Keywords: arm choice, chronic stroke, habits, reaching, value-based models of choice
Abstract
In neurotypical individuals, arm choice in reaching movements depends on expected biomechanical effort, expected success, and a handedness bias. Following a stroke, does arm choice change to account for the decreased motor performance, or does it follow a preinjury habitual preference pattern? Participants with mild-to-moderate chronic stroke who were right-handed before stroke performed reaching movements in both spontaneous and forced-choice blocks, under no-time, medium-time, and fast-time constraint conditions designed to modulate reaching success. Mixed-effects logistic regression models of arm choice revealed that expected effort predicted choices. However, expected success only strongly predicted choice in left-hemiparetic individuals. In addition, reaction times decreased in left-hemiparetic individuals between the no-time and the fast-time constraint conditions but showed no changes in right-hemiparetic individuals. Finally, arm choice in the no-time constraint condition correlated with a clinical measure of spontaneous arm use for right-, but not for left-hemiparetic individuals. Our results are consistent with the view that right-hemiparetic individuals show a habitual pattern of arm choice for reaching movements relatively independent of failures. In contrast, left-hemiparetic individuals appear to choose their paretic left arm more optimally: that is, if a movement with the paretic arm is predicted to be not successful in the upcoming movement, the nonparetic right arm is chosen instead.
NEW & NOTEWORTHY Although we are seldom aware of it, we constantly make decisions to use one arm or the other in daily activities. Here, we studied whether these decisions change following stroke. Our results show that effort, success, and side of lesion determine arm choice in a reaching task: whereas left-paretic individuals modified their arm choice in response to failures in reaching the target, right-paretic individuals showed a pattern of choice independent of failures.
INTRODUCTION
Consider the apparently simple task of reaching a target as fast as possible. Which arm should one choose? In a previous study of arm choice for horizontal movements in right-handed neurotypical individuals (1), we showed that, for a given target, the arm that both minimizes the expected effort and maximizes the expected success for the upcoming movement is chosen with higher probability than the other arm, with an overall handedness bias favoring the right arm. These results were consistent with previous research showing that motor decisions are based on the evaluation of both expected motor costs, such as effort, and expected rewards for the upcoming movement (2–6). Does this pattern of arm choice change in chronic stroke survivors who experience mostly unilateral motor deficits? In particular, is arm choice modified to account for the decreased performance in the paretic arm, or does it follow a preinjury pattern?
Here, we address these questions by analyzing the arm choice and movements performed by individuals with mild-to-moderate chronic stroke included in the Dose Optimization for Stroke Evaluation (DOSE) rehabilitation clinical trial (7). We predicted that effort strongly modulates arm choice because individuals poststroke often report effortful movements (8–10). In addition, this increase in effort has been proposed to increase arm nonuse (11), defined as the difference between what the individual can do when constrained to use the paretic arm and what the individual does when given a spontaneous choice to use either arm (12). We thus hypothesized that effort would play a greater role in arm choice in individuals poststroke compared with neurotypical individuals.
Similarly, we predicted that success modulates arm choice poststroke but with an interaction with the side of the lesion. Whereas overall use of the paretic arm decreases after stroke in premorbidly right-handed individuals, right-hemiparetic individuals (RH) use their paretic arm to a greater extent than left-hemiparetic (LH) individuals (13–16). A high level of practice is associated with the formation of habitual behavior (17, 18), defined as behavior that is insensitive to changes in the task goals. Thus, because RH individuals often use their paretic arm, we hypothesized that their arm choice will be habitual, that is, it will be less sensitive to induced failures to reach a target in our experimental setup. In contrast, because LH individuals seldom use their paretic arm, we hypothesized that their arm choice will be less habitual, that is, more sensitive to failures. Because short reaction times have been linked to habitual choices (19), we expected shorter reaction times in RH than in LH participants before a choice. Finally, we expected that arm choices in RH participants will better correlate with a clinical measure of habitual arm use, the Amount of Arm Use Test (AAUT).
To test these predictions, we modified an existing arm choice task (20) and a data analysis method that we developed to study arm choice in neurotypical individuals (1). Participants with mild-to-moderate chronic stroke performed arm movements to targets displayed on a table either in forced choice trials, in which participants were instructed to reach the targets with both arms in turn, and in spontaneous choice trials, in which either arm could be chosen for each target. Forced choice trials allowed us to estimate the effort and success rates for each arm for each target; these variables were then entered in a logistic regression model to predict arm choice in the spontaneous trials. To increase effort and failure rates, arm choices were made under three time-constrained conditions: no-, medium-, and fast-time constraint movement conditions.
MATERIALS AND METHODS
Participants
Data from 22 individuals poststroke with mild-to-moderate impairment, 12 with RH (1 female, mean ± SD aged 62.83 ± 13.95 yr) and 10 with LH (4 females, mean ± SD aged 56.90 ± 13.53 yr), were analyzed. The participants suffered a stroke from 0.47 to 14.38 yr before participation. In addition, 11 age-matched nondisabled participants (controls; 6 females, mean ± SD aged 54.36 ± 10.58 yr) were recruited for comparison.
Participants poststroke were a subset of those included in the DOSE rehabilitation clinical trial, for whom arm choice data were available. The DOSE trial was designed to investigate the effect of therapy dose on arm/hand function (NCT 01749358) (7). Here, we only analyzed arm choice and performance data at “baseline,” during which participants were tested three times with 2 wk of intervals before any therapy intervention. Detailed inclusion and exclusion criteria were previously reported (21). Briefly, participants poststroke were included if they had a stroke more than 5 mo ago, had mild-to-moderate motor impairment [upper extremity Fugl-Meyer motor (UEFM) > 19 out of 66], could reach with the paretic arm from the home position to a target 25 cm away within 5 s, had no arm/hand neglect as determined by Albert test, and self-reported premorbidly right-handed. Control participants were included if they reported no prior neurological disorders and self-reported to be right-handed. The study was approved by the Human Research and Review Committee of the University of Southern California, and each participant signed and received a copy of an informed consent. Clinical assessments for screening, UEFM, and Amount of Arm Use Test (AAUT), as well as arm choice tests, were performed by two trained and standardized experimenters.
Experimental Setup and Design
Experimental setup.
Details of the experimental setup, shown in Fig. 1A, can be found elsewhere (21). Briefly, participants sat on a wooden chair in front of a table with a restraining belt to minimize compensatory trunk motion during pointing movements. Two Mini-Bird magnetic sensors (Ascension Technology Corporation) were placed on the nail of the right and the left index fingers to measure hand trajectory and arm choice (sampling 100 Hz). At each trial, a target, appearing at one of the predefined 35 target locations, was projected on the table from an overhead projector. Participants were instructed to move their index finger from the home position to the target as rapidly and accurately as possible and to keep their finger on the target for at least 0.5 s before returning to the home position. A pleasant sound was provided following successful reaches, that is, when the target was reached within the given time; otherwise, an unpleasant sound was provided. In addition, participants received a “reward” point for each successful trial.
