Abstract
Women’s nutritional status during pregnancy can have long-term effects on children’s brains and cognitive development. Folate and choline are methyl-donor nutrients and are important for closure of the neural tube during fetal development. They have also been associated with brain and cognitive development in children. Animal studies have observed that prenatal folate and choline supplementation is associated with better cognitive outcomes in offspring and that these nutrients may have interactive effects on brain development. Although some human studies have reported associations between maternal folate and choline levels and child cognitive outcomes, results are not consistent, and no human studies have investigated the potential interactive effects of folate and choline. This lack of consistency could be due to differences in the methods used to assess folate and choline levels, the gestational trimester at which they were measured, and lack of consideration of potential confounding variables. This narrative review discusses and critically reviews current research examining the associations between maternal levels of folate and choline during pregnancy and brain and cognitive development in children. Directions for future research that will increase our understanding of the effects of these nutrients on children’s neurodevelopment are discussed.
Keywords: pregnancy, choline, folate, children, neurodevelopment, brain development, cognitive development
1. Introduction
A mother’s nutritional status, as defined by the intake and utilization of nutrients during pregnancy has a significant effect on the development of the fetal brain and subsequent cognitive outcomes later in life [1]. To support optimal fetal development and offspring cognitive function, it is recommended that pregnant women consume adequate, but not deficient or excessive, sources of nutrients (e.g., vitamin D, folate, choline, and iron), in addition to other food components (e.g., fibre, fat, and proteins) [2]. Notably, nutrient demands increase during pregnancy as maternal body mass and metabolic demands increase in conjunction with the development of the placenta and fetus [3,4]. Approximately 28 days after conception, the fetal neural plate folds and fuses, and forms the neural tube, which allows for the development of the fetal brain [5,6]. Certain neurodevelopmental processes, such as neural cell proliferation and cell migration, occur solely during gestation; however, other neurodevelopmental processes, such as neurogenesis, synapse formation, and myelination, begin during gestation and continue into adolescence and early adulthood [6]. Significant refinement of neural connections begins 24 weeks after conception and continues into the perinatal period [6,7]. Further, peak synapse development and significant brain growth in humans occurs between 34 weeks post conception and 2 years of age [7,8]. Maternal intake and utilization of nutrients provide the essential building blocks to support numerous cellular processes during fetal development, including cellular proliferation, DNA synthesis, and neurotransmitter and hormone metabolism [9,10,11,12]. Some nutrients, such as iron and long-chain polyunsaturated fatty acids, also enable axon myelination, synaptogenesis, and neurotransmitter transmission [9,12]. Therefore, the availability of nutrients to the developing fetus is likely to have long-lasting impacts on both the physical development of the brain as well as children’s cognitive development.
Cognition refers to the mental processes involved in the acquisition, retention, and use of knowledge, and the foundational aspects of cognitive development include attention, processing speed, representational competence (i.e., the ability to manipulate a mental image of an object or idea), and memory [13,14]. Nutritional research studies often assess children’s cognitive abilities using tests of attention, speed of information processing, learning and memory, executive functions (e.g., inhibitory control, cognitive flexibility, and working memory), and intelligence [15,16]. Children’s early cognitive performance on these types of assessments are predictive of later academic achievement and level of educational attainment [17,18,19]. Supporting healthy cognitive development in children begins in utero, as multi-micronutrient supplementation during the gestational period has been shown to be associated with improved cognitive outcomes in children at one and two years of age [20,21].
Two nutrients in particular, folate and choline, have been linked to the prevention of neural tube defects and to both health and cognitive outcomes in children [9,22,23,24,25,26]. Both folate and choline are methyl-donor nutrients, which means that they have been shown to alter DNA methylation and can have long-lasting impacts on gene expression and neuronal function; however, their effects on the development of children’s brains and cognition, and the mechanisms by which these effects occur have not been clearly established [9,27]. The objectives of this review are to discuss the current state of knowledge regarding the associations between maternal levels of folate and choline during pregnancy and children’s neurodevelopment, outline the limitations and knowledge gaps in the current literature, and suggest directions for future research. To address these objectives, we initially searched PubMed and Google Scholar for relevant articles between September 2020 and November 2020. In September 2021, we reran the search to determine if any additional relevant articles had been published. The keywords used were choline, folate, pregnancy, prenatal, child outcomes, cognitive outcomes, cognitive development, levels, concentrations, intake, mechanism, brain development, exposure, neurodevelopment, serum, plasma, red blood cell, food frequency questionnaires, supplementation, maternal, fetal development, offspring, infant, child, memory, intelligence, diet, nutrition, nutrients, dietary sources, deficiency, exposure, and gestational. The identified articles and relevant studies cited in the references of these articles were critically reviewed and information from 121 studies was included in this narrative review.
2. Gestational Folate and Fetal Brain Development
Folate, or vitamin B-9, is a water-soluble vitamin complex that is an essential nutrient [28]. It cannot be synthesized de novo by the body and must be obtained either from diet or supplementation [28]. Dietary folate is a naturally occurring nutrient found in foods such as leafy green vegetables, legumes, egg yolk, liver, and citrus fruit, whereas folic acid is a synthetic dietary supplement present in fortified foods and vitamins [29,30]. To increase the intake of folic acid in the general population and reduce the rate of neural tube defects, fortification of grain-based food products such as flour, cereal, and pasta with folic acid has been mandated in Canada and the United States since 1998 [30,31,32,33]. Currently, over 80 countries have mandated fortification of foods with folic acid, and it is recommended that men and non-pregnant women consume 320 to 400 μg of folate per day [30,34].
Compared to folate requirements of non-pregnant women, it is recommended that pregnant women consume at least 400 to 800 μg of folate per day to ensure healthy maternal, placental, and fetal tissue growth [34,35,36,37]. The placenta extracts folic acid from maternal circulation and concentrates it into fetal circulation [35,38,39,40]. This results in fetal levels that are two to four times higher than maternal levels [39,41,42,43,44,45]. In the fetus, folate is critically important for cellular proliferation, neural stem cell proliferation and differentiation, decreasing apoptosis, and altering and maintaining DNA synthesis [3,9,35]. Folate deficiency during pregnancy can result in megaloblastosis (i.e., large cells with arrested nuclear maturation) and cell death, particularly in rapidly proliferating somatic cells [35]. Embryonic neural tube and neural crest cells are highly proliferative and folate deficiency during the period of neural tube closure (21 to 28 days post conception) predisposes the fetus to neural tube defects [23,46].
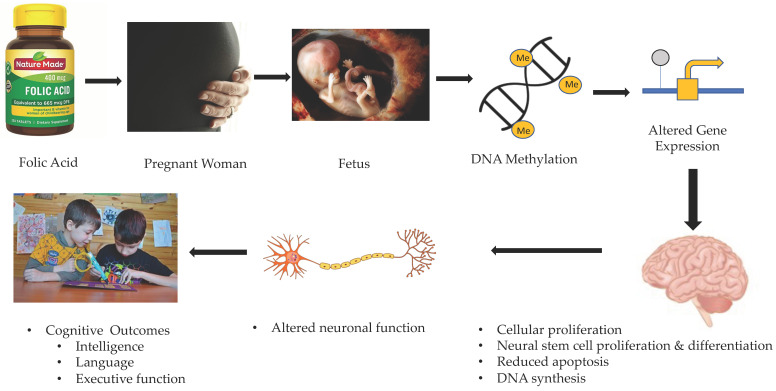
Given the ethical considerations regarding maternal nutrition during pregnancy, experimental research examining the effects of maternal folate deficiency on fetal brain development has been conducted in animals only. Craciunescu et al. found that maternal folate intake affected fetal forebrain progenitor cells during late gestation [47,48]. In these studies, pregnant mice were all fed the same standard diet containing 2 mg/kg of folic acid until day 11 of gestation and then folic acid was removed from the diets of the mice in the folate-deficient group [47,48]. Offspring of folate-deficient mice were found to have fewer replicating neural progenitor cells in the hippocampus, caudate, putamen, striatum, anterior and mid-posterior neocortex, and ventricular zones of the septum [47,48]. It was also found that apoptotic cells were more prevalent in the fetal septum and hippocampus of mice from mothers fed a folate-deficient diet as compared to the control mice [47,48]. Folic acid supplements before and during early pregnancy are essential for preventing neural tube defects; however, these animal studies suggest that folate availability throughout gestation is critically important for fetal brain development in both cortical and subcortical regions of the brain. Figure 1 provides a schema representing the potential mechanism of action through which folate is associated with children’s brain and cognitive development.
Figure 1.
Potential mechanism of action through which prenatal folate affects brain and cognitive outcomes in children.
3. Gestational Choline and Fetal Brain Development
Choline is also an essential nutrient but was only officially recognized as such in 1998 [49]. There are currently no mandates regarding choline supplementation of food in Canada or the United States [49,50,51]. Although choline is produced endogenously in the human liver, the amount of choline naturally produced is insufficient to meet the needs of the human body and thus adequate levels of choline can only be obtained through diet [52]. Animal food products such as beef, eggs, chicken, fish, and pork are major dietary sources of choline containing more than 60 mg of choline per 100 g [49,52,53,54]. Plant foods such as nuts, legumes, and cruciferous vegetables are also good dietary sources of choline containing at least 25 mg of choline per 100 g [53,54]. Choline can also be obtained through dietary supplements containing choline only, choline in combination with B-complex vitamins, or in some multivitamin products [54]. Choline dietary supplements typically contain between 10 mg and 250 mg of choline per dose and often provide choline in the form of choline bitartrate, phosphatidylcholine, or lecithin [54].
It is recommended that choline intake should increase from 425 mg/day in non-pregnant women to 450 mg/day in pregnant women [54]. Large amounts of choline from maternal diet are transferred across the placenta from the mother to the fetus [53,55,56,57]. Choline concentrations in the amniotic fluid are 10 times greater than in maternal blood, and plasma choline concentrations are 3 and 6 to 7 times greater in the umbilical cord and fetus, respectively, than they are in the maternal blood [44,45]. It is believed that choline plays a similar role to folate in brain development, including acting as a methyl donor in DNA methylation [9,51]. Like folate, choline is important for the prevention of neural tube defects as it is required for closure of the neural tube [9,22,58,59]. Choline also plays a significant role in hippocampal development and has been associated with the development of neural pathways and the expression of genes involved in memory processes [50,51].
