Abstract
Background
Retinovascular changes are reported on fundus imaging in schizophrenia (SZ). This is the first study to use swept-source optical coherence tomography angiography (OCT-A) to comprehensively examine retinal microvascular changes in SZ.
Methods
This study included 30 patients with SZ/schizoaffective disorder (8 early and 15 chronic) and 22 healthy controls (HCs). All assessments were performed at Beth Israel Deaconess Medical Center and Massachusetts Eye and Ear. All participants underwent swept-source OCT-A of right (oculus dextrus [OD]) and left (oculus sinister [OS]) eye, clinical, and cognitive assessments. Macular OCT-A images (6 × 6 mm) were collected with the DRI Topcon Triton for superficial, deep, and choriocapillaris vascular regions. Microvasculature was quantified using vessel density (VD), skeletonized vessel density (SVD), fractal dimension (FD), and vessel diameter index (VDI).
Results
Twenty-one HCs and 26 SZ subjects were included. Compared to HCs, SZ patients demonstrated higher overall OD superficial SVD, OD choriocapillaris VD, and OD choriocapillaris SVD, which were primarily observed in the central, central and outer superior, and central and outer inferior/superior, respectively. Early-course SZ subjects had significantly higher OD superficial VD, OD choriocapillaris SVD, and OD choriocapillaris FD compared to matched HCs. Higher bilateral (OU) superficial VD correlated with lower Positive and Negative Syndrome Scale (PANSS) positive scores, and higher OU deep VDI was associated with higher PANSS negative scores.
Conclusions and Relevance
These results suggest the presence of microvascular dysfunction associated with early-stage SZ. Clinical associations with microvascular alterations further implicate this hypothesis, with higher measures being associated with worse symptom severity and functioning in early stages and with lower symptom severity and better functioning in later stages.
Keywords: swept-source optical coherence tomography angiography, schizophrenia, microvascular, cognition, early-course schizophrenia
Introduction
Schizophrenia (SZ) is characterized by psychotic symptoms as well as cognitive, functional, and social deficits.1 While the cause of SZ is unknown, neuroimaging studies have identified cerebral gray matter loss and choroid plexus enlargement, and there is also evidence of higher peripheral inflammation.2–7 Additionally, cerebral blood flow deficits are found in early-course and chronic SZ.8–12 These neuroimaging findings may reflect inflammatory-mediated microvascular changes in SZ.13 Since the retina and brain develop from the anterior neural tube, they are homologous in structure and function,14 and retinal microvasculature can act as a “window into the brain”.15,16
Some studies in SZ have analyzed retinal microvasculature alterations using fundus imaging. Meier et al17 showed that SZ patients had wider retinal venules compared to controls and were associated with adult and childhood symptoms of psychosis.17 This study was replicated in a twin sample of youth, where wider retinal venules were predictive of one or more symptom(s) of psychosis as compared to controls or unaffected co-twins, with unaffected twins displaying intermediate width.18 Similarly, Appaji et al19,20 reported wider retinal venules and fractal dimension (FD) in SZ patients compared to controls. Also, greater venular caliber is associated with worse reaction time on a working memory task.19
These findings motivated examining microvascular pathology in early-course and chronic SZ, but studies have been limited by the poor resolution of brain and fundus imaging. Optical coherence tomography angiography (OCT-A) can provide noninvasive, rapid, and high-resolution imaging of the retina, the anterior most extension of the central nervous system, and, therefore, the brain.14 There is very limited literature on retinal microvasculature of SZ using OCT-A. Furthermore, most psychiatric clinics do not have access to cutting edge, research-based swept-source OCT-A technology. Thus, we aim to explore microvascular differences in the retina and choroid of SZ patients using swept-source OCT-A and to understand their associations with symptom severity, cognition, and functioning. In addition, we aim to explore differences between early-course and chronic patients to elucidate stage-specific microvascular changes in SZ.
Methods
Participants
This cross-sectional study was conducted and approved by the institutional review board at the Beth Israel Deaconess Medical Center and Massachusetts Eye and Ear. All participants were proficient in English and able to give written informed consent. The SZ group consisted of individuals with a diagnosis of SZ or schizoaffective disorder based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV21 (DSM-IV TR). A total of 52 participants, 22 healthy controls (HCs) and 30 SZ patients (21 with SZ and 9 with schizoaffective disorder), were recruited. Exclusion criteria included a history of (1) substance dependence/abuse within the past 6 months, (2) glaucoma, macular degeneration, retinal occlusions, ocular trauma, or myopia >4.0 diopters, (3) current pregnancy/breast feeding, (4) head injury with neurological sequelae, (5) intellectual disability, or a (6) history of neurologic disorders. HCs were excluded for a personal history of psychosis or major mood disorder, and a family history of psychosis. Height and weight were used to calculate body mass index (BMI). Cardiometabolic status was based on the presence of coronary artery disease, hypertension, diabetes, hyperlipidemia, obesity, and other related disorder. Thirty-day smoking was measured via the Fägerstrom Test for Cigarette Dependence.22 In SZ patients, illness duration, first- and/or second-generation antipsychotic use, and chlorpromazine (CPZ) equivalents were collected. Symptoms of psychosis and mania were evaluated using the Positive and Negative Syndrome Scale (PANSS) and Young Mania Rating Scale (YMRS), respectively.23,24 Cognition, global functioning, and social functioning were assessed with the Brief Assessment of Cognition in Schizophrenia (BACS), Global Assessment of Functioning (GAF), and Birchwood Social Functioning Scale (SFS), respectively.21,25,26
Best corrected visual acuity (BCVA) was calculated using the Snellen eye chart metric notation. Pupil dilation was performed when retinal image capturing was challenging. Retinal images were taken on Topcon DRI-OCT Triton Swept-Source OCT (Topcon, Japan) which has 100,000 A-scans/s, 4 repeated B-scans, and real-time eye tracking, and uses an innovative OCT-A Ratio Analysis algorithm for image segmentation.27 Two trained raters (D.B. and I.A.) assessed retinal layer segmentation accuracy from the automated tool according to the International Nomenclature for Optical Coherence Tomography Panel28 and manually modified improper segmentation. Fovea centered 6- × 6-mm2 images were extracted from ImageNET 6. These images were rated for image and motion artifacts: 0 (unusable), 1 (fair), and 2 (excellent) for each layer and eye.
