Abstract
BACKGROUND AND AIMS:
Endoscopic submucosal dissection (ESD) in Asia has been shown to be superior to endoscopic mucosal resection (EMR) and surgery for the management of selected early gastrointestinal cancers. We aimed to evaluate technical outcomes of ESD in North America.
METHODS:
We conducted a multicenter prospective study on ESD across 10 centers in the United States and Canada between April 2016 and April 2020. End points included rates of en bloc resection, R0 resection, curative resection, adverse events, factors associated with failed resection, and recurrence post-R0 resection.
RESULTS:
Six hundred and ninety-two patients (median age, 66 years; 57.8% were men) underwent ESD (median lesion size, 40 mm; interquartile range, 25–52 mm) for lesions in the esophagus (n = 181), stomach (n = 101), duodenum (n = 11), colon (n = 211) and rectum (n = 188). En bloc, R0, and curative resection rates were 91.5%, 84.2%, and 78.3%, respectively. Bleeding and perforation were reported in 2.3% and 2.9% of the cases, respectively. Only 1 patient (0.14%) required surgery for adverse events. On multivariable analysis, severe submucosal fibrosis was associated with failed en bloc, R0, and curative resection and higher risk for adverse events. Overall recurrence was 5.8% (31 of 532) at a mean follow-up of 13.3 months (range, 1–60 months).
CONCLUSIONS:
In this large multicenter prospective North American experience, we demonstrate that ESD can be performed safely, effectively, and is associated with a low recurrence rate. The technical resection outcomes achieved in this study are in line with the current established consensus quality parameters and further support the implementation of ESD for the treatment of select gastrointestinal neoplasms; ClinicalTrials.gov, Number: NCT02989818.
Keywords: Endoscopic Submucosal Dissection, Endoscopic Mucosal Resection, EMR, Polyps, Gastrointestinal Neoplasms
Endoscopic submucosal dissection (ESD) is a technique for the en bloc removal of dysplastic and early cancer lesions throughout the gastrointestinal (GI) tract.1 ESD carries distinct advantages over endoscopic mucosal resection (EMR) by providing the opportunity for accurate histopathologic evaluation of resection margins, a very low recurrence rate, and curative resection in selected neoplastic lesions. Furthermore, ESD is associated with lower morbidity and mortality, as well as higher patient quality of life compared with surgery.2–7 As a result, ESD has become a well-established procedure in Asian countries, and is being practiced increasingly in Europe. At the same time, adoption in the United States and Canada has been hampered by a multitude of factors related to the procedural degree of technical difficulty, device availability, training opportunities, lack of structured payer reimbursement, and longer procedure duration, coupled with higher adverse event rate compared with EMR.8,9 Importantly, the documented superior results with ESD reported from around the world have yet to be reproduced in high-quality prospective studies within North America. Therefore, we conducted a large prospective multicenter study to evaluate the technical outcomes of ESD when done as part of routine patient care in the United States and Canada.
Methods
Study Population
This was a prospective multicenter observational trial of consecutive patients 18 years or older who underwent ESD at 10 centers in the Unites States (n = 9) and Canada (n = 1) between April 2016 and April 2020. Patients scheduled to undergo ESD as part of their routine medical care were enrolled in the study. Patients with coagulopathy, contraindications to anesthesia or endoscopy, those who were pregnant, and those unable to provide informed consents were excluded. The study was approved by the Institutional Review Board for Human Research at each participating institution, with the University of Florida serving as the clinical coordinating center (ClinicalTrials.gov ID: NCT02989818). Signed procedure and research informed consent was obtained from all patients. All authors had access to the study data and reviewed and approved the final manuscript.
Endoscopic Submucosal Dissection Procedure
All cases were performed with intravenous moderate sedation, deep sedation, or general anesthesia with endotracheal intubation, at the discretion of the participating center. Carbon dioxide was used for insufflation in all cases. Lesions were examined under high-definition white light, near focus, and digital or dye-based chromoendoscopy. All lesions were categorized according to the Paris classification10 and their surface topography (granular or nongranular laterally spreading tumors), when applicable.11 Rectal lesions were defined as any lesion with an upper margin located within 18 cm of the anal verge and/or when >50% of the lesion was situated within 15 cm from the anal verge. ESD was completed as described previously (Figure 1).12–14 The degree of submucosal fibrosis was determined based on the findings identified at the time of ESD and classified as mild, moderate, or severe.15 Endoscopic closure of the ESD resection bed using clips and/or endoscopic suturing was performed at the discretion of the endoscopist.
Figure 1.
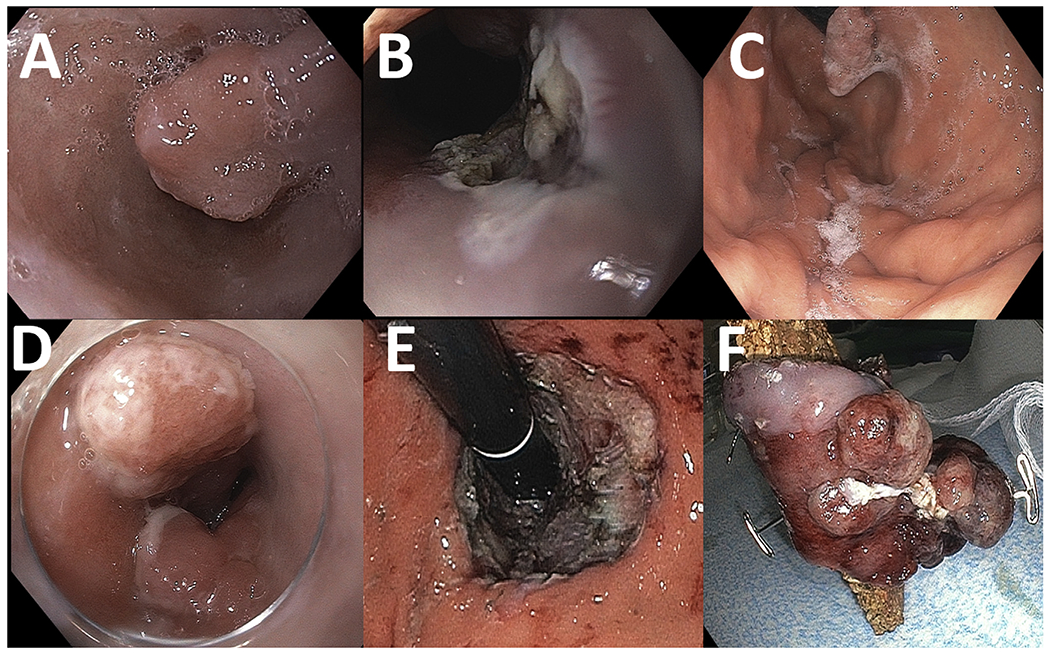
An 84-year-old patient with a 12-mm protruding lesion (Paris Is) in the background of Barrett’s esophagus is referred for endoscopy (A). The lesion is resected with EMR (B), with pathology confirming this to be a well-differentiated invasive adenocarcinoma with positive deep margins. The patient is deemed not a surgical candidate. He undergoes 4 sessions of cryotherapy and a second EMR due to recurrence of the nodule (C), with pathology demonstrating at least intramucosal adenocarcinoma with no lymphovascular invasion. The patient is subsequently referred for ESD. Two adjacent 10- to 15-mm nodules (Paris Is) were identified at the gastroesophageal junction on retroflexion (D). Successful circumferential ESD (E) with en bloc resection of a 60 × 30 mm tubular specimen (F). Pathology: Focal intramucosal adenocarcinoma with negative lateral and deep resection margins.