In human diets, the most common dietary form of choline is phosphatidylcholine (PC); however, it can also be found as free choline, phosphocholine, sphingomyelin, glycerophosphocholine, and lysophosphatidylcholine [53]. Choline is a component of sphingomyelin, which is a constituent of the myelin sheath of nerve axons and facilitates efficient transmission of nerve signals [51,53]. As a methyl donor in DNA methylation, choline is thought to regulate the expression of genes involved in regulating synaptic plasticity, learning, and memory [50,51]. As with folate, choline also plays a role in the conversion of homocysteine to methionine and helps regulate homocysteine concentrations in the body [55]. Further, it acts as a major constituent of phospholipids in cell membranes and signaling lipids in cells and is involved in the biosynthesis of lipoproteins [9,51,53,55]. It is also a constituent of the neurotransmitter acetylcholine (ACh), which acts in both the peripheral and central nervous systems [9,53,55,60]. ACh influences processes in the developing brain including progenitor cell proliferation and differentiation, neurogenesis, gliogenesis, cell survival, morphology and migration, and synaptic plasticity, and supports the development of the hippocampus [53]. ACh released from cholinergic neurons that extend into the hippocampus and cerebral cortex from the basal forebrain also regulates multiple processes including attention, learning and memory [61].
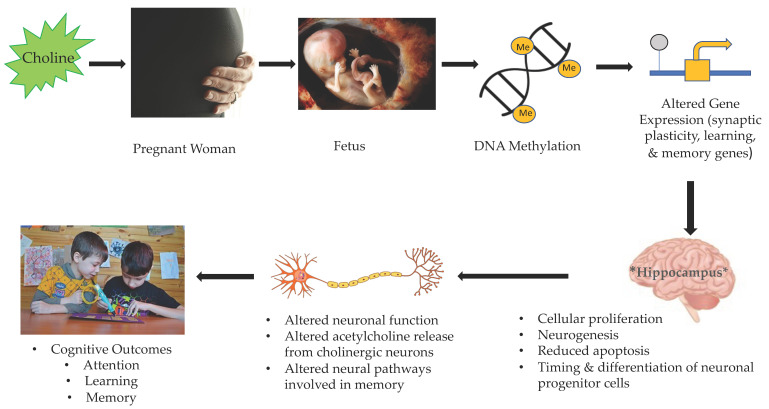
Like experimental studies examining the effects of folate deficiency on brain development in mice, animal models have also shown that gestational choline levels have significant effects on fetal brain development. Studies have found that maternal choline deficiency resulted in decreased progenitor cell proliferation and neurogenesis, and increased apoptosis in the fetal hippocampus [56,62,63]. Further, maternal choline supplementation stimulated hippocampal cell division and enhanced hippocampal neurogenesis [56,64]. Other studies have found that modification of choline intake levels in pregnant rats resulted in changes in the timing of migration and differentiation of neuronal progenitor cells in fetal hippocampal regions [62,65]. Thus, to support healthy brain differentiation and development, particularly in regions important for learning and memory such as the hippocampus, research suggests that it is important for a fetus to have access to adequate amounts of choline throughout gestation. Figure 2 provides a schema representing the potential mechanism of action through which choline is associated with brain and cognitive development in children.
Figure 2.
Potential mechanism of action through which prenatal choline affects brain and cognitive outcomes in children.
4. Potential Interactions between Gestational Folate and Choline
Few studies have examined the interactive effects of folate and choline on fetal brain development. Craciunescu et al. found choline supplementation may modify the effects that dietary folate availability has on neural progenitor cells in the fetal forebrain during late gestation [48]. Specifically, they reported that the rate of mitosis in folate-deficient choline-supplemented (FDCS) mice (i.e., 4.95 g choline chloride/kg diet, 0.0 mg folic acid/kg diet) was less than that of control mice who were supplemented with standard amounts of folate and choline (i.e., 1.1 g choline chloride/kg diet, 2 mg folic acid/kg diet) but greater than that of folate-deficient mice (i.e., 1.1 g choline chloride/kg diet, 0.0 mg folic acid/kg diet) [48]. Further, hippocampal apoptosis rates in FDCS mice were found to be significantly lower than those of folate-deficient mice, but the same as those in control mice [48]. As both folate and choline are methyl-donor nutrients that influence neurogenesis and apoptosis, it is possible that choline supplementation might mitigate the effects of folate deficiency on brain development, and this may be mediated by epigenetic events (i.e., methylation of DNA and histones important for epigenetic control of gene expression) [66,67]. Investigations of the interactive effects of folate and choline is important because they could provide a better understanding of how these nutrients work together to support brain and cognitive development. Future research may also provide further evidence that maternal gestational supplementation of these two nutrients is critical for optimal fetal brain development and subsequent cognitive outcomes in children.
5. The Current State of Knowledge on the Association between Maternal Folate and Offspring Cognitive Outcomes
Animal studies (see Table 1) suggest a relationship between maternal folate intake during pregnancy and offspring brain development; however, a limited number of studies have examined the effects of maternal folate levels on cognition. Jadavji et al. found that maternal folate deficiency in mice (i.e., 0.3 mg folic acid/kg diet) was associated with short-term memory impairment in offspring based on their poorer performance on the novel object recognition task and the Y-maze test compared to offspring of mice fed folate sufficient diets (i.e., 2 mg folic acid/kg diet) [68]. Additionally, Ferguson et al. found that maternal folate deficiency (e.g., 400 nmol of folic acid/kg diet) in mice produced offspring who exhibited more anxiety-related behavior on the elevated plus maze test [69]. These animal studies suggest that maternal gestational folate deficiency is associated with poorer cognitive and behavioural outcomes in offspring and that gestational folate availability is important for offspring cognitive and behavioural development.
Table 1.
Characteristics of the animal and human studies that examined associations between maternal prenatal folate and offspring cognitive outcomes.
| Identification | Location | Sample | Method Used to Determine Prenatal Folate Level | Maternal Folate Assessment | Dose of Folic Acid Intake or Mean Levels in Blood | Offspring’s/Children’s Age at the Time of Assessment | Offspring’s/Children’s Assessment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Animal Studies | ||||||||
| [48] Craciunescu et al., 2010 | United States | Fetal mice | Diet supplementation | Embryonic days 11–17 | Control diet: 2 mg folic acid/kg diet; 1.1 g choline chloride/kg diet |
Embryonic day 17 | Histological and immune-histochemical assays of fetal brain regions of interest | Folate-deficient mice had fewer neural progenitor cells undergoing mitosis in the septum and greater apoptosis rates in the septum and hippocampus compared to the control mice |
| Folate-deficient diet: 10.0 mg folic acid/kg diet, 1 g choline chloride/kg diet | ||||||||
| Folate-deficient choline-supplemented diet: 0.0 mg folic acid/kg diet, 4.95 g choline chloride/kg diet | ||||||||
| [68] Jadavji et al., 2015 | Canada | Methylenetetrahydrofolate reductase (MTHFR)-deficient male mice | Diet supplementation | 6 weeks prior to breeding until the end of lactation | Folate-deficient diet: 0.3 mg folic acid/kg diet | 3-week-old male mice | Novel object recognition task, Y-maze task | Maternal folate deficiency in mice was associated with short-term memory impairment in offspring |
| Folate sufficient diet: 2 mg folic acid/kg diet | ||||||||
| [69] Ferguson et al., 2005 | United States | 20 female mice per group. 4 males and 4 females/litter were retained for initial behavioral testing. 1 male and 1 female pup/litter were used in postweaning behavioral testing | Diet supplementation | 8 weeks prior to breeding until the end of gestation | Folate-deficient diets: (1) 400 nmol folic acid/kg diet and (2) 600 nmol folic acid/kg diet |
Postnatal days 4–83 | Righting reflex, negative geotaxis, forelimb hanging, motor coordination using Rotarod apparatus, open field activity, elevated plus maze | Maternal folate deficiency in mice produced offspring who exhibited more anxiety-related behavior in the elevated plus maze |
| Folate sufficient diet: 1200 nmol folic acid/kg diet | ||||||||
| Human Studies | ||||||||
| [70] Julvez et al., 2009 | Spain | 420 mother-child pairs | Maternal self-report questionnaire used to determine whether supplements containing folic acid were taken | First trimester | No or yes | 4 years of age | McCarthy Scales of Children’s Abilities, California Preschool Social Competence Scale | Verbal, motor-executive function, verbal-executive function, social competence and inattention symptom scores were positively associated with maternal use of folic acid supplements |
| [71] Wehby and Murray 2008 | United States | 6774 mother-child pairs | National survey that included data as to whether supplements containing folic acid had been taken | First trimester | No or yes | 3 years of age | 16 items from the Denver Developmental Screening Test | Prenatal folic acid supplementation had a positive effect on children’s overall cognitive and gross motor development |
| [72] McNulty et al., 2019 | United Kingdom | 37 mother-child pairs in the treatment group and 33 mother-child pairs in the placebo group | Supplements or placebos distributed to the women in 7-day pillboxes | 14 weeks gestation until the birth of the child | 400 ug/day supplement containing folic acid | 7 years of age | Wechsler Preschool and Primary Scale of Intelligence, Third Edition, UK Edition (WPPSI-III) | Children of mothers who were in the treatment group had higher scores on the WPPSI-III compared to the children of mothers who were given a placebo |
| Placebo containing no folic acid | ||||||||
| [74] Chatzi et al., 2012 | Greece | 553 mother-child pairs | A questionnaire was administered by a trained research nurse that asked whether the women had taken a folic acid supplement since they became pregnant. Supplement users reported the brand name, the dose, and the frequency of intake, which was converted into a measure of daily intake. | 14–18 weeks gestation | No folic acid intake from supplements | 18 months of age | Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) | Children of mothers who reported taking a daily supplement of 5 mg of folic acid or more had a 5 unit increase on receptive communication and a 3.5 unit increase in expressive communication |
| Daily intake of 5 mg of folic acid from supplements | ||||||||
| Daily intake of folic acid from supplements higher than 5 mg | ||||||||
| [75] Chen et al., 2021 | China | 32 cohort studies and 7 case–control studies | Systematic review and meta-analysis of research articles that examined the association between prenatal folic acid supplementation and postnatal neurodevelopmental outcomes. All studies discussed folic acid supplementation only | During pregnancy | Varied by study; some reported whether or not women were supplemented; others reported specific supplementation levels. | 18 months to 17 years |
Varied by study; different measures used to assess intelligence, risk of autistic traits, ADHD, behavior, language, and psychomotor problems | Appropriate maternal folic acid supplementation may have positive effects on children’s intelligence and development, and reduce the risk of autism traits, ADHD, and behavioral and language problems |
