Abstract
Osteoporosis, characterized by low bone mass and a disruption of bone microarchitecture, is traditionally treated using drugs or lifestyle modifications. Recently, several preclinical and clinical studies have investigated the effects of selenium on bone health, although the results are controversial. Selenium, an important trace element, is required for selenoprotein synthesis and acts crucially for proper growth and skeletal development. However, the intake of an optimum amount of selenium is critical, as both selenium deficiency and toxicity are hazardous for health. In this review, we have systematically analyzed the existing literature in this field to determine whether dietary or serum selenium concentrations are associated with bone health. In addition, the mode of administration of selenium as a supplement for treating bone disease is important. We have also highlighted the importance of using green-synthesized selenium nanoparticles as therapeutics for bone disease. Novel nanobiotechnology will be a bridgehead for clinical applications of trace elements and natural products.
Keywords: selenium, selenoprotein, bone mineral density, osteoporosis, therapeutics, nanoparticles, bone formation
1. Introduction
Bones play multiple roles in the body, including providing support to the body and an environment for generating blood cells, enabling movement, protecting vital organs, and storing minerals [1,2,3]. Thus, it is not surprising that the deterioration of bone health results in morbidity, disability, and occasionally worse outcomes [4,5]. Osteoporosis, which is characterized by low bone mass and a disruption of bone microarchitecture, is the most common bone disease in humans [6,7,8,9,10]. It is prevalent in menopausal women and elderly men and is a major risk factor for fractures [11,12]. With an increase in longevity and societal aging in many parts of the world, osteoporosis is becoming a global epidemic [13,14].
Several primary and secondary preventive strategies for osteoporosis have been recommended, including drugs, diet, and other lifestyle modifications [15,16]. Among dietary elements, most previous studies have focused on calcium and vitamin D supplementation [17,18,19,20,21,22,23,24], which have traditionally been provided as anti-resorptive treatments for every adult at risk; however, fractures can be prevented in their absence, and the effects of these agents on bone density appear to be negligible [25,26,27].
Selenium is a trace mineral and an indispensable component of various enzymes and proteins [28,29,30]. Selenoprotein is synthesized from selenocysteine, an essential amino acid residue important for DNA synthesis, which protects cells from damage and infection [31,32] and is involved in the reproduction and metabolism of thyroid hormones [33,34]. Although selenium is required in insignificant amounts, approximately 1 billion people reportedly have selenium deficiency worldwide [35]. Inadequate selenium intake may result from certain circumstances, in people living in places with suboptimal selenium content in the soil [36,37,38] or in patients with bowel disease [39,40,41] or chronic kidney disease [42,43,44]. Notably, the risk of developing selenium deficiency might increase because of climate change, which is known to reduce selenium content in soil [35].
Selenium has been reported to be important for skeletal development [45]. Recently, narrative review research [46] with several preclinical and clinical studies have investigated the effects of selenium on bone health, although the results are controversial [47,48,49,50]. In this review, we systematically analyzed the existing literature in this field to determine whether dietary or serum selenium concentrations are associated with bone health. The following Pubmed and Google Scholar keywords were used to review the above publications: selenium AND bone, selenium AND osteoporosis, selenoproteins AND bone metabolism, selenium AND bone cells, selenium AND bone in animal models, selenium AND bone in human study, and selenium AND randomized clinical trial. We expect that this review will contribute to the future development of the selenium-based therapeutics.
2. Selenium
2.1. Selenium Synthesis
The essential role of selenium in living organisms was identified 140 years after its discovery [51]. Selenium was discovered in 1817 by J. Berzelius and J. Gahn, who investigated a reddish brown residue after manufacturing sulfuric acid [52,53]. Selenium belongs to the family of chalcogens, including oxygen, sulfur, tellurium, and polonium and forms several allotropes that interconvert with changes in temperature [54,55].
Selenium performs its physiological functions principally as a constituent of selenoproteins [45]. Dietary sources of selenium uptake include inorganic forms such as selenate and selenite, as well as organic forms like selenocysteine (Sec) and selenomethionine (SeMet) [56,57]. Each of these forms can be metabolized to selenide, which is an intermediate in selenocysteine synthesis [58,59]. Selenocysteine biosynthesis is unique as it requires its own tRNA, named selenocysteine tRNA[Ser]Sec [60]. After selenide (H2Se) conversion to seryl-tRNA[Ser]Sec by seryl-tRNA synthetase, seryl-tRNA[Ser]Sec provides the backbone for selenocysteine biosynthesis. Subsequently, phosphoseryl-tRNA kinase transforms seryl-tRNA[Ser]Sec into phosphoseryl-tRNA[Ser]Sec, which acts as a substrate for selenocysteine synthase with selenophosphate. Selenophosphate is generated from selenide and ATP by selenophosphate synthase 2. Selenocysteine incorporation into proteins requires a cis-acting selenocysteine insertion sequence (SECIS) element at a specific UGA codon, which usually acts as a stop codon [61]. When a ribosome reaches the UGA codon, SECIS elements act as the factors that dictate the recoding of UGA as selenocysteine [62]. SECIS binding protein 2 forms a complex with ribosomes, SECIS elements, and a Sec-specific translation elongation factor, contributing to the efficient coding of UGA as selenocysteine [62]. As SECIS binding protein 2 is known to be a limiting factor for selenoprotein synthesis, the expression of SECIS binding protein 2 may regulate selenocysteine incorporation and selenoprotein production in cells [63,64]. The structure of Sec-specific translation elongation factor is similar to that of canonical elongation factor 1A, although its domain IV contains a unique COOH-terminal extension interacting with SECIS binding protein 2 and tRNA[Ser]sec [65,66]. In addition, additional SECIS-binding proteins such as ribosomal protein L30 [67], eukaryotic initiation factor 4a3 [68], and nucleolin [69] have been predicted to act as facilitators or modulators of selenoprotein synthesis.
2.2. Selenoproteins
In total, 24 selenoproteins have been identified in mice, and the targeted deletion of some of these proteins demonstrated their key functions in disease pathophysiology [70]. About 25 different selenoprotein-coding genes have been identified in humans so far, and these include those encoding glutathione peroxidases (GPx1, 2, 3, 4, 6), thioredoxin reductases (TrxR1, 2, 3), iodothyronine deiodinases (DIO1, 2, 3), selenophosphate synthetase (SPSHS2), methionine-R-sulfoxide reductase 1 (MSRB1), and selenoprotein H, I, K, M, N, O, P, R, S, T, V, and W (SELENOH, SELENOI, SELENOK, SELENOM, SELENON, SELENOO, SELENOP, SELENOR, SELENOS, SELENOT, SELENOV, and SELENOW) [71,72,73]. These can be classified into subfamilies according to their cellular functions, which include those involved in antioxidation (GPx1, 2, 3, 4), redox regulation (TrxR1, 2, 3, MSRB1, SELENOH, M, W), thyroid hormone metabolism (DIO1, 2, 3), selenium transport and storage (SELENOP), selenophosphate synthesis (SEPHS2), calcium metabolism (SELENOK, T), myogenesis (SELENON), protein folding (SELENOF, I, S), and protein AMPylation (SELENOO). The functions of other selenoproteins, such as GPx6 and SELENOV, remain unclear [74,75,76]. GPx requires selenoproteins when it reduces lipid hydroperoxides to their corresponding alcohols and protects the organism from oxidative damage [77]. TrxR is a family of selenium-containing pyridine nucleotide-disulfide oxidoreductases involved in defense against oxidative damage via oxygen metabolism [78]. Most thyroid hormone deiodinases, which activate or deactivate various thyroid hormones and their metabolites, use selenoproteins as cofactors [79]. Hashimoto’s thyroiditis is induced when thyroid cells are destroyed by autoantibodies. Although the effect of dietary selenium on thyroid autoantibodies has been controversial, there is evidence that selenium intake reduces anti-thyroid peroxidase antibodies in patients with this disease [80]. The liver produces and dissolves SELENOP (also known as SePP), which acts as the primary transporter of Se to peripheral tissues. SELENOP concentration in the serum or plasma has been shown to be a valuable diagnostic parameter for selenium deficiency [81,82,83]. Most selenoproteins are beneficial for human diseases, indicating that the occurrence of a deficiency condition may be associated with the development or progression of pathological diseases [84]. However, the role of individual proteins must be integrated within a complex biochemical environment characterized by antagonistic, activator, and synergistic effects. The major characteristics of human selenoproteins, their roles in human health, and their correlation with diseases are summarized in Table 1.
Table 1.
| Selenoprotein | Subcellular Location | Function | Effect on Health and Disease | Ref. |
|---|---|---|---|---|
| Glutathione Peroxidases (GPxs) | ||||
| GPx 1 | Cytoplasm Mitochondrial membrane |
Antioxidant. Cellular H2O2 ↓and lipid peroxide ↓. Cardioprotective and anti-angiogenic. |
Cardiovascular: CVD, hypertension, peripheral vascular disease, ICH. Cancer: lung, prostate, bladder, primary liver Keshan disease, Kashin–Beck disease. |
[91,92,93,94,95,96] |
| GPx2 | Cytoplasm | Antioxidant. Peroxide ↓ in the gut. Carcinogenesis. |
||
| GPx3 | Secreted abundantly in plasma |
Antioxidant. Peroxide ↓ in blood. Tumor suppressor. |
Ischemic stroke, differentiated thyroid cancer. | [97,98] |
| GPx4 | Cytoplasm Mitochondria Nuclei |
Antioxidant. Phospholipid peroxide ↓. Regulation of ferroptosis and phospholipid, H2O2 levels. Involved in spermiogenesis. |
Adenomatous polyps, male infertility. Cancer: colorectal cancer, breast cancer. |
[99,100] |
| GPx6 | Secreted protein | Cellular H2O2 ↓ in the olfactory epithelium. | - | |
| Iodothyronine Deiodinases (DIOs) | ||||
| DIO1 | Plasma membrane | Regulation of systemic circulating thyroid hormone levels. | Muscle strength, lean body mass. Cardiovascular diseases. |
[101] |
| DIO2 | ER membrane | Regulation of local muscular thyroid hormone levels. |
Type-2 diabetes and insulin resistance. Osteoporosis and osteoarthritis. Mental retardation. |
[102,103,104,105] |
| DIO3 | Plasma membrane | Inactivates thyroid hormone. | Osteoporosis and osteoarthritis. |
[106] |
| Thioredoxin Reductases (TrxRs) | ||||
| TrxR1 | Cytoplasm Nucleoplasm |
Antioxidant. Regenerates reduced thioredoxin. Embryonic development. |
Advanced colorectal adenoma. | [107] |
| TrxR2 | Mitochondrial membrane | Antioxidant. Catalyzes a variety of reactions, specific for Trx and GPx. Embryonic development. |
Gastric cancer. | [108] |
| TrxR3 | Cytoplasm Nucleoplasm |
Antioxidant. Oxidized ↓ of Trx and GPx2. Involved in spermiogenesis. |
- | |
|
Selenoprotein P (SEPP) |
Secreted protein | Selenium transport to peripheral tissues. | Prostate cancer, colorectal adenoma, colorectal cancer. Neurological disorders, type 2 diabetes. |
[100,107,109,110] |
|
Selenoprotein M (SELENOM) |
ER membrane | Protein folding in ER. Involved in redox regulation. |
Neurological disorders. | |
|
Selenoprotein N (SELENON) |
ER membrane | Regulation of muscle development. | Muscle disorders. | [111,112] |
|
Selenoprotein O (SELENOO) |
Mitochondrial membrane | Possibly involved in redox regulation. | - | |
|
Selenoprotein S (SELENOS) |
ER membrane | Involved in ER-associated degradation and immune response. Involved in protein folding. |
Cardiovascular disease. Cancer: gastric, colorectal, and rectal cancer. Inflammatory response. Pre-eclampsia. |
[100,113,114,115] |
|
Selenoprotein T (SELENOT) |
ER membrane Golgi-complex |
Involved in redox regulation and cell anchorage. | - | |
|
Selenoprotein V (SELENOV) |
Unclear | Unknown. | - | |
| Selenophosphate synthetase 2 (SEPHS2) | Nucleoplasm | Synthesis of selenophosphate. | - | |
|
MSRB1 Selenoprotein R (SELENOR) |
Cytoplasm Nucleoplasm |
Antioxidant. Regulation of actin polymerization. Involved in redox regulation. |
Cardiovascular diseases. | |
|
Selenoprotein W (SELENOW) |
ER membrane | Antioxidant. Involved in muscle growth and differentiation. Involved in redox regulation. |
Muscle disorders, neurological disorders. | |
|
Selenoprotein H (SELENOH) |
Nucleoplasm and nucleoli | Antioxidant. Involved in redox regulation. |
Neurological disorders. | |
|
Selenoprotein I (SELENOI) |
Plasma membrane | Involved in phospholipid biosynthesis. Involved in protein folding. |
- | |
|
Selenoprotein F (SELENOF) |
ER membrane | Involved in protein folding. Possibly implicated in cancer etiology. |
Cancer: Prostate cancer, lung cancer, and rectal cancer. | [113,116,117] |
|
Selenoprotein K (SELENOK) |
ER membrane | Modulates calcium metabolism. Involved in ER-associated degradation and immune response. |
- | |
Abbreviations: ER: endoplasmic reticulum; MSRB1: methionine sulfoxide reductase B1; H2O2: hydrogen peroxide; BAT: brown adipose tissue; ICH: intracerebral hemorrhage; ↓: reduced or decreased.
2.3. Selenium Deficiency and Toxicity
Selenium is a microelement that rarely occurs in its elemental form in nature; instead, organic and inorganic mineral forms of selenium, such as selenide, selenate, and selenite, have been detected in soil [118,119,120]. Previous studies have shown that humans can absorb organic compounds better than inorganic compounds [121,122,123]. Inorganic forms of selenium, such as selenite, are used as dietary supplements [124]. Selenate is the most common inorganic form of selenium in soil; it dissolves in water and reaches rivers and oceans [125]. Organic forms of selenium occur mostly in plant and animal food as selenomethionine and selenocysteine [120,126]. Many whole grains, dairy products, meat, and seafood are good sources of selenium, although geographic variation in selenium content in soil may determine the level of selenium in food [127].
The margin between selenium deficiency and toxicity is narrow. A small amount of selenium is necessary for cell homeostasis because of its antioxidant activities, while supra-nutritional levels of selenium act as pro-oxidants in vivo [128]. Selenium poisoning, termed selenosis, can develop if selenium intake exceeds the tolerable limit of 400 µg/day [129]. Selenosis is characterized by garlicky breath, gastrointestinal disorders, hair loss, nail discoloration, fatigue, irritability, and neurotoxic effects [130]. Recent studies have suggested that excess selenium intake may increase the risk of type 2 diabetes [131]. Hydrogen selenide, a corrosive gas, is the most toxic selenium compound [132]. Interestingly, consumption of lower doses of selenium, such as 300 µg/day, may also exert negative effects on the body. Long-term consumption of 300 μg/day in the form of selenium-enriched yeast increased mortality in the cohort of the Danish Prevention of Cancer by Intervention with Selenium (PRECISE) trial. Hence, high daily selenium supplementation or intake should be avoided [133].