Histopathology Evaluation
All ESD resected tissue specimens were pinned down with needles onto cork after removal and fixed in 10% formalin solution. Histologic assessment was performed by experienced GI pathologists from each respective participating center according to the World Health Organization classification of colorectal neoplasia and the Vienna classification.16,17
Follow-Up
Surveillance endoscopy was performed to evaluate for recurrence generally within 3–6 months after the index treatment based on the European Society of Gastrointestinal Endoscopy guidelines on ESD.18 During surveillance, the ESD scar was carefully interrogated with both high-definition white light and narrow band imaging to assess for recurrence. Biopsies were performed from the scar and/or of any visible lesions when recurrence was suspected on endoscopic assessment.
Data Collection
Data were prospectively recorded and entered into a central electronic database specifically created for the purpose of the study (REDCap). Data collection was separated into the following categories: baseline, procedural, and post-procedural data. Baseline data included patient demographic characteristics, lesion characteristics (size, morphology, and location), history of interventions before ESD. Procedure-related data included procedure time, presence of submucosal fibrosis, completion of endoscopic resection, and size of the resected specimen. Post-procedural data included adverse events, resection outcomes, ESD specimen histopathology, and lesion recurrence on follow-up.
Measured Outcomes
The primary outcomes were the proportions of ESD cases with en bloc, R0, and curative resection. En bloc resection was defined as excision of the visible targeted lesion in a single specimen. Complete resection (R0) was defined as en bloc resection with lateral and deep margins free of neoplasia on histologic evaluation. Curative resection was defined as R0 resection with favorable histology. Histologic criteria that were considered favorable were well to moderate degree of differentiation, absence of lymphovascular invasion, and absence of deep invasion or tumor budding.12,18,19 The depth of invasion was defined based on location and cancer specifics. Resected specimens were considered curative if submucosal invasion was <500 μm below the muscularis mucosa for esophageal and gastric adenocarcinoma and <1000 μm for colon adenocarcinoma.12,18,19 For esophageal squamous cell carcinoma, invasion of the muscularis mucosa (m3) or submucosal invasion were not considered curative, given the higher risk of lymph node metastasis in these lesions.18 Recurrence was defined as histologic confirmation of the initial lesion on surveillance endoscopy after index R0 resection. Adverse events were categorized according to the American Society of Gastrointestinal Endoscopy consensus criteria.20
Statistical Analysis
Descriptive statistics for each baseline variable was obtained and expressed as mean ± SD and median (interquartile range [IQR]). Chi-square or Fisher exact test for categorical variables and the t test for continuous variables were performed when indicated. Nominal P values are reported; P values <.05 were considered significant. Multivariate logistic regressions were modeled to determine independent variables associated with failed en bloc, R0, curative resection and increase in adverse events using the backwards modeling approach. The independent variables in the analysis included those that were considered clinically relevant based on the reported literature.12,18 Each clinically relevant independent variable was analyzed to the dependent variable listed above and subsequently removed from the model if they were not clinically or statistically significant. All statistical analysis was performed with the open source statistical software package R, version 3.5.0.
Results
Study Population and Baseline Characteristics
A total of 692 patients (median age, 66 years; IQR, 57–73 years; 57.8% were men) underwent ESD by 12 endoscopists across 10 centers in the United States (n = 9) and Canada (n = 1) between April 2016 and April 2020 (Table 1). Median lesion size was 40 mm (IQR, 25–52 mm). The most common ESD site was in the colon (211 of 692 [30.5%]), followed by rectum (188 of 692 [27.2%]), esophagus (181 of 692 [26.2%]), stomach (101 of 692 [14.6%]), and duodenum (11 of 692 [1.6%]). Polyp morphology based on the Paris classification is summarized in Table 2. Macroscopically, 50.9% (203 of 399) and 30.3% (121 of 399) of the colorectal lesions were classified as granular and nongranular laterally spreading tumors, respectively. Most of the lesions (632 of 692 [91.3%]) had been manipulated before ESD: 82.4% (570 of 692) were biopsied with cold forceps, 18.2% (126 of 692) had prior endoscopic resection attempts, and 62 of 692 (8.9%) had been tattooed underneath.
Table 1.