| [76] Villamor et al., 2012 | United States | 1210 mother-child pairs | Food frequency questionnaires | First and second trimester | Mean estimated folate intake of 949 ± 390 ug/day | 3 years of age | Peabody Picture Vocabulary Test, Third Edition (PPVT-III), Wide Range Assessment of Visual Motor Abilities | For each 600 μg per day increase in total folate intake during the first trimester of pregnancy there was a 1.6-point increase in scores on the PPVT-III in the children |
| [77] Boeke et al., 2013 | United States | 895 mother-child pairs | Food frequency questionnaires | First and second trimesters | Mean daily estimated folate intake of 972 ug/day in the first trimester and 1268 ug/day in the second trimesters | 7 years of age | Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), Kaufman Brief Intelligence Test, Second Edition (KBIT-2) |
No associations were found between maternal folate intake and child cognitive outcomes |
| [85] Veena et al., 2010 | India | 536 mother-child pairs | Maternal plasma/serum folate concentrations from blood samples | 28–32 weeks gestation | Mean plasma/serum folate concentrations of 34.7 nmol/L | 9 or 10 years of age | Kaufman Assessment Battery for Children | Positive association between maternal plasma/serum folate concentrations and children’s performance on the Kaufman Assessment Battery for Children |
| [88] Ars et al., 2019 | Netherlands | 256 mother-child pairs (62 in the low folate group and 194 in the normal folate group) | Maternal plasma/serum folate concentrations from venous blood samples | First trimester | Low folate group: plasma/serum folate levels below 8 nmol/L | 6 years of age | Neuroimaging and NEPSY-II-NL | Low maternal plasma/serum folate concentrations below 8 nmol/L were associated with smaller total brain volume and poorer language and visuospatial skills in children |
| Normal folate group: plasma/serum folate levels above 8 nmol/L | ||||||||
| [89] Wu et al., 2012 | United States | 154 mother-child pairs | Food frequency questionnaires and maternal plasma/serum folate concentrations from blood samples | 16 weeks gestation | Mean plasma/serum folate concentrations of 36.4 nmol/L | 18 months of age | Bayley Scales of Infant Development, Third Edition (Bayley-III) | No association found between maternal plasma/serum folate concentrations and child cognitive function |
| [90] Tamura et al., 2005 | United States | 355 mother-child pairs | Maternal red blood cell folate concentrations from blood samples | Second and third trimesters | Mean red blood cell folate concentrations were 873 nmol/L, 1070 nmol/L, and 1096 nmol/L at 19, 26, and 37 weeks gestation, respectively | 5 years of age | Differential Ability Scales, Visual and Auditory Sequential Memory Tests, Knox Cube Test, Gross Motor Scale, and Grooved Pegboard Test | No association found between maternal red blood cell folate concentrations and child cognitive function |
Human studies (see Table 1) have also examined the association between maternal folate levels during pregnancy and child cognitive outcomes; however, various methods have been used to measure maternal folate levels. Several studies considered maternal use of folic acid supplements only during pregnancy. Julvez et al. found that in four-year-old children, verbal, motor- executive function, and verbal-executive function scores on the McCarthy Scales of Children’s Abilities, and social competence and inattention symptom scores on the California Preschool Social Competence Scale were positively associated with maternal use of folic acid supplements during the first trimester of pregnancy [70]. Similarly, Wehby and Murray assessed the effect of using folic acid supplements at conception and/or during the first trimester of pregnancy on cognitive development at three years of age using 16 items from the Denver Developmental Screening Test and found that prenatal folic acid supplementation had a positive effect on children’s overall cognitive and gross motor development [71]. A study conducted in the United Kingdom reported that seven-year-old children of mothers who received 400 μg folic acid per day during the second and third trimester of pregnancy had higher scores on the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) as compared to the children of mothers who were given a placebo [72]. Further, compared to a nationally representative sample of children seven years of age, the children of the folate-supplemented mothers had higher verbal, performance, general language, and Full-Scale IQ scores on the WPPSI-III [72]. It is notable that the folic acid supplement given to the women in this study was at the low end of the recommended range for folic acid intake for pregnant women (e.g., 400–800 μg/day) [34,36,37,72,73]. In another study, Chatzi et al. examined neurodevelopmental outcomes on the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) in 18-month-old children of mothers who reported taking daily supplements of 5000 μg of folic acid or more throughout pregnancy compared to children of mothers who did not use a folic acid supplement throughout pregnancy [74]. They found that children of mothers who reported taking a daily supplement had a 5 unit increase on receptive communication and a 3.5 unit increase in expressive communication on the Bayley-III scales [74]. The maternal folic acid intake of the women in this study was well above the recommended intake for pregnant women (e.g., 400–800 ug/day) [34,36,37,73,74]. Finally, a recent meta-analysis conducted by Chen et al., which investigated the effect of maternal folic acid supplementation on children’s neurodevelopment, concluded that appropriate maternal folic acid supplementation may have positive effects on children’s intelligence and development and reduce the risk of language problems, ADHD, autism traits, and behavioral problems [75]. Cumulatively, the research evidence suggests that maternal folic acid supplementation, even at the lower end of the recommended range throughout pregnancy, is an important predictor of children’s cognitive outcomes. However, in the majority of these studies, women’s actual folate levels were not measured and folate intake from their diets was not considered.
In addition to the above supplementation studies, other studies have used food frequency questionnaires (FFQs) to estimate levels of maternal prenatal folic acid and examine associations with cognitive outcomes. For example, Villamor et al. found that for each 600 μg per day increase in total folate intake during the first trimester of pregnancy assessed using FFQs there was a 1.6-point increase in scores on the Peabody Picture Vocabulary Test, Third Edition (PPVT-III) in three-year-old children [76]. The women in this study had a mean estimated folate intake of 949 ± 390 μg/day, which was well above the recommended daily intake for pregnant women [76]. In contrast, Boeke et al. did not find an association between increased folate intake assessed using FFQs during the first and second trimesters of pregnancy and higher scores on the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2) or the Kaufman Brief Intelligence Test, Second Edition (KBIT-2) in children at age seven [77]. The women in this study had a mean daily estimated folate intake of 972 ± 392 μg/day and 1268 ± 381 μg/day in the first and second trimesters of pregnancy, respectively, which were well above the recommended intake levels for pregnant women [77]. The results of these studies suggest that maternal folic acid intake may be an important predictor of some areas of cognitive development (e.g., language) but not of other areas (e.g., memory and intelligence). The use of FFQs to quantify maternal folic acid intake accounts for most dietary sources of folic acid and thus provides an accurate estimation of women’s actual folate intakes; however, there can be variability among FFQs in the dietary sources of folic acid considered and participants’ recall can limit the accuracy of this method [78,79].
Studies have also measured maternal folate concentrations (i.e., plasma/serum folate, red blood cell (RBC) folate) directly from blood samples collected during pregnancy. Although plasma/serum and RBC folate concentrations are both associated with folic acid intake [20,21], they result from different biologic processes and are not interchangeable [80,81]. Plasma/serum folate concentrations reflect very recent intake, whereas RBC folate concentrations reflect the long-term average of intake over the life span of red blood cells and folate stores in the liver [82]. As noted above, it is recommended that pregnant women should consume at least 400 to 800 μg of folate per day; however, a corresponding plasma/serum folate level has not been established [34,35,36,37,83,84]. Therefore, the results of studies that have examined the associations between plasma/serum and RBC folate levels in maternal blood and child outcomes may not be comparable. A study in India that measured maternal plasma/serum folate concentrations at 28 to 32 weeks’ gestation (i.e., third trimester) reported a positive association with children’s performance on a test of cognitive function (i.e., Kaufman Assessment Battery for Children) [85]. The women in this study had mean plasma/serum folate concentrations of 34.7 ± 19.2 nmol/L, which were within the normal range (i.e., 7–46 nmol/L) for non-pregnant women [34,85,86,87]. Ars et al. found that low plasma/serum folate concentrations below 8 nmol/L during the first trimester of pregnancy were associated with smaller total brain volume and poorer language and visuospatial skills on the NEPSY-II-NL in children at six years of age [88]. In contrast, maternal plasma/serum folate concentrations at 16 and 36 weeks gestation (i.e., second and third trimesters) were not associated with scores on the Bayley-III at 18 months of age [89]. The women in this study had mean plasma/serum folate concentrations of 36.4 ± 8.08 nmol/L at 16 weeks gestation, which was in the normal range for non-pregnant women [34,86,87,89]. Tamura et al. examined the association between RBC folate status during the second and third trimesters of pregnancy and children’s neurodevelopment at age five years and found no associations [90]. Neurodevelopment was assessed using the Differential Ability Scales, Visual and Auditory Sequential Memory Tests, Knox Cube Test, Gross Motor Scale, and Grooved Pegboard Test [90]. In this study, the women’s mean RBC folate concentrations were 873 nmol/L, 1070 nmol/L, and 1096 nmol/L at 19, 26, and 37 weeks gestation, respectively, which were slightly above or slightly below the recommended RBC folate concentration of 906 nmol/L for pregnant women [34,37,90]. Like studies that used FFQs, which estimate recent folate levels, there was some support that maternal plasma/serum folate levels were associated with children’s cognitive outcomes. However, both FFQs and plasma/serum folate assess a woman’s folate levels at a given time point. They are not predictive of folate levels across pregnancy as levels may vary depending on when the blood sample was taken [91,92,93]. In contrast, the Tamura et al. study, which investigated the association between RBC folate (which is reflective of longer-term intake), and child outcomes did not report any associations, suggesting that the inconsistencies in the findings of human studies may be due to differences in the measure used to assess folate levels.