According to the World Health Organization (WHO) standards, the recommended daily intake of selenium for adults is 55 µg/day [51]. The optimal selenium concentration in plasma has been suggested to be 90–120 g/L, which is sufficient to saturate selenoproteins in blood [134]. People living in low selenium regions and those with compromised bowel absorption and undergoing chronic hemodialysis are at high risk of developing selenium deficiency [135]. Keshan disease, caused by a combination of dietary deficiency of selenium and Coxsackie virus infection, was first noted in people living in Northeast China, where soil contains low levels of selenium [136,137,138]. The affected patients suffer from congestive cardiomyopathy and pulmonary edema, often culminating in death [139,140]. Selenium deficiency has been suggested to be related to immune reactions against viruses [141,142]. People with Kashin-Beck disease (KBD) are distributed from Northeastern to Southwestern China. KBD is probably caused by the ingestion of fungal-contaminated grains [143] and is characterized by pain, limited motion in many joints of the body, and growth retardation in children, and selenium deficiency is considered a predisposing factor for the KBD [144]. Plasma selenium levels in children with KBD were lower than 27 ng/mL in the Tibet Autonomous Region, whereas appropriate selenium concentrations for optimal GPx activity are 80–100 ng/mL [33,145,146,147].
3. Roles of Selenoproteins in Bone Cells
Compared to boron, iron, zinc, and copper, selenium is a trace element present in bones that affects bone metabolism [1]. Bone has the second-highest proportion of body selenium (16%), followed by skeletal muscles (27.5%) [148]. Selenium deficiency is associated with low bone mass in male rats [149] and osteoarthropathy in KBD [150,151], as it adversely affects the biosynthesis of several antioxidant selenoproteins, which impairs bone metabolism [152].
3.1. Bone Remodeling
Healthy skeletal remodeling is maintained by an equilibrium between the activities of mesenchymal stem cell-derived osteoblasts and hematopoietic cell-derived osteoclasts on the bone surface [153]. The molecular triad of the receptor activator of the NF-κB ligand (RANKL) expressed by osteoblast lineage cells, osteoprotegerin (OPG) produced by osteoblastic or stromal cells, and the receptor activator of NF-κB (RANK) expressed by osteoclasts forms an important signaling link between osteoblasts and osteoclasts [154,155,156]. The RANK/RANKL pathway has been identified as the major signaling mechanism responsible for osteoclast differentiation. OPG is a non-signaling decoy receptor for RANKL that plays a critical regulatory role in osteoclast-mediated bone resorption by suppressing osteoclastogenesis and regulating mature osteoclasts [157,158]. Reactive oxygen species (ROSs) act as critical intracellular signaling mediators for RANKL-induced osteoclastic differentiation. Large amounts of ROSs are produced during bone remodeling, which are harmful for normal bone physiology, as they suppress osteoblastic differentiation and promote osteoclastic differentiation independent of NF-κB activation [159,160].
3.2. Selenoproteins in Bone Metabolism
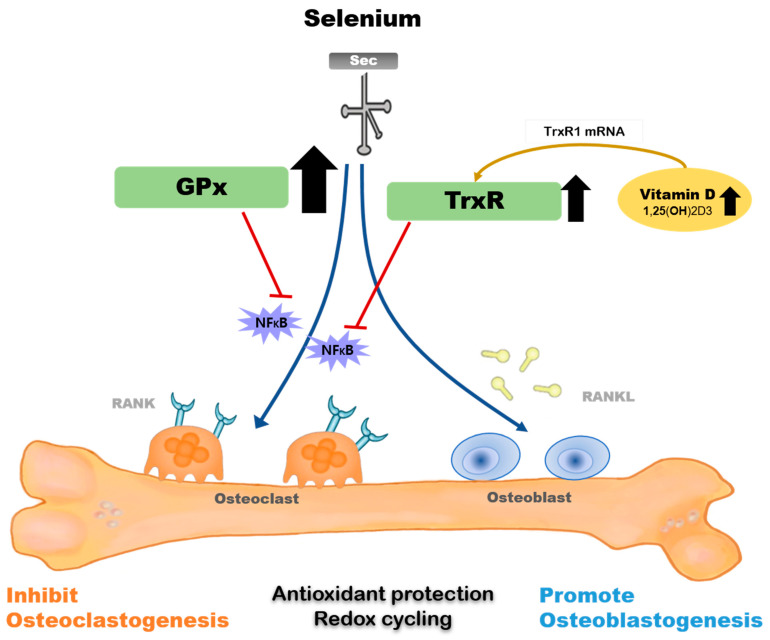
The majority of selenoprotein-related genes and at least nine selenoprotein biosynthesis-related genes have been identified in osteoblasts or osteoclasts [161,162,163,164]. Figure 1 shows the mechanism underlying the metabolic effect of selenium on osteoblasts and osteoclasts.
Figure 1.
Mechanism of selenium-related bone metabolism. Selenium supplement encourages the activity of GPx and TrxR, and GPx activity is more activated in those with a selenium-deficient status. GPx and TrxR suppress NFκB activation at supranutritional selenium levels and regulate osteoclastogenesis and osteoblastogenesis. Co-treatment of 1α,25(OH)2D3 and selenium synergistically elevates TrxR1 protein and activity. GPxs: glutathione peroxidases; TrxRs: thioredoxin reductases; RANK: receptor activator of NFκB; RANKL: receptor activator of NFκB ligand.
TrxR1 is a selenoprotein that is upregulated by 1,25(OH)2D3 and is expressed early in the osteoblast differentiation signaling cascade, highlighting a part of the mechanism by which selenium promotes bone formation [45]. 1,25(OH)2D3 did not increase TrxR activity in human fetal osteoblast (hFOB) cells cultured in selenium-deficient media. The co-treatment of 1,25(OH)2D3 and selenium increased TrxR1 protein and activity, suggesting that 1,25(OH)2D3-mediated osteoblastic differentiation and/or maintenance of the differentiation program would be impaired if selenium was deficient in bone [165]. GPx1 is a critical antioxidant enzyme in osteoclasts that can inhibit osteoclastogenesis when activated [45]. Bone marrow matrix cells cultured at low selenium concentrations poorly expressed GPx and TrxR, and showed signs of chromosomal damage, which were restored by selenium supplementation; in addition, basal selenoprotein activity was recovered [162].
Additionally, evidence indicates that supplementing with supranutritional selenium can modulate specific pathological changes via various mechanisms, including GPx and TrxR suppressing NF-κB activation and its inflammatory reactions. [166,167,168,169]. Genetic studies have shown that a single nucleotide polymorphism (SNP) at codon 198 of GPx1 is associated with low bone mineral density and increase in the levels of bone turnover markers [170].
Adequate selenium consumption appears to be important for osteoclast and osteoblast cell proliferation and differentiation, mostly via ROS regulation. Studies have shown that extracellular signal-regulated kinases, ERK1/2, mediate the inhibitory effect of hydrogen peroxide on osteoblast differentiation [171,172,173]. Selenium treatment has been shown to protect bone marrow stromal cells from the hydrogen-peroxide-induced suppression of osteoblastic differentiation by inhibiting oxidative stress and ERK activation [174,175,176]. In contrast, ROS levels increased when selenoprotein expression decreased, which was related to pathologically exacerbated signaling and enhanced osteoclast activity [163,177].
Recently, Kim et al. demonstrated that SELENOW contributes to osteoclastogenesis, but is downregulated via RANKL/RANK/tumor-necrosis-factor-receptor-associated factor 6/p38 signaling. They suggested that this RANK-dependent inhibition of SELENOW blocks overactive osteoclasts to prevent osteoporosis [178]. SELENOP is a major selenium transporter in plasma that is produced and released by hepatocytes and resorbed by bone cells via apolipoprotein receptor 2(ApoER2) [179,180,181]. In SELENOP-mediated tissue-specific selenium absorption, transcription of mRNA and LDL-receptor-related protein 8 (LRP8) protein expression has been proceeding. A crucial component of the intracellular selenium transportation system, the LRP8/ApoER2 complex is increased in chronic selenium deficiency, suggesting this process as important feedback to avoid chronically low SELENOP levels [46,161]. Interestingly, bones, brain, and testis are resistant to limited selenium supply, ensuring that the most essential selenium-dependent processes continue in specific tissues even under selenium deficiency due to increases in the expression of ApoER 2 [180,181,182].
4. Selenium and Bone: Animal Model Studies
Few studies have investigated the effects of selenium on bone metabolism in animal models [183,184,185]. Table 2 presents the results of animal studies regarding intervention with selenium supplementation.
Table 2.
Effects of selenium on bone health in in vivo studies.
| Author, Year [Ref] |
Study Design | Animal Model | Intervention (Dose, Formula, Duration) |
Result |
|---|---|---|---|---|
| Turan et al. 1997 [187] |
Assigned to 4 groups: Se- and vitamin-E-adequate/ Se- or vitamin-E-deficient Se-excess diet. |
New Zealand white rabbits | Normal Se diet, 0.5 mg/kg; excess Se diet, 10 mg/kg. Sodium selenite. 12 weeks. |
GPx activity ↑ or ↓: plasma selenium level ↑ or ↓. Light microscopic investigations of the bone tissues showed osteomalacia. Biomechanical strength of the bones ↓ in both Se-deficient and Se-excess diets. |
| Moreno-R et al. 2001 [149] |
Assigned to Se-deficient diet for two generations. |
Wistar rats | Se-deficient diet, 0.005 mg/kg; Se-adequate diet, 0.19 mg/kg. 72 days. |
Weight and tail length ↓. Plasma calcium ↓ and urinary calcium concentration ↑. PTH and 1,25(OH)2D3 2-fold ↑. Plasma osteocalcin and urinary deoxypyridoline ↓. BMD of the femur and tibia ↓. |
| Turan et al. 2003 [190] |
1000 IU/kg/day heparin for 4 weeks. Osteoporosis model. |
New Zealand white rabbits | Se diet, 0.05 mg/kg/day. Sodium selenite. 30 days. |
Combination of vitamins E and C with selenium prevented structural alterations in the long bones in an osteoporosis model. |
| Ren et al. 2007 [192] |
Assigned to 4 groups: Se- and iodine-sufficient/-deficient diets for two generations. |
Sprague-Dawley rats | Se-deficient diet, <0.02 μg/g; Se-adequate diet, 0.1–0.3 μg/g. 3 months. |
Tibial length, the thickness of the growth plate cartilage ↓ in Se- and iodine-deficient rats. PTHrP expression ↑. ColX expression ↓ in Se-deficient rats in both generations, independently of iodine deficiency. |
| Cao et al. 2012 [177] |
Assigned to 3 groups: purified, Se-deficient, and Se-adequate diet. |
C57BL/6J mice | Se-deficient diet, 0.9 μg Se/kg; Se-adequate diet, 100 μg Se/kg. SeMet or SeBean. 4 months. |
In selenium-deficient group: GPx1 activity and GPx1 mRNA in liver ↓. Femoral trabecular bone volume/total volume ↓, and trabecular separation ↑. Bone structural parameters ↔. Serum CRP, TRAP, and PTH ↑. |
| Yao et al. 2012 [191] |
Assigned to 4 groups: control, KBD diet, Se diet, and Se plus iodine diet. |
Wistar rats | KBD Se diet, 0.031 mg/kg; normal Se diet, 0.14 mg/kg. 12 weeks. |
Selenium supplement group and selenium plus iodine group: bone volume/tissue volume ratio (BV/TV), trabecular thickness, trabecular number ↑, trabecular separation ↓. KBD diet group: Tibial growth plate chondrocyte necrosis ↑, total serum protein and albumin ↓. |
| Min et al. 2015 [193] |
Assigned to 2 groups: Se-sufficient/-deficient diets for two generations. |
Dark Agouti rats |
Se-sufficient diet, 0.288 μg/g; Se-deficient diet, 0.018 μg/g. 2 months. |
In two generations of rats, gene expressions of COL II, GPx1, and GPx4 ↓ in Se-sufficient rats. Epiphyseal plate lesion ↓, cartilage type II collagen production ↓, and GPx1 activity ↓. |
Abbreviations: SeMet: Selenomethionine; SeBean: seleno pinto beans; Se: selenium; TRAP: tartrate-resistant acid phosphatase; KBD: Kashin-Beck disease; 1,25(OH)2D3: 1,25-dihydroxyvitamin D3; PTH: parathyroid hormone; Gpx: glutathione peroxidase; BMD: bone mineral density; PTHrP: parathyroid hormone-related peptide; COL II: type II collagen; ColX: type X collagen; ↑ indicates increase or upregulation; ↓ indicates decrease or downregulation; ↔ indicates no change.
Decades ago, Sasaki et al. discovered that a low-selenium diet of 3–11 months reduced bone volume, bone mineral density, and femur ash weight in rats [186]. Turan et al. [187] fed young rabbits of both sexes with a selenium- and vitamin-E-adequate diet (control group), a selenium- and vitamin-E-deficient diet, or excessive selenium for 12 weeks. They evaluated three-point bending, representing the risk of bone fracture [188]. In this study, plasma red blood cell GPx activity increased or decreased relative to plasma selenium levels in the group fed a selenium- and vitamin-E-deficient diet and the group fed a selenium-excess diet. Although selenium-deficient diets did not affect body weight or growth patterns, the biomechanical strength of the bones decreased significantly in the groups fed the selenium- and vitamin-E-deficient diet or the selenium-excess diet. Notably, bone histopathology observed in the selenium- and vitamin-E-deficient group was indicative of osteomalacia [187]. Osteomalacia is a disorder of decreased bone mineralization with a histological feature that is different from osteoporosis, which shows normal bone mineralization but an ongoing loss of bone matrix that leads to decreased bone mass [189]. Later, Turan et al. [190] developed a heparin-induced osteoporosis model in adult female New Zealand white rabbits. They were divided into three experimental and control groups, each on the basis of supplementation with deionized water, a combination of vitamin C and E, and sodium selenite plus a combination of vitamin C and E. The long bone tissue of rabbits from the group fed selenium plus combined vitamin supplementation showed almost the same structure as that of normal rabbits. [190]. Similarly, Cao et al. administered a four-month selenium-deficient or -adequate diet to adult male C57BL/6J mice and reported that bone microarchitecture was impaired in the selenium-deficient diet group, accompanied by increases in the levels of bone absorptive markers and decreases in GPx activity in the blood [177]. Yao et al. examined the effects of supplemented selenium combined with iodine, which was designed for a diet for the KBD endemic area, on the histology of bone and growth plate cartilage in 96 Wistar rats of both sexes [191]. Supplementation with selenium and iodine decreased necrosis of the chondrocytes in the growth plate and promoted trabecular bone formation in rats. Additionally, they observed increases in bone volume/tissue volume ratio, trabecular thickness, and trabecular number and decreases in trabecular separation [191].
The effects of a selenium diet on bone metabolism appear to be clear in animal studies conducted over generations [149,192,193]. Moreno-Reyes et al. provided a selenium-depleted diet to growing male rats for two generations [149] and observed growth retardation and low bone mineral density in the second-generation rats fed a selenium-depleted diet compared with the first-generation rats. The selenium-depleted diet was associated with reductions in the levels of pituitary growth hormone and insulin-like growth factor I [149].
Ren et al. designed an animal study where a selenium- and/or iodine-deficient diet was supplied for two generations and reported that the combination of selenium and iodine deficiency impaired bone and cartilage growth [192]. Additionally, Min et al. showed that selenium deficiency in rats fed a low-selenium diet over two generations can lead to epiphyseal plate thickness shortening, pathological changes, and decreased antioxidant activity [193].
