probe were used to calculate P-values. In the retrospective human study, all statistical computations were performed by using software SPSS v25, and results were declared significant with P-value < .05.
The human study was performed retrospectively, and DDW treatment started at different time points after remission, so MST was computed from the start of DDW treatment. The mean difference was declared significant with P-value < .05.
Results
Higher Deuterium/Hydrogen Ratio Stimulates Gene Expression
The results of the in vitro experiment are summarized in Table 1; expression in 77.5% of cancer-related genes (183 genes) and in 33% of kinase genes (177 genes) changed at least by 30%. The copy number exceeded 30 in 65.6% of cancer-related and in 69.7% of kinase genes. Finally, 124 (52.5%) out of 236 tumor-related genes, and 135 (25.3%) out of 536 kinase genes met both requirements.
Table 1.
Effect of altered D-concentration on gene expression.
| Changes in Gene Expression Exceeds 30% | Copy Number of Genes Exceeds 30 | Changes in Expression Exceeds 30% and the Copy Number Exceeds 30 | |
|---|---|---|---|
| 236 cancer-related genes | 183 (77.5%) | 155 (65.6%) | 124 (52.5%) |
| 536 kinase genes | 177 (33%) | 374 (69.7%) | 135 (25.3%) |
In Tables 2 and 3 the changes in expression are summarized for the 2 groups of genes.
Table 2.
Changes in the expression of 124 cancer-related genes.
| Deuterium-Depleted Medium (40-80 ppm D) | Control Medium (150 ppm D) | Deuterium-Enriched Medium (300 ppm D) |
|---|---|---|
| upregulation: 1 gene | 0 | upregulation: 1 gene |
| 0 | 0 | upregulation: 118 genes |
| downregulation: 5 genes | 0 | upregulation: 5 genes |
Table 3.
Changes in the expression of 135 kinase genes.
| Deuterium-Depleted Medium (40-80 ppm D) | Control Medium (150 ppm D) | Deuterium-Enriched Medium (300 ppm D) |
|---|---|---|
| downregulation: 1 gene | 0 | 0 |
| 0 | 0 | upregulation: 134 genes |
According to Table 2, there was only 1 gene (.8%) out of 124 cancer-related genes (meeting both requirements, see Table 1) which was upregulated both in D-depleted and D-enriched media, five genes (4%) were downregulated in D-depleted medium, upregulated in D-enriched medium, while 95.2% of the genes (118 genes) were upregulated only in the D-enriched medium.
Similar results were obtained with the 135 kinase genes (Table 3) where not more than 1 gene (.7%) responded to D-depletion and was downregulated, but 99.3% (134 genes) were upregulated in D-enriched media.
The data in Tables 2 and 3 show that, in the 2 different sets of genes, 97.3% of them responded to the higher-than-natural D-concentration.
When changes in gene expression were investigated irrespectively of the copy number, it was also confirmed that most of the genes responded to the higher D-concentration. In 69.9% (165) of the cancer-related genes and in 71.6% (384) of kinase genes the expression was stimulated at 300 ppm with over 30% compared to normal D-concentration. At the same time, the 40 ppm D-concentration exerted an inhibitory effect in 8.4% and 2.6% of the 2 gene groups, respectively.
Of the genes showing significant changes in expression, KRAS and TGFBR2 as cancer-related genes should be highlighted: their copy number doubled at 300 ppm D-concentration but remained unchanged in DDW. The most significant, 2.17-fold, increase in expression was detected for the CCND2 gene, which belongs to a highly conserved Cyclin family. From among the kinase genes, we highlight the SGK1 gene, representing the highest doubled-copy number at 300 ppm D-concentration.