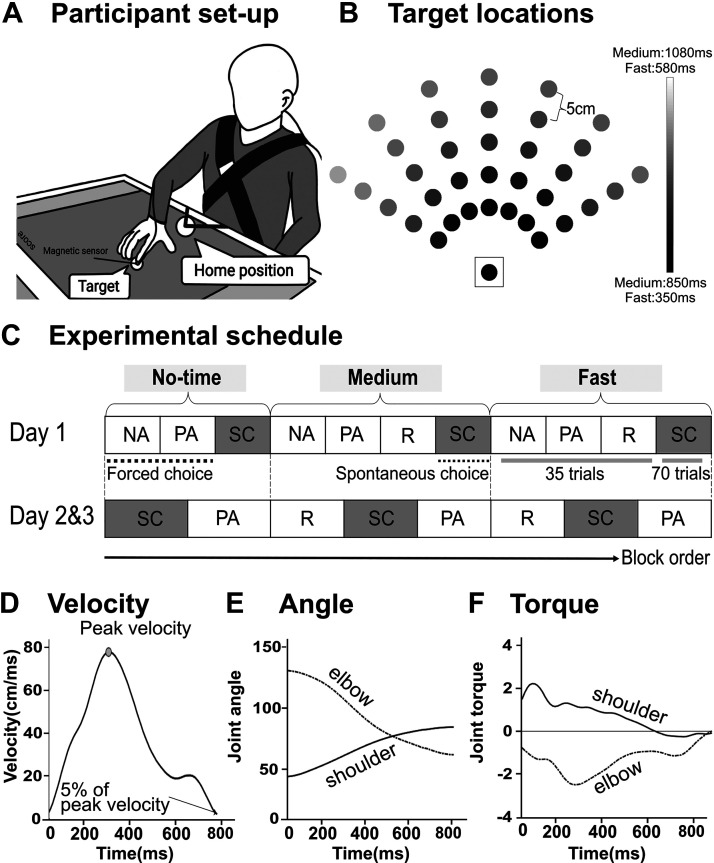
Figure 1.
Bilateral arm reaching test setup, target locations, and protocol. A: setup. The circles on the bottom and the middle of the touch screen show the home position and a target, respectively. At each trial, participants were instructed to reach to the target using either right or left index finger (depending on conditions) as quickly and accurately as possible. Magnetic sensors were attached to the index finger tips of both hands to record the choice of hand and kinematics. B: 35 targets displayed in the workspace (plus the home target, shown surrounded by a square). Gray shading shows the time constraint for each target in the fast condition. C: experimental protocol common for the stroke group. On day 1, the forced choice blocks (PA and NA) were always presented before the spontaneous choice blocks (SC) for familiarization purpose. On days 2 and 3, reminder sessions (R) were presented before the spontaneous choice blocks, followed by the forced choice blocks (PA) for the medium and fast conditions (see materials and methods). The spontaneous choice blocks used for the analysis of the paretic arm choice are shaded in gray. D: examples of velocity profile of a single reaching movements for one subject poststroke illustrating the computation of movement time. E: example of estimated joint angle. An inverse kinematics model was used to estimate shoulder and elbow joint angles from hand trajectories. F: example of shoulder and elbow joint torques estimated by an inverse dynamics model of the arm in 2 dimensions. Fast, fast-time constraint condition; Medium, medium-time constraint condition; NA, nonparetic arm only block; No-time, no-time constraint condition; PA, paretic arm only block; R, reminder block; SC, spontaneous choice block.
Experimental design.
Participants performed blocks of forced choice trials and spontaneous choice trials, in no-, medium-, and fast-time constraint conditions to an array of 35 targets (see Fig. 1B). In the forced choice blocks, participants reached each of the 35 targets once with each arm. In the spontaneous choice blocks, participants reached each target with the arm of their choice, with two trials per target (thus, for a total 70 targets, see Fig. 1C). In the spontaneous choice blocks, participants were reminded that there was no right or wrong answer in their choice of arm but were instructed to maximize the number of successful trials by moving fast or by using the nonparetic arm when expecting failure with the paretic arm.
In the no-time constraint condition, the targets were displayed until the participants’ index finger landed inside the target disk; in contrast, in the fast-time constraint condition, the targets disappeared ∼0.5 s after movement onset. Movement time limits in the fast-time constraint condition were estimated from previous aiming data in neurotypical right-handed participants (22) and ranged between 350 and 580 ms. Movement time limits were 500 ms longer for the medium condition (see Fig. 1B). The no-time constraint condition was given before the medium- and fast-time constraint condition (Fig. 1C), because we observed in a pilot study that stroke participants often did not select their paretic arm when they started with the more challenging condition first. Similarly, we included the medium-time constraint because we noticed in a second pilot study that some participants did not select their paretic arm for any target if the fast-time constraint condition directly followed the no-time constraint condition. Reminder sessions of 35 trials were included before the medium- and fast-time constraint conditions to remind the participants of the time limits for these conditions. These reminder sessions were similar to the spontaneous choice blocks except that the targets did not disappear, and “too slow” visual and auditory feedback was provided after every trial if the time criterion was not met.
Stroke participants.
Participants poststroke were tested with the arm-reaching task on days 1, 2, and 3, with at least 2 wk between testing days to minimize learning effects on the test. In the forced choice blocks on day 1, participants reached all 35 targets first with their nonparetic arm and then with their paretic arm. In the forced choice blocks on days 2 and 3, participants reached all targets with their paretic arm only. We previously demonstrated excellent test-retest reliability of arm choice in the spontaneous choice block of the fast-time constraint condition and moderate-to-good reliability in the spontaneous choice block of the medium- and no-time constraint conditions in the same stroke participants (21).
Control participants.
Control participants were tested on days 1 and 2, with 1 wk between testing days. In forced choice blocks on day 1, these participants reached to all 35 targets first with their right arm and then with their left arm, whereas on day 2, they reached the targets with their right arm only. Spontaneous choice blocks were given on days 1 and 2. Note that participants in the control group were only tested twice because their success rates were near 100% in all conditions; thus, unlike the stroke group, a third session was not necessary to estimate success rates for each target.
Data Measurement and Processing
Arm choice.
Choice of the right arm for each target in each condition was measured from the spontaneous choice blocks on days 1, 2, and 3 for the stroke group and on days 1 and 2 for the control group (Fig. 1C). For each trial, the target-specific arm choice was coded as 1 for the right arm and 0 for the left arm. Since there were two spontaneous choice trials per target per day, the six choice trials per target in the stroke group (4 in the control group) were used to develop the arm choice models (see Target-by-Target Arm Choice Analysis: Mixed-Effect Logistic Regression Models). In addition, the overall percentage of choice for the right arm was calculated by dividing the number of choices for this arm by the total number of the target appearance in each time condition and day. This percentage of the choice in each condition was then averaged across days.
Success in reaching.
Success to reach each target in each condition was measured from the forced choice blocks on days 1, 2, and 3 for the stroke group and on days 1 and 2 for the control group. The target-specific success rates were calculated by dividing the number of successes by the total number of the target appearance across testing days in each condition. For instance, if the stroke participants missed one target, success rate for this target was 66.7%, because a target only appeared once for each condition and testing day. This target-specific success rates were used to develop the arm choice models (see below). In addition, the overall success rate for the paretic arm (and the right arm for the control group) was calculated by taking the average over the 35 targets in the forced choice block in each condition.
Effort estimation.
