Supplemental digital content is available in the text.
KEY WORDS: Blood platelets, wounds and injuries, sequence analysis, RNA
Abstract
BACKGROUND
The earliest measurable changes to postinjury platelet biology may be in the platelet transcriptome, as platelets are known to carry messenger ribonucleic acids (RNAs), and there is evidence in other inflammatory and infectious disease states of differential and alternative platelet RNA splicing in response to changing physiology. Thus, the aim of this exploratory pilot study was to examine the platelet transcriptome and platelet RNA splicing signatures in trauma patients compared with healthy donors.
METHODS
Preresuscitation platelets purified from trauma patients (n = 9) and healthy donors (n = 5) were assayed using deep RNA sequencing. Differential gene expression analysis, weighted gene coexpression network analysis, and differential alternative splicing analyses were performed. In parallel samples, platelet function was measured with platelet aggregometry, and clot formation was measured with thromboelastography.
RESULTS
Differential gene expression analysis identified 49 platelet RNAs to have differing abundance between trauma patients and healthy donors. Weighted gene coexpression network analysis identified coexpressed platelet RNAs that correlated with platelet aggregation. Differential alternative splicing analyses revealed 1,188 splicing events across 462 platelet RNAs that were highly statistically significant (false discovery rate <0.001) in trauma patients compared with healthy donors. Unsupervised principal component analysis of these platelet RNA splicing signatures segregated trauma patients in two main clusters separate from healthy controls.
CONCLUSION
Our findings provide evidence of finetuning of the platelet transcriptome through differential alternative splicing of platelet RNA in trauma patients and that this finetuning may have relevance to downstream platelet signaling. Additional investigations of the trauma platelet transcriptome should be pursued to improve our understanding of the platelet functional responses to trauma on a molecular level.
Trauma-induced coagulopathy (TIC) is a potentially devastating acquired multiphenotypic failure of vascular homeostasis caused by severe injury and hemorrhagic shock, and is associated with bleeding, thrombosis, inflammatory complications, and death.1,2 Platelet dysfunction is often reported as a critical component of TIC and its complications. However, despite a large body of literature identifying platelet dysfunction in up to half of injured patients, the ex vivo evidence in support of this is discordant, with increased levels of activated platelets that contribute to hemostasis, yet paradoxically impaired platelet aggregation.1,3–5 Further, impaired platelet aggregation is commonly identified outside of TIC and in patients with minor injury, is not accounted for by thrombocytopenia, and cannot be rescued with platelet transfusion.5–8 Numerous factors have been implicated in this biology;9–14 however, given the contradictory findings and complexities in the ex vivo study of platelet function (related to the small size and reactive nature of platelets, as well as limitations of the available assays), it remains unknown whether the described platelet dysfunction in trauma is pathologic or not, and whether it requires treatment.5 As such, there is a clear need for improved methods of studying postinjury platelet biology.
Despite being small and anucleate, platelets participate in an array of physiological functions, including clot formation and breakdown, endothelial maintenance, and immune response.15 Platelets do not transcribe genes de novo, but they carry megakaryocyte derived messenger ribonucleic acids (RNAs) to support these various functions. There is evidence that this platelet transcriptome is stable in health.16 However, it has been identified that in some infectious and inflammatory disease states, platelet RNA can undergo differential alternative splicing in response to physiological signals as part of the tailored platelet response to that disease state.17–26 Therefore, transcriptomics is emerging as an attractive means of studying the platelet response to disease.25,27–30 Recently, we identified numerous RNAs potentially involved in platelet function in the plasma of traumatic brain injury patients31 and hypothesized that platelets themselves were the most likely source of this. However, it remains unknown if the physiology of trauma alters the platelet transcriptome or if differential alternative splicing of platelet RNA could be responsible for the described changes in platelet function after injury. Thus, the aim of this exploratory pilot study was to examine the platelet transcriptome and platelet RNA splicing signatures in trauma patients compared with healthy donors.
PATIENTS AND METHODS
Participant Enrollment and Sample Collection
Whole blood was collected in standard laboratory vacuum-sealed tubes containing 3.2% (0.109 mol/L) sodium citrate (Becton Dickinson, Franklin Lakes, NJ) from a convenience sample of trauma patients who met trauma activation criteria on arrival to the emergency department prior to resuscitation and from healthy donors over a 2-year period. From the citrated whole blood, platelets were immediately isolated for RNA sequencing, and functional assays of clot formation and platelet aggregation were performed by the below described techniques. Clinical characteristics of the trauma patients were collected in parallel; however, given the exploratory pilot nature of the study, no power calculations were performed, and no a priori clinical hypotheses were made. Healthy donors were recruited during the same 2-year period, underwent the same sampling, and limited clinical characteristics were collected in parallel (age, sex, and use of anticoagulants and antiplatelets). Samples were not analyzed if participants were determined to be younger than 18 years, pregnant, in custody, or receiving anticoagulant or antiplatelet medications. This study was approved by the institutional review board of the University of California, San Francisco (IRB 19-28933), and informed consent was obtained from all participants.
Platelet Isolation
Platelets were immediately isolated for RNA sequencing, based on previously described methods.18,22,24 Briefly, fresh whole blood was centrifuged at 200g for 20 minutes. The resultant platelet rich plasma supernatant was treated with Prostaglandin-E1 (PG-E1, 600 nM; Cayman Chemical, Ann Arbor, MI), and centrifuged at 800g for 20 minutes to pellet platelets. Platelets were resuspended in PIPES saline glucose buffer (PSG, 5 mM PIPES, 145 mM NaCl, 4 mM KCl, 50 μM Na2HPO4, 1 mM MgCl2·6H2O, 5.5 mM glucose, pH 6.8) with PG-E1 and incubated with magnetic bead-conjugated anti-CD45 (cat. no. 130-045-801; Miltenyi Biotec, Bergisch Gladbach, Germany). Leukocytes were negatively selected by passing the platelet anti-CD45 bead suspension through a magnetic column, and platelets were pelleted by centrifugation and resuspended in M199 medium (Lonza, Basel, Switzerland). Platelets were again pelleted by centrifugation, lysed in Trizol (Life Technologies, Carlsbad, CA), and stored at −80°C. All platelet isolates were stored for less than 2 years to limit storage or RNA degradation effects.