OCT-A Image Processing
Angiography and enface images were quality controlled to exclude images with significant artifacts or severe motion, leaving a total of 47 (26 SZ patients and 21 HCs) participants. A custom semi-automated algorithm was developed using MATLAB 2018b to process raw angiography images and to extract measures: vessel density (VD), skeletonized vessel density (SVD), vessel diameter index (VDI), and FD. Current vessel processing algorithms, while robust, lack intensity normalization, a feature that was built into our semi-automated algorithm using bias field correction. Larger superficial vessels that projected into the deep and choriocapillaris images were removed. Foveal avascular zone (FAZ) was manually traced for each OCT-A image using ImageJ and was masked out. The Early Treatment Diabetic Retinopathy Study (ETDRS) was used to extract regional data.
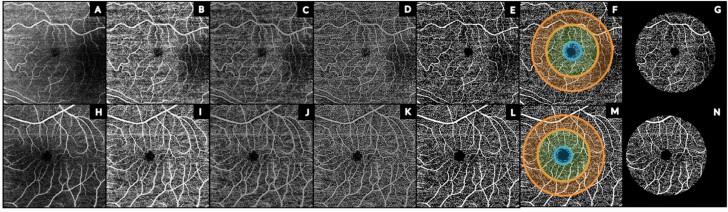
The semi-automated algorithm is illustrated in figure 1 for a HC and SZ eye. The raw OCT-A image (figure 1A and H) was converted to an 8-bit image. An exponential contrast-limited adaptive histogram equalization filter was used to enhance image contrast (figure 1B and I) followed by bias field correction29 to reduce intensity inhomogeneity (figure 1C and J). Top and bottom hat filtering (figure 1D and K) was performed for vessel enhancement using a disk window with a diameter of 2 pixels and the FAZ was masked (figure 1E and L). Larger penetrating vessels were masked from deep and choriocapillaris images. The image was binarized (figure 1E and L) and superimposed with a FAZ-centered ETDRS grid for central (d = 1 mm), inner ring (d = 3 mm), and outer ring (d = 4.7 mm) regions (figure 1F and K). Due to variation in foveal centering of the 6- × 6-mm scan, an outer ring diameter of 4.7 mm was used to ensure full image data capture for the outer ring. Retinal vascular measures were extracted from the ETDRS-masked image (figure 1G and N).
Fig. 1.
Processing pipeline of representative OS superficial complex OCT-A images from a proband (A–G) and healthy control (H–N). (A, H) Raw OCT-A image; (B,I) contrast-limited adaptive histogram equalization (CLAHE) filtered image; (C, J) bias field corrected image to reduce intensity inhomogeneity; (D, K) top and bottom hat filtered image for vessel enhancement; (E, L) binarized image; (F, M) FAZ-centered ETDRS grid used for masking OCT-A image for blue central (d = 1 mm), green parafoveal (d = 3 mm), and orange perifoveal (d = 4.7 mm) regions; (G, N) ETDRS-masked binarized image. OS = oculus sinister; OCT-A = optical coherence tomography angiography; FAZ = foveal avascular zone; ETDRS = Early Treatment Diabetic Retinopathy Study. For color, please see the figure online.
Microvascular measure extraction was based upon the methodology used by Kim et al30 on OCT-A images in diabetic retinopathy. VD was calculated as the ratio between the area encompassed by the vessels (number of white pixels) to the total area of the image (total number of pixels). Skeletonized images were generated by iteratively removing pixel until 1-pixel thickness was achieved along the length of the vessel. SVD was calculated as the total amount of white pixels divided by the total area of the image. VDI was calculated as VD divided by SVD. The box-counting method was performed on the skeletonized image to calculate FD.
