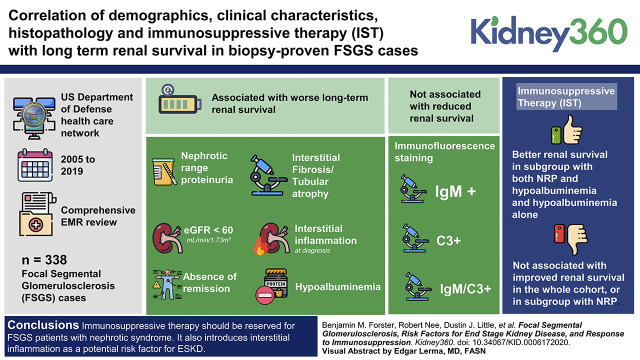
Visual Abstract
Keywords: glomerular and tubulointerstitial diseases, end stage kidney disease, estimated glomerular filtration rate, focal segmental glomerulosclerosis, immunosuppression, proteinuria, renal survival, risk factors
Abstract
Background
FSGS is a heterogeneic glomerular disease. Risk factors for kidney disease ESKD and the effect of immunosuppression treatment (IST) has varied in previously published cohorts. These cohorts were limited by relatively small case numbers, short follow-up, lack of racial/ethnic diversity, a mix of adult and pediatric patients, lack of renin-angiotensin-aldosterone system (RAAS) inhibition, or lack of subgroup analysis of IST.
Methods
We compared demographics, clinical characteristics, histopathology, and IST to long-term renal survival in a large, ethnically diverse, adult cohort of 338 patients with biopsy-proven FSGS with long-term follow-up in the era of RAAS inhibition using data from the US Department of Defense health care network.
Results
Multivariate analysis showed that nephrotic-range proteinuria (NRP), eGFR <60 ml/min per 1.73 m2, hypoalbuminemia, interstitial fibrosis and tubular atrophy, and interstitial inflammation at diagnosis and the absence of remission were all associated with worse long-term renal survival. IgM, C3, and a combination of IgM/C3 immunofluorescence staining were not associated with reduced renal survival. IST was not associated with improved renal survival in the whole cohort, or in a subgroup with NRP. However, IST was associated with better renal survival in a subgroup of patients with FSGS with both NRP and hypoalbuminemia and hypoalbuminemia alone.
Conclusions
Our study suggests that IST should be reserved for patients with FSGS and nephrotic syndrome. It also introduces interstitial inflammation as a potential risk factor for ESKD and does not support the proposed pathogenicity of IgM and complement activation.
Introduction
FSGS is a glomerular disease defined by histopathologic lesions. There is significant disease heterogeneity likely due to multiple mechanisms of disease, clinical, and demographic risk factors, and comorbidities (1–3). The incidence of FSGS has risen over the past decades for unclear reasons (1). Previous retrospective cohort studies have significant variability in their findings. Some reported that sex, proteinuria, serum creatinine level, and body mass index (BMI) at diagnosis were associated with long-term survival, whereas others did not (3–15). Immunosuppression was associated with improved renal survival in some cohorts but not others (4,6,7,9–11,13). Results likely differed due to study population and follow-up. Previous studies included both children and adults (4,6,8,11,13,14). A portion of studies included few Black people (6,7,11). Most studies included a large portion of patients diagnosed before 2000, many of which did not receive renin-angiotensin-aldosterone system (RAAS) blockade (5–7,9–13,15). Many studies included 100 patients or fewer (8–13,15). The follow-up period for the majority of cohorts was <6 years (4–10,12,13,15). Most used serum creatinine to describe renal function and not eGFR (5–13,15). Therefore, in this study we sought to assess the associations between demographics, clinical characteristics, histopathology, immunosuppression treatment (IST), and long-term kidney survival in a large, racially and ethnically diverse, adult FSGS cohort with long-term follow-up.
Materials and Methods
We queried the previously described US Department of Defense health care network for current and previous active-duty military members with the International Classification of Diseases, 9th Revision (ICD-9) and 10th Revision (ICD-10) codes 581.1, 582.1, and N04 between 2005 and 2019 (16). The health care network is comprised of 9.6 million people with 65 hospitals and 412 clinics worldwide that have shared a universal electronic medical record (EMR) since 2005. We next reviewed the comprehensive EMR for each potential case to compile the cohort of 338 patients with FSGS. FSGS diagnosis was confirmed by histopathology report or multiple notes from a nephrologist confirming biopsy-proven primary FSGS. Patients were excluded when there was evidence for a secondary cause of FSGS to include HIV, hepatitis B, hepatitis C, parvovirus B19, single kidney, reflux nephropathy, heroin use, and sickle cell disease.
Demographics, presenting clinical characteristics, laboratory, histopathologic, treatment, and outcome data were collected for each FSGS patient in the cohort from the time of biopsy-proven diagnosis to ESKD or last follow-up. Hypertension was defined by a prescription for antihypertensive medications or ≥2 systolic or diastolic BP readings of ≥140 mm Hg or ≥90 mm Hg, respectively, more than 2 weeks apart. Proteinuria was quantified by either 24-hour urine collection or spot urine protein-to-creatinine ratio (UPCR). eGFR was determined using the CKD Epidemiology Collaboration equation. ESKD was defined by the initiation of kidney replacement therapy or preemptive kidney transplant. Glucocorticoid use was defined by ≥30mg per day with the intent to treat for up to 4 months to achieve remission unless the risk of an adverse outcome outweighed the perceived benefit of therapy. RAAS inhibitions included any use of angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, or mineralocorticoid receptor antagonist. Interstitial inflammation and interstitial fibrosis and tubular atrophy (IFTA) were defined by either percent or description. “Scant” was assigned equivalence to <5%, “mild” or “patchy” to 5%–15%, “moderate” to 15%–25%, and “significant” or “severe” to >25%. Categorical variables were established for nephrotic-range proteinuria (NRP; ≥3.5 gm/d), CKD (eGFR at diagnosis <60ml/min per 1.73 m2), IST (any duration of glucocorticoids, calcineurin inhibitor, cyclophosphamide, and/or mycophenolate mofetil), IFTA (≥15% or a description of “moderate,” “severe,” or “significant”), interstitial inflammation (≥15% or a description of “moderate,” “severe,” or “significant”), serum albumin (<3.0 mg/dl), IgM staining on immunofluorescence (present), C3 staining on immunofluorescence (present), and achievement of remission. Complete remission was defined by a reduction in proteinuria to <0.3 gm/24 hours with <25% reduction in eGFR from baseline at biopsy diagnosis. Partial remission was defined by a 50% reduction in proteinuria to <3.5 gm/d or a reduction in proteinuria to <2.0 gm/d.