Molecular Biology and RNA Sequencing
From platelet lysates stored in Trizol, RNA was isolated using the Qiamp RNA Blood Mini Kit (Qiagen, Hilden, Germany). Deoxyribonucleic acid (DNA) contamination was removed with DNAse I, and sample quality was assessed by Bioanalyzer (Agilent, Santa Clara, CA). Complementary DNA libraries were generated with 1 ng to 2 ng of RNA using the Ovation random primed isothermal amplification system (NuGen, Redwood City, CA). Sequencing was performed at a depth of 100 to 400 million reads using a Novaseq 6000 (Illumina, San Diego, CA).
Transcriptomic Analyses
Differential Gene Expression Analysis: Platelet RNA Abundance in Trauma Patients Versus Healthy Donors
First, we performed differential gene expression analysis to compare platelet RNA abundance in trauma patients compared with healthy donors. The RNA sequencing data were aligned to the GRCh38 assembly of the human genome and mapped with release 100 of the Ensembl gene annotations of this genome using STAR Aligner (version 2.6.0c).32 Quality was checked using fastQC (version 0.11.5).33 To compare overall platelet RNA abundance, gene level expression values were then quantitated from mapped reads using featureCounts within the Subread package (version 2.0.0).34 Differential gene expression analysis was performed by a generalized linear model within EdgeR.35 We assessed for significance using a false discovery rate (FDR) of 0.05 under the Benjamini-Hochberg procedure.36,37
Weighted Gene Coexpression Network Analysis: Platelet RNA Module-Trait Relationships in Trauma Patients and Healthy Donors
Next, weighted gene coexpression network analysis (WGCNA)38,39 was used to further characterize platelet RNA abundance associated with trauma. Briefly, WGCNA constructs a network of genes from patterns of coexpression in an agnostic manner with no a priori patient characteristics. Sets of genes sharing coexpression patterns (i.e., modules) are identified in the resultant network. This approach allows for an independent unsupervised interpretation of the gene expression data.38 In doing so, a summary score of the expression of genes in these modules were evaluated for an association with platelet aggregation responses. Following identification of modules in our data, we performed a further analysis examining group membership using higher orders of biological organization, including gene ontology and protein-protein interaction analyses.
Following alignment, the log counts per million gene lists were filtered with a threshold of one count per million in at least five samples to remove the lowest expressed reads. From this, the resultant genes were used for WGCNA analysis. We selected an empirical soft power threshold of 17, which represented a strong model fit to a scale-free topology (signed R2 = 0.80) to generate the signed adjacency matrix. A clustered gene tree was generated using the “average” method. Genes with highly correlated expression were grouped into modules based on topological overlap. Each module was assigned a color, and this color label was used for identification in all subsequent analyses. The module eigengenes (i.e., the first principal component of variation among the coexpressed genes in that module) of each of the coexpression modules was tested for an association with platelet aggregation responses. Pearson's correlation coefficients and p values were calculated for each pair of eigengene and platelet aggregation response. A significant correlation was assessed using a p value less than 0.05. To evaluate for higher orders of biological organization of genes from modules where traits correlated with module expression levels, we evaluated for functional enrichment using pathway analysis (Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, and Reactome) using Metascape40 and the Search Tool for the Retrieval of Interacting Genes41 for protein-protein interaction elucidation. We assessed for significance of the functional enrichment pathway analysis using a p value less than 0.01.
Differential Alternative Splicing Analyses: Platelet RNA Splicing in Trauma Patients Versus Healthy Donors
Analysis of differential alternative platelet RNA splicing was performed using alternative splicing mapping tool.42 We assessed significance of the differential alternative splicing with an FDR cutoff of less than 0.001. For each splicing event, a ratio was calculated for each sample between transcripts found to include that splicing event and transcripts found specifically without that splicing event. Unsupervised principal component analysis was performed on the samples' splice junction inclusion ratios within R (version 4.1.1). K-means clustering was performed to group samples into clusters with maximum similarity, using the “kmeans” method in R. A pathway overrepresentation test was performed to identify pathways that are enriched for genes showing differential alternative splicing events using Gene Ontology Enrichment Analysis.43–45
Functional Assessment of Clot Formation and Platelet Aggregation
Rotational Thromboelastometry: Clot Formation
Rotational thromboelastometry (Werfen, Barcelona, Spain) was performed to assess global clot formation immediately on whole blood according to the manufacturer's directions. Extrinsic clotting pathway function was tested using Extem reagents. The clot formation time (CFT) in seconds, α angle in degrees, maximum clot formation in mm (MCF), and percent maximum lysis were quantified.
Multiple Impedance Platelet Aggregometry: Platelet Aggregation
Platelet aggregometry using a Multiplate multiple impedance aggregometer (Roche, Basel, Switzerland) was performed to assess platelet aggregation in response to stimulation immediately on whole blood according to manufacturer’s directions. The following platelet surface stimulants were used (all from Hart Biologicals, Hartlepool, UK): Adenosine diphosphate (final concentration 6.45 μM), thrombin analog (SFLLRN, final concentration 32.3 μM), or collagen (final concentration 3.23 μg/mL). An extensive panel of platelet aggregation responses to each surface stimulant were included: areas under the aggregation curve, maximal aggregation value (aggregation units), maximal aggregation velocity (velocity units), baseline (prestimulation) aggregation value (impedance units), and endpoint (poststimulation) aggregation value (impedance units). Deviation from the mean and correlation coefficient served as quality control values.
For all functional assessments of clot formation and platelet aggregation, Kruskal-Wallis tests were used to assess overall statistical significance and Wilcoxon signed rank tests were used to make pairwise comparisons.
RESULTS
Trauma Patients and Healthy Donors
We isolated platelets from a total of nine trauma patients and five healthy donors. The trauma patients suffered from a range of severity of injuries, but all had normal platelet counts (median and interquartile range of 267 and 224–309 × 109 platelets/L; additional clinical characteristic details in Supplementary Table 1, http://links.lww.com/TA/C189).
Transcriptomic Analyses
Differential Gene Expression Analysis: Platelet RNA Abundance in Trauma Patients Versus Healthy Donors
Forty-nine platelet RNAs were found with differing abundance in trauma patients compared with healthy donors (Table 1), primarily mitochondrial or annotated as such. All but two platelet RNAs were found to be present in lower abundance in trauma patients than in healthy donors.
TABLE 1.