Survival of mice exposed to chemical carcinogen
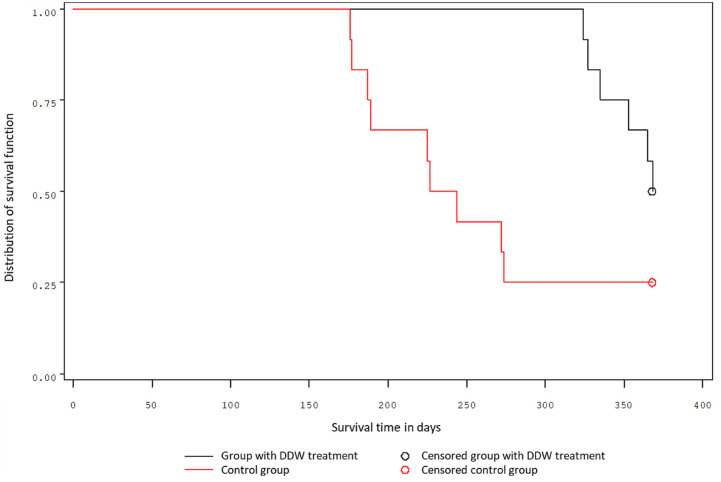
The one-year survival data, separate for males and females, are shown in Figures 1 and 2. In the group of male mice, the first animals in the control group perished after 156 days, and in the DDW-treated group after 334 days, following carcinogen exposure. A quarter of the treated male mice survived 344 days, while a quarter of control mice survived only 188 days. The probability of the 1-year survival was 58% for the treated mice and 25% for the control (Figure 1.). The 2 groups differed significantly (P = .0241 Log-rank test; P = .0051 Wilcoxon test).
Figure 1.
Survival of male mice.
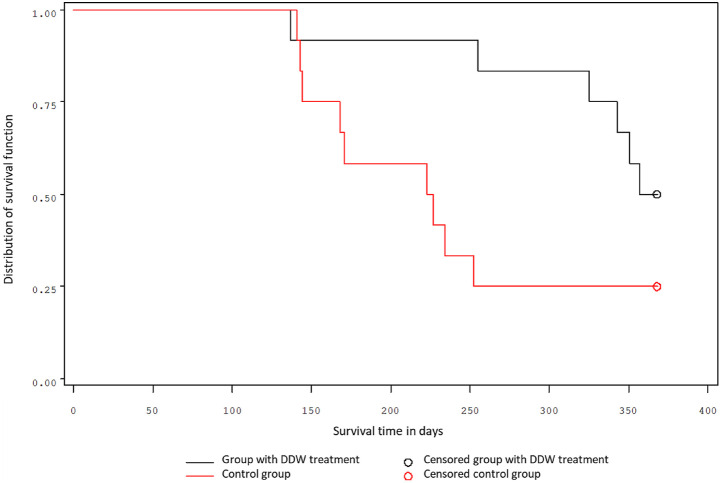
Figure 2.
Survival of female mice.
Similar results were obtained for the groups of female mice. A quarter of treated female mice survived 334 days, while a quarter of control mice survived only 156 days. The probability of the 1-year survival was 50% for treated mice and 25% for control (Figure 2.). The 2 groups differed significantly (P = .0515 Log-rank test; P = .00278 Wilcoxon test).
Preventive effect of deuterium depletion in 204 tumor-free cancer patients who previously received conventional therapies
The patients whose data were analyzed in this study had a broad variety of malignancies. The distribution of tumor types within the study population is summarized in Table 4.
Table 4.
Distribution of tumor types in the population of 204 cancer patients.
| Tumor Type | Number of Patients | % Of the Total |
|---|---|---|
| Female breast | 76 | 37.3 |
| Digestive system | 41 | 20.1 |
| Urinary | 23 | 11.3 |
| Gynecologic | 16 | 7.8 |
| Brain and nervous system | 12 | 5.9 |
| Lungs | 9 | 4.4 |
| Skin | 9 | 4.4 |
| Head and neck | 7 | 3.4 |
| Hematopoietic | 6 | 2.9 |
| Bone | 3 | 1.5 |
| Childhood | 1 | 0.5 |
| Not specified | 1 | 0.5 |
The cumulative follow-up time of the 204 patients (Table 5) was more than one thousand years (1024 years), so that the average follow-up time per patient was 5 years (median: 3.6 years). Median survival time (MST) was not calculable due to the extremely low death rate: only 13 patients died out of 204. Two deaths out of 13 were not cancer-related, so, considering the 1024 years of cumulative follow-up time and the number of death cases (11 cancer-related deaths) 5.4% of the study population was lost. It is important to note that the average time between diagnosis and the start of DDW-consumption was shorter than 1 year; 325 days (median 78 days).
Table 5.
