Abstract
Tick-borne ehrlichial pathogens of animals and humans require a mammalian reservoir of infection from which ticks acquire the organism for subsequent transmission. In the present study, we examined the strain structure of Anaplasma marginale, a genogroup II ehrlichial pathogen, in both an acute outbreak and in persistently infected cattle that serve as a reservoir for tick transmission. Using the msp1α genotype as a stable strain marker, only a single genotype was detected in a disease outbreak in a previously uninfected herd. In contrast, a diverse set of genotypes was detected in a persistently infected reservoir herd within a region where A. marginale is endemic. Genotypic diversity did not appear to be rapidly generated within an individual animal, because only a single genotype, identical to that of the inoculating strain, was detected at time points up to 2 years after experimental infection, and only a single identical genotype was found in repeat sampling of individual naturally infected cattle. Similarly, only a single genotype, identical to that of the experimentally inoculated St. Maries or South Idaho strain, was identified in the bloodmeal taken by Dermacentor andersoni ticks, in the midgut and salivary glands of the infected ticks, and in the blood of acutely infected cattle following tick transmission. The results show that mammalian reservoirs harbor genetically heterogeneous A. marginale and suggest that different genotypes are maintained by transmission within the reservoir population.
Pathogens in ehrlichial genogroups I and II, within the order Rickettsiales, are transmitted by ixodid ticks and cause acute disease in animals and humans (12, 20). Persistently infected mammals serve as reservoir hosts for ticks to acquire the pathogen and subsequently transmit infection to a new susceptible host, resulting in mild to severe illness that may progress to death (12, 20). Anaplasma marginale is a genogroup II ehrlichial pathogen that is maintained in persistently infected domestic cattle and transmitted to susceptible cattle within the same population (5, 12). Similar to the other tick-transmitted ehrlichiae, the well-characterized A. marginale strains have been isolated from acutely affected animals during a disease outbreak and, thus, represent successful transmission of a virulent genotype (1, 4, 13). In contrast, there is no information regarding the uniformity, or lack of it, of strain composition in the persistently infected cattle that serve as a reservoir for transmission. We hypothesized that, in comparison to acute disease outbreaks, persistently infected cattle would harbor a greater diversity of A. marginale genotypes. For the present study, we tested this hypothesis by using natural infections within a region of endemicity and in an acute disease outbreak, as well as by using experimental tick-borne transmission.
MATERIALS AND METHODS
Naturally infected animal populations.
Genotypic diversity was studied in two cattle populations. The first was a Hereford and Tarantais beef cattle herd (n = 235 animals) located within an area of eastern Oregon where A. marginale is endemic. This herd was selected based on a high prevalence of infection (64%) within the herd and a history of seasonal transmission (19). Sampling was done at two time points: prior to when feeding ticks are detected on cattle in this region (March) and following a minimum of 4 months of tick activity (August). The second population studied was a mixed-breed beef cattle herd (n = 120) in Platte, S.Dak. This herd had no previously reported cases of anaplasmosis in the 5 years prior to undergoing an acute outbreak at the time of this study. Sampling was done at a single time point during the acute outbreak. A. marginale infection of individual animals in both herds was detected by using an MSP5 competitive inhibition (CI)–enzyme-linked immunosorbent assay (ELISA) and was confirmed with the msp5 nested-PCR test. These test procedures and their specificity and sensitivity for detection in both acutely and persistently infected cattle have been previously reported in detail (9, 19).
Determination of the msp1α genotype.
