Abstract
Huntington’s disease (HD) is a devastating polyglutamine disorder characterized by extensive neurodegeneration and metabolic abnormalities at systemic, cellular and intracellular levels. Metabolic alterations in HD manifest as abnormal body weight, dysregulated biomolecule levels, impaired adipocyte functions, and defective energy state which exacerbate disease progression and pose acute threat to the health of challenged individuals in form of insulin resistance, cardiovascular disease, and energy crisis. To colossally mitigate disease symptoms, we tested the efficacy of curcumin in Drosophila model of HD. Curcumin is the bioactive component of turmeric (Curcuma longa Linn), well-known for its ability to modulate metabolic activities. We found that curcumin effectively managed abnormal body weight, dysregulated lipid content, and carbohydrate level in HD flies. In addition, curcumin administration lowered elevated reactive-oxygen-species levels in adult adipose tissue of diseased flies, and improved survival and locomotor function in HD flies at advanced disease stage. Altogether, these findings clearly suggest that curcumin efficiently attenuates metabolic derangements in HD flies and can prove beneficial in alleviating the complexities associated with HD.
Keywords: Body weight, curcumin, Huntington’s disease, lipid metabolism, neurodegenerative disease, reactive-oxygen-species
Impact statement
Huntington’s disease (HD) is a devastating neurodegenerative disorder with no known cure till date. We have shown that an effective dose of polyphenol curcumin when administered from early developmental stages amends metabolic alterations in Drosophila model of HD. Until now, current treatment strategies of HD remain inadequate in delaying the disease progression and impart multiple side-effects which fail to provide the much-needed relief to the challenged individuals. In this context, phytochemical curcumin can emerge as a safe and effective therapeutic option in gradual improvement of disease symptoms and amendment of metabolic defects linked with HD progression, with least side-effects. Our work in Drosophila HD model provides useful insights on significant amelioration of disease complexities by administration of pleiotropic phytochemicals such as curcumin, and these effects can further be screened in mammalian system and ultimately in humans with the final aim to improve quality of lives of HD patients.
Introduction
HD is a dominantly inherited, late-onset and progressive neurodegenerative disorder characterized by locomotor disturbances, cognitive decline, and psychiatric abnormalities. In humans, the causative gene for HD is located on chromosome 4p16.3 which comprises polymorphic trinucleotide cytosine-adenine-guanine (CAG)n repeats coding for ∼348 kD huntingtin (Htt) protein. Abnormal expansion of (CAG)n repeats beyond 35 results in HD in humans. 1
HD encompass an array of metabolic abnormalities which include cachexia, 2 persistent energy deficit, 3 hypothalamic-endocrine disturbances with peripheral hormonal dysregulation,4,5 and altered biomolecule metabolism, e.g. fatty acids, protein, cholesterol, glucose, etc.6–9 Multiple defects are found in peripheral tissue such as skeletal, 10 pancreas, 11 adipose, 12 and cardiac cells. 13 The multi-faceted metabolic alterations in conjunction with neurodegeneration aggravate the disease condition and augment risk of developing acute complications such as cardiovascular diseases, diabetes, pneumonia in HD patients thereby impacting their life expectancy.
The currently available target-specific synthetic drugs for HD offer marginal relief by suppressing the neurological symptoms but cause numerous side effects. 14 In this scenario, testing natural compounds with known therapeutic potential and minimum side-effects appear promising. Keeping this aspect as a major consideration, we tested phytochemical “curcumin,” a small polyphenolic compound and bioactive component of turmeric (Curcuma longa Linn) with strong antioxidant,15,16 anti-inflammatory,15,17,18 neuroprotective, 19 and metabolic properties18,20,21 in Drosophila model of HD.
Several studies have showcased the efficacy of curcumin in animal models of neurodegeneration such as Alzheimer’s,22–24 Parkinson’s, 25 and HD.26–28 Curcumin is shown to suppress inflammation, 29 increase lifespan, 25 ameliorate behavioral dysfunctions, 26 and improve disease phenotype 27 in these disease models. Besides alleviating neurodegeneration, curcumin also protects organs from toxicities 30 and effectively attunes several incapacitating metabolic conditions such as diabetes 31 and obesity.32,33
With pleiotropic effects of curcumin in mitigating diverse diseases, we aimed to assess the efficacy of curcumin in attenuation of metabolic derangements linked with HD progression. Drosophila model of HD expressing exon1 fragment of human huntingtin with expanded 93 glutamine repeats (Httex1p Q93) in all the neuronal populations recapitulates characteristic features of disease including late-onset nature, mutant Htt accumulation, progressive locomotor dysfunction, photoreceptor neurons degeneration, and reduced lifespan.34–37 The diseased flies also exhibit significant alterations in their body weight with extensive modulation in major energy reserves with HD progression. 36 More recently, the molecular mechanisms including fatbody-specific apoptosis, mitochondrial dysfunction, and calcium derangement, leading to altered metabolism were reported in the HD fly model. 38 In the present study, we tested specific doses of curcumin in the HD flies and evaluated the phytochemical’s efficacy to suppress behavioral and metabolic abnormalities.
By dosage studies, we found that an effective concentration of curcumin significantly attenuated dysregulated body weight and total lipid content in diseased flies at specific ages. Additionally, quantification of carbohydrate levels suggested that the same dose effectively regulated circulating trehalose levels and lowered elevated reactive-oxygen-species (ROS) level in adult adipose tissue of diseased flies. Interestingly, the feeding behavior of diseased larvae or flies reared on curcumin-supplemented food remain unchanged and matched those raised on normal food. Curcumin intake also resulted in an improvement in the median survival and locomotor function of diseased flies. Furthermore, expression profiling of metabolic regulators such as dSREBP or HLH106, bmm, and lipin genes revealed no change in their levels in diseased flies upon curcumin supplementation. Altogether, these findings suggest that curcumin proves immensely beneficial in the management of metabolic alterations and energetics in Drosophila model of HD. We, therefore, propose that curcumin can serve to be a safe and effective putative therapeutic option for the management of HD with minimum side effects. However, more emphasis will be required to elucidate the complex mechanism of curcumin actions.
Materials and methods
Drosophila stocks, crosses, and dietary condition
Transgenic fly lines w; P{UAS-Httex1p Q20}, w; P{UAS-Httex1p Q93}4F1, 36 and w; P{w+mW.hs = GawB}elavC155 (on X chromosome) were used in this study. Expression of polyglutamine (polyQ) containing peptides was carried out using the bipartite UAS-GAL4 expression system. 39 Virgins from stocks with polyQ constructs under the control of yeast UAS were crossed with males from driver line expressing yeast GAL4 transcriptional activator under neuronal elav promotor. The resulting progeny expressing polyQ peptides were reared on food mixed without and with 10, 15, and 20 µM concentration of curcumin (Sigma Aldrich), with equal supplementation of dimethyl sulfoxide (DMSO, Sigma Aldrich) in all the conditions. Treatment to different feeding conditions was maintained from early larval stages till day 15 post eclosion. Cultures were grown at 25 °C and 65% humidity under a 12 h:12 h light/dark cycle.
Survival assay
For survival analysis, a cohort of freshly emerged flies from curcumin unfed and fed conditions were collected and transferred to respective food conditions, without and with 10 µM concentration of curcumin. Flies were transferred to fresh vials every alternate day till 15 days and mortality in each condition was scored. Minimum 100 flies (five replicates with 20 flies/replicate) from each condition were used for the assay. Survival was analyzed using Kaplan-Meier estimator.
Food intake assay
The rate of food intake at larval and adult stages was ascertained using colorimetric dye intake assay. Synchronized feeding third instar female larvae were sorted and placed in a hole created at the center of an agar plate (3.3% wt/vol) filled with yeast paste containing 4% (w/v) FD&C Blue dye # 1 (Sigma Aldrich). The larvae were allowed to feed in the dyed-yeast paste for 2 h. After feeding, larvae were thoroughly washed with ice cold distilled water, pat-dried and homogenized in 200 µl of 1× PBS. The homogenate was centrifuged at 13,500 rpm for 10 min and the absorbance of supernatant was recorded at 625 nm. For each condition, minimum two replicates of 10 larvae each were used for the assay.