Platelet RNA Abundance in Trauma Patients Compared With Healthy Donors
| Gene | Gene Name | Fold Change* | p | FDR |
|---|---|---|---|---|
| MYL4 | Myosin light chain 4 | 5.82 | 3.53 × 10−5 | 0.01 |
| AC093809.1 | Mitochondrially encoded cytochrome C oxidase III pseudogene | 5.74 | 9.64 × 10−5 | 0.03 |
| AGGF1P10 | Angiogenic factor with G-patch and FHA domains 1 pseudogene 10 | 5.22 | 9.78 × 10−5 | 0.03 |
| REEP1 | Receptor accessory protein 1 | −3.65 | 2.12 × 10−4 | 0.04 |
| AC109635.3 | Pantothenate kinase 3 pseudogene | 3.32 | 1.17 × 10−4 | 0.03 |
| FBXO48 | F-Box protein 48 | 3.22 | 1.96 × 10−4 | 0.04 |
| IL6STP1 | interleukin 6 signal transducer pseudogene 1 | 2.99 | 2.31 × 10−5 | 0.01 |
| MTATP8P1 | Mitochondrially encoded ATP synthase 8 pseudogene 1 | 2.86 | 1.37 × 10−5 | 0.01 |
| MTND5P11 | Mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 5 pseudogene 11 | 2.84 | 2.53 × 10−8 | 0.00 |
| MTND6P4 | Mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 6 pseudogene 4 | 2.67 | 1.01 × 10−6 | 0.00 |
| AC107954.1 | Capping protein (actin filament) muscle Z-line, alpha 2 | 2.61 | 7.45 × 10−5 | 0.02 |
| SEPTIN7P3 | Septin 7 pseudogene 3 | 2.53 | 1.12 × 10−4 | 0.03 |
| H3P1 | H3 histone pseudogene 1 | 2.44 | 2.53 × 10−5 | 0.01 |
| MTND4LP30 | Mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4L pseudogene 30 | 2.41 | 2.44 × 10−5 | 0.01 |
| MTATP8P2 | Mitochondrially encoded ATP synthase 8 pseudogene 2 | 2.39 | 6.96 × 10−6 | 0.01 |
| FTH1P3 | Ferritin, heavy polypeptide 1 pseudogene 3 | 2.32 | 3.55 × 10−5 | 0.01 |
| RN7SL3 | RNA component of signal recognition particle 7SL3 | 2.32 | 2.48 × 10−5 | 0.01 |
| MTND4P12 | Mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4 pseudogene 12 | 2.25 | 2.56 × 10−7 | 0.00 |
| NAP1L1P1 | Nucleosome assembly protein 1-like 1 pseudogene 1 | 2.23 | 2.00 × 10−6 | 0.00 |
| MTRNR2L8 | Humanin-like 8 | 2.13 | 8.27 × 10−5 | 0.02 |
| PCNPP5 | PEST containing nuclear protein pseudogene 5 | 2.12 | 1.40 × 10−5 | 0.01 |
| MTCO3P12 | Mitochondrially encoded cytochrome c oxidase III pseudogene 12 | 2.11 | 1.84 × 10−6 | 0.00 |
| NOMO2 | NODAL modulator 2 | 2.07 | 1.26 × 10−4 | 0.03 |
| AP000763.2 | Mitochondrially encoded cytochrome c oxidase I pseudogene | 2.07 | 9.22 × 10−6 | 0.01 |
| MTCO1P40 | Mitochondrially encoded cytochrome c oxidase I pseudogene 40 | 2.03 | 8.90 × 10−6 | 0.01 |
| MTCYBP18 | Mitochondrially encoded cytochrome b pseudogene 18 | 1.98 | 9.12 × 10−5 | 0.03 |
| OVCH1-AS1 | OVCH1 antisense RNA 1 | 1.92 | 2.64 × 10−6 | 0.00 |
| FTH1P5 | Ferritin, heavy polypeptide 1 pseudogene 5 | 1.91 | 5.61 × 10−5 | 0.02 |
| YWHAZP5 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide pseudogene | 1.88 | 2.02 × 10−5 | 0.01 |
| ATF4P3 | Activating transcription factor 4C | 1.87 | 2.73 × 10−5 | 0.01 |
| MTCO2P12 | Mitochondrially encoded cytochrome c oxidase II pseudogene 12 | 1.82 | 2.62 × 10−5 | 0.01 |
| NDUFA4 | NADH-ubiquinone oxidoreductase MLRQ subunit | 1.81 | 5.39 × 10−6 | 0.01 |
| FUNDC1 | FUN14 domain-containing protein 1 | 1.76 | 1.04 × 10−4 | 0.03 |
| H3P47 | H3 histone pseudogene 47 | 1.72 | 2.20 × 10−4 | 0.04 |
| TLK1P1 | Tousled like kinase 1 pseudogene 1 | 1.72 | 4.31 × 10−5 | 0.02 |
| FTH1P8 | Ferritin, heavy polypeptide 1 pseudogene 8 | 1.67 | 1.36 × 10−5 | 0.01 |
| H3P16 | H3 histone pseudogene 16 | 1.66 | 6.08 × 10−5 | 0.02 |
| TTC1 | Tetratricopeptide repeat domain 1 | 1.63 | 9.52 × 10−6 | 0.01 |
| FTH1P23 | Ferritin, heavy polypeptide 1 pseudogene 23 | 1.63 | 3.83 × 10−5 | 0.01 |
| FTH1P16 | Ferritin heavy chain 1 pseudogene 16 | 1.57 | 1.55 × 10−4 | 0.04 |
| CCNYL1 | Cyclin Y like 1 | 1.55 | 1.72 × 10−4 | 0.04 |
| ITGB3BP | Integrin subunit beta 3 binding protein | 1.54 | 1.68 × 10−4 | 0.04 |
| DNAJC3 | DnaJ heat shock protein family (Hsp40) member C3 | 1.54 | 2.76 × 10−5 | 0.01 |
| SNN | Stannin | −1.48 | 2.30 × 10−4 | 0.04 |
| AC113404.3 | RAP1B like (pseudogene) | 1.48 | 1.21 × 10−5 | 0.01 |
| AC084824.2 | Nucleosome assembly protein 1-like 1 (NAP1L1) pseudogene | 1.48 | 2.08 × 10−4 | 0.04 |
| FTH1P11 | Ferritin heavy chain 1 pseudogene 11 | 1.47 | 9.91 × 10−5 | 0.03 |
| ROCK2 | Rho associated coiled-coil containing protein kinase 2 | 0.89 | 5.74 × 10−5 | 0.02 |
| RIT1 | Ras like without CAAX 1 | 0.89 | 1.46 × 10−4 | 0.03 |
WGCNA: Platelet RNA Module-Trait Relationships in Trauma Patients and Healthy Donors
Correlative module-trait relationships were identified for sets of coexpressed platelet RNAs and platelet aggregation responses. Multiple sets of coexpressed platelet RNAs correlated with measures of platelet aggregation responses (cyan, brown, light cyan; Fig. 1 and Supplementary Figures 1–12, http://links.lww.com/TA/C190).
