Abstract
Sugar-sweetened beverages (SSBs) are a major source of added sugars in the diet. A robust body of evidence has linked habitual intake of SSBs with weight gain and a higher risk (compared with infrequent SSB consumption) of type 2 diabetes mellitus, cardiovascular diseases and some cancers, which makes these beverages a clear target for policy and regulatory actions. This Review provides an update on the evidence linking SSBs to obesity, cardiometabolic outcomes and related cancers, as well as methods to grade the strength of nutritional research. We discuss potential biological mechanisms by which constituent sugars can contribute to these outcomes. We also consider global trends in intake, alternative beverages (including artificially-sweetened beverages) and policy strategies targeting SSBs that have been implemented in different settings. Strong evidence from cohort studies on clinical outcomes and clinical trials assessing cardiometabolic risk factors supports an aetiological role of SSBs in relation to weight gain and cardiometabolic diseases. Many populations show high levels of SSB consumption and in low-income and middle-income countries, increased consumption patterns are associated with urbanization and economic growth. As such, more intensified policy efforts are needed to reduce intake of SSBs and the global burden of obesity and chronic diseases.
Subject terms: Obesity, Risk factors
Evidence from cohort studies and clinical trials supports an aetiological role of sugar-sweetened beverage (SSB) intake in the development of obesity and related chronic diseases. This Review provides an up-to-date view, considering the evidence, potential mechanisms and policy actions to reduce the global intake of SSBs.
Key points
Sugar-sweetened beverages (SSBs) are consumed on a global scale, with intake levels above the recommended daily limits for free sugar in many high-income countries and on the rise in low-income and middle-income countries.
Prospective cohort studies of clinical outcomes and clinical trials assessing intermediate risk factors provide strong evidence for an aetiological relationship between SSBs and weight gain and the risk of related chronic diseases.
SSBs promote weight gain through adding additional liquid calories to the diet, from hyperinsulinaemia induced by the rapid absorption of glucose, and possibly from activation of the dopaminergic reward system.
SSBs contribute to chronic disease risk through weight gain, through development of risk factors precipitated by adverse glycaemic effects and through hepatic metabolism of excess fructose from sugars in SSBs.
Several policy and regulatory strategies exist across different levels of governance that can be adopted concurrently to change social norms and limit intake of SSBs among individuals and populations.
Given the consistency of the evidence across different populations and high intake levels globally, reducing intake of SSBs is one important step to improving overall diet quality and cardiometabolic health.
Introduction
The worldwide prevalence of overweight and obesity has nearly tripled over the past four decades, and represents one of the most serious unmet public health challenges of the 21st century. Pooled estimates from population-based studies from across the globe show that the prevalence of obesity increased between 1975 and 2016, from <1% to 6–8% among children, from 3% to >11% among men and from 6% to 15% among women1. Over 2.1 billion people, or nearly 30% of the global population, have overweight or obesity, giving rise to substantial health, social and economic costs2. Excess weight is the leading risk factor for type 2 diabetes mellitus (T2DM) and can also lead to a number of related chronic conditions, including coronary heart disease (CHD), stroke and many cancers. The global economic effect of obesity is estimated to be ~2 trillion US dollars, or 2.8% of global gross domestic product, which is roughly equivalent to the global economic effect of smoking or armed conflict3.
Obesity is a complex condition that results from various physiological, environmental, behavioural and sociopolitical factors, which all contribute to a positive energy balance. Maintaining a healthy weight is in large part a function of modifiable lifestyle choices4 that are shaped by the broader food environment, including availability, price and marketing5. Among these factors, sugar-sweetened beverages (SSBs) have emerged as an important risk factor, with a robust body of evidence linking SSBs to weight gain and risk of T2DM, cardiovascular disease (CVD) and certain cancers6. Collectively, SSBs are the largest source of added sugar in the diet; a typical 12 fl oz (355 ml) serving of soda delivers 35.0–37.5 g of sugar and 140–150 calories (Box 1). Numerous health authorities have called for reductions in SSB consumption. In addition, a number of public policies have been implemented to limit SSB intake in order to improve health and curtail escalating health-care costs.
In this Review, we provide an overview of the role of SSBs in the obesity epidemic. We consider global trends in intake, epidemiological evidence linking SSBs to obesity, T2DM, CVD and related cancers, and the strength of the evidence. We also highlight biological mechanisms, alternative beverage options and directions for policy.
Box 1 Composition and definitions of SSBs.
Currently there is no universal consensus on how sugar-sweetened beverages (SSBs) are defined. The most widely accepted definition used in research is to consider any beverage as an SSB if it contains caloric sweeteners such as sucrose, high-fructose corn syrup (HFCS) or fruit juice concentrates among others, which are added to the beverages by manufacturers, establishments or individuals. However, some authorities have developed more specific definitions based on sugar content per volume, which have been used for regulatory initiatives. For example, the New York City Board of Health defines SSBs as having ≥25 calories or 6.25 g of added sugar per 8 fl oz (~237 ml), whereas in the UK the definition for taxation is ≥5 g of added sugar per 100 ml. Unsweetened fruit juice is not considered an SSB, as the sugars in these beverages are naturally occurring and are not added. However, juice contains free sugars, defined as added sugars plus sugars that are naturally present in honey, syrup and fruit juices. The prevailing sugars used to flavour SSBs include HFCS (42–55% fructose and the remainder glucose) in the USA and sucrose (50% fructose and 50% glucose) in Europe.
SSB global intake trends
According to national survey data from the USA, intake of SSBs has declined modestly since the early 2000s7, but consumption levels remain high. Between 2011 and 2014, US adults were estimated to consume 145 kcal per day from SSBs, which corresponds to 6.5% of daily calories8. This intake level alone nearly meets the daily recommendation of no more than 10% of total calories coming from added sugar9 or free sugar (added sugars plus sugars that are naturally present in honey, syrup and fruit juices)10 suggested by multiple health authorities including the Dietary Guidelines for Americans and WHO. In the US population, higher intakes have been reported among youth and young adults, among non-Hispanic Black individuals and among Hispanic men and women, compared with other demographics8. These intake trends track with disparities in obesity and chronic disease prevalence. Similar trends of a decline or plateauing of SSB intake have been observed in other high-income countries. By contrast, intake of SSBs is increasing in many low-income and middle-income countries (LMICs), as widespread urbanization and economic development have increased the availability of these beverages5.
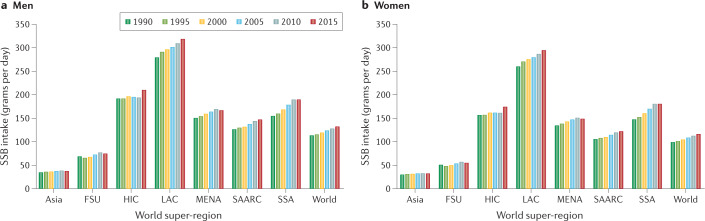
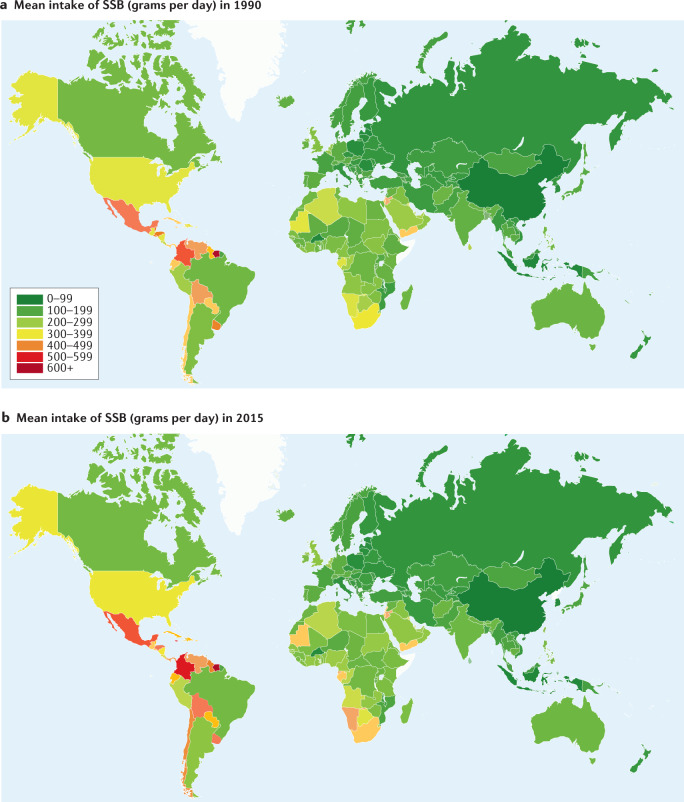
A survey of SSB consumption in adults in 187 countries found that intake was higher in middle-income countries compared with either high-income or low-income countries11. Of the seven super-regions (groupings of world regions) evaluated, SSB consumption was highest in Latin America and the Caribbean, with a steady rise in intake from 1990 to 2015, and intake was lowest in Asia, with generally higher intakes observed among men than among women in all global regions (Fig. 1). In comparing global intakes of SSBs in individual countries in 1990 and 2015, marked increases are observed in countries in South and Central America, and parts of southern and north Africa (Fig. 2).
Fig. 1. Global trends in sugar-sweetened beverage intake by sex.
Mean intake of sugar-sweetened beverages (SSBs) (grams of SSB per day) from 1990 to 2015 in seven world super-regions, that is, grouping of world regions that exhibit similar cause-of-death patterns. These are: Asia, comprising east and Southeast Asia, former Soviet Union countries (FSU), western high-income countries (HIC), Latin America and Caribbean countries (LAC), Middle East and north Africa (MENA), south Asia (SAARC), and sub-Saharan Africa (SSA). Data are shown separately for men (part a) and women (part b). For reference, 237 g of SSB is equivalent to 8 fl oz (237 ml) of SSB and 355 g of SSB is equivalent to 12 fl oz (355 ml) of SSB. Data are obtained from: Global Dietary Database.