DNA was extracted from blood, isolated tick midguts, or isolated tick salivary glands by using Puregene (Gentra Systems, Inc.) (7, 17). Amplification of the variable repeat region was done by PCR with Pwo DNA polymerase (Boehringer Mannheim) and primers in the conserved regions flanking the repeat sequences. The forward primer was 5′CATTTCCATATACTGTGCAG (nucleotide position −99 to −80 relative to the transcription start site; numbering based on the Florida strain msp1α sequence [1]; GenBank accession no. M32871, M32872), and the reverse primer was 5′-CTTGGAGCGCATCTCTCTTGCC (position +881 to +862). The number of repeats (each 87 or 84 bp long) can be determined from the size of the resulting amplicon, because the 268 bp preceding and the 17 bp following the repeats are conserved in all strains (1). The identity of the amplicon and the numbers and sequences of the repeats present were confirmed by sequencing. PCR products were ligated into pCR 2.1 by using the TA cloning kit (Invitrogen), and transformation of Escherichia coli XL-1 Blue was done as described previously (7). The presence of inserts in plasmids from transformed colonies was confirmed by restriction digestion or PCR (7). Plasmid insert DNA was sequenced in both directions by using an ABI PRISM (Applied Biosystems, Inc.) automated sequencer. Sequence analysis was performed on a VAX11/785 computer, by using the GCG (Genetics Computer Group) package, version 9. Full-length msp1α was amplified with primers flanking the complete open reading frame (1). The forward primer (5′-GTGCTTATGGCAGACATTTC) was derived from the sequence upstream of the transcriptional start site (positions −110 to −91), and the reverse primer (5′-GACTCTATCAAAGACCGGAAACTC) represents the complementary sequence to the last bases contained in the open reading frame (+2568 to +2544). The full-length gene was cloned and sequenced by the methods described above for repeat region amplicons.
To control for PCR- or cloning-induced artifacts in determination of the number of msp1α repeats, unamplified DNA was digested with EcoRII and, following agarose gel electophoresis, hybridized with a labeled msp1α probe spanning the repeat region. The probe was generated by PCR with the primers 5′CATTTCCATATACTGTGCAG (position −99 to −80) and 5′-CTTGGAGCGCATCTCTCTTGCC (position +881 to +862) and labeled with digoxigenin, as previously described (15). The EcoRII sites in msp1α are external to the repeat section (positions +81 to +85 and +1248 to +1252) and thus can be used to provide an independent estimation of the number of repeats (1). EcoRII digestion and Southern blotting were done by standard methods as previously described (16). A second control was determination of the apparent molecular size of the expressed MSP1a, which directly reflects the number of repeats (1, 14). A. marginale organisms were solubilized in sample buffer containing 2% (wt/vol) sodium dodecyl sulfate (SDS) and 5% β-mercaptoethanol (6). Following SDS-polyacrylamide gel electrophoresis and transfer to nitrocellulose, MSP1a was bound with monoclonal antibody Ana22B1 (13, 14). Detection of bound antibody by enhanced chemiluminescence and determination of apparent molecular size were done as described previously (6).
Experimental infection and tick transmission.
Three genotypically distinct strains of A. marginale, Florida, South Idaho, and St. Maries (1, 4,13), were used to examine genotype stability in infected cattle and during tick transmission. All calves were confirmed to be seronegative by the MSP5 CI-ELISA prior to experimental infection. Infected cattle were maintained in tick-free housing to prevent a second infection with an additional genotype. Three Holstein calves (animals 94B04, 94B05, and 94B06) were inoculated intravenously with the Florida strain. The msp1α genotype was determined at the following time points during persistent infection: 94B04, 616, 702, and 719 days postinfection; 94B05, 525 and 612 days postinfection; and 94B06, 525, 584, and 684 days postinfection. Two Holstein calves were inoculated intravenously with the South Idaho strain (animal 786) or the St. Maries strain (animal 787). Both strains are naturally transmitted by Dermacentor andersoni ticks and can be experimentally transmitted by adult males of the Reynolds Creek stock of D. andersoni (4, 5,17, 18). Following development of rickettsemia, 250 laboratory-reared adult male Dermacentor andersoni ticks were placed in an orthopedic stockinet and allowed to attach and feed on each calf for 7 days. The ticks were removed and were incubated for an additional 7 days at 26°C with 90 to 98% relative humidity and a 14-h photoperiod. A group of 50 ticks from each calf were dissected, and DNA was extracted from isolated midguts and salivary glands. Ticks from each feeding were then allowed to attach and transmission feed on individual uninfected recipient calves (animal 788 for South Idaho strain-infected ticks and animal 789 for St. Maries strain-infected ticks) for 3 days. Ticks were then removed, and salivary glands were obtained by dissection and used for isolation of infective-stage DNA, as previously described (17, 18).
Nucleotide sequence accession numbers.