Figure 1.
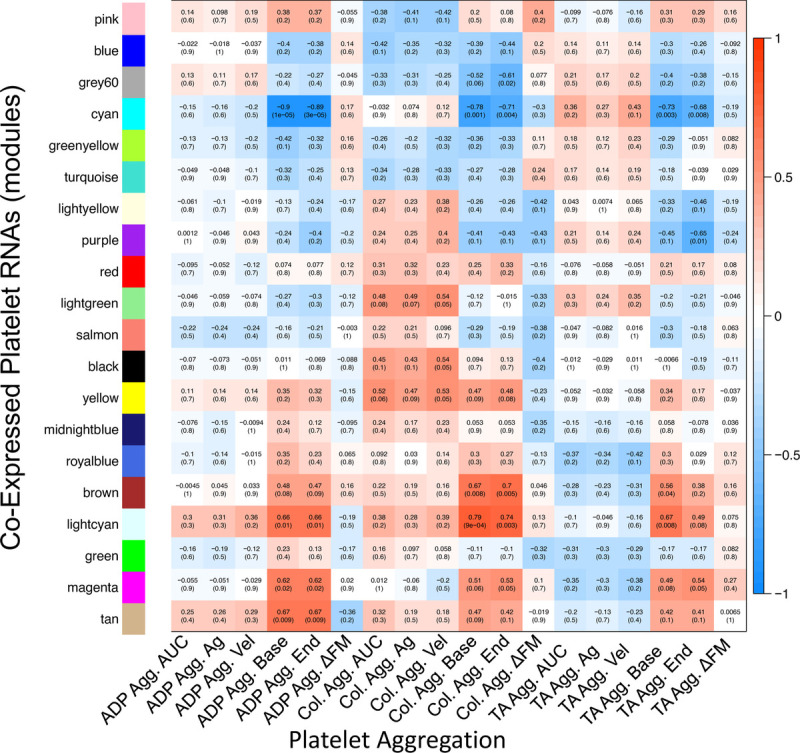
Heatmap of correlations of coexpressed platelet RNAs and platelet aggregation. Within each box, the top number indicates the calculated Pearson's correlation coefficient, and the bottom shows the p value. Positive correlations are depicted in red and negative correlations are depicted in blue. For example, the cyan set of coexpressed platelet RNAs correlated negatively with baseline aggregation for all three stimulants, suggesting its involvement in platelet aggregation. Agg., aggregometry; Ag, maximal aggregation value in Multiplate aggregometry; Vel, maximal aggregation velocity in multiplate aggregometry; base, baseline (prestimulation) aggregation value; end, endpoint (poststimulation) aggregation value; ΔFM, deviation from mean in aggregometry (quality control value describing deviation of duplicate aggregometry electrode pairs, included here as a control that appropriately should not correlate with gene expression); TA, thrombin analog.
Differential Alternative Splicing Analyses: Platelet RNA Splicing in Trauma Patients Versus Healthy Donors
We found 1,188 splicing events across 462 platelet RNAs (FDR < 0.001; Fig. 2A) in trauma patients compared with healthy donors. There was relatively uniform platelet RNA splicing across the healthy donors, but unsurprisingly, a higher degree of heterogeneity across the trauma patients. Using unsupervised principal components analysis, three main clusters formed: healthy donors (group 1), and two separate trauma clusters (group 2 and group 3; Fig. 2B). There was one outlying trauma patient (7) that did not cluster with others. These clusters were consistent with those identified by K-means clustering when applied to all 1,188 significant splicing events (Supplementary Fig. 13, http://links.lww.com/TA/C190). We performed a Gene Ontology enrichment analysis across the platelet RNAs that contained significant splicing events to identify associated biological processes (Fig. 2C). We found that these platelet RNAs were involved in coagulation, platelet activation, and wound healing, as well as a myriad of other processes, including immune response, posttranscriptional gene regulation, and other signaling and cell physiologic processes.
Figure 2.
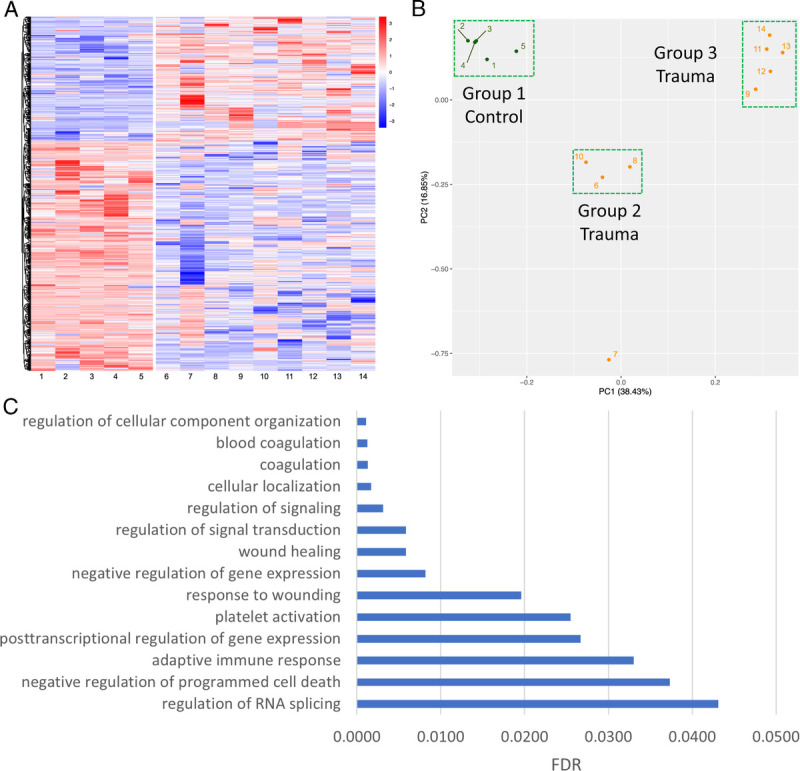
Differential alternative splicing of platelet RNA in trauma patients. (A) Heat map showing differential alternative splicing of platelet RNA in healthy donors (left) versus trauma patients (right). Blue corresponds to decreased inclusion ratio and red corresponds to increased inclusion ratio for each splicing event. In total, 1,188 splicing events across 462 platelet RNAs passed a FDR cutoff of less than 0.001. (B) Unsupervised principal component analysis reduced the data into two components explaining 16.85% and 38.43% of the data, respectively. Three main clusters were formed with unsupervised principal component analysis: healthy donors (Group 1), and two separate trauma clusters (Group 2 and Group 3). PC, principal component. C) Selected pathways from Gene Ontology enrichment analysis which are overrepresented among the platelet RNAs found to have differential alternative splicing between healthy donors and trauma patients, along with their FDR. Discovered pathways involve many common functions of platelets, including both coagulation and immune responses.