Effort for both arms was estimated from movement trajectories to each target in the forced choice block on day 1 via an inverse dynamics transformation as in our previous study (1). Position data from the magnetic sensors were filtered at 5 Hz with a Butterworth low-pass filter. We assumed that movements were planar and the shoulder was fixed; under these conditions, we can uniquely derive shoulder and elbow joint angles from the index finger position (x, y) using inverse kinematics of a two degrees of freedom arm model (Fig. 1E). We then differentiated the two joint angles into joint velocities and accelerations and estimated the shoulder and elbow torques using inverse dynamics (Fig. 1F). All arm parameters were taken from Van Beers et al. (23). Note that we computed “absolute effort” defined by summing the absolute torques at both joints from the movement start to the end, since such effort has recently been shown to better account for arm movement planning (4) and was a better predictor of choice than effort derived from summing the squared torques (1). The target-specific effort for each arm was used to develop the arm choice model (see below). In addition, the overall effort for the paretic arm (and the right arm for the control group) was calculated by taking the average over the 35 targets in the forced choice block in each condition.
Reaction times in spontaneous choice blocks.
The reaction time for each target was measured in each spontaneous choice block in all time constraint conditions across testing days. Reaction time was defined as the time between the target appearance and the time at which the index finger’s tangential velocity exceeded 5% of maximum velocity (Fig. 1D). Median value of reaction time for the 70 movements in each spontaneous choice block was computed for each condition and then averaged across testing days.
Clinical Assessment of Arm Use
We administered the Actual Amount of Use Test (AAUT) to the stroke participants to assess spontaneous use of the paretic arm and hand in the real world (24). In the AAUT, participants perform 14 upper-extremity daily tasks, such as opening a file folder and writing on and folding up a piece of paper with the hands of their choice (including bimanually) without any prompts. Note that the AAUT is a covert test—thanks to the use of scenario involving the above common daily activities, the participants were not aware that they were tested. A trained evaluator watched video recordings of the test and graded spontaneous arm use behaviors based on the quality of movement scale (QOM), which ranged from 0 to 5. The QOM score for each item was averaged over the 14 tasks; high-average scores indicated more- and good use of the paretic arm. If participants habitually use their paretic arm, then we expect arm choice measured by our reaching task should correlate with arm use as assessed by the covert AAUT (21).
Overall Behavioral and Clinical Measures Analyses
To investigate the effects of both groups and time constraints on all behavioral data (arm choice, success rates, effort, and reaction time), we developed linear mixed-effect models in which between-subject groups (RH, LH, and control) and within-subject conditions (no-, medium-, and fast-time constraints) were entered as fixed effects and participants as random intercepts. Log-likelihood ratio tests (LRT) were used to verify the need for inclusion of the random effect term. Visual inspection of residuals versus fits plots and qq-plots was performed to check for heteroscedasticity or non-normal distribution of the residuals. Post hoc analyses were performed using the Tukey’s test to correct for multiple comparisons. In addition, correlations between arm choice measured in the reaching task and arm use measured by AAUT in both stroke groups (LH and RH) were conducted using Spearman’s correlation after Shapiro–Wilk test for normality. Possible differences in age for RH, LH, and control groups were tested using the Kruskal–Wallis test. For the stroke groups (LH and RH), between-group differences in stroke onset and UEFM were analyzed using either independent t test or Wilcoxon rank-sum test after Shapiro–Wilk test for normality. All statistical analyses were performed with the R statistical package version 3.6.2 (25). Significance levels were set to P = 0.05 for these overall analyses, and all results are given in means ± standard deviation.
Target-by-Target Arm Choice Analysis: Mixed-Effect Logistic Regression Models
Right arm choice.
In a previous study with neurotypical individuals, we proposed a model of arm choice based on the well-accepted theoretical framework according to which reward-driven decision making occurs via the comparison of “action values,” that is, the expected rewards and cost for possible actions in a given state (26). We then used logistic regression models to show that between-arm differences in expected effort and in expected success accurately predicted arm choice (1). Here, similarly, we tested whether choice of the right arm is predicted by between-arm differences in expected effort and expected success rates in individuals poststroke. Because we do not have access to expected efforts and success variables used for the decision (which are internal to the participants), we used, as a proxy, the effort and success rates measured for each target in forced blocks for each arm, as in our previous study. Then using the target-by-target binary choice data in spontaneous choice blocks, we predicted the probability of right arm choice from these effort and success rate variables. To take into account the large differences in participants’ characteristics and for the repeated measurements, we used random slopes and intercepts. Note that for stroke participants, success of the paretic arm was highly predictive of condition: success was 100% in the no-time constraint condition but was largely reduced in the fast-time constraint condition. Thus, to reduce model complexity (i.e., the number of parameters to estimate) and avoid high collinearity between the predictors, we included success but not the time constraint condition as factor.
We built the mixed-effect logistic regression models for each group using a forward stepwise approach. We started with the simplest (base) model with mixed intercepts. Next, for the stroke participants, we added one predictor among effort and success. We then extended the model by including effort and success. For instance, for a LH stroke participant j, the model that gives the probability of choosing the right arm for a target i as a function of effort and success is given by the following equation:
where c is a constant parameter that biases overall arm choice for the subject j, and aj and bj are weighting parameters for differences between the right arm and left arm effort and success during the no-, medium-, and fast-time constraint conditions. The mixed-effect parameters for each participant j (aj, bj, and cj) are drawn from a Gaussian distribution centered on the mean of all participants (the fixed effects). The brackets < > indicate expected effort and success for movement to target i in subject j. Expected effort and success were computed from the movements to this target in forced choice blocks (see Data Measurement and Processing). The predictors were z-transformed before inclusion in the models such that the parameters could be compared. The probability of choosing the left arm is given by P(Lefti,j) = 1 − P(Righti,j).
We compared nested models using the Akaike information criteria (AIC) and with the likelihood ratio tests (LRT) using the anova() function in R. A total of 3.7% of choice data for the stroke group and 8% for the control group were missing, yielding totals of 13,350 and 4,250 useful data points, respectively. Missing data were due to the 1) inability to obtain the whole reaching trajectory when participants used the opposite arm by mistake in the forced choice block or 2) abnormally long movement duration, as detected by >3 standard deviations (such long durations were often due to unexpected movements such that the participants rubbed their eyes or noses).
RESULTS
Participant Demographics and Clinical Data
Participants in the RH, LH, and control groups did not differ in age (P = 0.272, Kruskal–Wallis test). The UEFM scores ranged from 19 to 57 and were not different between the LH and RH subgroups (LH: 41.6 ± 3.3, RH: 44.9 ± 3.2, Mann–Whitney U test, P = 0.453). There was no difference in time since the stroke onset between the LH and RH groups (LH: 3.4 ± 1.5 yr, RH: 2.2 ± 0.4 yr, Mann–Whitney U test, P = 0.496). Consistent with the entry criteria, all participants reported to be right-hand dominant before stroke.
