Abstract
Chlorella vulgaris from Al-Ahsa, KSA was proved to be an active silver and gold nanoparticle producer. Nanogold and nanosilver particles were characterized using UV-visible spectroscopy, Fourier-transform infrared spectroscopy, and scanning electronmicroscopy. Both nanoparticles were used in the antimicrobial bioassay. The two nanoparticles showed antibacterial activities, with the silver nanoparticles being the most effective. To investigate the argumentative nature of their biosynthesis (i.e., whether it is a biotic or abiotic process), we isolated total ribonucleic acid RNA as an indicator of vitality. RNA was completely absent in samples taken after one week of incubation with silver nitrate and even after one or two days. However, successful extraction was only achievable in samples taken after incubation for one and four hours with silver nitrate. Most importantly, the gel image showed recognizable shearing of the nucleic acid after 4 h as compared to the control. An assumption can be drawn that the synthesis of nanoparticles may start biotically by the action of enzyme(s) and abiotically by action of reducing entities. Nonetheless, with prolonged incubation, excessive nanoparticle accumulation can be deadly. Hence, their synthesis continues abiotically. From the RNA banding profile, we suggest that nanosilver production starts both biotically and abiotically in the first few hours of incubation and then continues abiotically. Nanosilver particles proved to have more of an antimicrobial impact than nanogold and hence are recommended for different applications as antibacterial agents.
Keywords: antibacterial bioassay, Chlorella vulgaris, nanogold, nanosilver, RNA extraction
1. Introduction
Microalgae are considered as significant bio-factories for the treatment of wastewater, the production of biofuel, the manufacturing of a variety of commercially valuable products, and the synthesis of nanoparticles. The synthesis of nanoparticles from biological sources has appeared recently as a potential substitute to the typical physical and chemical methods. Due to their cost-effectiveness and environmental friendliness, some microorganisms, such as bacteria, algae, and fungi, have been successfully implemented as prospective prolific source of eco-friendly biogenic nanoparticles. For example, different algal species belonging to different algal groups such as Chlorophyta, Haptophyta, and Ochrophyta were reported to biosynthesize nanoparticles [1,2]. According to a recent review by Jacob et al. [3], their ease of growth and ability to live in extreme conditions make them plausible candidates for large-scale synthesis of nanoparticles. The ability to biosynthesize nanoparticles is suggested to be a mechanism by which the microalgae detoxify excessive bulky metals in their niche which can otherwise be toxic and hazardous [4]. The biomass itself can be used as well as the cell free supernatants.
Microalgae can be regarded as a potent nano-factory, capable of manufacturing diverse nanoparticles such as silver, gold, cadmium, and platinum [5]. Several methods for generating metallic nanoparticles from microalgae and their related aqueous salt solutions have been proposed. For the biosynthesis of silver and gold nanoparticles, Li et al. [6] utilized a fine powder of Spirogyra insignis (Charophyta). It has been shown that cell-free supernatants of cyanobacteria and chlorophyta species can be used to synthesize silver nanoparticles [7]. Interestingly, Agarwal et al. pointed out in their review that gold shape-directing high molecular weight protein and silver nanoparticle low molecular weight proteins contained in the biomass of microalgae are responsible for reducing gold and silver ions into their neutral metallic nanoform [5]. In addition, silver nanoparticle manufacturing has been demonstrated in different algal species such as Chlorella vulgaris, Spirulina platensis, and Lyngbya majuscula [8].
The bio-based synthesis has been proposed as a means of producing nanoparticles. The basic concept of this kind of synthesis is to transform metal ions into nanoparticles or bioactive products with high reducing power via a cell factory [9]. Algae include a wide array of bioactive compounds (for example, polysaccharides, vitamins, proteins, lipids, and pigments) which can be used to synthesize various metals nanoparticles by activating the detoxifying machinery in both living cells and cellular extracts [6]. Several studies have addressed the synthesis of nanomaterials [10,11]. Metallic nanoparticles can be generated using one of two bio-based synthesis methods; the first one is an in vivo biosynthesis by which the metal ions are delivered into cells or onto the cell surface, where they are then transformed into metallic nanomaterials by a particular enzyme via the metallic detoxification machinery. The second biosynthesis method is an in vitro by which metallic ions are collected and converted into metallic nanomaterials utilizing bioactive component derived from microorganisms [12]. Nonetheless, there is no consensus in the literature about the exact route the biosynthesis can follow. Therefore, and in order to fill in the gap in our knowledge, we attempt to investigate the of the biotic and abiotic nanosilver synthesis.
With regard to antimicrobial activity of nanoparticles, Hafez (2013) showed that nano-silver particles produced by some microalgae had antimicrobial activity [13]. He used aqueous extract from the algae Chroococcus disperses and Chlorella vulgaris for production of nanosilver and tested them against two plant pathogens (Pseudomonas flavescens and Erwinia amylovora) in addition to several plant pathogenic fungi. The results showed that high antibacterial activity was obtained by the produced nanosilver and the inhibition zones exceeded that of control antibiotic disc. Similar results were obtained for the antifungal test. The fact that microalgae, such as Chlorella vulgaris, grow rapidly in relatively inexpensive growth media makes them a more plausible cost-effective candidates for nanoparticles biosynthesis. Here in the present study, we attempted to evaluate and compare the antimicrobial activity of both nanogold and nanosilver biosynthesized by Chlorella vulgaris and to further investigate the most active nanoparticles against more pathogens.
