Abstract
Due to age and impaired kidney function, older adults with kidney disease are at increased risk of medication-related problems and related hospitalizations. One proa ctive approach to minimize this risk is deprescribing. Deprescribing refers to the systematic process of reducing or stopping a medication. Aside from preventing harm, deprescribing can potentially optimize patients’ quality of life by aligning medications with their goals of care. For some patients, deprescribing could involve less aggressive management of their diabetes and/or hypertension. In other instances, deprescribing targets may include potentially inappropriate medications that carry greater risk of harm than benefit in older adults, medications that have questionable efficacy, including medications that have varying efficacy by degree of kidney function, and that increase medication regimen complexity. We include a guide for clinicians to utilize in deprescribing, the List, Evaluate, Shared Decision-Making, Support (LESS) framework. The LESS framework provides key considerations at each step of the deprescribing process that can be tailored for the medications and context of individu al patients. Patient characteristics or clinical events that warrant consideration of deprescribing include limited life expectancy, cognitive impairment, and health status changes, such as dialysis initiation or recent hospitalization. We acknowledge patient-, clinician-, and system-level challenges to the depre scribing process. These include patient hesitancy and challenges to discussing goals of care, clinician time constraints and a lack of evidence-based guidelines, and system-level challenges of interoperable electronic health records and limited incentives for deprescribing. However, novel evidence-based tools designed to facilitate deprescribing and future evidence on effectiveness of deprescribing could help mitigate these barriers. This review provides foundational knowledge on deprescribing as an emerging component of clinical practice and research within nephrology.
Keywords: geriatric and palliative nephrology, aged, deprescriptions, dialysis, kidney diseases
Introduction
More than half of older adults with kidney disease have at least one medication that is either renally inappropriate, or potentially inappropriate, for their age (1). The combination of older age and kidney disease, compared with either characteristic alone, confers heightened vulnerability to medication-related problems (2). Yet, medication-related harm can be prevented through improvements in the quality of medication decision making for older adults with kidney disease.
Several factors contribute to medication-related problems in older adults with kidney disease. Older age can cause alterations in medication pharmacokinetics and/or pharmacodynamics, compounding the effect of declining or minimal kidney function on the clearance of specific medications. Underlying reasons for these alterations include decreased muscle mass, reduced kidney mass and perfusion, and malnutrition (3). As a result, medication risk/benefit profiles differ in older adults because some medications carry greater risk of harm than benefit, due to increased risk of geriatric syndromes, including cognitive impairment or falls (4,5). In addition, the benefits or efficacy of some medications may be equivocal because of limited life expectancy or limited clinical trial data involving older adults. Older adults with kidney disease also often have multiple clinicians, subsequently more medication prescribers, a high prevalence of polypharmacy, and a higher risk of medication-related problems (6,7). Increased prevalence of care transitions and clinician changes among older adults, including hospitalizations and dialysis initiation, may predispose patients to prescribing cascades if they are executed without careful medication review (8). Given the accelerated aging inherent to kidney disease, it is important to acknowledge these factors may also exist in young patients with kidney disease (9).
Although an essential approach to prevent medication-related problems is to consider risks and benefits at medication initiation, it is also important to consider deprescribing medications that may carry new risk or limited efficacy with increasing age and/or declining kidney function. Deprescribing is the process of reducing or stopping a medication that can potentially improve clinical outcomes in older adults with kidney disease (7,10,11). Although deprescribing is typically done in reaction to a preventable medication-related problem (e.g., reducing insulin after hypoglycemia), proactive deprescribing is routine for nephrology practices when doses of medications are decreased as kidney function declines. However, proactive deprescribing of medications not primarily cleared by the kidneys should also be considered (12). Formally, deprescribing involves a systematic process of medication reconciliation, medication review, shared decision making, followed by implementation and monitoring (13).
Deprescribing leads to lower use and/or prescribing of medications that carry greater risk of harm than benefit in older adults (e.g., sedative hypnotics, first-generation antihistamines) (14–17). Deprescribing interventions are varied in methodology (e.g., clinician and/or patient education, clinician-led deprescribing, pharmacist medication review and recommendations), setting (e.g., hospital, nursing home, primary care), and approach (e.g., single or multiple medication targets). This variation in methodology and rigor has limited generalizability of existing evidence (18,19). However, meta-analyses suggest deprescribing involving medication review (often pharmacist led) may yield a reduction in all-cause mortality (26% relative risk reduction) among community-dwelling older adults and falls among nursing home residents (20,21). Other patient benefits of deprescribing include improved adherence, improved quality of life, and lower financial burden (22). Older adults with polypharmacy acknowledge their medication burden and would be willing to attempt deprescribing at least one medication if their physician said it was possible (23). Patients requiring dialysis also report satisfaction in a deprescribing quality improvement program (24). This evidence demonstrates the potential benefit of deprescribing for older adults with kidney disease.
This literature review attempts to promote integration of deprescribing into routine care in CKD and dialysis clinics by delineating three overarching areas for consideration of deprescribing: (1) less intensive management of exemplar chronic conditions (diabetes and hypertension), (2) less inappropriate prescribing, and (3) less medication regimen complexity. Then, this review provides a deprescribing framework to facilitate clinical encounters. Last, we address common deprescribing challenges and recommendations for future research.
Less Intensive Management of Diabetes and Hypertension
Glycemic control is standard of care for CKD management. Yet intensive glycemic control, defined as therapy yielding hypoglycemic events and/or hemoglobin A1c (A1C) <6%, can be harmful in older adults with kidney disease. The risk of sarcopenia and frailty also increases in older adults with diabetes and CKD, resulting in reduced insulin clearance, which may be particularly concerning because older adults may have less hypoglycemic awareness (25). Hypoglycemic events are independently a cause for substantial concern because of their severe sequalae, including dizziness, slurred speech, seizure, cognitive decline, functional decline, and confusion, or more acute events increasing the risk of cardiac ischemia or arrythmias (26,27). Overtreatment of diabetes has also been linked to increased short- and long-term mortality, fractures/head injuries, cognitive impairment, and increased health care utilization and expenditures (25,28). For example, in a study of older adults aged ≥75 years treated with at least one antihyperglycemic agent, there was a high prevalence of intensive control (A1C <7), and an increased risk of emergency department visits/hospitalizations or death within 30 days of reaching control using high risk agents (e.g., insulin, or specific sulfonylureas) (29). Furthermore, the risk of hypoglycemia increases with worsening eGFR stage and use of antihyperglycemic medications increases the risk approximately 40 times at each eGFR stage (compared with nonusers) (30).
The A1C target of <7 was established from studies including individuals aged <65 years, so clear guidance regarding appropriate A1C targets is lacking for older adults with kidney disease (31). However, A1C targets <8% may be considered among frail individuals and those with limited lifespan, propensity for hypoglycemic events, and limited resources (25). More specifically, the American Diabetes Association recommends a A1C <8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, and comorbid conditions, such as CKD (32). Considering these recommendations, we suggest clinicians balance the importance of avoiding hypoglycemic events associated with intensive treatment with avoidance of severe hyperglycemia, which can cause negative sequelae including dehydration, polyuria, and predisposition for urinary infections and cardiovascular events (25).