Effects of Time-Constraint Condition on Choice, Effort, Success Rate, and Reaction Time
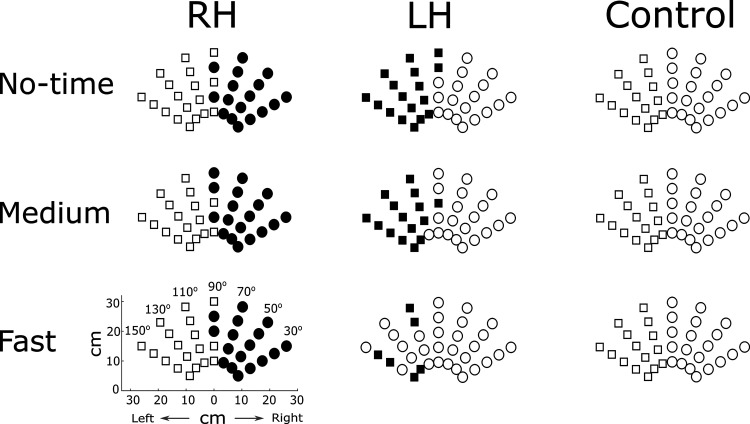
Figure 2 shows arm choice across the no-, medium-, and fast-time constraint conditions for one representative participant from each of the RH, LH, and control groups. Compared with the control participant, the RH participant showed an overall decrease in right arm choice for the midline target as expected because of the impairment due to stroke. Strikingly, the pattern of choice did not change for the RH group across time conditions, despite the increase in failures in the fast-time condition. In contrast, the LH participant, who presented a pattern of choice similar to the control participant in the no-time condition, showed a large decrease in arm choice in the fast-time constraint condition. As we will show below, these results for three individual participants hold true when the data from all participants are analyzed.
Figure 2.
Arm choice in the spontaneous choice block across no-time, medium, and fast conditions for 1 representative participant from each group. Circle markers represent right arm choice and square markers represent left arm choice. Black-shaded markers represent the paretic arm choice for the RH and the LH participants. Overall, the RH and LH participants chose their paretic arm less often than the nonparetic arm, whereas the (right-handed) participant in the control group chose the right arm more than the left arm. Across conditions, the participants in the RH and the control groups maintained similar arm choice patterns, whereas the LH participant largely decreased paretic arm choice in the fast condition. Control, neurotypical age-match participant; LH, participant with left hemiparesis; RH, participant with right hemiparesis.
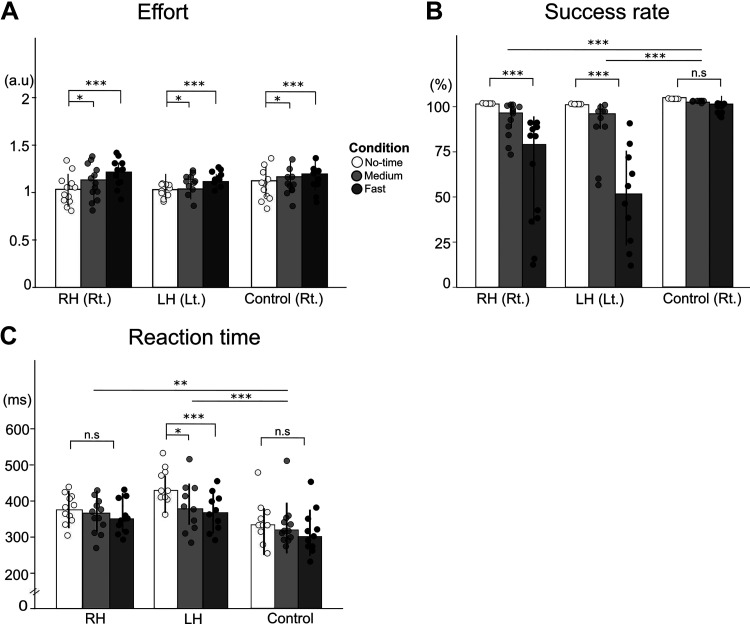
Figure 3, A and B, shows the effort and percentage of success of the paretic arm for the stroke group and the right arm for the control group. The overall reaction times for both the right and the left arms are shown in Fig. 3C. The linear mixed-effect models of effort with groups and time conditions show that condition is significant, but group and interaction group by conditions are not (condition: P = 0.001; Fig. 3A). All groups showed higher effort in the fast- and medium-time conditions than in the no-time condition (t = 4.11, P < 0.001; t = 2.60, P = 0.03, for the fast- and the medium-time constraint conditions, respectively; Fig. 3A). Thus, as expected, effort increased when the allowed movement time decreased, although as the figure shows, the changes were modest.
Figure 3.
Effort and success of the paretic arm and choice reaction times in RH, LH, and control groups. A and B: effort and success rates measured in the forced choice blocks of the paretic arm for RH and LH groups (and the right arm for control group) in the no-time (white), medium- (light gray), and fast- (dark gray) time constraint conditions. Dots indicate each individual. C: reaction times measured in the spontaneous choice blocks across the time-constraint conditions for all groups. a.u., arbitrary unit; LH, left hemiparesis; Lt., Left arm; n.s., not significant; RH, right hemiparesis; Rt., right arm; *P < 0.05, **P < 0.01, ***P < 0.001. Error bars show standard deviations.
The linear mixed-effect models of success rate with groups and time conditions show that group, condition, and interactions are all significant (all, P < 0.001, Fig. 3B). Success rate in the no-time condition was 100% in all group, which is not surprising given that reaching to the farthest target within 5 s with the paretic arm was an inclusion criterion. Success rates for both the RH and the LH groups were significantly lower than the success rates for the control group in the fast-time constraint conditions (t = 5.780, P < 0.0001 for RH and t = 7.788, P < 0.0001 for the LH; Fig. 3B). Although the control group success rates were close to 100% in all conditions, both the RH and the LH stroke groups showed a significant decrease in success rates of the paretic arm in the fast-time constraint condition (t = 7.369, P < 0.001 and t = 9.304, P < 0.001 for the RH and LH groups, respectively) compared with the no-time constraint condition. There was no difference in success (rates) in the medium- and the fast-time conditions between the RH and LH groups, although there was a trend for greater success rates in the fast-time constraint condition in the RH group than in the LH group, with a large between-subject variability (61.47 ± 31.79 vs. 46.71 ± 27.00 for the RH and LH groups, respectively, t = 2.312, P = 0.348).
The linear mixed-effect models of reaction time with groups and time conditions show that group and condition are significant (group, P = 0.008; condition P < 0.0001) with a trend for significance for interaction (P = 0.06). The LH group showed a decrease in reaction time from the no-time to the medium-time constraint condition (t = 3.629, P = 0.016) and to the fast-time constraint condition (t = 5.332, P < 0.0001). On contrary, neither the RH group nor the control group showed changes in reaction times across conditions, as their reaction times were already low in the no-time condition (P > 0.05; Fig. 3C).
