Abstract
Background & Aims: Previous results from observational, interventional studies and in vitro experiments suggest that certain micronutrients possess anti-viral and immunomodulatory activities. In particular, it has been hypothesized that zinc, selenium, copper and vitamin K1 have strong potential for prophylaxis and treatment of COVID-19. We aimed to test whether genetically predicted Zn, Se, Cu or vitamin K1 levels have a causal effect on COVID-19 related outcomes, including risk of infection, hospitalization and critical illness. Methods: We employed a two-sample Mendelian Randomization (MR) analysis. Our genetic variants derived from European-ancestry GWAS reflected circulating levels of Zn, Cu, Se in red blood cells as well as Se and vitamin K1 in serum/plasma. For the COVID-19 outcome GWAS, we used infection, hospitalization or critical illness. Our inverse-variance weighted (IVW) MR analysis was complemented by sensitivity analyses including a more liberal selection of variants at a genome-wide sub-significant threshold, MR-Egger and weighted median/mode tests. Results: Circulating micronutrient levels show limited evidence of association with COVID-19 infection, with the odds ratio [OR] ranging from 0.97 (95% CI: 0.87–1.08, p-value = 0.55) for zinc to 1.07 (95% CI: 1.00–1.14, p-value = 0.06)—i.e., no beneficial effect for copper was observed per 1 SD increase in exposure. Similarly minimal evidence was obtained for the hospitalization and critical illness outcomes with OR from 0.98 (95% CI: 0.87–1.09, p-value = 0.66) for vitamin K1 to 1.07 (95% CI: 0.88–1.29, p-value = 0.49) for copper, and from 0.93 (95% CI: 0.72–1.19, p-value = 0.55) for vitamin K1 to 1.21 (95% CI: 0.79–1.86, p-value = 0.39) for zinc, respectively. Conclusions: This study does not provide evidence that supplementation with zinc, selenium, copper or vitamin K1 can prevent SARS-CoV-2 infection, critical illness or hospitalization for COVID-19.
Keywords: COVID-19, SARS-CoV-2, copper, zinc, selenium, vitamin K1, Mendelian Randomization
1. Introduction
Highly transmissible and virulent in at-risk groups, SARS-CoV-2, the causal agent for COVID-19, has been sweeping the globe since December 2019. Despite intensive research, there are few effective prophylactic and early stage therapeutic interventions for COVID-19, with the exception of vaccines [1,2]. However, worldwide vaccine distribution remains highly inequitable, with less than 4% of the African population vaccinated as of September 2021 [3].
Corollary to drug repurposing efforts, the potential importance of micronutrients and their supplementation in preventing and alleviating COVID-19 has been proposed [4,5]. Supplements have some unique advantages as they are inexpensive, widely available over-the-counter, easily distributed and stored, generally well tolerated and well characterised in terms of safety. However, unlike drugs, the quality assurance of dietary supplements is not well-regulated, which can lead to risks associated with contamination, adulteration, fraudulent ingredients [6] and an incorrect, potentially toxic dosage [7,8]. Amongst vitamins and minerals, good mechanistic reasons for more research exist for zinc, selenium, copper and vitamin K1, chiefly due to the important roles of Ze, Se and Cu in immune and antiviral responses [4,9] while for vitamin K1 the primary focus is its role in coagulation, which is crucial for pathogenesis of severe COVID-19 [10].
Zinc (Zn) is an essential trace metal with structural roles in regulatory proteins, as an enzyme cofactor, and as a signalling molecule. Of relevance to COVID-19, zinc deficiency can lead to a dysfunctional immune response with reduced activity of innate immune cells [11,12], lymphopenia [13] and activation of NF-KB signalling inducing production of IL-6 and other cytokines involved in “cytokine storm”, characteristic of COVID-19 [14,15,16]. Furthermore, zinc has manifold antiviral properties in vitro and in vivo [17,18]. With respect to SARS-CoV, Zn combined with a ionophore was shown to inhibit its RNA polymerase and block virus replication in cell culture [19]. In SARS-CoV-2, Zn2+ inhibits the main protease (Mpro) which results in reduced viral replication in cell culture [20]. Limited evidence from randomized trials on common cold suggests beneficial effect of zinc supplementation on cold duration and less conclusively, incidence and severity [21], while adjuvant treatment in severe paediatric pneumonia reduced hospital stay [22].
Selenium (Se) is a constituent of 25 selenoproteins with functions in redox homeostasis, endoplasmic reticulum stress and the inflammatory response [23]. Overall, Se can be a partial determinant of viral virulence [24]. Sub-optimally low Se intake is associated with coxsackievirus infection in aetiology of Keshan disease [25]. Furthermore, immunocompetence for infection clearance with other viral diseases is decreased in Se deficiency [7] and marginal Se status [26]. In vitro, Se supplementation was shown to inhibit the replication of the porcine circovirus [27]. Ebselen, a synthetic organoselenium compound, was found to be one of the most effective SARS-CoV-2 main protease (Mpro) inhibitors by forming a selenyl sulphide bond with the protease’s catalytic dyad [28,29], which provides a potential mode of antiviral action for organic selenium molecules, similar to ionic zinc. In addition, ebselen is functionally related to glutathione peroxidase 1, a major selenoenzyme which has been also found to physically interact with Mpro [30]. Other mechanisms through which Se could assist in COVID-19 management is through control of ROS-driven endothelial damage [23], reduced IL-6 pathway response [31,32,33] and the stimulation of the innate immune system [34,35].
Copper (Cu) is indispensable for the processes of respiration, free-radical defence and immune regulation due to its structural role in cuproenzymes [9,36]. Similar to Zn, Cu plays an essential role in the antioxidant response activated during inflammation. Copper deficiency can result in neutropenia and immunosuppression via reduced T-cell proliferation [37]. The inactivation of viruses, including SARS-CoV-2 [38] on copper surfaces is widely exploited in clinical practice [39], but Cu2+ was also reported to decrease the infectivity of HIV [40], influenza [41] virus and SARS-CoV-2 [42] in mammalian cells. Copper can also exert antiviral properties potentially by stimulating autophagy [43].
Two vitamers of vitamin K exist: K1 (phylloquinone) and K2 (menaquinone) [10]. Vitamin K is necessary for activation of pro- and anti- clotting factors in the liver and peripheral tissues, respectively. Moreover, vitamin the K activates Matrix Gla protein (MGP) which inhibits elastic fibre degradation and vascular mineralisation. Extrahepatic vitamin K deficiency and low MGP activity have been found in hospitalised COVID-19 patients [44,45]. According to Janssen et al. (2021) [46], this could result from increased degradation of elastic fibres by SARS-CoV-2 promoting lung fibrosis and concomitant with predicted depletion of endothelial vitamin K-dependent anti-coagulant (protein S) lead to coagulopathy. Therefore, vitamin K could provide an adjunct therapy of thrombosis events which are characteristic of severe COVID-19 [47].
In the absence of well-powered randomized control trials (RCT) testing the prophylactic and therapeutic potential of these micronutrients, we decided to carry out a Mendelian Randomization (MR) assessment of their potential causal effects. MR is an established causal inference method that uses genetic variants as instrumental variables [48].
