Abstract
Pullorum disease caused by Salmonella Pullorum remains an important disease for the poultry industry due to high morbidity and mortality in many countries. Phage therapy is becoming an alternative strategy to control multidrug-resistant Salmonella infections in young chicks. However, how bacteriophages affect the growth performance of chicks infected with S. Pullorum remains poorly understood. Herein, we assessed the therapeutic efficacy of Salmonella phage CKT1 against hypervirulent arthritis-causing S. Pullorum. The results showed that single phage treatment after hypervirulent S. Pullorum infection significantly improved body weight loss of chicks. Compared with enlarged liver and spleen in only Salmonella challenged group, phage administration substantially reduced the liver/body and spleen/body weight ratios, bacterial loads in organs and the degree of hepatic sinusoidal dilatation and congestion. Moreover, phage CKT1 can enter the organs of chicks and stay for at least 3 d in liver and spleen, and promote higher serum levels of IL-6 production within 6 d postinfection, indicating phage-induced bacterial lysis may be involved in inflammatory immune response to S. Pullorum infection. Analysis of the microbiome of gastrointestinal tract of chicks demonstrated that Salmonella challenge significantly reduced the relative abundances of Lachnoclostridium and Blautia, resulting in remarkably increased Escherichia-Shigella and Klebsiella becoming the predominant bacterial taxa. In contrast, the use of phage CKT1 significantly reduced Escherichia-Shigella and Klebsiella populations in intestine, permitting the proliferation of beneficial microbiota in Firmicutes including Lachnoclostridium, Ruminococcus, Lactobacillus, and Pseudoflavonifractor. In addition, phage alone treatments did not affect the normal gut microbiota structure of chicks, and phage therapy on Salmonella infected chicks increased bacteria species richness in the cecum. These results suggest that Salmonella phage CKT1 could improve growth performance of chicks challenged with S. Pullorum by normalizing the abnormal intestinal microbiome.
Key words: Salmonella Pullorum, bacteriophage, chick, growth performance, intestinal microbiome
INTRODUCTION
Poultry, the largest livestock population in the world, represents important sources of food and income in the world in general and in China in particular. However, many bacterial diseases (Salmonellosis, Colibacillosis, Mycoplasmosis, etc.) comprise approximately half of the non–outbreak-related mortality in broiler breeders and commercial layers, causing high economic losses in poultry production (Thøfner and Christensen, 2021). Salmonellosis particularly pullorum disease (PD) and fowl typhoid are the most common diseases in poultry (Barrow and Freitas Neto, 2011). There are more than 2,600 serovars of Salmonella enterica; Salmonella enterica serovar Gallinarum biovar Pullorum (S. Pullorum), the causative agent of pullorum disease, could result in a high mortality rate among embryos and chicks, as well as weakness and white diarrhea in many developing countries (Barrow and Freitas Neto, 2011). Moreover, arthritis associated with S. Pullorum infection has become increasingly frequent in chicken flocks, especially in Chinese native chicken breeds (Guo et al., 2019). The hypervirulent arthritis-causing S. Pullorum isolated from Chinese native Qingjiaoma chicken breeds had greater negative effect on growth performance of chicks than white diarrhea-causing strain (Li et al., 2021). Due to the increased complexity and difficulty of salmonellosis control, new ways should be explored to reduce Salmonella contamination besides extensive testing and eradication programs in poultry farms.
For the increasing risk of antibiotic-resistant bacteria to public health, many countries have imposed a ban on the use of antibiotics as growth promoters in food-producing animals, resulting in more severe infections and great economic loss (Cui et al., 2021). Phage therapy, the use of bacteriophages for the treatment of bacterial infections, has gained a renewed interest as increased antibiotic resistance in bacteria (Young and Gill, 2015). Bacteriophages (phages) are viruses that are specific obligate bacterial parasites and usually possess high specificity for one bacterial species; thus, their use in treatment offers the specific targeting of a group of bacteria without affecting the normal microbiota (Kortright et al., 2019). Phages multiply inside the infected host cell in a so-called lytic infection cycle, and are released from host cells by bacteriolysis. It has been shown that phage has a profound impact on the dynamics of the gut microbiome, not only affecting certain species directly but also having a cascading effect on others (Hsu et al., 2019). In addition, phage- and bactierial-derived pathogen-associated molecular patterns released after bacterial lysis could stimulate local innate immune responses, thus promoting bacterial clearance (Roach et al., 2017; Krut and Bekeredjian-Ding, 2018).
Many trials with phages, including several commercially available Salmonella bacteriophages, have been conducted in poultry, and the positive effects of phage therapy have been observed in reducing mortality rate and Salmonella population in the intestinal tract of chicks (Nabil et al., 2018; Tie et al., 2018), combating horizontal infections induced by Salmonella in layer and broiler chickens (Lim et al., 2011, 2012; Hong et al., 2013), and reducing the contamination level of Salmonella in the environment and poultry carcasses (Henriques et al., 2013; Atterbury et al., 2020). It was also showed that phage treatment by coarse spray could significantly reduce the disease symptoms in the chicks and the incidence of Salmonella in the ceca (Borie et al., 2009; Henriques et al., 2013). However, how Salmonella phages shape the gut microbiome and the persistence of phages in organs of chicks remain poorly understood. We previously showed that a hypervirulent arthritis-causing S. Pullorum 20JS04 isolated from Chinese native Qingjiaoma chicken breeds had greater negative impact on the growth performance of chicks compared with the white diarrhoea-causing S. Pullorum standard strain (Li et al., 2021). The current study aims to assess the therapeutic efficacy of Salmonella phage CKT1 against the hypervirulent S. Pullorum infection in young SPF White Leghorn chicks, and investigate the effects of Salmonella phage particles on gut microbiota.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Hypervirulent S. Pullorum strain 20JS04 was isolated from Chinese native Qingjiaoma chicken breeders with arthritis symptoms. The standard strain of S. Pullorum CVCC526 used as Salmonella phage CKT1 host for phage propagation was purchased from the China Veterinary Culture Collection Center (Beijing, China). All Salmonella strains stored at −70°C were firstly streaked onto xylose lysine desoxycholate (XLD) agar plate and incubated at 37°C for 24 h for subsequent tests.