2. Materials and Methods
2.1. The Cyanobacterial Cells
The coccoid algal monospecific culture was collected and purified using standard microalgal culturing techniques. Mid-logarithmic cultures were used for the biosynthesis of silver and gold nanoparticles.
2.2. Silver Nanoparticles Biosynthesis
Silver nitrate (Sigma-Aldrich, St. Louis, MO, USA) was prepared at 10 mM concentration and kept in dark bottle. Cells of Chlorella vulgaris were washed with distilled water and centrifuged. The cells were incubated with silver nitrate solution for two weeks. The color of the outer solution turned gradually into yellow, pale brown and then dark brown. The formed silver nanoparticles were subjected to the following analyses (UV-visible spectroscopy and Fourier-Transform infrared spectrometer, FTIR).
2.3. Nanogold Particles Biosynthesis
Chlorella vulgaris mid-logarithmic culture was treated with 1500 mg/mL HAuCl4 solution containing Au3+. Immediately after the incubation, gradual slow color change was observed to occur until the complete change into a pink color. The nanoparticle suspensions produced were scanned using UV-visible spectroscopy (540 nm) and FTIR.
2.4. UV–Visible Spectroscopic (UV–vis) Analysis
The UV–vis spectrophotometer (Genesys10S UV–visible double beam spectrophotometer) was used. Scanned spectrum was recorded for the wavelength range of 200 nm to 600 nm.
2.5. FTIR Analysis
Characterization of silver nanoparticles was performed on dried biosamples of chlorella containing nanoparticles using Fourier-Transform infrared spectrometer (FTIR, Agilent Cory 630, Agilent Technologies, Santa Clara, CA, USA).
2.6. Scanning Electron Microscopy
Both aqueous external solutions containing nanosilver particles and nanogold particles were used in scanning electron microscopy. One drop of each solution was mounted on the metallic stub, left to air dry and fixed using double face adhesive carbon. The stubs were coated with gold three times using sputter, and the samples were then visualized using Joel JSM-5510LV scanning electron microscope.
2.7. Antibacterial Bioassay Using Disc Diffusion Method
The antibacterial potency of nanosilver and nanogold particles synthesized by Chlorella vulgaris, was evaluated against pathogenic bacterial strains namely; methicillin-resistant staphylococcus aureus (MRSA), Staphylococcus aureus and Streptococcus sp. These Gram-positive pathogenic bacteria were freshly sub-cultured prior to their assay. The sensitivity of pathogenic strains to the extract was assayed through modified Kirby Bauer disk diffusion susceptibility method [14]. Sterilized paper discs (6 mm in diameter) were saturated with 30 µL of equal concentrations of nanosilver and nanogold particles for antibacterial tests. Discs were dried and placed on the surface of inoculated nutrient agar medium with bacterial suspensions prepared in physiological saline (0.85 g NaCl in 100 mL distilled water). Plates were kept for 2 h at 4 °C to ensure diffusion of bioactive material, after that the plates were incubated at 37 °C. Control discs containing 30 µL of sterilized distilled water were left to dry, then used as negative control whereas positive control sets included discs of antibiotic Chloramphenicol at concentration of 5%. Plates were incubated for 24 h and diameter of inhibition zones (mm) were measured in triplicates and the average standard deviation was recorded. The results were compared, and the antimicrobial bioassay was further performed in a single experiment on Escherichia coli (gram negative bacterium) using both silver and gold nanoparticles to explore their broad-spectrum antibacterial bioactivity.
2.8. The Antibacterial Biovassay Using MIC/MBC
The minimum inhibitory concentration (MIC) of the synthesized AgNPs against four different bacterial pathogens (E. coli, S. aureus, MRSA, and Streptococcus sp.) was determined using a standard protocol [15]. Briefly, the compounds were dissolved in dimethyl sulfoxide (5% DMSO) and sterilized using a 0.2 μm Millipore membrane filter (Merck Millipore, Burlington, MA, USA). The solution was then serially diluted (two-fold in a 96-well microtiter plate) in the Mueller Hinton broth (MHB). An aliquot (5 μL) of the freshly prepared bacterial suspensions of the test organism (at 1.5 × 106 colony forming units (CFU) mL−1) was transferred into 200 mL of sterilized MHB. After mixing, the 96-well microtiter plates were covered with a sterile plate sealer and incubated at 37 °C for 18 h under shaking. After incubation, 5 mL of the microbial cells in each well were spotted on Mueller Hinton agar plates and incubated at 37 °C for 18 h. The visible growth was monitored to determine the MIC of the particles. The experiment was repeated three times to confirm the antibacterial activity of the silver nanoparticles.
2.9. Chlorella vulgaris RNA Extraction
20 mL of Chlorella vulgaris cultures were transferred to centrifuge tubes and centrifuged at 14,000 rpm for 10 min. Pelleted cells were disrupted in liquid nitrogen in a ceramic mortar and homogenized with 500 µL of Trizol® reagent (Life Technologies Corp., Carlsbad, CA, USA). Total RNA was extracted according to the manufacturer’s instructions for the reagent. Subsequently, the RNA concentrations were determined using NanoDrop™ 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of the extracted RNA was analyzed by electrophoresis in 1% agarose gel.