Since genetic variants are independently and randomly distributed at meiosis and established at conception, the risk of confounding and reverse causation is greatly reduced in MR [48]. This is especially important as any observational studies linking nutrients levels to COVID-19 outcomes are confounded by fact that COVID-19 at-risk groups (e.g., the elderly, high BMI individuals, diabetics [49]) have, on average, lower/suboptimal levels of many micronutrients [13,15,18,44,46,50,51,52] and, at the same time, suffer from poorer COVID-19 outcomes. MR analyses can help to clarify the causal pathway in such cases. As such, MR has been successfully applied in nutritional epidemiology [53], including in studies using our micronutrients of interest as exposure [54,55,56,57,58,59,60]. Here, we apply the MR framework to test whether genetically predicted Zn, Se, Cu or vitamin K1 levels are causally related to COVID-19 outcomes: risk of infection, hospitalization and critical illness.
2. Material & Methods
2.1. Selection of Genetic Instruments—Relevance MR Criterion
2.1.1. GWAS Studies
We searched the literature, in the OpenGWAS [61] and GWAS Catalog [62], for genetic instruments associated with zinc, copper, selenium and vitamin K1 levels in populations of European ancestry.
We evaluated genetic instruments from the published GWAS of zinc, copper and selenium content of erythrocytes in the Queensland Institute of Medical Research (QIMR) twin cohort and whole blood selenium in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort of pregnant women [63], measured using inductively coupled plasma mass spectrometry. The red blood concentrations of those trace elements generally represent the overall nutritional status well [64] and the total blood measurement is a standard biomarker [65]. GWAS was adjusted for the following covariates: the analysis batch, haemoglobin concentration and analytical QC data.
For selenium instruments, we used a published fixed-effects meta-analysis of toe-nail selenium concentration, measured using neutron activation analysis in four European-ancestry US cohorts (Coronary Artery Risk Development in Young Adults, Johnston County Osteoarthritis Project, Nurses’ Health Study, Health Professionals Follow-up Study) co-analysed with the QIMR & ALSPAC GWAS results [66]. Toe-nail Se GWAS was adjusted for the following covariates: age, smoking status, geography and top eigenvectors. Compared to the circulating selenium, toe-nail content reflects more long-term Se exposure.
Vitamin K instruments were derived from a GWAS of phylloquinone (vitamin K1), the primary circulating form of vitamin K, for which the measurements were available in two European ancestry CHARGE cohorts [67], namely, the Framingham Offspring Consortium and the Health, Aging and Body Composition study. Phylloquinone measurements were obtained in plasma/serum using reverse-phase high-performance liquid chromatography followed by fluorometric detection. Vitamin K1 concentration in plasma reflects recent intake [44]. The GWAS included the following covariates: age, sex and study-specific stratification, including population structure.
Further details regarding GWAS cohorts, sample collection, analysis and quality control are provided in the Supplementary Methods.
We do not have detailed information on supplementation in the exposure GWAS studies, other than for the toe-nail selenium analysis, and individuals with toenail Se concentrations > 2.0 μg/g were excluded, as this could “reflect exogenous contamination or considerable excess ingestion of Se supplements”. Genetic variants used as instrumental variables can influence life-long absorption, distribution and excretion of environmentally available micronutrient concentrations, whether dietary or supplemental, but within a natural range. Any substantial supplementation among the subjects of exposure GWAS could potentially introduce a measurement error and thereby dilute the effect seen in MR analysis and is one limitation of our study design.
For each micronutrient phenotype, we clumped the instruments using PLINK ver. v1.90b4.1 [68] and 1000 Genomes European reference panel [69], at the threshold of r2 < 0.05 and clumping distance of 10 Mbp. The final set of instruments was derived by combining one representative SNP from each clump selected based on lowest p-value and presence in the outcome dataset (Supplementary Figure S1).
2.1.2. Zinc Genetic Instruments
One of the two instruments for zinc was missing (rs1532423); however, we found a good proxy for the missing instrument using 1000 Genomes European population using the LDproxy web app [70]: rs2453868, situated 34,383 bp away with r2 of 0.93 and D′ of 1 (correlated alleles: rs1532423A = rs2453868C, rs1532423G = rs2453868T). Overall, rs2120019 and rs1532423 account for 4.6% of variance in red blood cell copper concentration (Supplementary Table S1).
2.1.3. Selenium Genetic Instruments
Out of the 12 genome-wide significant SNPs found at two loci in the meta-analysis, four of the SNPs survived LD-pruning. However, only two variants could be used as instruments (rs921943, rs6859667) as SNPs in the other two clumps (rs6586282, rs1789953, rs234709) or their proxies were not available in the COVID-19 GWAS. The two instruments in the meta-analysis accounted for only 2.25% of variance in the trait (Supplementary Table S1). The results of the meta-analysis were initially presented as Z-scores which we converted to betas using the formula in Taylor et al. (2016) [71].
2.1.4. Copper Genetic Instruments
Two genome-wide significant instruments were identified: rs1175550 and rs2769264; altogether they account for 4.6% of variance in red blood cell copper concentration (Supplementary Table S1).
2.1.5. Vitamin K1 Genetic Instruments
Overall, 11 signals at 6 loci were detected in the GWAS discovery stage at p-value < 5 × 10−5, with none of the SNPs reaching genome-wide significance. We found four SNPs of concern which were removed from downstream processing (Supplementary Methods). The three variants retained: rs4645543, rs4852146, rs6862071 (Supplementary Table S1) jointly explained 3.06% of variance in circulating phylloquinone concentration.
2.1.6. Sensitivity Analyses Using Subsignificant Hits
In addition to the main analysis, where we included only genome-wide significant hits at p-value 5 × 10−8 (with the exception of vitamin K1, for which no variants crossed the threshold), we ran sensitivity analyses including variants selected at a more liberal, sub-significant threshold of 5 × 10−5. This allowed us to enhance the statistical power of analysis at the increased risk of violation of core MR assumptions. For zinc (Supplementary Table S2), we used 12 SNPs (total R2 of 14%), while for selenium we only had access to results from individual cohorts: QIMR (15 SNPs, R2 = 13.26%) and ALSPAC (12 SNPs, R2 = 10.93%) and for copper we arrived at 7 SNPs (R2 = 10.03%).
2.1.7. Independence MR Criterion
We calculated the variance in each exposure, explained by each set of instruments (R2) and F-statistics using the formulas in Yarmolinsky et al. (2018) [57]. We did not observe any weak instrument bias, with the F-statistic ranging from 15 to 172 (Supplementary Tables S1 and S2).
2.1.8. Exclusion Restriction MR Criterion
Both PhenoScanner V2 [72] and OpenGWAS [61] were used to assess presence of horizontal pleiotropy among the candidate variants using default settings (Supplementary Tables S3 and S4, Supplementary Methods).
In addition to this, we conducted a leave-one-out analysis in our sensitivity checks which should minimize any possible confounding introduced by individual SNPs associated with height and RBC traits, whenever possible. Next, we calculated Cochran’s Q statistic and I2 to look for signs of heterogeneity, also indicative of pleiotropy. Finally, MR-Egger is one of the MR methods which we employed and which can detect directional horizontal pleiotropy if the intercept significantly deviates from 0 [73].
2.1.9. Selection of Outcomes
The largest publicly available GWAS to date on COVID-19, provided in the COVID-19 Host Genetics Initiative release 5, was selected [74]. This fixed-effect meta-analysis contains up to 49,562 COVID-19 patients and 2 million controls from 46 studies across 19 countries, however, we used results obtained in 35 European-only cohorts. The outcomes available were very severe (critical) COVID-19 (vs. population), hospitalized (vs. SARS-CoV-2 infected but non-hospitalized with COVID-19 or vs. population) and SARS-CoV-2 infection (vs. population) (Supplementary Table S5). The covariates used in the GWAS analysis were age and sex.
