Keywords: beamforming, cognitive impairment, magnetoencephalography, Parkinson’s, resting-state
Abstract
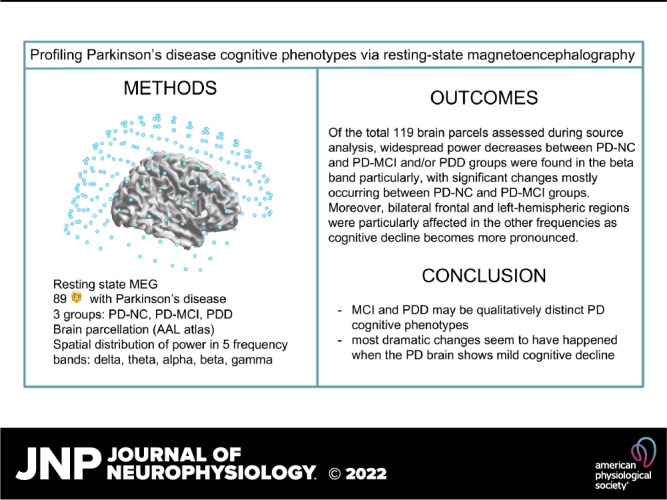
Aberrant brain oscillations are a hallmark of Parkinson’s disease (PD) pathophysiology and may be related to both motor and nonmotor symptoms. Mild cognitive impairment (MCI) affects many people with PD even at the time of diagnosis and conversion risks to PD dementia (PDD) are very high. Unfortunately, pharmacotherapies are not addressing cognitive symptoms in PD. Profiling PD cognitive phenotypes (e.g., MCI, PDD, etc.) may therefore help inform future treatments. Neurophysiological methods, such as magnetoencephalography (MEG), offer the advantage of observing oscillatory patterns, whose regional and temporal profiles may elucidate how cognitive changes relate to neural mechanisms. We conducted a resting-state MEG cross-sectional study of 89 persons with PD stratified into three phenotypic groups: normal cognition, MCI, and PDD, to identify brain regions and frequencies most associated with each cognitive profile. In addition, a neuropsychological battery was administered to assess each domain of cognition. Our data showed higher power in lower frequency bands (delta and theta) observed along with more severe cognitive impairment and associated with memory, language, attention, and global cognition. Of the total 119 brain parcels assessed during source analysis, widespread group differences were found in the beta band, with significant changes mostly occurring between the normal cognition and MCI groups. Moreover, bilateral frontal and left-hemispheric regions were particularly affected in the other frequencies as cognitive decline becomes more pronounced. Our results suggest that MCI and PDD may be qualitatively distinct cognitive phenotypes, and most dramatic changes seem to have happened when the PD brain shows mild cognitive decline.
NEW & NOTEWORTHY Can we better stage cognitive decline in patients with Parkinson’s disease (PD)? Here, we provide evidence that mild cognitive impairment, rather than being simply a milder form of dementia, may be a qualitatively distinct phase in its development. We suggest that the most dramatic neurophysiological changes may occur during the time the PD brain transitions from normal cognition to MCI, then compensatory changes further occur as the brain “switches” to a dementia state.
INTRODUCTION
Among a large list of nonmotor symptoms, cognitive dysfunction has a profound impact on Parkinson’s disease (PD) patient suffering and caregiver burden. It affects 20%–40% of patients even at the time of diagnosis (1) and up to 75%–83% of patients with PD eventually develop PD dementia (PDD) (2). PDD manifests as “dysexecutive” difficulties in planning, abstract thinking, memory, and visuospatial ability, with language relatively unaffected (2). It is typically preceded by mild cognitive impairment (MCI), observed in up to 38% of PD diagnoses (3). Because the diagnostic criteria for PD MCI were only adopted less than 10 years ago (4), older studies only distinguished between PDD and nondemented PD or cognitively normal PD patients (PD-NC). Operationalization of the PD MCI criteria and application in observational and interventional studies has increased over the past several years.
At the physiological level, pathological neural oscillations within cortico-basal ganglia-thalamic-cortical loops have been largely studied, and it is now widely agreed that aberrant synchronizations are linked to the motor symptoms of PD (5). As a motivating analogy, pathological beta oscillations in the subthalamic nucleus (6) are correlated with motor symptoms and deep brain stimulation surgery efficacy, indicating an intertwined relationship between neural rhythms, symptoms, and treatments. How impaired cortical and subcortical rhythms are tied to cognitive symptoms and cognitive stages is still unknown. The neuropathophysiology associated with cognitive stages can be studied at the oscillatory level via magnetoencephalography (MEG) (7), a neuroimaging technique that works by directly observing the magnetic fields that instantly accompany electric currents within the brain during neural activity. Uncovering the neurophysiologic basis of PD cognitive phenotypes is of high interest to improve diagnostics, track progression, and develop targeted treatments.
Previous MEG research has suggested that parkinsonian cognitive impairment may be accompanied by “spectral slowing,” an increase in the slower frequencies such as delta and theta power (3). This spectral slowing appears to be correlated with clinical progression of motor symptoms as well as global cognitive decline (8). In the beta-band, a shift in information flow from anterior to posterior is associated with poorer cognitive performance (9), and lower beta power is proposed to be the best predictor for future conversion to PDD in a longitudinal MEG study (10). Although the refinement of diagnostic criteria for staging cognitive decline in PD (4) has led to more rigor for research studies in PD, no MEG study so far has taken the advantage of looking at whole brain-based oscillatory dynamics in distinct cognitive subgroups.
In this study, we performed spectral power analyses on resting-state MEG data recorded on a large (n = 89), cross-sectional cohort of patients with PD, stratified by cognitive diagnosis according to the Movement Disorders Society (MDS) Task Force, e.g., PD-NC, PD MCI, and PDD. We aimed to confirm published findings related to oscillatory slowing, as well as refine these patterns in relationship with different stages of cognitive status in PD.
METHODS
Participants
The present study was designed to evaluate the changes that give rise to dementia/cognitive impairment within the context of parkinsonism. Therefore, our study design did not include a healthy control comparator group. Eighty-nine people with PD were recruited from the University of Colorado Anschutz Medical Campus Movement Disorders clinic and from campus-wide advertisements. All participants signed written informed consent to participate in the study approved by the Colorado Multiple Institution Review Board. Inclusion criteria included a diagnosis of probable PD according to the UK Brain Bank Criteria (11). Participants were excluded if they had features suggestive of other causes of parkinsonism, cerebrovascular disease or history of major head trauma, or if they had a history of deep brain stimulation or ablation surgery. All study visits were performed in the best dopaminergic “ON” state. Table 1 describes participants’ demographics and baseline clinical features.
Table 1.