Time periods for the study population of 204 tumor-free patients.
| Cumulative Time from the Diagnosis to the End of the Follow-Up | 1024 years |
|---|---|
| Median time between the date of diagnosis and the start of DDW consumption | 78 days |
| Median duration of DDW consumption | 334 days |
| Median time from the diagnosis to the end of the follow-up | 1309 days (3.6 years) |
| Number of patients who died from cancer | 11 (5.4%) |
| Median survival time | Not calculable |
| Cumulative follow-up period/Cancer-related deaths | 1024/11 = 93.1 years |
Although the death rate was very low, 45 patients (22%) relapsed within the follow-up period. To reveal and confirm the protective effect of deuterium depletion, it is important to know the details and to verify correlation between the long survival without recurrence of cancer and the possible reason for relapse.
For this purpose, 3 groups of patients were created (Table 6.). It was found that 159 patients (77.9%) remained cancer-free during a cumulative follow-up time of 803 years (average follow-up time 5 years, median: 3.3 years).
Table 6.
Death rates in the subgroups of the 204 patients associated with relapse.
| Relapse | Number of patients | Cumulative Follow-Up Time (years) | Average Follow-Up Time (years) | Cumulative Length of DDW Ingestion (years) | Number of Deaths |
|---|---|---|---|---|---|
| No relapse | 159 (77.9%) | 803 | 5.0 | 205 | 2 |
| Relapse/After finishing DDW treatment | 22 (11.8%) | 159 | 7.2 | 48 | 8 |
| Relapse/During DDW treatment | 23 (11.3%) | 62 | 2.7 | 42 | 3 |
The 45 patients who relapsed were divided into 2 subgroups: in 22 cases recurrence occurred long after stopping DDW consumption, and in 23 cases during DDW consumption. The average time until recurrence after finishing DDW consumption was 3.2 years (median: 2.56 years) and 8 death cases occurred in this group. In these patients, recurrence and death were probably related to the discontinuation of DDW consumption since these events happened long after finishing DDW. Cancer recurrence occurred during DDW consumption in 23 cases (11.2%) and in this subgroup 3 patients died.
The cases of 13 patients who died during the follow-up
In the group of patients who were in remission without relapse, 2 death cases occurred. One of the patients died 20 years after diagnosis; her death was due to her old age. The other patient died because of pulmonary embolism, so these 2 deaths were not cancer-related.
In Table 7, the 11 (8 + 3) cancer-related death cases are summarized, identified by type of cancer.
Table 7.
Distribution of the 11 deaths by tumor types.
| Tumor Type | Duration of DDW Consumption (in days) | Time of Death after Stopping Drinking DDW (in days) |
|---|---|---|
| Bladder | 91 | 2716 |
| Rectum | 140 | 762 |
| Esophagus | 1927 | 2060 |
| Uterine | 189 | 1259 |
| NHL | 313 | 1073 |
| Melanoma | 1101 | 2315 |
| Astrocytoma 3 | 1620 | 0 |
| Breast (a) | 539 | 0 |
| Breast (b) | 1527 | 76 |
| ALL | 245 | 0 |
| Colon | 1081 | 11 |
The 2 patients with bladder and rectum tumors consumed DDW only for 91 and 140 days, respectively. The patient with a bladder tumor started to consume DDW 4 months, the rectum cancer patient 2 years, after diagnosis, and they died 7.5 and 2 years, respectively, after stopping DDW consumption. We suppose that the short, insufficient duration of DDW consumption played a major role in their deaths.
The patients with esophagus cancer, uterine cancer, NHL, and melanoma consumed DDW for relatively long periods (1927 days, 189 days, 313 days, 1101 days), but they stopped it, and their deaths occurred 5.6, 3.4, 2.9, and 6.3 years later, respectively.
The patient with astrocytoma type 3 brain tumor did not relapse for 4 years after diagnosis. The patient repeated 16 courses over the follow-up period of longer than 8 years.
Breast-cancer patient (a) started consuming DDW immediately after diagnosis and surgery but being asymptomatic, she stopped taking DDW after 8 months. 2.5 years later, when metastases in the lung were diagnosed, she started DDW again, but she died 10 months later.
Breast-cancer patient (b) started taking DDW 3.5 years after diagnosis, allowing cancer cells to multiply within this long period of time. In this case, the disease progressed despite DDW consumption, metastases in the bone and lung developed. The patient died 8 months later.
The acute lymphoid leukemia (ALL) case happened in the early 1990s, when DDW was available with moderately reduced D-concentration (105-130 ppm D). The patient began taking DDW 16 months after diagnosis and died 8 months later.