The GenBank/EMBL accession numbers for the full-length msp1α nucleotide sequences are as follows: Florida, M32871, M32872; South Idaho, M32868; St. Maries, AF293062; Platte, S.Dak., AF293063; Baker, Oreg. (animal 2079), AF293064.
RESULTS
Genotype composition in a disease outbreak.
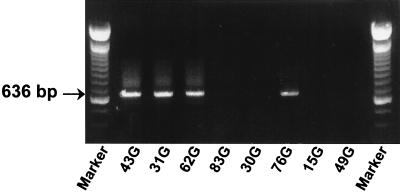
An acute outbreak of anaplasmosis was diagnosed in a beef cattle herd in Platte, S.Dak. There had been no reported cases of disease in the previous 5 years. In the outbreak, the presumptive diagnosis of acute A. marginale infection was based on clinical signs of severe anemia, fever, and lethargy. Infection was confirmed in 10 animals by the msp5 nested PCR and the MSP5 CI-ELISA (data not shown). Blood collected from each of these 10 animals during the period of high-level rickettsemia and acute clinical disease was used for DNA extraction and determination of the msp1α genotype. The msp1α genotypes identified in each of the 10 acutely rickettsemic cattle in the S.Dak. outbreak were identical. Amplicons from four of the infected animals are shown in Fig. 1. The number and sequence of the repeats within msp1α were determined by sequencing amplicons cloned into pCR 2.1. and expressed as the encoded amino acid sequence according to the convention described by Allred et al. (1) (Table 1). The full-length Platte, S.Dak., strain msp1α gene sequence (GenBank accession no. AF293063) confirmed the presence of four 87-bp repeats (Table 2). No additional genotypes were detected in this outbreak, and no amplicons were generated by using blood collected from five animals within the herd that were clinically normal and that remained seronegative in the MSP5 CI-ELISA.
FIG. 1.
Presence of a single msp1α genotype in a herd of cattle during an acute outbreak. Repeat region amplicons were derived from each of the acutely infected animals in the Platte, S.Dak., herd. Individual animal numbers are shown in the bottom margin. Animals 43G, 31G, 62G, and 76G were infected, and animals 83G, 30G, 15G, and 49G were uninfected. The size of the msp1α repeat region amplicon is indicated in the left margin.
TABLE 1.
Tandem repeat forms encoded by A. marginale msp1αa
| Repeat form |
Encoded sequence |
|---|---|
| A | DDSSSASGQQQESSVSSQS—EASTSSQLG— |
| B | ADSSSAGGQQQESSVSSQSDQASTSSQLG— |
| C | ADSSSAGGQQQESSVSSQSGQASTSSQLG— |
| D | ADSSSASGQQQESSVSSQS—EASTSSQLGG |
| E | ADSSSASGQQQESSVSSQS—EASTSSQLG— |
| F | TDSSSASGQQQESSVSSQSGQASTSSQLG— |
| G | DDSSSASGQQQESSVSSQSGQASTSSQSG— |
| H | TDSSSASGQQQESSVSSQSGQASTSSQSG— |
| I | DDSSSASGQQQESSVSSQSGQASTSSQLG— |
| J | ADSSLAGGQQQESSVSSQSDQASTSSQLG— |
Repeat forms A to E were identified by sequencing msp1α in the Florida, South Idaho, Virginia, and Washington-O strains of A. marginale and have been previously reported (1). Forms F to J were identified by sequencing full-length msp1α in the St. Maries strain or the entire repeat region of isolates from persistently infected cattle in the Baker, Oreg., herd. The boldface residues represent substitutions, insertions, or deletions.
TABLE 2.
msp1α genotypes of A. marginale strains and recent isolates from natural infectionsa
| Organism source | Genotype (order of each encoded repeat form) |
|---|---|
| Florida strain | 8 repeats (A/B/B/B/B/B/B) |
| St. Maries strain | 3 repeats (J/B/B) |
| South Idaho strain | 6 repeats (D/D/D/D/D/E) |
| Natural persistent infection (Baker, Oreg.) | 8 repeats (A/F/A/F/I/F/F/H) |
| 7 repeats (A/F/A/F/I/F/H) | |
| 5 repeats (A/F/A/F/H) | |
| 3 repeats (A/F/H) | |
| 2 repeats (A/H) | |
| 1 repeat (G) | |
| Natural acute infection (animal 3035; Baker, Oreg.) | 5 repeats (A/F/A/F/H) |
| Natural acute infection (Platte, S. Dak.) | 4 repeats (B/B/B/C) |
The repeat form structures of the Florida and South Idaho strains have been previously reported (1). The structures of the St. Maries strain and the isolates from the Baker, Oreg., and Platte, S. Dak., herds were determined by msp1α and amplicon sequencing.