Statistical Approach
All statistical analyses were performed using R statistical software (version 3.5.1). Group differences in sociodemographic variables were examined using analysis of variance (ANOVA) and chi-squared tests. ANOVA was used to examine the effects of various moderators (age, sex, race, BMI, systolic/diastolic blood pressure, BCVA, cardiometabolic status, smoking status, illness duration, antipsychotic status, and CPZ equivalents) on average retinal vascular measures. Significant predictors (P < .05) from these ANOVA examinations were used as covariates. Retinal vascular measures were pre-adjusted using the intercept adjustment method for age, sex, race, BCVA, BMI, OCT-A scanner (scanner software upgrade during the study), and image QC (supplementary table 1). Because some retinal vascular measures were not normally distributed, a nonparametric, univariate Kruskal-Wallis test was used to study group differences while using covariate adjusted measures. Cohen’s d effect sizes were calculated using pre-adjusted retinal measures. Clinical associations to vascular measures were analyzed via partial Spearman correlations using age, sex, and race as covariates for clinical data. The significance level was set to .05 for a 2-sided alternative hypothesis test. Multiple comparison testing was performed using the false discovery rate (FDR) method31 with significance set to .05. Sector group differences were corrected by eye (n = 2), layer (n = 3), and vascular measure (n = 4) for a total of 9 measures. Correlations were performed by vascular measures (n = 4) and clinical data (n = 9). Post-hoc analyses were performed between early-course (n = 8; illness durations <5 y) and chronic (n = 15; >5 y) SZ group and a median split for age was performed in the HC group to match the sample. Intraclass correlation coefficient (ICC) was used to measure inter-eye reliability for overall retinal vascular measures. Relative ICC was rated as poor (<0.50), fair (0.50 < ICC < 0.75), and excellent (>0.75) (supplementary table 2).
Results
Demographics
SZ and HC groups were matched for age, sex, race, BCVA, systolic/diastolic blood pressure, and cardiometabolic disease status (table 1). SZ patients had higher BMI (F = 4.76, P = .034) and smoking status (χ 2 = 8.5, P = .006), as well as lower SFS (F = 22.0, P < .001), GAF (F = 82, P < .001), and BACS (F = 5.59, P = .023) compared to HCs.
Table 1.
Clinical and Demographic Information Comparing SZ to HCs
| Variable | HC (n = 21) | SZ (n = 26, SZ = 18, SZA = 8) | Test Stat (χ2, F) | P |
|---|---|---|---|---|
| Age (years) | 38.0 (11.6) | 37.0 (12.7) | 0.07 | .79 |
| Sex (female/male) | 7/14 | 6/20 | 0.21 | .65 |
| Race (AA/CA/OT) | 2/15/04 | 9/13/04 | 4.11 | .13 |
| Visual acuity | 0.92 (0.24) | 0.83 (0.28) | 1.54 | .22 |
| BMI | 26.3 (4.0) | 30.5 (8.0) | 4.76 | .034* |
| Systolic blood pressure | 122.2 (10.0) | 124.0 (14.5) | 0.20 | .65 |
| Diastolic blood pressure | 77.4 (10.4) | 76.4 (13.4) | 0.08 | .78 |
| Cardiometabolic disorder (yes/no) | 6/14 | 7/19 | 0.05 | .82 |
| Smoking status (yes/no) | 0/20 | 8/18 | 8.5 | .006** |
| Duration of illness (months) | — | 163.6 (153.1) | — | — |
| Antipsychotic status (yes/no) | — | 22/3 | — | — |
| CPZ equivalents | — | 338.8 (256.3) | — | — |
| Social Functioning Scale | 153.3 (17.0) | 127.5 (19.6) | 22.0 | <.001*** |
| Global Assessment of Functioning | 81.9 (9.4) | 46.2 (15.5) | 82.2 | <.001*** |
| BACS composite score | −0.16 (0.73) | −0.69 (0.77) | 5.19 | .028* |
| PANSS total score | — | 55.5 (14.6) | — | — |
| PANSS positive symptoms score | — | 13.2 (4.7) | — | — |
| PANSS negative symptoms score | — | 13.7 (4.8) | — | — |
| Young Mania Rating Scale | — | 7.0 (6.5) | — | — |
Note: HC = healthy control; SZ = schizophrenia; SZA = schizoaffective disorder; AA = African American; CA = Caucasian; OT = other; BMI = body mass index; CPZ = chlorpromazine; BACS = Brief Assessment of Cognition in Schizophrenia; PANSS = Positive and Negative Syndrome Scale . Continuous data are given as mean (SD). Cardiometabolic disease variable accounts for whether person had either a cardiovascular (heart or vessels) or metabolic disorder. Smoking variable accounts for whether a person had smoked within the last 30 d.
Significant test statistics are in bold and denoted as *P < .05, **P < .01, and ***P < .005.
Microvascular Group Differences
The SZ group demonstrated higher right eye (oculus dextrus [OD]) superficial SVD (d = 0.65, P = .044), choriocapillaris VD (d = 0.65, P = .044), and choriocapillaris SVD (d = 0.79, P = .017) compared to HCs. No group differences were observed in bilateral (OU) or left eye (oculus sinister [OS]) measures (table 2).
Table 2.