Preparation of the Bacteriophage
Salmonella phage CKT1 (GenBank accession OK143508) was isolated from poultry sewage in Shandong province, China and exhibited a clear plaquing phenotype on both S. Pullorum host strain 20JS04 and CVCC526. To prepare Salmonella phage CKT1, fresh overnight culture of the host strain CVCC526 was inoculated at 1:10 into 3 mL of new LB media, then 0.1 mL of a 106 PFU/mL phage suspension was added to the tube. The culture was incubated at 37°C, 180 rpm for 4 h to allow phage propagation. Phage lysate was centrifuged at 10,000 g for 5 min, and the supernatant was filtered through a 0.22-μm filter. Phage titer was determined by decimally diluting the phage suspension in SM buffer (10 mM MgSO4, 100 mM NaCl, 0.01% w/v gelatin, 50 mM Tris-HCl, pH 7.5) and plating on the seeded top agar plates. Phage stocks can be stored at 4°C for 72 h before application.
Experimental Design of the SPF Chick Model
All animal experiments were carried out in strict accordance with animal protocols that were approved by the Ethical Committee of Animal Experiments of Shandong Agricultural University (permit number SDAUA-2018-027). As shown in Table 1, the SPF chicks under experiment were divided into 4 groups (20 chicks/group), and each group was housed in a separate air-filtered isolation cabinet in a well-ventilated room with 4 compartments of about 10 square meters. Chicks were provided with sterile feed and water ad libitum. The chicks in group 2 (only Salmonella challenged) and 4 (Salmonella challenged and phage treated) were challenged orally at d 2 with a single dose of 2 × 106 CFUs S. Pullorum in a total volume of 0.5 mL. The control group (Group 1) and group 3 (only phage treated) were orally inoculated with the same volume of bacteria free-buffered PBS. At 8 h postinfection, both group 3 and group 4 were orally treated with 0.5 mL of phage suspension at 107 PFU/chick, and the other 2 groups (Groups 1 and 2) were orally inoculated with the same volume of SM buffer. Chicks were maintained at an age-appropriate temperature for 7 d and monitored daily for clinical signs and mortalities.
Table 1.
Experimental design of bacteriophage treatment in SPF chicks challenged with S. Pullorum.
| Group | Treatment | Doses |
Age of Salmonella infection | Treatment schedule of phage | Time of euthanasia | |
|---|---|---|---|---|---|---|
| Salmonella | phage | |||||
| 1 | Nonchallenged and nontreated control negative | - | - | - | - | d 3 and 6 postinfection |
| 2 | Only Salmonella challenged | 0.5 mL 2 × 106 CFU/chick | - | 2nd day | - | |
| 3 | Only phage treated | - | 0.5 mL 2 × 107 PFU/chick | - | 8 h postinfection | |
| 4 | Salmonella challenged and phage treated | 0.5 mL 2 × 106 CFU/chick | 0.5 mL 2 × 107 PFU/chick | 2nd day | 8 h postinfection | |
Bacterial Burden, Phage Titer, and Histopathology
At d 3 and 6 postinfection, 6 randomly selected chicks from each group were weighed, and blood was collected from jugular vein. Then chicks were sacrificed by cervical dislocation, and subjected to gross lesions investigation. Samples of the liver and spleen of the chicks were taken to determine the number of Salmonella bacteria and bacteriophage titer. Briefly, the tissue samples were weighed and homogenized in 1 mL of PBS, and serial dilutions of the homogenates were plated onto XLD plates for counting of bacteria. Three suspected S. Pullorum colonies for each sample were identified by PCR assays using a specific target gene ipaJ (Xu et al., 2018). To determine phage titer, the homogenates were centrifuged at 10,000 rpm for 10 min and the supernatant was serially diluted and quantified by double agar overlay assay. The livers from each group at d 3 postinfection were fixed by 4% paraformaldehyde for 24 h at room temperature. Then the liver samples were embedded by paraffin, and slides were stained with hematoxylin-eosin (HE) stain.
Serological and Cytokine Testing in Blood Serum
Serum samples were collected by centrifugation and subjected to serological testing and enzyme-linked immunosorbent assay (ELISA) for interleukin-6 (IL-6) and interferon gamma (IFN-γ) (Cloud-Clone Corp., China). The S. Pullorum and S. Gallinarum multivalent antigen used for the agglutination test was purchased from Beijing Zhonghai Biotech Co. Ltd in China. The assays were performed as recommended by the manufacturers.
16S rRNA Amplicon Sequencing and Bioinformatic Analyses
Extraction of DNA from preweighed cecal content samples was performed using SDS method. V3-V4 hypervariable region of 16S rRNA gene was amplified using forward primer 341F and reverse primer 806R with dual-index barcodes by TruSeq DNA PCR-free sample preparation kit (Takahashi et al., 2014). The purified amplicons were quantified by using Qubit Fluorometer (ThermoFisher Scientific, Carlsbad, CA) and subjected to Illumina NovaSeq6000 desktop sequencer. Reads were filtered using Qiime and removed chimeric reads to obtain effective tags (Hall and Beiko, 2018). The above quality-controlled sequences were then clustered OTUs and annotated against SSUrRNA database using a 97% similarity cutoff (Quast et al., 2013). Alpha diversity (Shannon's diversity index and Faith's phylogenetic diversity) and beta diversity (weighted and unweighted UniFrac) were calculated using Qiime (Lozupone and Knight, 2005). The functional contribution of the bacteria was predicted based on OTUs using the Tax4Fun package in R software (Asshauer et al., 2015).