2.1.10. Statistical Analysis
We used the online mRnd power calculator to explore the limits of our MR analysis [75]. All the MR analyses were performed using TwoSampleMR [76] and MendelianRandomization [77] R packages. Our main method was the inverse variance weighted (IVW) random-effects meta-analysis of causal effects of individual instruments, as it is the most efficient; however, the IVW is biased in cases of unbalanced pleiotropy [78]. We complemented IVW with analyses using other MR methods: MR-Egger [73], weighted median-based and mode-based estimator. MR-Egger relaxes the assumption of balanced horizontal pleiotropy at the cost of reduced power, whereas weighted median estimator is still valid if only min. Of the total variants, 50% meet the three main MR assumptions [48]; mode-based estimator is similarly robust to outliers while being more conservative.
2.1.11. Ethics Statement
This study used publicly available summary data and no original data collection was undertaken for this manuscript. Evidence of ethical approval for all of the included GWAS studies can be found in previous publications. Our investigation is in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
3. Results
3.1. Power Analysis
In the main analysis, SARS-CoV-2 infection was the outcome with the greatest power due to the highest number of cases and controls in the outcome GWAS. The minimum detectable one-tailed odds ratio (OR) at an 80% power ranged from 0.91 for selenium to 0.93 for zinc and copper (Supplementary Table S6). Furthermore, a COVID-19 hospitalization analysis revealed lower power, ranging from OR of 0.82 for Se to 0.87 for Zn and Cu. Poor power was found for very severe COVID-19 (OR of 0.75 for Se to 0.81 for Zn and Cu). In order to increase the power in our sensitivity analyses, we used sets of genome-wide sub-significant (max p-value of 5 × 10−5) variants for zinc, selenium and copper (Supplementary Table S7). In the sub-significant instrument analyses, minimum detectable one-tailed odds ratios at 80% power were 0.95–0.96 for SARS-CoV-2 infection and 0.87–0.89 for very severe (critical) COVID-19.
3.2. Mendelian Randomization
3.2.1. Zinc
We found little evidence in favor of the genetically predicted circulating zinc concentration having a large effect on COVID-19 outcomes (Table 1, Figure 1). IVW odds ratios of SARS-CoV-2 infection were 0.97 (95% CI: 0.87–1.08, nominal p-value = 0.55) in the main analysis and 1.01 (95% CI: 0.98–1.05, nominal p-value = 0.49) in the sensitivity sub-significant (max p-value of 5 × 10−5) analysis, per 1 SD increase of circulating zinc. Hospitalization (ver. Population) showed weak evidence of association with zinc levels (OR = 1.06, 95% CI: 0.81–1.39, p-value = 0.66) in the main analysis and sub-significant analysis (OR = 0.98, 95% CI: 0.91–1.06, p-value = 0.62). Similar results were obtained for the hospitalized (ver. Non-hospitalized) outcome (Table 1, Figure 1). Lastly, we did not find a strong effect for zinc on all severe diseases included in the main analysis (OR = 1.21, 95% CI: 0.79–1.86, p-value = 0.39). A narrower but overlapping estimate was derived from the more liberal set of SNPs (OR = 0.92, 95% CI: 0.81–1.04, p-value = 0.16). Estimates in the sensitivity analyses using MR-Egger, median-weighted and mode-weighted estimator directionally matched the results from IVW (Figure 1, Supplementary Table S8).
Table 1.
Results of MR analysis studying the effect of circulating zinc (Zn), selenium (Se), copper (Cu) and vitamin K1 concentration on 3 COVID-19 outcomes. Inverse-variance weighted (IVW)-based odds ratios, their p-values along with Cochrane’s Q statistic and MR-Egger intercept are presented. We used two sets of instruments whenever possible: Zn/Se/Cu refers to instruments with p-values < 5 × 10−8 and Zn/Se/Cu/vit. K1 sub-significant refers to instruments with p-values < 5 × 10−5.
| Exposure | Outcome | n SNPs | IVW Odds Ratio (95% CI) | IVW p-Value 1 | Cochrane’s Q | Cochrane’s Q p-Value 1 | MR-Egger Intercept | MR-Egger Intercept p-Value 1 |
|---|---|---|---|---|---|---|---|---|
| Zn | SARS-CoV-2 infection | 2 | 0.97 (0.87–1.08) | 0.548 | 0.73 | 0.394 | NA 2 | NA 2 |
| Zn | Hospitalized (ver. non-hospitalized) | 2 | 0.99 (0.69–1.44) | 0.971 | 0.02 | 0.889 | NA 2 | NA 2 |
| Zn | Hospitalized (ver. population) | 2 | 1.06 (0.81–1.39) | 0.663 | 1.62 | 0.203 | NA 2 | NA 2 |
| Zn | Very severe COVID-19 | 2 | 1.21 (0.79–1.86) | 0.386 | 2.09 | 0.148 | NA 2 | NA 2 |
| Zn subsignificant | SARS-CoV-2 infection | 12 | 1.01 (0.98–1.05) | 0.489 | 7.32 | 0.772 | 0.898 | 0.468 |
| Zn subsignificant | Hospitalized (ver. non-hospitalized) | 12 | 0.97 (0.85–1.11) | 0.688 | 14.09 | 0.228 | 0.717 | 0.496 |
| Zn subsignificant | Hospitalized (ver. population) | 12 | 0.98 (0.91–1.06) | 0.623 | 13.40 | 0.268 | 0.108 | 0.424 |
| Zn subsignificant | Very severe COVID-19 | 12 | 0.92 (0.81–1.04) | 0.161 | 13.37 | 0.270 | 0.845 | 0.340 |
| Se meta-analysis | SARS-CoV-2 infection | 2 | 1.03 (0.95–1.11) | 0.506 | 1.68 | 0.195 | NA 2 | NA 2 |
| Se meta-analysis | Hospitalized (ver. non-hospitalized) | 2 | 0.91 (0.75–1.11) | 0.347 | 0.58 | 0.445 | NA 2 | NA 2 |
| Se meta-analysis | Hospitalized (ver. population) | 2 | 0.98 (0.87–1.10) | 0.715 | 0.28 | 0.599 | NA 2 | NA 2 |
| Se meta-analysis | Very severe COVID-19 | 2 | 0.99 (0.83–1.17) | 0.864 | 0.22 | 0.638 | NA 2 | NA 2 |
| Se ALSPAC subsignificant | SARS-CoV-2 infection | 12 | 0.99 (0.95–1.03) | 0.704 | 9.66 | 0.561 | 0.104 | 0.457 |