STATA MP/16.1 (College Station, Tx) was used for all statistical analysis. Univariate analyses were performed with chi-squared testing for categorical variables. The t‐test was used for continuous variables (Mann–Whitney test used for non-normally distributed variables). P<0.05 was considered statistically significant for all comparisons.
The probability of ESKD by a certain time point was estimated using the Kaplan-Meier method and log-rank test to assess the significant difference between the groups. We conducted both nonstratified and stratified Kaplan-Meier analyses by demographic and clinical variables. We also conducted Cox proportional hazards regression analyses to assess for association between each potential risk factor and renal survival. The categorical variables in the Cox models were age (<60 years old versus >60 years old), race (Black versus non-Black), serum albumin (≤3 gm/dl versus >3 gm/dl, proteinuria (>3.5 gm/d versus ≤3.5 gm/d), eGFR (<60 ml/min/1.732 versus ≥60 ml/min/1.732), remission state (complete versus partial versus no remission), IST (yes versus no), interstitial inflammation (≥15% versus <15%), and IFTA (≥15% versus <15%). Multivariate analyses were performed for the entire study cohort and were further stratified by race, eGFR, NRP, serum albumin, and by both NRP and hypoalbuminemia. We tested the proportional-hazards assumption on the basis of the graphical method of plotting estimates of −ln [−ln (survival probability)] versus ln (analysis time) and on the basis of testing of Schoenfeld residuals. We found no evidence that our specifications violated the proportional-hazards assumption. The follow-up period for the survival analyses were censored at the last patient follow-up or end of study in January 2020. This study was approved by the Walter Reed National Military Medical Center Institutional Review Board.
Results
The FSGS cohort was predominately Black, male, and <40 years old (Table 1). Nearly all patients with FSGS had hypertension at diagnosis (97%). NRP, a serum albumin ≤3.0 mg/dl, and an eGFR <60 ml/min/1.732 were present at biopsy-proven FSGS diagnosis in 46%, 32%, and 43% of patients, respectively (Table 1). Median follow-up after biopsy diagnosis was 9.5 years. At 5, 10, and 15 years after biopsy diagnosis, 67%, 42%, and 19% of initial patients with FSGS had clinical follow-up data available (Supplemental Figure 1). The complete renal biopsy report was available for review in 64% of patients with FSGS. Documentation by a nephrologist confirmed the other patients. The majority of biopsy reports did not list a specific variant (86%). RAAS inhibition was almost universal (97%), but only 42% of patients with FSGS received IST. Corticosteroids and calcineurin inhibitors were most common. In total, 27% of patients progressed to ESKD. Obesity (BMI >30) was not associated with progression to ESKD (22% [22/98] versus 24% [31/130], P=0.8).
Table 1.
Clinical background data for patients with FSGS at diagnosis
| Variable | All | No ESKD (%) | ESKD (%) | P value |
| Total, N | 338 | 247 | 91 | |
| Male, n (%) | 290 (86) | 210/247 (85) | 80/91 (88) | 0.50 |
| Race, n (%) | ||||
| Black | 216 (64) | 154/247 (62) | 62/91 (68) | 0.39 |
| White | 109 (32) | 84/247 (34) | 25/91 (28) | 0.31 |
| Asian | 11 (3) | 9/247 (4) | 2/91 (2) | 0.73 |
| American Indian | 2 (0.6) | 0/247 (0) | 2/91 (2) | 0.07 |
| Ethnicity | ||||
| Hispanic | 34 (10) | 21/247 (9) | 13/91 (14) | 0.15 |
| Non-Hispanic | 98 (29) | 72/247 (29) | 26/91 (29) | 1 |
| Missing | 206 (61) | 154/247 (62) | 52/91 (57) | 0.45 |
| Age (yr) | 35.7±12.6 | 35.8±12.4 | 35.5±13.0 | 0.88 |
| UPCR at diagnosis (gm) | 4.6±4.5 | 4.4±4.6 | 5.2±4.2 | 0.32 |
| Diagnosis proteinuria, n/N (%) | ||||
| <3.5 gm | 150/278 (54) | 120/210 (57) | 30/68 (44) | 0.07 |
| ≥3.5 gm | 128/278 (46) | 90/210 (43) | 38/68 (56) | 0.07 |
| Diagnosis creatinine (mg/dl) | 1.8±1.2 | 1.5±0.9 | 2.7±1.6 | <0.001 |
| Diagnosis eGFR (ml/min/1.732) | 65±31 | 73±30 | 43±23 | <0.001 |
| Diagnosis eGFR <60 m/min/1.732 | 121/281 (43) | 71/212 (34) | 50/69 (73) | <0.001 |
| Diagnosis albumin (g/dl) | 3.5±0.9 | 3.2±1.0 | 0.02 | |
| Diagnosis albumin, n/N (%) | ||||
| ≤3.0 g/dl | 87/271 (32) | 60/204 (29) | 27/67 (40) | 0.01 |
| ≤2.5 g/dl | 53/281 (19) | 34/204 (17) | 19/67 (28) | 0.04 |
| Immunosuppression, n/N (%) | 137/329 (42) | 104/246 (42) | 33/83 (40) | 0.69 |
| Prednisone | 133/329 (40) | 102/246 (42) | 31/83 (37) | 0.51 |
| CNI | 60/329 (18) | 45/246 (18) | 15/83 (18) | 0.96 |
| MMF | 28/329 (9) | 19/246 (8) | 9/83 (11) | 0.38 |
| Cyclophosphamide | 4/329 (1) | 3/246 (1) | 1/83 (1) | 1 |
| Rituximab | 3/329 (0.9) | 2/246 (0.8) | 1/83 (1) | 1 |
| Remission, n/N (%) | ||||
| Complete | 62/238 (26) | 59/169 (35) | 3/69 (5) | <0.001 |
| Partial | 60/238 (25) | 49/169 (29) | 11/69 (16) | 0.04 |
| None | 116/238 (49) | 61/169 (36) | 55/69 (80) | <0.001 |
| Follow-up months | 128±93.6 | |||
| Months to ESKD | 83.6±74.4 | |||
| Total glomeruli | 17.3±11.1 | 18.7±11.3 | 12.9±9.2 | 0.001 |
| Segmental glomeruli | 2.8±2.7 | 2.9±2.6 | 2.7±2.9 | 0.61 |
| Obsolescent glomeruli | 3.4±3.9 | 3.1±3.4 | 4.6±5.3 | 0.01 |
| IFTA, n/N (%) | ||||
| <15% or none/scant/mild | 133/215 (62) | 117/165 (71) | 16/50 (32) | <0.001 |
| ≥15% or moderate/significant | 82/215 (38) | 48/165 (29) | 34/50 (68) | <0.001 |
| Inflammation, n/N (%) | ||||
| <15% or none/scant/mild | 154/188 (82) | 126/145 (87) | 28/43 (65) | 0.002 |
| ≥15% or moderate/significant | 34/188 (18) | 19/145 (13) | 15/43 (35) | 0.002 |
| IgM staining | 129/187 (69) | 103/147 (70) | 26/40 (65) | 0.54 |
| C3 staining | 114/186 (61) | 87/147 (59) | 27/39 (70) | 0.25 |
| Biopsy variants, n/N (%) | ||||
| NOS | 186/215 (87) | 146/167 (87) | 40/48 (83) | 0.47 |
| Collapsing | 11/215 (5) | 6/167 (4) | 5/48 (10) | 0.07 |
| Tip | 12/215 (6) | 10/167 (6) | 2/48 (4) | 0.74 |
| Cellular | 4/215 (2) | 4/167 (2) | 0/48 (0) | 0.57 |
| Perihilar | 2/215 (0.9) | 1/167 (0.6) | 1/48 (2) | 0.40 |
| BMI at diagnosis (kg/m2) | 30.1±5.2 | 30.1±5.1 | 30.1±5.6 | 0.99 |
| HTN, prediagnosis, n/N (%) | 147/264 (56) | 107/198 (54) | 40/66 (60) | 0.21 |
| HTN, postdiagnosis, n/N (%) | 327/338 (97) | 237/247 (96) | 90/91 (99) | 0.18 |
| DM, n/N (%) | 63/337 (19) | 41/246 (17) | 22/91 (24) | 0.24 |
| IDDM, n/N (%) | 30/337 (9) | 20/246 (8) | 10/91 (11) | 0.59 |
| ACEi/ARB, n/N (%) | 329/338 (97) | 242/247 (98) | 87/91 (96) | 0.23 |
UPCR, urine protein-to-creatinine ratio; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; IFTA, interstitial fibrosis and tubular atrophy; NOS, not otherwise specified; BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; IDDM, insulin dependent diabetes mellitus; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker. Continuous variables presented with mean ± standard deviation.