6. The Current State of Knowledge on the Associations between Maternal Choline and Offspring Cognitive Outcomes
In rat and mice models (see Table 2), high maternal choline intake during pregnancy has been found to enhance the cognitive abilities of offspring; however, the optimal amount is not known [50]. Pregnant rats whose diet was supplemented with choline chloride were found to produce offspring with enhanced visuospatial memory skills as compared to non-choline-supplemented rats [94]. Choline supplementation in pregnant rats has also been linked to increased ability to retain a larger number of items in working memory, increased memory precision due to less proactive interference, an increased tendency for object exploration, and increased thresholds for implementing chunking strategies in offspring [94,95,96,97]. Further, choline supplementation in pregnant rats facilitates temporal memory and accelerates the maturation of relational cue processing in offspring [51,98]. These findings suggest that choline supplementation during pregnancy may be predictive of better cognitive function in offspring, particularly offspring memory.
Table 2.
Characteristics of the animal and human studies that examined the associations between maternal prenatal choline and offspring cognitive outcomes.
| Identification | Location | Sample | Method Used to Determine Prenatal Choline Level | Maternal Choline Assessment | Dose of Choline Intake or Mean Levels in Blood | Offspring’s/Children’s Age at the Time of Assessment | Offspring’s/Children’s Assessment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Animal Studies | ||||||||
| [94] Meck et al., 1988 | United States | 16 male albino rats from 8 pregnant female rats | Diet supplementation | 2 days prior to conception until birth of offspring | Control diet: 50 mM saccharin | 60 days of age | 12 and 18 arm radial maze task | Offspring of choline-supplemented rats had enhanced visuospatial memory skills as compared to offspring of non-choline-supplemented rats |
| Choline supplemented diet: 50 mM saccharin containing 5 mL/L choline chloride | ||||||||
| [95] Glenn et al., 2008 | United States | 20 rats (10 supplemented, 10 not supplemented) | Diet supplementation | Embryonic days 12–17 | Control diet: 1.1 g/kg choline chloride | 1 and 24 months of age | Open field exploration, novel object exploration, BrdU immuno-histochemistry and unbiased stereology to assess hippocampal plasticity markers | Prenatal choline supplementation was positively associated with exploratory behavior in rats and preserved some features of hippocampal plasticity in offspring over a 2-year time span |
| 8 male and 8 female pups from each experimental group were used in the behavioral tests | Choline-supplemented diet: 5 g/kg choline chloride | |||||||
| [96] Meck and Williams 1999 | United States | 30 offspring of 18 pregnant rats | Supplementation in drinking water | Embryonic days 11–18 | Choline-deficient diet: no choline or saccharin | 120 days of age | Twelve arm radial maze | Choline-supplemented and choline-deficient rats performed more accurately than control rats during spaced trials. Choline-supplemented rats displayed less proactive interference during massed trials compared to control and choline-deficient rats |
| Control diet: 50 mM saccharin | ||||||||
| Choline-supplemented diet: 50 mM saccharin and 25 mM choline chloride | ||||||||
| [97]. Meck and Williams 1997 | United States | 34 pregnant dams that produced 128 adult female rats | Supplementation in drinking water | Embryonic days 12–17 | Control diet: 50 mM saccharin | 60 days of age | 6, 12, 18, 24 radial arm mazes | Rats treated perinatally with choline had a higher threshold for implementing a chunking strategy in the radial arm maze tasks |
| Supplemented diet: 50 mM saccharin and 25 mM choline chloride | ||||||||
| [98] Meck and Williams 1997 | United States | 30 offspring of 18 pregnant rats | Supplementation in drinking water | Embryonic days 11–18 | Choline-deficient diet: no choline or saccharin | 4–6 months of age and 24–26 months of age | Peak-interval timing procedure | Prenatal choline supplementation was positively associated with cognitive function in offspring and choline deficiency was positively associated with impaired divided attention and accelerated age-related declines in temporal processing |
| Control diet: 50 mM saccharin | ||||||||
| Choline-supplemented diet: 50 mM saccharin and 25 mM choline chloride | ||||||||
| Human Studies | ||||||||
| [77] Boeke et al., 2013 | United States | 895 mother-child pairs | Food frequency questionnaires | First and second trimesters | Mean estimated daily choline intake was 328 mg/day |
7 years of age | Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), Kaufman Brief Intelligence Test, Second Edition (KBIT-2) |
Prenatal choline intake was positively associated with memory scores, but not intelligence scores |
| [89] Wu et al., 2012 | United States | 154 mother-child pairs | Food frequency questionnaires; measured maternal plasma-free choline concentrations from blood samples | 16 weeks gestation | Mean plasma-free choline concentration of the women was 7.07 umol/L; mean estimated daily choline intake was 383 mg/day | 18 months of age | Bayley Scales of Infant Development, Third Edition (Bayley-III) | Positive associations were found between maternal plasma free choline concentrations during pregnancy and infant cognitive development |
| [99] Caudill et al., 2018 | United States | 12 mother-child pairs in the control group and 12 mother-child pairs in the treatment group | Supplement mixed in juice consumed at the study facility | 27 weeks gestation until the birth of the offspring | 480 mg choline/day or 930 mg choline/day |
∼4, 7, 10, and 13 months of age |
Visual attention task | Infants born to women supplemented with 930 mg of choline chloride per day compared to infants of women, who received 480 mg of choline per day, displayed higher information processing speed |
| [100] Signore et al., 2008 | United States | 404 mother-child pairs | Maternal free and total serum choline concentrations from blood samples | 16 to 18 weeks, 24 to 26 weeks, 30 to 32 weeks, and 36 to 38 weeks gestation | Median free choline concentrations increased from 9.34 to 10.10 umol/L over the gestational period; median total choline concentrations increased from 2.57 to 2.75 mmol/L over the gestational period | 5 years of age | Wechsler Preschool and Primary Scales of Intelligence-Revised (WPPSI-R) | No associations found between maternal prenatal choline concentrations and offspring WISC-R Full-Scale IQ, visuospatial processing, or memory |
Enhanced cognitive function associated with increased maternal intake of choline during gestation has also been noted in human studies (see Table 2). The methods used to investigate choline levels in women were like those used in studies that examined maternal folate levels (i.e., supplementation, FFQs, measurements from blood samples). One study reported that infants born to women who were supplemented with 930 mg of choline chloride per day, compared to infants of women who were receiving 480 mg of choline per day, displayed improved information processing speed measured using a visual attention task [99]. It is of note that the choline levels in both groups were above adequate intake levels for pregnant women [34,54,99]. Another study used a FFQ to estimate choline intake during the first and second trimesters and found that prenatal choline intake was positively associated with memory scores on the WRAML2, but not intelligence scores on the KBIT-2 in seven-year-old children [77]. The women in this study had a mean estimated daily choline intake of 328 ± 63 mg/day, which was below the recommended adequate intake level for pregnant women (i.e., 450 mg/day) [34,54,77]. A study that directly measured maternal plasma-free choline at 16 weeks gestation reported positive associations with infant cognitive development at 18 months of age on the Bayley-III [89]. The mean plasma-free choline concentration of the women was 7.07 ± 1.78 umol/L and the mean estimated daily choline intake was 383 ± 98.6 mg/day at 16 weeks gestation, which was below the recommended adequate intake level for pregnant women [34,54,89]. However, in a study conducted by Signore et al., maternal gestational free and total serum choline concentrations measured at multiple timepoints throughout pregnancy (i.e., at 16 to 18 weeks, 24 to 26 weeks, 30 to 32 weeks, and 36 to 38 weeks gestation) were not associated with Wechsler Preschool and Primary Scales of Intelligence-Revised (WPPSI-R) Full-Scale IQ, visuospatial processing or memory in children at five years of age [100]. There are no recommendations regarding serum choline concentrations in maternal blood during pregnancy; therefore, no conclusions can be drawn as to whether these women were below, within, or above adequate recommended serum choline levels for pregnant women. The results from these studies suggest that maternal choline may be predictive of children’s memory, attention, and processing speed, even at low levels; however, future research is needed that examines these relationships when choline intake during pregnancy is at or above recommended intake levels. Additionally, the form of choline measured in studies should also be considered as one study found that plasma free choline concentrations were not predictive of dietary choline intake during pregnancy [101]. Considering the evidence from animal models supporting the importance of maternal choline for brain development and particularly hippocampal function, and that choline supplementation might mitigate the negative effects of folate deficiency on brain development, future research that examines folate and choline together and considers possible interactions between these two nutrients is needed.
7. Gaps in the Current State of Knowledge and Directions for Future Research
Animal studies have established that gestational availability of folate and choline influences numerous processes that are critical for healthy development of the fetal brain. The current literature also suggests that maternal folate and choline levels during pregnancy may be important predictors of children’s cognitive outcomes; however, there are inconsistencies in the findings that have yet to be resolved. These inconsistencies may be a result of several methodological constraints and confounding factors that have not been accounted for in the current literature.
One such methodological constraint is that the methods used to assess folate and choline levels in the current literature are not consistent across studies and some have inherent limitations that limit validity of the findings. For example, several studies have simply assessed whether women did or did not take folic acid or choline supplements during pregnancy and did not account for dietary sources of folate or choline [70,71,72,74,99]. FFQs were also used frequently to estimate women’s folate and choline intake; however, studies have shown that there can be significant variability between the dietary sources of these nutrients that are considered, and levels of these nutrients in the dietary sources [78,79]. This variability could influence the consistency of the results across studies that use this method. Other studies have assessed plasma/serum levels of folate or choline concentrations; however, there are currently no recommendations regarding their adequate or recommended levels in pregnant women [85,89,100]. Thus, classifying pregnant women as below, within, or above recommended ranges for these nutrients during pregnancy based on their concentrations in serum/plasma blood samples is not currently possible. The use of various methods to assess women’s folate and choline levels during pregnancy limit the possibility of comparing the results of the research conducted to date. Further, the lack of consistency in the findings reported in the human literature could be due to the different methods used to measure folate and choline levels. Future research is needed that investigates the biological relevance of folate and choline intake and plasma/serum levels and RBC folate levels on human brain development and their association with children’s cognitive outcomes.