The patient with colon cancer made a short break in DDW-consumption. Recurrence happened during this period, suggesting that the break started too early.
The data show that deaths did not specifically associate with one or other tumor type; on the contrary, the 11 cases represented 10 different tumors. The common features of deadly outcome were mostly the inappropriate DDW-consumption and the type and stage of cancer at the diagnosis.
Discussion
Despite all attempts to develop safe and effective drugs against malignant diseases, the incidence and mortality of cancer have been increasing dramatically. 1 There is no proven method to prevent cancer, conventional drugs cannot be widely used, and evidence on the true value of unconventional approaches is contradictory.
Since the recognition of the first tyrosine kinase gene encoding a protein identified as a potential oncogene,37,38 drug-discovery strategies have been genetic-linkage-based, dealing with cancer as a genetic disease. The target was a mutation in one particular gene, affecting one signaling pathway in cell proliferation. However, the failure of thousands of clinical trials, and the continuing lack of safe and effective drugs to treat and prevent cancer, indicate the weaknesses of this approach.
Recognition of the regulatory role of deuterium in cell cycle offers a new opportunity to influence the whole biochemical cascade of cell division.15,26 Gene expression studies22,31 proved that D/H ratios different from the natural ratio (1/6600, equivalent to 150 ppm) simultaneously modified the expression of hundreds of genes. A sub-molecular change, increasing/decreasing D/H ratio, is apparently transmitted to molecular level, where the activated/suppressed genes and their protein products regulate G1 phase progression, S phase initiation, and completion of the cell cycle. 39 This is in line with earlier findings 15 in which D-concentration exceeding 150 ppm stimulated cell growth, supporting the hypothesis that an altered D/H ratio can be one of the key regulatory factors. In the in vitro study presented here, D-depletion upregulated 1 and downregulated the expression of 5 cancer-related genes. These 6 genes and all the other 118 cancer-related genes (100%) were upregulated in the medium with elevated D level (300 ppm).
There was only one kinase gene downregulated in DDW while 134 (99.3%) were upregulated.
In contrast to most current drug development strategies, which aim to develop drugs targeting single genes, our study proves that alterations in the D/H ratio offer the useful opportunity to simultaneously affect the activity of a wide range of genes.
A previous short-term mouse study by our group22,31 confirmed that keeping the D-concentration of the organism below natural levels by administration of DDW can inhibit the cells exposed to a carcinogen from increasing gene expression (c-Myc, Ha-ras, p53, Kras, and Bcl2). In the present study, data on the mice’s survival proved that long-term consumption of DDW was able to protect the animals against cancer development.
This finding was in line with that of the follow-up of 204 tumor-free, previously operated and treated cancer patients who consumed DDW on a regular basis. It is well known that—depending on tumor type, cancer stage at diagnosis, and the conventional therapy applied—tumorous diseases can show frequent recurrence. According to the data summarized in Table 6, 77.9% of our patients did not relapse during the follow-up period, and 11.8% of them had recurrences years after stopping DDW, which finally means that almost 90% of the follow-up population had no relapse.
To prove the reduction of relapse rate suggested by these data, the rate of recurrence among our set of patients was compared to historical controls in case of colorectal, breast, prostate, and bladder cancer. These four tumor types represented 56.3% of our study population (115 patients) consuming DDW in remission. After standard treatment of colorectal cancer with curative intent, 30% to 40% of patients typically develop recurrent disease.40,41 For breast cancer,42,43 prostate 44 and bladder cancer, 45 the recurrence rates are 40%, 20–40%, and 45%, respectively, depending on the stage at diagnosis and the efficacy of the therapy. In our study population, 27 patients had previously colorectal tumor and 5 patients (18.5%) relapsed. Of the 76 breast-cancer patients, 9 relapsed (recurrence rate 11.8%). No relapse occurred in 5 prostate cancer patients (.0%) and only one relapsed (14.2%) out of the 7 bladder cancer patients. In summary, out of the 115 patients, 14% relapsed (15 patients), which was one third of the expected recurrence rate. The low relapse rates led to extremely low mortality rates: during the 1024 years cumulative follow-up, only 11 patients (5.4%) were lost. It is important to emphasize that 8 deaths of the 11 happened several years after cessation of DDW consumption, supporting the strong protective effect of deuterium depletion.