5. Clinical Studies on the Effects of Selenium on Bone Health
As observed in preclinical studies, selenium deficiency may potentially cause bone damage. Despite efforts to prove the association between selenium and bone health in humans, clinical studies have yielded controversial outcomes. An extensive literature search was done on correlations between bone health, selenium blood level, and selenium dietary intakes and on the effectiveness of selenium supplementation in humans. For the present section, we reviewed a total of 21 studies: 6 case–control studies, 10 cross-sectional studies, 2 cohort studies, 1 longitudinal study, and 2 randomized, double-blind, placebo-controlled studies. Table 3 and Table 4 compare the results of previous observational, clinical studies, and they were grouped according to the ethnicity of the population and are listed in order. Most of these observational studies assessed selenium status by measuring blood selenium levels alone or paired with selenoprotein P levels, or by using a questionnaire on selenium intake. The outcomes of bone health were considered by BMD or fracture history.
Table 3.
Studies on blood selenium level and bone health.
| Author, Year [Ref] |
Setting/ Study Design |
Country | Number of Subjects | Case/Control Mean Serum Selenium Status |
Outcomes Considered | Results | +/- | |
|---|---|---|---|---|---|---|---|---|
| Odabasi et al. 2008 [194] |
Case–control study | Turkey | 138 postmenopausal women |
Osteoporosis (n = 77) Control (n = 61) |
Se (ng/mL): 76.98 Se (ng/mL): 78.98 |
Trace element. With BMD. |
No significant difference between the osteoporosis and control group. | - |
| Arikan et al. 2011 [47] |
Case–control study | Turkey | 107 postmenopausal women |
Osteoporosis (n = 35) Osteopenia (n = 37) Healthy (n = 35) | Se (μg/L): 66.16 ± 12.1 Se (μg/L): 66.89 ± 15.5 Se (μg/L): 67.12 ± 11.6 |
Trace element. Lipid level. With BMD. |
No significant differences between the osteoporosis, osteopenia, and healthy groups. | - |
| Hoeg et al. 2012 [48] |
Population-based cohort study | Europe (OPUS study) |
1144 postmenopausal women |
Mean Se: 94.3 (μg/L) Mean SePP: 3.2 (mg/L) |
Se, SePP, TFT, bone turnover markers (OC, PINP, uNTX to Cr). With BMD. Vertebral, hip, nonvertebral fracture. |
Se and SePP statuses were inversely related to bone turnover markers. Positively correlated with BMD. Se and SePP statuses were not related to both non-vertebral and vertebral fracture. |
+ | |
| Beukhof et al. 2016 [195] |
Population-based cross-sectional study | The Netherlands | 387 elderly men |
Mean Se (μg/L): 91.9 Mean SePP (mg/L): 3.4 0.5% of subjects are Se-deficient |
Se, SePP. TFT. With total and femoral BMD. |
Se and SePP statuses were positively associated with total BMD and femoral trochanter BMD. | + | |
| Marta et al. 2021 [196] |
Cross-sectional, population-based cohort study | Spain (Hortega Study) |
1365 >20 yr adults |
Low BMD High BMD |
Se level: 82.8 μg/L Se level: 85.7 μg/L |
Se, As, and Cd. With calcaneus BMD and osteoporosis-related bone fractures (in >50 yr older subjects). |
Calcaneus BMD had non-linear dose–response; inverse below 105 μg/L and positive above 105 μg/L. Osteoporosis-related bone fractures show U-shape dose–response; positive above 100 μg/L, HR 2.25 (1.13–4.49). |
+ |
| Al-E-Ahmad et al. 2018 [197] |
Case–control study | Iran | 180 elderly adults |
Osteoporosis (n = 90) Healthy (n = 90) |
Se (μg/L): 57.58 ± 25.5 Se (μg/L): 81.09 ± 25.6 |
ALOX12 SNPs. Se level. With BMD. |
Se level was different among groups Positively correlation between serum Se and BMD. |
+ |
| Liu et al. 2009 [198] |
Cross-sectional study | China | 290 postmenopausal women |
Osteoporosis (n = 123) Osteopenia (n = 127) Healthy (n = 31) |
Se (mg/L): 0.067 ± 0.02 Se (mg/L): 0.069 ± 0.02 Se (mg/L): 0.065 ± 0.01 |
Serum macro-element and trace element. With BMD. |
No significant differences between the osteoporosis, osteopenia, and healthy groups. No correlation between BMD and selenium. |
- |
| Wang et al. 2015 [199] |
Case–control study | China | 91 elderly men |
Osteoporosis (n = 30) Osteopenia (n = 31) Healthy (n = 30) |
Se (ppb): 125.53 ± 22.8 Se (ppb): 144.88 ± 26.8 Se (ppb): 133.97 ± 29.0 |
Trace element. With BMD. |
No significant differences between the osteoporosis, osteopenia, and healthy groups. No correlation between BMD and selenium. |
- |
| Park et al. 2020 [200] |
Cross-sectional study | Korea | 1167 adults |
Low BMD group Normal group |
Se level: 0.05 μg/g Se level: 0.06 μg/g |
Hair Se. Quartile level. With BMD. |
Lower Se levels were associated with low BMD. | + |
Abbreviations: BMD: bone mineral density; DXA: dual energy X-ray absorptiometry; Se: selenium; SePP: selenoprotein P; TFT: thyroid hormone test; PINP: procollagentype I N-terminal propeptide; OC: osteocalcin; uNTX to Cr: urinary resorption marker N-terminal telopeptide of type I collagen to creatine; SNPs: single nucleotide polymorphisms; OPUS study: Osteoporosis and Ultrasound Study; + means positive correlation with bone health; - means no significant correlation.
Table 4.
Studies on selenium intake and bone health.
| Author, Year [Ref] |
Study Design | Country | Number of Subjects/Age |
Diet Assessment | Outcome Considered | Selenium Intake/Selenium Status | Results | +/- |
|---|---|---|---|---|---|---|---|---|
| Wolf et al. 2005 [49] |
Population-based cross-sectional study | USA, 3 clinical sites |
11,068 postmenopausal women 50–79 years |
Semi-quantitative FFQ | Intakes of antioxidants with total BMD | Antioxidant diet group Se: 85.9 ± 38.6 μg/d. Total group Se: 94.1 ± 43.2 μg/d. |
Selenium had no association with BMD after multiple adjustments. | - |
| Zhang et al. 2006 [201] |
Population-based case–control study | USA, Utah |
1215 hip fracture 1349 controls ≥ 50 years |
137-item FFQ | Intakes of antioxidants with hip fracture modified by smoking |
Quintile of antioxidant intake of Se: 58, 79, 99, 121, 162 μg/d. | Selenium was associated with reduced risk of osteoporotic hip fracture. Selenium hip fracture OR 0.27 (0.12- 0.58) in ever smokers. |
+ |
| Wu et al. 2021 [50] |
Cross-sectional, population-based cohort study | USA | 2983 Adults ≥ 40 years |
2-day food records FFQ |
Whole blood and serum Se with FN, LS BMD, and FRAX score | Mean dietary selenium intake: 101.5 μg/day. Mean whole blood selenium: 196.7 μg/L. Mean serum selenium: 131.1 μg/L. |
Increased Se status is correlated with an increased FN BMD, decreased FRAX scores, and reduced incidence of previous bone fracture history. | + |
| Ilich et al. 2008 [202] |
Cross-sectional study | Croatia | 120 postmenopausal women |
3-day food records FFQ |
Hip and LS BMD | Control group: 104.0 ± 27.1 μg/d. OP group: 96.5 ± 33.8 μg/d. |
There was no correlation between selenium deficiency and BMD. | - |
| Rivas et al. 2012 [203] |
Cross-sectional study | Spain | 280 women ≥ 18 years |
24-hr diet recall FFQ | Dietary antioxidants with calcaneous BMD | 18–35 yr group: Se: 60.21 μg/d. 36–45 yr group: Se: 69.56 μg/d. >45 yr group: Se: 75.81 μg/d. |
Positive association was observed among BMD and selenium. Higher antioxidant intake correlated with high BMD score. |
+ |
| P-Z et al. 2012 [204] |
Cross-sectional study |
Spain | 335 postmenopausal women | 7-day food records | Se and calcium intake status with Ad-SoS at the phalanges | Mean Se intake: 95.5 μg/d. | Elevated selenium intake negatively affected bone mass Ad-SOS score only in low calcium intake group. | + |
| Chan et al. 2009 [205] |
Cross-sectional study | China | 441 women ≥ 20–35 years |
5-day food records | Dietary intake with hip, FN, and LS BMD | Honkong Se: 80.6 ± 27.2 μg/d. Beijing Se: 46.4 ± 14.5 μg/d. |
Beijing had lower selenium intake. There was no association between selenium and BMD. |
- |
| Sun et al. 2014 [206] |
Case–control study | China | 726 hip fracture 726 controls |
Semi-quantitative FFQ | Dietart intake of antioxidant with hip fracture | Cases: Men: 43.5; Women: 40.8 (μg/d). Controls: Men: 48.3; Women: 47.7 (μg/d). |
Higher dietary intake of Se associated with a lower risk of hip fracture. OR of hip fracture: 0.43 (0·26–0·70). |
+ |
| Wang, Y. et al. 2019 [207] |
Cross-sectional study | China | 6267 Adults |
Validated semi-quantitative FFQ | BMD at the phalanges | OP: 39.1 ± 31.1 μg/d. Non-OP: 44.0 ± 23.3 μg/d. |
Lower levels of dietary Se intake associated with a higher prevalence of osteoporosis. OR of OP: 0.72 (0.55–0.94). |
+ |
| Zhang et al. 2021 [208] |
Longitudinal study | China | 17,150 Adults ≥ 20 years |
3-day food records | Self-reported history of fracture | Selenium intake quartile: Q1: 20 ± 5; Q2: 31.8 ± 3; Q3: 42.5 ± 4; Q4: 71.2 ± 45 (μg/d). |
Non-linear association between selenium intake and fracture. HRs for fracture Q1: 1.07 (0.86–1.33), Q2: 1 (ref), Q3: 1.25 (1.02–1.53), Q4: 1.33 (1.07–1.65). The U-shape dose–response of the association varies by gender and urbanization level. |
+ |
Abbreviations: BMD: bone mineral density; DXA: dual energy X-ray absorptiometry; FFQ: food frequency questionnaire; LS: lumbar spine; FN: femoral neck; Se: selenium; SePP: selenoprotein P; OP: osteoporosis; FRAX: Fracture Risk Assessment Tool; Ad-SoS: amplitude-dependent speed of sound; + means positive correlation with bone health; - means no significant correlation.
5.1. Association of Selenium Intake Levels with Bone Health: Observational Studies
Several case–control studies have been performed to determine the differences in selenium levels between patients with osteoporosis and healthy individuals. In a study which is the first of its kind, the differences in the concentrations of elements, including magnesium, zinc, copper, manganese, and selenium, were determined in 77 postmenopausal women with osteoporosis and 61 healthy controls. However, the plasma concentrations of selenium and other elements did not vary significantly between the patients and controls [194]. Liu et al. investigated the serum concentrations of macro and trace elements such as zinc, iron, copper, and selenium in 290 postmenopausal women in the Chinese urban area. Comparison of bone mineral density of healthy individuals with those with osteoporosis and osteopenia did not reveal any significant difference in the levels of these elements [198]. Wang et al. grouped 91 elderly Chinese men with osteoporosis and showed that plasma levels of Mn, Zn, Cu, Se, and Pb were not related to bone mineral density [199]. Some studies have documented that the levels of triglycerides and low-density lipoprotein cholesterol correlated negatively with selenium content in postmenopausal women [209,210]. There were no differences in the levels of trace elements such as selenium and lipids in 107 women who were divided into postmenopausal osteopenia, osteoporosis, and health disorder groups, and there was no direct correlation between selenium and bone mineral density [47].
Other population-based cohort studies have used large numbers of participants. A prospective study [48] that characterized the association between selenium status and bone mass among 1144 healthy euthyroid postmenopausal women demonstrated that plasma selenium levels or SELENOP concentrations correlated with hip and lumbar spine bone mineral density and bone turnover markers. Higher selenium status was related to low bone turnover markers and high bone mineral density, but was not related to a risk of vertebral fracture [48]. Beukhof et al. determined selenium content by measuring both plasma selenium and SELENOP levels in a cohort study of 387 healthy elderly men in the Netherlands. Selenium and SELENOP levels were positively associated with total and femoral trochanter bone mineral density in subjects without selenium deficiency [195]. Al-E-Ahmad et al. showed an association between selenium level and lumbar spine and femoral neck bone mineral density among elderly individuals living in Iran. As selenium possesses antioxidant activity, they investigated the correlation between SNPs in ROS-related genes [211,212,213] and selenium levels and revealed that selenium reduced ROS activity. In addition, selenium levels correlated positively with bone mineral density [197].
Recent studies have shown a positive correlation between selenium levels and bone mineral density. In a study on 1167 healthy Korean adults, Park et al. found differences in hair selenium levels between the low and normal bone mineral density groups. Participants with low bone mineral density had significantly lower hair selenium levels adjusted for osteoporosis-related risk factors compared to a healthy control [200]. In the population-based Hortega study [196] involving 1365 participants, a non-linear dose–response relationship of selenium was observed with reductions in bone mineral density, showing an inverse association below 105 μg/L, which became increasingly positive above 105 μg/L. While a U-shaped dose–response curve of selenium was observed with the incidence of osteoporosis-related bone fractures in a prospective analysis, the positive association above 105 μg/L was markedly stronger than that observed in the cross-sectional analysis [196]. In a study that did not directly evaluate selenium levels, Mendelian randomization analysis was performed on summary data from large Genome-wide Association Study (GWAS) datasets, and serum selenium levels were found to correlate positively with the heel bone mineral density. A nominally significant relationship was observed between serum selenium levels and forearm bone mineral density. However, selenium significantly affected only bone mineral density at specific skeletal sites such as heel [214].
Other studies have investigated selenium intake instead of measuring selenium levels in the blood. Wolf et al. measured bone mineral density in 11,068 postmenopausal women aged 50–79 years enrolled in the Women’s Health Initiative Observational Study and Clinical Trial at three clinics in the USA, where the level of selenium was estimated using a self-reported food-frequency questionnaire. However, dietary selenium intake and total bone mineral density were not associated [49]. Two more studies showed a lack of correlation between selenium intake and bone mineral density [202,205]. In contrast, several studies have shown a positive correlation with bone health. Zhang et al. constructed a case–control study and showed that selenium intake was inversely associated with a reduced risk of osteoporotic hip fracture in 2564 elderly smokers (OR = 0.27, 95% CI = 0.12–0.58) [201]. An earlier case–control study also showed that a higher dietary intake of selenium was associated with a lower risk of hip fracture (OR = 0.43, 95% CI = 0.26–0.70) [206]. Two Spanish studies of postmenopausal and non-menopausal women showed that a selenium diet was associated with bone mineral density at specific sites, including the calcaneus or phalanges [203,204].
In a cross-sectional study of 6267 Chinese people, the prevalence of osteoporosis was high (OR = 0.72, 95% CI = 0.55–0.94) in both men and women with lower dietary selenium intake levels. Although several confounding factors were considered in this study, unfortunately, detailed information on hormonal status is lacking [207]. Zhang et al. used data from 17,150 participants from the China Health and Nutrition Survey, in which the self-reported history of fractures showed a non-linear association with selenium intake [208]. Recently, Wu et al. analyzed the data of 2983 adults in the National Health and Nutrition Examination Survey (NHANES) 2013–2014 [50] and observed that a higher selenium level, measured in plasma and diet, correlated with a lower bone mineral density and a decreased FRAX score, which represents the 10-year fracture risk [50].