Baseline Characteristics
| Characteristic | Data |
|---|---|
| Age, y, median (IQR) | 66 (57–73) |
|
| |
| Sex, men/women, n | 400/292 |
|
| |
| ASA grade; n (%) | |
| I | 72 (10.4) |
| II | 335 (48.4) |
| III | 269 (38.9) |
| IV | 16 (2.3) |
|
| |
| Lesion size, mm, median (IQR) | 40 (25–52) |
|
| |
| Lesion site | |
| Esophagus, n (%) | 181 (26.2) |
| Distal esophagus/GEJ, n | 121 |
| Esophageal body, n | 60 |
| Stomach, n (%) | 101 (14.6) |
| Antrum, n | 37 |
| Body, n | 30 |
| Lesser curvature, n | 14 |
| Fundus, n | 4 |
| Cardia, n | 16 |
| Duodenum, n (%) | 11 (1.5) |
| Colon, n (%) | 399 (57.7) |
| Ileocecal valve, n | 11 |
| Cecum, n | 51 |
| Ascending colon, n | 81 |
| Transverse colon, n | 22 |
| Descending colon, n | 14 |
| Sigmoid colon, n | 32 |
| Rectum, n | 188 |
|
| |
| Polyp morphology based on Paris Classification, n (%) | |
| Is | 132 (19.1) |
| Ip | 17 (2.5) |
| IIa | 268 (38.7) |
| IIb | 48 (6.9) |
| IIa+IIc | 66 (9.5) |
| IIa+Is | 32 (4.6) |
| Is+IIa | 24 (3.5) |
|
| |
| Gross morphology colorectal lesions (n = 399), n (%) | |
| Laterally spreading tumor, granular | |
| Uniform | 36 (9) |
| Mixed | 167 (41.9) |
| Laterally spreading tumor, nongranular | |
| Flat | 79 (19.8) |
| Pseudodepressed | 42 (10.5) |
|
| |
| Interventions before ESD, n (%) | |
| None | 61 (8.8) |
| Tattoo underneath lesion | 62 (8.9) |
| Cold biopsy forceps | 570 (82.4) |
| Hot biopsy forceps | 5 (0.72) |
| Snare | 51 (7.4) |
| Argon plasma coagulation | 23 (3.3) |
| Radiofrequency ablation | 46 (6.6) |
| Submucosal injection only | 4 (0.58) |
| Endoscopic resection attempt | 126 (18.2) |
ASA, American Society of Anesthesiologists physical status classification; GE gastroesophageal junction.
Table 2.
Endoscopic Submucosal Dissection Procedural Characteristics
| Characteristics | All sites (n = 692) |
Esophagus (n = 181) |
Stomach (n = 101) |
Duodenum (n = 11) |
Colon (n = 211) |
Rectum (n = 188) |
|---|---|---|---|---|---|---|
| Resected specimen size, mm, median (IQR) | 45 (33.5–60) | 45 (35–57.3) | 39 (30–50) | 30 (19.8–38.8) | 50 (30–60) | 46 (24–60) |
|
| ||||||
| Submucosal fibrosis, n (%) | 323 (46.7) | 70 (38.7) | 48 (47.5) | 3 (27.3) | 120 (56.9) | 93 (49.7) |
| Mild | 100 (31) | 27 (38.6) | 20 (41.8) | 0 (0) | 35 (29.2) | 28 (30.1) |
| Moderate | 81 (25) | 17 (24.3) | 14 (29.1) | 2 (66.7) | 26 (21.7) | 22 (23.7) |
| Severe | 142 (44) | 26 (37.1) | 14 (29.1) | 1 (33.3) | 59 (49.1) | 43 (46.2) |
|
| ||||||
| Procedure time, min, range | 77.5 (15–553) | 75 (20–318) | 70 (22–315) | 71.5 (38–309) | 80 (18–553) | 80 (15–480) |
|
| ||||||
| Type of submucosal lifting solution, n (%) | ||||||
| Voluven (Fresenius Kabi Norge A.S.) | 409 (59.1) | 98 (54.2) | 51 (50.5) | 7 (63.7) | 131 (62.1) | 121 (64.7) |
| Orise (Boston Scientific) | 36 (5.2) | 12 (6.6) | 8 (7.9) | 1 (9.1) | 6 (2.8) | 7 (3.7) |
| Eleview (Medtronic) | 19 (2.7) | 8 (4.4) | 4 (4) | 0 | 3 (1.4) | 3 (1.6) |
| Gonak (Akorn) | 11 (1.6) | 6 (3.3) | 4 (4) | 0 | 0 | 1 (0.5) |
| Hespan (Braun Medical) | 19 (2.7) | 8 (4.4) | 4 (4) | 2 (18.2) | 0 | 3 (1.6) |
| Normal saline | 112 (16.2) | 27 (14.9) | 21 (20.8) | 1 (9.0) | 44 (20.9) | 19 (10.2) |
| Combination of saline and viscous solution | 86 (12.5) | 22 (12.2) | 9 (8.9) | 0 | 27 (12.8) | 33 (17.6) |
|
| ||||||
| Type of ESD electrocautery knife, n (%) | ||||||
| Dual knife (Olympus America) | 476 (68.8) | 126 (69.6) | 72 (71.3) | 7 (63.6) | 145 (68.7) | 125 (66.8) |
| IT knife (Olympus America) | 214 (30.9) | 90 (49.7) | 56 (55.4) | 1 (9.0) | 13 (6.2) | 53 (28.3) |
| Hybrid knife (ERBE) | 179 (25.9) | 44 (24.3) | 30 (29.7) | 1 (9.0) | 53 (25.1) | 51 (27.3) |
| Combination/other | 129 (18.6) | 27 (14.9) | 13 (12.9) | 9 (81.8) | 46 (21.8) | 61 (32.6) |
|
| ||||||
| Endoscopic retraction with clip/string/snare, n (%) | 53 (7.7) | 10 (5.5) | 13 (12.8) | 1 (9) | 23 (10.9) | 6 (3.2) |
|
| ||||||
| Elective closure, n (%) | 169 (24.4) | 14 (7.7) | 31 (30.7) | 8 (72.7) | 72 (34.1) | 45 (24.1) |
| Standard clips | 101 (59.8) | 12 (85.7) | 8 (25.8) | 1 (9.3) | 64 (88.9) | 16 (35.6) |
| Endoscopic suturing | 68 (40.2) | 2 (14.3) | 23 (74.2) | 7 (90.7) | 8 (11.1) | 29 (64.4) |
|
| ||||||
| Outpatient setting, n (%) | 463 (66.9) | 107 (59.1) | 67 (66.3) | 9 (81.8) | 150 (71.1) | 130 (69.5) |
|
| ||||||
| Hospital length of stay, d, mean ± SD | 1.33 ± 0.89 | 1.24 ± 0.63 | 1.57 ± 1.16 | 1 | 1.47 ± 1.1 | 1.13 ± 0.44 |
Procedural Characteristics
Procedural characteristics are summarized in Table 2. Median lesion and resected specimen sizes were 40 mm (IQR, 25–52 mm) and 45 mm (IQR, 33.5–60 mm), respectively. Submucosal fibrosis was encountered in nearly one-half of the cases (323 of 692 [46.7%]), of which 142 of 323 (44%) were classified as severe fibrosis, followed by mild (100 of 323 [31%]) and moderate (81 of 323 [25%]). Median procedure time was 77.5 minutes (range, 15–553 minutes). The most commonly used submucosal lifting solution was 6% hydroxyethyl starch (Voluven; Frsenius Kabi Norge A.S., Halden, Norway and Hespan; Braun Medical, Bethlehem, PA) (428 of 692 [61.8%]), whereas normal saline was used in 112 of 692 (16.2%) of the cases. Overall, the Dual-type knives (Olympus America, Center Valley, PA) was the most often used ESD knife (476 of 692 [68.8%]), followed by the IT-type knives (Olympus America, Center Valley, PA) (214 of 692 [30.9%]) and hybrid knife (ERBE USA, Marietta, GA) (179 of 692 [25.9%]). Traction and counter-traction during ESD was performed using string/clip/snare in 7.7% (53 of 692) of the cases. Elective closure of the ESD resection bed was performed in 169 of 692 cases (24.4%) using either standard clips (101 of 169 [59.8%]) or endoscopic suturing (68 of 169 [40.2%]). A total of 463 of 692 cases (66.9%) were performed in the outpatient setting and patients were discharged on the same day of the procedure. For the 229 patients (33.1%) who were admitted post procedure, mean ± SD hospital length of stay was 1.33 ± 0.89 days.