Genotype composition in a herd from an area of endemicity.
Six different genotypes (8, 7, 5, 3, 2, and 1 repeats) were detected within the Baker, Oreg., herd in March, prior to the transmission season (Table 2). Amplicons representing four different genotypes (8, 7, 5, and 1 repeats), each derived from a persistently infected animal, are shown in Fig. 2. Only a single msp1α genotype was detected in an individual animal, and isolates with the same number of repeats always had the same order of repeats and an identical nucleotide sequence. In addition to the five repeat forms previously identified by sequence analysis of the Florida, South Idaho, Virginia, and Washington-O strains of A. marginale (1), four new repeat forms, all encoded by 87-bp segments, were identified in the persistently infected animals in the Baker, Oreg., herd (Table 1). The variation among genotypes was limited to the number of repeats, the presence of single-codon deletions, or nonsynonymous nucleotide substitutions within a codon (Tables 1 and 2).
FIG. 2.
Presence of multiple msp1α genotypes in a Baker, Oreg., herd of cattle within a region of endemicity. Repeat region amplicons representing four different genotypes, each derived from a persistently infected animal, are shown. The animal number is shown in the bottom margin, and the positions of amplicons representing eight (984 bp), seven (897 bp), five (723 bp), or one (372 bp) repeat are indicated in the left and right margins.
The msp1α genotype was also determined in a previously uninfected animal (animal 3035) that developed acute rickettsemia following tick transmission within this herd from an area of endemicity in August. The msp1α amplicon was sequenced, and the presence of five 87-bp repeats was determined (Table 2). This sequence matched that detected in four other persistently rickettsemic animals (no. 786, 0055, 1223, and 4135) within the same Baker, Oreg., herd.
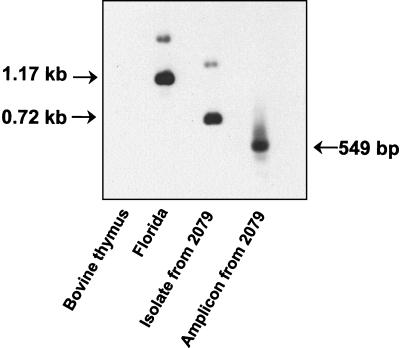
To confirm that the detected diversity in repeat numbers reflected true differences in the genotype rather than a PCR- or cloning-induced artifact, we determined the msp1α genotype without prior PCR amplification. Digestion of genomic DNA with EcoRII results in two fragments that can be hybridized with the probe (nucleotides −99 to +881): an internal msp1α fragment and a larger fragment composed of the 5′ end of msp1α and unknown upstream sequence. With the Florida strain used as a positive control, the msp1α probe hybridized strongly to the predicted 1.17-kb internal fragment (+83 to +1255), which includes one 84-bp repeat and seven 87-bp repeats (1), and weakly to the larger fragment containing the 5′ end (Fig. 3). EcoRII digestion of the isolate from animal 2079 of the Baker, Oreg., herd, shown by sequencing of the complete msp1α gene (GenBank accession no. AF293064) to contain one 84-bp repeat and two 87-bp repeats, resulted in a strongly hybridizing fragment of 0.72 kb (Fig. 3). This fragment is 435 bp smaller than the corresponding fragment in the Florida strain, consistent with the presence of only three repeats. A larger, weakly binding EcoRII fragment was also detected, as predicted, in the isolate from animal 2079.
FIG. 3.