For food intake estimation at adult stages, age-matched flies from different experimental conditions were collected and transferred to food containing 2.5% (w/v) FD&C Blue dye # 1 for 30 min of feeding. After feeding, the crop and midgut region was dissected and homogenized in 200 µl of ice-cold 1× PBS. The homogenate was centrifuged at 13,500 rpm for 10 min and absorbance of supernatant was recorded at 625 nm. Two replicates of 10 flies each, per condition were used and food intake was assessed at day 6, 8, and 12.
Protein quantification
Total protein content was estimated using Bicinchoninic acid (BCA) method; 4 flies/replicate were homogenized in 400 µl of 2% Na2SO4 and 0.05% Tween 20 (1:1); 80 µl of homogenate was aliquoted in fresh tubes and 500 µl of 0.15% sodium deoxycholate was added. The mixture was left on ice for 10 min and 1 ml of 3 M trichloroacetic acid was added. The samples were spun at 8500 rpm for 15 min at 4 °C. After discarding the supernatant, pellet was rinsed once with 1 ml of 1 M HCL, dried, and mixed with 1.6 ml of BCA (Sigma-Aldrich) standard working reagent. The mixture was then incubated at 60 °C for 10 min and cooled on ice to stop further color development; 100 µl of the supernatant was aliquoted and absorbance was recorded at 562 nm. BSA (1 µg/µl) was used as standard to calculate protein concentrations. For each condition, five replicates were used for protein estimation.
Glycogen estimation
For glycogen quantification, 40 4 flies/replicate were homogenized in 400 µl of 2% Na2SO4; 20 µl of homogenate was aliquoted and mixed with 46 µl of 2% Na2SO4 and 934 µl of chloroform/methanol (1:1). The tubes were spun at 13,500 rpm for 10 min to obtain glycogen containing pellets which was air dried for 10 min. Then, 500 µl of 0.2% (w/v) anthrone reagent (0.2% anthrone in 72% sulphuric acid) (Sigma Aldrich) was added and the mixture was heated at 90 °C for 20 min with mixing every 5 min. Samples were then cooled on ice for 10 min and returned to room temperature for 20 min; 100 µl of the supernatant was aliquoted and absorbance was recorded at 620 nm. D-glucose was used as standard to calculate carbohydrate concentration. Five replicates per condition were used for glycogen estimation.
Trehalose estimation
To measure trehalose content, 40 4 flies/replicate were homogenized in 500 µl of 70% ethanol and tubes were spun at 5000 rpm at 4 °C. The pellets were resuspended in 200 µl of 2% NaOH, heated at 100 °C for 10 min, and cooled on ice; 100 µl of mixture was then aliquoted and 750 µl of anthrone reagent was added. The samples were heated at 90 °C for 20 min with mixing every 5 min, cooled on ice for 10 min, and returned to room temperature for 20 min; 100 µl of the supernatant was aliquoted and absorbance was recorded at 620 nm. Trehalose concentration was calculated using D-glucose standard. For each condition, five replicates were used for the assay.
Lipid estimation
For each condition, groups of 10 freshly emerged flies were collected, etherized and weighed immediately in micrograms using Citizen CM11 microbalance to obtain fresh weight. The samples were dried afterwards in a preheated oven at 70 °C for 36 h and weighed again to obtain dry weight. The difference between fresh weight and dry weight represented water content of the flies. Subsequently, ether soluble lipids were extracted from the samples by adding 1 ml of diethyl ether to intact dry flies stored in 1.5 ml microcentrifuge tubes. The tubes were kept on a gel rocker and lipid extraction was carried out for 48 h with three ether changes at an interval of 12 h each. After the last ether change, flies were dried for 2 h at 30–35°C and weighed again to obtain lipid free weight of flies. Lipid content of the flies was calculated by subtracting lipid free weight from dry weight.41,42 Five replicates per condition were used and lipid content was assessed at 0, 3, 5, 7, 9, 11, and 13 days of age.
Lipid droplet staining
For lipid droplet staining in adult adipose tissue, age-matched flies were collected, etherized, and their abdomen was dissected in ice-cold 1× PBS. The abdomens were fixed in 4% formaldehyde in PBT for 20 min at room temperature. After fixation, sheets of fat body were detached from the dorsal abdominal area, fixed for additional 10 min, and washed thrice for 10 min each with 1× PBS. The tissue was stained with 1:2000 dilution of 0.5 mg/ml Nile Red (Sigma Aldrich, N3013) in PBS for 30 min. After staining, the tissue was rinsed twice with 1× PBS and mounted in Vectashield mounting medium (Vector Labs, H-1000). Images were acquired using Nikon Eclipse (Ni-E) fluorescence microscope and lipid droplets quantification was performed using NIS-Elements AR software. Minimum five samples per condition were analyzed for lipid droplets quantification.
ROS detection and imaging
Dihydroethidium (DHE) staining was used to monitor superoxide radicals in the adult adipose tissue of flies from different experimental conditions. 43 Age-matched flies were etherized and adult fat body was dissected from the dorsal surface of abdomen. To allow optimal respiration, tissue was dissected in 1× Schneider’s Drosophila medium (+) L-glutamine (GIBCO, 21720024) and any extraneous tissue was removed. A stock solution of 30 mM DHE (Invitrogen Molecular Probes, D11347) was reconstituted just before use in anhydrous DMSO (Sigma-Aldrich, 276855). For staining, the reconstituted dye was further diluted in 1× Schneider’s medium to obtain a final working concentration of 50 µM and vortexed to disperse the dye evenly. The tissue was incubated with the dye for 4 min in dark soon after dissection at room temperature. The samples were rinsed twice in 1× Schneider’s medium and images were acquired immediately using Nikon Eclipse (Ni-E) fluorescence microscope. ROS quantification was performed using NIS-Elements AR software. Minimum seven samples per condition were analyzed for ROS quantification.
Quantitative real time PCR expression profiling
For transcriptional analysis, flies from different experimental conditions were collected, snap frozen, and stored at –80°C until processing. Total RNA from these flies was isolated using TRIZOL® Reagent (Thermo Fisher Scientific, 15596–026) following standard protocol of RNA extraction from Drosophila. 44 After RNA extraction, 1 µg of the total RNA was used for cDNA synthesis with Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622). Thereafter, qRT-PCR reaction was performed on CFX Connect Real-Time System (Bio-Rad Laboratories) using SYBR-Green based detection system with QuantiNova SYBR green PCR Kit (Qiagen, 208052). Relative expression of mRNA was calculated through ΔΔCt method using the ribosomal protein RP49 RNA expression as internal control for each sample. Primers used in the qRT-PCR analysis were designed online using Primer3 software and evaluated on NCBI-Blast. Minimum six replicates per condition with 6 flies/replicate were used for the assay.
Statistical analysis
Statistical analysis was performed in IBM SPSS statistical package (version 22). The results are represented as mean ± S.E.M in all the graphs. Normality and homoscedasticity of the data were assessed using Shapiro-Wilk and Levene’s test, respectively. Data were analyzed using Mann-Whitney U test and multi-factor analysis of variance (ANOVA) followed by Tukey HSD post-hoc test for multiple comparisons and Test of Simple Effects for pairwise comparisons. Survival data were analyzed using Kaplan-Meier test.
Results
Curcumin supplementation slightly improves the median survival but not maximum life span in HD flies
In the present study, a dosage effect of curcumin, i.e. 0, 10, 15, and 20 µM was assessed on the locomotor performance as well as survival of HD Drosophila (10 µM dose was used for all other assays). A cohort of female flies expressing unexpanded (Httex1p Q20) and expanded glutamine repeats (Httex1p Q93) was collected and reared on food supplemented without and with different concentration of curcumin. Initially, their vertical locomotor performance was assessed at day 1, 3, 7, and 9 post-eclosion. Significant amelioration in behavioral dysfunction with curcumin supplementation in 7-day-old HD flies has previously been reported 26 ; however, in this study, we chose an additional time-point, i.e. day 9 to check whether curcumin shows beneficial effect at later stage when both disease symptoms and metabolic dysregulation become more distressing. We found that among different tested concentrations, 10 µM curcumin-fed diseased flies display significant improvement in their motor activity at day 7 and 9 (Tukey HSDα0.05, day 7, n = 20; P = 0.000073; day 9, n = 20; P = 0.023, Supplemental Tables 1 and 2) as compared to the age-matched diseased flies reared on normal food (Figure S1).