Although CKD guidelines support a systolic BP <120 mmHg (33), this goal may not be prudent for older adults with CKD. This intensive BP target was derived from the Systolic Blood Pressure Intervention Trial, which demonstrated reduced cardiovascular events and mortality benefit. However, evidence that this BP target slows kidney disease progression is lacking, thus making its universal applicability to older adults with CKD unclear (34,35). Furthermore, the Systolic Blood Pressure Intervention Trial excluded older adults having difficulty with physical and/or cognitive function (e.g., nursing-home residents, individuals with dementia) (34). Thus, the study is not generalizable to a sizable proportion of the older adults with CKD in which these conditions are common. In addition to a lack of clear efficacy, there is risk of harm from intensive BP management. Both hypotension and addition of antihypertensive agents increases older adults’ risk for falls, and related fractures and brain injury (36). Addition of antihypertensive agents contributes to medication burden, which can affect a patient’s quality of life (37). A clinical trial demonstrated no difference in adverse events between patients who had (versus those who did not) antihypertensives deprescribed, providing reassurance for deprescribing antihypertensives in older adults with moderate CKD (38).
Less Inappropriate Prescribing of Medications
Older adults with kidney disease may be inappropriately prescribed medications that: (1) have more potential risk of harm than benefit and/or (2) have questionable efficacy. Continued use of medications in either category as patients age and/or kidney disease progresses can lead to preventable adverse events. Care for patients with kidney disease tends to focus on deprescribing medications with greater risk than benefit due to impaired kidney function. In addition, clinicians should consider deprescribing potentially inappropriate medications (PIMs), or medications that carry substantial risk in older adults. The American Geriatrics Society developed the Beers criteria which provides guidance regarding a list of PIMs (39). Similar comprehensive guides with specific criteria exist worldwide (40,41). PIMs represent a broad range of medication categories (e.g., central nervous system (CNS)-active, antithrombotics, endocrine, cardiovascular). PIMs have been associated with falls, confusion, death, and hospitalizations in older community-dwelling populations (4,5). PIM use in older adults is also associated with higher healthcare utilization, including outpatient visits and emergency room visits (42). Notably, more than half of older adults with CKD have at least one PIM (43). Rates of PIM prescribing among older adults with advanced CKD is at least three times the prescribing rates of medications with primarily renal clearance (44). Among older adults receiving dialysis, nearly half have a PIM prescription presumably used for symptoms associated with ESKD, such as anxiety, muscle cramps, or pain (e.g., benzodiazepines, muscle relaxants, opioids, gabapentinoids) (45–49). Some epidemiologic studies of specific PIMs, predominantly CNS-active medications, demonstrate increased risk of harm, such as fall, fracture, or altered mental status, in patients with kidney disease. Additionally, PIMs carry critical theoretical risks of adverse events. For example, a patient receiving a CNS-active medication could experience lethargy leading to falls, confusion, long-term cognitive impairment, or motor vehicle crashes.
In contrast to PIMs, medications with questionable efficacy may not carry demonstrable benefit for older adults with kidney disease because commonly prescribed medications have been understudied in older adults (50). As kidney function declines, the efficacy of some medications also declines. For example, dialysis initiation can lead to loss of efficacy of diuretics over time. Medications can develop questionable efficacy because of health status changes. Patients with declining life expectancy (≤6 months) should have their medications reviewed for questionable efficacy (e.g., statins and other lipid-lowering medications) (51,52). Continuation of dual antiplatelet therapy beyond 6 or 12 months may need to be considered on an individual basis, given limited evidence for extended therapy in this population (53). Medications with questionable efficacy carry limited benefit, and could become burdensome and impair quality of life. Thus, if polypharmacy is concomitant with substantial pill burden or side effects, medications with little benefit should be considered for deprescribing (54). Our clinical vignette illustrates medication targets for less inappropriate prescribing (Figure 1) (51,55,56).
Figure 1.
Clinical vignette.
Less Complexity in Medication Regimens
Individuals with advanced CKD and ESKD receiving kidney replacement therapies have a high burden of medications, in part due to the comorbid conditions, including hypertension, diabetes, and cardiovascular disease. For example, individuals requiring dialysis take approximately 10–12 prescribed and over-the-counter medications and nearly 19 pills per day (57–60). Adding to the volume of medications, medication regimen complexity can increase on the basis of directions for use (e.g., before or after dialysis sessions), dosing frequencies (e.g., including at varying times of the day or after versus with meals), and complex dosage formulations that may be more difficult to use (e.g., injectables). These elements of complexity are included in the 65-item validated Medication Regimen Complexity Index (MRCI) (61). Higher MRCI scores have been linked to medication-related problems, poor medication adherence, mortality, and hospital readmissions (62,63). When the MRCI is applied to individuals with kidney failure, regimen complexity can increase with transplant or dialysis modalitychange (64). Individuals on home hemodialysis take fewer medications due to improved BP and lower serum phosphate, but medication-regimen complexity is increased due to the need for injections at home (e.g., erythropoietin-stimulating agent) and the need to manage dialysate for home hemodialysis (65). Changes in complexity can be especially critical among older adults with kidney disease for whom cognitive impairment is prevalent but poorly recognized, or for whom worsening dexterity may impair injection delivery or opening pill bottles (66). Although the MRCI is not practical for routine clinical use, its elements provide insight for clinicians on how to assess for and reduce medication complexity. Clinicians should be cognizant of medication changes affecting complexity and consider ways to reduce complexity when warranted (e.g., considering combination pills, reducing the frequency of medications, eliminating medications that are difficult to administer when possible). Although polypills have been suggested as a strategy to decrease pill burden, regimen complexity, and adherence concerns, few studies have examined this approach in older adults with kidney disease (67). Further, this strategy is limited by the difficulty of adjusting doses with kidney disease progression or occurrence of side effects with an individual medication within the polypill.
The List, Evaluate, Shared Decision-Making, Support Framework for Deprescribing
To identify opportunities for deprescribing, clinicians can use the List, Evaluate, Shared Decision-Making, Support framework (Figure 2). The List, Evaluate, Shared Decision-Making, Support framework acknowledges deprescribing should be individualized to a given patient. Therefore, clinicians should start with an accurate, current medication list through a structured medication reconciliation process (68).
Figure 2.