Southern blot confirmation of the msp1α repeat structure predicted by amplicon size and sequence. The undigested msp1α repeat region amplicon from persistently infected animal 2079 of the Baker, Oreg., herd and EcoRII-digested DNA extracted from the Florida strain, the isolate obtained from animal 2079, and, as a negative control, bovine thymus were Southern blotted with an msp1α probe. The sizes of the internal EcoRII fragments of msp1α in the Florida strain and isolate 2079 are indicated in the left margin, and the size of the amplicon from isolate 2079 is indicated in the right margin.
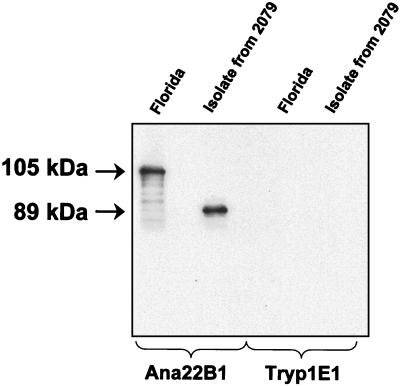
The Florida strain MSP1a protein was detected at an apparent molecular mass of 105 kDa (Fig. 4). The isolate from 2079 had an apparent molecular mass of 89 kDa (Fig. 4). This is the size predicted for MSP1a containing one 28-amino-acid repeat and two 29-amino-acid repeats and is approximately 16 kDa smaller than the Florida strain MSP1a (Fig. 4), representing the presence of five fewer 29-amino-acid repeats.
FIG. 4.
Western blot confirmation of the msp1α repeat structure predicted by amplicon size and sequence. Organisms of the Florida strain and the isolate obtained from persistently infected animal 2079 of the Baker, Oreg., herd were Western blotted with the anti-MSP1a monoclonal antibody Ana22B1 or, as a negative control, the anti-Trypanosoma brucei monoclonal antibody Tryp1E1. The sizes of MSP1a in the Florida strain and isolate 2079 are indicated in the left margin.
Stability of genotypes within persistently infected cattle.
Genotypes were examined at multiple time points of persistent Florida strain infection in each of three animals. The genotypes at all eight time points, ranging from 525 to 719 days after experimental inoculation, were identical to that of the inoculating Florida strain (Table 2). To test whether genotypes were also stable in naturally infected animals in a region of endemicity, 10 of the animals in the Baker, Oreg., herd for which genotypes were determined in March were also tested in August, following the tick transmission season. All 10 animals remained persistently infected during the period, and there was only a single genotype of A. marginale in each animal. The genotypes, in terms of both the number of repeats determined by PCR and the sequence, were identical for March and August.
Stability of genotypes during tick transmission.
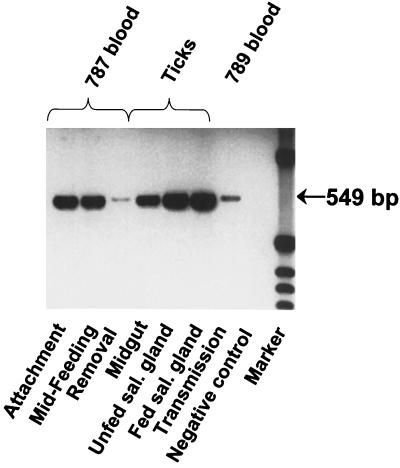
The stability of genotypes during tick transmission was tested with the South Idaho and St. Maries strains. The South Idaho strain msp1α gene contains six repeats (five of 87 bp and one of 84 bp) (Table 2), and the complete sequence has been reported previously (1). The full-length St. Maries msp1α gene was sequenced (GenBank accession no. AF293062), and three 89-bp repeats were identified, including a variant form, designated J, not present in the previously examined strains (Tables 1 and 2). Only a single genotype, identical to that of the inoculated St. Maries (Fig. 5) or South Idaho strain, was detected in the following samples obtained during tick transmission: (i) the blood of infected animals when ticks fed and acquired A. marginale, (ii) the infected tick (from the midgut and salivary gland prior to reattachment for transmission feeding and from the salivary gland after 3 days of transmission feeding), and (iii) the blood of infected animals following successful transmission. Sequencing of all the samples confirmed the invariance of the repeat structure.
FIG. 5.