Endoscopic Submucosal Dissection Resection Outcomes and Adverse Events
Overall, en bloc and R0 resection were achieved in 91.5% (633 of 692) and 84.2% (583 of 692) of the cases, respectively (Table 3). R1 resection (n = 109) was due to positive lateral resection margins in 41.3% (45 of 109), positive deep resection margins in 22% (24 of 109), or both in 13.8% (15 of 109). Curative resection was attained in 542 of 692 of the cases (78.3%) and ranged from 71.3% in the esophagus to 83.9% in the colon.
Table 3.
Endoscopic Submucosal Dissection Resection Outcomes
| Outcomes | All sites (n = 692) |
Esophagus (n = 181) |
Stomach (n = 101) |
Duodenum (n = 11) |
Colon (n = 211) |
Rectum (n = 188) |
|---|---|---|---|---|---|---|
| Resection outcomes | ||||||
| En bloc resection, n (%) | 633 (91.5) | 175 (96.7) | 99 (98) | 10 (91) | 181 (85.8) | 167 (88.8) |
| R0 resection, n (%) | 583 (84.2) | 156 (86.2) | 83 (82.2) | 8 (72.7) | 176 (83.4) | 161 (85.6) |
| R1 resection, n | 109 | 25 | 18 | 3 | 35 | 27 |
| Positive lateral resection margins, n (%) | 45 (41.3) | 5 (20) | 5 (27.8) | 1 (33.3) | 20 (57.1) | 14 (51.9) |
| Positive deep resection margins, n (%) | 24 (22) | 8 (32) | 9 (50) | 2 (66.7) | 0 | 5 (18.5) |
| Positive lateral and deep resection margins, n (%) | 15 (13.8) | 8 (32) | 3 (16.7) | 0 | 1 (2.9) | 3 (11.1) |
| Not specified, n (%) | 25 (22.9) | 4 (16) | 1 (5.5) | 0 | 14 (40) | 5 (18.5) |
| Curative resection, n (%) | 542 (78.3) | 129 (71.3) | 78 (77.2) | 8 (72.7) | 177 (83.9) | 150 (79.8) |
|
| ||||||
| Adverse events, n (%) | 70 (10.1) | 36 (19.9) | 4 (4) | 1 (9.1) | 18 (8.5) | 11 (5.9) |
| Severity | ||||||
| Mild | 35 (50) | — | — | — | — | — |
| Moderate | 28 (40) | — | — | — | — | — |
| Severe | 7 (10) | — | — | — | — | — |
| Type of adverse event | ||||||
| Delayed bleeding | 16 (2.3) | 3 (1.7) | 3 (3) | 1 (9.1) | 4 (1.9) | 5 (2.7) |
| Perforation | 20 (2.9) | 4 (2.2) | 1 (1) | 0 | 10 (4.7) | 5 (2.7) |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Anesthesia related | 0 | 0 | 0 | 0 | 0 | 0 |
| Stricture formation | 26 (3.8) | 26 (14.4) | 0 | 0 | 0 | 0 |
| Pleural effusions | 3 (0.43) | 2 (1.1) | 0 | 0 | 0 | 1 (0.5) |
| Odynophagia | 1 (0.14) | 1 (0.6) | 0 | 0 | 0 | 0 |
| Post-polypectomy syndrome | 1 (0.14) | 0 | 0 | 0 | 1 (0.5) | 0 |
| Abdominal pain | 3 (0.43) | 0 | 0 | 0 | 3 (1.4) | 0 |
Adverse events were reported in 70 of the 692 cases (10.1%), of which most were either mild (35 of 70 [50%]) or moderate (28 of 70 [40%]) in severity (Table 3). Case by case details on patients with severe adverse events (n = 10) is summarized in Supplementary Table 1. In aggregate, delayed bleeding occurred in 2.3% (16 of 692) of the patients. Of these 16 patients with delayed bleeding, 14 underwent endoscopic therapy, of which 3 also received blood transfusions. Two of the 16 patients with delayed bleeding were managed expectantly. Perforation was reported in 2.9% (20 of 692) of the cases. All cases of perforation were successfully closed endoscopically, except for 1 patient who presented with delayed perforation due to ischemic necrosis after ESD in a lesion in the ascending colon. The patient underwent right hemicolectomy with full recovery. In aggregate, the most commonly reported adverse event was stricture formation (26 of 692 [3.8%]). Stricture developed only after esophageal ESD, and accounted for 14.4% (26 of 181) of the esophageal cases. Of these, 76.9% (20 of 26) were symptomatic and required serial endoscopic dilations. One patient developed severe abdominal pain after colon ESD for a lesion in the cecum and was diagnosed with post-polypectomy syndrome in the absence of perforation on diagnostic laparoscopy.