Another potential methodological constraint is that the gestational trimester in which folate and choline levels are measured could be differentially associated with children’s cognitive outcomes [70]. Changes in concentrations of folate and choline throughout pregnancy and the impact of varying concentrations of these nutrients on children’s neurodevelopmental outcomes are areas of research in need of further investigation. Lewis et al. and Signore et al. both found that maternal free and total choline concentrations remain relatively constant throughout gestation [100,102]. However, Fayyaz et al. found that folate concentrations tend to increase throughout pregnancy [86]. Interestingly, Villamor et al. found that maternal folate intake during the first trimester, but not the second trimester of pregnancy, was associated with children’s scores on the PPVT-III at three years of age, suggesting that the timing of folate supplementation during pregnancy could influence children’s neurodevelopment [76]. However, the precise timing and the ideal concentrations of these nutrients at different time points during pregnancy to ensure optimal neurodevelopment are currently unknown.
Another issue of concern is that the research to date has examined a limited number of potential confounders. Demographic factors such as family socioeconomic status and maternal and paternal level of educational attainment have been associated with maternal nutrient use and children’s cognitive development [103,104]. Sample characteristics such as maternal pre-pregnancy body mass index (BMI) and child sex have also been found to be confounding factors that can influence children’s cognitive development [105,106]. Finally, other important micronutrients that are often supplemented during pregnancy such as vitamins B-12 and B-6, omega-3 long-chain polyunsaturated fatty acids, and iron have also been found to be associated with children’s cognitive outcomes [107,108,109,110,111,112,113,114,115]. These variables need to be considered as potential confounding factors when assessing the effects of folate and choline on children’s neurodevelopment.
In addition to the already identified methodological and analytical issues, it is important to note that there is an imbalance in the number of studies that considered the effects of maternal folate compared to maternal choline. More human studies have examined the association between maternal folate levels and children’s cognitive outcomes and no studies have investigated their interactive effects. The evidence from animal models suggesting that gestational choline supplementation may reduce the adverse effects of folate deficiency on fetal brain development, supports the conduct of human studies that investigate how these two nutrients interact to influence cognitive outcomes in children.
Further, limited research has investigated possible biological mechanisms (e.g., DNA methylation alterations) that may underlie these associations. It has been noted that folate and choline likely play similar roles in the development of the fetal brain and central nervous system and that their interaction may influence fetal brain development [9,48]. However, to date, no human studies have examined potential synergistic effects of maternal folate and choline levels during pregnancy and potential underlying biological mechanisms (i.e., DNA methylation) on children’s cognitive outcomes
One last point that needs to be considered in future research is the level of supplementation. Several studies have reported that supplementing with large amounts of folate or choline during pregnancy may result in adverse child health outcomes, including low birth weight and increased risk for developing autism spectrum disorder (ASD) or colitis [116,117,118,119,120,121]. However, there is little research on the association between very high maternal choline or folate supplementation and adverse child neurodevelopmental outcomes. Further, the precise levels above the daily recommended levels for folate and/or choline that would be harmful to children’s neurodevelopment have yet to be determined.
In summary, the findings from this narrative review suggest that future nutritional research examining the associations between maternal folate and choline levels and children’s cognitive outcomes should develop and use consistent and accurate methods to measure women’s folate and choline levels across the different trimesters of pregnancy and consider a wider range of potential confounding variables (e.g., demographic factors, levels of other nutrients) in order to reduce inconsistencies in results that could be attributable to discrepancies in methodology. Additionally, future research is needed that addresses the following issues: (a) What are the optimal intake levels/status of folate and choline needed during pregnancy to promote optimal child neurodevelopment? (b) Do the optimal levels/status vary across the different trimesters of pregnancy? and (c) Is there a risk to children’s neurodevelopment if maternal intake/status is above or below the optimal levels? Additionally, potential biological pathways (e.g., DNA methylation) should be examined to further understand the mechanistic basis for both folate’s and choline’s role in children’s brain and cognitive development and how these nutrients might interact within the context of early development to further support children’s neurodevelopment. It is important that future research address these methodological limitations and mechanistic questions to better understand how maternal folate, choline, and their interaction during pregnancy, influence brain and cognitive development in children.
8. Conclusions
Folate and choline are important methyl-donor nutrients that play critical roles in the development of the fetal brain. Animal studies indicate that gestational folate and/or choline levels are associated with structural and cellular alterations in various brain regions such as the cerebral cortex and hippocampus. The current human literature also suggests that maternal folate and choline concentrations during pregnancy may play a role in children’s cognitive development; however, there are some inconsistencies in published research. To address these inconsistencies, future research needs to use consistent methods to assess women’s folate and choline levels during pregnancy, and consider confounding factors (e.g., parental socioeconomic status, maternal pre-pregnancy BMI, infant birth weight, maternal levels of other micronutrients during pregnancy, gestational trimester), relative maternal folate and choline concentrations (e.g., below, at, or above recommended levels) and the potential synergistic effects that maternal folate and choline levels may have on fetal brain development and in turn children’s cognitive development.
Acknowledgments
Salary support was provided to Gillian England-Mason through a Postgraduate Fellowship in Health Innovation provided by Alberta Innovates, the Ministry of Economic Development, Trade and Tourism, and the Government of Alberta.
Author Contributions
Conceptualization, N.I., G.E.-M., C.J.F., D.D. and F.A.; methodology N.I., G.E.-M., C.J.F., D.D. and F.A.; writing—original draft preparation, N.I.; writing—review and editing, N.I., G.E.-M., C.J.F., D.D. and F.A.; supervision, G.E.-M., C.J.F., D.D. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mousa A., Naqash A., Lim S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients. 2019;11:443. doi: 10.3390/nu11020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kominiarek M.A., Rajan P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016;100:1199–1215. doi: 10.1016/j.mcna.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnton-Hill I., Mkparu U.C. Micronutrients in Pregnancy in Low- and Middle-Income Countries. Nutrients. 2015;7:1744–1768. doi: 10.3390/nu7031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker H., DeAngelis B., Holland B., Gittens-Williams L., Barrett T. Vitamin Profile of 563 Gravidas during Trimesters of Pregnancy. J. Am. Coll. Nutr. 2002;21:33–37. doi: 10.1080/07315724.2002.10719191. [DOI] [PubMed] [Google Scholar]
- 5.Couperus J.W., Nelson C.A. Blackwell Handbook of Early Childhood Development. John Wiley & Sons Ltd.; New York, NY, USA: 2006. Early Brain Development and Plasticity; pp. 85–105. [Google Scholar]
- 6.Raybaud C., Ahmad T., Rastegar N., Shroff M., Al Nassar M. The Premature Brain: Developmental and Lesional Anatomy. Neuroradiology. 2013;55:23–40. doi: 10.1007/s00234-013-1231-0. [DOI] [PubMed] [Google Scholar]
- 7.Levitt P. Structural and Functional Maturation of the Developing Primate Brain. J. Pediatr. 2003;143:35–45. doi: 10.1067/S0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 8.Huttenlocher P.R., Dabholkar A.S. Regional Differences in Synaptogenesis in Human Cerebral Cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Nyaradi A., Li J., Hickling S., Foster J., Oddy W.H. The Role of Nutrition in Children’s Neurocognitive Development, from Pregnancy through Childhood. Front. Hum. Neurosci. 2013;7:97. doi: 10.3389/fnhum.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X.-M., Huang G.-W., Tian Z.-H., Ren D.-L., Wilson J.X. Folate Stimulates ERK1/2 Phosphorylation and Cell Proliferation in Fetal Neural Stem Cells. Nutr. Neurosci. 2009;12:226–232. doi: 10.1179/147683009X423418. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J., Thompson C.B. Metabolic Regulation of Cell Growth and Proliferation. Nat. Rev. Mol. Cell Biol. 2019;20:436–450. doi: 10.1038/s41580-019-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgieff M.K. Nutrition and the Developing Brain: Nutrient Priorities and Measurement. Am. J. Clin. Nutr. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 13.Ardila A. Development of Metacognitive and Emotional Executive Functions in Children. Appl. Neuropsychol. Child. 2013;2:82–87. doi: 10.1080/21622965.2013.748388. [DOI] [PubMed] [Google Scholar]