Fig. 2. Global intake of sugar-sweetened beverages in 1990 and 2015.
Mean intake of sugar-sweetened beverages (SSBs) (grams of SSB per day) in 1990 (part a) and 2015 (part b). The colour of individual countries corresponds to SSB consumption level, with lower intakes shown in green (0–200 g per day) and higher intakes shown in red (500–600 g per day). Countries with no data are shown in white. Data are for men and women combined. For reference, 237 grams of SSB is equivalent to 8 fl oz (237 ml) of SSB and 355 grams of SSB is equivalent to 12 fl oz (355 ml) of SSB. Data are obtained from: Global Dietary Database.
Intake trends among youth have generally paralleled those in adults. Survey data from adolescents in 53 LMICs showed that SSB intake was highest in Central and South America, and lowest in Southeast Asia12. Of note, 54% of adolescents consumed SSBs at least once per day12. Daily intake of SSBs was shown to decrease between 2002 and 2018 among adolescents in eastern Europe, with larger declines observed in more affluent groups13. These downward trends are consistent with observations in western Europe14,15, the USA16 and Canada17.
SSBs and cardiometabolic disease
Obesity
An analysis of historical data from the USA illustrates parallel trends between the rise in consumption of added sugar (largely from SSBs) and epidemics of obesity and T2DM18. Obesity prevalence has not declined despite the decline in SSB intake since the early 2000s; however, this observation does not refute the association between SSBs and weight gain. Weight change is a gradual process and major reversals in obesity prevalence would not yet be expected at the population level. Moreover, since obesity and T2DM are complex conditions, causation cannot be inferred from time-trend data alone. Rather, we consider evidence from prospective cohort studies and randomized clinical trials (RCTs), which are able to establish temporality between SSB intake and weight change, or development of obesity and related sequelae.
A number of meta-analyses have synthesized the evidence on SSBs and weight gain and obesity. The majority have shown positive associations19–21, although a meta-analysis of studies among children found a null association22. However, this meta-analysis included estimates that were adjusted for total energy intake, which attenuated the overall association23. SSBs add calories to the diet and adjusting for total energy intake is equivalent to assessing SSB-induced effects on body weight that are independent of total energy intake. Our previous meta-analysis of cohort studies found that each serving per day increment in SSB consumption was associated with a weight gain of 0.12 kg (0.26 lb) in 1 year among adults and an increase in BMI of 0.05 kg/m2 in 1 year among children21. We included estimates that were not adjusted for total energy intake, which might explain some of the differences observed between studies. We also included studies that evaluated the change in intake of SSBs in relation to weight change. These studies are able to simulate quasi-experimental studies that are generalizable to a real-world setting. Although the results of our meta-analysis seem modest, weight gain is a gradual process, with an average weight gain in adults of about 0.45 kg (1 lb) per year24. Therefore, limiting SSB consumption could be an effective way to prevent age-related weight gain. Limiting SSB intake among children is an important strategy for them to develop healthy weight trajectories25.
Findings from cohort studies are supported by our previous analysis of gene–SSB interactions. Using data from three large cohorts, we found that people who consumed one or more SSB servings per day had a stronger genetic risk of having an elevated BMI and obesity; this risk was twice that in people who consumed less than one serving per month26. This finding suggests that individuals with a genetic predisposition to obesity compared with those without might be more susceptible to adverse associations between SSB consumption and weight gain. Further support for the link between SSBs and weight gain has been provided by studies at different points in the life-course. For example, findings from birth cohorts have shown positive associations between perinatal SSB intake and postpartum weight retention among mothers and offspring adiposity27,28.
The majority of RCTs assessing the effects of SSB intake have only evaluated short-term effects on weight change, rather than long-term patterns, owing to the logistical challenges and difficulties in maintaining participant adherence to assigned beverage regimens over time. In our previous meta-analysis of five trials among adults, we found that adding SSBs to the diet in hypercaloric trials statistically significantly increased body weight (weighted mean difference 0.85 kg, 95% CI 0.50–1.20 kg)21. Similarly, another meta-analysis of seven RCTs found a statistically significant dose-dependent increase in body weight when SSBs were added to participant diets (standardized mean difference 0.28 kg, 95% CI 0.12–0.44 kg)29. These studies demonstrate that additional calories from SSB intake are not spontaneously compensated for by a reduction in other sources of calories at subsequent meals, which realistically simulates what happens in human populations.
The evidence from hypercaloric trials has been limited to adults. By contrast, studies that reduce SSB consumption (hypocaloric trials) have been conducted among children and adults. Our meta-analysis of hypocaloric trials in children showed a reduction in BMI gain over time when SSB intake was reduced21. Of note, more pronounced benefits were observed among children with overweight and obesity compared with lean children21. Similar results were observed in a meta-analysis of hypocaloric trials in adults and children that showed an overall benefit of reducing SSB intake on weight that was also more pronounced among participants with overweight and obesity30. These findings are consistent with a 2020 trial that found that replacing SSBs with non-caloric beverages reduced body weight among adults with central adiposity31. These meta-analyses of hypocaloric trials included many studies with limitations, including small sample size, short duration, lack of blinding and poor adherence. The majority were also effectiveness trials of behaviour modification, which test methods of intervention rather than causal relations. As such, a lack of benefit does not preclude causality but rather that the specific intervention modality might not have been effective in changing behaviours.
Two of the most rigorous RCTs to date32,33, conducted in children and adolescents, have overcome many of the limitations of previous trials. These trials provided strong evidence for a benefit of replacing SSBs with non-caloric options on weight gain. Another meta-analysis of trials in adults found that under isoenergetic conditions where SSBs were replaced with other carbohydrates (isocaloric trials), no changes in body weight were observed. This finding suggests that SSBs contribute to weight change through changes in calories20.
Type 2 diabetes mellitus
A substantial body of literature has demonstrated that intake of SSBs is associated with a higher risk of T2DM, both through body weight gain and independently through other metabolic pathways. Experimental evidence from RCTs is lacking owing to the high cost of running such trials and other feasibility constraints; however, findings from prospective cohort studies have shown consistent associations. For example, a meta-analysis of 17 prospective cohort studies found that an increase in SSB intake of one serving per day was associated with an 18% higher risk of T2DM (95% CI 9–28%) when estimates that did not adjust for BMI were used in the analysis34. When estimates that did adjust for BMI were included, the association was attenuated to 13% (95% CI 6–21%), which suggests that BMI partially mediates the association (Table 1). Positive associations were also observed between intakes of fruit juice and artificially-sweetened beverages (ASBs) and T2DM risk, although associations were not as strong as those observed for SSBs34. This study also estimated that 8.7% (95% CI 3.9–12.9%) of T2DM cases in the USA and 3.6% (95% CI 1.7–5.6%) of T2DM cases in the UK predicted to occur over 10 years were attributable to SSBs, which suggests that high levels of consumption of SSBs over many years in a population could be related to a substantial number of new occurrences of T2DM34. These findings, along with the findings of previous meta-analyses35,36 and more recent studies published over the past 5 years in the Mexican Teacher’s Cohort37, the Northern Manhattan Study38 and the Women’s Health Initiative39, provide strong evidence that intake of SSBs is associated with increased risk of T2DM in different populations and in a dose–response manner.
Table 1.
Association between sugar-sweetened beverage intake and risk of T2DMa
| Cohort | Sample size (n) | Weightb (%) | Relative risk (95% CI) | Weightb (%) | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Unadjusted for adiposity | Adjusted for adiposity | ||||
| EPIC-InterAct | 11,684 | 9.9 | 1.21 (1.12–1.31) | 8.8 | 1.13 (1.05–1.22) |
| ARIC | 1,437 | 10.5 | 1.01 (0.96–1.06) | 9.4 | 1.01 (0.96–1.06) |
| Black WHS | 2,550 | 10.5 | 1.10 (1.05–1.16) | 8.3 | 1.06 (0.97–1.16) |
| NHS II | 5,121 | 10.4 | 1.17 (1.11–1.24) | 9.0 | 1.11 (1.04–1.19) |
| KIHD | 506 | 9.1 | 1.06 (0.95–1.18) | 7.8 | 1.05 (0.95–1.17) |
| NHS I | 7,300 | 10.2 | 1.39 (1.30–1.48) | 8.8 | 1.23 (1.14–1.32) |
| CARDIA | 174 | NA | NA | 7.9 | 1.03 (0.93–1.14) |
| HPFS | 3,229 | 9.5 | 1.31 (1.20–1.44) | 8.0 | 1.22 (1.10–1.35) |
| FOS | 303 | 6.0 | 1.12 (0.90–1.40) | 4.7 | 1.12 (0.90–1.39) |
| Iowa WHS | 999 | NA | NA | 6.2 | 1.49 (1.27–1.75) |
| MESA | 413 | NA | NA | 3.0 | 0.86 (0.63–1.18) |
| Occupation cohort, Japan | 170 | 6.3 | 1.08 (0.88–1.33) | 4.9 | 1.07 (0.87–1.32) |
| SCHS | 2,250 | 4.4 | 2.22 (1.64–3.00) | 3.2 | 1.95 (1.44–2.65) |
| HIPOP-OHP | 212 | 7.3 | 0.89 (0.75–1.06) | 5.8 | 0.89 (0.75–1.06) |
| E3N | 1,054 | 0.5 | 2.82 (0.87–9.17) | 0.3 | 2.70 (0.82–8.82) |
| JPHC | 676 | 5.6 | 1.25 (0.99–1.58) | 3.8 | 1.15 (0.88–1.50) |
| FMCHES | 175 | NA | NA | 0.1 | 15.0d |
| Overall figures | 38,253 | 100 | 1.18 (1.09–1.28); I2 = 89.0c | 100 | 1.13 (1.06–1.21); I2 = 79.8c |
ARIC, Atherosclerosis Risk in Communities study; CARDIA, Coronary Artery Risk Development in Young Adults study; EPIC, European Prospective Investigation into Cancer and Nutrition study; FMCHES, Finnish Mobile Clinic Health Examination Survey; FOS, Framingham Offspring Study; HIPOP-OHP, High-risk and Population Strategy for Occupational Health Promotion study; HPFS, Health Professional Follow-up Study; JPHC, Japan Public Health Center-based prospective study; MESA, Multi-Ethnic Study of Atherosclerosis; NA, not available; NHS, Nurses’ Health Study; SCHS, Singapore Chinese Health Study; T2DM, type 2 diabetes mellitus; WHS, Women’s Health Study. aProspective associations for an incremental increase in consumption of sugar-sweetened beverages (per one serving per day) with incident T2DM, unadjusted and adjusted for adiposity from 17 prospective cohort studies. Data are from a random effects meta-analysis34. bWeight (%) denotes the percentage weight that each study contributed to the overall estimate. cThe I2 value denotes the percentage of between-study heterogeneity in the overall estimate. dEstimates with 95% confidence intervals greater than 10 are not presented. Table 1 is adapted from ref.34, CC BY 4.0 (https://creativecommons.org/licences/by/4.0/).