Statistical Analysis
Statistical significance was determined based on one-way analysis of variance (ANOVA) in appropriate condition. Significance was determined at P < 0.05.
RESULTS
Phage Therapy Improved Body Weight Loss of Chicks Infected With S. Pullorum
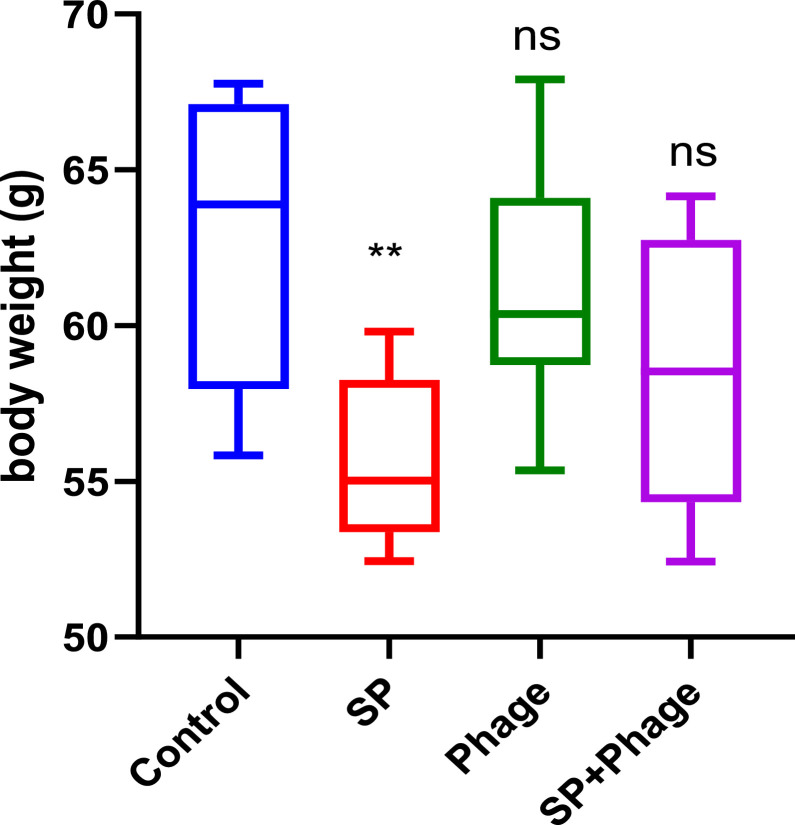
As shown in Figure 1, infection with 2 × 106 CFU/chick of the hypervirulent S. Pullorum strain 20JS04 had a significant negative effect on body weight of young SPF chicks (55.62 g ± 2.75, P < 0.01). Phage alone group did not show any abnormal behavior or obvious symptoms within 7 d, as comparing to chicks in control group (62.82 g ± 4.82), and Salmonella phage CKT1 had no significant negative effects on body weight of chicks (61.12 g ± 4.13). When the infected chicks were treated with a single dose of phage (107 PFU/chick) after S. Pullorum challenge, the body weight of chicks was markedly increased (58.49 g ± 4.62), close to the weight level of chicks in control group.
Figure 1.
Phage therapeutic effect on the body weight of SPF chicks infected with S. Pullorum. n = 6 chicks. ** P < 0.01 (One-way ANOVA); ns, no significance.
Phage Therapeutic Effect on the Liver and Spleen of Chicks Infected With S. Pullorum
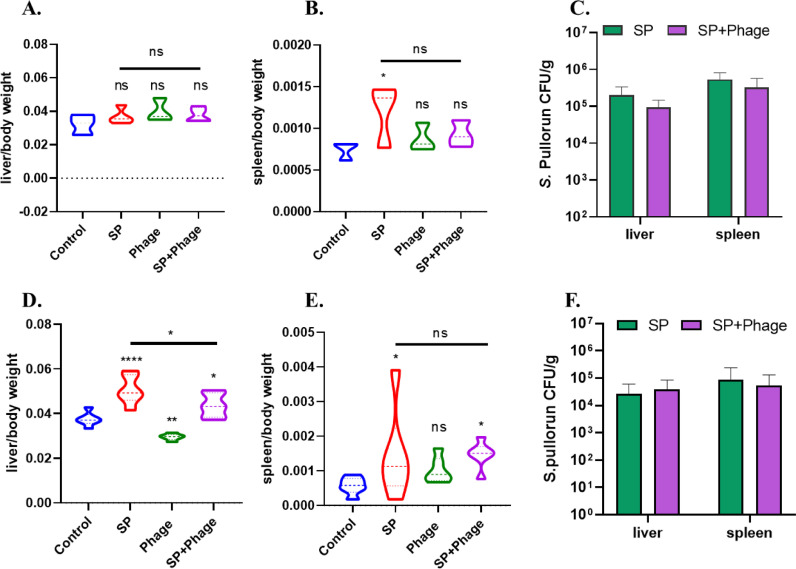
As shown in Figure 2, Salmonella infection did not affect liver/body weight ratio of chicks, but resulted in significantly increased spleen/body weight ratio (P < 0.05) on d 3 postinfection. However, chicks challenged with Salmonella showed significantly elevated both liver/body (P < 0.0001) and spleen/body (P < 0.05) weight ratios on d 6 postinfection compared with that of the control group, indicating that the liver and spleen of chicks might be enlarged (Figures 2D and 2E). For phage alone group, no difference was observed on both liver/body and spleen/body weight ratios on d 3 postinfection, but a significant reduction (P < 0.01) in liver/body weight ratio occurred on d 6 postinfection. For the infected chicks treated with phage, the liver/body weight ratio was significantly lower than those infected chicks without phage treatment (P < 0.01), but still higher than that of the control group on d 6 postinfection; the spleen/body weight ratio was also increased but showed no difference between Salmonella challenged chicks treated with or without phage CKT1 on d 6 postinfection. The average loads of S. Pullorum in liver and spleen reached 2 × 105 CFU/g and 5.4 × 105 CFU/g on d 3 postinfection, respectively, which were higher than that on d 6 postinfection (2.7 × 104 CFU/g and 8.9 × 104 CFU/g, respectively; Figures 2C and 2F). However, phage treatment just resulted in a reduction of 39.3% in bacterial loads of spleen (5.4 × 104 CFU/g) on d 6 postinfection, no significant difference in Salmonella numbers in both liver and spleen were found between infected chicks treated with or without phage CKT1 on d 6 postinfection.