- 14.Wilks T., Gerber R.J., Erdie-Lalena C. Developmental Milestones: Cognitive Development. Pediatr. Rev. 2010;31:364–367. doi: 10.1542/pir.31.9.364. [DOI] [PubMed] [Google Scholar]
- 15.Campbell J.M., Brown R.T., Cavanagh S.E., Vess S.F., Segall M.J. Evidence-Based Assessment of Cognitive Functioning in Pediatric Psychology. J. Pediatr. Psychol. 2008;33:999–1014. doi: 10.1093/jpepsy/jsm138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes D., Bryan J. The Assessment of Cognitive Performance in Children: Considerations for Detecting Nutritional Influences. Nutr. Rev. 2003;61:413–422. doi: 10.1301/nr.2003.dec.413-422. [DOI] [PubMed] [Google Scholar]
- 17.Tikhomirova T., Malykh A., Malykh S. Predicting Academic Achievement with Cognitive Abilities: Cross-Sectional Study across School Education. Behav. Sci. 2020;10:158. doi: 10.3390/bs10100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesayan A., Amani M., Asadi Gandomani R. Cognitive Profile of Children and Its Relationship with Academic Performance. Basic Clin. Neurosci. 2019;10:165–174. doi: 10.32598/bcn.9.10.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strenze T. Intelligence and Socioeconomic Success: A Meta-Analytic Review of Longitudinal Research. Intelligence. 2007;35:401–426. doi: 10.1016/j.intell.2006.09.004. [DOI] [Google Scholar]
- 20.Li Q., Yan H., Zeng L., Cheng Y., Liang W., Dang S., Wang Q., Tsuji I. Effects of Maternal Multimicronutrient Supplementation on the Mental Development of Infants in Rural Western China: Follow-up Evaluation of a Double-Blind, Randomized, Controlled Trial. Pediatrics. 2009;123:e685–e692. doi: 10.1542/peds.2008-3007. [DOI] [PubMed] [Google Scholar]
- 21.He Y., Gao J., Wang T., Liu C., Luo R. The Association between Prenatal Micronutrient Supplementation and Early Development of Children under Age Two: Evidence from Rural Guizhou, China. Child. Youth Serv. Rev. 2020;112:104929. doi: 10.1016/j.childyouth.2020.104929. [DOI] [Google Scholar]
- 22.Shaw G.M., Finnell R.H., Blom H.J., Carmichael S.L., Vollset S.E., Yang W., Ueland P.M. Choline and Risk of Neural Tube Defects in a Folate-Fortified Population. Epidemiol. Camb. Mass. 2009;20:714–719. doi: 10.1097/EDE.0b013e3181ac9fe7. [DOI] [PubMed] [Google Scholar]
- 23.Bestwick J.P., Huttly W.J., Morris J.K., Wald N.J. Prevention of Neural Tube Defects: A Cross-Sectional Study of the Uptake of Folic Acid Supplementation in Nearly Half a Million Women. PLoS ONE. 2014;9:e89354. doi: 10.1371/journal.pone.0089354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholl T.O., Hediger M.L., Schall J.I., Khoo C.S., Fischer R.L. Dietary and Serum Folate: Their Influence on the Outcome of Pregnancy. Am. J. Clin. Nutr. 1996;63:520–525. doi: 10.1093/ajcn/63.4.520. [DOI] [PubMed] [Google Scholar]
- 25.Czeizel A.E. Reduction of Urinary Tract and Cardiovascular Defects by Periconceptional Multivitamin Supplementation. Am. J. Med. Genet. 1996;62:179–183. doi: 10.1002/(SICI)1096-8628(19960315)62:2<179::AID-AJMG12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Tolarova M. Periconceptional Supplementation with Vitamins and Folic Acid to Prevent Recurrence of Cleft Lip. Lancet Lond. Engl. 1982;2:217. doi: 10.1016/S0140-6736(82)91063-7. [DOI] [PubMed] [Google Scholar]
- 27.McKee S.E., Reyes T.M. Effect of Supplementation with Methyl-Donor Nutrients on Neurodevelopment and Cognition: Considerations for Future Research. Nutr. Rev. 2018;76:497–511. doi: 10.1093/nutrit/nuy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naninck E.F.G., Stijger P.C., Brouwer-Brolsma E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019;10:502–519. doi: 10.1093/advances/nmy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan Y.-M., Bailey R., O’Connor D.L. Folate. Adv. Nutr. 2013;4:123–125. doi: 10.3945/an.112.003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partearroyo T., de Samaniego-Vaesken M.L., Ruiz E., Olza J., Aranceta-Bartrina J., Gil Á., González-Gross M., Ortega R.M., Serra-Majem L., Varela-Moreiras G. Dietary Sources and Intakes of Folates and Vitamin B12 in the Spanish Population: Findings from the ANIBES Study. PLoS ONE. 2017;12:e0189230. doi: 10.1371/journal.pone.0189230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray J.G. Folic Acid Food Fortification in Canada. Nutr. Rev. 2004;62:S35–S39. doi: 10.1111/j.1753-4887.2004.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 32.Government of Canada C.F.I.A. Nutrient Content Claims: Reference Information. [(accessed on 3 October 2021)]. Available online: https://inspection.canada.ca/food-label-requirements/labelling/industry/nutrient-content/reference-information/eng/1389908857542/1389908896254?chap=1%20%20https://www.inspection.gc.ca/food-label-requirements/labelling/industry/nutrient-content/reference-information/eng/1389908857542/1389908896254?chap=1.
- 33.Health Canada Regulations Amending the Food and Drug Regulations (1066) Can. Gaz. Part 1. 1997;131:3702–3737. [Google Scholar]
- 34.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US); Washington, DC, USA: 1998. The National Academies Collection. Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 35.Antony A.C. In Utero Physiology: Role of Folic Acid in Nutrient Delivery and Fetal Development. Am. J. Clin. Nutr. 2007;85:598S–603S. doi: 10.1093/ajcn/85.2.598S. [DOI] [PubMed] [Google Scholar]
- 36.Viswanathan M., Treiman K.A., Kish-Doto J., Middleton J.C., Coker-Schwimmer E.J.L., Nicholson W.K. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:190–203. doi: 10.1001/jama.2016.19193. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization Guideline: Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects. World Health Organization: Geneva, Switzerland. [(accessed on 10 January 2022)]. Available online: https://apps.who.int/iris/handle/10665/161988.
- 38.Bisseling T.M., Steegers E.a.P., van den Heuvel J.J.M., Siero H.L.M., van de Water F.M., Walker A.J., Steegers-Theunissen R.P.M., Smits P., Russel F.G.M. Placental Folate Transport and Binding Are Not Impaired in Pregnancies Complicated by Fetal Growth Restriction. Placenta. 2004;25:588–593. doi: 10.1016/j.placenta.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Giugliani E.R., Jorge S.M., Gonçalves A.L. Serum and Red Blood Cell Folate Levels in Parturients, in the Intervillous Space of the Placenta and in Full-Term Newborns. J. Perinat. Med. 1985;13:55–59. doi: 10.1515/jpme.1985.13.2.55. [DOI] [PubMed] [Google Scholar]
- 40.Stark K.D., Pawlosky R.J., Sokol R.J., Hannigan J.H., Salem N., Jr. Maternal Smoking Is Associated with Decreased 5-Methyltetrahydrofolate in Cord Plasma. Am. J. Clin. Nutr. 2007;85:796–802. doi: 10.1093/ajcn/85.3.796. [DOI] [PubMed] [Google Scholar]
- 41.Economides D.L., Ferguson J., Mackenzie I.Z., Darley J., Ware I.I., Holmes-Siedle M. Folate and Vitamin B12 Concentrations in Maternal and Fetal Blood, and Amniotic Fluid in Second Trimester Pregnancies Complicated by Neural Tube Defects. Br. J. Obstet. Gynaecol. 1992;99:23–25. doi: 10.1111/j.1471-0528.1992.tb14386.x. [DOI] [PubMed] [Google Scholar]
- 42.Guerra-Shinohara E.M., Morita O.E., Peres S., Pagliusi R.A., Sampaio Neto L.F., D’Almeida V., Irazusta S.P., Allen R.H., Stabler S.P. Low Ratio of S-Adenosylmethionine to S-Adenosylhomocysteine Is Associated with Vitamin Deficiency in Brazilian Pregnant Women and Newborns. Am. J. Clin. Nutr. 2004;80:1312–1321. doi: 10.1093/ajcn/80.5.1312. [DOI] [PubMed] [Google Scholar]
- 43.Obeid R., Munz W., Jäger M., Schmidt W., Herrmann W. Biochemical Indexes of the B Vitamins in Cord Serum Are Predicted by Maternal B Vitamin Status. Am. J. Clin. Nutr. 2005;82:133–139. doi: 10.1093/ajcn/82.1.133. [DOI] [PubMed] [Google Scholar]
- 44.Molloy A.M., Mills J.L., Cox C., Daly S.F., Conley M., Brody L.C., Kirke P.N., Scott J.M., Ueland P.M. Choline and Homocysteine Interrelations in Umbilical Cord and Maternal Plasma at Delivery. Am. J. Clin. Nutr. 2005;82:836–842. doi: 10.1093/ajcn/82.4.836. [DOI] [PubMed] [Google Scholar]
- 45.Thorand B., Pietrzik K., Prinz-Langenohl R., Hages M., Holzgreve W. Maternal and Fetal Serum and Red Blood Cell Folate and Vitamin B12 Concentrations in Pregnancies Affected by Neural Tube Defects. Z. Geburtshilfe Neonatol. 1996;200:176–180. [PubMed] [Google Scholar]
- 46.De Wals P., Tairou F., Van Allen M.I., Lowry R.B., Evans J.A., Van den Hof M.C., Crowley M., Uh S.-H., Zimmer P., Sibbald B., et al. Spina Bifida before and after Folic Acid Fortification in Canada. Birth Defects Res. Part A Clin. Mol. Teratol. 2008;82:622–626. doi: 10.1002/bdra.20485. [DOI] [PubMed] [Google Scholar]
- 47.Craciunescu C.N., Brown E.C., Mar M.-H., Albright C.D., Nadeau M.R., Zeisel S.H. Folic Acid Deficiency during Late Gestation Decreases Progenitor Cell Proliferation and Increases Apoptosis in Fetal Mouse Brain. J. Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 48.Craciunescu C.N., Johnson A.R., Zeisel S.H. Dietary Choline Reverses Some, but Not All, Effects of Folate Deficiency on Neurogenesis and Apoptosis in Fetal Mouse Brain. J. Nutr. 2010;140:1162–1166. doi: 10.3945/jn.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisel S.H., da Costa K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blusztajn J.K., Slack B.E., Mellott T.J. Neuroprotective Actions of Dietary Choline. Nutrients. 2017;9:815. doi: 10.3390/nu9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meck W.H., Williams C.L. Metabolic Imprinting of Choline by Its Availability during Gestation: Implications for Memory and Attentional Processing across the Lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/S0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 52.Wiedeman A.M., Barr S.I., Green T.J., Xu Z., Innis S.M., Kitts D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients. 2018;10:1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korsmo H.W., Jiang X., Caudill M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients. 2019;11:1823. doi: 10.3390/nu11081823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Office of Dietary Supplements—Choline. [(accessed on 3 October 2021)]; Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/