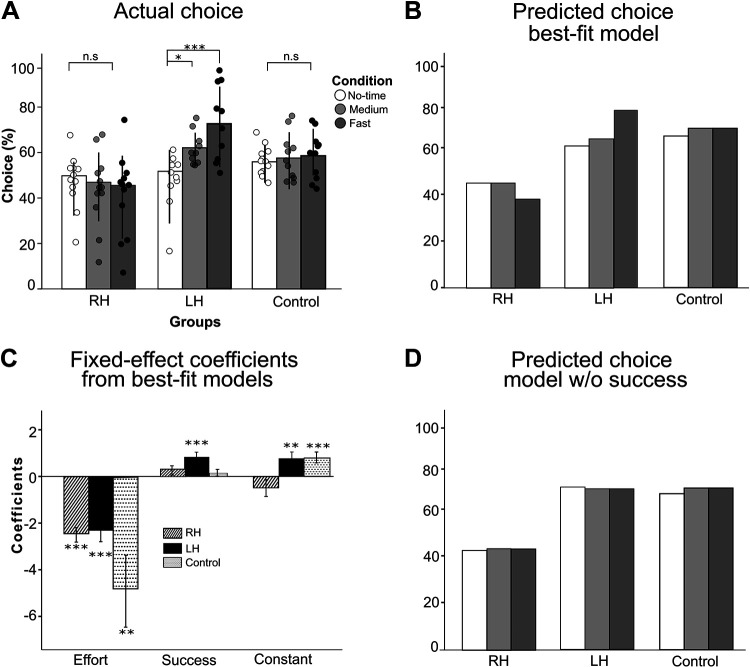
Figure 4A shows right arm choice across groups and conditions. The linear mixed-effect models of arm choice with groups and time conditions show that group and interaction group by conditions are highly significant [group: P = 0.003; group × condition: P < 0.0001; see Fig. 4A; note that because the figure shows the percentage of right arm choice, the percentage of (left) paretic arm choice for the LH group is given by 100% minus right arm choice]. The arm choice pattern was not different across time-constraint conditions in the RH and control groups (all comparisons P > 0.15). In contrast, the arm choice pattern was significantly different across conditions in the LH group: the paretic left arm choice was lower in the medium-time constraint (t = 3.267, P = 0.043) and in the fast-time constraint (t = 6.244, P < 0.001) conditions than in the no-time constraint condition (as illustrated in Fig. 2, middle and bottom).
Figure 4.
Right arm choice from actual data, best-fit models, and reduced models. A: right arm choice measured by the spontaneous choice block in the no-time (white), the medium- (light gray) and the fast- (dark gray) time constraint conditions for RH, LH, and control groups. Note that the percentage of (left) paretic arm choice for the LH group is given by 100% minus right arm choice. B: average choice predicted by the best-fit models with effort and success: the models well predict the average right arm choice in all time conditions (compare with the data in A). C: fixed-effect coefficients significantly different from zero are indicated by *P < 0.05, **P < 0.01, ***P < 0.001. The success term is only significant for the LH group. The constant terms that are statistically positive for the control and LH groups indicate the bias of using the right arm. D: average choice predicted by the reduced models with the effort terms but without the success terms (model 1 in Table 1): the model for the LH group does not account for the modulation of arm choice with the time condition (compare with B). LH, left hemiparesis; n.s., not significant; RH, right hemiparesis.
Expected Effort and Success Predicts Arm Choice but Success Differentially Influences Choice in the RH and LH Groups
We then tested whether 1) between-arm differences in expected effort and 2) between-arm differences in expected success accounted for right arm choice in the RH, LH, and control group. For all groups, model 1 with effort showed lower AIC than model 2 with success (see results in Table 1). Model 3 with effort and success improved on model 1 for the RH group (AIC = 5,748; comparison with model 1; LRT, P < 0.0001), LH group (AIC = 5,118; comparison with model 1; LRT, P < 0.0001), and control group (AIC = 3,629; comparison with model 1; LRT, P < 0.0001). Accuracy of the model calculated by comparing the actual to predicted arm choice for each target and using a probability threshold of 0.5 for the RH, LH, and control groups were 82.0 ± 6.0%, 80.1 ± 5.2%, and 79.4 ± 12.5%, respectively. Figure 4B shows that model 3 well predicts the mean right arm choice data.
Table 1.
Mixed-effect logistic regression models for the right arm choice in RH, LH, and control groups
| Right Arm Choice Mixed-Effect Logistic Regression Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
RH |
LH |
Control |
||||||
| Models\Variables | (1) | (2) | (3) | (1) | (2) | (3) | (1) | (2) | (3) |
| Constant | −0.407 (0.260) |
−0.241 (0.179) |
−0.390
(0.287) |
0.681** (0.219) |
0.561*** (0.132) |
0.816**
(0.272) |
0.835*** (0.169) |
0.300** (0.112) |
0.845***
(0.169) |
| Effort | −2.403*** (0.325) |
−2.441***
(0.313) |
−2.084*** (0.486) |
−2.299***
(0.482) |
−4.883** (1.636) |
−4.915**
(1.640) |
|||
| Success | 0.258* (0.121) |
0.217
(0.125) |
0.621*** (0.166) |
0.793***
(0.205) |
0.115* (0.059) |
0.113
(0.107) |
|||
| Observ. | 7,284 | 7,284 | 7,284 | 6,066 | 6,066 | 6,066 | 4,250 | 4,250 | 4,250 |
| LLH | −2,915 | −4,688 | −2,868 | −2,820 | −3,743 | −2,553 | −1,812 | −2,848 | −1,808 |
| AIC | 5,838 | 9,385 | 5,748 | 5,649 | 7,495 | 5,118 | 3,632 | 5,705 | 3,629 |
Model 3 (bold), which included both effort and success predictors, was the selected model for each group based on AIC and log-likelihood ratio tests. However, note that whereas the coefficient of the effort term is largely significant in all 3 models, the coefficient for success is only significant in the LH model (P < 0.001). AIC, Akaike information criterion; LH, left hemiparesis; LLH, log-likelihood; Observ., observation; RH, right hemiparesis.
*P < 0.05, **P < 0.01, ***P < 0.001.
Whereas the coefficient for success was significantly different from zero for the LH group (coefficient = 0.793, P < 0.001), it was not for the RH and or for the control groups (0.217, P > 0.05 and 0.113, P > 0.05, respectively; see Table 1). The importance of including success in the model for the LH group, but not for the RH and control groups, is further illustrated by Fig. 4D, in which we compared arm choice in models that do and do not include the success term (model 3 and model 1 in Table 1). Without the success term, predictions of arm choice largely deviate from the data in all time conditions for the LH group (Fig. 4, A, B, and D) but not for the RH and control groups.
The between-arm difference in effort strongly modulated choice in all groups in a similar manner in the RH and LH group. The fixed-effect coefficient of effort was different from zero (−2.441, P < 0.001 and −2.229, P < 0.001 for the RH and LH group from model 3; Table 1 and Fig. 4C). The effect of effort was, however, larger for the control group (−4.915, P < 0.001 from model 3).
As illustrated by the choice data of the representative participants in Fig. 2, right arm choice in the RH group was decreased compared with the control group, and similar in the LH and control groups. This is shown by the fixed-effect intercept being not different from zero for the RH group (−0.390, P > 0.05, see model 3, Table 1) but being positive for both the LH and control groups (LH: 0.816, P < 0.01 in model 3; Control: 0.845, P < 0.001 in model 3; Table 1 and Fig. 4C). Thus, whereas the LH and control groups used their right hands to reach targets near the midline, the RH groups used their left hand for these targets.
Note that a possible confound in the above analysis is that, in the fast-time constraint condition, the LH group exhibited a success rate with the paretic arm ∼15% lower on average than that of the RH group (see Fig. 3B). Thus, there is a possibility that the higher success rates with the paretic arm in RH participants diminished its influence on arm choice. We addressed this issue by matching participants in the two groups by success rates—this led to removal of two RH participants with the highest success rates (RH 1 and 6)—and reran the model fit for this group. Results did not change. Although the model 3 showed the lowest AIC (AIC for model 3 = 5,133), the coefficient of expected success was again not significant for this model (0.157, P = 0.281).