Overall, 5-, 10-, and 15-year renal survival were 86%, 75%, and 55%, respectively (Supplemental Figure 1). On Kaplan-Meier analysis, renal survival at 5, 10, 15 years was lower for patients with FSGS and NRP versus non-NRP, serum albumin ≤3.0 mg/dl versus >3.0 mg/dl, inflammation ≥15% versus <15%, eGFR <60 ml/min/1.732 versus ≥60 ml/min/1.732, and IFTA ≥15% versus <15% (Figures 1 and 2, Table 2). Black race, Hispanic ethnicity, sex, age, and BMI were not associated with worse long-term renal survival (Supplemental Figure 2).
Figure 1.
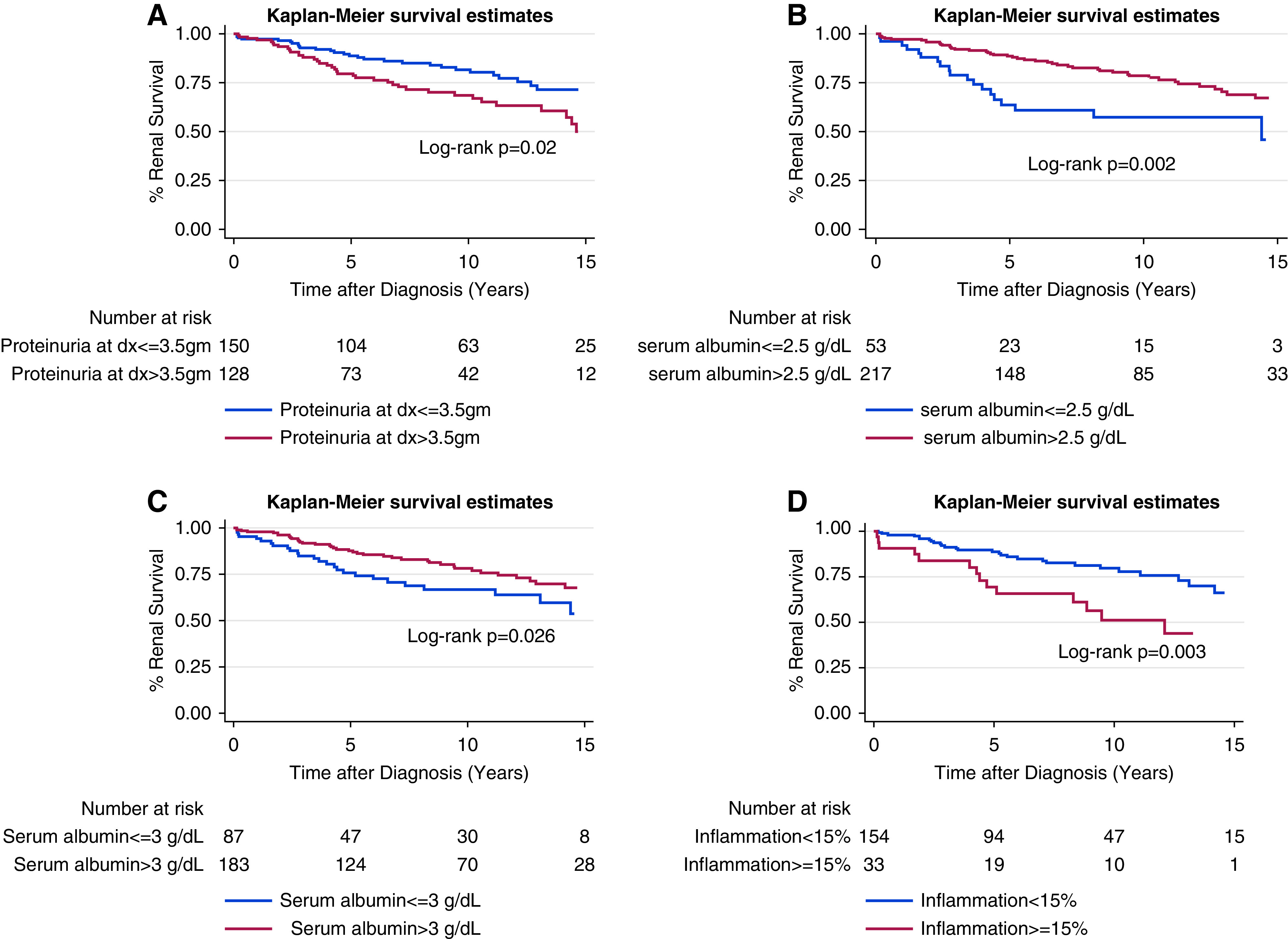
FSGS Kaplan-Meier curves for renal survival on the basis of disease acuity variables. These include (A) nephrotic range proteinuria versus non-nephrotic range proteinuria; (B) serum albumin ≤2.5 g/dl versus >2.5 g/dl; (C) serum albumin ≤3.0 mg/dl versus >3.0 mg/dl; and (D) interstitial inflammation ≥15% versus <15%. Dx, diagnosis.