Finally, given the identification of splicing of platelet RNAs involved in coagulation and platelet activation in trauma patients compared with healthy donors, we next examined if groups 1, 2, and 3 differed in clot formation and platelet aggregation responses (Fig. 3). Relative to group 1 (healthy donors), group 2 and group 3 (trauma) had trends toward more disordered clot formation (Fig. 3A). Specifically, CFT was prolonged, while α angle and MCT were decreased. Further, group 2 had trends toward the lowest platelet aggregation responses to Adenosine diphosphate, thrombin analog, and collagen stimulation (Fig. 3B).
Figure 3.
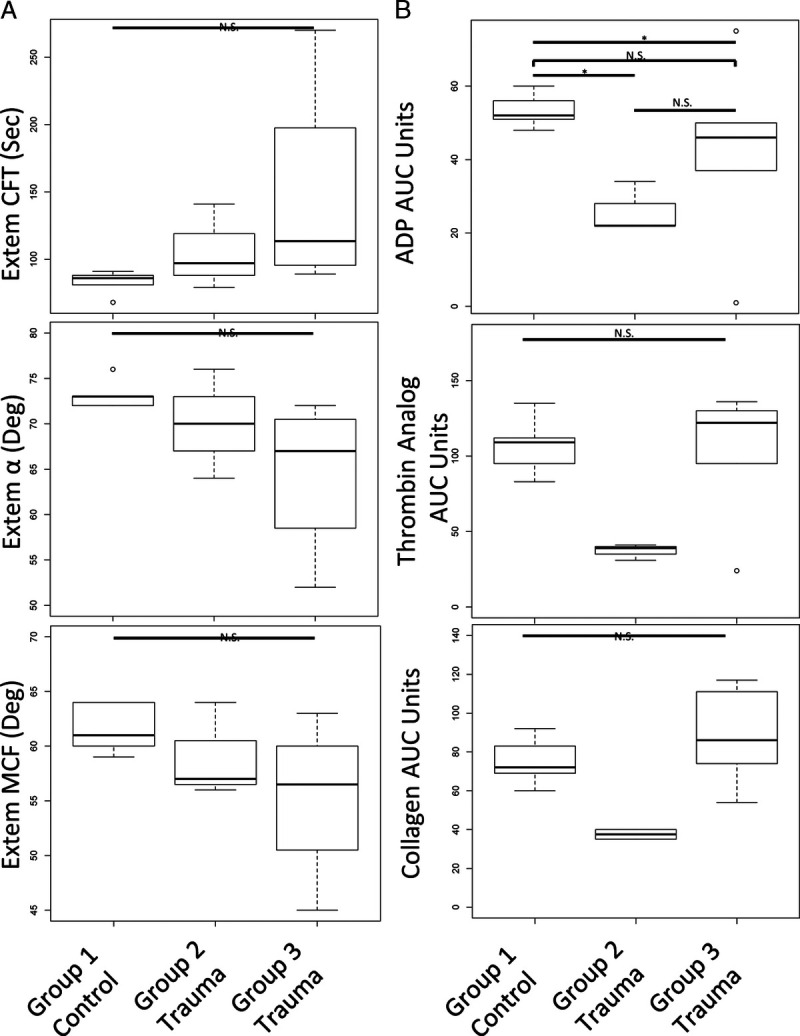
Platelet RNA splicing principal component groups have distinct clot formation and platelet aggregation responses. (A) Compared with group 1 (healthy donors, controls), group 3, and to a lesser extent group 2, had prolonged CFT, decreased α angle and decreased MCF by ROTEM. (B) Compared with group 1 (healthy donors, controls), group 2 had decreased platelet aggregation responses to Adenosine diphosphate, thrombin analog, and collagen stimulation by platelet aggregometry. Kruskal-Wallis tests were used to assess overall statistical significance and Wilcoxon signed rank tests were used to make pairwise comparisons; N.S., not statistically significant; *p ≤ 0.05. ROTEM, rotational thromboelastometry.
DISCUSSION
The results of this exploratory pilot study show promise for applying a transcriptomic approach to the study of postinjury platelet biology. Specifically, we have shown evidence of finetuning of the platelet transcriptome through differential alternative splicing of platelet RNA in trauma. Further, our results preliminarily support that both the static and dynamic platelet transcriptome after trauma has relevance to downstream platelet signaling, as has been identified in other disease states.25,26,28–30 In the case of the WGCNA analysis, our findings suggest that coexpressed platelet RNAs correlate with platelet aggregation responses that are specifically perturbed in the context of trauma physiology. In the case of the differential alternative splicing analyses, using unsupervised techniques two main clusters of trauma patients formed based on their platelet RNA splicing signatures, and these clusters appeared to differ in clot formation and platelet aggregation responses. Overall, we believe that these findings suggest that there may be biologic and physiologic relevance to finetuning of the platelet transcriptome in trauma patients, and substantiate that platelet transcriptomics may be valuable for improving our molecular understanding of the often-described but poorly understood postinjury platelet dysfunction.
The spliced platelet RNAs we identified in trauma patients are known to be involved with cytoskeletal function, vesicle fusion, cell signaling, and intriguingly, regulation of splicing. As such, we hypothesize that platelet RNA could be used to further activate or repress additional splicing and downstream functions. In fact, while the number of platelet RNA splicing events that passed very high statistical significance was large (FDR < 0.001, 1,188 splicing events), the overall percent change of the splicing ratios was relatively small (data not shown), suggesting a significant proportion of platelet RNA is not spliced in circulating platelets early following injury. This could mean that following any degree of injury, there are transcriptionally prepared circulating platelets, and a major signaling event (i.e., shock, intracranial hypertension, etc.) may be required to complete maturation of platelet RNA for translation into necessary gene products specific to the signal received.
