Significance
Because dengue viruses are spread by mosquitoes during biting, transmission capacity depends on mosquito-biting behavior. For this reason, it is critical to understand how infection in mosquitoes influences biting. To answer this question, we deployed a multidisciplinary approach including high-resolution, multivariate biting behavior monitoring on mice, in vivo transmission assay, and mathematical modeling. We demonstrated that infected mosquitoes are more attracted to mice and bite more often to get the same amount of blood as uninfected mosquitoes. While the effect of increased attraction to host on transmission capacity is trivial, we showed that increased number of bites results in successive transmission. Eventually, we calculated that the infection-induced behavior changes tripled transmission capacity of mosquitoes.
Keywords: mosquito, dengue virus, blood-feeding behavior, transmission, epidemiology
Abstract
Mosquito blood-feeding behavior is a key determinant of the epidemiology of dengue viruses (DENV), the most-prevalent mosquito-borne viruses. However, despite its importance, how DENV infection influences mosquito blood-feeding and, consequently, transmission remains unclear. Here, we developed a high-resolution, video-based assay to observe the blood-feeding behavior of Aedes aegypti mosquitoes on mice. We then applied multivariate analysis on the high-throughput, unbiased data generated from the assay to ordinate behavioral parameters into complex behaviors. We showed that DENV infection increases mosquito attraction to the host and hinders its biting efficiency, the latter resulting in the infected mosquitoes biting more to reach similar blood repletion as uninfected mosquitoes. To examine how increased biting influences DENV transmission to the host, we established an in vivo transmission model with immuno-competent mice and demonstrated that successive short probes result in multiple transmissions. Finally, to determine how DENV-induced alterations of host-seeking and biting behaviors influence dengue epidemiology, we integrated the behavioral data within a mathematical model. We calculated that the number of infected hosts per infected mosquito, as determined by the reproduction rate, tripled when mosquito behavior was influenced by DENV infection. Taken together, this multidisciplinary study details how DENV infection modulates mosquito blood-feeding behavior to increase vector capacity, proportionally aggravating DENV epidemiology. By elucidating the contribution of mosquito behavioral alterations on DENV transmission to the host, these results will inform epidemiological modeling to tailor improved interventions against dengue.
Dengue viruses (DENV) threaten almost half of the world’s population due to the geographic distribution of their mosquito vectors, primarily Aedes aegypti (1). Dengue symptoms range from flu-like to severe forms, including potentially lethal hemorrhage. Currently, there are no approved therapeutics, and the only licensed vaccine can increase the risk of severe disease in immunologically naïve patients, thereby restraining vaccine uptake in the population (2, 3). Furthermore, while vector control has been the sole widely deployed intervention, current control methods can only reduce epidemics and require sustained long-term endeavor (4, 5). Here, we want to improve the understanding of factors that influence DENV transmission by mosquitoes to calibrate existing intervention strategies.
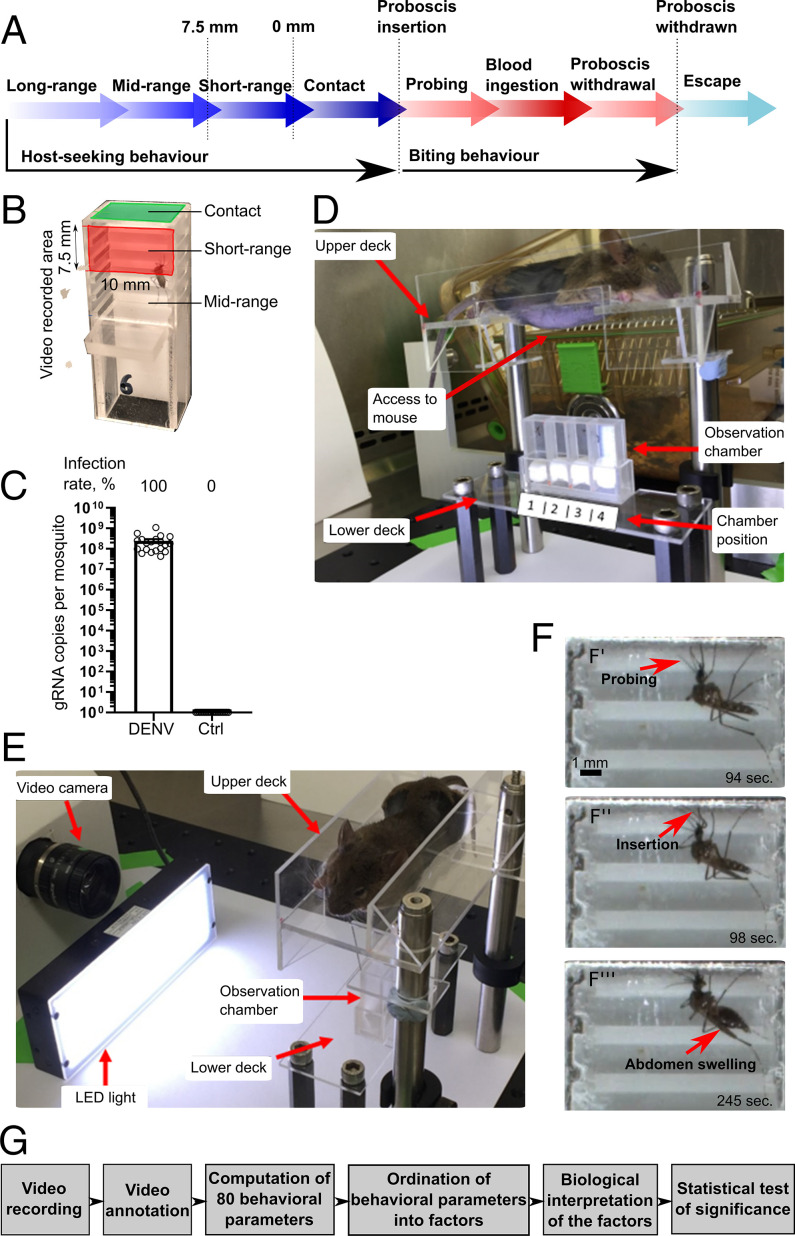
DENV transmission occurs during mosquito blood-feeding, which is composed of two behavioral stages: host-seeking and biting behaviors (6) (Fig. 1A). Host-seeking is initiated at a long-range distance (several meters) by the detection of host olfactory cues (7). Higher concentration of CO2, as seen during exhalation by humans, is sufficient to elicit the host-seeking behavior sequence in mosquitoes (8–10) that begins with their upwind flying to trace back the CO2 plume. They then narrow down the host target by sensing volatile, host-emitted odors. As the mosquitoes get closer to the host, they integrate visual and thermal cues (10, 11). At short-range distance, while CO2-sensing is no longer necessary, thermal, visual, humidity, and other olfactory cues are integrated into a multimodal sensory detection mechanism that guides mosquito-landing on their host (9, 10). Upon contact, mosquitoes evaluate host–surface chemistry using sensory appendages on their mouthparts and legs (6, 12, 13). The host-seeking behavior is completed when a mosquito inserts its proboscis (mouthparts) into the host skin (Fig. 1A).