Nonparametric Analysis of Retinal Vascular Measures Comparing SZ to HCs
| Layer | OU | OD | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | SZ | Cohen’s d | P | HC | SZ | Cohen’s d | P | HC | SZ | Cohen’s d | P | ||
| Superficial | VD | 0.33 (0.016) | 0.333 (0.018) | 0.19 | .81 | 0.315 (0.02) | 0.325 (0.019) | 0.52 | .14 | 0.352 (0.018) | 0.349 (0.022) | −0.12 | .72 |
| SVD | 0.088 (0.004) | 0.09 (0.005) | 0.38 | .36 | 0.085 (0.005) | 0.088 (0.005) | 0.65 | .044* | 0.094 (0.005) | 0.094 (0.006) | 0.038 | .84 | |
| VDI | 3.717 (0.093) | 3.687 (0.13) | −0.27 | .32 | 3.673 (0.10) | 3.652 (0.15) | −0.16 | .41 | 3.75 (0.10) | 3.717 (0.14) | −0.28 | .44 | |
| FD | 1.65 (0.007) | 1.653 (0.008) | 0.36 | .29 | 1.648 (0.009) | 1.653 (0.008) | 0.51 | .08~ | 1.657 (0.009) | 1.658 (0.01) | 0.10 | .57 | |
| Deep | VD | 0.268 (0.023) | 0.265 (0.026) | −0.14 | .35 | 0.233 (0.021) | 0.234 (0.037) | 0.036 | .91 | 0.308 (0.027) | 0.299 (0.028) | −0.32 | .18 |
| SVD | 0.09 (0.006) | 0.088 (0.007) | −0.16 | .30 | 0.08 (0.009) | 0.08 (0.01) | 0.008 | 1 | 0.101 (0.008) | 0.098 (0.008) | −0.34 | .14 | |
| VDI | 2.981 (0.062) | 2.976 (0.079) | −0.063 | .75 | 2.895 (0.079) | 2.90 (0.10) | 0.062 | .89 | 3.067 (0.078) | 3.055 (0.077) | −0.15 | .60 | |
| FD | 1.653 (0.012) | 1.652 (0.012) | −0.15 | .47 | 1.639 (0.017) | 1.638 (0.018) | −0.045 | .77 | 1.671 (0.015) | 1.668 (0.014) | −0.25 | .26 | |
| Choriocapillaris | VD | 0.378 (0.008) | 0.38 (0.009) | 0.34 | .29 | 0.362 (0.011) | 0.370 (0.013) | 0.65 | .044* | 0.394 (0.013) | 0.392 (0.012) | −0.18 | .57 |
| SVD | 0.119 (0.002) | 0.12 (0.002) | 0.44 | .18 | 0.115 (0.002) | 0.118 (0.003) | 0.79 | .017* | 0.123 (0.004) | 0.123 (0.003) | −0.12 | .71 | |
| VDI | 3.162 (0.023) | 3.160 (0.022) | −0.075 | .83 | 3.136 (0.037) | 3.141 (0.029) | 0.15 | .49 | 3.20 (0.025) | 3.188 (0.03) | −0.27 | .54 | |
| FD | 1.70 (0.003) | 1.702 (0.004) | 0.39 | .34 | 1.70 (0.003) | 1.701 (0.005) | 0.54 | .14 | 1.703 (0.005) | 1.704 (0.006) | 0.11 | .97 | |
Note: SZ = schizophrenia/schizoaffective; HC = healthy control; OU = oculus uterque; OD = oculus dexter; OS = oculus sinister; VD = vessel density; SVD = skeletonized vessel density; VDI = vessel diameter index; FD = fractal dimension. For both diagnostic groups, vascular measures are given as mean (SD). Retinal vascular measures are adjusted for age, sex, race, visual acuity, OCT-A scanner, image quality, and body mass index.
Trending test statistics are denoted as ~P < .1. Significant test statistics are in bold and denoted as *P < .05.
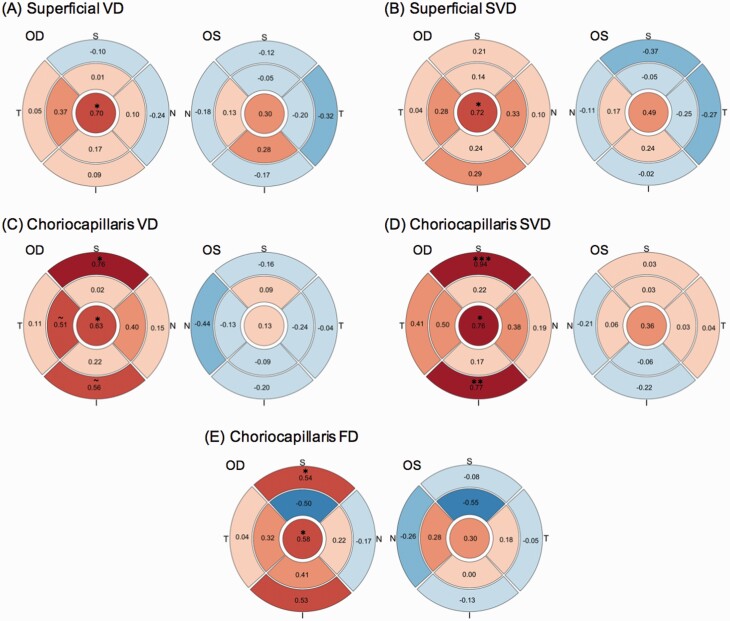
ETDRS sector measures were used to examine regional vascular differences (supplementary tables 3–5). Greater OU superficial central (d = 0.69, P = .02) and choriocapillaris central SVD (d = 0.66, P = .026) were observed in SZ group compared to HCs (figure 2A and B; supplementary table 3). In SZ group, OU FD in the inner superior sector was significantly lower for the superficial (d = −0.60, P = .042), deep (d = −0.78, P = .019), and choriocapillaris (d = −0.80, P = .015) regions compared to HCs (figure 2C–E). For OD, SZ group demonstrated higher superficial central VD (d = 0.69, P = .024) and SVD (d = 0.71, P = .019) compared to HCs (supplementary table 4). The SZ group displayed greater OD choriocapillaris VD in the central (d = 0.64, P = .03) and outer superior (d = 0.77, P = .025) sector. Higher OD choriocapillaris SVD in central (d = 0.76, P = .017), outer superior (d = 0.96, P = .004), and outer inferior (d = 0.75, P = .007) sectors were also observed in SZ group. Similarly, SZ OD choriocapillaris FD measures were greater in the central (d = 0.59, P = .012) and outer superior (d = 0.54, P = .039) sectors. No group differences were found for OS sector measures (supplementary table 5). OD choriocapillaris for the outer superior SVD (PFDR = .032) and outer inferior SVD (PFDR = .032) regions survived FDR correction.