Several limitations warrant consideration. First, while the sample size is small, this was an exploratory pilot transcriptomics study, and our results passed very stringent statistical cutoffs. Further, our sample size is compatible with other exploratory platelet transcriptomic studies.22,48,49 Second, although unsupervised analyses can be subject to unaccounted for confounders, the unbiased nature of our complementary DNA preps, mapping efficiency of using paired end Novaseq runs, and incorporation of high read depth of the samples maximized our opportunity to find the differential alternative spliced platelet RNAs in trauma patients. Third, we did not power the study to be able to examine differences in clinical characteristics between trauma patients and cannot make any conclusions on the clinical data of the patients. We recognize that the trauma patients studied were heterogeneous, including patients suffering from a range of severity of injury (from minor to severe), and patients with and without shock. Given platelet dysfunction is identified throughout the trauma literature in up to half of trauma patients regardless of injury severity or shock state, this representative cohort is relevant to an exploratory analysis of the platelet transcriptome in trauma patients compared with healthy donors. Our cohort included patients with low injury severity scores, suggesting that severe injury is not necessary to trigger alterations in the platelet transcriptome. This is relevant given the previous trauma literature has identified platelet dysfunction in patients with minor injury and may support our hypothesis that any amount of injury could create transcriptionally prepared circulating platelets. In addition, although the healthy donors and trauma patients were relatively well matched in age, there was sex variation in the healthy donors but not the trauma patients. It should be noted, however, that despite sex variation in the healthy donors, they clustered tightly together, an initial suggestion that the platelet transcriptome may be conserved between men and women. Hypotheses related to the effects of clinical differences of the trauma platelet transcriptome will be testable with large, diverse, and carefully phenotyped sample sets, now that our pilot data supports this line of investigation. Finally, there are known other RNA regulatory mechanisms that we did not examine that may also contribute to the trauma platelet transcriptome.50,51 Furthermore, while we did not perform proteomics, it can be reasonably assumed that a subset of the platelet RNAs we examined are for the purpose of production of protein products.52
In summary, we have shown evidence of finetuning of the platelet transcriptome through differential alternative splicing of platelet RNA in trauma patients, and that this finetuning may have relevance to downstream platelet signaling. Additional investigations of the trauma platelet transcriptome should be pursued to improve our understanding of the platelet functional responses to trauma on a molecular level and to uncover potential unique therapeutic targets for platelet-based contributions to hemorrhage, thrombosis, development of organ failure, and death after injury. Future studies with larger patient populations are necessary to allow for incorporation of more extensive clinical data and additional measures of postinjury platelet function.
Supplementary Material
AUTHORSHIP
A.T.F. designed and executed experiments, analyzed and interpreted data, article preparation. M.-C.L. assisted in the experimental design, supervised bioinformatic analyses, analyzed and interpreted data, and article preparation. F.M. performed molecular biology laboratory procedures and RNA sequencing. Y.A.S. performed bioinformatic analyses, analyzed and interpreted data, article preparation. C.M.V.B. performed bioinformatic analyses, provided information technology support, and analyzed and interpreted data. Z.A.M. prepared clinical data, assisted in experimental design, interpreted data, article preparation. R.A.C., M.S.P.H. interpreted data, article preparation. N.M. interpreted data, article preparation. J.C. interpreted data, article preparation. K.M.K. performed bioinformatic analyses, analyzed and interpreted data, article preparation. R.J.B. designed experiments, supervised bioinformatic analyses, analyzed and interpreted data, article preparation. L.Z.K. designed experiments, analyzed and interpreted data, article preparation.
ACKNOWLEDGMENTS
We wish to acknowledge the healthy donors and trauma patients and their loved ones who so graciously donated their blood to serve as the basis for this work. We wish to acknowledge Dr. Linus Tsai of the Broad Institute for his insightful and thought provoking discussions.
DISCLOSURE
This work was generously supported by NIH 1K23GM130892-01 (LZK), the University of California Irene Perstein Award (LZK), and the National Center for Advancing Translational Sciences of the NIH 5TL1TR001871-04 (ZAM).
The authors declare no conflicts of interest.
DISCUSSION
CARRIE SIMS, M.D. (Columbus, Ohio): Thank you, Dr. Reilly and the AAST for the privilege of discussing this paper. Understanding how platelets function after injury is vitally important in terms of identifying and treating trauma-induced coagulopathy.
Dr. Kornblith and colleagues’ paper adds to our understanding. But, like any good basic science paper, it raises more questions.
In this small, observational trial of nine trauma patients, it was demonstrated that injury results in an increase in the mRNA expression of 49 genes involved in the regulation of RNA splicing, program cell death, activation, wound healing, and mitochondrial function.
Moreover, healthy volunteers had a different RNA signature than blunt trauma or penetrating trauma. While this is intriguing, I have several questions.
Number 1. Your controls have three women, yet all of the patients are men. What was the rationale for not controlling for gender? Additionally, who were the volunteers? Do they match the socio-economic, racial or other parameters of your patients that might impact the platelet transcriptome?
Number 2. All three blunt patients had an ISS of one. Do you think if those patients had been more injured their transcriptome would have looked more like the penetrating trauma group? Are we seeing differences in degree of injury rather than a true phenotype based on injury mechanism?
Number 3. I am very intrigued by Patient Number 7 who was mis-categorized. By the numbers he should have clustered with penetrating group. Why do you think his clustering was so off? Does this call into question using such a small number to draw over-arching conclusions?
Number 4. Finally, do these differences in expression have clinical consequences? How many patients had clinical bleeding problems? Which of the gene differences or alternative splicing events is likely to be both clinically significant and easily measurable?
Once again, terrific paper and intriguing data. Looking forward to when we can discuss the paper in person and not by ZOOM.
JOHN B. HOLCOMB, M.D. (Birmingham, Alabama): Holcomb from Alabama. Lucy, great paper. I just have a question about the methodology. Why did you choose healthy controls? Wouldn’t a better control group would have been minimally-injured trauma patients who weren’t in shock or weren’t getting resuscitated? Thanks.
SCOTT BRAKENRIDGE, M.D. (Seattle, Washington): Congratulations, this is really interesting and novel work. I’m always excited to this lab on the program, because I learn something new about platelet biology every time they present their work.
My question for you is, is it possible or practical to look at the transcriptome within the megakaryocytes and then compare it to that in the platelets? The reason I ask that question, is that I am curious as to which compartment the presumed post-transcriptional modifications are actually happening.
Are the variations you are finding just a reflection of the megakaryocyte transcriptome itself? Or do you think these changes are actually happening independently in the platelets after they are circulating?
LUCY Z. KORNBLITH, M.D. (San Francisco, California): Thank you for the opportunity to be here virtually, and thank you to Dr. Sims for her expert thoughts and for sharing them with me in advance. I’m going to address them in order, and then I’ll address Dr. Holcomb’s and Dr. Brakenridge’s questions.