Figure 2.
Phage therapeutic effect on liver and spleen of chicks infected with S. Pullorum. Phage effect on the liver/body weight ratio (A) and spleen/body weight ratio (B) on d 3 postinfection. n = 6 chicks. (C) Bacterial loads in liver and spleen on d 3 postinfection. Phage effect on the spleen/body weight ratio (D) and spleen/body weight ratio (E) on d 6 postinfection. n = 6 chicks. (F) Bacterial loads in liver and spleen on day 6 post infection. *, P < 0.05 (One-way ANOVA); **, P < 0.01; ****, P < 0.0001; ns, no significance.
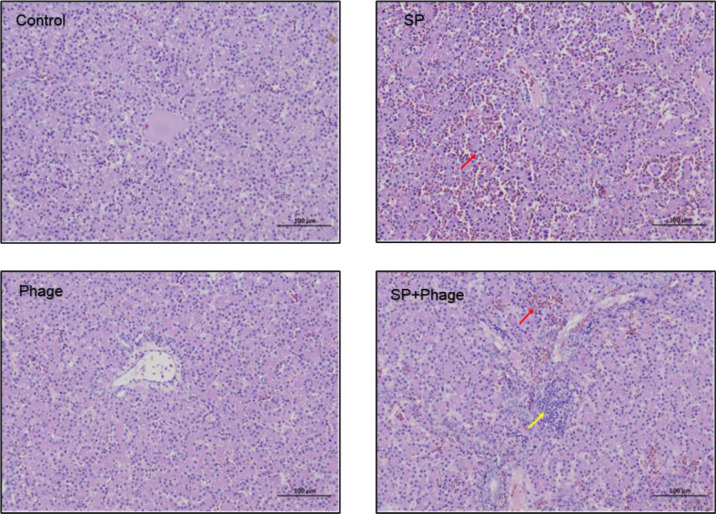
Compared with the normal liver of chicks in control group, enlarged liver in Salmonella challenged group displayed many white necrotic spots, while infected chicks treated with phage showed less necrosis. There were massive hepatic sinusoidal dilatation and congestion in only Salmonella challenged chicks. In contrast, besides small focal infiltration of inflammatory cells around the portal area in liver, only a moderate amount of hepatic sinusoidal dilatation and congestion were observed in infected chicks in phage treatment group (Figure 3). It was noteworthy that, the liver in phage control group had no obvious pathological changes but the liver size became smaller, which may be related to chicken wings injury due to unexpected air-filtered isolation cabinet failure.
Figure 3.
Phage therapeutic effect on pathological changes of liver. Massive hepatic sinusoidal dilatation and congestion (red arrow) in only Salmonella challenged chicks, whereas only a moderate amount of hepatic sinusoidal dilatation and congestion (red arrow) and small focal infiltration of inflammatory cells around the portal area in liver (yellow arrow) were observed in infected chicks after phage treatment.
Phage Persistence in Chicks
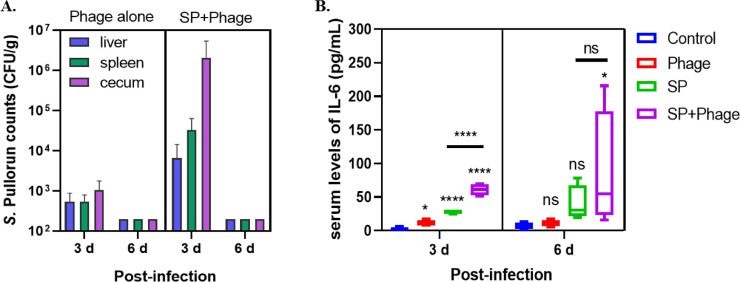
As shown in Figure 4A, phage particles can be detected in the liver and spleen of chicks in phage alone group, suggesting that Salmonella phage CKT1 was able to enter organs, although the phage titers in both liver and spleen were less than 103 PFU/g after 3 d administration. In addition to approximately 106 PFU/g of phage particles were remained in cecum of infected chicks after 3 d of phage administration, Salmonella phage CKT1 was also detected in both liver and spleen at concentrations of around 104 PFU/g for at least 3 d. However, phages could not be detected in cecum, liver, and spleen after 6 d of phage treatment. It is noteworthy that phage titers in organs of chicks in phage treated group were generally higher than that in phage alone group, indicating that phage CKT1 was propagated on colonized S. Pullorum in organs.
Figure 4.
(A) Salmonella phage CKT1 persistence in liver, spleen and cecum of chicks in both phage alone group and phage therapy group on d 3 and 6 postinfection. (B) Salmonella phage CKT1 effect on serum levels of IL-6 on d 3 and 6 postinfection. *, P < 0.05 (One-way ANOVA); ****, P < 0.0001; ns, no significance.