Cardiovascular disease
Consistent with the literature on weight gain and T2DM, accumulating evidence has also linked intake of SSBs to risk of CVD. For example, a meta-analysis of seven prospective cohort studies found that SSB consumption was associated with a 9% higher risk of CVD when comparing extreme categories of intake (none or less than one per month versus one or more per day) (relative risk (RR) 1.09, 95% CI 1.01–1.18)40. The association was linear, with each one-serving per day increase in SSB intake associated with an 8% higher risk of CVD (RR 1.08, 95% CI 1.02–1.14). In stratified analysis, each serving per day increment was associated with a 15% higher risk of CHD (RR 1.15, 95% CI 1.09–1.22), whereas no significant association was observed with stroke (RR 1.05, 95% CI 0.95–1.16)40. These results are consistent with those of a previous meta-analysis that also found a null association with stroke41. However, subgroup analyses in that study showed a significant positive association between SSB intake and ischaemic stroke among women (RR 1.33, 95% CI 1.07–1.66), whereas no associations were noted for men or for men or women with haemorrhagic stroke41. Similar to studies in T2DM, when estimates were adjusted for BMI, the association between SSB and CHD was somewhat attenuated, suggesting adiposity as a partial mediator42.
Evidence from cohort studies also supports a link between SSB intake and risk of the metabolic syndrome35,43,44, a precursor for cardiometabolic diseases. A review of observational studies and trials among children found consistent evidence that cardiometabolic risk increases as intake of SSBs increases, with strong evidence noted for risk of increased adiposity and dyslipidaemia45.
Findings from short-term RCTs in adults exploring the effects of SSBs or their constituent sugars on intermediate risk factors for T2DM and CHD provide mechanistic support for the associations observed in epidemiological studies. For example, a meta-analysis of 39 RCTS with at least 2 weeks of intervention found that high versus low consumption of SSBs and/or sugar significantly raised serum concentrations of triglycerides (mean difference (MD) 0.11 mmol/l, 95% CI 0.07–0.15 mmol/l), total cholesterol (MD 0.16 mmol/l, 95% CI 0.10–0.24 mmol/l) and LDL cholesterol (MD 0.12 mmol/l, 95% CI 0.05–0.19 mmol/l)46. Intake of SSBs and/or sugar was also shown to statistically significantly increase blood pressure of participants in studies that were at least 8 weeks in duration (systolic blood pressure, MD 6.9 mm Hg, 95% CI 3.4–10.3 mmol/l; diastolic blood pressure, 5.6 mm Hg, 95% CI 2.5–8.8 mmol/l)46, consistent with cohort studies that have found positive associations between intake of SSBs and hypertension47. In a 2-week parallel-arm trial in which participants consumed beverages containing 10%, 17.5% or 25% of energy requirements from high-fructose corn syrup (HFCS) statistically significantly increased serum concentrations of postprandial triglycerides, LDL-cholesterol and uric acid in a linear dose–response manner48. In a 10-week trial conducted among participants with overweight, a sucrose-rich diet statistically significantly increased postprandial glycaemia, insulinaemia and serum concentrations of lipids compared with a diet rich in artificial sweeteners49. A 3-week crossover trial among healthy-weight men found that consumption of moderate quantities of SSBs resulted in impaired glucose and lipid metabolism, and inflammation50. In other studies, the results on inflammatory markers have been inconsistent, possibly due to differences in study quality and duration51,52. Of note, a 2020 RCT by Ebbeling and colleagues found that replacing SSBs with ASBs or water for 12 months did not affect the triglyceride to HDL-cholesterol ratio or other cardiometabolic risk markers31. The authors posited that the null findings might have been due to compensatory changes in other dietary sources of carbohydrate, which are typically controlled for along with other measures of diet quality in cohort studies.
Non-alcoholic fatty liver disease
Accumulating evidence has linked intake of SSBs with development of non-alcoholic fatty liver disease (NAFLD), owing to metabolism of constituent sugars, particularly fructose moieties from sucrose or HFCS53. Meta-analyses of a limited number of epidemiological studies support a positive association between intake of SSBs and development of NAFLD in children and adults. Based on 12 studies (one cohort, two case–control and nine cross-sectional), Chen and colleagues found that each one serving per day increase in SSB intake was associated with a 39% higher risk of NAFLD (95% CI 29–50%)54. Similar findings in previous meta-analyses and qualitative reviews have been reported55,56. However, interpretation of these meta-analyses is complicated by the low quality of the included studies.
Meta-analyses of RCTs of fructose, sucrose and HFCS intake have found that among hypercaloric trials, providing excess energy of these sugars in healthy adults can raise intrahepatocellular lipids and serum concentrations of alanine transaminase, a biomarker of liver function57. However, in isocaloric trials, isocaloric exchange of fructose-containing sugars for other carbohydrates has been found not to induce NAFLD-associated changes, suggesting that the adverse effect of SSBs on the liver might be more attributable to excess calories than fructose57. Interestingly, isocaloric studies in animals have shown that fructose-fed rats develop features of the metabolic syndrome despite no differences in weight gain between groups and that hepatic steatosis can be induced with calorie restriction in the context of a high-sugar diet53. Larger, longer and higher-quality observational studies and RCTs on the relationship between SSBs and histopathological changes in NAFLD are required to address key research gaps.
SSBs and gout
Regular consumption of SSBs and fructose-containing sugars has also been associated in epidemiological studies with hyperuricaemia and gout. For example, a meta-analysis of three prospective cohort studies found a twofold higher risk of gout comparing the highest with the lowest intake of SSBs (RR 2.08, 95% CI 1.40–3.08)58. A dose–response association was also observed, with each serving per week increase in intake of SSBs associated with a 4% higher risk of gout (RR 1.04, 95% CI 1.02–1.07). In this study, a positive yet weaker association was observed between intake of fruit juice and gout, whereas no association was observed for intake of whole fruit58. These findings are consistent with cross-sectional studies linking SSB intake to hyperuricaemia59 and with RCTs that have demonstrated increases in serum concentrations of uric acid with intake of SSB or fructose60.
SSBs and cancer
Intake of SSBs might increase the risk of certain cancers through excess adiposity and cardiometabolic perturbations. Obesity, insulin resistance and T2DM are established risk factors for different cancers61. However, the epidemiological evidence for an association between SSB intake and the risk of cancer is limited and conflicting, which has precluded international health authorities from drawing firm conclusions62. This inconsistency is also reflected in meta-analyses and reviews. The most up-to-date meta-analysis, including 27 prospective cohort and case–control studies, found a positive association between SSB intake and breast cancer (RR 1.14, 95% CI 1.01–1.30) and prostate cancer (RR 1.18, 95% CI 1.10–1.27) and also between fruit juice intake and prostate cancer (RR: 1.03, 95% CI: 1.01–1.05), when comparing extreme categories of intake (as defined in the individual studies)63. A subgroup analysis also found a stronger association between SSB intake and the risk of premenopausal breast cancer, compared with the risk of postmenopausal breast cancer. A tendency also existed for positive associations between SSB intake and the risks of colorectal cancer and pancreatic cancer63. Associations were not observed between SSB intake and bladder or renal cell cancers.
Among the studies included in this meta-analysis63, the NutriNet-Santé study in a large French cohort found a positive association between SSB intake and overall cancer risk and breast cancer risk, with a stronger association with the risk of premenopausal breast cancer compared with postmenopausal breast cancer64. No associations were observed with prostate cancer or colorectal cancer risk, possibly owing to the limited number of cases as noted by the authors. In the Melbourne Collaborative Cohort Study, statistically significant positive associations were observed between SSB intake and postmenopausal breast cancer and colorectal cancer65. A positive association between SSB intake and postmenopausal breast cancer was also observed in the Seguimiento Universidad de Navarra (SUN) cohort in Spain66. In contrast, no associations were observed between sugary beverage (SSB or fruit juice) consumption and combined and site-specific (breast, prostate, colorectal) cancers associated with excess adiposity in the Framingham Offspring Cohort67. However, in a subgroup analysis, a positive association was observed between SSB consumption and cancer risk among participants with central adiposity67. A meta-analysis of food groups and risk of colorectal cancer found no association with SSBs68, which is consistent with an analysis in the California Teacher’s Study69. The evidence regarding SSBs and pancreatic cancer is mixed, with some studies finding positive associations overall70 or in subgroups71 and others finding no associations72.