Clinical Validation of Arm Choice with the AAUT
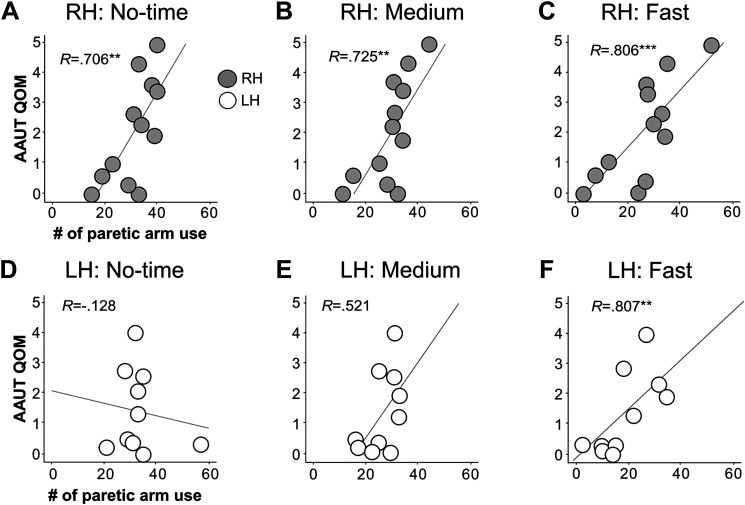
For the RH group, use of the paretic arm measured by AAUT was significantly correlated with the choice of the paretic arm expressed by the number of targets that participants successfully reached using the paretic arm under the no-, medium-, and fast-time constraint conditions (all Pearson’s correlation; r = 0.706, P = 0.01 for the no-time condition; r = 0.725, P = 0.005 for the medium-time condition; r = 0.806, P < 0.001 for the fast-time condition; Fig. 5, A–C). However, for the LH group, only the paretic arm choice in the fast-time constraint conditions correlated with AAUT (Pearson’s correlation; r = 0.807, P = 0.006; Fig. 5F), whereas choice in the medium- and no-time constraint conditions did not (Pearson’s correlation; r = 0.521, P = 0.293 for the medium-time condition; Spearman’s correlation, r = −0.128, P = 0.724 for the no-time condition; Fig. 5, D and E).
Figure 5.
Correlation analysis between AAUT and paretic arm choice for RH and LH groups in no-time, medium-, and fast-time constraint conditions. Top row shows the correlations between the paretic arm use measured by AAUT and the paretic arm choice measured by the reaching system in the no-time (A), the medium- (B), and the fast- (C) time constraint conditions in the RH group. Bottom row shows the correlations in the LH group. The RH group shows moderate-to-good correlations between clinical test and the paretic arm choice in all time conditions, whereas the LH group shows only good correlation under the fast- (F) time constraint conditions, but not under the no-time (D) or the medium- (E) time constraint conditions. **P < 0.01, ***P < 0.001. AAUT, Amount of Arm Use Test; LH, left hemiparesis; QOM, quality of movement scale; RH, right hemiparesis.
DISCUSSION
The arm choice models developed in this study show that individuals with mild-to-moderate chronic stroke who were right-handed before stroke, like neurotypical individuals (1), chose the arm that minimizes the expected effort and maximizes the expected success for the upcoming movement. However, a notable result of the present study is that the side of lesion modulates the choice patterns, with success strongly predicting choice only in LH individuals. LH individuals were more likely to switch their arm choice from the paretic to the nonparetic side when success rates decreased in the medium and fast conditions. Accordingly, a model without the success term did not account for the decrease in paretic arm choice for the medium- and fast-time condition in the LH group. Conversely, RH individuals were less likely to switch arm regardless of the time constraints even when success rates decreased. In addition, reaction times decreased in LH individuals between the no-time and the fast-time constraint conditions but showed no changes in RH individuals. Finally, the correlations between arm choice in the experiment and between a measure of habitual arm use in daily activities (the AAUT) were only strong in all time conditions in RH individuals. These results thus add to the body of research showing the influence of side of lesion on the patterns of arm use (13–16, 20), at least for lower impairment levels (27).
A contemporary decision framework, according to which human choices are driven by a combination of a goal-oriented system and a habitual system (28, 29), sheds additional light on our results. The habitual system develops with extensive practice and can be probed by testing the sensitivity to changes in task goals (17, 30, 31). The goal-directed system makes choices via mental simulation of the decision environment to evaluate the outcomes of possible actions. Thus, decisions governed by the goal-directed system can be quickly modified following environment changes to maintain optimal actions. However, the mental simulations require relatively long decision times (19). Thus, time pressure facilitates habitual choice (21, 32) via a shift from the goal-directed system to the habitual system (19). Although we did not design our task to directly pressure the decision time (since we manipulated movement time), the reaction time decreased across conditions in the LH group but were already low and showed no changes in RH group. Thus, overall, our results support the view of a more habitual pattern of choice in the RH group and a greater reliance of goal-directed choices in the LH group.
Thus, left-hemiparetic individuals appear to choose their paretic left arm more optimally: that is, if a movement with the paretic arm is predicted to be not successful in the upcoming movement, the nonparetic right arm is chosen instead. In contrast, in RH participants, because habitual choices result in fewer successes than could be achieved by choosing the nonparetic arm in RH participants, why these long-lasting changes do not remap following movement failures in our experiment? We can envision three possible nonexclusive possibilities that would counteract this remapping. First, the computational and time savings due to habitual actions in RH individuals could offset the advantages of otherwise more optimal decisions in our task (33). Second, because the unsuccessful movement of the paretic arm is often compensated with the nonparetic arm in a bimanual manner in RH individuals (14), remapping to avoid failures in daily activities might not be necessary. Third, repeated habitual choice in RH individuals can lead to (rewarding) improved function via “self-training” (26, 34), as suggested by the somewhat larger success rates in RH individuals compared with LH individuals, despite overall similar impairment levels between the two groups.
The effort parameter estimated in the choice model was the largest of all fixed parameters in all groups;1 because of the negative sign of this parameter, effort acts as a cost, as expected. Thus, our results confirm, and extend to stroke survivors, the results of our previous study showing that nondisabled participants prefer to select the arm associated with lower biomechanical effort (1). However, in contrast to the differential effect of expected success, the effect of the expected effort in arm choice was similar in RH and LH participants, and greater in control participants. This is in contrast to our hypothesis that effort would have a greater role in arm choice poststroke because individuals poststroke often report a greater sense of effort, which is consistent with preserved central motor commands but damage to efferent pathways (35), and is thought to play a role in nonuse poststroke (8–10). Note, however, that our estimation of effort poststroke may be underestimated because we did not take into account possible abnormal cocontractions, or muscle “synergies” (36–38) in the paretic arm, as cocontractions increase effort (39). Recording surface electromyography of muscles involved in the task could help identify cocontraction and could be used to update the effort model. Finally, estimation of effort can be improved in future work, as we estimated effort using a planar two-link arm (shoulder and elbow), although participants in our experiments could abduct their arm and rotate their shoulders internally or externally to some extent.