Figure 2.
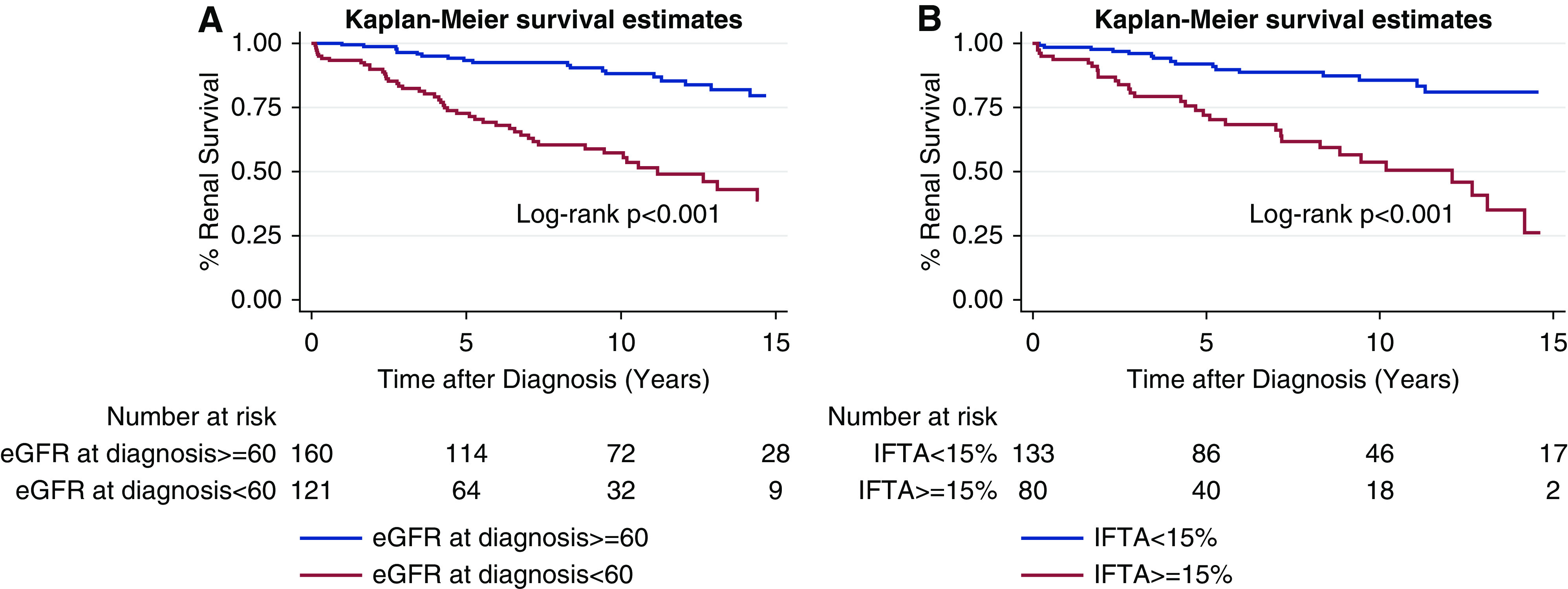
FSGS Kaplan-Meier curves for renal survival on the basis of disease chronicity variables. These include (A) eGFR ≥60 ml/min/1.732 versus <60 ml/min/1.732 at diagnosis; and (B) interstitial fibrosis and tubular atrophy (IFTA) ≥15% versus <15%.
Table 2.
The 5-, 10-, and 15-yr renal survival rates for various risk factors
| Kidney Survival (%) | 5 Yr | 10 Yr | 15 Yr |
| Overall | 86 | 75 | 55 |
| NRP | 80 | 69 | 50 |
| NNRP | 89 | 82 | 71 |
| Albumin <3.0 g/dl | 76 | 67 | 54 |
| Albumin >3.0 g/dl | 88 | 78 | 68 |
| eGFR <60 m/min/1.732 | 73 | 57 | 39 |
| eGFR >60 m/min/1.732 | 93 | 88 | 80 |
| IFTA ≥15% | 72 | 54 | 26 |
| IFTA <15% | 92 | 86 | 81 |
| Interstitial inflammation | 79 | 62 | 49 |
| ≥15% | |||
| Interstitial inflammation | 90 | 83 | 71 |
| <15% | |||
| Complete remission | 98 | 96 | 93 |
| Partial remission | 88 | 78 | 68 |
| No remission | 72 | 56 | 37 |
NRP, nephrotic-range proteinuria; NNRP, non-NRP; IFTA, interstitial fibrosis and tubular atrophy.
IgM and C3 staining on immunofluorescence were not individually associated with worse renal survival. The combination of both IgM and C3 staining was also not associated with reduced long-term renal survival to include analysis of both IgM+/C3+ (n=100) versus C3−/IgM− (n=44), and IgM+/C3+ (n=100) versus IgM−/C3− (n=44), IgM+/C3− (n=28), and IgM−/C3+ (n=14) (Supplemental Figure 3).
In total, 42% of patients received immunosuppression. Immunosuppression was not associated with improved renal survival for the whole FSGS cohort (P=0.99; Figure 3) or groups stratified by eGFR ≥60 ml/min per 1.73 m2 (P=0.96), eGFR <60 ml/min per 1.73 m2 (P=0.88), NRP (P=0.09), and non-NRP (P=0.78, Figure 3, Supplemental Figure 4). But, when stratified by albumin ≤3.0 mg/dl and ≤2.5 mg/dl, IST was associated with improved renal survival (P=0.01 and P<0.001, respectively; Figure 3). IST was also associated with increased renal survival in a subgroup stratified for both NRP and albumin ≤3.0 mg/dl (P=0.001) and a subgroup stratified for both NRP and albumin ≤2.5 mg/dl (Figure 3, P=0.03). When stratified by Black race (P=0.9), Hispanic ethnicity (P=0.79), age (0.80), sex (P=0.95), BMI (0.54), interstitial inflammation (P=0.34), IFTA (P=0.64), and IgM+/C3+ immunofluorescence (P=0.31), IST was not associated with improved renal survival (Supplemental Figures 5 and 6).
Figure 3.