Phage Therapy Affected Serum Cytokine Levels
Serological analysis showed that no antibodies to S. Pullorum was detected in any Salmonella challenged group on d 6 postinfection. Among three tested cytokines, the serum level of IFN-γ was very low, beyond the detection limit of ELISA kit used in this study. As shown in Figure 4B, infection with S. Pullorum caused a 11.9-fold (P < 0.0001) and 6.4-fold (P = 0.33) increase in the serum levels of IL-6 at d 3 and 6 postinfection. There was also a significant upregulation (4.8-fold, P < 0.05) of serum IL-6 following only phage administration on d 3 postinfection, but the IL-6 level was just 1.7-fold higher (P = 0.90) on d 6 postinfection. In terms of the serum levels of IL-6 in infected chicks after phage treatment, there was a 25.8-fold (P < 0.0001) and 13.8-fold (P < 0.05) increase on d 3 and 6 postinfection, respectively. Compared with the level of serum IL-6 of infected chicks without phage treatment, phage therapy significantly promoted higher levels of IL-6 production (2.2-fold, P < 0.0001) on d 3 post infection, and resulted in a 2.1-fold increase although no statistical difference on d 6 postinfection were found.
Phage Therapy Normalized Altered Intestinal Microbiome Caused by Hypervirulent S. Pullorum
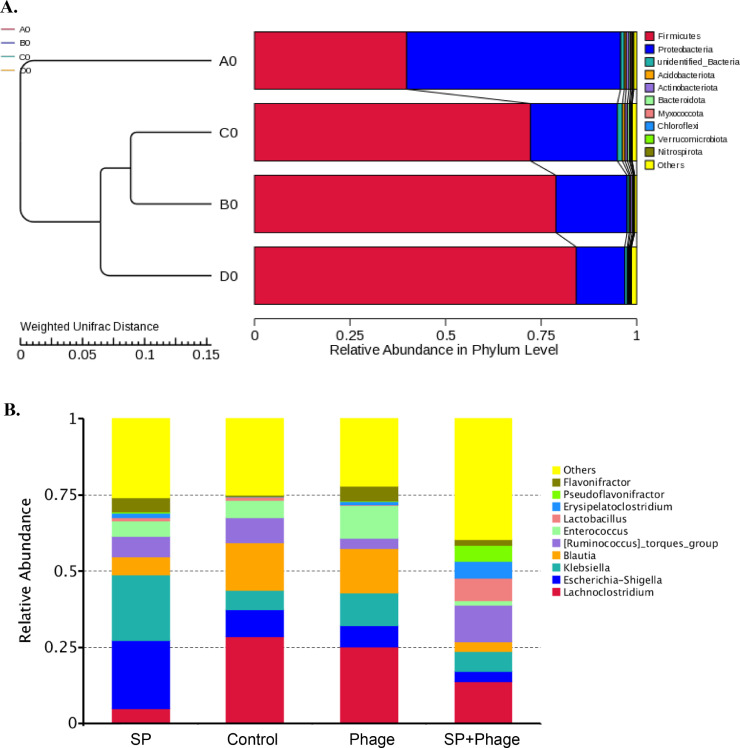
To determine whether phage treatment altered the gut metagenome of chicks, 16S rRNA gene profiling was carried out on cecal content samples. At the phylum level on d 6 postinfection, Firmicutes and Proteobacteria were predominant in gut microbiota of chicks from all groups. However, a higher relative abundance of Proteobacteria (0.56, P < 0.0001) and less abundant Firmicutes (0.41, P < 0.0001) was observed in only Salmonella challenged group than in the control group (0.19 and 0.79 respectively). In contrast, there was no significant difference in bacterial phyla between phage alone and phage therapy group (Figure 5A). The phylogenetic tree showed that the bacterial communities were divided into 2 major groups, Salmonella challenged group form a single branch, whereas phage alone, phage therapy and control groups were distributed on the same large branch (Figure 5A). Chicks between phage alone and control group showed a smaller weighted UniFrac distance, indicating single phage administration did not affect the normal gut microbiota structure of chicks. The beta diversity distance between only Salmonella challenged group and control group was statistically significantly larger compared to that between control group and other phage groups, implying Salmonella challenge significantly altered the gut microbiota structure of chicks (Figure 5A). Of the top 10 bacterial taxa at the genus level in control group, Lachnoclostridium (0.285) and Blautia (0.156) were predominant, followed by Escherichia-Shigella (0.089) and Ruminococcus (0.082), which was similar to that of phage alone group. In contrast, the relative abundances of Lachnoclostridium (0.048) and Blautia (0.059) in only Salmonella infected chicks were reduced substantially, resulting in significantly increased Escherichia-Shigella (0.225, P < 0.0001) and Klebsiella (0.216, P < 0.0001) becoming the predominant bacterial taxa (Figure 5B). Interestingly, the relative abundances of Escherichia-Shigella (0.034, P < 0.0001) and Klebsiella (0.064, P < 0.0001) decreased significantly after Salmonella phage treatment against infected chicks, accompanied by Lachnoclostridium, Ruminococcus, Lactobacillus and Pseudoflavonifractor increased remarkably compared to that of only Salmonella challenged group. However, the relative abundances of Blautia and Enterococcus in phage treatment group were still lower than that of control group (Figure 5B, Figure S1).
Figure 5.
(A) UPGMA tree based on weighted UniFrac distance at the phylum level. On the left is the UPGMA cluster tree structure, on the right is the species relative abundance distribution at the phylum level for each sample. A0: SP, B0: Control, C0: Phage, D0: SP + Phage; (B) Salmonella phage CKT1 therapeutic effect on the gut metagenome of chicks at the genus level. The relative abundances of the top 10 genera were displayed.