In any case, our results suggest that participants poststroke were able, as a group, to evaluate the effort for both arms for the upcoming arm choice. The computation of expected effort in advance of a movement decision has been related to a network comprised of regions involved in action planning and execution, notably the supplementary motor area (SMA) (40–43). In individuals with mild-to-moderate stroke, this network is often intact. Indeed, a lesion analysis of the participants in the ICARE clinical trial show that only modest subset (16%) had lesions of the sensorimotor cortex (44). Similarly, computation of expected reward and success in advance of a decision has been related to corticostriatal and nigrostriatal networks (28, 45–48). Here again, lesions that involve the striatum were rare among the ICARE participants (13%) (44). Thus, as for effort, participants in the DOSE trial, were presumably also able to estimate expected success for the upcoming movement, as neurotypical individuals do. Nonetheless, only the LH group appeared to have made use of such estimates in our experiment.
We note here three additional limitations and leave several open questions that need to be addressed in future works. First, our experimental task may limit the generalization of our findings. In particular, both the use of the restraining belt to minimize compensatory trunk movements and the reaching movements that do not require finger movements or object manipulation may influence spontaneous choice. Second, it may be argued that our number of participants poststroke (n = 22) was too small to select the best model of arm choice and to reliably test for the significance of two different regressors for the LH and RH groups. However, we believe that our results are valid because 1) the stroke group was relatively homogenous, with strict entry criteria for the DOSE trial; 2) the models were preplanned based on hypotheses derived from previous research; and 3) the stroke models were developed with multiple data points from all stroke participants with up to 630 arm choices per participants, which, accounting for missing data, yielded a total of 17,600 data points. Third, because of the relatively small number of participants, we could not link specific brain lesion characteristics to arm choice, nor could we include additional covariates in the models, such as impairment levels, pain, or the integrity of visuospatial memory (e.g., Ref. 49). In future work with larger datasets, such covariates could be included to improve predictions of arm choice to a larger and more diverse population of stroke survivors.
Implications of Our Findings for Neurorehabilitation
Our findings, if confirmed with additional studies including reaching and grasping movements, suggest new rehabilitation strategies that are differently targeted to individuals with RH and LH. Spontaneous use of the paretic arm in individuals poststroke is a crucial indicator of both recovery and effectiveness of rehabilitation (50, 51). Because stroke often leads to hemiparesis, individuals poststroke often choose to perform motor actions with the nonparetic arm. These individuals often exhibit at least some degree of nonuse (12, 20, 34, 52). Results from our arm choice models show that for both RH and LH individuals, effort is a large (negative) predictor of arm choice. Thus, for all participants, treatment strategy should aim to increase spontaneous use by decreasing muscle co-activation patterns of shoulder and elbow to reduce abnormal elbow/shoulder joint torque coupling (53, 54). In addition, because success largely influence choice in the LH individuals, additional treatment regimens will be needed for these individuals to increase the use of their paretic arm and to de-sensitize task failure in low-risk conditions (such as in this study) when the paretic arm is used. For instance, for LH individuals, task-oriented repetitive movements with virtual reality setups that encourage choice of the paretic arm (55, 56) can be helpful to increase use in the natural environment (57). In both cases, increased arm use may in turn further increase arm function via “self-training” (34); then the patient can enter a virtuous circle in which spontaneous arm use and motor performance reinforce each other (26, 58).
GRANTS
This work was funded by Grants NIH R01 HD065438 and R56 NS100528.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K., C.J.W., and N.S. conceived and designed research; S.K. performed experiments; S.K., C.E.H., B.K., and N.S. analyzed data; S.K., C.J.W., and N.S. interpreted results of experiments; S.K. prepared figures; S.K. and N.S. drafted manuscript; S.K., C.E.H., B.K., C.J.W., and N.S. edited and revised manuscript; S.K., C.E.H., B.K., C.J.W., and N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Computational Neuro-rehabilitation Laboratory, Rebecca Lewthwaite, John Monterosso, and Jim Gordon for comments on this work. We thank Shannon Massimo and Nicolo Betoni for help with data collection and Hyeshin Park for help with BART programming.
Footnotes
As a reminder, all variables were z-transformed so direct comparison of parameter values is meaningful.
REFERENCES
- 1.Schweighofer N, Xiao Y, Kim S, Yoshioka T, Gordon J, Osu R. Effort, success, and nonuse determine arm choice. J Neurophysiol 114: 551–559, 2015. doi: 10.1152/jn.00593.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cos I, Bélanger N, Cisek P. The influence of predicted arm biomechanics on decision making. J Neurophysiol 105: 3022–3033, 2011. doi: 10.1152/jn.00975.2010. [DOI] [PubMed] [Google Scholar]
- 3.Shadmehr R, Ahmed AA. Précis of vigor: neuroeconomics of movement control. Behav Brain Sci 44: E123, 2021. doi: 10.1017/S0140525X20000667. [DOI] [PubMed] [Google Scholar]
- 4.Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol 26: 1929–1934, 2016. doi: 10.1016/j.cub.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Lum PS, Shadmehr R, Lee SW. Perceived effort affects choice of limb and reaction time of movements. J Neurophysiol 125: 63–73, 2021. doi: 10.1152/jn.00404.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Xiao Y, Burdet E, Gordon J, Schweighofer N. The duration of reaching movement is longer than predicted by minimum variance. J Neurophysiol 116: 2342–2345, 2016. doi: 10.1152/jn.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage matters: a phase IIb randomized controlled trial of motor therapy in the chronic phase after stroke. Stroke 50: 1831–1837, 2019. doi: 10.1161/STROKEAHA.118.023603. [DOI] [PubMed] [Google Scholar]
- 8.Gandevia SC. The perception of motor commands or effort during muscular paralysis. Brain 105: 151–159, 1982. doi: 10.1093/brain/105.1.151. [DOI] [PubMed] [Google Scholar]
- 9.Rode G, Rossetti Y, Boisson D. Inverse relationship between sensation of effort and muscular force during recovery from pure motor hemiplegia: a single-case study. Neuropsychologia 34: 87–95, 1996. doi: 10.1016/0028-3932(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 10.Sunderland A, Tuke A. Neuroplasticity, learning and recovery after stroke: a critical evaluation of constraint-induced therapy. Neuropsychol Rehabil 15: 81–96, 2005. doi: 10.1080/09602010443000047. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist 8: 325–338, 2002. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews K, Steward J. Stroke recovery: he can but does he? Rheumatology 18: 43–48, 1979. doi: 10.1093/rheumatology/18.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RR, Birkenmeier RL, Lang CE. Real-world affected upper limb activity in chronic stroke: an examination of potential modifying factors. Top Stroke Rehabil 22: 26–33, 2015. doi: 10.1179/1074935714Z.0000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haaland KY, Mutha PK, Rinehart JK, Daniels M, Cushnyr B, Adair JC. Relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch Phys Med Rehabil 93: 1957–1962, 2012. doi: 10.1016/j.apmr.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain 136: 1288–1303, 2013. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani S, Przybyla A, Good DC, Haaland KY, Sainburg RL. Contralesional arm preference depends on hemisphere of damage and target location in unilateral stroke patients. Neurorehabil Neural Repair 28: 584–593, 2014. doi: 10.1177/1545968314520720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haith AM, Krakauer JW. The multiple effects of practice: skill, habit and reduced cognitive load. Curr Opin Behav Sci 20: 196–201, 2018. doi: 10.1016/j.cobeha.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardwick RM, Forrence AD, Krakauer JW, Haith AM. Time-dependent competition between goal-directed and habitual response preparation. Nat Hum Behav 3: 1252–1262, 2019. doi: 10.1038/s41562-019-0725-0. [DOI] [PubMed] [Google Scholar]