- 55.Zeisel S.H. Choline: Critical Role during Fetal Development and Dietary Requirements in Adults. Annu. Rev. Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craciunescu C.N., Albright C.D., Mar M.-H., Song J., Zeisel S.H. Choline Availability during Embryonic Development Alters Progenitor Cell Mitosis in Developing Mouse Hippocampus. J. Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun L.D., Cornford E.M., Oldendorf W.H. Newborn Rabbit Blood-Brain Barrier Is Selectively Permeable and Differs Substantially from the Adult. J. Neurochem. 1980;34:147–152. doi: 10.1111/j.1471-4159.1980.tb04633.x. [DOI] [PubMed] [Google Scholar]
- 58.Shaw G.M., Carmichael S.L., Yang W., Selvin S., Schaffer D.M. Periconceptional Dietary Intake of Choline and Betaine and Neural Tube Defects in Offspring. Am. J. Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 59.Zeisel S.H. The Fetal Origins of Memory: The Role of Dietary Choline in Optimal Brain Development. J. Pediatr. 2006;149:S131–S136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarter M., Parikh V. Choline Transporters, Cholinergic Transmission and Cognition. Nat. Rev. Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 61.Napoli I., Blusztajn J.K., Mellott T.J. Prenatal Choline Supplementation in Rats Increases the Expression of IGF2 and Its Receptor IGF2R and Enhances IGF2-Induced Acetylcholine Release in Hippocampus and Frontal Cortex. Brain Res. 2008;1237:124–135. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 62.Albright C.D., Friedrich C.B., Brown E.C., Mar M.H., Zeisel S.H. Maternal Dietary Choline Availability Alters Mitosis, Apoptosis and the Localization of TOAD-64 Protein in the Developing Fetal Rat Septum. Dev. Brain Res. 1999;115:123–129. doi: 10.1016/S0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 63.Mehedint M.G., Craciunescu C.N., Zeisel S.H. Maternal Dietary Choline Deficiency Alters Angiogenesis in Fetal Mouse Hippocampus. Proc. Natl. Acad. Sci. USA. 2010;107:12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glenn M.J., Gibson E.M., Kirby E.D., Mellott T.J., Blusztajn J.K., Williams C.L. Prenatal Choline Availability Modulates Hippocampal Neurogenesis and Neurogenic Responses to Enriching Experiences in Adult Female Rats. Eur. J. Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albright C.D., Tsai A.Y., Mar M.H., Zeisel S.H. Choline Availability Modulates the Expression of TGFbeta1 and Cytoskeletal Proteins in the Hippocampus of Developing Rat Brain. Neurochem. Res. 1998;23:751–758. doi: 10.1023/A:1022411510636. [DOI] [PubMed] [Google Scholar]
- 66.Niculescu M.D., Craciunescu C.N., Zeisel S.H. Dietary Choline Deficiency Alters Global and Gene-specific DNA Methylation in the Developing Hippocampus of Mouse Fetal Brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehedint M.G., Niculescu M.D., Craciunescu C.N., Zeisel S.H. Choline Deficiency Alters Global Histone Methylation and Epigenetic Marking at the Rel Site of the Calbindin 1 Gene. FASEB J. 2010;24:184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jadavji N.M., Deng L., Malysheva O., Caudill M.A., Rozen R. MTHFR Deficiency or Reduced Intake of Folate or Choline in Pregnant Mice Results in Impaired Short-Term Memory and Increased Apoptosis in the Hippocampus of Wild-Type Offspring. Neuroscience. 2015;300:1–9. doi: 10.1016/j.neuroscience.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson S.A., Berry K.J., Hansen D.K., Wall K.S., White G., Antony A.C. Behavioral Effects of Prenatal Folate Deficiency in Mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2005;73:249–252. doi: 10.1002/bdra.20111. [DOI] [PubMed] [Google Scholar]
- 70.Julvez J., Fortuny J., Mendez M., Torrent M., Ribas-Fitó N., Sunyer J. Maternal Use of Folic Acid Supplements during Pregnancy and Four-Year-Old Neurodevelopment in a Population-Based Birth Cohort. Paediatr. Perinat. Epidemiol. 2009;23:199–206. doi: 10.1111/j.1365-3016.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 71.Wehby G.L., Murray J.C. The Effects of Prenatal Use of Folic Acid and Other Dietary Supplements on Early Child Development. Matern. Child Health J. 2008;12:180–187. doi: 10.1007/s10995-007-0230-3. [DOI] [PubMed] [Google Scholar]
- 72.McNulty H., Rollins M., Cassidy T., Caffrey A., Marshall B., Dornan J., McLaughlin M., McNulty B.A., Ward M., Strain J.J., et al. Effect of Continued Folic Acid Supplementation beyond the First Trimester of Pregnancy on Cognitive Performance in the Child: A Follow-up Study from a Randomized Controlled Trial (FASSTT Offspring Trial) BMC Med. 2019;17:196. doi: 10.1186/s12916-019-1432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.US Preventive Services Task Force. Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., García F.A.R., Kemper A.R., Krist A.H., Kurth A.E., et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:183–189. doi: 10.1001/jama.2016.19438. [DOI] [PubMed] [Google Scholar]
- 74.Chatzi L., Papadopoulou E., Koutra K., Roumeliotaki T., Georgiou V., Stratakis N., Lebentakou V., Karachaliou M., Vassilaki M., Kogevinas M. Effect of High Doses of Folic Acid Supplementation in Early Pregnancy on Child Neurodevelopment at 18 Months of Age: The Mother-Child Cohort “Rhea” Study in Crete, Greece. Public Health Nutr. 2012;15:1728–1736. doi: 10.1017/S1368980012000067. [DOI] [PubMed] [Google Scholar]
- 75.Chen H., Qin L., Gao R., Jin X., Cheng K., Zhang S., Hu X., Xu W., Wang H. Neurodevelopmental Effects of Maternal Folic Acid Supplementation: A Systematic Review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2021:1–17. doi: 10.1080/10408398.2021.1993781. [DOI] [PubMed] [Google Scholar]
- 76.Villamor E., Rifas-Shiman S.L., Gillman M.W., Oken E. Maternal Intake of Methyl-Donor Nutrients and Child Cognition at 3 Years of Age. Paediatr. Perinat. Epidemiol. 2012;26:328–335. doi: 10.1111/j.1365-3016.2012.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boeke C.E., Gillman M.W., Hughes M.D., Rifas-Shiman S.L., Villamor E., Oken E. Choline Intake during Pregnancy and Child Cognition at Age 7 Years. Am. J. Epidemiol. 2013;177:1338–1347. doi: 10.1093/aje/kws395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouckaert K.P., Slimani N., Nicolas G., Vignat J., Wright A.J.A., Roe M., Witthöft C.M., Finglas P.M. Critical Evaluation of Folate Data in European and International Databases: Recommendations for Standardization in International Nutritional Studies. Mol. Nutr. Food Res. 2011;55:166–180. doi: 10.1002/mnfr.201000391. [DOI] [PubMed] [Google Scholar]
- 79.Messerer M., Johansson S.-E., Wolk A. The Validity of Questionnaire-Based Micronutrient Intake Estimates Is Increased by Including Dietary Supplement Use in Swedish Men. J. Nutr. 2004;134:1800–1805. doi: 10.1093/jn/134.7.1800. [DOI] [PubMed] [Google Scholar]
- 80.Yang Q., Cogswell M.E., Hamner H.C., Carriquiry A., Bailey L.B., Pfeiffer C.M., Berry R.J. Folic Acid Source, Usual Intake, and Folate and Vitamin B-12 Status in US Adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am. J. Clin. Nutr. 2010;91:64–72. doi: 10.3945/ajcn.2009.28401. [DOI] [PubMed] [Google Scholar]
- 81.Hopkins S.M., Gibney M.J., Nugent A.P., McNulty H., Molloy A.M., Scott J.M., Flynn A., Strain J.J., Ward M., Walton J., et al. Impact of Voluntary Fortification and Supplement Use on Dietary Intakes and Biomarker Status of Folate and Vitamin B-12 in Irish Adults. Am. J. Clin. Nutr. 2015;101:1163–1172. doi: 10.3945/ajcn.115.107151. [DOI] [PubMed] [Google Scholar]
- 82.Wu A., Chanarin I., Slavin G., Levi A.J. Folate Deficiency in the Alcoholic—Its Relationship to Clinical and Haematological Abnormalities, Liver Disease and Folate Stores. Br. J. Haematol. 1975;29:469–478. doi: 10.1111/j.1365-2141.1975.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 83.Chen M.-Y., Rose C.E., Qi Y.P., Williams J.L., Yeung L.F., Berry R.J., Hao L., Cannon M.J., Crider K.S. Defining the Plasma Folate Concentration Associated with the Red Blood Cell Folate Concentration Threshold for Optimal Neural Tube Defects Prevention: A Population-Based, Randomized Trial of Folic Acid Supplementation. Am. J. Clin. Nutr. 2019;109:1452–1461. doi: 10.1093/ajcn/nqz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cordero A.M., Crider K.S., Rogers L.M., Cannon M.J., Berry R.J. Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects: World Health Organization Guidelines. MMWR Morb. Mortal. Wkly. Rep. 2015;64:421–423. [PMC free article] [PubMed] [Google Scholar]
- 85.Veena S.R., Krishnaveni G.V., Srinivasan K., Wills A.K., Muthayya S., Kurpad A.V., Yajnik C.S., Fall C.H. Higher Maternal Plasma Folate but Not Vitamin B-12 Concentrations during Pregnancy Are Associated with Better Cognitive Function Scores in 9-10 Year Old Children in South-India- J. Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fayyaz F., Wang F., Jacobs R.L., O’Connor D.L., Bell R.C., Field C.J. APrON Study Team Folate, Vitamin B12, and Vitamin B6 Status of a Group of High Socioeconomic Status Women in the Alberta Pregnancy Outcomes and Nutrition (APrON) Cohort. Appl. Physiol. Nutr. Metab. 2014;39:1402–1408. doi: 10.1139/apnm-2014-0181. [DOI] [PubMed] [Google Scholar]