Participants’ demographics
| Demographics | PD-NC | PD-MCI | PDD | F/t/χ2 Value | P Value |
|---|---|---|---|---|---|
| Sex (No. Male) | 25/37 | 26/35 | 12/17 | 5.115 | 0.059 |
| Age | 69.74 ± 6.95 | 66.71 ± 6.86 | 75.78 ± 8.59 | 8.029 | 0.001 |
| Education, yr | 16.48 ± 4.05 | 15.76 ± 4.21 | 16.81 ± 3.03 | 0.523 | 0.568 |
| Levodopa equivalence | 374.17 ± 385.97 | 586.37 ± 421.17 | 753.61 ± 481.08 | 4.230 | 0.018 |
| UPDRS-III | 22.27 ± 9.03 | 23.75 ± 7.89 | 34.45 ± 6.28 | 8.469 | 0.0009 |
| Hoehn and Yahr | 2.16 ± 0.27 | 2.23 ± 0.56 | 2.75 ± 0.72 | 3.294 | 0.059 |
Values are expressed as means ± SD or fraction of participants. The high levodopa equivalence standard deviation indicates that the participants were spread out over a wide range of dosages. PDD, Parkinson’s disease dementia; PD-MCI, Parkinson’s disease mild cognitive impairment; PD-NC, Parkinson’s disease normal cognition; UPDRS, Unified Parkinson’s Disease Rating Scale. Bold type represents significant between-groups differences.
Cognitive Assessment and Classification
All participants underwent extensive neuropsychological assessments (Table 2), which included the Montreal Cognitive Assessment (MoCA) (12), the Boston Naming Test (BNT) (13), the Dementia Rating Scale-2 (DRS-2) (14), Trail Making Tests (TMT) A and B (15), the Brief Test of Attention (BTA) (16), the California Verbal Learning Test, Second Edition (CVLT-II) (17), the Symbol Digit Modality Test (SDMT) Oral (18), the Delis–Kaplan Executive Function System (DKEFS) Verbal Fluency/Letter Fluency task (19), and the Judgment of Line Orientation task (JLO) (20). This battery was chosen based on previous work operationalizing the PD MCI criteria (21).
Table 2.
Neuropsychological test scores by cognitive class
| Cognitive Domain Test | PD-NC | PD-MCI | PDD | F/t/χ2 Value | P Value |
|---|---|---|---|---|---|
| Global cognition | |||||
| DRS-2 total | 137.32 (5.03), 129–143 | 133.76 (4.97), 124–144 | 115.53 (15.67), 82–143 | 23.346 | 0.000003 |
| MoCA | 27.63 (1.21), 25–30 | 23.12 (4.02), 16–28 | 18.85 (6.22), 9–26 | 14.370 | 0.00004 |
| Global cognition composite score | 0.87 (.23), 0.03 to 1.11 | 0.09 (0.43), −0.04 to 1.03 | −1.07 (1.25), −4.00 to 0.47 | 17.684 | 0.000001 |
| Attention | |||||
| Trails A | 37.97 (16.02), 23.0–76.2 | 49.05 (15.16), 28.68–79.0 | 107.01 (109.72), 29–352 | 6.023 | 0.006 |
| BTA | 14.13 (2.97), 7–19 | 11.59 (4.78), 5–19 | 10.25 (7.13), 2–20 | 2.359 | 0.081 |
| Attention composite score | 0.33 (0.63), −0.72 to 1.09 | 0.28 (0.64), −1.31 to 1.04 | 0.25 (0.81), −1.48 to 1.02 | 1.465 | 0.092 |
| Language | |||||
| BNT | 57.12 (3.42), 47–60 | 55.47(3.13), 49–60 | 55.78 (3.68), 47–60 | 0.596 | 0.685 |
| Verbal fluency | 50.65 (11.61), 34–65 | 34.65 (13.95), 17–62 | 25.76 (11.35), 18–49 | 6.952 | 0.003 |
| Language composite score | 0.59 (0.42), −0.19 to 1.45 | −0.12 (1.07), −2.05 to 1.32 | −0.43 (0.86), −1.89 to 1.10 | 2.309 | 0.114 |
| Learning and memory | |||||
| CVLT (1–5) | 41.01 (6.21), 31–60 | 31.78 (7.25), 21–53 | 28.88 (16.25), 15–69 | 6.987 | 0.005 |
| CVLT long delay free recall | 9.45 (3.01), 4–14 | 6.78 (3.59), 0–13 | 3.25 (4.37), 0–12 | 6.844 | 0.003 |
| Learning and memory composite score | 0.54 (0.84), −0.92 to 2.12 | −0.18 (0.73), −1.70 to 1.07 | −0.15 (1.38), −2.11 to 1.17 | 6.821 | 0.004 |
| Executive function | |||||
| Trails B | 91.25 (26.18), 61–163.04 | 137.52 (57.25), 69–264.37 | 251.73 (153.87), 48–512 | 8.894 | 0.001 |
| SDMT oral | 50.54 (8.25), 24–67 | 35.44 (11.22), 15–58 | 28.97 (13.25), 16–53 | 8.703 | 0.001 |
| Executive function composite score | 0.59 (0.65), −0.97 to 1.11 | −0.10 (0.76), −1.34 to 1.45 | −0.29 (0.84), −1.86–1.09 | 6.875 | 0.002 |
| Visuospatial | |||||
| JLO | 29.27 (4.28), 21–30 | 25.52 (4.25), 17–30 | 24.60 (5.46), 12–29 | 1.479 | 0.081 |
Data presented as means (SD), range. BNT, Boston Naming Test; BTA, Brief Test of Attention; CVLT, California Verbal Learning Test; DRS-2, Dementia Rating Scale; JLO, Judgment of Line Orientation; MoCA, Montreal Cognitive Assessment; PDD, Parkinson’s disease dementia; PD-MCI, Parkinson’s disease mild cognitive impairment; PD-NC, Parkinson’s disease normal cognition; SDMT, Symbol Digit Modalities Test.
Cognitive data were checked for missingness and found to violate the assumption that data were missing completely at random (MCAR): Little’s MCAR Test, P = 0.007. Therefore, data were imputed to create five multiple imputations. The imputed data did not significantly differ from the original data (all P values >0.05). For data reduction purposes, cognitive composite scores were created using principal components analyses for each set of imputed data separately. Composites were created from two variables per domain as follows: Global cognition, MoCA and DRS-2 total scores; Attention, TMT-A and BTA; Language, BNT and verbal fluency; Learning and memory, CVLT trials 1–5 total score and CVLT long delay free recall; and Executive function, TMT-B and SDMT oral.
The JLO test was the only individual test of visuospatial abilities, so a composite score was not created for it.