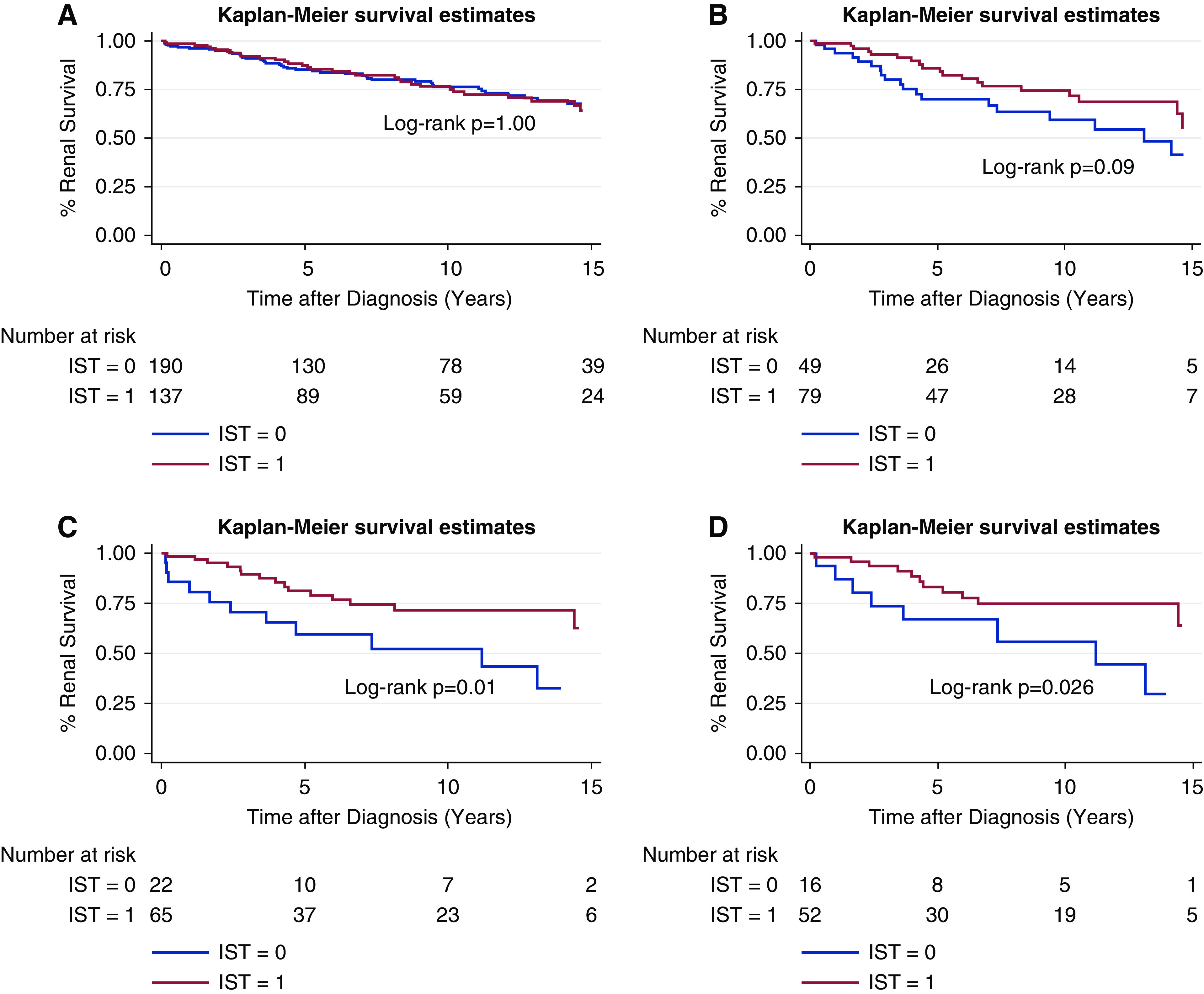
FSGS Kaplan-Meier curves for renal survival curves. These are for (A) immunosuppression treatment (IST) versus no IST; (B) IST versus no IST stratified by nephrotic-range proteinuria; (C) IST versus no IST stratified by albumin ≤3.0 mg/dl; and (D) IST versus no IST stratified by both nephrotic-range proteinuria (NRP) and albumin ≤3.0 mg/dl.
Multivariable Cox regression analysis found that serum albumin, eGFR, percent IFTA, percent inflammation, and NRP at biopsy-confirmed FSGS diagnosis all remained significantly associated with ESKD (Tables 3 and 4). Black race was not associated with ESKD. Two separate Cox regression analyses were performed, which included either IFTA or interstitial inflammation because of high clinical correlation. RAAS inhibition and hypertension were not included in the model because they were present in over 97% of patients with FSGS.
Table 3.
Multivariate analysis including IFTA
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
| Age | 1.03 (1.00 to 1.06) | 0.008 |
| Black race | 0.9 (0.5 to 1.8) | 0.77 |
| Albumin ≤3.0 mg/dl | 4.2 (1.7 to 11.1) | 0.003 |
| Proteinuria >3.5 gm | 3.1 (1.3 to 7.3) | 0.009 |
| eGFR >60 ml/min/1.732 | 0.23 (0.09 to 0.53) | 0.001 |
| IFTA ≥15% | 1.5 (1.0 to 2.2) | 0.05 |
| Complete remission | 0.06 (0.02 to 0.24) | <0.001 |
| Partial remission | 0.18 (0.07 to 0.48) | 0.001 |
| IST | 0.62 (0.26 to 1.5) | 0.28 |
Each variable is adjusted for the others listed in the table. IFTA, interstitial fibrosis and tubular atrophy; IST, immunosuppression treatment.
Table 4.
Multivariate analysis including interstitial inflammation
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
| Age | 1.05 (1.02 to 1.08) | 0.001 |
| Black race | 0.7 (0.3 to 1.6) | 0.38 |
| Albumin ≤3.0 mg/dl | 2.0 (0.7 to 5.5) | 0.19 |
| Proteinuria >3.5 gm | 5.7 (2.3 to 14.3) | <0.001 |
| eGFR >60 ml/min/1.732 | 0.17 (0.07 to 0.41) | <0.001 |
| Interstitial inflammation ≥15% | 2.6 (1.1 to 6.4) | 0.04 |
| Complete remission | 0.04 (0.01 to 0.18) | <0.001 |
| Partial remission | 0.12 (0.04 to 0.32) | <0.001 |
| IST | 0.55 (0.23 to 1.3) | 0.18 |
Each variable is adjusted for the others listed in the table. IST, immunosuppression treatment.