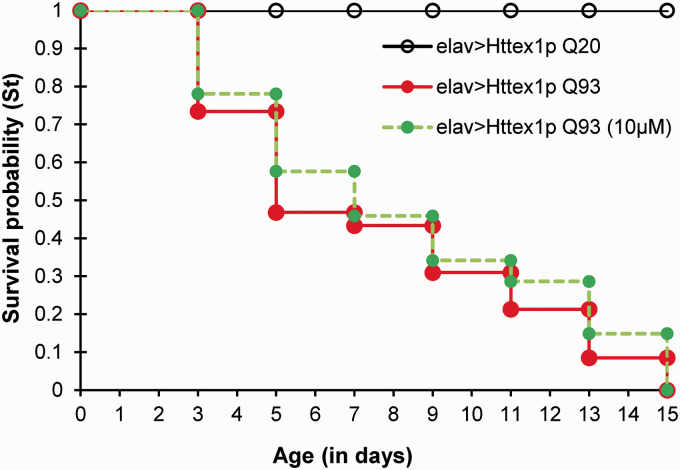
We further determined survivorship of diseased flies in response to 10 µM curcumin treatment. Lifespan and survival are multifaceted processes which can be influenced by several factors such as metabolic state, diet, physical activity, underlying disease, etc. After survival analysis, we found that although the survival curve of unfed (Httex1p Q93) and curcumin-fed diseased flies remain comparable to each other [log-rank test, Httex1p Q93 vs Httex1p Q93 (10 µM curcumin), P = 0.3049], the median survival of diseased flies receiving 10 µM dose of curcumin improved from ∼5 days (unfed) to ∼7 days (Supplemental Tables 3 and 4). Also, ∼75% death time of these flies was found to be increased, i.e. ∼13 days as compared to that of 11 days in unfed flies. Therefore, 10 µM dose of curcumin proved effective in slightly extending the median survival of diseased flies as compared to those receiving normal food although their maximum life span remained the same. The control flies (Httex1p Q20) exhibited no signs of mortality up to 15 days, suggesting that the transgene with unexpanded glutamine had no notable toxic effect (Figure 1). The higher doses of 15 µM and 20 µM were ineffective in improving locomotor disability and survival of the HD flies. Altogether, these results suggest that 10 µM dose of curcumin could extend the median survival of the diseased flies and improve their locomotor dysfunction.
Figure 1.
Curcumin slightly extends median survival in HD flies. Survival probability of flies with unexpanded (elav>Httex1p Q20) and expanded glutamine repeats (elav>Httex1pQ93) fed without and with 10 µM concentration of curcumin from larval stages. Slight but notable improvement was observed in the median survival time of 10 µM curcumin-fed diseased flies, as compared to the unfed controls. Median survival (50%) is ∼5 days for elav>Httex1pQ93 which increased upto ∼7 days for 10 µM curcumin-fed diseased flies. Interestingly, 75% death time for diseased condition is just ∼11 days whereas it extends upto ∼13 days for 10 µM curcumin-fed diseased flies. Survival data was analyzed using Kaplan-Meier method followed by log-rank test [elav>Httex1p Q93 vs. elav>Httex1p Q93 (10 µM), P = 0.3049]. For each condition, n = 100 (20 flies/replicate, 5 replicates/condition). (A color version of this figure is available in the online journal.)
Curcumin manages altered body weight, dry mass, and water content in HD flies
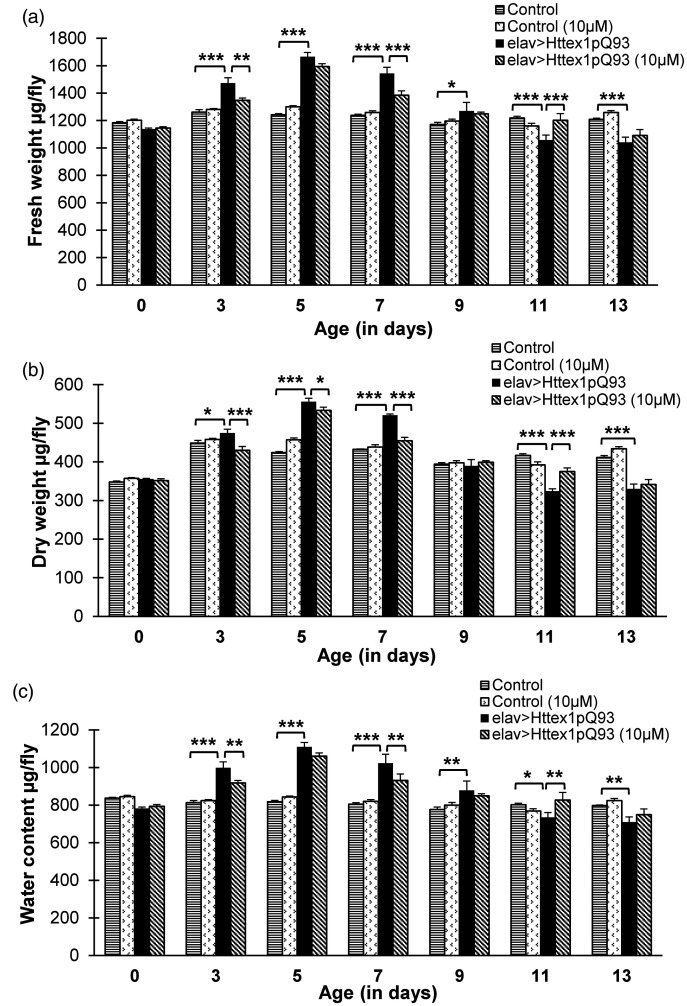
Previously, we have reported extensive metabolic alterations in HD flies as a consequence of neuronal expression of mutant Htt. 36 Regulation of metabolism can possibly muffle HD symptoms, particularly at advanced stages of the disease. HD flies exhibit significant weight modulation during entire course of the disease with excessive increase in weight during disease onset followed by significant reduction at terminal stage. 36 In an attempt to test the effect of curcumin in body weight management, newly emerged control and diseased flies were reared on food supplemented without and with 10 µM dose of curcumin from early larval stage till 13 days post-eclosion. Quantification of fresh body weight showed that 10 µM dose of curcumin significantly attenuates body weight alterations observed in HD flies not fed with curcumin with disease progression. Diseased flies fed with 10 µM curcumin exhibited a significant decline in their abnormally high body weight at day 3 (Tukey HSDα0.05, n = 50, P = 0.001) and 7 (Tukey HSDα0.05, n = 50, P = 0.000041) followed by significant improvement in deteriorating body weight at day 11 (Tukey HSDα0.05, n = 50, P = 0.000209) as compared to the age-matched diseased flies (Supplemental Table 5 and 6). Interestingly, we observed that body weight of diseased flies reared on 10 µM curcumin became comparable to age-matched flies with unexpanded glutamines at day 3, 7, and 11. Flies with unexpanded glutamines did not display any variation in their body weight upon receiving the same dose of curcumin for 13 days (Figure 2(a)). Body weight of an organism is majorly constituted by water, protein, carbohydrates, and lipids; hence, further understanding of the relative proportions of these molecules in control and curcumin-fed HD flies will help gain a deeper insight.
Figure 2.
Dietary curcumin modulates body weight, dry mass, and water content. Curcumin administration significantly regulates abnormally high and low body weight (a) at day 3, 7, and 11 (b) dry mass at day 3, 5, and 7 in diseased flies (c) dysregulated water content is managed at day 3, 7, and 13 in diseased flies upon curcumin feeding. Data was analyzed using multi-factor ANOVA followed by Tukey HSD post hoc test and Test of Simple Effects. Values are represented as mean ± S.E.M. Tukey HSDα0.05, *** P < 0.001; ** P < 0.01; * P < 0.05. For each condition, n = 50 (10 flies/replicate, 5 replicates/condition).
We assessed the impact of curcumin on dry weight and water content of HD flies. Dry weight assessment revealed that administration of 10 µM dose of curcumin significantly amend abnormally high dry weight in disease flies at day 3 (Tukey HSDα0.05, n = 50, P = 0.000032), 5 (Tukey HSDα0.05, n = 50, P = 0.030), and 7 (Tukey HSDα0.05, n = 50, P = 2.6012E-9), followed by significant improvement in severely low dry mass at day 11 (Tukey HSDα0.05, n = 50, P = 3.00E-6), as compared to the untreated diseased flies (Supplemental Tables 7 and 8). Previously, we had reported significant alterations in dry weight of HD flies through the entire course of disease. 36 Flies with unexpanded glutamines receiving 10 µM curcumin however exhibited modulation in dry weight at day 5 (Tukey HSDα0.05, n = 50, P = 0.002), 11 (Tukey HSDα0.05, n = 50, P = 0.018), and 13 (Tukey HSDα0.05, n = 50, P = 0.028) as compared to the age-matched Q20 flies (Figure 2(b)).