Fig. 1.
Description of the behavioral assay. (A) Mosquito blood-feeding behavioral stages. (B) Observation chamber. (C) Viral load in whole mosquitoes at 10 d postoral infection with DENV or postoral feeding on noninfectious blood meal (Ctrl). Bars show geometric means ± 95% CI from 20 mosquitoes in each condition. (D and E) Behavioral assay device. An anesthetized mouse with a shaved belly was positioned on the upper deck, before lowering the upper deck onto the lower deck, which contained four observation chambers. Mosquitoes were video recorded for 30 min. (F) Examples of video-recorded pictures showing probing initiation (F’), proboscis insertion (F’’), and blood ingestion with abdomen swelling (F’’’). (G) Schematic of the high-resolution, high-throughput behavioral assay.
The biting behavior begins with the mosquito proboscis probing through the host skin to locate a blood source (6) (Fig. 1A). Upon proboscis insertion, a retractable outer cover called labium bends to unravel a fascicle composed of six stylets (14). These include a pair of sharp mandibles and a pair of serrated maxilla that effortlessly pierce through the skin by vibration (15). The hypopharynx, which is enclosed within a sheath-like structure formed by the maxilla and mandibles, releases saliva along the proboscis path to limit bite-sensing, platelet aggregation, blood-clotting, and the immune response (16, 17). Also enclosed within this sheath is a hollow, pointed tube called labrum. Gustatory sensilla on the labrum guide the fascicle through the epidermis and dermis to locate a blood vessel (18). Once located, the labrum punctures the vessel and ingests blood upon activation of the cibarial pump (6). In the absence of any disturbance, mosquitoes ingest blood until abdominal stretch receptors signal repletion, which then induces proboscis withdrawal and mosquito escape flight (19). Alternatively, if the mosquitoes are disturbed, they resume an earlier step or interrupt the behavior. Hereafter, we define a successful bite as the completion of the biting behavior sequence up to blood ingestion and an unsuccessful bite as a biting sequence that does not result in blood ingestion.
DENV transmission occurs during a mosquito bite when the virus is expectorated with the saliva. Almost the entire viral load is deposited extravascularly in the skin (20–22). The keratinocytes and fibroblasts, which are the prevailing cell types in the skin, are productively infected (23–25). The ensuing inflammatory response attracts myeloid cells, which then become infected and carry the viruses to lymph nodes, triggering systemic infection (24, 26). Therefore, skin infection triggered by mosquito bites is solely responsible for the onset of systemic infection and transmission (20, 27). Topical interferon-based treatment soon after a mosquito bite was shown to block systemic infection, strongly supporting the requirement for replication in the skin (28). Overall, initial skin infection and subsequent systemic infection rely on mosquito blood-feeding behavior (29).
Despite the importance of mosquito blood-feeding behavior in determining DENV transmission efficiency, the impact of viral infection on the behavior remains inconclusive and sometimes controversial. On the one hand, it is well established that DENV infection increases mosquito locomotor activity (30–32), potentially augmenting its dispersal to facilitate encounters with a host. Combined with an enhanced sensitivity to synthetic human odors (31), infection is expected to increase attraction to the host. On the other hand, some studies have shown that DENV reduces attraction to the host (33) and slightly decreases probing initiation time (17), which is a proxy for host-seeking efficiency. During a bite, DENV infection extends probing duration at 5, 8, and 11 d postinfection (dpi), whereas it surprisingly has no impact on the same parameter at the interspersed 7, 9, 10, and 20 dpi (34). Another study reported no impact on probing duration but an extension of blood ingestion duration (35). Yet another study found that both the probing and blood-feeding durations were not affected in DENV-infected mosquitoes (36). We suggest that these inconsistencies probably originate from methodological and technical flaws, such as artificial infection route through inoculation (34, 36), low number of repeats (<10) (17, 36), use of dead mice (17), limited number of measured parameters (all cited studies quantified less than seven behavioral parameters), infection with laboratory-adapted virus strain (17, 33, 35, 36), or lack of high-resolution enlarged visuals (17, 30–36). These limitations may have led to biased observations of the intertwined behavioral sequences that take place during mosquito blood-feeding.
To determine the impact of DENV infection on A. aegypti blood-feeding behavior and thus on their viral transmission capacity to the host, we designed a high-resolution, close-up, video-recorded assay to analyze the final sequences of the host-seeking and the entire biting stage. We measured and timed mosquito-walking and immobile activities, mosquito position at midrange, short-range, and in contact with the host, proboscis insertion, insertion length, motions within the skin, wriggling, and grooming, and blood ingestion and the resultant abdominal swelling. After annotating the videos, we used a computerized algorithm to calculate 80 behavioral parameters and applied multivariate statistics to reveal the complex and interconnected behavioral changes. Using low-passage virus and anesthetized mice, we observed that DENV infection increased attraction to the host and decreased probing efficiency; the latter resulted in a higher number of unsuccessful bites to obtain a similar blood meal size as those obtained by uninfected mosquitoes. To demonstrate that the increased number of bites augmented transmission capacity, we showed that DENV is transmitted at each successive probes in immuno-competent mice. Using mathematical modeling, we calculated that by increasing host attraction and frequency of infectious probes, DENV-induced alterations of mosquito blood-feeding behavior tripled transmission capacity.
Results
Blood-Feeding Behavioral Assay.
To video-record mosquito blood-feeding behavior, we built a transparent observation chamber capped with a mesh (Fig. 1B, SI Appendix, Fig. S1A, and Methods). A. aegypti were orally infected with a low-passage DENV serotype 2 (DENV2) at an inoculum dose in the moderate-to-high range of what is quantified in patient blood (37), resulting in 100% infection rate in the mosquitoes (Fig. 1C). Control mosquitoes were fed uninfected blood. We started recording encaged mosquitoes as soon as the upper platform was lowered to position an anesthetized mouse in contact with the mesh of the observation chambers (Fig. 1 D and E and SI Appendix, Fig. S1B). To determine the transmission potential of a mosquito, we observed its behavior over a meaningful period of time (30 min; refer to example in Movie S1). During this time, the mosquitoes were able to complete host-seeking and biting behaviors or resume blood-feeding behavior if their previous attempts were not successful or blood repletion was not satisfactory (Fig. 1A). We recorded 65 control and 52 infected mosquitoes, as some of the mosquitoes did not survive the starvation.
The videos were manually annotated with the help of an in-house software that registered the time of occurrence of each activity and the lengths of the abdomen and inserted proboscis (SI Appendix, Table S1; refer to an example of annotation in Movie S2). Since the high-resolution camera could only record the top 7.5-mm region of the chamber, we arbitrarily chose to define this area as short-range distance and the region below (outside the video frame) as midrange distance (Fig. 1B).