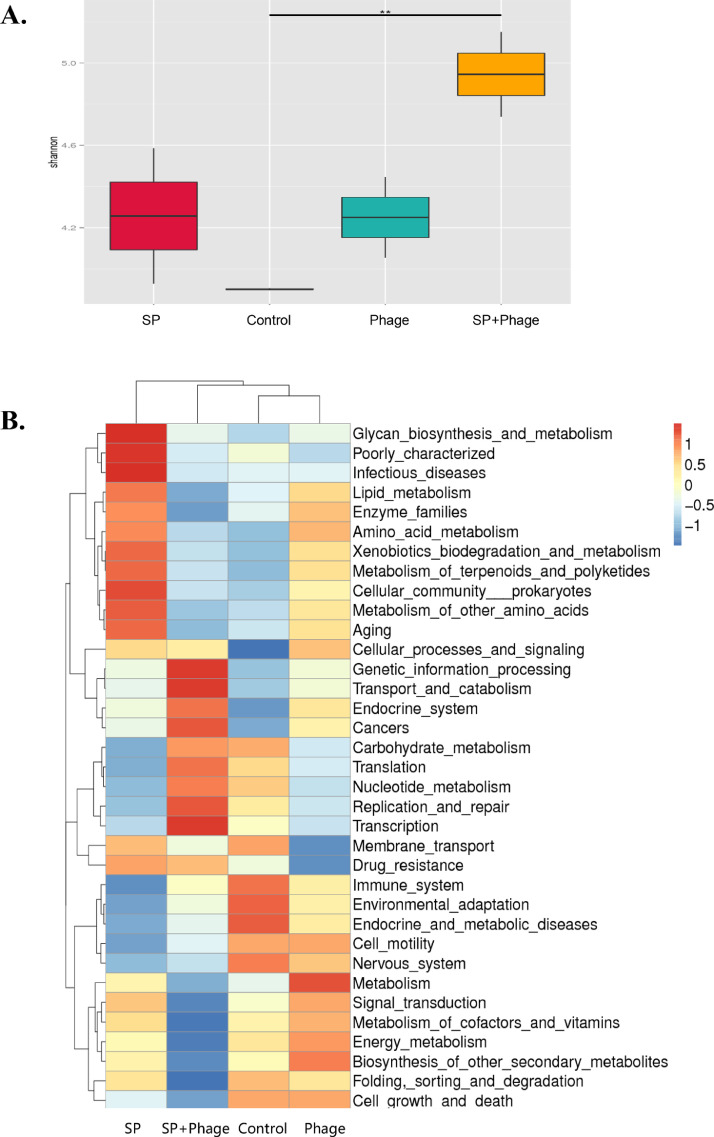
Alpha diversity comparison between Salmonella challenge and phage therapy groups showed significant differences in Shannon indices, indicating phage administration increased species richness in the cecum of chicks and made the distribution of species more even (Figure 6A). Cluster analysis of the metabolism functions in level 2 revealed that abundance of glycan biosynthesis and metabolism genes and infectious diseases genes in only Salmonella challenged group were increased greatly compared to other groups, followed by the increase of cellular community prokaryotes genes, amino acid metabolism genes, xenobiotics biodegradation and metabolism genes and terpenoids and polyketides metabolism genes. In contrast, above genes were reduced to the similar level as the control group after introduction of phage on infected chicks, which was also accompanied by an increase in genes mainly related to transport and catabolism, transcription, and replication and repair. The profile of expressed genes was general similar between phage alone group and the control group, and cell growth and death genes and cell motility genes were both highly expressed in both 2 groups (Figure 6B).
Figure 6.
(A) Alpha diversity comparison between Salmonella challenge, phage alone, phage therapy and control groups with a box plot of the Shannon index. (B) Cluster heatmap of annotated metabolism functions in level 2 by Tax4Fun. **, P < 0.01 (Wilcoxon rank-sum test).
DISCUSSION
Hypervirulent arthritis-causing S. Pullorum is widespread in Chinese native chicken flocks with swollen joint and lameness, leading to severe economic losses (Guo et al., 2019). The problems associated with multidrug-resistant Salmonella and the regulations concerning the use of antimicrobials in animal production have led to a resurgence of interest in phage therapy. In this study, we assessed the therapeutic efficacy of Salmonella phage CKT1 against hypervirulent S. Pullorum strain 20JS04 which were isolated from Chinese native chicken breeders with arthritis symptoms. We used the LD50 dose of Salmonella in Chinese native Qingjiaoma chicks to infect SPF White Leghorn chicks, although this dose did not cause arthritis symptoms, significant body weight loss was still observed in SPF chicks, similar to the symptom appeared in Qingjiaoma chicks (Li et al., 2021). Besides, this hypervirulent S. Pullorum strain also induced enlarged liver and spleen and massive hepatic sinusoidal dilatation and congestion in young chicks on d 6 postinfection. When bacteriophages were administrated to chicks after oral Salmonella infections, organ damage was effectively reduced. However, bacterial loads in liver and spleen were not significantly decreased after phage treatment. Many studies showed that frequent phage treatments or a single administration of high titer of phage, and especially prior to colonization of the intestinal tract by Salmonella, could result in effective bacterial reduction (Borie et al., 2008; Bardina et al., 2012). Tie et al. (2018) demonstrated that a single oral administration of Salmonella phage YSP2 2 h after bacteria challenge with approximately 109 PFU/chick could protect chickens against diarrhea and relieve hemorrhage in intestine and liver tissue significantly. Nabil et al. (2018) showed that successive phage treatments applied within a shorttime period infection were able to clear Salmonella from infected chicks. Our results suggested that a single phage administration after Salmonella infection was not sufficient to reduce bacterial loads in organs significantly. One reason may be that phage particles tend to be flushed from the organs of chicks over time by the immune system. Successive phage treatments may be efficacious in reducing S. Pullorum colonization in poultry.