The mechanism of the anti-tumor effect of deuterium depletion suggested the inhibitory effect of DDW on cell growth was due to reduction of D-concentration below the natural level. Regarding the mechanism of prevention, the recent gene expression research reveals inhibiting the D/H ratio from increasing over the natural concentration could be responsible for the preventive effect. Any change of D/H ratio will result in a new equilibrium in all molecules having hydrogen in an exchangeable position, which can simultaneously modify the behavior of numerous molecules, genes, and enzymes involved in cell division. The presence of D at a certain concentration is necessary for normal cell growth, 15 even more for the proliferation of neoplastic cells. The activation of the cell’s own Na+/H+ antiport generates an increase in D/H ratio in the intracellular space by preferentially eliminating the lighter hydrogen isotope. Similarly, fibroblasts, transformed with the H+-ATPase gene of yeast, showed not only increased intracellular pH but also tumorous phenotype.20,46 Putting together all the available information, we concluded that the cell cycle regulating system recognizes changes in the D/H ratio and when this ratio reaches a certain threshold it will trigger the cascading molecular processes which drive the cell cycle forward to enter its S phase. 15
The final D/H ratio of cells depends not only on the activated Na+/H+ antiport but on mitochondria-burning nutrients as carbon sources to store energy in ATP molecules, while producing metabolic water and carbon dioxide. Mitochondria, working properly in healthy cells, can produce deuterium-depleted metabolic water (DDMW). This depends on the D-concentration of the actual sources of carbon, which is close to 150 ppm for carbohydrates but significantly lower, 120 ppm for fats.47-49 So, the higher is the proportion of fat-related carbon sources, the lower is the D-content of metabolic water. When a growth hormone binds to the membrane receptor and stimulates the Na+/H+ antiport which prefers to eliminate the lighter isotope of H+, the D/H ratio in the cytoplasm will increase. However, in healthy cells mitochondria typically produce DDMW (as stated above) that retards the cell from reaching the D/H ratio necessary to initiate cell division; so that the probability of cell division is finally determined by the balance of these 2 opposing processes. If the tricarboxylic acid cycle of the mitochondria does not work properly, which is a hallmark of cancer cells, metabolic water production is restricted. This in turn drives the D/H ratio to more easily reach the threshold level. Then cells will multiply quickly.50,51 This submolecular regulatory system explains also why ketogenic diet can aid cancer therapy. The changes of metabolic parameters during such a diet, based on restricted carbohydrate intake, and its beneficial effects, are well documented.52-55 Moreover, the anticancer effect of Metformin, a drug used in type 2 diabetes, also results from enhancing mitochondrial function. 14
The effect of certain successful novel cancer drugs can also be explained as a result of a D/H ratio shift. Trastuzumab (Herceptin) administration in HER2-positive metastatic breast-cancer patients significantly prolonged overall survival (33 vs 26 months; p = .003) and led to a 49.8% reduction in death risk.56,57 Herceptin targets the product of the HER2 gene, which encodes the human epidermal growth factor family of receptor (EGFR) tyrosine kinases. In HER2-positive breast-cancer patients, either the amplification of the gene or its overexpression was confirmed. This increased the EGFR in the membrane by 40-100%. The increased number of EGFR molecules leads to an activated Na+/H+ antiport, which prefers to eliminate the light hydrogen isotope while increasing the D/H ratio in the intracellular space. Herceptin binding to EGFR inhibits the binding of growth hormone to trigger signal transduction pathways including the activation of the Na+/H+ antiport. In the absence of activation of the Na+/H+ antiport, the D/H ratio will not be increased, and all the genes with roles in cell proliferation will not be turned on.58,59
In summary, we suppose the membrane transport activity leads to a modified D/H ratio and this change triggers distinct molecular processes. Our studies revealed that a modified D/H ratio simultaneously strongly modifies the expression of hundreds of cancer-related and kinase genes with roles in cell-cycle regulation. Most genes were found to respond to an elevated D/H ratio, which is consistent with our former data that elevated D-concentration stimulates cell growth. Our animal studies proved that DDW, in the form of drinking water, inhibits cancer development after exposure to a chemical carcinogen. Presumably, this is by preventing the cells from increasing D/H ratio to the threshold necessary for inducing gene expression. Similar results were obtained in our follow-up study of 204 DDW-consuming cancer patients then in remission. The extremely low death rate (11 deaths out of 204 patients within the 1024 years cumulative follow-up time) confirmed that maintaining a D-concentration at sub-natural levels can prevent relapse of cancer patients who are in remission.