| Se ALSPAC subsignificant | Hospitalized (ver. non-hospitalized) | 12 | 1.01 (0.88–1.16) | 0.844 | 12.15 | 0.353 | 0.675 | 0.439 |
| Se ALSPAC subsignificant | Hospitalized (ver. population) | 12 | 1.03 (0.95–1.12) | 0.453 | 4.62 | 0.948 | 0.262 | 0.522 |
| Se ALSPAC subsignificant | Very severe COVID-19 | 12 | 1.06 (0.94–1.19) | 0.369 | 6.77 | 0.817 | 0.278 | 0.642 |
| Se QIMR subsignificant | SARS-CoV-2 infection | 15 | 1.00 (0.97–1.03) | 0.974 | 9.35 | 0.808 | 0.973 | 0.392 |
| Se QIMR subsignificant | Hospitalized (ver. non-hospitalized) | 15 | 1.04 (0.94–1.16) | 0.412 | 17.82 | 0.215 | 0.050 | 0.352 |
| Se QIMR subsignificant | Hospitalized (ver. population) | 15 | 1.06 (1.00–1.12) | 0.033 | 13.47 | 0.490 | 0.212 | 0.363 |
| Se QIMR subsignificant | Very severe COVID-19 | 15 | 1.07 (0.99–1.16) | 0.069 | 11.77 | 0.624 | 0.679 | 0.371 |
| Cu | SARS-CoV-2 infection | 2 | 1.07 (1.00–1.14) | 0.057 | 0.66 | 0.415 | NA 2 | NA 2 |
| Cu | Hospitalized (ver. non-hospitalized) | 2 | 0.98 (0.79–1.21) | 0.842 | 0.00 | 0.984 | NA 2 | NA 2 |
| Cu | Hospitalized (ver. population) | 2 | 1.07 (0.88–1.29) | 0.493 | 2.24 | 0.135 | NA 2 | NA 2 |
| Cu | Very severe COVID-19 | 2 | 1.13 (0.82–1.55) | 0.467 | 2.84 | 0.092 | NA 2 | NA 2 |
| Cu subsignificant | SARS-CoV-2 infection | 7 | 1.01 (0.96–1.07) | 0.662 | 11.30 | 0.080 | 0.022 | 0.227 |
| Cu subsignificant | Hospitalized (ver. non-hospitalized) | 7 | 0.98 (0.86–1.12) | 0.792 | 1.39 | 0.967 | 0.006 | 0.882 |
| Cu subsignificant | Hospitalized (ver. population) | 7 | 0.99 (0.91–1.08) | 0.816 | 5.09 | 0.532 | 0.018 | 0.493 |
| Cu subsignificant | Very severe COVID-19 | 7 | 0.94 (0.83–1.07) | 0.326 | 3.87 | 0.694 | 0.017 | 0.672 |
| vit. K1 subsignificant | SARS-CoV-2 infection | 3 | 0.99 (0.93–1.05) | 0.677 | 0.95 | 0.621 | 0.507 | 0.000 |
| vit. K1 subsignificant | Hospitalized (ver. non-hospitalized) | 3 | 1.06 (0.88–1.28) | 0.565 | 0.50 | 0.779 | 0.697 | 0.000 |
| vit. K1 subsignificant | Hospitalized (ver. population) | 3 | 0.98 (0.87–1.09) | 0.662 | 0.62 | 0.732 | 0.593 | 0.000 |
| vit. K1 subsignificant | Very severe COVID-19 | 3 | 0.93 (0.72–1.19) | 0.546 | 4.42 | 0.110 | 0.349 | 0.084 |
1—Nominal p-value, 2—Insufficient number of SNPs for MR-Egger analysis.
Figure 1.
MR estimates for the effect of circulating zinc (Zn) on three COVID-19 outcomes obtained using 4 different statistical methods. We used two sets of zinc instruments: Zn refers to instruments with p-values < 5 × 10−8 and sub-significant Zn refers to instruments with p-values < 5 × 10−5.
3.2.2. Selenium
We detected a weak causal effect of meta-analysed selenium levels (per 1 SD increment) on SARS-CoV-2 infection, COVID-19 hospitalization and critical illness (Table 1 and Figure 2). IVW odds ratio of SARS-CoV-2 infection using the instruments in the GWAS meta-analysis of Se blood and toe-nail levels was 1.03 (95% CI: 0.95–1.11, nominal p-value = 0.5), while in the ALSPAC and QIMR sub-significant (max p-value of 5 × 10−5) sensitivity analyses, we found narrower CI intervals also overlapping 1: OR = 0.99 (95% CI: 0.95–1.03, p-value = 0.70) and 1.00 (0.97–1.03, p-value = 0.97), accordingly. Similar results were found for protection against hospitalization (ver. population) using the main (OR = 0.98; 95% CI: 0.87–1.10, p-value = 0.71), ALSPAC (OR = 1.03, 95% CI: 0.95–1.12, p-value = 0.45) and QIMR (OR = 1.06, 95% CI: 1.00–1.12, p-value = 0.03) sub-significant sets of instruments. The nominally significant increased risk of hospitalization with COVID-19 in the QIMR cohort did not survive multiple-correction testing. Similar estimates were found in the hospitalized COVID-19 (vs. non-hospitalized) comparison; however, for this outcome the point-estimate ORs were <1 in the QIMR cohort using the following sensitivity methods: MR-Egger, weighted median and mode (Figure 2, Supplementary Table S8). Finally, increased genetically predicted selenium levels did not causally associate with COVID-19 severity using the meta-analysis (OR = 0.99, 95% CI: 0.83–1.17, p-value = 0.86), ALSPAC (OR = 1.06; 95% CI: 0.94–1.19, p-value = 0.37) or QIMR (OR = 1.07; 95% CI: 0.99–1.16, p-value = 0.07) sub-significant instruments.
Figure 2.
MR estimates for the effect of circulating selenium (Se) on three COVID-19 outcomes obtained using 4 different statistical methods. We used three sets of selenium instruments: Se refers to instruments with p-values < 5 × 10−8 and sub-significant Se refers to instruments with p-values < 5 × 10−5 in the ALSPAC and QIMR cohorts.
3.2.3. Copper
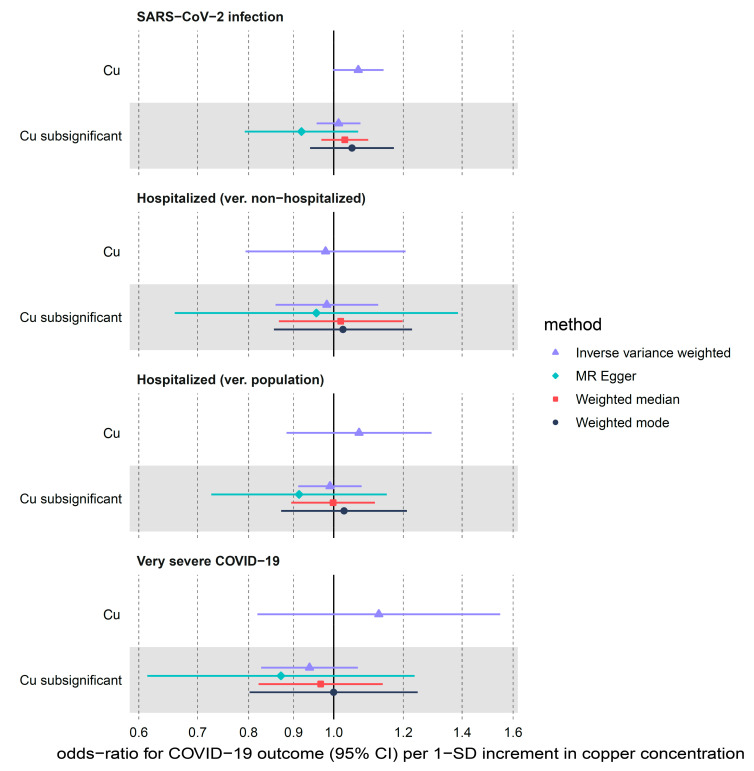
Limited evidence for the predicted increase in the risk of SARS-CoV-2 infection per 1 SD increment in circulating copper levels (IVW OR = 1.07, 95% CI: 1.00–1.14, nominal p-value = 0.06) was attenuated (Table 1, Figure 3) using the sensitivity sub-significant (max p-value of 5 × 10−5) instrument set (OR= 1.01; 95% CI: 0.96–1.07, p-value = 0.66). For the two other outcomes, we found non-significant > 1 OR point estimates in the main analysis (OR = 1.07, 95% CI: 0.88–1.29, p-value = 0.49 for hospitalized ver. population and OR = 1.13; 95% CI: 0.82–1.55, p-value = 0.47 for very severe COVID-19) which were flipped <1 in the sub-significant instrument analysis (OR = 0.99, 95% CI: 0.91–1.08, p-value = 0.82 and OR = 0.94, 95% CI: 0.83–1.07, p-value = 0.33, respectively) using the IVW method as well as MR-Egger and weighted median (Figure 3, Supplementary Table S8).