First, regarding the sex differences between the healthy donors and the trauma patients, we, of course, recognize the importance of understanding sex differences, which is why we collected both male and female sex in the healthy controls. We did intend to collect both sexes in the trauma patients, as well, but we met our planned end prior to obtaining any female sex samples. However, it is valuable to note that when examining the platelet RNA splicing differences, our healthy controls clustered very tightly together compared to trauma, which supports that sex was likely not a driver of splicing differences and that the platelet genome appears to be conserved regardless of sex in the healthy state. It is certainly possible, though, that with a physiologic insult like trauma there could be splicing differences of platelet RNA between male and female patients, despite seeing a lack of difference in the healthy state. There is, of course, data that supports differential functional responses of platelets based on sex, but it remains unknown whether this is driven by the megakaryocyte and platelet genome or somewhere on the journey from gene to protein product function. So as we continue to pursue platelet genomics in trauma we do expect we will be able to better characterize differences driven by sex.
Next, regarding the healthy volunteers, we have IRB collection for healthy donors for all our experiments. That is advertised in the same hospital in which the trauma patients are cared for. Through our IRB, we collect age, sex and antiplatelet medication use on the healthy donors so I can’t specifically comment on race, ethnicity or socio-economic status. We do know they were well-matched in age to the trauma patients, and we also know that the trauma patients clustered into two main separate groups. These groups had a mix of white, Caucasian, black, and ‘other’ in both of the clusters. So, as with sex, when the trauma platelet RNA was subject to this unbiased bioinformatics approach there was not clustering that we found by race or ethnicity. However, I do think race, ethnicity, and socio-economic status are very important to investigate as we further explore the role of the platelet genome in the response to trauma.
Next, we agree and hypothesize that the degree of injury likely contributes to the splicing of platelet RNA. That being said, half of the penetrating injury patients also had an ISS of one and did not cluster with the blunt trauma patients. So, as we continue to pursue this, we hypothesize that we will be able to see subgroups of platelet RNA splicing signatures emerge according to degree of injury and shock state. Regarding Trauma Patient Number 7, the outlier, we also are very intrigued by this patient. The RNA splicing signature was dramatically different than the other trauma clusters. Interestingly, we did find that outlier was the only trauma patient who tested positive to amphetamines, and we know that catecholamines do have effects on platelet function. It could be anything from pharmacology like that or genetic predisposition, sample acquisition, anything that could change the character of the platelets we sequenced. I think it remains an interesting mystery.
Finally, regarding Dr. Sims’s comment about whether differences in the platelet genome have clinical consequence, I think that’s our million dollar question. We didn’t power the study to look at clinical differences between patients or be able to control for any characteristics of patients. We first needed to know if we could see platelet genome differences between healthy controls and trauma patients, looking for big signals. Phenotyping the trauma patients is, of course, the ultimate goal. We feel our data supports that continued pursuit of this scientific path, and increasing sampling to address clinical questions might be able to resolve some of the ongoing mysteries regarding post-injury platelet function.
Regarding Dr. Holcomb’s question about methodology, it’s a very important point. I strongly agree that having sick control groups is always valuable. As mentioned, this first pass at this was really to see big signals, big differences, which is why we started with healthy donors versus trauma patients. We continue to pursue this methodology and will be able to have better controls and compare minimally-injured trauma controls to severely-injured.
Dr. Brakenridge’s comments about the megakaryocytes is so interesting. Actually, following the presentation immediately before me I was thinking their methodology would be a great way to get megakaryocytes from patients to sequence separately to compare the megakaryocyte genome to the circulating platelet genome.I think that is something that going forward could be pursued in a human model and, of course, in an animal model. There is strong evidence in other disease states – sepsis, MI, other inflammatory disease states – that the platelet genome is changing in circulation. I think these very early time point samples are likely to be evidence of a changing platelet genome rather than a changing megakaryocyte genome, but there is a lot more to come.
Thank you for the questions.
Footnotes
Published online: November 2, 2021.
This study was presented at the 2021 Annual Meeting of the American Association for the Surgery of Trauma; September 29 to October 2, 2021 in Atlanta, Georgia and received the 2021 American Association for the Surgery of Trauma Canizaro award.
A.T.F., M.-C.L., R.J.B., and L.Z.K. contributed equally to this study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Alexander T. Fields, Email: alexander.fields2@ucsf.edu.
Man-Cheung Lee, Email: Man-Cheung.Lee@ucsf.edu.
Fahima Mayer, Email: Fahima.Mayer@ucsf.edu.
Yale A. Santos, Email: Yale.Santos@ucsf.edu.
Cedric M.V. Bainton, Email: cbainton@g.hmc.edu.
Zachary A. Matthay, Email: Zachary.Matthay@ucsf.edu.
Rachael A. Callcut, Email: racallcut@ucdavis.edu.
Nasima Mayer, Email: mayern@berkeley.edu.
Joseph Cuschieri, Email: Joseph.Cuschieri@ucsf.edu.
Kord M. Kober, Email: Kord.Kober@ucsf.edu.
Roland J. Bainton, Email: Roland.Bainton@ucsf.edu.