Evaluation of water content revealed that diseased flies exhibited an altered pattern of water level from day 3 to 13 (Tukey HSDα0.05, n = 50; day 3, P = 1.634E-8; day 5, P = 3.567E-16; day 7, P = 1.223E-10, day 9, P = 0.001182; day 11, P = 0.03236; day 13, P = 0.00494), when compared to age-matched unexpanded flies, as reported previously. 36 Interestingly, when fed with 10 µM dose of curcumin, diseased flies exhibited a significant reduction in their elevated water levels at day 3 (Tukey HSDα0.05, n = 50; P = 0.009255) and 7 (Tukey HSDα0.05, n = 50; P = 0.0033913), followed by a significant improvement at day 11 (Tukey HSDα0.05, n = 50; P = 0.0033980) (Supplemental Tables 9 and 10) the period when their water content declines below normal levels (Figure 2(c)). No detectable effect of curcumin was observed on the water content of Q20 flies from day 0 till day 13. Modulation of dry weight and water content of HD flies by curcumin suggests that it is perhaps micromanaging the metabolic and/or catabolic processes in diseased condition.
Dietary curcumin has no effect on food intake in diseased flies
Feeding is an inherent characteristic of an organism with profound effect on whole body composition, energy stores, metabolic activity, and life span. Food intake quantification becomes critical while assessing behavior, nutrition, and drug administration.
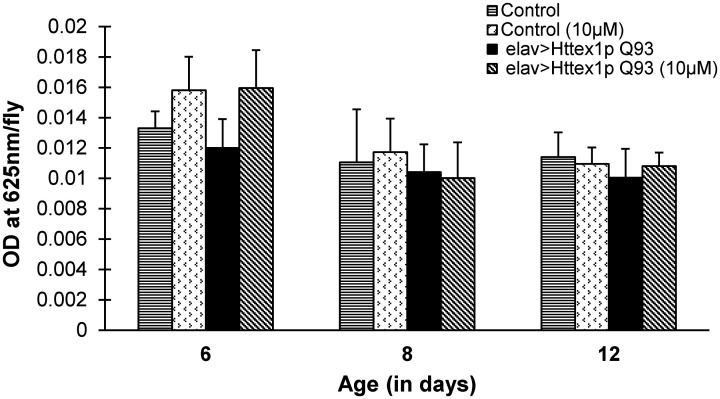
In order to ascertain the probable cause of weight modulation, we quantified food intake in normal and diseased condition, at both larval and adult stages, reared on diet without and with 10 µM curcumin. The synchronized feeding stage third instar larvae and adult flies, grown on standard or curcumin-supplemented medium were fed with blue dye. As shown previously, 26 we found that control (elav>Q20) and diseased (elav>Q93) larvae fed with 10 µM dose of curcumin show feeding behavior comparable to those fed diet without curcumin (Figure S2, two-way ANOVA, n = 20, F1, 4 = 0.080, P = 0.791, Supplemental Table 11). Similarly, at adult stage too, there was no difference in the food intake of 6, 8, and 12 day old Q20 and diseased flies due to curcumin supplementation (Figure 3, multi-factor ANOVA, n = 20, F2, 36 = 0.110, P = 0.896, Supplemental Tables 12 and 13). These results clearly suggest that curcumin modulates body weight in diseased flies either by amendment of systemic metabolism or cellular metabolic components instead of directly altering the feeding response. Therefore, we proceeded to ascertain which of the macromolecules, if any, curcumin modulates by quantifying proteins, glycogen, trehalose, and lipid levels in these flies.
Figure 3.
Feeding behavior of HD flies remain unchanged by curcumin supplementation in the food. Feeding of control (elav>Httex1p Q20) and diseased (elav>Httex1p Q93) female adult flies reared on control or 10 µM curcumin supplemented diet was measured using colorimetric dye intake assay. No difference was observed in food intake of 6-, 8-, and 12-day-old control and diseased females supplemented without or with effective concentration of curcumin. Data was analyzed using multi-factor ANOVA, F2, 36 = 0.110, P = 0.896. Values are represented as mean ± S.E.M and for each group, n = 20 flies (10 flies/replicate, 2 replicates/condition).
Curcumin superintends macromolecules involved in metabolism
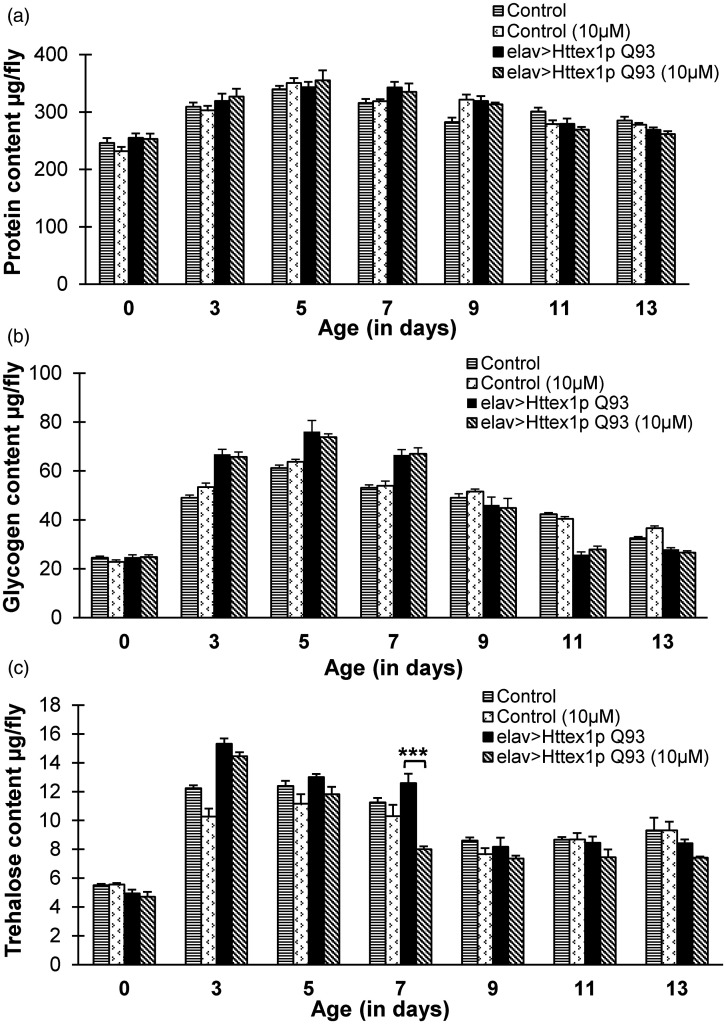
In an attempt to elucidate a detailed account of curcumin action on components involved in metabolism, we investigated the effect of curcumin feeding on total protein, glycogen, and circulating disaccharide trehalose levels in both control and diseased condition without or with curcumin supplementation. Surprisingly, we did not find any effect of 10 µM curcumin on the levels of total protein (Figure 4(a)) (Supplemental Tables 14 and 15) and glycogen content (Figure 4(b), Supplemental Tables 16 and 17) in Q20 or diseased flies. However, administration of 10 µM dose of curcumin resulted in significant (Tukey HSDα0.05, n = 20, P = 5.00E-6) decrease in the trehalose level in diseased flies at day 7. Further, no notable effect of curcumin on the trehalose levels of age-matched flies with unexpanded glutamine, except decrease at day 3 (Tukey HSDα0.05, n = 20, P = 0.034) (Figure 4(c)) (Supplemental Tables 18 and 19) was found. These findings further suggested that curcumin intake has no substantial effect on the protein or stored carbohydrate in unexpanded or HD flies; however, there is a noticeable effect of phytochemical on the circulating sugar trehalose levels at specific ages in both control and diseased flies.
Figure 4.