Although heterogeneity exists across these studies due to differences in population characteristics, study design and analysis strategies, the majority were adjusted for BMI. This fact suggests that pathways other than body weight gain might be implicated in the associations. Given the diverse aetiologies of site-specific cancers, additional research is warranted that explores intake of SSB by cancer type and in different ethnic groups, in which associations between SSB and cancer might vary61,63.
Mortality
Consistent with the literature on clinical outcomes, evidence has shown a link between SSB intake and mortality. Our previous analysis in the Nurses’ Health Study (NHS) and Health Professional Follow-Up study (HPFS) found a positive dose–response association between SSBs and mortality that was largely driven by CVD mortality, with stronger associations observed among women than among men73. Consuming two or more SSBs per day was associated with a 31% higher risk of death from CVD (RR 1.31, 95% CI 1.15–1.50) than consuming none or less than one SSB per month73. A 2021 meta-analysis of six cohort studies found an 8% higher risk of CVD mortality per one serving per day increment in SSB (RR 1.08, 95% CI 1.04–1.13)40. In 2019, the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, found that each additional serving per day of SSBs was associated with an 11% higher risk of all-cause mortality (RR 1.11, 95% CI 1.03–1.19)74. However, no association was observed for CHD mortality, possibly owing to the limited number of cases. Positive associations between SSB intake and mortality have also been found in the UK Biobank75 and the Western New York Exposures and Breast Cancer (WEB) Study76. In a 2020 study in Mexico, an estimated 6.9% (95% CI 5.4–8.5%) of adult deaths were attributable to SSBs, representing 40,842 excess deaths per year77. Similarly, based on NHANES data for 2012, an estimated 7.4% of all cardiometabolic-related deaths in the US were attributable to intake of SSBs78.
Causality and strength of the evidence
The majority of evidence considered in this review was obtained from meta-analyses and systematic reviews, which provide overall summaries of the evidence. Meta-analyses of trials and prospective cohort studies are increasingly used to inform dietary recommendations, public policies and clinical practice guidelines, underscoring the importance of quality and transparency in evidence synthesis. Best practices for meta-analysis require that authors conduct individual assessments of study quality, followed by an overall grading of the strength of the meta-evidence. The methods used to grade the evidence are critical, as findings are often used to guide recommendations or next steps in policy action. Multiple grading tools, which differ in how they rate evidence (particularly with respect to observational studies) are in common use (Box 2). This variation has led to some confusion regarding the strength of the evidence linking SSBs to weight gain and cardiometabolic outcomes. Given the constraints of RCTs in nutritional epidemiology, and the need for well-designed cohort studies of hard end points that are not feasible in trials (such as incident T2DM), evidence rating tools that support a variety of combinations of study designs will be the most informative for evidence grading in nutrition research.
Box 2 Evidence grading tools in nutrition research.
A systematic review identified 15 grading tools applicable to nutrition153. Among these, Grading of Recommendations, Assessments, Development and Evaluation (GRADE) was the most widely adopted. By default, GRADE rates evidence from randomized clinical trials (RCTs) as high quality, whereas evidence from observational studies starts at low quality. Certainty of the evidence is then upgraded or downgraded according to study characteristics applied to set criteria. RCTs are often not feasible in nutrition120 and a well-designed cohort study can simulate a trial when relevant confounders are accounted for154. Meta-analyses using GRADE have typically rated the evidence on SSBs and weight gain or type 2 diabetes mellitus (T2DM) from cohort studies to be of low or moderate quality20,34.
In the US, the Dietary Guidelines for Americans committee developed their own evidence grading system (National Evidence Library Grading Rubric) to guide conclusion statements153. This tool considers studies of strong and weak design but does not define the study designs, which affords some flexibility in considering different types of evidence. Developed as a GRADE alternative, NutriGrade takes factors into account that are specific to nutrition research, including effect size, dose–response for cohort studies, study design considerations for RCTs and funding bias155,156. In 2019, the Hierarchies of Evidence Applied to Lifestyle Medicine (HEALM) tool was proposed and provides a more flexible framework for evidence grading, recognizing that different types of studies make distinct contributions to knowledge153. Although not a formal evidence grading tool, the Bradford–Hill criteria for causality (commonly used in non-communicable disease epidemiology) comprise nine criteria to evaluate an evidence base to establish causal relationships. We previously illustrated that the evidence linking SSBs to weight gain and risk of T2DM meet the Bradford–Hill criteria for causality157,158. Based on our assessment, cohort studies showed strong and consistent associations, established temporality and demonstrated dose–response relationships, whereas short-term RCTs established biological rationale and causal relationships with intermediate biomarkers, thus meeting the key criteria for causality.
Underlying biological mechanisms
The leading biological mechanisms that link SSBs to weight gain include decreased satiety after consumption of SSBs than after consumption of solid food, and an incomplete compensatory reduction in energy intake6. This model is supported indirectly by short-term feeding trials in healthy adults showing that consumption of SSBs leads to greater energy intake79 and weight gain49,79–82 than consumption of ASBs. Some evidence has also been provided by a limited number of studies in healthy adults showing that isocaloric consumption of liquid sugars leads to greater energy intake and weight gain than consumption of solid food83–85. These studies suggest that calories consumed in the form of liquid beverages might not be satiating and might not be able to suppress intake of calories from foods consumed in subsequent meals to the level needed to maintain energy balance. Findings from studies in animals are consistent with this observation86. However, the underlying mechanisms of this lack of compensatory response remain unknown. Fructose in SSBs is thought to potentially promote weight gain through inducing reductions in resting energy expenditure87 and through the induction of leptin resistance88; however, further studies are needed to elucidate these pathways88. Early introduction of SSBs might be particularly detrimental in children, as it might promote sweet taste preference45.
Sugar addiction
Interest is growing in sugar addiction as a putative driver of excessive SSB intake89. Consumption of sugar has been shown to release endogenous opioids in the nucleus accumbens, a primary site for reinforced behaviours in the brain, and to activate the dopaminergic reward system89. Rats with intermittent access to sugar show the same decrease in levels of dopamine D2 receptor mRNA in the nucleus accumbens that occurs in morphine and cocaine addiction, and demonstrate characteristics of addiction such as escalation of intake, withdrawal and cravings90. These findings suggest that sugary foods and beverages are potentially rewarding and can trigger addictive-like behaviours, which might be responsible for over-consumption. However, a variety of reviews on this topic are conflicting and findings in humans are less consistent than findings from studies in animals91. Intake of sugars containing glucose and fructose has also been shown to induce the metabolic syndrome in mice in the absence of taste, probably due to over-consumption owing to post-ingestive reward signals92. Whether these findings are applicable to humans is unknown.
Glycaemic load
Consumption of SSBs might also promote weight gain through adverse effects on metabolism, through their ability to induce rapid spikes in blood levels of glucose and insulin93,94. In general, SSBs have moderate-to-high glycaemic index values95, which in combination with the large quantities consumed contribute to a high dietary glycaemic load. Diets with a high glycaemic load might promote weight gain by raising the postprandial ratio of serum concentrations of insulin to glucagon, resulting in increased hunger and decreased energy expenditure96. Individuals with increased central adiposity are more likely to have high insulin secretion in response to sugar consumption; thus, reducing glycaemic load among this group might have the most pronounced benefits on metabolism31.
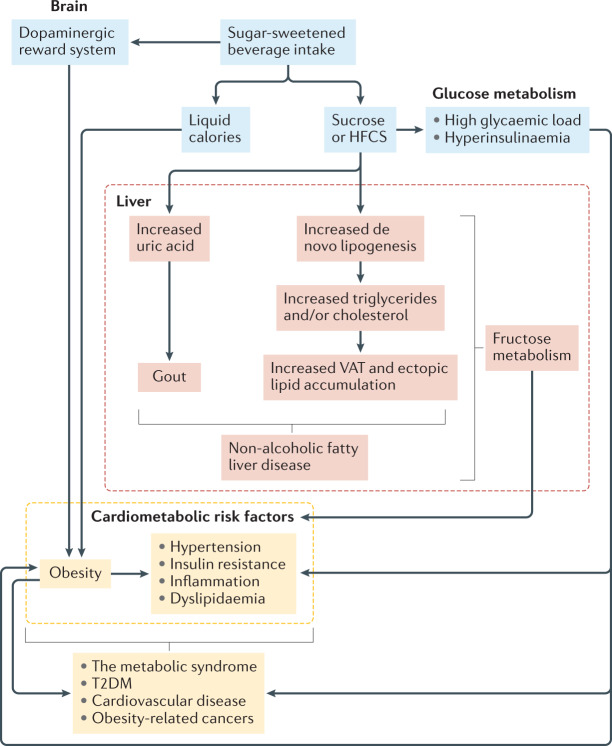
SSBs contribute to the development of cardiometabolic diseases and some cancers, in part through weight gain, but also through independent metabolic effects of glucose and fructose contained in constituent sugars (Fig. 3). Through their contribution to a high glycaemic load diet, SSBs can promote insulin resistance97, exacerbate inflammatory biomarkers98, and have been associated with increased risk of T2DM99,100 and CHD101. Habitual consumption of diets with a high glycaemic load might also influence cancer risk via hyperinsulinaemia and activation of the insulin-like growth factor axis102. Studies in animals have suggested that the metabolic effects of glucose might be partly driven by the conversion of glucose to fructose in the liver103. Thus, fructose might have a role in how diets with a high glycaemic load induce metabolic effects88.