Figure 3.
MR estimates for the effect of circulating copper (Cu) on three COVID-19 outcomes obtained using 4 different statistical methods. We used the following two sets of copper instruments: Cu refers to instruments with p-values < 5 × 10−8 and sub-significant Cu refers to instruments with p-values < 5 × 10−5.
3.2.4. Vitamin K1
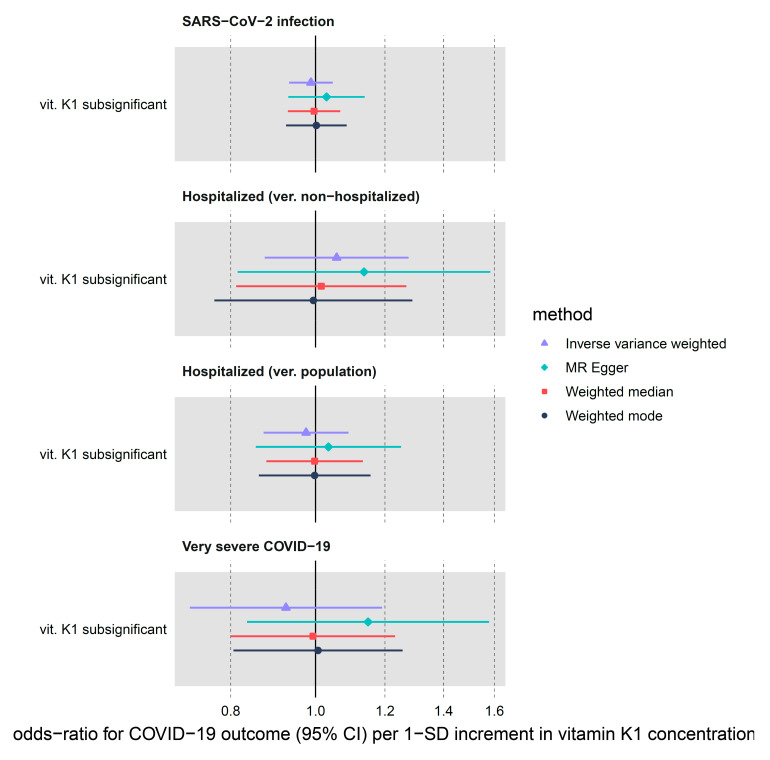
Using a limited set of three vitamin K1 genome-wide sub-significant (max p-value of 5 × 10−5) instruments, we were not able to detect any strong effect of genetically predicted circulating vitamin K1 on COVID-19 outcomes (Table 1, Figure 4). In the IVW analysis, vitamin K1 increment (per natural log transformed nmol/L) associated with OR of 0.99 (95% CI: 0.93–1.05, p-value = 0.68) for SARS-CoV-2 infection. Next, both hospitalization (ver. population) and very severe COVID-19 showed a lower OR for increased vitamin K1 exposure (OR = 0.98, 95% CI: 0.87–1.09, p-value = 0.66 and OR = 0.93, 95% CI: 0.72–1.19, p-value = 0.55, respectively) but the CI comfortably overlapped with OR on either side of 1. The confidence intervals for our sensitivity methods (MR-Egger, weighted median and mode) overlapped the IVW CI, although the OR point estimates sometimes differed in terms of direction of effect.
Figure 4.
MR estimates for the effect of circulating vitamin K1 on three COVID-19 outcomes obtained using 4 different statistical methods. We only had access to instruments with sub-significant p-values < 5 × 10−5.
3.2.5. Pleiotropic Bias
We did not detected a significant heterogeneity of effects between our variants using Cochrane’s Q and pleiotropy using MR-Egger intercept in the zinc, selenium and copper MR analysis (Table 1). However, for vitamin K1 we did find directional pleiotropy using MR-Egger, with the intercept differing significantly from 0. Therefore, causal estimates using this set of instruments are biased and need to be interpreted cautiously.
4. Discussion
Our analyses attempted to elucidate the potential of zinc, selenium, copper and vitamin K1 in prophylaxis and treatment of COVID-19 using MR techniques. However, we found little evidence of a causal association of genetically predicted micronutrient concentration on COVID-19 outcomes.
Zinc’s many antiviral, immunomodulatory and anti-inflammatory functions have generated a lot of interest for its potential use in COVID-19 management [13,18,51,52,79]. Consequently, over 50 RCTs, including zinc, albeit typically used as adjuvant treatment or prophylactic, have commenced (ClinicalTrials.Gov, (accessed on 1 September 2021)). One of the first to publish results, The COVID A to Z trial, which tested the direct effect of high-dose zinc supplementation, found no reduction in symptom duration in outpatients and was terminated early on [80]. In terms of prophylaxis, no beneficial effect of zinc supplementation was reported among the 370,000 British users of the COVID-19 Symptom Study app [81]. However, small observational studies reported lower serum Zn levels as a predictor of illness severity [82,83,84,85]. As a caveat, all the real life data and MR analyses do not include the application of ionophores, which may be necessary for zinc’s antiviral inhibition [19,20].
Moreover, a number of observational studies established a positive correlation between low serum selenium levels and COVID-19 severity and mortality [83,86,87,88]. An ecological study found a significant positive association between hair selenium concentration and COVID-19 recovery rate in different provinces of China [89] which was subsequently replicated using local soil selenium concentrations as the predictor variable [90].
In general, one method by which to partially reconcile our findings for selenium and zinc with general micronutrient deficit in hospitalized or severely ill COVID-19 patients found in small studies derives from previous observations in critically ill individuals. Therefore, the initial hypozincemia and hyposelenemia is thought to stem from disease-driven inflammatory process (acute phase response), is found chiefly in plasma (but not, e.g., erythrocytes) [18,91,92] and recovers over time in survivors. However, this does not mean that pre-existing deficiencies will not have a compounded negative impact at this stage [52].
A recently published study suggested that Cu status is correlated with the survival status of COVID-19 patients [36]. In contrast, another study from Skalny et al. [88] reported that plasma copper levels and the Cu/Zn ratio increased in more severe disease. This could reflect the fact that copper and zinc are antagonistically absorbed [93] and high serum Cu/Zn ratio is a marker for infection, as zinc gets redistributed to liver in the acute phase of the infection [94].
Despite evidence supporting the role of coagulation modulation by vitamin K in COVID-19 severity and poorer outcomes among hospitalized COVID-19 patients with lower vitamin K status [44,45], we did not detect any effects of circulating phylloquinone on very severe COVID-19 or other outcomes.
We conducted multiple sensitivity analyses, involving different methods (e.g., MR-Egger) and instrument selection, which revealed consistent results. In general, the inclusion of pleiotropic variants in MR is likely to skew the results away from the null, so it is reassuring that we find no effects also in the analyses involving variants selected at a more liberal sub-significant p-value threshold [78] and in vitamin K1 analyses showing directional pleiotropic bias.
MR studies have confirmed RCT findings for many known risk factors, such as blood pressure and low-density lipoproteins [95]. MR has also indicated no causal effect of high-density lipoproteins and C-reactive protein [96,97] on cardiovascular disease, which, had it been reported earlier, could have saved a lot of effort and the cost of developing failed drugs. MR evidence can therefore deliver considerable insight about the prospect of a therapeutic. Here, current MR analyses do not support causal pathway between Zn, Se, Cu, vitamin K1 blood levels and COVID-19 outcomes.