Classification of normal cognition (PD-NC; n = 35), mild cognitive impairment (PD-MCI; n = 37), or dementia (PDD; n = 17) was determined by consensus conference, attended by neurologists and neuropsychologists with experience in PD cognition. Before consensus conference meetings, raw scores were transformed to z scores based on normative data for each of the individual neuropsychological tests, drawn from either testing manuals (17, 20, 22–24) or additional normative studies (25–28). MDS Task Force Level II diagnostic guidelines for PDD (29) and PD-MCI (4) were applied, requiring impairment on two tests in one cognitive domain (PD-MCI) or one test in each of two cognitive domains (PD-MCI and PDD) for classification as cognitively impaired. Of note, we diverged from the MDS recommendations in that we had two neuropsychological tests for all but one domain tested (visuospatial abilities). Impairment was defined as performance of ≥2 standard deviations below age- and education-matched norms (21). PD-MCI was differentiated from PDD based on the results of the neurologist’s functional interview: if significant functional impairment related to cognitive symptoms was present based on clinical impression, a participant was classified as PDD.
MEG Data Acquisition and Preprocessing
Neuromagnetic resting-state data were acquired using a Magnes 3600 whole head MEG device with an array of 248 sensors (4D Neuroimaging, San Diego, CA) in a magnetically shielded room (ETS-Lindgren, Cedar Park, TX). Data were acquired continuously at sample rates of either 678.17 Hz (n = 57) or 290.64 Hz (n = 32) with an acquisition bandwidth of 0.1–200 Hz. First, location and orientation of the MEG coils relative to each subject’s head were determined by digitizing a set of fiducial reference points on the head using a magnetic digitizer (Polhemus 3SPACE). Then, data were acquired for a minimum of 4 min during which subjects were lying supine and were instructed to keep their eyes closed for the first half of the acquisition period and to open them at the halfway point when they heard a brief sound. Eyes-closed and eyes-open data were combined because eye opening and closing is a powerful modulator of the alpha frequency band power.
Processing of the time-series MEG data for spectral power and source estimates of brain activity was carried out in MATLAB 2016b (the MathWorks, Inc., Natick, MA) with the FieldTrip toolbox for MEG analysis (30), using a customized MATLAB scripted pipeline. Raw MEG data were first bandpass filtered between 0.5 and 55 Hz (4th order, phase-invariant Butterworth characteristic filter). Bad channels were determined from individual MEG sensors based on excessive variance in the signal intensity and spectral power, relative to their nearest neighbors (less than or greater than 2 SD from the neighborhood mean). Data segments with SQUID jump artifacts were identified and excluded from further analysis (FieldTrip ft_artifact_jump.m function). Eye blink/movement and cardiac artifacts were then identified and removed from the data by independent component analysis (ICA). The runica algorithm was used, with a number of components limited to 50. The ICA cleaned data were then formed into 2-s contiguous trials, with 0.5 s overlap, and any trials with amplitudes exceeding ±3 pT were excluded from further analysis. A mean of 253 ± 16 (for PD-NC data sets), 244 ± 26 (PD-MCI), and 302 ± 21 (PDD) epochs were subjected to further analysis.
Spectral power was then calculated on the cleaned 2-s trials. A discrete Fourier analysis was carried out to generate a power spectrum over 99 frequencies over the range of 0–50 Hz; this range encompasses the five main frequency bands (δ, 0–3.5 Hz; theta, 4–7 Hz; alpha, 8–12 Hz; beta, 13–30 Hz; and gamma, 31–55 Hz).
MEG Data Analyses
For experiments involving oscillatory power in each frequency band, sensor-level data were used with no three-dimensional (3-D) reconstruction, although interpolation was necessary in some cases where the Fourier processing had been set to generate a different number of frequencies over the 0–55 Hz interval (i.e., a spectrum of 173 frequencies had to be converted to 99 due to the two different sampling rates—see MEG Data Acquisition and Preprocessing).
For experiments involving 3-D source reconstruction, a standardized MRI headmodel (standard_singleshell.mat from FieldTrip) in Montreal Neurological Institute (MNI) space was used for all patients. An 8-mm cubic voxel, MNI space source model (standard_sourcemodel3d8mm.mat), and the MNI head model were coregistered to the individual subjects using the MEG fiducials gathered with each subject and corresponding MRI fiducials using the MATLAB SPM 12 toolbox function spm_eeg_inv_rigidreg.m (SPM12; http://www.fil.ion.ucl.ac.uk/spm/). The source model was then used to compute leadfields and source analysis was carried out using FieldTrip’s ft_sourceanalysis() function.
For studies of whole brain band power differences over the entire data acquisition period of each subject, a beamforming method (“pcc”) was employed. To achieve sufficient resolution to accurately apply brain parcellation, interpolation was performed on the source model for each trial, with the effect of increasing its dimensions from 20 × 22 × 25 to 91 × 91 × 109.
For the experiments involving whole brain power in the five frequency bands over the whole acquisition period, as well as in the analysis of significantly different parcel power intensity, normalization was carried out by dividing the power of the trials with above-median power by those with below-median power, and later referred to “ratio” (e.g., alpha ratio). This was performed because in distributed source analyses, there is a strong noise bias in the center of the imaging volume, and normalization by contrasting different data intervals is a common strategy for resolving the bias problem (31).
Volumetric models of brain activity were analyzed with respect to functionally organized brain “parcels” rather than individual voxels. Parcellation of the models was carried out with FieldTrip/MATLAB (AAL parcellation) (32). The anatomical automatic labeling (AAL) parcellation was chosen because it is readily available in FieldTrip, is well-validated, has been successfully used in prior PD studies (9), and because its parcel sizes are large enough to be resolvable at the spatial resolution typical of MEG reconstruction. Values assigned to each parcel were the average of the powers assigned to its constituent voxels.
Statistical Analyses
To investigate the possibility of oscillatory slowing with cognitive decline, we carried out univariate analysis of the whole brain power contained within each of the five bands at the sensor level. We then computed multivariate analysis of the distribution of power among individual frequencies within bands, looking for statistically significant differences between the three cognitive classes. For this subanalysis only, beta and gamma could not be investigated, because they contain more frequencies than there are patient samples in the PDD group, and multivariate ANOVA (MANOVA) becomes unreliable in such cases. We also performed multivariate analysis of the total power between the five bands. Last, we looked for associations between total power in each band and subareas of cognitive functioning, by implementing a set of linear regressions. For stepwise regression, the minimum and maximum number of predictors in the model were set as 2 and 8. A 10-fold cross validation was implemented to estimate the average root mean square error (RMSE) of prediction for each of the seven models. From the seven models, the best one corresponds to the smallest RMSE value. Then the best model was fitted to find covariates that are associated with outcome variables. Stepwise regression was carried out with “caret” statistical package in R (33) and significance was chosen at P < 0.05. Stepwise regression was used for variable selection before linear regression. Scores for JLO, global cognition, attention, language, memory, and executive function were outcome variables of interest, and whole brain power for each band was regarded as predictors separately.