Curcumin regulates circulating sugar trehalose in diseased flies. There is no notable effect of curcumin on the levels of (a) protein and (b) glycogen in HD flies. On the contrary, (c) significant reduction in otherwise increased levels of circulating sugar trehalose is observed in curcumin-fed diseased flies at day 7. Data was analyzed using multi-factor ANOVA followed by Tukey HSD post hoc test and Test of Simple Effects. Values are represented as mean ± S.E.M. Tukey HSDα0.05, *** P < 0.001; ** P < 0.01; * P < 0.05. For each condition, n = 20 (4 flies/replicate, 5 replicates/condition) for protein, glycogen, and trehalose content quantification.
Curcumin attunes total lipid and subcellular lipid content in HD flies
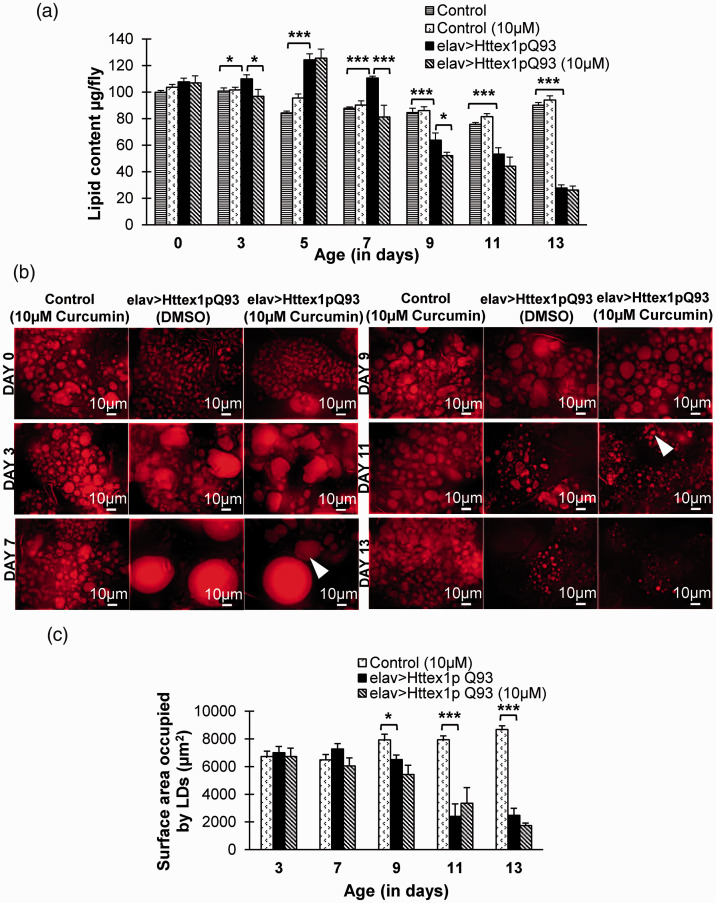
To unravel the effect of curcumin on major energy reserves, we quantified total lipid content in Q20 and diseased flies reared on diet without and with 10 µM curcumin up to 13 days. Lipids constitute a dominant fraction in organism’s body weight; therefore, any modulation in body weight indicates probable regulation in lipid metabolism. We have reported earlier that there is significant perturbation in lipid level in diseased flies through the entire course of disease which becomes initially high and then low (Tukey HSDα0.05, n = 50; day 5, P = 3.942E-11; day 7, P = 0.000060; day 9, P = 0.00020; day 11, P = 0.000080; and day 13, P = 0.0000) as compared to age-matched control flies with unexpanded glutamines. 36 Interestingly, in the present study with 10 µM curcumin supplementation, significant attenuation in dysregulated total lipid content of diseased flies was observed at day 3 (Tukey HSDα0.05, n = 50, P = 0.01682) and upon progression at day 7 (Tukey HSDα0.05, n = 50, P = 4.411E-7). However, decline in lipid levels at advanced disease stage, i.e. day 9 (Tukey HSDα0.05, n = 50, P = 0.03493) could not be reinstated by curcumin. We found no notable difference in lipid content by supplementation of curcumin in 0- to 13-day old Q20 flies with exception at day 5 (Tukey HSDα0.05, n = 50, P = 0.0404; Figure 5(a), Supplemental Tables 20 and 21). These results suggest that the administered dose of curcumin modulates altered lipid level in diseased flies at different ages which might be linked to their improved metabolic profile. However, at terminal disease stage as marked by day 11 or 13, the insidious disease progression seems to outweigh the phytochemical’s beneficial effects.
Figure 5.
Curcumin modulates total lipid content. (a) Dietary curcumin significantly decreases abnormally high lipid levels in diseased flies at day 3 and 7 which becomes comparable to those of age-matched flies with unexpanded glutamines. However, the lipid levels further decline significantly at day 9 in curcumin-fed diseased flies. (b) At sub-cellular level, curcumin intake improves distribution of intracellular lipid in lipid droplets (LDs) in abdominal adipose tissue of diseased flies at day 7 and 11. Arrowheads indicate presence of smaller LDs at day 7 and moderately improved LD distribution at day 11. Scale bar represents 10 µm. (c) Quantification of total surface area occupied by LDs suggests a considerable reduction in bigger LDs at day 7 and subsequent improvement in distribution at day 11 by curcumin intake. Lipid content data was analyzed using multi-factor ANOVA followed by Tukey HSD post hoc test and Test of Simple Effects and LD quantification were analyzed using two-way ANOVA. Values are represented as mean ± S.E.M. Tukey HSDα0.05, *** P < 0.001; ** P < 0.01; * P < 0.05. For each condition, n = 50 (10 flies/replicate, 5 replicates/condition) for total lipid content and n = 5 for LD quantification. (A color version of this figure is available in the online journal.)
Besides estimating total lipid content, to ascertain the probable effect of curcumin on subcellular lipids, we monitored distribution of lipid droplets (LDs), the storehouse of intracellular lipids which enrich Drosophila adipocytes. LDs are highly dynamic organelles with hydrophobic core comprising of neutral triacylglycerols (TAGs). We found that there was a noticeable regulation in LDs distribution in disease condition after supplementation of 10 µM curcumin. Large LDs present in adipose tissue in 7-day-old HD flies exhibited marked reduction in their size with 10 µM curcumin administration. In addition, at day 11, LD distribution improved upon curcumin supplementation as compared to untreated flies with expanded glutamines (Figure 5(b) and (c), Supplemental Table 22). However, 10 µM dose of curcumin had no observable effect on the abundance of LDs in adipose tissue of flies with unexpanded glutamines. Taken together, these results suggest that 10 µM dose of curcumin exhibits beneficial effects on the altered intracellular lipid abundance that ultimately reflects in the modulation of total lipid content and may have a beneficial impact on the maintenance of overall metabolic homeostasis in HD flies.
Curcumin suppresses oxidative stress in diseased condition
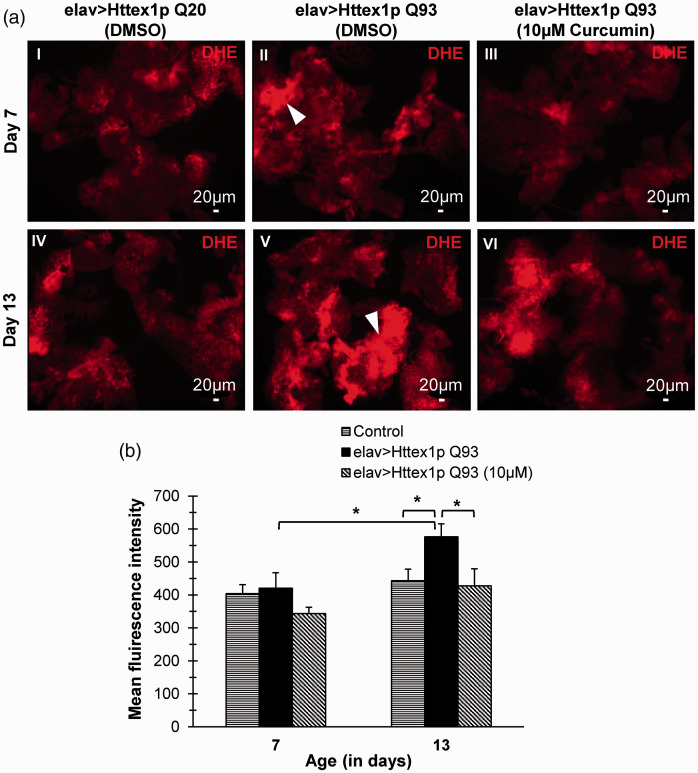
Curcumin acts as an excellent antioxidant and metabolic regulator, and by our results it is evident that curcumin effectively improves the overall metabolic condition and maintains energy balance, likely by impacting vital organs. Drosophila fat body is a key metabolic organ which performs multiple functions such as nutrient storage and mobilization; thus, we investigated the effect of curcumin on the fat body in control and HD flies and monitored their ROS levels.