To thoroughly describe blood-feeding behavior without preselecting parameters that will make presumptions based on the observations, we computed 80 behavioral parameters from the video annotations. The parameters included total duration of the activity, average duration per activity, count, time from start to the activity, and size of certain features for the activities observed at midrange, short-range, and contact distances and during probing, blood ingestion, body maintenance, and locomotor activities (SI Appendix, Tables S1 and S2). Within each stage, we interpreted the biological significance of the behavioral parameters as being significant for sensing, attraction to the host, or efficiency of the activity (SI Appendix, Table S1). We also categorized the parameters as belonging to the host-seeking or biting behavior stages (SI Appendix, Table S1). The behavioral assay allowed us to observe mosquito blood-feeding with unprecedented accuracy and to generate a large set of unbiased, statistically analyzable data (Fig. 1G).
Multivariate Statistical Analysis Uncovered Complex Behavioral Patterns.
We analyzed host-seeking and biting behavior stages separately. First, we analyzed host-seeking parameters for all mosquitoes, considering that their physiological status was homogenized by the behavioral assay design (e.g., same age, starvation condition, temperature, and humidity). Second, we analyzed biting parameters only for mosquitoes that probed and thus had completed the host-seeking stage. Positing that each of the behavioral parameters alone (SI Appendix, Table S1) did not represent the complex behaviors in mosquito blood-feeding, we used factor analysis based on correlation matrix to ordinate parameters into factors, thereby revealing patterns among the behavioral parameters (38). We then biologically interpreted the factors and statistically determined the impact of infection on the factor scores (Fig. 1G). Factor analysis for similar purposes is routinely used in social sciences (39) and psychology (40) studies, and we have also previously deployed it in an agricultural entomology study (41). There are three advantages to the multivariate approach: 1) it limits multiple-comparison error through statistical ordination of the parameters into fewer factors, 2) it reveals complex mosquito behaviors associated with multiple parameters, and 3) it combines the multicollinearity among single behavioral parameters to increase contrast and diminish variance between the conditions (control versus infected), thereby enhancing statistical resolution of the composite factors (42). Eventually, as a confirmatory approach, we also tested the impact of infection on the single behavioral parameters using univariate statistics.
Interpretation of Host-Seeking Behavior Factors.
We applied multivariate analysis to 44 behavioral parameters that are associated with the host-seeking stage (SI Appendix, Table S3) for 65 control and 52 infected mosquitoes. In total, 11 factors were generated, but only nine of them that explained more than 5% of the total variance were analyzed (SI Appendix, Table S3). Each factor had two to seven parameters with high loadings (SI Appendix, Tables S4 and S5), which were used for biological interpretation as detailed in Methods.
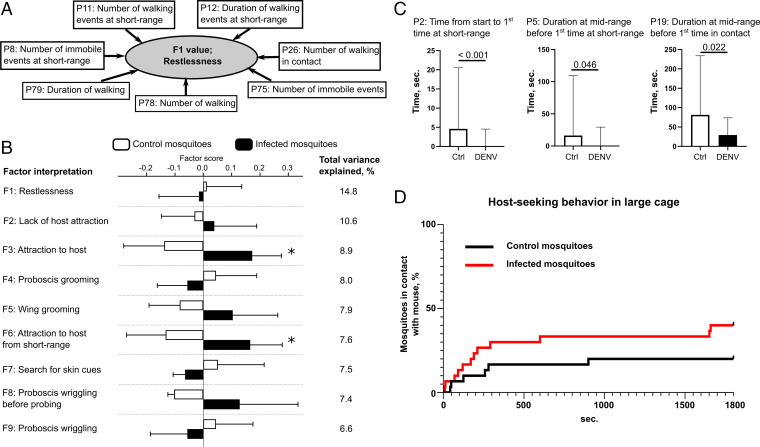
Factor 1, which clustered seven parameters related to locomotor activity and explained 14.8% of the total variance, was interpreted as “restlessness” (SI Appendix, Table S4 and Fig. 2 A and B). Factor 2, which clustered six parameters related to mosquito position, locomotor activity, and body maintenance and explained 10.6% of the total variance, was interpreted as “lack of host attraction.” Factor 3, which clustered six parameters related to mosquito position and explained 8.9% of the total variance, was interpreted as “attraction to host.” Factor 4, which clustered all the four parameters related to proboscis grooming and explained 8% of the total variance, was interpreted as “proboscis grooming.” Factor 5, which clustered all the four parameters related to wing grooming and explained 7.9% of the total variance, was interpreted as “wing grooming.” Factor 6, which clustered four parameters related to mosquito position and locomotor activity and explained 7.6% of the total variance, was interpreted as “attraction to host from short-range distance.” Factor 7, which clustered three parameters related to locomotor activity and explained 7.5% of the total variance, was interpreted as “search for skin cues.” Factor 8, which clustered all the three parameters related to proboscis wriggling before probing and explained 7.4% of the total variance, was interpreted as “proboscis wriggling before probing.” Factor 9, which clustered the two parameters related to proboscis wriggling and explained 6.6% of the total variance, was interpreted as “proboscis wriggling.”
Fig. 2.
DENV infection increases mosquito attraction to the host. (A) Schematic of the ordination of host-seeking behavioral parameters into biologically interpretable factors and example of Factor 1. (B) Factor analysis of host-seeking behavior. Bars show factor score means ± SEM. Variance explained by each factor is detailed. *P value < 0.055 as determined by unpaired t test. (C) Univariate analysis of host-seeking behavior. Bars indicate median ± 95% CI for three behavior parameters that were significantly (P value < 0.05 as determined by Mann–Whitney U test) different between infection (DENV) and control (Ctrl) conditions. (B and C) N infected = 52 and N control = 65. (D) Mosquito attraction to the mouse in large cages; N = 30 for each condition.
DENV Infection Increases Mosquito Attraction to the Host.
Analysis of the behavioral parameters revealed the impact of DENV infection on the host-seeking behavior. First, we observed that infection did not alter the probing rate: 70% versus 63% in control and infected mosquitoes, respectively (χ2 P value of 0.71; Table 1). As body size can influence feeding behavior (43), we measured the abdominal diameter before blood-feeding in infected and control mosquitoes. The abdomen was of the same size in both conditions (DENV = 0.77 ± 0.005; Control = 0.78 mm ± 0.006; t test, P value = 0.74; Dataset S1). The lack of effect on the probing rate suggests that DENV infection did not disturb the completion of host-seeking behavior within the time period. However, it is important to note that our experimental set-up allowed us to observe host-seeking behavior only at a short-range distance and did not account for long-range detection of the host. Other studies carried out using a larger cage that allows mosquito flight had shown that landing on a host mimic was accomplished in less than 120 s under uninfected condition (12). We posit that our longer observation time of 30 min may have provided multiple opportunities to the mosquitoes to initiate host-seeking, potentially minimizing the differences in the completion of host-seeking behavior, which is measured by probing rate, between infected and control mosquitoes.
Table 1.
Impact of DENV infection on rates of biting and blood ingestion
| Control mosquitoes | DENV-infected mosquitoes | P value* | |
| N observed | 65 | 52 | |
| N biting | 46 | 33 | |
| N blood ingesting | 40 | 27 | |
| Biting rate, percentage | 70.77 | 63.46 | 0.71 |
| Blood ingestion rate, percentage | 86.96 | 81.81 | 0.85 |
As determined by χ2 test.