Invariance of the msp1α genotype during tick transmission of the St. Maries strain of A. marginale. Repeat region amplicons were generated from A. marginale in the following samples: the blood of animal 787 at three time points during acquisition feeding by Dermacentor andersoni—tick attachment, midfeeding, and tick removal; infected D. andersoni tick midguts and salivary glands prior to transmission feeding (Unfed sal. gland) and after 3 days of transmission feeding (Fed sal. gland); and the blood of animal 789 during acute rickettsemia following transmission. PCR amplification without DNA template was done as a negative control. The size of the msp1α repeat region amplicon is indicated in the right margin.
DISCUSSION
The A. marginale genotypic diversity within a herd of persistently infected cattle was striking and demonstrates much broader genetic heterogeneity of ehrlichial pathogens in the mammalian reservoir than has been apparent based on characterization of isolates from acutely infected animal or human patients. Within the single Baker, Oreg., herd, we identified six distinct A. marginale genotypes as determined by the number and sequence of msp1α repeats. We conclude that the detected genotypes accurately represent true genotypic diversity, rather than errors introduced during PCR amplification or sequencing, based on the following observations: (i) only a single genotype was detected in any infected animal; (ii) samples collected at different times from the same naturally infected animals (Baker, Oreg., and Platte, S.Dak., herds) always gave rise to an identical genotype; (iii) samples collected at different times from the same experimentally infected animals (Florida and South Idaho strains) always gave rise to the same genotype, and the genotype was identical to that of the inoculating strain; (iv) Southern blotting of unamplified DNA identified EcoRII fragments predicted by the PCR-generated and -sequenced msp1α amplicon; and (v) Western blotting of the organism revealed the MSP1a protein of the size predicted by the PCR-generated and -sequenced amplicon.
How is this diversity of genotypes within the persistently infected herd generated? We considered the most likely possibility to be during the sequential cycles of replication that characterize persistent infection (8) or, alternatively, during replication in the midgut and salivary glands of transmitting ticks (10, 11). Examination of three experimentally infected animals at multiple time points during persistence revealed only a single genotype, identical to that of the inoculating Florida strain. This msp1α genotypic stability was supported by examination of 10 persistently infected individuals in the herd from an area of endemicity at two time points. In each of the 10 infected individuals, only a single, identical genotype was identified in both samples. Similarly, only a single msp1α genotype, with a repeat structure identical to that of the inoculating St. Maries or South Idaho strain, was detected within the tick midgut epithelium, where early replication takes place, and in the tick salivary gland, where final replication and development of infectivity occur (10, 11). Thus, using experimental infection with three genotypically distinct strains and by sequential sampling during natural infection, we were unable to identify emergence of new genotypes in the mammalian reservoir or in the tick vector.
The arrangement of alternating A and F repeat forms with a terminating H form in the Baker, Oreg., herd (Table 2) suggests that the diverse genotypes within this herd may have arisen from a common precursor strain. If so, this genotypic change appears to be an infrequent event, because generation of diversity was not detected in our studies of persistent infection and tick transmission. An alternative hypothesis is that the diversity represents separate transmission events, each introducing a new msp1α genotype into the herd that is then maintained by transmission within the herd. Importantly, there had been no new addition of animals from outside the Baker, Oreg., herd into the study population for several years. Regardless of the source, the infrequent generation of new genotypes suggests that the genotypes are maintained by transmission within the herd, a possibility supported by the identification of multiple animals with the same genotype. While it is unproven whether each of the detected genotypes represents a transmissible phenotype, the genotype represented by five repeats (three 87 bp and two 84 bp) was shown to be transmitted to animal 3035. Although this was a common msp1α genotype, we do not have sufficient data to determine whether this represents a genotype with enhanced transmissibility.
In contrast to the diversity of genotypes within the persistently infected Baker, Oreg., herd, only a single genotype was detected in the acute outbreak affecting the Platte, S.Dak. herd. The detection of only a single genotype in all of the affected individuals is suggestive of transmission from a point source—most likely introduction of an animal infected with this genotype into the herd.