Histopathology and Follow-up
Final ESD specimen histopathology is summarized in Table 4. Follow-up was available in 532 of the 692 patients (76.9%). The mean number of surveillance procedures was 1.8 (range, 1–9) over a mean follow-up of 13.3 months (range, 1–60 months). In aggregate, lesion recurrence was identified in 5.8% (31 of 532) of the cases: 8.9% (13 of 145) in the esophagus, 11.1% (9 of 81) in the stomach, 10% (1 of 10) in the duodenum, and 2.7% (8 of 296) in the colorectum. Histopathology, additional treatment, and outcomes of these patients are summarized in Supplementary Table 2.
Table 4.
Endoscopic Submucosal Dissection Histopathology
| ESD histopathology | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Esophagus | n | Stomach | N | Duodenum | n | Colon | N | Rectum | n |
| Nondysplastic esophageal mucosa | 2 | Nondysplastic gastric mucosa | 7 | Gastrinoma | 1 | Nondysplastic colon mucosa | 7 | Nondysplastic colon mucosa | 1 |
| Nondysplastic BE | 8 | Hyperplastic | 9 | Lipoma | 1 | Inflammatory polyp | 1 | Adenoma with LGD | 71 |
| BE with LGD | 5 | Pancreatic heterotopia | 1 | Adenoma with LGD | 5 | Adenoma with LGD | 102 | Adenoma with HGD | 47 |
| BE with HGD | 32 | Adenoma with LGD | 18 | Carcinoid | 4 | Adenoma with HGD | 59 | Intramucosal adenocarcinoma | 17 |
| Intramucosal adenocarcinoma | 59 | Adenoma with HGD | 12 | Intramucosal adenocarcinoma | 6 | Invasive adenocarcinoma | 20 | ||
| Invasive adenocarcinoma | 61 | Intramucosal adenocarcinoma | 23 | Invasive adenocarcinoma | 7 | Sessile serrated adenoma | 5 | ||
| Intramucosal SCC | 1 | Invasive adenocarcinoma | 16 | Sessile serrated adenoma | 22 | Condyloma | 1 | ||
| Invasive SCC | 2 | Carcinoid | 13 | Hyperplastic polyp | 14 | Lipoma | 1 | ||
| Leiomyoma | 1 | Leiomyoma | 1 | ||||||
| Granular cell tumor | 3 | Metastatic lung adenocarcinoma | 1 | ||||||
| Melanoma | 1 | ||||||||
BE, Barrett’s esophagus; HGD, high-grade dysplasia; LGD, low-grade dysplasia; SCC, squamous cell cancer.
Predictors of Resection Outcomes and Adverse Events
A multivariate logistic regression was performed to identify predictors of failed en bloc, R0, curative resection, and adverse events (Table 5). We included age, sex, lesion characteristics (size, morphology, and location), prior EMR attempt, tattoo at the ESD site, presence of submucosal fibrosis, and pre-ESD histology of invasive cancer as covariates. Colorectal ESD (as opposed to ESD in the esophagus, stomach, or duodenum) was a strong predictor for failed en bloc resection (odds ratio [OR], 8.03; 95% confidence interval [CI], 1.54–41.83; P = .01). Severe submucosal fibrosis was a predictor of failed en bloc (OR, 3.22; 95% CI, 1.09–9.53; P = .04), failed R0 (OR, 4.30; 95% CI, 2.20–8.41; P < .001), failed curative resection (OR, 2.59; 95% CI, 3.62-16.47; P < .001), and positively associated with a higher risk for adverse events (OR, 2.05; 95% CI, 1.00-4.19; P = .05). ESD on lesions with a depressed component on morphology (Paris IIc or Paris III) were associated with a higher likelihood for failed curative resection (OR, 1.91; 95% CI, 1.00–3.64; P = .05). Patient characteristics (age and sex), lesion size ≥40 mm (yes vs no), prior EMR or tattoo were not associated with any of the resection outcomes on multivariate logistic regression (Table 5).
Table 5.
Predictors of Failed En Bloc, R0, Curative Resection, and Adverse Events
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Predictors of failed en bloc resection | |||
| Age | 1.03 | 0.99–1.07 | .07 |
| Sex | 0.78 | 0.36–1.67 | .52 |
| Lesion size ≥40 mm (yes vs no) | 0.88 | 0.59–1.31 | .54 |
| Paris Classification IIc or III component (yes vs no) | 0.88 | 0.19–4.04 | .87 |
| Morphology (LST-G vs LST-NG) | 0.81 | 0.25–2.58 | .72 |
| Type of ESD | |||
| Colon | 8.03 | 1.54–41.83 | .01 |
| Esophagus | 1 | — | — |
| Stomach | 1 | — | — |
| Duodenum | 1 | — | — |
|
| |||
| Prior EMR attempt (yes vs no) | 2.28 | 0.43–11.93 | .92 |
|
| |||
| Tattoo at ESD site (yes vs no) | 0.82 | 0.20–3.3 | .78 |
|
| |||
| Submucosal fibrosis | |||
| Mild | 0.74 | 0.14–3.85 | .73 |
| Moderate | 1.03 | 0.20–5.34 | .97 |
| Severe | 3.22 | 1.09–9.53 | .04 |
|
| |||
| Predictors of failed R0 resection | |||
| Age | 0.99 | 0.96–1.01 | .47 |
| Sex | 1.58 | 0.92–2.72 | .09 |
| Lesion size ≥40 mm (yes vs no) | 0.80 | 0.49–1.28 | .35 |
| Paris Classification IIc or III component (yes vs no) | 1.10 | 0.54–2.24 | .81 |
| Morphology (LST-G vs LST-NG) | 0.98 | 0.42–2.28 | .97 |
| Type of ESD | |||
| Colon | 0.79 | 0.08–7.65 | .84 |
| Esophagus | 0.55 | 0.55–5.42 | .61 |
| Stomach | 1.17 | 0.12–11.16 | .89 |
| Duodenum | 1 | — | — |
| Prior EMR attempt (yes vs no) | 0.59 | 0.18–1.93 | .14 |
| Tattoo at ESD site (yes vs no) | 0.96 | 0.35–2.66 | .94 |
| Submucosal fibrosis | |||
| Mild | 0.68 | 0.22–2.10 | .51 |
| Moderate | 0.68 | 0.22–2.01 | .50 |
| Severe | 4.30 | 2.20–8.41 | <.001 |
|
| |||
| Predictors of failed curative resection | |||
| Age | 0.98 | 0.95–1.00 | .15 |
| Sex | 1.24 | 0.58–2.65 | .57 |
| Lesion size ≥40 mm (yes vs no) | 0.59 | 0.35–1.01 | .06 |
| Paris Classification IIc or III component (yes vs no) | 1.91 | 1.00–3.64 | .05 |
| Morphology (LST-G vs LST-NG) | 0.71 | 0.32–1.58 | .40 |
| Type of ESD | |||
| Colon | 1.13 | 0.12–10.69 | .91 |
| Esophagus | 1.83 | 0.21–15.96 | .58 |
| Stomach | 1.37 | 0.15–12.10 | .78 |
| Duodenum | 1 | — | — |
| Prior EMR attempt (yes vs no) | 0.30 | 0.88–1.04 | .06 |