Thanks to the MR framework’s use of genetic instrumental variables, the possibility of confounding and reverse causality was limited. Lately, some concern regarding the ubiquity of the collider bias in epidemiological investigations of COVID-19 was voiced [98]. However, the use of general-population control without a known COVID-19 infection in the outcome GWAS serves to decrease the likelihood of collider bias emerging, while not biasing effect size estimates in GWAS sensitivity analyses. We also included a hospitalized versus non-hospitalized GWAS outcome but even in these analyses at a higher risk of collider bias, the results broadly agreed with those from hospitalized versus population outcome. However, the ascertainment bias in the GWAS due to differential case reporting and varying SARS-CoV-2 exposure levels by, e.g., socio-economic status remains difficult to account for.
Another potential form of bias affecting the MR is population stratification. Since we included only European-ancestry samples in all our analyses, we limited the potential of this bias to skew the results. However, there are no strong biological reasons as to why our conclusions should not be generalizable to other ancestries.
The main limitation of our study is the relatively low power to detect modest effects of micronutrient levels on COVID-19 hospitalization and severity due to the few reliable genetic instruments available for micronutrients of interest and limited number of cases in the COVID-19 GWAS. This could be improved in the future as better-powered GWAS for both the exposures (particularly for vitamin K1, where no instruments reached genome-wide significance) and outcome become available. Since we used the same GWAS for instrument discovery and effect estimate, our analysis is likely to suffer from the “winners’ curse” and that it increased the weak instrument bias that would pull the results towards the null [78], which again could be rectified if new micronutrient GWASs are released.
Another limitation is that MR methods only used model linear effects within the normal range of micronutrient concentration, so any potential non-linear U/J-shaped, threshold effects will not be correctly estimated. Individual-level data for both exposure and outcome in the same population sample are required for such an analysis [78]. Additionally, MR cannot answer the question as to whether specific subgroups, such as micronutrient deficient individuals, can benefit from supplementation. Furthermore, the phenotypes used in an MR analysis typically correspond to lifelong exposure and small changes in micronutrient concentration, which does not exactly mirror intensive, high-dose clinical interventions.
5. Conclusions
In conclusion, we found little evidence of the effect of genetically predicted zinc, selenium, copper or vitamin K1 levels towards preventing infection with SARS-CoV-2, and disease progression, including hospitalization or developing very severe COVID-19. Similar MR findings were obtained for two other promising micronutrients, namely, vitamin C [99] and vitamin D [100,101], suggesting that the utility of dietary supplementation for general population in the COVID-19 pandemic may be limited.
Abbreviations
GWAS—Genome wide association study, MR—Mendelian Randomization, RCT—Randomized control trial.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14020233/s1, Supplementary Figure S1: Flowchart depicting instrument selection for the Mendelian Randomization analyses. Supplementary Table S1: Summary statistics of variants used as instrumental variables in the main study. Supplementary Table S2: Summary statistics of variants used as instrumental variables in the sensitivity analyses. Supplementary Table S3: Results of PhenoScanner and OpenGWAS pleiotropy scan for variants used in the main study. Supplementary Table S4: Results of PhenoScanner and OpenGWAS pleiotropy scan for variants used in the sensitivity analyses. Supplementary Table S5: Overview of COVID-19 GWAS used in the study. Supplementary Table S6: Power analysis results for the main study. Supplementary Table S7: Power analysis results for the sensitivity analyses. Supplementary Table S8: Full results of Mendelian Randomization analyses conducted. Supplementary File S1: Supplementary Methods. Supplementary File S2: STROBE-MR checklist.
Author Contributions
M.K.S. conceptualization, data curation, formal analysis, writing—original draft, writing—review and editing, T.R.G. funding acquisition, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the UK Medical Research Council [mc_uu_00011/4] and carried out in the MRC Integrative Epidemiology Unit.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All exposure data described in the article is provided in the supplementary tables. All outcome data is available from COVID-19 HGI website: https://www.COVID19hg.org/results/r5/ (accessed on 1 September 2021). Code for statistical analyses is available on: https://github.com/marynias/COVID19 (accessed on 5 January 2022).
Conflicts of Interest
TRG receives funding from Biogen for unrelated research.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siemieniuk R.A.C., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., Pardo-Hernandez H., Qasim A., Martinez J.P.D., Rochwerg B., et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ. 2020;370:1–18. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoszko J.J., Siemieniuk R.A.C., Kum E., Qasim A., Zeraatkar D., Ge L., Han M.A., Sadeghirad B., Agarwal A., Agoritsas T., et al. Prophylaxis against COVID-19: Living systematic review and network meta-analysis. BMJ. 2021;373 doi: 10.1136/bmj.n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Africa Africa Faces 470 Million COVID-19 Vaccine Shortfall in 2021. [(accessed on 16 September 2021)]. Available online: https://www.afro.who.int/news/africa-faces-470-million-COVID-19-vaccine-shortfall-2021.
- 4.Akhtar S., Das J.K., Ismail T., Wahid M., Saeed W., Bhutta Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2020;79:289–300. doi: 10.1093/nutrit/nuaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White C.M. Dietary Supplements Pose Real Dangers to Patients. Ann. Pharmacother. 2020;54:815–819. doi: 10.1177/1060028019900504. [DOI] [PubMed] [Google Scholar]
- 7.Rayman M.P. The argument for increasing selenium intake. Proc. Nutr. Soc. 2002;61:203–215. doi: 10.1079/PNS2002153. [DOI] [PubMed] [Google Scholar]
- 8.Maret W., Sandstead H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Fooladi S., Matin S., Mahmoodpoor A. Copper as a potential adjunct therapy for critically ill COVID-19 patients. Clin. Nutr. ESPEN. 2020;40:90–91. doi: 10.1016/j.clnesp.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudelko M., Yip T.F., Hei Law G.C., Lee S.M.Y. Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno. 2021;1:17–29. doi: 10.3390/immuno1010003. [DOI] [Google Scholar]
- 11.Keen C.L., Gershwin M.E. Zinc deficiency and immune function. Annu. Rev. Nutr. 1990;10:415–431. doi: 10.1146/annurev.nu.10.070190.002215. [DOI] [PubMed] [Google Scholar]
- 12.Dardenne M. Zinc and immune function. Eur. J. Clin. Nutr. 2002;56:S20–S23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- 13.Joachimiak M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021;15:e0008895. doi: 10.1371/journal.pntd.0008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020;146:518–534.e1. doi: 10.1016/j.jaci.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayor-Ibarguren A., Busca-Arenzana C., Robles-Marhuenda Á. A Hypothesis for the Possible Role of Zinc in the Immunological Pathways Related to COVID-19 Infection. Front. Immunol. 2020;11:1736. doi: 10.3389/fimmu.2020.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinni V., El-Khoury J., Perera M., Bellomo R., Jones D., Bolton D., Ischia J., Patel O. Zinc supplementation as an adjunct therapy for COVID-19: Challenges and opportunities. Br. J. Clin. Pharmacol. 2021;87:3737–3746. doi: 10.1111/bcp.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panchariya L., Khan W.A., Kuila S., Sonkar K., Sahoo S., Ghoshal A., Kumar A., Verma D.K., Hasan A., Das S., et al. Zinc2+ ion inhibits SARS-CoV-2 main protease and viral replication in vitro. Chem. Commun. 2021 doi: 10.1039/D1CC03563K. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 21.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst. Rev. 2013:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Brooks W.A., Yunus M., Santosham M., Wahed M.A., Nahar K., Yeasmin S., Black R.E. Zinc for severe pneumonia in very young children: Double-blind placebo-controlled trial. Lancet. 2004;363:1683–1688. doi: 10.1016/S0140-6736(04)16252-1. [DOI] [PubMed] [Google Scholar]