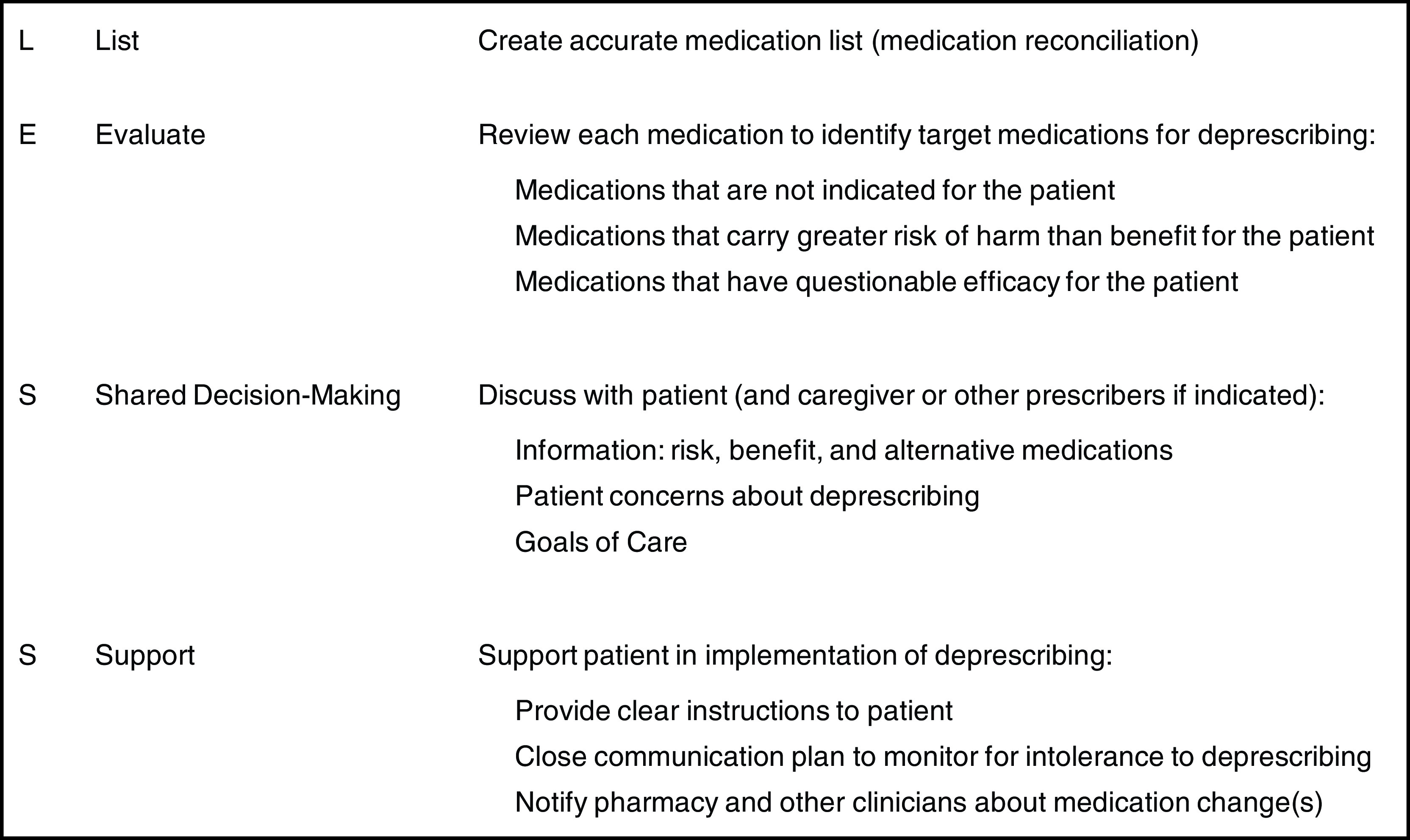
List Evaluate Shared Decision-Making Support (LESS) framework for the deprescribing process.
Next, clinicians should evaluate the medication list using a structured process (i.e., medication review) identifying medications having high risk-to-benefit profile, limited efficacy, those no longer indicated, and regimens too complex for safe use (examples in Table 1). In some instances, there is insufficient evidence on a medication’s risk in older adults with kidney disease. Clinicians can extrapolate from evidence obtained from cohort studies conducted in the general older adult population. This extrapolation is reasonable as individuals with impaired kidney function may have a narrower therapeutic window and/or uremic toxins that may affect hepatic drug metabolism (69). Clinicians can also rely on the patient’s preferences and goals of care to discern if a specific medication with unclear risks should be considered for deprescribing.
Table 1.
Deprescribing principles and examples of medications to deprescribe in older adults with kidney diseasea
| Deprescribing Principle | Medication | Considerations in Nondialysis Patients | Considerations in Dialysis |
| Deprescribe medication with risk exceeds desired benefit | First generation sulfonylurea - glyburide | No specific restrictions | Avoid due to higher risk of hypoglycemic events compared with other antiglycemic agents (88,89) |
| Dabigatran | No specific data (90) | bleed risk increases with GFR decline; safer agent available (apixaban) (91,92) | |
| Metformin | Discontinue use of metformin as it is excreted by the kidneys, and accumulation with reduced kidney function may increase risk of lactic acidosis (88) when eGFR is <30 per Food and Drug Administration standards (93) | Contraindicated in dialysis | |
| Baclofen and other muscle relaxants (e.g., dantrolene, metaxalone, carisoprodol, chlorzoxazone, cyclobenzaprine, methocarbamol, tizanidine, or orphenadrine) | Baclofen use is associated with encephalopathy among older adults with CKD at high doses (≥20 mg per day) (94) In older adults with CKD (eGFR <60), baclofen prescriptions at ≥20 mg per day were associated with higher risk of fall-related hospitalization and hypotension (vs <20 mg/day) (95) |
Muscle relaxant use is common in patients with ESKD on hemodialysis and associated with encephalopathy and falls (96) Baclofen should be avoided in individuals on dialysis because of the risk of hospitalization and encephalopathy (97) |
|
| Opiate (e.g., hydrocodone, oxycodone, tramadol, codeine, hydromorphone, fentanyl, methadone, meperidine, and morphine) | Among individuals on hemodialysis, all opiate agents were associated with a significantly higher hazard of altered mental status. Several agents were associated with a higher hazard of falls, and fracture in a dose-dependent manner, and risks were present even at lower dosing and for agents recommended for use in dialysis (98) | Opiate use was associated with 50% GFR reduction and kidney failure/hospitalization and prekidney failure death vs nonsteroidal anti-inflammatory drugs among individuals with CKD (99) | |
| Pregabalin and gabapentin | Data unavailable except for limited data showing effective use for chronic uremic pruritis (100) | Among individuals with ESKD on hemodialysis, gabapentin was associated with higher hazards of altered mental status, fall, and fracture, respectively, in the highest dose category; pregabalin was associated with up to 51% and 68% higher hazards of altered mental status and fall, respectively (101) | |
| Benzodiazepines | Limited data available | Codispensing opioids and short-acting benz odiazepines is common among individuals on dialysis and associated with a higher risk of death (102) | |
| Sedative hypnotics (zolpidem) | Limited data available | Individuals initiating zolpidem had an increased risk of fall related fractures vs trazodone among individuals on maintenance hemodialysis (103) | |
| Deprescribe medication that requires significant kidney function for efficacy | SGLT2 inhibitors (empagliflozin, canagliflozin, dapagliflozin) | Rapidly evolving guidelines suggest SGLT2 inhibition for patients with type 2 diabetes and eGFR as low as 30 mL/min per 1.73 m2, particularly if severely increased albuminuria is present, SGLT2 inhibitor withdrawal is not required if eGFR decreases to <30 ml/min per 1.73 m2, as per the CREDENCE protocol. I DAPA-CKD was stopped early for overwhelming efficacy, the eGFR cutoff could be reduced to 25 mL/min per 1.73 m2(104) | Discontinue use of SGLT2 inhibitors as their mechanism of action requires that they be filtered from the blood through the glomerulus to exhibit their inhibitory effects exclusively on the extracellular side of the proximal tubule plasma membrane (88) |
| Nitrofurantoin | Limited data available | Nitrofurantoin primarily renally excreted, and relies on urinary concentration to achieve its effect. It may be associated neurotoxicity and life-threatening pulmonary toxicity, it should be avoided in patients on dialysis (105) | |
| Deprescribe medication demonstrated to have limited or no documented benefit in patient with kidney disease | Tramadol extended release | Limited data available | Extended-release products are not necessary in ESKD products (106) |
| Fenofibrate | In patients with severe renal impairment (creatinine clearance ≤30 ml/min) there was 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid compared with that of healthy subjects (107) | Unclear benefit in patients on dialysis (108) |
This table demonstrates key principles to consider during medication review and examples of medications. It should not to be considered a complete list of medications for deprescribing.
CREDENCE, Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD).
Clinicians should also evaluate for specific patient characteristics, including presence of limited life expectancy and/or characteristics associated with increased risk of medication-related problems, such as frailty, cognitive impairment, or mobility impairment (70). Assessments identifying patients with limited life expectancy include the surprise question (i.e., “Would I be surprised if this patient died in the next year?”), a Karnofsky Performance status score, and/or activities of daily living assessment (71–74). Clinicians may also prioritize patients with recent care transitions, including dialysis initiation, hospitalization, or nursing home admission.