Although there are studies showing non-significant results between selenium and bone health [47,49,194,198,199,202,205], a positive correlation was observed between selenium and bone health, showing a positive relationship between selenium and bone mineral density [48,50,195,196,197,200,203,204,207]and a reduction in the risk of osteoporotic fracture [50,196,201,206,208]. The limitations of these observational studies include the fact that not all, but some, were conducted with sample sizes too small to achieve statistically valid results. The majority of studies found no evidence of a selenium deficiency, as demonstrated by the Recommended Daily Allowance (RDA).
5.2. Randomized Clinical Trial of Selenium Intake: Intervention Studies
Based on the positive results obtained in the above observational studies, two randomized clinical trials were performed. The characteristics of interventional studies are shown in Table 5. The effect of selenium level on bone health was tested for the first time in a randomized controlled trial by Walsh et al., where they completed a 6-month randomized, double-blind, placebo-controlled trial involving postmenopausal women aged >55 years with osteopenia or osteoporosis in the UK. An equal number of participants were randomly assigned to a placebo or to groups administered 50 μg or 200 μg of oral sodium selenite per day. Among the 120 participants recruited, 115 (96%) completed the follow-up. The primary endpoint was a biochemical marker of bone resorption (urinary N-telopeptide, uNTx) at 26 weeks, the levels of which did not differ significantly between the treatment groups. Secondary assessments of other markers of bone turnover, i.e., bone mineral density, muscular performance, and levels of glutathione peroxidase, highly sensitive C-reactive protein, and interleukin-6, revealed that there were no significant changes after selenium supplementation [215]. The authors of that study mentioned that the dietary selenium intake at baseline was higher than that expected in the UK population. Second, the study population included only postmenopausal women, suggesting that further studies investigating elderly people with selenium deficiency are required [215].
Table 5.
Selenium supplementation trials in humans with osteoporosis.
| Author, Year [Ref] |
Study Design | Study Population (Treated/Placebo) | Intervention Se Supplement | Intervention Duration and Follow-Up |
Primary Outcomes | Secondary Outcomes | Results | +/- |
|---|---|---|---|---|---|---|---|---|
| Walsh et al. 2021 [215] |
6-month randomized, double-blind, placebo-controlled trial | 120 Postmenopausal women ≥55 years with osteopenia or osteoporosis, UK. |
1:1:1 randomized trial; received 200 μg, 50 μg, or placebo per day of sodium selenite. | 26 weeks/ 6 month |
uNTX to Cr. | Serum Se SePP, BMD, other BTM, muscle function antioxidant, and inflammatory markers. | Urine NTx to creatinine ratio did not differ significantly between treatment groups at 26 weeks. None of the secondary or mechanistic endpoint measurements differed. |
- |
| Perri et al. 2021 [216] |
Single-center, randomized, double-blinded, placebo-controlled, multi-arm, parallel clinical trial | 490 Healthy adults, 60–74 years. PRECISE trial: (1998–2015), Denmark. |
1:1:1:1 Randomized 100, 200, 300 μg or placebo per day of selenium yeast | 6 Month/ Mean 15.9 years |
Bone turnover markers, osteocalcin, P1NP, carboxy-terminal collagen crosslinks, bone alkaline phosphatase. |
Serum Se, TFT, lipid level, antioxidant, and inflammatory markers. |
Selenium supplementation reduced P1NP at 6 months, but there were no significant effects on other BTM or after 5 years. | - |
Abbreviations: BMD: bone mineral density; DXA: dual energy X-ray absorptiometry; Se: selenium; SePP: selenoprotein P; PINP: procollagen type I N-terminal propeptide; OC: osteocalcin; uNTX to Cr: urinary resorption marker N-terminal telopeptide of type I collagen to creatine; BTM: bone turnover markers; - means no significant correlation with bone health.
A population with relatively low selenium level was investigated in a single-center, randomized, double-blinded, placebo-controlled, multi-arm, parallel clinical trial (PRECISE) in Denmark [216]. The 491 volunteers, aged 60–74 years, were randomly and equally assigned to treatment groups administered 100, 200, or 300 µg selenium per day via selenium-enriched yeast for 5 years (from randomization in 1998–1999) and were followed up for further 10 years. The plasma selenium level was within the optimal level during the follow-up period. The levels of bone turnover markers, measured at 6 months, showed a significant linear decrease in the level of procollagen type 1 N-terminal propeptide (P1NP), although this effect was not apparent at 5 years; no significant effect of selenium supplementation on any other bone formation markers was observed [216]. As P1NP is a marker of osteoblast function [217], the decrease in PINP level indicated a reduction in bone formation. Therefore, the effect of selenium on bone turnover and bone health remains to be determined on the basis of the results of these randomized clinical trials.
6. Novel Approach of Selenium Supplement
The contradictions in the results of clinical studies can be because of the differences in the levels of dietary selenium and selenium supplements [218]. Therefore, current studies have focused on developing various selenium-based therapeutic strategies instead of traditional selenium intake trials. Here, we have focused on various novel therapeutic strategies involving selenoproteins.
6.1. Selenium Nanoparticles
The amount of bioavailable selenium in the diet largely differs with the food and soil fodder to which humans and animals are exposed [219,220]. Moreover, in the environment, selenium is present in various oxidation states (2, 0, 4+, and 6+) that are cytotoxic. Owing to its high toxicity (Se4+), selenite is reduced to elemental selenium (Se0) by biogeochemical cycles [221,222,223]. Therefore, a high dose of selenium is harmful for humans and has a narrow safety-to-toxicity margin [224,225]. Selenium nanoparticles are potential candidates with low cytotoxicity [226,227] that can be used to circumvent the various problems associated with inorganic and organic selenium compounds, such as ROS generation and low redox activity [228,229,230,231].
6.1.1. Synthesis of Selenium Nanoparticles
Selenium nanoparticles can be synthesized chemically [232], physically [233], or even biologically using microorganisms or plant extracts, a process known as green synthesis [234,235]. Gao et al. [236] showed that hollow, chemically synthesized, spherical selenium nanoparticles possess antioxidant properties that can reduce selenium toxicity. Selenium nanoparticles are less toxic than inorganic and organic selenium. The lower hepatotoxicity and nephrotoxicity of these nanoparticles have been demonstrated in in vivo studies [226,237,238]. However, these chemically synthesized nanoparticles are not suitable for biomedical use, limiting their application. As harsh chemicals are used in chemical synthesis, even small amounts of these compounds in humans can cause systemic toxicity. Therefore, green synthesis is gaining popularity in the biomedical field [239,240,241].
Furthermore, oral supplementation with nanoparticles is considered to be the most appropriate and cost-effective method. However, absorption barriers in the digestive tract may render the absorption of nanoparticles difficult. For example, to metabolize nanoparticles, the mucus covering the intestinal mucosa should be overcome [242]. Thus, numerous compounds have been used to stabilize selenium nanoparticles, including amino acids, bovine serum albumin, polysaccharides such as chitosan [243,244,245], gum arabic [243,246], and polymers such as poly(lactic-co-glycolic acid) [247].
6.1.2. Effect of Selenium Nanoparticles on Bone Health
Selenium nanoparticles have been shown to possess therapeutic potential against various cancer cells, microbial pathogens, and viruses and protect from hepatic diseases [218,248,249,250], diabetes [251,252,253], and neurological diseases [254,255,256]. However, few studies have reported the therapeutic effects of selenium nanoparticles on bone-related conditions.
Experiments have been performed with green selenium nanoparticles. Polysaccharide-protein complex-coated selenium nanoparticles (PTR-SeNPs) synthesized using the mushroom polysaccharide isolated from Pleurotus tuberregium and selenium nanoparticles (Cs4-SeNPs) synthesized using the mushroom polysaccharide isolated from Cordyceps sinensis inhibited osteoclastogenesis while promoting osteoblastogenesis, thereby stimulating bone formation via preosteoblast differentiation [257,258]. The PTR-SeNP system has been rationally designed and identified as a potent bone-formation therapeutic that can antagonize osteoporosis. The nanosystem is incorporated by osteoblasts and significantly enhances bone formation in vitro and in vivo [259]. Fatima et al. [260] found that modest concentrations of selenium nanoparticles can improve human mesenchymal stem cell survival and osteogenic potential by reducing oxidative stress. Selenium nanoparticles may promote osteogenesis by activating the JNK/FOXO3 pathway. Another study also demonstrated increases in the osteogenic maturation of MC3T3-E1 cells and the downregulation of ROSs by hydrogen peroxide [261].
Many clinical studies have investigated the effects of cancer chemotherapy on osteoporosis. Anastrozole is used to treat breast cancer; however, bone toxicity limits its clinical use. In vitro studies on human osteoblasts have shown that selenium nanoparticles reduce cell death. At the examined doses, selenium nanoparticles significantly prevented decreases in bone density and increases in the levels of biochemical markers of bone resorption, eventually preventing anastrozole-induced osteoporosis [262]. Therefore, selenium nanoparticle co-supplementation might prevent bone loss by enhancing osteogenesis and may be an effective candidate for treating osteoporosis.
6.2. Implanted Selenium Therapeutics
Numerous metal ions, including selenium, possess osteoinductive properties. Hence, the effects of coating or implanting selenium and selenium nanoparticles on bone structures and tumors [263], or other orthopedic medical devices [264], have been investigated. The integration of selenium into biomaterials is an effective therapy for promoting bone remodeling [265,266].
Previous studies have shown that the local use of selenium correlates with bone formation and reduces the risk of osteoporosis due to its antioxidant property [175,267]. The effect of calcium phosphate ceramics on bone healing [268] and the strong affinity between selenium and calcium phosphate have been shown [266]. Li et al. [269] conducted a study with selenium-modified calcium phosphate cement (Se-CPC), CPC with 3% selenium nanoparticles, and scaffolds. These were implanted into the femoral epiphysis of an ovariectomized rat with osteoporosis and incubated for 12 weeks. The results of a reverse transcription-quantitative polymerase chain reaction revealed that the Se-CPC group had higher OPG and lower RANKL levels. This stimulated bone regeneration and bone formation by activating the OPG/RANKL pathway and inhibiting local oxidative stress [269]. Additional experiments should be performed in the future to expand the availability of localized selenium nanoparticles.
7. Conclusions and Future Prospects
The effects of selenium on human health are numerous and complex, and there are significant information gaps that have to be filled. In the present review, we highlighted the importance of selenium in maintaining bone health, especially in osteoporosis, which is one of the major diseases affecting bone health.
Osteoporosis contributes significantly to a social management burden due to the high mortality, morbidity, and treatment costs associated with the disease [270]. By 2020, the number of people suffering from osteoporosis worldwide exceeded 200 million, which will increase rapidly in successive years. The number of annual hip fractures will surpass 3 million, and the cost of treatment is estimated to be 25 billion USD by 2025 [13,271].
New drugs are available for the treatment and prevention of osteoporosis. As mentioned earlier, the daily intake of calcium- and vitamin-D-rich food is part of the traditional lifestyle modification therapy [272]. Only subtle adverse events have occurred with anti-osteoporotic drugs including romosozumab, calcium supplements, bisphosphonates, and selective estrogen receptor modulators, which are currently safely prescribed and widely used [273,274,275]. In addition to anti-osteoporotic drugs, natural products, such as trace elemental particles that are safe for human health, can be used in conjunctive therapy. Selenium may be used as an adjuvant for the treatment of osteoporosis, as some studies have demonstrated that selenium exerts cardiovascular protective and anti-osteoporotic effects [276,277,278,279,280].
Most observational studies have reported that the level of selenium correlates positively with bone health, as it increases bone mineral density or reduces the risk of osteoporotic fracture, while some studies have shown a lack of association between selenium status and bone health. However, there is a discrepancy between the results of randomized clinical trials and those of observational studies. The baseline selenium level or the formula of selenium supplement might be a key factor influencing the effect of selenium on bone health. Further research on the different functions and effects of selenium biomarkers and on more effective and safe methods of selenium supplementation are required. Therefore, the novel approach of synthesizing selenium supplements, such as selenium nanoparticles, is being investigated.
Nanobiotechnology has been a hot topic of research ever since its emergence in the biomedical domain. Green nanoparticles are produced using natural microbes, which can be optimal for human use. However, reports on green-synthesized selenium nanoparticles are limited. Further studies are required to understand the relationship between selenium and selenium nanoparticles, as well as the biological pathways underlying the differences in the therapeutic efficacies of selenium and selenium nanoparticles. In the future, the use of selenium nanoparticles should be expanded to treat bone health and other medical conditions. Finally, the effect of selenium on bone health is still an open area of investigation, and more studies on the role of selenium nanoparticles are required.