The data also proved that lower D-concentration triggers apoptosis, but also that the cells adapt to the decreased D level. Consequently, proper dosing of DDW is critical, because inappropriate reductions of D-concentration may not induce the apoptosis of cancer cells but might rather help them adapt to lower D-concentrations, thereby becoming resistant to DDW.
Literature data and own results led to the conclusion that replacing normal drinking water with deuterium-depleted water, a ketogenic diet, blocking EGFR with trastuzumab, or enhancing mitochondrial function with Metformin, all have the same effect: boosting the capacity of cells to maintain a lower D/H ratio.
So, deuterium depletion opens new perspectives in cancer treatment and prevention, offering a completely safe and non-invasive treatment modality.
Footnotes
Authors' contributions: Conceptualization, G.S., A.P., L.P.; methodology, L.P., L.I.N., Z.Gy., I.F., Gy.C.; software, L.I.N., Z.Gy., B.Zs.K.; investigation, G.S., L.P., L.I.N., Z.Gy.; resources, G.S., I.S.; data curation, B.Zs.K., L.P., L.I.N., Z.Gy., G.S, I.S.; writing—original draft preparation, B.Zs.K., I.S., G.S.; writing—review and editing, A.P., Z.Gy., I.S.; visualization, L.P., L.I.N., Z.Gy.; funding acquisition, G.S.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the HYD LLC for Cancer Research and Drug Development.
Ethical Approval: Animal study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Committee on Research of the University of Pécs (permit number: BA02/2000-24/2006) Ethical Approval to retrospective study is not applicable for this article.
Statement of informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs
István Fórizs https://orcid.org/0000-0003-0813-6079
Gábor Somlyai https://orcid.org/0000-0001-9116-6417
References
- 1.https://www.who.int/health-topics/cancer#tab=tab_1,” 2018
- 2.Ladanie A, Schmitt AM, Speich B, et al. Clinical trial evidence supporting us food and drug administration approval of novel cancer therapies between 2000 and 2016. JAMA Netw Open. 2020;3(11):e2024406. doi: 10.1001/jamanetworkopen.2020.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seyfied TN. Cancer as a Metabolic Disease. New Jersey, USA: John Wiley and Sons; 2012. [Google Scholar]
- 4.Bishop JM. Cancer: the rise of the genetic paradigm. Genes Dev. 1995;9(11):1309-1315. doi: 10.1101/gad.9.11.1309. [DOI] [PubMed] [Google Scholar]
- 5.Wishart DS. Is cancer a genetic disease or a metabolic disease? EBioMedicine. 2015;2(6):478-479. doi: 10.1016/j.ebiom.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12(5):496-503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 8.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9:498-509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 9.Aditya B, Imad MT, Cerhan KA, et al. Efficacy of antioxidant supplementation in reducing primary cancer incidence and Mortality: Systematic review and meta-analysis. Mayo Clin Proc. 2008;83:23-24. [DOI] [PubMed] [Google Scholar]
- 10.https://www.cancer.net/about-us/cancernet-editotial.board
- 11.Frey MK, Ellis AE, Koontz LM, et al. Ovarian cancer survivors' acceptance of treatment side effects evolves as goals of care change over the cancer continuum. Gynecol Oncol. 2017;146(2):386-391. doi: 10.1016/j.ygyno.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Colagiuri B, Dhillon H, Butow PN, Jansen J, Cox K, Jacquet J. Does Assessing Patients' Expectancies About Chemotherapy Side Effects Influence Their Occurrence? J Pain Symptom Manage. 2013;46(2):275-281. doi: 10.1016/j.jpainsymman.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93-126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 14.Najafi M, Cheki M, Rezapoor S, et al. Metformin: Prevention of genomic instability and cancer: A review. Mutat Res Genet Toxicol Environ Mutagen. 2018;827:1-8. doi: 10.1016/j.mrgentox.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Somlyai G, Jancsó G, Jákli G, et al. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS (Fed Eur Biochem Soc) Lett. 1993;317:1-4. doi: 10.1016/0014-5793(93)81479-J. [DOI] [PubMed] [Google Scholar]