Once medication(s) eligible for deprescribing are identified, shared decision making is a critical next step. The information sharing between patients and clinicians would include discussions about the risks, benefits, and alternatives of the medications considered for deprescribing, followed by collaborative decision making. The decision-making discussion can mirror an existing communication framework used for dialysis decision making: setup, perceptions and perspectives, invitation, recommendation, empathize, summarize, and strategize. This framework allows clinicians to formally introduce the decision to be made, to understand “their worries and hopes” related to deprescribing a medication, and develop an individualized plan (75). The shared decision making discussion can be further enhanced through incorporating five social-contextual factors: (1) clinical, (2) psychologic, (3) social, (4) financial, and (5) physical (Table 2) (76). Addressing these factors provides more context for patients’ medication use and goals of care. With this, clinicians can discern whether deprescribing is consistent with patient social contexts and preferences, and how to prioritize medications for deprescribing. Decision aids may be incorporated to increase the quality and efficiency of decision making (77,78).
Table 2.
Socio-contextual factors to consider when deprescribinga
| Determinant | Key Components of Assessment | Example |
| Clinical | Assess the complex comorbid conditions affecting a patient, the risks/benefits of medications used to treat each of these, and the adverse drug events exacerbated by specific agents. Identify medication benefits vs harms and expected time to benefit in the context of diagnosis, and symptom management goals (e.g., decreasing pruritis). Prescribing cascades (e.g., proton pump inhibitor for aspirin use) should also be noted along with medications that have equivocal evidence for benefit including preventive agents such as statins etc. Finally, available alternatives should be discussed. | Understand the role of each medication and assess its use in the context of patient circumstances, e.g., diuretics in an anuric patient. |
| Psychologic | Determine anxiety/worry about medications or conditions that affect deprescribing and assess perceptions and/or knowledge regarding treatments (e.g., perceptions of a need for intensive glucose or BP control, or intensive phosphate control). Any anxieties or distress that arises from possible discontinuation of certain medications should be addressed, and patient-identified prioritization of treatment goals. This includes an understanding of health literacy, cognitive function, goals of care (e.g., relief of symptoms, overall function), decisional self-efficacy etc. | Prioritizing volume management and dyspnea reduction over phosphate control; exploring anxieties regarding stroke and other cardiovascular event concerns in individuals with nonindicated long-term anticoagulant use. |
| Social | Assess caregiver and other loved ones’ effect on medication decision making, which may manifest as gatekeeping (e.g., concerns by family members regarding discontinuation of certain medications); assess other social support concerns and other social responsibilities (e.g., caring for another family member), which may limit time and opportunities for self-care. Family and other loved ones may need to serve as partners in deprescribing plans, while centering patient values and priorities in this process. | Concerns among caregivers that deprescribing agents such as sleep aids etc. will increase their caregiving needs. |
| Financial | Carefully assess costs of medications in the context of health insurance coverage and access including out of pocket costs for nonprescription medications are important provide reassurance that deprescribing should not be driven exclusively by cost-reduction incentives. | Consider when Tums could be safely substituted for more expensive phosphorous binders. |
| Physical | Assess frailty, changes in dexterity, vision, cognition, and the challenge of taking certain medications (e.g., those more complex to administer including injectables) is an important consideration among older adults. This also may include considerations of how changes in dexterity or memory may impair the ability to adhere to medications before and after dialysis or meals. | Considering prepackaged medications for each day of week. |
These five determinants of deprescribing were obtained from the deprescribing rainbow, a conceptual framework on the importance of patient context in deprescribing (76).
The final step is to deprescribe the medication(s) and provide adequate support to ensure the shared decision plan is executed. Support is demonstrated through providing patient-friendly instructions for medication tapering, education about potential withdrawal symptoms, a plan for alternative medications, and/or nonpharmacological approaches to symptom management (70,79). Similar to time-limited dialysis trials (80), deprescribing is time limited because a patient who does not achieve explicit goal during a deprescribing trial (e.g., lower dose provides symptom relief) can resume the original dose. Therefore, patient support includes close monitoring and resuming medication if intolerance occurs. Further, a clinician should notify the patient’s pharmacy and other clinicians to ensure medication(s) not be refilled or prescribed again. Table 3 includes deprescribing resources at the point of care, which clinicians and patients can use to evaluate and target medications for deprescribing, engage in shared decision making, and provide deprescribing support. Although most resources are not tailored for older adults with kidney disease, these tools can be extrapolated to this population.
Table 3.
Deprescribing resources at point of care
| Tool | Tool Platform And Population | Key Components | Website (If Available) |
| Deprescribing algorithms for patients on hemodialysis (109) | Designed for individuals on dialysis of all ages | Nine medication specific deprescribing algorithms provided for individuals on hemodialysis; these are medication specific and patient centered (e.g., loop diuretics, α-1 blockers, statins, benzodiazepines, metoclopramide, and gabapentinoids) | |
| MedStopper (110) | Web-based platform to aid clinicians and patients | Provides guidance via an ordered system to prioritize deprescribing on the basis of drug effect on symptoms, future effect on illness, and likelihood of causing harm Provides tapering suggestions and guidance regarding withdrawal system |
https://medstopper.com/about.php |
| Deprescribing.org | Older adults >65 and older Web-based platform |
Includes deprescribing guide for antihyperglycemics specifically, including A1C goal setting, and recommended deprescribing strategy and monitoring | https://deprescribing.org/wp-content/uploads/2017/11/AHG-deprescribing-algorithms-2017-English.pdf |
| Goal-directed medication review electronic decision support system (111) | Older adults Web-based platform |
A goal-directed medication review electronic decision support tool that identifies a patient’s specific deprescribing report on the basis of goals of care attitudes, and drug burden Access a goals of care management tool (videos and guide) to engage in shared decision-making regarding goals and priorities Embeds the drug burden index and the revised patients’ attitudes toward deprescribing questionnaire |
https://gmedss.com/landing |
| Medsafer | Older patients | An electronic deprescribing tool to improve safety Provides personalized screening of history and medications; guides physicians and pharmacists in safe and successful deprescribing using criteria to identify PIMS in hospitalized older adults Includes links to educational brochures regarding deprescribing (e.g., opiates, proton pump inhibitors, sedatives, sulfonylurea) |
https://www.medsafer.org/study-protocol-le-protocole-detude |
| Primary health Tasmania deprescribing resources | Older adult patients | Website includes principles of deprescribing and medication guides (e.g., bisphosphonates, antihypertensives, antihyperglycemics, proton pump inhibitors, statin, benzodiazepines) Contains links to videos that can aid deprescribing |
https://www.primaryhealthtas.com.au/resources/deprescribing-resources/
https://www.primaryhealthtas.com.au/for-health-professionals/programs/managing-medicines/ |
| PIMS Plus | All ages | Website with ability to search by medication to identify medication information for consumers and guidance regarding when to avoid use Provides patients a survey to help determine the benefit of a medication review |
https://www.pimsplus.org/ |
A1c, hemoglobin A1c; PIMS, potentially inappropriate medications.
Challenges of Deprescribing
Despite its merits, deprescribing may be challenged by patients and their caregivers, the care team, and system level barriers that include fragmentation of care (24,81–83). In a systematic review, major patient-level barriers to deprescribing included lack of support, time, or guidance from clinicians regarding the deprescribing decision, feeling a sense of pressure to continue taking the medication as prescribed, prior negative experiences from deprescribing, and generalized worries regarding coping, and other implications of deprescribing (including fear of withdrawal or symptom relapse) (84). Patients also may have concerns about changing a medication used long-term, discussing their goals of care and life expectancy, and/or asking their clinician questions about the decision to deprescribe.