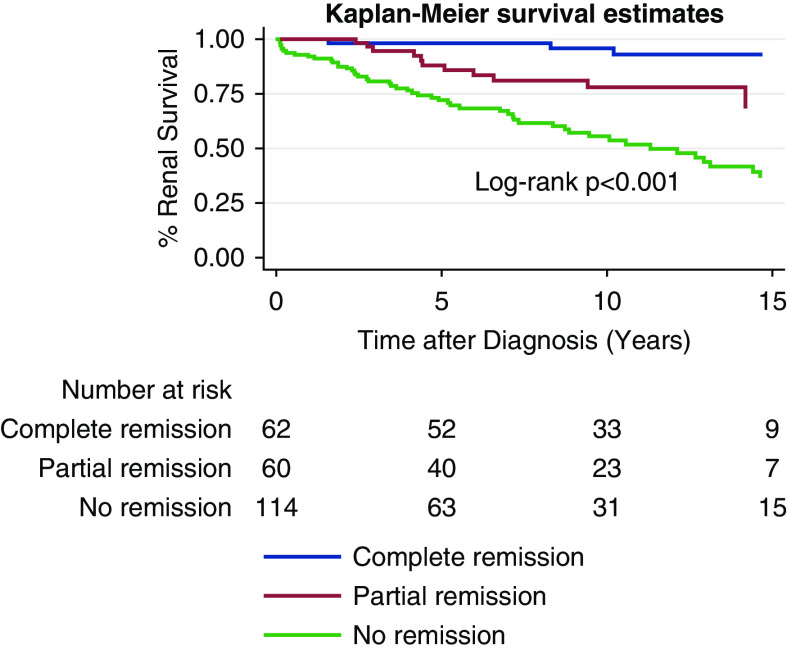
Complete and partial remission occurred in 26% and 25% of patients with FSGS, respectively. Renal survival was significantly better with complete and partial remission than no remission at 5, 10, and 15 years (Figure 4). Remission remained significantly associated with renal survival after adjustment for confounding variables (Table 3).
Figure 4.
FSGS Kaplan-Meier curves for renal survival curve for complete remission (blue), partial remission (red), and no remission (green).
Discussion
This large racially and ethnically diverse adult FSGS cohort with a long clinical follow-up period provides valuable prognostic and IST treatment outcome data to instruct patient counseling and care, and guide future research. Because of our large cohort size, we were able to demonstrate that NRP, eGFR <60 ml/min per 1.73 m2, hypoalbuminemia, IFTA, interstitial inflammation, and absence of remission were all associated with reduced renal survival even after adjusting for potential confounding variables (Tables 3 and 4). Previous studies reported mixed and sometimes conflicting results on these potential risk factors. Many did not have a large enough cohort for multivariate analysis (3–15). Similar to previous studies, Black and White race had similar long-term renal survival. We also report for the first time that Hispanic ethnicity had similar long-term renal survival to non-Hispanic patients with FSGS.
The overall 5-year renal survival in our cohort was similar to the cohort of 250 patients reported by Bohle et al. (86% versus 90%), but significantly better at 10 years (75% versus 50%) and 15 years (65% versus 35%) (Supplemental Table 1) (6). A similar pattern of improved renal survival was noted in between NRP subgroups at 10-year follow-up (69% versus 50%) and 15-year follow-up (50% versus 30%) and between CKD subgroups at 15-year follow-up (39% versus 25%) (Figure 1). In addition, patients in our cohort that did not achieve remission had a higher percent renal survival than the 281-patient cohort published by Troyanov et al. at 10-year (56% vs 40%) and 15-year (37% versus 20%) follow-up (4,5). The same trends were seen when comparing our cohort to other smaller cohorts (7,9–11,13). The most likely explanation for the higher observed long-term renal survival in these high-risk FSGS subgroups is the ubiquitous use of RAAS inhibition in our cohort (97%). However, we were limited in our ability to compare renal outcomes on the basis of RAAS inhibition because only nine patients were not treated. Previous retrospective FSGS studies found that RAAS inhibition reduced proteinuria, but did not affect renal survival (17–20). However, these studies were limited by their small cohorts and short follow-up periods. Three larger cohort studies documented 50%–90% RAAS inhibition but they neither specifically analyzed the association of RAAS inhibition and renal survival nor had overall renal survival data at 10 and 15 years (3,4,14). The studies that did have long-term renal survival data included patients predominantly from time periods before significant RAAS inhibition use (5–7,9–11,13). Although significantly limited by historical comparison, our study suggests RAAS blockade may provide some long-term renal survival benefit even in high-risk patients with FSGS, which supports current Kidney Disease: Improving Global Outcomes guidelines. Alternatively, our study had a larger number of patients still tracked at 10 and 15 years, which decreases bias in our estimates. In addition, we provide the first renal survival curves using eGFR at diagnosis which will provide more robust prognostic information for reduced renal function present at diagnosis.
Interstitial inflammation was independently associated with worse long-term renal survival (Figure 1, Table 4). The most likely explanation is that interstitial inflammation transitioned to fibrosis. However, IST was not associated with improved renal survival in an FSGS subgroup with interstitial inflammation. Given observed trends, it is possible that IST treatment in patients with FSGS and interstitial inflammation is associated with improved renal survival after 10 years, but statistical significance was not demonstrated due to the low number of patients at these time intervals (Supplemental Figure 5). Interstitial inflammation has not been previously assessed as a risk factor for progression to ESKD in an FSGS cohort. Our data suggest it could help assess kidney prognosis and be a topic for future study.
There has been renewed focus on the potential pathogenic role of IgM antibody and complement activity in FSGS (21,22). Strassheim et al. (21) reported compelling evidence from a murine model that IgM binds in the glomerulus and activates complement to cause increased proteinuria. Two FSGS cohort studies found no association between IgM staining on immunofluorescence and long-term renal survival in FSGS (23,24). C3 staining was associated with worse renal outcomes in one study but not another (23,25). The combination of IgM and C3 staining was associated with both renal dysfunction and poor renal survival previously (23,24). In our cohort, C3, IgM, or a combination of IgM/C3 staining on immunofluorescence were not associated with reduced renal survival (Supplemental Figure 3). Previous cohorts that investigated IgM/C3 staining included predominantly Asian patients, had significantly fewer patients, and a shorter follow-up than our cohort.