| Tattoo at ESD site (yes vs no) | 0.24 | 0.50–1.1 | .07 |
| Submucosal fibrosis | |||
| Mild | 1.20 | 0.54–2.67 | .66 |
| Moderate | 1.62 | 0.72–3.68 | .25 |
| Severe | 2.59 | 1.39–4.85 | .003 |
|
| |||
| Predictors of adverse events | |||
| Age | 1.01 | 0.85–1.33 | .62 |
| Sex | 0.91 | 0.45–4.88 | .44 |
| Lesion size ≥40 mm (yes vs no) | 1.21 | 0.82–1.79 | .34 |
| Paris Classification IIc or III component (yes vs no) | 0.89 | 0.36–2.19 | .81 |
| Morphology (LST-G vs LST-NG) | 0.77 | 0.33–1.82 | .56 |
| Type of ESD | |||
| Colon | 0.28 | 0.22–3.42 | .32 |
| Esophagus | 4.38 | 0.48–39.5 | .19 |
| Stomach | 1.17 | 0.12–11.16 | .89 |
| Duodenum | 1 | — | — |
| Submucosal fibrosis | |||
| Mild | 0.87 | 0.32–2.34 | .78 |
| Moderate | 2.23 | 0.95–5.26 | .06 |
| Severe | 2.05 | 1–4.19 | .05 |
LST-G, laterally spreading tumor-granular; LST-NG, laterally spreading tumor-nongranular.
Discussion
ESD is an established technique with a defined role within the spectrum of available therapies for dysplastic and early cancer lesions of the GI tract. Around the world, the acceptance of ESD as a primary modality for therapy of selected lesions has been facilitated by its well-documented advantages and its role has been further supported by recent professional society guidelines from Asia and Europe.12,17 At the same time, adoption of ESD in North America has lagged, and even the latest 2020 US Multi-Society Task Force guideline on endoscopic removal of colorectal lesions does not clearly endorse the utility of ESD.20 Multiple factors have contributed to that, but perhaps most importantly, it has been the lack of higher-quality studies documenting outcomes of ESD in North America.
In this large prospective multicenter study, we report the outcomes of ESD when performed as part of routine patient care across various centers in the United States and Canada. The overall proportion of en bloc, R0, and curative resections with ESD for lesions throughout the GI tract were 91.5%, 84.2%, and 78.3%, respectively, which compare favorably with current established quality benchmarks for ESD.21,22 Indeed, our results are in line with the recently proposed thresholds for ESD training outlined by the 2019 European Society of Gastrointestinal Endoscopy, which set the goal for en bloc resection at >90%, R0 resection at >80% and for curative resection at >75%.21 In addition, we also identified factors associated with poor ESD outcomes. Both tattooing underneath a lesion and prior incomplete endoscopic resection have been shown to cause submucosal fibrosis and complicate subsequent resection attempts.23,24 Similarly, in this study, submucosal fibrosis was commonly identified in patients with a tattoo underneath the lesion (51 of 62 [82.3%]) and in those with prior incomplete EMR attempts (91 of 99 [92%]). On multivariate analysis, presence of severe submucosal fibrosis was the strongest predictor for failed ESD; further highlighting the detrimental consequences of both of these actions before definitive endoscopic resection. Conversely, neither lesion size nor morphology was shown to impact ESD resection outcomes.
The R1 resection rate was relatively low in our study (15.8%). Noteworthy, on further analysis, most of these cases of incomplete resection were due to positive lateral margins (41.3%). These findings have important clinical implications. Our data reiterate the importance of careful lesion assessment and characterization before resection in order to improve resection outcomes and provides a target for future educational efforts for North American endoscopists performing ESD. Furthermore, we should also highlight that many patients in this study underwent ESD because they were not good operative candidates, even if curative resection was deemed unlikely based on preoperative criteria. We did not specifically capture the proportion of patients that underwent ESD as a palliative measure as part of this study and therefore cannot directly evaluate the impact of their inclusion on the overall R0 and curative resection reported in this study. Future studies are needed to evaluate the role of ESD in these poor surgical candidates and the long-term clinical outcomes of nonhistologically complete endoscopic resection.
As highlighted previously, the role of ESD has been heavily investigated, with the bulk of the studies coming from Japan. Results from this study not only assure that the high technical standards set by our Japanese counterparts can be achieved in North America, but also expand in areas where very few Japanese data are available. Management of Barrett’s-associated neoplasia continues to evolve, and our current study provides additional support to the emerging preliminary data on the high technical success rate and safety of ESD in the esophagus.25–28 Although stricture formation after esophageal ESD remains an ongoing concern, all cases in our series responded adequately to endoscopic therapy. Importantly, it should be emphasized that many of these patients who underwent ESD had already exhausted other currently available standard therapies, including radiofrequency ablation, cryoablation, and EMR. In many cases, ESD may have been the only remaining option available due to the high-risk patient characteristics precluding esophageal surgery (66.8% of the esophageal ESD patients were either American Society of Anesthesiologists physical status classification III or IV).