Cytokines are key communication molecules between host cells in the defense against Salmonella. Infection with Salmonella leads to the activation and recruitment of neutrophils and macrophages and the production of proinflammatory cytokines. The Th1 cytokine IFN-γ has important roles in innate and acquired immunity, such as inducing antimicrobial responses in macrophages (Schroder et al., 2004). ELISA results showed little or no induction of serum IFN-γ in both Salmonella challenged chicks and phage treated chicks. This observation illustrated that the low-level of IFN-γ may be permissive for systemic spread of this hypervirulent S. Pullorum strain. IL-6 is a pleiotropic cytokine that has proinflammatory activity via the induction of acute phase protein synthesis, and it is important in the development of adaptive immune responses (Tanaka et al., 2014). The serum level of IL-6 was remarkably increased in chicks by 3 d postinfection, suggesting that IL-6 was involved in triggering an inflammatory response to S. Pullorum infection. Further administration of phages on infected chicks promoted the production of serum IL-6, which may be related to phage-induced bacterial lysis. Many previous studies showed that antibacterial activity of phages combined with the host's own immune defenses led to asymptotic eradication of bacterial infections (Roach et al., 2017; Krut and Bekeredjian-Ding, 2018). Recent literature also provides evidence that even phages that target the same bacterial pathogen may yield different effects (Shiley et al., 2017). However, there are few studies focused on phage effects on the immunity of body, especially in food animals. To screen the best candidate phages for systemic infections of Salmonella in poultry, it will be essential in future studies to investigate the overall animal host immune response against the bacteriophage activities as well as responses at the cellular levels of the host.
The microbiome of the broiler chicken gastrointestinal tract has been amply demonstrated to be important for the health of host, as it has a positive impact on the immune system, the physiology of the gastrointestinal tract, and productivity (Clavijo and Florez, 2018). Although the use of phages could reduce the Salmonella load in the cecal content, how phages modulate the intestinal microbiome of chicken intestines is still unclear. Here we showed that Salmonella challenge disrupted the normal gut microbiota structure of chicks, and the use of phage CKT1 significantly reduced the Escherichia-Shigella and Klebsiella populations, permitting the proliferation of beneficial microbiota including Lachnoclostridium, Ruminococcus, Lactobacillus, and Pseudoflavonifractor. It should be noted that Salmonella phage CKT1 specifically infected Salmonella and body weight loss was not fully recovered after phage therapy. The still lower abundances of Blautia, a genus of anaerobic bacteria with probiotic characteristics, and opportunistic pathogen Enterococcus in phage treatment group suggested that phage therapy shaped the abnormal gut microbiome of chicks to a certain extent. Interestingly, Akkermansia, a mucin-degrading bacterium that inversely correlates with body weight in rodents and humans (Everard et al., 2013), is increased after Salmonella challenge, indicating that increased Akkermansia may also contribute to the body weight loss caused by Salmonella infection in young chicks. To the best of our knowledge, the inverse correlation of Akkermansia abundance with body weight in chicks has not been reported to date. Moreover, the increased levels of glycan biosynthesis and lipid metabolism genes and infectious diseases genes in only Salmonella challenged group reflected Salmonella infection and adaptation strategy to gain a growth advantage over the intestinal microbiota. Phage therapy led to a reduction of infectious diseases genes and an increase in genes mainly related to transport and catabolism, transcription, and replication and repair, suggesting reduced metabolic activity of pathogenic bacteria and alterations in bacterial diversity and catabolism of the other microbiota. Interestingly, Upadhaya et al. (2021) assessed the dietary usage of a bacteriophage cocktail in broiler chickens without bacterial challenge. They found that phage cocktail improved broiler weight and 0.05% phage addition was sufficient for supporting immune organs, bursa and spleen as well as enhancing gut microbiome. These results indicated the potential of phages as a substitute antibiotic growth promoter in broiler chickens.
In summary, we investigated the therapeutic efficacy of Salmonella phage CKT1 against hypervirulent arthritis-causing S. Pullorum in a chick model. The results showed that single phage treatment after S. Pullorum infection significantly improved the body weight loss of chicks, possibly by modulating the abnormal intestinal microbiome, indicating Salmonella phage CKT1 could be considered as a potential alternative to antimicrobial growth promoters on poultry farms.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by Shandong Provincial Agricultural Animal Breeding Project of China (2020LZGC013), Shandong Agricultural Major Application Technology Innovation Project (SD2019XM009), and Talents Gathering Project of Zaozhuang.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101668.
Appendix. Supplementary materials
Supplementary Figure S1. Heat maps of species abundance clustering at the genus level between Salmonella challenge, phage alone, phage therapy and control groups. Species annotated information is listed in the horizontal direction, and top 35 genera sample information is listed in the vertical direction. The species cluster tree is shown on the left-hand side, and the corresponding values of the heat map are the Z values obtained after the relative abundance of each row of species is standardized.