Fig. 2.
Plots illustrating regional group differences between schizophrenia (SZ) and healthy controls (HCs) in OD and OS retinal vascular measures for (A) superficial skeletonized vessel density, (B) choriocapillaris skeletonized vessel density, (C) superficial fractal dimension, (D) deep fractal dimension, and (E) choriocapillaris fractal dimension. Effect sizes are given in each section. Color signifies that, compared to HCs, probands demonstrated higher (red) or lower (blue) measures. Color intensity reflects the strength of the effect size. Test statistics are denoted as: trending, ~P < .1 and significant, *P < .05, **P < .01, and ***P < .005. OD = oculus dextrus; OS = oculus sinister. For color, please see the figure online.
Post-Hoc Analysis
This sample contained 8 early-course and 15 chronic SZ subjects, with 3 participants not having duration of illness information. Early-course SZ patients were younger (F = 8.48, P = .008) and had lower BMI (F = 4.47, P = .047), systolic blood pressure (F = 7.16, P = .016), and CPZ dosage (F = 5.13, P = .039) compared to chronic SZ patients (supplementary table 6). There were no significant clinical differences between HCs and early-course or chronic SZ patients (Supplementary tables 7 and 8). Compared to age-matched HCs, early-course SZ group demonstrated higher OU choriocapillaris SVD (d = 1.20, P = .026), driven by right eye measures (d = 1.15, P = .033), as well as increased choriocapillaris FD (d = 1.17, P = .033) and OD superficial VD (d = 1.23, P = .026) (supplementary table 9, supplementary figures 1 and 2). No differences were observed between HCs and chronic SZ or between early-course and chronic SZ patients (supplementary tables 10 and 11).
Clinical Correlations
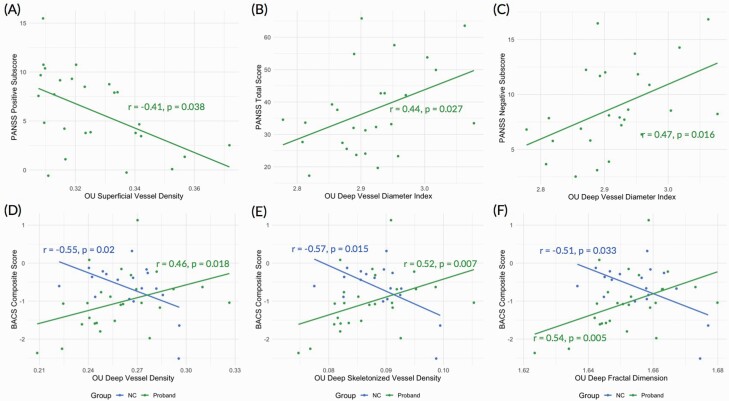
Clinical correlations are shown in supplementary table 12. Higher PANSS positive scores were correlated with lower superficial VD (d = −0.41, P = .038; figure 3A) and this effect was most prominent for suspiciousness (r = −.41, P = .039; supplementary table 15A). Higher PANSS total scores were associated with higher deep VDI (d = 0.44, P = .027; figure 3B). Higher PANSS negative scores were associated with greater deep VDI (d = 0.47, P = .016; figure 3C), primarily in the passive/apathetic social withdrawal (r = .36, P = .07) and difficulty in abstract thinking (r = .37, P = .06) domains (supplementary table 15B).
Fig. 3.
Scatter plots illustrating Spearman’s correlations between (A) OU superficial vessel density and PANSS positive subscore, (B) OU deep vessel diameter index and PANSS total score, (C) OU deep vessel diameter index and PANSS negative subscore, (D) OU deep vessel density and BACS composite score, (E) OU deep skeletonized vessel density and BACS composite score, and (F) OU deep fractal dimension and BACS composite score. Retinal vascular measures were adjusted for age, sex, race, visual acuity, body mass index, OCT-A scanner, and image QC. Clinical measures were adjusted for age, sex, and race, but BACS composite scores were adjusted for only race. OU = oculus uterque; PANSS = Positive and Negative Syndrome Scale; BACS = Brief Assessment of Cognition in Schizophrenia; OCT-A = optical coherence tomography angiography.
In the overall sample, no relationships were observed between retinal measures and SFS, GAF, or BACS. Within SZ, lower GAF scores were associated with lower superficial VD (d = 0.41, P = .041; supplementary table 13). In HCs, a better BACS score was associated with lower deep VD (d = −0.55, P = .02), deep SVD (d = −0.57, P = .015), and deep FD (d = −0.51, P = .033 (supplementary table 14). Conversely in SZ, a lower BACS score was associated with smaller deep VD (d = 0.46, P = .018), deep SVD (d = 0.52, P = .007), and deep FD (d = 0.54, P = .005). Correlations between BACS and deep SVD in HCs (PFDR = .035) and in SZ group (PFDR = .021) and between BACS and deep FD in SZ group (PFDR = .015) survived FDR correction. For correlations between BACS subscores and retinal measures in SZ and HCs, see supplementary table 15C and 15D, respectively.