Our study provides additional data on the current status of colorectal ESD in North America. A previous systematic review and meta-analysis on ESD for colorectal neoplasia demonstrated significant differences between non-Asian and Asian countries with regard to en bloc resection rate (81.2% vs 93%, respectively) and R0 resection rate (71.3% vs 85.6%).29 In contrast, colorectal ESD outcome measures in our series (en bloc and R0 resection rates of 88% and 85%, respectively) were more similar to the target benchmarks attained at Asian countries.29 These results may be indicative of both an increase in ESD training opportunities over recent years and the uptake of ESD at various centers across North America.8,30
Nonetheless, ESD is a technically challenging procedure and risks for serious adverse events have slowed its adoption in North America. In this large multicenter prospective study, ESD was associated with an adequate safety profile. Importantly, the serious adverse event rate in our series was low, and only 1 patient of 692 (0.14%) required surgery. Yet, ESD remains an effort-intensive procedure with higher complication rates compared with EMR, which brings legitimate concerns about the impact on health care cost. Worth noting is that most ESD cases in this North American study were safely performed in the outpatient setting, with only one-third requiring post-procedural hospital observation with a mean ± SD length of stay of 1.33 ± 0.89 days. As opposed to Asia, where patients are routinely admitted to the hospital after ESD for observation, our data suggest that this practice may not be required in North America; possibly curtailing health care costs. As reported previously, selective ESD, particularly for lesions highly suspicious for containing submucosal invasive cancer, remains a cost-effective strategy.31
There are several strengths to our study. This is the largest prospective study on ESD outcomes in North America. The external validity of our observations is augmented by including patients from multiple centers, incorporating lesions from throughout the GI tract, and enrolling patients as part of their routine care with very few exclusion criteria. Furthermore, the study outcomes include all defined quality indicators for ESD.21,22 Lastly, surveillance endoscopies were performed in the majority of patients after their index ESD procedure during a mean follow-up of 13.3 months. This, in turn, allowed us to determine the proportion of patients with recurrence after R0 resection (5.8%), which is in line with the pooled recurrence rate (5.2%; 95% CI, 3.3%–8.1%) reported among non-Asian countries in a recent systematic review and meta-analysis.29
Our study is not without limitations. A priori criteria for ESD were not defined in this observational prospective study, and therefore our results are subject to selection bias. In spite of the favorable ESD outcomes achieved in this study, many lesions could have been adequately treated with EMR techniques based on the retrospective assessment of the final ESD histology. Unfortunately, in real life, significant uncertainty exists regarding the most likely final pathology of a lesion in spite of our best efforts in performing extensive pre-procedure evaluation. Therefore, it is unbefitting to apply the results of the final ESD histology and stipulate that EMR may have been adequate to start with. A recent systematic review by Fuccio et al32 demonstrated that most ESD cases are indeed performed for lesions that could have potentially been removed by other means based on histology, which is consistent with our data. These findings support the need for a better preprocedure evaluation that can predict, with a higher degree of certainty, the nature of the lesion and thereby the most appropriate treatment strategy. Some Western data on preprocedure risk stratification for covert invasive cancer in the colorectum provide a very useful framework, yet we need to refine our approach.33 The primary aim of this study was to report on the outcomes of ESD when performed as part of routine patient care in order to incorporate a broad diverse patient group and thereby provide “real world” data. Therefore, it is important to recognize that observational studies of novel procedures with no narrow inclusion criteria and structured follow-up, such as ours, tend to overestimate success and minimize risks. In addition, we also acknowledge that histopathologic assessment of the ESD specimens were performed by GI pathologists at each participating center rather than at a central coordinating center, potentially introducing heterogeneity in the histopathologic interpretation of the specimens. Nonetheless, this issue is, to some extent, mitigated by the fact that all participating institutions were either academic or high-volume ESD centers with GI pathologists experienced in the evaluation of ESD colorectal specimens. We should further highlight that a stepwise regression model with backward elimination was chosen for the multivariate analysis, as starting with a full model allowed us to simultaneously consider the effects of all of the potential variables associated with ESD resection outcomes. Nonetheless, this statistical model is not without its limitations, which includes potential bias in parameter estimation, given the degree of freedom for analysis. These data should be interpreted with caution. Lastly, we recognize that, despite the high follow-up rate, the relatively short follow-up prohibits significant conclusions on longer-term outcomes. Additional studies with longer systematic follow-up are needed to expand on our preliminary findings and evaluate the long-term recurrence rates, and both cancer-specific and all-cause mortality.
In summary, in this North American experience, we demonstrated that ESD can be performed safely and effectively, and is associated with a low recurrence rate. The resection outcomes in this study achieved adequate levels of performance based on current established consensus quality parameters and further support the implementation of ESD for the management of select gastrointestinal neoplasms.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Endoscopic submucosal dissection is a well-established technique in Asia for the management of select gastrointestinal neoplasms. There are limited data on the performance of endoscopic submucosal dissection in the United States and Canada.
NEW FINDINGS
Endoscopic submucosal dissection can be performed safely, effectively, and is associated with a low recurrence rate.
LIMITATIONS
This was an observational study evaluating endoscopic submucosal dissection technical outcomes as part of routine patient care with variable clinical follow-up.
IMPACT
Resection outcomes in this study are in line with established consensus endoscopic submucosal dissection quality benchmarks and support the implementation of endoscopic submucosal dissection in North America for the management of selected gastrointestinal neoplasms.
Abbreviations used in this paper:
- CI
confidence interval
- EMR
endoscopic mucosal resection
- ESD
endoscopic submucosal dissection
- GI
gastrointestinal
- IQR
interquartile range
- OR
odds ratio
Footnotes
CRediT Authorship Contributions
Peter V. Draganov, MD (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Hiroyuki Aihara, MD (Data curation: Equal; Writing – review & editing: Supporting).
Michael S. Karasik, MD (Data curation: Equal; Writing – review & editing: Supporting).
Saowanee Ngamruengphong, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Abdul A. Aadam, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Mohamed O. Othman, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Neil Sharma, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Ian S. Grimm, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Alaa Rostom, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Joseph B. Elmunzer, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Salmaan A. Jawaid, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Donevan Westerveld, MD (Data curation: Supporting; Formal analysis: Lead; Writing – review & editing: Supporting).
Yaseen B. Perbtani, MD (Data curation: Lead).
Brenda J. Hoffman, MD (Data curation: Supporting; Writing – review & editing: Supporting).