- 16.Cong FS, Zhang YR, Sheng HC, Ao ZH, Zhang SY, Wang JY. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp Ther Med. 2010;1(2):277-283. doi: 10.3892/etm_00000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soleyman-Jahi S, Zendehdel K, Akbarzadeh K, Haddadi M, Amanpour S, Muhammadnejad S. In vitro assessment of antineoplastic effects of deuterium depleted water. Asian Pac J Cancer Prev. 2014;15(5):2179-2183. doi: 10.7314/apjcp.2014.15.5.2179. [DOI] [PubMed] [Google Scholar]
- 18.Pouyssegur J, Chambard JC, Franchi A, Paris S, Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci Unit States Am. 1982;79(13):3935-3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic PH. Proc Natl Acad Sci Unit States Am. 1984;81(15):4833-4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988;334(6181):438-440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- 21.Somlyai G, Laskay G, Berkényi T, et al. The biological effects of deuterium-depleted water, a possible new tool in cancer therapy. Zeitschrift für Onkologie (ger) Journal of Oncology. 1998;30:91-94. [Google Scholar]
- 22.Gyöngyi Z, Budán F, Szabó I, et al. Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutr Cancer. 2013;65(2):240-246. doi: 10.1080/01635581.2013.756533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovács A, Guller I, Krempels K, et al. Deuterium depletion may delay the progression of prostate cancer. J Cancer Ther. 2011;02:548-556. doi: 10.4236/jct.2011.24075. [DOI] [Google Scholar]
- 24.K K, I S, Z G. et al. A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies. J Cancer Res Ther. 2013;1(8):194-200. doi: 10.14312/2052-4994.2013-29. [DOI] [Google Scholar]
- 25.Zhang X, Gaetani M, Chernobrovkin A, Zubarev RA. Anticancer effect of deuterium depleted water - redox disbalance leads to oxidative stress. Mol Cell Proteomics. 2019;18(12):2373-2387. doi: 10.1074/mcp.RA119.001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Wang J, Zubarev RA. Slight deuterium enrichment in water acts as an antioxidant: Is deuterium a cell growth regulator? Mol Cell Proteomics. 2020;19(11):1790-1804. doi: 10.1074/mcp.RA120.002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ávila DS, Somlyai G, Somlyai I, Aschner M. Anti-aging effects of deuterium depletion on Mn-induced toxicity in a C. elegans model. Toxicol Lett. 2012;211(3):319-324. doi: 10.1016/j.toxlet.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Qin D, Yang H, et al. Neuroprotective effects of deuterium-depleted water (ddw) against H2O2-induced oxidative stress in differentiated PC12 cells through the PI3K/Akt signaling pathway. Neurochem Res 2020;45(5):1034-1044. doi: 10.1007/s11064-020-02978-4. [DOI] [PubMed] [Google Scholar]
- 29.Pomytkin IA and Kolesova OE. Relationship between natural concentration of heavy water isotopologs and rate of H2O2 generation by mitochondria. Bull Exp Biol Med 2006;142(5):570-572. doi: 10.1007/s10517-006-0420-9. [DOI] [PubMed] [Google Scholar]
- 30.Somlyai G. Let’s Defeat Cancer! Budapest, Hungary: AkadémiaiAcademia Kiadó; 2021. [Google Scholar]
- 31.Gyöngyi Z and Somlyai G. Deuterium depletion can decrease the expression of c-Myc, Ha-ras and p53 gene in carcinogen-treated mice. In vivo. 2000;14:437-439. PMID: 10904878. [PubMed]
- 32.G S BZs K I S A P LI N and LG P. Deuterium depletion inhibits lung cancer cell growth and migration in vitro and results in severalfold increase of median survival time of non-small cell lung cancer patients receiving conventional therapy. J Cancer Res Ther 2021;9(2):12-19. doi: 10.14312/2052-4994.2021-2. [DOI] [Google Scholar]
- 33.Fortina P, Surrey S. Digital mRNA profiling. Nat Biotechnol. 2008;26(3):293-294. doi: 10.1038/nbt0308-293. [DOI] [PubMed] [Google Scholar]
- 34.Percie du Sert N, Hurst V, Ahluwalia A, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. 2020;177(16):3617-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guide for the Care and Use of Laboratory Animals, 8th Edition’ 2011. https://journals.sagepub.com/doi/10.1258/la.2012.150312 [Google Scholar]
- 36.https://www.cancer.gov./about-cancer/understanding/statistics
- 37.Vogt PK. Cancer genes. West J Med. 1993;158(3):273-278. PMCID: PMC1311753. [PMC free article] [PubMed] [Google Scholar]