Last, for band power differences between parcels, instead of grand-averaging, significance at each parcel was assessed using ANOVA and MANOVA, with the cognitive impairment as independent variable. Multiple comparisons between parcel power values were controlled by the false-discovery rate (FDR), using the Benjamini–Hochberg procedure to limit FDR to α < 0.05. As an additional test of whether the observed parcel power significance might simply be due to chance, we randomly permuted the parcel identities for each patient and then ran ANOVA again. In this case, even after multiple repetitions, no parcels yielded P values under 0.05.
RESULTS
Whole Brain Power Differences between PD Cognitive Classes
Group differences in total power between bands.
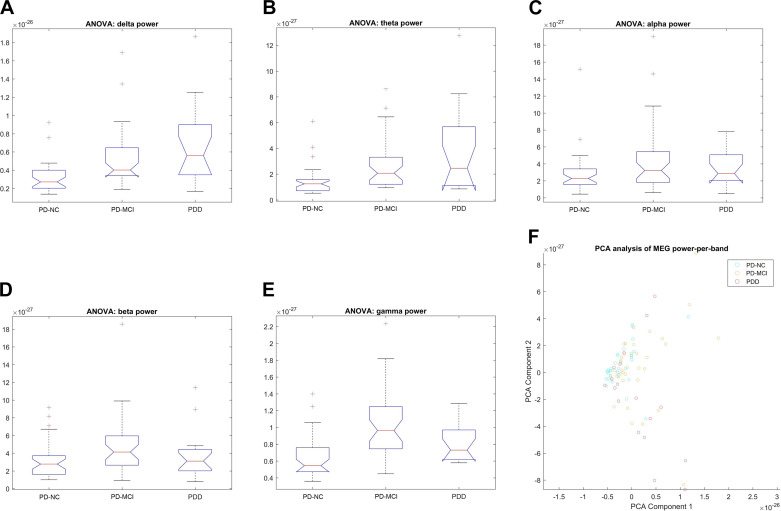
There was an exceptionally strong group difference in the gamma band (F = 16.06, P < 1.18 e−06), but also very significant in the theta and delta bands (F = 8.48, P < 0.0004; F = 9.67, P < 0.0002) (Table 3; Fig. 1, A–E). However, there appears to be a difference in the type of trend between the high- and the low-frequency bands: the overall power in the low-frequency bands (delta and theta ) increased from PD-NC to PD-MCI and from PD-MCI to PDD, whereas that for the high-frequency bands (beta and gamma) increased from PD-NC to PD-MCI but decreased from PD-MCI to PDD. The alpha band alone did not show a significant trend.
Table 3.
Whole brain power differences between individual bands
| Frequency Band | P Value | F |
|---|---|---|
| Delta | 0.0002 | 9.67 |
| Theta | 0.0004 | 8.48 |
| Alpha | 0.1206 | 2.17 |
| Beta | 0.0443 | 3.23 |
| Gamma | 1.18 e−06 | 16.06 |
We performed separate ANOVA tests on each of the whole brain bands power individually. Except for the alpha range, there is a significant between-group differences in all frequency bands. There is an exceptionally strong effect in the gamma band, but also very significant in the theta and delta bands. Bold type represents significant between-groups differences.
Figure 1.
Whole brain power difference between cognitive classes. ANOVA plots show power in the low‐frequency bands [delta (A), theta (B)] increased from cognitively normal Parkinson’s disease (PD) (PD‐NC) to PD‐mild cognitive impairment (MCI) and also from PD‐MCI to PD dementia (PDD), whereas power for the high-frequency bands [beta (D), gamma (E)] increases from PD‐NC to PD‐MCI but decreases from PD‐MCI to PDD. No change was observed in the alpha band (C). F: eigenvector plot showing a wedge-shaped overall distribution, with PD-NC datasets tightly clustered around the apex of the wedge, whereas PD-MCI cases are mostly distributed above and to the right of that spot, and PDD cases are mostly found below and to the right of the spot. MEG, magnetoencephalography.
Power distribution within bands.
Highly significant differences were found in the two lowest frequency bands (δ, P < 1.7 e−06; theta, P < 0.0006), but much less significant for alpha band (P < 0.039) (Table 4). Extremely high significances from a multivariate analysis can be a sign of issues in the MANOVA analysis, so we tried a different significance method (Pillai’s trace) that was recommended to check for such problems. This corrected approach still produced a significance level of P < 0.0055. Mahalanobis distances, calculated between the distributions of the three cognitive classes as part of the MANOVA analysis, likewise showed a greater separation between the PD-MCI and PDD distributions as between the PDD and PD-NC distributions, particularly in the alpha and δ bands. For the theta band, by contrast, the distance from PD-NC to PDD was roughly equal to the sum of the other two distances.
Table 4.
Mahalanobis distances between groups for within band analyses
| Frequency Band | PD-NC | PD-MCI | PDD |
|---|---|---|---|
| Alpha | |||
| PD-NC | 0 | 0.7037 | 1.2021 |
| PD-MCI | 0.7037 | 0 | 1.8277 |
| PDD | 1.2021 | 1.8277 | 0 |
| Delta | |||
| PD-NC | 0 | 1.6108 | 2.2343 |
| PD-MCI | 1.6108 | 0 | 2.1652 |
| PDD | 2.2343 | 2.1652 | 0 |
| Theta | |||
| PD-NC | 0 | 1.1173 | 2.123 |
| PD-MCI | 1.1173 | 0 | 2.123 |
| PDD | 2.123 | 2.123 | 0 |
Mahalanobis distances showing a greater separation between the Parkinson’s disease mild cognitive impairment (PD-MCI) and Parkinson’s disease dementia (PDD) distributions as between the PDD and Parkinson’s disease normal cognition (PD-NC) distributions, particularly in the alpha and delta bands. For the theta band, by contrast, the distance from PD-NC to PDD is roughly equal to the sum of the other two distances.
Power distribution between bands.
MANOVA yielded two highly significant (P < 1.7681 e−07; P < 0.0067) eigenvector pairs, which indicates that two dimensions can explain most of the variation between phenotypes (Fig. 1F). A multiplot of the data projected onto the 2-D subspace defined by these eigenvectors shows a wedge-shaped distribution with PD-NC data sets tightly clustered around the apex of the wedge, whereas PD-MCI cases are mostly distributed above and to the right of that spot, and PDD cases are mostly found below and to the right of the spot.
Associations between domains of cognitive function and whole brain power.