Clinician-level challenges include limited understanding regarding deprescribing and PIMs, inertia to act on the basis of low perceived value of deprescribing, low self-efficacy, and low feasibility of deprescribing (85). Clinician barriers also include managing alternate goals during the patient encounter (e.g., achieving glycemic goals to meet a metric or guideline), challenges communicating with other clinical team members, inadequate time and training on how to approach deprescribing, and a lack of clear evidence-based guidelines (85,86). Conflicts between the role of the pharmacist, primary care physician, and specialists may also impair deprescribing—this may be especially challenging if there is not an interoperable electronic health record (EHR) for communicating questions or concerns about medications (81,85).
System-level challenges include (1) limited resources (e.g., time and administrative support), (2) the absence of interoperable EHRs, and (3) the absence of policies promoting individualized medication management. With performance metrics not accounting for goals of care and tailored shared decision-making among older adults with kidney disease (e.g., guidelines for tight BP and glucose control), clinicians are more likely to make decisions poorly individualized to the needs, risks, and benefits for patients (81,85).
Despite these barriers, several potential solutions exist. At the patient level, shared decision-making tools may aid discussions about the appropriateness and benefits of deprescribing and enhance buy-in. Patients and their caregivers should be empowered through educational efforts to discuss concerns specific to medications identified for deprescribing. In clinics, there can be a structured approach to identifying patients eligible for deprescribing with clearly defined roles for each clinician involved (nurse, physician, pharmacist). At a system level, increasing financial incentives to enhance multidisciplinary collaboration (e.g., with specific roles and reimbursement incentives for community pharmacists and clinicians) for deprescribing efforts and increasing the interoperability of EHRs may reduce barriers. This should be coupled with renewed performance metrics that incentivize (1) tailored deprescribing (versus inappropriately penalizing deprescribing statins and other agents with uncertain efficacy) and (2) medication review at key care transitions.
Recommended Areas for Research
Nephrology clinicians need more evidence to guide deprescribing efforts; therefore, there is a need for additional pharmacoepidemiologic studies and consensus methods to identify potentially harmful medications and how to deprescribe them. Clinical trials are needed to assess the effect of deprescribing on hospitalizations and patient-reported outcomes, such as health-related quality of life, in patients across the continuum of kidney disease (87). Last, there is a need for observational studies to identify patient factors that may increase risk associated with specific medications.
With the growing number of older adults with kidney disease, the prevalence of medication-related problems is likely to increase. These medication-related problems are not limited to altered kidney clearance. Medication-related problems can also develop from intensive management of chronic conditions, use of PIMs, and patient challenges following complex medication regimens. Deprescribing could improve quality of life among older adults with kidney disease while minimizing pill burden and risk of harm, including hospitalizations. We are conducting studies to inform best practices to overcome challenges to deprescribing, identify risk attributable to certain medications in older adults with kidney disease, and demonstrate efficacy of deprescribing in this population. Although additional research is needed, this review provides a framework and resources for nephrology clinicians to proactively consider deprescribing in practice.
Disclosures
D. Mohottige report s having an ownership interest through their owning minimal stocks in Gilead and Pfizer totaling <$5,000; and reports other interests/relationships as a National Kidney Foundatoin Health Equity Advisory Committee member. R.K. Hall reports consultancy agreements with Bayer, Reata Pharmaceuticals, Tavere Pharmaceuticals, and United Health Group; and reports being a scientific advisor or member of the CJASN and Journal of American Geriatrics Society editorial boards. The remaining author has nothing to disclose.
Funding
This work is supported in part by the National Institute on Aging award number K76AG059930 and the American Society of Nephrology Foundation for Kidney Research (to R. Hall) and the Duke Center for Research to Advance Healthcare Equity (REACH Equity) (to D. Mohottige), which is supported by the National Institute on Minority Health and Health Disparities under award number U54MD012530.
Acknowledgments
The authors acknowledge Dr. Donna Crabtree for assistance with manuscript preparation.
Footnotes
Podcast available at: https://www.asn-online.org/media/podcast/K360/2021_09_30_KID0001942021.mp3
Author Contributions
R. Hall and D. Mohottige wrote the original draft; and all authors conceptualized the study, were responsible for the methodology, and reviewed and edited the manuscript.
References
- 1.Roux-Marson C, Baranski JB, Fafin C, Exterman G, Vigneau C, Couchoud C, Moranne O, Investigators PSPA: Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr 20: 87, 2020. 10.1186/s12877-020-1485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardone KE, Bacchus S, Assimon MM, Pai AB, Manley HJ: Medication-related problems in CKD. Adv Chronic Kidney Dis 17: 404–412, 2010. 10.1053/j.ackd.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Gallieni M, Cancarini G: Drugs in the elderly with chronic kidney disease: Beware of potentially inappropriate medications. Nephrol Dial Transplant 30: 342–344, 2015. 10.1093/ndt/gfu191 [DOI] [PubMed] [Google Scholar]
- 4.Jano E, Aparasu RR: Healthcare outcomes associated with Beers’ criteria: A systematic review. Ann Pharmacother 41: 438–447, 2007. 10.1345/aph.1H473 [DOI] [PubMed] [Google Scholar]
- 5.Fick DM, Mion LC, Beers MH, L Waller J: Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health 31: 42–51, 2008. 10.1002/nur.20232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beadles CA, Voils CI, Crowley MJ, Farley JF, Maciejewski ML: Continuity of medication management and continuity of care: Conceptual and operational considerations. SAGE Open Med 2: 2050312114559261, 2014. 10.1177/2050312114559261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battistella M, Ng P: Addressing polypharmacy in outpatient dialysis units. Clin J Am Soc Nephrol 16: 144–146, 2020. 10.2215/CJN.05270420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen PV, Spinelli C: Prescribing cascade in an elderly woman. Can Pharm J 149: 122–124, 2016. 10.1177/1715163516640811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P: Chronic kidney disease and premature ageing. Nat Rev Nephrol 10: 732–742, 2014. 10.1038/nrneph.2014.185 [DOI] [PubMed] [Google Scholar]
- 10.Thompson W, Farrell B: Deprescribing: What is it and what does the evidence tell us? Can J Hosp Pharm 66: 201–202, 2013. 10.4212/cjhp.v66i3.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triantafylidis LK, Hawley CE, Perry LP, Paik JM: The role of deprescribing in older adults with chronic kidney disease. Drugs Aging 35: 973–984, 2018. 10.1007/s40266-018-0593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott S, Wright DJ, Bhattacharya D: The role of behavioural science in changing deprescribing practice. Br J Clin Pharmacol 87: 39–41, 2021. 10.1111/bcp.14595 [DOI] [PubMed] [Google Scholar]
- 13.Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD: Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol 78: 738–747, 2014. 10.1111/bcp.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher PF, O’Connor MN, O’Mahony D: Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 89: 845–854, 2011. 10.1038/clpt.2011.44 [DOI] [PubMed] [Google Scholar]