Second, we compared the factor scores between the infected and control mosquitoes. Factor 3 and Factor 6, which were interpreted as “attraction to host” and as “attraction to host from short-range distance,” respectively, were increased by DENV infection (Fig. 2B). DENV infection did not affect the other factors that were indicative of “restlessness,” “lack of host attraction,” “proboscis grooming,” “search for skin cues,” and “proboscis wriggling.”
Third, we performed a “classical” univariate analysis on the 44 host-seeking parameters. “Time from start to first time at short-range” (parameter 2 [P2]), “duration at midrange before first time at short-range” (P5), and “duration at midrange before first time in contact” (P19) were significantly reduced upon infection (Fig. 2C and Dataset S2), confirming the multivariate analysis results.
Fourth, as the high-resolution behavioral assay only allowed analysis of mosquito behavior at a short-range distance in a small observation chamber that restricted mosquito flight, we monitored mosquito attraction to a mouse in a larger cage (30 × 30 × 30 cm), which allowed ample space for mosquito flight. Over the 30-min observation period, DENV-infected mosquitoes were more attracted to the mouse than the control mosquitoes (Fig. 2D; P value = 0.098 as determined by Mantel–Cox test). Altogether, the results show that DENV infection increases mosquito attraction to the host.
Interpretation of Biting Behavioral Factors.
Next, we applied multivariate analysis to the 36 behavioral parameters that are associated with the biting stage (Dataset S3) for 46 control and 33 infected mosquitoes that probed at least once. Eight factors were generated, but only five that explained more than 5% of the total variance were analyzed (SI Appendix, Table S6). Each factor included two to 10 parameters with high loadings (SI Appendix, Tables S7 and S8), which were used for biological interpretation as detailed in the methods section.
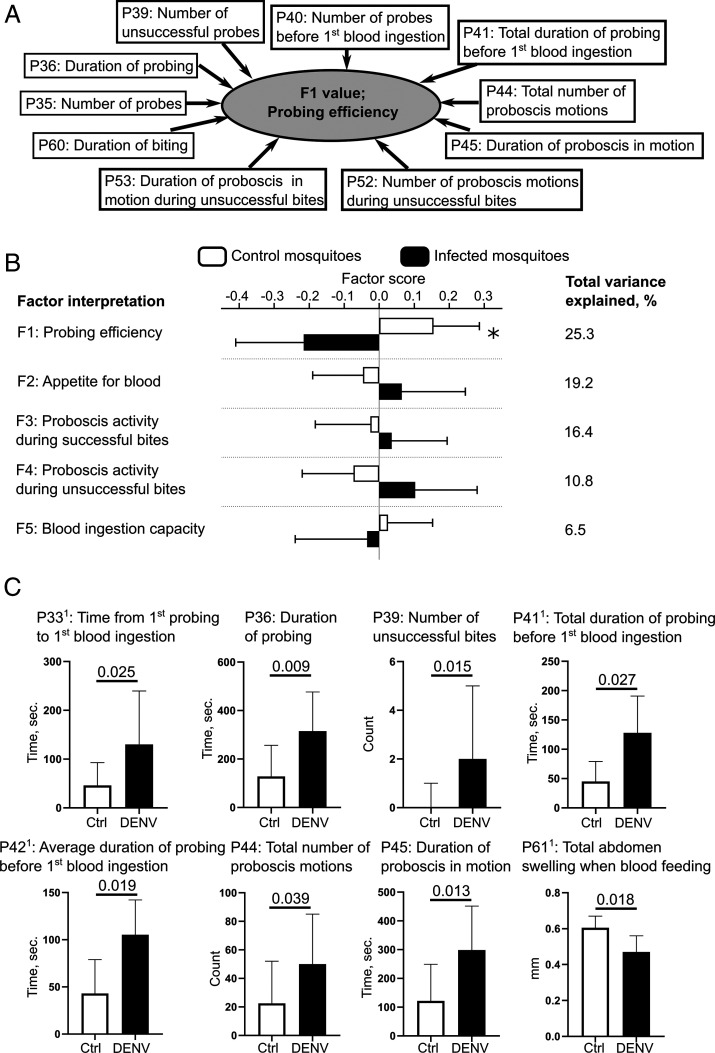
Factor 1, which clustered 10 parameters related to probing and explained 25.3% of the total variance (SI Appendix, Table S7 and Fig. 3 A and B), was interpreted as “probing efficiency.” Interestingly, parameters measuring average durations of probes (i.e., “average duration per probe” [P37], “average duration of probing before first blood ingestion” [P42], and “average duration of proboscis motions during unsuccessful bites” [P45]) were not correlated with factor 1 (SI Appendix, Table S8), suggesting that the duration of a single probe is not constrained by probing efficiency. In other words, when a bite is unsuccessful (i.e., does not lead to blood feeding), mosquitoes resume the biting-behavior sequence instead of extending the probing duration. Factor 2, which clustered nine parameters mostly related to blood ingestion but also to probing and mosquito position and explained 19.2% of the total variance, was interpreted as “appetite for blood.” Factor 3, which clustered seven parameters related to probing and explained 16.4% of the total variance, was interpreted as “proboscis activity during successful bites.” Factor 4, which clustered three parameters related to probing and explained 10.8% of the total variance, was interpreted as “proboscis activity during unsuccessful bites.” Factor 5, which clustered two parameters associated with blood ingestion and explained 6.5% of the total variance, was interpreted as “blood ingestion capacity.”
Fig. 3.
DENV-infection hinders probing efficiency. (A) Schematic of the ordination of biting behavioral parameters into biologically interpretable factors and example of Factor 1. (B) Factor analysis of the biting behavior. Bars show factor score means ± SEM. Variance explained by each factor is detailed. *P value < 0.055 as determined by unpaired t test. (C) Univariate analysis of the biting behavior. Bars indicate median ± 95% CI for eight behavior parameters that were significantly (P value < 0.05 as determined by Mann–Whitney U test) different between infection (DENV) and control (Ctrl) conditions. 1Parameters were calculated only with individuals that blood-fed. (B and C) N infected = 33 and N control = 46.
DENV Infection Increases the Number of Probes before Blood Ingestion.
Analysis of the behavioral parameters revealed the effects of DENV infection on the biting behavior. First, we observed that infection did not alter blood-feeding rate among biting mosquitoes −86% versus 81% for control and infected mosquitoes, respectively, with a χ2 P value of 0.85 (Table 1). This suggests that DENV-infected mosquitoes remain capable of completing biting by inserting proboscis, sensing a blood source, and imbibing blood.
Second, by comparing the factor scores between infected and control mosquitoes, we revealed that DENV infection reduced factor 1, which was interpreted as “probing efficiency” (Fig. 3B). In other words, DENV infection hampered the ability of mosquitoes to locate and/or perforate blood vessels. Inversely, “appetite for blood,” “proboscis activity during successful bites,” “proboscis activity during unsuccessful bites,” and “blood ingestion capacity” were not altered.