3. Results
3.1. UV-Visible Spectroscopy
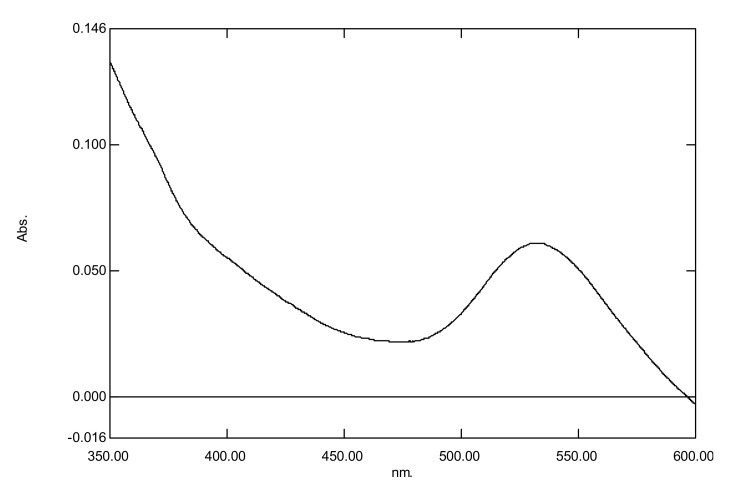
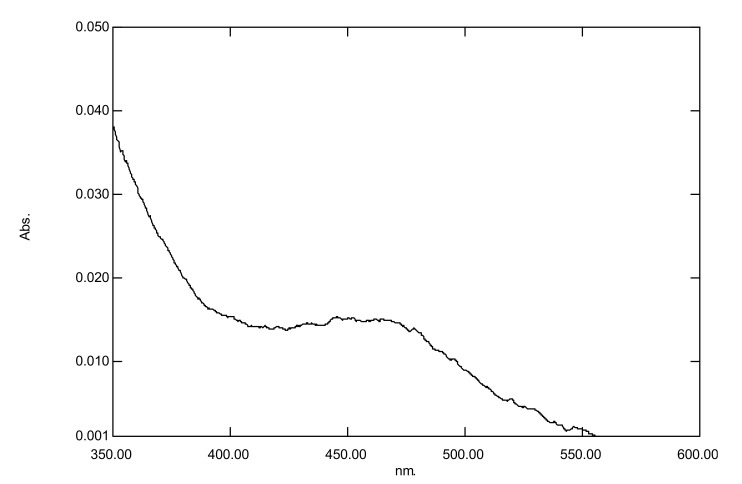
With regard to the UV-visible spectrum of nanogold, the surface plasmon band was detected within the range of 520–550 nm (Figure 1) similar to that reported by [16]. With regard to silver nanoparticles, the surface plasmon band was found within the range of 410–450 nm (Figure 2) similar to that reported by [17]. The results showed that the nanogold plasmon resonance band was detected in the range of 510–550 nm (which is in accordance with [17].
Figure 1.
Ultra violet-visible spectrum of nanogold.
Figure 2.
Ultra violet-visible spectrum of nanosilver.
3.2. FTIR
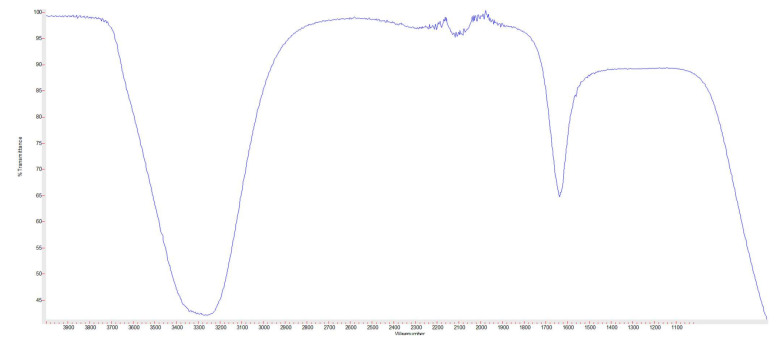
In addition, FTIR confirmed their biosynthesis by showing the characteristic bands of gold nanoparticles (Figure 3) which were similar to that reported in [17]. With regard to the FTIR, the characteristic bands of nanogold were detected at 3414.96, 2158.28, and 1640.60 cm−1.
Figure 3.
Fourier-Transform infrared (FTIR) spectrum of nanogold.
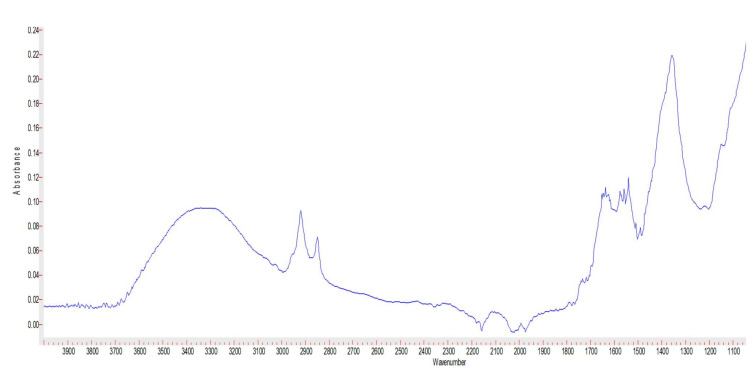
The FTIR spectra of the AgNPs. and the FTIR (Figure 4) showed the characteristic silver nanoparticles peaks at 2927, 1631, 1383 cm−1 and 3297 cm−1 [18].