Author Contributions
Conceptualization: S.L., T.Y. and K.-C.P.; investigation, writing, and original draft preparation: T.Y., S.-Y.L. and J.C.; writing, reviewing, and editing: S.-Y.L., S.L. and S.-H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Health Technology R&D Project of the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, South Korea (grant number HI16C1559), and by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (No. NRF-2021R1A4A3023587, 2021R1C1C1006856).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaffney-Stomberg E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019;188:26–34. doi: 10.1007/s12011-018-1583-8. [DOI] [PubMed] [Google Scholar]
- 2.Taylor T., Moore J. The effect of high and low levels of dietary inorganic phosphate on the pre-laying storage of calcium and phosphorus and on the composition of the medullary and cortical bone in pullets. Br. J. Nutr. 1958;12:35–42. doi: 10.1079/BJN19580007. [DOI] [PubMed] [Google Scholar]
- 3.Nishimuta M. The concept of intracellular-, extracellular-and bone-minerals. Biofactors. 2000;12:35–38. doi: 10.1002/biof.5520120106. [DOI] [PubMed] [Google Scholar]
- 4.Van Den Bergh J.P., Van Geel T.A., Geusens P.P. Osteoporosis, frailty and fracture: Implications for case finding and therapy. Nat. Rev. Rheumatol. 2012;8:163–172. doi: 10.1038/nrrheum.2011.217. [DOI] [PubMed] [Google Scholar]
- 5.Farhat G.N., Cauley J.A. The link between osteoporosis and cardiovascular disease. Clin. Cases Miner. Bone Metab. 2008;5:19–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Klibanski A., Adams-Campbell L., Bassford T., Blair S.N., Boden S.D., Dickersin K., Gifford D.R., Glasse L., Goldring S.R., Hruska K. Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc. 2001;285:785–795. [Google Scholar]
- 7.Kanis J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 8.Hampton T. Experts urge early investment in bone health. JAMA. 2004;291:811–812. doi: 10.1001/jama.291.7.811. [DOI] [PubMed] [Google Scholar]
- 9.Bundred N.J. Optimising bone health in survivors of breast cancer. Lancet Oncol. 2007;8:89–91. doi: 10.1016/S1470-2045(07)70007-4. [DOI] [PubMed] [Google Scholar]
- 10.Armas L.A., Recker R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012;41:475–486. doi: 10.1016/j.ecl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Wade S.W., Strader C., Fitzpatrick L.A., Anthony M.S., O’Malley C.D. Estimating prevalence of osteoporosis: Examples from industrialized countries. Arch. Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. [DOI] [PubMed] [Google Scholar]
- 12.Yeh Y.-T., Li P.-C., Wu K.-C., Yang Y.-C., Chen W., Yip H.-T., Wang J.-H., Lin S.-Z., Ding D.-C. Hysterectomies are associated with an increased risk of osteoporosis and bone fracture: A population-based cohort study. PLoS ONE. 2020;15:e0243037. doi: 10.1371/journal.pone.0243037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Miner. Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 14.Aspray T.J., Hill T.R. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019;91:453–476. doi: 10.1007/978-981-13-3681-2_16. [DOI] [PubMed] [Google Scholar]
- 15.Prestwood K.M., Pilbeam C.C., Raisz L.G. Treatment of osteoporosis. Annu. Rev. Med. 1995;46:249–256. doi: 10.1146/annurev.med.46.1.249. [DOI] [PubMed] [Google Scholar]
- 16.Lane J.M., Russell L., Khan S.N. Osteoporosis. Clin. Orthop. Relat. Res. 2000;372:139–150. doi: 10.1097/00003086-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Weaver C.M., Alexander D.D., Boushey C.J., Dawson-Hughes B., Lappe J.M., LeBoff M.S., Liu S., Looker A.C., Wallace T.C., Wang D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016;27:367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzoli R., Biver E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020;18:273–284. doi: 10.1007/s11914-020-00591-6. [DOI] [PubMed] [Google Scholar]
- 19.Paschalis E.P., Gamsjaeger S., Hassler N., Fahrleitner-Pammer A., Dobnig H., Stepan J.J., Pavo I., Eriksen E.F., Klaushofer K. Vitamin D and calcium supplementation for three years in postmenopausal osteoporosis significantly alters bone mineral and organic matrix quality. Bone. 2017;95:41–46. doi: 10.1016/j.bone.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Liu C., Kuang X., Li K., Guo X., Deng Q., Li D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020;11:10817–10827. doi: 10.1039/D0FO00787K. [DOI] [PubMed] [Google Scholar]
- 21.Heaney R.P. Calcium, dairy products and osteoporosis. J. Am. Coll. Nutr. 2000;19:83S–99S. doi: 10.1080/07315724.2000.10718088. [DOI] [PubMed] [Google Scholar]
- 22.Jackson R.D., LaCroix A.Z., Gass M., Wallace R.B., Robbins J., Lewis C.E., Bassford T., Beresford S.A., Black H.R., Blanchette P., et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 23.Zhu K., Bruce D., Austin N., Devine A., Ebeling P.R., Prince R.L. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J. Bone Miner. Res. 2008;23:1343–1348. doi: 10.1359/jbmr.080327. [DOI] [PubMed] [Google Scholar]
- 24.Daly R.M., Brown M., Bass S., Kukuljan S., Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: A 2-year randomized controlled trial. J. Bone Miner. Res. 2006;21:397–405. doi: 10.1359/JBMR.051206. [DOI] [PubMed] [Google Scholar]
- 25.Grant A.M., Avenell A., Campbell M.K., McDonald A.M., MacLennan G.S., McPherson G.C., Anderson F.H., Cooper C., Francis R.M., Donaldson C., et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): A randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/s0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 26.Salovaara K., Tuppurainen M., Kärkkäinen M., Rikkonen T., Sandini L., Sirola J., Honkanen R., Alhava E., Kröger H. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: A population-based 3-year randomized, controlled trial--the OSTPRE-FPS. J. Bone Miner. Res. 2010;25:1487–1495. doi: 10.1002/jbmr.48. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J.G., Zeng X.T., Wang J., Liu L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA. 2017;318:2466–2482. doi: 10.1001/jama.2017.19344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatfield D.L., Gladyshev V.N. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 30.Mehdi Y., Hornick J.L., Istasse L., Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013;18:3292–3311. doi: 10.3390/molecules18033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rayman M.P. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 34.Addinsall A.B., Wright C.R., Andrikopoulos S., van der Poel C., Stupka N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem. J. 2018;475:1037–1057. doi: 10.1042/BCJ20170920. [DOI] [PubMed] [Google Scholar]
- 35.Jones G.D., Droz B., Greve P., Gottschalk P., Poffet D., McGrath S.P., Seneviratne S.I., Smith P., Winkel L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA. 2017;114:2848–2853. doi: 10.1073/pnas.1611576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C., Yao H., Wu Y., Sun G., Yang W., Li Z., Shang L. Status and risks of selenium deficiency in a traditional selenium-deficient area in Northeast China. Sci. Total Environ. 2021;762:144103. doi: 10.1016/j.scitotenv.2020.144103. [DOI] [PubMed] [Google Scholar]
- 37.Tan J., Zhu W., Wang W., Li R., Hou S., Wang D., Yang L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002;284:227–235. doi: 10.1016/S0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- 38.Mehdi Y., Dufrasne I. Selenium in Cattle: A Review. Molecules. 2016;21:545. doi: 10.3390/molecules21040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisshof R., Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:576–581. doi: 10.1097/MCO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 40.Kudva A.K., Shay A.E., Prabhu K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G71–G77. doi: 10.1152/ajpgi.00379.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ala M., Kheyri Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition. 2021;85:111153. doi: 10.1016/j.nut.2021.111153. [DOI] [PubMed] [Google Scholar]
- 42.Zachara B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015;68:131–151. doi: 10.1016/bs.acc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Wu C.Y., Wong C.S., Chung C.J., Wu M.Y., Huang Y.L., Ao P.L., Lin Y.F., Lin Y.C., Shiue H.S., Su C.T., et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Hazard. Mater. 2019;375:224–232. doi: 10.1016/j.jhazmat.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 44.Omrani H., Golmohamadi S., Pasdar Y., Jasemi K., Almasi A. Effect of selenium supplementation on lipid profile in hemodialysis patients. J. Ren. Inj. Prev. 2016;5:179–182. doi: 10.15171/jrip.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Zhang J., Xiao J. Selenoproteins and selenium status in bone physiology and pathology. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014;1840:3246–3256. doi: 10.1016/j.bbagen.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Vescini F., Chiodini I., Palermo A., Cesareo R., De Geronimo V., Scillitani A., Gennari L., Falchetti A. Selenium: A Trace Element for a Healthy Skeleton—A Narrative Review. Endocr. Metab. Immune Disord. Drug Targets. 2021;21:577–585. doi: 10.2174/1871530320666200628030913. [DOI] [PubMed] [Google Scholar]
- 47.Arikan D.C., Coskun A., Ozer A., Kilinc M., Atalay F., Arikan T. Plasma selenium, zinc, copper and lipid levels in postmenopausal Turkish women and their relation with osteoporosis. Biol. Trace Elem. Res. 2011;144:407–417. doi: 10.1007/s12011-011-9109-7. [DOI] [PubMed] [Google Scholar]
- 48.Hoeg A., Gogakos A., Murphy E., Mueller S., Kohrle J., Reid D.M., Gluer C.C., Felsenberg D., Roux C., Eastell R., et al. Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women. J. Clin. Endocrinol. Metab. 2012;97:4061–4070. doi: 10.1210/jc.2012-2121. [DOI] [PubMed] [Google Scholar]
- 49.Wolf R.L., Cauley J.A., Pettinger M., Jackson R., Lacroix A., Leboff M.S., Lewis C.E., Nevitt M.C., Simon J.A., Stone K.L., et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women’s Health Initiative. Am. J. Clin. Nutr. 2005;82:581–588. doi: 10.1093/ajcn/82.3.581. [DOI] [PubMed] [Google Scholar]
- 50.Wu C.C., Wang C.K., Yang A.M., Lu C.S., Lin C.Y. Selenium status is independently related to bone mineral density, FRAX score, and bone fracture history: NHANES, 2013 to 2014. Bone. 2021;143:115631. doi: 10.1016/j.bone.2020.115631. [DOI] [PubMed] [Google Scholar]
- 51.Kieliszek M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules. 2019;24:1298. doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mojadadi A., Au A., Salah W., Witting P., Ahmad G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients. 2021;13:3256. doi: 10.3390/nu13093256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd R. Selenium stories. Nat. Chem. 2011;3:570. doi: 10.1038/nchem.1076. [DOI] [PubMed] [Google Scholar]
- 54.Fleischer H., Glang S., Schollmeyer D., Mitzel N.W., Bühl M. Experimental investigations and ab initio studies of selenium(II) dialkanethiolates, Se(SR)2. Dalton Trans. 2004:3765–3771. doi: 10.1039/B409726B. [DOI] [PubMed] [Google Scholar]
- 55.Tan K.H., Demco D.E., Fechete R., Pich A. Functional selenium modified microgels: Temperature-induced phase transitions and network morphology. Soft Matter. 2019;15:3227–3240. doi: 10.1039/C8SM02646G. [DOI] [PubMed] [Google Scholar]
- 56.Schrauzer G.N. Nutritional selenium supplements: Product types, quality, and safety. J. Am. Coll. Nutr. 2001;20:1–4. doi: 10.1080/07315724.2001.10719007. [DOI] [PubMed] [Google Scholar]
- 57.Ip C., Birringer M., Block E., Kotrebai M., Tyson J.F., Uden P.C., Lisk D.J. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J. Agric. Food Chem. 2000;48:2062–2070. doi: 10.1021/jf000051f. [DOI] [PubMed] [Google Scholar]
- 58.Sunde R.A., Raines A.M., Barnes K.M., Evenson J.K. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep. 2009;29:329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sunde R.A., Raines A.M. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv. Nutr. 2011;2:138–150. doi: 10.3945/an.110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K.H., Shim M.S., Kim J.Y., Jung H.K., Lee E., Carlson B.A., Xu X.M., Park J.M., Hatfield D.L., Park T., et al. Drosophila selenophosphate synthetase 1 regulates vitamin B6 metabolism: Prediction and confirmation. BMC Genom. 2011;12:426. doi: 10.1186/1471-2164-12-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014;94:739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tujebajeva R.M., Copeland P.R., Xu X.M., Carlson B.A., Harney J.W., Driscoll D.M., Hatfield D.L., Berry M.J. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Low S.C., Grundner-Culemann E., Harney J.W., Berry M.J. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papp L.V., Lu J., Striebel F., Kennedy D., Holmgren A., Khanna K.K. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol. Cell. Biol. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itoh Y., Chiba S., Sekine S., Yokoyama S. Crystal structure of human selenocysteine tRNA. Nucleic Acids Res. 2009;37:6259–6268. doi: 10.1093/nar/gkp648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zavacki A.M., Mansell J.B., Chung M., Klimovitsky B., Harney J.W., Berry M.J. Coupled tRNA(Sec)-dependent assembly of the selenocysteine decoding apparatus. Mol. Cell. 2003;11:773–781. doi: 10.1016/S1097-2765(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 67.Chavatte L., Brown B.A., Driscoll D.M. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat. Struct. Mol. Biol. 2005;12:408–416. doi: 10.1038/nsmb922. [DOI] [PubMed] [Google Scholar]
- 68.Budiman M.E., Bubenik J.L., Miniard A.C., Middleton L.M., Gerber C.A., Cash A., Driscoll D.M. Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation. Mol. Cell. 2009;35:479–489. doi: 10.1016/j.molcel.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miniard A.C., Middleton L.M., Budiman M.E., Gerber C.A., Driscoll D.M. Nucleolin binds to a subset of selenoprotein mRNAs and regulates their expression. Nucleic Acids Res. 2010;38:4807–4820. doi: 10.1093/nar/gkq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigó R., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 71.Gromer S., Eubel J., Lee B., Jacob J. Human selenoproteins at a glance. Cell. Mol. Life Sci. CMLS. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J., Holmgren A. Selenoproteins. J. Biol. Chem. 2009;284:723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 73.Reeves M., Hoffmann P. The human selenoproteome: Recent insights into functions and regulation. Cell. Mol. Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoytcheva Z.R., Berry M.J. Transcriptional regulation of mammalian selenoprotein expression. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009;1790:1429–1440. doi: 10.1016/j.bbagen.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitts M.W., Hoffmann P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018;70:76–86. doi: 10.1016/j.ceca.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sreelatha A., Yee S.S., Lopez V.A., Park B.C., Kinch L.N., Pilch S., Servage K.A., Zhang J., Jiou J., Karasiewicz-Urbańska M. Protein AMPylation by an evolutionarily conserved pseudokinase. Cell. 2018;175:809–821.e19. doi: 10.1016/j.cell.2018.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthukumar K., Nachiappan V. Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J. Biochem. Biophys. 2010;47:383–387. [PubMed] [Google Scholar]
- 78.Meyer Y., Buchanan B.B., Vignols F., Reichheld J.P. Thioredoxins and glutaredoxins: Unifying elements in redox biology. Annu. Rev. Genet. 2009;43:335–367. doi: 10.1146/annurev-genet-102108-134201. [DOI] [PubMed] [Google Scholar]