Fig. 3. Biological mechanisms for sugar-sweetened beverage intake and development of obesity, cardiometabolic risk and related chronic diseases.
Biological mechanisms linking intake of sugar-sweetened beverages (SSBs) to the development of obesity, intermediate cardiometabolic risk factors (such as non-alcoholic fatty liver disease, hypertension, insulin resistance, inflammation and dyslipidaemia) and related chronic diseases (the metabolic syndrome, type 2 diabetes mellitus (T2DM), cardiovascular disease and cancer). Mechanisms that promote weight gain and obesity include: an incomplete compensatory reduction in food intake in response to liquid calories provided by SSBs; hyperinsulinaemia induced by rapid absorption of glucose; and potential activation of the dopaminergic reward system in the brain. Obesity increases cardiometabolic risk and is associated with the development of related chronic diseases. Elevated risk of these outcomes also occurs independently of weight gain through the development of risk factors precipitated by adverse glycaemic effects and fructose metabolism in the liver. Excess fructose ingestion promotes uric acid production, hepatic de novo lipogenesis, accumulation of visceral adipose tissue (VAT) and ectopic lipid deposition, and can lead to the development of gout and non-alcoholic fatty liver disease. HFCS, high-fructose corn syrup. Adapted from ref.43, CC BY 4.0 (https://creativecommons.org/licences/by/4.0/).
Fructose
Fructose is consumed from SSBs as a component of sucrose or HFCS and is thought to contribute to cardiometabolic risk through its metabolic fate in the liver. The absorption of fructose in the gut is enhanced in the presence of glucose and this effect accounts for the rapid and complete absorption of both monoglycerides when ingested as sucrose or HFCS, as found in SSBs. Uptake of glucose in the liver is tightly regulated; however, the hepatic uptake and metabolism of fructose, which occurs through first-pass metabolism via fructokinase, is unregulated104. When consumed in moderation, fructose is converted to glucose, lactate and fatty acids in the liver for use as metabolic substrates104. However, when fructose is consumed in excess, an increase in hepatic de novo lipogenesis occurs that can lead to atherogenic dyslipidaemia and insulin resistance. The amount of fructose needed to increase blood lipid levels is debated; however, consuming HFCS-sweetened beverages containing 10–25% of total daily calorie requirements has been shown to produce notable linear increases in postprandial triglycerides. This finding suggests a dose–response relationship between fructose consumption and increases in triglycerides48. Increased concentrations of lipid in the liver can upregulate the production and secretion of VLDL, which leads to increased circulating levels of triglycerides. Excess intake of fructose has also been associated with the production of small dense LDL-C particles, resulting from increased levels of VLDL- induced lipoprotein remodelling48,105. Some studies have shown that intake of fructose can promote the accumulation of visceral adipose tissue and ectopic lipid deposition in humans106–109. The metabolism of fructose in the liver can also deplete intracellular ATP in hepatocytes, which can lead to an increase in uric acid production. These fructose-induced alterations to hepatic metabolism have implicated SSBs in the development of NAFLD and other metabolic complications110, possibly through cellular energy homeostasis and mitochondrial oxidative stress88. Studies in animals have suggested that fructose metabolism is largely responsible for the effects of SSBs on cardiometabolic diseases88,111,112.
Uric acid production
Fructose is the only sugar known to increase hepatic uric acid production. Hyperuricaemia is a precursor to gout113,114 and both gout and hyperuricaemia have been associated with hypertension, T2DM and CVD115,116. The development of hyperuricaemia has been shown to precede the onset of obesity and T2DM. Furthermore, hyperuricaemia might mediate the association between SSB intake and hypertension, possibly through the induction of renal disease, endothelial dysfunction and activation of the renin–angiotensin system115. Excess production of uric acid has also been linked to a reduction in nitric oxide levels in endothelial cells, which might partially explain the link between fructose-containing beverages and CHD115.
Alternative beverages
As public health measures continue to call for reductions in intake of SSBs to prevent weight gain and cardiometabolic diseases, interest is growing in alternative beverages. Among these, ASBs have attracted the most attention.
ASBs
Despite containing few calories and no sugar, some cohort studies among adults have found positive associations between intake of ASBs and weight gain and the risks of T2DM and CVD34,40,117, which has obscured dietary guidance. Interpretation of findings from these studies is complicated, due to potential residual confounding by unmeasured or poorly measured factors linked to ASB intake. Reverse causation is also a concern in these studies, since individuals with obesity or with other risk factors for T2DM, such as elevated blood glucose or insulin levels, might switch from SSBs to ASBs and this scenario might drive spurious positive associations118,119. Although it is difficult to address these biases in statistical analyses, studies with repeated assessments of diet are less prone to reverse causation, as they enable changes in intake over time to be examined120. These types of studies have shown marginal statistically non-significant associations between ASBs and weight gain and cardiometabolic outcomes24,73,121–123.
Substitution analyses in cohort studies have shown inverse associations with weight gain, T2DM and mortality when SSBs are replaced with ASBs73,124,125. These findings are consistent with those of short-term trials in different populations including healthy children, and adults with and without overweight and obesity that have shown modest benefits on body weight and metabolic risk factors when SSBs are replaced with ASBs21,126. However, a number of biological mechanisms have been proposed that might link ASBs to weight gain and adverse cardiometabolic health. For example, the intense sweetness of artificial sweeteners might condition towards a taste preference for sweets. Furthermore, ASBs stimulate sweet taste receptors and can activate the cephalic phase insulin response (CPIR). ASBs might also stimulate the release of gut hormones. In addition, neural responses to ASBs might exert a food reward response. Moreover, ASB consumption might modulate appetite regulation. Finally, ASBs might cause alterations in the gut microflora127. Although intriguing, these mechanisms are not well understood and different types of artificial sweeteners might elicit different physiological effects128. For example, saccharine and sucralose seemingly stimulate the CPIR, whereas aspartame and stevia do not128. A 2019 RCT in adults with overweight or obesity illustrated modest reductions in weight with intake of sucralose, modest weight gain with saccharine and no effect on weight with intake of aspartame or stevia, relative to sucrose129. Although the findings need to be replicated, this study suggests that some of the inconsistency observed in the epidemiological evidence might be due to combined effects of different artificial sweeteners.
Given that ultra-processed foods might increase weight gain independently of calories130, the processing of both SSBs and ASBs could potentially be implicated in obesity, although this hypothesis requires investigation. Further studies are needed to better understand the effects of individual sweeteners and the consequences of consuming ASBs over the life-course131. Based on the current evidence, consumption of ASBs in place of SSBs could be a helpful strategy to reduce cardiometabolic risk, with the ultimate goal of switching to water or other healthful beverages.
Fruit juice
Whether 100% fruit juice is an acceptable replacement for SSBs has also been a question of great interest. Fruit juice is often perceived as healthful, as most juices contain some vitamins and nutrients. However, some fruit juices contain similar amounts of calories and sugar to SSBs from the natural sugars present in fruit. Findings from cohort studies in the USA suggest that intake of fruit juice is associated with weight gain132 and the risk of T2DM133, while the opposite has been shown for whole fruit24,134. This finding can be explained by differences in the food matrix and effects on absorption. Sugars in juice are absorbed more quickly than those in whole fruit, owing in part to the fibre content of whole fruit, which slows the rate of absorption135,136. The rapid absorption of liquid fructose from juice, combined with the large volumes that are sometimes consumed, can lead to increased concentrations of fructose in the liver and could drive de novo lipogenesis and the production of lipids88,137. High intake of fruit juice has been associated with a higher risk of mortality compared with low intake74. However, some benefits of fruit juice on cardiometabolic risk have also been reported132,138. Further research on fruit juice is warranted, which should consider different types of juices that are consumed in different countries, as their nutrient profiles and sugar contents probably differ.
Water, tea and coffee
Water is free of sugar and calories and is considered the optimal beverage for hydration. In an analysis of the Harvard cohorts, we found that replacing one serving per day of SSBs with water was inversely associated with weight gain124 and risk of T2DM125. With the growing demand for water, different types of sparkling and flavoured options (some containing artificial sweeteners) are now available, which might help habitual SSB consumers switch to water. Ensuring access to potable water and limiting use of plastic bottled water for both environmental and health reasons will be important initiatives when promoting water intake in place of SSBs.
A number of studies have shown that moderate consumption (two to five cups per day) of regular or decaffeinated coffee or tea is associated with decreased risk of T2DM and CVD139,140, which is probably owing to the myriad of bioactive compounds in these beverages. In a substitution analysis, we found that replacing one serving per day of SSBs with coffee (decaffeinated and caffeinated; whether the coffee was sweetened was not specified) was associated with a 17% lower risk of T2DM122. Provided there are no contraindications and that the use of caloric sweeteners and creamers is limited, coffee and tea can be healthful alternatives so SSBs. Certain groups such as pregnant women and children should consume caffeine-containing beverages with caution, as little is known about their effects in children, and excess caffeine could be harmful during pregnancy.
Policies
Based on the current evidence, national and international organizations recommend limiting intake of SSBs141. The WHO, and US and Canadian dietary guidelines recommend an upper limit of 10% of total energy from all added sugar or free sugar; this recommendation is supported by numerous medical associations. Consistent with these recommendations, a number of public policies have been identified to help change SSB consumption patterns (Box 3). The most common include SSB taxation, banning of sales and vending in schools, government restrictions on marketing unhealthy foods or beverages to children, public health education campaigns and front-of-package warning labels142,143.