Figure 4.
FTIR spectrum of nanosilver.
3.3. Scanning Electron Microscopy
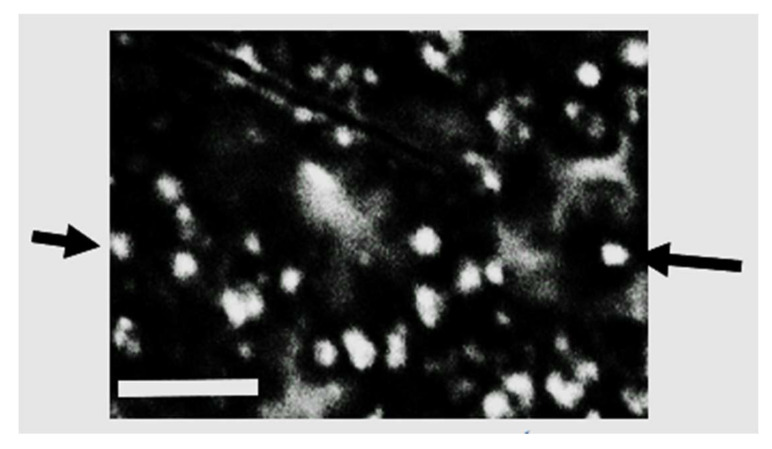
SEM revealed the presence of spherical nanogold particles (Figure 5) with size range of 20–25 nm of uniform spherical appearance. In case of silver nanoparticles, a noticeable aggregation was frequently noticed but the individual silver nanoparticles were also abundant in the size range of 50–80 nm (Figure 6) with spherical appearance although when aggregated the aggregated particles become angular.
Figure 5.
Scanning electromicrograph showing the spherical shape of biogenic nanogold particles (denoted by black arrows) (scale bar 100 nm).
Figure 6.
Scanning electromicrograph showing the spherical shape of biogenic nanosilver particles (denoted by black arrow) (Scale bar 100 nm).
3.4. Total RNA Extraction
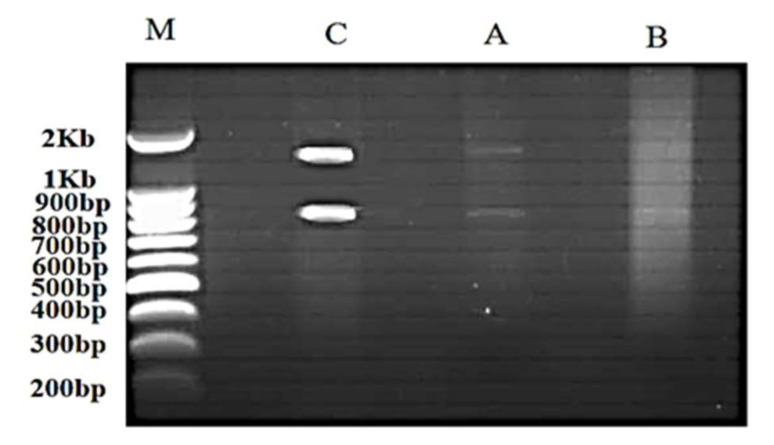
Total RNA was extracted from C. vulgaris in order to detect cells vitality. Electrophoresis gel has been used to analyze the integrity of extracted RNA. Intact extracted RNA was detected from the control sample that was untreated with silver nitrate. However, there was a fading band for sample taken after one hour and rather “ghost” bands after four hours. Indeed, nucleic acid smearing was faintly observed for one-hour sample and intensified for the four-hour incubation (Figure 7). Furthermore, none of the extracted RNA was detected from C. vulgaris that was incubated with silver nitrate for six and eight hours as well as those for one day, two days, and one week.
Figure 7.
Gel electrophoresis of RNA extracted from Chlorella vulgaris. Extracted RNA from: untreated sample with silver nitrate (control C), C. vulgaris incubated with silver nitrate for one hour (A), C. vulgaris incubated with silver nitrate for four hours with smear formation (B); (M), molecular size marker.