Third, we conducted a univariate analysis on the 36 biting parameters. To limit bias due to parameter approximations to accomodate a multivariate analysis (SI Appendix, Table S2), parameters related to blood ingestion were calculated only from mosquitoes that fed on blood (Dataset S3). We found that DENV infection increased several parameters related to probing duration, including “time from first probing to first blood ingestion” (P33), “total duration of probing before first blood ingestion” (P41), and “average duration of probing before first blood ingestion” (P42) (Fig. 3C), leading to an extended, overall probing duration to locate blood. Interestingly, “number of unsuccessful bites” (P39) was significantly increased by infection. As “average duration per probe” (P37) was only marginally impacted by infection (median [(95% CI], for Control = 73.24 [62 to 97.3] and for DENV = 89.4 [73.2 to 108.5]; P value = 0.098), we speculate that the extended, total probing duration before blood ingestion was due to the higher number of unsuccessful bites (Fig. 3B and Dataset S3). Similarly, the higher number of unsuccessful bites possibly explains the increase in “total duration of probing” (P36) caused by infection. Of note, “number of successful bites” (P38, P value = 0.32) and “number of blood ingestion events” (P57, P value = 0.12) were not altered upon infection (Dataset S3). Together, this indicates that infected mosquitoes have similar blood appetites compared to control mosquitoes and succeed in ingesting blood over the 30-min observation period. However, infected mosquitoes have to conduct more probing attempts before reaching the blood vessels than uninfected mosquitoes. In line with the multivariate analysis, the univariate analysis provided further insights into how DENV infection altered probing efficiency.
During mosquito probing, we observed upward and downward motions of the proboscis (Movie S1), which indicates directional changes while searching for blood (18). Upon DENV infection, “total number of proboscis motions” and “duration of proboscis motions” increased (Fig. 3C). However, as the “number of proboscis motions per probe” (P43) was not altered (P value = 0.265; Dataset S3), the increase in proboscis motion number and duration can be attributed to the extended probing duration (P36). As noted above about the probing duration, which is independent of bite output, these results point to mosquitoes following a behavior pattern with regards to their proboscis motions that does not depend on behavior output.
Intriguingly, when calculated from blood-fed mosquitoes only, “total abdomen swelling after blood ingestion” (P61) was reduced by infection (Fig. 3C). This may result from a combination of moderate reductions in “number of blood ingestion events” (P57) and “average abdomen swelling per blood ingestion event” (P62) (Dataset S3). The unfulfilled blood repletion that we observed over the 30-min period would likely stimulate further biting. Altogether, we observed that DENV infection in mosquitoes increased the number of probes necessary to achieve their blood satiety.
Successive Probing Results in Multiple Transmissions.
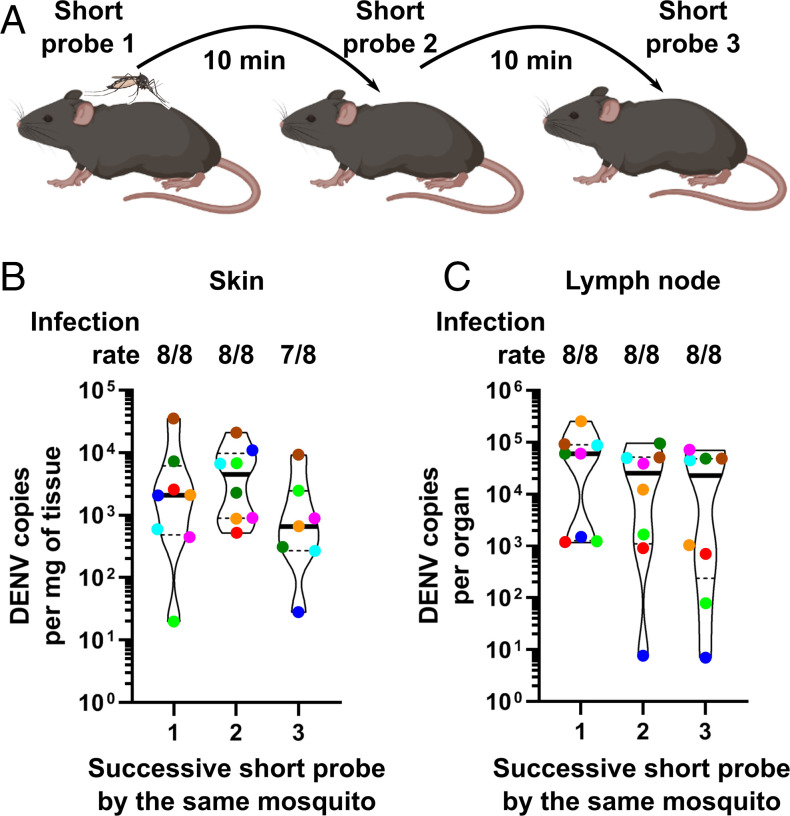
To determine the impact of the biting behavioral alterations on virus transmission, we artificially reproduced repeated probing (i.e., unsuccessful bites) by using short probes as a proxy for unsuccessful bites. To induce short probes, we let one infected mosquito bite one mouse and disturbed the probing 20 s after proboscis insertion. We chose this short time frame because in the behavioral analysis described above, only 6% (2 out of 33) of the infected mosquitoes achieved blood-feeding within 20 s of probing (P33 in Dataset S3). Accordingly, none of the short probes resulted in blood ingestion. Of note, as median for “time from first probing to first blood ingestion” (P33) was 53 and 172 s in uninfected and infected mosquitoes, respectively, the 20-s duration that we used represented a conservative measure of the transmission ability, which may vary with probing time. Following a 10-min rest period between bites, we then let the same mosquito conduct two successive short probes in two other mice (Fig. 4A). We chose to determine viral transmission over three short probes, as the median of unsuccessful bites for infected mosquitoes was two within 30 min (P39 in Dataset S3). Consistent with the behavioral assay, all eight tested mosquitoes achieved the three bites in less than 26 min (SI Appendix, Table S9). To evaluate virus multiplication and transmission, we quantified DENV in the skin and draining lymph nodes 24 h postprobing. Importantly, we used immuno-competent mice (wild-type C57BL/6) that we previously used to evaluate immune response to DENV inoculation (44, 45) but not as a model for virus transmission by mosquitoes. As DENV is susceptible to the murine immune response, its infectivity is usually evaluated in an immuno-compromised mouse model (46). However, immune deficiency can lead to overestimation of infectivity. For this reason, we used a wild-type animal model of mosquito transmission to quantify short-term DENV infectivity in an immune-relevant context and, thus, to accurately determine saliva infectivity.
Fig. 4.
Mosquitoes transmit DENV at each successive probe. (A) Schematic of the transmission assay. (B and C) DENV infection in skin (B) and the corresponding draining lymph nodes (C) from three mice successively bitten by the same infected mosquito. Infection rate indicates the number of infected tissues over the number of tissues bitten by different mosquitoes. Violin plots indicate median (thick line) and quartiles (dotted lines). Eight mosquitoes were analyzed, and each had bitten three different mice, totaling 24 different mice. Dots with the same color represent bites by the same mosquito.