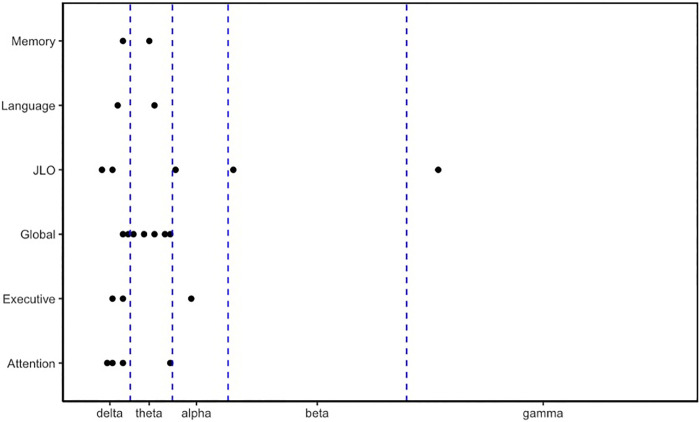
All areas of cognition were associated with the delta band (Fig. 2). In addition, all but visuospatial and executive functioning were associated with the theta band. Instead, those were associated with the alpha band. Last, the beta and gamma bands were also associated with visuospatial abilities.
Figure 2.
Associations between cognitive domains (y‐axis) and oscillatory power in each of the frequency bands (delta, theta, alpha, beta, and gamma) across all three groups. JLO, Judgment of Line Orientation task.
Regional Activation Differences between PD Cognitive Classes
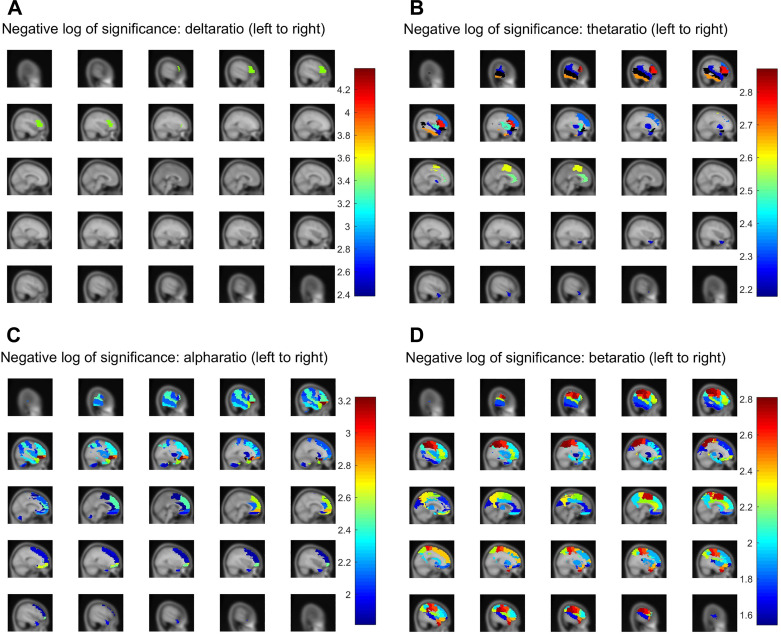
We found that 67 of the 116 parcels show group-wise ratio power differences at P < 0.01 in at least one frequency band (Table 5 and Fig. 3, A–D). Another 16 parcels were significant in more than one band, of which two (the orbital and triangular left frontal gyri) were significant in three of the five bands. We noticed a strong tendency for the most significantly class-dependent parcel powers to concentrate in the left hemisphere for the δ band (all parcels) as well as for the beta (∼2/3 parcels), theta, and alpha bands (∼3/4 parcels). Interestingly, significant changes occurred between the PD-NC and PD-MCI groups rather than with other group comparisons (bolded values with asterisks), which indicates most dramatic changes may happen when the PD brain shows mild cognitive decline. Of note, the most widespread whole brain group significances occurred in the beta range. No parcels reached α < 0.05 in the gamma band.
Table 5.
AAL parcels and between groups’ significance
| Left Hemisphere | Parcel Name | Delta | Theta | Alpha | Beta | Mean Significance Per Band | Right Hemisphere | Parcel Name | Delta | Theta | Alpha | Beta | Mean Siginificance Per Band |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal | Frontal_Inf_Tri_L | 0.00041 * | 0.00169 * | 0.00340 | 0.00529 * | 0.00067 | Frontal | Frontal_Med_Orb_R | 0.00161 | 0.00161 | |||
| Frontal_Inf_Orb_L | 0.00663 * | 0.00060 | 0.00643 * | 0.00152 | Supp_Motor_Area_R | 0.00187 * | 0.00187 | ||||||
| Frontal_Mid_L | 0.00465 * | 0.00464 * | 0.00990 * | 0.00213 | Precentral_R | 0.00276 * | 0.00276 | ||||||
| Frontal_Inf_Oper_L | 0.00135 * | 0.01010 | 0.00905 * | 0.00228 | Frontal_Sup_Medial_R | 0.00265 | 0.00916 * | 0.00295 | |||||
| Precentral_L | 0.00247 * | 0.00247 | Frontal_Inf_Orb_R | 0.00387 * | 0.00387 | ||||||||
| Supp_Motor_Area_L | 0.00257 * | 0.01445 | 0.00649 * | 0.00261 | Frontal_Sup_R | 0.01184 | 0.00401 * | 0.00396 | |||||
| Frontal_Sup_Orb_L | 0.00640 | 0.00524 * | 0.00291 | Frontal_Sup_Orb_R | 0.00230 | 0.01482 * | 0.00428 | ||||||
| Frontal_Mid_Orb_L | 0.00136 | 0.01146 * | 0.00320 | Frontal_Mid_R | 0.01381 | 0.01186 * | 0.00642 | ||||||
| Frontal_Sup_Medial_L | 0.00353 | 0.00353 | Frontal_Mid_Orb_R | 0.00318 | 0.02275 * | 0.00648 | |||||||
| Rectus_L | 0.01033 | 0.00995 * | 0.00507 | Frontal_Inf_Oper_R | 0.00716 * | 0.00716 | |||||||
| Frontal_Sup_L | 0.00802 | 0.01858 * | 0.00665 | Rectus_R | 0.01175 | 0.01868 * | 0.00761 | ||||||
| Frontal_Med_Orb_L | 0.00817 | 0.02019 * | 0.00709 | Frontal_Inf_Tri_R | 0.00955 * | 0.00955 | |||||||
| Parietal | SupraMarginal_L | 0.00576 * | 0.00380 * | 0.00247 * | 0.00134 | Parietal | SupraMarginal_R | 0.00175 * | 0.00175 | ||||
| Parietal_Inf_L | 0.00390 * | 0.00155 * | 0.00136 | Postcentral_R | 0.00219 * | 0.00219 | |||||||
| Parietal_Sup_L | 0.00164 * | 0.00164 | Paracentral_Lobule_R | 0.00436 * | 0.00436 | ||||||||
| Postcentral_L | 0.00835 * | 0.00177 * | 0.00253 | Angular_R | 0.00544 * | 0.00544 | |||||||
| Angular_L | 0.00887 * | 0.00834 * | 0.00430 | Parietal_Sup_R | 0.00581 * | 0.00581 | |||||||
| Precuneus_L | 0.00449 * | 0.00449 | Precuneus_R | 0.01062 * | 0.01062 | ||||||||
| Paracentral_Lobule_L | 0.00549 * | 0.00549 | Parietal_Inf_R | 0.01067 * | 0.01067 | ||||||||
| Temporal | Temporal_Sup_L | 0.00591 * | 0.00343 | 0.00583 * | 0.00169 | Temporal | Temporal_Pole_Mid_R | 0.00587 * | 0.01096 | 0.00196 * | 0.00209 | ||
| Temporal_Pole_Sup_L | 0.00565 * | 0.00261 * | 0.01057 * | 0.00209 | Temporal_Pole_Sup_R | 0.00313 * | 0.00313 | ||||||