3.5. Antimicrobial Bioassay Using Disc Diffusion
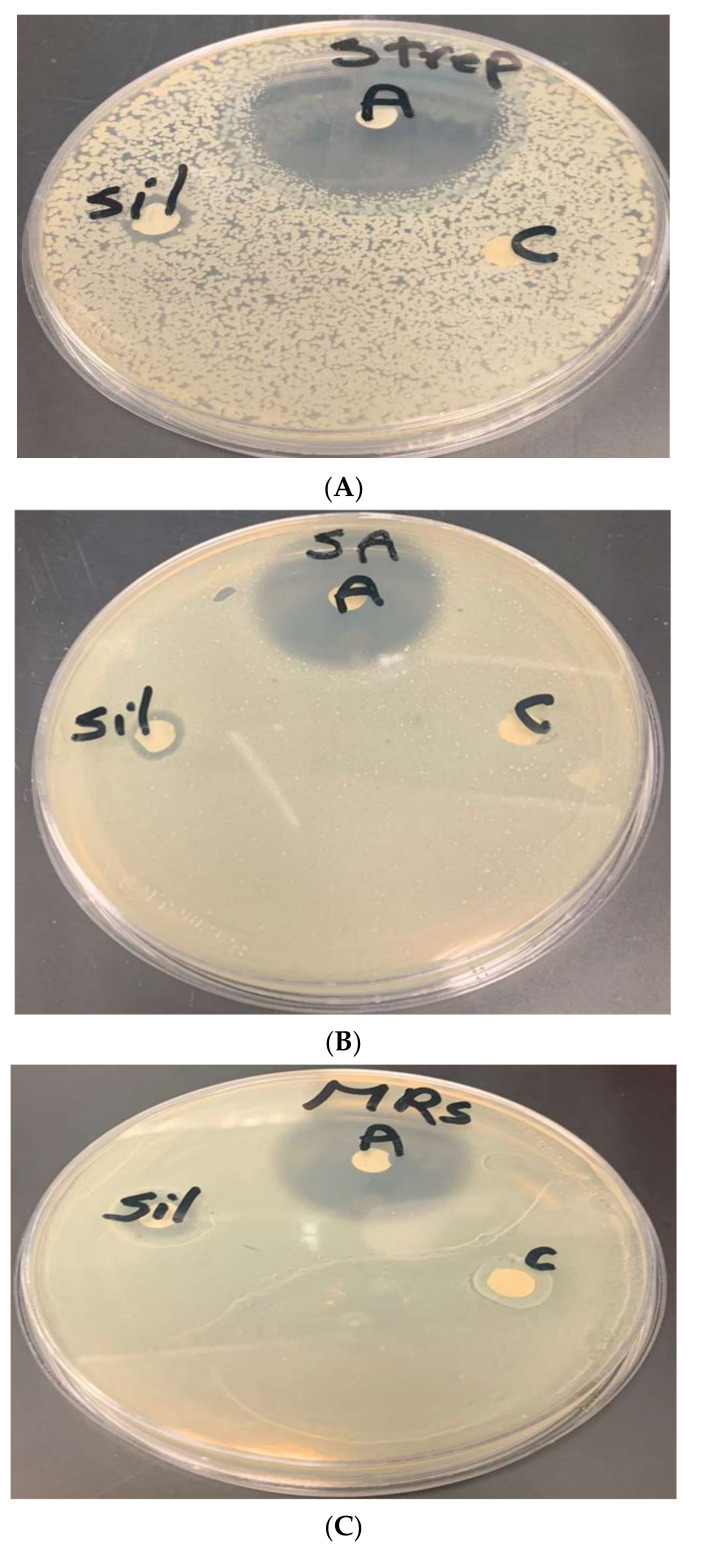
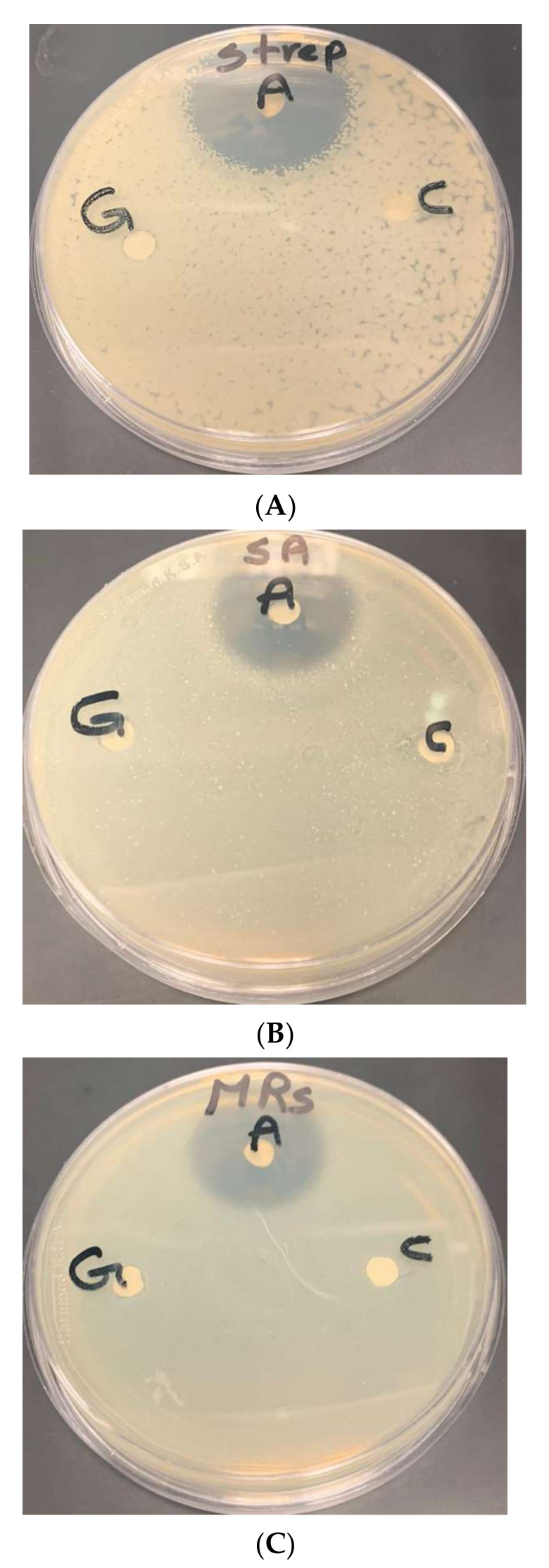
The antimicrobial bioassay results (Table 1) showed that the greatest inhibition zone was obtained for methicillin-resistant staphylococcus aureus whereas the smallest was for Streptococcus sp. (Figure 8). However, despite using low concentration silver of nanoparticles, they showed antimicrobial effect against the multidrug resistant strain of methicillin-resistant Staphylococcus aureus. The inhibition zone was 1.7 cm, whereas there were no obvious results on gold nanoparticles compared to silver nanoparticles in the same species (Figure 9). Gram negative E. coli growth was also inhibited (Figure 10).