Discussion
In this study, we found greater retinal microvascular measures principally localized to the right eye in individuals with SZ. Early-course SZ subjects demonstrated the greatest alterations compared to chronic SZ subjects. Relationships between retinal vascular measures with symptoms and cognition were also identified. The findings in this study add to the growing interest detecting pathophysiologic mechanisms underlying neuropsychiatric disorders via the retina.
To our knowledge, this is the first SZ study to comprehensively analyze macular retinal microvascular abnormalities using OCT-A. We identified greater VD and SVD in SZ subjects compared to HCs, and sector analyses demonstrated regional microvascular changes. Our results, largely driven by right eye measures, may reflect abnormal left hemispheric function and connectivity that has been previously reported in SZ patients.32 Min and Oh33 reported left hemisphere (right visual field) impairment during a visual word recognition task in SZ subjects. Additionally, reductions in leftward asymmetry of the integrity of the uncinate fasciculus34,35 and the inferior occipito-frontal fasciculus,35 connecting occipital and posterior temporal regions to the prefrontal cortex and involving attention, reading, and visual processing, have been reported in SZ patients. Studies correlating retinal microvascular alterations with brain structural and connectivity laterality indices are needed to further elucidate this potential connection.
We suspect that the observed increases in OD superficial and choriocapillaris VD and SVD reflect a pathologic angiogenesis pathway, potentially in response to a hypoxic environment,36 blood brain/retinal barrier disruption,37 and/or inflammation mediated microvascular disruption13 that results in the formation of longer and denser vessels. Reduced peripapillary and superficial macular vascular network density have been reported in SZ patients with longer durations of illness by Budakoglu et al38 and Silverstein et al,39 respectively, which differs from our findings of increased VD in a primarily early-course SZ group. These differences could arise from size variation in analyzed images, where Silverstein et al.39 utilized 3 x 3 mm2 OCTA scans vs our 4.7 x 4.7 mm2 scans, as well as from methodological differences when calculating vascular measures. Furthermore, their sample excluded individuals with systemic diseases such as diabetes or hypertension, whereas we did not since there was no moderator effect of systemic diseases on vascular measures (supplementary table 1). Research in Alzheimer’s disease (AD), a neuropsychiatric disorder that displays similar retinal structural deficits40 to SZ, has reported VD reductions in both the superficial and deep plexus,40,41 which differs from our findings of increased VD. Studies in mild cognitive impairment (MCI), a precursor to AD in some individuals, have observed smaller decreases in superficial and deep VD compared to HCs.42–44 Interestingly, van de Kreeke et al45 reported greater VD in the macular region as well as surrounding the optic nerve head in those with preclinical AD (cognitively healthy and positive for cerebral amyloid-beta). They hypothesized that the increased VD values were reflective of an inflammatory retinal state, mirroring the inflammatory nature of AD onset and amyloid-beta accumulation.45,46 With disease progression, continued inflammation results in a neurodegenerative process as evidenced by microvascular loss resulting in a decrease in VD measures. The pathophysiological changes observed in SZ may be similar to those seen in AD, but further research is needed to better understand this process.
We found that early-course SZ had the most prominent changes in overall OD retinal measures, displaying higher OD superficial and choriocapillaris VD and FD as well as higher OD choriocapillaris SVD compared to HCs. Silverstein et al39 observed no significant difference in superficial perfusion and vascular densities between individuals at early (defined as less than 2 y from their first single hospitalization) and established illness stages (greater than 2 y since onset with multiple hospitalizations) of SZ. Another study reported similar FD increases in psychosis albeit utilizing fundus imaging20 and in a sample with an average illness duration of 7 years. A previous report by our group analyzing peripheral angiogenic and inflammatory markers found greater levels of both vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase (sFlt-1), an anti-angiogenic factor that sequesters soluble VEGF,47 in individuals with familial high risk for psychosis. Furthermore, sFlt-1 and VEGF levels were positively correlated, and we posited that there was an “angiogenic cascade” within the familial high-risk group that persists in those with first-episode SZ.48,49 VEGF has also been noted to be higher in multi-episode SZ47 and was found to be increased in an inflammatory subtype of psychosis.37 Our group found that individuals with psychosis plus a higher inflammatory signature demonstrated cortical thickening and greater subcortical volumes compared to psychosis individuals with lower inflammatory marker levels.13 Additionally, some retinal structural studies in SZ have reported no group differences in retinal thickness between SZ and HCs. Within our sample, we previously reported no differences in retinal nerve fiber layer (RNFL) thicknesses between SZ and HCs, but did identify a thicker outer plexiform layer and a thinner outer nuclear layer.50 When correlating OD retinal structural and microvascular measures, we found a nonsignificant positive correlation between superficial VD and RNFL thickness in early-course SZ patients (r = .54, P = .26), while higher superficial VD correlated with lower RNFL thickness in chronic SZ patients (r = −.49, P = .13). Taken together, inflammation may either reflect pathological thickening in the retina/brain or be a proxy for microvascular dysfunction in SZ.