Several countries as well as some US cities have implemented excise taxes on SSBs as a strategy to curtail intake and generate revenue. In 2014, Mexico implemented a 10% excise tax (that is, 1 peso per litre) on SSBs, which sparked global interest. Two years after implementation, a 7.6% reduction in sales of SSBs was observed, while sales of untaxed beverages such as water increased by 2.1%144. Based on modelled data, the potential effect of the tax was estimated to have prevented ~200,000 people from developing obesity and to have saved International $980 million in health-care costs between 2013 and 2022 (ref.145). Berkeley, California, was the first US city to implement a penny per ounce excise tax on SSBs; comparing trends before and 1 year after tax implementation, a 9.6% reduction in sales of SSBs was observed while sales of untaxed beverages increased by 3.5%146. A meta-analysis of studies evaluating SSB taxes found that taxation resulted in decreases in sales, purchasing and consumption of taxed beverages147. More recently in 2018, the UK implemented a tiered tax on SSBs, based on sugar content (£0.24 per litre for drinks containing ≥8 g total sugar per 100 ml, and £0.18 per litre for drinks containing between 5 g and <8 g total sugar per 100 ml) that was designed to incentivize reformulation. Analyses of soft drink sugar content and sales illustrate the intended benefits following reformulation148,149. Compared with pre-tax trends, 1 year after implementation there was no change in the volume of soft drinks purchased but the quantity of sugar purchased in these beverages decreased by 30 g per household per week150. This finding suggests that a tiered tax might reduce sugar intake without harming industry sales. Whether these early benefits of SSB taxes will continue and translate into improvements in health will be important factors to monitor.
In the USA and Canada, the nutritional facts labels on packaged foods and beverages were recently revised (with respective compliance dates in 2021 and 2022) to require the added sugar (USA) and total sugar (Canada) content of packaged products to be displayed, with percentage daily values to help consumers meet sugar recommendations. Different types of front of package labels have been implemented in different countries, including traffic light labelling in the UK, the Nutri-Score in France, the star system in Australia and New Zealand, and the Nordic keyhole in Norway, Denmark and Sweden, to help consumers make informed choices151. Chile was the first country to implement a national front-of-package warning, along with marketing restrictions and banning products high in calories, sugar, sodium and saturated fat in schools. Early evaluation of these policies found a 23.7% reduction in purchases of high-in beverages (that is, containing added sugar, saturated fat, salt or calories exceeding thresholds) compared with pre-implementation trends152. The observed decrease was greater than changes in purchases following implementation of single standalone policies in Latin America, including SSB taxation152.
To change SSB consumption patterns, a combination of policies across different levels of governance is needed, together with widespread public health education to serve as an important step in changing social norms surrounding beverage habits. A key priority for researchers and policymakers will be the continued evaluation of these policies in relation to short-term behaviour changes and clinical outcomes to ensure their effectiveness over time.
Box 3 Policy actions to reduce consumption of sugar-sweetened beverages.
Financial incentives to reduce sugar-sweetened beverage (SSB) intake, such as taxation, should be considered by governments. A price increase of at least 10% based on volume sold or sugar content is recommended to influence purchasing behaviours.
Access to SSBs acquired through subsidized nutrition programmes should be limited, by subsidizing the sales of healthful beverages and foods.
Regulatory action to reduce marketing of unhealthy beverages and foods in the media and at sports events or other activities, particularly to children, should be considered.
Front-of-package labelling systems should be implemented to help guide consumers to make healthful beverage and food choices and stay within added sugar and free sugar targets.
Access of SSBs should be limited in schools, by adopting polices that restrict sales of SSBs and provision in school meal programmes. Access to potable water in schools should be prioritized.
Access to SSBs should be limited in health-care facilities, government institutions and other public spaces, by adopting polices that restrict sales of SSBs and provision in institutional meals. Access to potable water and healthful alternatives should be prioritized.
Healthful beverages, such as water, should be made the default choice by adopting policies that promote them, or by voluntary action in the hospitality and service industries.
Specific guidelines for healthful beverage consumption should be included in national and international dietary recommendations.
Health risks linked to the over-consumption of SSBs should be included in national and international programmes and reports that target prevention of obesity and chronic diseases.
Policies and regulatory action that ensure access to potable water in all communities should be adopted.
Implementation of multiple actions is expected to be more effective at reducing intake levels than single standalone policies. Policy actions should be accompanied by concurrent public health education campaigns to reinforce key messages and ensure effectiveness.
Conclusions
SSBs are consumed on a global scale, with intake levels above recommendations in many high-income countries and on the rise in LMICs. Based on the available evidence from prospective cohort studies that assessed long-term outcomes, as well as RCTs that assessed intermediate risk factors, strong evidence exists for an aetiological relationship between intake of SSBs and weight gain, and risk of T2DM, CHD and/or NAFLD. The evidence for other conditions, including stroke and specific types of cancer, is less consistent and further research is warranted. Although specific thresholds for intake of SSBs have not been identified as most observations are from dose–response analyses, clinically important weight gain and risk of attendant cardiometabolic conditions are associated with intake of SSBs at commonly consumed levels, such as one serving per day.
SSBs might promote weight gain through multiple mechanisms, including incomplete compensation for liquid calories by reductions in food intake at subsequent meals, hyperinsulinaemia induced by the rapid absorption of large amounts of sugar and possibly through neural pathways of food addiction. These beverages are thought to increase T2DM and cardiometabolic risk through weight gain. In addition, SSBs act independently of weight gain through a high glycaemic load and the unique metabolic effects of excess fructose in the liver, which has been linked to accumulation of visceral adipose tissue and ectopic lipid deposition, gout and NAFLD. Various policies and regulatory strategies to reduce intake of SSBs are in place or are being considered in several countries. Continued evaluation of these policies is needed in order to gauge their effectiveness over time. In addition, more and higher quality trials are required to identify new strategies or combinations of actions that are effective in reducing SSB intake at the individual and population level. Although policies targeting SSBs should not be considered a ‘magic bullet’ for obesity prevention, they can be effective in shifting consumption levels or social norms, which are important public health goals.
Key areas for which future research is warranted include examining the effects of different sugars on health outcomes over a broad range of doses, investigating the health effects of sugar consumed in solid form compared with liquid form and further elucidating biological mechanisms of energy compensation and sugar addiction. Important research gaps also exist regarding suitable alternative beverages, including the health effects of consuming ASBs over the life-course, examination of different types of juices and ensuring global access to potable water.
In the coming years, as the world grapples with rising obesity and chronic disease burdens alongside infectious diseases, such as the COVID-19 pandemic that has been worsened by obesity, there will be an urgent need for coordinated actions across all sectors of society to prioritize obesity prevention. These efforts should focus on nutrition policies and regulatory strategies aimed at improving overall diet quality, creating healthier food environments and reducing health disparities. Given the strength and consistency of the evidence across different populations and increased consumption patterns associated with nutrition transitions, SSBs present a clear target for policy action. With the high intake levels across the globe, reducing consumption of SSBs remains an important step in improving diet quality, which could have a measurable effect on weight control and in improving global health.
Glossary
- Sugar-sweetened beverages
(SSBs). Beverages that contain added sugar, including carbonated and non-carbonated soft drinks, fruit drinks, and sports and energy drinks that are typically low in nutritional quality.
- Artificially-sweetened beverages
(ASBs). Beverages that are flavoured with low-calorie sweeteners (aspartame, sucralose, acesulfame-potassium, saccharine or stevia) and contain few to no calories but retain a sweet taste.
- Non-alcoholic fatty liver disease
(NAFLD). This spectrum of pathological disorders includes simple hepatic steatosis, non-alcoholic steatohepatitis, fibrosis and cirrhosis and arises without alcohol consumption.
- Gout
A common form of inflammatory arthritis arising from deposition of uric acid in articular cartilage.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
V.S.M. is on a pro bono retainer for expert support for litigation related to sugar-sweetened beverages and has served as a consultant for the City of San Francisco for a case related to health warning labels on soda. There are no other financial or personal conflicts of interest to disclose that are related to the content of this paper. F.B.H. declares no competing interests.
Footnotes
Peer review information
Nature Reviews Endocrinology thanks K. Backholer, R. Johnson, L. Moreno and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Global dietary database: https://www.globaldietarydatabase.org/data-download
Contributor Information
Vasanti S. Malik, Email: vasanti.malik@utoronto.ca
Frank B. Hu, Email: nhbfh@channing.harvard.edu
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Obesity Collaborators et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobbs R. et al. Overcoming obesity: an initial economic analysis. McKinsey Global Institute. https://www.sportanddev.org/en/article/publication/overcoming-obesity-initial-economic-analysis (2014).
- 4.Hruby A, et al. Determinants and consequences of obesity. Am. J. Public Health. 2016;106:1656–1662. doi: 10.2105/AJPH.2016.303326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 6.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am. J. Clin. Nutr. 2011;94:726–734. doi: 10.3945/ajcn.111.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosinger, A., Herrick, K., Gahche, J. & Park, S. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief, no. 270 (National Center for Health Statistics, 2017). [PubMed]
- 9.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 dietary guidelines for Americans, 8th Edn. (U.S. Department of Health and Human Services, 2015).