- 23.Bermano G., Méplan C., Mercer D.K., Hesketh J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021;125:618–627. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzanares W., Moreira E., Hardy G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): Does selenium have a place? Nutrition. 2021;81:110989. doi: 10.1016/j.nut.2020.110989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loscalzo J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014;370:1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- 26.Broome C.S., McArdle F., Kyle J.A.M., Andrews F., Lowe N.M., Hart C.A., Arthur J.R., Jackson M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004;80:154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Ren F., Hesketh J., Shi X., Li J., Gan F., Huang K. Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free Radic. Biol. Med. 2012;53:395–405. doi: 10.1016/j.freeradbiomed.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Amporndanai K., Meng X., Shang W., Jin Z., Rogers M., Zhao Y., Rao Z., Liu Z.-J., Yang H., Zhang L., et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021;12:3061. doi: 10.1038/s41467-021-23313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 30.Seale L.A., Torres D.J., Berry M.J., Pitts M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020;112:447–448. doi: 10.1093/ajcn/nqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuji P.A., Carlson B.A., Anderson C.B., Seifried H.E., Hatfield D.L., Howard M.T. Dietary Selenium Levels Affect Selenoprotein Expression and Support the Interferon-γ and IL-6 Immune Response Pathways in Mice. Nutrients. 2015;7:6529–6549. doi: 10.3390/nu7085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walston J., Xue Q., Semba R.D., Ferrucci L., Cappola A.R., Ricks M., Guralnik J., Fried L.P. Serum Antioxidants, Inflammation, and Total Mortality in Older Women. Am. J. Epidemiol. 2005;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 33.Kim I.Y., Stadtman T.C. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc. Natl. Acad. Sci. USA. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiremidjian-Schumacher L., Roy M., Wishe H.I., Cohen M.W., Stotzky G. Supplementation with selenium and human immune cell functions. Biol. Trace Elem. Res. 1994;41:115. doi: 10.1007/BF02917222. [DOI] [PubMed] [Google Scholar]
- 35.Ravaglia G., Forti P., Maioli F., Bastagli L., Facchini A., Mariani E., Savarino L., Sassi S., Cucinotta D., Lenaz G. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged ≥90 y. Am. J. Clin. Nutr. 2000;71:590–598. doi: 10.1093/ajcn/71.2.590. [DOI] [PubMed] [Google Scholar]
- 36.Hackler J., Heller R.A., Sun Q., Schwarzer M., Diegmann J., Bachmann M., Moghaddam A., Schomburg L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients. 2021;13:1898. doi: 10.3390/nu13061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Percival S.S. Copper and immunity. Am. J. Clin. Nutr. 1998;67:1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 38.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano. 2020;14:12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 40.Sagripanti J.-L., Lightfoote M.M. Cupric and Ferric Ions Inactivate HIV. AIDS Res. Hum. Retrovir. 1996;12:333–336. doi: 10.1089/aid.1996.12.333. [DOI] [PubMed] [Google Scholar]
- 41.Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., Matsuda S., Mizota C., Tani M., Yamamoto Y., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008;153:1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez K., Saunier F., Rigaill J., Audoux E., Botelho-Nevers E., Prier A., Dickerscheit Y., Pillet S., Pozzetto B., Bourlet T., et al. Evaluation of in vitro activity of copper gluconate against SARS-CoV-2 using confocal microscopy-based high content screening. J. Trace Elem. Med. Biol. 2021;68:126818. doi: 10.1016/j.jtemb.2021.126818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang T., Posimo J.M., Gudiel A.A., Cicchini M., Feldser D.M., Brady D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020;22:412–424. doi: 10.1038/s41556-020-0481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linneberg A., Kampmann F.B., Israelsen S.B., Andersen L.R., Jørgensen H.L., Sandholt H., Jørgensen N.R., Thysen S.M., Benfield T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients. 2021;13:1985. doi: 10.3390/nu13061985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dofferhoff A.S.M., Piscaer I., Schurgers L.J., Visser M.P.J., van den Ouweland J.M.W., de Jong P.A., Gosens R., Hackeng T.M., van Daal H., Lux P., et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2020;73:e4039–e4046. doi: 10.1093/cid/ciaa1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen R., Visser M.P.J., Dofferhoff A.S.M., Vermeer C., Janssens W., Walk J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2021;126:191–198. doi: 10.1017/S0007114520003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haycock P.C., Burgess S., Wade K.H., Bowden J., Relton C., Davey Smith G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016;103:965–978. doi: 10.3945/ajcn.115.118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robberecht H., De Bruyne T., Davioud-Charvet E., Mackrill J., Hermans N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019;25:1694–1706. doi: 10.2174/1381612825666190701144709. [DOI] [PubMed] [Google Scholar]
- 51.Mossink J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr. Prev. Health. 2020;3:111–117. doi: 10.1136/bmjnph-2020-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wessels I., Rolles B., Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson S.C. Mendelian randomization as a tool for causal inference in human nutrition and metabolism. Curr. Opin. Lipidol. 2021;32:1–8. doi: 10.1097/MOL.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 54.Kodali H.P., Pavilonis B.T., Schooling C.M. Effects of copper and zinc on ischemic heart disease and myocardial infarction: A Mendelian randomization study. Am. J. Clin. Nutr. 2018;108:237–242. doi: 10.1093/ajcn/nqy129. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J., Liu C., Sun Y., Francis M., Ryu M.S., Grider A., Ye K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthr. Cartil. 2021;29:1029–1035. doi: 10.1016/j.joca.2021.02.564. [DOI] [PubMed] [Google Scholar]
- 56.Tsilidis K.K., Papadimitriou N., Dimou N., Gill D., Lewis S.J., Martin R.M., Murphy N., Markozannes G., Zuber V., Cross A.J., et al. Genetically predicted circulating concentrations of micronutrients and risk of colorectal cancer among individuals of European descent: A Mendelian randomization study. Am. J. Clin. Nutr. 2021;113:1490–1502. doi: 10.1093/ajcn/nqab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yarmolinsky J., Bonilla C., Haycock P.C., Langdon R.J.Q., Lotta L.A., Langenberg C., Relton C.L., Lewis S.J., Evans D.M., Consortium P., et al. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. JNCI J. Natl. Cancer Inst. 2018;110:1035–1038. doi: 10.1093/jnci/djy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zwakenberg S.R., Remmelzwaal S., Beulens J.W.J., Booth S.L., Burgess S., Dashti H.S., Imamura F., Feskens E.J.M., van der Schouw Y.T., Sluijs I. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes. 2019;68:220–225. doi: 10.2337/db18-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsson S.C., Traylor M., Markus H.S. Circulating Vitamin K1 Levels in Relation to Ischemic Stroke and Its Subtypes: A Mendelian Randomization Study. Nutrients. 2018;10:1575. doi: 10.3390/nu10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin S., Yang H. Ovarian cancer risk according to circulating zinc and copper concentrations: A meta-analysis and Mendelian randomization study. Clin. Nutr. 2021;40:2464–2468. doi: 10.1016/j.clnu.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Elsworth B., Lyon M., Alexander T., Liu Y., Matthews P., Hallett J., Bates P., Palmer T., Haberland V., Smith G.D., et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020 doi: 10.1101/2020.08.10.244293. [DOI] [Google Scholar]