Strikingly, all the eight mosquitoes transmitted DENV into the skin up to the second short probe and seven mosquitoes up to the third short probe (Fig. 4B). Among the infected skin samples, DENV genome copies were similar between the three successive short probes (P value = 0.24; Fig. 4B). To confirm active infection, we quantified DENV in the draining lymph nodes of these same mice, as the onset of infection here requires virus replication in the skin (28, 47). We detected DENV in lymph nodes (Fig. 4C), validating the immuno-competent mouse model to quantify bite-initiated transmission and demonstrating that the model replicates the expected natural route of infection. In contrast to what we observed in the skin, all three successive bites from the eight mosquitoes resulted in infection in the lymph nodes (Fig. 4C), including the third mosquito bite, which did not yield skin infection (red point, Fig. 4B). This might be explained by the viruses being carried by infected myeloid cells to the lymph nodes within 4 h postbiting (48). Similar to skin infection, there was no strong difference in viral load between the three short probes (P value = 0.06; Fig. 4C), confirming that each probe was equally infectious. It was previously shown that the saliva from DENV-infected mosquitoes remain infectious even after 20 successive probes on guinea pigs (49). However, the study was conducted using saliva that was artificially collected in capillaries—a method that biases the volume of saliva collected (20)—and infectivity was evaluated in qualitative terms in mosquitoes (i.e., detection of infection after injection in mosquitoes). In contrast, our assay quantified saliva infectivity in live mammals. Altogether, our results show that mosquitoes transmit DENV multiple times within a short period of time through short probes, thereby establishing a positive correlation between the number of bites and transmission events.
DENV-Altered Mosquito Blood-Feeding Behavior Increases Transmission Capacity.
Based on our observations of multiple DENV transmissions during successive short probes by infected mosquitoes, we assumed that DENV infection influences vector capacity by altering the host-seeking and biting behaviors of the mosquitoes. We also posited that each unsuccessful bite is conducted on a different host as mosquitoes fly away to escape being noticed after each probe (50, 51). To model the impact of behavioral alterations on dengue epidemiology, we calculated the basic reproduction rate (R0) for infected mosquitoes by incorporating “duration at midrange distance before first time in contact” (P19) and “number of unsuccessful bites” (P39) as proxies for host-seeking and biting behaviors, respectively, in a compartmental mathematical model. Both of these parameters were significantly altered by infection (Figs. 2C and 3C), and we ran the model using values proportional to the differences in means between control and infected mosquitoes (SI Appendix and Dataset S3). While we used classical parameter values to obtain a baseline R0 of 2.5 (R0 may vary between epidemiological settings) (52) with blood-feeding behavior of uninfected mosquitoes, infection-induced alterations of host-seeking and biting behaviors, separately, increased R0 to 3.15 and 4.19, respectively (Fig. 5). Importantly, when changes in host-seeking and biting behaviors were combined, the R0 was multiplied more than three times to 7.65. Altogether, these results suggest that DENV-induced changes in mosquito blood-feeding behavior dramatically aggravate DENV epidemiology.
Fig. 5.
R0 is increased by infection-induced changes on mosquito blood-feeding behavior. The impact of infection on host-seeking and biting behaviors separately and together was mathematically modeled.
Discussion
Mosquito blood-feeding behavior is a key determinant of vector capacity and thus transmission efficiency. Here, we deploy a reductionist multidisciplinary approach to detail how DENV infection alters blood-feeding behavior and how these behavioral alterations influence DENV epidemiology. To monitor mosquito blood-feeding behavior with unprecedented accuracy, we designed a close-up, video-based assay with mice. Using semiautomated methods to process the videos, we then generated a large amount of standardized and unbiased behavioral data, which we analyzed using multivariate statistics. The assay allowed behavioral observations close to the mouse skin, providing a high-resolution model of proximal host-seeking and biting. To expand our observations of the host-seeking behavior, we also monitored mosquito attraction to the host in larger cages. The behavioral analysis revealed that DENV infection augments mosquito attraction to the host and hinders its biting efficiency, the latter resulting in an increased number of unsuccessful bites to reach a similar level of blood repletion as seen in uninfected mosquitoes. Enhanced sensitivity to synthetic human odors (31) and increased mobility (30) upon DENV2 infection may have induced the increased attraction to the host. Altered probing efficiency was previously reported upon DENV3 infection (34), although not in as much detail as in the present study. While the impact of increased attraction to the host on vector capacity is trivial, we used an immuno-competent mouse model to show that successive short probes (as short as 20 s and representing a proxy for unsuccessful bites) result in repeated transmissions, thereby demonstrating that DENV-induced alteration of the biting behavior also increases vector capacity. Finally, our mathematical models indicate that the DENV-induced alterations of mosquito blood-feeding behavior triple transmission efficiency, which we expect would proportionally aggravate DENV epidemics.
This high-resolution analysis of mosquito blood-feeding behavior on mice supports the existence of a biting behavioral pattern independent of the behavior outcome (i.e., whether biting leads to blood ingestion or not). We observed that despite infected mosquitoes conducting significantly more “unsuccessful bites” (P39), “average duration per probe” (P37) was similar for infected and control mosquitoes (Dataset S3). Similarly, “average duration of proboscis in motion per probe” (P46) was not affected by infection, whereas “total number of proboscis in motion” (P44) and “duration of proboscis in motion” (P45) were increased by infection (Dataset S3). A biting pattern independent of the outcome was also revealed by the multivariate analysis, in which “average duration per probe” (P37) was not correlated with the factor describing biting efficiency.” Such a behavioral pattern was previously observed using an artificial skin mimic (12). In the skin mimic study, probing duration did not vary between mosquitoes that were stimulated to imbibe a watery solution by the addition of a phagostimulant and those that did not feed in the absence of the phagostimulant. It is likely that the probing duration is constrained by the associated risk of being noticed and killed by the host. However, the corollary of this behavioral constraint is that mosquitoes failing to imbibe a liquid have to resume biting and conduct multiple bites. This was observed in both the prior study using artificial skin (12) and the present study using mice. Because we showed that mosquitoes transmit DENV during successive probes, the current study suggests that the virus subverts a mosquito behavioral association between biting success and probing repetition to increase transmission efficiency.
Infection can alter biting success in three possible ways: 1) interfering with ingestion, 2) obscuring sensory cues, and/or 3) hindering proboscis progress (53). First, DENV did not seem to reduce ingestion capacity, as “average abdominal swelling per blood ingestion event” (P62) was unaffected (Dataset S3). Second, the phagoreceptors responsible for locating blood vessels are present in the brain and maxillary palps, both of which are infected by DENV (17, 54). In these chemosensory organs, DENV infection regulates gene expressions (31), including the regulation in opposite directions of two odorant-binding proteins (OBP) required for efficient blood-feeding behavior (17). The biting deficiency that we observed may be related to DENV-mediated inhibition of certain OBPs. Third, proboscis progression is facilitated by the secretion of saliva with functions in hemostasis, inflammation, and immunity (16). We and others have reported that DENV infection regulates the protein content in saliva (55) and the expression of genes involved in blood-feeding in the salivary glands (17, 56). Infection-induced biting hindrance may thus be caused by disruption of the sensory apparatus and saliva-mediated proboscis progression. Sensory disturbance can also result in increased mosquito attraction to the host upon infection (31). Alternatively, the metabolic cost associated with infection (57) may increase the need for the energy-rich blood, promoting attraction to the host. Regardless of the mechanism, the altered behavior is remarkably specific, as it did not impact body maintenance, locomotor activity, and blood ingestion capacity, all of which were also evaluated in our multivariate behavioral assay. Given the crucial role of blood-feeding in influencing DENV epidemiology, evolution is expected to strongly select for virus-induced manipulation of mosquito behavior that favors transmission (58).