Basal ROS level was detected in the adipose tissue of 7- and 13-day-old flies with unexpanded glutamines whereas ROS in abdominal fat body of 7 and 13 (Tukey HSDα0.05, n = 7, P = 0.024) day old diseased flies was considerably higher as compared to age-matched control flies. Further, diseased flies displayed significantly high ROS at day 13 (Tukey HSDα0.05, n = 7, P = 0.010) in comparison to 7-day-old counterparts which clearly indicated that ROS levels increase with disease progression. Interestingly, significant decline in the elevated ROS levels in adult adipose tissue of diseased flies reared on 10 µM curcumin was found at day 13 (Tukey HSDα0.05, n = 7, P = 0.021) as compared to those reared without curcumin (Figure 6(a) and (b), Supplemental Table 23).
Figure 6.
Curcumin reduces elevated ROS levels in adipose tissue of diseased flies. (a, I-VI) Evaluation of ROS production in abdominal fat body of 7- and 13-day-old control females (elav>Httex1p Q20; panels I, IV), diseased (elav>Httex1p Q93; panels II, V), and diseased flies supplemented with 10 µM curcumin (panel III, VI). Adult fat body from 7- and 13-day-old diseased condition displayed increased levels of ROS as compared to age-matched control flies. Interestingly, curcumin intake in diseased condition suppressed elevated levels of ROS in diseased condition. Arrowheads show areas in adult fat body producing higher ROS levels than neighboring cells. Red = dihydroethidium (DHE), scale bars represent 20 µm. (b) Quantification of mean fluorescence intensity of DHE staining in adult fat body of 7- and 13-day-old control, diseased and curcumin-fed flies revealed significant increase in ROS intensity in day 13 diseased condition as compared to day 7 diseased or age-matched control flies. Furthermore, there was a significant effect of curcumin treatment on ROS intensity levels (F1, 24 = 8.782, P = 0.007). Curcumin feeding results in significant decrease in elevated ROS levels in day 13 old diseased flies which becomes comparable to those of age-matched control flies. Data was analyzed using two-way ANOVA followed by Tukey HSD post hoc test. Values are represented as mean ± S.E.M. Tukey HSDα0.05, **P < 0.01. For each condition, n = 7 female flies. (A color version of this figure is available in the online journal.)
Collectively, these results indicated that high ROS levels detected in the adipose tissue in 7- and 13-day-old diseased flies might underlie the altered cellular functions that scale up to alter tissue function and in turn contribute to the persistent disruptions in systemic metabolic state as evident in HD flies. Administration of 10 µM curcumin lowers the free radical levels in adipose tissue at advanced stage of the disease thereby providing protection against increased oxidative insult. This may ultimately result in the fine tuning of major biomolecules and amelioration of disease symptoms in HD flies.
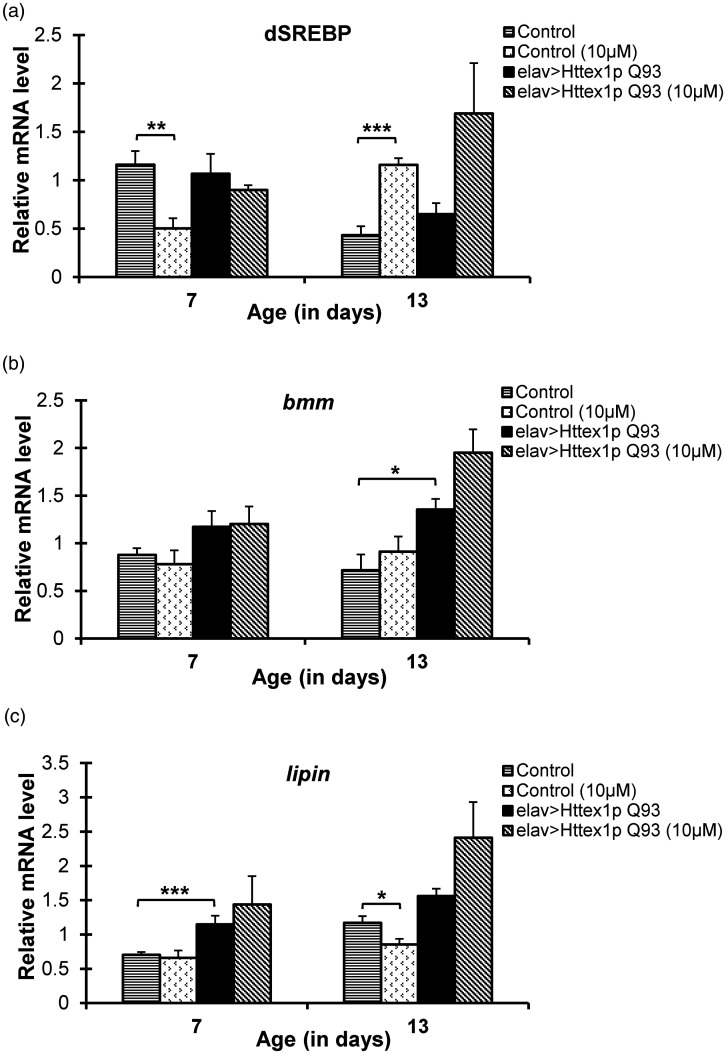
dSREBP, bmm, and lipin levels in curcumin-fed HD flies remain unaltered
Diseased flies showcased high ROS in adult adipose tissue which might deter the optimal organ functioning. Generally, ROS production and lipid metabolism pathways remain intricately entwined, and an abnormal lipid level may lead to increased oxidative stress and vice versa, ultimately disrupting the overall cellular performance. Therefore, improved ROS and lipid levels point towards a probable regulation of key metabolic regulators in curcumin-fed HD flies. dSREBP (HLH106), bmm, and lipin are few key effector genes which regulate lipogenesis, lipolysis, energy metabolism, and inflammation in Drosophila.
To decipher the basis of curcumin action in diseased condition, we monitored the expression of these genes in Q20 and diseased flies reared without and with curcumin. There was no change in the mRNA level of dSREBP, bmm, and lipin genes in diseased flies at day 7 and 13 with or without 10 µM curcumin administration (Figure 7). The variation in lipid or ROS levels did not correlate with the expression level of dSREBP, bmm, or lipin genes. In Q20 flies, however, we observed modulation in dSREBP expression with curcumin supplementation (Figure 7(a)). Significant decrease at day 7 (Student’s t-test, n = 36, P = 0.00454) followed by significant upregulation at day 13 (Student’s t-test, n = 36, P = 0.0195) in dSREBP mRNA levels was seen in curcumin-fed Q20 flies, but they did not correlate to their corresponding lipid levels at day 7 or 13.
Figure 7.
dSREBP, bmm, and lipin expression remain unmodulated in HD flies upon curcumin supplementation. (a) dSREBP or HLH106 mRNA levels in 7- and 13-day-old control (elav>Httex1p Q20) and diseased (elav>Httex1p Q93) flies reared on control or 10 µM curcumin supplemented diet was monitored using quantitative RT-PCR. Control flies exhibited significant decrease in dSREBP mRNA levels at day 7 followed by significant increase at day 13, whereas diseased flies did not display any change in the expression of dSREBP gene at both the ages without or with 10 µM curcumin supplementation. (b) bmm mRNA levels remain unchanged in 7- and 13-day-old Q20 and diseased flies reared without and with 10 µM curcumin. (c) No change in lipin mRNA levels was seen in 7- or 13-day-old diseased flies reared on 10 µM curcumin diet. Q20 flies showed significant decrease in lipin mRNA at day 13 upon curcumin feeding. Data was analyzed using Mann-Whitney U test. Values are represented as mean ± S.E.M. *** P < 0.001; ** P < 0.01. Sample size: 6 flies/replicate, 6 replicates/condition.