| Temporal_Pole_Mid_L | 0.00610 * | 0.00305 | 0.01383 * | 0.00255 | Temporal_Sup_R | 0.02131 | 0.02131 | ||||||
| Temporal_Inf_L | 0.00208 * | 0.00483 * | 0.02127 * | 0.00313 | Heschl_R | 0.02565 | 0.02565 | ||||||
| Temporal_Mid_L | 0.00664 * | 0.00758 * | 0.01643 * | 0.00341 | |||||||||
| Heschl_L | 0.01138 * | 0.01138 | |||||||||||
| Occipital | Occipital_Sup_L | 0.02138 * | 0.02138 | ||||||||||
| Cuneus_L | 0.02298 * | 0.02298 | |||||||||||
| Other | Insula_L | 0.00330 * | 0.00664 | 0.01269 * | 0.00251 | Other | Cingulum_Ant_R | 0.00762 | 0.00522 * | 0.00321 | |||
| Cerebellum_10_L | 0.00279 * | 0.00279 | Rolandic_Oper_R | 0.00610 * | 0.00610 | ||||||||
| Cingulum_Ant_L | 0.00307 * | 0.01502 | 0.02158 * | 0.00441 | Cingulum_Mid_R | 0.00634 * | 0.00634 | ||||||
| Rolandic_Oper_L | 0.00668 | 0.01272 * | 0.00485 | Caudate_R | 0.01068 * | 0.01068 | |||||||
| Putamen_L | 0.00570 * | 0.01283 | 0.02572 * | 0.00492 | Insula_R | 0.01270 * | 0.01270 | ||||||
| Pallidum_L | 0.00540 * | 0.00540 | Putamen_R | 0.01282 * | 0.01282 | ||||||||
| Cerebellum_7b_L | 0.00814 * | 0.00814 | Pallidum_R | 0.01506 * | 0.01506 | ||||||||
| Hippocampus_L | 0.00928 * | 0.00928 | Amygdala_R | 0.01752 * | 0.01752 | ||||||||
| ParaHippocampal_L | 0.01079 * | 0.01079 | Olfactory_R | 0.01971 * | 0.01971 | ||||||||
| Amygdala_L | 0.01541 | 0.02862 * | 0.01101 | ||||||||||
| Cingulum_Mid_L | 0.01164 * | 0.01164 | |||||||||||
| Cerebellum_8_L | 0.01251 * | 0.01251 | |||||||||||
| Thalamus_L | 0.01350 * | 0.01350 | |||||||||||
| Cingulum_Post_L | 0.02380 * | 0.02380 |
P values of parcels showing significant (P < 0.01) differences in each band power between cognitive phenotypes, grouped by brain lobe and hemisphere. Parcels are ranked in increasing order by average P value for that parcel divided by total number of bands achieving significance (far right column). Left frontal lobe areas, corresponding to speech centers of Broca’s area, as well as left temporal lobe areas, are particularly prominent, showing high significance in multiple bands. Beta power significance was the most widespread of the five bands, being substantial in parcels across the brain including the occipital lobe. Given that the parcellation used (AAL) contains 116 parcels, this is far above what would be expected by chance or random fluctuation. As an additional test of whether the observed parcel power significance might simply be due to chance, we randomly permuted the parcel identities for each patient and then ran ANOVA again. In this case, even after multiple repetitions, no parcels yielded P values under 0.05. AAL, anatomical automatic labeling.
Bolded values indicate significant changes occurring between the Parkinson’s disease normal cognition (PD-NC) and Parkinson’s disease mild cognitive impairment (PD-MCI) groups rather than other group comparisons.
Figure 3.
Parcel‐wise significance maps using the anatomical automatic labeling (AAL) parcellation. Parcels were subjected to false-discovery rate (FDR) correction of α < 0.05 and are colored by their estimated negative‐log significance, for four frequency bands: delta (A), theta (B), alpha (C), and beta (D).
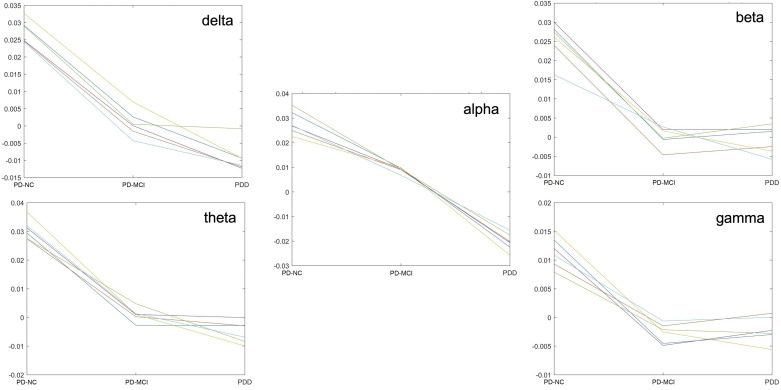
Investigating this further on the six most significant parcels per band, we found a power decrease between PD-NC and PD-MCI and/or PDD, but pair-wise comparisons showed the PD-MCI—>PDD difference was not always significant, because the PD-NC—>PD-MCI difference was much larger, except in the alpha range (Fig. 4).
Figure 4.
Ratio power of individual parcels of highest significance in the delta, theta, alpha, beta, and gamma bands showing the majority of changes occurring between normal cognition (NC) and mild cognitive impairment (MCI) states. PD, Parkinson’s disease; PDD, Parkinson’s disease dementia.
DISCUSSION
Altogether, our results suggest four main features of the PD brain related to cognitive decline: 1) dramatic change as the PD brain transitions to MCI, 2) slowing associated with cognitive decline, 3) hemispheric asymmetry, and 4) widespread abnormalities in beta oscillations. We discuss these features in the following sections.