Table 1.
Antibacterial activity of silver and gold nanoparticles of Chlorella vulgaris.
| Bacterial Species | Inhibition Zone for Nanogold | Inhibition Zone for Nanosilver |
|---|---|---|
| Staphylococcus aureus | 0.4 ± 0.2 | 1.2 ± 0.1 |
| Streptococcus sp. | 0.5 ± 0.1 | 1.0 ± 0.2 |
| Methicillin-resistant Staphylococcus aureus | 0.3 ± 0.1 | 1.7 ± 0.1 |
| Escherichia coli | 0.9 ± 0.1 | 1.9 ± 0.1 |
Figure 8.
Inhibition zone of silver nanoparticles of Chlorella vulgaris extract against (A) Streptococcus sp., (B) Staphylococcus aureus, and (C) methicillin-resistant Staphylococcus aureus.
Figure 9.
Inhibition zone of gold nanoparticles of Chlorella vulgaris extract against (A) Streptococcus sp., (B) Staphylococcus aureus, and (C) methicillin-resistant Staphylococcus aureus.
Figure 10.
Inhibition zone of silver and gold nanoparticles of Chlorella vulgaris against E. coli.
The discs labelled “A” denotes antibiotics; “Sil” denotes disc containing silver nanoparticles; and “c” denotes control disc (containing no silver nanoparticles).
The discs labelled “A” denotes antibiotics; “G“ denotes disc containing gold nanoparticles; and “c” denotes control disc (containing no gold nanoparticles).
The discs labelled “A” denotes antibiotics; “g” denotes disc containing gold nanoparticles; and “s” denotes silver nanoparticles (whereas “−“denotes control disc (containing no nanoparticles)).
3.6. Antimicrobial Bioassay Using MIC/MBC µg/mL
The silver and gold nanoparticle showed antibacterial activities with both bacteriostatic and bactericidal capabilities (Table 2). However, some variances were detected amongst the action of the silver and gold nanoparticle compounds. The silver nanoparticle was the most active, while gold nanoparticle showed the lowest activity.
Table 2.
Antimicrobial activity of silver and gold nanoparticle (MIC/MBC µg/mL). MIC stands for minimum inhibitory concentration whereas MBC stands for minimum bactericidal concentration.
| Bacteria | Nanosilver | Nanogold | Chloamphenicol 5% |
|---|---|---|---|
| E. coli | 62.5/125 | 125/250 | 62.5/125 |
| S. aureus | 125/250 | >250 | 62.5/125 |
| Streptococcus sp. | 250/250 | >1000 | >250 |
| Methicillin Resistant Staphylococcus aureus | 125/250 | 250/250 | 62.5/125 |
4. Discussion
Both nanosilver and nanogold particles showed the characteristic surface plasmon bands that conform with those reported in [16] for nanosilver and in [17] for nanogold. Moreover, the FTIR confirmed the formation of nanogold where the characteristic bands were detected that are attributed to carbonyl stretch and free-amide stretch vibrations in the amide linkages of the proteins. The carbonyl group and peptides of proteins have the ability to bind metal, such that the proteins can act as capping and stabilizing agent of gold nanoparticles to prevent their agglomeration. With regard to the bands of silver nanoparticles, they showed the characteristic peak as that reported by Al dayel et al. [18]. In agreement with this, Chlorella vulgaris has been reported to produce nano-silver [19]. However, the scanning electron microscopy showed the tendency of the silver nanoparticles to aggregate indicating the absence of enough stabilizers in the outer external solution. Unlike FTIR which was performed on dried cellular nanoparticles that showed the binding and association with several biomolecules and functional groups. This only indicates that incase of biogenic synthesis of silver nanoparticles, the intracellular nanoparticles are most likely to be stabilized and capped by several cellular bioactive molecules (unlike the extracellular nanosilver, which most likely tends to aggregate due to lack of external stabilizers).
It is worth noting that, unlike cyanobacteria which can secrete stabilizing and capping materials into external solution, the green alga Chlorella vulgaris is not able to do so.
Unlike silver nanoparticles, the nanogold particles were more uniform and homogenous and far less prone to aggregation as SEM revealed.
Inorganic nanoparticles of silver and gold are being used in many pharmaceutical applications due to their functionality and biocompatibility [20]. For example, they can be used for their antimicrobial action [21]). The reduction of gold ions is suggested to follow similar path to that to of silver ions, which can proceed through the reductase enzymes [22] and electron carriers such as quinones. Gold nanoparticles are also known for their potent antibacterial activity against acne or scurf and have commercial applications [23]. Zhang et al. (2015) have also reported gold nanoparticle-mediated growth inhibition of different Gram-positive and Gram-negative bacteria and fungi [23].