REFERENCES
- 1.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, Schochl H, Hunt BJ, Sauaia A. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128(8):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51(4):639–647. [DOI] [PubMed] [Google Scholar]
- 4.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vulliamy P, Kornblith LZ, Kutcher ME, Cohen MJ, Brohi K, Neal MD. Alterations in platelet behavior after major trauma: adaptive or maladaptive? Platelets. 2021;32(3):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017;83(3):388–397. [DOI] [PubMed] [Google Scholar]
- 7.Kornblith LZ, Decker A, Conroy AS, Hendrickson CM, Fields AT, Robles AJ, Callcut RA, Cohen MJ. It's about time: transfusion effects on post-injury platelet aggregation over time. J Trauma Acute Care Surg. 2019;87(5):1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirajuddin S, Valdez C, DePalma L, Maluso P, Singhal R, Schroeder M, Sarani B. Inhibition of platelet function is common following even minor injury. J Trauma Acute Care Surg. 2016;81(2):328–332. [DOI] [PubMed] [Google Scholar]
- 9.Vulliamy P, Gillespie S, Armstrong PC, Allan HE, Warner TD, Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A. 2019;116(35):17444–17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiener G, Moore HB, Moore EE, Gonzalez E, Diamond S, Zhu S, D’Alessandro A, Banerjee A. Shock releases bile acid inducing platelet inhibition and fibrinolysis. J Surg Res. 2015;195(2):390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verni CC, Davila AJ, Balian S, Sims CA, Diamond SL. Platelet dysfunction during trauma involves diverse signaling pathways and an inhibitory activity in patient-derived plasma. J Trauma Acute Care Surg. 2019;86(2):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields AT, Matthay ZA, Nunez-Garcia B, Matthay EC, Bainton RJ, Callcut RA, Kornblith LZ. Good platelets gone bad: the effects of trauma patient plasma on healthy platelet aggregation. Shock. 2021;55(2):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starr NE, Matthay ZA, Fields AT, Nunez-Garcia B, Callcut RA, Cohen MJ, Kornblith LZ. Identification of Injury and Shock Driven Effects on Ex Vivo Platelet Aggregometry: A Cautionary Tale of Phenotyping. J Trauma Acute Care Surg. 2020;89:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vulliamy P, Montague SJ, Gillespie S, Chan MV, Coupland LA, Andrews RK, Warner TD, Gardiner EE, Brohi K, Armstrong PC. Loss of GPVI and GPIbα contributes to trauma-induced platelet dysfunction in severely injured patients. Blood Adv. 2020;4(12):2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34(1):5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondina MT Voora D Simon LM Schwertz LM Harper JF Lee O Bhatlekar SC Li Q Eustes AS Montenont E, et al. Longitudinal RNA-Seq analysis of the repeatability of gene expression and splicing in human platelets identifies a platelet SELP splice QTL. Circ Res. 2020;126(4):501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyrich AS, Lindemann S, Tolley ND, Kraiss LW, Dixon DA, Mahoney TM, Prescott SP, McIntyre TM, Zimmerman GA. Change in protein phenotype without a nucleus: translational control in platelets. Semin Thromb Hemost. 2004;30(4):491–498. [DOI] [PubMed] [Google Scholar]
- 18.Denis M Tolley ND Bunting M Bunting M Schwertz H Jiang H Lindemann S Yost C Rubner F Albertine K Swoboda K, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwertz H Tolley ND Foulks JM Denis MM Risenmay BW Buerke M Tilley RE Rondina MT Harris EM Kraiss LW, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203(11):2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2008;122(1):47–57. [DOI] [PubMed] [Google Scholar]
- 21.Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104(13):3943–3948. [DOI] [PubMed] [Google Scholar]
- 22.Nassa G Giurato G Cimmino G Rizzo F Ravo M Salvati A Nyman TA Zhu Y Vesterlund M Lehtio J, et al. Splicing of platelet resident pre-mRNAs upon activation by physiological stimuli results in functionally relevant proteome modifications. Sci Rep. 2018;8:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang C, Wuren T, Ga Q, Bai Z, Guo L, Eustes AS, McComas KN, Rondina MT, Ge R. The human platelet transcriptome and proteome is altered and pro-thrombotic functional responses are increased during prolonged hypoxia exposure at high altitude. Platelets. 2020;31(1):33–42. [DOI] [PubMed] [Google Scholar]
- 24.Hottz ED, Medeiros-de-Moraes IM, Vieira-de-Abreu A, de Assis EF, Vals-de-Souza R, Castro-Faria-Neto HC, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol. 2014;193(4):1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manne BK Denorme F Middleton EA Portier I Rowley JW Stubben C Petrey AC Tolley ND Guo L Cody M Grissom CK Brown SM Beesley SJ Schwertz H Kosaka Y Manne BK Krauel K, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton EA Rowley JW Campbell RA, et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood. 2019;134(12):911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19(5):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eicher JD, Wakabayashi Y, Vitseva O, Esa N, Yang Y, Zhu J, Freedman JE, McManus DD, Johnson AD. Characterization of the platelet transcriptome by RNA sequencing in patients with acute myocardial infarction. Platelets. 2016;27(3):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, Sturfelt G, Erlinge D, Bengtsson AA. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116(11):1951–1957. [DOI] [PubMed] [Google Scholar]
- 30.Plé H, Maltais M, Corduan A, Rousseau G, Madore F, Provost P. Alteration of the platelet transcriptome in chronic kidney disease. Thromb Haemost. 2012;108(4):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornblith LZ Bainton CMV Fields AT Matthay ZA Magid NT Nunez-Garcia B Prakash A Kurien PA Callcut RA Cohen MJ, et al. A journey upstream: Fluctuating platelet-specific genes in cell-free plasma as proof-of-concept for using ribonucleic acid sequencing to improve understanding of postinjury platelet biology. J Trauma Acute Care Surg. 2020;88(6):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao Y, Smyth GK, Shi W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. [DOI] [PubMed] [Google Scholar]
- 35.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 38.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langfelder P, Horvath S. Fast R functions for robust correlations and hierarchical clustering. J Stat Softw. 2012;46(11):i11. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szklarczyk D Gable AL Nastou KC Lyon D Kirsch R Pyysalo S Doncheva NT Legeay M Fang T Bork P, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A. 2014;111(51):E5593–E5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashburner M Ball CA Blake JA Botstein D Butler H Cherry JM Davis AP Dolinski K Dwight SS Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gene Ontology Consortium . The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49(D1):D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seal RL, Chen LL, Griffiths-Jones S, Lowe TM, Mathews MB, O'Reilly D, Pierce AJ, Stadler PF, Ulitsky I, Wolin SL, Bruford EA. A guide to naming human non-coding RNA genes. EMBO J. 2020;39(6):e103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res. 2003;31(1):439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bray PF McKenzie SE Edelstein LC Nagalla S Delgrosso K Ertel A Kupper J Jing Y Londin E Loher P, et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 2013;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rondina MT, Schwertz H, Harris ES, Kraemer BF, Campbell RA, Mackman N, Grissom CK, Weyrich AS, Zimmerman GA. The septic milieu triggers expression of spliced tissue factor mRNA in human platelets. J Thromb Haemost. 2011;9(4):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddoch-Cardenas KM, Peltier GC, Chance TC, Nair PM, Meledeo MA, Ramasubramanian AK, Cap AP, Bynum JA. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion. 2021;61(1):178–190. [DOI] [PubMed] [Google Scholar]
- 51.Ple H, Landry P, Benham A, Coarfa C, Gunaratne PH, Provost P. The repertoire and features of human platelet microRNAs. PLoS One. 2012;7(12):e50746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowley JW, Weyrich AS. Coordinate expression of transcripts and proteins in platelets. Blood. 2013;121(26):5255–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