- 15.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C: Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: The D-PRESCRIBE Randomized Clinical Trial. JAMA 320: 1889–1898, 2018. 10.1001/jama.2018.16131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S: Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Intern Med 174: 890–898, 2014. 10.1001/jamainternmed.2014.949 [DOI] [PubMed] [Google Scholar]
- 17.Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA: Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother 2: 257–264, 2004. 10.1016/j.amjopharm.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Tjia J, Velten SJ, Parsons C, Valluri S, Briesacher BA: Studies to reduce unnecessary medication use in frail older adults: A systematic review. Drugs Aging 30: 285–307, 2013. 10.1007/s40266-013-0064-1 [DOI] [PubMed] [Google Scholar]
- 19.Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD: The feasibility and effect of deprescribing in older adults on mortality and health: A systematic review and meta-analysis. Br J Clin Pharmacol 82: 583–623, 2016. 10.1111/bcp.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomfield HE, Greer N, Linsky AM, Bolduc J, Naidl T, Vardeny O, MacDonald R, McKenzie L, Wilt TJ: Deprescribing for community-dwelling older adults: A systematic review and meta-analysis. J Gen Intern Med 35: 3323–3332, 2020. 10.1007/s11606-020-06089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kua CH, Mak VSL, Huey Lee SW: Health outcomes of deprescribing interventions among older residents in nursing homes: A systematic review and meta-analysis. J Am Med Dir Assoc 20: 362–372.e11, 2019. 10.1016/j.jamda.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Woodford HJ, Fisher J: New horizons in deprescribing for older people. Age Ageing 48: 768–775, 2019. 10.1093/ageing/afz109 [DOI] [PubMed] [Google Scholar]
- 23.Reeve E, Wolff JL, Skehan M, Bayliss EA, Hilmer SN, Boyd CM: Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med 178: 1673–1680, 2018. 10.1001/jamainternmed.2018.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre C, McQuillan R, Bell C, Battistella M: Targeted deprescribing in an outpatient hemodialysis unit: A quality improvement study to decrease polypharmacy. Am J Kidney Dis 70: 611–618, 2017. 10.1053/j.ajkd.2017.02.374 [DOI] [PubMed] [Google Scholar]
- 25.Clemens KK, O’Regan N, Rhee JJ: Diabetes management in older adults with chronic kidney disease. Curr Diab Rep 19: 11, 2019. 10.1007/s11892-019-1128-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson RTCE, Harris ND, Ireland RH, Lee S, Newman C, Heller SR: Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 52: 1469–1474, 2003. 10.2337/diabetes.52.6.1469 [DOI] [PubMed] [Google Scholar]
- 27.Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC: Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 4: 1121–1127, 2009. 10.2215/CJN.00800209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ: The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 17: 673–680, 2011 [PubMed] [Google Scholar]
- 29.Lega IC, Campitelli MA, Austin PC, Na Y, Zahedi A, Leung F, Yu C, Bronskill SE, Rochon PA, Lipscombe LL: Potential diabetes overtreatment and risk of adverse events among older adults in Ontario: A population-based study. Diabetologia 64: 1093–1102, 2021. 10.1007/s00125-020-05370-7 [DOI] [PubMed] [Google Scholar]
- 30.Hodge M, McArthur E, Garg AX, Tangri N, Clemens KK: Hypoglycemia incidence in older adults by estimated GFR. Am J Kidney Dis 70: 59–68, 2017. 10.1053/j.ajkd.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 31.Kidney Disease : Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 98:S1–S115, 2020 [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association : 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes 2019. Diabetes Care 42[Suppl 1]: S90–S102, 2019. 10.2337/dc19-S009 [DOI] [PubMed] [Google Scholar]
- 33.Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, Knoll GA, Muntner P, Pecoits-Filho R, Sarnak MJ, Tobe SW, Tomson CRV, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli M, Cheung M, Earley A, Mann JFE: Executive summary of the KDIGO (Kidney Disease Improving Global Outcomes 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO_BP_Exec_Summary_final.pdf. Accessed March 1, 2021 [Google Scholar]
- 34.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015. 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang AR, Lóser M, Malhotra R, Appel LJ: Blood pressure goals in patients with CKD: A review of evidence and guidelines. CJSAN 14: 161–169 2019. 10.2215/CJN.07440618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H: Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 174: 588–595, 2014. 10.1001/jamainternmed.2013.14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss J, Freeman M, Low A, Fu R, Kerfoot A, Paynter R, Motu'apuaka M, Kondo K, Kansagara D: Benefits and Harms of Intensive Blood Pressure Treatment in Adults Aged 60 Years or Older: A Systematic Review and Meta-analysis. Ann Intern Med. 2017. Mar 21;166(6):419–429. doi: 10.7326/M16-1754. Epub 2017 Jan 17. Erratum in: Ann Intern Med. 2018 Jan 16;168(2):159. Erratum in: Ann Intern Med. 2018 Apr 3;168(7):529–530. PMID: 28114673. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard JP, Burt J, Lown M, Temple E, Lowe R, Fraser R, Allen J, Ford GA, Heneghan C, Hobbs FDR, Jowett S, Kodabuckus S, Little P, Mant J, Mollison J, Payne RA, Williams M, Yu L-M, McManus RJ; OPTIMISE Investigators: Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: The OPTIMISE Randomized Clinical Trial. JAMA 323: 2039–2051, 2020. 10.1001/jama.2020.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.2019 American Geriatrics Society Beers Criteria® Update Expert Panel : American Geriatrics Society 2019 Updated AGS Beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 67: 674–694, 2019. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 40.O’Mahony D: STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: Origin and progress. Expert Rev Clin Pharmacol 13: 15–22, 2020. 10.1080/17512433.2020.1697676 [DOI] [PubMed] [Google Scholar]
- 41.Holt S, Schmiedl S, Thürmann PA: Potentially inappropriate medications in the elderly: The PRISCUS list. Dtsch Arztebl Int 107: 543–551, 2010. 10.3238/arztebl.2010.0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark CM, Shaver AL, Aurelio LA, Feuerstein S, Wahler RG Jr, Daly CJ, Jacobs DM: Potentially inappropriate medications are associated with increased healthcare utilization and costs. J Am Geriatr Soc 68: 2542–2550, 2020. 10.1111/jgs.16743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tesfaye WH, Wimmer BC, Peterson GM, Castelino RL, Jose MD, McKercher C, Zaidi STR: The effect of hospitalization on potentially inappropriate medication use in older adults with chronic kidney disease. Curr Med Res Opin 35: 1119–1126, 2019. 10.1080/03007995.2018.1560193 [DOI] [PubMed] [Google Scholar]
- 44.Molnar AO, Bota S, Jeyakumar N, McArthur E, Battistella M, Garg AX, Sood MM, Brimble KS: Potentially inappropriate prescribing in older adults with advanced chronic kidney disease. PLoS One 15: e0237868, 2020. 10.1371/journal.pone.0237868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009. 10.2215/CJN.00430109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondo N, Nakamura F, Yamazaki S, Yamamoto Y, Akizawa T, Akiba T, Saito A, Kurokawa K, Fukuhara S: Prescription of potentially inappropriate medications to elderly hemodialysis patients: Prevalence and predictors. Nephrol Dial Transplant 30: 498–505, 2015. 10.1093/ndt/gfu070 [DOI] [PubMed] [Google Scholar]