- 79.Pakdel F., Ghazavi R., Heidary R., Nezamabadi A., Parvizi M., Haji Safar Ali Memar M., Gharebaghi R., Heidary F. Effect of Selenium on Thyroid Disorders: Scientometric Analysis. Iran J. Public Health. 2019;48:410–420. doi: 10.18502/ijph.v48i3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazokopakis E.E., Papadakis J.A., Papadomanolaki M.G., Batistakis A.G., Giannakopoulos T.G., Protopapadakis E.E., Ganotakis E.S. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in Patients with Hashimoto’s thyroiditis. Thyroid. 2007;17:609–612. doi: 10.1089/thy.2007.0040. [DOI] [PubMed] [Google Scholar]
- 81.Brodin O., Hackler J., Misra S., Wendt S., Sun Q., Laaf E., Stoppe C., Björnstedt M., Schomburg L. Selenoprotein P as biomarker of selenium status in clinical trials with therapeutic dosages of selenite. Nutrients. 2020;12:1067. doi: 10.3390/nu12041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schomburg L., Orho-Melander M., Struck J., Bergmann A., Melander O. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients. 2019;11:1852. doi: 10.3390/nu11081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedrosa L.F., Motley A.K., Stevenson T.D., Hill K.E., Burk R.F. Fecal selenium excretion is regulated by dietary selenium intake. Biol. Trace Elem. Res. 2012;149:377–381. doi: 10.1007/s12011-012-9430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roman M., Jitaru P., Barbante C. Selenium biochemistry and its role for human health. Metallomics. 2013;6:25–54. doi: 10.1039/C3MT00185G. [DOI] [PubMed] [Google Scholar]
- 85.Davis C.D., Tsuji P.A., Milner J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012;32:73–95. doi: 10.1146/annurev-nutr-071811-150740. [DOI] [PubMed] [Google Scholar]
- 86.Gladyshev V.N., Arnér E.S., Berry M.J., Brigelius-Flohé R., Bruford E.A., Burk R.F., Carlson B.A., Castellano S., Chavatte L., Conrad M. Selenoprotein gene nomenclature. J. Biol. Chem. 2016;291:24036–24040. doi: 10.1074/jbc.M116.756155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qian F., Misra S., Prabhu K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Crit. Rev. Biochem. Mol. Biol. 2019;54:484–516. doi: 10.1080/10409238.2020.1717430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu W., Zhao C., Hu H., Yin S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients. 2021;13:1739. doi: 10.3390/nu13051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Radomska D., Czarnomysy R., Radomski D., Bielawska A., Bielawski K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients. 2021;13:1649. doi: 10.3390/nu13051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong Y.M., Mo X.Y., Zou X.Z., Song R.X., Sun W.Y., Lu W., Chen Q., Yu Y.X., Zang W.J. Association study between polymorphisms in selenoprotein genes and susceptibility to Kashin-Beck disease. Osteoarthr. Cartil. 2010;18:817–824. doi: 10.1016/j.joca.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Ratnasinghe D., Tangrea J.A., Andersen M.R., Barrett M.J., Virtamo J., Taylor P.R., Albanes D. Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res. 2000;60:6381–6383. [PubMed] [Google Scholar]
- 93.Lei C., Niu X., Wei J., Zhu J., Zhu Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin. Chim. Acta. 2009;399:102–108. doi: 10.1016/j.cca.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 94.Ichimura Y., Habuchi T., Tsuchiya N., Wang L., Oyama C., Sato K., Nishiyama H., Ogawa O., Kato T. Increased risk of bladder cancer associated with a glutathione peroxidase 1 codon 198 variant. J. Urol. 2004;172:728–732. doi: 10.1097/01.ju.0000130942.40597.9d. [DOI] [PubMed] [Google Scholar]
- 95.Ezzikouri S., El Feydi A.E., Afifi R., Benazzouz M., Hassar M., Pineau P., Benjelloun S. Polymorphisms in antioxidant defence genes and susceptibility to hepatocellular carcinoma in a Moroccan population. Free Radic. Res. 2010;44:208–216. doi: 10.3109/10715760903402906. [DOI] [PubMed] [Google Scholar]
- 96.Arsova-Sarafinovska Z., Matevska N., Eken A., Petrovski D., Banev S., Dzikova S., Georgiev V., Sikole A., Erdem O., Sayal A., et al. Glutathione peroxidase 1 (GPX1) genetic polymorphism, erythrocyte GPX activity, and prostate cancer risk. Int. Urol. Nephrol. 2009;41:63–70. doi: 10.1007/s11255-008-9407-y. [DOI] [PubMed] [Google Scholar]
- 97.Lin J.C., Kuo W.R., Chiang F.Y., Hsiao P.J., Lee K.W., Wu C.W., Juo S.H. Glutathione peroxidase 3 gene polymorphisms and risk of differentiated thyroid cancer. Surgery. 2009;145:508–513. doi: 10.1016/j.surg.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 98.Grond-Ginsbach C., Lichy C., Padovani A., Pezzini A. GPx-3 gene promoter variation and the risk of arterial ischemic stroke. Stroke. 2007;38:e23–e24. doi: 10.1161/STROKEAHA.106.479444. [DOI] [PubMed] [Google Scholar]
- 99.Udler M., Maia A.T., Cebrian A., Brown C., Greenberg D., Shah M., Caldas C., Dunning A., Easton D., Ponder B., et al. Common germline genetic variation in antioxidant defense genes and survival after diagnosis of breast cancer. J. Clin. Oncol. 2007;25:3015–3023. doi: 10.1200/JCO.2006.10.0099. [DOI] [PubMed] [Google Scholar]
- 100.Meplan C., Hughes D.J., Pardini B., Naccarati A., Soucek P., Vodickova L., Hlavata I., Vrana D., Vodicka P., Hesketh J.E. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis. 2010;31:1074–1079. doi: 10.1093/carcin/bgq076. [DOI] [PubMed] [Google Scholar]
- 101.Peeters R.P., van den Beld A.W., van Toor H., Uitterlinden A.G., Janssen J.A., Lamberts S.W., Visser T.J. A polymorphism in type I deiodinase is associated with circulating free insulin-like growth factor I levels and body composition in humans. J. Clin. Endocrinol. Metab. 2005;90:256–263. doi: 10.1210/jc.2004-1301. [DOI] [PubMed] [Google Scholar]
- 102.Mentuccia D., Proietti-Pannunzi L., Tanner K., Bacci V., Pollin T.I., Poehlman E.T., Shuldiner A.R., Celi F.S. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: Evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- 103.Guo T.W., Zhang F.C., Yang M.S., Gao X.C., Bian L., Duan S.W., Zheng Z.J., Gao J.J., Wang H., Li R.L., et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J. Med. Genet. 2004;41:585–590. doi: 10.1136/jmg.2004.019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dora J.M., Machado W.E., Rheinheimer J., Crispim D., Maia A.L. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: Case-control study and meta-analysis. Eur. J. Endocrinol. 2010;163:427–434. doi: 10.1530/EJE-10-0419. [DOI] [PubMed] [Google Scholar]
- 105.Canani L.H., Capp C., Dora J.M., Meyer E.L., Wagner M.S., Harney J.W., Larsen P.R., Gross J.L., Bianco A.C., Maia A.L. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 106.Meulenbelt I., Bos S.D., Chapman K., van der Breggen R., Houwing-Duistermaat J.J., Kremer D., Kloppenburg M., Carr A., Tsezou A., Gonzalez A., et al. Meta-analyses of genes modulating intracellular T3 bio-availability reveal a possible role for the DIO3 gene in osteoarthritis susceptibility. Ann. Rheum. Dis. 2011;70:164–167. doi: 10.1136/ard.2010.133660. [DOI] [PubMed] [Google Scholar]
- 107.Meplan C., Nicol F., Burtle B.T., Crosley L.K., Arthur J.R., Mathers J.C., Hesketh J.E. Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, se intake, and cancer status. Antioxid. Redox Signal. 2009;11:2631–2640. doi: 10.1089/ars.2009.2533. [DOI] [PubMed] [Google Scholar]
- 108.Wang J., Sun T., Yang M., Lin D.X., Tan W., Li K.J., Xiao Y. Association of genetic polymorphisms in selenoprotein GPX1 and TXNRD2 with genetic susceptibility of gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:511–514. [PubMed] [Google Scholar]
- 109.Peters U., Chatterjee N., Hayes R.B., Schoen R.E., Wang Y., Chanock S.J., Foster C.B. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol. Biomark. Prev. 2008;17:1144–1154. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 110.Cooper M.L., Adami H.O., Gronberg H., Wiklund F., Green F.R., Rayman M.P. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008;68:10171–10177. doi: 10.1158/0008-5472.CAN-08-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zorzato F., Jungbluth H., Zhou H., Muntoni F., Treves S. Functional effects of mutations identified in patients with multiminicore disease. IUBMB Life. 2007;59:14–20. doi: 10.1080/15216540601187803. [DOI] [PubMed] [Google Scholar]
- 112.Jurynec M.J., Xia R., Mackrill J.J., Gunther D., Crawford T., Flanigan K.M., Abramson J.J., Howard M.T., Grunwald D.J. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc. Natl. Acad. Sci. USA. 2008;105:12485–12490. doi: 10.1073/pnas.0806015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sutherland A., Kim D.H., Relton C., Ahn Y.O., Hesketh J. Polymorphisms in the selenoprotein S and 15-kDa selenoprotein genes are associated with altered susceptibility to colorectal cancer. Genes Nutr. 2010;5:215–223. doi: 10.1007/s12263-010-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shibata T., Arisawa T., Tahara T., Ohkubo M., Yoshioka D., Maruyama N., Fujita H., Kamiya Y., Nakamura M., Nagasaka M., et al. Selenoprotein S (SEPS1) gene -105G>A promoter polymorphism influences the susceptibility to gastric cancer in the Japanese population. BMC Gastroenterol. 2009;9:2. doi: 10.1186/1471-230X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moses E.K., Johnson M.P., Tommerdal L., Forsmo S., Curran J.E., Abraham L.J., Charlesworth J.C., Brennecke S.P., Blangero J., Austgulen R. Genetic association of preeclampsia to the inflammatory response gene SEPS1. Am. J. Obs. Gynecol. 2008;198:336.e1–336.e5. doi: 10.1016/j.ajog.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 116.Penney K.L., Schumacher F.R., Li H., Kraft P., Morris J.S., Kurth T., Mucci L.A., Hunter D.J., Kantoff P.W., Stampfer M.J., et al. A large prospective study of SEP15 genetic variation, interaction with plasma selenium levels, and prostate cancer risk and survival. Cancer Prev. Res. (Phila) 2010;3:604–610. doi: 10.1158/1940-6207.CAPR-09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jablonska E., Gromadzinska J., Sobala W., Reszka E., Wasowicz W. Lung cancer risk associated with selenium status is modified in smoking individuals by Sep15 polymorphism. Eur. J. Nutr. 2008;47:47–54. doi: 10.1007/s00394-008-0696-9. [DOI] [PubMed] [Google Scholar]
- 118.Fordyce F. Selenium geochemistry and health. Ambio. 2007;36:94–97. doi: 10.1579/0044-7447(2007)36[94:SGAH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 119.Lobinski R., Edmonds J.S., Suzuki K.T., Uden P.C. Species-selective determination of selenium compounds in biological materials (Technical Report) Pure Appl. Chem. 2000;72:447–461. doi: 10.1351/pac200072030447. [DOI] [Google Scholar]
- 120.Hoac T., Lundh T., Önning G., Åkesson B. Selenoproteins and Mimics. Springer; Berlin/Heidelberg, Germany: 2012. Selenoproteins and Selenium Speciation in Food; pp. 183–206. [Google Scholar]
- 121.Pérez-Corona M.T., Sánchez-Martínez M., Valderrama M.J., Rodríguez M.E., Cámara C., Madrid Y. Selenium biotransformation by Saccharomyces cerevisiae and Saccharomyces bayanus during white wine manufacture: Laboratory-scale experiments. Food Chem. 2011;124:1050–1055. doi: 10.1016/j.foodchem.2010.07.073. [DOI] [Google Scholar]
- 122.Guo X., Wu L. Distribution of free seleno-amino acids in plant tissue of Melilotus indica L. grown in selenium-laden soils. Ecotoxicol. Environ. Saf. 1998;39:207–214. doi: 10.1006/eesa.1997.1628. [DOI] [PubMed] [Google Scholar]
- 123.Thomson C.D., Robinson M.F., Butler J.A., Whanger P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993;69:577–588. doi: 10.1079/BJN19930057. [DOI] [PubMed] [Google Scholar]
- 124.Kang D., Lee J., Wu C., Guo X., Lee B.J., Chun J.-S., Kim J.-H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020;52:1198–1208. doi: 10.1038/s12276-020-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haug A., Graham R.D., Christophersen O.A., Lyons G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007;19:209–228. doi: 10.1080/08910600701698986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gawor A., Ruszczynska A., Czauderna M., Bulska E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals. 2020;10:808. doi: 10.3390/ani10050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hao L., Zhang J., Zhang S., Ma S., Li B., Long J., Fan J., Luo K. Distribution characteristics and main influencing factors of selenium in surface soil of natural selenium-rich area: A case study in Langao County, China. Environ. Geochem. Health. 2021;43:333–346. doi: 10.1007/s10653-020-00711-2. [DOI] [PubMed] [Google Scholar]
- 128.Razaghi A., Poorebrahim M., Sarhan D., Bjornstedt M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer. 2021;155:256–267. doi: 10.1016/j.ejca.2021.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Institute of Medicine (US) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press (US); Washington, DC, USA: 2000. Panel on dietary antioxidants and related compounds. [PubMed] [Google Scholar]
- 130.MacFarquhar J.K., Broussard D.L., Melstrom P., Hutchinson R., Wolkin A., Martin C., Burk R.F., Dunn J.R., Green A.L., Hammond R., et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010;170:256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qin H.B., Zhu J.M., Liang L., Wang M.S., Su H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013;52:66–74. doi: 10.1016/j.envint.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 132.Dauplais M., Lazard M., Blanquet S., Plateau P. Neutralization by metal ions of the toxicity of sodium selenide. PLoS ONE. 2013;8:e54353. doi: 10.1371/journal.pone.0054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rayman M.P., Winther K.H., Pastor-Barriuso R., Cold F., Thvilum M., Stranges S., Guallar E., Cold S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018;127:46–54. doi: 10.1016/j.freeradbiomed.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 134.Kipp A.P., Strohm D., Brigelius-Flohé R., Schomburg L., Bechthold A., Leschik-Bonnet E., Heseker H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015;32:195–199. doi: 10.1016/j.jtemb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 135.Ravaglia G., Forti P., Maioli F., Bastagli L., Facchini A., Mariani E., Savarino L., Sassi S., Cucinotta D., Lenaz G. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged ≥ 90 y. Am. J. Clin. Nutr. 2000;71:590–598. doi: 10.1093/ajcn/71.2.590. [DOI] [PubMed] [Google Scholar]
- 136.Prabhu K.S., Lei X.G. Selenium. Adv. Nutr. 2016;7:415–417. doi: 10.3945/an.115.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014;370:1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- 138.Li Q., Liu M., Hou J., Jiang C., Li S., Wang T. The prevalence of Keshan disease in China. Int. J. Cardiol. 2013;168:1121–1126. doi: 10.1016/j.ijcard.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 139.Vinceti M., Filippini T., Wise L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018;5:464–485. doi: 10.1007/s40572-018-0213-0. [DOI] [PubMed] [Google Scholar]
- 140.Bomer N., Grote Beverborg N., Hoes M.F., Streng K.W., Vermeer M., Dokter M.M., IJmker J., Anker S.D., Cleland J.G.F., Hillege H.L., et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2020;22:1415–1423. doi: 10.1002/ejhf.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J. Nutr. 2003;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 142.Ren L.Q., Li X.J., Li G.S., Zhao Z.T., Sun B., Sun F. Coxsackievirus B3 infection and its mutation in Keshan disease. World J. Gastroenterol. 2004;10:3299–3302. doi: 10.3748/wjg.v10.i22.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chasseur C., Suetens C., Nolard N., Begaux F., Haubruge E. Fungal contamination in barley and Kashin-Beck disease in Tibet. Lancet. 1997;350:1074. doi: 10.1016/S0140-6736(05)70453-0. [DOI] [PubMed] [Google Scholar]