Immunosuppression was not associated with better renal survival in the whole FSGS cohort (Figure 3). Immunosuppression was also not associated with a renal survival benefit in subgroups stratified separately by proteinuria and eGFR at diagnosis. However, it is possible that immunosuppression may have provided benefit in patients with FSGS and NRP (P=0.09) if the stratified cohort had been larger to provide more power. Interestingly, when further stratified by hypoalbuminemia and proteinuria together and hypoalbuminemia alone, immunosuppression was associated with improved renal survival (Figure 3). Specifically, the lower the serum albumin at diagnosis, the more IST was associated with improved long-term renal survival. Analysis of hypoalbuminemia alone is relevant because it is possible that some patients with FSGS with proteinuria ≤3.5 gm on spot urine protein-to-creatinine ratio would have had >3.5 gm if measured by the gold-standard 24-hour urine collection, and therefore met the criteria for nephrotic syndrome if accompanied by a serum albumin ≤3.0 g/dl. Our findings suggest that immunosuppression may only significantly benefit patients with FSGS and nephrotic syndrome and not FSGS of undetermined cause with significant proteinuria but preserved serum albumin. This is the strongest evidence to date to support the 2012 and 2020 draft Kidney Disease: Improving Global Outcomes guidelines for FSGS treatment, which were based in part on two previous publications, but acknowledges that treatment strategy for FSGS of undetermined cause is a “conundrum” (26,27). Laurin et al. found that immunosuppression was not associated with improved renal survival in the whole FSGS cohort. However, immunosuppression was beneficial in the subgroup of collapsing patients with FSGS, which had 300% higher mean proteinuria and significantly lower mean serum albumin to suggest a much higher incidence of nephrotic syndrome in that subpopulation (14). Schwartz et al. (13) also found that immunosuppression was associated with better renal survival in patients with NRP FSGS but not the whole FSGS cohort. However, serum albumin levels were not reported. The other IST outcome studies did not provide subgroup analysis of hypoalbuminemia or nephrotic syndrome. Most studies demonstrate that IST is associated with increased remission (5,7,8,10,11). Other studies show that remission is associated with improved long-term renal survival (5,7,8,10,11,13). However, this does not guarantee that IST is associated with long-term renal survival. At least three studies reported that IST was not associated with improved renal outcomes, despite it being associated with remission and remission being associated with renal survival (7,10,11). Two other studies found that IST was not associated with long-term renal survival, but did not also have robust analysis of both the IST/remission and the remission/renal survival relationships (6,13). One possible explanation for this pattern is that the renal survival benefit from of IST-induced remission in a nephrotic syndrome subpopulation could be negated by increased IST-related renal risk factors such as hypertension, hyperglycemia, increased BMI, and interstitial fibrosis within the whole cohort. When stratified by race, IST was not associated with improved renal survival in Black patients. Although we did not measure APOL1 genotype, this would suggest IST does not directly treat the specific pathophysiology of APOL1-associated FSGS (8,28,29). Therefore, developing more targeted therapy would likely be beneficial.
The strength of this study is that it is the largest racially and ethnically diverse adult FSGS cohort from the era of RAAS inhibition with the longest follow-up period. In addition, patients were predominantly managed within the same health care system. This minimized potential confounding due to differences in access to care, quality of care, and socioeconomic factors. It also reports on the effect of interstitial inflammation and IgM and C3 staining on immunofluorescence on renal survival.
Our study had limitations intrinsic to the retrospective cohort design. Specifically, there was significant variability in treatment strategies, follow-up intervals, and laboratory data. Some patients did not have all of the desired data available in the EMR for collection. A detailed histopathology report was not available for each patient nor was each biopsy reviewed independently by a study pathologist. The majority of detailed pathology reports did not comment on a specific FSGS lesion. Interstitial inflammation and IFTA severity were assigned by either reported percent or description. Both are estimates subject to pathologist interpretation and sample variability. Correlation between percent and descriptive report of interstitial inflammation and IFTA has not been previously investigated. These limitations could explain why we found no renal survival benefit for IST in patients with higher interstitial inflammation. Our cohort was predominantly male likely because it was derived from prior and current military members. Despite our large cohort with long median follow-up, we were not able to adjust for all potential confounding variables in multivariable analyses because it would reduce study power and significance. We therefore focused principally on variables with statistical significance in univariate analysis. Specifically, we did not include BMI in the multivariate analysis, because obesity was not associated with ESKD on univariate analysis or renal survival on Kaplan Meier analysis. We did not have genetic analysis for the study cohort (2). Uneven distribution of established FSGS risk mutations that are generally more resistant to steroid treatment, could have been a confounding variable in data analysis. However, inadvertent inclusion of steroid-resistant genetic causes of FSGS would have only reduced or negated the significance of our treatment comparisons. Specifically, we were not able to adjust for high-risk APOL1 genotype, but most clinicians do not currently have access to this information, so our data can still be instructive for real-time patient care. We did not record the exact duration of prednisone treatment for each patient due to the challenges of tracking the exact timing of not only cessation of treatment, but also the variable weaning strategies. However, any abridged steroid courses would most likely have only reduced the significance of our treatment analysis. We also cannot fully delineate between proteinuria and a negative acute phase reactant of systemic inflammation as the cause of hypoalbuminemia, although IST would theoretically improve both. Finally, Hispanic ethnicity data were missing from a large number of patients with FSGS, so data analysis in this group may have been underpowered to show existing significant differences. Future study of FSGS in the Hispanic population is warranted.
This large, racially and ethnically diverse adult FSGS cohort with long-term follow-up provides important prognostic information for clinicians and suggests IST should be reserved only for FSGS with nephrotic syndrome, but a prospective trial is required to confirm these findings.
Disclosures
D. Little and P. Greasley both work for AstraZeneca. They have no significant financial arrangements to produce or sell products that are the subject of the studies reported in this manuscript. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government.
Author Contributions
B.M. Forster and S.W. Olson were responsible for conception, design, analysis and interpretation of data, manuscript drafting, intellectual content, and final approval. D.J. Little, P.J. Greasley, J.B. Hughes, S.M. Gordon were responsible for the conception, design, manuscript revision, intellectual content, and final approval.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006172020/-/DCSupplemental.