- 10.World Health Organization. Guideline: Sugars intake for adults and children (WHO, 2015). [PubMed]
- 11.Singh GM, et al. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS ONE. 2015;10:e0124845. doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, et al. Consumption of carbonated soft drinks among young adolescents aged 12 to 15 years in 53 low- and middle-income countries. Am. J. Public Health. 2017;107:1095–1100. doi: 10.2105/AJPH.2017.303762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatelan A, et al. Time trends in consumption of sugar-sweetened beverages and related socioeconomic differences among adolescents in Eastern Europe: signs of a nutrition transition? Am. J. Clin. Nutr. 2021;114:1476–1485. doi: 10.1093/ajcn/nqab175. [DOI] [PubMed] [Google Scholar]
- 14.Fismen AS, et al. Trends in food habits and their relation to socioeconomic status among Nordic adolescents 2001/2002–2009/2010. PLoS ONE. 2016;11:e0148541. doi: 10.1371/journal.pone.0148541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrar I, Schmitting S, Della Corte KW, Buyken AE, Alexy U. Age and time trends in sugar intake among children and adolescents: results from the DONALD study. Eur. J. Nutr. 2020;59:1043–1054. doi: 10.1007/s00394-019-01965-y. [DOI] [PubMed] [Google Scholar]
- 16.Dai J, Soto MJ, Dunn CG, Bleich SN. Trends and patterns in sugar-sweetened beverage consumption among children and adults by race and/or ethnicity, 2003–2018. Public Health Nutr. 2021;24:2405–2410. doi: 10.1017/S1368980021001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AC, Kirkpatrick SI, Hammond D. Beverage consumption and energy intake among Canadians: analyses of 2004 and 2015 national dietary intake data. Nutr. J. 2019;18:60. doi: 10.1186/s12937-019-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am. J. Clin. Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 19.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am. J. Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 21.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am. J. Clin. Nutr. 2008;87:1662–1671. doi: 10.1093/ajcn/87.6.1662. [DOI] [PubMed] [Google Scholar]
- 23.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am. J. Clin. Nutr. 2009;89:438–439. doi: 10.3945/ajcn.2008.26980. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan L, et al. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics. 2014;134(Suppl 1):29–35. doi: 10.1542/peds.2014-0646F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q, et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahabamunuge J, et al. Association of sugar-sweetened beverage intake with maternal postpartum weight retention. Public Health Nutr. 2021;24:4196–4203. doi: 10.1017/S1368980020005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillman MW, et al. Beverage intake during pregnancy and childhood adiposity. Pediatrics. 2017;140:e20170031. doi: 10.1542/peds.2017-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes. Rev. 2013;14:620–633. doi: 10.1111/obr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes. Rev. 2011;12:346–365. doi: 10.1111/j.1467-789X.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebbeling CB, et al. Effects of sugar-sweetened, artificially sweetened, and unsweetened beverages on cardiometabolic risk factors, body composition, and sweet taste preference: a randomized controlled trial. J. Am. Heart Assoc. 2020;9:e015668. doi: 10.1161/JAHA.119.015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebbeling CB, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 34.Imamura F, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik VS, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.InterAct Consortium. et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56:1520–1530. doi: 10.1007/s00125-013-2899-8. [DOI] [PubMed] [Google Scholar]
- 37.Stern D, et al. Sugar-sweetened soda consumption increases diabetes risk among Mexican women. J. Nutr. 2019;149:795–803. doi: 10.1093/jn/nxy298. [DOI] [PubMed] [Google Scholar]
- 38.Gardener H, Moon YP, Rundek T, Elkind MSV, Sacco RL. Diet soda and sugar-sweetened soda consumption in relation to incident diabetes in the Northern Manhattan study. Curr. Dev. Nutr. 2018;2:nzy008. doi: 10.1093/cdn/nzy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, et al. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: the prospective Women’s Health Initiative observational study. Am. J. Clin. Nutr. 2017;106:614–622. doi: 10.3945/ajcn.116.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin J, et al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv. Nutr. 2021;12:89–101. doi: 10.1093/advances/nmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int. J. Clin. Pract. 2016;70:791–805. doi: 10.1111/ijcp.12841. [DOI] [PubMed] [Google Scholar]
- 42.Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis. 2014;234:11–16. doi: 10.1016/j.atherosclerosis.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 43.Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11:1840. doi: 10.3390/nu11081840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira-Pego C, et al. Frequent consumption of sugar- and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a Mediterranean population at high cardiovascular disease risk. J. Nutr. 2016;146:1528–1536. doi: 10.3945/jn.116.230367. [DOI] [PubMed] [Google Scholar]
- 45.Vos MB, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2016;135:e1017–e1034. doi: 10.1161/CIR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014;100:65–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, et al. Important food sources of fructose-containing sugars and incident hypertension: a systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2019;8:e010977. doi: 10.1161/JAHA.118.010977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanhope KL, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015;101:1144–1154. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raben A, et al. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr. Res. 2011;55:8326. doi: 10.3402/fnr.v55i0.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aeberli I, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am. J. Clin. Nutr. 2011;94:479–485. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am. J. Clin. Nutr. 2005;82:421–427. doi: 10.1093/ajcn/82.2.421. [DOI] [PubMed] [Google Scholar]
- 52.Kuzma JN, et al. No differential effect of beverages sweetened with fructose, high-fructose corn syrup, or glucose on systemic or adipose tissue inflammation in normal-weight to obese adults: a randomized controlled trial. Am. J. Clin. Nutr. 2016;104:306–314. doi: 10.3945/ajcn.115.129650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen T, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018;68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, et al. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: an updated systematic review and dose-response meta-analysis. Int. J. Env. Res. Public Health. 2019;16:2192. doi: 10.3390/ijerph16122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asgari-Taee F, et al. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur. J. Nutr. 2019;58:1759–1769. doi: 10.1007/s00394-018-1711-4. [DOI] [PubMed] [Google Scholar]
- 56.Chung M, et al. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100:833–849. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu S, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayoub-Charette S, et al. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2019;9:e024171. doi: 10.1136/bmjopen-2018-024171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar-sweetened beverages and serum uric acid concentrations: a systematic review and meta-analysis. J. Hum. Nutr. Diet. 2021;34:305–313. doi: 10.1111/jhn.12796. [DOI] [PubMed] [Google Scholar]
- 60.Wang DD, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J. Nutr. 2012;142:916–923. doi: 10.3945/jn.111.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makarem N, Bandera EV, Nicholson JM, Parekh N. Consumption of sugars, sugary foods, and sugary beverages in relation to cancer risk: a systematic review of longitudinal studies. Annu. Rev. Nutr. 2018;38:17–39. doi: 10.1146/annurev-nutr-082117-051805. [DOI] [PubMed] [Google Scholar]
- 62.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, and physical activity, and the prevention of cancer: a global perspective (AICR, 2007).
- 63.Llaha F, et al. Consumption of sweet beverages and cancer risk. A systematic review and meta analysis of observational studies. Nutrients. 2021;13:516. doi: 10.3390/nu13020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debras C, et al. Total and added sugar intakes, sugar types, and cancer risk: results from the prospective NutriNet-Sante cohort. Am. J. Clin. Nutr. 2020;112:1267–1279. doi: 10.1093/ajcn/nqaa246. [DOI] [PubMed] [Google Scholar]
- 65.Hodge AM, Bassett JK, Milne RL, English DR, Giles GG. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018;21:1618–1626. doi: 10.1017/S1368980017002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanos-Nanclares A, et al. Sugar-sweetened beverage consumption and incidence of breast cancer: the Seguimiento Universidad de Navarra (SUN) Project. Eur. J. Nutr. 2019;58:2875–2886. doi: 10.1007/s00394-018-1839-2. [DOI] [PubMed] [Google Scholar]
- 67.Makarem N, et al. Consumption of sugars, sugary foods, and sugary beverages in relation to adiposity-related cancer risk in the Framingham Offspring Cohort (1991–2013) Cancer Prev. Res. 2018;11:347–358. doi: 10.1158/1940-6207.CAPR-17-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwingshackl L, et al. Food groups and risk of colorectal cancer. Int. J. Cancer. 2018;142:1748–1758. doi: 10.1002/ijc.31198. [DOI] [PubMed] [Google Scholar]
- 69.Pacheco LS, et al. Sugar-sweetened beverages and colorectal cancer risk in the California Teachers Study. PLoS ONE. 2019;14:e0223638. doi: 10.1371/journal.pone.0223638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genkinger JM, et al. Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer Epidemiol. Biomark. Prev. Oncol. 2012;21:305–318. doi: 10.1158/1055-9965.EPI-11-0945-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schernhammer ES, et al. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol. Biomark. Prev. Oncol. 2005;14:2098–2105. doi: 10.1158/1055-9965.EPI-05-0059. [DOI] [PubMed] [Google Scholar]
- 72.Navarrete-Munoz EM, et al. Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Am. J. Clin. Nutr. 2016;104:760–768. doi: 10.3945/ajcn.116.130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik VS, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113–2125. doi: 10.1161/CIRCULATIONAHA.118.037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA. Association of sugary beverage consumption with mortality risk in US adults: a secondary analysis of data from the REGARDS Study. JAMA Netw. Open. 2019;2:e193121. doi: 10.1001/jamanetworkopen.2019.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson JJ, et al. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study. BMC Med. 2020;18:97. doi: 10.1186/s12916-020-01554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koyratty N, et al. Sugar-sweetened soda consumption and total and breast cancer mortality: the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Epidemiol. Biomark. Prev. Oncol. 2021;30:945–952. doi: 10.1158/1055-9965.EPI-20-1242. [DOI] [PubMed] [Google Scholar]
- 77.Braverman-Bronstein A, et al. Mortality attributable to sugar sweetened beverages consumption in Mexico: an update. Int. J. Obes. 2020;44:1341–1349. doi: 10.1038/s41366-019-0506-x. [DOI] [PubMed] [Google Scholar]
- 78.Micha R, et al. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. J. Am. Med. Assoc. 2017;317:912–924. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44:187–193. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am. J. Clin. Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 81.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am. J. Clin. Nutr. 1990;51:963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 82.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br. J. Nutr. 2007;97:193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 83.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int. J. Obes. Relat. Metab. Disord. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 84.Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:385–390. doi: 10.1097/MCO.0b013e328346df36. [DOI] [PubMed] [Google Scholar]