- 87.Pfeiffer C.M., Johnson C.L., Jain R.B., Yetley E.A., Picciano M.F., Rader J.I., Fisher K.D., Mulinare J., Osterloh J.D. Trends in Blood Folate and Vitamin B-12 Concentrations in the United States, 1988 2004. Am. J. Clin. Nutr. 2007;86:718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 88.Ars C.L., Nijs I.M., Marroun H.E., Muetzel R., Schmidt M., Steenweg-de Graaff J., van der Lugt A., Jaddoe V.W., Hofman A., Steegers E.A., et al. Prenatal Folate, Homocysteine and Vitamin B12 Levels and Child Brain Volumes, Cognitive Development and Psychological Functioning: The Generation R Study. Br. J. Nutr. 2019;122:S1–S9. doi: 10.1017/S0007114515002081. [DOI] [PubMed] [Google Scholar]
- 89.Wu B.T.F., Dyer R.A., King D.J., Richardson K.J., Innis S.M. Early Second Trimester Maternal Plasma Choline and Betaine Are Related to Measures of Early Cognitive Development in Term Infants. PLoS ONE. 2012;7:e43448. doi: 10.1371/journal.pone.0043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tamura T., Goldenberg R.L., Chapman V.R., Johnston K.E., Ramey S.L., Nelson K.G. Folate Status of Mothers during Pregnancy and Mental and Psychomotor Development of Their Children at Five Years of Age. Pediatrics. 2005;116:703–708. doi: 10.1542/peds.2004-2189. [DOI] [PubMed] [Google Scholar]
- 91.Han Y.H., Yon M., Hyun T.H. Folate Intake Estimated with an Updated Database and Its Association to Blood Folate and Homocysteine in Korean College Students. Eur. J. Clin. Nutr. 2005;59:246–254. doi: 10.1038/sj.ejcn.1602065. [DOI] [PubMed] [Google Scholar]
- 92.Houghton L.A., Sherwood K.L., O’Connor D.L. How Well Do Blood Folate Concentrations Predict Dietary Folate Intakes in a Sample of Canadian Lactating Women Exposed to High Levels of Folate? An Observational Study. BMC Pregnancy Childbirth. 2007;7:25. doi: 10.1186/1471-2393-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marshall J.R. Methodologic and Statistical Considerations Regarding Use of Biomarkers of Nutritional Exposure in Epidemiology. J. Nutr. 2003;133:881S–887S. doi: 10.1093/jn/133.3.881S. [DOI] [PubMed] [Google Scholar]
- 94.Meck W.H., Smith R.A., Williams C.L. Pre- and Postnatal Choline Supplementation Produces Long-Term Facilitation of Spatial Memory. Dev. Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 95.Glenn M.J., Kirby E.D., Gibson E.M., Wong-Goodrich S., Mellott T.J., Blusztajn J.K., Williams C.L. Age-Related Declines in Exploratory Behavior and Markers of Hippocampal Plasticity Are Attenuated by Prenatal Choline Supplementation in Rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meck W.H., Williams C.L. Choline Supplementation during Prenatal Development Reduces Proactive Interference in Spatial Memory. Dev. Brain Res. 1999;118:51–59. doi: 10.1016/S0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 97.Meck W.H., Williams C.L. Perinatal Choline Supplementation Increases the Threshold for Chunking in Spatial Memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 98.Meck W.H., Williams C.L. Simultaneous Temporal Processing Is Sensitive to Prenatal Choline Availability in Mature and Aged Rats. Neuroreport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 99.Caudill M.A., Strupp B.J., Muscalu L., Nevins J.E.H., Canfield R.L. Maternal Choline Supplementation during the Third Trimester of Pregnancy Improves Infant Information Processing Speed: A Randomized, Double-Blind, Controlled Feeding Study. FASEB J. 2018;32:2172–2180. doi: 10.1096/fj.201700692RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Signore C., Ueland P.M., Troendle J., Mills J.L. Choline Concentrations in Human Maternal and Cord Blood and Intelligence at 5 y of Age. Am. J. Clin. Nutr. 2008;87:896–902. doi: 10.1093/ajcn/87.4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mujica M., Lewis E., Jacobs R., Letourneau N., Bell R., Field C., Lamers Y. Plasma Free Choline Concentration Did Not Reflect Dietary Choline Intake in Early and Late Pregnancy: Findings from the APrON Study. Curr. Dev. Nutr. 2020;4:1825. doi: 10.1093/cdn/nzaa067_052. [DOI] [Google Scholar]
- 102.Lewis E.D., Subhan F.B., Bell R.C., McCargar L.J., Curtis J.M., Jacobs R.L., Field C.J. APrON team Estimation of Choline Intake from 24 h Dietary Intake Recalls and Contribution of Egg and Milk Consumption to Intake among Pregnant and Lactating Women in Alberta. Br. J. Nutr. 2014;112:112–121. doi: 10.1017/S0007114514000555. [DOI] [PubMed] [Google Scholar]
- 103.de Neubourg E., Borghans L., Coppens K., Jansen M. Explaining Children’s Life Outcomes: Parental Socioeconomic Status, Intelligence and Neurocognitive Factors in a Dynamic Life Cycle Model. Child Indic. Res. 2018;11:1495–1513. doi: 10.1007/s12187-017-9481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arkkola T., Uusitalo U., Pietikäinen M., Metsälä J., Kronberg-Kippilä C., Erkkola M., Veijola R., Knip M., Virtanen S.M., Ovaskainen M.-L. Dietary Intake and Use of Dietary Supplements in Relation to Demographic Variables among Pregnant Finnish Women. Br. J. Nutr. 2006;96:913–920. doi: 10.1017/BJN20061929. [DOI] [PubMed] [Google Scholar]
- 105.Ardila A., Rosselli M., Matute E., Inozemtseva O. Gender Differences in Cognitive Development. Dev. Psychol. 2011;47:984–990. doi: 10.1037/a0023819. [DOI] [PubMed] [Google Scholar]
- 106.Duncan G.J., Lee K.T.H., Rosales-Rueda M., Kalil A. Maternal Age and Child Development. Demography. 2018;55:2229–2255. doi: 10.1007/s13524-018-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor R.M., Fealy S.M., Bisquera A., Smith R., Collins C.E., Evans T.-J., Hure A.J. Effects of Nutritional Interventions during Pregnancy on Infant and Child Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2017;9:1265. doi: 10.3390/nu9111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhatnagar S., Taneja S. Zinc and Cognitive Development. Br. J. Nutr. 2001;85:S139–S145. doi: 10.1079/BJN2000306. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez S., Urrechaga E., Llorente-Ballesteros M., Escanero J. Psychiatry and Neuroscience Update. Springer; Cham, Switzerland: New York, NY, USA: 2015. The Role of Iron and Other Trace Elements on Mental Development and Cognitive Function; pp. 157–179. [Google Scholar]
- 110.Amorós R., Murcia M., Ballester F., Broberg K., Iñiguez C., Rebagliato M., Skröder H., González L., Lopez-Espinosa M.-J., Llop S. Selenium Status during Pregnancy: Influential Factors and Effects on Neuropsychological Development among Spanish Infants. Sci. Total Environ. 2018;610–611:741–749. doi: 10.1016/j.scitotenv.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 111.Amorós R., Murcia M., González L., Soler-Blasco R., Rebagliato M., Iñiguez C., Carrasco P., Vioque J., Broberg K., Levi M., et al. Maternal Copper Status and Neuropsychological Development in Infants and Preschool Children. Int. J. Hyg. Environ. Health. 2019;222:503–512. doi: 10.1016/j.ijheh.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Zeeshan F., Bari A., Farhan S., Jabeen U., Rathore A.W. Correlation between Maternal and Childhood VitB12, Folic Acid and Ferritin Levels. Pak. J. Med. Sci. 2017;33:162–166. doi: 10.12669/pjms.331.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tran P.V., Kennedy B.C., Pisansky M.T., Won K.-J., Gewirtz J.C., Simmons R.A., Georgieff M.K. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency–Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus123. J. Nutr. 2016;146:484–493. doi: 10.3945/jn.115.227561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Min J.-Y., Min K.-B. The Folate-Vitamin B12 Interaction, Low Hemoglobin, and the Mortality Risk from Alzheimer’s Disease. J. Alzheimers Dis. JAD. 2016;52:705–712. doi: 10.3233/JAD-151095. [DOI] [PubMed] [Google Scholar]
- 115.Fuller N.J., Evans P.H., Howlett M., Bates C.J. The Effects of Dietary Folate and Zinc on the Outcome of Pregnancy and Early Growth in Rats. Br. J. Nutr. 1988;59:251–259. doi: 10.1079/BJN19880032. [DOI] [PubMed] [Google Scholar]
- 116.Huot P.S.P., Dodington D.W., Mollard R.C., Reza-López S.A., Sánchez-Hernández D., Cho C.E., Kuk J., Ward W.E., Anderson G.H. High Folic Acid Intake during Pregnancy Lowers Body Weight and Reduces Femoral Area and Strength in Female Rat Offspring. J. Osteoporos. 2013;2013:154109. doi: 10.1155/2013/154109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raghavan R., Riley A.W., Volk H., Caruso D., Hironaka L., Sices L., Hong X., Wang G., Ji Y., Brucato M., et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018;32:100–111. doi: 10.1111/ppe.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li W., Xu B., Cao Y., Shao Y., Wu W., Zhou J., Tan X., Wu X., Kong J., Hu C., et al. Association of Maternal Folate Intake during Pregnancy with Infant Asthma Risk. Sci. Rep. 2019;9:8347. doi: 10.1038/s41598-019-44794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaible T.D., Harris R.A., Dowd S.E., Smith C.W., Kellermayer R. Maternal Methyl-Donor Supplementation Induces Prolonged Murine Offspring Colitis Susceptibility in Association with Mucosal Epigenetic and Microbiomic Changes. Hum. Mol. Genet. 2011;20:1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ross R.G., Hunter S.K., McCarthy L., Beuler J., Hutchison A.K., Wagner B.D., Leonard S., Stevens K.E., Freedman R. Perinatal Choline Effects on Neonatal Pathophysiology Related to Later Schizophrenia Risk. Am. J. Psychiatry. 2013;170:290–298. doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheatham C.L., Goldman B.D., Fischer L.M., da Costa K.-A., Reznick J.S., Zeisel S.H. Phosphatidylcholine Supplementation in Pregnant Women Consuming Moderate-Choline Diets Does Not Enhance Infant Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2012;96:1465–1472. doi: 10.3945/ajcn.112.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.