The pathogenic bacteria used in the antimicrobial bioassay are all of different characteristics and it is quite difficult to find one antimicrobial agent that has broad-spectrum activity against all of them. Indeed, the antimicrobial capability of both nanogold and nano silver particles biosynthesized by Chlorella vulgaris was experimentally proven. Nevertheless, the nanosilver particles were more potent antimicrobials than the nanogold particles. Moreover, the antibacterial action of biogenic nano-silver particles was effective against multidrug resistant Staphylococcus aureus (MRSA). Skulberg (2000) [24] illustrated that the need to exploit microalgae from rather underexplored areas for their applicable potential as they possess unique metabolites and bioactive compounds of wide array of utilizes and effect that can be effective against multidrug-resistant strains. With regard to their effect on Gram-negative Escherichia coli, both showed antimicrobial action but in case of gold nanoparticles it showed weak antibacterial impact unlike nanosilver particles which were more potent antibacterial agents. The cells of Gram-negative bacteria are hard to penetrate since there is a huge outer lipid membrane that protects it. Nonetheless, those cells were susceptible to silver nanoparticles. Overall, silver nanoparticles showed a broad-spectrum antimicrobial bioactivity against different pathogenic bacteria (both Gram-positive and Gram-negative) and even against multidrug-resistant bacterium. This only attests for the great antimicrobial potential of silver nanoparticles.
The Antimicrobial Bioassay Using Dilution Test
The mechanism of action of silver and gold nanoparticles exhibited both bacteriostatic and bactericidal action through the inhibition of bacterial enzymes by binding to DNA, as well as causing damage to both the bacterial cell wall and cytoplasmic membrane [25]. However, when comparing the antimicrobial effect of silver nanoparticles to that of gold nanoparticles, the superiority of silver nanoparticles is most evident with its wide-spectrum antimicrobial action against both Gram-positive and Gram-negative bacteria. In addition, the outer solution of Chlorella vulgaris may have numerous bioactive compounds such as flavonoids, tannin, phenolic compounds, terpenes, glycosides, and saponins (which has been reported, for example, by [26]. Those compounds may act as stabilizers/capping agents for those nanoparticles present in the outer solution of Chlorella sp. cells.
The biosynthetic mechanism of these nanoparticles is debatable and is thought to proceed both biotically via enzymes and abiotically by cellular reducing entities. Nonetheless, we detected the nearly complete loss of vitality, as indicated from the disintegration of total RNA, after four hours of incubation with silver nitrate and the complete loss of vitality for periods longer than this. Nonetheless, the accumulation of silver nanoparticles continued which is most likely through the abiotic pathway.
Overall, Chlorella vulgaris is a promising bio-source for antimicrobials most important of which are biogenic nanoparticles as silver nanoparticles. Nonetheless, the accumulation of these nanoparticles seems to be lethal to the algal itself but the accumulation of nanoparticles continues after its mortality possibly via functional groups within the non-living biomass. These results undoubtedly verify the strong antimicrobial potency of biogenic silver nanoparticles. Given the other merits of their green, cost-free, and easy synthesis, biogenic nanosilver represents the most plausible candidate for antimicrobial pharmaceuticals.
Therefore, we recommend the mass exploitation of the algal biomass for the purpose of nanoparticles pharmaceutical production.
Acknowledgments
The author acknowledges the Deanship of Scientific Research at King Faisal University for the financial support under Nasher Track Number NA000170. The author also would like to express their gratitude for Aziza Ibrahim AlIbrahim and AlAnoud Al Homaidan for their technical assistance with chemical analyses and Hany Alfrhan for technical assistance with molecular work. The authors would like to express their deepest gratitude for College of Veterinary Medicine for allowing the use of SEM facility under the guidance of Distinguished Dr Abud El Rahman Hreba.
Abbreviations
| AgNPs | Silver nanoparticles |
| KSA | Kingdom of Saudi Arabia |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| DNA | Deoxyribonucleic acid |
Author Contributions
The author contributed wholly to the manuscript. N.A.H.E.S. was responsible for conception, work design, microalgal isolation, and characterization, nanoparticles biosynthesis and characterization, electron microscopy as well as the writing up. She was also responsible for interpretation of data. M.F.A. was responsible for antimicrobial bioassay, MIC investigation, data interpretation, writing up and Obtaining fund for the research from Deanship of Scientific research, King Faisal University, Nasher track number NA000170. M.A.A.K. was responsible for RNA extraction, electron microscopy, work design, interpretation of data and writing up. All authors have read and agreed to the published version of the manuscript.