Conflicts of interest
Peter V. Draganov is a consultant for Boston Scientific, Olympus America, Cook Medical, Microtech, Steris, Merit, Fujifilm, and Lumendi. Dennis Yang is a consultant for Boston Scientific, Lumendi, and Steris Endoscopy. Saowanee Ngamruengphong is a consultant for Boston Scientific. B. Joseph Elmunzeis a consultant for Takeda Pharmaceuticals. Hiroyuki Aihara is a consultant for Boston Scientific, Olympus America, Fujifilm, Medtronic, Auris Health, Lumendi, and 3-D Matrix. Neil Sharma is a consultant for Boston Scientific, Steris, Mauna Kea, and Medtronic and serves on the advisory board for Endoscopynow. Ian S. Grimm is a consultant for Boston Scientific. Abdul Aziz Aadam is a consultant for Boston Scientific and Steris Endoscopy. Mohamed O. Othman is a consultant for Abbvie, Olympus, Lumendi, ConMed, and Boston Scientific. Alexander Schlachterman is a consultant for Lumendi, ConMed, and Medtronics. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2021.02.036.
References
- 1.Draganov PV, Wang AY, Othman MO, et al. AGA Institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16–25. [DOI] [PubMed] [Google Scholar]
- 2.Peery AF, Shaheen NJ, Cools KS, et al. Morbidity and mortality after surgery for nonmalignant colorectal polyps. Gastrointest Endosc 2018;87:243–250.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law R, Das A, Gregory D, et al. Endoscopic resection is cost-effective compared with laparoscopic resection in the management of complex colon polyps: an economic analysis. Gastrointest Endosc 2016;83:1248–1257. [DOI] [PubMed] [Google Scholar]
- 4.Jayanna M, Burgess NG, Singh R, et al. Cost analysis of laterally spreading colorectal lesions. Clin Gastroenterol Hepatol 2016;14:271–278. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Teriaky A, Sheh S, et al. Morbidity and mortality after surgery for nonmalignant colorectal polyps: a 10-year national analysis. Am J Gastroenterol 2019;114:1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang H, de Vos TotNederveen Cappel WH, van der Zwaan SMS, et al. Quality of life and fear of cancer recurrence in T1 colorectal cancer patients treated with endoscopic or surgical tumor resection. Gastrointest Endosc 2019;89:533–544. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura F, Saito Y, Haruyama S, et al. Short-term prospective questionnaire study of early postoperative quality of life after colorectal endoscopic submucosal dissection. Dig Dis Sci 2017;62:3325–3335. [DOI] [PubMed] [Google Scholar]
- 8.Kotzev AI, Yang D, Draganov PV. How to master endoscopic submucosal dissection in the USA. Dig Endosc 2019;31:94–100. [DOI] [PubMed] [Google Scholar]
- 9.Yang D, Wagh MS, Draganov PV. The status of training in new technologies in advanced endoscopy: from defining competence to credentialing and privileging. Gastrointest Endosc 2020;92:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Paris Endoscopic Classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(Suppl):S3–S43. [DOI] [PubMed] [Google Scholar]
- 11.Kudo S, Lamber R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008;68(Suppl):S3–S47. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015;27:417–434. [DOI] [PubMed] [Google Scholar]
- 13.Takezawa T, Hayashi Y, Shinozaki S, et al. The pocket-creation method facilitates colonic endoscopic submucosal dissection (with video). Gastrointest Endosc 2019;89:1045–1053. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Coman RM, Kahaleh M, et al. Endoscopic submucosal dissection for Barrett’s early neoplasia: a multicenter study in the United States. Gastrointest Endosc 2017;86:600–607. [DOI] [PubMed] [Google Scholar]
- 15.Kim EK, Han DS, Ro Y, et al. The submucosal fibrosis: what does it mean for colorectal endoscopic submucosal dissection? Intest Res 2016;14:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. WHO classification of tumours of the digestive system. J Clin Ultrasound 2014;00:1–6. [Google Scholar]
- 17.Schlemper RJ, Riddel RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829–854. [DOI] [PubMed] [Google Scholar]
- 19.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3–15. [DOI] [PubMed] [Google Scholar]
- 20.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446–454. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions: recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2020;115:435–464. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel-Nunes P, Pioche M, Albeniz E, et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2019;51:980–992. [DOI] [PubMed] [Google Scholar]
- 23.Grimm IS, McGill SK. Look but don’t touch: what not to do in managing large colorectal polyps. Gastrointest Endosc 2019;89:479–481. [DOI] [PubMed] [Google Scholar]
- 24.Im HG, Thosani N, Banerjee S, et al. Effect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesions. Gastrointest Endosc 2015;81:204–213. [DOI] [PubMed] [Google Scholar]
- 25.Fuccio L, Bhandari P, Maselli R, et al. Ten quality indicators for endoscopic submucosal dissection: what should be monitored and reported to improve quality. Ann Transl Med 2018;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Othman M, Draganov PV. Endoscopic mucosal resection vs endoscopic submucosal dissection for Barrett’s esophagus and colorectal neoplasia. Clin Gastroenterol Hepatol 2019;17:1019–1028. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Draganov PV. Expanding role of third space endoscopy in the management of esophageal diseases. Curr Treat Options Gastroenterol 2018;16:41–57. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018;87:1383–1393. [DOI] [PubMed] [Google Scholar]
- 29.Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74–86. [DOI] [PubMed] [Google Scholar]
- 30.McCarty TR, Aihara H. Current state of education and training for endoscopic submucosal dissection: translating strategy and success to the USA. Dig Endosc 2020;32:851–860. [DOI] [PubMed] [Google Scholar]
- 31.Bahin FF, Heitman SJ, Rasouli KN, et al. Wide-field endoscopic mucosal resection versus endoscopic submucosal dissection for laterally spreading colorectal lesions: a cost-effective analysis. Gut 2018;67:1965–1973. [DOI] [PubMed] [Google Scholar]
- 32.Fuccio L, Repicci A, Hassan C, et al. Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection. Gut 2018;67:1464–1474. [DOI] [PubMed] [Google Scholar]
- 33.Burgess NG, Hourigan LF, Zanati SA, et al. Risk stratification for covert invasive cancer among patients referred for colonic endoscopic mucosal resection: a large multicenter cohort. Gastroenterology 2017;153:732–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