There are several limitations to our experimental design that constrain the breadth of the conclusions. We used mice to monitor mosquito behavior. Although A. aegypti feeds on rodents in the field, humans are its preferred host (59), and mosquito attraction cues certainly differ between the two species. Similarly, we used a mouse model to quantify transmission. Although we used wild-type mice to obtain a stringent quantification of infectivity, saliva infectivity may differ in humans. As safety reasons preclude the use of humans with infected mosquitoes, it would be of interest to test our findings in nonhuman primates, which is the most-relevant animal model. Furthermore, we used a low-passage (<7) DENV2 strain throughout the study to obtain field-relevant results. However, mosquito behavior may be altered differently by the various DENV serotypes or even genotypes. In addition, other factors that may influence how DENV infection alters mosquito behavior include virulence level, inoculum dose, extrinsic incubation period, mosquito age, size, genetics and physiological status, number of previous blood feed, origin of the blood, temperature, humidity, light, and microbiota. Altogether, the reductionist approach that we undertook provided a supported answer to the long-standing question about the effect of DENV infection on mosquito transmission rate. Although the conclusions are relevant mainly within the boundaries of the model system, they have broader significance as they shed important light on the contribution of DENV-altered mosquito behavior in influencing dengue epidemiology.
The blood-feeding habits of A. aegypti make it a devastating vector (29). The mosquito takes multiple blood meals during one gonotrophic cycle (60–62), preferentially feeds on humans (59), and is a persistent biter that will seek hosts until it achieves blood repletion (63). The alterations in mosquito blood-feeding behavior that we observed upon DENV infection amplify vector capacity and shape its epidemiology. The enhanced transmission rate may explain why epidemics occur even with very low mosquito house index (5). Similarly, repeated infectious bites within a short period of time provide a rationale for the highly focal dengue clusters (defined as two or more cases within 150 m of each other in a period of 14 d) that are regularly observed (64–66). More strikingly, repeated infectious bites offer an explanation for the simultaneous occurrence of dengue symptoms in multiple people within the same household (67, 68). Altogether, this study demonstrates the contribution of DENV infection–induced modifications of mosquito behavior on disease epidemiology and supports the integration of infection-induced behavior alterations in epidemiological models to better inform public health authorities.
Methods
Material: Mosquito, Mouse, and Viruses.
Details are provided in SI Appendix, SI Methods.
Mosquito Oral Infection.
Female mosquitoes 3 to 5 d-old were orally infected by feeding on DENV-infectious blood. More information is provided in SI Appendix, SI Methods.
Absolute Quantification of DENV Load.
Viral genomic RNA was absolutely quantified by one-step qRT-PCR. More information is provided in SI Appendix, SI Methods.
The Behavior Video Recording.
A custom-made device allowed high-resolution video-recording of mosquito blood-feeding on mice for 30 min. More information is provided in SI Appendix, SI Methods.
Multivariate Statistical Analysis.
Based on the videos, mosquito behavior was semiautomatically annotated and used to calculate 80 behavioral parameters, which were then analyzed through factor analysis and univariate tests. More information is provided in SI Appendix, SI Methods.
Biological Interpretation of the Factors.
Factors related to host-seeking and biting behaviors were biologically interpreted based on their behavioral parameter loadings. More information is provided in SI Appendix, SI Methods.
Large-Cage Host-Seeking Behavioral Assay.
Mosquito host-seeking behavior in large cage was video-recorded, and the time from the start to the first contact with the mouse was calculated. More information is provided in SI Appendix, SI Methods.
Successive Bite Assay.
Single mosquitoes were let to bite three different mice sequentially. Virus was quantified in skin and lymph nodes of each mouse by one-step qRT-PCR. More information is provided in SI Appendix, SI Methods.
Mathematical Modeling of DENV Epidemiology.
The effect of DENV infection-induced behavioral changes on DENV transmission rate was calculated using a mathematical model. More information is provided in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank Professor Mariano Garcia-Blanco for his support and comments on an earlier version of the manuscript. We thank all staff, especially Menchie Manuel from the core insectary facility at Duke-NUS Medical School where the mosquito experiments were conducted. We thank the scientific editor Sruthi Jagannathan for the thorough editing. Research was supported by the Ministry of Education, Singapore (MOE 2015-T3-1-003 attributed to R.M.K. and J.P., MOE-T2EP30120-0011 to A.L.S., and MOE2019-T2-1-133 to A.C.-C.), by the Agence National de la Recherche, France (ANR-20-CE15-0006) attributed to J.P., and by the Duke-NUS Signature Research Programme Emerging Infectious Diseases funded by the Agency for Science, Technology and Research, Singapore.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117589119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Bhatt S., et al. , The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierson T. C., Diamond M. S., The continued threat of emerging flaviviruses. Nat. Microbiol. 5, 796–812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler D. J., Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11, 480–496 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler D. J., Cities spawn epidemic dengue viruses. Nat. Med. 10, 129–130 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Ooi E.-E., Goh K.-T., Gubler D. J., Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 12, 887–893 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements A. N., Biology of Mosquitoes: Development Nutrition and Reproduction (Chapman & Hall, 1992). [Google Scholar]
- 7.Gibson G., Torr S. J., Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol. 13, 2–23 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Dekker T., Geier M., Cardé R. T., Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208, 2963–2972 (2005). [DOI] [PubMed] [Google Scholar]
- 9.McMeniman C. J., Corfas R. A., Matthews B. J., Ritchie S. A., Vosshall L. B., Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Breugel F., Riffell J., Fairhall A., Dickinson M. H., Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 25, 2123–2129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corfas R. A., Vosshall L. B., The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife 4, e11750 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hol F. J., Lambrechts L., Prakash M., BiteOscope, an open platform to study mosquito biting behavior. eLife 9, e56829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jové V., et al. , Sensory discrimination of blood and floral nectar by Aedes aegypti mosquitoes. Neuron (2020) 10.1016/j.neuron.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasubramanian M. K., Barham O. M., Swaminathan V., Mechanics of a mosquito bite with applications to microneedle design. Bioinspir. Biomim. 3, 046001 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Gurera D., Bhushan B., Kumar N., Lessons from mosquitoes’ painless piercing. J. Mech. Behav. Biomed. Mater. 84, 178–187 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro J. M., et al. , An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8, 6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim S., Ramirez J. L., Dimopoulos G., Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog. 8, e1002631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choumet V., et al. , Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One 7, e50464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwadz R. W., Regulation of blood meal size in the mosquito. J. Insect Physiol. 15, 2039–2044 (1969). [DOI] [PubMed] [Google Scholar]