Another important observation from this study is the regional characteristic of microvascular changes. Group differences in retinal thickness measures, between individuals with SZ and HCs, have been observed to have regional variations in both peripapillary51 and macular52 measures. Therefore, we were interested to see whether the microvascular alterations we observed in SZ patients were region-specific. Increases in superficial VD and SVD were primarily impacted by the central region (FAZ removed). However, FAZ area was not significantly different between SZ group and HCs, with SZ group having smaller FAZ areas (OD: d = −0.43, P = .11; OS: d = −0.15, P = .76). These findings suggest that our microvascular measures are more sensitive to changes than FAZ area alone. Interestingly, Budakoglu et al38 found overall reductions in radial peripapillary VD but only temporal VD measures were significantly different between SZ group and HCs. This may suggest different pathologic processes occurring at the optic nerve head, where reductions in VD were observed, and at the macula, where we observed increased superficial VD. OD choriocapillaris VD, SVD, and FD were similarly higher in the central region, but also demonstrated increases in the outer superior (VD, SVD, and FD) and outer inferior (SVD) sectors. Choriocapillaris vascular abnormalities may be more widespread, occurring both centrally and perifoveally, compared to the superficial plexus. The greater implication of central vascular measures in SZ suggests that this area may be more sensitive for detecting “angiogenic cascade” activation as a result of inflammation of blood brain/retinal barrier-related deficits. OU FD was reduced across all 3 retinal plexi for the inner superior region, even though no differences in overall OU FD were detected between diagnostic groups. This is in contrast to the increases in FD reported in individuals with SZ and BD,20 although these differences may be due to the use of fundus vs OCT-A for measure generation.
With regard to clinical correlations, we found that higher positive symptoms correlated with lower superficial VD, which was found primarily in the chronic SZ group (r = −.54, P = .039), while early-course SZ group demonstrated a nonsignificant positive correlation (r = .58, P = .13). In chronic SZ group, greater superficial VD was associated with better GAF (r = .63, P = .015), while early-course SZ group showed an inverse relationship (r = −0.67, P = .067). Meier et al17 has reported links between greater psychosis symptoms and wider superficial venular diameter, but this observation was not replicated in the current study. Instead, greater deep VDI was associated with worse negative psychosis symptoms. Lastly cognitive relationships with retinal vascular measures were orthogonal between SZ group and HCs. Deep VD, SVD, and FD were positively correlated with BACS composite scores in SZ, but HCs had inverse associations. Literature in AD have reported similar positive correlations between superficial VD and cognitive measures in individuals with MCI and AD.53,54 These results suggest that retinal microvascular measures may potentially be a biomarker for symptom severity, functioning, and cognition in SZ patients and may be stage-specific.
This study has several strengths as well as limitations. Investigating retinal microvascular changes in SZ using OCT-A and their correlations with clinical and cognitive data is novel. We developed a robust semi-automated processing pipeline to analyze a set radius around the FAZ to reduce artifacts and extracted sector measures to analyze regional changes. This study was limited by relatively small sample size, heterogeneous SZ sample, and lack of intraocular pressure data. Despite this, retinal microvasculature may serve as a biomarker for symptom severity, functioning, and cognition in SZ. With current literature being small and demonstrating varying results, additional studies with larger samples are necessary to elucidate the disease mechanisms at play. In addition, future work should analyze stage-specific alterations in SZ retinal microvasculature to uncover how retinal pathologies change with disease progression.
Supplementary Material
Acknowledgments
We would like to acknowledge the patients who agreed to participate in this study. Also, we would like to acknowledge Megan Kasetty, Konstantinos Douglas, and Vivian Douglas from Massachusetts Eye and Ear for organizing and editing some of the retinal OCT-A images. We would like to thank the B-SNIP2 principal investigators Carol Tamminga, Brett Clementz, Elliot Gershon, and Godfrey Pearlson for their input and support of this study. All authors report no biomedical financial interests or potential conflicts of interest.
Funding
This work was supported in part by the Harvard Medical School Dupont Warren Fellowship and Livingston Grant; Sydney R. Baer Jr Foundation; National Institute of Health Harvard Catalyst (grant number 1KL2TR002542 to P.L.); and National Institute of Mental Health (grant number R01 78113 to M.K.).