- 19.Keramati M, Dezfouli A, Piray P. Speed/accuracy trade-off between the habitual and the goal-directed processes. PLoS Comput Biol 7: e1002055, 2011. doi: 10.1371/journal.pcbi.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han CE, Kim S, Chen S, Lai YH, Lee JY, Osu R, Winstein CJ, Schweighofer N. Quantifying arm nonuse in individuals poststroke. Neurorehabil Neural Repair 27: 439–447, 2013. doi: 10.1177/1545968312471904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Park H, Han CE, Winstein CJ, Schweighofer N. Measuring habitual arm use post-stroke with a bilateral time-constrained reaching task. Front Neurol 9: 883, 2018.doi: 10.3389/fneur.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Kim S, Winstein CJ, Gordon J, Schweighofer N. Short-duration and intensive training improves long-term reaching performance in individuals with chronic stroke. Neurorehabil Neural Repair 30: 551–561, 2016. doi: 10.1177/1545968315606990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beers RJ, Haggard P, Wolpert DM. The role of execution noise in movement variability. J Neurophysiol 91: 1050–1063, 2004. doi: 10.1152/jn.00652.2003. [DOI] [PubMed] [Google Scholar]
- 24.Taub E, Crago JE, Uswatte G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil Psychol 43: 152–170, 1998. doi: 10.1037/0090-5550.43.2.152. [DOI] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. https://www.R-project.org [Google Scholar]
- 26.Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol 4: e1000133, 2008. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav G, Haaland KY, Mutha PK. Laterality of damage influences the relationship between impairment and arm use after stroke. J Int Neuropsychol Soc 25: 470–478, 2019. doi: 10.1017/S1355617718001261. [DOI] [PubMed] [Google Scholar]
- 28.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron 69: 1204–1215, 2011. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron 63: 733–745, 2009. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson A. Actions and habits: the development of behavioural autonomy. Philos Trans R Soc London B Biol Sci 308: 67–78, 1985.doi: 10.1098/rstb.1985.0010. [DOI] [Google Scholar]
- 31.Dolan RJ, Dayan P. Goals and habits in the brain. Neuron 80: 312–325, 2013. doi: 10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wit S, Kindt M, Knot SL, Verhoeven AAC, Robbins TW, Gasull-Camos J, Evans M, Mirza H, Gillan CM. Shifting the balance between goals and habits: five failures in experimental habit induction. J Exp Psychol Gen 147: 1043–1065, 2018. doi: 10.1037/xge0000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidaka Y, Han CE, Wolf SL, Winstein CJ, Schweighofer N. Use it and improve it or lose it: Interactions between arm function and use in humans post-stroke. PLoS Comput Biol 8: e1002343, 2012. doi: 10.1371/journal.pcbi.1002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuppuswamy A, Clark EV, Turner IF, Rothwell JC, Ward NS. Post-stroke fatigue: a deficit in corticomotor excitability? Brain 138: 136–148, 2015. doi: 10.1093/brain/awu306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewald JPA, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 38.Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain 126: 2510–2527, 2003. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- 39.Huang HJ, Kram R, Ahmed AA. Reduction of metabolic cost during motor learning of arm reaching dynamics. J Neurosci 32: 2182–2190, 2012. doi: 10.1523/JNEUROSCI.4003-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croxson PL, Walton ME, O'Reilly JX, Behrens TEJ, Rushworth MFS. Effort-based cost-benefit valuation and the human brain. J Neurosci 29: 4531–4541, 2009. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein-Flügge MC, Kennerley SW, Friston K, Bestmann S. Neural signatures of value comparison in human cingulate cortex during decisions requiring an effort-reward trade-off. J Neurosci 36: 10002–10015, 2016. doi: 10.1523/JNEUROSCI.0292-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prévost C, Pessiglione M, Météreau E, Cléry-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. J Neurosci 30: 14080–14090, 2010. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zénon A, Sidibé M, Olivier E. Disrupting the supplementary motor area makes physical effort appear less effortful. J Neurosci 35: 8737–8744, 2015. doi: 10.1523/JNEUROSCI.3789-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwardson MA, Wang X, Liu B, Ding L, Lane CJ, Park C, Nelsen MA, Jones TA, Wolf SL, Winstein CJ, Dromerick AW. Stroke lesions in a large upper limb rehabilitation trial cohort rarely match lesions in common preclinical models. Neurorehabil Neural Repair 31: 509–520, 2017. doi: 10.1177/1545968316688799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66: 585–595, 2010. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preuschoff K, Bossaerts P, Quartz SR. neural differentiation of expected reward and risk in human subcortical structures. Neuron 51: 381–390, 2006. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 47.Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev 95: 853–951, 2015. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci 7: 887–893, 2004. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 49.Schweighofer N, Lee JY, Goh HT, Choi Y, Kim SS, Stewart JC, Lewthwaite R, Winstein CJ. Mechanisms of the contextual interference effect in individuals poststroke. J Neurophysiol 106: 2632–2641, 2011. doi: 10.1152/jn.00399.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors’ perspective. Disabil Rehabil 27: 1213–1223, 2005. doi: 10.1080/09638280500075717. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Wolf SL, Zhang Q, Thompson PA, Winstein CJ. Minimal detectable change of the actual amount of use test and the motor activity log: the excite trial. Neurorehabil Neural Repair 26: 507–514, 2012. doi: 10.1177/1545968311425048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW III, DeLuca SC, Miller NE. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav 61: 281–293, 1994. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JPA. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve 32: 170–178, 2005. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis MD, Lan Y, Yao J, Dewald JPA. Robotic quantification of upper extremity loss of independent joint control or flexion synergy in individuals with hemiparetic stroke: a review of paradigms addressing the effects of shoulder abduction loading. J Neuroeng Rehabil 13: 95, 2016. doi: 10.1186/s12984-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballester BR, Maier M, San Segundo Mozo RM, Castañeda V, Duff A, Verschure PFMJ. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil 13: 74, 2016. doi: 10.1186/s12984-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballester BR, Nirme J, Duarte E, Cuxart A, Rodriguez S, Verschure P, Duff A. The visual amplification of goal-oriented movements counteracts acquired non-use in hemiparetic stroke patients. J Neuroeng Rehabil 12: 50, 2015. [Erratum in J Neuroeng Rehabil 12: 106, 2015]. doi: 10.1186/s12984-015-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, Cen SY, Azen SP; Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA 315: 571–581, 2016. doi: 10.1001/jama.2016.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schweighofer N, Han CE, Wolf SL, Arbib MA, Winstein CJ. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the Extremity Constraint-Induced Therapy Evaluation (EXCITE) trial. Phys Ther 89: 1327–1336, 2009. doi: 10.2522/ptj.20080402. [DOI] [PMC free article] [PubMed] [Google Scholar]