- 62.Milano A., McMahon A., Welter D., Bowler E., Hastings E., Cunningham F., MacArthur J., Morales J., Gil L., Cerezo M., et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2016;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans D.M., Zhu G., Dy V., Heath A.C., Madden P.A.F., Kemp J.P., McMahon G., St Pourcain B., Timpson N.J., Golding J., et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013;22:3998–4006. doi: 10.1093/hmg/ddt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitoux D., Arnaud J., Chappuis P. Are Copper, Zinc and Selenium in Erythrocytes Valuable Biological Indexes of Nutrition and Pathology ? J. Trace Elem. Med. Biol. 1999;13:113–128. doi: 10.1016/S0946-672X(99)80001-7. [DOI] [PubMed] [Google Scholar]
- 65.Combs G.F., Jr. Biomarkers of Selenium Status. Nutrients. 2015;7:2209–2236. doi: 10.3390/nu7042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornelis M.C., Fornage M., Foy M., Xun P., Gladyshev V.N., Morris S., Chasman D.I., Hu F.B., Rimm E.B., Kraft P., et al. Genome-wide association study of selenium concentrations. Hum. Mol. Genet. 2014;24:1469–1477. doi: 10.1093/hmg/ddu546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dashti H.S., Shea M.K., Smith C.E., Tanaka T., Hruby A., Richardson K., Wang T.J., Nalls M.A., Guo X., Liu Y., et al. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am. J. Clin. Nutr. 2014;100:1462–1469. doi: 10.3945/ajcn.114.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience. 2015;4:s13742-015. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auton A., Abecasis G.R., Altshuler D.M., Durbin R.M., Bentley D.R., Chakravarti A., Clark A.G., Donnelly P., Eichler E.E., Flicek P., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machiela M.J., Chanock S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor A.E., Burgess S., Ware J.J., Gage S.H., Richards J.B., Davey Smith G., Munafò M.R. Investigating causality in the association between 25(OH)D and schizophrenia. Sci. Rep. 2016;6:26496. doi: 10.1038/srep26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamat M.A., Blackshaw J.A., Young R., Surendran P., Burgess S., Danesh J., Butterworth A.S., Staley J.R. PhenoScanner V2: An expanded tool for searching human genotype–phenotype associations. Bioinformatics. 2019;35:4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brion M.-J.A., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2012;42:1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R., et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yavorska O.O., Burgess S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017;46:1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burgess S., Davey Smith G., Davies N.M., Dudbridge F., Gill D., Glymour M.M., Hartwig F.P., Holmes M.V., Minelli C., Relton C.L., et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marreiro D.D.N., Cruz K.J.C., Oliveira A.D., Morais J.B.S., Freitas B.J.S.A., Melo S.R.D.S., Santos L.R., Cardoso B.E.P., Dias T.M.D.S. Antiviral and immunological activity of zinc and possible role in COVID-19. Br. J. Nutr. 2021:1–8. doi: 10.1017/S0007114521002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louca P., Murray B., Klaser K., Graham M.S., Mazidi M., Leeming E.R., Thompson E., Bowyer R., Drew D.A., Nguyen L.H., et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health. 2021;4:149–157. doi: 10.1136/bmjnph-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yasui Y., Yasui H., Suzuki K., Saitou T., Yamamoto Y., Ishizaka T., Nishida K., Yoshihara S., Gohma I., Ogawa Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—relationship between serum zinc level and critical illness of COVID-19. Int. J. Infect. Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heller R.A., Sun Q., Hackler J., Seelig J., Seibert L., Cherkezov A., Minich W.B., Seemann P., Diegmann J., Pilz M., et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38:101764. doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., Manoharan S., Ramani V., Narasimhan G., Kaliamoorthy I., et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogel-González M., Talló-Parra M., Herrera-Fernández V., Pérez-Vilaró G., Chillón M., Nogués X., Gómez-Zorrilla S., López-Montesinos I., Arnau-Barrés I., Sorli-Redó M.L., et al. Low Zinc Levels at Admission Associates with Poor Clinical Outcomes in SARS-CoV-2 Infection. Nutrients. 2021;13:562. doi: 10.3390/nu13020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Majeed M., Nagabhushanam K., Gowda S., Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition. 2021;82:111053. doi: 10.1016/j.nut.2020.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12:2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skalny A.V., Timashev P.S., Aschner M., Aaseth J., Chernova L.N., Belyaev V.E., Grabeklis A.R., Notova S.V., Lobinski R., Tsatsakis A., et al. Serum Zinc, Copper, and Other Biometals Are Associated with COVID-19 Severity Markers. Metabolites. 2021;11:244. doi: 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H.-Y., Zhang A.-R., Lu Q.-B., Zhang X.-A., Zhang Z.-J., Guan X.-G., Che T.-L., Yang Y., Li H., Liu W., et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021;21:452. doi: 10.1186/s12879-021-06167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stefanowicz F., Gashut R.A., Talwar D., Duncan A., Beulshausen J.F., McMillan D.C., Kinsella J. Assessment of plasma and red cell trace element concentrations, disease severity, and outcome in patients with critical illness. J. Crit. Care. 2014;29:214–218. doi: 10.1016/j.jcrc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 92.Nichol C., Herdman J., Sattar N., O’Dwyer P.J., O’Reilly D.S.J., Littlejohn D., Fell G. Changes in the Concentrations of Plasma Selenium and Selenoproteins after Minor Elective Surgery: Further Evidence for a Negative Acute Phase Response? Clin. Chem. 1998;44:1764–1766. doi: 10.1093/clinchem/44.8.1764. [DOI] [PubMed] [Google Scholar]
- 93.Oestreicher P., Cousins R.J. Copper and Zinc Absorption in the Rat: Mechanism of Mutual Antagonism. J. Nutr. 1985;115:159–166. doi: 10.1093/jn/115.2.159. [DOI] [PubMed] [Google Scholar]
- 94.Stafford S.L., Bokil N.J., Achard M.E.S., Kapetanovic R., Schembri M.A., McEwan A.G., Sweet M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013;33:e00049. doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burgess S., Malarstig A. Using Mendelian randomization to assess and develop clinical interventions: Limitations and benefits. J. Comp. Eff. Res. 2013;2:209–212. doi: 10.2217/cer.13.14. [DOI] [PubMed] [Google Scholar]
- 96.Voight B.F., Peloso G.M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M.K., Hindy G., Hólm H., Ding E.L., Johnson T., et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.C Reactive Protein Coronary Heart Disease Genetics Collaboration Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griffith G.J., Morris T.T., Tudball M.J., Herbert A., Mancano G., Pike L., Sharp G.C., Sterne J., Palmer T.M., Davey Smith G., et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hui L.L., Nelson E.A.S., Lin S.L., Zhao J.V. The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: A Mendelian randomisation study. Eur. J. Clin. Nutr. 2021 doi: 10.1038/s41430-021-00993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler-Laporte G., Nakanishi T., Mooser V., Morrison D.R., Abdullah T., Adeleye O., Mamlouk N., Kimchi N., Afrasiabi Z., Rezk N., et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med. 2021;18:1–14. doi: 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amin H.A., Drenos F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: A Mendelian randomisation study of open data. BMJ Nutr. Prev. Health. 2021;4:42–48. doi: 10.1136/bmjnph-2020-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All exposure data described in the article is provided in the supplementary tables. All outcome data is available from COVID-19 HGI website: https://www.COVID19hg.org/results/r5/ (accessed on 1 September 2021). Code for statistical analyses is available on: https://github.com/marynias/COVID19 (accessed on 5 January 2022).