- 38.Yarbro JW. Oncogenes and cancer suppressor genes. Semin Oncol Nurs. 1992;8(1):30-39. doi: 10.1016/0749-2081(92)90006-o. [DOI] [PubMed] [Google Scholar]
- 39.Yavari Kand Kooshesh L. Deuterium depleted water inhibits the proliferation of human MCF-7 breast cancer cell lines by inducing cell cycle arrest. Nutr Cancer 2019;71(6):1019-1029. doi: 10.1080/01635581.2019.1595048. [DOI] [PubMed] [Google Scholar]
- 40.Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival. Ann Surg. 2016;263(6):1143-1147. doi: 10.1097/SLA.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelmsen M, Kring T, Jorgensen LN, et al. Determinants of recurrence after intended curative resection for colorectal cancer. Scand J Gastroenterol. 2014;49(12):1399-1408. doi: 10.3109/00365521.2014.926981. [DOI] [PubMed] [Google Scholar]
- 42.Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34(9):927-935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209-219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurbegovic S, Berg KD, Thomsen FB, et al. The risk of biochemical recurrence for intermediate-risk prostate cancer after radical prostatectomy. Scandinavian Journal of Urology. 2017;51(6):450-456. doi: 10.1080/21681805.2017.1356369. [DOI] [PubMed] [Google Scholar]
- 45.Flaig TW, Spiess PE, Agarwal N, et al. NCCN guidelines insights: bladder cancer, version 5.2018. J Natl Compr Cancer Netw. 2018;16(9):1041-1053. doi: 10.6004/jnccn.2018.0072. [DOI] [PubMed] [Google Scholar]
- 46.Kotyk A, Dvořáková M, Koryta J. Deuterons cannot replace protons in active transport processes in yeast. FEBS (Fed Eur Biochem Soc) Lett. 1990;264:203-205. [DOI] [PubMed] [Google Scholar]
- 47.Billault I, Duan J-R, Guiet S, Robins RJ. Quantitative deuterium isotopic profiling at natural abundance indicates mechanistic differences for δ12-epoxidase and δ12-desaturase in vernonia galamensis. J Biol Chem. 2005;280(18):17645-17651. doi: 10.1074/jbc.M500909200. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler H, Osmond CB, Stichler W, Trimborn P. Hydrogen isotope discrimination in higher plants: Correlations with photosynthetic pathway and environment. Planta. 1976;128:85-92. [DOI] [PubMed] [Google Scholar]
- 49.Dzhimak SS, Basov AA, Baryshev MG. Content of deuterium in biological fluids and organs: influence of deuterium depleted water on D/H gradient and the process of adaptation. Dokl Biochem Biophys. 2015;465:370-373. doi: 10.1134/S1607672915060071. [DOI] [PubMed] [Google Scholar]
- 50.Somlyai G. Deuterium Depletion Results in Several-fold Increases in the Median Survival Time of Cancer Patients during Oncotherapy. Budapest, Hungary: 3rd International Congress on Deuterium Depletion; 2015. [Google Scholar]
- 51.Boros LG, D’Agostino DP, Katz HE, Roth JP, Meuillet EJ, Somlyai G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med Hypotheses. 2016;87:69-74. doi: 10.1016/j.mehy.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seyfried TN, Flores RE, Poff AM, D'Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35(3):515-527. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seyfried TN, Flores R, Poff AM, D’Agostino DP, Mukherjee P. Metabolic therapy: A new paradigm for managing malignant brain cancer. Cancer Lett. 2015;356(2 Pt A):289-300. doi: 10.1016/j.canlet.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 55.Miraghajani M, Khodadadi S, Sobhani N, et al. Tumor cells growth and survival time with the ketogenic diet in animal models: A systematic review. Int J Prev Med. 2017;8:35. doi: 10.4103/2008-7802.207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lv S, Wang Y, Sun T, et al. Overall survival benefit from trastuzumab-based treatment in HER2-positive metastatic breast cancer: A retrospective analysis. Oncology Research and Treatment. 2018;41:450-455. doi: 10.1159/000488202. [DOI] [PubMed] [Google Scholar]
- 57.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 58.Cho H-S, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the herceptin fab. Nature. 2003;421(6924):756-760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 59.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]