Dramatic Change as the PD Brain Transitions to MCI
In contrast with the increase in the lower-frequency range of oscillations, we observed a tendency in higher-frequency bands for the total power to increase between NC and MCI and to decrease from MCI to PDD. In addition, the fact that PD-MCI and PDD cases are found in partially overlapping but overall near-opposite regions of the PCA plot, with normal cognition cases midway, suggests distinct phenomena may occur during dementia progression. Besides spectral slowing, studies show compensatory or reversal effects as MCI proceeds to dementia, such as hyperconnectivity in early-moderate disease followed by loss of connectivity in low alpha band (34) or heightened beta power in early PD, followed by reduced power in late PD (7). This pattern, also called “activity-dependent degeneration” (35) has been described as a network-level attempt to recover or compensate for global damage. The question of whether PDD and PD-MCI may be truly distinct physiologic phenotypes is still unclear, due in part to the considerable heterogeneity of dementia and MCI symptoms (3). It has been hypothesized that cognitive impairment in PD can be separated into two distinct types affecting different brain areas: one involving anterior regions and associated with mild early impairment, and another, more posteriorly located and associated with worse prognosis (36). Here, we offer evidence that PD-MCI and PDD are distinct from both one another and from patients with normal cognition, but that most dramatic changes may happen when the PD brain shows mild cognitive decline. It may be possible that compensatory changes further occur as the brain switches to a dementia state. A longitudinal study would ideally address this hypothesis.
Slowing Associated with Cognitive Decline
Our results replicated the “oscillatory slowing” (7) as the PD brain transitions to MCI or from MCI to dementia. We also observed that those were significantly associated with each area of cognitive function throughout the three phenotypes with less reliance on oscillations of higher frequencies. This pattern is quite interesting given the preponderance of high-frequency oscillations contributing to cognitive tasks, such as “binding gamma,” a way to bind information processed in distributed neural circuits and/or cortical areas into a coherent cognitive processes (37). As cognitive symptoms appear, it is not surprising to see less “binding” phenomena accompanying large cognitive networks weakening.
Hemispheric Asymmetry
One of the most striking findings of our localization results is the considerable left-hemispheric preference among the parcels observed to show significant changes with cognitive status. A preferential left-hemispheric involvement in dementia progression was implied in several prior studies on patients with AD-dementia (35) or PDD (38). A possible explanation is that the battery used to characterize cognitive groups contained a number of relatively language-dependent tasks. Lateralization of language processing to the left hemisphere in most individuals is certainly among the most prominent functional brain asymmetries known (39). But, this phenomenon may not be limited to brain activation patterns, but to the rate of actual tissue loss. In a recent study (40), loss of gray matter in patients with PD was localized preferentially to the left hemisphere, particularly the left temporal and parietal lobes. Future studies investigating structural abnormalities with respect to cognitive groups will help clarify this possibility.
Widespread Abnormalities in Beta Oscillations
Widespread group significances in the beta range of oscillations were not unexpected given the numerous pieces of evidence of beta abnormalities in PD as well as the following idea. Aside from a sensorimotor role, frontal beta oscillations have especially been involved in diverse roles during memory (41) and executive control processes (42), as well as during prevention of distractions (43). Whether beta is generated in the basal ganglia or in the cortex, its propagation between those regions via thalamocortical loops is thought to be orchestrating both cognition and movement (44), which relies essentially to its suitability to long-distance communication between distinct regions (45). Various impairments in thalamocortical loops as well as in beta rhythms are widely observed in the PD brain. It is therefore possible that, as the disease progresses along with cognitive decline, beta abnormalities may be the range of oscillations where we can see the most widespread damage or “rewiring” as the brain adapts to utilized brain networks that are spared by the disease.
Strengths and Limitations
Our approach offers novelty in several respects, in using resting-state MEG data to find hallmarks of parkinsonian cognitive decline. Also, our sample size, with 89 subjects overall, makes our study one of the larger neuroimaging studies on PDD currently available. Studies that have focused on resting-state activity in PD have become increasingly common (40), but the large majority thus far have utilized fMRI methodology rather than MEG; those which have used MEG (9, 46) were generally performed with significantly smaller groups of subjects, did not distinguish between PDD and PD-MCI, or focused primarily on the motor symptoms of PD. The main limitation of our study is the cross-sectional nature of the data set, which means that the onset and progression of dementia within the PD brain cannot be tracked within the same brain, but only when looking at a grand average of brains with similar levels of cognitive decline. The second most limiting factor is the lack of assessment of sex differences, which are crucial given the largely dominant male representation in patients with PD. Our last limitation relates to normalization of oscillatory power values in different frequency bands. Although this is an interesting but controversial area of discussion, we believe adequate normalization should account for investigations in motor networks, given the cognitive and motor overlapping neurophysiopathology in cortico-basal ganglia-thalamo-cortical loops in addition to the preponderance and variability of motor symptoms in PD.
It is our hope that further MEG studies, as their numbers increase and as consistent methodologies are settled upon, will eventually converge upon a clearer understanding of the nature of PD-related cognitive decline. Given the many advantages of the resting-state MEG methodology compared with fMRI, with its much greater temporal resolution, its ability to resolve specific frequency ranges, and also in being far less complex, time-consuming, and stressful for the patient, we hope that these investigations will spark further interest in the application of MEG to neuroimaging-based diagnostics for dementia.
DATA AVAILABILITY
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
ETHICAL APPROVALS
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
GRANTS
All phases of this study were supported by three National Institutes of Health (NIH) Grants 1K02NS080885-01A1 (to PI: B. M. Kluger), 1R21NS093266-01A1 (to PI: B. M. Kluger), and K01 AT009894-01 (to PI: I. Buard); and a Michael J. Fox Foundation for Parkinson’s Research Grant 10879 (to PI: S. K. Holden). B. M. Kluger received funding from the National Institute of Aging Grant K02 AG062745; the National Institute of Nursing Research Grant R01NR016037; and the Patient Centered Outcomes Research Institute Grant DI-2019C2-17499. Also Honoraria from the American Academy of Neurology and from the World Parkinson Congress. D. Ghosh received funding from National Cancer Institute Grants U01 CA235488 and R01 CA129102, as well as from the National Science Foundation Grant DMS 1914937. I. Buard received funding from the National Center for Complementary and Integrative Health and an honorarium from the University of Arizona as Chair of Data Safety Monitoring board for an NIA-funded RCT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
B.M.K. and I.B. conceived and designed research; S.E.R., C.S.M., S.K.H., and I.B. performed experiments; O.B.S., D.C.R., and X.Y. analyzed data; O.B.S. and I.B. interpreted results of experiments; O.B.S. and X.Y. prepared figures; O.B.S. and I.B. drafted manuscript; D.C.R., D.G., X.Y., S.K.H., B.M.K., and I.B. edited and revised manuscript; O.B.S., D.C.R., D.G., X.Y., S.E.R., C.S.M., S.K.H., B.M.K., and I.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Candace Ellman and Nicole Leith for their assistance with manuscript review and submission, and also Dr. Eugene Kronberg for his contribution to the graphical abstract.