No change in bmm mRNA was observed either in Q20 or diseased flies, except at day 13 when diseased flies exhibited higher bmm mRNA levels in comparison to age-matched control flies (Figure 7(b)). However, no effect of curcumin on bmm expression was seen. Similarly, HD flies fed with curcumin did not show any modulation in their lipin expression at any age. Conversely, untreated diseased flies exhibited significantly high lipin mRNA levels at day 7 as compared to control flies. Q20 flies also showed significant decline in lipin mRNA levels at day 13 with curcumin supplementation (Figure 7(c), Supplemental Table 24). These results clearly indicated that curcumin did not modulate the action of these three genes in HD.
Discussion
Turmeric has a long history of traditional use in Asian population as food additive, herbal remedy, and medicine.15,18 Asian population also have the lowest prevalence of HD at 0.40/100,000, as compared to a high prevalence of 2.71/100,000 worldwide. 45 The toxicological profile of curcumin deems it extremely safe and it can be used a regular part of diet and medicine. 46 Several in vitro and in vivo studies have confirmed the activity of curcumin in biological system by either detecting curcumin or its bio-transformed metabolites in plasma, peripheral organs, and brain.23,47–49
In HD, extensive neuronal degeneration in major brain areas induce coordinated set of behavioral abnormalities in human patients as well as flies. Systemic metabolic derangements inflict additional burden on the health of already challenged individuals and gradually disables them. Although various strategies have been devised to slow down the disease progression, there are no effective treatment till now. Curcumin’s efficacy against HD has been tested against various parameters in different models and has highlighted mitigation of cell death, 26 clearance of aggregates, 27 transcriptional regulation, 28 and reduction of oxidative stress 50 as possible protective mechanisms. Interestingly, curcumin with multiple cellular targets, minimum side effects, and broad range of pharmacological activities can offer several advantages as a therapeutic choice over synthetic drugs with comparatively high toxicity and major side-effects.
Previously, the metabolic abnormalities seen in HD has been reiterated in in vivo transgenic Drosophila model expressing mutant human Htt. 36 Recently, the molecular basis of such metabolic derangements was explored which included transcriptional dysregulation of bmm and lipin, fatbody specific apoptosis, mitochondrial dysfunction, and calcium dysregulation. 38 In the present study, an effective concentration of 10 µM curcumin administered to diseased flies since larval stages is shown to attenuate their motor dysfunction,51,52 survival and metabolic abnormalities. We reinstated the effect of curcumin on locomotor functions in HD flies at an advanced stage (day 9) not reported earlier (Figure S1). 26 This could be explained by the protection of motor neurons from mutant Htt-induced damage owing to the strong anti-oxidant property of curcumin. Curcumin feeding also slightly improved the median survival in diseased flies which is again an indicator of enhanced protection from characteristic mutant Htt neurotoxicity and inflammation. Interestingly, curcumin feeding could mitigate the abnormal effects of weight gain (at day 3, 7) and subsequent loss (at day 11) in the disease condition and therefore could finely manage the body weight changes throughout the disease progression. However, once the disease becomes chronic, curcumin cannot mitigate them. It is possible that curcumin can recuperate the extent of neurodegenerative damages only up to a certain threshold of the disease. Curcumin targets or receptors might undergo progressive or deteriorative changes later and may become unavailable for further action. 53
Food intake quantification revealed that curcumin-fed HD larvae or flies did not display any prominent change in their feeding pattern which clearly indicated that weight modulation in these flies was not due to mere food intake but through modulation of metabolic process by curcumin. Amendment in dysregulated water, trehalose, and lipid levels further corroborated the same. Water balance in Drosophila is usually regulated by neurosecretory or similar cell types, and any impairment in their functioning can lead to disproportionate water levels. Curcumin efficiently regulated the fluctuating water levels in HD flies and this might be attributed to better functioning of these cells in response to curcumin treatment. Additionally, curcumin effectively countered hyperglycemia in 7-day-old HD flies. This effect could either be due to increased uptake of trehalose by cells or their controlled release into the circulation by source organs, apparently under the impact of curcumin. In contrast to these, no detectable effect of curcumin on other altered energy components, e.g. protein or glycogen in HD flies was seen. The limited effect of curcumin on such critical energy reserves might explain the acute energy crisis prevalent at terminal stage when all the energy sources are urgently required for long-term sustenance and functional outcomes.
Curcumin exhibited prominent systemic and intracellular hypolipidemic effect in HD flies upon disease progression. The hypolipidemic effect might be due to the interaction of curcumin with important regulators of lipid metabolism. Several evidences point towards such beneficial effect of curcumin in attenuation of altered body weight, fat gain, or glucose levels by regulation of multiple lipid metabolic genes.32,54 Based on these reports, we investigated the effect of curcumin on three key lipid regulators dSREBP, bmm, and lipin in diseased flies; however surprisingly, no effect of curcumin on the expression of these genes in HD flies was seen. These findings indicate towards other critical players in lipid metabolism such as protein levels, activities, post-translational modifications, downstream effectors, target genes, etc. a study of which will help elaborate the molecular action of curcumin in HD.
Chronic inflammatory conditions are often associated with neurodegenerative disorders and they can trigger numerous metabolic defects in the affected individuals. To elucidate the mode of action of curcumin in HD, its anti-inflammatory effect in HD flies was investigated. We noted an aggravated inflammatory state in HD flies, as denoted by the elevated ROS levels in adipose tissue, and 10 µM curcumin effectively amended the abnormally high superoxide levels at both initial (day 7) and advanced (day 13) disease stages. Hence, the potent free-radical scavenging and anti-inflammatory property of curcumin proved immensely beneficial in attenuation of inflammatory and oxidative insult in HD thereby ameliorating the disease state. It can be safely said that curcumin acts at the neuronal as well as peripheral levels to manage the overall disease pathology; however, the dosage and form of curcumin used would play a significant role in establishing clinical relevance.
Conclusions
These findings in Drosophila model of HD show that curcumin is beneficial in suppression of neurodegeneration with amelioration of metabolic dysregulation. This study adds to many other studies advocating the management of different pathological manifestations of the HD by curcumin. Therefore, with further consideration, curcumin may prove to be a safe and suitable treatment regimen for management of HD.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211046927 for Management of altered metabolic activity in Drosophila model of Huntington’s disease by curcumin by Kumari Aditi, Akanksha Singh, Mallikarjun N Shakarad and Namita Agrawal in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We thank Prof. J. Lawrence Marsh, University of California Irvine, CA, USA for providing transgenic Drosophila lines UAS-Httex1p Q20, UAS-Httex1p Q93, and elav-GAL4 driver. We are thankful to Dr. Ekta Kohli, Defense Institute of Physiology and Allied Sciences (DIPAS), Defense Research and Development Organisation (DRDO), Delhi, India for providing RT-PCR facility.
AUTHORS’ CONTRIBUTIONS: KA, AS, MNS, and NA conceptualized the idea, designed the research, interpreted and analyzed the results. KA and AS performed the experiments and carried out data collection. KA and AS wrote the manuscript. All authors read, critically reviewed and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: KA and AS acknowledge University Grants Commission (UGC) and Council of Scientific & Industrial Research (CSIR), respectively, for providing research fellowship.
SUPPLEMENTAL MATERIAL: Supplemental material for this article is available online.