- 47.Parker K, Aasebø W, Stavem K: Potentially inappropriate medications in elderly haemodialysis patients using the STOPP criteria. Drugs Real World Outcomes 3: 359–363, 2016. 10.1007/s40801-016-0088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf E, Kothari NR, Roberts JK, Sparks MA: Baclofen toxicity in kidney disease. Am J Kidney Dis 71: 275–280, 2018. 10.1053/j.ajkd.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 49.Winkelmayer WC, Mehta J, Wang PS: Benzodiazepine use and mortality of incident dialysis patients in the United States. Kidney Int 72: 1388–1393, 2007. 10.1038/sj.ki.5002548 [DOI] [PubMed] [Google Scholar]
- 50.Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA: Examining the evidence: A systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med 26: 783–790, 2011. 10.1007/s11606-010-1629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kutner JS, Blatchford PJ, Taylor DH Jr, Ritchie CS, Bull JH, Fairclough DL, Hanson LC, LeBlanc TW, Samsa GP, Wolf S, Aziz NM, Currow DC, Ferrell B, Wagner-Johnston N, Zafar SY, Cleary JF, Dev S, Goode PS, Kamal AH, Kassner C, Kvale EA, McCallum JG, Ogunseitan AB, Pantilat SZ, Portenoy RK, Prince-Paul M, Sloan JA, Swetz KM, Von Gunten CF, Abernethy AP: Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med 175: 691–700, 2015. 10.1001/jamainternmed.2015.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson J: Deprescribing in palliative care. Clin Med (Lond) 19: 311–314, 2019. 10.7861/clinmedicine.19-4-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mavrakanas TA, Chatzizisis YS, Gariani K, Kereiakes DJ, Gargiulo G, Helft G, Gilard M, Feres F, Costa RA, Morice M-C, Georges J-L, Valgimigli M, Bhatt DL, Mauri L, Charytan DM: Duration of dual antiplatelet therapy in patients with CKD and drug-eluting stents: A meta-analysis. CJSAN 14: 810–822, 2019. 10.2215/CJN.12901018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehling M: Multimorbidity and polypharmacy: How to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: Fit for the aged. J Am Geriatr Soc 57: 560–561, 2009. 10.1111/j.1532-5415.2009.02131.x [DOI] [PubMed] [Google Scholar]
- 55.Dong Z, Wang Z, Yang K, Liu Y, Gao W, Chen W: Tamsulosin versus terazosin for benign prostatic hyperplasia: A systematic review. Syst Biol Reprod Med 55: 129–136, 2009. 10.3109/19396360902833235 [DOI] [PubMed] [Google Scholar]
- 56.Langeard A, Saillant K, Charlebois Cloutier E, Gayda M, Lesage F, Nigam A, Bherer L, Fraser SA: Association between statin use and balance in older adults. Int J Environ Res Public Health 17: 4662, 2020. 10.3390/ijerph17134662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The USRDS (US Renal Data System) dialysis morbidity and mortality study: Wave 2. Am J Kidney Dis 30[Suppl 1]: S67–S85, 1997. 10.1016/S0272-6386(97)90181-5 [DOI] [PubMed] [Google Scholar]
- 58.Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS: Medication prescribing patterns in ambulatory haemodialysis patients: Comparisons of USRDS (United States Renal Data System) to a large not-for-profit dialysis provider. Nephrol Dial Transplant 19: 1842–1848, 2004. 10.1093/ndt/gfh280 [DOI] [PubMed] [Google Scholar]
- 59.St Peter WL: Management of polypharmacy in dialysis patients. Semin Dial 28: 427–432, 2015. 10.1111/sdi.12377 [DOI] [PubMed] [Google Scholar]
- 60.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009. 10.2215/CJN.00290109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George J, Phun YT, Bailey MJ, Kong DC, Stewart K: Development and validation of the Medication Regimen Complexity Index. Ann Pharmacother 38: 1369–1376, 2004. 10.1345/aph.1D479 [DOI] [PubMed] [Google Scholar]
- 62.Willson MN, Greer CL, Weeks DL: Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother 48: 26–32, 2014. 10.1177/1060028013510898 [DOI] [PubMed] [Google Scholar]
- 63.Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K: Medication regimen complexity and number of medications as factors associated with unplanned hospitalizations in older people: A population-based cohort study. J Gerontol A Biol Sci Med Sci 71: 831–837, 2016. 10.1093/gerona/glv219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marienne J, Laville SM, Caillard P, Batteux B, Gras-Champel V, Masmoudi K, Choukroun G, Liabeuf S: Evaluation of changes over time in the drug burden and medication regimen complexity in ESRD patients before and after renal transplantation. Kidney Int Rep 6: 128–137, 2020. 10.1016/j.ekir.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardone KE, Manley HJ, Grabe DW, Meola S, Hoy CD, Bailie GR: Quantifying home medication regimen changes and quality of life in patients receiving nocturnal home hemodialysis. Hemodial Int 15: 234–242, 2011. 10.1111/j.1542-4758.2011.00539.x [DOI] [PubMed] [Google Scholar]
- 66.Murray AM: Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis 15: 123–132, 2008. 10.1053/j.ackd.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wald NJ, Law MR: A strategy to reduce cardiovascular disease by more than 80%. BMJ 326: 1419, 2003. 10.1136/bmj.326.7404.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frament J, Hall RK, Manley HJ: Medication reconciliation: The foundation of medication safety for patients requiring dialysis. Am J Kidney Dis 76: 868–876, 2020. 10.1053/j.ajkd.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladda MA, Goralski KB: The effects of CKD on cytochrome P450-mediated drug metabolism. Adv Chronic Kidney Dis 23: 67–75, 2016. 10.1053/j.ackd.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 70.Scott I, Anderson K, Freeman C: Review of structured guides for deprescribing. Eur J Hosp Pharm Sci Pract 24: 51–57, 2017. 10.1136/ejhpharm-2015-000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt RJ, Landry DL, Cohen L, Moss AH, Dalton C, Nathanson BH, Germain MJ: Derivation and validation of a prognostic model to predict mortality in patients with advanced chronic kidney disease. Nephrol Dial Transplant 34: 1517–1525, 2019. 10.1093/ndt/gfy305 [DOI] [PubMed] [Google Scholar]
- 72.Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, Dalton C, MacKay K, Pellegrino B, Anantharaman P, Schmidt R: Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 3: 1379–1384, 2008. 10.2215/CJN.00940208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW: Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914–919, 1963. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 74.Lawton MP, Brody EM: Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179–186, 1969. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 75.Schell JO, Cohen RA: A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol 9: 2014–2021, 2014. 10.2215/CJN.02190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd A, Jansen J, Colvin J, McLachlan AJ: The deprescribing rainbow: A conceptual framework highlighting the importance of patient context when stopping medication in older people. BMC Geriatr 18: 295, 2018. 10.1186/s12877-018-0978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jhaveri A, Sibley RA, Spatz ES, Dodson J: Aspirin, statins, and primary prevention: Opportunities for shared decision making in the face of uncertainty. Curr Cardiol Rep 23: 67, 2021. 10.1007/s11886-021-01499-y [DOI] [PubMed] [Google Scholar]
- 78.Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC: Tools to promote shared decision making in serious illness: A systematic review. JAMA Intern Med 175: 1213–1221, 2015. 10.1001/jamainternmed.2015.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanlon JT, Semla TP, Schmader KE: Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc 63: e8–e18, 2015. 10.1111/jgs.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scherer JS, Holley JL: The role of time-limited trials in dialysis decision making in critically ill patients. Clin J Am Soc Nephrol 11: 344–353, 2016. 10.2215/CJN.03550315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerlach N, Michiels-Corsten M, Viniol A, Schleef T, Junius-Walker U, Krause O, Donner-Banzhoff N: Professional roles of general practitioners, community pharmacists and specialist providers in collaborative medication deprescribing: A qualitative study. BMC Fam Pract 21: 183, 2020. 10.1186/s12875-020-01255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huffmyer MJ, Keck JW, Harrington NG, Freeman PR, Westling M, Lukacena KM, Moga DC: Primary care clinician and community pharmacist perceptions of deprescribing. J Am Geriatr Soc 69: 1686–1689, 2021. 10.1111/jgs.17092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayward S, Hole B, Denholm R, Duncan P, Morris JE, Fraser SDS, Payne RA, Roderick P, Chesnaye NC, Wanner C, Drechsler C, Postorino M, Porto G, Szymczak M, Evans M, Dekker FW, Jager KJ, Caskey FJ; EQUAL Study investigators: International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: Results from the European Quality study. Nephrol Dial Transplant 36: 503–511, 2021. 10.1093/ndt/gfaa064 [DOI] [PubMed] [Google Scholar]