REFERENCES
- 1.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65: 1239–1245, 2005. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 2.Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson’s disease. J Neurol Sci 374: 26–31, 2017. doi: 10.1016/j.jns.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jimenez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov Disord 31: 861–881, 2016. doi: 10.1002/mds.26662. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27: 349–356, 2012. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmermann L, Florin E. Parkinson’s disease and pathological oscillatory activity: is the beta band the bad guy?—new lessons learned from low-frequency deep brain stimulation. Exp Neurol 233: 123–125, 2012. doi: 10.1016/j.expneurol.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, Hill BC, Kilbane C, Henderson JM, Brontë-Stewart H. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord. 30: 1750–1758, 2015. doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- 7.Boon LI, Geraedts VJ, Hillebrand A, Tannemaat MR, Contarino MF, Stam CJ, Berendse HW. A systematic review of MEG-based studies in Parkinson’s disease: the motor system and beyond. Hum Brain Mapp 40: 2827–2848, 2019. doi: 10.1002/hbm.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Cognitive decline in Parkinson's disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging 34: 408–418, 2013. doi: 10.1016/j.neurobiolaging.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Boon LI, Hillebrand A, Olde Dubbelink KTE, Stam CJ, Berendse HW. Changes in resting-state directed connectivity in cortico-subcortical networks correlate with cognitive function in Parkinson’s disease. Clin Neurophysiol 128: 1319–1326, 2017. doi: 10.1016/j.clinph.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Olde Dubbelink KTE, Hillebrand A, Twisk JWR, Deijen JB, Stoffers D, Schmand BA, Stam CJ, Berendse HW. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology 82: 263–270, 2014. doi: 10.1212/WNL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 11.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184, 1992. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RMA, Wadia P, Miyasaki J, Duff-Canning S, Lang AE, Marras C. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 23: 297–299, 2008. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger, 1983. [Google Scholar]
- 14.Mattis S. Rating Scale-2 (DRS-2). Psychological Assessment Resources Inc., 2002. [Google Scholar]
- 15.Army Individual Test Battery. Army Individual Test Battery 1944. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office, 1944. [Google Scholar]
- 16.Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the brief test of attention. Clin Neuropsychol 10: 80–89, 1996. doi: 10.1080/13854049608406666. [DOI] [Google Scholar]
- 17.Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test, (CVLT-II). The Psychological Corporation, 2000. [Google Scholar]
- 18.Pereira DR, Costa P, Cerqueira JJ. Repeated assessment and practice effects of the written symbol digit modalities test using a short inter-test interval. Arch Clin Neuropsychol 30: 424–434, 2015. doi: 10.1093/arclin/acv028. [DOI] [PubMed] [Google Scholar]
- 19.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS): Examiner's Manual. San Antonio, TX: The Psychological Corporation, 2001. [Google Scholar]
- 20.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol 35: 364–367, 1978. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 21.Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson’s disease. Mov Disord 28: 1972–1979, 2013. doi: 10.1002/mds.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt KS, Mattis PJ, Adams J, Nestor P. Test-retest reliability of the dementia rating scale-2: alternate form. Dement Geriatr Cogn Disord 20: 42–44, 2005. doi: 10.1159/000085073. [DOI] [PubMed] [Google Scholar]
- 23.Reitan RM, Wolfson D (Editors). The Trail-Making Test (2nd ed.). Tucson, AZ: Neuropsychology Press, 1993. [Google Scholar]
- 24.Wechsler D. Wechsler Memory Scale—Revised Manual. San Antonio, TX: The Psychological Corporation, 1987. [Google Scholar]
- 25.Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry Neuropsychol Behav Neurol 15: 39–43, 2002. [PubMed] [Google Scholar]
- 26.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills 76: 1219–1230, 1993. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 27.Tombaugh TN, Hubley AM. The 60-item Boston Naming Test: norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol 19: 922–932, 1997. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14: 167–177, 1999. [PubMed] [Google Scholar]
- 29.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: 1689–1707; quiz 1837, 2007. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 30.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross J, Baillet S, Barnes GR, Henson RN, Hillebrand A, Jensen O, Jerbi K, Litvak V, Maess B, Oostenveld R, Parkkonen L, Taylor JR, van Wassenhove V, Wibral M, Schoffelen JM. Good practice for conducting and reporting MEG research. Neuroimage 65: 349–363, 2013. doi: 10.1016/j.neuroimage.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolls ET, Huang C-C, Lin C-P, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage 206: 116189, 2020. doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn M. Building predictive models in R using the caret package. J Stat Softw 28, 2008. doi: 10.18637/jss.v028.i05. [DOI] [Google Scholar]
- 34.Olde Dubbelink KTE, Stoffers D, Deijen JB, Twisk JWR, Stam CJ, Hillebrand A, Berendse HW. Resting-state functional connectivity as a marker of disease progression in Parkinson’s disease: a longitudinal MEG study. Neuroimage Clin 2: 612–619, 2013. doi: 10.1016/j.nicl.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci 15: 683–695, 2014. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 36.Tessitore A, Cirillo M, De Micco R. Functional connectivity signatures of Parkinson’s disease. J Parkinsons Dis 9: 637–652, 2019. doi: 10.3233/JPD-191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5: 16–25, 2001. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 38.Bosboom JL, Stoffers D, Wolters E, Stam CJ, Berendse HW. MEG resting state functional connectivity in Parkinson's disease related dementia. J Neural Transm (Vienna) 116: 193–202, 2009. doi: 10.1007/s00702-008-0132-6. [DOI] [PubMed] [Google Scholar]
- 39.Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci USA 112: 2871–2875, 2015. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang LC, Wu PA, Lin SZ, Pang CY, Chen SY. Graph theory and network topological metrics may be the potential biomarker in Parkinson’s disease. J Clin Neurosci 68: 235–242, 2019. doi: 10.1016/j.jocn.2019.07.082. [DOI] [PubMed] [Google Scholar]
- 41.Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. Gamma and beta bursts underlie working memory. Neuron. 90: 152–164, 2016. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 29: 12675–12685, 2009. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanslmayr S, Matuschek J, Fellner MC. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr Biol 24: 904–909, 2014. doi: 10.1016/j.cub.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt R, Herrojo Ruiz M, Kilavik BE, Lundqvist M, Starr PA, Aron AR. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J Neurosci 39: 8231–8238, 2019. doi: 10.1523/JNEUROSCI.1163-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97: 1867–1872, 2000. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olde Dubbelink KTE, Hillebrand A, Stoffers D, Deijen JB, Twisk JWR, Stam CJ, Berendse HW. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain 137: 197–207, 2014. doi: 10.1093/brain/awt316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.