REFERENCES
- Asshauer K.P., Wemheuer B., Daniel R., Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015;31:2882–2884. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterbury R.J., Gigante A.M., Rubio Lozano M.S., Mendez Medina R.D., Robinson G., Alloush H., Barrow P.A., Allen V.M. Reduction of Salmonella contamination on the surface of chicken skin using bacteriophage. Virol. J. 2020;17:98. doi: 10.1186/s12985-020-01368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina C., Spricigo D.A., Cortes P., Llagostera M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012;78:6600–6607. doi: 10.1128/AEM.01257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P.A., Freitas Neto O.C. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- Borie C., Albala I., Sanchez P., Sanchez M.L., Ramirez S., Navarro C., Morales M.A., Retamales A.J., Robeson J. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008;52:64–67. doi: 10.1637/8091-082007-Reg. [DOI] [PubMed] [Google Scholar]
- Borie C., Sanchez M.L., Navarro C., Ramirez S., Morales M.A., Retamales J., Robeson J. Aerosol spray treatment with bacteriophages and competitive exclusion reduces Salmonella Enteritidis infection in chickens. Avian Dis. 2009;53:250–254. doi: 10.1637/8406-071008-Reg.1. [DOI] [PubMed] [Google Scholar]
- Clavijo V., Florez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Liu Q., Jiang Z., Song Y., Yi S., Qiu J., Hao G., Sun S. Characteristics of Salmonella from Chinese native chicken breeds fed on conventional or antibiotic-free diets. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.607491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., de Vos W.M., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Li Z., Zhou X., Huang C., Hu Y., Geng S., Chen X., Li Q., Pan Z., Jiao X. Induction of arthritis in chickens by infection with novel virulent Salmonella Pullorum strains. Vet. Microbiol. 2019;228:165–172. doi: 10.1016/j.vetmic.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Hall M., Beiko R.G. 16S rRNA gene analysis with QIIME2. Methods. Mol. Biol. 2018;1849:113–129. doi: 10.1007/978-1-4939-8728-3_8. [DOI] [PubMed] [Google Scholar]
- Henriques A., Sereno R., Almeida A. Reducing Salmonella horizontal transmission during egg incubation by phage therapy. Foodborne Pathog. Dis. 2013;10:718–722. doi: 10.1089/fpd.2012.1363. [DOI] [PubMed] [Google Scholar]
- Hong S.S., Jeong J., Lee J., Kim S., Min W., Myung H. Therapeutic effects of bacteriophages against Salmonella Gallinarum infection in chickens. J. Microbiol. Biotechnol. 2013;23:1478–1483. doi: 10.4014/jmb.1304.04067. [DOI] [PubMed] [Google Scholar]
- Hsu B.B., Gibson T.E., Yeliseyev V., Liu Q., Lyon L., Bry L., Silver P.A., Gerber G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25:803–814. doi: 10.1016/j.chom.2019.05.001. e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortright K.E., Chan B.K., Koff J.L., Turner P.E. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. 2019;25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Krut O., Bekeredjian-Ding I. Contribution of the immune response to phage therapy. J. Immunol. 2018;200:3037–3044. doi: 10.4049/jimmunol.1701745. [DOI] [PubMed] [Google Scholar]
- Li P., Zhang M., Hao G., Sun S. Research note: hypervirulent arthritis-causing Salmonella Pullorum isolated from Chinese native chicken breeds significantly decreased growth performance of chicks. Poult. Sci. 2021;101 doi: 10.1016/j.psj.2021.101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T.H., Kim M.S., Lee D.H., Lee Y.N., Park J.K., Youn H.N., Lee H.J., Yang S.Y., Cho Y.W., Lee J.B., Park S.Y., Choi I.S., Song C.S. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012;93:1173–1178. doi: 10.1016/j.rvsc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Lim T.H., Lee D.H., Lee Y.N., Park J.K., Youn H.N., Kim M.S., Lee H.J., Yang S.Y., Cho Y.W., Lee J.B., Park S.Y., Choi I.S., Song C.S. Efficacy of bacteriophage therapy on horizontal transmission of Salmonella Gallinarum on commercial layer chickens. Avian Dis. 2011;55:435–438. doi: 10.1637/9599-111210-Reg.1. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabil N.M., Tawakol M.M., Hassan H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018;8 doi: 10.1080/20008686.2018.1539056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach D.R., Leung C.Y., Henry M., Morello E., Singh D., Di Santo J.P., Weitz J.S., Debarbieux L. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe. 2017;22:38–47. doi: 10.1016/j.chom.2017.06.018. e34. [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Shiley J.R., Comfort K.K., Robinson J.B. Immunogenicity and antimicrobial effectiveness of Pseudomonas aeruginosa specific bacteriophage in a human lung in vitro model. Appl. Microbiol. Biotechnol. 2017;101:7977–7985. doi: 10.1007/s00253-017-8504-1. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Tomita J., Nishioka K., Hisada T., Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thøfner I., Christensen J.-P. In: Pages 199-227 in Advancements and Technologies in Pig and Poultry Bacterial Disease Control. Foster N., Kyriazakis I., Barrow P., editors. Academic Press; Cambridge, MA: 2021. Bacterial diseases in poultry. [Google Scholar]
- Tie K., Yuan Y., Yan S., Yu X., Zhang Q., Xu H., Zhang Y., Gu J., Sun C., Lei L., Han W., Feng X. Isolation and identification of Salmonella Pullorum bacteriophage YSP2 and its use as a therapy for chicken diarrhea. Virus Genes. 2018;54:446–456. doi: 10.1007/s11262-018-1549-0. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Ahn J.M., Cho J.H., Kim J.Y., Kang D.K., Kim S.W., Kim H.B., Kim I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:49. doi: 10.1186/s40104-021-00570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Liu Z., Li Y., Yin C., Hu Y., Xie X., Li Q., Jiao X. A rapid method to identify Salmonella enterica serovar Gallinarum biovar Pullorum using a specific target gene ipa J. Avian Pathol. 2018;47:238–244. doi: 10.1080/03079457.2017.1412084. [DOI] [PubMed] [Google Scholar]
- Young R., Gill J.J. Microbiology. Phage therapy redux–what is to be done? Science. 2015;350:1163–1164. doi: 10.1126/science.aad6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Heat maps of species abundance clustering at the genus level between Salmonella challenge, phage alone, phage therapy and control groups. Species annotated information is listed in the horizontal direction, and top 35 genera sample information is listed in the vertical direction. The species cluster tree is shown on the left-hand side, and the corresponding values of the heat map are the Z values obtained after the relative abundance of each row of species is standardized.