- 85.Mourao DM, Bressan J, Campbell WW, Mattes RD. Effects of food form on appetite and energy intake in lean and obese young adults. Int. J. Obes. 2007;31:1688–1695. doi: 10.1038/sj.ijo.0803667. [DOI] [PubMed] [Google Scholar]
- 86.Togo J, Hu S, Li M, Niu C, Speakman JR. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: liquid or solid. Mol. Metab. 2019;27:22–32. doi: 10.1016/j.molmet.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox CL, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur. J. Clin. Nutr. 2012;66:201–208. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sundborn G, et al. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity. 2019;27:879–887. doi: 10.1002/oby.22472. [DOI] [PubMed] [Google Scholar]
- 89.DiNicolantonio JJ, O’Keefe JH, Wilson WL. Sugar addiction: is it real? A narrative review. Br. J. Sports Med. 2018;52:910–913. doi: 10.1136/bjsports-2017-097971. [DOI] [PubMed] [Google Scholar]
- 90.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olszewski PK, Wood EL, Klockars A, Levine AS. Excessive consumption of sugar: an insatiable drive for reward. Curr. Nutr. Rep. 2019;8:120–128. doi: 10.1007/s13668-019-0270-5. [DOI] [PubMed] [Google Scholar]
- 92.Andres-Hernando A, et al. Sugar causes obesity and metabolic syndrome in mice independently of sweet taste. Am. J. Physiol. Endocrinol. Metab. 2020;319:E276–E290. doi: 10.1152/ajpendo.00529.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tey SL, Salleh NB, Henry J, Forde CG. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017;41:450–457. doi: 10.1038/ijo.2016.225. [DOI] [PubMed] [Google Scholar]
- 94.Solomi L, Rees GA, Redfern KM. The acute effects of the non-nutritive sweeteners aspartame and acesulfame-K in UK diet cola on glycaemic response. Int. J. Food Sci. Nutr. 2019;70:894–900. doi: 10.1080/09637486.2019.1585418. [DOI] [PubMed] [Google Scholar]
- 95.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern. Med. 2018;178:1098–1103. doi: 10.1001/jamainternmed.2018.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. J. Am. Med. Assoc. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 98.Liu S, et al. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 99.Bhupathiraju SN, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014;100:218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Livesey G, et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11:1280. doi: 10.3390/nu11061280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Livesey G, Livesey H. Coronary heart disease and dietary carbohydrate, glycemic index, and glycemic load: dose-response meta-analyses of prospective cohort studies. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019;3:52–69. doi: 10.1016/j.mayocpiqo.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am. J. Clin. Nutr. 2008;87:1793–1801. doi: 10.1093/ajcn/87.6.1793. [DOI] [PubMed] [Google Scholar]
- 103.Lanaspa MA, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun SZ, Empie MW. Fructose metabolism in humans–what isotopic tracer studies tell us. Nutr. Metab. 2012;9:89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goran, M. I., Tappy, L. & Lê, K-A. Dietary Sugars and Health (CRC Press, 2015).
- 106.Teff KL, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J. Clin. Endocrinol. Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stanhope KL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stanhope KL, et al. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am. J. Clin. Nutr. 2008;87:1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am. J. Clin. Nutr. 2008;88:1733S–1737S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tappy L, Rosset R. Health outcomes of a high fructose intake: the importance of physical activity. J. Physiol. 2019;597:3561–3571. doi: 10.1113/JP278246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andres-Hernando A, et al. Deletion of fructokinase in the liver or in the intestine reveals differential effects on sugar-induced metabolic dysfunction. Cell Metab. 2020;32:117–127.e3. doi: 10.1016/j.cmet.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andres-Hernando A, et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI Insight. 2021;6:e140848. doi: 10.1172/jci.insight.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304:2270–2278. doi: 10.1001/jama.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Richette P, Bardin TG. Gout. Lancet. 2010;375:318–328. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 116.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat. Clin. Pract. Nephrol. 2005;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 117.Azad MB, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189:E929–E939. doi: 10.1503/cmaj.161390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nettleton JA, et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32:688–694. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 120.Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015;6:5–18. doi: 10.3945/an.114.007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Koning L, et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011;93:1321–1327. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fung TT, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009;89:1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan A, et al. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int. J. Obes. 2013;37:1378–1385. doi: 10.1038/ijo.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pan A, et al. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am. J. Clin. Nutr. 2012;95:1454–1460. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malik VS. Non-sugar sweeteners and health. BMJ. 2019;364:k5005. doi: 10.1136/bmj.k5005. [DOI] [PubMed] [Google Scholar]
- 127.Swithers SE. Not so sweet revenge: unanticipated consequences of high-intensity sweeteners. Behav. Anal. 2015;38:1–17. doi: 10.1007/s40614-015-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunter SR, Reister EJ, Cheon E, Mattes RD. Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11:2717. doi: 10.3390/nu11112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Higgins KA, Mattes RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019;109:1288–1301. doi: 10.1093/ajcn/nqy381. [DOI] [PubMed] [Google Scholar]
- 130.Hall KD, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson RK, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138:e126–e140. doi: 10.1161/CIR.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 132.Schulze MB, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 133.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31:1311–1317. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muraki I, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ravn-Haren G, et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013;52:1875–1889. doi: 10.1007/s00394-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 136.Pepin A, Stanhope KL, Imbeault P. Are fruit juices healthier than sugar-sweetened beverages? A review. Nutrients. 2019;11:1006. doi: 10.3390/nu11051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lanaspa MA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghanim H, et al. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care. 2007;30:1406–1411. doi: 10.2337/dc06-1458. [DOI] [PubMed] [Google Scholar]
- 139.van Dam RM. Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Appl. Physiol. Nutr. Metab. 2008;33:1269–1283. doi: 10.1139/H08-120. [DOI] [PubMed] [Google Scholar]
- 140.Bhupathiraju SN, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am. J. Clin. Nutr. 2013;97:155–166. doi: 10.3945/ajcn.112.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.UConn Rudd Center for Food Policy and Health. Sugar-sweetened beverage taxes and sugar intake: policy statements, endorsements, and recommendations. https://uconnruddcenter.org/wp-content/uploads/sites/2909/2020/09/SugaryDrinkTaxStatements.pdf (2017).
- 142.Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4:174–186. doi: 10.1016/S2213-8587(15)00419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muth ND, et al. Public policies to reduce sugary drink consumption in children and adolescents. Pediatrics. 2019;143:e20190282. doi: 10.1542/peds.2019-0282. [DOI] [PubMed] [Google Scholar]
- 144.Colchero MA, Rivera-Dommarco J, Popkin BM, Ng SW. In Mexico, evidence of sustained consumer response two years after implementing a sugar-sweetened beverage tax. Health Aff. 2017;36:564–571. doi: 10.1377/hlthaff.2016.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanchez-Romero LM, et al. Projected impact of Mexico’s sugar-sweetened beverage tax policy on diabetes and cardiovascular disease: a modeling study. PLoS Med. 2016;13:e1002158. doi: 10.1371/journal.pmed.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Silver LD, et al. Changes in prices, sales, consumer spending, and beverage consumption one year after a tax on sugar-sweetened beverages in Berkeley, California, US: a before-and-after study. PLoS Med. 2017;14:e1002283. doi: 10.1371/journal.pmed.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Teng AM, et al. Impact of sugar-sweetened beverage taxes on purchases and dietary intake: systematic review and meta-analysis. Obes. Rev. 2019;20:1187–1204. doi: 10.1111/obr.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bandy LK, Scarborough P, Harrington RA, Rayner M, Jebb SA. Reductions in sugar sales from soft drinks in the UK from 2015 to 2018. BMC Med. 2020;18:20. doi: 10.1186/s12916-019-1477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scarborough P, et al. Impact of the announcement and implementation of the UK soft drinks industry levy on sugar content, price, product size and number of available soft drinks in the UK, 2015-19: a controlled interrupted time series analysis. PLoS Med. 2020;17:e1003025. doi: 10.1371/journal.pmed.1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pell D, et al. Changes in soft drinks purchased by British households associated with the UK soft drinks industry levy: controlled interrupted time series analysis. BMJ. 2021;372:n254. doi: 10.1136/bmj.n254. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Muller L, Ruffieux B. What makes a front-of-pack nutritional labelling system effective: the impact of key design components on food purchases. Nutrients. 2020;12:2870. doi: 10.3390/nu12092870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Taillie LS, Reyes M, Colchero MA, Popkin B, Corvalan C. An evaluation of Chile’s Law of Food Labeling and Advertising on sugar-sweetened beverage purchases from 2015 to 2017: a before-and-after study. PLoS Med. 2020;17:e1003015. doi: 10.1371/journal.pmed.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katz DL, et al. Hierarchies of Evidence Applied to Lifestyle Medicine (HEALM): introduction of a strength-of-evidence approach based on a methodological systematic review. BMC Med. Res. Methodol. 2019;19:178. doi: 10.1186/s12874-019-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J. Epidemiol. Commun. Health. 2006;60:578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schwingshackl L, et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv. Nutr. 2016;7:994–1004. doi: 10.3945/an.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schlesinger S, et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2019;10:205–218. doi: 10.1093/advances/nmy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr. Diabetes Rep. 2012;12:195–203. doi: 10.1007/s11892-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 158.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013;14:606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]