References
- 1. Haller CS, Padmanabhan JL, Lizano P, Torous J, Keshavan M. Recent advances in understanding schizophrenia. F1000Prime Rep. 2014;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeLisi LE, Szulc KU, Bertisch HC, Majcher M, Brown K. Understanding structural brain changes in schizophrenia. Dialogues Clin Neurosci. 2006;8(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly S, Guimond S, Pasternak O, et al. White matter microstructure across brain-based biotypes for psychosis—findings from the Bipolar-Schizophrenia Network for Intermediate Phenotypes. Psychiatry Res Neuroimaging. 2021;308:111234. [DOI] [PubMed] [Google Scholar]
- 4. Keshavan MS, Collin G, Guimond S, Kelly S, Prasad KM, Lizano P. Neuroimaging in schizophrenia. Neuroimaging Clin N Am. 2020;30(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen P, Chaddock CA, Egerton A, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173(4):392–399. [DOI] [PubMed] [Google Scholar]
- 9. Guimarães TM, Machado-de-Sousa JP, Crippa JAS, Guimarães MRC, Hallak JEC. Arterial spin labeling in patients with schizophrenia: a systematic review. Arch Clin Psychiatry São Paulo. 2016;43(6):151–156. [Google Scholar]
- 10. Kindler J, Schultze-Lutter F, Hauf M, et al. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr Bull. 2018;44(1):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng V, Lizano P, Bolo NR, et al. Altered cerebral perfusion in bipolar disorder: a pCASL MRI study. Bipolar Disord. 2021;23:130–140. [DOI] [PubMed] [Google Scholar]
- 12. Cui LB, Wang LX, Tian P, et al. Aberrant perfusion and its connectivity within default mode network of first-episode drug-naïve schizophrenia patients and their unaffected first-degree relatives. Sci Rep. 2017;7(1):16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lizano P, Lutz O, Xu Y, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry. 2020. doi: 10.1038/s41380-020-00914-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. London A, Benhar I, Schwartz M. The retina as a window to the brain—from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. [DOI] [PubMed] [Google Scholar]
- 15. Chu EM, Kolappan M, Barnes TR, Joyce EM, Ron MA. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res Neuroimaging. 2012;203(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meier MH, Shalev I, Moffitt TE, et al. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry. 2013;170(12):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meier MH, Gillespie NA, Hansell NK, et al. Retinal microvessels reflect familial vulnerability to psychotic symptoms: a comparison of twins discordant for psychotic symptoms and controls. Schizophr Res. 2015;164(1–3):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Appaji A, Nagendra B, Chako DM, et al. Relation between retinal vascular abnormalities and working memory impairment in patients with schizophrenia and bipolar disorder. Asian J Psychiatr. 2020;49:101942. [DOI] [PubMed] [Google Scholar]
- 20. Appaji A, Nagendra B, Chako DM, et al. Retinal vascular fractal dimension in bipolar disorder and schizophrenia. J Affect Disord. 2019;259:98–103. [DOI] [PubMed] [Google Scholar]
- 21. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 22. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 23. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 25. Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. [DOI] [PubMed] [Google Scholar]
- 26. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. [DOI] [PubMed] [Google Scholar]
- 27. Stanga PE, Tsamis E, Papayannis A, Stringa F, Cole T, Jalil A. Swept-source optical coherence tomography Angio™ (Topcon Corp, Japan): technology review. Dev Ophthalmol. 2016;56:13–17. [DOI] [PubMed] [Google Scholar]
- 28. Staurenghi G, Sadda S, Chakravarthy U, Spaide RF; International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel . Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121(8):1572–1578. [DOI] [PubMed] [Google Scholar]
- 29. Li C, Gore JC, Davatzikos C. Multiplicative intrinsic component optimization (MICO) for MRI bias field estimation and tissue segmentation. Magn Reson Imaging. 2014;32(7):913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362–OCT370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 32. Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 2014;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Min SK, Oh BH. Hemispheric asymmetry in visual recognition of words and motor response in schizophrenic and depressive patients. Biol Psychiatry. 1992;31(3):255–262. [DOI] [PubMed] [Google Scholar]
- 34. Kubicki M, Westin CF, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyata J, Sasamoto A, Koelkebeck K, et al. Abnormal asymmetry of white matter integrity in schizophrenia revealed by voxelwise diffusion tensor imaging. Hum Brain Mapp. 2012;33(7):1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moises HW, Wollschläger D, Binder H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl Psychiatry. 2015;5:e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pong S, Karmacharya R, Sofman M, Bishop JR, Lizano P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1–2):30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Budakoglu O, Ozdemir K, Safak Y, Sen E, Taskale B. Retinal nerve fibre layer and peripapillary vascular density by optical coherence tomography angiography in schizophrenia [published online ahead of print February 25, 2021]. Clin Exp Optom. doi: 10.1080/08164622.2021.1878816 [DOI] [PubMed] [Google Scholar]
- 39. Silverstein SM, Lai A, Green KM, Crosta C, Fradkin SI, Ramchandran RS. Retinal microvasculature in schizophrenia. Eye Brain. 2021;13:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song A, Johnson N, Ayala A, Thompson AC. Optical coherence tomography in patients with Alzheimer’s disease: what can it tell us? Eye Brain. 2021;13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pellegrini M, Vagge A, Ferro Desideri L, et al. Optical coherence tomography angiography in neurodegenerative disorders. J Clin Med. 2020;9(6):1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chua J, Hu Q, Ke M, et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimers Res Ther. 2020;12(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang H, Wei Y, Shi Y, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38(3):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020;98(6):e781–e787. [DOI] [PubMed] [Google Scholar]
- 45. van de Kreeke JA, Nguyen HT, Konijnenberg E, et al. Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol. 2020;104(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement Transl Res Clin Interv. 2018;4:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Misiak B, Stramecki F, Stańczykiewicz B, Frydecka D, Lubeiro A. Vascular endothelial growth factor in patients with schizophrenia: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:24–29. [DOI] [PubMed] [Google Scholar]
- 48. Lizano PL, Yao JK, Tandon N, Mothi SS, Montrose DM, Keshavan MS. Association of sFlt-1 and worsening psychopathology in relatives at high risk for psychosis: a longitudinal study. Schizophr Res. 2017;183:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lizano PL, Keshavan MS, Tandon N, et al. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: a preliminary study. Schizophr Res. 2016;170(1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bannai D, Lizano P, Kasetty M, et al. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: a preliminary study. Psychiatry Res Neuroimaging. 2020;299:111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lizano P, Bannai D, Lutz O, Kim LA, Miller J, Keshavan M. A meta-analysis of retinal cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull. 2020;46(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Samani NN, Proudlock FA, Siram V, et al. Retinal layer abnormalities as biomarkers of schizophrenia. Schizophr Bull. 2018;44(4):876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s disease on optical coherence tomography angiography. PLoS One. 2019;14(4):e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bulut M, Kurtuluş F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102(2):233–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.