In summary, we have identified genotypic diversity within a population of persistently infected cattle that serve as reservoirs for tick transmission of the ehrlichia A. marginale. These genotypes appear to be relatively stable, because we failed to detect change during experimental or naturally occurring infection and tick transmission. The association of differences in virulence, antigenicity, and transmissibility with specific A. marginale strains, isolated from acute cases and defined by the msp1α genotype (1–4, 13, 14, 18, 21), indicates that the diversity of genotypes present within an endemically infected reservoir population could also represent a diversity of biological phenotypes. Clearly, the transmissibility and virulence of genotypes within the reservoir population are important determinants of the incidence and severity of disease in the susceptible population. Testing whether the same diversity is present within the wildlife mammalian reservoirs of ehrlichial pathogens causing disease in humans and learning how this diversity is generated will be critical to improved understanding of ehrlichial transmission and disease.
ACKNOWLEDGMENTS
This work was supported by grants from USDA NRICGP (97–35204-4597 and 96–37204-3610) and NIH (R01 AI44005).
The isolates from the Platte, S.Dak., outbreak were provided by Jon Seagren, and the Baker, Oreg., isolates were collected with the assistance of Patricia Rasmussen, Susana Torioni de Echaide, and Odillon Vidotto. We acknowledge Teresa Harkins, Beverly Hunter, Emma Karel, Kay Morris, and Carla Robertson for excellent technical assistance.
REFERENCES
- 1.Allred D R, McGuire T C, Palmer G H, Leib S R, Harkins T M, McElwain T F, Barbet A F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci USA. 1990;87:3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown W C, Zhu D, Shkap V, McGuire T C, Blouin E F, Kocan K M, Palmer G H. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect Immun. 1998;66:5414–5422. doi: 10.1128/iai.66.11.5414-5422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camacho-Nuez M, Muñoz de Lourdes M, Suarez C E, McGuire T C, Brown W C, Palmer G H. Expression of polymorphic msp1β genes during acute Anaplasma marginale rickettsemia. Infect Immun. 2000;68:1946–1952. doi: 10.1128/iai.68.4.1946-1952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriks I S, Stiller D, Goff W L, Parish S M, McElwain T F, Palmer G H. Molecular and biological characterization of a new isolated Anaplasma marginale strain. J Vet Diagn Investig. 1994;6:435–441. doi: 10.1177/104063879400600406. [DOI] [PubMed] [Google Scholar]
- 5.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles D P, Torioni de Echaide S, Palmer G H, McGuire T C, Stiller D, McElwain T F. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996;34:2225–2230. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocan K M. Development of Anaplasma marginale in ixodid ticks: coordinated development of a rickettsial organism and its tick host. In: Sauer JR, Hair JA, editors. Morphology, physiology and behavioral ecology of ticks. Chichester, United Kingdom: Horwood; 1986. pp. 472–505. [Google Scholar]
- 11.Kocan K M, Goff W L, Stiller D, Edwards W, Ewing S A, Claypool P L, McGuire T C, Hair J A, Barron S J. Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am J Vet Res. 1993;54:107–112. [PubMed] [Google Scholar]
- 12.Losos G J. Anaplasmosis. In: Losos GJ, editor. Infectious tropical diseases of domestic animals. Essex, United Kingdom: Longman House; 1986. pp. 743–795. [Google Scholar]
- 13.McGuire T C, Palmer G H, Goff W L, Johnson M I, Davis W C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984;45:697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberle S M, Palmer G H, Barbet A F, McGuire T C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988;56:1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer G H, Abbott J R, French D M, McElwain T F. Persistence of Anaplasma ovis infection and conservation of the msp-2 and msp-3 multigene families within the genus Anaplasma. Infect Immun. 1998;66:6035–6039. doi: 10.1128/iai.66.12.6035-6039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rurangirwa F R, Stiller D, Palmer G H. Strain diversity in major surface protein expression during tick transmission of Anaplasma marginale. Infect Immun. 2000;68:3023–3027. doi: 10.1128/iai.68.5.3023-3027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torioni de Echaide S, Knowles D P, McGuire T C, Palmer G H, Suarez C E, McElwain T F. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay with recombinant major surface protein 5. J Clin Microbiol. 1998;36:777–782. doi: 10.1128/jcm.36.3.777-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker D H, Dumler S J. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickwire K B, Kocan K M, Barron S J. Infectivity of three Anaplasma marginale isolates for Dermacentor andersoni. Am J Vet Res. 1987;48:96–99. [PubMed] [Google Scholar]