- 144.McClure B.A., Hagen G., Brown C.S., Gee M.A., Guilfoyle T.J. Transcription, organization, and sequence of an auxin-regulated gene cluster in soybean. Plant Cell. 1989;1:229–239. doi: 10.1105/tpc.1.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xia Y., Hill K.E., Byrne D.W., Xu J., Burk R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 146.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J. Trace Elem. Med. Biol. 1995;9:65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 147.Duffield A.J., Thomson C.D., Hill K.E., Williams S. An estimation of selenium requirements for New Zealanders. Am. J. Clin. Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 148.Zachara B.A., Pawluk H., Bloch-Boguslawska E., Śliwka K.M., Korenkiewicz J., Skok Ź., Ryć K. Tissue level, distribution and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health Int. J. 2001;56:461–466. doi: 10.1080/00039890109604483. [DOI] [PubMed] [Google Scholar]
- 149.Moreno-Reyes R., Egrise D., Neve J., Pasteels J.L., Schoutens A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001;16:1556–1563. doi: 10.1359/jbmr.2001.16.8.1556. [DOI] [PubMed] [Google Scholar]
- 150.Moreno-Reyes R., Mathieu F., Boelaert M., Begaux F., Suetens C., Rivera M.T., Nève J., Perlmutter N., Vanderpas J. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am. J. Clin. Nutr. 2003;78:137–144. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 151.Suetens C., Moreno-Reyes R., Chasseur C., Mathieu F., Begaux F., Haubruge E., Durand M., Neve J., Vanderpas J. Epidemiological support for a multifactorial aetiology of Kashin-Beck disease in Tibet. Int. Orthop. 2001;25:180–187. doi: 10.1007/s002640100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ebert R., Jakob F. Selenium deficiency as a putative risk factor for osteoporosis. Int. Congr. Ser. 2007;1297:158–164. doi: 10.1016/j.ics.2006.08.001. [DOI] [Google Scholar]
- 153.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 154.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 155.Sims N.A., Gooi J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell. Dev. Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 156.Nakahama K. Cellular communications in bone homeostasis and repair. Cell. Mol. Life Sci. 2010;67:4001–4009. doi: 10.1007/s00018-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boyle W., Simonet W., Lacey D. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 158.Lacey D., Timms E., Tan H.-L., Kelley M., Dunstan C., Burgess T., Elliott R., Colombero A., Scully G.E.S., Hsu H. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 159.Wauquier F., Leotoing L., Coxam V., Guicheux J., Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 160.Manolagas S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pietschmann N., Rijntjes E., Hoeg A., Stoedter M., Schweizer U., Seemann P., Schomburg L. Selenoprotein P is the essential selenium transporter for bones. Metallomics. 2014;6:1043–1049. doi: 10.1039/C4MT00003J. [DOI] [PubMed] [Google Scholar]
- 162.Ebert R., Ulmer M., Zeck S., Meissner-Weigl J., Schneider D., Stopper H., Schupp N., Kassem M., Jakob F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24:1226–1235. doi: 10.1634/stemcells.2005-0117. [DOI] [PubMed] [Google Scholar]
- 163.Dreher I., Schütze N., Baur A., Hesse K., Schneider D., Köhrle J., Jakob F. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem. Biophys. Res. Commun. 1998;245:101–107. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- 164.Kohrle J., Jakob F., Contempré B., Dumont J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 165.Jakob F., Becker K., Paar E., Ebert-Duemig R., Schütze N. [13]—Expression and Regulation of Thioredoxin Reductases and Other Selenoproteins in Bone. In: Sies H., Packer L., editors. Methods in Enzymology. Volume 347. Academic Press; Cambridge, MA, USA: 2002. pp. 168–179. [DOI] [PubMed] [Google Scholar]
- 166.Prabhu K.S., Zamamiri-Davis F., Stewart J.B., Thompson J.T., Sordillo L.M., Reddy C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-kappaB in up-regulation. Biochem. J. 2002;366:203–209. doi: 10.1042/bj20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park J.M., Kim A., Oh J.H., Chung A.S. Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation mediated by MT1-MMP expression and further tumor invasion through suppression of NF-kappaB activation. Carcinogenesis. 2007;28:837–847. doi: 10.1093/carcin/bgl203. [DOI] [PubMed] [Google Scholar]
- 168.Christensen M.J., Nartey E.T., Hada A.L., Legg R.L., Barzee B.R. High selenium reduces NF-kappaB-regulated gene expression in uninduced human prostate cancer cells. Nutr. Cancer. 2007;58:197–204. doi: 10.1080/01635580701328701. [DOI] [PubMed] [Google Scholar]
- 169.Alwahaibi N.Y., Budin S.B., Mohamed J., Alhamdani A. Nuclear factor-kappa B as a promising target for selenium chemoprevention in rat hepatocarcinogenesis. J. Gastroenterol. Hepatol. 2010;25:786–791. doi: 10.1111/j.1440-1746.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- 170.Mlakar S.J., Osredkar J., Prezelj J., Marc J. The antioxidant enzyme GPX1 gene polymorphisms are associated with low BMD and increased bone turnover markers. Dis. Markers. 2010;29:71–80. doi: 10.1155/2010/354189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johnson R.H. Autonomic involvement in systemic diseases. Curr. Opin. Neurol. Neurosurg. 1992;5:468–475. [PubMed] [Google Scholar]
- 172.Bai X.-c., Lu D., Bai J., Zheng H., Ke Z.-y., Li X.-m., Luo S.-q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem. Biophys. Res. Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 173.Xu Z.-S., Wang X.-Y., Xiao D.-M., Hu L.-F., Lu M., Wu Z.-Y., Bian J.-S. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage—implications for the treatment of osteoporosis. Free Radic. Biol. Med. 2011;50:1314–1323. doi: 10.1016/j.freeradbiomed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 174.Subbaiah P.V., Chen C.H., Albers J.J., Bagdade J.D. Studies on the cofactor requirement for the acylation and hydrolysis reactions catalyzed by purified lecithin-cholesterol acyltransferase. Effect of low density lipoproteins and apolipoprotein A-I. Atherosclerosis. 1982;45:181–190. doi: 10.1016/0021-9150(82)90137-X. [DOI] [PubMed] [Google Scholar]
- 175.Liu H., Bian W., Liu S., Huang K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol. Trace Elem. Res. 2012;150:441–450. doi: 10.1007/s12011-012-9488-4. [DOI] [PubMed] [Google Scholar]
- 176.Wang L., Zhang Y.-G., Wang X.-M., Ma L.-F., Zhang Y.-M. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem.-Biol. Interact. 2015;242:255–261. doi: 10.1016/j.cbi.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 177.Cao J.J., Gregoire B.R., Zeng H. Selenium deficiency decreases antioxidative capacity and is detrimental to bone microarchitecture in mice. J. Nutr. 2012;142:1526–1531. doi: 10.3945/jn.111.157040. [DOI] [PubMed] [Google Scholar]
- 178.Kim H., Lee K., Kim J.M., Kim M.Y., Kim J.-R., Lee H.-W., Chung Y.W., Shin H.-I., Kim T., Park E.-S., et al. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021;12:2258. doi: 10.1038/s41467-021-22565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Burk R.F., Hill K.E., Olson G.E., Weeber E.J., Motley A.K., Winfrey V.P., Austin L.M. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J. Neurosci. 2007;27:6207–6211. doi: 10.1523/JNEUROSCI.1153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pietschmann N., Rijntjes E., Hoeg A., Schomburg L. On the importance of selenium for bone physiology. Bone Abstr. 2013;1:115. doi: 10.1530/boneabs.1.PP115. [DOI] [Google Scholar]
- 181.Burk R.F., Hill K.E., Motley A.K., Winfrey V.P., Kurokawa S., Mitchell S.L., Zhang W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014;28:3579–3588. doi: 10.1096/fj.14-252874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Olson G.E., Winfrey V.P., NagDas S.K., Hill K.E., Burk R.F. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J. Biol. Chem. 2007;282:12290–12297. doi: 10.1074/jbc.M611403200. [DOI] [PubMed] [Google Scholar]
- 183.Chung Y.W., Kim T.S., Lee S.Y., Lee S.H., Choi Y., Kim N., Min B.-M., Jeong D.-W., Kim I.Y. Selenite-induced apoptosis of osteoclasts mediated by the mitochondrial pathway. Toxicol. Lett. 2006;160:143–150. doi: 10.1016/j.toxlet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 184.Hiraoka K., Komiya S., Hamada T., Zenmyo M., Inoue A. Osteosarcoma cell apoptosis induced by selenium. J. Orthop. Res. 2001;19:809–814. doi: 10.1016/S0736-0266(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 185.Chen Y.-C., Sosnoski D.M., Gandhi U.H., Novinger L.J., Prabhu K.S., Mastro A.M. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis. 2009;30:1941–1948. doi: 10.1093/carcin/bgp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sasaki S., Iwata H., Ishiguro N., Habuchi O., Miura T. Low-selenium diet, bone, and articular cartilage in rats. Nutrition. 1994;10:538–543. [PubMed] [Google Scholar]
- 187.Turan B., Balcik C., Akkas N. Effect of dietary selenium and vitamin E on the biomechanical properties of rabbit bones. Clin. Rheumatol. 1997;16:441–449. doi: 10.1007/BF02238935. [DOI] [PubMed] [Google Scholar]
- 188.Turner C.H., Burr D.B. Basic biomechanical measurements of bone: A tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-K. [DOI] [PubMed] [Google Scholar]
- 189.Bhan A., Qiu S., Rao S.D. Bone histomorphometry in the evaluation of osteomalacia. Bone Rep. 2018;8:125–134. doi: 10.1016/j.bonr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Turan B., Can B., Delilbasi E. Selenium combined with vitamin E and vitamin C restores structural alterations of bones in heparin-induced osteoporosis. Clin. Rheumatol. 2003;22:432–436. doi: 10.1007/s10067-003-0809-z. [DOI] [PubMed] [Google Scholar]
- 191.Yao Y.F., Pei F.X., Li X.B., Yang J., Shen B., Zhou Z.K., Li L., Kang P.D. Preventive effects of supplemental selenium and selenium plus iodine on bone and cartilage development in rats fed with diet from Kashin-Beck disease endemic area. Biol. Trace Elem. Res. 2012;146:199–206. doi: 10.1007/s12011-011-9232-5. [DOI] [PubMed] [Google Scholar]
- 192.Ren F.L., Guo X., Zhang R.J., Wang Sh J., Zuo H., Zhang Z.T., Geng D., Yu Y., Su M. Effects of selenium and iodine deficiency on bone, cartilage growth plate and chondrocyte differentiation in two generations of rats. Osteoarthr. Cartil. 2007;15:1171–1177. doi: 10.1016/j.joca.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 193.Min Z., Zhao W., Zhong N., Guo Y., Sun M., Wang Q., Zhang R., Yan J., Tian L., Zhang F., et al. Abnormality of epiphyseal plate induced by selenium deficiency diet in two generation DA rats. APMIS. 2015;123:697–705. doi: 10.1111/apm.12404. [DOI] [PubMed] [Google Scholar]
- 194.Odabasi E., Turan M., Aydin A., Akay C., Kutlu M. Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann. Acad. Med. Singap. 2008;37:564–567. [PubMed] [Google Scholar]
- 195.Beukhof C.M., Medici M., van den Beld A.W., Hollenbach B., Hoeg A., Visser W.E., de Herder W.W., Visser T.J., Schomburg L., Peeters R.P. Selenium Status Is Positively Associated with Bone Mineral Density in Healthy Aging European Men. PLoS ONE. 2016;11:e0152748. doi: 10.1371/journal.pone.0152748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Galvez-Fernandez M., Grau-Perez M., Garcia-Barrera T., Ramirez-Acosta S., Gomez-Ariza J.L., Perez-Gomez B., Galan-Labaca I., Navas-Acien A., Redon J., Briongos-Figuero L.S., et al. Arsenic, cadmium, and selenium exposures and bone mineral density-related endpoints: The HORTEGA study. Free Radic. Biol. Med. 2021;162:392–400. doi: 10.1016/j.freeradbiomed.2020.10.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Al E.A.A., Parsian H., Fathi M., Faghihzadeh S., Hosseini S.R., Nooreddini H.G., Mosapour A. ALOX12 gene polymorphisms and serum selenium status in elderly osteoporotic patients. Adv. Clin. Exp. Med. 2018;27:1717–1722. doi: 10.17219/acem/75689. [DOI] [PubMed] [Google Scholar]
- 198.Liu S.Z., Yan H., Xu P., Li J.P., Zhuang G.H., Zhu B.F., Lu S.M. Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi’an urban area. Biol. Trace Elem. Res. 2009;131:205–214. doi: 10.1007/s12011-009-8363-4. [DOI] [PubMed] [Google Scholar]
- 199.Wang L., Yu H., Yang G., Zhang Y., Wang W., Su T., Ma W., Yang F., Chen L., He L., et al. Correlation between bone mineral density and serum trace element contents of elderly males in Beijing urban area. Int. J. Clin. Exp. Med. 2015;8:19250–19257. [PMC free article] [PubMed] [Google Scholar]
- 200.Park K.C., Kwon Y., Lee Y., Kim D.K., Jang Y., Lee S. Low selenium levels are associated with decreased bone mineral densities. J. Trace Elem. Med. Biol. 2020;61:126534. doi: 10.1016/j.jtemb.2020.126534. [DOI] [PubMed] [Google Scholar]
- 201.Zhang J., Munger R.G., West N.A., Cutler D.R., Wengreen H.J., Corcoran C.D. Antioxidant intake and risk of osteoporotic hip fracture in Utah: An effect modified by smoking status. Am. J. Epidemiol. 2006;163:9–17. doi: 10.1093/aje/kwj005. [DOI] [PubMed] [Google Scholar]
- 202.Ilich J.Z., Cvijetic S., Baric I.C., Cecic I., Saric M., Crncevic-Orlic Z., Blanusa M., Korsic M. Nutrition and lifestyle in relation to bone health and body weight in Croatian postmenopausal women. Int. J. Food Sci. Nutr. 2009;60:319–332. doi: 10.1080/09637480701780724. [DOI] [PubMed] [Google Scholar]
- 203.Rivas A., Romero A., Mariscal-Arcas M., Monteagudo C., Lopez G., Lorenzo M.L., Ocana-Peinado F.M., Olea-Serrano F. Association between dietary antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr. Hosp. 2012;27:1886–1893. doi: 10.3305/nh.2012.27.6.6039. [DOI] [PubMed] [Google Scholar]
- 204.Pedrera-Zamorano J.D., Calderon-Garcia J.F., Roncero-Martin R., Manas-Nunez P., Moran J.M., Lavado-Garcia J.M. The protective effect of calcium on bone mass in postmenopausal women with high selenium intake. J. Nutr. Health Aging. 2012;16:743–748. doi: 10.1007/s12603-012-0071-7. [DOI] [PubMed] [Google Scholar]
- 205.Chan R., Woo J., Lau W., Leung J., Xu L., Zhao X., Yu W., Lau E., Pocock N. Effects of lifestyle and diet on bone health in young adult Chinese women living in Hong Kong and Beijing. Food Nutr. Bull. 2009;30:370–378. doi: 10.1177/156482650903000408. [DOI] [PubMed] [Google Scholar]
- 206.Sun L.L., Li B.L., Xie H.L., Fan F., Yu W.Z., Wu B.H., Xue W.Q., Chen Y.M. Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: A case-control study. Br. J. Nutr. 2014;112:1706–1714. doi: 10.1017/S0007114514002773. [DOI] [PubMed] [Google Scholar]
- 207.Wang Y., Xie D., Li J., Long H., Wu J., Wu Z., He H., Wang H., Yang T., Wang Y. Association between dietary selenium intake and the prevalence of osteoporosis: A cross-sectional study. BMC Musculoskelet. Disord. 2019;20:585. doi: 10.1186/s12891-019-2958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang Y., Ye M., Zhao Y., Xiong Y., Shen S., Yu Q., Lu Y., Shi Z., Lei X. Higher Dietary Se Intake Is Associated With the Risk of New-Onset Fracture: A National Longitudinal Study for 20 Years. Front. Nutr. 2021;8:719147. doi: 10.3389/fnut.2021.719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Karita K., Yamanouchi Y., Takano T., Oku J., Kisaki T., Yano E. Associations of blood selenium and serum lipid levels in Japanese premenopausal and postmenopausal women. Menopause. 2008;15:119–124. doi: 10.1097/gme.0b013e31806bf32c. [DOI] [PubMed] [Google Scholar]
- 210.Hiller R., Seigel D., Sperduto R.D., Blair N., Burton T.C., Farber M.D., Gragoudas E.S., Gunter E.W., Haller J., Seddon J.M., et al. Serum zinc and serum lipid profiles in 778 adults. Ann. Epidemiol. 1995;5:490–496. doi: 10.1016/1047-2797(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 211.Zeng H., Cao J.J., Combs G.F., Jr. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients. 2013;5:97–110. doi: 10.3390/nu5010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ichikawa S., Koller D.L., Johnson M.L., Lai D., Xuei X., Edenberg H.J., Klein R.F., Orwoll E.S., Hui S.L., Foroud T.M., et al. Human ALOX12, but not ALOX15, is associated with BMD in white men and women. J. Bone Miner. Res. 2006;21:556–564. doi: 10.1359/jbmr.051212. [DOI] [PubMed] [Google Scholar]