FSGS Kaplan-Meier curves for renal survival in the whole cohort. Download Supplemental Figure 1, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) Black patients vs. White patients and B) Hispanic patients vs. Non-Hispanic patients C) Patients with BMI >30 vs. BMI <30. Download Supplemental Figure 2, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) IgM positive immunofluorescence versus IgM negative immunofluorescence; B) C3 positive immunofluorescence versus C3 negative immunofluorescence; C) IgM and C3 positive immunofluorescence versus IgM and C3 negative immunofluorescence. Download Supplemental Figure 3, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) Immunosuppression treatment (IST) versus no IST stratified by estimate glomerular filtration rate ≥ 60 ml/min; B) Immunosuppression treatment (IST) versus no IST stratified by estimate glomerular filtration rate < 60 ml/min. Download Supplemental Figure 4, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival for Immunosuppression treatment (IST) versus no IST stratified by interstitial inflammation ≥ 15%. Download Supplemental Figure 5, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curve for renal survival for immunosuppression treatment (IST) versus no IST stratified by Black race. Download Supplemental Figure 6, PDF file, 716 KB (715.1KB, pdf)
References
- 1.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012. 10.1681/ASN.2012040389 [DOI] [PubMed] [Google Scholar]
- 2.De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC: Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol 29: 759–774, 2018. 10.1681/ASN.2017090958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korbet SM, Schwartz MM, Lewis EJ: Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis 23: 773–783, 1994. 10.1016/S0272-6386(12)80128-4 [DOI] [PubMed] [Google Scholar]
- 4.Laurin LP, Gasim AM, Poulton CJ, Hogan SL, Jennette JC, Falk RJ, Foster BJ, Nachman PH: Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol 11: 386–394, 2016. 10.2215/CJN.07110615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group: Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Wehrmann M, Bohle A, Held H, Schumm G, Kendziorra H, Pressler H: Long-term prognosis of focal sclerosing glomerulonephritis. An analysis of 250 cases with particular regard to tubulointerstitial changes. Clin Nephrol 33: 115–122, 1990 [PubMed] [Google Scholar]
- 7.Stirling CM, Mathieson P, Boulton-Jones JM, Feehally J, Jayne D, Murray HM, Adu D: Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM 98: 443–449, 2005. 10.1093/qjmed/hci072 [DOI] [PubMed] [Google Scholar]
- 8.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ: Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 15: 2169–2177, 2004. 10.1097/01.ASN.0000135051.62500.97 [DOI] [PubMed] [Google Scholar]
- 10.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM: Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542, 1995. 10.1016/0272-6386(95)90120-5 [DOI] [PubMed] [Google Scholar]
- 11.Cattran DC, Rao P: Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32: 72–79, 1998. 10.1053/ajkd.1998.v32.pm9669427 [DOI] [PubMed] [Google Scholar]
- 12.Korbet SM, Schwartz MM, Lewis EJ: The prognosis of focal segmental glomerular sclerosis of adulthood. Medicine (Baltimore) 65: 304–311, 1986. 10.1097/00005792-198609000-00003 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MM, Evans J, Bain R, Korbet SM: Focal segmental glomerulosclerosis: prognostic implications of the cellular lesion. J Am Soc Nephrol 10: 1900–1907, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Laurin LP, Gasim AM, Derebail VK, McGregor JG, Kidd JM, Hogan SL, Poulton CJ, Detwiler RK, Jennette JC, Falk RJ, Nachman PH: Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol 11: 1752–1759, 2016. 10.2215/CJN.13091215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron JS, Turner DR, Ogg CS, Chantler C, Williams DG: The long-term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol 10: 213–218, 1978 [PubMed] [Google Scholar]
- 16.Gordon SM, Stitt RS, Nee R, Bailey WT, Little DJ, Knight KR, Hughes JB, Edison JD, Olson SW: Risk factors for future scleroderma renal crisis at systemic sclerosis diagnosis. J Rheumatol 46: 85–92, 2019. 10.3899/jrheum.171186 [DOI] [PubMed] [Google Scholar]
- 17.Korbet SM: Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol 23: 219–228, 2003. 10.1053/snep.2003.50020 [DOI] [PubMed] [Google Scholar]
- 18.Crenshaw G, Bigler S, Salem M, Crook ED: Focal segmental glomerulosclerosis in African Americans: effects of steroids and angiotensin converting enzyme inhibitors. Am J Med Sci 319: 320–325, 2000. 10.1016/S0002-9629(15)40759-1 [DOI] [PubMed] [Google Scholar]
- 19.Praga M, Hernández E, Montoyo C, Andrés A, Ruilope LM, Rodicio JL: Long-term beneficial effects of angiotensin-converting enzyme inhibition in patients with nephrotic proteinuria. Am J Kidney Dis 20: 240–248, 1992. 10.1016/S0272-6386(12)80696-2 [DOI] [PubMed] [Google Scholar]
- 20.Stiles KP, Abbott KC, Welch PG, Yuan CM: Effects of angiotensin-converting enzyme inhibitor and steroid therapy on proteinuria in FSGS: a retrospective study in a single clinic. Clin Nephrol 56: 89–95, 2001 [PubMed] [Google Scholar]
- 21.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanović D, Holers VM, Thurman JM: IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol 24: 393–406, 2013. 10.1681/ASN.2012020187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurman JM, Wong M, Renner B, Frazer-Abel A, Giclas PC, Joy MS, Jalal D, Radeva MK, Gassman J, Gipson DS, Kaskel F, Friedman A, Trachtman H: Complement activation in patients with focal segmental glomerulosclerosis. PLoS One 10: e0136558, 2015. 10.1371/journal.pone.0136558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YM, Gu QH, Huang J, Qu Z, Wang X, Meng LQ, Wang F, Liu G, Cui Z, Zhao MH: Clinical significance of IgM and C3 glomerular deposition in primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 11: 1582–1589, 2016. 10.2215/CJN.01190216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirioglu S, Caliskan Y, Ozluk Y, Dirim AB, Istemihan Z, Akyildiz A, Yazici H, Turkmen A, Kilicaslan I, Sever MS: Co-eposition of IgM and C3 may indicate unfavorable renal outcomes in adult patients with primary focal segmental glomerulosclerosis. Kidney Blood Press Res 44: 961–972, 2019. 10.1159/000501827 [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Cui Z, Gu QH, Zhang YM, Qu Z, Wang X, Wang F, Cheng XY, Meng LQ, Liu G, Zhao MH: Complement activation profile of patients with primary focal segmental glomerulosclerosis. PLoS One 15: e0234934, 2020. 10.1371/journal.pone.0234934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) glomerulonephritis work group, KDIGO clinical practice guideline for glomerulonephritis. 2020. Draft (unpublished)
- 27.National Kidney Foundation . KDIGO clinical practice guidelines for glomerulonephritis. Available at https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed March 1, 2020
- 28.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman BI, Limou S, Ma L, Kopp JB: APOL1-associated nephropathy: A key contributor to racial disparities in CKD. Am J Kidney Dis 72[Suppl 1]: S8–S16, 2018. 10.1053/j.ajkd.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FSGS Kaplan-Meier curves for renal survival in the whole cohort. Download Supplemental Figure 1, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) Black patients vs. White patients and B) Hispanic patients vs. Non-Hispanic patients C) Patients with BMI >30 vs. BMI <30. Download Supplemental Figure 2, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) IgM positive immunofluorescence versus IgM negative immunofluorescence; B) C3 positive immunofluorescence versus C3 negative immunofluorescence; C) IgM and C3 positive immunofluorescence versus IgM and C3 negative immunofluorescence. Download Supplemental Figure 3, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival: A) Immunosuppression treatment (IST) versus no IST stratified by estimate glomerular filtration rate ≥ 60 ml/min; B) Immunosuppression treatment (IST) versus no IST stratified by estimate glomerular filtration rate < 60 ml/min. Download Supplemental Figure 4, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curves for renal survival for Immunosuppression treatment (IST) versus no IST stratified by interstitial inflammation ≥ 15%. Download Supplemental Figure 5, PDF file, 716 KB (715.1KB, pdf)
FSGS Kaplan-Meier curve for renal survival for immunosuppression treatment (IST) versus no IST stratified by Black race. Download Supplemental Figure 6, PDF file, 716 KB (715.1KB, pdf)