Funding
The author acknowledges the financial and moral support and facilities provided by King Faisal University for the conduction of research. The research was funded by Deanship of Scientific research, King Faisal University, AlAhsa, KSA, Nasher trackNA000170.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Merin D.D., Prakash S., Bhimba B.V. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac. J. Trop. Med. 2010;3:797–799. doi: 10.1016/S1995-7645(10)60191-5. [DOI] [Google Scholar]
- 2.Dahoumane S.A., Djediat C., Yéprémian C., Couté A., Fiévet F., Coradin T., Brayner R. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J. Nanoparticle Res. 2012;14:883. doi: 10.1007/s11051-012-0883-8. [DOI] [Google Scholar]
- 3.Jacob J.M., Ravindran R., Narayanan M., Samuel S.M., Pugazhendhi A., Kumar G. Microalgae: A prospective low cost green alternative for nanoparticle synthesis. Curr. Opin. Environ. Sci. Health. 2021;20:100163. doi: 10.1016/j.coesh.2019.12.005. [DOI] [Google Scholar]
- 4.Makarov V., Love A.J., Sinitsyna O., Makarova S., Yaminsky I., Taliansky M., Kalinina N. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014;6:35–44. doi: 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal P., Gupta R., Agarwal N. Advances in Synthesis and Applications of Microalgal Nanoparticles for Wastewater Treatment. J. Nanotechnol. 2019;2019:7392713. doi: 10.1155/2019/7392713. [DOI] [Google Scholar]
- 6.Li S.N., Wang R.P., Ho S.H. Algae-mediated biosystems for metallic nanoparticle production: From synthetic mechanisms to aquatic environmental applications. J. Hazard. Mater. 2021;420:126625. doi: 10.1016/j.jhazmat.2021.126625. [DOI] [PubMed] [Google Scholar]
- 7.Patel V., Berthold D., Puranik P., Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015;5:112–119. doi: 10.1016/j.btre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sheekh Mostafa M., El-Kassas Hala Y. Algal production of nano-silver and gold: Their antimicrobial and cytotoxic activities: A review. J. Gene. Eng. Biotechnol. 2016;14:299–310. doi: 10.1016/j.jgeb.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SadowskiI Z., Maliszewska I.H., GrochowalskaR B., Polowczyk I., Kozlecki T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci. Pol. 2008;26:419–424. [Google Scholar]
- 10.Iravani S. Magnetic Nanostructures. Springer; Cham, Switzerland: 2019. Bio-based synthesis of magnetic nanoparticles and their applications; pp. 13–31. [Google Scholar]
- 11.Punjabi K., Choudhary P., Samant L., Mukherjee S., Vaidya S., Chowdhary A. Biosynthesis of nanoparticles: A review. Int. J. Pharm. Sci. Rev. Res. 2015;30:219–226. [Google Scholar]
- 12.Rehman F.U., Jiang H., Selke M., Wang X.M. Mammalian cells: A unique scaffold for in situ biosynthesis of metallic nanomaterials and biomedical applications. J. Mater. Chem. B. 2018;6:6501–6514. doi: 10.1039/C8TB01955J. [DOI] [PubMed] [Google Scholar]
- 13.Hafez E. Antimicrobial activity of nano-silver particles produced by microalgae. J. Pure Appl. Microbiol. 2013;7:35–42. [Google Scholar]
- 14.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1996;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 15.Golus J., Sawicki R., Widelski J., Ginalska G. The agar microdilution method: A new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016;121:1291–1299. doi: 10.1111/jam.13253. [DOI] [PubMed] [Google Scholar]
- 16.Yasin S., Liu Y. Biosynthesis of silver nanoparticles by bamboo leaves extract and their antimicrobial activity. J. Fiber Bioeng. Inform. 2013;6:77–84. [Google Scholar]
- 17.Bakir E., Younis N.S., Mohamed M.E., El Semary N. Cyanobacteria as nanogold factories: Chemical and Anti-Myocardial infarction properties of gold nanoparticles synthesized by Lyngbya majuscula. Mar. Drugs. 2018;16:217. doi: 10.3390/md16060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al dayel M.F., El Semary N.A., Al Amer K., Al Ali K.M. Investigating the applications of Chlorella vulgaris in agriculture and nanosilver production. J. Environ. Biol. 2020;41:1099–1104. doi: 10.22438/jeb/41/5/MRN-1395. [DOI] [Google Scholar]
- 19.Soleimani M., Habibi-Pirkoohi M. Biosynthesis of silver nanoparticles using Chlorella vulgaris and evaluation of antibacterial efficacy against Staphylococcus aureus. Avicenna J. Med. Biotechnol. 2017;9:120–125. [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C., Van Zalinge H., Pearson J.L., Glidle A., Cooper J.M., Cumming D.R.S., Haiss W., Yao J., Schiffrin D.J., Proupín-Pérez M., et al. A combined top-down bottom-up approach for introducing nanoparticle networks into nanoelectrode gaps. Nanotechnology. 2006;17:3333–3339. doi: 10.1088/0957-4484/17/14/001. [DOI] [PubMed] [Google Scholar]
- 21.Bindu M.R., Umadevi M. Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;128:37–45. doi: 10.1016/j.saa.2014.02.119. [DOI] [PubMed] [Google Scholar]
- 22.Brayner R., Barberousse H., Hemadi M., Djedjat C., Yéprémian C., Coradin T., Livage J., Fiévet F., Couté A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007;7:2696–2708. doi: 10.1166/jnn.2007.600. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Dasari T.P.S., Deng H., Yu H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C. 2015;33:286–327. doi: 10.1080/10590501.2015.1055161. [DOI] [PubMed] [Google Scholar]
- 24.Skulberg O.M. Microalgae as a source of bioactive molecules–experience from cyanophyte research. Environ. Boil. Fishes. 2000;12:341–348. doi: 10.1023/a:1008140403621. [DOI] [Google Scholar]
- 25.Naraginti S., Kumari P.L., Das R.K., Sivakumar A., Patil S.H., Andhalkar V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. 2016;62:293–300. doi: 10.1016/j.msec.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 26.Shabudeen S., Arasu A., Ponnuswamy I. The uses of Chlorella vulgaris as antimicrobial agent and as a diet: The presence of bioactive compounds which caters the vitamins, minerals in general. Int. J. Bio-Sci. Bio-Technol. 2015;7:185–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.