- 213.Chen C.J., Huang H.S., Chang W.C. Depletion of phospholipid hydroperoxide glutathione peroxidase up-regulates arachidonate metabolism by 12S-lipoxygenase and cyclooxygenase 1 in human epidermoid carcinoma A431 cells. FASEB J. 2003;17:1694–1696. doi: 10.1096/fj.02-0847fje. [DOI] [PubMed] [Google Scholar]
- 214.Qu Z., Yang F., Yan Y., Hong J., Wang W., Li S., Jiang G., Yan S. Relationship between Serum Nutritional Factors and Bone Mineral Density: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021;106:e2434–e2443. doi: 10.1210/clinem/dgab085. [DOI] [PubMed] [Google Scholar]
- 215.Walsh J.S., Jacques R.M., Schomburg L., Hill T.R., Mathers J.C., Williams G.R., Eastell R. Effect of selenium supplementation on musculoskeletal health in older women: A randomised, double-blind, placebo-controlled trial. Lancet Healthy Longev. 2021;2:e212–e221. doi: 10.1016/S2666-7568(21)00051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Perri G., Hill T., Mathers J.C., Walsh J., Gossiel F., Winther K., Frölich J., Folkestad L., Cold S., Eastell R. Effect of selenium supplementation on biomarkers of bone turnover. Proc. Nutr. Soc. 2021;80:E110. doi: 10.1017/S0029665121002330. [DOI] [Google Scholar]
- 217.Kuo T.-R., Chen C.-H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017;5:1–9. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Amin K.A., Hashem K.S., Alshehri F.S., Awad S.T., Hassan M.S. Antioxidant and hepatoprotective efficiency of selenium nanoparticles against acetaminophen-induced hepatic damage. Biol. Trace Elem. Res. 2017;175:136–145. doi: 10.1007/s12011-016-0748-6. [DOI] [PubMed] [Google Scholar]
- 219.Rayman M.P., Infante H.G., Sargent M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008;100:238–253. doi: 10.1017/S0007114508922522. [DOI] [PubMed] [Google Scholar]
- 220.Fairweather-Tait S.J., Collings R., Hurst R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010;91:1484S–1491S. doi: 10.3945/ajcn.2010.28674J. [DOI] [PubMed] [Google Scholar]
- 221.Menon S., Ks S.D., Santhiya R., Rajeshkumar S., Kumar V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces. 2018;170:280–292. doi: 10.1016/j.colsurfb.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 222.Fernandes A.P., Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta. 2015;1850:1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 223.Jayaweera G.R., Biggar J.W. Role of redox potential in chemical transformations of selenium in soils. Soil Sci. Soc. Am. J. 1996;60:1056–1063. doi: 10.2136/sssaj1996.03615995006000040014x. [DOI] [Google Scholar]
- 224.Srivastava N., Mukhopadhyay M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess. Biosyst. Eng. 2015;38:1723–1730. doi: 10.1007/s00449-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 225.Estevez H., Garcia-Lidon J.C., Luque-Garcia J.L., Camara C. Effects of chitosan-stabilized selenium nanoparticles on cell proliferation, apoptosis and cell cycle pattern in HepG2 cells: Comparison with other selenospecies. Colloids Surf. B Biointerfaces. 2014;122:184–193. doi: 10.1016/j.colsurfb.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 226.Wang H., Zhang J., Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 227.Benko I., Nagy G., Tanczos B., Ungvari E., Sztrik A., Eszenyi P., Prokisch J., Banfalvi G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 2012;31:2812–2820. doi: 10.1002/etc.1995. [DOI] [PubMed] [Google Scholar]
- 228.Forootanfar H., Adeli-Sardou M., Nikkhoo M., Mehrabani M., Amir-Heidari B., Shahverdi A.R., Shakibaie M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014;28:75–79. doi: 10.1016/j.jtemb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 229.Peng D., Zhang J., Liu Q., Taylor E.W. Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J. Inorg. Biochem. 2007;101:1457–1463. doi: 10.1016/j.jinorgbio.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 230.Shakibaie M., Khorramizadeh M.R., Faramarzi M.A., Sabzevari O., Shahverdi A.R. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol. Appl. Biochem. 2010;56:7–15. doi: 10.1042/BA20100042. [DOI] [PubMed] [Google Scholar]
- 231.Shi L., Xun W., Yue W., Zhang C., Ren Y., Shi L., Wang Q., Yang R., Lei F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011;96:49–52. doi: 10.1016/j.smallrumres.2010.11.005. [DOI] [Google Scholar]
- 232.Zhang S.-Y., Zhang J., Wang H.-Y., Chen H.-Y. Synthesis of selenium nanoparticles in the presence of polysaccharides. Mater. Lett. 2004;58:2590–2594. doi: 10.1016/j.matlet.2004.03.031. [DOI] [Google Scholar]
- 233.Quintana M., Haro-Poniatowski E., Morales J., Batina N. Synthesis of selenium nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2002;195:175–186. doi: 10.1016/S0169-4332(02)00549-4. [DOI] [Google Scholar]
- 234.Shoeibi S., Mashreghi M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017;39:135–139. doi: 10.1016/j.jtemb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 235.Ramamurthy C., Sampath K.S., Arunkumar P., Kumar M.S., Sujatha V., Premkumar K., Thirunavukkarasu C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess. Biosyst. Eng. 2013;36:1131–1139. doi: 10.1007/s00449-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 236.Gao X., Zhang J., Zhang L. Hollow sphere selenium nanoparticles: Their in-vitro anti hydroxyl radical effect. Adv. Mater. 2002;14:290–293. doi: 10.1002/1521-4095(20020219)14:4<290::AID-ADMA290>3.0.CO;2-U. [DOI] [Google Scholar]
- 237.Zhang J., Wang X., Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 238.Bhattacharjee A., Basu A., Bhattacharya S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus. 2019;62:259–268. doi: 10.1007/s13237-019-00303-1. [DOI] [Google Scholar]
- 239.Nikam A., Pagar T., Ghotekar S., Pagar K., Pansambal S. A review on plant extract mediated green synthesis of zirconia nanoparticles and their miscellaneous applications. J. Chem. Rev. 2019;1:154–163. [Google Scholar]
- 240.Ghotekar S. Plant extract mediated biosynthesis of Al2O3 nanoparticles-a review on plant parts involved, characterization and applications. Nanochem. Res. 2019;4:163–169. [Google Scholar]
- 241.Ghotekar S. A review on plant extract mediated biogenic synthesis of CdO nanoparticles and their recent applications. Asian J. Green Chem. 2019;3:187–200. [Google Scholar]
- 242.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bai K., Hong B., He J., Hong Z., Tan R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017;12:4527. doi: 10.2147/IJN.S129958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bai K., Hong B., Tan R., He J., Hong Z. Selenium nanoparticles-embedded chitosan microspheres and their effects upon alcohol-induced gastric mucosal injury in rats: Rapid preparation, oral delivery, and gastroprotective potential of selenium nanoparticles. Int. J. Nanomed. 2020;15:1187. doi: 10.2147/IJN.S237089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen W., Yue L., Jiang Q., Xia W. Effect of chitosan with different molecular weight on the stability, antioxidant and anticancer activities of well-dispersed selenium nanoparticles. IET Nanobiotechnol. 2019;13:30–35. doi: 10.1049/iet-nbt.2018.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yu B., Zhang Y., Zheng W., Fan C., Chen T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012;51:8956–8963. doi: 10.1021/ic301050v. [DOI] [PubMed] [Google Scholar]
- 247.Abd-Rabou A.A., Shalby A.B., Ahmed H.H. Selenium Nanoparticles Induce the Chemo-Sensitivity of Fluorouracil Nanoparticles in Breast and Colon Cancer Cells. Biol. Trace Elem. Res. 2019;187:80–91. doi: 10.1007/s12011-018-1360-8. [DOI] [PubMed] [Google Scholar]
- 248.Liu T., Zeng L., Jiang W., Fu Y., Zheng W., Chen T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine. 2015;11:947–958. doi: 10.1016/j.nano.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 249.Krishnan V., Loganathan C., Thayumanavan P. Green synthesized selenium nanoparticles using Spermacoce hispida as carrier of s-allyl glutathione: To accomplish hepatoprotective and nephroprotective activity against acetaminophen toxicity. Artif. Cells Nanomed. Biotechnol. 2019;47:56–63. doi: 10.1080/21691401.2018.1543192. [DOI] [PubMed] [Google Scholar]
- 250.Bai K., Hong B., He J., Huang W. Antioxidant capacity and hepatoprotective role of chitosan-stabilized selenium nanoparticles in concanavalin a-induced liver injury in mice. Nutrients. 2020;12:857. doi: 10.3390/nu12030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dkhil M.A., Zrieq R., Al-Quraishy S., Abdel Moneim A.E. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules. 2016;21:1517. doi: 10.3390/molecules21111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ahmed H.H., Abd El-Maksoud M.D., Moneim A.E.A., Aglan H.A. Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol. Trace Elem. Res. 2017;177:267–280. doi: 10.1007/s12011-016-0876-z. [DOI] [PubMed] [Google Scholar]
- 253.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 254.Sadek K.M., Lebda M.A., Abouzed T.K., Nasr S.M., Shoukry M. Neuro-and nephrotoxicity of subchronic cadmium chloride exposure and the potential chemoprotective effects of selenium nanoparticles. Metab. Brain Dis. 2017;32:1659–1673. doi: 10.1007/s11011-017-0053-x. [DOI] [PubMed] [Google Scholar]
- 255.Nazıroğlu M., Muhamad S., Pecze L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017;10:773–782. doi: 10.1080/17512433.2017.1324781. [DOI] [PubMed] [Google Scholar]
- 256.Yuan X., Fu Z., Ji P., Guo L., Al-Ghamdy A.O., Alkandiri A., Habotta O.A., Abdel Moneim A.E., Kassab R.B. Selenium Nanoparticles Pre-Treatment Reverse Behavioral, Oxidative Damage, Neuronal Loss and Neurochemical Alterations in Pentylenetetrazole-Induced Epileptic Seizures in Mice. Int. J. Nanomed. 2020;15:6339–6353. doi: 10.2147/IJN.S259134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chan C.H. Master’s Thesis. The Hong Kong Polytechnic University; Hong Kong, China: 2018. Investigation of Effects of Novel Selenium Nanoparticles Functionalized with Cordyceps sinensis on Osteoblastogenesis. [Google Scholar]
- 258.Luk K.H. Ph.D. Thesis. The Hong Kong Polytechnic University; Hong Kong, China: 2017. Selenium Nanoparticles Prepared by Myco-Fabrication: A Novel Bone-Forming Agent for Managing/Preventing Postmenopausal Osteoporosis. [Google Scholar]
- 259.Yu S., Luk K.H., Cheung S.T., Kwok K.W., Wong K.H., Chen T. Polysaccharide-protein complex-decorated selenium nanosystem as an efficient bone-formation therapeutic. J. Mater. Chem. B. 2018;6:5215–5219. doi: 10.1039/C8TB01084F. [DOI] [PubMed] [Google Scholar]
- 260.Fatima S., Alfrayh R., Alrashed M., Alsobaie S., Ahmad R., Mahmood A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021;16:331–343. doi: 10.2147/IJN.S285233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lee S.C., Lee N.H., Patel K.D., Jang T.S., Knowles J.C., Kim H.W., Lee H.H., Lee J.H. The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells. Nanomaterials. 2021;11:557. doi: 10.3390/nano11020557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vekariya K.K., Kaur J., Tikoo K. Alleviating anastrozole induced bone toxicity by selenium nanoparticles in SD rats. Toxicol. Appl. Pharmacol. 2013;268:212–220. doi: 10.1016/j.taap.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 263.Zhou Z.F., Sun T.W., Qin Y.H., Zhu Y.J., Jiang Y.Y., Zhang Y., Liu J.J., Wu J., He S.S., Chen F. Selenium-doped hydroxyapatite biopapers with an anti-bone tumor effect by inducing apoptosis. Biomater. Sci. 2019;7:5044–5053. doi: 10.1039/C9BM00953A. [DOI] [PubMed] [Google Scholar]
- 264.Tran P.A., O’Brien-Simpson N., Palmer J.A., Bock N., Reynolds E.C., Webster T.J., Deva A., Morrison W.A., O’Connor A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019;14:4613–4624. doi: 10.2147/IJN.S197737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang Y., Lv P., Ma Z., Zhang J. Enhanced healing of rat calvarial critical size defect with selenium-doped lamellar biocomposites. Biol. Trace Elem. Res. 2013;155:72–81. doi: 10.1007/s12011-013-9763-z. [DOI] [PubMed] [Google Scholar]
- 266.Vaquette C., Bock N., Tran P.A. Layered Antimicrobial Selenium Nanoparticle-Calcium Phosphate Coating on 3D Printed Scaffolds Enhanced Bone Formation in Critical Size Defects. ACS Appl. Mater. Interfaces. 2020;12:55638–55648. doi: 10.1021/acsami.0c17017. [DOI] [PubMed] [Google Scholar]
- 267.Mackinnon E., Rao A., Josse R., Rao L. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporos. Int. 2011;22:1091–1101. doi: 10.1007/s00198-010-1308-0. [DOI] [PubMed] [Google Scholar]
- 268.Xiao D., Zhang J., Zhang C., Barbieri D., Yuan H., Moroni L., Feng G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020;106:22–33. doi: 10.1016/j.actbio.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 269.Li T.L., Tao Z.S., Wu X.J., Yang M., Xu H.G. Selenium-modified calcium phosphate cement can accelerate bone regeneration of osteoporotic bone defect. J. Bone Miner. Metab. 2021;39:934–943. doi: 10.1007/s00774-021-01240-3. [DOI] [PubMed] [Google Scholar]
- 270.Johnell O., Kanis J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 271.Rashki Kemmak A., Rezapour A., Jahangiri R., Nikjoo S., Farabi H., Soleimanpour S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islamic Repub. Iran. 2020;34:154. doi: 10.47176/mjiri.34.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hodsman A., Papaioannou A., Cranney A. Clinical practice guidelines for the use of parathyroid hormone in the treatment of osteoporosis. CMAJ Can. Med. Assoc. J. 2006;175:48. doi: 10.1503/cmaj.060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.McFarlane S.I., Muniyappa R., Shin J.J., Bahtiyar G., Sowers J.R. Osteoporosis and cardiovascular disease: Brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 274.Fuggle N.R., Cooper C., Harvey N.C., Al-Daghri N., Brandi M.L., Bruyere O., Cano A., Dennison E.M., Diez-Perez A., Kaufman J.M., et al. Assessment of Cardiovascular Safety of Anti-Osteoporosis Drugs. Drugs. 2020;80:1537–1552. doi: 10.1007/s40265-020-01364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Watts N.B., Diab D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 276.Shalihat A., Hasanah A.N., Lesmana R., Budiman A., Gozali D. The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: A literature review. Biomed. Pharmacother. 2021;134:111125. doi: 10.1016/j.biopha.2020.111125. [DOI] [PubMed] [Google Scholar]
- 277.Okatan E.N., Tuncay E., Turan B. Cardioprotective effect of selenium via modulation of cardiac ryanodine receptor calcium release channels in diabetic rat cardiomyocytes through thioredoxin system. J. Nutr. Biochem. 2013;24:2110–2118. doi: 10.1016/j.jnutbio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 278.Ojeda M.L., Sobrino P., Rua R.M., Gallego-Lopez M.D.C., Nogales F., Carreras O. Selenium, a dietary-antioxidant with cardioprotective effects, prevents the impairments in heart rate and systolic blood pressure in adolescent rats exposed to binge drinking treatment. Am. J. Drug Alcohol Abuse. 2021;47:1–14. doi: 10.1080/00952990.2021.1973485. [DOI] [PubMed] [Google Scholar]
- 279.Gunes S., Sahinturk V., Karasati P., Sahin I.K., Ayhanci A. Cardioprotective Effect of Selenium Against Cyclophosphamide-Induced Cardiotoxicity in Rats. Biol. Trace Elem. Res. 2017;177:107–114. doi: 10.1007/s12011-016-0858-1. [DOI] [PubMed] [Google Scholar]
- 280.Alehagen U., Alexander J., Aaseth J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE. 2016;11:e0157541. doi: 10.1371/journal.pone.0157541. [DOI] [PMC free article] [PubMed] [Google Scholar]