ORCID iD: Kumari Aditi https://orcid.org/0000-0002-9733-4992
References
- 1.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosome. Cell 1993; 72:971–83 [DOI] [PubMed] [Google Scholar]
- 2.Sanberg PR, Fibiger HC, Mark RF. Body weight and dietary factors in Huntington’s disease patients compared with matched controls. Med J Aust 1981; 1:407–9 [DOI] [PubMed] [Google Scholar]
- 3.Gines S, Seong IS, Fossale E, Ivanova E, Trettel F, Gusella JF, Wheeler VC, Persichetti F, MacDonald ME. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum Mol Genet 2003; 12:497–508 [DOI] [PubMed] [Google Scholar]
- 4.Björkqvist M, Petersén A, Bacos K, Isaacs J, Norlén P, Gil J, Popovic N, Sundler F, Bates GP, Tabrizi SJ, Brundin P, Mulder H. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum Mol Genet 2006; 15:1713–21 [DOI] [PubMed] [Google Scholar]
- 5.Popovic V, Svetel M, Djurovic M, Petrovic S, Doknic M, Pekic S, Miljic D, Milic N, Glodic J, Dieguez C, Casanueva FF, Kostic V. Circulating and cerebrospinal fluid ghrelin and leptin: potential role in altered body weight in Huntington’s disease. Eur J Endocrinol 2004; 151:451–5 [DOI] [PubMed] [Google Scholar]
- 6.Chaves G, Özel RE, Rao NV, Hadiprodjo H, Da Costa Y, Tokuno Z, Pourmand N. Metabolic and transcriptomic analysis of Huntington’s disease model reveal changes in intracellular glucose levels and related genes. Heliyon 2017; 3:e00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS One 2007; 2:e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Underwood BR, Broadhurst D, Dunn WB, Ellis DI, Michell AW, Vacher C, Mosedale DE, Kell DB, Barker RA, Grainger DJ, Rubinsztein DC. Huntington disease patients and transgenic mice have similar pro-catabolic serum metabolite profiles. Brain 2006; 129:877–86 [DOI] [PubMed] [Google Scholar]
- 9.Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, Strand A, Tarditi A, Woodman B, Racchi M, Mariotti C, Di Donato S, Corsini A, Bates G, Pruss R, Olson JM, Sipione S, Tartari M, Cattaneo E. Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J Neurosci 2005; 25:9932–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi RK, Adhihetty P, Shukla S, Hennessy T, Calingasan N, Yang L, Starkov A, Kiaei M, Cannella M, Sassone J, Ciammola A, Squitieri F, Beal MF. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet 2009; 18:3048–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreassen OA, Dedeoglu A, Stanojevic V, Hughes DB, Browne SE, Leech CA, Ferrante RJ, Habener JF, Beal MF, Thomas MK. Huntington’s disease of the endocrine pancreas: insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol Dis 2002; 11:410–24 [DOI] [PubMed] [Google Scholar]
- 12.Fain JN, Del Mar NA, Meade CA, Reiner A, Goldowitz D. Abnormalities in the functioning of adipocytes from R6/2 mice that are transgenic for the Huntington’s disease mutation. Hum Mol Genet 2001; 10:145–52 [DOI] [PubMed] [Google Scholar]
- 13.Mihm MJ, Amann DM, Schanbacher BL, Altschuld RA, Bauer JA, Hoyt KR. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis 2007; 25:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroy A, Lithgow GJ, Alavez S. Curcumin and neurodegenerative diseases. Biofactors 2013; 39:122–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 2008; 65:1631–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsey NA, Wilkins HM, Linseman DA. Neutraceutical antioxidants as novel neuroprotective agents. Molecules 2010; 15:7792–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009; 30:85–94 [DOI] [PubMed] [Google Scholar]
- 18.Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med 1991; 57:1–7 [DOI] [PubMed] [Google Scholar]
- 19.Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ. Van der Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs 2012; 21:1123–40 [DOI] [PubMed] [Google Scholar]
- 20.Dixit VP, Jain P, Joshi SC. Hypolipidaemic effects of curcuma longa L and Nardostachys jatamansi, DC in triton-induced hyperlipidaemic rats. Indian J Physiol Pharmacol 1988; 32:299–304 [PubMed] [Google Scholar]
- 21.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr 1970; 100:1307–15 [DOI] [PubMed] [Google Scholar]
- 22.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging 2001; 22:993–1005 [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 2005; 280:5892–901 [DOI] [PubMed] [Google Scholar]
- 24.Deshpande P, Gogia N, Singh A. Exploring the efficacy of natural products in alleviating Alzheimer's disease. Neural Regen Res 2019; 14:1321–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddique YH, Naz F, Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson's disease. Biomed Res Int 2014; 2014:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anjalika C, Agrawal N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci Rep 2016; 6:18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elifani F, Amico E, Pepe G, Capocci L, Castaldo S, Rosa P, Montano E, Pollice A, Madonna M, Filosa S, Calogero A, Maglione V, Crispi S, Di Pardo A. Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington’s disease. Hum Mol Genet 2019; 28:4012–21 [DOI] [PubMed] [Google Scholar]
- 28.Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, Maiti P, Frautschy SA, Zeitlin S, Levine MS, Chesselet MF. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol Neurodegener 2012; 7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001; 21:8370–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M, Sasi P, Gupta VH, Rai G, Amarapurkar DN, Wangikar PP. Protective effect of curcumin, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro model. Hum Exp Toxicol 2012; 31:788–97 [DOI] [PubMed] [Google Scholar]
- 31.Su L, Wang Y, Chi H. Effect of curcumin on glucose and lipid metabolism, FFAs and TNF-α in serum of type 2 diabetes mellitus rat models. Saudi J Biol Sci 2017; 24:1776–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding L, Li J, Song B, Xiao X, Zhang B, Qi M, Huang W, Yang L, Wang Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol Appl Pharmacol 2016; 304:99–109 [DOI] [PubMed] [Google Scholar]
- 33.Weisberg S, Leibel R, Tortoriello DV. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr Diabetes 2016; 6:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh JL, Pallos J, Thompson LM. Fly models of Huntington’s disease. Hum Mol Genet 2003; 12:R187–R193 [DOI] [PubMed] [Google Scholar]
- 35.Marsh JL, Thompson LM. Can flies help humans treat neurodegenerative diseases? Bioessays 2004; 26:485–96 [DOI] [PubMed] [Google Scholar]
- 36.Aditi K, Shakarad MN, Agrawal N. Altered lipid metabolism in Drosophila model of Huntington’s disease. Sci Rep 2016; 6:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A, Irvine KD. Drosophila as a model for understanding development and disease. Dev Dyn 2012; 241:1–2 [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Agrawal N. Deciphering the key mechanisms leading to alteration of lipid metabolism in Drosophila model of Huntington's disease. Biochim Biophys Acta Mol Basis Dis 2021; 1867:166127. [DOI] [PubMed] [Google Scholar]
- 39.Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001; 413:739–43 [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Gopalacharyulu P, Seppänen-Laakso T, Ruskeepää AL, Aye CC, Carson BP, Mora S, Oresic M, Teleman AA. Insulin signaling regulates fatty acid catabolism at the level of CoA activation. PLoS Genet 2012; 8:e1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrashekara KT, Popli S, Shakarad MN. Curcumin enhances parental reproductive lifespan and progeny viability in Drosophila melanogaster. Age (Dordr) 2014; 36:9702–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handa J, Chandrashekara KT, Kashyap K, Sageena G, Shakarad MN. Gender based disruptive selection maintains body size polymorphism in Drosophila melanogaster. J Biosci 2014; 39:609–20 [DOI] [PubMed] [Google Scholar]
- 43.Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of reactive oxygen species. Protoc Exch 2008; 10:1–8 [Google Scholar]
- 44.Green MR, Sambrook J. Total RNA isolation from Drosophila melanogaster. Cold Spring Harb Protoc 2020; 2020:101675. [DOI] [PubMed] [Google Scholar]
- 45.Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and Meta-analysis. Mov Disord 2012; 27:1083–91 [DOI] [PubMed] [Google Scholar]
- 46.Ganiger S, Malleshappa HN, Krishnappa H, Rajashekhar G, Rao VR, Sullivan F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem Toxicol 2007; 45:64–9 [DOI] [PubMed] [Google Scholar]
- 47.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 2008; 326:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res 2001; 61:1058–64 [PubMed] [Google Scholar]
- 49.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 1999; 27:486–94 [PubMed] [Google Scholar]
- 50.Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington's disease. Neuromolecular Med 2014; 16:106–18 [DOI] [PubMed] [Google Scholar]
- 51.Marsh JL, Walker H, Theisen H, Zhu YZ, Fielder T, Purcell J, Thompson LM. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet 2000; 9:13–25 [DOI] [PubMed] [Google Scholar]
- 52.Marsh JL, Thompson LM. Drosophila in the study of neurodegenerative disease. Neuron 2006; 52:169–78 [DOI] [PubMed] [Google Scholar]
- 53.Phom L, Achumi B, Alone DP, Muralidhara, Yenisetti SC. Curcumin’s neuroprotective efficacy in Drosophila model of idiopathic Parkinson’s disease is phase specific: Implication of its therapeutic effectiveness. Rejuvenation Res 2014; 17:481–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, Lu H, Fantus IG, Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 2012; 7:e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211046927 for Management of altered metabolic activity in Drosophila model of Huntington’s disease by curcumin by Kumari Aditi, Akanksha Singh, Mallikarjun N Shakarad and Namita Agrawal in Experimental Biology and Medicine