- 20.Styer L. M., et al. , Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3, 1262–1270 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turell M. J., Spielman A., Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am. J. Trop. Med. Hyg. 47, 190–194 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Turell M. J., Tammariello R. F., Spielman A., Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J. Med. Entomol. 32, 563–568 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Lim P.-Y., Behr M. J., Chadwick C. M., Shi P.-Y., Bernard K. A., Keratinocytes are cell targets of West Nile virus in vivo. J. Virol. 85, 5197–5201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surasombatpattana P., et al. , Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect. Genet. Evol. 11, 1664–1673 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Bustos-Arriaga J., et al. , Activation of the innate immune response against DENV in normal non-transformed human fibroblasts. PLoS Negl. Trop. Dis. 5, e1420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duangkhae P., et al. , Interplay between keratinocytes and myeloid cells drives dengue virus spread in human skin. J. Invest. Dermatol. 138, 618–626 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Pingen M., et al. , Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 44, 1455–1469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryden S. R., et al. , Pan-viral protection against arboviruses by activating skin macrophages at the inoculation site. Sci. Transl. Med. 12, eaax2421 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Scott T. W., Takken W., Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 28, 114–121 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Lima-Camara T. N., et al. , Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS One 6, e17690 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallon A. K., et al. , Dengue infection modulates locomotion and host seeking in Aedes aegypti. PLoS Negl. Trop. Dis. 14, e0008531 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaburro J., et al. , Dengue virus infection changes Aedes aegypti oviposition olfactory preferences. Sci. Rep. 8, 13179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciel-de-Freitas R., Sylvestre G., Gandini M., Koella J. C., The influence of dengue virus serotype-2 infection on Aedes aegypti (Diptera: Culicidae) motivation and avidity to blood feed. PLoS One 8, e65252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt K. B., et al. , Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 57, 119–125 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Sylvestre G., Gandini M., Maciel-de-Freitas R., Age-dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) feeding behavior, survival, oviposition success and fecundity. PLoS One 8, e59933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam J. L., Scott T. W., Blood-feeding behavior of dengue-2 virus-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 52, 225–227 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Vaughn D. W., et al. , Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181, 2–9 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Quinn G. P., Keough M. J., Experimental Design and Data Analysis for Biologists (Cambridge University Press, 2002). [Google Scholar]
- 39.Mair P., “Factor analysis” in Modern Psychometrics with R, Use R! Mair P., Ed. (Springer International Publishing, 2018), pp. 17–61. [Google Scholar]
- 40.Truelove H. B., Gillis A. J., Perception of pro-environmental behavior. Glob. Environ. Change 49, 175–185 (2018). [Google Scholar]
- 41.Pompon J., Quiring D., Giordanengo P., Pelletier Y., Role of host-plant selection in resistance of wild Solanum species to Macrosiphum euphorbiae and Myzus persicae. Entomol. Exp. Appl. 137, 73–85 (2010). [Google Scholar]
- 42.Yoo W., et al. , A study of effects of multiCollinearity in the multivariable analysis. Int. J. Appl. Sci. Technol. 4, 9–19 (2014). [PMC free article] [PubMed] [Google Scholar]
- 43.Price D. P., Schilkey F. D., Ulanov A., Hansen I. A., Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit. Vectors 8, 252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St John A. L., et al. , Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. U.S.A. 108, 9190–9195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathore A. P. S., et al. , Immunological and pathological landscape of dengue serotypes 1-4 infections in immune-competent mice. Front. Immunol. 12, 681950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zellweger R. M., Shresta S., Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 5, 151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pingen M., Schmid M. A., Harris E., McKimmie C. S., Mosquito biting modulates skin response to virus infection. Trends Parasitol. 33, 645–657 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Schmid M. A., et al. , Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody-dependent enhancement. PLoS Pathog. 12, e1005676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Putnam J. L., Scott T. W., The effect of multiple host contacts on the infectivity of dengue-2 virus-infected Aedes aegypti. J. Parasitol. 81, 170–174 (1995). [PubMed] [Google Scholar]
- 50.Walker E. D., Edman J. D., The influence of host defensive behavior on mosquito, (Diptera: Culicidae) biting persistence. J. Med. Entomol. 22, 370–372 (1985). [DOI] [PubMed] [Google Scholar]
- 51.Canyon D. V., Hii J. L., Muller R., Multiple host-feeding and biting persistence of Aedes aegypti. Ann. Trop. Med. Parasitol. 92, 311–316 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., et al. , Reviewing estimates of the basic reproduction number for dengue, Zika and chikungunya across global climate zones. Environ. Res. 182, 109114 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Lefèvre T., Thomas F., Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector-borne diseases. Infect. Genet. Evol. 8, 504–519 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Salazar M. I., Richardson J. H., Sánchez-Vargas I., Olson K. E., Beaty B. J., Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 7, 9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chisenhall D. M., et al. , Infection with dengue-2 virus alters proteins in naturally expectorated saliva of Aedes aegypti mosquitoes. Parasit. Vectors 7, 252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury A., et al. , JNK pathway restricts DENV2, ZIKV and CHIKV infection by activating complement and apoptosis in mosquito salivary glands. PLoS Pathog. 16, e1008754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan T. X., Randall G., Flavivirus modulation of cellular metabolism. Curr. Opin. Virol. 19, 7–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lefèvre T., et al. , New prospects for research on manipulation of insect vectors by pathogens. PLoS Pathog. 2, e72 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponlawat A., Harrington L. C., Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 42, 844–849 (2005). [DOI] [PubMed] [Google Scholar]
- 60.McClelland G. A., Conway G. R., Frequency of blood feeding in the mosquito Aedes aegypti. Nature 232, 485–486 (1971). [DOI] [PubMed] [Google Scholar]
- 61.Scott T. W., et al. , Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J. Med. Entomol. 30, 94–99 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Yasuno M., Tonn R. J., A study of biting habits of Aedes aegypti in Bangkok, Thailand. Bull. World Health Organ. 43, 319–325 (1970). [PMC free article] [PubMed] [Google Scholar]
- 63.Klowden M. J., Lea A., Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.). Am. J. Trop. Med. Hyg. 27, 827–831 (1978). [DOI] [PubMed] [Google Scholar]
- 64.Liebman K. A., et al. , Spatial dimensions of dengue virus transmission across interepidemic and epidemic periods in Iquitos, Peru (1999-2003). PLoS Negl. Trop. Dis. 6, e1472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoddard S. T., et al. , House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. U.S.A. 110, 994–999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vazquez-Prokopec G. M., Kitron U., Montgomery B., Horne P., Ritchie S. A., Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl. Trop. Dis. 4, e920 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mammen M. P., et al. , Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 5, e205 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterman S. H., et al. , Dengue transmission in two Puerto Rican communities in 1982. Am. J. Trop. Med. Hyg. 34, 625–632 (1985). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.