- 84.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD: Patient barriers to and enablers of deprescribing: A systematic review. Drugs Aging 30: 793–807, 2013. 10.1007/s40266-013-0106-8 [DOI] [PubMed] [Google Scholar]
- 85.Linsky A, Zimmerman KM: Provider and system-level barriers to deprescribing: Interconnected problems and solutions. Public Policy Aging Rep 28: 129–133, 2018. 10.1093/ppar/pry030 [DOI] [Google Scholar]
- 86.Linsky A, Gellad WF, Linder JA, Friedberg MW: Advancing the science of deprescribing: A novel comprehensive conceptual framework. J Am Geriatr Soc 67: 2018–2022, 2019. 10.1111/jgs.16136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mclaren S, Jhamb M, Unruh M: Using patient-reported measures to improve outcomes in kidney disease. Blood Purif 50: 649–654, 2021. 10.1159/000515640 [DOI] [PubMed] [Google Scholar]
- 88.de Boer IH, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T, Tandon N, Tuttle KR, Wanner C, Wilkens KG, Zoungas S, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli M, Cheung M, Earley A, Rossing P: Executive summary of the 2020 KDIGO (Kidney Disease Improving Global Outcomes) Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int 98: 839–848, 2020. 10.1016/j.kint.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 89.Rydberg T, Jönsson A, Røder M, Melander A: Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care 17: 1026–1030, 1994. 10.2337/diacare.17.9.1026 [DOI] [PubMed] [Google Scholar]
- 90.Knauf F, Chaknos CM, Berns JS, Perazella MA: Dabigatran and kidney disease: A bad combination. Clin J Am Soc Nephrol 8: 1591–1597, 2013. 10.2215/CJN.01260213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lutz J, Jurk K, Schinzel H: Direct oral anticoagulants in patients with chronic kidney disease: Patient selection and special considerations. Int J Nephrol Renovasc Dis 10: 135–143, 2017. 10.2147/IJNRD.S105771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A: Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 9: 2168–2175, 2011. 10.1111/j.1538-7836.2011.04498.x [DOI] [PubMed] [Google Scholar]
- 93.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr: Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: A systematic review. Ann Intern Med 166: 191–200, 2017. 10.7326/M16-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muanda FT, Weir MA, Bathini L, Blake PG, Chauvin K, Dixon SN, McArthur E, Sontrop JM, Moist L, Garg AX: Association of baclofen with encephalopathy in patients with chronic kidney disease. JAMA 322: 1987–1995, 2019. 10.1001/jama.2019.17725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muanda FT, Blake PG, Weir MA, Bathini L, Chauvin K, Dixon SN, McArthur E, Sontrop JM, Moist L, Kim RB, Garg AX: Association of baclofen with falls and fractures in patients with CKD [published online ahead of print February 10, 2021]. Am J Kidney Dis 2021. 10.1053/j.ajkd.2020.12.017 [DOI] [PubMed] [Google Scholar]
- 96.Mina D, Johansen KL, McCulloch CE, Steinman MA, Grimes BA, Ishida JH: Muscle relaxant use among hemodialysis patients: Prevalence, clinical indications, and adverse outcomes. Am J Kidney Dis 73: 525–532, 2019. 10.1053/j.ajkd.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 97.Chauvin KJ, Blake PG, Garg AX, Weir MA, Bathini L, Dixon SN, McArthur E, Sontrop JM, Moist L, Kim RB, Muanda FT: Baclofen has a risk of encephalopathy in older adults receiving dialysis. Kidney Int 98: 979–988, 2020. 10.1016/j.kint.2020.04.047 [DOI] [PubMed] [Google Scholar]
- 98.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol 13: 746–753, 2018. 10.2215/CJN.09910917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhan M, St Peter WL, Doerfler RM, Woods CM, Blumenthal JB, Diamantidis CJ, Hsu CY, Lash JP, Lustigova E, Mahone EB, Ojo AO, Slaven A, Strauss L, Taliercio JJ, Winkelmayer WC, Xie D, Fink JC; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Patterns of NSAIDs use and their association with other analgesic use in CKD. Clin J Am Soc Nephrol 12: 1778–1786, 2017. 10.2215/CJN.12311216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shavit L, Grenader T, Lifschitz M, Slotki I: Use of pregabalin in the management of chronic uremic pruritus. J Pain Symptom Manage 45: 776–781, 2013. 10.1016/j.jpainsymman.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 101.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol 29: 1970–1978, 2018. 10.1681/ASN.2018010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muzaale AD, Daubresse M, Bae S, Chu NM, Lentine KL, Segev DL, McAdams-DeMarco M: Benzodiazepines, codispensed opioids, and mortality among patients initiating long-term in-center hemodialysis. Clin J Am Soc Nephrol 15: 794–804, 2020. 10.2215/CJN.13341019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assimon MM, Flythe JE: Zolpidem versus trazodone initiation and the risk of fall-related fractures among individuals receiving maintenance hemodialysis. Clin J Am Soc Nephrol 16: 88–97, 2020. 10.2215/CJN.10070620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tuttle KR, Brosius FC 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, Perreault L, Rosas SE, Rossing P, Sola L, Vallon V, Wanner C, Perkovic V: SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: Report of a scientific workshop sponsored by the National Kidney Foundation. Diabetes 70: 1–16, 2021. 10.2337/dbi20-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oplinger M, Andrews CO: Nitrofurantoin contraindication in patients with a creatinine clearance below 60 mL/min: Looking for the evidence. Ann Pharmacother 47: 106–111, 2013. 10.1345/aph.1R352 [DOI] [PubMed] [Google Scholar]
- 106.ULTRAM ER : (tramadol HCI) Extended-Release Tablets [package insert]. Steinbach, MB, Canada: Valeant Pharmaceuticals International, Inc.; 2017 [Google Scholar]
- 107.TRICOR : (fenofibrate) tablet [package insert]. North Chicago, IL, U.S.A.: Abbott Laboratories; 2010 [Google Scholar]
- 108.Kaysen GA: Lipid-lowering therapy in CKD: Should we use it and in which patients. Blood Purif 43: 196–199, 2017. 10.1159/000452727 [DOI] [PubMed] [Google Scholar]
- 109.Lefebvre MJ, Ng PCK, Desjarlais A, McCann D, Waldvogel B, Tonelli M, Garg AX, Wilson JA, Beaulieu M, Marin J, Orsulak C, Lloyd A, McIntyre C, Feldberg J, Bohm C, Battistella M: Development and validation of nine deprescribing algorithms for patients on hemodialysis to decrease polypharmacy. Can J Kidney Health Dis 7: 2054358120968674, 2020. 10.1177/2054358120968674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassels A: ‘Can I stop even one of these pills?’ The development of a tool to make deprescribing easier. Eur J Hosp Pharm Sci Pract 24: 3–4, 2017. 10.1136/ejhpharm-2016-001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kouladjian O’Donnell L, Sawan M, Reeve E, Gnjidic D, Chen TF, Kelly PJ, Bell JS, Hilmer SN: Implementation of the Goal-directed Medication review Electronic Decision Support System (G-MEDSS)© into home medicines review: A protocol for a cluster-randomised clinical trial in older adults. [Published correction appears in BMC Geriatr 2020, 20: 378.] BMC Geriatr 20: 51, 2020. 10.1186/s12877-020-1442-2 [DOI] [PMC free article] [PubMed] [Google Scholar]



